Supplemental Digital Content is Available in the Text.
Keywords: lightheadedness, human movement system, mental fogginess, refractory dizziness, syncope
Abstract
Background and Purpose:
Postural orthostatic tachycardia syndrome (POTS) is increasingly recognized as a complication affecting recovery from concussion. Individuals with POTS demonstrate refractory dizziness, lightheadedness, cognitive dysfunction, fatigue, headache, chronic pain, nausea and gastrointestinal dysmotility, activity and exercise intolerance, syncope, and tachycardia. Subtypes of POTS may include hypovolemia, hyperadrenergic states, autonomic neuropathy, and underlying autoimmunity, which may variably impact response to rehabilitation in varying ways. The subtle presentation of POTS postconcussion is often mistaken for underlying anxiety, conversion disorder, or lack of motivation for recovery. This article will present clinical features of POTS that may arise after concussion, and propose a role for physical therapists in the diagnosis and management of POTS during concussion recovery.
Summary of Key Points:
Data recorded and entered into a database during clinic visits from a large pediatric institution indicate that 11.4% of individuals diagnosed with POTS report onset of symptoms within 3 months of sustaining a concussion. Activation of the sympathetic nervous system can result in lightheadedness, shortness of breath, chest pain, tachycardia, palpitations on standing or with exertion, and activity and exercise intolerance. Identified comorbidities in people with POTS such as joint hypermobility and autoimmune disorders can further influence recovery.
Recommendations for Clinical Practice:
Physical therapists may identify signs and symptoms of POTS in a subset of individuals who remain refractory to typical interventions and who exhibit symptom exacerbation with orthostatic activity. Incorporation of an individualized POTS exercise program into current established concussion interventions may be useful, with emphasis on initial recumbent exercises and ongoing physical therapy assessment of exercise tolerance for dosing of activity intensity and duration.
Video Abstract available for more insights from the authors (see Supplemental Digital Content 1, available at: http://links.lww.com/JNPT/A211).
INTRODUCTION
The World Health Organization diagnostic criteria for postconcussion syndrome require that 3 or more symptoms of headache, dizziness, fatigue, irritability, difficulty with concentration and mental tasks, memory impairment, sleep disturbances, heightened emotional responses, and poor stress tolerance must be present and interfering with the ability to participate in life activities.1 Early reports of dizziness and mental fogginess have been associated with prolonged recovery following concussion in high school athletes.2,3 Physical therapists address symptoms related to pain, headache, dizziness, and imbalance, and should incorporate interventions targeted to restore the interpretation of vestibular, oculomotor, cervicothoracic, and somatosensory afferent input through activities that progressively challenge central sensory processing and motor control responses.4–6 Among people experiencing prolonged recovery from injury, symptoms over time become multifactorial and require astute clinical reasoning skills to differentiate underlying causes for symptom triggers and to develop customized intervention plans until biomarkers or imaging techniques offer stronger evidence to localize brain trauma and neurophysiological involvement.
The pathophysiology following concussion has been well described to include a series of metabolic events resulting in altered cerebral blood flow, inflammatory processes, and disrupted axonal communication.7,8 Diffuse axonal injury causes damage to cortical and subcortical regions of the brain, potentially impacting regulation of body temperature, sleep cycles, digestion, and other autonomic functions.9 A growing body of evidence demonstrates that there is a transient cardiovascular autonomic dysfunction during the acute and subacute period following sports-related concussions and blast exposures in service members.10–12 Heart rate variability studies have attempted to measure sympathetic nervous system reactivity following concussion, although the evidence in conflicting.13–15 Two recent studies combined heart rate variability with autonomic function testing during a 14-day subacute recovery period and at a protracted time frame as a mechanism to further study effects of altered autonomic function after concussive injuries.10,16 Ongoing efforts to develop imaging techniques and biomarkers to confirm and localize concussion injuries have contributed to the understanding that dysregulation of the autonomic nervous system can occur as a consequence of injury.17
Graded exercise testing has been proposed as a method to classify ongoing symptoms 3 weeks postinjury into 3 subtypes of neurological postconcussion disorders.18 Individuals unable to exercise at maximal exertion following sports-related concussion because of increased symptoms have been categorized as having physiological postconcussion disorders, likely related to ongoing reduction in cerebral blood flow and prolonged effects of the metabolic energy crisis as well as autonomic dysfunction.19 The recommendations for the management of identified physiologic postconcussion disorders include earlier initiation of subsymptom aerobic exercise alongside treatment for potential visual, vestibular, and somatosensory impairments.20 In a retrospective study, 20 consecutive patients who sustained a concussion injury were evaluated to determine whether dizziness described as lightheadedness during quiet standing and exercise could be attributed to dysautonomia. Results showed significant tachycardia on tilt-table testing, altered adrenergic function during baroreflex testing, and variable standing norepinephrine (NE) levels, providing evidence linking dizziness and autonomic nervous system dysfunction.21
Dizziness is a poorly defined term depicting multiple sensory experiences often resulting from altered peripheral sensory afferent input and complex central cortical and subcortical sensory-motor processing. The complexity of symptom etiology related to dizziness has been described to involve an intricate bilateral relay of neurological connections extending from the vestibular nuclei through the parietoinsular vestibular cortex and thalamus and across the corpus callosum.22 In this context, lightheadedness in upright postures may be an indicator of dysautonomia compared with other descriptors of dizziness, such as vertigo or a persistent sense of motion, that are likely more indicative of peripheral or central vestibular dysfunction.
