Abstract
Purpose:
In locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck (CSCC-HN), surgery followed by radiotherapy is standard but can be cosmetically and functionally devastating, and many patients will recur.
Experimental Design:
Newly diagnosed or recurrent stage III-IVA CSCC-HN patients amenable to curative-intent surgery received two cycles of neoadjuvant PD-1 inhibition. The primary endpoint was ORR per RECIST 1.1. Secondary endpoints included pathologic response [pathologic complete response (pCR) or major pathologic response (MPR; ≤10% viable tumor)], safety, DSS, DFS, and OS. Exploratory endpoints included immune biomarkers of response.
Results:
Of 20 patients enrolled, 7 had recurrent disease. While only 6 patients (30%; 95% CI: 11.9–54.3) had partial responses by RECIST, 14 patients (70%; 95% CI: 45.7–88.1) had a pCR (n=11) or MPR (n=3). No SAEs ocurred during or after the neoadjuvant treatment. At a median follow-up of 22.6 months (95% CI: 21.7–26.1), one patient progressed and died, one died without disease, and two developed recurrence. The 12-month DSS, DFS, and OS rates were 95% (95% CI: 85.9–100), 89.5% (95% CI: 76.7–100), and 95% (95% CI: 85.9–100) respectively. Gene expression studies revealed an inflamed tumor microenvironment in patients with pCR or MPR and CyTOF analyses demonstrated a memory CD8+ T-cell cluster enriched in patients with pCR.
Conclusions:
Neoadjuvant immunotherapy in locoregionally advanced, resectable CSCC-HN is safe and induces a high pathologic response rate. Pathologic responses were associated with an inflamed tumor microenvironment.
INTRODUCTION
Approximately 1,100,000 Americans1 are diagnosed yearly with cutaneous squamous cell carcinoma (CSCC), with the head and neck (HN) harboring up to 80% of cases.2 Because of increasing sun exposure and an aging population, the incidence of CSCC is rising.3 While most patients present with early-stage, highly curable disease, about 2–5% present with locoregionally advanced CSCC.4,5 Standard treatment of resectable locoregionally advanced CSCC is surgery followed by adjuvant radiotherapy, which can cause significant disfigurement and functional morbidity.6,7 Despite aggressive local treatment, approximately 30% of patients with locoregionally advanced, resectable CSCC will recur and eventually die of disease.8,9
Programed cell death 1 (PD-1) immune checkpoint inhibition has been shown to be highly effective in patients with metastatic or advanced CSCC not amenable to curative surgery and/or radiotherapy with response rates ranging 34–50%.10,11,12 Indeed, the rationale for using a PD-1 checkpoint inhibitor in CSCC is strong. The main risk factor for CSCC development is ultraviolet exposure, which induces DNA damage and leads to a high tumor mutational burden (TMB). High TMB has been associated with response to PD-1 inhibitors in a variety of cancer types.13 Furthermore, immunosuppression is also linked to CSCC development, with chronically immune-suppressed patients having more than 100-fold increased risk of developing CSCC, usually presenting with more aggressive disease and having an increased risk of mortality.14
Given the suboptimal cosmetic, functional and oncologic outcomes achieved with surgery and radiotherapy in locoregionally advanced, resectable CSCC-HN, there is a strong rationale for the use of neoadjuvant systemic therapy. Neoadjuvant therapy could permit less destructive surgery, reduce the need for adjuvant radiotherapy and allow for pathologic response to be used as a predictor of long-term outcome. Given the attractive safety profile of anti-PD-1 agents, we performed a pilot phase II study to assess the safety and activity of neoadjuvant immunotherapy in CSCC-HN patients with locoregionally advanced, resectable disease. In doing so, we explored potential biomarkers of response and examined the changes induced by PD-1 inhibition in the tumor microenvironment using gene expression profiling and single-cell mass cytometry (CyTOF).
METHODS
Patients
Eligible patients were 18 years of age or older and had either newly diagnosed or recurrent, resectable CSCC-HN stage III-IV (AJCC Cancer Staging Manual, 8th Ed.).15 For patients where a primary tumor was not assessable (Tx), tumor classification was determined by the multidisciplinary team as part of routine clinical practice. A Zubrod performance status of 0–1,16 normal organ function, and the presence of measurable disease per RECIST 1.117 or per direct clinical measurement in patients with a primary tumor were also required for study participation. Major exclusion criteria included other malignancies within 5 years of treatment (including acute and chronic leukemia or lymphoma), history or risk of severe autoimmune disease, history of HIV infection or active hepatitis (B or C) or active tuberculosis, and ongoing immunosuppressive therapy. There were no patient drop outs.
Study Design
This was an investigator-initiated, single-institution, pilot phase II study of neoadjuvant PD-1 inhibition (cemiplimab) prior to curative intent surgery for locoregionally advanced CSCC-HN. The study was approved by the MD Anderson Cancer Center Institutional Review Board (IRB), conducted in accordance with the Declaration of Helsinki and all patients provided written informed consent before registration. All tumor tissue specimens analyzed in this study were co-collected for correlative analyses under a separate IRB approved protocol. The study schema is shown in Supplementary Figure 1.
Procedures
Patients received two cycles of intravenous cemiplimab 350 mg every 3 weeks before surgical resection. Surgery was planned ≥21 days after the second cemiplimab dose. Imaging of the neck (computed tomography [CT] or magnetic resonance imaging) and chest (CT or positron emission tomography -CT), photography of the tumor area for those patients with a primary tumor, and specimen collection were performed at baseline and after completion of neoadjuvant immunotherapy. All patients underwent oncologic surgical resection according to the original clinical and radiologic extent of disease. Adjuvant radiotherapy was planned at baseline for all patients. However, given the impressive pathologic responses noted on study, adjuvant therapies were re-considered by the multidisciplinary team on a case-by-case basis after surgery. Patients were then offered observation, adjuvant radiotherapy or chemoradiation according to individual pathologic findings, patient and provider preference. Clinical surveillance with physical examination and cross-sectional imaging was prescribed every 3–4 months for the first two-years after completion of therapy (surgery or radiotherapy, whichever occurred last) per institutional guidelines.
