Abstract
Objective:
Hormone therapy (HT) is used to treat menopause-related conditions and symptoms. The small intestine plays key roles in metabolic and endocrine function, but the effects of HT on the small intestinal microbiome are unknown. Here, we characterize duodenal microbiome differences, and the effects of HT, in postmenopausal women.
Methods:
Female participants undergoing esophagogastroduodenoscopy who were postmenopausal and taking HT (HT+), postmenopausal but not taking HT (HT−), or of reproductive age and not taking exogenous hormones (RA), were identified and matched for body mass index (±3 kg/m2). DNAs were isolated from duodenal aspirates obtained during upper endoscopy. V3 and V4 libraries were used for 16S rRNA sequencing. Serum hormone levels were analyzed by Luminex FlexMap.
Results:
The core duodenal microbiome was different in HT− participants (n = 12) when compared with RA participants (n = 10), but more similar in HT+ (n = 13) and RA participants. HT− participants had increased Proteobacteria taxa, leading to greater microbial dysbiosis compared with HT+ participants, and had decreased prevalence of Bacteroidetes, which was associated with higher fasting glucose levels, lower duodenal microbial diversity, and lower testosterone levels. HT+ participants had significantly higher estradiol (P = 0.04) and progesterone (P = 0.04), and lower fasting glucose (P = 0.03), than HT− participants, and had increased relative abundance of Prevotella (P = 0.01), and decreased Escherichia (P = 1.12E-7), Klebsiella (P = 5.93E-7), and Lactobacillus (P = 0.02), all associated with lower cardiovascular disease risks.
Conclusions:
These findings support previous studies suggesting that HT may have beneficial effects following menopause, and although preliminary, may also support a beneficial effect of HT on the duodenal microbiome.
Keywords: Duodenal microbiome, Hormone therapy, Menopause
Menopause is defined by a complete year without menstrual bleeding, and involves decreasing estrogen levels from age-related loss of ovarian follicular cells.1 Common symptoms of menopause include, but are not limited to, hot flashes, migraines, vaginal atrophy, and depression.2-6 The postmenopausal state is also associated with long-term complications such as cardiovascular disease (CVD) and osteoporosis.7-10 The elevated prevalence of these symptoms and complications among postmenopausal women led to the development of various therapies to minimize the risks associated with menopause.11 One such treatment is hormone therapy (HT).
HT involves the administration of one or two major classes of hormones: estrogens and progestogens. For women with a uterus, estrogens are typically given in combination with a progestogen (estrogen plus progestogen therapy [EPT]), whereas estrogen-only therapy (ET) is more typically given if a woman does not have a uterus.12 While some still question the efficacy of HT as a treatment for menopause-associated conditions,13 research supports that HT may significantly reduce all-cause morbidity, all-cause mortality, and cardiac mortality, with these effects being more pronounced in younger HT initiators than in older initiators, particularly for coronary heart disease.14,15 It has been suggested that these effects of HT, particularly the effects on CVD risk, may be mediated through effects on inflammation, through modulation of pro- and anti-inflammatory cytokines and chemokines, including tumor necrosis factor α and Interleukin (IL)6.16 Interestingly, a recent review suggested that oral ET may have a more favorable effect on lipoprotein levels and CVD risk than combined EPT.17 Symptomatic benefits have been shown in libido,18 hot flashes,19 and quality of sleep20 (reviewed in Palacios et al21). While HT attenuates bone loss,22 other agents have proven more effective.23 In addition, it has been suggested that risks for stroke, transient ischemic attack, and systemic embolism remain elevated in HT users.15
Recent research reveals significant changes in the microbial compositions of the fecal, vaginal, and urinary tract microbiomes in postmenopausal women.24-26 Changes in the fecal microbiome include a marked decrease in microbial diversity and an increase in the Firmicutes/Bacteroidetes ratio.24,27 While studies in animal models have shown that exogenous hormone therapies affect the fecal microbiome,28 little is known of the effects of HT on the postmenopausal microbiome.
It is important to note that these previous studies used fecal samples for analyses of the gut microbiome. However, the microbiome varies substantially throughout the small and large intestine,29 making stool a poor surrogate for the entire gastrointestinal tract. As the processes of digestion and nutrient absorption primarily occur in the small bowel, alterations in its microbial populations and their metabolic functions may provide unique, clinically relevant insights into the health of the human host. The REIMAGINE (Revealing the Entire Intestinal Microbiota and its Associations with the Genetic, Immunologic, and Neuroendocrine Ecosystem) study was created to characterize the interrelationships between microbial populations of the small intestine and human health and disease.30
In this study, we used samples and data from a cohort of REIMAGINE participants to analyze and compare the duodenal microbiome, as well as potentially associated metabolic and inflammatory markers, in postmenopausal women taking HT, postmenopausal women not taking HT, and women of reproductive age not taking exogenous hormones.
METHODS
Study participants
The REIMAGINE study is an ongoing study conceived to explore the relationships between the small bowel microbial populations and different conditions and diseases.30 All individuals aged 18 to 80 years undergoing standard of care upper endoscopy (esophagogastroduodenoscopy [EGD]) without colon preparation are eligible for inclusion. Those who consent to participate complete a detailed study questionnaire (described below) and provide fasting blood samples prior to the EGD procedure. While there are no exclusion criteria, small bowel biopsies are not collected from participants with bleeding disorders or advanced cirrhosis of the liver, to minimize the risk of bleeding from the biopsy site. The study protocol is approved by the Institutional Review Board at Cedars-Sinai Medical Center, and participants provide informed written consent prior to participation.
The REIMAGINE study questionnaire includes self-documented family and medical history, medication use, use of alcohol and recreational drugs, travel history, and dietary habits and changes.30 Participants also report any known underlying conditions, and female participants are asked to complete a section that includes menstrual and pregnancy history, use of oral contraceptives, history of gynecologic surgeries including hysterectomy, menopausal status, and hormone therapy. All medical information provided by participants is verified through audits of medical records, and all data are deidentified prior to analysis.
For the purposes of this study, female REIMAGINE participants who were postmenopausal, either due to natural menopause or as a result of surgery, were identified based on verified questionnaire data. These postmenopausal participants were then further categorized as taking HT (HT+ group) or not taking HT (HT− group), again based on verified data. Postmenopausal participants who could not be definitively assigned to either group were excluded from the analysis. Female REIMAGINE participants who were of reproductive age and who were not taking exogenous hormones (RA group) were similarly identified, again based on verified questionnaire data. Participants who had taken antibiotics in the month prior to EGD were excluded from the analysis. Participants in all three groups were then matched for body mass index (BMI, ± 3 kg/m2). Only samples and data from female REIMAGINE participants who met all of these criteria were included in the present study. The final analysis included 13 participants in the HT+ group, 12 participants in the HT− group, and 10 participants in the RA group.
