Abstract
CCN6 is an extracellular matrix protein that exerts tumor suppressive functions in breast cancer, where its decreased expression is a feature of advanced disease. However, neither its role nor mechanism of action in breast cancer metastasis has been established. Bone morphogenetic proteins (BMPs), which constitute ligands of the TGF-β superfamily, are multifunctional cytokines that induce epithelial-mesenchymal transition (EMT), cell invasion and metastasis. In this study, we identify a CCN6-BMP4-TAK1 kinase signaling pathway that controls the ability of the p38 MAP kinase to regulate acinar morphogenesis and invasion of breast cells. ShRNA-mediated attenuation of CCN6 in human mammary epithelial (HME) cells led to BMP4 upregulation as a major response to exposure to the TGF-β superfamily. CCN6 attenuation also induced BMP4-mediated activation of the Smad-independent TAK1 and p38 kinases. Conversely, ectopic expression of CCN6 in breast cancer cells antagonized BMP4-mediated TAK1/p38 activation and invasive capacity, both by binding BMP4 protein as well as decreasing BMP4 protein levels. Effects on BMP4 and p38 were confirmed in vivo where they correlated with decreased metastasis. In clinical specimens, we found that CCN6 expression was inversely associated with BMP4 and phospho-p38 levels in 69% of invasive breast carcinomas examined, consistent with the functional results. Together our findings identify a novel modifier pathway through which CCN6 acts to limit breast cancer invasion and metastasis.
Keywords: CCN6, WISP3, invasion, metastasis, BMP4, p38 MAP kinase
INTRODUCTION
The interplay between cancer cells and their microenvironment exerts a powerful influence on breast cancer behavior. The microenvironment consists of structural components of the extracellular matrix (ECM) and ECM-associated but structurally unrelated proteins, called matricellular proteins (1-3). Matricellular proteins modulate signaling pathways and facilitate epithelial-stromal cross-talk (2, 3). CCN proteins (named after Cyr61, CTGF, and NOV) are conserved ECM-associated proteins with developmental functions (3-5). Recent studies have shown that CCN proteins play important roles in tumorigenesis (6-11). Our laboratory has reported that CCN6 is expressed in normal breast epithelium but is reduced or lost in 60% of invasive breast carcinomas and in 79% of inflammatory breast cancers (IBC), the most lethal form of locally advanced breast cancer (7, 12). The high frequency of reduction or loss of CCN6 in breast cancer with high metastatic potential suggests that CCN6 may exert a role in the invasion and/or metastatic progression of human breast cancer.
Bone morphogenetic proteins (BMPs), a large subgroup of ligands of the TGF-β superfamily, play crucial roles during embryonic development and tumorigenesis (13-15). BMPs activate type I and type II receptors and modulate the expression of target genes through a series of signal transduction pathways (13-15). The best characterized BMP signaling pathway is through the Smad proteins (Smad-dependent pathway), in which BMPs bind to type II receptor, thereby resulting in phosphorylation of the type I receptor. The latter then phosphorylates receptor-regulated Smads (R-Smads, Smad1, 5 and 8), which can bind to the co-Smad (Smad4). R-Smad/co-Smad complexes translocate to the nucleus where they act as transcription factors and participate in the regulation of target gene expression. Smad6 and Smad7 function as inhibitors (I-Smads) of the TGF-β/BMP pathway (13, 16). On the other hand, recent studies have confirmed that BMPs may function through a Smad-independent pathway, by which BMPs can directly activate the mitogen-activated protein kinase (MAPK)/p38 through phosphorylation of TGF-β activated kinase 1 (TAK1) (17-21).
The present study demonstrates that CCN6 suppresses invasion and metastasis of breast cancer in vivo. In the absence of CCN6, there is activation of the BMP4 –induced Smad-independent TAK1/p38 pathway to promote invasion. However, when present, CCN6 protein binds directly to BMP4 to antagonize BMP4-mediated activation of TAK1/p38 kinases and decreases the invasiveness of breast cancer cells. Taken together, this study identifies a novel CCN6/BMP4/TAK1 axis that controls p38 activity and breast cancer cell invasion.
MATERIALS AND METHODS
Cell culture
HME and SUM149 cells were obtained from S. Ethier laboratory (Karmanos Cancer Institute). Cell lines were authenticated by morphology and growth characteristics and tested for mycoplasma. HME and SUM149 and their stable cell lines were selected and cultured as previously reported (7, 8). MDA-MB-231 cells were purchased form ATCC and maintained following manufacturer’s instructions.
CCN6 cloning
CCN6 and its truncated mutants were first cloned into p3xFLAG-CMV-14 vector (Sigma, St. Louis, MO) containing a Flag-tag at the C-terminus. The Flag-tagged CCN6 and its mutants were further cloned into a lentiviral pLentiLox-RSV-puro vector. The lentiviruses were packaged at the University of Michigan Vector Core. Transductions were carried out as reported (8). siRNA inhibition experiments for TAK1 are described in the Supplementary Methods.
3D cultures and immunocytochemistry
Cells were grown on top of growth factor reduced (GFR) matrigel (Cat# 354230, BD transduction, Bedford, MA) for 15 days following published protocols (22). Phase contrast images were taken on Leica inverted microscope. Immunocytochemical analysis was performed as reported (22).
For rescue experiments with recombinant human CCN6 (rhCCN6), HME-Control and CCN6 KD cells were grown on 3D for 4 days followed by serum starvation for 16 h. On day 5, cells were treated with 500 ng/ml of rhCCN6 (Peprotech, Rocky Hill, NJ) in 0.1% FBS-F12 medium for 4 days with media refreshed every 2 days. On day 10, cells were put back into complete HME media and cultured for another 5 days. Phase contrast imaging, immunoblots and immunocytochemical analyses were carried out at day 15. The percentage of single round acinar structures was calculated by counting 100 structures from 3 wells of the 8-well chamber slide.