Pathologic Assessment
Pretreatment biopsy specimens were subjected to histopathologic assessment for confirmation of diagnosis and to permit morphologic comparison between tumor tissue before treatment and any residual tumor following therapy. Pathologic response was assessed in the posttreatment surgical specimens according to standard pathologic evaluation recommendations18 and re-reviewed by a dedicated dermatopathologist (PN) to standardize reporting (Supplementary Figure 2). Pathologic complete response (pCR) was defined as absence of viable tumor in the post-treatment surgical specimens and major pathologic response (MPR) was defined as ≤ 10% viable tumor. Pathologic partial response (pPR) was defined as > 10% but ≤ 50% viable tumor. Stable or progressive disease was defined as > 50% viable tumor persisting in the post-treatment surgical specimens. Typical histopathologic patterns of response including fibrosis and coagulative tumoral necrosis were noted in the tumor specimens in pCR or MPR cases. In addition, certain histologic features that may be specific to treated CSCC were noted, including accumulation of anucleate or nonviable keratinous material either as aggregates of colloid bodies, almost forming nodular keratinocyte-derived amyloid or nodules of variably lamellated keratinous material. Lack of viability was determined using immunohistochemical studies for p63, p40 or cytokeratin cocktail (AE1/AE3, MNF116, Zym5.2, and Cam5.2) as required. Also noted was accumulation of dense inflammatory infiltrate composed of lymphocytes and histiocytes with variably foamy cytoplasm (similar to tumoral melanosis seen in treated melanoma). The relative percentages of tumor bed occupied by viable tumor, fibrosis, keratin, tumoral necrosis, and inflammatory infiltrate were determined for each sample (Supplementary Figure 2). In patients with lymph node involvement, histopathologic evaluation was performed similar to that of the primary tumor. If multiple lymph nodes were involved, the percent response to therapy was estimated for each lymph node separately and then averaged to generate the final treatment response.
Outcomes
The primary endpoint was overall response rate (ORR) to neoadjuvant PD-1 inhibition per RECIST 1.1, by comparison between baseline and preoperative imaging after 2 cycles of cemiplimab.17 Secondary endpoints included pathologic response, safety and tolerability, 1-year disease-specific survival (DSS), disease free survival (DFS), overall survival (OS), time to recurrence, and patterns of failure.
Toxicity was monitored from treatment start until 30 days after the last dose of cemiplimab and graded according to the National Cancer Institute Common Terminology Criteria v.4.03.19 Also recorded and considered in the evaluation of safety was any delay in planned surgery greater than 48 hours.
DSS was defined as the time interval between the date of diagnosis and date of death for patients who died of disease, and was censored at the last follow-up date or date of death of unrelated disease for patients who were alive or died of other causes, respectively. DFS was defined as the time interval between the surgery date and recurrence or death date, whichever occurred first, and was censored at the last follow-up date for patients who neither recurred nor died. OS was defined as the time interval between the date of diagnosis and date of death of any cause, and was censored at the last follow-up date for patients who were alive.
Nanostring
RNA was isolated from formalin-fixed paraffin-embedded (FFPE) tumor sections by de-waxing using deparaffinization solution (Qiagen), and total RNA was extracted using the RecoverALL™ Total Nucleic Acid Isolation kit (Ambion) according to the manufacturer’s instructions. RNA purity was assessed on the ND-NanoDrop1000 spectrometer (Thermo Scientific). For the NanoString assay, 100 ng of RNA was used to detect immune gene expression using the nCounter PanCancer Immune Profiling panel along with a custom CodeSet. Counts of the reporter probes were tabulated for each sample with the nCounter Digital Analyzer, and raw data were imported into the nSolver data analysis package (http://www.nanostring.com/products/nSolver) for normalization and cell type deconvolution. Gene set enrichment analysis (GSEA) was performed with Qlucore Omics Explorer software, version 3.5 (Qlucore). GraphPad Prism 8 (GraphPad Software v-8.4.3) was used for plotting the data and Mann-Whitney test was used to evaluate the statistical significance between the groups. P-values less than 0.05 were considered significant.
CyTOF Analysis
Pretretment and post-treatment tumor specimens were dissociated into single-cell suspensions and fixed-frozen for CyTOF analysis. Cells were stained with 37 antibodies (Supplementary Table 1). Metal-conjugated antibodies were purchased from Fluidigm or purified unlabeled antibodies were metal-labeled in house. Normalization of CyTOF data was performed using normalizer in R package premessa. Normalized files for individual specimens were then used for downstream analyses. Regulatory T cells (CD3+CD4+FOXP3+) were manually gated using FlowJo based on the expression of lineage markers on the live CD45+ cells, and the frequencies are shown as percentage of live CD45+ cells. For the cluster identification, manually gated CD45+ cells were subjected to clustering analysis using an approach previously described as “CyTOF workflow”.20 Data from specimens with poor viability and insufficient events were excluded to avoid inaccurate clustering and frequency calculations.20 Data were analyzed with Prism 8 (GraphPad Software). Statistical significance was determined by two-tailed student’s t-test; P < 0.05 was considered statistically significant.
Statistical Analysis
The data cut-off date was April 5, 2021. The primary objective of the study was to estimate the ORR with neoadjuvant cemiplimab. On power analsysis, a sample size of 20 evaluable patients with stage III-IV CSCC-HN was chosen to ensure that the ORR could be estimated with a standard error no larger than 0.112. A stopping rule was established as a surgical delay rate > 0.2. Safety and responses were assessed in all eligible patients.
Patients’ demographics, clinical characteristics, and outcomes were summarized using descriptive statistics. The distribution of time-to-event endpoints was estimated using the Kaplan-Meier method. Fisher’s exact test was used to study the association between treatment responses criteria. Statistical analyses were conducted in R version 3.4.2. All tests were two-tailed, and P < 0.05 was considered statistically significant.
