Abstract
High-risk human papillomavirus (HPV)–associated squamous cell carcinomas of the oropharynx (SCCOP) are among the fastest growing cancers. After standard-of-care treatment, however, patients with HPV+ SCCOP have better overall and disease-specific survival than patients with HPV− SCCOP, suggesting the importance of HPV-specific immunity. We reasoned that therapeutic vaccination targeting the HPV-16 E6 and E7 oncogenes could elicit high-affinity, high-frequency tumor antigen–specific T-cell responses, which could then be augmented and shielded from suppression in the tumor microenvironment by immune checkpoint modulation. In this study, we used a preclinical syngeneic mouse model of oral cancer comprised of mouse tonsil-derived epithelial cells stably expressing HPV-16 E6 and E7 genes along with H-ras oncogene (mEER) to identify combinations of vaccination and checkpoint antibodies capable of promoting tumor regression. Intranasal HPV E6/E7 peptide vaccination and single checkpoint antibodies failed to elicit responses in more than half of animals; however, 4–1BB agonist antibody along with either CD40 agonist antibody or CTLA-4 blockade eliminated the majority of established mEER tumors. The combination of intranasal HPV peptide vaccine and α4–1BB and αCTLA-4 antibodies produced curative efficacy and a better safety profile against orally implanted mEER tumors. Correlates of protective immunity included enhanced intratumoral levels of CD8 T cells relative to immunosuppressive regulatory T cells and myeloid-derived suppressor cells. Overall, our results demonstrate combination vaccine-immunotherapy modalities as novel treatment options for HPV+SCCOP.
Introduction
High-risk human papillomavirus (HPV) infection drives the oncogenesis and progression of a subset of head-and-neck squamous cell carcinoma, particularly in the oropharynx (SCCOP). The dramatic increase in many of these cases is attributable to HPV-16 infection (1). The standard-of-care treatment for SCCOP combines surgery, radiotherapy, and chemotherapy that offers 80% recovery, specifically among those associated with HPV infection (2). Unfortunately, this high rate of remission is accompanied by poor quality of life and lack of therapeutic options to successfully treat recurrences (3). In this setting, more tolerable treatment options with lower rates of recurrence are sorely needed.
Vaccination and immune checkpoint modulation are the mainstays of cancer immunotherapy due to their ability to enhance innate and adaptive immune responses along with the potential to overcome the immunosuppressive tumor microenvironment (4). Immune checkpoint antibodies, such as αCTLA-4, αCD40, αOX40, and αPD-1 enhance antitumor T-cell responses by diverse mechanisms that include the inhibition of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), in addition to enhancing antigen presentation and immune effector mechanisms (5). Antagonistic monoclonal antibodies for CTLA-4 and PD-1, the most prevalent inhibitory receptors on activated T cells, are currently approved by the FDA to treat patients with melanoma (6). These antibodies expand effector T-cell populations, increase T-cell effector function, and decrease the density and/or suppressive capacity of Tregs (7, 8). Agonistic antibodies to OX40 and 4–1BB, key costimulatory receptors on T cells, enhance T-cell proliferation, survival, and cytotoxicity while promoting more efficient IFN-γ production and/or cytotoxic effector T cells (9, 10). Strikingly, α4–1BB has been shown to induce the expression of the transcription factor Eomesodermin (Eomes), which programs T cells to acquire enhanced cytotoxic capacity and elevated IFN-γ and TNF-α production (termed ThEO or TcEO; ref. 11). Although most of these immune modulatory antibodies predominantly target T cells, agonistic antibodies to CD40, the costimulatory molecule on myeloid cells indirectly induce T-cell activation and antitumor immunity, through enhancing antigen presentation and costimulatory capacity along with increasing M1 macrophage polarization (12).
Recent preclinical and clinical evaluations clearly demonstrated the potential advantages of the combinations of therapeutic antibodies, relative to monotherapies to provide superior antitumor efficacy and enhanced overall survival benefits (13). Even as monotherapies, these immune-modulatory antibodies can cause dose-limiting immune-related adverse events that can be substantially worsened in the context of combination therapy (14). Therefore, careful selection of checkpoint modulating antibodies with acceptable safety profiles and supplementing with well-designed vaccines are important strategies for efficient clinical cancer care management.
Therapeutic vaccines targeting the E6 and E7 oncoproteins of HPV have an established capacity to safely elicit tumor antigen-specific T-cell responses, which can regress premalignant HPV+ lesions in human clinical trials (15). Nevertheless, HPV vaccines lack the capacity to eradicate established invasive cancers (16). This is partly due to the abundance of Tregs, deficiency in antigen presentation, and exhausted effector T-cell responses within the immunosuppressive tumor microenvironment combined with limited trafficking of T cells to relevant mucosal tissues, which diminish the therapeutic potential of the vaccine-induced response (8).
We investigated the therapeutic potential and underlying immune biology of a vaccine-immunotherapy combination strategy in a preclinical HPV+ oropharyngeal tumor model derived from mouse tonsil epithelial cells (mEER; ref. 17). This cell line has been shown to share some characteristics with human HPV+ head and neck cancers, such as E6-dependent loss of p53. Malignant transformation of this cell line requires H-Ras and E6 or E7 expression (17). Although H-Ras mutations are rare in HPV+ HNSCC, it is hypothesized that this mutation is analogous to synergistic activity of HPV oncogenes and growth factor signaling, which is known to be activated in head and neck cancers (18, 19). We tested the therapeutic efficacy of a variety of immune checkpoint modulating antibodies individually or in combination along with an HPV-16 E6 and E7 peptide vaccine developed in our laboratory for the induction of systemic and mucosal antigen-specific immune responses after intranasal delivery (20). Our data support the concept that therapeutic cancer vaccines combined with immune checkpoint modulation may offer a safe and potentially curative therapy for HPV+ oropharyngeal tumors.
Materials and Methods
Animals
Male C57BL/6 mice (7–10 weeks) were purchased from the Jackson Laboratory and MD Anderson Cancer Center Experimental Radiation Oncology Department and were maintained in a pathogen-free environment. Animal studies were approved and conducted in accordance with University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee guidelines. Animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) mixture administered by the intraperitoneal route for immunizations and with isofluorane for tumor cell injections and blood draws. Animals were sacrificed according to institutional guidelines.
Cell line
Mouse tonsil epithelial cells expressing HPV-16 E6 and E7 and H-Ras (mEER) were a kind gift from Dr. J. Lee, the creator of this cell line (Sanford Health, Sioux Falls, South Dakota) who maintains an authenticated stock (21). On receipt, cells were frozen in a large single-passage bank to ensure preservation of the integrity of the line at our site. Cells were tested for Mycoplasma every 6 months. These cells were maintained in complete media as previously described (21), and subcultured at 80% confluency the day before tumor induction in mice.
