Abstract
Background
Flaxseed orbitides are homodetic plant cyclic peptides arising from ribosomal synthesis and post-translation modification (N to C cyclization), and lacking cysteine double bonds (Nat Prod Rep 30:108-160, 2013). Screening for orbitide composition was conducted on the flax core collection (FCC) grown at both Saskatoon, Saskatchewan and Morden, Manitoba over three growing seasons (2009-2011). Two flax (Linum usitatissimum L.) accessions ‘Hollandia’ (CN 98056) and ‘Z 11637’ (CN 98150) produce neither [1−9-NαC]-linusorb B2 (3) nor [1−9-NαC]-linusorb B3 (1). Mass spectrometry was used to identify novel compounds and elucidate their structure. NMR spectroscopy was used to corroborate structural information.
Results
Experimental findings indicated that these accessions produce a novel orbitide, identified in three oxidation states having quasimolecular ion peaks at m/z 1072.6 (18), 1088.6 (19), and 1104.6 (20) [M + H]+ corresponding to molecular formulae C57H86N9O9S, C57H86N9O10S, and C57H86N9O11S, respectively. The structure of 19 was confirmed unequivocally as [1−9-NαC]-OLIPPFFLI. PCR amplification and sequencing of the gene coding for 18, using primers developed for 3 and 1, identified the putative linear precursor protein of 18 as being comprised of the first three amino acid residues of 3 (MLI), four conserved amino acid residues of 3 and/or 1 (PPFF), and the last two residues of 1 (LI).
Conclusion
Comparison of gene sequencing data revealed that a 117 base pair deletion had occurred that resulted in truncation of both 3 and 1 to produce a sequence encoding for the novel orbitide precursor of 18. This observation suggests that repeat units of flax orbitide genes are conserved and suggests a novel mechanism for evolution of orbitide gene diversity. Orbitides 19 and 20 contain MetO and MetO2, respectively, and are not directly encoded, but are products of post-translation modification of Met present in 18 ([1−9-NαC]-MLIPPFFLI).
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1303-8) contains supplementary material, which is available to authorized users.
Keywords: Linum usitatissimum L., Orbitides, Cyclolinopeptides, Plant genetic resources, Mass spectrometry, Sequencing
Background
Orbitides can be found in plant families and genera other than Linaceae and Linum, including Annonaceae (Panax), Caryophyllaceae (Saponaria), Euphorbiaceae (Jatropha), and Rutaceae (Citrus) [1–3]. Although their biological function is unknown, they are thought to serve an important role due to their abundance and conserved nature. Orbitides, like other macrocylic peptides, are resistant to protease digestion due to covalently closed ends which may be critical for biological activity [4]. Potential biological activities of flax orbitides and their analogs have been investigated over the past two decades [5–16] and the results summarized in a review [17]. To date, eleven parent flaxseed orbitides (1, 2, 5, 7, 10, 14, 21, 23-25 and 27) have been discovered and the corresponding DNA sequences encoding precursor proteins have been identified [18–20] from the flax genome database [21] (Fig. 1, Additional file 1: Table S1). The remaining flax orbitides reported in the literature (3, 4, 6, 8, 9, 11-13, 15-17, 22, 26 and 28) result from methionine post-translational oxidation.
Fig. 1.
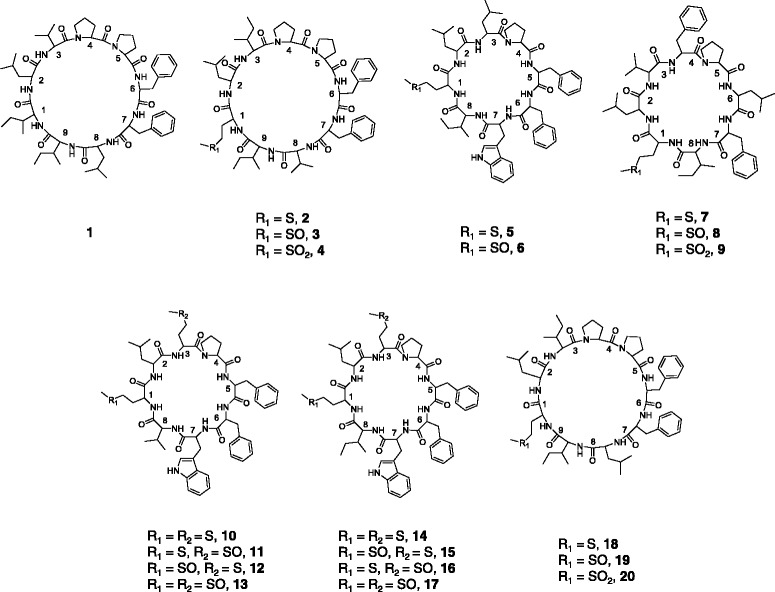
Structures of known and novel flaxseed orbitides observed in this study
All linear proteins containing orbitide domains are encoded by genes. These linear precursor proteins share structural similarities. In L. usitatissimum, orbitide domains are flanked predominantly by “DD”, “DG” or “SD” residues at the amino termini, and “FGK” or “VGK” residues at the carboxy termini. Additionally, some of these orbitide domains are present as tandem repeats in the proprotein carboxy terminal. The orbitide precursor protein of 5, 10, and 14 has been identified in L. usitatissimum chromosome Lu14 (National Center for Biotechnology Information (NCBI) GenBank™ CP027624.1 residues 17099153 to 17099545 [21]; as these orbitides are coded by the same gene it is hereafter named LINUSORB A, following our proposed systematic nomenclature [22]) for example. The function of the first exon of this gene is unknown, while the second exon encodes a linear precursor protein with five orbitide domains arranged in repeats in the order 14, 14, 5, 14, 10. The conserved nature of sequences flanking the orbitide coding region and occurrence of repeat units has allowed the identification of several other related putative orbitides in Citrus and Saponaria [2]. Additional gene sequences that appear to code for flax orbitides have been identified based on their conserved tandem repeat structure. However, detection of the actual orbitides remains to be established. It is not known in which tissue the gene may be expressed, or if the apparent genes are expressed at all.
Results
Recently, orbitide compositions of the flax core collection (FCC) harvested in 2009 were determined using high-performance liquid chromatography with reversed-phase monolithic HPLC columns and diode array detection (HPLC-DAD) [23]. The FCC is a subset of the Canadian flax world collection curated by Plant Gene Resources of Canada (PGRC) comprising 381 accessions [24]. The FCC was assembled to represent the phenotypic diversity and genetic variability of approximately 3500 flax accessions from around the world held in the Canadian collection. The FCC currently includes 407 accessions [25]. An additional ten cultivars were included in the study of orbitides in the FCC, namely ‘CDC Sorrel’, ‘CDC Mons’, ‘Lightning’, ‘Prairie Blue’, ‘Prairie Grande’, ‘Prairie Thunder’ and ‘Shape’ along with the checks ‘CDC Bethune’, ‘Hanley’, and ‘Macbeth’. Screening was limited to orbitides 1, 3, and 8 (encoded by the gene contained within L. usitatissimum chromosome Lu11; National Center for Biotechnology Information (NCBI) GenBank™ Sequence ID: CP027621.1 residues 2516493 to 2517107 [21]; Hereafter, LINUSORB B following our proposed systematic nomenclature [22]) and orbitides 6, 13, and 17 (encoded by LINUSORB A). These orbitides were chosen because of their high expression and known chromatographic separation methods. ‘Hollandia’ (CN 98056) and ‘Z 11637’ (CN 98150), both originated from The Netherlands, and were identified as accessions expressing the lowest total orbitide content and the lowest apparent concentration ratio of orbitides [1, 3, 8] to [6, 13, 17]. Closer evaluation revealed that the differences were attributed to a lack of 1 and 3, while the apparent concentration of 8 was comparable to the mean of the core collection. Expression of 6, 13 and 17 in these accessions was not significantly different from the rest of the core collection.
