Abstract
STUDY QUESTION
Can primary human uterine microvascular endothelial cells (UtMVECs) be used as a model to study uterine angiogenic responses in vitro that are relevant in pregnancy?
SUMMARY ANSWER
UtMVECs demonstrated angiogenic responses when stimulated with proangiogenic factors, including sphingosine 1-phosphate (S1P), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), physiological levels of wall shear stress (WSS), human chorionic gonadotropin (hCG) and various combinations of estrogen and progesterone.
WHAT IS KNOWN ALREADY
During sprouting angiogenesis, signaling from growth factors and cytokines induces a monolayer of quiescent endothelial cells (ECs) lining the vasculature to degrade the extracellular matrix and invade the surrounding tissue to form new capillaries. During pregnancy and the female reproductive cycle, the uterine endothelium becomes activated and undergoes sprouting angiogenesis to increase the size and number of blood vessels in the endometrium.
STUDY DESIGN, SIZE, DURATION
The study was designed to examine the angiogenic potential of primary human UtMVECs using the well-characterized human umbilical vein EC (HUVEC) line as a control to compare angiogenic potential. ECs were seeded onto three-dimensional (3D) collagen matrices, supplemented with known proangiogenic stimuli relevant to pregnancy and allowed to invade for 24 h. Sprouting responses were analyzed using manual and automated methods for quantification.
PARTICIPANTS/MATERIALS, SETTING, METHODS
RT-PCR, Western blot analysis and immunostaining were used to characterize UtMVECs. Angiogenic responses were examined using 3D invasion assays. Western blotting was used to confirm signaling responses after proangiogenic lipid, pharmacological inhibitor, and recombinant lentiviral treatments. All experiments were repeated at least three times.
MAIN RESULTS AND THE ROLE OF CHANCE
After ensuring that UtMVECs expressed the proper endothelial markers, we found that UtMVECs invade 3D collagen matrices dose-dependently in response to known proangiogenic stimuli (e.g. S1P, VEGF, bFGF, hCG, estrogen, progesterone and WSS) present during early pregnancy. Invasion responses were positively correlated with phosphorylation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and p42/p44 mitogen-activated protein kinase (ERK). Inhibition of these second messengers significantly impaired sprouting (P < 0.01). Gene silencing of membrane type 1-matrix metalloproteinase using multiple approaches completely abrogated sprouting (P < 0.001). Finally, UtMVECs displayed a unique ability to undergo sprouting in response to hCG, and combined estrogen and progesterone treatment.
LARGE SCALE DATA
Not applicable.
LIMITATIONS, REASONS FOR CAUTION
The study of uterine angiogenesis in vitro has limitations and any findings many not fully represent the in vivo state. However, these experiments do provide evidence for the ability of UtMVECs to be used in functional sprouting assays in a 3D environment, stimulated by physiological factors that are produced locally within the uterus during early pregnancy.
WIDER IMPLICATIONS OF THE FINDINGS
We show that UtMVECs can be used reliably to investigate how growth factors, hormones, lipids and other factors, such as flow, affect angiogenesis in the uterus.
STUDY FUNDING/COMPETING INTERESTS
This work was supported by NIH award HL095786 to K.J.B. The authors have no conflicts of interest.
Keywords: angiogenesis, human uterine microvascular endothelial cells, sphingosine 1-phosphate, hCG, wall shear stress, estrogen, progesterone
Introduction
During angiogenesis, endothelial cells (ECs) lining blood vessels are activated by proangiogenic stimuli to locally degrade the basement membrane and extracellular matrix (ECM), invade the surrounding tissue and form new blood vessels (Folkman and D’Amore, 1996; Carmeliet and Jain, 2011; Potente et al., 2011). While ECs are normally quiescent in adults, after activation of the angiogenic switch, they can be induced to undergo sprouting angiogenesis (Bergers and Benjamin, 2003). This activation occurs in the uterine endothelium during pregnancy and the female reproductive cycle and also occurs during wound repair (Folkman, 1995; Ribatti et al., 2007).
Proper cyclical angiogenesis and angiogenic development of capillaries and arterioles within the decidua early in pregnancy are critical for normal reproductive processes and placental development, respectively (Reynolds and Redmer, 1995; Reynolds et al., 2005a, b). Between gestational days 7 and 11 of human pregnancy, the vessels of the uterus respond to locally produced angiogenic factors to initiate angiogenesis and surround the syncytiotrophoblast layer (Burton et al., 1999). This early endometrial decidualization provides the developing fetus with adequate nutrition before maturation of the placenta where, in humans, the uteroplacental circulation opens around Week 12 (Hustin and Schaaps, 1987). Impaired decidual vascularization in response to proangiogenic stimuli and defective uterine spiral artery remodeling are associated with detrimental complications, including recurrent spontaneous abortions (RSAs), high-blood pressure, pre-eclampsia and fetal growth restriction, while excessive uterine angiogenesis early in pregnancy is thought to increase oxidative stress in the conceptus, leading to RSA (Quenby et al., 2009; Williams et al., 2009; Lyall et al., 2013). Thus, investigation of the mechanisms controlling uterine angiogenesis in humans is critical to completely understand key events required in early pregnancy.
Throughout pregnancy, various proangiogenic growth factors (GFs), including vascular endothelial GF (VEGF) and basic fibroblast GF (bFGF), are produced in utero by the decidua, placenta and endometrium, stimulating new blood vessel growth (Reynolds et al., 1992; Torry and Rongish, 1992; Zygmunt et al., 1998a, 2002, 2003; Hyder and Stancel, 1999; Li et al., 2001; Herr et al., 2003). In response to VEGF binding to the VEGF receptor 2 (VEGFR2), both the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Protein kinase B (Akt) pathways are activated through second messengers, inducing ECs to undergo increased proliferation, chemotaxis and reorganization of the actin cytoskeleton to increase vascular permeability (Ferrara and Henzel, 1989; Dvorak et al., 1991; Senger et al., 1993; Waltenberger et al., 1994; Hanahan and Folkman, 1996; Yoshida, 1996; Ferrara and Davis-Smyth, 1997; Takahashi et al., 1999, 2001; Ruan and Kazlauskas, 2012). VEGF expression increases 3-fold during the secretory phase of the uterine cycle (Torry et al., 1996). Additionally, VEGF isoforms and receptors are differentially expressed during implantation, suggesting a role for VEGF signaling during early pregnancy when sprouting angiogenesis is critical (Hyder and Stancel, 1999; Halder et al., 2000). bFGF acts as a potent mitogen (Shing et al., 1984, 1985; Friesel and Maciag, 1995), activating PI3K and downstream Akt signaling (Ong et al., 2001; Rieck et al., 2001) as well as the MAPK pathway (Eliceiri et al., 1998; Giuliani et al., 1999; Cross et al., 2002; Tanghetti et al., 2002), inducing the upregulation of matrix metalloproteinases (MMPs), as well as proliferation and migration of ECs (Taraboletti et al., 2002). Expression of bFGF is increased in the porcine uterine epithelium and conceptus during the peri-implantation period (days 10–14), but not during the corresponding days of the estrous cycle (Gupta et al., 1997). Further, in cultured ECs, VEGF and bFGF have synergistic effects on cell proliferation and gene expression of proangiogenic factors (Pepper and Mandriota, 1998). These findings highlight a key role for both VEGF and bFGF in successful pregnancy.
Sphingosine 1-phosphate (S1P) synergizes with VEGF and bFGF in vitro to induce angiogenesis (Bayless and Davis, 2003). S1P is a bioactive lysosphingolipid released by activated platelets or generated locally by sphingosine kinase. On ECs, S1P binds to the G-protein coupled receptors S1P1 and S1P3 (Garcia et al., 2001; Kluk and Hla, 2002). This interaction triggers Gi signaling leading to downstream activation of extracellular-signal regulated kinase (ERK) and PI3K signaling pathways to promote angiogenesis (Lee et al., 1999; Wang et al., 1999; Morales-Ruiz et al., 2001). S1P1 and S1P3 along with sphingosine kinase are upregulated in the uterine endothelium during pregnancy, and inhibition of S1P signaling leads to defects in endothelial sprouting responses (Skaznik-Wikiel et al., 2006; Kaneko-Tarui et al., 2007; Dunlap et al., 2010). Not only does S1P synergize with VEGF and bFGF in vitro to promote sprouting (Bayless and Davis, 2003), S1P also stimulates the activation and translocation of the membrane-bound MMP, MT1-MMP, to the plasma membrane (Nyalendo et al., 2007; Gingras et al., 2008; Kwak et al., 2012). Once localized to the plasma membrane, MT1-MMP degrades multiple basement membrane and ECM components, such as fibrin, fibronectin, and collagen type I, remodeling the environment surrounding the uterine microvasculature (Wewer et al., 1986; Aplin et al., 1988; Ohuchi et al., 1997; Vu and Werb, 2000; Koshikawa et al., 2005; Sato et al., 2005; Nyalendo et al., 2007). Thus, signaling responses downstream of the lysosphingolipid S1P are likely critical for successful angiogenic responses.
Although most ECs are quiescent after development, the uterine endothelium serves as a striking exception to this rule, becoming repeatedly activated during the female reproductive cycle and during pregnancy. Cycling reproductive hormones, estrogen and progesterone, and other pregnancy-stimulated lipids and proteins have been implicated in inducing the angiogenic switch in the uterine endothelium (Lebovic et al., 2000; Moller et al., 2001). One such hormone, human chorionic gonadotropin (hCG), is produced and secreted by placental trophoblasts before implantation (Muyan and Boime, 1997; Kovalevskaya et al., 2002; Handschuh et al., 2007; Guibourdenche et al., 2010). After binding to G-protein coupled LH-hCG receptors on uterine EC (Toth et al., 1994), hCG has been shown to signal in a pertussis toxin (PTx)- sensitive manner to induce sprouting angiogenesis in in vitro assays (Zygmunt et al., 2002; Berndt et al., 2006; Herr et al., 2007). Thus, like VEGF, bFGF and S1P, hCG is well recognized to stimulate angiogenic responses in ECs.
Along with these proangiogenic factors, ECs are also constantly exposed to wall shear stress (WSS) applied by blood flow. Fluid forces can combine with proangiogenic molecules to promote angiogenesis in post-capillary venules (Davies et al., 1995; Topper and Gimbrone, 1999), where WSS levels are between 1 and 8 dyn/cm2 (Ichioka et al., 1997; Koutsiaris et al., 2007). During pregnancy, the overall maternal cardiac output increases, facilitating an increase in uteroplacental blood flow (Lecarpentier et al., 2016). This increased blood flow promotes placental vascularization, which is critical for proper fetal development (Reynolds and Redmer, 1995; Magness, 1998). Multiple studies have uncovered the importance of WSS forces, caused by flow, for proper vascular remodeling in the developing fetus (Hogers et al., 1997; Ichioka et al., 1997; Hove et al., 2003; Isogai et al., 2003; Lucitti et al., 2007). Therefore, the application of WSS to uterine ECs is a critical component to include when modeling the factors that promote uterine angiogenesis.
In studying angiogenesis in vitro, human umbilical vein ECs (HUVECs) have been utilized as a model to investigate angiogenic sprouting events. When studying the physiological or pathological angiogenesis of specific organs, however, often ECs of that particular tissue are preferable, as there may be tissue specific stimuli and responses not seen in all ECs. In this study, we tested the potential of primary human uterine microvascular ECs (UtMVECs) to serve as a model cell line to study endometrial sprouting angiogenesis in three-dimensional (3D) type I collagen matrices. We observe that UtMVECs can respond to various proangiogenic stimuli relevant to pregnancy that promote invasion into collagen matrices, through activation of key signaling pathways known to be essential during angiogenesis. Importantly, these studies provide a quantifiable model to investigate and quantify 3D UtMVEC sprouting, which should serve as a useful approach to aid our understanding of uterine angiogenesis during pregnancy and the female reproductive cycle.
Materials and Methods
Reagents
D-erythro-sphingosine 1-phosphate (S1P), ascorbic acid, pertussis toxin (PTx), β-estradiol, progesterone and hCG were purchased from Sigma-Aldrich (MO, USA). TNFα protease inhibitor-2 (TAPI-2), UO126, and Akt Inhibitor-X (Akt-X) were obtained from Calbiochem (CA, USA). VEGF and bFGF were purchased from R&D Systems (MN, USA). Tissue culture flasks were obtained from Corning (NY, USA). Rat-tail collagen type I was isolated as described (Bornstein, 1958).
