Abstract
The activation of TLR-MyD88 (Toll-like receptor- Myeloid differentiation factor 88) signaling within T cells functions as a potent costimulatory signal that boosts antitumor and antiviral responses. However, the molecular mechanisms underlying the costimulatory processes are poorly understood. We compared microarray gene analysis data between TLR1–TLR2 stimulated and unstimulated T-cell receptor transgenic ‘pmel’ and MyD88−/− pmel CD8+ T cells and identified changes in the expression of several TNF family members. In particular, TLR-stimulation increased 4-1BB levels in pmel but not in MyD88−/−pmel T cells. A link between 4-1BB and TLR1–TLR2 signaling in CD8+ T cells was highlighted by the suboptimal responses of 4-1BB−/− T cells to TLR1–TLR2 agonist, but their normal response to CD28 or OX40 costimulation. Blocking 4-1BB signaling with antibodies also hindered the costimulatory effects of the TLR1–TLR2 agonist. The elevated levels of 4-1BB transcripts in TLR1–TLR2–stimulated cells were not due to increased mRNA stability nor increased histone activation, but instead were associated with increased binding of p65 and c-Jun to two distinct 4-1BB promoter sites. Combining TLR1–TLR2 ligand with an agonistic antibody to 4-1BB enhanced the antitumor activity in mice with established melanoma tumors. These studies reveal that the costimulatory effects of TLR1–TLR2 signaling in CD8+ T cells are in part mediated by 4-1BB and are important for mounting an effective antitumor immune response.
Keywords: TLR signaling, CD8+ T-cell costimulation, 4-1BB, tumor immunology, B16 melanoma treatment, MyD88
Introduction
Toll-like receptors (TLRs) play a central role in activating immune cells and clearing infectious entities by recognizing various molecules derived from microbial pathogens (1, 2). TLRs also bind a range of molecules released from dying or stressed cells (3). Myeloid Differentiation Factor-88 (MyD88) is an adapter molecule used by most TLRs and necessary for TLR-induced signaling. The activation of TLR-MyD88 signaling in CD4+ and CD8+ T cells prolongs their survival, augments T-cell expansion, and can enhance effector functions against tumors and infections (4–6). MyD88 signaling in T cells plays a vital role in T-cell survival even in the absence of exogenous TLR agonists (7). The mechanisms underlying the costimulatory effects of MyD88 signaling in T cells have yet to be defined.
A T cell’s ability to proliferate and persist in vivo is heavily influenced by the stimulation of various costimulatory receptors, such as the tumor necrosis factor receptor (TNFR) members 4-1BB, CD70, LTA, OX-40, and GITR (8–10). 4-1BB signaling in T cells enhances proliferation and promotes T-cell survival by increasing IL2 and by upregulating the expression of anti-apoptotic proteins. 4-1BB plays an important role in generating a responsive memory T-cell population. Preclinical models indicate that stimulating 4-1BB signaling on T or NK (Natural killer) cells with agonistic antibodies elicits potent antitumor responses. Clinical trials are examining the antitumor activity of 4-1BB agonists alone or when administered together with other anticancer agents such as PD-1 inhibitor in patients with melanoma, colorectal, head and neck cancer, or relapsed/refractory B-cell non-Hodgkin's lymphoma (NCT02179918, NCT00612664, NCT01775631, NCT02110082, NCT01307267). Preliminary data thus far demonstrate partial responses in melanoma patients and an increased frequency of activated CD8+ T cells in circulation.
To better understand how TLR-MyD88 signals enhanced CD8+ T-cell responses, we assessed changes in gene expression profiles of the CD8+ T-cell receptor transgenic “pmel” mice, which recognize the epitope gp10025–33 expressed on melanoma cells, and MyD88−/−pmel CD8+ T cells stimulated with or without the TLR1–TLR2 ligand (TLR1–TLR2L) Pam3CSK4. TLR1–TLR2 engagement on T cells increased the expression of 4-1BB, OX40, OX40L, GITR, and LTA. We found that 4-1BB played a central role in regulating the costimulatory effects of TLR1–TLR2 signaling in T cells. Combination therapy using an agonistic antibody to 4-1BB and TLR1–TLR2L enhanced antitumor responses in mice with established tumors. These studies offer insights into the molecular mechanisms through which TLR-TLR2 signals costimulate CD8+ T cells and highlight the biological significance of exploiting these signaling pathways to augment T-cell responses.
Materials and methods
Mice
C57BL/6 and MyD88−/−mice were purchased from Charles River, Maryland while, TLR2−/− and pmel (B6.Cg-Thy1/Cy Tg(TcraTcrb)8Rest/J) mice were purchased from the Jackson Laboratory. The IRAK4 kinase dead mice were a generous gift from Dr. Stefanie Vogel and 4-1BB−/− mice from Dr. Lieping Chen. 4-1BB−/−pmel and MyD88−/−pmel mice were obtained by crossing pmel with 4-1BB−/−and MyD88−/− mice and crossing offspring over nine generations. All the protocols were approved by the University of Maryland Institutional Animal Care and Use Committee.
T-cell isolation and stimulation
Mouse T cells were cultured in RPMI 1640 (Invitrogen) medium with fetal bovine serum (Gemini), NEAA, Penicillin, streptomycin and gentamycin (Invitrogen). CD8+ T cells were initially sorted using the negative enrichment kit followed by positive selection (Invitrogen). In some experiments, pmel T cells were stimulated with MyD88−/− splenocytes pulsed with mouse gp-100 peptide (10 ng/ml; EGSRNQDWL, GenScript Corp) at 37°C in 7% CO2 at 1:5 T cell:APC ratio, whereas WT (C57BL/6) CD8+ T cells were stimulated with plate bound anti-CD3ε (BD Biosciences) at 0.5 µg/ml, with or without the TLR1–TLR2 agonist Pam3CSK4 (1.5 µg/ml, InvivoGen). T-cell proliferation was determined by 3H-thymidine (1µCi/well) uptake. For in vivo T-cell survival/expansion studies, CD8+ pmel T cells were purified by negative selection (Invitrogen) from CD90.1−CD45.2+ pmel and CD90.1+CD45.2+MyD88−/− pmel mice and activated in vitro with mgp100 peptide-pulsed WT splenocytes and, 1 day later, were enriched by negative selection, mixed at a 1:1 ratio and i.v. injected into CD45.1+ mice. The number of transferred T cells was determined in different organs at different time points by staining cells with antibodies against CD8, CD45.2, and CD90.1. T cells were restimulated by vaccinating mice s.c. with 100 µg hgp100 peptide admixed in IFA and 10 µg CpG-ODN on day 20 after T cell transfer. IL1-agonist (IL1α, 10 ng/ml) was from Biolegend and the cIAP1/2 inhibitor GDC-0152 was purchased from Selleck Chemicals. Purified CD8+ T cells from WT, MyD88−/−, 4-1BB−/−, TLR2−/−, and IRAK4 KD mice were activated with anti-CD3ε (0.5 µg/ml), with or without Pam3CSK4 (0.5 µg/ml), 3H3 (1ug/ml; rat IgG2a agonistic mAb to mouse 4-1BB, kindly provided by Dr. R.S. Mittler, Emory University), IgG2a isotype control (1 µg/ml, 2A3, BioXcell).