For inhibitor studies, cells in 3D culture were treated with different inhibitors in complete medium, which was refreshed every 3 days. p38 kinase inhibitors SB203850 and SB202190, TAK1 inhibitor LLZ1640-2 or TI-2 and its inactive analog LLZ1640-4 or TI-4 were purchased from Calbiochem (EMD Chemicals, Newark, NJ). The BMP4 neutralizing antibody was purchased from R&D Systems (Minneapolis, MN). Details on the 3D cultures and immunocytochemistry are described in the Supplementary Methods.
TGF-β/BMP pathway specific PCR array, co-immunoprecipitation and immunoblot
RNAs from HME-Control and HME-CCN6 KD cells were isolated by RNeasy kit (Qiagen GmbH, Hilden) and corresponding cDNA was analyzed by TGF-β/BMP pathway specific PCR array (SA Biosciences, Frederick, MD).
Immunoprecipitation was done following published protocols (23, 24). Briefly, rhCCN6 protein (Peprotech, Rocky Hill, NJ) was incubated with disulfide linked homodimer of biotin labeled rhBMP4 (Bio-BMP4) protein (R&D systems, Minneapolis, MN) for 3 h on ice in 100 μl of saline buffer containing 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 1.5 mM CaCl2, 1.5 mM MgCl2, 0.1% Triton X-100, 0.1% Octyglucoside, 0.1% CHAPS, 5% glycerol and 0.1% BSA. Immobilized streptavidin bead conjugate (#3419, Cell Signaling) was used to pull down Bio-BMP4 by incubating at 4°C for 2 h with constant mixing, beads were pelleted at 14,000 rpm for 1 min at 4°C, resuspended in precooled 1 ml of IP buffer as above but without BSA and washed three times at 4°C for 5 mins. After centrifugation, the supernatants were removed and the CCN6-BMP4 complex was eluted by boiling with 1 volume of 2X laemmeli buffer with β-mercaptoethanol. The samples were subjected to SDS gel electrophoresis under reducing conditions. CCN6 was detected using anti-CCN6 antibody (# sc-25543, 1:1000, Santa Cruz Biotechnology). Antibodies for immunoblot analyses are listed in the Supplementary Methods.
Surface Plasmon Resonance
Real time binding experiments were carried out using Biacore 2000 apparatus (Biacore AB, Uppsala, Sweden). Full length rhCCN6 (Peprotech, Rocky Hill, NJ) was immobilized on the CM5 sensor chip (certified grade, Biacore AB), HBS-EP buffer (10 mM HEPES pH7.4, 150 mM sodium chloride, 3 mM EDTA with 0.005% surfactant P20) was used as running buffer. Disulfide linked homodimer of rhBMP4 (R&D systems, Minneapolis, MN) was flown over CCN6 immobilized on CM5 chip. Kinetic analysis was carried out using concentrations between 1 μM to 7.8 nM by simultaneous fitting model. KD, ka, and kd, were calculated by simultaneous nonlinear regression.
Invasion assay
In vitro invasion was performed using Matrigel Invasion Chambers (BD Biosciences, Bedford, MA) according to the manufacturer’s procedures, in triplicate. Invaded cells were counted under an inverted microscope after 24 h. Details on the treatment with Bio-BMP4 and anti-BMP4 antibody are described in the Supplementary Methods.
Spontaneous Metastasis Assay
Firefly luciferase expressing MDA-MB-231 cells expressing Flag-Vector or CCN6-Flag were injected orthotopically into the right inguinal mammary gland (#4) of anesthetized 5-wk-old athymic nude mice (Harlan Sprague-Dawley). Mice were euthanized when primary tumor volumes reached 2.0 cm. Metastases were monitored by firefly luciferase bioluminescence imaging by BLI. Details on injection, image acquisitions, and statistical analyses can be found in Supplementary Methods.
Human breast tissue and immunohistochemistry
A high-density tissue microarray (TMA) containing 71 primary invasive breast carcinomas developed and characterized by our group was employed (25). Expression of CCN6, BMP4 and phospho-p38 proteins was evaluated as either low or high based on intensity of staining and percentage of staining cells, following published literature (7, 8, 26). Further details are provided in the Supplementary Methods.
RESULTS
CCN6 regulates acinar-like organization and invasion in breast cells
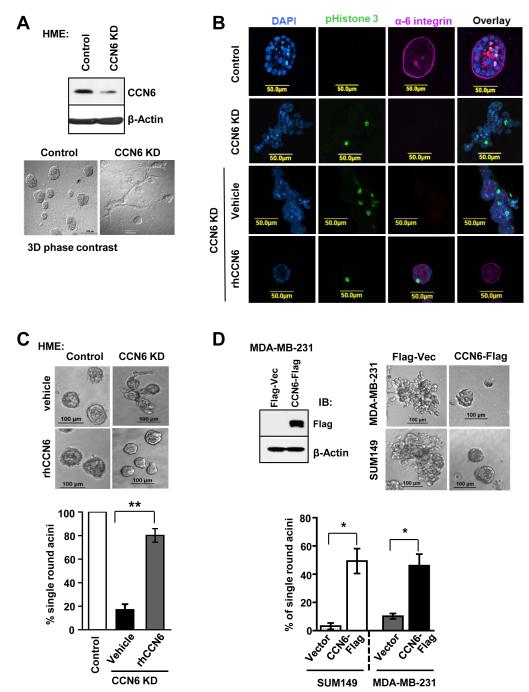
One of the earliest morphological alterations that distinguish benign breast acini from invasive carcinoma is the loss of cellular organization and invasion (27). These histopathological differences between benign glands and invasive carcinomas can be reproduced in three-dimensional (3D) culture, a physiologically relevant ex-vivo model (22, 28). Using this system, CCN6 knockdown (KD) non-tumorigenic human mammary epithelial (HME) cells are invasive compared with the polarized, self-organizing structures formed controls (Figure 1A). CCN6 KD cells lack acinar polarity with loss of the basal polarity marker α6-integrin, fail to growth arrest (positive P-Histone 3), and do not form a lumen (Figure 1B). As observed in vivo (7), CCN6 KD HME cells showed downregulation of E-cadherin expression at cell-cell borders in 3D (Supplementary Fig. S1).
