Abstract
Background: Distant metastasis is a rare occurrence in thyroid cancer, and it can be associated with poor prognosis. The genomic repertoires of various solid malignancies have previously been reported but remain underexplored in metastatic papillary thyroid cancer (PTC). Furthermore, whether distant metastases harbor distinct genetic alterations beyond those observed in primary tumors is unknown.
Methods: We performed whole-exome sequencing on 14 matched distant metastases, primary PTC tumors, and normal tissues. Point mutations, copy number alterations, cancer cell fractions, and mutational signatures were defined using the state-of-the-art bioinformatics methods. All likely deleterious variants were validated by orthogonal methods.
Results: Genomic differences were observed between primary and distant metastatic deposits, with a median of 62% (range 21–92%) of somatic mutations detected in metastatic tissues, but absent from the corresponding primary tumor sample. Mutations in known driver genes including BRAF, NRAS, and HRAS were shared and preferentially clonal in both sites. However, likely deleterious variants affecting DNA methylation and transcriptional repression signaling genes including SIN3A, RBBP1, and CHD4 were found to be restricted in the metastatic lesions. Moreover, a mutational signature shift was observed between the mutations that are specific or enriched in the metastatic and primary lesions.
Conclusions: Primary PTC and distant metastases differ in their range of somatic alterations. Genomic analysis of distant metastases provides an opportunity to identify potentially clinically informative alterations not detected in primary tumors, which might influence decisions for personalized therapy in PTC patients with distant metastasis.
Keywords: exome sequencing, papillary thyroid cancer, distant metastasis, DNA methylation
Introduction
Global incidence rates of thyroid cancer have rapidly increased in recent years (1). In Saudi Arabia, thyroid cancer accounts for 11.5% of all cancers and is the second most common cancer affecting Saudi females after breast cancer (2). Among different histological subtypes of thyroid cancer, papillary thyroid cancer (PTC) is the most common cancer, accounting for up to 80% of all newly diagnosed thyroid carcinoma cases (3).
Although metastasis to regional or local lymph nodes is common, distant metastasis is a less frequent event and occurs in less than 6% of patients (4–7). Furthermore, despite the usually favorable prognosis of PTC following appropriate treatment (8,9), distant metastasis is the most common cause of thyroid cancer-related mortality (10,11). Surgery and radioiodine therapy are the major treatment modalities in patients with 131I uptake (12,13), including patients demonstrating progressive disease and distant metastasis. Therefore, identifying new molecular targets and proposing new treatment modalities are clearly needed for patients with distant metastasis. There is a considerable lack of molecular profiling in metastatic PTC, where focus has typically been limited to primary thyroid lesions (14–16).
Exome sequencing of solid tumors has been previously used to investigate the clonal relationship between primary tumors and metastasis in several cancers, including breast, colorectal, endometrial, urothelial, and hepatocellular carcinomas (17–25), but has yet to be explored in thyroid cancer. Comparing distant metastasis with their corresponding primary tumor from PTC patients could uncover differences in their somatic genetic alterations. This can be relevant to a precision medicine approach in the clinical management of PTC patients with metastasis.
In this study, we undertook genomic sequence analysis of primary PTC and their distant metastatic deposits (lung, bone, kidney, and brain), in patients who presented with metastatic disease that was present at initial diagnosis before therapy, or metastases that developed after receiving initial therapy. Our aim was to determine differences in the repertoire of somatic mutations, copy number alterations (CNAs), and mutational signatures between primary PTC and their distant metastases.
Methods
Clinical samples
We performed whole-exome sequencing (WES) on 42 trios of patient-matched distant metastases, primary tumors, and normal samples, collected during the course of clinical care (e.g., for diagnosis, symptoms control, or restaging) from 14 patients at the King Faisal Specialist Hospital and Research Centre from the Department of Pathology. Nine of these patients presented with metastasis at initial diagnosis before therapy (cases: 1, 3, 4, 6, 7, 8, 10, 12, and 14), while five (cases: 2, 5, 9, 11, and 13) developed distant metastasis after a median of 37 months post-therapy (surgery followed by radioactive iodine). The clinicopathological details of these patients are summarized in Supplementary Table S1. This study was approved by the Research Advisory Council (RAC) of the hospital, under project RAC No. 2110 031.
DNA extraction and WES
DNA was extracted from formalin-fixed paraffin-embedded tissue blocks by performing punches in the corresponding tissue areas and extraction with the Gentra DNA isolation kit (Gentra, Minneapolis, MN) following the manufacturer's recommendations. WES was executed using SureSelectXT Target Enrichment (Agilent) on Illumina NovaSeq 6000. Sequencing reads were aligned to the human reference genome hg19 using Burrows-Wheeler Aligner v0.7.15 (26) algorithm with default settings, followed by local realignment and polymerase chain reaction duplicate marking via Picard tools (v1.119). Base-quality recalibration and calculation of coverage metrics were performed with GATK v3.8.0 (27). All cases passed internal quality control and quality matrix. By means of tumor-normal bam files, somatic single nucleotide variations (SNVs) were identified using MuTect v1.1.7 (28), while somatic small insertions and deletions (indels) were identified using VarScan v2.3.9 (29). Identified variants were annotated using ANNOVAR (30). In addition to passing the standard MuTect and VarScan filters, variants presenting with the following features were excluded: common single nucleotide polymorphisms (SNPs) with a minor allele frequency of >0.05, as documented in dbSNP, the National Heart, Lung, and Blood Institute exome sequencing project, 1000 Genomes, and in our in-house data from exome sequencing of ∼700 normal samples; noncoding region variants; and variants in repetitive regions. Mutations were also manually checked using the Integrated Genomics Viewer v2.4.10 to filter out false positives. All potentially deleterious variants were validated by Sanger sequencing as previously described (31).
Gene copy number profiling and cancer cell fraction
FACETS v0.5.13 (32) was used to determine CNAs and regions of loss of heterozygosity (LOH), while outputs generated by MuTect and VarScan were utilized to calculate mean allelic frequency. CNA and SNV data were analyzed by ABSOLUTE v1.0.6 (33) to define the integer copy number, tumor purity, and cancer cell fractions (CCFs). CNAs were further defined as gains, amplifications, or homozygous deletions, in relation to the average ploidy of all samples from a given patient using the modal copy number for each segment from ABSOLUTE. Copy number segments with a modal copy number greater than the average sample ploidy +1 or average sample ploidy +3 were considered gains and amplifications, respectively; while copy number segments with a modal copy number less than the average ploidy −1 were classified as losses, and modal copy numbers of 0 as homozygous deletions. CCF estimates for amplifications were disregarded as it increases exponentially with the number of absolute copy numbers.
Defining pathogenicity
To delineate the potential functional effect of the generated missense SNVs, different algorithms were used such as MutationTaster (34), CHASM (35), and FATHMM (36) for variant classification. Missense variants classified as nondeleterious by MutationTaster and CHASM (thyroid) were considered likely passengers. The missense variants predicted to be “driver” and/or “cancer” by CHASM (thyroid) and/or FATHMM, respectively, were classified as likely pathogenic. Neutral inframe indels established using MutationTaster and PROVEAN (37) were considered likely passengers. Inframe and frameshift indels, splice site, and nonsense mutations were considered likely pathogenic if these mutations were associated with loss of wild-type allele (i.e., LOH) or affected haploinsufficiency genes (38). SNVs occurring in hot spot residues (39) were also considered likely pathogenic. Cancer genes were annotated based on previously published studies (40–42). All mutations not defined as potentially pathogenic or likely passenger mutations were classified as indeterminate pathogenic.