Cells
UtMVECs (Lonza, MD, USA), were used at passages 4–7, and HUVECs (Lonza, MD, USA), were used at passages 2–6. Cells were cultured on gelatin-coated flasks in M199 (Gibco, Thermo Fisher, MA, USA) growth medium containing fetal bovine serum (Invitrogen, CA, USA), bovine hypothalamic extract (Maciag et al., 1979) and heparin (Sigma-Aldrich, MO, USA), as previously described (Bayless et al., 2009).
mRNA isolation, cDNA synthesis and RT-PCR
RNA was isolated from HUVECs and UtMVECs at Passage 5 using an RNeasy minikit (Qiagen, MD, USA). RNA was treated with RNase-Free DNase (Qiagen, MD, USA) for 10 min at 22°C. RNA (1.5 μg) was reverse transcribed into cDNA using SuperScript III First-Strand Synthesis System (Invitrogen, CA, USA) following manufacturer’s instructions. Primers were used to amplify human GAPDH (NM_002046.4, 228 bp; 5′-CGACCACTTTGTCAAGCTCA-3′ and 5′-AGGGGTCTACATGGCAACTG-3′), Claudin-5 (NM_001130861.1, 239 bp; 5′-GAGGCGTGCTCTACCTGTTT-3′ and 5′-GTACTTCACGGGGAAGCTGA-3′), Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) (M28526.1, 172 bp; 5′-ATGATGCCCAGTTTGAGGTC-3′ and 5′-ACGTCTTCAGTGGGGTTGTC-3′), vascular endothelial (VE)-cadherin (NM_001795.3, 182 bp; 5′-CCAGGTATGAGATCGTGGTG-3′ and 5′-AAACAGAGAGCCCACAGAGG-3′), Vimentin (NM_003380, 177 bp; 5′-GGGACCTCTACGAGGAGGAG-3′ 5′-AAGATTGCAGGGTGTTTTCG-3′), von Willebrand Factor (vWF) (K03028.1, 152 bp; 5′-AGATGTTTGCCTACGGCTTG-3′ and 5′-CAGCCTGTGACCCTCTTCTC-3′), Caveolin-1 (NM_001172897.1, 152 bp; 5′-GAGCTGAGCGAGAAGCAAGT-3′ and 5′-CAAATGCCGTCAAAACTGTG-3′), α-Smooth Muscle Actin (α–SMA, ×13839.1, 222 bp; 5′-TTCAATGTCCCAGCCATGTA-3′ and 5′-GAAGGAATAGCCACGCTCAG-3′), and cytokeratin-8 (M34225.1, 158 bp; 5′-GTGGAGAGCTGGCCATTAAG-3′ and 5′-CTTCCTGTAGGTGGCGATGT-3′). RT-PCR was performed using a 58°C annealing temperature and 30 s extension time for 25 cycles.
Immunostaining
UtMVECs (Passage 4) were seeded onto 12 mm2 coverslips (50 000 cells per coverslip) precoated with 50 μl of 20 μg/ml type I collagen in a 24-well plate. Cells were allowed to attach for 1 h at 37°C with 5% CO2 before adding 1 ml M199-based growth medium overnight. Cells were fixed with 4% paraformaldehyde in PBS for 20 min and washed two times for 15 min in Tris-Glycine buffer containing 0.3% tris and 1.5% glycine. After permeabilization for 20 min using 0.5% Triton X-100 in PBS, cells were blocked overnight in 0.1% Triton X-100, 1% bovine serum albumin (BSA) and 1% goat serum in TBS at 4°C. Coverslips were incubated with primary antibodies directed against PECAM-1 (1:200; 3528S, Cell Signaling, MA, USA), VE-cadherin (1:200; sc-9989 Santa Cruz Biotechnology, TX, USA), claudin-5 (1:100; 35–2500, Invitrogen, USA), vimentin (1:100; sc-6260, Santa Cruz Biotechnology), vWF (1:100; A0082, DAKO, CA, USA), α-SMA (1:200; A5228, Sigma-Aldrich), mouse IgG (1:100; 02–6502, Invitrogen), or rabbit IgG (1:100; 02–6102, Invitrogen) diluted in blocking buffer in a humidified chamber, at room temperature, for 3 h. Coverslips were washed three times for 20 min with 0.1% Triton X-100 in PBS before a 1 h incubation with goat Alexa Fluor 488-conjugated secondary antibodies (1:300; Molecular Probes, Thermo Fisher, MA, USA) diluted in blocking buffer. After washing three times for 20 min in 0.1% Triton X-100 in PBS, coverslips were incubated with 1 μM DAPI (Molecular Probes) for 10 min at room temperature with slight agitation before mounting and imaging with a Nikon TI A1R inverted confocal microscope. Using a numerical aperture of 1.4, 60× Z-stack images were taken, using eight 0.4 μm steps, compressed and analyzed using Nikon Elements Software (Media Cybernetics, MD, USA).
Invasion assays
Invasion assays were performed as described previously (Bayless et al., 2009). Briefly, 3D type I collagen matrices were prepared at a final concentration of 2.5 mg/ml containing specified concentrations of S1P (0–1 μM). A total of 28 μl of collagen was added per well of a half-area 96-well plate (Corning), and allowed to polymerize at 37°C with 5% CO2 for 30–45 min. Cells (30 000) were allowed to attach in a 50 μl volume of M199 containing 1× reduced serum II ((RSII), 500 μg/ml BSA, 5 μg/ml insulin, 5 μg/ml human holo-transferrin, 4.28 μg/ml oleic acid, 5 ng/ml sodium selenite; Sigma-Aldrich, USA). After 30 min, an additional 50 μl of M199 containing 1× RSII, 80 ng/ml VEGF, 80 ng/ml bFGF, and 100 μg/ml ascorbate was added (Final concentration: 1×RSII, 40 ng/ml VEGF, 40 ng/ml bFGF, 50 μg/ml ascorbate in 100 μl M199). Cells were allowed to invade for 24 h at 37°C with 5% CO2 before fixing in 3% glutaraldehyde (Sigma-Aldrich) in PBS and staining with 0.1% toluidine blue (Sigma-Aldrich) in 30% methanol.
In S1P dose–response experiments, collagen matrices were prepared as described above, containing 0, 10, 100 or 1000 nM S1P. In time-course experiments, ECs were seeded onto collagen matrices containing either 0 or 1 μM S1P for 30 min before collecting extracts. In the remaining wells, 50 μl of M199 containing RSII, GFs and ascorbate was added (as described above), and cells were allowed to invade for 8, 12, 16 and 24 h before being fixed, stained and quantified. In experiments with pharmacological inhibitors, ECs were pretreated with compounds for 20 min at 37°C. Vehicle controls diluted identically were included for PTx (50% (v/v) glycerol with 50 mM Tris, pH 7.5, 10 mM glycine, and 0.5 M NaCl), Akt-X (water), UO126 (DMSO) and TAPI-2 (DMSO). VEGF (40 ng/ml), bFGF (40 ng/ml), RSII and ascorbate (50 μg/ml) were added to cells before seeding onto collagen matrices. Cells were allowed to invade for 24 h before fixing in 3% glutaraldehyde in PBS and staining with 0.1% toluidine blue in 30% methanol.
In hCG dose–response experiments, hCG was reconstituted in sterile water at 4000 U/ml, aliquoted for one-time use, and stored at −80°C. Collagen matrices were prepared as described above, except with the addition of 0 or 300 nM S1P and 0, 20, 100, 200 or 400 U/ml hCG. Cells were seeded as described above and allowed to invade for 24 h, fixed in 3% glutaraldehyde and stained with 0.1% toluidine blue in 30% methanol.
Experiments delivering β-estradiol (E2) and progesterone (P4) were conducted in phenol red-free DMEM (Gibco). ECs were seeded onto collagen matrices, diluted in phenol red-free DMEM containing 300 nM S1P. After 30 min, cells were supplemented with 50 μl of phenol red-free DMEM, containing final concentrations of 1× RSII, 4 ng/ml VEGF and bFGF (10-fold less than in previously described experiments), 50 μg/ml ascorbate, and the following doses of hormones: 0 nM E2 and 0 nM P4, 0.1 nM E2 and 10 nM P4, 1 nM E2 and 100 nM P4, or 10 nM E2 and 1000 nM P4. Cells were allowed to invade for 24 h before being fixed, stained and quantified.
WSS experiments
WSS experiments were conducted as previously described (Duran et al., 2017). Briefly, collagen matrices were prepared at a final concentration of 3.75 mg/ml containing specified concentrations of S1P (0 or 1 μM) and GF (0 or 4 ng/ml VEGF and 0 or 4 ng/ml bFGF). Collagen (46 μl) was added per well (7 mm diameter) of an 8-well silicone mold pressed onto a 2 × 3 inch glass slide, and allowed to polymerize at 37°C with 5% CO2 for 1 h. Cells were seeded (50 000/100 μl M199) and allowed to attach for 2 h before assembling onto parallel plate flow chambers. A uniform flow rate of 5.3 dyn/cm2 WSS was applied using M199 supplemented with 1x RSII and 50 μg/ml ascorbate. Cells were allowed to invade for 24 h at 37°C with 5% CO2 before fixing in 3% glutaraldehyde in PBS and staining with 0.1% toluidine blue in 30% methanol.
Quantification of invasion responses
Invasion density was quantified manually as the average number of invading structures per 0.25 mm2 field using an eyepiece fitted with a 10 × 10 ocular grid. At least three wells were counted per treatment for three independent experiments. Invasion density was also quantified automatically by photographing individual wells at low power (4×). Images were captured using an Olympus CKX41 microscope with a Q-Color three camera, and one image was taken per well. A single depth was chosen and applied to all treatment groups to capture images. Images were imported into ImagePro Analyzer Software (Media Cybernetics, MD, USA). After correcting for background, a standardized region of interest was uniformly applied to every image to objectively quantify the number of invading structures per image. Identical settings were applied to all wells and treatment groups in each experiment. At least three replicate wells were analyzed and averaged per treatment from three independent experiments.
To visualize and measure invasion distance of sprouting ECs, toluidine blue stained samples were cut and imaged from the side using an Olympus CKX41 microscope with a Q-Color three camera. Invasion distance was quantified using ImagePro Analyzer Software using 4× magnification images taken of side views of invasion. The perpendicular distance from the cell monolayer to the leading edge of invading cells was recorded as invasion distance. At least 100 cells per treatment group were included in the analysis.
Western blotting
Whole cell extracts from invasion experiments were prepared by removing collagen matrices from wells at indicated time points and adding to boiling 1.5× Laemmli sample buffer containing 2% 2-mercaptoethanol and incubating at 100°C for 10 min before storing at −20°C. Proteins were separated on 8.5% sodium dodecyl sulfate polyacrylamide gels and transferred to Immobilon polyvinylidene difluoride membranes (EMD Millipore, MA, USA). After blocking for 1 h at room temperature in either 5% BSA in 0.1% Tween 20 in TBS for phospho-antibodies or 5% evaporated non-fat milk in 0.1% Tween-20 in TBS, membranes were incubated with antibodies directed against PECAM-1 (3528S, Cell Signaling), VE-cadherin (sc-52751, Santa Cruz Biotechnology), claudin-5 (35–2500, Invitrogen), caveolin-1 (32512, Cell Signaling), vimentin (sc-6260, Santa Cruz Biotechnology), vWF (A0082, DAKO), α-SMA (A5228, Sigma-Aldrich), MT1-MMP (MAB3328, Millipore), Akt (9272, Cell Signaling), pAkt (phosphorylated Ser473, 4060, Cell Signaling), ERK (4695S, Cell Signaling), pERK (phosphorylated Thr202, Tyr204, 9101, Cell Signaling), alpha-actin (CP01, Calbiochem), β2M (sc-15366 Santa Cruz Biotechnology), or GAPDH (ab8245, Abcam, MA, USA) overnight at 4°C. Membranes were washed three times for 5 min with 0.1% Tween-20 in TBS before incubating for 1 h with horse-radish peroxidase (HRP)-conjugated secondary antibodies (DAKO) directed against the appropriate species. Following three 5-min washes with 0.1% Tween-20 in TBS, membranes were incubated for 5 min with Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore, USA) before exposing HyBlot CL autoradiography film to membranes (Denville Scientific, MA, USA).
Lentiviral transduction
The enhanced green fluorescent protein (GFP) recombinant lentiviral vector construct has been previously described (Saunders et al., 2006; Lee et al., 2009; Su et al., 2010) and was used to transduce UtMVECs at Passage 5 using the VIRAPOWER lentiviral expression system, following manufacturer’s instructions (Invitrogen) as previously described in detail (Bayless et al., 2009; Su et al., 2010). Five days after transduction, cells were used for invasion assays containing RSII, VEGF, bFGF, ascorbate and 1 μM S1P, as described above. Invading cells were either fixed in 3% glutaraldehyde in PBS and stained with 0.1% toluidine blue, or fixed in 4% paraformaldehyde in PBS and stained with 1 μM DAPI (Molecular Probes). Samples were cut and mounted so that the monolayer was perpendicular to the imaging plane, which displayed a side view of each sample. Z-stacks with 1 μm step-size images of non-transduced and GFP-transduced cells were taken using a Nikon TI A1R inverted confocal microscope at 20× magnification.
