Abstract
Purpose
Two clinical-stage anticancer drugs, the Bcl-2 inhibitor ABT-263 and the MDM2 inhibitor SAR405838 achieve complete tumor regression in animal models of leukemia but also induce acquired resistance. Elucidation of acquired resistance mechanisms and development of strategies to overcome the resistance are critical for their successful clinical development.
Experimental Design
We employed RS4;11 and MV4;11 cell lines, two acute leukemia models, to investigate acquired resistance mechanisms for both drugs in vitro and in vivo and evaluated several treatment regimens in xenograft mouse models to improve long-term, complete tumor regression.
Results
Resistance to either SAR405838 or ABT-263 (or its analogue ABT-737) develops in acute leukemia models in vitro and in vivo. RS4;11 and MV4;11 tumors treated with SAR405838 acquire resistance to the drug by mutation of the p53 gene or compromise of p53 function. RS4;11 tumors treated with either ABT-263 or ABT-737 acquire resistance primarily through down-regulation of BAX but not BAK. When acute leukemia cells become highly resistant to the MDM2 inhibitor, they retain their sensitivity to the Bcl-2 inhibitors, or vice versa. Certain sequential or combination treatment of SAR405838 and ABT-263 can achieve longer-term tumor regression than treatment with either agent alone.
Conclusion
Our study provides new insights into the mechanisms of acquired resistance of Bcl-2 and MDM2 inhibitors in acute leukemia models and suggests that certain sequential or combination treatment of these two distinct classes of apoptosis-inducing agents should be tested as new treatment strategies for acute leukemia in the clinic.
Keywords: Resistance, MDM2, Bcl-2, Inhibitor, Leukemia
Introduction
Acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) remain incurable with conventional chemotherapy and new therapeutic strategies are needed (1-3). Targeting key apoptosis regulators to promote apoptosis in tumor cells is being pursued as a promising cancer therapeutic strategy (4).
Bcl-2 family proteins are a class of master regulators of apoptosis (5-9). Bcl-2, the founding member of the family, is overexpressed in patients with leukemia and is an attractive therapeutic target (10, 11). Intense research efforts have yielded potent small-molecule inhibitors of Bcl-2 such as ABT-737 (12) and its orally active analogue ABT-263 (navitoclax) (13). Both compounds have demonstrated impressive single-agent activity against leukemia (12-14). Both compounds bind to Bcl-2, Bcl-xL and Bcl-w with high affinities but Bcl-2 appears to be their most critical target in leukemia cells in vivo (15). Accordingly, we refer to them as Bcl-2 inhibitors in this study.
The tumor suppressor p53 is another attractive therapeutic target for leukemia (16-21). In about 90% of leukemias, p53 retains its wild-type status, but its function is effectively inhibited by its endogenous cellular antagonist MDM2 (22-26). Small molecules designed to block the p53-MDM2 interaction (MDM2 inhibitors) activate the tumor suppressor function of wild-type p53 (27-30). Several highly potent MDM2 inhibitors, such as RG7112 (29, 31) and SAR405838 (32) are now in clinical trials for cancer treatment.
While both ABT-263 (13) and SAR405838 (32) can achieve complete tumor regression in xenograft models of leukemia, tumors eventually regrew after termination of the treatment, suggesting the emergence of resistance to both classes of drugs. Such acquired resistance is a major cause of cancer drug failure in clinical trials (33). Although resistance mechanisms for Bcl-2 and MDM2 inhibitors have been investigated in cell culture models (34-39), no study of their in vivo acquired resistance mechanisms has been reported.
In this study, we have elucidated acquired resistance mechanisms for the Bcl-2 and MDM2 inhibitors in vitro and in vivo using the RS4;11 and the MV4;11 leukemia cell lines. The RS4;11 cell line was established from an acute lymphoblastic leukemia (ALL) patient, whereas the MV4;11 cell line was established from a patient with acute myeloid leukemia (AML). Both leukemia cell lines contain wild-type p53 and harbor a chromosomal t(4;11) translocation. While the RS4;11 cell line harbors wild-type FLT3, the MV4;11 cell line harbors a FLT3-ITD mutation, a common (25-30%) mutation associated with poor prognosis in AML patients (40-42). Both cell lines are sensitive to apoptosis induction by Bcl-2 and MDM2 inhibitors in vitro and are therefore excellent models to investigate the acquired resistance of leukemia cells to these two classes of apoptosis-inducing agents.
Our study has yielded new insights into the resistance mechanisms for both classes of drugs and resulted in novel therapeutic strategies.
Materials and Methods
Reagents and antibodies
SAR405838 was provided by Sanofi. ABT compounds were purchased from Selleck Chemicals (Houston, TX). Rabbit antibodies for caspase-3, PARP, Mcl-1 (D35A5), Bcl-xL (54H6) and mouse antibody for caspase-7 were obtained from Cell Signaling Technology (Danvers, MA); rabbit antibodies for GAPDH and BAK (G-23) and mouse antibodies for BAX (6A7 and 6D149) and Bcl-2 were from Santa Cruz Biotechnology (Dallas, TX); mouse antibody p53 (Ab-6) and MDM2 (Ab-1) and rabbit PUMA (Ab-1) were from Calbiochem (Millipore). Mouse antibody for p21 was from BD Pharminogen (San Jose, CA).
Cell Culture, cell viability, and apoptosis assays
RS4;11 and MV4;11 cell lines were purchased from American Type Culture Collection (ATCC), where authentication is performed by STR analysis, and cultured as recommended for a maximum of 3 months. All in vivo acquired resistant sublines were cultured for a maximum of 15 passages. Cell viability was evaluated by a WST-8 assay (Dojindo) (43). Apoptosis was analyzed using Annexin V-FLUOS staining kit (Roche Applied Science, Indianapolis, IN). Differences in mean values of cell apoptosis among different groups were analyzed by 2-way ANOVA using Prism, with a P value of <0.05 being considered significant.
Resistant Cell Lines
Both parental cell lines were treated with ABT-737 starting from 10 nM for 72 hrs. The cells were then rinsed and the remaining live cells were expanded in regular medium. This process was repeated with increased drug concentration till 10 μM and surviving cells were utilized for subsequent experiments. An identical protocol was utilized to obtain in vitro sublines resistant to SAR405838, with the exception of the final drug concentration being 20 μM. DMSO treated cell lines were generated as controls.
