Abstract
Purpose
Glioblastoma multiforme (GBM) is an aggressive adult brain tumor with a poor prognosis. One hallmark of GBM is the accumulation of immunosuppressive and tumor-promoting CD4+FoxP3+GITR+ regulatory T cells (Tregs). Here, we investigated the role of indoleamine 2,3 dioxygenase (IDO) in brain tumors and the impact on Treg recruitment.
Experimental Design
To determine the clinical relevance of IDO expression in brain tumors, we first correlated patient survival to the level of IDO expression from resected glioma specimens. We also used novel orthotopic and transgenic models of glioma to study how IDO affects Tregs. The impact of tumor-derived and peripheral IDO expression on Treg recruitment, GITR expression and long-term survival was determined.
Results
Downregulated IDO expression in glioma predicted a significantly better prognosis in patients. Co-incidently, both IDO -competent and -deficient mice showed a survival advantage bearing IDO-deficient brain tumors, when compared to IDO-competent brain tumors. Moreover, IDO-deficiency was associated with a significant decrease in brain-resident Tregs, both in orthotopic and transgenic mouse glioma models. IDO-deficiency was also associated with lower GITR expression levels on Tregs. Interestingly, the long-term survival advantage conferred by IDO-deficiency was lost in T cell-deficient mice.
Conclusions
These clinical and pre-clinical data confirm that IDO expression increases the recruitment of immunosuppressive Tregs which leads to tumor outgrowth. In contrast, IDO deficiency decreases Treg recruitment and enhances T cell-mediated tumor rejection. Thus, the data suggest a critical role for IDO-mediated immunosuppression in glioma and supports the continued investigation of IDO-Treg interactions in the context of brain tumors.
Keywords: immunosuppression, tryptophan, glioblastoma, metabolism, immunotherapy
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive form of primary brain cancer. Even after surgical resection, irradiation and chemotherapy, the median survival for patients with GBM remains at only 14.6 months, with 26% of patients alive after 2 years (1). Due to the invasiveness of GBM, it remains a significant challenge to resect all of the tumor cells during surgery. The incomplete surgical removal likely contributes to the high rate of relapse in patients. An additional factor that contributes to the poor prognosis in GBM is the potently immunosuppressive tumor environment. The induction and/or maintenance of immunosuppression in GBM is due in part to the infiltration and accumulation of the highly immunosuppressive regulatory T cells (Tregs; CD4+FoxP3+CD25+) (2–6).
The pathogenic role of Tregs in brain tumors has previously been reported by several independent groups (3, 5, 7–9). Tregs constitutively express high levels of the receptor, glucocorticoid-induced TNFR-related protein (GITR) (10), suppress the functions of antigen presenting cells (APCs) via the expression of immunosuppressive cytokines, interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) (11) and arise from thymus-derived (natural; nTreg) or induced (iTreg; converted from a CD4+FoxP3− conventional T cell) progenitor (12). Specific to GBM, Tregs accumulate at high levels (2, 7), universally express GITR (4) and are predominantly thymus-derived (5). Several factors have been implicated to be critical for recruiting Tregs to glioma including the Treg-recruiting chemokines, CCL2 and CCL22 (13), although there are other agents that may be involved (14). Moreover, while select chemokines control Treg recruitment, a ‘master’ central regulator orchestrating Treg infiltration and/or accumulation has yet to be implicated in the context of CNS malignancy.
Indoleamine 2,3 dioxygenase (IDO) is a cytoplasmic enzyme that catabolizes the essential amino acid, tryptophan, to the metabolite, kynurenine (15). In vitro, previous work has demonstrated that GBM cells can be induced to express IDO (16) and that this induction may be associated with tumorigenesis (17), though this had not been shown in vivo prior to this study. Moreover, IDO has been shown to potently inhibit T cell activation and/or proliferation (18), while also regulating the recruitment (19) expansion (20) and activation of Tregs (21) through an APC-dependent mechanism. Based on these observations, it is no surprise that dendritic cell-expressed IDO has been positively associated with promoting tumorigenesis in tumors that localize outside of the central nervous system (CNS) (22). However, given the immunospecialization of APCs in the brain (23), as well as previous evidence indicating that GBM cells express IDO (24), we hypothesized that IDO expression by glioma cells, rather than peripheral cells, would promote Treg accumulation and tumor outgrowth.
Herein, we present our comprehensive in vivo-based investigation demonstrating the role of IDO in brain tumors. We show that the downregulation of IDO in human glioma is associated with a significant survival advantage in patients. Using the GL261 orthotopic- and/or RasB8 transgenic- mouse glioma models, we also demonstrate the impact of IDO competency on tumor-infiltrating Tregs in WT and IDO-deficient (IDO−/−) mice bearing IDO -competent and -deficient brain tumors, in addition to the analysis of RasB8 mice with variable IDO competency. Orthotopically, we show that IDO-deficiency, specifically in tumor cells, is associated with a significant long-term survival advantage in immunocompetent mice, regardless of IDO expression in the periphery. This survival benefit is associated with a significant decrease in the level of brain tumor infiltrating Tregs. Similarly, the lack of IDO expression in RasB8 mice is also associated with decreased Treg recruitment to the brain, confirming that the effects of IDO expression on Treg recruitment are not exclusive to an orthotopic model. Furthermore, there is an association between the expression of tumor-derived IDO and GITR on infiltrating Tregs. Finally, we identify a contextual role for the effects of IDO on T cells since CD4-, CD8- and Rag1- deficient mouse models with IDO-deficient brain tumors lose the long-term survival advantage. This work advances our understanding of the role of IDO in human glioma and provides further understanding into the interaction between IDO and Tregs in the context of brain tumors.
Materials and Methods
Mice, procedures and murine glioma cell line
C57BL/6 (wild-type; Cat# 000664), IDO−/− (Cat# 005897), CD4−/− (Cat# 002663), CD8−/− (Cat# 002665) and Rag1−/− (Cat# 002216) mice were obtained from Jackson Laboratories (Bar Harbor, ME), maintained in the University of Chicago Carlson Barrier Facility and intracranially-injected between the ages of 6 and 8 weeks. Hemizygous transgenic RasB8 mice were obtained from Dr. Abjihit Guha (Hospital for Sick Children, Toronto, Canada), endogenously expressing a single allele of oncogenic V12HA-ras and maintained in our laboratory. The RasB8 mice were backcrossed to a wild-type (IDO+/+) or IDO-deficient (IDO−/−) background. RasB8 mice were analyzed at 5 months post-natal and/or when symptomatic onset occurred including abnormal fur coat, hunched posture, lack of normal motion/activity and weight loss. All mouse strains used in the included studies were on the C57BL/6 background and were provided autoclaved food pellets and water ad libitum. All surgical procedures were completed in accordance with NIH guidelines on the care and use of laboratory animals for research purposes. Mice were euthanized by cervical dislocation. GL261 cells were obtained from NCI Frederick National Tumor Repository Lab and cultured in DMEM supplemented with 10% fetal calf serum as well as streptomycin (100 mg/ml) and penicillin (100 U/ml) at 37°C in a humidified atmosphere of 95 % air/5 % CO2. No authentication of the cell line was performed. Details regarding the genetic modifications to the GL261 cell line are enclosed in the supplementary methods and Supplementary Figure 1. All cell culture products were purchased from Gibco Invitrogen (Carlsbad, CA). Intracranial injection procedures are described further in the supplementary methods.
Flow cytometry
T cell isolation and staining were performed as previously described (5, 6) (and detailed in the supplementary methods).
