Abstract
Women are especially predisposed to development of arterial stiffening secondary to obesity due to consumption of excessive calories. Enhanced activation of vascular mineralocorticoid receptors impairs insulin signaling, induces oxidative stress, inflammation and maladaptive immune responses. We tested whether a sub-pressor dose of mineralocorticoid receptor antagonist, spironolactone (1 mg•kg−1•day−1) prevents aortic and femoral artery stiffening in female C57BL/6J mice fed a high fat/high sugar western diet (WD) for four months (i.e., from 4–20 weeks of age). Aortic and femoral artery stiffness were assessed using ultrasound, pressurized vessel preparations and atomic force microscopy. WD induced weight gain and insulin resistance compared to control diet-fed mice and these abnormalities were unaffected by spironolactone. Blood pressures and heart rates were normal and unaffected by diet or spironolactone. Spironolactone prevented WD-induced stiffening of aorta and femoral artery as well as endothelial and vascular smooth muscle cells within aortic explants. Spironolactone prevented WD-induced impaired aortic protein kinase B/endothelial nitric oxide synthase signaling, as well as, impaired endothelium-dependent and –independent vasodilation. Spironolactone ameliorated WD-induced aortic medial thickening and fibrosis and the associated activation of the pro-growth extracellular receptor kinase 1/2 pathway. Finally, preservation of normal arterial stiffness with spironolactone in WD-fed mice was associated with attenuated systemic and vascular inflammation and an anti-inflammatory shift in vascular immune cell marker genes. Low-dose spironolactone may represent a novel prevention strategy to attenuate vascular inflammation, oxidative stress, and growth pathway signaling and remodeling to prevent development of arterial stiffening secondary to consumption of a WD.
Keywords: Aldosterone, spironolactone, vascular stiffness, obesity
INTRODUCTION
In the setting of obesity and/or diabetes women exhibit significant cardiovascular disease (CVD), more frequently and with higher severity than diabetic men.1, 2 This is in contrast to lean non-diabetic premenopausal women that have lower incidences of CVD relative to similar men. Increased aortic stiffness independently predicts future CVD, especially in women.3, 4 Aortic stiffening, which is a normal aging phenomenon, is inordinately deleterious in postmenopausal women compared to men.5, 6 Moreover, women struggling from obesity have elevated arterial stiffness and are more vulnerable to CVD compared to men.7–9 Epidemiological studies reveal that measurement of aortic stiffness has a superior predictive value for determining cardiovascular (CV) risk compared to classical CV risk factors and, as such, has emerged as an important “biomarker” predictive of end organ damage and overall CVD risk.3, 4 Given the ongoing epidemic of obesity, targeting arterial stiffness to reduce the risk for a first CV event10, 11 with pharmacological or lifestyle interventions is urgently needed, especially for women. Importantly, few studies address the impact of important environmental factors, such as diet, in the genesis of Cardiovascular Disease (CVD) in females. This has led to a call by the NIH and the American Heart Association to fill this void with more studies on CVD in females.12
Emerging evidence supports the notion that over-activation of vascular mineralocorticoid receptors (MRs) contribute to CVD.13–15 The gender related differences in CVD are due, in part, to steroid hormones.16 Increasing evidence of a role for aldosterone and MR signaling in development of arterial stiffness has emanated from multiple studies.17–20 A recent clinical study reported an association between elevated serum aldosterone concentrations and increased aortic stiffening in normotensive overweight and obese adults aged 20 to 45 years.21 Moreover, accumulating evidence suggests improvement in arterial elasticity occurs with administration of MR antagonists (MRA).22–24
Obesity-related vascular insulin resistance promotes endothelial cell (EC) dysfunction as a consequence of impaired insulin-mediated activation of endothelial nitric oxide synthase (eNOS) to reduce bioavailability of nitric oxide (NO).25 In obesity, impaired EC-mediated vascular relaxation could also result from MR signaling-mediated increased generation of reactive oxygen species (ROS) with subsequent NO scavenging and reduction in bioavailable NO, which normally reduces extracellular matrix (ECM) remodeling and stiffening.14, 15 Additionally, abnormal immune and inflammatory responses contribute to vascular dysfunction and stiffness.21 ECM remodeling underlies age-related aortic stiffening.10, 26
We recently reported that low dose spironolactone administered to female mice prevents western diet (WD)-induced development of myocardial stiffness and diastolic dysfunction.27 That study demonstrated an important role for the MR in promoting myocardial oxidative stress, fibrosis and impaired immunity associated with decreased left ventricular compliance resulting from consumption of a WD. The idea that an increase in vascular stiffness secondary to consumption of a WD can be mediated by excessive MR signaling is intriguing and raises the question whether utilizing a low (sub-pressor) dose of an MRA could improve compliance in conduit and muscular arteries (e.g., aorta and femoral artery). To test this hypothesis, we administered a low dose of the MRA, spironolactone (Sp) to female C57BL/6J mice fed a WD high in fat and the refined sugars, sucrose and high fructose corn syrup (HFCS). Herein, we report that vascular MR signaling plays an important role in inflammatory and immune responses and development of aortic and femoral artery stiffening secondary to consumption of a WD. Importantly, the development of these CV abnormalities can be prevented with a low dose of Sp.