Flow cytometry
In some experiments the expression of various molecules on pmel T cells was determined by flow cytometry after activation and analyzed using FlowJo software (Tree Star) at the indicated time points. Antibodies against 4-1BB, OX-40, OX-40L, CD25, CD44, CD62L, CD132, CD127, and GITR used in flow cytometry were purchased from E-biosciences or BD Biosciences. Blocking antibodies to CD28 and GITR were purchased from BD Pharmingen, OX-40L from R&D systems and 4-1BBL (19H3) from Dr. R.S. Mittler.
Whole genome gene expression
MyD88−/− splenocytes underwent two rounds of CD8 T cell depletion by CD8 positive selection. Splenocytes were pulsed with 10ng/ml of mgp100 for two hours at 37°C followed by addition of purified pmel or MyD88−/− pmel CD8 T with or without 10µg/ml of Pam3CSK4. Seventy-two hours post stimulation, pmel or MyD88−/− pmel CD8 T cells were selected by two rounds of negative enriched (Invitrogen, Dynal AS). Purity of CD3+CD8+ was found to exceed 97% in all three experiments conducted as determined by flow cytometry. Double stranded cDNA (dsDNA) is made from 200 ng of total RNA using oligo-dT, reverse transcriptase and DNA polymerase as recommended by the manufacturer (AMBION). In addition, the remaining RNA is digested with RNAse H. The dsDNA is purified trough columns and used as template to generate biotin-labeled RNA (cRNA). The GEO Submission is GSE79475 and NCBI tracking system number is 17812033.
For whole genome gene expression cRNA (1.25 µg in 10 µl) is mixed with hybridization buffer and processed and analyzed, as recommended by Illumina Inc, San Diego, CA. For data analysis the samples were normalized using the cubic spline algorithm, assuming that the distribution of transcripts is similar and the net expression was determined by subtracting the expression levels in the reference group (control samples) from the condition group (treatment samples). A differential expression is determined by comparison between the condition group and the reference group using an algorithm that assumes that target signal intensity (l) is normally distributed among replicates corresponding to some biological condition. This experiment was conducted three times and RNA samples from each group were used for the gene array. Changes in mRNA transcript levels observed between groups were confirmed by real-time PCR.
mRNA stability
CD8+T cells were activated in an anti-CD3ε (0.5 µg/ml) coated plate for 3 days, in the presence or absence of Pam3CSK4. mRNA was collected at 0, 2, 4, 12 and 16 hours after actinomycin D (Sigma) treatment (10 µg/ml). Reverse transcription was performed using the high capacity reverse transcription kit from Invitrogen. 4-1BB and β-actin mRNA levels were measured by qPCR using the SYBR green mix from BIORAD and the following primer sets synthesized by Integrated DNA technologies (IDT): 4-1BB, CCTTCCTGAAATTCAGGTGCTGCAG and GCAGCACAATGACCACCACGTTG; b-actin, GAAAAGATGACCCAGATCATG and ATCTTCATGAGGTAGTCCGTC.
Chromatin immunoprecipitation
CD8 T cells (106) were cultured in a 24 well plated coated with 1 µg/ml anti-CD3ε in RPMI, 10% FBS, 1% PenStrep, 1% NEAA and 0.1% gentamycin, for 24, 48, 72, and 96 hours in the presence or absence of Pam3CSK4 (1 µg/ml). At each time point, cells were collected and chromatin immunoprecipitation was done using the Magnetic ChIP kit from Thermo Scientific. Crosslinking of protein and DNA was performed by adding 37% formaldehyde to the culture solution to attain a final concentration of 1% formaldehyde. The cells were then lysed using lysis buffer provided. Chromatin was digested using the MNase enzyme and the nuclear membrane was disrupted using short pulses of sonication. The resulting chromatin was incubated overnight with antibodies to p65, c-Jun, RNA polymerase, and Isotype control from Cell Signaling Technologies and to H3K4me3 from SABiosciences EpiTech ChIP antibody kit. The immune complexes were isolated using Protein A/G coated magnetic beads and magnetic stand. The crosslinks were reversed and protein digested using Proteinase K. The purified DNA was then used in PCR to detect the promoter regions of 4-1BB. The following primer sets were used: P1, ACGTCCTAATGGGCAACAGCTG, GTGAGGTTCTGCCGCTCCAC; P2, TTGGCCACCACACCATGC, CAAGGGTTTCAAGGTCCCC. Densitometry data was obtained using Image J.
In vivo adoptive transfer experiments
For T-cell survival/expansion studies, CD8+ pmel T cells were purified by negative selection, (InvivoGen) from CD90.1−CD45.2+ pmel and CD90.1+CD45.2+MyD88−/− pmel mice and activated in vitro with mgp100 peptide-pulsed WT splenocytes and, 1 day later, were enriched by negative selection, mixed at a 1:1 ratio and i.v. injected into CD45.1+ mice. The number of transferred T cells was determined in different organs at different time points by staining cells with antibodies against CD8 (553033), CD45.2 (553772), and CD90.1(557266, BD Biosciences). T cells were restimulated by vaccinating mice s.c. with 100µg hgp100 peptide admixed in IFA and 10 µg CpG-ODN (tlrl-1826 InvivoGen) on day 20 after T cell transfer. CD90.1−CD45.2+ pmel and CD90.1+CD45.2+ MyD88−/− pmel CD8 T cells were activated using 1µg/ml of mgp10025–33 for 5 days then mixed at 1:1 and injected intravenously into CD45.1+ mice. For tumor growth experiments, 105 B16 F1 melanoma cells were injected subcutaneously into the flanks of C57B6 mice on day 0. B16 F1 melanoma cells were purchased from American Type Culture Collection (ATCC) in 2013 and were used within 3 years of receiving them. We authenticated that our B16 cells expressed the melanin pigment by visual inspection and microscopic inspection and that B16 cells were recognized as target by pmel CD8+ T cells. On day 9, when tumors were detected, the mice were sublethally irradiated (400rads) using a Cesium irradiator. After 24 hours, 5–7×106 pmel T cells which were activated three days prior with 2.5µg/ml hgp100 and 100U/ml IL2 (589106 Biolegend), were injected intravenously into all groups. On days 10, 14, 17 and 20 post tumor injection the mice were administered TLR1–TLR2 ligand (10µg), 3H3 (100µg, BE0239 BioXcell), TLR1–TLR2 ligand (10µg) and 3H3 (100µg), intraperitoneally or intravenously as indicated in the figure legend. Tumors were measured regularly with a caliper and mice were euthanized when tumor reached the set size limit or if the mice appeared moribund.
