Abstract
Although Rhesus (Rh) proteins are best known as antigens on human red blood cells, they are not restricted to red cells or to mammals, and hence their primary biochemical functions can be studied in more tractable organisms. We previously established that the Rh1 protein of the green alga Chlamydomonas reinhardtii is highly expressed in cultures bubbled with air containing high CO2 (3%), conditions under which Chlamydomonas grows rapidly. By RNA interference, we have now obtained Chlamydomonas rh mutants (epigenetic), which are among the first in nonhuman cells. These mutants have essentially no mRNA or protein for RH1 and grow slowly at high CO2, apparently because they fail to equilibrate this gas rapidly. They grow as well as their parental strain in air and on acetate plus air. However, during growth on acetate, rh1 mutants fail to express three proteins that are known to be down-regulated by high CO2: periplasmic and mitochondrial carbonic anhydrases and a chloroplast envelope protein. This effect is parsimoniously rationalized if the small amounts of Rh1 protein present in acetate-grown cells of the parental strain facilitate leakage of CO2 generated internally. Together, these results support our hypothesis that the Rh1 protein is a bidirectional channel for the gas CO2. Our previous studies in a variety of organisms indicate that the only other members of the Rh superfamily, the ammonium/methylammonium transport proteins, are bidirectional channels for the gas NH3. Physiologically, both types of gas channels can apparently function in acquisition of nutrients and/or waste disposal.
The Rhesus (Rh) blood group substance, one of the most abundant proteins in red cell membranes, was discovered over six decades ago (1). It is composed of the two antigenic Rh30 proteins and the Rh-associated glycoprotein, RhAG (2-9), which is most closely related to the ancestor of all other Rh proteins (ref. 8 and J. Peng and C.-H. Huang, personal communication). The biochemical function of Rh proteins, which are predicted to have 12 transmembrane-spanning segments, remains controversial (10-12). Human RhAG was reported to be an ammonium import/methylammonium export system when expressed in Saccharomyces cerevisiae (13) and an ammonium-(methylammonium)/proton exchanger in oocytes injected with RhAG cRNA (14). Moreover, Rhnull red blood cells were found to accumulate more of the ammonium analogue [14C]methylammonium than normal red cells and to lose it less rapidly after being preloaded, leading to the proposal that the Rh blood group substance was an ammonium(methylammonium) export system (15). Although inconsistent, all of the studies cited above concluded that Rh proteins, like their only known paralogues, the ammonium/methylammonium transport (Amt) proteins [also called methylammonium permeases (Mep) in Saccharomyces cerevisiae], were active transport systems for the ion 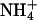 . Disruption of an Rh gene in the slime mold Dictyostelium discoideum yielded no phenotype (16).
. Disruption of an Rh gene in the slime mold Dictyostelium discoideum yielded no phenotype (16).
Contrary to views of others, we have proposed that Amt/Mep proteins are bidirectional channels for the uncharged species NH3 and constitute an example of biological gas channels (12, 17-20). There is now considerable genetic and physiological evidence to support this view in several microorganisms. We have proposed that Rh proteins are gas channels for CO2 (12), which like NH3 is a readily hydrated gas. As a first test of the latter hypothesis, we studied expression of the RH1 gene of Chlamydomonas reinhardtii, one of the few microorganisms to have Rh proteins. Chlamydomonas also has at least four AMT genes (K.-S. Kim and W.I., unpublished data). In support of our hypothesis, the RH1 gene is highly expressed under conditions of high CO2 availability with ammonium as nitrogen source, a condition of nitrogen excess, whereas Amt function is detected only under nitrogen-limiting conditions. Based on the association of erythrocyte Rh proteins with the  exchanger (band 3), Tanner and colleagues (21, 22) have recently concurred with our view that Rh proteins are gas channels but have speculated that they have broad substrate specificity (CO2, O2, NO). Forster and colleagues (23) obtained direct evidence that a protein might be involved in CO2 transport across the erythrocyte membrane.
exchanger (band 3), Tanner and colleagues (21, 22) have recently concurred with our view that Rh proteins are gas channels but have speculated that they have broad substrate specificity (CO2, O2, NO). Forster and colleagues (23) obtained direct evidence that a protein might be involved in CO2 transport across the erythrocyte membrane.
Given that homologous recombination has not been used successfully to target gene disruptions in C. reinhardtii, we sought to eliminate function of Rh1 by RNA interference (RNAi), a method that has been used previously to down-regulate gene expression in this organism (refs. 24-26 and J. Rohr, N. Sarkar, S. Balenger, B.-r. Jeong, and H. Cerutti, personal communication). By RNAi, we have now obtained three lines of C. reinhardtii with essentially no mRNA or protein for RH1 and have initiated their phenotypic characterization.
Materials and Methods
Strains and Growth Conditions. Strains 4A+, 17D-, 4A-, and CC125 were obtained from the laboratory of K. K. Niyogi (University of California, Berkeley). Strains 4A+ and 17D- were isolated in the laboratory of J.-D. Rochaix (University of Geneva, Geneva). They are nit1 nit2 derivatives of wild-type strain 137c (27) that were selected for rapid growth on acetate in the dark. The 4A- strain, a mating minus strain isogenic with 4A+, was selected after progeny of 4A+ × 17D- were backcrossed for five generations to 4A+. Strains were maintained at 24°C on TAP medium containing NH4Cl (10 mM) as nitrogen source (27) under continuous illumination (40 μmol photons m-2·s-1). Zeomycin (trade name Zeocin)-resistant transformants were maintained similarly with 5 μg/ml of Zeocin (Invitrogen) added to the medium and progeny from genetic crosses were scored at this Zeocin concentration. Cells were grown in liquid culture in TP medium (27) without Zeocin and were bubbled with air [0.035% (vol/vol) CO2] or with air enriched with 3% (vol/vol) CO2 (referred to as high CO2) (12). For shift experiments, cells were grown in TAP medium with vigorous shaking until they reached mid-exponential phase (chlorophyll a+b content of 4-8 μg/ml). They then were bubbled with high CO2 for 3 h. The nitrogen source was NH4Cl (10 mM) unless otherwise specified. Chlorophyll a+b content was estimated after extracting cells with 96% (vol/vol) ethanol (28).
