Contents:
Executive Summary
Introduction
Methodology
Definition, Epidemiology and Risk Factors
Diagnosis of Asthma
Management of Stable Asthma
Management of Acute Exacerbations of Asthma
Miscellaneous Issues in Asthma Management
EXECUTIVE SUMMARY
Asthma is defined as a chronic inflammatory disorder of the airways which manifests itself as recurrent episodes of wheezing, breathlessness, chest tightness and cough. It is characterized by bronchial hyper-responsiveness and variable airflow obstruction, that is often reversible either spontaneously or with treatment. The prevalence of asthma in India is about 2%, and asthma is responsible for significant morbidity. In India, the estimated cost of asthma treatment per year for the year 2015 has been calculated at about 139.45 billion Indian rupees.
1. When should a diagnosis of asthma be considered?
A clinical diagnosis of asthma should be suspected in the presence of recurrent/episodic wheezing, breathlessness, cough, and/or chest tightness with no alternative explanation for these symptoms. (1A)
None of the symptoms and signs are specific for asthma. (UPP)
Absence of signs and symptoms at the time of presentation does not rule out the presence of asthma. (1A)
2. What is the role of spirometry in the diagnosis of asthma?
Wherever available, spirometry is recommended for all patients suspected to have asthma for confirming diagnosis (3A), assessing severity of airflow limitation (1A) and monitoring asthma control. (2A)
A normal spirometry does not rule out asthma. (1A)
The ratio of forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) below the lower limit of normal (lower 5th percentile of values from reference population) should be preferentially used as the criterion to diagnose airflow obstruction. (1A)
When reference equations for lower limit of normal are not available a fixed cut off of FEV1/FVC <0.75 for older subjects and <0.8 for younger individuals may be used to diagnose airflow obstruction. (UPP)
3. What is the role of reversibility testing in asthma?
Bronchodilator reversibility is a useful investigation in the diagnostic workup for asthma and is recommended if spirometry demonstrates presence of airflow limitation. (2A)
If spirometry is not available, bronchodilator reversibility may be assessed with peak expiratory flow (PEF) meters. (3B)
Presence of bronchodilator reversibility is neither diagnostic of asthma nor its absence rules out asthma. (1A)
4. What is the role of PEF monitoring in asthma?
PEF measurements should not be used interchangeably with FEV1 measurements. (1A)
Self-monitoring of PEF by patients is recommended for better asthma control. (1A)
5. Do bronchoprovocative tests help in the diagnosis and management of asthma?
Bronchoprovocative testing is not recommended as a routine test in the diagnosis of asthma. (1A)
Methacholine challenge can be used to exclude asthma as a differential especially when spirometry is normal. (2A)
Tests for bronchial hyper-responsiveness are to be performed in specialized centers only. (UPP)
6. What is the role of chest radiography in asthma?
Chest radiograph is not routinely recommended for patients suspected to have asthma. (2A)
A chest radiograph in a stable asthmatic may be considered when alternate diagnosis or complication of asthma is suspected. (UPP)
7. What is the role of non-invasive markers of inflammation in asthma management?
Quantification of eosinophil count in sputum (<2% normal, >2% suggestive of eosinophilic inflammation) can guide inhaled corticosteroid (ICS) therapy, thereby reducing the risk of exacerbations in adults with moderate to severe asthma. (2A)
Measuring the exhaled breath fractional nitric oxide (FENO) is not recommended routinely in the management of asthma. (2A)
8. What is the role of testing the allergic status of an asthmatic patient?
Tests for allergic status by measurement of total IgE, specific IgE to various environmental allergens, and skin prick tests are not recommended routinely for the diagnosis or management of asthma. (UPP)
These tests may however be done in specialized centers when specific triggers are suspected. (UPP)
9. How to categorize the severity of stable asthma?
We do not recommend classifying asthma based on severity of asthma.
10. How to assess asthma control during follow up?
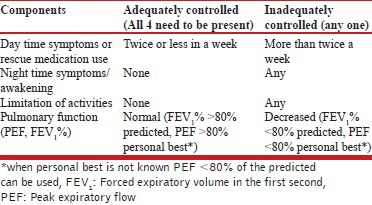
Asthma control should be classified as adequate or inadequate based on day time symptoms (or rescue medication use), night time symptoms/awakening, limitation of activities and pulmonary function (PEF, FEV1 %) as described in the Table below.
Level of current asthma control (over the preceding 4 weeks)
11. What is the role of inhaled corticosteroids (ICSs) in asthma?
ICSs are the controller medication of choice for management of stable asthma. (1A)
All the ICSs are equally efficacious when used in equipotent doses. (1A)
Most of the clinical benefit from ICS is obtained at low to moderate doses. Only a minority of patients benefit from increasing the dose beyond this. (1A)
ICS should be started at low to moderate dose (depending on the severity of symptoms at presentation) and used at lowest possible dose required. (1A)
High-dose ICS use should preferably be avoided to decrease the risk of side effects, both local and systemic. (1A)
We recommend the use of valved holding chambers/spacers whenever using moderate to high-dose ICS. (UPP)
12. What is the role of long-acting beta-2 agonists (LABA) in stable asthma?
LABA monotherapy should not be used in the management of stable asthma. (1A)
Addition of LABA to ICS is the preferred choice when symptoms are uncontrolled despite ICS monotherapy in moderate doses. (1A)
13. What is the role of leukotriene receptor antagonists (LTRAs) in stable asthma?
Monotherapy with LTRA is inferior to monotherapy with ICS. (1A)
Monotherapy with LTRA might be an alternative to ICS in patients with mild asthma if they are unwilling to use ICS or if they are not suitable for ICS therapy. (1B)
As add-on to ICS, LTRAs are inferior to LABA. (1A)
Addition of LTRA might be beneficial in patients whose asthma remain uncontrolled despite the ICS/LABA combination. (2B)
14. What is the role of long-acting anti-muscarinic agent tiotropium in the management of stable asthma?
Tiotropium may be used as add-on therapy if asthma remains uncontrolled despite moderate-to-high-dose ICS and LABA combination therapy. (1A)
15. What is the role of long-acting methylxanthines in the management of stable asthma?
Methylxanthine monotherapy is inferior to ICS monotherapy. (1A)
When stepping up from ICS monotherapy, addition of methylxanthine to ICS is as effective as doubling the dose of ICS (1A) but inferior to the ICS/LABA combination. (2A)
Methylxanthines may be used as an add-on therapy in patients who remain uncontrolled on a moderate to high ICS/LABA combination. (2B)
Whenever used as an add-on to ICS, we recommend using low dose (200-400 mg/day) sustained release formulations of theophylline. (UPP)
16. What is the role of short-acting beta-2 agonists (SABAs) in stable asthma?
SABA is the agent of choice for rescue medication in asthma. (UPP)
Short-acting muscarinic antagonist (SAMA) is a less preferred alternative/add-on to SABA as reliever medication. (UPP)
Formoterol monotherapy as a reliever should be avoided due to safety concerns with the use of LABA monotherapy. (1A)
Oral beta-agonists should not be used as rescue medications.(UPP)
17. What is the role of using a single inhaler for maintenance and reliever therapy?
We prefer the use of single inhaler therapy (SiT) using an ICS/LABA combination (formoterol-based) as both maintenance and reliever medication whenever feasible (steps 3-5, as described below). (1A)
Proposed strategy for the management of asthma in the Indian setting
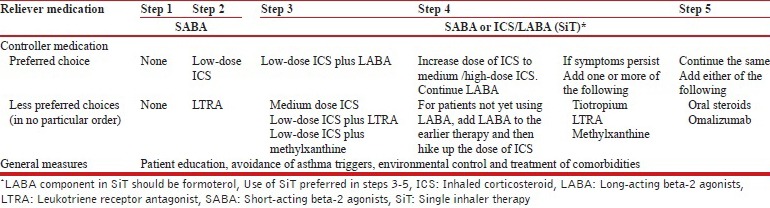
18. What should be the strategy for management of stable asthma in the Indian context?
We recommend a five-step approach for the management of stable asthma with an aim to achieve and maintain asthma control as shown in the Table below.
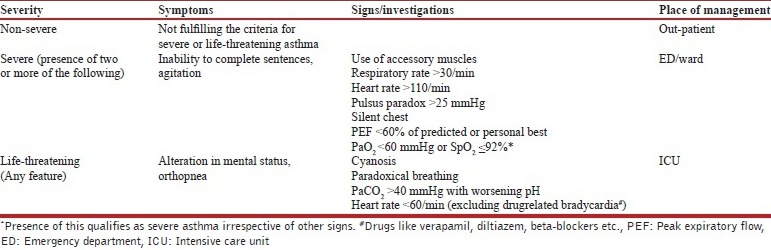
19. How is the severity of an asthma attack assessed?
The classification of acute asthma exacerbation and the site of management of an acute attack is shown in the Table below.
Assessment of the severity of acute asthma exacerbation
20. How should patients with an acute exacerbation of asthma be evaluated?
Oxygen saturation should be measured by pulse oximetry in all patients presenting with an acute attack of asthma. (UPP)
Non-severe exacerbation does not require any investigation in most instances, except PEF and pulse oximetry. (UPP)
Patient with a PEF less than 60% of predicted or personal best should be managed in the emergency department. (2A)
Patients with a saturation of less than 92% should be managed in the emergency department or hospital ward and investigated further with an arterial blood gas analysis, if available. (2A)
21. What is the role of oxygen in the management of severe acute asthma?
Oxygen should be used only in hypoxemic patients. (1A)
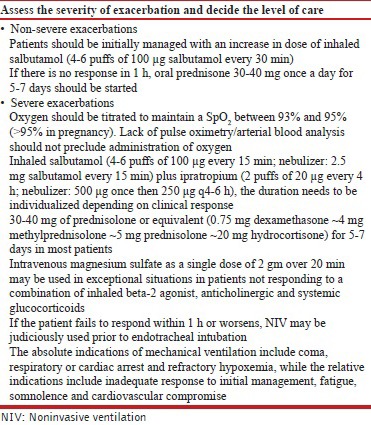
Oxygen should be titrated to maintain a SpO2 between 93% and 95% (>95% in pregnancy). (1A)
Lack of pulse oximetry/arterial blood analysis should not preclude administration of oxygen. (UPP)
In patients in whom there is a need of oxygen >8 L/min, PaCO2 should be closely monitored. (2A)
22. What is the role of bronchodilators in severe acute asthma?
Rapid-acting inhaled beta-2 agonists (salbutamol) are the bronchodilators of choice for managing acute exacerbation of asthma. (1A)
Combination of ipratropium bromide with salbutamol produces better bronchodilation than either drug alone. Ipratropium (500 μg once then 250 μg q4-6 h) should be used in all patients with severe exacerbations of asthma. (1A)
MDI with a spacer device is as effective as nebulizer in the management of acute asthma (1A). However, the dose required is higher with nebulizer with increased propensity for side-effects.
In patients unable to use MDI with spacer, drugs can be delivered via a nebulizer. Once stabilized patient should be switched over to spacer from nebulizer. (UPP)
Continuous (2.5 mg salbutamol every 15 min, or >4 nebulization per hour) nebulization is better than intermittent (2.5 mg salbutamol every 20 min, or ≤3 nebulization per hour) nebulization of rapid-acting SABA (1A). The subsequent dose of nebulized salbutamol should be 2.5 mg every 2-4 h depending on the clinical response. (UPP)
Levosalbutamol has similar efficacy and safety as compared to salbutamol in acute asthma, and has no additional benefit in the management of severe acute asthma. (1A)
Formoterol confers no added advantage over salbutamol, hence it is not recommended for routine use in acute asthma. (1A)
Parenteral beta-2 agonists and theophylline should not be used routinely as they do not confer any advantage over inhaled beta-2 agonists but are associated with increased adverse reactions (1A). However, they may be used in exceptional circumstances where inhaled medications are ineffective. (UPP)
23. What is the role of corticosteroids in management of severe acute asthma?
Systemic glucocorticoids should be used in all patients with severe acute asthma. (1A)
Oral route is as effective as parenteral route except in very sick patients or those with contraindications to enteral feeding. (1A)
Daily doses of glucocorticoids equivalent to 30-40 mg of prednisolone or equivalent (0.75 mg dexamethasone ~ 4 mg methylprednisolone ~ 5 mg prednisolone ~ 20 mg hydrocortisone) for 5-7 days are adequate in most patients. (1A)
Systemic steroids can be stopped without tapering if given for less than 3 weeks. (1A)
In non-severe exacerbations, patients should be initially managed with increase in dose of inhaled SABA (4-6 puffs of 100 μg salbutamol every 30 min). If there is no response in 1 h, oral prednisone 30-40 mg once a day for 5-7 days should be started. (UPP)
ICSs do not provide any additional benefit when used along with systemic corticosteroids and ICSs are hence not recommended in acute asthma. (1A)
The dose of inhaled steroids (in patients already on inhaled steroids) should be hiked up for 2-4 weeks at discharge from ED in addition to oral steroids. (2A)
24. What is the role of magnesium sulfate in the management of severe acute asthma?
There is no role of intravenous or inhaled magnesium sulfate in routine management of acute exacerbation of asthma. (1A)
Intravenous magnesium sulfate as a single dose of 2 gm over 20 min may be used in exceptional situations in those with severe asthma not responding to a combination of inhaled beta-2 agonist, anticholinergic and systemic glucocorticoids. (UPP)
25. What is the role of leukotriene inhibitors in severe acute asthma?
Leukotriene modifiers have no role in the management of patients with acute asthma. (1A)
26. What is the role of antibiotics in management of severe acute asthma?
Antibiotics should not be routinely used in acute asthma except in demonstrable bacterial infection. (1A)
27. What is the role of noninvasive ventilation (NIV) in severe acute asthma?
There is paucity of data on the role of NIV in acute asthma and hence it should be judiciously used in asthma exacerbation. (2B)
28. What is the role of heliox in the management of severe acute asthma?
Heliox should not be routinely used in treatment of acute asthma exacerbation. (1A)
29. What should be the strategy for management of acute exacerbation in the Indian context?
The first step is to decide the severity of the exacerbation, which guides the site for management of the exacerbation. Once the site has been identified, further management should be done as outlined in Table below.
30. What is the management of difficult-to-treat asthma?
Patients with difficult-to-treat asthma are defined as those whose symptoms are inadequately controlled despite optimal step 4 therapy for a period of 1-3 months. (UPP)
Patient compliance to drug adherence and inhaler technique should be checked at each visit. (UPP)
In patients with difficult-to-treat asthma, the possibility of asthma mimics (vocal cord dysfunction, tracheal tumors, and others) should be considered. (UPP)
Patients with difficult-to-treat asthma should also be evaluated for the presence of ABPA. (UPP)
Smoking cessation should be advised for all asthmatics who are smokers. (UPP)
Patients with difficult-to-treat asthma with features of associated comorbidities (like rhinitis, obesity, obstructive sleep apnea, and gastro-esophageal reflux disease) should be evaluated and treated accordingly. (UPP)
Addition of oral corticosteroids for difficult-to-treat asthma should be considered only if the patient's symptoms remain uncontrolled despite maximal step 4 therapy. (UPP)
When considered, oral corticosteroids should be used at the lowest possible dose for the shortest possible duration and patients should be simultaneously monitored for drug-related adverse effects. (UPP)
31. What is the role of anti-IgE in asthma?
Omalizumab may be considered as an adjunctive therapy to ICS in patients with moderate to severe asthma who have elevated serum IgE levels and a positive skin test to at least one perennial aero-allergen. (1B)
Practical management of asthma exacerbations
32. What is the role of bronchial thermoplasty in asthma?
As of now, good quality evidence is lacking for recommending bronchial thermoplasty in the routine management of bronchial asthma. (2A)
33. What is the role of immunotherapy in asthma?
Single allergen immunotherapy may provide a modest benefit to patients with mild-to-moderate asthma with demonstrable skin allergy to that antigen. (2B)
Multiple allergen immunotherapy cannot be recommended at the moment based on currently available evidence. (2A)
Immunotherapy carries the risk of severe reactions which can be life threatening. Therefore, it should be practiced only by well-trained personnel in centers experienced in performing the technique. (3A)
Immunotherapy should not be used in patients with severe or poorly controlled asthma, and in patients with FEV1 <70% because of significantly higher risk of fatal reactions. (3A)
34. What is the role of patient education in asthma?
Optimal self-management which involves a combination of patient education, self-monitoring, regular physician review, and self-management using a written asthma action plan is strongly recommended in the management of asthma. (1A)
35. What is the role of pulmonary rehabilitation in asthma?
Pulmonary rehabilitation therapy in asthmatics produces significant improvement in exercise capacity. (2A)
Pulmonary rehabilitation therapy in asthmatics improves asthma symptoms and quality of life. (3A)
36. What is the role of vaccination in the prevention of asthma exacerbations?
Current evidence is insufficient to recommend influenza or pneumococcal vaccination routinely for patients with asthma. (3A)
37. What is the role of antibiotics in the prevention of asthma exacerbations?
Available evidence does not suggest a role for antibiotics in the prevention of asthma exacerbations. (2A)
38. How should asthma be managed during pregnancy?
Poorly controlled asthma and asthma exacerbations are associated with adverse pregnancy outcomes, while well-controlled asthma is associated with normal pregnancy outcomes. (2A)
Most medications used for asthma have negligible effects on the fetus. (3A)
Adequate asthma control in pregnancy should be attempted with routinely available asthma medications as in the non-pregnant state (including systemic steroids whenever indicated). (3A)
Asthma during lactation should be managed similar to asthma during pregnancy. (3A)
Caution should be exercised while using theophyllines during pregnancy and lactation. (3A)
39. How should exercise-induced asthma (EIA) be managed?
Pretreatment with bronchodilator agents (SABA, SAMA, and LABA) as well as anti-inflammatory agents (LTRA but not ICS) is effective in attenuating the fall in FEV1 associated with EIA. (2A)
Regular use of ICS or LTRAs is effective in prevention of exercise-induced bronchospasm. (2A)
Regular use of LABA as prophylaxis for EIA should be avoided as long-term regular administration of LABA induces tolerance and may cause increase in adverse effects. (2A)
40. How should aspirin-induced asthma (AIA) be managed?
Patients with AIA should avoid all NSAIDs which can inhibit the enzyme cyclo-oxygenase 1 (COX-1). (3A)
COX-2 inhibitors can be safely used in patients with AIA. (3A)
Patients with AIA can have cross-reactions to paracetamol (esp. in doses ≥1000 mg); however, these reactions tend to be mild. (3A)
Aspirin desensitization may be useful in selected subjects with AIA. (3A)
There is no sufficient evidence to suggest that the management of AIA should be different from that of allergic asthma apart from avoidance of NSAIDs. (UPP)
41. What are the recommendations for occupational asthma?
Both removal of exposure and reduction of exposure improve symptoms of occupational asthma. Removal of exposure appears to be better than reduction of exposure. However, this should be considered against a background of increased risk of unemployment with the former. (2A)
A. INTRODUCTION
Bronchial asthma is a common respiratory disorder with prevalence ranging from 1-18% in different populations. It is an important public health problem in India with significant morbidity. The prevalence of asthma in India is about 2% with a burden of about 17 million asthmatic patients. Thus, asthma imposes a tremendous burden on the healthcare system and society of India due to loss of productivity, especially due to the fact that young individuals in the most efficient phase of their life, are affected. Several international guidelines for diagnosis and management of asthma are available, however there is a need for country-specific guidelines due to vast differences in availability and affordability of healthcare facilities across the globe.
The two foremost societies of Respiratory Medicine in India namely the Indian Chest Society (ICS) and the National College of Chest Physicians (NCCP) of India have collaborated to develop evidence-based guidelines with an aim to assist physicians at all levels of healthcare in diagnosis and management of asthma in a scientific manner.
Besides a systematic review of literature, the Indian studies were specifically analyzed to arrive at simple and practical recommendations. The evidence is presented under these five headings: (a) definitions, epidemiology and impact, (b) diagnosis, (c) pharmacologic management of stable disease, (d) management of acute exacerbations, and (e) nonpharmacologic management and special situations.
B. METHODOLOGY
The process of development of guidelines for diagnosis and management of patients of bronchial asthma in India was undertaken as a joint exercise of the two National Pulmonary Associations (Indian Chest Society and National College of Chest Physicians), by the Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh. The committee constituted for this purpose included representatives from the two associations, as well as experts from other institutes and medical colleges, including those from disciplines of Internal Medicine, Microbiology, and Pharmacology.
For the development of guidelines, an extensive initial desk review was followed by a joint workshop. The review of literature was performed by searching the electronic databases (PubMed, EmBase, and Cochrane). The major international guidelines, including those available from the Global Initiative for Asthma (GINA), British Thoracic Society (BTS) and National Asthma Education and Prevention Program of the National Heart, Lung, and Blood Institute, were also reviewed.
The search was conducted under five subgroups (a) definitions, epidemiology and impact, (b) diagnosis, (c) pharmacologic management of stable disease, (d) management of acute exacerbations, and (e) nonpharmacologic management and special situations. Important questions were framed on the basis of discussions on issues with reference to the Indian context. Literature review and discussions in each area were coordinated by Group Chairs and recorded by rapporteurs. The available evidence as well as the questions were circulated to all the group members before the joint workshop. Discussions for grading of evidence and recommendations were held independently in five parallel group sessions, and thereafter together in the joint meeting of all the groups. Final decisions in the joint group were based on a consensus approach.
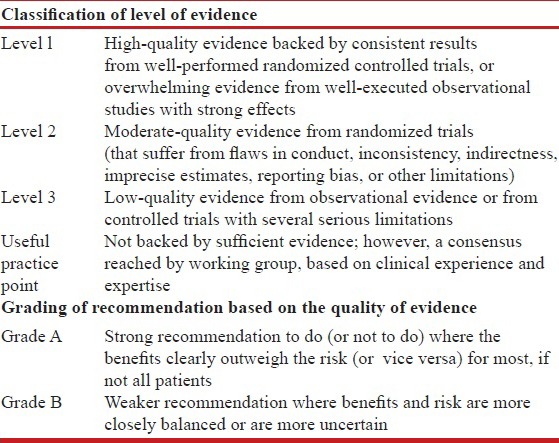
The modified GRADE system was used for classifying the quality of evidence as 1, 2, 3 or usual practice point (UPP) [Table 1].[1] The strength of recommendation was graded as A or B depending upon the level of evidence [Table 1]. Grade A recommendations in the guidelines should be interpreted as “recommended” and the grade B recommendations as “suggested.” While making a recommendation, the issues of practicality, costs, and feasibility in the country at different levels of healthcare were also taken into consideration.[2]
Table 1.
Classification of level of evidence and grading of recommendation based on the quality of evidence supporting the recommendation
The final document was reviewed by all the committee members, as well as by other external experts.
C. DEFINITION, EPIDEMIOLOGY AND RISK FACTORS
C1. What is the definition of asthma?
Asthma was first defined in 1959 as “a disease characterized by wide variation over short periods of time in resistance to flow in the airways of the lung.”[3] Several definitions have been laid down in different guidelines,[4,5,6] but the most widely accepted definition is the one proposed by Global Initiative for asthma.[5] This definition involves several components, which are difficult to establish in routine clinical practice, especially in a resource-limited country like India. Therefore, we recommend the following clinical definition of asthma: “Asthma is defined as a chronic inflammatory disorder of the airways which manifests itself as recurrent episodes of wheezing, breathlessness, chest tightness and cough. It is characterized by bronchial hyper-responsiveness and variable airflow obstruction, that is often reversible either spontaneously or with treatment.”[7]
C2. What is the prevalence of asthma?
Asthma is one of the most common chronic diseases worldwide. The global prevalence of asthma, using a definition of clinical asthma or treated asthma, is estimated to be about 4.5% (95% confidence intervals [CI], 4.4-4.6).[8,9,10] Using this prevalence figure, there are about 315 million people estimated to be suffering from asthma worldwide. Using a less rigorous definition for diagnosis of asthma, the global prevalence is approximately 8.6% (95% CI, 8.5-8.7) with a burden of 623 million asthmatic patients.[9] There has been an increase in prevalence of asthma over time, similar to other allergic disorders. Thus, an additional 100 million people worldwide are likely to develop asthma, by 2025.[10]
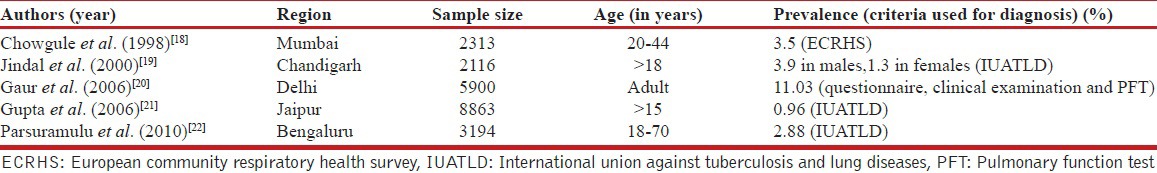
In studies from several single centers, the prevalence of asthma in children in India ranged from 2.3% to 11.9% [Table 2],[11,12,13,14,15,16,17] while the prevalence of asthma in adults varied from 0.96% to 11.03% [Table 3].[18,19,20,21,22] The major drawback of these studies is the small sample size; hence, these results cannot be used for the estimation of nationwide prevalence. Studies in special groups have reported prevalence ranging from 5.8% in petrol pump workers to 14.8% in industrial workers.[23,24] One study using data from the third National Family Health Survey (NFHS 3) found the prevalence of self-reported asthma to be 1.9%.[25] In a recently conducted World Health Survey, the prevalence of wheezing, clinical asthma and doctor-diagnosed asthma was 9.63%, 3.3% and 3.16%, respectively in Indian adults.[9] The Indian Study on Epidemiology of Asthma, Respiratory Symptoms and Chronic Bronchitis (INSEARCH) in adults, which involved 16 centers across the country in two phases is the largest, prospective multicenter study on the prevalence of asthma in Indian adults.[26,27] The prevalence of asthma in adults reported in this study, using a validated International Union against Tuberculosis and Lung Diseases questionnaire, was 2.05%, with an estimated burden of 17.23 million.[26,27,28] Currently, it is reasonable to accept a prevalence of asthma in India of at least 2% till systematic studies on physician-diagnosed asthma are available.
Table 2.
Prevalence of asthma in Indian children
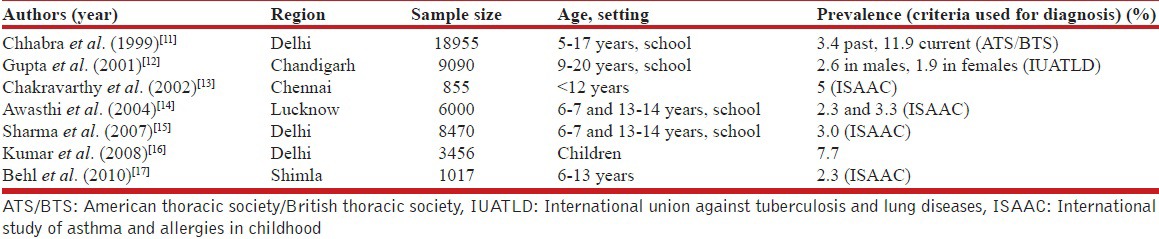
Table 3.
