Abstract
Meiotic crossover frequencies show wide variation among organisms. But most organisms maintain at least one crossover per homolog pair (obligate crossover). In Saccharomyces cerevisiae, previous studies have shown crossover frequencies are reduced in the mismatch repair related mutant mlh3Δ and enhanced in a meiotic checkpoint mutant pch2Δ by up to twofold at specific chromosomal loci, but both mutants maintain high spore viability. We analyzed meiotic recombination events genome-wide in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants to test the effect of variation in crossover frequency on obligate crossovers. mlh3Δ showed ∼30% genome-wide reduction in crossovers (64 crossovers per meiosis) and loss of the obligate crossover, but nonexchange chromosomes were efficiently segregated. pch2Δ showed ∼50% genome-wide increase in crossover frequency (137 crossovers per meiosis), elevated noncrossovers as well as loss of chromosome size dependent double-strand break formation. Meiotic defects associated with pch2∆ did not cause significant increase in nonexchange chromosome frequency. Crossovers were restored to wild-type frequency in the double mutant mlh3Δ pch2Δ (100 crossovers per meiosis), but obligate crossovers were compromised. Genetic interference was reduced in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. Triple mutant analysis of mlh3Δ pch2Δ with other resolvase mutants showed that most of the crossovers in mlh3Δ pch2Δ are made through the Mus81-Mms4 pathway. These results are consistent with a requirement for increased crossover frequencies in the absence of genetic interference for obligate crossovers. In conclusion, these data suggest crossover frequencies and the strength of genetic interference in an organism are mutually optimized to ensure obligate crossovers.
Keywords: crossover frequency, crossover assurance, meiotic chromosome segregation, genetic interference, genome wide recombination map
Meiosis is a reductional division that produces haploid gametes or spores from diploid progenitor cells. Ploidy reduction is achieved by one round of DNA replication, followed by two consecutive nuclear divisions (Meiosis I and II), producing four daughter cells (Roeder 1997). Crossovers promote the formation of chiasma which serves as a physical linkage between two homologs and opposes the spindle generated forces that pull apart the homolog pairs. This opposing set of forces provides the tension necessary to promote proper disjunction of homolog pairs at Meiosis I (Petronczki et al. 2003). Failure to maintain at least one crossover per homolog pair increases the probability of nondisjunction, resulting in aneuploid gametes (Serrentino and Borde 2012). Although crossovers are important for chromosome segregation, nonexchange chromosomes have been observed to segregate accurately forming viable gametes (Hawley et al. 1992; Davis and Smith 2003; Kemp et al. 2004; Newnham et al. 2010; Krishnaprasad et al. 2015).
Meiotic crossovers (and noncrossovers) are initiated in S. cerevisiae by 150–170 programmed double-strand breaks (DSBs) formed by an evolutionarily conserved topoisomerase-like protein Spo11 and other accessory proteins (reviewed in Neale and Keeney 2006). The frequency and distribution of crossovers on chromosomes is a tightly regulated process. Four known aspects of this regulation are crossover interference, obligate crossovers, crossover homeostasis, and crossover invariance. Crossover interference regulates the spatial patterning of crossovers, where the presence of a crossover prevents the formation of a second crossover in its vicinity. As a consequence, an even distribution of crossovers is ensured, and closely spaced crossovers are less frequently observed than expected from a random distribution (Berchowitz and Copenhaver 2010). An obligate crossover per homolog pair facilitates disjunction. The obligate crossover is not a specific type of crossover, but refers to the observation that process(es) that generate most of the crossovers result in at least one crossover per homolog pair (Barchi et al. 2008). The obligate crossovers are ensured in different organisms with different crossover frequencies. For example, organisms that have strong genetic interference like Caenorhabditis elegans, Drosophila, Arabidopsis, mouse, and humans ensure obligate crossovers, although the number of crossovers per bivalent pair is low (∼1–2) (Copenhaver et al. 2002; Hillers and Villeneuve 2003; Berchowitz and Copenhaver 2010; Libuda et al. 2013). But organisms with weaker interference, like S. cerevisiae, make ∼90 crossovers, or ∼6 times the number of bivalent pairs (16), to ensure an obligate event. Crossover homeostasis ensures that the number of crossovers is maintained (at the expense of noncrossovers) when the DSB precursors become limiting (Martini et al. 2006). This crossover buffering mechanism is also seen when the DSBs are increased (Cole et al. 2012). Crossover invariance is another mechanism that maintains the number of crossovers in organisms that lack interference. This phenomenon refers to the observation that, in Schizosaccharomyces pombe, the ratio of intersister to interhomolog repair is modulated in response to variations in DSB intensity to ensure uniform crossover distribution (Hyppa and Smith 2010). Recent studies suggest crossover interference, obligate crossovers, and crossover homeostasis are the outcomes of a single crossover patterning process regulated by the “mechanical stress relief” of chromosomes (Wang et al. 2015; Zickler and Kleckner 2016). As per this model, crossover interference and homeostasis are linked processes. But the designation of an obligate crossover is not dependent on either interference or homeostasis (Zhang et al. 2014; Wang et al. 2015).
The majority of the crossovers (Class I) in S. cerevisiae show interference, and are formed through a pathway involving the ZMM (Zip1, Zip2, Zip3, Zip4, Mer3, Spo16, and Msh4-Msh5) and STR (Sgs1, Rmi1, and Top3) procrossover factors and mismatch repair related proteins Exo1 and MutLγ (Mlh1-Mlh3) (Allers and Lichten 2001; Hunter and Kleckner 2001; Borner et al. 2004; Nishant et al. 2008; Shinohara et al. 2008; De Muyt et al. 2012; Zakharyevich et al. 2012). Cytological studies in the mouse have shown that the MutLγ complex acts downstream of Msh4-Msh5 (MutSγ) (Baker et al. 1996; Lipkin et al. 2002; Kolas et al. 2005). Further physical, genetic, and biochemical analysis in S. cerevisiae suggest MutLγ has an endonuclease activity that can generate crossovers from dHJs in a pathway comprising MutSγ, Exo1 and Sgs1 (Nishant et al. 2008; Hunter 2011; Zakharyevich et al. 2012; Rogacheva et al. 2014). Noninterfering crossovers are formed by a separate pathway involving the structure specific endonuclease Mus81-Mms4 (Class II crossovers). Mainly aberrant and multichromatid joint molecules are resolved by this pathway (de los Santos et al. 2003; Oh et al. 2008). The structure specific endonucleases Yen1 and Slx1-Slx4 have a minor role in meiotic crossover formation, which is observed in mms4Δ/mms4Δ sgs1Δ background (Zakharyevich et al. 2012; Higashide and Shinohara 2016). Genetic interference between crossovers is compromised in most ZMM mutants (zip1Δ, mer3Δ, msh4Δ, and msh5Δ) (Sym and Roeder 1994; Nakagawa and Ogawa 1999; Novak et al. 2001; Argueso et al. 2004; Zakharyevich et al. 2012), but the distribution of Zip3 foci (measured in physical distances), which mark DSB sites designated to be repaired as crossovers, show interference in zip1Δ and msh4Δ mutants (Zhang et al. 2014).
It is not clear why organisms have vastly different crossover frequencies and how this is related to ensuring an obligate crossover. In this study, we address the relationship of obligate crossovers with crossover frequency using mlh3Δ and pch2Δ mutants. In S. cerevisiae, both these mutants have very different crossover frequencies but show high spore viability. At specific chromosomal loci, mlh3Δ shows up to a 50% reduction in crossover frequency but with good spore viability ranging from 72% and up to 92% depending on the strain background (Nishant et al. 2008; Cotton et al. 2010; Sonntag Brown et al. 2013). Pch2 is an evolutionarily conserved hexameric ring AAA+ ATPase protein that has roles in meiotic checkpoint signaling triggered by unrepaired DSBs, remodeling the chromosome axis at the future crossover sites and interhomolog bias (San-Segundo and Roeder 1999; Borner et al. 2008; Ho and Burgess 2011; Zanders et al. 2011; Chen et al. 2014). pch2Δ mutants are observed to make 50% more crossovers than wild type at specific loci, and also have good viability (95%) (Zanders and Alani 2009; but see Joshi et al. 2009). The mlh3Δ and pch2Δ mutants are therefore useful to test the effect of a wide range of crossover frequencies on obligate crossovers. It is important to note that pleiotropic effects of pch2Δ could affect the interpretation of changes in crossover frequencies on the obligate crossover.
We performed genome-wide mapping of meiotic recombination events in mlh3Δ, pch2Δ and mlh3Δ pch2Δ using the S288c/YJM789 hybrid. Crossovers in all three mutants, mlh3Δ (64 crossovers per meiosis on average), pch2Δ (137 crossovers per meiosis on average) and mlh3Δ pch2Δ (100 crossovers per meiosis on average) showed reduced genetic interference. Noncrossovers were not affected in mlh3Δ, but increased in pch2Δ and mlh3Δ pch2Δ. Crossover and noncrossover data suggest loss of chromosome size dependent DSB formation in pch2Δ mutants. mlh3Δ pch2Δ showed uniform crossover density on all chromosomes consistent with loss of genetic interference. Nonexchange chromosomes were abundant in mlh3∆ (47% of meioses), but efficiently segregated. pch2Δ (7%) had a reduced percentage of meioses with nonexchange chromosomes, showing that high crossover frequencies can promote crossover assurance in the absence of genetic interference. Even though the mlh3Δ pch2Δ mutant made as many crossovers as wild type, 20% of meioses showed nonexchange chromosomes, consistent with noninterfering crossovers being inefficient in promoting crossover assurance.
Materials and Methods
Media and strains
All S. cerevisiae strains used in this study (S288c, YJM789, and SK1) were grown on either YPD (yeast extract, peptone, and dextrose) or synthetic complete medium at 30° (Rose et al. 1990). The drugs, geneticin (Invitrogen), nourseothricin (Werner BioAgents), and hygromycin (HiMedia) were added to YPD as described in Goldstein and McCusker (1999). Sporulation media for the genetic analysis was prepared as described in Argueso et al. (2004). For whole genome recombination analysis, S288c and YJM789 strains were used. For tetrad analysis and cytology, the SK1 strains, EAY1108/EAY1112, NHY1168/NHY1162, and their derivatives, were used (Argueso et al. 2004; Martini et al. 2006). All strains were transformed using standard techniques (Gietz et al. 1995). The mlh3Δ::kanMX4 strains were created using plasmid pEAI168. pch2Δ::natMX4, slx4Δ::kanMX4 and mlh3Δ::hphMX4 were made using deletion constructs amplified by PCR. The meiotic depleted allele of MMS4 (mms4-md) in EAY1108/EAY1112 was created by transformation with the kanMX-pCLB2-3HA-MMS4 PCR product generated from the haploid parents of MJL3172 (De Muyt et al. 2012). Strain information is given in Supplemental Material, Table S1.
