Abstract
Increased life expectancy combined with the aging Baby Boomer Generation has resulted in an unprecedented global expansion of the elderly population. The growing population of older adults and increased rate of age-related chronic illness has caused a substantial socio-economic burden. The gradual and progressive age-related decline in hormone production and action has a detrimental impact on human health by increasing risk for chronic disease and reducing life span. Here we review the age-related decline in hormone production, as well as age-related biochemical and body composition changes that reduce the bioavailability and actions of some hormones. The impact of hormonal changes on various chronic conditions including frailty, diabetes, cardiovascular disease and dementia are also discussed. Hormone replacement therapy has been attempted in many clinical trials to reverse and/or prevent the hormonal decline in aging to combat the progression of age-related diseases. Unfortunately, hormone replacement therapy is not a panacea, as it often results in various adverse events which outweigh its potential health benefits. Therefore, except in some specific individual cases, hormone replacement is not recommended. Rather, positive lifestyle modifications such as regular aerobic and resistance exercise programs and/or healthy calorically restricted diet can favorably affect endocrine and metabolic functions and act as countermeasures to various age-related diseases. Here we provide a critical review of the available data and offer recommendations which hopefully will form the groundwork for physicians/scientists to develop and optimize new endocrine-targeted therapies and lifestyle modifications that can better address age-related decline in heath.
Keywords: Hormones, Aging, Frailty, Diabetes, Cardiovascular Disease, Exercise, Dementia
Introduction
Aging is inevitable and is the single most important modulator of human life span and health span. The substantially increased morbidity and mortality associated with advancing age contributes to the higher socio-economic cost of the older population. An unavoidable consequence of increased life expectancy is an expansion of the older population. In 2012 it was estimated that there were approximately 43.1 million people aged 65 and older in the United States, and this number is projected to reach 83.7 million by the year 2050.1 Worldwide, the number of people aged 65 and older is projected to be 1.6 billion by 2050.2 The abrupt increase in lifespan that has occurred since the turn of the 20th century has prompted scientists, health care organizations, and national leadership to develop approaches aimed at extending quality-of-life and reducing late-onset diseases of aging.3 It is therefore critical to understand the “normal” age-related changes in human physiology and the underpinnings of these changes. Multiple age-related hormonal and metabolic changes greatly contribute to the principal age-related chronic diseases and decline in physiological functions which include atherosclerosis, hypertension, diabetes, hyperlipidemia, obesity, sarcopenia, osteoporosis, thrombogenesis, chronic inflammation and decline in immune functions. Another emerging health concern of aging is a decline in brain function, which is mostly related to the development of degenerative brain diseases that cause cognitive decline in the form of various types of dementias. Interestingly, the development of cognitive decline during aging is more prevalent in people with metabolic problems. Aging adversely affects not only hormonal secretions but also their biological availability (Eg; sex hormones) and their effects on targeted organs (Eg; insulin resistance). One of the most important questions is whether any or all of the hormonal and metabolic changes that occur with age are preventable and/or reversible. In addition, many metabolic changes, especially those related to hormonal actions, are related to lifestyle modifications that are common as people become older. In this review we will attempt to critically summarize the hormonal and metabolic changes that occur with age and whether and/or how these age-related alterations can be prevented or slowed down thus benefitting the welfare of humanity.
Hormone Changes with Age
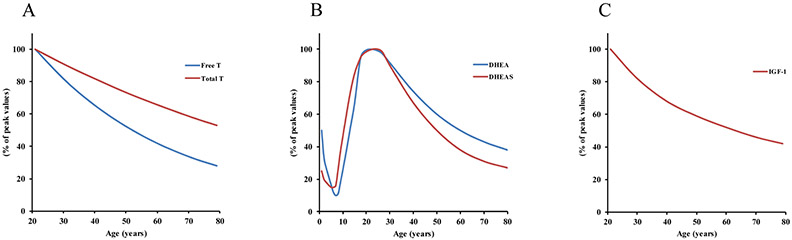
A number of terms have been used to describe the loss of hormone production and their secretory patterns as we age including menopause, andropause, adrenopause, and somatopause.4 Menopause is associated with an abrupt loss of estrogen and progesterone production in women at mid-age following the cessation of ovarian function.5 Although the sudden decline in female sex hormone production in menopause has a clear consequence on cardio-metabolic health, the current review will focus on the adverse health effects of the gradual loss of hormones during aging. For more information regarding the influence of menopause on metabolism in elderly women, we refer readers to other comprehensive reviews.6, 7 A gradual decline in testosterone (T), termed andropause, begins around 20-30 years of age in men and persists until death (Figure 1A). Women also experience decreased T with age, but the T level in women is approximately 10-times lower than in men,8 thus the effects of lower T during aging may be more detrimental in men. Because of the greater decline in T in men, a majority of the studies in this area have been performed in men, therefore generalizing of the effects of andropause across genders should be carefully considered by readers. Adrenopause is characterized by reduced secretion of dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) with advanced age (Figure 1B). Somatopause is the term used to describe a decline in pulsatile secretion of growth hormone (GH), resulting in reduced insulin-like growth factor 1 (IGF-1) that occurs with age (Figure 1C). It has been suggested that the altered hormonal profile that is associated with aging plays a critical role in the onset of many metabolic complications that also come with advancing age.9 Thus, identifying strategies to mitigate the deleterious effects of andropause, adrenopause, and somatopause remain a high priority as the aging population continues to grow.
Figure 1: Decline in hormone production with age.
A: There is a gradual and consistent decline in T production with each year of age in men beginning around the 3rd decade. Free T, the most biologically active form of T, declines at nearly twice the rate of total T. B: Plasma levels of DHEA and DHEA-S sharply decline at birth until the age of ~6-7. A sharp rise in the levels of DHEA and DHEA-S occurs until approximately the 3rd decade. DHEA and DHEA-S then gradually decline until death in men and women, with a slightly steeper decline in DHEA-S compared to DHEA. C: IGF-1 consistently decreases with age beginning around the 3rd decade in men and women. Pulsatile release of GH throughout the day maintains plasma IGF-1 levels through production and secretion from the liver. aT = testosterone; DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate; IGF-1 = insulin-like growth factor 1; GH = growth hormone.
A widely held notion is that approaches for combating the decline in endocrine function observed during aging may improve the quality of life of elderly people. Additionally, if strategies which successfully improve endocrine function in the elderly population can improve quality of life, then a substantial burden on the national and global economy should be lessened.10 In recent decades, hormone replacement therapy has garnered substantial attention due to promising findings, but apparently preventing an age-related decline in hormones by exogenous replacement is associated with increased risk for adverse effects in older adults.11-13 The controversies and conflicting results on hormone replacement have more recently discouraged physicians away from prescribing hormone therapy in most healthy older people. In contrast, the emerging data from multiple studies show the indisputable beneficial effects of lifestyle changes, especially exercise 14, 15 and caloric restriction,16-18 in mitigating many age-related physical and cognitive declines.
The current review presents an overview of the major metabolic consequences of normal aging, many of which are associated with the decline or alteration of endocrine function. Specifically, we address the metabolic outcomes of andropause, adrenopause, and somatopause. Further, we discuss the efficacy and complications of hormone replacement therapy and lifestyle changes, especially exercise, as interventions for treating the age-related declines in metabolic function. By summarizing the collective literature regarding the major hormone-associated metabolic complications that occur during aging, we hope to provide the groundwork for physicians and scientists to develop and optimize new endocrine-targeted therapies and lifestyle modifications that can better address metabolic health concerns during aging.
Reduction of Hormone Availability and Action Related to Age
Testosterone
The pulsatile secretion of gonadotropin-releasing hormone from the hypothalamus results in the release of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary.19 Luteinizing hormone then binds with specific high affinity to luteinizing hormone receptors on the plasma membrane of testicular Leydig cells in men and of theca cells in women, leading to a cascade of signaling events which results in T synthesis.20 Because T is a steroid hormone and cannot be stored in the cells where it is produced, it is immediately secreted into the circulation. Once in the circulation, approximately 98-99% of T associates with hydrophilic binding partners.21 The remaining 1-2% of free T is the most biologically active form of T. Sex hormone-binding globulin (SHBG) is the primary binding protein for T and reduces the transport of T into the cell, thus inhibiting its biological action.22 T also binds to albumin in the blood, but the movement of T is less restricted by albumin and reaches the intracellular compartment.23 After transport through the circulation, T exerts its effect by binding to the intracellular androgen receptor, which subsequently is transported as the androgen receptor-testosterone complex to the nucleus where it induces gene transcription.24 Activation of this hypothalamic-pituitary-gonadal axis has a robust anabolic effect, increasing muscle mass and strength, promoting muscle protein synthesis, and increasing bone mineral density.25-27 The term “hypogonadism” is used to describe the clinical condition of low levels of serum T and its associated symptoms of decreased libido, loss of muscle and bone mass, depression, and anemia.12 Some or all of these features of hypogonadism are present in a milder but variable extent in older men.
The anabolic effect of T is reduced during aging due to a gradual and consistent decline in circulating T that begins around the third to fourth decade in men,28, 29 also known as andropause. Approximately 40-50% of men over the over the age of 80 have T levels below that of normal healthy young individuals.28, 30 By the third decade, both men and pre-menopausal women experience a decline in DHEA and DHEA-S,31 which can serve as precursors for the production of androgenic hormones such as T.32 The decline in total and free T levels in men occurs at a rate of approximately 1% and 2% per year, respectively (Figure 1A).33, 34 Even though women have a considerably lower level of T, they too experience reductions in bioavailable T with age.35 In men, this decline in T has been suggested to be due to a combination of both defective gonadotropin-releasing hormone secretion and Leydig cell responsiveness.36 The biologically active forms of T (free T and albumin–bound T) decrease at a greater rate than SHBG-bound or total T during aging,37 likely because of the age-associated increase in SHBG.28, 38, 39 The increase in SHBG and SHBG-bound T reduces the mobility and effectiveness of endogenously produced T. Thus, not only is T production reduced during aging, but a greater proportion of the T that is produced is less effective.
Dehydroepiandrosterone
Approximately 75-90% of DHEA is produced in the adrenal cortex 40 and converted to DHEA-S, its sulfated form, by hydrosteroid sulfatases.41 The remaining ~10-25% of DHEA production occurs in the testes, ovaries, and brain.42 The secretion of DHEA-S, the most abundant circulating steroid hormone,43 is synchronized with the secretion of cortisol in response to corticotropin-releasing hormone and adrenocorticotropic hormone.44, 45 After traveling through the circulation and arriving at peripheral tissues, DHEA-S can be metabolized back to DHEA by sulfohydrolase.46 DHEA-S essentially serves as a large and stable plasma reservoir for later conversion to DHEA.42 DHEA serves as a precursor to many androgenic and estrogenic hormones.32, 47, 48 Thus, low levels of DHEA or DHEA-S result in an even greater dysregulation of the overall hormonal profile. In fact, around 30% of androgens in men and around 75% of estrogens in premenopausal women are produced from the conversion of DHEA/DHEA-S steroids.42
After birth DHEA and DHEA-S levels sharply decline and do not begin to increase until around 7-9 years of age.42, 49 By age 20-30 years, DHEA and DHEA-S levels reach their peak and steadily decline at a rate of approximately 2-3% per year in both men and women (Figure 1B).33, 50, 51 The age-related trend in DHEA is unique from any other steroid hormone and suggests that the signals to produce DHEA are distinct from signals for the production of other hormones.50 Men have around 2 times greater DHEA-S than women,32 but women have been reported to have greater levels of DHEA.42, 49 The cause of these gender related differences is unknown.
Growth Hormone and IGF-1
Also referred to as somatotropin, GH is a peptide hormone that is synthesized and secreted by the anterior pituitary which initiates signaling processes involved in growth of nearly all tissues in the human body. GH is released in a pulsatile fashion, with the largest peak in GH observed soon after slow wave sleep and numerous smaller peaks in GH observed shortly after meals.52-54 Negligible amounts of GH are produced in between the 10-15 secretory bursts that can occur during a 24-hour period.52 The primary positive and negative regulators of the pulsatile secretion of GH are GH-releasing hormone (GHRH) and somatostatin, respectively.55 Ghrelin, an endogenous ligand of the GH secretagogue receptor, is also a potent stimulant of GH secretion.56 GHRH is secreted by the hypothalamus in response to low levels of GH and IGF-1, and the response to GHRH is inhibited by IGF-1,57 providing a negative feedback loop for GH secretion.58
The magnitude of GH pulses peak during puberty and subsequently decline at a gradual rate of approximately 1-2% per year until death in men and women.59-61 Once released into the blood, GH signals the production and release of IGF-1 by binding to the GH receptor on the plasma membrane of liver and other peripheral tissues.62, 63 IGF-1 is a primary mediator of cellular growth 64 and a critical component of human post-natal development. Like GH, serum IGF-1 increases with age until puberty, and then gradually declines into old age (Figure 1C).61, 65 The reduction in GH and IGF-1 in healthy aging adults is primarily due to a reduction in the amplitude of the GH secretion, not a reduction in frequency of secretory bursts or the half-life of GH.66 The decrease in GH and IGF-1 with age has been termed “somatopause” and appears to have a robust impact on metabolic health.67, 68
Metabolic and Physical Performance Decline of Aging: Related to Hormone Changes and Lifestyle Changes
The reduction in hormone production that commonly occurs with age can influence a variety of metabolic processes (Table 1).