Figure 1. CCN6 regulates acinar-like organization and invasion in HME cells.
A. Immunoblot and phase contrast 3D images of non-tumorigenic human mammary epithelial (HME) cells with stable CCN6 knockdown (KD) and controls grown on matrigel for 15 days. Control cells form organized acinar-like structures. In contrast, CCN6 KD induces invasion in 3D. B. Representative confocal images show that CCN6 KD cells express higher phosphorylated H3 and inhibition of α-6 integrin, with invasive phenotype compared to controls. Treatment with rhCCN6 (500 ng/ml) rescues the above features. C. rhCCN6 rescues the invasive phenotype of CCN6 KD HME cells and induces the formation of acinar-like structures compared to vehicle treated cells. The bar graph shows the percentage of single round acinar structures ± SD obtained by counting 100 structures. D. Stable CCN6 overexpression reduced invasion in MDA-MB-231 and SUM149 breast cancer cells compared to controls. Immunoblot and representative 3D images are shown. The bar graph shows the percentage of single round acinar-like structures calculated as in C.
The invasiveness of CCN6 KD HME cells in 3D culture was completely reversed by treatment with recombinant human CCN6 (rhCCN6) protein (Figure 1B-C). Using a 500ng/ml dose as previously reported (29), rhCCN6 rescued the formation of acinar-like structures with restoration of α6-integrin at the basement membrane and decreased cell proliferation (P-Histone 3) (Figure 1B-C). Likewise, stable CCN6 overexpression in SUM149 and MDA-MB-231 breast cancer cells blocked 3D invasion and induced acinar formation (Figure 1D).
CCN6 regulates BMP4-mediated TAK1 and p38 activation
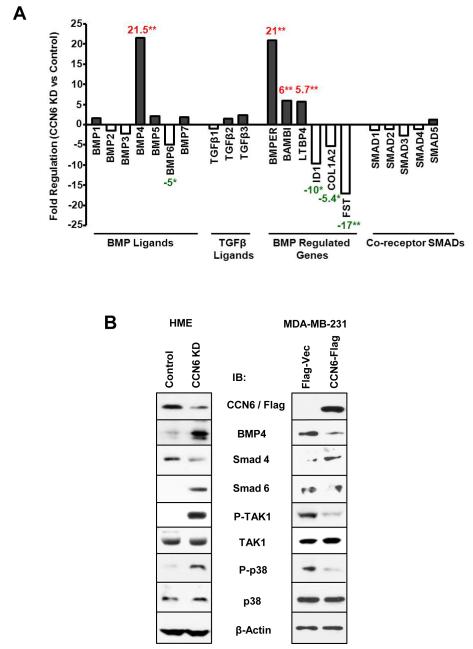
One of the major regulators of invasion in breast cancer is the TGF-β/BMP pathway (13-15). CCN proteins have been shown to modulate TGF-β/BMP activity (4, 30), but whether CCN6 can regulate this pathway in human cells and the underlying mechanism were unknown. The effect of CCN6 KD on the TGF-β/BMP pathway was first assessed utilizing a pathway specific PCR array comparing CCN6 KD HME cells and scrambled controls. CCN6 KD had no significant effect on the mRNA levels of TGF-β1, TGF-β2, or on BMP1, 2, 3, 5 and 6 genes, but induced a 21-fold increase in BMP4 mRNA levels (Figure 2A and Supplementary Table S1). Consistent with a specific role for CCN6 on BMP4 regulation, CCN6 KD led to a 6- and 25 -fold increase in BAMBI and BMPER mRNA levels, two known extracellular regulators of BMP4 (31-33). CCN6 KD also resulted in downregulation of Inhibitor of Differentiation 1 (ID1), a BMP4 target gene with functions in mammary gland development (34).
Figure 2. CCN6 regulates BMP4-mediated TAK1 and p38 activation.
A. Quantitative real time PCR array of TGF-β/BMP pathway genes reveal that CCN6 KD principally leads to upregulation of BMP4 and BMP-regulated genes in HME cells. No significant effect was observed on the mRNA levels of TGF-β1, TGF-β2, BMP1, 2, 3, 5 and 6 genes, or co-receptor Smads between HME CCN6 KD and controls. B. Immunnoblots of HME and MDA-MB-231 cells show that CCN6 mainly regulates BMP4 protein and its non-Smad signaling proteins P-TAK1 and P-p38 MAPK.
