Highlights:
-
•
A Dutch Quality Improvement Program for chemoradiation of LACC in was succesfully implemented.
-
•
EBRT and BT treatment planning results improved in time between workshops.
-
•
Homework and workshop activities provide a suitable platform for exchange of experience and quality improvement.
Keywords: Quality assurance, Quality improvement, Education & training
Abstract
Purpose
To report on the “Dutch Quality Improvement Project” regarding external beam (EBRT) and brachytherapy (BT) contouring and treatment planning for locally advanced cervical cancer (LACC).
Material and methods
Two rounds of three workshops were organized. Data from two patients with LACC were made available for homework exercises. Contouring and treatment planning was asked for according to the EMBRACE-II protocol. The submissions were analysed and the results were addressed during the workshops.
Results
Almost all invited centres participated. EBRT contouring guidelines were followed within acceptable range, with major effort needed with regard to the ITV concept. BT contouring was of good quality, with especially small discrepancies for centres already participating in EMBRACE.
EBRT treatment planning results improved between workshops with more centres being able to fulfil the planning aims. Guidance was especially necessary to improve the coverage probability planning for affected nodes.
For BT planning prioritizing between target coverage and OAR sparing improved over time; the variation in dose to vaginal points remained considerable, as did variation in loading patterns and spatial dose distribution.
The project was highly appreciated by all participants.
Conclusion
Homework and workshop activities provide a suitable platform for discussion, exchange of experience and improvement of quality and conformity. Due to this project, radiotherapy for LACC can be administered with better and more comparable quality throughout the Netherlands.
Introduction
The Dutch Society of Radiation Oncology established a Platform for Radiotherapy of Gynaecological Tumours (LPRGT) with members from all radiation oncology centres that treat patients with gynaecological tumours. The platform has a long standing experience in driving clinical multicentre studies like the PORTEC trials for endometrial cancer [1], [2], [3]. Additionally, the LPRGT is actively driving forward the clinical implementations of innovative technology. For locally advanced cervix cancer (LACC) brachytherapy is applied according to GEC-ESTRO recommendations [4], [5]. Concerning developments for external beam radiotherapy treatment (EBRT), the LPRGT adheres to contouring and planning as described in the EMBRACE-II protocol (embracestudy.dk). This protocol describes the current state-of-the-art (chemo)radiation and brachytherapy (BT), based on the results of RetroEMBRACE and EMBRACE-I studies, and other recent publications [6], [7], [8], [9], [10], [11], [12], [13]. Important aspects are the use of MRI information for EBRT and BT planning, intensity modulated radiotherapy (IMRT), simultaneously integrated boosts (SIB) with coverage probability planning for the treatment of regional lymph node metastases, daily image guided radiotherapy, and MRI guided adaptive brachytherapy (IGABT).
The fast developments of image-guided adaptive treatment of LACC induces changing ‘Patterns of care’ [14], [15], [16], [17], [18]. The importance of training, audits, and continuous education is widely recognized, and sharing experiences (joys and pitfalls) for chosen approaches can boost clinical implementation.
The LPRGT initiated a “Dutch Quality Improvement Project” to support the clinical implementation of state-of-the-art radiotherapy and to strengthen national collaboration. It consisted of contouring and treatment planning exercises and workshops for both EBRT and BT. The target audience consisted of teams of Radiation Oncologists (RO), Medical Physicists (MP) and Radiation Therapists (RTT). The aim of this paper is to report on this project, including the achievements in EBRT and BT contouring and treatment planning as well as organisational aspects.
Materials & methods
Organisation
The project group consisted of 3 RO and 3 MP from 4 centres and was initiated by Dutch members of the EMBRACE-II study core group. Fifteen of the 16 centres currently treating LACC patients with EBRT participated and all (11) BT centres. The contouring workshops aimed at ROs, the EBRT planning workshops were meant for MPs and RTTs, and the BT planning workshops for teams of ROs, MPs and RTTs.
A general invitation to participate in the program was sent to all 16 centres in the summer of 2015. The first series of homework exercises and full-day workshops was organized between October 2015 and March 2016. The number of participants for each workshop was limited to 30–40 persons, preferably in teams of 2 or 3 members per centre. The homework exercises were mandatory for participation in the workshops. The tasks were meant to evaluate the understanding of contouring and treatment planning principles and served as a basis for discussing agreements and discrepancies.