Blood collection and analysis
As part of the REIMAGINE protocol, circulating levels of total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, glucose, and total protein, as well as circulating cytokine and chemokine levels, are routinely measured in all participants. Total cholesterol, LDL, HDL, triglycerides, glucose, and total protein are analyzed on a Cobas Integra 400 (Roche Diagnostics, Rotkreuz, Switzerland). Circulating cytokine and chemokine levels are determined on a Luminex FlexMap 3D (Luminex Corporation, Austin, TX), using a bead-based multiplex panel that included the following analytes: granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon γ, IL10, IL12P70, IL13, IL1B, IL2, IL4, IL5, IL6, IL8, and tumor necrosis factor α (EDM Millipore Corp, Billerica, MA). For this study, circulating hormone levels were also determined, using a bead-based multiplex panel that included estradiol, progesterone, and total testosterone (MILLIPLEX MAP Multi-Species Hormone Magnetic Bead Panel, cat. MSHMAG-21K, EDM Millipore Corp, Burlington, MA). Assays were performed on the Luminex FlexMap 3D (Luminex Corporation). Approximately 10% cross-reactivity to cortisol is observed in the progesterone assay. In human samples, estradiol accuracy and sensitivity are 80% and 0.01 ng/mL respectively. Progesterone accuracy and sensitivity are 85% and 0.25 ng/mL respectively. Total testosterone accuracy and sensitivity are 96% and 0.15 ng/mL respectively. The assay precision for estradiol and progesterone is < 10% intra-assay and < 15% inter-assay. The assay precision for total testosterone is < 10% intra-assay and < 10% inter-assay. A standard curve and internal controls were included in the assay. The standard curve for estradiol ranged from 0.014 to 10 ng/mL, progesterone ranged from 0.14 to 100 ng/mL and testosterone ranged from 0.21 to 150 ng/mL. Of note, blood samples were not taken in a cycle-specific manner, which may contribute to variability in hormone levels in women of reproductive age.
Collecting luminal samples from the small intestine
Duodenal luminal fluid was collected during the EGD procedure with a custom-engineered sterile aspiration double-lumen catheter (Hobbs Medical, Inc, Stafford Springs, CT).30 A novel sterile inner catheter was used to obtain duodenal aspirates. This catheter was passed through a sterile bone wax cap once the endoscopist reached the second portion of the duodenum, as a means to prevent contamination from the mouth, esophagus, and stomach.30
Aspirate processing
Sterile 1× dithiothreitol was added to each duodenal aspirate (∼1 mL) in a 1:1 ratio and the mixtures were vortexed for 30 seconds until they were fully liquified as previously described.30 This was followed by centrifugation at maximum speed (>13,000 RPM) for 5 minutes. Upon removal of the supernatant, 1 mL of sterile All Protect reagent (Qiagen, Hilden, Germany) was added to the remaining microbial pellet. The All Protect covered pellet was then stored at −80°C until it was used for DNA isolation.30
DNA isolation
Duodenal aspirate pellets were thawed on ice and processed as described previously.30 The MagAttract PowerSoil DNA KF kit (Qiagen) was used to isolate microbial DNA from each sample on a KingFisher Duo (Thermo Fisher Scientific, Waltham, MA),30 followed by quantification with the Qubit ds high sensitivity DNA BR Assay kit (Invitrogen by Thermo Fisher Scientific) on a Qubit 4 Fluorometer (Invitrogen).
Library preparation and 16S rRNA gene sequencing and analysis
Specific primers (S-D-Bact-0341-b-S-17 and S-D-Bact-0785-a-A-21)31 containing Illumina sequencing adapters30 were used to amplify the 16S V3 and V4 regions. An Agilent 2100 Bioanalyzer System was used to assess library quality. Paired-end sequencing of amplicons (2 × 301 cycles) was performed on a MiSeq System (Illumina, San Diego, CA), using the MiSeq reagent v3 Kit, 600 cycles, and 5% to 10% PhiX (Illumina).
The Operational Taxonomic Unit (OTU) clustering tool available at CLC Genomics Workbench v.10.1.1 and CLC Microbial Genomics Module v.2.5 (Qiagen) software were used to perform reference based OTU clustering and taxonomic analyses against the Greengenes Database 2013 with 97% similarity.30 Default parameters were used for minimum occurrences and chimera crossover cost, and the creation of new OTUs was not allowed. Microbial alpha diversity indices including Shannon's, Simpson's, and Observed (which represents the count of unique OTUs in each group), were calculated after low depth samples (<5,000 sequences per sample) were removed from the analysis. Intersample variability (beta diversity) was calculated using the Bray-Curtis metric using the MicrobiomeAnalyst tool.32,33
Core microbiome analysis and correlation constructions were performed using the Core Microbiome tool and correlation network tool available at MicrobiomeAnalyst. Correlations and associations between microbial taxa were generated based on Spearman rank correlation, using a correlation threshold of at least 0.3 and P values lower than 0.05.
Statistical analyses
Predictions for significant differentially abundant OTUs between different groups were performed using a rarefied OTU table. Multiple comparisons, metabolic pathway analysis, and statistical analyses were performed using CLC Microbial Genomics Module v.2.5 (Qiagen) and MicrobiomeAnalyst.32,33 The OTU table was rarefied to the minimal number of reads assigned to a sample, and a Negative Binomial GLM model was used to obtain maximum likelihood estimates for the fold change (FC) of an OTU between different groups. The Wald test was used for determination of significance, and P values were corrected using False Discovery Rate. A P value < 0.05 after controlling for False Discovery Rate of 0.05 was considered to be statistically significant. Two-tailed Spearman r correlations, statistical tests and graph construction were carried out with rarefied OTU tables using GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA)34 and IBM SPSS Statistics Version 24. Mann–Whitney test and unpaired t test were used to compare levels of serum biomarkers between different groups. Kruskal-Wallis test was used to compared microbial diversity across all three groups. Microbial Dysbiosis Index (MD-Index), an index to measure dysbiosis (ie, the imbalance in a microbial community), was calculated during the Correlation Network analysis using MicrobiomeAnalyst.
RESULTS
Demographics and sex steroid hormone levels
Samples and data from a total of 35 female participants in the REIMAGINE study were included in this study. The following three groups were defined: postmenopausal participants who were taking HT (HT+, n = 13, mean age = 61 ± 8, mean BMI = 24.11 ± 3.35 kg/m2); postmenopausal participants who were not taking HT (HT−, n = 12, mean age = 61 ± 7, mean BMI = 26.36 ± 4.64 kg/m2); and participants of reproductive age who were RA (n = 10, mean age = 32 ± 4, mean BMI = 25.59 ± 9.59 kg/m2) (see Table 1). In the HT+ group, six participants were on EPT (five oral and one oral plus transdermal), and seven were on ET only (two oral, four transdermal, one oral plus transdermal). Four participants in the HT+ group had had hysterectomies, of which two included removal of the ovaries. Only one participant in the HR− group had had a hysterectomy. Alcohol consumption and educational status were not significantly different between groups (P > 0.05), but there were more smokers in the HT− group (66%) than in the RA group (0%) (P = 0.0046). No significant differences were identified between the HT− and HT+ groups (P = 0.12), or between the HT+ and RA groups (P = 0.11), with regard to smoking status. Participants who had taken antibiotics in the month prior to EGD were excluded from the analysis for all groups.
