Abstract
Purpose
Endocrine therapy, using tamoxifen or an aromatase inhibitor, remains first-line therapy for the management of estrogen receptor (ESR1) positive breast cancer. However, ESR1 mutations or other ligand-independent ESR1 activation mechanisms limit the duration of response. The clinical efficacy of fulvestrant, a Selective Estrogen Receptor Downregulator (SERD) that competitively inhibits agonist binding to ESR1 and triggers receptor downregulation, has confirmed that ESR1 frequently remains engaged in endocrine therapy resistant cancers. We evaluated the activity of a new class of Selective Estrogen Receptor Modulators (SERM)/SERD hybrids (SSHs) that downregulate ESR1 in relevant models of endocrine-resistant breast cancer. Building on the observation that concurrent inhibition of ESR1 and the cyclin dependent kinases 4 and 6 (CDK4/6) significantly increased progression free survival in advanced patients, we explored the activity of different SERD- or SSH-CDK4/6 inhibitor combinations in models of endocrine therapy resistant ESR1+ breast cancer.
Experimental Design
SERDs, SSHs, and the CDK4/6 inhibitor palbociclib were evaluated as single agents or in combination in established cellular and animal models of endocrine therapy resistant ESR1+ breast cancer.
Results
The combination of palbociclib with a SERDs or an SSH was shown to effectively inhibit the growth of MCF-7 cell or ESR-1 mutant patient derived tumor xenografts. In tamoxifen-resistant MCF7 xenografts the palbociclib/SERDor SSH combination resulted in an increased duration of response as compared to either drug alone.
Conclusion
A SERD- or SSH-palbociclib combination has therapeutic potential in breast tumors resistant to endocrine therapies or those expressing ESR1 mutations.
Keywords: Selective estrogen receptor downregulator, CDK4/6 inhibitor, endocrine resistant breast cancer
Introduction
Breast cancer remains the most commonly diagnosed cancer among women and a leading cause of cancer mortality in women (1). While targeted therapies such as the selective estrogen receptor modulator (SERM) tamoxifen and aromatase inhibitors (AIs) are initially effective in the treatment of estrogen receptor (ESR1) positive tumors, de novo and acquired resistance remain an impediment to durable clinical responses, particularly in the setting of advanced disease. Resistance to tamoxifen is most likely due to the selection, over time, of a population of breast cancer cells capable of recognizing this SERM as an agonist (2). This may be due to increased expression and/or activity of co-regulators that interact with and modulate ESR1 transcriptional activity or to the selection of cells expressing ESR1 mutants that alter the pharmacology of the receptor (3-5). There is little data to suggest that loss of ESR1 is a dominant mechanism of resistance, as ESR1 loss at recurrence is observed in less than 20% of patients (6, 7). Thus, ESR1 remains a therapeutic target in breast cancers that are resistant to both first and second line endocrine interventions (8, 9). This finding has prompted the development of SERMs mechanistically distinct from tamoxifen, and of selective estrogen receptor downregulators (SERDs), competitive antagonists whose interaction with ESR1 induces degradation. Fulvestrant, the only SERD approved for the treatment of metastatic breast cancer, has been shown to be effective in the relapsed/advanced setting, and recent data in the second- and first-line settings has shown a higher dose (500mg/month) than initially approved (250mg/month) can promote progression-free and overall survival (10-12).
The recent confirmation of ESR1 mutations, which occur in 10-20% of endocrine therapy resistant disease, is another impediment to durable response to endocrine therapy (4, 5). These mutations, most commonly at positions Y537 and D538, enable ESR1 to activate transcription in a ligand-independent manner (generating aromatase inhibitor resistance), and increase the partial agonist activity of tamoxifen (4, 5, 13). Interestingly, when evaluated in cellular models of breast cancer, it was observed that ESR1 mutants remain sensitive to the inhibitory activities of fulvestrant, albeit with considerably reduced potency (3). An increase in the dose of fulvestrant to compensate is possible but would require additional high-volume gluteal injections, which might prove impractical. Not surprisingly, there has been considerable interest in developing SERMs and SERDs that can be dosed at concentrations required to inhibit the activity of the most prevalent ESR1 mutants and are easier to administer than fulvestrant. From these efforts have emerged GW5638, BPN1 and BPL2 (ARN810), high affinity orally bioavailable drugs that downregulate ESR1 expression and that are currently being considered for, or already are, in clinical development (14, 15).
In addition to their utility in breast cancer treatment, there is significant interest in the development of improved SERMs, compounds whose relative agonist/antagonist activity can differ between cells, for the treatment of post-menopausal symptoms, including osteoporosis. Emerging from this development are unique SERM/SERD hybrids (SSHs) that function as agonists in bone, but also inhibit ESR1 action in the reproductive system by inducing receptor degradation in these tissues. Recently, we and others reported that bazedoxifene, an ESR1 ligand developed for the treatment of post-menopausal osteoporosis, exhibits useful SSH pharmaceutical properties and effectively inhibited the growth of both treatment-naïve and tamoxifen-resistant xenograft tumors in mice (16, 17). Bazedoxifene has been approved for clinical use in Europe and Japan; therefore, near-term clinical evaluation of its efficacy in breast cancer patients is a highly feasible proposition (18).
Regardless of the efficacy of SERMs or SERDs in breast cancer, it is likely that, when used as single agents in advanced disease, resistance will limit the response duration. Thus, there is considerable interest in developing drug regimens combining SERMs and/or SERDs with inhibitors of other pathways that impinge upon ESR1 signaling. The utility of this general approach was highlighted in the PALOMA-1 trial, in which the combination of the CDK 4/6 inhibitor palbociclib with the AI letrozole significantly increased progression free survival as compared to letrozole therapy alone (10 months vs. 20 months) in advanced ESR1+ breast cancer (19). These data have led to the accelerated approval of palbociclib by the US FDA (February, 2015). The hypothesis that active repression of ESR1 function, as opposed to attenuation of ESR1 signaling through reduction of estrogens, may further improve response, particularly in patients who have progressed during AI therapies, has led to the initiation of clinical trials evaluating tamoxifen or fulvestrant alone or in combination with palbociclib. However, the recent realization of the extent to which ESR1 mutations occur in relapsed breast cancers, coupled with the known reduced potency with which SERMs and SERDs target these mutant receptors, highlights the need to identify SERM- or SERD-palbociclib combinations that will be effective against these mutations. The objective of this study, therefore, was to evaluate the activity of the palbociclib alone or in combination with clinically relevant SSHs and SERDs in established models of advanced breast cancer as a means to select an appropriate combination(s) for further clinical evaluation.