Orbitide compositions of oxidized extracts from accessions ‘Hollandia’ and ‘Z 11637’ were compared in detail with orbitides of ‘CDC Bethune’. The HPLC-DAD chromatograms displayed elution peaks at approximately 2.5, 2.7, 2.8, 3.3, 3.6, and 4.3 min corresponding to 13, 17, 3, 8, 6, and 1, respectively (Fig. 2a). Orbitides 1 and 3 were either not detected or were detected at low concentrations in ‘Hollandia’ and ‘Z 11637’, while expression of 8 in these accessions was comparable to that of ‘CDC Bethune’. Hence, orbitide concentrations of 1:3:8 observed in ‘Hollandia’ and ‘Z 11637’ deviated from the 1:1:1 ratio expressed in ‘CDC Bethune’. These findings were consistent regardless of harvest location (Saskatoon, SK or Morden, MB) and year (2009, 2010, 2011) and corroborate observations made by Burnett et al. [23] (Table 1). Low level detection of 1 and 3 in ‘Hollandia’ and ‘Z 11637’ resulted from flaxseed samples that were contaminated with one or more other accessions within the FCC. Such contamination is not uncommon in field plot studies. In 2009, mean total orbitide contents of ‘Hollandia’, ‘Z 11637’ and ‘CDC Bethune’ were 139.2, 106.9 (Table 1) and 196.9 mAU [23]. Similar total orbitide concentrations in ‘Hollandia’ and ‘Z 11637’ were observed in 2010 and 2011 with mean 2010 values being lower probably due to poor seed quality from the Saskatoon plots that year. Plant maturity at harvest was atypical because of a short growing season (from delayed seeding) with excess moisture. Overall, the sum of apparent orbitide concentrations of 1, 3, and 8 (encoded by LINUSORB B), hereafter refered to as [1,3,8], were lower than those of 6, 13, and 17 (encoded by LINUSORB A), hereafter refered to as [6,13,17]. The mean apparent concentration ratio of [1,3,8] to [6,13,17] observed in accessions ‘Hollandia’ and ‘Z 11637’ over three growing seasons was 0.7 ± 0.2, while that observed in ‘CDC Bethune’ in 2009 was 1.9 ± 0.1 [23]. These ratios were consistent regardless of harvest location and reflected the apparent low total orbitide expression of these accessions.
Fig. 2.
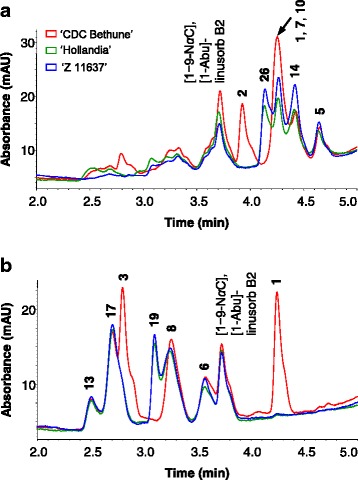
HPLC-DAD chromatograms of flaxseed (L. usitatissimum) ‘CDC Bethune’, ‘Hollandia’, and ‘Z 11637’ orbitide extracts. a unoxidized and b oxidized
Table 1.
Average orbitide distribution in oxidized aqueous methanolic extracts of ‘Hollandia’ and ‘Z 11637’ flaxseed accessions
| Orbitide (mAU) | ‘Hollandia’ | ‘Z 11637’ | ||||
|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2010 | 2011 | ||
| 1 | 2.0 ± 2.4 | 7.9 ± 9.2 | 10.7 ± 4.6 | 0.0 ± 0.0 | 0.9 ± 1.2 | 2.2 ± 2.5 |
| 3 | 1.7 ± 1.9 | 8.1 ± 9.5 | 10.1 ± 4.8 | 0.0 ± 0.0 | 1.3 ± 1.5 | 1.4 ± 1.6 |
| 6 | 19.1 ± 4.4 | 14.1 ± 0.6 | 17.0 ± 1.8 | 15.5 ± 3.2 | 13.7 ± 5.0 | 17.7 ± 1.9 |
| 8 | 48.2 ± 22.2 | 38.1 ± 1.3 | 39.8 ± 2.3 | 36.2 ± 5.8 | 34.3 ± 7.2 | 35.3 ± 2.8 |
| 13 | 16.9 ± 3.1 | 13.5 ± 1.2 | 15.2 ± 0.9 | 13.5 ± 2.2 | 9.8 ± 4.3 | 13.9 ± 1.0 |
| 17 | 51.2 ± 10.6 | 38.5 ± 2.8 | 43.8 ± 3.2 | 41.7 ± 6.3 | 31.9 ± 13.1 | 44.4 ± 2.3 |
| 19 | 38.6 ± 7.3 | 29.8 ± 2.5 | 28.3 ± 6.9 | 35.4 ± 5.3 | 29.4 ± 6.2 | 31.9 ± 2.7 |
| Total orbitide | 139.2 ± 33.4 | 121.4 ± 21.8 | 135.0 ± 6.5 | 106.9 ± 17.4 | 91.9 ± 26.9 | 114.9 ± 5.8 |
| [1,3,8]/[6,13,17] | 0.6 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.1 | 0.5 ± 0.0 | 0.7 ± 0.2 | 0.5 ± 0.0 |
Data are presented as mean values (peak area in mAU) from replicate analyses from named flaxseed accessions grown in Saskatoon, SK and Morden, MB plots; n = 4
An unidentified compound, at retention time 3.1 min, (18) was detected in HPLC-DAD chromatograms of oxidized orbitide extracts from ‘Hollandia’ and ‘Z 11637’, suggesting the presence of a novel compound (Fig. 2a). Orbitide extracts from these two accessions were analyzed without addition of hydrogen peroxide to determine whether the new peak corresponded to an oxidation product or to a compound unaffected by oxidation. The HPLC-DAD analyses of unoxidized extracts of ‘CDC Bethune’ showed the presence of 2, 14 and 5 eluting at 3.9, 4.4 and 4.7 min, respectively, along with the co-elution of 1, 7 and 10 at 4.3 min (confirmed by HPLC-ESI-MS and HPLC-ESI-MS/MS analyses) (Fig. 2b). Stefanowicz [26], Okinyo-Owiti et al. [20], and Burnett et al. [23] have reported similar observations for orbitide content of fresh flaxseed extracts. Unoxidized flaxseed extracts from ‘Hollandia’ and ‘Z 11637’ contained known orbitides 7, 10, 14, and 15 as found in ‘CDC Bethune’. However, in contrast to “CDC Bethune’, (i) no peak was observed for 2; (ii) the absence of 1 was confirmed by mass spectrometry; and (iii) an additional peak at retention time 4.2 min (18) was observed in extracts from ‘Hollandia’ and ‘Z 11637′ (Fig. 2b). The lack of 1 and 3 in oxidized extracts was supported by the absence of 1 and 2 in unoxidized extracts as 1 is unaffected by oxidation, while 3 is derived from post-translational oxidation of the methionine (Met) residue in 2 to methionine S-oxide (MetO). HPLC-DAD analyses of ‘Hollandia’ and ‘Z 11637′ grown at two locations over three years confirmed the consistent presence of the new peak at an apparent concentration of 32.1 ± 5.0 mAU. Signals in both oxidized and unoxidized extracts are consistent with a novel orbitide (18) and this orbitide is prone to oxidation.
