Abstract
Mutations of the insulin/IGF signaling (IIS) pathway extend Drosophila lifespan. Based on genetic epistasis analyses, this longevity assurance is attributed to downstream effects of the FOXO transcription factor. However, as reported FOXO accounts for only a portion of the observed longevity benefit, suggesting there are additional outputs of IIS to mediate aging. One candidate is target of rapamycin complex 1 (TORC1). Reduced TORC1 activity is reported to slow aging, whereas reduced IIS is reported to repress TORC1 activity. The eukaryotic translation initiation factor 4E binding protein (4E-BP) is repressed by TORC1, and activated 4E-BP is reported to increase Drosophila lifespan. Here we use genetic epistasis analyses to test whether longevity assurance mutants of chico, the Drosophila insulin receptor substrate homolog, require Drosophila d4eBP to slow aging. In chico heterozygotes, which are robustly long-lived, d4eBP is required but not sufficient to slow aging. Remarkably, d4eBP is not required or sufficient for chico homozygotes to extend longevity. Likewise, chico heterozygote females partially require d4eBP to preserve age-dependent locomotion, and both chico genotypes require d4eBP to improve stress-resistance. Reproduction and most measures of growth affected by either chico genotype are always independent of d4eBP. In females, chico heterozygotes paradoxically produce more rather than less phosphorylated 4E-BP (p4E-BP). Altered IRS function within the IIS pathway of Drosophila appears to have partial, conditional capacity to regulate aging through an unconventional interaction with 4E-BP.
Introduction
Mutations of the insulin/IGF-signaling (IIS) pathway in Drosophila and C. elegans slow aging when measured by adult survival and by the decline of age-associated traits [1–7]. In Drosophila, as in mammals, the insulin-like receptor is auto-phosphorylated upon ligand binding and subsequently recruits and phosphorylates the SHB2 domain-containing insulin receptor substrate (CHICO, the IRS1-4 homolog) [8–10]. Activated Drosophila IRS/CHICO transduces signaling through phosphatidylinositol-3-OH kinase PI(3)K which then regulates effector pathways including PKB/ AKT, Tsc2, PRAS40 and GSK [11–13]. Among these, AKT phosphorylates and represses FOXO. Genetic epistasis studies in worm and fly have examined whether the FOXO transcription factor is required for reduced IIS signaling to extend lifespan. In C. elegans, longevity assurance conferred by mutations of daf-2, the Insulin/IGF receptor ortholog, requires FOXO encoding daf-16 to extend lifespan [3,14,15]. Similarly, dfoxo is required for reduced IIS in Drosophila to extend life span [16,17]. But notably, null mutations of dfoxo in Drosophila only partially restore lifespan of IIS mutants toward wildtype. These observations suggest that IIS potentially signals through additional, unexplored factors to control aging.
One candidate involves target of rapamycin (TOR). PI3K/AKT can modulate TOR activity in mammalian and Drosophila cell culture [18,19], although the interaction of PI3K/AKT and TOR activity in Drosophila tissue is less clear [12,20]. In the standard view, activated AKT blocks the repressive effects of TSC1/2 upon target of rapamycin complex 1 (TORC1), permitting TORC1 to be autonomously activated by amino acids. TORC1 stimulates numerous growth-associated systems including protein translation machinery by phosphorylating the S6 ribosomal kinase (S6K) and the translation initiation factor inhibitory protein 4E-BP. PI3K/AKT also regulates TORC1 through PRAS40 (proline-rich Akt substrate of 40 kDa). AKT phosphorylates PRAS40 and thus reduces its ability to bind and inhibit TORC1 in cell culture and in Drosophila ovaries, but not so in somatic tissue [12].
Drosophila TOR and 4E-BP (encoded by thor, also denoted d4eBP) were seen to regulate aging in a number of studies. Lifespan was extended when TOR signaling was inhibited by over-expressing genes of the tuberous sclerosis complex (dTsc1, dTsc2), and by expressing dominant-negative forms of TOR and S6K [21]. The heteroallelic dTOR 7/P mutant genotype extended lifespan 20% and prevented age-dependent decline in cardiac performance [22]. Drosophila 4E-BP overexpressed in a constitutively activated form extended lifespan in adults fed a rich diet [23]. Expression of constitutively active 4E-BP in muscle is sufficient to extend lifespan and preserve age-associated muscle function, potentially because this systemically reduces circulating insulin-like peptides [24]. Likewise, ectopic expression of d4eBP specifically within the fly heart protects against age-related decline in myocardial function and cardiac stress resistance [25]. Finally, rapamycin increases adult Drosophila survival, although not in every reported case, presumably because this drug inhibits TOR [26–28]. Together these studies suggest that reduced TORC1 activity in Drosophila slows or retards aging, and that repression of 4E-BP might play a role in this process [29].
Based on these observations, it is natural to propose that reduced IIS modulates aging not just through activating FOXO but also by increasing the translation-inhibitory action of 4E-BP. Here we test this hypothesis by genetic epistasis analysis between mutants of chico (encoding the IRS1-4 homolog) and of d4eBP/thor. If reduced IIS increases 4E-BP activity (reduces p4E-BP) to affect longevity assurance, we expect the lifespan of chico d4eBP double mutants to be restored toward that of wildtype.
Homozygote and heterozygote genotypes of the chico 1 allele robustly extend Drosophila lifespan [2,30]. The chico 1 allele is a P-element P{ry11} insertion located in the insulin receptor substrate pleckstrin homology domain of CHICO [8,9,31]. Genetic analysis indicates chico 1 is a loss of function mutation [31]. Homozygotes of chico 1 (ch -/-) have been studied for how they affect growth, insulin/IGF signaling, development, oogenesis, and physiology [12,32]. Although the impact of chico 1 heterozygotes (ch +/-) remain relatively less explored, ch -/- and ch +/- produce striking differences despite their similar ability to extend lifespan: ch -/- are small, sterile and develop slowly while ch +/- are fecund, and have normal size and development time [2,31]. To facilitate analyses of chico 1 in aging, Tu et al. [30] crossed this allele into a cinnabar; rosy genetic background (cn 1/cn 1; ry 506/ry 506) so that stocks could be perpetually maintained by breading heterozygotes, and sibs would segregate all chico genotypes in a coisogenic background each generation (Among sibs: Heterozygotes are normal size and cinnabar eyes. Wildtype are normal size, apricot eyes by virtue of both cn and ry. Homozygotes are dwarf with cinnabar eyes).