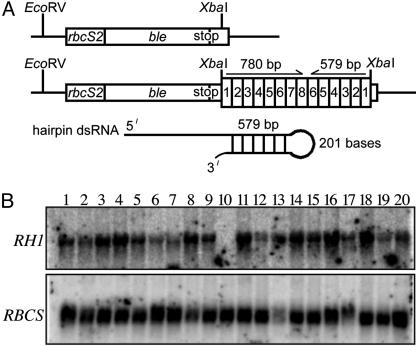
RNAi Construct. Plasmid pJES1459, which carries a long arm sense-short arm antisense construct for a portion of RH1 (Fig. 1), was constructed as follows. The 780-bp region containing exons 1-8 of RH1 and the 579-bp region containing exons 1-6 were amplified by PCR from cloned cDNA (GenBank accession number AY013257). This was done by using a single forward primer, RH1-XbaI (5′-CCTCTAGACCTCCCAAGATTCCCGC-3′), which introduced a unique XbaI restriction site, and a specific reverse primer for exon 8, RH1-Avr-Ex8 (5′-CACCTAGGGGCCAGTAGATGAAGAGG-3′), or exon 6, RH1-Avr-Ex6 (5′-AACCTAGGCAGCTGCTGGTTGAGAGC-3′), each of which introduced a unique AvrII site (restriction sites underlined in each case). PCRs were performed with Herculase Hot-Start polymerase (Stratagene) in a 100-μl volume containing 12 ng of RH1 cDNA, 200 nM of each primer, 200 μM dNTPs, and 2.5 units of Herculase Hot-Start polymerase. The conditions were: 2 min at 95°C, 30 cycles of amplification [denaturation (1 min at 94°C)/annealing (1 min at 60°C)/polymerization (1 min at 72°C)], and 10 min at 72°C. In each case, the unique fragment obtained was recovered from an agarose gel and cloned into the pT7-blue vector (Novagen) according to the manufacturer's recommendations. The 780-bp fragment (exons 1-8) was cloned in the forward orientation behind the T7 promoter of the vector to yield plasmid pJES1456, and the 579-bp fragment (exons 1-6) was cloned in the reverse orientation to yield pJES1457. Plasmid pJES1458 was obtained by cloning the ≈600-bp SmaI/AvrII fragment of pJES1457 into pJES1456 digested with SmaI and AvrII. Finally, the ≈1.3-kbp XbaI fragment of pJES1458, which carries the sense-antisense construct, was moved into NE451 linearized with XbaI, to yield pJES1459. NE451, a generous gift of H. Cerutti (University of Nebraska, Lincoln), was derived from pSP124S (ref. 29 and J. Rohr, N. Sarkar, S. Balenger, B.-r. Jeong, and H. Cerutti, personal communication) by removing the XbaI site upstream of the RBCS2 promoter, and hence it contains a unique XbaI site 20 bp downstream of the stop codon for the ble gene in the 3′ UTR of the RBCS2 gene (Fig. 1).
Fig. 1.
Construct yielding a double-stranded RNA hairpin for the RH1 gene (A) and Northern analysis of RH1 mRNA levels in Zeocin-resistant transformants of strain 4A+ (B). (A) Plasmid pJES1459 is a derivative of pSP124S (see Materials and Methods). The RBCS2 promoter drives expression of both the ble gene, which codes for Zeocin resistance, and a double-stranded RNA hairpin for a portion of the RH1 gene (double-stranded RNA RH1). (B) An example of a Northern blot for screening RH1 expression in Zeocin-resistant transformants. Cultures were grown on acetate with vigorous shaking until they reached the exponential growth phase and were then shifted up by bubbling with high CO2 for 3 h (see Materials and Methods). Two micrograms of total RNA was used in each lane. The membrane was hybridized to a probe for RH1 and then stripped and probed for RBCS. The sample for clone 22i is in lane 10.
DNA Transformation. Plasmid pJES1459 linearized with EcoRV (Fig. 1) was transformed into strain 4A+ by electroporation (30). The same was done for the vector NE451. Before electroporation, the cell wall of 4A+ was digested with an autolysin preparation obtained after mating strains 4A+ and 17D- (27). [Lytic activity was followed at OD435 after exposing treated cells to a mixture of Triton X-100 (0.075%) and EDTA (5 mM) (27).] A 150-ml culture of 4A+ grown on TAP medium to a chlorophyll a+b content of 6 μg/ml was treated with 6 ml of autolysin for 60 min at room temperature. Cells were then collected by low-speed centrifugation at 800 × g for 5 min at 10°C and suspended in 1.5 ml of TAP containing 40 mM sucrose at room temperature. A 250-μl portion of concentrated cells was mixed with 2.5 μg of linearized vector (in 40 μl) in an electroporation cuvette (0.4 cm wide) and chilled to 15°C. Electro-transformation was achieved by using a burst of 2,000 V with a capacitance of 10 μF without a resistance shunt. After shock, cells were left in the cuvette at 25°C for 30 min without agitation and were then transferred into a 150-ml flask containing 25 ml of TAP medium plus NH4Cl (10 mM) plus sucrose (40 mM) and incubated with shaking under light for 24 h. Before plating, cells were concentrated by centrifugation at 1,000× g for 5 min at room temperature and were suspended in 5 ml of TAP medium. Concentrated cells (0.5 ml) were mixed with melted agar (3.5 ml of 0.6% agar) that had been equilibrated at 42°C (31) and were quickly spread on TAP plates (2% agar) containing NH4Cl (10 mM) and Zeocin (5 μg/ml). Plates were incubated under light for 3 weeks before transformants were picked.