Classification and enrichment of mutations in primary and metastatic lesions
Mutations specific to metastatic lesions were defined as those present in metastatic biopsy, but absent in the corresponding primary tumor, whereas mutations enriched in the metastatic lesion were defined as those exhibiting a minimum 20% increase in metastatic CCF compared with the primary tumor. The opposite was applied for mutations specific to and enriched in the primary tumor.
Analysis of pathways associated with the metastatic process
Prospective pathways associated with the metastatic process were identified using Ingenuity Pathway Analysis (IPA) and g:Profiler (43)—by analyzing genes with likely pathogenic mutations specific to and/or enriched in the metastatic lesion, including those associated with LOH in metastasis but not associated with LOH in the matched primary tumor.
Mutational signatures
Mutational signatures were predicted using the deconstructSigs package (44) and illustrated according to the 96 substitution classification defined by the substitution classes (C>A, C>G, C>T, T>A, T>C, and T > G bins). The signatures were compared with 21 mutational signatures previously reported in different cancer types (45).
Statistical analyses
All statistical analyses were executed in IBM SPSS Statistics (v.21). Mann–Whitney U and Fisher's exact tests were utilized to compare continuous and categorical variables, respectively. Spearman's rank correlation tests were used to determine associations. All statistical tests were two-tailed, where p < 0.05 was considered statistically significant.
Results
Genetic heterogeneity in primary PTC and distant metastatic tissue
All primary tumors were surgically resected before the administration of any therapeutic regimen, followed by either the resection of therapy-naive metastatic tumor or post-therapy metastatic tumor. Hence, “treatment-naive” and “post-therapy” only refers to metastatic tumors, where nine treatment-naive and five post-therapy PTC patients with their corresponding treatment-naive primary tumors of different histological subtypes were tested. We first sought to determine whether the repertoire of somatic genetic alterations in biopsies of primary tumors and their respective metastases (bone, n = 6; lung, n = 2; and brain, n = 1) in treatment-naive patients would differ from biopsies of primary tumors and their respective metastases (bone, n = 3; kidney, n = 1; and lung, n = 1) after receiving systemic 131I therapy. WES was performed to a median depth of 169x (range 147x–198x) in primary biopsies, 167x (range 143x–185x) in metastases, and 173x (range 139x–517x) in matched normal tissues. The analysis revealed the presence of 356 primary and 351 metastatic somatic mutations. Interestingly, the mutation burden showed no difference between treatment-naive and post-therapy biopsies with the median number of mutations being 24.5 (range 3–49) in naive and 24.5 (range 10–61) in post-therapy samples (p = 0.724) (Fig. 1A). However, we observed a difference in hotspot mitogen-activated protein (MAP)-kinase mutations between treatment-naive and post-treatment metastases. A higher proportion of NRAS-mutant cases were observed with 55.5% found in treatment-naive metastatic tumors compared with only 20% found in post-treatment metastatic tumors. BRAF mutations were observed only in post-therapy metastases (14%). No significant association was noted between number of mutations and clinicopathological parameters such as age, stage, or histological subtype (Supplementary Table S2).
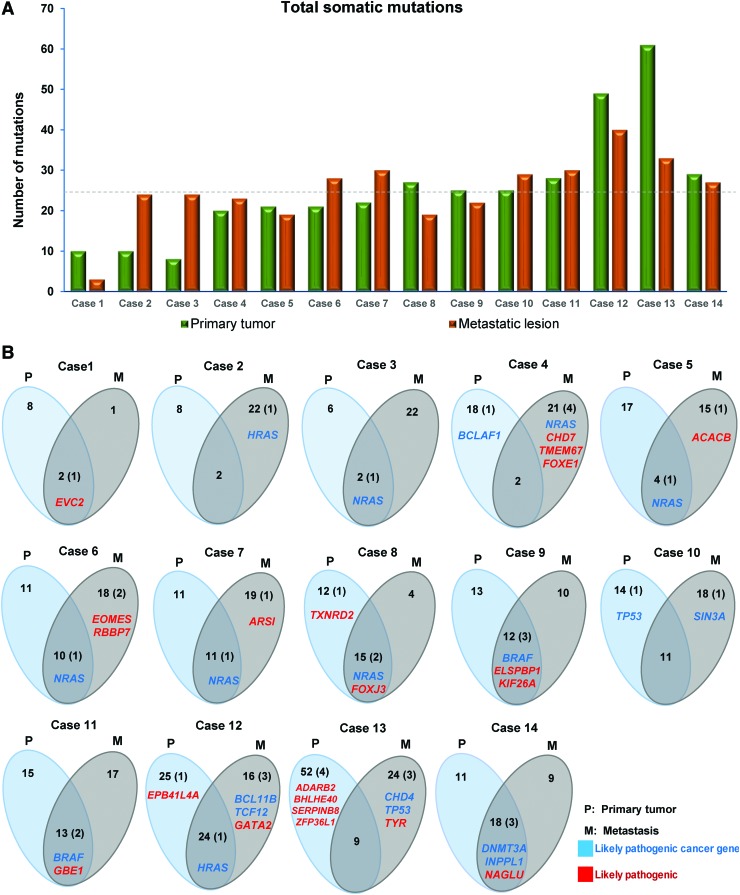
FIG. 1.
Heterogeneity and somatic mutations specific to primary, metastatic tumors, and shared between both lesions. (A) Number of somatic mutations identified in primary tumors and metastatic lesions. The dashed line shows a shared median of 24.5 mutations in both the primary tumors and the metastatic lesions. (B) Venn diagrams demonstrate the number of somatic mutations and likely pathogenic mutations specific to the primary tumor, metastases, and mutations present in both lesions. The number of likely pathogenic mutations is shown in parenthesis. Labeled genes with likely pathogenic mutations in noncancer genes are colored red, while those affecting cancer genes are colored blue. Color images are available online.
Mutations with a high likelihood of being pathogenic (31 mutations in primary and 36 in metastasis) were validated by Sanger sequencing, confirming 24 (77.4%) and 32 (89%) mutations in primary and metastatic deposits, respectively. We compared the range of mutations detected in the primary and metastatic lesions from each patient, which revealed a median of 41% (range 8–79%) of somatic mutations in common (Fig. 1B). Importantly, however, noticeable heterogeneity was observed with a median of 55% (38–90%) and a median of 62% (21–92%) of mutations restricted to the primary tumor and the metastasis, respectively (Fig. 1B). Furthermore, a median of 39% (range 6–61%) and 31% (range 5–50%) variant allele frequency (VAF) was observed for the somatic mutations found in the primary and metastasis, respectively (Supplementary Fig. S1).