METHODS
For detailed description of procedures, see Methods section in the online-only Data Supplement.
RESULTS
WD-induced increases in body and fat pad weights and systemic insulin resistance were not prevented by Sp
Body weights of 20-week old WDC and WDSp mice were similarly heavier compared to their lean counterparts (Table S1 in the online-only Data supplement). Percent body weight gain at the end of the study period was 71±8% and 97±12% for CDC and WDC (P=0.06), respectively, and 74±7% and 98±7% for CDSp and WDSp (P<0.05), respectively and weight gain was unaffected by Sp. Peri-reproductive and retroperitoneal fat pad masses were approximately 3- and 2-fold higher in WDC vs CDC (P<0.01), respectively and these changes were not altered by Sp (data not shown). Systemic glucose homeostasis, evaluated by intraperitoneal glucose tolerance, was impaired after WD feeding. The area under the curve (AUC) of the glycemic excursion after the intraperitoneal glucose challenge was increased in WD-fed mice versus CD-fed mice (P< 0.05; Figure S1). The AUC was unaffected by Sp in WD-fed mice.
Sp Prevents WD-induced Increases in Aortic PWV
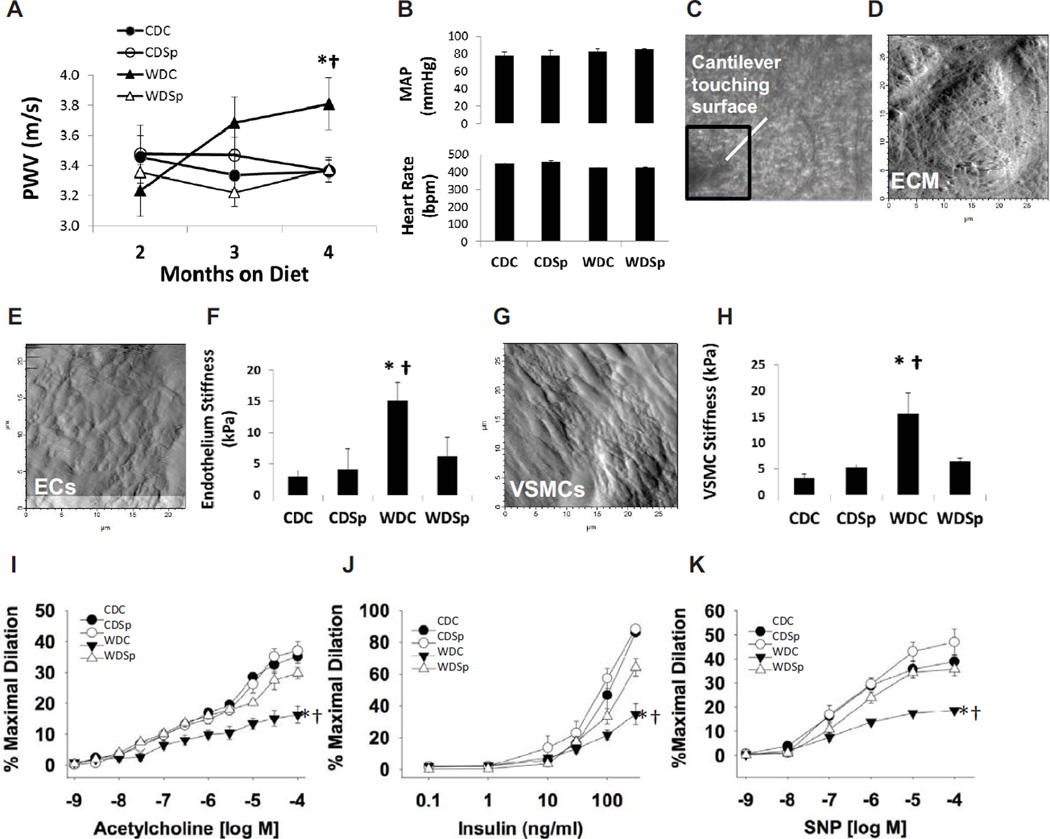
In vivo PWV, determined in mice following 2 and 3 months on CD or WD (Figure 1A and Table S2) was unaffected by diet or Sp; however, both WD and Sp affected PWV significantly at the 4 month time point. PWV was elevated in the WDC group compared to CDC (P=0.006) and Sp prevented the elevation in PWV (P<0.001). Mean arterial pressure (MAP) and heart rate was not different among groups at the end of the study (Figure 1B).
Figure 1.
