Abstract
Dopamine is well recognized as a neurotransmitter in the brain, and regulates critical functions in a variety of peripheral systems. Growing research has also shown that dopamine acts as an important regulator of immune function. Many immune cells express dopamine receptors and other dopamine related proteins, enabling them to actively respond to dopamine and suggesting that dopaminergic immunoregulation is an important part of proper immune function. A detailed understanding of the physiological concentrations of dopamine in specific regions of the human body, particularly in peripheral systems, is critical to the development of hypotheses and experiments examining the effects of physiologically relevant dopamine concentrations on immune cells. Unfortunately, the dopamine concentrations to which these immune cells would be exposed in different anatomical regions are not clear. To address this issue, this comprehensive review details the current information regarding concentrations of dopamine found in both the central nervous system and in many regions of the periphery. In addition, we discuss the immune cells present in each region, and how these could interact with dopamine in each compartment described. Finally, the review briefly addresses how changes in these dopamine concentrations could influence immune cell dysfunction in several disease states including Parkinson’s disease, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, as well as the collection of pathologies, cognitive and motor symptoms associated with HIV infection in the central nervous system, known as NeuroHIV. These data will improve our understanding of the interactions between the dopaminergic and immune systems during both homeostatic function and in disease, clarify the effects of existing dopaminergic drugs and promote the creation of new therapeutic strategies based on manipulating immune function through dopaminergic signaling.
Keywords: Dopamine, neuroimmunology, immune system, catecholamine
Graphical Abstract
Introduction
Dopamine, or 3-hydroxytyramine, is a catecholamine neurotransmitter that is associated with a variety of neurological processes, including motor control, cognition, learning and reward. In addition to these and other central nervous system (CNS) processes, dopamine influences numerous peripheral functions including gastrointestinal motility, hormone release, blood pressure and sodium balance. While dopamine was first synthesized in 1910 (Hornykiewicz 1986), it wasn’t until 1957 that dopamine was found in the human brain (Montagu 1957). Prior to this discovery, dopamine had only been found in peripheral tissues and body fluids of mammalian animals (Euler and Hellner 1951; Goodall 1951) and was assumed to be just a precursor to other catecholamines. This changed in 1958, when Arvid Carlsson found that dopamine acted as a neurotransmitter (Carlsson, Lindqvist, Magnusson, & Waldeck, 1958), and was primarily concentrated in the basal ganglia of both humans and rodents (Bertler and Rosengren 1959; Sano et al. 1959). Soon after, the development of fluorescent histochemical visualization of monoamines enabled observation of neuronal pathways containing dopamine (Carlsson et al. 1962), establishing an independent role for dopamine and leading to the identification of specific dopamine receptors (Kebabian et al. 1972; Seeman et al. 1976) and their signaling pathways (Kebabian and Calne 1979).
The immunomodulatory activities of dopamine were first proposed in the 1980’s and 1990’s, when a number of studies suggested immune cells contain components of the dopaminergic system (Cosentino et al. 1999; Le Fur et al. 1980; Musso et al. 1996; Santambrogio et al. 1993). Many studies now show that dopamine functions as an immunomodulatory regulator and is pivotal for neuroimmune communication, with recent studies finding dopamine-induced changes in the functions of lymphocytes, macrophages, neutrophils and monocytes (Calderon et al. 2017; Dos-Santos-Pereira et al. 2018; Fan et al. 2018; Gaskill et al. 2014; Kawano et al. 2018; Nolan et al. 2018). Significant progress has also been made in understanding the specific dopaminergic signaling mechanisms in a variety of cell types other than neurons, indicating that immune cells interact with dopamine centrally and peripherally, in both homeostatic and pathological conditions.
However, the physiological concentrations of dopamine in specific regions of the human body, particularly in peripheral systems, remain unclear due to a relative scarcity of data on this topic, and the large variability among those studies which have been done. Further, most studies comparing distinct effects of dopamine between tissues focus on the expression of dopamine receptors, but not the concentration of dopamine itself. This presents a significant problem in the field, as lack of information prevents the development of hypotheses and experiments examining the effects of physiologically relevant dopamine concentrations on immune function. The purpose of this review is to fill this knowledge gap, providing a comprehensive summary of the available data regarding dopamine concentrations and activities throughout the body in both humans and animal models. Recognizing the heterogeneity of dopamine concentrations and the cells that regulate it across distinct tissue milieu is critical to defining the complex role of this neurotransmitter in the immune response. Further, many dopaminergic drugs are currently in use as therapeutics for a variety of disorders including depression, Alzheimer’s disease, and Parkinson’s disease, so a more comprehensive understanding of the immunologic actions of dopamine could initiate drug repurposing and the development of new therapeutic strategies based on manipulating dopaminergic immunology.
Overview of the Dopaminergic System
Dopamine Receptors
Dopamine primarily mediates its effects through activation of dopamine receptors (DRs), which are members of the G protein-coupled receptor (GPCR) superfamily. Dopamine receptors are divided into 2 subgroups, D1-like (D1 and D5), and D2-like (D2, D3 and D4) (Beaulieu and Gainetdinov 2011; Missale et al. 1998), which have different affinities for dopamine (Mittal et al. 2017). Greengard and colleagues showed that dopamine acts on D1-like receptors to increase the formation of cAMP (Hemmings Jr et al. 1984), and this pathway now serves as the basis for the distinction between DR subtypes. The D1-like receptors couple to Gαs/olf and stimulate cAMP production, while D2-like DR couple to Gαi/o and inhibit cAMP production. In addition to regulating cAMP, DRs can act through several alternative signaling pathways. The most studied is the Gq/11 mediated activation of phospholipase C (PLC) which induces calcium release from the endoplasmic reticulum through activation of IP3 receptors (Felder et al. 1989; Jin et al. 2001; Wang et al. 1995). Dopamine also mediates β-arrestin 2 induced activation of Akt, and the transactivation of tyrosine receptor kinases (RTKs) such as BDNF and TrkB (Beaulieu et al. 2015). Additional signaling complexity is generated by the formation of oligomeric complexes with other GPCR, such as D2-D4 or D2 with the adenosine A2A receptor (Borroto-Escuela et al. 2011; Fiorentini et al. 2008; Fuxe et al. 2010; Lee et al. 2002; Łukasiewicz et al. 2016; Perreault et al. 2010; Perreault et al. 2014), although formation of some of these heteromers is controversial (Frederick et al. 2015). A more detailed discussion of DR signaling and pharmacology can be found in Beaulieu and Gainetidinov (Beaulieu and Gainetdinov 2011).
Dopamine Synthesis, Metabolism, Storage, and Transport
This has been covered extensively in other recent reviews (Arreola et al. 2016; Nolan and Gaskill 2018), so we will only briefly cover this topic. Dopamine is derived from a two-step process starting with the hydroxylation of L-tyrosine by the enzyme tyrosine hydroxylase (TH) (Meiser et al. 2013), followed by the decarboxylation of the resulting product, L-DOPA, by aromatic amino acid decarboxylase (AADC). This process primarily occurs in dopaminergic neurons, although immune cells (Nolan and Gaskill 2018) and other cells from peripheral tissues (Mezey et al. 1998; Nurse and Fearon 2002; Pilipović et al. 2008) also express enzymes for dopamine synthesis (Rubí and Maechler 2010; Ugrumov 2009). In neurons, once dopamine is produced, it is either stored in synaptic vesicles at high concentrations (mM) (Omiatek et al. 2013; Scimemi and Beato 2009) for future release, or hydroxylated to form norepinephrine if the cell contains dopamine-β-hydroxylase (DBH). Dopamine is released into the synaptic cleft upon neuronal excitation, and excess dopamine in the cleft is returned to the cell by reuptake through the dopamine transporter (DAT), located at the presynaptic membrane. The norepinephrine transporter (NET) can also take-up dopamine in areas where the concentration of DAT is low (Moron et al. 2002). After returning to the neuron, dopamine is translocated from the cytoplasm to storage vesicles by vesicular monoamine transporter 2 (VMAT2), located on vesicular membranes. Dopamine remaining in the cytoplasm is inactivated through multiple pathways including oxidative deamination by monoamine oxidase (MAO) and O-methylation by catechol-O-methyl transferase (COMT), leading to the formation of dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) (Kopin 1985; Korf et al. 1976). In addition, formation of dopamine sulfate, the predominant form of dopamine in circulation, is catalyzed by phenolsulfotransferases (PSTs) and glucuronidation is catalyzed by uridine diphosphoglucuronosyltransferases (UGTs) to form their respective inactive conjugates (Suominen et al. 2015; Suominen et al. 2013; Uutela et al. 2009). Dopamine is also susceptible to oxidation, producing reactive quinones and reactive oxygen species that can cause cell damage and neurodegeneration (Delcambre et al. 2016; Meiser et al. 2013).
Dopaminergic Interaction with Immune Cells
The immune and nervous systems participate in extensive bidirectional crosstalk, mediated by a wide array of neurotransmitters, hormones, cytokines, and other factors, including dopamine. Dopamine regulates a variety of immune functions including cytokine secretion, cell adhesion, cytotoxicity, and chemotaxis (Besser et al. 2005; Cosentino et al. 2007; Gaskill et al. 2012; Kipnis et al. 2004; Nolan et al. 2018; Watanabe et al. 2006a), and these immune functions in turn can affect dopaminergic signaling both centrally and peripherally (Kabiersch et al. 1998; Kumai et al. 2000; Song et al. 2006). The effects are likely mediated by activation of DRs, as both human and rodent immune cells express multiple DR subtypes, however, DRs on distinct immune cell types may have different sensitivities to dopamine (Ferrari et al. 2004). These cells may also respond to different dopamine concentrations than those required for classical dopamine signaling in neurons (Meredith et al. 2006). The specific effects of dopamine on immune function have been described in recent articles (Gaskill et al. 2013; Levite 2016; Nolan and Gaskill 2018; Pinoli et al. 2017), therefore this section will only briefly discuss the immune cells in the CNS and periphery which could respond to dopamine.
Dopamine-Immune Interactions in CNS Immune cells
In the CNS, microglia are the predominant immune effector cells and they express functional DRs (Farber et al. 2005; Huck et al. 2015; Kopec et al. 2017; Mastroeni et al. 2009), as well as other dopaminergic proteins (Fan et al. 2018; Myohanen et al. 2010). Microglia are heterogeneously located throughout the brain, and are likely to encounter dopamine in any brain region where it is elevated. In addition to microglia, different types of CNS macrophages including perivascular, juxtavascular, meningeal and choroid plexus macrophages are active in the CNS immune response in their cognate regions (Corraliza 2014; Nayak et al. 2012). Human monocyte-derived macrophages have been shown to express active DRs and other dopamine-related proteins, suggesting that the brain specific macrophages may also express dopaminergic machinery (Gaskill et al. 2009; Gaskill et al. 2012; Nolan et al. 2018).
Although they are not immune cells, astrocytes are the most abundant glial cells in the CNS and extensively modulate immune function within the brain. Astrocytes from different brain regions show heterogeneity in DR expression, with expression found in cells in dopaminergic areas like the basal ganglia or striatum but not in other regions such as the cerebellum (Bal et al. 1994; Khan et al. 2001; Reuss and Unsicker 2001; Zanassi et al. 1999). Astrocytes also express DAT (Takeda et al. 2002), MAO-B, and COMT (Levitt et al. 1982; Myohanen et al. 2010; Winner et al. 2017), suggesting that they can take up and metabolize dopamine.
Dopamine-Immune Interactions in Peripheral Immune Cells
T-lymphocytes were first shown to express DRs in 1980 (Le Fur et al. 1980), and since then many other studies have shown that T cells express all DRs (Besser et al. 2005; Huang et al. 2010; Kirillova et al. 2008; Levite et al. 2001; McKenna et al. 2002; Ricci et al. 1995; Santambrogio et al. 1993; Watanabe et al. 2006a). The binding profiles of dopaminergic ligands in these cells were similar to those in neuronal membranes, suggesting the receptors act similarly to those found in neurons (Takahashi et al. 1992). T-cells also express TH, DAT, VMAT2, and COMT, suggesting they have the capacity to take up, synthesize, store, and release dopamine (Bergquist et al. 1994; Cosentino et al. 2007; Qiu et al. 2004; Tsao et al. 1998). The particular expression and function of the T-cell dopaminergic system is heterogeneous among T cell subsets, and some studies show expression is dependent on activation state and/or differentiation (Cosentino et al. 2007; Mignini et al. 2013; Nakano et al. 2009), which is extensively reviewed elsewhere (Pacheco et al. 2009). There is much less data regarding the dopaminergic system in B-lymphocytes and natural killer cells, but both cell types have also been shown to express all subtypes of DRs (McKenna et al. 2002; Meredith et al. 2006; Santambrogio et al. 1993; Watanabe et al. 2006b) and B-cells have dopamine stores (Ferrari et al. 2004).
Human myeloid cells, such as monocytes and macrophages, also express all subtypes of DRs, as well as DAT, VMAT2, TH, and AADC (Coley et al. 2015; Gaskill et al. 2009; Gaskill et al. 2012; Nolan et al. 2018). Other studies have found that human monocytes/macrophages can store and produce dopamine as well (Cosentino et al. 2000; Flierl et al. 2009; Josefsson et al. 1996; Marino et al. 1999). Monocyte-derived dendritic cells express DRs, primarily D1-like DR, and expression MAO and VMAT2, while expression of DAT is not clear (Nakano et al. 2008). These cells were also shown to contain intracellular dopamine, which is released upon antigen presentation to T cells (Nakano et al. 2009). Fewer studies have focused on granulocytes, but all five DR subtypes have been found on neutrophils (Boneberg et al. 2006; McKenna et al. 2002; Sookhai et al. 1999) and eosinophils (McKenna et al. 2002). Neutrophils and eosinophils contain intracellular dopamine (Cosentino et al. 1999) and eosinophils can also release dopamine (Withers et al. 2017). Further, human neutrophils and eosinophils can respond to dopamine (Pinoli et al. 2017). To our knowledge, there is no data on DR expression in basophils or mast cells.
Neural-immune Interactions Between Central/Peripheral Dopaminergic Systems
In addition to directly responding to dopamine, immune cells can be indirectly influenced by dopaminergic regulation in distant tissues, including the CNS (Basu and Dasgupta 2000). For example, hypoactivation of central dopamine increases the risk of inflammation during infection or tissue injury (Engler et al. 2009), and animals with hyperdopaminergic systems showed increased lipopolysaccharide (LPS)-induced cytokine production in macrophages (Kavelaars et al. 2005; Teunis et al. 2004). In rats, elevation of CNS dopamine levels using L-DOPA caused peripheral T cells to exhibit similar characteristics to those of dopamine activated T cells in vitro (Ilani et al. 2004). In addition, direct activation of dopaminergic neurons in the mouse VTA using DREADDs led to enhanced phagocytic activity of splenic dendritic cells and macrophages (Ben-Shaanan et al. 2016). These data suggest dopaminergic neurotransmission is important to immunoregulation, and suggest that consideration of the immunologic impact of dopamine across the body is an important step in evaluating therapeutic efficacy of dopaminergic drugs.
Caveats Regarding the Comparison of Dopamine Concentrations
This review consolidates the data from a large number of studies describing dopamine concentrations both within the CNS and in the periphery. Despite the amount of research cited here, there were a number of additional studies that examined dopamine which were not included due to the inability to determine the precise dopamine concentrations being reported. For example, studies that only reported percent changes in dopamine relative to baseline (Dunn et al. 1987; Floresco et al. 2003; Hu et al. 2015; Jackson and Moghaddam 2001; Kao et al. 1994; Keefe et al. 1993; Tanda et al. 1997), only reported levels of dopamine metabolites (Dahlin et al. 2012; Geracioti et al. 1998; Kilpatrick et al. 1986), or found dopamine to be below the limit of detection (Markianos et al. 2009; Nagler et al. 2018) were not included. To more effectively compare dopamine concentrations between studies, all values were converted to relative molar concentrations by dividing original values by the molecular weight of dopamine (153.18 g/mol) if not already in a molar value, and multiplying the density of tissues or fluids which we averaged to be around 1 kg/L or kg/m3 for all tissues or fluids. Additionally, if the values reported were not usable in this calculation, for instance concentrations of dopamine over time or concentration of a tissue with undefined mass, these values were not included (Basson et al. 1997; Di Chiara and Imperato 1988; McCarty et al. 1986; Reith et al. 1997; Yoshimoto et al. 1992). All the calculated values are reported alongside the original measurements in Tables 1–4 for reference. While this enables a more standardized comparison, it does not account for substantial variability resulting from differences in species, age, cell type or sex (Arvidsson et al. 2014; Bourque et al. 2011; Cosentino et al. 2000; Pilipović et al. 2008; Wahlstrom et al. 2010). An additional consideration when comparing the concentrations of dopamine found in corresponding regions of different species, even though we limited reporting studies from only mammals, is that while dopamine pathways are functional similarly among rodent species (Bhagwandin et al. 2008; Calvey et al. 2016; Calvey et al. 2015; Kruger et al. 2012; Limacher et al. 2008), there are major variations between these pathways in different mammalian orders (Manger et al. 2004; Maseko et al. 2013). There may also be significant variation resulting from experimental differences such as detection technique, preparation of tissue, type of analysis used or physical state of the animal (i.e. freely moving versus anesthetized) (Jackowska and Krysinski 2013; Peaston and Weinkove 2004; Wanat et al. 2009; Wightman and Robinson 2002). Important examples include the fact that almost all researchers do not report free versus conjugated dopamine, and some experiments utilize additional reagents to increase dopamine to the level of detection (Hauber and Fuchs 2000; Ripley et al. 1997), which are useful in detecting small changes in dopamine in response to pharmacological agents, but give artificial values that confound our understanding of the true concentrations of dopamine that immune cells could be exposed to in a particular tissue. Further, research groups without experience examining dopamine tended to show more extreme values than those laboratories with extensive experience with studying this neurotransmitter, suggesting that research experience should also be considered when evaluating the cited studies.
Table 1: Dopamine Concentrations in the Central Nervous System.
A summary of human and animal studies that have examined concentrations of dopamine within the central nervous system. We have also included concentrations of dopamine in the retina and both anterior and posterior pituitary. Both original values and calculated relative molar values are reported to enable the comparison of dopamine concentrations between studies.
| Original Dopamine Concentration | Dopamine Concentration (M) | Location | Species | Method | Reference |
|---|---|---|---|---|---|
| 20 ng/g | 1.3 × 10−7 Ma | Amygdala | Human | HPLC | (Musshoff et al. 2000) |
| 0.11 pmol/mg | 1.1 × 10−7 Ma | Amygdala | Human | HPLC | (Ebinger et al. 1987) |
| 2.17 nmol/g | 2.2 × 10−6 Ma | Amygdala | Primate | HPLC | (Elchisak et al. 1983) |
| 1.9 ng/mgb | 1.2 × 10−5 Ma | Amygdala | Rat | HPLC | (Carvalho et al. 2005) |
| 1.7 ng/mgc | 1.1 × 10−5 Ma | Amygdala | Rat | HPLC | (Bradbury et al. 1985) |
| 0.15 fmol/μl | 1.5 × 10−10 Ma | Amygdala | Rat | Microdialysis | (Inglis and Moghaddam 1999) |
| 0.9 pg/10 μlb | 5.8 × 10−10 Ma | Amygdala | Rat | Microdialysis | (Tor-Agbidye et al. 2001) |
| 0.67 pg/μlc | 4.4 × 10−9 Ma | Amygdala | Rat | Microdialysis | (Adachi et al. 2013) |
| 0.79 nMc | 7.9 × 10−10 Ma | Amygdala | Rat | Microdialysis | (Weiss et al. 2000) |
| 16 ng/mgb | 1 × 10−4 Ma | Amygdala (Basolateral) | Rat | HPLC | (Fadok et al. 2010) |
| 7.95 pg/μg | 5.2 × 10−5 Ma | Arcuate Nucleus | Rat | HPLC | (MohanKumar e al. 1999) |
| 238.5 pg/mg | 1.6 × 10−6 Ma | Arcuate Nucleus | Rat | HPLC | (Bromek et al. 2013) |
| 2.92 pg/g log10 | 1 × 10−9 Ma | Basal Ganglia | Human | HPLC | (Kumar et al. 2009) |
| 116.1 pg/mg | 7.6 × 10−7 Ma | Brainstem | Rat | HPLC | (Rysz et al. 2015) |
| 65 pg/mgb | 4.2 × 10−7 Ma | Brainstem | Rat | HPLC | (Bromek et al. 2011) |
| 200 ng/gb | 1.3 × 10−6 Ma | Brainstem | Rat | HPLC | (Nikishina et al. 2016) |
| 201.1 ng/g | 1.3 × 10−6 Ma | Brainstem | Rat | HPLC | (Meshki Baf et al. 1994) |
| 3.43 ng/g | 2.2 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Wilson et al. 1996b) |
| 6.62 ng/mg | 4.3 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Wilson et al. 1996a) |
| 10,352 fmol/mg | 1 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Goldstein et al. 2011) |
| 3 ng/mgb | 2 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Davis and Sparks 1995) |
| 3.9 pg/g log10 | 2.9 × 10−6 Ma | Caudate Nucleus | Human | HPLC | (Kumar et al. 2009) |
| 4833 ng/g | 3.2 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Rajput et al. 2008) |
| 2470 ng/g | 1.6 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Musshoff et al. 2000) |
| 20.32 pmol/mg | 2 × 10−5 Ma | Caudate Nucleus | Human | HPLC | (Ebinger et al. 1987) |
| 127 ng/mg | 8.3 × 10−4 Ma | Caudate Nucleus | Primate | HPLC | (Elsworth et al. 2008) |
| 58.2 nmol/g | 5.8 × 10−5 Ma | Caudate Nucleus | Primate | HPLC | (Elchisak et al. 1983) |
| 200nM | 2 × 10−7 M | Caudate Nucleus (Synapse Interface) | Rat | FSCV | (Kawagoe et al. 1992) |
| 1.68 fmol/μl | 1.7 × 10−9 Ma | Caudate Nucleus | Rat | Microdialysis | (Inglis and Moghaddam 1999) |
| 10.9 μg/g | 7.1 × 10−5 Ma | Caudate Nucleus | Sheep | HPLC | (Juorio and Chedrese 1990) |
| 6 nM | 6 × 10−9 M | Caudate Nucleus (Extrasynaptic Region) | Rat | FSCV | (Kawagoe et al. 1992) |
| 1.2 nM | 1.2 × 10−9 M | Caudate/Putan en | Mouse | Microdialysis | (Bosse and Mathews 2011) |
| 168.4 pg/μg | 1.1 × 10−3 Ma | Caudate/Putamen | Rat | HPLC | (MohanKumar et al. 1999) |
| 8.4 ng/mgc | 5.5 × 10−5 Ma | Caudate/Putan en (Anterior) | Rat | HPLC | (Bradbury et al. 1985) |
| 5 ng/mgc | 3.3 × 10−5 Ma | Caudate/Putam en (Lateral) | Rat | HPLC | (Bradbury et al. 1985) |
| 200 nMb | 2 × 10−7 M | Caudate/Putam en (Phasic Value) | Rat | FSCV | (Venton et al. 2003) |
| 30 nM | 3 × 10−8 M | Caudate/Putam en (Tonic Value) | Rat | FSCV | (Venton et al. 2003) |
| 8 ng/g | 5.2 × 10−8 Ma | Cerebellum | Human | HPLC | (Musshoff et al. 2000) |
| 19 ng/g | 1.2 × 10−7 Ma | Cerebellum | Mouse | HPLC | (Sasa and Blank 1977) |
| 0.03 nmol/g | 3 × 10−8 Ma | Cerebellum | Primate | HPLC | (Elchisak et al. 1983) |
| 0.29 nmol/g | 2.9 × 10−7 Ma | Cerebellum | Rat | HPLC | (Swiercz et al. 2009) |
| 3.4 pg/mgb | 2.2 × 10−8 Ma | Cerebellum | Rat | HPLC | (Bromek et al. 2011) |
| 57.3 ng/g | 3.7 × 10−7 Ma | Cerebellum | Rat | HPLC | (Meshki Baf et al. 1994) |
| 1.19 ng/mg | 7.8 × 10−6 Ma | Cingulate Cortex | Primate | HPLC | (Elsworth et al. 2008) |
| 0.641 nmol/g | 6.4 × 10−7 Ma | Cingulate Gyrus | Primate | HPLC | (Elchisak et al. 1983) |
| 0.481 nmol/g | 4.8 × 10−7 Ma | Corpus Callosum | Primate | HPLC | (Elchisak et al. 1983) |
| 15 fmol/mg | 1.5 × 10−8 Ma | Cortex | Human | HPLC | (Goldstein et al. 2011) |
| 3.7 nmol/g | 3.7 × 10−7 Ma | Cortex | Rat | HPLC | (Swiercz et al. 2009) |
| 377.4 pg/mg | 2.5 × 10−6 Ma | Cortex | Rat | HPLC | (Rysz et al. 2015) |
| 0.01 ng/ml | 6.5 × 10−11 Ma | CSF | Human | HPLC | (Eldrup et al. 1995) |
| 89.4 pg/ml | 5.8 × 10−10 Ma | CSF | Human | HPLC | (Berger et al. 1994) |
| 15 pg/mlb | 9.8 × 10−11 Ma | CSF | Human | HPLC | (Scheller et al. 2010) |
| 0.05 nM | 5 × 10−11 M | CSF | Human | HPLC | (Andersen et al. 2017) |
| 0.32 ng/ml | 2.1 × 10−9 Ma | CSF | Human | HPLC | (Engelborghs el al. 2003) |
| 0.02 ng/ml | 1.3 × 10−10 Ma | CSF | Human | HPLC | (Raskind et al. 1999) |
| 2 log pg/ml | 1 × 10−9 Ma | CSF | Human (MDD patients) | HPLC | (King et al. 1986 |
| 0.91 nmol/g | 9.1 × 10−7 Ma | Diencephalon | Rat | HPLC | (Swiercz et al. 2009) |
| 19 ng/g | 1.2 × 10−7 Ma | Frontal cortex | Human | HPLC | (Musshoff et al. 2000) |
| 2.186 pg/g log10 | 1 × 10−9 Ma | Frontal Cortex | Human | HPLC | (Kumar et al. 2009) |
| 0.0975 nmol/g | 9.8 × 10−8 Ma | Frontal Cortex | Primate | HPLC | (Elchisak et al. 1983) |
| 291.7 pg/mg | 1.9 × 10−6 Ma | Frontal Cortex | Rat | HPLC | (Rysz et al. 2015) |
| 225 pg/mgb | 1.5 × 10−6 Ma | Frontal Cortex | Rat | HPLC | (Bromek et al. 2011) |
| 210.2 fmol/mg | 2.1 × 10−7 Ma | Frontal Cortex | Rat | HPLC | (Wisman et al. 2008) |
| 2.91 pg/g log10 | 1 × 10−9 Ma | Globus Pallidus | Human | HPLC | (Kumar et al. 2009) |
| 1.59 nmol/g | 1.6 × 10−6 Ma | Globus Pallidus | Primate | HPLC | (Elchisak et al. 1983) |
| 490 ng/g | 3.2 × 10−6 Ma | Globus Pallidus (External) | Human | HPLC | (Rajput et al. 2008) |
| 75 ng/g | 4.9 × 10−7 Ma | Globus Pallidus (Internal) | Human | HPLC | (Rajput et al. 2008) |
| 1.53 pmol/mg | 1.5 × 10−6 Ma | Globus Pallidus (Lateral) | Human | HPLC | (Ebinger et al. 1987) |
| 1.46 pmol/mg | 1.5 × 10−6 Ma | Globus Pallidus (Medial) | Human | HPLC | (Ebinger et al. 1987) |
| 11 ng/g | 7.2 × 10−8 Ma | Hippocampus | Human | HPLC | (Musshoff et al. 2000) |
| 0.27 pmol/mg | 2.7 × 10−7 Ma | Hippocampus | Human | HPLC | (Ebinger et al. 1987) |
| 9.06 pmol/mg | 9.1 × 10−6 Ma | Hippocampus (Dorsal) | Mouse | HPLC | (Kempadoo et al 2016) |
| 0.1 ng/mgb | 6.5 × 10−7 Ma | Hippocampus (Dorsal) | Rat | HPLC | (Carvalho et al. 2005) |
| 2.03 pg/μg | 1.3 × 10−5 Ma | Hippocampus | Rat | HPLC | (MohanKumar e al. 1999) |
| 116.7 ng/g | 7.6 × 10−7 Ma | Hippocampus | Rat | HPLC | (Meshki Baf et al. 1994) |
| 7.7 nmol/g | 7.7 × 10−7 Ma | Hippocampus | Rat | HPLC | (Swiercz et al. 2009) |
| 65.2 fmol/mg | 6.5 × 10−8 Ma | Hippocampus | Rat | HPLC | (Wisman et al. 2008) |
| 14.4 pg/mg | 9 × 10−8 Ma | Hippocampus | Rat | HPLC | (Rysz et al. 2015) |
| 0.11 nM | 1.1 × 10−10 M | Hippocampus | Rat | Microdialysis | (Borgkvist et al. 2012) |
| 186 ng/g | 1.2 × 10−6 Ma | Hypothalamus | Human | HPLC | (Musshoff et al. 2000) |
| 1301 pg/mgc | 8.5 × 10−6 Ma | Hypothalamus | Mouse | HPLC | (Nagler et al. 2018) |
| 111.9 ng/g | 7.3 × 10−7 Ma | Hypothalamus | Mouse | LC-MS | (Kim et al. 2014) |
| 2.71 nmol/g | 2.7 × 10−6 Ma | Hypothalamus | Primate | HPLC | (Elchisak et al. 1983) |
| 11 ng/mgb | 7.2 × 10−5 Ma | Hypothalamus | Rat | HPLC | (De Laurentiis e al. 2002) |
| 200 ng/gb | 1.3 × 10−6 Ma | Hypothalamus | Rat | HPLC | (Nikishina et al. 2016) |
| 241.2 pg/mg | 1.6 × 10−6 Ma | Hypothalamus | Rat | HPLC | (Rysz et al. 2015) |
| 304.8 ng/g | 2 × 10−6 Ma | Hypothalamus | Rat | HPLC | (Meshki Baf et al. 1994) |
| 32.85 ng/g | 2.1 × 10−7 Ma | Hypothalamus | Rat | HPLC | (Hu et al. 2014) |
