Abstract
Obesity has become a significant problem for developing countries, including Indonesia. High duration of sedentary activity and high intake of unhealthy foods were associated with high risk of overweight and obesity. The objective of this study was to compare the distributions of sedentary activity and dietary behavior with overweight/obesity risks between urban and rural areas among children and adolescents aged 10–18 years in Indonesia. This is a cross-sectional study. Data from a national survey in 33 Indonesian provinces (Basic Health Research /Riskesdas 2013) were analyzed. Multiple logistic regression models were used to calculate the odds ratio (OR) adjusted with all variables, such as age, gender, residency, education level, physical activity, and food intake. An urban–rural residence difference was found in the factors related to obesity. Daily caffeinated soft drinks and energy drinks consumption (OR = 1.12, 95% CI: 1.01–1.23) were related to risk of overweight and obesity in urban areas. Daily grilled foods (OR = 1.32, 95% CI: 1.22–1.42) and salty food (OR = 1.09, 95% CI: 1.04–1.15) consumption were significantly associated with obesity in rural areas but not in urban areas. Furthermore, sedentary activity was correlated with overweight and obesity among those who lived in urban and rural areas. Our findings suggest that education, environmental, and policy interventions may need to specifically target urban settings, where access is high to a wide range of processed and traditional high-sugar, high-fat snack foods and beverages.
Keywords: adolescents, overweight, obesity, urban, rural, diet, Indonesia
1. Introduction
Overweight and obesity among children and young adults have become emerging issues in every region of the world, including in low- and middle-income countries (LMICs) [1,2,3,4,5,6]. In the developing world, the prevalence of overweight and obesity was 28.8% and 2.3%–12.0%, respectively [4], similar to rates in higher-income countries [5]. Although there was a wide variation in overweight and obesity rates between countries, over the last 33 years, there has been no significant improvement in obesity reduction [7]. Additionally, in developing countries, the burden has doubled as obesity prevalence has risen alongside a persistent burden of undernutrition [1,8]. In Indonesia, overweight and obesity have become public health problems in parallel with undernutrition. The prevalence of adult overweight and obesity have increased from 8.6% and 10.5% in 2007 to 13.6% and 21.8% in 2018 [9], respectively. Among children aged 5–12 years, the proportion of overweight remained unchanged between 2013 and 2018 (10.8%), whereas the obesity prevalence slightly increased from 8.8% to 9.2% during these years [9,10]. Furthermore, central obese prevalence among >18 years population increased dramatically from 18.8% in 2007 to 31.0% in 2018 [9].
The determinants of overweight and obesity in LMICs are not well understood and could be driven by the changes in dietary behaviors and physical activity, as well as environmental factors, genetics and epigenetics, and stress. It has been suggested that diets have become higher in calories, sugar, fat, and salt, whereas physical activity level has reduced worldwide [1,7]. Maternal and household factors in early life may also bring demographic and socioeconomic changes, which can lead to higher body mass index (BMI) in later life [4,11].
In LMICs, the prevalence of overweight and obesity is typically higher in urban areas while underweight is typically higher in rural areas [12,13,14]. Children who live in urban areas tend to have a higher risk of overweight and obesity due to unhealthy dietary behaviors, such as high intake of sugar-sweetened beverages intake [15,16]. The dietary behaviors in urban areas are also more diverse compared to rural areas [17]. In contrast, among rural and semi-urban residents, salt is added almost regularly to cooking foods, and salted foods such as fish and meat are eaten frequently [18]. Subsequently, the association between dietary behavior and risk of overweight/obesity remains unclear after being stratified by rural and urban locale, and after adjustment for other factors.
To our knowledge, no study has reported differences in rates of overweight and obesity between urban- and rural-dwelling 10–18-year-old children and adolescents in Indonesia, along with the associated risk factors regarding dietary behavior. Therefore, our aim is to analyze the determinants of dietary behavior in children and adolescents in Indonesia. The second purpose of this study is to (1) examine the prevalence of overweight, and obesity; (2) explore the percentages of sedentary activity and dietary behavior; and (3) compare sedentary activity and dietary behavior with overweight/obese risk between urban and rural areas among children and adolescents aged 10–18 years in Indonesia. Given the knowledge that overweight and obesity proportions are higher in urban compared to rural areas, the findings of this study may contribute to the development of targeted overweight and obesity reduction programs in Indonesia based on rural–urban-specific causes.
2. Materials and Methods
2.1. Study Population and Design
We analyzed data from a cross-sectional, nationally representative survey (Indonesia Basic Health Research 2013/Riskesdas 2013/Riset Kesehatan Dasar 2013) conducted by the National Institute of Health Research Development (NIHRD), Ministry of Health, Indonesia in 2013. The methodology and the detailed protocol used are described elsewhere [10]. Briefly, the selection of participants was carried out by a two-stage stratified cluster sample drawn from across the country, including 33 provinces and 497 municipalities/districts in Indonesian. During a home visit, trained interviewers collected primary data on children and adolescents’ characteristics and other measurements. Well-trained interviewers collected anthropometric measurements (height and weight) using a standardized protocol. The inclusion criteria in this study were: (1) Children and adolescents aged 10–18 years old and (2) no missing data. The exclusion criteria were children and adolescents with missing data, such as weight, height, duration of sedentary behavior, and fruit vegetable intake. Data from 185,631 children and adolescents were available, however, there were 29,986 participants with missing data excluded from this study. Finally, 155,645 adolescents (83.85%) aged 10 to 18 years were included in this study.
2.2. Ethical Considerations
The Health Research Ethics Committee—University of Indonesia, Hasanuddin University, Airlangga University, and the National Institute of Health Research and Development (NIHRD), Ministry of Health Republic of Indonesia, reviewed and approved the protocol, design, data, and survey questionnaires (LB.02.01/5.2/KE.006/2013). No further ethical clearance was required for the secondary analysis of published public data. All of the participants were asked for their consent and signed the informed consent form in the survey.