Impaired aortic stiffness and aortic endothelial and smooth muscle cell function in untreated western diet-fed mice (WDC) are improved by MR antagonism (WDSp). (A) Aortic pulse wave velocity (PWV) measured after 2, 3 and 4 months on experimental diets. Values are mean ± SE; N=6–10 per group. (B) Mean (± SE) arterial pressure (n = 3–6/group) and heart rate (n = 6–10/group) at the end of the four month feeding trial did not differ among groups. The micrograph in panel (C) shows the AFM cantilever positioned over the surface of an aortic explant. Panel (D) shows the surface of the extracellular matrix (ECM) after removal of the surrounding adventitia. Panels (E) and (G) show the endothelial cell (EC) and VSMC surfaces, respectively, of intact aortic explants. The bar graphs in panels (F) and (H) demonstrate elevated stiffness in EC and VSMC of WD fed mice and improvement with Sp (n=4–5per group). Vasodilator responses of isolated aortic rings to the endothelium-dependent dilators acetylcholine (I) and insulin (J) and to the endothelium independent vasodilator, sodium nitropruside (SNP) (K). Values are mean ± SE; n=3–6 per group. Control Diet Control (CDC), Control Diet Sp (CDS), Western Diet Control (WDC), and Western Diet Sp (WDSp). Post-hoc comparisons within a time point; *P<0.05 CDC vs WDC; † P<0.05 WDC vs WDSp.
Sp Prevents WD-induced Increases in Aortic EC and Vascular Smooth Muscle Cell (VSMC) stiffness
To determine whether intact EC and VSMC stiffened in response to WD, we measured surface mechanical stiffness of EC and VSMC in aortic explants utilizing atomic force microscopy (AFM) (Figure 1C–H). Both EC and VSMC exhibited an approximately five-fold increase in surface stiffness (P<0.05 CDC vs WDC) and these effects were prevented by Sp administration (P<0.05 for WDC vs WDSp and P>0.05 for CDC vs WDSp).
Sp Prevents WD-Induced Endothelial Dysfunction in the Aorta
Endothelium-dependent vasodilatory responses to acetylcholine (ACh) were decreased in WDC compared to CDC (Emax = 16.3 ± 2.7% n=4 vs. 35.1 ± 2.2% n=6 and 4, respectively), and these defects were prevented in the WDSp group (Emax = 29.8 ± 1.8% n=4) (Figure 1I). Similarly, endothelium-dependent vasodilatory responses to insulin were also impaired in WDC mice (Emax = 34.8 ± 6.5% vs. 86.1 ± 1.7% n=6 and 4, respectively), and improved in the WDSp group (Emax = 64.2 ± 5.4% n=4) (Figure 1J). Endothelium-independent vasodilatory responses to sodium nitroprusside (SNP) were also decreased in WDC compared to CDC (Emax = 18.7 ± 0.7% n=4 vs. 38.9 ± 2.7% n=6 and 4, respectively), and these defects were abolished in the WDSp group (Emax = 35.7 ± 2.6% n=4) (Figure 1K). Collectively, these data are consistent with improved aortic EC and VSMC function in WDSp animals.
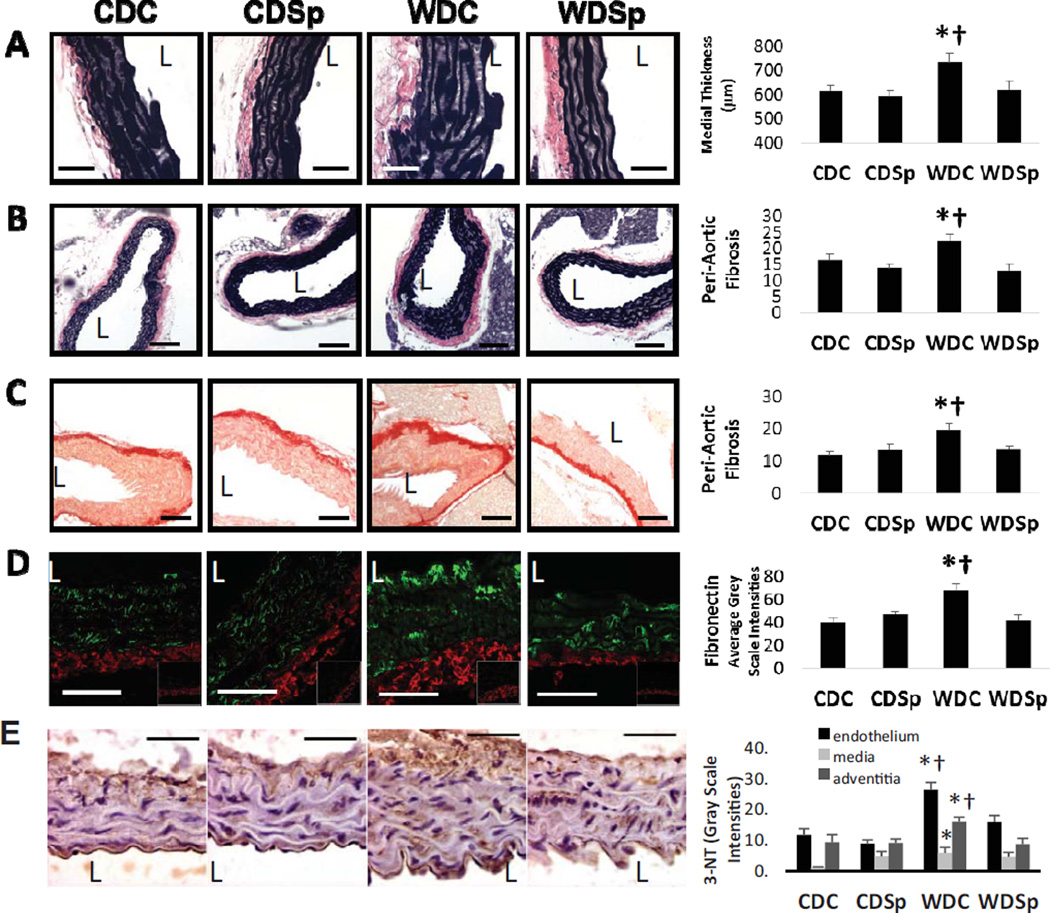
Sp Ameliorates WD-induced Aortic Remodeling
The medial layer of the aorta was 18% thicker in WD-fed mice compared to CD-fed mice (P<0.01) and thickening was prevented by Sp treatment (Figure 2A; P<0.05 WDC vs WDSp). Adventitial collagen accumulation was significantly enhanced in WD relative to CD-fed mice and this accumulation was prevented by Sp treatment (Figure 2B–C). Fibronectin accumulated predominately in the adventitia in all groups; however, compared to CD, WD induced an increase in adventitial fibronectin and Sp prevented this abnormality (Figure 2D).
