Abstract
Background
Recent data suggest that BRAFV600E-mutated metastatic colorectal cancer (mCRC) patients with right-sided tumours and ECOG-PS = 0 may achieve benefit from the triplet regimen differently than those with left-sided tumours and ECOG-PS > 0.
Methods
The predictive impact of primary sidedness and ECOG-PS was evaluated in a large real-life dataset of 296 BRAFV600E-mutated mCRC patients treated with upfront triplet or doublet ± bevacizumab. Biological differences between right- and left-sided BRAFV600E-mutated CRCs were further investigated in an independent cohort of 1162 samples.
Results
A significant interaction effect between primary sidedness and treatment intensity was reported in terms of both PFS (p = 0.010) and OS (p = 0.003), with a beneficial effect of the triplet in the right-sided group and a possible detrimental effect in the left-sided. No interaction effect was observed between ECOG-PS and chemo-backbone. In the MSS/pMMR population, a consistent trend for a side-related subgroup effect was observed when FOLFOXIRI ± bevacizumab was compared to oxaliplatin-based doublets±bevacizumab (p = 0.097 and 0.16 for PFS and OS, respectively). Among MSS/pMMR tumours, the BM1 subtype was more prevalent in the right-sided group (p = 0.0019, q = 0.0139). No significant differences were observed according to sidedness in the MSI-H/dMMR population.
Conclusions
Real-life data support the use of FOLFOXIRI ± bevacizumab only in BRAFV600E-mutated mCRC patients with right-sided tumours.
Subject terms: Oncology, Cancer
Background
BRAFV600E mutation is found in 8–12% of metastatic colorectal cancers (mCRC) and accounts for more than 95% of BRAF mutations in mCRC [1]. Its negative prognostic impact is well-established with a median overall survival (OS) of around 12 months [2, 3]. Given the low percentage of patients able to receive further therapies after progression to first-line treatment due to rapidly progressive and highly aggressive disease, the choice of upfront therapy is of paramount importance [4, 5].
Current international guidelines consider the intensified regimen FOLFOXIRI (fluorouracil, oxaliplatin and irinotecan) plus bevacizumab (bev) to be the preferred first-line option for patients with BRAFV600E mutant mCRC [6]. This recommendation mainly derives from a subgroup analysis of the phase III TRIBE study that compared FOLFOXIRI/bev with FOLFIRI (5-fluorouracil and irinotecan)/bev in the first-line setting of mCRC. In particular, among patients with BRAF mutant disease, the magnitude of the benefit from the triplet regimen was numerically higher compared with those with RAS mutant or RAS/BRAF wild-type tumours, even if a formal interaction effect was not observed [7, 8]. However, the phase III TRIBE2 trial comparing FOLFOXIRI/bev with FOLFOX (5-fluorouracil and oxaliplatin)/bev and a recent metanalysis of five randomised trials assessing the role of the triplet/bev with respect to the doublets (FOLFOX or FOLFIRI)/bev did not confirm these data [9, 10]. Although the reasons for such discordance are unknown, a subgroup effect according to primary tumour location was suggested both in the TRIBE2 study and in the above-mentioned metanalysis where patients with left-sided BRAF mutant tumours seemed to derive a detrimental effect from treatment intensification [9–11]. In addition, a recent study exploring clinical and gene expression markers in BRAF mutant patients enrolled in the TRIBE2 study, showed a significant interaction effect between the treatment arm and patients’ Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) in terms of progression-free survival (PFS) with a similar trend in OS, with no benefit from the triplet among those with ECOG-PS 1 [11].
Several studies, mainly focusing on RAS/BRAF wild-type tumours, showed that the molecular landscape of right- versus left-sided mCRC is different. Indeed, tumours located in the right colon have a higher rate of alterations potentially associated with poor prognosis [12, 13] and with resistance to anti-EGFR antibodies [14, 15]. It is currently unknown whether relevant differences exist in the molecular profile of right- versus left-sided tumours also among BRAFV600E-mutated mCRCs.
Drawing from these retrospective findings, we challenged the predictive impact of primary tumour location and ECOG-PS in a large real-life dataset of BRAFV600E mutant mCRC patients treated with upfront systemic therapies [16]. To further explore genomic and transcriptomic alterations in BRAFV600E-mutated CRCs according to primary tumour location, an independent database of patient tumours with comprehensive molecular profiling [17] was retrospectively reviewed.
Methods
Study populations
Consecutive patients with BRAFV600E-mutated mCRC referred to 22 oncology units between January 2005 and December 2016 included in the “BRAF BeCool” dataset were gathered [16]. Only patients who met the criteria of potential eligibility for the triplet regimen (aged 18–70 years with ECOG-PS of 2 or less, aged 71–75 years with an ECOG-PS of 0 and no previous oxaliplatin-based adjuvant therapy) [18] and received upfront doublet or triplet ± bev were deemed eligible for the present analysis (“BRAF-Be-Cool” population).
Tumours located in the cecum and the ascending and in the proximal two-thirds of the transverse colon were defined as right-sided, whereas those located in the distal third of the transverse colon, in the splenic flexure, descending colon, sigma and rectum were defined as left-sided [19].
In addition, a total of 1162 formalin-fixed paraffin-embedded (FFPE) tumour samples from BRAFV600E-mutated CRC patients were collected at various institutions in different countries and molecularly profiled by a commercial CLIA-certified laboratory (Caris Life Sciences, Phoenix, AZ) (“Comprehensive Genomic Profiling (CGP)” population) [17].
Genome, transcriptome and immunohistochemistry analyses of the CGP population
Next-generation sequencing (NGS) of DNA (592-gene panel or whole-exome) and RNA (whole-transcriptome) was performed using material isolated from FFPE samples [17].
Microsatellite (MS) and mismatch repair system (MMR) status were assessed with a combination method using immunohistochemistry (IHC), fragment analysis, and NGS, with the resulting status defined as either microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) or microsatellite stable (MSS)/ mismatch repair proficient (pMMR), as previously described [20, 21].
The consensus molecular subtype (CMS) classifier was developed using RNA sequencing data collected from the WTS platform, as previously described [22].
Tumour mutational burden (TMB) was measured by counting all non-synonymous missense, nonsense, inframe insertion/deletion and frameshift mutations found per tumour that had not been previously described as germline alterations in dbSNP151, Genome Aggregation Database (gnomAD) databases or benign variants identified by Caris geneticists. The threshold adopted for the definition of TMB-high (TMB-H) was ≥10 mutations per Megabase (Mb) based on the KEYNOTE- 158 trial showing higher clinical activity with pembrolizumab in patients with a TMB ≥ 10 mutations/Mb across several tumour types than patients with a TMB < 10 mutations/Mb [23]. Caris Life Sciences is a participant in the Friends of Cancer Research TMB Harmonization Project [24].