Distinct repertoire of genetic alterations in primary PTC and metastatic tissue
Previous genomic landscaping studies, including The Cancer Genome Atlas (TCGA), have only identified hotspot mutations or cancer genes in primary PTC tumors, but not in metastatic tissues. Therefore, our study focused on analyzing biopsies of primary PTC and distant metastases to compare the array of somatic mutations. Previously, TERT promoter mutations, in isolation and/or in combination with other driver mutations such as BRAFV600E, have been shown to play an important role in the development of a more aggressive phenotype and the development of metastases (46–48). The limitation of our exome sequencing approach limits the analyses to exonic mutations, not covering intronic or other regions. This prompted us to complement our study by analyzing the samples for the presence of TERT promoter mutations via Sanger sequencing in both primary PTC and their corresponding metastatic tumors. We found 50% of primary and 64.3% of metastatic tissues harboring a TERT 228C>T mutation (p = 0.45428), of which 22.2% of metastases also harbored BRAF mutations (p = 0.7764). The absence of significant difference in TERT promoter mutations between primary tumor and metastasis may be due to the limited cohort involved in this study to highlight the role of TERT mutations, which has been previously identified in large studies on advanced thyroid carcinomas (46–49).
We identified a median of 2 (range 0–4) likely pathogenic mutations per case (primary and/or metastasis), including a median of 1 (range 0–3) likely pathogenic mutation in cancer genes (Fig. 2 and Supplementary Fig. S2). Ten cases harbored a single hotspot mutation each, eight of which were shared between primary and metastasis, namely NRASQ61R (five cases), BRAFV600E (two cases), and HRASQ61R (one case). Moreover, NRAS and HRASQ61K mutations were found restricted to two cases of metastatic PTC. Similarly, TP53R342* mutation was found restricted in a single case of primary PTC, whereasTP53R248W was found restricted in a single case of metastatic PTC (Supplementary Table S3). Driver mutations tend to be clonal, that is, present in virtually 100% tumor cells (50,51). Among all likely pathogenic mutations affecting cancer genes, 20/29 (69%) were found in biopsies of both the primary tumor and the metastases, of which 16/29 (55%) were clonal in both (Fig. 3A, B). All BRAF (n = 2), HRAS (n = 1), and three cases of NRAS mutations were clonal in both biopsies. We also identified clonal mutations that are likely pathogenic, affecting the cancer genes DNMT3A and INPPL1 in a single pair of a primary tumor with its corresponding metastasis (Supplementary Table S3).
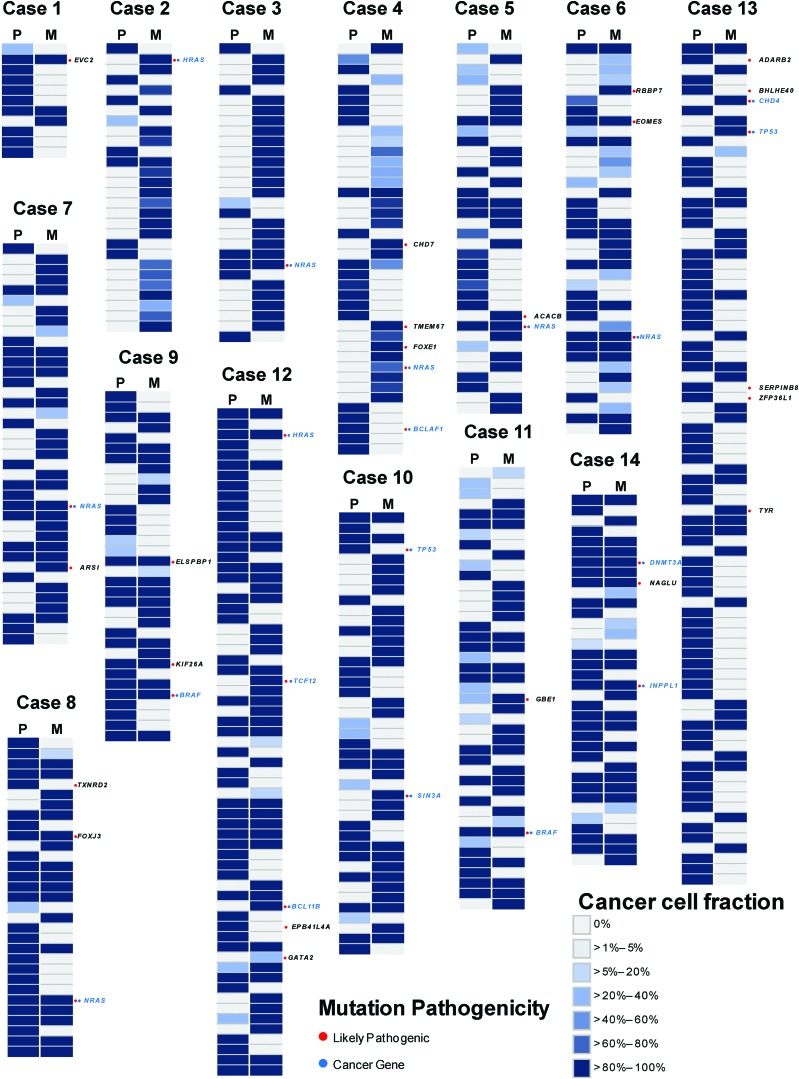
FIG. 2.
Genetic repertoire of somatic mutations in primary and metastatic lesions. Heat maps show the CCF of somatic mutations demonstrated by the ABSOLUTE algorithm with their presence (blue, see color key for the CCF percentage) or absence (light gray) in the primary and metastatic deposits. Likely pathogenic mutations are indicated by red circles, and the affected genes are shown for each lesion. Likely pathogenic mutations affecting cancer genes are indicated by blue circles in each lesion, labeled in blue. CCF, cancer cell fraction. Color images are available online.
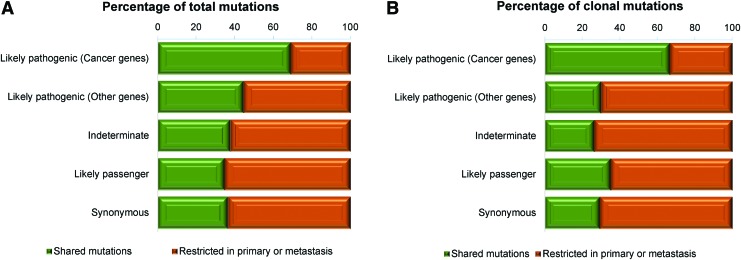
FIG. 3.
Clonality in primary and metastatic lesions of papillary thyroid carcinoma. (A) Figure showing the distribution of mutations shared in the category of likely pathogenic (cancer genes), likely pathogenic (other genes), indeterminate pathogenicity, likely passenger, and synonymous mutations between primary and metastatic lesions. (B) Figure displaying the distribution of clonal mutations shared in the category of likely pathogenic (cancer genes), likely pathogenic (other genes), indeterminate pathogenicity, likely passenger, and synonymous mutations between primary and metastatic lesions. Color images are available online.
Despite limited heterogeneity in the repertoire of hotspot mutations or mutations affecting known cancer genes between primary tumors and their respective metastases, two cases presented likely pathogenic mutations affecting cancer genes restricted to metastatic lesions, namely CHD4 (case 13) and SIN3A (case 10)—neither of which was associated with LOH (Fig. 2). Notably, these mutations were not identified in the primary tumors even with a second anatomically distinct biopsy (Supplementary Table S4).
Concurring with the hypothesis that heterogeneity would preferentially affect passenger alterations (52), we found that mutations of indeterminate pathogenicity, likely passenger and synonymous mutations, were less frequently shared between primary and metastasis comparing to the likely pathogenic mutations in cancer genes (37.6%, 34.7%, and 36.4% vs. 69% and p = 0.0010, p = 0.0006, and p = 0.0008, respectively) (Fisher's exact test; Fig. 3A, B). Interestingly, the likely pathogenic mutations (affecting a cancer gene) were more frequently synchronously present or clonal in biopsies of both lesions than indeterminate/likely passenger/synonymous mutations (69% vs. 36.5% and 55.2% vs. 21%, p = 0.0004 and p < 0.0001, respectively) (Fisher's exact test; Fig. 3A, B).
