Abstract
We present the first comparative plastome study of Pleurothallidinae with analyses of structural and molecular characteristics and identification of the ten most-variable regions to be incorporated in future phylogenetic studies. We sequenced complete plastomes of eight species in the subtribe and compared phylogenetic results of these to parallel analyses of their nuclear ribosomal DNA operon (26S, 18S, and 5.8S plus associated spacers) and partial mitochondrial genome sequences (29–38 genes and partial introns). These plastomes have the typical quadripartite structure for which gene content is similar to those of other orchids, with variation only in the composition of the ndh genes. The independent loss of ndh genes had an impact on which genes border the inverted repeats and thus the size of the small single-copy region, leading to variation in overall plastome length. Analyses of 68 coding sequences indicated the same pattern of codon usage as in other orchids, and 13 protein-coding genes under positive selection were detected. Also, we identified 62 polymorphic microsatellite loci and ten highly variable regions, for which we designed primers. Phylogenomic analyses showed that the top ten mutational hotspots represent well the phylogenetic relationships found with whole plastome sequences. However, strongly supported incongruence was observed among plastid, nuclear ribosomal DNA operon, and mitochondrial DNA trees, indicating possible occurrence of incomplete lineage sorting and/or introgressive hybridization. Despite the incongruence, the mtDNA tree retrieved some clades found in other analyses. These results, together with performance in recent studies, support a future role for mitochondrial markers in Pleurothallidinae phylogenetics.
1. Introduction
Neotropical Pleurothallidinae (Epidendreae, Epidendroideae) are the largest orchid subtribe, comprising more than 5,000 accepted species in 44 genera [1, 2]. These are mostly epiphytes and can occupy almost all habitat types from North America (Florida) and the Caribbean through southern South America (Argentina) [3], although most pleurothallid species have narrow endemic distributions, and, therefore, many are considered endangered (e.g. [4]).
Previously, circumscriptions of Pleurothallidinae genera were based on morphological characters, and so was the inference of evolutionary relationships: Luer [5] classified them into informal groups or “affinities”, relying on anther position and presence/absence of the annulus (a ring-like abscission zone on leaves). However, the first reclassification of Pleurothallidinae based on molecular evidence [6] highlighted several problems at generic and infrageneric levels (e.g. the polyphyletic “supergenus” Pleurothallis R.Br.), but some of the taxonomic changes that followed [7] were contested by Luer [8] due to the lack of morphological correlates, sampling problems, and the relatively low numbers of molecular markers used (i.e. often just nuclear ribosomal internal transcribed spacer, nrITS, plastid matK gene, and trnL-trnF intron/intergenic spacer).
This taxonomic controversy inspired more phylogenetic studies in the subtribe, mostly focused on specific genera and based almost only on nrITS (e.g. [9–17]). These studies initiated another round of reclassification in Pleurothallidinae [18], in which phylogenetic positions and generic classification were reassessed, providing a good framework for future studies. Karreman’s proposal [18] recognized nine genera affinities, but due to a large number of species and infrageneric categories in the subtribe, some relationships remained inconclusive because the compiled phylogenetic trees did not sample and fully resolve all Pleurothallidinae clades. Thus, some nomenclatural instability in the subtribe has continued and greatly affects regulations on international trade and conservation efforts that depend on Red Lists and population genetic studies.
In addition to nrITS, plastid genomes (plastomes) have been a source of good markers for Orchidaceae at various taxonomic levels [19]. In Pleurothallidinae, the matK gene and trnK −UUU intron are the main plastid markers used in combination with nrITS, but they are insufficiently variable to generate well resolved relationships for many genera. More recent molecular studies in the subtribe have used a wider number of plastid DNA markers (i.e. ycf1 [20], matK, psbD-trnT, rps16-trnQ, trnH-psbA, and trnS-trnG intergenic spacers [21]) in combination with nrITS, which improved tree resolution and support. However, the general utility of plastid DNA markers for the subtribe is still under-investigated, particularly which are the most variable and informative.
The advent of next-generation sequencing (NGS) has made plastome sequencing faster and more accessible [22], and they have become the main source of phylogenetic information for angiosperms [19, 23]. In Orchidaceae, plastome sequences have been compared among genera and species to find the most-variable regions, termed mutational hotspots [24–29]. Hence, comparative analyses of complete plastome sequences of Pleurothallidinae can indicate better molecular markers, but thus far only three plastomes of the subtribe are publicly available. Only the overall structure of these plastomes was analyzed and compared so far [30], with no analysis of which regions were more variable.
With this in view, we performed the first plastome study for Pleurothallidinae to identify mutational hotspots for use in future phylogenetic and population studies. We sequenced eight Pleurothallidinae plastomes and included two of those previously published in our analyses. We analyzed genome size and structure, gene content and order, and inverted repeats borders. We also compared codon usage and frequency and detected protein-coding genes under positive selection. In addition, we identified polymorphic microsatellite loci and the ten most-variable regions, for which we designed primers. Finally, we used a maximum likelihood phylogenetic approach to evaluate and compare relationships of pleurothallid genera [18] based on these plastomes, nuclear ribosomal DNA operons, mitochondrial data, and combined analyses.
2. Materials and methods
Species sampling
We included eight Pleurothallidinae species from individuals cultivated in the greenhouse of the Botany Department of Universidade Federal do Paraná (UFPR), Brazil. Fresh leaves were collected from these individuals for DNA extraction and NGS, and the vouchers were deposited at the UFPR herbarium (UPCB) [31]. We also used the plastome sequence of Anathallis obovata (Lindl.) Pridgeon & MW.Chase (MH979332), which was previously published by us [30], and obtained from GenBank the plastome sequence of Masdevallia picturata Rchb.f. (KJ566305), totaling ten species from subtribe Pleurothallidinae, representing seven of the nine generic affinity groups proposed by Karremans [18]. We also downloaded from GenBank the plastome sequences of two Laeliinae species to serve as the outgroup, according to the most recent classification of Orchidaceae [2]. Voucher and GenBank accession numbers of all species sampled are provided in S1 Table.
DNA extraction and NGS
We followed a plastid-enrichment procedure using 2 g of leaf tissue according to Sakaguchi et al. [32] and then extracted genomic DNA following the Doyle & Doyle [33] protocol, with reagent scaling to 2 mL microtubes and increasing the incubation time to 2−4 hours at 60°C. DNA was purified with DNA Clean and Concentrator kit (Zymo Research, Orange, CA) and sequenced on an Illumina MiSeq® using DNA Nextera XT Sample Prep kit (IlluminaTM) and MiSeq Reagent Kit V2 (IlluminaTM).
Genome assembly
The paired-end reads obtained from Illumina MiSeq sequencing (2 × 250 bp) were trimmed at 0.05 error probability limit and discarded when below 50 bp long with the CLC Genomics Workbench 8.0 (CLC Bio, Qiagen). Reads were then used in genome assembly with a mixed guided and de novo approach, performed in both CLC Genomics Workbench and Geneious Prime 2020.0.5 (Biomatters Ltd.). In the latter, we generated contigs from a de novo assembly of the reads using the MIRA 4.0 plugin, with the most accurate settings. In CLC Genomics Workbench, we mapped the reads in the complete genome sequence of Anathallis obovata (MH979332) using the default configuration and then generated a consensus sequence that was gapped in low-coverage areas (≤ 5×). These gaps were manually filled using the contigs, editing the former consensus sequence into a new one. The reads were mapped into the new consensus sequence with CLC Genomics Workbench, and the entire map was visually inspected to check for mismatches and assembly errors, which were manually corrected using the contigs. Sequencing information for each sample is available in S2 Table.
Genome annotation and graphical representations
Gene, coding sequence (CDS), ribosomal DNA (rDNA), and transport RNA (tRNA) annotations were imported from the complete plastome sequence of Anathallis obovata [30] in Geneious Prime. All annotations were manually verified and edited against those of the Dendrobium officinale Kimura & Migo (KC771275) and Masdevallia picturata (KJ566305) reference genomes. The IRs were identified and annotated using the Find Repeats tool of Geneious Prime and verified through REPuter online version (http://bibiserv.cebitec.uni-bielefeld.de/reputer). All sequences were submitted to GenBank through Geneious Prime. The.gb files of Pleurothallidinae plastomes were uploaded to Organellar Genome DRAW v1.1 [34] to make the genome maps and to IRscope [35] to produce the graphical representation of IR/LSC and IR/SSC junctions.