| 0.34 ng/g | 2.2 × 10−9 Ma | Hypothalamus | Rat | LC-MS/MS | (Tareke et al. 2007) |
| 0.21 ug/g | 1.4 × 10−6 Ma | Hypothalamus | Sheep | HPLC | (Juorio and Chedrese 1990’ |
| 150 pg/mgb | 9.8 × 10−7 Ma | Hypothalamus (Anterior Slices | Rat | HPLC | (Shintani et al. 1993) |
| 7.7 pg/μgb | 5 × 10−5 Ma | Medial Preoptk Area | Rat | HPLC | (MohanKumar e al. 1999) |
| 50.8 pg/μgb | 3.3 × 10−5 Ma | Median Eminence | Rat | HPLC | (MohanKumar e al. 1999) |
| 15 ng/mgb | 9.8 × 10−5 Ma | Median Eminence | Rat | HPLC | (Nagy et al. 1998) |
| 30 pg/μgb | 2 × 10−4 Ma | Median Eminence | Rat | HPLC | (Lafuente et al. 2005) |
| 127 ng/mgc | 8.3 × 10−4 Ma | Median Eminence | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 102 ng/g | 6.7 × 10−7 Ma | Medulla Oblongata | Human | HPLC | (Musshoff et al. 2000) |
| 0.259 nmol/g | 2.6 × 10−7 Ma | Medulla Oblongata | Primate | HPLC | (Elchisak et al. 1983) |
| 36.4 pg/mg | 2.4 × 10−7 Ma | Medulla Oblongata | Rat | HPLC | (Rysz et al. 2015) |
| 0.6 nmol/g | 6 × 10−7 Ma | Mesencephaloi | Rat | HPLC | (Swiercz et al. 2009) |
| 59.8 ng/g | 3.9 × 10−7 Ma | Midbrain | Mouse | LC-MS | (Kim et al. 2014) |
| 0.5 nmol/g | 5 × 10−7 Ma | Midbrain | Primate | HPLC | (Elchisak et al. 1983) |
| 104.76 ng/g | 6.8 × 10−7 Ma | Midbrain | Rat | HPLC | (Song et al. 2006) |
| 600 ng/gb | 3.9 × 10−6 Ma | Midbrain | Rat | HPLC | (Nikishina et al. 2016) |
| 180.2 ng/g | 1.2 × 10−6 Ma | Motor Cortex | Rat | HPLC | (Meshki Baf et al. 1994) |
| 2.44 ng/mg | 1.6 × 10−5 Ma | Nucleus Accumbens | Human | HPLC | (Wilson et al. 1996a) |
| 986 ng/g | 6.4 × 10−6 Ma | Nucleus Accumbens | Human | HPLC | (Musshoff et al. 2000) |
| 14.93 pmol/mg | 1.5 × 10−5 Ma | Nucleus Accumbens | Human | HPLC | (Ebinger et al. 1987) |
| 150 ng/mgb | 9.8 × 10−4 Ma | Nucleus Accumbens | Mouse | HPLC | (Winner et al. 2017) |
| 4.77 ng/mg | 3.1 × 10−5 Ma | Nucleus Accumbens | Mouse | HPLC | (Bergamini et al 2018) |
| 16.7 fmol/35 μl | 4.8 × 10−10 Ma | Nucleus Accumbens | Mouse | Microdialysis | (Airavaara et al. 2004) |
| 75.7 ng/mg | 4.9 × 10−6 Ma | Nucleus Accumbens | Primate | HPLC | (Elsworth et al. 2008) |
| 2 μM | 2 × 10−6 M | Nucleus Accumbens | Rat | FSCV | (Kutlu et al. 2018) |
| 0.59 μM | 5.9 × 10−7 M | Nucleus Accumbens | Rat | FSCV | (Wakabayashi e al. 2016) |
| 71.5 ng/mg | 4.7 × 10−4 Ma | Nucleus Accumbens | Rat | HPLC | (Fadok et al. 2010) |
| 11 ng/mg | 7.2 × 10−5 Ma | Nucleus Accumbens | Rat | HPLC | (Carvalho et al. 2005) |
| 4535.8 pg/mg | 3 × 10−5 Ma | Nucleus Accumbens | Rat | HPLC | (Rysz et al. 2015) |
| 45.51 ng/mg | 3 × 10−4 Ma | Nucleus Accumbens | Rat | HPLC | (Choi et al. 2012) |
| 8700 pg/mgb | 5.7 × 10−5 Ma | Nucleus Accumbens | Rat | HPLC | (Bromek et al. 2011) |
| 4.9 μg/gbd | 3.2 × 10−5 Ma | Nucleus Accumbens | Rat | HPLC | (Lucas and McMillen 2002) |
| 3.3 nMb | 3.3 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Hemby et al. 1995) |
| 1.48 fmol/μl | 1.5 × 10−9 Ma | Nucleus Accumbens | Rat | Microdialysis | (Yan 1999) |
| 0.14 pmol/25 μl | 5.6 × 10−9 Ma | Nucleus Accumbens | Rat | Microdialysis | (Anagnostakis and Spyraki 1994) |
| 0.01 pmol/20 μl | 5 × 10−10 Ma | Nucleus Accumbens | Rat | Microdialysis | (Pothos et al. 1991) |
| 3.45 nM | 3.5 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Weiss et al. 1996) |
| 1.14 nM | 1.1 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Borgkvist et al. 2012) |
| 7 pg/20 μl | 2.3 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Hernandez anc Hoebel 1988) |
| 9.75 nM | 9.8 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Pettit et al. 1990) |
| 58.7 fmol/40 μl | 1.5 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Chen et al. 1993) |
| 4.2 nM | 4.2 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Parsons and Justice 1992) |
| 10.8 nMb | 1.1 × 10−8 M | Nucleus Accumbens | Rat | Microdialysis | (Smith et al. 2006) |
| 17.4 nM | 1.7 × 10−8 M | Nucleus Accumbens | Rat | Microdialysis | (Moghaddam and Bunney 1989) |
| 2.4 nMc | 2.4 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Weiss et al. 2000) |
| 9.4 nM | 9.4 × 10−9 M | Nucleus Accumbens | Rat | Microdialysis | (Yim and Gonzales 2000) |
| 1.24 fmol/μl | 1.2 × 10−9 Ma | Nucleus Accumbens | Rat | Microdialysis | (Inglis and Moghaddam 1999) |
| 73.8 ng/mgc | 4.8 × 10−4 Ma | Nucleus Accumbens | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 50 nM | 5 × 10−8 M | Nucleus Accumbens (Core) | Rat | FSCV | (Wightman et al 2007) |
| 40 nM | 4 × 10−8 M | Nucleus Accumbens (Core) | Rat | FSCV | (Stuber et al. 2005) |
| 20 nM | 2 × 10−8 M | Nucleus Accumbens (Core) | Rat | FSCV | (Owesson-White et al. 2012) |
| 15 nMb | 1.5 × 10−8 M | Nucleus Accumbens (Core) | Rat | FSCV | (Vander Weele et al. 2014) |
| 20.4 nMb | 2 × 10−8 M | Nucleus Accumbens (Shell) | Rat | FSCV | (Roitman et al. 2008) |
| 41 nM | 4.1 × 10−8 M | Nucleus Accumbens (Shell) | Rat | FSCV | (Johnson et al. 2018) |
| 30 nMb | 3 × 10−8 M | Nucleus Accumbens (Shell) | Rat | FSCV | (Vander Weele et al. 2014) |
| 0.47 nM | 4.7 × 10−10 M | Nucleus Accumbens (Shell) | Rat | LC-MS/MS | (Hows et al. 2004) |
| 66.8 fmol/50μl | 1.3 × 10−9 Ma | Nucleus Accumbens (Shell) | Rat | Microdialysis | (Fadda et al. 2003) |
| 0.59 pg/μlc | 3.9 × 10−9 Ma | Nucleus Accumbens (Shell) | Rat | Microdialysis | (Adachi et al. 2013) |
| 0.884 nmol/g | 8.8 × 10−7 Ma | Occipital Cortex | Primate | HPLC | (Elchisak et al. 1983) |
| 40.6 ng/g | 2.7 × 10−7 Ma | Olfactory Bulb | Mouse | LC-MS | (Kim et al. 2014 |
| 0.192 μg/g | 1.3 × 10−6 Ma | Olfactory Bulb | Primate | HPLC | (Pifl et al. 2017) |
| 75 pg/mgb | 4.9 × 10−7 Ma | Olfactory Bulb | Rat | HPLC | (Bromek et al. 2011) |
| 6.87 nmol/g | 6.9 × 10−6 Ma | Olfactory Cortex | Primate | HPLC | (Elchisak et al. 1983) |
| 11 ng/g | 7.1 × 10−8 Ma | Olfactory Tubercle | Human | HPLC | (Musshoff et al. 2000) |
| 0.34 μM | 3.4 × 10−7 M | Olfactory Tubercle | Rat | FSCV | (Wakabayashi e al. 2016) |
| 4.1 ng/mgc | 2.7 × 10−5 Ma | Olfactory Tubercle | Rat | HPLC | (Bradbury et al. 1985) |
| 39.7 ng/mgc | 2.6 × 10−4 Ma | Olfactory Tubercle | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 6 ng/mgb | 3.9 × 10−5 Ma | Paraventricula Nucleus | Mouse | HPLC | (Sakic et al. 2002) |
| 14.4 pg/μg | 3.1 × 10−5 Ma | Paraventricula Nucleus | Rat | HPLC | (MohanKumar e al. 1999) |
| 346.2 pg/mg | 2.3 × 10−6 Ma | Paraventricula Nucleus | Rat | HPLC | (Bromek et al. 2013) |
| 0.01 ng/g | 6.5 × 10−11 Ma | Parietal Cortex | Rat | LC-MS/MS | (Tareke et al. 2007) |
| 48.9 ng/g | 3.2 × 10−7 Ma | Pituitary Gland | Mouse | LC-MS | (Kim et al. 2014 |
| 0.29 ng/g | 1.9 × 10−9 Ma | Pituitary Gland | Rat | LC-MS/MS | (Tareke et al. 2007) |
| 0.01 ng/mgb | 6.5 × 10−8 Ma | Pituitary Gland (Anterior Inner Zone) | Rat | HPLC | (Nagy et al. 1998) |
| 0.2 ng/mgb | 1.3 × 10−6 Ma | Pituitary Gland (Anterior Outer Zone) | Rat | HPLC | (Nagy et al. 1998) |
| 0.33 ng/mgb | 2.2 × 10−6 Ma | Pituitary Gland (Anterior) | Rat | HPLC | (De Laurentiis e al. 2002) |
| 0.45 ng/mgc | 2.9 × 10−6 Ma | Pituitary Gland (Anterior) | Rat | HPLC | (DeMaria et al. 1998) |
| 20 pg/μgb | 1.3 × 10−4 Ma | Pituitary Gland (Anterior) | Rat | HPLC | (Lafuente et al. 2005) |
| 0.2 ng/mgc | 1.3 × 10−6 Ma | Pituitary Gland (Anterior) | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 5 ng/mgb | 3.3 × 10−5 Ma | Pituitary Gland (Intermediate) | Rat | HPLC | (Nagy et al. 1998) |
| 25 ng/mgc | 1.6 × 10−4 Ma | Pituitary Gland (Intermediate) | Rat | HPLC | (DeMaria et al. 1998) |
| 7.5 ng/mgc | 4.9 × 10−5 Ma | Pituitary Gland (Posterior) | Rat | HPLC | (DeMaria et al. 1998) |
| 6 ng/mgb | 3.9 × 10−5 Ma | Pituitary Gland (Posterior) | Rat | HPLC | (De Laurentiis e al. 2002) |
| 1 ng/mgb | 6.5 × 10−6 Ma | Pituitary Gland (Posterior) | Rat | HPLC | (Nagy et al. 1998) |
| 6 pg/μgb | 3.9 × 10−5 Ma | Pituitary Gland (Posterior) | Rat | HPLC | (Lafuente et al. 2005) |
| 7.8 ng/mgc | 5.1 × 10−5 Ma | Pituitary Gland (Posterior) | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 0.176 nmol/g | 1.8 × 10−7 Ma | Pons | Primate | HPLC | (Elchisak et al. 1983) |
| 0.1 pmol/mg | 1 × 10−7 Ma | Postcentral Gyrus | Human | HPLC | (Ebinger et al. 1987) |
| 15 ng/g | 9.8 × 10−8 Ma | Precentral Gyrus | Human | HPLC | (Musshoff et al. 2000) |
| 0.08 pmol/mg | 8 × 10−8 Ma | Precentral Gyrus | Human | HPLC | (Ebinger et al. 1987) |
| 113.51 ng/g | 7.4 × 10−7 Ma | Prefrontal Cortex | Rat | HPLC | (Hu et al. 2014) |
| 0.08 ng/mgb | 5.2 × 10−7 Ma | Prefrontal Cortex | Rat | HPLC | (Carvalho et al. 2005) |
| 0.05 μg/gb | 3.3 × 10−7 Ma | Prefrontal Cortex | Rat | HPLC | (Lucas and McMillen 2002) |
| 79.95 nmol/l | 8 × 10−8 Ma | Prefrontal Cortex | Rat | Microdialysis | (Pistis et al. 2002) |
| 0.92 ng/mg | 6 × 10−6 Ma | Prefrontal Cortex (Dorsolateral) | Primate | HPLC | (Elsworth et al. 2008) |
| 0.475 ng/mg | 3.1 × 10−6 Ma | Prefrontal Cortex (Medial | Rat | HPLC | (Choi et al. 2012) |
| 2.7nM | 2.7 × 10−9 M | Prefrontal Cortex (Medial | Rat | Microdialysis | (Moghaddam and Bunney 1989) |
| 0.23 fmol/μl | 2.3 × 10−10 Ma | Prefrontal Cortex (Medial | Rat | Microdialysis | (Inglis and Moghaddam 1999) |
| 1.27 ng/mg | 8.3 × 10−6 Ma | Prelimbic Cortex | Primate | HPLC | (Elsworth et al. 2008) |
| 3.98 μg/g | 2.6 × 10−5 Ma | Putamen | Human | HPLC | (Pifl et al. 2014) |
| 4.04 ng/g | 2.6 × 10−5 Ma | Putamen | Human | HPLC | (Wilson et al. 1996b) |
| 7.16 ng/mg | 4.7 × 10−5 Ma | Putamen | Human | HPLC | (Wilson et al. 1996a) |
| 16.96 pmol/mg | 1.7 × 10−5 Ma | Putamen | Human | HPLC | (Ebinger et al. 1987) |
| 1170 ng/g | 7.6 × 10−6 Ma | Putamen | Human | HPLC | (Musshoff et al. 2000) |
| 15488 fmol/mg | 1.5 × 10−5 Ma | Putamen | Human | HPLC | (Goldstein et al. 2011) |
| 3.5 ng/mgb | 2.3 × 10−5 Ma | Putamen | Human | HPLC | (Davis and Sparks 1995) |
| 6475 ng/g | 4.2 × 10−5 Ma | Putamen | Human | HPLC | (Rajput et al. 2008) |
| 4.563 pg/g log10 | 1.1 × 10−9 Ma | Putamen | Human | HPLC | (Kumar et al. 2009) |
| 13.26 μg/g | 8.7 × 10−5 Ma | Putamen | Primate | HPLC | (Pifl et al. 2014) |
| 68.4 nmol/g | 6.8 × 10−5 Ma | Putamen | Primate | HPLC | (Elchisak et al. 1983) |
| 0.25 ng/mg | 1.6 × 10−6 Ma | Retina | Mouse | HPLC | (Wu et al. 2015) |
| 1.4 ng/mgb | 9.1 × 10−6 Ma | Retina | Mouse | HPLC | (Jackson et al. 2012) |
| 2.125 pg/mg | 1.4 × 10−8 Ma | Retina | Mouse | HPLC | (Lahouaoui et al 2016) |
| 0.128 nmol/g | 1.3 × 10−7 Ma | Retina | Primate | HPLC | (Elchisak et al. 1983) |
| 10,000 pg/mgb | 6.5 × 10−5 Ma | Striatum | Mouse | HPLC | (Batkowiec-Iskra et al. 2007) |
| 240 ng/mgb | 1.6 × 10−3 Ma | Striatum | Mouse | HPLC | (Winner et al. 2017) |
| 0.65 nmol/mg | 6.5 × 10−4 Ma | Striatum | Mouse | HPLC | (Kita et al. 2000) |
| 269.5 ng/mg | 1.8 × 10−3 Ma | Striatum | Mouse | HPLC | (Petzinger et al. 2007) |
| 3463 ng/g | 2.3 × 10−5 Ma | Striatum | Mouse | LC-MS | (Kim et al. 2014) |
| 4.2 nMb | 4.2 × 10−9 M | Striatum | Mouse | Microdialysis | (Zhang et al. 2009) |
| 5 fmol/μLb | 5 × 10−9 Ma | Striatum | Primate | Microdialysis | (Bradberry 2000 |
| 181 nM | 1.8 × 10−7 M | Striatum | Rat | FSCV | (Schwerdt et al. 2018) |
| 11.3 nmol/g | 1.1 × 10−5 Ma | Striatum | Rat | HPLC | (Swiercz et al. 2009) |
| 6553.8 pg/mg | 4.3 × 10−5 Ma | Striatum | Rat | HPLC | (Rysz et al. 2015) |
| 6 ng/mgb | 3.9 × 10−5 Ma | Striatum | Rat | HPLC | (Garrido-Gil et al. 2018b) |
| 7 ng/mgb | 4.6 × 10−5 Ma | Striatum | Rat | HPLC | (Villar-Cheda et al. 2014) |
| 1300 ng/gb | 8.5 × 10−6 Ma | Striatum | Rat | HPLC | (Nikishina et al. 2016) |
| 9500 pg/mgb | 6.2 × 10−5 Ma | Striatum | Rat | HPLC | (Bromek et al. 2011) |
| 1894.5 ng/g | 1.2 × 10−5 Ma | Striatum | Rat | HPLC | (Hu et al. 2014) |
| 10.4 ng/g | 6.8 × 10−8 Ma | Striatum | Rat | LC-MS/MS | (Tareke et al. 2007) |
| 20 nMb | 2 × 10−8 M | Striatum | Rat | Microdialysis | (Shou et al. 2006) |
| 146.4 ng/mgc | 9.6 × 10−4 Ma | Striatum | Rat | Radioenzymatic Assay | (Demarest et al. 1982) |
| 3.202 pg/g log10 | 1 × 10−8 Ma | Substantia Nigra | Human | HPLC | (Kumar et al. 2009) |
| 384 ng/g | 2.5 × 10−6 Ma | Substantia Nigra | Human | HPLC | (Musshoff et al. 2000) |
| 4.96 pmol/mg | 5 × 10−6 Ma | Substantia Nigra | Human | HPLC | (Ebinger et al. 1987) |
| 4.89 nmol/g | 4.9 × 10−6 Ma | Substantia Nigra | Primate | HPLC | (Elchisak et al. 1983) |
| 1000 pg/mgb | 6.5 × 10−6 Ma | Substantia Nigra | Rat | HPLC | (Bromek et al. 2011) |
| 0.41 ng/g | 2.7 × 10−9 Ma | Substantia Nigra | Rat | LC-MS/MS | (Tareke et al. 2007) |
| 1.04 ng/mg | 6.8 × 10−6 Ma | Supplementary Motor Area | Primate | HPLC | (Elsworth et al. 2008) |
| 43 ng/g | 2.8 × 10−7 Ma | Thalamus | Human | HPLC | (Musshoff et al. 2000) |
| 115.9 pg/mg | 7.6 × 10−7 Ma | Thalamus | Rat | HPLC | (Rysz et al. 2015) |
| 0.45 pmol/mg | 4.5 × 10−7 Ma | Thalamus (Anterior Nuclei) | Human | HPLC | (Ebinger et al. 1987) |
| 125 pmol/mgb | 1.3 × 10−4 Ma | Ventral Tegmental Area | Guinea Pig | HPLC | (Rice et al. 1994 |
| 9.2 ng/mg | 6 × 10−5 Ma | Ventral Tegmental Area | Rat | HPLC | (Salvatore et al. 2012) |
| 46.5 fmol/40 μl | 1.2 × 10−9 Ma | Ventral Tegmental Area | Rat | Microdialysis | (Chen et al. 1993) |
| 10 ng/g | 6.5 × 10−8 Ma | White Matter | Human | HPLC | (Musshoff et al. 2000) |
| 800 ng/g | 5.2 × 10−6 Ma | Whole brain | Mouse | GC-MS | (Weintraub et al 1975) |
| 6.27 pmol/mg | 6.3 × 10−6 Ma | Whole brain | Mouse | HPLC | (Quelhas-Santos et al. 2015) |
| 46.7 pmol/mg | 3 × 10−7 Ma | Whole brain | Mouse | HPLC | (Tsao et al. 1997) |
| 973 ng/g | 6.4 × 10−6 Ma | Whole brain | Mouse | HPLC | (Sasa and Blank 1977) |
Concentrations calculated by dividing values by the molecular weight of dopamine (153.18 g/mol) if not already in a molar value, and multiplying the density of tissues or fluids (kg/L or kg/m^3)
Estimate obtained from graph because no values given in text, or averaged values if multiple control values were given
Value obtained from one or an average of treatment groups other than control because no absolute control given
Averaged male and female control groups
Table 4: Dopamine Concentrations During Disease and Disease Models in the Central Nervous System and the Periphery.
A summary of human and animal studies that have examined concentrations of dopamine in disease states and animal models of disease in the central nervous system and periphery. Both original values and our calculated relative molar values are reported to easily compare concentrations of dopamine between studies.
| Original Value | Concentration of Dopamine (M) | Change From Baseline | Location | Species | Disease | Method | Reference |
|---|---|---|---|---|---|---|---|
| 133400 pmol/g | 1.3 × 10−4 Ma | No Change | Adrenal gland | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 130 pg/ugb | 8.5 × 10−4 Ma | Increased | Colon | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Levandis et al. 2015) |
| 57 pmol/g | 5.7 × 10−8 Ma | Decreased | Duodenum | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 35.7 fmol/mg | 3.6 × 10−8 Ma | Decreased | Frontal cortex | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Wisman et al. 2008) |
| 16 pmol/g | 1.6 × 10−8 Ma | Decreased | Heart | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 86 pmol/g | 8.6 × 10−8 Ma | No Change | Heart (Aorta) | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 17.2 fmol/mg | 1.7 × 10−8 Ma | Decreased | Hippocampus | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Wisman et al. 2008) |
| 165 pg/ugb | 1.1 × 10−3 Ma | No Change | Ileum | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Levandis et al. 2015) |
| 23 pmol/g | 2.3 × 10−8 Ma | Decreased | Kidney | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 6 pmol/g | 6 × 10−9 Ma | Decreased | Lung | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 68 pmol/g | 6.8 × 10−8 Ma | Decreased | Pancreas | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 27 pg/mgb | 1.8 × 10−7 Ma | Increased | Proximal colon | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Garrido-Gil et al. 2018b) |
| 43 pmol/g | 4.3 × 10−8 Ma | Decreased | Salivary gland | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 11 pmol/g | 1.1 × 10−8 Ma | Decreased | Spleen | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 104 pmol/g | 1 × 10−7 Ma | Decreased | Stomach | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 3 ng/mgb | 2 × 10−5 Ma | Decreased | Striatum | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Garrido-Gil et al. 2018b) |
| 14448 pmol/g | 1.4 × 10−5 Ma | No Change | Superior cervical ganglion | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 10 pmol/g | 1 × 10−8 Ma | No Change | Testis | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 7 pmol/g | 7 × 10−9 Ma | Decreased | Thymus | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 256 pmol/g | 2.6 × 10−7 Ma | Decreased | Vas deferens | Rat | 6-OHDA (Parkinson’s Disease Model) | HPLC | (Kawamura et al. 1999) |
| 1 ng/mg | 6.5 × 10−6 Ma | Decreased | Spinal cord (cervical dorsal horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 1.09 ng/mg | 7.1 × 10−6 Ma | No Change | Spinal cord (cervical ventral horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 0.82 ng/mg | 5.4 × 10−6 Ma | No Change | Spinal cord (lumbar dorsal horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 1.06 ng/mg | 6.9 × 10−6 Ma | No Change | Spinal cord (lumbar ventral horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 2.34 ng/mg | 1.5 × 10−5 Ma | No Change | Spinal cord (thoracic dorsal horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 3.1 ng/mg | 2 × 10−5 Ma | No Change | Spinal cord (thoracic lateral horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 2.17 ng/mg | 1.4 × 10−5 Ma | No Change | Spinal cord (thoracic ventral horn) | Rat | EAE (Multiple Sclerosis Model) | HPLC | (White et al. 1983) |
| 4,000 pg/mgb | 2.6 × 10−5 Ma | Decreased | Striatum | Mouse | EAE (Multiple Sclerosis Model) | HPLC | (Batkowiec-Iskra et al. 2007) |
| 2.102 pg/g log10 | 1 × 10−9 Ma | Decreased | Basal ganglia | Human | HIV | HPLC | (Kumar et al. 2009) |
| 2.086 pg/g log10 | 1 × 10−9 Ma | Decreased | Caudate | Human | HIV | HPLC | (Kumar et al. 2009) |
| 2.137 pg/g log10 | 1 × 10−9 Ma | No Baseline | CSF | Human | HIV | HPLC | (Kumar et al. 2009) |
| 1.9 pg/g log10 | 1 × 10−9 Ma | No Change | Frontal Cortex | Human | HIV | HPLC | (Kumar et al. 2009) |
| 2.198 pg/g log10 | 1 × 10−9 Ma | No Change | Globus pallidus | Human | HIV | HPLC | (Kumar et al. 2009) |
| 2.163 pg/g log10 | 1 × 10−9 Ma | Decreased | Putamen | Human | HIV | HPLC | (Kumar et al. 2009) |
| 1.747 pg/g log10 | 1 × 10−9 Ma | Decreased | Substantia Nigra | Human | HIV | HPLC | (Kumar et al. 2009) |
| 32.3 pg/mL | 2.1 × 10−10 Ma | Decreased | CSF | Human | HIV (Neurologically Asymptomatic) | HPLC | (Berger et al. 1994) |
| 18.97 pg/mL | 1.2 × 10−10 Ma | Decreased | CSF | Human | HIV (Neurologically Symptomatic) | HPLC | (Berger et al. 1994) |
| 25 pg/mLb | 1.6 × 10−10 Ma | Increased | CSF | Human | HIV (Therapy Naive, Asymptomatic) | HPLC | (Scheller et al. 2010) |
| 8.5 ng/g | 5.5 × 10−8 Ma | Decreased | Heart | Mouse | MPTP (Parkinson’s Disease Model) | HPLC | (Amino et al. 2008) |
| 0.46 pg/g | 3 × 10−6 Ma | Decreased | Putamen | Primate | MPTP (Parkinson’s Disease Model) | HPLC | (Pifl et al. 2014) |
| 5.2 pmol/mg | 5.2 × 10−6 Ma | Decreased | Spleen | Mouse | MPTP (Parkinson’s Disease Model) | HPLC | (Tsao et al. 1997) |
| 48 ng/mg | 3.1 × 10−4 Ma | Decreased | Striatum | Mouse | MPTP (Parkinson’s Disease Model) | HPLC | (Petzinger et al. 2007) |
| 6.9 pmol/mg | 6.9 × 10−6 Ma | Decreased | Thymus | Mouse | MPTP (Parkinson’s Disease Model) | HPLC | (Tsao et al. 1997) |
| 16.5 pmol/mg | 1.7 × 10−5 Ma | Decreased | Whole Brain | Mouse | MPTP (Parkinson’s Disease Model) | HPLC | (Tsao et al. 1997) |
| 0.3 ug/gb | 2 × 10−6 Ma | No Change | Olfactory Bulb | Primate | MPTP (Parkinson’s Disease Model) | HPLC | (Pifl et al. 2017) |
| 1.24 ng/mg | 8 × 10−6 Ma | Decreased | Caudate | Human | Parkinson’s Disease | HPLC | (Wilson et al. 1996b) |
| 2969 fmol/mg | 3 × 10−9 Ma | Decreased | Caudate | Human | Parkinson’s Disease | HPLC | (Goldstein et al. 2011) |
| 513 ng/g | 3.3 × 10−6 Ma | Decreased | Caudate nucleus | Human | Parkinson’s Disease | HPLC | (Rajput et al. 2008) |
| 83 fmol/mg | 8.3 × 10−8 Ma | No Change | Cortex | Human | Parkinson’s Disease | HPLC | (Goldstein et al. 2011) |
| 0.01 ng/ml | 6.5 × 10−11 Ma | No Change | CSF | Human | Parkinson’s Disease | HPLC | (Eldrup et al. 1995) |
| 1.22 ng/ml | 8 × 10−9 Ma | No Change | CSF | Human | Parkinson’s Disease | HPLC | (Engelborghs et al. 2003) |
| 0.01 nM | 1 × 10−11 M | No Change | CSF | Human | Parkinson’s Disease (No L-dopa Treatment) | HPLC | (Andersen et al. 2017) |
| 0.15 nM | 1.5 × 10−10 M | No Change | CSF | Human | Parkinson’s Disease (No L-dopa Treatment, Nondyskinetic) | HPLC | (Andersen et al. 2017) |
| 0.25 nM | 2.5 × 10−10 M | No Change | CSF | Human | Parkinson’s Disease (No L-dopa Treatment, Dyskinetic) | HPLC | (Andersen et al. 2017) |
| 89 ng/g | 5.8 × 10−7 Ma | Decreased | Globus pallidus (external) | Human | Parkinson’s Disease | HPLC | (Rajput et al. 2008) |
| 37 ng/g | 2.4 × 10−7 Ma | Decreased | Globus pallidus (Internal) | Human | Parkinson’s Disease | HPLC | (Rajput et al. 2008) |
| 0.02 ng/ml | 1.3 × 10−10 Ma | No Change | Plasma | Human | Parkinson’s Disease | HPLC | (Eldrup et al. 1995) |
| 1130 fmol/mg | 1.1 × 10−6 Ma | Decreased | Putamen | Human | Parkinson’s Disease | HPLC | (Goldstein et al. 2011) |
| 0.14 pg/g | 9 × 10−7 Ma | Decreased | Putamen | Human | Parkinson’s Disease | HPLC | (Pifl et al. 2014) |
| 0.21 ng/mg | 1.4 × 10−6 Ma | Decreased | Putamen | Human | Parkinson’s Disease | HPLC | (Wilson et al. 1996b) |
| 25 pg/ml | 1.6 × 10−10 Ma | No Baseline | Synovial fluid | Human | Rheumatoid Arthritis | HPLC | (Nakano et al. 2011) |
| 0.5 nMb | 5 × 10−10 M | No Baseline | TH+ Synovial Cells | Human | Rheumatoid Arthritis | HPLC | (Capellino et al. 2010) |
| 17 pmol/g | 1.7 × 10−8 Ma | Decreased | Colon | Rat | TNBS (Colitis Model) | HPLC | (Magro et al. 2004) |
| 28 pmol/g | 2.8 × 10−8 Ma | No Change | Ileum | Rat | TNBS (Colitis Model) | HPLC | (Magro et al. 2004) |
| 50 nmol/g | 5 × 10−8 Ma | Decreased | Colon | Human | Ulcerative Colitis and Crohn’s Disease | HPLC | (Magro et al. 2002) |
Concentrations calculated by dividing values by the molecular weight of dopamine (153.18 g/mol) if not already in a molar value, and multiplying the density of tissues or fluids (kg/L or kg/m^3)
Estimate obtained from graph because no values given in text, or averaged values if multiple control values were given
Value obtained from one or an average of treatment groups other than control because no absolute control given
Averaged male and female control groups
Dopamine in the Central Nervous System
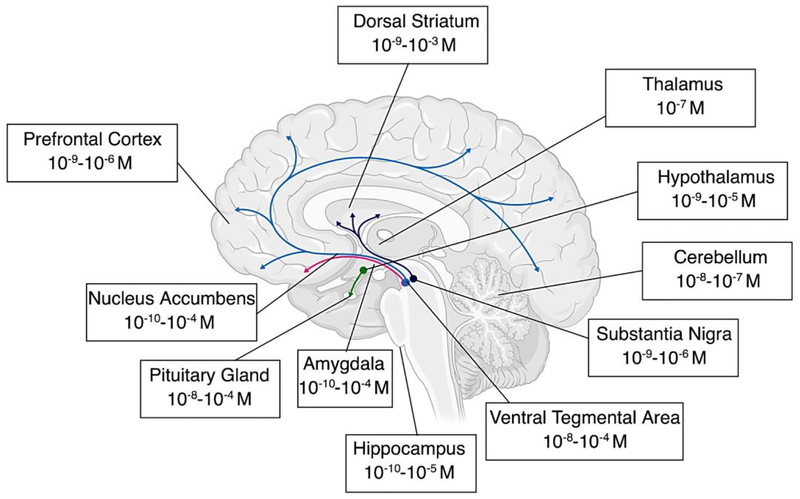
There are four main dopaminergic pathways in the mammalian brain; the nigrostriatal, mesolimbic, mesocortical, and tuberoinfundibular pathways. The nigrostriatal pathway is involved in motor control and starts in the substantia nigra (SbN), where dopaminergic neurons give rise to ascending fibers densely innervating the caudate and putamen (dorsal striatum). Both the mesolimbic pathway and mesocortical pathways are associated with the reward system (Wise 2004). The mesolimbic pathway connects the ventral tegmental area (VTA) to the limbic regions of the brain (nucleus accumbens, ventral striatum and amygdala), and the mesocortical pathway links the VTA to the cortex (medial, prefrontal, cingulate and entorhinal cortex). The tuberoinfundibular pathway is important in the inhibitory control of prolactin (Ben-Jonathan and Hnasko 2001) and runs from the arcuate and periventricular nuclei of the hypothalamus to the intermediate lobe of the pituitary and the median eminence. In addition to these regions, there are smaller amounts of dopamine in other areas in which immune cells are active, such as the CSF (Hubbard et al. 2009; Louveau et al. 2015) and the retina (Silverman and Wong 2018; Witkovsky 2004), but this review focuses on the major dopaminergic pathways.
Dopamine neurons represent only a fraction of the total CNS neuronal population, even within these regions, but they influence significant areas of the brain through networks of branching fibers and display diverse electrophysiological properties (Hauber 2010; Marinelli and McCutcheon 2014; Roeper 2013). These neurons operate in two distinct temporal modes, a “phasic” mode producing fast, transient dopamine release (seconds) through synchronized burst firing, and a tonic mode, which produces slow (minutes – hours), widespread dopamine release through non-synchronous spontaneous firing (Hauber 2010). Dopamine release is regulated by interactions with other neurons such as glutamatergic, cholinergic as well as GABAergic cells (Morikawa and Paladini 2011). The local dopamine concentration is also regulated by the relative rates of dopamine release and uptake, as they are regionally specific (Calipari et al. 2012; Cass and Gerhardt 1995; Cragg et al. 2000; Garris and Wightman 1994; Letchworth et al. 2001; Sulzer et al. 2016; Trout and Kruk 1992).
Dopamine neurons can communicate through either one-to-one synaptic wiring transmission, or through a one-to-many volume transmission. Modeling dopamine spillover during neurotransmission indicates that short distance volume transmission is the primary mode of dopamine-mediated communication (Agnati et al. 2010; Borroto-Escuela et al. 2018; Peters and Michael 2000; Venton et al. 2003). Additionally, the largest dimension of the dopamine synaptic cleft is small (300 nm) (Pickel et al. 1996), suggesting it was designed to promote dopamine efflux. This is also supported by ultrastructural studies showing many DRs and transporters are extrasynaptic (Caille et al. 1996; Levey et al. 1993; Nirenberg et al. 1996). These and other studies indicate that during volume transmission, a cloud of released dopamine spills out of the synapse in three dimensions and permeates the surrounding area (Cragg et al. 2001; Garris and Wightman 1994), exposing adjacent immune cells to elevated dopamine during neuronal communication. The dopaminergic tone in humans is unclear, but in rodents, tonic dopamine concentrations are commonly thought to be in the nanomolar range (Floresco et al. 2003; Keefe et al. 1993; Parsons and Justice 1992), while phasic dopamine concentrations can be as high as in the micromolar range (Garris et al. 1994; Kawagoe et al. 1992; Wanat et al. 2009). As the concentration of dopamine to which immune cells will be exposed depends on the regional dopaminergic tone, this section examines the concentrations of dopamine within these pathways (Table 1), the mechanisms contributing to these dopamine levels and the immune cells that could be exposed to dopamine in these regions under homeostatic and drug-using conditions.
Nigrostriatal, Mesolimbic and Mesocortical Dopamine Levels
The midbrain dopamine neurons making up the nigrostriatal, mesolimbic and mesocortical pathways are largely localized in the SbN and the VTA, with efferents reaching to the striatum, accumbens and several regions in the cortex. The basal dopamine levels in the rodent and primate striatum are thought to be around 10 – 30 nM (Owesson-White et al. 2012; Sulzer et al. 2016), although the estimates vary widely depending on the model and analytic technique used (Table 1, Figure 1). Although measuring the spatiotemporal dynamics of dopamine in vivo is difficult, models of dopamine release in the SbN suggest that during phasic firing, dopamine concentrations of 1 μM can be found up to 2 μm from the synapse, while concentrations of 10 nM can be found 8.2 um away (Cragg and Rice 2004). The distances in the striatum are suggested to be 2 – 7 μm for 1 uM dopamine, and 7 – 20 μm for 10 nM dopamine (Beyene et al. 2017; Cragg and Rice 2004; Staal et al. 2004; Sulzer et al. 2000), while models of the primate prefrontal cortex suggest that 10 nM dopamine can reach as far as 10 – 15 μm from the synapse during tonic firing, with concentrations as high as 90 nM during phasic output (Spühler and Hauri 2013). Examination of the nucleus accumbens suggests dopamine could reach 6 – 10 μm from the synapse at 10 nM concentrations (Cragg et al. 2001; Garris et al. 1994; Stamford et al. 1988). Some studies suggest that more extensive dopamine volume transmission may occur due to dopaminergic terminal-receptor mismatches in the retina, nucleus accumbens shell, and amygdala, reaching as far as 30 – 50 μm (Bjelke et al. 1996; Fuxe et al. 2003; Jansson et al. 1999).
