Abstract
T cell-based immunotherapies are a promising approach for patients with advanced cancers. However, various obstacles limit T cell efficacy, including suboptimal T cell receptor (TCR) activation and an immunosuppressive tumor environment. Here we developed a fusion protein by linking CD8α and MyD88 (CD8α:MyD88) to enhance CD8+ T cell responses to weakly immunogenic and poorly expressed tumor antigens. CD8α:MyD88-engineered T cells exhibited increased proliferation and expression of effector and co-stimulatory molecules in a tumor antigen-dependent manner. These effects were accompanied by elevated activation of TCR and Toll-like receptor (TLR) signaling-related proteins. CD8α:MyD88-expressing T cells improved anti-tumor responses in mice. Enhanced anti-tumor activity was associated with a unique tumor cytokine/chemokine signature, improved T cell infiltration, reduced markers of T cell exhaustion, elevated levels of proteins associated with antigen presentation, and fewer macrophages with an immunosuppressive phenotype in tumors. Given these observations, CD8α:MyD88 represents a unique and versatile approach to help overcome immunosuppression and enhance T cell responses to tumor antigens.
Keywords: Cancer immunotherapy, modified CD8 co-receptor, MyD88 signaling, T cell co-stimulation, lowering TCR threshold
Introduction
T cell-based immunotherapies are one of the most promising treatments for patients with advanced cancers, including melanoma. Several approaches have been developed to harness the anti-tumor activity of cytotoxic T cells, including vaccine-based therapies, adoptive cell transfer (ACT) of tumor infiltrating lymphocytes (TILs)(1,2) or T cells engineered to express a tumor-reactive T cell receptor (TCR)(3,4), and antibody-mediated checkpoint blockade(5). Despite encouraging clinical results demonstrating tumor regression and prolonged survival, durable anti-tumor responses are observed only in a subset of patients, highlighting the need to improve these therapies. Limitations include low tumor antigen-specific T cell precursor frequencies, suboptimal TCR responses due to low TCR affinity (or avidity) to weakly immunogenic tumor antigens, and low antigen expression and/or presentation on tumor cells(1,2,5). Furthermore, various suppressive mechanisms within the tumor microenvironment (TME) such as tumor-associated macrophage (TAM)(6) and Th2 cytokine accumulation hamper anti-tumor T cell responses. Moreover, chronic exposure to factors in the TME induces the expression of receptors that foster T cell exhaustion, such as Tim-3, Lag-3 and PD-1(7).
Various studies have demonstrated that activating MyD88 signaling in T cells via Toll-like receptor (TLR) engagement enhances cytokine production, cytotoxicity and proliferation(8–10). Yet, applying TLR co-stimulation specifically to T cells is challenging due to their low and transient expression of TLRs, insufficient intratumoral TLR agonist localization for T cell co-stimulation, and the potential for TLR engagement on tumor cells to promote tumor growth(10).
Here, we present a novel strategy to activate MyD88 signaling within CD8+ T cells in a TLR–independent but TCR-dependent fashion to overcome weak tumor antigenicity and an immunosuppressive TME. This strategy utilizes the CD8α chain – an endogenous TCR co-receptor that interacts with major histocompatibility complex I (MHC I). By fusing CD8α to MyD88 (CD8α:MyD88), we developed a novel platform that enhances various parameters of T cell function. CD8α:MyD88–expressing T cells respond to suboptimal levels of tumor antigen and display reduced levels of exhaustion markers and increased production of effector molecules. The enhanced anti-tumor activity of CD8α:MyD88–expressing T cells is associated with their ability to reshape various facets of the TME including the cytokine milieu as well as the phenotype of other immune cells, resulting in a TME that favors T cell infiltration and anti-tumor activity.
Materials and Methods
Plasmids, retroviral production and T cell transduction
The CD8α:MyD88 co-receptor was designed by fusing the murine CD8α extracellular (EC) and transmembrane (TM) domain sequences to the human MyD88 death domain (DD) and intermediate domain (ID) sequences (Supplementary Fig S1A). The CD8αΔIC construct lacked any intracellular domains. Genes were cloned into the pMIG-w vector containing a GFP reporter gene. Retroviral vector supernatants were produced from Phoenix Ampho and Eco packaging cell lines we have previously described(11). For generating engineered mouse T cells, pmel or OT-I T cells were activated for 48 hrs. using 1 µg/mL hgp10025–33 or SIINFEKL peptide, respectively, and 50 U/mL of IL-2 prior to transduction and efficiency was determined by assessing the frequency of GFP+ cells by flow cytometry. For generating DMF5-CD8α:MyD88 cells, human peripheral blood mononuclear cells (PBMCs) were engineered to express DMF5 as previously described(11), expanded with beads coated (Invitrogen) with HLA-A2/MART-127–35 (BD Pharmingen) and anti-CD28 antibody plus 100 U/mL IL-2 and then engineered to express the indicated CD8α vectors. Viable cells were enriched by ficoll-gradient prior to setting up all assays or injection into mice.
Mice, tumor model and ex vivo analysis
Studies were approved by the UMB Institutional Animal Care and Use Committee. C57BL/6J and pmel (B6.Cg-Thy1/Cy Tg(TcraTcrb)8Rest/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). T cells from IRAK-4 kinase dead mice were kindly provided by Dr. Stefanie Vogel at the University of Maryland, Baltimore, MD. C57BL/6J mice were injected with 2×105 B16-F1 melanoma cells subcutaneously on the right flank. Mice were irradiated with 550 radians on day 9 post tumor inoculation and intravenously injected with engineered pmel T cells on day 10. Mouse body weight and tumor size were monitored every 2–3 days. Tumor volume was calculated by the ellipsoid formula: length × width × height × (4/3)π. Specific tissues were harvested one week post T cell transfer for ex vivo analyses including flow cytometry and cytokine/chemokine Luminex. The following antibodies were used: CD8, Lag3, Tim3, I-A/I-E, CD86, CD11b, CD11c, F4/80, CD206, Gr-1, NK1.1 (BioLegend), CD45.2, CD8α, CD19 (BD Pharmingen), MHC class I H2 Kb + Db (Abcam).
