Abstract
We previously found that mice fed lutein accumulated its oxidative metabolites (3′-hydroxy-ε,ε-caroten-3-one and ε,ε-carotene-3,3′-dione) as major carotenoids, suggesting that mammals can convert xanthophylls to keto-carotenoids by the oxidation of hydroxyl groups. Here we elucidated the metabolic activities of mouse liver for several xanthophylls. When lutein was incubated with liver postmitochondrial fraction in the presence of NAD+, (3′R,6′R)-3′-hydroxy-β,ε-caroten-3-one and (6RS,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one were produced as major oxidation products. The former accumulated only at the early stage and was assumed to be an intermediate, followed by isomerization to the latter. The configuration at the C3′ and C6′ of the ε-end group in lutein was retained in the two oxidation products. These results indicate that the 3-hydroxy β-end group in lutein was preferentially oxidized to a 3-oxo ε-end group via a 3-oxo β-end group. Other xanthophylls such as β-cryptoxanthin and zeaxanthin, which have a 3-hydroxy β-end group, were also oxidized in the same manner as lutein. These keto-carotenoids, derived from dietary xanthophylls, were confirmed to be present in plasma of normal human subjects, and β,ε-caroten-3′-one was significantly increased by the ingestion of β-cryptoxanthin. Thus, humans as well as mice have oxidative activity to convert the 3-hydroxy β-end group of xanthophylls to a 3-oxo ε-end group.
Keywords: β-cryptoxanthin, keto-carotenoid, liver, lutein, mouse, oxidation, metabolism, zeaxanthin
Dietary carotenoids are an important source of vitamin A, and their antioxidant activities have been thought to benefit human health (1, 2). They can physically quench singlet oxygen and scavenge peroxy radicals, because they have a long chain of conjugated polyene (3, 4). Moreover, individual carotenoids have shown biological activities such as immune enhancement, anti-carcinogenesis, and anti-obesity activity (5–7). Carotenoids and their active metabolites must accumulate at a certain level in a target tissue in order to exert their biological activity. However, the processes of intestinal absorption, distribution, and metabolism up to accumulation in the target tissues have not yet been fully revealed (8, 9). With respect to metabolism, the cleavage reactions of carotenoids to small molecules have been well-characterized in terms of the responsible enzymes and genes, whereas other metabolic conversions without transformation of a carbon backbone remain to be clarified.
The central cleavage reaction, which oxidizes the central carbon-carbon double bond at C15-C15′ of provitamin A carotenoids to produce retinal (10), is catalyzed by a cytosolic enzyme, β-carotene 15,15′-oxygenase. Another cleavage enzyme, β-carotene 9′,10′-oxygenase, which is located in mitochondria, catalyzes the cleavage of the double bond at C9′-C10′ asymmetrically in various carotenoids, including nonprovitamin A carotenoids (11, 12). The genetic variants of these enzymes have been reported to be associated with the levels of carotenoids accumulated in the tissues of humans, cattle, and sheep (13–17). Thus, the cleavage reactions play an important role not only in producing the retinoids and apocarotenoids, but also in eliminating the carotenoids from the body.
Xanthophylls are a group of carotenoids with functional groups containing oxygen atoms. Lutein and zeaxanthin are noteworthy xanthophylls because they exclusively accumulate at high concentration in the macula lutea of the retina. Their consumption is thought to prevent age-related macular degeneration by reducing light-induced oxidative stress (18). Xanthophylls would be cleaved by the asymmetric cleavage enzyme to apocarotenoid and a small molecule, as a recombinant murine β-carotene 9′,10′-oxygenase in Escherichia coli was demonstrated to produce the specific cleavage products from lutein and zeaxanthin (12).
In fish and birds, diverse xanthophylls are synthesized from dietary carotenoids, and a metabolic conversion that retains a polyene backbone occurs in their tissues. For example, goldfinches oxidized hydroxyl groups of C3 and C3′ in lutein to 3-oxo carotenoids and cardinals oxidized C4 and C4′ of β-carotene and zeaxanthin to canthaxanthin and astaxanthin (19). Several 3-oxo carotenoids were detected in hen egg yolk (20). Fish have the same oxidative activity as birds and the reductive metabolism of astaxanthin to tunaxanthins was also reported in yellowtail (21). In human tissues, several keto-carotenoids derived from lutein and zeaxanthin were detected and assumed to be formed by a reaction with reactive oxygen species or by their enzymatic conversions (22).
The metabolic conversions have been elucidated mainly by identifying carotenoids that were not present in feed and by tracing labeled carotenoids (23). However, no enzyme reaction for the metabolic conversion of xanthophylls besides the cleavage reactions had been revealed until we found the NAD+-dependent oxidation of fucoxanthinol to amarouciaxanthin A by mouse liver microsomes (24). These two xanthophylls were the metabolites accumulated in the tissues of mice fed fucoxanthin, which is a major xanthophyll of edible brown algae. This conversion comprised the oxidation of a hydroxyl group at C3 of fucoxanthinol and the subsequent opening of 5,6-epoxide. We also found that mice fed lutein accumulated two keto-carotenoids as major carotenoids in the tissues (25), indicating that one of the two hydroxyl groups in lutein is oxidized to a carbonyl group in either a β-end group or ε-end group, and that the β-end group is isomerized to the ε-end group by double bond migration.
In the present study, we found that mouse liver has a metabolic activity to oxidize the 3-hydroxy β-end group in xanthophylls to a 3-oxo ε-end group via an intermediate of a 3-oxo β-end group, and that the same oxidative conversion occurs in humans.
MATERIALS AND METHODS
Materials
Lutein was isolated from a marigold extract suspended in safflower oil (FloraGLO; Kemin Industries, Des Moines, IA) and purified by TLC and reverse-phase HPLC to obtain zeaxanthin-free preparations. Zeaxanthin and β-cryptoxanthin were isolated from a paprika extract (ZEAGOLD; Kalsec, Kalamazoo, MI) and mandarin orange powder (Unitika, Osaka, Japan), respectively, and purified by TLC and reverse-phase HPLC after saponification. Lactucaxanthin ((3R,6R,3′R,6′R)-ε,ε-carotene-3,3′-diol) was prepared from lettuce (Lactuca sativa) as described (25). The (6R,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one and (6R,6′R)-ε,ε-carotene-3,3′-dione were prepared by the oxidation of lactucaxanthin with nickel peroxide (26). Lutein monomethyl ether was prepared as described (27). Other chemicals and solvents were of reagent grade.
Preparation of postmitochondrial fraction from mouse liver
Male ICR mice (6 weeks old; Clea Japan, Tokyo, Japan) were housed at 25°C with a 12 h light/dark cycle and acclimated with free access to a standard rodent chow (MF; Oriental Yeast Co., Tokyo, Japan) and tap water. After 7 days of feeding, the mice were anesthetized with isoflurane and euthanized by exsanguination. The livers were excised and rinsed with ice-cold saline. The livers were homogenized in a Potter-Elvehjem homogenizer with four volumes of ice-cold 50 mM HEPES-KOH buffer (pH 7.4) containing 0.154 M KCl, 1.0 mM EDTA, 1.0 mM EGTA, and 0.1 mM DTT. The postmitochondrial supernatant obtained by centrifugation at 10,000 g for 10 min was subjected to fractionation with a Sephadex G25 column. The protein fraction of the eluate was used as an enzyme source unless otherwise specified, and is here referred to as postmitochondrial fraction. All procedures involving mice were approved by the Animal Care Committee of the National Food Research Institute (approval # H23-035) and were conducted in accordance with the guidelines of the National Food and Agriculture Research Organization for laboratory animal studies.
Oxidation of xanthophylls by liver postmitochondrial fraction
The oxidation of several xanthophylls by postmitochondrial fraction of mouse liver was evaluated in the following conditions. The standard reaction mixture contained 20 μM xanthophyll, 2.4 mM NAD+, 0.1 M glycine-KOH buffer (pH 9.5), 0.5 mM DTT, 1 mM EDTA, 1 mM EGTA, 0.2% Tween 20, and postmitochondrial fraction (0.2–0.5 mg protein) in a total volume of 0.1 ml. Xanthophylls were solubilized in Tween 20 micelle before being added to the reaction mixture, as described (28). The reaction mixture was incubated at 37°C for 60 min, unless otherwise specified. The reaction was terminated by adding 0.5 ml ethanol containing 40 nmol/ml α-tocopherol and 35 μmol/ml acetic acid. Subsequently, 0.4 ml deionized water, 0.5 ml ethyl acetate, and 0.5 ml n-hexane were added and the mixture was vortexed after each addition. The upper phase was collected and the lower phase was mixed with the same volumes of ethyl acetate and n-hexane as noted above. The combined upper phase was evaporated to dryness in vacuo. The extract was subjected to an HPLC analysis after being dissolved in 400 μl of the eluting solvent.
Isomerization of 3′-hydroxy-β,ε-caroten-3-one
The isomerization of 3′-hydroxy-β,ε-caroten-3-one to 3′-hydroxy-ε,ε-caroten-3-one was evaluated in the following conditions. 12 μM 3′-Hydroxy-β,ε-caroten-3-one were incubated at 37°C for 30 min in the reaction mixture containing 0.1 M buffer, 5 mM DTT, 1 mM EDTA, 1 mM EGTA, and 0.2% Tween 20, either in the presence or in the absence of postmitochondrial fraction (0.32 mg protein) in a total volume of 0.1 ml. The pH of the reaction mixture was adjusted with the following buffer: MES-KOH buffer for pH 6.0, HEPES-KOH buffer for pH 7.0 and 7.4, TRICINE-KOH buffer for pH 8.0 and 8.5, and glycine-KOH buffer for pH 9.5. After the reaction was terminated, the extract was prepared and subjected to an HPLC analysis as described above.
Preparation of oxidation products of xanthophylls
The following preparative reactions were conducted to obtain sufficient amounts of the oxidation products to determine the chemical structure by NMR and circular dichroism (CD) spectroscopy. The reaction mixture contained 100 μM xanthophyll, 2.4 mM NAD+, 0.1 M glycine-KOH buffer (pH 9.5), 5 mM DTT, 1 mM EDTA, 1 mM EGTA, 0.5% Tween 20, and the postmitochondrial supernatant before fractionation with a Sephadex G25 column (120–300 mg protein) in a total volume of 60 ml.
