Abstract
Aims/hypothesis
At the same level of BMI, white people have less visceral adipose tissue (VAT) and are less susceptible to developing type 2 diabetes than Japanese people. No previous population-based studies have compared insulin resistance and insulin secretion between these two races in a standardised manner that accounts for VAT. We compared HOMA-IR, HOMA of beta cell function (HOMA-β%) and disposition index (DI) in US white men and Japanese men in Japan.
Methods
We conducted a population-based, cross-sectional study, comprising 298 white men and 294 Japanese men aged 40–49 years without diabetes. Insulin, glucose, VAT and other measurements were performed at the University of Pittsburgh. We used ANCOVA to compare geometric means of HOMA-IR, HOMA-β% and DI, adjusting for VAT and other covariates.
Results
White men had higher HOMA-IR, HOMA-β% and DI than Japanese men, and the difference remained significant (p<0.01) after adjusting for VAT (geometric mean [95% CI]): 3.1 (2.9, 3.2) vs 2.5 (2.4, 2.6), 130.8 (124.6, 137.3) vs 86.7 (82.5, 91.0), and 42.4 (41.0, 44.0) vs 34.8 (33.6, 36.0), respectively. Moreover, HOMA-IR, HOMA-β% and DI were significantly higher in white men even after further adjustment for BMI, impaired fasting glucose and other risk factors.
Conclusions/interpretation
The higher VAT-adjusted DI in white men than Japanese men may partly explain lower susceptibility of white people than Japanese people to developing type 2 diabetes. The results, however, should be interpreted with caution because the assessment of insulin indices was made using fasting samples and adjustment was not made for baseline glucose tolerance. Further studies using formal methods to evaluate insulin indices are warranted.
Keywords: Cross-sectional analysis, Epidemiology, Humans, Insulin resistance, Japanese, Pathophysiology, Race, White race, Type 2 diabetes, Visceral adipose tissue
Introduction
White people are less susceptible to developing type 2 diabetes than Asians (including Japanese) for any given level of BMI or waist circumference [1]. Moreover, white people have less visceral adipose tissue (VAT) than Japanese people in Japan for any given BMI or waist circumference [2]. In terms of pathophysiology, VAT is more strongly associated with increased insulin resistance than is BMI, because VAT is associated with excess fat deposition in the liver and enhanced pro-inflammatory cytokine production [3]. However, no previous population-based studies have compared insulin resistance or insulin secretion in white people in the USA and Japanese people in Japan after adjusting for VAT. A few volunteer-based studies have compared white and Japanese Americans [4–6] and one study compared white people in Denmark and Japanese people in Japan [7], after adjusting for BMI. However, the results of these studies were inconsistent. Most of these studies used HOMA indices for measuring insulin resistance and insulin secretion [4, 5, 7]. Despite of assessment of HOMA-IR and HOMA of beta cell function (HOMA-β%) from fasting samples, HOMA-IR and HOMA-β% are the most extensively used methods for evaluating insulin resistance and insulin secretion in epidemiological studies [8].
A recent meta-analysis indicated a similar disposition index (DI), i.e. insulin secretion and insulin resistance, among healthy glucose-tolerant participants of several races, including white people and East Asians (including Japanese) [9]. According to this meta-analysis, white people have higher insulin resistance and insulin secretion than East Asians that leads to a similar DI in the two races [9]. However, the sample sizes of the studies in East Asians were very small [9]. In this study, we hypothesise that white men are more insulin resistant, secrete more insulin and have higher DI than Japanese men at the same level of VAT. These differences may partly explain the lower susceptibility of white than Japanese people to type 2 diabetes. To test this hypothesis, we compared HOMA-IR, HOMA-β% and DI in population-based samples from 298 US white men and 294 Japanese men in Japan aged 40–49 years who were participants in the EBCT and Risk Factor Assessment among Japanese and U.S. Men in the Post World War II Birth Cohort (ERA JUMP) study.