Tetrad analysis
Diploids were sporulated following the zero growth mating protocol (Argueso et al. 2003). Haploid strains were mixed together on synthetic complete media. After incubation for 4 hr at 30°, the resulting diploids were patched on sporulation media. After 2 d of incubation at 30°, tetrads were dissected on synthetic complete medium using a Zeiss dissection microscope. The dissected tetrads were grown for 2 d, after which they were replica plated to various selective media. The replica plates were scored after 1 d of incubation. Marker segregation was analyzed using the recombination analysis software RANA (Argueso et al. 2004).
Meiotic time course
Wild type and pch2Δ diploids were streaked on YPD media, and a single colony was inoculated in 4 ml YPD and grown overnight at 30°. To obtain synchronized meiosis, cultures were grown in presporulation medium (SPS) (0.5% yeast extract, 1% peptone, 0.67% yeast nitrogen base, 1% potassium acetate, and 0.05 M potassium biphthalate) before transfer to the sporulation medium (2% potassium acetate, amino acids, and 0.001% polypropylene glycol) (Murakami et al. 2009).
Cytology
Meiotic cultures (5 ml) of wild type and pch2Δ from 2 to 8 hr postinduction into meiosis were collected at hourly intervals. Cultures were treated with Zymolyase 100T to obtain spheroplasts. Spheroplasts were washed and resuspended in MES/sorbitol solution (1 M sorbitol, 1 mM EDTA, 0.5 mM MgCl2, 0.1 M MES pH 6.5). Chromosome spreads were prepared on ethanol washed glass slides, using detergent (1% Lipsol) and 4% paraformaldehyde/3.4% sucrose as fixative. Slides were dried overnight and immunostained as described previously (Bishop 1994; Shinohara et al. 2000).
Analysis of Rad51 foci
Slides were stained with rabbit anti-Rad51 antibody (1:500) (a gift from Dr. Akira Shinohara), incubated overnight at 4°, followed by 2 hr incubation with secondary antibody anti-rabbit TRITC (1:1500) (Jackson ImmunoResearch). Immunofluorescence images of Rad51 foci were captured using 100× oil immersion objective on a Leica SP5 confocal microscope. Images were captured at 0.25 μm interval Z sections. Maximum intensity images were produced by projecting the optical sections (Z stacks). For each time point 100 images were analyzed in three independent experiments. Image analysis and 3D deconvolution was done using Leica Application Suite (LAS) Advanced Fluorescence (AF) Lite 2.8.0 software.
DNA extraction and whole genome sequencing of meiotic spores
Spore colonies from the four spore viable tetrads of mlh3∆, pch2∆, and mlh3∆ pch2∆ were grown overnight at 30° in 4 ml YPD broth. DNA was isolated from the culture using PrepEase DNA isolation kit (Affymetrix). Whole genome sequencing on Illumina platform was performed at Fasteris, Switzerland, as well as at the European Molecular Biology Laboratory (EMBL) Genomics Core Facilities (GeneCore), Heidelberg, Germany.
Annotation of recombination events
The raw reads were demultiplexed using the NGS QC Toolkit (version 2.3.3, http://www.nipgr.res.in/ngsqctoolkit.html), and processed for quality control (QC) using the trimmomatic tool (Version 0.36, http://www.usadellab.org/cms/index.php?page=trimmomatic) (Bolger et al. 2014). These QC filtered high quality reads were then mapped to the S. cerevisiae genome (version R64-1-1, 2011) using bowtie 2 (version 2.2.6) (Langmead and Salzberg 2012). Only uniquely mapped reads were considered for variant calling using Picard tools. Local realignment around indels was performed to reduce misalignment. Genotyping was done using GATK (UnifiedGenotyper, version 3.4-46) (McKenna et al. 2010; DePristo et al. 2011). From the alignment, an average of 59,215 SNPs was genotyped. Recombination events, such as crossovers, noncrossovers (type 0 gene conversions exhibiting 1:3 or 3:1 segregation of SNP markers), crossover independent gene conversions (type 0, 2, 3, and 4 gene conversions), and crossover associated gene conversions were annotated using the CrossOver program (v6.3) of the ReCombine program suite (v2.1) (Anderson et al. 2011). Parameters used for the CrossOver program were as described previously in Krishnaprasad et al. (2015). Custom R scripts were used to convert vcf files to segregation files (input file for the CrossOver program), to generate plots from the CrossOver program output and to perform various statistical tests. Detection of copy number variation was performed as described in Krishnaprasad et al. (2015).
Interference analysis
Interference analysis using the one pathway gamma model was performed as described previously (McPeek and Speed 1995; Krishnaprasad et al. 2015). For the two pathway analysis of interference, crossover distances between each crossover per chromosome was calculated for each tetrad, and converted to centimorgans. A gamma mixture model was fitted using an EM algorithm similar to that in the R package mixtools (Benaglia et al. 2009). The number of mixture components was fixed at two and the shape parameter for one of the components (Class II crossovers) was fixed at one. For the Coefficient of Coincidence (CoC) analysis, the genome was partitioned into 25 kb bins. The frequency of crossover events in each bin was calculated along with the expected and observed frequencies of double crossovers in all pairs of bins. The CoC (observed crossover frequency/expected crossover frequency) was analyzed for all bin pairs (with nonzero expected frequency) separated by a given interval (in kilobase) and averaged. Chi-square test was used to measure the statistical significance of observed and expected double crossover frequencies between wild type and the mutants.
Data availability
All strains listed in Table S1 are available upon request. Sequence data are available from the National Centre for Biotechnology Information Sequence Read Archive: Accession number SRP082254. The raw recombination data files and the custom R scripts are available online at the Dryad data repository (http://datadryad.org; http://dx.doi.org/10.5061/dryad.5vm4g).
Results
Genome-wide recombination maps for mlh3Δ, pch2Δ, and mlh3Δ pch2Δ by whole genome sequencing
mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutations were introduced in the S288c and YJM789 strains. Spore viability of mlh3Δ (85%) was similar to the wild-type S288c/YJM789 hybrid (Figure S1 and Table 1). Spore viability of pch2Δ (75%), in the S288c/YJM789 hybrid, was reduced compared to wild-type hybrid (P = 0.0001, Fischer’s exact test) (Figure S1 and Table 1). The mlh3Δ pch2Δ mutant also showed significantly less viability compared to wild type (59%, P = 7.9 × 10−21, Fischer’s exact test). We performed whole genome sequencing of spores from 54 four-viable spore tetrads of the S288c × YJM789 hybrid bearing mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutations (Table S2) (sequence data available from National Centre for Biotechnology Information Sequence Read Archive under accession number: SRP082254). High-resolution genome-wide recombination data were generated in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants by analyzing segregation of SNPs in the 54 tetrads (File S1). These include 19 tetrads in mlh3Δ, 15 tetrads in pch2Δ and 20 tetrads in mlh3Δ pch2Δ. Genome-wide recombination data for wild type were generated from 66 tetrads by combining previously published studies (Mancera et al. 2008; Krishnaprasad et al. 2015). Crossover and noncrossover counts for each of the 120 tetrads are shown in Table S3.
Table 1. Crossover (CO), noncrossover (NCO) counts and spore viability of mlh3Δ, pch2Δ, mlh3Δ pch2Δ mutants in the S288c/YJM789 hybrid.
| Genotype | N | S.V% | Tetrads Genotyped | Average CO Counts ± SD (Median) | Average NCO Counts ± SD (Median) |
|---|---|---|---|---|---|
| S288c × YJM789 | 180 | 84 | 66a | 93.4 ± 10.86 (94) | 46 ± 15.6 (43) |
| S288c × YJM789 mlh3Δ | 120 | 85 | 19 | 64.4 ± 8.5 (62) | 49.5 ± 11.6 (45) |
| S288c × YJM789 pch2Δ | 164 | 75 | 15 | 136.5 ± 25.6 (134) | 85.9 ± 25.9 (91) |
| S288c × YJM789 mlh3Δ pch2Δ | 120 | 59 | 20 | 99.8 ± 21.6 (96) | 93.6 ± 27.6 (86) |
Spore viability (S.V) data for wild type is from Krishnaprasad et al. (2015). N, number of tetrads analyzed for spore viability.
Merged data set from Krishnaprasad et al. (2015) and Mancera et al. (2008).
Crossover defects in mlh3Δ are distinct from msh4Δ
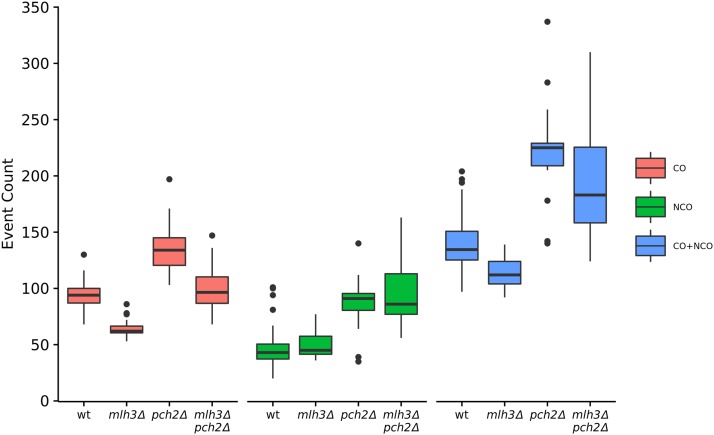
The mlh3Δ mutant showed a significant genome-wide reduction in crossovers compared to wild type (64 crossovers on average in mlh3Δ vs. 93 in wild type, P = 1.7 × 10−14, t-test) (Figure 1 and Table 1). These results are consistent with crossover data obtained from analysis of specific loci in mlh3Δ (Nishant et al. 2008; Sonntag Brown et al. 2013). Genome-wide crossover defects are therefore stronger in msh4Δ (average of 49 crossovers per meiosis) compared to mlh3Δ (P = 2.3 × 10−3, t-test), although the Msh4-Msh5, Mlh1-Mlh3 proteins are thought to act in the same pathway for making crossovers (Chen et al. 2008; Krishnaprasad et al. 2015). Previous work by Sonntag Brown et al. (2013) also showed stronger crossover defects in msh5Δ (37 cM) compared to mlh3Δ (54.5 cM) for chromosome XV in the SK1 EAY1108/EAY1112 background. Average noncrossover counts in mlh3Δ (50 per meiosis) were similar to wild type (46 per meiosis) (P = 0.29, t-test). Since the crossover defects in mlh3Δ were not associated with a concomitant increase in noncrossovers, perhaps in the absence of the Mlh3 protein DSBs that were not repaired as crossovers may be repaired using the sister chromatid.