Drosophila 4E-BP is encoded by d4eBP (also known as thor). This locus was originally identified as a P{lacW} insertion (thor 1) with defective innate immune function, and was proposed to produce 4E binding protein based on peptide homology [33,34]. P-element excision was used to produce a null allele (thor 2) by deleting ~1600 nucleotides in the regulatory and coding region, and to generate a perfect excision-revertant wildtype strain thor 1rv1 [33]. Although overexpression of d4E-BP antagonizes cell growth (wing discs, eyes) in response to PI(3)K/Akt signaling during development [19], homozygotes of thor 2 are largely viable, fertile and normal sized [35,36]. Phenotypes are most apparent under conditions of stress, where mutants show reduced resistance to hydrogen peroxide, and increased mortality during fasting that is associated with elevated rates of fat catabolism [35,37].
Materials and Methods
Fly husbandry
Flies were reared and maintained at 25°C, 40% relative humidity and 12-hour light/dark cycle. Standard cornmeal-sugar-yeast diet was used in all experiments (23 g/L SAF yeast (Lesaffre yeast corporation, Milwaukee, WI, USA), 100 g/L sucrose, 47 g/L cornmeal and 7 g/L agar, 0.2% Tegosep (methyl4-hydroxybenzoate, Sigma, St. Louis, MO, USA)).
Generation of d4eBP, chico double mutants
Drosophila d4eBP null mutants (Thor 2) were obtained from Dr. Deborah A. Kimbrell [33]. The chico mutant stock (y 1 ; chico 1 cn 1 ; ry 506) was previously made in our laboratory, and has been perpetually maintained by breeding heterozygotes [17,30]. Both chico and d4eBP are on the second chromosome (arm 2L), therefore, to introduce the d4eBP mutation into the chico-segregation stock we first backcrossed and recombined Thor 2 to the genotype y 1 ; cn 1 ; ry 506 to produce y 1 ; Thor 2 cn 1 ; ry 506. Subsequently, d4eBP, chico double mutants (y 1 ; Thor 2 chico 1 cn 1 ; ry 506) were produced through recombination between y 1 ; chico 1 cn 1 ; ry 506 and y 1 ; Thor 2 cn 1 ; ry 506. See supporting information (S1 Text and S1, S2 and S3 Figs) for details of genetic design.
Demography and survival analysis
Newly enclosed flies were allowed to mate for two days, then separated by sex and genotype, and assigned to replicate one-liter demography cages at a density of 125 flies per cage. Three independent cages were set-up per genotype. Two independent demography trials were performed and all cohorts within a trial were studied concurrently. Food was changed every two days, at which time dead flies were removed from the cage and counted. Survival analysis was conducted with JMP statistical software (SAS Institute, Cary, NC, USA), and data from replicate cages were combined. Mortality distributions were compared by Log-Rank and proportional hazard analyses.
Climbing
Climbing ability (negative geotaxis assay) was measured by tapping flies to the bottom of an empty vial and counting (with video recording) flies that climbed 8 vertical centimeters within 20 seconds. Forty females (10 per vial) were scored once a week for each genotype.
Fecundity
Females from each genotype were collected one day after eclosion and mated with wildtype males. Two days after mating, females were separated and kept in vials with standard fly food (ten vials per genotype, two females per vial). Over the following 10 days (beginning as females were 3 d old), females were passed to new vials daily, and eggs were counted.
Stress resistance
Starvation resistance was tested in 3-day-old female and male adults. Flies were transferred into glass vials containing 0.8% agar in PBS; dead flies were counted twice a day. Resistance to oxidative stress was tested in 3-day-old flies previously maintained on standard food then transferred into glass vials containing 1% agar, 5% sugar and 20mM paraquat (Sigma, St. Louis, MO, USA). Dead flies were counted twice a day. For both assays, a total of 50 flies were used for each genotype (10 flies per vial). Survival differences were analyzed by proportional hazard analysis.
Body weight and wing area
Eggs laid upon grape juice plates by 30 females were collected within a four-hour interval to measure larval development rate. Fifty eggs were transferred to a standard food vial; time to adult eclosion was recorded daily. Net adult body weight was measured from a pool of 10 flies, beginning three days after their eclosion from the controlled density vials, with four replicates per genotype and sex. From these same adults, total wing blade area was measured from images of slide-mounted tissue using ImageJ (imagej.nih.gov/ij). Fifteen flies were measured for each genotype, each sex.
Western blot
Antibodies for Akt (#9272), phospho-Akt (Ser505) (#4054), non-phospho-4E-BP1 (Thr46) (#4923), phospho-4E-BP1 (Thr37/46) (#2855) and β-actin (#4967) were purchased from Cell Signaling Technology (Danvers, MA, USA). Ten flies were homogenized in 100 μl RIPA buffer with protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and PhosSTOP phosphatase inhibitor cocktail (Roche, Nutley, NJ, USA). Supernatant was incubated with LDS loading buffer (Invitrogen, Grand Island, NY, USA) at 70°C for 10 min. Thirty micrograms of denatured protein was separated on 10% NuPAGE Novex 4–12% Bis-Tris precast polyacrylamide gels (Invitrogen, Grand Island, NY, USA) and transferred to PVDF membranes. Following incubation with primary and secondary antibodies, blots were visualized with Pierce ECL western blotting substrate (Thermo Fisher Scientific, Waltham, MA, USA). Three independent biological replicates were generated for all analyses, and samples for AKT and 4E-BP produced at different times. Band intensity was quantified with Image Lab software (Bio-Rad, Hercules, CA, USA).