2.3. Socio-Demographic and Dietary Behavior Questionnaire
Age, residency, gender, and education level were collected using a validated questionnaire and interview performed on children and adolescents [10,19]. A simple questionnaire and food cards were used to ask the frequency of consumption of 9 unhealthy food items, including sugar-sweetened beverages and foods, coffee and caffeinated soft drinks and energy drinks, fatty and fried foods, refined carbohydrates, salty foods, instant noodles, preserved meats, and grilled foods. Furthermore, this simple questionnaire also asked about the portions of fruits and vegetables consumed per day. The frequency of dietary behavior was evaluated by the question “how often do you consume?” with six possible answers: “>1 time/day”; “1 time/day”; “3–6 times/week”; “1–2 times/week”; “≤3 times/month”; and “never”. Then, in this study, dietary behavior was categorized into <1 time/day and ≥1 time/day.
The following is a description of unhealthy foods included in this study: (a) Refined carbohydrates included prepared flour products with added sugar, such as flavored bread; (b) fatty fried foods included fatty foods such as fatty meats, oxtail soup, fried foods, foods containing coconut milk and margarine, and high-cholesterol foods such as innards (intestines, tripe), eggs, and shrimps; and (c) sweet foods and drinks include high-sugar foods and beverages with additional natural sugar, such as cakes, canned fruit, artificial juice, soft drinks, syrups, and sweet tea. Soft drinks and sweetened drinks with zero calories and low sugar and diet drinks were excluded; (d) coffee; (e) caffeinated soft drink and energy drink, included caffeinated drinks except coffee, such as caffeinated soft drinks, and energy drink; (f) salty foods: Foods that are predominantly salty, like salted fish, fish pindang, salted eggs, salty snacks, foods containing shrimp paste, soy sauce, and sauce; (g) grilled foods: Foods that are processed by burning on fire directly, for example satay, grilled chicken, rolled goat, grilled fish; and (h) preserved meat: Beef or pork through processing and preservation use additives (nitrate). Preserved meat usually has red characteristics, for example, corned beef, sausages, meat burger, and smoked meat.
The definition of fruits and vegetables are all types of fruits and vegetables in Indonesia, both local and imported. In addition, the frequency of fruits and vegetable was categorized into <5 portion/day and ≥5portion/day [10].
2.4. Anthropometric Data
Height was measured by well-trained interviewers to the nearest 0.1 cm by a multi-function brand Stadiometer. A Camry digital weight scale with a capacity of 150 kg was used to measure body weight. Before use, the weight scale was calibrated daily. Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2). It was categorized using gender-specific of body mass index (BMI) per age according to CDC criteria: Underweight (less than 5th percentile), normal or healthy weight (5th–<85th percentile), overweight (85th–<95th percentile), and obese (≥95th percentile). Body mass index (BMI) per age based on the gender of the Indonesian children and adolescents are summarized in Table S1. Furthermore, adolescents were categorized as underweight, normal, overweight, or obese based on this national reference.
2.5. Physical Activity Questionnaire
An interview questionnaire was used to record types of sedentary activity. Participants were asked to recall all activities that started from waking up in the morning to going to sleep at night. Participants were asked how many hours or minutes they typically spent at work or home, sitting for transportation (including waiting for transport), sitting or lying down to watch TV or play electronic games, or sitting or lying down to carry out other activities (such as speaking, reading, etc.), not including sleeping. Total sedentary activity time (hours/day) was calculated based on time spent in all the above listed sedentary activity subcategories [10] and sedentary activities were coded as <2 h/day, 2–4 h/day, >4–6 h/day, >6–8 h/day, and >2 h/day.
2.6. Statistical Analysis
First, we examined the prevalence of overweight and obesity by using percentage. Second, chi-square tests were conducted to explore residence differences in overweight and obesity status, dietary behaviors, and other variables. Third, the odds ratio (OR) and 95% confidence intervals (CIs) for the association of factors related to overweight and obesity were estimated using logistic regression analysis. We controlled for all variables, such as residency, gender, age, education level, physical activity, and food intake in the regression analysis. In addition, we also stratified the models by gender and residency to investigate potential gender and residency difference in these associations. The data were analyzed using the SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA); p < 0.05 was deemed statistically significant.
3. Results
3.1. Prevalence of Obesity in Rural and Urban Areas
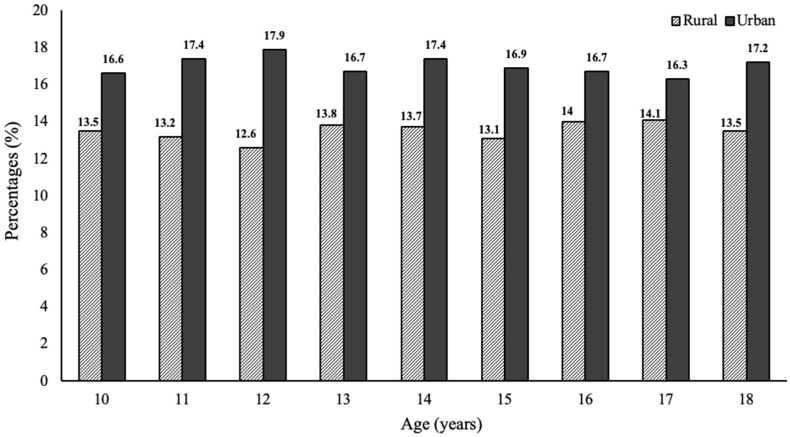
The prevalence of overweight and obesity among children and adolescent was higher in urban areas (17%) than in rural areas (13.5%). Moreover, the prevalence of obesity in urban areas was higher in every age category of children and adolescents (Figure 1).
Figure 1.
The percentage of overweight and obesity in rural and urban areas (%). p-value from Chi-square test was <0.0001 for each age, except 15–16 years old, where p-value was <0.05.
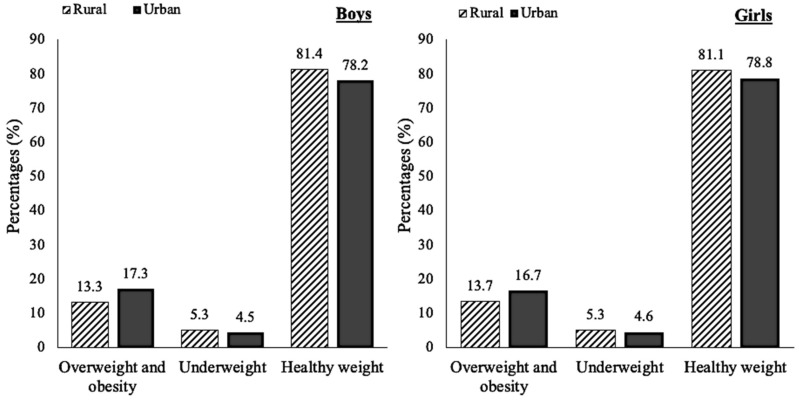
After stratifying by gender, boys (15.2%) had a slightly higher prevalence of overweight and obesity than girls (15.1%). In addition, among boys and girls, the prevalence of overweight and obesity was still higher in urban areas. Meanwhile, in urban areas, boys had a higher prevalence of overweight and obesity than girls (Figure 2).