Mitochondrial and nuclear sequences
From the sequencing output of the eight species sequenced here plus that of Anathallis obovata sequenced by us previously [30], we recovered the complete sequence of the nuclear ribosomal DNA (nrDNA) operon (26S, 18.S, and 5.8S plus the associated spacers, ITS1 and ITS2) and parts of the mitochondrial genome (S2 Table). We mapped the raw reads to the nuclear ribosomal DNA sequence of Phalaenopsis japonica (Rchb.f.) Kocyan & Schuit. (MN221419) and the complete mitochondrial genome of Allium cepa L. (NC_030100.1) with Geneious Prime, using BBmap, with the highest sensitivity and Kmer length = 8. Also, in Geneious Prime, we annotated the nrDNA operon based on Acianthera luteola (Lindl.) Pridgeon & M.W. Chase (KX495754) and using BLAST. For mitochondrial DNA (mtDNA), we extracted only regions with coverage depth ≥ 8× as consensus sequences and imported CDSs, rDNAs, and tRNAs annotations from the reference sequence (NC_030100.1), which were then verified manually (S3 Table). Consensus sequences without annotations were excluded from the analyses and GenBank submission.
Sequence alignments
All alignments were made in Geneious Prime. We performed a Mauve alignment of the ten complete Pleurothallidinae plastomes with the progressiveMauve algorithm [36] to compare their general structure. For all subsequent analyses, we removed one of IRs from the plastome sequences to avoid overrepresentation. All alignments were made using MAFFT v.7.450 [37] with the FFT-NS-2 algorithm.
Codon usage and molecular evolution analyses
Codon usage and molecular evolution analyses were performed in the R software environment (https://www.r-project.org/). For the codon usage analysis, we extracted all complete CDS annotations from each of the ten Pleurothallidinae plastomes using Geneious Prime tools. Relative synonymous codon usage (RSCU) and codon frequencies were calculated for each CDS set using the SeqinR package [38]. For the molecular evolution analysis, we extracted and aligned separately all CDSs in common to the ten Pleurothallidinae plastomes in Geneious Prime. This analysis consisted of the application of Tajima’s D neutrality test [39], from pegas package [40], in each CDS alignment. All codes and datasets used are available in S1 File.
Simple sequence repeats (SSRs)
Plastid SSRs were identified for the ten Pleurothallidinae plastomes through MISA-web online program [41], using the following search minimum parameters: ten repetitions for mononucleotide motifs, five repetitions for dinucleotide motifs, and three repetitions for tri-, tetra-, penta-, and hexanucleotide motifs. All SSRs were manually annotated to the sequences, which were then aligned (S2 File). We designed primers for polymorphic SSRs present in at least seven plastomes using Geneious Prime, with the following characteristics: 18−27 bp in length, guanine-cytosine (GC) content between 20−80%, melting temperature (Tm) of 57−63°C with a maximum variation of 1°C between primer pairs, and product sizes between 100−500 bp.
Sequence variability and indels events
We aligned the ten Pleurothallidinae plastome sequences and then extracted all introns and intergenic spacers (IGSs) with 150 bp minimum length using Geneious Prime tools. We also extracted molecular markers that have been commonly used in Orchidaceae phylogenetics, such as the trnH-psbA intergenic spacer, the matK CDS, and the 3’ portion of ycf1 CDS [19, 42]. All aligned sequences were uploaded to DnaSP v.6 software [43] to obtain the total number of variable sites and insertions/deletions (indels). These data were used to calculate the sequence variability (SV) sensu Shaw et al. [44] but considering indels as events instead of sites to reduce homoplasy in these alignments [45]. Therefore, we used the following Eq (1) to calculate SV, where l = total length in bp, m = total number of mutations, and i = number of indels events.
| (1) |
The ten sequences with the highest SV were selected as potential molecular markers for Pleurothalldinae. We designed primers for these sequences in Geneious Prime with the following characteristics: 18−27 bp in length, GC content between 20−80%, 57−63°C Tm, maximum variation of 2°C annealing temperature between primer pairs, and product size between 100−1,000 bp.
We also extracted the IR, LSC, and SSC regions of each Pleurothallidinae plastome using Geneious Prime tools and performed multiple and pairwise alignments using Masdevallia picturata as the reference. Back in DnaSP, we computed the number of indels events per region and each plastome.
Phylogenetic analyses
All phylogenetic analyses were performed with maximum likelihood (ML) using IQ-tree v.1.6.11 [46], with 1,000 ultra-fast bootstrap replicates and -bnni strategy to reduce the risk of overestimation [47–49]. The best nucleotide substitution model was set for each dataset under the AIC criterion using ModelFinder [50], implemented on IQ-tree. The resulting trees were visualized and edited using Figtree v.1.4.1 (http://tree.bio.ed.ac.uk) and CorelDRAW X8 (https://www.coreldraw.com/). We analyzed and compared the following aligned datasets including the twelve Epidendreae samples: complete plastome sequences (one IR), plastid CDSs, plastid non-coding sequences, and top ten plastid mutational hotspots. We also analyzed, compared, and combined the following datasets including nine Pleurothallidinae samples (excluding Masdevallia picturata): complete plastome sequences (one IR), mtDNA, and nrDNA operon. For partitioned datasets, we first defined the best substitution model for each partition before combining them using Geneious Prime tools. Bootstrap percentages (BP) above 95 were considered strongly supported [51]. The Epidendreae complete plastome dataset and the Pleurothallidinae combined dataset are available in S3 File.
3. Results and discussion
The overall characteristics of the plastomes analyzed are summarized in Table 1 and those of the mitochondrial DNA in Table 2. Pleurothallidinae plastomes varied from 148,246 to 157,905 bp in length, with Acianthera recurva (Lindl.) Pridgeon & M.W. Chase the shortest and Myoxanthus exasperatus (Lindl.) Luer the longest. GC content varied little (36.9−37.1%). They all possess the typical quadripartite structure of most angiosperms plastomes: LSC of 83,694−85,605 bp and SSC of 10,573−18,444 bp, interspersed by two IRs of 25,242−27,020 bp (S1 Fig) [52].
Table 1. General characteristics of Pleurothallidinae plastomes analyzed.
| Taxon | Length (bp) | LSC (bp) | SSC (bp) | IR (bp) | %GC |
|---|---|---|---|---|---|
| Acianthera recurva | 148,246 | 84,871 | 10,573 | 26,401 | 37.0 |
| Anathallis microphyta | 154,558 | 84,597 | 15,993 | 26,984 | 37.0 |
| Anathallis obovata | 155,515 | 86,694 | 17,923 | 26,949 | 37.1 |
| Dryadella lilliputiana | 156,807 | 84,943 | 17,992 | 26,936 | 37.1 |
| Masdevallia picturata | 156,045 | 84,948 | 18,029 | 26,534 | 36.9 |
| Myoxanthus exasperatus | 157,905 | 85,605 | 18,260 | 27,020 | 37.1 |
| Octomeria grandiflora | 155,284 | 84,916 | 17,874 | 26,247 | 36.9 |
| Pabstiella mirabilis | 150,317 | 83,699 | 16,134 | 25,242 | 37.1 |
| Stelis grandiflora | 157,535 | 85,205 | 18,444 | 26,943 | 36.9 |
| Stelis montserratii | 157,479 | 85,147 | 18,366 | 26,983 | 36.9 |
Table 2. General characteristics of mtDNA dataset for Pleurothallidinae.