Methods
Study population
We have previously described the method of participant selection in detail elsewhere [10]. Briefly, we randomly selected 310 white men from Allegheny County, Pennsylvania, USA and 313 Japanese men from Kusatsu, Shiga, Japan from 2002 to 2006. Exclusion criteria included coronary heart disease, stroke, type 1 diabetes and other severe diseases. Informed consent was obtained from all the participants. Our study was designed to examine differences in subclinical atherosclerosis measures, such as intima-media thickness of the carotid artery and coronary artery calcium, between white and Japanese people, with adequate power. For the current analyses, we excluded 31 participants (12 white and 19 Japanese men) with type 2 diabetes, defined as fasting glucose levels ≥7 mmol/l or use of medications for type 2 diabetes [11]. These individuals were excluded because HOMA-IR does not provide an accurate measure of insulin resistance among individuals with type 2 diabetes [12]. The final sample consisted of 592 men: 298 white men and 294 Japanese men. Of the 592 men, 348 (147 white men and 201 Japanese men) had impaired fasting glucose (IFG), defined as fasting glucose levels ≥5.6 mmol/l [11]. The study was approved by the Institutional Review Boards of the University of Pittsburgh, Pittsburgh, USA and the Shiga University of Medical Science, Otsu, Japan.
Study protocol
All participants underwent a physical examination and a laboratory assessment, and completed a self-administered questionnaire, as described previously [10]. Body weight and height were measured while the participants were wearing light clothing without shoes. BMI was calculated as weight (kg)/height squared (m2). Blood pressure was measured in the right arm of the seated participants after they emptied their bladder and sat quietly for 5 min, using an automated sphygmomanometer (BP-8800; Colin Medical Technology, Komaki, Japan) and an appropriate sized cuff. The average of two measurements was used in the analyses. Participants were considered smokers if they reported current use of cigarettes or having stopped smoking within the past 30 days. Participants were considered alcohol drinkers if they consumed alcohol ≥2 days per week. Physical activity was defined as participants exercising for ≥1 h per week. Participants were considered to have a family history of type 2 diabetes if either their father or mother had self-reported type 2 diabetes. Venipuncture was performed early in the clinic visit after a 12 h fast. Serum and plasma samples were stored at −80°C and shipped to the University of Pittsburgh. Serum/plasma samples were assayed for glucose, insulin, lipids (including triacylglycerol, LDL-cholesterol and HDL-cholesterol), C-reactive protein (CRP) and adiponectin as described previously [10].
VAT was determined at the level between the fourth and fifth lumbar vertebrae, using CT images obtained with the same apparatus at each site using a GE-Imatron C150 scanner (GE Medical Systems, South San Francisco, CA, USA) [13]. All CT images were analysed at the University of Pittsburgh by one trained reader using image analysis software (AccuImage Diagnostic, San Francisco, California). To determine the respective area of VAT and subcutaneous adipose tissue, a separation line was drawn manually using a cursor along the abdominal wall musculature in continuity with fascia of the paraspinal muscle. The reproducibility of the scans had an intraclass correlation of 0.90.
Insulin resistance was estimated from fasting samples as HOMA-IR using the approximated equation of Matthews et al [14]. Insulin secretion (beta cell function) was also obtained from fasting samples, as HOMA-β% using the approximated equation of Matthews et al [14]. At any given time, insulin secretion depends on the level of insulin resistance; therefore, DI, estimated as HOMA-β%/HOMA-IR, provides a better assessment of insulin secretion than only considering HOMA-β% [15].
Statistical analyses
We calculated race-specific means (±SD), medians (interquartile range) or geometric means (95% CI) for continuous variables based on their distributions. Proportions were estimated for categorical variables. Means, geometric means, medians and proportions of the variables were then compared between white and Japanese men using t tests, Mann-Whitney U tests or χ2 tests as appropriate. CRP and adiponectin were log converted for non-normal distributions. We used ANCOVA to compare geometric means of HOMA-IR, HOMA-β% and DI between white and Japanese men, adjusting for VAT. Insulin resistance is determined by factors other than VAT, such as smoking [16], alcohol consumption [16], physical activity [16], lipid medications [17], and levels of CRP [18] and adiponectin [19]. Moreover, individuals with IFG have higher insulin resistance and insulin secretion than individuals without IFG [20]. Therefore, we compared HOMA-IR, HOMA-β% and DI between white and Japanese men after further adjustment for BMI, smoking, alcohol, physical activity, lipid medications, IFG, and levels of CRP and adiponectin. We also performed sensitivity analyses on participants with normoglycaemia and IFG to examine how HOMA-IR, HOMA-β% and DI differ between the two races in these subgroups. All p values were two tailed. P values of <0.01 were considered significant. All statistical analyses were performed using SAS software version 9.3 (Cary, NC, USA).
