Abstract
PKC comprises a large family of serine/threonine kinases that share a requirement for allosteric activation by lipids. While PKC isoforms have significant homology, functional divergence is evident among subfamilies and between individual PKC isoforms within a subfamily. Here, we highlight these differences by comparing the regulation and function of representative PKC isoforms from the conventional (PKCα) and novel (PKCδ) subfamilies. We discuss how unique structural features of PKCα and PKCδ underlie differences in activation and highlight the similar, divergent, and even opposing biological functions of these kinases. We also consider how PKCα and PKCδ can contribute to pathophysiological conditions and discuss challenges to targeting these kinases therapeutically.
Keywords: PKC, cell cycle, differentiation, apoptosis, migration, cancer
Abbreviations: BIM, BCL-2-like protein 11; BCL-2, B cell lymphoma 2 apoptosis regulator; CDCP1, CUB domain–containing protein 1; CDK, cyclin-dependent kinase; CF, catalytic fragment; cPKC, conventional PKC; DAG, diacylglycerol; DLG, discs large MAGUK (membrane-associated guanylate kinase homologs) scaffold protein; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; ERK, extracellular signal–regulated kinase; FGF, fibroblast growth factor; IP3, inositol 3-phosphate; MEK, mitogen-activated protein kinase kinase; MMP, matrix metalloproteinase; MSK1, mitogen- and stress-activated protein kinase 1; mTORC2, mammalian target of rapamycin complex 2; nPKC, novel PKC; NSCLC, non–small cell lung cancer; PIP2, phosphatidylinositol 4,5-bisphosphate; PP2A, protein phosphatase 2A; PTB, phosphotyrosine-binding motif; ROS, reactive oxygen species; RTK, receptor tyrosine kinase; TKI, tyrosine kinase inhibitor; TNF, tumor necrosis factor; ZEB, zinc finger E-box-binding homeobox
PKC was discovered nearly 45 years ago based on its unique dependence on lipids and Ca2+ for activation (1, 2). Further studies revealed 10 PKC isoforms that are founding members of the larger AGC (collective name for cAMP-dependent PKA, cGMP-dependent protein kinase G, and PKC) superfamily of protein kinases (3, 4, 5). PKC subfamilies have been defined based on specific requirements for activation by lipids and Ca2+. These subfamilies include conventional PKCs (cPKCs; PKCα, PKCβ, and PKCγ), which require diacylglycerol (DAG) and Ca2+ for activation, novel PKCs (nPKCs; PKCδ, PKCε, PKCη, and PKCθ), which are Ca2+ independent, and atypical PKCs (PKCζ and PKCι), which do not require DAG or Ca2+ and are activated by protein–protein interactions (6). As many isoforms are ubiquitously expressed, targeting these kinases in disease has been daunting due in part to concerns about specificity and redundancy. This is the first review we are aware of that compares activation and function of representative isoforms of the cPKC (PKCα) and nPKC (PKCδ) subfamilies. Our goal is to highlight novel and unique aspects of the regulation and signaling functions of these isoforms to encourage their exploration as drug targets in cancer and other diseases.
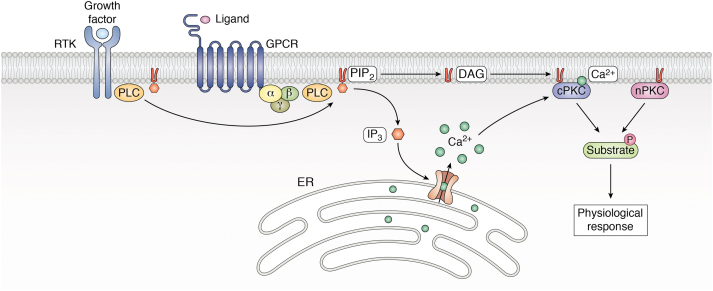
PKC isoforms participate in “outside–in” signaling by transducing signals from a variety of cell surface receptors including receptor tyrosine kinases (RTKs) and G protein–coupled receptors. Indeed, the identification of lipid-regulated kinases such as PKC was a turning point that linked hydrolysis of membrane inositol lipids, described decades earlier, to regulation of intracellular functions (7, 8). These receptors, as well as other physiologic activators of PKC, were shown to stimulate breakdown of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the signaling lipids DAG and inositol 3-phosphate (IP3) (9) (Fig. 1). DAG tethers PKC to the membrane, whereas IP3 induces release of Ca2+ from the endoplasmic reticulum (ER). Interaction of PKCs with the membrane induces conformational changes that lead to release of autoinhibition and activation. Thus, membrane localization is considered the hallmark of PKC activation (10) (see later and Refs. (4, 11) for a detailed description of PKC activation events).
Figure 1.
Diagrammatic representation of PKC-mediated signal transduction. Activation of phospholipase C (PLC) through ligand binding of receptor tyrosine kinases (RTKs) or G protein–coupled receptors (GPCRs) results in hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2), generating diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 releases Ca2+ stored in the endoplasmic reticulum (ER). The accumulation of DAG and Ca2+ results in membrane recruitment of conventional PKC isozymes (cPKCs), whereas recruitment of novel PKCs (nPKCs) is DAG dependent but Ca2+ independent. Activated PKCs phosphorylate their substrates to trigger downstream physiological responses.
All PKC isoforms have highly conserved C-terminal catalytic domains and similar N-terminal regulatory domains (4). However, divergence in critical motifs results in differences in cofactor requirements, mode of membrane recruitment, mechanisms of noncanonical activation, spatial distribution, desensitization, and protein–protein interactions. These differences underly the divergent functions that have been ascribed to PKC subfamilies and to isozymes within subfamilies (Fig. 2A). Later, we will discuss the unique structural features and known functions of PKCα (conventional subfamily) and PKCδ (novel subfamily) in diverse biological processes, highlighting contexts in which these kinases have contrasting and similar roles.
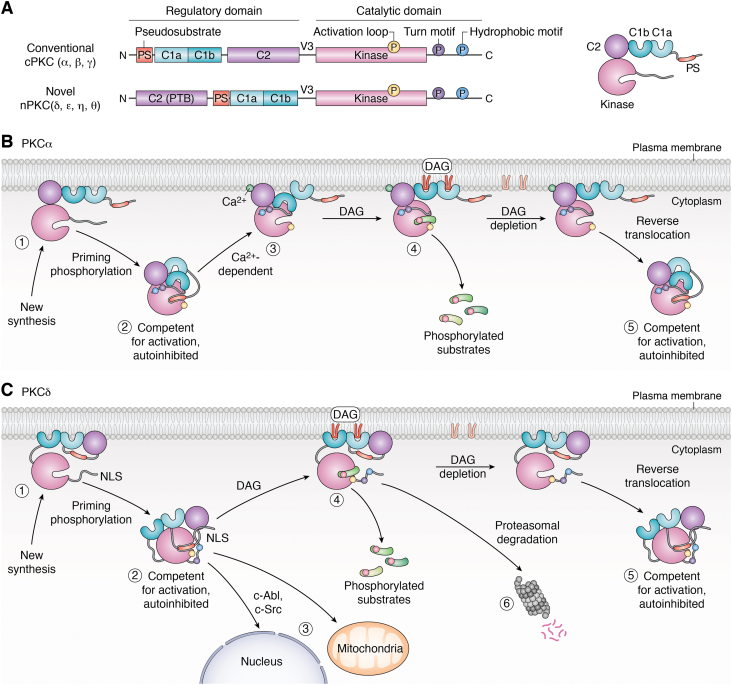
Figure 2.
Structural features and mechanisms of activation of PKCα and PKCδ.A, protein domains of the cPKC and nPKC isoform subfamilies. The N-terminal regulatory domain contains the pseudosubstrate domain (PS, brick red), the lipid-binding C1 domain (C1a and C1b, light and dark green), and the lipid-binding C2 domain (purple), which in PKCα also binds Ca2+. The phosphotyrosine-binding (PTB) motif in the C2 domain of PKCδ is indicated. The C-terminal catalytic domain contains the kinase core (pink) and phosphorylation sites in the activation loop (yellowcircle), the turn motif (purple circle), and the hydrophobic motif (blue circle). Phosphorylation at these sites is required for catalytic competence. The regulatory domain and the catalytic domain are connected by the flexible V3 hinge region, which contains a protease cleavage site. B and C, newly synthesized PKCα (B) and PKCδ (C) are in an open conformation at the plasma membrane (step 1). Upon phosphorylation at three “priming” sites, they are released into the cytosol. Cytosolic PKCα and PKCδ are competent for activation but in an autoinhibited conformation with the PS embedded in the substrate-binding site (step 2). B, activation of PKCα in response to physiological signals involves a two-step process. Initial interaction of PKCα with the plasma membrane is mediated by Ca2+-dependent lipid binding of the C2 domain (step 3), which enables interaction with DAG (redU shape) through the C1 domain (step 4). DAG-induced membrane interaction results in a conformational change allowing PKCα to phosphorylate its substrates. Upon DAG depletion, PKCα undergoes reverse translocation into the cytosol where it assumes its autoinhibited state and is available for reactivation (step 5). C, PKCδ is directly recruited to the plasma membrane or other subcellular organelles in response to DAG or other activation signals such as tyrosine phosphorylation by c-Abl and c-Src (steps 3 and 4). Membrane interaction induces a conformational change that results in PKCδ activation and phosphorylation of substrates (step 4). Signal termination of PKCδ can occur through reverse translocation following DAG depletion (step 5). Alternatively, long-term inactivation of PKCδ signaling can occur through proteasomal degradation of the enzyme (step 6). cPKC, conventional PKC; DAG, diacylglycerol; nPKC, novel PKC.
Distinct structural features of PKCα and PKCδ
Unique structural features and modes of activation
The N-terminal domain of cPKC and nPKC isoforms includes a tandem repeat C1 domain comprising C1a and C1b subdomains that bind DAG, albeit with varying affinity, a membrane lipid–binding C2 domain, and a pseudosubstrate motif that blocks access to the substrate-binding pocket (12). However, PKCs differ in the nature and arrangement of these domains. For example, in PKCα, the C2 domain lies between the C1 and catalytic domains, whereas in PKCδ, the C2 domain lies between the N terminus and the C1 domain (Fig. 2A). Elegant studies by several groups have revealed differences in the maturation, activation, and downregulation (inactivation) of PKCα and PKCδ that are thought to contribute to specification of function.
Kinase phosphorylation and maturation
Many AGC kinases share a requirement for serine/threonine phosphorylation at three conserved sites in the C-terminal domain for activity (3, 13). In PKCs, constitutive phosphorylation in the activation loop by the PIP3-regulated kinase, 3-phosphoinositide-dependent protein kinase 1, transphosphorylation at the “turn” motif, typically by mammalian target of rapamycin complex 2 (mTORC2), and autophosphorylation at the hydrophobic motif (13, 14, 15) is required for catalytic competence and protection from degradation. It is important to emphasize that, in contrast to other AGC kinases that are acutely activated by phosphorylation (e.g., Akt (13)), phosphorylation of the three “priming” sites is seen in inactive PKC (Fig. 2, B and C, step 2) and is, therefore, not indicative of PKC activation per se. Instead, membrane localization and substrate phosphorylation are the only reliable indicators of kinase activation. Comparison of the regulation and function of PKCα and PKCδ priming phosphorylation has revealed two important differences. First, unlike PKCα, which is dependent on activation loop phosphorylation for activity (14), the T505A activation loop mutant of PKCδ is still partially active (16). This difference may have consequences for modulation of kinase activity as well as kinase degradation. Second, a recent study from the Newton laboratory has identified a fourth priming phosphorylation motif, the mTOR interaction motif, in some mTORC2–regulated AGC kinases, including PKCα (17, 18). Phosphorylation of this motif (S631 in PKCα) by the mTORC2 complex allosterically regulates PIP3-regulated kinase, 3-phosphoinositide-dependent protein kinase 1 binding, activation loop phosphorylation, and autophosphorylation of the hydrophobic motif. Curiously, select nPKC isoforms, including PKCδ, are mTORC2 independent for priming and lack this conserved threonine (19). Additional serine and threonine phosphorylation events may fine-tune activation of PKCδ in response to specific signals (20). As discussed later, tyrosine phosphorylation may in addition play a role in modulating the activity of PKCα and PKCδ.
PKCα and PKCδ have unique C1, C2, and phosphotyrosine-binding domains
Divergence in the C2 and C1 domains of PKCα and PKCδ accounts for important differences in Ca2+ dependence and mechanism of activation (Fig. 2). C2 domains are evolutionary conserved lipid-and protein-binding motifs (21). PKCα has a topology I (S family) C2 domain that requires Ca2+ for lipid binding (21, 22). Since membrane binding of the C2 domain is required for membrane recruitment of PKCα by DAG, PKCα activation is Ca2+ dependent. In contrast, PKCδ has a topology II (P family) C2 domain that lacks the critical structural requirements for Ca2+ binding (21, 22); thus, PKCδ activation is Ca2+ independent (23, 24). The C2 domain of PKCδ also differs from that of PKCα in its ability to mediate protein–protein interactions (22, 23, 24). Benes et al. (22) have identified a novel high-affinity phosphotyrosine-binding (PTB) motif in the PKCδ C2 domain, which is not found in PKCα or other PKC isozymes. This PTB domain is distinct from both Src-homology 2 and previously described PTB domains in that it interacts with residues in the phosphorylated peptide both C-terminal and N-terminal to pTyr (22). Binding of the C2 PTB domain to phosphotyrosine-containing proteins in trans could drive the formation of PKCδ-specific signaling modules, whereas cis interactions could contribute to regulation of PKCδ by binding to tyrosine-phosphorylated residues within the kinase domain, for example, as induced by hydrogen peroxide (25).
cPKC and nPKC C1a and C1b domains differ in their affinity for DAG and play unique roles in isoform activation (26). In PKCα, the C1a domain has a higher affinity for DAG compared with the C1b domain (26). However, the C1a domain is masked in unstimulated PKCα by interaction with the C2 and catalytic domains and is only released following Ca2+-dependent interaction of the C2 domain with anionic lipids in the plasma membrane (27, 28, 29, 30, 31) (Fig. 2B). Thus, activation of PKCα requires a multistep process in which the C2 domain initially interacts with the membrane, with subsequent release of the C1a domain for membrane penetration and DAG binding (30, 31, 32). Notably, although the C2 domain of PKCα has low intrinsic affinity for Ca2+, its Ca2+ binding is enhanced by PIP2, phosphatidylserine, and DAG in the plasma membrane, allowing for enzyme activation by DAG even at subphysiological intracellular Ca2+ levels (21, 29, 33, 34, 35). Nonetheless, while activation of PKCα by DAG does not require release of intracellular calcium stores, elevated intracellular Ca2+ concentrations increase the rate of PKCα activation in the presence of DAG (29); thus, PKCα activation may be targeted to local areas of Ca2+ generation, consistent with spatially restricted signaling (36).
The mechanism of PKCδ activation is similar to that of PKCα, except that PKCδ does not bind calcium and is targeted to the membrane primarily through high-affinity binding of the C1 domains to membrane DAG (37) (Fig. 2C). However, the relative contribution of the C1a and C1b domains to membrane DAG binding remains to be resolved. It has been reported that, as in PKCα, the C1a domain of PKCδ has high affinity for DAG, whereas the C1b domain fails to bind DAG but has high affinity for phorbol esters (38). In contrast, other studies have shown that the C1b domain of PKCδ binds to DAG with an affinity that is two orders of magnitude greater than that of cPKCs, and that mutation of the C1a domain has minimal effects on binding of PKCδ to DAG-containing membranes (37, 39). Nonetheless, the high affinity of the C1 domain of PKCδ for DAG compensates for the lack of membrane binding of its C2 domain, allowing for direct C2 domain–independent membrane recruitment and activation of the kinase by signal-generated DAG (37). DAG-independent functions of the C1b domain may also contribute to targeting and activation of PKCδ (37). For instance, Wang et al. (40) have shown that the C1b domain of PKCδ mediates its association with the Golgi/ER protein, p23/Tmp21, to regulate apoptosis.
Noncanonical activation of PKC
In addition to the plasma membrane, it is now clear that PKC isoforms can be activated in a variety of subcellular locations and can respond to stimuli that do not promote hydrolysis of membrane lipids (41). One well-documented mechanism of noncanonical activation of both cPKCs and nPKCs is through reactive oxygen species (ROS) (42). The cysteine-rich zinc-binding finger of C1 domains is highly sensitive to oxidation by ROS, which destroys the conformation of the DAG-binding site. For PKC, oxidation by ROS typically relieves autoinhibition and activates the kinase (43). While redox-dependent conformational changes can activate both cPKCs and nPKCs, PKCδ can also be regulated by oxidative stress through changes in phosphorylation of specific tyrosine residues unique to this isoform (25, 44). There are at least two explanations for redox regulation via tyrosine phosphorylation (42). First, cysteine residues in the active site of protein tyrosine phosphatases are very sensitive to redox inactivation, and the inhibition of dephosphorylation manifests as an overall increase in tyrosine phosphorylation (45). Second, redox activation of RTKs (e.g., epidermal growth factor receptor [EGFR]) and non-RTKs (e.g., c-Abl, c-Src, and Src-family kinases) results in increased phosphorylation on tyrosine residues in PKCδ (42, 46). The most extensively studied of these residues are Tyr311 (rodent; 313 human), Tyr155 (rodent and human), and Tyr64 (rodent and human), which can be phosphorylated by c-Lck, c-Abl, and c-Src (25, 42, 44, 47, 48). PKCα is also tyrosine phosphorylated in response to oxidative stress (25), and tyrosine phosphorylation of Tyr195, Tyr285/286, Tyr365, Tyr504, Tyr512, and Tyr515 (human) has been reported in multiple studies https://www.phosphosite.org/proteinAction.action?id=1773.
Tyrosine phosphorylation is essential for activation of PKCδ in response to death signals. The Reyland laboratory has described a noncanonical activation scheme for PKCδ in which progressive phosphorylation at Tyr155 and Tyr64 by c-Abl and c-Src, respectively, allosterically activates the kinase while also promoting its nuclear translocation (48, 49). Tyrosine phosphorylation reveals a cryptic bipartite nuclear localization signal in the C terminus of PKCδ allowing importin-α binding and nuclear import (47, 50, 51) (Fig. 2C; also see the "Apoptosis" section). Phosphorylation of PKCδ at Tyr155 and Tyr64 appears to be a general response to agents that cause DNA damage (48). Phosphorylation at Tyr311 and Tyr187 can also promote the apoptotic function of PKCδ (52, 53, 54), suggesting that multiple tyrosine phosphorylation events may coordinate activation of this function of the kinase.
Other examples of noncanonical activation include Ca2+ overload in the ischemic heart, where the cysteine protease calpain cleaves PKCα in the V3 region to generate a constitutively active cytosolic C-terminal catalytic fragment (CF) that negatively regulates myocardial function (55). Likewise, PKCδ can be activated by caspase-3-mediated cleavage (56). In both cases, cleavage activates the kinase by releasing the CF from inhibitory interactions with the regulatory domain. Finally, in the context of cell migration, PKCα can be activated by oligomerized syndecan-4 (57), a transmembrane proteoglycan that serves as a receptor for heparan sulphate–binding growth factors and extracellular matrix (ECM) components such as fibronectin and vitronectin (see later).
Signal termination
PKC signal termination is mediated by acute inactivation and long-term desensitization mechanisms. For PKCα and PKCδ, this is accomplished largely through rapid metabolism of DAG (58), which leads to activity-dependent dissociation of PKC from the membrane and restoration of the autoinhibited protein in the cytosol (Fig. 2, B and C, step 5, and (59, 60)). However, prolonged activation by ligands that are not readily metabolized (e.g., phorbol esters, bryostatin) and, in the case of PKCδ, by physiological stimuli (e.g., growth factors), can result in activity-dependent downregulation/loss of PKCα and PKCδ protein, with loss of associated signaling in the continued presence of agonists (Fig. 2C, step 6). Multiple mechanisms of downregulation have been implicated, with subcellular localization playing an important role in dictating the engagement of processing pathways (61). While ubiquitin-mediated proteasomal degradation is a major mechanism of PKC downregulation (62, 63, 64, 65), endomembrane trafficking and lysosomal processing have also been shown to play a role, at least for PKCα (66, 67, 68).
Multisite dephosphorylation of PKC by PH domain leucine-rich repeat protein phosphatase (69) and protein phosphatase 2A (PP2A) (65, 70), which appears to occur in an intracellular compartment (66, 67, 68, 70), may serve as a trigger for PKC degradation in some contexts (63, 70). For cPKCs, peptidyl-prolyl isomerization of the turn motif–priming site by peptidyl-prolyl cis/trans isomerase (PIN1) is required for dephosphorylation of priming sites (71). However, fully phosphorylated mature PKC (Fig. 2, B and C, step 5) is the major substrate for the proteasome in many cell types (61, 65, 67, 72, 73). Parker et al. (73) showed that hyperphosphorylated PKCδ is rapidly degraded in phorbol ester–treated cells and at the G1/S boundary during the cell cycle. The Black laboratory confirmed degradation of fully phosphorylated PKCδ using short-chain DAGs such as 1,2-dioctanoyl-sn-glycerol (DiC8) (61). In the case of PKCα, proteasomal degradation of the fully primed active form following prolonged activation with phorbol esters or bryostatins requires the molecular chaperone heat shock protein 70 (72), which can, paradoxically, also serve to stabilize the dephosphorylated enzyme by binding to the turn motif, promoting its rephosphorylation and reentry into the pool of signaling competent enzyme (74). Maintenance of priming site phosphorylation on activated PKCα is also facilitated by heat shock protein 90 (72), with nucleotide occupancy of the active site within these kinases further contributing to phosphatase resistance (75). Although few studies have addressed lysosomal pathways of PKC downregulation, the Black group and others have determined that PKCα is targeted to lysosomes by phorbol esters via at least two distinct lipid raft–dependent pathways, and that the fully primed protein is also the major target for lysosomal degradation (66, 67, 68).