Figure 2.
The percentage of overweight and obesity stratified by gender and locale. p-values from chi-square test for girls in urban and rural areas were <0.0001; also, boys in urban and rural areas were <0.0001.
3.2. Sedentary Activity and Dietary Behaviour in Rural and Urban Areas
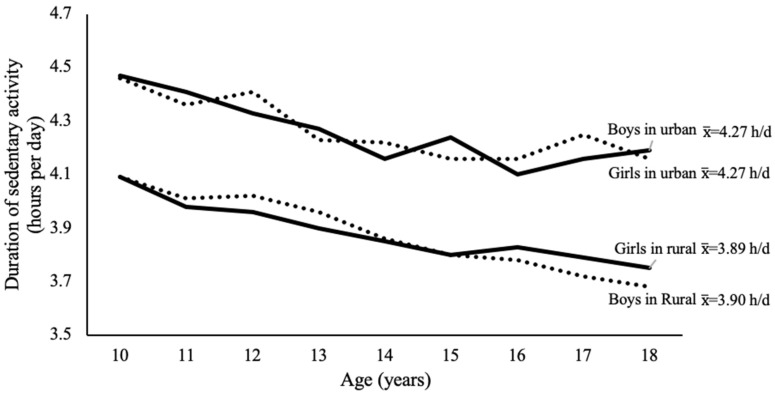
Sedentary activity was more prevalent in urban areas than in rural areas. Urban areas had higher mean hours of sedentary activity per day than in rural areas (Figure 3). However, sedentary activity was associated with a higher risk of overweight and obesity both in urban (OR = 1.09, 95% CI: 1.02–1.16) and in rural (OR = 1.10, 95% CI: 1.04–1.17) areas (Table 1).
Figure 3.
Duration of daily sedentary activity (hours/day) in each age among girls and boys in urban and rural areas. p-values from t-test for girls in urban and rural area, as well as boys in urban and rural areas, were <0.0001.
Table 1.
Multivariate analysis of overweight and obese risks.
| Variables (Ref vs. Risk) | Overweight and Obese | ||||||
|---|---|---|---|---|---|---|---|
| Rural and Urban (n = 155,645) | Rural (n = 83,820) | Urban (n = 71,825) | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Residency | Rural | ref | |||||
| Urban | 1.32 *** | 1.28–1.35 | - | - | - | - | |
| Gender | Girls | ref | ref | ref | |||
| Boys | 1.01 | 0.98–1.04 | 0.97 | 0.93–1.01 | 1.05 * | 1.01–1.10 | |
| Age | 15–18 years | ref | ref | ref | |||
| 10–14 years | 1.06 * | 1.02–1.10 | 1.03 | 0.97–1.08 | 1.10 * | 1.04–1.17 | |
| Education level | |||||||
| High | ref | ref | ref | ||||
| Low (not completed in primary school) | 0.92 * | 0.89–0.96 | 0.93 * | 0.87–0.98 | 0.91 * | 0.86–0.97 | |
| Sedentary activity | |||||||
| <2 h/day | ref | ref | ref | ||||
| 2–4 h/day | 1.12 *** | 1.08–1.18 | 1.10 * | 1.03–1.17 | 1.11 * | 1.04–1.19 | |
| >4–6 h/day | 1.09 *** | 1.05–1.14 | 1.09 * | 1.03–1.16 | 1.06 | 0.99–1.14 | |
| >6–8h/day | 1.09 * | 1.02–1.16 | 1.07 | 0.98–1.18 | 1.05 | 0.95–1.15 | |
| >8 h/day | 1.15 *** | 1.08–1.22 | 1.06 | 0.96–1.16 | 1.13 * | 1.04–1.23 | |
| Sugar-sweetened beverages and foods | |||||||
| <1 time/day | ref | ref | ref | ||||
| ≥1 time/day | 0.97 | 0.94–1.00 | 0.98 | 0.94–1.02 | 0.95 * | 0.92–0.99 | |
| Coffee consumption | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.01 | 0.96–1.06 | 1.04 | 0.98–1.11 | 0.96 | 0.89–1.03 | |
| Caffeinated soft drinks and energy drinks consumption | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.11 * | 1.03–.19 | 1.10 | 0.99–1.22 | 1.12 * | 1.01–1.23 | |
| Fatty fried foods | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.00 | 0.96–1.03 | 0.96 | 0.92–1.01 | 1.03 | 0.99–1.07 | |
| Refined carbohydrate | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.01 | 0.97–1.05 | 0.98 | 0.92–1.03 | 1.04 | 0.99–1.09 | |
| Salty foods | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.03 | 0.99–1.07 | 1.09 * | 1.04–1.15 | 0.98 | 0.93–1.03 | |
| Instant noodle | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 0.95 * | 0.91–0.99 | 0.95 | 0.89–1.01 | 0.95 | 0.90–1.01 | |
| Preserved meats | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 0.95 | 0.89–1.01 | 0.93 | 0.83–1.03 | 0.97 | 0.89–1.06 | |
| Grilled foods | <1 time/day | ref | ref | ref | |||
| ≥1 time/day | 1.19 *** | 1.12–1.26 | 1.32 * | 1.22–1.42 | 1.03 | 0.94–1.14 | |
| Vegetables and fruits | ≥5 portion/day | ref | ref | ref | |||
| <5 portion/day | 0.92 * | 0.88–0.98 | 0.96 | 0.89–1.04 | 0.90 * | 0.83–0.96 | |
Reference group: Sedentary activity: <2 h/day, dietary behavior: <1 time/day, * p < 0.05, *** p-value < 0.0001.