Isolation and Detection of RNA and Protein, Assays for Methylammonium Sensitivity and Transport, and Genetic Crosses. Isolation of RNA, Northern analysis, Western analysis, and [14C]methylammonium uptake assays were performed as described (12). The mtCA (LIP-21) (32) and LIP-36 (33) proteins were detected with rabbit polyclonal antibodies directed against the intact proteins (generous gift of M. H. Spalding, Iowa State University, Ames). Sensitivity to methylammonium (concentrations of 50, 100, and 1,000 μM) was determined on solid TAP medium with arginine (2.5 mM) as the nitrogen source. The lowest concentration to which 4A+ is sensitive is 50 μM. With ammonium as nitrogen source strain, 4A+ is resistant to methylammonium at concentrations up to 5 mM. Genetic analysis was performed as described (34). The RNAi strains described in this study had few or no flagellated cells, which was problematic for successful mating. Various isolation, growth conditions, and enrichment procedures were attempted to encourage mating. Five isolates of each RNAi line were mated 10 times each. Clone 28i never produced zygotes. Clone 24i produced a few apparent zygotes that failed to germinate. Only clone 22i, in two matings, produced the three tetrads described in Results. To increase the chances of obtaining viable progeny after zygotes were germinated, products were allowed to grow in place before single colonies were isolated.
Results
RNAi Decreases Amounts of RH1 mRNA. The C. reinhardtii parental strain 4A+ was transformed with plasmid pJES1459, a construct expressing an inverted repeat of the first six exons of RH1. This construct should yield a double-stranded RNA hairpin with an arm length of ≈600 bp and a loop of ≈200 bases constituted by exons 7 and 8 of RH1 (Fig. 1 A) (ref. 26 and J. Rohr, N. Sarkar, S. Balenger, B.-r. Jeong, and H. Cerutti, personal communication). At the recommendation of H. Cerutti, we made a transcriptional fusion of the DNA coding for the RH1 hairpin to the ble gene (codes for Zeocin resistance) in such a way that both were under control of the RBCS2 promoter (Fig. 1). This was done to increase the probability that Zeocin-resistant transformants, which should carry stable insertions in the genome, would express the RH1 hairpin. Transformants were selected on acetate, a condition under which RH1 expression is very low in the parental strain and Rh1 protein is presumably not required (12).
Zeocin-resistant transformants of 4A+ obtained with pJES1459 (n = 120) or with the vector NE451 (n = 50) were screened by Northern blot hybridization for levels of RH1 mRNA (Fig. 1B). Clones were grown to mid-exponential phase in medium containing acetate and were then subjected to an upshift by bubbling with high CO2 for 3 h. For three clones (3% of the transformants), designated 22i (Fig. 1B, lane 10), 24i, and 28i (not shown), RH1 mRNA was not detected. These clones had normal amounts of RBCS mRNA and no apparent growth defect before shift. We noted that among the 120 experimental transformants, ≈10% grew very slowly on acetate. Many of these had parental amounts of RH1 mRNA. Those that had low amounts of RH1 and RBCS mRNA were not studied further. Clones obtained by transformation with the vector NE451 expressed RH1 at normal levels and one of them, clone 2, was chosen as a control for subsequent experiments.
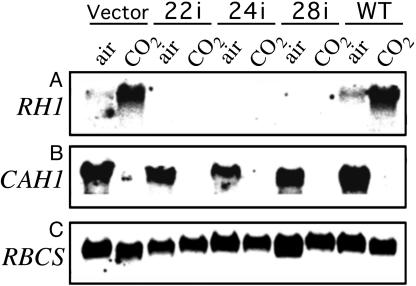
To confirm that RH1 mRNA was not present in clones 22i, 24i, and 28i, even under steady-state conditions of high CO2 availability, cells were grown in medium without acetate and bubbled with high CO2 (12). Under this condition, both the parental strain 4A+ and control clone 2 expressed RH1 strongly, whereas clones 22i, 24i, and 28i had no detectable RH1 mRNA (Fig. 2A). Expression of the periplasmic carbonic anhydrase gene CAH1, which is known to be induced only under conditions of limiting carbon availability (35-37), was detected in all strains grown in air but not in high CO2 (Fig. 2B). The expected lack of detection of CAH1 mRNA in the cultures bubbled with high CO2 indicated that clones 22i, 24i, and 28i perceived this condition as one of CO2 excess. These clones had normal amounts of mRNA for RBCS (Fig. 2C).
Fig. 2.
Northern analysis of RH1 mRNA levels in Zeocin-resistant transformants. Five micrograms of total RNA was used for each lane. Membranes were hybridized to probes for RH1 (A), CAH1 (B), or RBCS (C). Samples were from cells grown in air or high CO2 as indicated. In all cases, the nitrogen source was 10 mM NH4Cl. Clone 2 is a transformant obtained with the vector alone, clones 22i, 24i, and 28i are rh1 RNAi lines, and 4A+ is the parental strain.