Researchers and clinicians recognize exercise intolerance in some individuals postconcussion despite graded exercise, Return to Play and Return to Duty exertion training programs that provide parameters for progressive cardiovascular training zones.23–25 Autonomic nervous system dysregulation could account for failure to complete exertion training successfully in some cases due to orthostatic and activity intolerance.10,26 Adjustments to heart rate (HR) training parameters should be made to disrupt the perpetuated sympathetic nervous system activity, with supplementary strength and resistance training in semirecumbent positions. In such cases, the presence of a type of dysautonomia referred to as postural orthostatic tachycardia syndrome (POTS) could preclude concussion exertion program participation due to new or easily provoked symptoms. Identification of POTS requires additional patient education for personal health management to avoid severe exacerbation of a myriad of symptoms and, in some cases, syncopal events that could create risk of further head trauma. The purpose of this article it to present clinical features of POTS that may arise in some individuals postconcussion, to propose physical therapy screening for POTS as routine care after concussion injuries, and to introduce a modification to currently established concussion exercise training programs in the presence of POTS.
OVERVIEW OF POTS
Postural orthostatic tachycardia syndrome is a form of dysautonomia characterized by sustained tachycardia and variable complex symptoms that occur upon standing.27 It is estimated that 1 to 3 million Americans have POTS with a similar prevalence internationally. Onset of POTS typically occurs in adolescent girls and women of childbearing age, and women are affected more often than men by 4.5:1.28,29 Associated symptoms include dizziness, headache, nausea, visceral pain, heaviness of the extremities, reduced mental clarity, and generalized fatigue.28,30,31 Postural orthostatic tachycardia syndrome is also often accompanied by disturbed sleep, poor body temperature regulation, and gastrointestinal complaints of bloating, constipation, abdominal pain, and early satiety. Peripheral edema and skin discoloration patterns including livedo reticularis or acrocyanosis are common; in some cases, bladder dysfunction can occur.
In a retrospective study of 722 patients with POTS enrolled in a pediatric patient database, 11.4% of patients identified a temporal link to a concussive event precipitating their POTS symptoms.32 In another study, a vestibular questionnaire issued to 50 consecutive patients diagnosed with POTS revealed that 100% of subjects experienced lightheadedness upon standing, 46% reported vertigo, 98% experienced unstable gait, and 40% identified with a “swimming” sensation, while 48% reported a “rocking” sensation.33 In this cohort, a mean composite autonomic symptom score (COMPASS) score of 52 in combination with vestibular symptoms indicated the potential for peripheral and central neurophysiology as well as cerebral hypoperfusion contributing to symptoms of dizziness. While there are many potential triggers of POTS, there is growing awareness that head trauma and rapid deceleration injuries can be a stimulus for deregulation of the autonomic nervous system.
PATHOPHYSIOLOGY OF POTS
Understanding the heterogeneous pathophysiology of POTS subtypes may improve medical and rehabilitative management. It is important to note that people with POTS may fall under multiple phenotypes and have underlying conditions affecting central and peripheral pathophysiology. Because of the numerous comorbidities associated with POTS noted in Table 1, a detailed interdisciplinary medical workup is indicated.34–37 There are currently 3 clinical subtypes described in the literature: hypovolemic, neuropathic, and hyperadrenergic POTS.
Table 1. Comorbidities of Postural Orthostatic Tachycardia Syndrome.
| Antiphospholipid syndrome |
| Anxiety disorders |
| Autoimmune thyroiditis |
| Celiac disease |
| Chiari malformation |
| Depression disorders |
| Diabetes |
| Eosinophilic esophagitis |
| Fibromyalgia |
| Hypermobile Ehlers-Danlos syndrome |
| Hypermobility spectrum disorders |
| Lyme disease |
| Mast cell activation syndrome |
| Median arcuate ligament syndrome |
| Migraine |
| Mitochondrial diseases |
| Multiple sclerosis |
| Myalgic encephalomyelitis/Chronic fatigue syndrome |
| Neurocardiogenic syncope |
| Neuromyelitis optica |
| Paraneoplastic syndrome |
| Porphyria |
| Sarcoidosis |
| Sjögren's syndrome |
| Small fiber neuropathy |
| Syringomyelia |
| Systemic lupus erythematous |
Adapted with permission from Pavlik D et al.34
Hypovolemia is often a contributing factor in POTS, as plasma volume, red cell volume, and total blood volume are reduced compared with control subjects in studies.28,38 In POTS, the renin-angiotensin-aldosterone system responsible for the regulation of plasma volume does not function properly, impairing the typical water and sodium retention needed to build plasma volume.39
Approximately 50% of people with POTS experience a partial sympathetic denervation of blood vessels in the lower extremities, resulting in a reduced vasoconstriction response during upright posture, sometimes referred to as neuropathic POTS.30,40 Impaired venous return upon standing causes blood pooling in the lower limbs and within the abdominal cavity, which lowers stroke volume and results in compensatory tachycardia and reduced exercise capacity. This leads to a hyperdynamic blood flow, also termed “high flow” POTS.41
A smaller subset of individuals with POTS develop excessive plasma NE greater than 600 pg/mL upon standing and profound sympathetic activity that often results in anxiety, palpitations, tachycardia, tremulousness, and sometimes postural hypertension with greater than 10-mm Hg systolic BP increase upon standing.42 The excessive sympathetic tone is considered to be of central origin in hyperadrenergic POTS, but smaller increases in plasma NE upon standing, which are not uncommon in POTS, may also result as a compensatory response to hypovolemia and peripheral autonomic neuropathy. In some patients with POTS, a deficiency in the NE transporter protein may also lead to an excessive NE response.43,44
MANAGEMENT OF POTS
