Abstract
Fusarium verticillioides is an agriculturally important fungus because of its association with maize and its propensity to contaminate grain with toxic compounds. Some isolates of the fungus harbor a meiotic drive element known as Spore killer (SkK) that causes nearly all surviving meiotic progeny from an SkK × Spore killer-susceptible (SkS) cross to inherit the SkK allele. SkK has been mapped to chromosome V but the genetic element responsible for meiotic drive has yet to be identified. In this study, we used cleaved amplified polymorphic sequence markers to genotype individual progeny from an SkK × SkS mapping population. We also sequenced the genomes of three progeny from the mapping population to determine their single nucleotide polymorphisms. These techniques allowed us to refine the location of SkK to a contiguous 102 kb interval of chromosome V, herein referred to as the Sk region. Relative to SkS genotypes, SkK genotypes have one extra gene within this region for a total of 42 genes. The additional gene in SkK genotypes, herein named SKC1 for Spore Killer Candidate 1, is the most highly expressed gene from the Sk region during early stages of sexual development. The Sk region also has three hyper-variable regions, the longest of which includes SKC1. The possibility that SKC1, or another gene from the Sk region, is an essential component of meiotic drive and spore killing is discussed.
Keywords: genomic conflict, mapping, meiotic drive, fungi, spore killing
Fusarium verticillioides is an ascomycete fungus that can exhibit both endophytic and pathogenic growth on maize (Bacon and Hinton 1996; White 1998). The fungus is of agricultural concern because of its ability to cause maize ear and stalk rot and also because it can contaminate maize kernels with a group of mycotoxins known as fumonisins (Marasas 2001; (Munkvold and Desjardins 1997). The problems caused by F. verticillioides and its myoctoxins were noticed as early as 1970, when the fungus was correlated with an outbreak of equine leukoencephalomalacia (ELEM) in South Africa (Kellerman et al. 1972). Since then, fumonisins have been confirmed to cause ELEM in horses (Marasas et al. 1988; Kellerman et al. 1990), pulmonary edema in pigs (Harrison et al. 1990), and liver cancer in laboratory rats (Gelderblom et al. 1988, 1991). Fumonisin-contaminated grain has also been linked to neural tube defects in humans (Missmer et al. 2006). Thus, the potential presence of fumonisins, even in apparently healthy grain, makes it necessary to aggressively screen grain for these mycotoxins. Contaminated grain must be destroyed, resulting in crop losses worth millions of dollars per yr (Wu 2007).
The primary sources of inoculum for endophytic and pathogenic growth of F. verticillioides on maize are asexual spores known as macroconidia and microconidia (Munkvold 2003). Sexual spores, called ascospores, may also be important to the F. verticillioides life cycle (Reynoso et al. 2009). Sexual reproduction in this heterothallic fungus begins when the immature fruiting bodies of one strain are fertilized by a strain of opposite mating type (Martin et al. 2011). Fruiting bodies are called perithecia and meiosis occurs in spore sacs, called asci, within the perithecia. At the start of meiosis, a single diploid nucleus is formed from the haploid genomes of both parents. This diploid nucleus separates into four haploid nuclei through the standard reductional and equational division stages of meiosis, and each of the four haploid nuclei undergoes a postmeiotic mitosis to form eight nuclei. Each nucleus is then incorporated into an ascospore through a process known as ascospore delimitation. A phenotypically normal ascus should thus contain eight ascospores at maturity in F. verticillioides.
During normal development, asci break down and ascospores are exuded from perithecia in gelatinous masses. It is possible, however, to examine ascospores within intact asci by perithecial dissection. Kathariou and Spieth (1982) used this technique during their discovery of an allele named Spore killer (SkK). In SkK × SkS crosses, where the latter stands for Spore killer-susceptible, asci contain four instead of eight ascospores (Kathariou and Spieth 1982). While early stages of ascus development are cytologically normal in these crosses, four ascospores degenerate shortly after ascospore delimitation (Raju 1994). The four surviving ascospores are almost always of the SkK genotype (Kathariou and Spieth 1982; Xu and Leslie 1996). Unlike SkK × SkS crosses, SkK × SkK crosses produce asci with eight ascospores (Kathariou and Spieth 1982). The SkK allele is thus sufficient for both spore killing and resistance to killing.
The ability of SkK-ascospores to kill SkS-ascospores suggests that SkK could be the predominant allele in some F. verticillioides populations. Although the literature is limited on this subject, a screen of 225 F. verticillioides isolates from 24 fields across Europe and North America found the SkK allele to be present in 81% of isolates (Kathariou and Spieth 1982). Shortly after the discovery of SkK in F. verticillioides, a spore killing allele was also discovered in F. subglutinans (Sidhu 1984). While this allele was also named Spore killer, it was given the slightly different notation of SKk. Sidhu (1984) presented evidence that SKk was present in 10 of 15 F. subglutinans isolates obtained from a single maize field. Therefore, SkK and SKk appear to be the predominant alleles in at least some populations of F. verticillioides and F. subglutinans.
The relationship between SkK and SKk is still unclear. To our knowledge, the only other significant research involving either allele was performed by Xu and Leslie (1996) during construction of an F. verticillioides genetic map. During this study, SkK was mapped between two restriction fragment length polymorphism (RFLP) markers on chromosome V. These markers, named RFLP1 and 11p18, are located 2.5 cM and 8.6 cM from SkK, respectively (Xu and Leslie 1996). Xu and Leslie’s mapping population was derived from an SkK × SkS cross, and, interestingly, only one of more than 100 progeny did not inherit the SkK allele (Xu and Leslie 1996), demonstrating that SkK can achieve transmission rates of over 99% in laboratory crosses.
An F. verticillioides reference genome, derived from an SkS strain known as Fv149-SkS, was published 14 yr after the initial mapping of SkK (Ma et al. 2010). Here, we advance Fusarium Spore killer research by delineating physical borders for SkK with respect to this reference genome. Our data place SkK within a 102 kb contiguous sequence of DNA, which we refer to as the Sk region. Notable differences exist between this region in the SkK and SkS strains examined in this study. These are described and discussed below.
Materials and Methods
Strains, media, and culture conditions
Key strains are listed in Table 1. Vegetative propagation was performed on V8 juice agar (VJA) (Tuite 1969) in 16 mm test tubes at room temperature on a laboratory benchtop. Carrot agar (CA), which was originally described by Klittich and Leslie (1988), was prepared as follows: 200 g of organic peeled baby cut carrots were autoclaved in 200 ml of water, pureed with a blender, then adjusted to 500 ml with sterile water to create a 1× stock. Diluted CA (e.g., 0.1× and 0.25×) was prepared by mixing the appropriate volumes of 1× CA stock and sterile water before adding agar to a final concentration of 2% and autoclaving. Liquid Vogel’s Minimal medium (VMM, Vogel 1956) or GYP medium (2% glucose, 1% peptone, and 0.3% yeast extract) were used to produce mycelia for genomic DNA isolation. Liquid cultures for genomic DNA isolation were incubated at 28° in the dark without agitation.
Table 1. Key strains used in this study.
| Namea | Genotype | Alternate Names |
|---|---|---|
| Fv149 | SkS, MAT-1 | FGSC 7600,b M-3125,c A00149c |
| Fv999 | SkK, MAT-2 | FGSC 7603,b A00999d |
| RJP98.75 | SkK, MAT-2 | This study |
| RJP98.111 | SkK, MAT-2 | This study |
| RJP98.118 | SkK, MAT-2 | This study |
| RBM43.31 | SkK, MAT-1 | This study |
Throughout the text, the suffix -SkK or -SkS is added to each strain name to denote genotype with respect to Sk.