While PKCα and PKCδ are both degraded in response to prolonged activation by phorbol esters, studies with other PKC agonists have identified at least two important differences in activation-induced desensitization of these isoforms. PKCδ is readily degraded in response to prolonged activation by membrane-permeant short-chain DAGs (61) or physiological signals that stimulate the production of DAG (e.g., gonadotropin-releasing enzyme (76), bombesin (77), and platelet-activated growth factor (77)). In contrast, chronic activation of PKCα by DiC8 (61) or physiological agonists (e.g., thyrotropin-releasing enzyme (78), angiotensin II (79)) fails to engage desensitization mechanisms, such as dephosphorylation, ubiquitination, internalization, or degradation, with the enzyme remaining membrane associated and able to support downstream signaling for prolonged periods (e.g., 12 h (61, 77)). Differences in Ca2+ sensitivity and DAG affinity of PKCα and PKCδ failed to explain the selective resistance of PKCα. Thus, although an effect of different membrane domains was observed, underlying mechanisms remain to be determined (61). Another notable difference is seen in the response of these isoforms to bryostatin 1. While concentrations from 0.1 to 1 μM bryostatin promote proteasomal and lysosomal degradation of PKCα (61, 63), these doses fail to downregulate PKCδ (80, 81). Interestingly, these low concentrations of bryostatin also block the ability of phorbol esters to induce PKCδ degradation when the two agonists are coapplied, suggesting that bryostatin directs PKCδ to a subcellular compartment that is not accessible to phorbol esters.
Subcellular localization of PKCα and PKCδ
The differential effects of PKC agonists on PKCα and PKCδ may reflect their activation at different cellular locations (82), with PKCα translocating mainly to the plasma membrane, whereas PKCδ accumulates in a variety of additional compartments, including the plasma membrane, Golgi membranes, the ER, mitochondria, and the nucleus (50, 83, 84). Localization-specific functions of PKC may be regulated by spatially restricted generation of second messengers such as Ca2+ and/or DAG or by protein–protein interactions that facilitate access to unique substrates (85), among other mechanisms.
The almost exclusive localization of activated PKCα at the plasma membrane (65, 86, 87, 88) may explain the important role of this isoform in regulating cell growth, differentiation, and migration. As discussed further, extensive evidence supports the ability of both PKCα and PKCδ to regulate the activity of RTKs and downstream effectors that reside at the plasma membrane (89, 90, 91, 92, 93, 94, 95, 96, 97). PKCα also coordinately regulates plasma membrane–associated Rho-GTPases, as well as integrins and the actin cytoskeleton, to regulate cell spreading, focal contact formation, and migration (see later).
The wide subcellular distribution of activated PKCδ is consistent with its diverse functions in proliferation, migration, DNA repair, apoptosis, and metabolism. Agonist-induced changes in the intracellular distribution and activity of PKCδ have been investigated using FRET-based fluorescence reporters (41, 83). These studies demonstrate a unique two-step mechanism for recruitment and retention of PKCδ at the mitochondria, where the kinase regulates respiration and promotes apoptosis (98, 99, 100). In other studies, Gomel et al. (101) targeted exogenous active PKCδ to the cytosol, ER, nucleus, or mitochondria. Their studies showed that ER-targeted PKCδ is antiapoptotic, whereas nuclear-, cytoplasmic-, and mitochondrial-targeted PKCδ is proapoptotic.
The Reyland laboratory has shown that nuclear localization of PKCδ is highly regulated and linked to cell death signals such as activation of c-Abl and caspase-3 (47, 50). Nuclear localization is in addition controlled by c-Src and Src-family kinases (83), suggesting a potential link between growth factor signaling and cell death. In addition to PKCδ, caspase-3 also accumulates in the nucleus in response to apoptotic signals, where it can cleave PKCδ in the V3 region to generate a constitutively activated CF (PKCδ CF) (51, 56), which is also constitutively nuclear because of exposure of its nuclear localization signal. Whether PKCδ CF is functionally distinct from full-length PKCδ is unclear since both forms of PKCδ can induce apoptosis when overexpressed, albeit with different kinetics (51, 102).
Proliferation and differentiation
In-depth studies of the growth regulatory functions of PKCα in normal cells have been performed in the hematopoietic system and in regenerating epithelial tissues, including the intestinal epithelium, epidermis, and endometrium. While PKCα signaling can promote (103, 104, 105) or inhibit (106, 107) proliferation in cells of the immune system, the kinase is predominantly antiproliferative and prodifferentiation in regenerating epithelia (86, 87, 88, 108, 109, 110, 111, 112, 113, 114, 115). PKCδ can also promote (116, 117, 118, 119, 120, 121) or inhibit (122, 123, 124, 125, 126) proliferation in normal and cancer cells (127, 128, 129). Curiously, in some cases, PKCα and PKCδ regulate similar cell functions, albeit with different outcomes. For example, while cell cycle arrest by PKCα can lead to cell differentiation (86, 87, 88, 108, 109, 110, 111, 112, 113, 114, 115), cell cycle arrest by PKCδ is more likely to drive cell death (130, 131). PKCα and PKCδ diverge significantly in their regulation of growth factor signaling, with PKCα inhibiting growth factor receptor activity and downstream pathways, and PKCδ generally propagating growth factor signaling by regulating downstream pathways such as the MEK (mitogen-activated protein kinase kinase)–ERK (extracellular signal–regulated kinase) cascade (102, 132, 133). Here, we will discuss what is known about mechanism(s) underlying the overlapping and divergent effects of these PKC isozymes on growth regulatory targets.
Cell cycle regulation
Compelling evidence for growth-suppressive and differentiation-inducing functions of PKCα comes from immunohistochemical analysis of unperturbed epithelial tissues. PKCα is cytosolic and inactive in proliferating cells of intestinal and colonic crypts (88, 110), the basal layer of the epidermis (112, 113), and the endometrium (86). However, coincident with cell growth arrest and differentiation in these tissues, PKCα robustly localizes to the plasma membrane, a hallmark of PKC activation (10). More specifically, PKCα is cleared from the cytosol and appears at the plasma membrane in the upper crypt region of the intestinal epithelium, the first suprabasal layer (spinous layer) of the epidermis, and in nonproliferating estrus phase endometrial cells. Remarkably, PKCα remains membrane associated and presumably active for prolonged periods in postmitotic cells, for example, 2 to 3 days in the intestine and 8 to 10 days in the skin, consistent with the requirement for sustained activation of the enzyme for maintenance of physiological responses such as growth arrest and differentiation (134, 135). Strikingly, in vivo studies in genetically engineered mice largely support a growth suppressive role for PKCα. While PKCα knockout mice are viable, fertile, and have no overt phenotype, Oster and Leitges (136) reported increased crypt cell mitotic index in the intestinal epithelium of these mice. The Farese group further showed that insulin signaling through PI3K is enhanced in PKCα-deficient skeletal muscle and adipocytes and that PKCα acts as a physiological feedback inhibitor of the insulin pathway (137), a finding that has been confirmed in multiple systems (138, 139, 140).
A direct role for PKCα activity in driving growth arrest was established by in vitro studies in intestinal crypt–like cells (108, 109) and keratinocytes (87, 112), which demonstrated that PKCα can trigger hallmark events of cell cycle withdrawal into G0. Evidence from these and other systems points to D-type cyclins and the cyclin-dependent kinase (CDK) inhibitory proteins, p21Cip1 and p27Kip1, as critical cell cycle regulatory targets of PKCα (Fig. 3A). PKCα induces rapid downregulation of cyclins D1, D2, and D3 and/or induction of p21Cip1/p27Kip1, inhibition of G1/S cyclin/CDK complex activity, and changes in the pocket proteins, p107, pRb, and p130, characteristic of G1 arrest and cell cycle withdrawal in multiple cell types (87, 108, 109, 112, 141, 142). PKCα-induced downregulation of D-type cyclins is mediated by at least two mechanisms: inhibition of cyclin D translation through PP2A-mediated activation of the translational repressor 4E-BP1 (143, 144, 145), and transcriptional repression, likely through a MEK–ERK-dependent mechanism (135, 143, 145). PKCα induces p21Cip1 at the level of transcription via p53-dependent (146) and p53-independent mechanisms (141, 143). Consistent with its ability to trigger cell cycle withdrawal, activation of PKCα can induce p21Cip1-dependent cell senescence (147). Studies in lung cancer cells by the Kazanietz group showed that activation of PKCα in S phase results in irreversible G2/M cell cycle arrest, which was not observed when PKCα was activated in G1 phase (147).
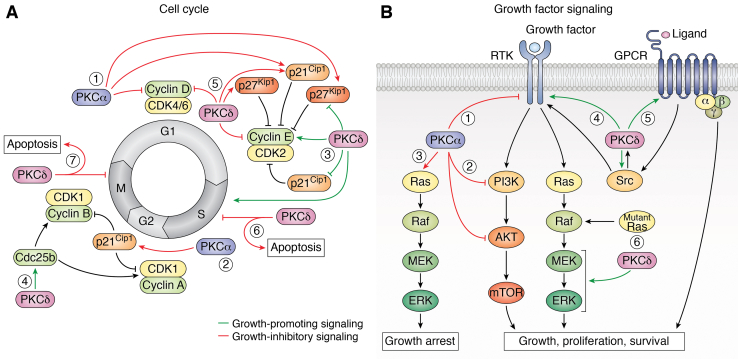
Figure 3.
Growth regulation by PKCα and PKCδ.A, PKCα signaling predominantly inhibits cell cycle progression. In G1, PKCα induces cell cycle withdrawal by inhibiting CDK4/6 and CDK2 activity through downregulation of cyclin D and upregulation of p21Cip1 and p27Kip1 (1). PKCα activation in S phase induces senescence as a result of p21Cip1 upregulation in G2/M (2). In contrast, PKCδ both promotes and inhibits cell cycle progression. Positive effects of PKCδ on G1/S progression involve cyclin E upregulation and downregulation of p21Cip1 and p27Kip1 (3). PKCδ can also promote S-phase transit (3) and can enhance the G2/M transition by activating CDK1 through phosphorylation of Cdc25b (4). Conversely, PKCδ inhibits G1 progression by downregulating cyclin D and cyclin E and upregulating p21Cip1 and p27Kip1 (5). PKCδ can also inhibit cell cycle progression in S phase (6) and G2/M (7), which results in apoptosis. B, PKCα can inhibit proliferation by suppressing the activity of receptor tyrosine kinases (RTKs) (1) such as EGFR, and by inhibiting multiple steps in the PI3K–AKT pathway (2). In addition, PKCα induces growth-inhibitory ERK signaling (3) that is dominant over growth-promoting signals. In contrast, PKCδ promotes proliferation and survival through positive regulation of receptor and non-RTKs (4) and G protein–coupled receptors (GPCRs) (5) and by promoting oncogenic ERK signaling downstream of mutant K-Ras (6). CDK, cyclin-dependent kinase; EGFR, epidermal growth factor receptor; ERK, extracellular signal–regulated kinase.
In contrast to PKCα, which largely restrains cell growth and cell cycle progression, PKCδ can promote or suppress proliferation depending on the context. Perhaps not surprisingly, PKCδ regulates many of the same targets as PKCα. Studies from Kitamura et al. (130) demonstrate biphasic activation of PKCδ in response to serum and a requirement for PKCδ activation for DNA synthesis. PKCδ can promote cell cycle progression in G1 by increasing cyclin levels or by reducing expression or nuclear levels of p21Cip1 and/or p27Kip1 (130, 131), and can enhance G2/M transition through phosphorylation of Cdc25b (148) (Fig. 3A). In contrast, vascular endothelial cells and B cells derived from PKCδ knockout mice show an increase in proliferation (149, 150) consistent with studies by Watanabe et al. (122, 151, 152, 153) that have linked PKCδ to growth arrest. As shown for PKCα, induction of G1 or G1/S arrest can occur through upregulation of p21Cip1 or p27Kip1 or through downregulation of cyclin D and/or cyclin E (123, 131, 151, 154, 155, 156, 157, 158). Several mechanisms have been identified for regulation of p21Cip1 by PKCδ including (a) transcriptional induction through KLF4 (124), (b) regulation of p21Cip1 phosphorylation (159), and (c) regulation of the interaction of p21Cip1 with CDK2 (160). PKCδ can in addition regulate the interaction of p27Kip1 with CDK4 to inhibit proliferation (123).
Cell cycle arrest by PKCδ may have important implications for cell death decisions. Notably, in some studies, PKCδ promotes G1 transition and causes cells to arrest in S or G2/M (130, 131). Using a PKCδ overexpression model, Ohno et al. (130) showed that phosphorylation of PKCδ at Thr505 is required for the serum-induced transition from G1 to S phase; however, in the same study, PKCδ induced a block in G2/M progression. Similarly, expression of the PKCδ CF induced a strong G2/M block in primary human keratinocytes and immortalized HaCaT cells coincident with induction of apoptosis (161). Other studies show that overexpression of PKCδ stimulates G1 → S transition, but the cells then arrest in S phase and undergo apoptosis (131). Thus, G1 → S promotion under these conditions is not proproliferative but proapoptotic.
Finally, like PKCα, increasing evidence points to a role for PKCδ in cell senescence (161, 162, 163, 164, 165). Studies in adipocyte stem cells show that PKCδ can induce senescence through regulation of human telomerase reverse transcriptase (162, 163). Similar studies identified PKCδ as a mediator of transforming growth factor-β–induced senescence through repression of human telomerase reverse transcriptase (165) or inactivation of glycogen synthase kinase 3β (166). PKCδ can also function in cell senescence downstream of p16INK4a and Rb (164). An interesting question is whether induction of senescence by PKCδ is a survival mechanism under conditions where DNA repair is inhibited (see the “Cell survival and cell death” section).
Regulation of growth factor signaling
Inhibitory effects of PKCα on cell cycle progression also reflect its ability to suppress the activity of tyrosine kinase receptors, including EGFR (89, 90, 91, 92, 93, 94, 95), ErbB2 (Erb-B2 RTK 2) (HER2 [human epidermal growth factor receptor 2]/neu) (93, 96, 97), c-Met (hepatocyte growth factor receptor) (167), and RET (rearranged during transfection) (168) (Fig. 3B). PKCα can inhibit tyrosine kinase receptor signaling by reducing ligand-binding affinity though direct receptor phosphorylation and by altering cell surface expression of receptors by modulating receptor trafficking (93, 94, 95, 167, 168, 169, 170, 171, 172, 173, 174, 175). PKCα also regulates cell proliferation by acting downstream of growth factor receptors, as exemplified by its ability to suppress insulin action through inhibition of PI3K–Akt and MEK–ERK signaling downstream of the insulin receptor (138, 139, 140). PKCα-mediated inhibition of PI3K–Akt signaling has been observed in multiple systems (e.g., (86, 145, 176, 177)) and can be accomplished via distinct mechanisms, including suppression of the catalytic activity of PI3K by phosphorylation of the p84α regulatory subunit (177) or direct phosphorylation of the catalytic subunit (178) and by PP2A-dependent dephosphorylation of Akt (86). Interestingly, PKCα can also induce growth arrest via strong and sustained activation, rather than inhibition, of the MEK–ERK pathway (135). PKCα-induced growth inhibitory ERK signaling is dominant over proproliferative ERK signaling from serum-regulated growth factors (111, 135, 179, 180, 181), further supporting the ability of PKCα to drive growth arrest in epithelial tissues.
In direct contrast to PKCα, PKCδ promotes signaling through tyrosine kinase and G protein–coupled receptors, including receptors for EGF, fibroblast growth factor (FGF), hepatocyte growth factor, insulin-like growth factor 1, and vascular endothelial growth factor (102, 132, 133, 182, 183, 184, 185, 186, 187, 188) (Fig. 3B). PKCδ can drive growth factor signaling as part of an active receptor complex, through regulation of A disintegrin and metalloprotease 17–mediated shedding of ligands such as EGF (189), and by controlling recycling and degradation of activated cell surface receptors (170, 190, 191, 192). Ligand binding to EGFR and other receptors in this family frequently results in activation of a PKCδ–Src–ERK pathway (127, 132, 133, 193). The Reyland laboratory has shown that in Her2/ErbB2-positive breast cancer cells, loss of PKCδ disrupts the association of Src with ErbB2 and inhibits ERK activation (127). In addition to acute regulation, PKCδ contributes to sustained ERK activation both downstream of growth factors and through growth factor–independent mechanisms (118, 190, 194, 195, 196, 197). For example, sustained activation of ERK in response to DNA damage is PKCδ dependent but EGFR independent (197). PKCδ is required for maintenance of ERK activation downstream of mutant K-Ras in some non–small cell lung cancer (NSCLC) cells and may be a mechanism of resistance to tyrosine kinase inhibitors (TKIs) (128, 198, 199, 200). PKCδ can also activate MEK–ERK by inhibiting Raf kinase inhibitory protein (RKIP), a negative regulator of ERK activation (201). Regulation of growth factor signaling by PKCδ could potentially also be mediated by its ability to bind phosphotyrosine and assemble signaling complexes (22).
Cell differentiation
Elegant studies by the Fields laboraotory showed that PKCα promotes cytostasis and megakaryocytic differentiation of K562 human erythroleukemia cells and that this effect is mediated by isozyme-specific sequences within the catalytic domain (202, 203, 204). The Schwende group (205) demonstrated the ability of PKCα to promote differentiation of THP-1 monocyte-like cells into macrophage-like cells, and Nishizuka et al. (134) showed that sustained activation of PKCα is required for differentiation of HL-60 promyelocytic leukemia cells into macrophages. The link between PKCα signaling and cell differentiation is clearly illustrated by studies in keratinocytes. Early work by Yuspa et al. identified PKCα as a major player in the induction of differentiation markers during Ca2+-induced keratinocyte differentiation (114), findings that were subsequently confirmed by others (87, 113, 115). Using human keratinocyte organotypic raft cultures, Denning et al. (87) demonstrated that PKCα deficiency results in decreased cell differentiation, indicated by reduced expression of the late granular layer differentiation marker, loricrin, and impaired epidermal stratification. PKCα has also been shown to play a major role in the differentiation program of mouse keratinocytes by promoting Ca2+-dependent activation of AP-1 transcription factors (206). Studies from the Rosato laboratory (207) investigated the involvement of PKCα and PKCδ in FGF receptor 2b–induced keratinocyte differentiation. Their work showed that PKCδ is necessary at the onset of differentiation, whereas PKCα is necessary for the terminal stages of differentiation. Consistent with this role, PKCδ promotes differentiation in a variety of cells and tissue types (208, 209, 210).
Cell survival and cell death
While PKCα provides a strong antiproliferative signal, multiple studies have also linked PKCα to cell survival. In contrast, while PKCδ supports cell survival in some contexts, it is more commonly a regulator of cell death (211). Interestingly, PKCα and PKCδ have been shown to have opposing effects on cell survival in the same cell (212, 213, 214). For example, in salivary acinar cells, apoptosis induced by loss of PKCα requires PKCδ activity (214).
Cell survival
An early report by Parker et al. (215) described a causal relationship between loss of PKCα function and induction of apoptosis in COS cells, pointing to the ability of PKCα to mediate survival signaling. Subsequent studies in a broad range of cell types further support this role (214, 216, 217, 218). The prosurvival functions of PKCα are modulated primarily through Bcl-2 (B cell lymphoma 2 apoptosis regulator) family members (Fig. 4A). Induction of cell death in glioma cell lines and rat hepatic epithelial cells by PKCα knockdown is associated with significant downregulation of the survival protein, Bcl-xL (B cell lymphoma–extra large apoptosis regulator) (216, 219). Ruvolo et al. (220) determined that PKCα can phosphorylate Bcl-2 on Ser70 in vitro, a modification linked to enhanced Bcl-2 antiapoptotic activity (221), and PKCα-mediated phosphorylation of Bcl-2 was subsequently confirmed in multiple studies using a variety of cell types (220, 222, 223, 224, 225). Importantly, PKCα can associate with mitochondrial membranes (221, 222) via a mechanism that may involve anchorage by protein interacting with PRKCA 1 (PICK1) (222), for appropriate positioning to regulate Bcl-2. Additional mechanisms associated with the prosurvival effects of PKCα include (a) suppression of apoptosis mediators FEM1b and apoptotic protease activating factor-1 (APAF-1) in T-cell acute lymphoblastic leukemia cells (226), (b) modulation of the proapoptotic BH3 protein, BAD (BCL-2-associated death promoter), in lymphoma cells (227), (c) induction of nuclear translocation of NFκB in bladder cancer cells (228), (d) cytoplasmic localization of p53 in melanoma cells (229), and (e) upregulation of Dicer in bladder cancer cells (230).