Table 1:
Potential age-related metabolic consequences of reduced testosterone (andropause), DHEA (adrenopause), and growth hormone (somatopause) based on both human observational studies and rodent studies. ↑, increased; ↓ decreased.
| Potential Age-related Metabolic Consequences of: | ||
|---|---|---|
| Reduced Testosterone | Reduced DHEA | Reduced Growth Hormone |
| • ↑ subcutaneous and visceral fat • ↑ risk for obesity • ↓ insulin sensitivity • ↑ risk for type 2 diabetes • ↑ high blood pressure • ↑ triglycerides • ↑ risk of metabolic syndrome • ↓ muscle mass • ↓ strength • ↓ bone density |
• ↑ body fat mass • ↑ waist to hip ratio • ↓ lean body mass • ↓ VO2 max • ↑ risk of cardiovascular disease • ↑ risk of ischemic heart disease • ↓ bone density |
• ↑ risk for obesity • ↑ visceral adipose tissue • ↓ lean body mass • ↓ strength • ↑ risk of metabolic syndrome • ↑ risk of cardiovascular disease • ↓ bone density |
As a consequence, physiological outcomes which are major risk factors for diabetes and metabolic abnormalities are negatively affected. Moreover, the habitual decline in physical activity that occurs with aging can exacerbate these metabolic abnormalities (Figure 2). In this section we touch on some of the physiological outcomes related to the hormonal changes of aging. However, the association between hormonal changes and these physiological outcomes cannot be fully delineated from the influence that lifestyle changes (i.e. physical activity and diet) may also have on these outcomes. Therefore, we also address the influence of positive lifestyle modifications, such as increased physical activity and reduced caloric intake, on important health-related outcomes during aging.
Figure 2: Schematic diagram of the aging-related influence on metabolism.
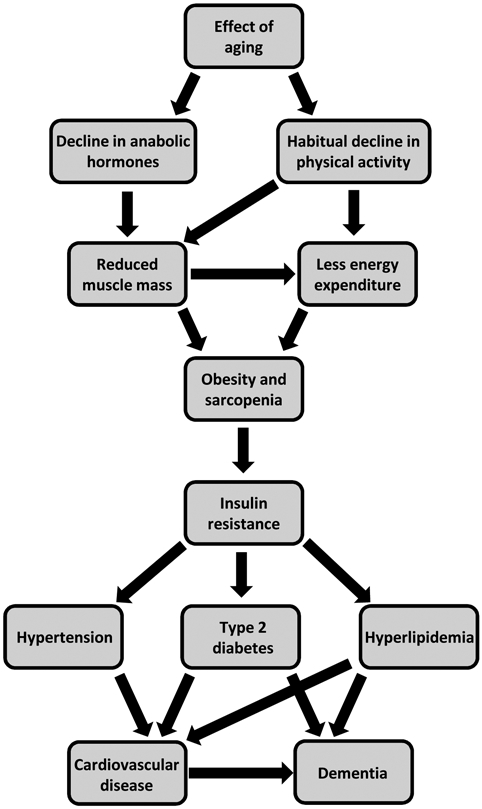
Aging results in a decline in anabolic hormone production and, as is true for a large sum of the older population, a habitual decline in physical activity. This decrease in physical activity results in lower daily energy expenditure, leading to increased rates of obesity in the older population. Moreover, both hormonal dysregulation and reduced physical activity influence the reduction in muscle mass that occurs with age, also known as sarcopenia. The increased rates of obesity in aging combined with the development of sarcopenia can have devastating consequences on metabolism. After the development of insulin resistance, obesity and sarcopenia can result in an increased risk for type 2 diabetes, hypertension, and hyperlipidemia. These metabolic disturbances are known to lead to the development of cardiovascular disease and dementia. Importantly, positive lifestyle modifications such as regular exercise and healthy diet can combat multiple nodes in this process and are critical for healthy aging and the prevention of metabolic disease.
Body Composition / Obesity
Percent fat mass is an important predictor of metabolic disease.69, 70 Altered body composition, particularly loss of lean tissue (especially muscle mass), and increased obesity (accumulation of body fat) become more evident with age and can have profound effects on metabolism. The decline in hormone production that is associated with age may play a critical role in the increased fat mass and decrease in lean tissue that occurs with age. For example, it has been observed in elderly (60-80 year-old) men with subnormal T levels that subcutaneous and visceral fat mass are elevated when compared to elderly men with normal T levels.71 A significant negative association has also been observed between obesity and T levels by multiple groups,72-74 emphasizing the metabolic importance of maintaining T production with age. Lower DHEA-S levels have been associated with greater body fat,75 increased waist to hip ratio,76 and decreased percent lean body mass 77 in men over the age of 60. An inverse correlation between DHEA and BMI has also been found, suggesting DHEA may increase lipolytic capacity and decrease body fat.78 It is logical to speculate that because DHEA serves as a precursor to multiple androgens, such as T, that the beneficial effects of higher endogenous DHEA may be due, at least in part, to an elevation in T production. Increased adiposity 79, 80 is also associated with reduced GH secretion during aging. In fact, 40 weeks of GH administration decreases visceral adipose volume in obese subjects.81 Taken together these data highlight the roles of T, DHEA, and GH in substrate metabolism and storage, and suggest that dysregulation of these important hormones in aging might result in deleterious effects on body composition, an important indicator of metabolic health.
Caloric restriction (CR) has been shown to robustly improve body composition and reduce obesity.82-84 Thus, CR has been touted as an exceptional strategy for improving health and lifespan. In fact, some researchers have explored the feasibility of performing long-term CR studies in humans to assess its effect on multiple health parameters.85, 86 In rodents it has been well established that CR can improve metabolic health and significantly extend lifespan.87, 88 CR can also improve hormonal regulation. In obese male subjects who were calorically restricted for 3 months, total T levels are significantly increased, concomitant with a large decrease in body fat.89 In obese subjects who lost ~30kg of body mass, GH secretion was more than two-fold greater than obese subjects who did not lose weight.90 These findings suggest that CR is a modifiable lifestyle factor which may improve hormonal regulation, resulting in improved body composition, a major risk factor for metabolic disease. However, it is unclear if the beneficial effect of CR on body composition requires the improvement in hormonal production. If hormonal improvements are necessary for the CR effect on metabolism, then future therapies and drugs aimed at improving body composition via CR-related mechanisms should also target these important hormones for an optimal improvement in metabolic health.
Insulin Sensitivity
Reduced insulin sensitivity is an essential precursor in the development of type 2 diabetes. Insulin resistance, type 2 diabetes, and associated clustering of cardio-metabolic changes including dyslipidemia, hypertension, and increased thrombogenesis are significant risk factors for cardiovascular disease and all-cause mortality. The rates of type 2 diabetes and insulin resistance are significantly greater in the elderly population compared to young adults,91, 92 leading to greater risk for cardiovascular events. It is logical to speculate that the reduction in anabolic hormone production that occurs with age may play a role in the reduction in insulin sensitivity that is also commonly observed with age. In elderly men, lower T levels are associated with reduced insulin sensitivity as indicated by higher glucose levels during an oral glucose tolerance test,71 reduced quantitative insulin sensitivity check index score,93, 94 and lower HOMA-IR values.95 Additionally, endogenous GH levels are positively associated with insulin sensitivity in elderly subjects.96, 97 The decline in T production and reduction in GH with age, therefore, may have a significant influence on reducing insulin sensitivity. Low levels of both DHEA and DHEA-S are associated with elevated risk of cardiovascular disease.98-100 Feldman et al. reported that in 40-70 year old men in the lowest quartile for plasma DHEA or DHEA-S there was a significantly greater risk for ischemic heart disease.101 Reduced endogenous GH secretion during aging gives rise to a number of negative metabolic outcomes that collectively result in elevated risk for cardio-metabolic morbidity and mortality, which are deleterious consequences of aging. However, the mechanisms which are responsible for the decline in insulin sensitivity and increased cardiovascular disease risk with age are not entirely clear.
Increasing physical activity level is a simple lifestyle modification that can have a robust impact on health in older populations. Exercise training can significantly improve insulin sensitivity.102 In fact, even a single bout of exercise can enhance insulin-stimulated glucose uptake in whole muscle tissue 103-105 and individual muscle fibers.106, 107 Unfortunately, insulin sensitivity is typically reduced with age.108 In older adults, those who partake in aerobic exercise (AE) ≥ 5 days per week have greater insulin sensitivity than those who exercise ≤ 1 day per week.109 In a separate study, our group previously found that insulin-induced glucose disposal was greater in aerobically trained old and young subjects than in their sedentary counterparts.110 Importantly, this study found that that there were no age-related differences in insulin sensitivity, suggesting that the commonly observed age-related reduction in insulin sensitivity is likely due to reductions in physical activity rather than aging per se. Furthermore, insulin sensitivity can be improved, irrespective of age, by either AE or resistance exercise (RE).111 These data support the provocative idea that simply maintaining activity levels in old age can completely prevent the commonly observed reductions in insulin sensitivity with age. Of course, aging may introduce other symptoms which limit an individual’s ability to remain physically active, indirectly affecting insulin sensitivity. Since it has been well documented that exercise/physical activity can help to maintain normal hormone production with age,112, 113 it is possible that the maintenance of insulin sensitivity in old subjects by exercise is mediated by the maintenance of hormonal production.
Another lifestyle change which can help to maintain insulin sensitivity in aging is CR. Reducing caloric intake to ~75-80% of baseline energy requirements has been shown to maintain insulin sensitivity in overweight middle-aged humans.114, 115 Two separate studies have shown that even in the absence of improvements in mitochondrial content and oxidative capacity, CR designed to reduce body weight by 10% within 16 weeks in obese humans can significantly improve insulin sensitivity.116, 117 Another study demonstrated that insulin sensitivity is negatively associated with percent body fat and waist-to-hip-ratio, but age is not significantly associated with insulin sensitivity,118 suggesting that weight loss (which can be aggressively achieved by CR) may be crucial for improving insulin sensitivity with age. A number of studies have demonstrated that life span is extended and insulin sensitivity is improved in old rodents which are subjected to ~40% CR.119-121 Long-term CR in rats results in elevated Leydig cell production of T in old age,122 suggesting that hormone production mediates the effect of CR on insulin sensitivity in old age. However, since these studies in rats typically employ ~40% CR, which is unfeasible for most humans, it will be critical to determine if the same effects are observed in humans with modest reductions in caloric intake that do not reduce mood and quality of life. It will also be important to determine if CR-induced alterations in hormone production or CR per se are responsible for improvements in human insulin sensitivity.
Aerobic Capacity
Age is associated with a decline in aerobic capacity (VO2max). VO2max is highly dependent on both the amount of mitochondria and the oxidative capacity of the mitochondria in skeletal muscle.123-125 Multiple groups have reported a decrease in skeletal muscle mitochondrial content and function with age.126-128 The aging-associated decrease in mitochondrial content and function can result in reduced VO2max, which is a strong predictor of early mortality.129 In old men, low T is associated with low VO2max and low levels of muscle oxidative phosphorylation genes, suggesting that low T induces reductions in mitochondrial capacity.130 Corroborating this, mice that are treated with exogenous T have transcriptionally upregulated mitochondrial biogenesis.131 Further, low T levels in humans are associated with elevated mitochondrial reactive oxygen species (H2O2) production and enhanced inflammatory markers.132 Together these results support the notion that the maintenance of T levels during aging augments VO2max via maintenance of mitochondrial function. Higher levels of DHEA are also associated with increased VO2max during aging,77 which may be due to elevated mitochondrial biogenesis in response to high levels of T.133 Because DHEA is the primary precursor for conversion to T,134 the beneficial effect of DHEA on aerobic capacity during aging may be due to the maintenance of T levels. Interestingly, although IGF-1 levels decline with age, at least one group has shown that VO2max is not independently associated with IGF-1 levels during aging.135 Four weeks of GH administration at either high or low doses does not alter VO2max in healthy young volunteers.136 However, acute GH administration in healthy young humans, which increases IGF-1, promotes an increase in mitochondrial oxidative capacity and the abundance of various mitochondrial genes in skeletal muscle.137 Therefore, the influence of GH/IGF-1 and aerobic capacity is unclear, since mitochondrial adaptations to GH are present in the absence of any detectable changes in functional aerobic capacity. However, the influences of other anabolic hormones, especially T, clearly have a robust impact on VO2max.
Despite the reduction in VO2max with age, exercise training can prevent the loss of aerobic capacity in older adults. In a study by Holloszy’s group, VO2max was measured in older adults (~62 years old) who were either sedentary or aerobically trained master athletes before and after an 8-year follow up. The reduction in VO2max in sedentary individuals was approximately 12% per decade, whereas in age-matched aerobically trained master athletes only a 5.5% reduction in VO2max per decade was observed.138 Since aging is highly associated with reduced mitochondrial function due to decreasing mitochondrial DNA (mtDNA) and increased DNA oxidation,126 it has been suggested that the declining capacity of mitochondria to produce ATP during aging may contribute to insulin resistance and reduced physical function that occur with age.126 However, AE training (AET) can prevent the loss in mitochondrial function with age. Lanza et al. demonstrated that the normal age related decline in mitochondrial oxidative capacity is not present in AE trained older individuals.110 In fact, much of the decline in mitochondria, especially mitochondrial content and respiration, can be reversed by 3 months of high-intensity interval training (HIIT).111 However, despite the improvements in mitochondria in older trained individuals, the beneficial effect of exercise cannot completely maintain VO2max at levels observed in young subjects.111 The age-associated decline in maximal heart rate is typically reported to be unaltered by exercise training,139-142 but some evidence suggests that the decline in maximal heart rate with age can be attenuated by vigorous exercise.143-145 It appears that stroke volume and oxygen extraction can be maintained by exercise training during aging.140, 146 Thus, there is a possible dissociation between aging-associated declines in VO2max and cardiac output/mitochondrial function. It will be important to clearly identify which factors prevent chronic exercise training from completely reversing the decline in VO2max. Furthermore, it will also be important to know if the declines in these cardiac functions are irreversible.