Results
Table 1 shows the characteristics of the study population. White men had significantly higher BMI, VAT, fasting insulin levels and CRP levels than Japanese men. White men were significantly more physically active, had higher adiponectin levels and lipid medication use than Japanese men. In addition, white men had a significantly lower prevalence of smoking, alcohol consumption, hypertension and IFG, and lower levels of glucose than Japanese men. Electronic Supplementary Material [ESM] Table 1 shows the characteristics of the participants with normoglycaemia and ESM Table 2 shows the characteristics of the participants with IFG.
Table 1.
Characteristics of the ERA JUMP participants
| Characteristic | White men | Japanese men | p value |
|---|---|---|---|
| Participants (n) | 298 | 294 | |
| Age (years) | 45.0 (2.8) | 45.1 (2.8) | NS |
| BMI (kg/m2) | 27.8 (4.3) | 23.5 (2.9) | <0.01 |
| VAT (cm2) | 171.0 (73.5) | 131.6 (51.2) | <0.01 |
| Systolic BP (mmHg) | 122.5 (11.3) | 124.6 (16.0) | NS |
| Current smokersa (%) | 7.4 | 50.3 | <0.01 |
| Alcohol drinkersb (%) | 44.6 | 67.0 | <0.01 |
| Hypertensionc (%) | 14.4 | 24.5 | <0.01 |
| Glucose (mmol/l) | 5.5 (5.2, 5.8) | 5.7 (5.4, 6.1) | <0.01 |
| Insulin (pmol/l) | 88.9 (71.5, 120.8) | 65.3 (50.0, 84.7) | <0.01 |
| Triacylglycerol (mmol/l) | 1.4 (1.0, 2.1) | 1.6 (1.1, 2.1) | NS |
| LDL-cholesterol (mmol/l) | 3.5 (0.87) | 3.4 (0.93) | NS |
| HDL-cholesterol (mmol/l) | 1.2 (0.33) | 1.4 (0.33) | <0.01 |
| CRP (nmol/l) | 9.2 (8.2, 10.3) | 3.7 (3.3, 4.1) | <0.01 |
| Adiponectin (mg/l) | 10.1 (9.6, 10.7) | 6.1 (5.6, 6.5) | <0.01 |
| IFGd(%) | 49.3 | 68.4 | <0.01 |
| Hypertension medication (%) | 7.7 | 5.1 | NS |
| Lipid medication (%) | 12.1 | 3.1 | <0.01 |
| Physical activitye(%) | 72.8 | 26.1 | <0.01 |
| Family history of type 2 diabetesf (%) | 12.8 | 11.6 | NS |
Values are means (SD) unless specified otherwise
Current smokers were defined as having reported current use of cigarettes or having stopped smoking within the past 30 days
Alcohol drinkers were defined as those who consumed alcohol ≥2 times/week
Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications
IFG was defined as fasting serum glucose level ≥5.6 mmol/l
Physical activity was defined as exercise ≥1 h in a week
Family history of type 2 diabetes was defined as either father or mother of participant having type 2 diabetes
NS, not significant
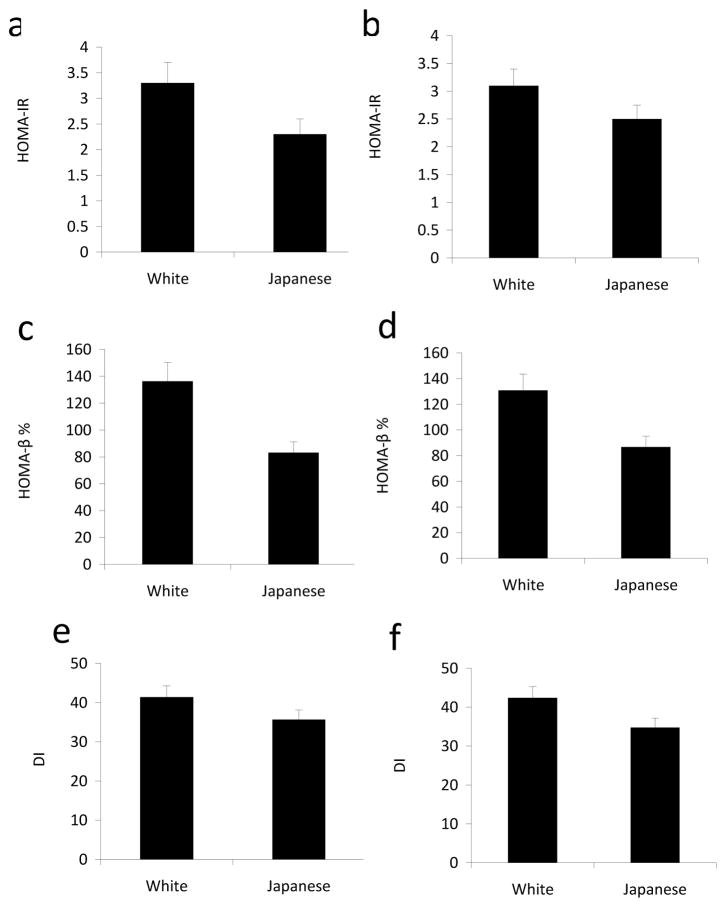
White men had significantly higher HOMA-IR (Fig. 1.0 a,b), HOMA-β% (Fig. 1.0 c,d) and DI (Fig. 1.0 e,f) than Japanese men, both before and after adjusting for VAT. Even after further adjustment for BMI, smoking, alcohol, physical activity, lipid medications, IFG and levels of CRP and adiponectin, white men had higher HOMA-IR, HOMA-β% and DI than Japanese men (Table 2).
Fig. 1.
HOMA-IR, HOMA-β% and DI by race without (a, c and e, respectively) and with (b, d and f, respectively) adjustment for VAT in the ERA JUMP study. Data are geometric means ± 95% CI. HOMA-IR, HOMA-β% and DI were significantly different (p<0.01) between white men and Japanese men before and after adjustment for VAT
Table 2.
Comparison of HOMA-IR, HOMA-β% and DI between white and Japanese men in the ERA JUMP studya
| White men (n=298) | Japanese men (n=294) | p value | |
|---|---|---|---|
| Insulin resistance marker | |||
| HOMA-IR | 3.1 (2.9, 3.2) | 2.5 (2.4, 2.7) | <0.01 |
| Insulin secretion markers | |||
| HOMA-β% | 121.9 (114.9, 129.3) | 93.5 (88.0, 99.3) | <0.01 |
| DI | 40.0 (38.8, 41.1) | 37.0 (35.9, 38.1) | <0.01 |
Values are geometric means (95% CI)
Adjusted for visceral adipose tissue, BMI, current smokers, alcohol drinkers, physical activity, lipid medication, IFG, C-reactive protein and adiponectin
In sensitivity analyses, HOMA-IR, HOMA-β% and DI were significantly higher in white than Japanese men in both subgroups (normoglycaemia and IFG) before (ESM Fig. 1.0 a,c,e) and after adjusting for VAT (ESM Fig. 1.0 b,d,f). After further adjustment for BMI, smoking, alcohol, physical activity, lipid medications, and levels of CRP and adiponectin, HOMA-IR, HOMA-β% and DI were still higher in white men than Japanese men in both subgroups, but the difference was not statistically significant (ESM Tables 3 and 4).