Reagents
The E744–62 peptide, Q19D (QAEPDRAHYNIVTFCCKCD); E749–57 peptide, R9F (RAHVYNIVTIF); E643–57 peptide, Q15L (QLLRREVYDFAFRDL); and E649–58 peptide, V10C (VYDFAFRDLC) were purchased from Elim Biopharma. The glycolipid α-galactosylceramide (αGalCer) adjuvant was purchased from DiagnoCine. APC-labeled H-2Db epitope E749–57 (RAHYNIVTF)-containing tetramer was procured from the MHC tetramer production facility at Baylor College of Medicine (Houston, TX). The tumor-infiltrating lymphocytes (TIL) were analyzed by multi-parametric flow cytometry using the antibodies described in the Supplementary Methods. The following antibodies for immunotherapy were purchased from BioXcell and used at the concentrations shown: α4–1BB (LOB12.3 at 350 μg per dose), αCD40 (FGK4.5 at 100 μg per dose), αCTLA-4 (9H10 at 100 μg per dose), αPD-1 (RMP1–14 at 250 μg per dose), and αOX-40 (OX-86 at 100 μg per dose).
In vivo tumor challenge
Mice were injected with 1 × 106 mEER cells subcutaneously in 200 μL PBS on the right flank. Tumor growth was measured using a caliper and mice were sacrificed when the tumor area reached 200 mm2. For oral HPV+ tumor studies, 4 × 104 mEER cells in 50 μL PBS were injected into the base of the tongue. Mice were monitored closely and sacrificed when a necrotic tumor was observed and/or when the mice lost 20% or more of their initial weight.
Characterization of TIL was performed as described in Supplementary Methods.
Vaccination and immunotherapy
Between days 5 and 7 after tumor challenge, mice were immunized under anesthesia with the HPV vaccine (100 μg each of the four peptides with 2 μg of α-GalCer) via the intranasal route twice at 6-day intervals as described previously (20). Immunized animals also received intraperitoneal injection of therapeutic antibodies starting on the day of intranasal immunization and 2 additional times at 3-day intervals. Control animals were untreated. For in vivo depletion of CD8+ T cells, we injected 350 μg per mouse of αCD8 mAb (clone 2.43, BioXCell) by the intraperitoneal route on the day before and the day after tumor implantation, and every 3 days until mice were sacrificed.
Flow cytometry
For characterization of TILs, mice were sacrificed at day 15 after tumor challenge. Subcutaneous tumors were collected and digested as previously described (20). For tongue tumors, we isolated lymphocytes from the oral mucosa following a published protocol (22) with some modifications as described in Supplementary Methods. The gating strategy to identify the different immune cell populations is depicted in Supplementary Fig. S1.
Liver function assessment
Blood was collected from anesthetized mice through retro-orbital plexus at day 14 after tumor challenge for the analysis of the serum enzymes: aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels and Alkaline Phosphatase by the Clinical Pathology Laboratory in the Veterinary Medicine and Surgery Department at MD Anderson Cancer Center.
Magnetic resonance imaging
Mice were imaged at day 19 after tumor challenge on the 1T Bruker ICON at the MD Anderson Cancer Center Small Animal Imaging Facility as described in Supplementary Methods (23).
Statistical analysis
All statistics were calculated using GraphPad Prism version 6 for Windows. Statistical significance was determined using one-way ANOVA to test differences between multiple groups and the Mantel–Cox analysis for survival. P values less than 0.05 were considered significant.
Results
Therapeutic HPV vaccination enhances antitumor efficacy of immune checkpoint therapy
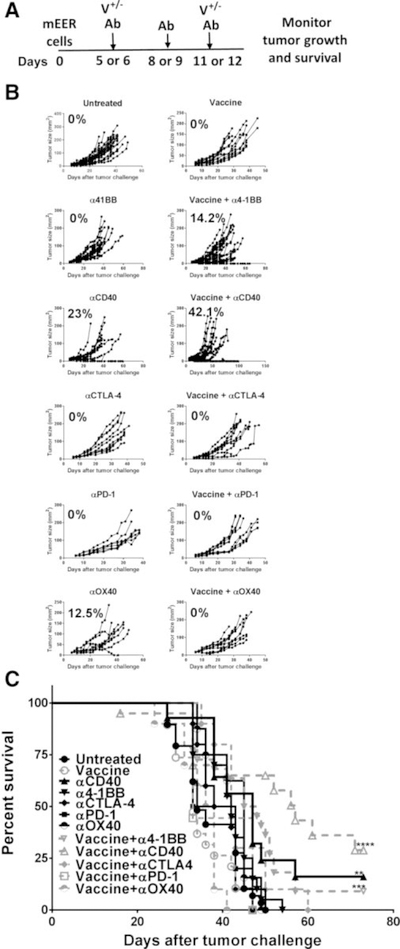
The mEER tumor model has been established as a preclinical surrogate for HPV+ oropharyngeal cancers (21). We tested a number of costimulatory agonists and coinhibitory blocking antibodies along with or without coadministration of an intranasal vaccine comprised of HPV-16 E6 and E7 peptides admixed with the NKT cell ligand αGalCer as adjuvant for the treatment of mice subcutaneously implanted with 1 × 106 mEER tumor cells. The selected antibodies included those that targeted TNF-receptor family members such as 4–1BB, CD40, and OX40, and others directed against the inhibitory immune checkpoint molecules CTLA-4 and PD-1 (4, 24). Separate groups of mice with established subcutaneous mEER tumors were injected with these checkpoint modulating antibodies on days 6, 9, and 12 after tumor challenge along with or without intranasal HPV peptide vaccination on days 6 and 12 as described earlier (20), and monitored for tumor growth (Fig. 1A). Among the different antibodies tested, treatment with αCD40 and αOX40 resulted in 23% and 12.5% of mice exhibiting tumor regression, respectively (Fig. 1B). Vaccination when combined with αCD40 treatment further significantly delayed tumor growth, resulting in more than 40% mice exhibiting tumor regression and extended survival (Fig. 1C). The combination of HPV peptide vaccination and α4–1BB treatment was effective to a lesser extent showing tumor regression in 14% of mice. None of the other checkpoint antibody treatments along with or without intranasal HPV peptide vaccination yielded any significant therapeutic benefit in this model. Furthermore, we observed that administration of vaccine and checkpoint antibodies, α4–1BB, αCD40 or αPD-1 sequentially was not effective, relative to HPV peptide vaccine and immune checkpoint modulation delivered concurrently (Supplementary Fig. S2).
Figure 1.
Intranasal HPV peptide vaccination enhances the therapeutic efficacy of α4–1BB and αCD40 antibodies. A, Mice were injected subcutaneously with mEER cells (1 × 106) and when the tumors were palpable, received intranasal HPV peptide vaccination on days 6 and 12 along with intraperitoneal injections of α4–1BB, αCD40, αCTLA-4, αPD-1, or αOX40 at days 6, 9, and 12. The tumor growth (B) and survival (C) were monitored over time. The percentage of mice showing tumor regression is noted for each treatment (B). A Mantel–Cox test was performed to determine the significance of survival for each of the treatment groups relative to untreated group; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005. Results represent pooled data from multiple experiments (n = 7–27 mice/group).
Combinations of immune checkpoint modulating antibodies along with intranasal HPV peptide vaccination promote mEER tumor rejection
A potential modality to enhance the efficacy of immunotherapy is through the activation of T cells and antigen-presenting cells (APC) at the same time. Agonistic antibodies against CD40 (on APCs) and 4–1BB (mostly on T cells) target these two arms of the immune system. Because αCD40 and α4–1BB as monotherapies demonstrated the highest therapeutic synergy with HPV vaccination, we reasoned that a combination of all three immune therapies might further enhance antitumor responses. It has also been demonstrated previously that costimulatory activation of tumor-specific cells can be significantly enhanced when they are free from the limitations of checkpoint inhibition (13). Therefore, we selected α4–1BB as the activating T-cell antibody and combined it with antibodies that would block T-cell coinhibitory molecules and/or qualitatively or quantitatively diminish regulatory T cells (αCTLA-4, αPD-1, and αOX40), all in the presence or absence of intranasal HPV peptide vaccination.