TABLE 1.
Demographic characteristics of the different groups, including circulating sex steroid hormone levels, lipid profiles, and levels of pro- and anti-inflammatory cytokines and chemokines
| Reproductive age, not taking exogenous hormones (RA) | Postmenopausal, taking HT (HT+) | Postmenopausal, not taking HT (HT−) | P value (HT+ vs HT−) | |
| N per group | 10 | 13 | 12 | |
| Age (y) ± SD | 32 ± 4 | 61 ± 8 | 61 ± 7 | 0.61 |
| BMI (kg/m2) ± SD | 25.59 ± 9.59 | 24.11 ± 3.35 | 26.36 ± 4.64 | 0.25 |
| Fasting glucose mg/dL | 89.07 ± 9.89 | 94.75 ± 9.31 | 108.85 ± 16.88a,b | 0.03b |
| Cholesterol mg/dL | 166.16 ± 31.61 | 189.88 ± 33.43 | 174 ± 44.3 | 0.65 |
| HDL mg/dL | 62.91 ± 16.78 | 64.91 ± 16.69 | 55.14 ± 13.79 | 0.16 |
| LDL mg/dL | 87.32 ± 27.29 | 99.98 ± 19.42 | 98.4 ± 41.69 | 0.89 |
| Triglycerides mg/dL | 73.84 ± 24.16 | 113.95 ± 86.96 | 152.45 ± 82.57 | 0.08 |
| Total protein g/dL | 7.23 ± 0.85 | 6.95 ± 0.62 | 6.59 ± 0.42 | 0.23 |
| GM-CSF pg/mL | 7.93 ± 8.15 | 7.37 ± 7.26 | 5.54 ± 2.1 | 0.83 |
| IFNγ pg/mL | 6.37 ± 9.26 | 12.26 ± 22.34 | 19.21 ± 42.86 | 0.97 |
| IL10 pg/mL | 10.33 ± 6.51 | 7.56 ± 4.72 | 10 ± 7.49 | 0.57 |
| IL12p70 pg/mL | 4.31 ± 1.14 | 6.35 ± 6.78 | 3.9 ± 0.68 | 0.57 |
| IL13 pg/mL | 62.46 ± 158.78 | 17.04 ± 37.13 | 5.93 ± 5.04 | 0.62 |
| IL1β pg/mL | 2.69 ± 0.95 | 2.56 ± 0.69 | 2.27 ± 0.32 | 0.31 |
| IL2 pg/mL | 1.41 ± 0.77 | 1.79 ± 2.46 | 1.08 ± 0.68 | 0.57 |
| IL4 pg/mL | 132.56 ± 350.61 | 20.55 ± 54.21 | 4.48 ± 6.11 | 0.66 |
| IL5 pg/mL | 8.44 ± 15.75 | 4.6 ± 4.08 | 3.61 ± 2.62 | 0.50 |
| IL6 pg/mL | 37.25 ± 96.4 | 12.06 ± 29.68 | 1.98 ± 5.18 | 0.45 |
| IL8 pg/mL | 18.29 ± 32.48 | 10.82 ± 7.52 | 12.86 ± 14.58 | 0.94 |
| TNFα pg/mL | 12.53 ± 5.98 | 13.61 ± 2.89 | 15.79 ± 5.85 | 0.55 |
| Reasons for endoscopy | Abdominal pain, bloating, diarrhea, irritable bowel syndrome (IBS), workup of celiac disease, Crohn disease, anemia, GI bleed, etc. | |||
Measurements are presented as mean ± SD.
BMI, body mass index; GM-CSF, granulocyte-macrophage colony-stimulating factor; HDL, high-density lipoprotein; IFNγ, interferon gamma; IL, interleukin; LDL, low-density lipoprotein; TNFα, tumor necrosis factor alpha.
Denotes a significant difference between the HT− group and the RA group.
When participants with diabetes/taking diabetes medications were removed from the analysis, the differences between the HT− and RA groups remained significant, but the differences between the HT− and HT+ groups did not remain significant. There were no significant differences between the HT+ group and the RA group for any of the parameters tested.
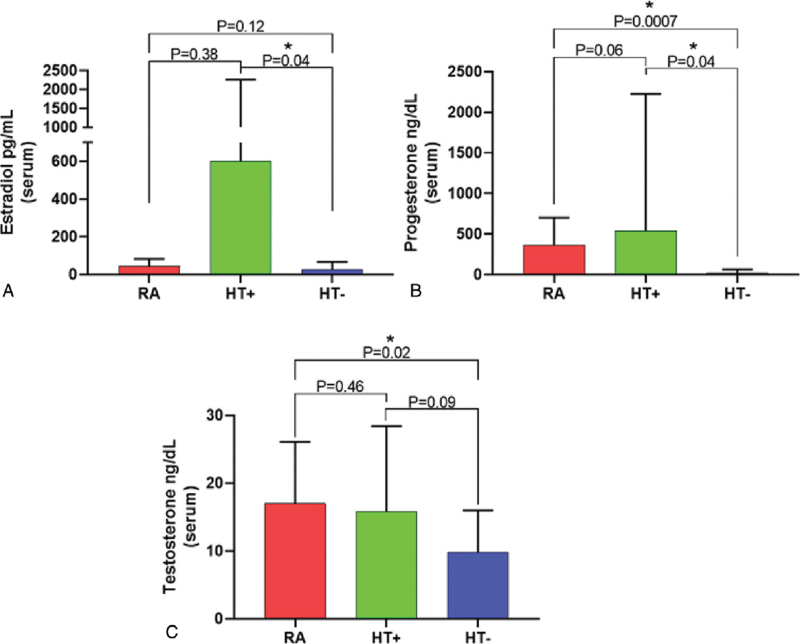
Serum levels of the sex steroid hormones estradiol and total testosterone were not significantly different in postmenopausal women taking HT (the HT+ group) when compared with reproductive age women who were not taking exogenous hormones (the RA group) (Fig. 1). We note that blood samples were not taken in a cycle-specific manner, which could have affected progesterone levels in women of reproductive age. In contrast, serum levels of estradiol (P = 0.04) and progesterone (P = 0.04) were significantly lower in postmenopausal women who were not taking HT (the HT− group) when compared with the HT+ group, and serum progesterone levels were also significantly lower in the HT− group when compared with the RA group (P = 0.0007, Fig. 1). Total testosterone levels were significantly lower in the HT− group when compared with the RA group (P = 0.02), but were not significantly different when compared with the HT+ group (P = 0.09, Fig. 1).
FIG. 1.
Mean serum levels of estradiol (A), progesterone (B), and total testosterone (C) in reproductive age women not taking exogenous hormones (RA), postmenopausal women taking HT (HT+), and postmenopausal women not taking HT (HT−). ∗ denotes significant differences (P < 0.05). Mann-Whitney test was used to compare differences in estradiol levels between groups. Unpaired t test with Welch's correction was used to compare differences in progesterone and testosterone levels between groups.