Short hairpin RNA (shRNA) interferences
Short 19-bp hairpins for generating RNA interference: BAX (nucleotides 239-257, Genbank NM138761), BAK (nucleotides 535-553, Genbank NM001188) and p53 (nucleotides 611-629 Genbank NM000546) (35). The oligonucleotides were annealed and ligated into a self-inactivating lentiviral vector under the control of the H1 promoter (44). The vector also carried the GFP reporter gene under control of the human ubiquitin-C promoter to monitor infection efficiency. A scrambled shRNA construct was utilized as a control (35). Lentiviral shRNA virus-containing supernatant, generated by the University of Michigan Vector Core, was used to infect RS4;11 and MV4;11 cells. 96 h post infection, the infected cells were sorted for GFP fluorescence.
p53 Mutation analysis
Primers to amplify and sequence genomic DNA for exons 2 to 11 of human p53 were used according to Hauser et al. (45). Primers to amplify and sequence cDNA for exons 2 to 11 of human p53 were used according to Aziz et al. (36). Mutation surveyor (SoftGenetics LLC) was used to compare experimental sequences against Refseq GenBank as well as by visual inspection of sequence tracings.
In vivo xenograft studies
To develop xenograft tumors, 5 × 106 tumor cells with 50% Matrigel were injected subcutaneously on the dorsal side of SCID mice. For efficacy experiments, tumor sizes and animal weights were measured 2-3 times per week with tumor volume (mm3) = (length × width2)/2. Significance (P) was calculated by unpaired two-tailed t test using Prism. P value of < 0.05 being considered significant.
Results
Establishment and characterization of RS4;11 sublines resistant to the Bcl-2 inhibitors
We first employed the RS4;11 cell line to investigate the acquired resistance mechanisms to the Bcl-2 inhibitors.
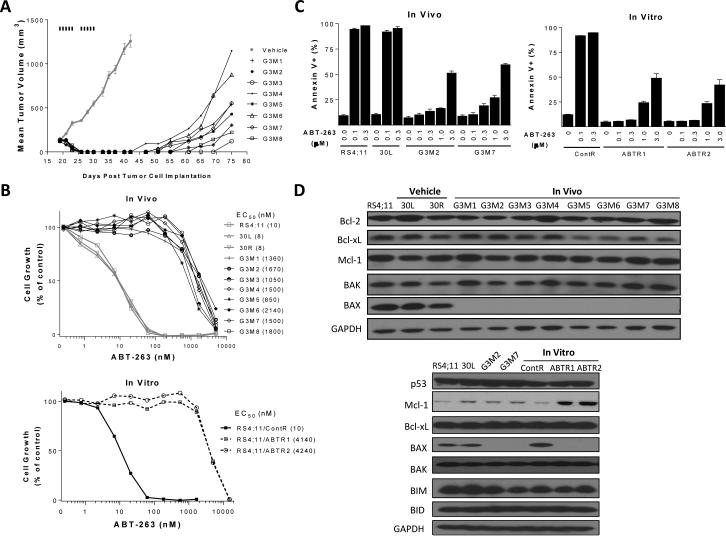
Treatment of RS4;11 xenograft tumors in mice with ABT-737 yielded complete tumor regression (Fig. 1A), but all tumors regrew after termination of the treatment. We isolated RS4;11 tumor cells from the regrown tumors and established 8 sublines (G3M1-G3M8). While the RS4;11 parental cell line and 2 representative sublines (30L and 30R) established from the vehicle-treated tumors are equally sensitive to both ABT-737 and ABT-263 in a cell growth assay, all 8 sublines obtained from the ABT-737 treated tumors become resistant to ABT-263 and ABT-737, with an average IC50 value >100-fold higher than the control cell lines (Fig. 1B and SI Fig. S1A). ABT-737 and ABT-263 also become ineffective in induction of apoptosis in the G3M1-G3M8 sublines (Fig. 1C and SI Fig. S1B).
Figure 1.
Establishment and characterization of in vitro and in vivo RS4;11 sublines resistant to ABT-263/ABT-737. A, Establishment of in vivo RS4;11 sublines. RS4;11 xenograft tumors were treated with vehicle or ABT-737 at 100 mg/kg, i.p. for 5 days per week for 2 weeks. Vehicle treated tumors and ABT-737-treated, regrown tumors were harvested and cultured to generate sublines. B, Sensitivity of in vivo and in vitro RS4;11 sublines to ABT-263 in a cell growth assay. Parental, two representative vehicle-treated sublines (30L and 30R) and 8 sublines (G3M1-G3M8) generated from ABT-treated tumors were treated with ABT-263 or vehicle control for 4 days and cell viability was determined by a WST assay. C, Sensitivity of in vivo and in vitro RS4;11 sublines to ABT-263 in an apoptosis assay. Parental RS4;11 cell line and sublines established from RS4;11 xenografts (30L, vehicle-treated; G3M2 and G3M7, SAR405838-treated) and RS4;11 sublines established in vitro (ContR, vehicle-treated; ABTR1 and ABTR2, SAR405838-treated) were treated with ABT-263 or vehicle control for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining. Data (mean ± SD) are from triplicates, including both early (Annexin V-positive/PI-negative) and late (Annexin V-positive/PI-positive) apoptotic cells. D, Immunoblotting of Bcl-2 family proteins for in vitro and in vivo RS4;11 resistant sublines and control lines. GAPDH was used as the loading control.
Upregulation of the Mcl-1 protein was identified as the primary acquired resistance mechanism to ABT-737 (34, 35). Western blotting, however, showed that the resistant G3M1-G3M8 and the sensitive control cell lines have similar levels of Mcl-1 expression (Fig. 1D). Profiling of other Bcl-2 family members showed that compared to the control cell lines, BAX protein is profoundly down-regulated in all the 8 resistant cell lines (Fig. 1D), but other Bcl-2 proteins, including BAK, have similar levels between resistant and sensitive cell lines (Fig. 1D and SI Fig. S1C).
To investigate the acquisition of resistance to the Bcl-2 inhibitors in vitro, the RS4;11 cells were exposed to gradually increasing concentrations of ABT-737. This yielded two sublines (ABTR1 and ABTR2), which exhibit >100-fold resistance to ABT-263 compared to the parental RS4;11 cell line in a cell growth assay (Fig. 1B) and are also insensitive to apoptosis induction by ABT-263 (Fig. 1C). Western blotting showed that the BAX protein, but not BAK, is also greatly down-regulated in ABTR1 and ABTR2 sublines when compared to the parental cell line. However, in contrast to the lack of Mcl-1 upregulation in all the 8 in vivo resistant sublines, Mcl-1 is up-regulated in the two in vitro resistant sublines when compared to the control cell lines (Fig. 1D).
