Abstract
Obesity has reached epidemic proportions and its prevalence is climbing. Obesity is characterized by hypertrophied adipocytes with a dysregulated adipokine secretion profile, increased recruitment of inflammatory cells, and impaired metabolic homeostasis that eventually results in the development of systemic insulin resistance, a phenotype of type 2 diabetes. Nitric oxide synthase (NOS) is an enzyme that converts L-arginine to nitric oxide (NO), which functions to maintain vascular and adipocyte homeostasis. Arginase is a ureohydrolase enzyme that competes with NOS for L-arginine. Arginase activity/expression is upregulated in obesity, which results in diminished bioavailability of NO, impairing both adipocyte and vascular endothelial cell function. Given the emerging role of NO in the regulation of adipocyte physiology and metabolic capacity, this review explores the interplay between arginase and NO, and their effect on the development of metabolic disorders, cardiovascular diseases, and mitochondrial dysfunction in obesity. A comprehensive understanding of the mechanisms involved in the development of obesity-induced metabolic and vascular dysfunction is necessary for the identification of more effective and tailored therapeutic avenues for their prevention and treatment.
Keywords: Obesity, Diabetes, Adipokines, Inflammation, Insulin Resistance, Cardiovascular Disease, Review
2. INTRODUCTION
Obesity, a condition characterized by the excessive accumulation and storage of fat in the body, is generally defined as a body mass index (BMI: weight-lbs/(height-inches)2 × 703) of 30 or greater. Obesity is considered the core of metabolic disorders and an independent risk factor for all-cause mortality in the general population, particularly from cardiovascular disease (1). The vast majority of patients with type 2 diabetes (T2D) exhibit obesity and insulin resistance (2, 3). According to the World Health Organization, obesity is now considered a serious health problem worldwide, with its prevalence nearly tripling over the past 40 years due to overnutrition and reduced physical activity (4). A key function of adipose, or fat, tissue is energy homeostasis. Adipose tissue stores excess nutrients (ie: glucose and fatty acids) through the process of lipogenesis. In conditions of nutrient deficiency, it ensures a stable supply of energy to all organs and tissues through lipolysis (5).
Adipose tissue is the largest endocrine organ in the body, consisting mainly of adipocytes which are capable of secreting a variety of cell signaling cytokines, known as adipokines (6). These adipokines, particularly those in visceral adipose tissue (VAT), can regulate local and systemic inflammation as well as energy homeostasis (7). Healthy adipocytes are insulin sensitive, a trait essential for adipocyte glucose uptake and for the prevention of hepatic gluconeogenesis, which allows for the maintenance of normal blood glucose levels (8). Insulin resistance is an important feature of metabolically unhealthy obesity, a condition which differs from healthy obesity in terms of fat distribution. Metabolically healthy obese individuals exhibit increased subcutaneous adipose tissue (SAT) mass with less inflammation, less VAT and ectopic (liver and skeletal muscle) fat accumulation, and a normal adipokine secretion profile compared to metabolically unhealthy obese individuals (9). Studies from many groups have led to our current understanding that vascular pathology and dysfunction of obesity-related metabolic dysfunction develops through a chronic and progressive inflammatory process (10–12).
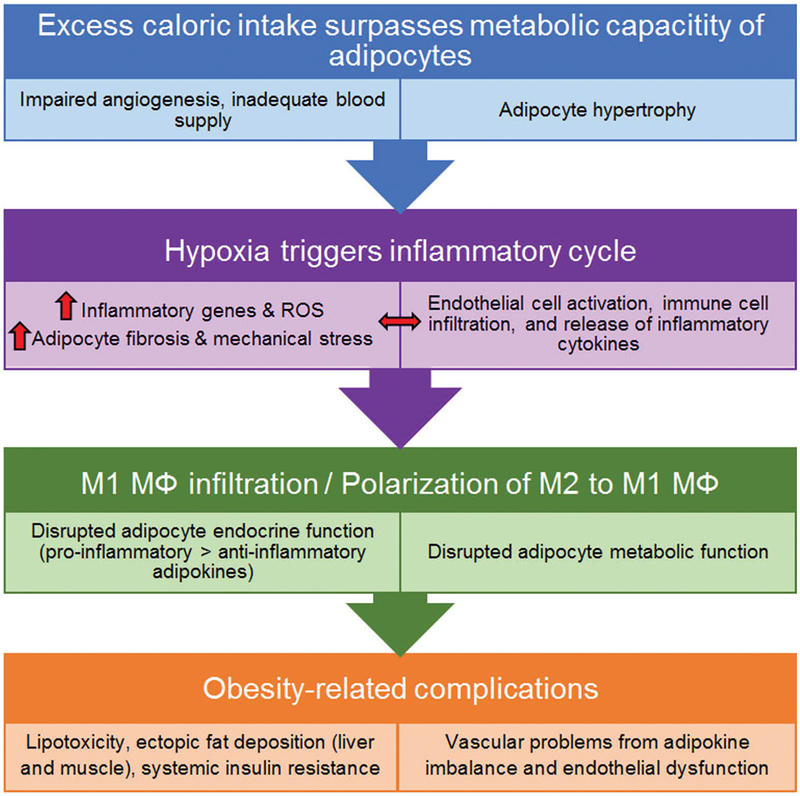
The pathogenesis of obesity is far more complex than just lipid accumulation and involves interactions among many cell types (Figure 1). With expansion of the VAT, hypertrophy of adipocytes, and inadequate vascularity (impaired angiogenesis), hypoxia occurs, causing the release of inflammatory cytokines and chemokines. These factors ‘activate’ endothelial cells by enhancing leukocyte and monocyte adhesion to the endothelium and inducing tissue infiltration by pro-inflammatory macrophages. This further elevates levels of inflammatory factors, triggering a vicious cycle of inflammation (13, 14). Nitric oxide (NO) has been recognized as a key regulator of body composition, energy metabolism, and vascular function. NO is produced from L-arginine by three NO synthase (NOS) isoforms: endothelial NOS (eNOS/NOS3), inducible (iNOS/NOS2), and neuronal NOS (nNOS, NOS1) (15, 16). NO produced by eNOS (nanomolar range) relaxes vascular smooth muscle cells and prevents their excessive proliferation, increases blood flow, and suppresses platelet aggregation (17–19). This eNOS-produced NO also prevents ‘activation’ of endothelial cells by suppressing the release of factors that trigger migration and adhesion of leukocytes and monocytes to the endothelium, preventing infiltration of inflammatory macrophages. Endothelial NO concentration and production are suppressed in obesity (20, 21). Inducible NOS (iNOS), in contrast, produces much higher and toxic levels of NO (micromolar range) and is found in adipocytes and pro-inflammatory macrophages. NO production by iNOS is elevated in obesity (22).
Figure 1.
Schematic illustrating the development of metabolic and cardiovascular dysfunctions in obesity.
With the exception of nNOS, the genes related to the NO system (eNOS, iNOS, subunits of the soluble guanylate cyclase (sGC), and both genes encoding cGMP-dependent protein kinases) are expressed in subcutaneous human adipose tissue and isolated adipocytes. Under physiological conditions, eNOS appears to be the predominant NOS isoform in human adipocytes (23). Expression of eNOS has been reported in human, rat, and mouse adipose tissue (24). eNOS synthesizes NO through the oxidation of the semi-essential amino acid, L-arginine (25, 26). NO signaling mechanisms involve either the activation of sGC, which increases the levels of the secondary messenger cGMP, or the posttranslational modification of the cysteine thiol group (S-nitrosylation) of various proteins to form nitrosothiols (SNO), directly affecting signal transduction (27, 28).
Reactive oxygen species (ROS) are often greatly elevated in obesity and hyperglycemia and can have serious pathological effects. ROS include hydrogen peroxide (H2O2), superoxide (O2−), hydroxyl radical (OH), high levels of nitric oxide (NO), and peroxynitrite (ONOO−). These ROS are products of numerous enzymatic reactions that occur within various subcellular compartments. Chronic hypernutrition induces the production of superoxide from NADPH oxidases, mitochondrial oxidative phosphorylation, and endothelial dysfunction/eNOS uncoupling (29–32). Chronic inflammation in adipose tissue can further perpetuate the vicious cycle of inflammation by promoting the infiltration of pro-inflammatory, ROS-producing macrophages (33, 34). Obesity also is associated with the depletion or decreased activity of antioxidant defense enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (33, 35). Acute changes in ROS concentration are important for cellular homeostasis and normal physiological processes where the ROS contribute to protective immune responses and act as intracellular signaling molecules that can induce insulin secretion and insulin sensitivity (36, 37). However, if not properly managed, ROS accumulation that exceeds the cellular antioxidant capacity may lead to maladaptive responses that result in metabolic dysfunction and inflammation (38, 39).
3. OBESITY-INDUCED ADIPOSE TISSUE DYSFUNCTION AND METABOLIC DYSREGULATION
3.1. Impaired adipogenesis
Adipose tissue expansion occurs through enlargement of existing adipocytes (hypertrophy) and/or through increased number of adipocytes (hyperplasia/adipogenesis). Adipogenesis occurs in two consecutive phases: first, mesenchymal stem cells commit to the formation of preadipocytes, which is then followed by terminal differentiation (40). The signaling mechanisms driving adipogenesis are not clearly understood. What is known is that the commitment step involves repression of zinc-finger protein 521 (ZNF521) and bone morphogenetic protein 4 (BMP4), which ultimately leads to the activation of ZNF423 and its downstream target PPAR gamma. The process of adipogenesis also involves the sequential activation of several C/EBP transcription factors, C/EBP beta, sigma, and alpha. Activated PPAR gamma and C/EBP alpha then drive the terminal differentiation of preadipocytes (41–43).
It has been suggested that a causal relationship exists between adipocyte size and the formation of new adipocytes. Individuals with large subcutaneous adipocytes have poor differentiation capacity, either due to elevated dedifferentiation signals or downregulation of differentiation factors, both of which are associated with a high risk of T2D (44–46). Hypertrophied adipocytes showed reduced potential to recruit mesenchymal stem cells and promote their terminal differentiation into new adipocytes, secondary to impaired PPAR gamma activation and adipocyte differentiation (46, 47). Mature, healthy adipocytes secrete BMP4 during adipogenesis, causing mesenchymal stem cell commitment to an adipogenic phenotype. In an attempt to avoid dysregulation, hypertrophied adipocytes secrete higher levels of BMP4 to recruit preadipocytes. However, this process becomes futile due to increased secretion of endogenous BMP4 antagonist, Gremlin1. Inhibition of Gremlin1 has been shown to enhance the process of adipogenesis, restoring some of the buffering functionality of these adipocytes (48).
3.2. Enhanced inflammatory response and disrupted adipokine profile
Adipokines are cytokines secreted from adipose tissue that play an important role in maintaining energy homeostasis (49). Additionally, their immunomodulatory activities contribute to the chronic low-grade inflammation associated with obesity (50). During obesity, there is increased secretion of several pro-inflammatory adipokines that occurs in tandem with downregulation of anti-inflammatory adipokines (51). This adipokine imbalance is pivotal in the development of metabolic disorders and cardiovascular disease (52). Obesity disrupts adipose tissue homeostasis through deregulation of adipogenesis, reduced angiogenesis, and localized hypoxia, creating an environment of high cellular stress (53). Adipose tissue of obese subjects, primarily their VAT and to a lesser extent, their SAT, has been shown to sustain a state of chronic low-grade inflammation, which has been linked to the development of insulin resistance (54). The physiological response to this elevated adipocyte stress is the release of inflammatory cytokines and chemokines, chiefly, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-alpha), which recruit more inflammatory cells, thus further perpetuating the cycle of cellular stress, inflammation, and impaired macrophage emigration (55, 56). On the molecular level, hypertrophied adipocytes secrete saturated fatty acids which activate the toll-like receptor (TLR)-4 on macrophages. This TLR-4 activation results in increased activity of the transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB), which upregulates expression of TNF-alpha (57, 58). TNF-alpha increases adipocyte lipolysis, producing more free fatty acids, and also promotes adipocyte expression of intracellular adhesion molecule-1 (ICAM-1) and MCP-1. These proteins recruit circulating inflammatory monocytes and promote their differentiation into macrophages, exacerbating inflammation. Hypertrophied adipocytes also express lower levels of adiponectin, an anti-inflammatory adipokine that inhibits TLR-activated NF-kappaB, allowing the expression of TNF-alpha to proceed relatively unhindered (59–61).
Adipose tissue macrophages represent about 40% of all adipose tissue cells during metabolic stress (62, 63). In healthy adipose tissue, the resident macrophages are primarily polarized towards the anti-inflammatory, reparative M2 phenotype. These cells secrete anti-inflammatory cytokines, like IL-10, and perform immune surveillance and lipid buffering functions to maintain a state of insulin sensitivity (64, 65). However, under obese conditions, hypoxic adipocytes secrete chemotactic molecules to recruit inflammatory monocytes, activated T cells, and B cells. The presence of activated T cells coupled with the reduced number of regulatory T cells (Tregs), the immunosuppressive subpopulation of T cells that downregulate the activation and proliferation of effector T cells, leads to a phenotypic shift in the macrophage population towards the pro-inflammatory, M1 phenotype (66). The M1 macrophages secrete mainly pro-inflammatory cytokines such as TNF-alpha and IL-6 (67). The degree of insulin resistance positively correlates with the amount of infiltrating M1-like macrophages in adipose tissue (68). It has been proposed that the key link between inflammatory stimuli and insulin resistance is the intracellular activation and nuclear translocation of NF-kappaB in response to increased pro-inflammatory stimuli (69). Anti-inflammatory drugs, such as salicylates, have been shown to inhibit NF-kappaB and improve insulin sensitivity in obese rodents and diabetic patients (70). In adipose tissue of obese mice, the c-Jun N-terminal kinase (JNK)–activator protein-1 (AP-1) pathway is activated. Activation of the JNK/AP-1 pathway results in phosphorylation of insulin receptor substrate-1 (IRS-1) at its negative regulatory site, preventing interaction with the insulin receptor and inhibiting the insulin signaling pathway (71). Our recent studies showed that systemic administration of an arginase inhibitor or deletion of endothelial arginase 1, an isoform of arginase that competes with eNOS for available L-arginine, protected mice against obesity-induced inflammatory responses, indicating protective functions of NO (72, 73). Another study showed that arginase 2 activity promoted a pro-inflammatory macrophage response through increased generation of mitochondrial oxidative stress. The formation of excess mitochondrial ROS contributed to the development of insulin resistance and atherosclerosis (74).
3.3. Adipokines
Adipokines are a group of proteins composed of cytokines, chemokines, and hormones that are secreted from adipose tissue. They play important roles in the maintenance of energy homeostasis, appetite, glucose and lipid metabolism, insulin sensitivity, angiogenesis, immunity and inflammation, hemostasis, and blood pressure (75). Adipokines are classified as either pro-inflammatory or anti-inflammatory. The former has been shown to be elevated at the expense of the latter in obesity. This adipokine imbalance is believed to be the link between obesity, metabolic disorders, and cardiovascular diseases. Pro-inflammatory adipokines include leptin, resistin, TNF-alpha, retinol binding protein 4, lipocalin 2, angiopoietin-like protein 2, and visfatin. Anti-inflammatory adipokines include adiponectin, omentin, and adipolin (51, 76).
3.3.1. Leptin
Leptin is considered a pro-inflammatory adipokine since it induces production of ROS, TNF-alpha, and IL-6 by macrophages and monocytes, which in turn initiate the production of more leptin (77). However, leptin has many beneficial roles outside of its inflammatory effects. Leptin is a 16 kDa adipokine produced primarily in adipocytes from the LEP gene, the human homologue of the murine obese (ob) gene (78). Leptin receptors are produced from the diabetes (db) gene (79). Leptin regulates appetite and food intake by communicating energy status to the central nervous system (80). Leptin enhances glucose utilization and insulin sensitivity under normal conditions and ameliorates hyperlipidemia as shown in both experimental and clinical studies (81, 82). In mouse models, severe obesity can be induced by mutations in either the ob or db genes (78, 83). However, hyperleptinemia is common in clinical settings and administration of exogenous leptin does not result in weight loss, indicating that leptin resistance could be due to downregulation of its receptor or impairment of signal transduction (84, 85). The form of leptin resistance seen primarily in obesity occurs through inhibition of JAK2/STAT3 signaling, which is normally activated once leptin binds its receptor (86). Increased activity of the protein suppressor of cytokine signaling 3 (SOCS3) inhibits activation of the JAK/STAT3 pathway, reducing leptin signal transduction (87–89).
The cardiovascular effects of leptin are controversial. Elevated leptin levels are associated with hypertension caused by chronic activation of the sympathetic nervous system (90, 91). Additionally, studies that investigated the metabolic effects of insulin showed that prolonged exposure of rat adipocytes to high leptin concentrations (>1 nM), resulted in dose-dependent inhibition of insulin-stimulated glucose uptake, which was paralleled by decreased lipogenesis (92). Inhibition of insulin-stimulated glucose uptake and downregulation of lipogenesis are key events that can lead to the development of insulin resistance and cardiovascular dysfunction. Increased serum levels of leptin and arginase 1 have been found in obese patients (93). In contrast, leptin-mediated vasodilatory effects from increased NO production are impaired under pathological conditions, including obesity and metabolic syndrome (94). Leptin-deficient mice showed significant elevation in arginase activity in wounded skin, which correlated with impaired skin repair, likely due to decreases in endothelial cell-derived NO needed for angiogenic repair and unchecked inflammatory responses (95). The impaired tissue repair in leptin-deficient mice was abolished with administration of exogenous leptin (96).