Results
Aging and reproduction
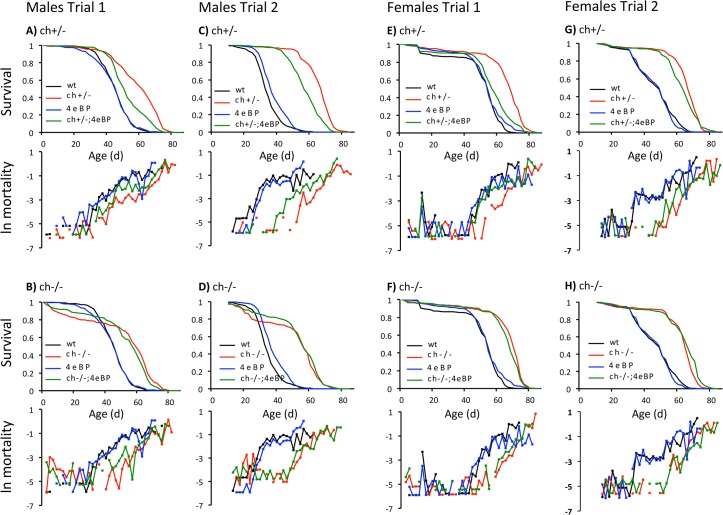
Life tables from single sex cohorts produced consistent outcomes in two independent demography trials. Adult survival of ch +/- (chico +/chico 1) was robustly increased as a result of uniformly reduced age-specific mortality (Fig 1A, 1C, 1E and 1G). The ch +/- genotype reduced mortality approximately 4-fold relative to wildtype (Table 1). The double mutant ch +/- d4eBP partially ameliorated this longevity assurance by increasing age specific mortality relative to ch +/- about 2-fold in three cases and 18% in one case (Table 1). The d4eBP mutation produced little or no effect on mortality relative to wildtype, indicating that loss of d4eBP in the double mutant reduces longevity assurance of this genotype through genetic interactions (epistasis) rather than by independent effects. Together these data demonstrate that longevity assurance conferred by ch +/- requires 4E-BP to some extent in both males and females.
Fig 1. Survivorship and mortality of male and female adult Drosophila with single and combined mutations of chico and d4eBP.
Cohorts of all genotypes were aged concurrently in two independent trials. Deaths in Trial 2 were recorded beginning at 10 days of age. Mortality rate is plotted as ln(μ x), estimated as ln(-ln(1-q x)) where q x is age-specific mortality. Panels A, C, E and G plot chico heterozygotes ch +/- relative to wildtype, d4eBP null mutant and the double mutant ch +/- d4eBP. Panels B, D, F and H plot chico homozygotes ch -/- relative to wildtype, d4eBP null mutant and the double mutant ch -/- d4eBP.
Table 1. Life table and proportional hazard survival analysis statistics of adult Drosophila wildtype, chico, d4eBP and chico d4eBP genotypes.
Independent replicate trials, sexes (males & once mated females) maintained as separate cohorts. Number: adults for combined cages of synchronous cohorts. Upper (UL) and lower (LL) 95% confidence intervals for median lifespan. Relative risk estimated from Cox proportional hazard analyses for each genotype (row) relative to chico genotype (column); probability > χ 2 based on log-likelihood (***) for p < 0.0001. Relative risk indicates fold change of the row genotype relative to the column chico genotype. Relative risk less than one (significant estimates in italics) indicate reduced mortality relative to the column chico genotype. Relative risk greater than one (significant estimates underlined) indicates increased mortality relative to the column chico genotype.
| Trial 1 | Males | ||||||
| Relative risk with respect to chico genotype: | |||||||
| Genotype | Number | Mean (d) | Median (d) | (LL, UL) | wildtype | ch +/- | ch -/- |
| wildtype | 356 | 44.8 | 46 | (44, 46) | |||
| d4eBP | 481 | 43.8 | 46 | (44, 46) | 1.03 (0.730) | ||
| ch +/- | 361 | 58.7 | 62 | (60, 64) | 0.264 (***) | ||
| ch -/- | 367 | 50.8 | 60 | (58, 62) | 0.331 (***) | ||
| ch +/- d4eBP | 250 | 52.2 | 52 | (50, 52) | 0.481 (***) | 1.84 (***) | |
| ch -/- d4eBP | 356 | 51.2 | 58 | (56, 60) | 0.403 (***) | 1.22 (0.0167) | |
| Trial 1 | Females | ||||||
| Relative risk with respect to chico genotype: | |||||||
| Genotype | Number | Mean (d) | Median (d) | (LL, UL) | wildtype | ch +/- | ch -/- |
| wildtype | 370 | 49.4 | 54 | (54, 56) | |||
| d4eBP | 370 | 52.3 | 54 | (54, 54) | 0.684 (***) | ||
| ch +/- | 457 | 64 | 68 | (68, 70) | 0.228 (***) | ||
| ch -/- | 373 | 64.7 | 70 | (70, 70) | 0.213 (***) | ||
| ch +/- d4eBP | 383 | 54.8 | 56 | (56, 58) | 0.486 (***) | 2.12 (***) | |
| ch -/- d4eBP | 251 | 63.2 | 68 | (66, 68) | 0.250 (***) | 1.17 (0.0501) | |
| Trial 2 | Males | ||||||
| Relative risk with respect to chico genotype: | |||||||
| Genotype | Number | Mean (d) | Median (d) | (LL, UL) | wildtype | ch +/- | ch -/- |
| wildtype | 328 | 34.1 | 34 | (32, 34) | |||
| d4eBP | 375 | 37.7 | 36 | (36, 38) | 0.759 (0.003) | ||
| ch +/- | 304 | 64.6 | 68 | (66, 68) | 0.0516 (***) | ||
| ch -/- | 204 | 49.8 | 56 | (54, 58) | 0.139 (***) | ||
| ch +/- d4eBP | 357 | 56.8 | 56 | (56, 58) | 0.108 (***) | 2.09 (***) | |
| ch -/- d4eBP | 140 | 52.3 | 56 | (54, 58) | 0.123 (***) | 0.884 (0.261) | |
| Trial 2 | Females | ||||||
| Relative risk with respect to chico genotype: | |||||||
| Genotype | Number | Mean (d) | Median (d) | (LL, UL) | wildtype | ch +/- | ch -/- |
| wildtype | 353 | 45.7 | 48 | (44, 50) | |||
| d4eBP | 392 | 45.6 | 48 | (46, 50) | 1.12 (0.113) | ||
| ch +/- | 368 | 63.9 | 66 | (66, 68) | 0.181 (***) | ||
| ch -/- | 284 | 61.2 | 64 | (64, 66) | 0.237 (***) | ||
| ch +/- d4eBP | 351 | 61 | 64 | (62, 64) | 0.212 (***) | 1.18 (0.0315) | |
| ch -/- d4eBP | 266 | 62.4 | 66 | (64, 68) | 0.182 (***) | 0.768 (0.002) | |
This demographic outcome for ch +/- contrasts to results from chico homozygotes (ch -/-, chico 1/chico 1) (Fig 1B, 1D, 1F, and 1H). As with chico heterozygotes, ch -/- consistently increases survival relative to wildtype and d4eBP by uniformly reducing age-specific mortality approximately 4-fold (Table 1). Remarkably, there is no appreciable difference in survival between ch -/- and the double mutant ch -/- d4eBP. 4E-BP is not required to realize longevity assurance of the ch -/- genotype.