The many effects that CR has on metabolism have prompted researchers to study the impact of CR on longevity. Because aerobic capacity is a strong predictor of lifespan,147 it is reasonable to speculate that CR is also related to VO2max. In fact, 2 years of 25% CR in humans results in a significant increase in relative VO2max (mL/kg/min) compared to ad libitum controls.148 In rodents, 40% CR prevents the age-associated decline in muscle mitochondrial function 149, 150 and aerobic capacity.150 Moreover, 40% CR in rodents also leads to lower heart mitochondrial H2O2 production,151 reducing mitochondrial damage and extending life span. Lifelong CR in mice can completely prevent the age-related loss of mitochondrial oxidative capacity and efficiency without increasing mitochondrial content,152 suggesting that CR preserves mitochondrial function by maintaining its existing components, not by replacing damaged mitochondria with new mitochondria. However, it is important to make the distinction that CR has not been shown to improve mitochondrial function, but rather CR may prevent the age-associated decline in mitochondria. Understanding the mechanisms by which CR maintains mitochondrial integrity during aging will be important for optimizing the therapeutic potential of this robust lifestyle practice.
Muscle Mass and Strength
Both muscle mass and strength decline with age. Postmortem studies performed in relatively healthy people in Sweden originally reported lower muscle cross sectional area in older people. In particular, this study reported a significant reduction in the number of muscle fibers expressing the type II myosin heavy chain (known as fast-twitch muscle fibers) in older subjects compared to young subjects.153 Cross-sectional data in 60-90 year-old men and women show a significant age-related decline in muscle mass and strength, which corresponds to a reduction in T.154 Longitudinal data show that when older men (~65 years old) were evaluated after a 12-year follow up (at ~77 years old), there was a significant decline in muscle cross sectional area and strength,155 and T consistently declines during this age range.28, 29 In elderly men, low T levels are associated with reduced muscle strength.156 Orchiectomised rats, which have drastically reduced T, display muscle atrophy and reduced muscle ribosome content, but treating orchiectomised rats with T recovers muscle ribosome content to normal values.157 Therefore, at least in rodents, T plays an important role in maintaining muscle mass during aging through regulating ribosomal content, which is critical for protein synthesis. However, the effects of low T in elderly human populations on ribosomal biogenesis, capacity, and content have yet to be evaluated. It also remains to be determined whether maintaining T levels during aging is critical for the conservation of muscle mass and strength via ribosomal biogenesis. Reductions in GH secretion also result in a loss of lean body mass 79, 158 and decreased strength.158 The loss of GH production in aging results in reduced circulating IGF-1, which is an important regulator of muscle mass and strength during aging.159 IGF-1 regulates muscle mass via Akt-mediated signaling, inhibiting forkhead box type O (FoxO) transcription factors and the ubiquitin-proteasome system.160 Further, IGF-1 increases mTOR signaling, resulting in increased ribosomal translation of transcriptome to proteins.161 Thus, both T and GH are important regulators of muscle mass and strength during aging.
Sarcopenia, the loss of muscle mass with age, and reduced strength are well known symptoms of normal aging.162, 163 Thankfully, RE can attenuate and partially reverse the decline in muscle mass that is observed in older adults,164, 165 although high intensity interval training (HIIT; aerobic exercise) can also modestly increase muscle mass. In fact, RE training (RET) can increase muscle mass and strength in old individuals who were previously sedentary, but not to the same extent as younger people.164, 165 Although HIIT modestly increases muscle mass, it is not nearly as effective at improving muscle strength as RET.111 Factors which restrict the capacity of older adults to partake in RET such as increased soreness, risk for injury, and joint pain may be critical barriers which limit benefits of RET. Although reduced muscle mass and strength may be partially due to reduced activity in aging, inactivity does not completely explain muscle loss with age.
Bone Health
After reaching peak bone mineral density (BMD) by the third decade, a consistent decline in bone mass and BMD occurs in both men and women with advancing age, with a steeper decline in women, especially after menopause.166, 167 The declines in bone mass and density with age are accompanied by a drastically increased risk for fractures,168 which are associated with increasingly greater risk for mortality after the age of 60.169 The correlation between bone loss and declining hormone production with age has sparked investigations into the influence of hormones such as T, DHEA, and GH on the maintenance of bone health with age. Although estrogen deficiency in post-menopausal women is clearly linked to increased osteoporosis with age,170, 171 the influence of T, DHEA, and GH on BMD in aging men is not as clear. Although Meier et al. found no relationship between T levels and BMD in healthy older men,172 others have reported a positive association between T and BMD in elderly men 173 and post-menopausal women.174 However, Slemenda et al. have reported that both T and DHEA are negatively associated with BMD in older men.175 These confounding results leave uncertainty in the relationship between T levels in aging and bone health. However, at least in hypogonadal men, T is clearly significantly correlated with BMD.176 Furthermore, in GH-deficient adults, levels of GH are significantly associated with BMD.177 Therefore, since hormone deficiency is increasingly prevalent in older adults and bone loss occurs more rapidly with T or GH deficiency, positive lifestyle strategies for combating declines in hormonal secretion should be considered for the maintenance of bone health.
Consistent lifelong exercise has been known to build and maintain bone health. In childhood, the loading impact of physical exercise has been shown to have a significant impact on the increase in BMD.178-180 Although exercise may only exert minimal increases in BMD in adulthood, it can certainly attenuate the decline in BMD that is associated with age.181 Aerobic exercise, especially exercise which produces a physical loading impact on bone, such as running, has been shown to maintain bone health.182, 183 Resistance exercise training, especially that which encompasses the both upper and lower body exercises has a profoundly positive impact on BMD.181, 184 Thus, for the maintenance of bone health and reducing the risk of fractures in aging, both aerobic and resistance exercise are highly recommended for older individuals.
Cognitive Processes
Aging is associated with cognitive decline, even in the absence of dementia.185 Although the mechanisms responsible for this decline are not completely understood, mounting evidence continues to point towards metabolic derangements in the brain as the culprit for cognitive declines associated with age. Brain glucose metabolism significantly declines in old age 186 and can initiate a chain of deleterious metabolic derangements in the brain which may highly impact cognition. Increased oxidative protein damage in the brain 187 and decreased brain mitochondrial enzyme activity 188 are both associated with aging. Moreover, neuroinflammation has also been highly associated with cognitive aging.189 These, and other, age-related effects in the brain are likely due to altered fuel metabolism. When brain glucose metabolism is disturbed in mice using an insulin receptor antagonist, brain mitochondrial structure and function are dramatically impaired.190 It is likely that maintenance of metabolism, specifically mitochondrial metabolism, in the brain can combat the age related decline in cognitive function. As with other deleterious aging-associated outcomes, positive lifestyle modifications have been shown to prevent or reduce the cognitive decline with age.
Exercise is convincingly beneficial for cognitive health with age. For example, Rogers et al. showed in a 4-year prospective longitudinal study that older adults approaching retirement (~65 years of age) who either continued working or retired and began a regular physical activity routine had significantly better cognitive test scores than those who retired and did not remain physically active.191 Older adults who were underwent AET for 3 months increased functional capacity of key attentional aspects of the brain, but the sedentary control group did not.192 What are the mechanisms responsible for the beneficial effect of AET on cognition in older adults? Some suggest that improved blood flow and oxygen delivery are responsible for these changes.193, 194 Others have provided evidence that improved brain mitochondrial function following AET can improve brain metabolism,190 potentially leading to improved cognition. Perhaps multiple mechanisms are responsible for the cognitive improvements following AET. It is also important to note that RET has also been shown to improve cognitive functioning in older adults,195, 196 but the mechanisms of its effect are even less clear than AET. AE and RE can independently improve cognition, but the brain signaling processes that occur after each type of exercise are distinct,197 suggesting AET and RET have different mechanisms of action. Understanding how both RET and AET can improve/maintain brain function with aging will be a critical step for prescribing therapies and creating drugs which mitigate the effects of aging on the brain.
CR is another lifestyle modification which can improve cognitive function in older adults. Witte et al. showed in healthy older people (mean age 60.5 years) that 3 months of 30% CR significantly improved verbal memory scores.16 Age-dependent cognitive deficits that are observed in ad libitum-fed mice are absent in mice that are 30% calorically restricted.198 In these same mice it was observed that hippocampal autophagy processes were upregulated during CR,198 suggesting the process of removing damaged and dysfunctional proteins is crucial for maintaining cognitive function during aging. It is reasonable to speculate that these processes of improved protein turnover in the brain can enhance brain mitochondrial structure and function. Supporting this idea, Sanz et al. have demonstrated that 40% CR in old rats results in reduced brain mitochondrial H2O2 production and lower oxidative damage to nuclear DNA.199 Thus, the metabolic processes which occur in the brain in response to CR can have a substantial beneficial effect during aging and therefore may reduce cognitive decline.
Impact of Hormone Replacement in Aging
T replacement therapy has been introduced as a mode for treating many of the metabolic deficiencies that come with age. Various methods of T replacement such as oral tablets, mucoadhesives, injections, transdermal patches or cream, and subdermal implants have been used and are reported to provide multiple health benefits to hypogonadal men.200 Of course the various forms of T replacement have distinct advantages and disadvantages. For example, injectable T is relatively inexpensive, but the prescribed weekly injections result in peaks in T soon after the injections that are supraphysiological and dips in T by the end of the week. Transdermal patches or cream provide a steady and consistent lower dose of T, but may result in skin irritation or inadequate absorption. Regardless of the administration method, T replacement has been shown to provide a variety of health benefits. The Testosterone Trials, a multi-center set of randomized trials across 12 clinical sites, tested the effect of T administration in 790 elderly men on 7 different primary outcomes (sexual function, physical function, vitality, cardiovascular health, bone health, cognitive function, and anemia).201 These trials and other independent studies found that T administration in elderly men resulted in improvements in sexual function,202, 203 lean body mass,204, 205 physical function,206 strength,25, 205, 207 protein synthesis,25 cholesterol,204, 205, 208 and bone density.209 Potentially explaining some of the positive effects of T on human health, studies using cell culture and rodent models reveal that T administration increases the activity of glycolytic enzymes (hexokinase, phosphofructokinase, and glycogen synthase) and upregulates the expression of genes and proteins involved in glucose metabolism (IRS-1, IRS-2, GLUT4, PPARγ).210-213 However, some groups have shown that T replacement in old men does not provide significant benefits in strength 214-217 or cognition,218 increases coronary artery plaque formation,219 and is consistently shown to have no effect on insulin sensitivity.216, 220 The disparate findings regarding efficacy of T replacement and its effect on metabolic health have created some controversy, especially considering the potential risks associated with T replacement treatment. Some of the risks associated with T therapy include exacerbation of prostate cancer, cardiovascular-related events, hepatotoxicity, erythrocytosis, sleep apnea, and dermatological issues.12, 13 A meta-analysis analyzing adverse events of T therapy reported that in those who received T replacement therapy compared to placebo, the odds of developing a prostate event or having a hematocrit >50% were 1.78 and 3.69 times greater, respectively.221 Presumably, lower doses of T may not exert such adverse health effects, but unfortunately low-dose T does not have physiologically relevant beneficial effects on health.216 The duration of T replacement in most previous studies ranges from approximately 1-36 months, but the side effects of longer duration T replacement therapy have yet to be assessed. Moreover, most studies evaluating the effect of T replacement are performed in relatively healthy elderly men. However, those who likely stand to benefit the most from T replacement therapy are the frail elderly, yet the beneficial and adverse effects of T replacement in this population are largely unknown.222
Although the efficacy of T replacement therapy remains under question, some have postulated that treatment with the T precursor, DHEA, may provide improvements in health without negative side effects. Studies in cultured skeletal muscle cells and rodents have suggested that DHEA administration can increase the expression of the glucose transporter GLUT4 and key glycolytic enzymes, phosphofructokinase and hexokinase.212, 223 However, studies examining the influence of DHEA administration in humans are less promising. DHEA has been introduced as an “anti-aging” therapy via ingestible tablets or transdermal patches. Although both of these modes of administration of DHEA clearly elevate plasma DHEA and DHEA-S levels,224 the beneficial effect on metabolism in the elderly population is underwhelming. In old men and postmenopausal women, although DHEA administration has been shown to produce very minor elevations in bone mineral density,216, 225, 226 these increases are not as large as those produced by other therapies. The collective literature suggests that DHEA therapy has no significant effect on muscle mass and strength 227, 228 or insulin sensitivity.48, 216, 229-231 Long term (24 month) DHEA administration in old people, which elevated DHEA levels to that in the high-normal range for young people, did not result in improvements in body fat or muscle mass.216 Conflicting findings regarding DHEA administration and cholesterol have been reported. One group has shown that DHEA administration seems to lower HDL in postmenopausal women,40 but another group has shown elevated HDL, and decreased LDL and plasma triglycerides in postmenopausal women.232 It has been suggested that DHEA supplementation may improve vascular endothelial function and cardiovascular disease, but no long term (multiple year) studies have evaluated the impact of DHEA therapy on cardiovascular health.233 The current state of the literature suggests that DHEA may have minor metabolic health benefits, but long term adverse side effects are not completely known. Thus, DHEA supplementation should be approached with caution, and should be immediately terminated at the onset of any side effects.