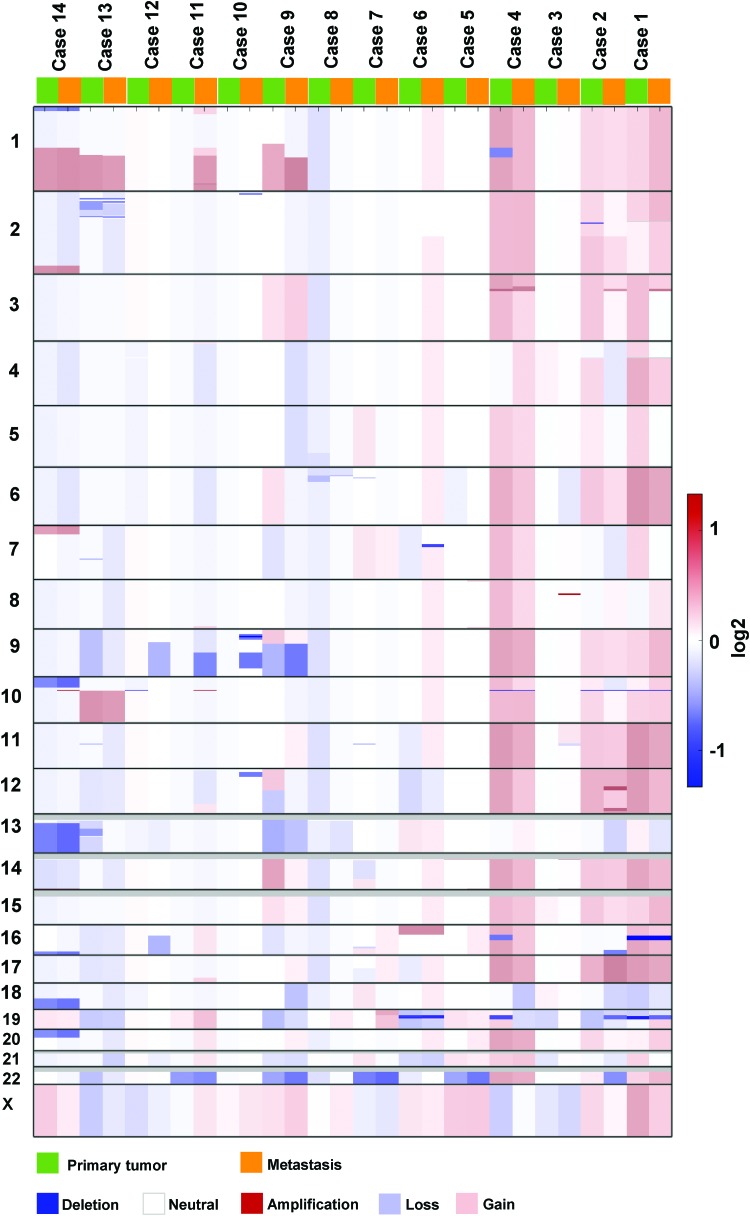
Copy number analysis indicated sharing of CNAs in 42% (22/52) of focal amplifications and homozygous deletions in both lesions, including amplifications affecting AKT1 (case 14), CARD11 (case 14), and BAP1 (case 4). A biallelic deletion of the SHCBP1 gene was observed in a single case (case 1); it was shared between the primary tumor and its metastasis (Fig. 4, Supplementary Fig. S3, and Supplementary Table S5). Noticeable differences between the primary tumor and the metastasis were observed with 7.4% (2/27) and 55.6% (15/27) amplifications, and 36% (9/25) and 16% (4/25) biallelic deletions specific for the primary and metastatic lesions, respectively (Supplementary Table S6).
FIG. 4.
CNAs in primary and metastatic deposits. Figure demonstrates CNAs between primary and metastatic lesions highlighted with different color keys (blue: deletion, light blue: loss; white: neutral; light red: gain; red: amplification). CNAs, copy number alterations. Color images are available online.
Pathway analysis of genes restricted to metastases
In seven cases, likely pathogenic mutations were found restricted to the metastasis, affecting the ACACB, CHD4, EOMES, FOXE1, GATA2, HRAS, NRAS, RBBP7, SIN3A, TMEM67, TP53, and TYR genes (Supplementary Table S4). The mutations in these genes were absent in second anatomically distinct biopsies. These genes were investigated for the potential association with specific biological pathways. Using IPA, genes with likely pathogenic mutations specific to or enriched in metastasis demonstrated that DNA Methylation Pathway genes were significantly associated with the metastatic process (p < 0.0001; Fig. 5 and Supplementary Table S7). Genes restricted to the DNA Methylation Pathway were found in three cases, including CHD4 (case 13), SIN3A (case 10), and RBBP7 (case 6), all of which were completely absent in the two distinct primary biopsies. A further three genes (NRAS, HRAS, and TP53), showing greater than 1% frequency in primary thyroid cancer in TCGA, were filtered out, and the remaining nine genes were analyzed for pathway association. Remarkably, DNA Methylation Pathway remained the prime candidate in both IPA and g:Profiler (p < 0.0001 and 0.0269).
FIG. 5.
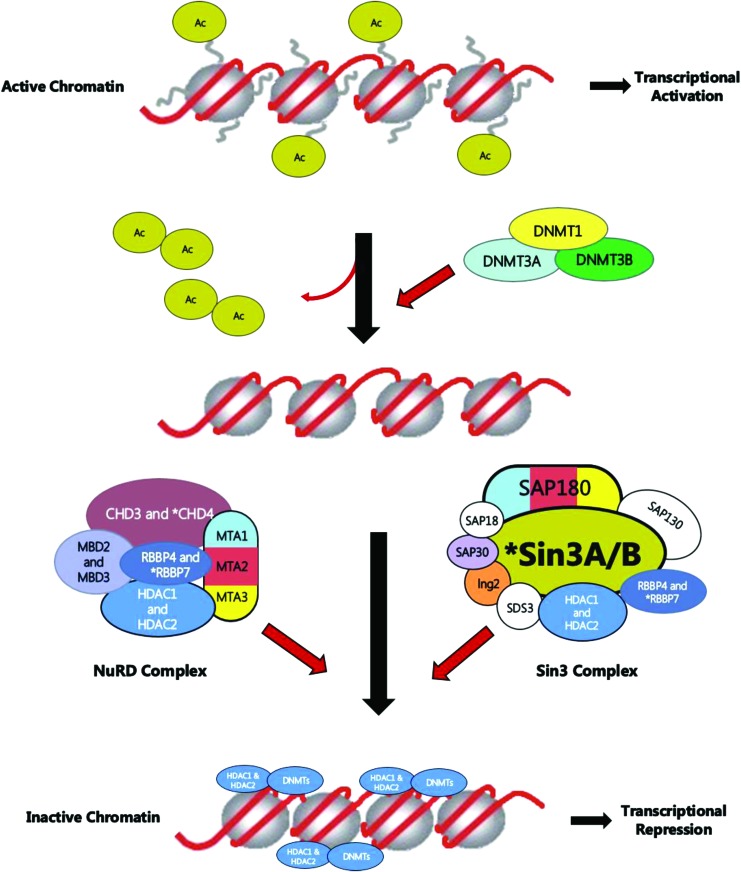
Likely pathogenic mutations restricted in the metastases affect DNA methylation and transcriptional repression signaling pathway. Figure demonstrating the mechanism of genes involved in DNA methylation and repression signaling. The asterisk indicates mutated genes restricted to metastasis in the CHD4 and RBBP7 genes of the NuRD complex and SIN3A of the Sin3 complex. Color images are available online.