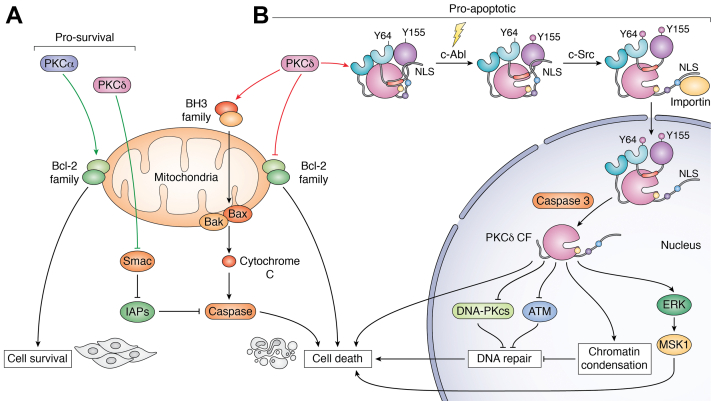
Figure 4.
Regulation of cell survival and apoptosis by PKCα and PKCδ.A, PKCα predominantly promotes cell survival through activation of Bcl-2 family proteins. While PKCδ is primarily a proapoptotic kinase, it can drive cell survival by sequestering Smac, thus allowing IAPs to inhibit caspase activation. B, PKCδ drives cell death by downregulating antiapoptotic Bcl-2 family proteins or by upregulating the proapoptotic BH3 family proteins, which allows the formation of Bax/Bak channels, release of cytochrome c, and activation of caspase. Upon DNA-damaging signals (yellowlightning bolt), PKCδ undergoes noncanonical activation involving sequential phosphorylation of Y155 and Y64 by c-Abl and c-Src, respectively. These phosphorylation events result in a conformational change that releases the C-terminal tail from the C2 and kinase domains, revealing a cryptic NLS to allow importin binding and nuclear transport. In the nucleus, active caspase-3 cleaves PKCδ to generate active C-terminal catalytic domain (PKCδ CF). Potential mechanisms of PKCδ-dependent cell death include suppression of the DNA damage response, alterations in chromatin structure, and inhibition of DNA repair. The ERK–MSK1 pathway is important for regulation of the proapoptotic response of PKCδ. Prosurvival proteins are in green, and proapoptotic proteins are in orange. CF, catalytic fragment; ERK, extracellular signal–regulated kinase; IAP, inhibitor-of-apoptosis protein; MSK1, mitogen- and stress-activated protein kinase 1; NLS, nuclear localization signal.
Prosurvival signaling by PKCδ is somewhat surprising given its well-established role in regulating cell death. In particular, many studies have shown an essential role for PKCδ in the survival of cells that are dependent on activated K-Ras. These include neuroendocrine tumor cells (116), cancer stem cells in pancreatic and prostate tumors (117), and a subset of lung cancer cells (117, 128, 198). In NIH 3T3 cells engineered to express activated K-Ras (199) and pancreatic cancer cell lines with activated K-Ras (186), PKCδ is required to maintain Akt prosurvival signaling. However, in K-Ras–addicted NSCLC cells, PKCδ is required for ERK activation downstream of mutant K-Ras but not Akt activation (128, 198, 199). PKCδ supports the survival of cancer stem cells in multiple human tumor types (117, 121, 231) and facilitates maintenance of tumor-initiating cells (232). Muselli et al. (233) have recently shown that PKCδ regulates expression of the protein Bmi1 (B cell–specific Moloney murine leukemia virus integration site 1), which is required for stem cell renewal. Other prosurvival mechanisms include sequestration of Smac, an antagonist of inhibitor-of-apoptosis proteins (Fig. 4A) (234, 235), and regulation of the phosphorylation and inactivation of the proapoptotic protein Bim (BCL-2-like protein 11) (236, 237) (186, 198, 237).
Contrary to its prosurvival roles, overexpression of PKCδ is associated with decreased survival in colon cancer cells, keratinocytes, and many nontransformed cells (124, 125, 238). Consistent with a dual role for PKCδ in regulation of cell viability, functional proteomic analysis of PKCδ-depleted salivary epithelial cells reveals upregulation of signaling pathways that promote cell survival as well as cell death (239). In particular, ERK signaling, energy sensing, the DNA damage response, and apoptosis were identified as key pathways dependent on PKCδ (187, 194, 195, 196, 197, 240, 241, 242). These seemingly paradoxical functions of PKCδ may reflect cell- and context-specific integration of different signaling pathways.
Apoptosis
The preponderance of evidence supports opposite roles for PKCα and PKCδ in cell death, with PKCα suppressing and PKCδ inducing apoptosis (211, 238) (Fig. 4B). However, PKCα can also play a proapoptotic role. In hormone-dependent LNCaP prostate cancer cells, prolonged association of PKCα with non-nuclear membranes leads to apoptosis (243), and PKCα mediates apoptosis induced by DAG-lactones (244). In renal tubular cells, PKCα mediates cell death induced by polychlorinated biphenyls, via a mechanism that involves downregulation of Bcl-2 and activation of caspase-3 (245).
Extensive studies from the Reyland laboratory and others have shown that PKCδ plays a central role in activation of cell death pathways under conditions of cell stress and DNA damage (149, 246, 247) (Fig. 4B). Depletion of PKCδ suppresses DNA damage–induced cell death in most nontransformed cells and some cancer cells, PKCδ knockout mice are protected from irradiation-induced damage to the salivary gland and thymus, and salivary epithelial cells from PKCδ knockout mice are resistant to multiple apoptotic stimuli (211, 247). PKCδ also regulates apoptosis through TRAIL (tumor necrosis factor (TNF)–related apoptosis-inducing ligand) and TNFα (248, 249). In this context, PKCδ can regulate secretion of death receptor ligands in response to phorbol ester (250) and death receptor expression in the context of ER stress (251, 252).
Nuclear PKCδ is the primary inducer of cell death, although localization of PKCδ to the mitochondria may also contribute to apoptosis (50, 51, 253, 254). Nuclear localization of PKCδ is tightly regulated to prevent inappropriate activation of cell death pathways (see the “Distinct structural features of PKCα and PKCδ” section) (47, 50). Once in the nucleus, PKCδ is cleaved by nuclear caspase-3 to generate a 42 kD CF (PKCδ CF), which is constitutively nuclear and a potent inducer of apoptosis (51). Interestingly, caspase-3 cleavage of PKCδ per se is not required for this mechanism of apoptosis, but nuclear translocation of PKCδ and PKCδ kinase activity are essential (51). Defining the critical role tyrosine phosphorylation plays in activating PKCδ in response to DNA damage led to the prediction that TKIs could be protective against irradiation damage. The Reyland laboratory has subsequently shown that TKIs block PKCδ activation and provide robust protection against radiation-induced damage to the salivary gland in vivo (49, 255). Furthermore, PKCδ-targeted siRNAs reduce cytotoxin-induced renal cell injury in mice as well as irradiation-induced salivary gland damage (256, 257). Thus, PKCδ-targeted therapies could be used to provide protection from damage during radiation and/or chemotherapy treatment.
Mechanistically, PKCδ can regulate the apoptotic machinery through downregulation of prosurvival Bcl-2 proteins, such as Mcl-1 (myeloid cell leukemia-1 antiapoptotic BCL-2 family protein), or upregulation of proapoptotic Bcl-2 proteins, including BIM, BAD, BAX (BCL-2 associated X protein), and BAK (BCL-2 antagonist/killer 1) (236, 258, 259, 260, 261). However, Bcl-2 proteins have not been identified as direct substrates of PKCδ. More likely, PKCδ regulates these apoptotic players indirectly through p38, c-Jun N-terminal kinase, ERK, and mitogen- and stress-activated protein kinase 1 (MSK1), Akt, and other pathways that control cell survival in response to damage (176, 196, 197, 262, 263). In addition, there is evidence that PKCδ regulates the DNA damage response and cell cycle checkpoints (264, 265, 266, 267, 268, 269, 270). For example, PKCδ can regulate activation of the DNA damage sensors DNA-PK (DNA-dependent protein kinase) and ATM (ataxia–telangiectasia mutated) as well as phosphorylation of RAD9 and γH2AX (264, 265, 266, 268, 269). In the case of DNA-PK, PKCδ inactivates the catalytic subunit, suppressing DNA repair and inducing apoptosis (265). Phosphorylation of histone H3 Ser10 by PKCδ has also been described, which is accompanied by chromatin condensation and increased apoptosis (271). Similarly, the Reyland laboratory has shown that MSK1 is required for apoptosis through a PKCδ → ERK → MSK1 pathway (197). Cell cycle checkpoints in S phase and G2/M phase may also be targets for induction of cell death by PKCδ (266, 267, 269) as loss of the G2/M checkpoint is associated with increased radiation sensitivity and apoptosis (267). Whether these checkpoints are directly regulated by PKCδ or induce cell death secondary to accumulation of DNA damage has not been fully elucidated.
Regulation of cell motility and migration
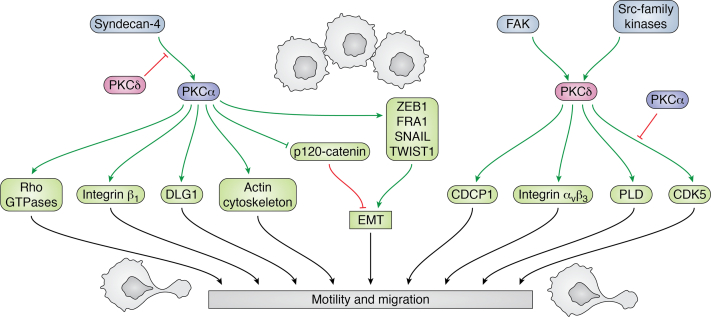
While the biological effects of PKCα and PKCδ often diverge, a notable exception is seen in the regulation of cell motility and migration where both isoforms generally act as positive regulators, although the mechanisms underlying this regulation often differ (Fig. 5). A large body of evidence points to PKCα as a positive regulator of epithelial-to-mesenchymal transition (EMT), a dynamic process in which polarized epithelial cells assume a mesenchymal phenotype characterized by enhanced migratory capacity and invasiveness. EMT plays important roles during normal development as well as pathological processes such as tumor metastasis (272, 273, 274, 275). PKCα regulates the expression of transcription factors that play key roles in EMT, including FRA1 (276), SNAIL (274, 277), TWIST1 (277, 278), ZEB1 (zinc finger E-box–binding homeobox 1) (273), and ZEB2 (277). For example, TWIST1 is a PKCα substrate, and PKCα-mediated phosphorylation leads to accumulation of TWIST1 through inhibition of its ubiquitination and proteasomal degradation (278). Knockdown of PKCα leads to downregulation of ZEB1 and accompanying upregulation of E-cadherin mRNA, as well as inhibition of cell migration and invasion (273). PKCα is also likely to affect EMT and E-cadherin expression through negative regulation of p120-catenin by direct inhibitory phosphorylation (279, 280). In contrast to PKCα, few studies have reported regulation of EMT by PKCδ; however, PKCδ activity has been linked to EMT based on its ability to mediate effects of EGF on E-cadherin and adherens junctions in primary keratinocytes (281).
Figure 5.
Effects of PKCα and PKCδ on motility and migration. PKCα and PKCδ have predominantly positive effects on cell motility and migration, although they generally regulate these processes through different mechanisms. Activation of PKCα following its interaction with syndecan-4 promotes migration through regulation of Rho-GTPases. PKCα can also enhance migration through regulation of the Scribble–LGL–DLG polarity complex and remodeling of the actin cytoskeleton. PKCα regulation of ZEB1, FRA1, SNAIL, and TWIST1 enhances EMT and promotes cellular motility. PKCδ mediates effects of FAK and Src family kinases on migration, acting through PLD, CDK5, and CDCP1. Integrin signaling represents a common target for PKCα and PKCδ, although the specific integrins targeted differ. Finally, syndecan-4 and CDK5 represent nodes where PKCα and PKCδ can regulate each other’s effects on migration. Positive effects are shown in green arrows, and negative effects are shown in red. CDCP1, CUB domain–containing protein 1; CDK5, cyclin-dependent kinase 5; DLG, discs large MAGUK (membrane-associated guanylate kinase homologs) scaffold protein; EMT, epithelial-to-mesenchymal transition; FAK, focal adhesion kinase ; LGL, lethal giant larvae protein; PLD, phospholipase D; ZEB1, zinc finger E-box–binding homeobox 1.
In addition to regulating EMT, PKCα has direct effects on cell migration and invasion, predominantly mediated by coordinated regulation of Rho-GTPases (282). PKCα binds to syndecan-4, a ubiquitously expressed heparan sulphate proteoglycan that acts as a receptor for growth factors such as FGF2, vascular endothelial growth factor, and PGDF, and ECM components, including fibronectin and vitronectin (283). Following engagement with heparan sulphate–binding proteins, syndecan-4 undergoes PIP2-dependent oligomerization, which results in activation of PKCα through a noncanonical mechanism that is independent of DAG and Ca2+ (57, 282, 284, 285, 286). In the resting state, syndecan-4 represses the activity of Rho-family small GTPases, RhoA and Rac1, through interaction with and activation of the Rho GDP–dissociation inhibitor, RhoGDI1. However, following activation, syndecan-4-bound PKCα phosphorylates RhoGDI1, leading to release and activation of RhoA and Rac1 (287, 288, 289, 290). Thus, syndecan-4-mediated activation of PKCα supports directional migration through activation of RhoA and Rac1 at sites of matrix and/or growth factor binding (291). PKCα also supports migration through phosphorylation of p190RhoGAP (GTPase-activating protein) (292), which leads to inhibition of RhoA but not Rac1, and thus regulates the phasic activation of Rho-GTPases that is required for migration (292, 293). Notably, PKCδ signaling can oppose the effects of PKCα on RhoA and Rac1. While PKCδ does not interact with syndecan-4 (282), the kinase can phosphorylate syndecan-4 on Ser183, which inhibits PIP2 binding and oligomerization to prevent PKCα activation by heparan sulphate–binding proteins (294).
Modulation of migration by PKCα can also involve direct effects on components of the actin cytoskeleton. For example, phosphorylation by PKCα protects the actin crosslinking protein, filamin A, from degradation and enhances cytoskeletal remodeling and cell migration (295, 296). PKCα also binds and phosphorylates vinculin (297) and fascin (298) to regulate cell spreading and focal adhesion. The Scribble–LGL (lethal giant larvae protein)–DLG (discs large MAGUK [membrane-associated guanylate kinase homologs] scaffold protein) polarity complex has also been implicated in the ability of PKCα to control directed migration (299, 300, 301). PKCα interacts with DLG1 at the leading edge, and blockade of this interaction reduces the ability of PKCα to promote migration (299). Notably, regulation of DLG1 is unique to PKCα since it is mediated by a PDZ ligand motif in the C terminus of the kinase that is not present in PKCδ or other PKC isozymes.
Both PKCα and PKCδ regulate motility and invasion through modulation of integrin function. PKCα interacts with the cytoplasmic tail of β1 integrin to promote its internalization, and inhibition of this interaction suppresses migration (302, 303). Effects on integrin internalization can be mediated by PKCα phosphorylation of formin-like receptor 2 in lamellipodia and filopodia to promote actin-dependent internalization of formin-like receptor 2/α integrin/β1 integrin complexes (304, 305). While PKCα regulates integrin recycling, PKCδ is important for linking integrins to downstream effectors including phospholipase D, Src, focal adhesion kinase, and mitogen-activated protein kinase (306, 307, 308). In addition, PKCδ can control migration through regulation of integrin expression (309, 310).
PKCδ also has effects on migration that are distinct from those of PKCα. Limited evidence supports a role for PKCδ in controlling the stability of the actin cytoskeleton and enhancing migration through phosphorylation of myosin light chain (311, 312, 313). PKCδ also plays an important role in regulating smooth muscle cell and neutrophil migration (314, 315, 316, 317). Soroush et al. (318, 319) have shown that PKCδ regulation of neutrophil–endothelial cell interactions and neutrophil migration is dependent on phosphorylation of PKCδ on Tyr155. A role for PKCδ in cortical neuron migration has also been shown, with PKCδ promoting cell migration by stabilizing the CDK5 activator, p35 (320). In contrast, PKCα has been reported to be a negative regulator of CDK5 activity (321). Another mechanism unique to PKCδ involves regulation of cell migration through interaction of the enzyme with the transmembrane CUB domain–containing protein 1 (CDCP1) (322, 323, 324). As discussed previously, this protein interacts with the PTB in the C2 domain of PKCδ (22). Upregulation of CDCP1 is seen in many types of cancer where it is associated with progressive disease and poor survival (323, 325). In pancreatic cancer, CDCP1 regulates cell migration, invasion, and ECM degradation through a mechanism that requires association with PKCδ and recruitment of Src (323). Hepatocellular carcinoma and renal carcinoma cells can upregulate CDCP1 via a hypoxia-inducible factor–dependent mechanism to drive PKCδ-dependent migration (322, 324), and a novel small-molecule inhibitor that blocks association of PKCδ and CDCP1 was shown to inhibit metastasis of gastric carcinoma cells (326).
PKCα and PKCδ in disease
Functions of PKCα and PKCδ in cell proliferation, cell survival, cell death, and migration are often subverted in disease. Disease phenotypes likely reflect the sum of individual phenotypes, in the context of their relative contribution to the specific disease process. Here, we will discuss what is known about the contribution of PKCα and PKCδ to cancer, immune, and neurodegenerative disorders.
Roles in tumorigenesis
Analysis of mouse models of cancer clearly reveals opposing phenotypes for PKCα and PKCδ in tumor development and progression. The antiproliferative activity of PKCα (see the “Proliferation and differentiation” section) is reflected in a predominantly tumor suppressive role of the enzyme, supported by frequent downregulation of PKCα mRNA and protein in most cancer types (327) and by the fact that PKCα knockout or inhibition enhances tumorigenesis in all murine cancer models reported to date (i.e., models of intestinal (136), skin (328), endometrial (86), and lung (329) cancer as well as B-CLL (330)). Oster and Leitges (136) showed that loss of PKCα in the ApcMin/+ mouse model of intestinal neoplasia enhances tumor formation in the intestine, with lesions displaying a more aggressive histopathological phenotype and mice dying significantly earlier than their PKCα-expressing littermates. Hara et al. (328) showed that PKCα knockout mice subjected to the two-stage protocol of skin carcinogenesis develop significantly more papillomas, and the Black laboratory has shown that PKCα deficiency accelerates tumor formation in a mutant PTEN (phosphatase and tensin homolog)–driven model of endometrial tumorigenesis (86). Similarly, genetic deletion of PKCα in three murine lung adenocarcinoma models (LSL-Kras, LA2-Kras, and urethane exposure) by the Fields group (329) significantly increased tumor number, size, and aggressiveness, while promoting progression from adenoma to carcinoma and reducing mouse survival. Consistent with a role of PKCα in regulating cell senescence (147), PKCα deficiency resulted in bypass of oncogene-induced senescence in these lung cancer models (329). Loss of PKCα also resulted in expansion of the tumor-initiating bronchioalveolar stem cell population, facilitated by enhanced expression of inhibitor of DNA binding proteins 1–3 (Id1–3), an effect of PKCα deficiency also identified by the Black group (86, 111) in intestinal epithelial and endometrial cells. Finally, Michie et al. (330) reported that deficiency of PKCα in hematopoietic progenitor cells results in B-CLL-like disease in mice. It should be noted that, aside from studies in hematopoietic progenitor cells (330), all PKCα-deficient mouse models referenced here harbored organism-wide loss of the kinase. Thus, additional studies are needed to exclude a role for PKCα deficiency in tumor-associated stromal and immune cells in observed tumor-suppressive effects. Nevertheless, analysis of patient tumors supports findings in animal models, with PKCα loss correlating with advanced disease in many human tumor types (86, 329, 331, 332). In addition, while mutations in PKCα are rare in tumors, a loss-of-function PKCα mutant (D463H) is a hallmark of chordoid gliomas, present in 100% of cases examined to date (333, 334).
Both increased (16, 17) and decreased (335, 336, 337, 338, 339) expression of PKCδ has been observed in human tumors; however, functional genomic alterations of PKCδ are rare, and none have been mechanistically linked to cancer (340). While increased expression of PKCδ correlated with poor prognosis in a subset of human breast tumors (127, 341), expression of PKCδ was shown to decrease with increasing tumor grade in urinary bladder cancer (336). The variable expression of PKCδ in human tumors argues for context-dependent roles in tumor promotion and tumor suppression, consistent with the dual functions of PKCδ in proliferation and cell death that have been demonstrated in vitro. This contrasts with the finding that PKCδ largely functions as a tumor promoter in mouse models of cancer, including mammary gland, pancreatic, and lung (127, 128, 129). An exception are studies in transgenic mice where overexpression of PKCδ suppresses phorbol ester– but not UV irradiation–induced skin cancer (342, 343).
It should be noted that the PKCδ-deficient mouse cancer models in which PKCδ functions as a tumor promoter are all likely to be K-Ras dependent and, as discussed previously, PKCδ is required for survival of cells with activated K-Ras (128, 129, 186, 199) (see the “Cell survival and cell death” section). This is in direct contrast to PKCα, which functions as a tumor suppressor in K-Ras-driven tumor models (329). Studies by the Reyland laboratory in K-Ras mutant NSCLC cell lines support the notion that PKCδ function may be dependent on oncogenic context. In these studies, PKCδ function was investigated in two subpopulations of K-Ras mutant NSCLC cells defined based on dependence on K-Ras for survival (128, 198). NSCLC cells functionally dependent on K-Ras were found to require PKCδ for survival, whereas those not functionally dependent on K-Ras used PKCδ for apoptosis (128, 198). Studies in additional cancer models are clearly needed to delineate the roles of PKCδ in tumorigenesis and to understand how oncogenic context contributes to the function of this kinase.