Figure 1.
Crossover, noncrossover and total events per meiosis for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. The box plots show minimum, first quantile, median, third quantile, and maximum count.
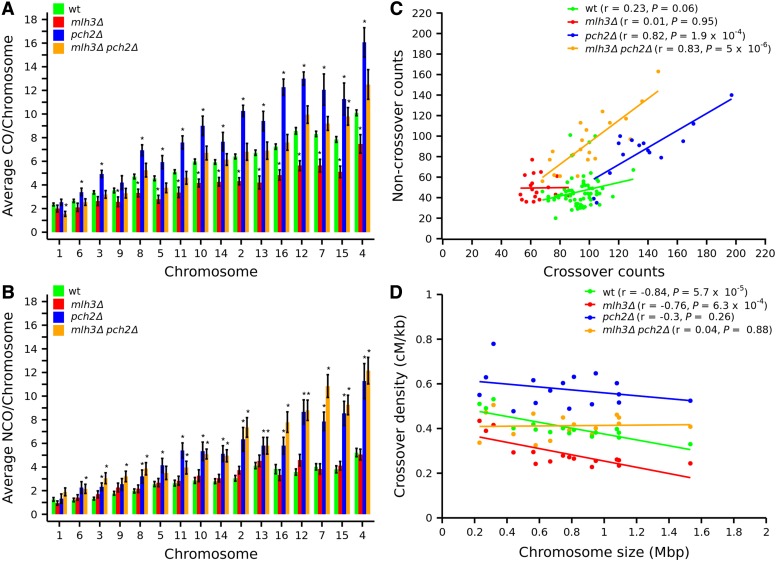
The effects of chromosome size on crossover distribution were analyzed using average crossover counts per chromosome (Figure 2A and Table S4). The mlh3Δ mutant showed a significant decrease in crossovers on medium and large chromosomes (Figure 2A and Figure S2A). Crossovers on small chromosomes also showed a decrease in mlh3Δ, but these were not significant except for chromosome IX. Crossover defects in mlh3Δ are therefore different from msh4Δ and msh4-R676W, which had significant crossover reduction on all (small, medium, and large) chromosomes (Krishnaprasad et al. 2015). Noncrossover counts in mlh3Δ did not show significant differences on any of the chromosomes compared to wild type (Figure 2B and Figure S2D). The noncrossover data in mlh3∆ are different from msh4Δ, where average noncrossover counts were increased on several chromosomes (Krishnaprasad et al. 2015). When the noncrossover counts are regressed against the crossover counts, we observed no correlation in wild type and mlh3Δ (wild type, r = 0.23, P = 0.06; mlh3Δ, r = 0.01, P = 0.95; Figure 2C).
Figure 2.
Crossover and noncrossover distribution on chromosomes for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. (A, B) Bar plot showing average crossover and noncrossover counts per chromosome for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. The asterisk symbol (*) shows chromosomes with significant difference (P < 0.05, t-test) in crossover or noncrossover counts compared to wild type. Chromosomes are arranged according to size from left to right. Error bars are “mean ± SE.” (C) Crossover vs. noncrossover scatter plot with correlation coefficient (r) and P values for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. (D) Crossover density (centimorgans per kilobase) plotted as a function of chromosome length (megabase) for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ along with the correlation coefficient (r) and P values.
Wild-type cells show variation in crossover density (centimorgan per kilobase) with chromosome size (Figure 2D, and see Pan et al. 2011). Previous studies have shown that high crossover density on small chromosomes is mostly a consequence of higher DSB density on small chromosomes (Pan et al. 2011). We plotted crossover density vs. chromosome length for wild type, and observed a pattern similar to that observed for DSB density (Figure S3, and see Pan et al. 2011). First, the crossover density decreases with chromosome size, indicating that larger chromosomes have fewer crossovers than expected for their physical length (assuming crossover density is equal on all chromosomes). Second, if we take into account this inverse relationship between chromosome length and crossover density, we observed that the smaller chromosomes deviate a lot from the regression line, indicating that they have a much higher crossover density than expected (Figure S3). mlh3Δ showed a significant negative correlation coefficient (r = −0.76, P = 6.3 × 10−4), implying an inverse relation between crossover density and chromosome size (Figure 2D). The correlation coefficient for mlh3Δ was comparable to wild type (r = −0.84, P = 5.7 × 10−5). Crossover density was higher on smaller chromosomes in wild type and mlh3Δ, even when the analysis excluded the three smallest chromosomes (I, III, and VI), which have the highest DSB densities (Figure S4A, and see Sun et al. 2015). Noncrossover density, as well as crossover plus noncrossover density, also showed a significant negative correlation with chromosome size in wild type and mlh3Δ (Figure S4, B–D). The relationship between crossover or noncrossover density and chromosome size was therefore similar between wild type and mlh3Δ. These results suggest the regulation of DSB distribution based on chromosome size is intact in mlh3Δ like in wild type. Further, since most crossovers in wild type and mlh3Δ are Class I and Class II, respectively, these results suggest the chromosome size effect seen for crossover density variation is dependent on DSB density, and not on the recombination pathway as shown previously (Pan et al. 2011). It is important to note that using crossover and noncrossover density as a proxy for DSBs does not consider the effect of any changes in homolog bias in these mutants. Crossover and noncrossover distributions at centromere and telomere regions were comparable to wild type, but noncrossovers were elevated within 10 kb of centromere region in mlh3Δ (File S2). Chromosome-wide crossover distribution for mlh3Δ is shown in Figure S5.
Genome-wide increase in crossovers and noncrossovers in pch2Δ
Average crossovers in pch2Δ (137) were significantly more than wild type (P = 1.15 × 10−5, t-test) (Figure 1). The increase in crossovers was statistically significant on all the medium and large chromosomes, and on two of the smaller chromosomes (III and VI) (Figure 2A and Figure S2B). This result is consistent with increased crossovers observed at specific loci on medium and large chromosomes in SK1 background (Zanders and Alani 2009), but different from Joshi et al. (2009) who observed no increase in crossovers across nine intervals on three chromosomes. The increase in crossovers on chromosome III is different from the results observed by Zanders and Alani (2009) and San-Segundo and Roeder (1999). Such differences may be because of the limited number of chromosomal loci analyzed in the previous studies (San-Segundo and Roeder 1999; Joshi et al. 2009; Zanders and Alani 2009). Noncrossovers in pch2Δ (average of 86 per meiosis) were also significantly higher than wild type (46, P = 2.9 × 10−5, t-test) (Figure 1 and Table 1). Similar to the trend seen for crossovers, increased noncrossovers in pch2∆ were significant for all medium and large chromosomes (Figure 2B and Figure S2E). These results suggest that specific regulation of DSB formation on medium and large chromosomes is affected in pch2∆. Among the small chromosomes, Chromosome III was exceptional in showing an increase in both crossovers and noncrossovers. This might be due to an enhanced distribution of DSBs on chromosome III in pch2Δ mutants. An increase in DSB formation at the HIS4::LEU2 locus has been observed previously in pch2Δ (Farmer et al. 2012). Interestingly Borner et al. (2008) reported increased noncrossovers and decreased crossovers for the HIS4::LEU2 locus on chromosome III for pch2Δ. When the noncrossover counts are regressed against the crossover counts, we observed a well correlated linear relationship (Figure 2C). pch2Δ cells with more crossovers also had more noncrossovers (r = 0.82, P = 1.9 × 10−4). Although pch2Δ showed a negative correlation coefficient (r = −0.30) between crossover density and chromosome size, it was not significant (P = 0.26), consistent with the enhanced crossovers on large and medium chromosomes (Figure 2D). Unlike wild type, pch2Δ did not show a significant negative correlation between chromosome size and crossover density, noncrossover density, as well as crossover plus noncrossover density (Figure S4).
Crossover and noncrossover distributions within 10 kb of the centromere were comparable between wild type and pch2Δ (File S2). But crossover and noncrossover distributions in pch2Δ were suppressed for an extended distance from the telomere (up to 80 kb) compared to the wild type (File S2). These differences may reflect changes in DSB distribution near telomeres. Chromosome-wide analysis of crossover distribution showed distinct crossover peaks compared to wild type, including elevated crossovers around the rDNA locus (Figure S6). This is consistent with the observation of increased DSBs and recombination at the outermost rDNA repeats in pch2Δ and the role of Pch2 in maintaining the integrity of the rDNA cluster (Vader et al. 2011).