Since the seminal publication nearly 30 years ago in the New England Journal of Medicine by Rudman et al. showing that GH replacement in elderly men resulted in increased lean body mass and decreased fat mass,234 multiple groups have evaluated the efficacy of GH replacement in old men and women. Subsequently, other groups have also shown that GH therapy can improve body composition 235 and cholesterol 236 in GH-deficient older adults. These initial promising findings in GH-deficient adults prompted the promotion of GH replacement by the medical industry in old adults (without clinical GH deficiency) with little regard for the potential negative side effects. GH replacement has been associated with increased risk for adverse events such as soft tissue edema, carpal tunnel syndrome, glucose intolerance, type 2 diabetes, joint pain, and gynecomastia in healthy older adults.237 Moreover, although GH-replacement may provide minor benefits in body composition to healthy elderly subjects, GH-replacement does not improve strength, VO2max, bone mineral density, lipid levels, or fasting glucose.237, 238 It has also been suggested that the improvements in lean body mass following GH administration may be due to elevated water retention, which artificially increases values for lean body mass using certain methods for computing lean body mass. This is corroborated by the fact that although increases in lean body mass are observed after GH treatment, no effect of muscular strength is often observed in healthy older subjects.215, 217, 238-240 Thus, the efficacy and safety of GH replacement in the healthy aging population remains controversial. Based on the collective literature, the use of GH replacement for non-medical conditions such as aging is now strongly discouraged by the American Association of Clinical Endocrinologists.241
Impact of Lifestyle Modifications on Hormone Production in Aging
Aging is associated with a decrease in physical activity levels.242 In general, this change in physical activity with age appears to be at least partially due to declines in occupational activity which are not offset by increases in leisure activity, especially upon retirement.243 Unlike hormone replacement therapies, increased physical activity levels and calorically restricted diets in older adults rarely results in negative side effects. Although fear of injury is a commonly reported barrier to exercise in the older population, multiple studies have reported that older adults are not at an increased risk for exercise-related injuries.244, 245 Frailty is a commonly reported side effect of caloric restriction in the aging population, which can likely be offset by maintaining dietary protein intake.246 Frailty in older adults can also be drastically reduced by combined aerobic and resistance exercise.247 The minimal risks that are posed by exercise or CR in the aging population are greatly outweighed by the positive impact that these lifestyle modifications can have on overall health. In particular, the following section describes the influence that regular physical exercise can have on hormone production in the aging population.
Acute RE has been shown to increase endogenous T production in old men,248-253 but this effect is worn of by ~2hr post-exercise.254 It appears that a low intensity or low volume of acute RE does not result in as robust of an effect as high intensity or high volume exercise on T levels.253, 255-258 Not surprisingly, the beneficial effects of RE (improved muscle mass and strength, elevated muscle protein synthesis, and increased bone mineral density) are similar to the primary reported functions of T on metabolism. Compared to old men, young men have a greater increase in total and free T in response to an acute bout of RE,248 possibly explaining the larger absolute increases in strength that are observed in young men following RET compared to old men.259, 260 However, RET, which increases muscle strength, does not result in elevated basal levels of T in middle-aged or old men.249, 256, 261-263 RET does appear, however, to increase the effect of an acute bout of high intensity exercise on free and total T.264 Thus, the repeated effect of multiple acute bouts of RE on T level, rather than RET per se, may underlie the beneficial effect of RE on metabolic health in old men. Table 2 summarizes the notable literature that assesses the effects of acute or chronic RE on endogenous T production in old men and women.
Table 2:
A summary of notable studies that examined the acute and training effect of resistance exercise on endogenous testosterone levels in old men and women.a T= testosterone; RE= resistance exercise; RET=resistance exercise training; AE= aerobic exercise; ↔= unchanged; ↑= increased; 1RM= 1-rep maximum; 10RM=10-rep maximum.
| Author, Year | Exercise Duration | Exercise details | Gender | Age | Effect of exercise on T |
|---|---|---|---|---|---|
| Craig, 1989 263 | Training (12 wk) | Progressive whole body RE | male | 63 ± 1 | ↔ in resting T following RET. |
| Hakkinen, 1994 262 | Training (12 wk) | Progressive whole body RE | male and female | male:64-73 female:66-73 | ↔ in free or total resting T post-RET in old subjects. |
| Hakkinen, 1995 255 | Acute | 5 sets of 10 reps (at ~10RM) for 3 exercises (bench press, leg press, sit-ups) | male and female | male:68 ± 3 female:69 ± 3 | ↔ in T immediately following RET in old subjects. |
| Nicklas, 1995 256 | Both Acute and Training (16 wk) | Progressive whole body RE | male | 60 ± 4 | Resting T ↔ after RET. In both trained and untrained men, T was ↔ after an acute bout of RE. |
| Hakkinen, 1998 253 | Acute | Upper body, lower body, or both upper and lower body RE | male | 70 ± 4 | ↑ in total and free T only occurred when lower body exercises were included. |
| Kraemer, 1998 248 | Acute | 4 sets of 10RM squats | male | 62 ± 3.2 | T was ↑ immediately and up to 30 min following RE. |
| Hakkinen, 2000 249 | Both Acute and Training (24 wk) | Progressive whole body RE | male and female | male:72 ± 3 female:67 ± 3 | Trained and untrained men ↑ total and free T after acute RE. Only trained women increase free T after acute RE. Resting T is ↔ by RET. |
| Hakkinen, 2002 261 | Training (24 wk) | Progressive whole body RE | male and female | male:65 ± 5 female:64 ± 4 | ↔ in basal T concentrations in old women or men after RET. |
| Kostka, 2003 257 | Acute | low-volume RE: 6-16 reps of leg extensions at 30-70% of 1RM | male and female | male:71 ± 5 female:71 ± 4 | ↔ in T concentrations in old women or men immediately after acute low-volume RE. |
| Baker, 2006 251 | Acute | Whole body: 3 sets, 10 reps at 80% of 1RM for 6 exercises | male | 65 ± 1 | Total and free T ↑ immediately post-RE, but was returned to baseline by 15 min post-RE. |
| Smilios, 2007 252 | Acute | Whole body: 3 sets, 15 reps at 60% 1RM for 6 exercises | male | 69 ± 5 | T was ↑ immediately post-RE and at 15 min post-RE. |
| Roberts, 2009 258 | Acute | Lower body only (squat, leg press, leg extension): 3 sets, 10 reps at 80% 1RM | male | 68 ± 1 | Free T was ↔ at 5min post-RE. |
| Ahtiainen, 2011 250 | Both Acute and Training (21 wk) | Progressive whole body RE | male | 60-65 | ↑ during acute RE in both trained and untrained. |
| Lovell, 2012 264 | Training (16 wk) | Progressive lower body | male | 70-80 | ↑ in free and total T in response to acute AE following RET. |
| Paunksnis, 2018 254 | Acute | constant intensity: 3 sets, 10 reps, at 75% 1RM variable intensity: 3 sets, 8-12 reps, at 67-80% 1RM | male | 65 ± 3 | ↔ T at 2h or 24h post-exercise. Trend for ↑ T at 2h post-exercise. Did not report T immediately post-exercise. |
Interestingly, when T therapy is combined with RET, there was no additive effect of T therapy on upper or lower body strength.265 However, RET plus T therapy showed a greater reduction in fat mass compared to RET alone,265 suggesting T therapy in combination with RET may elicit some additional health benefits, such as body composition, but not functional improvements (muscle strength). Currently, it appears that RET is the most effective and safe approach for older adults to attenuate the deleterious effects of age-associated declines in T on metabolism. T replacement therapy in elderly people should be used with extreme caution, and may only be an acceptable therapy for clinical cases of hypogonadism if prescribed by a physician.
DHEA and DHEA-S are generally found to be increased following exercise. Tremblay et al. observed that acute RE, but not AE, increased DHEA-S in resistance trained 18-55 year-old men.266 Copeland et al. corroborated this finding in women by showing that acute RE, but not AE, increased DHEA in young and old women.267 Others have found either elevated268 or unchanged 269 DHEA or DHEA-S in response to acute sub-maximal exercise in older adults. When RET was performed for 4 months in old men and women, Villareal and Holloszy found that there was no change in endogenous levels of DHEA.228 Hersey et al. corroborated this finding by showing in 70-79 year-old men and women that 6 months of either AET or RET did not result in elevated plasma DHEA.270 Though few studies have been conducted, it appears that increases in DHEA are present following an acute bout of exercise in elderly subjects if the intensity and volume of exercise are robust enough to elicit a response. However, like the response of T to exercise training, DHEA is not altered following exercise training in elderly subjects. Table 3 summarizes the notable literature assessing the effects of acute exercise and exercise training on endogenous DHEA production in elderly men and women.
Table 3:
A summary of notable studies that examined the acute and training effect of exercise on endogenous DHEA levels in old men and women. a DHEA= dehydroepiandrosterone; DHEA-S=Dehydroepiandrosterone sulfate; RE= resistance exercise; RET=resistance exercise training; AE=aerobic exercise; AET=aerobic exercise training; ↔= unchanged; ↑= increased; 1RM=1-rep maximum; 10RM=10-rep maximum; HRmax= maximum heart rate.
| Author, Year | Exercise Duration | Exercise details | Gender | Age | Effect of exercise on DHEA |
|---|---|---|---|---|---|
| Hersey, 1994 270 | Training | RET: progressive whole body AET: progressive treadmill walking/jogging |
male and female | 70-79 | ↔ resting DHEA following 6 months of RET or AET. |
| Copeland, 2002 267 | Acute | RE: 8 whole body exercises 3 sets, 10 reps at 10RM AE: 40 min cycling at 75% of HRmax |
female | mean 62.3 | RE, but not AE, ↑ DHEA immediately post-exercise. DHEA values returned to normal by 30 min post-exercise. |
| Villareal, 2006 228 | Training | Whole body progressive RET: 2-3 sets, 6-12 reps at 65-85% 1RM for 4 months |
male and female | 65-78 | ↔ resting DHEA following 4 months of RET. |
| Aldred, 2009 269 | Acute | AE: 50% of maximal treadmill workload for 30 min | male and female | 65-75 | ↔ DHEA or DHEA-S immediately post-exercise. |
| Heaney, 2013 268 | Acute | AE: incremental submaximal walk test, terminated at 75% HRmax | male and female | 60-77 | DHEA was ↑ immediately post-exercise and was ↔ by 1 hr post-exercise. |
Although DHEA administration alone has been widely shown to have little to no beneficial effect on metabolic health, some groups have assessed the combined effect of DHEA supplementation with exercise training on human health. In 65-78 year-old men and women it was shown that daily DHEA replacement therapy in addition to 4 months of heavy RET had a greater effect than placebo + RET on muscle size and strength.228 Another group found that DHEA supplementation with light AE or yoga exercise in >70 year old women resulted in increased lower body strength compared to the group which did not receive DHEA.271 However, Igwebuike discovered that when DHEA was administered during combined AET and RET in post-menopausal women that DHEA therapy provided no additional benefits in physical performance, body composition, or insulin sensitivity compared to training without DHEA.272 These limited results suggest that DHEA in combination with exercise training may have a beneficial effect on some aspects of human health. However, the modest amount of studies in this area limits the interpretations that can be made regarding the influence of DHEA in combination with exercise training. Of note, none of the aforementioned studies provide a mechanistic explanation for the additional benefit that DHEA potentially provides when combined with exercise training. Since the underlying mechanisms regarding any beneficial effect of DHEA in combination with exercise training remain unclear, we currently do not recommend this strategy for improving metabolic health. Rather, exercise alone (without exogenous hormone therapy) currently remains the most robust and safe option for improving metabolic health in aging populations.
Exercise remains an exciting therapeutic tool that may be used to mitigate the aging-associated dysregulation of the GH/IGF-1 axis. It has long been known in young-middle aged subjects that during an acute bout of exercise there is a sharp rise in GH concentrations in the blood.273-275 GH is also increased in old subjects in response to acute heavy RE,256, 276, 277 but in the post-exercise recovery period the reversal of GH back to resting values occurs at a quicker rate in old subjects compared to young.248, 278 The increase in GH during exercise appears to be dependent on exercise intensity.273 In particular, heavy RE or very high intensity AE will result in the largest GH response.279 In old men and women, exercise needs to be performed above lactate threshold for a significant increase in GH to occur, but younger subjects can experience increased GH at lower intensities of exercise.280 Table 4 summarizes the notable studies which assess the acute effect of exercise on GH secretion in old subjects.