We found significant differences in dietary behaviors among children depending on locale (Table 2). There was a significant association between sugar-sweetened beverages, coffee, caffeinated soft drink and energy drink, fatty fried foods, refined carbohydrate, preserved meat, and grilled foods consumption with locale (rural and urban). In this study, we also reported that there was no significant difference in intakes of salty foods and instant noodles between rural and urban areas. However, only among boys, lower intake of vegetables and fruits consumption was significantly higher in rural than urban areas.
Table 2.
Percentages of dietary behaviors of children and adolescents stratified by locale and sex, n = 155,645.
| Variables | Boys | p-Value | Girls | p-Value | ||
|---|---|---|---|---|---|---|
| Rural (n = 43,207) | Urban (n = 36,219) | Rural (n = 40,613) | Urban (n = 35,606) | |||
| Sugar-sweetened beverages and foods | ||||||
| <1 time/day | 51.2 | 44.0 | <0.0001 | 53.2 | 46.1 | <0.0001 |
| ≥1 time/day | 48.8 | 56.0 | 46.8 | 53.9 | ||
| Coffee consumption | ||||||
| <1 time/day | 83.7 | 88.9 | <0.0001 | 90.8 | 94.9 | <0.0001 |
| ≥1 time/day | 16.3 | 11.1 | 9.2 | 5.1 | ||
| Caffeinated soft drinks and energy drinks consumption | ||||||
| <1 time/day | 95.6 | 95.0 | <0.0001 | 96.6 | 96.2 | 0.0011 |
| ≥1 time/day | 4.4 | 5.0 | 3.4 | 3.8 | ||
| Fatty fried foods | ||||||
| <1 time/day | 70.3 | 62.0 | <0.0001 | 69.3 | 61.2 | <0.0001 |
| ≥1 time/day | 29.7 | 38.0 | 30.7 | 38.8 | ||
| Refined carbohydrate | ||||||
| <1 time/day | 84.6 | 76.8 | <0.0001 | 83.2 | 75.9 | <0.0001 |
| ≥1 time/day | 15.4 | 23.2 | 16.8 | 24.1 | ||
| Salty foods | ||||||
| <1 time/day | 78.9 | 79.1 | 0.5847 | 78.9 | 78.3 | 0.0536 |
| ≥1 time/day | 21.1 | 20.9 | 21.1 | 21.7 | ||
| Instant noodle | ||||||
| <1 time/day | 85.5 | 85.2 | 0.3081 | 85.4 | 85.6 | 0.3937 |
| ≥1 time/day | 14.5 | 14.8 | 14.6 | 14.4 | ||
| Preserved meats | ||||||
| <1 time/day | 96.0 | 93.8 | <0.0001 | 95.9 | 93.5 | <0.0001 |
| ≥1 time/day | 4.0 | 6.2 | 4.1 | 6.5 | ||
| Grilled foods | ||||||
| <1 time/day | 92.8 | 95.1 | <0.0001 | 93.1 | 94.9 | <0.0001 |
| ≥1 time/day | 7.2 | 4.9 | 6.9 | 5.1 | ||
| Vegetables and fruits | ||||||
| ≥5 portion/day | 6.7 | 7.4 | 0.0001 | 6.8 | 7.0 | 0.2831 |
| <5 portion/day | 93.3 | 92.6 | 93.2 | 93.0 | ||
Percentage (%) and p-value from chi square.
Furthermore, children and adolescents who lived in rural areas had a higher percentage of coffee and grilled foods consumption, while those in urban areas had a higher percentage of sugar-sweetened beverages, caffeinated soft drink and energy drink, fatty fried foods, refined carbohydrate, and preserved meat consumption.
3.3. Overweight and Obesity Risk in Rural and Urban Areas
There was a significant difference found in the risk of overweight and obesity between urban and rural areas. In a multivariate logistic regression model analysis, after controlling for all variables, children and adolescents living in urban areas (OR = 1.32, 95% CI: 1.28–1.35), with sedentary activity (OR = 1.10, 95% CI: 1.05–1.14), consuming daily caffeinated soft drinks and energy drinks consumption (OR = 1.11, 95% CI: 1.03–1.19), and daily grilled foods intakes (OR = 1.19, 95% CI: 1.12–1.26) were all significantly associated with a higher risk of overweight and obesity. In contrast, low education level (OR = 0.92, 95% CI: 0.89–0.96) and low intake of daily fruits and vegetable consumption (<5 portions/day) (OR = 0.92, 95% CI: 0.88–0.98) significantly reduced overweight and obesity risk in rural and urban areas (Table 1).
Sedentary activity was associated with overweight and obesity risk, not only in rural areas, but also in urban areas. In rural areas, there was no significant association of gender and age with overweight and obesity risk, but, there was a significant association between low education level (OR = 0.93, 95%CI: 0.87–0.98), sedentary activity 2–4 h/day (OR = 1.10, 95% CI: 1.03–1.17), daily salty foods consumption (OR = 1.09, 95% CI: 1.04–1.15), and daily grilled foods consumption (OR = 1.31, 95% CI: 1.22–1.42) with overweight and obesity risks. Furthermore, in urban areas, being a boy (OR = 1.05, 95%CI: 1.01–1.10), of younger age (OR = 1.10, 95%CI: 1.04–1.17), with sedentary activity 2–4 h/day (OR = 1.11, 95%CI: 1.04–1.19) and frequently consuming caffeinated soft drink and energy drink (OR = 1.12, 95%CI: 1.01–1.23) was significantly associated with overweight and obesity risk.
4. Discussion
This is the first nationally representative study in Indonesia exploring the differences between weight status, and dietary and physical activity behaviors among children and adolescents in urban and rural areas. We found that urban residence was associated with greater obesity risk. Studies in developing countries, such as Vietnam, Malaysia, Indonesia, and Thailand, have also shown that the prevalence of overweight and obesity is higher in urban areas [13,14,20,21], agreeing with our findings. In developing countries, the economic growth resulting from the nutrition transition affects individuals and families, increasing their access to processed and fast food [22,23,24,25].