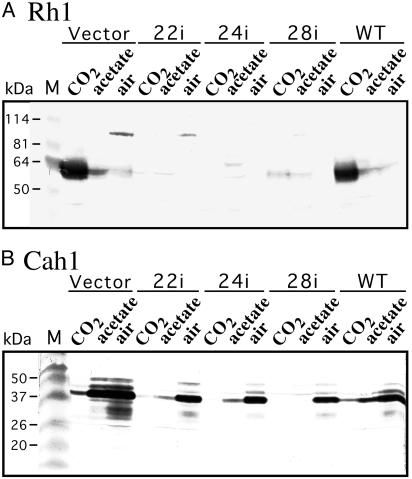
RNAi Lines Lack Rh1 Protein. Western analysis using affinity-purified antibody specific to Rh1 (12) established that clones 22i, 24i, and 28i produced negligible amounts of Rh1 protein when grown in high CO2 (Fig. 3A). Control strains had large amounts of Rh1 protein under these conditions. All strains had large amounts of Cah1 protein (38) when grown in air (Fig. 3B).
Fig. 3.
Western analysis of levels of Rh1 protein (A) and the periplasmic carbonic anhydrase Cah1 (B). Whole cell samples were prepared, subjected to SDS/PAGE (8.5% gel in A and 12% gel in B), and analyzed as described in Materials and Methods. Extract corresponding to 4 μg of chlorophyll a+b was used. The nitrocellulose membrane was hybridized with antiserum directed against a unique C-terminal peptide of Rh1 (A) or against Cah1 (B). Sera were diluted 1,000-fold. Cells were grown on high CO2, acetate bubbled with air, or air, as indicated. Clone 2 carries the vector alone, clones 22i, 24i, and 28i are rh1 RNAi lines, and 4A+ is the parental strain. Molecular mass standards (Benchmark Prestained, GIBCO/BRL) are on the left.
Rh1 Protein Is Required for Optimal Growth at High CO2. To determine the function of the Rh1 protein, we measured the growth rates of the clones lacking it under three conditions (Table 1). When bubbled with high CO2, the three rh1 RNAi lines grew slower than their parental strain 4A+ and control clone 2 (Table 1), although their doubling times differed. Growth defects were apparently specific to high CO2 because the three RNAi lines grew as well as control strains on air or acetate bubbled with air, conditions under which RH1 is expressed at very low levels (Fig. 3A).
Table 1.
Growth of rh1 RNAi lines
| Doubling time, h
|
||||
|---|---|---|---|---|
| Strain | 3% CO2* | Acetate† | Air* | |
| 4A+ | (Parent) | 5 | 8 | 22 |
| Vector (clone # 2) | (Control) | 5 | 8 | 20 |
| 22i | (RNAi) | 7 | 8 | 21 |
| 24i | (RNAi) | 8 | 8 | 21 |
| 28i | (RNAi) | 10 | 8 | 21 |
TP medium with 10 mM NH4Cl as nitrogen source, bubbled with air enriched with 3% CO2 (3% CO2) or air (0.035% CO2)
TAP medium with 10 mM NH4Cl as nitrogen source, bubbled with air
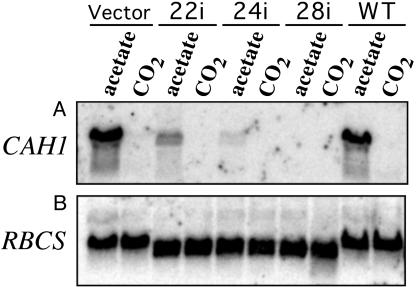
Lack of Rh1 Protein Affects Expression of Three CO2-Responsive Genes Involved in the Carbon Concentrating Mechanism (CCM). Many Chlamydomonas genes are highly expressed during growth in air, a circumstance under which the so-called CCM is activated (36, 39-44). These include genes coding for a periplasmic carbonic anhydrase Cah1 (36, 38), the mitochondrial carbonic anhydrase mtCA (32, 45), and the chloroplast envelope protein LIP-36 (33, 46). We have found that these genes are also highly expressed when the two parental strains that we tested, 4A+ and CC125, are grown on acetate and bubbled with air (Fig. 3B, Western analysis; Fig. 4A, Northern analysis; and data not shown). This therefore appears to be another condition of low CO2 availability (but see ref. 35). Although all three rh1 RNAi lines had high levels of CAH1 mRNA when grown in air (Fig. 2B; see above), they had low, albeit somewhat different, levels of CAH1 mRNA when grown on acetate with air (Fig. 4A). Likewise, they had low levels of Cah1 protein (Fig. 3B) and of the mtCA and LIP-36 proteins (Fig. 6, which is published as supporting information on the PNAS web site). The three rh1 RNAi lines had higher levels of the mtCA and LIP-36 proteins when grown in air.
Fig. 4.
Northern analysis of CAH1 mRNA levels in acetate-grown cells. Five micrograms of total RNA was used for each lane. Membranes were hybridized to probes for CAH1 (A)or RBCS (B). Cells were grown on acetate and bubbled with air or on high CO2 as indicated. In all cases, the nitrogen source was 10 mM NH4Cl. Clone 2 carries the vector alone, clones 22i, 24i, and 28i are rh1 RNAi lines, and 4A+ is the parental strain.