The cardiovascular manifestations of POTS include reduced stroke volume, reduced blood and plasma volume, and decreased cardiac chamber size mimicking prolonged bed rest despite many individuals with POTS participating actively in sports and recreation prior to the onset of POTS symptoms.45,46 Initial medical management of POTS typically includes specific guidance to increase daily fluid intake and sodium consumption and to wear compression stockings to encourage peripheral vasoconstriction. Pharmacological support often includes agents such as fludrocortisone to expand blood volume, β-blockers to reduce tachycardia, midodrine to reduce orthostatic hypotension, pyridostigmine to increase venous return and decrease tachycardia, and stimulants to reduce mental clouding, often referred to as brain fog.28,28
Some individuals with POTS respond favorably to progressive exercise training, resulting in gradual increase in blood volume, exercise capacity, and stroke volume along with improved left ventricular diastolic function.47–50 Recommendation for exercise to manage POTS stems from a study that engaged primary care physicians in the distribution of a 3-month unsupervised exercise program, referred to as the Levine protocol, for patients with POTS.51 Of the 41% who completed the program, 71% reported significant reduction in POTS symptoms. The majority of subjects (59%) failed to complete the program, with 24% reporting other medical problems limiting their ability to complete the program, 16% reporting difficulty gaining access to needed exercise equipment, and 40% reporting that the exercise program was too physically challenging.
A single case report describes the physical therapy evaluation and intervention of an adult who previously overcame her POTS diagnosis through medical management and exercise but had a relapse following brief fever and malaise.52 The physical therapist was able to confirm the exacerbation with a systems review, test of upright tolerance monitoring HR and blood pressure (BP), and a Rockport Fitness Test (1-Mile Track Walk Test) to estimate maximal aerobic capacity (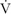 O2max).53 Based on the subject's previous success overcoming POTS using submaximal exercise and presenting health status, cardiovascular reconditioning with target HR ranges between 60% and 85% HRmax and a semirecumbent strengthening program were conducted for 4 weeks, with weekly PT sessions to provide assessment and modulation of activity recommendations. At the time of discharge, the subject's exercise tolerance improved and she no longer met the diagnostic criteria for POTS. In a telephone follow-up 4 weeks postdischarge, she reportedly maintained improvements, though the reported estimated
O2max).53 Based on the subject's previous success overcoming POTS using submaximal exercise and presenting health status, cardiovascular reconditioning with target HR ranges between 60% and 85% HRmax and a semirecumbent strengthening program were conducted for 4 weeks, with weekly PT sessions to provide assessment and modulation of activity recommendations. At the time of discharge, the subject's exercise tolerance improved and she no longer met the diagnostic criteria for POTS. In a telephone follow-up 4 weeks postdischarge, she reportedly maintained improvements, though the reported estimated 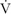 O2max increased only to 60% to 70% that of her gender and age-matched peers, perhaps reflective of a persistent mild autonomic dysfunction in some individuals that can result in ongoing lesser symptoms and the need for persistent pharmacological management.54
O2max increased only to 60% to 70% that of her gender and age-matched peers, perhaps reflective of a persistent mild autonomic dysfunction in some individuals that can result in ongoing lesser symptoms and the need for persistent pharmacological management.54
POTS AND CONCUSSION: DIFFERENTIAL DIAGNOSIS
Orthostatic intolerance and activity or exercise intolerance after concussion may be key indicators of POTS. Table 2 contains a detailed review of systems that could be performed alongside the COMPASS 31, a symptom questionnaire that identifies nonspecific autonomic dysfunction in the domains of orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor functions.55,56 Scores on the COMPASS 31 range from 0 to 100, with higher scores indicating greater severity. This tool has been shown to have preliminary clinical utility in quantifying symptom burden in individuals who have been diagnosed with POTS via autonomic function testing as compared with healthy controls, particularly related to the orthostatic intolerance and pupillomotor domains.57 Future research investigating the validity of the COMPASS 31 in people with POTS and concussion injuries would allow recommendations for clinical application, improved communication to primary care providers, and identification of the need for specialty care referrals involved in concussion management.
Table 2. Review of Systems for Postural Orthostatic Tachycardia Syndrome and Concussion.
| Cardiovascular/Pulmonary system | Assess heart rate, respiratory rate, blood pressure, and presence of edema. |
| Integumentary system | Note altered skin coloration such as acrocyanosis or livedo reticularis and general skin integrity. |
| Musculoskeletal system | Observe gross abnormalities in range of motion including any apparent joint hypermobility; inquire regarding general areas of musculoskeletal pain or inflammation. |
| Neuromuscular system | Inquire regarding any changes in sensation or signs of neuropathy, especially in the distal extremities. Assess presence of muscle spasm/fasciculation. |
| Mental status | Inquire regarding any cognitive changes or loss of consciousness from fainting. |
Orthostatic testing offers objective data for clinical decision making. Baseline HR and BP should be obtained after 10 minutes of quiet supine rest. Immediate transition to standing is provocative and requires monitoring for symptoms of dizziness, unsteadiness, headache, nausea, tremulousness, sweating, and syncope while recording symptom provocation, HR, and BP after 3, 5, 7, and 10 minutes of standing. According to the 2015 Heart Rhythm Society International Consensus statement,58 POTS is defined as variable constellation of symptoms that occur with standing, and the diagnostic criteria for POTS presented in Table 3 are based on Tilt Table Testing.58–60 The active 10-minute standing test has not been validated as a diagnostic test; thus, individuals with sustained significant HR elevation and/or orthostatic symptoms during a standing orthostatic test should be referred for medical evaluation. Ideally, a diagnosis of POTS should be confirmed with passive Head Up Tilt testing, though patient access to autonomic testing can be challenging.61
Table 3. Age-related Diagnostic Criteria for POTS58–60.