Urbanization is associated with a dramatic reduction in physical activity and changes in dietary behavior [26]. Rural-to-urban migration may change dietary behaviors from consuming main staples, such as grains and legumes, to regularly consuming energy-dense and animal-source foods. The pattern increases with wealth in urban areas [27]. On the contrary, among lower-wealth families, poor diet and limited access for physical activity may increase their vulnerability of becoming overweight or obese [28]. However, living in urban areas may also present certain challenges related to physical activity due to the residential and public transport density, and the availability of public parks [29]. Urban areas may be considered obesogenic environments [30], with advertisements prominently delivering messages to increase fast foods, processed foods, and beverages consumption that are high in carbohydrates, sugar, fat, and salt [31].
There was no association between sex and overweight or obesity when being analyzed for both rural and urban. Nonetheless, urban boys were more likely to become overweight and obese than urban girls. In this study, boys tended to become more obese than girls likely due to having a higher intake of sugar-sweetened beverages and caffeinated soft drink and energy drink than girls. In terms of sedentary activity, both in boys and girls in urban or rural, sedentary activity has a significant association with overweight and obesity. However, a previous study found that girls tend to be less active than boys at all ages [32,33,34]. Fear of injury, sense of safety, and reliance on others for everyday mobility could be the reason that girls participated less than boys [35]. On the other hand, there was no difference between boys and girls living in rural areas in regard to overweight and obesity risk. A study in Portugal reported that boys who were living in urban areas participated in various sports activities compared to those living in rural areas [36]. It seems that rural children and adolescents of both sexes have more difficulty in accessing sport or fitness centers and any other facilities for performing physical activity compared to urban-living children and adolescents.
A previous study reported that children and adolescents consuming any sweet drink or soft drink was >70% and the proportion remained steady across time [37]. A cross-sectional study that included 75 countries found that soft drink consumption is significantly associated with overweight and obesity, especially in low- and middle-income countries [38]. In particular, higher soda drink consumption was associated with weight gain, contributing approximately 8–9% of child and adult total energy intakes, thus leading to obesity [39]. In this study, daily caffeinated beverages consumption, which includes energy drinks and caffeinated soft drinks, are also a risk factor for overweight and obesity, particularly in urban areas, a finding in line with other studies [40]. While caffeine is the key ingredient in most energy drinks, most products available on the market also contain a high amount of glucose or artificial sweetener [41], which may later contribute to higher energy intake. Moreover, the consumption of energy drinks presumably leads to higher carbohydrate oxidation and lower lipid oxidation [42]. Regarding soft drinks, besides a high level of added sugar, poor satiety and insufficient total energy compensation may influence weight gain [39]. Finally, soft drink consumption can be used as an indicator of poor diet as it is linked to increased calorie intake and may displace nutritious food [43]. For regular caffeinated sugar-sweetened beverage consumption, caffeine content was found to be additive, thus promoting repeated consumption of sugar-sweetened beverages themselves, thus leading to weight gain [44]. Children and adolescents in urban areas may be better able to find caffeinated beverages than in rural area. A policy to limit caffeinated beverages consumption in urban areas is needed, particularly targeting regulations in the advertisement, sale, and location of food retailers. School- and family-level supports are required to help children and adolescents make healthy food choices.
In rural Indonesia, participants with higher daily salty foods and grilled foods consumptions had higher risk factors for overweight and obesity. Salty snacks were associated with higher increases in mean daily energy for children [45]. A previous study showed that long-term consumption of a high salt diet is associated with increased frequency of obesity [46]. Longitudinal studies have also shown that high salt consumption predicts the development of obesity, even when total energy intake or consumption of sugary drinks has been controlled [47]. Furthermore, a previous study also found that regular servings of energy-dense snack foods, which have high sodium, had a direct relationship with body mass index (BMI) z-score among adolescents [48].
In this study, grilled foods, including grilled beef, chicken, goat, and fish, were related to meat consumption. A previous prospective study found that total consumption of meat was positively associated with weight gain [49]. A cohort of British adults study also reported that red or processed meat consumption significantly predicted increased BMI and waist circumference [50]. Red and processed meats are part of the western dietary pattern, which is considered an obesity-inducing dietary pattern [51,52,53]. Red and processed meats contain high saturated fatty acid and cholesterol [54]. They are also categorized as high-energy-density food, which is directly associated with obesity [55,56]. Red meat intake is also positively related to some gut microbiota, which play a role in increasing obesity [57]. Furthermore, future studies should investigate why daily grilled foods consumption has a significant association with overweight and obesity, and why this relationship appears to be more prominent in rural areas. The type of foods and grilling methods used in urban and rural areas may be different and may explain the association with overweight and obesity.
Surprisingly, one factor that was found to have a protective effect against overweight and obesity was low educational attainment. Greater education may be associated with greater income and therefore greater access to high fat and high sugar snack foods. One earlier study also found an inverse relationship between educational level and overweight-obesity, agreeing with our finding [58]. The association between sociodemographic factors, including education level, depends on the development level of the country, wherein gender appeared to modify the effect [58,59]. Cohen et al. (2013) also suggests that the association between education and obesity may be affected by age [58]. As this study used data from a 10–18-year-old population, this may disguise the association between education and overweight/obesity as this population has roughly the same level of education. Moreover, formal educational attainment does not always reflect the level of knowledge in nutrition. It is possible that children and adolescents with lower educational attainment have more knowledge in nutrition, thus practicing a healthy diet and being more active, subsequently making them less likely to develop overweight and obesity. Previous studies have revealed that nutritional knowledge of either children or adolescents is related to overweight and obesity [60,61]. Parental factors such as knowledge, perception, and behavior might also play a role in childhood overweight and obesity [62,63].
There are some limitations to this study. First, the cross-sectional design prevents strong causal inferences. Second, no information was collected regarding total calories, macronutrients, and micronutrients from certain foods, thus disallowing us to examine whether the foods that children and adolescents consumed meet their daily nutritional needs. Third, the majority of data in this study were originally in categorical form, so that we were unable to transform them into a numeric scale.
Despite its limitations, our research provides suggestions for the development of effective prevention policies on the issue of the nutrition transition, which has been shown to increase the frequency of unhealthy foods consumption, obesity, and non-communicable diseases both in urban and rural areas. This study provides details on the types of food and food frequency, making it easier to recognize common foods that should be a concern for overweight and obesity in urban and rural areas. In addition, only a few questions about the foods were asked, and the help of food cards can reduce the bias in the interview process.