PD-L1 expression was tested via IHC using SP142 antibody (Spring Biosciences). The staining intensity on the tumour cell membrane was assessed on a semiquantitative scale: 0 for no staining,
1+ for weak staining, 2+ for moderate staining and 3+ for strong staining. Tumours exhibiting >5% of tumour cells stained as 2+ or 3+ were considered PD-L1 positive.
The Microenvironment Cell Population-counter (MCP-counter) was used for quantification of the immune and stromal cell population abundance using WTS data. Gene expression levels were analysed for each subgroup, with the fold change in median expression calculated for comparison [25].
Based on a previously published classifier of BRAFV600E mutant tumours using a 44-gene set signature, a composite score was derived from the difference in average z-scores of genes associated with BM1 or BM2 subtype (note: four genes from the original gene set did not correlate with remaining genes as previously reported and were removed) [26].
Wnt signalling activation was defined as ligand-independent (LI) in case of APC or CTNNB1 mutations and ligand-dependent (LD) in case of RNF43 mutation or RSPO3 fusion [27].
Homologous recombination deficiency (HRD) was defined as the presence of one or more pathogenic or presumed pathogenic mutations, categorised according to the American College of Medical Genetics and Genomics standards, in any of Homologous Recombination-related genes, as previously described [28].
In a cohort of 331 BRAFV600E-mutated CRC samples profiled by whole-exome sequencing (WES), the genomic loss of heterozygosity (LOH), defined as the percentage of examined genomic segments (max 552) with an average SNP variant frequency skewed ≥15% from the expected heterozygous frequency (50%) was assessed. Tumours with a LOH ≥ 16% of examined segments were regarded as LOH-high, while tumours with a LOH < 16% as LOH-low [29, 30].
Statistics
Descriptive statistics was used to summarise clinicopathological characteristics. PFS and OS were defined as the time from the start of first-line chemotherapy to the first evidence of disease progression or death, whichever occurred first, and as the time from randomisation to death due to any cause, respectively. Survival curves were estimated by the Kaplan–Meier method and compared with the log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CI) were estimated with a Cox proportional hazards model. Subgroup analyses according to primary tumour location and ECOG-PS for PFS and OS were carried out using an interaction test. Chi-square test, Fisher’s exact test or Mann–Whitney test were used whenever appropriate to compare clinical and molecular baseline characteristics between patients treated with triplet and doublet chemotherapy and between right- and left-sided tumours. The impact of primary tumour location, ECOG-PS and other prognostic factors on PFS and OS was firstly assessed in univariate analyses. Significantly prognostic covariates (p ≤ 0.10) were included in a multivariable Cox proportional hazard model. The data cut-off for the present analysis was July 13, 2020.
Statistical significance was set at p = 0.05 for a bilateral test. To adjust p-values for multiple hypothesis testing, the q values were calculated using the Benjamini–Hochberg method. All analyses were carried out in SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and Python 3.9.7 (Python Software Foundation).
Results
BRAF-Be-Cool population
Of 647 patients included in the “BRAF-Be-Cool” dataset, 296 fulfilled the pre-specified age and ECOG-PS inclusion criteria. Overall, 124 patients (42%) were treated with triplet ± bev and 172 (58%) were treated with doublet ± bev (134 oxaliplatin-based and 38 irinotecan-based) (Supplementary Fig. 1). Patients’ characteristics are listed in Supplementary table 1. As expected, most of the patients had an ECOG-PS of 0 (73%) and a right-sided primary tumour (60%). Microsatellite status was available only for 192 (65%) patients with 160 pMMR/MMS (83%) and 32 dMMR/MSI-H (17%).
No prognostic differences were observed between right- and left-sided tumours in terms of PFS and OS (Supplementary Fig. 2A and B).
Among patients with a right-sided primary tumour, those treated with FOLFOXIRI ± bev had more frequently ECOG-PS = 0 (p = 0.032), a lower median age (p = 0.002) and received a bev-containing regimen (p < 0.001) compared with patients treated with doublet ± bev. In the left-sided group, patients treated with FOLFOXIRI ± bev had more frequently pMMR/MSS (p = 0.0085) tumours and received a bev-containing regimen (p = 0.0078) (Table 1).
Table 1.
Patients’ characteristics of BRAF-BeCool population based on tumour sidedness.
| Right sided N = 179 | Left sided and rectum N = 116 | |||||
|---|---|---|---|---|---|---|
| Triplet+/−bev N = 72 n (%) | Doublet+/−bev N = 107 n (%) | p | Triplet+/−bev N = 52 n (%) | Doublet+/−bev N = 64 n (%) | p | |
| Age (years) | 0.002 | 0.23 | ||||
| Median | 59 | 64 | 59 | 61 | ||
| Range (min–max) | 27–74 | 28–74 | 34–74 | 24–75 | ||
| Sex | 0.84 | 0.80 | ||||
| Male | 30 (42%) | 43 (40%) | 28 (54%) | 36 (56%) | ||
| Female | 42 (58%) | 64 (60%) | 24 (46%) | 28 (44%) | ||
| ECOG-PS | 0.032 | 0.18 | ||||
| 0 | 60 (83%) | 74 (69%) | 40 (77%) | 42 (66%) | ||
| 1–2 | 12 (17%) | 33 (31%) | 12 (23%) | 22 (34%) | ||
| Microsatellite status | 0.32 | 0.0085 | ||||
| pMMR/MSS | 48 (81%) | 45 (74%) | 43 (100%) | 24 (83%) | ||
| dMMR/MSI-H | 11 (19%) | 16 (26%) | 0 (0%) | 5 (17%) | ||
| NA | 13 | 46 | 9 | 35 | ||
| Resected primary tumour | 0.69 | 0.71 | ||||
| Yes | 57 (79%) | 82 (77%) | 35 (67%) | 41 (64%) | ||
| No | 15 (21%) | 25 (23%) | 17 (33%) | 23 (36%) | ||
| Liver only disease | 0.093 | 0.49 | ||||
| Yes | 22 (31%) | 21 (20%) | 16 (31%) | 16 (25%) | ||
| No | 50 (69%) | 86 (80%) | 36 (69%) | 48 (75%) | ||
| Number of metastatic sites | 0.58 | 0.94 | ||||
| 1 | 38 (53%) | 52 (49%) | 24 (46%) | 30 (47%) | ||
| >1 | 34 (47%) | 55 (51%) | 28 (54%) | 34 (53%) | ||
| R0/R1 resection of metastases | 0.20 | 0.33 | ||||
| Yes | 17 (24%) | 17 (16%) | 7 (13%) | 13 (20%) | ||
| No | 55 (76%) | 90 (84%) | 45 (87%) | 51 (80%) | ||
| Time to metastases | 0.41 | 0.20 | ||||
| Synchronous | 68 (94%) | 96 (90%) | 47 (90%) | 52 (81%) | ||
| Metachronous | 4 (6%) | 11 (10%) | 5 (10%) | 12 (19%) | ||
| Semplified risk score | 0.30 | 0.68 | ||||
| High | 12 (17%) | 25 (24%) | 9 (17%) | 15 (24%) | ||
| Intermediate | 23 (32%) | 37 (36%) | 19 (37%) | 20 (31%) | ||
| Low | 37 (51%) | 42 (40%) | 24 (46%) | 29 (45%) | ||
| NA | – | 3 | – | – | ||
| Bevacizumab-based treatment | <0.001 | 0.0078 | ||||
| Yes | 70 (97%) | 72 (67%) | 48 (92%) | 46 (72%) | ||
| No | 2 (3%) | 35 (33%) | 4 (8%) | 18 (28%) | ||
Bold values indicate statistical significance p < 0.05.