Oxidized and unoxidized extracts of ‘Hollandia’, ‘Z 11637’ and ‘CDC Bethune’ were subjected to extensive HPLC-ESI-MS and HPLC-ESI-MS/MS analyses. The unidentified peak (18) occurring in unoxidized extracts possessed a quasimolecular ion at m/z 1072.6246 ([M + H]+, tR = 7.4 min). Tandem mass spectrometry displayed ring opening at the amide nitrogen of proline followed by sequential loss of neutral single amino acid residues typical of b fragmentation. The proposed mass fragmentation pattern of 18 is: Leu/Ile-Leu/Ile-Met-Leu/Ile-Leu/Ile-Phe to m/z 342.18 ([M + H]+ which is attributed to the tripeptide fragment [Phe-Pro-Pro] (Fig. 3a). The HPLC-ESI-MS spectra of oxidized extracts from the anomalous accessions showed novel orbitide 19 (m/z 1088.6211 ([M + H]+, tR = 3.7 min), in addition to low apparent concentration of 20 (m/z 1104.6150 ([M + H]+, tR = 5.7 min). Both 19 and 20 showed similar fragmentation patterns to that of 18, with the exception of the third, methionine-containing, residue (Figs. 3b and c). The third cleavage residue observed in these spectra corresponded to a loss of 147.04 Da and 163.03 Da in 19 and 20, respectively. The difference (15.9949 Da) between 18 and 19, and between 19 and 20, suggests that 19 and 20 were derived from oxidative modification of Met present in 18 to MetO and MetO2, respectively. Oxidation of the Met residue to MetO and subsequently to MetO2 arose from addition of H2O2 to the extracts. Based on these assumptions, the molecular weights and tandem mass spectra, the proposed mass fragmentation pattern of 19 is Leu/Ile-Leu/Ile-MetO-Leu/Ile-Leu/Ile-Phe-[Phe-Pro-Pro], while that of 20 is Leu/Ile-Leu/Ile-MetO2-Leu/Ile-Leu/Ile-Phe-[Phe-Pro-Pro].
Fig. 3.
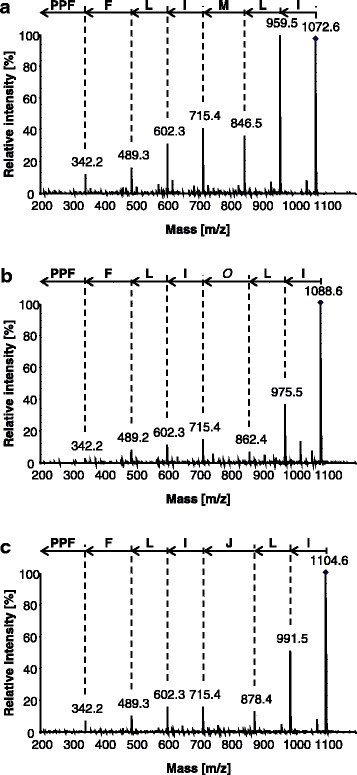
Product ion spectra of ions derived from 18, 19, and 20. a [1−9-NαC]-MLIPPFFLI (18, m/z 1072.6246) at tR 7.4 min, b [1−9-NαC]-OLIPPFFLI (19, m/z 1088.6211) at tR 3.7 min, and c [1−9-NαC]-JLIPPFFLI (20, m/z 1104.6150) at tR 5.7 min
Orbitide 19 was isolated in sufficient quantities for further characterization via nuclear magnetic resonance (NMR). NMR spectra were conducted in deuterated chloroform and recorded at 308 K (Additional file 2: Figure S1, Additional file 3: Figure S2, Additional file 4: Figure S3, Additional file 5: Figure S4, Additional file 6: Figure S5, Additional file 7: Figure S6, Additional file 8: Figure S7 and Additional file 9: Figure S8). Sequential assignment of α, β, γ and δ protons were performed using 1H-1H correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY) and Nuclear Overhauser effect spectroscopy (NOESY) experiments. In addition, heteronuclear multiple quantum correlation (HMQC) was performed to assign carbon atoms attached to protons. Resonances arising from protons attached to heteroatoms were assigned using 1H NMR data and their coupling to amide protons and carbonyl carbons employed NOE and heteronuclear multiple-bond correlation (HMBC) spectroscopy, all of which collectively confirmed the orbitide amino acid sequence. The NMR spectral data of 19 (Fig. 4, Table 2) showed remarkable similarities to that of 3 which is not surprising as the calculated molecular weight of 19 (m/z 1088.6213 ([M + H]+, C57H86N9O10S) is 14.0157 Da higher than that of 3 (m/z 1074.6056 ([M + H]+, C56H84N9O10S, [1−9-NαC]-OLIPPFFVI) (Additional file 1: Table S1), thereby suggesting the presence of an additional methylene moiety [9]. 1H NMR data of 19 displayed most resonances also observed in that of 3. However, the 1H NMR spectrum of 19, unlike 3, displayed two sharp singlets at δ 2.54 and 2.56 ppm and carbon signals at δ 38.17 and 38.73 ppm that correspond to methyl of a MetO moiety. Note that 3 in deuterated dimethyl sulfoxide exhibited only one MetO singlet at δ 2.45 ppm and a carbon signal at δ 37.6 ppm [9]. These observations suggest that 19 existed in two stable conformers under the NMR experimental conditions employed and that the MetO exists in R or S diastereomeric forms. The 1H NMR revealed seven amide proton signals (δ 6.74, 6.95, 7.45, 7.48, 7.65 (2), and 7.76 ppm) (Table 2). The 13C NMR spectrum showed two sets of signals for each carbon atom corresponding to two conformers; signals from the major conformer are shown in Table 2. Nine amide carbonyl signals (δ 170.69, 170.77, 171.14, 171.40, 171.50, 172.42, 172.74, 172.98, and 173.36 ppm) were observed in 13C NMR indicating that 19 is a nonapeptide. NMR analyses (Table 2) showed the presence of Leu in 19 instead of Val as detected in 3 [9]. The NMR spectroscopic data clearly distinguishes between Leu and Ile residues and further corroborated mass spectral analyses thus confirming the structure of 19 as: Ile-Leu-MetO-Ile-Leu-Phe-Phe-Pro-Pro.