5. Conclusions
The current findings of the study identified the differences in overweight and obesity risk factors in rural and urban areas. Those living in urban areas had a higher percentage of overweight and obesity. Sedentary activity had a significant association with overweight and obesity not only in rural areas, but also in urban areas. However, dietary behavior has a different association with overweight and obesity risk in rural and urban areas.
Strategies to improve the nutritional status of children and adolescents in Indonesia should focus on developing approaches that vary by locale. Through understanding the risk factors both in rural and urban settings in Indonesia, these data can inform targeted community-based interventions for children and adolescent obesity prevention programs. In addition, our findings suggest that education, environmental, and policy interventions may need to specifically target urban settings, where access is high to a wide range of processed and traditional high-sugar, high-fat snack foods and beverages. Policies should be enacted that target access to these foods both within and immediately near school grounds. For example, sugar-sweetened beverages and caffeinated soft drinks and energy drinks should not be provided both at home and at school canteens in urban areas. Moreover, there is a need for policy-based strategies to limit the sugar-sweetened beverages and caffeinated soft drinks and energy drink exposure from the media. For both children and adolescents living in rural and urban areas, nutrition education should be integrated into the curriculum as a school-wide strategy that involves discussion on healthy eating and promotion of physical activity. This program should also involve parents so that healthy behaviors can continue after school.
In addition, in urban areas, sports centers could provide the opportunity for youth to join organized sports which are safe and gender friendly. Parks and public spaces should also enable children and adolescents in both genders to participate in various forms of physical activities. In rural settings where physical activity and sports facilities are lacking, we suggest a community approach to incorporating physical activity into everyday life in the natural environment (e.g., the use of walking trails, playing traditional games). The promotion of local-based foods that are high in fruits and vegetables and low in ultra-processed ingredients may also be advantageous.
Acknowledgments
The authors thank The National Institute of Health Research and Development (NHIRD), Ministry of Health Republic of Indonesia for providing us with the 2013 Riskesdas data. This work was supported by Taipei Medical University and Alma Ata University. The second author would also like to acknowledge the financial support from the Ministry of Research and Technology and Higher Education, the Republic of Indonesia through the grant of World Class Professor Scheme B, for the year of 2019. The authors would also like to thank Emma C. Lewis, M.S. for checking the grammar of this manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/11/2813/s1, Table S1. Body mass index (BMI) per age for Indonesian populations based on gender.
Author Contributions
E.N. and C.-H.B. analyzed data and interpreted the results. E.N. and B.A.P. wrote the manuscript. H.H., J.-S.C., J.C.-J.C., B.A.P., and J.G. contributed to interpreting the results. All authors participated in the critical revision and approved the final manuscript.
Funding
Taipei Medical University, Indonesian Ministry of Health and The University of Alma Ata.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ford N.D., Patel S.A., Narayan K.V. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health. 2017;38:145–164. doi: 10.1146/annurev-publhealth-031816-044604. [DOI] [PubMed] [Google Scholar]
- 2.Jaacks L.M., Kavle J., Perry A., Nyaku A. Programming maternal and child overweight and obesity in the context of undernutrition: current evidence and key considerations for low- and middle-income countries. Public Health Nutr. 2017;20:1286–1296. doi: 10.1017/S1368980016003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaacks L.M., Slining M.M., Popkin B.M. Recent Underweight and Overweight Trends by Rural–Urban Residence among Women in Low- and Middle-Income Countries. J. Nutr. 2014;145:352–357. doi: 10.3945/jn.114.203562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poobalan A., Aucott L. Obesity among Young Adults in Developing Countries: A Systematic Overview. Curr. Obes. Rep. 2016;5:2–13. doi: 10.1007/s13679-016-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popkin B.M., Slining M.M. New dynamics in global obesity facing low- and middle-income countries. Obes. Rev. 2013;14:11–20. doi: 10.1111/obr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 7.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., De Onis M., Ezzati M., Grantham-McGregor S., Katz J., Martorell R., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Health Research and Development . The Main Results of Riskesdas 2018. Ministry of Health; Jakarta, Indonesia: 2018. [Google Scholar]
- 10.National Institute of Health Research and Development . Riset Kesehatan Dasar 2013. Ministry of Health; Jakarta, Indonesia: 2013. [Google Scholar]
- 11.Oddo V.M., Mueller N.T., Pollack K.M., Surkan P.J., Bleich S.N., Jones-Smith J.C. Maternal employment and childhood overweight in low- and middle-income countries. Public Health Nutr. 2017;20:2523–2536. doi: 10.1017/S1368980017001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiuchi Y., Kusama K., Kanha S., Yoshiike N., FIDR Research and the FIDR Research Team Urban-Rural Differences in Nutritional Status and Dietary Intakes of School-Aged Children in Cambodia. Nutrients. 2018;11:14. doi: 10.3390/nu11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Nguyen B.K., Le Thi H., Thuy N.T., Huu C.N., Do T.T., Deurenberg P., Khouw I. Double burden of undernutrition and overnutrition in Vietnam in 2011: Results of the SEANUTS study in 0.5–11-year-old children. Br. J. Nutr. 2013;110:S45–S56. doi: 10.1017/S0007114513002080. [DOI] [PubMed] [Google Scholar]