3.3.2. Resistin
Resistin is a pro-inflammatory adipokine produced primarily from adipocytes in rodents, and monocytes and macrophages in humans (97). Elevated serum levels of resistin are associated with metabolic disorders and diabetic microvascular complications mediated by endothelial dysfunction (98). Interestingly, obesity is still seen in resistin-deficient mice, despite improved glucose tolerance and insulin sensitivity (99). Pro-inflammatory cytokines such as IL-1, IL-6, and TNF-alpha induce transcription of the resistin gene (RETN) in human mononuclear cells, which leads to the expression of more pro-inflammatory cytokines, resulting in the precipitation of inflammation (100). Resistin activates SOCS3, an inhibitor of the insulin signaling pathway, thereby inducing insulin resistance (101). In vivo supplementation of eNOS substrate, L-arginine, to mice fed a high-fat diet (HFD), enhanced insulin sensitivity without affecting resistin levels (102). Previous reports have shown that inflammatory stimuli produce high levels of iNOS-generated NO which promotes resistin expression, while iNOS inhibition reduces resistin expression, confirming the deleterious effect of high NO levels (103, 104).
3.3.3. Tumor necrosis factor-alpha
Tumor Necrosis Factor-alpha (TNF-alpha) is a pro-inflammatory cytokine, which in obesity, is heavily produced by monocytes and macrophages present in the stromal vascular fraction of adipose tissue. TNF-alpha levels have been found to positively correlate with obesity and T2D (54). TNF-alpha plays a central role in the development of insulin resistance and inflammation by inducing a repressive form of insulin receptor substrate-1 (IRS-1), effectively halting the insulin signaling pathway (105). Interestingly, short-term treatment (~4 weeks) with TNF-alpha blockers in obese diabetic patients and patients with metabolic syndrome reduced inflammatory responses, but did not improve insulin signaling suppression (54, 106). However, patients with metabolic syndrome that were treated with TNF-alpha blockers for a prolonged period (~6 months), showed lower fasting glucose levels, indicating improvement in insulin resistance and glucose uptake (107). In addition to its non-vascular effects, TNF-alpha has been shown to induce impairment of NO-mediated vasodilation in the small arteries found in the visceral fat of obese patients (108). It also has been shown that TNF-alpha activity impairs NO-induced vascular endothelial vasorelaxation through upregulation of arginase 1 expression/activity in ischemia-reperfusion injuries (109). In addition, TNF-alpha functions to reduce the levels of the anti-inflammatory adipokine, adiponectin, and increase the level of the pro-inflammatory adipokine, visfatin/NAMPT (110).
3.3.4. Retinol binding protein 4 (RBP4)
Retinol binding protein 4 (RBP4) is a blood transporter for retinol (vitamin A) secreted by the liver, adipose tissue, and macrophages (111). RBP4 serum level positively correlates with metabolic disorders, obesity, insulin resistance, and pro-atherogenic conditions (112). RBP4 induces insulin resistance by preventing insulin-initiated phosphorylation of insulin receptor substrate 1 (IRS-1) (113). RBP4 levels can be used to determine the predisposition of patients to atherosclerosis due to its positive correlation with obesity and pro-atherogenic markers (112). Mice lacking RBP4 exhibit reduced systolic blood pressure through enhanced eNOS phosphorylation and NO-mediated vasodilation (114).
3.3.5. Lipocalin 2
Lipocalin 2 is a carrier of retinoids, arachidonic acid, steroids, leukotriene B4, and platelet activating factor. Lipocalin 2 is produced primarily by adipocytes and macrophages upon activation of NF-kappaB. Elevated serum levels of lipocalin 2 positively correlate with metabolic disorders and inflammation (115, 116). Lipocalin-2 has been shown to cause M1 macrophage polarization while suppressing formation of the M2 macrophage phenotype, thereby increasing expression of iNOS and decreasing arginase 1 activity in macrophages (117). Inhibition of iNOS, pharmacologically or via gene silencing, prevents IL-1beta and IFN-gamma-induced lipocalin 2 expression (118). Paradoxically, lipocalin 2 knockout mice showed increased body weight, adipose tissue weight, and insulin resistance compared to wild type mice (119). Also at odds with its association with metabolic disorders, lipocalin 2 was recently reported to interact synergistically with insulin and retinoic acid in the activation of beige adipocytes with a resultant thermogenesis (120). The mechanisms behind these apparent contrasting effects of lipocalin 2 have yet to be elucidated.
3.3.6. Angiopoietin-like protein 2 (ANGPTL2)
Angiopoietin-like protein 2 (ANGPTL2) is an adipokine produced mainly from adipocytes, macrophages, and endothelial cells and is involved in the development of insulin resistance and inflammation (121). Serum and adipose tissue levels of ANGPTL2 positively correlate with metabolic disorders and inflammation (122). ANGPTL2 transgenic mice have been shown to have reduced eNOS expression, which is indicative of impaired NO-mediated vasorelaxation (123). This is in contrast to another study which showed that ANGPTL2 improves insulin sensitivity and lipid profile in genetically diabetic mice (124). The reasons for these differing effects of ANGPTL2 activity have not been resolved.
3.3.7. Visfatin
Visfatin, also known as cytokine pre-B cell colony enhancing factor (PBEF), or nicotinamide phosphoribosyltransferase (NAMPT), is produced primarily in adipocytes and macrophages (125, 126). This adipokine was initially thought to have insulin-mimetic effects, but this response has not been observed in humans (125, 127). However, administration of visfatin has been shown to ameliorate glucose intolerance and improve hepatic insulin sensitivity (128). The controversy over visfatin function was highly debated but more recent data indicate that serum levels of visfatin are higher in obese and T2D patients. This study suggested that visfatin-induced the release of pro-inflammatory cytokines, like TNF-alpha, which contributed to the onset of insulin resistance (129, 130). This pro-inflammatory role is reported to involve activation of p38 mitogen-activated protein kinase (p38 MAPK) and extracellular signal-regulated kinase (ERK) pathways (131). It also has been reported that circulating levels of visfatin are markedly elevated during atherosclerosis and that this increase was closely associated with decreased levels of L-arginine and NO, and increased levels of an endogenous inhibitor of NOS, asymmetric dimethylarginine (ADMA) (132).
The contrasting characterizations of visfatin as pro-inflammatory versus anti-inflammatory may be due to the differences between the extracellular and intracellular actions of visfatin, and whether its function is mediated by enzyme activity or by activation of the unknown visfatin receptor (126). Intracellular visfatin/NAMPT produces NAD+. NAD+ is essential for the activity of sirtuin1 (SIRT1), a protein and histone deacetylase, which exerts many beneficial effects on cellular metabolism and vascular function (133). SIRT1 induces eNOS activity and NO production and thus improves cardiovascular function (134).
3.3.8. Adiponectin
Adiponectin is an anti-inflammatory adipokine synthesized only by adipocytes. Compared to most other adipokines, the healthy plasma concentration of adiponectin is high (~3−30 μg/mL) (135–137). Adiponectin enhances insulin sensitivity by increasing glucose and fatty acid metabolism through the activation of AMP Kinase (AMPK) and PPAR alpha (138–140). Conditions that adversely affect adiponectin concentrations are hypoxia, pro-inflammatory cytokines, and oxidative stress (141). The plasma adiponectin levels in obese subjects negatively correlate with plasma lipid peroxidation, a marker of oxidative stress (33). Overexpression of adiponectin in ob/ob mice results in healthy adipogenesis with expansion of the subcutaneous adipose tissue and insulin sensitivity similar to that of lean mice (142).
The effects of adiponectin on cellular metabolism and insulin sensitivity are important for the maintenance of good health (52). Adiponectin exerts an anti-inflammatory effect by repressing TNF-alpha production and promoting eNOS activity (143). Moreover, adiponectin inhibits toll-like receptor-induced activation of NF-kappaB and limits macrophage polarization to pro-inflammatory M1 macrophages, while simultaneously increasing the number of anti-inflammatory M2 macrophages (144).
Adequate adiponectin levels are associated with proper eNOS function. eNOS-deficient mice showed reduced adiponectin levels while mice overexpressing dimethylarginine dimethylaminohydrolase (DDAH), the enzyme responsible for the degradation of endogenous eNOS inhibitor, ADMA, showed higher adiponectin levels (145, 146). In turn, adiponectin can enhance NO levels by increasing eNOS mRNA stability and eNOS phosphorylation (147, 148). Adiponectin has also been shown to impede NO degradation through suppression of superoxide anion formation (149). Additionally, global deletion of the mitochondrial arginase isoform, arginase 2, induced a significant increase in adiponectin expression in epididymal adipose tissue with no significant effect on circulating adiponectin or hepatic levels, suggestive of a local autocrine effect (150).
3.3.9. Omentin
Omentin is an anti-inflammatory adipokine produced in adipose tissue that exhibits insulin-sensitizing properties through activation of the Akt signaling pathway (151). It has been shown that circulating levels of omentin are decreased in obese patients with insulin resistance (152). Omentin expression in both visceral and subcutaneous adipose tissue was found to correlate positively with the expression of neuropeptide Y (NPY), the most potent appetite stimulating peptide, suggesting that omentin may play a role in appetite modulation (153, 154).
In addition, omentin has been associated with reduced inflammation, improved lipid metabolism and vasodilation, and a reduction in the development of obesity-related cardiovascular disease and atherosclerosis. Omentin induces adiponectin expression, resulting in improved fatty acid breakdown and increased insulin-mediated glucose uptake (155). Omentin also stimulates endothelial-derived NO production, resulting in vasorelaxation, maintained endothelial barrier function, and reduced inflammation (156, 157). In addition to its positive regulatory roles, omentin has been shown to suppress TNF-alpha production (158). These various functions of omentin protect against atherosclerosis and obesity-related cardiovascular disorders.
3.3.10. Adipolin
Adipolin is an anti-inflammatory, insulin-sensitizing adipokine, primarily secreted from adipose tissue. Adipolin levels are reduced in obese mice and are negatively correlated with insulin resistance (159). Adipolin reduces inflammation through the inhibition of macrophage recruitment and secretion of pro-inflammatory cytokines (160).
3.4. Premature cellular senescence in adipose tissue
Cellular senescence is a state of irreversible replicative arrest that is associated with aging. It is initiated by a variety of factors including progressive telomere shortening through many cell cycles, buildup of reactive oxygen species (ROS), DNA damage, growth factors, and other metabolic and mitogenic stressors (161). This process is not only a stress response to severe cellular damage designed to protect against the proliferation of aberrant cells, but is also involved in development (162). The accumulation of these senescence-inducing factors triggers the upregulation of cyclin-dependent kinase inhibitors, p16INK4a and p53/p21, which arrest the cell cycle (163). However, premature senescence has been observed in the preadipocytes and adipocytes of the visceral adipose tissue (VAT) of young obese humans and animals (164). This phenomenon is promoted by nutrient excess, which contributes to oxidative stress, adipose tissue metabolic dysregulation, and inflammation (33, 61, 65, 165).
The marked accumulation of senescent cells in the visceral adipose tissue of obese humans and animals, compared to their age-matched counterparts, is correlated with compromised adipose tissue and mitochondrial function (166). The effect of obesity-induced senescence is particularly evident in preadipocytes, where it contributes to the reduction of adipogenesis and lipogenesis, leading to lipotoxicity and inflammation (167). Although senescent cells cannot divide, they remain metabolically active. Especially in the VAT, senescent cells readily produce and release pro-inflammatory cytokines, chemokines, and growth factors. This process, which has been termed the senescence-associated secretory phenotype (SASP), enhances inflammation and adipose tissue dysfunction (166). The SASP release of MCP-1 further exacerbates the inflammatory state by promoting pro-inflammatory macrophage infiltration (168, 169). Additionally, the accumulation of senescent cells in the VAT can induce senescence in neighboring cells in a feed-forward mechanism (161).
In addition to the deleterious effects of obesity-induced preadipocyte and adipocyte senescence, senescence in VAT endothelial cells (EC) also plays a key role in VAT dysfunction (170). The process of transporting fatty acids (FAs) into adipocytes requires the microvascular endothelium. Senescence in these VAT EC has been reported to block fatty acid transport into adipocytes by a mechanism involving reduced PPAR gamma expression and activity (171, 172). The failure of adipocytes to take up free FAs results in their inability to store FAs in the VAT, leading to ectopic fat deposition and toxicity in the skeletal muscle and liver. Endothelial cells from the VAT of obese subjects also have been shown to exhibit the SASP, possessing inflammatory and angiogenic secretory profiles (170).
Another cell type found in VAT that can be forced into senescence by overnutrition are T cells. T cell senescence has also been noted in the VAT of diet-induced obese mice. These senescent CD4+-associated T cells appear to enhance the inflammatory environment in obese VAT by releasing large amounts of osteopontin (173). Osteopontin, an inflammatory cytokine, has been reported to be elevated in the blood of obese diabetic and insulin-resistant patients. This is correlated with the severity of coronary artery disease, and plays a causative role in VAT inflammation, macrophage infiltration, and insulin resistance (174, 175).
4. IMPAIRED GLUCOSE METABOLISM AND INSULIN SENSITIVITY
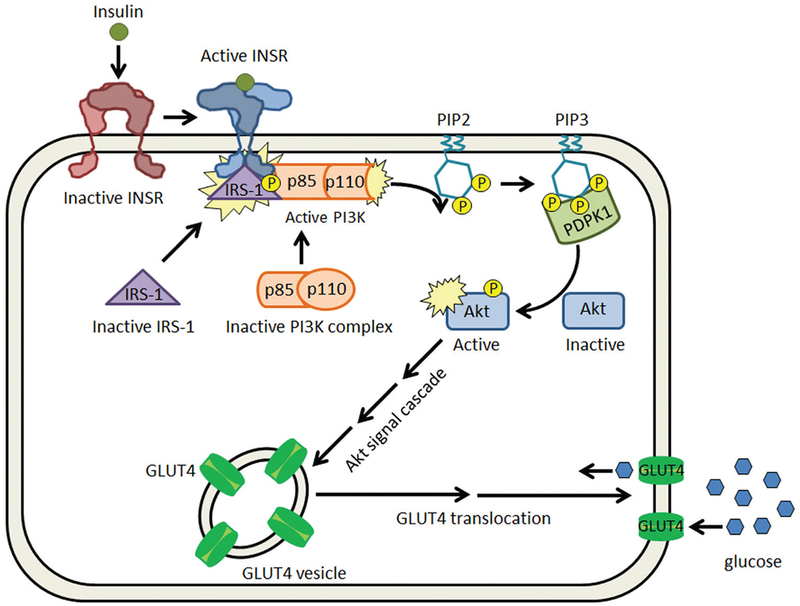
The metabolic effects of insulin are mediated through a signal cascade initiated by the binding of insulin to its receptor (INSR) (Figure 2). Insulin binding triggers a conformational change in the receptor, leading to activation of the tyrosine kinase domain through autophosphorylation (176). The activated receptor phosphorylates insulin receptor substrates (IRSs), which in turn, bind phosphoinositide 3’ kinase (PI3K). PI3K-IRS-1 phosphorylates its plasma membrane-bound substrate, phosphatidylinositol (3,4)-bisphosphate (PIP2) forming secondary messenger, phosphatidylinositol (3,4,5)-triphosphate (PIP3). PIP3 is required for the activation of protein kinase B (PBK or Akt), which once activated, mediates the translocation of glucose transporter 4 (GLUT4) to the plasma membrane (176). In addition to promoting cellular glucose uptake, insulin also increases the expression of transcription factor adipocyte determination and differentiation-dependent factor 1 (ADD1). ADD1 upregulates expression of two genes: fatty acid synthase (FAS), an enzyme critical in lipogenesis, and leptin, which is responsible for appetite suppression (177).
Figure 2.
Insulin signaling pathway. Insulin binds to the inactive insulin receptor (INSR) and elicits a conformational change. This allows IRS-1 to bind to the intracellular domain of the INSR, where it is phosphorylated and subsequently able to bind to p85, the regulatory domain of PI3K. The binding of the p85 domain in PI3K activates its kinase domain, p110, which phosphorylates PIP2, producing PIP3. PIP3 is bound by phosphoinositide-dependent kinase-1 (PDPK1), which activates Akt through phosphorylation. Activated Akt acts through a signaling cascade to promote GLUT4 translocation to the plasma membrane to facilitate glucose uptake.
Obesity is associated with a decrease in insulin-dependent GLUT4 expression and membrane translocation, which results in reduced glucose uptake and the subsequent development of hyperglycemia (178–180). The mechanism for the development of insulin resistance is not fully understood, but it is postulated that insulin resistance manifests as a defensive response from hypertrophied adipocytes (181). The onset of insulin resistance is an initial step in the development of T2D (181). Cell starvation from the lack of intracellular glucose results in an increase in pancreatic insulin secretion, triggered by increased fat metabolism and the production of ketones (182, 183). This increase in insulin results in hyperinsulinemia because the insulin receptors no longer respond properly to insulin levels. Sustained, elevated insulin release leads to dysfunction in its production and pancreatic beta-cell failure, with ensuing hypoinsulinemia and hyperglycemia (184).