Single center studies from India reporting the population prevalence of asthma
C3. What are the implications of asthma on morbidity and mortality?
Asthma is responsible for significant morbidity worldwide. It is the 25th leading cause of disability adjusted life years (DALYs) lost per year accounting for an estimated 15 million DALYs lost (about 1% of all lost DALYs).[10,29] This is comparable to other common diseases like diabetes mellitus and schizophrenia. Asthma accounts for 1 of 250 deaths worldwide, however most of these deaths are preventable with appropriate management.[10] No data is available from India on mortality and morbidity.
C4. What is the economic impact of asthma?
In Europe, the estimated direct costs of asthma treatment are about 17.7 billion Euros every year while the indirect cost due to loss of productivity is about 9.8 billion Euros annually.[30] Similarly in the United States, the total additional cost of asthma to society was 56 billion dollars, with loss of productivity due to morbidity accounting for 3.8 billion dollars and productivity losses due to mortality amounting to 2.1 billion dollars.[31]
In India, the estimated cost of asthma treatment per year for the year 2015 has been calculated at about 139.45 billion Indian rupees (approximately 2.3 billion US dollars). Interestingly, it has been deduced that this cost is likely to come down to about 48.5 billion Indian rupees if all asthmatics receive treatment according to evidence-based guidelines.[32] It is noteworthy that this estimate does not include the indirect costs of asthma.[32]
C5. What are the risk factors for asthma?
Several factors have been found to have a strong association with development of asthma and are considered as risk factors. However, no cause and effect relationship has been established for any of the etiological factors and development of asthma.
I. Non-modifiable risk factors
Age and gender: In two multicentre studies from India, the prevalence of asthma increased with advancing age. However, this association is likely the result of mathematic coupling of age rather than a true risk factor. Female gender has consistently been associated with higher prevalence of asthma in adults.[25,26,27] In children, slight male predominance has been reported,[11] which is consistent with reports worldwide.
Atopy: Atopy is production of abnormal amounts of IgE antibodies in response to common environmental allergens. A history of atopy is the strongest risk factor for development of asthma with an adjusted odds ratio of 12.3 (95% CI, 11.1-13.7).[26]
Family history of asthma and/or atopy: A family history of atopy and/or asthma is strongly associated with development of asthma.[33] In INSEARCH I and II, the adjusted OR for asthma in those with family history of asthma was 6.1 (95% CI, 5.4-6.9) and 8.8 (95% CI, 8.1-9.6), respectively.[26,27]
Genetic risk factors: Several genetic factors have been implicated in different studies, however no cause and effect relation has been established.[34] Genome-wide association studies have identified a locus on chromosome 17q12-21 as a risk factor for childhood-onset asthma, but not for atopy or adult-onset asthma.[35] Studies from India have described polymorphism in different genes such as GSTM 1, GSTT 1, MBL2 and others,[36,37,38,39,40,41,42,43] to be associated with asthma. However, there are no systematic genome-wide association studies on asthma from the Indian subcontinent.
II. Modifiable risk factors
-
Tobacco smoke: The association between tobacco smoke exposure and asthma has been established in numerous studies.[44,45,46,47] Several studies from India, both in children and adults, have consistently reported higher prevalence of asthma in those exposed to tobacco smoke, both active and passive.[25,26] Dose–response relationship has been reported for both active and passive/environmental (ETS) tobacco smoke exposure suggesting causal relationship.[44,48,49] In fact, tertiary smoking defined as fetus exposed to tobacco smoke as a consequence of mother being exposed to ETS has also been reported to increase the risk of development of asthma.[50]
Tobacco smoke exposure not only increases risk of asthma but also affects the course of asthma, for example, by increasing the risk of acute exacerbations.
Biomass exposure: Indoor air pollution due to combustion of solid fuels for cooking and heating has been shown to significantly increase the risk of asthma.[16,27,51,52,53,54,55] In the INSEARCH study, the odds of having asthma in those with exposure to biomass combustion were 1.3-1.6.[27]
Infections: Respiratory viral infections early in life, especially those due to respiratory syncytial virus (RSV) and para-influenza virus, have been associated with increased incidence of asthma.[56,57] In a long-term follow-up study, as many as 40% of RSV-infected infants have been reported to develop asthma,[56] although there has been no reported effect of respiratory viral infections on asthma in adults.[58] On the other hand, the “hygiene hypothesis” of asthma is based on the assumption that recurrent infections early in life modulate the immune system to a non-allergic pathway, thereby decreasing the risk of asthma and other allergic diseases.[59] At present, the evidence regarding infections and asthma is conflicting and clear conclusions are not possible.
Occupational exposures: More than 300 substances have been reported to predispose to occupational asthma.[60] Data from India is limited and the prevalence reported in industrial workers is slightly higher than that in general population.[23,24]
Formula feed and cow milk in infancy: Duration of exclusive breast-feeding is inversely associated with incidence of wheezing in childhood and atopic asthma in later life. On the contrary, infants fed with formula feed (cow milk or soy protein) have been reported to have a higher incidence of wheezing illnesses in early childhood.[61,62]
Diet: A history of perceived worsening of asthma in relation to dietary items is fairly common among Indian asthmatics ranging from 60% to 90%.[63] However, the skin prick test (SPT)-proven sensitization is seen in only a small fraction ranging from 1.7% for black gram to 6.2% for rice. Moreover, confirmation by re-challenge is positive only in 1.7%,[64,65,66] and hence the exact relationship between diet and asthma remains unclear.
Obesity: Asthma is more common in obese individuals especially those with body mass index (BMI) >30 kg/m2, with a clear dose–response relationship.[67] Obese patients also have multiple co-morbidities making treatment of asthma difficult.[68,69]
C6. What are the triggers for asthma?
Several factors have been known to precipitate asthma symptoms including cold air, extreme emotional arousal, physical exercise, aspirin and other NSAIDs, beta-blockers, indoor allergens (house dust mites in bedding, carpets and stuffed furniture, pet dander), outdoor allergens (especially molds and pollen), tobacco smoke, chemical irritants in the workplace and air pollution.[70]
C7. What are the factors protective against asthma?
Some studies suggest exclusive breast feeding,[14] regular intake of fruits and green leafy vegetables,[14,25] to have protective role against asthma. Similarly, one study from India suggested a protective role of Bacille Calmette-Guérin (BCG) vaccination, however others found negligible benefit.[71]
D. DIAGNOSIS OF ASTHMA
D1. When should a diagnosis of asthma be considered?
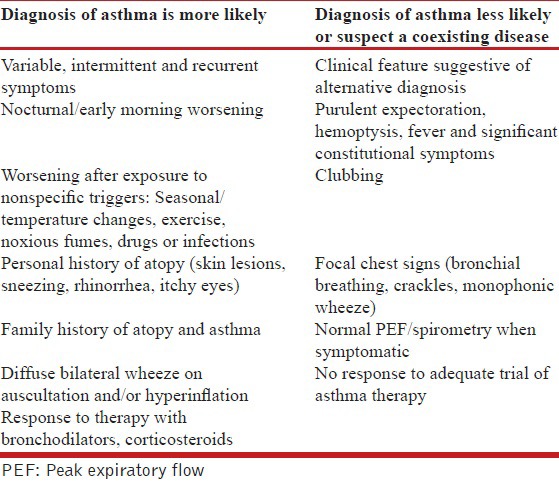
The diagnosis of asthma remains largely clinical due to the absence of a gold standard. The classical symptoms of asthma (wheezing, breathlessness, cough, and chest tightness) tend to be variable, seasonal, recurrent and/or nocturnal. Cough may be the only manifestation of asthma (cough variant asthma). None of these symptoms are however specific for the diagnosis of asthma, and patients may be completely asymptomatic at the time of initial evaluation. Presence of atopy, family history of asthma in a first degree relative, and/or symptomatic worsening after exposure to non-specific triggers support a diagnosis of asthma. Physical examination further helps in the diagnosis, and in exclusion of asthma mimics [Table 4]. The presence of expiratory polyphonic wheeze is a typical finding, and hyperinflated chest may suggest long-standing disease. However, respiratory system examination may be completely normal when performed during an asymptomatic period. Tachycardia, tachypnea, use of accessory muscles of respiration suggest an asthma exacerbation. Patients may present de novo with an exacerbation. When the exacerbation is very severe, marked airflow limitation and air trapping may result in a ‘silent chest’ accompanied by signs of respiratory failure.
Table 4.
Clinical features favoring a diagnosis of asthma
Recommendations
A clinical diagnosis of asthma should be suspected in the presence of recurrent/episodic wheezing, breathlessness, cough, and/or chest tightness with no alternative explanation for these symptoms. (1A)
None of the symptoms and signs are specific for asthma. (UPP)
Absence of signs and symptoms at the time of presentation does not rule out the presence of asthma. (1A)
D2. What are the differential diagnoses of asthma?
Many clinical conditions can either mimic or coexist with asthma.[72] The differential diagnosis of asthma broadly includes disorders causing chronic cough, wheezing and/or airflow limitation on spirometry.[7,73]
Chronic obstructive pulmonary disease (COPD) is a common condition that mimics asthma and may sometimes co-exist with it. A meticulous clinical history is important in differentiating asthma from COPD. COPD predominantly afflicts older adults with significant exposure to risk factors, particularly smoking and biomass fuel exposure.[74] Asthmatic patients worsen in response to certain triggers, but generally return to their normal baseline status over a short period (and may even become asymptomatic). On the other hand, symptoms of cough, wheeze and breathlessness in COPD patients are likely to persist even between periods of symptomatic worsening. Symptom-based questionnaires aimed at differentiating asthma from COPD found that increasing age, greater tobacco exposure, worsening cough, and persistent sputum production were all significant predictors for diagnosing COPD.[75] Though classically described as having fixed obstruction on spirometry, reversibility and airway hyper responsiveness may occasionally be noted in COPD as well. It may be difficult to distinguish these two entities in some patients, and in a minor proportion both diseases may coexist, the so-called “asthma-COPD overlap syndrome” (ACOS).[76]
Disorders such as tuberculosis and bronchiectasis should be considered in patients having hemoptysis, chest pain and/or constitutional symptoms of fever and weight loss. In any patient presenting with cough and expectoration for more than 2 weeks, tuberculosis should be considered and relevant investigations such as sputum smear examination for acid-fast bacilli and chest radiography should be performed. This is particularly important in India where the burden of tuberculosis is still high.[7] Presence of focal signs on chest examination should raise a suspicion of pneumonia, tuberculosis or bronchiectasis, which may occur in an asthmatic as well.
Gastro-esophageal reflux disease (GERD) is another important consideration that apart from mimicking asthma can also cause poor asthma control.[77] Presence of stridor, localized monophonic wheeze may suggest an intrathoracic or extrathoracic airway obstruction from a variety of causes (such as foreign body aspiration or benign/malignant airway tumors).[78] Cystic fibrosis sometimes mimics asthma, and presence of features suggestive of chronic malnutrition, failure to thrive and diarrhea in a young individual point toward this diagnosis. Early morning/nocturnal cough with wheezing and chest tightness is typical of asthma. However, chronic nocturnal cough may also be a feature of upper airway cough syndrome.[79]
Spirometry would however be normal in upper airway cough syndrome. Cardiac failure and tropical eosinophilia are other causes of nocturnal cough. The chronic cough syndromes are particularly difficult to distinguish from cough variant asthma. Cough variant asthma may sometimes precede typical asthma and is associated with BHR and airway eosinophilia. Chronic cough can also be drug-induced (as with angiotensin-converting enzyme inhibitors used for hypertension).[79]
Paradoxical vocal cord motion, also known as vocal cord dysfunction, is an important asthma mimic, which may also coexist with asthma. Vocal cord dysfunction should be suspected when patients fail to demonstrate adequate control despite high doses of bronchodilators and ICS. Hyper-responsiveness of larynx to various intrinsic and extrinsic triggers possibly results in vocal cord dysfunction. The diagnosis can be confirmed on video-laryngoscopy.[80] In some reports, up to 50% of individuals with vocal cord dysfunction had asthma as well.[81]
In a real world scenario, a therapeutic trial is warranted in patients in whom the diagnosis of asthma is highly likely. If the response to treatment (documented by improvement in clinical symptoms and preferably lung function) is good, it is continued. However, if the diagnosis remains uncertain after initial clinical evaluation, spirometry should be performed to look for airflow obstruction. Further differential diagnosis need to be considered based on the presence or absence of airflow obstruction [Table 5].[73]
Table 5.
Differential diagnosis of asthma based on presence or absence of airflow obstruction on spirometry
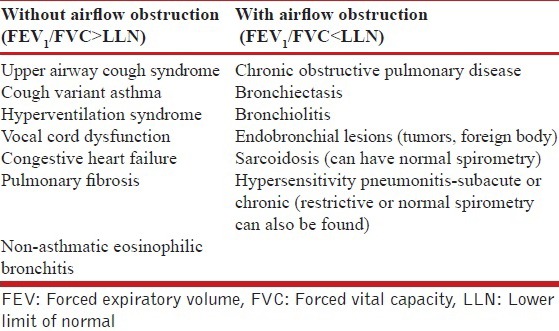
D3. What is the role of spirometry in the diagnosis of asthma?
A normal spirometry does not exclude asthma however the demonstration of obstruction and/or bronchodilator reversibility supports a clinical diagnosis of asthma. Wherever feasible, a clinical diagnosis of asthma should be supported by demonstration of variability of airway obstruction on pulmonary function testing over a period, even if not demonstrated at a point.[76] Therefore, pulmonary function testing by spirometry should be used in diagnosis, classification and severity assessment of asthma.[4,76] Even asymptomatic asthmatics may have a reduction in FEV1 and this reduction is more severe in individuals with severe disease. Patients with long-standing asthma, as well as elderly, are in particular poor perceivers, in whom the role of pulmonary function is even more important.[82,83] In a population study, only 34% of individuals with reduced FEV1 and symptoms suggestive of asthma sought medical attention, and among these 21% were missed by clinicians.[84]
Since spirometry is an effort-dependent procedure, it should be performed according to standard guidelines.[85,86] The ratio of forced expiratory volume in first second to the forced vital capacity (FEV1/FVC) should be calculated, and a reduced value interpreted as evidence of airflow limitation. The threshold for this purpose is not clearly defined. A FEV1/FVC ratio below 0.75-0.80 (arbitrary cut-off) is often used to diagnose airflow obstruction in adults.[87] However, this might underestimate and overestimate obstruction in the young and the elderly, respectively.[88] It appears more appropriate to statistically define lower limits of normality for FEV1, FVC and FEV1/FVC ratio using regression equations derived from healthy individuals from various geographical locations (e.g. lower fifth percentile of values from reference population). Such reference equations for Indian population are available.[89,90,91,92,93,94] Severity of airflow limitation can be quantified by expressing FEV1 as a percentage of its predicted value (FEV1 %). FEV1 % is an important factor determining risk of exacerbations and long-term outcome in asthmatics.[95,96]
Caution needs to be exercised to avoid spread of infection through spirometry testing. If any patient is suspected to have pulmonary tuberculosis, it is prudent to obtain sputum smear for acid-fast bacilli before subjecting him/her to lung function testing.
Recommendations
Wherever available, spirometry is recommended for all patients suspected to have asthma for confirming diagnosis (3A), assessing severity of airflow limitation (1A) and monitoring asthma control. (2A)
A normal spirometry does not rule out asthma. (1A)
The ratio of forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) below the lower limit of normal (lower fifth percentile of values from reference population) should be preferentially used as the criterion to diagnose airflow obstruction. (1A)
When reference equations for lower limit of normal are not available a fixed cut off of FEV1/FVC <0.75 for older subjects and <0.8 for younger individuals may be used to diagnose airflow obstruction. (UPP)
D4. What is the role of reversibility testing in asthma?
Variability and reversibility are two important characteristics of asthma. Variability is the change in lung function or symptoms with time, whether diurnal or seasonal, and such history favors a diagnosis of asthma. Variability in lung function demonstrated from time to time is also indicative of asthma, and should preferably be documented in all patients. Demonstration of bronchodilator reversibility on spirometry also favors the diagnosis of bronchial asthma. This can be easily done after performing baseline spirometry, by administering 400 μg of inhaled salbutamol (or equivalent) and repeating the test after 15-20 minutes. An improvement in FEV1 and/ or FVC of at least 12% and 200 mL compared to the baseline value indicates a positive bronchodilator response.[85] Despite its usefulness, a lack of bronchodilator reversibility does not rule out the presence of asthma. If spirometry is not available, PEF meters may be used for this purpose, although it is less sensitive and specific.[97]
When spirometry is not available, response to bronchodilator can be assessed using peak expiratory flow (PEF) measurements. An increase in baseline peak expiratory flow by 60 L/min (and/or 20%) following inhalation of 400 μg of salbutamol or equivalent is considered a positive bronchodilator response.[76] In one study, a post-bronchodilator increase in PEF ≥60 L/min correlated with an absolute increase in FEV1 of >190 mL and FEV1 % predicted of > 9%.[98] Although changes in PEF following bronchodilator administration correlates with changes in FEV1, the sensitivity is poor.[99] Hence, demonstration of airway obstruction as well as bronchodilator reversibility is preferably done with spirometry.
Recommendations
Bronchodilator reversibility is a useful investigation in the diagnostic workup for asthma and is recommended if spirometry demonstrates presence of airflow limitation. (2A)
If spirometry is not available, bronchodilator reversibility may be assessed with PEF meters. (3B)
Presence of bronchodilator reversibility is neither diagnostic of asthma nor its absence rules out asthma. (1A)
D5. What is the role of PEF monitoring in asthma?
Peak expiratory flow varies with race, height, age of the patients, and has a wide “normal” range.[100,101,102] Although a normal value of PEF cannot be assigned, it is possible for an individual patient to determine his/her normal or best PEF. Therefore, it is useful in individual patients to monitor asthma control, and the patient's personal best PEF appears to be a better comparator than the predicted PEF.[103] Peak expiratory flow measurements are available for different populations and can be used for monitoring in case personal best is not available.[104,105,106,107] PEF measurements are known to be affected by the technique and the equipment by which it is measured, and thus proper instructions and training are essential for the patient.[108,109] PEF measurements have been used in the past as a surrogate to FEV1 measurements, but the correlation between them is poor and hence they should not be used interchangeably.[110,111,112,113]
Peak expiratory flow is known to exhibit a circadian rhythm and this variability is exaggerated in individuals with bronchial hyper-reactivity.[114] Diurnal PEF variability can be calculated using the amplitude percent mean method (difference between maximum and minimum PEF of the day expressed as a percentage of mean PEF). When done daily, this can help patients recognize when the disease control starts getting poor. A diurnal variation in PEF more than 20% is suggestive of poor control of bronchial asthma.[115,116,117] The optimum number of daily measurements required to document PEF variability is also not clear.[118] Alternatively, PEF can be monitored once daily by taking this measurement first thing in the morning (before taking any drugs) and comparing the reading to a known personal best.[76,119] If PEF falls below 80% of personal best, it is suggestive of inadequate disease control.
Recommendations
PEF measurements should not be used interchangeably with FEV1 measurements. (1A)
Self-monitoring of PEF by patients is recommended for better asthma control. (1A)
D6. Do bronchoprovocative tests help in the diagnosis and management of asthma?
Excessive narrowing of the airway in response to a physical or chemical stimulus is called bronchial hyper-responsiveness (BHR). It is common in asthmatics, but even apparently healthy individuals may sometimes demonstrate airway hyper-responsiveness.[120] BHR may occur as a transient phenomenon in viral upper respiratory infections or may be persistent in few conditions like sarcoidosis.[121] Hence, the specificity of this test in the diagnosis of asthma is poor.
Bronchoprovocative tests can be performed using either direct or indirect stimuli. Pharmacologic agents active on bronchial smooth muscles (e.g., methacholine) are direct stimulants. On the other hand, indirect stimuli (like exercise) cause release of biological mediators such as histamine and prostaglandins, which in turn constrict the airway. The bronchoprovocative test results are expressed as the provocative dose of the agonist required to cause a 20% fall in FEV1.[76] A methacholine challenge test may be helpful in excluding asthma objectively, owing to its high sensitivity even at low doses.[122] Clinical asthma is unlikely when methacholine challenge at a dose of 16 mg/mL does not produce a 20% fall in FEV1.[123,124] In patients with normal chest radiograph, clinical examination and spirometry, methacholine challenge test had the highest sensitivity to diagnose asthma (85.7%) when compared with PEF variability, blood and sputum eosinophil counts.[125] However, a negative methacholine challenge test alone may under-diagnose asthma, particularly in exercise-induced bronchoconstriction that may precede typical bronchial asthma.[126,127] The test has a significant risk of precipitating an acute attack of asthma, and is therefore performed only at a few laboratories with experienced personnel and sufficient facilities for resuscitation. Studies comparing clinical questionnaires with BHR have reported higher sensitivity with former compared to latter; however, the use of BHR led to a greater specificity (97%) compared to questionnaire alone (90%).[128,129]
Recommendations
Bronchoprovocative testing is not recommended as a routine test in the diagnosis of asthma. (1A)
Methacholine challenge can be used to exclude asthma as a differential especially when spirometry is normal. (2A)
Tests for bronchial hyper-responsiveness are to be performed in specialized centers only. (UPP)
D7. What is the role of chest radiography in asthma?
There is no role for routine chest radiography in a newly diagnosed patient of asthma. A chest radiograph may be warranted when additional complications like allergic bronchopulmonary aspergillosis (ABPA), or an alternative diagnosis like tuberculosis, are under consideration.[130,131] A study done in children with newly diagnosed asthma found that 85% of them had a normal chest radiograph, and even among those with abnormal findings, the abnormalities were transient.[132,133] Presence of increased bronchovascular markings and low diaphragm are few features noted in asthmatics more commonly than normal individuals. However, these do not differentiate asthma from other pulmonary disorders.[134] Other imaging modalities like CT, single-photon emission computerized tomography and positron emission tomography are being investigated to understand the pathophysiologic and anatomic abnormalities in asthmatics.[135] Currently they have no role in the routine diagnosis or management of asthma.
Recommendations
Chest radiograph is not routinely recommended for patients suspected to have asthma. (2A)
A chest radiograph in a stable asthmatic may be considered when alternate diagnosis or complication of asthma is suspected. (UPP)
D8. What is the role of non-invasive markers of inflammation in asthma management?
Assessment of airway inflammation may have therapeutic implications in asthma as it is a chronic inflammatory disease of the airways. Endo-bronchial biopsy may enable identification of the nature of inflammation and subsequent modification of therapy; however, it is an invasive procedure.[136] Various non-invasive markers of inflammation have been investigated in the last decade. These include sputum differential cytology, exhaled breath pH and nitric oxide, exhaled breath proteins (like IL-6, IL-8), and serum proteins (like eosinophilic cationic protein, adiponectin, and periostin).[137,138,139] Two of these markers, fractional exhaled nitric oxide (FENO) and sputum differential eosinophil count (also called sputum inflammometry) have shown some promise.[140,141] Both reflect eosinophilic airway inflammation and in turn corticosteroid responsiveness.
For sputum inflammometry, sputum (either spontaneously produced or induced with 3% saline) is processed as per the recommendations of the working group of European Respiratory Society.[142,143] Differential cell count is reported as the percentage of total non-squamous cells (after a minimum of 400 non-squamous cells are counted).[144] Induced sputum from healthy volunteers predominantly contains macrophages and neutrophils, whereas eosinophils constituted only a small proportion (0.6 ± 0.8%).[145] In asthmatics, the sputum eosinophil count may be elevated up to 50% or more. Sputum eosinophil count of > 1% showed sensitivity and specificity of 72% and 80%, respectively for diagnosis of asthma.[146] A meta-analysis of three trials concluded that sputum eosinophil count-guided therapy significantly reduced number of exacerbations as compared with symptom-based tailoring of therapy.[147] Quantification of eosinophil count in sputum can guide ICS therapy, thereby reducing the risk of exacerbations in adults with moderate to severe asthma.[148]
Nitric oxide (NO) exhibits physiological effects of vasodilation, bronchodilation and immune enhancement at low concentrations, and acts as an inflammatory agent at higher concentrations.[149,150,151] NO levels are elevated in exhaled breath of asthmatics,[152,153] more specifically in a subset of patients with eosinophilic inflammation.[154] The non-invasive nature of the test and its repeatability has made FENO a potentially useful investigation in asthma management. However, the test is not freely available, and a reference value for FENO is difficult to establish owing to the variations noted with age, gender, smoking status, use of anti-inflammatory medications and measurement techniques.[155] In general, FENO <25 parts per billion (ppb) is suggestive of non-eosinophilic inflammation unlikely to be steroid responsive. FENO >50 ppb is more suggestive of eosinophilic airway inflammation and steroid responsiveness.[155] A systematic review of trials comparing FENO-guided and symptom-guided therapy concluded that the former had only moderate benefit in improving asthma outcomes.[156] Also, the number of exacerbations in adults did not differ significantly whether the treatment was FENO guided or symptom guided.[147] Therefore, measurement of FENO is not routinely recommended for diagnosis or management of asthma.
Recommendations
Quantification of eosinophil count in sputum (<2% normal, >2% suggestive of eosinophilic inflammation) can guide ICS therapy, thereby reducing the risk of exacerbations in adults with moderate to severe asthma. (2A)
Measuring the exhaled breath FENO is not recommended routinely in the management of asthma. (2A)
D9. What is the role of testing the allergic status of an asthmatic patient?
Asthma has been traditionally classified as atopic and non-atopic. In the last decade, asthma has also been categorized based on clinical features into various phenotypes by using statistical approaches and cluster analysis.[157,158,159] These phenotypes have varying degrees of association with atopy. Studies in the pediatric population suggest strong evidence demonstrating the relationship between asthma severity and atopy.[160,161,162] However, in adult asthmatics, atopy although prevalent need not correlate with disease severity.[163,164] Tests for determining allergic status (skin prick tests, total and antigen specific IgE) are not useful for all patients with asthma as the presence of a positive test neither confirms presence of allergy nor proves causality. Therefore, these tests may be performed only when specific allergy is suspected such as allergic aspergillosis,[165] and specific treatment is contemplated (as discussed in section E).
Recommendations
Tests for allergic status by measurement of total IgE, specific IgE to various environmental allergens, and skin prick tests are not recommended routinely for the diagnosis or management of asthma. (UPP)
These tests may however be done in specialized centers when specific triggers are suspected. (UPP)
D10. How to categorize the severity of stable asthma?
Asthma severity could mean the severity of airway obstruction, severity of symptoms or the disease severity off-treatment. A classification of asthma severity, widely used earlier, was based on the pre-treatment disease characteristics. A similar classification was adopted in the previous Indian guidelines on asthma.[7] This classification was originally meant for treatment naïve patients. However, clinicians in practice as well as various researchers began using this scheme also for patients already on treatment.[166] For patients already on treatment, asthma control rather than severity, should be assessed. Asthma control and severity are not synonymous. Severity means the intrinsic intensity of the illness, while control reflects the extent to which the symptoms can be controlled with treatment. The confusion between the two arises primarily because both severity and control classifications employ the same parameters while assessing a patient. Moreover, severity classification has major drawbacks. For instance, a patient having severe asthma at presentation may improve with the initial treatment given and may remain well controlled for years with low-intensity treatment alone. On the other hand, patients who had mild asthma at presentation may remain poorly controlled if he/she is not compliant with therapy or there are other environmental triggers precluding good control. Therefore, the classification based on severity at presentation is neither able to predict the response to treatment nor the long-term prognosis as to how the disease will behave in future. The current approach is to classify asthma based on control irrespective of the effects of treatment.[167]
Recommendations
We do not recommend classifying asthma based on severity of asthma.