| Taxon | Total length (bp) | %GC | # genes | # CDSs | # tRNAs | # rDNAs |
|---|---|---|---|---|---|---|
| Acianthera recurva | 62,237 | 48.3 | 38 | 26 | 9 | 3 |
| Anathallis microphyta | 32,417 | 46.5 | 32 | 25 | 4 | 3 |
| Anathallis obovata | 46,959 | 47.0 | 33 | 25 | 5 | 3 |
| Dryadella lilliputiana | 83,225 | 47.2 | 36 | 25 | 8 | 3 |
| Myoxanthus exasperatus | 61,363 | 47.4 | 37 | 26 | 8 | 3 |
| Octomeria grandiflora | 63,381 | 48.2 | 33 | 25 | 6 | 2 |
| Pabstiella mirabilis | 43,635 | 48.4 | 29 | 25 | 1 | 3 |
| Stelis grandiflora | 49,861 | 47.4 | 34 | 25 | 6 | 3 |
| Stelis montserratii | 73,468 | 47.4 | 38 | 26 | 9 | 3 |
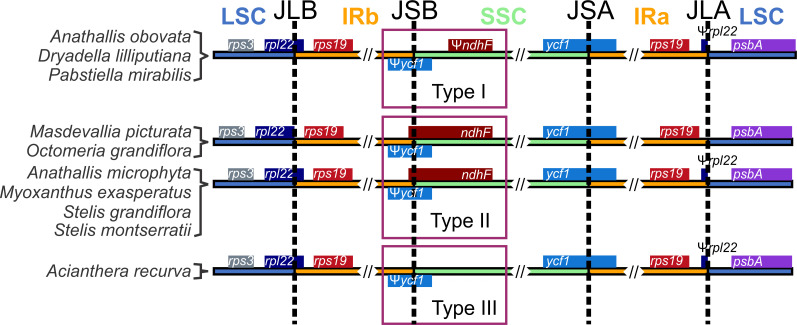
Gene composition is similar among the Pleurothallidinae plastomes analyzed: 102 genes are shared, being 68 CDSs, 4 rDNAs, and 30 tRNAs (S4 Table), and a variable set of ndh genes (S2 Fig). Of the 11 ndh genes, only ndhB, ndhD, and ndhH are present in all plastomes, but those with complete reading frames include only Myoxanthus exasperatus, Octomeria grandiflora Lindl., Stelis grandiflora Lindl., and S. montserratii (Porsch) Karremans. The Masdevallia picturata and Octomeria grandiflora plastomes are the only ones that have all 11 ndh genes intact, whereas Acianthera recurva has the fewest of these genes, only five, none with complete CDSs. Despite the variation observed in ndh gene content, the Mauve alignment showed no inversions or other rearrangements (S3 Fig). Complete losses and pseudogenization of ndh genes are common in Orchidaceae and have occurred independently [53–58]. These events have been linked to IR/SSC border instability and IR expansion [54, 57, 58], which we observed for the IR borders among these Pleurothallidinae plastomes (Fig 1). The IRa/SSC junction (JSA) is located within the ycf1 gene, producing a partial copy of this gene in IRb, but there are three types of IRb/SSC junctions (JSB) in Pleurothallidinae that can be classified into types I, II, and III sensu Luo et al. [25]. Type I occurs in Anathallis obovata, Dryadella lilliputiana (Cogn.) Luer, and Pabstiella mirabilis and is characterized by the presence of ndhF in the SSC without overlapping the JSB. Type II was the most common and detected in Anathallis microphyta, Masdevallia picturata, Myoxanthus exasperatus, Octomeria grandiflora, Stelis grandiflora, and S. montserratii, in which ndhF overlaps the JSB. Finally, type III, ndhF deletion, was found only in Acianthera recurva.
Fig 1. Inverted repeats (IRs) borders of the ten Pleurothallidinae plastomes analyzed.
IRs/SSC junction types sensu Luo et al. [25].
We also observed a significant reduction of the ycf1 partial copy in Pabstiella mirabilis due to a 1,000 bp shift of the gene into the SSC. Size reduction of ycf1 in IRs was highlighted as one possible outcome of IRs/SSC junction instability promoted by deletion/retention of ndh genes, especially ndhF [54]. Pabstiella mirabilis has only 15 bp of ndhF, but other Pleurothallidinae with this gene missing or pseudogenized did not suffer alterations in ycf1 size at the IRs. Thus, IR/SSC junction instability appears to have many attributes not exclusively dependent on the ndh gene content. The specific complement of ndh genes in P. mirabilis could have produced some IR reduction, but this was too small to alter gene content.
The LSC/IRb junction (JLB) varied among Pleurothallidinae as well. In Octomeria grandiflora and Masdevallia picturata, the JLB is situated in the rpl22-rps19 IGS and rps19 gene, respectively, producing a partial copy of the rps19 gene at IRa in this case (Fig 1). However, in the other Pleurothallidinae, the JLB is located in the rpl22 gene, producing a partial copy of this gene in IRa (Fig 1). We believe that retention of complete CDSs of all 11 ndh genes in Masdevallia picturata and Octomeria grandiflora may have caused a slight IR expansion, thus generating the variation observed in JLB position in these two plastomes relative to the others. Despite this, all ten plastomes have the trnH-GUG and rps19 genes in both IRs, even though rps19 is truncated in the IRa of Masdevallia picturata, which is the type III JLB, conserved among monocots [59].
IR expansion/retraction is considered one of the main causes of length variation in angiosperm plastomes [60–62]. However, the first study in Dendrobium Sw. showed a major contribution of the LSC to plastome total size variation due to the presence of large number of indels in this region [27]. This same large LSC contribution was also observed in Pleurothallidinae: of the 2,417 indel events, 1,899 (78.57%) are in the LSC, 384 (15.89%) in the SSC, and 134 (5.54%) in the IR (S5 Table). Nonetheless, when we analyzed indels events in each plastome, we found no correlation between the number of LSC indels and genome size (R = −0.52, p = 0.16), but we observed such a correlation with indels in the IR (R = −0.69, p = 0.04). We also found a correlation between genome size and LSC size (R = 0.70, p = 0.04), SSC size (R = 0.93, p < 0.01), and ndh CDSs (R = 0.68, p = 0.05). SSC size, in turn, is sensitive to ndh gene content (R = 0.66, p = 0.05) and their CDSs (R = 0.77, p = 0.01). Correlation plots are presented in S4 Fig. These results contradict, in part, the findings of Niu et al. [27] but also show that plastome size is affected by a combination of factors, for which the relative importance varies among clades. For Pleurothallidinae plastomes, which have few generic differences, indels in the most conservative region, the IR, have a greater impact on length than LSC indels, despite their predominance in the latter. Also, the sizes of LSC and SSC contribute unequally to Pleurothallidinae genome size because both varied more than the IR, but SSC size varied the most: about four times the range observed for LSC and IR (7,871 bp, against 1,906 bp of LSC and 1,778 bp of IR). In addition, seven of the 11 ndh genes are located in SSC, so it is not surprising that ndh gene composition greatly influences the length of this region and, consequently, plastome size because it is the main distinctive feature among Pleurothallidinae genomes. This influence of ndh gene deletion/retention on the SSC size has been widely reported in the literature [53, 54, 63, 64], but the importance of this region for orchid plastome size variation has only been observed for Pleurothallidinae and Bulbophyllum Thouars [29] thus far.
Protein-coding genes
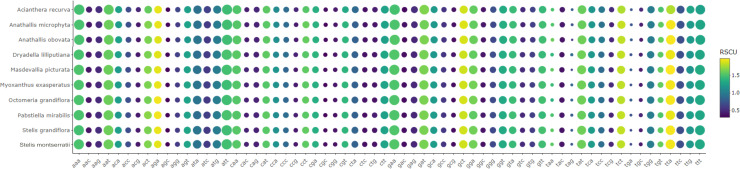
We observed that relative codon frequencies are similar among Pleurothallidinae (Fig 2, S6 Table). The most common were AAA (lysine) in Acianthera recurva, Anathallis microphyta, and Pabstiella mirabilis, GAA (glutamate) in Dryadella lilliputiana, and ATT (isoleucine) in the remaining species, whereas TGC (cysteine) was the rarest after stop codons. Among amino acids, leucine was the most frequent, and cysteine the least, similar to that observed in Bulbophyllum plastomes [29]. ATG (methionine) was the main start codon, but there is GTG in rps19, ACG in ndhD and rps2, CTG in ndhC (just Dryadella lilliputiana), and ATT in matK, except in Pabstiella mirabilis, which has an alternative start codon [65]. For relative synonymous codon usage, we identified a preference for codons that end in A/T (AT3) instead of G/C (GC3), a bias also observed for other angiosperms, including Bulbophyllum, and likely correlated with the high AT content of plastomes [29, 66–68].
Fig 2. Heatmap of relative synonymous codon usage (RSCU) and codon relative frequency observed for Pleurothallidinae CDSs.
The lowest frequency is indicated by purple and highest yellow. Relative frequencies are proportional to circle sizes.
To assess selection in protein-coding genes, we applied Tajima’s D test in the 68 CDSs shared among Pleurothallidinae. The results indicated that 13 genes are under positive selection: accD, atpB, petB, psbB, psbT, rbcL, rpl22, rpl32, rpl33, rpoC1, rps18, ycf1, and ycf2 (S7 Table). These genes have point mutations (SNPs), alignment gaps, and size variation. One of the most informative markers for land plants, ycf1 is the second largest gene in the plastome and has been suggested as a potential DNA barcode [42]. The accD, rbcL, rpl22, rpl32, rpoC1, and ycf2 genes also appear to be under positive selection in other Orchidaceae, perhaps related to their adaptative capacities [29, 68, 69].