We next investigated if BMP4 signaling was activated in the invasive CCN6 KD HME cells. Western blots showed that CCN6 KD cells exhibited increased expression of BMP4 and the R-Smad, P-Smad1 (Figure 2B and Supplementary Figure S2A). However, no significant difference in invasion was found upon Smad1 siRNA downregulation in CCN6 KD cells compared to scrambled controls (Supplementary Figure S2B). Moreover, CCN6 KD led to downregulation of another R-Smad, P-Smad5, and co-receptor Smad4. I-Smads, Smad6 and 7, function as inhibitors of TGF-β family signaling, with Smad6 preferentially blocking BMP signaling (35). We found that Smad6 was upregulated in CCN6 KD cells compared to controls (Figure 2B). These data suggest that in the setting of CCN6 KD, the Smad-dependent pathway may not necessary for BMP4-induced invasion.
In contrast, CCN6 KD increased P-TAK1 and its downstream molecule P-p38 MAPK, both of which are components of the BMP4 Smad-independent pathway (Figure 2B). Further supporting the effect of CCN6 on p38 activation, rhCCN6 protein rescued the upregulation of P-p38 caused by CCN6 KD (Supplementary Figure S3A). We also tested the effect of ectopic CCN6 on BMP4/TAK1/p38 signaling. Overexpression of CCN6 decreased BMP4 protein, P-TAK1 and P-p38 proteins in MDA-MB-231 cells compared to controls (Figure 2B). Together, these data suggest that CCN6 regulates BMP4 signaling through the TAK1 and p38 axis.
Activation of BMP4/TAK1/p38 signaling is required for the invasive morphology change due to CCN6 knockdown in benign breast cells
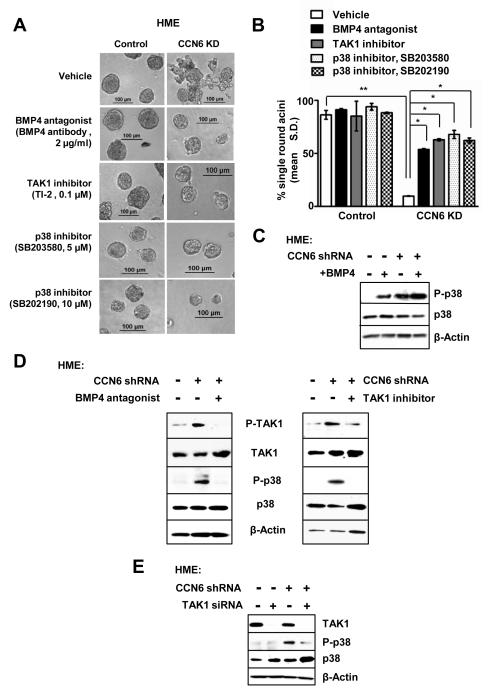
We next determined the specific contribution of the BMP4/TAK1/p38 signaling pathway to the invasive CCN6 KD phenotype. Towards this end, individual pathway proteins were inhibited in cells growing in 3D using pharmacological inhibitors, function blocking antibodies, and siRNA-mediated knockdown. Specific inhibition of BMP4, TAK1, or p38 reduced invasion and restored acinar-like organization of CCN6 KD cells (Figure 3A-B and Supplementary Figure S3B). p38 inhibitors were sufficient to decrease the invasive morphology of MDA-MB-231 and SUM149 breast cancer cells (Supplementary Figure S3C).
Figure 3. Inhibition of BMP4/TAK1/p38 pathway components reverses the invasive morphological change of HME CCN6 knockdown cells.
A. HME CCN6 KD and controls were grown in 3D with or without BMP4 blocking antibodies (2 μg/ml), TAK1 inhibitor LLZ1640-2 (TI-2, 0.1μM), or p38 kinase inhibitors SB203850 (5 μM or 20 μM) and SB202190 (10 μM), respectively. Anti-BMP4, TAK1 inhibitor, and p38 inhibitors rescue the 3D invasive activity conferred by CCN6 KD. B. Bars show the mean percentage of single round acini ± SD of three independent experiments. C. Immunoblot of HME CCN6 KD cells and controls in the absence or presence of recombinant BMP4 protein (100ng/ml). CCN6 KD enhances BMP4-induced upregulation of phosphorylated p38 protein compared to controls. D. Immunoblots of HME CCN6 KD cells and controls in the presence or absence of BMP4 blocking antibodies or TAK1 inhibitor. BMP4 inhibition reverses the upregulation of P-TAK1 and P-p38 induced by CCN6 KD (Left). Specific TAK1 inhibition rescues the increased P-p38 levels conferred by CCN6 KD (Right). E. Immunoblot of HME CCN6 KD cells and controls shows that TAK1 siRNA inhibition reverses the upregulation of P-p38 induced by CCN6 KD compared scramble controls.
CCN6 KD enhanced BMP4-mediated phosphorylation of p38 (Figure 3C). Consistent with a crucial role for BMP4 in the invasive activity of CCN6 KD, BMP4 inhibition using a function blocking antibody was sufficient to block p38 phosphorylation in CCN6 KD cells (Figure 3D left panel). Likewise, treatment with TAK1 siRNAs or TAK1 kinase inhibitor TI-2 blunted p38 phosphorylation due to CCN6 KD (Figure 3D right panel, and 3E). Together, these data demonstrate that specific activation of the BMP4/TAK1/p38 signaling cascade is required for the invasiveness triggered by CCN6 KD. Furthermore, the observed P-p38 upregulation due to CCN6 KD requires BMP4 and TAK1 kinase activation.