Many factors are associated with the development of insulin resistance in obesity including adipocyte dysfunction, elevated oxidative stress, and high levels of NO produced by iNOS. Adipocyte hypertrophy occurs as a buffering response to chronic overnutrition that protects other tissues from lipotoxicity (185). The development of adipocyte hypertrophy has systemic deleterious effects, in addition to promoting adipocyte dysfunction. In fact, genetic deletion of GLUT4 from adipose tissue resulted in systemic insulin resistance similar to that seen with the same deletion in skeletal muscle, tissue crucial for glucose uptake. In obese women, adipose tissue expression of GLUT4 and IRS-1 were found to be significantly reduced, indicating that obesity-induced adipocyte hypertrophy is linked to development of insulin resistance (186). This decrease in GLUT4 expression in subcutaneous adipocytes was also seen in patients who developed T2D (187). Obesity has also been shown to induce systemic and local oxidative stress, which is suggested to be critical in the pathogenesis of metabolic syndrome. Reactive oxygen species (ROS) are produced from many sources in response to the increase in fatty acids present in visceral adipose tissue under obese conditions (33, 188). A transient increase of intracellular ROS is essential for insulin signaling and glucose uptake. However, chronic elevation of intracellular ROS induces insulin resistance through suppression of the insulin signaling pathway (33, 178–180, 189). Another mechanism that contributes to the pathogenesis of insulin resistance is abnormal eNOS and iNOS activity. Insulinstimulated glucose uptake in adipose tissue and skeletal muscle is NO-dependent, and these physiological levels of NO are produced by constitutively active eNOS (190, 191). In obesity, proper eNOS function is compromised (192). Mice fed a high-fat diet (HFD) become obese, but supplementation with L-arginine, the substrate for eNOS, increased NO production and improved their insulin sensitivity (102). Inducible NOS (iNOS) has a pro-inflammatory role in the immune response and is upregulated in obesity, diminishing insulin sensitivity (193). Interestingly, inhibition of all NOS isoforms restored adipocyte insulin sensitivity, suggesting that inhibition of the detrimental iNOS activity is more important than the beneficial, constitutive activity of eNOS in regards to the maintenance of the homeostatic insulin response (103, 194, 195). These compounding effects promoted in obesity contribute to the type 2 diabetic state consisting of impaired glucose metabolism and insulin resistance.
5. IMPAIRED PROTEIN AND MUSCLE METABOLISM
In contrast to the increased adiposity seen in obesity and T2D, muscle mass in these conditions decreases due to impaired protein synthesis and increased muscle degradation in a process known as sarcopenia. The process of sarcopenia is accelerated with aging. In healthy subjects, insulin stimulates protein anabolism by simultaneously promoting protein synthesis and reducing protein catabolism (196). A large survey reported an inverse relationship between the skeletal muscle (SM) index (ratio of SM mass to body weight) and insulin resistance, indicating that patients with insulin resistance were likely to have increased muscle atrophy (197). Furthermore, muscle from obese and/or T2D patients has been found to exhibit increased levels of myostatin, a hormone known to reduce skeletal muscle mass (198, 199). Additionally, obese women have been shown to display more resistance to insulin-stimulated protein anabolism than lean women (200). Ectopic fat deposition in the skeletal muscle, or myosteatosis, has been shown to contribute to impaired protein anabolism and muscle function (201, 202). Diets high in protein and essential branched-chain amino acids (BCAA - leucine, isoleucine and valine) are very important for promotion of protein anabolism and the maintenance of muscle mass (203). Activation of mammalian target of rapamycin complex 1 (mTORC1) is believed to be centrally involved in this process.
Adipocytes also catabolize BCAAs to produce precursors required for fatty acid and sterol biosynthesis. This catabolic process increases dramatically with adipogenesis indicating that homeostasis of protein metabolism is distorted in obesity (204). Mice deficient in the enzyme responsible for BCAA catabolism showed decreased adiposity despite higher food intake and enhanced energy expenditure (205). Mice fed a leucinedeficient diet showed reduced adipose tissue weight and lipogenesis, with enhanced lipolysis and energy expenditure (206).
6. IMPAIRED LIPID METABOLISM
One of the primary functions of adipocytes is the storage of lipids in the form of triacylglycerol (TAG), which constitutes about 90% of adipocyte volume. The effect of insulin on lipid metabolism is highly coordinated. It simultaneously stimulates fatty acid anabolism (lipogenesis) through upregulation of lipogenic enzymes like acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), while preventing lipid catabolism (lipolysis) by inhibiting the phosphorylation and activation of hormone-sensitive lipase (HSL) (207–209). In obesity, adipocytes are abnormally enlarged and the usually, tightly regulated effects of insulin on lipid metabolism are lost. Basal lipolysis is increased in obesity, resulting in hyperlipidemia due to increased secretion of lipolytic adipokines, serum amyloid A (SAA), IL-6, and TNF-alpha, from the hypertrophied adipocytes (210, 211). Concurrently, these dysfunctional adipocytes exhibit lower rates of lipogenesis as the rates of lipolysis increase, a phenotype commonly seen in insulin resistance (212). During obesity, the increased levels of free fatty acids and cholesterol result in myosteatosis and hepatosteatosis, or the build of ectopic lipid deposits in the skeletal muscle and liver (213–215).
Lipoprotein lipase (LPL) is an enzyme critical for the hydrolysis of TAG in circulating chylomicrons and very low-density lipoproteins (VLDL). LPL hydrolyzes TAG into two free fatty acids and one monoacylglycerol (216). LPL is expressed in adipocytes and subsequently transported to the capillary endothelium (217, 218). In adipose tissue, LPL influences fatty acid (FA) uptake for lipid storage and in skeletal and cardiac muscle, LPL induces FA uptake to provide these energetically-active cells with fuel (219, 220). Previous studies in mice on a high-fat diet (HFD) have shown that the skeletal muscle-specific deletion of LPL reduced lipid deposition and increased insulin sensitivity in the muscles (221, 222). This result indicates that LPL-induced uptake of fatty acids in skeletal muscle is detrimental in obesity due to the extreme excess of circulating lipids. When transgenic mice, overexpressing LPL in their adipose tissue, were challenged with a HFD, they exhibited elevated adiponectin levels, improved glucose and insulin tolerance, and increased energy expenditure when compared to control mice on the same diet (223). The positive, anti-inflammatory effect of LPL seen in adipocytes was suppressed in cultured adipocytes treated with TNF-alpha due to the activation of inducible nitric oxide synthase (iNOS), which produced toxic levels of nitric oxide (224). Other in vitro studies demonstrated that both a NO-releasing compound and the NOS substrate (hydroxylamine and L-arginine, respectively) increased LPL activity as seen by adipocyte differentiation and lipid accumulation (225). These data indicate that the toxic levels of NO from iNOS have a deleterious effect on LPL function, while moderate levels of NO promote LPL activity.
In addition to LPL dysfunction, mitochondrial lipid metabolism also is adversely affected by obesity. In lean conditions, fatty acids are internalized and transported into mitochondria, where they undergo beta-oxidation to produce acetyl-CoA. Acetyl-CoA is subsequently shuttled into the citric acid cycle to produce energy for the cell. In obesity, the mitochondria in white adipose tissue are inundated with excess lipids and incomplete beta-oxidation occurs. Incomplete beta-oxidation coupled with lipid overloading results in accumulation of toxic lipid intermediates and ROS, which promote insulin resistance (226, 227).
7. ROLE OF MITOCHONDRIAL DYSFUNCTION AND ER STRESS IN OBESITY
Mitochondria are dynamic organelles, critical for the maintenance of energy homeostasis. The mitochondria produce ATP, a form of cellular energy currency, through a sequence of processes that terminate with the electron transport chain (ETC): the citric acid cycle (CAC), pyruvate decarboxylation, fatty acid beta-oxidation, branched chain amino acid degradation, and oxidative phosphorylation (228). Mitochondria also internalize Ca2+, an important physiological process that influences mitochondria metabolism, intracellular Ca2+ signaling, and under conditions of oxidative stress, this uptake leads to initiation of apoptosis, or programmed cell death (229).
7.1. Mitochondrial dysfunction
In response to increased energy expenditure, healthy cells undergo mitochondrial biogenesis, a process where cells increase their mitochondrial mass in order to increase their individual ATP production. Mitochondrial biogenesis is closely associated with the process of adipogenesis, indicating the importance of mitochondria to healthy adipocyte physiology and function (230–234). In adipose tissue, eNOS-produced NO plays an important role in mitochondrial biogenesis by increasing oxygen consumption and energy expenditure, inducing gene expression, and promoting protein kinase G (PKG)-dependent phosphorylation of AMP-activated protein kinase (AMPK) (20, 235). Activation of AMPK promotes expression of PPAR gamma coactivator 1 alpha (PGC-1 alpha), leading to increased expression of PPAR gamma, a protein that upregulates adipogenesis (236, 237).
Mitochondrial dysfunction, a process defined by poor ATP production, often occurs in obesity. There is still some debate on whether mitochondrial dysfunction is a cause or consequence of obesity and overnutrition. However, there have been data revealing that an excess of nutrients, as seen in obesity and type 2 diabetes, overwhelms the handling capacity of the mitochondrial metabolic processes, resulting in mitochondrial dysfunction (238). In the mouse preadipocyte cell line, 3T3-L1, mitochondrial dysfunction was manifested as reduced fatty acid oxidation, which resulted in TAG accumulation and increased glucose uptake, that latter of which is suggested to increase glycerol 3-phosphate synthesis, leading to further lipid accumulation. Increased lipid accumulation in adipocytes leads to the eventual loss of the lipotoxicity buffering capacity of these cells. The excess free fatty acids are released into the bloodstream resulting in ectopic fat deposition, which is believed to be the underlying cause of the development of insulin resistance in obesity (239).
In addition to the adverse effects seen from systemic lipid inundation and the subsequent steatosis, mitochondrial dysfunction also results in increased ROS production, as seen in both clinical and experimental studies (240, 241). The electron transport chain (ETC), primarily complexes I, II, and III, are considered to be major sources of ROS generation due to the capacity for electron leakage (242–244). Electron leakage directly correlates with mitochondrial membrane potential (245). Activation of the uncoupler protein (UCP) by ROS serves as a feedback mechanism to lower membrane potential (246). These detrimental by-products of metabolism can induce metabolic dysfunction, inflammation (through upregulation of TNF-alpha), tissue damage, and the development of insulin resistance (38, 39). ROS have also been shown to increase expression of activating transcription factor 3 (ATF-3), a protein responsible for the downregulation of adiponectin expression (33). Though adipocytes, unlike other cell types, can endure high levels of ROS without sustaining substantial damage, chronic ROS elevation is detrimental and decreases adiponectin expression (247). In contrast, increased mitochondrial biogenesis has been correlated with increased adiponectin levels, reduced oxidative stress, improved mitochondrial function, and increased insulin sensitivity (33, 248).
These findings suggest an important link between oxidative stress, mitochondrial dysfunction, and metabolic dysregulation during obesity. Thus, further investigation is warranted to combat obesity and obesity-related dysregulations by targeting any or all of these disorders.
Paradoxically, mild mitochondrial dysfunction, in the absence of oxidative stress, protects against obesity as seen in mice with a fat-specific deletion of the mitochondrial transcription factor A (TFAM). These mice exhibited decreased mitochondrial DNA (mtDNA) copy number and altered expression of ETC proteins, with decreased expression of complex I, the main site of superoxide formation in the ETC (249). These mice also exhibited a compensatory increase in complex II, which resulted in increased oxygen consumption, uncoupling, and decreased ROS production (250). The overall effect of the fat-specific deletion of TFAM in mice challenged with a HFD was higher energy expenditure and protection from diet-induced obesity, insulin resistance, and steatosis (250).
7.2. Compromised mitochondrial dynamics
Mitochondria are highly dynamic organelles, exhibiting fission, fusion, and mitophagy. Mitochondrial fusion is the process by which two mitochondria physically merge their inner and outer membranes to form a larger, mitochondrion (251). This process is controlled by several proteins, including the dynamin-related GTPases, mitofusin-1 and mitofusin-2 (MFN-1 and MFN-2), and optic atrophy protein 1 (OPA-1), the former are responsible for the outer mitochondrial membrane fusion and the latter is required for the fusion of the inner mitochondrial membranes (252). Mitochondrial fission is the opposite of fusion, and is where a mitochondrion divides to form two mitochondria (251). Fission is controlled by dynamin-related protein 1 (DRP1), a protein recruited from the cytosol to the mitochondrion where it can then bind its receptors, fission 1 (FIS1) and mitochondrial fission factor (MFF) (253). The processes of fusion and fission occur cyclically. Fusion allows two mitochondria to mix their components and is typically followed within minutes by fission, which returns the fused mitochondria back into two distinct organelles (254). This dynamic process of mitochondrial fusion and fission in healthy cells is believed to be critical for cell health and aberrant function of this process is associated with several disease states (255, 256). Mitochondria with high membrane potential will continue the fission/fusion cycle while those with a low potential will remain depolarized until recovery (254). A continuous, but precisely controlled, cycle of fission and fusion is important for the proper distribution of mitochondria throughout the cell, repair of damaged mitochondria, and for mitochondrial quality control (256). Mitochondria that are damaged/depolarized and unable to recover, do not undergo fusion and are not incorporated into the healthy mitochondrial network (257). They form autophagosomes that eventually undergo mitophagy (258). Mitophagy is a catabolic process in which damaged mitochondria are degraded by lysosomes (258). This process is initiated by PARKIN and (PTEN)-induced putative kinase 1 (PINK1), which induce ubiquitination and degradation of fusion-promoting proteins MFN-1 and 2 (259).
Mitochondrial dynamics represent cellular adaptation to fluctuations in metabolic demand. Increased energy demand and decreased supply of nutrients are both associated with inhibition of mitochondrial fission and the promotion of fusion, or increased mitochondrial elongation, which allows respiration to be coupled to ATP synthesis (260). During conditions of increased energy expenditure, expression of MFN-2 is increased in skeletal muscles and brown adipose tissue (261, 262). Moreover, exercise improves insulin sensitivity in skeletal muscles of insulin resistant patients and is associated with decreased DRP1 and increased MFN-1 and MFN-2 expression (263). On the flipside, mitochondrial fragmentation and uncoupled respiration predominate under conditions of excess nutrient supply, where the promotion of thermogenesis is necessary to dispose of the caloric excess (264). Increased DRP1 activity in brown adipocytes is associated with increased levels of uncoupling protein-1 (UCP-1) (265). In fact, brown/beige adipocytes rely greatly on fission to enhance the uncoupling process, allowing for increased oxygen consumption. Though mitochondrial fission is considered to be a physiological adaptation to bioenergetic stressors rather than a harmful process, excessive mitochondrial fission events can be deleterious for the cell (264).
Disruption of the tightly regulated mitochondrial dynamics is associated with metabolic disorders and insulin resistance seen in diabetic and obese patients (264). One study showed that mice lacking DRP1 or MFN-1 in the liver were resistant to high-fat diet-induced obesity and insulin resistance (266). Another study reported that increased hepatic levels of PINK1, a protein that promotes mitophagy, were positively correlated with increased insulin sensitivity. This effect is likely due to successful degradation of dysfunctional mitochondria, which can produce elevated levels of ROS (267–269). In pancreatic beta-cells, exposure to hyperglycemia and high levels of palmitate (obese conditions) resulted in reduced mitochondrial fusion (270). This environment also caused the same effect in leukocytes, which led to their enhanced adhesion to endothelial cells and subsequent inflammation (271). Skeletal muscle of Zucker obese rats showed decreased mitochondrial size, mitochondrial volume/unit of mitochondrial surface ratio, and MFN-2 expression (272). MFN-2 expression was found to be downregulated in human obese and type 2 diabetic patients (273, 274). MFN-2-deficient mice exhibited impaired insulin signaling in the liver and muscle, and increased ER stress through a mechanism involving increased ROS and JNK activation (272). Additionally, adipocyte-specific deletion of MFN-2, but not MFN-1, was associated with brown adipose tissue dysfunction and impaired lipid metabolism (275). Given the importance of proper mitochondrial dynamics in the maintenance of cell health and insulin sensitivity, the mitochondrial fission/fusion cycle is a promising therapeutic target for combating metabolic disorders (276).
7.3. Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is the organelle where protein synthesis, folding, and maturation occurs (277, 278). Accumulation of misfolded proteins in the ER lumen is problematic and can eventually lead to cell death. During ER stress, mammalian cells trigger the unfolded protein response (UPR), a highly conservative response system intended to rectify the aggregation of misfolded proteins in the ER (279). The UPR begins with the activation of signaling pathways involved in either suppression of protein translation, to prevent more proteins from being misfolded, or the upregulation of chaperone protein expression, to coordinate and regulate proper protein folding (280–282). Hypoxia and ROS can increase the production of free fatty acids in adipocytes, oxidize proteins, and decrease calcium levels in ER lumen; all processes that impair ER protein folding in adipocytes and lead to ER stress (33, 283–286). Inflammatory cytokines also trigger ER stress by promoting ROS formation, or by increasing iNOS activity to pathological levels, which impedes the function of the ER Ca2+ pump (281, 287–289). Indicators of ER stress have been shown to be elevated in adipose tissue of obese mice and humans (290, 291). Administration of chemical chaperones that block ER stress, like 4-PBA, to mice on a high-fat diet (HFD), reduced adipose tissue inflammation, increased insulin sensitivity, and suppressed HFD-induced weight gain (292). Additionally, weight loss has been shown to be associated with reduced expression of ER stress markers (293).
In addition to triggering the unfolded protein response, ER stress has been shown to disrupt lipid metabolism. This mechanism involves activation of sterol regulatory element-binding protein (SREBP), which induces transcription of fatty acid synthase (FAS) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-COA), genes involved in lipid and cholesterol synthesis, respectively. Upregulation of these genes leads to excessive lipid production, resulting in fatty acid accumulation in the liver (294). Interestingly, deletion of ER stress sensor inositol-requiring enzyme 1 alpha (IRE1 alpha) in mouse adipose tissue macrophages halted the progression of obesity, insulin resistance, and hepatic steatosis when the mice were challenged with a HFD (295). This mutation also increased energy expenditure by inducing the browning of white adipose tissue, increased the metabolic activity of brown adipose tissue, and promoted macrophage polarization to the anti-inflammatory, M2 phenotype (295). Prevention of ER stress activation may be an effective therapeutic strategy for the treatment of metabolic syndrome (294).
