Abstract
In the context of the coronavirus disease 2019 pandemic, myocardial injury is a relatively frequent finding. Progression to cardiogenic shock has been rarely described, especially in healthy young patients. The underlying mechanisms are to date controversial. A previously healthy 18‐year‐old female teenager affected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) developed fulminant cardiogenic shock requiring a prompt extracorporeal membrane oxygenation support. Cardiac involvement was predominant compared with the pulmonary one. Myocardial biopsies were performed; and in order to clarify the pathophysiology of the acute heart failure, optical and transmission electron microscopy study was realized. Two additional immunohistology techniques were developed in order to (i) detect a SARS‐CoV‐2 recombinant fusion nucleoprotein by using a specific antibody and (ii) study fractalkine expression induced by activated endothelium because this molecule is well known to be elevated in patients with severe cytokine release syndrome. SARS‐CoV‐2 genome was not detected in the myocardium. Even if the clinical presentation, laboratory markers, and cardiac imaging techniques strongly suggested fulminant myocarditis, histology and immunohistology were not fully consistent with this diagnosis according to the Dallas criteria. Although rare suspected coronavirus particles were found by transmission electron microscopy in the cardiac endothelium, neither significant immunoreactivity for the viral nucleocapsid protein nor image suggestive of endotheliitis was detected. Intense endothelial immunoreactivity pattern for fractalkine expression was observed. From a clinical point of view, the left ventricular systolic function gradually improved, and the patient survived after a long stay in the intensive care unit. Our observations suggest that a massive cytokine storm induced by SARS‐CoV‐2 infection was the main cause of the cardiogenic shock, making a direct viral injury pathway very unlikely.
Keywords: SARS‐CoV‐2, Cytokine storm, Cardiogenic shock, Teenager, Extracorporeal membrane oxygenation, Myocardial injury
Introduction
The frequency of myocardial injury (defined by increased cardiac troponin levels) is variable among hospitalized coronavirus disease 2019 (COVID‐19) patients, with a reported prevalence of 7–28%. 1 , 2 , 3 , 4 However, progression to cardiogenic shock (CS) is uncommonly reported in adults and exceptionally described in healthy children or adolescent patients. 5 Prognosis in this setting is often ominous, and therapeutic options are limited. We described a case of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐related CS in a surviving female teenager who required an extracorporeal membrane oxygenation (ECMO) support in the acute phase.
Case report
A previously healthy 18‐year‐old woman was admitted to the emergency department of our hospital complaining of nausea and vomiting. These symptoms occurred the day before she was admitted. No fever was reported. Upon admission, clinical features of impending shock were observed: body temperature was 32°C and blood pressure 85/50 mmHg, with cold extremities and confusion. Electrocardiogram revealed sinus tachycardia at 140 b.p.m., with subtle diffuse ST segment elevation and PR segment depression (Figure 1 A ). Arterial blood gas analysis showed severe metabolic acidosis with serum lactates at 11 mmol/L (n.v. <1 mmol/L). Laboratory tests revealed marked leucocytosis and neutrophilia, with a C‐reactive protein raised at 29 mg/L (n.v. <5 mg/L). Cardiac markers were elevated: creatine kinase (CK) 1400 U/L, CK‐MB 92 U/L, and