Fig. 4.
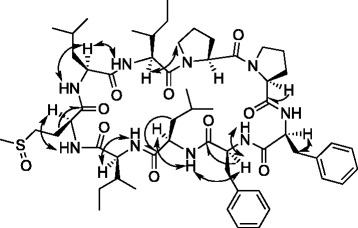
Structure of 19 ([1−9-NαC]-OLIPPFFLI). Double arrows show selected NOESY correlations, while half arrows show selected HMBC correlations
Table 2.
1H and 13C NMR assignments for [1−9-NαC]-OLIPPFFLI (19)
| Assignment | δH | δC | Assignment | δH | δC | ||
|---|---|---|---|---|---|---|---|
| MetO1 | Phe6 | ||||||
| α | 4.78 (1H, m) | 52.11 | α | 4.78 (1H, m) | 54.78 | ||
| β | 2.25 (2H, m) | 29.93 | β | 2.95 (2H, m) | 36.01 | ||
| γ | 3.02 (2H, m) | 50.09 | γ | 136.61 | |||
| ε Me | 2.54 (3H, s) | 38.17 | δ | 129.71 | |||
| NH | 7.76 (1H, s) | ε | 129.02 | ||||
| C=O | 173.36 | ζ | 126.84 | ||||
| Leu2 | NH | 7.45 (1H, m) | |||||
| α | 4.51 (1H, m) | 55.82 | C=O | 172.74 | |||
| β | 2.25 (1H, m) | 40.38 | Phe7 | ||||
| 1.97 (1H, m) | α | 4.87 (1H, m) | 54.12 | ||||
| γ | 1.62 (1H, m) | 28.55 | β | 3.08 (2H, m) | 34.59 | ||
| δ Me | 0.93 (3H, m) | 23.21 | γ | 136.95 | |||
| 0.88 (3H, m) | 21.56 | δ | 129.97 | ||||
| NH | 6.95 (1H, m) | ε | 129.05 | ||||
| C=O | 170.77 | ζ | 127.24 | ||||
| Ile3 | NH | 7.65 (1H, m) | |||||
| α | 4.58 (1H, m) | 56.15 | C=O | 171.14 | |||
| β | 1.94 (1H, m) | 36.89 | Leu8 | ||||
| γ | 1.69 (2H, m) | 24.34 | α | 4.08 (1H, m) | 54.98 | ||
| γ Me | 1.07 (3H, m) | 15.65 | β | 2.15 (1H, m) | 38.73 | ||
| δ Me | 0.93 (3H, m) | 11.31 | 1.91 (1H, m) | ||||
| NH | 8.06 (1H, br.s) | γ | 1.62 (1H, m) | 25.03 | |||
| C=O | 171.40 | δ Me | 0.97 (3H, m) | 23.34 | |||
| Pro4 | 0.94 (3H, m) | 21.74 | |||||
| α | 3.98 (1H, m) | 59.03 | NH | 7.65 (1H, br.s) | |||
| β | 2.28 (1H, m) | 28.78 | C=O | 171.50 | |||
| 2.06 (1H, m) | |||||||
| γ | 1.94 (1H, m) | 24.68 | |||||
| 1.69 (1H, m) | Ile9 | ||||||
| δ | 3.94 (1H, m) | 48.06 | α | 4.34 (1H, m) | 58.71 | ||
| 3.69 (1H, m) | β | 1.99 (1H, m) | 36.73 | ||||
| C=O | 170.69 | γ | 1.51 (2H, m) | 25.37 | |||
| Pro5 | γ Me | 0.94 (3H, m) | 16.02 | ||||
| α | 4.13 (1H, m) | 61.28 | δ Me | 0.88 (3H, m) | 11.78 | ||
| β | 1.94 (2H, m) | 32.11 | NH | 6.74 (1H, m) | |||
| γ | 1.51 (1H, m) | 21.99 | C=O | 172.42 | |||
| 1.14 (1H, m) | |||||||
| δ | 3.30 (2H, m) | 47.39 | |||||
| C=O | 172.98 | ||||||
Orbitides, by definition, are ribosomally synthesized and post-translationally modified N-to-C linked peptides. Consequently, compounds 18, 19, and 20 cannot be unequivocally classified as orbitides until their corresponding biosynthetic precursor is identified. Based on the mass fragmentation pattern of 19 and its homology with 3, the proposed sequence of the mature genetically encoded 18 is [1−9-NαC]-MLIPPFFLI. Based on the recently proposed systematic nomenclature of flax orbitides [17, 22], novel orbitides 18 ([1−9-NαC]-MLIPPFFLI), 19 ([1−9-NαC]-OLIPPFFLI), and 20 ([1−9-NαC]-JLIPPFFLI) are designated [1−9-NαC]-linosorb F1, [1−9-NαC]-[1-MetO]-linosorb F1, [1−9-NαC]-[1-MetO2]-linosorb F1, respectively. Although an assembly of the entire L. usitatissimum genome has been published [21], the genomic data is constructed only from the cultivar ‘CDC Bethune’. Nevertheless, we conducted TBLASTN [27, 28] searches using default algorithm parameters with a putative precursor for 18, MLIPPFFLI, as the initial query in addition to all possible linear transcripts for 18. The searches returned no hits which is not surprising given that the observed changes in orbitide production in ‘Hollandia’ and ‘Z 11637’ indicate that these accessions may contain different sequences and/or may differ in their transcription, translation, and/or post-translational modification of LINUSORB B compared to ‘CDC Bethune’.
LINUSORB B encodes a gene that contains one copy of each of the linear precursor orbitides of 1, 3 and 8. These orbitides are expressed equally in ‘CDC Bethune’, as in all other accessions within the FCC with the exception of ‘Hollandia’ and ‘Z 11637’ [23]. We studied the orbitide encoding region of LINUSORB B to determine if an alteration in this gene was responsible for the unusual apparent concentration ratio of orbitides and low total orbitide content observed in ‘Hollandia’ and ‘Z 11637’. Sequencing of the orbitide encoding region of this gene in ‘Hollandia’ and ‘Z 11637’ revealed a 117 bp deletion (Additional file 10). The transcribed gene encodes an orbitide with amino acid sequence MLIPPFFLI and corresponds to the deduced structure of 18. As the size of the orbitide peptides repeat unit is 117 bp, the deletion maintains the reading frame of the gene and results in the synthesis of two orbitides (18 and 7) instead of three (1, 2 and 7).
Discussion
The deletion precludes expression of intact 1 and 2, but results in the joining of the 5′ fragments of 2 and the 3′ portion of 1 to form the novel orbitide 18. Orbitides 1 and 2 have fourteen bp in common (TCCCCCCCTTCTTT); the beginning and end of the deletion occurs within this shared sequence for 2 and 1, respectively (Fig. 5). It is not possible to determine the exact nucleotide at which the deletion starts and ends. However, the peptide sequences of Orbitides 1, 2 and 18 are conserved at this location for the ‘Hollandia’, ‘Z11637’ and other genotypes. This means that the lesion resulting in Orbitide 18 occurred at the same nucleotide in this region for Orbitides 1 and 2 (Fig. 5).