We analyzed the mRNA levels for both BAX and Mcl-1 in these resistant and control cell lines by qPCR. Our data showed that the mRNA levels of BAX are reduced by approximately 70% in each of these resistant sublines obtained from in vitro and in vivo ABT-737 treatment compared to the RS4;11 parental and vehicle control treated cell lines (SI Fig. S1D). However, the Mcl-1 mRNA levels are very similar in these two resistant cell lines compared to the RS4;11 parental cell line (SI Fig. S1D). Hence, while the reduced transcription of BAX clearly contributes to the reduction in BAX protein in these in vitro and in vivo resistant sublines, the increase of Mcl-1 protein in these two in vitro resistant sublines is not due to a change in Mcl-1 transcription.
Establishment and characterization of RS4;11 resistant sublines to the MDM2 inhibitor
The MDM2 inhibitor SAR405838 inhibits cell growth with IC50 = 140 nM and effectively induces apoptosis in the RS4;11 cell line (Fig. 2).
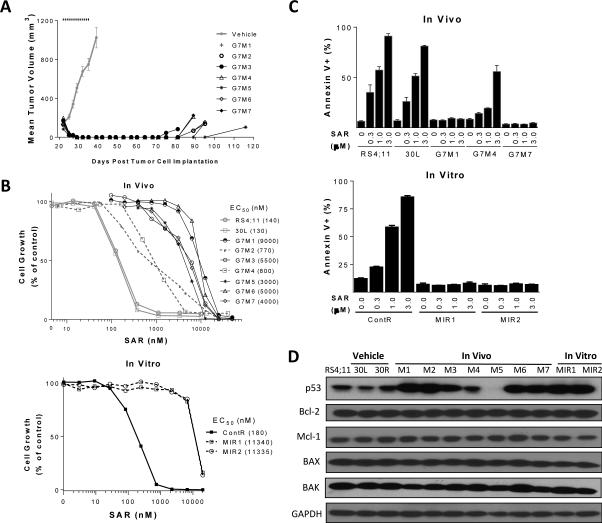
Figure 2.
Establishment and characterization of RS4;11 sublines obtained from in vitro and in vivo treatment with SAR405838. A, Establishment of in vivo RS4;11 sublines. RS4;11 xenograft tumors were treated with vehicle or 200 mg/kg of SAR405838 orally for 21 days and SAR405838-treated tumors (G7M1-G7M7) were harvested when the regressed tumors regrew to approximately 100 mm3 and then cultured to establish sublines. B, Sensitivity of in vivo and in vitro RS4;11 sublines to SAR405838 in a cell growth assay. Parental RS4;11 cell line and sublines established from RS4;11 xenografts (30L, vehicle-treated; G7M1-G7M7, SAR405838-treated) and RS4;11 sublines established in vitro (ContR, vehicle-treated; MIR1 and MIR2, SAR405838-treated) were treated with SAR405838 and cell viability was determined by a WST assay. C, Sensitivity of in vivo and in vitro RS4;11 sublines to SAR405838 in an apoptosis assay. RS4;11 parental and established sublines were treated with SAR405838 for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining. D, Immunoblotting of representative Bcl-2 family proteins and p53 for in vitro and in vivo RS4;11 sublines and control lines, with GAPDH as the loading control.
Treatment of the RS4;11 xenograft tumors in mice with SAR405838 induced rapid and complete tumor regression, which persisted for >30 days, but eventually the tumors returned (Fig. 2A). Harvesting and culturing all regrown tumors treated with SAR405838 established 7 sublines (G7M1-G7M7). A cell growth assay showed that while two sublines, G7M2 and G7M4, exhibited 5-fold greater resistance to SAR405838 compared with the parental and vehicle treated cell lines, the other 5 sublines showed >35-fold resistance to SAR405838 (Fig. 2B). The moderate resistance to SAR405838 in the G7M4 subline and more significant resistance in the G7M1 and G7M7 sublines were confirmed in an apoptosis assay (Fig. 2C and SI Fig. S2A).
We also established two RS4;11 sublines (MIR1 and MIR2) in vitro by gradually increasing the concentration of SAR405838 in the cell culture. Both the MIR1 and MIR2 sublines are >60-fold less sensitive to SAR405838 than the control cell lines in a cell growth assay (Fig. 2B) and their resistance was confirmed in an apoptosis assay (Fig. 2C).
Since the activity of SAR405838 depends upon wild-type p53 (32), we analyzed the p53 mutation by sequencing exons 2-11 in all the in vitro and in vivo sublines obtained with SAR405838 treatment. Both the MIR1 and MIR2 in vitro sublines and 5 out of 7 of the in vivo sublines harbor p53 mutation(s) (SI Table S1).
Immunoblotting showed that, compared to the control cell lines, basal p53 protein expression is increased in all the sublines harboring p53 mutation(s) (G7M1-3, 6-7 and MIR1-2) (Fig. 2D). qPCR analysis showed that p53 mRNA levels are not significantly changed in G7M1, G7M2, MIR1 and MIR2 sublines with a mutated p53 compared to the parental RS4;11 cell line but much lower in the G7M5 subline with a low level of p53 protein (SI Fig. S2B). In contrast to the loss of BAX expression in the resistant sublines obtained from the ABT-737/ABT-263 treatment, similar levels of BAX protein were found in all the sublines obtained from treatment with SAR405838 and in the parental cell line (Fig. 2D).
To investigate if any of these sublines obtained from SAR405838 treatment still retains functional p53, we examined p21, a p53-regulated protein, accumulation of p53 protein and PARP cleavage in these sublines treated with SAR405838 (SI Fig. S2C and S2D). In the G7M4 subline lacking p53 mutation, SAR405838 induces a dose-dependent increase of p21 and p53 proteins and cleavage of PARP, indicative of p53 activation and apoptosis induction, but with a reduced potency as compared to that in the parental cell line (SI Fig. S2C). In G7M5, which has no expression of p53 at the basal level (Fig. 2D), SAR405838 fails to induce p53 and p21 upregulation and PARP cleavage (SI Fig. S2C). No significant induction of p21 protein, PARP cleavage or p53 accumulation is observed in the sublines harboring p53 mutation (G7M1, G7M3 and G7M7) when treated with SAR405838, indicative of the absence of p53 activation (SI Fig. S2D).