The reaction mixture was placed in a conical flask with a screw cap and was incubated at 37°C under anaerobic conditions by displacing the air with argon gas to eliminate the oxidative degradation of xanthophylls during incubation. The reaction times for the preparations of 3′-hydroxy-β,ε-caroten-3-one, 3′-hydroxy-ε,ε-caroten-3-one, and ε,ε-carotene-3,3′-dione from lutein were 1.5, 9.5, and 24 h, respectively. The reaction times for the preparations of β,β-caroten-3-one and β,ε-caroten-3′-one from β-cryptoxanthin were 20 min and 9.5 h, respectively. The reaction times for the preparations of 3-hydroxy-β,ε-caroten-3′-one and ε,ε-carotene-3,3′-dione from zeaxanthin were 7.5 and 32 h, respectively. The reactions were terminated by adding 60 ml ethanol containing 20 nmol/ml α-tocopherol. Subsequently, 60 ml ethyl acetate and 60 ml n-hexane were added and vigorously mixed. The upper phase was collected and the lower phase was mixed with the same volumes of ethyl acetate and n-hexane as above. The combined upper phase was evaporated to dryness and subjected to the purification of oxidation products as described in the supplementary Methods.
Liver samples of mice fed lutein
The liver samples of mice fed lutein esters were obtained in our previous study (25) and stored at −80°C until the isolation of the metabolites of lutein. The liver samples were homogenized as described in the section above and mixed well with an equal volume of the following solvents: ethanol, ethyl acetate, and n-hexane. The upper phase was collected and the lower phase was extracted with the same volume of ethyl acetate and n-hexane, as noted above. The combined upper phase was subjected to fractionation on an open column of silicic acid. The fractions rich in keto-carotenoids were purified by semi-preparative HPLC on a cyanopropyl column of Inertsil CN-3 (10 × 250 mm; GL Sciences, Tokyo, Japan) attached to an Inertsil CN-3 guard column (7.6 × 30 mm) with the following mobile phase at a flow rate of 5.0 ml/min: n-hexane:dichloromethane:methanol (75:25:0.15, by volume). The eluates were further purified by reverse-phase HPLC on a Wakopak Navi C30-5 column (100 × 250 mm; Wako Pure Industries, Osaka, Japan), with methanol containing 0.05% ammonium acetate as a mobile phase.
HPLC analyses of keto-carotenoids
We analyzed the extract of the reaction mixture with xanthophylls and liver postmitochondrial fraction by conducting normal-phase HPLC with an HP-1100 system (Agilent Technologies, Palo Alto, CA) equipped with a photodiode array detector and a column oven at 25°C. A cyanopropyl column of Inertsil CN-3 (2.1 × 250 mm; GL Sciences) attached to an Inertsil CN-3 guard column (1.5 × 10 mm) was used with the following mobile phase at a flow rate of 0.2 ml/min: n-hexane:dichloromethane:methanol:N,N-diisopropylethylamine (75:25:0.15:0.1, by volume) for lutein, zeaxanthin, and lactucaxanthin and their oxidation products, and n-hexane:dichloromethane:methanol:N,N-diisopropylethylamine (95:5:0.15:0.1, by volume) for β-cryptoxanthin and its oxidation products.
The eluate was monitored at the absorption maxima of the xanthophylls in the mobile phase. The concentrations were calculated from the calibration curve of each purified xanthophyll. The concentration of the standard solutions was determined from their maximum absorption of UV-visible (VIS) spectra in ethanol using the following absorption coefficients : lutein, 1.45 × 105; zeaxanthin, 1.41 × 105; and β-cryptoxanthin, 1.36 × 105 (29).
As the absorption coefficients of β,β-caroten-3-one, β,ε-caroten-3′-one, 3′-hydroxy-β,ε-caroten-3-one, 3-hydroxy-β,ε-caroten-3′-one, 3′-hydroxy-ε,ε-caroten-3-one, ε,ε-carotene-3,3′-dione, and lactucaxanthin were not available, those of the following carotenoids that have the same chromophores were used: β-cryptoxanthin, lutein, lutein, lutein, ε,ε-carotene (1.56 × 105) (29), ε,ε-carotene, and ε,ε-carotene, respectively.
The oxidation products of the xanthophylls were purified and analyzed by separating them into their steric isomers by normal-phase HPLC with the HP-1100 system equipped with a UV-VIS detector and a column oven at 25°C. A chiral column (SUMICHIRAL OA-2000, 4.6 × 250 mm; Sumika Chemical Analysis Service, Osaka, Japan) was used with the following mobile phase at a flow rate of 1.0 ml/min: n-hexane:dichloromethane:ethanol (76:24:0.5, by volume) for 3′-hydroxy-ε,ε-caroten-3-one, and n-hexane:dichloromethane:ethanol (81:19:0.5, by volume) for ε,ε-carotene-3,3′-dione.
Analysis of keto-carotenoids in human plasma
Four healthy volunteers (mean age of 43.3 years) were recruited from the National Food Research Institute (Tsukuba, Japan). None of the participants had consumed any supplement containing carotenoids during the 3 months before the study was started or during the study period. The human study was approved by the Ethics Committee of the National Food Research Institute and all of the participants signed the informed consent form.
The participants ingested canned mandarin orange juice (190 g) containing 1.9 mg of β-cryptoxanthin at lunch or dinner each day for 13 consecutive days. Blood was collected by venipuncture into vacutainer tubes with sodium EDTA as an anticoagulant from overnight-fasted participants in the morning on the first day and on the day after the final day. The blood was centrifuged at 1,000 g for 15 min at 4°C and the plasma was collected and stored at −80°C.
Xanthophylls in 0.4 ml of the plasma were extracted by the modified procedures described (30), using lutein monomethyl ether as an internal standard. The plasma extract was dissolved in 200 μl of n-hexane:dichloromethane:methanol (95:5:0.15, v/v/v) and an aliquot was subjected to HPLC analysis on the cyanopropyl column as described above, except that the gradient elution was performed at a flow rate of 0.15 ml/min, using solvent A, n-hexane:dichloromethane:methanol:N,N-diisopropylethylamine (95:5:0.15:0.1, by volume) and solvent B, n-hexane:dichloromethane:methanol:N,N-diisopropylethylamine (75:25:0.15:0.1, by volume) as follows: 0–25 min at 100% solvent A; 25–50 min, a linear gradient to 100% solvent B; and 50–80 min at 100% solvent B. The recovery of each analyte spiked to human plasma in this procedure was more than 96%, and the limit of quantification was 1.2 nmol/l plasma.
Effect of keto-carotenoids on nitric oxide production by RAW 264 cells
RAW 264 mouse macrophage cells (RIKEN BioResource Center, Tsukuba, Japan) were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 4 mmol/l L-glutamine, 40,000 units/l penicillin, and 40 mg/l streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. The cells were maintained by passing every 2 days. The cells were seeded at a density of 3 × 104 cells per well in a 96-well plate and incubated for 4 h. Thereafter, the cells were cultured with the medium (0.1 ml) in which xanthophylls and keto-carotenoids were solubilized by the solvent evaporation method, as described (31). After 24 h cultivation, lipopolysaccharide (LPS) from E. coli (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the medium (final concentration of 40 ng/ml). After incubation for an additional 24 h, the cell supernatants were collected and subjected to an analysis of nitrite by using Griess reagent (Promega, Tokyo, Japan) as a measure of nitric oxide (NO) formation. The cellular protein and the cytotoxic effects (lactate dehydrogenase leakage) of cell treatments were evaluated as described (32).
We used Western blotting to examine the effect of keto-carotenoids on the protein expression of inducible NO synthase (iNOS). The RAW 264 cells were seeded at a density of 23 × 104 cells per well in a 12-well plate containing 1 ml of medium per well, and treated with β,ε-caroten-3′-one and then with LPS, as described above. The cells washed with Hanks’ balanced salt solution were recovered with a lysis buffer (50 mM Tris-HCl, 1 mM EDTA, and 1 mM phenylmethanesulfonyl fluoride). The recovered cells were homogenized on ice by sonication and then centrifuged (10,000 g, 15 min). The supernatants were collected and their protein concentrations were determined by the BCA method. Equal amounts of protein from each sample were subjected to SDS-PAGE and then electroblotted on a polyvinylidene difluoride membrane. Rabbit iNOS antibody (1:1,000, #2982; Cell Signaling Technology Japan, Tokyo, Japan) and β-actin antibody (1:1,000, #4967; Cell Signaling Technology Japan) were used as primary antibodies. The secondary immunoreaction and chromogenic reaction were carried out using a WesternBreeze chromogenic Western blot immunodetection kit (Life Technologies Japan, Tokyo, Japan).
Spectroscopic analysis
High-resolution mass spectra were recorded on a Fourier-transform ion cyclotron resonance mass spectrometer with an interface of ESI (Apex II 70e; Bruker Daltonics, Billerica, MA) and on a Kingdon trap-type mass spectrometer with an interface of atmospheric pressure chemical ionization (Orbitrap Veros Pro ETD; Thermo Fisher Scientific, Waltham, MA). The 1H NMR (800 MHz) and 13C NMR (201 MHz) spectra were recorded on a Bruker AVANCE 800 instrument at 4°C in CDCl3 with tetramethylsilane as an internal standard. The CD spectra of the xanthophylls and oxidation products were measured with a J-820 CD system (Jasco, Tokyo, Japan) at 20°C in ethanol.
Statistical analysis
Values are expressed as means and SDs. The data were analyzed by one-way ANOVA, followed by the Tukey-Kramer test and the paired Student’s t-test. P values <0.05 were considered significant.