Microsatellites (SSRs)
Simple sequence repeats (SSRs), also known as short tandem sequences or microsatellites, are 1−6 bp repeats common in all genomes [70, 71]. We identified 1,290 SSRs in Pleurothallidinae, of which 170 (13%) are unique and varied 113−143 per species (S8 Table). They were most common in the LSC (71%) and IGSs (55.6%) and least abundant in the IR (13.5%) and introns (16.2%). This distribution is as expected: the LSC is the largest region [22], and IGSs are the most common and variable plastome category [22, 72]. This is similar to what was found in Bulbophyllum [29] and Dendrobium [27] plastomes. Mono- and trinucleotide repeats are the most common and hexanucleotides the rarest, the latter absent in some species (S8 Table). As for other Orchidaceae [27, 29, 68, 73], A/T repeats predominate, followed by AAG/CTT. Most trinucleotide microsatellites were found in CDSs and the IR, the latter possessing nearly all of these. Indeed, tri- and hexanucleotide SSRs were previously reported to be more frequent in CDSs than any other plastome category [74], undoubtedly due to maintenance of protein function [74, 75].
Plastid microsatellites are excellent tools for genotyping, genetic mapping, and population studies [76–78]. Therefore, we designed 54 primers that cover 62 polymorphic SSR loci present in at least seven Pleurothallidinae (S9 Table). These repeats consist of 17 polyTs (10−19x), nine polyAs (10−19x), three di- (4−23x), 31 tri- (3−10x), one tetra- (3x), and one pentanucleotide repeat (3−4x). We hope that providing this set of microsatellite primers will encourage more population and species delimitation studies in Pleurothallidinae.
Mutation hotspots
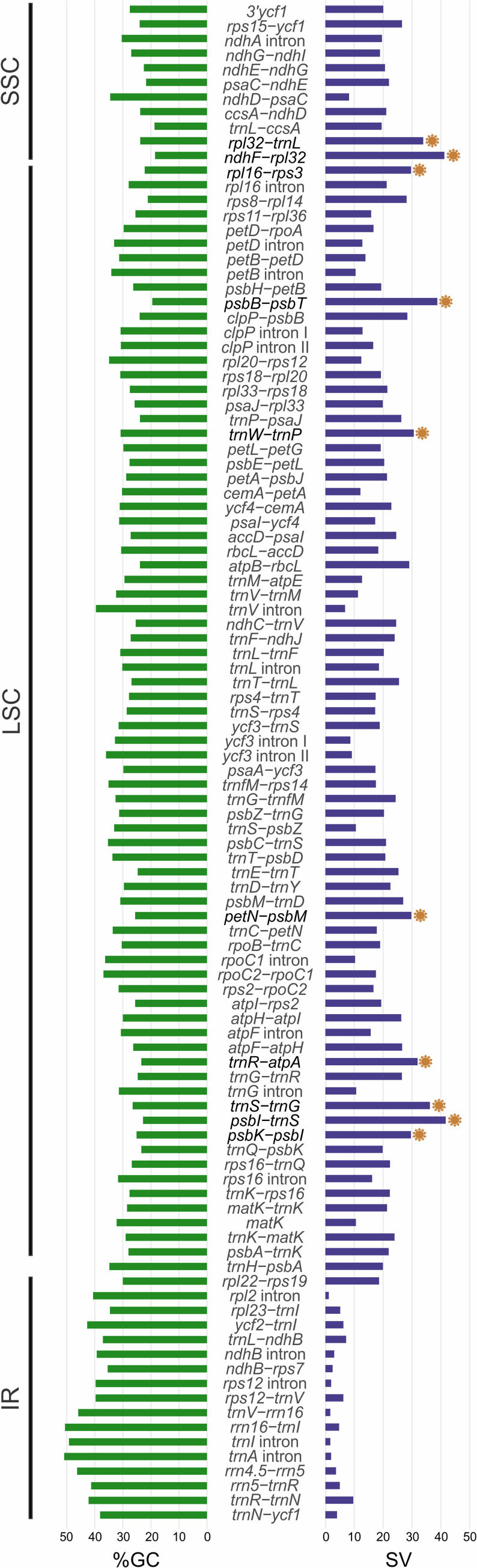
We analyzed levels of variation in 104 sequences more than 150 bp long, comprising IGSs, introns, and two CDSs (S10 Table). The most variable sequences (higher SV) are IGSs that have low GC content (Fig 3). This inverse relation between SV and %GC was expected and previously reported in Bulbophyllum and Dendrobium, as AT-rich sequences have higher mutation rates [27, 29, 68]. Seven of the ten sequences with highest SV found (ndhF-rpl32, psbB-psbT, psbK-psbI, rpl16-rps3, rpl32-trnL, trnR-atpA, and trnS-trnG) were previously identified in various other orchid clades [24, 26–29, 64, 68], but we identify here for the first time in orchids the following: petN-psbM, psbI-trnS, and trnW-trnP.
Fig 3. Graph of sequence variability (SV) and %GC for 104 Pleurothallidinae plastid sequences.
The ten most-variable sequences are highlighted.
Most phylogenetic studies in Pleurothallidinae have used nrITS, sometimes combined with the trnKUUU intron or matK (e.g. [6, 14]). Other markers such as ycf1 and trnH-psbA were included more recently [20, 21]. These all have SV below 25%, in contrast with 29.63−41.67% for the ten hotspots (S10 Table). Therefore, we designed subtribe-specific primers for the top ten to assist future phylogenetic analyses in Pleurothallidinae (S11 Table).
Phylogenomics
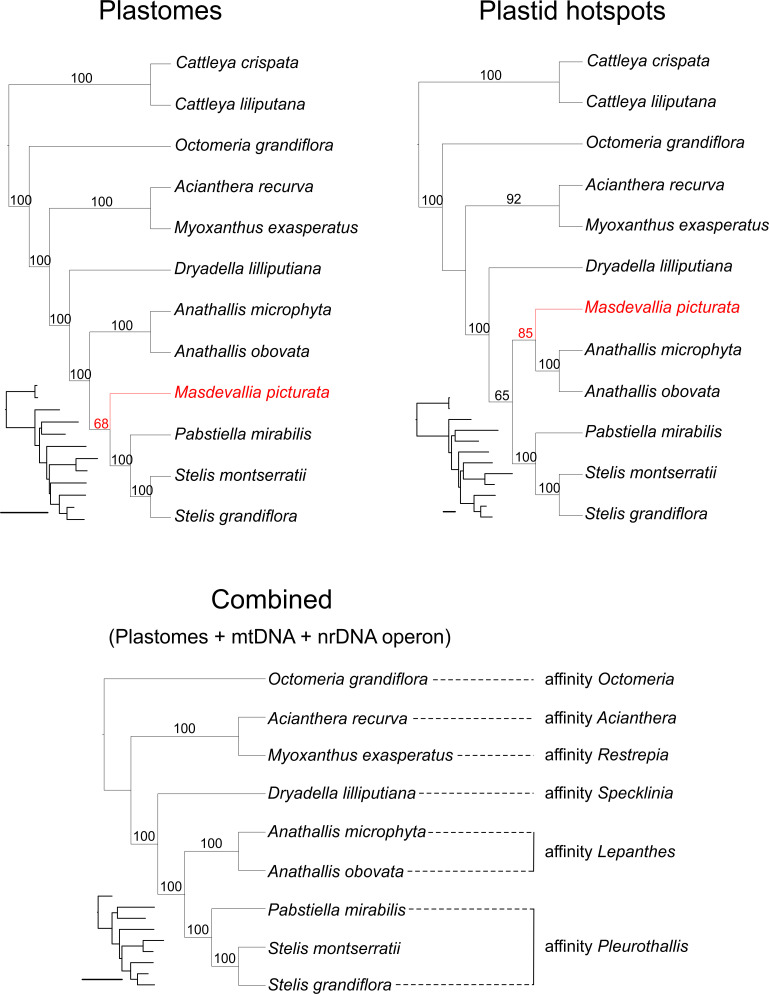
The aligned matrix of Epidendreae plastome sequences consisted of 142,507 bp, ~10% of which are variable and of these 41.47% are potentially parsimony-informative (PIC). As expected, non-coding regions had more variable characters (13.69%) and proportionally fewer PICs, whereas protein-coding loci are more conservative (Table 3) [79]. Tree topology based on plastid hotspots was the same as plastid non-coding DNA (NC-DNA) analysis (Figs 4 and S5). When comparing ML trees from the plastome datasets, the only disagreement was the position of Masdevallia picturata, but not with strong support (Fig 4).