D11. How to assess asthma control during follow up?
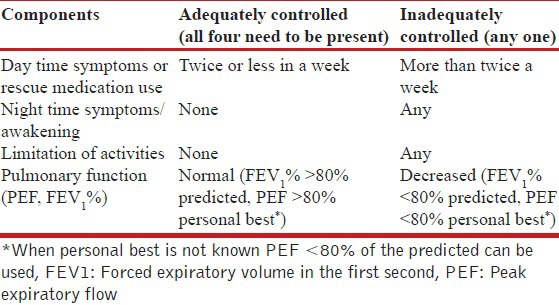
The GINA guidelines classify asthma into well controlled, not controlled and partially controlled based on four parameters: Presence of day-time symptoms, nocturnal awakening due to symptoms, the need for rescue medications and limitation of activities. Lung function is used for assessment of future risk. These parameters are assessed during every visit usually at intervals of 1-3 months. There seems to be little use of three-tier classification as the treatment option remains the same for asthma that is either partly controlled or uncontrolled. Hence, we propose a modified scheme wherein asthma will be categorized as either adequately controlled or inadequately controlled by assessing four parameters—day time symptoms (or rescue medication use), night-time symptoms/awakening, limitation of activities, and lung function (PEF, FEV1 %) [Table 6].
Table 6.
Level of current asthma control (over the preceding 4 weeks)
Asthma control includes both the level of current control and assessment of future risk. Assessment of future risk includes evaluation of the propensity for exacerbations, decline in lung function and treatment-related side effects. These should also be evaluated along with the assessment of current control. Poor clinical control, frequent exacerbations, critical care admissions, requirement of high dose of therapy to achieve adequate control, non-compliance with treatment, tobacco smoking, and rapid decline in FEV1 are some poor prognostic features.[76]
Recommendations
Asthma control should be classified as adequate or inadequate based on day time symptoms (or rescue medication use), night time symptoms/awakening, limitation of activities and pulmonary function (PEF, FEV1 %) as described in Table 6. The assessment of asthma control includes current status as well as future risk.
E. MANAGEMENT OF STABLE ASTHMA
E1. What are the goals for the management of stable asthma?
The goals of asthma management include relief of patient's current symptoms and prevention of further disease progression [Table 7]. The aim should be to achieve a level of asthma control, which would enable the individual to carry out all his activities (day to day, occupational and recreational) without any functional impairment. It is also important to prevent exacerbations and avoid any side-effects, which might arise from the medications used for treatment.
Table 7.
Goals of management of stable asthma (adapted from GINA guidelines)

E2. What are the drugs available for the treatment of stable asthma?
The drugs available for management of asthma can be divided into two broad categories—controller medications and reliever medications [Table 8]. Controller medications need to be taken regularly (irrespective of symptoms) and are primarily meant to prevent and control symptoms, reduce airway inflammation and/or decrease the risk of exacerbations. These include anti-inflammatory drugs (ICSs, leukotriene antagonists, mast cell stabilizers) and long-acting bronchodilators. On the other hand, reliever medications (also known as rescue medications) are fast-acting bronchodilators that are taken as and when needed to relieve the acute symptoms. There is no role of antihistamines, expectorants or mucolytics in the routine management of asthma.
Table 8.
Commonly used drugs and their doses for management of stable asthma

E3. What are the benefits and current role of ICSs for management of stable asthma?
ICSs are the cornerstone in management of stable asthma.[7,76,168,169] They suppress airway inflammation, which is the root cause of asthma symptoms. Four different Cochrane reviews published over the last decade have concluded that budesonide, beclomethasone, fluticasone and ciclesonide are clearly superior to placebo at all doses, and significantly improve lung function, symptom scores and quality of life, as well as decrease the risk of exacerbations and need for reliever medications.[170,171,172,173]
Inhaled steroids have also been shown to be superior to other controller medicines. The SOCS trial (Salmeterol or Corticosteroids Study) showed that ICS monotherapy is superior to long-acting beta-agonist (LABA) monotherapy and patients well controlled on ICS cannot be switched to LABA monotherapy without losing asthma control.[174] A Cochrane review comparing ICS monotherapy to LTRAs concluded that ICS monotherapy is superior to LTRA monotherapy in decreasing asthma exacerbations, daytime and nocturnal symptoms and rescue medication use, as well as improving quality of life, lung function and patient satisfaction.[175] Several randomized-controlled trials (RCTs) comparing oral methylxanthine therapy (theophylline) with ICS monotherapy concluded that the latter was clearly superior.[176,177,178,179,180,181] Thus, ICS are the first-choice controller medication in the management of stable asthma.
E4. What is the optimal dose of ICS to be used? Is any ICS preferred over others in the management of asthma?
Several formulations of ICS are currently available in India [Table 9], and their corresponding equipotent doses when using a hydro-fluoro-alkane (HFA)-based inhaler (adapted from GINA guidelines) are also listed [Table 9].[76]
Table 9.
Commonly available inhaled corticosteroids and their equipotent doses (in μg)
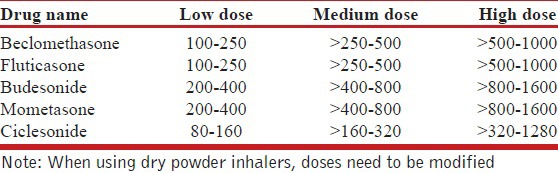
Inhaled steroids have a narrow therapeutic index.[182] Most clinical benefits are achieved at low doses, and increasing the ICS dose beyond the medium-dose range [Table 9] generally increases adverse effects without necessarily increasing clinical efficacy. The MICE (Measuring Inhaled Corticosteroids Efficacy) study showed that near maximal FEV1 and PC20 responses occurred at low to medium doses for both beclomethasone and fluticasone.[183] Significant inter-subject variability was noticed in the response to ICS; hence, some severe asthmatics might benefit from the use of high-dose ICS. A meta-analysis on the dose response relationship of fluticasone showed that peak clinical benefit was achieved at 500-600 μg/day (medium dose), and that 80-90% of the maximum clinical benefit was achieved at doses as low as 100-200 μg/day.[184] Another review of eight studies concluded that increasing the dose of fluticasone beyond 200 μg/day does not increase the magnitude of clinical benefit.[185] Similarly, three Cochrane reviews assessed the dose-effect responses of fluticasone and budesonide.[186,187,188] All these reviews suggest a little clinical difference between low and moderate doses, and between moderate and high doses. Also, it has been shown that initiating treatment with low-dose ICS is as effective as starting from an initial high dose and later stepping down.[187]
Inhaled steroids have also been used on an as-needed basis in adults with mild persistent asthma. This is also known as “Symptom-Based Controller” (SBC) approach. The BASALT (Best Adjustment Strategy for Asthma in the Long Term) trial showed that as needed symptom-based use of ICS was not inferior to regular daily use.[189] Also, patients in the as-needed ICS arm had lesser days of missed work, cumulative ICS exposure and seasonal exacerbations. In the IMPACT (IMProving Asthma Control Trial) trial, symptom scores were better in the daily ICS group but the exacerbation rates and change in PEF (primary outcome) were similar between the two groups.[190] The BEST (BEclomethasone plus Salbutamol Treatment) trial also concluded that as needed ICS use was as effective as daily ICS use in patients with mild asthma.[191] A Cochrane meta-analysis, which included six trials on adults and children, concluded that there was low quality evidence suggesting equivalence of intermittent and daily regimens in management of mild asthma.[192] Thus, in well-selected and motivated patients, as-needed ICS therapy can yield comparable asthma outcomes with reduced exposure to ICS. The approach also empowers patients to appropriate self-management. However, further studies are needed before this approach can be routinely recommended for all patients with mild asthma.
Inhaled steroids differ from each other in several pharmacokinetic and pharmacodynamic aspects such as potency, oral bioavailability, metabolism and serum protein binding.[193] However, in terms of clinical efficacy, all are equally effective. Several Cochrane reviews have shown that all ICS, when given at equipotent doses, lead to similar clinical outcomes.[194,195,196]
E5. What are the adverse effects of ICS when used in management of stable asthma?
Local and systemic side effects are a cause of major concern with long-term ICS use. The most common local side effects are oral candidiasis and dysphonia. Other less frequent side effects include perioral dermatitis, pharyngitis, reflex cough, sensation of thirst, and tongue hypertrophy.[197] Factors that influence the development of local side effects include: (a) proportion of drug deposited in oropharynx (which in turn depends on the inhalational technique, type of inhaler used, use of spacer devices, and nature of propellant); (b) type of drug used (prodrug vs. active drug); (c) frequency of ICS use; and, (d) dose of ICS.[197,198] Using ICS at the lowest possible dose and frequency, rinsing mouth and oropharynx by gargling every time after ICS inhalation, and using a spacer device are important measures to reduce local side effects from ICS use. Ciclesonide, being a prodrug (not in active from in the pharynx), has been shown to have the least incidence of oro-pharyngeal side effects among all ICS.[197,199]
When used in high doses, ICS can cause systemic side effects, the most worrisome being the suppression of hypothalamo-pituitary-adrenal (HPA) axis.[200,201] Current use of high-dose ICS (beclomethasone dipropionate [BDP] equivalent ≥ 1000 μg/day) increases the risk of developing adrenal crisis.[201] Because of its unique pharmacokinetic properties (such as extensive first pass metabolism, extra hepatic metabolism and high protein binding), ciclesonide has an oral bio-availability of < 1% and has not been shown to cause significant HPA axis suppression.[202,203] In a large retrospective cohort study, a dose–response relationship between the dose of ICS and risk of pneumonia, lower respiratory infection and tuberculosis was noted.[204,205] Other systemic side effects reported with ICS include suppression of growth (a serious concern when ICS are used in children), reduced bone mineral density, ocular side effects (glaucoma and cataract), skin thinning and bruising, and increased risk of infections.[202,206,207]
Recommendations
ICSs are the controller medication of choice for management of stable asthma. (1A)
All the ICSs are equally efficacious when used in equipotent doses. (1A)
Most of the clinical benefit from ICS is obtained at low to moderate doses. Only a minority of patients benefit from increasing the dose beyond this. (1A)
ICS should be started at low to moderate dose (depending on the severity of symptoms at presentation) and used at lowest possible dose required. (1A)
High-dose ICS use should preferably be avoided to decrease the risk of side effects, both local and systemic. (1A)
We recommend the use of valved holding chambers/spacers whenever using moderate to high-dose ICS. (UPP)
E6. What are the benefits of using LABA in the management of asthma? Can LABA monotherapy be used for the management of stable asthma?
The two most commonly used LABA are salmeterol and formoterol. Both are highly selective and potent beta-2 adrenergic receptor agonists, cause smooth muscle relaxation up to 12 h, and are given twice a day. Formoterol differs from salmeterol in having a faster onset of action (5 min vs. 15 min) and higher intrinsic receptor affinity.[208] Hence, it can also be used as reliever medication for symptom relief. A recent Cochrane meta-analysis of 62 studies involving 42,333 participants has shown that LABA are superior to placebo in improving lung function, symptom and quality of life (QoL) scores, and decreasing exacerbations and rescue medication use.[209]
LABA monotherapy controls asthma symptoms but does not effectively suppress airway inflammation. It can therefore cause a masking effect by suppressing airway symptoms but allowing inflammation to progress subclinically. Two large RCTs, the SMART (Salmeterol Multicenter Asthma Research Trial) study and the SNS (Serevent Nationwide Surveillance) study have shown that salmeterol use, either as a monotherapy (SNS study) or as add-on drug (SMART study), increases the risk of asthma-related deaths and life-threatening exacerbations.[210,211] Also, the SOCS study and the SLIC (salmeterol ± ICSs) study have shown that switching the patient from ICS monotherapy to LABA (salmeterol) monotherapy leads to loss of clinical control.[174,212] A recent meta-analysis suggested that that salmeterol monotherapy increases the risk of asthma-related deaths, but this risk is decreased with concomitant use of ICS.[213] The use of formoterol was not associated with any increase in asthma-related mortality or hospitalizations.[214] A recently published overview of Cochrane reviews also concluded that the risk of non-fatal serious adverse reactions was more common with salmeterol monotherapy when compared to formoterol monotherapy and ICS/LABA combination therapy.[215] In view of these data, the use of LABA monotherapy especially salmeterol, is strongly discouraged.
E7. What are the benefits of adding LABA to ICS monotherapy?
The OPTIMA (Oxis and Pulmicort Turbuhaler in Management of Asthma study) and the FACET (Formoterol and Corticosteroid Establishing Trial) trials showed that among patients poorly controlled on low to moderate dose ICS therapy, addition of LABA significantly decreased the risk of exacerbations and improved asthma control.[216,217] Another large RCT, the GOAL (Gaining Optimal Asthma controL) study comprising of 3421 patients, showed that asthma control is more often achieved with the ICS/LABA combination as compared to ICS monotherapy; patients receiving the ICS/LABA combination had lesser exacerbations and better health status.[218] The SLIC study also showed that addition of LABA in patients poorly controlled on ICS monotherapy improved asthma control and allowed ICS dose to be reduced by 50%.[212] A Cochrane review of 71 studies comparing the ICS/LABA combination to same dose ICS monotherapy concluded that adding LABA to ICS decreased the risk of exacerbations requiring oral corticosteroids by 28%, with a number needed to treat (NNT) to prevent one exacerbation being 41. The addition of LABA further improved lung function, symptom scores and decreased the recue medication use without causing any increase in adverse reactions.[219] Two Cochrane reviews comparing the efficacy and adverse effects of ICS/salmeterol and ICS/formoterol combination therapies concluded that there was no statistically significant difference between the two.[220,221]
Addition of LABA to ICS is also superior to doubling the dose of ICS in patients uncontrolled on low-dose ICS monotherapy. A Cochrane meta-analysis of 48 studies including 15,000 participants concluded that adding LABA to ICS is better than increasing the dose of ICS in reducing the number of exacerbations requiring oral corticosteroids.[222] The ICS/LABA combination was also superior in improving lung function and asthma symptoms. The steroid-related local side effects were also less in the combination arm. The ICS/LABA combination is also superior to the ICS/LTRA combination and the ICS/methylxanthine combination in treatment of asthma.[223,224]
E8. What are the novel beta agonists for the management of stable asthma?
Several new bronchodilators are being tried in asthma. One such group of drugs are the ultra-long-acting beta agonists (vilanterol, indacaterol, and olodaterol) which need only once a day dosing. Vilanterol (25 μg/day) is used in combination with fluticasone furoate (100-200 μg/day) in once a day dosing. This combination has been shown to be superior to fluticasone furoate monotherapy and non-inferior to the salmeterol/fluticasone combination.[225,226,227] These benefits are similar with either morning or evening dosing.[228] This combination also has been shown to have good safety profile.[229,230,231] Indacaterol and olodaterol are less well studied in asthma.[232,233,234]
Recommendations
LABA monotherapy should not be used in the management of stable asthma. (1A)
Addition of LABA to ICS is the preferred choice when symptoms are uncontrolled despite ICS monotherapy in moderate doses. (1A)
E9. What is the role of anti-leukotriene agents in the management of stable asthma?
Cysteinyl leukotrienes LTC4, LTD4 and LTE4 are regarded as among the most potent inflammatory mediators in asthma. They are produced by the 5-lipoxygenase pathway of the arachidonic acid metabolism. Their actions are not blocked by corticosteroids and hence the anti-inflammatory effects of the anti-leukotriene agents are complementary to those produced by corticosteroids.[235] The available anti-leukotriene agents are cysteinyl leukotriene-1 receptor antagonists (LTRAs), namely montelukast, zafirlukast and pranlukast, and a 5-lipoxygenase inhibitor (zileuton). These drugs are given orally and hence cause anti-inflammatory effects beyond the airways by also decreasing symptoms of coexisting allergic rhinitis and conjunctivitis. Also, they can be used in patients unwilling/unable to use inhaled medications. Oral montelukast is the most commonly used anti-leukotriene agent.
These drugs can be used as monotherapy (in patients with mild asthma) or as add-on to ICS monotherapy or ICS/LABA combination therapy.[236] LTRA monotherapy is inferior to ICS monotherapy in patients with mild to moderate asthma. Treatment with LTRA is more likely to result in acute exacerbations requiring systemic corticosteroids. This risk is even higher when used in patients with moderately severe asthma. Inhaled steroids are also superior to LTRA in improving patient's symptoms, quality of life and lung function.[175] Even in patients with coexisting asthma and allergic rhinitis, treatment with combined inhaled and intranasal corticosteroids is superior to treatment with oral LTRAs.[237,238,239] In contrast to the results from these studies, RCTs conducted in a real world scenario, which compared montelukast with ICS monotherapy in patients with mild asthma have shown that both treatments were equally effective.[240]
A recent Cochrane review of 16 RCTs has shown that LTRAs, when added to ICS, result in a non-significant decrease in the risk of exacerbations requiring oral corticosteroids.[241] Although not significant, dose reduction of ICS was possible, and patient withdrawals due to poor asthma control were decreased. Several large observational studies have shown that in patients uncontrolled on ICS monotherapy or ICS/LABA combination therapy, addition of LTRA improves the asthma control and quality of life.[242,243,244,245]
LTRAs are inferior to LABA as add-on therapy to ICS. A Cochrane review of 17 RCTs showed the ICS/LABA combination to be superior to the ICS/LTRA combination in preventing exacerbations requiring oral corticosteroids.[224] ICS/LABA was better than ICS/LTRA in improving the lung function and quality of life scores as well. Similar results were seen in other studies also.[246,247]
E10. What is the safety profile of anti-leukotriene agents when used to manage stable asthma?
Anti-leukotriene agents are generally well tolerated and do not cause significant drug reactions.[244,248] Zileuton is associated with liver toxicity and monitoring of liver function tests is recommended with its use. Churg-Strauss syndrome was earlier reported as a complication of LTRA use. However, this was likely due to steroid withdrawal, and no significant association was found after controlling for asthma drug use.[249]
Recommendations
Monotherapy with LTRA is inferior to monotherapy with ICS. (1A)
Monotherapy with LTRA might be an alternative to ICS in patients with mild asthma if they are unwilling to use ICS or if they are not suitable for ICS therapy. (1B)
As add-on to ICS, LTRAs are inferior to LABA. (1A)
Addition of LTRA might be beneficial in patients whose asthma remain uncontrolled despite the ICS/LABA combination. (2B)
E11. What is the role of long-acting anti-muscarinic agent tiotropium in the management of stable asthma?
Many asthmatics remain poorly controlled despite using ICS or the ICS/LABA combination. There is increasing interest in the role of tiotropium in the management of such difficult to treat asthma.[250] Several RCTs have addressed the role of tiotropium in uncontrolled asthma. In two studies, tiotropium was added to ICS monotherapy, and the clinical benefit was similar as that achieved with addition of LABA.[251,252] However, further studies are needed before tiotropium can be recommended as an alternative to LABA, when adding on to ICS monotherapy. In studies where tiotropium was added to ICS/LABA combination therapy, there was improvement in lung function and decrease in the number of exacerbations.[253,254]. A meta-analysis of six trials (three published and three unpublished) using tiotropium 5 μg once daily delivered via a soft-mist inhaler showed that addition of tiotropium to ICS or ICS/LABA therapy improves lung function.[255] Thus, tiotropium may be considered as add-on therapy when patients remain uncontrolled despite the ICS/LABA combination, and in those with ACOS.
Recommendation
Tiotropium may be used as add-on therapy if asthma remains uncontrolled despite moderate-to-high-dose ICS and LABA combination therapy. (2A)
E12. What are the benefits with the use of methylxanthines in the management of asthma? What is their role in the management of stable asthma?
Theophylline (dimethylxanthine) continues to be a commonly prescribed drug for management of asthma, especially in the developing world, because of its low cost, easy availability and ease of administration. When used in standard doses, the weak bronchodilator action of theophylline is attributed to non-specific phosphodiesterase (PDE) inhibition and adenosine receptor antagonism seen at blood levels of 10-20 mg/L. However, the anti-inflammatory action of theophylline (from histone deacetylase activation) occurs at much lower blood levels of 5-10 mg/L, when the drug is used in low doses.[256] At such low doses the adverse effects of theophylline are also minimal.
Two meta-analyses found LABA monotherapy to be superior to theophylline in improving lung function and symptoms, with lesser incidence of adverse drug reactions.[257,258] Several RCTs comparing ICS monotherapy found it to be superior to theophylline in improving symptoms, lung function and decreasing exacerbations.[176,177,178,179,180,181] Hence, theophylline therapy, in general, is inferior to both ICS and LABA monotherapy.
Many RCTs have compared addition of theophylline to low-dose ICS (ICS/Theo) versus doubling the dose of ICS.[259,260,261,262] All these studies uniformly showed both to be equally effective. The only RCT which compared the ICS/LABA combination (salmeterol + fluticasone) with the ICS/Theo combination found the ICS/LABA combination to be better.[223] In patients who remain uncontrolled despite the ICS/LABA combination, addition of theophylline improves lung function and symptom scores, and decreases exacerbations.[263,264]
Doxophylline is a newer alternative to theophylline. It is postulated to cause lesser side effects due to lack of adenosine receptor antagonism and calcium channel receptor blocking ability.[265] Two RCTs comparing theophylline (standard dose) with doxophylline found both the drugs to be equally effective, with doxophylline causing lesser adverse drug reactions.[266,267] There is no study comparing low-dose theophylline with doxophylline in asthma.
Recommendations
Methylxanthine monotherapy is inferior to ICS monotherapy. (1A)
When stepping up from ICS monotherapy, addition of methylxanthine to ICS is as effective as doubling the dose of ICS (1A) but inferior to the ICS/LABA combination. (2A)
Methylxanthines may be used as an add-on therapy in patients who remain uncontrolled on the moderate to high ICS/LABA combination. (2B)
Whenever used as an add-on to ICS, we recommend using low-dose (200-400 mg/day) sustained release formulations of theophylline. (UPP)
E13. What are the available reliever medications? Which drug is preferred for use as a reliever?
Inhaled drugs used as reliever medications include short-acting beta agonists (SABAs), like salbutamol and levosalbutamol, rapidly acting LABA (formoterol), short-acting anti-muscarinic agent (SAMAs), like ipratropium and oxitropium or a combination of SABA and SAMA. SABAs are the most effective drugs for relief of symptoms in patients with asthma. Although formoterol is as effective as SABA, it is not recommended as a rescue medication owing to safety concerns with LABA monotherapy.[268] SAMAs have a slightly delayed onset of action compared to SABA, and hence are not the preferred rescue drugs. They may still be useful in a subset of patients who cannot tolerate SABA therapy because of side effects. There is no head to head comparison between SABA and SAMA in asthma. A Cochrane review concluded that although SAMA is better than placebo, there is no added advantage of adding SAMA to SABA therapy in patients with stable asthma.[269]
We also strongly recommend against using oral beta agonists, which continue to be available in the Indian market, as reliever medications because of their unfavourable risk-benefit ratio.
Recommendations
SABA is the agent of choice for rescue medication in asthma. (UPP)
SAMA is a less preferred alternative/add-on to SABA as reliever medication. (UPP)
Formoterol monotherapy as a reliever should be avoided due to safety concerns with the use of LABA monotherapy. (1A)
Oral beta agonists should not be used as rescue medications. (UPP)
E14. What is the role of using a single inhaler for maintenance and reliever therapy?
A combination of an ICS and a fast-acting LABA (formoterol) in a single inhaler, as both controller and reliever medication, is increasingly being used in management of asthma.[270] This is referred to as single inhaler therapy (SiT) or SMART (single agent for maintenance and reliever therapy) approach. We prefer not using the term SMART, as it was originally coined to describe a proprietary inhaler device for maintenance and reliever therapy. Although majority of the studies till date have assessed either budesonide (as turbuhaler) or beclomethasone (as MDI) in SiT, the components in the SiT approach may be any available ICS (budesonide, fluticasone, beclomethasone and others) and any fast-acting LABA (formoterol, indacaterol, vilanterol and others) delivered through any type of device (MDI, turbuhaler, DPI, etc.).
The SiT approach has several advantages over conventional therapy. It is more patient friendly as the patient needs to use only single inhaler. As the ICS and LABA components are combined through a single delivery device, patient cannot use LABA alone, thus reducing the complications of LABA monotherapy. Use of the SiT approach is also associated with reduction in asthma exacerbations.[270] During periods of worsening asthma control which precede an asthma exacerbation, patients tend to take increasing doses of reliever medication. In SiT, an extra dose of ICS is also delivered with each reliever dose used. These additional doses of ICS delivered at the time of worsening prevent the impending asthma exacerbation. Also, the SiT approach is more cost effective.[271] It decreases the cumulative corticosteroid dose used, by decreasing the daily dose requirement of ICS and by decreasing the exposure to systemic steroids during exacerbations by decreasing the frequency of exacerbations.
Numerous RCTs have compared the SiT approach with other forms of conventional therapy (i.e. separate devices for reliever and controller medications). Most of these studies used the formoterol plus budesonide turbuhaler. Only a few studies have used a formoterol plus beclomethasone combination, and none have used the formoterol plus fluticasone combination.[272,273] These studies can be categorized into three broad groups based on the controller medication used in the control arm: (a) SiT vs. ICS monotherapy (b) SiT vs. fixed dose ICS/LABA and (c) SiT vs. conventional best practice.
Various studies have compared the SiT approach (formoterol plus budesonide) with ICS monotherapy (budesonide monotherapy plus reliever medication).[274,275,276,277] Use of the SiT approach decreased number of exacerbations, delayed time to first exacerbation and improved symptoms, asthma control and lung function. In all these studies, ICS dose used in the SiT group was significantly lower than that in the conventional arm. Many studies have compared the SiT approach with a fixed-dose ICS/LABA combination.[271,272,273,277,278,279,280,281,282,283] The results again were similar with most studies reporting decrease in exacerbations and improvement in symptoms and lung function with the SiT approach. The dose of ICS used was either same or lower in the SiT arm in these studies. Several studies also compared the SiT approach with conventional guideline-based best practice.[284,285,286,287,288] In these studies, though asthma control was better in the SiT group, number of exacerbations, lung function and symptoms were similar between the two arms. The dose of ICS used was however significantly lower with the SiT approach.