Figure 1 -. Concentrations of Dopamine Throughout the Central Nervous System.
Range of dopamine concentrations found throughout the central nervous system, based on the summary of literature in Table 1. These values represent the range of calculated absolute molar values, which provide a simplified way to compare relative physiologically relevant concentrations across the brain. The dopaminergic pathways of the brain in which dopamine concentrations are the highest are highlighted; the nigrostriatal pathway starts in the substantia nigra and innervates the dorsal striatum (purple), the mesocortical pathway connects the ventral tegmental area to the cortex (blue), the mesolimbic pathway connects the ventral tegmental area to the limbic regions of the brain such as the amygdala and hippocampus (red), and the tuberoinfundibular pathway which runs from the hypothalamus to the pituitary (green). Concentrations in these regions change significantly during the use of illicit drugs (Table 2) and in different disease states (Table 4). For clarity, data showing concentrations that were outliers in the calculated range of concentrations for each region are excluded.
These “spheres of influence” are significantly affected by DAT function in these regions (Sulzer et al. 2016), and in the case of diseases that dysregulate DAT function, such as Parkinson’s Disease (Mackie et al. 2018) or HIV (Gaskill et al. 2017), the area exposed to dopamine could be much larger. An important caveat to these models is that they generally assume the only DAT taking up dopamine are those on dopaminergic neurons, whereas numerous studies have shown DAT is also present on immune cells and astrocytes, which may also influence dopamine concentrations. Further, the distances and concentrations modeled here are based on quantal release from a single synapse, and depending on the stimulus, the firing pattern and the number of synapses involved, the concentration of dopamine could be significantly greater (Arbuthnott and Wickens 2007). For instance, dopamine neurons projecting to the dorsal striatum and the nucleus accumbens shell show classical slow firing properties, whereas dopamine neurons in the medial VTA projecting to the amygdala or nucleus accumbens core have unconventional fast-firing properties that include an almost doubled basal firing rate and maximal firing rate (Hauber 2010; Lammel et al. 2008).
Thus, when microglia and macrophages in these regions are in close proximity to dopaminergic neurons, they would be exposed to dopamine concentrations ranging from 10 nM to 1 μM or higher. Microglia are particularly likely to encounter elevated dopamine in this way, as the density of these cell is particularly high in the SbN (Kim et al. 2000; Lawson et al. 1990; Yang et al. 2013), and both ultrastructural analysis and two-photon imaging studies show microglial processes contact neuronal cell bodies and dendritic spines (Tremblay et al. 2010; Wake et al. 2009). Similarly, all types of CNS macrophages have been shown to interact with neurons in different brain regions (Faraco et al. 2017). By participating in the ‘tripartite’ synapse (Farhy-Tselnicker and Allen 2018), astrocytes can also regulate synapses by direct contact (Hama et al. 2004; Nishida and Okabe 2007), and are interconnected with each other to expand the range and magnitude of synaptic regulation. Localization of substantial D1R on fine processes of astrocytes within the SbN and D2R in the prefrontal cortex suggest that they are a likely recipient for dopamine (Khan et al. 2001; Nagatomo et al. 2017), and that dopamine could impact large astroglial networks within these regions.
Tuberoinfundibular Dopamine
Studies indicate that the regions in this pathway contain high levels of dopamine, ranging from 1 μM to around 100 μM in the in both the hypothalamus and the pituitary (Table 1). While most neurons release dopamine into the synaptic cleft and bind to postsynaptic receptors, the majority of tuberoinfundibular dopaminergic neurons (TIDA) lack true synaptic contacts and are categorized as secretory neurons (Ben-Jonathan and Hnasko 2001). As such, dopamine diffuses away from the terminals through the perivascular space and is transported by portal blood to the pituitary. The rate of dopamine release from neurons of this pathway appears to be slower than from classical neurons, but the basal activity is high, making the dopaminergic environment within this pathway quite unique. Maintaining low circulating prolactin levels requires a continuous high input of dopamine and a high but sustainable rate of synthesis, but also a mechanism to allow for rapid decreases in dopamine to enable prolactin release during situations that result in massive changes in hormones like pregnancy. This is accomplished by hypothalamic TH activity that is basally constitutive but can be transiently inactivated, unlike TH in most tissues, which can rapidly generate dopamine for immediate release (Haycock and Haycock 1991). Both microglia and macrophages are active in the hypothalamus, mediating the inflammatory response to obesity (Valdearcos et al. 2017). Studies have also identified both dendritic cells (Glennon et al. 2015) and macrophages (Fujiwara et al. 2017) in the pituitary that may play a role in communicating immune activation to the hypothalamic pituitary adrenal (HPA) axis. As local dopamine concentrations fluctuate to regulate prolactin production (Lyons et al. 2012; Stagkourakis et al. 2016) or in response to diet (Volkow et al. 2011), immune cells located in this pathway could be exposed to significant dopamine fluctuations.
CNS Dopamine During Drug Abuse
The effects of drug abuse on CNS dopamine has been discussed in detail in a number of excellent recent reviews (Fox and Wightman 2017; Nutt et al. 2015; Solinas et al. 2018; Volkow and Morales 2015).
Therefore, this section will only briefly discuss these effects, focusing particularly on how drug abuse changes regional dopamine concentrations and the impact this may have on immune cell interactions. This is important, as recent research has revealed that CNS immune signaling may substantially contribute to dopamine signaling induced by drugs of abuse (Hutchinson and Watkins 2014; Lacagnina et al. 2017). The dopaminergic system, particularly the mesolimbic and mesocortical pathways, is activated by many of types of drugs of abuse, including psychostimulants, opioids, nicotine and alcohol (Di Chiara and Imperato 1988; Pierce and Kumaresan 2006; Volkow and Morales 2015). The specific pharmacological effects of these drugs are wide-ranging but many act, at least partially, by interfering with dopamine reuptake through antagonism or reversal of DAT (Sulzer 2011; Torres et al. 2003; Volkow et al. 1997). Despite the differences in mechanisms, all drugs of abuse increase extracellular DA levels, generally to the high nanomolar to low micromolar range (Table 2).
Table 2: Dopamine Concentrations in the Central Nervous System in Response to Drugs of Abuse.
A summary of human and animal studies that have examined concentrations of dopamine within the central nervous system in response to drugs of abuse. Both original values and our calculated relative molar values are reported to easily compare concentrations of dopamine between studies.
| Original Dopamine Concentration | Concentration of Dopamine (M) | Change from Baseline | Location | Species | Drug | Method | Reference |
|---|---|---|---|---|---|---|---|
| 900 pg/10 μlb | 5.9 × 10−7 Ma | Increased | Amygdala | Rat | Amphetamine | Microdialysis | (Tor-Agbidye et al. 2001) |
| 147 pg/20μl | 4.8 × 10−8 Ma | Increased | Nucleus Accumbens | Rat | Amphetamine | Microdialysis | (Hernandez and Hoebel 1988) |
| 70nMb | 7 × 10−8 M | Increased | Nucleus Accumbens | Rat | Amphetamine | Microdialysis | (Moghadda m and Bunney 1989) |
| 7nMb | 7 × 10−9 M | Increased | Prefrontal Cortex (Medial) | Rat | Amphetamine | Microdialysis | (Moghadda m and Bunney 1989) |
| 300 nMb (Phasic) | 3 × 10−7 M | Increased | Caudate/Putamen | Rat | Cocaine | FSCV | (Venton et al. 2003) |
| 110 nM (Tonic) | 1.1 × 10−7 M | Increased | Caudate/Putamen | Rat | Cocaine | FSCV | (Venton et al. 2003) |
| 4175 fmol/35 μlb | 1.2 × 10−7 Ma | Increased | Nucleus Accumbens | Mouse | Cocaine | Microdialysis | (Airavaara et al. 2004) |
| 65 nM | 6.5 × 10−8 M | Increased | Nucleus Accumbens | Rat | Cocaine | FSCV | (Phillips et al. 2003) |
| 36 nMb | 3.6 × 10−8 M | Increased | Nucleus Accumbens | Rat | Cocaine | Microdialysis | (Smith et al. 2006) |
| 122nMb | 1.2 × 10−7 M | Increased | Nucleus Accumbens | Rat | Cocaine | Microdialysis | (Moghadda m and Bunney 1989) |
| 85 pg/20 μlb | 2.8 × 10−8 Ma | Increased | Nucleus Accumbens | Rat | Cocaine | Microdialysis | (Hernandez and Hoebel 1988) |
| 2.62 μM | 2.6 × 10−6 M | Increased | Nucleus Accumbens | Rat | Cocaine | Microdialysis | (Pettit et al. 1990) |
| 70 nM | 7 × 10−8 M | Increased | Nucleus Accumbens (Core) | Rat | Cocaine | FSCV | (Wightman et al. 2007) |
| 70 nM | 7 × 10−8 M | Increased | Nucleus Accumbens (Core) | Rat | Cocaine | FSCV | (Stuber et al. 2005) |
| 0.83 nMb | 8.3 × 10−10 M | Increased | Nucleus Accumbens (Shell) | Rat | Cocaine | LC-MS/MS | (Hows et al. 2004) |
| 250 fmol/50μl | 4.9 × 10−7 Ma | Increased | Nucleus Accumbens (Shell) | Rat | Cocaine | Microdialysis | (Fadda et al. 2003) |
| 8nMb | 8 × 10−9 M | No Change | Prefrontal Cortex (Medial) | Rat | Cocaine | Microdialysis | (Moghadda m and Bunney 1989) |
| 35 fmol/μlb | 3.5 × 10−8 Ma | Increased | Striatum | Primate | Cocaine | Microdialysis | (Bradberry 2000) |
| 60 nMb | 6 × 10−8 M | Increased | Striatum | Rat | Cocaine | Microdialysis | (Shou et al. 2006) |
| 3.6 nMb | 3.6 × 10−9 M | Increased | Caudate/Putamen | Mouse | Ethanol | Microdialysis | (Bosse and Mathews 2011) |
| 2.96 fmol/μlb | 3 × 10−9 Ma | Increased | Nucleus Accumbens | Rat | Ethanol | Microdialysis | (Yan 1999) |
| 6.21 nM | 6.2 × 10−9 M | Increased | Nucleus Accumbens | Rat | Ethanol | Microdialysis | (Weiss et al. 1996) |
| 13.2 nM | 1.3 × 10−8 M | Increased | Nucleus Accumbens | Rat | Ethanol | Microdialysis | (Yim and Gonzales 2000) |
| 14 nMb | 1.4 × 10−8 M | No Change | Nucleus Accumbens | Rat | Heroin | Microdialysis | (Smith et al. 2006) |
| 8.8 nMb | 8.8 × 10−9 M | Increased | Nucleus Accumbens | Rat | Heroin | Microdialysis | (Hemby et al. 1995) |
| 2.97 ng/mg | 1.9 × 10−5 Ma | Decreased | Caudate | Human | Methamphetamine | HPLC | (Wilson et al. 1996a) |
| 1.48 ng/mg | 1 × 10−5 Ma | Decreased | Nucleus Accumbens | Human | Methamphetamine | HPLC | (Wilson et al. 1996a) |
| 3.49 ng/mg | 2.3 × 10−5 Ma | Decreased | Putamen | Human | Methamphetamine | HPLC | (Wilson et al. 1996a) |
| 0.1 nmol/mg | 1 × 10−4 Ma | Decreased | Striatum | Mouse | Methamphetamine | HPLC | (Kita et al. 2000) |
| 50 nMb | 5 × 10−8 M | Increased | Nucleus Accumbens (Core) | Rat | Morphine | FSCV | (Vander Weele et al. 2014) |
| 0.02 pmol/20 μl | 1 × 10−9 Ma | Increased | Nucleus Accumbens | Rat | Morphine | Microdialysis | (Pothos et al. 1991) |
| 0.21 pmol/25 μlb | 8.4 × 10−9 Ma | No Change | Nucleus Accumbens | Rat | Morphine | Microdialysis | (Anagnosta kis and Spyraki 1994) |
| 50 nMb | 5 × 10−8 M | Increased | Nucleus Accumbens (Shell) | Rat | Morphine | FSCV | (Vander Weele et al. 2014) |
| 200 fmol/50μl | 4 × 10−7 Ma | Increased | Nucleus Accumbens (Shell) | Rat | Morphine | Microdialysis | (Fadda et al. 2003) |
| 250 nMb | 2.5 × 10−7 M | Increased | Nucleus Accumbens (Core) | Rat | Oxycodone | FSCV | (Vander Weele et al. 2014) |
| 500 nMb | 5 × 10−7 M | Increased | Nucleus Accumbens (Shell) | Rat | Oxycodone | FSCV | (Vander Weele et al. 2014) |
| 1.5 nM | 1.5 × 10−9 M | Decreased | Striatum | Mouse | Oxycodone | Microdialysis | (Zhang et al. 2009) |
| 88 fmol/40 μlb | 2.2 × 10−9 Ma | No Change | Nucleus Accumbens | Rat | THC | Microdialysis | (Chen et al. 1993) |
| 134 nmol/Lb | 1.3 × 10−7 Ma | Decreased | Prefrontal Cortex | Rat | THC | Microdialysis | (Pistis et al. 2002) |
| 604.5 fmol/40 μlb | 1.5 × 10−8 Ma | Increased | Ventral Tegmental Area | Rat | THC | Microdialysis | (Chen et al. 1993) |
Concentrations calculated by dividing values by the molecular weight of dopamine (153.18 g/mol) if not already in a molar value, and multiplying the density of tissues or fluids (kg/L or kg/m^3)
Estimate obtained from graph because no values given in text, or averaged values if multiple control values were given
Value obtained from one or an average of treatment groups other than control because no absolute control given
Averaged male and female control groups
These increases in dopamine concentrations would expand the volume of the brain permeated by dopamine, and also increase the distance from the synapse at which higher concentrations of dopamine are present (Peters and Michael 2000; Spühler and Hauri 2013; Venton et al. 2003). This could substantially increase the number of immune cells which interact with dopamine, with larger increases in tissues that have proportionately greater responses to drug use. The largest drug-induced elevations in dopamine concentrations generally occur within the basal ganglia, specifically in the striatum and nucleus accumbens (Fadda et al. 2003; Shou et al. 2006; Stuber et al. 2005; Wightman et al. 2007). In both striatum and prefrontal cortex, increased dopamine concentrations induced by blockade of dopamine reuptake using cocaine, methylphenidate or nomifensine enhance the diffusion of dopamine, increasing the volume of tissue exposed to this neurotransmitter by as much as 50% (Peters and Michael 2000; Spühler and Hauri 2013; Venton et al. 2003). Changes in dopamine reuptake could also enhance heterogeneity in dopaminergic tone, and create local “hot spots” with unusually high dopamine concentrations (Peters and Michael 2000; Spühler and Hauri 2013). Another factor influencing the interaction of dopamine with immune cells is that different types of drugs have a regionally distinct impact on dopamine diffusion and reuptake, suggesting the changes in immune cell exposure could differ in magnitude across the brain (Cass et al. 1992; Cragg and Greenfield 1997; Jones et al. 1995; Porrino et al. 2004; Salinas et al. 2016). There are also regional differences in the homeostatic rate of uptake that could affect immune cell responses. For example, cocaine mediated inhibition of uptake in a region where it tightly controls extracellular DA such as the striatum would have a different effect on extracellular levels than in a brain region where uptake does not regulate DA as closely such as in the nucleus accumbens shell (Wu et al. 2001).
Drug-induced increases in dopamine likely have a large impact on CNS immune cells and astrocytes, as many drugs of abuse increase expression of microglial and astrocytic markers, increase cytokine/chemokine release, and promote pro-inflammatory glial phenotypes (Alfonso-Loeches et al. 2010; Cadet and Bisagno 2014; Schwarz and Bilbo 2013; Wang et al. 2012). Specifically, glial inhibitors and cytokines can augment drug-induced dopamine release (Bland et al. 2009; Hutchinson et al. 2008; Nakajima et al. 2004; Zhang et al. 2006), demonstrating that CNS immune cells could modulate the effects of drugs of abuse and interact with dopamine during drug exposure. Synaptic remodeling may also occur during increased exposure to dopamine during drug abuse, which could contribute to the persistent behavioral effects typical of substance abuse disorders (Coller and Hutchinson 2012; Kovacs 2012). Importantly, the changes in dopaminergic tone evoked by drug abuse will also depend on the timing, method of delivery and length of drug exposure. There are large differences in dopamine response between chronic drug abusers and intermittent or naive users (Sklair-Tavron et al. 1996; Volkow et al. 2010; Wu and French 2000), and some studies show that chronic drug use decreases drug-induced dopamine release (Volkow et al. 1996; Wilson et al. 1996a). Thus, it is important to consider not only the neurological effects of the drug being used, but also the epidemiological context of the substance abuser in order to develop a complete picture of how the changes in CNS dopamine induced in a particular drug abuser impact the immune cells in the CNS.
Dopamine in Peripheral Systems
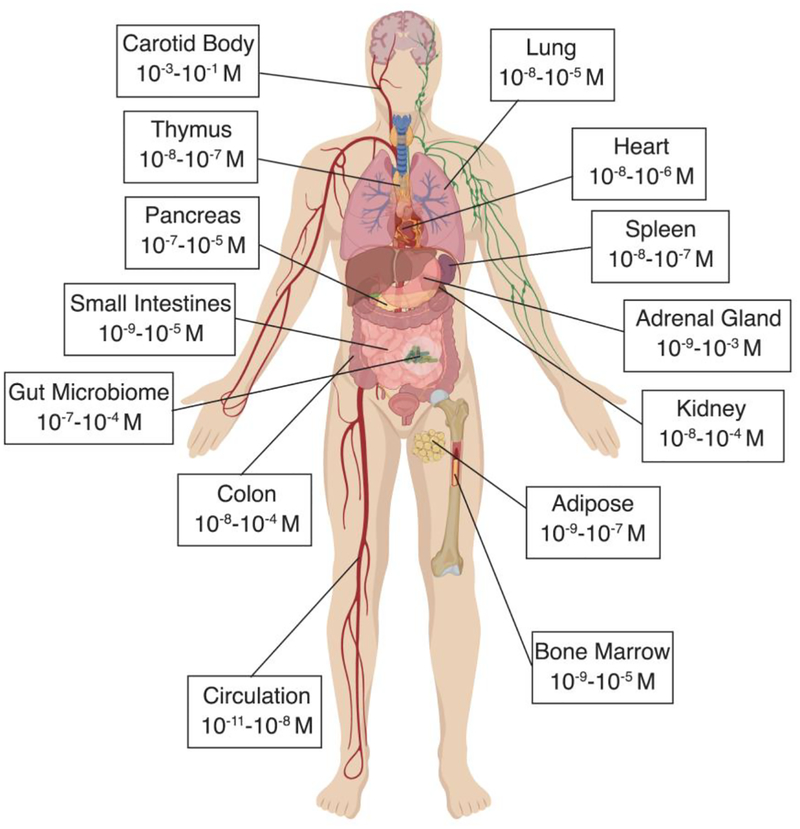
It has been more than five decades since a peripheral role for dopamine was first described (Goldberg 1972), and while dopamine is most often studied in the context of its actions in the CNS, this neurotransmitter is also present throughout the periphery. Peripheral dopamine plays an important regulatory role in a variety of functions including hormone secretion, vascular tone, sympathetic regulation, immune activation, gastrointestinal motility, blood pressure, respiration, and renal functions (Arreola et al. 2016; Goldstein et al. 1995; Rubí and Maechler 2010). Dopamine can be released from sympathetic nerves and the adrenal medulla, as well as from other peripheral organs, where dopamine can act as an autocrine/paracrine regulator of local organ function. This section focuses on the available research showing the concentrations of dopamine in peripheral compartments (Table 3), and discusses how the dopaminergic machinery found in peripheral systems affects the amount of dopamine seen by immune cells in these regions. Specifically, the tissues discussed here are those in which multiple studies have reported measurable dopamine concentrations that could interact with resident immune cells. Tissues that are not discussed may also express sufficient dopamine to affect immune cells, but either the reports of this were scarce or to our knowledge it has not be demonstrated yet. For example, very few studies have reported dopamine concentrations in the liver. However, the liver receives both parasympathetic and sympathetic input (Yi et al. 2010), and is known to have some of the highest expression of COMT in the body (Männistö and Kaakkola 1999; Myohanen et al. 2010), suggesting it plays a major role in metabolizing dopamine (Eisenhofer et al. 1995). Therefore, more research is warranted to further characterize additional sources of dopamine and dopaminergic regulation throughout the body.
Table 3: Dopamine Concentrations in the Periphery.
A summary of human and animal studies that have examined concentrations of dopamine within the periphery. Both original values and our calculated relative molar values are reported to easily compare concentrations of dopamine between studies.
| Original Dopamine Concentration | Concentration of Dopamine (M) | Location | Species | Method | Reference |
|---|---|---|---|---|---|
| 30 ng/gb | 2 × 10−7 Ma | Adipose (Brown) | Mouse | HPLC | (Griggio et al. 1992) |
| 1 pg/mgc | 6.5 × 10−9 Ma | Adipose (Epididymal White) | Mouse | HPLC | (Nagler et al. 2018) |
| 100 pg/mgb | 7 × 10−7 Ma | Adipose (Mesenteric Tissue Cells) | Rat | HPLC | (Vargovic et al. 2011) |
| 0.0853 nmol/g | 8.5 × 10−8 Ma | Adrenal Gland | Primate | HPLC | (Elchisak et al. 1983) |
| 430 ng/kg | 2.8 × 10−9 Ma | Adrenal Gland | Rat | HPLC | (Snider and Kuchel 1983) |
| 94600 pmol/g | 9.5 × 10−5 Ma | Adrenal Gland | Rat | HPLC | (Kawamura et al. 1999) |
| 51 nmol/g | 5.1 × 10−5 Ma | Adrenal Gland (Cortex) | Rat | HPLC | (Hannah et al. 1984) |
| 0.5 ng/mlb | 3.3 × 10−9 Ma | Adrenal Gland (Medulla) | Mouse | HPLC | (Torres-Rosas et al. 2014) |
| 6 nmol/g | 6 × 10−6 Ma | Adrenal Gland (Medulla) | Rat | HPLC | (Hannah et al. 1984) |
| 0.37 mg/g | 2.4 × 10−3 Ma | Adrenal Gland (Medulla) | Rat | HPLC | (Ortega-Saenz et al. 2016) |
| 24 pmol/mg | 2.4 × 10−5 Ma | Adrenal Gland (Medulla) | Rat | HPLC | (Favre et al. 1986) |
| 6.1 ug/g | 4 × 10−5 Ma | Adrenal Gland (Medulla) | Rat | HPLC | (Fhaneret al. 2013) |
| 1.1 ng/mlc | 7 × 10−9 Ma | Amniotic Fluid | Human | HPLC | (Jonathan and Munsick 1980) |
| 45 pmol/g | 4.5 × 10−8 Ma | Aorta | Rat | HPLC | (Kawamura et al. 1999) |
| 0.15 μg/g | 9.8 × 10−7 Ma | Aorta | Sheep | HPLC | (Juorio and Chedrese 1990) |
| 0.043 μg/g | 3 × 10−7 Ma | Artery (Mesenteric) | Rat | HPLC | (Bell and Gillespie 1981) |
| 0.15 pmol/mg | 1.5 × 10−7 Ma | Bladder | Rat | HPLC | (Favre et al. 1986) |
| 2 nMb | 2 × 10−9 M | Bone Marrow | Mouse | HPLC | (Maestroni et al. 1998) |
| 250 pg/gb | 1.6 × 10−9 Ma | Bone Marrow | Mouse | HPLC | (Marino et al. 1997) |
| 35 ng/mgb | 2.3 × 10−5 Ma | Bone Marrow | Mouse | HPLC | (Chakroborty et al. 2008) |
| 202 ng/gb | 1.3 × 10−3 Ma | Carotid Body | Human (infant) | HPLC | (Perrin et al. 1984) |
| 209.6 pmol/mg | 2.1 × 10−1 Ma | Carotid Body | Rat | HPLC | (Vicario et al. 2000) |
| 84.04 mg/g | 5.5 × 10−1 Ma | Carotid Body | Rat | HPLC | (Ortega-Saenz et al. 2016) |
| 3.3 nmol/mg | 3.3 × 10−3 Ma | Carotid Body | Rat | HPLC | (Hanbauer et al. 1981) |
| 256.2 pmol/mg | 2.6 × 10−1 Ma | Carotid Body | Rat | HPLC | (Prieto-Lloret et al. 2015) |
| 129 pmol/mg | 1.3 × 10−1 Ma | Carotid Body | Rat | HPLC | (Favre et al. 1986) |
| 115.4 ng/g | 7.5 × 10−7 Ma | Cecum | Mouse | HPLC | (Asano et al. 2012) |
| 140 pmol/gb | 1.4 × 10−7 Ma | Colon | Human | HPLC | (Magro et al. 2002) |
| 177 ng/g | 1.2 × 10−6 Ma | Colon | Mouse | HPLC | (Asano et al. 2012) |
| 26 pmol/g | 3 × 10−8 Ma | Colon | Rat | HPLC | (Magro et al. 2004) |
| 60 pg/μgb | 3.9 × 10−4 Ma | Colon | Rat | HPLC | (Levandis et al. 2015) |
| 100 pg/mgb | 6.5 × 10−7 Ma | Colon (Ascending) | Mouse | HPLC | (Garrido-Gil et al. 2018a) |
| 15 pg/mg | 9.8 × 10−8 Ma | Colon (Proximal) | Rat | HPLC | (Garrido-Gil et al. 2018a) |
| 12 pg/mgb | 7.8 × 10−8 Ma | Colon (Proximal) | Rat | HPLC | (Garrido-Gil et al. 2018b) |
| 239 pmol/g | 2.4 × 10−7 Ma | Duodenum | Rat | HPLC | (Kawamura et al. 1999) |
| 3.67 μg/g | 2.4 × 10−5 Ma | Duodenum | Sheep | HPLC | (Juorio and Chedrese 1990) |
| 276 pg/ml | 1.8 × 10−9 Ma | Duodenum (Juice) | Rat | HPLC | (Mezey et al. 1996) |
| 13 pmol/mg | 1.3 × 10−5 Ma | Ganglion (Coeliac) | Rat | HPLC | (Favre et al. 1986) |
| 15 pmol/mg | 1.5 × 10−5 Ma | Ganglion (Mesenteric) | Rat | HPLC | (Favre et al. 1986) |
| 26 pmol/mg | 2.6 × 10−5 Ma | Ganglion (Superior Cervical) | Rat | HPLC | (Favre et al. 1986) |
| 8082 pmol/g | 8.1 × 10−6 Ma | Ganglion (Superior Cervical) | Rat | HPLC | (Kawamura et al. 1999) |
| 14 pmol/mg | 1.4 × 10−5 Ma | Ganglion (Superior Cervical) | Rat | HPLC | (Prieto-Lloret et al. 2015) |
| 0.52 pg/μg | 3 × 10−6 Ma | Heart | Human | HPLC | (Regitz et al. 1990) |
| 15 ng/gb | 1 × 10−7 Ma | Heart | Mouse | HPLC | (Griggio et al. 1992) |
| 55.9 ng/g | 3.6 × 10−7 Ma | Heart | Mouse | HPLC | (Amino et al. 2008) |
| 30 ng/g | 2 × 10−7 Ma | Heart | Mouse | HPLC | (Wagner et al. 1979b) |
| 30 ng/gb | 2 × 10−7 Ma | Heart | Pig | HPLC | (Schoeneman n et al. 1990) |
| 12 ng/gb | 8 × 10−8 Ma | Heart | Rat | HPLC | (Schoeneman n et al. 1990) |
| 15 ng/g | 1 × 10−7 Ma | Heart | Rat | HPLC | (Snider and Kuchel 1983) |
| 10 ng/gb | 6.5 × 10−8 Ma | Heart | Rat | HPLC | (Eldrup 2004) |
| 0.11 pmol/mg | 1.1 × 10−7 Ma | Heart | Rat | HPLC | (Favre et al. 1986) |
| 170 ng/gb | 1.1 × 10−6 Ma | Heart (Atrium) | Dog | HPLC | (Mohanty et al. 1986) |
| 0.58 nmol/g | 5.8 × 10−7 Ma | Heart (Atrium) | Primate | HPLC | (Elchisak et al. 1983) |
| 0.135 μg/g | 9 × 10−7 Ma | Heart (Atrium) | Mouse | HPLC | (Bell and Gillespie 1981) |
| 143 ng/g | 9.3 × 10−7 Ma | Heart (Myocardium) | Human | HPLC | (Pierpont et al. 1987) |
| 120 ng/gb | 7.8 × 10−7 Ma | Heart (Ventricle) | Dog | HPLC | (Mohanty et al. 1986) |
| 0.549 nmol/g | 5.5 × 10−7 Ma | Heart (Ventricle) | Primate | HPLC | (Elchisak et al. 1983) |
| 15.7 ng/g | 1 × 10−7 Ma | Ileum | Mouse | HPLC | (Asano et al. 2012) |
| 30 pmol/g | 3 × 10−8 Ma | Ileum | Rat | HPLC | (Magro et al. 2004) |
| 160 pg/μgb | 1 × 10−3 Ma | Ileum | Rat | HPLC | (Levandis et al. 2015) |
| 0.03 pmol/g | 3 × 10−8 Ma | Jejunum | Mouse | HPLC | (Quelhas-Santos et al. 2015) |
| 36 pmol/gb | 3.6 × 10−8 Ma | Jejunum (Epithelial cells) | Rat | HPLC | (Vieira-Coelho et al. 1998) |
| 41 pmol/g | 4.1 × 10−8 Ma | Jejunum (Mucosa) | Rat | HPLC | (Vieira-Coelho et al. 1998) |
| 61 pmol/g | 6.1 × 10−8 Ma | Jejunum (Mucosa) | Rat | HPLC | (Finkel et al. 1994) |
| 13.3 ng/g | 8.6 × 10−8 Ma | Kidney | Mouse | HPLC | (Wagner et al. 1979a) |
| 115 ng/mgb | 7.5 × 10−4 Ma | Kidney | Mouse | HPLC | (Zhang et al. 2011a) |
| 0.5 ng/mgb | 3.3 × 10−6 Ma | Kidney | Mouse | HPLC | (Weinman et al. 2011) |
| 33 pmol/mg | 3.3 × 10−5 Ma | Kidney | Rat | HPLC | (Wahbe et al. 1982) |
| 5 ng/gb | 3 × 10−8 Ma | Kidney | Rat | HPLC | (Snider and Kuchel 1983) |
| 50 pmol/g | 5 × 10−8 Ma | Kidney | Rat | HPLC | (Kawamura et al. 1999) |
| 0.04 pmol/mg | 4 × 10−8 Ma | Kidney | Rat | HPLC | (Favre et al. 1986) |
| 0.846 nmol/g | 8.5 × 10−7 Ma | Kidney (Cortex) | Primate | HPLC | (Elchisak et al. 1983) |
| 0.017 μg/g | 1 × 10−7 Ma | Kidney (Cortex) | Rat | HPLC | (Bell and Gillespie 1981) |
| 0.225 nmol/g | 2.3 × 10−7 Ma | Kidney (Medulla) | Primate | HPLC | (Elchisak et al. 1983) |
| 0.092 nmol/g | 9.2 × 10−8 Ma | Liver | Primate | HPLC | (Elchisak et al. 1983) |
| 0.011 pmol/mg | 1.1 × 10−8 Ma | Liver | Rat | HPLC | (Favre et al. 1986) |
| 1.697 μg/g | 1.1 × 10−5 Ma | Lung | Cow | HPLC | (Eyre 1971) |
| 0.11 μg/g | 7 × 10−7 Ma | Lung | Human | HPLC | (Aviado and Sadavongviva d1970) |
| 0.0446 nmol/g | 4.5 × 10−8 Ma | Lung | Primate | HPLC | (Elchisak et al. 1983) |
| 40 ng/g | 2.6 × 10−7 Ma | Lung | Rat | ELISA | (Hampl et al. 2015) |
| 34 pmol/g | 3.4 × 10−8 Ma | Lung | Rat | HPLC | (Kawamura et al. 1999) |
| 58.8 pmol/g | 5.9 × 10−8 Ma | Lung | Rat | HPLC | (Scarcella and Bryan-Lluka 1995) |
| 9.96 μg/g | 6.5 × 10−5 Ma | Lung | Sheep | HPLC | (Juorio and Chedrese 1990) |
| 0.021 pmol/mg | 2.1 × 10−8 Ma | Lung/Trachea | Rat | HPLC | (Favre et al. 1986) |
| 0.5 μmol/kgb | 5 × 10−7 Ma | Microbiome (Biomass) | E coli | HPLC | (Shishov et al. 2009) |
| 0.1 μmol/kgb | 1 × 10−7 Ma | Microbiome (Supernatant) | E coli | HPLC | (Shishov et al. 2009) |
| 79.4 μg/mlb | 5.2 × 10−4 Ma | Microbiome (Supernatant) | E faecium | HPLC | (Villageliu and Lyte 2018) |
| 0.73 mg/L | 4.8 × 10−6 Ma | Microbiome (Supernatant) | Hafnia alvei | HPLC | (Özoğul 2004) |
| 1.06 mg/L | 6.9 × 10−6 Ma | Microbiome (Supernatant) | Klebsiella pneumoniae | HPLC | (Özoğul 2004) |
| 2.46 mg/L | 1.6 × 10−5 Ma | Microbiome (Supernatant) | Morganella morganii | HPLC | (Özoğul 2004) |
| 29 pg/mgc | 1.8 × 10−7 Ma | Pancreas | Mouse | HPLC | (Nagler et al. 2018) |
| 0.218 nmol/g | 2.2 × 10−7 Ma | Pancreas | Primate | HPLC | (Elchisak et al. 1983) |
| 84 pmol/mg | 8.4 × 10−5 Ma | Pancreas | Rat | HPLC | (Mezey et al. 1996) |
| 103 pmol/g | 1 × 10−7 Ma | Pancreas | Rat | HPLC | (Kawamura et al. 1999) |
| 8 μmol/kg | 8 × 10−6 Ma | Pancreas (Islets) | Golden hamster | HPLC | (Zern et al. 1980) |
| 10 pg/ml | 6.5 × 10−11 Ma | Plasma | Human | HPLC | (Saha et al. 2001) |
| 17 pg/ml | 3.5 × 10−8 Ma | Plasma | Human | HPLC | (Mitchell et al. 2018) |
| 10 pg/ml | 6.5 × 10−11 Ma | Plasma | Human | HPLC | (Gardner and Shoback 2007) |
| 10 pg/ml | 6.5 × 10−11 Ma | Plasma | Human | HPLC | (Lechin et al. 1990) |
| 38.9 pg/ml | 2.5 × 10−l0 Ma | Plasma | Human | HPLC | (Scozzi et al. 2012) |
| 22.14 ng/L | 1.4 × 10−10 Ma | Plasma | Human | HPLC | (Ambade et al. 2009) |
| 0.02 ng/ml | 1.3 × 10−l0 Ma | Plasma | Human | HPLC | (Eldrup et al. 1995) |
| 55.5ng/L | 3.6 × 10−10 Ma | Plasma | Human | HPLC | (Iwen et al. 2017) |
| 0.76 ng/ml | 5 × 10−9 Ma | Plasma | Mouse | HPLC | (Kavelaars et al. 2005) |
| 0.9 ng/ml | 5.9 × 10−9 Ma | Plasma | Mouse | HPLC | (Alaniz et al. 1999) |
| 75 pg/mlb | 4.9 × 10−10 Ma | Plasma | Mouse | HPLC | (Kanemi et al. 2005) |
| 7 pmol/ml | 7 × 10−9 Ma | Plasma | Mouse | HPLC | (Quelhas-Santos et al. 2015) |
| 0.1 ng/mlb | 7 × 10−10 Ma | Plasma | Rat | HPLC | (De Laurentiis et al. 2002) |
| 0.15 ng/mlb | 9.8 × 10−10 Ma | Plasma | Rat | HPLC | (Snider and Kuchel 1983) |
| 0.32 nmol/L | 3.2 × 10−10 Ma | Plasma (arterial) | Human | HPLC | (Goldstein et al. 1999) |
| 0.23 nmol/L | 2.3 × 10−10 Ma | Plasma (Hepatic Vein) | Human | HPLC | (Goldstein et al. 1999) |
| 0.93 nmol/L | 9.3 × 10−10 Ma | Plasma (Portal Vein) | Human | HPLC | (Goldstein et al. 1999) |
| 2 ng/ml | 1.3 × 10−10 Ma | Plasma (Umbilical Cord) | Human | HPLC | (Jonathan and Munsick 1980) |
| 18 ng/g | 1.8 × 10−7 Ma | Salivary Gland | Rat | HPLC | (Snider and Kuchel 1983) |
| 39 ng/gb | 2.5 × 10−7 Ma | Salivary Gland | Rat | HPLC | (Tomassoni et al. 2015) |
| 211 pmol/g | 2.1 × 10−7 Ma | Salivary Gland | Rat | HPLC | (Kawamura et al. 1999) |
| 1 pmol/mg | 1 × 10−6 Ma | Seminal Vesicles | Rat | HPLC | (Favre et al. 1986) |
| 0.032 pmol/mg | 3.2 × 10−8 Ma | Small intestine | Rat | HPLC | (Favre et al. 1986) |
| 1.31 ng/mg | 8.6 × 10−6 Ma | Spinal Cord (Cervical Dorsal Horn) | Rat | HPLC | (White et al. 1983) |
| 0.65 ng/mg | 4.2 × 10−6 Ma | Spinal Cord (Cervical Ventral Horn) | Rat | HPLC | (White et al. 1983) |
| 0.79 ng/mg | 5.2 × 10−6 Ma | Spinal Cord (Lumbar Dorsal Horn) | Rat | HPLC | (White et al. 1983) |
| 0.67 ng/mg | 4.4 × 10−6 Ma | Spinal Cord (Lumbar Ventral Horn) | Rat | HPLC | (White et al. 1983) |
| 2.18 ng/mg | 1.4 × 10−5 Ma | Spinal Cord (Thoracic Dorsal Horn) | Rat | HPLC | (White et al. 1983) |
| 2.67 ng/mg | 1.7 × 10−5 Ma | Spinal Cord (Thoracic Lateral Horn) | Rat | HPLC | (White et al. 1983) |
| 1.88 ng/mg | 1.2 × 10−5 Ma | Spinal Cord (Thoracic Ventral Horn) | Rat | HPLC | (White et al. 1983) |
| 13.5 pmol/g | 1 × 10−7 Ma | Spleen | Mouse | HPLC | (Tsao et al. 1997) |
| 50 ng/gb | 3 × 10−7 Ma | Spleen | Pig | HPLC | (Schoeneman n et al. 1990) |
| 0.173 nmol/g | 1.7 × 10−7 Ma | Spleen | Primate | HPLC | (Elchisak et al. 1983) |
| 10 ng/gb | 7 × 10−8 Ma | Spleen | Rat | HPLC | (Schoeneman n et al. 1990) |
| 0.011 μg/g | 7 × 10−8 Ma | Spleen | Rat | HPLC | (Bell and Gillespie 1981) |
| 0.084 pmol/mg | 8.4 × 10−8 Ma | Spleen | Rat | HPLC | (Favre et al. 1986) |
| 152 pmol/g | 1.5 × 10−7 Ma | Spleen | Rat | HPLC | (Kawamura et al. 1999) |
| 0.078 pmol/mg | 7.8 × 10−8 Ma | Stomach | Rat | HPLC | (Favre et al. 1986) |
| 185 pmol/g | 1.9 × 10−7 Ma | Stomach | Rat | HPLC | (Kawamura et al. 1999) |
| 20 ng/gb | 1.3 × 10−7 Ma | Stomach | Rat | HPLC | (Eldrup 2004) |
| 32.9 ng/g | 3 × 10−7 Ma | Stomach (Gastric Corpus Mucosa) | Guinea pig | HPLC | (Shichijo et al. 1997) |
| 95.8 ng/g | 6 × 10−7 Ma | Stomach (Gastric Corpus Muscle) | Guinea pig | HPLC | (Shichijo et al. 1997) |
| 5.41 ng/ml | 4 × 10−8 Ma | Stomach (Gastric Juice) | Human | HPLC | (Christensen and Brandsborg 1974) |
| 1.02 nmol/g | 1 × 10−6 Ma | Testis | Primate | HPLC | (Elchisak et al. 1983) |
| 5 pmol/g | 5 × 10−9 Ma | Testis | Rat | HPLC | (Kawamura et al. 1999) |
| 12.7 pmol/mg | 1 × 10−7 Ma | Thymus | Mouse | HPLC | (Tsao et al. 1997) |
| 15 pmol/g | 1.5 × 10−8 M | Thymus | Rat | HPLC | (Kawamura et al. 1999) |
| 25 ng/gd | 1.6 × 10−7 Ma | Thymus | Rat | HPLC | (Pilipovic et al. 2008) |
| 0.352 μg/g | 2.3 × 10−6 Ma | Vas Deferens | Rat | HPLC | (Bell and Gillespie 1981) |
| 2.1 pmol/mg | 2.1 × 10−6 Ma | Vas Deferens | Rat | HPLC | (Favre et al. 1986) |
| 2466 pmol/g | 2.5 × 10−6 Ma | Vas Deferens | Rat | HPLC | (Kawamura et al. 1999) |
Con cent ratio ns calculated by dividing values by the molecular weight of dopamine (153 .18 g/m ol) if not already in a molar value, and multiplying the density of tissues or fluid s (kg/L or kg/m^3)
Estimate obtained from graph because no values given in text, or averaged values if multiple control values were given
Value obtained from one or an average of treatment groups other than control because no absolute control given
Averaged male and female control groups
Adrenals
The adrenal glands are one of the more well-known sources of peripheral dopamine, and express both D1-like and D2-like DRs (Pivonello et al. 2004). Specifically, the adrenal medulla, located at the center of the gland and surrounded by the cortex, is innervated by the greater splanchnic nerve and regulates secretion of catecholamines into systemic circulation (Bloom et al. 1988). However, both medulla and cortex seem to be important for dopamine production (McCarty et al. 1986). Adrenocortical dopamine appears to derive from DOPA removed from the circulation and decarboxylated in non-catecholaminergic cells (Buu and Lussier 1990). Recently, studies showed that electroacupuncture in the sciatic nerve of mice increased the production of dopamine in the adrenal medulla, and vagotomy abolishes this, suggesting that dopamine from the adrenals relies on both neuronal and non-neuronal inputs (Torres-Rosas et al. 2014). Many studies have shown that resident neuroendocrine chromaffin cells release dopamine (Fhaner et al. 2013; Leszczyszyn et al. 1991; Podvin et al. 2015), and the vesicular concentration of dopamine in these cells has been estimated to be as high as 300 mM (Wightman et al. 1991) which is comparable to that found in midbrain neurons (Pothos et al. 1998), although a range of studies estimate the dopamine concentration in the adrenals to be in the nanomolar to milimolar range (Table 3).