Immunofluorescence and Histology
IDO−/− mice were ic. 4 × 105 Vc or IDOkd GL261 cells, euthanized at 2 weeks post-injection, followed by brain extraction and flash freezing [in 62.5% n-Butyl Bromide (Fisher Scientific, Pittsburgh, PA) + 37.5% 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Frozen brains were sectioned on the Microm cryostat with vacutome system (Thermo Scientific, Walldorf, Germany) with a temperature of −24°C and immersed into Tissue Teck O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) at 8 μm intervals and thaw-mounted onto pre-cleaned SuperFrost slides (Fisher Scientific). Sections were post-fixed with 4% paraformaldehyde, blocked for non-specific staining with 10% bovine serum albumin (A4503; Sigma-Aldrich; Saint Louis, MO) in PBS for 1 hr. Sections were incubated overnight with mouse anti-GFAP alexa fluor 488 (131–17719; Invitrogen; Carlsbad, CA) and rat anti-CD3-AF647 (145-2C11; Ebioscience; San Diego, CA) in PBS with 1% BSA at 4°C. Sections were washed extensively (PBS), one drop of glycerol was added and the slides were cover-slipped. Images of antibody-stained sections were captured using the Sp5 Tandem Scanner 2-photon confocal microscope (Leica Microsystems, Buffalo Grove, IL) running LAS AF software (Leica Microsystems) using the argon and red NeHe laser lines. Fluorescent images were captured using the 20× objective. For details on the method of tumor quantification, see the supplementary methods.
Patient Datasets and Data Analysis
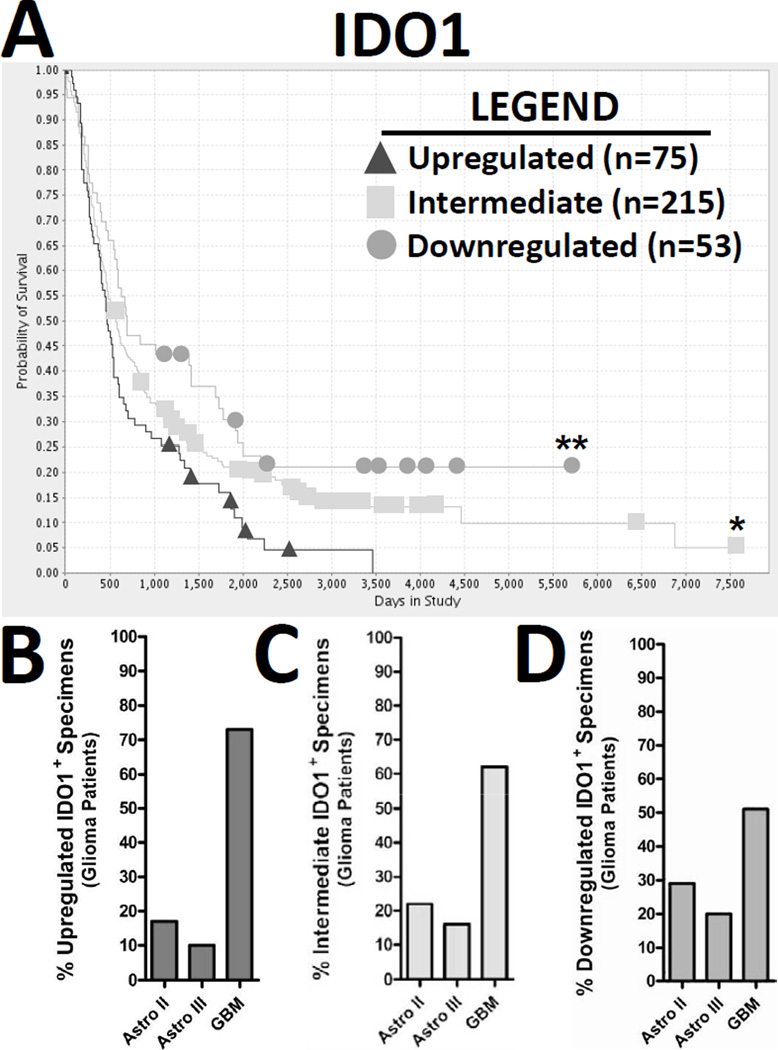
All glioma patient data was publicly available in de-identified form and obtained from the NCI Repository for Molecular Brain Neoplasia Data (REMBRANDT) database (https://caintegrator.nci.nih.gov/rembrandt/) (25), using the data available on April 18th, 2012. Thus, no Institutional Review Board approval was needed. There were 343 glioma patient samples that were correlated between IDO expression and overall survival. Although all of the patient samples are represented in the Kaplan-Meier Survival Plot, not all of the patients in the database were identified for glioma subtype. Thus, only those patients that were positively identified as astrocytoma or GBM were used in the analysis for calculating the percentage of patients with upregulated, intermediate or downregulated IDO expression. The differences between groups was analyzed by Log-Rank p-value. The graphs were created using Rembrandt data for Affymetrix probes 210029 using the Lowest Geometric Mean Intensity and associated survival data. Up- or down- regulation among glioma specimens refers to a 2-fold (or greater) change in IDO expression, when compared to specimens from non-glioma patients.
Statistical analysis
Survival curves were calculated according to the method of Kaplan–Meier. Overall survival is defined as the time from injection of GL261 cells to day 150 of the time course. The p-value was obtained by log-rank statistical analysis and was considered significant when p ≤ 0.05. For non-survival curves, data are presented as ± SEM and were analyzed by two-way ANOVA, one-way ANOVA or the 2-tailed Student’s t test and a p value ≤ 0.05 were considered significant. Post-hoc analyses were performed using the Newman-Keuls method for multi-comparison procedures. All analyses were performed using GraphPad Prism version 4.00 (GraphPad Software, Inc.).
Results
Upregulation of IDO in glioma is associated with a poor prognosis
Previous work has demonstrated that the upregulation of glioma-expressed tryptophan dioxygenase, an enzyme associated with mediating tryptophan catabolism, is positively correlated to an earlier time of death in patients (26). However, whether other enzymes that mediate tryptophan catabolism, such as IDO, have a similar correlation between patient survival and expression pattern has not been determined. Using the REpository of Molecular BRAin Neoplasia DaTa (REMBRANDT) and based on a sample size of data taken from 343 glioma patients, we analyzed the molecular expression levels for IDO in glioma specimens and correlated those data to patient survival (Fig. 1A). IDO expression was upregulated (triangles; n=75), intermediately-expressed (squares; n=215) and downregulated (circles; n=53) in 75, 215 and 53 patients, respectively. The overall survival of patients with upregulated-, intermediate- and downregulated- expression in glioma specimens was 24.9 ± 2.76, 34 ± 2.71 and 44.3 ± 6.21 months, respectively. Moreover, glioma upregulated for IDO was significantly correlated to an earlier average time of death (post-diagnosis), when compared to patients with intermediate (p<0.05)- and downregulated (p<0.005)- expressing glioma. To better understand whether the increased IDO expression in glioma was associated with a particular grade of astrocytoma, we also investigated the percentage of grade II, III and IV astrocytoma associated with upregulated-, intermediate- and downregulated- IDO expression. Figure 1B demonstrates that of the IDO-upregulated specimens, 17%, 10% and 73% of specimens were associated with astrocytoma grade II, III and IV, respectively. Figure 1C demonstrates that of the IDO-intermediately-expressed specimens, 22%, 16% and 62% of specimens were associated with astrocytoma grade II, III and IV, respectively. Figure 1D demonstrates that of the IDO-downregulated specimens, 29%, 20% and 50% were associated with astrocytoma grade II, III and IV, respectively. Further analysis correlating the absolute expression level of IDO with survival, age of diagnosis and Karnofsky score, among grade(s) II, III and IV astrocytoma, is presented in Supplementary Figure 2. Collectively, the data demonstrate that upregulated IDO expression in glioma predicts a poor prognosis in patients and that this expression level tends to predominate in high grade glioma; although it is not an absolute indicator of a GBM diagnosis.