| Age, y | Criteria |
|---|---|
| Adolescents: 12-19 | HR increase > 40 bpm or sustained orthostatic HR > 120 bpm; <20/10 mm Hg change in BP |
| Adults: >19 | HR increase >30 bpm or sustained orthostatic HR >120 bpm; <20/10 mm Hg change in BP |
Abbreviations: BP, blood pressure; bpm, beats per minute; HR, heart rate.
Clinical features for the differential diagnosis of POTS in individuals who demonstrate activity and exercise intolerance postconcussion are illustrated in Supplemental Digital Content 2 Appendix 1, International Classification of Functioning, Disability and Health Model for Identification POTS, available at: http://links.lww.com/JNPT/A212 . The presented International Classification of Functioning, Disability and Health model elucidates the similarities between symptoms of POTS and concussion and the shared medical history contributions that are often associated with prolonged recovery from concussion. Standard performance of a 10-minute standing test on initial physical therapy evaluation postconcussion would allow gathering of data regarding the incidence of orthostatic intolerance, early identification of the potential presence of autonomic dysfunction, and the possibility that POTS may be a poor prognostic factor in poor concussion recovery. Subsequent performance of orthostatic testing at monthly reassessment intervals for those with symptoms of unclear etiology, who exhibit poor response to conventional treatment or develop new-onset syncope, might reveal individuals who initially fall underneath the sustained elevation in HR diagnostic criteria but develop POTS over time. A schematic of the proposed integration of orthostatic testing in physical therapy management in people who sustain concussion or whiplash injuries is illustrated in Supplemental Digital Content 3 Appendix 2, Recommendations for the Management of POTS, available at: http://links.lww.com/JNPT/A213. Identification of POTS could more effectively identify individuals who benefit from a modified physical therapy intervention plan and allow a more accurate prognosis of recovery time frames.
EXERCISE TOLERANCE IN INDIVIDUALS WITH POTS AND CONCUSSION
Exercise training for individuals diagnosed with POTS and concussion must be monitored and individualized. A clinician guide to the development of a POTS exercise program for pediatric and adolescent patients was produced at the Children's Hospital of Philadelphia (CHOP) and is based on the Levine protocol.51 The underlying premise of the adapted exercise program is that individuals with POTS benefit from initial exercise training in a recumbent or seated position due to orthostatic intolerance that elicits tachycardia and excessive symptom provocation. The exercise guidelines, available through the Dysautonomia International Web site, include calculations for HR parameters and recommendations for rate of perceived exertion (RPE) during activity.62 The suggested preliminary Base Pace training zone for use in people with POTS is derived from age-related HR calculations and provides an adjustment to the HR training zones of current return to sport and activity concussion exertion training programs. It is anticipated that those individuals requiring preliminary recumbent exercises in a Base Pace training zone will require a protracted length of time to successfully tolerate 20 minutes of activity at this low intensity as it will be challenging to remain within the prescribed HR parameters due to sympathetic nervous system activation.
Heart rate training zone calculations should be modified for individuals with POTS due to persistent tachycardia causing exercise intolerance. Maximal HR is determined using the standard calculation (220 − age), but in the presence of POTS, the resting heart rate (RHR) must be determined by the quiet supine rest of orthostatic testing, as orthostatic postures would produce an elevated RHR. Subtracting RHR from the maximal HR determines heart rate reserve (HRR). In typical exercise training, a percentage of HRR is used to identify a targeted training zone. In the presence of POTS, the maximal steady state (MSS) is first calculated as 75% of the HRR plus the RHR (MSS = 0.75 [HRR] + RHR) to account for heightened sympathetic activity, ±5 beats per minute (bpm) for the MSS training zone.
Beginning exercise in the MSS training zone for those with POTS often results in elevated symptoms, tachycardia, and increased RPE. A base pace training zone offers a further reduction in training intensity and is determined as 75% to 85% of the MSS training zone; anything below the base pace becomes the recovery zone. Table 4 demonstrates calculated HR training zones for a 20-year-old with POTS and concussion who has a supine RHR of 76 bpm. As age increases and maximal HR reduces, the calculations for HR training zones will require further adjustment with appropriate clinical assessment of symptom provocation during supervised cardiovascular training.