Simplified score as described by Loupakis et al. [12].
N number, ECOG-PS Eastern Cooperative Oncology Group Performance Status, NA not available, pMMR proficient mismatched repair, MSS microsatellite stable, dMMR deficient mismatched repair, MSI-H microsatellite instability high.
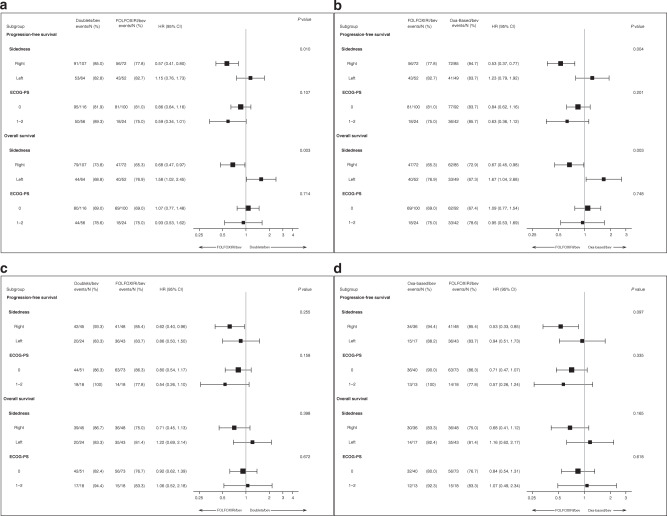
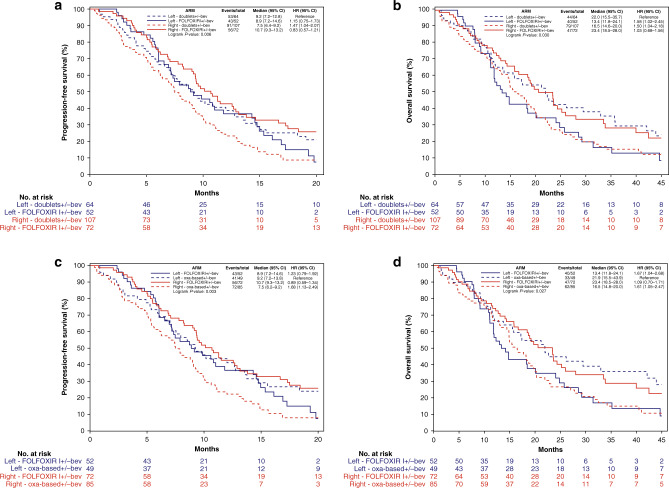
A significant interaction effect was shown between sidedness and the chemo-backbone (FOLFOXIRI ± bev versus doublet ± bev) in terms of both PFS (p for interaction = 0.010) and OS (p for interaction = 0.003) with a clear benefit and a possible detrimental effect from the triplet in right- and left-sided tumours, respectively (Figs. 1a; 2a and b). These findings were confirmed after excluding patients treated with irinotecan-containing doublet chemotherapy (p for interaction of 0.004 and 0.003 for PFS and OS, respectively) (Figs. 1b; 2a and b). When the population was restricted to the MSS/pMMR group (N = 160) (Fig. 1c and d; Supplementary Fig. 3), a similar trend was observed comparing FOLFOXIRI ± bev versus oxaliplatin-based doublet ± bev (p for interaction of 0.097 and 0.16 for PFS and OS, respectively).
Fig. 1. Forest plot according to sidedness and ECOG-PS of PFS and OS.
Overall BRAF-BeCool population (a), overall BRAF-BeCool population excluding irinotecan-containing doublet chemotherapy (b), MSS/pMMR BRAF-BeCool population (c), MSS/pMMR BRAF-BeCool population excluding irinotecan-containing doublet chemotherapy (d).
Fig. 2. Kaplan–Meier curves of PFS and OS according to sidedness and treatment in the BRAF-BeCool population.
PFS in BRAF-BeCool population (a), OS in BRAF-BeCool population (b), PFS in BRAF-BeCool population excluding irinotecan-containing doublet chemotherapy (c), OS in BRAF-BeCool population excluding irinotecan-containing doublet chemotherapy (d).
Patients with ECOG-PS 0 reported longer PFS (9.8 versus 5.1 months; HR: 1.73, 95% CI: 1.30–2.29; p < 0.001) and OS (20.6 versus 11.2; HR: 1.90, 95% CI: 1.41–22.57; p < 0.001) compared with those with ECOG-PS 1 or 2 (Supplementary Fig. 4A and B). In the multivariable model, the better prognosis for patients with ECOG-PS = 0 was confirmed in terms of both PFS (p = 0.008) and OS (p = 0.014) (Supplementary Table 2). No interaction effect was observed between ECOG-PS and treatments in terms of both PFS and OS (Fig. 1).
Of note, patients with MSI-H/dMMR tumours (N = 32), showed a better prognosis than those with MSS/pMMR tumours (N = 160) at uni- and multi-variate analyses. Seven (22%) of these patients received checkpoint inhibitors in subsequent lines (Supplementary Table 2).