RESULTS
Patients and Treatment
From July 2018 through February 2019, 20 patients enrolled in the study; 13 (65%) with newly diagnosed and seven (35%) with recurrent CSCC-HN. Nine (45%) patients presented with regional nodal metastases and without a defined primary tumor. None of the patients had received prior radiotherapy to the site of presenting disease. All patients received the two planned doses of neoadjuvant cemiplimab. Patient characteristics are summarized in Table 1. A CONSORT patient flow diagram is provided in Supplementary Figure 3. The median number of comorbidites among the cohort was 4 (range 1–9). The most common comorditites were hypertension (16, 80%), hyperlipidemia (13, 65%), gastroesophageal reflux (8, 40%), obstructive sleep apnea (5, 25%), diabetes mellitus (4, 20%), coronary artery disease (4, 20%), atrial fibrillation (4, 20%) and chronic renal insufficiency (4, 20%).
Table 1.
Patient characteristics
| Patient Characteristics | N=20 | |
|---|---|---|
| Age, Average (SD) | 68.4 (10.9) | |
| Gender, No. (%) | ||
| Female | 2 (10) | |
| Male | 18 (90) | |
| Location, No. (%) | ||
| Cheek | 2 (10) | |
| External auditory canal | 1 (5) | |
| Forehead | 3 (15) | |
| Neck | 1 (5) | |
| Neck nodes | 7 (35) | |
| Nose | 1 (5) | |
| Parotid | 2 (10) | |
| Scalp | 3 (15) | |
| Recurrent disease, No. (%) | ||
| No | 13 (65) | |
| Yes | 7 (35) | |
| T classification, No. (%) | ||
| 1 | 2 (10) | |
| 2 | 1 (5) | |
| 3 | 6 (30) | |
| 4 | 2 (10) | |
| x | 9 (45) | |
| N classification, No. (%) | ||
| 0 | 5 (25) | |
| 1 | 4 (20) | |
| 2b | 9 (45) | |
| 2c | 1 (5) | |
| 3b | 1 (5) | |
| Clinical stage, No. (%)* | ||
| III | 8 (40) | |
| IV | 12 (60) |
AJCC Cancer Staging Manual, 8th Ed.
Safety and Feasibility
Treatment-related adverse events (TRAE) of any grade occurred in 7 patients (35%) and are summarized in Table 2. There were no serious adverse events and all TRAE resolved fully. All patients underwent the proposed surgery with no delays related to neoadjuvant therapy, thereby never triggering the pre-defined stopping rule. The median time from last dose of cemiplimab to surgery was 30 days (range, 21–50), allowing for medical clearance and coordination with plastic surgery (as needed) given the elderly population with co-mordidities and advanced disease frequently requiring complex reconstruction. Surgery was performed in all patients according to the original extent of disease with the intent of an R0 resection. Details of the surgical procedures performed are listed in Supplementary Table 2. Among the 11 patients with primary tumors, frozen section histologic assessment confirmed negative surgical margins in 9/11 (82%) patients; in the two patients with positive margins, the disease at the margin involved the dura over the sagittal sinus (n=1) and the intracranial portion of the trigeminal nerve (n=1). Extensive reconstruction was frequently required, including microvascular free tissue transfer in 10 (50%) patients. One (5%) surgical complication, a hematoma, was observed for the entire cohort. With a median follow up of 22.6 months, we have not observed late immune-related adverse events.
Table 2.
Summary of adverse events (AE) possibly, probably, or definitely related to the study drug
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Total (%) |
|---|---|---|---|---|
| Diarrhea | 0 | 0 | 1 | 1 (5%) |
| Fatigue | 0 | 1 | 0 | 1 (5%) |
| Joints pain | 0 | 1 | 0 | 1 (5%) |
| Myalgia | 0 | 1 | 0 | 1 (5%) |
| Pruritus | 6 | 0 | 0 | 6 (30%) |
| Maculo-papular rash | 3 | 0 | 0 | 3 (15%) |
| Hypothyroidism | 0 | 1 | 0 | 1 (5%) |
Efficacy
All 20 patients were evaluable for clinical and pathologic response. The ORR per RECIST was 30% (6 of 20; 95% CI: 11.9–54.3); all imaging responses were partial. Two (10%) patients had radiographic evidence of progression per RECIST. Notably, 70% (14/20; 95% CI: 45.7–88.1) of patients achieved either a pCR (11, 55%) or MPR (3, 15%) [Figure 1A]. Representative clinical, radiographic, and histologic images of a responder with a MPR are shown in Figure 1B. The responses per RECIST are summarized in Supplementary Table 3. We observed no association between imaging response and pathologic response (Odds ratio [OR]=2.65; p=0.61) [Supplementary Table 4].
Figure 1. Efficacy of neoadjuvant cemiplimab.