A second series of homework exercises and 3 half-day workshops were arranged between January and March 2017, in which the same cases were used. Preparation was less labour intensive, as the relevant data (images, contours) were already available at the centres.
The project was funded by the Quality Project Program of the Dutch Society of Radiation Oncology, and supported by Elekta Brachytherapy (Veenendaal, The Netherlands).
Patient data
External beam case
For the EBRT case we used data of a 41 year old patient with stage Ib2 squamous cell cancer and two pathological nodes in the right pelvis, a single node in the left pelvis, and two nodes left and right in the common iliac region. Reference contours for targets and OAR were created by two expert ROs.
Brachytherapy case
For the BT case we used data of a 59 year old patient with stage IIb squamous cell cancer with involvement of the proximal 20 mm of vagina, both parametria and uterine corpus. The patient received two BT applications (with 2 HDR fractions each) with the Utrecht applicator (Elekta, Veenendaal, Netherlands), the first with 3 interstitial needles, and the second with 4 interstitial needles. Data of the first application was used. The reference contours were generated by the same experts.
Data handling and homework exercises.
All imaging data were anonymised and sent in DICOM format to the participants for importation into their local contouring and planning software. Both patients had given informed consent for using their data for educational purposes. Additionally, we sent a set of instructions for the exercises as well as the relevant chapters from the EMBRACE-II protocol, clinical information of the cases and a digital form to report contouring/planning parameters.
Contouring for EBRT and BT
Seven weeks before the workshop, the images were transferred: for EBRT, CT, PET and diagnostic MR scans, along with the registration between CT and MR; for BT, three T2w (axial, sagittal, coronal) MR data sets. Contouring of the following target and organ at risk (OAR) structures was asked for the EBRT case: GTV-Tinit, CTV-T-HRinit, CTV-T-LRinit, ITV-T-LRinit (standard margin approach EMBRACE II protocol), CTV-E, ITV45, PTV45, for each of the 5 separate nodes GTV-N, CTV-N, and PTV-N, as well as bladder, rectum, sigmoid, and bowel ([19] and Appendix A). For the BT case, contours of the GTVres, CTVHR, and CTVIR [4], [19] were requested. DICOM structure sets and volumes as calculated by the centre were to be returned.
EBRT planning
After the contouring workshop, the reference contours for targets and OAR were distributed for both EBRT and BT planning exercises. The assignment was to generate an IMRT or VMAT (Volumetric Arc Therapy) plan, including a SIB for the 5 lymph nodes, with aims and constraints based on the EMBRACE II protocol: 45 Gy to the elective PTV; and 55 Gy and 57.5 Gy to the nodes (depending on the position), in 25 fractions. The planning aim, new in EMBRACE II, was that the volume of PTV45 receiving 95% of the planning aim dose (PTV45 V95%) should be higher than 95% (hard constraint) but close to 95% (soft constraint, see Table 3 in [13]). With respect to affected lymph nodes the concept of coverage probability (CoP) planning was introduced [12], including the three hard constraints (CTV-N D98 > 100%, PTV-N D98 > 90%, Dmax < 107%) and one soft constraint (CTV-N D50 > 102%). The dose distribution (in DICOM), the relevant DVH parameters, specific parameters of the planning technique and preferably results of in-house plan QA measurements were to be returned.
BT planning
For the BT planning, an Oncentra Brachy (Elekta, Veenendaal, Netherlands) RTplan file with applicator reconstruction was provided allowing all participants to use the same applicator reconstruction and thereby facilitating a fair plan comparison. All participants except one used Oncentra Brachy in their clinic.
The following dose points were to be placed: points A, ICRU bladder, Lateral Vaginal points at 5 mm, and Recto-Vaginal Reference Point (RVRP) [19], [20]. Three plans were required: (1) a generic “standard plan”, consisting of a set of pre-determined dwell times and positions, (2) a centre-specific “centre standard plan” (not optimized), and (3) an optimized plan according to the aims and constraints of the EMBRACE-II protocol. DVH parameters for all plans, DICOM RTdose and RTplan for the optimized plan were returned. For the second workshop the coordinates of the dose points were distributed and only an optimized plan was requested.
Analysis
For visualization, sanity checks and further evaluation, all DICOM objects were centrally collected. All contours were displayed simultaneously to assess the location of delineation variations. The EBRT planning DVH parameters and compliance with aims and constraints were evaluated. For BT, DVH parameters and doses to points were evaluated, as well as the contribution of the different applicator components.