The HT− group also had significantly increased fasting glucose levels when compared with both the HT+ group (P = 0.03) and the RA group (P = 0.01) (Table 1). After correcting the analysis for participants with diabetes and/or taking medication for diabetes, the HT− group still had significantly increased fasting glucose levels when compared with the RA group (P = 0.014), but no differences were observed when comparing to the HT+ group (P = 0.18). In contrast, fasting glucose levels in the HT+ group were similar to the levels in the RA group (P = 0.24), even after correcting the analysis for participants with diabetes and/or taking medication for diabetes (P = 0.23). There were no significant differences in circulating levels of cholesterol, HDL, LDL, triglycerides, or total protein between any of the groups (Table 1). Given the suggestions in the literature that some effects of HT may be mediated through effects on inflammatory cytokines and chemokines, levels of a panel of cytokines and chemokines were also compared between groups, but there were no significant differences between the groups in the levels of any of the cytokines or chemokines tested (Table 1).
Decreased duodenal microbial diversity in postmenopausal women is associated with higher fasting blood glucose and lower total testosterone levels
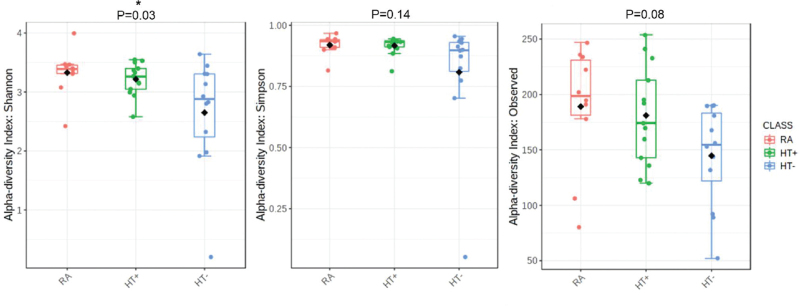
Duodenal microbial alpha diversity was analyzed using different indices: Shannon, Simpson, and Observed (Observed represents the count of unique OTUs in each group). We identified a statistically significant decrease in duodenal microbial diversity in the HT− group when compared with the HT+ and RA groups for Shannon index (P = 0.03), but not for Simpson index (P = 0.14) (Fig. 2). In contrast, duodenal microbial diversity in the HT+ group was more similar to that in the RA group (Fig. 2). While Simpson's index gives more weight to common or dominant species (ie, is more sensitive to species evenness), Shannon index assumes all species (including rare species) are represented in a sample (ie, is more sensitive to species richness).35 Only Shannon's index was decreased in the HT− group, suggesting that changes in duodenal microbial diversity in these participants were primarily due to changes in overall species richness, including rare taxa.
FIG. 2.
Duodenal microbial alpha diversity in the RA, HT+, and HT− groups, as determined using three different indices, Shannon's, Simpson's, and Observed. P values were obtained by comparing differences across all groups using Kruskal-Wallis test.
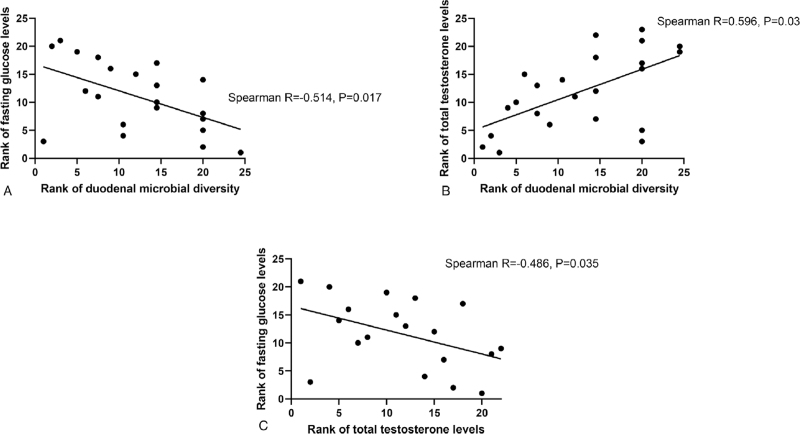
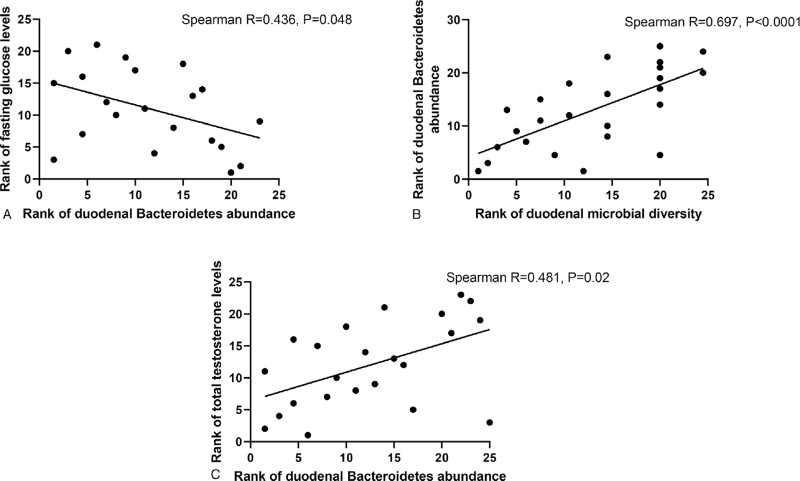
Duodenal microbial diversity appeared to exhibit an inverse association with fasting glucose levels (Spearman R = −0.373, P = 0.05), ie, decreased diversity was associated with increased fasting glucose levels. To account for potential effects of the differences in age between the postmenopausal groups and the RA group, the analysis was repeated without the RA group, which resulted in a stronger association between duodenal microbial diversity and fasting glucose levels (Spearman R = −0.514, P = 0.017, Fig. 3A).
FIG. 3.
Spearman rank correlations between duodenal microbial diversity, fasting glucose levels, and total testosterone in postmenopausal women (HT+ and HT− groups).
In postmenopausal women (ie, across both the HT+ and HT− groups), decreased duodenal microbial diversity was also strongly associated with decreased total testosterone levels (Spearman R = 0.596, P = 0.03, Fig. 3B), and decreased testosterone levels were also correlated with increased fasting glucose levels (Spearman R = −0.486, P = 0.035, Fig. 3C). When the RA group was included in this analysis, the association between total testosterone and duodenal microbial diversity remained significant (Spearman R = 0.491, P = 0.005), as did the association between total testosterone and fasting glucose levels (Spearman R = −0.456, P = 0.025) (data not shown). No differences in beta diversity were observed between groups (see Figure, Supplemental Digital Content 1).