Results
MyD88 promotes CD8+ T-cell survival and changes TNF family member expression
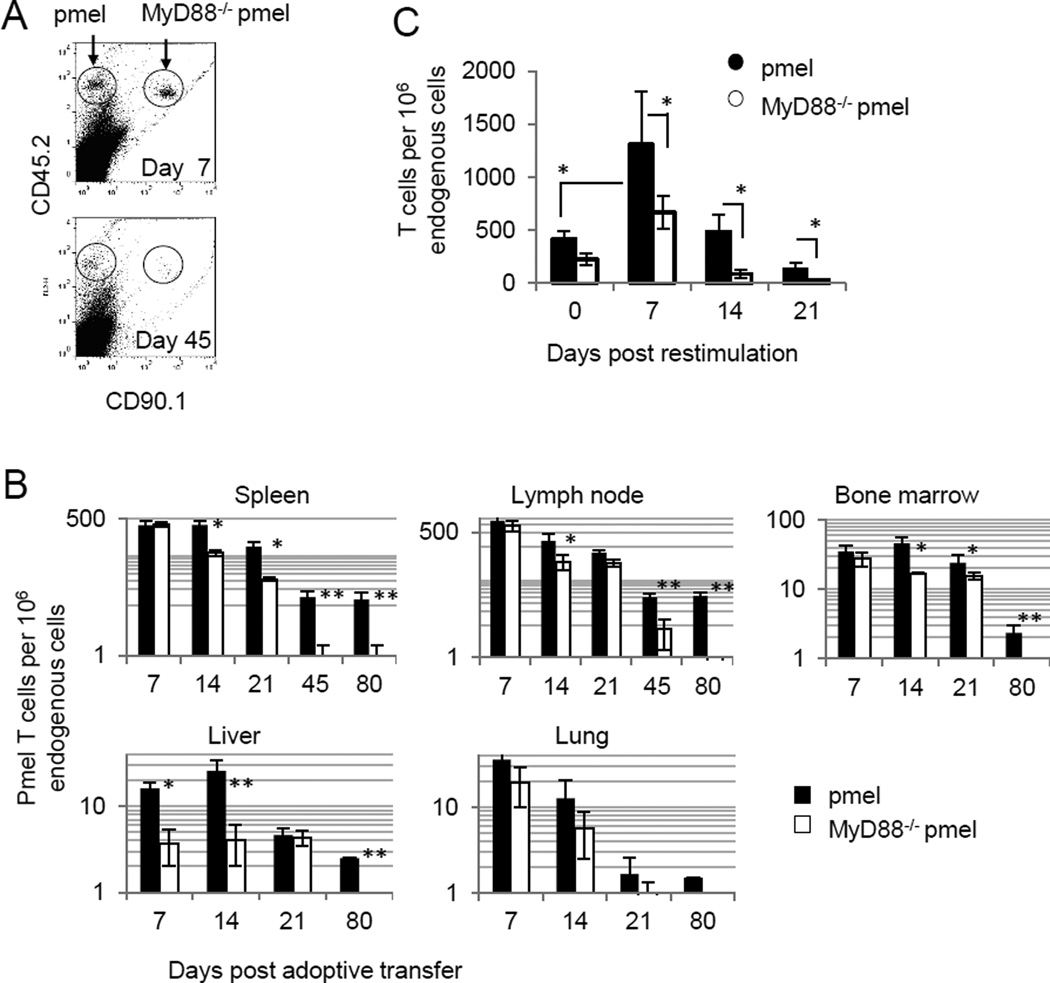
We examined the biological significance of MyD88 signaling in T-cell survival. An equal number of antigen-activated pmel (CD45.2+CD90.1−) and MyD88−/−pmel (CD45.2+ CD90.1+) CD8+ T cells were co-injected into CD45.1+ mice and T-cell numbers in various organs were compared at different time points after cell transfer. Fig. 1A shows a representative dot plot of pmel and MyD88−/−pmel CD8+ T cells 7 and 45 days post transfer. The number of pmel T cells and MyD88−/−pmel T cells were similar on day 7 in the spleen, lymph node, and bone marrow (Fig. 1B). However, more pmel T cells were recovered from each of the different organs starting on day 14. T-cell expansion and contraction kinetics in response to antigen restimulation were also assessed 20 days after T cell transfer. Pmel cells exhibited a greater potential to expand and persist than did MyD88−/−pmel cells(Fig. 1C). These data indicate that MyD88 signaling in T cells provides a distinct survival and/or proliferative advantage over MyD88-deficient pmel T cells.
Figure 1. MyD88 in CD8+ T cells plays an important role for T-cell persistence and expansion.
A, Antigen-activated pmel (CD45.2+ CD90.1−) or MyD88−/− pmel (CD45.2+ CD90.1+) T cells were injected into C57BL6 mice. Blood samples were analyzed 45 days after transfer by flow cytometric staining for specific congenic markers. B, The number of transferred cells in the spleen, lymph nodes, bone marrow, liver and lung were analyzed by flow cytometry at the indicated time points after transfer. C, 20 days after adoptive transfer of pmel or MyD88−/− pmel T cells, mice were vaccinated with gp100, CpG-ODN in IFA and the number of pmel and MyD88−/− pmel CD8+ T cells in the blood were measured by flow cytometric analysis at the indicated time points after vaccination. Statistics were generated by student T test comparing pmel and MyD88−/−pmel T cells, *P <0.05, **P <0.01.