Many types of immune cells, in particular macrophages, dendritic cells, mast cells, and lymphocytes, can be found in the adrenals (Kanczkowski et al. 2016; Schober et al. 1998). Close cell–cell localization between immune cells and surrounding adrenocortical, chromaffin, or endothelial cells has been observed (Gonzalez-Hernandez et al. 1994; Wolkersdorfer et al. 1999), indicating that immune cells are likely to be in close contact with dopamine-releasing cells and exposed to high concentrations of dopamine. The catecholaminergic machinery in immune cells themselves can also be altered by the adrenal environment, as adrenalectomy increases TH and decreases MAO-A expression in macrophages (Stanojevic et al. 2013). The dopamine released from the adrenals is likely to influence other peripheral regions as well, as the hormones and catecholamines they release can regulate cytokine expression and immune cell activation (Deak 2008; Kanczkowski et al. 2016). Further, mature dendritic cells exposed to dopamine in the adrenals migrate from their residence in the adrenal cortex into the bloodstream and lymph nodes to present antigen to lymphocytes (Deak 2008).
Bone Marrow
The bone marrow microenvironment is critical in the maintenance of hematopoietic stem cells (HSCs), from which immune cells are derived through hematopoiesis. Signals from the sympathetic nervous system, including dopamine release, have been shown to regulate HSC development and function (Cosentino et al. 2015; Madden 2017; Mercier et al. 2011). Dopamine specifically enhances a number of cellular functions including cell polarity, migration, colony formation and metalloproteinase secretion, through stimulation of DRs expressed on these cells (Basu et al. 1993; Chakroborty et al. 2008; Spiegel et al. 2007). These and other studies suggest dopamine plays an active role in the bone marrow, a hypothesis supported by data showing nanomolar to micromolar ranges of dopamine in this compartment (Chakroborty et al. 2008; Maestroni et al. 1998; Marino et al. 1997), which is substantially more than what is typically found in circulation. Interestingly, bone marrow dopamine levels display rhythmicity, which could be disrupted by chemical sympathectomy, thereby indicating the possible role of this rhythmicity in regulation of hematopoiesis (Maestroni et al. 1998). The dopamine found in this compartment can also act on the many other immune cells present in the bone marrow, including macrophages and osteoclasts, several types of T-cells, B-cells and myeloid-derived suppressor cells. These cells are found throughout the bone marrow, circulating through the capillary network permeating this region (Mercier et al. 2011; Zhao et al. 2012), and all respond to dopamine. Thus, the dopamine present in the bone marrow could directly influence both the development of immune cell precursors, as well as indirectly affect hematopoiesis and ancillary functions, by acting on the mature immune cells hosted in this compartment. Further characterization of the role of dopamine in hematopoiesis is needed to better define how dopamine affects immune cell development, and to determine how dopaminergic effects on mature immune cells influence this process. This is of particular clinical significance as bone marrow derived stem cells are being utilized for therapies in Parkinson’s disease (Fu et al. 2015) as well as Alzheimer’s disease (Fang et al. 2018).
Carotid bodies
Carotid bodies, small clusters of chemoreceptors (type I cells) located near the bifurcation of the carotid artery, are one of the major groups of peripheral chemoreceptors in the body (Kumar and Prabhakar 2012). These cells are similar to the chromaffin cells of the adrenal medulla, and express both pre- and post-synaptic DRs (Almaraz et al. 1991; Bairam et al. 1998; McQueen et al. 1984) as well as dense core vesicles where TH has been localized (Karasawa et al. 1982; Nurse and Fearon 2002), indicating the storage of dopamine. Although these vesicles contain approximately 20-fold less catecholamine per vesicle than larger vesicles in chromaffin cells (Wightman et al. 1991), they have around 5-fold more dopamine than the smaller vesicles found in sympathetic ganglia (Zhou and Misler 1995). These cells are synaptically connected to nerve terminals of the petrosal ganglion neurons (Iturriaga and Alcayaga 2004), and release dopamine in response to changes in the oxygenation, pH, and temperature of arterial blood (Kumar and Prabhakar 2012). The literature suggests that the amount of dopamine in this region is substantial, in the micromolar to millimolar range (Table 3). The carotid bodies contain large numbers of monocytes and macrophages (Dvorakova et al. 2000), and carotid bodies exposed to chronic hypoxia, which increases dopamine (Hanbauer et al. 1981), also show invasion of macrophages and subsequent upregulation of proinflammatory cytokines (Lam et al. 2012). Carotid bodies can also respond to a wide variety of blood-borne stimuli, including cytokines, with IL-6 inducing catecholamine release from chemoreceptors in a dose-dependent manner via elevation in intracellular Ca2+ (Fan et al. 2009). The dopamine concentrations found in carotid bodies are sufficient to increase macrophage-mediated inflammation, including production of IL-6 (Nolan et al. 2018), suggesting that bidirectional interaction between inflammatory stimuli and changes in chemoreceptor dopamine release could stimulate immune activation and inflammation in these regions.
Circulation
Dopamine plays an important role in the circulatory system, potentiating vasodilation in systemic arteries (Amenta et al. 2000) and enhancing blood flow in skeletal muscles (Eliasen et al. 1989). Clinically, dopamine has long been used to treat cardiovascular complications arising from shock, trauma, and sepsis (Zhang and Chen 2016), suggesting that dopamine is involved in the regulation of vascular pathologies. Circulating dopamine can originate from diet and other physiological sources that can be independent or dependent of the nervous system (Eisenhofer and Goldstein 2004; Goldstein et al. 1999). Conditions that increase sympathetic nervous system activity can increase plasma dopamine levels (Van Loon 1983), and similarly, loss of sympathetic nerve function can decrease plasma dopamine (Goldstein and Holmes 2008). Dopamine is also released directly into the circulation from chromaffin cells of the adrenal medulla, amine precursor uptake decarboxylase (APUD) cells found predominantly in the kidney and pancreas (Rubi and Maechler 2010; Wolfovitz et al. 1993), and possibly from other, as yet undefined peripheral sources. Overall, peripheral dopamine synthesis and metabolism may currently be underestimated, as the high levels of dopamine in the plasma and the urinary excretion rates of dopamine metabolites and conjugated dopamine are not well accounted for by the known sources of peripheral dopamine.
Free (non-sulfated or glucuronated) dopamine levels in the circulation are relatively low compared to the rest of the body, comprising only 5% of dopamine in plasma (Kuchel and Kuchel 1991). Most studies report free dopamine concentrations in the picomolar range, but nanomolar levels have also been found (Table 3). These levels seem to vary widely among individuals (Eisenhofer et al. 2005), and can be substantially altered during normal activity. For instance, ingestion of a meal can increase dopamine plasma concentrations by more than 5000% (Eisenhofer and Goldstein 2004). The circulatory system is home to a wide variety of immune cells, many of which actively respond to dopamine (Scheiermann et al. 2015; Shi and Pamer 2011). Within circulation, these immune cells, including T-cells, granulocytes or monocytes, are likely to encounter large fluctuations in dopamine, with only small regions concentrated with enough dopamine to mediate the effects described for these cells (Gaskill et al. 2013; Pinoli et al. 2017). In particular, pockets of higher dopamine concentrations present on blood vessels and other tissue barriers may be critical for extravasation and trafficking of T cells and monocytes, as dopamine has been shown to both promote adhesion to extracellular matrix components and enhance chemokinesis and transmigration (Calderon et al. 2017; Coley et al. 2015; Levite et al. 2001; Watanabe et al. 2006a). Encounters with elevated dopamine could also slow down or diminish the immediate response to pathogenic insults, as dopamine decreases neutrophil adherence, phagocytosis, ROS formation and migration while increasing the apoptosis of these cells (Sookhai et al. 1999; Trabold et al. 2007; Wenisch et al. 1996).
The majority of circulating dopamine is conjugated with sulfates or glucuronides, rendering it biologically inactive (Yoneda et al. 1983). In humans, sulfation is the more important metabolic pathway (Claustre et al. 1983), Dopamine sulfate has a half-life of a few hours, compared to a few minutes for free dopamine (Eldrup 2004). Its concentration is relatively independent of sympathetic nerves and more dependent on diet and conjugation of dopamine in the gastrointestinal tract (Eldrup et al. 1997; Goldstein et al. 1999). Unlike inactivation of dopamine by deamination or O-methylation, glucuronidation and sulfoconjugation are reversible by the enzymes β-glucuronidase (Pellock and Redinbo 2017) and arylsulfatase A (ARSA) (Strobel et al. 1990), respectively, which are found in the both the CNS and peripheral tissues (Antunes et al. 2012; Borcherding et al. 2011; Richard et al. 2001; Sperker et al. 2000). The function of dopamine conjugation remains unclear. One hypothesis is that conjugation sequesters dopamine to reduce its bioactivity and prevent catecholamine buildup in circulation, while another proposes that it acts as a reservoir for free dopamine (Goldstein et al. 1999; Yamamoto et al. 1996). Higher levels of plasma dopamine are associated with congenital heart defects (Yoshizumi et al. 1998) and are a risk factor for future coronary events in patients with coronary artery disease (Abe et al. 2007), so sequestration of free dopamine might be a protective mechanism. It is not clear that immune cells have the capability to reverse sulfation or glucuronidation, but these processes would prevent circulating leukocytes from encountering high levels of free dopamine in the blood stream, thereby reducing dopamine-mediated inflammation.
Heart
Within the heart, the measured concentrations of dopamine are generally found to be in the high nanomolar range (Table 3), suggesting that dopamine could be synthesized in this compartment non-neuronally. This is supported by studies indicating that dopamine can be synthesized in the heart independent of noradrenergic nerves (Mohanty et al. 1986), possibly via chromaffin cells found in the paraganglia of the heart (Chumasov et al. 2011; Scheuermann 1993). These concentrations of dopamine are high enough to elicit activity from the immune cells present in the heart, which include resident cardiac macrophages, T-cells and mast cells, as well as lesser numbers of neutrophils and eosinophils (Frieler and Mortensen 2015; Lavine et al. 2014). Recent studies have shown that immune cells play an important role in both homeostatic heart function and in cardiac pathology. Distinct subsets of cardiac macrophages, both directly and indirectly facilitate tissue repair after cardiac injury, through phagocytosis and the production of cytokines such as IL-1β, IL-10 and IL-6, which are important regulators of cardiomyocyte and fibroblast function (Epelman et al. 2014; Frieler and Mortensen 2015). Recent studies show that heart-resident macrophages and cardiomyocytes can be physically connected by gap junctions to allow for synchronous propagation of electrical signals that drive the heart to contract during a normal heartbeat (Hulsmans et al. 2017). Monocyte derived macrophages are also recruited to the heart after injury, and have been show to promote inflammation (Lavine et al. 2014). Different subtypes of T-cells are involved in cardiac remodeling, altering cardiac physiology and both promoting and suppressing hypertrophy (Hamrell et al. 1995; Tang et al. 2012). Mast cells are also associated with maladaptive cardiac remodeling, potentially through interactions with fibroblasts (Zhang et al. 2011b). As dopamine has been shown to regulate cytokine production, phagocytosis and chemotaxis in both macrophages and T-cells, and to inhibit Treg function (Gaskill et al. 2013), the interactions of immune cells with dopamine in the heart could certainly promote or exacerbate cardiac inflammation and pathology. Exogenous changes in dopamine levels due to therapeutics or drug abuse could also disrupt non-pathologic cardiac function.
Kidney
Dopamine regulation in the kidney is one of the better characterized systems in the periphery, with dopamine levels reaching nanomolar to micromolar concentrations in this organ (Table 3). DRs in the kidney contribute to the control of renal electrolyte balance and blood pressure, as well as renin production (DiBona 1990; Gildea 2009; Harris and Zhang 2012; Hussain and Lokhandwala 2003). The primary source of dopamine in the kidney are renal peritubular cells (RPTs). These cells express AADC but not TH or DBH, so dopamine can only be produced from L-DOPA and can’t be converted into norepinephrine. Peritubular L-DOPA is transported into the RPTs via the Na+-independent and pH-sensitive L-type amino acid transporters (LAT) or related to b0,+ amino acid transporters (rBAT) from the circulation or following filtration at the glomerulus (Harris and Zhang 2012). This dopamine can then be secreted into the lumen from the same transporter, acting as a paracrine agent along nephron segments, circulating throughout the kidney (Carey 2001; Hussain and Lokhandwala 2003) or metabolized due to the high activity of COMT in this organ (Vieira-Coelho and Soares-da-Silva 1996). In addition to metabolizing dopamine, recent research has identified another enzyme, a FAD/NADH-dependent amine oxidase known as renalase, that is expressed in the kidney and secreted into blood, where it may metabolize catecholamines (Luft 2005). Renalase knockout mice have increased urinary and circulating dopamine, which is thought to result from an enhanced availability/uptake of l-DOPA in RPTs (Quelhas-Santos et al. 2015; Sizova et al. 2013). Interestingly, renalase is expressed in other tissues such as the brain, heart, and pancreas (Fedchenko et al. 2013; Guo et al. 2016), so although it is not well known it could be hypothesized that this enzyme has an important regulatory role in metabolizing dopamine throughout the body.
The primary immune cells in a healthy kidney are several discrete subpopulations of tissue resident macrophages, resident dendritic cells and a small number of lymphocytes and mast cells. The dendritic cells are found in the interstitium and extend dendrites into the tubular lumen, while the macrophages are also found in the interstitium, as well as the renal medulla and capsule and to a lesser extent in the glomeruli. The lymphocytes and mast cells can also be found in the interstitium (Kawakami et al. 2013; Kurts et al. 2013; Weisheit et al. 2015). High levels of dopamine are present in all these regions; therefore, kidney immune cells are likely to regularly interact with high levels of this neurotransmitter. Because of this regular interaction, it is likely that the impact of dopamine on the function of these cells is part of homeostatic kidney function. Indeed, mice with intrarenal dopamine deficiency show increased oxidative stress and inflammatory infiltration, and reduced intrarenal dopamine synthesis is associated with increased detrimental effects of angiotensin II on renal injury (Yang et al. 2012; Zhang et al. 2011a).
Lung
A number of cells within the lung may be capable of producing dopamine, including alveolar type II epithelial cells (Adir et al. 2004) and pulmonary neuroendocrine cells (Scheuermann et al. 1988). Additionally, dopamine is taken up and metabolized in lungs from rats (Bryan-Lluka et al. 1992; Scarcella and Bryan-Lluka 1995) and humans (Russell et al. 1982). There is evidence that dopamine is physiologically produced and distributed in the lungs in a process similar to that found in the kidney, although stimulation of DRs has opposite effects in these organs, increasing lung Na+ absorption but increasing kidney Na+ excretion (Barnard et al. 1999; Bertorello and Sznajder 2005). Further, while pulmonary endothelial cells are a site of very rapid metabolism by MAO and COMT (Russell et al. 1982) there is a lack of conversion of dopamine to norepinephrine, unlike other peripheral tissues (Scarcella and Bryan-Lluka 1995). DRs are found in airway smooth muscle (Mizuta et al. 2013), lung epithelial cells (Matsuyama et al. 2018), and lung arteries (Kobayashi et al. 1995), and lung dopamine levels are substantial enough to influence these receptors in a dose-dependent fashion (Ciarka et al. 2007). These concentrations are in nanomolar to micromolar levels (Table 3), and together these data suggest dopamine is involved in a number of pulmonary functions. Indeed, this neurotransmitter can modulate respiratory function through carotid bodies, and influence pulmonary circulation, neuromodulation of sensory pulmonary nerves, and lung water clearance (Chamorro-Marín et al. 2008; Prieto-Lloret et al. 2015; Vohra et al. 2012).
The role of the immune system in the lungs is critical, as they represent the environment most frequently targeted by pathogens (Lloyd and Marsland 2017). Alveolar macrophages make up a significant portion of immune cells within this organ during steady state, and an increase in specialized lymphocytes and neutrophils are recruited during bouts of inflammation (Cho et al. 2016; Hussell and Bell 2014). Lung immune activation can be triggered by the pulmonary neuroendocrine cells, which are the only innervated airway epithelial cells (Branchfield et al. 2016), suggesting dopamine could play a role in regulating this process. Expression of MAO and COMT in murine alveolar macrophages is regulated by LPS, suggesting lung inflammation changes their response to dopamine (Flierl et al. 2007). Pretreatment with dopamine ameliorated LPS-mediated edema formation and lowered neutrophil infiltration in a murine lung injury model (Vohra et al. 2012). In humans, inhaled dopamine induces bronchodilation during bronchial obstruction in asthmatic patients (Cabezas et al. 2003). However, D1-like receptor antagonists suppress Th17-mediated neutrophilic airway inflammation resulting from severe asthma (Nakagome et al. 2011). As with the kidney, the relatively high baseline dopamine levels in the lung suggest that dopamine-immune interactions are a regular part of pulmonary function, and seem to have an anti-inflammatory and therapeutically beneficial effect. However, in individuals with certain conditions, or under conditions of aberrant dopamine regulation, the alterations in immune function resulting from interaction with dopamine could be dangerous (Ciarka et al. 2004).
Gastrointestinal System
Dopaminergic mechanisms are important for regulation of gastrointestinal motility, likely through stimulation of DRs found along the gastrointestinal tract (Glavin and Hall 1995; Li et al. 2006; Mittal et al. 2017). The expression and activity of TH is high throughout the gastrointestinal system, and it is thought to be a significant source of dopamine metabolism. The sources of dopamine include the enteric nervous system as well as non-neuronal cells such as stomach epithelial cells, cells in the lamina propria and gut resident immune cells (Eisenhofer et al. 1997; Mezey et al. 1998). The microbiome may also be involved in the production of gut dopamine. Germ free animals have decreased dopamine concentrations in the small intestine (Asano et al. 2012), and germ free mice also display an increased turnover rate of dopamine in the brain (Diaz Heijtz et al. 2011). Additionally, microbiome depletion by antibiotics decreases TH in the gut and cytokine inhibition in invariant NKT cells, which could be reversed by replenishing the microbiome or treating with the D1-like receptor agonist A68,930 (Xue et al. 2018). Recent data indicate that bacteria produce and recognize neurochemicals (Lyte 2013; Strandwitz 2018), and have shown micromolar concentrations of dopamine that can be detected in the bacteria themselves and their culture fluid (Nagler et al. 2018; Özoğul 2004; Shishov et al. 2009). In addition, bacteria themselves, in particular Clostridium species, have been shown to express β-glucuronidase, which could significantly contribute to the generation of free dopamine in the gut (Asano et al. 2012). Overall, studies show nanomolar to micromolar dopamine throughout the gastrointestinal tissues and fluids including gastric and duodenal juice, stomach, small intestine, and colon (Table 3).
The gut contains the largest number of immune cells in the body, include multiple subsets of T-cells, macrophages and dendritic cells, as well as a variety of granulocytes, many of which are specifically adapted to the GI tract (Huffnagle and Noverr 2008; Wu and Wu 2012). These cells are found throughout the gastrointestinal system, and the ubiquity of these cells suggests that they could encounter dopamine anywhere within the gastrointestinal system. Large numbers of macrophages accumulate around the submucosal and myenteric plexuses (Bogunovic et al. 2009), which contain nerve terminals for both sympathetic and parasympathetic nerve fibers connected to the CNS. Mast cells have also been shown to surround nerve terminals in these regions (Schemann and Camilleri 2013). The mucosal plexus in the mucosal layer contains nerve endings in close proximity to a high concentration of immune and epithelial cells (Benarroch 2007). In addition, a substantial number of immune cells in the lamina propria express mRNA for DRs and TH but not DBH and PNMT, suggesting that these cells are exclusively dopaminergic (Mezey and Palkovits 1992). This demonstrates that many of the immune cells in this compartment are specifically adapted to respond to dopamine. The close proximity of other immune cells to nerve fibers and other sources of dopamine indicate many gastrointestinal immune populations interact with dopamine on a regular basis. Thus, dopamine-mediated changes in immune function are likely important to maintaining gut homeostasis, and disruptions in the gut dopaminergic system could be involved the development or exacerbation of a number of gastrointestinal disorders (Magro et al. 2004; Pacheco et al. 2014; Rooks et al. 2014; Tolstanova et al. 2015), as discussed later in this review.
Lymphoid Organs
Primary and secondary lymphoid organs, including the thymus, spleen, and lymph nodes, are massively innervated by sympathetic nerves that store a large amount of dopamine (Mignini et al. 2009; Weihe et al. 1991). Another source of dopamine for these regions is thought to be autocrine and paracrine secretion of dopamine by immune cells, primarily T-cells, as both regulatory CD4+CD25+ T-cells (Cosentino et al. 2007) and T follicular helper cells (Papa et al. 2017) contain and release dopamine. In addition, parasympathetic efferent nerves could also contribute to dopamine production, but their presence and function is not clear (Nance and Sanders 2007; Schafer et al. 1998). Many of the nerve fibers within the secondary lymphoid tissues are in close contact with blood vessels (Mignini et al. 2014), and DRs, TH and VMATs can be found on sympathetic nerve endings in the medulla, cortico-medullary junction, and thymic epithelial cell compartments of the thymus (Pilipović et al. 2008). Dopaminergic proteins are also found in the white pulp border and to a lesser extent in the red pulp in the spleen (Mignini et al. 2003; Mignini et al. 2009). These and other studies indicate that dopamine is present throughout the lymphoid organs, that some of it may derive from the CNS, and that dopamine is involved in the function of the lymphoid tissues. This is supported by data showing that the destruction of dopaminergic terminals in the nucleus accumbens and striatum results in depression of spleen natural killer cells and lymphocytes (Deleplanque et al. 1994).
The lymphoid organs have been shown to contain high nanomolar concentrations of dopamine, although the amounts vary between tissues (Table 3). The presence of both dopamine and dopaminergic proteins at the border of the thymic medulla, where most of single-positive (CD4+ and CD8+) lymphocytes reside, suggests that these immune cells may be directly exposed to dopamine from nerve terminals. Further, in the splenic white pulp, sympathetic nerve terminals are in direct apposition to T-cells and adjacent to both dendritic cells and B-cells, with a neuroimmune junction approximately 6 nm wide (Felten et al. 1987; Felten et al. 1985). These data indicate an extremely close interaction between sympathetic terminals and immune cells in these organs, demonstrating that interactions with dopamine are essential to proper tissue homeostasis and suggesting that stimuli which disrupt dopamine concentrations in these tissues could significantly impair immune function.
Pancreas
Concentrations of dopamine in the pancreas are relatively high, with reports showing dopamine content in the low micromolar range (Table 3). Dopamine in this organ is derived from sympathetic innervation and the circulation, as well as from pancreatic cells and resident immune cells (Rodriguez-Diaz et al. 2011; Zern et al. 1980). Within the endocrine tissues of the pancreas, dopamine can directly regulate insulin production (Farino et al. 2019; Rubí and Maechler 2010). In addition, dopamine release from the hypothalamus can indirectly regulate insulin and dopamine production through effects of prolactin on islet cells, which express TH (Teitelman et al. 1981) and DRs (Chen et al. 2014; Rubi et al. 2005). Dopamine from the exocrine tissues is released into the duodenum to protect it from harmful digestive enzymes. Although pancreatic exocrine cells can respond to and produce dopamine, the dopamine released from these cells likely does not contribute to dopamine in the general circulation, as there is an absence of DAT in endothelial cells of arteries but abundance in excreting ducts and veins (Mezey et al. 1996). While chemical sympathectomy does not significantly alter dopamine levels, suggesting a non-neuronal source of pancreatic dopamine (Mezey et al. 1996), neurons from the gut can send axonal projections to the pancreas (Kirchgessner and Gershon 1990), and there is a central vagal connection through the paraventricular nucleus of the hypothalamus (Davis and Smith 1985).
While the role of pancreatic immune cells is still being defined, T-cells, macrophages and dendritic cells are all though to play important roles in the development and function of this organ (Carrero et al. 2017; Hawkins et al. 1996; Homo-Delarche and Drexhage 2004). The endocrine pancreas has a highly specialized islet structure and microvascular cells that facilitates T cell infiltration, bringing these lymphocytes into close contact with dopamine-producing islet cells. The resident macrophages and dendritic cells in the islets are in close contact with blood vessels where they could encounter dopamine (Boldison and Wong 2016; Homo-Delarche and Drexhage 2004). While these and other studies indicate dopamine-immune interaction may be part of normal pancreatic function, dopamine mediated changes to insulin levels, combined with the inflammatory phenotype of dopamine-exposed immune cells, suggests that changes in pancreatic dopamine levels could contribute to development of pancreatic pathology. Further, dopamine/insulin crosstalk can occur in other organs, such as the brain (Mebel et al. 2012; Stouffer et al. 2015; Williams et al. 2007), so the pancreatic influence on dopamine concentrations could also affect immune cells in other tissues.