T cell proliferation assays, cytokine measurements, and intracellular staining
Splenocytes from C57BL/6J mice were irradiated (3,000 radians) and pulsed with varying concentrations of hgp10025–33 or SIINFEKL peptide for 2 hours at 37°C. Pmel or OT-I T cells were co-cultured with peptide-pulsed splenocytes at a 1:1 ratio and supernatant was collected after 24 hours. Alternatively, B16-F1 cells (ATTC CRL-6323, obtained within three years of using them) were irradiated (20,000 radians) and plated at various cell numbers together with 1×105 transduced T cells for 48 hours. Cytokine concentrations were determined by ELISA (eBioscience) or the Milliplex Cytokine/Chemokine Kit (Millipore). IFN-γ and TNF-α production by DMF5 T cells was evaluated at 2:1 and 1:2 T cell to PBMC ratio after 4 days of stimulation. We assessed T cell proliferation by adding 1 µCi/well of tritiated-thymidine (methyl-3H, Perkin Elmer) per well and measured thymidine-incorporation 24 hours later. Intracellular levels of signaling proteins were evaluated by flow cytometry. Briefly, cells were permeabilized in BD Pharmingen Phosflow Perm Buffer III (BD Bioscience) and stained with anti-p-p65 and anti-rabbit IgG F(ab')2 Fragment-PE (Cell Signaling), or anti-p-ERK1/2-Pacific Blue and anti-p-JNK-PE, or anti-p-p38-Pacific Blue and anti-p-Zap70-PE (BD Bioscience). For flow cytometry-based proliferation assays, transduced T cells were pulsed with cell proliferation dye eFluor 450 (eBioscience), washed, and co-cultured with hgp10025–33-pulsed splenocytes at a 1:1 ratio for 72 hours. In other experiments, T cells were co-incubated with TAg-pulsed splenocytes (mouse pmel or OT-I T cells) or autolougous PBMCs (human DMF5 T cells) for 48 or 96 hours with Brefeldin A added the last 6 hours of incubation prior to staining. For evaluating human T cell proliferation, transduced T cells were co-cultured with Malme-3M melanoma cell (HLA-A2+MART-1+) or with A375 melanoma cells (HLA-A2+) pulsed or unpulsed with 10µg of MART-127–35 peptide at a ratio of 1:1 T cell to tumor cell ratio. For the phenotyping screen, transduced T cells were co-cultured with 0.12 µg/mL of hgp10025–33-pulsed splenocytes at a 1:2 splenocyte to T cell ratio for 48 hours, stained with the Zombie Aqua viability dye (BioLegend), anti-CD45.2 and anti-CD8α antibodies, and stained with the LegendScreen Mouse Cell Screening (PE) Kit (BioLegend). Malme-3M and A375 cell lines were obtained from ATCC and used were tested for Mycoplasma within three years of purchasing them. All cell lines within the last 3 months. All flow cytometry was performed on the BD LSRII at the Greenebaum Comprehensive Cancer Center Flow Cytometry Shared Service Lab and analyzed by FlowJo (Tree Star).
Statistical Analysis
Proliferation and ELISA experiments were performed in triplicate in at least two independent experiments and analyzed by one-way ANOVA. Animal studies contained 8 to 10 animals per group for growth and survival, and 5 per group for ex vivo flow cytometry analysis. For flow cytometry and the cytokine arrays, the values and error bars represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p≤ 0.001; one-way ANOVA with Tukey’s Multiple Comparison Test; n=3 experimental replicates and are representative of at least two independent experiments. Tumor sizes was analyzed by a mixed model repeated measures with AR(1) covariance structure (yielding smallest BIC), followed by Sidak-adjusted comparisons at each time.
Results
CD8α:MyD88 lowers the threshold of T cell activation resulting in increased proliferation and cytokine production
The extracellular and transmembrane domains of CD8α were fused to the human MyD88 death and intermediate domains to generate a CD8α:MyD88 co-receptor (Supplementary Fig S1A). As controls, we generated CD8α lacking the intracellular domain (CD8αΔIC), or an empty vector control. Each vector also contained a GFP reporter to distinguish transduced T cells (Supplementary Fig S1B). CD8α:MyD88 expression was confirmed by western blot and imaging flow cytometry (Supplementary Fig S1C & S1D).
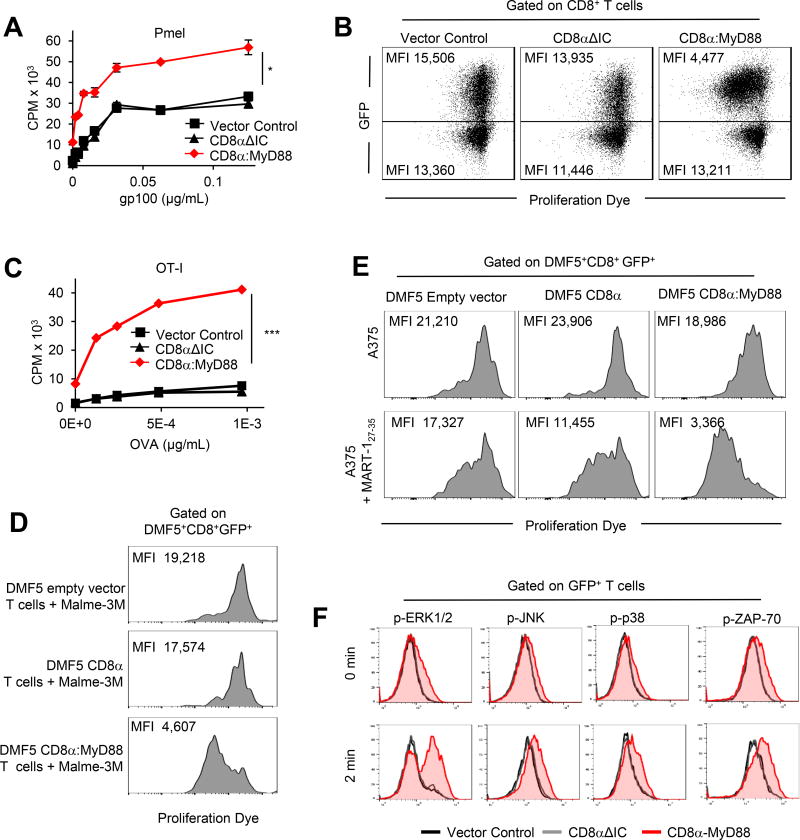
Gp10025–33-specific TCR transgenic pmel CD8+ T cells were engineered to express CD8α:MyD88, CD8αΔIC, or only GFP using retroviral vectors and stimulated with gp10025–33–pulsed splenocytes. Pmel T cells expressing CD8α:MyD88 exhibited a lower activation threshold as demonstrated by increased proliferation in response to lower tumor antigen (TAg) levels compared to control T cell groups (Figure 1A). Notably, while only half of the CD8+ T cells expressed CD8α:MyD88, proliferation was more than doubled at most antigen concentrations, Figure 1A.). The ability for T cells to recognize exceedingly low TAg levels is relevant as tumor cells can downregulate TAg or MHC I expression to evade T cell detection. In the absence of TAg, CD8α:MyD88 T cells were not activated, indicating that the enhancement of TCR responses by MyD88 is TCR-dependent.
