Abstract
Objective: To prepare efficient antibacterial carvacrol (CAR) and thymol (THY)-loaded electrospun polycaprolactone (PCL)-based wound dressings.
Approach: Using electrospinning we were able to prepare wound dressings with antimicrobial action thanks to their large surface per volume ratio, which allows their loading with therapeutic amounts of active principles. By nuclear magnetic resonance we demonstrated that the antimicrobial compounds are donors of hydrogen bonds to the ester functional group in PCL, which acts as acceptor and that intermolecular interaction is responsible for the high drug loading achieved.
Results: Those mats loaded with CAR and THY without the use of solubilizing agents were able to completely eradicate both Gram-positive (Staphylococcus aureus ATCC 25923) and Gram-negative (Escherichia coli S17 strain) bacteria at doses inferior to the ones needed when using the free nonsupported compounds. A superior antimicrobial action was observed for THY and CAR against Gram-negative bacteria than against Gram-positive bacteria, despite the higher hydrophilicity of the outer layer of Gram-negative bacteria.
Innovation: We demonstrate that a direct contact between the bacteria and the dressing is required to elicit antimicrobial action. We also evaluated drug loadings by gas chromatography coupled with mass spectrometry and nuclear magnetic resonance validating a new analytical approach. Finally we were able to visualize the pathogenic bacteria on the dressings by confocal microscopy.
Conclusion: The interaction between the PCL-based mat and the pathogenic bacteria is a key issue to achieve complete pathogen eradication. Under no-contact conditions, released CAR or THY from the electrospun mats did not exert any antimicrobial action at the doses tested.
Keywords: electrospinning, essential oils, bacteria, antimicrobial, wound dressings
Manuel Arruebo, PhD.

Introduction
Wound-associated infections generate a tremendous burden for health care systems. Either acute (caused by trauma or surgery) or chronic wounds (pressure, diabetic, or vascular ulcers) all are susceptible to bacterial colonization. The application of antimicrobial drugs and even surgery might be needed to eradicate those infections.
Antimicrobial wound dressings with no cytotoxicity against human cells can represent a novel nonsystemic alternative to current treatments. Mats composed of electrospun nanofibers (NFs) have been widely used as structural materials in wound dressings thanks to their large surface per volume ratio, which allows their loading with therapeutic amounts of active principles, including antimicrobials, regenerative compounds, or anti-inflammatory drugs. Their porous structure allows also gas exchange and an adequate permeability of water vapor to maintain the required wound moisture facilitating the perfusion in the wound of the host immune cells. Several antibiotics, drugs, and antiseptics have been loaded within electrospun NFs to prevent wound infection. Some works exploit the inhibitory activity of natural origin polymers to prevent bacterial colonization. In this regard, chitosan1- and sericin/chitosan2-based electrospun NFs have been prepared and their antimicrobial action demonstrated against Gram-positive and Gram-negative bacteria. Other authors use inorganic antimicrobial nanomaterials such as ZnO3 or Ag4,5 incorporated into the precursor polymeric solution as colloidal suspensions before electrospinning, rendering fibers with the antimicrobial material loaded as filler.
Antibiotics such as fusidic acid6 (a steroid antibiotic commonly used in topical creams) have been incorporated within poly(lactic-co-glycolic acid)-based electrospun NFs to obtain a mat able to eliminate methicillin-resistant Staphylococcus aureus (MRSA) strains in vitro. Neomycin, an aminoglycoside antibiotic, has been adsorbed on the surface of electrospun mats composed of poly(styrene sulfonic acid-co-maleic acid) blended with polyvinyl alcohol and their antimicrobial and regenerative action evaluated in vitro and in vivo, respectively.7 Those authors showed a faster wound closure in the first days when using the antibiotic-releasing mats compared to commercial passive dressings and similar results to the ones obtained using commercial antibacterial gauze dressings. Tetracycline-loaded triple-layered electrospun micro/NF matrices of polycaprolactone (PCL) and poly(ethylene-co-vinyl acetate) showed a complete bacterial eradication of biofilm forming strains of S. aureus MRSA252.8 Those mats not only prevented the wound from biofilm formation but also were able to eliminate mature dense biofilms.
Bactericidal drugs such as nitrofurazone have been loaded into poly(l-lactide) (PLLA)/sericin NFs fabricated by electrospinning and their cytocompatibility on murine fibroblasts guaranteed while showing an enhanced wound healing closure in vivo compared to the use of commercial nonwoven dressings.9 Electrospun mats composed also by PLLA releasing two drugs of different nature, including a hydrophilic antibiotic (polymyxin B sulfate) and a hydrophobic anti-inflammatory drug (dexamethasone), have demonstrated antibacterial ability against Gram-positive and Gram-negative bacteria showing complete wound healing in vivo of infected full-thickness burns and wounds.10
However, in all those previous cases the presence of antibiotic resistant strains and the cytotoxicity associated to some of those inorganic antimicrobial nanomaterials or to their released ions against eukaryotic cells (i.e., Ag11) have imposed the urgent need of developing new antimicrobials or to reformulate some of the existing drugs. The EU 2017 Action Plan12 highlights the need to develop new therapeutics and alternative treatments to face antimicrobial resistance, indicating that more research is needed to advance in the repurposing of old antimicrobials, to improve their activity and to develop new combination therapies.
Old antimicrobials such as natural origin compounds (i.e., essential oils and plant extracts) have shown antimicrobial properties minimizing the development of resistances and susceptibilities due to their multiple mechanisms of action,13,14 whereas antibiotics usually have a single target site. When multiple mechanisms of action take place against a pathogenic microorganism, simultaneous gene mutations are difficult to occur and consequently the development of resistances is less likely. Combinations of natural origin compounds present in essential oils have also shown synergistic effects against both Gram-negative and Gram-positive bacteria either alone15 or in combination with antibiotics.16 Their antimicrobial action is usually explained by the effect on the membrane wall integrity and also on the leakage of intracellular components thanks to the interaction of the essential oils with the cellular lipid bilayer.17 Therefore, revisiting old naturally occurring compounds can provide new insights in the development of antimicrobial materials preventing the development of resistances and avoiding the cytotoxicity of many nanoparticles and their associated released ions against human cells.