The core duodenal microbiome is similar in postmenopausal women taking HT and reproductive age women
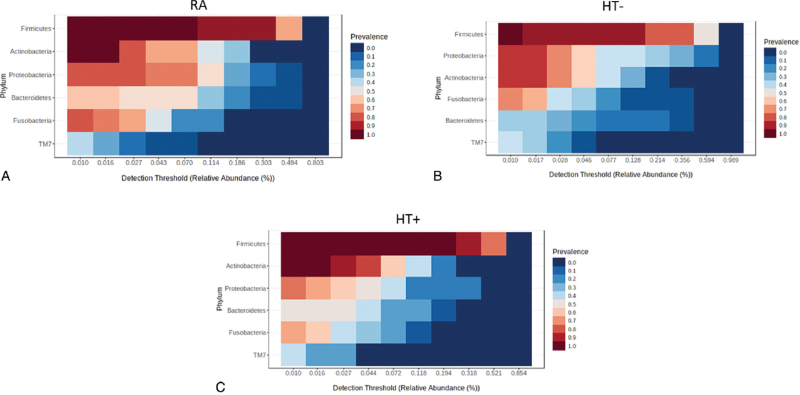
The core duodenal microbiome was defined as the most widespread microbial components of the microbiome found across all participants.36 The most prevalent phylum in the core duodenal microbiome of reproductive age women who were not taking exogenous hormones (RA) was Firmicutes, followed by Actinobacteria, Proteobacteria, Bacteroidetes, Fusobacteria, and TM7 (Fig. 4A). In postmenopausal women not taking HT (HT−), Firmicutes remained the most prevalent phylum, but Proteobacteria moved up in rank to the second most prevalent phylum, and Actinobacteria dropped to third most prevalent, followed by Fusobacteria as fourth most prevalent, and Bacteroidetes was decreased to 5th most prevalent (Fig. 4B). In contrast, the core duodenal profile in postmenopausal women taking HT (HT+, Fig. 4C) was more similar to that in the RA group, with the same order of prevalence for all phyla.
FIG. 4.
Core duodenal microbiome profiles at the phylum level in the RA group (A), postmenopausal HT− group (B), and postmenopausal HT+ group (C).
The duodenal microbiome shows fewer associations between taxa and greater dysbiosis in postmenopausal women not taking HT
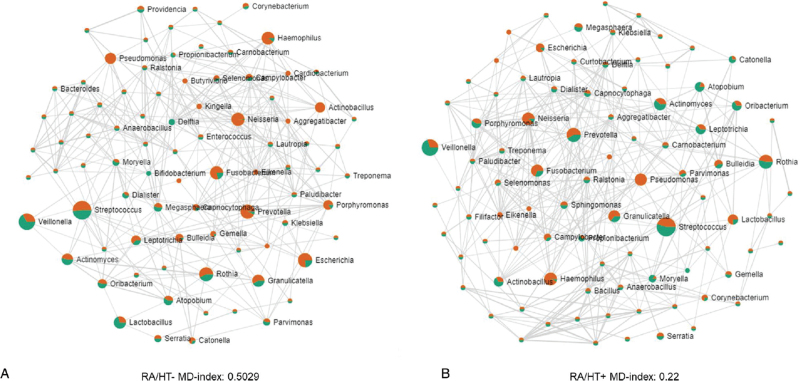
The changes identified in the core duodenal microbiome of the HT− group (Fig. 4A) were driven primarily, but not solely, by the phylum Proteobacteria, which had a strong negative correlation both with phylum Firmicutes (Spearman R = −0.820, P < 0.0001) and with phylum Actinobacteria (Spearman R = −0.343, P = 0.04) in postmenopausal women, resulting in a loss of associations between phyla (see Figure, Supplemental Digital Content 2). This loss of associations between taxa in the HT− group was also evident when analysis was performed at genus level, using the RA group as controls, with a higher level of dysbiosis (indicated by the MD-Index) in the HT- group (MD-Index = 0.5029) when compared with the HT+ group (MD-Index = 0.22) (Fig. 5).
FIG. 5.
(A) Spearman correlations and relative abundances in the HT− group (orange) as compared with the RA group (green) at the genus level. (B) Spearman correlations and relative abundances in the HT+ group (orange) as compared with the RA group (green) at the genus level. A higher microbial dysbiosis (MD) index is indicative of a greater degree of dysbiosis.
Although no direct association was identified between phylum Proteobacteria and phylum Bacteroidetes, the relative abundance of phylum Bacteroidetes was significantly decreased in the HT− group when compared with both the RA group (FC = −4.33, P = 0.045) and the HT+ group (FC = −1.34, P = 0.02) (see Figure, Supplemental Digital Content 3).
Lower relative abundance of Bacteroidetes in postmenopausal women is associated with increased fasting glucose levels, decreased microbial diversity, and decreased testosterone levels
The phyla Proteobacteria, Firmicutes, and Actinobacteria did not exhibit any direct associations with serum sex hormone levels or with fasting glucose levels in postmenopausal women (ie, across both the HT+ and HT− groups). In contrast, a lower relative abundance of the phylum Bacteroidetes in the duodenal microbiome of postmenopausal women was associated with higher fasting glucose levels (Spearman R = −0.436, P = 0.048, Fig. 6A), lower duodenal microbial diversity (Spearman R = 0.697, P < 0.0001, Fig. 6B), and decreased serum testosterone levels (Spearman R = 0.481, P = 0.02, Fig. 6C).
FIG. 6.
Correlations between relative abundance of phylum Bacteroidetes in the duodenal microbiome and fasting glucose (A), duodenal microbial diversity (B), and total testosterone (C), in postmenopausal women (both the HT+ and HT− groups).
Within phylum Bacteroidetes, one of the most important taxa which contributed to these associations was the genus Prevotella, the relative abundance of which exhibited positive correlations with duodenal microbial diversity (Spearman R = 0.658, P < 0.0001), and to a lesser extent with total testosterone levels (Spearman R = 0.378, P = 0.075), in postmenopausal women. The relative abundance of Prevotella was decreased in the duodenal microbiome of the HT− group when compared with the RA group (FC = −4.58, P = 0.01) (see Figure, Supplemental Digital Content 4). In contrast, the relative abundance of Prevotella in the HT+ group was closer to that in the duodenal microbiome of the RA group (see Figure, Supplemental Digital Content 4).