Cost-effectiveness analyses have also showed that direct and indirect costs of therapy were less if the SiT approach was used.[271,280,284,287] The SMARTASIA study, an open-labelled observational study which also included patients from India, also showed that there was improvement in asthma control, asthma symptoms and rescue medication use when SiT therapy was initiated.[289] In patients poorly controlled despite using moderate dose ICS, the SiT approach using formoterol/budesonide 4.5/160 μg two inhalations twice a day was better than using the same inhaler one inhalation twice a day.[290] Several meta-analyses that assessed the SiT approach also concluded that this strategy led to a statistically significant reduction in asthma exacerbations requiring oral corticosteroids.[270,291,292] It can therefore be concluded that the SiT approach is clearly superior to ICS monotherapy and fixed dose ICS/LABA combination therapy. When compared with guideline-based conventional best practice, the SiT approach is equally effective but the benefits are seen at a lesser daily dose of ICS.
Recommendations
We prefer the use of SiT using an ICS/LABA combination (formoterol-based) as both maintenance and reliever medication whenever feasible (steps 3-5, as described later). (1A)
E15. What should be the strategy for management of stable asthma in the Indian context?
We recommend a five-step approach for the management of stable asthma with an aim to achieve and maintain asthma control. Health education, identification and avoidance of asthma triggers, environmental control and treatment of asthma comorbidities are general measures which are recommended for all patients of asthma irrespective of the severity of disease. The pharmacologic management of each of the five steps is outlined in Table 10. The step at which the treatment is to be initiated depends on the severity and frequency of symptoms at presentation as described in Table 11.
Table 10.
Proposed strategy for the management of asthma in the Indian setting
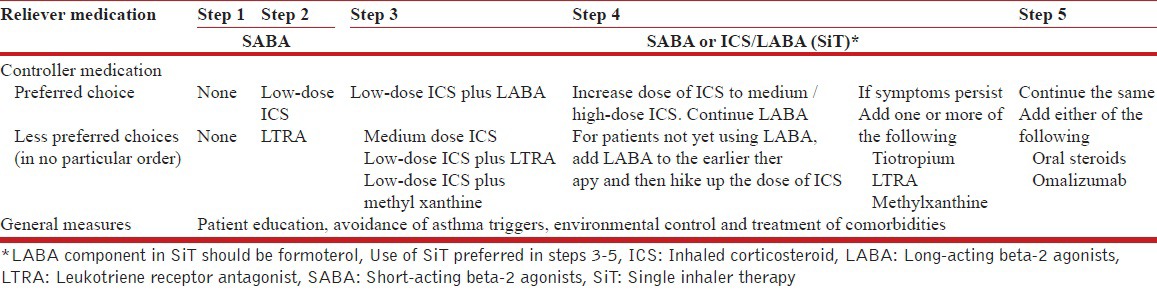
Table 11.
Practical considerations while initiating therapy in asthma patients

E16. How should patients of asthma be monitored in the outpatient setting?
The frequency of follow-up visit for patients with asthma depends on the severity of symptoms at the earlier visit. The optimal follow-up frequency is not clearly defined, and in general the follow-up duration is between 1 and 3 months. For patients with asthma exacerbation, the first follow-up visit should be at 1-2 weeks (UPP). At each visit the patients should be assessed for asthma control and it is imperative that the inhaler technique be checked at every visit [See Appendix].
APPENDIX.
Steps for using a pressurized metered dose inhaler device

Steps for using a pressurized metered dose inhaler device with spacer

Care of the spacer

Stepping Up Therapy: Patients whose asthma remains inadequately controlled on the existing treatment need to augment therapy with an aim of achieving adequate asthma control. The optimal time period after which such augmentation needs to be considered is not defined. The GOAL study showed that each criterion used to assess asthma control took a different time to get controlled. Improvement in nocturnal symptoms and improvement in PEF occurred rapidly, whereas daytime symptoms were the last to respond.[293] It was also observed that the proportion of patients getting controlled rose steadily over 1 year even when the patients were maintained on a steady dose of medication. We suggest considering stepping up of therapy at intervals of 1-3 months [Table 12]. However, this decision needs to be individualized (UPP).
Table 12.
Practical considerations when stepping up and stepping down asthma therapy
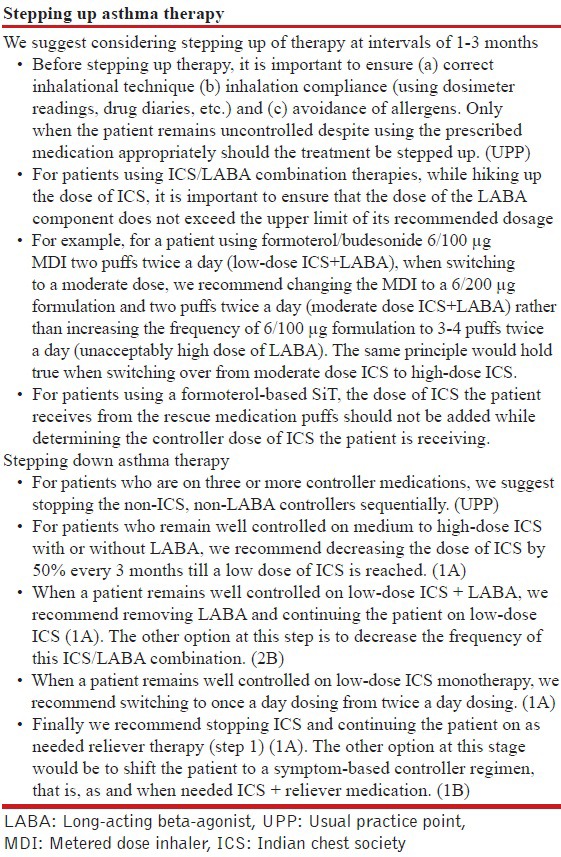
Stepping Down Therapy: Patients who remain well controlled on treatment require step down of their treatment to maintain control at the lowest possible step. However, the optimal timing and sequence of stepping down is debatable.[294,295] We suggest stepping down every 2-3 months [2B, Table 12]. For patients with seasonal exacerbations, we suggest not to step down treatment at the time when asthma control is likely to be poor. (UPP) Patients who are being stepped down should be closely monitored for any loss of asthma control (UPP).
Studies have demonstrated that patients controlled on high-dose ICS may be switched to lower dose ICS without any loss of asthma control.[296,297,298] It is also been observed that decreasing the dose of ICS is superior to removing LABA in maintaining asthma control when stepping down from ICS + LABA therapy.[299,300,301] There is possibility of worsening asthma control when LABA is removed from the ICS/LABA combination, and patients should be carefully monitored.[302,303,304] Inhaled steroids especially budesonide, ciclesonide and formoterol can be safely given once daily. A meta-analysis of nine studies has shown that budesonide given once a day is as effective as when given twice a day.[305] Finally, it has been shown that patients who stop ICS are at an increased risk of having an exacerbation and should be carefully observed.[306]
F. MANAGEMENT OF ACUTE EXACERBATIONS OF ASTHMA
F1. What is the definition of acute exacerbation of asthma?
An exacerbation of asthma is characterized by worsening of one or more of the asthma symptoms (cough, wheezing, chest tightness, dyspnea), leading either to increased need for rescue medications or hospitalization. It is usually associated with a decline in lung function (PEF or FEV1).[7,307] The risk factors for a severe attack of asthma are enumerated in Table 13.
Table 13.
Risk factors for severe attack of asthma

F2. How is the severity of an asthma attack assessed?
The severity of an asthma exacerbation is defined on a combination of signs and symptoms and the extent of accompanying cardiorespiratory dysfunction into non-severe, severe and life-threatening [Table 14]. Sweating, use of accessory muscles, paradoxical pulse and inability to communicate in complete sentences suggest significant airway obstruction.[308,309,310,311] Changes in mental status point toward a life-threatening exacerbation.[311] Hypercarbia occurs in the presence of arterial oxygen desaturation, severe obstruction and/or ventilatory depression.[312,313,314,315] Room air oxygen saturation ≥92% is uncommonly associated with complications.[316,317,318] Patients classified as having a severe asthma attack are best managed in a hospital setting [Figure 1], while those classified as having non-severe asthma exacerbations can be safely managed on an outpatient basis.
Table 14.
Assessment of the severity of acute asthma exacerbation
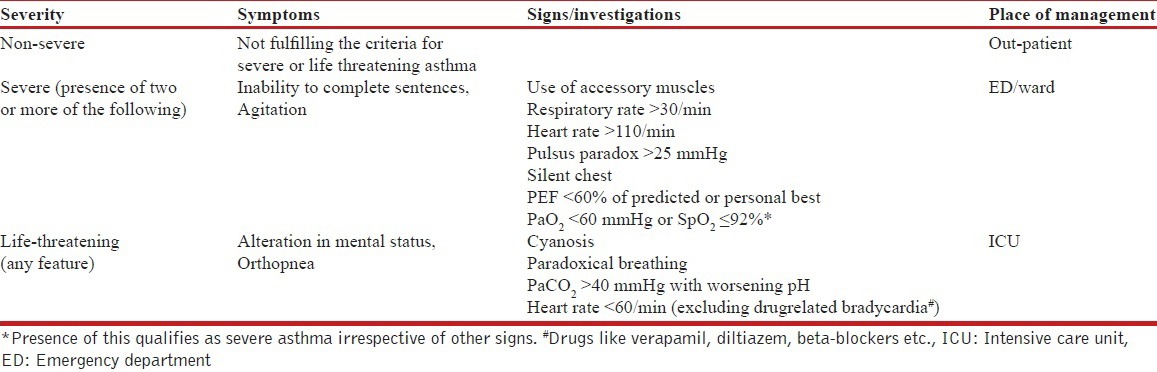
Figure 1.
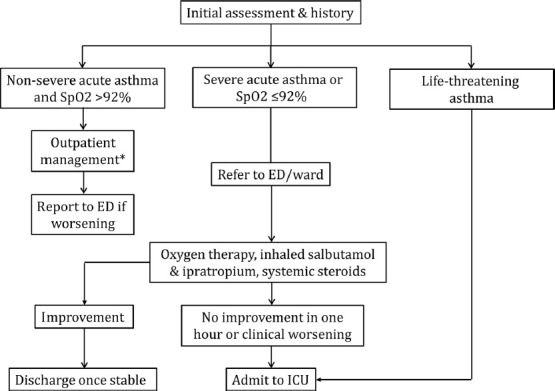
Algorithm for the evaluation and management of an acute asthma exacerbation
F3. What is the differential diagnosis of an acute severe attack of asthma?
Conditions that mimic asthma exacerbation include exacerbation of chronic obstructive pulmonary disease, acute heart failure, pulmonary thromboembolism, pneumothorax, hyperventilation (panic attacks/uremia), vocal cord dysfunction, and foreign body inhalation.
F4. How should patients with an acute exacerbation of asthma be evaluated?
Clinical history regarding the medications currently used, compliance with asthma medication, presence of comorbidities should be elicited. Clinical signs signifying a severe exacerbation should be sought. The current PEF should be noted and a PEF value less than 60% of predicted (or personal best) is an indication for referral to emergency department. Oxygen saturation should be measured using pulse oximetry and a value <92% generally signifies a severe exacerbation. Apart from PEF and pulse oximetry, no additional laboratory investigations are required for non-severe exacerbation. Arterial blood gas analysis should be performed in those with oxygen saturation < 92%, whose PEF does not improve to 40-45% of predicted or personal best or who worsen during or after treatment. All hospitalized patient should be evaluated with an arterial blood gas analysis.[319,320] Besides, patients should be investigated to rule out an alternate diagnosis, if clinically indicated (complete blood count, electrolytes, creatinine, urea, electrocardiogram, chest radiograph, echocardiogram and others).
Recommendation
Oxygen saturation should be measured by pulse oximetry in all patients presenting with an acute attack of asthma. (UPP)
Non-severe exacerbation does not require any investigation in most instances, except PEF and pulse oximetry. (UPP)
Patient with a PEF less than 60% of predicted or personal best should be managed in the emergency department. (2A)
Patients with a saturation of less than 92% should be managed in the emergency department or hospital ward and investigated further with an arterial blood gas analysis, if available. (2A)
F5. What are the goals of treatment of severe acute asthma?
The goals of managing acute exacerbation of asthma include relief of symptoms, adequate oxygenation, reversal of bronchial obstruction, and prevention of the next episode of exacerbation. Recovery from wheezing and normalization of pulmonary mechanics take longer time and are not the immediate goals of acute management.[309,311,319] Unlike COPD exacerbation, patients with asthma exacerbation should be able to return to their baseline activity after the acute event abates.[321]
F6. What is the role of oxygen in the management of severe acute asthma?
Oxygen therapy may be required during an exacerbation to maintain normal arterial oxygenation. The dose of oxygen should be based on achieving and maintaining target arterial oxygen saturation by pulse oximetry between 93% and 95%.[322,323] Higher doses of oxygen may be deleterious and administration of 100% oxygen has been demonstrated to result in a significant increase in PaCO2 and a decrease in PEF compared to FiO2 of 28%.[322]
Recommendation
Oxygen should be used only in hypoxemic patients. (1A)
Oxygen should be titrated to maintain a SpO2 between 93% and 95% (>95% in pregnancy). (1A)
Lack of pulse oximetry/arterial blood analysis should not preclude administration of oxygen. (UPP)
In patients in whom there is a need of oxygen >8 L/min, PaCO2 should be closely monitored. (2A)
F7. What is the role of bronchodilators in management of exacerbation of asthma?
Inflammation with resultant increased bronchiolar smooth muscle tone cause progressive narrowing of the airways during an asthma attack. This causes increased resistance to flow, pulmonary hyperinflation and ventilation/perfusion (V/Q) mismatch.[324] Persistence of airway obstruction thus leads to respiratory failure by increasing the work of breathing, inefficient gas exchange and respiratory muscle fatigue. Thus, relieving airway obstruction is of utmost importance in the management of acute asthma exacerbation,[325] and bronchodilators are the primary agents for achieving this goal.
F8. What is the role of beta agonists in management of severe acute asthma?
Short-acting beta agonists are the first-line agents for achieving bronchodilation due to their rapidity of action.[76] Inhaled salbutamol, levosalbutamol, formoterol, terbutaline and adrenaline have all been used in the management of severe acute asthma. Salbutamol is the most commonly used inhaled beta agonist. One dose-finding study found no significant difference in outcomes (lung function test, hospital admission rates, and adverse effects) between either 2.5 mg or 7.5 mg of inhaled salbutamol administered every 20 min for a total of three doses.[326]
In a meta-analysis comparing inhaled salbutamol with inhaled adrenaline, there was some benefit of inhaled adrenaline over inhaled salbutamol; however, dose per dose, 2 mg of adrenaline was inferior to 2.5 or 5 mg dose of salbutamol.[327]
Racemic salbutamol is a 1:1 mixture of R and S enantiomers of salbutamol. The R isomer levosalbutamol is responsible for the bronchodilator effect of racemic salbutamol while the S component causes the detrimental effects.[328,329,330,331] In a multicenter study of 627 patients, the use of levosalbutamol significantly reduced hospitalization, led to a greater improvement in FEV1, particularly among patients not on steroids and those with higher serum levels of S-salbutamol at presentation. However, time to discharge criteria did not differ among the two groups.[332] In a recent meta-analysis (seven trials with 1,625 patients), levosalbutamol was not found superior to salbutamol either with regards to efficacy or safety in severe acute asthma.[333]
Formoterol is another agent with quick onset of action and has been investigated for its efficacy in acute asthma. A meta-analysis (576 participants) comparing high-dose formoterol with salbutamol found no significant difference in primary (lung function) or secondary outcomes (serum potassium, heart rate and QT interval) between the two groups.[334] Also, the additional use of formoterol had no synergistic activity over salbutamol. In a study comparing nebulized adrenaline with terbutaline, improvement in lung function was similar in the two groups but the PaO2 was significantly higher after first dose of terbutaline compared to adrenaline; also, there was no synergistic effect between terbutaline and adrenaline.[335]
Beta-2 agonists should be judiciously used as they are associated with significant dose-dependent side effects like nausea, vomiting, headache, tremors and hypokalemia. Around 30% of patients may experience these side effects although they are usually mild.[336]
F9. What is the role of parenteral bronchodilators in acute asthma?
In general, there is no role of parenteral bronchodilators in the management of severe acute asthma, and inhalation route is the preferred route of delivering beta-2 agonists in acute asthma. In a meta-analysis of 15 trials there was no benefit of using intravenous beta-2 agonist compared to either inhaled beta-2 agonist or intravenous methylxanthines.[337] The use of intravenous aminophylline is widespread in the Indian subcontinent. However, a recent meta-analysis found no reduction in hospital admissions with the use of intravenous aminophylline as compared to standard care.[338] Not only there was no improvement in pulmonary function tests, patients in the aminophylline group patient also had higher episodes of arrhythmias and vomiting.[338] Therefore, the current evidence does not favor the use of intravenous aminophylline or intravenous beta-2 agonist in addition to inhaled beta-2 agonist in severe acute asthma.[338]
Although parenteral beta-2 agonist or theophylline confer no additional benefit, the expert group felt that they may be used in exceptional circumstances such as type 2 brittle asthma or patients on mechanical ventilation with intense bronchospasm causing ineffective delivery of nebulized drugs.
F10. What should be the mode of administering inhaled bronchodilators?
During an asthma attack, inhaled drugs can be delivered either by a nebulizer or a metered dose inhaler (MDI) with spacer. There is no difference in outcomes based on the method of administration.[339] Infection control and costs however favor the use of MDIs. In severe exacerbations the decision to use nebulizers or MDI is based on several other factors. Patients who are severely dyspneic, those who do not tolerate MDIs or have altered mental status are best administered inhaled drugs with a nebulizer.
There are two strategies of delivering nebulized bronchodilators namely continuous or intermittent. In the intermittent approach, beta-2 agonist is given every 20-30 min or ≤3 nebulization per hour, whereas in the continuous strategy the drug is given every 15 min or ≥4 nebulization per hour. A Cochrane review comparing intermittent with the continuous approach (8 RCTs, 461 patients) found the latter to be more beneficial in reducing the number of hospitalizations especially in those with severe airway obstruction.[340] There was also a small but significant improvement in the pulmonary functions in the continuous strategy group. Continuous treatment was well tolerated with no difference in the adverse effects.[340]
F11. What is the role of anticholinergics in the management of severe acute asthma?
Cholinergic mechanisms may be important in regulating acute bronchomotor responses by provoking bronchoconstriction via vagal pathways.[341] Anticholinergic medications antagonize transmission at the muscarinic receptors and appear to relieve bronchoconstriction primarily in larger airways. Studies comparing inhaled salbutamol with anti-cholinergics for achieving bronchodilatation found no difference between the two. However, addition of ipratropium to salbutamol provided additional bronchodilatation compared to either agent alone.[342,343,344] A meta-analysis of 32 (adults = 16, children = 16) randomized controlled trials comprising 3611 patients showed a significant reduction in hospitalization in both children and adults and significant improvement of lung function, when ipratropium was combined with salbutamol. Further, the use of two or more doses of ipratropium led to better outcomes compared to lesser doses.[345] Although few studies have shown that combined therapy had no benefit, these were limited by their small sample size.[346,347]
Recommendations
Rapid-acting inhaled beta-2 agonists (salbutamol) are the bronchodilators of choice for managing acute exacerbation of asthma. (1A)
Combination of ipratropium bromide with salbutamol produces better bronchodilation than either drug alone. Ipratropium (500 μg once then 250 μg q4-6 h) should be used in all patients with severe exacerbations of asthma. (1A)
MDI with a spacer device is as effective as nebulizer in the management of acute asthma (1A). However, the dose required is higher with nebulizer with increased propensity for side-effects.
In patients unable to use MDI with spacer, drugs can be delivered via a nebulizer. Once stabilized patient should be switched over to spacer from nebulizer. (UPP)
Continuous (2.5 mg salbutamol every 15 min, or >4 nebulization per hour) nebulization is better than intermittent (2.5 mg salbutamol every 20 min, or ≤3 nebulization per hour) nebulization of rapid-acting SABA (1A). The subsequent dose of nebulized salbutamol should be 2.5 mg every 2-4 h depending on the clinical response. (UPP)
Levosalbutamol has similar efficacy and safety as compared to salbutamol in acute asthma, and has no additional benefit in the management of severe acute asthma. (1A)
Formoterol confers no added advantage over salbutamol, hence it is not recommended for routine use in acute asthma. (1A)
Parenteral beta-2 agonists and theophylline should not be used routinely as they do not confer any advantage over inhaled beta-2 agonists but are associated with increased adverse reactions. (1A) However, they may be used in exceptional circumstances where inhaled medications are ineffective. (UPP)
F12. What is the role of corticosteroids in management of severe acute asthma?
Anti-inflammatory agents like glucocorticoids most effectively resolve the airway inflammation associated with severe acute asthma. A Cochrane review (six trials; 374 patients) found that the use of corticosteroids was associated with significant reduction in the number of relapses, hospitalization rates and use of SABAs.[348,349] In another review of 12 studies involving 863 patients, administration of corticosteroids within 1 h of presentation was associated with a significant reduction in hospital admission rates with a number needed to treat of eight, with no increase in adverse effects.[350] In a meta-analysis of six trials (344 adult patients) comparing the dose and route of administration of corticosteroids in hospitalized patients, it was found that low-dose systemic steroids (≤80 mg/day of methylprednisolone or ≤400 mg/day of hydrocortisone or <100 mg/day of prednisolone) were equally effective when compared to either medium-dose (>80 mg ≤360 mg/day of methylprednisolone or > 400 ≤1800 mg/day of hydrocortisone, or >100mg ≤450 mg/day of prednisolone) or high-dose (>360/day of methylprednisolone or >1800 mg/day of hydrocortisone or 450 mg/day of prednisolone) corticosteroids.[351] A 5-7 day course of oral steroids is as effective as 10-14 day therapy.[352,353] In patients receiving systemic steroids, the dose of steroids need not be tapered unless the duration of treatment lasts for more than three weeks.[354,355]
Inhaled steroids have also been investigated in acute asthma exacerbations. When compared to placebo, ICSs were associated with lesser hospital admissions in patients with mild or moderate exacerbations of asthma. However, in combination with systemic corticosteroids there is no additional benefit of ICSs.[356] A single center trial comparing the use of oral versus ICSs found no benefit with the use of inhaled steroids.[357] In a Cochrane review (909 patients) studying the role of ICSs in addition to systemic corticosteroids at discharge from emergency department, no benefit was seen with the addition of ICS therapy with standard care of inhaled bronchodilators and systemic steroids.[358] However, patients who are already are on ICS for control of asthma symptoms should not stop taking their medication during the acute attack.
Recommendations
Systemic glucocorticoids should be used in all patients with severe acute asthma. (1A)
Oral route is as effective as parenteral route except in very sick patients or those with contraindications to enteral feeding. (1A)
Daily doses of glucocorticoids equivalent to 30-40 mg of prednisolone or equivalent (0.75 mg dexamethasone ~ 4 mg methylprednisolone ~ 5 mg prednisolone ~ 20 mg hydrocortisone) for 5-7 days are adequate in most patients. (1A)
Systemic steroids can be stopped without tapering in those receiving treatment for less than 3 weeks. (1A)
In non-severe exacerbations, patients should be initially managed with increase in dose of inhaled SABA (4-6 puffs of 100 μg salbutamol every 30 min). If there is no response in 1 h, oral prednisone 30-40 mg once a day for 5-7 days should be started. (UPP)
Inhaled steroids do not provide any additional benefit when used along with systemic corticosteroids and inhaled steroids are hence not recommended in acute asthma. (1A)
The dose of inhaled steroids (in patients already on inhaled steroids) should be hiked up for 2-4 weeks at discharge from ED in addition to oral steroids. (2A)
F13. What is the role of magnesium sulfate in the management of severe acute asthma?
Magnesium acts primarily by inhibiting bronchial smooth muscle contraction.[359] In a study, 248 adult patients with severe asthma and FEV1 <30% predicted were randomized to receive two grams of intravenous magnesium sulfate or placebo within 30 min of arrival in the emergency department. There was a trend towards improvement in FEV1 in the magnesium group[360] A Cochrane review also concluded that intravenous magnesium sulfate had limited role in management of acute severe asthma and its use should only be reserved for patients with severe acute asthma.[361] However, in the recent multicenter 3Mg trial, 1109 patients were randomized to receive nebulized magnesium sulfate, intravenous magnesium sulfate or placebo. There was no difference in outcome between the three groups.[362] A review of 16 studies (adults, seven; children, nine) studying the effect of inhaled magnesium sulfate in acute severe asthma in addition to standard care involving inhaled beta-2 agonist, anticholinergics, and systemic corticosteroids found no significant improvement in pulmonary functions or decline in hospital admission when magnesium sulfate was used in addition to beta-2 agonist.[363]
Recommendations
There is no role of intravenous or inhaled magnesium sulfate in routine management of acute exacerbation of asthma. (1A)
Intravenous magnesium sulfate as a single dose of 2 gm over 20 min may be used in exceptional situations in those with severe asthma not responding to a combination of inhaled beta-2 agonist, anticholinergic and systemic glucocorticoids. (UPP)
F14. What is the role of leukotriene inhibitors in severe acute asthma?
Leukotriene inhibitors have also been studied in patients with severe acute asthma.[364] In one study of 70 patients with acute asthma, the oral montelukast (plus prednisolone) and the prednisolone group had significant improvement in PEF compared to placebo. However, the difference in PEF was not significant when montelukast (plus prednisolone) was compared with prednisolone.[365] In a randomized trial, 73 adult patients with severe acute asthma requiring hospitalization were given either 10 mg of oral montelukast or placebo on admission and then once daily for 4 weeks. Though the PEF was significantly higher in the morning following the first dose, the PEF at discharge or at 4 weeks was not different between the two groups.[366] In another randomized double blind trial, patients with acute asthma received either 7 mg or 14 mg of montelukast or placebo with standard care. There was significant improvement in lung functions in the montelukast group as compared to placebo. However, there was no significant difference between the groups with regards to the frequency of visits to the emergency, hospitalizations, doctor visits, or the need for rescue corticosteroids in the 14-day period after discharge.[367] In two different trials, there was significant improvement in lung function in the montelukast group at 60 min but there was no difference in other outcomes.[368,369]
Recommendation
Leukotriene modifiers have no role in the management of patients with acute asthma. (1A)
F15. What is the role of antibiotics in management of severe acute asthma?
The literature on the use of antibiotics in patients with severe acute asthma is scarce. In a Cochrane review (two studies), the use of antibiotics did not produce significant difference in lung functions, length of hospital stay, time taken for 50% improvement in symptoms when compared to the placebo group.[370] The use of antibiotics in asthmatic patients with pneumonia should be according to the recently published pneumonia guidelines.[371]
Recommendation
Antibiotics should not be routinely used in acute asthma except in demonstrable bacterial infection. (1A)
F16. What is the role of noninvasive ventilation (NIV) in severe acute asthma?
The role of NIV in acute exacerbation of COPD is well established. However, the role of NIV in acute asthma remains uncertain. Although both asthma and COPD are obstructive lung diseases, it is incorrect to extrapolate the data on the use of NIV in COPD for asthma. In fact, it would be dangerous to use NIV routinely in hypercapnic asthma patients while it is a common practice in COPD. This is due to the fact that the primary pathophysiology in COPD is dynamic hyperinflation due to loss of elastic recoil of the lungs while in asthma the dynamic hyperinflation is due to acute bronchospasm and tachypnea.