Fig. 5.

Open reading frame of LINUSORB B, which encodes Orbitides 1, 2, and 7. Sequence arranged so that repeats within gene are aligned (nucleotide lines 3-5). Dashes are inserted to allow alignment of repeats. Bolded: Orbitides 7, 2 and 1 from top to bottom, respectively. Green text: location of PCR primers. Red text: Deleted sequence in ‘Hollandia’/‘Z11637’. Underlined: identical sequence between Orbitides 2 and 1 indicating the region for the start and end of the 117 bp deletion. Lowercase: Differences between sequence following Orbitide 2 and sequence following Orbitide 1 up to the primer binding site
Evidence that the 117 bp deletion occurred between the sequences for Orbitides 2 and 1 is that the sequence of the PCR fragment from ‘Hollandia’ and ‘Z11637’ situated 5′ of Orbtide 2 and 3′ of Orbitide 1 are identical to that of the reference gene sequence from CDC Bethune and from PCR fragments amplified from 16 other genotypes (data not shown). Furthermore, sequence differences unique to the deleted fragment (7 nucleotide differences and a 3 bp and a 9 bp insertion) were not observed in the sequence of the PCR product from ‘Hollandia’ and ‘Z11637’. Taken together, the simplest explaination for the observed DNA and orbitide sequence in ‘Hollandia’ and ‘Z11637’ is a 117 bp deletion starting in the common 14 bp region of Orbitide 2 and ending at the same nucleotide in Orbitide 1. Alternative explanations are possible, but are not as parsimonious as the one suggested here as they need to explain the conserved sequence identity in the rest of the PCR fragment and the absence of the unique sequence differences located between the Orbitide 2 and 1 coding regions.
At the amino acid level, the putative linear transcript in ‘Z 11637’ consists of a fusion of the first three amino acid residues of 2 (MLI), the conserved residues PPFF, contributed either entirely from 1 or 2 or as a portion from both, and the last two residues of 1 (LI) (Fig. 5). The amplified fragment in ‘Hollandia’ is similar to that from ‘Z 11637’ and, as a result, the alignment of ‘CDC Bethune’ with ‘Hollandia’ is identical to that with ‘Z11637’. There were no mutations observed in the DNA coding fragment of 8 which explains the comparable expression of 8 among ‘Hollandia’, ‘Z 11637’ and ‘CDC Bethune’. The putative precursor of 1, 7 and 18 is flanked by “DG” and “FGK” amino acid residues at the amino and carboxy termini, respectively. New orbitide 18 is the result of the removal of a whole repeat unit suggesting that orbitide expression is conserved and that a mechansim(s) to delete fragments function(s) to maintain the integrity of the orbitide repeat units. A search for similar, whole orbitide unit, insertions/deletions in the other main orbitide gene (LINUSORB A) to determine whether a consistent mechanism for orbitide gene recombination exists is underway.
The mutation event occurring in ‘Hollandia’ and ‘Z 11637’ was determined to be heritable by crossing ‘Hollandia’ with ‘CDC Sorrel’ and ‘Hollandia’ with ‘CDC Sanctuary’. The sequence of LINUSORB B in ‘CDC Sorrel’ and ‘CDC Sanctuary’ is identical to that of ‘CDC Bethune’, and, as such, these cultivars expressed 1, 2, 5, 7, 10 and 14 at similar concentrations to the check ‘CDC Bethune’. Inheritance of the altered LINUSORB B gene was observed both phenotypically (via HPLC-DAD and mass spectral analyses) and genotypically (PCR and qPCR) in F3 seed. The expected Mendelian ratios for monogenetic inheritance were observed in this population. No distortion of the expected phenotypic ratios was observed in reciprocal crosses in these populations, indicating that maternal factors do not affect orbitide expression.
Although it is difficult to determine the timeline of the DNA variation, we infer that the deletion event probably occurred in a common ancestor of ‘Hollandia’ and ‘Z 11637’ as both orignated from the Netherlands. Rapid changes in the flax genome as a response to growth environment has been reported in which DNA variants can be stable or unstably inherited [29]. ‘Hollandia’ is a flax cultivar in which stable genomic variations have been observed, others including ‘Stormont Cirrus’, ‘Rembrandt’, and ‘Liral Monarch’ [30]. CA Cullis [30] has suggested that organization of the flax genome contributes to widespread genomic variations that may be observed in flax within a generation. We speculate that the deletion event described here could be an example of this type of genomic reorganization induced by environmental stress. It is also intriguing to speculate on the role of orbitides in flax physiology and agronomic selection. All flax cultivars and accessions examined in the FCC expressed orbitides in their seed [23], however, as demonstrated in this paper, significant alterations in their composition and total concentration are possible.
Conclusions
The unidentified peak observed in HPLC-DAD chromatograms of extracts from ‘Hollandia’ and ‘Z 11637’ flax cultivars is attributed to novel orbitide 18. Mass spectrometry (high resolution HPLC-ESI-MS and HPLC-ESI-MS/MS) and NMR spectroscopy revealed the structure of 18 as [1−9-NαC]-OLIPPFFLI with m/z 1072.6. The analysis of the gene sequence of LINUSORB B in ‘Hollandia’ and ‘Z 11637’ explains the apparently reduced total orbitide concentration by the lack of 1 and 2, and the expression of 18, as compared with ‘CDC Bethune’. Sequencing results also corroborate the amino acid sequence of 18 deduced from spectroscopy. Future work will include recovery of sequences with similarity to LINUSORB B and LINUSORB A from the entire FCC [24] which covers approximately 95% of the genetic variation in flax. Correlations between gene structure and orbitide expression will be made once this data is obtained.
Methods
Flaxseed
Seed from the cultivars ‘Hollandia’ (CN 98056), ‘Z 11637’ (CN 98150), and ‘CDC Bethune’ were regenerated at Kernen Crop Research Farm, Saskatoon, SK and Morden Research Station, Morden, MB in 2009, 2010, and 2011. Flaxseed was kindly donated by Dr. Scott Duguid (Morden Research Station, MB) and Drs. Helen Booker and Gordon Rowland (Crop Development Centre, Saskatoon SK).
Sample preparation for orbitide composition analysis
Approximately 120 mg of flaxseed from each accession for each growing season was weighed and placed directly into wells (~ 1.8 mL volume) of 96-well plates. Each sample was prepared according to Burnett et al. [23] in quadruplicate to enable replicate composition analyses for oxidized and unoxidized (native) extracts. Specifically, pre-heated H2O (60 °C) was added to each well at a seed˗water ratio of 1:10 (w/v) and wells were covered with a 96-well plate sealing mat (square cap). Covered plates were incubated at 60 °C for 2 h and then gum extracts were removed via pipette. The degummed seeds were ground at 1400 strokes per min for 10 min using a 2010 Geno/grinder (SPEX CertiPrep, Inc., Methucen, NJ) and spherical 5-mm zirconia grinding media (1 per well). A 100 μL aliquot of internal standard [1−9-NαC],[1-Abu]-linusorb B2 (0.1 mg/mL in MeOH), prepared as previously reported, [31] was added to each well. Subsequently, 860 μL of methanol:water (78:22, v/v) was added to each well to make a final dilution of 1:8 (w/v). Samples were mixed for 2 min at 1400 strokes per min and were subsequently incubated at 60 °C for 2 h. Samples were centrifuged at 1760×g for 20 min and a 400 μL aliquote of the resulting supernatant was transferred to a 96-well filter plate.