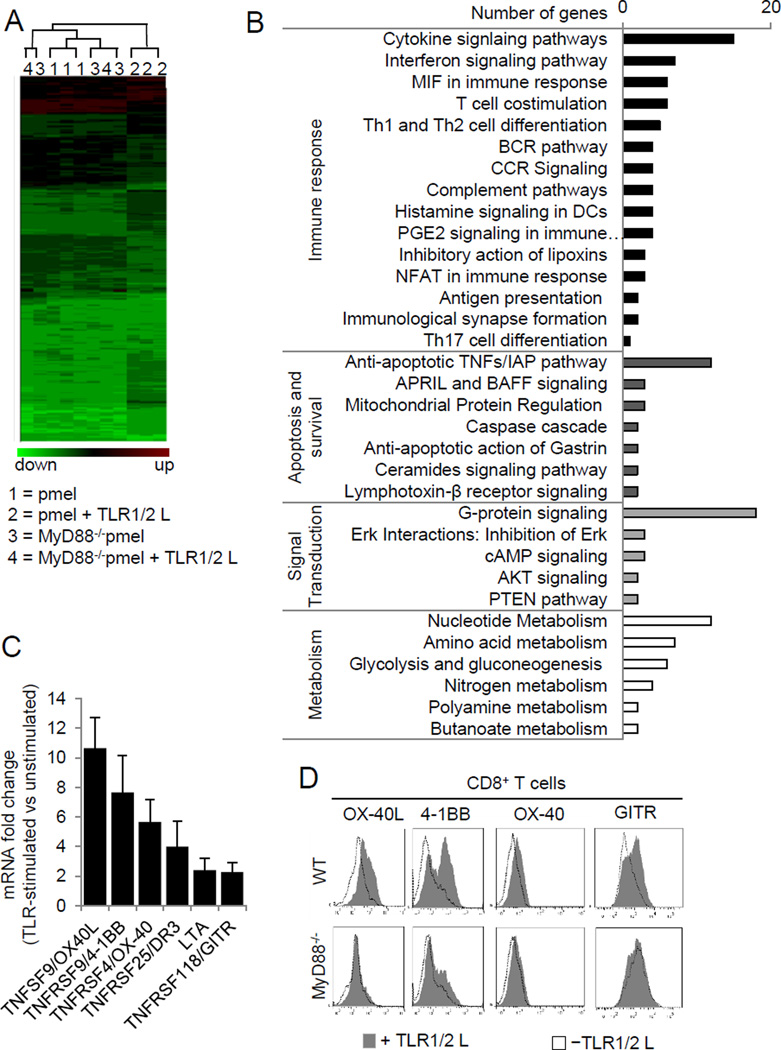
Microarray gene expression profiles were compared between TLR1–TLR2–stimulated and unstimulated pmel and MyD88−/−pmel CD8+ T cells. Gene expression in the pmel, MyD88−/−pmel, and MyD88−/−pmel + TLR1–TLR2L groups were related, whereas TLR1–TLR2–stimulated pmel cells were only distantly related (Fig. 2A). TLR stimulation enhanced the expression of immune response genes and genes regulating apoptosis and survival, signal transduction pathways, and metabolism (Fig. 2B). Under the classification of T-cell costimulation, expression of the TNF family members TNFSF9/OX40L, TNFRSF9/4-1BB, TNFRSF4/OX-40, TNFRSF25/DR3, LTA and TNFRSF118/GITR were most prominently enhanced after TLR1–TLR2 stimulation. Expression of the genes was confirmed by quantitative RT-PCR (Fig. 2C). Surface expression of OX40L, 4-1BB, and GITR were increased in response to TLR1–TLR2 stimulation and correlated with the RNA transcript data (Fig. 2D). OX40 surface expression was moderately increased. In contrast, the expression of each of these proteins remained similar in TLR1–TLR2–stimulated and unstimulated MyD88−/− T cells (Fig. 2D, bottom panel). These data highlight a previously unappreciated association between TLR–MyD88 signaling and TNFR family member expression in CD8+ T cells.
Figure 2. Gene expression analysis of TLR2-stimulated CD8+ T cells show an enhanced expression of TNFRSF members.
A, Gene expression analysis was conducted on purified CD8+ T cells from pmel and MyD88−/− pmel mice, activated with hgp10025–33–pulsed MyD88−/− APCs with or without TLR1–TLR2L for three days. Genes colored green are under-expressed, while red indicates over-expression. B, Classification of genes upregulated by TLR stimulation in panel (A). C, Changes in mRNA transcript levels between TLR-stimulated and non-TLR-stimulated (±s.d.) T cells were determined by real time PCR. D, Surface expression of TNFRSF members on WT and MyD88−/− CD8+ T cells 3 days after activation with anti-CD3ε in the presence or absence of TLR1–TLR2 ligand were confirmed by flow cytometric analysis. The data shown are representative of three independent experiments.
Costimulatory effects depend upon 4-1BB expression on CD8+ T cells
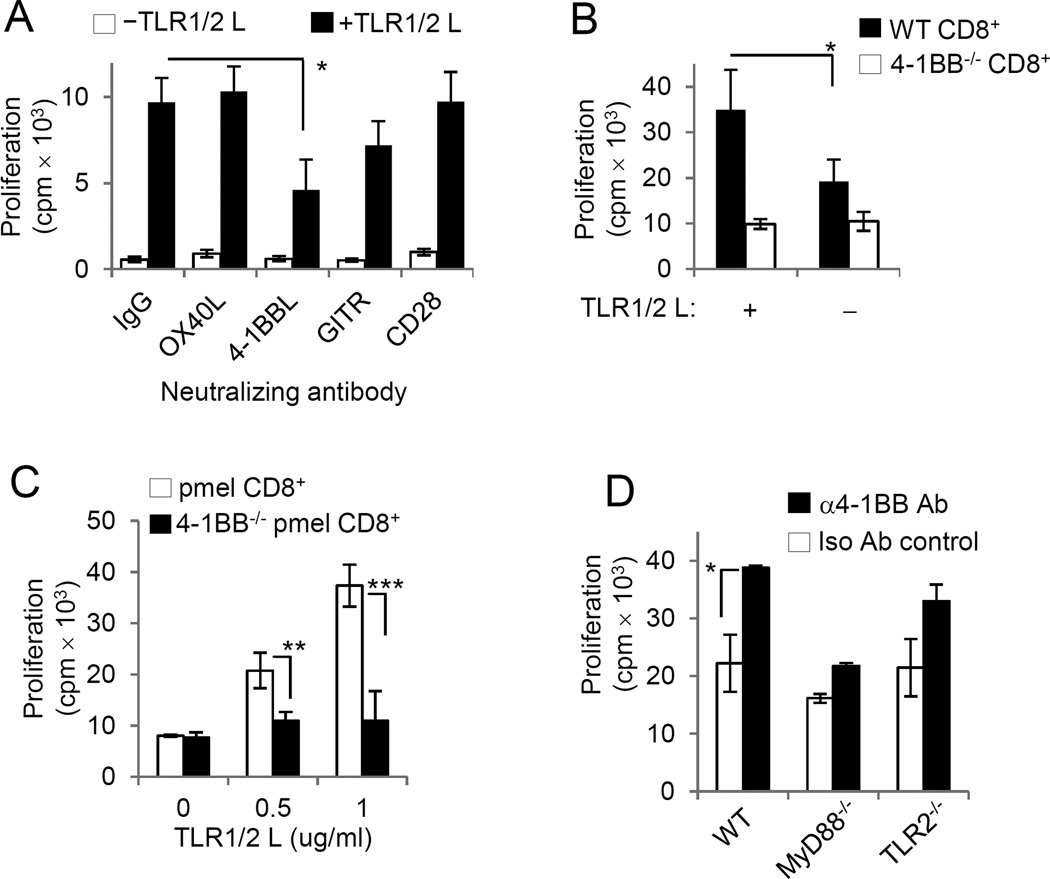
We examined the effect that blocking 4-1BB, OX-40, and GITR had on the costimulatory effects of TLR1–TLR2 stimulation. Pmel T cells were activated with mgp100-pulsed MyD88−/− splenocytes in the presence or absence of TLR1–TLR2L, with or without blocking antibodies to OX-40L, 4-1BBL, GITR, or CD28. Blocking 4-1BB signaling reduced the costimulatory effects of TLR1–TLR2 ligand (Fig. 3A). Blocking GITR also modestly reduced TLR2 signals. In contrast, blocking OX40L or CD28 did not impair TLR1–TLR2 signaling, suggesting that although upregulated after TLR stimulation, these receptors do not appear to modulate the costimulatory effects of TLR1–TLR2 signaling in CD8+ T cells. The costimulatory effect of TLR1–TLR2 stimulation on T cells was influenced by 4-1BB expression, so we assessed TLR1–TLR2L’s proliferative effects on wild-type (WT) and 4-1BB−/− CD8+ T cells. TLR1–TLR2L increased WT CD8+ T-cell proliferation but did not alter 4-1BB−/− CD8+ T-cell proliferation (Fig. 3B). Likewise, TLR1–TLR2 engagement increased antigen-driven pmel T-cell expansion but did not augment 4-1BB−/−pmel T-cell proliferation (Fig. 3C).