- 14.Sandjaja S., Budiman B., Harahap H., Ernawati F., Soekatri M., Widodo Y., Sumedi E., Rustan E., Sofia G., Syarief S.N., et al. Food consumption and nutritional and biochemical status of 0.5-12-year-old Indonesian children: the SEANUTS study. Br. J. Nutr. 2013;110:S11–S20. doi: 10.1017/S0007114513002109. [DOI] [PubMed] [Google Scholar]
- 15.Jia P., Li M., Xue H., Lu L., Xu F., Wang Y. School environment and policies, child eating behavior and overweight/obesity in urban China: The childhood obesity study in China megacities. Int. J. Obes. 2017;41:813–819. doi: 10.1038/ijo.2017.2. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez V.R., Gonzalez R.K., Correa B.J.E., Meneses E.J.F., Martinez T.J. Demographic and socioeconomic differences in consumption of sugar-sweetened beverages among Colombian children and adolescents. Nutr. Hosp. 2015;31:2479–2486. doi: 10.3305/nh.2015.31.6.8986. [DOI] [PubMed] [Google Scholar]
- 17.Wang H., Liu C., Fan H., Tian X. Rising food accessibility contributed to the increasing dietary diversity in rural and urban China. Asia Pac. J. Clin. Nutr. 2017;26:738–747. doi: 10.6133/apjcn.052016.03. [DOI] [PubMed] [Google Scholar]
- 18.Kerry S.M., Emmett L., Micah F.B., Martin-Peprah R., Antwi S., Phillips R.O., Plange-Rhule J., Eastwood J.B., Cappuccio F.P. Rural and semi-urban differences in salt intake, and its dietary sources, in Ashanti, West Africa. Ethn. Dis. 2005;15:33–39. [PubMed] [Google Scholar]
- 19.National Institute of Health Research and Development . Indonesia Health Profile 2013. Ministry of Health; Jakarta, Indonesia: 2013. [Google Scholar]
- 20.Rojroongwasinkul N., Kijboonchoo K., Wimonpeerapattana W., Purttiponthanee S., Yamborisut U., Boonpraderm A., Kunapan P., Thasanasuwan W., Khouw I. SEANUTS: The nutritional status and dietary intakes of 0.5–12-year-old Thai children. Br. J. Nutr. 2013;110:S36–S44. doi: 10.1017/S0007114513002110. [DOI] [PubMed] [Google Scholar]
- 21.Poh B.K., Ng B.K., Haslinda M.D., Shanita S.N., Wong J.E., Budin S.B., Ruzita A.T., Ng L.O., Khouw I., Norimah A.K. Nutritional status and dietary intakes of children aged 6 months to 12 years: Findings of the Nutrition Survey of Malaysian Children (SEANUTS Malaysia) Br. J. Nutr. 2013;110:S21–S35. doi: 10.1017/S0007114513002092. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Lobstein T. Worldwide trends in childhood overweight and obesity. Pediatr. Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui S.T., Kandala N.B., Stranges S. Urbanisation and geographic variation of overweight and obesity in India: A cross-sectional analysis of the Indian Demographic Health Survey 2005–2006. Int. J. Public Health. 2015;60:717–726. doi: 10.1007/s00038-015-0720-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen M., Liu W., Tao X. Evolution and assessment on China’s urbanization 1960–2010: Under-urbanization or over-urbanization? Habitat Int. 2013;38:25–33. doi: 10.1016/j.habitatint.2012.09.007. [DOI] [Google Scholar]
- 25.Dong Y., Ma Y., Dong B., Zou Z., Hu P., Wang Z., Yang Y., Song Y., Ma J. Geographical variation and urban-rural disparity of overweight and obesity in Chinese school-aged children between 2010 and 2014: Two successive national cross-sectional surveys. BMJ Open. 2019;9:e025559. doi: 10.1136/bmjopen-2018-025559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbanya J.C., Unwin N.C., Minkoulou E.M., Tournoux C., Sobngwi E., Porcher R., Kengne A.P., Fezeu L., Gautier J.F., Aspray T.J., et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int. J. Epidemiol. 2004;33:769–776. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- 27.Peters R., Amugsi D.A., Mberu B., Ensor T., Hill A.J., Newell J.N., Elsey H. Nutrition transition, overweight and obesity among rural-to-urban migrant women in Kenya. Public Health Nutr. 2019;22:3200–3210. doi: 10.1017/S1368980019001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobstein T., Baur L., Uauy R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004;5:4–85. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 29.Sallis J.F., Cerin E., Conway T.L., Adams M.A., Frank L.D., Pratt M., Salvo D., Schipperijn J., Smith G., Cain K.L., et al. Physical activity in relation to urban environments in 14 cities worldwide: A cross-sectional study. Lancet. 2016;387:2207–2217. doi: 10.1016/S0140-6736(15)01284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder H., Bawaked R.A., Ribas-Barba L., Izquierdo-Pulido M., Roman-Viñas B., Fíto M., Serra-Majem L. Cumulative Effect of Obesogenic Behaviours on Adiposity in Spanish Children and Adolescents. Obes. Facts. 2017;10:584–596. doi: 10.1159/000480403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amanzadeh B., Sokal-Gutierrez K., Barker J.C. An interpretive study of food, snack and beverage advertisements in rural and urban El Salvador. BMC Public Health. 2015;15:521. doi: 10.1186/s12889-015-1836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welk G.J., Calabro M.A., Russell D.W., Nicklay E., Hensley L.D., Joens-Matre R.R. Rural–Urban Differences in Physical Activity, Physical Fitness, and Overweight Prevalence of Children. J. Rural. Health. 2008;24:49–54. doi: 10.1111/j.1748-0361.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 33.Fisher M., Juszczak L., Friedman S.B. Sports participation in an urban high school: Academic and psychologic correlates. J. Adolesc. Health. 1996;18:329–334. doi: 10.1016/1054-139X(95)00067-3. [DOI] [PubMed] [Google Scholar]
- 34.Reimers A.K., Wagner M., Alvanides S., Steinmayr A., Reiner M., Schmidt S., Wöll A. Proximity to Sports Facilities and Sports Participation for Adolescents in Germany. PLoS ONE. 2014;9:e93059. doi: 10.1371/journal.pone.0093059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Hecke L., Deforche B., Van Dyck D., De Bourdeaudhuij I., Veitch J., Van Cauwenberg J. Social and Physical Environmental Factors Influencing Adolescents’ Physical Activity in Urban Public Open Spaces: A Qualitative Study Using Walk-Along Interviews. PLoS ONE. 2016;11:e0155686. doi: 10.1371/journal.pone.0155686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado-Rodrigues A.M., Coelho-E-Silva M.J., Mota J., Padez C., Martins R.A., Cumming S.P., Riddoch C., Malina R.M. Urban-rural contrasts in fitness, physical activity, and sedentary behaviour in adolescents. Health Promot. Int. 2012;29:118–129. doi: 10.1093/heapro/das054. [DOI] [PubMed] [Google Scholar]