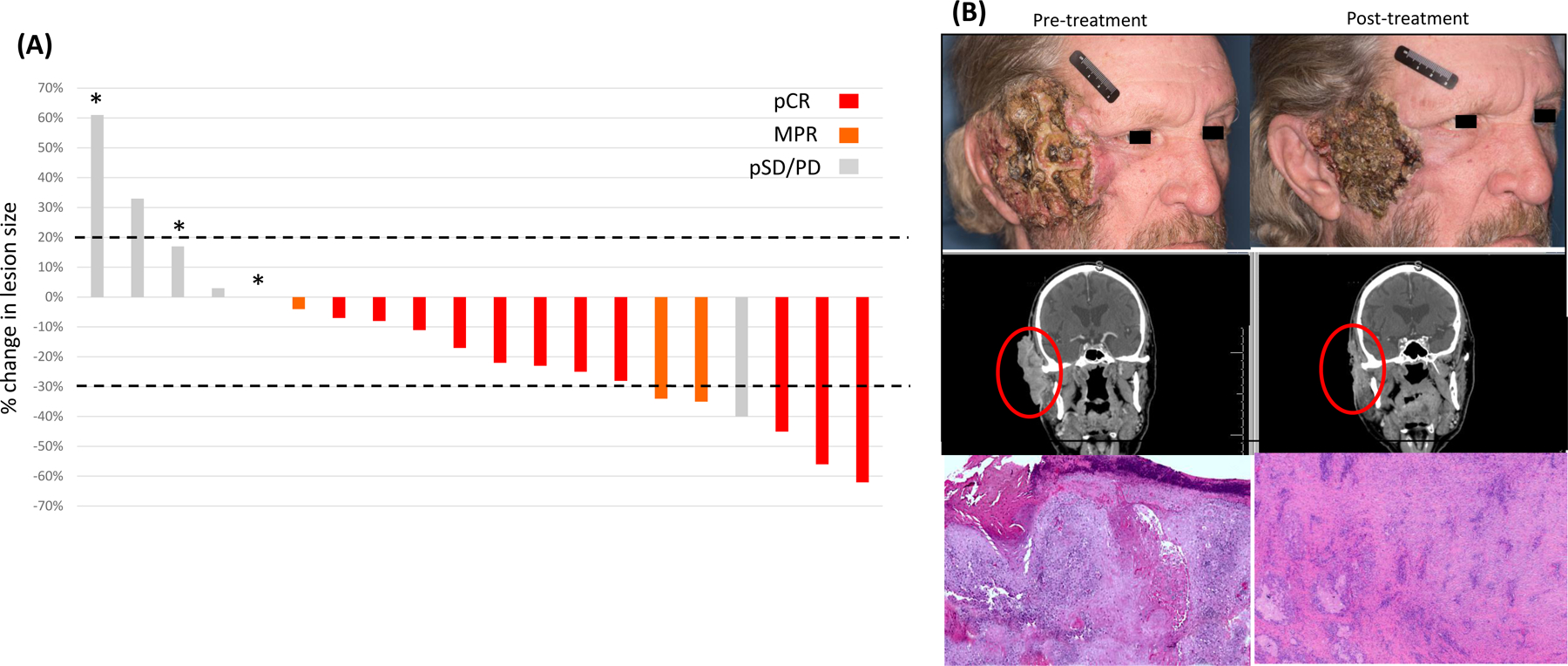
A) Waterfall plot of percentage change from baseline in target lesions per RECIST 1.1 and color-coded for pathologic response in the same subject; *, indicates recurrence after surgery and radiation. B) Representative pretreatment (left) and post-treatment (right) photographs, coronal computed tomography (CT) images, and micrographs of tumor specimens of a patient who achieved a major pathologic response (6-mm foci of residual viable tumor) following neoadjuvant cemiplimab treatment. pCR, pathologic complete response; MPR, major pathologic response; SD/PD, stable or progressive disease.
On the basis of pathologic response, 11 (55%) patients were not recommended previously planned adjuvant radiotherapy and an additional patient (5%) declined adjuvant therapy. None of these patients developed recurrence. Eight (40%) patients received postoperative radiotherapy, including 2 (10%) who received adjuvant chemoradiation for positive surgical margins (n=1) or extranodal extension (n=1). Of these, two (25%) did not complete adjuvant therapy because of disease progression (n=1) or toxicity (n=1).
At a median follow-up time of 22.6 months (95% CI: 21.7–26.1), 3 of 20 (15%) patients recurred, none of whom achieved either an imaging response or pathologic response (pCR or MPR) [Figure 1A]. Two of the patients who recurred had aggressive primary tumors involving the calvarium or skull base deemed resectable by the treating surgeon. One patient had T4aN2b disease involving the skull base at presentation, appeared stable during neoadjuvant immunotherapy but was found to have positive margins intracranially at the time of surgery. This patient progressed during adjuvant chemoradiation and died of disease 4.7 months after surgery. Another patient had recurrent T4aN0 disease involving the calvarium at presentation, experienced disease progression during neoadjuvant immunotherapy and was also found to have positive margins intracranially at the time of surgery. This patient received adjuvant radiotherapy but recurred 11.5 months after surgery. Finally, a third patient with TxN3b disease involving the parotid gland did not respond to neoadjuvant immunotherapy. He was treated with negative margin surgery and radiation therapy but recurred 17.5 months after surgery. At this writing, both patients are alive with disease and receiving palliative systemic therapy. Two (10%) patients died during follow-up; one of disease as described above and one who achieved a pCR and was not treated with adjuvant radiotherapy but subsequently developed a secondary malignancy (T-cell lymphoma) and died of treatment complications of lymphoma 11 months after surgery. An interval event chart depicting individual patient outcomes is provided in Figure 2.