CGP population
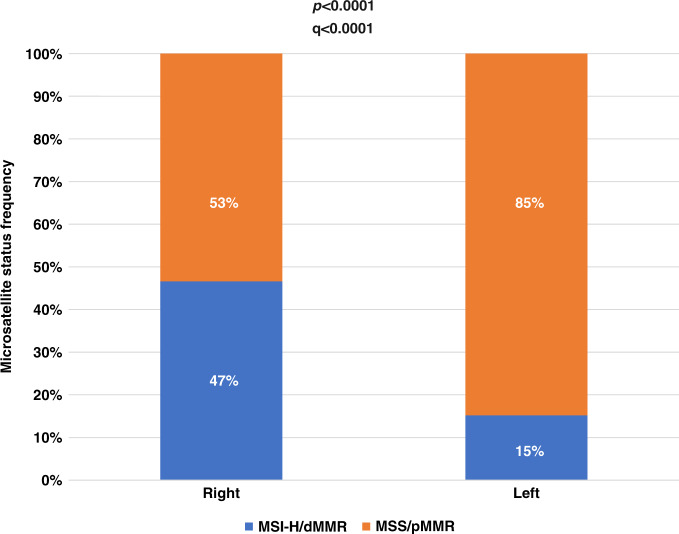
Of 1162 BRAFV600E mutant patients included in the platform, 165 tumours had an unknown primary location and were excluded from the analysis. Among the 997 remaining cases, 766 (76.8%) and 231 (23.1%) were right- and left-sided, respectively. Overall, 392 (39%) and 604 (61%) were MSI-H/dMMR and MSS/pMMR, respectively, and in 1 tumour microsatellite status was not available. Right-sided tumours were enriched in MSI-H/dMMR compared to left-sided tumours (47% vs 15%, p and q < 0.0001) (Fig. 3).
Fig. 3.
Frequency of microsatellite/mismatch repair status in both right- and left-sided tumours of the CGP population.
Considering the different biological characteristics between MSI-H/dMMR and MSS/pMMR tumours, the analyses were separately conducted in these two groups.
MSS/pMMR cohort
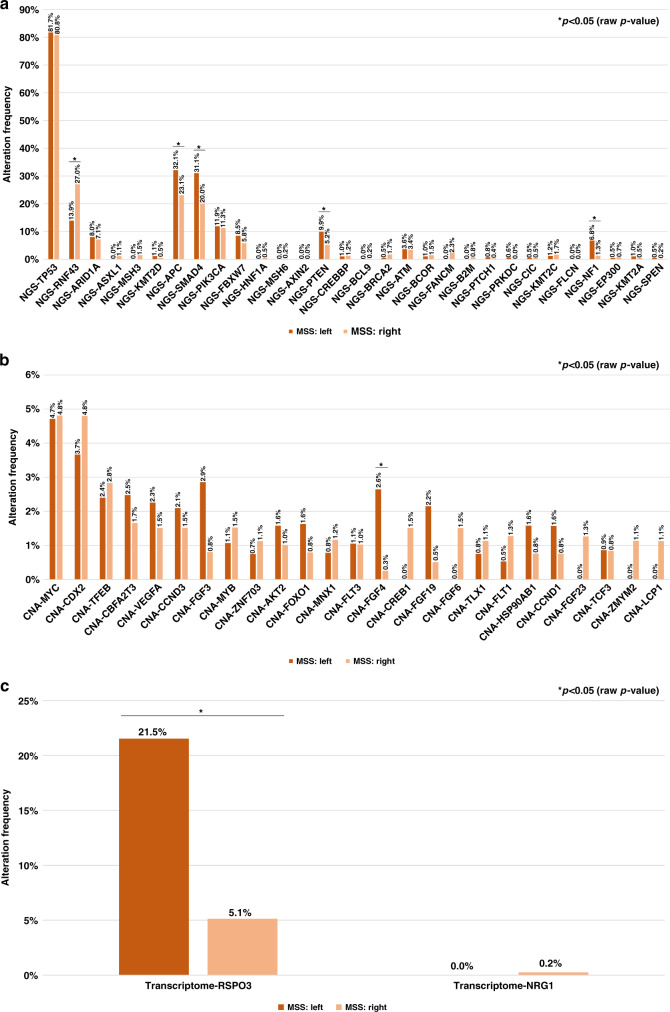
The most frequently mutated genes (>5%) were TP53, RNF43, ARID1A, APC, SMAD4, PIK3CA, FBXW7, PTEN and NF1. Among these, mutations in APC, SMAD4, PTEN and NF1 genes were more frequent in left-sided tumours, while RNF43 mutation was more common in right-sided ones (p < 0.05) (Fig. 4a). Amplification and fusion events were uncommon overall, but higher frequencies of FGF4 amplifications and RSPO3 fusions were observed in left-sided tumours (p < 0.05) (Fig. 4b and c).
Fig. 4. Frequency of most common alterations in MSS/pMMR cohort of the CGP population.
Mutations (a), amplifications (b), fusions (c).
Patients’ characteristics are summarised in Table 2. Patients with right-sided tumours were more frequently females (p = 0.018, q = 0.067) and older (p and q < 0.0001) compared with those with left-sided CRCs. TMB-high tumours were uncommon, while PD-L1 was expressed in the 15% of tumours without any difference according to sidedness. In addition, no difference in the distribution of CMS subtypes was observed between right- and left-sided tumours, with a limited prevalence of the CMS2 subtype overall.
Table 2.
Patients’ characteristics in the CGP population.