Sexual crosses
Crosses were performed on CA in a manner similar to previously described methods (Klittich and Leslie 1988). Asexual spores (conidia) from the female parent were transferred to the center of a 60 mm petri dish containing 20 ml of CA or diluted CA. The female parent was then cultured for 10−14 d before fertilization with a suspension of conidia from the male parent. Conidial suspensions were prepared by adding 2.0 ml of 0.001% Tween-20 to a 10- to 14-d-old test-tube culture of the male parent and dislodging the conidia with a pipette tip. Fertilization was performed by transferring 1.0 ml of this conidial suspension to the surface of a culture of the female parent. The conidial suspension was spread over the surface of the female culture with a glass rod. Crosses were incubated in a culture chamber that alternated between 23.0° (12 hr, light) and 22.5° (12 hr, dark). Light was provided by white (Philips F34T12/CW/RS/EW/ALTO) and black (General Electric F40T12BL) fluorescent lamps.
The Fv999-SkK × Fv149-SkS mapping population
In F. verticillioides, ascospores are exuded from mature perithecia in a hair-like structure called a cirrus. To obtain an SkK mapping population, Fv999-SkK was crossed with Fv149-SkS and cirri were isolated from the tops of a few perithecia with a sterile needle, dispersed in sterile water, and spread onto a plate of 4% water agar. Germinating ascospores were transferred to VJA in 16 mm test tubes.
Microscopy
Asci were dissected from perithecia in 25% glycerol under magnification. A Vanguard 1433Phi light microscope and attached digital camera (Amscope MU1000) were used for imaging. The condenser and aperture diaphragm were set for high contrast, which allowed for the number of ascospores in mature asci to be determined without tissue staining.
DNA methods
Genomic DNA was prepared using one of three methods. In method one, strains were cultured in 25 ml liquid VMM at 28° in the dark for 3 d. Mycelia were washed with 0.9% NaCl and dried by lyophilization before extraction with IBI Scientific’s Genomic DNA Mini Kit for Plants. Method two, based on Henderson et al. (2005), was used as an inexpensive and time-efficient alternative to method one. A 6-inch plain-tipped wood applicator was used to transfer ≤10 mg of conidia to 200 µl of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0). The suspension was boiled at 105° in a heat block for 12 min, incubated on ice for 2 min, then vortexed for 5 sec. Insoluble material was pelleted at 15,000 × g for 10 min at room temperature, after which 25 µl of supernatant was transferred to a new vial and frozen at −20° for storage. In our hands, this method works well for the amplification of polymerase chain reaction (PCR) products shorter than 1 kb. Method three was used for the preparation of genomic DNA for high-throughput sequencing. Strains were cultured in 25 ml of liquid GYP at 28° for 48 hr. Mycelia were washed with water and DNA was extracted with the Zymo Research Fungal DNA Miniprep Kit.
PCR assays were performed with MidSci Bullseye Taq DNA Polymerase or New England Biolabs Phusion High-Fidelity DNA Polymerase.
Genome sequencing
DNA libraries for MiSeq sequencing (Illumina) were constructed from 1 ng of genomic DNA using the Illumina Nextera XT DNA Library Preparation Kit. Sequencing was performed with MiSeq Reagent Kit Version 3. Adapters were removed and low-quality reads were trimmed with CLC Genomics Workbench (Version 8.0). The datasets were deposited in the National Center for Biotechnology Information’s (NCBI) Sequence Read Archive (Leinonen et al. 2011). They can be obtained with the following accession numbers: SRR3271586 (Fv999-SkK), SRR3273544 (JP98.75-SkK), SRR3273545 (JP98.111-SkK), and SRR3273546 (JP98.118-SkK). The datasets are of high quality. For example, draft genomes were assembled with CLC Genomics Workbench and all assemblies had N50 values over 90 kb with coverage levels between 49- and 73-fold (Table 2).
Table 2. Draft genome assembly statistics.
| Name | Totala | Contigs | Avg. Lengthb | # Reads | N50 | Coveragec |
|---|---|---|---|---|---|---|
| Fv999-SkK | 41,922,529 | 855 | 255 | 10,956,620 | 109,413 | 67 |
| RPJ98.75-SkK | 41,848,894 | 985 | 257 | 8,087,199 | 91,461 | 50 |
| RPJ98.111-SkK | 41,892,017 | 841 | 242 | 12,538,869 | 120,565 | 72 |
| RPJ98.118-SkK | 41,868,129 | 914 | 268 | 9,009,572 | 88,867 | 58 |
Total, the total length of each genome assembly.
Avg. length, the average length of each read in the assembly.
Coverage, calculated by multiplying the number of reads by the average read length and dividing the product by 41.9 Mb, the genome size of Fv149-SkS (NCBI ASM14955v1).
CAPS markers
Cleaved amplified polymorphic sequence (CAPS) markers (Konieczny and Ausubel 1993) were used to help refine the location of SkK (Table 3). CAPS markers were identified by first aligning genome sequencing reads from strain Fv999-SkK to the Fv149-SkS reference genome (NCBI, ASM14955v1) with Bowtie 2 (Langmead and Salzberg 2012) and then visually scanning aligned reads with Tablet (Milne et al. 2010) for polymorphisms in GGCC sites. This four-base sequence is cleaved by the restriction endonuclease HaeIII. Six CAPS markers on chromosome V were chosen for this study, along with one CAPS marker on each of chromosomes I, VII, and XI (Table 3). PCR primers for each CAPS marker were designed to amplify a short product (<500 bp) from both Fv999-SkK and Fv149-SkS sequences. The PCR primers used for amplification of each CAPS marker are described in Supplemental Material, Table S1.
Table 3. Marker locations.
| Markera | Chromosome | Position (Mb) |
|---|---|---|
| CAPS-1 | V | 1.47 |
| CAPS-2 | V | 1.42 |
| CAPS-3 | V | 0.78 |
| CAPS-4 | V | 0.43 |
| CAPS-5 | V | 0.02 |
| CAPS-6 | V | 2.12 |
| CAPS-9 | XI | 0.07 |
| CAPS-10 | VII | 1.05 |
| CAPS-11 | I | 0.73 |
| RFLP-11p18 | V | 1.67 |
Marker locations on chromosomes I, V, VII, and XI of F. verticillioides Fv149-SkS (NCBI ASM14955v1).
The following protocol was used to analyze the segregation patterns of CAPS markers in each individual of the Fv999-SkK × Fv149-SkS mapping population. Genomic DNA was isolated from each progeny, CAPS markers were amplified by PCR, and PCR products were digested with HaeIII. The digested products were then examined for Fv999-SkK or Fv149-SkS cleavage patterns by gel electrophoresis on 2% agarose-TAE (40 mM Tris, 20 mM acetic acid, and 1 mM EDTA) gels. The HaeIII-based DNA digest patterns of each CAPS marker for Fv999-SkK and Fv149-SkS alleles are listed in Table S2.
Single nucleotide polymorphisms (SNPs)
Reads from each MiSeq dataset were aligned to the reference genome of strain Fv149-SkS with Bowtie 2 (Langmead and Salzberg 2012). SAMtools 1.3 and BCFtools 1.3 were then used to report SNPs in variant call format (VCF) (Li et al. 2009; Danecek et al. 2011). Only reads that aligned to a single region of Fv149-SkS with <10 mismatches were used to produce the VCF files. Custom Perl scripts were then used to extract significant SNPs from the VCF files. Significant SNPs were defined as those which were supported by at least 90% of reads at each position. Only positions covered by more than nine reads were considered. Polymorphisms caused by insertions or deletions were ignored.
Gene predictions
Protein-coding genes within the Sk region of Fv999-SkK were predicted as follows: first, the sequence of the Sk region in Fv999-SkK was obtained by de novo assembly of MiSeq reads (Table 2); second, the coding sequences of annotated genes from the Sk region of Fv149-SkS were obtained from GenBank (CM000582.1); third, the Fv149-SkS coding sequences were aligned to the Sk region of Fv999-SkK with Clustal Omega (Sievers et al. 2011; Li et al. 2015); and fourth, the alignments were used to manually annotate protein-coding sequences within the Fv999-SkK Sk region. These steps identified 41 putative protein-coding genes within the Sk region of Fv999-SkK. Augustus 3.2.1 (Stanke and Waack 2003) was then used for de novo prediction of protein-coding genes. Augustus predictions matched our manual annotation with the notable exception of an additional gene, SKC1 (described below), within the Sk region of Fv999-SkK. The complete sequence and annotation of the 102 kb SkK region from Fv999-SkK can be downloaded from GenBank with accession number KU963213.