RESULTS
Formation of 3′-hydroxy-ε,ε-caroten-3-one from lutein
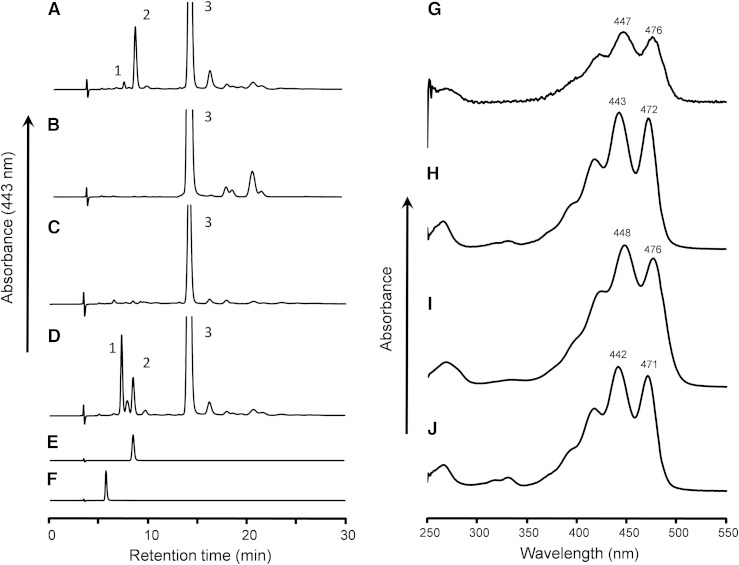
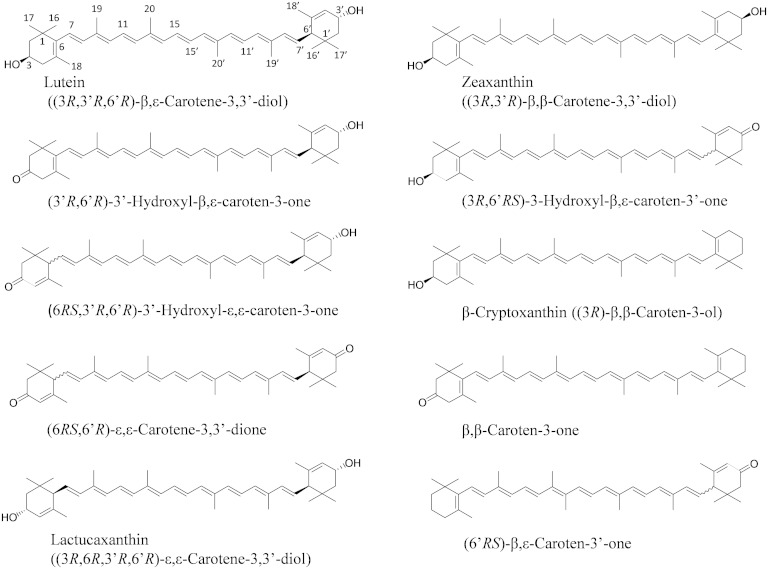
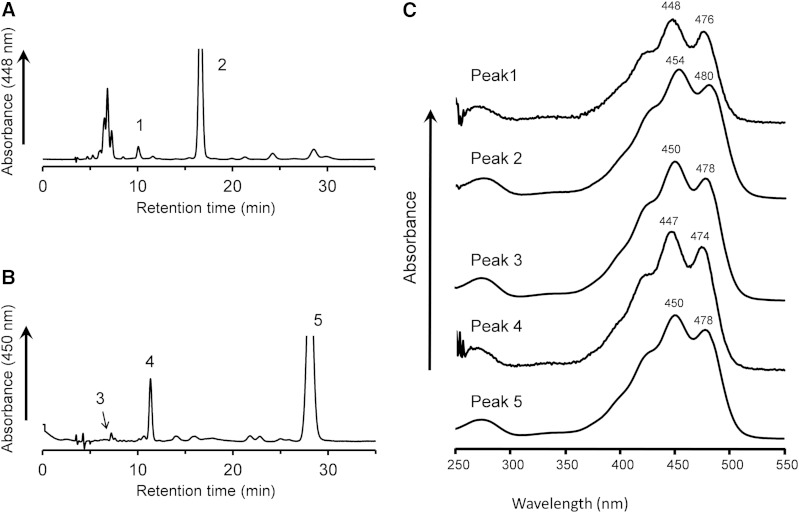
After the incubation of lutein with postmitochondrial fraction of mouse liver in the presence of coenzyme NAD+ for 60 min, the extract of the reaction mixture showed an unknown peak (peak 2, 8.5 min) before that of lutein (peak 3, 14.1 min) in the elution profile of a normal-phase HPLC on a cyanopropyl column (Fig. 1A). Peak 2 was not detected in the reaction mixtures containing no NAD+ (Fig. 1B) and the postmitochondrial fraction treated with protease (Fig. 1C). The peak 2 formation was optimum at pH 9.5, and the reaction dependent on NADP+ was only 6.6% of that on NAD+. These results indicate that peak 2 was formed from lutein by a NAD+-dependent dehydrogenase reaction. Its UV-VIS spectrum showed the maximum absorption at 443 nm with well-defined vibrational bands (Fig. 1H), suggesting the loss of one double bond in the conjugated polyene structure of lutein. The retention time and UV-VIS spectrum of peak 2 were identical to those of 3′-hydroxy-ε,ε-caroten-3-one that was prepared by the oxidation of lactucaxanthin with nickel peroxide (Fig. 1E, J). The oxidation product of lutein (peak 2) was identified as 3′-hydroxy-ε,ε-caroten-3-one, based on the high-resolution MS m/z 589.4011 [M + Na]+ (calculated for C40H54O2Na: 589.4016) and 1H and 13C NMR data as shown in supplementary Table 1. Structures of xanthophylls and their oxidation products described in this study are shown in Fig. 2.
Fig. 1.
Normal-phase HPLC profiles of extracts from incubation mixtures of lutein with postmitochondrial fraction of mouse liver. A, D: Complete reaction mixtures. B: Minus NAD+. C: Protease-treated postmitochondrial fraction. E: 3′-Hydroxy-ε,ε-caroten-3-one prepared from lactucaxanthin. F: ε,ε-Carotene-3,3′-dione prepared from lactucaxanthin. The reaction mixtures were incubated at 37°C for 60 min, except that mixture (D) was incubated for 20 min. Peaks were identified as 3′-hydroxy-β,ε-caroten-3-one (peak 1), 3′-hydroxy-ε,ε-caroten-3-one (peak 2), or all-trans-lutein (peak 3). The UV-VIS spectra of the major peaks in the HPLC eluate of n-hexane:dichloromethane:methanol:diisopropylethylamine (75:25:0.15:0.1, by volume) are shown: peak 1 (G), peak 2 (H), peak 3 (I), and 3′-hydroxy-ε,ε-caroten-3-one prepared from lactucaxanthin (J). The three-digit numbers attached to the spectral curves indicate the wavelength (in nanometers) of absorption peaks.
Fig. 2.
Structures of xanthophylls and their oxidation products.
The chemical shifts of H-2′, H-4′, H-6′, H-7′, H-16′, and H-17′ of the 3′-hydroxyl ε-end group with 3′R,6′R and 3′S,6′S configurations are different from those of 3′S,6′R or 3′R,6′S (33). The chemical shifts of these protons of isolated 3′-hydroxy-ε,ε-caroten-3-one were consistent with those reported for lutein ((3R,3′R,6′R)-β,ε-carotene-3,3′-diol) (34), indicating that its configuration of the 3′-hydroxyl ε-end group can be assumed to be 3′R,6′R or 3′S,6′S.
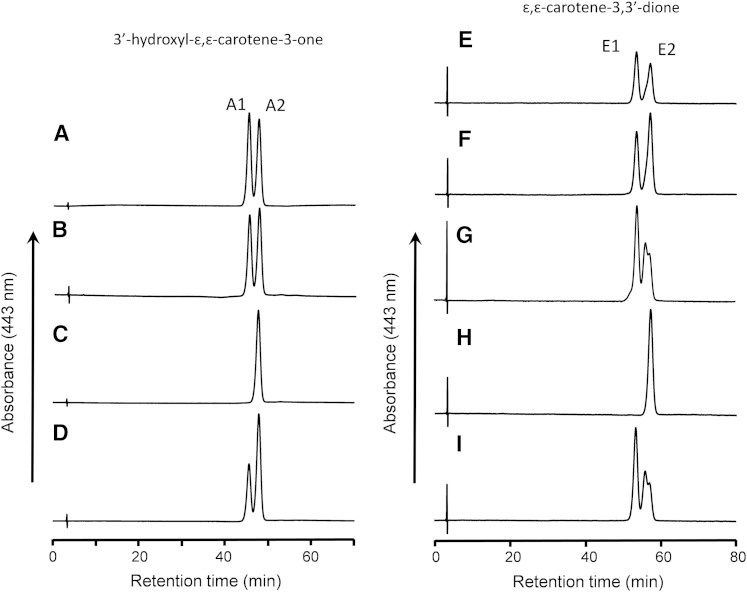
The isolated 3′-hydroxy-ε,ε-caroten-3-one was further separated to two isomers of peaks A1 and A2 on a chiral column as shown in Fig. 3A. Peak A2 had the same retention time as (6R,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one that was prepared by the oxidation of lactucaxanthin ((3R,6R,3′R,6’R)-ε,ε-carotene-3,3′-diol) with nickel peroxide (Fig. 3C). Moreover, peak A2 had the CD spectrum: λext 242 nm (Δε −9), 250 (0), 266 (+39), which is identical to that of (6R,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one: λext 241 nm (Δε −8), 249 (0), 266 (+39) (supplementary Fig. 1). Thus, the configuration of peak A2 was assigned as 6R,3′R,6′R.
Fig. 3.
HPLC profiles of 3′-hydroxy-ε,ε-caroten-3-one (A–D) and ε,ε-carotene-3,3′-dione (E–I) isolated from different sources on a chiral column. A, E: Incubation mixture of lutein with postmitochondrial fraction of mouse liver. B, F: Liver extract of mice fed lutein. C: 3′-Hydroxy-ε,ε-caroten-3-one prepared from lactucaxanthin. D: A mixture of (A) and (C). G: Incubation mixture of zeaxanthin with postmitochondrial fraction. H: ε,ε-Carotene-3,3′-dione prepared from lactucaxanthin. I: Egg yolk. Peak A1, (6S,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one; peak A2, (6R,3′R,6′R)-3′-hydroxy-ε,ε-caroten-3-one; peak E1, meso-ε,ε-carotene-3,3′-dione; peak E2, (6R,6′R)-ε,ε-carotene-3,3′-dione.