| Characteristics | Overall population N = 997 n (%) | MSS/pMMR population N = 604 | MSI-H/dMMR population N = 392 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Right-sided N = 409 (68%) n (%) | Left-sided N = 195 (32%) n (%) | p | q | Right-sided N = 357 (91%) n (%) | Left-sided N = 35 (9%) n (%) | p | q | ||
| Age | N = 997 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| Median | 70 | 68 | 60 | <0.0001a | <0.0001a | 76 | 78 | 0.60a | 0.78a |
| Range | 19–90 | 34–90 | 25–90 | 19–90 | 56–90 | ||||
| Sex | N = 997 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| Male | 375 (38%) | 168 (41%) | 100 (51%) | 0.018b | 0.067b | 94 (26%) | 12 (34%) | 0.31b | 0.56b |
| Female | 622 (62%) | 241 (59%) | 95 (49%) | 263 (74%) | 23 (66%) | ||||
| MS/MMR status | N = 996 | / | / | / | / | ||||
| MSI-H/dMMR | 392 (39%) | / | / | / | / | / | / | / | / |
| MSS/pMMR | 604 (61%) | / | / | / | / | ||||
| NA | 1 | / | / | / | / | ||||
| TMB-high (≥10 mut/Mb) | N = 997 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| Yes | 393 (39%) | 9 (2%) | 1 (1%) | 0.13b | 0.31b | 349 (98%) | 34 (97%) | 0.82b | 0.82b |
| No | 604 (61%) | 400 (98%) | 194 (99%) | 8 (2%) | 1 (3%) | ||||
| TMB (mut/Mb) | N = 997 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| Median | 6.5 | 5 | 4 | 0.15a | 0.34a | 39 | 34 | 0.33a | 0.56a |
| Range | 0–179 | 0–57 | 1–11 | 8–179 | 6–73 | ||||
| PD-L1 | N = 955 | N = 388 | N = 189 | N = 343 | N = 35 | ||||
| Yes | 203 (21%) | 54 (14%) | 29 (15%) | 0.65b | 0.79b | 108 (31%) | 12 (34%) | 0.73b | 0.80b |
| No | 752 (79%) | 334 (86%) | 160 (85%) | 235 (69%) | 23 (66%) | ||||
| NA | 42 | 21 | 6 | 14 | – | ||||
| CMS subtypes | N = 964 | N = 400 | N = 188 | N = 343 | N = 33 | ||||
| CMS1 | 584 (61%) | 177 (44%) | 80 (43%) | 0.50b | 0.69b | 297(87%) | 30 (91%) | 0.76b | 0.80b |
| CMS2 | 11 (1%) | 6 (1%) | 5 (3%) | 0 (0%) | 0 (%) | ||||
| CMS3 | 145 (15%) | 74 (19%) | 42 (22%) | 27 (8%) | 2 (6%) | ||||
| CMS4 | 224 (23%) | 143 (36%) | 61 (32%) | 19 (5%) | 1 (3%) | ||||
| NA | 33 | 9 | 7 | 14 | 2 | ||||
| BM subtypes | N = 996 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| BM1 | 385 (39%) | 215 (53%) | 85 (44%) | 0.0019b | 0.0139b | 82 (23%) | 3 (9%) | 0.046b | 0.17b |
| BM2 | 436 (44%) | 119 (29%) | 85 (44%) | 206 (58%) | 26 (74%) | ||||
| Unclear | 175 (17%) | 75 (18%) | 25 (12%) | 69 (19%) | 6 (17%) | ||||
| NA | 1 | – | – | – | – | ||||
| WNT pathway | N = 994 | N = 407 | N = 194 | N = 357 | N = 35 | ||||
| LD | 477 (48%) | 128 (31%) | 69 (36%) | 0.28b | 0.56b | 256 (72%) | 24 (69%) | 0.12b | 0.32b |
| LI | 191 (19%) | 92 (23%) | 63 (32%) | 30 (8%) | 6 (17%) | ||||
| Both | 55 (6%) | 3 (1%) | 0 (0%) | 48 (14%) | 4 (11%) | ||||
| Neither | 271 (27%) | 184 (45%) | 62 (32%) | 23 (6%) | 1 (3%) | ||||
| NA | 3 | 2 | 1 | – | – | ||||
| HD pathway | N = 997 | N = 409 | N = 195 | N = 357 | N = 35 | ||||
| HRD | 390 (39%) | 58 (14%) | 19 (10%) | 0.13b | 0.31b | 283 (79%) | 30 (86%) | 0.36b | 0.57b |
| HDP | 607 (61%) | 351 (86%) | 176 (90%) | 74 (21%) | 5 (14%) | ||||
| LOH | N = 330 | N = 131 | N = 58 | N = 128 | N = 12 | ||||
| High (≥16%) | 40 (12%) | 29 (22%) | 10 (17%) | 0.44b | 0.65b | 1 (1%) | 0 (0%) | 0.76b | 0.80b |
| Low (<16%) | 290 (88%) | 102 (78%) | 48 (83%) | 127 (99%) | 12 (100%) | ||||
| NA | 667 | 278 | 137 | 229 | 23 | ||||
| LOH | N = 330 | N = 131 | N = 58 | N = 128 | N = 12 | ||||
| Median | 6% | 10% | 8% | <0.0001a | <0.0001a | 3% | 3.5% | 0.0046a | 0.023a |
| Range | 0–33% | 0–33% | 2–22% | 0–15% | 1–14% | ||||
Bold values indicate statistical significance p < 0.05.
NA not available, N number, mut mutation, MS microsatellite, MMR mismatch repair, MSI-H microsatellite instability high, dMMR deficient mismatch repair, MSS microsatellite stable, pMMR proficient mismatch repair, TMB tumour mutational burden, Mb megabase, CMS consensus molecular subtype, BM BRAFV600E mutant, HD homologous recombination, HRD homologous recombination deficiency, HRP homologous recombination proficiency, LOH loss of heterozigosity.
aMann–Whitney test.
bChi-square test.
In left-sided tumours, a trend for higher TOP2A expression, target of irinotecan, was observed (p = 0.067), which was not significant after adjustment for multiple hypotheses testing. The expression of ERCC1 and ERCC2, targets of oxaliplatin, was not significantly different according to primary location (Supplementary Fig. 5).
MCP-counter analysis of the tumour microenvironment showed minimal differences based on sidedness, with an increase of some immune and stromal cell populations in right-sided CRC that were not significant after adjustment for multiple hypothesis testing (Supplementary Fig. 6A).
BM1/BM2 subtypes were equally distributed in left-sided tumours, whereas right-sided CRC had increased prevalence of BM1 (53% versus 44%, p = 0.0019, q = 0.0139) (Supplementary Fig. 7).
Among alterations implicated in the WNT pathway, RNF43 mutation, APC mutation and RPSO3 fusion were strongly mutually exclusive with each other (p < 0.05). CTNNB1 mutations had a tendency toward mutual exclusivity with these alterations but occurred at a very low frequency. Other WNT pathway alterations (BCL9, AMER1, CTNNA1 and AXIN2 mutations) frequently co-occurred with RNF43 mutation, but not with APC mutation, and were mutually exclusive with RSPO3 fusion (Supplementary Fig. 8A). LD alterations occurred at similar rates in left- and right-CRC, while LI alterations were numerically higher in left-sided CRC (32% versus 23%, p = 0.28, q = 0.56). Tumours harbouring both LD and LI alterations were rare in both left- and right-sided tumours.