Specific components present in essential oils (i.e., thymol [THY]) have been included in nanofibrous mats made of PCL, polylactic acid, and blends and their antimicrobial and regenerative action demonstrated in vitro and in vivo, respectively. Those drug-eluting dressings produced a faster wound closure compared to commercial passive dressings and compared to the physiological healing process due to an improved vapor permeability and due to the presence of the phenolic monoterpenoid.18 Another natural phenol (2,3-dihydroxybenzoic acid; DHBA) incorporated within electrospun poly(d,l-lactide)/polyethylene oxide (PEO) NFs inhibited the growth of Gram-positive and Gram-negative bacteria while preventing hemolysis.19 Caffeic acid has also been loaded within poly(3-hydroxybutyrate) electrospun mats and its antibacterial and cytostatic efficiency demonstrated against Gram-positive bacteria (i.e., S. aureus) and Gram-negative bacteria (i.e., Escherichia coli) and against human cervical HeLa tumoral cells, respectively.20 Olive oil released from electrospun biodegradable PEO/chitosan/PCL NFs has demonstrated antimicrobial action against Gram-negative bacteria (E. coli) and Gram-positive (S. aureus) with no cytotoxicity on human epidermal growth factor receptor 2-expressing fibroblast.21
Of the different components of essential oils derived from plants, the phenolic monoterpenoid isomers THY and carvacrol (CAR) have demonstrated antimicrobial effect against Gram-positive and Gram-negative bacteria.22 It has also been demonstrated that they act as efflux pump inhibitors in foodborne pathogens.23 They both act as membrane permeabilizers producing intracellular leakage affecting pH homeostasis and interfering with the intracellular ionic equilibrium.24 The alkyl incorporation into the phenol nucleus in CAR and THY increases the antimicrobial action of the phenol and it is responsible for altering the distribution ratio between hydrophilic and hydrophobic phases of the bacterial wall reducing the surface tension and also altering the species selectivity.22 The presence of the hydroxyl group and a delocalized electron system is essential for the antimicrobial activity17; in this regard, surprisingly, the same molecule without the hydroxyl group (i.e., p-cymene) did not show antimicrobial action at the same doses than THY or CAR did against Gram-negative bacteria.22
Results from our laboratory have shown superior antimicrobial effects of CAR and THY compared to other previously reported antimicrobial components present in essential oils, including squalene, rosmarinic acid, tyrosol, eugenol, and β-caryophyllene.25 In this regard, even though CAR and THY showed higher minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) than topical antibiotics (i.e., mupirocin) against planktonic and biofilm-forming S. aureus strains26 and also they showed higher MICs and MBCs than the ones required when using commercial antiseptics (i.e., chlorhexidine), they, on the contrary, showed a reduced cytotoxicity against human cells, including human dermal fibroblasts, macrophages, and keratinocytes.27 In addition to their biocidal action those components have also shown anti-inflammatory activity in vivo during wound healing reducing edema formation and peritonitis.28 With this aim, in this work we have developed PCL electrospun nanofibrous mats loaded with either THY or CAR and we have challenged them against Gram-positive bacteria (i.e., S. aureus) and Gram-negative bacteria (i.e., E. coli). Special emphasis has been placed on the importance of the contact between the mats and the bacteria and on the interaction between the active principle and the polymeric substrate.
Clinical Problem Addressed
Infected wounds place a significant burden on health care systems. Acute wounds caused by trauma or chronic wounds such as pressure, diabetic, or vascular ulcers are all commonly colonized by bacteria. A specific wound care, the application of antimicrobial drugs, and even surgery might be needed to eradicate those infections. Still, those infections can prolong the hospital stay, increase the antibiotic resistance or susceptibility, increase the long-term patient disability, and cause elevated costs for health care systems.
Materials and Methods
Materials
PCL (Mn = 80,000 Da), CAR (food grade, ≥98%), (S)-(−)-limonene (food grade, ≥95%), naproxen sodium salt (98–102%), phosphate-buffered saline (PBS), and Tween 80 were purchased from Sigma-Aldrich (Madrid, Spain). Dichloromethane (DCM, >99%) and N,N-dimethylformamide (DMF, >99%) were purchased from Fisher Scientific. THY (99%) was purchased from Acros Organics. Tryptone soy broth (TSB) and tryptone soy agar (TSA) were purchased from Laboratorios Conda-Pronadisa SA (Madrid, Spain). Acetonitrile (≥99.9%), formic acid (98–100%), and deuterated chloroform (99.8% D) were purchased from VWR (Barcelona, Spain). All reagents were used as received without any further purification.
Preparation of PCL NFs, CAR-loaded PCL NFs, and THY-loaded PCL NFs
A solution of PCL (10% w/w) was prepared in a mixture of DCM and DMF (at a 1:1 volume ratio). The solution was stirred overnight at room temperature. For the preparation of CAR-loaded and THY-loaded NFs, appropriate amounts of the select active compound (20 w/w% referred to the PCL mass) were added to the polymer solution and stirred for 30 min before electrospinning.
A Yflow 2.2 D500 electrospinner equipped with a flat plate as collector was used to obtain the bare CAR- and THY-loaded NFs. The collection plate was covered with aluminum foil for facilitating the recovery of the electrospun mats. The electrospinner was equipped with a 22-gauge needle, and the pump was working at a flow rate of 1.0 mL/h. The distance between the tip of the needle and the collector plate was fixed at 18 cm. The voltage applied to the collector plate was −4.00 kV, and the voltage applied to the needle varied from +6.62 to +10.22 kV to obtain a stable Taylor cone (Table 1). All NFs were obtained at room temperature and with a relative humidity of 30–50%.
Table 1.
Experimental conditions for the synthesis of the bare and drug-loaded electrospun mats and chemical characterization
| Initial Active Component (% w/w) | Voltage (kV) | Fiber Diameter (nm) | Drug Loading by GC (% w/w) | Drug Loading by 1H NMR (% w/w) | Encapsulation Efficiency (%)a | Active Component Release after 24 h (% of Loaded Drug)a | |
|---|---|---|---|---|---|---|---|
| PCL | 0 | 12.94 | 263 ± 68 | — | — | — | — |
| PCL-CAR | 20 | 14.22 | 299 ± 69 | 15.40 ± 1.44 | 16.54 ± 0.30 | 77.00 ± 7.18 | 5.78 ± 0.93 |
| PCL-THY | 20 | 10.62 | 259 ± 47 | 17.13 ± 1.61 | 17.46 ± 0.60 | 85.65 ± 8.05 | 7.33 ± 2.56 |
To achieve a stable cone, jet voltage was modified depending on each polymeric system produced.
Calculated from the GC-MS data. CAR and THY (data shown for drug loading, encapsulation efficiency, and release correspond with mean and standard deviation of three independent experiments for each material).
CAR, carvacrol; GC, gas chromatography; GC-MS, gas chromatography coupled with mass spectrometry; NMR, nuclear magnetic resonance; PCL, polycaprolactone; THY, thymol.
For the evaluation of the influence of the contact between the drug-loaded mats and the bacteria to achieve a complete biocidal effect, several mats were also prepared by alternating a bare PCL mat with a drug-loaded mat and a top bare PCL mat in a “sandwich type” form, in such a way that the antimicrobial mat was sandwiched between bare PCL mats. The mass proportion of those layers was 1:2:1.
Physicochemical characterization of the electrospun NFs
For the morphological study of the electrospun mats scanning electron microscopy (SEM) was used. Samples were covered with an Au/Pd layer before electronic visualization in an Inspect F-50 SEM microscope. NF diameters and standard deviations were measured using the ImageJ software.
CAR and THY concentrations were determined by gas chromatography coupled with mass spectrometry (GC-MS). A calibration curve was prepared from 1 to 50 ppm. Totally 5 ppm of (S)-(−)-limonene was added as internal standard. Samples were prepared dissolving 10 mg of the corresponding NF mat in a mixture of DCM and acetonitrile 1:1 (10 mL), diluting the sample with the mixture (to fit the sample concentration to the calibration curve) and adding 5 ppm of the internal standard. GC-MS measurements were carried out in a Shimadzu 2010 SE GC-MS chromatograph equipped with an AOC 20i injector. A Zebron ZB-50 capillary column (30 m × 0.25 mm, 0.25 μm thickness; Phenomenex) was used. The initial oven temperature was 50°C for 1 min and then increased to 160°C at 10°C/min with a final rise to 200°C at 20°C/min. Helium was used as carrier gas at a constant flow rate of 1 mL/min. The detector temperature was 250°C, and the transfer line and ion source temperatures were both set at 200°C. For analysis, 1 μL of sample was injected, working with a split ratio of 1:10.