Figure 2.
Aortic remodeling in untreated western diet-fed mice (WDC) mice is prevented by MR antagonism (WDSp). (A) Representative micrographs show medial wall thickening, peri-aortic fibrosis by (B) VVG and (C) picrosirius red staining and (D) adventitial accumulation of fibronectin. (E) Immunostaining analysis for 3-nitrotyrosine staining. Abbreviations and symbols are the same as in Figure 2 legend. The vessel lumen is indicated by the letter L. Values are mean ± SE; n=5 per group.
WD-Induced Vascular Oxidative Stress Was Improved by Sp
WD induced increases in oxidant stress, assessed by 3-NTY staining, in each layer of the aorta compared to CDC (P<0.05 for each layer) (Figure 2E). Staining was most intense in the endothelium, modest in the adventitia and relatively minimal in the medial layer. Sp largely prevented 3-NTY accumulation in the endothelium and adventitia (P<0.05 WDC vs WDSp for both layers).
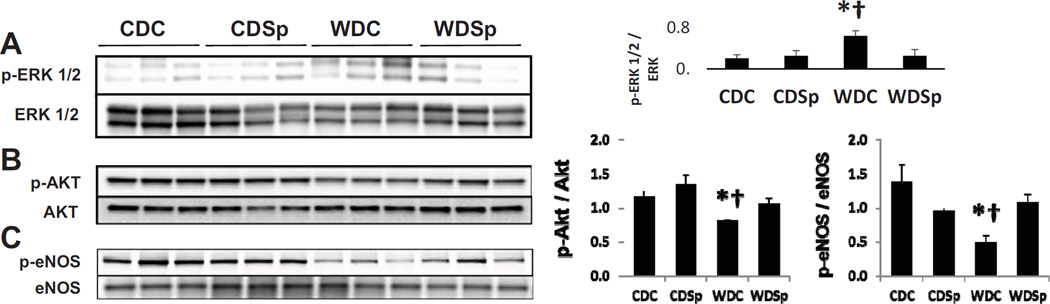
Sp Prevented WD-induced Activation of ERK1/2 and Impairment in protein kinase B (Akt)/eNOS in the aorta
WD induced a 3-fold increase in ERK1/2 activation (P<0.05 CDC vs WDC) that was prevented by Sp (P<0.05 WDC vs WDSp; Figure 3A). WD induced decreases in the phosphorylation (p) of both p-AktSer473 and p-eNOSSer1177 in aorta protein extracts compared to those from CDC mice (p<0.05 for both proteins) (Figure 3B, C). Sp preserved the normal activation levels in both p-AktSer473 and p-eNOSSer1177 protein compared with WDC group (p<0.05 for each protein).
Figure 3.
Immunoblot analysis of (A) activation of ERK1/2, (B) p-Akt and (C) eNOS expression relative to total Akt and eNOS expression, respectively. Abbreviations and symbols are the same as in Figure 2. Values are mean ± SE; n=3 per group.
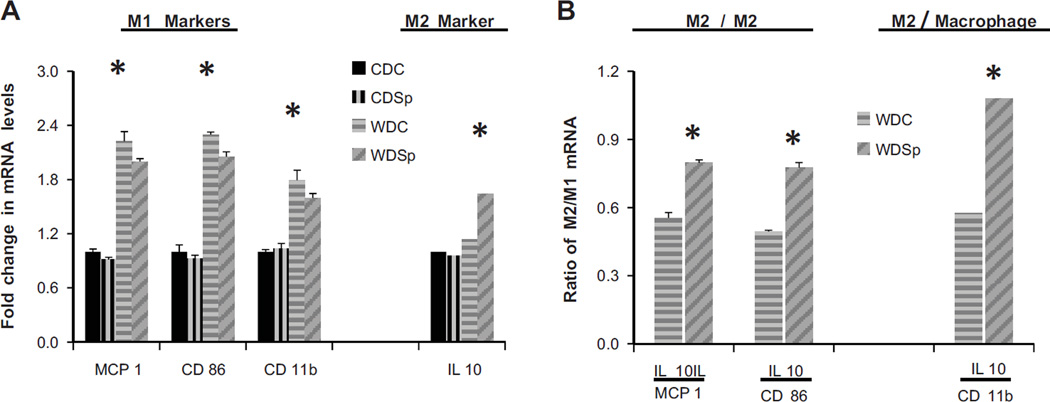
Sp Ameliorates Immune and Inflammatory Changes in the Aorta
Inflammatory markers
Levels of the pro-inflammatory M1 marker transcripts, MCP-1 and CD 86, were elevated in aortic extracts of WDC and WDSp versus CDC mice (Figure 4A). The transcript level of interleukin (IL) 10, an anti-inflammatory M2 marker, was elevated only in WDSp. The ratios of M2 to M1 expression illustrates that there was a relative increase in macrophage polarization favoring M2 in the WDSp group compared to WDC (Figure 4B). Additionally, the aorta of WDC mice exhibited significantly elevated levels of the macrophage marker, CD 11b which was not affected by Sp. Nonetheless, there was a relative increase in macrophage polarization favoring M2 in the WDSp group compared to WDC as indicated by the significantly higher IL 10/CD 11b ratio (Figure 4B).