Figure 1. The upregulation of IDO in glioma is associated with a decreased overall lifespan in patients.
Data was isolated from the Repository of Molecular Brain Neoplasia Data (REMBRANDT) and represents the analysis of 343 different glioma patients. (A) The Kaplan-Meier survival curve for IDO1 was plotted for upregulated (triangle), intermediate (square) and downregulated (circle) expression over time. (B) Upregulated, (C) intermediate and (D) downregulated IDO expression in glioma was correlated to the percentage of grade II (Astro II), grade III (Astro III) or glioblastoma multiforme [(GBM) grade IV astrocytoma]. *p < 0.05; **p < 0.005
Peripheral IDO expression neither affects survival nor T cell levels in brain tumors
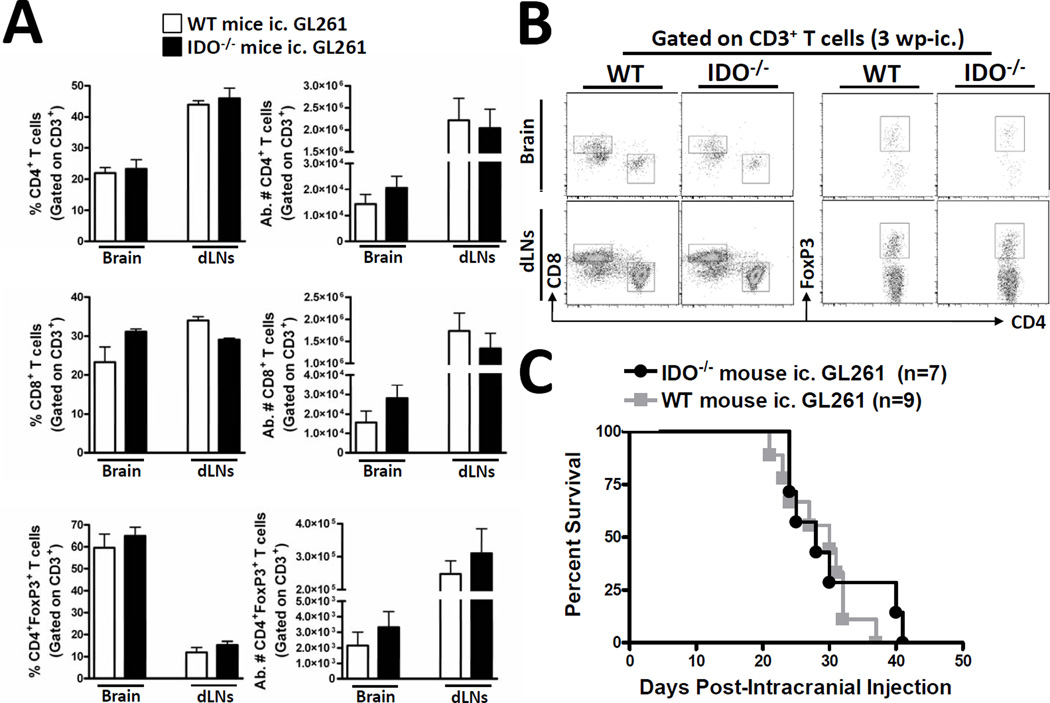
Previous work has demonstrated that IDO expression by dendritic cells (DCs) plays a critical role in regulating the anti-tumor immune response (22). Co-incidentally, it has also been demonstrated that CNS-resident glia express IDO under neuroinflammatory conditions (27–29). However, no investigation has determined whether peripheral IDO is a critical source of immune regulation in the context of brain tumors. To investigate whether non-tumor-derived IDO plays a critical role in regulating T cell levels in brain tumors, we intracranially-injected (ic.) GL261 cells into the brain of WT or IDO-deficient (IDO−/−) mice. Figure 2A,B indicates that peripheral (non-tumor-derived) IDO did not significantly affect the frequency nor absolute number of CD4+-, CD8+- or CD4+FoxP3+ regulatory- T cells in the brain or draining cervical lymph nodes (dLNs) at 3 weeks post-ic. To determine whether peripheral IDO expression is required to affect overall survival from brain tumors, we ic. GL261 cells into the brain of WT or IDO-deficient (IDO−/−) mice and found an average survival time of 30- and 28- days, respectively (Fig. 2C). Collectively, these data suggest that the peripheral expression of IDO neither affects the T cell response in the brain tumor and draining lymph nodes nor does it affect the overall survival.
Figure 2. Peripheral IDO deficiency has no effect on brain tumor-infiltrating T cell levels and survival.
Wild-type (WT) (white bars) or indoleamine 2,3 dioxygenase 1-deficient (IDO−/−; black bars) mice were intracranially-injected 4×105 normal GL261 cells. The frequency and absolute numbers of (A) total CD4+ T cells, CD8+ T cells and CD4+FoxP3+ regulatory T cells isolated from the brain and bilateral deep and superficial cervical draining lymph nodes (dLN) of tumor-bearing mice were analyzed at 3 weeks post-intracranial injection (wp-ic). All T cell populations were initially identified by the expression of CD3. (B) Representative flow cytometric plots demonstrate the gating strategy utilized for the identification of total CD3+CD4+, CD3+CD8+ and CD3+CD4+FoxP3+ T cells. Bar graphs in figure A are shown as mean ± SEM and are representative of two independent experiments (n = 3 – 5 mice/group). (C) The Kaplan-Meier curve represents mouse survival times over a time course of 50 days (n = 7 – 9 mice/group).