Nuclear magnetic resonance (NMR) spectra were recorded on a NMR spectrometer Bruker DPX 400 (Bruker Daltonik GmbH, Rheinstetten, Germany). Twenty milligrams of each compound (PCL and CAR- and THY-loaded NFs) or 40 mg of a physical mixture (PCL with CAR or PCL with THY, 50/50 w/w in both cases) were dissolved in 700 μL of CDCl3, and resulting solutions were transferred to 5 mm OD NMR tubes. Samples for calibration curves were prepared by successive addition of 20 μL of a solution of CAR (or THY) in CDCl3 (20 mg/mL) to a 5 mm OD NMR tube, which contained 500 μL of a solution of PCL in CDCl3 (20 mg/mL). The range for concentrations varied from 1.96% to 20.63% w/w for CAR and 1.60% to 17.80% w/w for THY. 1H NMR spectra were recorded at 400 MHz. They were recorded over a spectral width of 0–13 ppm, chemicals shift values (δ) were reported in ppm, and coupling constants (J) were retrieved in Hz. The quantification was based on the integration ratio between the methylene (-OCH2-) of PCL at 4.04 ppm (triplet, J = 6.6 Hz) and H-3 signal of CAR or THY at 7.06 ppm (doublet, J = 7.6 Hz) and 7.08 ppm (doublet, J = 7.6 Hz), respectively.
13C NMR spectra were recorded at 100 MHz using the same spectrophotometer. Broadband proton decoupled 13C NMR spectra were recorded over a spectral width of 0–250 ppm, and chemicals' shift values (δ) were reported in ppm.
Drug release studies were carried out in a continuous mode by flushing PBS (with Tween 80, 2% w/v) at 37°C with a flow rate of 1 mL/min through a fixed bed composed of the electrospun mats using a Shimadzu LC-10AT VP syringe pump. Flushed solutions were collected in 1.5 mL Eppendorf vials at different time intervals (1–10 min: 1 sample/min; 20–180 min: 1 sample/30 min; 4–8 h: 1 sample/60 min). Collected samples were analyzed using an ACQUITY UPLC® Waters liquid chromatography equipped with a column heater, a photodiode array detector ACQ-PDA, a quaternary solvent manager ACQ-QSM, and a sample manager ACQ-FTN, controlled by Waters® Empower™ chromatographic software. An ACQUITY UPLC Waters BEH C18 column (2.1 × 50 mm, 1.7 μm particle diameter) protected by a 0.2 μm stainless steel in-line filter was used. For the quantification of CAR and THY, a calibration curve from 2.5 to 300 ppm was prepared. Twenty-five ppm of naproxen sodium salt was added to the different samples as internal standard. Analyses were run at 40°C under isocratic condition, with a mobile phase composed by a mixture of Milli-Q water (with formic acid, 0.1% v/v) and acetonitrile (50:50 v/v). The injection volume was 1 μL at a flow rate of 0.3 mL/min. CAR, THY, and naproxen sodium salt were detected and quantified using the PDA detector set at 275 nm wavelength. Water vapor transmission rates through the mats were measured according to ASTM Standard E96/E96M-10.
MIC and MBC determination
The antibacterial activity of the loaded NF mats against E. coli S17 strain (which was kindly donated by Dr. Jose Antonio Ainsa) and S. aureus ATCC 25923 was investigated according to the ASTM E-2180 norm.29 To this end, 50 mL of warm TSA (47°C) was inoculated with 50 μL of a stationary culture of the selected bacteria. NF mats were cut, with the required weight to achieve MIC and MBC, and placed in 12-well plates after sterilization under UV light (30 min for each side). Then, each well was filled with 1 mL of the inoculated TSA and incubated in a closed box with water (to keep an adequate humidity) at 37°C for 24 h. Afterward, each sample was transferred to a Falcon type flask, and 10 mL of TSB was added. The corresponding sample (both mat and supernatant) was sonicated for 1 min and vortexed for 1 min. Colony forming units per milliliter (CFU/mL) were measured from the resulting suspensions using the standard microdilution method. MIC was defined as necessary concentration of mats in the culture medium to achieve a 3 log10 reduction in bacterial growth, and MBC was defined as necessary concentration of mats to achieve the complete eradication of bacteria in growth medium.
The influence of the contact was studied by analyzing the antimicrobial action of the products released from the mats at different time points. Initially we chose mats with weights that correspond to the needed mass to reach MBC doses against each specific pathogen (Table 2). Then we placed those mats in culture medium without bacteria for specific times (1, 2, and 7 days). For each time point we separated the immersed mats from their corresponding supernatants (labeled as “exudates”), and we independently challenged the immersed mat and those exudates against bacteria (106 CFU/mL). CFU/mL were measured for the mats and for the exudates using the standard microdilution method.
Table 2.
Minimum inhibitory concentration and minimum bactericidal concentration values for the thymol- and carvacrol loaded electrospun nanofibers against Gram negative (Escherichia coli) and Gram positive (Staphylococcus aureus) bacteria
| MIC | MBC | ||||
|---|---|---|---|---|---|
| Bacteria | Material | NF Concentration (mg/mL) | Active Component Released After 24 h (mg/mL) | NF Concentration (mg/mL) | Active Component Released After 24 h (mg/mL) |
| Escherichia coli | PCL-CAR | 2.10 | 0.02 | 15.00 | 0.13 |
| PCL-THY | 7.50 | 0.09 | 10.00 | 0.12 | |
| Staphylococcus Aureus | PCL-CAR | 10.00 | 0.09 | 25.00 | 0.22 |
| PCL-THY | 10.00 | 0.12 | 30.00 | 0.38 | |
The concentration of the electrospun mats in the biological media, as well as the total concentration of the corresponding active component (total active component concentration), and the concentration measured after 24 h flushing through (active component released) are depicted (data shown correspond with mean and standard deviation of four independent experiments for each combination of material and bacteria).
MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; NF, nanofiber.
The influence of the volatility of the antimicrobial natural compounds was also evaluated. One well of a six-well plate was filled with 2 mL of warm TSA (47°C) inoculated with 106 CFU/mL of the corresponding bacteria. Another well was filled with necessary amount of the compound to reach the MIC and MBC (each concentration and growth controls were placed in independent plates to avoid any biocidal action potentially attributed to the originated volatile compounds). Samples were incubated for 24 h at 37°C and treated in the same way as MIC and MBC evaluation samples.
Confocal analysis
For the visualization of the bacteria supported on the NF mats, the LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Invitrogen™) was used. NF-based mats were incubated with 1 mL of inoculated TSB with the corresponding microorganism (106 CFU/mL) for 24 h at 37°C. Mats were kept on the bottom of the well using CellCrown™ inserts (Scaffdex). Then, TSB was removed, and mats were cleaned with saline solution. After cleaning, samples were stained according to the manufacturer's instructions and rinsed with saline solution (to remove the excess of stain) before their visualization with a Leica TCS SP2 confocal microscope.
Statistical analysis
One-way analysis of variance with a significance level of p < 0.05 was used for a completely randomized statistical analysis to determine significant differences between controls and treated samples. When significant differences were found, Tukey's Honestly Significant Difference multiple comparison test was used with a confidence interval of 95%. Statgraphics Centurion XV software was used for statistical analysis.