Hence, these data show that treatment of the RS4;11 cell line in vitro and in vivo by SAR405838 yielded acquired resistant sublines, which have either inactive, mutated p53 or p53 with compromised function.
RS4;11 sublines resistant to the Bcl-2 inhibitors retain their sensitivity to the MDM2 inhibitor
Exons 2-11 were sequenced in all the RS4;11 resistant sublines to ABT compounds. No p53 mutation could be detected, suggesting that these sublines may contain wild-type p53 and could be still sensitive to MDM2 inhibitors.
Indeed, SAR405838 potently inhibits cell growth and induces dose-dependent apoptosis in the ABTR1 and ABTR2 sublines (Fig. 3), albeit with modestly reduced potency compared to that in the control RS4;11 cell line.
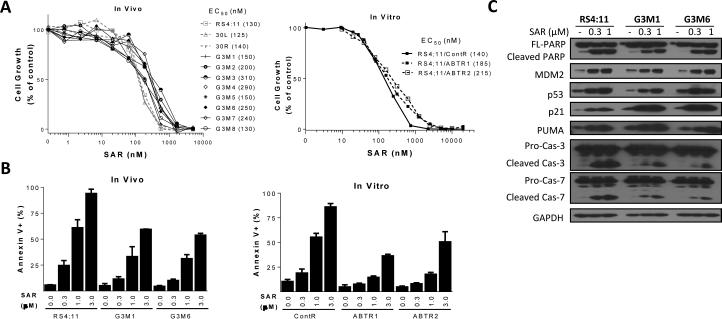
Figure 3.
Evaluation of SAR405838 in RS4;11 xenografts or sublines treated with Bcl-2 inhibitors. A, RS4;11 parental and sublines previously exposed to ABT-737 in vivo (G3M1-G3M8) and in vitro (ABTR1 and ABTR2) were treated for 4 days with SAR405838 and cell viability was determined by a WST assay. B, RS4;11 parental and representative sublines were treated with SAR405838 for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining. C, RS4;11 parental and two representative sublines (G3M1 and G3M6) were treated with SAR405838 for 24 h for immunoblotting of p53 and p53-regulated proteins and biochemical markers of apoptosis.
In the 8 in vivo sublines (G3M1-G3M8) resistant to ABT compounds, SAR405838 is still effective in inhibition of cell growth (Fig 3A). Apoptosis induction by SAR405838 is attenuated by only 2-3 times in the G3M1 and G3M6 sublines compared to the RS4;11 parental and vehicle-treated cell lines (Fig. 3B). In the RS4;11 parental, and the G3M1 and G3M6 sublines, SAR405838 dose-dependently induces activation of p53 and apoptosis, as shown by upregulation of p53, p21, MDM2 and PUMA proteins, and cleavage of caspase-3/-7 and PARP (Fig. 3C).
Hence, when the RS4;11 cells acquire profound resistance to the Bcl-2 inhibitors, the cells are still sensitive to the MDM2 inhibitor, thus lacking cross-resistance.
RS4;11 sublines resistant to SAR405838 retain sensitivity to ABT-263
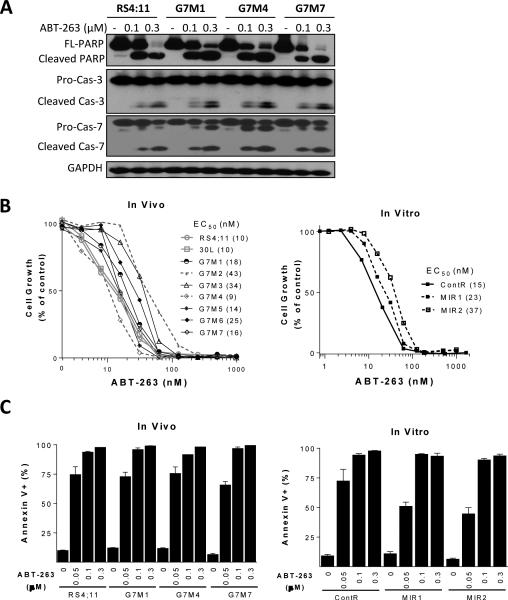
We next evaluated ABT-263 for its effectiveness in the RS4;11 sublines obtained with SAR405838 treatment. In cell growth and apoptosis assays, both in vitro (MIR1 and MIR2) and in vivo (G7M1-G7M7) sublines show similar sensitivity to ABT-263, as compared to the RS4;11 parental cell line (Fig. 4). Based upon cleavage of caspase-3, caspase-7 and PARP (Fig. 4A), ABT-263 is equally effective in inhibiting cell growth and induction of apoptosis in representative sublines such as G7M1, G7M4 and G7M7 and the parental cell line (Fig. 4B and 4C). Hence, while p53 mutation or compromised p53 function greatly diminishes the activity of the MDM2 inhibitor, it has no effect on the activity of the Bcl-2 inhibitor.
Figure 4.
Evaluation of ABT-263 in RS4;11 xenografts or sublines treated with SAR405838. A, Representative RS4;11 sublines were treated with ABT-263 for 24 h and biochemical markers of apoptosis were analyzed by immunoblotting. B, RS4;11 parental and sublines were treated with ABT-263 for 4 days and cell viability was determined by a WST assay. C, Cells were treated with ABT-263 for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining.
Different roles of BAX in the activity of Bcl-2 and MDM2 inhibitors
When the RS4;11 cell line was treated with ABT-737 in vitro and in vivo, BAX, but not BAK is profoundly down-regulated (Fig. 1D), and the cells become resistant to ABT-737 and ABT-263 but retain their sensitivity to SAR405838 (Fig. 3). This suggested that BAX and BAK play different roles in the activity of these two different classes of drugs, which was further investigated.