Tumor-derived IDO decreases survival and increases Treg recruitment in brain tumors
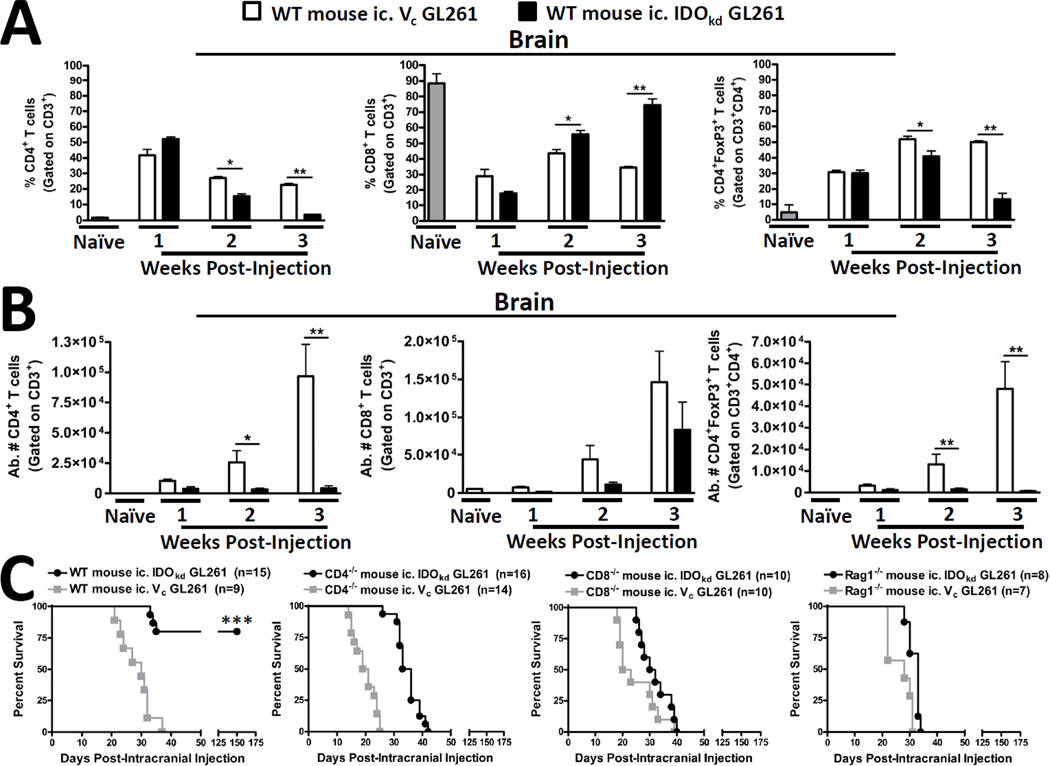
Since our previous data demonstrate that peripheral IDO is unimportant to the level of T cell infiltration in brain tumors, we next determined whether tumor-derived IDO was involved. We compared wild-type mice ic. with IDO-competent GL621 cells [transduced with a scrambled shRNA (vector control; Vc)] with IDO-deficient GL261 cells [transduced with IDO-specific shRNA (IDOkd)]. As shown in Figure 3A,B, the inhibition of IDO had profound effects on the levels of brain tumor-infiltrating T cell levels. Since the ratio of Tregs:effector T cells has been shown to reflect the overall likelihood of an effective anti-tumor response, we analyzed both the frequency of-, as well as the absolute numbers of- T cells in the brain. In the first week post-ic., there were no significant changes in the frequency and absolute cell number of T cells in the brain. However, by 2 weeks post-ic., the level of Tregs were significantly increased in the IDO competent, when compared to IDO-deficient brain tumors (p<0.05). This difference was further increased at 3 weeks post-ic. (p<0.01). However, this effect was highly localized since there were no significant differences in Treg levels in the tumor-draining lymph nodes or spleen. (Supplementary Figure 3). Also, in contrast to the increased level of Tregs in the brain, the frequency of CD8+ T cells decreased at 2 and 3 weeks post-ic. in IDO-competent, when compared to the IDO-deficient brain tumors. Collectively, these data suggest that the expression of IDO by brain tumors increases the ratio of immunosuppressive Tregs to cytotoxic CD8+ T cells.
Figure 3. The presence or absence of brain tumor-derived IDO contextually regulates T cell infiltration and long-term survival in T cell -competent, but not -deficient mice.
Wild-type (WT) mice were intracranially-injected (ic.) 4×105 GL261 cells transduced with -scrambled shRNA (vector control, Vc; white bars) or -shRNA specific to IDO (IDO knockdown, IDOkd; black bars). Naïve (control; grey bars) mice were used as a baseline of normal T cell levels in the brain. The (A) frequency and (B) absolute numbers of total CD4+ T cells, CD8+ T cells and CD4+FoxP3+ regulatory T cells isolated from the brain of tumor-bearing mice were analyzed from naïve, or at 1, 2 and 3 weeks post-injection. All T cell populations were initially identified by the expression of CD3. Bar graphs in figures A – B are shown as mean ± SEM and are representative of two independent experiments (n = 4 – 5 mice/group). Kaplan-Meier survival curves for (C) WT, CD4-deficient (CD4−/−; lack CD4+ T cells), CD8-deficient (CD8−/−; lack CD8+ T cells) and recombinase activating gene 1-deficient (Rag1−/−; lack all functional T or B cells) mice ic. 4×105 Vc or IDOkd GL261 cells. Mouse survival was analyzed for up to 150 days (n = 7 – 16 mice/group) and reflects the pooled data resulting from 2 – 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
To understand the functional importance of the T cell infiltration, we also studied the interaction between IDO and T cells using wild-type (WT), CD4-deficient (CD4−/−; CD4+ T cell-deficient), CD8-deficient (CD8−/−; CD8+ T cell-deficient) and recombinase activating gene 1-deficient (Rag1−/−; deficient for functional T and B cells) mice with IDO-competent and IDO-deficient brain tumors. Figure 3C demonstrates that WT (immunocompetent) mice with IDO-competent brain tumors have an average survival of 30 days post-ic., whereas most WT mice ic. with IDO-deficient tumors survive until 150 days post-ic. (when the study was terminated) (p<0.001). In contrast, T cell-deficient mice were incapable of mediating long-term survival. CD4−/− mice with IDO-competent and IDO-deficient brain tumors survive an average of 20 and 34.5 days post-ic., respectively. Similarly, CD8−/− mice with IDO-competent and IDO-deficient brain tumors survive an average of 21.5 and 31 days post-ic., respectively. Finally, Rag1−/− mice with IDO-competent and IDO-deficient brain tumors survive an average of 28 and 33 days post-ic., respectively. Taken together, these data demonstrate that brain tumor-derived IDO increases Tregs while simultaneously decreasing the frequency of CD8+ T cells which suppresses tumor rejection and leads to mortality. Conversely, the inhibition of IDO is associated with a reduction of Tregs and an increased frequency of CD8+ T cells leading to effective tumor rejection and long-term tumor immunity. Moreover, this protective immune-mediated response is critically-dependent on the coordinated response by both CD4+ and CD8+ T cells since it is abrogated by both types of T cell-deficiency.