Figure 3. 4-1BB contributes to the costimulatory effects of TLR1–TLR2 ligand.
A, Purified pmel T cells were cultured with gp10025–33–pulsed MyD88−/− APCs in the presence of blocking antibodies to different TNFRSF members. B, WT and 4-1BB−/− CD8+ T cells were activated with plate bound anti-CD3ε and cultured in the presence or absence of TLR1–TLR2 ligand. C, Purified pmel and 4-1BB−/−pmel CD8+ T cells were activated with gp10025–33–pulsed pulsed irradiated MyD88−/− splenocytes D, WT, MyD88−/− and TLR2−/−CD8+ T cells were activated with plate bound anti-CD3ε antibody and cultured in the presence of agonistic 4-1BB antibody, 3H3 or isotype antibody. Proliferation was assessed by measuring 3H-thymidine uptake at 72 hours post activation in A–D. Statistics were generated by student T test, *P <0.05, **P <0.01, ***P <0.001.
We examined whether differences in the expression of cytokine receptors could help explain the changes in T-cell expansion between 4-1BB signaling competent and 4-1BB deficient T cells. TLR1–TLR2 stimulation increased the expression of CD25 (the α–chain of the IL2 receptor), CD132 (common-γ chain) and CD127 (IL7 receptor subunit) on WT and 4-1BB−/− T cells (Supplementary Fig. S1). Wild-type and 4-1BB−/−T cells also expressed more of the activation markers CD69, CD44, and CD62L and the costimulatory molecule CD28 after TLR stimulation. However, TLR1–TLR2L did not alter the expression of these molecules in MyD88−/−CD8+ T cells. Thus, the costimulatory effect of TLR1–TLR2 engagement likely occurs via mechanisms that do not involve these proteins.
To further understand the association between 4-1BB and TLR1–TLR2 signaling, we investigated how the absence of MyD88 or TLR2 altered the costimulatory effects of 4-1BB signaling. Purified WT, MyD88−/−, or TLR2−/− CD8+ T cells were activated with −CD3ε antibody and agonistic 4-1BB antibody. Whereas wild-type and TLR2−/− CD8+ T cells proliferated more in response to 4-1BB stimulation, MyD88−/− T cells did not respond (Fig. 3D). These results suggest a potential role for MyD88 in 4-1BB mediated costimulation. We tested whether the lack of 4-1BB on T cells might have a global impact and prevent T cells from responding to other common costimulatory signals. We observed that although 4-1BB−/− T cells did not respond to TLR1–TLR2 L costimulation, they did respond to both to CD28 and OX40 (Supplementary Fig S2), indicating that 4-1BB deficiency does not globally impact all costimulatory signals. Instead, the contribution of 4-1BB to modulating TLR1–TLR2 costimulation is somewhat specific.
TLR signals enhance 4-1BB expression through increased transcription factor binding
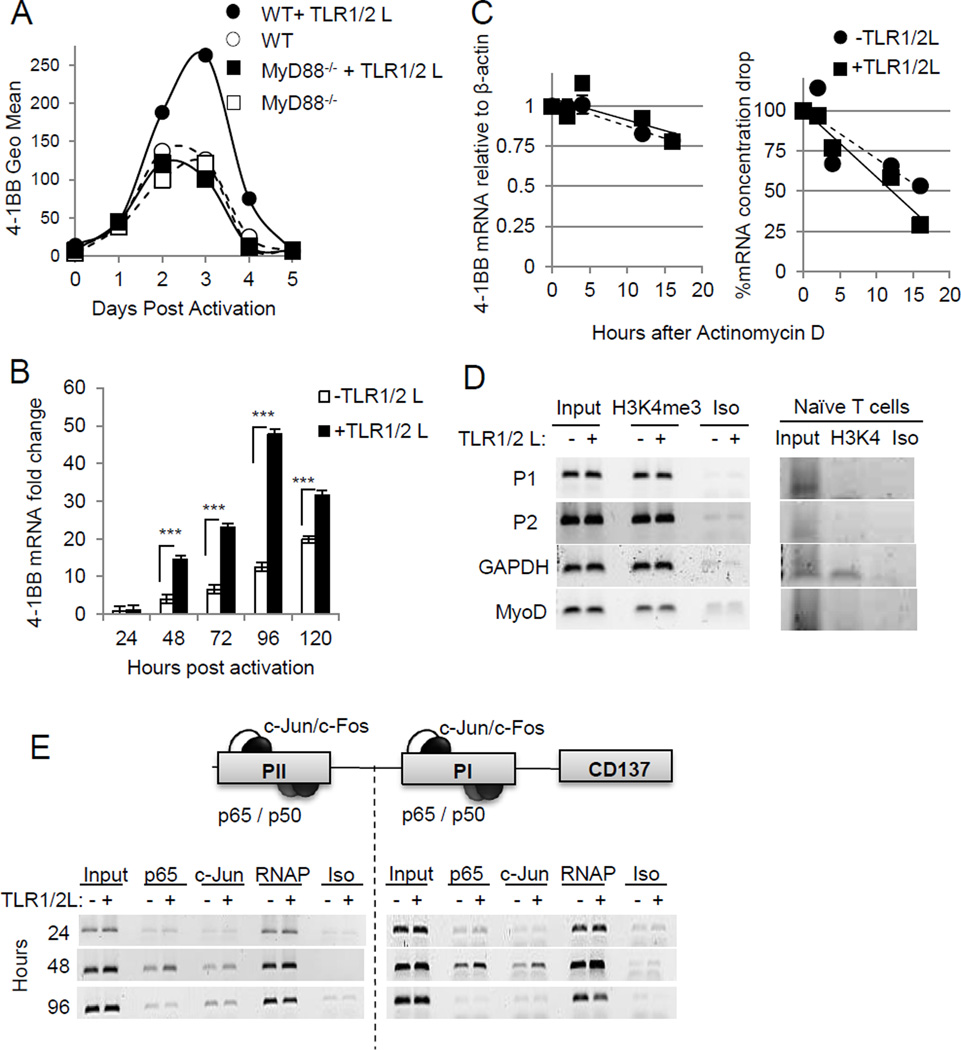
We assessed 4-1BB expression kinetics in WT and MyD88−/− CD8+ T cells over a period of 5 days with or without TLR1–TLR2L. As reported by others (19), 4-1BB expression on CD8+ T cells increased upon T-cell activation, peaking between 2 and 3 days after activation, and returning to basal levels by day 4 in non-TLR–stimulated T cells. TLR1–TLR2L increased 4-1BB expression over non-TLR-stimulated WT T cells between days 2 and 5. However, 4-1BB expression on MyD88−/− CD8+ T cells was not affected by TLR1–TLR2L (Fig. 4A). The increase in 4-1BB surface expression in response to TLR1–TLR2 stimulation correlated with increase transcripts, as assessed by quantitative real-time PCR (RT-PCR) (Fig. 4B).