Short hairpin RNA (shRNA) backbone vectors containing sequences directed against MT1-MMP (MMP14) and beta 2 microglobulin (β2M) were purchased from Sigma-Aldrich (TRCN0000050855; TRCN0000057254). Lentiviral particles were generated as previously described using the VIRAPOWER lentiviral packaging mix (Invitrogen) (Dave et al., 2016). The lentiviral particles were harvested at 72 h and centrifuged at 1000 × g for 10 min. The viral supernatant was concentrated using Lenti-X Concentrator (Clontech, CA, USA) per manufacturer’s instructions. UtMVECs and HUVECs at 30–45% confluency in 25cm2 flasks were transduced with viral supernatant and polybrene (12 μg/ml; Sigma-Aldrich) and incubated for 5 h before removing lenti-containing media and feeding with 5 ml endothelial growth medium. ECs were cultured for 4 days before use in invasion assays.
siRNA silencing
siRNA sequences against MT1-MMP (siMT1-MMP; siRNA ID: s8879) and an siRNA control (siControl; Negative Control No. 2 siRNA) were purchased from Thermo Fisher. ECs were seeded at 50–60% confluency in T25 flasks and transfected with 12.5 μl siPORT Amine (Thermo Fisher) and 20 nM of each siRNA, following manufacturer’s instructions. ECs were cultured for 3 days and used in invasion assays on the fourth day.
Statistical analyses
All graphs show average values ±SD or SEM, as indicated. Statistical analyses were performed using Prism 6 (GraphPad Software, Inc., CA, USA). Individual tests are described in detail in the figure legends. All data shown are representative of at least three individual experiments. An unpaired Student’s t-test was used on data comparing two groups. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for multiple comparisons. P-values of <0.05 were considered significant. Letters indicate statistical differences between groups.
Results
UtMVECs express EC markers and characteristics consistent with ECs in culture
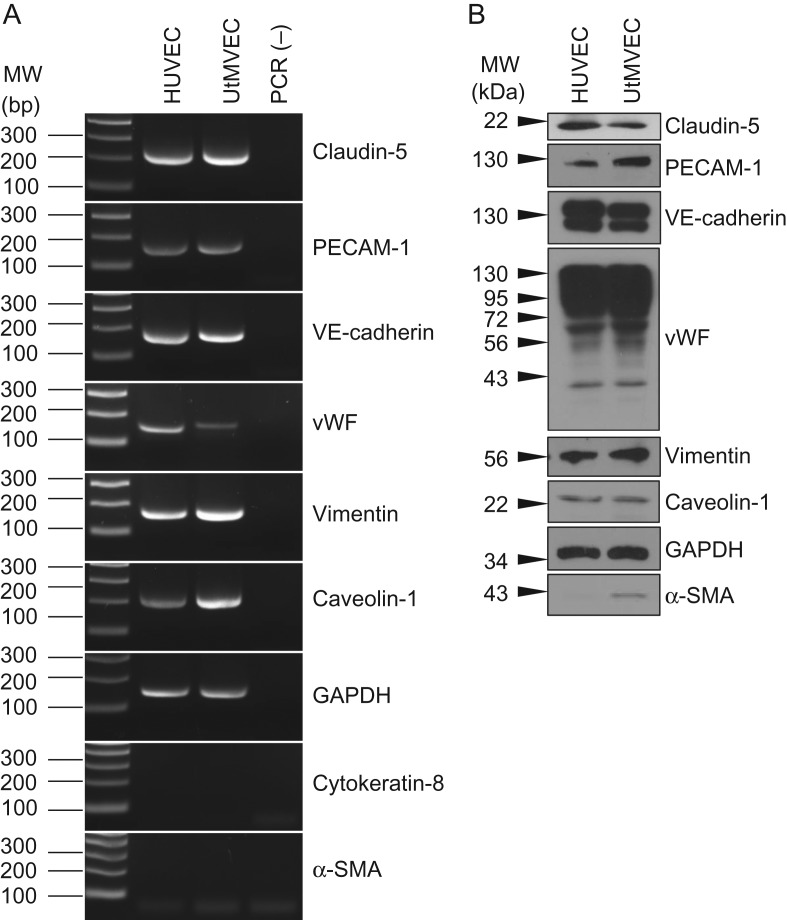
We first confirmed that UtMVECs in culture express EC markers. Total RNA was isolated from UtMVECs, along with HUVECs as a control. Both UtMVECs and HUVECs were found to express mRNA for the EC markers claudin-5, PECAM-1, VE-cadherin and vWF. Along with these EC markers, UtMVECs and HUVECs expressed the mesenchymal cell marker vimentin. Both HUVECs and UtMVECs were positive for caveolin-1 and GAPDH mRNA and exhibited no detectable expression of the epithelial cell marker cytokeratin-8 nor the smooth muscle cell and pericyte marker, α-SMA (Fig. 1A). Western blotting performed on total cell lysates demonstrated the expression of claudin-5, PECAM-1, VE-cadherin, vWF, vimentin, caveolin-1, and GAPDH, as well as low levels of α-SMA protein in both cell types, corroborating the mRNA data (Fig. 1B).
Figure 1.
UtMVECs express EC markers. (A) RNA was isolated from HUVECs and UtMVECs (Passage 5) and reverse transcribed into cDNA. RT-PCR was performed using primers specific for Claudin-5, platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular endothelial-cadherin (VE-cadherin), von Willebrand factor (vWF), Vimentin, Caveolin-1, human GAPDH, cytokeratin-8 and smooth muscle actin (α-SMA). (B) Protein extracts were analyzed by Western blotting using antibodies directed to Claudin-5, PECAM-1, VE-cadherin, vWF, Vimentin, Caveolin-1, GAPDH and α-SMA.
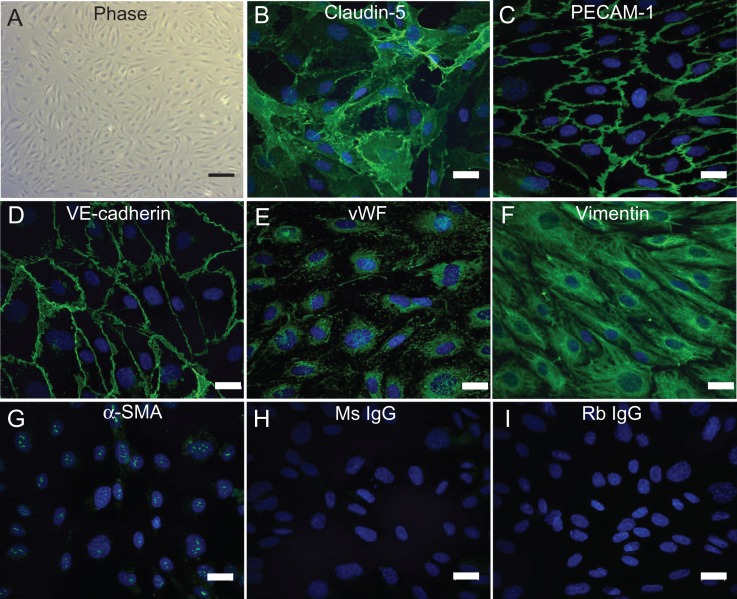
To reinforce findings from expression analysis, we also looked at EC morphology and the expression of protein markers in adherent UtMVECs. Blood vessels are lined by a single layer of ECs with a cobblestone appearance, connected by adherens and tight junctions, forming a selective barrier between blood and surrounding tissues (Dejana, 2004). Cultured UtMVECs exhibited the characteristic EC cobblestone morphology (Fig. 2A). Cell–cell junctions stained positively for the tight junction protein, claudin-5 and the adherens junction proteins PECAM-1 and VE-cadherin (Fig. 2B–D). UtMVECs also demonstrated punctate, cytoplasmic staining for vWF, a glycoprotein synthesized in Weibel-Palade bodies found exclusively in ECs (Fig. 2E), as well as filamentous staining for vimentin, the major intermediate filament in ECs (Fig. 2F). Neither α-SMA nor the isotype controls (Ms IgG and Rb IgG) displayed significant positive staining (Fig. 2G–I). Collectively, Figs 1 and 2 demonstrate that UtMVECs express the expected EC markers in culture and do not express high levels of smooth muscle cell or epithelial cell markers.
Figure 2.
Immunofluorescence staining confirms UtMVECs express known EC markers. (A) Phase contrast image of cultured UtMVECs demonstrating EC morphology. (B–I) Indirect immunofluorescence imaging of UtMVECs stained with primary antibodies directed to (B) Claudin-5, (C) PECAM-1, (D) VE-cadherin, (E) vWF, (F) Vimentin, (G) α-SMA, and isotype controls for (H) mouse (Ms) IgG and (I) rabbit (Rb) IgG. All groups (B–I) were incubated with Alexa-488-conjugated secondary antibodies (green) recognizing corresponding species. Nuclei were stained with DAPI (blue). Coverslips were imaged using a Nikon TI A1R inverted confocal microscope. Scale bar in (A) = 200 μm. Scale bars in (B–I) = 10 μm.
S1P and GFs stimulate dose-dependent UtMVEC invasion
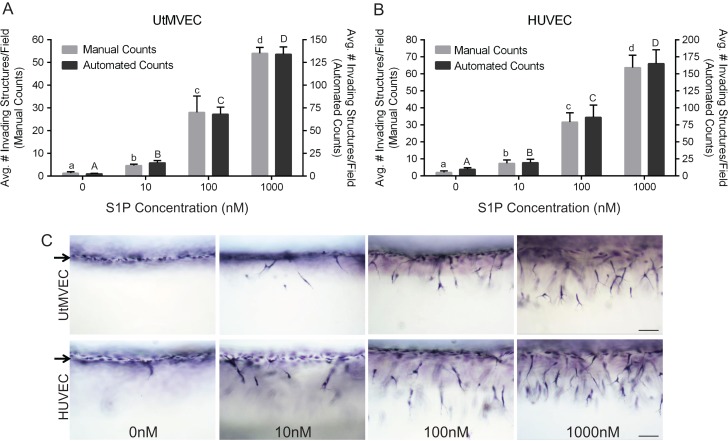
To examine their angiogenic potential, UtMVECs were seeded onto 3D collagen matrices and allowed to invade in the presence of proangiogenic factors known to be important in pregnancy (Reynolds et al., 1992; Torry and Rongish, 1992; Hyder and Stancel, 1999; Zygmunt et al., 2003; Skaznik-Wikiel et al., 2006; Dunlap et al., 2010). In this experiment, collagen matrices contained increasing doses of S1P in the presence of constant levels of VEGF and bFGF. Little UtMVEC invasion occurred in the presence of GFs alone (0 nM), and invasion responses increased dose-dependently with the concentration of S1P present (Fig. 3A). The number of invading structures was quantified manually, as well as automatically (as described in Materials and Methods), revealing agreement between manual and automated counts (Fig. 3A). Images of invasion illustrate the observed dose-dependent UtMVEC invasion responses (Fig. 3C, upper panels). For comparison, we also included results from the well-characterized HUVEC line (Bayless and Davis, 2003; Bayless et al., 2009), and like UtMVECs, we observed a dose-dependent increase in invasion with increasing levels of S1P present (Fig. 3B and C). Thus, these experiments reveal that proangiogenic factors produced locally during uterine angiogenesis are capable of eliciting robust invasion responses in UtMVECs.
Figure 3.
Sphingosine 1-phosphate and GFs stimulate dose-dependent UtMVEC and HUVEC invasion. (A) UtMVECs and (B) HUVECs were seeded onto 2.5 mg/ml collagen matrices containing 0–1000 nM sphingosine 1-phosphate (S1P) in the presence of 40 ng/ml bFGF and VEGF. Cells were allowed to invade 24 h. Invasion density quantification was performed as described in the Materials and Methods section and is presented on the y-axis of panels (A) and (B), with manual and automated counts depicted to the left and right, respectively. At least six wells were averaged per treatment. Data in both panels show average values (±SD) from a representative experiment of three independent experiments performed. (C) Photographs of UtMVEC and HUVEC invasion responses (side view) at 24 h with indicated concentration of S1P. Scale bars represent 100 μm. Arrows indicate the cell monolayer. Statistical significance in panels (A) and (B) was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to all other letters. Lower and upper case letters compare manual and automated counts, respectively.