Adipose
As dopamine has direct inhibitory effects on the secretion of insulin, it follows that dopamine also participates in glucose homeostasis and body weight. Alterations in dopaminergic markers such as DAT are decreased in postmortem brain samples from obese subjects (Wu et al. 2017) and dopamine is decreased in the brains of obese rats (Cone et al. 2013). Dopamine can also directly affect differentiation and proliferation of adipocytes themselves (Borcherding et al. 2011). While concentrations of dopamine in adipose tissue appears to be in the lower nanomolar range (Table 3), adipocytes express DRs (Wang et al. 2018), TH (Zhu et al. 2014) and ARSA, indicative of an active sulfoconjugation mechanism in adipose tissues (Borcherding et al. 2011). And while the adrenal medulla on average had 1000 fold more TH expression than adipose tissues, considering the size of the adrenal medulla compared to all of the adipose depots within the body, the dopamine production in adipose tissue may be comparable (Vargovic et al. 2011).
The immune composition of adipose tissue fluctuations based on the obesity of the individual and thus metabolic state of the tissue. In general, adipose tissue macrophages, which are divided into several distinct subsets, constitute 5% of the cells in adipose tissue, although the ratio of the subsets changes with body composition (Ortega Martinez de Victoria et al. 2009). In addition, dendritic cells, neutrophils, and mast cells are all relatively sparse in the adipose tissue of lean individuals, but much more common in the adipose tissue of obese individuals (Bertola et al. 2012; Elgazar-Carmon et al. 2008; Liu et al. 2009). This increase is also seen in T-cells, which are the second most common immune cell in adipose after macrophages (Wu et al. 2007). Some studies suggest that adipocytes act as antigen presenting cells during inflammation (Deng et al. 2013; Morris et al. 2013), meaning that T-cells in adipose make direct contact with dopamine-producing adipocytes. As dopamine can enhance inflammatory activity in immune cells, the immune cells in adipose that are exposed to dopamine released from adipocytes and innervating nerve fibers could be contributing to the inflammatory milieu that is now commonly associated with metabolic disorders and obesity (Huh et al. 2014). Further research on the impact of changes in dopamine on adipose tissue is important to more effectively use dopaminergic drugs to treat obesity and metabolic disorders, as well as manage metabolic symptoms associated with chronic treatment with dopaminergic drugs such as antipsychotics (Panariello et al. 2011).
Peripheral Dopamine and Drug Abuse
There is little data on the effects of drugs of abuse on peripheral dopamine concentrations, although a mouse study using positron emission tomography (PET) imaging and quantitative whole-body autoradiography (QWBAR) with [18F]FDOPA indicates that use of ketamine, cocaine and methamphetamine increase dopamine levels in the gastrointestinal tract and kidney (Yeh et al. 2014). Many of the drugs that produce increases in CNS dopamine, such as cocaine, methamphetamine, and ethanol, are associated with significant pathology in peripheral organs with higher dopamine levels, such as the lung, gut, or kidney (Dimitrijevic et al. 2008; Lineberry and Bostwick 2006; Tiwari et al. 2006). Drug induced increases in the dopamine content of these tissues could disrupt homeostatic function and/or promote inflammation through dopamine modulation of resident macrophages and other immune cells. Indeed, drugs of abuse such as opioids have been shown to modulate both peripheral innate and adaptive immune cells (Roy et al. 2011). Further, DR expression in peripheral lymphocytes is affected by cocaine (Faraj et al. 1991), opioids (Goodarzi et al. 2009), alcohol (Biermann et al. 2007), and heroin (Czermak et al. 2004) during abuse and abstinence phases. These effects could also be induced by therapeutic agents that increase dopamine or act on DRs, such as Bromocriptine, L-DOPA, Emsam or Wellbutrin (Brannan et al. 1993; Lamensdorf et al. 1999; Stahl et al. 2004). Future studies should address the impact of drugs on peripheral dopamine, as it is clear that dopamine is present throughout the periphery, and the actions of both immune cells and dopamine are necessary for proper function in many different tissues.
Clinical Implications of Dopamine Stimulation of Immune Cells
Disturbances in central and peripheral dopamine production are involved in many pathological conditions, including but not limited to Parkinson’s disease, NeuroHIV, multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease. All these pathologies are also influenced by changes in immune function, suggesting that communication between the dopaminergic system and immune cells plays a substantial role in their etiology. This section briefly discusses these interactions, examining the role dopamine in several pathologies in which dopamine-mediated alterations in the immune system might impact the development of disease.
Parkinson’s Disease
Parkinson’s disease (PD) is the most well-known disease associated with dopaminergic dysfunction. Characterized by James Parkinson in 1817, this disease is characterized by the degeneration of dopaminergic neurons in the substantia nigra, pathological protein aggregates known as Lewy bodies, reduced CNS extracellular DA concentrations particularly in the striatum, neuroinflammation, and motor disturbances (Table 4). In addition to these neurological symptoms, Parkinson’s symptoms include duodenal ulcers, GI absorptive motile functions, and generation of Lewy bodies in the gut (Glavin and Szabo 1990; Singaram et al. 1995). It is not clear whether this connection is due to dysregulation of the local dopamine levels or the influence of the damaged CNS dopaminergic system on innervated peripheral organs, although both may contribute (Pellegrini et al. 2016; Singaram et al. 1995). Of note, PD has been positively correlated with inflammatory bowel diseases (Lin et al. 2016), another group of disorders where dopamine may be an important regulator of disease progression, and it has recently been hypothesized that T-cell driven inflammation, which mediates dopaminergic neurodegeneration, is triggered in the gut (Campos-Acuña et al. 2019). Although PD itself reduces dopaminergic tone by destroying dopamine neurons in the nigrostriatal pathway, treatment for this disease involves the administration of L-DOPA and/or DR agonists, potentially leading to stimulation of DRs on immune cells. Indeed, both in both humans and Parkinson’s rodent models, changes in dopamine concentrations have been found in the periphery as well as in the CNS (Eldrup et al. 1995; Kawamura et al. 1999; Winner et al. 2017). Dopamine has been shown to affect phagocytosis in myeloid cells (Gaskill et al. 2013), and microglia show greater phagocytic activity in the substantia nigra of the brain of PD patients has been reported (Barcia 2013).
Peripherally, the correlations between central and peripheral dopamine in PD patients are inconsistent (Buttarelli et al. 2009; Pontieri and Colosimo 2010), although significant correlations are more often found in patients undergoing dopamine therapy. A number of studies have reported increased dopamine in the intestines accompanying decreased CNS dopamine after treatment with 6-OHDA (Garrido-Gil et al. 2018b; Levandis et al. 2015), which is used to model Parkinson’s in rodents. With regards to the specific dopamine-immune effects in PD and PD models, lymphocytes in these systems show reductions in intracellular dopamine content, as well as changes in expression of TH, DAT, and DRs (Barbanti et al. 1999; Caronti et al. 2001; Kustrimovic et al. 2016; Nagai et al. 1996). CD4+ T-cells seem to play more of a role in disease progression, as these cells can infiltrate into the substantia nigra during PD, and depletion of CD4+ T-cells attenuates dopaminergic neurodegeneration in animal models of PD (Benner et al. 2008; Brochard et al. 2009). Very recently, it has also been shown that oxidized forms of α-synuclein, one of the main constituents of Lewy bodies, specifically drive CD4+ T-cell responses in PD patients (Sulzer et al. 2017). A case study additionally demonstrated that treatment with L-DOPA led to neutropenia and changes in neutrophil TH and DR expression (Cordano et al. 2015). These and other studies show that PD has a significant interaction with immune function (Mackie et al. 2018), and suggest that the role of dopamine in this interaction should be studied further.
NeuroHIV
The connection between HIV infection of the CNS and dopaminergic dysfunction has been recently reviewed (Gaskill et al. 2013; Nolan and Gaskill 2018), and will therefore only be briefly discussed here. This virus attacks immune cells, primarily CD4+ T cells and myeloid cells, and in the CNS the primary targets for HIV are myeloid cells such as microglia and macrophages. Once infected these cells can produce new virus, act as viral reservoirs, and release neurotoxic factors that contribute to the constellation of pathologies and behavioral cognitive and motor symptoms known as NeuroHIV. Prior to the use of combined antiretroviral therapy (cART), greater amounts of viral DNA and elevated neuropathology were seen in regions of the brain innervated by dopaminergic neurons (Aylward et al. 1993; Fujimura et al. 1997; Kieburtz et al. 1996). With cART, the effects are subtler, but changes in the dopaminergic system are still present (Cassol et al. 2014; Gaskill et al. 2017). Drugs which increase dopamine levels exacerbate SIV-associated neuropathology in the Rhesus macaque model of NeuroAIDS (Czub et al. 2004; Czub et al. 2001), and individuals infected with HIV show alterations in CNS and CSF dopamine (Table 4). During HIV infection, dopamine could increase monocyte transmigration into the CNS (Calderon et al. 2017; Coley et al. 2015), as well as increase HIV infection of macrophages (Gaskill et al. 2009; Gaskill et al. 2014) and promote an inflammatory environment, even in infected individuals on cART (Nolan et al. 2018). As drug abuse is a common comorbidity in HIV infection (Mathers et al. 2008), and many HIV-infected individuals also suffer from neuropsychiatric disorders (Dubé et al. 2005; Gallego et al. 2011), changes in dopamine concentrations in response to both illicit and psychiatric drugs could exacerbate the impact of dopamine on HIV associated neuropathology. Overall, these and other studies indicate that the bidirectional interactions between dopamine and HIV-infected myeloid cells are an important driver of NeuroHIV.
Multiple Sclerosis
Multiple sclerosis (MS) is a progressive, neurodegenerative disease characterized by progressive loss of neurological function due to the destruction of sheath axonal myelin throughout the brain and spinal cord (Cosentino and Marino 2013; Marino and Cosentino 2016). The heightened inflammatory processes present in the CNS during MS increase the entry of circulating immune cells (Engelhardt 2006; Larochelle et al. 2011). As dopamine can increase the activation and production of inflammatory mediators, changes in CNS dopamine levels during increased immune cell entry could contribute to MS pathology. In experimental mouse models of MS such as experimental autoimmune encephalomyelitis (EAE), changes in CNS dopamine alter the progression of disease (Bałkowiec-Iskra et al. 2007). While there are no changes in the dopamine concentrations in the immune cells of MS patients (Cosentino et al. 2002; Rajda et al. 2002), there are substantial differences in DR expression in these cells, particularly D1-like DRs (Cosentino et al. 2014; Giorelli et al. 2005; Prado et al. 2018). In an EAE model of MS, these changes promoted the production of inflammatory cytokines IL-12 and IL-23, and disrupted the balance of activity between Treg and Th17 cells, while the depletion of dopamine decreased the severity of the disease (Nakano et al. 2008; Prado et al. 2012; Prado et al. 2018). While other innate immune cells that are responsive to dopamine, including monocytes, NK cells, mast cells, and neutrophils also play a role in MS (Chanvillard et al. 2013; Hernández-Pedro et al. 2013; Hertwig et al. 2016; Naegele et al. 2012), the specific dopaminergic modulation of these cells in the context of MS is not clear. One of the most common treatments for MS, IFN-β, induces the production of dopamine in human lymphocytes (Cosentino et al. 2005), and a longitudinal study in relapsing remitting MS patients undergoing IFN-β treatment for 12 months found increased D5 and decreased D2 in lymphocytes compared to untreated patients (Zaffaroni et al. 2008). Thus, IFN-β may alter the response to dopamine in these cells, promoting D1-like responses and suggesting a potential benefit for dopaminergic agents in MS (Cosentino and Marino 2013). Thus, the amount of dopamine immune cells encounter in the brains of MS patients could substantially alter the progression of disease by changing the inflammatory milieu in the CNS.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease in which the destruction of bone tissue and the articular structures of joints leads to progressive disability. Macrophages that infiltrate the synovial tissues play a crucial role in the progression of this disease, and dendritic cells (Gierut et al. 2010; Lutzky et al. 2007), neutrophils, and NK cells (Falgarone et al. 2005) in the synovial fluid also contribute to the development of the pathology. The dopaminergic system strongly influences the progression of RA (Pacheco et al. 2014), although dopamine levels in synovial fluid are relatively low, in the picomolar to nanomolar range (Table 4). Dopamine released from dendritic cells mediates IL-6 dependent differentiation of Th17 lymphocytes, resulting in exacerbated cartilage destruction that was blocked by the a D1-like receptor antagonist (SCH23390) (Nakano et al. 2011). In addition, the D2 antagonist haloperidol, and the DR agonist cabergoline have also been shown to ameliorate disease progression (Fahmy Wahba et al. 2015; Mobini et al. 2011). These effects may be mediated through effects on synovial fibroblasts in RA patients, which express both DRs and TH, (Capellino et al. 2014; Capellino et al. 2010), or through the effect of dopamine on osteoblasts, which also express these receptors and TH (Capellino et al. 2016). Expression of TH in osteoblasts suggests dopamine locally synthesized in the bone could influence disease progression, and that dopamine may be involved in not only bone formation (Lee et al. 2015), but also bone remodeling and joint erosion in RA.
Inflammatory Bowel Diseases
The autoimmune disorders known as inflammatory bowel diseases (IBDs) are categorized as chronic inflammatory conditions of the gastrointestinal system, with the two main categories including Crohn’s disease and ulcerative colitis. In both rodent models and humans with IBD, dopamine is decreased in the colon (Magro et al. 2004; Magro et al. 2002) (Table 4), and rodent models of IBD show the disease is enhanced by treatment with the peripheral dopamine antagonist domperidone, and ameliorated by the dopamine agonist bromocriptine (Herak-Perkovic et al. 2001). A number of similar studies in rodent models show that treatment with D2 agonists (Tolstanova et al. 2015), D2 antagonists (Kim D, Kim W, et. al, 2019), and the herbal alkaloid berberine, which acts as a pan-DR antagonist (Kawano et al. 2015) all decreased the severity of IBD. The link between the dopaminergic system on IBD is also supported by a rat study showing that ulcerative colitis correlated with enhanced inflammatory and damaging effects of LPS on dopaminergic neurons in the nigrostriatal pathway (Villaran et al. 2010). In human IBD patients, impaired synthesis or cellular storage of dopamine was observed in enteroendocrine cells and the enteric nervous system, suggesting aberrant intestinal cellular synthesis and storage of dopamine could stimulate inflammation in nearby immune cells (Magro et al. 2002). These data show that the inflammatory responses triggering IBD are connected to both local and distal dopaminergic systems, and that changes in dopamine may be central to disease development.
Conclusion
The data presented here demonstrate that the dopaminergic system is active in many tissues, both in the CNS and the periphery. This activity is seen not only in the concentrations of dopamine found in these tissues, but in the variety of functions that are affected by this neurotransmitter. Dopamine has been shown to regulate immune cells in the brain and throughout the periphery, and dopamine displays complex regulatory effects on immune responses, depending on dopamine concentration, time of exposure, subtype of receptors, type of immune cells and immune cell activation state. The concentrations required to induce these effects on immunity are found in many compartments, where dopamine and the immune cells it can affect contribute to both homeostatic function and pathological responses. A better understanding of dopaminergic regulation of immune function is critical to understanding its role in both tissue homeostasis and many disease states associated with abnormal dopaminergic signaling and an altered/imbalanced immune response. Overall, a better appreciation of the broad immunomodulatory effects of this neurotransmitter is critical to the advancement of a number of fields, as it will likely demonstrate new pathways and mechanisms involved in many seemingly well understood processes, along with the development of new and better therapeutics and health strategies.
Figure 2 -. Concentrations of Dopamine Throughout the Periphery.
A graphical representation of the various concentrations of dopamine throughout the periphery, based on the summary literature contained in findings in Table 3. These values represent the range of calculated absolute molar values, which provide a simplified way to compare relative physiologically relevant concentrations across peripheral systems. These concentrations can change during different disease states (Table 4). For clarity, data showing concentrations that were outliers in the calculated range of concentrations for each region are excluded
Acknowledgements
We would like to state our tremendous appreciation to all the members of the Gaskill laboratory and the department of Pharmacology and Physiology for their insights and critical feedback during the preparation of this manuscript. This work was supported by a grant from the National Institutes of Drug Abuse, DA039005 (PJG), as well as support from the Drexel University College of Medicine.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography
- Abe M et al. (2007) Association of high levels of plasma free dopamine with future coronary events in patients with coronary artery disease Circulation journal : official journal of the Japanese Circulation Society 71:688–692 [DOI] [PubMed] [Google Scholar]
- Adachi S, Endo Y, Mizushige T, Tsuzuki S, Matsumura S, Inoue K, Fushiki T (2013) Increased levels of extracellular dopamine in the nucleus accumbens and amygdala of rats by ingesting a low concentration of a long-chain Fatty Acid Bioscience, biotechnology, and biochemistry 77:2175–2180 doi: 10.1271/bbb.130234 [DOI] [PubMed] [Google Scholar]
- Adir Y et al. (2004) Augmentation of Endogenous Dopamine Production Increases Lung Liquid Clearance American Journal of Respiratory and Critical Care Medicine 169:757–763 doi: 10.1164/rccm.200207-744OC [DOI] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K (2010) Understanding wiring and volume transmission Brain Res Rev 64:137–159 doi: 10.1016/j.brainresrev.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Airavaara M, Planken A, Gäddnäs H, Piepponen TP, Saarma M, Ahtee L (2004) Increased extracellular dopamine concentrations and FosB/ΔFosB expression in striatal brain areas of heterozygous GDNF knockout mice European Journal of Neuroscience 20:2336–2344 doi: 10.1111/j.1460-9568.2004.03700.x [DOI] [PubMed] [Google Scholar]
- Alaniz RC, Thomas SA, Perez-Melgosa M, Mueller K, Farr AG, Palmiter RD, Wilson CB (1999) Dopamine β-hydroxylase deficiency impairs cellular immunity Proceedings of the National Academy of Sciences 96:2274–2278 doi: 10.1073/pnas.96.5.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage J Neurosci 30:8285–8295 doi: 10.1523/jneurosci.0976-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz L, Teresa Pérez-García M, González C (1991) Presence of D1 receptors in the rabbit carotid body Neuroscience Letters 132:259–262 doi: 10.1016/0304-3940(91)90315-K [DOI] [PubMed] [Google Scholar]
- Ambade V, Arora MM, Singh P, Somani BL, Basannar D (2009) Adrenaline, Noradrenaline and Dopamine Level Estimation in Depression : Does it Help? Medical journal, Armed Forces India 65:216–220 doi: 10.1016/S0377-1237(09)80006-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F, Barili P, Bronzetti E, Felici L, Mignini F, Ricci A (2000) Localization of dopamine receptor subtypes in systemic arteries Clinical and experimental hypertension (New York, NY : 1993) 22:277–288 [DOI] [PubMed] [Google Scholar]
- Amino T, Uchihara T, Tsunekawa H, Takahata K, Shimazu S, Mizusawa H, Orimo S (2008) Myocardial nerve fibers are preserved in MPTP-treated mice, despite cardiac sympathetic dysfunction Neuroscience research 60:314–318 doi: 10.1016/j.neures.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Anagnostakis Y, Spyraki C (1994) Effect of morphine applied by intrapallidal microdialysis on the release of dopamine in the nucleus accumbens Brain research bulletin 34:275–282 doi: 10.1016/0361-9230(94)90064-7 [DOI] [PubMed] [Google Scholar]
- Andersen AD et al. (2017) Cerebrospinal fluid levels of catecholamines and its metabolites in Parkinson’s disease: effect of l-DOPA treatment and changes in levodopa-induced dyskinesia Journal of Neurochemistry 141:614–625 doi: 10.1111/jnc.13997 [DOI] [PubMed] [Google Scholar]
- Antunes IF et al. (2012) 18F-FEAnGA for PET of beta-glucuronidase activity in neuroinflammation Journal of nuclear medicine : official publication, Society of Nuclear Medicine 53:451–458 doi: 10.2967/jnumed.111.096388 [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J (2007) Space, time and dopamine Trends in neurosciences 30:62–69 doi: 10.1016/j.tins.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Arreola R et al. (2016) Immunomodulatory Effects Mediated by Dopamine Journal of immunology research 2016:3160486 doi: 10.1155/2016/3160486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson E, Viereckel T, Mikulovic S, Wallén-Mackenzie Å (2014) Age- and Sex-Dependence of Dopamine Release and Capacity for Recovery Identified in the Dorsal Striatum of C57/Bl6J Mice PLOS ONE 9:e99592 doi: 10.1371/journal.pone.0099592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y et al. (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice Am J Physiol Gastrointest Liver Physiol 303:G1288–1295 doi: 10.1152/ajpgi.00341.2012 [DOI] [PubMed] [Google Scholar]
- Aviado DM, Sadavongvivad C (1970) Pharmacological significance of biogenic amines in the lungs: Noradrenaline and dopamine British Journal of Pharmacology 38:374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD (1993) Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging Neurology 43:2099–2104 [DOI] [PubMed] [Google Scholar]
- Bairam A, Frenette J, Dauphin C, Carroll JL, Khandjian EW (1998) Expression of dopamine D1-receptor mRNA in the carotid body of adult rabbits, cats and rats Neuroscience research 31:147–154 [DOI] [PubMed] [Google Scholar]
- Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, Feuerstein C (1994) Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies Molecular Brain Research 23:204–212 doi: 10.1016/0169-328X(94)90227-5 [DOI] [PubMed] [Google Scholar]
- Bałkowiec-Iskra E et al. (2007) MPTP-induced central dopamine depletion exacerbates experimental autoimmune encephalomyelitis (EAE) in C57BL mice Inflammation Research 56:311–317 doi: 10.1007/s00011-007-6128-0 [DOI] [PubMed] [Google Scholar]
- Barbanti P et al. (1999) Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease Movement disorders : official journal of the Movement Disorder Society 14:764–771 [DOI] [PubMed] [Google Scholar]
- Barcia C (2013) Glial-mediated inflammation underlying parkinsonism Scientifica 2013:357805–357805 doi: 10.1155/2013/357805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard ML et al. (1999) Stimulation of the dopamine 1 receptor increases lung edema clearance Am J Respir Crit Care Med 160:982–986 doi: 10.1164/ajrccm.160.3.9812003 [DOI] [PubMed] [Google Scholar]
- Basson H, Bairam A, Cottet-Emard JM, Pequignot JM, Marchal F (1997) Carotid body dopamine content and release by short-term hypoxia: effect of haloperidol and alpha methyl paratyrosine Archives of physiology and biochemistry 105:3–9 doi: 10.1076/apab.105.1.3.13145 [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS (2000) Dopamine, a neurotransmitter, influences the immune system J Neuroimmunol 102:113–124 [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Lahiri T, Chowdhury JR (1993) Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice Life sciences 53:415–424 [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Espinoza S, Gainetdinov RR (2015) Dopamine receptors – IUPHAR Review 13 British Journal of Pharmacology 172:1–23 doi: 10.1111/bph.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors Pharmacological reviews 63:182–217 doi: 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- Bell C, Gillespie JS (1981) Dopamine and noradrenaline levels in peripheral tissues of several mammalian species J Neurochem 36:703–706 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R (2001) Dopamine as a Prolactin (PRL) Inhibitor Endocrine reviews 22:724–763 doi: 10.1210/edrv.22.6.0451 [DOI] [PubMed] [Google Scholar]
- Ben-Shaanan TL et al. (2016) Activation of the reward system boosts innate and adaptive immunity Nat Med 22:940–944 doi: 10.1038/nm.4133 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2007) Enteric nervous system Neurology 69:1953 doi: 10.1212/01.wnl.0000281999.56102.b5 [DOI] [PubMed] [Google Scholar]
- Benner EJ et al. (2008) Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons PLoS One 3:e1376 doi: 10.1371/journal.pone.0001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G et al. (2018) Chronic social stress induces peripheral and central immune activation, blunted mesolimbic dopamine function, and reduced reward-directed behaviour in mice Neurobiology of stress 8:42–56 doi: 10.1016/j.ynstr.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B (1994) Cerebrospinal fluid dopamine in HIV-1 infection AIDS 8:67–72 [DOI] [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A (1994) Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop Proc Natl Acad Sci U S A 91:12912–12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertler A, Rosengren E (1959) Occurrence and distribution of catechol amines in brain Acta Physiol Scand 47:350–361 [PubMed] [Google Scholar]
- Bertola A et al. (2012) Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients Diabetes 61:2238–2247 doi: 10.2337/db11-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello AM, Sznajder JI (2005) The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks American journal of respiratory cell and molecular biology 33:432–437 doi: 10.1165/rcmb.2005-0297TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser MJ, Ganor Y, Levite M (2005) Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both J Neuroimmunol 169:161–171 doi: 10.1016/j.jneuroim.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Beyene A, McFarlane IR, Pinals R, Landry M (2017) Stochastic Simulation Of Dopamine Neuromodulation For Implementation Of Fluorescent Neurochemical Probes In The Striatal Extracellular Space bioRxiv:144436 doi: 10.1101/144436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwandin A, Fuxe K, Bennett NC, Manger PR (2008) Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brains of two species of African mole-rat Journal of chemical neuroanatomy 35:371–387 doi: 10.1016/j.jchemneu.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Biermann T, Bonsch D, Reulbach U, Kornhuber J, Bleich S (2007) Dopamine and N-methyl-D-aspartate receptor expression in peripheral blood of patients undergoing alcohol withdrawal Journal of neural transmission (Vienna, Austria : 1996) 114:1081–1084 doi: 10.1007/s00702-007-0661-4 [DOI] [PubMed] [Google Scholar]
- Bjelke B et al. (1996) Dopaminergic transmission in the rat retina: evidence for volume transmission Journal of chemical neuroanatomy 12:37–50 [DOI] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW (2009) The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release Brain, behavior, and immunity 23:492–497 doi: 10.1016/j.bbi.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SR, Edwards AV, Jones CT (1988) The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves J Physiol 397:513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M et al. (2009) Origin of the lamina propria dendritic cell network Immunity 31:513–525 doi: 10.1016/j.immuni.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldison J, Wong FS (2016) Immune and Pancreatic beta Cell Interactions in Type 1 Diabetes Trends Endocrinol Metab 27:856–867 doi: 10.1016/j.tem.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Boneberg EM, von Seydlitz E, Propster K, Watzl H, Rockstroh B, Illges H (2006) D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+ -T cells J Neuroimmunol 173:180–187 doi: 10.1016/j.jneuroim.2005.11.018 [DOI] [PubMed] [Google Scholar]
- Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, Ben-Jonathan N (2011) Dopamine Receptors in Human Adipocytes: Expression and Functions PLoS ONE 6:e25537 doi: 10.1371/journal.pone.0025537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgkvist A, Malmlof T, Feltmann K, Lindskog M, Schilstrom B (2012) Dopamine in the hippocampus is cleared by the norepinephrine transporter The international journal of neuropsychopharmacology 15:531–540 doi: 10.1017/s1461145711000812 [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO et al. (2018) Brain Dopamine Transmission in Health and Parkinson’s Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors Frontiers in synaptic neuroscience 10:20–20 doi: 10.3389/fnsyn.2018.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO et al. (2011) Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions Biochem Biophys Res Commun 404:928–934 doi: 10.1016/j.bbrc.2010.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse KE, Mathews TA (2011) Ethanol-induced increases in extracellular dopamine are blunted in brain-derived neurotrophic factor heterozygous mice Neuroscience letters 489:172–176 doi: 10.1016/j.neulet.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T (2011) Male/Female differences in neuroprotection and neuromodulation of brain dopamine Frontiers in endocrinology 2:35–35 doi: 10.3389/fendo.2011.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW (2000) Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use J Neurosci 20:7109–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AJ, Costall B, Domeney AM, Naylor RJ (1985) Laterality of dopamine function and neuroleptic action in the amygdala in the rat Neuropharmacology 24:1163–1170 doi: 10.1016/0028-3908(85)90149-2 [DOI] [PubMed] [Google Scholar]
- Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X (2016) Pulmonary neuroendocrine cells function as airway sensors to control lung immune response Science (New York, NY) 351:707–710 doi: 10.1126/science.aad7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan T, Martinez-Tica J, Di Rocco A, Yahr MD (1993) Low and high dose bromocriptine have different effects on striatal dopamine release: an in vivo study Journal of neural transmission Parkinson’s disease and dementia section 6:81–87 [DOI] [PubMed] [Google Scholar]
- Brochard V et al. (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease J Clin Invest 119:182–192 doi: 10.1172/jci36470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Golembiowska K, Daniel WA (2011) Cytochrome P450 mediates dopamine formation in the brain in vivo J Neurochem 118:806–815 doi: 10.1111/j.1471-4159.2011.07339.x [DOI] [PubMed] [Google Scholar]
- Bromek E, Wojcikowski J, Daniel WA (2013) Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus in the central noradrenergic regulation of liver cytochrome P450 Biochem Pharmacol 86:1614–1620 doi: 10.1016/j.bcp.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Bryan-Lluka LJ, Westwood NN, O’Donnell SR (1992) Vascular uptake of catecholamines in perfused lungs of the rat occurs by the same process as Uptake1 in noradrenergic neurones Naunyn-Schmiedeberg’s archives of pharmacology 345:319–326 [DOI] [PubMed] [Google Scholar]
- Buttarelli FR et al. (2009) Central and peripheral dopamine transporter reduction in Parkinson’s disease Neurol Res 31:687–691 doi: 10.1179/174313209x383259 [DOI] [PubMed] [Google Scholar]
- Buu NT, Lussier C (1990) Origin of dopamine in the rat adrenal cortex Am J Physiol 258:F287–291 doi: 10.1152/ajprenal.1990.258.2.F287 [DOI] [PubMed] [Google Scholar]
- Cabezas GA, Israili ZH, Velasco M (2003) The actions of dopamine on the airways American journal of therapeutics 10:477–486 [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V (2014) Glial-neuronal ensembles: partners in drug addiction-associated synaptic plasticity Frontiers in Pharmacology 5 doi: 10.3389/fphar.2014.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B (1996) Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation Brain Res 730:17–31 [DOI] [PubMed] [Google Scholar]
- Calderon TM et al. (2017) Dopamine Increases CD14(+)CD16(+) Monocyte Transmigration across the Blood Brain Barrier: Implications for Substance Abuse and HIV Neuropathogenesis J Neuroimmune Pharmacol 12:353–370 doi: 10.1007/s11481-017-9726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Huggins KN, Mathews TA, Jones SR (2012) Conserved dorsal-ventral gradient of dopamine release and uptake rate in mice, rats and rhesus macaques Neurochemistry international 61:986–991 doi: 10.1016/j.neuint.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvey T et al. (2016) Nuclear organisation of some immunohistochemically identifiable neural systems in five species of insectivore-Crocidura cyanea, Crocidura olivieri, Sylvisorex ollula, Paraechinus aethiopicus and Atelerix frontalis Journal of chemical neuroanatomy 72:34–52 doi: 10.1016/j.jchemneu.2015.12.012 [DOI] [PubMed] [Google Scholar]
- Calvey T, Patzke N, Kaswera-Kyamakya C, Gilissen E, Bertelsen MF, Pettigrew JD, Manger PR (2015) Organization of cholinergic, catecholaminergic, serotonergic and orexinergic nuclei in three strepsirrhine primates: Galago demidoff, Perodicticus potto and Lemur catta Journal of chemical neuroanatomy 70:42–57 doi: 10.1016/j.jchemneu.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Acuña J, Elgueta D, Pacheco R (2019) T-Cell-Driven Inflammation as a Mediator of the Gut-Brain Axis Involved in Parkinson’s Disease Frontiers in Immunology 10 doi: 10.3389/fimmu.2019.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellino S et al. (2014) Increased Expression of Dopamine Receptors in Synovial Fibroblasts From Patients With Rheumatoid Arthritis: Inhibitory Effects of Dopamine on Interleukin-8 and Interleukin-6 Arthritis & Rheumatology 66:2685–2693 doi: 10.1002/art.38746 [DOI] [PubMed] [Google Scholar]
- Capellino S, Cosentino M, Wolff C, Schmidt M, Grifka J, Straub RH (2010) Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target Annals of the rheumatic diseases 69:1853–1860 doi: 10.1136/ard.2009.119701 [DOI] [PubMed] [Google Scholar]
- Capellino S, Frommer KW, Rickert M, Steinmeyer J, Rehart S, Müller-Ladner U, Neumann E (2016) OP0149 Dopamine Pathway and Bone Metabolism in Rheumatoid Arthritis Annals of the rheumatic diseases 75:112 doi: 10.1136/annrheumdis-2016-eular.2873 [DOI] [Google Scholar]
- Carey RM (2001) Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure Hypertension 38:297–302 [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA (1962) Cellular localization of brain monoamines Acta physiologica Scandinavica Supplementum 56:1–28 [PubMed] [Google Scholar]
- Caronti B, Antonini G, Calderaro C, Ruggieri S, Palladini G, Pontieri FE, Colosimo C (2001) Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease Journal of neural transmission (Vienna, Austria : 1996) 108:803–807 doi: 10.1007/s007020170030 [DOI] [PubMed] [Google Scholar]