Lactucaxanthin and (6R,6′R)-ε,ε-carotene-3,3′-dione prepared from lactucaxanthin with nickel peroxide showed CD spectra: λext 210 nm (Δε +19), 266 (+27), 301 (0), and λext 242 nm (Δε −22), 251 (0), 267 (+50), respectively (supplementary Fig. 2). These two reference compounds and 3′-hydroxy-ε,ε-caroten-3-one prepared by enzyme reaction showed a characteristic Cotton effect at 266 nm. Considering the NMR data for 3′-hydroxy-ε,ε-caroten-3-one formed by enzyme reaction, its four possible configurations are 6R,3′R6′R, 6S,3′R,6′R, 6S,3′S,6′S, and 6R,3′S,6′S. According to the additivity rule in CD spectra of carotenoids (35), the Δε values of the four isomers at 266 nm are calculated from the Δε values of the above two reference compounds as follows: 6R,3′R,6′R, +39; 6S,3′R,6′R, −12; 6S,3′S,6′S, −39; and 6R,3′S,6′S, +12.
The peak A2 and (6R,6′R,3′R)-3′-hydroxy-ε,ε-caroten-3-one showed the Δε value of +39 at 266 nm, which is consistent with the calculated value of 6R,3′R,6′R isomer. Thus, the peak A2 configuration was confirmed to be 6R,3′R,6′R as already assigned above. Peak 1A had the CD spectrum: λext 222 nm (Δε +5), 244 (+16), 255 (0), 269 (−16) (supplementary Fig. 1), and its configuration was assigned as 6S,3′R,6′R because the Δε at 269 nm was close to the calculated Δε value for 6S,3′R,6′R. Thus, 3′-hydroxy-ε,ε-caroten-3-one formed from lutein by liver postmitochondrial fraction comprised two diastereomers of 6S,3′R,6′R (peak A1) and 6R,3′R,6′R (peak A2). The configuration of the 3′-hydroxy ε-end group was identical to that of lutein and the newly formed 3-oxo ε-end group had both the R and S configurations at C6.
The preparation of 3′-hydroxy-ε,ε-caroten-3-one isolated from livers of mice fed lutein was also separated to two peaks on a chiral column (Fig. 3B). They had the same retention times as those prepared by the oxidation of lutein with postmitochondrial fraction. The forward peak had the same CD spectrum: λext 220 nm (Δε +3), 244 (+17), 256 (0), 267 (−15) as peak A1 and the backward peak had the same CD spectrum: λext 238 nm (Δε −8), 250 (0), 266 (+39) as peak A2. Thus, the liver of the mice fed lutein contained the same two diastereomers of 3′-hydroxy-ε,ε-caroten-3-one as those observed in the reaction mixture of lutein with postmitochondrial fraction.
Formation of 3′-hydroxy-β,ε-caroten-3-one
The extract of the reaction mixture of lutein and postmitochondrial fraction after 60 min showed a small peak (peak 1, 7.8 min) before that of 3′-hydroxy-ε,ε-caroten-3-one (peak 2) as shown in Fig. 1A, whereas peak 1 was a prominent product peak after a short incubation (20 min) (Fig. 1D). The UV-VIS spectrum of the peak 1 was identical to that of lutein (Fig. 1G, I), suggesting that peak 1 had the same conjugated polyene structure as lutein. The oxidation product of lutein (peak 1) was identified as 3′-hydroxy-β,ε-caroten-3-one, based on high-resolution MS m/z 565.40451 [M-H]− (calculated for C40H53O2: 565.4051) and 1H and 13C NMR data (supplementary Table 1). The chemical shifts of protons in the ε-end group were consistent with those reported for lutein, indicating that the configuration of ε-end group would be 3′R,6′R or 3′S,6′S.
The isolated 3′-hydroxy-β,ε-caroten-3-one gave a single peak in the HPLC profile on a chiral column and had the CD spectrum: λext 212 nm (Δε +10), 270 (+10), 309 (0) (supplementary Fig. 2). The CD spectrum showed nearly the same extremum wavelength and signs as that of lactucaxanthin. The Δε at 270 nm in 3′-hydroxy-β,ε-caroten-3-one was approximately one-half of that in lactucaxanthin. As lactucaxanthin has a configuration of 6R,3R in two ε-end groups, the configuration of ε-end group in 3′-hydroxy-β,ε-caroten-3-one can be assigned as 6′R,3′R, but not 6′S,3′S. These results indicate that lutein was converted to (6′R,3′R)-3′-hydroxy-β,ε-caroten-3-one simply by the oxidation of the 3-hydroxyl β-end group to a 3-oxo β-end group.
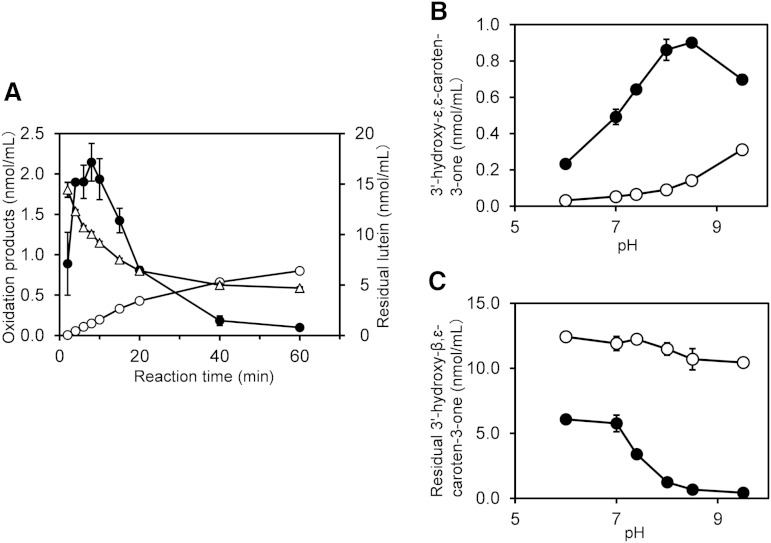
The time courses of the oxidation products during the oxidation of lutein by postmitochondrial fraction showed that 3′-hydroxy-β,ε-caroten-3-one reached a maximum of 2.14 nmol/ml at 8 min and then declined, whereas 3′-hydroxy-ε,ε-caroten-3-one increased steadily up to 0.8 nmol/ml after a 60 min incubation. Lutein, of which the initial concentration was 20 nmol/ml, decreased to 4.7 nmol/ml after the 60 min incubation (Fig. 4A).
Fig. 4.
Time course of lutein oxidation by postmitochondrial fraction of mouse liver and the isomerization of 3′-hydroxy-β,ε-caroten-3-one. A: Time course of lutein oxidation by liver postmitochondrial fraction under standard conditions. Closed circles, 3′-hydroxy-β,ε-caroten-3-one; open circles, 3′-hydroxy-ε,ε-caroten-3-one; open triangles, lutein. B: 3′-Hydroxy-ε,ε-caroten-3-one formed from 3′-hydroxy-β,ε-caroten-3-one by isomerization in the incubation mixtures described in the section entitled “Isomerization of 3′-hydroxy-β,ε-caroten-3-one” in the Materials and Methods, either in the presence (closed circle) or in the absence (open circle) of postmitochondrial fraction. C: Residual 3′-hydroxy-β,ε-caroten-3-one in the above incubation mixtures. Data are expressed as mean ± SD of triplicate incubations.
Isomerization of 3′-hydroxy-β,ε-caroten-3-one to 3′-hydroxy-ε,ε-caroten-3-one
We incubated 12 μM of 3′-hydroxy-β,ε-caroten-3-one with postmitochondrial fraction of mouse liver to examine its isomerization to 3′-hydroxy-ε,ε-caroten-3-one (Fig. 4B). The isomerization in the presence of postmitochondrial fraction was confirmed to occur with the optimum pH of 8.5. Even in the absence of the postmitochondrial fraction, a spontaneous isomerization occurred under an alkaline condition. The residual concentration of 3′-hydroxy-β,ε-caroten-3-one in the absence of postmitochondrial fraction was more than 10 μM, whereas it was remarkably low in the presence of postmitochondrial fraction, particularly in an alkaline condition (Fig. 4C). At pH 7.4, 72% of the 3′-hydroxy-β,ε-caroten-3-one was lost during incubation with postmitochondrial fraction, and only 5.3% was isomerized to 3′-hydroxy-ε,ε-caroten-3-one.
Formation of ε,ε-carotene-3,3′-dione from lutein
An unknown peak (peak 1) in addition to 3′-hydroxy-ε,ε-caroten-3-one was detected in a HPLC profile of the reaction mixture of lutein and liver postmitochondrial fraction after incubation for >18 h (supplementary Fig. 3A). The isolated unknown peak was identified as ε,ε-carotene-3,3′-dione, based on high-resolution MS m/z 565.4035 [M+H]+ (calculated for C40H53O2: 565.4040) and 1H and 13C NMR data (supplementary Table 1). The ε,ε-carotene-3,3′-dione isolated was separated to two peaks (Fig. 3E) with the equal peak area on a chiral column. Under the HPLC conditions, the ε,ε-carotene-3,3′-dione prepared from egg yolk was separated to three peaks (Fig. 3I), which represented all of the possible configurations: meso, 6S,6′S, and 6R,6′R isomers (20). Peak E1 showed no signal in CD (supplementary Fig. 4), indicating the meso isomer. Peak E2 had the same retention time as (6R,6′R)-ε,ε-carotene-3,3′-dione prepared from lactucaxanthin by oxidation with nickel peroxide (Fig. 3H). Peak E2 showed the CD spectrum: λext 241 nm (Δε −21), 251 (0), 266 (+40), which was identical to that of (6R,6′R)-ε,ε-carotene-3,3′-dione (supplementary Fig. 4). Thus, we assigned peaks E1 and E2 as meso and 6R,6′R isomers, respectively. The ε,ε-carotene-3,3′-dione isolated from the liver of mice fed lutein showed the two peaks on a chiral column with the same retention times (Fig. 3F) and CD spectra as the ε,ε-carotene-3,3′-dione prepared from lutein with postmitochondrial fraction.