In a randomized trial from India, 53 patients with severe acute asthma were treated with standard care or NIV. Except for the dose of inhaled bronchodilators, which was significantly lesser in the NIV arm, there was no difference in either the primary or the secondary outcomes.[372] In a recent Cochrane review (six trials, 206 patients) also, no benefit was seen with the use of NIV in acute asthma.[373]
Recommendation
There is paucity of data on the role of NIV in acute asthma and hence it should be judiciously used in asthma exacerbation. (2B)
F17. What is the role of heliox in the management of severe acute asthma?
Heliox is a mixture of oxygen and helium that has lower density and a higher viscosity than air-oxygen mixtures. These physical properties reduce the Reynolds number and may transform areas with turbulent flow into areas of laminar flow, thereby decreasing the work of breathing.[374] A meta-analysis conducted in 2003 had concluded that there was no role of heliox in the routine management of acute asthma; however, a recent review did find marginal benefit with the use of heliox.[375,376] As the benefit with the use of heliox is not substantial and the equipment is not widely available in India, the expert group found no clear role of heliox in acute asthma.
Recommendation
Heliox should not be routinely used in treatment of acute asthma exacerbation. (1A)
F18. What are the indications of invasive mechanical ventilation (MV) in severe acute asthma?
The absolute indications of MV in severe acute asthma include coma, respiratory or cardiac arrest and refractory hypoxemia while the relative indications include inadequate response to initial management, hypercapnia, fatigue, somnolence and cardiovascular compromise. The initial ventilatory strategy in the management of severe acute asthma is outlined in Table 15.
Table 15.
Initial ventilator settings in patients with acute asthma
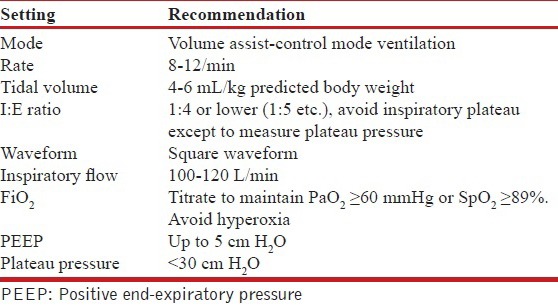
F19. What should be the strategy for management of acute exacerbation in the Indian context?
The first step is to decide the severity of the exacerbation, which guides the site for management of the exacerbation [Figure 1]. Once the site has been identified, further management should be done as outlined in Table 16.
Table 16.
Practical management of asthma exacerbations
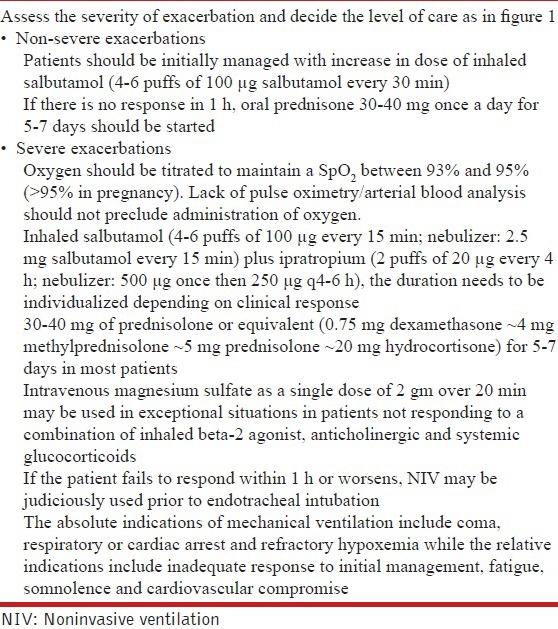
F20. What are the hospital discharge criteria?
A patient with severe acute asthma is considered fit for discharge from the medical facility when he/she is able to return to the previous state of health and in general should be clinically stable for at least 24 hours. The patient should be able to eat and get adequate sleep, should be able to comfortably use the inhaled medication with requirement of inhaled short-acting drugs no more than every 4 hours.
G. MISCELLANEOUS ISSUES IN ASTHMA MANAGEMENT
G1. What is the management of difficult-to-treat asthma?
Patients whose asthma symptoms are inadequately controlled, despite optimal step 4 therapy for a period of 1-3 months, can be considered to have difficult-to-treat asthma. Poor adherence to therapy and poor inhaler technique are often overlooked but one of the commonest reasons for poor asthma control.[377,378,379] Therefore, these should be checked at each patient visit. In patients who have poorly controlled symptoms despite good adherence and technique, an alternative diagnosis should be ruled out (such as COPD, vocal cord dysfunction, tracheal tumors, and others) as it can mimic asthma.[380,381,382,383,384] Around 13% of asthma patients attending special clinics might also be suffering from allergic bronchopulmonary aspergillosis.[385] There is some evidence to suggest that current smoking reduces the effectiveness of inhaled and oral corticosteroids.[386,387] All asthmatics who continue to smoke should be advised to quit smoking. Avoidance of exposure to allergens is paramount for patients with refractory asthma and should be re-emphasized. Some patients with asthma have associated comorbidities (gastro-esophageal reflux disease, obesity, obstructive sleep apnea, allergic rhinitis, and others) which may contribute to or exacerbate the symptoms of asthma.[388] Although there is no definite evidence to suggest improved asthma control by treatment of these comorbidities, some data suggest that treating these comorbidities may be of some benefit.[389,390,391]
The management of difficult-to-treat asthma should be individualized and is best done by clinicians who have experience in this area. Besides addressing the aforementioned factors, judicious use of a combination of available modalities of treatment is required. Although oral corticosteroids have not been studied in any RCT involving patients with difficult-to-control asthma, they are the most potent drugs for asthma and should be considered when the patients’ symptoms are uncontrolled despite maximal step 4 therapy. When used, they should be employed at the lowest possible dose for the shortest possible duration required to achieve asthma control in view of their many adverse effects.
Recommendations
Patients with difficult-to-treat asthma are defined as those whose symptoms are inadequately controlled despite optimal step 4 therapy for a period of 1-3 months. (UPP)
Patient compliance to drug adherence and inhaler technique should be checked at each visit. (UPP)
In patients with difficult-to-treat asthma, the possibility of asthma mimics (COPD, vocal cord dysfunction, tracheal tumors, and others) should be considered. (UPP)
Patients with difficult-to-treat asthma should be evaluated for presence of ABPA. (UPP)
Smoking cessation should be advised for all asthmatics who are smokers. (UPP)
Patients with difficult-to-treat asthma with features of associated comorbidities (like rhinitis, obesity, obstructive sleep apnea, and gastro-esophageal reflux disease) should be evaluated and treated accordingly. (UPP)
Addition of oral corticosteroids for difficult-to-treat asthma should be considered only if the patient's symptoms remain uncontrolled despite maximal step 4 therapy. (UPP)
When considered, oral corticosteroids should be used at the lowest possible dose for the shortest possible duration and patients should be simultaneously monitored for drug-related adverse effects. (UPP)
G2. What is the role of anti-IgE in asthma?
Meta-analyses of trials of anti-IgE therapy have shown that the use of omalizumab as an adjunct to ICS in moderate to severe asthma reduces the number of asthma exacerbations and allows dose reduction and withdrawal of ICS.[392,393] Patients who were on oral steroids had reduction in their maintenance dosage after initiation of omalizumab and the proportion of patients taking maintenance oral steroids were markedly lower at 2 years compared to baseline.[392,393] Most of these trials used omalizumab for a duration of 16-28 weeks in asthmatics who had elevated serum IgE levels (140-1300 IU/mL in various trials) and a positive skin test to aero-allergens. Analysis of pooled data from seven trials showed that most patients who responded to omalizumab therapy did so by the end of 12 weeks.[394] Therefore, a minimum duration of 12-24 weeks is advisable before judging the therapeutic response. Some experts advocate indefinite therapy with omalizumab as there is some evidence to suggest that cessation of anti-IgE therapy after successful long-term therapy may cause severe asthma exacerbations.[395] The most common and well-documented adverse effect with omalizumab is injection site reaction. Considering the possibility of increased risk of parasitic infections and malignancy, more data is needed on the safety of long-term therapy with omalizumab before its widespread adoption.
Recommendations
Omalizumab may be considered as an adjunctive therapy to ICS in patients with moderate to severe asthma who have elevated serum IgE levels and a positive skin test to at least one perennial aero-allergen. (1B)
G3. What is the role of bronchial thermoplasty in asthma?
Bronchial thermoplasty is a procedure that consists of ablation of airway smooth muscle by delivering controlled radiofrequency energy via a catheter introduced into the bronchial tree through a flexible bronchoscope. The role of bronchial thermoplasty in asthma has been studied in randomized controlled trials.[396,397,398] The AIR trial was a sham-controlled study that included patients on LABA and ICS who worsened after withdrawal of LABA; two other trials (AIR2, RISA) included patients who were symptomatic despite LABA and high-dose ICS and were not sham controlled. The patients included in the study had airway hyper-responsiveness to methacholine and a pre-bronchodilator FEV1 ≥ 50-60%. Although AIR2 demonstrated improvement in quality of life with a reduction in severe exacerbations and healthcare use after the treatment, the trial suffered from several methodological flaws.[399] A Cochrane review of the trials found only modest clinical benefit in quality of life and lower rates of asthma exacerbation, but no significant difference in asthma control scores.[400] Further, these studies excluded patients who had refractory and relatively more severe form of asthma. Therefore, the results of these trials cannot be extrapolated to this subset of patients for whom this costly treatment is being marketed.[399] Also, bronchial thermoplasty can itself lead to an increased risk of exacerbations during the treatment period.
Recommendation
As of now, good quality evidence is lacking for recommending bronchial thermoplasty in the routine management of bronchial asthma. (2A)
G4. What is the role of immunotherapy in asthma?
A systematic review has shown that the use of subcutaneous immunotherapy (SCIT) with a single allergen extract decreases asthma symptom scores, asthma medication use and bronchial hyperreactivity.[401] Most of these studies were performed in patients with mild to moderate asthma having evidence of allergy to one or few antigens. However, the magnitude of benefit appears to be only modest with treatment effects comparable to those observed with inhaled bronchodilators and cromones, and lesser than that of low-dose ICS.[402,403] Evidence comparing the efficacy of immunotherapy with single allergen versus immunotherapy with multiple allergen is scarce. No good-quality large-scale study has demonstrated a benefit in validated asthma scores or medication use with SCIT using multiple allergens.[401,404,405] Evidence to support sublingual immunotherapy (SLIT) in asthma is even weaker.[406] A randomized, double-blind, placebo-controlled trial with SLIT comparing monotherapy versus multi-allergen extract found no significant differences in medication or symptom scores in either treatment group compared with placebo.[407] Sublingual and subcutaneous multiple allergen immunotherapy in poly-sensitized patients’ needs more supporting evidence to corroborate its efficacy in practice.[404] Analysis of safety data indicates that systemic reactions are more common in asthmatics as compared to patients with other allergic diseases, and fatalities are more common among patients with severe or poorly controlled asthma.[408,409] Limited evidence however suggests SLIT to be safer than SCIT.[408]
Recommendations
Single allergen immunotherapy may provide a modest benefit to patients with mild-to-moderate asthma with demonstrable skin allergy to that antigen. (2B)
Multiple allergen immunotherapy cannot be recommended at the moment based on currently available evidence. (2A)
Immunotherapy carries the risk of severe reactions which can be life-threatening. Therefore, it should be practiced only by well-trained personnel in centers experienced in performing the technique. (3A)
Immunotherapy should not be used in patients with severe or poorly controlled asthma, and in patients with FEV1 <70% because of significantly higher risk of fatal reactions. (3A)
G5. What is the role of patient education in asthma?
Patient education when used alone does not improve health outcomes in asthma[410] However, when used as part of an optimal self-management plan (along with self-monitoring of symptoms or peak expiratory flow, regular physician review and self-management using a written asthma action plan) it significantly contributes to reduction in asthma-related hospitalizations and other adverse health outcomes.[411]
Recommendation
Optimal self-management which involves a combination of patient education, self-monitoring, regular physician review, and self-management using a written asthma action plan is strongly recommended in the management of asthma. (1A)
G6. What is the role of pulmonary rehabilitation in asthma?
Pulmonary rehabilitation in asthmatic patients may provide additional important benefits such as improvement in exercise capacity and dyspnea, and might help in improving quality of life.[412,413] A meta-analysis of 21 RCTs found that physical training in asthmatics produced significant improvement in cardiopulmonary fitness as measured by maximal oxygen consumption (VO2 max); however there was no significant benefit in maximal ventilation at peak exercise (VEmax), FEV1, FVC or PEF.[413] Although most of the studies in this meta-analysis suggested an improvement in asthma symptoms and quality of life, the results could not be pooled due to heterogeneity between the included studies. There was also an insignificant increase in 6MWD in one included study.
Recommendations
Pulmonary rehabilitation therapy in asthmatics produces significant improvement in exercise capacity. (2A)
Pulmonary rehabilitation therapy in asthmatics improves asthma symptoms and quality of life. (3A)
G7. What is the role of vaccination in the prevention of asthma exacerbations?
No good quality studies have examined the role of pneumococcal or influenza vaccination in preventing asthma exacerbations in adults. The limited available evidence does not suggest a reduction in asthma exacerbations after influenza vaccination in asthmatics of any age group.[414] However, pneumococcal vaccine might decrease asthma exacerbations in asthmatic children who are prone to recurrent otitis media.[415] No data is available on the role of pneumococcal vaccination in asthmatic adults.
Recommendation
Current evidence is insufficient to routinely recommend influenza or pneumococcal vaccination for patients with asthma. (3A)
G8. What is the role of antibiotics in the prevention of asthma exacerbations?
In a randomized controlled trial that compared therapy with thrice-weekly low-dose azithromycin (250 mg) for 6 months against placebo in subjects with exacerbation-prone severe asthma, azithromycin did not reduce the number of exacerbations or the number of lower respiratory tract infections requiring antibiotic therapy.[416] Moreover, azithromycin use was associated with increased oropharyngeal carriage of macrolide-resistant streptococci.
Recommendation
Available evidence does not suggest a role for antibiotics in the prevention of asthma exacerbations. (2A)
G9. What is the management of asthma in the following special situations?
Asthma and pregnancy: The prevalence of asthma during pregnancy is approximately 4-8%.[417] Among pregnant asthmatics, approximately one third of women have worsening disease, one-third show improvement, and the remaining one-third show no change in disease severity.[418] Poorly controlled asthma during pregnancy can have numerous adverse consequences, both for the mother (pre-eclampsia, placenta previa, need for caesarian delivery) and the fetus (preterm delivery, post-term delivery, low birth weight, congenital anomalies, increased infant mortality).[419,420] If asthma is adequately managed during pregnancy, pregnancy outcomes in these women are largely similar to non-asthmatics.[421] Exacerbations requiring medical intervention occur in about 20%, and exacerbations requiring hospitalization occur in about 6% of pregnant asthmatics. Most of these exacerbations occur in the late second trimester. Pregnant asthmatics experiencing exacerbations are at a significantly higher risk of having a low birth weight baby.[422]
Available evidence suggests that the effect of most commonly used asthma medications on pregnancy outcomes is negligible.[423,424,425,426] Use of theophyllines can be associated with increased risk of preterm delivery.[427] Oral steroids may also be associated with an increased risk of pre-eclampsia, gestational diabetes and cleft palate.[420,425,428,429] However, this data is confounded by the fact that patients on these drugs also had more severe asthma.
Asthma during lactation should be managed similar to asthma during pregnancy. Prednisone, theophylline, antihistamines, ICS, beta-2 agonist, and cromolyn are not contraindicated during breastfeeding. However, maternal use of theophylline may cause irritability in sensitive infants.[428,430]
Recommendations
Poorly controlled asthma and asthma exacerbations are associated with adverse pregnancy outcomes while well-controlled asthma is associated with normal pregnancy outcomes. (2A)
Most medications used for asthma have negligible effects on the fetus. (3A)
Adequate asthma control in pregnancy should be attempted with routinely available asthma medications as in the non-pregnant state (including systemic steroids whenever indicated). (3A)
Asthma during lactation should be managed similar to asthma during pregnancy.(3A)
Caution should be exercised while using theophyllines during pregnancy and lactation. (3A)
Exercise-induced asthma: Exercise-induced bronchoconstriction/exercise-induced asthma (EIB/EIA) has been defined as a fall in FEV1 of 10% or greater on an exercise challenge test (4-6 min of exercise at near-maximum targets with a total duration of exercise of 6-8 min).[431,432] Pretreatment with any one of the bronchodilator agents (SABA, SAMA, LABA) or anti-inflammatory agents (LTRA, mast cell stabilizers, but not ICS) has been shown to be effective in preventing EIA.[431,433] Daily use of ICS, LTRA or LABA has also been shown to decrease the fall in FEV1 associated with exercise.[434] However, regular use of LABA can induce tachyphylaxis and may be associated with increased mortality (when used without ICS).[61,431]
Recommendation
Pretreatment with bronchodilator agents (SABA, SAMA, and LABA) as well as anti-inflammatory agents (LTRA but not ICS) is effective in attenuating the fall in FEV1 associated with EIA. (2A)
Regular use of ICS or LTRAs is effective in prevention of exercise-induced bronchospasm. (2A)
Regular use of LABA as prophylaxis for EIA should be avoided as long-term regular administration of LABA induces tolerance and may cause increase in adverse effects. (2A)
Aspirin-induced asthma (AIA): Aspirin-induced asthma occurs because of the inhibition of the enzyme cyclo-oxygenase 1 (COX-1) by aspirin and other similar non-steroidal anti-inflammatory drugs (NSAIDs) which can cross-react with aspirin.[435] The diagnosis of AIA can be established by oral, nasal or bronchial challenge testing with aspirin in patients with a suggestive history.[438,439,440] However, such testing is potentially dangerous as it can produce life-threatening complications. COX-2 inhibitors have been shown to be safe in AIA in numerous studies.[441,442,443,444] Oral challenge testing studies have shown that paracetamol in doses less than 1000 mg appears to be relatively safe in these patients.[445] Although cross-reaction tends to occur at higher doses, most of the reactions usually consist of mild bronchospasm and naso-ocular reactions.[445] Limited evidence also suggests that the chronic administration of aspirin following desensitization may improve symptoms of asthma and rhinosinusitis and may reduce the need for OCS and nasal surgeries.[446,447] However, chronic therapy with aspirin may be associated with gastritis. LTRAs like montelukast improve control of asthma in AIA when added to ICS therapy.[448] However, existing evidence is insufficient to suggest a separate line of management for asthma in AIA compared to allergic asthma.
Recommendation
Patients with AIA should avoid all NSAIDs which can inhibit the enzyme cyclo-oxygenase 1 (COX-1). (3A)
COX-2 inhibitors can be safely used in patients with AIA. (3A)
Patients with AIA can have cross-reactions to paracetamol (esp. in doses ≥1000 mg); however, these reactions tend to be mild. (3A)
Aspirin desensitization may be useful in selected subjects with AIA. (3A)
There is no sufficient evidence to suggest that the management of AIA should be different from that of allergic asthma apart from avoidance of NSAIDs.(UPP)
Occupational Asthma: Occupational asthma is defined as new onset asthma symptoms or definite worsening of previously quiescent asthma after employment, along with presence of history of occupational exposure to known or suspected sensitizing agents. In view of the enormity of its economic impact, the diagnosis of occupational asthma should be supported by objective criteria and should not be made on the basis of history alone. A meta-analysis of 21 trials has shown that both removal and reduction of exposure appear to be effective in occupational asthma.[449] In this meta-analysis, both removal and reduction of exposure increased the likelihood of reporting absence of asthma symptoms; however, only removal of exposure was associated with an improvement in FEV1. However, removal of exposure was associated with a higher risk of unemployment.
Recommendation
Both removal of exposure and reduction of exposure improve symptoms of occupational asthma. Removal of exposure appears to be better than reduction of exposure. However, this should be considered against a background of increased risk of unemployment with the former. (2A)
Allergic bronchopulmonary aspergillosis (ABPA): ABPA is an allergic pulmonary disorder caused by hypersensitivity to the fungus Aspergillus fumigatus, and clinically manifests as chronic asthma, recurrent pulmonary infiltrates, and bronchiectasis.[130] The prevalence of ABPA is speculated to be about 2.5% in the general population,[450] while the prevalence in asthma clinics is about 12.9%.[385] With increasing knowledge of this disorder the number of cases diagnosed may continue to rise.[451] Evidence from cohort studies suggests that long-term steroids may help in symptom control and prevent relapses.[452,453] Hence, it appears prudent to evaluate patients with difficult-to-treat asthma for evaluation of ABPA.
Footnotes
Source of Support: Nill.
Conflict of Interest: None declared.
REFERENCES
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Rennie D, Meade MO, Cook DJ. 2nd ed. New York: McGraw Hill; 2008. Users’ Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice. [Google Scholar]
- 3.Ciba Guest Symposium. Terminology, definitions and classification of chronic pulmonary emphysema and related conditions. Thorax. 1959;14:286–99. [Google Scholar]
- 4.National Heart, Lung and Blood Institute. Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program. [Last accessed on 2014 Mar 5]. Available from: http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf .
- 5.Vancouver (WA): Global Initiative for Asthma (GINA); 2012. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; p. 110. [Google Scholar]
- 6.Levy ML, Thomas M, Small I, Pearce L, Pinnock H, Stephenson P. Summary of the 2008 BTS/SIGN British Guideline on the management of asthma. Prim Care Respir J. 2009;18(Suppl 1):S1–16. doi: 10.3132/pcrj.2008.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindal SK, Gupta D, Aggarwal AN, Agarwal R. World Health Organization; Government of India. Guidelines for management of asthma at primary and secondary levels of health care in India (2005) Indian J Chest Dis Allied Sci. 2005;47:309–43. [PubMed] [Google Scholar]
- 8.Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 9.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Global Asthma Report. International Union against Tuberculosis and Lung Disease, Paris, 2011. [Last accessed on 2014 Mar 5]. Available from: http://www.globalasthmanetwork.org/publications/Global_Asthma_Report_2011.pdf .
- 11.Chhabra SK, Gupta CK, Chhabra P, Rajpal S. Risk factors for development of bronchial asthma in children in Delhi. Ann Allergy Asthma Immunol. 1999;83:385–90. doi: 10.1016/S1081-1206(10)62835-9. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Aggarwal AN, Kumar R, Jindal SK. Prevalence of bronchial asthma and association with environmental tobacco smoke exposure in adolescent school children in Chandigarh, north India. J Asthma. 2001;38:501–7. doi: 10.1081/jas-100105871. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy S, Singh RB, Swaminathan S, Venkatesan P. Prevalence of asthma in urban and rural children in Tamil Nadu. Natl Med J India. 2002;15:260–3. [PubMed] [Google Scholar]
- 14.Awasthi S, Kalra E, Roy S, Awasthi S. Prevalence and risk factors of asthma and wheeze in school-going children in Lucknow, north India. Indian Pediatr. 2004;41:1205–10. [PubMed] [Google Scholar]
- 15.Sharma SK, Banga A. Prevalence and risk factors for wheezing in children from rural areas of north India. Allergy Asthma Proc. 2007;28:647–53. doi: 10.2500/aap.2007.28.3059. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Nagar JK, Raj N, Kumar P, Kushwah AS, Meena M, et al. Impact of domestic air pollution from cooking fuel on respiratory allergies in children in India. Asian Pac J Allergy Immunol. 2008;26:213–22. [PubMed] [Google Scholar]
- 17.Behl RK, Kashyap S, Sarkar M. Prevalence of bronchial asthma in school children of 6-13 years of age in Shimla city. Indian J Chest Dis Allied Sci. 2010;52:145–8. [PubMed] [Google Scholar]
- 18.Chowgule RV, Shetye VM, Parmar JR, Bhosale AM, Khandagale MR, Phalnitkar SV, et al. Prevalence of respiratory symptoms, bronchial hyperreactivity and asthma in a megacity. Results of the European community respiratory health survey in Mumbai (Bombay) Am J Respir Crit Care Med. 1998;158:547–54. doi: 10.1164/ajrccm.158.2.9708064. [DOI] [PubMed] [Google Scholar]
- 19.Jindal SK, Gupta D, Aggarwal AN, Jindal RC, Singh V. Study of the prevalence of asthma in adults in North India using a standardized field questionnaire. J Asthma. 2000;37:345–51. doi: 10.3109/02770900009055458. [DOI] [PubMed] [Google Scholar]
- 20.Gaur SN, Gupta K, Rajpal S, Singh AB, Rohatgi A. Prevalence of bronchial asthma and allergic rhinitis among urban and rural adult population of Delhi. Indian J Allergy Asthma Immunol. 2006;20:90–7. [Google Scholar]
- 21.Gupta PR, Mangal DK. Prevalence and risk factors for bronchial asthma in adults in Jaipur district of Rajasthan (India) Lung India. 2006;23:53–8. [Google Scholar]
- 22.Parasuramalu BG, Huliraj N, Rudraprasad BM, Prashanth Kumar SP, Ramesh Masthi NR. Gangaboraiah. Prevalence of bronchial asthma and its association with smoking habits among adult population in rural area. Indian J Public Health. 2010;54:165–8. doi: 10.4103/0019-557X.75742. [DOI] [PubMed] [Google Scholar]
- 23.Gaur SN, Kumar R, Sinha R. Prevalence of bronchial asthma in petrol pump workers of Delhi. Indian J Occup Environ Med. 2003;7:13–6. [Google Scholar]
- 24.Gaur SN, Gupta K, Rajpal S, Agarwal N, Singh AB, Rohatgi A. Prevalence of bronchial asthma and allergic rhinitis among industrial workers in Delhi, India. Intern Med J Thai. 2005;21:13–8. [Google Scholar]
- 25.Agrawal S, Pearce N, Ebrahim S. Prevalence and risk factors for self-reported asthma in an adult Indian population: A cross-sectional survey. Int J Tuberc Lung Dis. 2013;17:275–82. doi: 10.5588/ijtld.12.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, Jindal SK, et al. Asthma Epidemiology Study Group. Prevalence and risk factors for bronchial asthma in Indian adults: A multicentre study. Indian J Chest Dis Allied Sci. 2006;48:13–22. [PubMed] [Google Scholar]
- 27.Jindal SK, Aggarwal AN, Gupta D, Agarwal R, Kumar R, Kaur T, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH) Int J Tuberc Lung Dis. 2012;16:1270–7. doi: 10.5588/ijtld.12.0005. [DOI] [PubMed] [Google Scholar]
- 28.Jindal SK. Burden of asthma in India. Int J Tuberc Lung Dis. 2013;17:145. doi: 10.5588/ijtld.12.0970. [DOI] [PubMed] [Google Scholar]
- 29.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–57. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 30.The European Lung White Book. The first comprehensive survey on respiratory health in Europe, 2003. [Last accessed on 2014 March 05]. Available from: http://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/
- 31.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Murthy KJR, Sastry JG. Economic burden of asthma. Back-ground papers; Burden of disease in India. [Last accessed on 2014 Mar 5]. Available from: http://www.who.int/macrohealth/action/NCMH_Burden%20of%20disease_%2829%20Sep%202005%29.pdf .