Figure 4. TLR signals enhance 4-1BB expression by increasing transcription factor binding to the 4-1BB promoter.
A, 4-1BB surface expression was analyzed by flow cytometry after activation of WT and MyD88−/− CD8+ T cells by plate bound anti-CD3ε, in the presence or absence of TLR1–TLR2 ligand at the indicated time points and is representative of at least two independent experiments. B, 4-1BB mRNA expression in CD8+ T cells was analyzed by qPCR at the indicated time points after activation. mRNA levels were normalized to β-actin. C, CD8+ T cells were activated for 72 hours and then treated with actinomycin D for 2, 4, 12 and 16 hours. mRNA was isolated and 4-1BB expression analyzed by qPCR and normalized to β-actin. D and E, Chromatin immunoprecipitation was conducted using the indicated antibodies to analyze histone modifications at the promoter regions of 4-1BB at indicated time points (D) or transcription factor binding (E) after activation with anti-CD3ε, with or without TLR ligand. Amplification of the GAPDH promoter site served as a control for transcriptionally active euchromatin and the MyoD promoter as a control for transcriptionally inactive euchromatin. RNA polymerase (RNAP) binding and isotype antibody served as positive and negative controls, respectively. Statistics were generated by student T test, ***P <0.001.
MyD88 signaling can enhance IFNγ mRNA stability (20), and we thus assessed whether TLR engagement increased 4-1BB transcripts by increasing mRNA stability. The decay rate of 4-1BB transcripts was the same in TLR–stimulated and unstimulated T cells (Fig. 4C, left), indicating that the increase in 4-1BB transcripts in TLR-stimulated T cells was not a result of enhanced mRNA stability, with both TLR-stimulated or unstimulated T cells maintaining more than 80% of the starting amount of 4-1BB mRNA. In contrast, the total mRNA dropped over 75% in both TLR-stimulated and unstimulated T cells (Fig. 4C, right).
We investigated whether TLR stimulation altered the amount of transcription factors bound to the 4-1BB promoter. The 4-1BB gene has three distinct promoter regions (PI, PII, and PIII), of which PI and PII each have an AP-1 and NF-κB binding site (21). We used a chromatin immunoprecipitation assay to assess NF-κB (p65) and AP-1(cJun) binding to each the PI and PII promoter regions at different time points after T-cell activation. Both p65 and c-Jun bound to both the PI and PII regions as early as 24 hours after T-cell activation (Fig. 4D). However, binding of p65 to the PI and PII regions in TLR-stimulated cells were increased 48 hours after T-cell activation (Fig. 4D). In TLR-stimulated cells c-Jun bound to the PI but not PII region. Because histone 3 lysine 4 trimethylation (H3K4me3) is associated with transcriptionally active genes, we also assessed whether TLR1–TLR2L regulated 4-1BB transcription by increasing H3K4me3binding (Fig. 4E). H3K4me3 was undetectable on PI and PII in naïve T cells and were similar in TLR-stimulated and unstimulated CD8+ T cells. Thus, increased 4-1BB transcripts were primarily regulated by enhanced binding of p65 and c-Jun to the PI and PII.