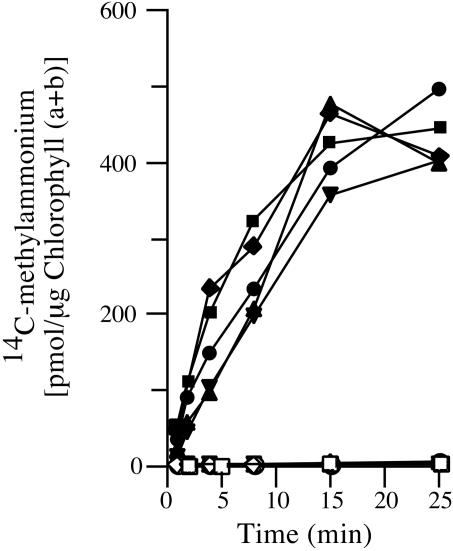
Lack of Rh1 Protein Does Not Affect Sensitivity to or Transport of Methylammonium. C. reinhardtii, like a number of other organisms, is sensitive to methylammonium and accumulates [14C]methylammonium only when grown under nitrogen-limiting conditions (see Materials and Methods) (12). Expression of three of the four AMT genes of this organism is highly induced under these conditions (K.-S. Kim and S.K., unpublished observations). We tested our rh1 RNAi lines for uptake of [14C]methylammonium when they were grown with either arginine or ammonium as nitrogen source. Like control strains, all accumulated large amounts of [14C]methylammonium when grown with arginine, a nitrogen-limiting condition, and failed to do so when grown with ammonium, a nitrogen-excess condition. This was true both for cells grown at high CO2 (Fig. 5) and cells grown on acetate (not shown). Likewise, all rh1 RNAi lines proved as sensitive to toxic effects of methylammonium as parental strain 4A+ or the vector control strain (data not shown; see Materials and Methods).
Fig. 5.
Uptake of [14C]methylammonium. Cells were grown in high CO2 with arginine (filled symbols) or NH4Cl (open symbols) as nitrogen source. They were exposed to methylammonium at an initial concentration of 6 μM. Clone 2 (vector), diamonds; clones 22i, 24i, and 28i (rh1 RNAi lines), circles, triangles, and inverted triangles, respectively; 4A+ (parent), squares.
Genetic Crosses with rh1 RNAi Lines. Crosses with RNAi line 22i provided evidence that Zeocin-resistance, loss of RH1 expression, and an alteration in CAH1 expression on acetate could be coinherited. Results were in accord with the view that clone 22i carried a Zeocin insertion(s) at a single locus in a nuclear chromosome.
Control clones 2 and 12, which carried the vector with no insert, yielded Zeocin-resistant and Zeocin-sensitive progeny 2:2. However, all three rh1 RNAi lines had few to no flagella, and only clone 22i, which had ≈1% flagellated cells in a typical culture, yielded progeny in a cross (see Materials and Methods). In crosses to strain 4A-, only three tetrads were obtained, one parental ditype and two tetratypes with respect to mating type (Table 2). With one exception (22i-34), the eight Zeocin-resistant progeny that we tested, like clone 22i itself, lacked Rh1 protein when grown at high CO2, indicating that it was possible to recover the Rh1 phenotype associated with Zeocin resistance. These progeny also had low levels of Cah1 protein on acetate. The four Zeocin-sensitive progeny that we tested, which came from the tetratype tetrad in which all genotypes were recovered, had Rh1 protein, like strains 4A+, 4A- and control clone 2. Unfortunately a Zeocin-resistant strain obtained from the complete tetratype tetrad (22i-29, mt+), mated poorly and failed to yield Zeocin-resistant progeny in a backcross to strain 4A-. The Zeocin-resistant strain mentioned above as an exception, 22-34i, was also obtained from the complete tetratype tetrad. This strain expressed RH1 and also expressed CAH1 on acetate. Hence, it had apparently lost the capacity to silence RH1. Strain 22i-34 mated well in additional crosses.
Table 2. Progeny from crosses between RNAi line 22i and 4A-.
| Cross | No. of colonies |
|---|---|
| Tetrad 1 (tetratype) | |
| ZR*† mt+ | 2 |
| ZR‡ mt- | 1 |
| ZS§ mt+ | 3 |
| ZS mt- | 2 |
| Tetrad 2 (parental ditype) | |
| ZR* mt+ | 5 |
| ZS mt- | 4 |
| Tetrad 3 (tetratype) | |
| ZR mt+ | 0 |
| ZR¶ mt- | 1 |
| ZS mt+ | 1 |
| ZS mt- | 6 |
ZR, Zeocin-resistant; ZS, Zeocin-sensitive; mt+, mating type plus; mt-, mating type minus.
All ZR progeny from tetrads 1 and 2 were tested for Rh1 and Cah1 proteins by Western blot. Cells were grown at high CO2 or on acetate, respectively
One of these was designated 22i-29
Clone 22i-34 appears to have lost the ability to silence RH1
Four of the five ZS progeny from tetrad 1 were tested for Rh1 protein. Cells were grown at high CO2
Clone 22i-19 took several days longer to grow on plates containing Zeocin and was less resistant than the other eight clones that were studied
In the parental ditype tetrad and the complete tetratype tetrad obtained from crosses between clone 22i and 4A-, Zeocin-resistance and sensitivity segregated 2:2. This was also the case for the many tetrads obtained by crossing strain 22i-34 (mt-), described above, to strain 4A+. Thus, Zeocin-resistance in clone 22i behaved as if it was associated with a single nuclear locus.
Discussion
Based on the homology of Rh proteins to Amt/Mep proteins, the abundance of the Rh blood group substance, and the organismal, organ, and tissue distribution of Rh proteins, we speculated that they were biological gas channels for CO2 (12). In agreement with this view, we found that expression of the RH1 gene of C. reinhardtii, one of the few microbes to have Rh proteins, was greatly increased at high CO2 (air supplemented with 3% CO2). By RNAi, we have now isolated three lines of C. reinhardtii epigenetic mutants that fail to express RH1 mRNA or protein. These are among the first rh mutants in an organism other than humans (16).