Figure 4.
Effect of Sp on macrophage polarization. (A) mRNA expression of M1 markers MCP-1 and CD 86, as well as the total macrophage cell marker, CD11b, in aorta. (B) mRNA expression of the M2 marker, IL10, and the ratios of M2/M1 and M2/total macrophage expression in aorta. Values are mean ± SE; n=3 per group; p<0.05.
ACh-induced Vasodilation and Arterial Structure in Femoral Arteries are Significantly Improved by Sp
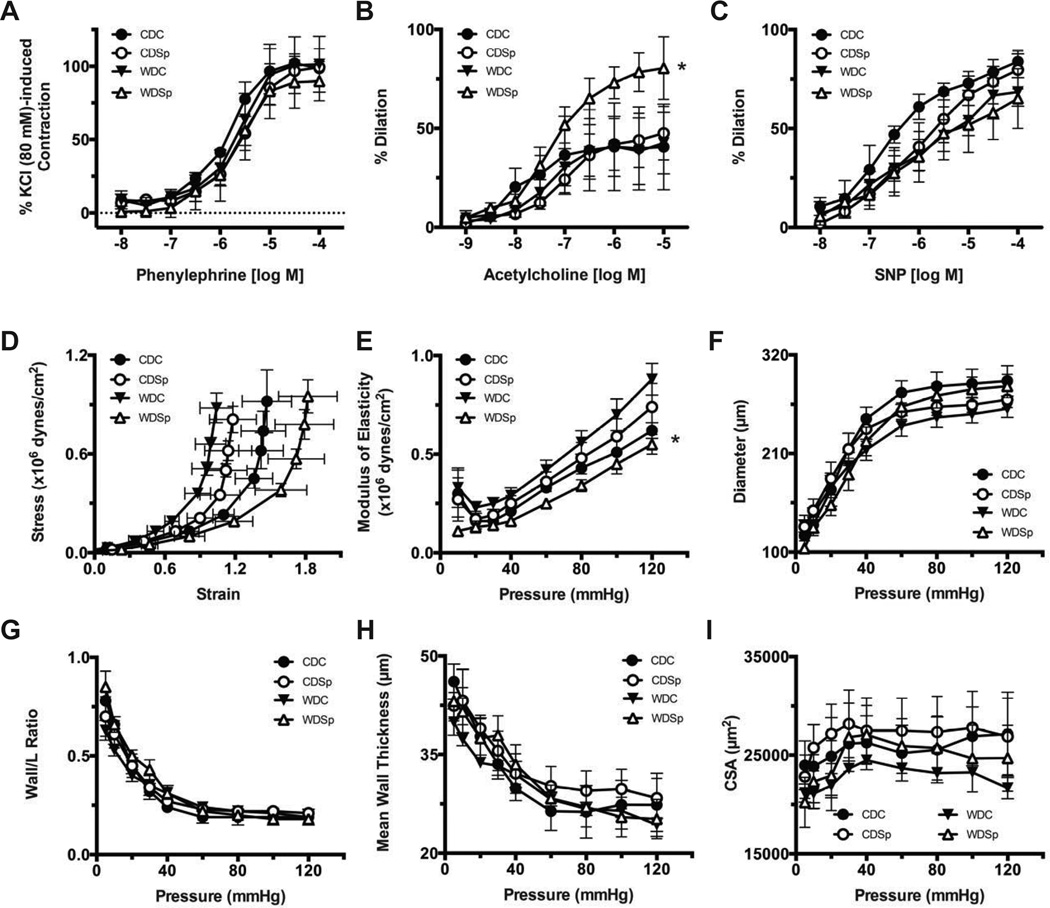
No significant effects of diet or in vivo Sp were observed on KCl- or Phenylephrine-induced constrictions in femoral arteries (Figure 5A). Endothelium-dependent ACh-induced vasodilation responses were not significantly affected by WD feeding. However, Sp significantly increased ACh-induced vasodilatory responses in WD-fed mice, WDSp vs WDC (Emax = 80.5 ± 15.9% n=4 vs. 42.6 ± 15.6% n=5, respectively; P<0.05). This greater relaxation response associated with Sp was not observed in CD-fed mice (Figure 5B). No significant differences were found for the endothelium-independent vasodilatory responses to SNP between any of the groups (Figure 5C).