- Carrero JA et al. (2017) Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice Proceedings of the National Academy of Sciences of the United States of America 114:E10418–E10427 doi: 10.1073/pnas.1713543114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MC, Albrechet-Souza L, Masson S, Brandao ML (2005) Changes in the biogenic amine content of the prefrontal cortex, amygdala, dorsal hippocampus, and nucleus accumbens of rats submitted to single and repeated sessions of the elevated plus-maze test Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 38:1857–1866 doi:/S0100-879x2005001200014 [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA (1995) In Vivo Assessment of Dopamine Uptake in Rat Medial Prefrontal Cortex: Comparison with Dorsal Striatum and Nucleus Accumbens Journal of Neurochemistry 65:201–207 doi:doi: 10.1046/j.1471-4159.1995.65010201.x [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR (1992) Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration J Neurochem 59:259–266 [DOI] [PubMed] [Google Scholar]
- Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D (2014) Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment AIDS (London, England) 28:1579–1591 doi: 10.1097/QAD.0000000000000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S (2008) Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization The Journal of Clinical Investigation 118:1380–1389 doi: 10.1172/JCI33125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-Marín V, García-Delgado M, Touma-Fernández A, Aguilar-Alonso E, Fernández-Mondejar E (2008) Intratracheal dopamine attenuates pulmonary edema and improves survival after ventilator-induced lung injury in rats Critical Care 12:R39 doi: 10.1186/cc6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC (2013) The role of natural killer cells in multiple sclerosis and their therapeutic implications Frontiers in immunology 4:63–63 doi: 10.3389/fimmu.2013.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Marmur R, Pulles A, Paredes W, Gardner EL (1993) Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana’s psychoactive ingredient Brain Research 621:65–70 doi: 10.1016/0006-8993(93)90298-2 [DOI] [PubMed] [Google Scholar]
- Chen Y, Hong F, Chen H, Fan RF, Zhang XL, Zhang Y, Zhu JX (2014) Distinctive expression and cellular distribution of dopamine receptors in the pancreatic islets of rats Cell Tissue Res 357:597–606 doi: 10.1007/s00441-014-1894-9 [DOI] [PubMed] [Google Scholar]
- Cho JL et al. (2016) Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation Science translational medicine 8:359ra132 doi: 10.1126/scitranslmed.aag1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat Neuroscience 210:243–248 doi: 10.1016/j.neuroscience.2012.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen NJ, Brandsborg O (1974) Dopamine in human gastric juice determined by a sensitive double-isotope-derivative technique Scandinavian journal of clinical and laboratory investigation 34:315–320 [DOI] [PubMed] [Google Scholar]
- Chumasov EI, Petrova ES, Korzhevskii DE (2011) The immunomorphological analysis of innervation of paraganglian chromaffin cells of mammalian arteries and heart Journal of Evolutionary Biochemistry and Physiology 47:381 doi: 10.1134/S0022093011040104 [DOI] [PubMed] [Google Scholar]
- Ciarka A, Rimacchi R, Vincent JL, Velez-Roa S, Dumonceaux M, Leeman M, van de Borne P (2004) Effects of low-dose dopamine on ventilation in patients with chronic obstructive pulmonary disease European journal of clinical investigation 34:508–512 doi: 10.1111/j.1365-2362.2004.01375.x [DOI] [PubMed] [Google Scholar]
- Ciarka A, Vincent JL, van de Borne P (2007) The effects of dopamine on the respiratory system: friend or foe? Pulmonary pharmacology & therapeutics 20:607–615 doi: 10.1016/j.pupt.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Claustre J, Serusclat P, Peyrin L (1983) Glucuronide and sulfate catecholamine conjugates in rat and human plasma J Neural Transm 56:265–278 [DOI] [PubMed] [Google Scholar]
- Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW (2015) Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis PLoS One 10:e0117450 doi: 10.1371/journal.pone.0117450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence Pharmacol Ther 134:219–245 doi: 10.1016/j.pharmthera.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF (2013) Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression PloS one 8:e58251–e58251 doi: 10.1371/journal.pone.0058251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordano C, Pardini M, Cellerino M, Schenone A, Marino F, Cosentino M (2015) Levodopa-induced neutropenia Parkinsonism & related disorders 21:423–425 doi: 10.1016/j.parkreldis.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Corraliza I (2014) Recruiting specialized macrophages across the borders to restore brain functions Frontiers in cellular neuroscience 8:262–262 doi: 10.3389/fncel.2014.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M et al. (2000) HPLC-ED measurement of endogenous catecholamines in human immune cells and hematopoietic cell lines Life sciences 68:283–295 [DOI] [PubMed] [Google Scholar]
- Cosentino M et al. (2007) Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop Blood 109:632–642 doi: 10.1182/blood-2006-01-028423 [DOI] [PubMed] [Google Scholar]
- Cosentino M, Marino F (2013) Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: teaching old drugs new tricks? J Neuroimmune Pharmacol 8:163–179 doi: 10.1007/s11481-012-9410-z [DOI] [PubMed] [Google Scholar]
- Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G (1999) Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils Life sciences 64:975–981 [DOI] [PubMed] [Google Scholar]
- Cosentino M, Marino F, Maestroni GJM (2015) Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives Frontiers in cellular neuroscience 9:302–302 doi: 10.3389/fncel.2015.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M et al. (2005) Interferon-gamma and interferon-beta affect endogenous catecholamines in human peripheral blood mononuclear cells: implications for multiple sclerosis J Neuroimmunol 162:112–121 doi: 10.1016/j.jneuroim.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Cosentino M, Zaffaroni M, Marino F (2014) Levels of mRNA for dopaminergic receptor D5 in circulating lymphocytes may be associated with subsequent response to interferon-β in patients with multiple sclerosis Journal of Neuroimmunology 277:193–196 doi: 10.1016/j.jneuroim.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Cosentino M et al. (2002) Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis Journal of Neuroimmunology 133:233–240 doi: 10.1016/S0165-5728(02)00372-7 [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Greenfield SA (1997) Differential Autoreceptor Control of Somatodendritic and Axon Terminal Dopamine Release in Substantia Nigra, Ventral Tegmental Area, and Striatum The Journal of Neuroscience 17:5738 doi: 10.1523/JNEUROSCI.17-15-05738.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA (2000) Dopamine Release and Uptake Dynamics within Nonhuman Primate Striatum <em>In Vitro</em> The Journal of Neuroscience 20:8209 doi: 10.1523/JNEUROSCI.20-21-08209.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME (2001) Dopamine-Mediated Volume Transmission in Midbrain Is Regulated by Distinct Extracellular Geometry and Uptake Journal of Neurophysiology 85:1761–1771 doi: 10.1152/jn.2001.85.4.1761 [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME (2004) DAncing past the DAT at a DA synapse Trends in neurosciences 27:270–277 doi: 10.1016/j.tins.2004.03.011 [DOI] [PubMed] [Google Scholar]
- Czermak C et al. (2004) Reduced dopamine D4 receptor mRNA expression in lymphocytes of long-term abstinent alcohol and heroin addicts Addiction (Abingdon, England) 99:251–257 [DOI] [PubMed] [Google Scholar]
- Czub S et al. (2004) Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs Acta Neuropathol 107:216–226 doi: 10.1007/s00401-003-0801-3 [DOI] [PubMed] [Google Scholar]
- Czub S et al. (2001) Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs Acta Neuropathol 101:85–91 [DOI] [PubMed] [Google Scholar]
- Dahlin M, Månsson J-E, Åmark P (2012) CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy Epilepsy Research 99:132–138 doi: 10.1016/j.eplepsyres.2011.11.003 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Smith PH (1985) Effects of substantia nigra lesions on the volumes of A, B, and D cells and the content of insulin and glucagon in the rat pancreas Diabetologia 28:756–762 doi: 10.1007/BF00265024 [DOI] [PubMed] [Google Scholar]
- Davis DG, Sparks DL (1995) Dopaminergic and serotonergic neurotransmitters in bone marrow transplant patients Journal of the Neurological Sciences 130:95–103 doi: 10.1016/0022-510X(95)00012-Q [DOI] [PubMed] [Google Scholar]
- De Laurentiis A, Pisera D, Caruso C, Candolfi M, Mohn C, Rettori V, Seilicovich A (2002) Lipopolysaccharide- and tumor necrosis factor-alpha-induced changes in prolactin secretion and dopaminergic activity in the hypothalamic-pituitary axis Neuroimmunomodulation 10:30–39 doi: 10.1159/000064412 [DOI] [PubMed] [Google Scholar]
- Deak T (2008) Immune cells and cytokine circuits: toward a working model for understanding direct immune-to-adrenal communication pathways Endocrinology 149:1433–1435 doi: 10.1210/en.2008-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcambre S, Nonnenmacher Y, Hiller K (2016) Dopamine Metabolism and Reactive Oxygen Species Production In: Buhlman LM (ed) Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease. Springer International Publishing, Cham, pp 25–47. doi: 10.1007/978-3-319-42139-1_2 [DOI] [Google Scholar]
- Deleplanque B, Vitiello S, Le Moal M, Neveu PJ (1994) Modulation of immune reactivity by unilateral striatal and mesolimbic dopaminergic lesions Neurosci Lett 166:216–220 [DOI] [PubMed] [Google Scholar]
- Demarest KT, Moore KE, Riegle GD (1982) Dopaminergic neuronal function, anterior pituitary dopamine content, and serum concentrations of prolactin, luteinizing hormone and progesterone in the aged female rat Brain Research 247:347–354 doi: 10.1016/0006-8993(82)91260-4 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME (1998) Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat Neuroendocrinology 67:377–383 doi: 10.1159/000054336 [DOI] [PubMed] [Google Scholar]
- Deng T et al. (2013) Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation Cell Metab 17:411–422 doi: 10.1016/j.cmet.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats Proc Natl Acad Sci U S A 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R et al. (2011) Normal gut microbiota modulates brain development and behavior Proceedings of the National Academy of Sciences of the United States of America 108:3047–3052 doi: 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF (1990) Renal dopamine containing nerves. What is their functional significance? American journal of hypertension 3:64s–67s [DOI] [PubMed] [Google Scholar]
- Dimitrijevic I, Kalezic N, Ristic J, Bojovic O, Dimitrijevic N (2008) Digestive system damage caused by substance abuse Acta chirurgica Iugoslavica 55:133–138 [DOI] [PubMed] [Google Scholar]
- Dos-Santos-Pereira M et al. (2018) Microglial glutamate release evoked by alpha-synuclein aggregates is prevented by dopamine Glia 66:2353–2365 doi: 10.1002/glia.23472 [DOI] [PubMed] [Google Scholar]
- Dubé B, Benton T, Cruess DG, Evans DL (2005) Neuropsychiatric manifestations of HIV infection and AIDS Journal of psychiatry & neuroscience : JPN 30:237–246 [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Powell ML, Moreshead WV, Gaskin JM, Hall NR (1987) Effects of Newcastle disease virus administration to mice on the metabolism of cerebral biogenic amines, plasma corticosterone, and lymphocyte proliferation Brain, Behavior, and Immunity 1:216–230 doi: 10.1016/0889-1591(87)90024-9 [DOI] [PubMed] [Google Scholar]
- Dvorakova M, Hohler B, Vollerthun R, Fischbach T, Kummer W (2000) Macrophages: a major source of cytochrome b558 in the rat carotid body Brain Res 852:349–354 [DOI] [PubMed] [Google Scholar]
- Ebinger G, Bruyland M, Martin JJ, Herregodts P, Cras P, Michotte Y, Gommé L (1987) Distribution of biogenic amines and their catabolites in brains from patients with Alzheimer’s disease Journal of the Neurological Sciences 77:267–283 doi: 10.1016/0022-510X(87)90128-6 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G et al. (1997) Substantial production of dopamine in the human gastrointestinal tract The Journal of clinical endocrinology and metabolism 82:3864–3871 doi: 10.1210/jcem.82.11.4339 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Hooper D, Holmes C, Goldstein DS, Friberg P (1995) Production and metabolism of dopamine and norepinephrine in mesenteric organs and liver of swine Am J Physiol 268:G641–649 doi: 10.1152/ajpgi.1995.268.4.G641 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS (2004) 45 - Peripheral Dopamine Systems In: Robertson D, Biaggioni I, Burnstock G, Low PA (eds) Primer on the Autonomic Nervous System (Second Edition). Academic Press, San Diego, pp 176–177. doi: 10.1016/B978-012589762-4/50046-3 [DOI] [Google Scholar]
- Eisenhofer G et al. (2005) Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine The Journal of clinical endocrinology and metabolism 90:2068–2075 doi: 10.1210/jc.2004-2025 [DOI] [PubMed] [Google Scholar]
- Elchisak MA, Cosgrove SE, Ebert MH, Stanley Burns R (1983) Distribution of free and conjugated dopamine in monkey brain, peripheral tissues and cerebrospinal fluid determined by high-performance liquid chromatography Brain Research 279:171–176 doi: 10.1016/0006-8993(83)90175-0 [DOI] [PubMed] [Google Scholar]
- Eldrup E (2004) Significance and origin of DOPA, DOPAC, and dopamine-sulphate in plasma, tissues and cerebrospinal fluid Danish medical bulletin 51:34–62 [PubMed] [Google Scholar]
- Eldrup E, Mogensen P, Jacobsen J, Pakkenberg H, Christensen NJ (1995) CSF and plasma concentrations of free norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylalanine (DOPA), and epinephrine in Parkinson’s disease Acta Neurologica Scandinavica 92:116–121 doi: 10.1111/j.1600-0404.1995.tb01023.x [DOI] [PubMed] [Google Scholar]
- Eldrup E, Moller SE, Andreasen J, Christensen NJ (1997) Effects of ordinary meals on plasma concentrations of 3,4-dihydroxyphenylalanine, dopamine sulphate and 3,4-dihydroxyphenylacetic acid Clinical science (London, England : 1979) 92:423–430 [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V, Rudich A, Hadad N, Levy R (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding Journal of lipid research 49:1894–1903 doi: 10.1194/jlr.M800132-JLR200 [DOI] [PubMed] [Google Scholar]
- Eliasen P, Klemp P, Vagn Nielsen H, Crone P (1989) Subcutaneous and muscle blood flow during dopamine infusion in man Scandinavian journal of clinical and laboratory investigation 49:43–47 [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Morrow BA, Redmond DE Jr., Roth RH (2008) Clozapine normalizes prefrontal cortex dopamine transmission in monkeys subchronically exposed to phencyclidine Neuropsychopharmacology 33:491–496 doi: 10.1038/sj.npp.1301448 [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Marescau B, De Deyn PP (2003) Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson’s disease Neurochem Res 28:1145–1150 [DOI] [PubMed] [Google Scholar]
- Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the blood-brain barrier Journal of neural transmission (Vienna, Austria : 1996) 113:477–485 doi: 10.1007/s00702-005-0409-y [DOI] [PubMed] [Google Scholar]
- Engler H et al. (2009) Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease Brain Behav Immun 23:518–526 doi: 10.1016/j.bbi.2009.01.018 [DOI] [PubMed] [Google Scholar]
- Epelman S et al. (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation Immunity 40:91–104 doi: 10.1016/j.immuni.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler US, Hellner S (1951) Excretion of Noradrenaline, Adrenaline, and Hydroxytyramine in Urine Acta Physiologica Scandinavica 22:161–167 doi: 10.1111/j.1748-1716.1951.tb00765.x [DOI] [PubMed] [Google Scholar]
- Eyre P (1971) Release of dopamine from bovine lung by specific antigen and by compound 48–80 British journal of pharmacology 42:423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W (2003) Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat Synapse (New York, NY) 50:1–6 doi: 10.1002/syn.10238 [DOI] [PubMed] [Google Scholar]
- Fadok JP, Darvas M, Dickerson TMK, Palmiter RD (2010) Long-Term Memory for Pavlovian Fear Conditioning Requires Dopamine in the Nucleus Accumbens and Basolateral Amygdala PLOS ONE 5:e12751 doi: 10.1371/journal.pone.0012751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy Wahba MG, Shehata Messiha BA, Abo-Saif AA (2015) Ramipril and haloperidol as promising approaches in managing rheumatoid arthritis in rats European journal of pharmacology 765:307–315 doi: 10.1016/j.ejphar.2015.08.026 [DOI] [PubMed] [Google Scholar]
- Falgarone G, Jaen O, Boissier MC (2005) Role for innate immunity in rheumatoid arthritis Joint, bone, spine : revue du rhumatisme 72:17–25 doi: 10.1016/j.jbspin.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Fan J et al. (2009) Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells J Neurosci Res 87:2757–2762 doi: 10.1002/jnr.22107 [DOI] [PubMed] [Google Scholar]
- Fan Y, Chen Z, Pathak JL, Carneiro AMD, Chung CY (2018) Differential Regulation of Adhesion and Phagocytosis of Resting and Activated Microglia by Dopamine Frontiers in cellular neuroscience 12:309–309 doi: 10.3389/fncel.2018.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Gao T, Zhang B, Pu J (2018) Recent Advances: Decoding Alzheimer’s Disease With Stem Cells Frontiers in aging neuroscience 10:77–77 doi: 10.3389/fnagi.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Park L, Anrather J, Iadecola C (2017) Brain perivascular macrophages: characterization and functional roles in health and disease Journal of molecular medicine (Berlin, Germany) 95:1143–1152 doi: 10.1007/s00109-017-1573-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj BA, Olkowski ZL, Jackson RT (1991) Binding of [3H]-Dopamine to Human Lymphocytes: Possible Relationship to Neurotransmitter Uptake Sites Pharmacology 42:135–141 doi: 10.1159/000138790 [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H (2005) Dopamine and noradrenaline control distinct functions in rodent microglial cells Mol Cell Neurosci 29:128–138 doi: 10.1016/j.mcn.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, Allen NJ (2018) Astrocytes, neurons, synapses: a tripartite view on cortical circuit development Neural Development 13:7 doi: 10.1186/s13064-018-0104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farino ZJ et al. (2019) New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion Molecular psychiatry doi: 10.1038/s41380-018-0344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre R, de Haut M, Dalmaz Y, Pequignot JM, Peyrin L (1986) Peripheral distribution of free dopamine and its metabolites in the rat Journal of Neural Transmission 66:135–149 doi: 10.1007/BF01260909 [DOI] [PubMed] [Google Scholar]
- Fedchenko V, Globa A, Buneeva O, Medvedev A (2013) Renalase mRNA levels in the brain, heart, and kidneys of spontaneously hypertensive rats with moderate and high hypertension Medical science monitor basic research 19:267–270 doi: 10.12659/MSMBR.889540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Jose PA, Axelrod J (1989) The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase The Journal of pharmacology and experimental therapeutics 248:171–175 [PubMed] [Google Scholar]
- Felten DL et al. (1987) Noradrenergic sympathetic neural interactions with the immune system: structure and function Immunological reviews 100:225–260 [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S (1985) Noradrenergic and peptidergic innervation of lymphoid tissue J Immunol 135:755s–765s [PubMed] [Google Scholar]
- Ferrari M, Cosentino M, Marino F, Bombelli R, Rasini E, Lecchini S, Frigo G (2004) Dopaminergic D1-like receptor-dependent inhibition of tyrosine hydroxylase mRNA expression and catecholamine production in human lymphocytes Biochem Pharmacol 67:865–873 [DOI] [PubMed] [Google Scholar]
- Fhaner MJ, Galligan JJ, Swain GM (2013) Increased catecholamine secretion from single adrenal chromaffin cells in DOCA-salt hypertension is associated with potassium channel dysfunction ACS chemical neuroscience 4:1404–1413 doi: 10.1021/cn400115v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel Y, Eklof AC, Granquist L, Soares-da-Silva P, Bertorello AM (1994) Endogenous dopamine modulates jejunal sodium absorption during high-salt diet in young but not in adult rats Gastroenterology 107:675–679 [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C (2008) Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization Molecular pharmacology 74:59–69 doi: 10.1124/mol.107.043885 [DOI] [PubMed] [Google Scholar]
- Flierl MA et al. (2007) Phagocyte-derived catecholamines enhance acute inflammatory injury Nature 449:721 doi:10.1038/nature0618510.1038/nature06185https://www.nature.com/articles/nature06185-supplementary-informationhttps://www.nature.com/articles/nature06185-supplementary-information [DOI] [PubMed] [Google Scholar]
- Flierl MA et al. (2009) Upregulation of Phagocyte-Derived Catecholamines Augments the Acute Inflammatory Response PLOS ONE 4:e4414 doi: 10.1371/journal.pone.0004414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission Nature Neuroscience 6:968 doi: 10.1038/nn1103 [DOI] [PubMed] [Google Scholar]
- Fox ME, Wightman RM (2017) Contrasting Regulation of Catecholamine Neurotransmission in the Behaving Brain: Pharmacological Insights from an Electrochemical Perspective Pharmacological reviews 69:12–32 doi: 10.1124/pr.116.012948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AL et al. (2015) Evidence against dopamine D1/D2 receptor heteromers Molecular psychiatry 20:1373–1385 doi: 10.1038/mp.2014.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieler RA, Mortensen RM (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling Circulation 131:1019–1030 doi: 10.1161/circulationaha.114.008788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M-H et al. (2015) Stem cell transplantation therapy in Parkinson’s disease SpringerPlus 4:597–597 doi: 10.1186/s40064-015-1400-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, Shapshak P (1997) HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 16:146–152 [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Yatabe M, Tofrizal A, Jindatip D, Yashiro T, Nagai R (2017) Identification of M2 macrophages in anterior pituitary glands of normal rats and rats with estrogen-induced prolactinoma Cell Tissue Res 368:371–378 doi: 10.1007/s00441-016-2564-x [DOI] [PubMed] [Google Scholar]
- Fuxe K, Jacobsen KX, Hoistad M, Tinner B, Jansson A, Staines WA, Agnati LF (2003) The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation Neuroscience 119:733–746 [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Leo G, Agnati LF (2010) Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis Current opinion in pharmacology 10:14–22 doi: 10.1016/j.coph.2009.10.010 [DOI] [PubMed] [Google Scholar]
- Gallego L, Barreiro P, Lopez-Ibor JJ (2011) Diagnosis and clinical features of major neuropsychiatric disorders in HIV infection AIDS reviews 13:171–179 [PubMed] [Google Scholar]
- Gardner DG, Shoback DM (2007) Greenspan’s basic & clinical endocrinology. McGraw-Hill Medical; New York:, [Google Scholar]
- Garrido-Gil P, Dominguez-Meijide A, Moratalla R, Guerra MJ, Labandeira-Garcia JL (2018a) Aging-related dysregulation in enteric dopamine and angiotensin system interactions: implications for gastrointestinal dysfunction in the elderly Oncotarget 9:10834–10846 doi: 10.18632/oncotarget.24330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gil P, Rodriguez-Perez AI, Dominguez-Meijide A, Guerra MJ, Labandeira-Garcia JL (2018b) Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models Molecular neurobiology 55:7297–7316 doi: 10.1007/s12035-018-0937-8 [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994) Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain J Neurosci 14:6084–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM (1994) Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study J Neurosci 14:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Coley JS, Berman JW (2013) Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND J Neuroimmune Pharmacol 8:621–642 doi: 10.1007/s11481-013-9443-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse Am J Pathol 175:1148–1159 doi: 10.2353/ajpath.2009.081067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW (2012) Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse J Neuroinflammation 9:203 doi: 10.1186/1742-2094-9-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H (2017) HIV, Tat and dopamine transmission Neurobiol Dis 105:51–73 doi: 10.1016/j.nbd.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW (2014) Dopamine receptor activation increases HIV entry into primary human macrophages PLoS One 9:e108232 doi: 10.1371/journal.pone.0108232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti TD Jr. et al. (1998) Continuous covariability of dopamine and serotonin metabolites in human cerebrospinal fluid Biological psychiatry 44:228–233 [DOI] [PubMed] [Google Scholar]
- Gierut A, Perlman H, Pope RM (2010) Innate immunity and rheumatoid arthritis Rheumatic diseases clinics of North America 36:271–296 doi: 10.1016/j.rdc.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea JJ (2009) Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance Current opinion in nephrology and hypertension 18:28–32 doi: 10.1097/MNH.0b013e32831a9e0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorelli M, Livrea P, Trojano M (2005) Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-beta Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 25:395–406 doi: 10.1089/jir.2005.25.395 [DOI] [PubMed] [Google Scholar]
- Glavin GB, Hall AM (1995) Central and peripheral dopamine D1/DA1 receptor modulation of gastric secretion and experimental gastric mucosal injury General pharmacology 26:1277–1279 [DOI] [PubMed] [Google Scholar]
- Glavin GB, Szabo S (1990) Dopamine in gastrointestinal disease Digestive Diseases and Sciences 35:1153–1161 doi: 10.1007/BF01537589 [DOI] [PubMed] [Google Scholar]
- Glennon E, Kaunzner UW, Gagnidze K, McEwen BS, Bulloch K (2015) Pituitary dendritic cells communicate immune pathogenic signals Brain Behav Immun 50:232–240 doi: 10.1016/j.bbi.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Goldberg LI (1972) Cardiovascular and renal actions of dopamine: potential clinical applications Pharmacological reviews 24:1–29 [PubMed] [Google Scholar]
- Goldstein DS, Holmes C (2008) Neuronal Source of Plasma Dopamine Clinical chemistry 54:1864–1871 doi: 10.1373/clinchem.2008.107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Mezey E, Yamamoto T, Aneman A, Friberg P, Eisenhofer G (1995) Is there a third peripheral catecholaminergic system? Endogenous dopamine as an autocrine/paracrine substance derived from plasma DOPA and inactivated by conjugation Hypertension research : official journal of the Japanese Society of Hypertension 18 Suppl 1:S93–99 [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC (2011) Catechols in post-mortem brain of patients with Parkinson disease European journal of neurology 18:703–710 doi: 10.1111/j.1468-1331.2010.03246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS et al. (1999) Sources and physiological significance of plasma dopamine sulfate The Journal of clinical endocrinology and metabolism 84:2523–2531 doi: 10.1210/jcem.84.7.5864 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Bornstein SR, Ehrhart-Bornstein M, Geschwend JE, Adler G, Scherbaum WA (1994) Macrophages within the human adrenal gland Cell Tissue Res 278:201–205 [DOI] [PubMed] [Google Scholar]
- Goodall M (1951) Studies of adrenaline and noradrenaline in mammalian heart and suprarenals Acta physiologica Scandinavica Supplementum 24:7–51 [PubMed] [Google Scholar]
- Goodarzi A, Vousooghi N, Sedaghati M, Mokri A, Zarrindast MR (2009) Dopamine receptors in human peripheral blood lymphocytes: changes in mRNA expression in opioid addiction European journal of pharmacology 615:218–222 doi: 10.1016/j.ejphar.2009.04.060 [DOI] [PubMed] [Google Scholar]
- Griggio MA, Richard D, Leblanc J (1992) Effects of fasting and food restriction on sympathetic activity in brown adipose tissue in mice Journal of comparative physiology B, Biochemical, systemic, and environmental physiology 162:602–606 [DOI] [PubMed] [Google Scholar]
- Guo X et al. (2016) Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer Sci Rep 6:22996 doi: 10.1038/srep22996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Hara C, Yamaguchi K, Miyawaki A (2004) PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes Neuron 41:405–415 [DOI] [PubMed] [Google Scholar]
- Hampl V et al. (2015) Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1–7/G-protein-coupled Mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia Physiological research 64:25–38 [DOI] [PubMed] [Google Scholar]
- Hamrell BB, Huber SA, Leslie KO (1995) Depressed unloaded sarcomere shortening velocity in acute murine coxsackievirus myocarditis: myocardial remodelling in the absence of necrosis or hypertrophy Eur Heart J 16 Suppl O:31–35 [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Karoum F, Hellstrom S, Lahiri S (1981) Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body Neuroscience 6:81–86 doi: 10.1016/0306-4522(81)90245-1 [DOI] [PubMed] [Google Scholar]
- Hannah JAM, Inglis GC, Connell JMC, Davies DL, Fraser R, Ball SG (1984) Dopamine, Noradrenaline and Adrenaline in the Adrenal Medulla and Cortex of the Rat Clinical Science 66:65P [Google Scholar]
- Harris RC, Zhang M-Z (2012) Dopamine, the kidney, and hypertension Current hypertension reports 14:138–143 doi: 10.1007/s11906-012-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W (2010) Dopamine release in the prefrontal cortex and striatum: temporal and behavioural aspects Pharmacopsychiatry 43 Suppl 1:S32–41 doi: 10.1055/s-0030-1248300 [DOI] [PubMed] [Google Scholar]
- Hauber W, Fuchs H (2000) Dopamine release in the rat globus pallidus characterised by in vivo microdialysis Behavioural brain research 111:39–44 [DOI] [PubMed] [Google Scholar]
- Hawkins TA, Gala RR, Dunbar JC (1996) The lymphocyte and macrophage profile in the pancreas and spleen of NOD mice: percentage of interleukin-2 and prolactin receptors on immunocompetent cell subsets Journal of reproductive immunology 32:55–71 [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA (1991) Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes J Biol Chem 266:5650–5657 [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE (1995) The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis Journal of Pharmacology and Experimental Therapeutics 273:591. [PubMed] [Google Scholar]
- Hemmings HC Jr, Greengard P, Tung HYL, Cohen P (1984) DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1 Nature 310:503 doi: 10.1038/310503a0 [DOI] [PubMed] [Google Scholar]
- Herak-Perkovic V, Grabarevic Z, Banic M, Anic B, Novosel V, Pogacnik M (2001) Effects of dopaminergic drugs on inflammatory bowel disease induced with 2,4-dinitrofluorbenzene in BALB/c mice Journal of veterinary pharmacology and therapeutics 24:267–273 [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis Life sciences 42:1705–1712 doi: 10.1016/0024-3205(88)90036-7 [DOI] [PubMed] [Google Scholar]
- Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, Pineda B, Sotelo J (2013) Initial immunopathogenesis of multiple sclerosis: innate immune response Clinical & developmental immunology 2013:413465–413465 doi: 10.1155/2013/413465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig L et al. (2016) Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica Mult Scler 22:160–173 doi: 10.1177/1352458515586084 [DOI] [PubMed] [Google Scholar]
- Homo-Delarche F, Drexhage HA (2004) Immune cells, pancreas development, regeneration and type 1 diabetes Trends Immunol 25:222–229 doi: 10.1016/j.it.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O (1986) A Quarter Century of Brain Dopamine Research In: Woodruff GN, Poat JA, Roberts PJ (eds) Dopaminergic Systems and their Regulation. Palgrave Macmillan; UK, London, pp 3–18. doi: 10.1007/978-1-349-07431-0_1 [DOI] [Google Scholar]
- Hows MEP, Lacroix L, Heidbreder C, Organ AJ, Shah AJ (2004) High-performance liquid chromatography/tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples Journal of Neuroscience Methods 138:123–132 doi: 10.1016/j.jneumeth.2004.03.021 [DOI] [PubMed] [Google Scholar]
- Hu L et al. (2014) A new stress model, a scream sound, alters learning and monoamine levels in rat brain Physiology & behavior 123:105–113 doi: 10.1016/j.physbeh.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu X, Qiao D (2015) Increased extracellular dopamine and 5-hydroxytryptamine levels contribute to enhanced subthalamic nucleus neural activity during exhausting exercise Biology of sport 32:187–192 doi: 10.5604/20831862.1150299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Qiu AW, Peng YP, Liu Y, Huang HW, Qiu YH (2010) Roles of dopamine receptor subtypes in mediating modulation of T lymphocyte function Neuro endocrinology letters 31:782–791 [PubMed] [Google Scholar]
- Hubbard KE, Wells A, Owens TS, Tagen M, Fraga CH, Stewart CF (2009) Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection Biomedical Chromatography 24:626–631 doi: 10.1002/bmc.1338 [DOI] [PubMed] [Google Scholar]
- Huck JHJ et al. (2015) De novo expression of dopamine D2 receptors on microglia after stroke Journal of Cerebral Blood Flow & Metabolism 35:1804–1811 doi: 10.1038/jcbfm.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G, Noverr MC (2008) GI microbiota and regulation of the immune system. Preface Adv Exp Med Biol 635:v–vi [PubMed] [Google Scholar]
- Huh JY, Park YJ, Ham M, Kim JB (2014) Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity Molecules and cells 37:365–371 doi: 10.14348/molcells.2014.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M et al. (2017) Macrophages Facilitate Electrical Conduction in the Heart Cell 169:510–522.e520 doi: 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T, Lokhandwala MF (2003) Renal dopamine receptors and hypertension Experimental biology and medicine (Maywood, NJ) 228:134–142 [DOI] [PubMed] [Google Scholar]
- Hussell T, Bell TJ (2014) Alveolar macrophages: plasticity in a tissue-specific context Nat Rev Immunol 14:81–93 doi: 10.1038/nri3600 [DOI] [PubMed] [Google Scholar]
- Hutchinson MR et al. (2008) Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia Brain Behav Immun 22:1248–1256 doi: 10.1016/j.bbi.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Watkins LR (2014) Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76 Pt B:218–227 doi: 10.1016/j.neuropharm.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Strous RD, Fuchs S (2004) Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells Faseb j 18:1600–1602 doi: 10.1096/fj.04-1652fje [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B (1999) Dopaminergic innervation of the amygdala is highly responsive to stress J Neurochem 72:1088–1094 [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J (2004) Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals Brain research Brain research reviews 47:46–53 doi: 10.1016/j.brainresrev.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Iwen KA et al. (2017) Cold-Induced Brown Adipose Tissue Activity Alters Plasma Fatty Acids and Improves Glucose Metabolism in Men The Journal of Clinical Endocrinology & Metabolism 102:4226–4234 doi: 10.1210/jc.2017-01250 [DOI] [PubMed] [Google Scholar]
- Jackowska K, Krysinski P (2013) New trends in the electrochemical sensing of dopamine Analytical and Bioanalytical Chemistry 405:3753–3771 doi: 10.1007/s00216-012-6578-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR et al. (2012) Retinal Dopamine Mediates Multiple Dimensions of Light-Adapted Vision The Journal of Neuroscience 32:9359 doi: 10.1523/JNEUROSCI.0711-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B (2001) Amygdala Regulation of Nucleus Accumbens Dopamine Output is Governed by the Prefrontal Cortex The Journal of Neuroscience 21:676 doi: 10.1523/JNEUROSCI.21-02-00676.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A et al. (1999) On the distribution patterns of D1, D2, tyrosine hydroxylase and dopamine transporter immunoreactivities in the ventral striatum of the rat Neuroscience 89:473–489 doi: 10.1016/S0306-4522(98)00317-0 [DOI] [PubMed] [Google Scholar]
- Jin L-Q, Wang H-Y, Friedman E (2001) Stimulated D1 dopamine receptors couple to multiple Gα proteins in different brain regions Journal of Neurochemistry 78:981–990 doi: 10.1046/j.1471-4159.2001.00470.x [DOI] [PubMed] [Google Scholar]
- Johnson JA, Rodeberg NT, Wightman RM (2018) Measurement of Basal Neurotransmitter Levels Using Convolution-Based Nonfaradaic Current Removal Analytical chemistry 90:7181–7189 doi: 10.1021/acs.analchem.7b04682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan NB, Munsick RA (1980) Dopamine and Prolactin in Human Pregnancy* The Journal of Clinical Endocrinology & Metabolism 51:1019–1025 doi: 10.1210/jcem-51-5-1019 [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM (1995) Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens Journal of Pharmacology and Experimental Therapeutics 274:396. [PubMed] [Google Scholar]