Invading UtMVECs display increased Akt and ERK activation in response to S1P and GFs
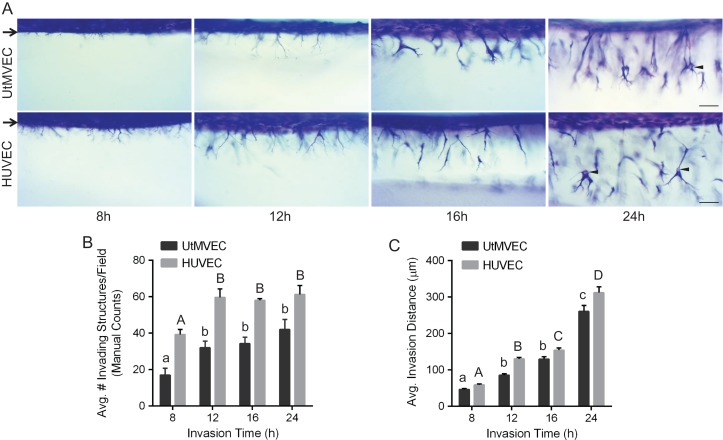
To illustrate UtMVEC invasion responses over time, invading cultures were fixed, stained and imaged from the side at 8, 12, 16, and 24 h (Fig. 4A). At 8 h, UtMVECs began to extend processes from the monolayer into the collagen matrix and the structures continued to increase in complexity at 12 h. Between 16 and 24 h of invasion, UtMVECs invaded deeper into the collagen and formed visible lumens (see arrowheads), which was comparable to the control HUVEC line (Fig. 4A). Quantification of the number of invading structures at all time points revealed the maximal number of invading UtMVEC structures was attained at 12 h (Fig. 4B). As seen from the images in panel Fig. 4A, after the 12 h time point, the invading structures increased in complexity and exhibited greater invasion distances that continued to increase, becoming maximal for both cell types at the 24 h time point (Fig. 4C).
Figure 4.
Temporal UtMVEC angiogenic responses are comparable to HUVEC controls. UtMVECs and HUVECs were allowed to invade for 8, 12, 16 and 24 h. (A) Photographs of invasion responses (side view) at each time point. Scale bars represent 100 μm. Arrows point to cell monolayer, while arrowheads indicate lumens in invading cells. (B) Quantification of invasion density with time, recorded manually as the average number of invading cells in a 0.25 mm2 field. Data shown represent average values (±SEM). (C) Invasion distance was quantified by measuring the distance from the cell monolayer to the tips of invading structures (n > 100 structures per treatment group). Data shown represent average invasion distances (±SEM). Statistical significance in panels (B) and (C) was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents significant statistical difference at P < 0.05 compared to other letters. Lower and upper case letters indicate differences between UtMVECs and HUVECs, respectively.
UtMVECs invade in response to physiological levels of WSS
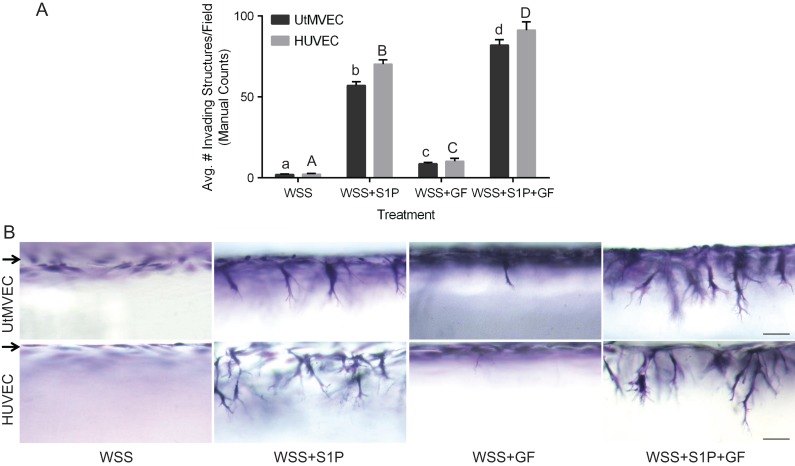
In addition to GFs and lipids, ECs are continuously exposed to WSS from fluid flow. WSS is required for successful vascular development and remodeling, and can induce endothelial quiescence or angiogenesis, depending on the context. During pregnancy, elevated shear stresses coincide with vascular remodeling and induction of angiogenesis in the uterus and developing placenta (Reynolds and Redmer, 1992; Kaufmann et al., 2004; Lucitti et al., 2007). We have previously shown that 5.3 dyn/cm2 WSS combined with 1000 nM S1P induces robust sprouting responses in HUVECs (Duran et al., 2017). To test the effect of WSS on UtMVEC sprouting, we combined the application of WSS with various combinations of S1P and GFs to promote UtMVEC invasion of collagen matrices (Fig. 5). Quantification of UtMVEC invasion revealed that WSS alone or WSS combined with GFs (WSS+GF) had little ability to induce UtMVEC sprouting (Fig. 5A). In contrast, S1P synergized with WSS to stimulate sprouting in the presence (WSS+S1P+GF) and the absence of GF (WSS + S1P), with WSS+S1P+GF promoting the greatest sprouting responses. Responses in HUVEC controls were comparable (Fig. 5A). Side view images of the invasion responses after 24 h demonstrate almost no induction of sprouting in WSS and WSS+GF treatment groups, and strong elicitation of sprouting in WSS+S1P and WSS+S1P+GF treatment groups (Fig. 5B). Comparison of manual and automated quantification methods revealed agreement between both methods (Supplementary Fig. 1A and B). These data show that physiological levels of shear stress combine with proangiogenic factors to promote UtMVEC sprouting into 3D collagen matrices.
Figure 5.
Wall shear stress synergizes with S1P and GFs to stimulate UtMVEC invasion. UtMVECs and HUVEC controls were seeded onto 3D collagen matrices containing nothing (wall shear stress: WSS), 1 μM S1P (WSS+S1P), 4 ng/ml VEGF and bFGF (WSS+GF), or 1 μM S1P and 4 ng/ml VEGF and bFGF (WSS+S1P+GF). In all treatment groups, 5.3 dyn/cm2 WSS was applied and cells were allowed to invade for 24 h. (A) Average invasion density (±SD) of UtMVECs and HUVEC controls after 24 h of invasion was quantified manually. Statistical significance was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Lower and upper case letters compare UtMVECs and HUVECs, respectively. (B) Photographs of invasion responses. Scale bars = 50 μm. Arrows indicate the cell monolayer.
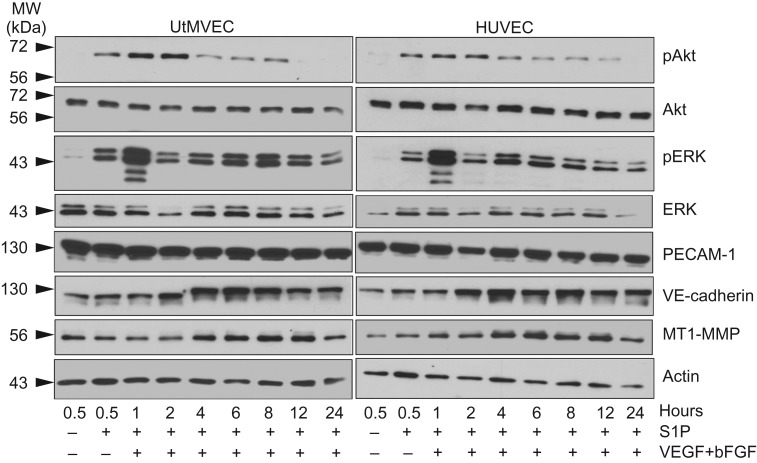
To further investigate the UtMVEC sprouting response and the activation of critical signaling pathways known to be important during angiogenesis, protein extracts of invading cultures, treated with or without S1P and GFs, were collected over time (Fig. 6). Western blots of invading cultures demonstrate that UtMVECs and HUVEC controls exhibited increased Akt phosphorylation (pAkt, Ser473), between 0.5 and 2 h, while total Akt levels remained relatively constant throughout invasion. S1P also increased activation of ERK signaling (pERK) through phosphorylation of Thr202 and Tyr204 at 0.5 h; however, GFs combined with S1P to potently induce ERK phosphorylation at 1 h of invasion, while total ERK remained relatively constant. The EC-specific adherens junction protein VE-cadherin was upregulated with time in both UtMVECs and HUVECs, similar to the trend in protein levels seen with MT1-MMP. No significant changes in actin or PECAM-1 levels were observed. Altogether, the expected time-dependent increases in UtMVEC invasion were observed that correlated with increases in Akt and ERK activation.
Figure 6.
Invading UtMVECs display increased signaling responses following treatment with S1P and GFs. UtMVECs and HUVEC controls were seeded onto 2.5 mg/ml type I collagen matrices in the presence (+) or the absence (−) of 1 μM S1P and 40 ng/ml VEGF and bFGF. Extracts of invading cells were made at the indicated times. Western blotting was performed with antibodies directed to activated Akt (pAkt, phosphorylated at Ser473), total Akt, activated ERK (pERK, phosphorylated at Thr202, Tyr204), total ERK, PECAM-1, VE-cadherin, membrane type 1-matrix metalloproteinase (MT1-MMP) and actin. Western blots from a representative experiment (n = 3) are shown.
UtMVEC invasion requires S1P, Akt, ERK and MMP signaling
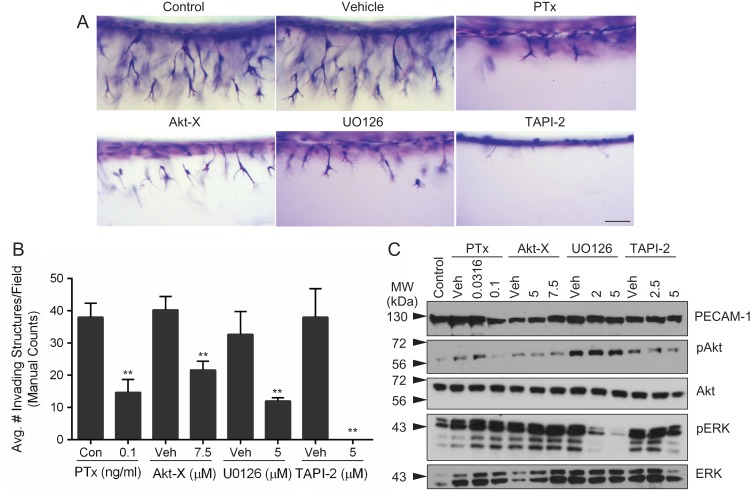
The studies above show that S1P dose-dependently increased UtMVEC invasion into collagen (Fig. 3) and stimulated critical proangiogenic signaling pathways, including Akt and ERK activation, as well as MT1-MMP upregulation (Fig. 6). To demonstrate the functional involvement of S1P, Akt, ERK and MMPs in UtMVEC invasion, we treated UtMVECs with pertussis toxin (PTx), Akt Inhibitor-X (Akt-X), UO126 and TAPI-2, respectively. ECs respond to S1P via G-protein coupled receptors S1P1 and S1P3 (Kluk and Hla, 2002), which activate Gi signaling (Wang et al., 1999) to promote sprouting angiogenesis (Lee et al., 1999). PTx, which blocks Gi activation, was used to inhibit S1P signaling (Kaslow and Burns, 1992). Akt-X inhibits the phosphorylation of Akt at Ser473, downstream of GF-activated PI3K signaling, preventing Akt stimulation of cell migration and cytoskeletal remodeling, which is important for the initiation of angiogenesis (Shiojima and Walsh, 2002; Zhu et al., 2002). GFs also activate the MAP kinase signaling cascade, where ERK is phosphorylated at Thr202 and Tyr204 by MEK1/2, leading to increased gene transcription, proliferation and migration (Zhu et al., 2002; Wietecha and DiPietro, 2013). MEK1/2 activity is non-competitively inhibited by UO126, preventing downstream activation of ERK. TAPI-2 was used to inhibit all MMPs. We observed that UtMVEC invasion was significantly inhibited by PTx, Akt-X and UO126 treatment and completely blocked by TAPI-2 (Fig. 7A). Quantification of invasion responses demonstrated that PTx, Akt-X, UO126 and TAPI-2 significantly inhibited invasion compared to vehicle controls (Fig. 7B). As in previous quantifications, automated counts agreed well with manual counts (Supplementary Fig. 1 C). To confirm the efficacy of the compounds tested, protein extracts were made from invading cultures to examine the activation of Akt and ERK signaling after incubation with inhibitors. Expression of total Akt, ERK and PECAM-1 proteins were relatively constant in all treatment groups. As expected, at 1 h of invasion, pAkt and pERK protein levels decreased with increasing doses of Akt-X and UO126, respectively, compared to the vehicle control (Fig. 7C). Together, these data indicate that S1P, Akt, ERK and MMP activity are required for successful UtMVEC invasion in 3D collagen matrices.