8. NITRIC OXIDE SYNTHASE (NOS) DYSFUNCTIONS
Nitric oxide (NO) is well known for its vasodilatory, anti-thrombotic, anti-proliferative, and anti-inflammatory effect in the vasculature (296–299). NO production and effects occur in a variety of cells and tissues. NO is produced from L-arginine by endothelial, neuronal, and inducible NO synthase (eNOS, nNOS, and iNOS). NO triggers a signal cascade by binding soluble guanylate cyclase (sGC), initiating the conversion of guanosine 5’-triphosphate (GTP) to the secondary messenger, cyclic 3’,5’-monophosphate (cGMP) (300). cGMP activates cGMP-dependent protein kinase (PKG) which then phosphorylates target proteins involved in mediating the vasodilatory response (301).
Adipose tissue (AT) from mice lacking eNOS (eNOS−/−) was reported to exhibit increased pro-inflammatory gene expression and macrophages, in addition to increased ROS and decreased mitochondrial biogenesis and adiponectin levels (146, 299). In contrast to these findings, another study reported that the lack of eNOS in mice did not promote AT inflammation (302). Use of a phosphodiesterase-5 (PDE5) inhibitor, such as sildenafil, blunted obesity-induced adipose tissue inflammation and macrophage infiltration, through the prevention of PDE5-mediated cGMP degradation (299). PDE5 inhibition resulted in enhanced NO-cGMP-PKG signaling, promotion of vasorelaxation, increased energy expenditure, and elevated insulin sensitivity (303). Other studies have demonstrated that eNOS expression and activity are tightly regulated in adipose tissue and muscle, and that eNOS is necessary for caloric restriction-induced upregulation of SIRT1, a protein involved in promotion of insulin sensitivity and amplification of eNOS activity (235, 304, 305). Further, eNOS−/− mice failed to exhibit the beneficial effects of swim training-induced increases in mitochondrial biogenesis, mtDNA copy number, and glucose uptake in the subcutaneous adipose tissue as compared with wild-type mice. In the same study, the NO donor, DETA-NO, was found to promote mitochondrial biogenesis, glucose uptake, and increased GLUT4 membrane density in cultured murine and human adipocytes (306). These results indicate that physiological levels of NO play a pivotal role in maintaining healthy metabolic function of adipose tissue.
Data from both human and animal studies have shown reduced expression and activity of eNOS and NO production under obese conditions (307–310). Suggested mechanisms for decreased NO levels include upregulation of cell membrane caveolin1 (CAV1), a negative regulator of eNOS activity, and increased levels of ceramide, a disruptor of the eNOS/Akt/HSP-90 complex (311, 312). However, a mechanism involved in the obesity-induced reduction of eNOS-produced NO supported by substantial evidence is the elevation of arginase expression/activity. Arginase is an enzyme that competes with NOS for their common substrate, L-arginine (313). Reduced availability of L-arginine leads to decreased NO production and NOS uncoupling. NOS uncoupling results in production of the ROS superoxide (O2−), which can subsequently react with NO to form peroxynitrite (ONOO−), another toxic oxidative species (313). Several studies that used HFD and rodent models of obesity have shown prominent involvement of arginase in both visceral adipose inflammation and vascular dysfunction and inflammation through genetic deletion of arginase or use of arginase inhibitors (14, 73, 314). Our lab also found that mice specifically lacking arginase 1 in endothelial cells were protected from high-fat diet-induced systemic vascular dysfunction, hypertension, reduced vascular NO, elevated ROS levels, adipose tissue inflammation, fibrosis, and reduced vascularity (73, 313).
NO from endothelial or neuronal NOS at low to moderate concentrations stimulates glucose and fatty acid oxidation and inhibits synthesis of glucose, triacylglycerol, and low-density lipoproteins. These beneficial effects are linked to increased mitochondrial biogenesis and oxidative phosphorylation, as well as development and activity of brown adipose tissue (16). NOS function in mitochondria, along with cytoplasmic NO production, have been shown to induce mitochondrial biogenesis (235, 315). An in vitro study showed that NO acutely inhibits brown adipocyte proliferation but stimulates adipogenesis as shown by increased expression of peroxisome proliferator-activated receptor gamma (PPAR gamma) and uncoupling protein 1 (UCP 1) (316). In spite of the multitude of beneficial effects of NO at low to moderate concentrations, high concentrations of NO produced by iNOS is cytotoxic and can generate detrimental peroxynitrite and hydroxyl radicals (317). Under conditions of low L-arginine bioavailability, such as increased arginase activity, providing supplemental L-arginine restored NO production (318). In Zucker obese/diabetic rats, dietary supplementation with L-arginine suppressed weight gain and other features of metabolic syndrome, while elevating the respiratory exchange ratio (RER) and heat production (319, 320). L-arginine supplementation also improved metabolic disturbances by increasing insulin sensitivity in mice challenged with a low protein diet (321). Human studies also reported the effectiveness of supplemental L-arginine in the improvement of insulin sensitivity in patients with metabolic syndrome (165, 322).
Summaries of studies that have investigated means of enhancing constitutive NO production from NOS to prevent or reduce obesity-induced metabolic and vascular dysfunctions are provided in Table 1.
Table 1.
Therapeutic interventions applied to obesity models and the observed effects
| Intervention | Model | Effect |
|---|---|---|
| Treatment with arginase inhibitor, Nω-hydroxy-nor-L-arginine (nor-NOHA) | C57BL/6 mice fed high-fat diet (HFD) | Prevented HFD-induced increases in body weight, hepatic metabolic abnormalities (323), endothelial dysfunction (324) and adipose tissue inflammation (314). |
| Zucker obese rats | Prevented obesity-induced hypertension and endothelial dysfunction (318). | |
| Treatment with arginase inhibitor, S-(2-boronoethyl)-L-cysteine (BEC) | Zucker obese rats | Prevented obesity-induced hypertension and endothelial dysfunction (318). |
| Treatment with arginase inhibitor, 2-(S)-amino-6-boronohexanoic acid (ABH) | C57BL/6 mice fed high-fat diet (HFD) | Prevented obesity-induced bone loss (325), endothelial dysfunction, hypertension (73) and visceral adipose tissue (VAT) inflammation (14). No effect on body weight. |
| Endothelial specific Arginase 1 knockout (eNOS−/−) | C57BL/6 mice fed high-fat diet (HFD) | Prevented obesity-induced endothelial dysfunction, hypertension (73) and visceral adipose tissue (VAT) inflammation (14). There was no effect on body weight. |
| Global deletion of Arginase 2° | C57BL/6 mice fed high-fat diet (HFD) | Prevented obesity-induced renal oxidative stress and inflammation (326), endothelial dysfunction (327), hepatic steatosis (150), insulin resistance, adipose tissue inflammation (74), and pancreatic ductal adenocarcinoma growth (328). |
| Supplement with sepiapterin and L-citrulline; precursor of BH4 (tetrahydrobiopterin) and substrate for L-arginine synthesis, respectively | Db/db mice | Prevented cardiomyopathy in obese T2D mice (329). |
| L-arginine supplementation (potential mechanism of increased NO availability) | Zucker obese rats | Anti-inflammatory effects in obese rats (330) and decreased macrophage inflammatory response (331). |
| Prevented obesity-induced hypertension and endothelial dysfunction (318). | ||
| Healthy patients with metabolic syndrome | Improved endothelial function and glucose metabolism in metabolic syndrome patients (332). | |
| C57BL/6 mice fed high-fat diet (HFD) | Anti-obesity effects, reduced white fat mass and plasma lipids, increased skeletal muscle and brown fat, insulin sensitivity, and increased energy expenditure (321, 333). | |
| Sodium nitrite treatment | Streptozotocin (STZ)-induced diabetic rats fed HFD | Decreased body weight and induction of white adipose tissue browning (334). |
| Global iNOS−/− knockout | Ob/ob obese mice given high dose of insulin via implanted insulin pump | Prevented hyperinsulinemia-induced inflammation, fibrosis and insulin resistance in adipose tissue (335, 336). |
| Phosphodiesterase-5 inhibitor (Sildenafil) | C57BL/6 mice fed high-fat diet (HFD) | Long term treatment (12 weeks): anti-obesity and insulin sensitizing (337). |
| C57BL/6 mice | Short term (7 days): browning of white adipose tissue (338). | |
| Global iNOS−/− knockout mice | Model of metabolic syndrome - Isolated muscle mitochondria | Lower metabolic activity, reduced mitochondrial density and muscle fatty acid oxidation (339). |
| Global iNOS−/− knockout mice supplemented with sodium nitrate | Model of metabolic syndrome | Reduced visceral fat mass, circulating triglyceride and glucose levels (340). |
| eNOS transgenic mice | C57BL/6 mice fed high-fat diet (HFD) | Enhanced fatty acid oxidation in adipose tissue, resistant to diet induced obesity without affecting insulin resistance (20). |
9. PERSPECTIVE/SUMMARY
Historically, adipocytes were considered to be inert lipid reservoirs, however, recent studies have shown the important, systemic endocrine function of adipocytes, which is intimately involved in the regulation of insulin sensitivity, energy homeostasis, and cardiovascular function. The rapidly growing prevalence of obesity worldwide affects individuals of all genders, ages, ethnic groups, and socioeconomic levels. Obesity greatly increases the risk of developing various comorbidities, indicating the dire need to better understand the intricate mechanisms behind obesity-induced metabolic and cardiovascular dysfunctions. At physiological levels, the vasoprotective molecule, NO, plays a prominent role in maintaining adipocyte and vascular function. However, expression of inducible NOS leads to high levels of NO, which are detrimental to metabolic and cardiovascular function. In obesity, arginase and NOS and are dysregulated. Given the deleterious effects of elevated arginase activity/expression seen in obesity-related metabolic and cardiovascular disorders, targeting this enzyme could be a possible therapeutic strategy in the treatment of obesity-induced diseases. Complicating this potential strategy is the fact that arginase has two isoforms, A1 and A2, and not enough is known about the ability and effect of preferentially targeting them. Additionally, the effect of systemic arginase inhibition in the presence of comorbidities or on other organ systems is not well known. Currently, there are only a few clinical trials testing the efficacy of arginase inhibition in different pathologies. Exploring tissue-specific, cell-specific or isoform-specific arginase inhibitors or modulators may prove to be an effective therapeutic strategy for combating obesity-related disorders. However, further studies of the complex mechanisms behind the development of metabolic and cardiovascular disease induced by obesity are required to address future treatment strategies for this ever-growing health problem.
10. ACKNOWLEDGEMENTS
Funding was provided by the U.S. National Institute of Health grants: R01 HL070215 (RWC), R01 EY01176 (RBC and RWC). The work was also supported by the American Heart Association) grants (17PRE33660321) (RTA) and 13SDG17410007 (HAT) and U.S. Veterans Administration Merit Review Award I01BX003221 (RBC).
Abbreviations:
- ACC
acetyl-CoA carboxylase
- ADD1
adipocyte determination and differentiation-dependent factor 1
- ADMA
asymmetric dimethylarginine
- AMPK
5’ adenosine monophosphate-activated protein kinase
- ANGPTL2
angiopoietin-like protein 2
- AT
adipose tissue
- ATF-3
activating transcription factor 3
- BCAA
branched chain amino acid
- BMI
body mass index
- BMP4
bone morphogenic protein 4
- C/EBP
CCAAT-enhancer-binding protein
- CAC
citric acid cycle
- CAV1
caveolin1
- DDAH
dimethylarginine dimethylaminohydrolase
- DRP1
dynamin-related protein 1
- eNOS
endothelial nitric oxide synthase
- ETC
electron transport chain
- FA
fatty acid
- FAS
fatty acid synthase
- FIS1
mitochondrial fission protein 1
- HFD
high-fat diet
- HMG-COA
3-hydroxy-3-methylgutaryl-CoA reductase
- HSL
hormone-sensitive lipase
- ICAM-1
intracellular adhesion molecule 1
- IFN-gamma
interferon gamma
- iNOS
inducible nitric oxide synthase
- INSR
insulin substrate receptor
- IRE1 alpha
inositol-requiring enzyme 1 alpha
- IRS-1
insulin receptor substrate 1
- IL-1, 6, 10
interleukin 1, interleukin 6, interleukin 10
- JNK/AP-1
c-Jun N-terminal kinase–activator protein-1
- LPL
lipoprotein lipase
- MCP-1
monocyte chemoattractant protein
- MFF
mitochondrial fission factor
- MFN-1, 2
mitofusin-1, mitofusin-2
- mTORC1
mammalian target of rapamycin complex 1
- NAD+
nicotinamide adenine dinucleotide
- NF-kappaB
nuclear factor kappa-light-chain-enhancer of activated B cells
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- OPA-1
optic atrophy protein 1
- PDE5
phosphodiesterase-5
- PI3K
phosophinositide 3’ kinase
- PINK1
PTEN-induced putative kinase 1
- PIP2
phosphatidylinositol (3,4)-bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-triphosphate (PIP3)
- PKG
protein kinase G
- PPARgamma
peroxisome proliferator-activated receptor gamma
- RBP4
retinol binding protein 4
- ROS
reactive oxygen species
- SAA
serum amyloid A
- SAT
subcutaneous adipose tissue
- sGC
soluble guanylate cyclase
- SIRT1
sirtuin 1
- SOCS3
suppressor of cytokine signaling 3
- SREBP
sterol regulatory element-binding protein
- T2D
type II diabetes
- TAG
triacylglycerol
- TFAM
transcription factor A, mitochondrial
- TLR-4
toll-like receptor 4
- TNF-alpha
tumor necrosis factor alpha
- Treg
regulatory T cell
- UCP-1
uncoupling protein 1
- VAT
visceral adipose tissue
- VLDL
very low density lipoprotein
- ZNF
zinc-finger protein
11. REFERENCES
- 1.Brant LC, Wang N, Ojeda FM, LaValley M, Barreto SM, Benjamin EJ, Mitchell GF, Vasan RS, Palmisano JN and Münzel T: Relations of Metabolically Healthy and Unhealthy Obesity to Digital Vascular Function in Three Community-Based Cohorts: A Meta-Analysis. Journal of the American Heart Association, 6(3), e004199 (2017) DOI: 10.1161/JAHA.116.004199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JW, Kendall CW and Jenkins DJ: Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. Journal of the American college of nutrition, 22(5), 331–339 (2003) DOI: 10.1080/07315724.2003.10719316 [DOI] [PubMed] [Google Scholar]
- 3.Daly A: Diabesity: the deadly pentad disease. The Diabetes Educator, 20(2), 156–162 (1994) DOI: 10.1177/014572179402000212 [DOI] [PubMed] [Google Scholar]
- 4.Hruby A and Hu FB: The epidemiology of obesity: a big picture. Pharmacoeconomics, 33(7), 673–689 (2015) DOI: 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe SS, Huh JY, Hwang IJ, Kim JI and Kim JB: Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Frontiers in endocrinology, 7, 30 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehr S, Hartwig S and Sell H: Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics-Clinical Applications, 6(1–2), 91–101 (2012) [DOI] [PubMed] [Google Scholar]
- 7.Mancuso P: The role of adipokines in chronic inflammation. ImmunoTargets and therapy, 5, 47 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newsholme P, Cruzat V, Arfuso F and Keane K: Nutrient regulation of insulin secretion and action. Journal of Endocrinology, 221(3), R105–R120 (2014) [DOI] [PubMed] [Google Scholar]
- 9.Blüher M: Mechanisms in Endocrinology: Are metabolically healthy obese individuals really healthy? European journal of endocrinology, 171(6), R209–R219 (2014) [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM and Gonzalez FJ: Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet–fed mice. Diabetes, 60(10), 2484–2495 (2011) DOI: 10.2337/db11-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’rourke R, White A, Metcalf M, Olivas A, Mitra P, Larison W, Cheang E, Varlamov O, Corless C and Roberts C: Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia, 54(6), 1480–1490 (2011) DOI: 10.1007/s00125-011-2103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, Khalyfa AA, Andrade J and Khalyfa A: Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. American journal of respiratory cell and molecular biology, 56(4), 477–487 (2017) DOI: 10.1165/rcmb.2016-0243OC [DOI] [PubMed] [Google Scholar]
- 13.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA and Ravichandran KS: Pannexin 1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. American Journal of Physiology-Lung Cellular and Molecular Physiology (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao L, Bhatta A, Xu Z, Chen J, Toque HA, Chen Y, Xu Y, Bagi Z, Lucas R, Huo Y, Caldwell RB and Caldwell RW: Obesity-induced vascular inflammation involves elevated arginase activity. Am J Physiol Regul Integr Comp Physiol, 313(5), R560–R571 (2017) DOI: 10.1152/ajpregu.00529.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansbury BE and Hill BG: Regulation of obesity and insulin resistance by nitric oxide. Free radical biology and medicine, 73, 383–399 (2014) DOI: 10.1016/j.freeradbiomed.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW and Wu G: Nitric oxide and energy metabolism in mammals. Biofactors, 39(4), 383–391 (2013) DOI: 10.1002/biof.1099 [DOI] [PubMed] [Google Scholar]
- 17.Tsao PS, McEvoy LM, Drexler H, Butcher EC and Cooke JP: Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation, 89(5), 2176–2182 (1994) DOI: 10.1161/01.CIR.89.5.2176 [DOI] [PubMed] [Google Scholar]
- 18.Tsao PS, Wang B.-y., Buitrago R, Shyy JY-J and Cooke JP: Nitric oxide regulates monocyte chemotactic protein-1. Circulation, 96(3), 934–940 (1997) DOI: 10.1161/01.CIR.96.3.934 [DOI] [PubMed] [Google Scholar]
- 19.Li H, Xia N and Förstermann U: Nitric Oxide Synthesis in Vascular Physiology and Pathophysiology In: Endothelial Signaling in Development and Disease. Springer, (2015) [Google Scholar]
- 20.Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M and Bhatnagar A: Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circulation research, 111(9), 1176–1189 (2012) DOI: 10.1161/CIRCRESAHA.112.266395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang K, Huang Y, Frankel J, Addis C, Jaswani L, Wehner PS, Mangiarua EI and McCumbee WD: The short-term consumption of a moderately high-fat diet alters nitric oxide bioavailability in lean female Zucker rats. Canadian journal of physiology and pharmacology, 89(4), 245–257 (2011) DOI: 10.1139/y11-016 [DOI] [PubMed] [Google Scholar]