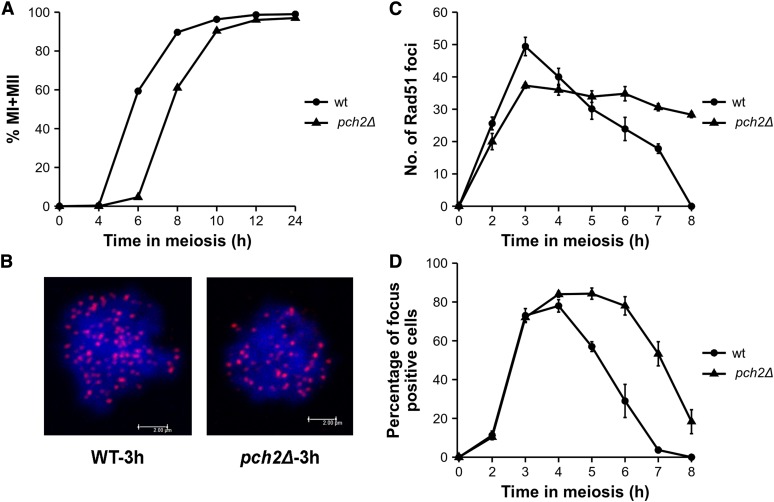
Genome-wide recombination analysis showed a correlated increase in both crossovers and noncrossovers in pch2Δ (Figure 1 and Figure 2C). The simplest explanation is that pch2Δ makes more DSBs. Rad51 focus analysis can provide information on whether pch2Δ mutants show increased DSBs. We measured Rad51 focus counts in wild type and pch2Δ mutants in three independent meiotic time courses to determine changes in cellular DSB frequency. pch2Δ mutants showed a 2 hr delay in meiotic divisions, as observed previously (Wu and Burgess 2006; Zanders and Alani 2009) (Figure 3A). We analyzed Rad51 foci in 1 hr increments from 2 to 7 hr time points in wild type, and from 2 to 8 hr time points in pch2Δ. For wild type and pch2Δ, maximum Rad51 foci were detected at 3 hr, and representative images are shown in Figure 3B. The maximum (average) Rad51 focus counts for pch2Δ (37) were reduced compared to wild type (49). However, unlike wild type, where the Rad51 foci peaked at 3 hr and could not be detected after 7 hr, pch2Δ showed a gradual decline of Rad51 foci over an extended time from 3 hr until 8 hr (Figure 3C). In wild type, the percentage of cells that show Rad51 foci was negligible (3.7%) at 7 hr, while, in pch2Δ, almost 50% of the cells showed Rad51 foci (Figure 3D). The persistent presence of DSBs may be due to ongoing formation and repair of new DSBs (turnover), which may explain the increased crossovers and noncrossovers observed in pch2Δ. In addition, such a pattern may be caused by delayed repair of DSBs, which may cause more DSBs, and, in turn, result in more crossovers and noncrossovers (Hochwagen et al. 2005; Borner et al. 2008). The cytological observations are consistent with a prophase delay in pch2Δ for a variable amount of time, during which additional DSBs are made asynchronously, so that there is a much greater range in the total number of crossover and noncrossover events (Figure 1, Figure 2C, and Table 1). Persistent DSBs in the pch2∆ mutant have been observed previously at the HIS4::LEU2 locus by Southern blot analysis, and also from analysis of Rad51 foci in pch2Δ ndt80Δ mutants (Hochwagen et al. 2005; Subramanian et al. 2016).
Figure 3.
Rad51 foci analysis in wild type and pch2Δ mutants. (A) Meiotic time course assay for wild type and pch2Δ. Data are averaged across three independent meiotic time courses. (B) Representative image of Rad51 foci in wild type and pch2Δ at the 3 hr time point. (C) Average Rad51 foci counts with SE from three independent experiments in wild type and pch2Δ. Rad51 foci from 100 images were counted for each time point and each experiment. (D) Percentage of cells in a field that are positive for Rad51 foci at each time point. Cells with >5 Rad51 foci are counted as positive for Rad51 foci. Data are averaged across three independent experiments and plotted with SE measurements.
The mlh3Δ pch2Δ mutant has wild-type crossover frequency and uniform crossover density on all chromosomes
Since pch2Δ shows increased crossovers in wild-type background as well as in crossover mutants like msh5Δ and mms4Δ (Zanders and Alani 2009), we were curious to see if a pch2Δ mutation in the mlh3Δ background would restore crossovers to wild-type frequencies. mlh3Δ pch2Δ made, on average, 100 crossovers per meiosis, comparable to the wild-type S288c × YJM789 hybrid (Figure 1 and Table 1) (P = 0.22, t-test). None of the chromosomes (except chromosomes I and XV) in mlh3Δ pch2Δ showed significant differences in crossovers compared to wild type (Figure 2A and Figure S2C). Noncrossovers in mlh3Δ pch2Δ (average of 94 per meiosis) were significantly more than wild type (P = 1.84 × 10−7, t-test) and comparable to pch2Δ (P = 0.40, t-test). These results suggest most of the increase in noncrossovers in pch2Δ mutants comes from the activity of the structure-specific endonucleases, which are less biased toward resolution of the dHJs into crossovers. The increase in noncrossovers was observed across small (VI, III, and IX), and all medium and large chromosomes (except V) (Figure 2B and Figure S2F). Similar to the pattern observed in pch2Δ, crossover, and noncrossover counts in mlh3Δ pch2Δ were positively correlated (r = 0.83, P = 5 × 10−6) (Figure 2C).
The crossover density in mlh3Δ pch2Δ was uniform across all chromosome sizes and the correlation coefficient (r = 0.04, P = 0.88) was close to zero (Figure 2D). A similar lack of correlation was seen for crossover, noncrossover, and crossover plus noncrossover density with chromosome size (including/excluding the smallest chromosomes I, III, and VI) (Figure S4). Like pch2Δ, crossover and noncrossover distributions were suppressed for an extended distance from the telomere (up to 80 kb) in mlh3Δ pch2Δ (File S2). Chromosome-wide distribution showed distinct crossover peaks in mlh3Δ pch2Δ compared to wild type, especially around the rDNA locus (Figure S7).
Enhanced gene conversion frequencies and long gene conversion tracts in pch2Δ mutants
Most markers (>97.5%) showed 2:2 segregation in wild type and mlh3Δ mutant (Table S5). pch2Δ mutants showed higher gene conversion frequencies than wild type or mlh3Δ mutant. The percentage of SNP markers segregating 2:2 was 94.7% in pch2∆, compared to >97.5% in wild type and mlh3Δ. Like the pch2Δ single mutant, the mlh3Δ pch2Δ mutant also showed higher gene conversion frequencies than wild type (94% of markers segregating 2:2). Increases in gene conversion frequencies in pch2Δ at specific loci have been previously observed by Zanders and Alani (2009) and Joshi et al. (2009). The median gene conversion tract lengths associated with crossovers (2.4, 2.7, and 3.9 kb) were significantly longer than the noncrossover gene conversion tract lengths (1.7, 1.8, and 2.2 kb) for the mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants (Figure S8, A–C and Table S5). These results are similar to observations in wild type and other crossover mutants (Terasawa et al. 2007; Chen et al. 2008; Mancera et al. 2008; Oke et al. 2014; Krishnaprasad et al. 2015). Both crossover- and noncrossover-associated gene conversion tract lengths were significantly longer in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ compared to wild type (Figure S8, A–C and Table S5). In addition, long gene conversion tracts associated with crossovers (>4 kb) and noncrossovers (>2 kb) were over-represented in pch2Δ and mlh3Δ pch2Δ compared to wild type and mlh3Δ (Figure S8, B and C, see Discussion).
Most crossovers in mlh3Δ pch2Δ are made by the Mus81-Mms4 pathway
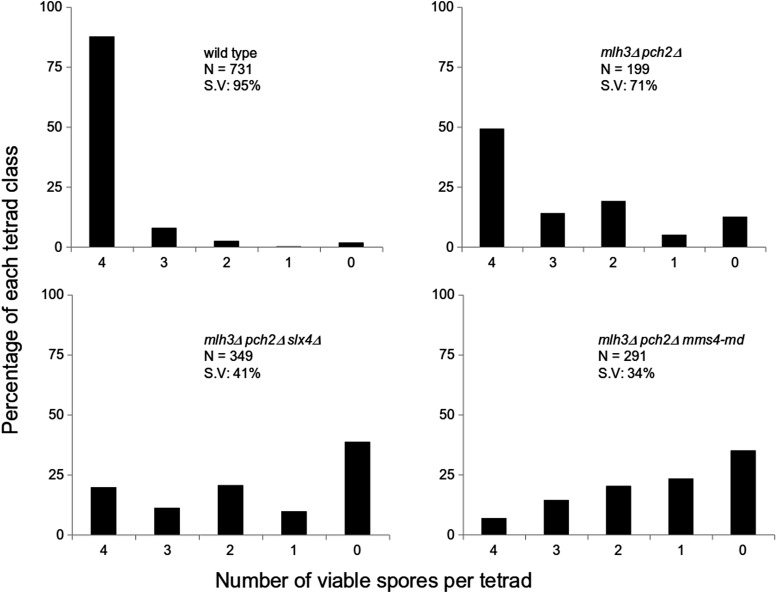
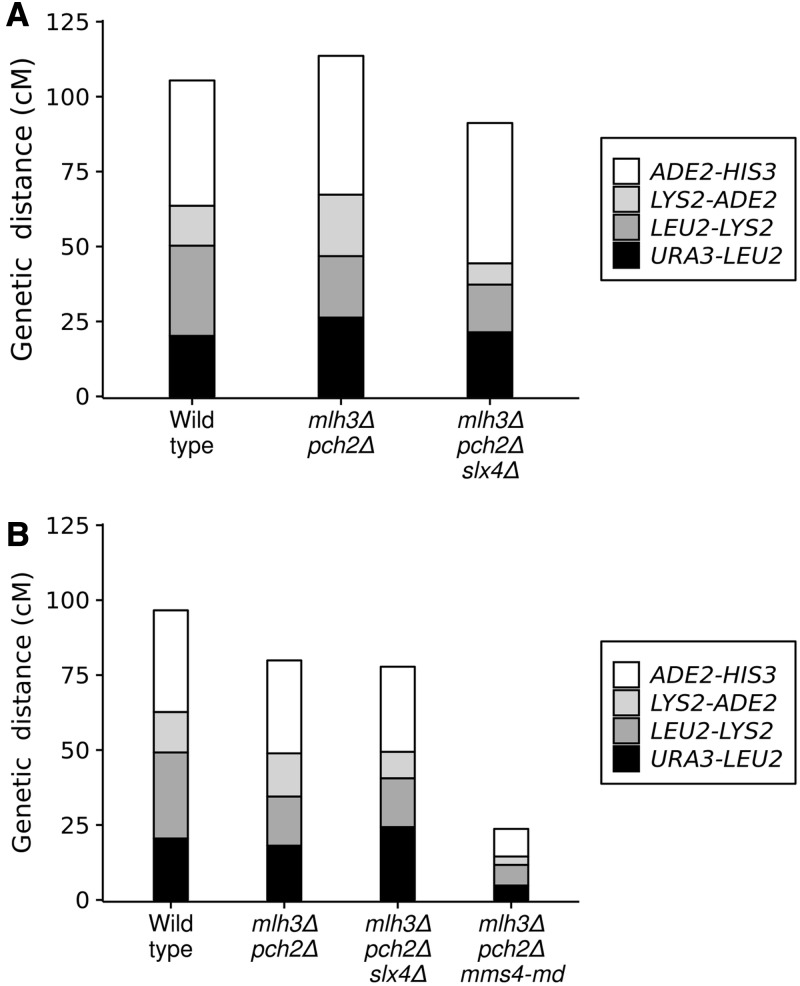
Although mlh3Δ has ∼30% reduction in crossovers, mlh3Δ pch2Δ has wild-type crossover frequency. In S. cerevisiae, the major pathway for resolving joint molecules into crossovers involves Mlh1-Mlh3/Exo1 proteins (De Muyt et al. 2012; Zakharyevich et al. 2012). Joint molecules are also resolved into crossovers by minor pathways involving the structure selective nucleases Mus81-Mms4, Yen1, and Slx1-Slx4 (De Muyt et al. 2012; Zakharyevich et al. 2012). Yen1 is thought to process joint molecules that escape Mus81-Mms4 (Matos et al. 2011). We were curious to know how wild-type frequency of crossovers is possible in mlh3Δ pch2Δ in the absence of the major resolvase (Mlh3). We tested the role of the two joint molecule resolvases, Mus81-Mms4 and Slx1-Slx4, in making crossovers in the mlh3Δ pch2Δ background. For testing the role of Mus81-Mms4, a meiotic depleted allele of MMS4 (mms4-md) was used (De Muyt et al. 2012). Spore viability and genetic map distances of mlh3Δ pch2Δ mms4-md, and mlh3Δ pch2Δ slx4Δ, were compared with mlh3Δ pch2Δ in the EAY1108/EAY1112 background (Argueso et al. 2004). mlh3Δ pch2Δ has 71% spore viability in the EAY1108/EAY1112 background and has a spore viability pattern (4, 2, and 0 viable spore tetrad class >3 and 1 viable spore tetrad class) suggesting Meiosis I nondisjunction (Figure 4). Introduction of mms4-md and slx4Δ mutations reduced the spore viability to 34% and 41%, respectively (Figure 4). Genetic map distances in four intervals in mlh3Δ pch2Δ (113.6 cM), based on four-viable spore tetrads was similar to wild type (105.4 cM), in agreement with the whole genome sequencing data from the S288c/YJM789 hybrid (Figure 1 and Figure 5A). However, map distances when all viable spores were considered showed a reduction in mlh3Δ pch2Δ (79.9 cM) relative to wild type (96.6 cM) (Figure 5B). These results suggest restoration of crossovers in mlh3Δ pch2Δ to wild-type frequency occurs through an increase in both single crossovers measured as tetratypes (TT), as well as double crossovers measured as nonparental ditypes (NPDs). The increase in double crossovers (NPDs) contributes to map distances from tetrad data but not from spore data (Table S6 and Table S7). More NPD tetrads are also observed in pch2Δ mutants relative to wild type (Joshi et al. 2009; Zanders and Alani 2009).