- 33.Geneva, Switzerland: 2013. [Last accessed on 2014 Mar 5]. WHO Fact sheet No. 307, 2013, Updated November. Available from: http://www.who.int/mediacentre/factsheets/fs307/en/ [Google Scholar]
- 34.Vercelli D. Gene-environment interactions in asthma and allergy: The end of the beginning? Curr Opin Allergy Clin Immunol. 2010;10:145–8. doi: 10.1097/ACI.0b013e32833653d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijk FN, de Jongste JC, Postma DS, Koppelman GH. Genetics of onset of asthma. Curr Opin Allergy Clin Immunol. 2013;13:193–202. doi: 10.1097/ACI.0b013e32835eb707. [DOI] [PubMed] [Google Scholar]
- 36.Birbian N, Singh J, Jindal SK. High risk association of IL-1 receptor antagonist (IL-1RN) VNTR polymorphism with asthma in a north Indian population: A pilot study. Cytokine. 2013;62:389–94. doi: 10.1016/j.cyto.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Birbian N, Singh J, Jindal SK, Joshi A, Batra N, Singla N. Association of the wild-type A/A genotype of MBL2 codon 54 with asthma in a north Indian population. Dis Markers. 2012;32:301–8. doi: 10.3233/DMA-2012-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang S, Wei X, Gong C, Wei J, Chen Z, Chen X, et al. Significant association between asthma risk and the GSTM1 and GSTT1 deletion polymorphisms: An updated meta-analysis of case-control studies. Respirology. 2013;18:774–83. doi: 10.1111/resp.12097. [DOI] [PubMed] [Google Scholar]
- 39.Nagarkatti R, B Rao C, Rishi JP, Chetiwal R, Shandilya V, Vijayan V, et al. Association of IFNG gene polymorphism with asthma in the Indian population. J Allergy Clin Immunol. 2002;110:410–2. doi: 10.1067/mai.2002.127859. [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, Batra J, Mabalirajan U, Sharma S, Nagarkatti R, Aich J, et al. A genetic variation in inositol polyphosphate 4 phosphatase a enhances susceptibility to asthma. Am J Respir Crit Care Med. 2008;177:712–9. doi: 10.1164/rccm.200705-781OC. [DOI] [PubMed] [Google Scholar]
- 41.Birbian N, Singh J, Jindal SK. Protective role of IL-18 -137G/C polymorphism in a North Indian population with asthma: A pilot study. Cytokine. 2013;61:188–93. doi: 10.1016/j.cyto.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Birbian N, Singh J, Jindal SK, Singla N. Association of ß(2)-adrenergic receptor polymorphisms with asthma in a north Indian population. Lung. 2012;190:497–504. doi: 10.1007/s00408-012-9407-7. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Nagarkatti R, B-Rao C, Niphadkar PV, Vijayan V, Sharma SK, et al. A_16_C haplotype in the FcepsilonRIbeta gene confers a higher risk for atopic asthma in the Indian population. Clin Genet. 2004;66:417–25. doi: 10.1111/j.1399-0004.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim O, Kim BH. Association of asthma symptoms with cigarette smoking and alcohol consumption in Korean adolescents. Nurs Health Sci. 2013;15:65–72. doi: 10.1111/j.1442-2018.2012.00737.x. [DOI] [PubMed] [Google Scholar]
- 45.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: Longitudinal and case. control studies. Thorax. 1998;53:204–12. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pokharel PK, Kabra SK, Kapoor SK, Pandey RM. Risk factors associated with bronchial asthma in school going children of rural Haryana. Indian J Pediatr. 2001;68:103–6. doi: 10.1007/BF02722022. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell EA, Beasley R, Keil U, Montefort S, Odhiambo J ISAAC Phase Three Study Group. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: Analyses from phase three of the ISAAC programme. Thorax. 2012;67:941–9. doi: 10.1136/thoraxjnl-2011-200901. [DOI] [PubMed] [Google Scholar]
- 48.Ehrlich RI, Du Toit D, Jordaan E, Zwarenstein M, Potter P, Volmink JA, et al. Risk factors for childhood asthma and wheezing. Importance of maternal and household smoking. Am J Respir Crit Care Med. 1996;154:681–8. doi: 10.1164/ajrccm.154.3.8810605. [DOI] [PubMed] [Google Scholar]
- 49.He QQ, Wong TW, Du L, Jiang ZQ, Yu TS, Qiu H, et al. Environmental tobacco smoke exposure and Chinese schoolchildren's respiratory health: A prospective cohort study. Am J Prev Med. 2011;41:487–93. doi: 10.1016/j.amepre.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 50.De Luca G, Olivieri F, Melotti G, Aiello G, Lubrano L, Boner AL. Fetal and early postnatal life roots of asthma. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):80–3. doi: 10.3109/14767058.2010.509931. [DOI] [PubMed] [Google Scholar]
- 51.Gupta D, Aggrawal AN, Chaudary J, Jindal SK. Domestic fuel combustion and morbidity from asthma among non-smoking women. Lung India. 1999;17:10–4. [Google Scholar]
- 52.Agrawal S. Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: Findings from a nationwide large-scale cross-sectional survey. J Asthma. 2012;49:355–65. doi: 10.3109/02770903.2012.663030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra V. Effect of indoor air pollution from biomass combustion on prevalence of asthma in the elderly. Environ Health Perspect. 2003;111:71–8. doi: 10.1289/ehp.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trevor J, Antony V, Jindal SK. The effect of biomass fuel exposure on the prevalence of asthma in adults in India-review of current evidence. J Asthma. 2014;51:136–41. doi: 10.3109/02770903.2013.849269. [DOI] [PubMed] [Google Scholar]
- 55.Guddattu V, Swathi A, Nair NS. Household and environment factors associated with asthma among Indian women: A multilevel approach. J Asthma. 2010;47:407–11. doi: 10.3109/02770903.2010.481343. [DOI] [PubMed] [Google Scholar]
- 56.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 57.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–5. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 59.Robinson DS, Larché M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malo JL, Lemière C, Gautrin D, Labrecque M. Occupational asthma. Curr Opin Pulm Med. 2004;10:57–61. doi: 10.1097/00063198-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Silvers KM, Frampton CM, Wickens K, Pattemore PK, Ingham T, Fishwick D, et al. New Zealand Asthma and Allergy Cohort Study Group. Breastfeeding protects against current asthma up to 6 years of age. J Pediatr. 2012;160:991–6.e1. doi: 10.1016/j.jpeds.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 62.Friedman NJ, Zeiger RS. The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115:1238–48. doi: 10.1016/j.jaci.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 63.Kumar R, Kumari D, Srivastava P, Khare V, Fakhr H, Arora N, et al. Identification of IgE-mediated food allergy and allergens in older children and adults with asthma and allergic rhinitis. Indian J Chest Dis Allied Sci. 2010;52:217–24. [PubMed] [Google Scholar]
- 64.Kumar R, Srivastava P, Kumari D, Fakhr H, Sridhara S, Arora N, et al. Rice (Oryza sativa) allergy in rhinitis and asthma patients: A clinico-immunological study. Immunobiology. 2007;212:141–7. doi: 10.1016/j.imbio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Kumari D, Kumar R, Sridhara S, Arora N, Gaur SN, Singh BP. Sensitization to blackgram in patients with bronchial asthma and rhinitis: Clinical evaluation and characterization of allergens. Allergy. 2006;61:104–10. doi: 10.1111/j.1398-9995.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 66.Kasera R, Singh BP, Lavasa S, Prasad KN, Sahoo RC, Singh AB. Kidney bean: A major sensitizer among legumes in asthma and rhinitis patients from India. PLoS One. 2011;6:e27193. doi: 10.1371/journal.pone.0027193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta P. Asthma in the obese: Yet another reason to lose weight. Lung India. 2008;25:1–3. doi: 10.4103/0970-2113.44128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brashier B, Salvi S. Obesity and asthma: Physiological perspective. J Allergy (Cairo) 2013. 2013 doi: 10.1155/2013/198068. 198068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vernon MK, Wiklund I, Bell JA, Dale P, Chapman KR. What do we know about asthma triggers? A review of the literature. J Asthma. 2012;49:991–8. doi: 10.3109/02770903.2012.738268. [DOI] [PubMed] [Google Scholar]
- 71.Singh M, Das RR, Kumar L, Kumar R. Bacille Calmette-Guérin vaccination is associated with lower prevalence of allergic diseases in Indian children. Am J Rhinol Allergy. 2013;27:e107–12. doi: 10.2500/ajra.2013.27.3940. [DOI] [PubMed] [Google Scholar]
- 72.Ryu JH, Scanlon PD. Obstructive lung diseases: COPD, asthma, and many imitators. Mayo Clin Proc. 2001;76:1144–53. doi: 10.4065/76.11.1144. [DOI] [PubMed] [Google Scholar]
- 73.British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2008;63(Suppl 4):iv1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 74.Gupta D, Agarwal R, Aggarwal AN, Maturu VN, Dhooria S, Prasad KT, et al. S K Jindal for the COPD Guidelines Working Group. Guidelines for diagnosis and management of chronic obstructive pulmonary disease: Joint ICS/NCCP (I) recommendations. Lung India. 2013;30:228–67. doi: 10.4103/0970-2113.116248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D, et al. Symptom-based questionnaire for differentiating COPD and asthma. Respiration. 2006;73:296–305. doi: 10.1159/000090141. [DOI] [PubMed] [Google Scholar]
- 76.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2014. [Last accessed on 2014 Jun 24]. Available from: http://www.ginasthma.org/
- 77.Mathew JL, Singh M, Mittal SK. Gastro-oesophageal reflux and bronchial asthma: Current status and future directions. Postgrad Med J. 2004;80:701–5. doi: 10.1136/pgmj.2004.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jindal A, Bal A, Agarwal R. Inflammatory myofibroblastic tumor of the trachea in the pediatric age group: Case report and systematic review of the literature. J Bronchology Interv Pulmonol. 2015;22:58–65. doi: 10.1097/LBR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 79.Pratter MR. Overview of common causes of chronic cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl):59s–62s. doi: 10.1378/chest.129.1_suppl.59S. [DOI] [PubMed] [Google Scholar]
- 80.Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17:45–9. doi: 10.1097/MCP.0b013e32834130ee. [DOI] [PubMed] [Google Scholar]
- 81.Newman KB, Mason UG, 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152:1382–6. doi: 10.1164/ajrccm.152.4.7551399. [DOI] [PubMed] [Google Scholar]
- 82.Killian KJ, Watson R, Otis J, St Amand TA, O’Byrne PM. Symptom perception during acute bronchoconstriction. Am J Respir Crit Care Med. 2000;162:490–6. doi: 10.1164/ajrccm.162.2.9905079. [DOI] [PubMed] [Google Scholar]
- 83.Ekici M, Apan A, Ekici A, Erdemoğlu AK. Perception of bronchoconstriction in elderly asthmatics. J Asthma. 2001;38:691–6. doi: 10.1081/jas-100107547. [DOI] [PubMed] [Google Scholar]
- 84.van Schayck CP, van Der Heijden FM, van Den Boom G, Tirimanna PR, van Herwaarden CL. Underdiagnosis of asthma: Is the doctor or the patient to blame? The DIMCA project. Thorax. 2000;55:562–5. doi: 10.1136/thorax.55.7.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 86.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 87.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 88.Aggarwal AN. How appropriate is the gold standard for diagnosis of airway obstruction? Lung India. 2008;25:139–41. doi: 10.4103/0970-2113.45276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain SK, Ramiah TJ. Spirometric studies in healthy women 15-40 years age. Indian J Chest Dis. 1967;9:1–12. [PubMed] [Google Scholar]
- 90.Jain SK, Ramiah TJ. Normal standards of pulmonary function tests for healthy Indian men 15-40 years old: Comparison of different regression equations (prediction formulae) Indian J Med Res. 1969;57:1453–66. [PubMed] [Google Scholar]
- 91.Jindal SK, Wahi PL. Pulmonary function laboratory in the tropics, needs, problems, and solutions. In: Sharma OP, editor. Lung Disease in the Tropics. New York: Marcel Dekker; 1991. pp. 523–42. [Google Scholar]
- 92.Udwadia FE, Sunavala JD, Shetye VM. Lung function studies in healthy Indian subjects. J Assoc Physicians India. 1987;35:491–6. [PubMed] [Google Scholar]
- 93.Vijayan VK, Kuppurao KV, Venkatesan P, Sankaran K, Prabhakar R. Pulmonary function in healthy young adult Indians in Madras. Thorax. 1990;45:611–5. doi: 10.1136/thx.45.8.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aggarwal AN, Gupta D, Jindal SK. Development of a simple computer program for spirometry interpretation. J Assoc Physicians India. 2002;50:567–70. [PubMed] [Google Scholar]
- 95.Kupczyk M, Kupry I, Górski P, Kuna P. Long-term deterioration of lung function in asthmatic outpatients? Respiration. 2004;71:233–40. doi: 10.1159/000077420. [DOI] [PubMed] [Google Scholar]
- 96.Kitch BT, Paltiel AD, Kuntz KM, Dockery DW, Schouten JP, Weiss ST, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest. 2004;126:1875–82. doi: 10.1378/chest.126.6.1875. [DOI] [PubMed] [Google Scholar]
- 97.Pino JM, García-Río F, Prados C, Alvarez-Sala R, Díaz S, Villasante C, et al. Value of the peak expiratory flow in bronchodynamic tests. Allergol Immunopathol (Madr) 1996;24:54–7. [PubMed] [Google Scholar]
- 98.Dekker FW, Schrier AC, Sterk PJ, Dijkman JH. Validity of peak expiratory flow measurement in assessing reversibility of airflow obstruction. Thorax. 1992;47:162–6. doi: 10.1136/thx.47.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aggarwal AN, Agarwal R, Gupta D, Jindal SK. Use of peak expiratory flow for assessing bronchodilator responsiveness. Prim Care Respir J. 2009;18:50–2. doi: 10.3132/pcrj.2008.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ray D, Rajaratnam A, Richard J. Peak expiratory flow in rural residents of Tamil Nadu, India. Thorax. 1993;48:163–6. doi: 10.1136/thx.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaur H, Singh J, Makkar M, Singh K, Garg R. Variations in the peak expiratory flow rate with various factors in a population of healthy women of the malwa region of Punjab, India. J Clin Diagn Res. 2013;7:1000–3. doi: 10.7860/JCDR/2013/5217.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittal S, Gupta S, Kumar A, Singh KD. Regression equations for peak expiratory flow in healthy children aged 7 to 14 years from Punjab, India. Lung India. 2013;30:183–6. doi: 10.4103/0970-2113.116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A. A comparison of the individual best versus the predicted peak expiratory flow in patients with chronic asthma. J Asthma. 2001;38:33–40. doi: 10.1081/jas-100000019. [DOI] [PubMed] [Google Scholar]
- 104.Malik SK, Jindal SK, Banga N, Sharda PK, Gupta HD. Peak expiratory flow rate of healthy north Indian teachers. Indian J Med Res. 1980;71:322–4. [PubMed] [Google Scholar]
- 105.Malik SK, Jindal SK, Sharda PK, Banga N. Peak expiratory flow rates of school age girls from Punjab (Second report) Indian Pediatr. 1982;19:161–4. [PubMed] [Google Scholar]
- 106.Malik SK, Jindal SK. Pulmonary function tests in healthy children. Indian Pediatr. 1985;22:677–81. [PubMed] [Google Scholar]
- 107.Vijayan VK, Reetha AM, Kuppurao KV, Venkatesan P, Thilakavathy S. Pulmonary function in normal south Indian children aged 7 to 19 years. Indian J Chest Dis Allied Sci. 2000;42:147–56. [PubMed] [Google Scholar]
- 108.Bongers T, O’Driscoll BR. Effects of equipment and technique on peak flow measurements. BMC Pulm Med. 2006;6:14. doi: 10.1186/1471-2466-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malik SK, Jindal SK, Banga N. Experience with Wright's new mini peak flow meter for monitoring airways obstruction. Indian J Chest Dis Allied Sci. 1980;22:220–3. [PubMed] [Google Scholar]
- 110.Slieker MG, van der Ent CK. The diagnostic and screening capacities of peak expiratory flow measurements in the assessment of airway obstruction and bronchodilator response in children with asthma. Monaldi Arch Chest Dis. 2003;59:155–9. [PubMed] [Google Scholar]
- 111.Aggarwal AN, Gupta D, Jindal SK. The relationship between FEV1 and peak expiratory flow in patients with airways obstruction is poor. Chest. 2006;130:1454–61. doi: 10.1378/chest.130.5.1454. [DOI] [PubMed] [Google Scholar]
- 112.Llewellin P, Sawyer G, Lewis S, Cheng S, Weatherall M, Fitzharris P, et al. The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology. 2002;7:333–7. doi: 10.1046/j.1440-1843.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 113.Palma-Carlos AG, Palma-Carlos ML. Correlation between clinical classification, PEF and FEV1: Guidelines and reality. Eur Ann Allergy Clin Immunol. 2003;35:130–2. [PubMed] [Google Scholar]
- 114.Hetzel MR. The pulmonary clock. Thorax. 1981;36:481–6. doi: 10.1136/thx.36.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980;35:732–8. doi: 10.1136/thx.35.10.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aggarwal AN, Gupta D, Chaganti S, Jindal SK. Diurnal variation in peak expiratory flow in healthy young adults. Indian J Chest Dis Allied Sci. 2000;42:15–9. [PubMed] [Google Scholar]
- 117.Aggarwal AN, Gupta D, Kumar V, Jindal SK. Assessment of diurnal variability of peak expiratory flow in stable asthmatics. J Asthma. 2002;39:487–91. doi: 10.1081/jas-120004911. [DOI] [PubMed] [Google Scholar]
- 118.Gupta D, Aggarwal AN, Chaganti S, Jindal SK. Reducing the number of daily measurements results in poor estimation of diurnal variability of peak expiratory flow in healthy individuals. J Postgrad Med. 2000;46:262–4. [PubMed] [Google Scholar]
- 119.Higgins BG, Britton JR, Chinn S, Jones TD, Jenkinson D, Burney PG, et al. The distribution of peak expiratory flow variability in a population sample. Am Rev Respir Dis. 1989;140:1368–72. doi: 10.1164/ajrccm/140.5.1368. [DOI] [PubMed] [Google Scholar]
- 120.Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis. 1980;121:389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- 121.Aggarwal AN, Gupta D, Chandrasekhar G, Jindal SK. Bronchial hyperresponsiveness in patients with sarcoidosis. J Assoc Physicians India. 2004;52:21–3. [PubMed] [Google Scholar]
- 122.Perpiñá Tordera M, García Río F, Álvarez Gutierrez FJ, Cisneros Serrano C, Compte Torrero L, Entrenas Costa LM, et al. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Guidelines for the study of nonspecific bronchial hyperresponsiveness in asthma. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Arch Bronconeumol. 2013;49:432–46. doi: 10.1016/j.arbres.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009;103:363–72. doi: 10.1016/S1081-1206(10)60353-5. 400. [DOI] [PubMed] [Google Scholar]
- 124.Cockcroft DW. Direct challenge tests: Airway hyperresponsiveness in asthma: Its measurement and clinical significance. Chest. 2010;138(Suppl):18s–24s. doi: 10.1378/chest.10-0088. [DOI] [PubMed] [Google Scholar]
- 125.Goldstein MF, Veza BA, Dunsky EH, Dvorin DJ, Belecanech GA, Haralabatos IC. Comparisons of peak diurnal expiratory flow variation, postbronchodilator FEV (1) responses and methacholine inhalation challenges in the evaluation of suspected asthma. Chest. 2001;119:1001–10. doi: 10.1378/chest.119.4.1001. [DOI] [PubMed] [Google Scholar]
- 126.Anderson SD, Charlton B, Weiler JM, Nichols S, Spector SL, Pearlman DS A305 Study Group. Comparison of mannitol and methacholine to predict exercise-induced bronchoconstriction and a clinical diagnosis of asthma. Respir Res. 2009;10:4. doi: 10.1186/1465-9921-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ernst P, Ghezzo H, Becklake MR. Risk factors for bronchial hyperresponsiveness in late childhood and early adolescence. Eur Respir J. 2002;20:635–9. doi: 10.1183/09031936.02.00962002. [DOI] [PubMed] [Google Scholar]
- 128.Remes ST, Pekkanen J, Remes K, Salonen RO, Korppi M. In search of childhood asthma: Questionnaire, tests of bronchial hyperresponsiveness and clinical evaluation. Thorax. 2002;57:120–6. doi: 10.1136/thorax.57.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pekkanen J, Pearce N. Defining asthma in epidemiological studies. Eur Respir J. 1999;14:951–7. doi: 10.1034/j.1399-3003.1999.14d37.x. [DOI] [PubMed] [Google Scholar]
- 130.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135:805–26. doi: 10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 131.Agarwal R, Khan A, Garg M, Aggarwal AN, Gupta D. Pictorial essay: Allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging. 2011;21:242–52. doi: 10.4103/0971-3026.90680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hederos CA, Janson S, Andersson H, Hedlin G. Chest X-ray investigation in newly discovered asthma. Pediatr Allergy Immunol. 2004;15:163–5. doi: 10.1046/j.1399-3038.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 133.Gershel JC, Goldman HS, Stein RE, Shelov SP, Ziprkowski M. The usefulness of chest radiographs in first asthma attacks. N Engl J Med. 1983;309:336–9. doi: 10.1056/NEJM198308113090603. [DOI] [PubMed] [Google Scholar]
- 134.Rubenstein HS, Rosner BA, LeMay M, Neidorf R. The value of the chest X-ray in making the diagnosis of bronchial asthma. Adolescence. 1993;28:505–16. [PubMed] [Google Scholar]
- 135.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Lung imaging in asthmatic patients: The picture is clearer. J Allergy Clin Immunol. 2011;128:467–78. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Good JT, Jr, Kolakowski CA, Groshong SD, Murphy JR, Martin RJ. Refractory asthma: Importance of bronchoscopy to identify phenotypes and direct therapy. Chest. 2012;141:599–606. doi: 10.1378/chest.11-0741. [DOI] [PubMed] [Google Scholar]
- 137.Tseliou E, Bessa V, Hillas G, Delimpoura V, Papadaki G, Roussos C, et al. Exhaled nitric oxide and exhaled breath condensate pH in severe refractory asthma. Chest. 2010;138:107–13. doi: 10.1378/chest.09-1257. [DOI] [PubMed] [Google Scholar]
- 138.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) Study Group. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54.e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wadsworth S, Sin D, Dorscheid D. Clinical update on the use of biomarkers of airway inflammation in the management of asthma. J Asthma Allergy. 2011;4:77–86. doi: 10.2147/JAA.S15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bush A, Eber E. The value of FeNO measurement in asthma management: The motion for Yes, it's NO--or, the wrong end of the stick! Paediatr Respir Rev. 2008;9:127–31. doi: 10.1016/j.prrv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 141.Nair P. Update on clinical inflammometry for the management of airway diseases. Can Respir J. 2013;20:117–20. doi: 10.1155/2013/602936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Djukanoviæ R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002;37:1s–2s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 143.Paggiaro PL, Chanez P, Holz O, Ind PW, Djukanoviæ R, Maestrelli P, et al. Sputum induction. Eur Respir J Suppl. 2002;37:3s–8s. doi: 10.1183/09031936.02.00000302. [DOI] [PubMed] [Google Scholar]
- 144.Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 2002;37:19s–23s. doi: 10.1183/09031936.02.00001902. [DOI] [PubMed] [Google Scholar]
- 145.Spanevello A, Confalonieri M, Sulotto F, Romano F, Balzano G, Migliori GB, et al. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med. 2000;162:1172–4. doi: 10.1164/ajrccm.162.3.9908057. [DOI] [PubMed] [Google Scholar]
- 146.Hunter CJ, Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. A comparison of the validity of different diagnostic tests in adults with asthma. Chest. 2002;121:1051–7. doi: 10.1378/chest.121.4.1051. [DOI] [PubMed] [Google Scholar]
- 147.Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, et al. A systematic review and meta-analysis: Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) Thorax. 2012;67:199–208. doi: 10.1136/thx.2010.135574. [DOI] [PubMed] [Google Scholar]
- 148.Hargreave FE, Nair P. Point: Is measuring sputum eosinophils useful in the management of severe asthma? Yes. Chest. 2011;139:1270–3. doi: 10.1378/chest.11-0618. [DOI] [PubMed] [Google Scholar]
- 149.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 150.Stewart L, Katial RK. Exhaled nitric oxide. Immunol Allergy Clin North Am. 2012;32:347–62. doi: 10.1016/j.iac.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 151.Nathan C, Xie QW. Nitric oxide synthases: Roles, tolls and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 152.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6:1368–70. [PubMed] [Google Scholar]
- 153.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98:2622–7. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee onInterpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006340.pub3. CD006340. [DOI] [PubMed] [Google Scholar]
- 157.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National Heart, Lung and Blood Institute's Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–56. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 159.Weinmayr G, Keller F, Kleiner A, du Prel JB, Garcia-Marcos L, Batllés-Garrido J, et al. Asthma phenotypes identified by latent class analysis in the ISAAC phase II Spain study. Clin Exp Allergy. 2013;43:223–32. doi: 10.1111/cea.12035. [DOI] [PubMed] [Google Scholar]
- 160.Schwindt CD, Tjoa T, Floro JN, McLaren C, Delfino RJ. Association of atopy to asthma severity and medication use in children. J Asthma. 2006;43:439–46. doi: 10.1080/02770900600758234. [DOI] [PubMed] [Google Scholar]
- 161.Carroll WD, Lenney W, Child F, Strange RC, Jones PW, Whyte MK, et al. Asthma severity and atopy: How clear is the relationship? Arch Dis Child. 2006;91:405–9. doi: 10.1136/adc.2005.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for hospital admission for asthma from childhood to young adulthood: A longitudinal population study. J Allergy Clin Immunol. 2002;110:220–7. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- 163.Ponte EV, Souza-Machado A, Souza-Machado C, Franco R, Cruz AA. Atopy is not associated with poor control of asthma. J Asthma. 2012;49:1021–6. doi: 10.3109/02770903.2012.733989. [DOI] [PubMed] [Google Scholar]
- 164.Ozol D, Koca C, Mete E, Yigitoðlu R. Influence of atopy on asthma severity in adult female patients. J Investig Allergol Clin Immunol. 2008;18:36–40. [PubMed] [Google Scholar]
- 165.Agarwal R, Chakrabarti A. Allergic bronchopulmonary aspergillosis in asthma: Epidemiological, clinical and therapeutic issues. Future Microbiol. 2013;8:1463–74. doi: 10.2217/fmb.13.116. [DOI] [PubMed] [Google Scholar]
- 166.Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32:545–54. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 167.Cockcroft DW, Swystun VA. Asthma control versus asthma severity. J Allergy Clin Immunol. 1996;98:1016–8. doi: 10.1016/s0091-6749(96)80185-0. [DOI] [PubMed] [Google Scholar]
- 168.Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society Asthma Clinical Assembly. Canadian Thoracic Society 2012 guideline update: Diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19:127–64. doi: 10.1155/2012/635624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Executive Committee Gema 2009. GEMA 2009 (Spanish guideline on the management of asthma) J Investig Allergol Clin Immunol. 2010;20(Suppl 1):1–59. [PubMed] [Google Scholar]
- 170.Adams NP, Bestall JC, Jones P. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database Syst Rev. 1999 CD003274. [Google Scholar]
- 171.Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD003135.pub3. CD003135. [DOI] [PubMed] [Google Scholar]
- 172.Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD002738.pub2. CD002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006217.pub2. CD006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Jr, Sorkness CA, et al. Asthma Clinical Research Network for the National Heart, Lung and Blood Institute. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: A randomized controlled trial. JAMA. 2001;285:2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 175.Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314. doi: 10.1002/14651858.CD002314.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Youngchaiyud P, Permpikul C, Suthamsmai T, Wong E. A double-blind comparison of inhaled budesonide, long-acting theophylline and their combination in treatment of nocturnal asthma. Allergy. 1995;50:28–33. doi: 10.1111/j.1398-9995.1995.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 177.Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild-to-moderate asthma. The American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. J Allergy Clin Immunol. 1998;101:14–23. doi: 10.1016/s0091-6749(98)70187-3. [DOI] [PubMed] [Google Scholar]