The workshops
Participation was accredited for all disciplines by their professional societies. The contouring- and brachy planning workshops were organised at a location with 12 workstations.
A major part of each workshop was in-depth explanation of the concepts, followed by a summary of results and feedback of the exercises, especially focussing on discrepancies and errors.
For the contour workshop, standard ITV-T-LRinit pre-workshop contours with isotropic margins were discussed and the difference with respect to ITV-T-LRinit with individualized margins based on the anatomy changes as found at different imaging time points was highlighted. The participants practised live contouring, based on MRI, CT and PET-CT information with different fillings of bladder and rectum.
Furthermore, the participants contributed with short presentations on various subjects: the patterns-of-care study in the Netherlands [15], the use of ‘library of plans’/’plan of the day’ approaches for EBRT, and practical workflows for brachy treatment. There was ample time for discussion and exchange of tips and tricks. Participants were asked to fill in evaluation sheets.
Results
Contouring
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tipsro.2018.10.001.
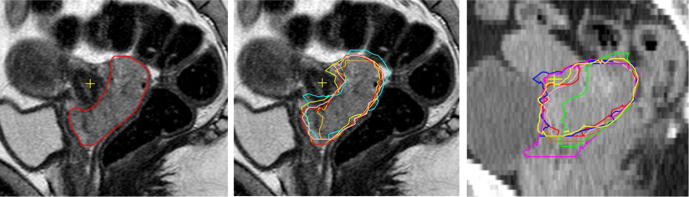
Thirty participants from 14 centres attended the first workshop, 13 centres sent in delineations for EBRT. Five centres contoured on MRI, whereas eight still on CT, according to their clinical practice. MRI derived target volumes were overall smaller with less variation (Fig. 1). The mean volumes of GTV-T and CTV-T-LRinit were 64 (SD 6)/198 (SD 20) cm3 on MRI and 93 (SD23)/280 (SD 36) cm3 on CT, respectively. Contouring discrepancies for CTV-T-LRinit were mainly in the parametria, cranially (peritoneal border), laterally (border obturator lymph node region) and caudally (parametria to paracolpia). Additionally, several centres did not accomplish the upper border of the lymphatic region according to the pre-defined risk profile. Additionally, the extent of contours varied especially around the aorta and vena cava, the caudal border of the pre-sacral space, the caudal expansion of external iliac vessels and obturator region. BT contouring was done by 10 of the 11 participating BT centres. Agreement for the target contours was quite good; however there was a clear difference between the 5 centres already participating in the EMBRACE-I study versus the others. The former generated smaller GTVres (mean 15 (SD 19) versus 22 (SD 42) cm3), larger CTVHR (mean 45 (SD 16) versus 39 (SD 26) cm3) with less variation. Discrepancies were mainly present for remaining cervix and vaginal extension (Fig. S1).
Fig. 1.
Contours for External Beam Planning. Sagittal slices showing the GTV-Tinit. (A) MRI with the expert contour. (B) MRI with contours from centres contouring on MRI. (C) CT with contours from centres contouring on CT.
Supplementary Fig. S1.
Contours for Brachy Planning. Sagittal T2w MRI slice showing inter-observer variation in CTVHR. The main differences are found in the cranial and caudal region.
In the second contouring workshop a new group of ROs was trained, from 10 centres. Overall the results were quite comparable with the ones from the first workshop with slight improvement for the ITV-T-LR concept.