Figure 2. Oncologic outcomes following neoadjuvant cemiplimab and surgery.
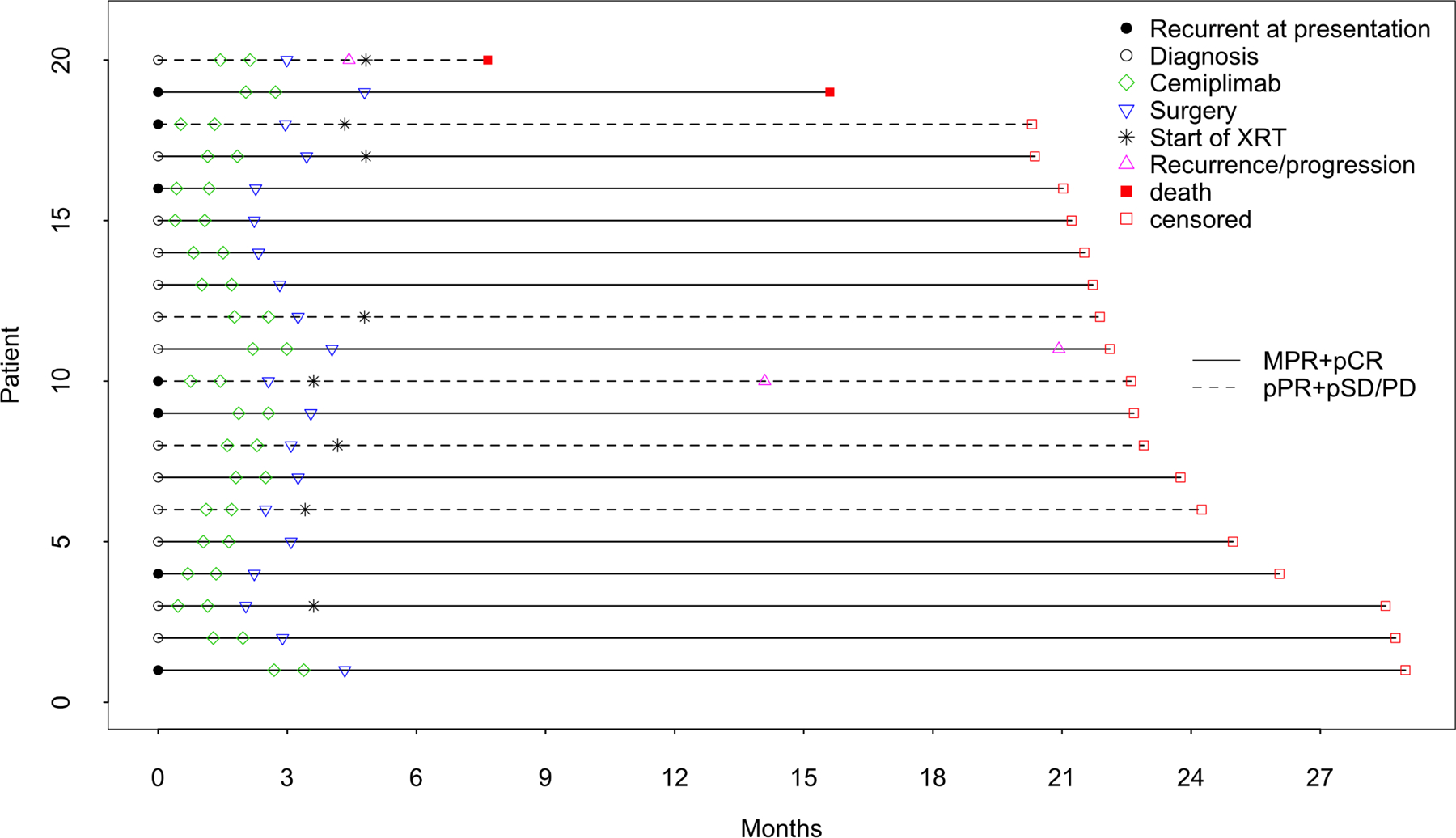
Interval event chart aligned by diagnosis date indicating treatment procedures and patient outcomes following surgery.
At 12 months, the DSS rate was 95% (95% CI: 85.9–100); the DFS rate was 89.5% (95% CI: 76.7–100) and OS rate was 95% (95% CI: 85.9–100). Kaplan-Meier estimates of DFS and OS are despicted in Supplementary Figure 4.
Favorable Immune Microenvironment in Pretreatment Tumor Tissues is Associated with Pathologic Responses
We compared gene expression in pretreatment tumor specimens of pathologic responders (R), defined as patients with pCR or MPR, and pathologic non-responders (NR), defined as patients with stable or progressive disease (pSD/PD). We observed that an immune cell inflamed tumor microenvironment was associated with favorable pathologic responses (Figure 3A). Pathologic responders (R) had significantly higher infiltration of T cells, including CD8 T cells and Th1 cells, along with higher PD-1 and PD-L1 expression (Figure 3B). This was accompanied by a significantly higher expression of IFNG and related immune genes including IDO1, CXCL9, CD274, GZMK, and ICOS involved in TCR and PD1 signaling pathways (Figure 3C–D) indicating a favorable microenvironment for effective anti-PD1 therapy.
Figure 3. Favorable immune microenvironment prior to cemiplimab treatment is associated with pathological responses.
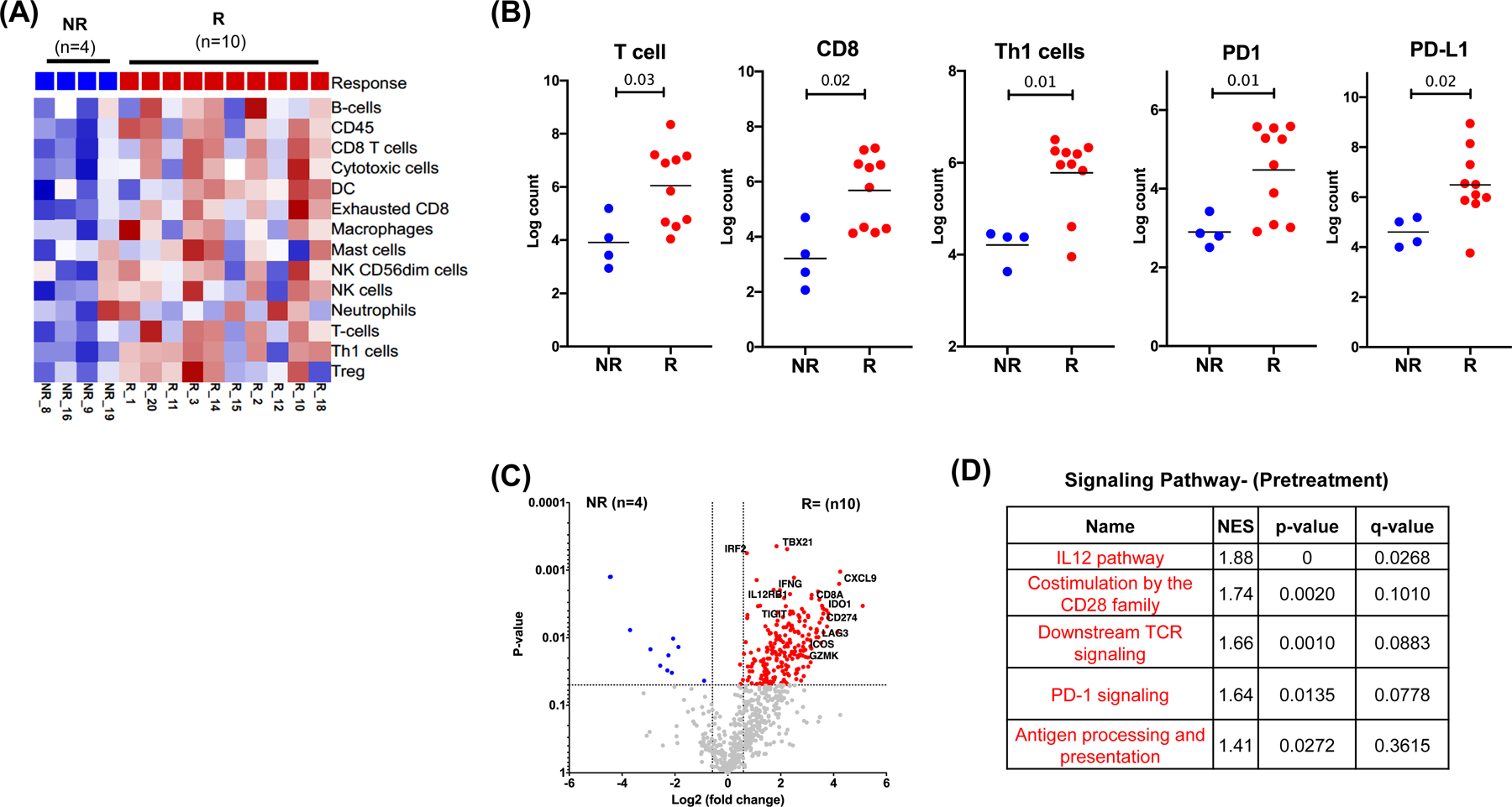
Pretreatment tumor specimens from pathological responders (R, red, n=10) and pathological non-responders (NR, blue, n=4) were analyzed for gene expression using a custom NanoString panel. (A) Heat map showing supervised clustering of immune cell infiltrates by response. (B) Scatter plots showing significantly different immune cell phenotypes between NR and R patients. Asterisks denote statistically significant differences. (C) Volcano plot of differentially expressed genes (Log2 FC>1.5 and P<0.05) by response. (D) Immune pathways with significantly different expression between R and NR patients per gene set enrichment analysis. NES, normalized enrichment score.