Involvement in resistance to cancer therapeutics
Interestingly, both PKCα and PKCδ can promote resistance of cancer cells to chemotherapeutic agents, perhaps reflecting mechanistic overlap in their signaling functions. The ability of these kinases to protect cells from the cytotoxic effects of chemotherapeutic agents likely reflects their well-established prosurvival functions (see the “Cell survival and cell death” section). Increased expression of PKCα confers resistance of tumor cells to adriamycin (344), tamoxifen (345), etoposide, and cytosine arabinoside (220, 223), and protective effects have been linked to enhanced Bcl-2 phosphorylation in some contexts (220, 223). Conversely, reduced levels of PKCα sensitize T-acute lymphoblastic leukemia cells to vincristine and prednisone by preventing the downregulation of proapoptotic factors, FEM1b and Apaf-1 (226). Reduced expression of PKCα also sensitizes tumor cells to cisplatin, taxol (224, 346, 347), erlotinib (277), and mitomycin-C plus 5-fluorouracil (217). The clinical relevance of these findings is highlighted by evidence that PKCα expression correlates with resistance to antiestrogen therapy in breast cancer patients (348).
As might be expected, PKCδ has been validated as a synthetic lethal target in some cancers with mutant K-Ras (200, 349) and is required for resistance to TKIs in a subset of K-Ras mutant NSCLC cells (116, 186, 199). In addition, a recent study by Chen et al. (350) showed that PKCδ contributes to acquired resistance to EGFR inhibitors by stabilizing interaction of sodium/glucose cotransporter 1 (SGLT1) with EGFR and increasing glucose uptake. PKCδ expression is increased in NSCLC cells that are dependent on K-Ras for survival, and this correlates with increased nuclear abundance of PKCδ and resistance to chemotherapy-induced apoptosis (198). In this model, nuclear accumulation of PKCδ correlates with resistance to TKIs (200) rather than apoptosis.
Roles in invasion and metastasis
Consistent with their ability to promote cell migration (see the “Regulation of cell motility and migration” section), both PKCα and PKCδ have been implicated in tumor cell invasion and metastasis. Evidence points to PKCα, but not PKCδ, acting as a positive regulator of EMT through regulation of transcription factors such as TWIST and SNAIL (274, 277, 278). PKCα can also regulate Rho-GTPases and the actin cytoskeleton to promote cell spreading, migration and invasion, and both isoforms can regulate degradation of the ECM. PKCα promotes tumor cell invasion in multiple cancer types, including colon cancer (351), hepatocellular carcinoma (352), pancreatic cancer (274), endometrial cancer (353), melanoma (354), and glioblastoma (355). Furthermore, reduced expression/activity of PKCα inhibits metastasis in xenograft models of breast cancer (356, 357), ovarian cancer (358), and melanoma (355). In addition to effects on migration, this activity is associated with the ability of PKCα to increase matrix metalloproteinase (MMP) secretion in breast cancer (357), glioblastoma (355), and lung cancer cells (359). Interestingly, PKCδ suppresses MMP9 secretion and migration in breast and colon cancer cells (360, 361, 362); however, it is required for chemomigration and MMP9 expression in prostate cancer cells (363) and for MMP9 expression in thrombin-stimulated astrocytes (364).
Early studies by the Jaken laboratory suggested a relationship between high expression of PKCδ and metastasis (361). They showed that mammary tumor cells that are engineered to overexpress the regulatory domain of PKCδ, which inhibits the activity of endogenous PKCδ, have reduced metastasis when transplanted into the mammary fat pad of mice (361). More recently, PKCδ has been linked to metastatic phenotypes such as migration and invasion in many in vitro models, including thyroid (365), hepatocellular (322), breast (127, 366), lung (128, 310), prostate (363), pancreatic (323), and renal cell (324) cancer. While confirmation using in vivo models and human tumors is needed, therapeutic targeting of PKCα or PKCδ may hold promise for suppression of metastatic disease; however, the potential role for these kinases as tumor suppressors would need to be taken into consideration in any clinical applications.
Contribution to autoimmune disease, inflammation, and neurodegeneration
PKCδ knockout mice develop a lupus-like autoimmune disease with age, which has been linked to a defect in the establishment of B-cell tolerance and aberrant accumulation of subpopulations of B cells (126, 150, 195, 367, 368). Notably, a similar autoimmune phenotype has been described in a patient with a rare loss-of-function mutation in the PRKCD gene (369, 370). In vivo studies reveal a role for PKCδ in promoting inflammation, as PKCδ knockout mice show defects in macrophage function (371, 372), platelet activation (373), and expression of proinflammatory cytokines (374, 375). Limited evidence also supports a role for PKCα in regulation of inflammatory responses, which can be positive or negative in different contexts. PKCα overexpression in mouse epidermis promotes marked intraepidermal neutrophilic inflammation and expression of inflammation-related genes such as cyclooxygenase 2 and TNFα (376, 377). Conversely, PKCα is protective against lipopolysaccharide-induced lung inflammation likely through inhibition of proinflammatory cytokine release by macrophages (378).
Given its role in inflammation, it is not surprising that disruption of PKCδ in vivo can be protective in tissue injury models and mouse models of disease. For example, PKCδ knockout mice show improved pathogen clearance and increased survival in rat models of sepsis (379, 380, 381). Both PKCα and PKCδ have been implicated in neurodegenerative disease (248, 382, 383, 384, 385). The discovery of rare gain-of-function PKCα variants in families with late-onset Alzheimer’s disease supports a causative role for PKCα in the disease (386). In the case of PKCδ, loss or downregulation is protective in Alzheimer’s and Parkinson’s disease and is associated with increased survival in Huntington’s disease (248, 383, 384, 385). In some cases, these effects are associated with alterations in cell death, consistent with the known role of PKCδ in promoting apoptosis (247, 384, 385).
Summary and perspective
In this review, we have compared the structure, activation, and subcellular localization of PKCα and PKCδ and discussed their unique and sometimes opposing functions (Fig. 6). Our goal was to identify important gaps in knowledge and to stimulate new questions, particularly as they relate to human disease and therapy. Here, we highlight major themes in the regulation of biological functions by PKCα and PKCδ and discuss implications for the pathogenesis of diseases such as cancer.
Figure 6.
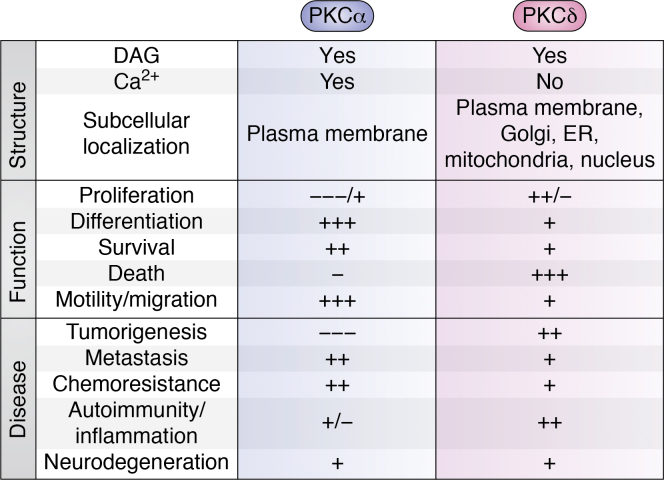
Summary of the regulation and functions of PKCα and PKCδ signaling. The regulation and predominant roles of PKCα and PKCδ are indicated. + and – indicate positive and negative effects on the indicated cellular processes, with multiple +/− symbols indicating the relative prevalence of indicated effects based on the literature.
PKCα and PKCδ can regulate the same or similar biological functions but with opposing outcomes
The contrasting functions of PKCα and PKCδ are often mediated by regulation of the same target molecules or pathway. This dichotomy is well exemplified by the growth-regulatory functions of these kinases. While PKCα is primarily involved in antiproliferative and prodifferentiation activities, PKCδ has both proproliferative and antiproliferative functions. Remarkably, both kinases regulate the same cell cycle proteins and growth factor signaling pathways, albeit in different ways. In the case of growth factor signaling, the specific pathway targets diverge, with PKCα regulating ligand binding, receptor trafficking, and downstream pathways, whereas PKCδ primarily regulates the activation of proproliferative pathways downstream of growth factor receptors. The observation that PKCα and PKCδ can control similar (or the same) biological pathways with different outcomes indicates that output is likely to be highly dependent on tissue, cellular, and signaling context.
PKCα and PKCδ have largely opposing roles in cell survival and cell death
In most, but not all cases, PKCα activates prosurvival pathways, whereas PKCδ promotes cell death. In some contexts, contrasting functions are mediated by opposing effects on the same target proteins, with one example being the Bcl-2 family proteins. However, PKCδ can exert proapoptotic roles through regulation of targets not shared by PKCα, as seen in response to cell stress or DNA damage. The ability of PKCδ to regulate cell proliferation and cell death raises the important question of how these functions are segregated. Evidence points to subcellular localization of PKCδ as a determinant of function. When primarily cytoplasmic, PKCδ appears to be proproliferative, whereas nuclear translocation is tightly associated with cell death. However, some cancer cells may have high levels of nuclear PKCδ but not induce apoptosis, consistent with evasion of apoptosis being a hallmark of cancer. An interesting question is whether proproliferative signaling and cell death signaling by PKCδ are linked, and if so, how. A possible connection is EGFR and MEK–ERK, which can regulate both biological outcomes. Studies in cells with specific oncogenic drivers and lessons from drug resistance models suggest that PKCδ “rewiring” is likely an adaptive response to promote tumor cell survival.
In mouse models of tumorigenesis, PKCα is uniformly tumor suppressive, whereas PKCδ usually functions as a tumor promoter
The largely tumor-suppressive effects of PKCα suggest that antiproliferative signaling is dominant over the prosurvival functions of the kinase. The largely tumor-promoting effects of PKCδ, on the ther hand, point to dominant effects of PKCδ-regulated proproliferative and prosurvival pathways. It is notable that both PKCα and PKCδ generally act as positive regulators of migration and invasion, and both kinases have been implicated in tumor metastasis. The well-documented ability of PKCα to promote cell motility, EMT, invasion, and survival likely explains the seemingly contradictory tumor-promoting activity of this kinase in some cancer types, such as breast tumors. It should be appreciated that in vivo cancer models are largely limited to studies in whole animal knockouts of PKCα or PKCδ and to a select group of tumor models. Analysis of the role of these kinases in models with tissue-specific gene disruption, and in the context of other cancer driving mutations, will be required to more fully understand how these enzymes contribute to tumorigenesis and tumor progression.
The well-documented roles of PKCα and PKCδ in disease pathogenesis support their potential as therapeutic targets
While efforts are underway to target individual PKC isoforms, isozyme-specific targeting is a challenging task because of structural similarity and overlapping functions. There are also important concerns about whether therapeutic strategies should focus on rescuing or inhibiting PKC signaling given the complex phenotypes observed. In addition, both PKCα and PKCδ can promote resistance of cancer cells to the effects of chemotherapeutic agents, further complicating approaches to targeting these enzymes in cancer. A deeper understanding of the functions of each isoform in specific disease settings will be essential for the development of effective drug strategies.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
J. D. B., T. A., A. R. B., and M. E. R. conceptualization; J. D. B., T. A., A. R. B., and M. E. R. writing–original draft; J. D. B., T. A., A. R. B., and M. E. R. writing–review & editing; J. D. B., T. A., A. R. B., and M. E. R. visualization; J. D. B. and M. E. R. supervision; J. D. B. and M. E. R. project administration; J. D. B. and M. E. R. funding acquisition.
Funding and additional information
This work was supported by the National Institutes of Health R01 grants DE027517 and DE015648, T32 CA190216, and F32 DE029116 (to M. E. R.) and by the National Institutes of Health R01 Grant CA054807, R21 Grant CA191894, Cancer Center Core Grant CA036727, and P20 Grant GM121316 and DOD award W81XWH-20-1-0590 (to J. E. D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Alex Toker
Contributor Information
Jennifer D. Black, Email: Jennifer.Black@unmc.edu.
Mary E. Reyland, Email: Mary.Reyland@cuanschutz.edu.
References
- 1.Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- 2.Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J. Biol. Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 3.Leroux A.E., Schulze J.O., Biondi R.M. AGC kinases, mechanisms of regulation and innovative drug development. Semin. Cancer Biol. 2018;48:1–17. doi: 10.1016/j.semcancer.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Newton A.C. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 5.Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J. Biol. Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- 6.Mellor H., Parker P.J. The extended protein kinase C superfamily. Biochem. J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hokin L.E., Hokin M.R. The incorporation of 32P into the nucleotides of ribonucleic acid in pigeon pancreas slices. Biochim. Biophys. Acta. 1953;11:591–592. doi: 10.1016/0006-3002(53)90105-x. [DOI] [PubMed] [Google Scholar]
- 8.Meldrum E., Parker P.J., Carozzi A. The PtdIns-PLC superfamily and signal transduction. Biochim. Biophys. Acta. 1991;1092:49–71. doi: 10.1016/0167-4889(91)90177-y. [DOI] [PubMed] [Google Scholar]
- 9.Ganong B.R., Loomis C.R., Hannun Y.A., Bell R.M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft A.S., Anderson W.B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- 11.Rosse C., Linch M., Kermorgant S., Cameron A.J., Boeckeler K., Parker P.J. PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 12.House C., Kemp B.E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 13.Newton A.C. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh D.B., Ziegler W., Parker P.J. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Good J.A., Ziegler W.H., Parekh D.B., Alessi D.R., Cohen P., Parker P.J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 16.Stempka L., Girod A., Muller H.J., Rincke G., Marks F., Gschwendt M., et al. Phosphorylation of protein kinase Cdelta (PKCdelta) at threonine 505 is not a prerequisite for enzymatic activity. Expression of rat PKCdelta and an alanine 505 mutant in bacteria in a functional form. J. Biol. Chem. 1997;272:6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- 17.Baffi T.R., Lorden G., Wozniak J.M., Feichtner A., Yeung W., Kornev A.P., et al. mTORC2 controls the activity of PKC and Akt by phosphorylating a conserved TOR interaction motif. Sci. Signal. 2021;14 doi: 10.1126/scisignal.abe4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baffi T.R., Newton A.C. mTOR regulation of AGC kinases: new twist to an old tail. Mol. Pharmacol. 2021;101:213–218. doi: 10.1124/molpharm.121.000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J., Holewinski R.J., Van Eyk J.E., Steinberg S.F. A novel phosphorylation site at Ser130 adjacent to the pseudosubstrate domain contributes to the activation of protein kinase C-delta. Biochem. J. 2015;473:311–320. doi: 10.1042/BJ20150812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbalan-Garcia S., Gomez-Fernandez J.C. Signaling through C2 domains: more than one lipid target. Biochim. Biophys. Acta. 2014;1838:1536–1547. doi: 10.1016/j.bbamem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Benes C.H., Wu N., Elia A.E., Dharia T., Cantley L.C., Soltoff S.P. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Smallwood N.D., Hausman B.S., Wang X., Liedtke C.M. Involvement of NH2 terminus of PKC-delta in binding to F-actin during activation of Calu-3 airway epithelial NKCC1. Am. J. Physiol. Cell Physiol. 2005;288:C906–C912. doi: 10.1152/ajpcell.00484.2004. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Lluch G., Bird M.M., Canas B., Godovac-Zimmerman J., Ridley A., Segal A.W., et al. Protein kinase C-delta C2-like domain is a binding site for actin and enables actin redistribution in neutrophils. Biochem. J. 2001;357:39–47. doi: 10.1042/0264-6021:3570039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi H., Tanaka M., Takemura Y., Matsuzaki H., Ono Y., Kikkawa U., et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Ziemba B.P., Falke J.J., Voth G.A. Interactions of protein kinase C-alpha C1A and C1B domains with membranes: a combined computational and experimental study. J. Am. Chem. Soc. 2014;136:11757–11766. doi: 10.1021/ja505369r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farah C.A., Sossin W.S. The role of C2 domains in PKC signaling. Adv. Exp. Med. Biol. 2012;740:663–683. doi: 10.1007/978-94-007-2888-2_29. [DOI] [PubMed] [Google Scholar]
- 28.Slater S.J., Seiz J.L., Cook A.C., Buzas C.J., Malinowski S.A., Kershner J.L., et al. Regulation of PKC alpha activity by C1-C2 domain interactions. J. Biol. Chem. 2002;277:15277–15285. doi: 10.1074/jbc.M112207200. [DOI] [PubMed] [Google Scholar]
- 29.Stahelin R.V., Wang J., Blatner N.R., Rafter J.D., Murray D., Cho W. The origin of C1A-C2 interdomain interactions in protein kinase Calpha. J. Biol. Chem. 2005;280:36452–36463. doi: 10.1074/jbc.M506224200. [DOI] [PubMed] [Google Scholar]
- 30.Medkova M., Cho W. Interplay of C1 and C2 domains of protein kinase C-α; in its membrane binding and activation ∗. J. Biol. Chem. 1999;274:19852–19861. doi: 10.1074/jbc.274.28.19852. [DOI] [PubMed] [Google Scholar]
- 31.Ziemba B.P., Li J., Landgraf K.E., Knight J.D., Voth G.A., Falke J.J. Single-molecule studies reveal a hidden key step in the activation mechanism of membrane-bound protein kinase C-alpha. Biochemistry. 2014;53:1697–1713. doi: 10.1021/bi4016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipp P., Reither G. Protein kinase C: the "masters" of calcium and lipid. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egea-Jiménez A.L., Pérez-Lara A., Corbalán-García S., Gómez-Fernández J.C. Phosphatidylinositol 4,5-bisphosphate decreases the concentration of Ca2+, phosphatidylserine and diacylglycerol required for protein kinase C α to reach maximum activity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antal C.E., Violin J.D., Kunkel M.T., Skovsø S., Newton A.C. Intramolecular conformational changes optimize protein kinase C signaling. Chem. Biol. 2014;21:459–469. doi: 10.1016/j.chembiol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igumenova T.I. Dynamics and membrane interactions of protein kinase C. Biochemistry. 2015;54:4953–4968. doi: 10.1021/acs.biochem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reither G., Schaefer M., Lipp P. PKCalpha: a versatile key for decoding the cellular calcium toolkit. J. Cell Biol. 2006;174:521–533. doi: 10.1083/jcb.200604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giorgione J.R., Lin J.H., McCammon J.A., Newton A.C. Increased membrane affinity of the C1 domain of protein kinase Cdelta compensates for the lack of involvement of its C2 domain in membrane recruitment. J. Biol. Chem. 2006;281:1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahelin R.V., Digman M.A., Medkova M., Ananthanarayanan B., Rafter J.D., Melowic H.R., et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cdelta. J. Biol. Chem. 2004;279:29501–29512. doi: 10.1074/jbc.M403191200. [DOI] [PubMed] [Google Scholar]
- 39.Dries D.R., Gallegos L.L., Newton A.C. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J. Biol. Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Xiao L., Kazanietz M.G. p23/Tmp21 associates with protein kinase Cdelta (PKCdelta) and modulates its apoptotic function. J. Biol. Chem. 2011;286:15821–15831. doi: 10.1074/jbc.M111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott J.D., Newton A.C. Shedding light on local kinase activation. BMC Biol. 2012;10:61. doi: 10.1186/1741-7007-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg S.F. Mechanisms for redox-regulation of protein kinase C. Front. Pharmacol. 2015;6:128. doi: 10.3389/fphar.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konishi H., Yamauchi E., Taniguchi H., Yamamoto T., Matsuzaki H., Takemura Y., et al. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiarugi P., Taddei M.L., Ramponi G. Oxidation and tyrosine phosphorylation: synergistic or antagonistic cues in protein tyrosine phosphatase. Cell Mol. Life Sci. 2005;62:931–936. doi: 10.1007/s00018-004-4448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truong T.H., Carroll K.S. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry. 2012;51:9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adwan T.S., Ohm A.M., Jones D.N., Humphries M.J., Reyland M.E. Regulated binding of importin-alpha to protein kinase Cdelta in response to apoptotic signals facilitates nuclear import. J. Biol. Chem. 2011;286:35716–35724. doi: 10.1074/jbc.M111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphries M.J., Ohm A.M., Schaack J., Adwan T.S., Reyland M.E. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene. 2008;27:3045–3053. doi: 10.1038/sj.onc.1210967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wie S.M., Adwan T.S., DeGregori J., Anderson S.M., Reyland M.E. Inhibiting tyrosine phosphorylation of protein kinase Cdelta (PKCdelta) protects the salivary gland from radiation damage. J. Biol. Chem. 2014;289:10900–10908. doi: 10.1074/jbc.M114.551366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeVries T.A., Neville M.C., Reyland M.E. Nuclear import of PKCdelta is required for apoptosis: Identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeVries-Seimon T.A., Ohm A.M., Humphries M.J., Reyland M.E. Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J. Biol. Chem. 2007;282:22307–22314. doi: 10.1074/jbc.M703661200. [DOI] [PubMed] [Google Scholar]