The duodenal microbiome of postmenopausal women not taking HT is enriched for microbes associated with increased risk of cardiovascular disease (CVD)
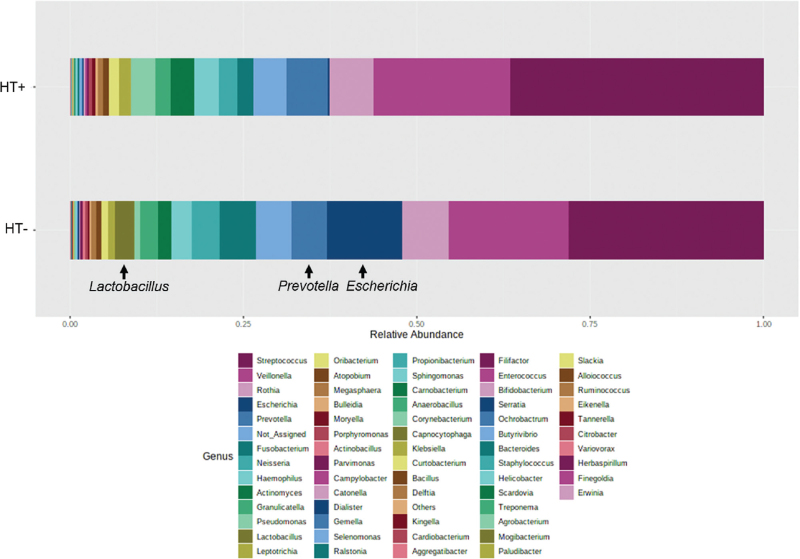
As lower relative abundances of both phylum Bacteroidetes and genus Prevotella have been associated with increased risks for CVD,37-39 we also determined the relative abundance of other microbes associated with CVD risk in the duodenal microbiome of the HT− and HT+ groups. Analysis at the genus level revealed that the duodenal relative abundance of Escherichia37,39 (phylum Proteobacteria, FC = 8.73, P = 1.12E-7), Klebsiella37,39 (phylum Proteobacteria, FC = 8.67, P = 5.93E-7), and Lactobacillus40 (phylum Firmicutes) (FC = 4.13, P = 0.02) was increased in the HT− group when compared with the HT+ group (Fig. 7).
FIG. 7.
Relative abundance of different genera in the duodenal microbiome of the HT− and HT+ groups. Genus Klebsiella is not visible due to its low relative abundance.
DISCUSSION
In this study, we demonstrate that the core duodenal microbiome at phylum level is significantly different in postmenopausal women when compared with women of reproductive age, and that these differences are lessened in postmenopausal women taking hormone therapy. We found that the core duodenal microbiome in postmenopausal women not taking HT (HT− group) had increased prevalence of phylum Proteobacteria, and decreased prevalence of phylum Bacteroidetes, when compared with women of reproductive age who were not taking exogenous hormones (RA group). Changes in taxa within Proteobacteria in the HT− group negatively impacted phylum Firmicutes, resulting in greater dysbiosis and fewer associations between taxa in the duodenal microbiome, whereas the decreased relative abundance of Bacteroidetes was associated with higher fasting glucose levels, lower duodenal microbial diversity, and lower serum testosterone levels. In contrast, postmenopausal women who were taking HT (HT+ group) had a core microbiome that was more similar to that in the RA group, with similar prevalence of Proteobacteria and Bacteroidetes. The HT+ group also had significantly higher levels of serum estradiol and progesterone, and significantly lower fasting glucose levels, than the HT− group, as well as a lesser degree of duodenal dysbiosis. Lastly, the relative abundances of several taxa associated with CVD risks37-39 were different in the HT− group when compared with the HT+ group, including decreased relative abundance of Bacteroidetes and genus Prevotella, and increased relative abundance of Escherichia, Klebsiella, and Lactobacillus. Together, these findings suggest that there may be positive changes in the duodenal microbiome of postmenopausal women taking HT.
Some previous studies have examined the gut microbiome in postmenopausal women, and found decreased microbial diversity and increased Firmicutes/Bacteriodetes ratios.24,27 However, it is important to note that these studies, like the majority of gut microbiome studies, were performed using fecal samples. Although less frequently studied due to the cost and difficulty of obtaining samples,41 the small intestine plays key roles in metabolic and endocrine function.42 Therefore, we undertook to explore the roles of the small intestinal microbiome in human health and disease.29,30,43,44 Our studies have not only shown that the small intestine contains greater numbers of microbes than previously thought,29 but have also demonstrated that the microbial populations of the small bowel are significantly different from those found in stool.29 Given the critical roles played by the small intestine in metabolic homeostasis and endocrine function, including the release from enteroendocrine cells of peptides that regulate food intake and hepatic glucose production,45 it is essential to study small intestinal microbial populations directly, rather than relying on stool as a surrogate, to identify microbial taxa with causal roles in metabolic and endocrine function.
In this study, we found that in the RA group, Firmicutes was the most prevalent phylum in the core duodenal microbiome, followed by Actinobacteria, Proteobacteria, and Bacteroidetes. In the HT− group, Firmicutes remained the most prevalent phylum, but Proteobacteria was the second most prevalent, and Actinobacteria was decreased to the 3rd most prevalent, and Bacteroidetes to the 5th most prevalent. These findings are consistent with our previous finding that the duodenal relative abundance of Proteobacteria increases, and Bacteroidetes decreases, with increasing age.46 In contrast, the core duodenal microbiome profile in the HT+ group was far more similar to the RA group, with lower prevalence of Proteobacteria and increased prevalence of Bacteroidetes than in the HT− group, which may suggest that HT preserves the duodenal microbiome despite increasing age. Changes in taxa within phylum Proteobacteria in the HT− group severely impacted phylum Firmicutes, and there were significantly fewer associations between duodenal microbial taxa. These disruptions were also evident at the genus level, with lesser dysbiosis in the HT+ than in the HT− group, suggesting that HT may have beneficial effects on the duodenal microbiome.