Table 4:
A summary of notable studies assessing the acute effect of exercise on growth hormone secretion in old men and women. aGH= growth hormone; RE= resistance exercise; AE=aerobic exercise; ↔= unchanged; ↑=increased; 1RM=1-rep maximum; 5RM= 5-rep maximum; 10RM=10-rep maximum; LT= lactate threshold.
| Author, Year | Exercise Type | Exercise details | Gender | Age | Effect of exercise on GH |
|---|---|---|---|---|---|
| Craig, 1989 263 | Resistance | 8 exercises, 1-3 sets, 8-10 reps | male | 63 ± 1 | Acute RE ↑ GH in old men immediately and 15min post-exercise. |
| Pyka, 1992 276 | Resistance | 13 exercises, 3 sets, 8 reps at 70% of 1RM | male and female | 72 ± 1 | Acute RE resulted in a small but significant ↑ in GH in old men and women. |
| Pyka, 1994 281 | Resistance | 12 exercises, 3 sets, 8 reps at 85% of 1RM | male and female | 70 ± 1 | Acute RE ↑ GH in old men and women. The same effect was observed regardless of training status. |
| Nicklas, 1995 256 | Resistance | 14 exercises, 1-2 sets, starting at ~5RM with decreasing weight until 15 reps achieved | male | 60 ± 4 | Acute RE ↑ GH in old men. The same effect was observed regardless of training status. |
| Kraemer, 1998 248 | Resistance | Squats, 4 sets, 10 reps at 70% of 1RM | male | 62 ± 3 | Acute RE ↑ GH in old men immediately- and 5min-post exercise. GH returned to sedentary values by 15min post-exercise. |
| Kraemer, 1999 278 | Resistance | Squats, 4sets, 10reps at ~10RM | male | 62 ± 3 | Acute RE ↔ GH in old men immediately-post exercise. Data appear to show trend for ↑ GH, but not statistically significant. |
| Weltman, 2006 280 | Aerobic | Treadmill run at 75% of the difference between LT and VO2max | male and female | male:64 ± 2 female:66 ± 4 | Acute AE approached a significant ↑ in GH for old men (P=0.07) and women (P=0.09). |
| Manini, 2012 277 | Resistance | Knee extension, 4 sets at 80% of 1RM until volitional fatigue | male | 67 ± 5 | Acute RE ↑ GH in old men. |
AET appears to attenuate the acute effect of exercise on GH secretion,282 but RET does not change the acute effect of exercise on GH release in young283 or old281 subjects. Though the mechanisms for the exercise-mediated increase in GH concentration are not completely understood, it has been hypothesized that nitric oxide, afferent neural stimulation, or blood lactate play significant roles in the elevation of GH post-exercise.284 The beneficial metabolic effects of exercise may partially be due to elevations of GH concentration, but a causal link between post-exercise GH and metabolism will be difficult to explicitly demonstrate. The combined effect of exercise and GH replacement has provided some insight into the effect of GH on metabolism post-exercise. Yarasheski et al. showed that RET plus GH replacement versus RET without GH replacement had a greater increase in fat free mass and protein synthesis, but not muscle strength in young men285. The authors attributed the lack of an additive effect of GH on functional capacity of skeletal muscle to increased non-skeletal muscle lean tissue.285 This finding in young men resulted in a follow up study by the same group in old men. Very similar results were reported,286 suggesting a lack of an additional functional benefit of GH therapy when combined with RET. Thus, GH replacement does not seem to enhance the exercise benefit on muscle strength and is not recommended as a therapy for aging individuals. Exercise remains the most optimal method for improving metabolic health in older individuals.
Twelve-weeks of CR increases total T in obese men by improving testicular function and reducing conversion of T to β-estradiol by aromatase in the adipose tissue.89 However, this young population of unhealthy subjects is known to have lower than normal T levels. In another study in young but healthy subjects, CR actually reduced T levels.287 Additionally, CR significantly reduces T production in healthy young rats in a dose response fashion.288 However, when rats which have been calorically restricted early in life (reducing T production) are aged, the Leydig cell production of T is significantly greater than in ad libitum-fed controls in older age.122 Perhaps the reduced T production at young ages and subsequently increased T production with advancing age serves as a protective effect of CR. However, in late-middle aged (~50 year-old) humans who had consistently performed CR for ~7.5 years, T production is significantly reduced compared to control subjects that were not calorically restricted.289 The conflicting findings make it unclear how/if CR can exert an effect on metabolism by regulating T production. In obese subjects who have lost ~30kg of weight, GH secretion is significantly greater than obese subjects who remain weight stable.90 However, as is the case with T, CR does not appear to alter GH production in normal weight subjects. Two of the only long-term CR studies in humans (CALERIE and Biosphere 2) both reported negligible effects on other important hormones discussed in this review (DHEA-S, GH, and IGF-1),290 providing convincing evidence that CR does not influence the production of these hormones in normal weight subjects. To the best of our knowledge, no comprehensive studies have been performed in older healthy subjects that assess the effect of long term CR on anabolic hormone production. It is important to note that because older adults display a significantly lower anabolic response to low dose dietary protein than younger subjects246 that caloric restriction in the aging population should limit the reduction in protein intake. Interestingly, Levine et al. reported a significant reduction in all-cause mortality in older adults that consumed a high protein diet (≤ 20% kcal protein) compared to older adults consuming a low protein diet (< 10% kcal protein).291 Others have even suggested that to avoid developing frailty that aging adults who partake in CR should ingest as much as 30-35% of their total caloric intake in protein.292 However, potential adverse effects of high protein intake in older adults, especially on renal function, will need further investigation.293 It will be important to determine the long term effect of CR in elderly subjects on anabolic hormone production, especially when the CR includes an optimal dosage of protein. Until the effect of CR is more clearly described in the elderly population, exercise training currently exerts a more convincing impact on hormone production than CR.
Conclusion
The aging process is quite complex, and can affect a variety of hormones which are important for physical performance, body composition, metabolic health, and cognition. A host of metabolic derangements are associated with the decline in endogenous production of T, DHEA, and GH/IGF-1 during aging (Table 1). As a result, extensive research has examined various approaches for combating the detrimental metabolic impact of andropause, adrenopause, and somatopause. Hormone replacement therapy often results in either very minor benefit and/or increased risk for adverse events in the healthy aging population. Thus, for aging adults that do not have clinical indications of hormone deficiency based on careful assessment, hormone replacement therapy is not advised. Rather, probably the most effective and modifiable lifestyle factors that result in health benefits during aging are exercise and CR. CR and exercise can aid in preventing excess fat accumulation and maintaining muscle mass, critical factors for preventing age-related frailty and cardio-metabolic risks. As discussed throughout this review, exercise and CR can affect the regulation of multiple hormones that are important for healthy aging. Unlike hormone replacement therapy, exercise or CR rarely result in detectable negative side effects. Additionally, besides the influence that exercise and CR have on endocrine regulation during aging, there are a variety of additional health benefits that these lifestyle modifications can provide. However, the mechanisms responsible for their robust effects on endocrine regulation in older adults are still not completely understood. It will be crucial to further understand how exercise and CR exert such powerful effects on hormonal regulation of metabolism in order to more effectively treat chronic age-related metabolic disease. Because many aging adults are unable to exercise or cannot perform exercise at a high enough intensity to experience significant benefit, the development of drugs or therapies which target the exercise-mediated molecular events involved in hormonal regulation could prove to be quite valuable.
Alphabetical List of Abbreviations
- AE
aerobic exercise
- AET
aerobic exercise training
- BMD
bone mineral density
- CR
caloric restriction
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone Sulfate
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- HIIT
high intensity interval training
- IGF-1
insulin-like growth factor 1
- RE
resistance exercise
- RET
resistance exercise training
- SHBG
sex hormone-binding globulin
- T
testosterone
Footnotes
Potential Competing Interests: All authors report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States: United States Census Bureau, Economics and Statistics Administration, US; 2014. [Google Scholar]
- 2.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016: US Department of Commerce, Economics and Statistics Administration, US; 2018. [Google Scholar]
- 3.Olshansky SJ. From lifespan to healthspan. Jama. 2018;320:1323–1324. [DOI] [PubMed] [Google Scholar]
- 4.Lamberts SW, Van den Beld AW, Van Der Lely A-J. The endocrinology of aging. Science. 1997;278:419–424. [DOI] [PubMed] [Google Scholar]
- 5.Greendale GA, Lee NP, Arriola ER. The menopause. The Lancet. 1999;353:571–580. [DOI] [PubMed] [Google Scholar]
- 6.Stachowiak G, Pertyński T, Pertyńska-Marczewska M Metabolic disorders in menopause. Przeglad menopauzalny= Menopause review. 2015;14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Seminars in reproductive medicine. Vol 28: © Thieme Medical Publishers; 2010:426–434. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LW, Cureton KJ, Thompson FN. Comparison of serum testosterone and androstenedione responses to weight lifting in men and women. European journal of applied physiology and occupational physiology. 1983;50:413–419. [DOI] [PubMed] [Google Scholar]
- 9.Guarner-Lans V, Rubio-Ruiz ME, Pérez-Torres I, de MacCarthy GB. Relation of aging and sex hormones to metabolic syndrome and cardiovascular disease. Experimental gerontology. 2011;46:517–523. [DOI] [PubMed] [Google Scholar]
- 10.Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health affairs. 2013;32:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes SJ, Shalet SM. Which adults develop side effects of growth hormone replacement? Clinical endocrinology. 1995;43:143–149. [DOI] [PubMed] [Google Scholar]
- 12.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. New England Journal of Medicine. 2004;350:482–492. [DOI] [PubMed] [Google Scholar]
- 13.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. New England Journal of Medicine. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen JA. Impact of physical exercise on endocrine aging. Sports Endocrinology. Vol 47: Karger Publishers; 2016:68–81. [DOI] [PubMed] [Google Scholar]
- 15.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocrine reviews. 2012;33:314–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witte A, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proceedings of the National Academy of Sciences. 2009;106:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. Journal of gerontology. 1987;42:78–81. [DOI] [PubMed] [Google Scholar]
- 18.Fontán-Lozano Á, Sáez-Cassanelli JL, Inda MC, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. Journal of Neuroscience. 2007;27:10185–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HH. Regulation of gonadotropin-releasing hormone gene expression. Seminars in reproductive medicine. Vol 25: Copyright© 2007 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New: …; 2007:313–325. [DOI] [PubMed] [Google Scholar]
- 20.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Molecular and cellular endocrinology. 2009;299:23–31. [DOI] [PubMed] [Google Scholar]
- 21.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training. Sports medicine. 2010;40:1037–1053. [DOI] [PubMed] [Google Scholar]
- 22.Hobbs CJ, Jones RE, Plymate SR. The effects of sex hormone binding globulin (SHBG) on testosterone transport into the cerebrospinal fluid. The Journal of steroid biochemistry and molecular biology. 1992;42:629–635. [DOI] [PubMed] [Google Scholar]
- 23.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier: primary role of albumin-bound hormone. The Journal of clinical investigation. 1979;64:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobinet J, Poujol N, Sultan C. Molecular action of androgens. Molecular and cellular endocrinology. 2002;198:15–24. [DOI] [PubMed] [Google Scholar]
- 25.Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. American Journal of Physiology-Endocrinology And Metabolism. 1995;269:E820–E826. [DOI] [PubMed] [Google Scholar]
- 26.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. New England Journal of Medicine. 1996;335:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. The Journal of Clinical Endocrinology & Metabolism. 1999;84:1966–1972. [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2001;86:724–731. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:M76–M99. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Bailliere's clinical endocrinology and metabolism. 1997;11:289–309. [DOI] [PubMed] [Google Scholar]
- 31.Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. The Journal of Clinical Endocrinology & Metabolism. 1995;80:1429–1430. [DOI] [PubMed] [Google Scholar]
- 32.Labrie F, Bélanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. The Journal of Clinical Endocrinology & Metabolism. 1997;82:2403–2409. [DOI] [PubMed] [Google Scholar]
- 33.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of Clinical Endocrinology & Metabolism. 2002;87:589–598. [DOI] [PubMed] [Google Scholar]
- 34.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. The Journal of Clinical Endocrinology & Metabolism. 2006;92:549–555. [DOI] [PubMed] [Google Scholar]
- 35.Fabbri E, An Y, Gonzalez-Freire M, et al. Bioavailable testosterone linearly declines over a wide age spectrum in men and women from the Baltimore Longitudinal Study of Aging. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2016;71:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. European Journal of Endocrinology. 1999;141:257–266. [DOI] [PubMed] [Google Scholar]
- 37.Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. The Journal of Clinical Endocrinology & Metabolism. 2006;91:1336–1344. [DOI] [PubMed] [Google Scholar]
- 38.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. The Journal of Clinical Endocrinology & Metabolism. 1991;73:1016–1025. [DOI] [PubMed] [Google Scholar]