Figure 1. CD8α:MyD88 expression enhances T cell responses to tumor antigen.
A) Pmel T cells were engineered with the empty vector control, CD8αΔIC or CD8α:MyD88. T cell proliferation was measured by 3H-thymidine incorporation after stimulation with gp10025–33-pulsed splenocytes for 48 hours. B) Flow cytometry of pmel T cells co-cultured with splenocytes pulsed with 0.12 µg/mL gp10025–33 peptide at 72 hours. C) Proliferation of OT-I T cells co-cultured with OVA (SIINFEKL)-pulsed splenocytes measured by 3H-thymidine incorporation at 48 hours. D–E) DMF5 empty vector control, DMF5 CD8α or DMF5 CD8α:MyD88 human T cells were labeled with proliferation dye and cultured with HLA-A2+ MART-1+ Malme-3M or with HLA-A2+ MART-1− A375 melanoma pulsed or not with 10µg/ml/106 cells MART-127–35 cells at a 1:1 ratio. T cell proliferation was measured by flow cytometry after 5 days. F) Intracellular staining of phosphorylated proteins in transduced T cells stimulated with peptide-pulsed splenocytes and fixed at given timepoints. Values and error bars represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p≤ 0.001 A and C, one-way ANOVA with Tukey’s Multiple Comparison Test; n=3 experimental replicates; representative of at least two independent experiments. D and E are representative of three independent experiments.
To determine whether the increased proliferation observed in CD8α:MyD88 T cells was due to their ability to promote the division of non-transduced T cells, we compared cell division of CD8α:MyD88 (GFP+) and non-transduced (GFP−) T cells. CD8α:MyD88 T cells loaded with a proliferation dye expanded to a greater degree than non-transduced T cells, as indicated by a lower median fluorescence intensity (MFI) in GFP+ cells (Figure 1B, right panel). The increased proliferation by CD8α:MyD88 T cells was more pronounced at sub-optimal TAg concentrations that were too low to activate control T cells (Supplementary Fig S1E). In addition, the degree of cell division between the GFP− T cells from each of the groups was comparable. These data indicate that the co-stimulatory effects of CD8α:MyD88 occur within engineered T cells and do not impact the proliferation of neighboring T cells.
One of the potential advantages to using CD8α:MyD88 is the ability to boost responses with any given TCR. This was confirmed using OT-I TCR transgenic CD8+ T cells specific for an ovalbumin (OVA) peptide presented on MHC I, which also showed increased proliferation to antigen stimulation in a dose-dependent manner (Figure 1C). Likewise, human T cells engineered to express fully human CD8α:MyD88 and the DMF5 TCR, which recognizes the MART-127–35 TAg(12), demonstrated greater proliferation than control T cells following stimulation with MART-1+ Malme-3M melanoma cells (Figure 1D). In addition, DMF5 CD8α:MyD88–expressing T cells proliferated in response to MART-127–35 peptide-pulsed but not unpulsed A375 (HLA-A2+ MART-1−) melanoma cells (Figure 1E), further highlighting that the enhancing effects of CD8α:MyD88 are strictly regulated by tumor antigen expression and are effective in multiple tumor and antigen models.
MyD88 transduces signals by recruiting IL-1 receptor associated kinase-4 (IRAK-4). In the absence of functional IRAK-4, CD8α:MyD88 did not enhance proliferation (Supplementary Fig S1F), demonstrating the dependence of CD8α:MyD88 co-stimulation on the canonical MyD88 pathway. CD8α:MyD88 led to the enhanced and more rapid activation (phosphorylation) of downstream signaling proteins ERK1/2, JNK, and p38 (Figure 1F). Increased ERK1/2 activation is advantageous as ERK2 drives CD8+ T cell proliferation and survival(13). CD8α:MyD88 also enhanced the activation of the proximal TCR protein ZAP-70, a CD3 receptor–associated protein tyrosine kinase (Figure 1F).
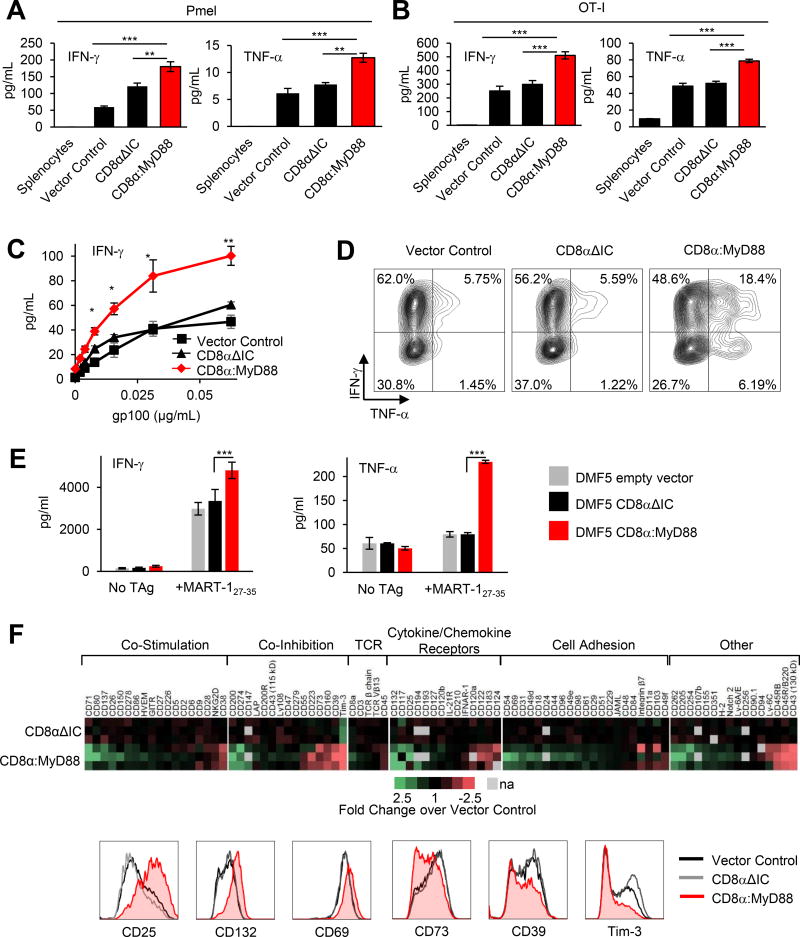
CD8α:MyD88 expression in pmel and OT-I T cells also increased IFN-γ and TNF-α production over CD8αΔIC and empty vector T cells (Figure 2A and 2B) and occurred in an antigen concentration–dependent fashion (Figure 2C). Increased cytokine levels were in part due to the ability of CD8α:MyD88 to increase the frequency of T cells capable of producing both IFN-γ and TNF-α or TNF-α alone (Figure 2D). In addition, increases in IFN-γ–producing CD8α:MyD88 T cells were more apparent at lower TAg levels (Supplementary Fig S2A). MART-127–35–specific human DMF5 CD8+ T cells also showed enhanced IFN-γ and TNF-α production in response to stimulation with TAg (Figure 2E).