Evolutionary dynamics of somatic mutations and CNAs
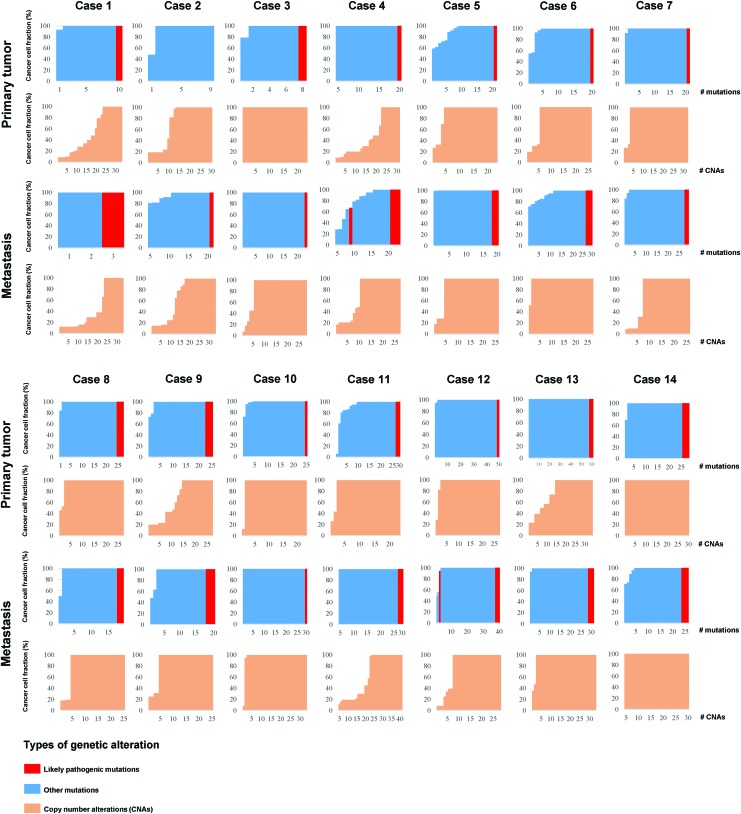
To explore evolutionary patterns of somatic SNVs and CNAs, the ABSOLUTE algorithm was employed to calculate the CCF of SNVs and CNAs. A median of 23% (range 4–60%) and 40% (range 0–65%) of subclonal SNVs were found in primary and metastatic tumors, respectively (Fig. 6 and Supplementary Fig. S4). Overall, no significant difference was observed in the mutation burden of subclonal mutations between primary and metastatic lesions (p = 0.265, Mann–Whitney U test), although we note that a nominally significant difference was observed in 12/14 cases (p = 0.039, Mann–Whitney U test). Very limited heterogeneity was observed for CNAs (excluding amplifications; see the Methods section), with a median of 87% (range 29–100%) and 83% (30–100%) found to be clonal in the primary and the metastatic tissues, respectively (Fig. 6). No significant difference or association was observed between the proportion of subclonal mutations or of subclonal CNAs within primary-metastasis pairs (all p > 0.05, Mann–Whitney U and Spearman's correlation tests). Furthermore, no significant difference or association was observed between the VAFs of primary and the metastatic samples (p > 0.05, Mann–Whitney U and Spearman's correlation tests). A proportion analysis of subclonal mutations and CNAs revealed no difference or correlation between different clinicopathological characteristics—such as treatment (naive and post-therapy), metastatic site biopsied (bone vs. lung), histological subtype (follicular vs. tall cell), age (<45 vs. ≥45 years), and sex (all p > 0.05, Mann–Whitney U and Spearman's correlation tests). These results suggest that the evolutionary patterns of SNVs and CNAs are distinct.
FIG. 6.
Dynamics of somatic mutations and CNAs. The figure shows the CCFs of the somatic mutations (red and blue) and of the CNAs (orange, excluding amplifications; see the Methods section). CCFs of the somatic mutations and the CNAs are sorted in increasing order. Color images are available online.
Mutational signature shift
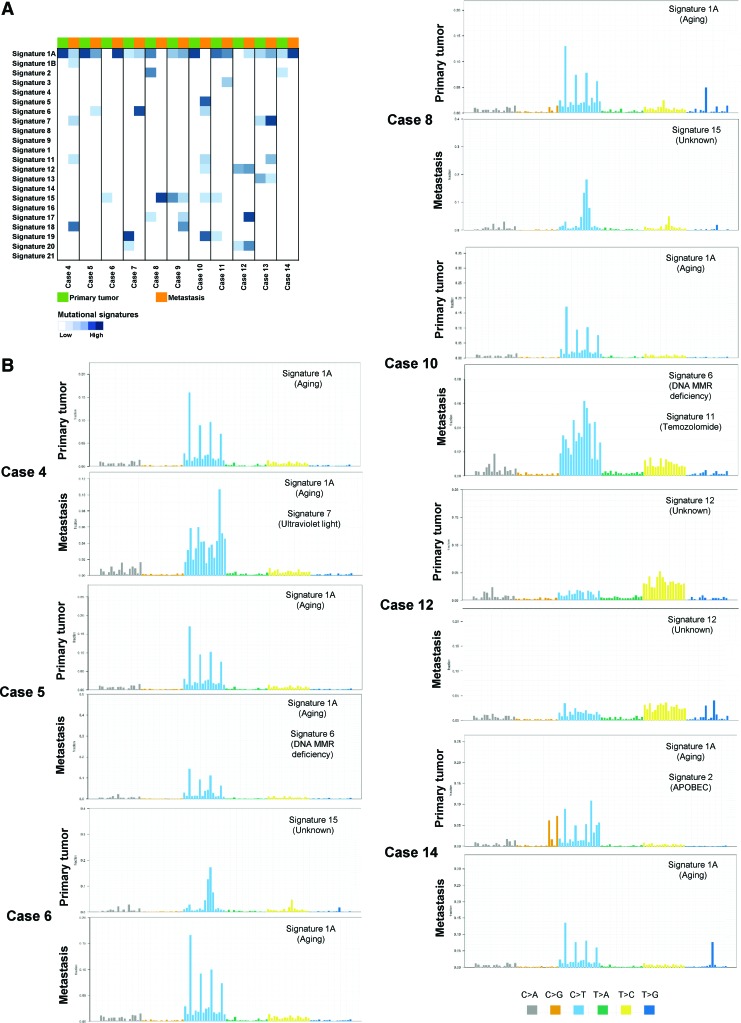
We compared the mutational signatures for mutations specific to or enriched in primary biopsies and mutations specific to or enriched in the metastatic tumors, to 21 mutational signatures previously reported in different cancer types (45), for 11 cases (3 cases were excluded from signature analysis due to insufficient number of mutations [<15] to perform this analysis). A shift was observed in the most dominant signature (signature 1a associated with aging) in eight cases. Signature 6 associated with DNA mismatch repair (MMR) deficiency and signature 11 associated with temozolomide were found specific to the metastatic process in five cases. Signature 15 was observed in two cases found specific to primary and metastatic lesions, respectively (Fig. 7 and Supplementary Fig. S5). Mutational signatures were not consistent, as signature shifts did not occur in any particular direction, with observed patterns varying between mutations specific to or enriched in the primary and mutations specific to or enriched in metastasis.