Materials and Methods
Reagents
ESR1 ligands included 17β-estradiol (Sigma), ICI 182,780 (Tocris), and 4-hydroxytamoxifen (Sigma). Palbociclib, pipendoxifene and bazedoxifene were provided by Pfizer. (S)-3-(3-hydroxyphenyl)-4-methyl-2-(4-((S)-2-((R)-3-methylpyrrolidin-1-yl)propoxy)phenyl)-2H-chromen-6-ol (BPN1) and (E)-3-(4-((E)-2-(2-chloro-4-fluorophenyl)-1-(1H-indazol-5-yl)but-1-en-1-yl)phenyl)acrylic acid (BPL2) were synthesized as described (14, 15). Ligands were dissolved in ethanol or DMSO.
Cell culture
MCF7 cells were provided by Dr. Kenneth Korach (NIEHS) in 2004. TamR cells were derived from a tamoxifen-resistant MCF7 xenograft tumor (20) in 2001. Both cell lines were maintained in DMEM/F12 media (Invitrogen) supplemented with 8% fetal bovine serum (FBS) (Gemini), non-essential amino acids (Invitrogen), and sodium pyruvate (Invitrogen), with 100nM 4OHT added for TamR cells. Unless otherwise indicated, cells were plated for experiments in phenol red free (PRF) media supplemented with 8% charcoal stripped FBS (CFS) (Gemini). LTED MCF7 cells, derived in vitro as previously described (21), were maintained and plated for experiments in PRF DMEM/F12 media supplemented with 8% CFS. 48 hours after plating, cells were treated as indicated, and were harvested for immunoblot or real time quantitative PCR analysis 24 hours later. MCF7, LTED and TamR cell lines were authenticated by STR analysis in 2013. T47D cells were received from ATCC in 2007 (authenticated by STR analysis) and were maintained in DMEM media + 10% FBS.
Immunoblot analysis
Protein expression was analyzed as described (22) using antibodies from Cell Signaling (pRb ab6075) and Santa Cruz Biotechnology (cytokeratin 18 sc-6259), (lamin A, sc-20680), (α-tubulin sc-5546), (ESR1 sc-8005, sc-543).
In-cell western analysis
MCF7 cells (2.5×104/well) were plated in clear bottom 96-well black plates for 24 hrs prior to addition of ligand for 18 hrs. Fixation, detection of ESR1 (sc-543) and analysis were performed per LI-COR manufacturer's protocol using the LiCOR ODYSSEY infra-red imaging system. Data was normalized to DNA content (DRAQ5).
RNA isolation and real time quantitative PCR
RNA isolation and analysis was performed as described (23). mRNA abundance was calculated using the ΔΔCT method (22). Primer sequences are available upon request.
Proliferation assays
Proliferation assays were performed essentially as previously described (17) and analyzed using FluoReporter (Gibco) or Cell Titer Glo (Promega) kits per manufacturer's instructions.
In vivo studies
All procedures were approved by the Institutional Animal Care and Use Committee at Duke University or Washington University in St. Louis. The human tissues for the experiments were processed in compliance with NIH regulations and institutional guidelines, and approved by the institutional review board at Washington University in St. Louis.
TamR xenograft tumor study
Tamoxifen-stimulated TamR tumors were initiated in the mammary gland of tamoxifen treated (5 mg sc pellet, Innovative Research of America) ovariectomized 6-week old female NU/NU mice by serial transfer as previously described (17). Tumors were measured 3× weekly by caliper (volume = (A2 × B)/2, where A is the shorter axis). At ~0.1cm3 tumor volume, mice were randomized to treatment (28 days) with vehicle, BZA (5 or 10 mg/kg/day), PIP (5 or 10 mg/kg/day) or ICI (200 mg/kg weekly). Treatment groups were further subdivided into vehicle or palbociclib (100 mg/kg/day p.o.) treatment. SERDs were dissolved in corn oil and injected sc. Palbociclib was dissolved in 10 mM sodium lactate, pH 4. Following euthanasia, tissues were cryopreserved for analysis.
PDX tumor studies
6- to 8-week-old female SCID Biege (Charles River) mice were used in therapeutic efficacy studies as previously described (13). 2-3 ×106 tumor cells were subcutaneously injected into each mouse. Tumor size was monitored every three days. Tumor-bearing mice were randomized at 50-150 mm3 tumor volume. BZA (10 mg/kg/day) and palbociclib (125 mg/kg/day p.o.) were administered as above. At euthanasia, tumors were fixed in 10% buffered formalin.
Analysis of animal tissues
Frozen tissues were pulverized prior to protein extraction essentially as above. Total RNA was extracted using the Direct-zol RNA isolation kit (Genessee Scientific) per manufacturer's instructions. mRNA expression was detected as above. IHC analysis was conducted as described (13). Using antibodies purchased from Thermo Scientific (ESR1, RM-9101-S), (Ki67, RM-9106-S1) and Cell Signaling (pRb S807/811, 8516).
Statistical analyses
Tumor growth was analyzed (GraphPad Prism 6) by exponential growth curve analysis and by 2-way ANOVA of matched values followed by Bonferroni multiple comparisons to establish significance (p<0.05) between groups at each day of treatment.