Filter plates were covered and placed on top of receiving 96-well plates and assembled plates were placed in a centrifuge for filtration at 1760×g for 10 min. Filtered extracts (100 μL aliquots) were transferred to 96-well HPLC trays and were subsequently oxidized with H2O2 (50 μL, 4.5% in H2O, v/v) or diluted with H2O (50 μL) such that each cultivar per harvest year had replicates for each treatment. After 1 h at room temperature, the oxidized extracts were quenched with Na2S2O3 (150 μL, 0.2 M) in 70% aq. MeOH, whereas 70% aq. MeOH (150 μL) was added to the diluted extracts. The 96-well trays were placed in an HPLC-DAD, as described below, with an autoinjector for sample analyses. Extracts were also analyzed via high resolution HPLC Electrospray Ionization mass spectrometry (HR-HPLC-ESI-MS and HPLC-ESI–MS/MS).
Extraction and isolation
Orbitides were extracted from ‘Hollandia’ (CN 98056) and ‘Z 11637’ (CN 98150) seed regenerated in 2009. Seed samples from Saskatoon, SK and Morden, MB, were combined based on flax accession (total mass of extracted flaxseed was 80 g) and were ground prior to extraction with 70% MeOH (1:20 v/v, × 2). Each sample was incubated at 60 °C for 2 h. Aliquots of each aqueous methanolic extract were filtered through 0.45 μm PTFE membranes for HPLC-DAD and HPLC-ESI-MS analyses. Then, extracts were freed of solvent using a rotary evaporator to yield crude orbitide extracts (total mass 4.95 g). Mass spectra of all ‘Hollandia’ and ‘Z 11637’ extracts indicated the presence of 18 possessing quasimolecular ion peak at m/z 1072.6, in addition to other known orbitides.
Conversion of methionine-containing orbitides to their methionine S-oxide-containing form is necessary to simplify orbitide peak chromatographic separation, and increase the concentration of novel orbitides in a single oxidation state. Consequently, crude orbitide extracts were oxidized with H2O2 prior to flash column chromatography as follows. Extracts were suspended in MeOH (5 mL, × 2), filtered through 0.45 μm PTFE membranes, and subsequently combined with H2O2 (1 mL, 30% in H2O, v/v). Oxidation was monitored by HPLC-DAD and upon conversion of 18 to 19, the reaction mixture was quenched with saturated Na2S2O3 in 70% aq. MeOH (10 mL), then filtered. The sample was freed of solvent using a rotary evaporator (dry mass of sample 4.56 g). The oxidized extract was subjected to flash column chromatography on silica gel 60 (40-63 μm particle size, EMD Chemicals). Sequential elution with the following solvent systems was conducted (each 200 mL, with each eluent being divided into two fractions): (a) n-hexane; (b) 20% EtOAc in n-hexane; (c) 50% EtOAc in n-hexane; (d) 80% EtOAc in n-hexane; (e) 100% EtOAc; (f-j) 2% MeOH in CH2Cl2 to 10% MeOH in CH2Cl2 using 2% MeOH increments. Each fraction collected was analyzed via HPLC-DAD and HPLC-ESI-MS. Fractions determined to contain substantial amounts of 19 (m/z 1088.6) were purified using preparative reversed-phase chromatography.
HPLC-DAD analyses
Analytical
Analyses of FCC orbitide composition were performed on a 1200 series HPLC system (Agilent Technologies Canada, Mississauga, ON) equipped with a quaternary pump, autosampler, photodiode-array detector (wavelength range 190-300 nm), and a degasser. Chromatographic separations were carried out on 50 mm × 4.6 mm i.d., reversed phase Chromolith SpeedRod RP-18e columns (Merck KGaA, Darmstadt, Germany) equipped with in-line filters. The mobile phase consisted of a linear gradient of H2O and acetonitrile (CH3CN) (70:30 to 30:70 in 4 min, to 10:90 in 0.5 min, to 70:30 in 0.5 min, to equilibration for 1 min) at a flow rate of 2 mL/min [31]. All analyses were conducted at 23 °C using 10 μL injection volumes and recording absorbance over the entire UV spectrum. Peak area integration was conducted at a wavelength of 214 nm with a 10 nm bandwidth. All samples analyzed via HPLC-DAD and HPLC-MS were previously filtered through 0.45 μm PTFE membranes. The molar extinction coefficients at 214 nm of all orbitides discussed in this manuscript are similar and, therefore, A214 is proportional to orbitide concentration. Pure orbitide standards were not used to determine either matrix effects or concentration. All concentrations are compared based on their A214 during elution from an HPLC column.
Preparative
Preparative reversed phase chromatography was performed on an Agilent 1200 series HPLC system (Agilent Technologies Canada, Mississauga, ON) equipped with a Chromolith® SemiPrep RP-18e column (100 × 10 mm i.d.), and a UV/VIS detector operating at a wavelength of 214 nm. The mobile phase consisted of H2O–CH3CN (55:45 for 12 min, to 5:95 in 1.0 min, to 55:45 in 1.0 min, to equilibration for 6 min) at a flow rate of 3 mL/min. All preparative separations were performed at ambient temperature.
Mass spectral analyses
High resolution HPLC-ESI-MS and HPLC-ESI–MS/MS analyses were performed on an Agilent 1200 series HPLC system connected directly to a micrOTOF-Q II hybrid quadrupole time of flight MS/MS (Bruker Daltonik GmbH, Bremen, Germany) with Apollo II electrospray ionization (ESI) ion source at a capillary voltage of -4500 V, nebulizer gas at 4.0 bar, and drying gas temperature held at 200 °C. Chromatographic separation for MS analyses was achieved at ambient temperature using a Chromolith® FastGradient RP-18e column (50 mm × 2.0 mm i.d., Merck KGaA, Darmstadt, Germany). The mobile phase consisted of a linear gradient of 0.1% formic acid in H2O and 0.1% formic acid in CH3CN (60:40 for 2 min, to 10:90 in 8 min, to 60:40 in 0.5 min, to equilibration for 5.5 min) at a flow rate of 0.4 mL/min [16]. HPLC-ESI-MS/MS analyses were conducted on a Bruker micrOTOF-Q II Mass Spectrometer using identical parameters to those described for HR-HPLC-ESI-MS.