The global loss of IDO significantly decreases Treg recruitment to the brain
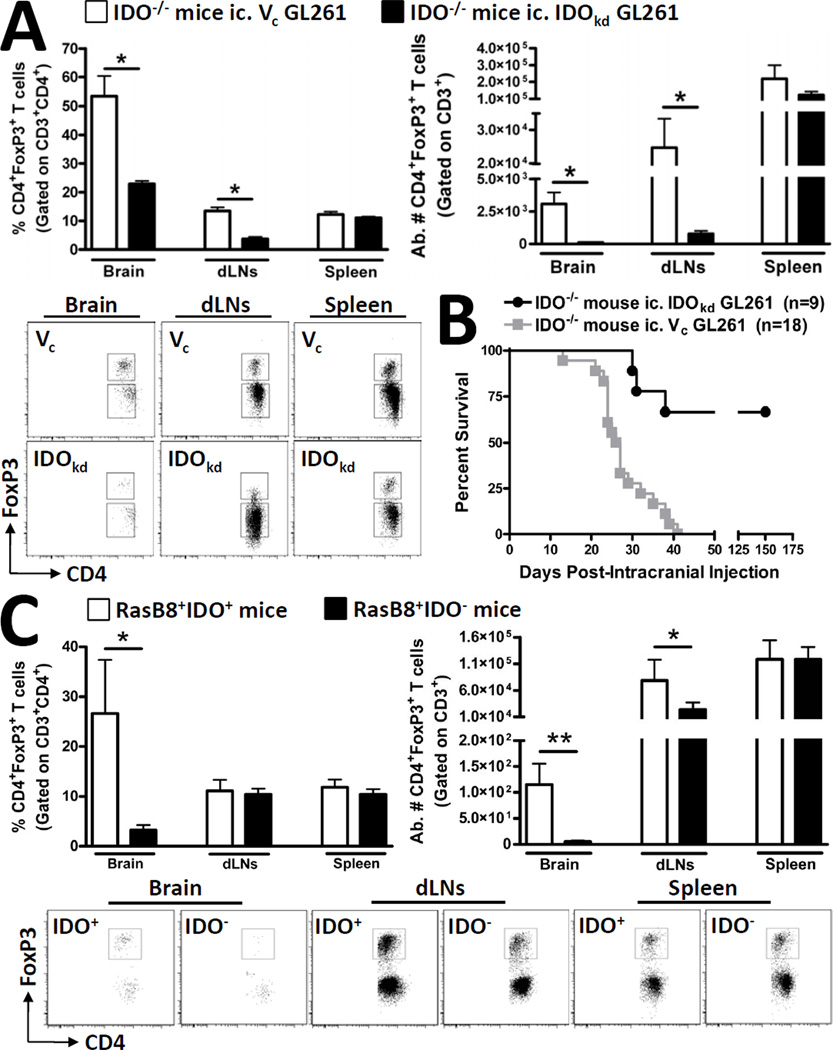
Although we established that tumor-derived IDO expression significantly affects the tumor-resident immune response, we sought to further determine the effects of global IDO-deficiency on Tregs, given the previous work demonstrating the critical impact of peripheral IDO-expression on immune-mediated tumor immunity (22). Similarly to what was observed in WT mice, IDO−/− mice had significantly fewer Tregs in IDO-deficient brain tumors, when compared to IDO-competent tumors (p<0.05) (Fig. 4A). Interestingly, we also observed that Tregs were also decreased in the cervical dLNs of IDO−/− with IDO-deficient brain tumors, which had not been observed in WT mice (Supplementary Figure 3) (p<0.05) or in the spleens of tumor-bearing mice. This specific interaction suggests that IDO expressing cells in tumor draining lymph nodes, as has previously been ascribed to APCs, play a critical role in Treg expansion outside of the tumor microenvironment (21), yet have little impact on Treg recruitment to the actual malignancy. Accordingly, IDO−/− mice with IDO-competent brain tumors survive an average of 26.5 days post-ic., whereas most IDO−/− mice with IDO-deficient brain tumors survive until 150 days post-ic. (when the study was terminated) (p<0.002) (Fig. 4B). Importantly, in vitro analysis of apoptosis and proliferation attributes demonstrated no differences between the Vc and IDOkd GL261 cell lines (Supplementary Figure 4). To assess whether the effects of global IDO-deficiency were restricted to an orthotopic tumor model, we also analyzed the RasB8 transgenic glioma model that was on an IDO-competent or IDO-deficient background. IDO competent RasB8 mice showed an increased Treg frequency (p<0.05) and absolute cell number (p<0.01) in the brain, when compared to IDO-deficient counterparts (Fig. 4C). Also in accordance with what was observed in the orthotopic model, Treg levels were decreased in the draining lymph nodes of IDO−/− RasB8 mice, confirming the critical role of IDO in this anatomical location; although this effect was not as exaggerated in the RasB8 transgenic model, when compared to the GL261 orthotopic model. Collectively, these data show a series of local interactions between IDO expression and Treg accumulation when comparing the brain tumor with the periphery. However, it is important to note that brain tumor expression of IDO overrides the peripheral contribution of IDO in so far as determining the level of brain-resident Tregs, as well as overall impact on survival.
Figure 4. Global IDO-deficiency affects Treg levels in brain tumors and draining lymph nodes without effects in the spleen.
Indoleamine 2,3 dioxygenase 1-deficient (IDO−/−) mice were intracranially-injected (ic.) 4×105 GL261 cells transduced with -scrambled shRNA (vector control, Vc; white bars) or -shRNA specific to IDO (IDO knockdown, IDOkd; black bars). The (A) frequency and absolute numbers of CD4+FoxP3+ regulatory T cells isolated from brain, ipsilateral deep and superficial cervical draining lymph nodes (dLN) and spleen of tumor-bearing mice were analyzed at 3 weeks post-intracranial injection (wp-ic). Representative flow cytometric plots demonstrate the gating strategy utilized for the identification of total CD3+CD4+FoxP3+ Tregs in the GL261-cell based orthotopic glioma mouse model. (B) The Kaplan-Meier curve represents mouse survival times over a time course of 150 days (n = 9 – 18 mice/group) and represent the combined data from 3 independent experiments. (C) RasB8 mice that were IDO -competent (white bars) or -deficient were analyzed for the frequency and (E) absolute numbers of Tregs. Representative flow cytometric plots demonstrate the gating strategy utilized for the identification of total CD3+CD4+FoxP3+ Tregs in the RasB8 transgenic glioma mouse model. For all experiments, Tregs were initially first identified for the co-expression of CD3 and CD4, prior to FoxP3. Bar graphs in figures A and C are shown as mean ± SEM and are representative of two independent experiments (n = 3 – 5 mice/group). *p < 0.05; **p < 0.01; ***p < 0.001.
IDO deficiency in brain tumors is associated with decreased T cell infiltration
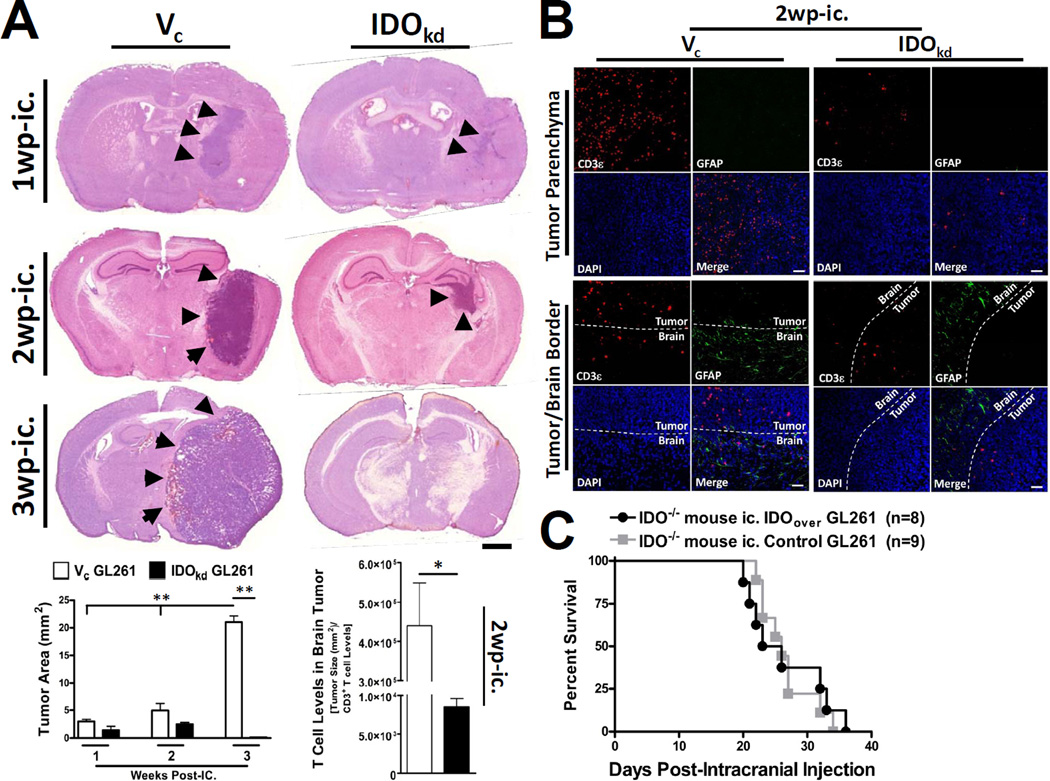
Our data demonstrate that T cell levels increase in IDO-competent brain tumors over time. However it was not clear whether T cell infiltration was simply due to a function of increasing tumor size or if IDO expression endowed the tumor with special T cell-recruiting capabilities. To determine this, we chose to quantify the average size of IDO competent and IDO-deficient brain tumors at 1, 2 and 3 weeks post-ic. As shown in Figure 5A, tumor size is significantly larger in IDO-competent, when compared to IDO-deficient brain tumors throughout the duration of the time course. Moreover, the IDO-competent tumor is significantly larger at 3wp-ic., when compared to all other groups and time points (p<0.001). However, when the tumor size was taken into consideration, the number of infiltrating T cells was still significantly increased in IDO-competent brain tumors, when compared to IDO-deficient tumors (p<0.01). To confirm this observation, IDO -competent and -deficient brain tumors were analyzed for infiltrating T cells at 2 weeks post-ic. in IDO−/− mice; a time point where the brain tumors were the most similar in size throughout the time course. As shown in Figure 5A,B, there was a robust level of T cell infiltration in IDO-competent, when compared to IDO-deficient brain tumors. Finally, to determine whether increasing the level of IDO expression would negatively impact survival from brain tumors, we compared the survival of mice ic. with (pEF6) control and IDO-over-expressing brain tumors cells and found no differences between overall lifespans (Fig. 5C). Collectively, these data suggest that IDO expression by brain tumors significantly impacts factors that recruit T cells. Additionally, these data imply that although IDO promotes tumorigenesis, increasing IDO expression beyond a certain threshold does not impact survival.