The rh1 RNAi lines had growth defects specifically at high CO2, a condition under which wild type strains grow rapidly. Thus, Rh1, which is a membrane protein and hence would function before the CO2-fixing enzyme ribulose-bisphosphate carboxylase, apparently allows rapid equilibration of CO2 when it is readily available. The growth defect of the RNAi lines provides further evidence that Rh1 is a gas channel for CO2. At high CO2, the CCM is not induced (36, 39-44) and there is no need to prevent CO2 regenerated near Rubisco from leaking away. (It is CO2, rather than 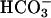 , which is the substrate for this enzyme, but probably
, which is the substrate for this enzyme, but probably 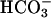 that is accumulated and transferred to the chloroplast by cells grown in air). To our knowledge, the rh1 RNAi lines are the first strains of C. reinhardtii with growth defects specifically at high CO2. We have maintained these lines on acetate, a condition under which RH1 is poorly expressed by parental strains, and their phenotype has been stable for >1 year. Unfortunately, it was difficult to do genetic crosses with the RNAi lines, and the Rh1 phenotype was coinherited with Zeocin resistance only among progeny that had the same problems with flagellar synthesis and mating as the original RNAi line (see Results).
that is accumulated and transferred to the chloroplast by cells grown in air). To our knowledge, the rh1 RNAi lines are the first strains of C. reinhardtii with growth defects specifically at high CO2. We have maintained these lines on acetate, a condition under which RH1 is poorly expressed by parental strains, and their phenotype has been stable for >1 year. Unfortunately, it was difficult to do genetic crosses with the RNAi lines, and the Rh1 phenotype was coinherited with Zeocin resistance only among progeny that had the same problems with flagellar synthesis and mating as the original RNAi line (see Results).
The rh1 RNAi lines also have an interesting regulatory defect. When grown on acetate, they express three proteins involved in the CCM poorly, whereas parental strains express them well. Like parental strains, the rh1 RNAi lines express these three proteins, Cah1, mtCA, and LIP-36 (32, 33, 36, 38, 45, 46), well when grown in air, indicating that they can sense the absence of high CO2. The failure of rh1 RNAi lines to express the CCM-associated proteins on acetate is parsimoniously explained if the small amounts of Rh1 protein present in parental strains increase leakage of CO2 generated from acetate and hence contribute to a condition of internal CO2 limitation. In other words, Rh1 appears to be a bidirectional channel for CO2. We have previously provided evidence that the Amt protein of Salmonella typhimurium, a paralogue of Rh proteins, is a bidirectional channel for NH3 (20). It appears to allow leakage of NH3 generated internally when S. typhimurium is grown on arginine as sole nitrogen source. Physiologically, biological gas channels can apparently function in nutrient acquisition and/or waste disposal.
We obtained no evidence that Rh1 was involved in transport of methylammonium (CH3NH2 or 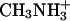 ) (Fig. 5 and Results), a finding that is not surprising given that C. reinhardtii has at least four AMT genes and mRNA levels for three are increased specifically under nitrogen-limiting conditions. By contrast, the largest class of Chlamydomonas mutants selected for resistance to methylammonium (1 mM) has lesions in AMT4, and the amt4 mutants have marked defects in uptake of [14C]methylammonium (6 μM) (K.-S. Kim and W.I., unpublished results). We have previously discussed internal inconsistencies in the original evidence that human Rh proteins expressed in Saccharomyces cerevisiae play a role in ammonium/methylammonium transport (13, 18). Studies in oocytes (14) indicated that methylammonium uptake by human RhAG protein was 50% inhibited at an ammonium concentration >1 mM, which is 10-20 times higher than the maximum concentration found in blood (47). Although these studies provide biochemical evidence that Rh proteins may transport ammonium and its analogue methylammonium, they fail to provide evidence that this is their physiological function. Finally, despite the controls presented, we think that differences in [14C]methylammonium accumulation between Rhnull red blood cells and normal cells (2-fold after 30 min at the very high external concentration of 31 mM) (15) must be interpreted with caution. The Rhnull syndrome in humans, which is very rare (<1/106), is associated with pronounced morphological and biochemical abnormalities of the red cell (2-4, 6, 9, 48-50). These include different degrees of stomatocytosis (bending to form a mouth-like opening), increased osmotic fragility, altered phospholipid asymmetry, altered cell volume, defective cation fluxes, and elevated Na+/K+ ATPase activity. Differences in the accumulation of a weak base such as methylammonium could be the consequence of one or more of these pleiotropic secondary abnormalities rather than a direct result of loss of the primary function of the Rh blood group substance.
) (Fig. 5 and Results), a finding that is not surprising given that C. reinhardtii has at least four AMT genes and mRNA levels for three are increased specifically under nitrogen-limiting conditions. By contrast, the largest class of Chlamydomonas mutants selected for resistance to methylammonium (1 mM) has lesions in AMT4, and the amt4 mutants have marked defects in uptake of [14C]methylammonium (6 μM) (K.-S. Kim and W.I., unpublished results). We have previously discussed internal inconsistencies in the original evidence that human Rh proteins expressed in Saccharomyces cerevisiae play a role in ammonium/methylammonium transport (13, 18). Studies in oocytes (14) indicated that methylammonium uptake by human RhAG protein was 50% inhibited at an ammonium concentration >1 mM, which is 10-20 times higher than the maximum concentration found in blood (47). Although these studies provide biochemical evidence that Rh proteins may transport ammonium and its analogue methylammonium, they fail to provide evidence that this is their physiological function. Finally, despite the controls presented, we think that differences in [14C]methylammonium accumulation between Rhnull red blood cells and normal cells (2-fold after 30 min at the very high external concentration of 31 mM) (15) must be interpreted with caution. The Rhnull syndrome in humans, which is very rare (<1/106), is associated with pronounced morphological and biochemical abnormalities of the red cell (2-4, 6, 9, 48-50). These include different degrees of stomatocytosis (bending to form a mouth-like opening), increased osmotic fragility, altered phospholipid asymmetry, altered cell volume, defective cation fluxes, and elevated Na+/K+ ATPase activity. Differences in the accumulation of a weak base such as methylammonium could be the consequence of one or more of these pleiotropic secondary abnormalities rather than a direct result of loss of the primary function of the Rh blood group substance.