Figure 2. CD8α:MyD88 T cells exhibit enhanced cytokine production and alter the expression of co-stimulatory and co-inhibitory molecules in response to tumor antigen.
IFN-γ and TNF-α levels measured by ELISA in supernatants from A) pmel T cells co-cultured with gp10025–33-pulsed splenocytes and B) OT-I T cells with OVA (SIINFEKL)-pulsed splenocytes at 24 hours. C) IFN-γ production by engineered pmel T cells co-cultured with gp10025–33--pulsed splenocytes. D) Intracellular cytokine staining of pmel T cells stimulated with splenocyte pulsed with 5 µg/mL gp10025–33. E) Cytokine production by DMF5 empty vector, DMF5 CD8α or DMF5 CD8α:MyD88 human T cells in response to stimulation with MART-127–35-pulsed PBMCs as measured by ELISA 4 days later. Data from three independent experiments with three replicates per reaction is shown. Values represent mean ± s.e.m.; *** p≤ 0.001, one-way ANOVA; F) Flow cytometry screen of pmel T cells co-cultured for 48 hours with splenocytes pulsed with 0.12 µg/mL gp100. The fold change of the median fluorescence intensity (MFI) of CD8αΔIC and CD8α:MyD88 T cells over control vector T cells from three independent experiments is displayed in a heat map. Gray boxes represent data not available, na. Representative histograms are shown of the expression of most upregulated and downregulated molecules. Values and error bars represent mean ± s.e.m.. *p≤0.05, **p≤0.01, ***p≤0.001 A–C) One-way ANOVA with Tukey’s Multiple Comparison Test at each concentration; n=3 experimental replicates; representative of at least two independent experiments.
CD8α:MyD88 upregulates cytokine receptors and co-stimulatory molecules while reducing molecules associated with T cell exhaustion
To better understand changes induced by CD8α:MyD88, we conducted a flow cytometry-based protein expression array evaluating the levels of 252 surface proteins. Changes in the expression of 89 surface proteins were identified comparing the fold change between vector control and CD8α:MyD88 or CD8α:ΔIC T cells (Figure 2F). CD8α:MyD88 increased expression of IL-2 receptor chains CD25 and CD132, co-stimulatory molecules involved in T cell proliferation and cytolytic function such as CD71, CD26, and CD137 (4-1BB)(14), and various activation-associated adhesion molecules including CD44 and CD69. CD8α:MyD88 also decreased the expression of co-inhibitory molecules including CD160, CD73, Tim3, and CD39 (Figure 2F). Notably, CD39 has been reported to be a marker of terminally exhausted CD8+ T cells and was considerably reduced in CD8α:MyD88 T cells(15). The most differentially-expressed proteins fell into a wide variety of classes, but changes by CD8α:MyD88 universally favored improved T cell function (Figure 2F, bottom panels and Supplementary Fig S2B).
CD8α:MyD88 increases T cell responses to weakly immunogenic B16-F1 melanoma cells
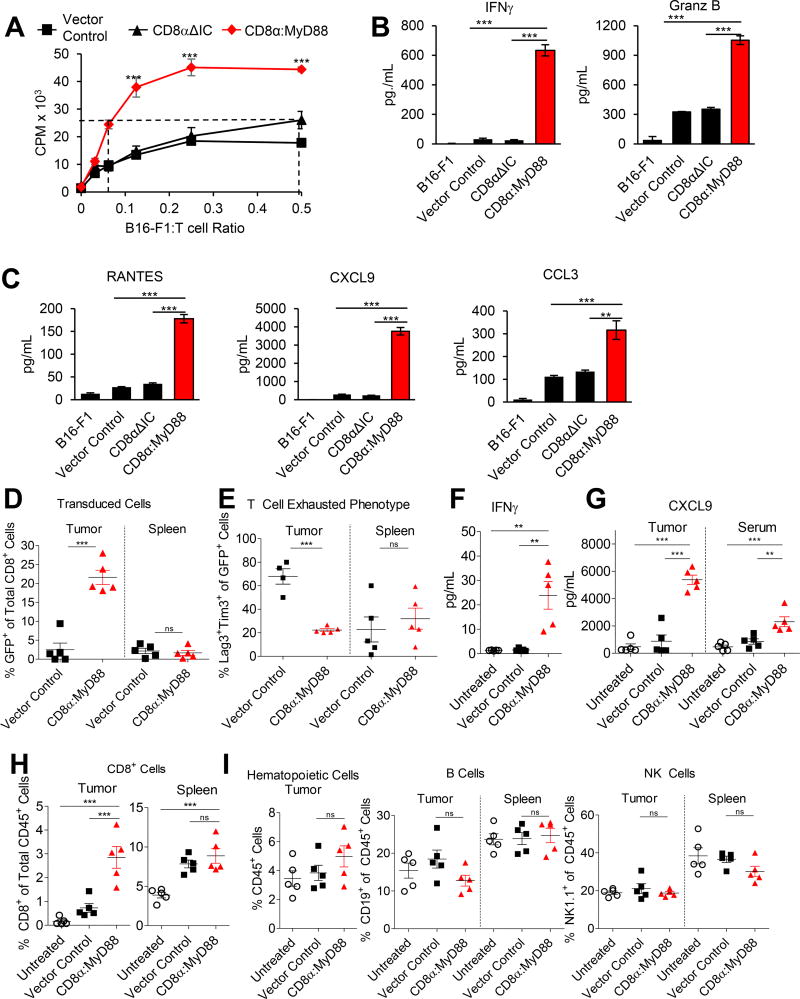
Cancer cells, including the melanoma cell line B16-F1, often carry alterations in antigen-presentation machinery(16,17) that make them poor T cells targets. Despite having nearly undetectable MHC I levels (Supplementary Fig S3A), CD8α:MyD88 substantially enhanced T cell proliferation following co-culture with B16-F1 as compared with control T cells (Figure 3A). Proliferation occurred in a tumor cell number–dependent manner and T cells did not proliferate in the absence of tumor cells. Notably, CD8α:MyD88–expressing T cells required nearly ten-fold fewer melanoma cells to achieve the same level of proliferation as control T cells (Figure 3A, hashed lines). CD8α:MyD88 T cells produced higher levels of IFN-γ and granzyme B than control T cells in response to tumor cells (Figure 3B). Further, CD8α:MyD88 T cells produced IFN-γ at tumor cell numbers at which control T cells could not, emphasizing the propensity of CD8α:MyD88 to lower the TCR activation threshold, a beneficial feature for destroying weakly immunogenic tumor cells. CD8α:MyD88 also induced multiple chemokines important for immune cell recruitment including RANTES, CXCL9, and CCL3 (Figure 3C and Supplementary Fig S3B). In addition, we evaluated changes in the levels of other cytokines/chemokines following stimulation with B16-F1 melanoma cells and found that CD8α:MyD88 increased CXCL1, CXCL2, CCL4, CXCL10, GM-CSF, IL-3 and LIF levels in tumors and serum (Supplementary Fig S4A and S4B).