high‐sensitivity troponin T 1650 pg/mL (n.v. <14 pg/mL). Serum creatinine elevation at 1.9 mg/dL and mild hyperkalaemia were compatible with concomitant acute kidney injury (Figure 2). Urgent transthoracic echocardiogram (TTE) showed left ventricular (LV) wall thickening without dilation and a speckled myocardial appearance suggesting myocardial oedema as well as mild inferior pericardial effusion and severe bi‐ventricular dysfunction with LV ejection fraction (LVEF) of 10% (Figure 1 B and Videos S1 and S2 ). Chest X‐ray was unremarkable (Figure 1 C ). Fulminant myocarditis was suspected, and the patient was transferred to the intensive care unit (ICU). Increased infusion rates of dobutamine and additional vasopressors were needed. Unfortunately, despite pharmacological treatment, the patient remained in shock and developed acute respiratory failure. Mechanical ventilation was started; and considering refractory CS, a percutaneous venoarterial ECMO (VA‐ECMO) was inserted 8 h after admission. TTE showed an aortic valve hardly opening in systole. Therefore, an intra‐aortic balloon pump (IABP) was inserted. A coronary angiogram showed normal coronary arteries, and ventriculography confirmed a very poor LV contractility ( Video S3 ). Endovascular myocardial biopsies showed a low density of inflammatory cells without myocyte degeneration or necrosis (Figure 3 A–C ). The patient was extensively evaluated for any underlying infectious or autoimmune aetiologies (Table 1 ). Multiple blood cultures were negative. SARS‐CoV‐2 nucleic acid was present in the nasopharyngeal swab. Transmission electron microscopy (TEM) was performed on the myocardial tissue, demonstrating suspected coronavirus particles in endothelial cell cytoplasm (Figure 4 A, B ). Upon admission, the patient was treated empirically with hydroxychloroquine, (400 mg b.i.d. at Day 1 and then 200 mg b.i.d. from Day 2 to 5), high doses of methylprednisolone (200 mg/day from Day 1 to 8), and intravenous immunoglobulin (cumulative dose 2 mg/kg, from Day 1 to 3) and antibiotics. Her ICU stay was complicated by the following: (i) pulmonary oedema and acute respiratory distress syndrome requiring a switch in the ECMO configuration from VA‐ECMO to venoartero‐venous ECMO (VAV‐ECMO) on Day 5; (ii) bilateral significant pleural effusion requiring drainage (Days 7 and 9); (iii) severe coagulopathy (fibrinogen 160 mg/dL and platelets 27 × 103/mm3); and (iv) severe rhabdomyolysis (CK level up to >30 000 U/L) possibly related to ECMO‐induced haemolysis. After 12 days of mechanical circulatory support, bedside TTE demonstrated a gradual improvement in cardiac contractility, with an LV outflow tract velocity time integral of 14 cm (Figure 5). The arterial VAV‐ECMO cannula was removed the same day, and the patient was weaned from VV‐ECMO and IABP on Day 17. The ICU stay lasted 34 days. Before discharge, 45 days after admission, a cardiac magnetic resonance revealed a non‐dilated LV with moderate global hypokinesia and LVEF close to 40% ( Videos S4 , S5 ). A non‐ischaemic pattern of late gadolinium enhancement was obvious in basal to mid‐inferior and inferoseptal segments (Figure 6 A ). Although the patient had stable resting oxygen saturation in the range of 95–97% while breathing room air, chest computed tomography still showed diffuse, bilateral infiltrates, suggestive of moderate persistent lung involvement (Figure 6 B and C). An evidence‐based, guideline‐recommended heart failure medical therapy was started. The TTE performed 2 months after discharge confirmed progressive improvement in LV systolic function with triplane LVEF of 48% ( Video S6 ).
Figure 1.