- 39.Leifke E, Gorenoi V, Wichers C, Von Zur Mühlen A, Von Büren E, Brabant G. Age-related changes of serum sex hormones, insulin like growth factor 1 and sex hormone binding globulin levels in men: cross sectional data from a healthy male cohort. Clinical endocrinology. 2000;53:689–695. [DOI] [PubMed] [Google Scholar]
- 40.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. The Journal of clinical endocrinology and metabolism. 2011;96:1642–1653. [DOI] [PubMed] [Google Scholar]
- 41.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEAD-S: a review. The Journal of Clinical Pharmacology. 1999;39:327–348. [DOI] [PubMed] [Google Scholar]
- 42.Mannella P, Simoncini T, Caretto M, Genazzani A. Dehydroepiandrosterone and cardiovascular disease. Vitamins and hormones. Vol 108: Elsevier; 2018:333–353. [DOI] [PubMed] [Google Scholar]
- 43.Montanini V, Simoni M, Chiossi G, et al. Age-related changes in plasma dehydroepiandrosterone sulphate, cortisol, testosterone and free testosterone circadian rhythms in adult men. Hormones. 1988;29:1–6. [DOI] [PubMed] [Google Scholar]
- 44.Pavlov EP, Harman SM, Chrousos GP, Loriaux DL, Blackman MR. Responses of plasma adrenocorticotropin, cortisol, and dehydroepiandrosterone to ovine corticotropin-releasing hormone in healthy aging men. The Journal of Clinical Endocrinology & Metabolism. 1986;62:767–772. [DOI] [PubMed] [Google Scholar]
- 45.Parker C Jr, Azziz R, Potter H, Boots L. Adrenal androgen production in response to adrenocorticotropin infusions in men. Endocrine research. 1996;22:717–722. [DOI] [PubMed] [Google Scholar]
- 46.Kishimoto Y, Hoshi M. Ddhydroepiandrosterone Sulphate in Rat Brain: Incorporation From Blood and Metabolism in vivo. Journal of neurochemistry. 1972;19:2207–2215. [DOI] [PubMed] [Google Scholar]
- 47.Young J, Couzinet B, Nahoul K, et al. Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. The Journal of Clinical Endocrinology & Metabolism. 1997;82:2578–2585. [DOI] [PubMed] [Google Scholar]
- 48.Mortola J, Yen S. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 1990;71:696–704. [DOI] [PubMed] [Google Scholar]
- 49.Šulcová J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. Journal of Endocrinology. 1997;154:57–62. [DOI] [PubMed] [Google Scholar]
- 50.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends in Endocrinology & Metabolism. 2002;13:234–239. [DOI] [PubMed] [Google Scholar]
- 51.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. The Journal of Clinical Endocrinology & Metabolism. 1984;59:551–555. [DOI] [PubMed] [Google Scholar]
- 52.Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in humans. American Journal of Physiology-Endocrinology And Metabolism. 1991;260:E101–E110. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi Y, Kipnis D, Daughaday W. Growth hormone secretion during sleep. The Journal of clinical investigation. 1968;47:2079–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho KY, Veldhuis JD, Johnson ML, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. The Journal of clinical investigation. 1988;81:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tannenbaum GS, Ling N. The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology. 1984;115:1952–1957. [DOI] [PubMed] [Google Scholar]
- 56.Khatib N, Gaidhane S, Gaidhane AM, et al. Ghrelin: ghrelin as a regulatory Peptide in growth hormone secretion. Journal of clinical and diagnostic research: JCDR. 2014;8:MC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartman ML, Clayton PE, Johnson ML, et al. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. The Journal of clinical investigation. 1993;91:2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roelfsema V, Clark RG. The growth hormone and insulin-like growth factor axis: its manipulation for the benefit of growth disorders in renal failure. Journal of the American Society of Nephrology. 2001;12:1297–1306. [DOI] [PubMed] [Google Scholar]
- 59.Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. Age-related change in the twenty-four hour spontaneous secretion of growth hormone. The Journal of Clinical Endocrinology & Metabolism. 1972;35:665–670. [DOI] [PubMed] [Google Scholar]
- 60.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. The Journal of Clinical Endocrinology & Metabolism. 1991;73:1081–1088. [DOI] [PubMed] [Google Scholar]
- 61.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocrine reviews. 1993;14:20–39. [DOI] [PubMed] [Google Scholar]
- 62.Le Roith D, Bondy C, Yakar S, Liu J-L, Butler A. The somatomedin hypothesis: 2001. Endocrine reviews. 2001;22:53–74. [DOI] [PubMed] [Google Scholar]
- 63.Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocrine reviews. 2009;30:494–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. Journal of Applied Physiology. 2002;93:1159–1167. [DOI] [PubMed] [Google Scholar]
- 65.Laron Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency. Hormones. 2008;7:24–27. [DOI] [PubMed] [Google Scholar]
- 66.Veldhuis JD, Erickson D, Iranmanesh A, Miles JM, Bowers CY. Sex-steroid control of the aging somatotropic axis. Endocrinology and Metabolism Clinics. 2005;34:877–893. [DOI] [PubMed] [Google Scholar]
- 67.Lombardi G, Tauchmanova L, Di CS, et al. Somatopause: dismetabolic and bone effects. Journal of endocrinological investigation. 2005;28:36–42. [PubMed] [Google Scholar]
- 68.Anawalt BD, Merriam GR. Neuroendocrine aging in men: andropause and somatopause. Endocrinology and metabolism clinics of North America. 2001;30:647–669. [DOI] [PubMed] [Google Scholar]
- 69.Zeng Q, Dong S-Y, Sun X-N, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Brazilian Journal of Medical and Biological Research. 2012;45:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosy-Westphal A, Geisler C, Onur S, et al. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. International journal of obesity. 2006;30:475. [DOI] [PubMed] [Google Scholar]
- 71.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. International journal of impotence research. 2008;20:378. [DOI] [PubMed] [Google Scholar]
- 72.Kelly D, Jones T. Testosterone and obesity. Obesity Reviews. 2015;16:581–606. [DOI] [PubMed] [Google Scholar]
- 73.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. The Journal of Clinical Endocrinology & Metabolism. 1977;45:1211–1219. [DOI] [PubMed] [Google Scholar]
- 74.Allan CA, McLachlan RI. Androgens and obesity. Current Opinion in Endocrinology, Diabetes and Obesity. 2010;17:224–232. [DOI] [PubMed] [Google Scholar]
- 75.Hernández-Morante JJ, Pérez-de-Heredia F, Luján JA, Zamora S, Garaulet M. Role of DHEA-S on body fat distribution: gender-and depot-specific stimulation of adipose tissue lipolysis. Steroids. 2008;73:209–215. [DOI] [PubMed] [Google Scholar]
- 76.Haffner S, Valdez R, Stern M, Katz M. Obesity, body fat distribution and sex hormones in men. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1993;17:643–649. [PubMed] [Google Scholar]
- 77.Abbasi A, Duthie EH Jr, Sheldahl L, et al. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community dwelling older men and women. Journal of the American Geriatrics Society. 1998;46:263–273. [DOI] [PubMed] [Google Scholar]
- 78.De Pergola G, Giagulli V, Garruti G, et al. Low dehydroepiandrosterone circulating levels in premenopausal obese women with very high body mass index. Metabolism-Clinical and Experimental. 1991;40:187–190. [DOI] [PubMed] [Google Scholar]
- 79.Salomon F, Cuneo RC, Hesp R, Sönksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. New England Journal of Medicine. 1989;321:1797–1803. [DOI] [PubMed] [Google Scholar]
- 80.Binnerts A, Swart GR, Wilson JP, et al. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition. Clinical endocrinology. 1992;37:79–87. [DOI] [PubMed] [Google Scholar]
- 81.Attallah H, Friedlander AL, Nino-Murcia M, Hoffman AR. Effects of growth hormone and pioglitazone in viscerally obese adults with impaired glucose tolerance: a factorial clinical trial. PLoS clinical trials. 2007;2:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Redman LM, Heilbronn LK, Martin CK, et al. Effect of calorie restriction with or without exercise on body composition and fat distribution. The Journal of Clinical Endocrinology & Metabolism. 2007;92:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the national Academy of Sciences. 2004;101:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rochon J, Bales CW, Ravussin E, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2010;66:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ravussin E, Redman LM, Rochon J, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. The Journals of Gerontology: Series A. 2015;70:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. [DOI] [PubMed] [Google Scholar]
- 88.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition. 1986;116:641–654. [DOI] [PubMed] [Google Scholar]
- 89.Schulte D, Hahn M, Oberhäuser F, et al. Caloric restriction increases serum testosterone concentrations in obese male subjects by two distinct mechanisms. Hormone and Metabolic Research. 2014;46:283–286. [DOI] [PubMed] [Google Scholar]
- 90.Rasmussen M, Hvidberg A, Juul A, et al. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. The Journal of Clinical Endocrinology & Metabolism. 1995;80:1407–1415. [DOI] [PubMed] [Google Scholar]
- 91.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 92.Boyle JP, Honeycutt AA, Narayan KV, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US. Diabetes care. 2001;24:1936–1940. [DOI] [PubMed] [Google Scholar]
- 93.Muller M, Grobbee DE, Den Tonkelaar I, Lamberts SW, Van Der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. The Journal of Clinical Endocrinology & Metabolism. 2005;90:2618–2623. [DOI] [PubMed] [Google Scholar]
- 94.Patel SM, Ratcliffe SJ, Reilly MP, et al. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism. 2009;94:4776–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeap BB, Chubb SP, Hyde Z, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. European Journal of Endocrinology. 2009;161:591–598. [DOI] [PubMed] [Google Scholar]
- 96.Johansson J-O, Fowelin J, Landin K, Lager I, Bengtsson B-Å. Growth hormone-deficient adults are insulin-resistant. Metabolism. 1995;44:1126–1129. [DOI] [PubMed] [Google Scholar]
- 97.Hew F, Koschmann M, Christopher M, et al. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. The Journal of Clinical Endocrinology & Metabolism. 1996;81:555–564. [DOI] [PubMed] [Google Scholar]
- 98.Mitchell LE, Sprecher DL, Borecki IB, Rice T, Laskarzewski PM, Rao D. Evidence for an association between dehydroepiandrosterone sulfate and nonfatal, premature myocardial infarction in males. Circulation. 1994;89:89–93. [DOI] [PubMed] [Google Scholar]
- 99.Herrington DM. Dehydroepiandrosterone and coronary atherosclerosis. Annals of the New York Academy of Sciences. 1995;774:271–280. [DOI] [PubMed] [Google Scholar]
- 100.Herrington DM, Gordon GB, Achuff SC, et al. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. Journal of the American College of Cardiology. 1990;16:862–870. [DOI] [PubMed] [Google Scholar]
- 101.Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts Male Aging Study. American journal of epidemiology. 2001;153:79–89. [DOI] [PubMed] [Google Scholar]
- 102.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. Journal of applied physiology. 2004;96:101–106. [DOI] [PubMed] [Google Scholar]
- 103.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. The Journal of clinical investigation. 1982;69:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy J. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. American Journal of Physiology-Endocrinology And Metabolism. 1989;256:E494–E499. [DOI] [PubMed] [Google Scholar]
- 105.Wojtaszewski J, Hansen BF, Kiens B, Markuns J, Goodyear L, Richter E. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. [DOI] [PubMed] [Google Scholar]
- 106.Cartee GD, Arias EB, Yu CS, Pataky MW. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. American Journal of Physiology-Endocrinology and Metabolism. 2016;311 :E818–E824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pataky MW, Yu CS, Nie Y, et al. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. American Journal of Physiology-Endocrinology and Metabolism. 2019;316:E695–E706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davidson MB. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism. 1979;28:688–705. [DOI] [PubMed] [Google Scholar]
- 109.Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes care. 2009;32:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell metabolism. 2017;25:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hurel S, Koppiker N, Newkirk J, et al. Relationship of physical exercise and ageing to growth hormone production. Clinical Endocrinology. 1999;51:687–691. [DOI] [PubMed] [Google Scholar]
- 113.Copeland JL, Chu SY, Tremblay MS. Aging, physical activity, and hormones in women—a review. Journal of aging and physical activity. 2004;12:101–116. [DOI] [PubMed] [Google Scholar]
- 114.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes care. 2006;29:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. The American journal of clinical nutrition. 2006;84:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson ML, Distelmaier K, Lanza IR, et al. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes. 2016;65:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menshikova EV, Ritov VB, Dube JJ, et al. Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. The Journals of Gerontology: Series A. 2017;73:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. The Journal of Clinical Endocrinology & Metabolism. 1992;75:1125–1132. [DOI] [PubMed] [Google Scholar]
- 119.Masoro E, Shimokawa I, Yu B. Retardation of the aging processes in rats by food restriction. Annals of the New York Academy of Sciences. 1991;621:337–352. [DOI] [PubMed] [Google Scholar]
- 120.Zheng Y, Zhang W, Pendleton E, et al. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. The Journal of endocrinology. 2009;203:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharma N, Castorena CM, Cartee GD. Greater insulin sensitivity in calorie restricted rats occurs with unaltered circulating levels of several important myokines and cytokines. Nutrition & metabolism. 2012;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H, Luo L, Liu J, Brown T, Zirkin BR. Aging and caloric restriction: effects on Leydig cell steroidogenesis. Experimental gerontology. 2005;40:498–505. [DOI] [PubMed] [Google Scholar]
- 123.Hoppeler H, Weibel E. Limits for oxygen and substrate transport in mammals. Journal of Experimental Biology. 1998;201:1051–1064. [DOI] [PubMed] [Google Scholar]
- 124.Lindstedt SL, Wells DJ. Skeletal muscle mitochondria: the aerobic gate? Oxygen Transfer from Atmosphere to Tissues: Springer; 1988:207–213. [DOI] [PubMed] [Google Scholar]
- 125.Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier RL. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo. 2000;13:14–27. [DOI] [PubMed] [Google Scholar]
- 126.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences. 2005;102:5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochromec oxidase gene expression in rat skeletal muscle, liver, and heart. Journal of Biological Chemistry. 2000;275:3343–3347. [DOI] [PubMed] [Google Scholar]