Figure 7.
UtMVEC invasion is dependent on S1P, Akt, ERK and MMP signaling. UtMVECs were pretreated with indicated concentrations of inhibitors or vehicle controls (Veh) for 20 min, before being seeded onto 2.5 mg/ml collagen type I matrices containing 1 μM S1P, with 40 ng/ml VEGF and bFGF in the media. (A) Side view photographs of invasion responses at 24 h following no treatment (Control), DMSO (Vehicle), 0.1 ng/ml Pertussis Toxin (PTx), 7.5 μM Akt Inhibitor-X (Akt-X), 5 μM UO126 or 5 μM TNFα protease inhibitor-2 (TAPI-2). Scale bar represents 100 μm. (B) Average invasion density (±SD) after 24 h of invasion was quantified manually. Statistical significance was determined using a Student’s t-test, comparing each inhibitor to its respective control, where ** represents a significant statistical difference at P < 0.01 compared to the control. (C) Western blotting was performed using extracts collected after 1 h of invasion using antibodies directed to PECAM-1 (loading control), pAkt, Akt, pERK and ERK. Western blots from a representative experiment (n = 3) are shown.
Silencing of MT1-MMP inhibits UtMVEC sprouting
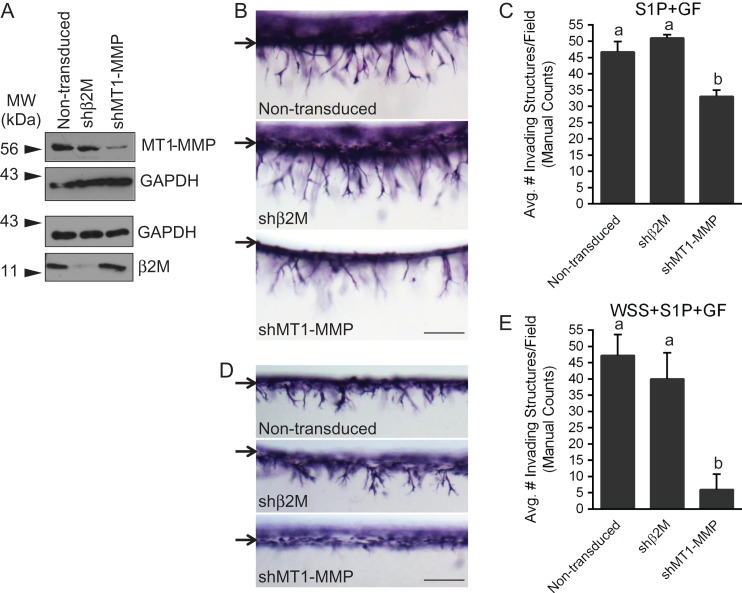
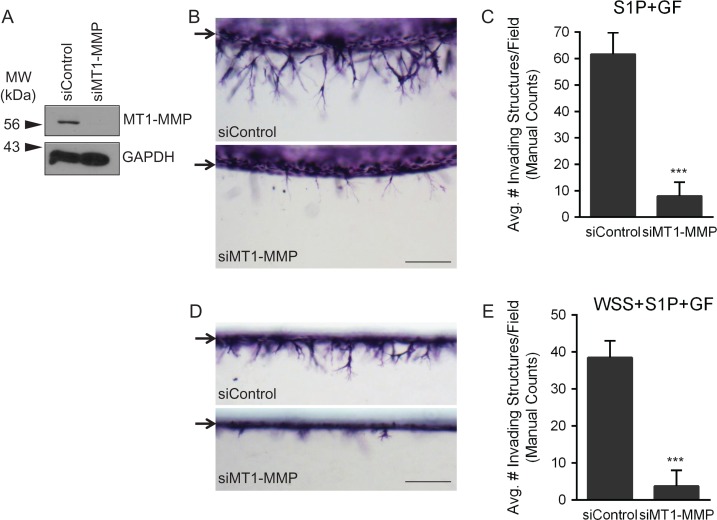
Although pharmacological inhibition of various signaling pathways provides support for the involvement of a variety of molecules during endothelial sprouting, these drugs do not specifically inhibit a single protein. The complementary approach of targeting expression of a specific gene is a more precise method to clearly determine the involvement of individual molecules in the process. However, when using such an approach, it is critical that insertion of the gene to be expressed or activation of the RNA-induced silencing complex does not alter the invasion response. The above results with TAPI-2 suggest the general involvement of MMPs in sprouting responses. As proof of principle to identify a single MMP that controls sprouting, we chose to silence expression of MT1-MMP, a protein known to be critical for endothelial angiogenesis in vivo (Zhou et al., 2000; Chun et al., 2004). UtMVECs were not transduced (non-transduced) or transduced with lentiviruses delivering shRNA to beta 2 microglobulin (β2M), a negative control, or MT1-MMP. Western blots demonstrate successful knockdown of MT1-MMP and β2M (Fig. 8A). We performed dual invasion assays with these cells, using the S1P and GF (S1P+GF) system (described in Figs 3 and 4), or the WSS, S1P, and GF (WSS+S1P+GF) system (described in Fig. 5). In both experiments, knockdown of MT1-MMP inhibited invasion responses, shown in side view images (Fig. 8B and D). Quantification of invasion density demonstrated significantly decreased invasion when MT1-MMP expression was knocked down, while silencing of β2M did not affect invasion density compared to non-transduced controls (Fig. 8C and E). Similar results were observed in HUVEC invasion controls (Supplementary Fig. 2), and agreement occurred between both manual and automated counting methods (Supplementary Fig. 3A and B).
Figure 8.
Silencing MT1-MMP with shRNA in UtMVECs significantly decreases invasion responses. UtMVECs were either not transduced (non-transduced) or transduced with lentiviruses delivering shRNA directed to beta 2 microglobulin (shβ2M) or MT1-MMP (shMT1-MMP). After 4 days, UtMVECs were allowed to invade in the presence of S1P+GF (B and C) or WSS+S1P+GF (D and E). (A) Western blot analysis of MT1-MMP and β2M expression along with corresponding loading controls for each. Photographs of UtMVEC invasion responses observed in each treatment group when stimulated with (B) S1P+GF or (D) WSS+S1P+GF. Arrows indicate the original monolayer. Scale bars equal 200 μm. Invasion density (±SD) of cells was quantified manually in experiments applying (C) S1P+GF or (E) WSS+S1P+GF. Statistical significance of invasion density between Non-transduced, shβ2M and shMT1-MMP treatments was analyzed using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Results from a representative experiment (n = 3) are shown.
To confirm the results from the shRNA experiments, we also delivered siRNA targeted to a scrambled control (siControl) or MT1-MMP (siMT1-MMP). Western blot analysis confirmed successful MT1-MMP protein silencing (Fig. 9A). UtMVECs transfected with either siRNA were allowed to invade in response to S1P and GF (S1P+GF) (Fig. 9B) or WSS, S1P, and GF (WSS+S1P+GF) (Fig. 9D). In agreement with shRNA experiments, siRNA-mediated silencing of MT1-MMP successfully decreased corresponding invasion responses (Fig. 9B and D). Quantification of invasion density confirmed that silencing of MT1-MMP decreased invasion (Fig. 9C and E), and this was reinforced using both manual and automated counts (Supplementary Fig. 3C and D).
Figure 9.
Silencing of MT1-MMP with siRNA in UtMVECs significantly decreases invasion responses. UtMVECs were either transfected with a scrambled siRNA control (siControl) or siRNA directed against MT1-MMP (siMT1-MMP). After 4 days, UtMVECs were allowed to invade in the presence of S1P+GF (B and C) or WSS+S1P+GF (D and E). (A) Western blot analysis of MT1-MMP expression along with GAPDH loading control. Photographs of UtMVEC invasion responses observed in each treatment group when stimulated with (B) S1P+GF or (D) WSS+S1P+GF. Arrows represent the original monolayer. Scale bars equal 200 μm. Invasion density (±SD) of cells was quantified manually in experiments applying (C) S1P+GF or (E) WSS+S1P+GF. Statistical significance of invasion density between siControl and siMT1-MMP treatments was analyzed using a Student’s t-test where *** represents a significant statistical difference at P < 0.001 compared to the siControl. Results from a representative experiment (n = 3) are shown.
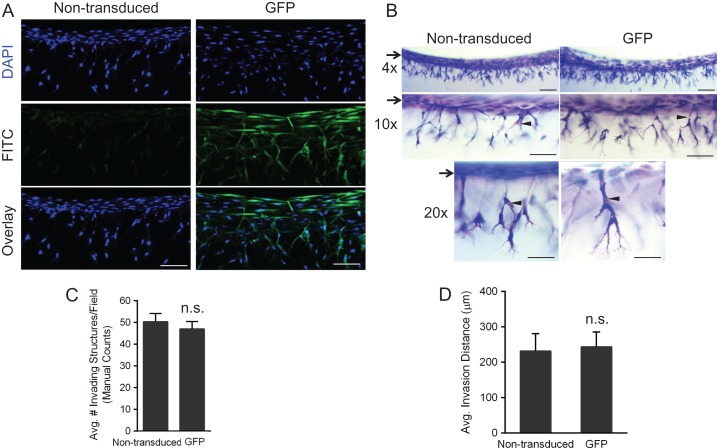
UtMVECs demonstrate comparable invasion responses after lentiviral transduction of a control gene
The above results indicate that UtMVECs are amenable to lentiviral transduction for specific gene knockdown. In the converse experiment, we assessed the ability of UtMVECs to overexpress a gene while maintaining the same angiogenic potential as non-transduced cells. UtMVECs were either not transduced (non-transduced) or transduced with lentiviruses expressing enhanced GFP. On the fifth day of transduction, cells were allowed to invade into collagen matrices for 24 h. Cells were DAPI-stained and imaged from the side using confocal microscopy to confirm GFP expression in UtMVECs transduced with GFP-delivering lentiviruses (Fig. 10A). To visualize angiogenic responses after GFP-transduction (GFP) or not (non-transduced), collagen gels of invading UtMVECs were also stained and imaged at increasing magnifications (Fig. 10B). The number of invading structures and the invasion distance from the monolayer, indicated by arrows, was comparable between treatment groups. The morphology of invading structures appeared unaffected by lentiviral transduction, as non-transduced and GFP-transduced cells formed comparable overall structures with lumens (indicated by arrowheads) (Fig. 10B). Quantification of invasion density and invasion distance from the monolayer (Fig. 10C and D) revealed that invasion responses were not significantly different between the two treatment groups. Automated quantification of invasion density was in agreement with the manual quantifications (Supplementary Fig. 4). These results indicate UtMVECs can be transduced with recombinant lentiviruses to overexpress a protein, while still maintaining angiogenic capabilities.
Figure 10.
UtMVECs demonstrate comparable invasion responses after lentiviral transduction. UtMVECs were either not transduced (Non-transduced) or transduced with green fluorescent protein lentiviruses (GFP) for 5 days and seeded onto 2.5 mg/ml collagen type I matrices containing 1 μM S1P in the presence of 40 ng/ml VEGF and bFGF and allowed to invade 24 h. (A) Cells were stained with DAPI to label cell nuclei prior to mounting on the side. Z-stacks with 1 μm step-size images of non-transduced and GFP-transduced cells were taken using a Nikon TI A1R inverted confocal microscope at 20× magnification. Note successful overexpression of GFP in GFP-transduced cells. Scale bars = 100 μm. (B) Toluidine blue stained collagen matrices were imaged from the side at 4×, 10× and 20× magnification to demonstrate comparable invasion distance and density in both groups. Scale bars equal 200 μm, 100 μm and 50 μm, respectively. Arrows indicate the original cell monolayer, while the arrowheads point to lumens in invading cells. (C) Invasion density (±SD) of invading cells was quantified manually. Statistical significance of invasion density between non-transduced and GFP-transduced cells was analyzed using a Student’s t-test, finding no significant differences (n.s.) between the groups with P < 0.05 as the limit for significance. (D) Invasion distance was quantified by measuring the distance from the cell monolayer to the tips of invading structures (n > 100 structures per treatment group). Data shown represent average invasion distances (±SD) from a representative experiment (n = 3). Statistical significance of invasion density between non-transduced and GFP-transduced cells was analyzed using a Student’s t-test. No significant differences (n.s.) between the groups were observed with P < 0.05 considered significant.