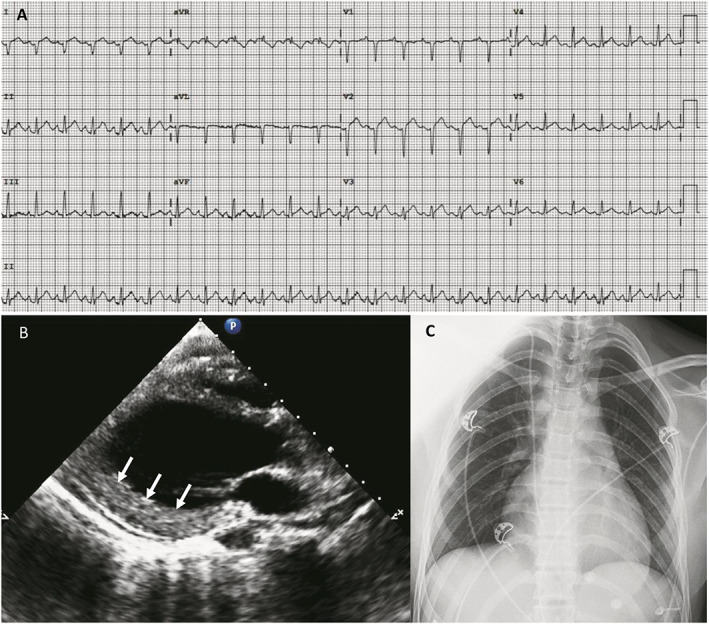
Electrocardiogram (EKG), transthoracic echocardiogram (TTE), and chest X‐ray at admission. (A) EKG showing sinus tachycardia, inframillimetric ST segment elevation, and PR depression. (B) Parasternal long axis view of left ventricular (LV) demonstrating non‐dilated LV (end‐diastolic diameter 42 mm) and wall thickening of inferolateral wall (13 mm) with granulated myocardial appearance (white arrows). (C) Absence of lung infiltrates.
Figure 2.
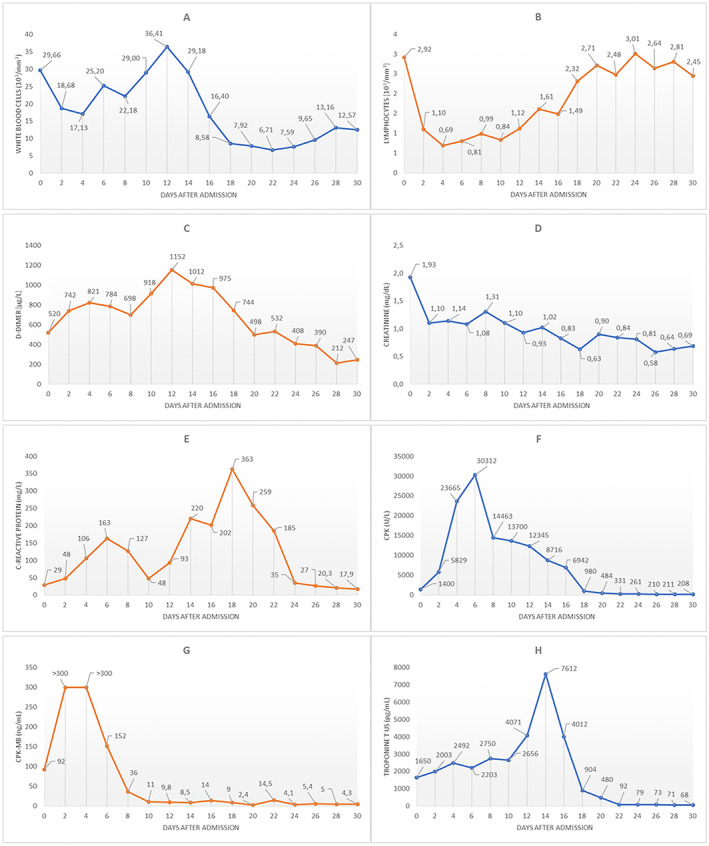
2Temporal changes in laboratory markers from admission: white blood cells (A), lymphocytes (B), D‐dimer (C), serum creatinine (D), C‐reactive protein (E), creatine kinase (CK) (F), CK‐MB (G), and high‐sensitivity cardiac troponin T (H). The second troponin peak observed at Day 14 is probably multifactorial and mainly explained by some changes in the extracorporeal membrane oxygenation (ECMO) configuration and by an Enterococcus faecium sepsis [as suggested by white blood cell (WBC) and C‐reactive protein kinetics].
Figure 3.