Rates of HRD and LOH-High, as dichotomous variables, were similar in left- and right-sided CRC, although right-sided tumours had a higher median LOH (10% versus 8%, p and q < 0.0001).
MSI-H/dMMR cohort
Consistent with the high mutational burden associated with MSI-H/dMMR tumours, several genes were mutated, but no significant differences were observed based on sidedness (Supplementary Fig. 9A). On the other hand, amplification and fusion events were generally absent (Supplementary Fig. 9B and C).
Patients’ characteristics were listed in Table 2. Overall, 97%, 33% and 89% of MSI-H/dMMR tumours were TMB-high, PD-L1 positive and CMS1, respectively. CMS2 subtype was not found. No significant differences were reported for these molecular characteristics based on sidedness.
In left-sided tumours, a higher expression was observed for TOP2A (p = 0.0065, q = 0.030) and ERCC1 (p = 0.0049, q = 0.030) with a trend for ERCC2 (p = 0.087, q = 0.16) compared with right-sided ones (Supplementary Fig. 5).
MCP-counter analysis showed minimal differences based on sidedness with an increase of some immune and stromal cell populations in right-sided CRC that were not significant after adjustment for multiple hypothesis testing (Supplementary Fig. 6B).
Overall, MSI-H/dMMR tumours were enriched in BM2 subtype, with a higher rate of BM2 in left-sided tumours (74% versus 58%, p = 0.046, q = 0.17) (Supplementary Fig. 7).
Regarding WNT pathway alterations, APC and RNF43 mutations were mutually exclusive (p < 0.05). RSPO3 fusion had a tendency toward mutual exclusivity with other alterations but occurred at a very low frequency (Supplementary Fig. 8B). LD alterations were more common overall, with a similar frequency based on sidedness, while LI alterations were numerically higher in left-sided CRC (17% versus 8%, p = 0.12, q = 0.32). MSI-H/dMMR tumours harbouring both alterations were more common than MSS/pMMR ones, with a similar frequency between right- and left-sided CRC.
HRD was much more prevalent among MSI-H/dMMR tumours with respect to MSS/pMMR ones, yet no differences based on sidedness were observed. Median LOH was low in both right- and left-sided CRC, and LOH-high was identified in only one MSI-H/dMMR tumour.
Discussion
BRAFV600E-mutated CRCs were initially considered a homogeneous entity with distinct clinicopathological, molecular and prognostic features [2, 3, 5, 31–35]. Conversely, it has been recently shown that these tumours show heterogeneous characteristics both from a clinical and a molecular perspective. In fact, the median OS of BRAFV600E-mutated mCRC ranges from 6 to 30 months [16], and at least two distinct molecular subtypes (BM1 and BM2) have been identified [26], as well as two different mechanisms of Wnt signalling pathway activation (LD and LI) [27].
From a therapeutical point of view, although the combination of a BRAF inhibitor with an anti-EGFR agent has clearly demonstrated its efficacy in advanced lines of treatment of mCRC [36, 37] and is under investigation in the first-line setting, chemotherapy with or without bevacizumab is currently the standard upfront treatment [38, 39]. However, the use of the triplet versus doublets of chemotherapy is still debated, with recent data from the TRIBE2 randomised trial suggesting significant interaction effects between chemo-intensity and both primary tumour location and ECOG-PS. Indeed, even if limited by retrospective nature and limited sample size, a possible detrimental effect of triplet chemotherapy in BRAF-mutated patients with left-sided primary tumours or an ECOG-PS of 1 was observed [11].
Considering the limitations of these data, we challenged these findings in a larger real-world population of 296 BRAF-mutated mCRC patients treated with upfront doublet or triplet ± bev [16]. To mitigate the intrinsic clinical selection bias of this series, only patients potentially eligible for an intensified regimen according to their age and ECOG-PS (i.e. aged 18–70 years with ECOG-PS of 2 or less, aged 71–75 years with an ECOG-PS of 0 and no previous oxaliplatin-based adjuvant therapy) were included [18]. The interaction effect was confirmed for primary tumour location, but not for ECOG-PS. In particular, patients with right-sided primary tumours showed a clear benefit from FOLFOXIRI ± bev, while those with left-sided tumours showed a possible detrimental effect from the triplet. These findings were more pronounced when the analysis was restricted to patients treated with an oxaliplatin-based doublet in the control arm. Taking into account the association of BRAFV600E mutation and MSI-H/dMMR status [3, 33], and the new first-line standard, i.e. the anti-PD1 pembrolizumab, for patients with MSI-H/dMMR tumours irrespective of BRAF mutation [40], we restricted the analysis to the subgroup with MSS/pMMR tumours. Although this analysis was hampered by reduced sample size for the lack of microsatellite status in about one-third of cases, a similar trend was observed when comparing the triplet with the oxaliplatin-based doublet.
In right-sided tumours, the higher prevalence of younger patients and with ECOG-PS of 0 in the group treated with FOLFOXIRI ± bev may have contributed to the better outcome observed in this group. However, considering the lack of any interaction effect between chemo-intensity and ECOG-PS and the median age abundantly under 70 years in both groups, this hypothesis seems unlikely.
Subsequently, we evaluated whether the different efficacy of treatments based on primary tumour location was underpinned by relevant molecular differences using a large dataset of 997 BRAFV600E-mutated CRC samples that underwent comprehensive molecular profiling. Again, considering the association between BRAFV600E mutation and MSI-H/dMMR status, the biological differences between MSI-H/dMMR and MSS/pMMR tumours, and the higher frequency of MSI-high/dMMR status in the right side of the colon [41], we separately analysed these two populations.
Overall, a different molecular profile between MSS/pMMR and MSI-H/dMMR was confirmed in BRAF-mutated tumours, as expected. In the MSI-H/dMMR group, no substantial differences were observed based on sidedness. On the other hand, a few differences between right- and left-sided tumours were reported in the MSS/pMMR group, including both clinical and biological parameters. The higher expression of TOP2A in left-sided tumours may cause decreased activity of irinotecan and consequently lower efficacy of FOLFOXIRI compared with FOLFOX in left- versus right-sided colon. However, this difference was not statistically significant after adjustment for multiple hypotheses testing, and this hypothesis would hardly explain the potential detrimental effect of treatment intensification in left-sided tumours. In the right-sided group, the higher representation of BM1 subtypes may justify the better efficacy of the triplet. Indeed, similar findings were also reported in the subgroup analysis of BRAF-mutated patients included in the TRIBE2 study where an interaction effect, albeit not statistically significant probably due to the small sample size, was suggested between chemo-intensity and BM subtypes, with a better outcome for BM1 tumours treated with FOLFOXIRI and bev [11]. However, the lack of a biological rational supporting a reduced efficacy of irinotecan in BM2 subtype limits the above interpretation.