Figure 3. CD8α:MyD88 T cells display improved responses to melanoma in vitro and in vivo.
A) Proliferation of engineered pmel T cells stimulated with irradiated B16-F1 tumor cells for 72 hours. B–C) ELISA and Luminex analysis of cytokines and chemokines in the supernatant of T cells co-cultured with B16-F1 tumor cells for 48 hours. D–I) Mice bearing established B16-F1 tumors were exposed to a sublethal dose of irradiation (550 rads) followed by transfer of T cells by intraveneous injection one day later. Tissues were harvested and analyzed by flow cytometry one week after T cell transfer. D) The frequency of GFP+ cells in the tumor and spleen. E) The percentage of CD8+GFP+ cells expressing exhaustion markers Tim-3 and Lag-3. F) IFN-γ levels in tumor tissue measured by ELISA of tumor homogenate. G) Protein levels of CXCL9 in the tumor and serum. H) Frequency of CD8+ T cells in the tumor and spleen. I) Frequency of CD45+ cells, CD19+ cells, and NK1.1+ cells in the tumor and spleen. Values and error bars represent mean ± s.e.m. * p ≤ 0.05, ** p ≤ 0.01, *** p≤ 0.001 A–C) One-way ANOVA with Tukey’s Multiple Comparison Test at each concentration; n=3 experimental replicates or n=5 for D–I.
CD8α:MyD88 expression increases the infiltration of T cells into the tumor and reduces T cell exhaustion phenotype
On the basis that MyD88 activation in T cells enhanced proliferation and production of various cytokines/chemokines, we evaluated T cells in the tumor and spleen seven days after adoptive transfer into tumor-bearing mice. CD8α:MyD88–expressing T cells comprised a greater proportion of the CD8+ T cell population within the tumor as compared with vector control T cells (Figure 3D). However, the numbers of control and CD8α:MyD88–expressing T cells were similar in the spleen (Figure 3D). These data suggest that the co-stimulatory effect of CD8α:MyD88 signaling occurs in a manner that depends strictly on TCR engagement in the tumor and does not occur in a non-specific manner in other tissues. Furthermore, in agreement with data demonstrating a reduction of exhaustion markers on CD8α:MyD88 T cells (Figure 2F), there were nearly four times fewer Lag3+Tim3+ CD8α:MyD88–expressing T cells in tumors as compared with control T cells, but similar proportions in spleens (Figure 3E). Lag3+Tim3+ expression on T cells is associated with a reduced ability to produce effector molecules including IFN-γ. Fittingly, CD8α:MyD88 T cells induced higher levels of IFN-γ in tumors, whereas IFN-γ was nearly undetectable in tumors of control–treated or untreated mice (Figure 3F). IFN-γ is known to induce CXCL9, a potent chemoattractant that recruits T cells to the tumor and skews responses towards a Th1 phenotype(18,19). Indeed, CXCL9 was elevated in tumors and serum of CD8α:MyD88 T cells–treated mice (Figure 3G). Accordingly, the percentage of CD8+ T cells was higher in the tumors, but not the spleens, of mice treated with CD8α:MyD88 T cells (Figure 3H). Differences were not detected in the number of CD45+ hematopoietic cells, B cells, or NK cells in the tumors or spleens of mice (Figure 3I).
CD8α:MyD88 T cells alter the tumor microenvironment and induce tumor regression
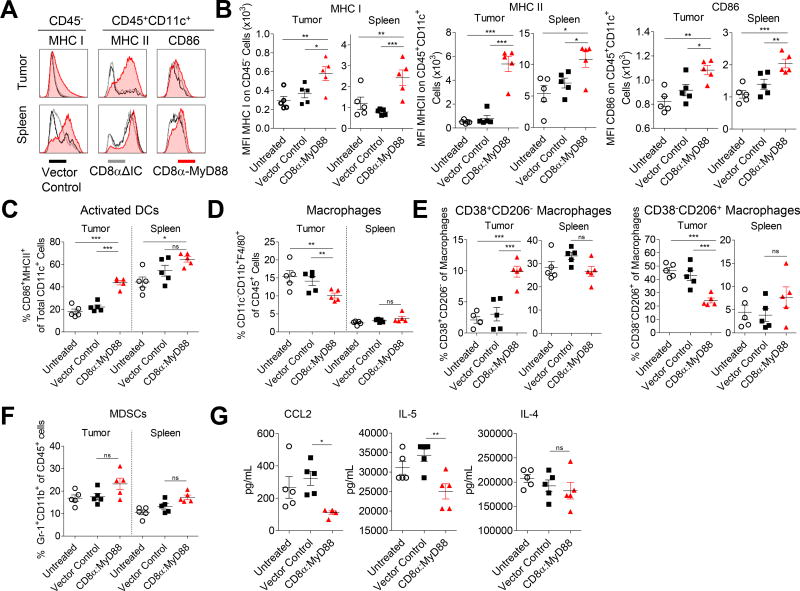
In accordance with increased IFN-γ levels, MHC I expression on non-lymphoid (CD45−) cells was increased in mice treated with CD8α:MyD88 T cells (Figure 4A and 4B). Treatment with CD8α:MyD88 T cells also increased the expression of MHC II and CD86 on dendritic cells (DCs) (Figure 4A and 4B). The percentage of activated DCs co-expressing MHC II and CD86 was also elevated in the tumors, but not spleens, of CD8α:MyD88 T cell–treated mice (Figure 4C).