- 22.Jang JE, Ko MS, Yun J-Y, Kim M-O, Kim JH, Park HS, Kim A-R, Kim H-J, Kim BJ and Ahn YE: Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes, db151624 (2016) [DOI] [PubMed] [Google Scholar]
- 23.Engeli S, Janke J, Gorzelniak K, Böhnke J, Ghose N, Lindschau C, Luft FC and Sharma AM: Regulation of the nitric oxide system in human adipose tissue. Journal of lipid research, 45(9), 1640–1648 (2004) DOI: 10.1194/jlr.M300322-JLR200 [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Eto M, Ito Y, Mochizuki S, Son B-K, Ogawa S, Iijima K, Kaneki M, Kozaki K and Toba K: Suppressive role of PPARγ-regulated endothelial nitric oxide synthase in adipocyte lipolysis. PloS one, 10(8), e0136597 (2015) DOI: 10.1371/journal.pone.0136597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncada S, Palmer RM and Higgs EA: The biological significance of nitric oxide formation from L-arginine In: Portland Press Limited, (1989) [DOI] [PubMed] [Google Scholar]
- 26.Stamler JS and Meissner G: Physiology of nitric oxide in skeletal muscle. Physiological reviews, 81(1), 209–237 (2001) DOI: 10.1152/physrev.2001.81.1.209 [DOI] [PubMed] [Google Scholar]
- 27.Stamler JS: Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell, 78(6), 931–936 (1994) DOI: 10.1016/0092-8674(94)90269-0 [DOI] [PubMed] [Google Scholar]
- 28.Stamler JS, Lamas S and Fang FC: Nitrosylation: the prototypic redox-based signaling mechanism. Cell, 106(6), 675–683 (2001) DOI: 10.1016/S0092-8674(01)00495-0 [DOI] [PubMed] [Google Scholar]
- 29.Savini I, Catani M, Evangelista D, Gasperi V and Avigliano L: Obesity-associated oxidative stress: strategies finalized to improve redox state. International journal of molecular sciences, 14(5), 10497–10538 (2013) DOI: 10.3390/ijms140510497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra D, Mera P, Malandrino MI, Mir JF and Herrero L: Mitochondrial fatty acid oxidation in obesity. Antioxidants & redox signaling, 19(3), 269–284 (2013) DOI: 10.1089/ars.2012.4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dandona P, Ghanim H, Chaudhuri A, Dhindsa S and Kim SS: Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Experimental & molecular medicine, 42(4), 245 (2010) DOI: 10.3858/emm.2010.42.4.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabit CE, Chung WB, Hamburg NM and Vita JA: Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Reviews in Endocrine and Metabolic Disorders, 11(1), 61–74 (2010) DOI: 10.1007/s11154-010-9134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and Shimomura I: Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation, 114(12), 1752–1761 (2017) DOI: 10.1172/JCI21625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C and Morales-González JA: Inflammation, oxidative stress, and obesity. International journal of molecular sciences, 12(5), 3117–3132 (2011) DOI: 10.3390/ijms12053117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi A and Chamari M: Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atheroscler, 2(4) (2010) [Google Scholar]
- 36.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B and Ooms LM: Reactive oxygen species enhance insulin sensitivity. Cell metabolism, 10(4), 260–272 (2009) DOI: 10.1016/j.cmet.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani A-L, Ktorza A, Casteilla L and Pénicaud L: Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes, 58(3), 673–681 (2009) DOI: 10.2337/db07-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMurray F, Patten DA and Harper ME: Reactive oxygen species and oxidative stress in obesity—recent findings and empirical approaches. Obesity, 24(11), 2301–2310 (2016) DOI: 10.1002/oby.21654 [DOI] [PubMed] [Google Scholar]
- 39.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q and Griendling KK: Reactive oxygen species in metabolic and inflammatory signaling. Circulation Research, 122(6), 877–902 (2018) DOI: 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cristancho AG and Lazar MA: Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews Molecular cell biology, 12(11), 722 (2011) DOI: 10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A and Smith U: Insulin resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism, 26(4), 193–200 (2015) DOI: 10.1016/j.tem.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 42.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR and Spiegelman BM: Transcriptional control of preadipocyte determination by Zfp423. Nature, 464(7288), 619 (2010) DOI: 10.1038/nature08816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F and Baron R: Direct transcriptional repression of Zfp423 by Zfp521 mediates a BMP-dependent osteoblast versus adipocyte lineage commitment switch Molecular and cellular biology, MCB; 00185–14 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talchai C, Xuan S, Lin HV, Sussel L and Accili D: Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell, 150(6), 1223–1234 (2012) DOI: 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith U and Kahn BB: Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. Journal of internal medicine, 280(5), 465–475 (2016) DOI: 10.1111/joim.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafson B and Smith U: The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes, 61(5), 1217–1224 (2012) DOI: 10.2337/db11-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arner P, Arner E, Hammarstedt A and Smith U: Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PloS one, 6(4), e18284 (2011) DOI: 10.1371/journal.pone.0018284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafson B, Hammarstedt A, Hedjazifar S, Hoffmann JM, Svensson P-A, Grimsby J, Rondinone C and Smith U: BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes, db141127 (2015) [DOI] [PubMed] [Google Scholar]
- 49.Al-Suhaimi EA and Shehzad A: Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. European journal of medical research, 18(1), 12 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilg H and Moschen AR: Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Reviews Immunology, 6(10), 772 (2006) [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K, Fuster JJ and Walsh K: Adipokines: a link between obesity and cardiovascular disease. Journal of cardiology, 63(4), 250–259 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouchi N, Parker JL, Lugus JJ and Walsh K: Adipokines in inflammation and metabolic disease. Nature Reviews Immunology, 11(2), 85–97 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel P and Abate N: Body fat distribution and insulin resistance. Nutrients, 5(6), 2019–2027 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotamisligil GS, Shargill NS and Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 259(5091), 87–91 (1993) [DOI] [PubMed] [Google Scholar]
- 55.Reilly SM and Saltiel AR: Adapting to obesity with adipose tissue inflammation. Nature Reviews Endocrinology, 13(11), 633 (2017) [DOI] [PubMed] [Google Scholar]
- 56.Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M and Spiro W: Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nature medicine, 20(4), 377 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suganami T, Nishida J and Ogawa Y: A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arteriosclerosis, thrombosis, and vascular biology, 25(10), 2062–2068 (2005) [DOI] [PubMed] [Google Scholar]
- 58.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K and Aoe S: Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid–induced inflammatory changes in the interaction between adipocytes and macrophages. Arteriosclerosis, thrombosis, and vascular biology, 27(1), 84–91 (2007) [DOI] [PubMed] [Google Scholar]
- 59.Ruan H, Hacohen N, Golub TR, Van Parijs L and Lodish HF: Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-κB activation by TNF-α is obligatory. Diabetes, 51(5), 1319–1336 (2002) [DOI] [PubMed] [Google Scholar]
- 60.Permana PA, Menge C and Reaven PD: Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochemical and biophysical research communications, 341(2), 507–514 (2006) [DOI] [PubMed] [Google Scholar]
- 61.Hajer GR, van Haeften TW and Visseren FL: Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal, 29(24), 2959–2971 (2008) [DOI] [PubMed] [Google Scholar]
- 62.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL and Ferrante AW Jr.: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest, 112(12), 1796–808 (2003) DOI: 10.1172/jci19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL and Ferrante AW Jr.: CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest, 116(1), 115–24 (2006) DOI: 10.1172/jci24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K and Urakaze M: Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes, 58(11), 2574–2582 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutens L and Stienstra R: Adipose tissue macrophages: going off track during obesity. Diabetologia, 59(5), 879–894 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Issazadeh-Navikas S, Teimer R and Bockermann R: Influence of dietary components on regulatory T cells. Molecular Medicine, 18(1), 95 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Exley MA, Hand L, O’Shea D and Lynch L: Interplay between the immune system and adipose tissue in obesity. Journal of Endocrinology, 223(2), R41–R48 (2014) [DOI] [PubMed] [Google Scholar]
- 68.Otto TC and Lane MD: Adipose development: from stem cell to adipocyte. Critical reviews in biochemistry and molecular biology, 40(4), 229–242 (2005) [DOI] [PubMed] [Google Scholar]
- 69.Lackey DE and Olefsky JM: Regulation of metabolism by the innate immune system. Nature Reviews Endocrinology, 12(1), 15 (2016) [DOI] [PubMed] [Google Scholar]
- 70.Shoelson S, Lee J and Yuan M: Inflammation and the IKKβ/IκB/NF-κB axis in obesity-and diet-induced insulin resistance. International journal of obesity, 27(S3), S49 (2003) [DOI] [PubMed] [Google Scholar]
- 71.Gual P, Le Marchand-Brustel Y and Tanti J-F: Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie, 87(1), 99–109 (2005) [DOI] [PubMed] [Google Scholar]
- 72.Yao L, Bhatta A, Xu Z, Chen J, Toque HA, Chen Y, Xu Y, Bagi Z, Lucas R and Huo Y: Obesity-induced vascular inflammation involves elevated arginase activity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 313(5), R560–R571 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatta A, Yao L, Xu Z, Toque HA, Chen J, Atawia RT, Fouda AY, Bagi Z, Lucas R and Caldwell RB: Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovascular Research, 113(13), 1664–1676 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ming XF, Rajapakse AG, Yepuri G, Xiong Y, Carvas JM, Ruffieux J, Scerri I, Wu Z, Popp K and Li J: Arginase II promotes macrophage inflammatory responses through mitochondrial reactive oxygen species, contributing to insulin resistance and atherogenesis. Journal of the American Heart Association, 1(4), e000992 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aguilar-Valles A, Inoue W, Rummel C and Luheshi GN: Obesity, adipokines and neuroinflammation. Neuropharmacology, 96, 124–134 (2015) [DOI] [PubMed] [Google Scholar]
- 76.Shibata R, Ouchi N, Ohashi K and Murohara T: The role of adipokines in cardiovascular disease. Journal of cardiology, 70(4), 329–334 (2017) [DOI] [PubMed] [Google Scholar]
- 77.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C and Fantuzzi G: Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proceedings of the National Academy of Sciences, 97(5), 2367–2372 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L and Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425 (1994) [DOI] [PubMed] [Google Scholar]
- 79.Münzberg H and Morrison CD: Structure, production and signaling of leptin. Metabolism-Clinical and Experimental, 64(1), 13–23 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Friedman JM: Leptin, leptin receptors, and the control of body weight. Nutrition reviews, 56(2), S38 (1998) [DOI] [PubMed] [Google Scholar]
- 81.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML and Taylor SI: Leptin-replacement therapy for lipodystrophy. New England Journal of Medicine, 346(8), 570–578 (2002) [DOI] [PubMed] [Google Scholar]
- 82.Dong M and Ren J: What fans the fire: insights into mechanisms of leptin in metabolic syndrome-associated heart diseases. Current pharmaceutical design, 20(4), 652–658 (2014) [DOI] [PubMed] [Google Scholar]
- 83.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, More KJ and Breitbart RE: Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell, 84(3), 491–495 (1996) [DOI] [PubMed] [Google Scholar]
- 84.Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW and Klein S: Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes, 60(5), 1474–1477 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Francisco V, Pino J, Gonzalez-Gay MA, Mera A, Lago F, Gómez R, Mobasheri A and Gualillo O: Adipokines and inflammation: is it a question of weight? British journal of pharmacology (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myers MG, Cowley MA and Münzberg H: Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol, 70, 537–556 (2008) [DOI] [PubMed] [Google Scholar]
- 87.Bjørbæk C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS and Myers MG: SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. Journal of Biological Chemistry, 275(51), 40649–40657 (2000) [DOI] [PubMed] [Google Scholar]
- 88.Rahmouni K, Correia ML, Haynes WG and Mark AL: Obesity-associated hypertension new insights into mechanisms. Hypertension, 45(1), 9–14 (2005) [DOI] [PubMed] [Google Scholar]
- 89.Bjørbæk C, Elmquist JK, Frantz JD, Shoelson SE and Flier JS: Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular cell, 1(4), 619–625 (1998) [DOI] [PubMed] [Google Scholar]
- 90.Allison MA, Ix JH, Morgan C, McClelland RL, Rifkin D, Shimbo D and Criqui MH: Higher leptin is associated with hypertension: the Multi-Ethnic Study of Atherosclerosis. Journal of human hypertension, 27(10), 617 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng R, Xu C-H, Xu Y-N, Wang Y.-l. and Wang M: Association of leptin levels with pathogenetic risk of coronary heart disease and stroke: a meta-analysis. Arquivos Brasileiros de Endocrinologia & Metabologia, 58(8), 817–823 (2014) [DOI] [PubMed] [Google Scholar]
- 92.Müller G, Ertl J, Gerl M and Preibisch G: Leptin impairs metabolic actions of insulin in isolated rat adipocytes. Journal of Biological Chemistry, 272(16), 10585–10593 (1997) [DOI] [PubMed] [Google Scholar]
- 93.Tanrikulu-Küçük S, Koçak H, Öner-İyidoğan Y, Seyithanoğlu M, Topparmak E and Kayan-Tapan T: Serum fetuin-A and arginase-1 in human obesity model: Is there any interaction between inflammatory status and arginine metabolism? Scandinavian journal of clinical and laboratory investigation, 75(4), 301–307 (2015) [DOI] [PubMed] [Google Scholar]
- 94.Payne GA, Kohr MC and Tune JD: Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. British journal of pharmacology, 165(3), 659–669 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frank S, Stallmeyer B, Kampfer H, Kolb N and Pfeilschifter J: Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest, 106(4), 501–9 (2000) DOI: 10.1172/jci9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kämpfer H, Pfeilschifter J and Frank S: Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. Journal of Investigative Dermatology, 121(6), 1544–1551 (2003) [DOI] [PubMed] [Google Scholar]
- 97.Kaser S, Kaser A, Sandhofer A, Ebenbichler C, Tilg H and Patsch J: Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochemical and biophysical research communications, 309(2), 286–290 (2003) DOI: 10.1016/j.mehy.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 98.Blaslov K, Bulum T and Duvnjak L: The role of endothelial dysfunction driven by adipocitokines in the development and progression of microvascular complications in patients with type 1 and type 2 diabetes. Medical hypotheses, 84(6), 593–595 (2015) DOI: 10.1016/j.mehy.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 99.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A and Scherer PE: Regulation of fasted blood glucose by resistin. Science, 303(5661), 1195–1198 (2004) DOI: 10.1126/science.1092341 [DOI] [PubMed] [Google Scholar]
- 100.Bokarewa M, Nagaev I, Dahlberg L, Smith U and Tarkowski A: Resistin, an adipokine with potent proinflammatory properties. The Journal of Immunology, 174(9), 5789–5795 (2005) DOI: 10.4049/jimmunol.174.9.5789 [DOI] [PubMed] [Google Scholar]
- 101.Steppan CM, Wang J, Whiteman EL, Birnbaum MJ and Lazar MA: Activation of SOCS-3 by resistin. Molecular and cellular biology, 25(4), 1569–1575 (2005) DOI: 10.1128/MCB.25.4.1569-1575.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szulinska M, Musialik K, Suliburska J, Lis I and Bogdanski P: The effect of L-arginine supplementation on serum resistin concentration in insulin resistance in animal models. Eur Rev Med Pharmacol Sci, 18(4), 575–580 (2014) [PubMed] [Google Scholar]
- 103.Juan C, Chang C, Chuang T, Huang S, Kwok C and Ho L: Insulin sensitivity and resistin expression in nitric oxide-deficient rats. Diabetologia, 49(12), 3017–3026 (2006) DOI: 10.1007/s00125-006-0403-4 [DOI] [PubMed] [Google Scholar]
- 104.Lin S-Y, Sheu WH-H, Chen W-Y, Lee F-Y and Huang C-J: Stimulated resistin expression in white adipose of rats with bile duct ligation-induced liver cirrhosis: relationship to cirrhotic hyperinsulinemia and increased tumor necrosis factor-alpha. Molecular and cellular endocrinology, 232(1–2), 1–8 (2005) [DOI] [PubMed] [Google Scholar]
- 105.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF and Spiegelman BM: IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science, 271(5249), 665–8 (1996) DOI: 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 106.Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS and Grinspoon SK: Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. American Journal of Physiology-Endocrinology and Metabolism, 293(1), E102–E109 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS and Grinspoon SK: TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 96(1), E146–E150 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Virdis A, Duranti E, Rossi C, Dell’Agnello U, Santini E, Anselmino M, Chiarugi M, Taddei S and Solini A: Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. European heart journal, 36(13), 784–794 (2014) DOI: 10.1093/eurheartj/ehu072 [DOI] [PubMed] [Google Scholar]
- 109.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM and Zhang C: TNF-α contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arteriosclerosis, thrombosis, and vascular biology, 27(6), 1269–1275 (2007) DOI: 10.1161/ATVBAHA.107.142521 [DOI] [PubMed] [Google Scholar]