- 52.Lu W., Finnis S., Xiang C., Lee H.K., Markowitz Y., Okhrimenko H., et al. Tyrosine 311 is phosphorylated by c-Abl and promotes the apoptotic effect of PKCdelta in glioma cells. Biochem. Biophys. Res. Commun. 2007;352:431–436. doi: 10.1016/j.bbrc.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blass M., Kronfeld I., Kazimirsky G., Blumberg P.M., Brodie C. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol. Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomonaco S.L., Kahana S., Blass M., Brody Y., Okhrimenko H., Xiang C., et al. Phosphorylation of protein kinase cdelta on distinct tyrosine residues induces sustained activation of Erk1/2 via down-regulation of MKP-1: role in the apoptotic effect of etoposide. J. Biol. Chem. 2008;283:17731–17739. doi: 10.1074/jbc.M801727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang M.Y., Zhang Y., Matkovich S.J., Diwan A., Chishti A.H., Dorn G.W., 2nd Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ. Res. 2010;107:903–912. doi: 10.1161/CIRCRESAHA.110.220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghayur T., Hugunin M., Talanian R.V., Ratnofsky S., Quinlan C., Emoto Y., et al. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horowitz A., Simons M. Phosphorylation of the cytoplasmic tail of syndecan-4 regulates activation of protein kinase Calpha. J. Biol. Chem. 1998;273:25548–25551. doi: 10.1074/jbc.273.40.25548. [DOI] [PubMed] [Google Scholar]
- 58.Eichmann T.O., Lass A. DAG tales: the multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell Mol. Life Sci. 2015;72:3931–3952. doi: 10.1007/s00018-015-1982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gould C.M., Newton A.C. The life and death of protein kinase C. Curr. Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng X., Hannun Y.A. An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem. 1998;273:26870–26874. doi: 10.1074/jbc.273.41.26870. [DOI] [PubMed] [Google Scholar]
- 61.Lum M.A., Barger C.J., Hsu A.H., Leontieva O.V., Black A.R., Black J.D. Protein kinase Calpha (PKCalpha) is resistant to long term desensitization/down-regulation by prolonged diacylglycerol stimulation. J. Biol. Chem. 2016;291:6331–6346. doi: 10.1074/jbc.M115.696211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H.W., Smith L., Pettit G.R., Smith J.B. Bryostatin 1 and phorbol ester down-modulate protein kinase C-alpha and -epsilon via the ubiquitin/proteasome pathway in human fibroblasts. Mol. Pharmacol. 1997;51:439–447. [PubMed] [Google Scholar]
- 63.Lee H.W., Smith L., Pettit G.R., Vinitsky A., Smith J.B. Ubiquitination of protein kinase C-alpha and degradation by the proteasome. J. Biol. Chem. 1996;271:20973–20976. [PubMed] [Google Scholar]
- 64.Lu Z., Hunter T. Degradation of activated protein kinases by ubiquitination. Annu. Rev. Biochem. 2009;78:435–475. doi: 10.1146/annurev.biochem.013008.092711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leontieva O.V., Black J.D. Identification of two distinct pathways of protein kinase Calpha down-regulation in intestinal epithelial cells. J. Biol. Chem. 2004;279:5788–5801. doi: 10.1074/jbc.M308375200. [DOI] [PubMed] [Google Scholar]
- 66.Melnikov S., Sagi-Eisenberg R. Down-regulating protein kinase C alpha: functional cooperation between the proteasome and the endocytic system. Cell Signal. 2009;21:1607–1619. doi: 10.1016/j.cellsig.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Lum M.A., Pundt K.E., Paluch B.E., Black A.R., Black J.D. Agonist-induced down-regulation of endogenous protein kinase c alpha through an endolysosomal mechanism. J. Biol. Chem. 2013;288:13093–13109. doi: 10.1074/jbc.M112.437061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevostel C., Alice V., Joubert D., Parker P.J. Protein kinase C(alpha) actively downregulates through caveolae-dependent traffic to an endosomal compartment. J. Cell Sci. 2000;113:2575–2584. doi: 10.1242/jcs.113.14.2575. [DOI] [PubMed] [Google Scholar]
- 69.Gao T., Brognard J., Newton A.C. The phosphatase PHLPP controls the cellular levels of protein kinase C. J. Biol. Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 70.Hansra G., Garcia-Paramio P., Prevostel C., Whelan R.D., Bornancin F., Parker P.J. Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem. J. 1999;342:337–344. [PMC free article] [PubMed] [Google Scholar]
- 71.Abrahamsen H., O'Neill A.K., Kannan N., Kruse N., Taylor S.S., Jennings P.A., et al. Peptidyl-prolyl isomerase Pin1 controls down-regulation of conventional protein kinase C isozymes. J. Biol. Chem. 2012;287:13262–13278. doi: 10.1074/jbc.M112.349753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lum M.A., Balaburski G.M., Murphy M.E., Black A.R., Black J.D. Heat shock proteins regulate activation-induced proteasomal degradation of the mature phosphorylated form of protein kinase C. J. Biol. Chem. 2013;288:27112–27127. doi: 10.1074/jbc.M112.437095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivastava J., Procyk K.J., Iturrioz X., Parker P.J. Phosphorylation is required for PMA- and cell-cycle-induced degradation of protein kinase Cdelta. Biochem. J. 2002;368:349–355. doi: 10.1042/BJ20020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao T., Newton A.C. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J. Biol. Chem. 2002;277:31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- 75.Cameron A.J., Escribano C., Saurin A.T., Kostelecky B., Parker P.J. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat. Struct. Mol. Biol. 2009;16:624–630. doi: 10.1038/nsmb.1606. [DOI] [PubMed] [Google Scholar]
- 76.Maccario H., Junoy B., Poulin B., Boyer B., Enjalbert A., Drouva S.V. Protein kinase Cdelta as gonadotropin-releasing hormone target isoenzyme in the alphaT3-1 gonadotrope cell line. Neuroendocrinology. 2004;79:204–220. doi: 10.1159/000078102. [DOI] [PubMed] [Google Scholar]
- 77.Olivier A.R., Parker P.J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J. Biol. Chem. 1994;269:2758–2763. [PubMed] [Google Scholar]
- 78.Kiley S.C., Parker P.J., Fabbro D., Jaken S. Differential regulation of protein kinase C isozymes by thyrotropin-releasing hormone in GH4C1 cells. J. Biol. Chem. 1991;266:23761–23768. [PubMed] [Google Scholar]
- 79.Chiu T., Santiskulvong C., Rozengurt E. ANG II stimulates PKC-dependent ERK activation, DNA synthesis, and cell division in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G1–G11. doi: 10.1152/ajpgi.00419.2002. [DOI] [PubMed] [Google Scholar]
- 80.Szallasi Z., Denning M.F., Smith C.B., Dlugosz A.A., Yuspa S.H., Pettit G.R., et al. Bryostatin 1 protects protein kinase C-delta from down-regulation in mouse keratinocytes in parallel with its inhibition of phorbol ester-induced differentiation. Mol. Pharmacol. 1994;46:840–850. [PubMed] [Google Scholar]
- 81.Szallasi Z., Smith C.B., Pettit G.R., Blumberg P.M. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J. Biol. Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- 82.Newton A.C. Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kajimoto T., Sawamura S., Tohyama Y., Mori Y., Newton A.C. Protein kinase C {delta}-specific activity reporter reveals agonist-evoked nuclear activity controlled by Src family of kinases. J. Biol. Chem. 2010;285:41896–41910. doi: 10.1074/jbc.M110.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kajimoto T., Shirai Y., Sakai N., Yamamoto T., Matsuzaki H., Kikkawa U., et al. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J. Biol. Chem. 2004;279:12668–12676. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- 85.Jaken S., Parker P.J. Protein kinase C binding partners. Bioessays. 2000;22:245–254. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 86.Hsu A.H., Lum M.A., Shim K.S., Frederick P.J., Morrison C.D., Chen B., et al. Crosstalk between PKCalpha and PI3K/AKT signaling is tumor suppressive in the endometrium. Cell Rep. 2018;24:655–669. doi: 10.1016/j.celrep.2018.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jerome-Morais A., Rahn H.R., Tibudan S.S., Denning M.F. Role for protein kinase C-alpha in keratinocyte growth arrest. J. Invest. Dermatol. 2009;129:2365–2375. doi: 10.1038/jid.2009.74. [DOI] [PubMed] [Google Scholar]
- 88.Saxon M.L., Zhao X., Black J.D. Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ. J. Cell Biol. 1994;126:747–763. doi: 10.1083/jcb.126.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedman B., Frackelton A.R., Jr., Ross A.H., Connors J.M., Fujiki H., Sugimura T., et al. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc. Natl. Acad. Sci. U. S. A. 1984;81:3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao J., Alroy I., Waterman H., Schejter E.D., Brodie C., Gruenberg J., et al. Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J. Biol. Chem. 2000;275:26178–26186. doi: 10.1074/jbc.M002367200. [DOI] [PubMed] [Google Scholar]
- 91.Lin C. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986;44:839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- 92.Hunter T., Ling N., Cooper J.A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984;311:480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- 93.Santiskulvong C., Rozengurt E. Protein kinase Cα mediates feedback inhibition of EGF receptor transactivation induced by Gq-coupled receptor agonists. Cell Signal. 2007;19:1348–1357. doi: 10.1016/j.cellsig.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Koese M., Rentero C., Kota B.P., Hoque M., Cairns R., Wood P., et al. Annexin A6 is a scaffold for PKCα to promote EGFR inactivation. Oncogene. 2013;32:2858–2872. doi: 10.1038/onc.2012.303. [DOI] [PubMed] [Google Scholar]
- 95.Macdonald-Obermann J.L., Pike L.J. The intracellular juxtamembrane domain of the epidermal growth factor (EGF) receptor is responsible for the allosteric regulation of EGF binding. J. Biol. Chem. 2009;284:13570–13576. doi: 10.1074/jbc.M109.001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ouyang X., Gulliford T., Zhang H., Huang G.C., Epstein R. Human cancer cells exhibit protein kinase C-dependent c-erbB-2 transmodulation that correlates with phosphatase sensitivity and kinase activity. J. Biol. Chem. 1996;271:21786–21792. doi: 10.1074/jbc.271.36.21786. [DOI] [PubMed] [Google Scholar]
- 97.Ouyang X., Gulliford T., Epstein R.J. The duration of phorbol-inducible ErbB2 tyrosine dephosphorylation parallels that of receptor endocytosis rather than threonine-686 phosphorylation: implications for the physiological role of protein kinase C in growth factor receptor signalling. Carcinogenesis. 1998;19:2013–2019. doi: 10.1093/carcin/19.11.2013. [DOI] [PubMed] [Google Scholar]
- 98.Li L., Lorenzo P.S., Bogi K., Blumberg P.M., Yuspa S.H. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol. Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acin-Perez R., Hoyos B., Gong J., Vinogradov V., Fischman D.A., Leitges M., et al. Regulation of intermediary metabolism by the PKCdelta signalosome in mitochondria. FASEB J. 2010;24:5033–5042. doi: 10.1096/fj.10-166934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu-Zhang A.X., Murphy A.N., Bachman M., Newton A.C. Isozyme-specific interaction of protein kinase Cdelta with mitochondria dissected using live cell fluorescence imaging. J. Biol. Chem. 2012;287:37891–37906. doi: 10.1074/jbc.M112.412635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomel R., Xiang C., Finniss S., Lee H.K., Lu W., Okhrimenko H., et al. The localization of protein kinase Cdelta in different subcellular sites affects its proapoptotic and antiapoptotic functions and the activation of distinct downstream signaling pathways. Mol. Cancer Res. 2007;5:627–639. doi: 10.1158/1541-7786.MCR-06-0255. [DOI] [PubMed] [Google Scholar]
- 102.Cheng J., He S., Wang M., Zhou L., Zhang Z., Feng X., et al. The caspase-3/PKCδ/Akt/VEGF-A signaling pathway mediates tumor repopulation during radiotherapy. Clin. Cancer Res. 2019;25:3732–3743. doi: 10.1158/1078-0432.CCR-18-3001. [DOI] [PubMed] [Google Scholar]
- 103.Iwamoto T., Hagiwara M., Hidaka H., Isomura T., Kioussis D., Nakashima I. Accelerated proliferation and interleukin-2 production of thymocytes by stimulation of soluble anti-CD3 monoclonal antibody in transgenic mice carrying a rabbit protein kinase Cϵ. J. Biol. Chem. 1992;267:18644–18648. [PubMed] [Google Scholar]
- 104.Pfeifhofer C., Gruber T., Letschka T., Thuille N., Lutz-Nicoladoni C., Hermann-Kleiter N., et al. Defective IgG2a/2b class switching in PKCα-/- mice. J. Immunol. 2006;176:6004–6011. doi: 10.4049/jimmunol.176.10.6004. [DOI] [PubMed] [Google Scholar]
- 105.Haslauer M., Baltensperger K., Porzig H. Erythropoietin- and stem cell factor-induced DNA synthesis in normal human erythroid progenitor cells requires activation of protein kinase Cα and is strongly inhibited by thrombin. Blood. 1999;94:114–126. [PubMed] [Google Scholar]
- 106.Pierce A., Heyworth C.M., Nicholls S.E., Spooncer E., Dexter T.M., Lord J.M., et al. An activated protein kinase C α gives a differentiation signal for hematopoietic progenitor cells and mimicks macrophage colony-stimulating factor-stimulated signaling events. J. Cell Biol. 1998;140:1511–1518. doi: 10.1083/jcb.140.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mischak H., Pierce J.H., Goodnight J., Kazanietz M.G., Blumberg P.M., Mushinski J.F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ and not by protein kinase CβII, -ε, -ζ, and -η. J. Biol. Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 108.Frey M.R., Clark J.A., Leontieva O., Uronis J.M., Black A.R., Black J.D. Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J. Cell Biol. 2000;151:763–778. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frey M.R., Saxon M.L., Zhao X., Rollins A., Evans S.S., Black J.D. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21waf1/cip1 and p27kip1 and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J. Biol. Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 110.Verstovsek G., Byrd A., Frey M.R., Petrelli N.J., Black J.D. Colonocyte differentiation is associated with increased expression and altered distribution of protein kinase C isozymes. Gastroenterology. 1998;115:75–85. doi: 10.1016/s0016-5085(98)70367-1. [DOI] [PubMed] [Google Scholar]
- 111.Hao F., Pysz M.A., Curry K.J., Haas K.N., Seedhouse S.J., Black A.R., et al. Protein kinase Cα signaling regulates inhibitor of DNA binding 1 in the intestinal epithelium. J. Biol. Chem. 2011;286:18104–18117. doi: 10.1074/jbc.M110.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tibudan S.S., Wang Y., Denning M.F. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J. Invest. Dermatol. 2002;119:1282–1289. doi: 10.1046/j.1523-1747.2002.19625.x. [DOI] [PubMed] [Google Scholar]
- 113.Bollag W.B. Protein kinase Cα puts the hand cuffs on epidermal keratinocyte proliferation. J. Invest. Dermatol. 2009;129:2330–2332. doi: 10.1038/jid.2009.165. [DOI] [PubMed] [Google Scholar]
- 114.Denning M.F., Dlugosz A.A., Williams E.K., Szallasi Z., Blumberg P.M., Yuspa S.H. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- 115.Yang L.C., Ng D.C., Bikle D.D. Role of protein kinase C α in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J. Cell Physiol. 2003;195:249–259. doi: 10.1002/jcp.10248. [DOI] [PubMed] [Google Scholar]
- 116.Chen Z., Forman L.W., Miller K.A., English B., Takashima A., Bohacek R.A., et al. Protein kinase Cdelta inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer. 2011;18:759–771. doi: 10.1530/ERC-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Z., Forman L.W., Williams R.M., Faller D.V. Protein kinase C-δ inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer. 2014;14:90. doi: 10.1186/1471-2407-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garavello N.M., Pena D.A., Bonatto J.M., Duarte M.L., Costa-Junior H.M., Schumacher R.I., et al. Activation of protein kinase C delta by psideltaRACK peptide promotes embryonic stem cell proliferation through ERK 1/2. J. Proteomics. 2013;94:497–512. doi: 10.1016/j.jprot.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 119.Grossoni V.C., Falbo K.B., Kazanietz M.G., de Kier Joffe E.D., Urtreger A.J. Protein kinase C delta enhances proliferation and survival of murine mammary cells. Mol. Carcinog. 2007;46:381–390. doi: 10.1002/mc.20287. [DOI] [PubMed] [Google Scholar]
- 120.Lei Z., Wang J., Sun W., Chen X., Jiao W., Zhang H., et al. PKCδ reveals a tumor promoter function by promoting cell proliferation and migration in somatotropinomas. Int. J. Clin. Exp. Pathol. 2018;11:208–215. [PMC free article] [PubMed] [Google Scholar]
- 121.Berardi D.E., Flumian C., Rodriguez C.E., Bessone M.I., Cirigliano S.M., Joffé E.D., et al. PKCδ inhibition impairs mammary cancer proliferative capacity but selects cancer stem cells, involving autophagy. J. Cell Biochem. 2016;117:730–740. doi: 10.1002/jcb.25358. [DOI] [PubMed] [Google Scholar]
- 122.Cerda S.R., Bissonnette M., Scaglione-Sewell B., Lyons M.R., Khare S., Mustafi R., et al. PKC-delta inhibits anchorage-dependent and -independent growth, enhances differentiation, and increases apoptosis in CaCo-2 cells. Gastroenterology. 2001;120:1700–1712. doi: 10.1053/gast.2001.24843. [DOI] [PubMed] [Google Scholar]
- 123.Cerda S.R., Mustafi R., Little H., Cohen G., Khare S., Moore C., et al. Protein kinase C delta inhibits Caco-2 cell proliferation by selective changes in cell cycle and cell death regulators. Oncogene. 2006;25:3123–3138. doi: 10.1038/sj.onc.1209360. [DOI] [PubMed] [Google Scholar]
- 124.Chew Y.C., Adhikary G., Wilson G.M., Reece E.A., Eckert R.L. Protein kinase C (PKC) delta suppresses keratinocyte proliferation by increasing p21(Cip1) level by a KLF4 transcription factor-dependent mechanism. J. Biol. Chem. 2011;286:28772–28782. doi: 10.1074/jbc.M110.205245. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Hernández-Maqueda J.G., Luna-Ulloa L.B., Santoyo-Ramos P., Castañeda-Patlán M.C., Robles-Flores M. Protein kinase C delta negatively modulates canonical Wnt pathway and cell proliferation in colon tumor cell lines. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 127.Allen-Petersen B.L., Carter C.J., Ohm A.M., Reyland M.E. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene. 2014;33:1306–1315. doi: 10.1038/onc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Symonds J.M., Ohm A.M., Carter C.J., Heasley L.E., Boyle T.A., Franklin W.A., et al. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011;71:2087–2097. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mauro L.V., Grossoni V.C., Urtreger A.J., Yang C., Colombo L.L., Morandi A., et al. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas. 2010;39:e31–e41. doi: 10.1097/MPA.0b013e3181bce796. [DOI] [PubMed] [Google Scholar]
- 130.Kitamura K., Mizuno K., Etoh A., Akita Y., Miyamoto A., Nakayama K., et al. The second phase activation of protein kinase C delta at late G1 is required for DNA synthesis in serum-induced cell cycle progression. Genes Cells. 2003;8:311–324. doi: 10.1046/j.1365-2443.2003.00635.x. [DOI] [PubMed] [Google Scholar]
- 131.Santiago-Walker A.E., Fikaris A.J., Kao G.D., Brown E.J., Kazanietz M.G., Meinkoth J.L. Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J. Biol. Chem. 2005;280:32107–32114. doi: 10.1074/jbc.M504432200. [DOI] [PubMed] [Google Scholar]
- 132.Hsieh H.L., Sun C.C., Wang T.S., Yang C.M. PKC-delta/c-Src-mediated EGF receptor transactivation regulates thrombin-induced COX-2 expression and PGE(2) production in rat vascular smooth muscle cells. Biochim. Biophys. Acta. 2008;1783:1563–1575. doi: 10.1016/j.bbamcr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 133.Hsieh H.L., Tung W.H., Wu C.Y., Wang H.H., Lin C.C., Wang T.S., et al. Thrombin induces EGF receptor expression and cell proliferation via a PKC(delta)/c-Src-dependent pathway in vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 2009;29:1594–1601. doi: 10.1161/ATVBAHA.109.185801. [DOI] [PubMed] [Google Scholar]