The abnormalities of structure in Rhnull red cells, which are accompanied by a compensated hemolytic anemia, indicate that the Rh blood group substance plays a role in maintaining the flattened (biconcave discoid) shape of the red cell (3-6, 9, 51, 52). This cytoskeletal function increases the surface area to volume ratio relative to a sphere and hence the rate of diffusion of gases. Given that the Rh1 protein of the microbe C. reinhardtii appears to be a gas channel for CO2, the primary biochemical function of Rh proteins, including the Rh blood group substance, is presumably to facilitate CO2 diffusion. Hence, the structural role of the Rh blood group substance appears to be a related secondary function. Beckmann et al. (51) have recent evidence that the Rh30 proteins may be particularly important to the cytoskeletal role of the Rh blood group substance. These uniquely erythroid proteins, which constitute a heterooligomer with RhAG, give rise to the immunological differences between Rh-positive and -negative people (2-9). The Rh30 proteins are evolving more rapidly than RhAG and are apparently being subjected to positive selective pressure (refs. 6-8 and 53-55, and J. Peng and C.-H. Huang, personal communication). Based on the reasoning above, and congruent with views of Huang et al. (53), we propose that the function driving rapid evolution of the Rh30 proteins is their structural role in helping to maintain the flexible discoid shape of the red blood cell.
Supplementary Material
Acknowledgments
We thank H. Cerutti, B. Chin, A. Melis, K. K. Niyogi, and M. H. Spalding for valuable materials and advice, and B. Buchanan, H. Cerutti, L. Csonka, C.-H. Huang, J. Ingraham, B. Magasanik, S. Merchant, and D. Weeks for insightful criticisms of the manuscript. We are grateful to C.-H. Huang for sharing unpublished results from his laboratory and for his ongoing interest in this work. This work was supported by a grant from the Torrey Mesa Research Institute, Syngenta Research and Technology, San Diego (to S.K.).
Abbreviations: Rh, Rhesus; RNAi, RNA interference; Amt, ammonium/methylammonium transport; Mep, methylammonium permease; CCM, carbon concentrating mechanism.
See Commentary on page 7497.
References
- 1.Levine, P. & Stetson, R. E. (1939) J. Am. Med. Assoc. 113, 126-127. [Google Scholar]
- 2.Agre, P. & Cartron, J. P. (1991) Blood 78, 551-563. [PubMed] [Google Scholar]
- 3.Avent, N. D. & Reid, M. E. (2000) Blood 95, 375-387. [PubMed] [Google Scholar]
- 4.Cartron, J. P. (1999) Baillieres Clin. Haematol. 12, 655-689. [DOI] [PubMed] [Google Scholar]
- 5.Cartron, J.-P. & Agre, P. (1993) Semin. Hematol. 30, 193-208. [PubMed] [Google Scholar]
- 6.Huang, C.-H., Liu, P. Z. & Cheng, J. G. (2000) Semin. Hematol. 37, 150-165. [DOI] [PubMed] [Google Scholar]
- 7.Huang, C. H. (1997) Curr. Opin. Hematol. 4, 94-103. [DOI] [PubMed] [Google Scholar]
- 8.Huang, C. H. & Liu, P. Z. (2001) Blood Cells Mol. Dis. 27, 90-101. [DOI] [PubMed] [Google Scholar]
- 9.Walensky, L. D., Narla, M. & Lux, S. E., IV (2003) in Blood: Principles and Practice of Hematology, eds. Handin, R. I., Lux, S. E., IV, & Stossel, T. P. (Lippincott Williams & Wilkins, Philadelphia), pp. 1709-1858.