- 110.Hector J, Schwarzloh B, Goehring J, Strate T, Hess U, Deuretzbacher G, Hansen-Algenstaedt N, Beil F-U and Algenstaedt P: TNF-α alters visfatin and adiponectin levels in human fat. Hormone and Metabolic Research, 39(04), 250–255 (2007) DOI: 10.1055/s-2007-973075 [DOI] [PubMed] [Google Scholar]
- 111.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V and Gottesman ME: Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. The EMBO journal, 18(17), 4633–4644 (1999) DOI: 10.1093/emboj/18.17.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohapatra J, Sharma M, Acharya A, Pandya G, Chatterjee A, Balaraman R and Jain MR: Retinol-binding protein 4: a possible role in cardiovascular complications. British journal of pharmacology, 164(8), 1939–1948 (2011) DOI: 10.1111/j.1476-5381.2011.01492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Öst A, Danielsson A, Lidén M, Eriksson U, Nystrom FH and Strålfors P: Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. The FASEB journal, 21(13), 3696–3704 (2007) DOI: 10.1096/fj.07-8173com [DOI] [PubMed] [Google Scholar]
- 114.Kraus BJ, Sartoretto JL, Polak P, Hosooka T, Shiroto T, Eskurza I, Lee S-A, Jiang H, Michel T and Kahn BB: Novel role for retinol-binding protein 4 in the regulation of blood pressure. The FASEB Journal, fj. 14–266064 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowland JB, Muta T and Borregaard N: IL-1β-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IκB-ζ. The Journal of Immunology, 176(9), 5559–5566 (2006) DOI: 10.4049/jimmunol.176.9.5559 [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Wu Y, Zhang Y, LeRoith D, Bernlohr DA and Chen X: The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Molecular Endocrinology, 22(6), 1416–1426 (2008) DOI: 10.1210/me.2007-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng L, Xing H, Mao X, Li L, Li X and Li Q: Lipocalin 2 Promotes M1 Macrophages Polarization in a Mouse Cardiac Ischaemia–Reperfusion Injury Model. Scandinavian journal of immunology, 81(1), 31–38 (2015) DOI: 10.1111/sji.12245 [DOI] [PubMed] [Google Scholar]
- 118.Chang S-Y, Kim D-B, Ko S-H, Jang H-J, Jo Y-H and Kim M-J: The level of nitric oxide regulates lipocalin-2 expression under inflammatory condition in RINm5F beta-cells. Biochemical and biophysical research communications, 476(1), 7–14 (2016) DOI: 10.1016/j.bbrc.2016.05.110 [DOI] [PubMed] [Google Scholar]
- 119.Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA and Chen X: Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes, 59(6), 1376–1385 (2010) DOI: 10.2337/db09-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deis JA, Guo H, Wu Y, Liu C, Bernlohr DA and Chen X: Lipocalin 2 regulates retinoic acid-induced activation of beige adipocytes. Journal of Molecular Endocrinology, JME-18-0017 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H and Tazume H: Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell metabolism, 10(3), 178–188 (2009) DOI: 10.1016/j.cmet.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 122.Tian Z, Miyata K, Tazume H, Sakaguchi H, Kadomatsu T, Horio E, Takahashi O, Komohara Y, Araki K and Hirata Y: Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. Journal of molecular and cellular cardiology, 57, 1–12 (2013) DOI: 10.1016/j.yjmcc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 123.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M and Tabata M: Role of Endothelial Cell–Derived Angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arteriosclerosis, thrombosis, and vascular biology, 34(4), 790–800 (2014) DOI: 10.1161/ATVBAHA.113.303116 [DOI] [PubMed] [Google Scholar]
- 124.Kitazawa M, Nagano M, Masumoto K.-h., Shigeyoshi Y, Natsume T and Hashimoto S: Angiopoietin-like 2, a circadian gene, improves type 2 diabetes through potentiation of insulin sensitivity in mice adipocytes. Endocrinology, 152(7), 2558–2567 (2011) DOI: 10.1210/en.2010-1407 [DOI] [PubMed] [Google Scholar]
- 125.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C and Townsend RR: Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell metabolism, 6(5), 363–375 (2007) DOI: 10.1016/j.cmet.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T and Kiess W: Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nature Reviews Endocrinology, 11(9), 535–546 (2015) DOI: 10.1038/nrendo.2015.117 [DOI] [PubMed] [Google Scholar]
- 127.Wang T, Zhang X, Bheda P, Revollo JR, Imai S.-i. and Wolberger C: Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nature Structural and Molecular Biology, 13(7), 661 (2006) DOI: 10.1038/nsmb1114 [DOI] [PubMed] [Google Scholar]
- 128.Yoshino J, Mills KF, Yoon MJ and Imai S.-i.: Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell metabolism, 14(4), 528–536 (2011) DOI: 10.1016/j.cmet.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Olszanecka-Glinianowicz M, Kocełak P, Nylec M, Chudek J and Zahorska-Markiewicz B: Circulating visfatin level and visfatin/insulin ratio in obese women with metabolic syndrome. Arch Med Sci, 8(2), 214–218 (2012) DOI: 10.5114/aoms.2012.28547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panidis D, Farmakiotis D, Rousso D, Katsikis I, Delkos D, Piouka A, Gerou S and Diamanti-Kandarakis E: Plasma visfatin levels in normal weight women with polycystic ovary syndrome. European journal of internal medicine, 19(6), 406–412 (2008) DOI: 10.1016/j.ejim.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 131.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H and Tilg H: Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. The Journal of Immunology, 178(3), 1748–1758 (2007) DOI: 10.4049/jimmunol.178.3.1748 [DOI] [PubMed] [Google Scholar]
- 132.Kusku-Kiraz Z, Genc S, Bekpinar S, Unlucerci Y, Olgac V, Uysal M and Gurdol F: Circulating levels of apelin, glucagon-like peptide and visfatin in hypercholesterolemic–hyperhomocysteinemic guinea-pigs: their relation with NO metabolism. Molecular and cellular biochemistry, 400(1–2), 69–75 (2015) [DOI] [PubMed] [Google Scholar]
- 133.Imai S and Yoshino J: The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab, 15 Suppl 3, 26–33 (2013) DOI: 10.1111/dom.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borradaile NM and Pickering JG: NAD+, sirtuins, and cardiovascular disease. Current pharmaceutical design, 15(1), 110–117 (2009) DOI: 10.2174/138161209787185742 [DOI] [PubMed] [Google Scholar]
- 135.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y and Walsh K: Obesity, adiponectin and vascular inflammatory disease. Current opinion in lipidology, 14(6), 561–566 (2003) DOI: 10.1097/00041433-200312000-00003 [DOI] [PubMed] [Google Scholar]
- 136.Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y and Funahashi T: Adiponectin as a biomarker of the metabolic syndrome. Circulation Journal, 68(11), 975–981 (2004) DOI: 10.1253/circj.68.975 [DOI] [PubMed] [Google Scholar]
- 137.Li S, Shin HJ, Ding EL and van Dam RM: Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama, 302(2), 179–188 (2009) DOI: 10.1001/jama.2009.976 [DOI] [PubMed] [Google Scholar]
- 138.Kadowaki T, Yamauchi T and Kubota N: The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS letters, 582(1), 74–80 (2008) DOI: 10.1016/j.febslet.2007.11.070 [DOI] [PubMed] [Google Scholar]
- 139.Yamauchi T, Kamon J, Minokoshi Y. a., Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S and Ueki K: Adiponectin stimulates glucose utilization and fattyacid oxidation by activating AMP-activated protein kinase. Nature medicine, 8(11), 1288–1295 (2002) DOI: 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 140.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K and Tsunoda M: Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature, 423(6941), 762–769 (2003) DOI: 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- 141.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R and Matsuda M: Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes, 56(4), 901–911 (2007) DOI: 10.2337/db06-0911 [DOI] [PubMed] [Google Scholar]
- 142.Kim J-Y, Van De Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H and Li G: Obesity-associated improvements in metabolic profile through expansion of adipose tissue. The Journal of clinical investigation, 117(9), 2621–2637 (2007) DOI: 10.1172/JCI31021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu G-Z, Liang B, Lau WB, Wang Y, Zhao J, Li R, Wang X, Yuan Y, Lopez BL and Christopher TA: High glucose/High Lipids impair vascular adiponectin function via inhibition of caveolin-1/AdipoR1 signalsome formation. Free Radical Biology and Medicine, 89, 473–485 (2015) DOI: 10.1016/j.freeradbiomed.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamaguchi N, Argueta JGM, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S and Yamashita Y: Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS letters, 579(30), 6821–6826 (2005) DOI: 10.1016/j.febslet.2005.11.019 [DOI] [PubMed] [Google Scholar]
- 145.Razny U, Kiec-Wilk B, Wator L, Polus A, Dyduch G, Solnica B, Malecki M, Tomaszewska R, Cooke JP and Dembinska-Kiec A: Increased nitric oxide availability attenuates high-fat diet metabolic alterations and gene expression associated with insulin resistance. Cardiovascular diabetology, 10(1), 68 (2011) DOI: 10.1186/1475-2840-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koh EH, Kim M, Ranjan K, Kim HS, Park H-S, Oh KS, Park I-S, Lee WJ, Kim M-S and Park J-Y: eNOS plays a major role in adiponectin synthesis in adipocytes. American Journal of Physiology-Endocrinology and Metabolism, 298(4), E846–E853 (2010) [DOI] [PubMed] [Google Scholar]
- 147.Xi W, Satoh H, Kase H, Suzuki K and Hattori Y: Stimulated HSP90 binding to eNOS and activation of the PI3–Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochemical and biophysical research communications, 332(1), 200–205 (2005) DOI: 10.1016/j.bbrc.2005.04.111 [DOI] [PubMed] [Google Scholar]
- 148.Hattori Y, Suzuki M, Hattori S and Kasai K: Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia, 46(11), 1543–1549 (2003) DOI: 10.1007/s00125-003-1224-3 [DOI] [PubMed] [Google Scholar]
- 149.Motoshima H, Wu X, Mahadev K and Goldstein BJ: Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochemical and biophysical research communications, 315(2), 264–271 (2004) DOI: 10.1016/j.bbrc.2004.01.049 [DOI] [PubMed] [Google Scholar]
- 150.Liu C, Rajapakse AG, Riedo E, Fellay B, Bernhard M-C, Montani J-P, Yang Z and Ming X-F: Targeting arginase-II protects mice from high-fat-diet-induced hepatic steatosis through suppression of macrophage inflammation. Scientific reports, 6, 20405 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang R-Z, Lee M-J, Hu H, Pray J, Wu H-B, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC and Gong D-W: Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. American Journal of Physiology-Endocrinology and Metabolism, 290(6), E1253–E1261 (2006) [DOI] [PubMed] [Google Scholar]
- 152.de Souza Batista CM, Yang R-Z, Lee M-J, Glynn NM, Yu D-Z, Pray J, Ndubuizu K, Patil S, Schwartz A and Kligman M: Omentin plasma levels and gene expression are decreased in obesity. Diabetes, 56(6), 1655–1661 (2007)DOI: 10.2337/db06-1506 [DOI] [PubMed] [Google Scholar]
- 153.Brunetti L, Orlando G, Ferrante C, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F and Ricciuti A: Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides, 44, 66–74 (2013) DOI: 10.1016/j.peptides.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 154.Nway NC, Sitticharoon C, Chatree S and Maikaew P: Correlations between the expression of the insulin sensitizing hormones, adiponectin, visfatin, and omentin, and the appetite regulatory hormone, neuropeptide Y and its receptors in subcutaneous and visceral adipose tissues. Obesity research & clinical practice, 10(3), 256–263 (2016) DOI: 10.1016/j.orcp.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 155.Herder C, Ouwens DM, Carstensen M, Kowall B, Huth C, Meisinger C, Rathmann W, Roden M and Thorand B: Adiponectin may mediate the association between omentin, circulating lipids and insulin sensitivity: results from the KORA F4 study. European Journal of Endocrinology, 172(4), 423–432 (2015) DOI: 10.1530/EJE-14-0879 [DOI] [PubMed] [Google Scholar]
- 156.Yamawaki H, Tsubaki N, Mukohda M, Okada M and Hara Y: Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochemical and biophysical research communications 393(4), 668–672 (2010) DOI: 10.1016/j.bbrc.2010.02.053 [DOI] [PubMed] [Google Scholar]
- 157.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M and Hara Y: Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochemical and biophysical research communications 408(2), 339–343 (2011) DOI: 10.1016/j.bbrc.2011.04.039 [DOI] [PubMed] [Google Scholar]
- 158.Kazama K, Usui T, Okada M, Hara Y and Yamawaki H: Omentin plays an anti-inflammatory role through inhibition of TNF-alpha-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol, 686(1–3), 116–23 (2012) DOI: 10.1016/j.ejphar.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 159.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC and Wong GW: C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. Journal of Biological Chemistry 287(13), 10301–10315 (2012) DOI: 10.1074/jbc.M111.303651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T and Ouchi N: Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. Journal of Biological Chemistry, 286(40), 34552–34558 (2011) DOI: 10.1074/jbc.M111.277319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C and von Zglinicki T: A senescent cell bystander effect: senescence-induced senescence. Aging Cell, 11(2), 345–9 (2012) DOI: 10.1111/j.1474-9726.2012.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Deursen JM: The role of senescent cells in ageing. Nature, 509(7501), 439–446 (2014) DOI: 10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J, 22(16), 4212–22 (2003) DOI: 10.1093/emboj/cdg417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD and Kirkland JL: Fat tissue, aging, and cellular senescence. Aging cell, 9(5), 667–684 (2010) DOI: 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bogdanski P, Suliburska J, Grabanska K, Musialik K, Cieslewicz A, Skoluda A and Jablecka A: Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur Rev Med Pharmacol Sci, 16(6), 816–23 (2012) [PubMed] [Google Scholar]
- 166.Schafer MJ, Miller JD and LeBrasseur NK: Cellular senescence: Implications for metabolic disease. Mol Cell Endocrinol, 455, 93–102 (2017)DOI: 10.1016/j.mce.2016.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tchkonia T, Zhu Y, van Deursen J, Campisi J and Kirkland JL: Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest, 123(3), 966–72 (2013) DOI: 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sell H, Dietze-Schroeder D, Kaiser U and Eckel J: Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology, 147(5), 2458–67 (2006) DOI: 10.1210/en.2005-0969 [DOI] [PubMed] [Google Scholar]
- 169.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M and Kirkland JL: Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes, 64(7), 2289–98 (2015) DOI: 10.2337/db14-1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL and Bouloumie A: Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes, 59(11), 2755–63 (2010) DOI: 10.2337/db10-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Briot A, Decaunes P, Volat F, Belles C, Coupaye M, Ledoux S and Bouloumie A: Senescence Alters PPARgamma (Peroxisome Proliferator-Activated Receptor Gamma)-Dependent Fatty Acid Handling in Human Adipose Tissue Microvascular Endothelial Cells and Favors Inflammation. Arterioscler Thromb Vasc Biol, 38(5), 1134–1146 (2018) DOI: 10.1161/ATVBAHA.118.310797 [DOI] [PubMed] [Google Scholar]
- 172.Mehrotra D, Wu J, Papangeli I and Chun HJ: Endothelium as a gatekeeper of fatty acid transport. Trends Endocrinol Metab, 25(2), 99–106 (2014) DOI: 10.1016/j.tem.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N and Sano M: Obesity accelerates T cell senescence in murine visceral adipose tissue. The Journal of Clinical Investigation, 126(12), 4626–4639 (2016) DOI: 10.1172/JCI88606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yan X, Sano M, Lu L, Wang W, Zhang Q, Zhang R, Wang L, Chen Q, Fukuda K and Shen W: Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol, 9, 70 (2010) DOI: 10.1186/1475-2840-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trifari S, Kaplan CD, Tran EH, Crellin NK and Spits H: Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol, 10(8), 864–71 (2009) DOI: 10.1038/ni.1770 [DOI] [PubMed] [Google Scholar]
- 176.Miranda DN, Coletta DK, Mandarino LJ and Shaibi GQ: Increases in insulin sensitivity among obese youth are associated with gene expression changes in whole blood. Obesity, 22(5), 1337–1344 (2014) DOI: 10.1002/oby.20711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB and Spiegelman BM: Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. The Journal of clinical investigation, 101(1), 1–9 (1998) DOI: 10.1172/JCI1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H and Bashan N: Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes, 47(10), 1562–1569 (1998) DOI: 10.2337/diabetes.47.10.1562 [DOI] [PubMed] [Google Scholar]
- 179.Tirosh A, Potashnik R, Bashan N and Rudich A: Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-Kinase in 3T3-L1 adipocytes A Putative Cellular Mechanism For Impaired Protein Kinase B activation and glut4 translocation. Journal of Biological Chemistry, 274(15), 10595–10602 (1999) DOI: 10.1074/jbc.274.15.10595 [DOI] [PubMed] [Google Scholar]
- 180.Maddux BA, See W, Lawrence JC, Goldfine AL, Goldfine ID and Evans JL: Protection Against Oxidative Stress—Induced Insulin Resistance in Rat L6 Muscle Cells by Micromolar Concentrations of α-Lipoic Acid. Diabetes, 50(2), 404–410 (2001) DOI: 10.2337/diabetes.50.2.404 [DOI] [PubMed] [Google Scholar]
- 181.Al-Goblan AS, Al-Alfi MA and Khan MZ: Mechanism linking diabetes mellitus and obesity. Diabetes, metabolic syndrome and obesity: targets and therapy, 7, 587 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thompson JR and Wu G: The effect of ketone bodies on nitrogen metabolism in skeletal muscle. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 100(2), 209–216 (1991) DOI: 10.1016/0305-0491(91)90363-I [DOI] [PubMed] [Google Scholar]