Figure 5. The expression of IDO in brain tumors is associated with increased T cell infiltration.
(A) Indoleamine 2,3 dioxygenase 1-deficient (IDO−/−) mice were intracranially-injected (ic.) 4×105 GL261 cells transduced with -scrambled shRNA (vector control, Vc; white bars) or -shRNA specific to IDO (IDO knockdown, IDOkd; black bars). Mice were euthanized at 1, 2 or 3 weeks post-intracranial injection (wp-ic.), brains were flash frozen and systematically sectioned throughout the tumor injection site. Brain sections were stained with hematoxylin and eosin and scanned by the CRi Pannoramic Whole Slide Scanner. In each section, tumors (black arrows) were traced using the Pannoramic Viewer software and the area was digitally calculated. Scale bar = 1mm. Bar graphs representing the Vc (white bars) and IDOkd (black bars) median tumor volume are represented for the 1, 2 and 3 wp-ic. time points. The 2 wp-ic. tumor volumes were then divided by the absolute amount of CD3+ T cells based on flow cytometric quantification. Bar graphs are shown as mean±SEM (n=4 mice/group). (B) Fluorescent microscopy for CD3 and glial fibrillary acidic protein (GFAP) expression, as well as nuclear DAPI staining, was performed in IDO−/− mice ic. Vc and IDOkd GL261 cells at 2 wp-ic. Photomicrographs of the deep tumor parenchyma (top panel) or at the tumor/brain border (bottom panel) were imaged at 20× magnification. Bars, 50 μm. (C) IDO−/− mice were intracranially-injected (ic.) 4×105 GL261 cells transfected the pEF6 vector alone (control) or the pEF6 vector with an inserted IDO cassette (IDO-overexpressing, IDOover). *p < 0.05; **p < 0.01.
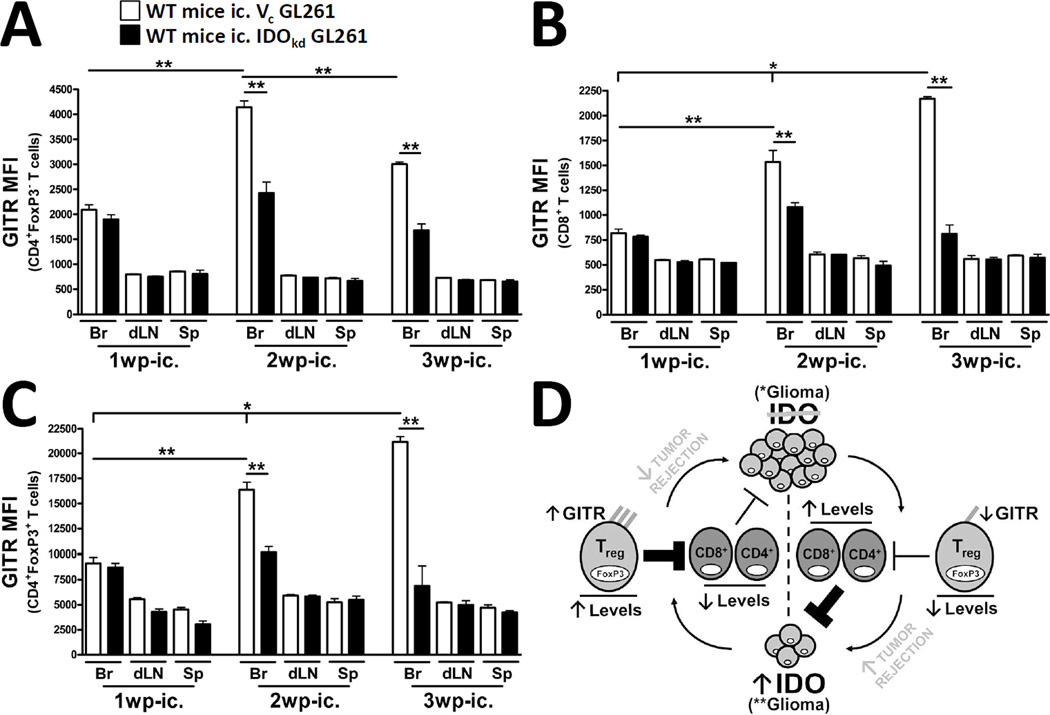
Brain tumor-derived IDO upregulates GITR on infiltrating T cells
We have previously demonstrated that GITR-expressing Tregs are preferentially increased in brain tumors, when compared to Tregs of the spleen (5). To determine whether IDO plays a role in this regulation, we determined the relative level of expression for GITR on CD4+FoxP3−-, CD8+- and CD4+FoxP3+ regulatory- T cells in the brain, dLNs and spleen of WT mice at 1, 2 and 3 wp-ic. with IDO-competent or IDO-deficient brain tumors. As shown in Figure 6A-C, mice bearing IDO-competent tumors were associated with upregulated GITR expression on all types of infiltrating T cells in the brain, when compared to mice bearing IDO-deficient tumors at 2 and 3 wp-ic. However, the actual level of expression for GITR varied widely and depended on the T cell subtype. Figure 6A demonstrates that for CD4+FoxP3− effector T cells, GITR expression was 2088 ± 103 in the brain of IDO-competent tumor-bearing mice at 1 wp-ic. and significantly upregulated to 4142 ± 126 and 3003 ± 42 at 2 and 3 wp-ic., respectively (p<0.01). In contrast, the level of GITR expression by CD4+FoxP3− effector T cells in the brains of IDO-deficient tumor-bearing mice was 1899 ± 95, 2425 ± 223 and 1678 ± 128 at 1, 2 and 3 wp-ic., respectively, and not significantly different from one another. A similar trend in the regulation of GITR on CD8+ T cells was observed, albeit, at a lower overall level of relative expression (Fig. 6B). Specifically, GITR expression was 817.8 ± 41 in the brain of IDO-competent tumor-bearing mice at 1 wp-ic. and significantly upregulated to 1536.2 ± 116 and 2169 ± 22 at 2 and 3 wp-ic., respectively (p<0.01), while the level of GITR expression by CD8+ cytotoxic T cells in the brain of IDO-deficient tumor-bearing mice was 781 ± 18, 1079 ± 45 and 810 ± 90 at 1, 2 and 3 wp-ic., respectively, and not significantly different from one another. In accordance with previous reports, Tregs expressed a much higher level of GITR when compared with CD4+FoxP3− and CD8+ T cells (10). Specifically, Figure 6C shows that GITR expression was 9108 ± 584 in the brain of IDO-competent tumor-bearing mice at 1 wp-ic. and upregulated to 16360 ± 747 at 2 wp-ic. (p<0.01) and further upregulated to 21133 ± 504 at 3 wp-ic. (p<0.01). In contrast, the level of GITR expression by Tregs in the brains of IDO-deficient tumor-bearing mice was 8692 ± 420, 10132 ± 573 and 6869 ± 1974 at 1, 2 and 3 wp-ic., respectively, and not significantly different from one another. Notably, GITR levels were not significantly changed in tumor-draining lymph nodes or the spleen throughout the time course. Collectively, these data suggest that IDO expression plays a critical role in the regulation of GITR on tumor-infiltrating T cells and particularly with regard to Tregs.