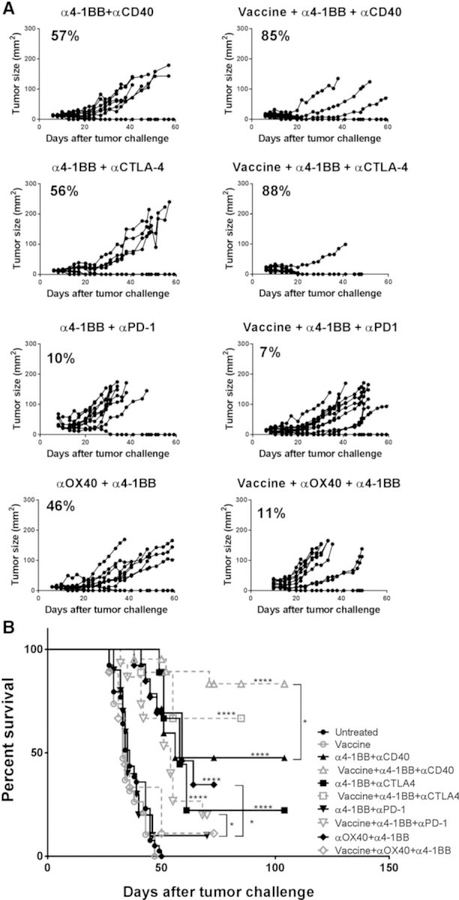
After establishing subcutaneous mEER tumors, mice were administered the intranasal HPV vaccine, in conjunction with intraperitoneal injection of the indicated therapeutic antibody combinations as per the scheme shown in Fig. 1A. Among the different antibodies tested, treatment with the combinations of α4–1BB and αCTLA-4, as well as α4–1BB, and αCD40 significantly reduced tumor growth in addition to inducing tumor regression in 56% and 57% of mice that was further significantly enhanced to 88% and 85%, respectively, by coadministration of the HPV peptide vaccine (Fig. 2A). Vaccine-mediated enhancement of tumor regression in these groups of mice also correlated with increases in survival (Fig. 2B). Also, the mixture of α4–1BB and αOX40 antibodies was effective in inducing tumor regression in 46% of mice, and the addition of the HPV peptide vaccine did not further enhance this response rate. In contrast, the combination of α4–1BB and αPD-1, with or without the HPV peptide vaccine, was largely ineffective. These results in the preclinical model of HPV+ oropharyngeal tumors demonstrate the protective efficacy of selected combinations of immune-modulatory antibodies (α4–1BB/αCD40 and α4–1BB/αCTLA-4) and that the addition of HPV peptide vaccine to these antibody combinations provides a further therapeutic advantage.
Figure 2.
Combination of the HPV peptide vaccine with α4–1BB and αCD40 is the most potent treatment for inducing long-term tumor regression. Mice bearing subcutaneous mEER tumors and treated with different combinations of immune modulator antibodies in the presence or absence of the intranasal HPV peptide vaccine as described in Fig. 1 legend were monitored for tumor growth (A) and survival (B) over time. The percentage of mice showing tumor regression is noted for each treatment (A). A Mantel–Cox test was performed to determine the significance of survival for each of the treatment groups relative to untreated group (shown on top of the line) and also between the antibody combinations in the presence or absence of the HPV peptide vaccination. *, P < 0.05; ****, P < 0.00005. Results represent pooled data from multiple experiments (n = 5–20 mice/group).
Coactivation of 4–1BB and CD40 with HPV vaccination induces elevated CD8 T-cell infiltration
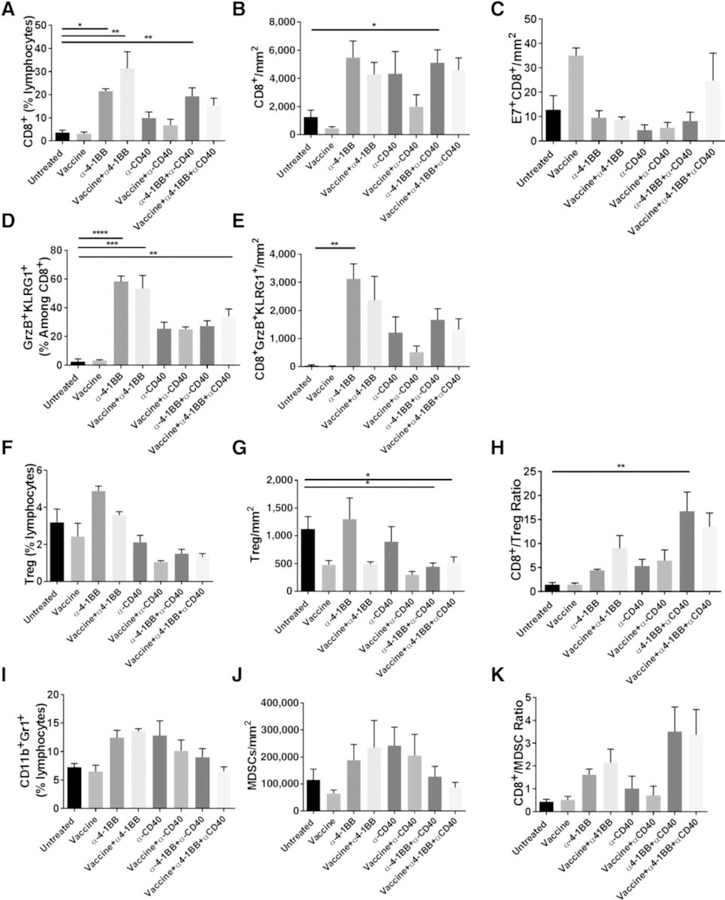
We investigated the correlates of protection against HPV+ mEER tumors afforded by the mixture of α4–1BB and αCD40 antibodies along with and without the HPV peptide vaccine. We isolated TIL 15 days after tumor implantation when significant differences in tumor growth were evident between mice in the various treatment groups versus in untreated mice. In general, mice treated with α4–1BB alone or in conjunction with αCD40 and/or the HPV peptide vaccine showed higher percentages, as well as absolute numbers, of total CD8 T cells relative to control untreated mice (Fig. 3A and B). In parallel, we also observed higher numbers of E7-specific CD8 T cells in mice receiving the vaccine alone or in combination with the mixture of α4–1BB and αCD40 antibodies (Fig. 3C). Furthermore, α4–1BB treatment showed higher percentages and frequencies of CD8 T cells expressing the activation and cytotoxic markers KLRG1 and Granzyme B (GzmB) relative to untreated mice (Fig. 3D and E). Another characteristic feature of α4–1BB treatment is its capacity to upregulate the expression of the transcription factor Eomes that imparts uniquely enhanced cytotoxic potential to CD8 T cells (11). Consistent with this finding, mice treated with α4–1BB in the presence or absence of the HPV peptide vaccine showed the highest expression of Eomes+KLRG1+ TcEO CD8 T cells (Supplementary Figure S3). The dependence of triple-combination therapy on the activity of CD8 T cells was further demonstrated by the complete loss of protection in the context of CD8 T-cell depletion (Supplementary Fig. S4). Mice cured of initial tumor challenge following treatment with the combination of αCD40 and α4–1BB, with and without HPV vaccination, were rechallenged at 7 weeks and followed for tumor growth for an additional 30 days. None of the rechallenged mice developed mEER tumors demonstrating the capacity of this combination to induce protective immune memory (Supplementary Fig. S5).