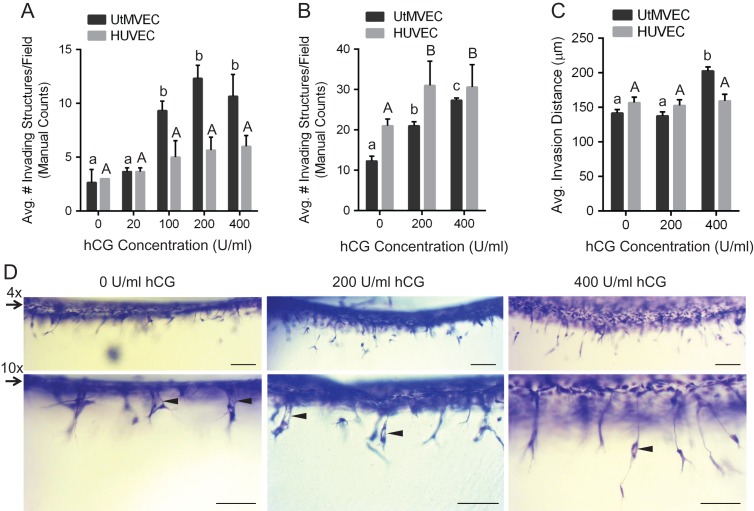
UtMVECs respond dose-dependently to hCG in invasion assays
hCG has been previously reported as a proangiogenic factor (Zygmunt et al., 2002; Berndt et al., 2006; Herr et al., 2007). hCG is synthesized by placental trophoblasts during early gestation (Muyan and Boime, 1997; Kovalevskaya et al., 2002; Handschuh et al., 2007; Guibourdenche et al., 2010), leading to decreased uterine vascular permeability in vivo (Toth et al., 2001) and increased EC sprouting in aortic ring assays (Zygmunt et al., 2002; Berndt et al., 2006; Herr et al., 2007). The uterine endothelium has been shown to express the LH-hCG receptor at both the mRNA and protein levels in vivo (Toth et al., 1994). To test whether hCG promoted UtMVEC sprouting in this model, UtMVECs were seeded in media containing GFs onto collagen matrices containing 0–400 U/ml hCG and allowed to invade for 24 h. No S1P was added to the matrix. Quantification of invasion density showed that as the concentration of hCG in the matrix increased, UtMVEC invasion also increased, with significantly more invasion at 100, 200 and 400 U/ml hCG compared to 0 and 20 U/ml hCG (Fig. 11A). Under the same conditions, hCG did not significantly stimulate HUVEC invasion (Fig. 11A). We next tested the UtMVEC responses to hCG in the presence of low levels of S1P stimulation. Cells were seeded onto collagen matrices containing 300 nM S1P and either 0, 200 or 400 U/ml hCG, and allowed to invade for 24 h. UtMVECs responded dose-dependently to the hCG addition (Fig. 11B). HUVEC invasion was also slightly increased with 200 and 400 U/ml hCG (Fig. 11B). While UtMVEC invasion density was slightly lower than HUVEC invasion density overall, UtMVEC invasion was more significantly enhanced with hCG treatment (Fig. 11B). Interestingly, quantitation of invasion distance revealed that 400 U/ml hCG significantly elevated UtMVEC but not HUVEC invasion distance (Fig. 11C). This was evident from side view images of UtMVEC invasion shown at two magnifications (Fig. 11D). These data indicate that hCG not only promotes and enhances UtMVEC sprouting responses but also that hCG selectively increases UtMVEC invasion distance.
Figure 11.
hCG increases UtMVEC invasion responses in a dose-dependent manner. (A) UtMVECs (Lonza Cat. CC-2564, Lot 2F1740) and HUVECs (Lonza Cat. C2517A, Lot 444770) derived from female donors, were seeded onto 2.5 mg/ml collagen matrices containing 0, 20, 100, 200 or 400 U/ml hCG in the presence of 40 ng/ml VEGF and bFGF. No S1P was added. Cells were allowed to invade for 24 h. Invasion density was quantified manually as the average number of invading cells in a 0.25 mm2 field (±SD). At least three wells were counted and averaged per treatment. Results shown are from a representative experiment (n = 3). Statistical significance was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Lower and upper case letters compare UtMVECs and HUVECs, respectively. (B) UtMVECs and HUVECs were seeded onto 2.5 mg/ml collagen matrices containing 300 nM S1P and either 0, 200 or 400 U/ml hCG in the presence of 40 ng/ml VEGF and bFGF. Cells were allowed to invade for 24 h. Invasion density (±SD) was quantified manually from three wells. Data from a representative experiment (n = 3) are shown. Statistical significance was determined using a one-way ANOVA with Tukey’s post hoc test, where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Upper and lower case letters compare HUVECs and UtMVECs, respectively. (C) Samples from (B) were used to quantify invasion distance, where n > 100 structures from a single experiment (n = 3) were included per treatment group for the analysis. Data shown represent average invasion distances (±SEM). Statistical significance was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Upper and lower case letters compare HUVECs and UtMVECs, respectively. (D) Photographs of UtMVEC invasion assays from panel B. Two magnifications are shown at 4× (upper panels) and 10× (lower panels). Scale bars = 200 μm and 100 μm, respectively for upper and lower panels. Arrows indicate the original cell monolayer, while arrowheads point to lumens in invading cells.
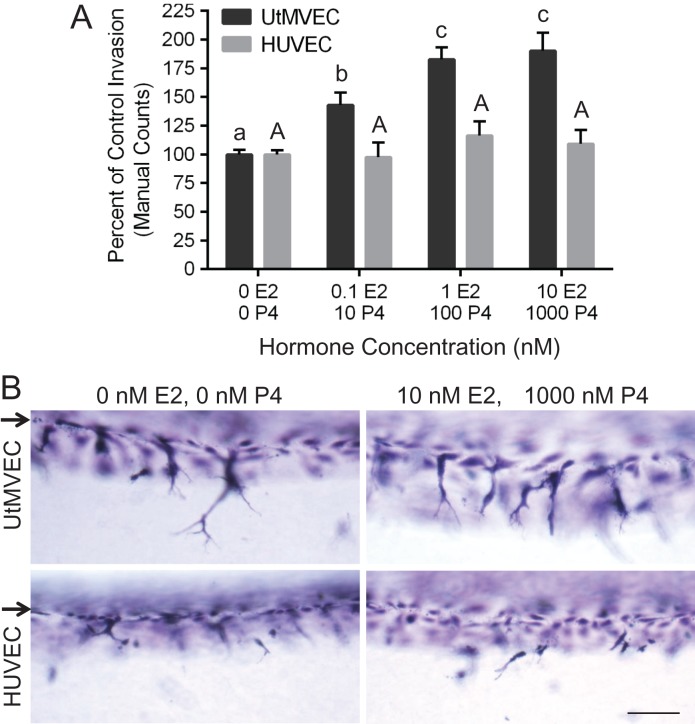
UtMVEC invasion is dose-dependently enhanced by estrogen and progesterone
During pregnancy, estrogen and progesterone combine to promote sprouting angiogenesis in the uterus (Das et al., 2009; Scott et al., 2012; Wetendorf and DeMayo, 2012; Goddard et al., 2014). To determine the influence of these key hormones on UtMVEC sprouting using this model, we added increasing doses of estrogen and progesterone in the presence of submaximal levels of S1P and VEGF and bFGF. Quantification of invasion revealed that combined treatment of estrogen and progesterone promoted UtMVEC sprouting in a dose-dependent manner, while no enhancement in HUVEC invasion responses was observed (Fig. 12A). Side view images of invasion demonstrate the ability of combined estrogen and progesterone treatment to selectively stimulate UtMVEC sprouting (Fig. 12B).
Figure 12.
Combined treatment with estrogen and progesterone selectively enhances UtMVEC invasion. (A) UtMVECs and HUVECs were seeded onto 2.5 mg/ml collagen matrices containing 300 nM S1P in the presence of 4 ng/ml VEGF and bFGF. Estrogen (E2) and progesterone (P4) were added at the following concentrations: 0 nM E2 and 0 nM P4, 0.1 nM E2 and 10 nM P4, 1 nM E2 and 100 nM P4, or 10 nM E2 and 1000 nM P4. Cells were allowed to invade for 24 h. At least three wells were counted per treatment and pooled from three independent experiments. The number of invading structures per well was normalized relative to the average control invasion (0 E2, 0 P4), (±SD). Statistical significance was determined using a one-way ANOVA with Tukey’s post hoc test where each letter represents a significant statistical difference at P < 0.05 compared to other letters. Lower and upper case letters compare UtMVECs and HUVECs, respectively. (B) Photographs of UtMVEC and HUVEC invasion responses after treatment with 0 nM E2 and 0 nM P4, or 10 nM E2 and 1000 nM P4. Arrows indicate the original monolayer. Scale bar equals 100 μm.
Discussion
We find here that human UtMVECs invade 3D collagen matrices in response to proangiogenic stimuli that are present during pregnancy and the female reproductive cycle. This model can be used to interrogate the necessity or consequence of other biological factors or genes on uterine angiogenesis. Further, we have developed an automated method to objectively quantify sprouting responses, which agrees with manual methods. We found that human UtMVECs were a pure EC population, free of epithelial and smooth muscle cells, and that UtMVECs responded similarly to HUVECs when activated with proangiogenic stimuli and WSS. Finally, UtMVECs were amenable to overexpression and gene silencing studies. Altogether, these studies establish UtMVECs as a useful and relevant cell type to determine the specific molecular events elicited by relevant pregnancy-associated factors to promote or inhibit uterine EC angiogenesis.
This model has the potential to further our understanding of signaling pathways that control uterine angiogenesis. It is well accepted that an adequate supply of nutrients and oxygen are delivered via the vasculature and are required for successful pregnancy. In mice and humans, the uterine decidua supports early pregnancy prior to placental formation, and the uterine vasculature similarly adapts to the rising needs of the fetus (Rockwell et al., 2002; Sengupta et al., 2007; Douglas et al., 2009). Three main changes occur within the vasculature, including vasodilation, increased permeability, and uterine angiogenesis (Reynolds et al., 1992; Torry and Rongish, 1992; Zygmunt et al., 2003). In humans, improper vascular development in the decidua correlates with miscarriage, abnormal placenta associated with pre-eclampsia, and intrauterine growth restriction (IUGR) (Maynard et al., 2008; Plaisier, 2011). An array of signals must converge for successful uterine remodeling and fetal development during pregnancy. The model system presented here isolates the responses of primary human UtMVECs, allowing the study of specific influences (e.g. hormones, proangiogenic GFs, S1P and WSS) on downstream signaling pathways relevant to angiogenesis during pregnancy. Because ECs are phenotypically heterogeneous and their gene expression can vary even within portions of the vascular loop in the same organ (Aird, 2006, 2007), developing this model to study the influence of pregnancy-associated factors on UtMVEC sprouting in a 3D environment is relevant to better understand the key events needed for vital angiogenic responses in pregnancy.
Multiple biochemical signals must converge within the uterus for a successful pregnancy. Several of the key stimuli present include VEGF, bFGF, placental GF (PlGF) and hCG. It is well accepted that these factors are present and produced in utero by the decidua, placenta, endometrium and trophoblasts (Reynolds et al., 1992; Torry and Rongish, 1992; Zygmunt et al., 1998a, 2002, 2003; Hyder and Stancel, 1999; Li et al., 2001; Herr et al., 2003). VEGF is produced in the placenta by villous trophoblasts and stromal macrophages (Jackson et al., 1994; Clark et al., 1998). In addition to GFs, kinases that generate active S1P, and phosphatases that turn over the proangiogenic lipid, are upregulated during decidualization (Jeng et al., 2007; Mizugishi et al., 2007; Brunnert et al., 2014) and in gravid versus non-gravid horns in pregnant sheep (Dunlap et al., 2010). The S1P receptors are also upregulated in mouse decidua at embryonic Day 4.5–7.5, supporting a role for the S1P signaling pathway in decidualization and successful angiogenic responses (Skaznik-Wikiel et al., 2006; Kaneko-Tarui et al., 2007). Uterine ECs must continually sense and respond to biochemical cues from GFs and lipids to appropriately instigate angiogenesis. These proangiogenic factors potently stimulate angiogenesis of human endometrial cells during in vitro assays (Zygmunt et al., 2003) and new blood vessel growth in vivo (Carmeliet and Collen, 2000; English et al., 2001; Carmeliet, 2003; Hla, 2004). We demonstrate here that UtMVECs respond similarly to the previously described HUVEC line in invasion assays stimulated by S1P+GF as well as WSS+S1P+GF (Bayless and Davis, 2003; Bayless et al., 2009; Duran et al., 2017). S1P dose-dependently promoted both UtMVEC and HUVEC invasion responses, where the latter cell type has been previously described (Bayless and Davis, 2003). Monitoring signaling responses induced by VEGF, bFGF and S1P stimulation in UtMVECs (Fig. 5) gave the expected increase in both Akt and ERK1/2 phosphorylation (L’Allemain et al., 1991; Gerber et al., 1998; Gupta et al., 1999; Igarashi and Michel, 2001; Zubilewicz et al., 2001). Consistent with a previously reported role for these molecules in sprouting and angiogenic responses (Eliceiri et al., 1998; Yang et al., 2003), pharmacological inhibition of Akt and ERK significantly decreased the sprouting responses of UtMVECs, as expected. Our model demonstrates synergism between S1P, VEGF, bFGF and hCG, demonstrating that the functional endothelial responses can be monitored in tandem with stimulation by various physiological factors that promote angiogenesis during pregnancy.