Figure 6. Brain tumor-expressed IDO upregulates GITR levels on tumor-infiltrating T cells.
Wild-type (WT) mice were intracranially-injected (ic.) 4×105 GL261 cells transduced with -scrambled shRNA (vector control, Vc; white bars) or -shRNA specific to IDO (IDO knockdown, IDOkd; black bars). The mean fluorescence intensity of GITR expressed by (A) CD4+FoxP3− T cells, (B) CD8+ T cells and (C) CD4+FoxP3+ regulatory T cells isolated from the brains, ipsilateral cervical draining lymph nodes (dLN) and spleen of tumor-bearing mice were analyzed at 1, 2 and 3 weeks post-injection. All T cell populations were initially identified by the expression of CD3. Bar graphs in figures A – C are shown as mean ± SEM and are representative of two independent experiments (n = 5 mice/group). (D) When IDO is absent from brain tumors, GITR expression remains low on infiltrating T cells. Moreover, while Treg levels remain decreased, CD4+ non-Tregs and CD8+ cytotoxic T cells are increased, resulting in effective tumor rejection. Reciprocally, when IDO is expressed, GITR expression is upregulated on infiltrating T cells; this is particularly evident on Tregs. This is co-incident with a significant increase in the ratio of Tregs, when compared to CD4+ non-Tregs and CD8+ cytotoxic T cells. The increased fraction of immunosuppressive Tregs is then associated with decreased tumor rejection and increased outgrowth. *p < 0.05; **p < 0.01.
Discussion
The potent immunosuppression induced by glioblastoma multiforme (GBM) is one of the primary obstacles to finding effective immunotherapies (30, 31). This immunosuppression is associated with a significant accumulation of Tregs within the tumor microenvironment (2–4, 32, 33) and is considered to be one of the primary obstacles inhibiting the tumor rejection functions of CD8+ cytotoxic T cells. It is therefore important that future immunotherapies simultaneously arm effector CD8+cytotoxic T cells, while simultaneously inhibiting immunosuppressive mechanisms. Relevant to this requirement, indoleamine 2,3 dioxygenase (IDO) is a molecule that has been shown to serve as a central regulator of both immunosuppression, as well as T cell function (18, 34). However, prior to this investigation, a comprehensive analysis of IDO in tumors of the central nervous system (CNS) had not been performed. Here, we elucidate the role of IDO in brain tumors and the overall impact on tumor immunity.
In the CNS, the expression of IDO is normally maintained at low to undetectable levels. However, inflammatory processes activated by bacterial byproducts or the pro-inflammatory cytokine, IFN-γ, rapidly upregulate IDO expression by glia (35). Moreover, IDO activity contributes to the suppression of pro-inflammatory T cell responses in the CNS, based on studies demonstrating the exacerbation of pathology related to the experimental autoimmune encephalomyelitis model (EAE; mouse model of multiple sclerosis) in the context of pharmacological IDO inhibition (28, 36). Although this effect would be undesirable in an autoimmune disorder of the CNS, it would be greatly appreciated in the context of CNS tumors, whereby IDO inhibition would lead to decreased immunosuppression and subsequently more effective immune-mediated tumor rejection. However, prior to this investigation, the in vivo role of IDO in the context of CNS malignancy had not been investigated comprehensively.
Here, we have demonstrated that IDO expression by brain tumors, alone, promotes tumor outgrowth in an orthotopic mouse model. This was based on the analysis of the orthotopic GL261 cell-based tumor model that was deficient for-, normal for- or overexpressed- IDO in WT and/or IDO−/− mice. Using these models, we found a reciprocal relationship whereby IDO-competent brain tumors were infiltrated by an increased frequency of Tregs, co-incident with a decreased frequency of CD8+ Tc cells, when compared to IDO-deficient brain tumors. These changes were co-incidental with a significant reduction of overall T cell levels in IDO-deficient-, when compared to IDO- competent brain tumors, even when taking into account the difference in overall tumor size. These data were further supported by the analysis in the spontaneously-developing RasB8 mouse glioma model whereby global IDO competency led to a significant increase in the overall level of Tregs found in the brain, suggesting that this IDO-regulated Treg accumulation is a generalizable phenomenon and not exclusive to the GL261-based brain tumor model. More importantly, our data demonstrate that IDO-deficient brain tumors lead to a significant and long-term survival advantage with clinically-relevant benefits. It’s also important to understand that this survival benefit is maintained both in the presence and absence of peripheral IDO competency. This suggests that the tumor, rather than tumor-draining lymph node APCs, plays an overarching role in regulating the anti-tumor immune response. Although determining if this effect is specific to CNS malignancies requires further investigation.
Our data further elucidate IDO-T cell-mediated mechanisms through the use of T cell-deficient mice whereby CD4+- and/or CD8+- T cell-deficiency abrogates the long-term survival mediated by IDO deficiency in immunocompetent mice. Finally, we reveal the impact of IDO on GITR expression levels by demonstrating a strong upregulation on IDO-competent tumor-infiltrating T cells, potentially providing additional future therapeutic value since it has recently been shown that targeting Treg-expressed GITR can positively impact tumor immunity (37, 38). It was interesting to find that all T cell subtypes upregulated GITR expression, although Tregs demonstrated the highest level of expression (5–10× higher than that of CD4+FoxP3−- and CD8+- T cells). Collectively, our data are summarized schematically in Figure 6D.
Our results from animal models parallel the glioma patient data showing that the upregulation of IDO expression by brain tumors is associated with a significantly worse prognosis in patients. It is interesting that the upregulation of IDO was more common among GBM patients, when compared to lower tumor grades that were more associated with downregulated IDO expression. This suggests that one of the selective pressures brain tumors may use to transit from earlier to later stages of astrocytoma may be through the upregulation of IDO. Furthermore, these data also implicate IDO as a future biomarker for the use in predicting the course of treatment (ie. whether or not to administer an IDO inhibitor), as well as predicting the overall survival in patients. However, it is not yet known whether patients with upregulated and downregulated IDO expression will possess similar responses to pharmacological IDO inhibitors, such as 1-methyl tryptophan (1-MT). Clinical trials aimed at IDO inhibition are currently recruiting patients with solid tumors. Perhaps an interesting retrospective follow-up to this trial will be whether patients with upregulated IDO expression perform better when administered a pharmacological IDO inhibitor compared to those patients with downregulated IDO expression.