Memory CD8 T Cells Correlate with Response While Regulatory T Cells and CD68+ Myeloid Cells Expressing VISTA Correlate with Resistance to Therapy
Based on CyTOF analyses, we found that a subset of memory CD8+ T cells, CD8+EOMES+CD45RO+, were significantly more abundant in post-treatment tumor specimens of pathologic responders (R) than in pretreatment tumor specimens (Figure 4A). In addition, we identified potential resistance mechanisms including CD3+CD4+FOXP3+ regulatory T cells and CD68+ myeloid cells expressing the inhibitory checkpoint VISTA, which were significantly higher in post-treatment tumor specimens of pathologic non-responders (NR) patients than in the post-treatment tumor specimens of pathologic responders (R) (Figure 4B–C, Supplementary Figure 5). VISTA+ myeloid cells have previously been shown to correlate with resistance to immune checkpoint therapy.21
Figure 4. Association of changes in tumor immune microenvironment after cemiplimab treatment with pathological responses.
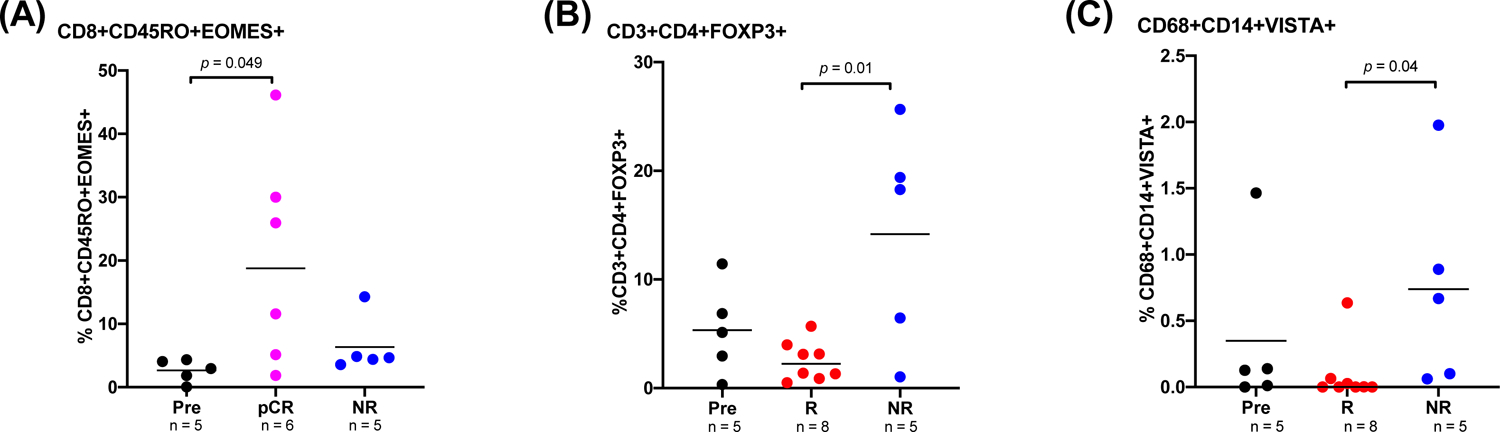
CyTOF analysis of pre-and post-treatment tumor specimens. (A) Dot plot showing percentage of a memory CD8-T cell subset (CD8+Eomes+CD45RO+) in pre-and post-treatment tumor specimens of patients with pathologic complete response (pCR) and pathologic nonresponders (NR, pathologic stable or progressive disease). (B–C) Dot plots showing percentages of (B) regulatory T cells (CD3+CD4+FOXP3+) and (C) a myeloid cell subset expressing inhibitory marker VISTA (CD68+CD14+VISTA+) in pre-and post-treatment tumor specimens of patients with pathologic response and NR. P<0.05 denotes statistically significant changes.
DISCUSSION
This is the first study to report the safety and efficacy of PD-1 inhibition before surgery in locoregionally advanced, resectable CSCC-HN. Despite an elderly patient population with competing comorbidities, neoadjuvant immunotherapy was generally well tolerated and did not delay curative-intent surgery. Notably, while the response rate by RECIST was 30%, the pathologic response rate (pCR and MPR) was substantially higher at 70%. Further, we observed no association between imaging response and pathologic response. These data, in the context of the growing body of literature, suggest that imaging responses my substantially underestimate pathologic responses.