Results
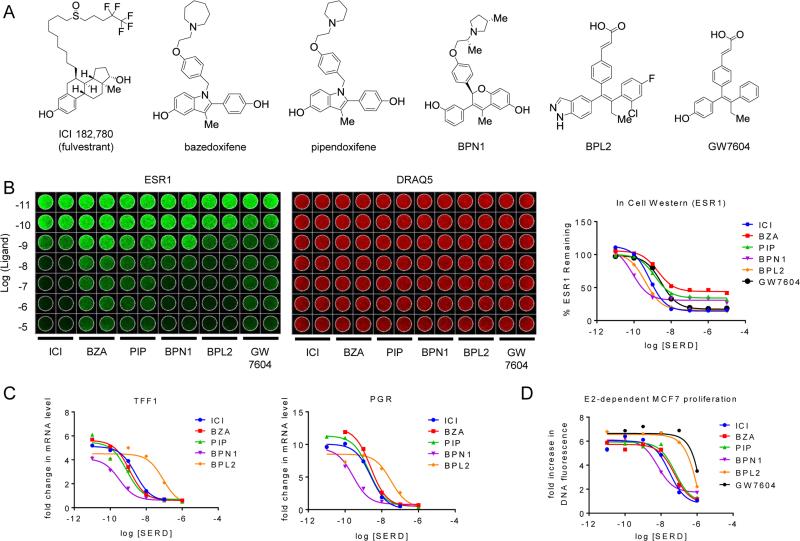
As an initial step in these studies, we performed a comparative analysis of the in vitro pharmacology of (a) the SERD fulvestrant (ICI 182,780; ICI) and (b) SSHs that have either been evaluated in humans or are currently being evaluated for clinical development: GW5638/DPC974 (active metabolite GW7604), bazedoxifene (BZA), pipendoxifene (PIP; ERA-923), and the benzopyran (S)-3-(3-hydroxyphenyl)-4-methyl-2-(4-((S)-2-((R)-3-methylpyrrolidin-1-yl)propoxy)phenyl)-2H-chromen-6-ol (BPN1, described in US patent 20140107095A1), and benzopyrazole (E)-3-(4-((E)-2-(2-chloro-4-fluorophenyl)-1-(1H-indazol-5-yl)but-1-en-1-yl)phenyl)acrylic acid (BPL2, described in US patent 20130231333A1) (Figure 1A) (20, 24, 25). In this study, the ability of each compound to (a) induce ESR1 degradation, (b) reverse 17β-estradiol (E2) dependent regulation of target gene transcription and (c) inhibit the growth of ESR1+ MCF7 breast cancer cells in response to E2 or growth factors was assessed. When analyzed using an in-cell western or by standard western immunoblotting (not shown), all of the compounds tested were found to quantitatively downregulate ESR1, albeit with differences in both efficacy and potency (Figure 1B, quantitated in right panel). However, these drugs exhibited similar efficacy, despite differences in potency, when assessed for their ability to (a) inhibit E2 dependent induction of the mRNAs encoding the progesterone receptor (PGR) and trefoil factor 1 (TFF1) (Figure 1C) and (b) inhibit E2-dependent proliferation of MCF-7 cells (Figure 1D). Interestingly, we noted discrepancies in the efficacy and potency with which SERD/SSHs inhibit ESR1 action when compared to their ability to downregulate ESR1 expression. Specifically, although BZA exhibited the least efficacy in assays of receptor degradation, it inhibited ESR1 activity (gene transcription and cell proliferation) with efficacy and potency similar to ICI, the most efficacious antagonist. Conversely, BPL2 potently induced ESR1 degradation, but was the least potent of the compounds tested when evaluated in ESR1 activity assays. Previously, we have shown that although receptor degradation is a desirable trait of ESR1 antagonists, the primary inhibitory activity of SERDs and SSHs with regard to ESR1 action relates to their ability to function as high affinity competitive antagonists that drive the receptor into a conformation that precludes interaction with coactivators (17, 22). Growth factor mediated activation of ESR1 has been implicated as a mechanism by which breast cancer cells may overcome the inhibitory effects of tamoxifen (26). Therefore, we next analyzed the ability of these compounds to attenuate insulin-stimulated proliferation of MCF7 cells, an estrogen-independent activity that requires ESR1 (not shown). This assay enabled the SERDs/SSHs tested to be differentiated, as we observed that while all of the drugs tested inhibited insulin-stimulated proliferation, the most effective downregulators of ESR1 (ICI, BPN1 and BPL2) exhibited inverse-agonist activity (Suppl. Figure S1). 4-hydroxytamoxifen did not attenuate the activity of insulin when analyzed under these conditions. Thus, it is likely that the SERD activity reinforces ESR1 inhibition, although the relative importance of receptor degradation vs. inhibition on the overall pharmacological activity of these drugs remains an unresolved issue.
Figure 1. SERDs and SERM/SERD hybrids inhibit ESR1 action with similar efficacy despite differences in potency and efficiency of estrogen receptor turnover.
A) Chemical structures of SERM/SERD hybrids (SSHs) and SERDs evaluated. B) MCF7 cells were incubated with increasing concentrations (10−11–10−5 M) SERD ICI 182,780 (ICI), or SSHs bazedoxifene (BZA), pipendoxifene (PIP), BPN1, or BPL2 for 18 hrs. ESR1 protein levels (left) were assessed using an In Cell Western. Data was normalized to DNA content using DRAQ5 (center) and quantitated (average of duplicate wells) using GraphPad Prism 6 (right). C) MCF7 breast cancer cells were treated for 24 hours with 1 nM E2 in the presence of increasing concentrations (10−11 – 10−6 M) of antagonist. mRNA levels of ESR1 target genes progesterone receptor (PGR) and trefoil factor 1 (TFF1) were assessed using real time quantitative PCR (RT qPCR) following RNA isolation. mRNA expression was normalized to the 36B4 housekeeping gene, and expression levels are presented as fold change as compared to an untreated control. D) MCF7 cells were plated in phenol red free media supplemented with charcoal stripped FBS 24 hours prior to treatment, and were treated with 1nM E2 as well as with the indicated ligands (10−11 – 10−6 M) on days 1, 4, and 6 of an 8 day proliferation assay. DNA content, as assessed by fluorescence (FluoReporter assay), was measured as a surrogate for cell proliferation. The relative increase in DNA fluorescence was calculated by normalizing to baseline values detected in a duplicate plate of cells that was harvested on day 1 prior to the initial treatment. Data are representative of at least 3 independent experiments.
These studies demonstrate that, when corrected for minor differences in potency, the antagonist efficacy of the SERDs/SSHs tested were very similar. Thus, we selected fulvestrant (ICI), bazedoxifene (BZA) and pipendoxifene (PIP) for further study as single agents and in combination with palbociclib as (a) ICI is currently available for clinical use, (b) BZA is approved for clinical use in Europe and Japan, and (c) PIP was evaluated in a previously completed phase II clinical trial in patients with advanced breast cancer. Further studies will be undertaken with BPN1 and BPL2, (third generation SERDs currently in clinical development), as information on their clinical activities emerges. No further studies were performed with GW7604/DPC974 as its development has been halted.