Table 4. Sample Heart Rate Training Zone Calculations.
| Training Zone | Heart Rate, bpm | Expected Rate of Perceived Exertion |
|---|---|---|
| CHOP POTS Exercise Program62 | ||
| Base pace training zone | 127-144 | 2-4 |
| 0.75 (MSS) = 0.75(169) + 76 | ||
| 0.85 (MSS) = 0.85(169) + 76 | ||
| Maximal steady state | 164-174 | 5-8 |
| MSS = 0.75 (HRR) + RHR = 0.75 (124) + 76 | ||
| MSS training zone = (MSS) ± 5 bpm | ||
| Race pace = Above MSS | 175-185 | 7-9 |
| Recovery = Below base pace | <125 | 0-2 |
| UPMC 5-Stage Exertion Protocol for Concussion23 | ||
| Stage 1: 30%-40% maximal exertion | 113-126 | Light aerobic conditioning |
| 0.3 (HRR) + RHR = 0.3 (124) +76 | ||
| Stage 2: 40%-60% maximal exertion | 126-150 | Light to moderate aerobic conditioning |
| 0.4 (HRR) + RHR = 0.4 (124) + 76 | ||
| Stage 3: 60%-80% maximal exertion | 150-175 | Moderately aggressive aerobic exercise |
| 0.6 (HRR) + RHR = 0.6 (124) + 76 | ||
| Stage 4: 80% maximal exertion | 175 | 80% exertion avoiding contact |
| 0.8 (HRR) + RHR = 0.8(124) + 76 | ||
| Stage 5: 100% | 200 | Full exertion for sports with contact |
| HRR + RHR = 124 + 76 |
Abbreviations: HRR, heart rate reserve; MSS, maximal steady state; RHR, resting heart rate; UPMC, University of Pittsburgh Medical Center.
Concussion exertion training protocols offer parameters for submaximal HR training zones and simultaneous balance and strengthening activities congruent with the recommendations in the CHOP POTS Exercise Program.23,62 However, for the aforementioned 20-year-old without evidence of POTS, the target HR training zone of stage 1 (see Table 4) in the UPMC Exertion Protocol for Concussion is 30% to 40% of maximal exertion. Table 4 offers a comparison of the HR training zone parameters, illustrating that the current recommendations for exertion training may not be tolerated if someone had undetected POTS. The initial base pace HR training zone recommended for those with POTS is slightly elevated at 125 to 145 bpm to accommodate for the anticipated sympathetic tone. Base pace exercise training appears to be aligned with stage 2 and 40% to 60% of maximal exertion in the UPMC protocol, indicating that the person with POTS would likely be working at a higher RPE or workload than would be desired in early recovery if an adjusted training zone was not utilized.
The challenge for individuals with POTS will be to find a recumbent exercise that permits sustained exercise for 3 to 5 minutes without exceeding the recommended HR range, symptom exacerbation, or elevation of RPE. As noted in the CHOP POTS Exercise Program, it may take 4 months of very gradual progression in the base pace training zone to tolerate 30 minutes of exercise before being able to attempt training in the MSS training zone, which correlates with stage 3 and 60% to 80% maximal exertion in the typical postconcussion exertion training programs. This protracted recovery time results in profound reduction in activity participation and can cause much personal stress and anxiety. Failure to recognize POTS as a contributing factor to exercise and activity intolerance can be misinterpreted by health care providers as a psychological overlay or noncompliance with recommended activity.63
Instruction in base pace training and development of individual warm-up, cooldown, and recovery techniques should help avoid excessive rapid rise in HR associated with POTS. A critical element involves initial training in mode 1 using a semirecumbent rowing machine, recumbent cycle or stepper, or in a swimming pool to prevent excessive HR rise outside of the base pace HR training zone. Activity progression and dosing should be addressed similarly to exertion training in standard postconcussion care, as demonstrated in Table 5, though it may take considerable time to progress to 15 to 20 minutes of exercise tolerance. A person is likely to remain in mode 1, tolerating less than 10 minutes of base pace activity for the first month or longer as the level of fatigue and deconditioning associated with POTS is comparable with that of chronic obstructive pulmonary disease or congestive heart failure.27 It is not uncommon for people with POTS to have exacerbations and variable response to recumbent exercise, with the need to repeat a previous mode(s) over prolonged time frames due to variant phenotypes of POTS, medication changes, hormonal influences, viral illnesses, or simply balancing work or school responsibilities with exercise through profound fatigue.46,52,64
Table 5. Progressive Exercise Training Modes51,62.
| Mode 1 | Any recumbent activity (rowing, cycle, stepper, kicking in a pool) |
| Mode 2 | Upright stationary cycle |
| Mode 3 | Treadmill walking 0% grade; elliptical trainer (arms stationary) |
| Mode 4 | Treadmill walking with incline; elliptical trainer (arms moving); jogging 0% grade |
| Interval training | Initiate early interval training on recumbent equipment and gradually progress to upright postures |
Strengthening exercises to target postural control, core strength, and joint stabilization are prescribed alongside exercise training to overcome deconditioning and musculoskeletal pain that occur with prolonged inactivity. Necessary balance and sensory processing activities that involve somatosensory, visual, and vestibular conflict are beyond the scope of this article but are integral to the overall wellness and return to activity in patients recovering from prolonged inactivity associated with concussion and POTS.65 Monthly calendars, available as part of the CHOP exercise program available online through Dysautonomia International, offer structure and guidance to conduct cardiovascular exercise training in specific HR training zones 3 times per week, with twice-weekly strength training.62 Regular interdisciplinary communication with medical providers regarding activity tolerance provides necessary feedback regarding efficacy of potential POTS pharmacological interventions and fosters ongoing collaborative patient medical management.