Discussion
This population-based study is the first to compare VAT-adjusted HOMA-IR, HOMA-β% and DI in US white men and Japanese men in Japan in a standardised manner. White men were more insulin resistant than Japanese men, with a significantly higher HOMA-IR both before and after adjusting for VAT. White men also had higher insulin secretion with a significantly higher HOMA-β%, and higher insulin secretion relative to insulin resistance with a significantly higher DI than Japanese men, both before and after adjusting for VAT.
Most of the studies evaluating differences in insulin indices between white and Japanese individuals have examined differences between white people from the USA and Japanese Americans [4–6]. However, it is important to make a distinction between Japanese Americans and Japanese in Japan because insulin resistance is determined not only by genetics [21] but also by metabolic factors such as obesity [22] and abdominal fat deposition [3]. Japanese Americans have a more Westernised lifestyle and thus have much higher BMI, VAT and prevalence of type 2 diabetes than Japanese living in Japan [23, 24].
Previous comparisons of insulin resistance between white and Japanese individuals have produced inconsistent results. In accordance with our findings, one study reported higher insulin resistance in US white people than Japanese Americans, measured using HOMA-IR among first degree relatives of individuals with type 2 diabetes, both before and after adjusting for BMI [4]. Similarly, a recent hospital-based study found higher insulin resistance in white people from Denmark than Japanese in Japan, as estimated by HOMA-IR and the Matsuda index in the unadjusted analyses [7]. However, after adjusting for BMI in this study, the significant difference in insulin resistance between the two races attenuated and became nonsignificant. In contrast to our findings, two studies reported lower insulin resistance in US white people than Japanese Americans. One study reported lower insulin resistance in white than Japanese American women without diabetes, as measured by the HOMA index [5]. Similarly, another study reported lower insulin resistance in white people than Japanese Americans, measured by the hyperglycaemic clamp among normal glucose tolerant participants [6]. However, all the above-mentioned studies were volunteer-based; most of the studies were small, and adjusted for only BMI in the comparison of insulin resistance between the races. The interpretations of these studies are thus limited.
We observed higher HOMA-β% (beta cell response or insulin secretion) in white than Japanese men both before and after adjusting for VAT. Our finding of higher insulin secretion is in accordance with higher insulin secretion in white than Japanese Americans in two earlier studies [4, 5]. In disagreement to our study findings, two studies found similar levels of insulin secretion in white and Japanese people [6, 7]. Smoking has been inversely associated with impaired insulin secretion in Japanese individuals in a prospective study [25]. Similarly, we found a significant inverse association of pack years of smoking with HOMA-β% in Japanese but not in white men (data not shown). Furthermore, in contrast to our study, previous studies did not find any difference in DI between white and Japanese people [4, 6, 7]. Nevertheless, as mentioned earlier, these studies were volunteer-based.
After adjusting for VAT and other covariates, the higher DI in white than Japanese men suggests a better compensation of insulin resistance in white individuals. This difference may partly explain the lower susceptibility of white than Japanese people to type 2 diabetes. However, higher DI in white than Japanese people suggests that white people have longer periods of exposure to hyperinsulinaemia before onset of diabetes than Japanese people. As hyperinsulinaemia is associated with increased atherosclerosis [26], longer exposure to hyperinsulinaemia in white than Japanese people before onset of type 2 diabetes would translate into higher incidence of CHD in white than Japanese individuals. In line with this reasoning, white people with diabetes have a higher incidence of CHD than Japanese people with type 2 diabetes who live in Japan [27, 28].
The strengths of our study include that it is the first population-based international study that compared VAT-adjusted insulin resistance and secretion between white people in the USA and Japanese in Japan in a standardised manner. Our study has some limitations. The participants were men aged 40–49 years. Therefore, it is unknown whether our results can be generalised to other age groups or women. As HOMA-IR and HOMA-β% are determined from fasting samples, these essentially provide an estimate of hepatic insulin resistance and nonstimulated insulin secretion. Moreover, HOMA-IR provides a better assessment of insulin resistance in overweight and obese participants than normal-weight participants without diabetes [29]. Insulin secretion measured using HOMA-β% does not account for factors other than glucose that determine beta cell function, such as amino acids, nonesterified fatty acids, cortisol, growth hormone, etc. [30]. Furthermore, HOMA-β% underestimates insulin secretion, especially in individuals with IFG or impaired glucose tolerance (IGT) [31]. Despite the limitations, HOMA-IR and HOMA-β% are the most extensively used markers of insulin resistance and beta cell function in epidemiological studies and are widely used for comparing insulin resistance and insulin secretion among various races in population-based studies [32–34].