It is important to note that, given the dramatic pathologic responses to neoadjuvant immunotherapy, the majority of patients (60%) did not receive adjuvant radiotherapy as was planned for all patients prior to study participation. None of these patients developed recurrence. During the study, the application of adjuvant radiotherapy was re-considered by the multidisciplinary team after surgery on a case-by-case basis. For example, one patient presented with recurrent CSCC-HN of the vertex scalp with bilateral parotid metastases. She was observed to have a pCR at the primary tumor and bilateral parotid glands after neoadjuvant cemiplimab. The original plan for adjuvant radiotherapy would have included the entire anterior scalp and bilateral parotid and cervical nodal basins. In her case, the expected morbidity of radiotherapy outweighed concern over the risk of locoregional recurrence. At this writing, the patient remains free of disease >23 months after surgery and without radiation.
It is well established that pCR or MPR to systemic therapy is a much better predictor of long-term patient outcomes than clinical or radiologic response.22,23–27 In patients with CSCC-HN, survival is not the only outcome of interest. The proximity of disease to critical structures, such as the eyes, ears, or mouth, can lead to substantial functional loss and deformity for patients with CSCC-HN. Thus, the omission of adjuvant radiotherapy with or without concurrent chemotherapy in patients who achieve a pathologic response to neoadjuvant cemiplimab could have a major positive impact on patient quality of life. In this study, the surgical plan was not altered by response to neoadjuvant therapy. However, it is easy to imagine a scenario in which surgery could be tailored or even avoided according to response, offering even greater improvement in patient quality of life. This warrants further investigation.
The efficacy of neoadjuvant PD-1 inhibition in CSCC-HN compares favorably with that of neoadjuvant epidermal growth factor receptor (EGFR) inhibitor in a similar patient population. In a study of neoadjuvant gefitinib in 22 patients with CSCC, albeit only 22% with nodal metastasis, the ORR was 45% and the pCR rate was 14%. Five (23%) patients had recurrence within 12 months yielding a 12-month DFS rate of 64%.25
PD-1 inhibition has been studied in metastatic and locally advanced, unresectable CSCC.11,12 In a phase II study including 78 patients, 26% were considered to have technically unresectable disease and 13% had previously received radiotherapy and no further radiation was considered acceptable. The ORR to cemiplimab by RECIST was 44%, which exceeded our imaging response rate. However, in that study 15 of 34 (44%) responses occurred after 2 months of treatment and imaging responses have been shown to appear for up to 12 months on therapy. The estimated progression-free survival rate at 12 months was 58%, significantly lower than the 89.5% 12-month DFS rate observed in our study, in which patients were treated with curative intent. Even so, our study included a high-risk, advanced-stage population represented exclusively by HN primaries, 75% with nodal involvement, 40% with T3 or T4 primary tumors, and 35% with recurrent disease.
While limited in size, our study results are encouraging and, if confirmed by ongoing trials, may herald a dramatic shift in clinical practice for locoregionally advanced, resectable CSCC-HN. Although some patients may be cured with immunotherapy alone, for now surgery followed by radiotherapy remains the standard of care. In a recent large prospective trial, the 2-year DFS in high-risk advanced, resectable CSCC treated with surgery and radiation was 78%.28 Surgery also remains critical for assessing pathologic response and may remove remnant clones resistant to systemic therapy. PD-1 inhibition is already approved for metastatic or unresectable CSCC. In our trial, importantly, the three (15%) patients who developed recurrence or progression had neither an imaging nor pathologic response to neoadjuvant cemiplimab. Further, two of these patients could be viewed in hindsight as having “borderline” resectable disease. So the importance of careful consideration of resectability remains paramount in patient selection.
Understanding cancer biology is essential to the successful application of personalized therapy. A distinct advantage of the neoadjuvant approach used in our study is the opportunity for paired tumor sampling before and after treatment, where correlatives could be explored. We focused our biomarker analysis on the characterization of the tumor immune microenvironment using gene expression and CyTOF for immune cell subsets. Despite the limitation of small sample size, we observed an inflamed immune tumor microenvironment comprising CD8+ T cells and Th1 cells in the pre-treatment tumor specimens of patients who had a pathologic response (pCR or MPR). These findings are in concordance with previous studies in which responses to targeting the PD-1/PD-L1 axis were associated with higher CD8+T-cell densities and Th1-type gene expression in melanoma, lung, and other cancers.29,30 Furthermore, evaluation of post-treatment tumor specimens identified potential mechanisms of resistance, including regulatory T cells and myeloid cells expressing VISTA.
Limitations of this study include the lack of randomization, small sample size, limited follow-up period, and performance in a single reference academic center. Nevertheless, the safety and efficacy results presented herein demonstrate the feasibility of the proposed therapeutic strategy.
In conclusion, neoadjuvant cemiplimab is safe and effective in locoregionally advanced, resectable CSCC-HN. To our knowledge, the pathologic response rate (pCR and MPR) of 70% is the highest reported with neoadjuvant anti-PD-1 therapy in solid tumors.20 Analysis of biological specimens revealed an inflamed tumor immune microenvironment in pretreatment tumor specimens of patients who achieved pathological responses (pCR and MPR) and suggests that patients with pCR may have memory CD8+ T cells expressing CD45RO and EOMES driving complete tumor regression. In contrast, an immunosuppressive immune tumor microenvironment was present in patients without a pathologic response (pSD and PD) and suggests additional combination therapies targeting the inhibitory markers expressed on myeloid cells may improve outcomes for this set of patients. Based on these preliminary findings, we have added an expansion cohort to include stage II disease (n=20) where baseline tissue specimens would be uniformly expected (NCT03565783). Our clinical findings are also currently being validated in a larger, multicenter phase II study (NCT04154943).
Supplementary Material
Statement of Translational Relevance.
Programed cell death 1 (PD-1) immune checkpoint inhibition has been shown to be highly effective in patients with metastatic or advanced cutaneous squamous cell carcinoma (CSCC) not amenable to curative therapy. In this study, we have demonstrated a 70% pathologic response rate after 2 doses of cemiplimab prior to surgery in resectable CSCC of the head and neck. At a median follow up of 22.6 months, no recurrences have been observed among responders most of whom were treated without adjuvant therapy. Analyses of paired specimens before and after neoadjuvant immunotherapy suggest that patients with an inflamed tumor microenvironment are more likely to benefit from PD-1 inhibition. We further report a subset of memory CD8+ T cells enriched in the pretreatment specimens of responders. Further investigation of this treatment approach, and underlying mechanisms of response, is warranted in CSCC.