Previous experience reported a differential response to oxaliplatin and irinotecan based on CMS classification. In particular, patients with CMS2 tumours achieve a higher response to oxaliplatin, while CMS4 tumours benefit more from irinotecan compared to other subtypes [42–44]. However, in our study, no difference distribution of CMS subtypes was shown between right- and left-sided tumours in both MSS/pMMR and MSI-H/dMMR populations.
Overall, the molecular similarity between right- and left-sided cancers could justify the comparable prognosis found in the BRAF-Be-Cool cohort, differently from what is reported in the literature for RAS/BRAF wild-type mCRC [12, 13].
The lack of information about the stage of disease, administered treatment and survival outcome of patients included in CGP population prevents us from drawing any predictive or prognostic conclusions. In particular, both the frequency of gene alterations and their clinical impact may differ in the early and in the metastatic disease as already reported for several markers [45–48]. Indeed, the restriction of molecular analyses to mCRC patients would have made the BRAF-Be-Cool and CGP populations more clinically similar and would have increased the possibility of finding molecular differences according to sidedness. Furthermore, the CpG islands methylation phenotype, features associated with BRAFV600E mutant tumours [49], was not assessed in this analysis for the lack of methylation profiling.
In conclusion, clinical data suggest a different efficacy of triplet and doublet chemotherapy based on primary tumour location in BRAF-mutated mCRC, although in the absence of a clear biological rationale.
Although considering the high sample size of the BRAF-Be-Cool cohort and the adoption of clinical criteria to select only patients potentially eligible for the triplet, the present findings are exploratory and would require prospective evaluation. Moreover, results of the ongoing BREAKWATER trial, assessing the combination of anti-EGFR and anti-BRAF agents alone or with FOLFOX in first-line will probably change the treatment landscape of BRAFV600E mutant mCRC, thus making sidedness less relevant in the future clinical practice. However, waiting for these results [39], our findings may be useful to refine the choice of the upfront treatment in the MSS population.
Supplementary information
reproducibility checklist that details key elements of the experimental and analytical design of the submission
Supplementary Table 1_patients characteristics
Supplementary Table 2_uni- e multivariate analysis
Supplementary Figure 2_PFS and OS based on primary tumor location
Supplementary Figure 3_PFS and OS in the MSS population
Supplementary Figure 4_PFS and OS based on ECOG-PS
Supplementary Figure 5_expression of TOP2A-ERCC1-ERCC2
Supplementary Figure 6_immune-stromal cells
Supplementary Figure 8_WNT co-occurrence
Supplementary Figure 9_alteration frequency in MSI-H
Acknowledgements
We are grateful to the “BRAF BeCool” investigators from the participating Italian centres.
Author contributions
Study concepts: RM, DR, CC, AE, WMK. Study design: RM, DR, CC, AE, WMK. Data acquisition: RM, DR, SL, PB, RI, VC, FP, AS-B, CA, CR, MS, MS, NP, MAC, MC, FC, GM. Quality control of the data and algorithms: RM, DR, PB. Data analysis and interpretation: RM, DR, CC, AE, PB. Statistical analysis: DR, AE, PB. Paper preparation: RM, DR, CC, AE. Paper editing: all authors. Paper review: all authors.
Funding
The present work was partially funded by Regione Veneto—grant RP-2014-00000395 and by Italian Health Ministry Grant 2019 GR-2019-12368903.
Data availability
Datasets supporting the results of this work are available to editors, referees and readers promptly upon request.
Competing interests
AE: Employee of Caris Life Sciences. PB: Employee of Caris Life Sciences. FP: honoraria for speaker activities and participation in advisory boards from Sanofi, Amgen, Bayer, Merck-Serono, Lilly, Astrazeneca, Servier, Organon, MSD; and research grants from BMS and Astrazeneca. AS-B: Honoraria: Amgen, Bayer, MSD, Servier. Consulting or advisory role—Amgen, Bayer, Novartis, Sanofi, Servier. MS: Advisory board, speakers’ bureau MERCK, MSD, Sanofi, Amgen, BMS, EISAI, Servier. GM: Honraria—Amgen, F. HoffmaneLa Roche, Bayer, Merk Serono, Sirtex. SL: Speakers’ Bureau—Amgen, Merck, Roche, Lilly, Bristol-Myers Squibb, Pierre-Fabre, GSK and Servier. Consulting or advisory role—Amgen, MSD, Merck Serono, Lilly, Astra Zeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, Servier, Research funding to Institution—Bayer, Merck, Amgen, Roche, Lilly, Astra Zeneca Bristol-Myers Squibb. WMK: Consultant—Merck. Employee of Caris Life Sciences. CC: honoraria—Amgen, Bayer, Merck, Roche and Servier. Consulting or advisory role—Amgen, Bayer, MSD, Roche. Speakers’ Bureau—Servier. Research funding—Bayer, Merck, Servier. Travel, accommodations and expenses—Roche and Servier. The remaining authors declare no competing interests.
Ethics approval and consent to participate
BRAF BeCool study was conducted in accordance with the Declaration of Helsinki. Approval for BRAF BeCool protocol was obtained from local ethics committees of participating sites.
Consent to publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Roberto Moretto, Andrew Elliott.
These authors jointly supervised this work: Michael Korn, Chiara Cremolini.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01852-0.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–7. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 3.Tran B, Kopetz S, Tie J, Gibbs P, Jiang Z-Q, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris V, Overman MJ, Jiang Z-Q, Garrett C, Agarwal S, Eng C, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164–71. doi: 10.1016/j.clcc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. 2017;28:562–8. doi: 10.1093/annonc/mdw645. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 7.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–18. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 8.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 9.Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 10.Cremolini C, Antoniotti C, Stein A, Bendell J, Gruenberger T, Rossini D, et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J Clin Oncol. 2020. 10.1200/JCO.20.01225 [DOI] [PubMed]
- 11.Moretto R, Giordano M, Poma AM, Passardi A, Boccaccino A, Pietrantonio F, et al. Exploring clinical and gene expression markers of benefit from FOLFOXIRI/bevacizumab in patients with BRAF-mutated metastatic colorectal cancer: subgroup analyses of the TRIBE2 study. Eur J Cancer. 2021;153:16–26. doi: 10.1016/j.ejca.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Arnold D, Lueza B, Douillard J-Y, Peeters M, Lenz H-J, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29. doi: 10.1093/annonc/mdx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. doi: 10.1016/j.ejca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Cremolini C, Morano F, Moretto R, Berenato R, Tamborini E, Perrone F, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28:3009–14. doi: 10.1093/annonc/mdx546. [DOI] [PubMed] [Google Scholar]
- 15.Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol. 2019;37:3099–110. doi: 10.1200/JCO.19.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loupakis F, Intini R, Cremolini C, Orlandi A, Sartore-Bianchi A, Pietrantonio F, et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: the “BRAF BeCool” study. Eur J Cancer. 2019;118:121–30. doi: 10.1016/j.ejca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Caris Life Sciences. Caris Molecular Intelligence Tumor Profiling—Enabling Precision Medicine n.d. 2021. https://www.carismolecularintelligence.com/. Accessed Nov 2021.