- 37.Jensen B.W., Nichols M., Allender S., de Silva-Sanigorski A., Millar L., Kremer P., Lacy K., Swinburn B. Consumption patterns of sweet drinks in a population of Australian children and adolescents (2003–2008) BMC Public Health. 2012;12:771. doi: 10.1186/1471-2458-12-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S., McKee M., Galea G., Stuckler D. Relationship of Soft Drink Consumption to Global Overweight, Obesity, and Diabetes: A Cross-National Analysis of 75 Countries. Am. J. Public Health. 2013;103:2071–2077. doi: 10.2105/AJPH.2012.300974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik V.S., Schulze M.B., Hu F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider M.B., Benjamin H.J., Bhatia J.J., Abrams S.A., De Ferranti S.D., Silverstein J., Stettler N., Thomas D.W., Daniels S.R., Greer F.R., et al. Sports drinks and energy drinks for children and adolescents: Are they appropriate? Pediatrics. 2011;127:1182–1189. doi: 10.1542/peds.2011-0965. [DOI] [PubMed] [Google Scholar]
- 41.Alsunni A.A. Energy Drink Consumption: Beneficial and Adverse Health Effects. Int. J. Health Sci. 2015;9:468–474. doi: 10.12816/0031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rush E., Schulz S., Obolonkin V., Simmons D., Plank L. Are energy drinks contributing to the obesity epidemic? Asia Pac. J. Clin. Nutr. 2006;15:242–244. [PubMed] [Google Scholar]
- 43.Vartanian L.R., Schwartz M.B., Brownell K.D. Effects of Soft Drink Consumption on Nutrition and Health: A Systematic Review and Meta-Analysis. Am. J. Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keast R.S.J., Swinburn B.A., Sayompark D., Whitelock S., Riddell L.J. Caffeine increases sugar-sweetened beverage consumption in a free-living population: A randomised controlled trial. Br. J. Nutr. 2015;113:366–371. doi: 10.1017/S000711451400378X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taillie L.S., Afeiche M.C., Eldridge A.L., Popkin B.M. Increased Snacking and Eating Occasions Are Associated with Higher Energy Intake among Mexican Children Aged 2–13 Years. J. Nutr. 2015;145:2570–2577. doi: 10.3945/jn.115.213165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi Y., Lee J.E., Chang Y., Kim M.K., Sung E., Shin H., Ryu S. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br. J. Nutr. 2016;116:1447–1456. doi: 10.1017/S0007114516003391. [DOI] [PubMed] [Google Scholar]
- 47.Lanaspa M.A., Kuwabara M., Andres-Hernando A., Li N., Cicerchi C., Jensen T., Orlicky D.J., Roncal-Jimenez C.A., Ishimoto T., Nakagawa T., et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA. 2018;115:3138–3143. doi: 10.1073/pnas.1713837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson N.I., Miller J.M., Watts A.W., Story M.T., Neumark-Sztainer D.R. Adolescent Snacking Behaviors Are Associated with Dietary Intake and Weight Status. J. Nutr. 2016;146:1348–1355. doi: 10.3945/jn.116.230334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vergnaud A.C., Norat T., Romaguera D., Mouw T., May A.M., Travier N., Luan J., Wareham N., Slimani N., Rinaldi S., et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am. J. Clin. Nutr. 2010;92:398–407. doi: 10.3945/ajcn.2009.28713. [DOI] [PubMed] [Google Scholar]
- 50.Wagemakers J.J., Prynne C.J., Stephen A.M., Wadsworth M.E. Consumption of red or processed meat does not predict risk factors for coronary heart disease; results from a cohort of British adults in 1989 and 1999. Eur. J. Clin. Nutr. 2009;63:303–311. doi: 10.1038/sj.ejcn.1602954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esmaillzadeh A., Azadbakht L. Major Dietary Patterns in Relation to General Obesity and Central Adiposity among Iranian Women. J. Nutr. 2008;138:358–363. doi: 10.1093/jn/138.2.358. [DOI] [PubMed] [Google Scholar]
- 52.Schulze M.B., Hu F.B. Dietary patterns and risk of hypertension, type 2 diabetes mellitus, and coronary heart disease. Curr. Atheroscler. Rep. 2002;4:462–467. doi: 10.1007/s11883-002-0051-1. [DOI] [PubMed] [Google Scholar]
- 53.Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Schulze M.B., Manson J.E., Willett W.C., Hu F.B. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia. 2003;46:1465–1473. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- 55.Xu F., Yin X.M., Tong S.L. Association between excess bodyweight and intake of red meat and vegetables among urban and rural adult Chinese in Nanjing, China. Asia Pac. J. Public Health. 2007;19:3–9. doi: 10.1177/101053950701900302. [DOI] [PubMed] [Google Scholar]
- 56.Rouhani M.H., Mirseifinezhad M., Omrani N., Esmaillzadeh A., Azadbakht L. Fast Food Consumption, Quality of Diet, and Obesity among Isfahanian Adolescent Girls. J. Obes. 2012;2012:1–8. doi: 10.1155/2012/597924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen A.K., Rai M., Rehkopf D.H., Abrams B. Educational attainment and obesity: A systematic review. Obes. Rev. 2013;14:989–1005. doi: 10.1111/obr.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajian-Tilaki K.O., Heidari B. Association of educational level with risk of obesity and abdominal obesity in Iranian adults. J. Public Health. 2009;32:202–209. doi: 10.1093/pubmed/fdp083. [DOI] [PubMed] [Google Scholar]
- 60.Al Yateem N., Rossiter R. Nutritional knowledge and habits of adolescents aged 9 to 13 years in Sharjah, United Arab Emirates: A crosssectional study. East. Mediterr. Health J. 2017;23:551–558. doi: 10.26719/2017.23.8.551. [DOI] [PubMed] [Google Scholar]
- 61.Trichesa R.M., Giuglianib E.R.J. Obesity, eating habits and nutritional knowledge among school children. Rev. Saude Publica. 2005;39:541. doi: 10.1590/s0034-89102005000400004. [DOI] [PubMed] [Google Scholar]
- 62.Sigmund E., Sigmundová D., Badura P., Gecková A.M. Health-related parental indicators and their association with healthy weight and overweight/obese children’s physical activity. BMC Public Health. 2018;18:676. doi: 10.1186/s12889-018-5582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson E., Sutin A.R. Parents’ Perceptions of Their Children as Overweight and Children’s Weight Concerns and Weight Gain. Psychol. Sci. 2017;28:320–329. doi: 10.1177/0956797616682027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.