CCN6 overexpression decreases invasion and binds to BMP4 in breast cancer cells
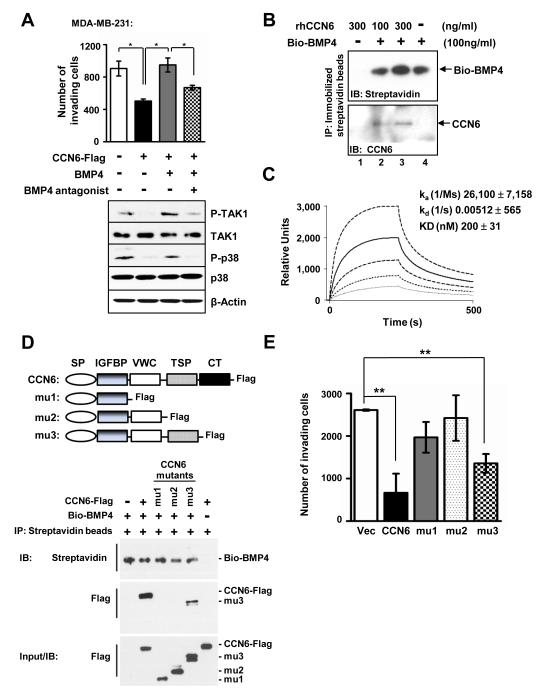
Consistent with the results of 3D cultures, CCN6 overexpression decreased invasion in MDA-MB-231 cells. This effect was rescued by rhBMP4. In contrast, addition of a BMP4 blocking antibody significantly inhibited BMP4-induced invasion (Figure 4A). CCN6 overexpression decreased BMP4, P-TAK1 and P-p38 proteins. BMP4 treatment rescued P-TAK1 and P-p38 levels, while the BMP4 antagonist had the opposite effect (Figure 2B and 4A).
Figure 4. CCN6 protein decreases invasion and physically interacts with BMP4 in MDA-MB-231 cells.
A. Invasion ability of MDA-MB-231CCN6-Flag cells and controls (Flag-Vector) untreated or treated with BMP4 protein (100ng/ml) or BMP4 protein plus anti-BMP4 antibody (used in Figure 2B). BMP4 protein rescues invasion of CCN6-Flag cells, which is blocked in the presence of BMP4 antibody. Immunoblots were performed with P-TAK1, TAK1, P-p38 and p38 antibodies. B. Immunoblot of rhCCN6 bound to Bio-BMP4 after immunoprecipitation (IP) with immobilized Streptavidin beads. The IP efficiency was monitored by anti-streptavidin antibody to detect biotin labeled BMP4. C. Kinetic and steady state parameters for Bio-BMP4 binding to immobilized CCN6 protein were determined by surface plasmon resonance. The sensorgrams indicate the responses when varying concentrations (from bottom to top, 31, 62, 125, 250 and 500 nM) of BMP4 were injected over immobilized CCN6. KD, ka, and kd, were calculated by simultaneous nonlinear regression. D. Structure of CCN6 and its three truncated mutants with C-terminal Flag-tag. CCN6 protein contains 4 motifs: IGFBP, VWC, TSP, and CT with an N-terminal signal peptide (SP). MDA-MB-231 breast cancer cells were stably transduced with lentivirus containing CCN6 and mutants. Similar to Figure 4B, anti-Streptavidin (binding with Bio-BMP4) immunoprecipitates were analyzed by immunoblotting with anti-Streptavidin or anti-flag antibody. Data show that the TSP motif of CCN6 is necessary for binding to BMP4 in MDA-MB-231 cells. E. Full-length CCN6 and its mutant 3 (mu3) decreased invasion in MDA-MB-231 cells, while no significant effect was observed for mu1 and mu2.
CCN6 shares the highly conserved protein motifs of the CCN family (3). It contains four structural modules: insulin growth factor binding protein (IGFBP), von Willebrand factor C (VWC), thrombospondin type I (TSP), and cysteine knot (CT), as well as an amino terminal signal peptide. The VWC and TSP motifs have been reported to facilitate binding of CCN proteins to BMP and TGF-β (30, 36, 37). These reported data together with the results shown in Figure 4A led us to hypothesize that CCN6 may bind to BMP4 to antagonize BMP4-mediated TAK1/p38 activation leading to decreased invasion. To investigate if CCN6 and BMP4 can interact directly we used independent and complementary methods: co-immunoprecipitation and surface plasmon resonance (SPR)-based binding assay. Figure 4B shows that in vitro purified full-length rhCCN6 protein co-immunoprecipitated with purified rhBMP4 (Bio-BMP4). SPR-based binding assay confirmed the binding of immobilized rhCCN6 protein to different concentrations of Bio-BMP4 with a dissociation constant (Kd) of 200 nM (Figure 4C).
To demonstrate the interaction between CCN6 and BMP4 in breast cancer cells, and to elucidate which CCN6 motif is important for BMP4 binding, MDA-MB-231 cells were transduced with Flag-tagged full-length CCN6, Flag-tagged CCN6 truncated mutants, or the empty vector control. Immunoprecipitation was carried out with Bio-BMP4 specifically bound to Streptavidin beads. Immunoblot with anti-Streptavidin and anti-Flag antibodies confirmed that only ectopic full-length CCN6 and its mutant 3 (mu3) bind to Bio-BMP4 in MDA-MB-231 cells, while no binding was observed for mutants 1 and 2 (mu1 and mu2) (Figure 4D). These data show that the TSP motif of CCN6 is necessary for binding to BMP4 in MDA-MB-231 cells. The relevance of these studies to human breast cancer is further supported by simultaneous double immunostaining showing that CCN6 and BMP4 proteins co-localize in human breast cancer tissues (Supplementary Figure S4). Next, we investigated if the ability of CCN6 to co-precipitate BMP4 is associated with inhibition of invasion. Full-length CCN6 and its mutant 3 (mu3) decreased invasion in MDA-MB-231 cells, while no effect was observed for mu1 and mu2, which were unable to bind to BMP4 (Figure 4E).