- 183.Asmat U, Abad K and Ismail K: Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J, 24(5), 547–553 (2016) DOI: 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kasuga M: Insulin resistance and pancreatic β cell failure. The Journal of Clinical Investigation, 116(7), 1756–1760 (2006) DOI: 10.1172/JCI29189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN and O’Rourke RW: Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring), 24(3), 597–605 (2016) DOI: 10.1002/oby.21377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Veilleux A, Blouin K, Rhéaume C, Daris M, Marette A and Tchernof A: Glucose transporter 4 and insulin receptor substrate–1 messenger RNA expression in omental and subcutaneous adipose tissue in women. Metabolism, 58(5), 624–631 (2009) DOI: 10.1016/j.metabol.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 187.Carvalho E, Jansson P-A, Nagaev I, Wenthzel A-M and Smith U: Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. The FASEB Journal, 15(6), 1101–1103 (2001) DOI: 10.1096/fj.00-0435fje [DOI] [PubMed] [Google Scholar]
- 188.Krieger-Brauer H and Kather H: Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochemical Journal, 307(Pt 2), 549 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Krieger-Brauer H and Kather H: Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. The Journal of clinical investigation, 89(3), 1006–1013 (1992) DOI: 10.1172/JCI115641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roy D, Perreault M and Marette A: Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. American Journal of Physiology-Endocrinology And Metabolism, 274(4), E692–E699 (1998) [DOI] [PubMed] [Google Scholar]
- 191.Bergandi L, Silvagno F, Russo I, Riganti C, Anfossi G, Aldieri E, Ghigo D, Trovati M and Bosia A: Insulin stimulates glucose transport via nitric oxide/cyclic GMP pathway in human vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology, 23(12), 2215–2221 (2003) DOI: 10.1161/01.ATV.0000107028.20478.8e [DOI] [PubMed] [Google Scholar]
- 192.Rudyk O and Eaton P: Examining a role for PKG Ialpha oxidation in the pathogenesis of cardiovascular dysfunction during diet-induced obesity. Free Radic Biol Med, 110, 390–398 (2017) DOI: 10.1016/j.freeradbiomed.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Silva JF, Correa IC, Diniz TF, Lima PM, Santos RL, Cortes SF, Coimbra CC and Lemos VS: Obesity, Inflammation, and Exercise Training: Relative Contribution of iNOS and eNOS in the Modulation of Vascular Function in the Mouse Aorta. Front Physiol, 7, 386 (2016) DOI: 10.3389/fphys.2016.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Butler R, Morris A and Struthers A: Systemic nitric oxide synthase inhibition increases insulin sensitivity in man. Clinical Science, 94(2), 175–180 (1998) DOI: 10.1042/cs0940175 [DOI] [PubMed] [Google Scholar]
- 195.McGrowder D, Ragoobirsingh D and Dasgupta T: Effects of S-nitroso-N-acetyl-penicillamine administration on glucose tolerance and plasma levels of insulin and glucagon in the dog. Nitric Oxide, 5(4), 402–412 (2001) DOI: 10.1006/niox.2001.0360 [DOI] [PubMed] [Google Scholar]
- 196.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C and Nair KS: Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. American Journal of Physiology-Endocrinology and Metabolism, 291(4), E729–E736 (2006) DOI: 10.1152/ajpendo.00003.2006 [DOI] [PubMed] [Google Scholar]
- 197.Srikanthan P and Karlamangla AS: Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab, 96(9), 2898–903 (2011) DOI: 10.1210/jc.2011-0435 [DOI] [PubMed] [Google Scholar]
- 198.Brandt C, Nielsen AR, Fischer CP, Hansen J, Pedersen BK and Plomgaard P: Plasma and muscle myostatin in relation to type 2 diabetes. PLoS One, 7(5), e37236 (2012) DOI: 10.1371/journal.pone.0037236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Consitt LA and Clark BC: The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J Frailty Aging, 7(1), 21–27 (2018) DOI: 10.14283/jfa.2017.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chevalier S. p., Marliss EB, Morais JA, Lamarche M and Gougeon R. j.: Whole-body protein anabolic response is resistant to the action of insulin in obese women. The American Journal of Clinical Nutrition, 82(2), 355–365 (2005) DOI: 10.1093/ajcn/82.2.355 [DOI] [PubMed] [Google Scholar]
- 201.Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier J-F, Giraudet C, Patrac V, Bertrand-Michel J, Migne C, Collin M-L, Chardigny J-M, Boirie Y and Walrand S: Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell, 13(6), 1001–1011 (2014) DOI: 10.1111/acel.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ and Sinacore DR: Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther, 88(11), 1336–44 (2008) DOI: 10.2522/ptj.20080079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dodd KM and Tee AR: Leucine and mTORC1: a complex relationship. American Journal of Physiology-Endocrinology and Metabolism, 302(11), E1329–E1342 (2012) DOI: 10.1152/ajpendo.00525.2011 [DOI] [PubMed] [Google Scholar]
- 204.Kitsy A, Carney S, Vivar JC, Knight MS, Pointer MA, Gwathmey JK and Ghosh S: Effects of leucine supplementation and serum withdrawal on branched-chain amino acid pathway gene and protein expression in mouse adipocytes. PloS one, 9(7), e102615 (2014) DOI: 10.1371/journal.pone.0102615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ and Hutson SM: Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell metabolism, 6(3), 181–194 (2007) DOI: 10.1016/j.cmet.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F and Guo F: Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes, 59(1), 17–25 (2010) DOI: 10.2337/db09-0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E and Holm C: Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. Journal of Biological Chemistry, 273(1), 215–221 (1998) DOI: 10.1074/jbc.273.1.215 [DOI] [PubMed] [Google Scholar]
- 208.Eckel RH: Insulin resistance: an adaptation for weight maintenance. The Lancet, 340(8833), 1452–1453 (1992) DOI: 10.1016/0140-6736(92)92633-Q [DOI] [PubMed] [Google Scholar]
- 209.Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes, 46(1), 3–10 (1997) DOI: 10.2337/diab.46.1.3 [DOI] [PubMed] [Google Scholar]
- 210.Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Eric Paulson K and Greenberg AS: TNF-α induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. Journal of cellular biochemistry, 89(6), 1077–1086 (2003) DOI: 10.1002/jcb.10565 [DOI] [PubMed] [Google Scholar]
- 211.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Møller K, Saltin B and Febbraio MA: Interleukin-6 stimulates lipolysis and fat oxidation in humans. The Journal of Clinical Endocrinology & Metabolism, 88(7), 3005–3010 (2003) DOI: 10.1210/jc.2002-021687 [DOI] [PubMed] [Google Scholar]
- 212.Strawford A, Antelo F, Christiansen M and Hellerstein M: Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. American Journal of Physiology-Endocrinology and Metabolism, 286(4), E577–E588 (2004) [DOI] [PubMed] [Google Scholar]
- 213.Larter CZ, Yeh MM, Van Rooyen DM, Teoh NC, Brooling J, Hou JY, Williams J, Clyne M, Nolan CJ and Farrell GC: Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice. Journal of gastroenterology and hepatology, 24(10), 1658–1668 (2009) DOI: 10.1111/j.1440-1746.2009.05996.x [DOI] [PubMed] [Google Scholar]
- 214.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E and Jensen MD: Subcutaneous adipocyte size and body fat distribution–. The American journal of clinical nutrition, 87(1), 56–63 (2008) DOI: 10.1093/ajcn/87.1.56 [DOI] [PubMed] [Google Scholar]
- 215.Haczeyni F, Barn V, Mridha AR, Yeh MM, Estevez E, Febbraio MA, Nolan CJ, Bell-Anderson KS, Teoh NC and Farrell GC: Exercise improves adipose function and inflammation and ameliorates fatty liver disease in obese diabetic mice. Obesity, 23(9), 1845–1855 (2015) DOI: 10.1002/oby.21170 [DOI] [PubMed] [Google Scholar]
- 216.Kersten S: Physiological regulation of lipoprotein lipase. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1841(7), 919–933 (2014) DOI: 10.1016/j.bbalip.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 217.Greenwood M: The relationship of enzyme activity to feeding behavior in rats: lipoprotein lipase as the metabolic gatekeeper. International journal of obesity, 9, 67–70 (1985) [PubMed] [Google Scholar]
- 218.Wang H and Eckel RH: Lipoprotein lipase: from gene to obesity. American Journal of Physiology-Endocrinology and Metabolism, 297(2), E271–E288 (2009) [DOI] [PubMed] [Google Scholar]
- 219.Merkel M, Heeren J, Dudeck W, Rinninger F, Radner H, Breslow JL, Goldberg IJ, Zechner R and Greten H: Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem, 277(9), 7405–11 (2002) DOI: 10.1074/jbc.M107914200 [DOI] [PubMed] [Google Scholar]
- 220.Goldberg JI, Eckel H Robert, Abumrad, A. Nada: Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. Journal of Lipid Research, 50:(Supplement), S86–S90 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W and Goldberg IJ: Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proceedings of the National Academy of Sciences, 98(13), 7522–7527 (2001) DOI: 10.1073/pnas.121164498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang H, Knaub LA, Jensen DR, Jung DY, Hong E-G, Ko H-J, Coates AM, Goldberg IJ, Becky A and Janssen RC: Skeletal muscle–specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes, 58(1), 116–124 (2009) DOI: 10.2337/db07-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Walton RG, Zhu B, Unal R, Spencer M, Sunkara M, Morris AJ, Charnigo R, Katz WS, Daugherty A and Howatt DA: Increasing adipocyte lipoprotein lipase improves glucose metabolism in high-fat diet-induced obesity. Journal of Biological Chemistry, 290(18), 11547–11556 (2015) DOI: 10.1074/jbc.M114.628487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Uchida Y, Tsukahara F, Ohba K.-i., Ogawa A, Irie K, Fujii E, Yoshimoto T, Yoshioka T and Muraki T: Nitric oxide mediates down regulation of lipoprotein lipase activity induced by tumor necrosis factor-α in brown adipocytes. European journal of pharmacology, 335(2–3), 235–243 (1997) [DOI] [PubMed] [Google Scholar]
- 225.Yan H, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M and Lau DC: Nitric oxide promotes differentiation of rat white preadipocytes in culture. Journal of lipid research, 43(12), 2123–2129 (2002) DOI: 10.1194/jlr.M200305-JLR200 [DOI] [PubMed] [Google Scholar]
- 226.Muoio DM and Neufer PD: Lipid-induced mitochondrial stress and insulin action in muscle. Cell metabolism, 15(5), 595–605 (2012) DOI: 10.1016/j.cmet.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kusminski CM and Scherer PE: Mitochondrial dysfunction in white adipose tissue. Trends in endocrinology & metabolism, 23(9), 435–443 (2012) DOI: 10.1016/j.tem.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH and Woychik R: TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Molecular cell, 61(2), 199–209 (2016) DOI: 10.1016/j.molcel.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Duchen MR: Mitochondria and calcium: from cell signalling to cell death. The Journal of physiology, 529(1), 57–68 (2000) DOI: 10.1111/j.1469-7793.2000.00057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M and Corvera S: Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Molecular and cellular biology, 23(3), 1085–1094 (2003) DOI: 10.1128/MCB.23.3.1085-1094.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Boubekeur S, Bunoust O, Camougrand N, Castroviejo M, Rigoulet M and Guérin B: A Mitochondrial Pyruvate Dehydrogenase Bypass in the YeastSaccharomyces cerevisiae. Journal of Biological Chemistry, 274(30), 21044–21048 (1999) DOI: 10.1074/jbc.274.30.21044 [DOI] [PubMed] [Google Scholar]
- 232.Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernández-Fernández C and Mouriño-Bayolo D: Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion (2018) [DOI] [PubMed] [Google Scholar]
- 233.Brosnan JT and Brosnan ME: Branched-chain amino acids: enzyme and substrate regulation. The Journal of nutrition, 136(1), 207S–211S (2006) [DOI] [PubMed] [Google Scholar]
- 234.DiMauro S and Schon EA: Mitochondrial respiratory-chain diseases. New England Journal of Medicine, 348(26), 2656–2668 (2003) DOI: 10.1056/NEJMra022567 [DOI] [PubMed] [Google Scholar]
- 235.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S and Clementi E: Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proceedings of the National Academy of Sciences of the United States of America, 101(47), 16507–16512 (2004) DOI: 10.1073/pnas.0405432101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jobgen WS, Fried SK, Fu WJ, Meininger CJ and Wu G: Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. The Journal of nutritional biochemistry, 17(9), 571–588 (2006) DOI: 10.1016/j.jnutbio.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 237.Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, Pagotto U, Carruba MO, Vettor R and Nisoli E: Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gao C-L, Zhu C, Zhao Y-P, Chen X-H, Ji C-B, Zhang C-M, Zhu J-G, Xia Z-K, Tong M-L and Guo X-R: Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Molecular and cellular endocrinology, 320(1–2), 25–33 (2010) [DOI] [PubMed] [Google Scholar]
- 239.Hardy OT, Czech MP and Corvera S: What causes the insulin resistance underlying obesity? Current opinion in endocrinology, diabetes, and obesity, 19(2), 81 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Talior I, Yarkoni M, Bashan N and Eldar-Finkelman H: Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin-resistant mice. American Journal of Physiology-Endocrinology And Metabolism, 285(2), E295–E302 (2003) [DOI] [PubMed] [Google Scholar]
- 241.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B and Li W: The hyperglycemia-induced inflammatory response in adipocytes the role of reactive oxygen species. Journal of Biological Chemistry, 280(6), 4617–4626 (2005) DOI: 10.1074/jbc.M411863200 [DOI] [PubMed] [Google Scholar]
- 242.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS and Beal MF: Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. Journal of Neuroscience, 24(36), 7779–7788 (2004) DOI: 10.1523/JNEUROSCI.1899-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL and Brand MD: Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox biology, 1(1), 304–312 (2013) DOI: 10.1016/j.redox.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR and Brand MD: Mitochondrial proton and electron leaks. Essays in biochemistry, 47, 53–67 (2010) DOI: 10.1042/bse0470053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J and Wieckowski MR: Relation between mitochondrial membrane potential and ROS formation In: Mitochondrial Bioenergetics. Springer, (2012) [DOI] [PubMed] [Google Scholar]
- 246.Cheng J, Nanayakkara G, Shao Y, Cueto R, Wang L, Yang WY, Tian Y, Wang H and Yang X: Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases In: Mitochondrial Dynamics in Cardiovascular Medicine. Springer, (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang T, Si Y, Shirihai OS, Si H, Schultz V, Corkey RF, Hu L, Deeney JT, Guo W and Corkey BE: Respiration in adipocytes is inhibited by reactive oxygen species. Obesity, 18(8), 1493–1502 (2010) DOI: 10.1038/oby.2009.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW and Cooney GJ: Insulin resistance is a cellular antioxidant defense mechanism. Proceedings of the National Academy of Sciences, 106(42), 17787–17792 (2009) DOI: 10.1073/pnas.0902380106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Grivennikova VG and Vinogradov AD: Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta, 1757(5–6), 553–61 (2006) DOI: 10.1016/j.bbabio.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 250.Vernochet C, Mourier A, Bezy O, Macotela Y, Boucher J, Rardin MJ, An D, Lee KY, Ilkayeva OR and Zingaretti CM: Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell metabolism, 16(6), 765–776 (2012) DOI: 10.1016/j.cmet.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Roy M, Reddy PH, Iijima M and Sesaki H: Mitochondrial division and fusion in metabolism. Curr Opin Cell Biol, 33, 111–8 (2015) DOI: 10.1016/j.ceb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Otera H and Mihara K: Molecular mechanisms and physiologic functions of mitochondrial dynamics. The Journal of Biochemistry, 149(3), 241–251 (2011) DOI: 10.1093/jb/mvr002 [DOI] [PubMed] [Google Scholar]
- 253.Losón OC, Song Z, Chen H and Chan DC: Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Molecular biology of the cell, 24(5), 659–667 (2013) DOI: 10.1091/mbc.e12-10-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kim I, Rodriguez-Enriquez S and Lemasters JJ: Selective degradation of mitochondria by mitophagy. Archives of biochemistry and biophysics, 462(2), 245–253 (2007) DOI: 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen H and Chan DC: Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet, 18(R2), R169–76 (2009) DOI: 10.1093/hmg/ddp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S and Las G: Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal, 27(2), 433–446 (2008) DOI: 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pickles S, Vigié P and Youle RJ: Mitophagy and quality control mechanisms in mitochondrial maintenance. Current Biology, 28(4), R170–R185 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ding WX and Yin XM: Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem, 393(7), 547–64 (2012) DOI: 10.1515/hsz-2012-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Youle RJ and Narendra DP: Mechanisms of mitophagy. Nature reviews Molecular cell biology, 12(1), 9 (2011) DOI: 10.1038/nrm3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Youle RJ and van der Bliek AM: Mitochondrial fission, fusion, and stress. Science, 337(6098), 1062–5 (2012) DOI: 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Dériaz O and Zorzano A: Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. The Journal of physiology, 567(1), 349–358 (2005) DOI: 10.1113/jphysiol.2005.092031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Soriano FX, Liesa M, Bach D, Chan DC, Palacín M and Zorzano A: Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator–activated receptor-γ coactivator-1α, estrogen-related receptor-α, and mitofusin 2. Diabetes, 55(6), 1783–1791 (2006) DOI: 10.2337/db05-0509 [DOI] [PubMed] [Google Scholar]
- 263.Fealy CE, Mulya A, Lai N and Kirwan JP: Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. Journal of Applied Physiology, 117(3), 239–245 (2014) DOI: 10.1152/japplphysiol.01064.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liesa M and Shirihai OS: Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell metabolism, 17(4), 491–506 (2013) DOI: 10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pisani DF, Barquissau V, Chambard J-C, Beuzelin D, Ghandour RA, Giroud M, Mairal A, Pagnotta S, Cinti S and Langin D: Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Molecular metabolism (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kulkarni SS, Joffraud M, Boutant M, Ratajczak J, Gao AW, Maclachlan C, Hernandez-Alvarez MI, Raymond F, Metairon S and Descombes P: Mfn1 deficiency in the liver protects against diet-induced insulin resistance and enhances the hypoglycemic effect of metformin. Diabetes, db151725 (2016) [DOI] [PubMed] [Google Scholar]