Figure 5.
Effect of Sp on WD-induced femoral artery endothelial dysfunction and vascular stiffness. Femoral artery constriction to phenylephrine (A) and vasodilation to ACh (B) and sodium nitroprusside (SNP; C), (D) femoral artery strain-stress relationship and (E) femoral artery modulus of elasticity-pressure curve. Femoral artery remodeling is indicated by pressure-diameter relationships, (F), wall/lumen-pressure relationships (G), mean wall thickness-pressure relationship (H) and cross sectional area-pressure relationship (I). Values are mean ± SE; n=3–7 per group; †p<0.05 WDSp vs WDC.
Effects of Sp and diet on femoral artery structure
Femoral arteries from WD-fed mice were less distensible than those of CD-fed mice as indicated by a significant leftward shift in their circumferential wall stress-strain curves (Figure 5D). Femoral arteries of WDSp mice were significantly more distensible than those of WDC. The modulus of elasticity, used as an index of stiffness, was significantly elevated in femoral arteries of WDC mice compared to those of CDC and WDSp (Figure 5E). No significant differences were observed for the internal passive pressure-diameter relationships, an index of remodeling, nor were there differences in wall to lumen ratios, mean wall thickness, and wall cross-sectional areas between any of the groups (Figure 5F–I).
DISCUSSION
Collectively, the results of this investigation support the hypothesis that MR play an important role in the development of aortic stiffness in female mice in the setting of WD-induced insulin resistance. Herein, we demonstrate that aortic PWV is increased in female mice fed a diet high in fat and refined sugars (sucrose/HFCS). Although MR blockade had no effect on reducing excess body or fat pad weight, it completely prevented the increase in PWV observed in the untreated mice fed WD. AFM was utilized to further assess potential cellular contributions to aortic stiffness using ex vivo aortic explants to measure EC and VSMC stiffness. Intact explants enable measurement of stiffness of EC and VSMC that are still in contact with native ECM proteins. Our findings in both types of preparations identified abnormally high stiffness in WD-fed mice that was prevented with Sp.
Abnormalities in the insulin signaling cascade in the aorta, including decreased Akt activation, have been documented in models of obesity and insulin resistance.28, 29 In the present study, we observed decreased vascular Akt phosphorylation (activation) in untreated WD-fed mice compared to their CD-fed counterparts. We also examined phosphorylation of eNOS as a downstream target of insulin-Akt signaling. Compared to untreated mice fed CD, we observed a marked decrease in phosphorylated-eNOS1177 and the ratio of p-eNOS1177/total eNOS in WD-fed female mice. Therefore, decreased eNOS phosphorylation may account, in part, for the impairment of aortic endothelial function and this may contribute to the observed aortic stiffening. A recent study indicated that aldosterone contributes importantly to a phenomenon known as “stiff endothelial cell syndrome” (SECS) mediated by increased EC expression of the epithelial sodium channel (ENac) and MR in concert with impaired generation of NO, and that Sp can prevent its manifestation and improve endothelial function.22 Therefore, therapies that increase EC NO bioavailability and improve EC function could potentially reduce aortic stiffening in overweight individuals.11
Inflammation due to maladaptive immune responses has recently been implicated in playing a role in development of vascular disease, including vascular stiffness.15, 30 One of the immune mechanisms related to inflammation is polarization of macrophages.25 The activation of an M1 macrophage phenotype leads to a pro-inflammatory response and conversely, activation of M2 macrophages mount an opposing anti-inflammatory response. Nonetheless, no studies have linked consumption of a high fat/high sucrose/HFCS diet with aortic stiffening, dysfunction and remodeling and an abnormal immune and inflammatory response and macrophage polarization. We observed increases in MCP-1, and CD86 in the aorta of WD-fed mice suggestive of an inflammatory response. Even though Sp did not prevent the WD-induced increase in these pro-inflammatory markers it significantly increased the M2 macrophage marker, IL-10, leading to improvement in the M1/M2 ratio suggesting suppression of the inflammatory response. Recent evidence indicates that IL-10 may contribute to improved cardiovascular insulin sensitivity.31 Additionally, IL-10 released from regulatory T cells (Tregs) has also been shown to improve endothelial function by suppressing NADPH oxidase mediated oxidative stress.32 Therefore, the apparent improvement in M2 macrophage polarization observed in this study may contribute to prevention of vascular injury by MR blockade. Although insulin metabolic signaling through Akt/eNOS pathway is impaired, growth factor signaling through activation of ERK1/2 pathway is either not affected or stimulated.14 In this regard, ERK1/2 activation modulates vascular remodeling and inflammatory response.14 Thus, ERK activation may contribute to vascular inflammation, remodeling and arterial stiffness, as observed in this study.
In addition to the aorta we also examined the function and structure of the femoral artery. As established by the recent Hoorn study, local arterial stiffness measures, specifically in the femoral and carotid arteries, but not brachial artery, independently predict CVD risk.33 This is the first study to show that WD-feeding results in impairments in femoral artery distensibility and stiffness and that low dose MRA prevents these impairments. In this study, endothelium-dependent and independent vasodilatory responses to ACh and SNP in the femoral artery were not affected by WD feeding, but Sp treatment significantly enhanced endothelial-dependent vasodilation in femoral arteries (Figure 5).