DI is assumed to be hyperbolic (the constant product of insulin sensitivity and insulin secretion) for individuals with the same degree of glucose tolerance [35]. However, we are unable to comment on the glucose tolerance state of the participants, as we did not perform the OGTT. Thus, it is possible that some of the participants with normoglycaemia may have had IGT, which would not be accounted for by adjusting for IFG [20]. Furthermore, the estimation of DI using HOMA-β% and HOMA-IR provides a less credible index of beta cell function than DI obtained using dynamic methods because both HOMA-β% and HOMA-IR are estimated from fasting samples and thus are interdependent [35]. Therefore, the results should be interpreted with caution.
We did not measure autoimmune antibodies against pancreatic beta cells. These antibodies reduce insulin secretion by destroying beta cells and may be purported as one of the reasons for significantly different DI between white and Japanese people. However, the estimated prevalence of these antibodies is similar among patients with type 2 diabetes in these races [36, 37]. We did not evaluate genetic differences related to insulin resistance or insulin secretion between white and Japanese individuals. To date, there is no conclusive evidence in regard to genetic differences between the two races [7, 21].
In summary, white men appear to have higher insulin resistance, insulin secretion and DI than Japanese men at the same level of VAT and on the basis of fasting levels of glucose and insulin. The higher DI in white than Japanese people may partly explain the lower susceptibility of white than Japanese individuals to type 2 diabetes. The reason for the difference in DI between white and Japanese individuals warrants further investigation. These findings need to be confirmed using formal methods for assessing insulin resistance and insulin secretion, such as the hyperinsulinaemic clamp and the intravenous glucose tolerance test.
Supplementary Material
Acknowledgments
Funding
This research was funded by the National Institute of Health (RO1 HL68200) and from the Japanese Ministry of Education, Culture, Sports, Science and Technology (B16790335 and A13307016).
Abbreviations
- CRP
C-reactive protein
- DI
Disposition index
- ERA JUMP
EBCT and Risk Factor Assessment among Japanese and U.S. Men in the Post World War II Birth Cohort
- HOMA-β%
HOMA of beta cell function
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- VAT
Visceral adipose tissue
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
VA, TK, RWE, AKad, TO, AF, EJWB-M, TH, AV, KM, HM, AKash, LHK, HU, AE-S and AS contributed to conception and design. TK, TO, EJWB-M, HM, AKash, HU and AE-S collected the data. VA, SREK and AS analysed and interpreted the data. VA drafted the article. All authors critically revised the article for intellectual content. All authors approved the final version. VA and AS are guarantors of this work.