Figure 4.
Spore viability analysis of wild type, mlh3Δ pch2Δ, mlh3Δ pch2Δ slx4Δ, and mlh3Δ pch2Δ mms4-md in the EAY1108/EAY1112 genetic background. The viable spores per tetrad (x axis) and the percentage of each tetrad class (y axis) are shown. N, number of tetrads dissected; S.V, percentage spore viability.
Figure 5.
Crossovers in mlh3Δ pch2Δ are made predominantly by the Mus81-Mms4 pathway. Genetic map distances for complete tetrads (A) and total spores (B) are shown for a set of four intervals on chromosome XV in the EAY1108/EAY1112 genetic background. Raw data are shown in Table S6 and Table S7 for complete tetrads and total spores, respectively.
mlh3Δ pch2Δ slx4Δ (91.2 cM) showed reduced genetic map distances compared to mlh3Δ pch2Δ (113.6 cM) in measures based on complete tetrads, but the genetic map distances from spore data were similar: 77.8 cM in mlh3Δ pch2Δ slx4Δ compared to 79.9 cM in mlh3Δ pch2Δ (Figure 5, A and B and Table S6, and Table S7). These results suggest Slx1-Slx4 does not significantly contribute to increased crossovers in mlh3Δ pch2Δ except for contributing to the NPD class. This is consistent with the meiotic role of the Slx1-Slx4 complex in the non-ZMM pathway characterized by multi-chromatid joint molecules (Zakharyevich et al. 2012; Kaur et al. 2015). mlh3Δ pch2Δ mms4-md mutants showed strong reduction in genetic map distances (23.7 cM) compared to mlh3Δ pch2Δ (79.9 cM) (Figure 5B and Table S7). Map distances from tetrad data could not be accurately estimated in mlh3Δ pch2Δ mms4-md due to the low frequency of four-spore viable tetrads and the strong reduction in the map distances. These results suggest that the majority of the crossovers in mlh3Δ pch2Δ are made through the Mus81-Mms4 pathway.
Genetic interference is reduced across a range of crossover frequencies in the mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants
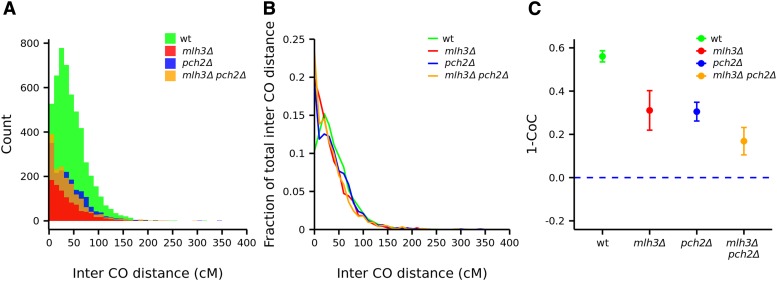
Interference limits crossover number and ensures that multiple crossovers along the chromosome are widely spaced (Muller 1916; Hillers 2004; Kleckner et al. 2004; Stahl et al. 2004). Intercrossover distances modeled using a gamma distribution can be used to determine the strength of genetic interference: γ = 1 corresponds to no interference, while γ > 1 indicates positive interference (Anderson et al. 2011). Intercrossover distances in physical units were converted into genetic distances (centimorgans) in wild type, pch2Δ, mlh3Δ, mlh3Δ pch2Δ, to account for differences in crossover frequencies (Materials and Methods) (Figure 6A). To account for differences in the number of tetrads analyzed, we also converted the counts for intercrossover distances as a fraction of the total for wild type and each mutant (Figure 6B). For the wild-type strain, the γ value was 1.83. Interference was reduced in pch2Δ (γ = 1.33), mlh3Δ (γ = 1.29) and mlh3Δ pch2Δ (γ = 1.13) compared to wild type. Interference analysis using the two pathway model that incorporates contribution from both interfering and noninterfering pathways also showed reduced interference in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ (File S3, and see Copenhaver et al. 2002; Housworth and Stahl 2003). We also analyzed interference from the genome wide crossover data using the CoC method (Materials and Methods, and see Anderson et al. 2015). Statistical significance of the difference between observed and expected frequency of double crossovers was estimated using the chi-square test. Interference (1 – CoC) was significantly reduced in mlh3Δ (P = 0.00033), pch2Δ (P = 4.36 × 10−6), and mlh3Δ pch2Δ (P = 1.85 × 10−9) relative to the wild type (Figure 6C).
Figure 6.
Analysis of crossover interference in wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. (A) Histogram showing actual count of intercrossover distances. (B) Line plot showing intercrossover distances as a fraction of the total for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. (C) Interference (1–CoC) in wild type (0.56), mlh3Δ (0.31), pch2Δ (0.30), and mlh3Δ pch2Δ (0.17) plotted for 0–25 kb adjacent intervals.
The reduced interference in mlh3Δ is consistent with the two pathway model for crossover formation (Copenhaver et al. 2002; Stahl et al. 2004; Getz et al. 2008). pch2Δ has been shown previously to lack genetic interference (Joshi et al. 2009; Zanders and Alani 2009). However, analysis of Zip3 foci that mark DSB sites that will be repaired as crossovers, suggests that interference is observed in pch2Δ at the crossover designation stage (Zhang et al. 2014). The loss of genetic interference in pch2Δ was thought to be due to the misregulation of the crossover/noncrossover decision step, and not because of the excess crossovers (Zanders and Alani 2009). We observe an increase in both crossovers and noncrossovers in pch2Δ mutants and Zip3 localization is known to be maintained in pch2Δ (Table 1, Zhang et al. 2014). Therefore, the reduced interference in pch2Δ is most likely due to the excess crossovers made by the MutLγ-dependent interfering, and the Mus81-Mms4-dependent noninterfering crossover pathways.
Since mlh3Δ pch2Δ also makes more noncrossovers, it is unlikely that the loss of interference is due to impairment of the crossover/noncrossover decision. The loss of interference is most likely because nearly all crossovers in mlh3Δ pch2Δ are made through the Mus81-Mms4 pathway (Figure 5B). Loss of interference in the mlh3Δ pch2Δ mutant is also supported from the uniform crossover density observed across all chromosome sizes (Figure 2D). The loss of genetic interference in mlh3Δ pch2Δ is particularly interesting as it makes as many crossovers on average as wild type. In summary, genetic interference is reduced across all three categories of crossover frequencies (reduced, equivalent, and greater than wild-type crossovers for mlh3Δ, mlh3Δ pch2Δ, and pch2Δ, respectively). No chromatid interference was observed among wild type, mlh3Δ, and pch2Δ mutants (P > 0.05, chi-square test), but negative chromatid interference characterized by a slight excess of two strand crossovers may be present in mlh3Δ pch2Δ mutants (0.01 < P < 0.05, chi-square test) (Table S8).