Table 3. Number of sequences, length, nucleotide variation, and best substitution model for each dataset and partitions.
| Dataset | # sequences | Length (bp) | Variable characters | PIC | Best model (AIC) |
|---|---|---|---|---|---|
| Whole plastomes | 12 | 142,507 | 14,003 (09.82%) | 5,807 (41.47%) | GTR+F+R2 |
| 9 | 139,059 | 11,045 (07.94%) | 3,330 (30.15%) | GTR+F+R2 | |
| Coding sequences | 12 | 58,979 | 3,672 (06.22%) | 1,596 (43.46%) | GTR+F+R2 |
| Non-coding sequences | 12 | 76,868 | 10,526 (13.69%) | 3,971 (37.72%) | TIM+F+R2 |
| Hotspots | 12 | 6,656 | 1,676 (25.18%) | 717 (42.78%) | Partitioned |
| ndhF-rpl32 | 12 | 1,146 | 370 (32.28%) | 135 (36.48%) | TVM+F+G4 |
| petN-psbM | 12 | 1,042 | 231 (22.17%) | 102 (44.15%) | TIM+F+G4 |
| psbB-psbT | 12 | 611 | 170 (27.82%) | 66 (38.82%) | K3Pu+F+G4 |
| psbI-trnSGCU | 12 | 165 | 45 (27.27%) | 21 (46.67%) | GTR+F |
| psbK-psbI | 12 | 617 | 127 (20.58%) | 54 (42.52%) | TVM+F+G4 |
| rpl16-rps3 | 12 | 199 | 48 (24.12%) | 25 (52.08%) | TVM+F+I |
| rpl32-trnLUAG | 12 | 988 | 260 (26.31%) | 116 (44.61%) | TVM+F+R2 |
| trnRUCU-atpA | 12 | 231 | 59 (25.54%) | 25 (42.37%) | K3Pu+F+G4 |
| trnSGCU-trnGUCC | 12 | 1,456 | 319 (21.91%) | 147 (46.08%) | K3Pu+F+G4 |
| trnWCCA-trnPUGG | 12 | 201 | 47 (23.38%) | 26 (55.32%) | K3Pu+F+R3 |
| mtDNA | 9 | 94,117 | 6,048 (06.43%) | 899 (14.86%) | TVM+F+I |
| nrDNA operon | 9 | 5,865 | 335 (05.71%) | 152 (45.37%) | GTR+F+R2 |
| Combined * | 9 | 239,041 | 17,506 (07.32%) | 4,381 (25.68%) | Partitioned |
PIC = parsimony-informative characters.
*Plastome + mtDNA + nrDNA operon.
Fig 4. Maximum likelihood trees based on complete plastomes (one IR), plastid hotspots, and combined analyses (plastomes (one IR) + mtDNA + nrDNA operon).
Numbers on branches are the bootstrap percentages; a tree with proportional branch lengths is on the left of each tree, which bars represent 0.02 nucleotide substitutions per site.
Based on our results (Fig 4), Octomeria grandiflora (Octomeria affinity sensu Karremans [18]) is sister to the rest of the sampled subtribe, followed next by the clade of Acianthera recurva (Acianthera affinity) and Myoxanthus exasperatus (Restrepia affinity). Then Dryadella lilliputiana (Specklinia affinity) is sister to the last two clades. The first is composed of Anathallis microphyta and A. obovata (Lepanthes affinity), and the second by Pabstiella mirabilis sister to Stelis grandiflora and S. montserratii (Pleurothallis affinity). Masdevallia picturata (Masdevallia affinity) is sister to the Pleurothallis affinity clade in whole plastome and CDS analyses, and sister to the Lepanthes affinity clade in NC-DNA and hotspots analyses, with higher support in the latter.
Comparing this topology with Karremans’ proposal [18], the positions of the Octomeria, Restrepia, and Acianthera affinities in this order agree. However, the Masdevallia and Specklinia affinities were not positioned accordingly. The classification of Pleurothallidinae genera into affinities is a compilation of phylogenetic results based almost exclusively on nrITS data, so this disagreement may be due to genomic incongruence. In agreement, the nuclear ribosomal DNA (nrDNA) operon analysis recovered the Specklinia+Pleurothallis relationship (S6 Fig). The mtDNA analysis, on the other hand, recovered only the Lepanthes and Acianthera+Myoxanthus clades, with the former sister of the rest (S6 Fig). It must be noted that such a sparse sample of genera does not constitute a robust evaluation of Karremans’ ideas of relationships, and our aim was to describe and compare the large amount of molecular data presented here.
Plastid molecular markers are widely used in phylogenetic studies of land plants due to their abundance in cells and easy amplification/sequencing [19, 80, 81]. The nuclear ribosomal internal transcribed spacers (nrITS) have poorer sequencing success than plastid markers, but they hold the majority of nrDNA operon molecular variability and have great discriminant power at the species level for most angiosperms [19, 82]. Hence, plastid markers are often combined with nrITS, specially in orchid phylogenetic studies (e.g. [6, 14, 21, 25, 83, 84]). Highly supported discordance between plastid and nuclear trees is uncommon in Orchidaceae but has been detected in Epidendroideae [55], especially in Catasetinae [85] and here in Pleurothallidinae. Such cytonuclear discordance can be due to divergence in evolutionary history between nuclear and plastid genomes and is usually attributed to introgression, incomplete lineage sorting, and hybridization [85–88]. Determining the causes of the observed cytoplasmic discordance is beyond our scope due to limited sampling, but these newly sequenced plastomes are a good starting point for future investigations on this subject in the Pleurothallidinae.
Mitochondrial molecular markers were not considered as potential plant DNA barcodes due to their low substitution rates [19, 89, 90]. Complete mitochondrial genome sequences are also difficult to assemble in plants because their overall structure is not as conserved as those of plastomes and contains horizontally inherited nuclear and plastid sequences [91]. Still, some mitochondrial markers have been analyzed in the past for use in orchid analyses [92], such as nad1 and cox1 introns [93, 94], but their low variability compared to plastid markers discouraged their use. More recently, several mitochondrial CDSs were included in a phylogenomic study in the family [95], which produced a highly supported tree with some incongruence with the plastid analysis. Our mtDNA analysis included mostly CDSs and partial introns and also produced a well-supported tree, but it significantly diverged from the plastid and nuclear results (S6 Fig). It is noteworthy that there is no orchid complete mitochondrial genome available, so we recovered the mtDNA from raw reads based on a reference sequence that is phylogenetically distant from Pleurothallidinae. In addition, sequencing for each species varied in read number, length, and quality, which directly impacted mtDNA length and content (Table 2). We believe that these two main factors generated high sequence heterogeneity among species that might have influenced the results, so they must be interpreted with care. Despite the odd topology, the mtDNA tree retrieved the Lepanthes affinity and Acianthera+Myoxanthus clades found in other analyses. Also, a high-throughput analysis for the Lepanthes horrida Rchb.f. species complex revealed that some mitochondrial markers were among the ten best performing markers [96], supporting a future role in Pleurothallidinae phylogenetics.
When the three datasets were combined (plastome, nrDNA operon, and mtDNA), we got the same topology as the plastome analysis with higher bootstrap percentages (Fig 4). In fact, applying standard methods to concatenated multigene data often improves tree resolution and support in phylogenetic estimates (e.g. as seen in Pleurothallidinae [21, 96]). Simulation-based studies have shown that phylogenetic accuracy increases as more genes are included to the dataset [97, 98]. With this in view, we hope that the molecular markers highlighted here as plastid hotspots will be used in future phylogenetic studies in Pleurothallidinae in conjunction with nrITS, mtDNA, and, if possible, with morphological and ecological data as well, so that more robust phylogenetic trees are produced.
4. Conclusions
The eight new plastomes of Pleurothallidinae sequenced here greatly increased orchid subtribal representation in GenBank. Their overall structure and codon usage are conserved, and gene content is similar, with variation only in the ndh gene composition. Protein-coding genes under positive selection were detected, and a complete set of primers were provided for microsatellites and the top ten most-variable markers, thus increasing molecular resources available to future evolutionary research in the subtribe at various scales, from genes to species. In particular, the top ten markers are more variable than any plastid markers previously used in Pleurothallidinae phylogenetics, and their tree topology is similar to that obtained with whole plastomes, reinforcing their potential as suitable molecular markers for the subtribe. However, strongly supported incongruence among plastid, nuclear ribosomal DNA, and mtDNA topologies suggests putative divergence in the evolutionary histories of these genomes, a topic that needs future investigation. The well-supported mitochondrial tree, together with the performance in recent studies, suggests that the inclusion of mitochondrial markers in phylogenetic studies of Pleurothallidinae could be useful. The combined analysis of the three genomes, in addition to improving the support, could be expected to circumvent partly the problems associated with individual analyses.
Supporting information
Genes are represented by rectangles, for which functions are identified by colors as shown in the legend. Genes placed inside the circle are transcribed clockwise, and those outside the circle are transcribed counter-clockwise. The gray inner circle is the GC content graph. Images of the sequenced species were taken by Eric C. Smidt except Marcelo Rodrigues for Myoxanthus exasperatus.
(TIF)
White squares = complete gene losses, light-grey squares = truncated reading frames (pseudogenes), and dark-grey squares = complete CDSs.