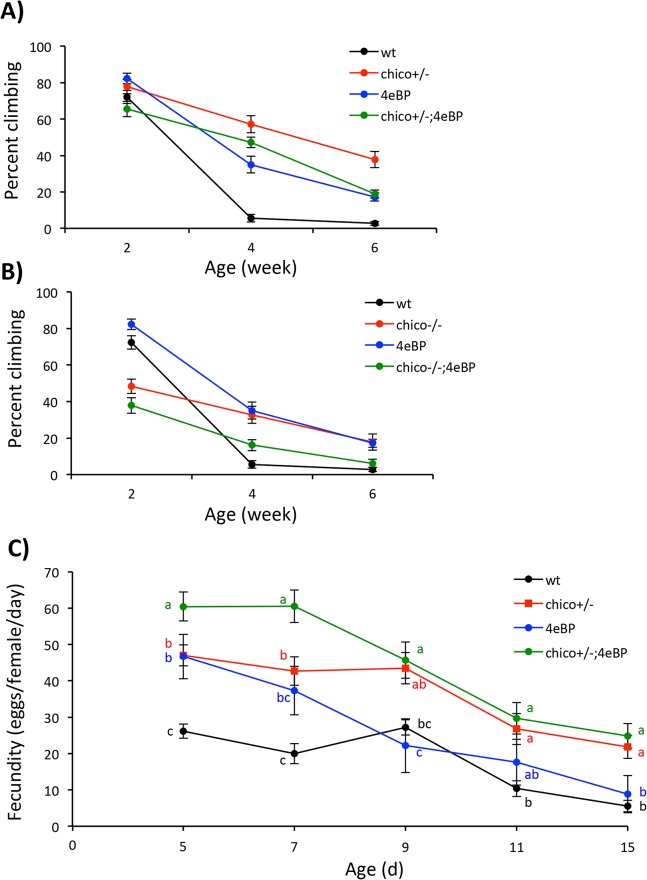
Aside from survival, decline in performance with age is a central feature of animal senescence [38,39]. The percentage of wildtype flies able to adeptly climb declines from about 70% at two weeks old to less than 10% by four weeks old (Fig 2). Chico heterozyogotes are robust climbers at two weeks and loose this ability only at a slow rate through age six weeks (Fig 2A). From weeks two to four the climbing index of ch +/- d4eBP declines at the same rate as ch +/- but with slightly lower overall performance. Statistical analysis for an effect of d4eBP upon the rate of change in the ch +/- background from weeks two to four indicates there is no significant interaction between these genes as they affect decline in climbing (general linear model with log likelihood ratio test, βgenotype x age(2–4) = 4.17, χ2 = 1.01, p = 0.32); there is no genetic epistasis in this time frame. Across weeks four and six there is significant genetic interaction; the double mutant looses climbing ability faster than predicted by the ch +/- mutant alone (βgenotype x age(4–6) = -8.32, χ2 = 4.53, p = 0.033). At least in one age category, ch +/- appears to partially require d4eBP to retard functional senescence.
Fig 2. Age dependent decline in climbing and egg production of Drosophila with single and combined mutations of chico and d4eBP.
Vertical climbing was measured from cohorts simultaneously aged to three time points (2, 4, 6 weeks). A) Percent climbing for chico heterozygotes ch +/- relative to wildtype, d4eBP null mutant and the double mutant ch +/- d4eBP. B) Percent climbing for chico homozygotes ch -/- relative to wildtype, d4eBP null mutant and the double mutant ch-/- d4eBP. C) Per capita egg production (with s.e. means) at ages five through 13 days old for chico heterozygotes ch +/- relative to wildtype, d4eBP null mutant and the double mutant ch +/- d4eBP. Within each age, differences in egg production evaluated by ANOVA with Tukey post-hoc test to resolve means that differ significantly, indicated by discordant letters.
Similar analyses with ch -/- did not reveal any genetic interaction with d4eBP (Fig 2B). While the ch -/- females were relatively weak climbers at young ages, they loose this capacity slowly as they age. Although starting at a low ability at young age, the rate of decline in the double mutant is similar to ch -/- (βgenotype x age(2–4) = -3.06, χ2 = 0.367, p = 0.544; βgenotype x age(4–6) = 2.50, χ2 = 0.479, p = 0.489) As with demographic aging there is no evidence that retarded functional aging conferred by ch -/- requires 4E-BP.
Egg production declines with age in mated female Drosophila (Fig 2C). From age 5 to 13 days old, wildtype fecundity declined from about 25–30 eggs per female per day to about 5–10 eggs per day. Chico heterozyogotes are more fecund than wildtype even though they are longer-lived (note: ch -/- are sterile). The rate of decline in egg production is similar for ch +/- and wildtype, suggesting that reproductive aging is not retarded by ch +/-. The interaction between ch +/- and d4eBP for egg production is complex. The genotypes have additive effects at days five and seven where the double mutant is more fecund than each single genotype. By day nine and thereafter, the fecundity of d4eBP is similar to that of wildtype while the double mutant is similar to that of ch +/-. At these later ages the d4eBP mutation no longer contributes to fecundity in the double mutant. Loss of 4E-BP generates extra fecundity only in young females and this is additive to the impact of ch +/-. Overall, ch +/- does not retard reproductive aging or interact genetically with d4eBP to affect egg production.