Efficient shRNA knock-down of BAX expression in the RS4;11 parental cell line renders the cells >100-times less sensitive to ABT-263 in a cell growth assay and unresponsive to apoptosis induction by ABT-263 (Fig. 5A and 5B). However, efficient shRNA knock-down of BAK expression in the RS4;11 parental cell line has little or no effect on the activity of ABT-263 in both cell growth and apoptosis assays (Fig. 5B). For SAR405838, efficient knock-down of BAX expression in the RS4;11 parental cell line results in only a ~3-fold reduction in sensitivity and efficient knock-down of BAK expression has no effect on the activity of SAR405838 (Fig. 5C).
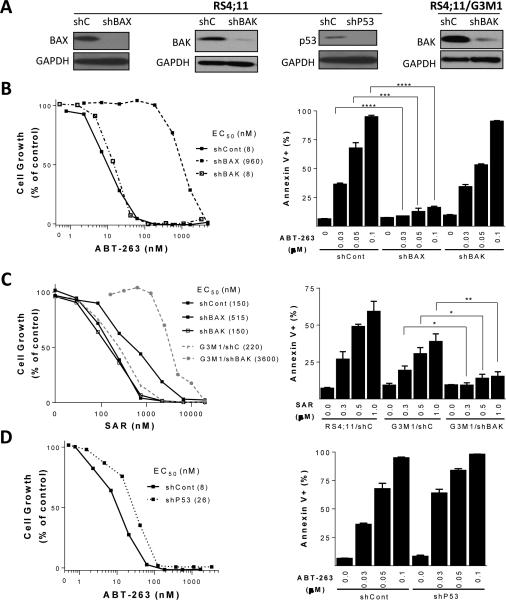
Figure 5.
Investigation of the role of BAK, BAX and p53 in the activity of ABT-263 and SAR405838 in the RS4;11 cell line. A, Immunoblots illustrating the efficiency of the shRNA-lentiviral approach in RS4;11 and RS4;11/G3M1 cells. B, BAX or BAK was knocked down by shRNA in the RS4;11 parental cell line and cells were treated with ABT-263 for 4 days for cell viability analysis by a WST assay or treated with ABT-263 for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining (***, P <0.001; ****, P <0.0001; t test). C, BAK or BAX was efficiently knocked down by shRNA in the RS4;11 parental cell line or BAK was knocked down in G3M1 subline with a very low level of BAX. Cells were treated with SAR405838 for 4 days for cell viability analysis using a WST assay. Apoptosis analysis by flow cytometry with Annexin V/P.I. double staining. RS4;11/shControl, G3M1/shControl and G3M1/shBAK cells were treated with SAR405838 for 24 h (*, P <0.05; **, P <0.01; t test). D, p53 was knocked down by shRNA in the RS4;11 parental cell line and the cells were treated with ABT-263 for 4 days for cell viability analysis in a WST assay or for 24 h for apoptosis analysis by flow cytometry with Annexin V/P.I. double staining.
Since BAX was down-regulated in all the RS4;11 sublines obtained from in vitro and in vivo treatment of the Bcl-2 inhibitors (Fig. 1, SI Fig. S3A and S3B), we knocked down BAK in one of the sublines (G3M1) (Fig. 5A) and evaluated the sensitivity of the cells to SAR405838. Efficient knockdown of BAK in the G3M1 subline results in a >15-fold increase in the IC50 value for SAR405838 in the cell growth assay and greatly reduces the apoptotic response to SAR405838 (Fig. 5C).
These data show that while BAX, but not BAK, plays a dominant role for the activity of ABT-263 in the RS4;11 cell line, the presence of either BAX or BAK is sufficient for effective apoptosis induction by SAR405838.
A minimal role of p53 in the activity of ABT-263
ABT-263 is effective in RS4;11 sublines harboring mutated p53, suggesting that p53 has a minimal role in the activity of Bcl-2 inhibitors. To further examine this, we stably knocked down p53 in the RS4;11 parental cell line using lenti-virus shRNA (Fig. 5A). While efficient knock-down of p53 dramatically reduces the activity of SAR40583 (32), it has only a modest effect on the activity of ABT-263 (Fig. 5D). Hence, p53 has a minimal role in the activity of ABT-263 in the RS4;11 cell line.
RS4;11 xenograft tumors lack cross-resistance in vivo to Bcl-2 and MDM2 inhibitors
When RS4;11 cells acquire resistance to the Bcl-2 inhibitors, they retain their sensitivity to the MDM2 inhibitor in vitro (Fig. 3). Conversely, when the RS4;11 cells acquire resistance to SAR405838, they remain sensitive to ABT-263 in vitro (Fig. 4). These data suggest that when the RS4;11 tumors acquire resistance to one class of apoptosis-inducing agent, they may still be susceptible in vivo to the second class of apoptosis-inducing agent. We directly examined this possibility in mice.
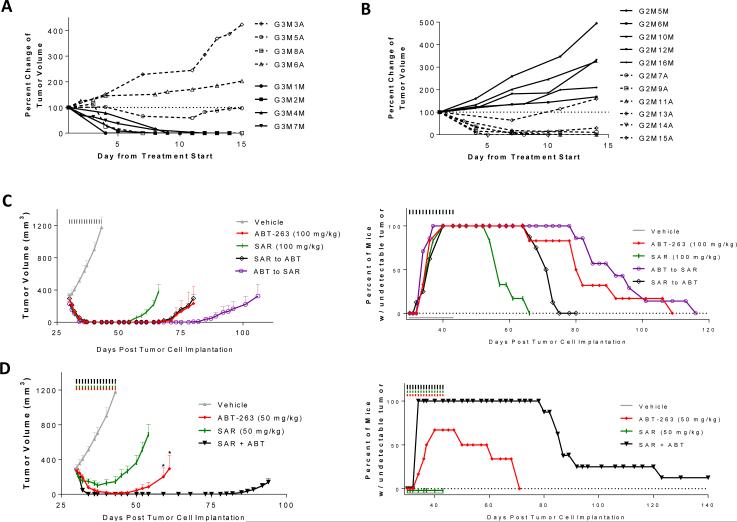
When RS4;11 xenograft tumors were treated with ABT-263, all tumors rapidly became undetectable (SI Fig. S3C), but regrew after the treatment was ended. When the regrown tumors reached an average volume of 200 mm3, they were randomized into two groups and treated with the maximum tolerated dose of ABT-263 (150 mg/kg) or SAR405838 at 100 mg/kg (Fig. 6A and SI Fig. 3C). While one of the 4 tumors treated with ABT-263 regressed, the other three tumors had no regression. In comparison, all 4 regrown tumors treated with SAR405838 completely regressed (Fig. 6A).