Structural analysis via nuclear magnetic resonance (NMR)
Proton NMR spectra were recorded on a 600 MHz Bruker Avance spectrometer (5 mm PABBO BB-probe head; TopSpin 3.2 software). The 1H NMR spectra (600 MHz) chemical shifts (δ) values are reported in parts per million (ppm) relative to the internal standard TMS. The δ values are referenced to CDCl3 at 7.26 ppm, and multiplicities are indicated by the following symbols: s = singlet, d = doublet, dd = doublet of doublets, m = multiplet, and br = broad. For 13C NMR (125.8 MHz), the chemical shift (δ) values were referenced to CDCl3 (77.23 ppm).
Orbitide gene sequence analyses
Primers were designed to amplify the portions of LINUSORB B which encoded for Orbitides 1, 3, and 8. The sequence of this gene has been confirmed in previous work [1–3], as well as other sources, and the identifier, LINUSORB B, is used throughout this paper to maintain consistency with our proposed systematic nomenclature [22]. Flax accessions and cultivars examined for sequence analysis were ‘Hollandia’, ‘Z 11637’, and ‘CDC Bethune’. A quick alkaline lysis method was used to extract crude total DNA from leaves of 14 day old seedlings [32]. Crude extracts (1 μL) were used as templates for PCR amplification of a 369 bp fragment using forward (5’ATTTCTGGAAAGGATGGCGG3’) and reverse (5’CTTGTCACCCTGCTGCTC3’) primers with annealing temperatures of 58.2 and 58.0 °C, respectively. The sizes of the fragments from different accessions were compared using agarose gel electrophoresis. The fragments from two PCRs (each 25 μL) were purified using a Qiagen DNAesy PCR purification kit and were then sequenced using the amplification primers or one of two internal primers (5’CGGCATTATTATACTTGTGGCCG3’ or 5’CGGCCACAAGTATAATAATGCCG3’). PCR fragment sequencing was performed by the National Research Council (NRC) Canada, Saskatoon, DNA Sequencing Facility. PCR product sequences were aligned against the LINUSORB B sequence from ‘CDC Bethune’ using Geneious 6.0 (Biomatters Inc., Auckland, New Zealand).
Additional files
Table S1. Calculated flaxseed orbitide masses and sequences. (DOCX 22 kb)
Figure S1. 1H NMR spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 178 kb)
Figure S2. 13C NMR spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 145 kb)
Figure S3. 1H-1H COSY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 479 kb)
Figure S4. DEPT spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 145 kb)
Figure S5. 1H-13C HMBC spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 189 kb)
Figure S6. 1H-13C HSQC spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 229 kb)
Figure S7. 1H-1H NOESY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 476 kb)
Figure S8. 1H-1H TOCSY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 763 kb)
Hollandia,Bethune_Orbitide_seq.fa sequence obtained from Sanger sequencing of the Hollandia/Z11637 orbitide [18, 8] PCR fragment and the CDC Bethune orbitide [1, 3, 8] PCR fragment. (FA 717 bytes)
Acknowledgments
Funding
The work was part of the Total Utilization Flax GENomics (TUFGEN) project #1309 funded by Genome Canada and the Saskatchewan Flax Development Commission (SFDC). Financial support for the author’s work was also provided by the Saskatchewan Ministry of Agriculture - Agricultural Development Fund (Grants 20080205, 20120099, and 20120146).
Availability of data and materials
The majority of the data generated or analyzed during this study are included in this published article and its supplementary information files. Excluded datasets are available from the corresponding author on a reasonable request.
Abbreviations
- CDC
Crop Development Centre
- CN
Canadian National
- COSY
Correlation spectroscopy
- FCC
Flax core collection
- HMBC
Heteronuclear multiple-bond correlation
- HMQC
Heteronuclear multiple quantum correlation
- HPLC-DAD
High performance liquid chromatography with diode array detector
- HPLC-ESI-MS/MS
High resolution high performance liquid chromatography with electrospray ionization tandem mass spectrometry
- HR-HPLC-ESI-MS
High resolution high performance liquid chromatography with electrospray ionization mass spectrometry
- NCBI
National Center for Biotechnology Information
- NMR
Nuclear Magnetic Resonance
- NOESY
Nuclear Overhauser effect spectroscopy
- PGRC
Plant Gene Resources of Canada
- TOCSY
Total correlation spectroscopy
Authors’ contributions
PB acquired and interpreted mass spectral data, isolated orbitide for subsequent analyses, conducted analyses on F3 seed, and was a major contributor in writing the manuscript. LY performed all gene sequence analyses of the orbitides, interpreted the corresponding data generated and co-wrote the manuscript. CO conducted orbitide profiling of the cultivars as part of her M.Sc. thesis. PJ performed NMR characterization and DO was involved in acquisition and interpretation of mass spectral data. MR supervised the work performed and contributed intellectually to the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1303-8) contains supplementary material, which is available to authorized users.
Contributor Information
Peta-Gaye Gillian Burnett, Phone: +1 306 966 8840, Email: pete.burnett@usask.ca.
Lester Warren Young, Email: lester.young@usask.ca.
Clara Marisa Olivia, Email: cmo539@mail.usask.ca.
Pramodkumar Dinkar Jadhav, Email: prj883@mail.usask.ca.
Denis Paskal Okinyo-Owiti, Email: denis.okinyo-owiti@usask.ca.
Martin John Tarsisius Reaney, Phone: +1 306 966 5027, Email: martin.reaney@usask.ca.
References
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30(1):108–60. [DOI] [PMC free article] [PubMed]
- 2.Condie JA, Nowak G, Reed DW, Balsevich JJ, Reaney MJT, Arnison PG, Covello PS. The biosynthesis of Caryophyllaceae-like cyclic peptides in Saponaria vaccaria L. from DNA-encoded precursors. Plant J. 2011;67(4):682–690. doi: 10.1111/j.1365-313X.2011.04626.x. [DOI] [PubMed] [Google Scholar]
- 3.Covello PS, Datla RSS, Stone SL, Balsevich JJ, Reaney MJ, Arnison PG, Condie JA. DNA sequences encoding caryophyllaceae and caryophyllaceae-like cyclopeptide precursors and methods of use. 2010; US Patent Application 2012/0058905A1.