Stress resistance
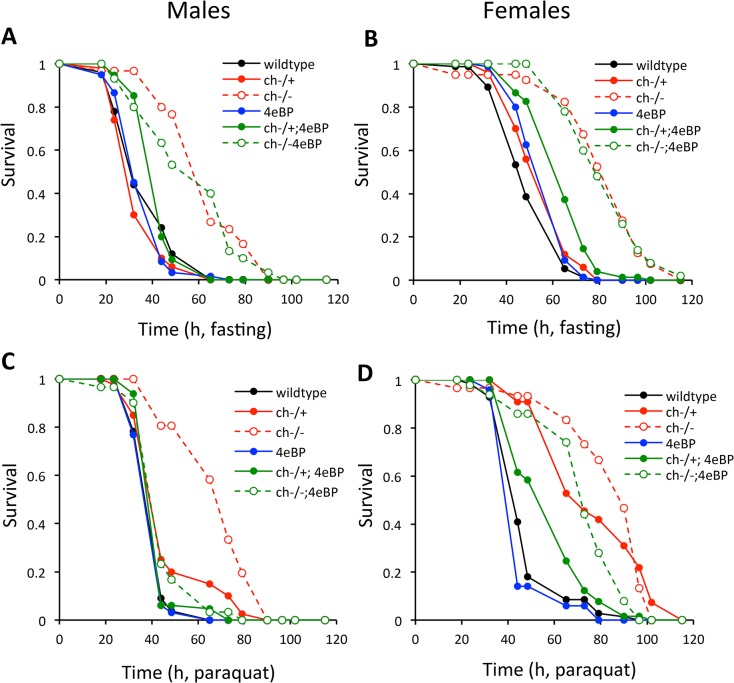
Genes that confer longevity assurance often increase stress resistance. As has been previously documented for mutants of Drosophila insulin/IGF signaling including chico [2], ch -/- strongly extends fasting survival relative to wildtype and to d4eBP (Fig 3A and 3B) (Table 2). In contrast with earlier reports where loss of d4eBP reduces fasting survival [35,37], here d4eBP mutants alone modestly increase female fasting survival and is neutral in males. The improved fasting survival of ch -/- is modestly epistatic to the loss of d4eBP in double mutant females, but not so in ch -/- males. Chico heterozygotes again present different interactions (Fig 3A and 3B) (Table 2). Chico heterozygosity modestly increases fasting survival in females and not in males, but in males the loss of d4eBP in the ch +/- background synergistically increases fasting survival more than expected from the combined effects of single mutants.
Fig 3. Resistance to acute stress-challenge by Drosophila with single and combined mutations of chico and d4eBP.
Survival of adult A) males and B) females when fasting with water. Survival of adult C) males and D) females when exposed to paraquat.
Table 2. Proportional hazard analysis for survival during paraquat exposure and starvation.
Proportional hazard modeled for ch +/- and d4eBP as single and double mutants relative to wildtype, and ch -/- and d4eBP as single and double mutants, with likelihood ratio test. Coefficient β for single loci: when significantly less than zero indicates reduction in mortality, estimates greater than zero indicate elevated mortality. Double mutants: coefficient β when significantly different from zero indicates gene interaction where the effect of the double mutant differs from expectation from product of single mutants. Epistasis inferred when significant gene interaction increases mortality (positive β) relative to expected product of chico and d4eBP; synergy inferred when gene interaction reduces mortality (negative β) relative to product of chico and d4eBP genotypes. Female and male survivorship when exposed to paraquat. Female and male survivorship during fasting.
| β | χ2 | Prob. | Inference | |
|---|---|---|---|---|
| Paraquat females | ||||
| d4eBP | 0.343 | 29.32 | < 0.0001 | Increases mortality |
| ch +/- | -0.448 | 38.04 | < 0.0001 | Reduces mortality |
| ch -/- | -0.677 | 59.44 | < 0.0001 | Reduces mortality |
| ch +/- d4eBP | 0.137 | 4.12 | 0.0423 | Epistasis |
| ch -/- d4eBP | 0.168 | 4.43 | 0.0354 | Epistasis |
| Paraquat males | ||||
| d4eBP | 0.0591 | 0.717 | 0.397 | Not significant |
| ch +/- | -0.147 | 4.313 | 0.0378 | Reduces mortality |
| ch -/- | -0.521 | 32.21 | < 0.0001 | Reduces mortality |
| ch +/- d4eBP | 0.0459 | 0.433 | 0.510 | No epistasis |
| ch -/- d4eBP | 0.305 | 12.46 | 0.0004 | Epistasis |
| Fasting females | ||||
| d4eBP | -0.243 | 10.16 | 0.0014 | Reduces mortality |
| ch +/- | -0.264 | 11.94 | 0.0005 | Reduces mortality |
| ch -/- | -0.978 | 107.7 | < 0.0001 | Reduces mortality |
| ch +/- d4eBP | 0.0119 | 0.0212 | 0.884 | No Epistasis |
| ch -/- d4eBP | 0.177 | 5.81 | 0.0159 | Epistasis |
| Fasting males | ||||
| d4eBP | -0.0477 | 0.582 | 0.445 | Not significant |
| ch +/- | -0.0299 | 0.203 | 0.652 | Not significant |
| ch -/- | -0.626 | 46.66 | < 0.0001 | Reduces mortality |
| ch +/- d4eBP | -0174 | 6.653 | 0.0099 | Synergy |
| ch -/- d4eBP | 0.00599 | 0.0054 | 0.941 | No epistasis |
Resistance to paraquat, a generator of oxidative stress, is elevated in both ch +/- and ch -/- females, and both chico genotypes modestly require d4eBP for this benefit (Fig 3C and 3D) (Table 2). In males, ch -/- produces a large benefit upon paraquat challenge that largely requires d4eBP. Male ch +/- produce a small survival benefit and provide no evidence for genetic interaction with d4eBP. Overall, resistance to paraquat is improved by both genotypes of chico and this requires 4E-BP.