Figure 3.
Immune correlates for tumor protection in mice treated with HPV peptide vaccine along with or without α4–1BB and αCD40. Mice bearing subcutaneous mEER tumors were either untreated or treated with intranasal HPV peptide vaccine, α4–1BB, or αCD40 individually or in combinations as described in Fig. 1 legend. Tumors were harvested at day 15 and the isolated leukocytes were characterized by flow cytometry after staining for different surface and intracellular markers. The figure shows percentages of CD8+ T cells (A), frequencies of CD8+ T cells as number of cells per tumor area (B), frequencies of E7-specific CD8+ T cells (C), percentages of CD8+ T cells expressing GrzB and KLRG1 (D), frequencies of CD8+ GrzB+KLRG1+ cells (E), percentages of CD4+FoxP3+ Tregs (F), frequencies of CD4+FoxP3+ Tregs (G), CD8+ T cells to Treg ratio (H), the percentages of CD11b+Gr1+ MDSCs (I), frequencies of CD11b+Gr1+ MDSCs (J), and the CD8+ T cells to MDSC ratio after every treatment (K). Data are represented as means ± SEM (n = 2–11 mice/group). Results represent pooled data from multiple experiments. Statistical significance was calculated using one-way ANOVA. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005.
The combination of 4–1BB and CD40 agonist antibodies increases the intratumoral ratio of CD8 T cells to immunosuppressive populations
Tregs and MDSC are critical mediators of T-cell immune suppression and tumor immune privilege, which must be overcome by CD8 T cells for immunotherapy to succeed (25, 26). In accordance with this, we observed that the treatment comprised of HPV vaccination along with α4–1BB or αCD40 antibodies significantly reduced the intratumoral frequency of Tregs (CD4+FoxP3+) compared with treatment with either antibody alone (Fig. 3F and G). When used in combination, α4–1BB and αCD40 with or without HPV peptide vaccination showed the highest reduction of intratumoral Tregs and the highest ratio of CD8 T cells relative to Tregs (Fig. 3H).
Although none of the studied treatments showed a significant decrease in the frequency of intratumoral MDSC (CD11b+Gr1+; Fig. 3I and J), we found that the ratio of CD8 T cells relative to MDSC was the highest in mice treated with the combination of α4–1BB and αCD40 both with and without HPV vaccination (Fig. 3K). These results demonstrate that increased ratios of CD8 T cells to Tregs as well as MDSC are potential correlates for the observed therapeutic efficacy in mice receiving the α4–1BB/αCD40 combination in the presence or absence of HPV peptide vaccine (Fig. 2).
One important feature of the HPV peptide vaccine employed in this investigation is the unique capacity of the αGalCer adjuvant to promote antigen-presentation and enhanced antigen-specific T-cell responses as a downstream outcome of pro-inflammatory cytokine release from αGalCer-responsive NKT cells (27, 28). Consistent with this, when we restimulated splenocytes with a pool of E6 and E7 peptides from the vaccine, we detected an increase in IFNγ+ CD8 T cells from mice receiving the vaccine along with α4–1BB/αCD40, but not from those isolated from mice that received the same combination without the vaccine (Supplementary Fig. S6). These data support the effectiveness of the HPV peptide vaccine incorporating the αGalCer adjuvant to promote DC-mediated T-cell activation in secondary lymphoid organs concurrent with antitumor efficacy.
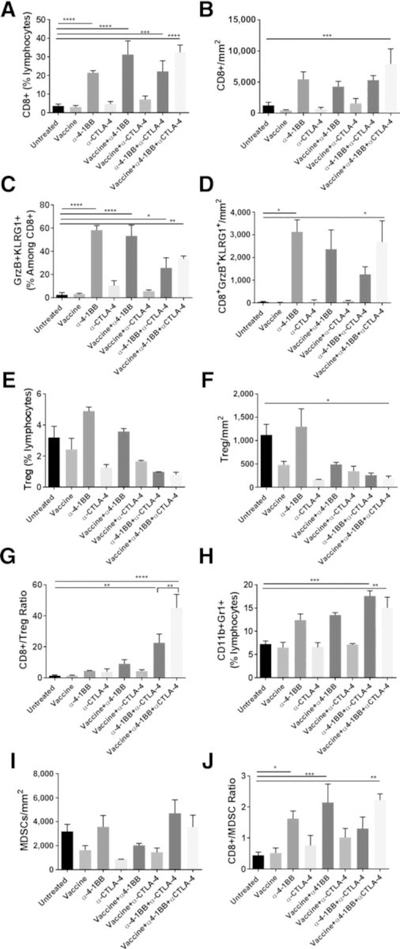
Intranasal HPV peptide vaccination combined with α4–1BB and αCTLA-4 antibodies significantly drive CD8 T-cell expansion and reduced Treg density
As shown in Fig. 2, in addition to the combination of α4–1BB and αCD40, we observed significant therapeutic efficacy for the α4–1BB/αCTLA-4 combination that was further enhanced by the peptide vaccine. Mice treated with this combination of HPV vaccine and α4–1BB/αCTLA-4 showed the highest percentage and frequency of CD8 T cells in the tumor (Fig. 4A and B). High levels of activated cytotoxic CD8 T cells (GzmB+KLRG1+) were observed in the tumors of mice in all groups that received α4–1BB (Fig. 4C and D). Also, consistent with the known effectiveness of the αGalCer adjuvant to promote both mucosal and systemic immunity to coadministered antigens (27, 28), we observed HPV E7 tetramer+ CD8 T cells in the blood of mice receiving the intranasal vaccination (Supplementary Fig. S7).
Figure 4.
Combination of α4–1BB and αCTLA-4 provides tumor protection by increasing the ratios of CD8+ T cells to immunosuppressive populations. Mice bearing subcutaneous mEER tumors were either untreated or treated with intranasal HPV peptide vaccine, α4–1BB, or αCTLA-4 individually or in combinations as described in Fig. 1 legend. Tumors were harvested at day 15 and the isolated leukocytes were characterized by flow cytometry after staining for different surface and intracellular markers. The figure shows percentages of CD8+T cells (A), frequencies of CD8+ T cells as number of cells per tumor area (B), percentages of CD8+ T cells expressing GrzB and KLRG1 (C), frequencies of CD8+GrzB+KLRG1+ cells (D), percentages of CD4+FoxP3+ Tregs (E), frequencies of CD4+FoxP3+ Tregs (F), CD8+ T cells to Treg ratio (G), percentages of CD11b+Gr1+ MDSCs (H), frequencies of CD11b+Gr1+ MDSCs (I), and the CD8+ T cells to MDSC ratio (J). Data are represented as means ± SEM. Results represent pooled data from multiple experiments (n = 2–11 mice/group). Statistical significance was calculated using one-way ANOVA. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005.