- 10.Ludewig, U., von Wiren, N., Rentsch, D. & Frommer, W. B. (2001) Genome Biol. 2, 1010.1-1010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakhoul, N. L. & Hamm, L. L. (2004) Pflugers Arch. 447, 807-812. [DOI] [PubMed] [Google Scholar]
- 12.Soupene, E., King, N., Feild, E., Liu, P., Niyogi, K. K., Huang, C. H. & Kustu, S. (2002) Proc. Natl. Acad. Sci. USA 99, 7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini, A.-M., Matassi, G., Raynal, V., Andre, B., Cartron, J.-P. & Cherif-Zahar, B. (2000) Nat. Genet. 26, 341-344. [DOI] [PubMed] [Google Scholar]
- 14.Westhoff, C. M., Ferreri-Jacobia, M., Mak, D. O. & Foskett, J. K. (2002) J. Biol. Chem. 277, 12499-12502. [DOI] [PubMed] [Google Scholar]
- 15.Hemker, M. B., Cheroutre, G., van Zwieten, R., Maaskant-van Wijk, P. A., Roos, D., Loos, J. A., van der Schoot, C. E. & von dem Borne, A. E. (2003) Br. J. Haematol. 122, 333-340. [DOI] [PubMed] [Google Scholar]
- 16.Benghezal, M., Gotthardt, D., Cornillon, S. & Cosson, P. (2001) Immunogenetics 52, 284-288. [DOI] [PubMed] [Google Scholar]
- 17.Soupene, E., He, L., Yan, D. & Kustu, S. (1998) Proc. Natl. Acad. Sci. USA 95, 7030-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soupene, E., Ramirez, R. M. & Kustu, S. (2001) Mol. Cell. Biol. 21, 5733-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soupene, E., Chu, T., Corbin, R. W., Hunt, D. F. & Kustu, S. (2002) J. Bacteriol. 184, 3396-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soupene, E., Lee, H. & Kustu, S. (2002) Proc. Natl. Acad. Sci. USA 99, 3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce, L. J., Beckmann, R., Ribeiro, M. L., Peters, L. L., Chasis, J. A., Delaunay, J., Mohandas, N., Anstee, D. J. & Tanner, M. J. (2003) Blood 101, 4180-4188. [DOI] [PubMed] [Google Scholar]
- 22.Tanner, M. J. (2002) Curr. Opin. Hematol. 9, 133-139. [DOI] [PubMed] [Google Scholar]
- 23.Forster, R. E., Gros, G., Lin, L., Ono, Y. & Wunder, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15815-15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerutti, H. (2003) Trends Genet. 19, 39-46. [DOI] [PubMed] [Google Scholar]
- 25.Koblenz, B., Schoppmeier, J., Grunow, A. & Lechtreck, K. F. (2003) J. Cell Sci. 116, 2635-2646. [DOI] [PubMed] [Google Scholar]
- 26.Sineshchekov, O. A., Jung, K. H. & Spudich, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 8689-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, E. H. (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide To Biology and Laboratory Use (Academic, San Diego). [DOI] [PubMed]
- 28.Wintermans, J. F. & de Mots, A. (1965) Biochim. Biophys. Acta 109, 448-453. [DOI] [PubMed] [Google Scholar]
- 29.Lumbreras, V., Stevens, D. R. & Purton, S. (1998) Plant J. 14, 441-448. [Google Scholar]
- 30.Shimogawara, K., Fujiwara, S., Grossman, A. & Usuda, H. (1998) Genetics 148, 1821-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kindle, K. L. (1990) Proc. Natl. Acad. Sci. USA 87, 1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geraghty, A. M. & Spalding, M. H. (1996) Plant Physiol. 111, 1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramazanov, Z., Mason, C. B., Geraghty, A. M., Spalding, M. H. & Moroney, J. V. (1993) Plant Physiol. 101, 1195-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine, R. P. & Ebersold, W. T. (1960) Annu. Rev. Microbiol. 14, 197-216. [DOI] [PubMed] [Google Scholar]
- 35.Fett, J. P. & Coleman, J. R. (1994) Plant Physiol. 106, 103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuzawa, H., Fujiwara, S., Yamamoto, Y., Dionisio-Sese, M. L. & Miyachi, S. (1990) Proc. Natl. Acad. Sci. USA 87, 4383-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawat, M. & Moroney, J. V. (1995) Plant Physiol. 109, 937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, C. S. & Spalding, M. H. (1995) Plant Mol. Biol. 29, 303-315. [DOI] [PubMed] [Google Scholar]
- 39.Badger, M. R. & Price, G. D. (1994) Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 369-392. [Google Scholar]
- 40.Fukuzawa, H., Miura, K., Ishizaki, K., Kucho, K. I., Saito, T., Kohinata, T. & Ohyama, K. (2001) Proc. Natl. Acad. Sci. USA 98, 5347-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan, A., Helman, Y., Tchernov, D. & Reinhold, L. (2001) Proc. Natl. Acad. Sci. USA 98, 4817-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan, A. & Reinhold, L. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 539-570. [DOI] [PubMed] [Google Scholar]
- 43.Moroney, J. V. & Somanchi, A. (1999) Plant Physiol. 119, 9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang, Y., Zhang, J. & Weeks, D. P. (2001) Proc. Natl. Acad. Sci. USA 98, 5341-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eriksson, M., Karlsson, J., Ramazanov, Z., Gardestrom, P. & Samuelsson, G. (1996) Proc. Natl. Acad. Sci. USA 93, 12031-12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen, Z. Y., Lavigne, L. L., Mason, C. B. & Moroney, J. V. (1997) Plant Physiol. 114, 265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huizenga, J. R., Tangerman, A. & Gips, C. H. (1994) Ann. Clin. Biochem. 31, 529-543. [DOI] [PubMed] [Google Scholar]
- 48.Ballas, S. K., Clark, M. R., Mohandas, N., Colfer, H. F., Caswell, M. S., Bergren, M. O., Perkins, H. A. & Shohet, S. B. (1984) Blood 63, 1046-1055. [PubMed] [Google Scholar]
- 49.Kuypers, F., van Linde-Sibenius-Trip, M., Roelofsen, B., Tanner, M. J., Anstee, D. J. & Op den Kamp, J. A. (1984) Biochem. J. 221, 931-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nash, R. & Shojania, A. M. (1987) Am. J. Hematol. 24, 267-275. [DOI] [PubMed] [Google Scholar]
- 51.Beckmann, R., Smythe, J. S., Anstee, D. J. & Tanner, M. J. (2001) Blood 97, 2496-2505. [DOI] [PubMed] [Google Scholar]
- 52.Ridgwell, K., Tanner, M. J. & Anstee, D. J. (1984) FEBS Lett. 174, 7-10. [DOI] [PubMed] [Google Scholar]
- 53.Huang, C. H., Liu, Z., Apoil, P. A. & Blancher, A. (2000) J. Mol. Evol. 51, 76-87. [DOI] [PubMed] [Google Scholar]
- 54.Kitano, T. & Saitou, N. (2000) Immunogenetics 51, 856-862. [DOI] [PubMed] [Google Scholar]
- 55.Matassi, G., Cherif-Zahar, B., Pesole, G., Raynal, V. & Cartron, J.-P. (1999) J. Mol. Evol. 48, 151-159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.