COMORBIDITIES OF POTS AFFECTING EXERCISE TOLERANCE
Numerous comorbidities have been associated with POTS, some of which may interfere with successful exercise training. One-third of people with POTS have comorbid Ehlers-Danlos syndrome, a heterogeneous group of inherited connective tissues disorders associated with joint laxity, joint subluxations and dislocations, and arthralgia.66,67 The diagnosis of hypermobile Ehlers-Danlos syndrome, the most common form of Ehlers-Danlos syndrome seen in POTS, remains clinical as there is no genetic marker yet identified.68–70 The correlation between POTS and hereditary connective tissue disorders is particularly important in the postconcussion population as development of craniocervical instability, craniocervical dyskinesia, and Chiari brainstem compression requires critical medical attention and can be primarily related to concussion or whiplash injuries as well as hypermobile Ehlers-Danlos syndrome.71,72 Patients with POTS and comorbid hypermobility spectrum disorders should be instructed in exercise approaches that emphasize joint stabilization and core control. Excessive stretching and hyperextension of joints should be avoided.
In some individuals, including those with postconcussive onset of POTS symptoms, POTS may be the consequence of an autoimmune mediated autonomic neuropathy, which may require treatment targeted to the autoimmune condition in addition to exercise and other standard POTS treatments. A study of 100 consecutive patients with POTS found that 31% of patients had 1 or more antibody markers and 20% had comorbid autoimmune disorders such as Hashimoto's thyroiditis, rheumatoid arthritis, or systemic lupus erythematosus.35 Postural orthostatic tachycardia syndrome has also been reported in association with Sjögren's syndrome and celiac disease, both of which are known to cause autonomic neuropathies.73,74
Postural orthostatic tachycardia syndrome can occur in association with mast cell activation disorders, in which mast cells degranulate inappropriately and release mediators or proliferate to produce inflammatory responses that can occur throughout the body, as mast cells are present in all types of tissues.75 Exercise can be a trigger for mast cell degranulation in these patients and in severe cases may result in exercise-induced anaphylaxis.76 Some individuals with POTS and concussion also experience chronic fatigue and disturbed sleep and can benefit from a variety of supportive therapies due to prolonged recovery time and reduced engagement in life activities.30
For individuals with known or suspected autoimmune diseases or mast cell activation disorders, further medical management and medication support may be necessary to suppress pain and inflammatory responses associated with exercise.37,75 This can include a wide spectrum of interventions from regular antihistamine use to immunomodulation infusion therapies. Current research efforts are targeted at better understanding antibody receptor and neurotransmitter activity involved in immunological responses and autonomic nervous system function.17 For some individuals, return to previous sport and intensity of activities may be inhibited by the development of autonomic small fiber neuropathies.
SUMMARY AND IMPLICATIONS FOR PRACTICE
Postural orthostatic tachycardia syndrome is a syndrome encompassing a cluster of symptoms following a wide range of triggering events resulting in a persistent dysfunction of autonomic nervous system regulation.30,77 Numerous symptoms of POTS and concussion can be indistinguishable, requiring astute history taking for subjective complaints correlated with provoking activities. While transient dysautonomia is recognized as a consequence of concussion, there is growing evidence that protracted elevation of sympathetic activity can result in exercise intolerance and poor response to physical therapy interventions.78 Routine collection of data related to orthostatic testing upon initial evaluation and at regular intervals will confirm the incidence of POTS related to concussion and whiplash-related deceleration injuries. Early identification of POTS allows necessary communication with medical providers for refinement of the diagnosis, patient education, medication support, and referral to specialists as needed. Monitoring of HR and BP during exertion training postconcussion is imperative to prevent misinterpretation of exercise intolerance with symptom exacerbation. Failure to recognize POTS as a cause for dizziness and protracted recovery disregards a major complication that could result in elevated symptoms, stress, anxiety, and depression due to poor response to vestibular and neuromuscular physical therapy interventions.
In the case in which transient dysautonomia is detected but does not resolve, or POTS is identified in protracted recovery with heavy symptom overlay, the COMPASS 31 may be a useful screening tool to identify the need to refer to medical providers for further diagnostic testing. Medical management in complex cases involving POTS often requires a series of medication trials to modulate a variety of gastrointestinal, hormonal, neurological, immunological, and allergy-related symptoms. Feedback to prescribing providers may help determine medication success or failure as related to exercise and activity tolerance in physical therapy, though development of an appropriate measurement tool is needed. At present, the Patient Specific Function Scale could aid in assessing progress toward reduction of impairments and designing patient-directed goals for return to graded participation in specific activities.79
Finally, research studies should be conducted in physical therapy to determine whether exercise tolerance improves and whether recovery from POTS can be achieved with physical therapy oversight of exercise training progressing from recumbent to upright activity in conjunction with core and joint stabilization as well as strength training. Studies including submaximal exercise training in subjects who have sustained known concussion and whiplash injuries with and without evidence of dysautonomia are necessary to determine the effect of addressing POTS in physical therapy on overall recovery from injury. Physical therapy involvement in management of POTS would assist in identification of comorbidities such as joint hypermobility, inflammatory responses, and neurological findings such as neuropathy and neuropathic pain that further limit exercise tolerance in a subset of individuals with a complicated and protracted recovery pattern.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jnpt.org).
REFERENCES
- 1.Postconcussional syndrome. International Statistical Classification of Diseases and Related Health Problems 10th Revision Web site. http://apps.who.int/classifications/icd10/browse/2016/en#/F07.2 Published 2008. Updated 2016. Accessed April 3, 2018.