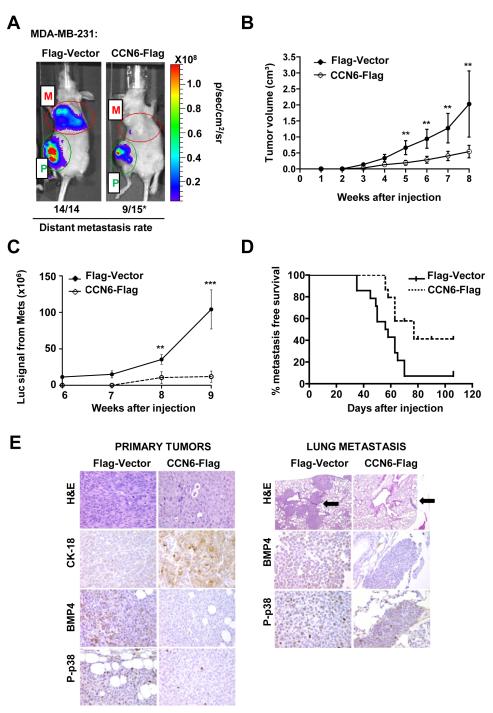
CCN6 overexpression is sufficient to reduce distant metastasis
To investigate the effect of CCN6 overexpression in metastasis, we injected MDA-MB-231 expressing CCN6-Flag or Flag-Vector into the mammary fat pads of nude mice. The development of primary tumors and distant metastases was monitored by firefly luciferase bioluminescence imaging (Figure 5A). Even though CCN6 overexpression reduced invasion of MDA-MB-231 and SUM149 cells (Figure 1), MDA-MB-231 cells were chosen for the metastasis experiment given their ability to form spontaneous metastases compared to SUM149 cells. CCN6 overexpression decreased tumor volume compared to controls (Figure 5B and Supplementary Figure S5). Bioluminescence analyses revealed that all Flag-Vector mice developed metastasis (n=14), compared to 9 of 15 (60%) of CCN6-Flag mice. The metastatic burden was significantly higher in Flag-Vector compared to CCN6-Flag mice (Figure 5C). Kaplan Meier survival analysis showed that CCN6-Flag mice had a median metastasis free survival of 77 days as compared to 57 days for Flag-Vector mice (p= 0.005, Figure 5D).
Figure 5. CCN6 overexpression in MDA-MB-231 cells is sufficient to decrease distant metastasis.
A. MDA-MB-231-CCN6-Flag-Luc and Flag-Vector-Luc were injected in the mammary fat pads of nude mice, n=15 mice/group. Representative pictures of luciferase imaging showing primary tumors (P) and metastasis (M). B. CCN6-Flag cells formed significantly smaller primary tumors compared to controls (mean tumor volume ± SD). C. CCN6 overexpression significantly decreased the metastatic burden. D. CCN6 significantly improved metastasis-free survival. The median survival was 57 days vs. 77 days for Flag-Vector and CCN6-Flag mice, respectively (Kaplan Meier log rank p=0.005). E. Photomicrographs of xenografts and lung metastasis of CCN6-Flag and Flag-Vector mice. CCN6 overexpression changed the tumor morphology from mesenchymal-like to epithelial, with upregulation of the epithelial marker cytokeratin-18 (CK-18). CCN6-Flag primary tumors and lung metastasis exhibit decreased BMP4 and P-p38 proteins. Black arrows show metastases in the lung of CCN6-Flag and Flag-Vector mice (200x magnification).
Consistent with the findings in 3D cultures, CCN6 overexpression induced in vivo phenotypic changes from mesenchymal-like to epithelial. Flag-Vector tumors contained spindled cells with low cytokeratin-18 (CK-18) and high vimentin levels. In contrast, CCN6-Flag tumors exhibited an epithelial morphology and high CK-18 expression (Figure 5E). Providing in vivo relevance to our in vitro observations and mechanistic studies, CCN6-Flag primary tumors and lung metastasis exhibited decreased BMP4 and P-p38 staining compared to Vector-Flag tumors and metastases (Figure 5E).
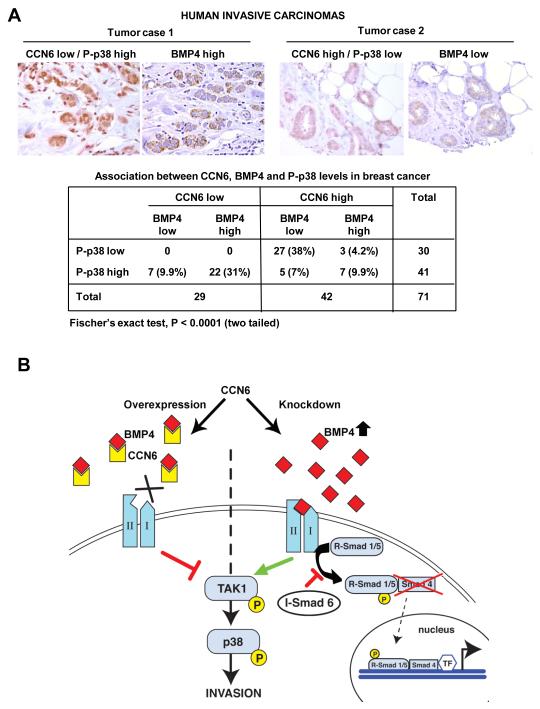
Low CCN6 protein is associated with high BMP4 and P-p38 in human invasive breast carcinomas
The significance of our novel findings to human breast cancer was validated by testing the expression of CCN6, BMP4 and P-p38 proteins in 71 primary invasive breast carcinoma tissue samples arrayed in a tissue microarray (25). Double immunostaining was performed to detect CCN6 and P-p38 proteins. When present, CCN6 protein localized predominantly to the cytoplasm and P-p38 protein localized to the nuclei. BMP4 protein was expressed mainly in the cytoplasm. CCN6, BMP4 and P-p38 were scored as high when over 10% of the cancer cells showed moderate or strong staining, and as low when staining was present in less than 10% of the tumor cells. We found a significant association between CCN6, BMP4 and P-p38 proteins. Of the 71 tumors, 22 (31%) had low CCN6 coupled with high BMP4/P-p38 expression; and 27 (38%) had high CCN6 in association with low BMP4/P-p38 (two-tailed Fisher’s exact test, P<0.0001) (Figure 6A).