4-1BB antibody plus TLR1–TLR2 ligand augments T-cell antitumor activity
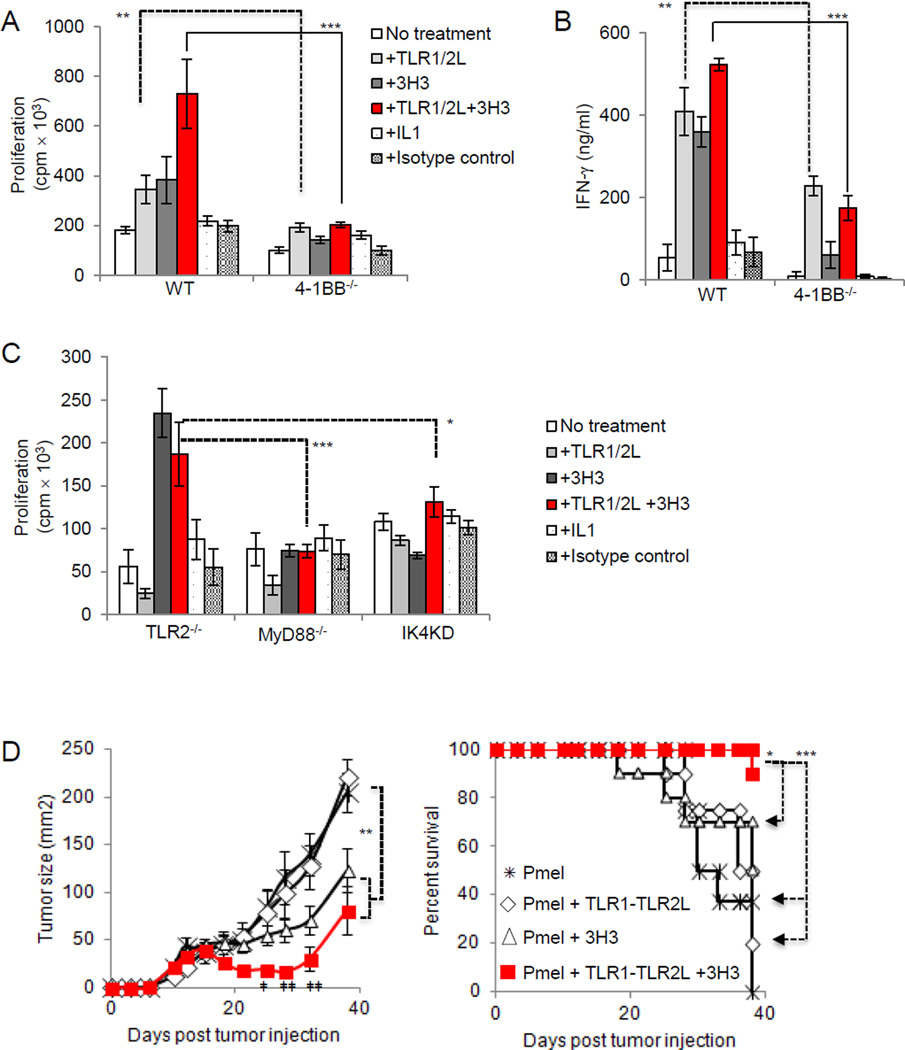
We assessed whether combined stimulation of 4-1BB and TLR1–TLR2 signals enhanced CD8+ T-cell responses above each of these signals alone. Purified WT or 4-1BB−/− CD8+ T cells were activated by plate bound CD3ε antibody and treated with agonistic 4-1BB antibody (3H3), TLR1–TLR2L, or both. We also treated cells with IL1α to rule out that the activation of MyD88 occurred via engagement of the IL1R. WT CD8+ T cells stimulated with 3H3 or TLR1–TLR2L proliferated more than untreated T cells or those treated with an isotype control antibody (Fig. 5A). Combining 3H3 with TLR1–TLR2L further enhanced T-cell proliferation over 3H3 or TLR1–TLR2 L (Fig. 5A). IL1α did not impact T-cell proliferation. Costimulatory effects of TLR1–TLR2L or 3H3 were not observed in 4-1BB−/− CD8+ T cells (Fig. 5A). Engagement of TLR1–TLR2 on both WT and 4-1BB−/− CD8+ T cells increased IFNγ production (Fig. 5B), suggesting that although the ability of TLR1–TLR2 stimulation to augment T proliferation is dependent on 4-1BB, it is not required to enhance IFNγ production.
Figure 5. Combining TLR1–TLR2 ligand and 4-1BB agonistic antibody increases T-cell proliferation and improves anti-tumor activity against melanoma in mice.
A and B, Purified WT and 4-1BB−/−, or (C),TLR2−/−, MyD88−/− and IRAK4 kinase dead CD8+ T cells were activated by CD3ε Ab-coated plates in the presence of TLR1–TLR2L, 3H3, TLR1–TLR2L and 3H3, IL1, or isotype antibody. Proliferation (±s.d.) was assessed by measuring 3H-thymidine uptake, whereas IFN-γ production was measured by ELISA 48 hours after activation. Student t test, ** P <0.01, *** P <0.001. Data from (A–C) are representative of three independent experiments. D, B16-F1 melanoma cells were injected subcutaneously into C57B6 mice. When palpable tumors were detected mice were irradiated and injected intravenously with 5 × 106–7 × 106 activated pmel T cells that had been exposed to TLR1–TLR2 ligand 24 hours before injection. TLR1–TLR2 ligand and 3H3 were injected intraperitoneally. Error bars represent standard error from the mean of 10 mice per group. Statistics were generates by repeated measures ANOVA-Tukey post test, *P <0.05, **P <0.01, ***P <0.001. Survival statistics were generated by log rank test. Student t test was conducted between 3H3 and TLR1–TLR2L + 3H3. ‡P <0.05, ‡‡P <0.01.
We assessed the effects of the above mentioned treatments on TLR2−/−, MyD88−/− and IRAK4 kinase dead (IRAK4-KD) CD8+ T cells (Fig. 5C). As expected, none of these T cells responded to TLR2 stimulation. However, 4-1BB stimulation augmented TLR2−/− CD8+ T-cell proliferation, but did not impact MyD88−/− or IRAK4-KD CD8+ T-cell proliferation. These data highlight that the proliferative effects of 4-1BB signaling in CD8+ T cells depend to some degree on both MyD88 and IRAK-4. However, although the costimulatory effects of TLR1–TLR2 ligand depend on 4-1BB, the costimulatory effects of 4-1BB do not rely on the expression of TLR2.
To further explore a link between TLR2 and 4-1BB signaling, we evaluated the effects of inhibiting the cellular inhibitors of apoptosis 1 and 2 (c-IAP1/2). c-IAP1/2 function as positive regulators of the canonical NF-κB signaling pathway and are essential in both TLR2 and 4-1BB signaling. Treating T cells with a small molecule inhibitor of c-IAP1 and c-IAP2, GDC-0152, impeded the effects of combination TLR1–TLR2 and 4-1BB stimulation and decreased the costimulatory effects of each separately (Supplementary Fig. S3). Thus, the costimulatory effects of TLR1–TLR2 and 4-1BB required TRAF signaling and NF-κB activation was required for the costimulatory effects of combination TLR1–TLR2 and 4-1BB signaling.
4-1BB antibody plus TLR1–TLR2 ligand augments T-cell antitumor activity
Combined treatment of mice with TLR1–TLR2L and agonistic 4-1BB antibody provided greater antitumor activity than did pmel T cells or TLR1–TLR2L alone and to a smaller extent over mice treated with 4-1BB antibody alone (Fig. 5D, left). Transient tumor regression was observed over the course of 2 weeks in mice receiving TLR1–TLR2L plus anti-4-1BB or anti-4-1BB alone. Combinatorial TLR1–TLR2L and anti-4-1BB treatment induced stronger antitumor responses than did 3H3 alone. The tumors in 4 of 10 mice treated with TLR1–TLR2L plus anti-4-1BB extensively regressed and three mice remained tumor free (Supplementary Fig S4). Combination TLR1–TLR2L plus anti-4-1BB treatment also significantly enhanced mouse survival over mice treated with 3H3 (P <0.05) or TLR1–TLR2 L (P <0.001). These results indicate that the costimulatory effects of TLR1–TLR2 signaling in CD8+ T cells are in part mediated by 4-1BB and can be exploited to augment antitumor immune response.