Fulvestrant, bazedoxifene and pipendoxifene inhibit ESR1 activity in relevant models of endocrine therapy resistant breast cancer
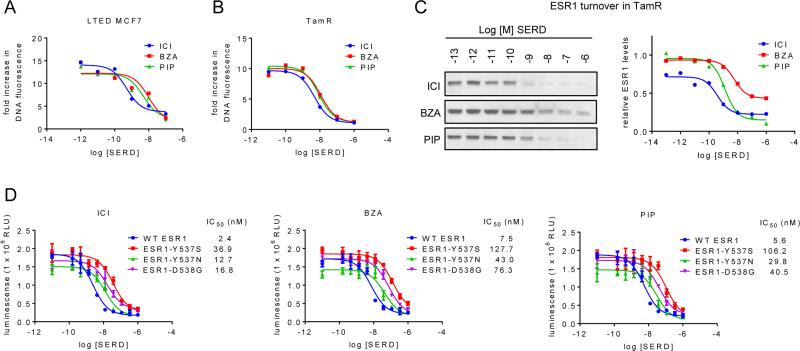
SERDs/SSHs are likely to be utilized and to have specific advantages in the setting of relapsed/resistant tumors in patients who have already progressed during tamoxifen and/or AI therapy. Thus, it was important to evaluate the efficacy of ICI, BZA and PIP in cellular models of endocrine therapy resistant breast cancer. Therefore, in vitro experiments similar to those in Figure 1 were conducted in MCF7 sublines that were adapted to grow under conditions of estrogen deprivation (LTED – a validated model of aromatase resistance (27-29)) or which were derived from tamoxifen-resistant xenografts (TamR (20)). As was observed for the endocrine therapy sensitive cells, ICI, BZA and PIP inhibited proliferation of the LTED (Figure 2A) and TamR (Figure 2B) cells with similar potency, and their ability to downregulate ESR1 in these resistant models mirrored that observed in the parental MCF7 cells (Figure 2C, Suppl. Figure S2A and not shown). Further, several mutations of ESR1 have recently been identified that are likely to contribute to disease progression (3-5). In agreement with prior studies, we observed that several of these mutants exhibit ligand-independent activation of ESR1 target gene transcription (data not shown). While these mutations reduce the potency of ICI, BZA, and PIP, all three compounds inhibited the proliferation of MCF7 cells expressing these mutations with similar efficacy (Figure 2D). In addition, in MCF7 cells expressing an ESR1-Y537N/D538G double mutation (recently identified in a fulvestrant-resistant patient tumor (5)), we observed a dramatically reduced sensitivity to SSHs and SERDs (Suppl. Figure S2B). However, it is important to note that these compounds effectively inhibited the activity of all of the ESR1 mutants tested, suggesting that their inhibition in patients will be possible if the tumor exposure of the drug(s) is sufficient to offset the decreased potency observed.
Figure 2. Fulvestrant, bazedoxifene, and pipendoxifene inhibit ESR1 activity in relevant models of endocrine therapy resistant breast cancer.
A) LTED MCF7 cells were plated in phenol red free media supplemented with FBS that was stripped of growth factors twice using charcoal. Cells were treated with ICI, BZA, and PIP (10−12 – 10−7 M) on days 1, 4, and 6 of an 8 day proliferation assay and analyzed as in Figure 1. B) TamR cells were plated in media supplemented with CFS 24 hours prior to treatment, and were treated with 1nM E2 as well as with ICI, BZA, and PIP (10−11 – 10−6 M) on days 1, 4, and 6 of an 8 day proliferation assay. Cell proliferation was quantitated as in Figure 1. C) TamR cells were plated in phenol red free media supplemented with charcoal stripped FBS 48 hours prior to treatment with ICI, BZA, or PIP (10−13 – 10−6 M) for 24 hours. Expression of ESR1 (C) and loading control cytokeratin 18 (CK18 – Suppl. Figure 2A) in whole cell extracts were detected by immunoblot (left). ESR1 levels relative to CK18 were quantitated by densitometry using Adobe Photoshop (right). D) MCF7 cells engineered to express wt ESR1 or Y537S, Y537N, or D538G mutations of ESR1 were treated for 7 days with increasing (10−11 – 10−6 M) concentrations of ICI, BZA, or PIP. DNA content, as assessed by luminescence (Cell Titer Glo assay), serves as an indicator of cell number in each condition. Data represent average detection +/− SD of triplicate wells. Data are representative of at least 3 independent experiments. IC50 values listed in the figure legend were calculated by non-linear curve regression using GraphPad Prism 6.
SERDs/SSHs and CDK4/6 inhibitors impact breast cancer growth by distinct mechanisms
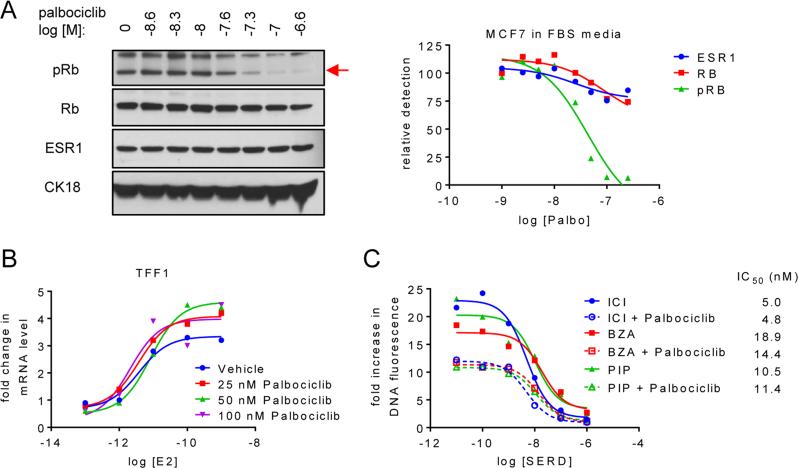
Previous studies have demonstrated that ESR1+ breast cancer cell models are particularly sensitive to CDK4/6 inhibitors (30). Given this observation and other work that has highlighted the significant convergence of the ESR1 and the cyclinD1/Rb/E2F1 pathways, it was considered likely that there would be a therapeutic advantage to combining CDK4/6 inhibitors with ESR1 antagonists. Thus, we performed a series of in vitro studies to evaluate the relative efficacies of ESR1 modulators (ICI, BZA or PIP) and the CDK4/6 inhibitor palbociclib as single agents or in combination in cellular models of ESR1-dependent breast cancer.
Treatment of MCF7 cells with increasing doses of palbociclib reduced Rb phosphorylation, but did not significantly change endogenous ESR1 expression (Figure 3A and Suppl. Figure S3A and B). Similarly, analysis of ESR1-dependent target gene expression revealed that neither the efficacy, nor the potency of 17β-estradiol was influenced by cotreatment with increasing doses of palbociclib (Figure 3B). Together, these data suggest that CDK4/6 inhibition itself is unlikely to directly impact ESR1 activity. Not surprisingly, using pRb as a readout in MCF-7 cells, we observed that when compared to the IC50 (43 nM) observed in Figure 3A using growth factor replete media, the IC50 of palbociclib was significantly left-shifted by growing cells in growth factor depleted media (a) alone (2 nM) or (b) supplemented with E2 (13 nM) (Suppl. Figure S3A and B, respectively). These findings underscore the importance of administering palbociclib concurrently with other agents that inhibit key cell growth pathways. A comparison of the ability of palbociclib to inhibit the proliferation of MCF7, LTED, and TamR cells revealed that all exhibited a similar sensitivity to CDK4/6 inhibition (Suppl. Figure S3C). As expected, E2-dependent MCF7 cell proliferation was attenuated by the addition of palbociclib (Figure 3C). Importantly, palbociclib did not negatively impact the anti-proliferative activity of ICI, BZA or PIP; nor did it change the IC50 of these drugs in this assay (Figure 3C). A similar response to SERD/SSH-palbociclib combinations was observed in the LTED and TamR cells (not shown). Considering these data, and that which has already been published on these drugs, it is likely that their antiproliferative activities, while converging on common growth-stimulatory pathways, occur by distinct mechanisms. Importantly, we observed that the expression of the Y537S, Y537N, or D538G ESR1 mutations in T47D breast cancer cells did not negatively impact the efficacy of the BZA-palbociclib combination (Suppl. Figure S3D).