Noninterfering crossovers are less efficient in promoting crossover assurance
We tested the effect of a wide range of crossover frequencies on obligate crossovers using data from genome-wide analysis of meiotic recombination in the mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants. We determined the frequency of meiosis with nonexchange events in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants. The percentage of meiosis with 1 or >1 nonexchange chromosome was 3% for wild type, 7% for pch2Δ, 47% for mlh3Δ, and 20% for mlh3Δ pch2Δ (Table S3). mlh3Δ, which showed a decrease in both genetic interference and crossover frequencies, had the highest number of meioses with nonexchange events. In 5% of the meioses, the mlh3Δ mutant also had >1 nonexchange chromosome. However, mlh3Δ had an overall lower amount of nonexchange events among four-spore viable tetrads (47%) compared to msh4Δ (72%) but similar to msh4-R676W (42%, Krishnaprasad et al. 2015). These results suggest that, even though mlh3Δ makes 64 crossovers, the obligate crossover is not maintained. These findings reinforce our earlier observations that simultaneous reductions in crossover frequencies and genetic interference compromise the obligate crossover (Krishnaprasad et al. 2015). In pch2Δ, the number of meioses with nonexchange events (7%) was comparable to wild type (3%) (P = 0.37, Binomial test). This is likely due to the excess crossovers made in pch2Δ that compensate for the loss of genetic interference. Previously, we had estimated that, if the crossovers are distributed randomly, a wild-type yeast cell would require up to ∼200 crossovers to ensure (with probability >0.98) the absence of nonexchange chromosomes (Krishnaprasad et al. 2015). The observations with the pch2Δ mutant support the theoretical predictions that genetic interference reduces the number of crossovers required for crossover assurance. The results also suggest that pch2Δ does not significantly increase nonexchange chromosome frequency through other mechanisms. The mlh3Δ pch2Δ mutant was most interesting, since, although it made as many crossovers as wild type, the percentage of meiosis with nonexchange events (20%) was significantly more than wild type (3%) (P = 2.7 × 10−3, Binomial test). These data experimentally demonstrate that if the wild-type frequency of crossovers were randomly distributed in S. cerevisiae, there would be more nonexchange chromosomes.
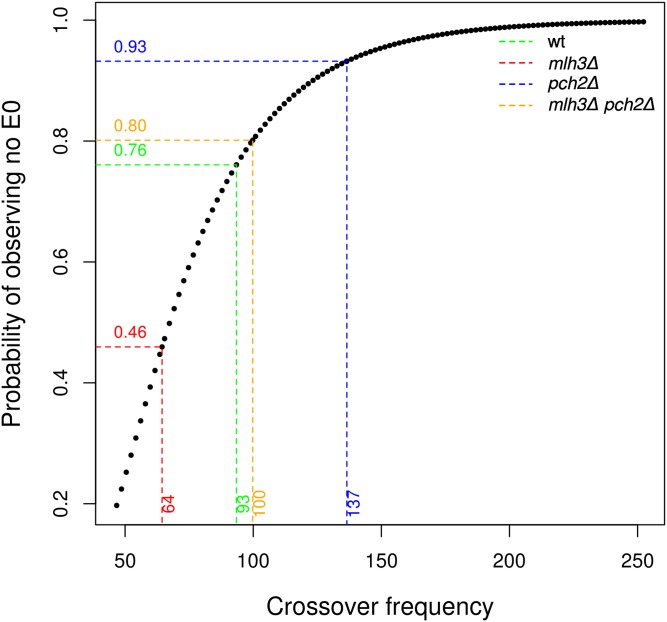
To further test how crossover frequency affects obligate crossovers, we estimated the percentage of meiosis with no nonexchange chromosomes expected for mlh3Δ, pch2Δ, and mlh3Δ pch2Δ by modeling the crossovers as a Poisson distribution (the Poisson distribution assumes the crossover events are independent) (Figure 7). The expected percentage of meiosis with no nonexchange chromosomes, 46% (mlh3Δ), 80% (mlh3Δ pch2Δ), 93% (pch2Δ) matched well with the experimental observations: 53% (mlh3Δ), 80% (mlh3Δ pch2Δ) and 93% (pch2Δ). Crossover distributions in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ, therefore, approximate a Poisson process. For wild type, the percentage of meiosis with nonexchange chromosomes (3%) is significantly lower than expected (24%) due to the presence of interference. These observations are consistent with the requirement for higher crossover frequencies in the absence of genetic interference to ensure obligate crossovers (Krishnaprasad et al. 2015; Berchowitz and Copenhaver 2010; Kaback et al. 1999).
Figure 7.
Probability of observing no E0 (chromosomes with zero crossovers per meiosis) for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. The probability of observing no E0 events is plotted against the crossover frequency modeled as a Poisson distribution. The dotted lines indicate the probabilities for finding no E0s in the absence of genetic interference for the experimentally observed average crossover counts per cell (mlh3Δ: 64; wild type: 93; mlh3Δ pch2Δ: 100; pch2Δ: 137).
In mutants with reduced genetic interference, adjacent crossovers may be closely spaced. As a result, they could be annotated as double noncrossovers inflating the number of nonexchange chromosomes. We inspected each nonexchange chromosome in the wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants for ambiguity in the annotation for double crossovers and double noncrossovers, and did not find any significant difference in the estimate of nonexchange chromosomes (Table S9). All nonexchange chromosomes, except one, had gene conversion events (Table S9). This observation suggests that lack of an obligate crossover was not a consequence of the absence of recombination interactions between the homologs, but rather a failure to convert even one of them into a crossover.
Small chromosomes are most sensitive to loss of the obligate crossover
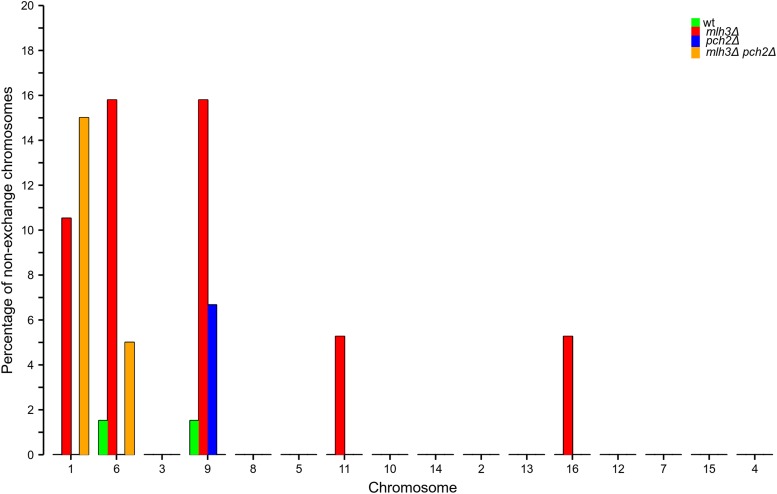
We analyzed the influence of chromosome size on the occurrence of nonexchange chromosomes in the wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants (Figure 8). For mlh3Δ, nonexchange chromosomes included small (I, VI, and IX), medium (XI), and large chromosomes (XVI). This observation is similar to the distribution of nonexchange chromosomes in msh4Δ observed previously, except that the number of nonexchange chromosomes is much less in mlh3Δ (Krishnaprasad et al. 2015). For pch2Δ, the only nonexchange chromosome was a small chromosome (IX). For mlh3Δ pch2Δ, only small chromosomes (I and VI) were nonexchange. These observations suggest that small chromosomes are particularly susceptible to the loss of the obligate crossover when there are crossover and/or interference defects. A stronger reduction in crossover frequencies, as observed in mlh3Δ mutants, is required for the loss of obligate crossovers on medium and large chromosomes. These observations further consolidate our previous results showing that small chromosomes are particularly sensitive to variations in crossover frequency (Krishnaprasad et al. 2015). A high frequency of nonexchange has been observed for smaller chromosomes in other crossover defective mutants, and also in human meiosis (Chen et al. 2008; Fledel-Alon et al. 2009). Among the small chromosomes, we observed a lack of E0s for the chromosome III. A similar phenomenon was seen in Krishnaprasad et al. (2015), where chromosome III had the fewest E0s among the small chromosomes. Analysis of copy number variation using read depth information did not detect aneuploidy in any of the mlh3Δ, pch2Δ, and mlh3Δ pch2Δ sequenced tetrads (Figure S9).
Figure 8.
The percentage of meiosis that are nonexchange for each chromosome for wild type, mlh3Δ, pch2Δ, and mlh3Δ pch2Δ. Chromosomes are shown in increasing order of size.
Discussion
We previously analyzed genome-wide crossovers in a msh4 hypomorph to demonstrate that a random distribution of reduced number of crossover events can increase nonexchange chromosome frequencies (Krishnaprasad et al. 2015). Our current work generalizes this observation to a larger context by analyzing nonexchange events when there is a random distribution of reduced, wild-type, and greater than wild-type frequency of crossovers using the mlh3Δ, pch2Δ, and mlh3Δ pch2Δ mutants.
Obligate crossover is sensitive to crossover frequencies and genetic interference
Genetic interference is affected in mlh3Δ, pch2Δ, and mlh3Δ pch2Δ due to increased contributions from the noninterfering pathway. Interference is also compromised in mlh3Δ and mlh3Δ pch2Δ because of defects in converting crossover designated sites into actual crossovers. The pch2Δ results suggest that mutants with reduced genetic interference can maintain an obligate crossover if crossover frequencies are significantly higher than in wild type (Figure 7). If the crossover frequency is reduced compared to wild type, there is a loss of the obligate crossover, as in the case of mlh3Δ. In mlh3Δ pch2Δ, we observed crossover frequencies equivalent to wild type (100 crossovers per meiosis), but an increased proportion of nonexchange chromosomes. Three lines of evidence support enhanced nonexchange chromosome frequency in mlh3Δ pch2Δ. First, we observe nonexchange chromosomes in 20% of meiosis in mlh3Δ pch2Δ in the S288c/YJM789 hybrid (Figure 8 and Table S3). Second, theoretical predictions by modeling the mlh3Δ pch2Δ crossover distribution as a Poisson process also support higher nonexchange chromosome frequencies consistent with the experimental results (Figure 7). Third, mlh3Δ pch2Δ showed spore viability pattern typical of Meiosis I nondisjunction in the SK1 EAY1108/1112 background (Figure 4). Since most crossovers in mlh3Δ pch2Δ are made by the Mus81-Mms4 pathway (Figure 5B), these observations are consistent with noninterfering crossovers being less efficient in supporting an obligate crossover. The higher E0 frequency in mlh3∆ pch2∆ also supports the prediction that S. cerevisiae will require up to 200 crossovers in the absence of genetic interference to have >0.98% probability of an obligate crossover on every homolog pair (Krishnaprasad et al. 2015).
Triple mutant analysis with another resolvase (Slx4) showed that genetic map distances measured from spore data were not significantly different between mlh3Δ pch2Δ (79.9 cM) and mlh3Δ pch2Δ slx4Δ (77.8 cM). However, map distances from tetrad data showed significant differences between mlh3Δ pch2Δ (113.6 cM) and mlh3Δ pch2Δ slx4Δ (91.2 cM). These results suggest Slx4 only makes a minor contribution to crossovers in the mlh3Δ pch2Δ mutant. Slx4 may be involved in resolving a fraction of closely spaced double crossovers that contribute to the NPD class of tetrads.