The oxidation of zeaxanthin
The oxidation of zeaxanthin with liver postmitochondrial fraction gave a more complicated HPLC elution profile compared with that of lutein (Fig. 5A). Several peaks appeared at 6–8 min, with a bell-shaped UV-VIS absorption spectrum. After these unknown peaks, a small peak appeared at 10 min, having a UV-VIS spectrum with a hypochromic shift of 6 nm from that of zeaxanthin (Fig. 5C), suggesting the loss of one double bond in the conjugated polyene structure of zeaxanthin. This peak was isolated and identified as 3-hydroxy-β,ε-caroten-3′-one, based on high-resolution MS m/z 567.4192 [M+H]+ (calculated for C40H55O2: 567.4197) 1H and 13C NMR data (supplementary Table 1). The isolated 3-hydroxy-β,ε-caroten-3′-one had the CD spectrum: λext 233 nm (Δε +4), 252 (0), 278 (−4) without the intense CD signal observed at 267 nm in (6R,6′R)-ε,ε-carotene-3,3′-dione, indicating that the configuration of the asymmetric center C6′ was racemic (supplementary Fig. 5). A mixture of (3R,6′R) and (3R,6′S) isomers of 3-hydroxy-β,ε-caroten-3′-one isolated from egg yolk had the CD spectrum: λext 282 nm (Δε −3.2) (20), very close to that of 3-hydroxy-β,ε-caroten-3′-one isolated in the present study. Moreover, the CD spectrum was similar to that of β-cryptoxanthin: λext 247 nm (Δε +6), 264 (0), 286 (−9), although the latter showed a bathochromic shift because of its longer chromophore compared with that of 3-hydroxy-β,ε-caroten-3′-one. Therefore, the configuration at C3 was assigned to be R, as in the case of β-cryptoxanthin and zeaxanthin. The 3-hydroxy-β,ε-caroten-3′-one formed from zeaxanthin was a mixture of the two diastereomers of (3R,6′R) and (3R,6′S). Indeed, they were separated to two peaks which were eluted at close retention times with the equal peak height in the HPLC profile on a chiral column.
Fig. 5.
Normal-phase HPLC profiles of extracts from incubation mixtures of zeaxanthin and β-cryptoxanthin with postmitochondrial fraction of mouse liver. The reaction mixtures containing zeaxanthin (A) and β-cryptoxanthin (B) were incubated at 37°C for 60 min in the standard condition. Peaks were identified as 3-hydroxy-β,ε-caroten-3′-one (peak 1), all-trans-zeaxanthin (peak 2), β,β-caroten-3-one (peak 3), β,ε-caroten-3′-one (peak 4), and all-trans-β-cryptoxanthin (Peak 5). UV-VIS spectra of the peaks in the HPLC mobile phases are shown in (C). The three-digit numbers attached to the spectral curves indicate the wavelength (in nanometers) of absorption peaks.
ε,ε-Carotene-3,3′-dione was detected in the HPLC profile of the reaction mixture of zeaxanthin and liver postmitochondrial fraction after incubation for >18 h (supplementary Fig. 3B). It was separated into three possible steric isomers on a chiral column as in case of the ε,ε-carotene-3,3′-dione isolated from egg yolk (Fig. 3G, I).
The oxidation of β-cryptoxanthin
In a normal phase HPLC profile, the extract from the reaction mixture of β-cryptoxanthin with postmitochondrial fraction of mouse liver gave two peaks before β-cryptoxanthin was eluted (Fig. 5B). The UV-VIS spectrum of peak 3 showed a maximum absorption at 450 nm (Fig. 5C), consistent with that of β-cryptoxanthin (peak 5). The level of peak 3 was increased to the maximum at the early stage of the reaction and then declined in a way similar to 3′-hydroxy-β,ε-caroten-3-one in the reaction mixture of lutein. We identified peak 3 as β,β-caroten-3-one, based on high-resolution MS m/z 551.4245 [M+H]+ (calculated for C40H55O: 551.4247) and 1H and 13C NMR data (supplementary Table 1). The peak 4 showed a UV-VIS spectrum with a maximum absorption at 447 nm (Fig. 5C). We identified peak 4 as β,ε-caroten-3′-one, based on high-resolution MS m/z 551.4246 [M+H]+ (calculated for C40H55O: 551.4247) and 1H and 13C NMR data (supplementary Table 1). The isolated β,ε-caroten-3′-one showed no CD signal, and it was separated to two peaks which were eluted at the close retention times with the equal peak height in the HPLC profile on a chiral column. The isolated β,ε-caroten-3′-one was thus a racemic mixture of 6′R and 6′S isomers.
Substrate specificity
The oxidative activity of the liver postmitochondrial fraction against several xanthophylls under the standard condition is shown in Table 1. β-Cryptoxanthin, lutein, zeaxanthin, and 3-hydroxy-β,ε-caroten-3′-one, which have a hydroxyl group in their β-end group, were converted to the corresponding keto-carotenoids with 3-oxo ε-end groups. In contrast, only a small amount of keto-carotenoids with 3-oxo ε-end groups were formed from lactucaxanthin and 3′-hydroxy-ε,ε-caroten-3-one, of which hydroxyl groups bind only to ε-end groups. Oxidation of the 3′-hydroxy ε-end group in lutein can produce 3-hydroxy-β,ε-caroten-3′-one, whereas its formation was not confirmed on an HPLC profile due to overlapping with other peaks.
TABLE 1.
Oxidation of xanthophylls by postmitochondrial fraction of mouse liver
| Substrate | Complete Incubation | Minus NAD+ Control | |
| Product (μM) | Residual Substrate(μM) | Residual Substrate (μM) | |
| β-Cryptoxanthin | β,ε-Caroten-3′-one | ||
| 0.274 ± 0.007 | 3.75 ± 0.08 | 13.74 ± 0.12 | |
| Lutein | 3′-Hydroxy-ε,ε-caroten-3-one | ||
| 0.700 ± 0.017 | 7.17 ± 0.55 | 14.03 ± 0.30 | |
| Zeaxanthin | 3-Hydroxy-β,ε-caroten-3′-one | ||
| 0.170 ± 0.006 | 3.13 ± 0.25 | 13.78 ± 0.13 | |
| 3-Hydroxy-β,ε-caroten-3′-one | ε,ε-Carotene-3,3′-dione | ||
| 0.408 ± 0.019 | 9.66 ± 0.31 | 15.16 ± 0.23 | |
| Lactucaxanthin | 3′-Hydroxy-ε,ε-caroten-3-one | ||
| 0.014 ± 0.007 | 16.53 ± 1.43 | 13.13 ± 0.33 | |
| 3′-Hydroxy-ε,ε-caroten-3-one | ε,ε-Carotene-3,3′-dione | ||
| 0.011 ± 0.003 | 17.30 ± 0.31 | 16.38 ± 0.13 | |
Xanthophyll (20 μM) was incubated at 37°C for 60 min in the standard reaction mixture. Values are mean ± SD of triplicate incubations.
The residual amount of the xanthophylls in the minus NAD+ control was >66% of the initial level after a 60 min incubation. In a complete incubation, the xanthophylls with a β-end group markedly decreased, while the other xanthophylls without β-end group were kept >83% of the initial level. The amount of keto-carotenoids formed from the xanthophylls with a β-end group was far lower than the decreased amount of xanthophylls. For example, the lutein concentration was decreased by 6.86 μM dependently on NAD+, whereas 3′-hydroxy-ε,ε-caroten-3-one produced from lutein was 0.7 μM. Only 10% of the consumed lutein was converted to 3′-hydroxy-ε,ε-caroten-3-one.
Keto-carotenoids in human plasma
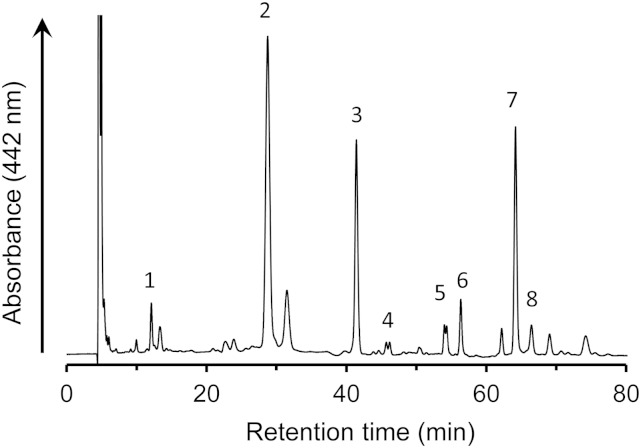
β,ε-Caroten-3′-one, 3-hydroxy-β,ε-caroten-3′-one, 3′-hydroxy-ε,ε-caroten-3-one, and ε,ε-carotene-3,3′-dione were detected in human plasma (Fig. 6, Table 2). 3′-Hydroxy-ε,ε-caroten-3-one and ε,ε-carotene-3,3′-dione were apparently separated into two peaks, indicating the presence of diastereomers. The combined amount of the four keto-carotenoids was approximately 20% of total xanthophylls before the intake of mandarin orange juice. β,ε-Caroten-3′-one, which was previously detected in egg yolk, was found to be present in human plasma for the first time. Its concentration was about one-seventh that of the β-cryptoxanthin. After the intake of mandarin orange juice, the levels of β-cryptoxanthin and β,ε-caroten-3′-one increased significantly by 6.8-fold and 2.5-fold, respectively, whereas the levels of other xanthophylls and keto-carotenoids derived from them were not changed.
Fig. 6.
HPLC profile of xanthophylls in human plasma extract. The extract was prepared from the plasma of one subject after intake of mandarin orange juice. Peak 1, β,ε-caroten-3′-one; peak 2, β-cryptoxanthin; peak 3, internal standard; peak 4, ε,ε-carotene-3,3′-dione; peak 5, 3′-hydroxy-ε,ε-caroten-3-one; peak 6, 3-hydroxy-β,ε-caroten-3′-one; peak 7, lutein; and peak 8, zeaxanthin.
TABLE 2.
Xanthophylls and their metabolites in human plasma after intake of mandarin orange juice
| Xanthophyll | Before (nM) | After (nM) | Difference (nM) |
| β-Cryptoxanthin | 154.8 ± 35.3 | 1045.3a ± 91.9 | 890.6 ± 74.4 |
| Lutein | 296.7 ± 182.9 | 279.2 ± 147.2 | −17.6 ± 50.9 |
| Zeaxanthin | 53.3 ± 25.7 | 52.6 ± 20.6 | −0.8 ± 6.9 |
| β,ε-Caroten-3′-one | 24.0 ± 9.2 | 60.0a ± 18.9 | 36.0 ± 12.1 |
| 3-Hydroxy-β,ε-caroten-3′-one | 60.6 ± 36.3 | 58.3 ± 31.2 | −2.4 ± 5.2 |
| 3′-Hydroxy-ε,ε-caroten-3-one | 42.0 ± 24.3 | 41.4 ± 27.0 | −0.6 ± 3.2 |
| ε,ε-Carotene-3,3′-dione | 17.0 ± 10.7 | 18.0 ± 11.5 | 0.9 ± 2.2 |
| Total keto-carotenoid | 143.8 ± 77.0 | 177.9a ± 76.8 | 34.0 ± 12.8 |
Values are mean ± SD of four subjects.