In this study, we have used a low dose of Sp, and the rationale for this is threefold. First, we previously established that this dose does not affect blood pressure.34 Second, in addition to the MR, Sp binds to androgen, progesterone and glucocorticoid receptors; however binding to non-MR is likely to require much higher doses of Sp to induce anti-androgenic or progesterone actions.35 Finally, addition of low dose of a MR antagonist to standard therapy has been shown to reduce morbidity and mortality among patients with heart failure.36 Thus, it could be reasoned that addition of low dose MRAs could prevent further progression of vascular stiffening and the associated risk of heart failure in obese females regardless of diabetic status and with minimal risk of side effects. Moreover, it is likely that the anti-stiffening effects of MRAs relate largely to preventing aldosterone, as opposed to glucocorticoid-mediated, MR signaling in the vasculature. This is because both vascular endothelial and smooth muscle cells express 11-β hydroxysteroid dehydrogenase, an enzyme that limits glucocorticoid signaling through the MR receptor.37
In contrast to our study, others have reported resistance to the metabolic and CV complications associated with high fat diets in female mice,38 suggesting estrogen-mediated CV protection.39 Such studies have mostly utilized high fat diets rather than diets high in both fat and refined sugars like the diet used in this investigation. The abrogation of CV protection, manifested in our model as an increase in aortic stiffness is consistent with clinical studies reporting increases in aortic PWV in overweight women.49, 50 Data from the Framingham Heart Study indicate that women have higher serum aldosterone concentrations that correlate with a pattern of LV concentric remodeling.16 We previously reported that young female C57BL6 mice exhibit higher serum levels of aldosterone compared with males (~50% higher in females), but WD had no effect on aldosterone levels in males or females.7 Therefore, we speculate that the interaction of higher aldosterone levels in females and consumption of a WD high in fat, sucrose and HFCS may act synergistically to promote the observed increases in oxidative stress, inflammation and vascular stiffness. On the other hand, a recent in vitro study showed that estrogen can suppress aldosterone mediated expression of genes in EC that contribute to CV dysfunction and disease.40 Thus, it is possible that this antagonistic effect of estrogen on deleterious MR signaling in the vasculature may be abrogated with high fat/sucrose/HFCS diet.
The results of this study indicate that the effects of Sp on prevention of WD-induced vascular stiffness occurred in the absence of an increase in MAP and HR. Moreover, aortic stiffness was not increased in control mice at the end of the feeding trial, therefore the effects seen in WD are not due to normal aging processes. The potential influence of the female estrous cycle on PWV is a potential limitation of this study. Nonetheless, a previous report demonstrated that PWV does not vary over the course of the menstrual cycle in women.41
In conclusion, we have elucidated a role for the MR in development of aortic stiffness and the associated abnormalities in immune responses, oxidative stress, endothelial/smooth muscle function and structural remodeling in a clinically relevant female model of weight gain due to consumption of a WD high in fat, HFCS and sucrose. Importantly, aortic stiffness was detected not only in vivo, but also on the aortic EC and VSMC surfaces of ex vivo preparations. These findings may be particularly relevant to overweight and obese women who may be at increased risk for development of aortic stiffness and CVD.
Perspectives
Results of this investigation suggest, not only that MR play a critical role in development of WD-induced aortic stiffness, but that a low dose of Sp exerts profound prophylaxis to maintain vascular health in individuals consuming a high fat/sucrose/HFCS diet. Targeting the MR with adjunctive low dose Sp may be an effective strategy to limit vascular disease progression in individuals struggling from overweight/obesity that are at risk for a first CV event. Our results suggest that this strategy could minimize the likelihood of side-effects associated with Sp (hypokalemia or hypotension) and maximize the efficacy of MRA. Thus, the rationale for this strategy parallels the rationale for use of a low dose of Sp to treat severe heart failure reported in RALES (Randomized Aldactone Evaluation Study).15
Supplementary Material
Novelty and Significance.
What is new
These data indicate that consumption of a Western Diet, high in fat and refined sugars promotes vascular insulin resistance and stiffness in female mice. Development of this vascular phenotype can be prevented by a low dose of the mineralocorticoid receptor blocker, spironolactone.
What is relevant
Vascular stiffness is a strong predictor for cardiovascular events and under insulin resistant conditions women have greater stiffness compared with men. In a very translational model of insulin resistance, activation of the mineralocorticoid receptor is important for development of vascular stiffness in females.
Summary
Results of this investigation suggest that enhanced mineralocorticoid receptor activation plays an integral role in development of vascular resistance that is promoted by consumption of a Western Diet in females.
Acknowledgements
The authors gratefully acknowledge Brenda Hunter for editorial assistance, Nathan Rehmer, Alex Meuth, Terry L. Carmack and Lisa D. Watkinson for technical support. This work was supported with resources and facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO including the Biomolecular Imaging Center (MRI) and the Small Animal Ultrasound Imaging Center (Echocardiography), as well as the Atomic Force Microscopy Center in the Dalton Cardiovascular Research Center.