Results
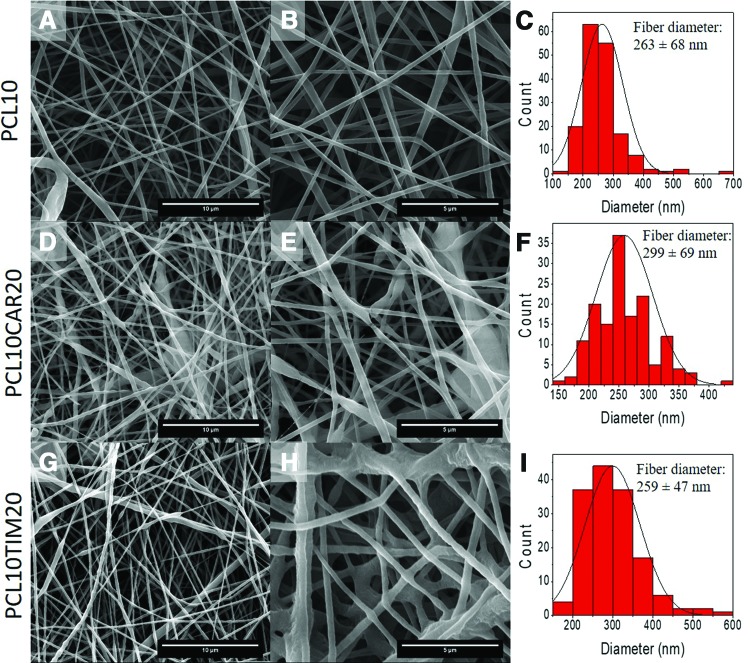
Bare PCL and THY and CAR-loaded NFs synthesized in this work showed submicrometric sizes with high porosity and elevated tortuosity (Fig. 1). The incorporation of the monoterpenoids did not substantially affect the morphology of the NFs. The great advantages of using electrospinning were that no leftovers of organic solvents were present on the electrospun fibers and that room temperatures were used for the fabrication of the fibers with the consequent reduction in the potential loss of the highly volatile organic biocidal compounds THY and CAR (2.13 and 3.95 Pa vapor pressure, respectively, at room temperature). Table 1 shows the synthesis conditions used and the average diameters of the NFs measured by SEM (N = 100). Water vapor transmission rates of 3,267 ± 62 and 3,189 ± 58 g/m2/day were obtained at a 75% humidity relative gradient with THY and CAR-loaded mats, respectively. NMR analysis was performed not only to quantify drug loadings and encapsulation efficiencies but also to understand the nature of the chemical bond between CAR and THY and the PCL substrate. It is reported that values of carbon chemical shifts in a 13C NMR spectrum are reproducible with variation less than or equal to 0.05 ppm (sweep width 250 ppm). It is also known that carbon chemical shifts are sensitive to hydrogen-bond formation.30 The extent of intermolecular hydrogen bonding is decreased by dilution with nonpolar solvents; however, NMR spectroscopy in solution has been widely used to study intra- and intermolecular hydrogen bonds.31,32
Figure 1.
Morphological characterization of the resulting electrospun mats by SEM. (A–C) Bare PCL NFs; (D–F) CAR-loaded PCL NFs; (G–I) THY-loaded PCL NFs; (J, K) picture of electrospun NFs. Scale bar in the left column: 10 μm; scale bar in the center column 5 μm. CAR, carvacrol; NF, nanofiber; PCL, polycaprolactone; SEM, scanning electron microscopy; THY, thymol. Color images are available online.
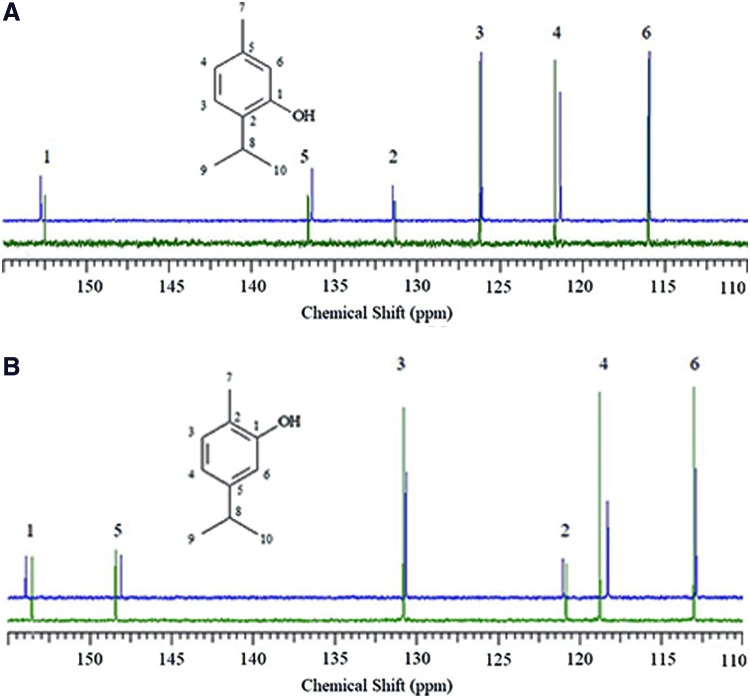
The 13C NMR spectra of THY and a mixture of PCL and THY (50:50 w/w) are shown in Fig. 2A. Physical mixtures of the corresponding drug and the polymer were initially prepared to demonstrate the linearity of the method and to understand the chemical bonding. The 13C NMR spectra of CAR and a mixture of PCL and CAR (50:50 w/w) are shown in Fig. 2B. CDCl3 was chosen as nonpolar solvent due to its null tendency to form hydrogen bonds. It should be noticed that according to the 13C chemical shifts recorded, the largest variations were observed for C-1, bearing the hydroxyl group (+0.36 and +0.25 ppm), carbon C-4, in para position (−0.48 and −0.33 ppm), and carbon C-5, bearing the alkyl groups (−0.33 and −0.24 ppm), for CAR and THY, respectively (Supplementary Tables S1 and S2). Carbon C-1 was deshielded, while C-4 and C-5 were shielded. These shifts were attributed to the formation of hydrogen bonds between the ester functional group in PCL and the hydroxyl group in CAR and THY. As we discussed in the Materials and Methods section 1H NMR spectra were recorded to quantify the drug loading in the NFs. The quantification was based on the integration ratio between the methylene (-OCH2-) of PCL at 4.04 ppm (triplet, J = 6.6 Hz) and H-3 signal of CAR or THY at 7.06 ppm (doublet, J = 7.6 Hz) and 7.08 ppm (doublet, J = 7.6 Hz), respectively (Supplementary Figs. S1B and S2B, respectively). Drug loadings (Table 1) were expressed as weigh percentage of the amount of CAR or THY loaded in the PCL NFs after solving the integral ratio (Supplementary Table S3).
Figure 2.
(A) THY structure and region of interest 13C NMR spectrum of THY (green line) and THY/PCL mixture (blue line). (B) CAR structure and region of interest of 13C NMR spectrum of CAR (green line) and CAR/PCL mixture (blue line). NMR, nuclear magnetic resonance. Color images are available online.