Significantly changed after intake of mandarin orange juice (P < 0.05; paired Student’s t-test).
Effect of keto-carotenoids on NO production by RAW 264 cells
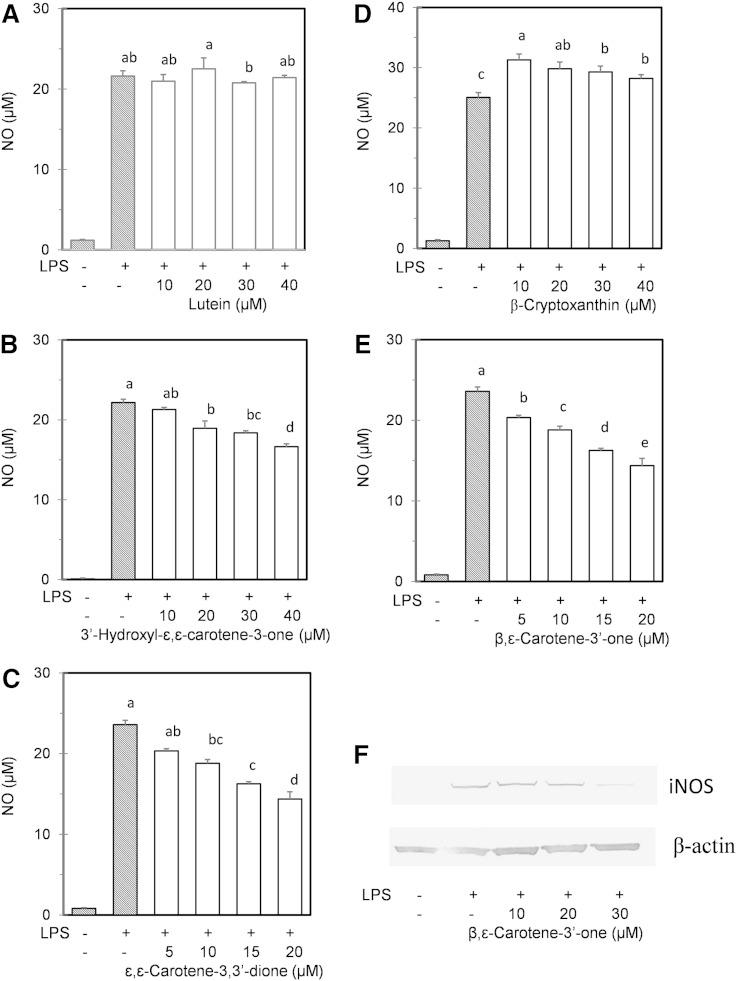
We found that the treatment of murine macrophage RAW 264 cells with lutein and β-cryptoxanthin did not suppress the NO production induced by LPS, whereas keto-carotenoids such as 3′-hydroxy-ε,ε-caroten-3-one, ε,ε-carotene-3,3′-dione, and β,ε-caroten-3′-one repressed the NO production in a dose-dependent manner (Fig. 7). In particular, β,ε-caroten-3′-one significantly repressed NO production at a low concentration (5 μM) and reduced the protein level of iNOS. Cytotoxic effects and growth inhibition were not observed in the cells treated with the xanthophylls and their oxidation products.
Fig. 7.
Effects of xanthophylls and their oxidation products on NO formation by RAW 264 mouse macrophage cells. The cells were cultured with the media containing the xanthophylls and their oxidation products for 24 h. Lutein (A), 3′-hydroxy-ε,ε-caroten-3-one (B), ε,ε-carotene-3,3′-dione (C), β-cryptoxanthin (D), and β,ε-caroten-3′-one (E). ε,ε-Carotene-3,3′-dione was prepared from zeaxanthin oxidation. Thereafter, LPS was added to the media and the incubation was continued. After 24 h incubation, the cell supernatants were evaluated for NO formation. Values not sharing common letters in the cells treated with LPS are significantly different by the Tukey-Kramer test (P < 0.05). F: Western blot analysis of inducible nitrate synthase expressed in the RAW 264 cells treated with β,ε-caroten-3′-one for 24 h. Replicate experiments showed similar trends.
DISCUSSION
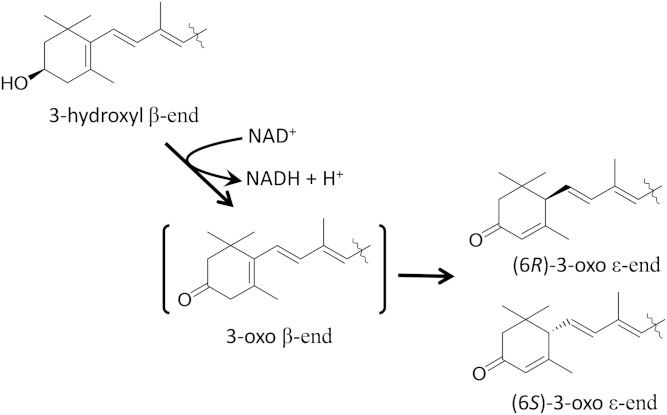
We previously found that mice fed lutein accumulated 3′-hydroxy-ε,ε-caroten-3-one and ε,ε-carotene-3,3′-dione in their tissues (25). In the present study, several oxidation products were successfully prepared from xanthophylls using the reaction system with liver postmitochondrial fraction. From their structures, we elucidated the oxidative metabolism of xanthophylls by liver enzymes in mammals. The most important study points in the conversion of lutein to 3′-hydroxy-ε,ε-caroten-3-one are the determination of which hydroxyl group is oxidized in lutein and how double bond migration occurs. From the configuration of the oxidation products of lutein, we could specify a hydroxyl group oxidized by liver enzyme. Lutein was oxidized to two diastereomers of (6R,3′R,6′R)- and (6S,3′R,6′R 3′)-hydroxy-ε,ε-caroten-3-one by postmitochondrial fraction. These configurations indicated that the (3′R,6′R)-3′-hydroxy ε-end group of lutein was retained intact in the oxidation product and that the β-end group of lutein was converted to (6R)- and (6S)-3-oxo ε-end groups by both dehydrogenation and the migration of a double bond.
From the reaction mixture at the early stage of lutein oxidation, we could isolate (6′R,3′R)-3′-hydroxy-β,ε-caroten-3-one, which was simply a dehydrogenation product of the 3-hydroxy β-end group in lutein. 3′-Hydroxy-β,ε-caroten-3-one was isomerized to 3′-hydroxy-ε,ε-caroten-3-one by incubation with postmitochondrial fraction. Therefore, 3′-hydroxy-β,ε-caroten-3-one is an intermediate in the oxidative conversion of lutein to 3′-hydroxy-ε,ε-caroten-3-one. The isomerization of 3′-hydroxy-β,ε-caroten-3-one also spontaneously occurred in the absence of postmitochondrial fraction, particularly under alkaline condition. It was uncertain whether the isomerization occurred by liver enzymes. The asymmetric center of C6 produced by double bond migration had both 6R and 6S configurations in 3′-hydroxy-ε,ε-caroten-3-one. The nonspecific configurations at C6 would favor the nonenzymatic migration of a double bond, which might be due to the unstable structure of the 3-oxo β-end group as described below. Thus, we demonstrated for the first time that the 3-hydroxy β-end group in lutein was converted to a 3-oxo ε-end group via the 3-oxo β-end group by a NAD+-dependent dehydrogenase of postmitochondrial fraction of mouse liver (Fig. 8). The two diastereomers of 3′-hydroxy-ε,ε-caroten-3-one were also found in the liver of mice fed lutein, indicating that the oxidative reaction of lutein found in vitro certainly worked in vivo.
Fig. 8.
Dehydrogenation and migration of a double bond in a 3-hydroxy β-end group.
Zeaxanthin, which has two 3-hydroxy β-end groups, was oxidized to two diastereomers of (3R,6′R)- and (3R,6′S)-3-hydroxy-β,ε-caroten-3′-one and finally to ε,ε-carotene-3,3′-dione with all of the possible configurations: meso, (6S,6′S), and (6R,6′R) by postmitochondrial fraction. The formation of these products from zeaxanthin was consistent with the 3-hydroxy β-end group to 3-oxo ε-end group scheme shown in Fig. 8. β-Cryptoxanthin, which has one 3-hydroxy β-end group, was oxidized by postmitochondrial fraction to a racemic mixture of (6′R)- and (6′S)-β,ε-caroten-3′-one, directly indicating the conversion of the 3-hydroxy β-end group to a 3-oxo ε-end group. β,β-Caroten-3-one, which was simply a dehydrogenation product of β-cryptoxanthin, reached a maximum at the early stage of the reaction. Considering the lutein oxidation, β-cryptoxanthin would be converted to a racemic mixture of β,ε-caroten-3′-one via β,β-caroten-3-one as an intermediate by dehydrogenation and the subsequent migration of a double bond.
We previously found that fucoxanthinol was oxidized to amarouciaxanthin A NAD+-dependently by microsomes of mouse liver (24). This oxidative conversion followed two reactions: the dehydrogenation of the 5,6-epoxy-3-hydroxy-5,6-dihydro-β-end group of fucoxanthinol and the isomerization to an ε-end group by cleavage of the epoxide. The oxidations of lutein and fucoxanthinol differed in isomerization, which was caused by double bond migration and by the cleavage of epoxide, respectively. The intermediates produced by dehydrogenation would have unstable conformations, which led to isomerization to an ε-end group by double-bond migration or the cleavage of epoxide. Thus, these two reactions would be mediated by the same microsomal dehydrogenase.