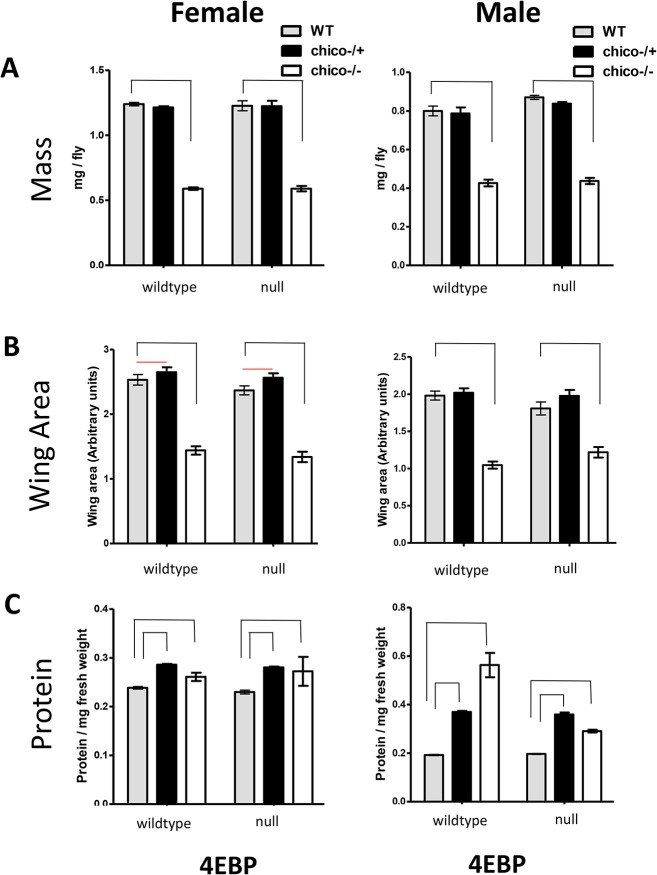
Size
Chico homozygotes are diminutive (Fig 4). In female ch -/-, the d4eBP mutant alone did not affect mass or wing area (ANOVA two-way interaction, p > 0.53). In males, the d4eBP mutant slightly reduced the impact of ch -/- upon wing area (p = 0.016) but not upon mass. Protein content was marginally elevated in ch -/- females and independent of d4eBP (p = 0.529), but strongly so in males and in a d4eBP-dependent manner (p < 0.0001). Thus, while growth is affected by ch -/-, these traits are independent of 4E-BP in females but somewhat dependent on 4E-BP in males.
Fig 4. Impact of chico and d4eBP alone and together upon measures of adult body size.
Females in left columns, males in right columns. Within each panel, chico genotypes are plotted for categories of d4eBP (wildtype and null mutant). A) Fresh body mass. B) Total wing area. C) Protein content per fresh body mass. Statistically significant differences among chico genotypes within d4eBP genotypes are indicated by brace-lines: p < 0.05. Statistics for interaction effects between genotypes are described in main text.
As noted, size is not a determinant of longevity assurance since the long-lived chico heterozygotes are no smaller than wildtype. In fact, the wing area of female ch +/-, but not males, was slightly larger than wildtype (Fig 4B). No interaction between ch +/- and d4eBP was detected in either sex (ANOVA two-way interaction p > 0.38). Mass of ch +/- did not differ from wildtype in either sex, or depend on d4eBP (p > 0.63). Protein content was elevated in ch +/- males and females (Fig 4C), but again independent of d4eBP (p > 0.14).
Signaling
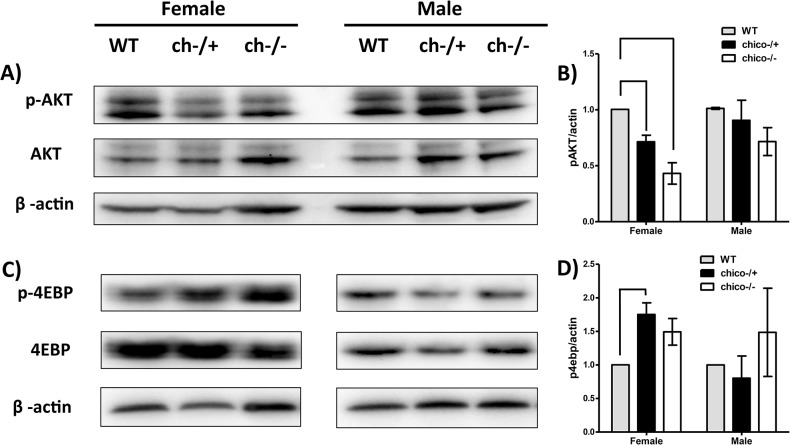
Drosophila insulin receptor substrate CHICO recruits and activates PI3K (p110) at the cell membrane, which in turn acts with PtdIns(3,4,5)P 3 to recruit and phosphorylate AKT [40,41]. Chico homozygotes were reported to interact with mutants of the insulin receptor locus (InR) to control growth [31], and further genetic study between dPKB (AKT) and dPTEN (p110 PI3K) loss-of-function mutants implicated Drosophila AKT as the major mediator of PI3K control of growth [42,43]. Here, consistent with observations for larvae [44], AKT phosphorylation is reduced in females for both chico genotypes, while only a small, non-significant reduction in pAKT is observed for males (Fig 5A and 5B). In cell culture, pAKT phosphorylates and inactivates tuberous sclerosis complex 2, thereby preventing TSC1/2 repression of TORC1 and permitting this kinase to phosphorylate and inactive 4E-BP. As noted, the relevance of this activity of AKT upon TORC1 is debated in the context of Drosophila physiology [12]. Pallares-Cartes [45] demonstrated that chico 1 homozygotes, via PRAS40, modulate TORC1 only within ovarian tissue and not in somatic tissue. Here, from whole females, we observe that the level of 4E-BP phosphorylation (inactive state) is increased (rather than decreased) in all chico genotypes, and significantly so in ch +/- (Fig 5C and 5D). In males we detect no significant differences in p4E-BP between wildtype and either chico genotype. Contrary to expectation, the inactive p4E-BP form is not decreased in animal tissue of chico mutant Drosophila.
Fig 5. Abundance of AKT, pAKT, 4E-BP and p4E-BP in whole adults of each chico genotype.
A) and C) Western blots with actin loading control. B) and D) Means (s.e.) from quantified replicate blots (n = 3) relative to actin within each matched sample. Significant differences (ANOVA, post hoc test) indicated by brace-lines: p < 0.05.
Discussion
These results reveal a complex of unexpected outcomes (summarized in S1 Table). Neither chico genotype decreases the phosphorylation of 4E-BP. This observation contrasts with results for how insulin/IGF signaling impacts TOR in cell culture [19,46] but not with in vivo analyses of Drosophila somatic tissue of chico homozygotes [45,47–49]. Furthermore, ch +/- actually elevates p4E-BP sampled from whole ch +/- females, suggesting these adults have elevated rather than repressed TORC1 activity.