ACKNOWLEDGEMENTS
Supported by Regeneron, the American Head and Neck Society / American Academy of Otolaryngology Head and Neck Surgery Foundation Surgeon Scientist Combined award and by the National Cancer Institute award P30CA016672. We thank the patients and their families for participating in this study and Stephanie P Deming from the Research Medical Library for editing services.
Footnotes
Presented during a poster discussion at the 2019 European Society for Medical Oncology Annual Congress, Barcelona, Spain, September 28, 2019.
Clinicaltrials.gov identifier: NCT03565783
Declaration of interests:
RF reports personal fees from Regeneron-Sanofi, Ayala Pharma, Klus Pharma, Medscape, Cellestia Biotech, Carevive, Prelude and Bicara; grants from AstraZeneca, Merck, Gennentech, Pfizer, Oropharynx Program Stiefel clinical trials, ASCO Career Development Award, and MD Anderson Khalifa Award within the past two years.
PS reports consulting, advisory roles, and/or stocks/ownership for Achelois, Apricity Health, BioAlta, Codiak BioSciences, Constellation, Dragonfly Therapeutics, Forty-Seven Inc., Hummingbird, ImaginAb, Jounce Therapeutics, Lava Therapeutics, Lytix Biopharma, Marker Therapeutics, Oncolytics, Infinity Pharma, BioNTech, Adaptive Biotechnologies, and Polaris; and owns a patent licensed to Jounce Therapeutics.
JA reports consulting, advisory roles, and/or stocks/ownership for Achelois, Apricity Health, BioAlta, Codiak BioSciences, Dragonfly Therapeutics, Forty-Seven Inc., Hummingbird, ImaginAb, Jounce Therapeutics, Lava Therapeutics, Lytix Biopharma, Marker Therapeutics, Polaris, BioNTech, and Adaptive Biotechnologies; and owns a patent licensed to Jounce Therapeutics.
BE served on advisory board for Roche/Genentech and for Seattle Genetics in the last 12 months.
NG received research funding from Regeneron. Received advisory board and consulting fees from PDS Biotechnology, Shattuck Labs, and Genzyme.
All other authors declare no competing interest.
REFERENCES
- 1.Ward RE, Ali SA, Kuhar M. Epithelioid malignant mesothelioma metastatic to the skin: A case report and review of the literature. J Cutan Pathol 2017;44(12):1057–63 doi 10.1111/cup.13026. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 2015;151(10):1081–6 doi 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 2010;146(3):283–7 doi 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 2013;68(6):957–66 doi 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Roscher I, Falk RS, Vos L, Clausen OPF, Helsing P, Gjersvik P, et al. Validating 4 Staging Systems for Cutaneous Squamous Cell Carcinoma Using Population-Based Data: A Nested Case-Control Study. JAMA Dermatol 2018;154(4):428–34 doi 10.1001/jamadermatol.2017.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer 2015;51(14):1989–2007 doi 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 7.Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R, et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns-Results of a non-interventional study of the DeCOG. Eur J Cancer 2018;96:34–43 doi 10.1016/j.ejca.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 8.Givi B, Andersen PE, Diggs BS, Wax MK, Gross ND. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck 2011;33(7):999–1004 doi 10.1002/hed.21574. [DOI] [PubMed] [Google Scholar]
- 9.Moore BA, Weber RS, Prieto V, El-Naggar A, Holsinger FC, Zhou X, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 2005;115(9):1561–7 doi 10.1097/01.mlg.0000173202.56739.9f. [DOI] [PubMed] [Google Scholar]
- 10.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018;379(4):341–51 doi 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 11.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020;21(2):294–305 doi 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grob JJ, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J Clin Oncol 2020;38(25):2916–25 doi 10.1200/JCO.19.03054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377(25):2500–1 doi 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam S, Yao CMK, Amit M, Gajera M, Luo X, Treistman R, et al. Association of Immunosuppression With Outcomes of Patients With Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol Head Neck Surg 2019. doi 10.1001/jamaoto.2019.3751. [DOI] [PMC free article] [PubMed]
- 15.Amin MBES, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. . AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017. [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5(6):649–55. [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, Blank CU, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 2018;29(8):1861–8 doi 10.1093/annonc/mdy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health NCI. Revised National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 for adverse event reporting. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40.
- 20.Nowicka M, Krieg C, Crowell HL, Weber LM, Hartmann FJ, Guglietta S, et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 2017;6:748 doi 10.12688/f1000research.11622.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017;23(5):551–5 doi 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, et al. Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol 2016;2(4):508–16 doi 10.1001/jamaoncol.2015.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong LP, Zhang CP, Ren GX, Guo W, William WN Jr., Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol 2013;31(6):744–51 doi 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licitra L, Grandi C, Guzzo M, Mariani L, Lo Vullo S, Valvo F, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial. J Clin Oncol 2003;21(2):327–33 doi 10.1200/JCO.2003.06.146. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CM, Glisson BS, Feng L, Wan F, Tang X, Wistuba, II, et al. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res 2012;18(5):1435–46 doi 10.1158/1078-0432.CCR-11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25(3):454–61 doi 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menzies AM, Rozeman EA, Amaria RN, Huang ACC, Scolyer RA, Tetzlaff MT, et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Journal of Clinical Oncology 2019;37(15_suppl):9503- doi 10.1200/JCO.2019.37.15_suppl.9503. [DOI] [PubMed] [Google Scholar]
- 28.Porceddu SV, Bressel M, Poulsen MG, Stoneley A, Veness MJ, Kenny LM, et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. J Clin Oncol 2018;36(13):1275–83 doi 10.1200/JCO.2017.77.0941. [DOI] [PubMed] [Google Scholar]
- 29.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515(7528):568–71 doi 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515(7528):563–7 doi 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