- 2.Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–2318. [DOI] [PubMed] [Google Scholar]
- 3.Iverson GL, Gaetz M, Lovell MR, Collins MW. Relation between subjective fogginess and neuropsychological testing following concussion. J Int Neuropsychol Soc. 2004;10(6):904–906. [DOI] [PubMed] [Google Scholar]
- 4.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87–93. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski P, Wilson J, Walker A, Enz D, Wang S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: a retrospective analysis on safety and feasibility. Phys Ther Sport. 2017;23:22–30. [DOI] [PubMed] [Google Scholar]
- 6.Gurley JM, Hujsak BD, Kelly JL. Vestibular rehabilitation following mild traumatic brain injury. Neurorehabilitation. 2013;32(3):519–528. [DOI] [PubMed] [Google Scholar]
- 7.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(suppl 4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. [DOI] [PubMed] [Google Scholar]
- 9.Toledo E, Lebel A, Becerra L, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Biobehav Rev. 2012;36(6):1510–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson JL, Yarbrough MB, Perez J, Evans K, Buckley T. Sport-related concussion induces transient cardiovascular autonomic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R575–R584. [DOI] [PubMed] [Google Scholar]
- 11.Kobeissy F, Mondello S, Tümer N, et al. Assessing neuro-systemic & behavioral components in the pathophysiology of blast-related brain injury. Front Neurol. 2013;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Fountaine MF, Toda M, Testa AJ, Hill-Lombardi V. Autonomic nervous system responses to concussion: arterial pulse contour analysis. Front Neurol. 2016;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conder RL, Conder AA. Heart rate variability interventions for concussion and rehabilitation. Front Psychol. 2014;5:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj. 2016;30(2):132–145. [DOI] [PubMed] [Google Scholar]
- 15.Esterov D, Greenwald BD. Autonomic dysfunction after mild traumatic brain injury. Brain Sci. 2017;7(8):pii:E100. 10.3390/brainsci7080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilz MJ, Liu M, Koehn J, et al. Valsalva maneuver unveils central baroreflex dysfunction with altered blood pressure control in persons with a history of mild traumatic brain injury. BMC Neurol. 2016;16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dambinova SA, Maroon JC, Sufrinko AM, Mullins JD, Alexandrova EV, Potapov AA. Functional, structural, and neurotoxicity biomarkers in integrative assessment of concussions. Front Neurol. 2016;7:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis MJ, Leddy J, Willer B. Multi-disciplinary management of athletes with post-concussion syndrome: an evolving pathophysiological approach. Front Neurol. 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30(1):33–48, vii. [DOI] [PubMed] [Google Scholar]
- 20.Baker JG, Freitas MS, Leddy JJ, Kozlowski KF, Willer BS. Return to full functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabil Res Pract. 2012;2012:705309. 10.1155/2012/705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman B, Vargas B, Dodick D. Autonomic nervous system dysfunction in concussion (P01. 265). Neurology. 2013;80(7 suppl):P01–265. [Google Scholar]
- 22.Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221(3):1291–1308. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds E, Collins MW, Mucha A, Troutman-Ensecki C. Establishing a clinical service for the management of sports-related concussions. Neurosurgery. 2014;75(suppl 4):S71–S81. [DOI] [PubMed] [Google Scholar]
- 24.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, November 2012. J Athl Train. 2013;48(4):554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DCoE clinical recommendation: progressive return to activity following acute concussion/mild TBI clinical suite. Defense and Veteran Brain Injury Center and Defense Centers of Excellence Web site. https://dvbic.dcoe.mil/material/progressive-return-activity-following-acute-concussionmild-tbi-clinical-suite. Published January 2014. Accessed May 1, 2017.
- 26.Heyer GL, Fischer A, Wilson J, et al. Orthostatic intolerance and autonomic dysfunction in youth with persistent postconcussion symptoms: a head-upright tilt table study. Clin J Sport Med. 2016;26(1):40–45. [DOI] [PubMed] [Google Scholar]
- 27.Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117(21):2814–2817. [DOI] [PubMed] [Google Scholar]
- 28.Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 29.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kizilbash SJ, Ahrens SP, Bruce BK, et al. Adolescent fatigue, POTS, and recovery: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care. 2014;44(5):108–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyer GL, Fedak EM, LeGros AL. Symptoms predictive of postural tachycardia syndrome (POTS) in the adolescent headache patient. Headache. 2013;53(6):947–953. [DOI] [PubMed] [Google Scholar]
- 32.Boris JR, Bernadzikowski T. Demographics of a large pediatric postural orthostatic tachycardia syndrome program. Cardiology in the Young. 2018;28(5):668–674. [DOI] [PubMed] [Google Scholar]
- 33.Goodman B, Dhawan P, Bogle J, Barrs D, Hoffman-Snyder C. Vestibular symptoms are common in postural tachycardia syndrome (PoTS) (P1. 281). Neurology. 2015;84(14 suppl):P1–281. [Google Scholar]
- 34.Pavlik D, Agnew D, Stiles L, Ditoro R. Recognizing postural orthostatic tachycardia syndrome. JAAPA. 2016;29(4):17–23. [DOI] [PubMed] [Google Scholar]
- 35.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. 2015;24(13):1364–1369. [DOI] [PubMed] [Google Scholar]
- 36.Grigoriou E, Boris JR, Dormans JP. Postural orthostatic tachycardia syndrome (POTS): association with Ehlers-Danlos syndrome and orthopaedic considerations. Clin Orthop Relat Res. 2015;473(2):722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afrin LB, Butterfield JH, Raithel M, Molderings GJ. Often seen, rarely recognized: mast cell activation disease—a guide to diagnosis and therapeutic options. Ann Med. 2016;48(3):190–201. [DOI] [PubMed] [Google Scholar]