FIG. 7.
Mutational signatures in primary tumor and metastasis. (A) Mutational signatures of the tumor samples analyzed for the mutations enriched in the primary tumor (green) and mutations enriched in the metastatic lesions (orange). The similarity of each mutational signature is annotated according to the signature fraction and is indicated in blue according to the color key. (B) Plots demonstrating the mutational signatures of the mutations enriched in the primary tumor and the metastatic lesion of cases 4, 5, 6, 8, 10, 12, and 14. The figure shows each mutational signature according to the 96 substitution classification defined by the substitution classes (C>A, C>G, C>T, T>A, T>C, and T > G bins). Color images are available online.
Discussion
Precision medicine in cancer involves tailoring the best therapy based on specific genomic alterations in the patient's tumor (53). WES analysis of the primary tumor and their matched metastasis can potentially identify novel metastasis-associated mutations and pathways. Although PTC is a typically curable cancer with distant metastasis occurring in less than 6%, it is the most frequent cause of thyroid cancer-related deaths (6).
Previous studies have attempted to identify reliable markers for predicting the metastatic spreading of PTC (54,55); however, none of these studies were based on simultaneous analysis of paired primary PTCs and their metastases using WES. The comparison analysis with TCGA data (31) showed a median of 24.5 (range 8–62) mutations in our primary cases, while a median of 15 (range 2–87) mutations were found in TCGA data. We found a median of 2 (range 1–5) and 1 (range 1–7) likely pathogenic mutations in our data and TCGA, respectively. With respect to mutations in cancer genes, a median of 2 (range 1–5) and 1 (range 1–6) were found in our primary cases and TCGA, respectively. Known driver gene (BRAF, NRAS, HRAS, and TP53) alterations were observed in 64.3% in our primary PTC and 71.6% in TCGA. We present the first WES analysis of genetic changes in primary PTC and their distant metastases.
Striking heterogeneity was observed between biopsies of paired primary tumors and metastases, with a median of only 40.5% mutations shared between any two biopsies. We also demonstrate that pathogenic mutations affecting MAP kinase pathway genes were present in 57% of primary tumors and their respective metastases, with only two cases harboring metastasis-restricted MAP kinase mutations (HRASQ61K [n = 1] and NRASQ61K [n = 1]). However, likely pathogenic mutations in the ACACB, CHD4, EOMES, FOXE1, GATA2, RBBP7, SIN3A, TMEM67, TP53, and TYR genes were found only in the metastases that were present at initial diagnosis before therapy, despite a second anatomically distinct diagnostic biopsy of the respective primary tumors. Taken together, these findings suggest that while most of the divergence between metastasis and their corresponding primary PTC tumors is attributed to passenger mutations, a few mutational driver events may play a role in metastasis development and evolution. We have previously reported the role of somatic TG mutations in metastatic PTC tissues (31). de Biase et al. have shown that despite the clinical aggressiveness of distant metastatic PTCs, they tend to have a low mutational load, comparable to nonmetastatic PTCs. They also found frequent mutations in the MED12 and MET genes in metastatic PTCs (54). Other genes reported to play a role in metastatic behavior of PTCs include TERT, ATM, RAS, and RET/PTC (8,56–58). Gandolfi et al. in their study of metastatic PTC found that the THYT1 genetic signature, comprising of duplications in chromosomal regions 1q and 5p (harboring the TERT genomic locus), and TERT promoter mutations, was a highly specific marker of distant metastasis in PTCs (59).
We did not observe any specific pattern in terms of the number of mutations or mutational signature in the nine bone metastases or three lung metastases. Neither was there any significant association between the number of mutations and clinicopathological parameters, such as age, stage, or histological subtype. Furthermore, no significant difference was found in the number of mutations in therapy-naive metastases and post-therapy metastases. Interestingly, the lack of correlation among the repertoire of subclonal mutations and CNAs, between primary PTC and metastases, might indicate that somatic mutations and CNAs evolve independently. Several whole-exome studies have identified the increased association between mutational burden and aggressive clinical behavior in several solid tumors.
Previous genomic landscaping of PTC in the TCGA (60) has identified no correlation between mutation burden and any aggressive clinical features such as advanced stage, high-grade tumor, or aggressive histological subtypes. This is in concordance with our previous PTC landscape study (31) where the mutation burden had no effect on any clinical or pathological phenotype, which could be due to the quietness of this tumor and the overall low mutation burden involved in the tumorigenesis.
Importantly, we have identified likely pathogenic metastasis-restricted mutations such as CHD4, RBBP7, and SIN3A that affect DNA methylation and transcriptional repression signaling pathways, which raises the possibility of their involvement in tumor dissemination. The role of epigenetic deregulation in cancer has been well established (61–65); however, with the use of next-generation sequencing technologies, the underlying epigenetic mechanisms pertaining to dysfunction are becoming further evident. Indeed, several exome sequencing studies have demonstrated an important role of mutations in genes encoding epigenetic modifiers in many cancers such as renal cell carcinoma and adenoid cystic carcinoma (66,67). Many tumor suppressor genes are silenced through synergistic layers of epigenetic regulation including abnormal DNA hypermethylation of promoter CpG islands, repressive chromatin modifications, and enhanced nucleosome deposition over transcription start sites (68). Two main forms of chromatin are present in the human genome, either in a condensed transcriptionally inactive form known as heterochromatin or as the transcriptionally active version, that is, euchromatin. Nucleosomes, which are octametric structures composed of two types of histones (H3, H4) with 147 bp of DNA wrapped around them, are the functional units of chromatin (69). Chromatin remodeling and histone modification have emerged as the main mechanism of gene expression regulation. Transcriptionally active chromatin is associated with hyperacetylation of histones (70), while the chromatin inactive region is enriched in deacetylated histones (71). The acetylation status of histones at specific DNA regulatory sequences depends on the recruitment of histone acetyltransferases or histone deacetylase activities, usually as part of large multiprotein complex of coactivators or corepressors, respectively (71).
Several corepressor complexes, capable of interacting with several transcriptional repressors, have been identified, such as the Sin3 complex and NuRD complex (containing CHD4, RBB4/7, and MTA-1,-2,-3 subunits) (72,73). Along with altered epigenomes, often only a few yet potent mutations in selected genes drive tumorigenesis in several neoplasms. While many cancers demonstrate genetic alterations in histone modifiers, some cancers harbor mutations in DNA methylation regulators, whereas others such as glioma and leukemia show a high prevalence of alterations in chromatin modifiers (74). The development of metastatic lesions has not yet been associated with any specific recurrent mutation. This raises the interesting possibility that the perturbation of only a selected number of pathways, such as the repression of select signaling pathways and alteration of DNA methylation, may be involved in metastatic dissemination of a tumor, as suggested by our data.