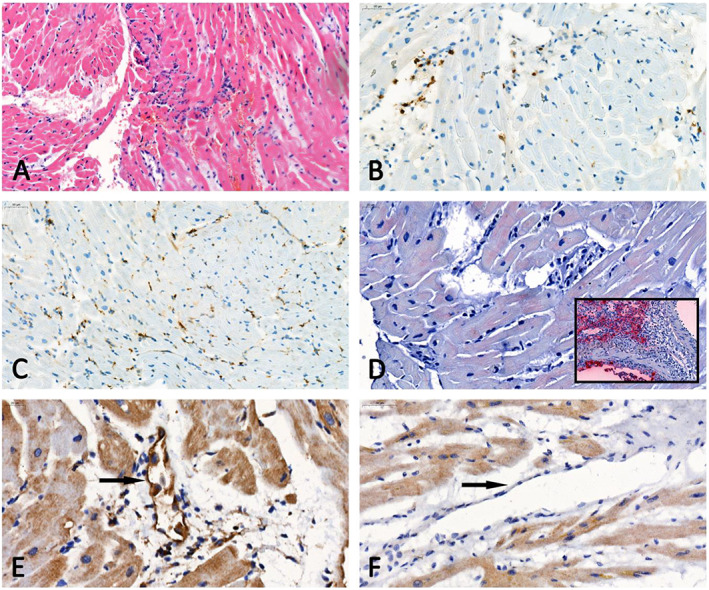
Optical microscopy of heart tissue. (A) Haematoxylin–eosin section showing a low density of mononuclear inflammatory cells (open arrows) in the absence of myocyte degeneration or necrosis. (B, C) Representative examples of CD3 (B) and CD68 (C) immunostaining demonstrating < 14 T lymphocytes or macrophages/mm2. (D) Absence of immunoreactivity for the viral nucleocapsid protein in the biopsy of our patient, which contrasts with the intense staining in the positive control [inset; lung tissue specimen of a severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected hamster]. (E, F) Immunohistology sections for fractalkine expression showing respectively an endothelial immunoreactivity in the biopsy of our patient (E) and no staining in the myocardium of a patient who died from a coronavirus disease 2019 (COVID‐19)‐unrelated cause (F).
Table 1.
Viral, bacterial, and autoimmune diagnostic
| Value | Normal value | |
|---|---|---|
| Adenovirus serology | Negative | Negative |
| Influenza virus serology | Negative | Negative |
| HBV and HCV serology | Negative | Negative |
| CMV serology | Negative | Negative |
| B19V serology | Negative | Negative |
| Echo/Coxsackie virus serology | Negative | Negative |
| EBV serology | Negative | Negative |
| Rubella virus serology | Negative | Negative |
| Measles virus serology | Negative | Negative |
| Rickettsia serology | Negative | Negative |
| Mycoplasma pneumoniae serology | Negative | Negative |
| Chlamydia serology | Negative | Negative |
| Borrelia serology | Negative | Negative |
| EMB PCR Panel a | Negative | Negative |
| Nasopharyngeal swab PCR SARS‐CoV‐2 | Positive | Negative |
| ANA (titre) | 1:80 | <1:80 |
| Anti‐ds‐DNA | 9.8 UI/mL | < 27 UI/mL |
| ANCA (p‐ANCA and c‐ANCA) | <0.20/ <0.20 UI/mL | <3.50/ <2.00 UI/mL |
| Rheumatoid factor | 10.7 U/mL | <14 U/mL |
Abbreviations: ANA, anti‐nuclear antibody; ANCA, anti‐neutrophil cytoplasmic antibodies; Anti‐ds‐DNA, anti‐double stranded DNA antibody; B19V, parvovirus B19; CMV, cytomegalovirus; EBM, endomyocardial biopsy; EBV, Epstein–Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; PCR, polymerase chain reaction.
EMB PCR Panel: enterovirus, adenovirus, CMV, B19V, and SARS‐CoV‐2 genome were searched.
Figure 4.