Discussion
Stimulation of CD8+ T cells with TLR ligands lead to enhanced proliferation and effector functions (24). Studies from our group have shown that MyD88-deficient CD8+ T cells have an impaired ability to survive long in vivo. How MyD88 within CD8+ T cells contributes to survival is undefined. The current studies demonstrate that TLR stimulation altered the expression of about 200 genes, including 4-1BB, OX-40, OX-40L, GITR, and DR3, which are known to costimulate activated CD8+ T cells (26–29). 4-1BB plays a crucial role in enhancing the function (30) and survival of CD8+ T cells (13, 14, 29, 31, 32). We present another vital role of 4-1BB as a mediator of the costimulatory effects of TLR1–TLR2 signals on CD8 T cells. TLR1–TLR2 engagement on T cells failed to costimulate T-cell expansion in the absence of 4-1BB or when blocking 4-1BB using antibodies. Although both TLR and 4-1BB signals promote CD8+ T-cell expansion and survival, combining these signals increased T-cell expansion over each individual treatment alone. The costimulatory effects of TLR1–TLR2L and the ability for 4-1BB blockade to inhibit the costimulatory properties of TLR1–TLR2 were heavily influenced by the amount of TCR (T-cell receptor) signal. Too high a concentration of CD3ε antibody or peptide-pulsed APCs (antigen presentation cells) bypassed the costimulatory effects of TLR1–TLR2 agonist or 4-1BB agonistic antibodies. Our analyses focused on CD8+ T-cells; however, the effects observed in CD8+ T cells might also occur in CD4+ T cells.
We demonstrated here that the combination of both TLR ligand and 4-1BB signals enhanced T-cell proliferation and IFNγ production in vitro and augmented antitumor responses in mice to a greater extent than either treatment alone. However, in vivo 4-1BB stimulation on different cells types can generate varied responses. For example, activating 4-1BB signals on DCs in a mouse model of HSV-1 (Herpes simplex virus-1) infection lead to the IFNγ dependent accumulation of IDO (indoleamine-pyrrole 2,3-dioxygenase) and subsequent suppression of the immune response. Additionally, 4-1BB signals enhanced immunity against influenza in a CD8+ T cell–dependent manner. (33). Depending on the in vivo model, 4-1BB can either promote or block CD4+CD25+ T regulatory cell activity. The transfer of 4-1BB+ CD8+ T cells generates an effective antitumor response when administered with agonistic 4-1BB antibody therapy. That 4-1BB stimulation can elicit varied immune responses highlights a potential advantage to the targeting of therapy to specific cell subsets.
These studies reveal that the costimulatory effects of TLR1–TLR2 signaling in CD8+ T-cell expansion are in part mediated by 4-1BB, that 4-1BB signaling in T cells depends in part on the presence of MyD88, and combination therapy using TLR ligand and agonistic 41BB antibodies can be exploited to enhance antitumor immune response. The proposed model through which the T-cell receptor, TLR2, and 4-1BB signaling are believed to interact is shown in Supplementary Fig. S5.
Supplementary Material
Acknowledgments
Grant support
These studies were supported by the R01CA140917 (E. Davila), University of Maryland, Marlene and Stewart Greenebaum Cancer (E. Davila), P30CA134274 (E. Davila), and the T32 AI007540 (A.M. Joseph). J. Zabaleta has been partially supported by grants from the National Institute of General Medical Sciences (NIGMS P20GM103501, P30GM114732, U54GM104940-01), and the National Institute on Minority Health and Health Disparities (NIMHD P20MD004817, U54MD008176-01)
Footnotes
Disclosures of Conflicts of Interest
No potential conflicts of interests were disclosed.
Reference List
- 1.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1(6):949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73(5):555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 4.Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. When toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effectors function. Blood. 2010 doi: 10.1182/blood-2010-02-268169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008 doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman AH, Cui W, LaRosa DF, Taylor DK, Zhang J, Goldstein DR, et al. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181(6):3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol Rev. 2013;255(1):125–148. doi: 10.1111/imr.12086. [DOI] [PubMed] [Google Scholar]
- 9.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25(2):230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180(12):8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 12.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 13.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168(8):3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 14.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol. 2004;172(2):981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 15.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3(6):536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 16.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 17.Sin JI, Kim H, Ahn E, Jeon YH, Park WS, Lee SY, et al. Combined stimulation of TLR9 and 4.1BB augments Trp2 peptide vaccine-mediated melanoma rejection by increasing Ag-specific CTL activity and infiltration into tumor sites. Cancer Lett. 2013;330(2):190–199. doi: 10.1016/j.canlet.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Sznol M, Hodi FS, Margolin K, McDermott DF, Ernstoff MS, Kirkwood JM, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) Journal of Clinical Oncology. 2008;26(15S) Ref Type: Abstract. [Google Scholar]
- 19.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150(3):771–781. [PubMed] [Google Scholar]
- 20.Sun D, Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol. 2006;7(4):375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- 21.Kim JD, Kim CH, Kwon BS. Regulation of mouse 4-1BB expression: multiple promoter usages and a splice variant. Mol Cells. 2011;31(2):141–149. doi: 10.1007/s10059-011-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng PH, Matsuzawa A, Zhang W, Mino T, Vignali DA, Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. 2010;11(1):70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10(8):561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 24.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93(6):847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng D, Zheng L, Srivastava R, Riker AI, Velasco-Gonzales C, Markovic SN, et al. Amplifying TLR-MyD88 signals within tumor-specific T-cells enhances antitumor activity to suboptimal levels of weakly-immunogenic tumor-antigens. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slebioda TJ, Rowley TF, Ferdinand JR, Willoughby JE, Buchan SL, Taraban VY, et al. Triggering of TNFRSF25 promotes CD8(+) T-cell responses and anti-tumor immunity. Eur J Immunol. 2011;41(9):2606–2611. doi: 10.1002/eji.201141477. [DOI] [PubMed] [Google Scholar]
- 27.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stockl J, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38(10):2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172(8):4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 29.Snell LM, Lin GH, McPherson AJ, Moraes TJ, Watts TH. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol Rev. 2011;244(1):197–217. doi: 10.1111/j.1600-065X.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187(11):1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS ONE. 2010;5(6):e11003. doi: 10.1371/journal.pone.0011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi BK, Kim YH, Choi JH, Kim CH, Kim KS, Sung YC, et al. Unified immune modulation by 4-1BB triggering leads to diverse effects on disease progression in vivo. Cytokine. 2011;55(3):420–428. doi: 10.1016/j.cyto.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173(4):2428–2434. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 35.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75(5):785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.