In addition, we observe that MT1-MMP (MMP14) protein levels are upregulated during EC invasion. MMPs are widely studied in angiogenesis and function to degrade the matrix to form new capillaries (Carmeliet and Jain, 2011). Although multiple classes of MMPs have been implicated in angiogenic events, MT1-MMP is essential for endothelial invasion within a 3D environment. Throughout pregnancy, MT1-MMP is expressed in the decidua as well as in fetoplacental ECs (Plaisier et al., 2008, 2009; van Hinsbergh and Koolwijk, 2008; Anacker et al., 2011; Hiden et al., 2012, 2013). In studies with mice lacking MT1-MMP, neovessel formation and collagenolysis were completely inhibited in MT1-MMP null tissue explants and microvascular cells, (Chun et al., 2004) and loss of MT1-MMP was associated with defective angiogenesis (Zigrino et al., 2012). In vivo, MT1-MMP expression is localized to tip cells within angiogenic vessels (Yana et al., 2007). MT1-MMP is also required for lumen formation in vitro (Stratman et al., 2009). Upregulation of MT1-MMP during UtMVEC sprouting is consistent with a requirement for MT1-MMP in angiogenesis. Further, silencing of MT1-MMP in UtMVECs abrogated angiogenic sprouting responses. Altogether, these data support a critical role for MT1-MMP in UtMVEC invasion of the ECM.
In addition to proangiogenic stimuli, the vasculature is constantly subjected to blood flow, which applies WSS to ECs. These mechanical forces are anticipated to work in combination with biochemical cues to correctly regulate angiogenesis in vivo (Davies et al., 1995; Topper and Gimbrone, 1999). Numerous examples in developmental models reveal that flow contributes to vascular remodeling and can alter the vasculature (Olson and Srivastava, 1996; Hogers et al., 1997; Isogai et al., 2003; Lucitti et al., 2007). In pregnancy, the rates of placental blood flow are dependent on placental vascularization, supporting that angiogenesis is ultimately needed for the development of healthy offspring (Reynolds and Redmer, 1995; Magness, 1998). In both maternal and fetal tissues, nitric oxide is a key factor needed for vascularization and the endothelial form of nitric oxide synthase (eNOS) is known to be activated by WSS in ECs (Rizzo et al., 1998; Fisslthaler et al., 2000). Mice lacking eNOS (NOS3) have decreased reproductive performance (van der Heijden et al., 2005) and impaired uteroplacental adaptations to pregnancy (Kulandavelu et al., 2013). Similarly, blocking NO production with L-NAME induces pre-eclampsia in a mouse model (Motta et al., 2015), indicating that lack of NO production has detrimental effects on pregnancy. In agreement with these studies, molecules such as arginine (a precursor for NO) and sildenafil (which inhibits NO degradation) have beneficial effects on conditions such as IUGR and pre-eclampsia in humans (von Dadelszen et al., 2011; Wu et al., 2013) and other species (Li et al., 2007; Wu et al., 2013). Together, these data confirm that flow is needed for proper vascular development in both maternal and fetal circulations. Further, although shear stress is sensed by all ECs in vivo, the study of endothelial responses to mechanical forces has chiefly been performed with two-dimensional cell cultures (Davies et al., 1995; Li et al., 2004; Sprague et al., 2010), with a notable exception in the mouse embryo (Lucitti et al., 2007). WSS levels in post-capillary venules have been estimated at 1–8 dyn/cm2 in various models, where angiogenesis initiates (Ichioka et al., 1997; Kim and Sarelius, 2003; Jones et al., 2004; Boisseau, 2005). In the present study, flow is demonstrated to stimulate angiogenic responses. We have modified a defined 3D system (Duran et al., 2017) where 5.3 dyn/cm2 WSS synergizes with S1P and GFs to promote sprouting responses in primary human UtMVECs. We find that WSS, S1P and GFs relevant to pregnancy synergize to promote UtMVEC sprouting, which mimics physiological stimuli present within the uterus.
The model presented here has a number of expedient features. The entire system is serum-free. This platform is amenable to additional testing of hormones and other factors that are physiologically relevant in pregnancy. As shown here, this approach allowed us to detect differential responses to hCG, estrogen and progesterone between UtMVECs and HUVECs. The use of primary human ECs is likely of benefit in this model because the timing and magnitude of angiogenic responses in humans differs from all other species studied (Herr et al., 2010). Thus, if the ultimate goal is to better understand the molecular and cellular events required for a successful human pregnancy, then this model is a step closer to realizing that goal. The system developed here quantifies primary human endothelial sprouting in a 3D environment and can simultaneously monitor signaling events with functional sprouting responses. We report here that S1P, hCG, hormones, VEGF, bFGF and WSS significantly enhance UtMVEC sprouting responses. Finally, we have developed an automated quantification system that agrees closely with manual counts, which not only reduces the time needed to quantify larger sample sizes but also removes bias and subjectivity from the process of quantification. Altogether, this approach offers increased utility over other approaches that model angiogenesis during pregnancy because it is serum-free, uses primary human uterine microvascular ECs, models a 3D, physiological environment, and can be quantified in an unbiased manner.
We have initiated these studies with VEGF, bFGF, hCG, hormones, S1P and WSS and the conditions established provide a suitable platform upon which to test additional proangiogenic factors known to be critical during normal and diseased pregnancy; for example, placental GF, prostaglandins, VEGFR1 and others. Because this system is amenable to manipulation of gene expression, we can coordinate signaling responses with changes in sprouting. In addition, we have developed an automated system to quantify sprouting, which opens up the possibility of applications such as high-throughput screening to look for factors that enhance angiogenic responses to overcome limitations with vascular development and angiogenesis. Additionally, with the ability to manipulate flow rates, we can examine the consequences of changing WSS levels (e.g. lower or higher than normal or pulsatile) on EC sprouting to mimic the effects of pre-eclampsia or high-blood pressure. Although the studies reported here use only uterine ECs, the experimental model could be expanded to include comparisons between uterine, placental, ovarian and endometrial ECs. The approach could also incorporate stromal and mural cells in co-culture studies, or trophoblasts to study the interplay between ECs in vascular remodeling events early in pregnancy. Altogether, these studies establish a model to objectively study the effects of a multitude of factors on uterine EC angiogenesis, which could aid our understanding of angiogenesis during pregnancy.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Authors’ roles
CLD and KJB designed the studies and CLD and CAA executed the studies. CLD, CAA and KJB analyzed data and CLD and KJB drafted the manuscript.
Funding
This work was supported by National Institutes of Health Public Health Service award HL095786 to K.J.B.
Conflict of interest
No conflicts of interest are declared.
Supplementary Material
Acknowledgements
The authors would like to thank our colleagues at Texas A&M University, including Drs Qinglei Li, Tom Welsh and J. Will Frank for helpful discussions.
References
- Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res 2006;98:159–162. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100:174–190. [DOI] [PubMed] [Google Scholar]
- Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, Kammerer U. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod 2011;17:637–652. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Charlton AK, Ayad S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res 1988;253:231–240. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun 2003;312:903–913. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat Protoc 2009;4:1888–1898. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003;3:401–410. [DOI] [PubMed] [Google Scholar]
- Berndt S, Perrier d’Hauterive S, Blacher S, Pequeux C, Lorquet S, Munaut C, Applanat M, Herve MA, Lamande N, Corvol P et al. . Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J 2006;20:2630–2632. [DOI] [PubMed] [Google Scholar]
- Boisseau MR. Roles of mechanical blood forces in vascular diseases. A clinical overview. Clin Hemorheol Microcirc 2005;33:201–207. [PubMed] [Google Scholar]
- Bornstein MB. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest 1958;7:134–137. [PubMed] [Google Scholar]
- Brunnert D, Sztachelska M, Bornkessel F, Treder N, Wolczynski S, Goyal P, Zygmunt M. Lysophosphatidic acid and sphingosine 1-phosphate metabolic pathways and their receptors are differentially regulated during decidualization of human endometrial stromal cells. Mol Hum Reprod 2014;20:1016–1025. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999;181:718–724. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 2003;4:710–720. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann NY Acad Sci 2000;902:249–262. discussion 262–244. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 2004;167:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol 1998;159:459–467. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Lu L, Magnusson P, Nyqvist D, Holmqvist K, Welsh M, Claesson-Welsh L. The Shb adaptor protein binds to tyrosine 766 in the FGFR-1 and regulates the Ras/MEK/MAPK pathway via FRS2 phosphorylation in endothelial cells. Mol Biol Cell 2002;13:2881–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA 2009;106:12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave JM, Abbey CA, Duran CL, Seo H, Johnson GA, Bayless KJ. Hic-5 mediates the initiation of endothelial sprouting by regulating a key surface metalloproteinase. J Cell Sci 2016;129:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Barbee KA, Lal R, Robotewskyj A, Griem ML. Hemodynamics and atherogenesis. Endothelial surface dynamics in flow signal transduction. Ann NY Acad Sci 1995;748:86–102. discussion 102–103. [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 2004;5:261–270. [DOI] [PubMed] [Google Scholar]
- Douglas NC, Tang H, Gomez R, Pytowski B, Hicklin DJ, Sauer CM, Kitajewski J, Sauer MV, Zimmermann RC. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology 2009;150:3845–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KA, Kwak HI, Burghardt RC, Bazer FW, Magness RR, Johnson GA, Bayless KJ. The sphingosine 1-phosphate (S1P) signaling pathway is regulated during pregnancy in sheep. Biol Reprod 2010;82:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran CL, Kaunas R, Bayless KJ. S1P synergizes with wall shear stress and other angiogenic factors to induce endothelial cell sprouting responses. Methods Mol Biol 2017;1697:99–115. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 1991;174:1275–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol 1998;140:1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D, Garcia JG, Brindley DN. Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovasc Res 2001;49:588–599. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989;161:851–858. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand 2000;168:81–88. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996;87:1153–1155. [DOI] [PubMed] [Google Scholar]
- Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J 1995;9:919–925. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336–30343. [DOI] [PubMed] [Google Scholar]
- Gingras D, Michaud M, Di Tomasso G, Beliveau E, Nyalendo C, Beliveau R. Sphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cells. FEBS Lett 2008;582:399–404. [DOI] [PubMed] [Google Scholar]
- Giuliani R, Bastaki M, Coltrini D, Presta M. Role of endothelial cell extracellular signal-regulated kinase1/2 in urokinase-type plasminogen activator upregulation and in vitro angiogenesis by fibroblast growth factor-2. J Cell Sci 1999;112:2597–2606. [DOI] [PubMed] [Google Scholar]
- Goddard LM, Murphy TJ, Org T, Enciso JM, Hashimoto-Partyka MK, Warren CM, Domigan CK, McDonald AI, He H, Sanchez LA et al. . Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell 2014;156:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, Brion DE, Fournier T. Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocrinol Metab 2010;95:E240–E244. [DOI] [PubMed] [Google Scholar]
- Gupta A, Bazer FW, Jaeger LA. Immunolocalization of acidic and basic fibroblast growth factors in porcine uterine and conceptus tissues. Biol Reprod 1997;56:1527–1536. [DOI] [PubMed] [Google Scholar]
- Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 1999;247:495–504. [DOI] [PubMed] [Google Scholar]
- Halder JB, Zhao X, Soker S, Paria BC, Klagsbrun M, Das SK, Dey SK. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis 2000;26:213–224. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–364. [DOI] [PubMed] [Google Scholar]
- Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta 2007;28:175–184. [DOI] [PubMed] [Google Scholar]
- Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, Preissner KT, Zygmunt M. HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta 2007;28:S85–S93. [DOI] [PubMed] [Google Scholar]
- Herr F, Baal N, Widmer-Teske R, McKinnon T, Zygmunt M. How to study placental vascular development? Theriogenology 2010;73:817–827. [DOI] [PubMed] [Google Scholar]
- Herr F, Liang OD, Herrero J, Lang U, Preissner KT, Han VK, Zygmunt M. Possible angiogenic roles of insulin-like growth factor II and its receptors in uterine vascular adaptation to pregnancy. J Clin Endocrinol Metab 2003;88:4811–4817. [DOI] [PubMed] [Google Scholar]
- Hiden U, Ghaffari-Tabrizi N, Gauster M, Tam-Amersdorfer C, Cetin I, Dieber-Rotheneder M, Lang U, Desoye G. Membrane-type matrix metalloproteinase 1 regulates trophoblast functions and is reduced in fetal growth restriction. Am J Pathol 2013;182:1563–1571. [DOI] [PubMed] [Google Scholar]
- Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, Lang U, Desoye G. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab 2012;97:3613–3621. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 2004;15:513–520. [DOI] [PubMed] [Google Scholar]
- Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res 1997;80:473–481. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003;421:172–177. [DOI] [PubMed] [Google Scholar]
- Hustin J, Schaaps JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987;157:162–168. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Stancel GM. Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol Endocrinol 1999;13:806–811. [DOI] [PubMed] [Google Scholar]
- Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. Effects of shear stress on wound-healing angiogenesis in the rabbit ear chamber. J Surg Res 1997;72:29–35. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem 2001;276:36281–36288. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development 2003;130:5281–5290. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Carney EW, Lye SJ, Ritchie JW. Localization of two angiogenic growth factors (PDECGF and VEGF) in human placentae throughout gestation. Placenta 1994;15:341–353. [DOI] [PubMed] [Google Scholar]
- Jeng YJ, Suarez VR, Izban MG, Wang HQ, Soloff MS. Progesterone-induced sphingosine kinase-1 expression in the rat uterus during pregnancy and signaling consequences. Am J Physiol Endocrinol Metab 2007;292:E1110–E1121. [DOI] [PubMed] [Google Scholar]
- Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol 2004;287:H1561–H1569. [DOI] [PubMed] [Google Scholar]
- Kaneko-Tarui T, Zhang L, Austin KJ, Henkes LE, Johnson J, Hansen TR, Pru JK. Maternal and embryonic control of uterine sphingolipid-metabolizing enzymes during murine embryo implantation. Biol Reprod 2007;77:658–665. [DOI] [PubMed] [Google Scholar]
- Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J 1992;6:2684–2690. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004;25:114–126. [DOI] [PubMed] [Google Scholar]
- Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation 2003;10:167–178. [DOI] [PubMed] [Google Scholar]
- Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta 2002;1582:72–80. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem 2005;280:88–93. [DOI] [PubMed] [Google Scholar]
- Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 2007;44:375–386. [PubMed] [Google Scholar]
- Kovalevskaya G, Genbacev O, Fisher SJ, Caceres E, O’Connor JF. Trophoblast origin of hCG isoforms: cytotrophoblasts are the primary source of choriocarcinoma-like hCG. Mol Cell Endocrinol 2002;194:147–155. [DOI] [PubMed] [Google Scholar]
- Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension 2013;61:259–266. [DOI] [PubMed] [Google Scholar]
- Kwak HI, Kang H, Dave JM, Mendoza EA, Su SC, Maxwell SA, Bayless KJ. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis 2012;15:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Allemain G, Pouyssegur J, Weber MJ. p42/mitogen-activated protein kinase as a converging target for different growth factor signaling pathways: use of pertussis toxin as a discrimination factor. Cell Regul 1991;2:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, Taylor RN. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 2000;15:67–77. [DOI] [PubMed] [Google Scholar]
- Lecarpentier E, Bhatt M, Bertin GI, Deloison B, Salomon LJ, Deloron P, Fournier T, Barakat AI, Tsatsaris V. Computational fluid dynamic simulations of maternal circulation: wall shear stress in the human placenta and its biological implications. PLoS One 2016;11:e0147262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, Kim KW, Kwon YG. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun 1999;264:743–750. [DOI] [PubMed] [Google Scholar]
- Lee PF, Yeh AT, Bayless KJ. Nonlinear optical microscopy reveals invading endothelial cells anisotropically alter three-dimensional collagen matrices. Exp Cell Res 2009;315:396–410. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Zhang L, Ding G, Gang Zhang Z, Li Q, Ewing JR, Lu M, Panda S, Ledbetter KA et al. . Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res 2007;1132:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A et al. . Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 2001;86:1823–1834. [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng J, Bird IM, Magness RR. Mechanisms of shear stress-induced endothelial nitric-oxide synthase phosphorylation and expression in ovine fetoplacental artery endothelial cells. Biol Reprod 2004;70:785–796. [DOI] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 2007;134:3317–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 2013;62:1046–1054. [DOI] [PubMed] [Google Scholar]
- Maciag T, Cerundolo J, Ilsley S, Kelley PR, Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci USA 1979;76:5674–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness RR. Maternal cardiovascular and other physiological responses to the endocrinology of pregnancy In: Bazer FW (ed).. The Endocrinology of Pregnancy. Totowa, NJ: Humana Press, 1998:507–539. [Google Scholar]
- Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med 2008;59:61–78. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest 2007;117:2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller B, Rasmussen C, Lindblom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod 2001;7:65–72. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem 2001;276:19672–19677. [DOI] [PubMed] [Google Scholar]
- Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A, Romanini MC. Effect of sildenafil on pre-eclampsia-like mouse model induced by L-name. Reprod Domest Anim 2015;50:611–616. [DOI] [PubMed] [Google Scholar]
- Muyan M, Boime I. Secretion of chorionic gonadotropin from human trophoblasts. Placenta 1997;18:237–241. [DOI] [PubMed] [Google Scholar]
- Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, Beliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem 2007;282:15690–15699. [DOI] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 1997;272:2446–2451. [DOI] [PubMed] [Google Scholar]
- Olson EN, Srivastava D. Molecular pathways controlling heart development. Science 1996;272:671–676. [DOI] [PubMed] [Google Scholar]
- Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci USA 2001;98:6074–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Mandriota SJ. Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp Cell Res 1998;241:414–425. [DOI] [PubMed] [Google Scholar]
- Plaisier M. Decidualisation and angiogenesis. Best Pract Res Clin Obstet Gynaecol 2011;25:259–271. [DOI] [PubMed] [Google Scholar]
- Plaisier M, Dennert I, Rost E, Koolwijk P, van Hinsbergh VW, Helmerhorst FM. Decidual vascularization and the expression of angiogenic growth factors and proteases in first trimester spontaneous abortions. Hum Reprod 2009;24:185–197. [DOI] [PubMed] [Google Scholar]
- Plaisier M, Koolwijk P, Willems F, Helmerhorst FM, van Hinsbergh VW. Pericellular-acting proteases in human first trimester decidua. Mol Hum Reprod 2008;14:41–51. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873–887. [DOI] [PubMed] [Google Scholar]
- Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 2009;24:45–54. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Redmer DA, Caton JS. Placental angiogenesis in sheep models of compromised pregnancy. J Physiol 2005. a;565:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta 2005. b;26:689–708. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J 1992;6:886–892. [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol Reprod 1992;47:698–708. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci 1995;73:1839–1851. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev 2007;59:185–205. [DOI] [PubMed] [Google Scholar]
- Rieck PW, Cholidis S, Hartmann C. Intracellular signaling pathway of FGF-2-modulated corneal endothelial cell migration during wound healing in vitro. Exp Eye Res 2001;73:639–650. [DOI] [PubMed] [Google Scholar]
- Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem 1998;273:34724–34729. [DOI] [PubMed] [Google Scholar]
- Rockwell LC, Pillai S, Olson CE, Koos RD. Inhibition of vascular endothelial growth factor/vascular permeability factor action blocks estrogen-induced uterine edema and implantation in rodents. Biol Reprod 2002;67:1804–1810. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Kazlauskas A. VEGF-A engages at least three tyrosine kinases to activate PI3K/Akt. Cell Cycle 2012;11:2047–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci 2005;96:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 2006;175:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CA, van Huyen D, Bany BM. Angiopoietin-like gene expression in the mouse uterus during implantation and in response to steroids. Cell Tissue Res 2012;348:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993;12:303–324. [DOI] [PubMed] [Google Scholar]
- Sengupta J, Lalitkumar PG, Najwa AR, Charnock-Jones DS, Evans AL, Sharkey AM, Smith SK, Ghosh D. Immunoneutralization of vascular endothelial growth factor inhibits pregnancy establishment in the rhesus monkey (Macaca mulatta). Reproduction 2007;133:1199–1211. [DOI] [PubMed] [Google Scholar]
- Shing Y, Folkman J, Haudenschild C, Lund D, Crum R, Klagsbrun M. Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem 1985;29:275–287. [DOI] [PubMed] [Google Scholar]
- Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 1984;223:1296–1299. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 2002;90:1243–1250. [DOI] [PubMed] [Google Scholar]
- Skaznik-Wikiel ME, Kaneko-Tarui T, Kashiwagi A, Pru JK. Sphingosine-1-phosphate receptor expression and signaling correlate with uterine prostaglandin-endoperoxide synthase 2 expression and angiogenesis during early pregnancy. Biol Reprod 2006;74:569–576. [DOI] [PubMed] [Google Scholar]
- Sprague B, Chesler NC, Magness RR. Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: experimental studies and hemodynamic models of shear stresses on endothelial cells. Int J Dev Biol 2010;54:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 2009;114:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Maxwell SA, Bayless KJ. Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J Biol Chem 2010;285:40624–40634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 1999;18:2221–2230. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J 2001;20:2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanghetti E, Ria R, Dell’Era P, Urbinati C, Rusnati M, Ennas MG, Presta M. Biological activity of substrate-bound basic fibroblast growth factor (FGF2): recruitment of FGF receptor-1 in endothelial cell adhesion contacts. Oncogene 2002;21:3889–3897. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 2002;160:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper JN, Gimbrone MA Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 1999;5:40–46. [DOI] [PubMed] [Google Scholar]
- Torry DS, Holt VJ, Keenan JA, Harris G, Caudle MR, Torry RJ. Vascular endothelial growth factor expression in cycling human endometrium. Fertil Steril 1996;66:72–80. [PubMed] [Google Scholar]
- Torry RJ, Rongish BJ. Angiogenesis in the uterus: potential regulation and relation to tumor angiogenesis. Am J Reprod Immunol 1992;27:171–179. [DOI] [PubMed] [Google Scholar]
- Toth P, Li X, Rao CV, Lincoln SR, Sanfilippo JS, Spinnato JA II, Yussman MA. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab 1994;79:307–315. [DOI] [PubMed] [Google Scholar]
- Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors--a review. Reprod Biol 2001;1:5–11. [PubMed] [Google Scholar]
- van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 2005;72:1161–1168. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res 2008;78:203–212. [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D et al. . Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG 2011;118:624–628. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 2000;14:2123–2133. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994;269:26988–26995. [PubMed] [Google Scholar]
- Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 1999;274:35343–35350. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol 2012;357:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer UM, Damjanov A, Weiss J, Liotta LA, Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation 1986;32:49–58. [DOI] [PubMed] [Google Scholar]
- Wietecha MS, DiPietro LA. Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv Wound Care (New Rochelle) 2013;2:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction 2009;138:177–184. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013;45:241–256. [DOI] [PubMed] [Google Scholar]
- Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S-I, Aoki T, Sato H et al. . Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci 2007;120:1607–1614. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem 2003;278:32124–32131. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Effects of basic fibroblast growth factor on the development of mouse preimplantation embryos. Nihon Sanka Fujinka Gakkai Zasshi 1996;48:170–176. [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA 2000;97:4052–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WH, MacIntyre A, Nicosia RF. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am J Pathol 2002;161:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigrino P, Ayachi O, Schild A, Kaltenberg J, Zamek J, Nischt R, Koch M, Mauch C. Loss of epidermal MMP-14 expression interferes with angiogenesis but not with re-epithelialization. Eur J Cell Biol 2012;91:748–756. [DOI] [PubMed] [Google Scholar]
- Zubilewicz A, Hecquet C, Jeanny JC, Soubrane G, Courtois Y, Mascarelli F. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene 2001;20:1403–1413. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Hahn D, Munstedt K, Bischof P, Lang U. Invasion of cytotrophoblastic JEG-3 cells is stimulated by hCG in vitro. Placenta 1998. a;19:587–593. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab 2002;87:5290–5296. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003;110:S10–S18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.