Segregation of nonexchange chromosomes in mlh3Δ
Since mlh3Δ and wild type have identical spore viability in the S288c/YJM789 hybrid and similar proportion of the four-viable spore tetrad class, we tested the association of spore viability with the nonexchange chromosome frequency. Mlh3 acts downstream of Msh4/5 at the final steps of Holliday Junction resolution (Baker et al. 1996; Lipkin et al. 2002; Snowden et al. 2004; Kolas et al. 2005). mlh3Δ mutants, therefore, provide an advantage in relating variations in crossover frequency with the loss of obligate crossovers without the confounding effects that may arise in other mutants that have an early role in the crossover/noncrossover decision step or that affect DSB formation. It is also important to recognize that the data for the nonexchange chromosome frequency comes from the analysis of the four viable spore tetrads only. mlh3Δ has high spore viability (85%), strong crossover defects (64 crossovers per meiosis), and abundant single nonexchange chromosomes (Figure 8 and Table 1). These data are similar to the pattern seen with msh4-R676W earlier (Krishnaprasad et al. 2015). The mlh3Δ mutant reinforces the idea that a reduction in crossover frequency results in loss of the obligate crossover, even though the average crossover number is still four times the number of homologs. The high spore viability of mlh3Δ, suggests these single nonexchange chromosomes are efficiently segregated as observed with msh4-R676W (Krishnaprasad et al. 2015), supporting the hypothesis that crossovers facilitate but are not essential for chromosome segregation. Other mechanisms, such as distributive segregation, spindle checkpoint, and centromere pairing may also contribute to the segregation of achiasmate chromosomes in S. cerevisiae (Guacci and Kaback 1991; Shonn et al. 2000; Kemp et al. 2004). These observations also suggest spore viability is a poor measure of the status of obligate crossovers. The mlh3Δ mutant showed 64 crossovers genome-wide compared to 49 reported in msh4Δ (Krishnaprasad et al. 2015). These results provide support to previous observation on Msh4/5 dependent but Mlh1/3 independent crossover pathways (Argueso et al. 2004). It is also expected if, in mlh3Δ, some of the crossover precursor joint molecules are resolved by structure selective nucleases as both crossovers and noncrossovers.
Recombination maps in pch2Δ capture DSB variation
We observed a simultaneous increase in crossovers and noncrossovers in pch2Δ, which suggested that DSBs are increased in pch2Δ. Previous studies have shown a complex role for Pch2 in DSB formation, with increased DSB formation at certain loci, such as the rDNA locus (Vader et al. 2011), and a general reduction in DSBs at other loci (Farmer et al. 2012). Pch2 also has a role in the processing of early DSBs (Joshi et al. 2015). These studies suggest overall DSB levels may remain constant in pch2Δ, and the effects need to be examined on a per locus/chromosome basis (Farmer et al. 2012). High-resolution crossover and noncrossover data in pch2Δ provide an indirect readout of variation in DSB formation. For example, pch2Δ mutants do not show negative correlation of chromosome size with crossover plus noncrossover density. We observe more crossovers around the rDNA locus in pch2Δ mutants. pch2Δ, as well as mlh3Δ pch2Δ, also show suppression of both crossovers and noncrossovers near telomeres, which may suggest an altered DSB distribution near telomeric regions. Also, we observed increased crossovers as well as noncrossovers on Chr III, consistent with increased DSB formation on chr III (Farmer et al. 2012). Such differences in DSB distribution, possibly by regulating Hop1 function (Borner et al. 2008; Wojtasz et al. 2009), and the use of the Mus81-Mms4 pathway, may be responsible for the differences in crossover and noncrossover patterns in pch2Δ and mlh3Δ pch2Δ compared to wild type (Figure S4, Figure S6, and Figure S7).
Although Rad51 foci analyses do not give information on regional DSB variation, it can provide information on total cellular DSB levels and its turnover. We did not observe peak Rad51 focus counts in pch2Δ to be greater than wild type. This is consistent with the physical analysis of DSBs, which suggests that, at least at specific loci, there is no increase in DSB formation in pch2Δ mutants (Zanders et al. 2011; Farmer et al. 2012; Joshi et al. 2015). Instead, pch2Δ showed the persistence of peak DSB foci over an extended period of the meiotic time course from 3 to 8 hr. It is possible there is DSB turnover in pch2Δ, so that DSBs are made and repaired and the process is repeated, leading to an overall increase in crossover and noncrossovers. Such a possibility is supported by previous observations of the accumulation of Rad51 foci in pch2Δ mutants (Subramanian et al. 2016). The only other alternative is that there is more repair of DSBs by the interhomolog pathway in pch2Δ mutants, which may account for increased crossovers and noncrossovers. But all available evidence suggests that in pch2Δ there is more intersister recombination than in wild type (Zanders et al. 2011; Joshi et al. 2015). Defects in DSB processing (e.g., longer resection) may also result in longer ssDNA filaments stabilized by Rad51 that may account for the long gene conversion tracts observed in pch2Δ mutants (Figure S8, B and C). The role of Pch2 in DSB regulation requires further investigation.
In conclusion, the mlh3Δ pch2Δ data provides experimental support from genome-wide analysis that wild-type crossover frequencies distributed randomly cannot maintain an obligate crossover on all homolog pairs (Kaback et al. 1999; Berchowitz and Copenhaver 2010; Krishnaprasad et al. 2015). The distribution of crossovers and noncrossovers suggest that chromosome-size dependent DSB formation is affected in pch2∆. pch2Δ mutants also show that obligate crossovers can be ensured through a random distribution of excess crossovers. These results are consistent with MutL gamma dependent interfering crossovers being more efficient in promoting homolog disjunction compared to the Mus81-Mms4 dependent noninterfering crossovers.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.040071/-/DC1.
Acknowledgments
We thank Eric Alani and Michael Lichten for helpful comments on the manuscript. We are also thankful to Michael Lichten and Scott Keeney for the gift of strains used in this study. This study was technically supported by the EMBL GeneCore. Part of this work was performed under the International Cooperative Research Program of Institute for Protein Research, Osaka University, ICRa-16-03. N.K.T. is supported by a Wellcome Trust-DBT India Alliance Intermediate fellowship (IA/I/11/2500268) and Indian Institute of Science Education and Research-Thiruvananthapuram (IISER-TVM) intramural funds. N.K.T. was also supported by a Department of Science & Technology-Japan Society for the Promotion of Science (DST-JSPS) Exploratory Exchange visit under the India-Japan Cooperative Science Program. The study was financially supported by a European Research Council Advanced Investigator Grant (AdG-294542) to L.M.S., and A.S. was supported by JSPS (KAKENHI Grant Number 22125002 and 15H05973). P.C. and A.V.P. are supported by a University Grants Commission fellowship. A.D. is supported by a fellowship from IISER-TVM. K.G.N. is supported by a fellowship from the Council for Scientific and Industrial Research, New Delhi.
Footnotes
Communicating editor: B. J. Andrews
Literature Cited
- Allers T., Lichten M., 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106: 47–57. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Chen S. Y., Dimon M. T., Oke A., DeRisi J. L., et al. , 2011. ReCombine: a suite of programs for detection and analysis of meiotic recombination in whole-genome datasets. PLoS One 6: e25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. M., Oke A., Yam P., Zhuge T., Fung J. C., 2015. Reduced crossover interference and increased ZMM-independent recombination in the absence of Tel1/ATM. PLoS Genet. 11: e1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Kijas A. W., Sarin S., Heck J., Waase M., et al. , 2003. Systematic mutagenesis of the Saccharomyces cerevisiae MLH1 gene reveals distinct roles for Mlh1p in meiotic crossing over and in vegetative and meiotic mismatch repair. Mol. Cell. Biol. 23: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Wanat J., Gemici Z., Alani E., 2004. Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics 168: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. M., Plug A. W., Prolla T. A., Bronner C. E., Harris A. C., et al. , 1996. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13: 336–342. [DOI] [PubMed] [Google Scholar]
- Barchi M., Roig I., Di Giacomo M., de Rooij D. G., Keeney S., et al. , 2008. ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet. 4: e1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaglia T., Chauveau D., Hunter D., Young D., 2009. mixtools: an R package for analyzing finite mixture models. J. Stat. Softw. 32: 1–29. [Google Scholar]
- Berchowitz L. E., Copenhaver G. P., 2010. Genetic interference: don’t stand so close to me. Curr. Genomics 11: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., 1994. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79: 1081–1092. [DOI] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B., 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner G. V., Kleckner N., Hunter N., 2004. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117: 29–45. [DOI] [PubMed] [Google Scholar]
- Borner G. V., Barot A., Kleckner N., 2008. Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis. Proc. Natl. Acad. Sci. USA 105: 3327–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Jomaa A., Ortega J., Alani E. E., 2014. Pch2 is a hexameric ring ATPase that remodels the chromosome axis protein Hop1. Proc. Natl. Acad. Sci. USA 111: E44–E53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Tsubouchi T., Rockmill B., Sandler J. S., Richards D. R., et al. , 2008. Global analysis of the meiotic crossover landscape. Dev. Cell 15: 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F., Kauppi L., Lange J., Roig I., Wang R., et al. , 2012. Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver G. P., Housworth E. A., Stahl F. W., 2002. Crossover interference in Arabidopsis. Genetics 160: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton V. E., Hoffmann E. R., Borts R. H., 2010. Distinct regulation of Mlh1p heterodimers in meiosis and mitosis in Saccharomyces cerevisiae. Genetics 185: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L., Smith G. R., 2003. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163: 857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos T., Hunter N., Lee C., Larkin B., Loidl J., et al. , 2003. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 164: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muyt A., Jessop L., Kolar E., Sourirajan A., Chen J., et al. , 2012. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol. Cell 46: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo M. A., Banks E., Poplin R., Garimella K. V., Maguire J. R., et al. , 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S., Hong E. J., Leung W. K., Argunhan B., Terentyev Y., et al. , 2012. Budding yeast Pch2, a widely conserved meiotic protein, is involved in the initiation of meiotic recombination. PLoS One 7: e39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledel-Alon A., Wilson D. J., Broman K., Wen X., Ober C., et al. , 2009. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 5: e1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz T. J., Banse S. A., Young L. S., Banse A. V., Swanson J., et al. , 2008. Reduced mismatch repair of heteroduplexes reveals “non”-interfering crossing over in wild-type Saccharomyces cerevisiae. Genetics 178: 1251–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., Willems A. R., Woods R. A., 1995. Studies on the transformation of intact yeast cells by the LiAc/ss‐DNA/PEG procedure. Yeast 11: 355–360. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Guacci V., Kaback D. B., 1991. Distributive disjunction of authentic chromosomes in Saccharomyces cerevisiae. Genetics 127: 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., Irick H., Zitron A. E., Haddox D. A., Lohe A., et al. , 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Higashide M., Shinohara M., 2016. Budding yeast SLX4 contributes to the appropriate distribution of crossovers and meiotic double-strand break formation on bivalents during meiosis. G3 Bethesda 6: 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillers K. J., 2004. Crossover interference. Curr. Biol. 14: R1036–R1037. [DOI] [PubMed] [Google Scholar]