EBRT planning
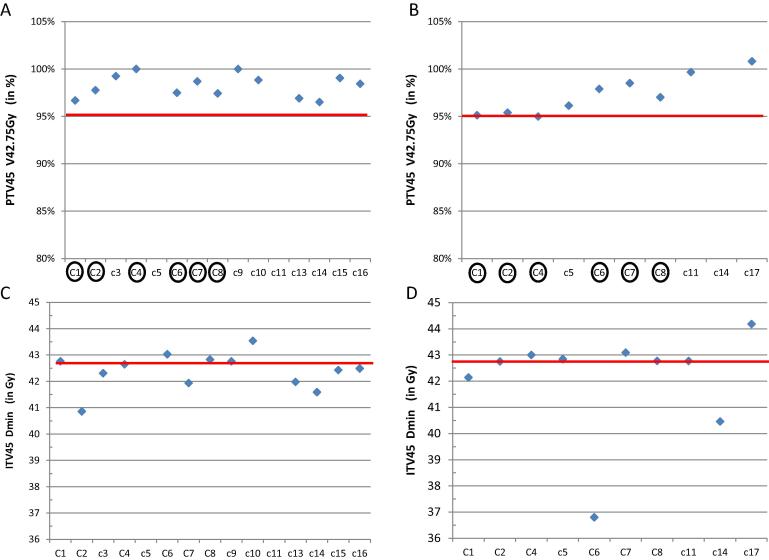
The first EBRT planning workshop had 31 participants (MPs and RTTs) from 14 centres. Three (step and shoot) IMRT, 11 VMAT and 1 Tomotherapy plan were submitted. None of these 15 plans met all 36 soft and hard dose constraints. Two plans were incorrect, the centres decided for field borders not fitting the (given) PTV45: for centre C5 too high, for C11 too low. In 5 plans, the constraints for 32 parameters or more were achieved; three plans met 23 or more of 25 hard dose constraints. Problematic parameters were minimum dose to ITV45 (9/15 violations), maximum dose to bladder (10/15 violations) and bowel (7/15 violations). The maximum dose value allowed within 10 mm from the CTV-T-HRinit (dose region of brachytherapy) was exceeded in all plans, due to the proximity of a pelvic node which needed boosting. The median PTV45 V95% was 98.4% (range 97–100%, Fig. 2), excluding the data from the incorrect plans. The average PTV45 dose conformity (V43Gy/PTV45) was 1.19 (SD 0.09) for 13 plans.
Fig. 2.
DVH values from the External Beam plans: Upper part: Volume of the PTV45 receiving 95% of the planning aim dose for the separate centres in % for the first (A) and the second (B) External Beam Planning workshop. Lower part, the minimum dose to the ITV45 in Gy for the first (C) and the second (D) workshop. Horizontal (red) lines: hard constraints. For A and B the soft constraint is that the values should be close to 95%. Note that not all centres submitted plans for two workshops, and that some centres reported erroneous values. The encircled centres submitted evaluable plans for the two workshops. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the second workshop 10 plans were submitted. Two met ≥ 32 of 36 constraints. The ITV45 minimum dose constraint was achieved in 7 of the 10 plans, an improvement over the first round. Results for PTV45 V95% improved to median 96.5% (range 95.0 – 99.7%) (Fig. 2).
A direct comparison of PTV V95% for the 6 centres that provided 2 plans (C1, C2, C4, C6, C7, C8) between the first and second was average 98% (SD 1.2%) and 96.4% (SD 1.3%), respectively.
With respect to CoP planning for affected lymph nodes, in the first workshop 7/15 plans met all nodal criteria, 13 showed a maximum of 3 violations. In the second, 5/10 were flawless and 7 showed a maximum of 3 violations. A considerable variation in dose distribution is possible, while still all nodal planning criteria were fulfilled. For example Fig. S2 shows in one case a body V50Gy = 86 cm3 and in the other 313 cm3, while both were meeting the constraints for the lymph nodes.
Supplementary Fig. S2.
Illustration of External Beam dose distributions in a coronal slice for two centres with the same colour mapping. Both centres fulfilled the hard constraints. Note the differences in coverage and body V50Gy (left V50Gy = 86 cm3, right V50Gy = 313 cm3).
BT planning
The first BT planning workshop had 34 participants from 11 centres, teams of MP, RO and RTT. Ten out of 11 centres submitted their plan in time for analysis. During the workshop, the priorities of aims and constraints for targets and OAR were the main issue. The “standard plan” is the same for all centres, allowing to evaluate the effect of placement of the dose points on the reported dose.
For the RVRP this resulted in a mean dose of 100 (range 80–128, SD 15%) Gy EQD2.
For the “centre-standard plans” we found two ‘schools’ in the Netherlands, as the ratio of the contribution of tandem/riright ovoid/left ovoid was 60/20/20% for 5 centres and 40/30/30% for 3 centres, and 50/25/25% for one centre. One centre did not provide a centre-standard plan.