Gene expression analysis
Sikhakolli et al. (2012) analyzed transcriptional changes in F. verticillioides during fruiting body development in Fv999-SkK × Fv149-SkS crosses by RNA sequencing (RNAseq) and deposited the datasets in NCBI’s SRA database (Leinonen et al. 2011). The following datasets were downloaded from SRA: 2 hr post fertilization (hpf) (SRR1592416), 24 hpf (SRR1592417), 48 hpf (SRR1592418), 72 hpf (SRR1592419), 96 hpf (SRR1592420), and 144 hpf (SRR1592421). Reads were aligned to coding sequences of each predicted gene from the Sk region, plus flanking genes FVEG_03199 and FVEG_03163 , with Bowtie 2 (Langmead and Salzberg 2012). Reads per kilobase exon model per thousand mapped reads (RPKK), a variation upon RPKM as described by Mortazavi et al. (2008), were calculated for each coding sequence. Because of sequence differences between SkK-linked and SkS-linked alleles of genes in the Sk region, alignments and RPKK calculations were performed separately for SkK-linked and SkS-linked alleles. Thus, reported RPKK values are averages except for SKC1, which is only found in SkK genotypes.
Data availability
Data deposition: genome sequencing data are available through NCBI’s Sequence Read Archive under accession numbers SRR3271586, SRR3273544, SRR3273545, and SRR3273546. Other sequence data are available from GenBank (KU963213).
Results
Increased production of fruiting bodies by strain Fv999-SkK on diluted CA
Directional crosses are often used to investigate sexual reproduction in heterothallic ascomycete fungi. In this type of cross, one parent is designated as the female and the other is designated as the male. The female provides protoperithecia, i.e., immature fruiting bodies, which are fertilized by conidia from the male. After fertilization, protoperithecia develop into perithecia.
With F. verticillioides, CA is commonly used as a growth medium to study sexual processes. Its preparation essentially involves purchasing carrots from a local market, followed by peeling, blending, and autoclaving a predetermined weight of carrot within a specified volume of water (Leslie and Summerell 2006). Because we were curious about how the amount of carrot in CA influences the productivity of Fv999-SkK × Fv149-SkS crosses, Fv999-SkK was cultured on 0.001×, 0.01×, 0.1×, 0.25×, 0.5×, and 1.0× CA, then fertilized with Fv149-SkS conidia. Crosses performed on 0.1× and 0.25× CA resulted in three- to six-fold more perithecia than crosses performed on 0.5× and 1.0× CA (Figure 1). No perithecia were produced when crosses were performed on 0.001× and 0.01× CA (Figure 1). Additionally, less conidia were produced on 0.1× CA than on 0.25× CA (data not shown). Since conidia can be a source of cross-contamination, 0.1× CA was used as the crossing medium for the remainder of this study.
Figure 1.
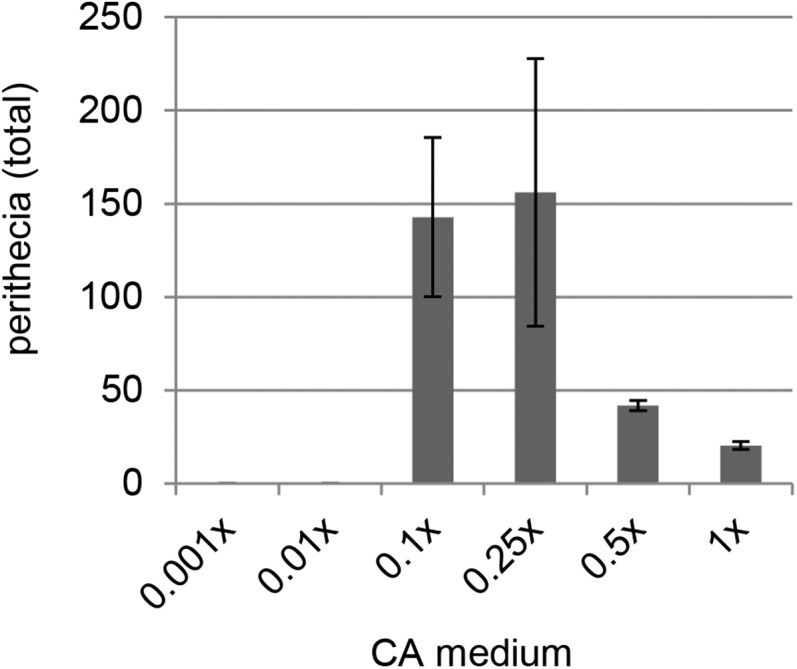
Perithecial production on different concentrations of carrot in CA medium. Cultures of Fv999-SkK were grown on CA containing different concentrations of carrot and fertilized with conidia from strain Fv149-SkS. The data represent the total number of perithecia observed 25 d after fertilization. Crosses were performed in triplicate. Error bars are standard deviation.
RFLP 11p18 overlaps with FVEG_02851 on chromosome V
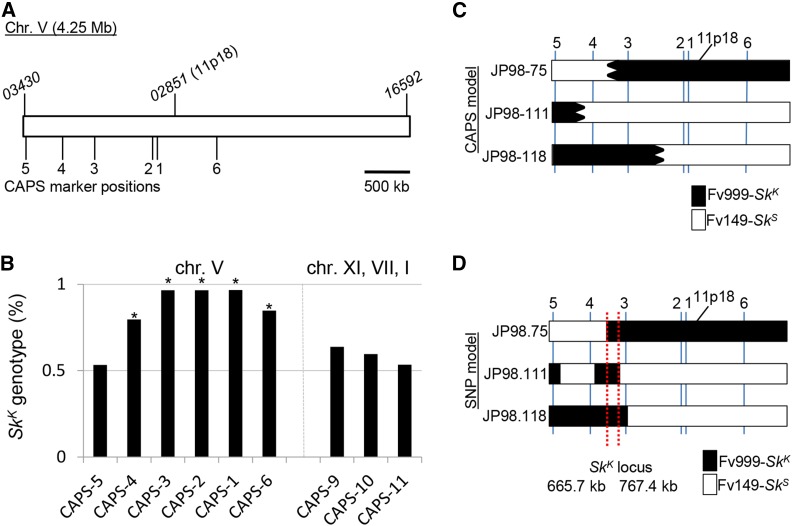
Although the sequence of RFLP1 was not available, we were able to obtain the sequence of RFLP 11p18 from the Fusarium Comparative Database. We used this sequence as the query in a Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990) search of the F. verticillioides reference genome. This search identified positions 1,672,753 to 1,673,602 on chromosome V (CM000582.1) as a match to 11p18. This region overlaps with most of the predicted coding sequence of gene FVEG_02851 , which spans positions 1,672,886 to 1,673,739 and encodes a protein of unknown function. This position on chromosome V was used as a reference point to design CAPS markers near the Sk locus (Figure 2A).
Figure 2.