- 134.Aihara H., Asaoka Y., Yoshida K., Nishizuka Y. Sustained activation of protein kinase C is essential to HL-60 cell differentiation to macrophage. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11062–11066. doi: 10.1073/pnas.88.24.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clark J.A., Black A.R., Leontieva O.V., Frey M.R., Pysz M.A., Kunneva L., et al. Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J. Biol. Chem. 2004;279:9233–9247. doi: 10.1074/jbc.M312268200. [DOI] [PubMed] [Google Scholar]
- 136.Oster H., Leitges M. Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 2006;66:6955–6963. doi: 10.1158/0008-5472.CAN-06-0268. [DOI] [PubMed] [Google Scholar]
- 137.Leitges M., Plomann M., Standaert M.L., Bandyopadhyay G., Sajan M.P., Kanoh Y., et al. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol. Endocrinol. 2002;16:847–858. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- 138.Oriente F., Andreozzi F., Romano C., Perruolo G., Perfetti A., Fiory F., et al. Protein kinase C-α regulates insulin action and degradation by interacting with insulin receptor substrate-1 and 14-3-3ε. J. Biol. Chem. 2005;280:40642–40649. doi: 10.1074/jbc.M508570200. [DOI] [PubMed] [Google Scholar]
- 139.Caruso M., Miele C., Oriente F., Maitan A., Bifulco G., Andreozzi F., et al. In L6 skeletal muscle cells, glucose induces cytosolic translocation of protein kinase C-α and trans-activates the insulin receptor kinase. J. Biol. Chem. 1999;274:28637–28644. doi: 10.1074/jbc.274.40.28637. [DOI] [PubMed] [Google Scholar]
- 140.Nawaratne R., Gray A., Jorgensen C.H., Downes C.P., Siddle K., Sethi J.K. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol. Endocrinol. 2006;20:1838–1852. doi: 10.1210/me.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Detjen K.M., Brembeck F.H., Welzel M., Kaiser A., Haller H., Wiedenmann B., et al. Activation of protein kinase Cα inhibits growth of pancreatic cancer cells via p21cip-mediated G1 arrest. J. Cell Sci. 2000;113:3025–3035. doi: 10.1242/jcs.113.17.3025. [DOI] [PubMed] [Google Scholar]
- 142.Sun X.G., Rotenberg S.A. Overexpression of protein kinase Cα in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ. 1999;10:343–352. [PubMed] [Google Scholar]
- 143.Pysz M.A., Leontieva O.V., Bateman N.W., Uronis J.M., Curry K.J., Threadgill D.W., et al. PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 2009;315:1415–1428. doi: 10.1016/j.yexcr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hizli A.A., Black A.R., Pysz M.A., Black J.D. Protein kinase C α signaling inhibits cyclin D1 translation in intestinal epithelial cells. J. Biol. Chem. 2006;281:14596–14603. doi: 10.1074/jbc.M601959200. [DOI] [PubMed] [Google Scholar]
- 145.Guan L., Song K., Pysz M.A., Curry K.J., Hizli A.A., Danielpour D., et al. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J. Biol. Chem. 2007;282:14213–14225. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 146.Wu T.T., Hsieh Y.H., Hsieh Y.S., Liu J.Y. Reduction of PKCα decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J. Cell Biochem. 2008;103:9–20. doi: 10.1002/jcb.21378. [DOI] [PubMed] [Google Scholar]
- 147.Oliva J.L., Caino M.C., Senderowicz A.M., Kazanietz M.G. S-Phase-specific activation of PKC alpha induces senescence in non-small cell lung cancer cells. J. Biol. Chem. 2008;283:5466–5476. doi: 10.1074/jbc.M707576200. [DOI] [PubMed] [Google Scholar]
- 148.Liu Y., Deng X., Wu D., Jin M., Yu B. PKCdelta promotes fertilization of mouse embryos in early development via the Cdc25B signaling pathway. Exp. Ther. Med. 2019;18:3281–3290. doi: 10.3892/etm.2019.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leitges M., Mayr M., Braun U., Mayr U., Li C., Pfister G., et al. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J. Clin. Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mecklenbrauker I., Saijo K., Zheng N.Y., Leitges M., Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–865. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- 151.Nakagawa M., Oliva J.L., Kothapalli D., Fournier A., Assoian R.K., Kazanietz M.G. Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cdelta-dependent induction of p21. J. Biol. Chem. 2005;280:33926–33934. doi: 10.1074/jbc.M505748200. [DOI] [PubMed] [Google Scholar]
- 152.Watanabe T., Ono Y., Taniyama Y., Hazama K., Igarashi K., Ogita K., et al. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koike K., Fujii T., Nakamura A.M., Yokoyama G., Yamana H., Kuwano M., et al. Activation of protein kinase C delta induces growth arrest in NPA thyroid cancer cells through extracellular signal-regulated kinase mitogen-activated protein kinase. Thyroid. 2006;16:333–341. doi: 10.1089/thy.2006.16.333. [DOI] [PubMed] [Google Scholar]
- 154.Perletti G., Marras E., Dondi D., Osti D., Congiu T., Ferrarese R., et al. p21(Waf1/Cip1) and p53 are downstream effectors of protein kinase C delta in tumor suppression and differentiation in human colon cancer cells. Int. J. Cancer. 2005;113:42–53. doi: 10.1002/ijc.20535. [DOI] [PubMed] [Google Scholar]
- 155.Ranta F., Leveringhaus J., Theilig D., Schulz-Raffelt G., Hennige A.M., Hildebrand D.G., et al. Protein kinase C delta (PKCdelta) affects proliferation of insulin-secreting cells by promoting nuclear extrusion of the cell cycle inhibitor p21Cip1/WAF1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soh J.W., Weinstein I.B. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J. Biol. Chem. 2003;278:34709–34716. doi: 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- 157.Ashton A.W., Watanabe G., Albanese C., Harrington E.O., Ware J.A., Pestell R.G. Protein kinase Cdelta inhibition of S-phase transition in capillary endothelial cells involves the cyclin-dependent kinase inhibitor p27(Kip1) J. Biol. Chem. 1999;274:20805–20811. doi: 10.1074/jbc.274.30.20805. [DOI] [PubMed] [Google Scholar]
- 158.Vucenik I., Ramakrishna G., Tantivejkul K., Anderson L.M., Ramljak D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res. Treat. 2005;91:35–45. doi: 10.1007/s10549-004-6456-5. [DOI] [PubMed] [Google Scholar]
- 159.Oh Y.T., Chun K.H., Park B.D., Choi J.S., Lee S.K. Regulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1 by protein kinase Cdelta-mediated phosphorylation. Apoptosis. 2007;12:1339–1347. doi: 10.1007/s10495-007-0066-8. [DOI] [PubMed] [Google Scholar]
- 160.Oh Y.T., Chun K.H., Oh J.I., Park J.A., Kim Y.U., Lee S.K. PKCdelta modulates p21WAF1/CIP1 ability to bind to Cdk2 during TNFalpha-induced apoptosis. Biochem. Biophys. Res. Commun. 2006;339:1138–1147. doi: 10.1016/j.bbrc.2005.11.121. [DOI] [PubMed] [Google Scholar]
- 161.Lee S.L., Hong S.W., Shin J.S., Kim J.S., Ko S.G., Hong N.J., et al. p34SEI-1 inhibits doxorubicin-induced senescence through a pathway mediated by protein kinase C-delta and c-Jun-NH2-kinase 1 activation in human breast cancer MCF7 cells. Mol. Cancer Res. 2009;7:1845–1853. doi: 10.1158/1541-7786.MCR-09-0086. [DOI] [PubMed] [Google Scholar]
- 162.Patel R., Apostolatos A., Carter G., Ajmo J., Gali M., Cooper D.R., et al. Protein kinase C delta (PKCdelta) splice variants modulate apoptosis pathway in 3T3L1 cells during adipogenesis: identification of PKCdeltaII inhibitor. J. Biol. Chem. 2013;288:26834–26846. doi: 10.1074/jbc.M113.482638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patel R.S., Carter G., El Bassit G., Patel A.A., Cooper D.R., Murr M., et al. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: role of protein kinase C delta (PKCδ) in adipose stem cell niche. Stem Cell Investig. 2016;3:2. doi: 10.3978/j.issn.2306-9759.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Takahashi A., Ohtani N., Yamakoshi K., Iida S., Tahara H., Nakayama K., et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 165.Katakura Y., Udono M., Katsuki K., Nishide H., Tabira Y., Ikei T., et al. Protein kinase C delta plays a key role in cellular senescence programs of human normal diploid cells. J. Biochem. 2009;146:87–93. doi: 10.1093/jb/mvp046. [DOI] [PubMed] [Google Scholar]
- 166.Byun H.O., Jung H.J., Kim M.J., Yoon G. PKCdelta phosphorylation is an upstream event of GSK3 inactivation-mediated ROS generation in TGF-beta1-induced senescence. Free Radic. Res. 2014;48:1100–1108. doi: 10.3109/10715762.2014.929120. [DOI] [PubMed] [Google Scholar]
- 167.Kermorgant S., Zicha D., Parker P.J. PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J. 2004;23:3721–3734. doi: 10.1038/sj.emboj.7600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andreozzi F., Melillo R.M., Carlomagno F., Oriente F., Miele C., Fiory F., et al. Protein kinase Cα activation by RET: evidence for a negative feedback mechanism controlling RET tyrosine kinase. Oncogene. 2003;22:2942–2949. doi: 10.1038/sj.onc.1206475. [DOI] [PubMed] [Google Scholar]
- 169.Morrison P., Takishima K., Rosner M.R. Role of threonine residues in regulation of the epidermal growth factor receptor by protein kinase C and mitogen-activated protein kinase. J. Biol. Chem. 1993;268:15536–15543. [PubMed] [Google Scholar]
- 170.Bailey T.A., Luan H., Tom E., Bielecki T.A., Mohapatra B., Ahmad G., et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J. Biol. Chem. 2014;289:30443–30458. doi: 10.1074/jbc.M114.608992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bakker J., Spits M., Neefjes J., Berlin I. The EGFR odyssey - from activation to destruction in space and time. J. Cell Sci. 2017;130:4087–4096. doi: 10.1242/jcs.209197. [DOI] [PubMed] [Google Scholar]
- 172.Idkowiak-Baldys J., Becker K.P., Kitatani K., Hannun Y.A. Dynamic sequestration of the recycling compartment by classical protein kinase C. J. Biol. Chem. 2006;281:22321–22331. doi: 10.1074/jbc.M512540200. [DOI] [PubMed] [Google Scholar]
- 173.Lund K.A., Lazar C.S., Chen W.S., Walsh B.J., Welsh J.B., Herbst J.J., et al. Phosphorylation of the epidermal growth factor receptor at threonine 654 inhibits ligand-induced internalization and down-regulation. J. Biol. Chem. 1990;265:20517–20523. [PubMed] [Google Scholar]
- 174.Wang X.Q., Yan Q., Sun P., Liu J.W., Go L., McDaniel S.M., et al. Suppression of epidermal growth factor receptor signaling by protein kinase C-alpha activation requires CD82, caveolin-1, and ganglioside. Cancer Res. 2007;67:9986–9995. doi: 10.1158/0008-5472.CAN-07-1300. [DOI] [PubMed] [Google Scholar]
- 175.Elnakat H., Gonit M., Salazar M.D., Zhang J., Basrur V., Gunning W., et al. Regulation of folate receptor internalization by protein kinase C alpha. Biochemistry. 2009;48:8249–8260. doi: 10.1021/bi900565t. [DOI] [PubMed] [Google Scholar]
- 176.Tanaka Y., Gavrielides M.V., Mitsuuchi Y., Fujii T., Kazanietz M.G. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 177.Hoshino D., Jourquin J., Emmons S.W., Miller T., Goldgof M., Costello K., et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCα invasive signaling Axis. Sci. Signal. 2012;5 doi: 10.1126/scisignal.2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sipeki S., Bander E., Parker P.J., Farago A. PKCα reduces the lipid kinase activity of the p110α/p85α PI3K through the phosphorylation of the catalytic subunit. Biochem. Biophys. Res. Commun. 2006;339:122–125. doi: 10.1016/j.bbrc.2005.10.194. [DOI] [PubMed] [Google Scholar]
- 179.Pysz M.A., Hao F., Hizli A.A., Lum M.A., Swetzig W.M., Black A.R., et al. Differential regulation of cyclin D1 expression by protein kinase C α and ε signaling in intestinal epithelial cells. J. Biol. Chem. 2014;289:22268–22283. doi: 10.1074/jbc.M114.571554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaur N., Lum M.A., Lewis R.E., Black A.R., Black J.D. A novel antiproliferative PKCα-Ras-ERK signaling axis in intestinal epithelial cells. J. Biol. Chem. 2022;298:102121–102142. doi: 10.1016/j.jbc.2022.102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wen-Sheng W., Jun-Ming H. Activation of protein kinase C alpha is required for TPA-triggered ERK (MAPK) signaling and growth inhibition of human hepatoma cell HepG2. J. Biomed. Sci. 2005;12:289–296. doi: 10.1007/s11373-005-1210-5. [DOI] [PubMed] [Google Scholar]
- 182.Cummings R., Zhao Y., Jacoby D., Spannhake E.W., Ohba M., Garcia J.G., et al. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J. Biol. Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 183.Jackson T.A., Koterwas D.M., Morgan M.A., Bradford A.P. Fibroblast growth factors regulate prolactin transcription via an atypical Rac-dependent signaling pathway. Mol. Endocrinol. 2003;17:1921–1930. doi: 10.1210/me.2003-0167. [DOI] [PubMed] [Google Scholar]
- 184.Jamison J., Wang J.H., Wells A. PKCδ regulates force signaling during VEGF/CXCL4 induced dissociation of endothelial tubes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Redig A.J., Platanias L.C. The protein kinase C (PKC) family of proteins in cytokine signaling in hematopoiesis. J. Interferon Cytokine Res. 2007;27:623–636. doi: 10.1089/jir.2007.0007. [DOI] [PubMed] [Google Scholar]
- 186.Xia S., Chen Z., Forman L.W., Faller D.V. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009;21:502–508. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen Y.J., Tsai R.K., Wu W.C., He M.S., Kao Y.H., Wu W.S. Enhanced PKCδ and ERK signaling mediate cell migration of retinal pigment epithelial cells synergistically induced by HGF and EGF. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu Y., Jamieson L., Brasier A.R., Fields A.P. NF-kappaB/RelA transactivation is required for atypical protein kinase C iota-mediated cell survival. Oncogene. 2001;20:4777–4792. doi: 10.1038/sj.onc.1204607. [DOI] [PubMed] [Google Scholar]
- 189.Kveiborg M., Instrell R., Rowlands C., Howell M., Parker P.J. PKCalpha and PKCdelta regulate ADAM17-mediated ectodomain shedding of heparin binding-EGF through separate pathways. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hu C.T., Cheng C.C., Pan S.M., Wu J.R., Wu W.S. PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2. Cell Signal. 2013;25:1457–1467. doi: 10.1016/j.cellsig.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 191.Llado A., Tebar F., Calvo M., Moreto J., Sorkin A., Enrich C. Protein kinaseCdelta-calmodulin crosstalk regulates epidermal growth factor receptor exit from early endosomes. Mol. Biol. Cell. 2004;15:4877–4891. doi: 10.1091/mbc.E04-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Park M., Kim W.K., Song M., Park M., Kim H., Nam H.J., et al. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clin. Cancer Res. 2013;19:4961–4971. doi: 10.1158/1078-0432.CCR-13-0131. [DOI] [PubMed] [Google Scholar]
- 193.Jia G., Wang R., Yue Y., Dai H. Activation of protein kinase cdelta contributes to the induction of Src/EGF receptor/ERK signaling in Ammonia-treated astrocytes. J. Mol. Neurosci. 2020;70:1110–1119. doi: 10.1007/s12031-020-01517-8. [DOI] [PubMed] [Google Scholar]
- 194.Caunt C.J., Rivers C.A., Conway-Campbell B.L., Norman M.R., McArdle C.A. Epidermal growth factor receptor and protein kinase C signaling to ERK2: spatiotemporal regulation of ERK2 by dual specificity phosphatases. J. Biol. Chem. 2008;283:6241–6252. doi: 10.1074/jbc.M706624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Limnander A., Depeille P., Freedman T.S., Liou J., Leitges M., Kurosaki T., et al. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat. Immunol. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Noh K.T., Son K.H., Jung I.D., Kang H.K., Hwang S.A., Lee W.S., et al. Protein kinase C delta (PKCdelta)-extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade regulates glycogen synthase kinase-3 (GSK-3) inhibition-mediated interleukin-10 (IL-10) expression in lipopolysaccharide (LPS)-induced endotoxemia. J. Biol. Chem. 2012;287:14226–14233. doi: 10.1074/jbc.M111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ohm A.M., Affandi T., Reyland M.E. EGF receptor and PKCdelta kinase activate DNA damage-induced pro-survival and pro-apoptotic signaling via biphasic activation of ERK and MSK1 kinases. J. Biol. Chem. 2019;294:4488–4497. doi: 10.1074/jbc.RA118.006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ohm A.M., Tan A.C., Heasley L.E., Reyland M.E. Co-Dependency of PKCdelta and K-ras: inverse association with cytotoxic drug sensitivity in KRAS mutant lung cancer. Oncogene. 2017;36:4370–4378. doi: 10.1038/onc.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xia S., Forman L.W., Faller D.V. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J. Biol. Chem. 2007;282:13199–13210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee P.C., Fang Y.F., Yamaguchi H., Wang W.J., Chen T.C., Hong X., et al. Targeting PKCdelta as a therapeutic strategy against heterogeneous mechanisms of EGFR inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell. 2018;34:954–969.e4. doi: 10.1016/j.ccell.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mandal J.P., Shiue C.N., Chen Y.C., Lee M.C., Yang H.H., Chang H.H., et al. PKCdelta mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free Radic. Biol. Med. 2021;163:69–87. doi: 10.1016/j.freeradbiomed.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 202.Hocevar B.A., Morrow D.M., Tykocinski M.L., Fields A.P. Protein kinase C isotypes in human erythroleukemia cell proliferation and differentiation. J. Cell Sci. 1992;101:671–679. doi: 10.1242/jcs.101.3.671. [DOI] [PubMed] [Google Scholar]
- 203.Murray N.R., Baumgardner G.P., Burns D.J., Fields A.P. Protein kinase C isotypes in human erythroleukemia (K562) cell proliferation and differentiation. Evidence that βII protein kinase C is required for proliferation. J. Biol. Chem. 1993;268:15847–15853. [PubMed] [Google Scholar]
- 204.Walker S.D., Murray N.R., Burns D.J., Fields A.P. Protein kinase C chimeras: catalytic domains of α and βII protein kinase C contain determinants for isotype-specific function. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9156–9160. doi: 10.1073/pnas.92.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dieter P., Schwende H. Protein kinase C-α and -β play antagonistic roles in the differentiation process of THP-1 cells. Cell Signal. 2000;12:297–302. doi: 10.1016/s0898-6568(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 206.Rutberg S.E., Saez E., Glick A., Dlugosz A.A., Spiegelman B.M., Yuspa S.H. Differentiation of mouse keratinocytes is accompanied by PKC-dependent changes in AP-1 proteins. Oncogene. 1996;13:167–176. [PubMed] [Google Scholar]
- 207.Rosato B., Ranieri D., Nanni M., Torrisi M.R., Belleudi F. Role of FGFR2b expression and signaling in keratinocyte differentiation: sequential involvement of PKCδ and PKCα. Cell Death Dis. 2018;9:565. doi: 10.1038/s41419-018-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deszo E.L., Brake D.K., Cengel K.A., Kelley K.W., Freund G.G. CD45 negatively regulates monocytic cell differentiation by inhibiting phorbol 12-myristate 13-acetate-dependent activation and tyrosine phosphorylation of protein kinase Cdelta. J. Biol. Chem. 2001;276:10212–10217. doi: 10.1074/jbc.M010589200. [DOI] [PubMed] [Google Scholar]
- 209.Feng X., Zhang J., Smuga-Otto K., Tian S., Yu J., Stewart R., et al. Protein kinase C mediated extraembryonic endoderm differentiation of human embryonic stem cells. Stem Cells. 2012;30:461–470. doi: 10.1002/stem.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Smyth D.C., Takenaka S., Yeung C., Richards C.D. Oncostatin M regulates osteogenic differentiation of murine adipose-derived mesenchymal progenitor cells through a PKCdelta-dependent mechanism. Cell Tissue Res. 2015;360:309–319. doi: 10.1007/s00441-014-2099-y. [DOI] [PubMed] [Google Scholar]
- 211.Reyland M.E., Jones D.N. Multifunctional roles of PKCdelta: opportunities for targeted therapy in human disease. Pharmacol. Ther. 2016;165:1–13. doi: 10.1016/j.pharmthera.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mandil R., Ashkenazi E., Blass M., Kronfeld I., Kazimirsky G., Rosenthal G., et al. Protein kinase Calpha and protein kinase Cdelta play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 2001;61:4612–4619. [PubMed] [Google Scholar]
- 213.Halder K., Banerjee S., Bose A., Majumder S., Majumdar S. Overexpressed PKCdelta downregulates the expression of PKCalpha in B16F10 melanoma: induction of apoptosis by PKCdelta via ceramide generation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091656. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Reyland M.E., Anderson S.M., Matassa A.A., Barzen K.A., Quissell D.O. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J. Biol. Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 215.Whelan R.D., Parker P.J. Loss of protein kinase C function induces an apoptotic response. Oncogene. 1998;16:1939–1944. doi: 10.1038/sj.onc.1201725. [DOI] [PubMed] [Google Scholar]
- 216.Leirdal M., Sioud M. Ribozyme inhibition of the protein kinase Cα triggers apoptosis in glioma cells. Br. J. Cancer. 1999;80:1558–1564. doi: 10.1038/sj.bjc.6690560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jiang X.H., Tu S.P., Cui J.T., Lin M.C., Xia H.H., Wong W.M., et al. Antisense targeting protein kinase C α and β1 inhibits gastric carcinogenesis. Cancer Res. 2004;64:5787–5794. doi: 10.1158/0008-5472.CAN-03-1172. [DOI] [PubMed] [Google Scholar]