- 4.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery--an underexploited structural class. Nat Rev Drug Discov. 2008;7(7):608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 5.Wieczorek Z, Bengtsson B, Trojnar J, Siemion IZ. Immunosuppressive activity of cyclolinopeptide A. Peptide Res. 1991;4(5):275–83. [PubMed] [Google Scholar]
- 6.Kessler H, Klein M, Müller A, Wagner K, Bats JW, Ziegler K, Frimmer M. Conformational prerequisites for the in vitro inhibition of cholate uptake in hepatocytes by cyclic analogues of antamanide and somatostatin. Angew Chem Int Ed Engl. 1986;25(11):997–999. doi: 10.1002/anie.198609971. [DOI] [Google Scholar]
- 7.Gaymes TJ, Cebrat M, Siemion IZ, Kay JE. Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Lett. 1997;418(1-2):224–7. doi: 10.1016/S0014-5793(97)01345-8. [DOI] [PubMed] [Google Scholar]
- 8.Drygała P, Olejnik J, Mazur A, Kierus K, Jankowski S, Zimecki M, Zabrocki J. Synthesis and immunosuppressive activity of cyclolinopeptide A analogues containing homophenylalanine. Eur J Med Chem. 2009;44(9):3731–8. doi: 10.1016/j.ejmech.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Morita H, Shishido A, Matsumoto T, Itokawa H, Takeya K. Cyclolinopeptides B-E, new cyclic peptides from Linum usitatissimum. Tetrahedron. 1999;55(4):967–976. doi: 10.1016/S0040-4020(98)01086-2. [DOI] [Google Scholar]
- 10.Siemion IZ, Cebrat M, Wieczorek Z. Cyclolinopeptides and their analogs--a new family of peptide immunosuppressants affecting the calcineurin system. Arch Immunol Ther Exp. 1999;47(3):143–153. [PubMed] [Google Scholar]
- 11.Górski A, Kasprzycka M, Nowaczyk M, Wieczoreck Z, Siemion IZ, Szelejewski W, Kutner A. Cyclolinopeptide: a novel immunosuppressive agent with potential anti-lipemic activity. Transplant Proc. 2001;33(1):553. doi: 10.1016/S0041-1345(00)02139-4. [DOI] [PubMed] [Google Scholar]
- 12.Benedetti E, Pedone C. Cyclolinopeptide A: inhibitor, immunosuppressor or other? J Pept Sci. 2005;11(5):268–72. [DOI] [PubMed]
- 13.Picur B, Cebrat M, Zabrocki J, Siemion IZ. Cyclopeptides of Linum usitatissimum. J Pept Sci. 2006;12:569–574. doi: 10.1002/psc.779. [DOI] [PubMed] [Google Scholar]
- 14.Morita H, Takeya K. Bioactive cyclic peptides from higher plants. Heterocycles. 2010;80:739–764. doi: 10.3987/REV-09-SR(S)7. [DOI] [Google Scholar]
- 15.Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol. 2014;51(9):1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okinyo-Owiti DP, Jadhav PD, Bauer R, Reaney MJT, Dong Q, Ling B, Yang J, Maley JM, Sammynaiken R. Evaluating the cytotoxicity of flaxseed orbitides for potential cancer treatment. Toxicol Rep. 2015;2:1014–1018. doi: 10.1016/j.toxrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJT. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends Food Sci Technol. 2014;38(1):5–20. doi: 10.1016/j.tifs.2014.03.011. [DOI] [Google Scholar]
- 18.Burnett P-GG, Jadhav PD, Okinyo-Owiti DP, Poth AG, Reaney MJT. Glycine-containing flaxseed orbitides. J Nat Prod. 2015;78(4):681–688. doi: 10.1021/np5008558. [DOI] [PubMed] [Google Scholar]
- 19.Gui B, Shim YY, Datla RSS, Covello PS, Stone SL, Reaney MJT. Identification and quantification of cyclolinopeptides in five flaxseed cultivars. J Agric Food Chem. 2012;60(35):8571–8579. doi: 10.1021/jf301847u. [DOI] [PubMed] [Google Scholar]
- 20.Okinyo-Owiti DP, Young L, Burnett PGG, Reaney MJT. New flaxseed orbitides: detection, sequencing, and 15 N incorporation. Pept Sci. 2014;102(2):168–175. doi: 10.1002/bip.22459. [DOI] [PubMed] [Google Scholar]
- 21.You FM, Xiao J, Li P, Yao Z, Jia G, He L, Zhu T, Luo MC, Wang X, Deyholos MK, Cloutier S. Chromosome‐scale pseudomolecules refined by optical, physical, and genetic maps in flax. Plant J. 2018. [DOI] [PubMed]
- 22.Shim YY, Young LW, Arnison PG, Gilding E, Reaney MJT. Proposed Systematic Nomenclature for Orbitides. J Natural Products. 2015;78 (4):645-52. [DOI] [PubMed]
- 23.Burnett P-GG, Olivia CM, Okinyo-Owiti DP, Reaney MJT. Orbitide composition of the flax Core collection (FCC) J Agric Food Chem. 2016;64:5197–5206. doi: 10.1021/acs.jafc.6b02035. [DOI] [PubMed] [Google Scholar]
- 24.Diederichsen A, Kusters P, Kessler D, Bainas Z, Gugel R. Assembling a core collection from the flax world collection maintained by plant gene resources of Canada. Genet Resour Crop Ev. 2013;60(4):1479–1485. doi: 10.1007/s10722-012-9936-1. [DOI] [Google Scholar]
- 25.Soto-Cerda BJ, Diederichsen A, Ragupathy R, Cloutier S. Genetic characterization of a core collection of flax (Linum usitatissimum L.) suitable for association mapping studies and evidence of divergent selection between fiber and linseed types. BMC Plant Biol. 2013;13(78):1–15. doi: 10.1186/1471-2229-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanowicz P. Detection and sequencing of new cyclic peptides from linseed by electrospray ionization mass spectrometry. Acta Biochim Pol. 2001;48(4):1125–1129. [PubMed] [Google Scholar]
- 27.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gertz EM, Yu Y-K, Agarwala R, Schäffer AA, Altschul SF. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cullis CA. DNA differences between flax genotrophs. Nature. 1973;243:515–516. doi: 10.1038/243515a0. [DOI] [PubMed] [Google Scholar]
- 30.Cullis CA. Mechanisms and control of rapid genomic changes in flax. Ann Bot. 2005;95(1):201–206. doi: 10.1093/aob/mci013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivia CM, Burnett P-GG, Okinyo-Owiti DP, Shen J, Reaney MJT. Rapid reversed-phase liquid chromatography separation of cyclolinopeptides with monolithic and microparticulate columns. J Chromatogr B. 2012;904:128–134. doi: 10.1016/j.jchromb.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Young L, Hammerlindl J, Babic V, McLeod J, Sharpe A, Matsalla C, Bekkaoui F, Marquess L, Booker H. Genetics, structure, and prevalence of FP967 (CDC Triffid) T- DNA in flax. SpringerPlus. 2015;4(1):1–9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Calculated flaxseed orbitide masses and sequences. (DOCX 22 kb)
Figure S1. 1H NMR spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 178 kb)
Figure S2. 13C NMR spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 145 kb)
Figure S3. 1H-1H COSY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 479 kb)
Figure S4. DEPT spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 145 kb)
Figure S5. 1H-13C HMBC spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 189 kb)
Figure S6. 1H-13C HSQC spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 229 kb)
Figure S7. 1H-1H NOESY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 476 kb)
Figure S8. 1H-1H TOCSY spectrum of [1−9-NαC]-OLIPPFFLI (19). (PDF 763 kb)
Hollandia,Bethune_Orbitide_seq.fa sequence obtained from Sanger sequencing of the Hollandia/Z11637 orbitide [18, 8] PCR fragment and the CDC Bethune orbitide [1, 3, 8] PCR fragment. (FA 717 bytes)
Data Availability Statement
The majority of the data generated or analyzed during this study are included in this published article and its supplementary information files. Excluded datasets are available from the corresponding author on a reasonable request.