- 178.Morali T, Yilmaz A, Erkan F, Akkaya E, Ece F, Baran R. Efficacy of inhaled budesonide and oral theophylline in asthmatic subjects. J Asthma. 2001;38:673–9. doi: 10.1081/jas-100107545. [DOI] [PubMed] [Google Scholar]
- 179.Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64–77. [PubMed] [Google Scholar]
- 180.Dahl R, Larsen BB, Venge P. Effect of long-term treatment with inhaled budesonide or theophylline on lung function, airway reactivity and asthma symptoms. Respir Med. 2002;96:432–8. doi: 10.1053/rmed.2001.1280. [DOI] [PubMed] [Google Scholar]
- 181.Yurdakul AS, Taci N, Eren A, Sipit T. Comparative efficacy of once-daily therapy with inhaled corticosteroid, leukotriene antagonist or sustained-release theophylline in patients with mild persistent asthma. Respir Med. 2003;97:1313–9. doi: 10.1016/j.rmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 182.Stoloff SW, Kelly HW. Updates on the use of inhaled corticosteroids in asthma. Curr Opin Allergy Clin Immunol. 2011;11:337–44. doi: 10.1097/ACI.0b013e328348a813. [DOI] [PubMed] [Google Scholar]
- 183.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Asthma Clinical Research Network of the National Heart Lung, and Blood Institute. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–8. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 184.Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: Meta-analysis. BMJ. 2001;323:253–6. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Masoli M, Weatherall M, Holt S, Beasley R. Clinical dose-response relationship of fluticasone propionate in adults with asthma. Thorax. 2004;59:16–20. [PMC free article] [PubMed] [Google Scholar]
- 186.Adams N, Bestall J, Jones PW. Budesonide at different doses for chronic asthma. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD003271. CD003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004109.pub2. CD004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Adams NP, Bestall JC, Jones P, Lasserson TJ, Griffiths B, Cates CJ. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD003534.pub3. CD003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: The BASALT randomized controlled trial. JAMA. 2012;308:987–97. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. BEST Study Group. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356:2040–52. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- 191.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. National Heart, Lung and Blood Institute's Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 192.Chauhan BF, Chartrand C, Ducharme FM. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database Syst Rev. 2013;2:CD009611. doi: 10.1002/14651858.CD009611.pub3. [DOI] [PubMed] [Google Scholar]
- 193.Baptist AP, Reddy RC. Inhaled corticosteroids for asthma: Are they all the same? J Clin Pharm Ther. 2009;34:1–12. doi: 10.1111/j.1365-2710.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 194.Adams N, Bestall JM, Jones PW. Inhaled beclomethasone versus budesonide for chronic asthma. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD003530. CD003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Adams N, Lasserson TJ, Cates CJ, Jones PW. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002310.pub4. CD002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus other inhaled steroids for chronic asthma in children and adults. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD007031. CD007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Buhl R. Local oropharyngeal side effects of inhaled corticosteroids in patients with asthma. Allergy. 2006;61:518–26. doi: 10.1111/j.1398-9995.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- 198.Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: Current understanding and review of the literature. Chest. 2004;126:213–9. doi: 10.1378/chest.126.1.213. [DOI] [PubMed] [Google Scholar]
- 199.Berger WE. Ciclesonide: A closer look at its systemic and oropharyngeal safety profile. Curr Drug Saf. 2006;1:265–70. doi: 10.2174/157488606777934422. [DOI] [PubMed] [Google Scholar]
- 200.Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87:457–61. doi: 10.1136/adc.87.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lapi F, Kezouh A, Suissa S, Ernst P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur Respir J. 2013;42:79–86. doi: 10.1183/09031936.00080912. [DOI] [PubMed] [Google Scholar]
- 202.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–17. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 203.Vogelmeier CF, Hering T, Lewin T, Sander P, Bethke TD. Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care. Respir Med. 2011;105:186–94. doi: 10.1016/j.rmed.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 204.McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: A case-control study. Chest. 2013;144:1788–94. doi: 10.1378/chest.13-0871. [DOI] [PubMed] [Google Scholar]
- 205.Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68:1105–13. doi: 10.1136/thoraxjnl-2012-203175. [DOI] [PubMed] [Google Scholar]
- 206.Sharma PK, Malhotra S, Pandhi P, Kumar N. Effect of inhaled steroids on bone mineral density: A meta-analysis. J Clin Pharmacol. 2003;43:193–7. doi: 10.1177/0091270002239829. [DOI] [PubMed] [Google Scholar]
- 207.Uboweja A, Malhotra S, Pandhi P. Effect of inhaled corticosteroids on risk of development of cataract: A meta-analysis. Fundam Clin Pharmacol. 2006;20:305–9. doi: 10.1111/j.1472-8206.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 208.Khianey R, Oppenheimer J. Controversies regarding long-acting ß2-agonists. Curr Opin Allergy Clin Immunol. 2011;11:345–54. doi: 10.1097/ACI.0b013e328348a82e. [DOI] [PubMed] [Google Scholar]
- 209.Walters EH, Gibson PG, Lasserson TJ, Walters JA. Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001385.pub2. CD001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 211.Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: Comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ. 1993;306:1034–7. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lemanske RF, Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Asthma Clinical Research Network for the National Heart, Lung, and Blood Institute. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: A randomized controlled trial. JAMA. 2001;285:2594–603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 213.Weatherall M, Wijesinghe M, Perrin K, Harwood M, Beasley R. Meta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapy. Thorax. 2010;65:39–43. doi: 10.1136/thx.2009.116608. [DOI] [PubMed] [Google Scholar]
- 214.Nelson H, Bonuccelli C, Radner F, Ottosson A, Carroll KJ, Andersson TL, et al. Safety of formoterol in patients with asthma: Combined analysis of data from double-blind, randomized controlled trials. J Allergy Clin Immunol. 2010;125:390–6. e8. doi: 10.1016/j.jaci.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 215.Cates CJ, Wieland LS, Oleszczuk M, Kew KM. Safety of regular formoterol or salmeterol in adults with asthma: An overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;2:CD010314. doi: 10.1002/14651858.CD010314.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: The OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–7. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 217.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 218.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 219.Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD005535.pub2. CD005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: Serious adverse events. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD007694.pub2. CD007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lasserson TJ, Ferrara G, Casali L. Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD004106.pub4. CD004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD005533.pub2. CD005533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Adachi M, Aizawa H, Ishihara K, Ohta K, Sano Y, Taniguchi H, et al. Comparison of salmeterol/fluticasone propionate (FP) combination with FP+sustained release theophylline in moderate asthma patients. Respir Med. 2008;102:1055–64. doi: 10.1016/j.rmed.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 224.Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD003137.pub4. CD003137. [DOI] [PubMed] [Google Scholar]
- 225.O’Byrne PM, Bleecker ER, Bateman ED, Busse WW, Woodcock A, Forth R, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J. 2014;43:773–82. doi: 10.1183/09031936.00064513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bateman ED, O’Byrne PM, Busse WW, Lötvall J, Bleecker ER, Andersen L, et al. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014;69:312–9. doi: 10.1136/thoraxjnl-2013-203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Woodcock A, Bleecker ER, Lötvall J, O’Byrne PM, Bateman ED, Medley H, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest. 2013;144:1222–9. doi: 10.1378/chest.13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kempsford RD, Oliver A, Bal J, Tombs L, Quinn D. The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med. 2013;107:1873–80. doi: 10.1016/j.rmed.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 229.Lipworth B. Systemic safety of fluticasone furoate/vilanterol combination. Thorax. 2013;68:1165. doi: 10.1136/thoraxjnl-2013-203910. [DOI] [PubMed] [Google Scholar]
- 230.Allen A, Schenkenberger I, Trivedi R, Cole J, Hicks W, Gul N, et al. Inhaled fluticasone furoate/vilanterol does not affect hypothalamic-pituitary-adrenal axis function in adolescent and adult asthma: Randomised, double-blind, placebo-controlled study. Clin Respir J. 2013;7:397–406. doi: 10.1111/crj.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Busse WW, O’Byrne PM, Bleecker ER, Lötvall J, Woodcock A, Andersen L, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the ß2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: A randomised trial. Thorax. 2013;68:513–20. doi: 10.1136/thoraxjnl-2012-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kanniess F, Boulet LP, Pierzchala W, Cameron R, Owen R, Higgins M. Efficacy and safety of indacaterol, a new 24-hour beta2-agonist, in patients with asthma: A dose-ranging study. J Asthma. 2008;45:887–92. doi: 10.1080/02770900802348321. [DOI] [PubMed] [Google Scholar]
- 233.Sugihara N, Kanada S, Haida M, Ichinose M, Adachi M, Hosoe M, et al. 24-h bronchodilator efficacy of single doses of indacaterol in Japanese patients with asthma: A comparison with placebo and salmeterol. Respir Med. 2010;104:1629–37. doi: 10.1016/j.rmed.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 234.Gibb A, Yang LP. Olodaterol: First global approval. Drugs. 2013;73:1841–6. doi: 10.1007/s40265-013-0137-9. [DOI] [PubMed] [Google Scholar]
- 235.Vignola AM. Effects of inhaled corticosteroids, leukotriene receptor antagonists, or both, plus long-acting beta2-agonists on asthma pathophysiology: A review of the evidence. Drugs. 2003;63(Suppl 2):35–51. doi: 10.2165/00003495-200363002-00004. [DOI] [PubMed] [Google Scholar]
- 236.Bel EH. Clinical practice. Mild asthma. N Engl J Med. 2013;369:549–57. doi: 10.1056/NEJMcp1214826. [DOI] [PubMed] [Google Scholar]
- 237.Barnes ML, Menzies D, Fardon TC, Burns P, Wilson AM, Lipworth BJ. Combined mediator blockade or topical steroid for treating the unified allergic airway. Allergy. 2007;62:73–80. doi: 10.1111/j.1398-9995.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 238.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31:616–24. doi: 10.1046/j.1365-2222.2001.01088.x. [DOI] [PubMed] [Google Scholar]
- 239.Wilson AM, Orr LC, Sims EJ, Dempsey OJ, Lipworth BJ. Antiasthmatic effects of mediator blockade versus topical corticosteroids in allergic rhinitis and asthma. Am J Respir Crit Care Med. 2000;162:1297–301. doi: 10.1164/ajrccm.162.4.9912046. [DOI] [PubMed] [Google Scholar]
- 240.Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695–707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 241.Ducharme F, Schwartz Z, Hicks G, Kakuma R. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003133.pub2. CD003133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dupont L, Potvin E, Korn D, Lachman A, Dramaix M, Gusman J, et al. Singulair as Complementary Therapy to Fixed Association in Real life Study Group. Improving asthma control in patients suboptimally controlled on inhaled steroids and long-acting beta2-agonists: Addition of montelukast in an open-label pilot study. Curr Med Res Opin. 2005;21:863–9. doi: 10.1185/030079905X46304. [DOI] [PubMed] [Google Scholar]
- 243.Korn D, Van den Brande P, Potvin E, Dramaix M, Herbots E, Peche R. Efficacy of add-on montelukast in patients with non-controlled asthma: A Belgian open-label study. Curr Med Res Opin. 2009;25:489–97. doi: 10.1185/03007990802667937. [DOI] [PubMed] [Google Scholar]
- 244.Virchow JC, Bachert C. Efficacy and safety of montelukast in adults with asthma and allergic rhinitis. Respir Med. 2006;100:1952–9. doi: 10.1016/j.rmed.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 245.Virchow JC, Mehta A, Ljungblad L, Mitfessel H MONICA Study Group. Add-on montelukast in inadequately controlled asthma patients in a 6-month open-label study: The MONtelukast In Chronic Asthma (MONICA) study. Respir Med. 2010;104:644–51. doi: 10.1016/j.rmed.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 246.Sadatsafavi M, Lynd L, Marra C, Bedouch P, Fitzgerald M. Comparative outcomes of leukotriene receptor antagonists and long-acting ß-agonists as add-on therapy in asthmatic patients: A population-based study. J Allergy Clin Immunol. 2013;132:63–9. doi: 10.1016/j.jaci.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 247.Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: A systematic review. Thorax. 2008;63:453–62. doi: 10.1136/thx.2007.081596. [DOI] [PubMed] [Google Scholar]
- 248.Barnes N, Thomas M, Price D, Tate H. The national montelukast survey. J Allergy Clin Immunol. 2005;115:47–54. doi: 10.1016/j.jaci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 249.Price D. Tolerability of montelukast. Drugs. 2000;59(Suppl 1):35–45. doi: 10.2165/00003495-200059001-00006. [DOI] [PubMed] [Google Scholar]
- 250.Guyer AC, Long AA. Long-acting anticholinergics in the treatment of asthma. Curr Opin Allergy Clin Immunol. 2013;13:392–8. doi: 10.1097/ACI.0b013e328362a775. [DOI] [PubMed] [Google Scholar]
- 251.Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128:315–22. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 252.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: A randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–14. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 254.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 255.Tian JW, Chen JW, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: A meta-analysis. Respir Care. 2014;59:654–66. doi: 10.4187/respcare.02703. [DOI] [PubMed] [Google Scholar]
- 256.Barnes PJ. Theophylline. Am J Respir Crit Care Med. 2013;188:901–6. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- 257.Tee AK, Koh MS, Gibson PG, Lasserson TJ, Wilson AJ, Irving LB. Long-acting beta2-agonists versus theophylline for maintenance treatment of asthma. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001281.pub2. CD001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Davies B, Brooks G, Devoy M. The efficacy and safety of salmeterol compared to theophylline: Meta-analysis of nine controlled studies. Respir Med. 1998;92:256–63. doi: 10.1016/s0954-6111(98)90105-6. [DOI] [PubMed] [Google Scholar]
- 259.Dempsey OJ, Fowler SJ, Wilson A, Kennedy G, Lipworth BJ. Effects of adding either a leukotriene receptor antagonist or low-dose theophylline to a low or medium dose of inhaled corticosteroid in patients with persistent asthma. Chest. 2002;122:151–9. doi: 10.1378/chest.122.1.151. [DOI] [PubMed] [Google Scholar]
- 260.Evans DJ, Taylor DA, Zetterstrom O, Chung KF, O’Connor BJ, Barnes PJ. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med. 1997;337:1412–8. doi: 10.1056/NEJM199711133372002. [DOI] [PubMed] [Google Scholar]
- 261.Lim S, Jatakanon A, Gordon D, Macdonald C, Chung KF, Barnes PJ. Comparison of high dose inhaled steroids, low dose inhaled steroids plus low dose theophylline, and low dose inhaled steroids alone in chronic asthma in general practice. Thorax. 2000;55:837–41. doi: 10.1136/thorax.55.10.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ukena D, Harnest U, Sakalauskas R, Magyar P, Vetter N, Steffen H, et al. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J. 1997;10:2754–60. doi: 10.1183/09031936.97.10122754. [DOI] [PubMed] [Google Scholar]
- 263.American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 264.Nie H, Zhang G, Liu M, Ding X, Huang Y, Hu S. Efficacy of theophylline plus salmeterol/fluticasone propionate combination therapy in patients with asthma. Respir Med. 2013;107:347–54. doi: 10.1016/j.rmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 265.Shukla D, Chakraborty S, Singh S, Mishra B. Doxofylline: A promising methylxanthine derivative for the treatment of asthma and chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2009;10:2343–56. doi: 10.1517/14656560903200667. [DOI] [PubMed] [Google Scholar]
- 266.Goldstein MF, Chervinsky P. Efficacy and safety of doxofylline compared to theophylline in chronic reversible asthma -- A double-blind randomized placebo-controlled multicentre clinical trial. Med Sci Monit. 2002;8:CR297–304. [PubMed] [Google Scholar]
- 267.Melillo G, Balzano G, Jodice F, De Felice A, Campisi V, Capone M, et al. Treatment of reversible chronic airways obstruction with doxofylline compared with slow-release theophylline: A double-blind, randomized, multicentre trial. Int J Clin Pharmacol Res. 1989;9:397–405. [PubMed] [Google Scholar]
- 268.Welsh EJ, Cates CJ. Formoterol versus short-acting beta-agonists as relief medication for adults and children with asthma. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD008418.pub2. CD008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Westby M, Benson M, Gibson P. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003269.pub2. CD003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Agarwal R, Khan A, Aggarwal AN, Gupta D. Is the SMART approach better than other treatment approaches for prevention of asthma exacerbations. A meta-analysis? Monaldi Arch Chest Dis. 2009;71:161–9. doi: 10.4081/monaldi.2009.348. [DOI] [PubMed] [Google Scholar]
- 271.Ställberg B, Ekström T, Neij F, Olsson P, Skoogh BE, Wennergren G, et al. SHARE Trial Group. A real-life cost-effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma. Respir Med. 2008;102:1360–70. doi: 10.1016/j.rmed.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 272.Patel M, Pilcher J, Beasley R. Combination ICS/fast-onset LABA inhaler as maintenance and reliever therapy: The future for uncontrolled adult asthma? Expert Rev Respir Med. 2013;7:451–4. doi: 10.1586/17476348.2013.837777. [DOI] [PubMed] [Google Scholar]
- 273.Papi A, Corradi M, Pigeon-Francisco C, Baronio R, Siergiejko Z, Petruzzelli S, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: A double-blind, randomised controlled trial. Lancet Respir Med. 2013;1:23–31. doi: 10.1016/S2213-2600(13)70012-2. [DOI] [PubMed] [Google Scholar]
- 274.Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: Can using a single inhaler containing budesonide and formoterol help? Br J Gen Pract. 2008;58:37–43. doi: 10.3399/bjgp08X263802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20:1403–18. doi: 10.1185/030079904X2051. [DOI] [PubMed] [Google Scholar]
- 276.Rabe KF, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: A randomized, double-blind trial. Chest. 2006;129:246–56. doi: 10.1378/chest.129.2.246. [DOI] [PubMed] [Google Scholar]
- 277.O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–36. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 278.Vogelmeier C, D’Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: An effective asthma treatment option? Eur Respir J. 2005;26:819–28. doi: 10.1183/09031936.05.00028305. [DOI] [PubMed] [Google Scholar]
- 279.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: A randomised controlled, double-blind study. Lancet. 2006;368:744–53. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 280.Lundborg M, Wille S, Bjermer L, Tilling B, Lundgren M, Telg G, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: An efficacy and cost-effectiveness study. Curr Med Res Opin. 2006;22:809–21. doi: 10.1185/030079906X100212. [DOI] [PubMed] [Google Scholar]
- 281.Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61:725–36. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101:2437–46. doi: 10.1016/j.rmed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 283.Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, et al. SMART Study Group. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: A randomised controlled trial. Lancet Respir Med. 2013;1:32–42. doi: 10.1016/S2213-2600(13)70007-9. [DOI] [PubMed] [Google Scholar]
- 284.Sears MR, Boulet LP, Laviolette M, Fitzgerald JM, Bai TR, Kaplan A, et al. Budesonide/formoterol maintenance and reliever therapy: Impact on airway inflammation in asthma. Eur Respir J. 2008;31:982–9. doi: 10.1183/09031936.00104007. [DOI] [PubMed] [Google Scholar]
- 285.Riemersma RA, Postma D, van der Molen T. Budesonide/formoterol maintenance and reliever therapy in primary care asthma management: Effects on bronchial hyperresponsiveness and asthma control. Prim Care Respir J. 2012;21:50–6. doi: 10.4104/pcrj.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Søes-Petersen U, Kava T, Dahle R, Lei Y, Dam N. Budesonide/formoterol maintenance and reliever therapy versus conventional best standard treatment in asthma in an attempted ‘real life’ setting. Clin Respir J. 2011;5:173–82. doi: 10.1111/j.1752-699X.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 287.Louis R, Joos G, Michils A, Vandenhoven G. A comparison of budesonide/formoterol maintenance and reliever therapy vs. conventional best practice in asthma management. Int J Clin Pract. 2009;63:1479–88. doi: 10.1111/j.1742-1241.2009.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Demoly P, Louis R, Søes-Petersen U, Naya I, Carlsheimer A, Worth H, et al. Budesonide/formoterol maintenance and reliever therapy versus conventional best practice. Respir Med. 2009;103:1623–32. doi: 10.1016/j.rmed.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 289.Zhong N, Lin J, Mehta P, Ngamjanyaporn P, Wu TC, Yunus F. Real-life effectiveness of budesonide/formoterol maintenance and reliever therapy in asthma patients across Asia: SMARTASIA study. BMC Pulm Med. 2013;13:22. doi: 10.1186/1471-2466-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Aubier M, Buhl R, Ekström T, Ostinelli J, van Schayck CP, Selroos O, et al. Comparison of two twice-daily doses of budesonide/formoterol maintenance and reliever therapy. Eur Respir J. 2010;36:524–30. doi: 10.1183/09031936.00022010. [DOI] [PubMed] [Google Scholar]
- 291.Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;4:CD007313. doi: 10.1002/14651858.CD007313.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;12:CD009019. doi: 10.1002/14651858.CD009019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bateman ED, Clark TJ, Frith L, Bousquet J, Busse WW, Pedersen SE Goal Investigators Group. Rate of response of individual asthma control measures varies and may overestimate asthma control: An analysis of the goal study. J Asthma. 2007;44:667–73. doi: 10.1080/02770900701554821. [DOI] [PubMed] [Google Scholar]
- 294.Rogers L, Reibman J. Stepping down asthma treatment: How and when. Curr Opin Pulm Med. 2012;18:70–5. doi: 10.1097/MCP.0b013e32834db017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bacharier LB. Step-down therapy in asthma: A focus on treatment options for patients receiving inhaled corticosteroids and long-acting beta-agonist combination therapy. Allergy Asthma Proc. 2012;33:13–8. doi: 10.2500/aap.2012.33.3493. [DOI] [PubMed] [Google Scholar]
- 296.Papi A, Nicolini G, Crimi N, Fabbri L, Olivieri D, Rossi A, et al. Step-down from high dose fixed combination therapy in asthma patients: A randomized controlled trial. Respir Res. 2012;13:54. doi: 10.1186/1465-9921-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hawkins G, McMahon AD, Twaddle S, Wood SF, Ford I, Thomson NC. Stepping down inhaled corticosteroids in asthma: Randomised controlled trial. BMJ. 2003;326:1115. doi: 10.1136/bmj.326.7399.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hagan JB, Samant SA, Volcheck GW, Li JT, Hagan CR, Erwin PJ, et al. The risk of asthma exacerbation after reducing inhaled corticosteroids: A systematic review and meta-analysis of randomized controlled trials. Allergy. 2014;69:510–6. doi: 10.1111/all.12368. [DOI] [PubMed] [Google Scholar]
- 299.Bateman ED, Jacques L, Goldfrad C, Atienza T, Mihaescu T, Duggan M. Asthma control can be maintained when fluticasone propionate/salmeterol in a single inhaler is stepped down. J Allergy Clin Immunol. 2006;117:563–70. doi: 10.1016/j.jaci.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 300.Godard P, Greillier P, Pigearias B, Nachbaur G, Desfougeres JL, Attali V. Maintaining asthma control in persistent asthma: Comparison of three strategies in a 6-month double-blind randomised study. Respir Med. 2008;102:1124–31. doi: 10.1016/j.rmed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 301.Reddel HK, Gibson PG, Peters MJ, Wark PA, Sand IB, Hoyos CM, et al. Down-titration from high-dose combination therapy in asthma: Removal of long-acting beta (2)-agonist. Respir Med. 2010;104:1110–20. doi: 10.1016/j.rmed.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 302.Koenig SM, Ostrom N, Pearlman D, Waitkus-Edwards K, Yancey S, Prillaman BA, et al. Deterioration in asthma control when subjects receiving fluticasone propionate/salmeterol 100/50 mcg Diskus are “stepped-down”. J Asthma. 2008;45:681–7. doi: 10.1080/02770900802168695. [DOI] [PubMed] [Google Scholar]
- 303.Berger WE, Bleecker ER, O’Dowd L, Miller CJ, Mezzanotte W. Efficacy and safety of budesonide/formoterol pressurized metered-dose inhaler: Randomized controlled trial comparing once- and twice-daily dosing in patients with asthma. Allergy Asthma Proc. 2010;31:49–59. doi: 10.2500/aap.2010.31.3309. [DOI] [PubMed] [Google Scholar]
- 304.Brozek JL, Kraft M, Krishnan JA, Cloutier MM, Lazarus SC, Li JT, et al. Long-acting ß2-agonist step-off in patients with controlled asthma. Arch Intern Med. 2012;172:1365–75. doi: 10.1001/archinternmed.2012.3250. [DOI] [PubMed] [Google Scholar]
- 305.Masoli M, Weatherall M, Holt S, Beasley R. Budesonide once versus twice-daily administration: Meta-analysis. Respirology. 2004;9:528–34. doi: 10.1111/j.1440-1843.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 306.Rank MA, Hagan JB, Park MA, Podjasek JC, Samant SA, Volcheck GW, et al. The risk of asthma exacerbation after stopping low-dose inhaled corticosteroids: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;131:724–9. doi: 10.1016/j.jaci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 307.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 308.Rebuck AS, Pengelly LD. Development of pulsus paradoxus in the presence of airways obstruction. N Engl J Med. 1973;288:66–9. doi: 10.1056/NEJM197301112880203. [DOI] [PubMed] [Google Scholar]