Figure 4. CD8α:MyD88 T cells alter the tumor microenvironment.
A–G) Mice bearing an established B16-F1 tumor (~50mm2) were exposed to a sublethal dose of irradiation (550 rads) followed by transfer of the CD8α:MyD88 or vector control pmel T cells (6×106) by intraveneous injection one day later. Tissues were harvested and analyzed by flow cytometry one week after T cell transfer. Transduction efficiency of CD8α:MyD88 T cells was 50%. Each data point represented one mouse. A) Median fluorescence intensity (MFI) of MHC I on CD45− cells and MHC II and CD86 on CD11c+ dendritic cells (DCs). B and C) Frequency of CD11c+ DCs co-expressing activation markers CD86 and MHC class II in the tumor and spleen. D) Frequency of macrophages in the tumor and spleen as defined by CD11c−CD11b+F4/80+ cells. E) Frequency of M1 (CD38+CD206−) and M2 (CD38−CD206+) macrophages in the tumor and spleen. F) Frequency of myeloid-derived suppressor cells (MDSCs) in the tumor and spleen as defined by co-expression of Gr1 and CD11b. G) Luminex analysis of CCL2, IL-4, and IL-5 in tumor homogenate.
The percentage of intratumoral CD45+CD11c−CD11b+F4/80+ macrophages was decreased in mice treated with CD8α:MyD88 T cells as compared with control-treated or untreated mice (Figure 4D). Further analysis of the macrophage population revealed an increase in the percentage of macrophages with an M1-like phenotype (CD38+CD206−), typically associated with anti-tumor responses, following administration of CD8α:MyD88 T cells (Figure 4E, left panel). The rise of M1-like macrophages in the tumor was associated with reduced M2-like pro-tumor macrophages (CD38−CD206+) (Figure 4E, right panel). In contrast, the percentage of M1-like and M2-like macrophages remained similar in the spleens of all groups. We did not detect any differences in the number of CD45+Gr1+CD11b+ myeloid-derived suppressor cells (MDSCs) (Figure 4F).
We characterized the levels of cytokines and chemokines associated with myeloid cell migration and differentiation. CCL2, which is involved in monocyte recruitment, Th2 polarization, and tumor progression(20,21), was decreased nearly three-fold in the tumors of CD8α:MyD88 T cell–treated mice (Figure 4G, left panel). Likewise, IL-5, which has been associated with Th2 responses and pro-tumor effects(22), was decreased following treatment with CD8α:MyD88 T cells (Figure 4G, middle panel). IL-4 levels remained similar in all groups (Figure 4G, middle panel). These studies highlight the propensity for CD8α:MyD88 to alter different facets of the TME including the cellular composition, cell surface protein expression, and cytokine profile toward one that can support CD8+ T cell expansion and anti-tumor activity.
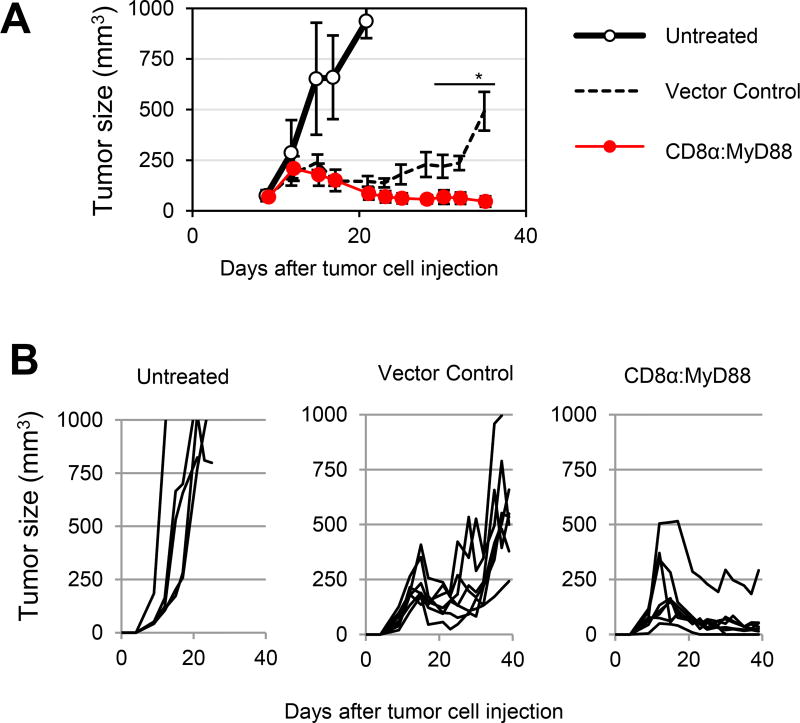
Given these findings, we assessed the anti-tumor activity of CD8α:MyD88–expressing T cells in tumor-bearing mice. Mice harboring established B16-F1 tumors (~30 mm2) were treated with control pmel or CD8α:MyD88–expressing pmel T cells. Two injections of CD8α:MyD88 pmel T cells were sufficient to delay tumor growth as compared with mice treated with control T cells or untreated mice. Importantly, while control T cells temporarily delayed or reversed tumor growth, CD8α:MyD88 T cells induced and maintained tumor regression of large established tumors (Figure 5A and 5B). All mice treated with CD8α:MyD88 T cells exhibited tumor regression and 50% (4 of 8) of mice cleared tumor. Notably, these anti-tumor responses were accomplished in the absence of additional T cell support such as TAg vaccination, cytokines, or checkpoint blockade, emphasizing the potent anti-tumor effects of activating MyD88 signaling in T cells. A single injection of CD8α:MyD88 T cells moderately delayed tumor growth as compared with mice treated with vector control T cells or untreated mice (Supplementary Fig 5A). Furthermore, we observed tumor control, and tumor regression in some animals lasting up to one week after cell transfer (Supplementary Fig S5B). In sharp contrast, tumor growth rates and mouse survival of the control T cell-treated group were similar with those of the untreated group (Supplementary Fig S5A–S5C), indicating that control tumor-specific T cells were ineffective at the given dose, exemplifying the potent anti-tumor effects of CD8α:MyD88 T cells. We also evaluated whether check point blockade using anti-PD-L1 antibodies or antibodies to stimulate 4-1BB signaling would potentiate antitumor T cell responses. We observed that while blocking PD-L1 had a negligible effect stimulating 4-1BB increased the antitumor activity of control T cells but it did not improve CD8α:MyD88 T cell responses (Supplementary Fig S6A and S6B). These data suggest that the co-stimulatory signals activated by 4-1BB signaling might be redundant to those activated by CD8α:MyD88.