Figure 3. SERDs or SERM/SERD hybrids and CDK4/6 inhibitors impact breast cancer growth by distinct mechanisms.
A) MCF7 cells were plated in media supplemented with FBS prior to 24 hours treatment with increasing concentrations of palbociclib (10−8.6 – 10−6.6 M). Levels of pRb, Rb, ESR1, and CK18 were detected by immunoblot of whole cell extracts (left) followed by densitometry analysis and normalization (right) as in Figure 2C. Red arrow (→) indicates protein band corresponding to pRb. Protein levels were normalized to the control (no palbociclib treatment) present in the first lane. B) MCF7 breast cancer cells were treated for 24 hours with increasing concentrations (10−13 – 10−9 M) E2 in the presence of palbociclib (0, 25, 50 or 100 nM). mRNA expression of TFF1 was analyzed as in Figure 1. C) Proliferation of MCF7 cells was analyzed as in Figure 1 after 8 days treatment with 1nM E2 as well as increasing concentrations of SSH/SERD (ICI, BZA, or PIP) and palbociclib (0 or 25 nM). Data are representative of at least 3 independent experiments.
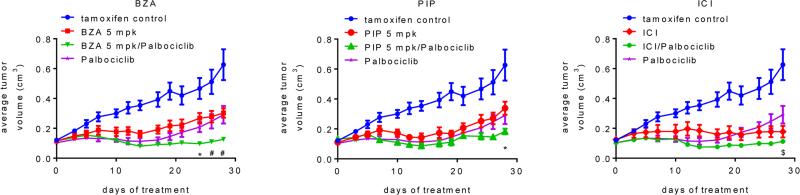
Palbociclib increases the efficacy with which SERDs inhibit the growth of tamoxifen-resistant breast cancer xenografts
Previously we have reported the development and characterization of an in vivo derived xenograft model of tamoxifen resistance. When engrafted in mice, this xenograft exhibits tamoxifen-dependent growth, an activity that can be attributed, at least in part, to increased FOXA1 activity as has been reported in endocrine therapy resistant breast tumors ((20, 31) and unpublished data). The ability of palbociclib, BZA and PIP to inhibit the tamoxifen-stimulated growth of TamR xenograft tumors in vivo as mono-therapies or as SERD/SSH-palbociclib combination therapies was next examined. For comparative purposes, we also included ICI and ICI-palbociclib arms in this study, although the dose of ICI used, and that which we and others have found to be required to inhibit tumor growth (~200 mg/kg weekly), far exceeds that administered to breast cancer patients (12, 32). Importantly, when corrected for species equivalency by body surface area (33), BZA and PIP were administered at doses similar to those previously evaluated in the clinic (18, 24, 34). Due to the high number of experimental groups, the data are presented in several panels to facilitate relevant comparisons. In this well-validated model of tamoxifen-resistant breast cancer (20, 35), we noted that palbociclib, BZA, PIP and ICI, when evaluated as mono-therapies, were similarly effective at inhibiting tumor growth (Figure 4 and Suppl. Figure S4A). The inhibition of tumor growth was similar in animals treated with 5 mg/kg BZA or PIP as compared to 10 mg/kg (Figure 4 and Suppl. Figure S4A). Of the combinations evaluated, only BZA-palbociclib significantly extended the duration of response as compared to either treatment alone, a result observed using either 5 or 10 mg/kg BZA (Suppl. Figure S4B and not shown). Interestingly, tumor regression (an uncommon response in this model) was initially observed for palbociclib treatment, followed by robust resistance in a subset of animals after 2-3 weeks of treatment (Figure 4). This variability in response to palbociclib correlated with the level of pRb apparent in the progressing tumors assessed at sacrifice (Suppl. Figure S4C), demonstrating resistance to palbociclib without loss of Rb expression. The mechanism by which these tumors develop resistance to palbociclib is currently under investigation. , We noted that pRb levels were reduced in the ICI, BZA and PIP treated animals, likely because cyclin D1 is a direct transcriptional target of ESR1 (Suppl. Figure S4C). However, whereas the impact of BZA, PIP and ICI, on tumor growth was statistically similar, higher levels of pRb were observed in the tumors of animals treated with PIP alone (Suppl. Figure S4C). Regardless, co-administration of palbociclib resulted in a sustained inhibition of Rb phosphorylation in all of the SSH/SERD-palbociclib treated tumors (Suppl. Figure S4C). Consistent with our in vitro analyses, it was observed that all of these compounds reduced the levels of intratumoral ESR1 whereas palbociclib treatment had no significant effect on receptor expression (Suppl. Figure S4C). Finally, evaluation of the intratumoral expression of the tamoxifen-induced genes AGR2 and KRT13 indicated that a) palbociclib treatment alone had little effect on ESR1 target gene activation, and b) all SSHs/SERDs efficiently inhibited ESR1 transcriptional activity regardless of palbociclib treatment (Suppl. Figure S4D). Overall, these findings highlight the efficacy of SSH/SERD-palbociclib combined treatment in a clinically relevant model of endocrine therapy resistant breast cancer.
Figure 4. Palbociclib increases the efficacy with which SERDs or SERM/SERD hybrids inhibit the growth of tamoxifen-resistant breast tumor xenografts.
TamR tumors were implanted into tamoxifen-treated mice. When Tam-stimulated tumors attained ~0.1cm3 tumor volume, animals were randomized (7-9 mice per group) to receive continued tamoxifen treatment as well as vehicle or SSH/SERD (BZA, 5 or 10 mg/kg/day s.c.; PIP, 5 or 10 mg/kg/day s.c.; or ICI, 5 mg/mouse 1× weekly i.m.), and also vehicle or palbociclib (100 mg/kg/day, p.o.). Tumor growth for each group (separated by SERD treatment for legibility) is presented as average tumor volume +/− SEM per study arm at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Tam control and palbociclib only treatments presented on each graph are identical. Tumor growth for animals treated with 5 mg/kg BZA or PIP are shown above, while measurements for animals treated with 10 mg/kg BZA or PIP are depicted in Suppl. Figure 4C. By day 14, responses to all treatments were significant as compared to the tam treatment only control (p < 0.01). Significant differences (p < 0.05) between combination treatments and SERD only (*), palbociclib only ($), or both single treatments (#) are indicated at appropriate time points.