References
- 1.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2008;9(Suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than White men in the same levels of waist circumference in a population-based study. International journal of obesity (2005) 2006;30:1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 3.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 4.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51:2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 5.Torrens JI, Skurnick J, Davidow AL, et al. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN) Diabetes care. 2004;27:354–361. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 6.Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes care. 2000;23:1353–1358. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
- 7.Moller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Whites. Diabetes care. 2014;37:796–804. doi: 10.2337/dc13-0598. [DOI] [PubMed] [Google Scholar]
- 8.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 9.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekikawa A, Ueshima H, Kadowaki T, et al. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. American journal of epidemiology. 2007;165:617–624. doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ADA. Standards of medical care in diabetes--2013. Diabetes care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard G, Bergman R, Wagenknecht LE, et al. Ability of alternative indices of insulin sensitivity to predict cardiovascular risk: comparison with the “minimal model”. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Annals of epidemiology. 1998;8:358–369. doi: 10.1016/s1047-2797(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. Journal of applied physiology. 2004;97:948–954. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M. Measuring and estimating insulin resistance in clinical and research settings. Nutr Metab Cardiovasc Dis. 2010;20:79–86. doi: 10.1016/j.numecd.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrecher A, Morimoto Y, Heak S, et al. The preventable proportion of type 2 diabetes by ethnicity: the multiethnic cohort. Annals of epidemiology. 2011;21:526–535. doi: 10.1016/j.annepidem.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zafrir B, Jain M. Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications. Cardiovasc Drugs Ther. 2014;28:361–377. doi: 10.1007/s10557-014-6534-9. [DOI] [PubMed] [Google Scholar]
- 18.Meng YX, Ford ES, Li C, et al. Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic US from National Health and Nutrition Examination Survey 1999–2002. Clinical chemistry. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of clinical endocrinology and metabolism. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Current diabetes reports. 2009;9:193–199. doi: 10.1007/s11892-009-0032-7. [DOI] [PubMed] [Google Scholar]
- 21.Fu D, Cong X, Ma Y, et al. Genetic polymorphism of glucokinase on the risk of type 2 diabetes and impaired glucose regulation: evidence based on 298,468 subjects. PloS one. 2013;8:e55727. doi: 10.1371/journal.pone.0055727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. The Medical clinics of North America. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto WY, Leonetti DL, Kinyoun JL, et al. Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes. 1987;36:721–729. doi: 10.2337/diab.36.6.721. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi S, Okubo M, Yoneda M, Jitsuiki K, Yamane K, Kohno N. A comparison between Japanese-Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomedicine & pharmacotherapy. 2004;58:571–577. doi: 10.1016/j.biopha.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto A, Tatsumi Y, Deura K, Mizuno S, Ohno Y, Watanabe S. Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men: The Saku Study. Journal of diabetes investigation. 2013;4:274–280. doi: 10.1111/jdi.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. Follow-up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia. 2001;44(Suppl 2):S3–13. doi: 10.1007/pl00002936. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama H, Matsushima M, Kawai K, et al. Low incidence of cardiovascular events in Japanese patients with type 2 diabetes in primary care settings: a prospective cohort study (JDDM 20) Diabetic medicine. 2011;28:1221–1228. doi: 10.1111/j.1464-5491.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes care. 2004;27:1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]
- 30.Boyko EJ, Jensen CC. Do we know what homeostasis model assessment measures? If not, does it matter? Diabetes care. 2007;30:2725–2728. doi: 10.2337/dc07-1248. [DOI] [PubMed] [Google Scholar]
- 31.Festa A, Haffner SM, Wagenknecht LE, Lorenzo C, Hanley AJ. Longitudinal decline of beta-cell function: comparison of a direct method vs a fasting surrogate measure: the Insulin Resistance Atherosclerosis Study. The Journal of clinical endocrinology and metabolism. 2013;98:4152–4159. doi: 10.1210/jc.2013-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Manson JE, Tinker L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes care. 2007;30:1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning F, Qiao Q, Tuomilehto J, et al. Does abnormal insulin action or insulin secretion explain the increase in prevalence of impaired glucose metabolism with age in populations of different ethnicities? Diabetes/metabolism research and reviews. 2010;26:245–253. doi: 10.1002/dmrr.1078. [DOI] [PubMed] [Google Scholar]
- 34.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 35.Mari A, Ahren B, Pacini G. Assessment of insulin secretion in relation to insulin resistance. Current opinion in clinical nutrition and metabolic care. 2005;8:529–533. doi: 10.1097/01.mco.0000171130.23441.59. [DOI] [PubMed] [Google Scholar]
- 36.Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes/metabolism research and reviews. 2012;28(Suppl 2):40–46. doi: 10.1002/dmrr.2345. [DOI] [PubMed] [Google Scholar]
- 37.Takeda H, Kawasaki E, Shimizu I, et al. Clinical, autoimmune, and genetic characteristics of adult-onset diabetic patients with GAD autoantibodies in Japan (Ehime Study) Diabetes care. 2002;25:995–1001. doi: 10.2337/diacare.25.6.995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.