Figure 5. CD8α:MyD88 T cells induce tumor regression of established melanoma tumors.
A and B) Mice bearing a established B16-F1 tumors were exposed to a sublethal dose of irradiation (550 rads) followed by transfer of 6×106 pmel T cells engineered to express CD8α:MyD88 or vector control-GFP by intravenous injection one day and eight days later. Average tumor volume as well as tumor volumes for individual mice are shown. Values and error bars represent mean ± s.e.m. * p ≤ 0.05 starting on day 28 and onward. tumor growth was analyzed by a mixed model repeated measures with AR(1) covariance structure, followed by Sidak-adjusted comparisons at each time.
Discussion
Through these studies, we present a novel strategy in which expressing the fusion protein CD8α:MyD88 activated MyD88 signaling in cytotoxic CD8+ T cells in a TLR–independent but TCR–dependent fashion, resulting in enhanced responses to suboptimal TAg levels and in an ability to reshape the TME toward one favoring anti-tumor immune responses. Notably, the enhancement of TCR responses by MyD88 was strictly dependent on TCR stimulation with the tumor antigen. To the best of our knowledge, this is the first strategy designed to boost TCR responses against weakly immunogenic or lowly expressed antigens without the need to alter the TCR sequence or identify stronger MHC I epitopes.
The use of CD8α:MyD88 offers several unique strategies to control tumor growth. First, the costimulatory effects of CD8α:MyD88 are not limited to a single tumor antigen nor are they MHC-restricted, as CD8α interacts with the α3 domain on all human MHC I complexes. Therefore, immunotherapy using CD8α:MyD88–expressing T cells could be utilized as a method to enhance TCR responses against a variety of tumor antigens and thus could be applied to patients with various malignancies regardless of HLA haplotype or tumor-antigen specificity. Furthermore, ongoing studies in our laboratory suggest that CD4:MyD88 extends CD4 T cells a similar costimulatory effect and could be used as a strategy to potentiate responses against tumor antigens expressed on MHC II molecules.
One limitation to T cell-based therapies is low TCR affinity and/or avidity(3,4). T cells with high affinity towards self antigens, such as tumor antigens, are eliminated in the thymus during development, leaving behind low-affinity or low avidity tumor-reactive T cells that recognize subdominant epitopes and weakly immunogenic antigens including neoantigens. Therefore, a second benefit of expressing CD8α:MyD88 is that it lowers the TCR activation threshold, providing the potential to expand both high and low affinity TCR T cells while preserving endogenous TCR signaling. The ability to enhance weak TCR responses is especially relevant when redirecting T cells towards neoantigens which can be expressed at low levels and/or have lower affinity than non-mutated self antigens. Amplifying T cell responses towards neoantigens are important for developing effective cancer therapies as they offer the ability to target bona fide tumor-specific antigens. Furthermore, it is estimated that up to 20% of cancers are associated with oncogenic viruses. Therefore, another attractive strategy to specifically target cancer while eliminating off-target effects are to develop CD8α:MyD88 T cells reactive towards viral antigens expressed by tumor cells.
Another important and potentially clinically valuable use of CD8α:MyD88 is its ability to enhance the function of T cells engineered to express tumor-reactive TCRs such as those targeting MART-1 or Ny-Eso(3,4). For example, our data demonstrating that CD8α:MyD88 expression augmented the responses of DMF5 TCR–engineered T cells (Figure 1D and 1E) highlights the opportunity to use CD8α:MyD88 to increase responses to a variety of engineered TCRs currently in clinical testing(3,4). Furthermore, the ability for T cells to recognize tumor cells expressing suboptimal MHC levels (in addition to low TAg levels) is an important feature as tumor cells can downregulate MHC I expression or antigen expression to evade detection by T cells. A third advantage offered by CD8α:MyD88 is the ability to activate MyD88 signaling specifically in transduced T cells. Tumor-specific cells can be isolated and activated against the tumor prior to introduction of the CD8α:MyD88 to limit immune-related adverse events. This is an important quality as it indicates that the boosting effects of CD8α:MyD88 occur only in gene-modified T cells and do not non-specifically co-stimulate non-transduced T cells such as auto-reactive T cells. Importantly, the ability to activate MyD88 signaling in engineered T cells would eliminate the need to provide systemic TLR agonists, which could have the undesirable effect of promoting tumor growth by stimulating TLRs on cancer cells(10).