Figure 6. CCN6 expression is inversely associated with BMP4 and P-p38 expression in human breast tissue samples.
A. Human breast cancer tissues (n=71) co-immunostained for CCN6 (red) and P-p38 (brown). Same tissues were immunostained for BMP4 (brown). Tumor case 1 shows an invasive carcinoma with low CCN6 and high P-p38 and BMP4 proteins. Tumor case 2 exhibits high CCN6, and low P-p38 and BMP4 proteins. The table shows the distribution of CCN6, BMP4 and P-p38. We discovered a significant association between CCN6 with BMP4 and P-p38 proteins, Fisher’s exact test, P<0.0001 (two-tailed). B. Our data support a model in which CCN6 antagonizes BMP4-mediated activation of TAK1 and p38 proteins to suppress breast cancer invasion. In the presence of CCN6, CCN6 binds to BMP4 and reduces BMP4 levels, with resulting decreased BMP-mediated TAK1/p38 signaling and inhibition of invasion. When CCN6 is reduced, as it occurs in inflammatory breast cancer, there is upregulation of BMP4 protein and enhanced activity of the BMP4/TAK1/p38 axis to promote invasion.
DISCUSSION
The data presented in this study reveal that CCN6 downregulation disrupts acinar morphogenesis and promotes invasion of mammary epithelial cells. In contrast, CCN6 overexpression reduces invasion and distant metastasis of breast cancer cells. Our data point to a previously unrecognized mechanism of CCN6 function by which CCN6 interacts directly with BMP4 protein in breast cancer cells to antagonize BMP4-mediated signaling through TAK1 and p38 MAPK.
A fundamental difference between normal and cancer cells is that normal breast cells are organized with the apical cytoplasm towards a central lumen, and the basal portion of the cytoplasm towards the basement membrane (27). In the breast, normal cells are organized into acini. CCN6 KD was sufficient to disrupt acinar organization and to induce a branching, disorganized, and invasive phenotype. To elaborate these conclusions we investigated the effect of CCN6 on acinar organization utilizing 3D cultures, a system that recapitulates the normal architecture of the human breast (22, 28). Treatment with recombinant CCN6 protein completely reversed the invasiveness of CCN6 KD cells and induced the formation of well-organized acini with restoration of apical-basal cell polarity, decreased cellular proliferation, and deposition of a basement membrane.
Our group has previously reported that CCN6 loss is associated with a highly metastatic form of invasive breast carcinoma termed inflammatory breast cancer, as well as with non-inflammatory invasive breast cancers with lymph node metastases (7, 12). Data presented here show for the first time that CCN6 overexpression decreases distant metastases in vivo, and improves survival. We observed that CCN6 upregulation was sufficient to reprogram the phenotype of MDA-MB-231 cells from a spindle to an epithelial morphology with upregulation of cytokeratin-18. The ability of CCN6 to promote a mesenchymal-to-epithelial transition and its dependency on BMP4/TAK1/p38 signaling is a novel finding with potential therapeutic implications.
The mechanisms implicated in CCN6 tumor suppressor activity have been elusive, and CCN6 binding partners in human breast cancer were unknown. Here, we demonstrate that CCN6 binds to BMP4 in human breast cancer MDA-MB-231 cells, and this novel interaction is important for the suppressive role of CCN6 on invasion. We found that CCN6 and BMP4 proteins co-localize in situ, as they were detected simultaneously in both the stroma and the cancer cells of human breast cancer tissues. Further supporting our results, the CCN6 zebrafish ortholog (zwisp3) was found to immunoprecipitate with BMP4, and CCN6 overexpression antagonized BMP and Wnt signaling in developing zebrafish (37). Another CCN family member, CTGF (also called CCN2), has been shown to physically interact with BMP4 in vitro, antagonizing BMP4 activity by preventing its binding to BMP receptors (30). However, the consequences of these interactions on downstream signal transduction pathways as related to cancer progression have not been investigated. Likewise, the possibility that CCN proteins would directly control BMP4-mediated TAK1 and p38 signaling had not been considered previously.
BMP4, an extracellular signaling protein that belongs to the TGFβ superfamily, is essential for development since Bmp4 null mice are embryonic lethal (38). In addition, BMP4 upregulation is a common event in the pathogenesis of human carcinomas, including breast cancer (39-41). The best studied mechanism of BMP4 action is by activating Smad-dependent signaling pathways through Smad1/5/8 (13, 16). In recent years, BMP4 has been shown to activate Smad-independent pathways, including p38 MAPK through activation of TAK1, a MAPKKK family member (17-20); but the responsible mechanisms are still ill defined. Through a combination of knockdown and rescue strategies, we have attempted to dissect the key signaling components linking CCN6 to BMP4 signaling pathways. The results reported here indicate that CCN6 binds to BMP4 in human breast cancer cells and that the TSP motif of CCN6 is required for this interaction. Our data show that the principal consequence of the interaction between CCN6 and BMP4 proteins in breast cancer is the inhibition of the BMP4/TAK1/p38 axis leading to reduced invasion. Conversely, in the absence of CCN6, BMP4 is upregulated leading to activation of TAK1 and p38 proteins to promote invasion. Our working model is shown in Figure 6B.