- 218.Zhu B.H., Yao Z.X., Luo S.J., Jiang L.M., Xiao J.W., Liu S.C., et al. Effects of antisense oligonucleotides of PKC-α on proliferation and apoptosis of HepG2 in vitro. Hepatobiliary Pancreat. Dis. Int. 2005;4:75–79. [PubMed] [Google Scholar]
- 219.Hsieh Y.C., Jao H.C., Yang R.C., Hsu H.K., Hsu C. Suppression of protein kinase Cα triggers apoptosis through down-regulation of Bcl-xL in a rat hepatic epithelial cell line. Shock. 2003;19:582–587. doi: 10.1097/01.shk.0000065705.84144.ed. [DOI] [PubMed] [Google Scholar]
- 220.Ruvolo P.P., Deng X., Carr B.K., May W.S. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 221.Ruvolo P.P., Deng X., May W.S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- 222.Wang W.L., Yeh S.F., Huang E.Y., Lu Y.L., Wang C.F., Huang C.Y., et al. Mitochondrial anchoring of PKCα by PICK1 confers resistance to etoposide-induced apoptosis. Apoptosis. 2007;12:1857–1871. doi: 10.1007/s10495-007-0098-0. [DOI] [PubMed] [Google Scholar]
- 223.Jiffar T., Kurinna S., Suck G., Carlson-Bremer D., Ricciardi M.R., Konopleva M., et al. PKC α mediates chemoresistance in acute lymphoblastic leukemia through effects on Bcl2 phosphorylation. Leukemia. 2004;18:505–512. doi: 10.1038/sj.leu.2403275. [DOI] [PubMed] [Google Scholar]
- 224.Villar J., Quadri H.S., Song I., Tomita Y., Tirado O.M., Notario V. PCPH/ENTPD5 expression confers to prostate cancer cells resistance against cisplatin-induced apoptosis through protein kinase Cα-mediated Bcl-2 stabilization. Cancer Res. 2009;69:102–110. doi: 10.1158/0008-5472.CAN-08-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Joniova J., Misuth M., Sureau F., Miskovsky P., Nadova Z. Effect of PKCα expression on Bcl-2 phosphorylation and cell death by hypericin. Apoptosis. 2014;19:1779–1792. doi: 10.1007/s10495-014-1043-7. [DOI] [PubMed] [Google Scholar]
- 226.Lei J., Li Q., Gao Y., Zhao L., Liu Y. Increased PKCα activity by Rack1 overexpression is responsible for chemotherapy resistance in T-cell acute lymphoblastic leukemia-derived cell line. Sci. Rep. 2016;6:33717. doi: 10.1038/srep33717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cerioni L., Palomba L., Brune B., Cantoni O. Peroxynitrite-induced mitochondrial translocation of PKCα causes U937 cell survival. Biochem. Biophys. Res. Commun. 2006;339:126–131. doi: 10.1016/j.bbrc.2005.10.193. [DOI] [PubMed] [Google Scholar]
- 228.Zheng J., Kong C., Yang X., Cui X., Lin X., Zhang Z. Protein kinase C-α(PKCα) modulates cell apoptosis by stimulating nuclear translocation of NF-kappa-B p65 in urothelial cell carcinoma of the bladder. BMC Cancer. 2017;17:432. doi: 10.1186/s12885-017-3401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Smith S.D., Enge M., Bao W., Thullberg M., Costa T.D., Olofsson H., et al. Protein kinase Cα (PKCα) regulates p53 localization and melanoma cell survival downstream of integrin alphav in three-dimensional collagen and in vivo. J. Biol. Chem. 2012;287:29336–29347. doi: 10.1074/jbc.M112.341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jiang Z., Kong C., Zhang Z., Zhu Y., Zhang Y., Chen X. Reduction of protein kinase C α (PKC-α) promote apoptosis via down-regulation of Dicer in bladder cancer. J. Cell Mol. Med. 2015;19:1085–1093. doi: 10.1111/jcmm.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yuan Y., Yangmei Z., Rongrong S., Xiaowu L., Youwei Z., Sun S. Sotrastaurin attenuates the stemness of gastric cancer cells by targeting PKCδ. Biomed. Pharmacother. 2019;117:109165. doi: 10.1016/j.biopha.2019.109165. [DOI] [PubMed] [Google Scholar]
- 232.Kim R.K., Suh Y., Hwang E., Yoo K.C., Choi K.S., An S., et al. PKCδ maintains phenotypes of tumor initiating cells through cytokine-mediated autocrine loop with positive feedback. Oncogene. 2015;34:5749–5759. doi: 10.1038/onc.2015.29. [DOI] [PubMed] [Google Scholar]
- 233.Muselli F., Mourgues L., Morcos R., Rochet N., Nebout M., Guerci-Bresler A., et al. Combination of PKCdelta inhibition with conventional TKI treatment to target CML models. Cancers (Basel) 2021;13:1693. doi: 10.3390/cancers13071693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Masoumi K.C., Cornmark L., Lonne G.K., Hellman U., Larsson C. Identification of a novel protein kinase Cdelta-Smac complex that dissociates during paclitaxel-induced cell death. FEBS Lett. 2012;586:1166–1172. doi: 10.1016/j.febslet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 235.Holmgren C., Cornmark L., Lonne G.K., Masoumi K.C., Larsson C. Molecular characterization of protein kinase C delta (PKCdelta)-Smac interactions. BMC Biochem. 2016;17:11. doi: 10.1186/s12858-016-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Quadros M.R., Connelly S., Kari C., Abrams M.T., Wickstrom E., Rodeck U. EGFR-dependent downregulation of Bim in epithelial cells requires MAPK and PKC-delta activities. Cancer Biol. Ther. 2006;5:498–504. doi: 10.4161/cbt.5.5.2567. [DOI] [PubMed] [Google Scholar]
- 237.Webster C.R., Johnston A.N., Anwer M.S. Protein kinase Cdelta protects against bile acid apoptosis by suppressing proapoptotic JNK and BIM pathways in human and rat hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G1207–G1215. doi: 10.1152/ajpgi.00165.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Reyland M.E. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front. Biosci. (Landmark Ed.) 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Speidel J.T., Affandi T., Jones D.N.M., Ferrara S.E., Reyland M.E. Functional proteomic analysis reveals roles for PKCδ in regulation of cell survival and cell death: implications for cancer pathogenesis and therapy. Adv. Biol. Regul. 2020;78:100757. doi: 10.1016/j.jbior.2020.100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Basu A., Tu H. Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cdelta. Biochem. Biophys. Res. Commun. 2005;334:1068–1073. doi: 10.1016/j.bbrc.2005.06.199. [DOI] [PubMed] [Google Scholar]
- 241.Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 242.Ginnan R., Guikema B.J., Singer H.A., Jourd'heuil D. PKC-delta mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2006;290:C1583–C1591. doi: 10.1152/ajpcell.00390.2005. [DOI] [PubMed] [Google Scholar]
- 243.Powell C.T., Brittis N.J., Stec D., Hug H., Heston W.D., Fair W.R. Persistent membrane translocation of protein kinase C α during 12-0-tetradecanoylphorbol-13-acetate-induced apoptosis of LNCaP human prostate cancer cells. Cell Growth Differ. 1996;7:419–428. [PubMed] [Google Scholar]
- 244.Garcia-Bermejo M.L., Leskow F.C., Fujii T., Wang Q., Blumberg P.M., Ohba M., et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCα. J. Biol. Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 245.Santiago M.F., Perez-Reyes P.L., Lopez-Aparicio P., Recio M.N., Perez-Albarsanz M.A. Differential effects of PCBs on the induction of apoptosis machinery and PKCα translocation in rat renal tubular cell cultures. Toxicol. Lett. 2006;163:91–100. doi: 10.1016/j.toxlet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 246.Allen-Petersen B.L., Miller M.R., Neville M.C., Anderson S.M., Nakayama K.I., Reyland M.E. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis. 2010;1:e17. doi: 10.1038/cddis.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Humphries M.J., Limesand K.H., Schneider J.C., Nakayama K.I., Anderson S.M., Reyland M.E. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J. Biol. Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 248.Gordon R., Anantharam V., Kanthasamy A.G., Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J. Neuroinflammation. 2012;9:82. doi: 10.1186/1742-2094-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xu L., Su L., Liu X. PKCdelta regulates death receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis in human lung cancer cells. Mol. Cancer Ther. 2012;11:2174–2182. doi: 10.1158/1535-7163.MCT-12-0602. [DOI] [PubMed] [Google Scholar]
- 250.Gonzalez-Guerrico A.M., Kazanietz M.G. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J. Biol. Chem. 2005;280:38982–38991. doi: 10.1074/jbc.M506767200. [DOI] [PubMed] [Google Scholar]
- 251.Goncalves G.L., Costa-Pessoa J.M., Thieme K., Lins B.B., Oliveira-Souza M. Intracellular albumin overload elicits endoplasmic reticulum stress and PKC-delta/p38 MAPK pathway activation to induce podocyte apoptosis. Sci. Rep. 2018;8:18012. doi: 10.1038/s41598-018-36933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu C., Li H., Zheng H., Zhai M., Lu F., Dong S., et al. CaSR activates PKCdelta to induce cardiomyocyte apoptosis via ER stressassociated apoptotic pathways during ischemia/reperfusion. Int. J. Mol. Med. 2019;44:1117–1126. doi: 10.3892/ijmm.2019.4255. [DOI] [PubMed] [Google Scholar]
- 253.Qi X., Mochly-Rosen D. The PKCdelta -Abl complex communicates ER stress to the mitochondria - an essential step in subsequent apoptosis. J. Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- 254.Zheng H., Liu J., Liu C., Lu F., Zhao Y., Jin Z., et al. Calcium-sensing receptor activating phosphorylation of PKCdelta translocation on mitochondria to induce cardiomyocyte apoptosis during ischemia/reperfusion. Mol. Cell Biochem. 2011;358:335–343. doi: 10.1007/s11010-011-0984-1. [DOI] [PubMed] [Google Scholar]
- 255.Wie S.M., Wellberg E., Karam S.D., Reyland M.E. Tyrosine kinase inhibitors protect the salivary gland from radiation damage by inhibiting activation of protein kinase C-delta. Mol. Cancer Ther. 2017;16:1989–1998. doi: 10.1158/1535-7163.MCT-17-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Arany S., Benoit D.S., Dewhurst S., Ovitt C.E. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol. Ther. 2013;21:1182–1194. doi: 10.1038/mt.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pabla N., Dong Z. Curtailing side effects in chemotherapy: a tale of PKCdelta in cisplatin treatment. Oncotarget. 2012;3:107–111. doi: 10.18632/oncotarget.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Choi S.Y., Kim M.J., Kang C.M., Bae S., Cho C.K., Soh J.W., et al. Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J. Biol. Chem. 2006;281:7049–7059. doi: 10.1074/jbc.M512000200. [DOI] [PubMed] [Google Scholar]
- 259.Murriel C.L., Churchill E., Inagaki K., Szweda L.I., Mochly-Rosen D. Protein kinase cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J. Biol. Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 260.Sitailo L.A., Tibudan S.S., Denning M.F. Bax activation and induction of apoptosis in human keratinocytes by the protein kinase C delta catalytic domain. J. Invest. Dermatol. 2004;123:434–443. doi: 10.1111/j.0022-202X.2004.23403.x. [DOI] [PubMed] [Google Scholar]
- 261.Sitailo L.A., Tibudan S.S., Denning M.F. The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J. Biol. Chem. 2006;281:29703–29710. doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Anderson S.M., Reyland M.E., Hunter S., Deisher L.M., Barzen K.A., Quissell D.O. Etoposide-induced activation of c-jun N-terminal kinase (JNK) correlates with drug-induced apoptosis in salivary gland acinar cells. Cell Death Differ. 1999;6:454–462. doi: 10.1038/sj.cdd.4400507. [DOI] [PubMed] [Google Scholar]
- 263.Yoshida K., Miki Y., Kufe D. Activation of SAPK/JNK signaling by protein kinase Cdelta in response to DNA damage. J. Biol. Chem. 2002;277:48372–48378. doi: 10.1074/jbc.M205485200. [DOI] [PubMed] [Google Scholar]
- 264.Arango D., Parihar A., Villamena F.A., Wang L., Freitas M.A., Grotewold E., et al. Apigenin induces DNA damage through the PKCdelta-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem. Pharmacol. 2012;84:1571–1580. doi: 10.1016/j.bcp.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bharti A., Kraeft S.K., Gounder M., Pandey P., Jin S., Yuan Z.M., et al. Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Mol. Cell Biol. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.LaGory E.L., Sitailo L.A., Denning M.F. The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint. J. Biol. Chem. 2010;285:1879–1887. doi: 10.1074/jbc.M109.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lee Y.J., Soh J.W., Dean N.M., Cho C.K., Kim T.H., Lee S.J., et al. Protein kinase Cdelta overexpression enhances radiation sensitivity via extracellular regulated protein kinase 1/2 activation, abolishing the radiation-induced G(2)-M arrest. Cell Growth Differ. 2002;13:237–246. [PubMed] [Google Scholar]
- 268.Li B., Wang X., Rasheed N., Hu Y., Boast S., Ishii T., et al. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev. 2004;18:1824–1837. doi: 10.1101/gad.1223504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Soriano-Carot M., Quilis I., Bano M.C., Igual J.C. Protein kinase C controls activation of the DNA integrity checkpoint. Nucl. Acids Res. 2014;42:7084–7095. doi: 10.1093/nar/gku373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yoshida K., Wang H.G., Miki Y., Kufe D. Protein kinase Cdelta is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. EMBO J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Park C.H., Kim K.T. Apoptotic phosphorylation of histone H3 on Ser-10 by protein kinase Cdelta. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Qi H., Sun B., Zhao X., Du J., Gu Q., Liu Y., et al. Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase Cα in epithelial ovarian cancer. Oncol. Rep. 2014;32:771–779. doi: 10.3892/or.2014.3229. [DOI] [PubMed] [Google Scholar]
- 273.Llorens M.C., Rossi F.A., Garcia I.A., Cooke M., Abba M.C., Lopez-Haber C., et al. PKCα modulates epithelial-to-mesenchymal transition and invasiveness of breast cancer cells through ZEB1. Front. Oncol. 2019;9:1323. doi: 10.3389/fonc.2019.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kyuno D., Kojima T., Yamaguchi H., Ito T., Kimura Y., Imamura M., et al. Protein kinase Cα inhibitor protects against downregulation of claudin-1 during epithelial-mesenchymal transition of pancreatic cancer. Carcinogenesis. 2013;34:1232–1243. doi: 10.1093/carcin/bgt057. [DOI] [PubMed] [Google Scholar]
- 275.Black A.R., Black J.D. The complexities of PKCα signaling in cancer. Adv. Biol. Regul. 2021;80 doi: 10.1016/j.jbior.2020.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tam W.L., Lu H., Buikhuisen J., Soh B.S., Lim E., Reinhardt F., et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–364. doi: 10.1016/j.ccr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Abera M.B., Kazanietz M.G. Protein kinase Cα mediates erlotinib resistance in lung cancer cells. Mol. Pharmacol. 2015;87:832–841. doi: 10.1124/mol.115.097725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tedja R., Roberts C.M., Alvero A.B., Cardenas C., Yang-Hartwich Y., Spadinger S., et al. Protein kinase Cα-mediated phosphorylation of Twist1 at Ser-144 prevents Twist1 ubiquitination and stabilizes it. J. Biol. Chem. 2019;294:5082–5093. doi: 10.1074/jbc.RA118.005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Vandenbroucke St Amant E., Tauseef M., Vogel S.M., Gao X.-P., Mehta D., Komarova Y.A., et al. PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ. Res. 2012;111:739–749. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brown M.V., Burnett P.E., Denning M.F., Reynolds A.B. PDGF receptor activation induces p120-catenin phosphorylation at serine 879 via a PKCalpha-dependent pathway. Exp. Cell Res. 2009;315:39–49. doi: 10.1016/j.yexcr.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Singh R., Lei P., Andreadis S.T. PKC-δ binds to E-cadherin and mediates EGF-induced cell scattering. Exp. Cell Res. 2009;315:2899–2913. doi: 10.1016/j.yexcr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 282.Lim S.T., Longley R.L., Couchman J.R., Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase Cα (PKCα) increases focal adhesion localization of PKC alpha. J. Biol. Chem. 2003;278:13795–13802. doi: 10.1074/jbc.M208300200. [DOI] [PubMed] [Google Scholar]
- 283.Elfenbein A., Simons M. Syndecan-4 signaling at a glance. J. Cell Sci. 2013;126:3799–3804. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Oh E.S., Woods A., Couchman J.R. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- 285.Koo B.K., Jung Y.S., Shin J., Han I., Mortier E., Zimmermann P., et al. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 2006;355:651–663. doi: 10.1016/j.jmb.2005.09.087. [DOI] [PubMed] [Google Scholar]
- 286.Horowitz A., Murakami M., Gao Y., Simons M. Phosphatidylinositol-4,5-bisphosphate mediates the interaction of syndecan-4 with protein kinase C. Biochemistry. 1999;38:15871–15877. doi: 10.1021/bi991363i. [DOI] [PubMed] [Google Scholar]
- 287.Elfenbein A., Rhodes J.M., Meller J., Schwartz M.A., Matsuda M., Simons M. Suppression of RhoG activity is mediated by a syndecan 4-synectin-RhoGDI1 complex and is reversed by PKCα in a Rac1 activation pathway. J. Cell Biol. 2009;186:75–83. doi: 10.1083/jcb.200810179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Dovas A., Choi Y., Yoneda A., Multhaupt H.A., Kwon S.H., Kang D., et al. Serine 34 phosphorylation of rho guanine dissociation inhibitor (RhoGDIα) links signaling from conventional protein kinase C to RhoGTPase in cell adhesion. J. Biol. Chem. 2010;285:23296–23308. doi: 10.1074/jbc.M109.098129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Knezevic N., Roy A., Timblin B., Konstantoulaki M., Sharma T., Malik A.B., et al. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol. Cell Biol. 2007;27:6323–6333. doi: 10.1128/MCB.00523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Katoh H., Hiramoto K., Negishi M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 291.Bass M.D., Roach K.A., Morgan M.R., Mostafavi-Pour Z., Schoen T., Muramatsu T., et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lévay M., Settleman J., Ligeti E. Regulation of the substrate preference of p190RhoGAP by protein kinase C-mediated phosphorylation of a phospholipid binding site. Biochemistry. 2009;48:8615–8623. doi: 10.1021/bi900667y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bidaud-Meynard A., Biname F., Lagree V., Moreau V. Regulation of Rho GTPase activity at the leading edge of migrating cells by p190RhoGAP. Small GTPases. 2017;10:99–110. doi: 10.1080/21541248.2017.1280584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Murakami M., Horowitz A., Tang S., Ware J.A., Simons M. Protein kinase C (PKC) δ regulates PKCα activity in a Syndecan-4-dependent manner. J. Biol. Chem. 2002;277:20367–20371. doi: 10.1074/jbc.M202501200. [DOI] [PubMed] [Google Scholar]
- 295.Urra H., Henriquez D.R., Canovas J., Villarroel-Campos D., Carreras-Sureda A., Pulgar E., et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol. 2018;20:942–953. doi: 10.1038/s41556-018-0141-0. [DOI] [PubMed] [Google Scholar]
- 296.Zhou A.X., Hartwig J.H., Akyurek L.M. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 297.Ziegler W.H., Tigges U., Zieseniss A., Jockusch B.M. A lipid-regulated docking site on vinculin for protein kinase C. J. Biol. Chem. 2002;277:7396–7404. doi: 10.1074/jbc.M110008200. [DOI] [PubMed] [Google Scholar]
- 298.Anilkumar N., Parsons M., Monk R., Ng T., Adams J.C. Interaction of fascin and protein kinase Cαa: a novel intersection in cell adhesion and motility. EMBO J. 2003;22:5390–5402. doi: 10.1093/emboj/cdg521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.O'Neill A.K., Gallegos L.L., Justilien V., Garcia E.L., Leitges M., Fields A.P., et al. Protein kinase Cα promotes cell migration through a PDZ-dependent interaction with its novel substrate discs large homolog 1 (DLG1) J. Biol. Chem. 2011;286:43559–43568. doi: 10.1074/jbc.M111.294603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Saito Y., Desai R.R., Muthuswamy S.K. Reinterpreting polarity and cancer: the changing landscape from tumor suppression to tumor promotion. Biochim. Biophys. Acta Rev. Cancer. 2018;1869:103–116. doi: 10.1016/j.bbcan.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 301.Marziali F., Dizanzo M.P., Cavatorta A.L., Gardiol D. Differential expression of DLG1 as a common trait in different human diseases: an encouraging issue in molecular pathology. Biol. Chem. 2019;400:699–710. doi: 10.1515/hsz-2018-0350. [DOI] [PubMed] [Google Scholar]
- 302.Ng T., Shima D., Squire A., Bastiaens P.I., Gschmeissner S., Humphries M.J., et al. PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Parsons M., Keppler M.D., Kline A., Messent A., Humphries M.J., Gilchrist R., et al. Site-directed perturbation of protein kinase C- integrin interaction blocks carcinoma cell chemotaxis. Mol. Cell Biol. 2002;22:5897–5911. doi: 10.1128/MCB.22.16.5897-5911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wang Y., Arjonen A., Pouwels J., Ta H., Pausch P., Bange G., et al. Formin-like 2 promotes β1-integrin trafficking and invasive motility downstream of PKCα. Dev. Cell. 2015;34:475–483. doi: 10.1016/j.devcel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 305.Zhong B., Wang K., Xu H., Kong F. Silencing Formin-like 2 inhibits growth and metastasis of gastric cancer cells through suppressing internalization of integrins. Cancer Cell Int. 2018;18:79. doi: 10.1186/s12935-018-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chae Y.C., Kim K.L., Ha S.H., Kim J., Suh P.G., Ryu S.H. Protein kinase Cdelta-mediated phosphorylation of phospholipase D controls integrin-mediated cell spreading. Mol. Cell Biol. 2010;30:5086–5098. doi: 10.1128/MCB.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Xue Z.H., Zhao C.Q., Chua G.L., Tan S.W., Tang X.Y., Wong S.C., et al. Integrin alphaMbeta2 clustering triggers phosphorylation and activation of protein kinase C delta that regulates transcription factor Foxp1 expression in monocytes. J. Immunol. 2010;184:3697–3709. doi: 10.4049/jimmunol.0903316. [DOI] [PubMed] [Google Scholar]