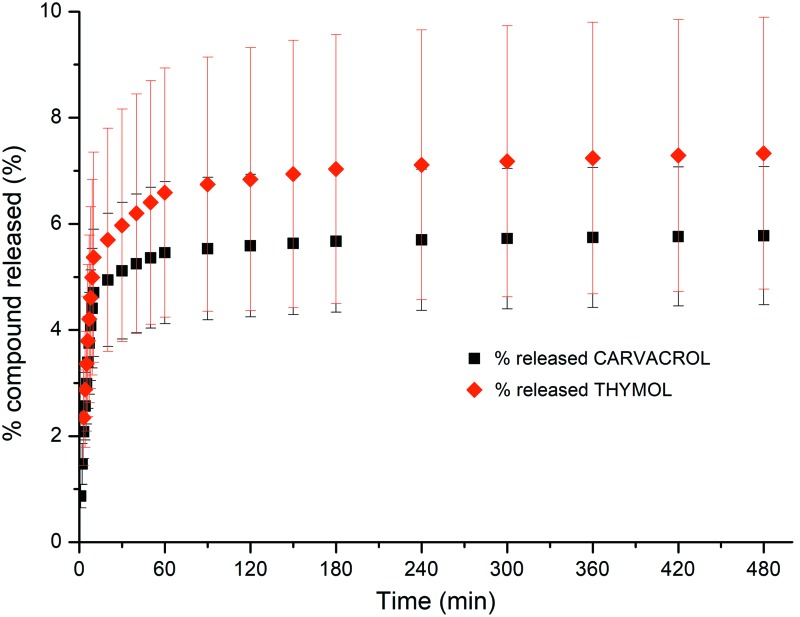
Drug release kinetics (Fig. 3 and Table 1) indicated that besides the initial burst release, only a 5.78% and a 7.33% of the total drug loaded were released after 24 h from the CAR and THY-loaded PCL NFs, respectively. This reduced release might indicate that the corresponding drugs are evenly distributed across the polymeric NFs, and the slow biodegradation of PCL would provide with a controlled release of the encapsulated drug over time. Linear regression analysis (r2 of 0.9 for CAR and 0.9 for THY) was performed to fit drug release kinetics to the Lindner and Lippold exponential model, which is indicative of an initial drug diffusion from the matrix and a subsequent matrix controlling erosion.33
Figure 3.
Drug release kinetics obtained by flushing through a fixed bed composed of the electrospun mats of PBS (with Tween 80, 2% w/v) at 37°C with a flow rate of 1 mL/min (data shown correspond with mean and standard deviation of three independent experiments for each material). PBS, phosphate-buffered saline. Color images are available online.
MIC and MBC values for the THY- and CAR-loaded electrospun mats are depicted in Table 2. In our previous work we calculated MIC and MBC values for those free monoterpenoids using Tween 80 as solubilizing agent.25 Against E. coli free CAR showed a MIC and MBC value of 0.2 and 0.4 mg/mL, respectively, whereas against S. aureus free CAR showed a MIC and MBC of 0.2 and 0.3 mg/mL, respectively. In addition, against E. coli free THY showed a MIC and MBC value of 0.2 and 0.3 mg/mL, respectively; the same concentrations were found for free THY against S. aureus. However, from the electrospun mats the doses released after 24 h in contact with the release medium were way below the MICs needed to inhibit the bacterial growth obtained with the free drugs (Table 2). In addition, lower concentrations were needed to completely eradicate the bacteria compared to the administration of the free drug. This is indicative of the importance of the contact effect of the mats and the bacteria to promote their eradication. In addition it is important to point out that in the case of the mats no solubilizing agent (i.e., Tween 80) was used.
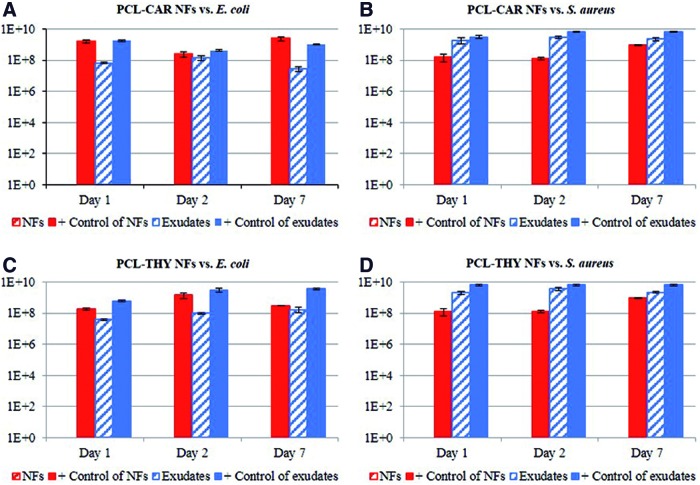
Not only the contact angle of any surface plays a role to favor contact between the biocidal and the bacteria but also we observed that the exudates released from the THY- and CAR-loaded electrospun fibers were not efficient in eliminating bacteria (Fig. 4). Initially we performed antimicrobial assays against E. coli and against S. aureus with mats in which corresponding weights according to Table 2 exerted a total eradication of bacteria (MBCs). Subsequently we placed fresh drug-loaded mats of the same weights in separated tubes containing culture medium. At specific time periods (1, 2, and 7 days), we separated the mats from the medium, and we independently challenged those mats and released exudates against bacteria. Figure 4 shows that over the time period studied the mats retained their antimicrobial action, whereas the exudates released from the corresponding mats did not produce any antimicrobial action. It is important to point out that the controls used in the experiment with mats and with exudates are different due to the differences in the experimental protocols when evaluating solids (mats) and liquids (exudates) (inoculum of 106 CFU/mL growth in TSA and 106 CFU/mL growth in TSB, respectively, incubated for 24 h at 37°C). The antimicrobial action was total against S. aureus and against E. coli for both THY and CAR-loaded mats (Fig. 4). Even though the water solubility of CAR is higher than the one of THY (1.25 and 0.9 mg/L, respectively), looking at the MBC the latter was more efficient than CAR in reducing E. coli cell counts (Table 2).
Figure 4.
Bacterial cell counts (CFU/mL) of the different drug-loaded NFs and their corresponding exudates retrieved from the immersion of the mats in culture media for different times. (A) CAR-loaded electrospun fibers and exudates against Escherichia coli; (B) CAR-loaded electrospun fibers and exudates against Staphylococcus aureus; (C) THY-loaded electrospun fibers and exudates against E. coli; and (D) THY-loaded electrospun fibers and exudates against S. aureus. Note: all the drug-loaded mats produced a complete bacterial eradication (data shown correspond with mean and standard deviation of four independent experiments for each combination of material and bacteria). Positive controls represent bacterial growth counts on nontreated PCL mats. CFU/mL, colony forming units per milliliter. Color images are available online.
To evaluate possible “cross effect” between samples placed in different wells due to the evaporation of the THY or CAR, we tested the effect of pure compounds against bacteria placed in a separated plate. The results showed bacterial growth in the same order of magnitude than controls (≈109 CFU/mL for S. aureus and ≈108 CFU/mL for E. coli). These results suggest that, even in the case of a volatile compound released to the air, under the conditions tested the vapor pressure achieved would not produce any antimicrobial effect; therefore, the antimicrobial action observed was solely attributed to the direct contact between the mats and the corresponding bacteria.
We also evaluated the antimicrobial action of the “sandwich type” films, which were prepared by alternating a bare PCL mat with a drug-loaded mat and a top bare PCL mat in such a way that the antimicrobial mat was sandwiched between bare PCL mats. The mass proportion of those layers was 1:2:1. The results showed bacterial counts after 24 h in the same order of magnitude than the controls (≈109 CFU/mL for S. aureus and ≈108 CFU/mL for E. coli), which indicates that contact is needed to provide with an antimicrobial action and that the reduced amount of THY and CAR released was not enough to exert any antimicrobial action even though the mass of the antimicrobial inner layer was enough to completely eradicate bacteria (concentration to produce MBCs (Table 2) under the experimental conditions tested without the top and bottom bare PCL layers).