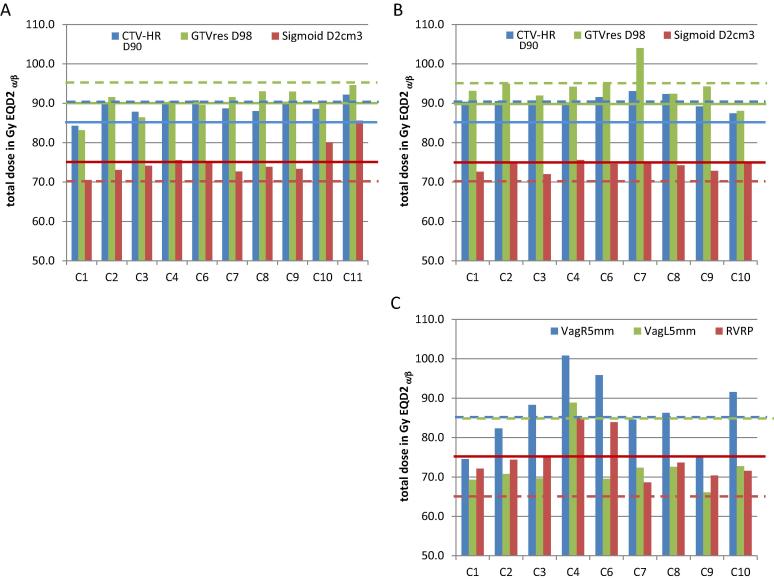
For the optimized plans 3 centres did not achieve the minimal planning aim doses for D90 and D98 CTVHR, D98 GTVres, and D98 CTVIR, while this should have been the highest treatment planning priority; four centres had unnecessary high OAR doses (Figs. 3 and S3). The spatial dose distributions differed, especially in the caudal and cranial part (Fig. S4).
Fig. 3.
DVH values from the brachy plans for the separate centres: D90 CTVHR, D98 GTVres and D2cm3 sigmoid of the first plan (A) and second plan (B), and dose to the Vaginal points 5 mm left and 5 mm right at the level of the vaginal sources (VagL5, VagR5) and the recto-vaginal reference point (RVRP) for the second plan (C). Horizontal (red) lines: hard constraints, dashed lines: soft constraints. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Supplementary Fig. S3.
Values of the main DVH parameters for the brachy plans of the participating centres both for the first (upper) and second (lower) BT planning workshop. All values in Gy EQD2 ( a/b = 10 for target, a/b = 3 OAR). Red= not achieving the hard constraint, orange= not achieving the soft constraint, green = achieving the aim (soft constraint). The plans for the first workshop miss the dose to the Recto Vaginal Reference Point (RVRP) as that point was not uniquely defined. Note that C11 did not submit a plan for the second workshop.
Supplementary Fig. S4.
Added volumes of 7.8 Gy isodose surface volume(the physical dose resulting in the aim dose of 85 Gy EQD2 for the D90 CTVHR). Discrete colour scale showing the number of centres achieving the 7.8 Gy in the given region. Each step in the discrete colour scale signifies one centre less, starting with dark red, all centres, to light blue, one centre. Contours visible are GTVres (magenta) CTVHR (pink) CTVIR (blue) and bladder rectum and bowel. The figure illustrates the differences in the cranial and caudal part. Left: first plan Right: second plan.
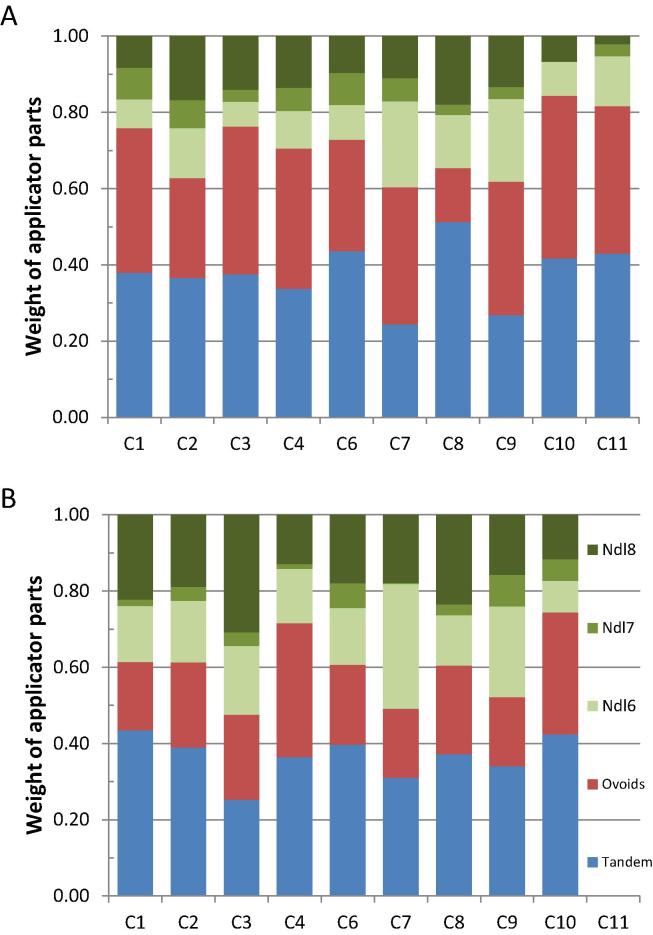
The relative contribution of tandem/ovoid/needles to the total dose distribution differed between centres (Fig. 4). E.g. total ovoid loading varied between 14 and 43%, needle contribution between 16 and 40%, with one exceptionally varying between 7–22%.