Mutational signatures in the cancer genome provide indication of mechanisms underlying neoplastic transformation (75,76). According to Alexandrov et al., molecular signature 2 and 5 are common in thyroid cancer (45). However, in our data, these signatures were only found in 2 of the primary PTC and a single metastatic case, with mutational signature 1 and 15 being most common in both primary tumors and their metastases. Furthermore, signature 6 (DNA MMR deficiency) and signature 11 (temozolomide) were observed in metastatic deposits and absent from their primary tissues in five patients. Particularly, mutational signature shifts in defective DNA repair seen in metastatic cases are of high importance. However, our study lacks the presence of multiple samples that could give us a better understanding of this shift between trunk mutations (mutations present in all biopsies of a patient) and non-trunk mutations (mutations present in selected biopsies of a patient). Currently, the genomic evolution of PTC, by using multiple biopsies at different time points to generate phylogenies, is being investigated in our laboratory.
Whether patients presenting with distant metastatic disease should have metastases biopsied to aid in therapeutic interventions is controversial (77). Due to the challenging and invasive nature of many metastatic disease sites, bias is seen in our cohort toward sites with increased level of accessibility.
Conclusions
As in any cancer, PTC relentlessly exploits a vast mutational repertoire and series of gene pathways to ensure tumor development, survival, and ability to invade distant regions of the body. This leads to considerable variability in interpatient genomic profiles. Recruiting a large prospective cohort of patients with metastatic disease is crucial to map this complexity, along with the incorporation of transcriptional epigenomic and clinical associations. Our data demonstrate that such endeavor would potentially provide insights into the mechanisms of the metastatic process and identify pathways that could represent new therapeutic targets. Finally, our study confirms the interaction between cancer genomics and epigenetics, in support of ongoing developments in new epigenetic therapies.
Supplementary Material
Acknowledgment
We thank the patients for their participation.
Authors' Contributions
T.M. and A.K.S. performed WES, critical data analyses, interpretation of results, and wrote the article. S.S., S.A., Z.Q., and W.N.A. performed DNA extraction, analyzed the data, and carried out statistical analyses. S.K.P., S.S.A., and F.A. contributed samples and analyzed clinical data. F.S.A. and K.S.A. designed, implemented the study, wrote and critically reviewed the article. This is to confirm that all authors read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This project was conducted by a generous fund from the Saudi Government to the Thyroid Cancer Genome Project as part of the International Cancer Genome Consortium (ICGC).
Supplementary Material
References
- 1. Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. 2015. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195 [DOI] [PubMed] [Google Scholar]
- 2. Saudi Cancer Registry. Cancer Incidence Report, Saudi Arabia 2015. Riyadh (KSA): Saudi Cancer Registry; 2018. Available at https://nhic.gov.sa/eServices/Documents/E%20SCR%20final%206%20NOV.pdf (accessed January10, 2019) [Google Scholar]
- 3. Schneider DF, Chen H. 2013. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin 63:373–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, Lee KE, Park YJ, Yi KH, Park DJ. 2016. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 122:1370–1379 [DOI] [PubMed] [Google Scholar]
- 5. Fraser S, Go C, Aniss A, Sidhu S, Delbridge L, Learoyd D, Clifton-Bligh R, Tacon L, Tsang V, Robinson B. 2016. BRAFV600E mutation is associated with decreased disease-free survival in papillary thyroid cancer. World J Surg 40:1618–1624 [DOI] [PubMed] [Google Scholar]
- 6. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, Patel SG, Ganly I. 2012. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 22:884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goffredo P, Sosa JA, Roman SA. 2013. Differentiated thyroid cancer presenting with distant metastases: a population analysis over two decades. World J Surg 37:1599–1605 [DOI] [PubMed] [Google Scholar]
- 8. Vuong HG, Altibi AM, Duong UN, Ngo HT, Pham TQ, Tran HM, Oishi N, Mochizuki K, Nakazawa T, Hassell L. 2017. Role of molecular markers to predict distant metastasis in papillary thyroid carcinoma: promising value of TERT promoter mutations and insignificant role of BRAF mutations—a meta-analysis. Tumor Biol 39:1010428317713913 [DOI] [PubMed] [Google Scholar]
- 9. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL. 2002. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885 [DOI] [PubMed] [Google Scholar]
- 10. Mazzaferri EL, Kloos RT. 2001. Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1447–1463 [DOI] [PubMed] [Google Scholar]
- 11. Melmed S, Polonsky K, Larsen P, Kronenberg H. 1897. Williams Textbook of Endocrinology, 2011. Saunders Elsevier, Philadelphia [Google Scholar]
- 12. Smith H. 1990. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am 19:685–718 [PubMed] [Google Scholar]
- 13. Robbins RJ, Schlumberger MJ. 2005. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. J Nucl Med 46:28S–37S [PubMed] [Google Scholar]
- 14. Finley DJ, Arora N, Zhu B, Gallagher L, Fahey III TJ. 2004. Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab 89:3214–3223 [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Rodero S, Fernández AF, Fernández-Morera JL, Castro-Santos P, Bayon GF, Ferrero C, Urdinguio RG, Gonzalez-Marquez R, Suarez C, Fernández-Vega I. 2013. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J Clin Endocrinol Metab 98:2811–2821 [DOI] [PubMed] [Google Scholar]
- 16. Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Lee SM, Forster MD, Ahmad T, Hiley CT, Abbosh C, Falzon M, Borg E, Marafioti T, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Dentro S, Taniere P, O'Sullivan B, Lowe HL, Hartley JA, Iles N, Bell H, Ngai Y, Shaw JA, Herrero J, Szallasi Z, Schwarz RF, Stewart A, Quezada SA, Le Quesne J, Van Loo P, Dive C, Hackshaw A, Swanton C, Consortium TR. 2017. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376:2109–2121 [DOI] [PubMed] [Google Scholar]
- 17. Ng CK, Bidard F-C, Piscuoglio S, Geyer FC, Lim RS, De Bruijn I, Shen R, Pareja F, Berman SH, Wang L. 2017. Genetic heterogeneity in therapy-naïve synchronous primary breast cancers and their metastases. Clin Cancer Res 23:4402–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yates LR, Knappskog S, Wedge D, Farmery JH, Gonzalez S, Martincorena I, Alexandrov LB, Van Loo P, Haugland HK, Lilleng PK. 2017. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32:169–184. e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertucci F, Finetti P, Guille A, Adélaïde J, Garnier S, Carbuccia N, Monneur A, Charafe-Jauffret E, Goncalves A, Viens P. 2016. Comparative genomic analysis of primary tumors and metastases in breast cancer. Oncotarget 7:27208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schrijver WA, Jiwa LS, van Diest PJ, Moelans CB. 2015. Promoter hypermethylation profiling of distant breast cancer metastases. Breast Cancer Res Treat 151:41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson WJ, Hoivik EA, Halle MK, Taylor-Weiner A, Cherniack AD, Berg A, Holst F, Zack TI, Werner HM, Staby KM. 2016. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet 48:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faltas B, Beltran H, Prandi D, Eng K, Pauli C, Robinson BD, Mosquera JM, Nanus DM, Tagawa ST, Elemento O. 2015. Whole exome sequencing to reveal chemotherapy-driven evolution of platinum-resistant metastatic urothelial cancer. J Clin Oncol 33(15_suppl):4513–4513 [Google Scholar]