Reduced microbial diversity is associated with increased dysbiosis,47,48 and is a hallmark of many gut-related diseases and conditions.49,50 We have previously shown that duodenal microbial diversity is decreased in conditions such as small intestinal bacterial overgrowth,43 and with increasing age.46 In this study, we found that following menopause, duodenal microbial diversity was decreased in the HT− group when compared with the RA group. Decreased microbial diversity was associated with increased fasting glucose levels in postmenopausal women, an effect which was independent of age, and both decreased duodenal diversity and increased fasting glucose levels were associated with decreased serum testosterone levels. Conversely, the HT+ group exhibited similar fasting blood glucose levels, and similar serum testosterone levels, to the RA group. These findings are consistent with many previous studies suggesting that HT decreases the risk of type 2 diabetes in postmenopausal women,51 including specific reductions in fasting glucose levels. The effects of testosterone on glucose levels and insulin resistance in postmenopausal women are less clear, but the 2019 Global Consensus Position Statement on the Use of Testosterone Therapy for Women concluded that testosterone therapy, in doses that approximate physiological testosterone concentrations for premenopausal women, is not associated with increases in blood glucose in postmenopausal women.52 In addition, studies have shown that testosterone can have beneficial effects in postmenopausal women, including reduced symptoms and improved quality of life, and does not adversely affect blood glucose levels.53
Across the postmenopausal groups (ie, in both the HT− and HT+ groups), decreased duodenal diversity, increased fasting glucose levels, and decreased serum testosterone levels, were associated with decreased relative abundance of the phylum Bacteroidetes in the duodenal microbiome. Changes in the relative abundance of Bacteroidetes were largely due to the genus Prevotella, which was significantly reduced in the duodenal microbiome of the HT− group. In contrast, the relative abundance of Bacteroidetes and Prevotella was more comparable in the HT+ and RA groups. These findings are of interest as studies using fecal samples have shown that lower relative abundances of phylum Bacteroidetes and genus Prevotella are associated with increased risks for CVD37,38—for example, a lower abundance of Prevotella was identified in individuals with atherosclerotic cardiovascular disease.39 Consistent with this, consumption of a Western diet has been associated with decreased relative abundance of Prevotella in fecal samples,54 whereas those consuming diets considered to be heart-healthy, such as vegetarian and Mediterranean diets, have increased relative abundance of Bacteroidetes, including Prevotella.55 Further, Prevotella has been shown to protect against Bacteroides-induced glucose intolerance, and mice gavaged with a live P copri strain exhibited improved glucose tolerance when compared with controls.56 It should be noted that Prevotella is a large genus, and the benefits and risks for CVD may vary depending on the different species and strains present57—for example, one study suggested that a Prevotella-enriched enterotype might be associated with increased risks for hypertension,58 and another suggested that Prevotella 7 was associated with an increased lifetime CVD risk.59
In addition to decreased relative abundance of Bacteroidetes and Prevotella, postmenopausal women not taking HT exhibited increased relative abundance of genera that are positively associated with increased risks for CVD, including Escherichia and Klebsiella (phylum Proteobacteria) as well as Lactobacillus (phylum Firmicutes).37,39 Increased relative abundance of Escherichia coli, Klebsiella spp., and Lactobacillus salivarius have been identified in fecal samples from atherosclerotic cardiovascular disease participants when compared with controls, and K oxytoca showed a positive correlation with serum levels of aspartate transaminase, which may be a marker for acute myocardial infarction as well as other conditions.39 In addition, the order Lactobacillales was increased, and phylum Bacteroidetes decreased, in participants with coronary artery disease when compared with healthy controls.40 Taken together, our findings of increased relative abundance of Bacteroidetes and Prevotella, and decreased Escherichia, Klebsiella and Lactobacillus, in the duodenal microbiome of postmenopausal women taking HT are consistent with a beneficial effect of HT on CVD risk. Moreover, these findings may suggest that these benefits of HT may be mediated, at least in part, through effects on the duodenal microbiome, although further interventional studies would be required to determine this.
This study does have limitations. These participants were undergoing upper endoscopy for the assessment of intestinal complaints, as well as for screening purposes due to familial and other risk factors, and as such they may not be fully representative of normal healthy individuals. The groups were also small, and for this reason we included both women on combined therapy (including transdermal therapy, oral therapy, or a combination of both) and women who had had hysterectomies and who were on estrogen only in the HT+ group. Further, as this was a retrospective review of an ongoing study, data on the duration of HT therapy was not available for all participants in the HT+ group, and blood samples were not taken in a cycle-specific manner, which could have affected progesterone levels in women of reproductive age. Larger studies will be required to the effects of natural versus surgical menopause, and of different HT therapies, on the small intestinal microbiome. All three groups were matched for BMI, to preclude the confounding effects of obesity on the small intestinal microbiome, and the HT− and HT+ groups were also matched for age. We note that our results reflect associations and correlations with duodenal microbial taxa, and further work is needed to define causal effects. No differences in inflammatory markers were seen between the groups, but future studies will include analyses of CVD markers such as trimethylamine N-oxide. Our next steps will include a larger study with greater numbers of participants, and analyses of microbial metabolic pathways involved in CVD risks.
Potential clinical value
Our results indicate that the duodenal microbiome in postmenopausal women not taking HT is significantly different from that seen in women of reproductive age, whereas the duodenal microbiome in postmenopausal women taking HT is more similar to that of reproductive age women. While the functions of many gut microbes remain to be determined, we note that some of the microbes with increased relative abundance in postmenopausal women taking HT have been associated with decreased CVD risks. Although further work is required to determine the mechanistic roles of these microbes, our findings may support a beneficial role for HT.
CONCLUSIONS
This is the first study to examine changes in the duodenal microbiome in postmenopausal women, and the potential impacts of hormone therapy. Our results show that postmenopausal women not taking HT exhibit a significantly different core microbiome characterized by lower relative abundance of phylum Bacteroidetes, and associated with decreased microbial diversity, increased fasting glucose levels, and decreased testosterone levels. Postmenopausal women not taking HT also exhibit increased relative abundance of taxa from phylum Proteobacteria, which results in increased duodenal microbial dysbiosis, and increased relative abundance of taxa previously associated with increased risks for CVD. In contrast, the duodenal microbiome in postmenopausal women taking HT is far more similar to that of reproductive age women, with increased relative abundance of phylum Bacteroidetes and genus Prevotella, both of which are associated with decreased CVD risks, and also had similar microbial diversity, fasting glucose levels, and testosterone levels when compared with women not taking HT. Although preliminary, these findings support previous studies suggesting that HT has beneficial effects following menopause, and may also suggest a beneficial effect of HT on the duodenal microbiome.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Dr Ali Rezaie, Dr Nipaporn Pichetshote, and Dr Bianca Chang for their assistance in obtaining samples, and Dr Maria Jesus Villanueva-Milan, Shreya Celly, Maritza Sanchez, Stacy Weitsman MS, and Walter Morales for their assistance with sample processing and analysis. They would also like to thank Frank Lee, the Monica Lester Charitable Trust and the Elias, Genevieve, and Georgianna Atol Charitable Trust for their generous support of the MAST program.
Footnotes
Funding/support: This study was supported in part by funds from the Monica Lester Charitable Trust and the Elias, Genevieve, and Georgianna Atol Charitable Trust.
Financial disclosures/conflicts of interest: None reported.
The datasets generated during this study are available at the National Center for Biotechnology Information (NCBI) BioProject Repository (https://www.ncbi.nlm.nih.gov/bioproject) under BioProject ID PRJNA725496.
Supplemental digital content is available for this article.