The most efficacious CTLA-4 antibodies in mice can efficiently deplete intratumoral Tregs (29, 30). Consistent with these reports, we found reduced levels of intratumoral Tregs in all groups of mice that received αCTLA-4 treatment (Fig. 4E and F). Consequently, when combined with the observed high levels of CD8 T-cell infiltration, mice receiving αCTLA-4 treatment combined with 4–1BB agonist showed the highest ratio of CD8 T cells relative to Tregs in the tumor (Fig. 4G). The addition of HPV vaccine to this dual combination significantly increased this ratio. On the other hand, the frequency of intratumoral MDSCs in mice treated with the αCTLA-4/α4–1BB combination in the presence or absence of the HPV peptide vaccine was higher relative to untreated mice (Fig. 4H and I). Nevertheless, owing to the high frequencies of infiltrating CD8 T cells from this combination treatment, the ratios of CD8 T cells to MDSCs were significantly elevated compared with untreated mice (Fig. 4J). Interestingly, mice responding to vaccine along with αCTLA-4/α4–1BB combination showed an enhanced infiltration of CD8+ T cells and lower expression of the immunosuppressive molecule arginase by MDSCs in their tumors compared with nonresponding and untreated mice (Supplementary Fig. S8). These results demonstrate that an increased ratio of CD8 T cells relative to Tregs, and, to a lesser extent, of CD8 T cells to MDSC, correlates with therapeutic efficacy of the combination treatment of intranasal HPV peptide vaccination with αCTLA-4/α4–1BB antibodies.
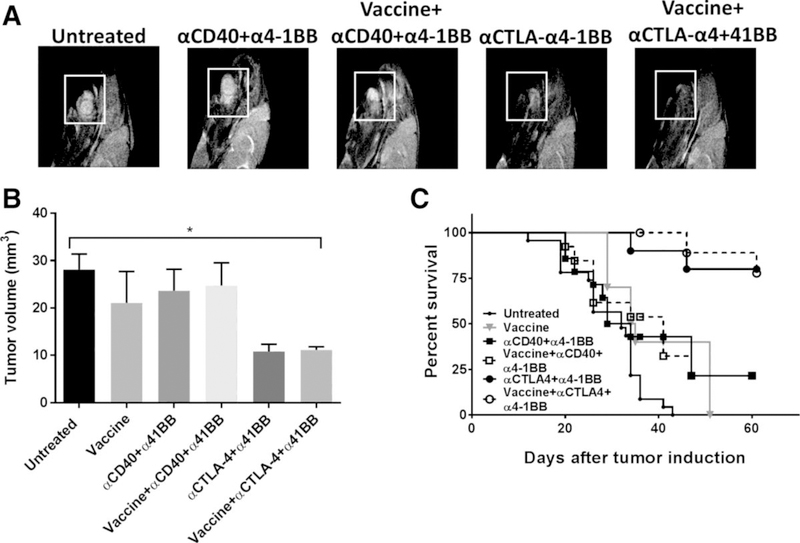
Combination HPV vaccine and α4–1BB/αCTLA-4 therapy cures oral HPV+ mEER tumors
Although subcutaneous tumor models are a useful tool for rapid screening of potential interventions and generation of early mechanistic insights, robust preclinical evaluation of immunotherapeutics targeting HPV+ malignancies requires their implantation in their natural mucosal environment (31, 32). As nearly half of oropharyngeal squamous cell carcinomas occur in the tongue (33), we investigated the two most efficacious immunomodulatory antibody combinations from our flank tumor studies (i.e., α4–1BB/αCD40 and α4–1BB/αCTLA-4) with or without HPV peptide vaccination against HPV+ mEER tumors orthotopically implanted in the tongue. Mice received intranasal peptide vaccination on days 5 and 11 and the indicated antibodies on days, 5, 8, and 11 after tumor challenge. At day 19, we used MRI to determine the tumor volume. Relative to untreated mice, those receiving the α4–1BB/αCTLA-4 combination, with or without vaccine, exhibited significantly reduced tumor growth (Fig. 5A and B) along with significant survival advantage (Fig. 5C). The combination of α4–1BB and αCD40, with or without vaccine, which showed significant protective efficacy for treating subcutaneous mEER tumors (Fig. 2) was largely ineffective at controlling the growth of the tumors in the tongue. At day 60, 80% of mice that received α4–1BB/αCTLA-4 with or without the HPV vaccine were alive whereas only 36% of mice that received α4–1BB/αCD40 were alive. These results demonstrate that the combination of α4–1BB and αCTLA-4 is a superior therapy for orthotopic HPV+ mEER tumors.
Figure 5.
Combination of HPV peptide vaccine, α4–1BB and αCTLA-4 induces regression of HPV+ tongue tumors. Mice were challenged with mEER tumor cells (40,000) in the tongue and treated with the different vaccine and immunotherapy combinations starting on day 5 as described in Fig. 1. A representative MRI (T2-weighted sagittal image) of mouse tongue (A) and tumor volume measured by MRI (B) at day 19 after tumor implantation are shown (data shown are means ± SEM; n = 5–12 mice/group). Survival curves of mice bearing tongue tumors treated with different immunotherapy combinations are shown (C; n = 10 – 23 mice/group). Statistical significance was calculated using one-way ANOVA. *, P < 0.05. Results represent pooled data from multiple experiments.
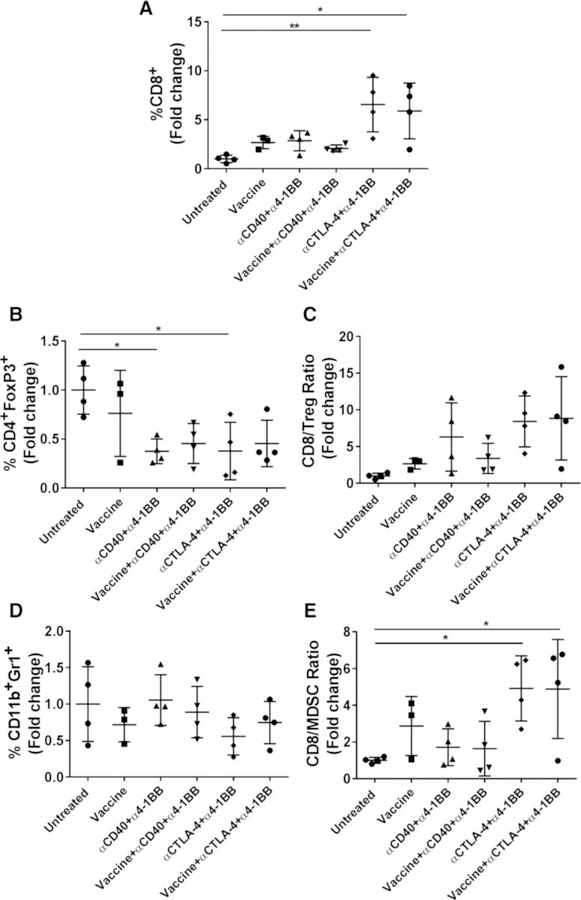
Immune infiltration of orally implanted HPV+ tumors
We analyzed the TIL in tongue tumors to understand the immune correlates for the observed therapeutic efficacy of the mixture of α4–1BB and αCD40 antibodies relative to that for the combination of α4–1BB and αCTLA-4 antibodies, in the presence or absence of the intranasal HPV peptide vaccine. Because of the limited size of tumors from the tongue, cells from 3 to 4 individual tumors in each treatment group were pooled for the analyses of immune infiltrates and the comparative differences for each treatment group relative to untreated mice were expressed as fold changes. In two separate experiments (with group sizes of 6–8 mice in each experiment) we observed the percentage of CD8 T cells to be significantly higher (over 5-fold) in mice receiving the combination of α4–1BB and αCTLA-4 versus untreated mice consistent with their reduced tumor burden and higher survival (Fig. 6A). The enhanced levels of CD8 T cells within the tumors of mice treated with the combination of α4–1BB and αCTLA-4 antibodies in the presence or absence of HPV peptide vaccination also coincided with relatively lower levels of Tregs (Fig. 6B) and overall improvements in the CD8 T-cell to Treg ratios (Fig. 6C). The wide variation may be attributed to the pool of responders versus nonresponder mice. In addition, although we did not observe any differences in the levels of MDSC in any of the treatment groups, relative to untreated controls, there was an increase in the ratio of CD8 T cells to MDSC for the treatment regimen combining α4–1BB and αCTLA-4 antibodies with and without the HPV peptide vaccine compared with untreated mice (Fig. 6D and E). These results support the conclusion that the combination of α4–1BB and αCTLA-4 antibodies effectively modulates the tumor microenvironment to enhance antitumor responses while decreasing immunosuppressive Treg and MDSC in HPV+ oral tumors.