- 23. Wei Q, Ye Z, Zhong X, Li L, Wang C, Myers R, Palazzo J, Fortuna D, Yan A, Waldman S. 2017. Multiregion whole-exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Ann Oncol 28:2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ouyang L, Lee J, Park C-K, Mao M, Shi Y, Gong Z, Zheng H, Li Y, Zhao Y, Wang G. 2014. Whole-genome sequencing of matched primary and metastatic hepatocellular carcinomas. BMC Med Genomics 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goswami RS, Patel KP, Singh RR, Meric-Bernstam F, Kopetz ES, Subbiah V, Alvarez RH, Davies MA, Jabbar KJ, Roy-Chowdhuri S. 2015. Hotspot mutation panel testing reveals clonal evolution in a study of 265 paired primary and metastatic tumors. Clin Cancer Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M. 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G. 2013. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang K, Li M, Hakonarson H. 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siraj AK, Masoodi T, Bu R, Beg S, Al-Sobhi SS, Al-Dayel F, Al-Dawish M, Alkuraya FS, Al-Kuraya KS. 2016. Genomic profiling of thyroid cancer reveals a role for thyroglobulin in metastasis. Am J Hum Genet 98:1170–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen R, Seshan VE. 2016. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res 44:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA. 2012. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol 30:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. 2010. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7:575. [DOI] [PubMed] [Google Scholar]
- 35. Carter H, Chen S, Isik L, Tyekucheva S, Velculescu VE, Kinzler KW, Vogelstein B, Karchin R. 2009. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res 69:6660–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, Day IN, Gaunt TR. 2013. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 34:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. 2012. Predicting the functional effect of amino acid substitutions and indels. PLoS One 7:e46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dang VT, Kassahn KS, Marcos AE, Ragan MA. 2008. Identification of human haploinsufficient genes and their genomic proximity to segmental duplications. Eur J Hum Genet 16:1350. [DOI] [PubMed] [Google Scholar]
- 39. Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB. 2016. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol 34:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR. 2004. A census of human cancer genes. Nat Rev Cancer 4:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA. 2013. Mutational landscape and significance across 12 major cancer types. Nature 502:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. 2014. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, Vilo J. 2016. g:Profiler—a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res 44:W83–W89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C. 2016. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol 17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale A-L. 2013. Signatures of mutational processes in human cancer. Nature 500:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Biase D, Gandolfi G, Ragazzi M, Eszlinger M, Sancisi V, Gugnoni M, Visani M, Pession A, Casadei G, Durante C, Costante G, Bruno R, Torlontano M, Paschke R, Filetti S, Piana S, Frasoldati A, Tallini G, Ciarrocchi A. 2015. TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid 25:1013–1019 [DOI] [PubMed] [Google Scholar]
- 47. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, Castro P, Prazeres H, Lima J, Amaro T, Lobo C, Martins MJ, Moura M, Cavaco B, Leite V, Cameselle-Teijeiro JM, Carrilho F, Carvalheiro M, Maximo V, Sobrinho-Simoes M, Soares P. 2014. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 99:E754–E765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, Al-Obaisi KAS, Victoria IG, Al-Sobhi SS, Al-Dawish M, Al-Dayel F, Al-Kuraya KS. 2018. Telomerase reverse transcriptase mutations are independent predictor of disease-free survival in Middle Eastern papillary thyroid cancer. Int J Cancer 142:2028–2039 [DOI] [PubMed] [Google Scholar]
- 49. Liu R, Xing M. 2016. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23:R143–R155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, Aas T, Alexandrov LB, Larsimont D, Davies H. 2015. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med 21:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murugaesu N, Wilson GA, Birkbak NJ, Watkins TB, McGranahan N, Kumar S, Abbassi-Ghadi N, Salm M, Mitter R, Horswell S. 2015. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov 5:821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Waters J, Leung ML, Unruh A, Roh W, Shi X, Chen K, Scheet P, Vattathil S, Liang H, Multani A, Zhang H, Zhao R, Michor F, Meric-Bernstam F, Navin NE. 2014. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 512:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beltran H, Eng K, Mosquera JM, Sigaras A, Romanel A, Rennert H, Kossai M, Pauli C, Faltas B, Fontugne J. 2015. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol 1:466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Biase D, Torricelli F, Ragazzi M, Donati B, Khun E, Visani M, Acquaviva G, Pession A, Tallini G, Piana S, Ciarrocchi A. 2018. Not the same thing: metastatic PTCs have a different background than ATCs. Endocr Connect pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. 2013. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gu Y, Liu X, Yu Y, Shi J, Ai L, Sun H, Kanu JS, Wang C, Liu Y. 2014. Association of ATM gene polymorphism with PTC metastasis in female patients. Int J Endocrinol 2014:370825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gandolfi G, Ragazzi M, Frasoldati A, Piana S, Ciarrocchi A, Sancisi V. 2015. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur J Endocrinol 172:403–413 [DOI] [PubMed] [Google Scholar]
- 58. Xu B, Tuttle RM, Sabra MM, Ganly I, Ghossein R. 2017. Primary thyroid carcinoma with low-risk histology and distant metastases: clinicopathologic and molecular characteristics. Thyroid 27:632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gandolfi G, Ragazzi M, de Biase D, Visani M, Zanetti E, Torricelli F, Sancisi V, Gugnoni M, Manzotti G, Braglia L. 2018. Genome-wide profiling identifies the THYT1 signature as a distinctive feature of widely metastatic papillary thyroid carcinomas. Oncotarget 9:1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cancer Genome Atlas Research N 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feinberg AP, Vogelstein B. 1983. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301:89. [DOI] [PubMed] [Google Scholar]
- 62. Teodoridis JM, Strathdee G, Brown R. 2004. Epigenetic silencing mediated by CpG island methylation: potential as a therapeutic target and as a biomarker. Drug Resist Updat 7:267–278 [DOI] [PubMed] [Google Scholar]
- 63. Boyes J, Byfield P, Nakatani Y, Ogryzko V. 1998. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 396:594. [DOI] [PubMed] [Google Scholar]
- 64. Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W. 2004. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A 101:2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K. 2008. A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol 10:1291. [DOI] [PubMed] [Google Scholar]
- 66. Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C. 2010. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT. 2013. The mutational landscape of adenoid cystic carcinoma. Nat Genet 45:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cai Y, Geutjes E-J, De Lint K, Roepman P, Bruurs L, Yu L, Wang W, Van Blijswijk J, Mohammad H, De Rink I. 2014. The NuRD complex cooperates with DNMTs to maintain silencing of key colorectal tumor suppressor genes. Oncogene 33:2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Margueron R, Reinberg D. 2010. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Strahl BD, Allis CD. 2000. The language of covalent histone modifications. Nature 403:41. [DOI] [PubMed] [Google Scholar]
- 71. Peinado H, Ballestar E, Esteller M, Cano A. 2004. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24:306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ahringer J. 2000. NuRD and SIN3: histone deacetylase complexes in development. Trends Genet 16:351–356 [DOI] [PubMed] [Google Scholar]
- 73. Jepsen K, Rosenfeld MG. 2002. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698 [DOI] [PubMed] [Google Scholar]
- 74. Roy DM, Walsh LA, Chan TA. 2014. Driver mutations of cancer epigenomes. Protein Cell 5:265–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alexandrov LB. 2015. Understanding the origins of human cancer. Science 350:1175–1177 [DOI] [PubMed] [Google Scholar]
- 76. Helleday T, Eshtad S, Nik-Zainal S. 2014. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 15:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H, Andre F. 2015. Precision medicine for metastatic breast cancer—limitations and solutions. Nat Rev Clin Oncol 12:693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.