Fig. 4.
Contribution of different parts of the applicator configuration to the Total Reference Air Karma for the brachy plans of the separate centres. Tandem (blue)/total of right and left ovoid (red)/3 separate needles (green). (A) First plan, (B) Second plan. Note that C11 did not submit a plan for the second workshop. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The plans for the second round had improved DVHs (Figs. 3 and S3). However, the doses to the vaginal points still varied considerably: 3 centres failed to meet the hard constraint for the RVRP (Fig. 3C). The contribution of tandem/ovoid/needles remained different (Fig. 4). The spatial dose distributions varied among centres. E.g., the plans of 3 centres had quite remarkable differences, especially in the cranial direction, although the 85 Gy EQD2 isodose surface volumes and DVHs were rather similar. Furthermore, variations in relative needle contribution, resulted in quite some variation with respect to position and level of high dose volumes.
Finally, an on-site conducted survey concerning the centres’ practices for BT applications and planning was performed using an electronic voting system. This survey revealed that the majority of centres used an IC/IS technique in most of their applications with in more than half of the centres 3–6 needles being applied.
Discussion
To our knowledge this is the first descriptive report concerning a National Quality Assurance Programme for state of the art curative radiotherapy for patients with LACC. The program was intended to improve the joint efforts off all three professions within radiotherapy for delivering comparable EBRT and BT treatments throughout the country. In the Netherlands about 350 patient with LACC are treated with curative intent per year, spread over 16 centres for EBRT and 11 centres for BT, all organized within LPRGT. Concurrent chemotherapy (or hyperthermia) is part of a curative LACC treatment approach in the Netherlands but has not been considered in this project.
Organising quality assurance programs like this, requires the exchange of patient data sets, delineations, treatment plans and dose distributions taking into account safety and privacy aspects. This has become feasible over the last decade due to the increased use of DICOM formats, although not easily achievable for all contouring and treatment planning systems. The program, including homework exercises and live workshops for EBRT and BT contouring and treatment planning was highly appreciated by all participants. Results of the participation survey were such that “good/very good” were scored in 90–100% for all relevant questions as e.g. on the value of issues they learned for the daily practise, the organisation, the set-up of the workshops, and the value of the combination of participants for the discussion. The joint effort of explaining and discussing modern concepts for contouring and treatment planning aims with their respective investigational backgrounds, helped to guide the process of national change. Having gained experience through homework exercises before participating in workshops especially helped to achieve active and interactive participation. Reflecting the centres own results in relation to results of other participants and expert’s opinions was valuable, as well as appreciating the differences between the centres in general, such as the use of MR.
Modern radiotherapy target concepts are complex with initial tumour targets at time of EBRT treatment planning (initial GTV, high and low risk CTVs and ITV) and residual tumour targets at time of BT. These targets have different levels of complexity and ask for understanding the anatomical situations at time of diagnoses and the situation at time of BT. Despite written guidelines [4], [5], [19], [21], [22] and on-going fine tuning [13], [23] we still see essential differences in understanding target concepts and planning aims. In parallel, the complexity in treatment planning is increasing. The quality of IMRT/VMAT plans is highly dependent on the previously defined planning aims and the same holds true for image guided brachytherapy plans. Furthermore, we have to deal with complex anatomical changes during treatment asking for individualized ITVs, improvements in daily position verification and “plan of the day” treatment strategies.