- 309.McFadden ER, Jr, Kiser R, DeGroot WJ. Acute bronchial asthma. Relations between clinical and physiologic manifestations. N Engl J Med. 1973;288:221–5. doi: 10.1056/NEJM197302012880501. [DOI] [PubMed] [Google Scholar]
- 310.Brenner BE, Abraham E, Simon RR. Position and diaphoresis in acute asthma. Am J Med. 1983;74:1005–9. doi: 10.1016/0002-9343(83)90802-1. [DOI] [PubMed] [Google Scholar]
- 311.McFadden ER., Jr Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740–59. doi: 10.1164/rccm.200208-902SO. [DOI] [PubMed] [Google Scholar]
- 312.Tai E, Read J. Blood-gas tensions in bronchial asthma. Lancet. 1967;1:644–6. doi: 10.1016/s0140-6736(67)92541-x. [DOI] [PubMed] [Google Scholar]
- 313.Weng TR, Langer HM, Featherby EA, Levison H. Arterial blood gas tensions and acid-base balance in symptomatic and asymptomatic asthma in childhood. Am Rev Respir Dis. 1970;101:274–82. doi: 10.1164/arrd.1970.101.2.274. [DOI] [PubMed] [Google Scholar]
- 314.Miyamoto T, Mizuno K, Furuya K. Arterial blood gases in bronchial asthma. J Allergy. 1970;45:248–54. doi: 10.1016/0021-8707(70)90135-8. [DOI] [PubMed] [Google Scholar]
- 315.Rees HA, Millar JS, Donald KW. A study of the clinical course and arterial blood gas tensions of patients in status asthmaticus. Q J Med. 1968;37:541–61. [PubMed] [Google Scholar]
- 316.Carruthers DM, Harrison BD. Arterial blood gas analysis or oxygen saturation in the assessment of acute asthma? Thorax. 1995;50:186–8. doi: 10.1136/thx.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jones J, Heiselman D, Cannon L, Gradisek R. Continuous emergency department monitoring of arterial saturation in adult patients with respiratory distress. Ann Emerg Med. 1988;17:463–8. doi: 10.1016/s0196-0644(88)80237-3. [DOI] [PubMed] [Google Scholar]
- 318.Geelhoed GC, Landau LI, Le Souëf PN. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23:1236–41. doi: 10.1016/s0196-0644(94)70347-7. [DOI] [PubMed] [Google Scholar]
- 319.McFadden ER, Jr, Elsanadi N, Dixon L, Takacs M, Deal EC, Boyd KK, et al. Protocol therapy for acute asthma: Therapeutic benefits and cost savings. Am J Med. 1995;99:651–61. doi: 10.1016/s0002-9343(99)80253-8. [DOI] [PubMed] [Google Scholar]
- 320.Strauss L, Hejal R, Galan G, Dixon L, McFadden ER., Jr Observations on the effects of aerosolized albuterol in acute asthma. Am J Respir Crit Care Med. 1997;155:454–8. doi: 10.1164/ajrccm.155.2.9032178. [DOI] [PubMed] [Google Scholar]
- 321.Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O’Byrne PM, et al. Exacerbations of asthma: A descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160:594–9. doi: 10.1164/ajrccm.160.2.9811100. [DOI] [PubMed] [Google Scholar]
- 322.Rodrigo GJ, Rodriquez Verde M, Peregalli V, Rodrigo C. Effects of short-term 28% and 100% oxygen on PaCO2 and peak expiratory flow rate in acute asthma: A randomized trial. Chest. 2003;124:1312–7. doi: 10.1378/chest.124.4.1312. [DOI] [PubMed] [Google Scholar]
- 323.Perrin K, Wijesinghe M, Healy B, Wadsworth K, Bowditch R, Bibby S, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66:937–41. doi: 10.1136/thx.2010.155259. [DOI] [PubMed] [Google Scholar]
- 324.Rodriguez-Roisin R. Acute severe asthma: Pathophysiology and pathobiology of gas exchange abnormalities. Eur Respir J. 1997;10:1359–71. doi: 10.1183/09031936.97.10061359. [DOI] [PubMed] [Google Scholar]
- 325.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: A review. Chest. 2004;125:1081–102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 326.Emerman CL, Cydulka RK, McFadden ER. Comparison of 2.5 vs 7.5 mg of inhaled albuterol in the treatment of acute asthma. Chest. 1999;115:92–6. doi: 10.1378/chest.115.1.92. [DOI] [PubMed] [Google Scholar]
- 327.Rodrigo GJ, Nannini LJ. Comparison between nebulized adrenaline and beta2 agonists for the treatment of acute asthma. A meta-analysis of randomized trials. Am J Emerg Med. 2006;24:217–22. doi: 10.1016/j.ajem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 328.Morley J. Anomalous effects of albuterol and other sympathomimetics in the guinea pig. Clin Rev Allergy Immunol. 1996;14:65–89. doi: 10.1007/BF02772204. [DOI] [PubMed] [Google Scholar]
- 329.Page CP, Morley J. Contrasting properties of albuterol stereoisomers. J Allergy Clin Immunol. 1999;104:S31–41. doi: 10.1016/s0091-6749(99)70271-x. [DOI] [PubMed] [Google Scholar]
- 330.Handley DA, Anderson AJ, Koester J, Snider ME. New millennium bronchodilators for asthma: Single-isomer beta agonists. Curr Opin Pulm Med. 2000;6:43–9. doi: 10.1097/00063198-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 331.Templeton AG, Chapman ID, Chilvers ER, Morley J, Handley DA. Effects of S-salbutamol on human isolated bronchus. Pulm Pharmacol Ther. 1998;11:1–6. doi: 10.1006/pupt.1998.0110. [DOI] [PubMed] [Google Scholar]
- 332.Nowak R, Emerman C, Hanrahan JP, Parsey MV, Hanania NA, Claus R, et al. XOPENEX Acute Severe Asthma Study Group. A comparison of levalbuterol with racemic albuterol in the treatment of acute severe asthma exacerbations in adults. Am J Emerg Med. 2006;24:259–67. doi: 10.1016/j.ajem.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 333.Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26:239–48. doi: 10.1016/j.pupt.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 334.Rodrigo GJ, Neffen H, Colodenco FD, Castro-Rodriguez JA. Formoterol for acute asthma in the emergency department: A systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2010;104:247–52. doi: 10.1016/j.anai.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 335.Adoun M, Frat JP, Doré P, Rouffineau J, Godet C, Robert R. Comparison of nebulized epinephrine and terbutaline in patients with acute severe asthma: A controlled trial. J Crit Care. 2004;19:99–102. doi: 10.1016/j.jcrc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 336.Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest. 1996;110:42–7. doi: 10.1378/chest.110.1.42. [DOI] [PubMed] [Google Scholar]
- 337.Travers AH, Rowe BH, Barker S, Jones A, Camargo CA., Jr The effectiveness of IV beta-agonists in treating patients with acute asthma in the emergency department: A meta-analysis. Chest. 2002;122:1200–7. doi: 10.1378/chest.122.4.1200. [DOI] [PubMed] [Google Scholar]
- 338.Nair P, Milan SJ, Rowe BH. Addition of intravenous aminophylline to inhaled beta (2)-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2012;12:CD002742. doi: 10.1002/14651858.CD002742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052. doi: 10.1002/14651858.CD000052.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Camargo CA, Jr, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD001115. CD001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Fryer AD, Jacoby DB. Effect of inflammatory cell mediators on M2 muscarinic receptors in the lungs. Life Sci. 1993;52:529–36. doi: 10.1016/0024-3205(93)90311-p. [DOI] [PubMed] [Google Scholar]
- 342.Leahy BC, Gomm SA, Allen SC. Comparison of nebulized salbutamol with nebulized ipratropium bromide in acute asthma. Br J Dis Chest. 1983;77:159–63. doi: 10.1016/0007-0971(83)90022-0. [DOI] [PubMed] [Google Scholar]
- 343.Ward MJ, Fentem PH, Smith WH, Davies D. Ipratropium bromide in acute asthma. Br Med J (Clin Res Ed) 1981;282:598–600. doi: 10.1136/bmj.282.6264.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Summers QA, Tarala RA. Nebulized ipratropium in the treatment of acute asthma. Chest. 1990;97:425–9. doi: 10.1378/chest.97.2.425. [DOI] [PubMed] [Google Scholar]
- 345.Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax. 2005;60:740–6. doi: 10.1136/thx.2005.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Salo D, Tuel M, Lavery RF, Reischel U, Lebowitz J, Moore T. A randomized, clinical trial comparing the efficacy of continuous nebulized albuterol (15 mg) versus continuous nebulized albuterol (15 mg) plus ipratropium bromide (2 mg) for the treatment of acute asthma. J Emerg Med. 2006;31:371–6. doi: 10.1016/j.jemermed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 347.Aggarwal P, Singh O, Wali JP, Handa R, Dwivedi SN, Biswas A, et al. Efficacy of nebulized ipratropium in acute bronchial asthma. J Indian Acad Clin Med. 2002;3:353–9. [Google Scholar]
- 348.Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000195.pub2. CD000195. [DOI] [PubMed] [Google Scholar]
- 349.Sherman MS, Verceles AC, Lang D. Systemic steroids for the treatment of acute asthma: Where do we stand? Clin Pulm Med. 2006;13:315–20. [Google Scholar]
- 350.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD002178. CD002178. [DOI] [PubMed] [Google Scholar]
- 351.Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001740. CD001740. [DOI] [PubMed] [Google Scholar]
- 352.Hasegawa T, Ishihara K, Takakura S, Fujii H, Nishimura T, Okazaki M, et al. Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Intern Med. 2000;39:794–7. doi: 10.2169/internalmedicine.39.794. [DOI] [PubMed] [Google Scholar]
- 353.Jones AM, Munavvar M, Vail A, Aldridge RE, Hopkinson L, Rayner C, et al. Prospective, placebo-controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med. 2002;96:950–4. doi: 10.1053/rmed.2002.1369. [DOI] [PubMed] [Google Scholar]
- 354.O’Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993;341:324–7. doi: 10.1016/0140-6736(93)90134-3. [DOI] [PubMed] [Google Scholar]
- 355.Lederle FA, Pluhar RE, Joseph AM, Niewoehner DE. Tapering of corticosteroid therapy following exacerbation of asthma. A randomized, double-blind, placebo-controlled trial. Arch Intern Med. 1987;147:2201–3. [PubMed] [Google Scholar]
- 356.Edmonds ML, Milan SJ, Camargo CA, Jr, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD002308. doi: 10.1002/14651858.CD002308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med. 2005;171:1231–6. doi: 10.1164/rccm.200410-1415OC. [DOI] [PubMed] [Google Scholar]
- 358.Edmonds ML, Milan SJ, Brenner BE, Camargo CA, Jr, Rowe BH. Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database Syst Rev. 2012;12:CD002316. doi: 10.1002/14651858.CD002316.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Rowe BH, Camargo CA., Jr The role of magnesium sulfate in the acute and chronic management of asthma. Curr Opin Pulm Med. 2008;14:70–6. doi: 10.1097/MCP.0b013e3282f19867. [DOI] [PubMed] [Google Scholar]
- 360.Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J, et al. AcuteAsthma/Magnesium Study Group. IV magnesium sulfate in the treatment of acute severe asthma: A multicenter randomized controlled trial. Chest. 2002;122:489–97. doi: 10.1378/chest.122.2.489. [DOI] [PubMed] [Google Scholar]
- 361.Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA., Jr Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001490. CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T. 3Mg Research Team. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): A double-blind, randomised controlled trial. Lancet Respir Med. 2013;1:293–300. doi: 10.1016/S2213-2600(13)70070-5. [DOI] [PubMed] [Google Scholar]
- 363.Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp-Sihota JA, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898. doi: 10.1002/14651858.CD003898.pub5. [DOI] [PubMed] [Google Scholar]
- 364.Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: Correlation with airflow limitation. Thorax. 2004;59:100–4. doi: 10.1136/thorax.2003.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Cýllý A, Kara A, Ozdemir T, Oðüþ C, Gülkesen KH. Effects of oral montelukast on airway function in acute asthma. Respir Med. 2003;97:533–6. doi: 10.1053/rmed.2003.1479. [DOI] [PubMed] [Google Scholar]
- 366.Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: A randomised, double-blind, placebo-controlled trial. Thorax. 2011;66:7–11. doi: 10.1136/thx.2010.135038. [DOI] [PubMed] [Google Scholar]
- 367.Camargo CA, Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003;167:528–33. doi: 10.1164/rccm.200208-802OC. [DOI] [PubMed] [Google Scholar]
- 368.Camargo CA, Jr, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, et al. A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol. 2010;125:374–80. doi: 10.1016/j.jaci.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 369.Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, et al. The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. J Asthma. 2012;49:649–56. doi: 10.3109/02770903.2012.690479. [DOI] [PubMed] [Google Scholar]
- 370.Graham V, Lasserson T, Rowe BH. Antibiotics for acute asthma. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD002741. CD002741. [DOI] [PubMed] [Google Scholar]
- 371.Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, et al. Pneumonia Guidelines Working Group. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: Joint ICS/NCCP (I) recommendations. Lung India. 2012;29(Suppl 2):S27–62. doi: 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55:536–43. [PubMed] [Google Scholar]
- 373.Lim WJ, Mohammed Akram R, Carson KV, Mysore S, Labiszewski NA, Wedzicha JA, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360. doi: 10.1002/14651858.CD004360.pub4. [DOI] [PubMed] [Google Scholar]
- 374.Manthous CA, Morgan S, Pohlman A. Heliox in the treatment of airflow obstruction: A critical review of the literature. Respir Care. 1997;42:1034–42. [Google Scholar]
- 375.Rodrigo GJ, Rodrigo C, Pollack CV, Rowe B. Use of helium-oxygen mixtures in the treatment of acute asthma: A systematic review. Chest. 2003;123:891–6. doi: 10.1378/chest.123.3.891. [DOI] [PubMed] [Google Scholar]
- 376.Rodrigo GJ, Castro-Rodriguez JA. Heliox-driven ß2-agonists nebulization for children and adults with acute asthma: A systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;112:29–34. doi: 10.1016/j.anai.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 377.Celano M, Geller RJ, Phillips KM, Ziman R. Treatment adherence among low-income children with asthma. J Pediatr Psychol. 1998;23:345–9. doi: 10.1093/jpepsy/23.6.345. [DOI] [PubMed] [Google Scholar]
- 378.Cerveri I, Locatelli F, Zoia MC, Corsico A, Accordini S, de Marco R. International variations in asthma treatment compliance: The results of the European Community Respiratory Health Survey (ECRHS) Eur Respir J. 1999;14:288–94. doi: 10.1034/j.1399-3003.1999.14b09.x. [DOI] [PubMed] [Google Scholar]
- 379.Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–8. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 380.Tano BD. All that wheezes is not asthma: Misclassification, comorbidities and treatment outcomes in acute asthma exacerbations. J Allergy Clin Immunol. 2008;121(Suppl 1):S162. [Google Scholar]
- 381.Koul PA, Khan UH, Shah TH, Dar AM. All that wheezes is not asthma. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2013-202369. pii: bcr2013202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.McSharry DG, McElwaine P, Segadal L, McNicholas WT. All that wheezes is not asthma. Lancet. 2007;370:800. doi: 10.1016/S0140-6736(07)61384-1. [DOI] [PubMed] [Google Scholar]
- 383.Koul PA, Wahid A, Bhat TA, Hussain T. Whistle in the bronchus. Ann Thorac Med. 2007;2:124–5. doi: 10.4103/1817-1737.33702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.De S, De S. Post intubation tracheal stenosis. Indian J Crit Care Med. 2008;12:194–7. doi: 10.4103/0972-5229.45081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:936–44. [PubMed] [Google Scholar]
- 386.Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57:226–30. doi: 10.1136/thorax.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med. 2003;168:1308–11. doi: 10.1164/rccm.200304-503OC. [DOI] [PubMed] [Google Scholar]
- 388.Guven SF, Dursun AB, Ciftci B, Erkekol FO, Kurt OK. The prevalence of obstructive sleep apnea in patients with difficult-to-treat asthma. Asian Pac J Allergy Immunol. 2014;32:153–9. doi: 10.12932/AP0360.32.2.2013. [DOI] [PubMed] [Google Scholar]
- 389.Chan WW, Chiou E, Obstein KL, Tignor AS, Whitlock TL. The efficacy of proton pump inhibitors for the treatment of asthma in adults: A meta-analysis. Arch Intern Med. 2011;171:620–9. doi: 10.1001/archinternmed.2011.116. [DOI] [PubMed] [Google Scholar]
- 390.Eneli IU, Skybo T, Camargo CA., Jr Weight loss and asthma: A systematic review. Thorax. 2008;63:671–6. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 391.Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: A meta-analysis. Allergy. 2013;68:569–79. doi: 10.1111/all.12124. [DOI] [PubMed] [Google Scholar]
- 392.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: A systematic review. Chest. 2011;139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 393.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559. doi: 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–8. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 395.Kupry-Lipiñska I, Kuna P. Loss of asthma control after cessation of omalizumab treatment: Real life data? Postepy Dermatol Alergol. 2014;31:1–5. doi: 10.5114/pdia.2014.40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: A multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–24. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–37. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 398.Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–91. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 399.Iyer VN, Lim KG. Bronchial thermoplasty: Reappraising the evidence (or lack thereof) Chest. 2014;146:17–21. doi: 10.1378/chest.14-0536. [DOI] [PubMed] [Google Scholar]
- 400.Torrego A, Solà I, Munoz AM, Roqué I, Figlus M, Yepes-Nuñez JJ, Alonso-Coello P, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014;3:CD009910. doi: 10.1002/14651858.CD009910.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001186.pub2. CD001186. [DOI] [PubMed] [Google Scholar]
- 402.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 403.Kemp JP, Cook DA, Incaudo GA, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Salmeterol Quality of Life Study Group. J Allergy Clin Immunol. 1998;101:188–95. doi: 10.1016/s0091-6749(98)70383-5. [DOI] [PubMed] [Google Scholar]
- 404.Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: Looking at the published evidence. J Allergy Clin Immunol. 2012;129:929–34. doi: 10.1016/j.jaci.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 405.Srivastava D, Singh BP, Arora N, Gaur SN. Clinico-immunologic study on immunotherapy with mixed and single insect allergens. J Clin Immunol. 2009;29:665–73. doi: 10.1007/s10875-009-9307-7. [DOI] [PubMed] [Google Scholar]
- 406.Calamita Z, Saconato H, Pelá AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: Systematic review of randomized-clinical trials using the cochrane collaboration method. Allergy. 2006;61:1162–72. doi: 10.1111/j.1398-9995.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- 407.Amar SM, Harbeck RJ, Sills M, Silveira LJ, O’Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: Monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009;124:150–6. doi: 10.1016/j.jaci.2009.04.037. e-5. [DOI] [PubMed] [Google Scholar]
- 408.Borchers AT, Keen CL, Gershwin ME. Fatalities following allergen immunotherapy. Clin Rev Allergy Immunol. 2004;27:147–58. doi: 10.1385/CRIAI:27:2:147. [DOI] [PubMed] [Google Scholar]
- 409.Nettis E, Giordano D, Ferrannini A, Tursi A. Systemic reactions to allergen immunotherapy: A review of the literature. Immunopharmacol Immunotoxicol. 2003;25:1–11. doi: 10.1081/iph-120018279. [DOI] [PubMed] [Google Scholar]
- 410.Gibson PG, Powell H, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD001005. CD001005. [DOI] [PubMed] [Google Scholar]
- 411.Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD001117. CD001117. [DOI] [PubMed] [Google Scholar]
- 412.Bindroo SK, Kumar R, Gaur SN. Effect of home-based pulmonary rehabilitation programme on disability in patients with persistent bronchial asthma. Indian J Allergy Asthma Immunol. 2002;18:63–71. [Google Scholar]
- 413.Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;9:CD001116. doi: 10.1002/14651858.CD001116.pub4. [DOI] [PubMed] [Google Scholar]
- 414.Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev. 2013;2:CD000364. doi: 10.1002/14651858.CD000364.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Sheikh A, Alves B, Dhami S. Pneumococcal vaccine for asthma. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002165. CD002165. [DOI] [PubMed] [Google Scholar]
- 416.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): A multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–9. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 417.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: Estimates from national health surveys. Ann Epidemiol. 2003;13:317–24. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 418.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: A prospective analysis. J Allergy Clin Immunol. 1988;81:509–17. [PubMed] [Google Scholar]
- 419.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158:1091–5. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 420.Källén B, Rydhstroem H, Aberg A. Asthma during pregnancy--a population based study. Eur J Epidemiol. 2000;16:167–71. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- 421.Schatz M, Zeiger RS, Hoffman CP, Harden K, Forsythe A, Chilingar L, et al. Perinatal outcomes in the pregnancies of asthmatic women: A prospective controlled analysis. Am J Respir Crit Care Med. 1995;151:1170–4. doi: 10.1164/ajrccm/151.4.1170. [DOI] [PubMed] [Google Scholar]
- 422.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: Incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tata LJ, Lewis SA, McKeever TM, Smith CJ, Doyle P, Smeeth L, et al. Effect of maternal asthma, exacerbations and asthma medication use on congenital malformations in offspring: A UK population-based study. Thorax. 2008;63:981–7. doi: 10.1136/thx.2008.098244. [DOI] [PubMed] [Google Scholar]
- 424.Schatz M, Zeiger RS, Harden K, Hoffman CC, Chilingar L, Petitti D. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100:301–6. doi: 10.1016/s0091-6749(97)70241-0. [DOI] [PubMed] [Google Scholar]
- 425.Bakhireva LN, Jones KL, Schatz M, Klonoff-Cohen HS, Johnson D, Slymen DJ, et al. Organization of Teratology Information Specialists Collaborative Research Group. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007;119:618–25. doi: 10.1016/j.jaci.2006.12.618. [DOI] [PubMed] [Google Scholar]
- 426.Sarkar M, Koren G, Kalra S, Ying A, Smorlesi C, De Santis M, et al. Montelukast use during pregnancy: A multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol. 2009;65:1259–64. doi: 10.1007/s00228-009-0713-9. [DOI] [PubMed] [Google Scholar]
- 427.Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP. Asthma symptoms, severity, and drug therapy: A prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102:739–52. doi: 10.1016/s0029-7844(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 428.Working Group Report on Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment. National Asthma Education and Prevention Program, National Heart, Lung, and Blood Institute. [Last accessed on 2004 Mar 05]. Available form: https://www.nhlbi.nih.gov/files/docs/resources/lung/astpreg_full.pdf .
- 429.Perlow JH, Montgomery D, Morgan MA, Towers CV, Porto M. Severity of asthma and perinatal outcome. Am J Obstet Gynecol. 1992;167:963–7. doi: 10.1016/s0002-9378(12)80020-2. [DOI] [PubMed] [Google Scholar]
- 430.American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–89. [PubMed] [Google Scholar]
- 431.Dryden DM, Spooner CH, Stickland MK, Vandermeer B, Tjosvold L, Bialy L, et al. Exercise-induced bronchoconstriction and asthma. Evid Rep Technol Assess (Full Rep) 2010:1–154. v-vi. [PMC free article] [PubMed] [Google Scholar]
- 432.Godfrey S, Springer C, Bar-Yishay E, Avital A. Cut-off points defining normal and asthmatic bronchial reactivity to exercise and inhalation challenges in children and young adults. Eur Respir J. 1999;14:659–68. doi: 10.1034/j.1399-3003.1999.14c28.x. [DOI] [PubMed] [Google Scholar]
- 433.Bonini M, Di Mambro C, Calderon MA, Compalati E, Schünemann H, Durham S, et al. Beta2-agonists for exercise-induced asthma. Cochrane Database Syst Rev. 2013;10:CD003564. doi: 10.1002/14651858.CD003564.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. American Thoracic Society Subcommittee on Exercise-induced Bronchoconstriction. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187:1016–27. doi: 10.1164/rccm.201303-0437ST. [DOI] [PubMed] [Google Scholar]
- 435.Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000;118:1470–6. doi: 10.1378/chest.118.5.1470. [DOI] [PubMed] [Google Scholar]
- 436.Stevenson DD. Oral challenges to detect aspirin and sulfite sensitivity in asthma. N Engl Reg Allergy Proc. 1988;9:135–42. doi: 10.2500/108854188778995020. [DOI] [PubMed] [Google Scholar]
- 437.Simon RA. Oral challenges to detect aspirin and sulfite sensitivity in asthma. Allerg Immunol (Paris) 1994;26:216–8. [PubMed] [Google Scholar]
- 438.Bush RK, Zoratti E, Taylor SL. Diagnosis of sulfite and aspirin sensitivity. Clin Rev Allergy. 1990;8:159–78. doi: 10.1007/BF02914443. [DOI] [PubMed] [Google Scholar]
- 439.Nizankowska E, Bestyñska-Krypel A, Cmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J. 2000;15:863–9. doi: 10.1034/j.1399-3003.2000.15e09.x. [DOI] [PubMed] [Google Scholar]
- 440.Milewski M, Mastalerz L, Nizankowska E, Szczeklik A. Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol. 1998;101:581–6. doi: 10.1016/S0091-6749(98)70163-0. [DOI] [PubMed] [Google Scholar]
- 441.Dahlén B, Szczeklik A, Murray JJ Celecoxib in Aspirin-Intolerant Asthma Study Group. Celecoxib in patients with asthma and aspirin intolerance. The Celecoxib in Aspirin-Intolerant Asthma Study Group. N Engl J Med. 2001;344:142. doi: 10.1056/NEJM200101113440215. [DOI] [PubMed] [Google Scholar]
- 442.El Miedany Y, Youssef S, Ahmed I, El Gaafary M. Safety of etoricoxib, a specific cyclooxygenase-2 inhibitor, in asthmatic patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2006;97:105–9. doi: 10.1016/S1081-1206(10)61378-6. [DOI] [PubMed] [Google Scholar]
- 443.Stevenson DD, Simon RA. Lack of cross-reactivity between rofecoxib and aspirin in aspirin-sensitive patients with asthma. J Allergy Clin Immunol. 2001;108:47–51. doi: 10.1067/mai.2001.116290. [DOI] [PubMed] [Google Scholar]
- 444.Woessner KM, Simon RA, Stevenson DD. Safety of high-dose rofecoxib in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2004;93:339–44. doi: 10.1016/S1081-1206(10)61392-0. [DOI] [PubMed] [Google Scholar]
- 445.Settipane RA, Schrank PJ, Simon RA, Mathison DA, Christiansen SC, Stevenson DD. Prevalence of cross-sensitivity with acetaminophen in aspirin-sensitive asthmatic subjects. J Allergy Clin Immunol. 1995;96:480–5. doi: 10.1016/s0091-6749(95)70290-3. [DOI] [PubMed] [Google Scholar]
- 446.Kowalski ML. Management of aspirin-sensitive rhinosinusitis-asthma syndrome: What role for aspirin desensitization? Allergy Proc. 1992;13:175–84. doi: 10.2500/108854192778817202. [DOI] [PubMed] [Google Scholar]
- 447.Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: Long-term outcomes. J Allergy Clin Immunol. 1996;98:751–8. doi: 10.1016/s0091-6749(96)70123-9. [DOI] [PubMed] [Google Scholar]
- 448.Dahlén SE, Malmström K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:9–14. doi: 10.1164/ajrccm.165.1.2010080. [DOI] [PubMed] [Google Scholar]
- 449.de Groene GJ, Pal TM, Beach J, Tarlo SM, Spreeuwers D, Frings-Dresen MH, et al. Workplace interventions for treatment of occupational asthma. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006308.pub3. CD006308. [DOI] [PubMed] [Google Scholar]
- 450.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361–70. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 451.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850–73. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 452.Patterson R, Greenberger PA, Halwig JM, Liotta JL, Roberts M. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986;146:916–8. doi: 10.1001/archinte.146.5.916. [DOI] [PubMed] [Google Scholar]
- 453.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–8. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]