(TIF)
(TIF)
(TIF)
Numbers on branches are the bootstrap percentages; a tree with proportional branch lengths is on the left of each tree, which bars represent 0.02 nucleotide substitutions per site.
(TIF)
Numbers on branches refer to bootstrap percentages; a tree with proportional branch lengths is on the left of each tree, which bars represent 0.02 nucleotide substitutions per site.
(TIF)
Accessions in boldface are sequences generated in this study. All vouchers provided are deposited at UPCB herbarium.
(PDF)
*See Mauad et al. [30].
(PDF)
*Genes with introns.
(PDF)
aGenes with introns, bduplicated genes (in IRs), cpartially duplicated genes, *pseudogenes.
(PDF)
The plastome of Madevallia picturata was the reference for all indels.
(PDF)
Species names were abbreviated as follows: ACIRE = Acianthera recurva, ANAMI = Anathallis microphyta, ANAOB = A. obovata, DRYLI = Dryadella lilliputiana, MASPI = Masdevallia infracta, MYOEX = Myoxanthus exasperatus, OCTGR = Octomeria grandiflora, PABMI = Pabstiella mirabilis, STEGR = Stelis grandiflora, and STEMO = Stelis montserratii.
(PDF)
D > 0 = purifying selection, and D < 0 = positive selection. Significant results (p ≤ 0.05) were highlighted in bold. *Tajima’s D test was not computed due to the lack of variable sites.
(PDF)
(PDF)
SSR type in brackets with repetition frequency following. Loci amplified by the same primer separated by semicolons. Slanting bars (/) indicate polymorphism in repeat units. Tm = primer melting temperature.
(PDF)
In total, 104 sequences were analyzed, comprising intergenic spacers (IGS), introns, and popular molecular markers for Orchidaceae such as the trnH-psbA IGS, the matK CDS, and the 3’ portion of ycf1 CDSs. IGS = inter-genic spacer, CDS = protein-coding sequence.
(PDF)
SV = sequence variability, Tm = primer melting temperature. *Primers previously published but with mismatches in Pleurothallidinae. For these cases, subtribe-specific primers for the same regions are provided.
(PDF)
(ZIP)
(ZIP)
(ZIP)
Acknowledgments
We thank the Universidade Federal do Paraná for providing the infrastructure for the realization of this study. AVSRM especially thanks Michelle Zavala Páez for helping with the statistical analyses and the reviewers for improving the manuscript.
Data Availability
All DNA sequences generated are available from the GenBank database. GenBank accession numbers of all species sampled are provided in S1 Table. Alignment matrices and trees are available from the TreeBase database (accession 27774). All other relevant data are within Supporting Information files.
Funding Statement
AVSRM - 88887.311411/2018-00 - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - https://www.gov.br/capes/pt-br ECS - 311001/2014-9 and 203304/2018-7 - Conselho Nacional de Desenvolvimento Científico e Tecnológico - https://www.gov.br/cnpq/pt-br The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.van den Berg C, Goldman DH, Freudenstein JV, Pridgeon AM, Cameron KM, Chase WM. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). Am J Bot. 2005; 92(4): 613–24. doi: 10.3732/ajb.92.4.613 [DOI] [PubMed] [Google Scholar]
- 2.Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, van den Berg C, et al. An updated classification of Orchidaceae. J Linn Soc, Bot. 2015; 177: 151–174. doi: 10.1111/boj.12234 [DOI] [Google Scholar]
- 3.Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN. Genera Orchidacearum IV: Epidendroidae (Part One). Oxford: Oxford University Press; 2005. [Google Scholar]
- 4.Martinelli G, Moraes MA. Livro Vermelho da Flora do Brasil. Rio de Janeiro: Andrea Jakobsson, Instituto de Pesquisas Jardim Botânico do Rio de Janeiro; 2013. [Google Scholar]
- 5.Luer CA. Icones Pleurothallidinarum III: Systematics of Pleurothallis (Orchidaceae). Monogr Syst Bot Mo Bot Gard. 1986; 20. [Google Scholar]
- 6.Pridgeon AM, Solano R, Chase MW. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. Am J Bot. 2001; 88(12): 2286–2308. doi: 10.2307/3558390 [DOI] [PubMed] [Google Scholar]
- 7.Pridgeon AM, Chase MW. Phylogenetic reclassification of Pleurothallidinae (Orchidaceae). Lindleyana. 2001; 16(4): 235–271. [Google Scholar]
- 8.Luer CA. A systematic method of classification of the Pleurothallidinae versus a strictly phylogenetic method. Selbyana. 2002; 23(1): 57–110. doi: 10.2307/41760106 [DOI] [Google Scholar]
- 9.Endara L, Williams NH, Whitten WM. Filogenia molecular preliminar de Scaphosepalum (Orchidaceae: Pleurothallidinae). Lankesteriana. 2011; 11(3): 245–252. doi: 10.15517/lank.v11i3.18279 [DOI] [Google Scholar]
- 10.Chiron GR, Guiard J, van den Berg C. Phylogenetic relationships in Brazilian Pleurothallis sensu lato (Pleurothallidinae, Orchidaceae): evidence from nuclear ITS rDNA sequences. Phytotaxa. 2012; 46: 34–58. doi: 10.11646/phytotaxa.46.1.5 [DOI] [Google Scholar]
- 11.Chiron GR, Karremans AP, van den Berg C. Nomenclatural notes in the Pleurothallidinae (Orchidaceae): Phloeophila. Phytotaxa. 2016; 270(1): 56–62. doi: 10.11646/phytotaxa.270.1.6 [DOI] [Google Scholar]
- 12.Karremans AP. Lankesteriana, a new genus in the Pleurothallidinae (Orchidaceae). Lankesteriana. 2014; 13(3): 319–332. doi: 10.15517/lank.v13i3.14368 [DOI] [Google Scholar]
- 13.Karremans AP, Rincón-González M. Nomenclatural notes in the Pleurothallidinae (Orchidaceae): Apoda-prorepentia. Phytotaxa. 2015; 238(2): 174–182. doi: 10.11646/phytotaxa.238.2.5 [DOI] [Google Scholar]
- 14.Karremans AP, Bakker FT, Pupulin F, Solano-Gómez R, Smulders MJM. Phylogenetics of Stelis and closely related genera (Orchidaceae: Pleurothallidinae). Plant Syst Evol. 2013. a; 299: 151–176. doi: 10.1007/s00606-012-0712-7 [DOI] [Google Scholar]
- 15.Karremans AP, Pupulin F, Gravendeel B. Taxonomy, molecular phylogenetics, reproductive isolation, and niche differentiation of the Specklinia endotrachys species complex (Orchidaceae: Pleurothallidinae). Lankesteriana. 2013. b; 13(1–2): 132–133. doi: 10.15517/lank.v0i0.11556 [DOI] [Google Scholar]
- 16.Karremans AP, Albertazzi FJ, Bakker FT, Bogarín D, Eurlings MCM, Pridgeon AM, et al. Phylogenetic reassessment of Specklinia and its allied genera in the Pleurothallidinae. Phytotaxa. 2016; 272(1): 1–36. doi: 10.11646/phytotaxa.272.1.1 [DOI] [Google Scholar]
- 17.Wilson M, Frank GS, Jost L, Pridgeon AM, Vieira-Uribe S, Karremans A.P. Phylogenetic analysis of Andinia (Pleurothallidinae; Orchidaceae) and a systematic re-circumscription of the genus. Phytotaxa. 2017; 295(2): 101–131. doi: 10.11646/phytotaxa.295.2.1 [DOI] [Google Scholar]
- 18.Karremans AP. Genera pleurothallidinarum: An updated phylogenetic overview of Pleurothallidinae. Lankesteriana. 2016; 16(2): 219–241. doi: 10.15517/lank.v16i2.26008 [DOI] [Google Scholar]
- 19.Vu THT, Le TL, Nguyen TK, Tran DD, Tran HD. Review on molecular markers for identification of orchids. Vietnam J Sci Technol. 2017; 59(2): 62–75. doi: 10.31276/VJSTE.59(2).62 [DOI] [Google Scholar]
- 20.Pérez-Escobar OA, Chomicki G, Condamine FL, Karremans AP, Bogarín D, Matzke NJ, et al. Recent origin and rapid speciation of Neotropical orchids in the world’s richest plant biodiversity hotspot. New Phytol. 2017; 215: 891–905. doi: 10.1111/nph.14629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez N, Toscano de Brito ALV, Mauad AVSR, Smidt EC. Molecular phylogeny and biogeography of Pabstiella (Pleurothallidinae: Orchidaceae) highlight the importance of Atlantic Forest in the speciation of the genus. J Linn Soc, Bot. Forthcoming 2020. doi: 10.1093/botlinnean/boaa092 [DOI] [Google Scholar]
- 22.Voelkerding KV, Dames SA, Durtschi JD. Next-generation sequencing: from basic research to diagnostics. Clin Chem. 2009; 55: 641–658. doi: 10.1373/clinchem.2008.112789 [DOI] [PubMed] [Google Scholar]