F. verticillioides Sk is located within a 102 kb region of chromosome V. (A) A diagram of the 4.25 Mb sequence of chromosome V from Fv149-SkS is shown. The annotated chromosome V sequence was downloaded from NCBI (CM000582.1). The positions of the first (FVEG_03430 ) and last (FVEG_16592 ) genes on the chromosome are indicated in the diagram. The approximate location of RFLP marker 11p18 is also shown. Marker 11p18 overlaps most of gene FVEG_02851. (B) A total of 60 progeny were isolated from a cross between Fv999-SkK and Fv149-SkS. These progeny were genotyped with nine different CAPS markers. Markers CAPS-1 through CAPS-6 are located on chromosome V. Markers CAPS-9, CAPS-10, and CAPS-11 are found on chromosomes XI, VII, and I, respectively. Each bar represents the percentage of Fv999-SkK genotypes recovered for each marker. An asterisk is placed above all markers whose recovery deviated significantly from a Mendelian ratio of 1:1 according to a χ2 test with P < 0.01. The biased transmission of Fv999-SkK sequences was detected for all markers on chromosome V, except for CAPS-5. (C) The marker patterns for progeny JP98.75-SkK, JP98.111-SkK, and JP98.118-SkK suggest that cross-over events occurred between CAPS-3 and CAPS-4 (for JP98.75-SkK), CAPS-4 and CAPS-5 (for JP98.111-SkK), and CAPS-2 and CAPS-3 (for JP98.118-SkK) (Table S3). These cross-overs did not identify an Fv999-SkK-derived region of chromosome V common to all three progeny. The predicted Fv999-SkK- and Fv149-SkS-inherited regions are shown in black and white, respectively. Irregular borders are used to indicate that exact cross-over positions could not be determined from the CAPS marker data. (D) SNP analysis was used to more accurately define cross-over positions for progeny JP98.75-SkK, JP98.111-SkK, and JP98.118-SkK. In addition to refining the location of the cross-overs identified by CAPS analysis, two additional cross-overs were identified between CAPS-3 and CAPS-4 on chromosome V of JP98.111-SkK. These findings helped delineate a single contiguous interval between position 665.7 kb and 767.4 kb on chromosome V as the only Fv999-SkK-derived chromosome V sequence common to all three progeny (red dotted lines). Therefore, this interval is referred to as the Sk region.
Loci linked to SkK drive through meiosis
To begin refining the location of SkK on chromosome V, Fv999-SkK was crossed with Fv149-SkS and the segregation patterns of nine CAPS markers were examined in 60 progeny. Both Fv999-SkK and Fv149-SkS patterns were inherited in a 1:1 ratio for chromosomes XI, VII, and I (Figure 2B and Table S3). In contrast, Fv999-SkK patterns of CAPS markers on chromosome V were inherited more frequently than expected (≥79.6%; Figure 2B and Table S3). The only exception was CAPS-5, which is located ∼20 kb from a telomere (Figure 2A and Table 3). Overall, these results mark the first independent confirmation of Xu and Leslie’s (1996) original findings on the biased recovery of molecular markers linked to SkK in SkK × SkS crosses.
Genome sequencing refines Sk to a 102 kb region of chromosome V
To delineate the position of SkK on chromosome V, we narrowed our focus to progeny JP98.75-SkK, JP98.111-SkK, and JP98.118-SkK. Even though CAPS marker analysis failed to identify an Fv999-SkK region of chromosome V that was inherited by all three of these progeny (Figure 2C, black shaded regions), all were determined to carry the SkK allele by phenotypic analysis (Figure 3 and data not shown). Therefore, their genomes were sequenced (Table 2) and their SNPs along chromosome V were examined. This analysis allowed us to identify two additional recombination events. Both of these recombination events occurred between CAPS-3 and CAPS-4 in the ascus that produced progeny JP98.111-SkK (Figure 2D), explaining why they were not identified by our CAPS marker analysis. More importantly, the SNP profiles of all three progeny require that SkK be located between positions 665,669 and 767,411 (Figure 2D, red lines) with respect to the reference sequence (GenBank, CM000582.1). We refer to this 102-kb contiguous sequence as the Sk region (GenBank, KU963213).
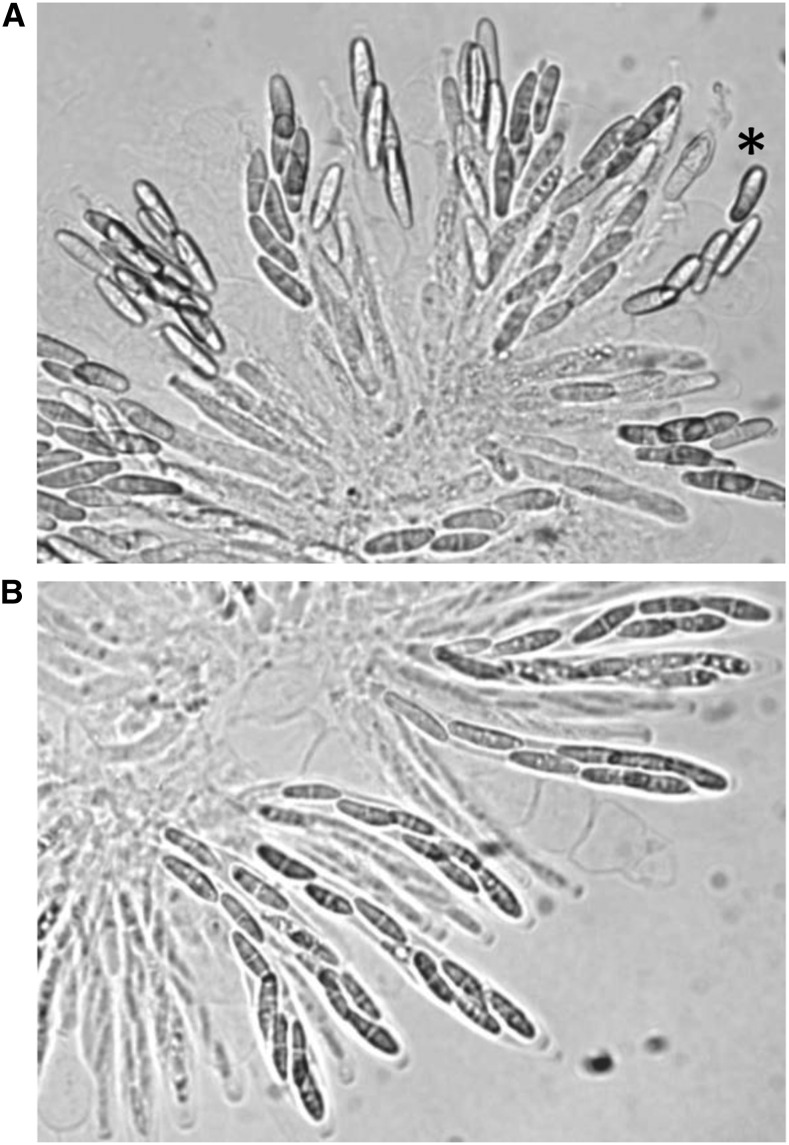
Figure 3.
Representative images of spore killing and resistance to spore killing in F. verticillioides asci. (A) Asci from a cross of progeny JP98.111-SkK with Fv149-SkS. Only four ascospores are detected in most of the mature asci, confirming that JP98.111-SkK carries the SkK allele. The asterisk denotes an ascus where there appears to be five ascospores. Two possible explanations are that this 5th ascospore escaped killing, or it strayed from another ascus during the dissection and imaging process. (B) Asci from a cross of progeny JP98.111-SkK with BM43.31-SkK. Eight ascospores can be detected in most of the mature asci, again confirming that progeny JP98.111-SkK carries the SkK allele.
SkK strains carry a unique gene in the Sk region
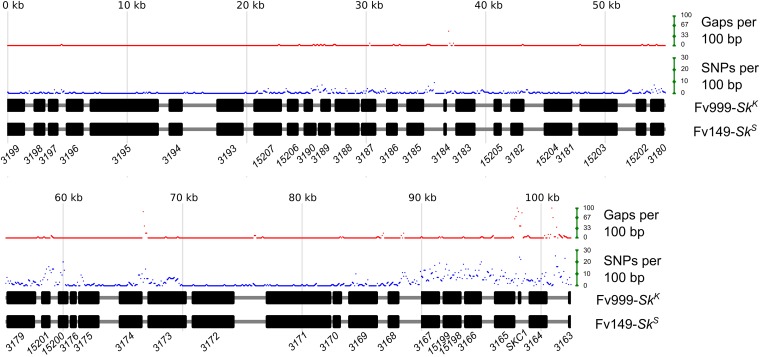
The Sk region spans 102,256 bases in Fv999-SkK, but only 101,743 bases in Fv149-SkS. A Clustal W-based alignment (Thompson et al. 1994) of the sequences from both strains is 102,557 bases long, with 301 gaps in the Fv999-SkK sequence and 814 gaps in the Fv149-SkS sequence (Figure 4). To identify specific regions in SkK and SkS strains with high levels of SNPs or gaps, the number of SNPs or gaps were calculated for each 100-base window across the alignment. A qualitative analysis of these results finds at least three hyper-variable intervals (Figure 4). One of the short hyper-variable intervals spans genes FVEG_03180 to FVEG_03175 , while another spans genes FVEG_03174 and FVEG_03173 (Figure 4). The longest hyper-variable interval is ∼14 kb long, spans gene FVEG_03167 , and extends to the right border of the Sk region (Figure 4).