Education through ESTRO and ASTRO teaching courses and workshops on national and international level intend to globally improve understanding and conformity of these concepts. Efforts as described for dummy run experiences within international studies like EMBRACE I [24] show that international agreement needs guidance but is achievable. The Dutch effort for LACC radiotherapy presented here supports this impression. Centres participating in EMBRACE I better fulfil the BT contouring demands compared to centres not having this experience. Dutch BT centres participating in EMBRACE I had to adopt GEC-ESTRO gyn BT guidelines ∼7 years ago and have been using them in clinical routine since then.
In the first round, achieving acceptable EBRT plans was difficult due to the novelty of the concepts. In the second round, institutes already using the new concepts clinically showed considerable improvement. Since most centres are willing to adopt these new concepts, it is to be expected that these results will improve overall as well. An important observation was that DVH parameters do not tell the whole story for treatment plan evaluation. Awareness for spatial dose distribution differences is also important and will help fine -tuning treatment planning results. A measure for globally judging the quality of a treatment plan is the conformity index, but checking dose distributions visually still helps to identify dose regions where improvements in terms of target or organ dose might be preferable and feasible (Fig. S2).
At the moment of writing 8 Dutch centres have passed QA requirements and dummy run procedures for EBRT and BT contouring and treatment planning, are on their way to entering patients into the EMBRACE II data base. This will help strengthen clinical evidence for modern LACC radiotherapy concepts.
Within the Dutch LPRGT it is felt that guidelines based on international consensus and repeated training help in developing a common sense necessary for making appropriate choices in the currently developing complexity. Organizing this sort of training and cooperation on a national level was time and resource consuming but helped to accomplish better treatment conformity among the treating centres.
Conclusion
Current LACC radiotherapy concepts for EBRT and BT target contouring and treatment planning are highly complex. Efforts as presented here for the “Dutch Quality Improvement Project” help to achieve more conformity among centres. The concept including homework and workshop activities provides a suitable platform for discussion, exchange of experience and improvement of conformity over time and is highly appreciated by all participating centres.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
We appreciate the financial support of this program by SKMS and we thank Elekta for providing the possibility to perform contouring and BT planning workshops.
Appendix A.
Glossary
| GTV-Tinit | Initial Gross Tumour Volume of the primary Tumour |
| CTV-T-HRinit | Initial High Risk Clinical Target Volume of the primary Tumour |
| CTV-T-LRinit | Initial Low Risk Clinical Target Volume of the primary Tumour |
| ITV-T-LRinit | Initial Internal Target Volume of the primary Tumour |
| GTV-N | Gross Tumour Volume of a individual pahtologic lymph Node |
| CTV-N | Clinical Target Volume of a individual pahtologic lymph Node |
| PTV-N | Planning Target Volume of a individual pahtologic lymph Node |
| CTV-E | Clinical Target Volume of the elective nodal region, including pathological lymph nodes if present |
| ITV45 | ITV-T-LR + CTV-E for 45 Gy |
| PTV45 | Planning Target Volume for 45 Gy |
| GTVres | Residual Gross Tumour Volume of the primary Tumour |
| CTVHR | Adaptive Hight Risk Clinical Target Volume of the primary Tumour |
| CTVIR | Intermediate Risk Clinical Target Volume of the primary Tumour |
Appendix B. Participating centres
AMC Amsterdam, Catharina Ziekenhuis Eindhoven, Erasmus MC Rotterdam, Isala Zwolle, LUMC Leiden, MAASTRO Maastricht, Medisch Spectrum Twente Enschede, Netherlands Cancer Institute Amsterdam, Radboudumc Nijmegen, Radiotherapiegroep Arnhem, Radiotherapiegroep Deventer, RIF Leeuwarden, UMC Groningen, UMC Utrecht, ISALA Zwolle, Haaglanden MC Den Haag, all in The Netherlands.