Secondly, chico heterozygotes and homozygotes appear to regulate aging in different ways. Retarded demographic aging by ch -/- does not require d4eBP. Although ch -/- reduces pAKT, whether foxo is required for ch -/- to extend lifespan has not been reported to date, and it remains an open question as to what downstream pathways are required for ch -/- to slow aging. On the other hand, previous reports show that ch +/- requires foxo to fully extend lifespan [16,17], and that ch +/- in part modulates longevity assurance through activin signaling that is repressed by chico/FOXO [50]. Here we see that ch +/- also partially requires d4eBP to slow demographic and functional aging.
Strikingly, ch +/- females have increased fecundity. This is somewhat unexpected since insulin/IGF signaling drives germline stem cell maintenance, proliferation, and niche organization, but could be explained if ch +/- increases TORC1 activity and thus increases pS6K [51–53]. In a further twist, d4eBP-null increases fecundity in the youngest females and independently of chico. These reproductive effects contrast with chico homozygotes, which are completely sterile. Besides regulating aging in different ways, chico genotypes appear to differentially modulate reproduction.
Thirdly, while ch +/- partially requires d4eBP to slow aging, at least in females this chico genotype increases the inactive form of the translation inhibitor (p4E-BP). 4E-BP binds and sequesters eIF4E away from eIF4G to inhibit cap-dependent translation, while phosphorylated binding protein (p4E-BP) disassociates from eIF4G to permit formation of the active translational complex [54–56]. We note that substantial non-phosphorylated 4E-BP remains in chico heterozygotes. Based on our genetic data we propose that CHICO can interact (directly or indirectly) with this residual, active 4E-BP to regulate longevity assurance. In this model, ch +/- changes some quality of insulin/IGF signaling to amplify the impact of the residual, active 4E-BP. This idea may explain why ectopic expression of constitutively active 4E-BP increases Drosophila survival [23], and that rapamycin fed to long-lived IIS mutants of Drosophila is still able to extend lifespan [26]. Similarly, expression of activated 4E-BP1 in mouse skeletal muscle protects against age-related decline in insulin-sensitivity and metabolic rate, apparently through muscle autonomous increase in translation of peroxisome-proliferation-activated receptor-γ coactivator-1α (PGC-1 α) [57].
This model assumes that ch +/- can regulate 4E-BP activity independent of TORC1 (S4 Fig). Drosophila 4E-BP interacts with eIF4E through canonical and non-canonical binding motifs, which permits even low concentrations of 4E-BP to efficiently compete against eIF4G [58]. While TORC1-independent interactions between IRS and 4E-BP are currently unknown, we can entertain several possibilities. Chico heterozygotes may increase the duration of 4E-BP binding with eIF4E in mRNP (messenger ribonucleoprotein) granules and thus reinforce translation repression [59]. Alternatively, downstream consequences of translation repression might interact with effects of FOXO-mediated transcription. Finally, 4E-BP typically functions in the cytoplasm [60], but some of this protein interacts with eIF4E in mammalian nuclei [61]. While the function of nuclear 4E-BP is unknown, it might be sensitive to IRS. Although these suggestions are speculative, they reflect a need to explore ways IRS can affect 4E-BP function through mechanisms that do not involve its presumed control through PI3K/AKT/TSC/TORC1.
Supporting Information
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(DOCX)
(DOCX)
Acknowledgments
Aleksandra Norton ably managed our demography core for life table analyses. Rochele Yamamoto provided expert advice and guidance in our genetic designs.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health/National Institute on Aging (www.nia.nih.gov) grant R37 AG024360 to MT. SP was supported by National Institutes of Health/National Institute on Aging (www.nia.nih.gov) training grant T32 AG 41688. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292: 107–110. [DOI] [PubMed] [Google Scholar]
- 2. Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen H, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292: 104–106. [DOI] [PubMed] [Google Scholar]
- 3. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366: 461–464. [DOI] [PubMed] [Google Scholar]
- 4. Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans . Genetics. 1995;141: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans . Science. 1997;277: 942–946. [DOI] [PubMed] [Google Scholar]
- 6. Lapierre LR, Hansen M. Lessons from C . elegans signaling pathways for longevity. Trends Endocrin Metab. 2012;23: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kannan K, Fridell YW. Functional implications of insulin-like peptides in metabolism, aging, and dietary restriction. Front Physiol. 2013;4: 288 10.3389/fphys.2013.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poltilove RM, Jacobs AR, Haft CR, Xu P, Taylor SI. Characterization of Drosophila insulin receptor substrate. J Biol Chem. 2000;275: 23346–23354. [DOI] [PubMed] [Google Scholar]
- 9. Marin-Hincapie M, Garofalo RS. Drosophila insulin receptor: Lectin-binding properties and a role for oxidation-reduction of receptor thiols in activation. Endocrinology. 1995;136: 2357–2366. [DOI] [PubMed] [Google Scholar]
- 10. Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, et al. SHB2 regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11: 427–37. 10.1016/j.cmet.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garofalo RS. Genetic analysis fo insulin signaling in Drosophila. Trends in Endocrinology and Metabolism. 2002;13: 156–162. [DOI] [PubMed] [Google Scholar]
- 12. Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J. 2010;425: 13–26. [DOI] [PubMed] [Google Scholar]
- 13. Lizcano JM, Alrubaie S, Kieloch A, Deak M, Leevers SJ, Alessi DR. Insulin-induced Drosophila S6 kinase activation requires phosphoinositide 3-kinase and protein kinase B. Biochem J. 2003;374: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans .Nature. 1997;389: 994–999. [DOI] [PubMed] [Google Scholar]
- 15. Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38: 251–257. [DOI] [PubMed] [Google Scholar]
- 16. Slack C, Giannakou ME, Foley A, Goss M, Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell. 2011;10: 735–748. 10.1111/j.1474-9726.2011.00707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto R, Tatar M. Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila lifespan. Aging Cell. 2011;10: 729–732. 10.1111/j.1474-9726.2011.00716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- 19. Miron M, Verdu J, Lachance PD, Birnbaum MJ, Lasko PF, Sonenberg N. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nature Cell Biology. 2001;3: 596–601. [DOI] [PubMed] [Google Scholar]