Figure 4.
Strain Fv999-SkK carries a unique gene within a hyper-variable region of the Sk region. The Sk regions from Fv999-SkK (GenBank, KU963213) and Fv149-SkS (GenBank, CM000582.1, positions 665,669 to 767,411) were imported into BioEdit (Version 7.2.5) (Hall 1999) and aligned with Clustal W. Custom Perl scripts were used to examine base mismatches and gap positions, and to generate the diagram. The total number of mismatches (SNPs) between the two sequences was calculated for each 100-base nonoverlapping window of the alignment. The total number of gaps was also calculated for each 100-base nonoverlapping window. A gap position was not considered an SNP. By this definition, a window with 100 gaps cannot have SNPs. Black rectangles represent the coding regions of predicted genes. Gene names have been abbreviated according to their identification tags in the Fv149-SkS annotation. For example: FVEG_03199 was shortened to 3199. A striking feature of the alignment is the presence of three hyper-variable intervals; spanning FVEG_03180 to FVEG_03175 , FVEG_03174 to FVEG_03173 , and FVEG_03167 to the right border of the Sk region. A second striking feature is the presence of a unique gene, SKC1, in Fv999-SkK only.
The Sk region in strain Fv149-SkS includes 41 putative protein-coding genes (Figure 4 and Table 4). The Sk region in Fv999-SkK carries the same 41 genes as well as one additional protein-coding gene, SKC1 (for Spore Killer Candidate 1), which is located between FVEG_03165 and FVEG_03164 (Figure 4). A BLASTP search (Altschul et al. 1997) of the NCBI nonredundant protein database with the predicted 70 amino acid sequence of Skc1 identified hypothetical proteins with Expect values ranging from 2 × 10−49 to 8 × 10−04 from various formae speciales of the F. oxysporum species complex (Figure S1). No significant hits were identified in other species. A search of the NCBI Conserved Domain Database (Marchler-Bauer et al. 2010) failed to identify a domain within Skc1.
Table 4. Comparison of predicted protein sequences within the Sk region of Fv999-SkK and Fv149-SkS.
| Name | Fv999-SkK Length | Fv149-SkS Length | Alignment Length | Identity (%) | Similarity (%) |
|---|---|---|---|---|---|
| 3198 | 279 | 279 | 279 | 99 | 99 |
| 3197 | 224 | 224 | 224 | 99 | 99 |
| 3196 | 462 | 462 | 462 | 100 | 100 |
| 3195 | 1786 | 1786 | 1786 | 100 | 100 |
| 3194 | 359 | 359 | 359 | 100 | 100 |
| 3193 | 655 | 655 | 655 | 100 | 100 |
| 15207 | 634 | 666 | 692 | 93 | 94 |
| 15206 | 274 | 274 | 274 | 100 | 100 |
| 3190 | 146 | 242 | 242 | 99 | 100 |
| 3189 | 246 | 336 | 336 | 89 | 91 |
| 3188 | 599 | 599 | 599 | 99 | 100 |
| 3187 | 327 | 327 | 327 | 98 | 99 |
| 3186 | 234 | 234 | 234 | 100 | 100 |
| 3185 | 409 | 409 | 409 | 100 | 100 |
| 3184 | 65 | 46 | 65 | 96 | 98 |
| 3183 | 513 | 513 | 513 | 100 | 100 |
| 15205 | 174 | 174 | 174 | 99 | 100 |
| 3182 | 379 | 355 | 379 | 97 | 98 |
| 15204 | 88 | 88 | 88 | 100 | 100 |
| 3181 | 667 | 667 | 667 | 100 | 100 |
| 15203 | 1029 | 1085 | 1085 | 99 | 99 |
| 15202 | 194 | 194 | 194 | 100 | 100 |
| 3180 | 359 | 359 | 359 | 97 | 99 |
| 3179 | 784 | 736 | 784 | 97 | 97 |
| 15201 | 227 | 227 | 227 | 89 | 90 |
| 15200 | 288 | 288 | 288 | 93 | 98 |
| 3176 | 139 | 139 | 139 | 100 | 100 |
| 3175 | 496 | 496 | 496 | 99 | 100 |
| 3174 | 620 | 620 | 620 | 96 | 97 |
| 3173 | 964 | 964 | 964 | 97 | 98 |
| 3172 | 1018 | 1018 | 1018 | 100 | 100 |
| 3171 | 1724 | 1724 | 1724 | 100 | 100 |
| 3170 | 230 | 212 | 230 | 98 | 98 |
| 3169 | 716 | 716 | 716 | 99 | 99 |
| 3168 | 299 | 299 | 299 | 100 | 100 |
| 3167 | 460 | 460 | 460 | 97 | 98 |
| 15199 | 133 | 133 | 133 | 80 | 86 |
| 15198 | 323 | 319 | 323 | 93 | 95 |
| 3166 | 466 | 466 | 466 | 90 | 95 |
| 3165 | 532 | 531 | 532 | 98 | 99 |
| Skc1 | 70 | Missing | na | na | na |
| 3164 | 476 | 479 | 479 | 98 | 99 |
The sequences of predicted proteins within the Sk region of Fv999-SkK and Fv149-SkS were imported into Bioedit 7.2.5 (Hall 1999) and aligned with Clustal W (Thompson et al. 1994). Sequence alignments were then analyzed for percent identity and percent similarity. Gapped-positions were excluded from the identity and similarity calculations. na, not applicable.
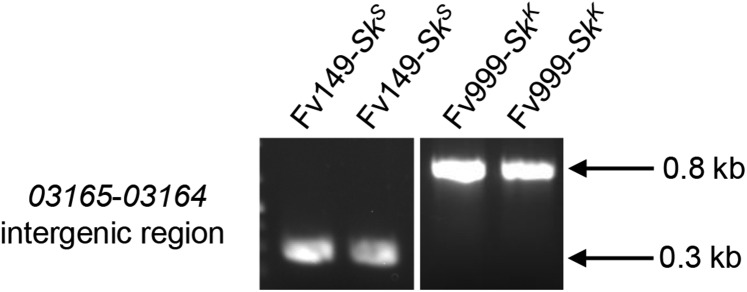
To confirm that SKC1 is absent in Fv149-SkS, the sequences corresponding to the FVEG_03165 -FVEG_03164 intergenic region from Fv999-SkK and Fv149-SkS were aligned and examined. This intergenic region consists of 1234 and 755 bases in Fv999-SkK and Fv149-SkS respectively (Figure S2). The alignment revealed that this difference in length was due to the presence of SKC1 in Fv999-SkK and its absence from Fv149-SkS (Figure S2). To confirm that the absence of SKC1 in Fv149-SkS was not due to an error in the reference genome sequence, we examined the lengths of the FVEG_03165 -FVEG_03164 intergenic region in our laboratory stocks of Fv999-SkK and Fv149-SkS by PCR. The PCR product lengths were consistent with the presence of SKC1 in Fv999-SkK and its absence from Fv149-SkS (Figure 5).
Figure 5.
The Sk region in Fv149-SkS is missing SKC1. To confirm that SKC1 is missing from the Sk region in Fv149-SkS, genomic DNA was isolated from two liquid cultures of Fv149-SkS and two liquid cultures of Fv999-SkK. All four genomic DNA samples were used as templates in standard PCR reactions with the following oligonucleotide primers: 5′ CGAATGACCTGGGGAGCCATAA 3′ and 5′ TCTCTCCACCACCTCCATCAGC 3′, which amplify the FVEG_03165 -FVEG_03164 intergenic regions from both Fv999-SkK and Fv149-SkS. PCR products were visualized by ethidium bromide staining after electrophoresis through a 1% agarose-TAE gel. We observed PCR products with lengths that were consistent with absence of SKC1 from Fv149-SkS (287 bp) and presence of SKC1 in Fv999-SkK (767 bp).