- 128.Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. Reduced amount of mitochondrial DNA in aged human muscle. Journal of applied physiology. 2003;94:1479–1484. [DOI] [PubMed] [Google Scholar]
- 129.Ladenvall P, Persson CU, Mandalenakis Z, et al. Low aerobic capacity in middle-aged men associated with increased mortality rates during 45 years of follow-up. European journal of preventive cardiology. 2016;23:1557–1564. [DOI] [PubMed] [Google Scholar]
- 130.Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes care. 2005;28:1636–1642. [DOI] [PubMed] [Google Scholar]
- 131.Usui T, Kajita K, Kajita T, et al. Elevated mitochondrial biogenesis in skeletal muscle is associated with testosterone-induced body weight loss in male mice. FEBS letters. 2014;588:1935–1941. [DOI] [PubMed] [Google Scholar]
- 132.Rovira-Llopis S, Bañuls C, de Marañon AM, et al. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radical Biology and Medicine. 2017;108:155–162. [DOI] [PubMed] [Google Scholar]
- 133.Guo W, Wong S, Li M, et al. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS One. 2012;7:e51180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. [DOI] [PubMed] [Google Scholar]
- 135.Haydar ZR, Blackman MR, Tobin JD, Wright JG, Fleg JL. The Relationship Between Aerobic Exercise Capacity and Circulating IGF-1 Levels In Healthy Men and Women. Journal of the American Geriatrics Society. 2000;48:139–145. [DOI] [PubMed] [Google Scholar]
- 136.Berggren A, Ehrnborg C, Rosén T, Ellegård L, Bengtsson B-Ak, Caidahl K. Short-term administration of supraphysiological recombinant human growth hormone (GH) does not increase maximum endurance exercise capacity in healthy, active young men and women with normal GH-insulin-like growth factor I axes. The Journal of Clinical Endocrinology & Metabolism. 2005;90:3268–3273. [DOI] [PubMed] [Google Scholar]
- 137.Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. The Journal of Clinical Endocrinology & Metabolism. 2008;93:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rogers MA, Hagberg JM, Martin W 3rd, Ehsani A, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. Journal of Applied Physiology. 1990;68:2195–2199. [DOI] [PubMed] [Google Scholar]
- 139.Hawkins SA, Wiswell RA. Rate and mechanism of maximal oxygen consumption decline with aging. Sports medicine. 2003;33:877–888. [DOI] [PubMed] [Google Scholar]
- 140.Heath G, Hagberg J, Ehsani AA, Holloszy J. A physiological comparison of young and older endurance athletes. Journal of Applied Physiology. 1981;51:634–640. [DOI] [PubMed] [Google Scholar]
- 141.Pollock ML, Foster C, Knapp D, Rod JL, Schmidt DH. Effect of age and training on aerobic capacity and body composition of master athletes. Journal of Applied Physiology. 1987;62:725–731. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the american college of cardiology. 2001;37:153–156. [DOI] [PubMed] [Google Scholar]
- 143.Gries KJ, Raue U, Perkins RK, et al. Cardiovascular and skeletal muscle health with lifelong exercise. Journal of Applied Physiology. 2018;125:1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Everman S, Farris JW, Bay RC, Daniels JT. Elite Distance Runners: A 45-Year Follow-up. Medicine and science in sports and exercise. 2018;50:73–78. [DOI] [PubMed] [Google Scholar]
- 145.Trappe S, Hayes E, Galpin A, et al. New records in aerobic power among octogenarian lifelong endurance athletes. Journal of applied physiology. 2012;114:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hagberg JM, Allen WK, Seals DR, Hurley B, Ehsani A, Holloszy J. A hemodynamic comparison of young and older endurance athletes during exercise. Journal of Applied Physiology. 1985;58:2041–2046. [DOI] [PubMed] [Google Scholar]
- 147.Koch LG, Kemi OJ, Qi N, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation research. 2011;109:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Racette SB, Rochon J, Uhrich ML, et al. Effects of two years of calorie restriction on aerobic capacity and muscle strength. Medicine and science in sports and exercise. 2017;49:2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hepple RT, Baker DJ, McConkey M, Murynka T, Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation research. 2006;9:219–222. [DOI] [PubMed] [Google Scholar]
- 150.Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. The FASEB Journal. 2005;19:1320–1322. [DOI] [PubMed] [Google Scholar]
- 151.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. The FASEB Journal. 2001;15:1589–1591. [DOI] [PubMed] [Google Scholar]
- 152.Lanza IR, Zabielski P, Klaus KA, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell metabolism. 2012;16:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 1983;6:588–595. [DOI] [PubMed] [Google Scholar]
- 154.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mechanisms of ageing and development. 1999;107:123–136. [DOI] [PubMed] [Google Scholar]
- 155.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. Journal of applied physiology. 2000;88:1321–1326. [DOI] [PubMed] [Google Scholar]
- 156.Häkkinen K, Pakarinen A. Muscle strength and serum testosterone, cortisol and SHBG concentrations in middle-aged and elderly men and women. Acta Physiologica Scandinavica. 1993;148:199–207. [DOI] [PubMed] [Google Scholar]
- 157.Mobley C, Mumford P, Kephart W, et al. Effects of testosterone treatment on markers of skeletal muscle ribosome biogenesis. Andrologia. 2016;48:1055–1065. [DOI] [PubMed] [Google Scholar]
- 158.Cuneo RC, Salomon F, Wiles M, Sönksen P. Skeletal muscle performance in adults with growth hormone deficiency. Hormone Research in Paediatrics. 1990;33:55–60. [DOI] [PubMed] [Google Scholar]
- 159.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. TRENDS in Genetics. 2002;18:295–301. [DOI] [PubMed] [Google Scholar]
- 160.Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular cell. 2004;14:395–403. [DOI] [PubMed] [Google Scholar]
- 161.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nature reviews Molecular cell biology. 2009;10:307. [DOI] [PubMed] [Google Scholar]
- 162.Doherty TJ. Invited review: aging and sarcopenia. Journal of applied physiology. 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- 163.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. Journal of the neurological sciences. 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 164.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Medicine & Science in Sports & Exercise. 2011;43:1177–1187. [DOI] [PubMed] [Google Scholar]
- 165.Kosek DJ, Kim J-s, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. Journal of applied physiology. 2006;101:531–544. [DOI] [PubMed] [Google Scholar]
- 166.Riggs B, Wahner H, Dunn W, Mazess R, Offord K, Melton Lr. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. The Journal of clinical investigation. 1981;67:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Riggs B, Wahner H, Seeman E, et al. Changes in bone mineral density of the proximal femur and spine with aging: differences between the postmenopausal and senile osteoporosis syndromes. The Journal of clinical investigation. 1982;70:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cummings SR, Browner W, Black D, et al. Bone density at various sites for prediction of hip fractures. The Lancet. 1993;341:72–75. [DOI] [PubMed] [Google Scholar]
- 169.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. Bmj. 1993;307:1248–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pouilles J, Tremollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcified Tissue International. 1993;52:340–343. [DOI] [PubMed] [Google Scholar]
- 171.Ahlborg H, Johnell O, Nilsson B, Jeppsson S, Rannevik G, Karlsson M. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28:327–331. [DOI] [PubMed] [Google Scholar]
- 172.Meier DE, Orwoll ES, Keenan EJ, Fagerstrom RM. Marked decline in trabecular bone mineral content in healthy men with age: lack of association with sex steroid levels. Journal of the American Geriatrics Society. 1987;35:189–197. [DOI] [PubMed] [Google Scholar]
- 173.Murphy S, Khaw K-t, Cassidy A, Compston JE. Sex hormones and bone mineral density in elderly men. Bone and mineral. 1993;20:133–140. [DOI] [PubMed] [Google Scholar]
- 174.van Geel TA, Geusens PP, Winkens B, Sels J-PJ, Dinant G-J. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. European Journal of Endocrinology. 2009;160:681. [DOI] [PubMed] [Google Scholar]
- 175.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. The Journal of clinical investigation. 1997;100:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kenny AM, Prestwood KM, Marcello KM, Raisz LG. Determinants of bone density in healthy older men with low testosterone levels. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55:M492–M497. [DOI] [PubMed] [Google Scholar]
- 177.Carroll PV, Christ the members of Growth Hormone Research Society Scientific Committee ER, Bengtsson BA, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. The Journal of Clinical Endocrinology & Metabolism. 1998;83:382–395. [DOI] [PubMed] [Google Scholar]
- 178.Bass S, Pearce G, Bradney M, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. Journal of Bone and Mineral Research. 1998;13:500–507. [DOI] [PubMed] [Google Scholar]
- 179.McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. The Journal of pediatrics. 2000;136:156–162. [DOI] [PubMed] [Google Scholar]
- 180.Grimston SK, Willows ND, Hanley DA. Mechanical loading regime and its relationship to bone mineral density in children. Medicine and science in sports and exercise. 1993;25:1203–1210. [PubMed] [Google Scholar]
- 181.Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. Journal of Aging and Health. 2009;21:519–527. [DOI] [PubMed] [Google Scholar]
- 182.Michel B, Lane NE, Björkengren A, Bloch D, Fries J. Impact of running on lumbar bone density: a 5-year longitudinal study. The Journal of rheumatology. 1992;19:1759–1763. [PubMed] [Google Scholar]
- 183.MacDougall J, Webber C, Martin J, et al. Relationship among running mileage, bone density, and serum testosterone in male runners. Journal of Applied Physiology. 1992;73:1165–1170. [DOI] [PubMed] [Google Scholar]
- 184.Layne JE, Nelson ME. The effects of progressive resistance training on bone density: a review. Medicine & science in sports & exercise. 1999;31:25–30. [DOI] [PubMed] [Google Scholar]
- 185.Levy R. Aging-associated cognitive decline. International Psychogeriatrics. 1994;6:63–68. [PubMed] [Google Scholar]
- 186.Bentourkia Mh, Bol A, Ivanoiu A, et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. Journal of the neurological sciences. 2000;181:19–28. [DOI] [PubMed] [Google Scholar]
- 187.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proceedings of the National Academy of Sciences. 1996;93:4765–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. American Journal of Physiology-Cell Physiology. 2007;292:C670–C686. [DOI] [PubMed] [Google Scholar]
- 189.Ownby RL. Neuroinflammation and cognitive aging. Current psychiatry reports. 2010;12:39–45. [DOI] [PubMed] [Google Scholar]
- 190.Ruegsegger GN, Vanderboom PM, Dasari S, et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. Journal of the American Geriatrics Society. 1990;38:123–128. [DOI] [PubMed] [Google Scholar]
- 192.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101:3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guiney H, Lucas SJ, Cotter JD, Machado L. Evidence cerebral blood-flow regulation mediates exercise–cognition links in healthy young adults. Neuropsychology. 2015;29:1. [DOI] [PubMed] [Google Scholar]
- 194.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of aging. 2010;31:2047–2057. [DOI] [PubMed] [Google Scholar]
- 195.Chang Y-K, Pan C-Y, Chen F-T, Tsai C-L, Huang C-C. Effect of resistance-exercise training on cognitive function in healthy older adults: a review. Journal of aging and physical activity. 2012;20:497–517. [DOI] [PubMed] [Google Scholar]
- 196.Best JR, Chiu BK, Hsu CL, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. Journal of the international neuropsychological society. 2015;21:745–756. [DOI] [PubMed] [Google Scholar]
- 197.Cassilhas R, Lee K, Fernandes J, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. [DOI] [PubMed] [Google Scholar]
- 198.Yang F, Chu X, Yin M, et al. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behavioural brain research. 2014;264:82–90. [DOI] [PubMed] [Google Scholar]
- 199.Sanz A, Caro P, Ibañez J, Gómez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. Journal of bioenergetics and biomembranes. 2005;37:83–90. [DOI] [PubMed] [Google Scholar]
- 200.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Therapeutics and clinical risk management. 2009;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. New England Journal of Medicine. 2016;374:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cunningham GR, Stephens-Shields AJ, Rosen RC, et al. Testosterone treatment and sexual function in older men with low testosterone levels. The Journal of Clinical Endocrinology & Metabolism. 2016;101:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cunningham GR, Stephens-Shields AJ, Rosen RC, et al. Association of sex hormones with sexual function, vitality, and physical function of symptomatic older men with low testosterone levels at baseline in the testosterone trials. The Journal of Clinical Endocrinology & Metabolism. 2015;100:1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tenover JS. Effects of testosterone supplementation in the aging male. The Journal of Clinical Endocrinology & Metabolism. 1992;75:1092–1098. [DOI] [PubMed] [Google Scholar]
- 205.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. The Journal of Clinical Endocrinology & Metabolism. 2005;90:1502–1510. [DOI] [PubMed] [Google Scholar]
- 206.Bhasin S, Ellenberg SS, Storer TW, et al. Effect of testosterone replacement on measures of mobility in older men with mobility limitation and low testosterone concentrations: secondary analyses of the Testosterone Trials. The lancet Diabetes & endocrinology. 2018;6:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sih R, Morley JE, Kaiser FE, Perry HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. [DOI] [PubMed] [Google Scholar]
- 208.Mohler ER III, Ellenberg SS, Lewis CE, et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. The Journal of Clinical Endocrinology & Metabolism. 2018;103:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA internal medicine. 2017;177:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen X, Li X, Huang H, Lin J. Effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression in cells sensitive to insulin. Zhonghua Yi Xue Za Zhi. 2006;86:1474–1477. [PubMed] [Google Scholar]
- 211.Salehzadeh F, Rune A, Osler M, Al-Khalili L. Testosterone or 17-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol. 2011;210:219–229. [DOI] [PubMed] [Google Scholar]
- 212.Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2008;294:E961–E968. [DOI] [PubMed] [Google Scholar]
- 213.Bergamini E, Bombara G, Pellegrino C. The effect of testosterone on glycogen metabolism in rat levator ani muscle. Biochimica et Biophysica Acta (BBA)-General Subjects. 1969;177:220–234. [DOI] [PubMed] [Google Scholar]