- 308.Bordeleau F., Galarneau L., Gilbert S., Loranger A., Marceau N. Keratin 8/18 modulation of protein kinase C-mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol. Biol. Cell. 2010;21:1698–1713. doi: 10.1091/mbc.E09-05-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lin T.H., Liu H.H., Tsai T.H., Chen C.C., Hsieh T.F., Lee S.S., et al. CCL2 increases αvβ3 integrin expression and subsequently promotes prostate cancer migration. Biochim. Biophys. Acta. 2013;1830:4917–4927. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 310.Symonds J.M., Ohm A.M., Tan A.C., Reyland M.E. PKCdelta regulates integrin alphaVbeta3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget. 2016;7:17905–17919. doi: 10.18632/oncotarget.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liedtke C.M., Hubbard M., Wang X. Stability of actin cytoskeleton and PKC-δ binding to actin regulate NKCC1 function in airway epithelial cells. Am. J. Physiol. Cell Physiol. 2003;284:C487–C496. doi: 10.1152/ajpcell.00357.2002. [DOI] [PubMed] [Google Scholar]
- 312.Romanova L.Y., Alexandrov I.A., Blagosklonny M.V., Nordan R.P., Garfield S., Acs P., et al. Regulation of actin cytoskeleton in lymphocytes: PKC-Δ disrupts IL-3–induced membrane ruffles downstream of Rac1. J. Cell Physiol. 1999;179:157–169. doi: 10.1002/(SICI)1097-4652(199905)179:2<157::AID-JCP6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 313.Iwabu A., Smith K., Allen F.D., Lauffenburger D.A., Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C δ-dependent pathway ∗. J. Biol. Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- 314.Li C., Wernig F., Leitges M., Hu Y., Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–2108. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- 315.Liu B., Ryer E.J., Kundi R., Kamiya K., Itoh H., Faries P.L., et al. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J. Vasc. Surg. 2007;45:160–168. doi: 10.1016/j.jvs.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mondrinos M.J., Zhang T., Sun S., Kennedy P.A., King D.J., Wolfson M.R., et al. Pulmonary endothelial protein kinase C-delta (PKCδ) regulates neutrophil migration in acute lung inflammation. Am. J. Pathol. 2014;184:200–213. doi: 10.1016/j.ajpath.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tang Y., Soroush F., Sun S., Liverani E., Langston J.C., Yang Q., et al. Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. J. Neuroinflammation. 2018;15:309. doi: 10.1186/s12974-018-1342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kostyak J.C., Bhavanasi D., Liverani E., McKenzie S.E., Kunapuli S.P. Protein kinase C delta deficiency enhances megakaryopoiesis and recovery from thrombocytopenia. Arterioscler Thromb. Vasc. Biol. 2014;34:2579–2585. doi: 10.1161/ATVBAHA.114.304492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Soroush F., Tang Y., Guglielmo K., Engelmann A., Liverani E., Patel A., et al. Protein kinase C-delta (PKCδ) tyrosine phosphorylation is a critical regulator of neutrophil-endothelial cell interaction in inflammation. Shock. 2019;51:538–547. doi: 10.1097/SHK.0000000000001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhao C.T., Li K., Li J.T., Zheng W., Liang X.J., Geng A.Q., et al. PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21353–21358. doi: 10.1073/pnas.0812872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sahin B., Hawasli A.H., Greene R.W., Molkentin J.D., Bibb J.A. Negative regulation of cyclin-dependent kinase 5 targets by protein kinase C. Eur. J. Pharmacol. 2008;581:270–275. doi: 10.1016/j.ejphar.2007.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Cao M., Gao J., Zhou H., Huang J., You A., Guo Z., et al. HIF-2α regulates CDCP1 to promote PKCδ-mediated migration in hepatocellular carcinoma. Tumour Biol. 2016;37:1651–1662. doi: 10.1007/s13277-015-3527-7. [DOI] [PubMed] [Google Scholar]
- 323.Miyazawa Y., Uekita T., Hiraoka N., Fujii S., Kosuge T., Kanai Y., et al. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- 324.Razorenova O.V., Finger E.C., Colavitti R., Chernikova S.B., Boiko A.D., Chan C.K., et al. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{delta}-driven migration. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1931–1936. doi: 10.1073/pnas.1011777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Khan T., Kryza T., Lyons N.J., He Y., Hooper J.D. The CDCP1 signaling hub: a target for cancer detection and therapeutic intervention. Cancer Res. 2021;81:2259–2269. doi: 10.1158/0008-5472.CAN-20-2978. [DOI] [PubMed] [Google Scholar]
- 326.Nakashima K., Uekita T., Yano S., Kikuchi J.I., Nakanishi R., Sakamoto N., et al. Novel small molecule inhibiting CDCP1-PKCdelta pathway reduces tumor metastasis and proliferation. Cancer Sci. 2017;108:1049–1057. doi: 10.1111/cas.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Black A.R., Black J.D. The complexities of PKCalpha signaling in cancer. Adv. Biol. Regul. 2021;80:100769. doi: 10.1016/j.jbior.2020.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hara T., Matsumura S., Hakuno F., Takahashi S., Chida K. PKCα suppresses 7,12-dimethylbenz[a]anthracene-induced skin tumor formation. Anticancer Res. 2012;32:3097–3101. [PubMed] [Google Scholar]
- 329.Hill K.S., Erdogan E., Khoor A., Walsh M.P., Leitges M., Murray N.R., et al. Protein kinase Calpha suppresses Kras-mediated lung tumor formation through activation of a p38 MAPK-TGFbeta signaling axis. Oncogene. 2014;33:2134–2144. doi: 10.1038/onc.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nakagawa R., Soh J.W., Michie A.M. Subversion of protein kinase C α signaling in hematopoietic progenitor cells results in the generation of a B-cell chronic lymphocytic leukemia-like population in vivo. Cancer Res. 2006;66:527–534. doi: 10.1158/0008-5472.CAN-05-0841. [DOI] [PubMed] [Google Scholar]
- 331.Milani G., Rebora P., Accordi B., Galla L., Bresolin S., Cazzaniga G., et al. Low PKCδ expression within the MRD-HR stratum defines a new subgroup of childhood T-ALL with very poor outcome. Oncotarget. 2014;5:5234–5245. doi: 10.18632/oncotarget.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Suga K., Sugimoto I., Ito H., Hashimoto E. Down-regulation of protein kinase C-α detected in human colorectal cancer. Biochem. Mol. Biol. Int. 1998;44:523–528. doi: 10.1080/15216549800201552. [DOI] [PubMed] [Google Scholar]
- 333.Goode B., Mondal G., Hyun M., Ruiz D.G., Lin Y.H., Van Ziffle J., et al. A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat. Commun. 2018;9:810. doi: 10.1038/s41467-018-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rosenberg S., Simeonova I., Bielle F., Verreault M., Bance B., Le Roux I., et al. A recurrent point mutation in PRKCA is a hallmark of chordoid gliomas. Nat. Commun. 2018;9:2371. doi: 10.1038/s41467-018-04622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.D'Costa A.M., Robinson J.K., Maududi T., Chaturvedi V., Nickoloff B.J., Denning M.F. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2006;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 336.Langzam L., Koren R., Gal R., Kugel V., Paz A., Farkas A., et al. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy. Am. J. Clin. Pathol. 2001;116:377–385. doi: 10.1309/1VKK-HWH7-YVJN-7UF7. [DOI] [PubMed] [Google Scholar]
- 337.Yadav V., Yanez N.C., Fenton S.E., Denning M.F. Loss of protein kinase C delta gene expression in human squamous cell carcinomas: a laser capture microdissection study. Am. J. Pathol. 2010;176:1091–1096. doi: 10.2353/ajpath.2010.090816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Su C.M., Weng Y.S., Kuan L.Y., Chen J.H., Hsu F.T. Suppression of PKCdelta/NF-kappaB signaling and apoptosis induction through extrinsic/intrinsic pathways are associated magnolol-inhibited tumor progression in colorectal cancer in vitro and in vivo. Int. J. Mol. Sci. 2020;21:3527. doi: 10.3390/ijms21103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Reno E.M., Haughian J.M., Dimitrova I.K., Jackson T.A., Shroyer K.R., Bradford A.P. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum. Pathol. 2008;39:21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Antal C.E., Hudson A.M., Kang E., Zanca C., Wirth C., Stephenson N.L., et al. Cancer-associated protein kinase C mutations reveal kinase's role as tumor suppressor. Cell. 2015;160:489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.McKiernan E., O'Brien K., Grebenchtchikov N., Geurts-Moespot A., Sieuwerts A.M., Martens J.W., et al. Protein kinase Cdelta expression in breast cancer as measured by real-time PCR, western blotting and ELISA. Br. J. Cancer. 2008;99:1644–1650. doi: 10.1038/sj.bjc.6604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Reddig P.J., Dreckschmidt N.E., Ahrens H., Simsiman R., Tseng C.P., Zou J., et al. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- 343.Aziz M.H., Wheeler D.L., Bhamb B., Verma A.K. Protein kinase C delta overexpressing transgenic mice are resistant to chemically but not to UV radiation-induced development of squamous cell carcinomas: a possible link to specific cytokines and cyclooxygenase-2. Cancer Res. 2006;66:713–722. doi: 10.1158/0008-5472.CAN-05-2684. [DOI] [PubMed] [Google Scholar]
- 344.Kong C., Zhu Y., Liu D., Yu M., Li S., Li Z., et al. Role of protein kinase C-alpha in superficial bladder carcinoma recurrence. Urology. 2005;65:1228–1232. doi: 10.1016/j.urology.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 345.Tian F., Wu H., Li Z., Wang N., Huang J., Li C., et al. Activated PKCα/ERK1/2 signaling inhibits tamoxifen-induced apoptosis in C6 cells. Cancer Invest. 2009;27:802–808. doi: 10.1080/07357900802672720. [DOI] [PubMed] [Google Scholar]
- 346.Zhao L.J., Xu H., Qu J.W., Zhao W.Z., Zhao Y.B., Wang J.H. Modulation of drug resistance in ovarian cancer cells by inhibition of protein kinase C-alpha (PKC-α) with small interference RNA (siRNA) agents. Asian Pac. J. Cancer Prev. 2012;13:3631–3636. doi: 10.7314/apjcp.2012.13.8.3631. [DOI] [PubMed] [Google Scholar]
- 347.Mohanty S., Huang J., Basu A. Enhancement of cisplatin sensitivity of cisplatin-resistant human cervical carcinoma cells by bryostatin 1. Clin. Cancer Res. 2005;11:6730–6737. doi: 10.1158/1078-0432.CCR-05-0450. [DOI] [PubMed] [Google Scholar]
- 348.Assender J.W., Gee J.M., Lewis I., Ellis I.O., Robertson J.F., Nicholson R.I. Protein kinase C isoform expression as a predictor of disease outcome on endocrine therapy in breast cancer. J. Clin. Pathol. 2007;60:1216–1221. doi: 10.1136/jcp.2006.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wilson F.H., Johannessen C.M., Piccioni F., Tamayo P., Kim J.W., Van Allen E.M., et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27:397–408. doi: 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Chen C.H., Wang B.W., Hsiao Y.C., Wu C.Y., Cheng F.J., Hsia T.C., et al. PKCdelta-mediated SGLT1 upregulation confers the acquired resistance of NSCLC to EGFR TKIs. Oncogene. 2021;40:4796–4808. doi: 10.1038/s41388-021-01889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Batlle E., Verdu J., Dominguez D., del Mont Llosas M., Diaz V., Loukili N., et al. Protein kinase C-α activity inversely modulates invasion and growth of intestinal cells. J. Biol. Chem. 1998;273:15091–15098. doi: 10.1074/jbc.273.24.15091. [DOI] [PubMed] [Google Scholar]
- 352.Hsieh Y.H., Wu T.T., Huang C.Y., Hsieh Y.S., Hwang J.M., Liu J.Y. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007;67:4320–4327. doi: 10.1158/0008-5472.CAN-06-2486. [DOI] [PubMed] [Google Scholar]
- 353.Haughian J.M., Bradford A.P. Protein kinase C alpha (PKCα) regulates growth and invasion of endometrial cancer cells. J. Cell Physiol. 2009;220:112–118. doi: 10.1002/jcp.21741. [DOI] [PubMed] [Google Scholar]
- 354.Putnam A.J., Schulz V.V., Freiter E.M., Bill H.M., Miranti C.K. Src, PKCα, and PKCδ are required for αvβ3 integrin-mediated metastatic melanoma invasion. Cell Commun. Signal. 2009;7:10. doi: 10.1186/1478-811X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Lin C.W., Shen S.C., Chien C.C., Yang L.Y., Shia L.T., Chen Y.C. 12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCα/ERK/NF-κB-dependent MMP-9 expression. J. Cell Physiol. 2010;225:472–481. doi: 10.1002/jcp.22226. [DOI] [PubMed] [Google Scholar]
- 356.Humphries B., Wang Z., Oom A.L., Fisher T., Tan D., Cui Y., et al. MicroRNA-200b targets protein kinase Cα and suppresses triple-negative breast cancer metastasis. Carcinogenesis. 2014;35:2254–2263. doi: 10.1093/carcin/bgu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kim J., Thorne S.H., Sun L., Huang B., Mochly-Rosen D. Sustained inhibition of PKCα reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011;30:323–333. doi: 10.1038/onc.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jiang Y., Berk M., Singh L.S., Tan H., Yin L., Powell C.T., et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin. Exp. Metastasis. 2005;22:369–376. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- 359.Cooke M., Casado-Medrano V., Ann J., Lee J., Blumberg P.M., Abba M.C., et al. Differential regulation of gene expression in lung cancer cells by diacyglycerol-lactones and a phorbol ester via selective activation of protein kinase C isozymes. Sci. Rep. 2019;9:6041. doi: 10.1038/s41598-019-42581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jackson D., Zheng Y., Lyo D., Shen Y., Nakayama K., Nakayama K.I., et al. Suppression of cell migration by protein kinase Cδ. Oncogene. 2005;24:3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- 361.Kiley S.C., Clark K.J., Goodnough M., Welch D.R., Jaken S. Protein kinase C δ involvement in mammary tumor cell metastasis. Cancer Res. 1999;59:3230–3238. [PubMed] [Google Scholar]
- 362.Bessa C., Soares J., Raimundo L., Loureiro J.B., Gomes C., Reis F., et al. Discovery of a small-molecule protein kinase Cδ-selective activator with promising application in colon cancer therapy. Cell Death Dis. 2018;9:23. doi: 10.1038/s41419-017-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yu H.S., Lin T.H., Tang C.H. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCdelta/c-Src dependent signaling pathway. Prostate. 2013;73:89–100. doi: 10.1002/pros.22544. [DOI] [PubMed] [Google Scholar]
- 364.Lin C.C., Lee I.T., Chi P.L., Hsieh H.L., Cheng S.E., Hsiao L.D., et al. C-Src/Jak2/PDGFR/PKCδ-dependent MMP-9 induction is required for thrombin-stimulated rat brain astrocytes migration. Mol. Neurobiol. 2014;49:658–672. doi: 10.1007/s12035-013-8547-y. [DOI] [PubMed] [Google Scholar]
- 365.Li N., Du Z.X., Zong Z.H., Liu B.Q., Li C., Zhang Q., et al. PKCδ-mediated phosphorylation of BAG3 at Ser187 site induces epithelial-mesenchymal transition and enhances invasiveness in thyroid cancer FRO cells. Oncogene. 2013;32:4539–4548. doi: 10.1038/onc.2012.466. [DOI] [PubMed] [Google Scholar]
- 366.Tang T., Zhu Q., Li X., Zhu G., Deng S., Wang Y., et al. Protease Nexin I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer cells metastasis and stemness. Cell Death Dis. 2019;10:649. doi: 10.1038/s41419-019-1882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Banninger G.P., Cha S., Said M.S., Pauley K.M., Carter C.J., Onate M., et al. Loss of PKCdelta results in characteristics of Sjogren's syndrome including salivary gland dysfunction. Oral Dis. 2011;17:601–609. doi: 10.1111/j.1601-0825.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gorelik G., Sawalha A.H., Patel D., Johnson K., Richardson B. T cell PKCdelta kinase inactivation induces lupus-like autoimmunity in mice. Clin. Immunol. 2015;158:193–203. doi: 10.1016/j.clim.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Belot A., Kasher P.R., Trotter E.W., Foray A.P., Debaud A.L., Rice G.I., et al. Protein kinase cdelta deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65:2161–2171. doi: 10.1002/art.38008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kuehn H.S., Niemela J.E., Rangel-Santos A., Zhang M., Pittaluga S., Stoddard J.L., et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121:3117–3125. doi: 10.1182/blood-2012-12-469544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Duan Y.T., Bi K.Y., Ma Y.S. PKC delta gene can induce macrophages to release inflammatory factors against Mycobacterium tuberculosis infection. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4228–4237. doi: 10.26355/eurrev_201807_15417. [DOI] [PubMed] [Google Scholar]
- 372.Parihar S.P., Ozturk M., Marakalala M.J., Loots D.T., Hurdayal R., Beukes D., et al. Protein kinase C-delta (PKCdelta), a marker of inflammation and tuberculosis disease progression in humans, is important for optimal macrophage killing effector functions and survival in mice. Mucosal Immunol. 2018;11:579–580. doi: 10.1038/mi.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zaid Y., Senhaji N., Darif Y., Kojok K., Oudghiri M., Naya A. Distinctive roles of PKC delta isozyme in platelet function. Curr. Res. Transl. Med. 2016;64:135–139. doi: 10.1016/j.retram.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 374.Niino Y.S., Kawashima I., Iguchi Y., Kanda H., Ogura K., Mita-Yoshida K., et al. PKCdelta deficiency inhibits fetal development and is associated with heart elastic fiber hyperplasia and lung inflammation in adult PKCdelta knockout mice. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ren J., Wang Q., Morgan S., Si Y., Ravichander A., Dou C., et al. Protein kinase C-delta (PKCdelta) regulates proinflammatory chemokine expression through cytosolic interaction with the NF-kappaB subunit p65 in vascular smooth muscle cells. J. Biol. Chem. 2014;289:9013–9026. doi: 10.1074/jbc.M113.515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Wang H.Q., Smart R.C. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J. Cell Sci. 1999;112:3497–3506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- 377.Cataisson C., Ohman R., Patel G., Pearson A., Tsien M., Jay S., et al. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319–328. doi: 10.1158/0008-5472.CAN-08-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wang M., Zhong H., Zhang X., Huang X., Wang J., Li Z., et al. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Li X., Cullere X., Nishi H., Saggu G., Durand E., Mansour M.K., et al. PKC-delta activation in neutrophils promotes fungal clearance. J. Leukoc. Biol. 2016;100:581–588. doi: 10.1189/jlb.4A0915-405R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Liverani E., Mondrinos M.J., Sun S., Kunapuli S.P., Kilpatrick L.E. Role of Protein Kinase C-delta in regulating platelet activation and platelet-leukocyte interaction during sepsis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Liverani E., Tursi S.A., Cornwell W.D., Mondrinos M.J., Sun S., Buttaro B.A., et al. Protein kinase C-delta inhibition is organ-protective, enhances pathogen clearance, and improves survival in sepsis. FASEB J. 2020;34:2497–2510. doi: 10.1096/fj.201900897R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lorden G., Newton A.C. Conventional protein kinase C in the brain: repurposing cancer drugs for neurodegenerative treatment? Neuronal. Signal. 2021;5 doi: 10.1042/NS20210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Du Y., Zhao Y., Li C., Zheng Q., Tian J., Li Z., et al. Inhibition of PKCdelta reduces amyloid-beta levels and reverses Alzheimer disease phenotypes. J. Exp. Med. 2018;215:1665–1677. doi: 10.1084/jem.20171193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Jin H., Kanthasamy A., Harischandra D.S., Kondru N., Ghosh A., Panicker N., et al. Histone hyperacetylation up-regulates protein kinase cdelta in dopaminergic neurons to induce cell death: Relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. J. Biol. Chem. 2014;289:34743–34767. doi: 10.1074/jbc.M114.576702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rué L., Alcalá-Vida R., López-Soop G., Creus-Muncunill J., Alberch J., Pérez-Navarro E. Early down-regulation of PKCδ as a pro-survival mechanism in Huntington's disease. Neuromolecular Med. 2014;16:25–37. doi: 10.1007/s12017-013-8248-8. [DOI] [PubMed] [Google Scholar]
- 386.Alfonso S.I., Callender J.A., Hooli B., Antal C.E., Mullin K., Sherman M.A., et al. Gain-of-function mutations in protein kinase Calpha (PKCalpha) may promote synaptic defects in Alzheimer's disease. Sci. Signal. 2016;9:ra47. doi: 10.1126/scisignal.aaf6209. [DOI] [PMC free article] [PubMed] [Google Scholar]