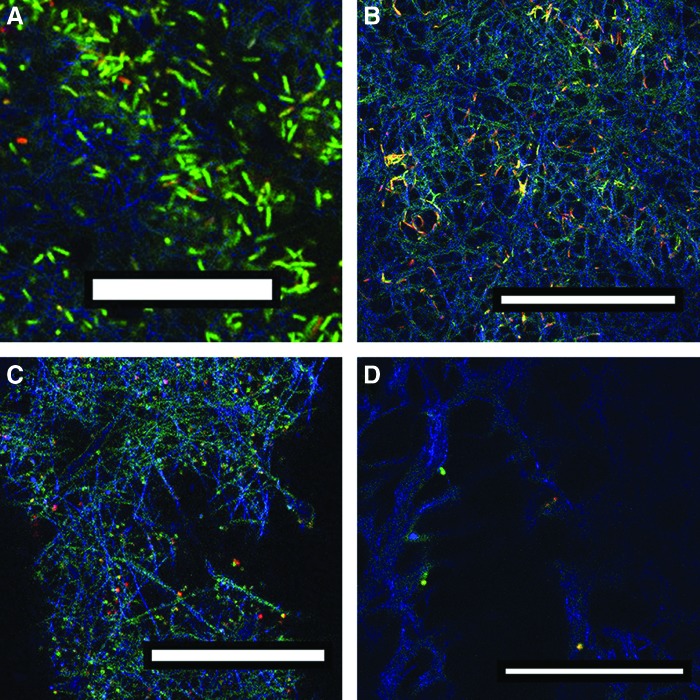
Finally, we qualitatively observed the viability of the pathogenic bacteria tested against the different bare and drug-loaded mats using confocal microscopy. As shown in Fig. 5, PCL fibers were observed by reflection on the confocal microscope and depicted in blue, live bacteria were visualized in green, and dead bacteria in red using the LIVE/DEAD BacLight Bacterial Viability Kit. Figure 5A and C shows that E. coli and S. aureus bacteria remained alive on the plain PCL mats with a characteristic viability of a stationary phase culture (some green cells and red cells could be observed indicative of a general bacterial population in stationary phase growth). However, when mats containing the antimicrobial compound (CAR) at MIC doses were challenged against bacteria a reduced number of dead bacteria stained in red (Fig. 5B, D) were observed for those CAR-loaded mats against E. coli and S. aureus, respectively. The reflection produced by the PCL fibers (in blue) was clearly observed which might be indicative of the prevention of bacterial attachment caused by the CAR-loaded mats. Some green fluorescence was also observed (in the case of E. coli) due to the projection of the live bacteria due to the presence of MIC concentrations, and several overlaps between red and green were also observed (labeled as yellow) indicative of the initial stages of apoptotic processes. In the case of S. aureus almost no cells were visualized, which might be indicative of the prevention of the bacterial adhesion on CAR-loaded mats. In addition, with these images we can observe cell membrane damage, in agreement with our previous results when using free CAR and THY.25
Figure 5.
Confocal microscopy images of: (A) PCL NFs against Escherichia coli; (B) CAR-loaded NFs against E. coli; (C) PCL NFs against Staphylococcus Aureus; and (D) CAR-loaded NFs against S. aureus (scale bars: 30 μm). Color images are available online.
Discussion
The porosity of the resulting NFs here obtained was potentially adequate to assure gas exchange and a sufficient water vapor permeability to maintain the required wound moisture while acting as a barrier to microorganisms. In addition, the large surface area per volume ratio of those <300 nm in diameter NFs guarantees also large surface contact with the bacteria. Drug (i.e., THY and CAR) loadings (measured by both GC-MS and by NMR) and encapsulation efficiencies reported in Table 1 highlight the efficiency of the electrospinning process where the solvent is rapidly evaporated by the electrical field when the polymeric jet travels toward the counter electrode precipitating and entrapping the dissolved drug.
The NMR results corroborated the drug loadings obtained from the GC-MS analysis and also demonstrated that intermolecular hydrogen bonding is responsible for the interaction between the CAR and THY and the PCL NFs. CAR and THY as donors of hydrogen bonds interact with the ester functional group in PCL acting as acceptor. Therefore, NMR can be an analytical technique used not only to evaluate drug loadings but also to understand the chemical interaction between the loaded drug and the encapsulating matrix.
Drug release kinetics showed an initial burst release, which is desirable to prevent an initial bacterial colonization after wounding; also, in this work, a polymer with slow biodegradation rates (PCL) was chosen because wound dressings are not intended to favor cell infiltration or colonization; instead a frequent replacement of the dressings is common to prevent microbial contamination, to absorb exudates and debris and to facilitate blood clogging while avoiding wound maceration.
It has been previously demonstrated that contact plays an important role to achieve antimicrobial action. In this regard, Regiel et al.34 demonstrated that chitosan films filled with Ag nanoparticles were able to completely eliminate S. aureus strains (ATCC 6538 and 9213) when a direct contact between the films and the bacteria was established following the ASTM E2180-07 standard test method29; however, the lixiviates retrieved from the same antimicrobial films after immersion in PBS for 24 h produced only a 4 log10 reduction in the bacterial load. With this aim, some commercial wound dressings such as ACTISORB™ incorporate activated carbon together with the biocidal material (i.e., Ag), acting the carbon as a sink for the bacteria present in the exudate promoting the antimicrobial action of the silver placed underneath.35 Kamimura et al.36 promoted the contact between CAR and E. coli K12 and Salmonella enterica serovar Typhimurium LT2 by encapsulating the phenolic terpenoid within hydrophilic cyclodextrin enhancing consequently its antimicrobial action.
Our results are in agreement with the previous literature where superior antimicrobial action was observed for THY against Gram-negative bacteria than against Gram-positive bacteria attributed to the higher hydrophilicity of the outer layer of Gram-negative bacteria compared to Gram-positive ones.37 This hydrophilicity would potentially hinder the contact between the nonpolar monoterpenoids and the bacteria in the absence of any surfactant because due to the structure of Gram-negative bacteria, those should be more resistant to hydrophobic drugs than Gram-positive ones because the transport inside the cell occurs through hydrophilic porins and therefore uptake of highly hydrophobic drugs is impeded.38 However, less essential oil loaded fibers are required to eliminate E. coli than S. aureus pointing to the importance of the contact between bacteria and mat and that in this case the hydrophilic/hydrophobic rate does not play a crucial role in the antimicrobial action observed. The antimicrobial action is attributed to functional and structural alterations in the inner and outer bacteria membranes interacting with intracellular moieties and membrane proteins.39 Our previous work also pointed out toward cell membrane damage, intracellular leakage, and cell disruption as the main mechanisms of action for both THY and CAR.25
Probably, the amphipathic character of the peptidoglycan outer layer of Gram-positive bacteria (mainly attributed to lipoteichoic acids) or the amphipathic character of the outer membrane (composed of lipopolysaccharides and proteins) of Gram-negative bacteria (being lipopolysaccharide the main amphipathic component of the membrane) promotes the interaction with the nonpolar THY and CAR.40
It is known that generally bacteria prefer to grow on a surface rather than in the surrounding aqueous phase.41 Moreover, irregularities of polymeric surfaces, such as those found in a fibrous mat, promote bacterial adhesion, especially when having a surface roughness in the nanometer scale.42 The preference of bacteria for adhesion on the mat surface would locate them in close contact with the highest concentration of antimicrobial compounds (that located in the mat itself).