Figure 6.
Immune correlates for vaccine immunotherapy of HPV+ tongue tumors. TILs isolated at day 15 after tongue tumor challenge and different immunotherapies were analyzed by flow cytometry. The fold changes of CD8+ T-cell percentages (A), CD4+FoxP3+ Treg percentages (B), CD8+ T cells to Treg ratios (C), CD11b+Gr1+ MDSCs (D), and CD8+ T cells to MDSC ratios (E) in the different treatment groups compared with untreated mice were calculated. Data for individual mice are shown along with mean ± SEM. Individual data points represent pools of 3 to 4 tumors in two separate experiments (n = 6–8 mice/group). Statistical significance was calculated using one-way ANOVA. *, P < 0.05; **, P < 0.005.
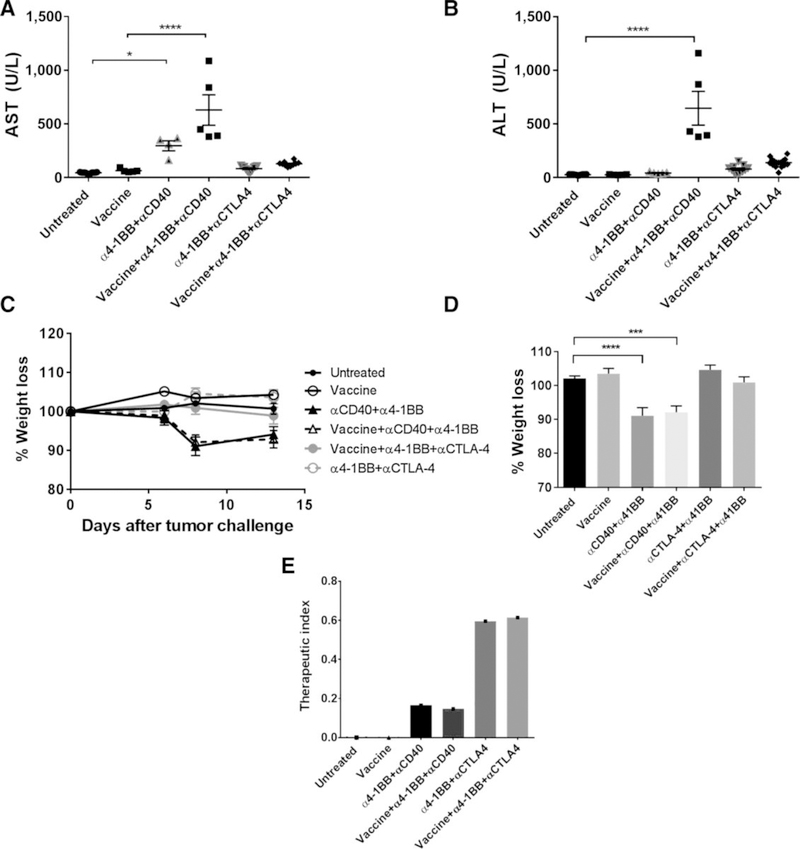
Combination of HPV vaccine, α4–1BB, and αCTLA-4 causes minimal liver toxicity
Agonist antibodies against 4–1BB have the potential to induce liver inflammation characterized by an elevation in the serum levels of liver transaminases (36). To evaluate the safety of our two most efficacious combination therapies, we tested whether any regimen involving α4–1BB, which was active against HPV+ mEER tumors, demonstrated toxicity. Mice treated with the α4–1BB/αCD40 and α4–1BB/αCTLA-4 combinations, both with and without HPV peptide vaccine were bled at day 14 (2 days after last treatment) and their sera were evaluated for AST and ALT levels. This serum analysis revealed the levels of AST and ALT to be significantly higher in mice receiving the α4–1BB/αCD40 combination with or without vaccine, compared with untreated mice (Fig. 7A and B). In contrast, mice receiving α4–1BB/α-CTLA-4, with or without the HPV vaccine, showed no differences in AST or ALT levels relative to control untreated mice. On the basis of a published report (37), these liver enzyme concentrations in mice treated with α4–1BB and αCD40 could represent a serious immune-related adverse event.
Figure 7.
Combination treatment consisting of α4–1BB and αCTLA-4 in the presence and absence of HPV peptide vaccine does not exhibit liver toxicities. Serum samples from mice treated with different vaccine and immunotherapy combinations were collected at day 14 after tumor challenge and analyzed for AST (A; n = 5–16 mice/group) and ALT levels (B). C, The weight of mice was monitored before and after tumor challenge and throughout treatment (n = 5–15). D, The percentage of weight loss was calculated at day 7 after tumor challenge. E, The therapeutic index for the different treatments was calculated by dividing the percentage regression of mice observed per group and the average AST number and multiplying this by 100. Results represent pooled data from multiple experiments. Data for individual mice are shown along with mean ± SEM. Statistical significance was calculated using one-way ANOVA. *, P < 0.05; ***, P < 0.005; ****, P < 0.0005.
We also observed that the liver toxicity in mice treated with the combination of α4–1BB and αCD40 was accompanied by weight loss relative to untreated mice (Fig. 7C). The onset of weight loss occurred shortly after initiation of α4–1BB/αCD40 therapy (2 days) and failed to normalize during the treatment period (Fig. 7D). On the other hand, no weight loss was observed in mice bearing HPV+mEER tumors treated with the combination of α4–1BB and αCTLA-4 in the presence or absence intranasal HPV peptide vaccination. These results support the conclusion that immunotherapy combining α4–1BB and αCTLA-4 is a safer treatment, relative to that combining α4–1BB and αCD40. Thus, the therapeutic efficacy in the absence of signs of liver toxicity for the combination treatment using α4–1BB and αCTLA-4 with and without the HPV peptide indicates a favorable therapeutic index (Fig. 7E).