Lutein has 3-hydroxy β-end and 3′-hydroxy ε-end groups. The former was preferentially oxidized, but it is uncertain whether the latter was oxidized to produce 3-hydroxy-β,ε-caroten-3′-one. However, a small amount of 3′-hydroxy-ε,ε-caroten-3-one and lactucaxanthin, in which hydroxyl groups bind only to ε-end groups, were oxidized to the corresponding keto-carotenoids NAD+-dependently by postmitochondrial fraction, although the oxidative activities were much lower than that for lutein.
The oxidation of the 3′-hydroxy ε-end group was also supported by the following results. A small amount of ε,ε-carotene-3,3′-dione that comprised the two diastereomers of (6S,6′R) and (6R,6′R) was obtained after a prolonged incubation of lutein with postmitochondrial fraction. These configurations were consistent with those predicted from the oxidation of the 3′-hydroxy ε-end group in the two diastereomers of 3′-hydroxy-ε,ε-caroten-3-one. ε,ε-Carotene-3,3′-dione isolated from the liver of mice fed lutein also comprised the two diastereomers of (6S,6′R) and (6R,6′R), indicating the oxidation of the 3′-hydroxy ε-end group in vivo. Further characterizations of ε-end group oxidation are needed to fully understand the oxidative metabolism of xanthophylls.
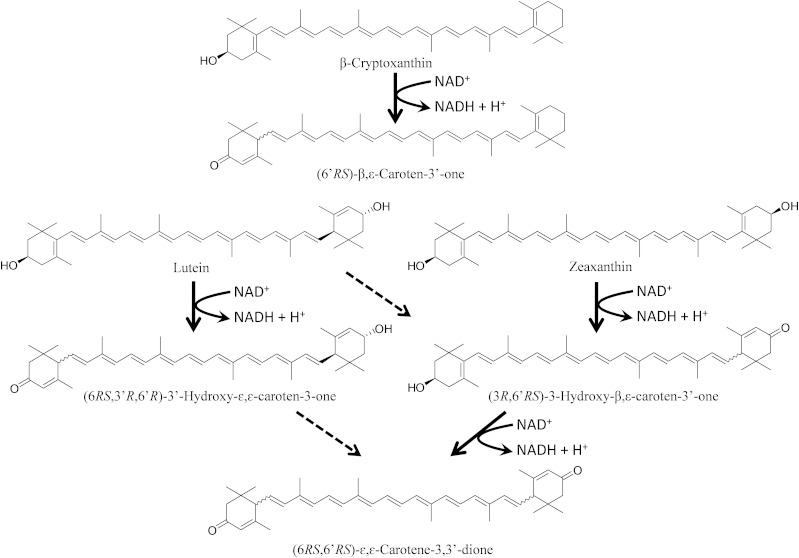
Based on the metabolic activity of postmitochondrial fraction of mouse liver observed in the present study, we speculate that the oxidation pathways of several xanthophylls in mammals are as shown in Fig. 9. Following the oxidative conversion of a 3-hydroxy β-end group to a 3-oxo ε-end group, β-cryptoxanthin and lutein are converted to β,ε-caroten-3′-one and 3′-hydroxy-ε,ε-caroten-3-one, respectively. Two steps of oxidation of zeaxanthin give 3-hydroxy-β,ε-caroten-3′-one and ε,ε-carotene-3,3′-dione. As mentioned, the oxidation of an ε-end group also occurred to produce ε,ε-carotene-3,3′-dione via 3′-hydroxy-ε,ε-caroten-3-one from lutein and possibly to produce 3-hydroxy-β,ε-caroten-3′-one from lutein.
Fig. 9.
Proposed oxidation pathway of xanthophylls in mammals. The solid arrows indicate the oxidation conversion of a 3-hydroxy β-end group to a 3-oxo ε-end group. The dashed arrows indicate the oxidative conversion of a 3-hydroxy ε-end group to a 3-oxo ε-end group. The latter reaction was not characterized in the present study.
We have analyzed xanthophylls and their metabolites in human plasma by normal-phase HPLC on a cyanopropyl column and the results confirmed the presence of the keto-carotenoids shown in the proposed pathway. We found for the first time that β,ε-caroten-3′-one was present in the human plasma and that its concentration increased significantly after the intake of mandarin orange juice rich in β-cryptoxanthin. Therefore, the presence of β,ε-caroten-3′-one in human plasma certainly indicates that the oxidative conversion of a 3-hydroxy β-end group to a 3-oxo ε-end group occurs in human tissues.
The oxidation of a β-end group in humans is also supported by the reported increase of 3-hydroxy-β,ε-caroten-3′-one in the plasma of humans and rhesus monkeys fed zeaxanthin (36, 37). In particular, the presence of equal amounts of two diastereomers (3R,6′R and 3R,6′S) of 3-hydroxy-β,ε-caroten-3′-one in the plasma of rhesus monkeys was quite consistent with the oxidation of a β-end group proposed in the present study.
In contrast to mouse plasma, the relative levels of keto-carotenoids to parental xanthophylls were apparently low in human plasma. The ratio of 3′-hydroxy-ε,ε-caroten-3-one to lutein in the plasma of mice fed lutein was 0.57 (25), whereas that of human subjects before the intake of mandarin orange juice in the present study was 0.14. This apparent difference in the ratio between the two species suggests that mice have higher oxidative activity of 3-hydroxy β-end groups to 3-oxo ε-end groups compared with humans.
In human plasma, the level of 3-hydroxy-β,ε-caroten-3′-one was higher than that of 3′-hydroxy-ε,ε-caroten-3-one. The former is produced from zeaxanthin, and the latter is produced from lutein, according to the oxidation pathway of the 3-hydroxy β-end group to the 3-oxo ε-end group. However, the level of 3-hydroxy-β,ε-caroten-3′-one is assumed to be lower than that of 3′-hydroxy-ε,ε-caroten-3-one, considering that the lutein levels of the human diet and plasma are much greater than those of zeaxanthin. This discrepancy suggests that a significant portion of lutein is oxidized to 3-hydroxy-β,ε-caroten-3′-one through the oxidation of a 3′-hydroxy ε-end group of lutein to a 3′-oxo ε-end group in human tissues.
This assumption is supported by the previous report that the plasma level of 3-hydroxy-β,ε-caroten-3′-one was increased in humans and rhesus monkeys fed lutein (37, 38). However, the oxidative activity of the β-end group in zeaxanthin is suggested to be higher than that of the ε-end group in lutein, because the formation rate constant of 3-hydroxy-β,ε-caroten-3′-one from zeaxanthin was found to be higher than that from lutein in Cohn and colleagues’ kinetic study of lutein and zeaxanthin orally administered to human subjects (36, 38). Thus, these results indicate that the oxidation of both the 3-hydroxy β-end group and the 3-hydroxy ε-end group occur in human tissues as in mouse, as mentioned above.
Regarding the bioavailability of xanthophylls, the oxidative metabolism of xanthophylls, as described above, would naturally decrease the level of intact xanthophylls in tissues. The 3-oxo ε-end group in the oxidation products has a hydrogen atom at C6, which is unstable to oxidation particularly under alkaline condition (39), and its α,β-unsaturated carbonyl moiety is highly reactive with nucleophilic molecules of biological tissues (40). Therefore, the oxidation products might be readily decreased in the biological tissues by oxidative degradation and reaction with nucleophiles, suggesting that the oxidative metabolism might decrease the level of total xanthophylls in tissues.
Moreover, the intermediate (3-oxo β-end group) formed by the dehydrogenation of a 3-hydroxy β-end group is extremely unstable due to steric hindrance of the β-end group. A 3-oxo-β-end group cannot form a stable half-chair conformation because of the presence of a carbonyl group at C3 and a double bond between C4 and C5. Moreover, methylene protons at C2 and C4 in a 3-oxo-β-end group are easily eliminated in basic medium because of the presence of a carbonyl group at neighbor position of C3. Therefore, xanthophylls with a 3-oxo-β-end group are very labile.
In fact, the isomerization of the intermediate (3′-hydroxy-β,ε-caroten-3-one) to a 3-oxo ε-end group in the incubation mixtures was not stoichiometric. Only a small portion of the intermediate was isomerized, and a large part disappeared (probably due to degradation to smaller molecules). The residual amounts of xanthophylls with a 3-hydoxy β-end group after incubation with liver postmitochondrial fraction were remarkably lower than those of xanthophylls with a hydroxyl group only in the ε-end group. The amounts of the products with a 3-oxo ε-end group were far less than those of the consumed substrates. These results indicate that the oxidation of the 3-hydroxy β-end group in xanthophylls might cause the oxidative degradation of xanthophylls, accompanied by a little conversion to an ε-end group. Therefore, the oxidation of 3-hydroxy β-end group in xanthophylls might participate in the elimination of xanthophylls from the body.
The genetic variants related to the intestinal absorption, distribution, and metabolism of carotenoids have been found to be associated with the carotenoid level in human plasma (17). The postprandial response of lutein in chylomicrons showed large interindividual differences. The responses were related to single nucleotide polymorphisms in 15 genes known to participate in the intestinal absorption of lutein and chylomicron metabolism (41). It is most likely that genetic variants of an unknown dehydrogenase responsible for the oxidation of lutein to 3′-hydroxy-ε,ε-caroten-3-one affect the long-term accumulations of lutein and zeaxanthin in human plasma, macula lutea, and other tissues. The characterization of the responsible enzyme and its gene would help clarify the causes of the interindividual variation in xanthophyll accumulation.
The oxidative metabolism of dietary xanthophylls has the potential to affect not only their bioavailability but also their biological activity. All of the oxidative metabolites of xanthophylls, as described above, have a characteristic structure of α,β-unsaturated carbonyl, which is highly reactive to nucleophiles to form a Michel-type adduct. These carbonyl compounds have diverse biological activities by reacting with sulfhydryl groups of target proteins. Various phytochemicals and medicines with an α,β-unsaturated carbonyl structure were reported to have biological activity such as anti-tumor, anti-viral, and anti-inflammatory activities (42–44). In the present study, three oxidative metabolites of xanthophylls were found to suppress the NO production by mouse macrophage RAW 264 cells stimulated with LPS. The suppression would be caused by reducing the expression of inducible nitric acid synthase by treatment of the oxidative metabolites. This biological activity of the oxidative metabolites was consistent with that of terpenes with an α,β-unsaturated carbonyl group, which suppressed NO production by inhibiting iNOS expression through the Narft-2 pathway. Thus, we suggest that the metabolites of xanthophylls reduce the formation of reactive oxygen species by macrophages and ameliorate oxidative damage by inflammation.