Palbociclib and BZA inhibit the growth of xenograft tumors derived from patients resistant to endocrine interventions
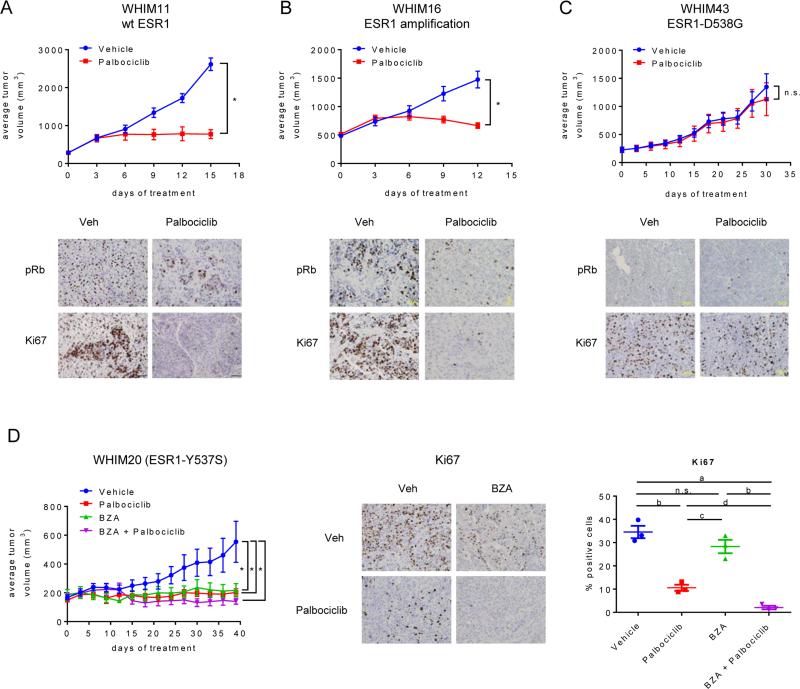
We next sought to evaluate the activity of selected ESR1-modulators and palbociclib as monotherapy or combination in patient derived xenograft (PDX) tumor models derived from biopsies of ESR1+ tumors that had progressed during or after endocrine therapies. Because palbociclib had not previously been evaluated in PDX models, we initially assessed the efficacy of palbociclib treatment alone in tumors expressing wt ESR1 (WHIM11), having amplified ESR1 expression (WHIM16), or expressing the ESR1-D538G mutation (WHIM43). Palbociclib significantly inhibited the growth of the WHIM11 and WHIM16 tumors, but was without effect on the WHIM43 tumors (Figure 5A-C). IHC analysis revealed that this lack of response was likely due to loss of Rb expression in this tumor (Figure 5C).
Figure 5. Palbociclib and BZA inhibit the growth of xenograft tumors derived from patients resistant to endocrine interventions.
A-C) WHIM11 (A), WHIM16 (B), or WHIM43 (C) PDX tumors were implanted into intact NSG mice. Animals bearing tumors of equivalent size (200-500 mm3 depending on the tumor model) were randomized (7-10 mice per group) to treatment with vehicle or palbociclib (125 mg/kg/day, p.o.). Upper panels: Tumor growth for each group is presented as average tumor volume +/− SEM per study arm at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Lower panels: pRb and Ki67 were detected by IHC analysis of representative tumors for each group. A-D) Asterisk (*) denotes significance (p < 0.0001) as compared to vehicle control. D) WHIM20 PDX tumors were implanted as above. When tumors reached ~200 mm3 volume, animals (4 mice per group) were randomized to palbociclib (125 mg/kg/day, p.o) or BZA (10 mg/kg/day sc) alone or in combination. Left: Tumor growth for each group is presented as average tumor volume +/− SEM per study arm at each day of treatment, with the initial day of treatment at randomization considered to be day 0. Center: Ki67 expression in one representative tumor per treatment group as detected by IHC analysis. Right: 38 sectors per tumor were quantitated by duplicate manual scoring of 3 tumors per group. Mean % positive cells/tumor of individual tumors, as well as the average (+/− SEM) per treatment group, are depicted in the right panel. Significant differences between treatments were detected by ANOVA followed by Holm-Sidak multiple comparison test and are indicated: a - p < 0.0001, b - p < 0.001, c - p < 0.01, d - p < 0.05, n.s. - comparison did not detect significant difference.
Having demonstrated the activity of palbociclib as a monotherapy in these clinically relevant models, we next evaluated its activity when administered in combination with BZA. From the ESR1+ PDXs available to us, we selected the WHIM20 tumor for these studies as it expresses ESR1-Y537S, an ESR1-mutation that exhibits significant SSH/SERD resistance (Figure 2D). In this model it was observed that BZA, palbociclib, or the combination were similarly effective in blocking tumor growth (Figure 5D). IHC analysis of these tumors revealed that the BZA-palbociclib combination resulted in a more complete suppression of Ki67 expression as compared to either treatment alone (Figure 5D). Together these data confirm the likely utility of BZA-palbociclib combination in achieving maximal suppression of tumor growth and may explain why, in the xenograft studies described in Figure 4, the duration of the response to the BZA-palbociclib combination is longer than that observed in tumors treated with each drug alone.
Discussion
Tamoxifen and aromatase inhibitors remain the first-line interventions of choice for the treatment of ESR1+ breast cancers (36). Further, the SERMs tamoxifen and raloxifene and the AI exemestane are active chemopreventative agents (37, 38). The enduring presence of tamoxifen and aromatase inhibitors in contemporary breast cancer treatment regimens attests to the central importance of ESR1 as a driver of tumor growth and progression. However, resistance to endocrine therapy is nearly universal in advanced disease, and ESR1+ tumors remain the majority cause of death from breast cancer. Although the mechanisms underlying resistance to endocrine therapy are complex and varied, loss of dependence on ESR1 and its downstream signaling pathways is an infrequent event. Therefore, ESR1 and the estrogen signaling axis remain important therapeutic targets, an observation that has driven the search for agents that target this pathway by new mechanisms. In this study, we demonstrate that ICI, BZA and PIP, high-affinity competitive antagonists of ESR1 that downregulate the receptor to varying degrees, are effective in relevant models of endocrine therapy resistant disease. Importantly, these modulators inhibited the activity of clinically relevant ESR1 mutations with efficacy similar to that observed for the wt ESR1 despite significantly reduced potency. Most importantly, we observed in both endocrine-resistant breast cancer xenograft tumors, and in PDX tumors expressing a relevant mutation of ESR1 (Y537S), that the SSH/SERD bazedoxifene in combination with palbociclib resulted in significant tumor growth control.