- Josefsson E, Bergquist J, Ekman R, Tarkowski A (1996) Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis Immunology 88:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juorio AV, Chedrese PJ (1990) The concentration of dopamine and related monoamines in arteries and some other tissues of the sheep Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 95:35–37 doi: 10.1016/0742-8413(90)90079-O [DOI] [PubMed] [Google Scholar]
- Kabiersch A, Furukawa H, del Rey A, Besedovsky HO (1998) Administration of interleukin-1 at birth affects dopaminergic neurons in adult mice Ann N Y Acad Sci 840:123–127 [DOI] [PubMed] [Google Scholar]
- Kanczkowski W, Sue M, Bornstein SR (2016) Adrenal Gland Microenvironment and Its Involvement in the Regulation of Stress-Induced Hormone Secretion during Sepsis Frontiers in endocrinology 7:156–156 doi: 10.3389/fendo.2016.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemi O, Zhang X, Sakamoto Y, Ebina M, Nagatomi R (2005) Acute stress reduces intraparenchymal lung natural killer cells via beta-adrenergic stimulation Clinical and experimental immunology 139:25–34 doi: 10.1111/j.1365-2249.2005.02672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TY, Chio CC, Lin MT (1994) Hypothalamic dopamine release and local cerebral blood flow during onset of heatstroke in rats Stroke 25:2483–2486; discussion 2486–2487 [DOI] [PubMed] [Google Scholar]
- Karasawa N, Kondo Y, Nagatsu I (1982) Immunohistocytochemical and immunofluorescent localization of catecholamine-synthesizing enzymes in the carotid body of the bat and dog Archivum histologicum Japonicum = Nihon soshikigaku kiroku 45:429–435 [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Cobelens PM, Teunis MAT, Heijnen CJ (2005) Changes in innate and acquired immune responses in mice with targeted deletion of the dopamine transporter gene Journal of Neuroimmunology 161:162–168 doi: 10.1016/j.jneuroim.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum Neuroscience 51:55–64 [DOI] [PubMed] [Google Scholar]
- Kawakami T et al. (2013) Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions Journal of immunology (Baltimore, Md : 1950) 191:3358–3372 doi: 10.4049/jimmunol.1300342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M et al. (1999) Differential effects of chemical sympathectomy on expression and activity of tyrosine hydroxylase and levels of catecholamines and DOPA in peripheral tissues of rats Neurochem Res 24:25–32 [DOI] [PubMed] [Google Scholar]
- Kawano M, Takagi R, Kaneko A, Matsushita S (2015) Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses Journal of Neuroimmunology 289:43–55 doi: 10.1016/j.jneuroim.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Kawano M, Takagi R, Saika K, Matsui M, Matsushita S (2018) Dopamine regulates cytokine secretion during innate and adaptive immune responses International immunology 30:591–606 doi: 10.1093/intimm/dxy057 [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB (1979) Multiple receptors for dopamine Nature 277:93–96 [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Petzold GL, Greengard P (1972) Dopamine-Sensitive Adenylate Cyclase in Caudate Nucleus of Rat Brain, and Its Similarity to the “Dopamine Receptor” Proceedings of the National Academy of Sciences of the United States of America 69:2145–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe KA, Zigmond MJ, Abercrombie ED (1993) In vivo regulation of extracellular dopamine in the neostriatum: influence of impulse activity and local excitatory amino acids Journal of neural transmission General section 91:223–240 [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory Proceedings of the National Academy of Sciences of the United States of America 113:14835–14840 doi: 10.1073/pnas.1616515114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS (2001) An astroglia-linked dopamine D2-receptor action in prefrontal cortex Proceedings of the National Academy of Sciences of the United States of America 98:1964–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieburtz K et al. (1996) Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection Archives of neurology 53:155–158 [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Johnson BJ, Cornwall J, Phillipson OT (1986) Thalamic control of dopaminergic functions in the caudate-putamen of the rat—II. Studies using ibotenic acid injection of the parafascicular-intralaminar nuclei Neuroscience 19:979–990 doi: 10.1016/0306-4522(86)90310-6 [DOI] [PubMed] [Google Scholar]
- Kim T-H, Choi J, Kim H-G, Kim HR (2014) Quantification of Neurotransmitters in Mouse Brain Tissue by Using Liquid Chromatography Coupled Electrospray Tandem Mass Spectrometry Journal of Analytical Methods in Chemistry 2014:11 doi: 10.1155/2014/506870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-G, Mohney RP, Wilson B, Jeohn G-H, Liu B, Hong J-S (2000) Regional Difference in Susceptibility to Lipopolysaccharide-Induced Neurotoxicity in the Rat Brain: Role of Microglia The Journal of Neuroscience 20:6309 doi: 10.1523/JNEUROSCI.20-16-06309.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RJ, Mefford IN, Wang C, Murchison A, Caligari EJ, Berger PA (1986) CSF dopamine levels correlate with extraversion in depressed patients Psychiatry Research 19:305–310 doi: 10.1016/0165-1781(86)90123-X [DOI] [PubMed] [Google Scholar]
- Kipnis J et al. (2004) Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration J Neurosci 24:6133–6143 doi: 10.1523/jneurosci.0600-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Gershon MD (1990) Innervation of the pancreas by neurons in the gut J Neurosci 10:1626–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM (2008) Dopamine Receptors in Human Lymphocytes: Radioligand Binding and Quantitative RT-PCR Assays Journal of neuroscience methods 174:272–280 doi: 10.1016/j.jneumeth.2008.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T et al. (2000) Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 18:521–530 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ricci A, Amenta F, Cavallotti C, Hattori K (1995) Localization of dopamine receptors in the rabbit lung vasculature Journal of vascular research 32:200–206 doi: 10.1159/000159094 [DOI] [PubMed] [Google Scholar]
- Kopec A, Smith CJ, Ayre NR, Sweat SC, Bilbo SD (2017) Microglial elimination of dopamine D1 receptors defines sex-specific changes in nucleus accumbens development and social play behavior during adolescence bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ (1985) Catecholamine metabolism: basic aspects and clinical significance Pharmacological reviews 37:333–364 [PubMed] [Google Scholar]
- Korf J, Grasdijk L, Westerink BHC (1976) EFFECTS OF ELECTRICAL STIMULATION OF THE NIGROSTRIATAL PATHWAY OF THE RAT ON DOPAMINE METABOLISM Journal of Neurochemistry 26:579–584 doi: 10.1111/j.1471-4159.1976.tb01514.x [DOI] [PubMed] [Google Scholar]
- Kovacs K (2012) Microglia and Drug-Induced Plasticity in Reward-Related Neuronal Circuits Frontiers in Molecular Neuroscience 5 doi: 10.3389/fnmol.2012.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger JL, Patzke N, Fuxe K, Bennett NC, Manger PR (2012) Nuclear organization of cholinergic, putative catecholaminergic, serotonergic and orexinergic systems in the brain of the African pygmy mouse (Mus minutoides): organizational complexity is preserved in small brains Journal of chemical neuroanatomy 44:45–56 doi: 10.1016/j.jchemneu.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Kuchel OG, Kuchel GA (1991) Peripheral dopamine in pathophysiology of hypertension. Interaction with aging and lifestyle Hypertension 18:709–721 [DOI] [PubMed] [Google Scholar]
- Kumai T, Tateishi T, Tanaka M, Watanabe M, Shimizu H, Kobayashi S (2000) Effect of interferon-alpha on tyrosine hydroxylase and catecholamine levels in the brain of rats Life sciences 67:663–669 [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains Journal of neurovirology 15:257–274 doi: 10.1080/13550280902973952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR (2012) Peripheral chemoreceptors: function and plasticity of the carotid body Comprehensive Physiology 2:141–219 doi: 10.1002/cphy.c100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Panzer U, Anders HJ, Rees AJ (2013) The immune system and kidney disease: basic concepts and clinical implications Nat Rev Immunol 13:738–753 doi: 10.1038/nri3523 [DOI] [PubMed] [Google Scholar]
- Kustrimovic N et al. (2016) Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease Sci Rep 6:33738 doi: 10.1038/srep33738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG et al. (2018) Granulocyte colony stimulating factor enhances reward learning through potentiation of mesolimbic dopamine system function The Journal of Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD (2017) Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42:156–177 doi: 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente A, González-Carracedo A, Romero A, Cabaleiro T, Esquifino AI (2005) Toxic effects of cadmium on the regulatory mechanism of dopamine and serotonin on prolactin secretion in adult male rats Toxicology Letters 155:87–96 doi: 10.1016/j.toxlet.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Lahouaoui H, Coutanson C, Cooper HM, Bennis M, Dkhissi-Benyahya O (2016) Diabetic retinopathy alters light-induced clock gene expression and dopamine levels in the mouse retina Molecular vision 22:959–969 [PMC free article] [PubMed] [Google Scholar]
- Lam S-Y, Liu Y, Ng K-M, Lau C-F, Liong EC, Tipoe GL, Fung M-L (2012) Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways Histochemistry and cell biology 137:303–317 doi: 10.1007/s00418-011-0900-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamensdorf I, Porat S, Simantov R, Finberg JP (1999) Effect of low-dose treatment with selegiline on dopamine transporter (DAT) expression and amphetamine-induced dopamine release in vivo Br J Pharmacol 126:997–1002 doi: 10.1038/sj.bjp.0702389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system Neuron 57:760–773 doi: 10.1016/j.neuron.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Larochelle C, Alvarez JI, Prat A (2011) How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Letters 585:3770–3780 doi: 10.1016/j.febslet.2011.04.066 [DOI] [PubMed] [Google Scholar]
- Lavine KJ et al. (2014) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart Proceedings of the National Academy of Sciences 111:16029 doi: 10.1073/pnas.1406508111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain Neuroscience 39:151–170 [DOI] [PubMed] [Google Scholar]
- Le Fur G, Phan T, Uzan A (1980) Identification of stereospecific [3H]spiroperidol binding sites in mammalian lymphocytes Life sciences 26:1139–1148 doi: 10.1016/0024-3205(80)90653-0 [DOI] [PubMed] [Google Scholar]
- Lechin F et al. (1990) Plasma neurotransmitters and cortisol in duodenal ulcer patients. Role of stress Dig Dis Sci 35:1313–1319 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Tseng HC, Wong SW, Wang Z, Deng M, Ko C-C (2015) Dopaminergic effects on in vitro osteogenesis Bone research 3:15020–15020 doi: 10.1038/boneres.2015.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ et al. (2002) Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor Cell 111:219–230 [DOI] [PubMed] [Google Scholar]
- Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ Jr., Near JA, Wightman RM (1991) Secretion of catecholamines from individual adrenal medullary chromaffin cells J Neurochem 56:1855–1863 [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ (2001) Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys J Neurosci 21:2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandis G et al. (2015) Response of colonic motility to dopaminergic stimulation is subverted in rats with nigrostriatal lesion: relevance to gastrointestinal dysfunctions in Parkinson’s disease Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 27:1783–1795 doi: 10.1111/nmo.12691 [DOI] [PubMed] [Google Scholar]
- Levey AI et al. (1993) Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies Proc Natl Acad Sci U S A 90:8861–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M (2016) Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases Acta physiologica (Oxford, England) 216:42–89 doi: 10.1111/apha.12476 [DOI] [PubMed] [Google Scholar]
- Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L (2001) Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function Eur J Immunol 31:3504–3512 doi:10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f [DOI] [PubMed] [Google Scholar]
- Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons Proceedings of the National Academy of Sciences of the United States of America 79:6385–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD (2006) Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice J Neurosci 26:2798–2807 doi: 10.1523/jneurosci.4720-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limacher A, Bhagwandin A, Fuxe K, Manger PR (2008) Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the Cape porcupine (Hystrix africaeaustralis): increased brain size does not lead to increased organizational complexity Journal of chemical neuroanatomy 36:33–52 doi: 10.1016/j.jchemneu.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH (2016) Association Between Parkinson’s Disease and Inflammatory Bowel Disease: a Nationwide Taiwanese Retrospective Cohort Study Inflamm Bowel Dis 22:1049–1055 doi: 10.1097/mib.0000000000000735 [DOI] [PubMed] [Google Scholar]
- Lineberry TW, Bostwick JM (2006) Methamphetamine Abuse: A Perfect Storm of Complications Mayo Clinic Proceedings 81:77–84 doi: 10.4065/81.1.77 [DOI] [PubMed] [Google Scholar]
- Liu J et al. (2009) Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice Nat Med 15:940–945 doi: 10.1038/nm.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Marsland BJ (2017) Lung Homeostasis: Influence of Age, Microbes, and the Immune System Immunity 46:549–561 doi: 10.1016/j.immuni.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Louveau A, Harris TH, Kipnis J (2015) Revisiting the Mechanisms of CNS Immune Privilege Trends Immunol 36:569–577 doi: 10.1016/j.it.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LA, McMillen BA (2002) Differences in brain area concentrations of dopamine and serotonin in Myers’ High Ethanol Preferring (mHEP) and outbred rats Journal of neural transmission (Vienna, Austria : 1996) 109:279–292 doi: 10.1007/s007020200023 [DOI] [PubMed] [Google Scholar]
- Luft FC (2005) Renalase, a catecholamine-metabolizing hormone from the kidney Cell Metabolism 1:358–360 doi: 10.1016/j.cmet.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Łukasiewicz S, Błasiak E, Szafran-Pilch K, Dziedzicka-Wasylewska M (2016) Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics – in vitro studies Journal of Neurochemistry 137:549–560 doi: 10.1111/jnc.13582 [DOI] [PubMed] [Google Scholar]
- Lutzky V, Hannawi S, Thomas R (2007) Cells of the synovium in rheumatoid arthritis. Dendritic cells Arthritis research & therapy 9:219 doi: 10.1186/ar2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DJ, Hellysaz A, Broberger C (2012) Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis J Neurosci 32:8074–8083 doi: 10.1523/jneurosci.0129-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M (2013) Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior PLOS Pathogens 9:e1003726 doi: 10.1371/journal.ppat.1003726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie P, Lebowitz J, Saadatpour L, Nickoloff E, Gaskill P, Khoshbouei H (2018) The dopamine transporter: An unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s Disease Brain Behav Immun 70:21–35 doi: 10.1016/j.bbi.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KS (2017) Sympathetic neural-immune interactions regulate hematopoiesis, thermoregulation and inflammation in mammals Developmental & Comparative Immunology 66:92–97 doi: 10.1016/j.dci.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Cosentino M, Marino F, Togni M, Conti A, Lecchini S, Frigo G (1998) Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis? Experimental hematology 26:1172–1177 [PubMed] [Google Scholar]
- Magro F, Fraga S, Ribeiro T, Soares-da-Silva P (2004) Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-γ upon L-DOPA uptake Acta Physiologica Scandinavica 180:379–386 doi: 10.1111/j.1365-201X.2004.01260.x [DOI] [PubMed] [Google Scholar]
- Magro F, Vieira-Coelho MA, Fraga S, Serrao MP, Veloso FT, Ribeiro T, Soares-da-Silva P (2002) Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease Dig Dis Sci 47:216–224 [DOI] [PubMed] [Google Scholar]
- Manger PR, Fuxe K, Ridgway SH, Siegel JM (2004) The distribution and morphological characteristics of catecholaminergic cells in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus) Brain, behavior and evolution 64:42–60 doi: 10.1159/000077542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S (1999) Catechol-<em>O</em>-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors Pharmacological reviews 51:593. [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE (2014) Heterogeneity of dopamine neuron activity across traits and states Neuroscience 282:176–197 doi: 10.1016/j.neuroscience.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino F, Cosentino M (2016) Multiple sclerosis: Repurposing dopaminergic drugs for MS--the evidence mounts Nature reviews Neurology 12:191–192 doi: 10.1038/nrneurol.2016.33 [DOI] [PubMed] [Google Scholar]
- Marino F, Cosentino M, Bombelli R, Ferrari M, Lecchini S, Frigo G (1999) Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells Experimental hematology 27:489–495 [DOI] [PubMed] [Google Scholar]
- Marino F et al. (1997) Measurement of catecholamines in mouse bone marrow by means of HPLC with electrochemical detection Haematologica 82:392–394 [PubMed] [Google Scholar]
- Markianos M, Koutsis G, Evangelopoulos ME, Mandellos D, Karahalios G, Sfagos C (2009) Relationship of CSF neurotransmitter metabolite levels to disease severity and disability in multiple sclerosis J Neurochem 108:158–164 doi: 10.1111/j.1471-4159.2008.05750.x [DOI] [PubMed] [Google Scholar]
- Maseko BC, Patzke N, Fuxe K, Manger PR (2013) Architectural organization of the african elephant diencephalon and brainstem Brain, behavior and evolution 82:83–128 doi: 10.1159/000352004 [DOI] [PubMed] [Google Scholar]
- Mastroeni D et al. (2009) Microglial responses to dopamine in a cell culture model of Parkinson’s disease Neurobiology of aging 30:1805–1817 doi: 10.1016/j.neurobiolaging.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM et al. (2008) Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review Lancet 372:1733–1745 doi: 10.1016/s0140-6736(08)61311-2 [DOI] [PubMed] [Google Scholar]
- Matsuyama N et al. (2018) The dopamine D1 receptor is expressed and induces CREB phosphorylation and MUC5AC expression in human airway epithelium Respiratory Research 19:53 doi: 10.1186/s12931-018-0757-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty R, Kirby RF, Carey RM (1986) Effects of dietary sodium on dopamine content of rat adrenal cortex Physiology & behavior 37:785–789 [DOI] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ (2002) Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study J Neuroimmunol 132:34–40 [DOI] [PubMed] [Google Scholar]
- McQueen DS, Mir AK, Brash HM, Nahorski SR (1984) Increased sensitivity of rabbit carotid body chemoreceptors to dopamine after chronic treatment with domperidone European journal of pharmacology 104:39–46 doi: 10.1016/0014-2999(84)90366-2 [DOI] [PubMed] [Google Scholar]
- Mebel DM, Wong JC, Dong YJ, Borgland SL (2012) Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake Eur J Neurosci 36:2336–2346 doi: 10.1111/j.1460-9568.2012.08168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism Cell Communication and Signaling 11:34 doi: 10.1186/1478-811X-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT (2011) The bone marrow at the crossroads of blood and immunity Nat Rev Immunol 12:49–60 doi: 10.1038/nri3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith EJ, Holder MJ, Rosen A, Lee AD, Dyer MJ, Barnes NM, Gordon J (2006) Dopamine targets cycling B cells independent of receptors/transporter for oxidative attack: Implications for non-Hodgkin’s lymphoma Proc Natl Acad Sci U S A 103:13485–13490 doi: 10.1073/pnas.0605993103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshki Baf MH, Subhash MN, Lakshmana KM, Sridhara Rama Rao BS (1994) Alterations in monoamine levels in discrete regions of rat brain after chronic administration of carbamazepine Neurochemical Research 19:1139–1143 doi: 10.1007/BF00965147 [DOI] [PubMed] [Google Scholar]
- Mezey É, Eisenhofer G, Hansson S, Hunyady B, Hoffman BJ (1998) Dopamine Produced by the Stomach May Act as a Paracrine/Autocrine Hormone in the Rat Neuroendocrinology 67:336–348 doi: 10.1159/000054332 [DOI] [PubMed] [Google Scholar]
- Mezey E, Eisenhofer G, Harta G, Hansson S, Gould L, Hunyady B, Hoffman BJ (1996) A novel nonneuronal catecholaminergic system: exocrine pancreas synthesizes and releases dopamine Proceedings of the National Academy of Sciences of the United States of America 93:10377–10382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Palkovits M (1992) Localization of targets for anti-ulcer drugs in cells of the immune system Science 258:1662–1665 [DOI] [PubMed] [Google Scholar]
- Mignini F, Sabbatini M, Capacchietti M, Amantini C, Bianchi E, Artico M, Tammaro A (2013) T-cell subpopulations express a different pattern of dopaminergic markers in intra- and extra-thymic compartments Journal of biological regulators and homeostatic agents 27:463–475 [PubMed] [Google Scholar]
- Mignini F, Sabbatini M, Mattioli L, Cosenza M, Artico M, Cavallotti C (2014) Neuro-immune modulation of the thymus microenvironment (review) International journal of molecular medicine 33:1392–1400 doi: 10.3892/ijmm.2014.1709 [DOI] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F (2003) Autonomic innervation of immune organs and neuroimmune modulation Autonomic & autacoid pharmacology 23:1–25 [DOI] [PubMed] [Google Scholar]
- Mignini F, Tomassoni D, Traini E, Amenta F (2009) Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen J Neuroimmunol 206:5–13 doi: 10.1016/j.jneuroim.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function Physiological reviews 78:189–225 doi: 10.1152/physrev.1998.78.1.189 [DOI] [PubMed] [Google Scholar]
- Mitchell UH, Obray JD, Hunsaker E, Garcia BT, Clarke TJ, Hope S, Steffensen SC (2018) Peripheral Dopamine in Restless Legs Syndrome Frontiers in neurology 9:155–155 doi: 10.3389/fneur.2018.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R et al. (2017) Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis Journal of cellular physiology 232:2359–2372 doi: 10.1002/jcp.25518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K, Zhang Y, Xu D, Mizuta F, D’Ovidio F, Masaki E, Emala CW (2013) The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle Respiratory research 14:89–89 doi: 10.1186/1465-9921-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini M, Kashi Z, Mohammad Pour AR, Adibi E (2011) The effect of cabergoline on clinical and laboratory findings in active rheumatoid arthritis Iranian Red Crescent medical journal 13:749–750 [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS (1989) Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine Synapse (New York, NY) 4:156–161 doi: 10.1002/syn.890040209 [DOI] [PubMed] [Google Scholar]
- MohanKumar SMJ, MohanKumar PS, Quadri SK (1999) Lipopolysaccharide-induced changes in monoamines in specific areas of the brain: blockade by interleukin-1 receptor antagonist1Preliminary results were presented at the Experimental Biology meeting, Atlanta, GA, USA, 1995.1 Brain Research 824:232–237 doi: 10.1016/S0006-8993(99)01206-8 [DOI] [PubMed] [Google Scholar]
- Mohanty PK, Sowers JR, Thames MD, Beck FW, Kawaguchi A, Lower RR (1986) Myocardial norepinephrine, epinephrine and dopamine concentrations after cardiac autotransplantation in dogs J Am Coll Cardiol 7:419–424 [DOI] [PubMed] [Google Scholar]
- Montagu KA (1957) Catechol Compounds in Rat Tissues and in Brains of Different Animals Nature 180:244 doi: 10.1038/180244a0 [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA (2011) Dynamic Regulation of Midbrain Dopamine Neuron Activity: Intrinsic, Synaptic, and Plasticity Mechanisms Neuroscience 198:95–111 doi: 10.1016/j.neuroscience.2011.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines J Neurosci 22:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL et al. (2013) Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice Diabetes 62:2762–2772 doi: 10.2337/db12-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musshoff F, Schmidt P, Dettmeyer R, Priemer F, Jachau K, Madea B (2000) Determination of dopamine and dopamine-derived (R)-/(S)-salsolinol and norsalsolinol in various human brain areas using solid-phase extraction and gas chromatography/mass spectrometry Forensic science international 113:359–366 [DOI] [PubMed] [Google Scholar]
- Musso NR, Brenci S, Setti M, Indiveri F, Lotti G (1996) Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes The Journal of clinical endocrinology and metabolism 81:3553–3557 doi: 10.1210/jcem.81.10.8855800 [DOI] [PubMed] [Google Scholar]
- Myohanen TT, Schendzielorz N, Mannisto PT (2010) Distribution of catechol-O-methyltransferase (COMT) proteins and enzymatic activities in wild-type and soluble COMT deficient mice J Neurochem 113:1632–1643 doi: 10.1111/j.1471-4159.2010.06723.x [DOI] [PubMed] [Google Scholar]
- Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M (2012) Neutrophils in multiple sclerosis are characterized by a primed phenotype J Neuroimmunol 242:60–71 doi: 10.1016/j.jneuroim.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T (1996) Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson's disease Neurology 46:791 doi: 10.1212/WNL.46.3.791 [DOI] [PubMed] [Google Scholar]
- Nagatomo K, Suga S, Saitoh M, Kogawa M, Kobayashi K, Yamamoto Y, Yamada K (2017) Dopamine D1 Receptor Immunoreactivity on Fine Processes of GFAP-Positive Astrocytes in the Substantia Nigra Pars Reticulata of Adult Mouse Frontiers in Neuroanatomy 11 doi: 10.3389/fnana.2017.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler J, Schriever SC, De Angelis M, Pfluger PT, Schramm KW (2018) Comprehensive analysis of nine monoamines and metabolites in small amounts of peripheral murine (C57Bl/6 J) tissues Biomedical chromatography : BMC 32 doi: 10.1002/bmc.4151 [DOI] [PubMed] [Google Scholar]
- Nagy GM, DeMaria JE, Freeman ME (1998) Changes in the local metabolism of dopamine in the anterior and neural lobes but not in the intermediate lobe of the pituitary gland during nursing Brain Research 790:315–317 doi: 10.1016/S0006-8993(97)01559-X [DOI] [PubMed] [Google Scholar]
- Nakagome K et al. (2011) Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin antigen-induced neutrophilic airway inflammation J Immunol 186:5975–5982 doi: 10.4049/jimmunol.1001274 [DOI] [PubMed] [Google Scholar]
- Nakajima A et al. (2004) Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity J Neurosci 24:2212–2225 doi: 10.1523/jneurosci.4847-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S (2008) Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis Biochem Biophys Res Commun 373:286–291 doi: 10.1016/j.bbrc.2008.06.012 [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S (2009) Dopamine released by dendritic cells polarizes Th2 differentiation International immunology 21:645–654 doi: 10.1093/intimm/dxp033 [DOI] [PubMed] [Google Scholar]
- Nakano K et al. (2011) Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model J Immunol 186:3745–3752 doi: 10.4049/jimmunol.1002475 [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM (2007) Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun 21:736–745 doi: 10.1016/j.bbi.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Zinselmeyer BH, Corps KN, McGavern DB (2012) In vivo dynamics of innate immune sentinels in the CNS Intravital 1:95–106 doi: 10.4161/intv.22823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikishina YO, Sapronova AY, Ugrumov MV (2016) The Effect of Dopamine Secreted by the Brain into the Systemic Circulation on Prolactin Synthesis by the Pituitary gland in Ontogenesis Acta naturae 8:111–117 [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM (1996) The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons J Neurosci 16:436–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Okabe S (2007) Direct astrocytic contacts regulate local maturation of dendritic spines J Neurosci 27:331–340 doi: 10.1523/jneurosci.4466-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R, Gaskill PJ (2018) The role of catecholamines in HIV neuropathogenesis Brain Res doi: 10.1016/j.brainres.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan RA, Muir R, Runner K, Haddad EK, Gaskill PJ (2018) Role of Macrophage Dopamine Receptors in Mediating Cytokine Production: Implications for Neuroinflammation in the Context of HIV-Associated Neurocognitive Disorders Journal of Neuroimmune Pharmacology doi: 10.1007/s11481-018-9825-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA, Fearon IM (2002) Carotid body chemoreceptors in dissociated cell culture Microscopy Research and Technique 59:249–255 doi: 10.1002/jemt.10199 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA (2015) The dopamine theory of addiction: 40 years of highs and lows Nature Reviews Neuroscience 16:305 doi: 10.1038/nrn3939 [DOI] [PubMed] [Google Scholar]
- Omiatek DM, Bressler AJ, Cans A-S, Andrews AM, Heien ML, Ewing AG (2013) The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry Scientific Reports 3:1447 doi: 10.1038/srep01447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW Jr., Krakoff J (2009) Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians Diabetes 58:385–393 doi: 10.2337/db08-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Saenz P et al. (2016) Selective accumulation of biotin in arterial chemoreceptors: requirement for carotid body exocytotic dopamine secretion J Physiol 594:7229–7248 doi: 10.1113/jp272961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA et al. (2012) Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens Journal of Neurochemistry 121:252–262 doi: 10.1111/j.1471-4159.2012.07677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özoğul F (2004) Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method European Food Research and Technology 219:465–469 doi: 10.1007/s00217-004-0988-0 [DOI] [Google Scholar]
- Pacheco R, Contreras F, Zouali M (2014) The dopaminergic system in autoimmune diseases Frontiers in immunology 5:117–117 doi: 10.3389/fimmu.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco R, Prado CE, Barrientos MJ, Bernales S (2009) Role of dopamine in the physiology of T-cells and dendritic cells J Neuroimmunol 216:8–19 doi: 10.1016/j.jneuroim.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Panariello F, De Luca V, de Bartolomeis A (2011) Weight gain, schizophrenia and antipsychotics: new findings from animal model and pharmacogenomic studies Schizophrenia research and treatment 2011:459284–459284 doi: 10.1155/2011/459284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa I et al. (2017) TFH-derived dopamine accelerates productive synapses in germinal centres Nature 547:318–323 doi: 10.1038/nature23013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB Jr. (1992) Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis J Neurochem 58:212–218 [DOI] [PubMed] [Google Scholar]
- Peaston RT, Weinkove C (2004) Measurement of catecholamines and their metabolites Annals of clinical biochemistry 41:17–38 doi: 10.1258/000456304322664663 [DOI] [PubMed] [Google Scholar]
- Pellegrini C et al. (2016) Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 28:1781–1791 doi: 10.1111/nmo.12933 [DOI] [PubMed] [Google Scholar]
- Pellock SJ, Redinbo MR (2017) Glucuronides in the gut: Sugar-driven symbioses between microbe and host J Biol Chem 292:8569–8576 doi: 10.1074/jbc.R116.767434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML et al. (2010) The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia J Biol Chem 285:36625–36634 doi: 10.1074/jbc.M110.159954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR (2014) Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance Neuropsychopharmacology 39:156–168 doi: 10.1038/npp.2013.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin DG, Cutz E, Becker LE, Bryan AC, Madapallimatum A, Sole MJ (1984) Sudden infant death syndrome: increased carotid-body dopamine and noradrenaline content Lancet 2:535–537 [DOI] [PubMed] [Google Scholar]
- Peters JL, Michael AC (2000) Changes in the kinetics of dopamine release and uptake have differential effects on the spatial distribution of extracellular dopamine concentration in rat striatum J Neurochem 74:1563–1573 [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan H-T, Parsons LH, Justice JB (1990) Extracellular Concentrations of Cocaine and Dopamine Are Enhanced During Chronic Cocaine Administration Journal of Neurochemistry 55:798–804 doi: 10.1111/j.1471-4159.1990.tb04562.x [DOI] [PubMed] [Google Scholar]
- Petzinger GM et al. (2007) Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury J Neurosci 27:5291–5300 doi: 10.1523/jneurosci.1069-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking Nature 422:614–618 doi: 10.1038/nature01476 [DOI] [PubMed] [Google Scholar]
- Pickel VM, Nirenberg MJ, Milner TA (1996) Ultrastructural view of central catecholaminergic transmission: immunocytochemical localization of synthesizing enzymes, transporters and receptors Journal of neurocytology 25:843–856 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews 30:215–238 doi: 10.1016/j.neubiorev.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Pierpont GL et al. (1987) Heterogeneous myocardial catecholamine concentrations in patients with congestive heart failure Am J Cardiol 60:316–321 [DOI] [PubMed] [Google Scholar]
- Pifl C et al. (2014) Is Parkinson's Disease a Vesicular Dopamine Storage Disorder? Evidence from a Study in Isolated Synaptic Vesicles of Human and Nonhuman Primate Striatum The Journal of Neuroscience 34:8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Reither H, Del Rey NL-G, Cavada C, Obeso JA, Blesa J (2017) Early Paradoxical Increase of Dopamine: A Neurochemical Study of Olfactory Bulb in Asymptomatic and Symptomatic MPTP Treated Monkeys Frontiers in neuroanatomy 11:46–46 doi: 10.3389/fnana.2017.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipović I, Vidić-Danković B, Perišić M, Radojević K, Čolić M, Todorović V, Leposavić G (2008) Sexual dimorphism in the catecholamine-containing thymus microenvironment: A role for gonadal hormones Journal of Neuroimmunology 195:7–20 doi: 10.1016/j.jneuroim.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Pinoli M, Marino F, Cosentino M (2017) Dopaminergic Regulation of Innate Immunity: a Review J Neuroimmune Pharmacol 12:602–623 doi: 10.1007/s11481-017-9749-2 [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P (2002) Δ9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study Brain Research 948:155–158 doi: 10.1016/S0006-8993(02)03055-X [DOI] [PubMed] [Google Scholar]
- Pivonello R et al. (2004) Dopamine receptor expression and function in human normal adrenal gland and adrenal tumors The Journal of clinical endocrinology and metabolism 89:4493–4502 doi: 10.1210/jc.2003-031746 [DOI] [PubMed] [Google Scholar]
- Podvin S, Bundey R, Toneff T, Ziegler M, Hook V (2015) Profiles of secreted neuropeptides and catecholamines illustrate similarities and differences in response to stimulation by distinct secretagogues Molecular and cellular neurosciences 68:177–185 doi: 10.1016/j.mcn.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Colosimo C (2010) Dopaminergic system in peripheral blood mononuclear cells in Parkinson’s disease Movement Disorders 25:125–126 doi: 10.1002/mds.22742 [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine Self-Administration Produces a Progressive Involvement of Limbic, Association, and Sensorimotor Striatal Domains The Journal of Neuroscience 24:3554 doi: 10.1523/JNEUROSCI.5578-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG (1991) Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment Brain Research 566:348–350 doi: 10.1016/0006-8993(91)91724-F [DOI] [PubMed] [Google Scholar]
- Pothos EN, Davila V, Sulzer D (1998) Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size J Neurosci 18:4106–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado C et al. (2012) Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity J Immunol 188:3062–3070 doi: 10.4049/jimmunol.1103096 [DOI] [PubMed] [Google Scholar]