Discussion
The use of immune checkpoint modulation to enhance antitumor efficacy, through promoting antitumor responses and minimizing immunosuppressive populations within tumors, has proven to be highly successful for the treatment of many cancers. However, many of the checkpoint antibodies also exhibit systemic toxicity, which limits their clinical application as monotherapies and makes some potentially efficacious combinations untenable in practice. Using a preclinical model of HPV+ oropharyngeal cancer (mEER), we present evidence in support of a combination treatment comprised of the mixture of 4–1BB agonist antibody and CTLA-4 antagonist antibody supplemented with intranasal HPV peptide vaccination as a safe and highly effective therapeutic strategy for inducing sustained regression of HPV+ tumors implanted subcutaneously or in the tongue of C57BL/6 mice. This vaccine-checkpoint antibody combination induced infiltration into and activation of CD8 T cells inside the tumor while reducing Treg density. In addition, we identified the combination of HPV peptide vaccine and agonistic antibodies targeting 4–1BB and CD40 to be effective for treating subcutaneous, but not orally implanted, HPV+ mEER tumors. Despite its efficacy, the translational potential of this combination might be limited by the significant elevation of liver enzymes coupled with weight loss, which it evoked in treated mice.
Intriguingly, αPD-1 therapy was not effective at treating HPV+ subcutaneous tumors. We hypothesize that PD-1 expression on CD8+ T cells may be at a threshold lower than that needed for αPD-1 therapy to be effective in this model, as it has been observed in patients with cancer (38, 39). This hypothesis is currently being tested in our laboratory.
Combining vaccination with immune checkpoint modulation creates a synergistic boost to antitumor immunity where the vaccine-induced T cells provide an on-target substrate that can then be amplified and protected from attenuation in the tumor microenvironment by the antibodies. The HPV-16 E6 and E7 peptide vaccine incorporating the αGalCer adjuvant, we developed is efficient at inducing systemic as well as mucosal HPV-specific CD4 and CD8 T-cell responses that effectively traffic to mucosal tissues after intranasal delivery (28). Data from this investigation demonstrated that the intranasal HPV-16 peptide vaccine significantly enhanced the therapeutic efficacy of the combinations of immune checkpoint antibodies against subcutaneous HPV+ mEER tumors, but its additive effect was only modest against tongue-implanted tumors. It has been reported that sublingual and buccal mucosa contain a small repertoire of immune cells, but tongue-infiltrating lymphocytes have not been clearly studied after vaccination (40, 41). Therefore, the lack of increased efficacy of the added intranasal HPV peptide vaccine over what was observed with the checkpoint modulating antibody mixtures to treat HPV+ mEER tumors in the tongue may be due to limited trafficking of antigen-specific T cells to this location. Additional routes of vaccine administration may enhance immune cell trafficking to this immune-privileged site and this is an area of current investigation in our laboratory. Alternatively, mEER tumors may not be truly dependent on E7 and may contain strong neoantigens that are immunologically dominant in the tongue limiting the benefits of augmenting the antigen-specific T-cell repertoire through immunization with the E6 and E7 peptides included in the vaccine (17). Future studies will attempt to investigate this possibility
It has been reported that immune modulatory antibodies can induce epitope spreading of tumor antigens, which broadens the repertoire of tumor-specific T cells (42). Thus, the limited vaccine-induced infiltration of HPV+ T cells into the tongue-implanted mEER tumors could have been offset by additional T-cell specificities enriched from the combinations of α4–1BB/αCD40 and α4–1BB/αCTLA-4 antibodies. The generation of a broader repertoire of T cells, in addition to HPV specificity, is potentially advantageous, and the identification of T-cell receptor specificities of T cells driving antitumor responses resulting from the combination of these immune checkpoint antibodies is a promising area for further investigation.
We previously reported that agonistic 4–1BB antibody treatment along with administration of the intranasal HPV peptide vaccine, used in this article, produced durable regression of vaginal HPV+TC-1 tumors, through its ability to generate highly cytotoxic TcEO cells11, (20). Consistent with these properties of α4–1BB antibody treatment, we observed that in mice harboring HPV+ mEER tumors treatments incorporating α4–1BB antibody induced high levels of cytotoxic CD8 T cells and efficient antitumor responses. However, α4–1BB therapy with or without HPV peptide vaccination was largely ineffective at rejecting HPV+ mEER tumors. The phenotype of the two tumor models, as well as the vaginal versus oral sites for the tumor growth, could potentially be the reasons for the differential efficacies of α4–1BB antibody treatment. In this regard, an RNA-seq analysis of HPV+ tumors from patients with cancer revealed differential gene-expression profiles between oropharynx and other sites, and some of the significant differences were associated with regulators of cell cycle and T-cell infiltration (43). We also observed that the combination of HPV vaccine with α4–1BB and αCD40 was highly effective for treating mEER tumors implanted subcutaneously but had limited efficacy against tongue tumors. The tumor microenvironment at these two locations may be comprised of distinct spectrums of immune cells and danger signals that may also affect the trafficking as well as functioning of immune cells (31, 32).
Immune modulatory antibodies, although effective for treating different types of cancers, also exhibit a wide range of toxicities, such as hepatotoxicity resulting from infiltration of proinflammatory myeloid cells in the liver noted for the CD40 agonist antibody in clinical trials (44–47). Similarly, 4–1BB agonist antibody induces liver toxicities due to myeloid activation and subsequent infiltration of activated T cells into the liver (48, 36). Consistent with these reports we observed an increase in serum transaminases in mice treated with these two agonistic antibodies together, despite their strong antitumor efficacy (Fig. 7). Gene therapy approaches locally delivering recombinant ligand constructs could potentially harness the therapeutic efficacy of targeting these costimulatory molecules without inducing toxicity based on reports showing that intratumoral CD40L and 4–1BBL gene delivery caused minor toxicities while inducing effective tumor-specific immunity in clinical and preclinical studies of urinary bladder cancer and melanoma (49–51).
The combination of α4–1BB and αCTLA-4 treatment of HPV+ mEER tumors in our studies showed significant antitumor efficacy and a significant survival advantage while inducing negligible levels of the liver enzymes ALT and AST. This combination has also been reported to be effective in preclinical models of melanoma and colon adenocarcinoma (26, 52). Importantly, multiple studies have demonstrated that combining α4–1BB and αCTLA-4 ameliorated the side effects of each monotherapy (36, 52). Although a Phase I trial of Ipilimumab and Urelumab was withdrawn before opening enrollment due to liver toxicity observed in the 4–1BB monotherapy trial (20), careful testing of the combination of α4–1BB and αCTLA-4 antibodies could prove beneficial for the clinical management of patients with HPV+ oropharyngeal cancer.
Current therapies for SCCOP, such as chemotherapeutic agents, radiation, and surgical resection, although useful in reducing tumor burden and extending survival of patients, often cause significant local and systemic toxicities, resulting in poor quality of life. Systematic assessment of the combination of cancer vaccines and immunotherapies promises the identification of safe and effective treatment options for HPV+ tumors. We propose that a therapeutic HPV peptide vaccine delivered along with the combination of 4–1BB agonist and CTLA-4 antagonist antibodies could offer a novel treatment option for HPV+ SCCOP.
Supplementary Material
Significance.
Combinations of vaccine and checkpoint modulation are effective and safe treatment options for HPV+ oral cancers.