Clustal W alignments were used to investigate the level of polymorphism between proteins encoded by the Sk region in Fv999-SkK and Fv149-SkS strains (Table 4). Analysis of these pairwise alignments revealed that Fveg_15199 is the most polymorphic protein of the region. Only 80% of the 133 amino acids of Fveg_15199 are identical between the two strains. This is a remarkably high level of polymorphism given that 34 of 41 proteins within the Sk region of Fv999-SkK and Fv149-SkS are >94% identical, and 40 of 41 are >88% identical (Table 4). A BLAST search of the NCBI nonredundant protein database identified homologs of Fveg_15199 in several Fusarium, Neonectria, Scedosporium, and Aspergillus species (Figure S3). A search of the NCBI Conserved Domain Database failed to identify a domain within Fveg_15199.
To shed light upon the transcriptional profile of protein-coding genes within the Sk region, we analyzed five F. verticillioides RNAseq datasets from NCBI’s Sequence Read Archive. These datasets were for five time points following the induction of sexual development in a cross of Fv999-SkK × Fv149-SkS (Sikhakolli et al. 2012). Of the genes within the Sk region, FVEG_03171 exhibited the greatest increase in expression from the first time point to the last time point (Table 5, 2.95 and 94.58 RPKK), suggesting that this gene may have an important role during later stages of sexual development. The corresponding protein, Fveg_03171 , includes a WSC-domain (pfam01822), which has been linked to carbohydrate-binding, cell wall integrity, and stress response. The gene FVEG_03194 exhibited the greatest fold change when comparing differences between all five time points (>1050×) (Table 5). The Fveg_03194 protein does not contain recognized domains, although putative homologs can be found in Fusarium, Nectria, Acremonium, and Trichoderma fungi (Figure S4). Interestingly, SKC1 reached the highest level of expression of all examined genes 2 hr after fertilization (Table 5). SKC1 transcript levels were also second only to FVEG_03197 at the last time point (144 hr, Table 5). Fveg_03197 appears to be from a family of proteins widely conserved among bacteria and eukaryotes (Figure S5), but a function for it or its homologs is unknown.
Table 5. Expression analysis of genes from the Sk region during sexual development.
| ID | 2 h | 24 h | 48 h | 72 h | 96 h | 144 h | Mina | Maxb | Fold Changec |
|---|---|---|---|---|---|---|---|---|---|
| 3198 | 39.17 | 150.92 | 38.40 | 24.98 | 28.10 | 20.10 | 20.10 | 150.92 | 7.51 |
| 3197 | 256.38 | 368.62 | 217.04 | 122.34 | 103.97 | 231.66 | 103.97 | 368.62 | 3.55 |
| 3196 | 0.83 | 0.13 | 0.87 | 0.56 | 0.83 | 0.87 | 0.13 | 0.87 | 6.59 |
| 3195 | 2.49 | 0.57 | 2.48 | 0.62 | 5.80 | 0.79 | 0.57 | 5.80 | 10.12 |
| 3194 | 0.25 | 0.12 | 0.30 | 14.02 | 124.53 | 1.20 | 0.12 | 124.53 | 1050.12 |
| 3193 | 138.42 | 94.82 | 71.69 | 43.35 | 80.19 | 75.41 | 43.35 | 138.42 | 3.19 |
| 15207 | 1.41 | 1.35 | 16.98 | 1.71 | 2.05 | 2.46 | 1.35 | 16.98 | 12.55 |
| 15206 | 0.78 | 1.40 | 5.23 | 1.29 | 0.76 | 1.08 | 0.76 | 5.23 | 6.86 |
| 3190 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.23 | 2.32 |
| 3189 | 0.00 | 0.00 | 0.00 | 0.05 | 0.50 | 0.00 | 0.00 | 0.50 | 4.95 |
| 3188 | 1.73 | 0.36 | 1.58 | 0.48 | 2.72 | 0.40 | 0.36 | 2.72 | 7.65 |
| 3187 | 1.90 | 0.97 | 1.34 | 31.36 | 42.16 | 0.71 | 0.71 | 42.16 | 59.47 |
| 3186 | 1.45 | 0.55 | 0.81 | 0.77 | 0.37 | 0.61 | 0.37 | 1.45 | 3.91 |
| 3185 | 5.41 | 3.74 | 6.17 | 2.61 | 1.72 | 1.51 | 1.51 | 6.17 | 4.09 |
| 3184 | 0.58 | 0.00 | 1.19 | 0.84 | 9.94 | 0.79 | 0.00 | 9.94 | 99.38 |
| 3183 | 59.62 | 22.63 | 32.99 | 13.12 | 29.15 | 21.10 | 13.12 | 59.62 | 4.54 |
| 15205 | 4.06 | 1.68 | 6.07 | 3.12 | 10.00 | 1.23 | 1.23 | 10.00 | 8.15 |
| 3182 | 0.30 | 0.00 | 1.19 | 0.00 | 0.29 | 0.00 | 0.00 | 1.19 | 11.93 |
| 15204 | 5.10 | 3.36 | 84.28 | 5.20 | 12.73 | 5.45 | 3.36 | 84.28 | 25.10 |
| 3181 | 2.16 | 0.50 | 5.24 | 0.50 | 1.93 | 0.66 | 0.50 | 5.24 | 10.57 |
| 15203 | 4.74 | 0.12 | 3.02 | 1.00 | 4.72 | 0.95 | 0.12 | 4.74 | 39.22 |
| 15202 | 1.05 | 0.44 | 8.61 | 7.61 | 9.45 | 0.74 | 0.44 | 9.45 | 21.57 |
| 3180 | 2.20 | 0.00 | 3.14 | 0.14 | 0.98 | 0.17 | 0.00 | 3.14 | 31.38 |
| 3179 | 1.28 | 0.06 | 1.14 | 0.10 | 2.70 | 0.09 | 0.06 | 2.70 | 47.85 |
| 15201 | 4.11 | 0.57 | 4.26 | 1.33 | 4.46 | 0.49 | 0.49 | 4.46 | 9.01 |
| 15200 | 1.02 | 0.55 | 0.32 | 0.36 | 0.29 | 0.24 | 0.24 | 1.02 | 4.16 |
| 3176 | 126.00 | 50.26 | 80.75 | 15.66 | 31.67 | 29.31 | 15.66 | 126.00 | 8.05 |
| 3175 | 48.87 | 12.93 | 38.03 | 9.13 | 19.60 | 13.41 | 9.13 | 48.87 | 5.35 |
| 3174 | 4.53 | 1.43 | 8.61 | 0.94 | 4.68 | 1.29 | 0.94 | 8.61 | 9.18 |
| 3173 | 1.04 | 0.17 | 2.86 | 0.42 | 1.90 | 0.42 | 0.17 | 2.86 | 17.29 |
| 3172 | 1.01 | 0.00 | 3.78 | 0.45 | 2.16 | 0.70 | 0.00 | 3.78 | 37.81 |
| 3171 | 2.95 | 37.34 | 38.55 | 117.07 | 44.37 | 94.58 | 2.95 | 117.07 | 39.66 |
| 3170 | 0.14 | 0.19 | 0.00 | 0.81 | 0.13 | 0.44 | 0.00 | 0.81 | 8.09 |
| 3169 | 5.48 | 0.61 | 5.38 | 3.02 | 7.40 | 2.61 | 0.61 | 7.40 | 12.05 |
| 3168 | 4.33 | 21.39 | 2.58 | 13.90 | 7.76 | 2.19 | 2.19 | 21.39 | 9.77 |
| 3167 | 0.17 | 0.71 | 0.13 | 0.21 | 0.12 | 0.04 | 0.04 | 0.71 | 19.47 |
| 15199 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.71 |
| 15198 | 1.34 | 0.12 | 0.56 | 0.21 | 0.31 | 0.19 | 0.12 | 1.34 | 11.68 |
| 3166 | 0.99 | 0.17 | 1.43 | 0.24 | 0.31 | 0.22 | 0.17 | 1.43 | 8.36 |
| 3165 | 20.77 | 11.51 | 13.49 | 9.68 | 8.41 | 4.09 | 4.09 | 20.77 | 5.07 |
| SKC1 | 481.58 | 392.59 | 227.42 | 211.66 | 360.52 | 97.65 | 97.65 | 481.58 | 4.93 |
| 3164 | 16.11 | 30.78 | 16.09 | 6.78 | 16.48 | 15.70 | 6.78 | 30.78 | 4.54 |
The minimum RPKK in the table.