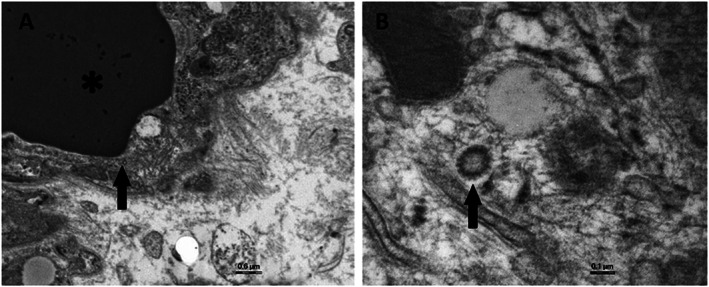
Electron microscopy of heart tissue. (A) Transmission electron microscopy representative examples showing viral particles (arrow) in the cytoplasm of endothelial cells around a vascular lumen containing red cells (asterisk). (B) The morphology associating a dense circular rim and a clear centre (arrow) suggests coronavirus particles.
Figure 5.
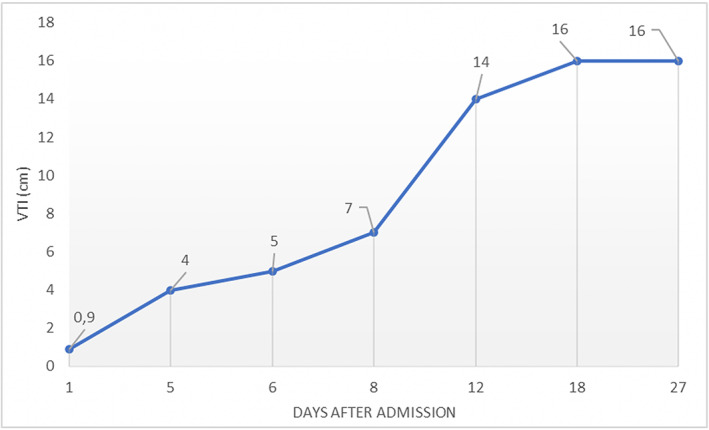
5Temporal changes in LVOT‐VTI. Progressive improvement of left ventricular outflow tract velocity time integral (LVOT‐VTI) evaluated by transthoracic echocardiogram (TTE). The arterial venoartero‐venous extracorporeal membrane oxygenation (VAV‐ECMO) cannula was removed at Day 12.
Figure 6.
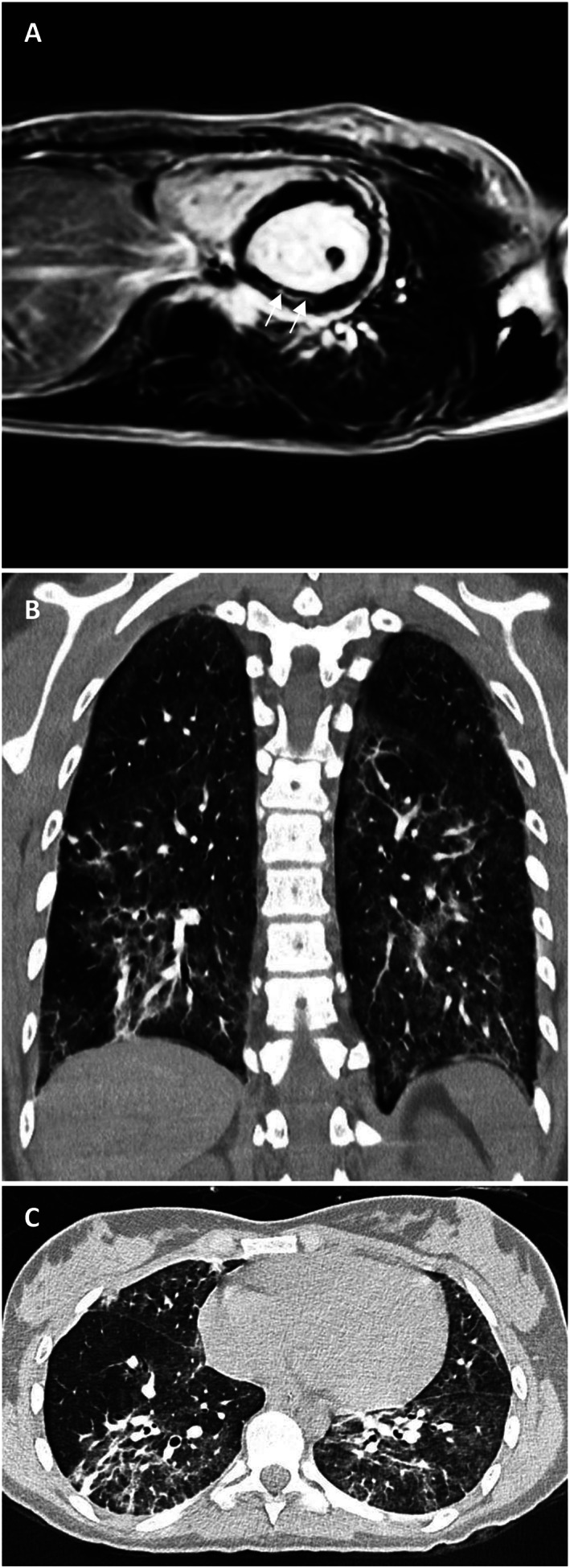
Pre‐discharge cardiovascular magnetic resonance and chest computed tomography (CT) imaging. (A) Short‐axis image of the mid‐ventricle demonstrating late gadolinium enhancement of the mid‐wall and sub‐epicardial regions of the myocardium (white arrows). (B, C) Non‐contrast‐enhanced thin slice volume CT: coronal (B) and axial (C) views. Bilaterally multiple patchy ground glass opacities and crazy paving are seen. The posterior segments of lower lobes and the periphery of the lungs are predominantly involved.
Discussion
The pathophysiology of cardiac involvement in COVID‐19 remains unclear but likely involves increased cardiac stress due to respiratory failure/hypoxaemia 6 and indirect injury related to cytokine storm and systemic inflammatory response. 7 , 8 SARS‐CoV‐2 binds to the transmembrane angiotensin‐converting enzyme 2 protein. Because this receptor is largely expressed in the cardiovascular system (mainly in the pericytes and endothelial cells), direct cardiomyocyte infection is a third postulated mechanism. 9 , 10 , 11 However, direct evidence confirming the last mechanism is still lacking. We presented a case of CS successfully supported by ECMO in a teenage patient affected by SARS‐CoV‐2 infection. Rare suspected coronavirus particles were found in the cardiac endothelium. Despite that their morphology consistent with that of Coronaviridae, we were unable to