In conclusion, we found that the 3-hydroxy β-end group of xanthophylls was oxidized to a 3-oxo ε-end group via an unstable intermediate with a 3-oxo β-end group by a NAD+-dependent dehydrogenase of mouse liver. The increase of β,ε-caroten-3′-one in human plasma by the ingestion of β-cryptoxanthin indicated that the oxidative conversion found in mouse liver also occurred in humans.
Supplementary Material
Footnotes
Abbreviations:
- CD
- circular dichroism
- iNOS
- inducible nitric oxide synthase
- LPS
- lipopolysaccharide
- NO
- nitric oxide
- VIS
- visible
This work was supported by a grant for scientific research (number 23580189) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and by a grant from Kieikai Research Foundation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures, one table, and methods.
REFERENCES
- 1.Stahl W., Sies H. 2005. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta. 1740: 101–107. [DOI] [PubMed] [Google Scholar]
- 2.Elliott R. 2005. Mechanisms of genomic and non-genomic actions of carotenoids. Biochim. Biophys. Acta. 1740: 147–154. [DOI] [PubMed] [Google Scholar]
- 3.Foote C. S., Denny R. W. 1968. Chemistry of singlet oxygen. VII. Quenching by beta-carotene. J. Am. Chem. Soc. 90: 6233–6235. [Google Scholar]
- 4.Burton G. W., Ingold K. U. 1984. beta-Carotene: an unusual type of lipid antioxidant. Science. 224: 569–573. [DOI] [PubMed] [Google Scholar]
- 5.Chew B. P., Park J. S. 2004. Carotenoid action on the immune response. J. Nutr. 134: 257S–261S. [DOI] [PubMed] [Google Scholar]
- 6.Nishino H., Murakoshi M., Ii T., Takemura M., Kuchide M., Kanazawa M., Mou X. Y., Wada S., Masuda M., Ohsaka Y., et al. 2002. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 21: 257–264. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. 2005. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 332: 392–397. [DOI] [PubMed] [Google Scholar]
- 8.Yonekura L., Nagao A. 2007. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 51: 107–115. [DOI] [PubMed] [Google Scholar]
- 9.Nagao A. 2011. Absorption and metabolism of dietary carotenoids. Biofactors. 37: 83–87. [DOI] [PubMed] [Google Scholar]
- 10.Olson J. A., Hayaishi O. 1965. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. USA. 54: 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276: 14110–14116. [DOI] [PubMed] [Google Scholar]
- 12.Amengual J., Lobo G. P., Golczak M., Li H. N. M., Klimova T., Hoppel C. L., Wyss A., Palczewski K., von Lintig J. 2011. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25: 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Våge D., Boman I. 2010. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian R., Pitchford W. S., Morris C. A., Cullen N. G., Bottema C. D. K. 2010. Genetic variation in the beta, beta-carotene-9 ’, 10 ’-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 41: 253–259. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson S. J., Hazra A., Chen C., Eliassen A. H., Kraft P., Rosner B. A., Willett W. C. 2012. β-Carotene 15,15’-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am. J. Clin. Nutr. 96: 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lietz G., Oxley A., Leung W., Hesketh J. 2012. Single nucleotide polymorphisms upstream from the β-carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr. 142: 161S–165S. [DOI] [PubMed] [Google Scholar]
- 17.Borel P. 2012. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 56: 228–240. [DOI] [PubMed] [Google Scholar]
- 18.Landrum J. T., Bone R. A., Moore L. L., Gomez C. M. 1999. Analysis of zeaxanthin distribution within individual human retinas. Methods Enzymol. 299: 457–467. [DOI] [PubMed] [Google Scholar]
- 19.McGraw K. J., Hill G. E., Stradi R., Parker R. S. 2001. The influence of carotenoid acquisition and utilization on the maintenance of species-typical plumage pigmentation in male American goldfinches (Carduelis tristis) and Northern cardinals (Cardinalis cardinalis). Physiol. Biochem. Zool. 74: 843–852. [DOI] [PubMed] [Google Scholar]
- 20.Matsuno T., Hirono T., Ikuno Y., Maoka T., Shimizu M., Komori T. 1986. Isolation of three new carotenoids and proposed metabolic pathways of carotenoids in hen’s egg yolk. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 84: 477–481. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno T. 2001. Aquatic animal carotenoids. Fish. Sci. 67: 771–783. [Google Scholar]
- 22.Khachik F., Beecher G. R., Goli M. B., Lusby W. R., Smith J. C., Jr 1992. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal. Chem. 64: 2111–2122. [DOI] [PubMed] [Google Scholar]
- 23.Hudon J. 1994. Biotechnological applications of research on animal pigmentation. Biotechnol. Adv. 12: 49–69. [DOI] [PubMed] [Google Scholar]
- 24.Asai A., Sugawara T., Ono H., Nagao A. 2004. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 32: 205–211. [DOI] [PubMed] [Google Scholar]
- 25.Yonekura L., Kobayashi M., Terasaki M., Nagao A. 2010. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 140: 1824–1831. [DOI] [PubMed] [Google Scholar]
- 26.Liaaen-Jensen S., Hertzberg S. 1966. Selective preparation of the lutein monomethyl ethers. Acta Chem. Scand. 20: 1703–1709. [Google Scholar]
- 27.Bone R. A., Landrum J. T. 1992. Distribution of macular pigment components, zeaxanthin and lutein, in human retina. Methods Enzymol. 213: 360–366. [DOI] [PubMed] [Google Scholar]
- 28.During A., Nagao A., Hoshino C., Terao J. 1996. Assay of beta-carotene 15,15’-dioxygenase activity by reverse-phase high-pressure liquid chromatography. Anal. Biochem. 241: 199–205. [DOI] [PubMed] [Google Scholar]
- 29.Britton G. 1995. UV/visible spectroscopy. In Carotenoids: Spectroscopy. Vol. 1B. G. Britton, S. Liaaen-Jensen, and H. Pfander, editors. Birkhäuser Verlag, Basel, Switzerland. 13–62. [Google Scholar]
- 30.Barua A. B., Olson J. A. 2001. Xanthophyll epoxides, unlike beta-carotene monoepoxides, are not detectibly absorbed by humans. J. Nutr. 131: 3212–3215. [DOI] [PubMed] [Google Scholar]
- 31.Jaswir I., Kobayashi M., Koyama T., Kotake-Nara E., Nagao A. 2012. Antioxidant behaviour of carotenoids highly accumulated in HepG2 cells. Food Chem. 132: 865–872. [Google Scholar]
- 32.Kotake-Nara E., Nagao A. 2012. Effects of mixed micellar lipids on carotenoid uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 76: 875–882. [DOI] [PubMed] [Google Scholar]
- 33.Englert G. 1995. NMR spectoscopy. In Carotenoids: Spectroscopy. Vol. 1B. G. Britton, S. Liaaen-Jensen, and H. Pfander, editors. Birkhäuser Verlag, Basel, Switzerland. 147–260. [Google Scholar]
- 34.Mayer H., Rüttimann A. 1980. Synthese von optisch aktiven, natürlichen carotinoiden und strukturell verwandten naturprodukten. IV. Synthese von (3R,3′R,6’R)-lutein. Vorläufige mitteilung. Helv. Chim. Acta. 63: 1451–1455. [Google Scholar]
- 35.Richard B., Klaus N. 1995. Circular dichroism. In Carotenoids: Spectroscopy. Vol. 1B. G. Britton, S. Liaaen-Jensen, and H. Pfander, editors. Birkhäuser Verlag, Basel, Switzerland. 63–116. [Google Scholar]
- 36.Hartmann D., Thurmann P. A., Spitzer V., Schalch W., Manner B., Cohn W. 2004. Plasma kinetics of zeaxanthin and 3′-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am. J. Clin. Nutr. 79: 410–417. [DOI] [PubMed] [Google Scholar]
- 37.Albert G. I., Hoeller U., Schierle J., Neuringer M., Johnson E. J., Schalch W. 2008. Metabolism of lutein and zeaxanthin in rhesus monkeys: Identification of (3R,6’R)- and (3R,6’R)-3 ’-dehydro-lutein as common metabolites and comparison to humans. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 151: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thürmann P. A., Schalch W., Aebischer J. C., Tenter U., Cohn W. 2005. Plasma kinetics of lutein, zeaxanthin, and 3 ’-dehydro-lutein after multiple oral doses of a lutein supplement. Am. J. Clin. Nutr. 82: 88–97. [DOI] [PubMed] [Google Scholar]
- 39.Buchecker R., Eugster C. H. 1979. Search for the presence in egg yolk, in flowers of Caltha palustris and in autumn leaves of 3′-epilutein (=(3R,3′S,6’R)-β,ε-carotene-3,3′-diol) and 3′,O-didehydrolutein (=(3R,6’R)-3-hydroxy-β,ε-carotene-3′-one). Helv. Chim. Acta. 62: 2817–2824. [Google Scholar]
- 40.LoPachin R. M., Barber D. S., Gavin T. 2008. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 104: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borel P., Desmarchelier C., Nowicki M., Bott R., Morange S., Lesavre N. 2014. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 100: 168–175. [DOI] [PubMed] [Google Scholar]
- 42.El-Subbagh H. I., Abu-Zaid S. M., Mahran M. A., Badria F. A., Al-Obaid A. M. 2000. Synthesis and biological evaluation of certain α,β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 43: 2915–2921. [DOI] [PubMed] [Google Scholar]
- 43.Murakami A., Takahashi D., Kinoshita T., Koshimizu K., Kim H., Yoshihiro A., Nakamura Y., Jiwajinda S., Terao J., Ohigashi H. 2002. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha,beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 23: 795–802. [DOI] [PubMed] [Google Scholar]
- 44.Gerhäuser C., Klimo K., Hümmer W., Hölzer J., Petermann A., Garreta-Rufas A., Böhmer F. D., Schreier P. 2009. Identification of 3-hydroxy-beta-damascone and related carotenoid-derived aroma compounds as novel potent inducers of Nrf2-mediated phase 2 response with concomitant anti-inflammatory activity. Mol. Nutr. Food Res. 53: 1237–1244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.