The maximum RPKK in the table.
Fold change was calculated by dividing the maximum RPKK by the minimum RPKK. If the minimum RPKK for a gene was 0 it was arbitrarily assigned a minimum RPKK of 0.1 to approximate a fold change value. Unrounded maximum and minimum RPKK values (not shown) were used to calculate fold change values.
Discussion
The segregation of alternate alleles into separate gametes during meiosis encourages genetic conflict (Burt and Trivers 2008). Evidence for this is found in meiotic drive, which occurs when an allele is transmitted through meiosis in a biased manner. Meiotic drive elements are found in a diverse range of fungi, where they achieve biased transmission through sexual reproduction by killing meiospores carrying an alternate allele (Turner and Perkins 1979; Kathariou and Spieth 1982; Raju 1994; Dalstra et al. 2003; Grognet et al. 2014). Therefore, fungal meiotic drive elements are often referred to as Spore killers. The molecular basis of meiotic drive by most Spore killers is unknown.
The existence of meiotic drive by spore killing in Fusarium was first recognized in 1982. More than three decades later, we still do not understand the molecular mechanism that mediates this process. This is similar to the situation in Neurospora, where three distinct Spore killers, namely Sk-1, Sk-2, and Sk-3, were identified in 1979 (Turner and Perkins 1979). A breakthrough in Sk-2 research was recently made by refining the location of an Sk-2 resistance gene to a 52 kb sequence of DNA (Hammond et al. 2012a). This provided the necessary foundation to clone and characterize this resistance gene (Hammond et al. 2012b), which in turn allowed for the isolation of killer-less Sk-2 mutants (Harvey et al. 2014). Here, we have taken a similar approach toward identifying the genetic basis of SkK in F. verticillioides by refining the position of SkK to a 102 kb region of chromosome V.
As with previous work on SkK (Xu and Leslie 1996), we observed a biased transmission of SkK-linked molecular markers (Figure 2B). This result was expected since the driving ability of SkK should also affect the transmission of alleles linked to SkK. This phenomenon is referred to as genetic hitchhiking (Lyttle 1991). The closer a hitchhiker is to a meiotic driver, the more likely it is to be transmitted to the next generation through the sexual cycle. In our study, patterns of CAPS markers from the parent Fv999-SkK were inherited at a higher frequency than could be attributed to chance alone for five of six markers on chromosome V, and the transmission bias generally decreased with increasing distance from the Sk region (Figure 2B). Surprisingly, CAPS-1 and CAPS-2 demonstrated higher levels of hitchhiking than CAPS-4 (Figure 2B), despite the latter being closer to the Sk region. One explanation for this could be the existence of a recombination hotspot between CAPS-4 and the Sk region; however, we do not have additional data to support this hypothesis.
While a recombination hotspot could explain the relatively low level of hitchhiking by CAPS-4, the opposite phenomenon of recombination-suppression is often associated with meiotic drive elements (reviewed by Lyttle 1991). For example, Neurospora Sk-2 requires specific alleles of at least two genes to mediate drive, a resistance gene called rsk and a killer gene called rfk (Hammond et al. 2012b; Harvey et al. 2014). Each gene is located on a different arm of chromosome III (Harvey et al. 2014). For Sk-2 to succeed as a meiotic driver, it is imperative that an Sk-2 ascospore inherit both rsk and rfk alleles because separation can lead to a self-killing genotype (Hammond et al. 2012b). This helps explain why Sk-2 is associated with a 30 cM “recombination-blocked” interval of chromosome III (Campbell and Turner 1987; Harvey et al. 2014). By suppressing recombination between rsk and rfk, Sk-2 can prevent these critical components of drive from separating during meiosis. With respect to F. verticillioides SkK, recombination-suppression, if it exists at all, does not appear to be a significant phenomenon. For example, our CAPS marker analysis identified 12 recombination events between CAPS-3 and CAPS-4 (n = 59, Table S3), and two recombination events between CAPS-2 and CAPS-3 (n = 59, Table S3). This relatively high number of recombination events near the Sk region argues against an Sk-2-like recombination block for SkK.
If F. verticillioides SkK requires multiple genes to function as a meiotic drive element, all should be found within the 102 kb Sk region defined by this study. For example, SNP-profiling of progeny JP98.75-SkK, JP98.111-SkK, and JP98.118-SkK indicates that only this region of chromosome V is common between these three progeny and their Fv999-SkK parent (Figure 2D and Figure 4). Assuming killing is mediated by two or more distinct genes within the Sk region, the close proximity of these genes may negate the requirement for a Neurospora Sk-2-like recombination block. Alternatively, meiotic drive and spore killing may be mediated by a single gene. A precedent for this is seen in the het-s spore killing mechanism of Podospora anserina (Coustou et al. 1997; Saupe 2011). This system is controlled by two alternate alleles of a single gene, named het-s and het-S. The former allele, het-s, encodes the HET-s prion and is the meiotic driver, while the latter allele, het-S, encodes the HET-S protein. Interaction of the HET-s prion with the HET-S protein causes HET-S to relocate to cell membranes, resulting in cell death, presumably through loss of membrane integrity (Seuring et al. 2012). Ascospores with het-s genotypes escape cell death because they do not produce the HET-S protein, thus the het-s prion is not toxic to them. This is just one example of how meiotic drive and spore killing can be mediated by alternate alleles of a single gene in fungi. Grognet et al. (2014) have recently identified spok1 and spok2, two additional spore killing genes in P. anserina, both of which also appear to be single-gene-based meiotic drive systems.
In the current study, comparative sequence analysis revealed multiple differences between the 102 kb Sk region in SkK and SkS strains of F. verticillioides. Presumably, one or more of these differences is the genetic basis for the different Sk phenotypes exhibited by the strains. An analysis of SNPs and gaps identified at least three hyper-variable intervals within the Sk region (Figure 4). The interspersed nature of these intervals is somewhat surprising. The most striking difference, however, is the presence of the SKC1 gene in SkK strains. Differences between the hyper-variable intervals and SKC1 in SkK vs. SkS strains raise at least two key questions: (1) are the hyper-variable intervals and/or SKC1 responsible for killing, and (2) do they correspond to the SkK allele that was previously defined by phenotypic analysis (Kathariou and Spieth 1982). The high level of expression of SKC1 throughout sexual development is consistent with its involvement in sexual reproduction. However, in addition to SKC1 and the hyper-variable intervals, there are less dramatic sequence differences between genes and intergenic sequences of the Sk region in SkK and SkS strains. The current data do not rule out the possibility that one or more of these differences is responsible for the SkK and SkS phenotypes. As a result, our efforts are now focused on targeted deletions of SKC1 and other sequences within the Sk region to determine the genetic basis of the spore killing phenotype in F. verticillioides.