Figure 6.
Antitumor activity of ABT-263 and SAR405838 alone, sequential treatment of the two drugs and their combination in the RS4;11 xenograft model. A, Antitumor activity of ABT-263 and SAR405838 in regrown tumors initially treated with ABT-263. When regressed tumors initially treated with ABT-263 daily for 21 days at 100 mg/kg via oral gavage (SI Fig. 3A) regrew to 100-200 mm3, they were retreated with either SAR405838 at 100 mg/kg (solid lines) or ABT-263 (dashed lines) at 150 mg/kg, both via oral gavage, daily for 14 days. Data are presented as percent change of the tumor volume for each mouse. B, Antitumor activity of ABT-263 and SAR405838 in regrown tumors initially treated with SAR405838. When regressed tumors initially treated with SAR405838 for 21 days at 100 mg/kg (SI Fig. 3D) regrew to 100-200 mm3, they were retreated with either ABT-263 at 100 mg/kg (dashed lines) or SAR405838 (solid lines) at 200 mg/kg for 14 days. Data are presented as percent change of the tumor volume for each mouse. C, Antitumor activity of single agents and sequential treatment. Mice were treated via oral gavage with vehicle, ABT-263 or SAR405838 for 14 days, or with one drug for 7 days, immediately followed with the other drug for an additional 7 days. Percentage of tumor-free survival of mice in the RS4;11 efficacy experiment shown on the left. D, Antitumor activity of ABT-263, SAR405838 and their combination. When tumors grew to approximately 300 mm3, mice were treated with vehicle, ABT-263, SAR405838 or their combination at 50 mg/kg for 14 days via oral gavage (*, P <0.05; t test for comparison of ABT-263 single agent treatment at 50 mg/kg to the combination treatment). Percentage of tumor-free survival of mice in the RS4;11 efficacy experiment shown on the left.
Similarly, the RS4;11 tumors regressed rapidly when treated with SAR405838 at 100 mg/kg in mice (SI Fig. S3D), but all tumors regrew after termination of the treatment. When the regrown tumors reached an average volume of 200 mm3, they were randomized into two groups and treated with either ABT-263 at 100 mg/kg or SAR405838 at 200 mg/kg (Fig. 6B and SI Fig. S3D). All tumors treated with SAR405838 at 200 mg/kg continued to grow, but 5 out of 6 tumors treated with ABT-263 regressed (Fig. 6B).
Our data show that when regrown RS4;11 xenograft tumors become resistant to the initial class of drug, they are still very responsive to the second class of drug, thus lacking cross-resistance.
Lack of cross-resistance for MDM2 and Bcl-2 inhibitors in the MV4;11 acute leukemia cell line
Using the RS4;11 cell line, we showed that when the cells develop profound acquired resistance to one class of apoptosis-inducing agents, they retain their sensitivity to the other class of apoptosis-inducing agents. We next extended our studies both in vitro and in vivo to the MV4;11 cell line.
The MV.ABT-R cell line was generated by treating the MV4;11 cell line with ABT-263 in vitro. MV.ABT-R is >18-fold less sensitive to ABT-263 than the parental cell line, but retains its sensitivity to SAR405838 (SI Fig. S4A and 4B). Western blotting revealed that the MV.ABT-R subline shows no decrease in expression of BAX but has increased expression of Mcl-1 protein compared to the control lines (SI Fig. S4C). Nevertheless, stable knock-down of BAX expression in the parental MV4;11 cell line also drastically attenuates cell growth inhibition and apoptosis induction by ABT-263 compared to the control cell line, but the cell line remains sensitive to SAR405838 (SI Fig. S5). In contrast, efficient knock-down of BAK expression in the MV4;11 cell line has little or no effect on the activity of ABT-263 or SAR405838 (SI Fig. S5).
We generated the MV.MI-R cell line by treating the MV4;11 cell line with SAR405838 in vitro. MV.MI-R is >20-times less sensitive to SAR405838 than the control cell line in a cell growth assay but has the same sensitivity as the parental cell line to ABT-263 (SI Fig. S4). Sequencing of exons 2-11 reveals that p53 has a homozygous mutation, R248W, in the MV.MI-R cell line. To further test the role of p53 on the activity of ABT-263 and SAR405838, we stably knocked down p53 in the MV4;11 cell line (SI Fig. S6A), which greatly reduces the activity of SAR405838 but has no effect on the activity of ABT-263 (SI Fig. S6B-D).
We then evaluated both SAR405838 and ABT-263 in mice bearing MV4;11 xenograft tumors. Although ABT-263 is quite potent in vitro (IC50 = ~50 nM in a cell growth assay), it at 100 mg/kg only modestly inhibits growth of MV4;11 xenograft tumors (SI Fig. 7A). In comparison, SAR405838 at 100 mg/kg induces complete but transient tumor regression of all MV4;11 xenograft tumors (SI Fig. S7A). When regressed tumors treated with SAR405838 regrew, they were harvested and cultured to establish 6 sublines (G3M1-G3M6). Two representative tumors treated with vehicle were also harvested and cultured yielding two sublines (G1M3 and G1M7). Cell growth assay showed that while two sublines (G3M2 and G3M5) exhibit moderate resistance to SAR405838 compared with the parental and vehicle treated cell lines, the other 4 sublines are highly resistant to SAR405838 (SI Fig. S7B). SAR405838 becomes ineffective in activation of p53 and induction of apoptosis in these resistant sublines (SI Fig. S7C and S7D). Sequencing exons 2-11 showed that p53 has a R248W homozygous mutation in all of the highly resistant SAR405838 sublines but harbors a R248W heterozygous mutation in those moderately resistant sublines (G3M2 and G3M5). MV4;11 in vivo sublines resistant to SAR405838 are still sensitive to ABT-263 in both cell growth and apoptosis assays and exhibit little or no drug resistance compared to the control cell lines (SI Fig. S8).
Sequential strategies improve tumor-free survival
Although both Bcl-2 and MDM2 inhibitors as single agents yield complete regression of the RS4;11 xenograft tumors, tumors regrow shortly after cessation of their respective treatment. We investigated strategies to improve tumor-free survival of mice in the RS4;11 xenograft model.