It has been established that IDO-expressing tumors systemically alter the accumulation of tryptophan catabolites (39). The first catabolic by-product of IDO catabolism, kynurenine, was recently highlighted as a critical catabolite involved in mediating immunosuppression in glioma (26). Co-incidentally, kynurenine has been associated with the expansion of Tregs via interaction with the aryl hydrocarbon receptor (Ahr) in CD4+ T cells (40). It was therefore interesting to find that Treg levels were simultaneously decreased in brain tumors and dLN, but not in the spleen, in the context of global IDO-deficiency. This may reflect a combination of factors including the lack of locally draining tumor-produced kynurenine, the limited migration pattern of brain tumor APCs, as well as the involvement of primarily thymus-derived, rather than tumor-induced Tregs. Notably, the effects of kynurenine on Ahr-expressing thymus-derived Tregs is currently unknown and is thus a future target of investigation.
The Treg accumulation in IDO-competent brain tumors was associated with a significant and time-dependent upregulation of GITR. The selective increase in GITR expression on tumor-infiltrating-, but not dLN- and spleen- resident Tregs, may predispose those tumor-resident Tregs to low-dose GITR mAb-mediated therapy; potentially minimizing the immunotoxic effects created by systemic administration. In support of this hypothesis are data from a mouse melanoma model demonstrating that GITR mAb decreases the stability and intratumoral accumulation of Tregs, co-incident with the regression of small established tumors (38). More recently, the phosphorylation status of c-Jun N-terminal kinase (JNK) was identified to be a critical regulator of immunosuppressiveness in Tregs, as well as a downstream target of activated GITR; potentially providing additional therapeutic value (41).
Collectively, our data provide the foundation and rationale for further investigation into determining the role of IDO in brain tumors. Future directions include the therapeutic targeting of IDO with the pharmacological inhibitor, 1-MT, as well as other agents identified to have the potential for IDO modulation such as Imatinib (42) and Acyclovir (43). Downstream catabolite production (ie. kynurenine), Treg levels and T cell-expressed GITR will be among the outcome measures analyzed. Finally, to provide better pre-clinical tools for future therapeutic testing, we are currently developing transgenic models that faithfully recapitulate high grade glioma and are conditionally deficient for IDO.
Inhibiting immunosuppression in glioma is one of the most critical barriers to effective and long-lasting anti-tumor immunity (30, 31). Here, we have demonstrated that the upregulation of IDO expression in patient glioma strongly predicts a poor prognosis. This association from glioma patients was then applied to mouse models confirming that tumor-derived IDO expression contributes to a decreased lifespan in a T cell-dependent manner. Moreover, the IDO expression in brain tumors was co-incident with increased Treg recruitment and upregulated GITR expression levels. Collectively, these data implicate IDO as central player in regulating brain tumor immunity, independently of peripheral sources, as well as providing the rationale for increased clinical and pre-clinical efforts for understanding its full impact in brain tumors.
Supplementary Material
Translational Relevance.
Effective immunotherapy of glioblastoma multiforme (GBM) remains a significant challenge due to the strong immunosuppression induced by the tumor. One of the immunosuppressive barriers in GBM is the result of a significant recruitment and accumulation of CD4+FoxP3+ regulatory T cells (Tregs). Moreover, since indoleamine 2,3 dioxygenase (IDO) has been implicated in the regulation of Treg function and expansion, we sought to determine its impact in the context of glioma, given the progressive accumulation of Tregs during brain tumor progression. Here, we show that IDO expression is a prognostic factor in glioma patients. Likewise, tumor-derived-, rather than peripheral- IDO increases Treg recruitment and decreases survival. Reciprocally, IDO deficiency in glioma leads to long-term survival associated with a significant decrease in Treg infiltration. This work provides both clinical and pre-clinical rationale for the therapeutic inhibition of IDO in brain tumors as a future treatment for patients with incurable forms of brain cancer.
Acknowledgements
We thank Ms. Lingjiao Zhang, M.S. for her expertise in the statistical analysis of our studies. We also thank Professor Robert Dantzer, D.V.M./Ph.D. (MD Anderson Cancer Center) for his suggestions and critical comments during the preparation of this work.
Funding: This work was supported by the NIH grants R01 CA138587 (M.S.L.), NIH R01 CA122930 (M.S.L.), NIH U01 NS069997 (M.S.L.), and NIH F32 NS073366 (D.A.W.).
Footnotes
Conflict of Interest Disclosure: The authors declare that no competing interests exist.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl Jour Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–437. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 4.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 5.Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wainwright DA, Sengupta S, Han Y, Ulasov IV, Lesniak MS. The presence of IL-17A and T helper 17 cells in experimental mouse brain tumors and human glioma. PLoS One. 2010;5:e15390. doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14:584–595. doi: 10.1093/neuonc/nos014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James CD. Stimulating anti-tumor immune response: the problem of regulatory T-cells. Neuro Oncol. 2011;13:1261. doi: 10.1093/neuonc/nor201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphries W, Wei J, Sampson JH, Heimberger AB. The role of tregs in glioma-mediated immunosuppression: potential target for intervention. Neurosurg Clin Nor Amer. 2010;21:125–137. doi: 10.1016/j.nec.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 11.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, et al. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–1312. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara M, Shibata M, Watanabe Y, Nukiwa T, Hirata F, Mizuno N, et al. Indoleamine 2,3-dioxygenase. Formation of L-kynurenine from L-tryptophan in cultured rabbit fineal gland. J Biol Chem. 1978;253:6081–6085. [PubMed] [Google Scholar]
- 16.Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, et al. Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. J Neurosurg. 2009;111:230–237. doi: 10.3171/2008.10.JNS081141. [DOI] [PubMed] [Google Scholar]
- 17.Avril T, Saikali S, Vauleon E, Jary A, Hamlat A, De Tayrac M, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol. 2010;225:22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. Journal Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 23.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 24.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. REMBRANDT home page. [Accessed 2012 April 18th];2005 [ http://rembrandt.nci.nih.gov]
- 26.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 27.Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Kohler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J Immunol. 1997;159:419–426. [PubMed] [Google Scholar]
- 28.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lawson MA, Kelley KW, Dantzer R. Primary murine microglia are resistant to nitric oxide inhibition of indoleamine 2,3-dioxygenase. Brain Behav and Immun. 2010;24:1249–1253. doi: 10.1016/j.bbi.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega EA, Graner MW, Sampson JH. Combating immunosuppression in glioma. Future Oncol. 2008;4:433–442. doi: 10.2217/14796694.4.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17:181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 33.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 35.Saito K, Markey SP, Heyes MP. Chronic effects of gamma-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res. 1991;546:151–154. doi: 10.1016/0006-8993(91)91171-v. [DOI] [PubMed] [Google Scholar]
- 36.Kwidzinski E, Bunse J, Kovac AD, Ullrich O, Zipp F, Nitsch R, et al. IDO (indolamine 2,3-dioxygenase) expression and function in the CNS. Adv Exp Med Biol. 2003;527:113–118. doi: 10.1007/978-1-4615-0135-0_13. [DOI] [PubMed] [Google Scholar]
- 37.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fotopoulou C, Sehouli J, Pschowski R, VONH S, Domanska G, Braicu EI, et al. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res. 2011;31:2629–2635. [PubMed] [Google Scholar]
- 40.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joetham A, Ohnishi H, Okamoto M, Takeda K, Schedel M, Domenico J, et al. Loss of T regulatory cell suppression following signaling through glucocorticoid-induced tumor necrosis receptor (GITR) is dependent on c-Jun N-terminal kinase activation. J Bio Chem. 2012;287:17100–17108. doi: 10.1074/jbc.M111.316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soderlund J, Erhardt S, Kast RE. Acyclovir inhibition of IDO to decrease Tregs as a glioblastoma treatment adjunct. J Neuroinflamm. 2010;7:44. doi: 10.1186/1742-2094-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.