- 20. Kockel L, Kerr KS, Melnick M, Bruckner K, Hebrok M, Perrimon N. Dynamic switch of negative feedback regulation in Drosophila Akt-TOR signaling. PLoS Genet. 2010;6: e1000990 10.1371/journal.pgen.1000990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapahi P, Zid BM, Harper D, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Current Biology. 2004;14: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, et al. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4: 133–142. [DOI] [PubMed] [Google Scholar]
- 23. Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139: 149–160. 10.1016/j.cell.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143: 813–825. 10.1016/j.cell.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, et al. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8: 542–552. 10.1111/j.1474-9726.2009.00504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster . Cell Metab. 2010;11: 35–46. 10.1016/j.cmet.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison B, Tran TT, Taylor D, Lee S-D, Min K-J. Effect of rapamycin on lifespan in Drosophila. Geriatrics and Gerontology International. 2010;10: 110–112. 10.1111/j.1447-0594.2009.00569.x [DOI] [PubMed] [Google Scholar]
- 28. Villa-Cuesta E, Holmbeck MA, Rand DM. Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. J Cell Sci. 2014;127: 2282–2290. 10.1242/jcs.142026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster . Exp Gerontol. 2011;46: 382–390. 10.1016/j.exger.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tu M-P, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homolog chico. Aging Cell. 2002;1: 75–80. [DOI] [PubMed] [Google Scholar]
- 31. Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97: 865–875. [DOI] [PubMed] [Google Scholar]
- 32. Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7: 907–916. [DOI] [PubMed] [Google Scholar]
- 33. Bernal A, Kimbrell DA. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc Natl Acad Sci USA. 2000;97: 6019–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez A, Zhou Z, Tang ML, Meller S, Chen J, Bellen H, et al. Identification of immune system and response genes, and novel mutations causing melanotic tumor formation in Drosophila melanogaster . Genetics. 1996;143: 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teleman AA, Chen Y-W, Cohen SM. 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 2005;19: 1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernal AR, Schoenfeld K, Kleinhesselink, Kimbrell DA . Loss of Thor, the single 4E-BP gene of Drosophila, does not result in lethality. Drosophila Information Service. 2004;87: 81–84. [Google Scholar]
- 37. Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19: 1840–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fulop T, Larbi A, Witkowski J, McElhaney J, Loeb M, Mitniski A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11: 547–563. 10.1007/s10522-010-9287-2 [DOI] [PubMed] [Google Scholar]
- 39. Tatar M. Can we develop genetically tractable models to assess healthspan (rather than life span) in animal models? J Gerontol A Biol Sci Med Sci. 2009;64A: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. The Drosophila phosphoinositide 3-kinae Dp110 promotes cell growth. EMBO Journal. 1996;15: 6584–6594. [PMC free article] [PubMed] [Google Scholar]
- 41. Weinkove D, Neufeld TP, Twardzik T, Waterfield MD, Leevers SJ. Regulation of imaginal disc cell size, cell number and organ size by Drosophila class IA phosphoinositide 3-kinase and its adaptor. Current Biology. 1999;9: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 42. Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, et al. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science. 2002;295: 2088–2091. [DOI] [PubMed] [Google Scholar]
- 43. Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends in Cell Biology. 2003;13: 79–85. [DOI] [PubMed] [Google Scholar]
- 44. Werz C, Kohler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5: e1000596 10.1371/journal.pgen.1000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pallares-Cartes C, Cakan-Akdogan G, Teleman AA. Tissue-specific coupling between insulin/IGF and TORC1 signaling via PRAS40 in Drosophila. Dev Cell. 2012;22: 172–182. 10.1016/j.devcel.2011.10.029 [DOI] [PubMed] [Google Scholar]
- 46. Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4: 658–665. [DOI] [PubMed] [Google Scholar]
- 47. Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000;14: 2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dong J, Pan D. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 2004;18: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schleich S, Teleman AA. Akt phosphorylates both Tsc1 and Tsc2 in Drosophila, but neither phosphorylation is required for normal animal growth. PLoS One. 2009;4: e6305 10.1371/journal.pone.0006305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bai H, Kang P, Hernandez AM, Tatar M. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 2013;9: e1003941 10.1371/journal.pgen.1003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drummond-Barbosa D, Spradling AC. Stem cell and their progeny respond to nutritinoal changes during Drosophila oogenesis. Developmental Biology. 2001;231: 265–278. [DOI] [PubMed] [Google Scholar]
- 52.LaFever L.The role of diet in the regulation of Drosophila ovarian stem cells and their progeny. Doctoral Thesis, Vanderbilt University. 2010.
- 53. Gancz D, Gilboa L. Insulin and target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila. Development. 2013;140: 4145–4154. 10.1242/dev.093773 [DOI] [PubMed] [Google Scholar]
- 54. Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994;371: 762–767. [DOI] [PubMed] [Google Scholar]
- 55. Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68: 913–963. [DOI] [PubMed] [Google Scholar]
- 56. Lavoie CA, Lachance PE, Sonenberg N, Lasko P. Alternatively spliced transcripts from the Drosophila eIF4E gene produce two different Cap-binding proteins. J Biol Chem. 1996;271: 16393–16398. [DOI] [PubMed] [Google Scholar]
- 57.Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, et al. Activated muscle 4E-BP1 signaling improves metabolic parameters during aging and obesity. J Clin Invest. 2015; In press. [DOI] [PMC free article] [PubMed]
- 58. Igreja C, Peter D, Weiler C, Izaurralde E. 4E-BPs require non-canonical 4E-binding motifs and a lateral surface of eIF4E to repress translation. Nat Commun. 2014;5: 4790 10.1038/ncomms5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buchan JR. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014;11: 1019–1030. 10.4161/15476286.2014.972208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kleijn M, Scheper GC, Wilson ML, Tee AR, Proud CG. Localisation and regulation of the eIF4E-binding protein 4E-BP3. FEBS Lett. 2002;532: 319–323. [DOI] [PubMed] [Google Scholar]
- 61. Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, et al. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA. 2008;14: 1318–1327. 10.1261/rna.950608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.