- Prado C et al. (2018) Dopaminergic Stimulation of Myeloid Antigen-Presenting Cells Attenuates Signal Transducer and Activator of Transcription 3-Activation Favouring the Development of Experimental Autoimmune Encephalomyelitis Frontiers in Immunology 9 doi: 10.3389/fimmu.2018.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Lloret J et al. (2015) Hypoxic pulmonary vasoconstriction, carotid body function and erythropoietin production in adult rats perinatally exposed to hyperoxia The Journal of physiology 593:2459–2477 doi: 10.1113/JP270274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YH, Peng YP, Jiang JM, Wang JJ (2004) Expression of tyrosine hydroxylase in lymphocytes and effect of endogenous catecholamines on lymphocyte function Neuroimmunomodulation 11:75–83 doi: 10.1159/000075316 [DOI] [PubMed] [Google Scholar]
- Quelhas-Santos J et al. (2015) Renalase regulates peripheral and central dopaminergic activities American Journal of Physiology-Renal Physiology 308:F84–F91 doi: 10.1152/ajprenal.00274.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajda C, Bencsik K, Vécsei LL, Bergquist J (2002) Catecholamine levels in peripheral blood lymphocytes from multiple sclerosis patients Journal of Neuroimmunology 124:93–100 doi: 10.1016/S0165-5728(02)00002-4 [DOI] [PubMed] [Google Scholar]
- Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O (2008) Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation Neurology 70:1403–1410 doi: 10.1212/01.wnl.0000285082.18969.3a [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Holmes C, Goldstein DS (1999) Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease Biological psychiatry 46:756–765 doi: 10.1016/S0006-3223(99)00008-6 [DOI] [PubMed] [Google Scholar]
- Regitz V, Bossaller C, Strasser R, Schuler S, Hetzer R, Fleck E (1990) Myocardial catecholamine content after heart transplantation Circulation 82:620–623 [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS (1997) Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers Psychopharmacology 134:309–317 [DOI] [PubMed] [Google Scholar]
- Reuss B, Unsicker K (2001) Atypical neuroleptic drugs downregulate dopamine sensitivity in rat cortical and striatal astrocytes Mol Cell Neurosci 18:197–209 doi: 10.1006/mcne.2001.1017 [DOI] [PubMed] [Google Scholar]
- Ricci A, Veglio F, Amenta F (1995) Radioligand binding characterization of putative dopamine D3 receptor in human peripheral blood lymphocytes with [3H]7-OH-DPAT J Neuroimmunol 58:139–144 [DOI] [PubMed] [Google Scholar]
- Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA (1994) Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro Experimental Brain Research 100:395–406 doi: 10.1007/BF02738400 [DOI] [PubMed] [Google Scholar]
- Richard K, Hume R, Kaptein E, Stanley EL, Visser TJ, Coughtrie MW (2001) Sulfation of thyroid hormone and dopamine during human development: ontogeny of phenol sulfotransferases and arylsulfatase in liver, lung, and brain The Journal of clinical endocrinology and metabolism 86:2734–2742 doi: 10.1210/jcem.86.6.7569 [DOI] [PubMed] [Google Scholar]
- Ripley TL, Jaworski J, Randall PK, Gonzales RA (1997) Repeated perfusion with elevated potassium in in vivo microdialysis--A method for detecting small changes in extracellular dopamine J Neurosci Methods 78:7–14 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren P-O, Caicedo A (2011) Innervation patterns of autonomic axons in the human endocrine pancreas Cell metabolism 14:45–54 doi: 10.1016/j.cmet.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J (2013) Dissecting the diversity of midbrain dopamine neurons Trends in neurosciences 36:336–342 doi: 10.1016/j.tins.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM (2008) Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli Nature neuroscience 11:1376–1377 doi: 10.1038/nn.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG et al. (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission Isme j 8:1403–1417 doi: 10.1038/ismej.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S et al. (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections J Neuroimmune Pharmacol 6:442–465 doi: 10.1007/s11481-011-9292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P (2005) Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion J Biol Chem 280:36824–36832 doi: 10.1074/jbc.M505560200 [DOI] [PubMed] [Google Scholar]
- Rubi B, Maechler P (2010) Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance Endocrinology 151:5570–5581 doi: 10.1210/en.2010-0745 [DOI] [PubMed] [Google Scholar]
- Rubí B, Maechler P (2010) Minireview: New Roles for Peripheral Dopamine on Metabolic Control and Tumor Growth: Let’s Seek the Balance Endocrinology 151:5570–5581 doi: 10.1210/en.2010-0745 [DOI] [PubMed] [Google Scholar]
- Russell WJ, Frewin DB, Jonsson JR (1982) Pulmonary extraction of catecholamines in critically ill patients Anaesthesia and intensive care 10:319–323 [DOI] [PubMed] [Google Scholar]
- Rysz M, Bromek E, Haduch A, Sadakierska-Chudy A, Daniel WA (2015) Damage to the Brain Serotonergic System Increases the Expression of Liver Cytochrome P450 Drug Metabolism and Disposition 43:1345. [DOI] [PubMed] [Google Scholar]
- Saha B, Mondal AC, Basu S, Dasgupta PS (2001) Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis Int Immunopharmacol 1:1363–1374 [DOI] [PubMed] [Google Scholar]
- Sakic B, Lacosta S, Denburg JA, Szechtman H (2002) Altered neurotransmission in brains of autoimmune mice: pharmacological and neurochemical evidence Journal of Neuroimmunology 129:84–96 doi: 10.1016/S0165-5728(02)00171-6 [DOI] [PubMed] [Google Scholar]
- Salinas AG, Davis MI, Lovinger DM, Mateo Y (2016) Dopamine dynamics and cocaine sensitivity differ between striosome and matrix compartments of the striatum Neuropharmacology 108:275–283 doi: 10.1016/j.neuropharm.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Dempsey C, Fields V (2012) Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area Journal of visualized experiments : JoVE:10.3791/4171 4171 doi: 10.3791/4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano I, Gamo T, Kakimoto Y, Taniguchi K, Takesada M, Nishinuma K (1959) Distribution of catechol compounds in human brain Biochim Biophys Acta 32:586–587 [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Lipartiti M, Bruni A, Toso RD (1993) Dopamine receptors on human T- and B-lymphocytes Journal of Neuroimmunology 45:113–119 doi: 10.1016/0165-5728(93)90170-4 [DOI] [PubMed] [Google Scholar]
- Sasa S, Blank CL (1977) Determination of serotonin and dopamine in mouse brain tissue by high performance liquid chromatography with electrochemical detection Analytical chemistry 49:354–359 [DOI] [PubMed] [Google Scholar]
- Scarcella DL, Bryan-Lluka LJ (1995) A kinetic investigation of the pulmonary metabolism of dopamine in rats shows marked differences compared with noradrenaline Naunyn-Schmiedeberg’s archives of pharmacology 351:491–499 [DOI] [PubMed] [Google Scholar]
- Schafer MK, Eiden LE, Weihe E (1998) Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system Neuroscience 84:361–376 [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Frenette PS, Hidalgo A (2015) Regulation of leucocyte homeostasis in the circulation Cardiovascular research 107:340–351 doi: 10.1093/cvr/cvv099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C et al. (2010) Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses Journal of neural transmission (Vienna, Austria : 1996) 117:699–705 doi: 10.1007/s00702-010-0415-6 [DOI] [PubMed] [Google Scholar]
- Schemann M, Camilleri M (2013) Functions and imaging of mast cell and neural axis of the gut Gastroenterology 144:698–704.e694 doi: 10.1053/j.gastro.2013.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann DW (1993) Comparative morphology, cytochemistry and innervation of chromaffin tissue in vertebrates Journal of anatomy 183 (Pt 2):327–342 [PMC free article] [PubMed] [Google Scholar]
- Scheuermann DW, Stilman C, De Groodt-Lasseel MHA (1988) Microspectrofluorimetric analysis of the formaldehyde-induced fluorophores of 5-hydroxytryptamine and dopamine in intrapulmonary neuroepithelial bodies after administration ofl-hydroxytryptophan andl-DOPA Histochemistry 88:219–225 doi: 10.1007/BF00570277 [DOI] [PubMed] [Google Scholar]
- Schober A, Huber K, Fey J, Unsicker K (1998) Distinct populations of macrophages in the adult rat adrenal gland: a subpopulation with neurotrophin-4-like immunoreactivity Cell Tissue Res 291:365–373 [DOI] [PubMed] [Google Scholar]
- Schoenemann HM, Failla ML, Rosebrough RW (1990) Cardiac and splenic levels of norepinephrine and dopamine in copper deficient pigs and rats Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 97:387–391 doi: 10.1016/0742-8413(90)90159-7 [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD (2013) Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction J Neurosci 33:961–971 doi: 10.1523/jneurosci.2516-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdt HN et al. (2018) Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model Communications Biology 1:144 doi: 10.1038/s42003-018-0147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Beato M (2009) Determining the Neurotransmitter Concentration Profile at Active Synapses Molecular neurobiology 40:289–306 doi: 10.1007/s12035-009-8087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scozzi D et al. (2012) Increased levels of plasmatic dopamine in human small cell lung cancer European Respiratory Journal 40 [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K (1976) Antipsychotic drug doses and neuroleptic/dopamine receptors Nature 261:717–719 [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation Nature reviews Immunology 11:762–774 doi: 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichijo K, Sakurai-Yamashita Y, Sekine I, Taniyama K (1997) Neuronal release of endogenous dopamine from corpus of guinea pig stomach Am J Physiol 273:G1044–1050 [DOI] [PubMed] [Google Scholar]
- Shintani F et al. (1993) Interleukin-1 beta augments release of norepinephrine, dopamine, and serotonin in the rat anterior hypothalamus J Neurosci 13:3574–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishov VA, Kirovskaya TA, Kudrin VS, Oleskin AV (2009) Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12 Applied Biochemistry and Microbiology 45:494–497 doi: 10.1134/S0003683809050068 [DOI] [PubMed] [Google Scholar]
- Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT (2006) Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence Analytical chemistry 78:6717–6725 doi: 10.1021/ac0608218 [DOI] [PubMed] [Google Scholar]
- Silverman SM, Wong WT (2018) Microglia in the Retina: Roles in Development, Maturity, and Disease Annual review of vision science 4:45–77 doi: 10.1146/annurev-vision-091517-034425 [DOI] [PubMed] [Google Scholar]
- Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM (1995) Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation Lancet 346:861–864 [DOI] [PubMed] [Google Scholar]
- Sizova D, Velazquez H, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Desir GV (2013) Renalase regulates renal dopamine and phosphate metabolism Am J Physiol Renal Physiol 305:F839–844 doi: 10.1152/ajprenal.00616.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ (1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons Proc Natl Acad Sci U S A 93:11202–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ (2006) Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31:139–150 doi: 10.1038/sj.npp.1300786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SR, Kuchel O (1983) Dopamine: an important neurohormone of the sympathoadrenal system. Significance of increased peripheral dopamine release for the human stress response and hypertension Endocrine reviews 4:291–309 doi: 10.1210/edrv-4-3-291 [DOI] [PubMed] [Google Scholar]
- Solinas M, Belujon P, Fernagut PO, Jaber M, Thiriet N (2018) Dopamine and addiction: what have we learned from 40 years of research Journal of neural transmission (Vienna, Austria : 1996) doi: 10.1007/s00702-018-1957-2 [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE (2006) The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats Pharmacopsychiatry 39:88–99 doi: 10.1055/s-2006-941557 [DOI] [PubMed] [Google Scholar]
- Sookhai S, Wang JH, McCourt M, O’Connell D, Redmond HP (1999) Dopamine induces neutrophil apoptosis through a dopamine D-1 receptor–independent mechanism Surgery 126:314–322 doi: 10.1016/S0039-6060(99)70171-6 [DOI] [PubMed] [Google Scholar]
- Sperker B et al. (2000) Expression and function of beta-glucuronidase in pancreatic cancer: potential role in drug targeting Naunyn-Schmiedeberg’s archives of pharmacology 362:110–115 [DOI] [PubMed] [Google Scholar]
- Spiegel A et al. (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling Nat Immunol 8:1123–1131 doi: 10.1038/ni1509 [DOI] [PubMed] [Google Scholar]
- Spühler IA, Hauri A (2013) Decoding the Dopamine Signal in Macaque Prefrontal Cortex: A Simulation Study Using the Cx3Dp Simulator PLOS ONE 8:e71615 doi: 10.1371/journal.pone.0071615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal RG, Mosharov EV, Sulzer D (2004) Dopamine neurons release transmitter via a flickering fusion pore Nat Neurosci 7:341–346 doi: 10.1038/nn1205 [DOI] [PubMed] [Google Scholar]
- Stagkourakis S, Kim H, Lyons DJ, Broberger C (2016) Dopamine Autoreceptor Regulation of a Hypothalamic Dopaminergic Network Cell reports 15:735–747 doi: 10.1016/j.celrep.2016.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004) A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor Primary care companion to the Journal of clinical psychiatry 6:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Palij P, Millar J (1988) Diffusion and uptake of dopamine in rat caudate and nucleus accumbens compared using fast cyclic voltammetry Brain Res 448:381–385 [DOI] [PubMed] [Google Scholar]
- Stanojevic S, Dimitrijevic M, Kustrimovic N, Mitic K, Vujic V, Leposavic G (2013) Adrenal hormone deprivation affects macrophage catecholamine metabolism and beta2-adrenoceptor density, but not propranolol stimulation of tumour necrosis factor-alpha production Experimental physiology 98:665–678 doi: 10.1113/expphysiol.2012.070524 [DOI] [PubMed] [Google Scholar]
- Stouffer MA et al. (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward Nature communications 6:8543–8543 doi: 10.1038/ncomms9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota Brain Res 1693:128–133 doi: 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G, Werle E, Weicker H (1990) Isomer specific kinetics of dopamine beta-hydroxylase and arylsulfatase towards catecholamine sulfates Biochemistry international 20:343–351 [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration Neuropsychopharmacology 30:853–863 doi: 10.1038/sj.npp.1300619 [DOI] [PubMed] [Google Scholar]
- Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission Neuron 69:628–649 doi: 10.1016/j.neuron.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D et al. (2017) T cells from patients with Parkinson’s disease recognize α-synuclein peptides Nature 546:656 doi:10.1038/nature2281510.1038/nature22815https://www.nature.com/articles/nature22815-supplementary-informationhttps://www.nature.com/articles/nature22815-supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D et al. (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles Proceedings of the National Academy of Sciences 97:11869 doi: 10.1073/pnas.97.22.11869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME (2016) Striatal dopamine neurotransmission: regulation of release and uptake Basal ganglia 6:123–148 doi: 10.1016/j.baga.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen T, Piepponen TP, Kostiainen R (2015) Permeation of Dopamine Sulfate through the Blood-Brain Barrier PLoS ONE 10:e0133904 doi: 10.1371/journal.pone.0133904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen T et al. (2013) Determination of Serotonin and Dopamine Metabolites in Human Brain Microdialysis and Cerebrospinal Fluid Samples by UPLC-MS/MS: Discovery of Intact Glucuronide and Sulfate Conjugates PLoS One 8:e68007 doi: 10.1371/journal.pone.0068007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz R, Grzelinska Z, Gralewicz S, Wasowicz W (2009) Catecholamine levels in the brain of rats exposed by inhalation to benzalkonium chloride International journal of occupational medicine and environmental health 22:107–113 doi: 10.2478/v10001-009-0012-9 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nagai Y, Ueno S, Saeki Y, Yanagihara T (1992) Human peripheral blood lymphocytes express D5 dopamine receptor gene and transcribe the two pseudogenes FEBS Lett 314:23–25 [DOI] [PubMed] [Google Scholar]
- Takeda H, Inazu M, Matsumiya T (2002) Astroglial dopamine transport is mediated by norepinephrine transporter Naunyn-Schmiedeberg’s archives of pharmacology 366:620–623 doi: 10.1007/s00210-002-0640-0 [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Chiara GD (1997) Cannabinoid and Heroin Activation of Mesolimbic Dopamine Transmission by a Common μ<sub>1</sub> Opioid Receptor Mechanism Science 276:2048 doi: 10.1126/science.276.5321.2048 [DOI] [PubMed] [Google Scholar]
- Tang TT et al. (2012) Regulatory T cells ameliorate cardiac remodeling after myocardial infarction Basic research in cardiology 107:232 doi: 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- Tareke E, Bowyer JF, Doerge DR (2007) Quantification of rat brain neurotransmitters and metabolites using liquid chromatography/electrospray tandem mass spectrometry and comparison with liquid chromatography/electrochemical detection Rapid Communications in Mass Spectrometry 21:3898–3904 doi: 10.1002/rcm.3295 [DOI] [PubMed] [Google Scholar]
- Teitelman G, Joh TH, Reis DJ (1981) Transformation of catecholaminergic precursors into glucagon (A) cells in mouse embryonic pancreas Proc Natl Acad Sci U S A 78:5225–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis MA, Heijnen CJ, Cools AR, Kavelaars A (2004) Reduced splenic natural killer cell activity in rats with a hyperreactive dopaminergic system Psychoneuroendocrinology 29:1058–1064 doi: 10.1016/j.psyneuen.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Tiwari A, Moghal M, Meleagros L (2006) Life threatening abdominal complications following cocaine abuse Journal of the Royal Society of Medicine 99:51–52 doi: 10.1258/jrsm.99.2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstanova G et al. (2015) Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease Dig Dis Sci 60:2963–2975 doi: 10.1007/s10620-015-3698-5 [DOI] [PubMed] [Google Scholar]
- Tomassoni D, Traini E, Mancini M, Bramanti V, Mahdi SS, Amenta F (2015) Dopamine, vesicular transporters, and dopamine receptor expression in rat major salivary glands Am J Physiol Regul Integr Comp Physiol 309:R585–593 doi: 10.1152/ajpregu.00455.2014 [DOI] [PubMed] [Google Scholar]
- Tor-Agbidye J, Yamamoto B, Bowyer JF (2001) Seizure Activity and Hyperthermia Potentiate the Increases in Dopamine and Serotonin Extracellular Levels in the Amygdala during Exposure to d-Amphetamine Toxicological Sciences 60:103–111 doi: 10.1093/toxsci/60.1.103 [DOI] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG (2003) Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter J Biol Chem 278:2731–2739 doi: 10.1074/jbc.M201926200 [DOI] [PubMed] [Google Scholar]
- Torres-Rosas R et al. (2014) Dopamine mediates vagal modulation of the immune system by electroacupuncture Nature medicine 20:291–295 doi: 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabold B, Gruber M, Frohlich D (2007) Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines Scandinavian cardiovascular journal : SCJ 41:59–64 doi: 10.1080/14017430601085948 [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK (2010) Microglial interactions with synapses are modulated by visual experience PLoS biology 8:e1000527 doi: 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout SJ, Kruk ZL (1992) Differences in evoked dopamine efflux in rat caudate putamen, nucleus accumbens and tuberculum olfactorium in the absence of uptake inhibition: influence of autoreceptors British Journal of Pharmacology 106:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT (1997) Effect of dopamine on immune cell proliferation in mice Life sciences 61:Pl 361–371 [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT (1998) Inhibition of immune cell proliferation with haloperidol and relationship of tyrosine hydroxylase expression to immune cell growth Life sciences 62:Pl 335–344 [DOI] [PubMed] [Google Scholar]
- Ugrumov MV (2009) Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance Journal of chemical neuroanatomy 38:241–256 doi: 10.1016/j.jchemneu.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Uutela P, Reinila R, Harju K, Piepponen P, Ketola RA, Kostiainen R (2009) Analysis of intact glucuronides and sulfates of serotonin, dopamine, and their phase I metabolites in rat brain microdialysates by liquid chromatography-tandem mass spectrometry Analytical chemistry 81:8417–8425 doi: 10.1021/ac901320z [DOI] [PubMed] [Google Scholar]
- Valdearcos M et al. (2017) Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility Cell Metab 26:185–197.e183 doi: 10.1016/j.cmet.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon GR (1983) Plasma dopamine: regulation and significance Federation proceedings 42:3012–3018 [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ (2014) Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery Eur J Neurosci 40:3041–3054 doi: 10.1111/ejn.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R (2011) Adipocytes as a new source of catecholamine production FEBS Letters 585:2279–2284 doi: 10.1016/j.febslet.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM (2003) Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing J Neurochem 87:1284–1295 [DOI] [PubMed] [Google Scholar]
- Vicario I, Rigual R, Obeso A, Gonzalez C (2000) Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro American journal of physiology Cell physiology 278:C490–499 doi: 10.1152/ajpcell.2000.278.3.C490 [DOI] [PubMed] [Google Scholar]
- Vieira-Coelho MA, Soares-da-Silva P (1996) Ontogenic aspects of liver and kidney catechol-O-methyltransferase sensitivity to tolcapone British journal of pharmacology 117:516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Coelho MA, Teixeira VA, Finkel Y, Soares-Da-Silva P, Bertorello AM (1998) Dopamine-dependent inhibition of jejunal Na+-K+-ATPase during high-salt diet in young but not in adult rats Am J Physiol 275:G1317–1323 [DOI] [PubMed] [Google Scholar]
- Villageliu D, Lyte M (2018) Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential PLoS One 13:e0207038 doi: 10.1371/journal.pone.0207038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Cheda B, Dominguez-Meijide A, Valenzuela R, Granado N, Moratalla R, Labandeira-Garcia JL (2014) Aging-related dysregulation of dopamine and angiotensin receptor interaction Neurobiology of aging 35:1726–1738 doi: 10.1016/j.neurobiolaging.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Villaran RF et al. (2010) Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease J Neurochem 114:1687–1700 doi: 10.1111/j.1471-4159.2010.06879.x [DOI] [PubMed] [Google Scholar]
- Vohra PK, Hoeppner LH, Sagar G, Dutta SK, Misra S, Hubmayr RD, Mukhopadhyay D (2012) Dopamine inhibits pulmonary edema through the VEGF-VEGFR2 axis in a murine model of acute lung injury American journal of physiology Lung cellular and molecular physiology 302:L185–192 doi: 10.1152/ajplung.00274.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow Nora D, Morales M (2015) The Brain on Drugs: From Reward to Addiction Cell 162:712–725 doi: 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity Trends in cognitive sciences 15:37–46 doi: 10.1016/j.tics.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R (2010) Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit BioEssays : news and reviews in molecular, cellular and developmental biology 32:748–755 doi: 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy Nature 386:827–830 doi: 10.1038/386827a0 [DOI] [PubMed] [Google Scholar]
- Volkow ND et al. (1996) Cocaine uptake is decreased in the brain of detoxified cocaine abusers Neuropsychopharmacology 14:159–168 doi: 10.1016/0893-133x(95)00073-m [DOI] [PubMed] [Google Scholar]
- Wagner J, Palfreyman M, Zraika M (1979a) Determination of dopa, dopamine, dopac, epinephrine, norepinephrine, alpha-monofluoromethyldopa and alpha-difluoromethyldopa in various tissues of mice and rats using reversed-phase ion-pair liquid chromatography with electrochemical detection Journal of chromatography 164:41–54 [DOI] [PubMed] [Google Scholar]
- Wagner J, Palfreyman M, Zraika M (1979b) Determination of DOPA, dopamine, DOPAC, epinephrine, norepinephrine, α-monofluoromethyldopa and α-difluoromethyldopa in various tissues of mice and rats using reversed-phase ion-pair liquid chromatography with electrochemical detection Journal of Chromatography B: Biomedical Sciences and Applications 164:41–54 doi: 10.1016/S0378-4347(00)81570-4 [DOI] [PubMed] [Google Scholar]
- Wahbe F, Hagege J, Loreau N, Ardaillou R (1982) Endogenous dopamine synthesis and dopa-decarboxylase activity in rat renal cortex Mol Cell Endocrinol 27:45–54 [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M (2010) Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment Brain and cognition 72:146–159 doi: 10.1016/j.bandc.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Bruno MJ, Bass CE, Park J (2016) Application of fast-scan cyclic voltammetry for the in vivo characterization of optically evoked dopamine in the olfactory tubercle of the rat brain The Analyst 141:3746–3755 doi: 10.1039/c6an00196c [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals J Neurosci 29:3974–3980 doi: 10.1523/jneurosci.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE (2009) Phasic dopamine release in appetitive behaviors and drug addiction Current drug abuse reviews 2:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Undie AS, Friedman E (1995) Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation Molecular pharmacology 48:988–994 [PubMed] [Google Scholar]
- Wang X et al. (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin Proceedings of the National Academy of Sciences of the United States of America 109:6325–6330 doi: 10.1073/pnas.1200130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Villar VA, Tiu A, Upadhyay KK, Cuevas S (2018) Dopamine D2 receptor upregulates leptin and IL-6 in adipocytes Journal of lipid research 59:607–614 doi: 10.1194/jlr.M081000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y et al. (2006a) Dopamine Selectively Induces Migration and Homing of Naive CD8<sup>+</sup> T Cells via Dopamine Receptor D3 The Journal of Immunology 176:848. [DOI] [PubMed] [Google Scholar]
- Watanabe Y et al. (2006b) Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3 J Immunol 176:848–856 [DOI] [PubMed] [Google Scholar]
- Weihe E, Nohr D, Michel S, Muller S, Zentel HJ, Fink T, Krekel J (1991) Molecular anatomy of the neuroimmune connection The International journal of neuroscience 59:1–23 [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Biswas R, Steplock D, Wang P, Lau YS, Desir GV, Shenolikar S (2011) Increased renal dopamine and acute renal adaptation to a high-phosphate diet Am J Physiol Renal Physiol 300:F1123–1129 doi: 10.1152/ajprenal.00744.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub ST, Stavinoha WB, Pike RL, Morgan WW, Modak AT, Koslow SH, Blank L (1975) Evaluation of the necessity for rapid inactivation of brain enzymes prior to analysis of norepinephrine dopamine and serotonin in the mouse Life sciences 17:1423–1427 doi: 10.1016/0024-3205(75)90162-9 [DOI] [PubMed] [Google Scholar]
- Weisheit CK, Engel DR, Kurts C (2015) Dendritic Cells and Macrophages: Sentinels in the Kidney Clinical journal of the American Society of Nephrology : CJASN 10:1841–1851 doi: 10.2215/cjn.07100714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens Proceedings of the National Academy of Sciences of the United States of America 97:4321–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats J Neurosci 16:3474–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenisch C et al. (1996) High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production Clin Diagn Lab Immunol 3:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Bhatnagar RK, Bardo MT (1983) Norepinephrine Depletion in the Spinal Cord Gray Matter of Rats with Experimental Allergic Encephalomyelitis Journal of Neurochemistry 40:1771–1773 doi: 10.1111/j.1471-4159.1983.tb08156.x [DOI] [PubMed] [Google Scholar]
- Wightman RM et al. (2007) Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens Eur J Neurosci 26:2046–2054 doi: 10.1111/j.1460-9568.2007.05772.x [DOI] [PubMed] [Google Scholar]
- Wightman RM et al. (1991) Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells Proc Natl Acad Sci U S A 88:10754–10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL (2002) Transient changes in mesolimbic dopamine and their association with ‘reward’ J Neurochem 82:721–735 [DOI] [PubMed] [Google Scholar]
- Williams JM et al. (2007) Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine PLoS biology 5:e274 doi: 10.1371/journal.pbio.0050274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM et al. (1996a) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users Nat Med 2:699–703 [DOI] [PubMed] [Google Scholar]
- Wilson JM et al. (1996b) Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease Neurology 47:718–726 [DOI] [PubMed] [Google Scholar]
- Winner BM, Zhang H, Farthing MM, Karchalla LM, Lookingland KJ, Goudreau JL (2017) Metabolism of Dopamine in Nucleus Accumbens Astrocytes Is Preserved in Aged Mice Exposed to MPTP Frontiers in Aging Neuroscience 9:410 doi: 10.3389/fnagi.2017.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation Nature Reviews Neuroscience 5:483 doi: 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]
- Wisman LAB, Sahin G, Maingay M, Leanza G, Kirik D (2008) Functional Convergence of Dopaminergic and Cholinergic Input Is Critical for Hippocampus-Dependent Working Memory The Journal of Neuroscience 28:7797 doi: 10.1523/JNEUROSCI.1885-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers SB et al. (2017) Eosinophils are key regulators of perivascular adipose tissue and vascular functionality Scientific Reports 7:44571 doi:10.1038/srep4457110.1038/srep44571https://www.nature.com/articles/srep44571-supplementary-informationhttps://www.nature.com/articles/srep44571-supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P (2004) Dopamine and retinal function Documenta Ophthalmologica 108:17–39 doi: 10.1023/B:DOOP.0000019487.88486.0a [DOI] [PubMed] [Google Scholar]
- Wolfovitz E, Grossman E, Folio CJ, Keiser HR, Kopin IJ, Goldstein DS (1993) Derivation of urinary dopamine from plasma dihydroxyphenylalanine in humans Clinical science (London, England : 1979) 84:549–557 [DOI] [PubMed] [Google Scholar]
- Wolkersdorfer GW et al. (1999) Lymphocytes stimulate dehydroepiandrosterone production through direct cellular contact with adrenal zona reticularis cells: a novel mechanism of immune-endocrine interaction The Journal of clinical endocrinology and metabolism 84:4220–4227 doi: 10.1210/jcem.84.11.6110 [DOI] [PubMed] [Google Scholar]
- Wu C, Garamszegi SP, Xie X, Mash DC (2017) Altered Dopamine Synaptic Markers in Postmortem Brain of Obese Subjects Frontiers in human neuroscience 11:386–386 doi: 10.3389/fnhum.2017.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H et al. (2007) T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity Circulation 115:1029–1038 doi: 10.1161/circulationaha.106.638379 [DOI] [PubMed] [Google Scholar]
- Wu H-J, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity Gut microbes 3:4–14 doi: 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA (2001) Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry Journal of Neuroscience Methods 112:119–133 doi: 10.1016/S0165-0270(01)00459-9 [DOI] [PubMed] [Google Scholar]
- Wu X, French ED (2000) Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment Neuropharmacology 39:391–398 [DOI] [PubMed] [Google Scholar]
- Wu X-H et al. (2015) Unaltered Retinal Dopamine Levels in a C57BL/6 Mouse Model of Form-Deprivation Myopia Investigative Ophthalmology & Visual Science 56:967–977 doi: 10.1167/iovs.13-13362 [DOI] [PubMed] [Google Scholar]
- Xue R et al. (2018) Peripheral Dopamine Controlled by Gut Microbes Inhibits Invariant Natural Killer T Cell-Mediated Hepatitis Frontiers in immunology 9:2398–2398 doi: 10.3389/fimmu.2018.02398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Polinsky RJ, Goldstein DS, Baucom CE, Kopin IJ (1996) Plasma sulfoconjugated dopamine levels are normal in patients with autonomic failure The Journal of laboratory and clinical medicine 128:488–491 [DOI] [PubMed] [Google Scholar]
- Yan Q-S (1999) Extracellular Dopamine and Serotonin After Ethanol Monitored With 5-Minute Microdialysis Alcohol (Fayetteville, NY) 19:1–7 doi: 10.1016/S0741-8329(99)00006-3 [DOI] [PubMed] [Google Scholar]
- Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC (2012) Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury Am J Physiol Renal Physiol 302:F742–749 doi: 10.1152/ajprenal.00583.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Lin C, Hsu CT, Wang TF, Ke FY, Kuo YM (2013) Differential distribution and activation of microglia in the brain of male C57BL/6J mice Brain structure & function 218:1051–1060 doi: 10.1007/s00429-012-0446-x [DOI] [PubMed] [Google Scholar]
- Yeh SH-H et al. (2014) Evaluation of Inhibitory Effect of Recreational Drugs on Dopaminergic Terminal Neuron by PET and Whole-Body Autoradiography BioMed Research International 2014:11 doi: 10.1155/2014/157923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C-X, la Fleur SE, Fliers E, Kalsbeek A (2010) The role of the autonomic nervous liver innervation in the control of energy metabolism Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1802:416–431 doi: 10.1016/j.bbadis.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA (2000) Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition Alcohol (Fayetteville, NY) 22:107–115 [DOI] [PubMed] [Google Scholar]
- Yoneda S, Alexander N, Vlachakis ND (1983) Enzymatic deconjugation of catecholamines in human and rat plasma and red blood cell lysate Life sciences 33:935–942 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK (1992) Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens Alcohol (Fayetteville, NY) 9:17–22 [DOI] [PubMed] [Google Scholar]
- Yoshizumi M et al. (1998) Changes in plasma free and sulfoconjugated dopamine in patients with congenital heart disease who underwent cardiac operation The Japanese journal of thoracic and cardiovascular surgery : official publication of the Japanese Association for Thoracic Surgery = Nihon Kyobu Geka Gakkai zasshi 46:651–656 [DOI] [PubMed] [Google Scholar]
- Zaffaroni M et al. (2008) Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis Exp Neurol 214:315–321 doi: 10.1016/j.expneurol.2008.08.015 [DOI] [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S (1999) Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture J Neurosci Res 58:544–552 [DOI] [PubMed] [Google Scholar]
- Zern RT, Bird JL, Feldman JM (1980) Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster Diabetologia 18:341–346 [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K (2006) Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine Progress in neuro-psychopharmacology & biological psychiatry 30:1381–1393 doi: 10.1016/j.pnpbp.2006.05.015 [DOI] [PubMed] [Google Scholar]
- Zhang MZ et al. (2011a) Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice J Clin Invest 121:2845–2854 doi: 10.1172/jci57324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W et al. (2011b) The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions Circulation 124:2106–2116 doi: 10.1161/CIRCULATIONAHA.111.052399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ (2009) Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:912–922 doi: 10.1038/npp.2008.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen K (2016) Vasoactive agents for the treatment of sepsis Ann Transl Med 4:333 doi: 10.21037/atm.2016.08.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E et al. (2012) Bone marrow and the control of immunity Cellular & molecular immunology 9:11–19 doi: 10.1038/cmi.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Misler S (1995) Amperometric detection of stimulus-induced quantal release of catecholamines from cultured superior cervical ganglion neurons Proc Natl Acad Sci U S A 92:6938–6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H (2014) Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation) Physiology & behavior 125:21–29 doi: 10.1016/j.physbeh.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]