It is generally accepted that sub-optimal pharmaceutical properties of the only currently approved SERD, fulvestrant, limit the achievable tumor exposure (12, 39-41). Despite these difficulties, higher doses of fulvestrant have improved overall survival in two trials, highlighting the promise of more effective ESR1 targeting. The identification of treatment-associated ESR1 mutations emphasizes the need for improved SERDs able to inhibit these mutations at therapeutically achievable levels. Fortunately, most of the newer, third generation ESR1 antagonists have improved pharmaceutical properties and their efficacy should not be limited by drug exposure. One SERD, ARN810 (BPL2), is currently being evaluated in a phase I/IIa trial enrolling relapsed/recurred breast cancer patients (42). However, BZA, a low-toxicity drug that has already been approved for the treatment and prevention of osteoporosis, may have immediate utility in advanced disease (16, 17).
Several new SERMs and SERDs will be available for clinical use in the next five years, and, by virtue of their distinct mechanisms of action, it may be possible to sequence their use to increase the duration of treatment response. However, more durable responses will likely be achieved by appropriate combination of SERDs/SERMs with other drugs that inhibit pathways of importance in cancer. Recently, we reported that the Notch signaling pathway is activated in tamoxifen-resistant breast cancers and that the growth of resistant xenografts could be inhibited using γ-secretase inhibitors; thus, the combination of a SERM/SERD with a Notch inhibitor may be beneficial (43). . There is also considerable experimental data that supports the combination of ESR1 modulators with inhibitors of ERK or PI3K signaling, although toxicities observed with the latter two classes of drug may limit their use (44). The most provocative data thus far on combination use have come from the PALOMA-1 trial in which the duration of progression free survival was nearly doubled in patients receiving a palbociclib-letrozole combination vs. letrazole alone (19).
Although AI-palbociclib combinations are in late-stage clinical development, there remains considerable enthusiasm for strategies in which palbociclib is combined with a SERD or SERM. One important advantage of a SERM or SSH/SERD over an AI is that its competitive activity will prevent the activation of ESR1, either independent of ligand or by endogenous steroidal estrogens or other non-steroidal compounds that exhibit estrogenic activity (e.g. 27-hydroxycholesterol). Indeed, several trials have been opened evaluating palbociclib combined with fulvestrant or tamoxifen in patients who have progressed after endocrine therapies. As reported here, and by others in the past, resistance to endocrine therapy can be attributed in some instances to somatic mutations in ESR1 that reduce the IC50 of the known SERMs and SERDs (3-5, 13). The pharmaceutical properties of fulvestrant are likely to limit its utility in treating tumors having ESR1 mutations. Likewise, pre-existing resistance to tamoxifen in advanced breast cancers makes it unsuitable for the combined therapy. Importantly, when appropriately dose corrected, all of the SERDs/SSHs evaluated in this study were able to inhibit the activity of the common receptor mutants. Given the pharmaceutical properties of BZA, it is reasonable to expect that this compound would have efficacy, as a single agent, in tumors expressing ESR1 mutations. Our studies also highlight the potential utility of palbociclib and BZA-palbociclib combinations in endocrine therapy resistant tumors. The mechanistic basis for the favorable activity of SERM/SERD-palbociclib combinations is currently under investigation.
In conclusion, there is substantial data highlighting the multiple points of convergence between the ESR1 and cyclin D1/E2F1/Rb signaling pathway. The results of the studies presented herein highlight how knowledge of these interactions has led to the development of useful strategies to inhibit multiple key steps points in this pathway to yield useful responses in preclinical models of advanced breast cancer. These findings underscore the need for the near-term clinical evaluation of SERD-palbociclib combinations in patients with advanced breast cancer.
Supplementary Material
Translational Relevance.
Resistance to endocrine therapies is a significant clinical issue in patients with estrogen receptor (ESR1) positive breast tumors. In refractory disease, however, ESR1 remains active and engaged, providing the impetus to evaluate third generation Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Downregulators (SERDs) as therapeutic interventions. In this study, we demonstrate in both cell-line derived and patient-derived xenograft models of endocrine therapy resistant breast cancer that the efficacy of the SERM/SERD hybrid (SSH) molecules bazedoxifene and pipendoxifene, or the SERD fulvestrant, were increased when co-administered with palbociclib, an inhibitor of the cyclin-dependent kinases 4 and 6. Importantly, these drug combinations were also effective in tumors expressing ESR1 ligand binding domain mutations associated with resistance to endocrine therapy. Bazedoxifene and fulvestrant are approved for the treatment of osteoporosis or breast cancer, respectively; thus, an evaluation of their activity in combination with palbociclib is a priority for clinical investigation.
Acknowledgements
The authors thank Dr. D. Clay Rouse, DVM (Duke University) for surgical expertise.
Grant support
Supporting funding provided by the NIH (2R01DK048807) (SEW, HMA, and DPM), Susan G Komen Grants KG090422, BCTR0707808 and Promise Grant PG 12220321 (all to MJE), Washington University Institute for Clinical and Translational Sciences (NIH/NCRR 5UL1RR02499203 to MJE and SL) and Siteman Cancer Center (NIH/NCI P30 CA91842) support for the Washington University HAMLET core, Brown Shoe, Theresa's Research Foundation, and research grants from Pfizer Pharmaceuticals (DPM, SL).
Supporting funding provided by the NIH (R37DK048807) (SEW, HMA, and DPM), Susan G Komen Grants KG090422, BCTR0707808 and Promise Grant PG 12220321 (all to MJE), Washington University Institute for Clinical and Translational Sciences (NIH/NCRR 5UL1RR02499203 to MJE and SL) and Siteman Cancer Center (NIH/NCI P30 CA91842) support for the Washington University HAMLET core), Brown Shoe, Theresa's Research Foundation, and research grants from Pfizer Pharmaceuticals (SEW, HMA, DPM, SL).
Footnotes
Disclosure summary: D.P.M, M.J.E. and S.E.W. have in the past served as paid consultants for Pfizer, Inc.
S.G.D, K.E., K.T.A. and T.V. are employed by Pfizer, Inc.