Therefore, we have demonstrated that electrospun dressings based on PCL containing antimicrobials of natural origin (CAR and THY) are efficient antimicrobial materials against Gram-positive and Gram-negative bacteria. A clear bacterial antiadhesive effect on those drug-loaded mats was observed by means of confocal microscopy using the LIVE/DEAD Kit, which shows how compromised membranes that are considered to be dead or dying will stain red, whereas cells with an intact membrane will stain green. As we mentioned before, antibiotic resistance and cytotoxicity on human cell lines are also reported for different common topical antimicrobial agents.43–45 Not only antibiotics but also metallic nanoparticles or their released ions have been used to treat infected wounds. However, despite the demonstrated antimicrobial action, metallic nanoparticles (i.e., Ag or ZnO) show large cytotoxicity against human cells, potential allergenic and inflammatory induction, and DNA damage,46,47 and even bacterial resistances to the metallic released ions and their ability to even promote antibiotic-associated resistances have been reported.48,49 Therefore, “old” antimicrobials such as CAR and THY can overcome those limitations preventing wound bacterial colonization, and they can be postulated as efficient antimicrobials when included in electrospun dressings. The lack of biocidal action of the exudates released from the mats also guarantees a reduced systemic release, and consequently, the designed mats would have a localized antibacterial action.
Finally, it has been reported that in infected wounds retrieved from animal models a bacterial cell count of 105 CFU/g of infected tissue (which is a quantitative indication of infection) is equivalent to 103 CFU/mL.50 Consequently, herein we have demonstrated that a few milligrams of THY and CAR-loaded electrospun mats (Table 2) are able to eliminate bacterial cell counts with loads up to 106 CFU/mL.
In summary, antimicrobial PCL-based mats fabricated by electrospinning incorporating natural origin biocidal compounds such as THY and CAR are able to eliminate stationary phase concentrations of pathogenic Gram-positive (S. aureus ATCC 25923) and Gram-negative (E. coli S17 strain) bacteria. A sustained release of the antimicrobial compound from the mats after an initial burst release would allow maintaining high antimicrobial action over time against both bacteria. This loading is here attributed to the presence of hydrogen bonds between the PCL fibers and the THY or CAR. Direct contact between the pathogens and the mats is needed to exert antibacterial action. The exudates released from the mats did not produce any antimicrobial action, which also is indicative of the local nonsystemic toxicity of the developed electrospun mats. High drug loadings and encapsulation efficiencies justify the use of electrospinning for the fabrication of antimicrobial wound dressings providing also with the required porous structure to allow gas exchange and an adequate water vapor permeability to maintain the required wound moisture.
Innovation
This work demonstrates that a direct contact between the pathogenic bacteria and the dressing is required to elicit antimicrobial action. Those dressings would not produce an uncontrolled drug release. This work also validates a novel analytical approach by combining the results of GC-MS and NMR, which provides with not only drug loadings but also a complete understanding on the interaction between the drug and the dressing. This work also shows optically how bacteria are able to spread on bare wound dressings made of PCL but fails in colonizing CAR-loaded wound dressings due to the prevention of bacterial attachment caused by the loaded mats.
Key Finding
Efficient antimicrobial wound dressings can be prepared by electrospinning.
CAR and THY are excellent antimicrobial compounds against both Gram+ and Gram− bacteria.
Direct contact between the bacteria and the dressing is required to elicit antimicrobial action.
By GC-MS and NMR it is possible to calculate not only drug loadings but also to understand the interaction between the drug and the drug-loaded mat.
Supplementary Material
Acknowledgments and Funding Sources
Financial support from the Spanish Ministry of Economy and Competitiveness (grant no. CTQ2014-52384-R) is gratefully acknowledged. The authors also acknowledge the financial support of the ERC Consolidator Grant program (ERC-2013-CoG-614715). CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions and financed by the Instituto de Salud Carlos III (Spain) with assistance from the European Regional Development Fund. We acknowledge the LMA-INA and Cell Culture Core Units from University of Zaragoza and IACS/IIS Aragon, respectively, for their continuous support.
Abbreviations and Acronyms
- CAR
carvacrol
- CFU/mL
colony forming units per milliliter
- DCM
dichloromethane
- DMF
N,N-dimethylformamide
- GC-MS
gas chromatography coupled with mass spectrometry
- MBC
minimum bactericidal concentration
- MIC
minimum inhibitory concentration
- NF
nanofiber
- NMR
nuclear magnetic resonance
- PBS
phosphate-buffered saline
- PCL
polycaprolactone
- PEO
polyethylene oxide
- PLLA
poly(l-lactide)
- SEM
scanning electron microscopy
- THY
thymol
- TSA
tryptone soy agar
- TSB
tryptone soy broth
Author Disclosure and Ghostwriting
All authors contributed toward writing this article, and no ghostwriters were used to write this article.
About the Authors
Enrique Gámez is a PhD student and graduated with a degree in chemistry in 2010. Gracia Mendoza, PhD, graduated with a degree in biology in 2009, and is a senior researcher of the University of Zaragoza. Sofía Salido, PhD, is an Associate Professor in the Department of Organic Chemistry in the University of Jaen. Manuel Arruebo, PhD and Silvia Irusta, PhD are Associate Professors in the Department of Chemical Engineering of the University of Zaragoza.