References
- 1.Nout R., Smit V., Putter H., Jürgenliemk-Schulz I., Jobsen J., Lutgens L. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 2.Creutzberg C.L., Nout R.A., Lybeert M.L.M., Wárlám-Rodenhuis C.C., Jobsen J.J., Mens J.W.M. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for Endometrial Carcinoma. Int J Radiat Oncol Biol Phys. 2011;81 doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haie-Meder C., Pötter R., Van Limbergen E., Briot E., De Brabandere M., Dimopoulos J. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Pötter R., Haie-Meder C., Van Limbergen E., Barillot I., De Brabandere M., Dimopoulos J. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy - 3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiolo. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Sturdza A., Pötter R., Fokdal L.U., Haie-Meder C., Tan L.T., Mazeron R. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicentre cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Fokdal L., Sturdza A., Mazeron R., Haie-Meder C., Tan L.T., Gillham C. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: Analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Pötter R., Georg P., Dimopoulos J.C.A., Grimm M., Berger D., Nesvacil N. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomden C.N., De Leeuw A.A.C., Van Limbergen E., De Brabandere M., Nulens A., Nout R.A. Multicentre treatment planning study of MRI-guided brachytherapy for cervical cancer: Comparison between tandem-ovoid applicator users. Radiother Oncol. 2013;107 doi: 10.1016/j.radonc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Rijkmans E.C., Nout R.A., Rutten I.H.H.M., Ketelaars M., Neelis K.J., Laman M.S. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro I., Janssen H., De Brabandere M., Nulens A., De Bal D., Vergote I. Long term experience with 3D image guided brachytherapy and clinical outcome in cervical cancer patients. Radiother Oncol. 2016;120:447–454. doi: 10.1016/j.radonc.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Lindegaard J.C., Assenholt M.S., Ramlov A., Fokdal L.U., Alber M., Tanderup K. Early clinical outcome of coverage probability based treatment planning in locally advanced cervical cancer for simultaneous integrated boost of nodes. Acta Oncol (Madr) 2017:1–8. doi: 10.1080/0284186X.2017.1349335. [DOI] [PubMed] [Google Scholar]
- 13.Pötter R., Tanderup K., Kirisits C., de Leeuw A., Kirchheiner K., Nout R. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018 doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan L.T. Implementation of image-guided brachytherapy for cervix cancer in the UK: progress update. Clin Oncol. 2011;23:681–684. doi: 10.1016/j.clon.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 15.De Boer P., Jürgenliemk-Schulz I.M., Westerveld H., de Leeuw A.A.C., Dávila-Fajardo R., Rasch C.R.N. Patterns of care survey: Radiotherapy for women with locally advanced cervical cancer. Radiother Oncol. 2017;123:306–311. doi: 10.1016/j.radonc.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Grover S., Harkenrider M.M., Cho L.P., Erickson B., Small C., Small W. Image guided cervical brachytherapy: 2014 survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2016;94:598–604. doi: 10.1016/j.ijrobp.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Phan T., Mula-Hussain L., Pavamani S., Pearce A., D’Souza D., Patil N.G. The changing landscape of brachytherapy for cervical cancer: a Canadian practice survey. Curr Oncol. 2015;22:356–360. doi: 10.3747/co.22.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim K., van Dyk S., Khaw P., Veera J., Mileshkin L., Ohanessian L. Patterns of practice survey for brachytherapy for cervix cancer in Australia and New Zealand. J Med Imaging Radiat Oncol. 2017:1–8. doi: 10.1111/1754-9485.12614. [DOI] [PubMed] [Google Scholar]
- 19.ICRU. Report 89. J ICRU 2016;13. 10.1093/jicru/ndw042. [DOI]
- 20.Westerveld H., Pötter R., Berger D., Dankulchai P., Dörr W., Sora M.C. Vaginal dose point reporting in cervical cancer patients treated with combined 2D/3D external beam radiotherapy and 2D/3D brachytherapy. Radiother Oncol. 2013;107:99–105. doi: 10.1016/j.radonc.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Lim K., Small W., Portelance L., Creutzberg C., Jürgenliemk-Schulz I.M., Mundt A. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan A.N., Erickson B., Gaffney D.K., Beriwal S., Bhatia S.K., Lee Burnett O. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2014;90:320–328. doi: 10.1016/j.ijrobp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cibula D., Pötter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Haie Meder C. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Kirisits C., Federico M., Nkiwane K., Fidarova E., Jürgenliemk-Schulz I.-M., de Leeuw A. Quality assurance in MR image guided adaptive brachytherapy for cervical cancer: Final results of the EMBRACE study dummy run. Radiother Oncol. 2015;117:548–554. doi: 10.1016/j.radonc.2015.08.001. [DOI] [PubMed] [Google Scholar]