certify that those particles corresponded to actual Coronaviridae in the absence of associated cytoplasmic vacuoles and immunogold techniques applied on TEM sections. In addition, we developed an immunohistology technique using an antibody against a SARS‐CoV‐2 recombinant fusion nucleoprotein and a lung tissue specimen of an infected hamster as positive control. No significant immunoreactivity (in either myocytes or endothelial cells) was detected (Figure 3 D ). Interestingly, also in other myocarditis reports, SARS‐CoV‐2 genome was not detected in the myocardium. 12 , 13 , 14 Even if the clinical presentation, laboratory markers, and cardiac imaging techniques strongly suggested fulminant myocarditis, histology and immunohistology were not fully consistent with this diagnosis according to the Dallas and World Health Organization/International Society and Federation of Cardiology criteria. 15 , 16 , 17 Although recent findings suggest that SARS‐CoV‐2 infection might induce endotheliitis as a direct consequence of viral involvement, endotheliitis signs were not found in our patient. 18 We therefore assumed that a massive cytokine storm and inadequate host inflammatory response leading to diffuse myocardial oedema were probably the main causes of CS. To support this hypothesis, we analysed fractalkine expression by immunohistology on myocardial biopsy. The chemokine receptor CX3CR1 is able to bind to fractalkine, which is expressed by activated endothelium in response to TNFα and IFN‐γ. 19 This molecule is well known to be elevated in patients with severe cytokine release syndrome, 20 and CX3CR1‐mediated inflammation has been demonstrated in several inflammatory disorders. 21 Immunohistology sections showed an endothelial immunoreactivity in our patient and no staining in the myocardium of a patient who died from a COVID‐19‐unrelated cause (Figure 3 E and F ). CS is a rare and life‐threatening presentation of SARS‐CoV‐2 infection that might require a mechanical circulatory support and a very long ICU stay. Our case adds another element to the pathophysiology understanding of the SARS‐CoV‐2‐related myocardial injury.