- 267.Cang X, Wang X, Liu P, Wu X, Yan J, Chen J, Wu G, Jin Y, Xu F and Su J: PINK1 alleviates palmitate induced insulin resistance in HepG2 cells by suppressing ROS mediated MAPK pathways. Biochemical and biophysical research communications, 478(1), 431–438 (2016) DOI: 10.1016/j.bbrc.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 268.Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D and Chu CT: Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. Journal of Biological Chemistry (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Exner N, Treske B, Paquet D, Holmström K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken H-H and Gasser T: Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. Journal of Neuroscience, 27(45), 12413–12418 (2007) DOI: 10.1523/JNEUROSCI.0719-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cerqueira FM, Chausse B, Baranovski BM, Liesa M, Lewis EC, Shirihai OS and Kowaltowski AJ: Diluted serum from calorie-restricted animals promotes mitochondrial β-cell adaptations and protect against glucolipotoxicity. The FEBS journal, 283(5), 822–833 (2016) DOI: 10.1111/febs.13632 [DOI] [PubMed] [Google Scholar]
- 271.Diaz-Morales N, Rovira-Llopis S, Bañuls C, Escribano-Lopez I, Lopez-Domenech S, Orden S, Roldan-Torres I, Alvarez A, Veses S and Jover A: Are Mitochondrial Fusion and Fission Impaired in Leukocytes of Type 2 Diabetic Patients? Antioxidants & redox signaling, 25(2), 108–115 (2016) [DOI] [PubMed] [Google Scholar]
- 272.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M and Zierath JR: Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism A novel regulatory mechanism altered in obesity. Journal of Biological Chemistry, 278(19), 17190–17197 (2003) DOI: 10.1074/jbc.M212754200 [DOI] [PubMed] [Google Scholar]
- 273.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y and Wallberg-Henriksson H: Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor α and interleukin-6. Diabetes, 54(9), 2685–2693 (2005) DOI: 10.2337/diabetes.54.9.2685 [DOI] [PubMed] [Google Scholar]
- 274.Hernández-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C and Naon D: Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/mitofusin-2 regulatory pathway in response to physical activity. Diabetes care (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Boutant M, Kulkarni SS, Joffraud M, Ratajczak J, Valera-Alberni M, Combe R, Zorzano A and Cantó C: Mfn2 is critical for brown adipose tissue thermogenic function. The EMBO journal, 36(11), 1543–1558 (2017) DOI: 10.15252/embj.201694914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M and Victor VM: Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox biology, 11, 637–645 (2017) DOI: 10.1016/j.redox.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gething M-J and Sambrook J: Protein folding in the cell. Nature, 355(6355), 33 (1992) DOI: 10.1038/355033a0 [DOI] [PubMed] [Google Scholar]
- 278.Ellgaard L, Molinari M and Helenius A: Setting the standards: quality control in the secretory pathway. Science, 286(5446), 1882–1888 (1999) DOI: 10.1126/science.286.5446.1882 [DOI] [PubMed] [Google Scholar]
- 279.Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF and Lavandero S: Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol, 301, 215–90 (2013) DOI: 10.1016/B978-0-12-407704-1.00005-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M and Ron D: Regulated translation initiation controls stress-induced gene expression in mammalian cells. Molecular cell, 6(5), 1099–1108 (2000) DOI: 10.1016/S1097-2765(00)00108-8 [DOI] [PubMed] [Google Scholar]
- 281.Li WW, Alexandre S, Cao X and Lee AS: Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca (2+)-ATPase inhibitor thapsigargin. Journal of Biological Chemistry, 268(16), 12003–12009 (1993) [PubMed] [Google Scholar]
- 282.Yoshida H, Haze K, Yanagi H, Yura T and Mori K: Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins Involvement of basic leucine zipper transcription factors. Journal of Biological Chemistry, 273(50), 33741–33749 (1998) DOI: 10.1074/jbc.273.50.33741 [DOI] [PubMed] [Google Scholar]
- 283.Zhang K and Kaufman RJ: From endoplasmic-reticulum stress to the inflammatory response. Nature, 454(7203), 455 (2008) DOI: 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cnop M, Foufelle F and Velloso LA: Endoplasmic reticulum stress, obesity and diabetes. Trends in molecular medicine, 18(1), 59–68 (2012) DOI: 10.1016/j.molmed.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 285.Jiao P, Ma J, Feng B, Zhang H, Alan-Diehl J, Eugene-Chin Y, Yan W and Xu H: FFA-Induced Adipocyte Inflammation and Insulin Resistance: Involvement of ER Stress and IKKβ Pathways. Obesity, 19(3), 483–491 (2011) DOI: 10.1038/oby.2010.200 [DOI] [PubMed] [Google Scholar]
- 286.Malhotra JD and Kaufman RJ: Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling, 9(12), 2277–2294 (2007) [DOI] [PubMed] [Google Scholar]
- 287.Tsujino M, Hirata Y, Imai T, Kanno K, Eguchi S, Ito H and Marumo F: Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiocytes. Circulation, 90(1), 375–383 (1994) DOI: 10.1161/01.CIR.90.1.375 [DOI] [PubMed] [Google Scholar]
- 288.Griendling KK, Sorescu D and Ushio-Fukai M: NAD (P) H oxidase: role in cardiovascular biology and disease. Circulation research, 86(5), 494–501 (2000) DOI: 10.1161/01.RES.86.5.494 [DOI] [PubMed] [Google Scholar]
- 289.Xue X, Piao J-H, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H and Nakano H: Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. Journal of Biological Chemistry, 280(40), 33917–33925 (2005) DOI: 10.1074/jbc.M505818200 [DOI] [PubMed] [Google Scholar]
- 290.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P and Merali S: Increase in endoplasmic reticulum stress–related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes, 57(9), 2438–2444 (2008) DOI: 10.2337/db08-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gregor MF and Hotamisligil GS: Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. Journal of lipid research, 48(9), 1905–1914 (2007) DOI: 10.1194/jlr.R700007-JLR200 [DOI] [PubMed] [Google Scholar]
- 292.Kawasaki N, Asada R, Saito A, Kanemoto S and Imaizumi K: Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Scientific reports, 2, 799 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS and Klein S: Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes, 58(3), 693–700 (2009) DOI: 10.2337/db08-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zha BS and Zhou H: ER stress and lipid metabolism in adipocytes. Biochemistry research international, 2012 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S and Yang L: The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nature immunology, 18(5), 519 (2017) DOI: 10.1038/ni.3709 [DOI] [PubMed] [Google Scholar]
- 296.Liu VW and Huang PL: Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovascular research, 77(1), 19–29 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A and Schwartz MW: Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arteriosclerosis, thrombosis, and vascular biology, 28(11), 1982–1988 (2008) DOI: 10.1161/ATVBAHA.108.169722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Huang PL: eNOS, metabolic syndrome and cardiovascular disease. Trends in Endocrinology & Metabolism, 20(6), 295–302 (2009) DOI: 10.1016/j.tem.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Handa P, Tateya S, Rizzo NO, Cheng AM, Morgan-Stevenson V, Han C-Y, Clowes AW, Daum G, O’Brien KD and Schwartz MW: Reduced vascular nitric oxide–cGMP signaling contributes to adipose tissue inflammation during highfat feeding. Arteriosclerosis, thrombosis, and vascular biology, 31(12), 2827–2835 (2011) DOI: 10.1161/ATVBAHA.111.236554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Denninger JW and Marletta MA: Guanylate cyclase and the NO/cGMP signaling pathway. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1411(2), 334–350 (1999) DOI: 10.1016/S0005-2728(99)00024-9 [DOI] [PubMed] [Google Scholar]
- 301.Feil R, Lohmann SM, de Jonge H, Walter U and Hofmann F: Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res, 93(10), 907–16 (2003) DOI: 10.1161/01.RES.0000100390.68771.CC [DOI] [PubMed] [Google Scholar]
- 302.Jurrissen TJ, Sheldon RD, Gastecki ML, Woodford ML, Zidon TM, Rector RS, Vieira-Potter VJ and Padilla J: Ablation of eNOS does not promote adipose tissue inflammation. Am J Physiol Regul Integr Comp Physiol, 310(8), R744–51 (2016)DOI: 10.1152/ajpregu.00473.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Colombo G, Colombo MD, Schiavon Lde L and d’Acampora AJ: Phosphodiesterase 5 as target for adipose tissue disorders. Nitric Oxide, 35, 186–92 (2013) DOI: 10.1016/j.niox.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 304.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S and Carruba MO: Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science, 310(5746), 314–7 (2005) DOI: 10.1126/science.1117728 [DOI] [PubMed] [Google Scholar]
- 305.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X and Zhai Q: SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab, 6(4), 307–19 (2007) DOI: 10.1016/j.cmet.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 306.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, Nisoli E and Vettor R: Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes, 63(8), 2800–11 (2014) DOI: 10.2337/db13-1234 [DOI] [PubMed] [Google Scholar]
- 307.Moncada S, Palmer R and Gryglewski RJ: Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proceedings of the National Academy of Sciences, 83(23), 9164–9168 (1986) DOI: 10.1073/pnas.83.23.9164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gryglewski R, Moncada S and Palmer R: Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. British journal of pharmacology, 87(4), 685–694 (1986) DOI: 10.1111/j.1476-5381.1986.tb14586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Palmer RM, Ferrige A and Moncada S: Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature, 327(6122), 524 (1987)DOI: 10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- 310.Melikian N, Seddon MD, Casadei B, Chowienczyk PJ and Shah AM: Neuronal nitric oxide synthase and human vascular regulation. Trends in cardiovascular medicine, 19(8), 256–262 (2009) DOI: 10.1016/j.tcm.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Förstermann U and Sessa WC: Nitric oxide synthases: regulation and function. European heart journal, 33(7), 829–837 (2011) DOI: 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang Q-J, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B and Gale D: Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes, 61(7), 1848–1859 (2012) DOI: 10.2337/db11-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP and Caldwell RB: Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiological Reviews, 98(2), 641–665 (2018) DOI: 10.1152/physrev.00037.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hu H, Moon J, Chung JH, Kim OY, Yu R and Shin MJ: Arginase inhibition ameliorates adipose tissue inflammation in mice with diet-induced obesity. Biochem Biophys Res Commun, 464(3), 840–7 (2015) DOI: 10.1016/j.bbrc.2015.07.048 [DOI] [PubMed] [Google Scholar]
- 315.Navarro A and Boveris A: The mitochondrial energy transduction system and the aging process. American Journal of Physiology-Cell Physiology, 292(2), C670–C686 (2007) [DOI] [PubMed] [Google Scholar]
- 316.Nisoli E, Clementi E, Tonello C, Sciorati C, Briscini L and Carruba MO: Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. British journal of pharmacology, 125(4), 888–894 (1998) DOI: 10.1038/sj.bjp.0702131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Beckman JS and Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. American Journal of Physiology-Cell Physiology, 271(5), C1424–C1437 (1996) [DOI] [PubMed] [Google Scholar]
- 318.Johnson FK, Peyton KJ, Liu XM, Azam MA, Shebib AR, Johnson RA and Durante W: Arginase promotes endothelial dysfunction and hypertension in obese rats. Obesity (Silver Spring), 23(2), 383–90 (2015) DOI: 10.1002/oby.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ and Wu G: Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr, 135(4), 714–21 (2005) DOI: 10.1093/jn/135.4.714 [DOI] [PubMed] [Google Scholar]
- 320.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL and Meininger CJ: Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr, 137(12), 2680–5 (2007) DOI: 10.1093/jn/137.12.2680 [DOI] [PubMed] [Google Scholar]
- 321.Clemmensen C, Madsen AN, Smajilovic S, Holst B and Bräuner-Osborne H: L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino acids, 43(3), 1265–1275 (2012) DOI: 10.1007/s00726-011-1199-1 [DOI] [PubMed] [Google Scholar]
- 322.Monti LD, Setola E, Lucotti PC, Marrocco-Trischitta MM, Comola M, Galluccio E, Poggi A, Mammi S, Catapano AL, Comi G, Chiesa R, Bosi E and Piatti PM: Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab, 14(10), 893–900 (2012) DOI: 10.1111/j.1463-1326.2012.01615.x [DOI] [PubMed] [Google Scholar]
- 323.Moon J, Do HJ, Cho Y and Shin M-J: Arginase inhibition ameliorates hepatic metabolic abnormalities in obese mice. PLoS One, 9(7), e103048 (2014) DOI: 10.1371/journal.pone.0103048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Chung JH, Moon J, Lee YS, Chung H-K, Lee S-M and Shin M-J: Arginase inhibition restores endothelial function in diet-induced obesity. Biochemical and biophysical research communications, 451(2), 179–183 (2014) DOI: 10.1016/j.bbrc.2014.07.083 [DOI] [PubMed] [Google Scholar]
- 325.Bhatta A, Sangani R, Kolhe R, Toque HA, Cain M, Wong A, Howie N, Shinde R, Elsalanty M and Yao L: Deregulation of arginase induces bone complications in high-fat/high-sucrose diet diabetic mouse model. Molecular and cellular endocrinology, 422, 211–220 (2016) DOI: 10.1016/j.mce.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Huang J, Rajapakse A, Xiong Y, Montani J-P, Verrey F, Ming X-F and Yang Z: Genetic targeting of arginase-ii in mouse prevents renal oxidative stress and inflammation in diet-induced obesity. Frontiers in physiology, 7, 560 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yu Y, Rajapakse AG, Montani J-P, Yang Z and Ming X-F: p38 mitogen-activated protein kinase is involved in arginase-II-mediated eNOS-Uncoupling in Obesity. Cardiovascular diabetology, 13(1), 113 (2014) DOI: 10.1186/s12933-014-0113-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zaytouni T, Tsai P-Y, Hitchcock DS, DuBois CD, Freinkman E, Lin L, Morales-Oyarvide V, Lenehan PJ, Wolpin BM and Mino-Kenudson M: Critical role for arginase 2 in obesity-associated pancreatic cancer. Nature communications, 8(1), 242 (2017) DOI: 10.1038/s41467-017-00331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Baumgardt SL, Paterson M, Leucker TM, Fang J, Zhang DX, Bosnjak ZJ, Warltier DC, Kersten JR and Ge Z-D: Chronic co-administration of sepiapterin and l-citrulline ameliorates diabetic cardiomyopathy and myocardial ischemia/reperfusion injury in obese type 2 diabetic mice. Circulation. Heart Failure, 9(1), e002424 (2016) DOI: 10.1161/CIRCHEARTFAILURE.115.002424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bonhomme S, Belabed L, Blanc M-C, Neveux N, Cynober L and Darquy S: Arginine-supplemented enteral nutrition in critically ill diabetic and obese rats: a dose-ranging study evaluating nutritional status and macrophage function. Nutrition, 29(1), 305–312 (2013) DOI: 10.1016/j.nut.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 331.Breuillard C, Belabed L, Bonhomme S, Blanc-Quintin M-C, Neveux N, Couderc R, De Bandt J-P, Cynober L and Darquy S: Arginine availability modulates arginine metabolism and TNFα production in peritoneal macrophages from Zucker Diabetic Fatty rats. Clinical nutrition, 31(3), 415–421 (2012) DOI: 10.1016/j.clnu.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 332.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E and Piatti P: L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism, 62(2), 255–264 (2013) DOI: 10.1016/j.metabol.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 333.Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK and Wu G: Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. The Journal of nutrition, 139(2), 230–237 (2008) DOI: 10.3945/jn.108.096362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Varzandi T, Abdollahifar MA, Rohani SAH, Piryaei A, Zadeh-Vakili A, Jeddi S and Ghasemi A: Effect of long-term nitrite administration on browning of white adipose tissue in type 2 diabetic rats: A stereological study. Life sciences, 207, 219–226 (2018) DOI: 10.1016/j.lfs.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 335.Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, Rajan S, Srivastava A, Vishwakarma AL and Gaikwad AN: Chronic hyperinsulinemia promotes meta-inflammation and extracellular matrix deposition in adipose tissue: Implications of nitric oxide. Molecular and cellular endocrinology (2018) [DOI] [PubMed] [Google Scholar]
- 336.Becerril S, Rodríguez A, Catalán V, Méndez-Giménez L, Ramírez B, Sáinz N, Llorente M, Unamuno X, Gómez-Ambrosi J and Frühbeck G: Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. International Journal of Obesity, 1 (2018) [DOI] [PubMed] [Google Scholar]
- 337.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT and Wasserman DH: Chronic treatment with sildenafil improves energy balance and insulin action in high-fat–fed conscious mice. Diabetes, 56(4), 1025–1033 (2007) DOI: 10.2337/db06-0883 [DOI] [PubMed] [Google Scholar]
- 338.Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, Haas B, Sassmann A, Pfeifer A and Kilić A: Increased cGMP promotes healthy expansion and browning of white adipose tissue. The FASEB Journal, 27(4), 1621–1630 (2013) DOI: 10.1096/fj.12-221580 [DOI] [PubMed] [Google Scholar]
- 339.Gouill EL, Jimenez M, Binnert C, Jayet P-Y, Thalmann S, Nicod P, Scherrer U and Vollenweider P: Endothelial Nitric Oxide Synthase (eNOS) Knockout Mice Have Defective Mitochondrial ß-0xidation. Diabetes, 56(11) (2007) [DOI] [PubMed] [Google Scholar]
- 340.Carlström M, Larsen FJ, Nyström T, Hezel M, Borniquel S, Weitzberg E and Lundberg JO: Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proceedings of the National Academy of Sciences, 107(41), 17716–17720 (2010) DOI: 10.1073/pnas.1008872107 [DOI] [PMC free article] [PubMed] [Google Scholar]