- 18.Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015;12:607–19. doi: 10.1038/nrclinonc.2015.129. [DOI] [PubMed] [Google Scholar]
- 19.Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:dju427. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805–12. doi: 10.1158/1541-7786.MCR-17-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7:746–56. doi: 10.1002/cam4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham JP, Magee D, Cremolini C, Antoniotti C, Halbert DD, Xiu J, et al. Clinical validation of a machine-learning-derived signature predictive of outcomes from first-line oxaliplatin-based chemotherapy in advanced colorectal cancer. Clin Cancer Res. 2021;27:1174–83. doi: 10.1158/1078-0432.CCR-20-3286. [DOI] [PubMed] [Google Scholar]
- 23.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 24.Merino DM, McShane LM, Fabrizio D, Funari V, Chen S-J, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8:e000147. doi: 10.1136/jitc-2019-000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becht E, Reyniès A, de, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–66. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 26.Barras D, Missiaglia E, Wirapati P, Sieber OM, Jorissen RN, Love C, et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res. 2017;23:104–15. doi: 10.1158/1078-0432.CCR-16-0140. [DOI] [PubMed] [Google Scholar]
- 27.Kleeman SO, Koelzer VH, Jones HJ, Vazquez EG, Davis H, East JE, et al. Exploiting differential Wnt target gene expression to generate a molecular biomarker for colorectal cancer stratification. Gut. 2020;69:1092–103. doi: 10.1136/gutjnl-2019-319126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moretto R, Elliott A, Zhang J, Arai H, Germani MM, Conca V, et al. Homologous recombination deficiency alterations in colorectal cancer: clinical, molecular, and prognostic implications. J Natl Cancer Inst. 2021. 10.1093/jnci/djab169. [DOI] [PMC free article] [PubMed]
- 29.Coleman RL, Swisher EM, Oza AM, Scott CL, Giordano H, Lin KK, et al. Refinement of prespecified cutoff for genomic loss of heterozygosity (LOH) in ARIEL2 part 1: a phase II study of rucaparib in patients (pts) with high grade ovarian carcinoma (HGOC) J Clin Oncol. 2016;34:5540–5540. doi: 10.1200/JCO.2016.34.15_suppl.5540. [DOI] [Google Scholar]
- 30.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91. doi: 10.1016/S1470-2045(17)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–72. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy C, Burke JP, Kalady MF, Coffey JC. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e711–718. doi: 10.1111/codi.12427. [DOI] [PubMed] [Google Scholar]
- 33.Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–30. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19. doi: 10.1016/S1470-2045(16)30500-9. [DOI] [PubMed] [Google Scholar]
- 35.Loupakis F, Moretto R, Aprile G, Muntoni M, Cremolini C, Iacono D, et al. Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br J Cancer. 2016;114:30–6. doi: 10.1038/bjc.2015.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381:1632–43. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 37.Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E–mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–84. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grothey A, Tabernero J, Taieb J, Yaeger R, Yoshino T, Maiello E, et al. LBA-5 ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2020;31:S242–3. doi: 10.1016/j.annonc.2020.04.080. [DOI] [Google Scholar]
- 39.Kopetz S, Grothey A, Yaeger R, Ciardiello F, Desai J, Kim TW, et al. BREAKWATER: Randomized phase 3 study of encorafenib (enco) + cetuximab (cet) ± chemotherapy for first-line treatment (tx) of BRAF V600E-mutant (BRAFV600) metastatic colorectal cancer (mCRC) J Clin Oncol. 2022;40:TPS211–TPS211. doi: 10.1200/JCO.2022.40.4_suppl.TPS211. [DOI] [Google Scholar]
- 40.André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 41.Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19–26. doi: 10.1016/j.ctrv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19:619–25. doi: 10.1038/nm.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song N, Pogue-Geile KL, Gavin PG, Yothers G, Kim SR, Johnson NL, et al. Clinical Outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol. 2016;2:1162–9. doi: 10.1001/jamaoncol.2016.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aderka D, Stintzing S, Heinemann V. Explaining the unexplainable: discrepancies in results from the CALGB/SWOG 80405 and FIRE-3 studies. Lancet Oncol. 2019;20:e274–83. doi: 10.1016/S1470-2045(19)30172-X. [DOI] [PubMed] [Google Scholar]
- 45.Zaanan A, Bachet J-B, André T, Sinicrope FA. Prognostic impact of deficient DNA mismatch repair and mutations in KRAS, and BRAFV600E in patients with lymph node-positive colon cancer. Curr Colorectal Cancer Rep. 2014;10:346–53. doi: 10.1007/s11888-014-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 47.Taieb J, Shi Q, Pederson L, Alberts S, Wolmark N, Van Cutsem E, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30:1466–71. doi: 10.1093/annonc/mdz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Formica V, Sera F, Cremolini C, Riondino S, Morelli C, Arkenau H-T, et al. KRAS and BRAF mutations in stage II/III colon cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2021. 10.1093/jnci/djab190. [DOI] [PMC free article] [PubMed]
- 49.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
reproducibility checklist that details key elements of the experimental and analytical design of the submission
Supplementary Table 1_patients characteristics
Supplementary Table 2_uni- e multivariate analysis
Supplementary Figure 2_PFS and OS based on primary tumor location
Supplementary Figure 3_PFS and OS in the MSS population
Supplementary Figure 4_PFS and OS based on ECOG-PS
Supplementary Figure 5_expression of TOP2A-ERCC1-ERCC2
Supplementary Figure 6_immune-stromal cells
Supplementary Figure 8_WNT co-occurrence
Supplementary Figure 9_alteration frequency in MSI-H
Data Availability Statement
Datasets supporting the results of this work are available to editors, referees and readers promptly upon request.