- 214.Harman SM, Blackman MR. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Hormone Research in Paediatrics. 2003;60:121–124. [DOI] [PubMed] [Google Scholar]
- 215.Giannoulis MG, Jackson N, Shojaee-Moradie F, et al. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: a randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2008;93:3066–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. New England Journal of Medicine. 2006;355:1647–1659. [DOI] [PubMed] [Google Scholar]
- 217.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2006;91:477–484. [DOI] [PubMed] [Google Scholar]
- 218.Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. Jama. 2017;317:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. Jama. 2017;317:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Basu R, Dalla Man C, Campioni M, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes care. 2007;30:1972–1978. [DOI] [PubMed] [Google Scholar]
- 221.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:1451–1457. [DOI] [PubMed] [Google Scholar]
- 222.Tenover JL. Experience with testosterone replacement in the elderly. Mayo Clinic Proceedings. Vol 75: Elsevier; 2000:S77–S82. [PubMed] [Google Scholar]
- 223.Sato K, Iemitsu M, Aizawa K, Ajisaka R. DHEA improves impaired activation of Akt and PKC ζ/λ-GLUT4 pathway in skeletal muscle and improves hyperglycaemia in streptozotocin-induced diabetes rats. Acta physiologica. 2009;197:217–225. [DOI] [PubMed] [Google Scholar]
- 224.Minghetti P, Cilurzo F, Casiraghi A, Montanari L, Santoro A. Development of patches for the controlled release of dehydroepiandrosterone. Drug development and industrial pharmacy. 2001;27:711–717. [DOI] [PubMed] [Google Scholar]
- 225.Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. The Journal of Clinical Endocrinology & Metabolism. 2008;93:4767–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clinical endocrinology. 2000;53:561–568. [DOI] [PubMed] [Google Scholar]
- 227.Dayal M, Sammel MD, Zhao J, Hummel AC, Vandenbourne K, Barnhart KT. Supplementation with DHEA: effect on muscle size, strength, quality of life, and lipids. Journal of women×s health. 2005;14:391–400. [DOI] [PubMed] [Google Scholar]
- 228.Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. American Journal of Physiology-Endocrinology and Metabolism. 2006;291:E1003–E1008. [DOI] [PubMed] [Google Scholar]
- 229.Casson PR, Santoro N, Elkind-Hirsch K, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertility and Sterility. 1998;70:107–110. [DOI] [PubMed] [Google Scholar]
- 230.Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–245. [DOI] [PubMed] [Google Scholar]
- 231.Basu R, Dalla Man C, Campioni M, et al. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56:753–766. [DOI] [PubMed] [Google Scholar]
- 232.Lasco A, Frisina N, Morabito N, et al. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. European journal of endocrinology. 2001;145:457–461. [DOI] [PubMed] [Google Scholar]
- 233.Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. The Journal of Clinical Endocrinology & Metabolism. 2003;88:3190–3195. [DOI] [PubMed] [Google Scholar]
- 234.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. New England Journal of Medicine. 1990;323:1–6. [DOI] [PubMed] [Google Scholar]
- 235.Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth hormone on body composition in elderly men. Hormone Research in Paediatrics. 1991;36:73–81. [DOI] [PubMed] [Google Scholar]
- 236.Feldt-Rasmussen U, Wilton P, Jonsson P. Aspects of growth hormone deficiency and replacement in elderly hypopituitary adults. Growth Hormone & IGF Research. 2004;14:51–58. [DOI] [PubMed] [Google Scholar]
- 237.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Annals of internal medicine. 2007;146:104–115. [DOI] [PubMed] [Google Scholar]
- 238.Lange KHW, Andersen JL, Beyer N, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. The Journal of Clinical Endocrinology & Metabolism. 2002;87:513–523. [DOI] [PubMed] [Google Scholar]
- 239.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Annals of internal medicine. 1996;124:708–716. [DOI] [PubMed] [Google Scholar]
- 240.Blackman MR, Sorkin JD, Münzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. Jama. 2002;288:2282–2292. [DOI] [PubMed] [Google Scholar]
- 241.Cook D, Yuen K, Biller B, Kemp S, Vance M. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients-2009 update. Endocrine practice. 2009;15:1–29. [DOI] [PubMed] [Google Scholar]
- 242.Bijnen FC, Feskens EJ, Caspersen CJ, Mosterd WL, Kromhout D. Age, period, and cohort effects on physical activity among elderly men during 10 years of follow-up: the Zutphen Elderly Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53:M235–M241. [DOI] [PubMed] [Google Scholar]
- 243.Verbrugge LM, Gruber-Baldini AL, Fozard JL. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1996;51:S30–S41. [DOI] [PubMed] [Google Scholar]
- 244.Little RM, Paterson DH, Humphreys DA, Stathokostas L. A 12-month incidence of exercise-related injuries in previously sedentary community-dwelling older adults following an exercise intervention. BMJ open. 2013;3:e002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Stathokostas L, Theou O, Little RM, Vandervoort A, Raina P. Physical activity-related injuries in older adults: a scoping review. Sports medicine. 2013;43:955–963. [DOI] [PubMed] [Google Scholar]
- 246.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American Journal of Physiology-Endocrinology and Metabolism. 2006;291:E381–E387. [DOI] [PubMed] [Google Scholar]
- 247.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. New England Journal of Medicine. 2017;376:1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kraemer WJ, Häkkinen K, Newton RU, et al. Acute hormonal responses to heavy resistance exercise in younger and older men. European journal of applied physiology and occupational physiology. 1998;77:206–211. [DOI] [PubMed] [Google Scholar]
- 249.Hakkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. Journals of Gerontology-Biological Sciences and Medical Sciences. 2000;55:B95. [DOI] [PubMed] [Google Scholar]
- 250.Ahtiainen JP, Hulmi JJ, Kraemer WJ, et al. Heavy resistance exercise training and skeletal muscle androgen receptor expression in younger and older men. Steroids. 2011;76:183–192. [DOI] [PubMed] [Google Scholar]
- 251.Baker JR, Bemben MG, Anderson MA, Bemben DA. Effects of age on testosterone responses to resistance exercise and musculoskeletal variables in men. Journal of Strength and Conditioning Research. 2006;20:874. [DOI] [PubMed] [Google Scholar]
- 252.Smilios I, Pilianidis T, Karamouzis M, Parlavantzas A, Tokmakidis S. Hormonal responses after a strength endurance resistance exercise protocol in young and elderly males. International journal of sports medicine. 2007;28:401–406. [DOI] [PubMed] [Google Scholar]
- 253.Häkkinen K, Pakarinen A, Newton R, Kraemer W. Acute hormone responses to heavy resistance lower and upper extremity exercise in young versus old men. European journal of applied physiology and occupational physiology. 1998;77:312–319. [DOI] [PubMed] [Google Scholar]
- 254.Paunksnis MR, Evangelista AL, La Scala Teixeira CV, et al. Metabolic and hormonal responses to different resistance training systems in elderly men. The Aging Male. 2018;21:106–110. [DOI] [PubMed] [Google Scholar]
- 255.Häkkinen K, Pakarinen A. Acute hormonal responses to heavy resistance exercise in men and women at different ages. International journal of sports medicine. 1995;16:507–513. [DOI] [PubMed] [Google Scholar]
- 256.Nicklas B, Ryan A, Treuth M, et al. Testosterone, growth hormone and IGF-I responses to acute and chronic resistive exercise in men aged 55-70 years. International journal of sports medicine. 1995;16:445–450. [DOI] [PubMed] [Google Scholar]
- 257.Kostka T, Patricot MC, Mathian B, Lacour J-R, Bonnefoy M. Anabolic and catabolic hormonal responses to experimental two-set low-volume resistance exercise in sedentary and active elderly people. Aging clinical and experimental research. 2003;15:123–130. [DOI] [PubMed] [Google Scholar]
- 258.Roberts MD, Dalbo VJ, Hassell SE, Kerksick CM. The expression of androgen-regulated genes before and after a resistance exercise bout in younger and older men. The Journal of Strength & Conditioning Research. 2009;23:1060–1067. [DOI] [PubMed] [Google Scholar]
- 259.Newton RU, Häkkinen K, Häkkinen A, McCormick M, Volek J, Kraemer WJ. Mixed-methods resistance training increases power and strength of young and older men. Medicine & Science in Sports & Exercise. 2002;34:1367–1375. [DOI] [PubMed] [Google Scholar]
- 260.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. American Journal of Physiology-Endocrinology And Metabolism. 1995;268:E422–E427. [DOI] [PubMed] [Google Scholar]
- 261.Häkkinen K, Kraemer WJ, Pakarinen A, et al. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60-75-year-old men and women. Canadian Journal of Applied Physiology. 2002;27:213–231. [DOI] [PubMed] [Google Scholar]
- 262.Häkkinen K, Pakarinen A. Serum hormones and strength development during strength training in middle Gaged and elderly males and females. Acta Physiologica Scandinavica. 1994;150:211–219. [DOI] [PubMed] [Google Scholar]
- 263.Craig B, Brown R, Everhart J. Effects of progressive resistance training on growth hormone and testosterone levels in young and elderly subjects. Mechanisms of ageing and development. 1989;49:159–169. [DOI] [PubMed] [Google Scholar]
- 264.Lovell DI, Cuneo R, Wallace J, McLellan C. The hormonal response of older men to sub-maximum aerobic exercise: The effect of training and detraining. Steroids. 2012;77:413–418. [DOI] [PubMed] [Google Scholar]
- 265.Hildreth KL, Barry DW, Moreau KL, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. The Journal of Clinical Endocrinology & Metabolism. 2013;98:1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. Journal of Applied Physiology. 2004;96:531–539. [DOI] [PubMed] [Google Scholar]
- 267.Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:B158–B165. [DOI] [PubMed] [Google Scholar]
- 268.Heaney JL, Carroll D, Phillips AC. DHEA, DHEA-S and cortisol responses to acute exercise in older adults in relation to exercise training status and sex. Age. 2013;35:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Aldred S, Rohalu M, Edwards K, Burns V. Altered DHEA and DHEAS response to exercise in healthy older adults. Journal of aging and physical activity. 2009;17:77–88. [DOI] [PubMed] [Google Scholar]
- 270.Hersey WC III, Graves JE, Pollock ML, et al. Endurance exercise training improves body composition and plasma insulin responses in 70-to 79-year-old men and women. Metabolism. 1994;43:847–854. [DOI] [PubMed] [Google Scholar]
- 271.Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. Journal of the American Geriatrics Society. 2010;58:1707–1714. [DOI] [PubMed] [Google Scholar]
- 272.Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2008;93:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sutton J, Lazarus L. Growth hormone in exercise: comparison of physiological and pharmacological stimuli. Journal of Applied Physiology. 1976;41:523–527. [DOI] [PubMed] [Google Scholar]
- 274.Raynaud J, Capderou A, Martineaud J, Bordachar J, Durand J. Intersubject viability in growth hormone time course during different types of work. Journal of Applied Physiology. 1983;55:1682–1687. [DOI] [PubMed] [Google Scholar]
- 275.Lassarre C, Girard F, Durand J, Raynaud J. Kinetics of human growth hormone during submaximal exercise. Journal of Applied Physiology. 1974;37:826–830. [DOI] [PubMed] [Google Scholar]
- 276.Pyka G, Wiswell RA, Marcus R. Age-dependent effect of resistance exercise on growth hormone secretion in people. The Journal of Clinical Endocrinology & Metabolism. 1992;75:404–407. [DOI] [PubMed] [Google Scholar]
- 277.Manini TM, Yarrow JF, Buford TW, Clark BC, Conover CF, Borst SE. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Hormone & IGF Research. 2012;22:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kraemer WJ, Häkkinen K, Newton RU, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. Journal of applied physiology. 1999;87:982–992. [DOI] [PubMed] [Google Scholar]
- 279.Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A. Growth hormone release during acute and chronic aerobic and resistance exercise. Sports medicine. 2002;32:987–1004. [DOI] [PubMed] [Google Scholar]
- 280.Weltman A, Weltman JY, Roy CP, et al. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. Journal of applied physiology. 2006;100:1623–1629. [DOI] [PubMed] [Google Scholar]
- 281.Pyka G, Taaffe D, Marcus R. Effect of a sustained program of resistance training on the acute growth hormone response to resistance exercise in older adults. Hormone and Metabolic research. 1994;26:330–333. [DOI] [PubMed] [Google Scholar]
- 282.Weltman A, Weltman JY, Womack CJ, et al. Exercise training decreases the growth hormone (GH) response to acute constant-load exercise. Medicine and science in sports and exercise. 1997;29:669–676. [DOI] [PubMed] [Google Scholar]
- 283.McCall GE, Byrnes WC, Fleck SJ, Dickinson A, Kraemer WJ. Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Canadian Journal of applied physiology. 1999;24:96–107. [DOI] [PubMed] [Google Scholar]
- 284.Godfrey RJ, Madgwick Z, Whyte GP. The exercise-induced growth hormone response in athletes. Sports medicine. 2003;33:599–613. [DOI] [PubMed] [Google Scholar]
- 285.Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy J, Bier D. Effect of growth hormone and resistance exercise on muscle growth in young men. American Journal of Physiology-Endocrinology And Metabolism. 1992;262:E261–E267. [DOI] [PubMed] [Google Scholar]
- 286.Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. American Journal of Physiology-Endocrinology And Metabolism. 1995;268:E268–E276. [DOI] [PubMed] [Google Scholar]
- 287.Karila T, Sarkkinen P, Marttinen M, Seppälä T, Mero A, Tallroth K. Rapid weight loss decreases serum testosterone. International journal of sports medicine. 2008;29:872–877. [DOI] [PubMed] [Google Scholar]
- 288.Levay EA, Tammer AH, Penman J, Kent S, Paolini AG. Calorie restriction at increasing levels leads to augmented concentrations of corticosterone and decreasing concentrations of testosterone in rats. Nutrition research. 2010;30:366–373. [DOI] [PubMed] [Google Scholar]
- 289.Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging cell. 2010;9:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Redman LM, Ravussin E. Endocrine alterations in response to calorie restriction in humans. Molecular and cellular endocrinology. 2009;299:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell metabolism. 2014;19:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Baum JI, Kim I-Y, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Walrand S, Short KR, Bigelow ML, Sweatt AJ, Hutson SM, Nair KS. Functional impact of high protein intake on healthy elderly people. American Journal of Physiology-Endocrinology and Metabolism. 2008;295:E921–E928. [DOI] [PMC free article] [PubMed] [Google Scholar]