The in vivo relevance of the mechanistic link between CCN6, BMP4 and P-p38 proteins was determined by testing their expression in xenografts and human tissue samples. CCN6 overexpression decreased the levels of BMP4 and P-p38 proteins in primary and metastatic tumors. In human breast cancer, 22 of 29 (76%) invasive carcinomas expressing low CCN6 were positive for BMP4 and P-p38; while 27 of 42 (64%) tumors with high CCN6 were negative for BMP4 and P-p38 proteins. From a clinical perspective, the role of CCN6 as a regulator of the BMP4/TAK1/p38 cascade is of particular interest because autocrine or paracrine activation of this pathway could be detectable and targetable in tumors. For example, a recent study in colon cancer showed that TAK1 inhibition using a synthetic TAK1 inhibitor decreased tumor progression in preclinical models of TAK1-dependent cancers (42, 43). Based on our data, CCN6 emerges as a logical target to inhibit BMP4-mediated activation of TAK1 and p38 in CCN6 deficient tumors with increased activity of this pathway.
In conclusion, our results demonstrate a previously undescribed mechanism of CCN6 tumor suppression in breast cancer. These data show that CCN6 protein has metastasis suppressor functions and uncover an underlying molecular mechanism by which CCN6 influences the BMP4/TAK1/p38 axis to regulate invasion. Our data provide evidence that CCN6 may be a rational therapeutic target for development of treatments to halt breast cancer metastasis and improve clinical outcome.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by NIH grants R01 CA107469, R01 CA125577 and U01CA154224 (to CGK), the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592).
Footnotes
Conflict of Interest: none
REFERENCES
- 1.Arendt LM, Rudnick JA, Keller PJ, Kuperwasser C. Stroma in breast development and disease. Semin Cell Dev Biol. 2009;21:11–8. doi: 10.1016/j.semcdb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallacchi V, Rodolfo M. Regulatory role of CCN3 in melanoma cell interaction with the extracellular matrix. Cell Adh Migr. 2009;3 doi: 10.4161/cam.3.1.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–73. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–63. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perbal B. The CCN3 protein and cancer. Adv Exp Med Biol. 2006;587:23–40. doi: 10.1007/978-1-4020-5133-3_3. [DOI] [PubMed] [Google Scholar]
- 6.Bleau AM, Planque N, Perbal B. CCN proteins and cancer: two to tango. Front Biosci. 2005;10:998–1009. doi: 10.2741/1594. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Zhang Y, Varambally S, Chinnaiyan AM, Banerjee M, Merajver SD, et al. Inhibition of CCN6 (Wnt-1-induced signaling protein 3) down-regulates E-cadherin in the breast epithelium through induction of snail and ZEB1. Am J Pathol. 2008;172:893–904. doi: 10.2353/ajpath.2008.070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleer CG, Zhang Y, Pan Q, Merajver SD. WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia. 2004;6:179–85. doi: 10.1593/neo.03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleer CG, Zhang Y, Pan Q, van Golen KL, Wu ZF, Livant D, et al. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene. 2002;21:3172–80. doi: 10.1038/sj.onc.1205462. [DOI] [PubMed] [Google Scholar]
- 10.Franzen CA, Chen CC, Todorovic V, Juric V, Monzon RI, Lau LF. Matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis. Mol Cancer Res. 2009;7:1045–55. doi: 10.1158/1541-7786.MCR-09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY, Lin BR, et al. Involvement of hypoxia-inducing factor-1alpha-dependent plasminogen activator inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell invasion. J Biol Chem. 2008;283:15807–15. doi: 10.1074/jbc.M708933200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–9. [PubMed] [Google Scholar]
- 13.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15:169–90. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550–3. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 18.Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–9. doi: 10.1016/j.biocel.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–84. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–24. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 22.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–30. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–98. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stal O, Perez-Tenorio G, Akerberg L, Olsson B, Nordenskjold B, Skoog L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003;5:R37–44. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen P. P. Rosen’s Breast Pathology. In: Rosen P, editor. Rosen’s Breast Pathology. Third ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. p. 325. P. [Google Scholar]
- 28.Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–51. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzatti G, Huang W, Pal A, Cabanillas AM, Kleer CG. CCN6 (WISP3) decreases ZEB1-mediated EMT and invasion by attenuation of IGF-1 receptor signaling in breast cancer. J Cell Sci. 2011;124:1752–8. doi: 10.1242/jcs.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–12. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 32.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, et al. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–79. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–5. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 34.Desprez PY, Sumida T, Coppe JP. Helix-loop-helix proteins in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003;8:225–39. doi: 10.1023/a:1025957025773. [DOI] [PubMed] [Google Scholar]
- 35.Ishisaki A, Yamato K, Hashimoto S, Nakao A, Tamaki K, Nonaka K, et al. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. 1999;274:13637–42. doi: 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- 36.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Weidinger G, Liang JO, Aquilina-Beck A, Tamai K, Moon RT, et al. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J Clin Invest. 2007;117:3075–86. doi: 10.1172/JCI32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 39.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–19. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–56. [PubMed] [Google Scholar]
- 41.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat Rev Genet. 2003;4:763–73. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 42.Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–50. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melisi D, Xia Q, Paradiso G, Ling J, Moccia T, Carbone C, et al. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst. 2011;103:1190–204. doi: 10.1093/jnci/djr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.