- 23.Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011; 76: 273–297. doi: 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Tang M, Li H, Zhang Z, Li D. Complete chloroplast genome of the genus Cymbidium: lights into the species identification, phylogenetic implications and population genetic analyses. BMC Evol Biol. 2013; 13: 84. doi: 10.1186/1471-2148-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Hou B, Niu Z, Liu W, Xue Q, Ding, X. Comparative chloroplast genomes of photosynthetic orchids: insights into evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PLoS One. 2014; 9: e99016. doi: 10.1371/journal.pone.0099016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Z, Xue Q, Zhu S, Sun J, Liu W, Ding X. The complete plastome sequences of four orchid species: insights into the evolution of Orchidaceae and the utility of plastomic mutational hotspots. Front Plant Sci. 2017; 8: 715. doi: 10.3389/fpls.2017.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Z, Zhu S, Pan J, Li L, Sun J, Ding X. Comparative analysis of Dendrobium plastomes and utility of plastomic mutational hotspots. Sci Rep. 2017; 7: 1–11. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smidt EC, Zavala-Páez M, Vieira LN, Viruel J, Baura VA, Balsanelli E, et al. Characterization of sequence variability hotspots in Cranichideae plastomes (Orchidaceae, Orchidoideae). PLoS One. 2020; 15(1): e0227991. doi: 10.1371/journal.pone.0227991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zavala-Páez M, Vieira LN, Baura VA, Balsanelli E, Souza EM, Cevallos MC, et al. Comparative Plastid Genomics of Neotropical Bulbophyllum (Orchidaceae; Epidendroideae). Front Plant Sci. 2020; 11: 799. doi: 10.3389/fpls.2020.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauad AVSR Vieira LN, Bolson M Baura VA, Balsanelli E Souza EM, et al. Complete chloroplast genome of Anathallis obovata (Orchidaceae: Pleurothallidinae). Rev. Bras. Bot. 2019; 42: 345–352. doi: 10.1007/s40415-019-00524-3 [DOI] [Google Scholar]
- 31.Thiers B. Index Herbariorum. A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. 2016 onward. Available from: http://sweetgum.nybg.org/science/ih (accessed 02 February 2020)
- 32.Sakaguchi S, Ueno S, Tsumura Y, Setoguchi H, Ito M, Hattori C, et al. Application of a simplified method of chloroplast enrichment to small amounts of tissue for chloroplast genome sequencing. Appl Plant Sci. 2017; 5(5): 1700002. doi: 10.3732/apps.1700002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 1987; 19(1): 11–15. [Google Scholar]
- 34.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007; 52: 267–274. doi: 10.1007/s00294-007-0161-y [DOI] [PubMed] [Google Scholar]
- 35.Amiryousefi A, Hyvönen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018; 34: 3030–3031. doi: 10.1093/bioinformatics/bty220 [DOI] [PubMed] [Google Scholar]
- 36.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004; 14: 1394–1403. doi: 10.1101/gr.2289704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013; 30(4): 772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charif D, Lobry JR. SeqinR 1.0–2: A contributed package to the R Project for Statistical Computing Devoted to Biological Sequences Retrieval and Analysis. In: Bastolla U, Porto M, Roman HE, Vendruscolo M, editors. Structural approaches to sequence evolution. New York: Springer; 2007. p.1–26. doi: 10.1007/978-3-540-35306-5 [DOI] [Google Scholar]
- 39.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989; 123(3): 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paradis E. pegas: an R package for population genetics with an integrated–modular approach. Bioinformatics. 2010; 26: 419–420. doi: 10.1093/bioinformatics/btp696 [DOI] [PubMed] [Google Scholar]
- 41.Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017; 33: 2583–2585. doi: 10.1093/bioinformatics/btx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong W, Xu C, Li C, Sun J, Zuo Y, Shi S, et al. ycf1, the most promising plastid DNA barcode of land plants. Sci Rep. 2015; 5: 8348. doi: 10.1038/srep08348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP v6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol. 2017; 34: 3299–3302. doi: 10.1093/molbev/msx248 [DOI] [PubMed] [Google Scholar]
- 44.Shaw J, Shafer HL, Leonard OR, Kovach MJ, Schorr M, Morris AB. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: The tortoise and the hare IV. Am J Bot. 2014; 101: 1987–2004. doi: 10.3732/ajb.1400398 [DOI] [PubMed] [Google Scholar]
- 45.Ingvarsson PK, Ribstein S, Taylor DR. Molecular evolution of insertions and deletion in the chloroplast genome of Silene. Mol Biol Evol. 2003; 20: 1737–1740. doi: 10.1093/molbev/msg163 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol. 2014; 32: 268–274. doi: 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minh BQ, Nguyen MAT, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 2013; 30: 1188–1195. doi: 10.1093/molbev/mst024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chernomor O, von Haeseler A, Minh BQ. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 2016; 65: 997–1008. doi: 10.1093/sysbio/syw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: Improving the ultrafast bootstrap approximation. Mol Biol Evol. 2017; 35(2): 518–522. doi: 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017; 14: 587. doi: 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985; 39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 52.Bock R. Cell and Molecular Biology of Plastids. New York: Springer; 2007. [Google Scholar]
- 53.Chang CC, Lin HC, Lin IP, Chow TY, Chen HH, Chen WH, et al. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): Comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Mol Biol Evol. 2006; 23: 279–291 doi: 10.1093/molbev/msj029 [DOI] [PubMed] [Google Scholar]
- 54.Kim HT, Kim JS, Moore MJ, Neubig KM, Williams NH, Whitten WM, et al. Seven new complete plastome sequences reveal rampant independent loss of the ndh gene family across orchids and associated instability of the inverted repeat/small single-copy region boundaries. PLoS One. 2015; 10: 1–18. doi: 10.1371/journal.pone.0142215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YK, Jo S, Cheon SH, Joo MJ, Hong JR, Kwak M, et al. Plastome evolution and phylogeny of Orchidaceae, with 24 new sequences. Front Plant Sci. 2020; 11: 22. doi: 10.3389/fpls.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HT, Chase MW. Independent degradation in genes of the plastid ndh gene family in species of the orchid genus Cymbidium (Orchidaceae; Epidendroideae). PLoS One. 2017; 12: 1–23. doi: 10.1371/journal.pone.0187318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin CS, Chen JJW, Huang YT, Chan MT, Daniell H, Chang WJ, et al. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci Rep. 2015; 5: 1–10. doi: 10.1038/srep09040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu Z, Pan J, Zhu S, Li L, Xue Q, Liu W, et al. Comparative analysis of the complete plastomes of Apostasia wallichii and Neuwiedia singapureana (Apostasioideae) reveals different evolutionary dynamics of IR/SSC boundary among photosynthetic orchids. Front Plant Sci. 2017; 8: 1–11. doi: 10.3389/fpls.2017.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang RJ, Cheng CL, Chang CC, Wu CL, Su TM, Chaw SM. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol Biol. 2008; 8: 36. doi: 10.1186/1471-2148-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chumley TW, Palmer JD, Mower JP, Fourcade HM, Calie PJ, Boore JL, et al. The complete chloroplast genome sequence of Pelargonium ×hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol. 2006; 23: 2175–2190. doi: 10.1093/molbev/msl089 [DOI] [PubMed] [Google Scholar]
- 61.Green BR. Chloroplast genomes of photosynthetic eukaryotes. Plant J. 2011; 66: 34–44. doi: 10.1111/j.1365-313X.2011.04541.x [DOI] [PubMed] [Google Scholar]
- 62.Weng ML, Ruhlman TA, Jansen RK. Expansion of inverted repeat does not decrease substitution rates in Pelargonium plastid genomes. New Phytol. 2017; 214: 842–851. doi: 10.1111/nph.14375 [DOI] [PubMed] [Google Scholar]