- 38.Stewart JM, Taneja I, Medow MS. Reduced central blood volume and cardiac output and increased vascular resistance during static handgrip exercise in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293(3):H1908–H1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–1582. [DOI] [PubMed] [Google Scholar]
- 40.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308–313. [DOI] [PubMed] [Google Scholar]
- 41.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr. 2004;145(6):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J. 2011;18(5):527–531. [DOI] [PubMed] [Google Scholar]
- 43.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342(8):541–549. [DOI] [PubMed] [Google Scholar]
- 44.Khan AW, Ziemann M, Corcoran SJ, et al. NET silencing by let-7i in postural tachycardia syndrome. JCI Insight. 2017;2(6):e90183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Q, Vangundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55(25):2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pianosi PT, Schroeder DR, Fischer PR. Cardiac responses to exercise distinguish postural orthostatic tachycardia syndrome variants. Physiol Rep. 2016;4(22):pii: e13040. 10.14814/phy2.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res. 2011;21(2):73–80. [DOI] [PubMed] [Google Scholar]
- 49.Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590(15):3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masuki S, Eisenach JH, Schrage WG, et al. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103(4):1128–1135. [DOI] [PubMed] [Google Scholar]
- 51.George SA, Bivens TB, Howden EJ, et al. The international POTS registry: Evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13(4):943–950. [DOI] [PubMed] [Google Scholar]
- 52.Richardson MV, Nordon-Craft A, Carrothers L. Using an exercise program to improve activity tolerance in a female with postural orthostatic tachycardia syndrome: a case report. Physiother Theory Pract. 2017;33(8):670–679. [DOI] [PubMed] [Google Scholar]
- 53.Kline GM, Porcari JP, Hintermeister R, et al. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc. 1987;19(3):253–259. [PubMed] [Google Scholar]
- 54.Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr. 2016;173:149–153. [DOI] [PubMed] [Google Scholar]
- 55.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treister R, O'Neil K, Downs HM, Oaklander AL. Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy. Eur J Neurol. 2015;22(7):1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rea NA, Campbell CL, Cortez MM. Quantitative assessment of autonomic symptom burden in postural tachycardia syndrome (POTS). J Neurol Sci. 2017;377:35–41. [DOI] [PubMed] [Google Scholar]
- 58.Sheldon RS, Grubb BP II, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. [DOI] [PubMed] [Google Scholar]
- 60.Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127(23):2336–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plash WB, Diedrich A, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond). 2013;124(2):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Exercises for dysautonomia patients: CHOP modified Dallas protocol. Dysautonomia International Web site. http://www.dysautonomiainternational.org/exercise. Published June 2017. Accessed April 3, 2018.
- 63.Masuki S, Eisenach JH, Johnson CP, et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol. 2007;102(3):896–903. [DOI] [PubMed] [Google Scholar]
- 64.Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010;56(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kristjansson E, Treleaven J. Sensorimotor function and dizziness in neck pain: implications for assessment and management. J Orthop Sports Phys Ther. 2009;39(5):364–377. [DOI] [PubMed] [Google Scholar]
- 66.Chelimsky G, Kovacic K, Nugent M, Mueller A, Simpson P, Chelimsky TC. Comorbid conditions do not differ in children and young adults with functional disorders with or without postural tachycardia syndrome. J Pediatr. 2015;167(1):120–124. [DOI] [PubMed] [Google Scholar]
- 67.Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32(16):1339–1345. [DOI] [PubMed] [Google Scholar]
- 68.Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017;175(1):148–157. [DOI] [PubMed] [Google Scholar]
- 69.Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. e1-e4. [DOI] [PubMed] [Google Scholar]
- 70.Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):8–26. [DOI] [PubMed] [Google Scholar]
- 71.Engelbert RH, Juul-Kristensen B, Pacey V, et al. The evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175(1):158–167. [DOI] [PubMed] [Google Scholar]
- 72.Castori M, Morlino S, Ghibellini G, Celletti C, Camerota F, Grammatico P. Connective tissue, Ehlers-Danlos syndrome(s), and head and cervical pain. Am J Med Genet C Semin Med Genet. 2015;169C(1):84–96. [DOI] [PubMed] [Google Scholar]
- 73.Goodman BP. Immunoresponsive autonomic neuropathy in Sjögren syndrome-case series and literature review [published online ahead of print March 31, 2017]. Am J Ther. 10.1097/MJT.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 74.Penny HA, Aziz I, Ferrar M, et al. Is there a relationship between gluten sensitivity and postural tachycardia syndrome? Eur J Gastroenterol Hepatol. 2016;28(12):1383–1387. [DOI] [PubMed] [Google Scholar]
- 75.Molderings GJ, Haenisch B, Brettner S, et al. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(7):671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jennings S, Russell N, Jennings B, et al. The Mastocytosis Society survey on mast cell disorders: patient experiences and perceptions. J Allergy Clin Immunol Pract. 2014;2(1):70–76. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal AK, Garg R, Ritch A, Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83(981):478–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. J Athl Train. 2013;48(5):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fairbairn K, May K, Yang Y, Balasundar S, Hefford C, Abbott JH. Mapping Patient-Specific Functional Scale (PSFS) items to the International Classification of Functioning, Disability and Health (ICF). Phys Ther. 2012;92(2):310–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