Supplementary Material
References
- 1. Ignatova M, Manolova N, Markova N, Rashkov I. Electrospun non-woven nanofibrous hybrid mats based on Chitosan and PLA for wound-dressing applications. Macromol Biosci 2009;1:102–111 [DOI] [PubMed] [Google Scholar]
- 2. Zhao R, Li X, Sun B, et al. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol 2014;68:92–97 [DOI] [PubMed] [Google Scholar]
- 3. Shalumon KT, Anulekha KH, Nair SV, et al. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol 2011;49:247–254 [DOI] [PubMed] [Google Scholar]
- 4. Dashdorj U, Reyes MK, Unnithan AR, et al. Fabrication and characterization of electrospun zein/Ag nanocomposite mats for wound dressing applications. Int J Biol Macromol 2015;80:1–7 [DOI] [PubMed] [Google Scholar]
- 5. Rath G, Hussain T, Chauhan G, Garg T, Goyal AK. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J Drug Target 2016;6:520–529 [DOI] [PubMed] [Google Scholar]
- 6. Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. Antimicrobial PLGA ultrafine fibers: Interaction with wound bacteria. Eur J Pharm Biopharm 2011;79:108–118 [DOI] [PubMed] [Google Scholar]
- 7. Nitanan T, Akkaramongkolporn P, Rojanarata T, Ngawhirunpat T, Opanasopit P. Neomycin-loaded poly(styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int J Pharm 2013;448:71–78 [DOI] [PubMed] [Google Scholar]
- 8. Alhusein N, De Bank PA, Blagbrough IS, Bolhuis A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly(ethylene-co-vinyl acetate). Drug Deliv Transl Res 2013;6:531–541 [DOI] [PubMed] [Google Scholar]
- 9. Zhao R, Li X, Sun B, Tong Y, Jiang Z, Wang C. Nitrofurazone-loaded electrospun PLLA/sericin-based dual-layer fiber mats for wound dressing applications. RSC Adv 2015;22:16940–16949 [Google Scholar]
- 10. Zhang X, Guo R, Xu J, et al. Poly(l-lactide)/halloysite nanotube electrospun mats as dual-drug delivery systems and their therapeutic efficacy in infected full-thickness burns. J Biomater Appl 2015;5:512–525 [DOI] [PubMed] [Google Scholar]
- 11. AshaRani PV, Low KM, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009;2:279–290 [DOI] [PubMed] [Google Scholar]
- 12. European Commission. EU One Health Action Plan against AMR. https://ec.europa.eu/health/amr/action_eu_en (last accessed February10, 2019)
- 13. Hammer KA, Carson CF, Rileya TV. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother 2012;2:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh SE, Maillard J-Y, Russell AD, Catrenich CE, Charbonneau DL, Bartolo RG. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J Hosp Infect 2003;2:98–107 [DOI] [PubMed] [Google Scholar]
- 15. Bassolé HR, Juliani HN. Essential oils in combination and their antimicrobial properties. Molecules 2012;17:3989–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langeveld WT, Veldhuizen EJA, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 2014;1:76–94 [DOI] [PubMed] [Google Scholar]
- 17. Betancourt L, Phandanauvong V, Patiño R, Ariza-Nieto C, Afanador-Téllez G. Composition and bactericidal activity against beneficial and pahogenic bacteria of oregano essential oils from four chemotypes of origanum and lippia genus. Rev la Fac Med Vet Zootec 2012;1:21–31 [Google Scholar]
- 18. Karami Z, Rezaeian I, Zahedi P, Abdollahi M. Preparation and performance evaluations of electrospun poly(ɛ-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J Appl Polym Sci 2013;2:756–766 [Google Scholar]
- 19. Ahire JJ, Neppalli R, Heunis TDJ, van Reenen AJ, Dicks LMT. 2,3-Dihydroxybenzoic acid electrospun into poly(d,l-lactide) (PDLLA)/poly(ethylene oxide) (PEO) nanofibers inhibited the growth of gram-positive and gram-negative bacteria. Curr Microbiol 2014;5:587–593 [DOI] [PubMed] [Google Scholar]
- 20. Ignatova MG, Manolova NE, Rashkov IB, et al. Poly(3-hydroxybutyrate)/caffeic acid electrospun fibrous materials coated with polyelectrolyte complex and their antibacterial activity and in vitro antitumor effect against HeLa cells. Mater Sci Eng C Mater Biol Appl 2016;65:379–392 [DOI] [PubMed] [Google Scholar]
- 21. Zarghami A, Irani M, Mostafazadeh A, Golpour M, Heidarinasab A, Haririan I. Fabrication of PEO/chitosan/PCL/olive oil nanofibrous scaffolds for wound dressing applications. Fibers Polym 2015;6:1201–1212 [Google Scholar]
- 22. Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000;2:308–316 [DOI] [PubMed] [Google Scholar]
- 23. Miladi H, Zmantar T, Chaabouni Y, et al. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb Pathog 2016;99:95–100 [DOI] [PubMed] [Google Scholar]
- 24. Lambert RJW, Skandamis PN, Coote PJ, Nychas G-JE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 2001;3:453–462 [DOI] [PubMed] [Google Scholar]
- 25. García-Salinas S, Elizondo-Castillo H, Arruebo M, Mendoza G, Irusta S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018;23:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kifer D, Mužinić V, Šegvić Klarić M. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J Antibiot (Tokyo) 2016;69:689–696 [DOI] [PubMed] [Google Scholar]
- 27. Wiatkowska MW-S, Urbaniak P, Szkaradkiewicz A, Jankun J, Kotwicka M. Effects of chlorhexidine, essential oils and herbal medicines (Salvia, Chamomile, Calendula) on human fibroblast in vitro. Cent Eur J Immunol 2016;2:125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riella KR, Marinho RR, Santos JS, et al. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol 2012;143:656–663 [DOI] [PubMed] [Google Scholar]
- 29. ASTM International. ASTM E2180-18, Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) In Polymeric or Hydrophobic Materials. https://www.astm.org/Standards/E2180 (last accessed February10, 2019)
- 30. Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric Identification of Organic Compounds. Hoboken, New Jersey: John Wiley & Sons, 2014 [Google Scholar]
- 31. Castola V, Mazzoni V, Corticchiato M, Bighelli A, Casanova J. Hydrogen-bonding effect on carbon-13 NMR chemical shifts of naturally occurring phenols. Can J Anal Sci Spectrosc 1997;3:90–94 [Google Scholar]
- 32. Charisiadis P, Kontogianni VG, Tsiafoulis CG, Tzakos AG, Siskos M, Gerothanassis IP. 1H-NMR as a structural and analytical tool of intra- and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014;9:13643–13682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindner WD, Lippold BC. Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress. Pharm Res An Off J Am Assoc Pharm Sci 1995;11:1781–1785 [DOI] [PubMed] [Google Scholar]
- 34. Regiel A, Irusta S, Kyziol A, Arruebo M, Santamaria J. Preparation and characterization of chitosan-silver nanocomposite films and their antibacterial activity against Staphylococcus aureus. Nanotechnology 2013;24:015101. [DOI] [PubMed] [Google Scholar]
- 35. Kerihuel JC. Charcoal combined with silver for the treatment of chronic wounds. J Wounds Care 2009;3:87–93 [Google Scholar]
- 36. Kamimura JA, Santos EH, Hill LE, Gomes CL. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT Food Sci Technol 2014;57:701–709 [Google Scholar]
- 37. Trombetta D, Castelli F, Sarpietro MG, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 2005;6:2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benz R, Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negativ bacteria. Eur J Biochem 1988;1:1–19 [DOI] [PubMed] [Google Scholar]
- 39. Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013;12:1451–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Tejada GM, Sáchez-Gómez S, Kowalski I, et al. Bacterial cell wall compounds as promising targets of antimicrobial agents I. Antimicrobial peptides and lipopolyamines. Curr Drug Targets 2012;9:1121–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Katsikogianni M, Missirlis YF, Harris L, Douglas J. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cells Mater 2004;37–57 [DOI] [PubMed] [Google Scholar]
- 42. Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 2010;10:3824–3846 [DOI] [PubMed] [Google Scholar]
- 43. Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother 1998;1:11–18 [DOI] [PubMed] [Google Scholar]
- 44. Cooper ML, Boyce ST, Hansbrough JF, Foreman TJ, Frank DH. Cytotoxicity to cultured human keratinocytes of topical antimicrobial agents. J Surg Res 1990;48:190–195 [DOI] [PubMed] [Google Scholar]
- 45. Hiron A, Falord M, Valle J, Débarbouillé M, Msadek T. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol Microbiol 2011;3:602–622 [DOI] [PubMed] [Google Scholar]
- 46. Vandebriel RJ, W. De Jong H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl 2012;5:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nguyen KC, Richards L, Massarsky A, Moon TW, Tayabali AF. Toxicological evaluation of representative silver nanoparticles in macrophages and epithelial cells. Toxicol Vitro 2016;163–173 [DOI] [PubMed] [Google Scholar]
- 48. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005;1:1–7 [DOI] [PubMed] [Google Scholar]
- 49. Sütterlin S, Tano E, Bergsten A, Tallberg A-B, Melhus A. Effects of silver-based wound dressings on the bacterial flora in chronic leg ulcers and its susceptibility in vitro to silver. Acta Derm Venereol 2012;1:34–39 [DOI] [PubMed] [Google Scholar]
- 50. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;2:244–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.