Since these two classes of drugs lack cross-resistance, we tested two sequential treatment strategies in vivo: (1) ABT-263 daily for one week followed by SAR405838 daily for a second week or (2) treatments in reverse order (Fig. 6C). Both strategies showed no signs of toxicity (SI Fig. S9A). The 100% tumor-free survival time of mice was 12 days when treated with SAR405838 alone for 2 weeks, and was 24 days when treated with ABT-263 alone for 2 weeks. In comparison, the 100% tumor-free survival time was 41 days with ABT-263 first for one week, followed by SAR405838 for another week, and 24 days for the reverse scheme (Fig. 6C). Hence, sequential treatment with ABT-263 first, followed by SAR405838 is the most effective of the four treatment schemes in achieving tumor-free survival in 100% of mice.
Combination strategies improve tumor-free survival
We investigated the synergistic effect combining ABT-263 and SAR405838 on cell growth and induction of apoptosis using a fixed-ratio design. Our data showed that the combination of these two drugs is highly synergistic based upon the combination index (CI) values (46) in the RS4;11 and MV4;11 cell lines (SI Tables S2 and S3).
We next investigated if the combination of ABT-263 and SAR405838 can further improve the tumor-free survival of mice in the RS4;11 xenograft model. The MTD in RS4;11 tumor-bearing mice of the combination was determined to be 50 mg/kg for each agent. SAR405838 as a single agent at 50 mg/kg fails to yield complete tumor regression in any animal, but ABT-263 as a single agent at 50 mg/kg achieves tumor regression in 4 out of 6 mice. The combination of the two drugs both at 50 mg/kg, however, results in complete tumor regression in 100% of mice for a period of 44 days (Fig. 6D) with no sign of toxicity (SI Fig. S9B). Therefore, the combination of these two drugs at 50 mg/kg each is much more effective than either drug as a single agent at 100 mg/kg in achieving complete tumor regression in 100% of mice.
Discussion
Although molecularly targeted anticancer drugs can achieve complete clinical response, such responses are typically short-lived and patients relapse quickly (2, 47, 48). It is therefore critical to identify mechanism(s) of acquired resistance and to develop strategies to combat such resistance. In the present study, we elucidated the mechanisms of acquired resistance of two classes of apoptosis-inducing agents, the Bcl-2 inhibitors ABT-737/ABT-263 and the MDM2 inhibitor SAR405838 in two acute leukemia models in vitro and in vivo.
ABT-737 and ABT-263 effectively induce apoptosis in the RS4;11 cell line in vitro, and achieve complete regression of RS4;11 xenograft tumors in mice. Both in vitro and in vivo treatments, however, lead to the development of sublines that possess profound acquired resistance to this class of drugs. BAX, but not BAK, is consistently down-regulated in each of these resistant sublines obtained from in vitro and in vivo treatments with the Bcl-2 inhibitors (Fig. 1D, SI Fig. S3A and S3B), suggesting a critical role for BAX. Indeed, knock-down of BAX, but not BAK, in the parental RS4;11 cell line dramatically reduces the sensitivity of the tumor cells to ABT-263, confirming that BAX is a key mediator for apoptosis induction by ABT-263 (Fig. 5). Interestingly, while Mcl-1 is not upregulated in the resistant sublines obtained from in vivo treatment of RS4;11 tumors in mice with ABT-737, it is increased in the resistant sublines obtained from in vitro treatment of ABT-737 (Fig. 1D). While Mcl-1 upregulation has been shown to be a primary mechanism of resistance to ABT-737 based upon in vitro data (34, 35), our study shows that loss of BAX, rather than upregulation of Mcl-1, is the primary acquired resistance mechanism when the RS4;11 tumors are treated with ABT-737/ABT-263 in animals.
For the MDM2 inhibitor SAR405838, in vitro and in vivo treatments of the RS4;11 cell line with the drug also result in acquired resistance. Each of these resistant sublines either harbors a nonfunctional mutated p53 gene for its transcriptional activity or contains p53 with compromised transcriptional activity. Consequently, SAR405838 becomes either much less effective or completely ineffective in activation of p53 in these resistant cell lines (Fig. 2 and SI Fig. 2).
When all RS4;11 sublines obtained from in vitro and in vivo treatments with either ABT-737 or ABT-263 become highly resistant to ABT compounds, they retain their sensitivity in vitro to SAR405838 (Fig. 3). When regressed RS4;11 tumors treated with ABT-263 regrow, these relapsed tumors become much less responsive to even a higher dose of ABT-263 but undergo rapid tumor regression when treated with SAR405838 (Fig. 6A). Conversely, ABT-263 is still very effective against RS4;11 resistant sublines generated by both in vitro and in vivo treatment of SAR405838 (Fig. 4). Further, when RS4;11 tumors initially treated with SAR405838 regrow, these relapsed tumors become unresponsive to the second round of SAR405838 treatment but undergo rapid tumor regression when treated with ABT-263 (Fig. 6B).
ABT-199 is a new, highly potent and selective Bcl-2 inhibitor currently in Phase III clinical development for the treatment of acute leukemia (49). We evaluated ABT-199 in the RS4;11 parental cell line and sublines obtained with either ABT-737 or SAR405838 treatment (SI Fig. S10 and S11). Similar to ABT-737 and ABT-263, ABT-199 is highly effective not only in the RS4;11 parental cell line, but also in sublines obtained from either in vitro or in vivo treatment with SAR405838. ABT-199 is ineffective in all sublines obtained from in vitro or in vivo treatment with ABT-737. Because ABT-199 is a selective Bcl-2 inhibitor, our data show that Bcl-2 is the primary cellular target for ABT-737 and ABT-263 in the RS4;11 cell line.
The lack of cross-resistance of the RS4;11 cell line to Bcl-2 and MDM2 inhibitors suggests that p53 and BAX play different roles in the activities of these two classes of apoptosis-inducing agents. Indeed, knock-down of p53 in the parental RS4;11 cell line dramatically diminishes the activity of SAR405838 but has a minimal effect on the activity of ABT-263 (Fig. 5). In contrast, knock-down of BAX profoundly reduces the potency of ABT-263 but only has a modest effect on the activity of SAR405838.