Acknowledgments
We thank Dr. J. Lee for kindly providing us the mEER cell line. The Small Animal Imaging Facility at MD Anderson Cancer Center is supported by the NIH/NCI under award number P30CA016672. The Flow Cytometry Core at MD Anderson Cancer Center is supported by the Cancer Center Support Grant NCI# P30 CA16672. This work was supported by the HPV-related Cancers Moon Shot of the University of Texas MD Anderson Cancer Center (to K.J. Sastry and M. Curran).
Footnotes
Disclosure of Potential Conflicts of Interest
M.A. Curran and K.J. Sastry report receiving a commercial research grant from Agenus, Inc. and M.A. Curran is a consultant/advisory board member for Inovio Pharmaceuticals, Inc. K.J. Sastry is a consultant/advisory board member for Phoenix Biotech, Inc. No potential conflicts of interest were disclosed by the other authors.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dok R, Nuyts S. HPV positive head and neck cancers: molecular pathogenesis and evolving treatment strategies. Cancers 2016;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose P, Brockton NT, Dort JC. Head and neck cancer: from anatomy to biology. Int J Cancer 2013;133:2013–23. [DOI] [PubMed] [Google Scholar]
- 4.Arina A, Corrales L, Bronte V. Enhancing T cell therapy by overcoming the immunosuppressive tumor microenvironment. Semin Immunol 2016. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol 2009;157:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14: 463–82. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185–202. [DOI] [PubMed] [Google Scholar]
- 9.Clouthier DL, Watts TH. Cell-specific and context-dependent effects of GITR in cancer, autoimmunity, and infection. Cytokine Growth Factor Rev 2014;25:91–106. [DOI] [PubMed] [Google Scholar]
- 10.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52: 50–66. [DOI] [PubMed] [Google Scholar]
- 11.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, et al. Systemic 4–1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 2013;210:743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan SB, Sorensen JF, Olsen BN, Pedersen AE. Anti-CD40-mediated cancer immunotherapy: an update of recent and ongoing clinical trials. Immunopharmacol Immunotoxicol 2014;36:96–104. [DOI] [PubMed] [Google Scholar]
- 13.Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother 2015;64:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer 2017;41:125–8. [DOI] [PubMed] [Google Scholar]
- 15.Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines 2016;15:1327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang A, Farmer E, Wu TC, Hung C-F. Perspectives for therapeutic HPV vaccine development. J Biomed Sci 2016;23:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover AC, Spanos WC, Harris GF, Anderson ME, Klingelhutz AJ, Lee JH. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch Otolaryngol Head Neck Surg 2007;133:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiaris H, Spandidos DA, Jones AS, Vaughan ED, Field JK. Mutations, expression and genomic instability of the H-ras proto-oncogene in squamous cell carcinomas of the head and neck. Br J Cancer 1995;72:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 2004;64:55–63. [DOI] [PubMed] [Google Scholar]
- 20.Bartkowiak T, Singh S, Yang G, Galvan G, Haria D, Ai M, et al. Unique potential of 4–1BB agonist antibody to promote durable regression of HPVþ tumors when combined with an E6/E7 peptide vaccine. Proc Natl Acad Sci U S A 2015;112:E5290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPVþ and HPV- HNSCC in mice: an immune clearance of HPVþ HNSCC. Head Neck 2009;31:911–8. [DOI] [PubMed] [Google Scholar]
- 22.Pandiyan P, Bhaskaran N, Zhang Y, Weinberg A. Isolation of T cells from mouse oral tissues. Biol Proced Online 2014;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandoval F, Terme M, Nizard M, Badoual C, Bureau M-F, Freyburger L, et al. Mucosal imprinting of vaccine-induced CD8(þ) T cells is crucial to inhibit the growth of mucosal tumors. Sci Translat Med 2013;5:172ra120–172ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. 2017;47:765–79. [DOI] [PubMed] [Google Scholar]
- 25.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4–1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE 2011;6:e19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med 2003;198:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney AN, Thapa P, Singh S, Wishahy AM, Zhou D, Sastry J. Intranasal but not intravenous delivery of the adjuvant alpha-galactosylceramide permits repeated stimulation of natural killer T cells in the lung. Eur J Immunol 2011;41:3312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32–42. [DOI] [PubMed] [Google Scholar]
- 30.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensel JA, Khattar V, Ashton R, Lee C, Siegal GP, Ponnazhagan S. Location of tumor affects local and distant immune cell type and number. Immun Inflamm Dis 2017;5:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Li L, Starr TK, Subramanian S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget 2017;8:54775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin 2015;65:401–21. [DOI] [PubMed] [Google Scholar]
- 34. Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8(þ) T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Investigat 2005;115:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol 2011;140:130–41. [DOI] [PubMed] [Google Scholar]
- 36.Bartkowiak T, Jaiswal AR, Ager CR, Chin R, Chen CH, Budhani P, et al. Activation of 4–1BB on liver myeloid cells triggers hepatitis via an interleukin-27-dependent pathway. Clin Cancer Res 2018;24:1138–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi RE, Parisi I, Despott EJ, Burroughs AK, O’Beirne J, Conte D, et al. Antitumour necrosis factor agent and liver injury: literature review, recommendations for management. World J Gastroenterol 2014;20:17352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: friend or foe for immunotherapy? Oncoimmunology 2018;7:e1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu R-Q, Zhang D-F, Tu E, Chen Q-M, Chen W. The mucosal immune system in the oral cavity—an orchestra of T cell diversity. Int J Oral Sci 2014;6:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J-Y, Chung H, Choi Y, Park J-H. Phenotype and tissue residency of lymphocytes in the murine oral mucosa. Front Immunol 2017;8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardwick N, Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy 2011; 3:731–3. [DOI] [PubMed] [Google Scholar]
- 43.The Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol 2009;27:4371–7. [DOI] [PubMed] [Google Scholar]
- 45.Ruter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther 2010;10:983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science (New York, N.y.) 2011;331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapanadze T, Medina-Echeverz J, Gamrekelashvili J, Weiss JM, Wiltrout RH, Kapoor V, et al. CD40 dependent exacerbation of immune mediated hepatitis by hepatic CD11b(þ) Gr-1(þ) myeloid derived suppressor cells in tumor bearing mice. Eur J Immunol 2015;45:1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin GH, Snell LM, Wortzman ME, Clouthier DL, Watts TH. GITR-dependent regulation of 4–1BB expression: implications for T cell memory and anti-4–1BB-induced pathology. J Immunol 2013;190:4627–39. [DOI] [PubMed] [Google Scholar]
- 49.Malmstrom PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, et al. AdCD40L immunogene therapy for bladder carcinoma—the first phase I/IIa trial. Clin Cancer Res 2010;16:3279–87. [DOI] [PubMed] [Google Scholar]
- 50.Loskog A, Maleka A, Mangsbo S, Svensson E, Lundberg C, Nilsson A, et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br J Cancer 2016;114:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragonnaud E, Andersson AM, Pedersen AE, Laursen H, Holst PJ. An adenoviral cancer vaccine co-encoding a tumor associated antigen together with secreted 4–1BBL leads to delayed tumor progression. Vaccine 2016;34:2147–56. [DOI] [PubMed] [Google Scholar]
- 52.Kocak E, Lute K, Chang X, May KF Jr, Exten KR, Zhang H, et al. Combination therapy with anti-CTL antigen-4 and anti-4–1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res 2006;66: 7276–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.