Sources of Funding: This work was supported by the Department of Medicine Research Council (VGD), National Institutes of Health (R01-HL073101 RO1-HL107910 to JRS, R01 HL088105 LAM-L and P01 HL095486 to GAM), Department of Veterans Affairs Biomedical Laboratory Research & Development (CDA-2 IK2 BX002030 to SBB, 0018 to JRS).
Footnotes
Disclosures: The authors have declared that no conflicts of interest exist.
References
- 1.Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28:323–333. doi: 10.3109/07853899608999089. [DOI] [PubMed] [Google Scholar]
- 2.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–968. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Alivon M, Beaussier H, Boutouyrie P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Ann Med. 2012;44(Suppl 1):S93–S97. doi: 10.3109/07853890.2011.653398. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 7.Manrique C, Demarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a western diet. Endocrinology. 2013;154:3632–3642. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryden Ahlgren A, Lanne T, Wollmer P, Sonesson B, Hansen F, Sundkvist G. Increased arterial stiffness in women, but not in men, with IDDM. Diabetologia. 1995;38:1082–1089. doi: 10.1007/BF00402179. [DOI] [PubMed] [Google Scholar]
- 9.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 10.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 11.Kearney TM, Murphy MH, Davison GW, O'Kane MJ, Gallagher AM. Accumulated brisk walking reduces arterial stiffness in overweight adults: Evidence from a randomized control trial. J Am Soc Hypertens. 2014;8:117–126. doi: 10.1016/j.jash.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGraw AP, McCurley A, Preston IR, Jaffe IZ. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep. 2013;15:340. doi: 10.1007/s11883-013-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–319. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrario CM, Schiffrin EL. Role of mineralocorticoid receptor antagonists in cardiovascular disease. Circ Res. 2015;116:206–213. doi: 10.1161/CIRCRESAHA.116.302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. doi: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 17.Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–2405. doi: 10.1097/HJH.0b013e32831286fd. [DOI] [PubMed] [Google Scholar]
- 18.Strauch B, Petrak O, Wichterle D, Zelinka T, Holaj R, Widimsky J., Jr Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. 2006;19:909–914. doi: 10.1016/j.amjhyper.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Rosa J, Somloova Z, Petrak O, Strauch B, Indra T, Senitko M, Zelinka T, Holaj R, Widimsky J., Jr Peripheral arterial stiffness in primary aldosteronism. Physiol Res. 2012;61:461–468. doi: 10.33549/physiolres.932344. [DOI] [PubMed] [Google Scholar]
- 20.Collier SR, Sandberg K, Moody AM, Frechette V, Curry CD, Ji H, Gowdar R, Chaudhuri D, Meucci M. Reduction of plasma aldosterone and arterial stiffness in obese pre- and stage1 hypertensive subjects after aerobic exercise. J Hum Hypertens. 2015;29:53–57. doi: 10.1038/jhh.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper JN, Tepper P, Barinas-Mitchell E, Woodard GA, Sutton-Tyrrell K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin Exp Hypertens. 2012;34:63–70. doi: 10.3109/10641963.2011.618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstadt H, Oberleithner H, Kliche K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–3659. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 23.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Nehme J, Mercier N, Labat C, Benetos A, Safar ME, Delcayre C, Lacolley P. Differences between cardiac and arterial fibrosis and stiffness in aldosterone-salt rats: effect of eplerenone. J Renin Angiotensin Aldosterone Syst. 2006;7:31–39. doi: 10.3317/jraas.2006.004. [DOI] [PubMed] [Google Scholar]
- 25.Demarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 27.Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique Acevedo C, Sowers JR. Mineralocorticoid Receptor Blockade Prevents Western Diet-induced Diastolic Dysfunction in Female Mice. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 29.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.114.04912. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial Stiffening Precedes Systolic Hypertension in Diet-Induced Obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63:1739–1747. doi: 10.1016/j.jacc.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Habibi J, DeMarco VG, Ma L, Pulakat L, Rainey WE, Whaley-Connell AT, Sowers JR. Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression. Am J Physiol Heart Circ Physiol. 2011;300:H1484–H1491. doi: 10.1152/ajpheart.01000.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, Kraehenbuehl C, Biollaz M, Grob J, Schmidlin J, Weiland P, Wehrli HU. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987;240:650–656. [PubMed] [Google Scholar]
- 36.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 37.Deuchar GA, McLean D, Hadoke PW, Brownstein DG, Webb DJ, Mullins JJ, Chapman K, Seckl JR, Kotelevtsev YV. 11beta-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe-/- mice. Endocrinology. 2011;152:236–246. doi: 10.1210/en.2010-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, Enriori PJ. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology. 2014;155:4447–4460. doi: 10.1210/en.2014-1342. [DOI] [PubMed] [Google Scholar]
- 39.Louwe MC, van der Hoorn JW, van den Berg SA, Jukema JW, Romijn JA, van Dijk KW, Rensen PC, Smit JW, Steendijk P. Gender-dependent effects of high-fat lard diet on cardiac function in C57Bl/6J mice. Appl Physiol Nutr Metab. 2012;37:214–224. doi: 10.1139/h11-153. [DOI] [PubMed] [Google Scholar]
- 40.Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155:4461–4472. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.