References
- 1.Carter D. New global survey shows an increasing cancer burden. Am J Nurs. 2014;114:17. doi: 10.1097/01.NAJ.0000444482.41467.3a. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Kobayashi Y, Wada O, Tateishi Y, Kitada L, Yamamoto Y, et al. Full activation of estrogen receptor alpha activation function-1 induces proliferation of breast cancer cells. J Biol Chem. 2003;278:26704–14. doi: 10.1074/jbc.M301031200. [DOI] [PubMed] [Google Scholar]
- 3.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–67. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–45. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang YF, Liao YY, Yang M, Peng NF, Xie SR, Xie YF. Discordances in ER, PR and HER2 receptors between primary and recurrent/metastatic lesions and their impact on survival in breast cancer patients. Med Oncol. 2014;31:214. doi: 10.1007/s12032-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 7.Drury SC, Detre S, Leary A, Salter J, Reis-Filho J, Barbashina V, et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer. 2011;18:565–77. doi: 10.1530/ERC-10-0046. [DOI] [PubMed] [Google Scholar]
- 8.Perey L, Paridaens R, Hawle H, Zaman K, Nole F, Wildiers H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00). Ann Oncol. 2007;18:64–9. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 9.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256:1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson J, Llombart-Cussac A, Feltl D, Dewar J, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II ‘FIRST’ study.. San Antonio Breast Cancer Symposium; San Antionio, Texas, USA. December 9-13, 2014.2014. p. Abstract S6-04. [Google Scholar]
- 11.Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–5. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 12.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell reports. 2013;4:1116–30. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahraman M, Govek S, Nagasawa J, Smith N, Seragon Pharmaceuticals, Inc Estrogen receptor modulators and uses thereof. 2014 Apr 17; United States patent US 201407095A1.
- 15.Smith N, Kahraman M, Govek S, Nagasawa J, Lai A, Julien J, et al. Aragon Pharmaceuticals, Inc. Estrogen receptor modulators and uses thereof. 2013 Sep 5; United States patent US 20130231333A1.
- 16.Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor alpha and cyclin D1. Mol Pharmacol. 2011;80:610–20. doi: 10.1124/mol.111.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19:2420–31. doi: 10.1158/1078-0432.CCR-12-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christiansen C, Chesnut CH, 3rd, Adachi JD, Brown JP, Fernandes CE, Kung AW, et al. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled Phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet Disord. 2010;11:130. doi: 10.1186/1471-2474-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RS. Final results of a randomized phase II study of PD0332991, a cyclin-dependent kinase (CDK)-4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1; TRIO-18) [abstract].. Proc Ann Meeting AACR.2014. p. CT101. [Google Scholar]
- 20.Connor CE, Norris JD, Broadwater G, Willson TM, Gottardis MM, Dewhirst MW, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917–22. [PubMed] [Google Scholar]
- 21.Jeng M-H, Shupnik MA, Bender TP, Westin EH, Bandyopadhyay D, Kumar R, et al. Estrogen Receptor Expression and Function in Long-Term Estrogen-Deprived Human Breast Cancer Cells. Endocrinology. 1998;139:4164–74. doi: 10.1210/endo.139.10.6229. [DOI] [PubMed] [Google Scholar]
- 22.Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor alpha by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82:122–30. doi: 10.1016/j.bcp.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardell SE, Nelson ER, McDonnell DP. From empirical to mechanism-based discovery of clinically useful Selective Estrogen Receptor Modulators (SERMs). Steroids. 2014;90:30–8. doi: 10.1016/j.steroids.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotreau MM, Stonis L, Dykstra KH, Gandhi T, Gutierrez M, Xu J, et al. Multiple-dose, safety, pharmacokinetics, and pharmacodynamics of a new selective estrogen receptor modulator, ERA-923, in healthy postmenopausal women. J Clin Pharmacol. 2002;42:157–65. doi: 10.1177/00912700222011193. [DOI] [PubMed] [Google Scholar]
- 25.Wong H, Grossman SJ, Bai SA, Diamond S, Wright MR, Grace JE, Jr., et al. The chimpanzee (Pan troglodytes) as a pharmacokinetic model for selection of drug candidates: model characterization and application. Drug Metab Dispos. 2004;32:1359–69. doi: 10.1124/dmd.104.000943. [DOI] [PubMed] [Google Scholar]
- 26.Johnston SR, Head J, Pancholi S, Detre S, Martin LA, Smith IE, et al. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res. 2003;9:524S–32S. [PubMed] [Google Scholar]
- 27.Martin LA, Ghazoui Z, Weigel MT, Pancholi S, Dunbier A, Johnston S, et al. An in vitro model showing adaptation to long-term oestrogen deprivation highlights the clinical potential for targeting kinase pathways in combination with aromatase inhibition. Steroids. 2011;76:772–6. doi: 10.1016/j.steroids.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995;80:2918–25. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- 29.Yue W, Fan P, Wang J, Li Y, Santen R. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–10. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast cancer research : BCR. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chisamore MJ, Wilkinson HA, Flores O, Chen JD. Estrogen-related receptor-alpha antagonist inhibits both estrogen receptor-positive and estrogen receptor-negative breast tumor growth in mouse xenografts. Mol Cancer Ther. 2009;8:672–81. doi: 10.1158/1535-7163.MCT-08-1028. [DOI] [PubMed] [Google Scholar]
- 33.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 34.Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525–35. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 35.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–8. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20:596–604. doi: 10.2174/092986713804999303. [DOI] [PubMed] [Google Scholar]
- 37.Decensi A, Dunn BK, Puntoni M, Gennari A, Ford LG. Exemestane for breast cancer prevention: a critical shift? Cancer Discov. 2012;2:25–40. doi: 10.1158/2159-8290.CD-11-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prev Res (Phila Pa) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linden H, Kurland B, Livingston R, Sandararajan L, Peterson L, Schubert E, et al. PET FES Measures Uterine and Tumor In Vivo Pharmacodynamics Of Endocrine Therapy. Meeting abstracts: San Antonio Breast Cancer Meeting. 2009 [Google Scholar]
- 40.Robertson J, Nicholson R, Bundred N, Anderson E, Rayter Z, Dowsett M, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61:6739–46. [PubMed] [Google Scholar]
- 41.Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of Fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 42.Mayer I, Bardia A, Dickler M, Manning H, Mahmood U, Ulaner G, et al. Abstract OT3-2-07: Phase I study of ARN-810, a novel selective estrogen receptor degrader, in post-menopausal women with locally advanced or metastatic estrogen receptor positive breast cancer. Cancer Res. 2013;73 Abstract nr OT3-2-07. [Google Scholar]
- 43.Martz C, Ottina K, Singleton K, Jasper J, Wardell S, Peraza-Penton A, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Science Signaling. 2014 doi: 10.1126/scisignal.aaa1877. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–62. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.