- 63.Jheng CF, Chen TC, Lin JY, Chen TC, Wu WL, Chang CC. The comparative chloroplast genomic analysis of photosynthetic orchids and developing DNA markers to distinguish Phalaenopsis orchids. Plant Sci. 2012; 190: 62–73. doi: 10.1016/j.plantsci.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 64.Zhu S, Niu Z, Xue Q, Wang H, Xie X, Ding X. Accurate authentication of Dendrobium officinale and its closely related species by comparative analysis of complete plastomes Acta Pharm Sin B. 2018; 8(6): 969–980. doi: 10.1016/j.apsb.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barthet MM, Moukarzel K, Smith KN, Patel J, Hilu KW. Alternative translation initiation codons for the plastid maturase matK: unraveling the pseudogene misconception in the Orchidaceae. BMC Evol Biol. 2015; 15: 210. doi: 10.1186/s12862-015-0491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morton BR. Strand asymmetry and codon usage bias in the chloroplast genome of Euglena gracilis. Proc Natl Acad Sci USA. 1998; 96(9): 5123–5128. doi: 10.1073/pnas.96.9.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian W, Yang JR, Pearson NM, Maclean C, Zhang J. Balanced codon usage optimizes eukaryotic translational efficiency. PLoS Genet. 2012; 83: e1002603. doi: 10.1371/journal.pgen.1002603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu Z, Xue Q, Wang H, Xie X, Zhu S, Liu W, et al. Mutational biases and GC-biased gene conversion affect GC content in the plastomes of Dendrobium genus. Int J Mol Sci. 2017; 18(11): 2307. doi: 10.3390/ijms18112307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong WL, Wang RN, Zhang NY, Fan WB, Fang MF, Li ZH. Molecular evolution of chloroplast genomes of orchid species: Insights into phylogenetic relationship and adaptive evolution. Int J Mol Sci. 2018; 19(3): 716. doi: 10.3390/ijms19030716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tautz D, Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984; 12: 4127–4138. doi: 10.1093/nar/12.10.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li YC, Korol AB, Fahima T, Beiles A, Nevo E. Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Mol Ecol. 2002; 11: 2453–2465. doi: 10.1046/j.1365-294x.2002.01643.x [DOI] [PubMed] [Google Scholar]
- 72.Gielly L, Taberlet P. The use of chloroplast DNA to resolve plant phylogenies—Noncoding versus rbcL sequences. Mol Biol Evol. 1994; 11(5): 769–77. doi: 10.1093/oxfordjournals.molbev.a040157 [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Du L, Liu A, Chen J, Wu L, Hu W, et al. The complete chloroplast genome sequences of five Epimedium species: lights into phylogenetic and taxonomic analyses. Front Plant Sci. 2016; 7: 306. doi: 10.3389/fpls.2016.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgante M, Hanafey M, Powell W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat Genet. 2002; 30(2): 194–200. doi: 10.1038/ng822 [DOI] [PubMed] [Google Scholar]
- 75.Metzgar D, Bytof J, Wills C. Selection against frameshift mutations limits microsatellite expansion in coding DNA. Genome Res. 2000; 10(1): 72–80. doi: 10.1101/gr.10.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Provan J, Powell W, Hollingsworth PM. Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol Evol. 2001; 16(3): 142–147. doi: 10.1016/s0169-5347(00)02097-8 [DOI] [PubMed] [Google Scholar]
- 77.Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004; 5(6): 435–445. doi: 10.1038/nrg1348 [DOI] [PubMed] [Google Scholar]
- 78.Gemayel R, Vinces MD, Legendre M, Verstrepen KJ. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet. 2010; 44: 445–477. doi: 10.1146/annurev-genet-072610-155046 [DOI] [PubMed] [Google Scholar]
- 79.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in Angiosperms: The tortoise and the hare III. Am J Bot. 2007; 94(3): 275–288. doi: 10.3732/ajb.94.3.275 [DOI] [PubMed] [Google Scholar]
- 80.Lahaye R, van der Bank M, Bogarín D, Warner J, Pupulin F, Gigot G, et al. DNA barcoding the floras of biodiversity hotspots. Proc Natl Acad Sci USA. 2008; 105(8): 2923–2928. doi: 10.1073/pnas.0709936105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian X, Li DZ. Application of DNA sequences in plant phylogenetic study. Acta Bot Yunnanica. 2002; 24: 170–184. [Google Scholar]
- 82.Group CPBOL, Li D-Z, Gao L-M, Li H-T, Wang H, Ge X-J, et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci USA. 2011; 108(49): 19641–19646. doi: 10.1073/pnas.1104551108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smidt EC, Borba E, Gravendeel B, Fischer GA, van den Berg C. Molecular phylogeny of the Neotropical sections of Bulbophyllum (Orchidaceae) using nuclear and plastid spacers. Taxon. 2011; 60(4): 1050–1064. doi: 10.1002/tax.604009 [DOI] [Google Scholar]
- 84.Gonçalvez GF, Mauad AVSR, Taques G, Smidt EC. Molecular and morphological phylogenetic analysis and taxonomic revision of the genus Orleanesia (Laeliinae, Epidendroideae, Orchidaceae). Phytotaxa. 2019; 392(1): 1–18. doi: 10.11646/phytotaxa.392.1.1 [DOI] [Google Scholar]
- 85.Pérez-Escobar OA, Balbuena JA, Gottschling M. Rumbling orchids: how to assess divergent evolution between chloroplast endosymbionts and the nuclear host. Syst Biol. 2016; 65(1): 51–65. doi: 10.1093/sysbio/syv070 [DOI] [PubMed] [Google Scholar]
- 86.Rieseberg LH, Soltis DE. Phylogenetic consequences of cytoplasmic gene flow in plants. Evol Trends Plants. 1991; 5: 65–84. [Google Scholar]
- 87.Tsitrone A, Kirkpatrick M, Levin DA. A model for chloroplast capture. Evolution. 2007; 57(8): 1776–1782. doi: 10.1111/j.0014-3820.2003.tb00585.x [DOI] [PubMed] [Google Scholar]
- 88.Fehrer J, Gemeinholzer B, Chrtek J, Bräutigam S. Incongruent plastid and nuclear DNA phylogenies reveal ancient intergeneric hybridization in Pilosella hawkweeds (Hieracium, Cichorieae, Asteraceae). Mol Phylogenet Evol. 2007; 42: 347–361. doi: 10.1016/j.ympev.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 89.Wolfe KH, Li WH, Sharp PM. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast and nuclear DNA. Proc Natl Acad Sci USA. 1987; 84: 9054–9058. doi: 10.1073/pnas.84.24.9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drouin G, Daoud H, Xia J. Relative rates of synonymous substitutions in the mitochondrial, chloroplast and nuclear genomes of seed plants. Mol Phyl Evol. 2008; 49(3): 827–31. doi: 10.1016/j.ympev.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 91.Cho Y, Qiu YL, Kuhlman P, Palmer JD. Explosive invasion of plant mitochondria by a group I intron. Proc Natl Acad Sci USA. 1998; 95(24): 14244–14249. doi: 10.1073/pnas.95.24.14244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freudenstein JV, Senyo DM, Chase MW. Mitochondrial DNA and relationships in the Orchidaceae. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution.Collingwood: CSIRO Publishing; 2000. p. 421–429. [Google Scholar]
- 93.Freudenstein JV, Chase M. Analysis of mitochondrial nad1b-c intron sequences in Orchidaceae: utility and coding of length-change characters. Syst Bot. 2001; 26: 643–657. doi: 10.1043/0363-6445-26.3.643 [DOI] [Google Scholar]
- 94.Inda LA, Pimentel M, Chase MW. Contribution of mitochondrial cox1 intron sequences to the phylogenetics of tribe Orchideae (Orchidaceae). Does the distribution and sequence of this intron in orchids tell us something about its evolution? Taxon. 2010; 59: 1053–1064. doi: 10.2307/20773976 [DOI] [Google Scholar]
- 95.Li YX, Li ZH, Schuiteman A, Chase MW, Li JW, Huang WC, et al. Phylogenomics of Orchidaceae based on plastid and mitochondrial genomes. Mol Phyl Evol. 2019; 139: 106540. doi: 10.1016/j.ympev.2019.106540 [DOI] [PubMed] [Google Scholar]
- 96.Bogarín D, Pérez-Escobar OA, Groenenberg D, Holland SD, Karremans AP, Lemmon EM, et al. Anchored hybrid enrichment generated nuclear, plastid and mitochondrial markers resolve the Lepanthes horrida (Orchidaceae: Pleurothallidinae) species complex. Mol Phyl Evol. 2018; 129: 27–47. doi: 10.1016/j.ympev.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 97.Rokas A, Carroll SB. More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Mol Biol Evol. 2005; 22: 1337–1344. doi: 10.1093/molbev/msi121 [DOI] [PubMed] [Google Scholar]
- 98.Gadagkar SR, Rosenberg MS, Kumar S. Inferring species phylogenies from multiple genes: Concatenated sequence tree versus consensus gene tree. J Exp Zool. 2005; 304B: 64–74. doi: 10.1002/jez.b.21026 [DOI] [PubMed] [Google Scholar]