The lack of cross-resistance for these two classes of apoptosis-inducing agents observed in the RS4;11 model was extended into the MV4;11 model both in vitro and in vivo. The MV4;11 cells develop profound acquired resistance in vitro upon treatment with either ABT-263 or SAR405838. When the MV4;11 cells develop acquired resistance to the one class of drug, they retain their sensitivity to the second class of drug. In mice, treatment of the MV4;11 xenografts with SAR405838, but not ABT-263, leads to complete tumor regression but the regressed tumors regrow after the treatment is terminated (SI Fig. S7). Sublines established from the regressed MV4;11 tumors treated with SAR405838 become highly resistant to SAR405838 but retain their sensitivity to ABT-263 in vitro. Mechanistically, MV4;11 cells develop profound acquired resistance to SAR405838 by a homozygous mutation of R248W of p53. Since ABT-263 failed to induce complete tumor regression of the MV4;11 tumors in vivo, we could not establish in vivo resistant MV4;11 sublines to ABT-263. However, treatment of the MV4;11 cell line in vitro with ABT-263 resulted in acquired resistance. Interestingly, upregulation of Mcl-1 protein, but not decrease of BAX, is observed in the two in vitro resistant sublines.
Since both ABT-263 and SAR405838 can completely regress the RS4;11 tumors in mice and all the cell lines cultured from relapsed tumors lack cross-resistance to these two classes of drugs, we tested two sequential and one combination treatment strategies for their effectiveness in achieving tumor-free survival. Sequential treatment with ABT-263 100 mg/kg daily for a week, followed by SAR405838 100 mg/kg daily for another week, can achieve 71% longer tumor-free survival in 100% of mice (41 days) than ABT-263 daily for 2 weeks at 100 mg/kg (24 days) and 240% longer than SAR405838 daily for 2 weeks at 100 mg/kg (12 days) (Fig. 6C). However, the alternative sequential treatment is only equally effective, as compared to ABT-263 daily for 2 weeks in achieving tumor-free survival in 100% of mice (Fig. 6C). Combination of ABT-263 and SAR405838 at 50 mg/kg for both drugs achieves complete tumor regression in 100% of the mice for 44 days in the RS4;11 xenograft model (Fig. 6D), much longer than both drugs as single agents at 100 mg/kg. Both single agent treatments at 50 mg/kg fail to achieve tumor regression in 100% of mice.
In summary, our study shows that acute leukemia cells develop acquired resistance in vitro and in vivo when treated with either a Bcl-2 inhibitor or an MDM2 inhibitor. The mechanisms of acquisition of resistance for these two classes of apoptosis-inducing agents are, however, completely different. New insights into the mechanisms of acquired resistance led us to develop both sequential and combination treatment strategies with these two classes of apoptosis-inducing agents to effectively improve the tumor-free survival of mice over the single-agent treatments, which should be evaluated for the treatment of acute leukemia in the clinic.
Supplementary Material
Translational Relevance.
Acquired resistance of tumor cells to anticancer drugs is a major cause of the failure of clinical cancer therapy. We have investigated the mechanisms of acquired resistance for two classes of novel anticancer drugs currently in clinical trials, ABT-263, a Bcl-2 inhibitor, and SAR405838, an MDM2 inhibitor. While both drugs yield rapid and complete tumor regression in an acute leukemia xenograft model in mice, tumors regrow after cessation of treatment. Tumors that become highly resistant to SAR405838 retain sensitivity to ABT-263 and vice versa, and sequential treatment with ABT-263 first followed by SAR405838, or their combination was found to achieve longer-term tumor regression than either single agent. This suggests that sequential treatment or a combination of these two classes of novel apoptosis-inducing agents should be explored as a clinical strategy to overcome acquired drug resistance in acute leukemia patients.
Acknowledgments
Financial support: This work was supported in part with funding from the National Institutes of Health (R01CA121279)
Footnotes
Conflict of interest disclosure statement: SAR405838 has been licensed by Ascenta and Sanofi from the University of Michigan for clinical development and SW, DM. YZ, and WS are inventors on the patents and receive royalties. SW owns stock in Ascenta.
References
- 1.Moore AS, Kearns PR, Knapper S, Pearson AD, Zwaan CM. Novel therapies for children with acute myeloid leukaemia. Leukemia. 2013;27:1451–60. doi: 10.1038/leu.2013.106. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 7.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 9.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 10.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–6. [PubMed] [Google Scholar]
- 11.Robinson BW, Behling KC, Gupta M, Zhang AY, Moore JS, Bantly AD, et al. Abundant anti-apoptotic BCL-2 is a molecular target in leukaemias with t(4;11) translocation. Br J Haematol. 2008;141:827–39. doi: 10.1111/j.1365-2141.2008.07100.x. [DOI] [PubMed] [Google Scholar]
- 12.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 13.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 14.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–16. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilev LT. p53 Activation by small molecules: application in oncology. J Med Chem. 2005;48:4491–9. doi: 10.1021/jm058174k. [DOI] [PubMed] [Google Scholar]
- 17.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 18.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 19.el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–57. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 20.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 21.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 22.Mitani N, Niwa Y, Okamoto Y. Surveyor nuclease-based detection of p53 gene mutations in haematological malignancy. Ann Clin Biochem. 2007;44:557–9. doi: 10.1258/000456307782268174. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–32. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 25.Momand J, Wu HH, Dasgupta G. MDM2--master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 26.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 27.Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14:5318–24. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13:23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 30.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–97. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barriere C, et al. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014;74:5855–65. doi: 10.1158/0008-5472.CAN-14-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 34.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–13. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Aziz MH, Shen H, Maki CG. Acquisition of p53 mutations in response to the non-genotoxic p53 activator Nutlin-3. Oncogene. 2011;30:4678–86. doi: 10.1038/onc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Loschmann N, et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima K, Duvvuri S, Ruvolo V, Samaniego F, Younes A, Andreeff M. Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737. Cancer. 2012;118:1023–31. doi: 10.1002/cncr.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 41.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 42.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–93. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–72. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 45.Hauser U, Balz V, Carey TE, Grenman R, Van Lierop A, Scheckenbach K, et al. Reliable detection of p53 aberrations in squamous cell carcinomas of the head and neck requires transcript analysis of the entire coding region. Head Neck. 2002;24:868–73. doi: 10.1002/hed.10128. [DOI] [PubMed] [Google Scholar]
- 46.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 47.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zauli G, Voltan R, Tisato V, Secchiero P. State of the art of the therapeutic perspective of sorafenib against hematological malignancies. Curr Med Chem. 2012;19:4875–84. doi: 10.2174/092986712803341548. [DOI] [PubMed] [Google Scholar]
- 49.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.