Supplementary Material
Acknowledgments
Dr. John Leslie (Kansas State University) and Dr. Frances Trail (Michigan State University) kindly donated strains Fv999-SkK and Fv149-SkS. We thank the Fungal Genetics Stock Center (McCluskey et al. 2010) for other key strains and the Broad Institute of Harvard and the Massachusetts Institute of Technology (www.broad.mit.edu) for access to the Fusarium Comparative Database. Chris McGovern and Nathane Orwig of the National Center for Agricultural Utilization Research provided much appreciated technical assistance. We thank Dr. Sven Saupe (Université de Bordeaux) for sharing his knowledge of the het-s spore killing mechanism. T.M.H. was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (1R15HD076309-01). Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The US Department of Agriculture is an equal opportunity provider and employer.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.029728/-/DC1
Communicating editor: K. Dawe
Literature Cited
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., et al. , 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C. W., Hinton D. M., 1996. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 74: 1195–1202. [Google Scholar]
- Burt A., Trivers R., 2008. Genes in Conflict: The Biology of Selfish Genetic Elements. Harvard University Press; Cambridge, MA. [Google Scholar]
- Campbell J. L., Turner B. C., 1987. Recombination block in the Spore killer region of Neurospora. Genome 29: 129–135. [DOI] [PubMed] [Google Scholar]
- Coustou V., Deleu C., Saupe S., Begueret J., 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. USA 94: 9773–9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalstra H. J. P., Swart K., Debets A. J. M., Saupe S. J., Hoekstra R. F., 2003. Sexual transmission of the [Het-S] prion leads to meiotic drive in Podospora anserina. Proc. Natl. Acad. Sci. USA 100: 6616–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C. A., Banks E., et al. , 2011. The variant call format and VCFtools. Bioinformatics 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom W. C., Jaskiewicz K., Marasas W. F., Thiel P. G., Horak R. M., et al. , 1988. Fumonisins – novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54: 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom W. C., Kriek N. P., Marasas W. F., Thiel P. G., 1991. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 12: 1247–1251. [DOI] [PubMed] [Google Scholar]
- Grognet P., Lalucque H., Malagnac F., Silar P., 2014. Genes that bias Mendelian segregation. PLoS Genet. 10: e1004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. A., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95–98. [Google Scholar]
- Hammond T. M., Rehard D. G., Harris B. C., Shiu P. K. T., 2012a Fine-scale mapping in Neurospora crassa by using genome-wide knockout strains. Mycologia 104: 321–323. [DOI] [PubMed] [Google Scholar]
- Hammond T. M., Rehard D. G., Xiao H., Shiu P. K. T., 2012b Molecular dissection of the Neurospora Spore killer elements. Proc. Natl. Acad. Sci. USA 109: 12093–12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. R., Colvin B. M., Greene J. T., Newman L. E., Cole J. R., Jr, 1990. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Invest. 2: 217–221. [DOI] [PubMed] [Google Scholar]
- Harvey A. M., Rehard D. G., Groskreutz K. M., Kuntz D. R., Sharp K. J., et al. , 2014. A critical component of meiotic drive in Neurospora is located near a chromosome rearrangement. Genetics 197: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T., Eariss G. A., Catcheside D., 2005. Reliable PCR amplification from Neurospora crassa genomic DNA obtained from conidia. Fungal Genet. Newsl. 52: 24. [Google Scholar]
- Kathariou S., Spieth P. T., 1982. Spore killer polymorphism in Fusarium moniliforme. Genetics 102: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman T. S., Marasas W. F., Pienaar J. G., Naudé T. W., 1972. A mycotoxicosis of equidae caused by Fusarium moniliforme sheldon. A preliminary communication. Onderstepoort J. Vet. Res. 39: 205–208. [PubMed] [Google Scholar]
- Kellerman T. S., Marasas W. F., Thiel P. G., Gelderblom W. C., Cawood M., et al. , 1990. Leukoencephalomalacia in two horses induced by oral dosing of fumonisin B1. Onderstepoort J. Vet. Res. 57: 269–275. [PubMed] [Google Scholar]
- Klittich C., Leslie J. F., 1988. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A., Ausubel F. M., 1993. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4: 403–410. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R., Sugawara H., Shumway M., 2011. The sequence read archive. Nucleic Acids Res. 39: D19–D21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. F., Summerell B. A., 2006. The Fusarium Laboratory Manual. Blackwell Publishing, Ames, IA. [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Cowley A., Uludag M., Gur T., McWilliam H., et al. , 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 43: W580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle T. W., 1991. Segregation distorters. Annu. Rev. Genet. 25: 511–557. [DOI] [PubMed] [Google Scholar]
- Ma L. J., van der Does H. C., Borkovich K. A., Coleman J. J., Daboussi M. J., et al. , 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas W. F. O., 2001. Discovery and occurrence of the fumonisins: a historical perspective. Environ. Health Perspect. 109(Suppl 2): 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas W. F., Kellerman T. S., Gelderblom W. C., Coetzer J. A., Thiel P. G., et al. , 1988. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 55: 197–203. [PubMed] [Google Scholar]
- Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., et al. , 2010. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39: D225–D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. H., Wingfield B. D., Wingfield M. J., Steenkamp E. T., 2011. Structure and evolution of the Fusarium mating type locus: new insights from the Gibberella fujikuroi complex. Fungal Genet. Biol. 48: 731–740. [DOI] [PubMed] [Google Scholar]
- McCluskey K., Wiest A., Plamann M., 2010. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J. Biosci. 35: 119–126. [DOI] [PubMed] [Google Scholar]
- Milne I., Bayer M., Cardle L., Shaw P., Stephen G., et al. , 2010. Tablet – next generation sequence assembly visualization. Bioinformatics 26: 401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer S. A., Suarez L., Felkner M., Wang E., Merrill A. H., Jr, et al. , 2006. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 114: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B., 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- Munkvold G. P., 2003. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 109: 705–713. [Google Scholar]
- Munkvold G. P., Desjardins A. E., 1997. Fumonisins in maize: can we reduce their occurrence? Plant Dis. 81: 556–565. [DOI] [PubMed] [Google Scholar]
- Raju N. B., 1994. Ascomycete spore killers: Chromosomal elements that distort genetic ratios among the products of meiosis. Mycologia 86: 461–473. [Google Scholar]
- Reynoso M. M., Chulze S. N., Zeller K. A., Torres A. M., Leslie J. F., 2009. Genetic structure of Fusarium verticillioides populations isolated from maize in Argentina. Eur. J. Plant Pathol. 123: 207–215. [Google Scholar]
- Saupe S. J., 2011. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin. Cell Dev. Biol. 22: 460–468. [DOI] [PubMed] [Google Scholar]
- Seuring C., Greenwald J., Wasmer C., Wepf R., Saupe S. J., et al. , 2012. The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol. 10: e1001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu G. S., 1984. Genetics of Gibberella fujikuroi V. Spore killer alleles in G. fujikuroi. J. Hered. 75: 237–238. [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., et al. , 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikhakolli U. R., López-Giráldez F., Li N., Common R., Townsend J. P., et al. , 2012. Transcriptome analyses during fruiting body formation in Fusarium graminearum and Fusarium verticillioides reflect species life history and ecology. Fungal Genet. Biol. 49: 663–673. [DOI] [PubMed] [Google Scholar]
- Stanke M., Waack S., 2003. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19(Suppl 2): 215–225. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J., 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite J., 1969. Plant Pathological Methods: Fungi and Bacteria. Burgess Publishing Company, Minneapolis. [Google Scholar]
- Turner B. C., Perkins D. D., 1979. Spore killer, a chromosomal factor in Neurospora that kills meiotic products not containing it. Genetics 93: 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J., 1956. A convenient growth medium for Neurospora (Medium N). Microb. Genet Bull 13: 42–43. [Google Scholar]
- White D. G., 1998. Compendium of Corn Diseases. APS Press, St. Paul, MN. [Google Scholar]
- Wu F., 2007. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 137: 363–374. [Google Scholar]
- Xu J. R., Leslie J. F., 1996. A genetic map of Gibberella fujikuroi mating population A (Fusarium moniliforme). Genetics 143: 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. R., Yan K., Dickman M. B., Leslie J. F., 1995. Electrophoretic karyotypes distinguish the biological species of Gibberella fujikuroi (Fusarium Section Liseola). Mol. Plant Microbe Interact. 8: 74–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data deposition: genome sequencing data are available through NCBI’s Sequence Read Archive under accession numbers SRR3271586, SRR3273544, SRR3273545, and SRR3273546. Other sequence data are available from GenBank (KU963213).