- Hillers K. J., Villeneuve A. M., 2003. Chromosome-wide control of meiotic crossing over in C. elegans. Curr. Biol. 13: 1641–1647. [DOI] [PubMed] [Google Scholar]
- Ho H. C., Burgess S. M., 2011. Pch2 acts through Xrs2 and Tel1/ATM to modulate interhomolog bias and checkpoint function during meiosis. PLoS Genet. 7: e1002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwagen A., Tham W. H., Brar G. A., Amon A., 2005. The FK506 binding protein Fpr3 counteracts protein phosphatase 1 to maintain meiotic recombination checkpoint activity. Cell 122: 861–873. [DOI] [PubMed] [Google Scholar]
- Housworth E. A., Stahl F. W., 2003. Crossover interference in humans. Am. J. Hum. Genet. 73: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., 2011. Double duty for Exo1 during meiotic recombination. Cell Cycle 10: 2607–2609. [DOI] [PubMed] [Google Scholar]
- Hunter N., Kleckner N., 2001. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106: 59–70. [DOI] [PubMed] [Google Scholar]
- Hyppa R. W., Smith G. R., 2010. Crossover invariance determined by partner choice for meiotic DNA break repair. Cell 142: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N., Barot A., Jamison C., Borner G. V., 2009. Pch2 links chromosome axis remodeling at future crossover sites and crossover distribution during yeast meiosis. PLoS Genet. 5: e1000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N., Brown M. S., Bishop D. K., Borner G. V., 2015. Gradual implementation of the meiotic recombination program via checkpoint pathways controlled by global DSB levels. Mol. Cell 57: 797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Barber D., Mahon J., Lamb J., You J., 1999. Chromosome size-dependent control of meiotic reciprocal recombination in Saccharomyces cerevisiae: the role of crossover interference. Genetics 152: 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., De Muyt A., Lichten M., 2015. Top3-Rmi1 DNA single-strand decatenase is integral to the formation and resolution of meiotic recombination intermediates. Mol. Cell 57: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B., Boumil R. M., Stewart M. N., Dawson D. S., 2004. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18: 1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Zickler D., Jones G. H., Dekker J., Padmore R., et al. , 2004. A mechanical basis for chromosome function. Proc. Natl. Acad. Sci. USA 101: 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolas N. K., Svetlanov A., Lenzi M. L., Macaluso F. P., Lipkin S. M., et al. , 2005. Localization of MMR proteins on meiotic chromosomes in mice indicates distinct functions during prophase I. J. Cell Biol. 171: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaprasad G. N., Anand M. T., Lin G., Tekkedil M. M., Steinmetz L. M., et al. , 2015. Variation in crossover frequencies perturb crossover assurance without affecting meiotic chromosome segregation in Saccharomyces cerevisiae. Genetics 199: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libuda D. E., Uzawa S., Meyer B. J., Villeneuve A. M., 2013. Meiotic chromosome structures constrain and respond to designation of crossover sites. Nature 502: 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin S. M., Moens P. B., Wang V., Lenzi M., Shanmugarajah D., et al. , 2002. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat. Genet. 31: 385–390. [DOI] [PubMed] [Google Scholar]
- Mancera E., Bourgon R., Brozzi A., Huber W., Steinmetz L. M., 2008. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E., Diaz R. L., Hunter N., Keeney S., 2006. Crossover homeostasis in yeast meiosis. Cell 126: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos J., Blanco M. G., Maslen S., Skehel J. M., West S. C., 2011. Regulatory control of the resolution of DNA recombination intermediates during meiosis and mitosis. Cell 147: 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., et al. , 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek M. S., Speed T. P., 1995. Modeling interference in genetic recombination. Genetics 139: 1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., 1916. The mechanism of crossing-over. Am. Nat. 50: 284–305. [Google Scholar]
- Murakami H., Borde V., Nicolas A., Keeney S., 2009. Gel electrophoresis assays for analyzing DNA double-strand breaks in Saccharomyces cerevisiae at various spatial resolutions. Methods Mol. Biol. 557: 117–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Ogawa H., 1999. The Saccharomyces cerevisiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis. EMBO J. 18: 5714–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. J., Keeney S., 2006. Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham L., Jordan P., Rockmill B., Roeder G. S., Hoffmann E., 2010. The synaptonemal complex protein, Zip1, promotes the segregation of nonexchange chromosomes at meiosis I. Proc. Natl. Acad. Sci. USA 107: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant K. T., Plys A. J., Alani E., 2008. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J. E., Ross-Macdonald P. B., Roeder G. S., 2001. The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158: 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. D., Lao J. P., Taylor A. F., Smith G. R., Hunter N., 2008. RecQ helicase, Sgs1, and XPF family endonuclease, Mus81-Mms4, resolve aberrant joint molecules during meiotic recombination. Mol. Cell 31: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke A., Anderson C. M., Yam P., Fung J. C., 2014. Controlling meiotic recombinational repair - specifying the roles of ZMMs, Sgs1 and Mus81/Mms4 in crossover formation. PLoS Genet. 10: e1004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Sasaki M., Kniewel R., Murakami H., Blitzblau H. G., et al. , 2011. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144: 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Siomos M. F., Nasmyth K., 2003. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell 112: 423–440. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11: 2600–2621. [DOI] [PubMed] [Google Scholar]
- Rogacheva M. V., Manhart C. M., Chen C., Guarne A., Surtees J., et al. , 2014. Mlh1-Mlh3, a meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J. Biol. Chem. 289: 5664–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F. M., Heiter P., 1990. Methods in Yeast Genetics: A Laboratory Course Manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- San-Segundo P. A., Roeder G. S., 1999. Pch2 links chromatin silencing to meiotic checkpoint control. Cell 97: 313–324. [DOI] [PubMed] [Google Scholar]
- Serrentino M. E., Borde V., 2012. The spatial regulation of meiotic recombination hotspots: are all DSB hotspots crossover hotspots? Exp. Cell Res. 318: 1347–1352. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Gasior S. L., Bishop D. K., Shinohara A., 2000. Tid1/Rdh54 promotes colocalization of rad51 and dmc1 during meiotic recombination. Proc. Natl. Acad. Sci. USA 97: 10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M., Oh S. D., Hunter N., Shinohara A., 2008. Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat. Genet. 40: 299–309. [DOI] [PubMed] [Google Scholar]
- Shonn M. A., McCarroll R., Murray A. W., 2000. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289: 300–303. [DOI] [PubMed] [Google Scholar]
- Snowden T., Acharya S., Butz C., Berardini M., Fishel R., 2004. hMSH4-hMSH5 recognizes Holliday junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol. Cell 15: 437–451. [DOI] [PubMed] [Google Scholar]
- Sonntag Brown M., Lim E., Chen C., Nishant K. T., Alani E., 2013. Genetic analysis of mlh3 mutations reveals interactions between crossover promoting factors during meiosis in baker’s yeast. G3 Bethesda 3: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Foss H. M., Young L. S., Borts R. H., Abdullah M. F., et al. , 2004. Does crossover interference count in Saccharomyces cerevisiae? Genetics 168: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V. V., MacQueen A. J., Vader G., Shinohara M., Sanchez A., et al. , 2016. Chromosome synapsis alleviates Mek1-dependent suppression of meiotic DNA repair. PLoS Biol. 14: e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Huang L., Markowitz T. E., Blitzblau H. G., Chen D., et al. , 2015. Transcription dynamically patterns the meiotic chromosome-axis interface. Elife 4: e07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sym M., Roeder G. S., 1994. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell 79: 283–292. [DOI] [PubMed] [Google Scholar]
- Terasawa M., Ogawa H., Tsukamoto Y., Shinohara M., Shirahige K., et al. , 2007. Meiotic recombination-related DNA synthesis and its implications for cross-over and non-cross-over recombinant formation. Proc. Natl. Acad. Sci. USA 104: 5965–5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G., Blitzblau H. G., Tame M. A., Falk J. E., Curtin L., et al. , 2011. Protection of repetitive DNA borders from self-induced meiotic instability. Nature 477: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zickler D., Kleckner N., Zhang L., 2015. Meiotic crossover patterns: obligatory crossover, interference and homeostasis in a single process. Cell Cycle 14: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasz L., Daniel K., Roig I., Bolcun-Filas E., Xu H., et al. , 2009. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet. 5: e1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Burgess S. M., 2006. Two distinct surveillance mechanisms monitor meiotic chromosome metabolism in budding yeast. Curr. Biol. 16: 2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K., Tang S., Ma Y., Hunter N., 2012. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders S., Alani E., 2009. The pch2Delta mutation in baker’s yeast alters meiotic crossover levels and confers a defect in crossover interference. PLoS Genet. 5: e1000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders S., Sonntag Brown M., Chen C., Alani E., 2011. Pch2 modulates chromatid partner choice during meiotic double-strand break repair in Saccharomyces cerevisiae. Genetics 188: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang S., Yin S., Hong S., Kim K. P., et al. , 2014. Topoisomerase II mediates meiotic crossover interference. Nature 511: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Kleckner N., 2016. A few of our favorite things: pairing, the bouquet, crossover interference and evolution of meiosis. Semin. Cell Dev. Biol. 54: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains listed in Table S1 are available upon request. Sequence data are available from the National Centre for Biotechnology Information Sequence Read Archive: Accession number SRP082254. The raw recombination data files and the custom R scripts are available online at the Dryad data repository (http://datadryad.org; http://dx.doi.org/10.5061/dryad.5vm4g).