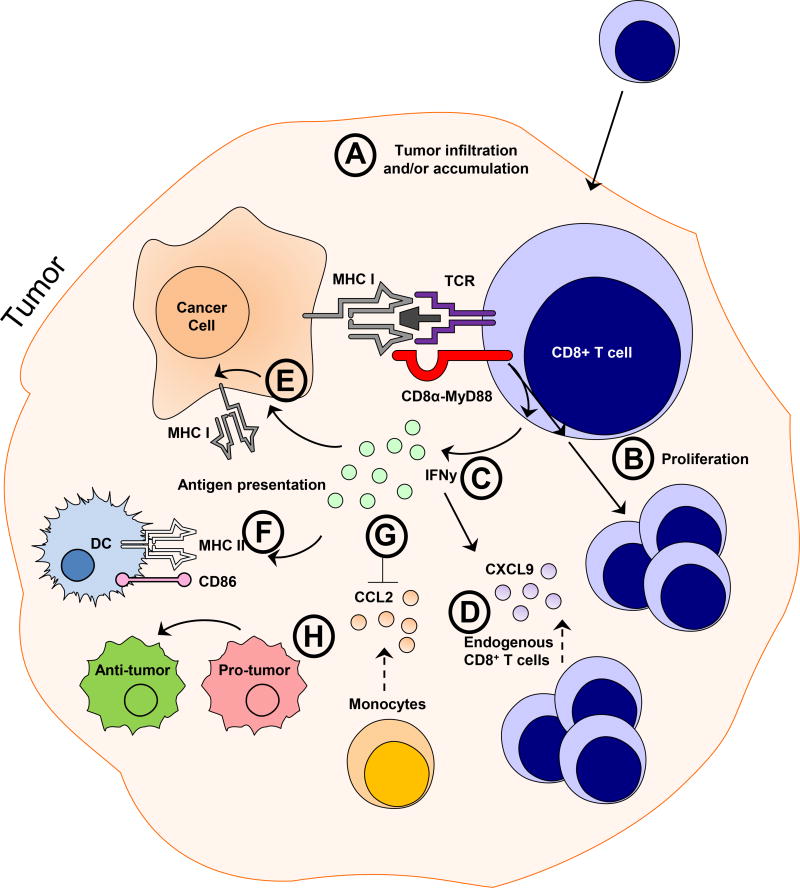
Fourth, CD8α:MyD88–expressing T cells exhibited a reduced ability to become exhausted in vitro and in vivo. This was exemplified by reduced frequencies of Lag3+Tim3+CD8+ T cells and an ability for CD8α:MyD88–expressing T cells to produce higher amounts of IFN-γ (Fig 3B and 3F) and/or granzyme B (Fig 3B) than control T cells which, did not produce detectable IFN-γ levels under those stimulation conditions. At present, we are unsure as to the molecular mechanisms underpinning this important observation and is an important part of our ongoing investigations. In addition to demonstrating a reduced tendency to become exhausted, CD8α:MyD88–expressing T cells altered various facets of the TME toward one that favors anti-tumor immune responses. These changes included increased T cell infiltrates, an altered cytokine/chemokine milieu, increased expression of stimulatory molecules on DCs, and a skewing of the macrophage population from a pro-tumor to an anti-tumor population. The proposed mechanisms by which CD8α:MyD88 T cells enhance anti-tumor responses are provided in Figure 6.
Figure 6. Proposed cellular processes impacted by CD8α:MyD88-expressing T cell in the tumor microenvironment.
A) We observed an increased frequency of CD8α:MyD88 T cells in the tumor, indicating enhanced infiltration, expansion, and/or survival of CD8α:MyD88–transduced T cells. B) CD8α:MyD88 T cells exhibit enhanced proliferation in response to tumor antigen and tumor cells in culture; an effect that we speculate might be occurring in vivo. C) CD8α:MyD88 T cells produce increased levels of IFN-γ. D) Increased levels of the IFN-γ can lead to induction of the T cell chemokine CXCL9 which were detected in the tumor and could contribute to the enhanced CD8+ T cell numbers observed in the tumor. E) IFN-γ has the ability to influence the antigen presentation machinery and is reflected by an enhanced expression of MHC I on non-hematopoietic (CD45−) cells, presumably tumor cells, and F) increased MHC II and CD86 expression on DCs. G) There were decreased levels of CCL2 in the tumor, which is a monocyte chemoattractant protein. Monocytes are the precursors of macrophages, and accordingly, an overall decreased number of macrophages were detected in the tumor of CD8α:MyD88 T cell-treated mice. H) The macrophages that were present were skewed from a pro-tumorigenic phenotype towards a phenotype favoring anti-tumor immunity.
In summary, the use of CD8α:MyD88 represents a universal approach to potentiate T cell responses in cancer patients, regardless of the HLA type. Moreover, that the TCR enhancing effects of CD8α:MyD88 occur strictly upon antigen recognition is innovative and highly suitable for self-regulating T cell responses.
Supplementary Material
Statement of Significance.
Findings highlight a unique method to lower the T cell receptor recognition threshold to any antigen and the ability to re-shape the tumor environment to one that favors antitumor immunity independent of HLA type.
Acknowledgments
We thank S.Vogel for providing us IRAK-4 kinase dead mice.
Additional information. This research was supported by the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, National Cancer Institute R01CA140917, P30CA134274, VA Merit Award BX002142 and National Institute of Allergy and Infectious Diseases 2T32AI007540-17.
Footnotes
Conflict of interest. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Yee C. Adoptive T-Cell Therapy for Cancer: Boutique Therapy or Treatment Modality? Clin Cancer Res. 2013;19:4550–2. doi: 10.1158/1078-0432.CCR-13-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abad JD, Wrzensinski C, Overwijk W, De Witte MA, Jorritsma A, Hsu C, et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. J Immunother. 2008;31:1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell. 2015;161:205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng D, Zheng L, Srivastava R, Velasco-Gonzalez C, Riker A, Markovic SN, et al. Amplifying TLR-MyD88 signals within tumor-specific T cells enhances antitumor activity to suboptimal levels of weakly immunogenic tumor antigens. Cancer Res. 2010;70:7442–54. doi: 10.1158/0008-5472.CAN-10-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–18. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–63. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng D, Kaczanowska S, Tsai A, Younger K, Ochoa A, Rapoport AP, et al. TLR5 ligand-secreting T cells reshape the tumor microenvironment and enhance antitumor activity. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-14-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–59. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poltorak M, Arndt B, Kowtharapu BS, Reddycherla AV, Witte V, Lindquist JA, et al. TCR activation kinetics and feedback regulation in primary human T cells. Cell Commun Signal. 2013;11 doi: 10.1186/1478-811X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph AM, Srivastava R, Zabaleta J, Davila E. Cross-talk between 4-1BB and TLR1–TLR2 signaling in CD8+ T cells regulate TLR2's costimulatory effects. Cancer Immunol Res. 2016 doi: 10.1158/2326-6066.CIR-15-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, et al. CD39 Expression Identifies Terminally Exhausted CD8(+) T Cells. Plos Pathog. 2015;11 doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Research. 2001;61:1095–9. [PubMed] [Google Scholar]
- 17.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–S98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Hu JM, Bernatchez C, Xia XQ, Xu ZH, Hwu P, Li SL. CXCL9, CXCL10 and IFN gamma favor the accumulation of infused T cells in tumors following IL-12 plus doxorubicin treatment. J Immunol. 2016;196 [Google Scholar]
- 19.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 20.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 21.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Cantor H. CD4 T-cell Subsets and Tumor Immunity: The Helpful and the Not-so-Helpful. Cancer Immunol Res. 2014;2:91–8. doi: 10.1158/2326-6066.CIR-13-0216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.