Conflict of interest
Giovanni Garau, Sabrina Joachim, Guy‐Loup Duliere, Maria Melissopoulou, Sandrine Boccar, Vincent Fraipont, Christophe Dugauquier, Pierre Troisfontaines, Olivier Hougrand, Philippe Delvenne, and Etienne Hoffer declare that they have no conflict of interest.
Supporting information
Video S1. PLAX acute phase.
Video S2. SAX acute phase.
Video S3. Ventriculography.
Video S4. CMR Cine.
Video S5. CMR Cine bis.
Video S6. Triplane recovery phase.
Acknowledgements
The authors are grateful to Dr D. Desmecht and M. Sarlet (Animal Medicine Faculty, Liège University) for providing the infected‐hamster lung control tissue specimens.
Garau, G. , Joachim, S. , Duliere, G.‐L. , Melissopoulou, M. , Boccar, S. , Fraipont, V. , Dugauquier, C. , Troisfontaines, P. , Hougrand, O. , Delvenne, P. , and Hoffer, E. (2021) Sudden cardiogenic shock mimicking fulminant myocarditis in a surviving teenager affected by severe acute respiratory syndrome coronavirus 2 infection. ESC Heart Failure, 8: 766–773. 10.1002/ehf2.13049.
References
- 1. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis 2020; 63: 390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet 2020; 11: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garot J, Amour J, Pezel T, Dermoch F, Messadaa K, Felten ML, Raymond V, Baubillier E, Sanguineti F, Garot P. SARS‐CoV‐2 fulminant myocarditis. J Am Coll Cardiol Case Rep 2020; 2: 1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin GM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani LR, Schwartz A, Uriel N. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation 2020; 141: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 7. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol 2020; 5: 751–753. [DOI] [PubMed] [Google Scholar]
- 9. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation 2005; 111: 2605–2610. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates a new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res 2020; 116: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Sawatis K, Marelli‐Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020; 116: 1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020; 22: 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Capelletti AM, Basso C, Godino C. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J 2020; 41: 1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996; 93: 841–842. [DOI] [PubMed] [Google Scholar]
- 16. Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, Olsen EG, Schoen FJ. Myocarditis: a histopathologic definition and classification. Am J Cardiol Pathol 1985; 1: 1–10. [PubMed] [Google Scholar]
- 17. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648. [DOI] [PubMed] [Google Scholar]
- 18. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepback RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID 19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schulz C, Schäfer A, Stolla M, Kerstan S, Lorenz M, von Brühl ML, Schiemann M, Bauersachs J, Gloe T, Busch DH, Gawaz M, Massberg S. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood. Circulation 2007; 116: 764–773. [DOI] [PubMed] [Google Scholar]
- 20. Murthy H, Iqbal M, Chavez JC, Kharfan‐Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther 2019; 8: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ge X‐Y, Fang S‐P, Zhou M, Luo J, Wei J, Wen X‐P, Yan X‐D, Zou Z. TLR4‐dependant internalization of CX3CR1 aggravates sepsis‐induced immunoparalysis. Am J Transl Res 2016; 8: 5696–5705. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. PLAX acute phase.
Video S2. SAX acute phase.
Video S3. Ventriculography.
Video S4. CMR Cine.
Video S5. CMR Cine bis.
Video S6. Triplane recovery phase.