REFERENCES
- 1.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011; 38:425–440. doi:10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RE, Kalilani L, Britt DiBenedetti D, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 2008; 11:32–43. doi:10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009; 6:2133–2142. doi:10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 4.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN). Arch Gen Psychiatry 2010; 67:598–607. doi:10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry 2006; 63:385–390. doi:10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 6.Martin VT, Pavlovic J, Fanning KM, et al. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention Study. Headache 2016; 56:292–305. doi:10.1111/head.12763. [DOI] [PubMed] [Google Scholar]
- 7.Greendale GA, Sowers MF, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res 2012; 27:111–118. doi:10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlamangla AS, Burnett-Bowie SM, Crandall CJ. Bone Health During the Menopause Transition and Beyond. Obstet Gynecol Clin North Am 2018; 45:695–708. doi:10.1016/j.ogc.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrinier N, Cournot M, Dallongeville J, et al. Menopause and modifiable coronary heart disease risk factors: a population based study. Maturitas 2010; 65:237–243. doi:10.1016/j.maturitas.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Gohlke-Barwolf C. Coronary artery disease–is menopause a risk factor? Basic Res Cardiol 95 Suppl 2000; 1:177–183. doi:10.1007/s003950070014. [DOI] [PubMed] [Google Scholar]
- 11.Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol 2015; 126:859–876. doi:10.1097/AOG.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fait T. Menopause hormone therapy: latest developments and clinical practice. Drugs Context 2019; 8:212551.doi:10.7573/dic.212551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagnacci A, Venier M. The controversial history of hormone replacement therapy. Medicina (Kaunas) 2019; 55: doi:10.3390/medicina55090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol 2012; 13:476–486. doi:10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nudy M, Chinchilli VM, Foy AJ. A systematic review and meta-regression analysis to examine the ’timing hypothesis’ of hormone replacement therapy on mortality, coronary heart disease, and stroke. Int J Cardiol Heart Vasc 2019; 22:123–131. doi:10.1016/j.ijcha.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh KK, Yoon BK. Controversies regarding hormone therapy: Insights from inflammation and hemostasis. Cardiovasc Res 2006; 70:22–30. doi:10.1016/j.cardiores.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Shufelt CL, Manson JE. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J Clin Endocrinol Metabolism 2021; 106:1245–1254. doi:10.1210/clinem/dgab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods NF, Mitchell ES, Smith-Di Julio K. Sexual desire during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt) 2010; 19:209–218. doi:10.1089/jwh.2009.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal R, Aggarwal N. Menopausal hot flashes: a concise review. J Midlife Health 2019; 10:6–13. doi:10.4103/jmh.JMH_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cintron D, Lipford M, Larrea-Mantilla L, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 2017; 55:702–711. doi:10.1007/s12020-016-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palacios S, Stevenson JC, Schaudig K, Lukasiewicz M, Graziottin A. Hormone therapy for first-line management of menopausal symptoms: practical recommendations. Womens Health 2019; 15: 1745506519864009. doi:10.1177/1745506519864009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int 2018; 29:1049–1055. doi:10.1007/s00198-018-4414-z. [DOI] [PubMed] [Google Scholar]
- 23.Zaheer S, LeBoff MS. Osteoporosis: Prevention and Treatment. 2018 Nov 26. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000. [Google Scholar]
- 24.Zhao H, Chen J, Li X, Sun Q, Qin P, Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett 2019; 593:2655–2664. doi:10.1002/1873-3468.13527. [DOI] [PubMed] [Google Scholar]
- 25.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas 2016; 91:42–50. doi:10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Curtiss N, Balachandran A, Krska L, Peppiatt-Wildman C, Wildman S, Duckett J. Age, menopausal status and the bladder microbiome. Eur J Obstet Gynecol Reprod Biol 2018; 228:126–129. doi:10.1016/j.ejogrb.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018; 116:43–53. doi:10.1016/j.maturitas.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Chen KLA, Liu X, Zhao YC, et al. Long-term administration of conjugated estrogen and bazedoxifene decreased murine fecal beta-glucuronidase activity without impacting overall microbiome community. Sci Rep 2018; 8:8166.doi:10.1038/s41598-018-26506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leite GGS, Weitsman S, Parodi G, et al. Mapping the segmental microbiomes in the human small bowel in comparison with stool: A REIMAGINE Study. Dig Dis Sci 2020; 65:2595–2604. doi:10.1007/s10620-020-06173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leite GGS, Morales W, Weitsman S, et al. Optimizing microbiome sequencing for small intestinal aspirates: validation of novel techniques through the REIMAGINE study. BMC Microbiol 2019; 19:239.doi:10.1186/s12866-019-1617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013; 41:e1.doi:10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong J, Liu P, Zhou G, Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 2020; 15:799–821. doi:10.1038/s41596-019-0264-1. [DOI] [PubMed] [Google Scholar]
- 33.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 2017; 45:W180–W188. doi:10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss S, Treuren WV, Lozupone C, et al. Correlation detection strategies in microbial data sets vary widely in sensitivity and precision. ISME J 2016; 10:1669–1681. doi:10.1038/ismej.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson KV, Burnet PW. Microbiome: should we diversify from diversity? Gut microbes 2016; 7:455–458. doi:10.1080/19490976.2016.1241933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risely A. Applying the core microbiome to understand host-microbe systems. J Anim Ecol 2020; 89:1549–1558. doi:10.1111/1365-2656.13229. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida N, Yamashita T, Hirata KI. Gut microbiome and cardiovascular diseases. Diseases 2018; 6:56.doi:10.3390/diseases6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Ames NP, Tun HM, et al. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol 2016; 7:129.doi:10.3389/fmicb.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jie Z, Xia H, Zhong S-L, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017; 8:845.doi:10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb 2016; 23:908–921. doi:10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Aidy S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol 2015; 32:14–20. doi:10.1016/j.copbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Leser TD, Molbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ Microbiol 2009; 11:2194–2206. doi:10.1111/j.1462-2920.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 43.Leite G, Morales W, Weitsman S, et al. The duodenal microbiome is altered in small intestinal bacterial overgrowth. PLoS One 2020; 15:e0234906.doi:10.1371/journal.pone.0234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weitsman S, Celly S, Leite G, et al. Effects of proton pump inhibitors on the small bowel and stool microbiomes. Dig Dis Sci 2021; doi:10.1007/s10620-021-06857-y. [DOI] [PubMed] [Google Scholar]
- 45.Duca FA, Waise TMZ, Peppler WT, Lam TKT. The metabolic impact of small intestinal nutrient sensing. Nat Commun 2021; 12:903.doi:10.1038/s41467-021-21235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leite G, Pimentel M, Barlow GM, et al. Age and the aging process significantly alter the small bowel microbiome. Cell Rep 2021; 36:109765.doi:10.1016/j.celrep.2021.109765. [DOI] [PubMed] [Google Scholar]
- 47.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol 2018; 44:34–40. doi:10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkins LJ, Monga M, Miller AW. Defining dysbiosis for a cluster of chronic diseases. Sci Rep 2019; 9:12918.doi:10.1038/s41598-019-49452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019; 7:14.doi:10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016; 8:51.doi:10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev 2017; 38:173–188. doi:10.1210/er.2016-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metabol 2019; 104:4660–4666. doi:10.1210/jc.2019-01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glaser R, York AE, Dimitrakakis C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas 2011; 68:355–361. doi:10.1016/j.maturitas.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Amato KR, Yeoman CJ, Cerda G, et al. Variable responses of human and non-human primate gut microbiomes to a Western diet. Microbiome 2015; 3:53.doi:10.1186/s40168-015-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016; 65:1812–1821. doi:10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 56.Kovatcheva-Datchary P, Nilsson A, Akrami R, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 2015; 22:971–982. doi:10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Ley RE. Gut microbiota in 2015: prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol 2016; 13:69–70. doi:10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017; 5:14.doi:10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly TN, Bazzano LA, Ajami NJ, et al. Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa heart study participants. Circ Res 2016; 119:956–964. doi:10.1161/circresaha.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.