Abstract
Frailty is a complex syndrome affecting a growing sector of the global population as medical developments have advanced human mortality rates across the world. Our current understanding of frailty is derived from studies conducted in the laboratory as well as the clinic, which have generated largely phenotypic information. Far fewer studies have uncovered biological underpinnings driving the onset and progression of frailty, but the stage is set to advance the field with preclinical and clinical assessment tools, multiomics approaches together with physiological and biochemical methodologies. In this article, we provide comprehensive coverage of topics regarding frailty assessment, preclinical models, interventions, and challenges as well as clinical frameworks and prevalence. We also identify central biological mechanisms that may be at play including mitochondrial dysfunction, epigenetic alterations, and oxidative stress that in turn, affect metabolism, stress responses, and endocrine and neuromuscular systems. We review the role of metabolic syndrome, insulin resistance and visceral obesity, focusing on glucose homeostasis, adenosine monophosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), and nicotinamide adenine dinucleotide (NAD+) as critical players influencing the age-related loss of health. We further focus on how immunometabolic dysfunction associates with oxidative stress in promoting sarcopenia, a key contributor to slowness, weakness, and fatigue. We explore the biological mechanisms involved in stem cell exhaustion that affect regeneration and may contribute to the frailty-associated decline in resilience and adaptation to stress. Together, an overview of the interplay of aging biology with genetic, lifestyle, and environmental factors that contribute to frailty, as well as potential therapeutic targets to lower risk and slow the progression of ongoing disease is covered.
Introduction
“Today, for the first time in history most people can expect to live into their 60s and beyond (2015)” (623). This increase in life expectancy is reflected in the current world population of approximately 7.7 billion (end of 2020) whereby approximately 1.0 billion people (13%) are over the age of 60 years. This age group, 60 years and older, is expected to grow significantly to 1.6, 2.1, and 3.1 billion in 2035, 2050, and 2100, respectively, and there will be a concomitant growth in many serious health concerns such as an increased risk for chronic and metabolic diseases [e.g., cardiovascular disease (CVD), cancer, and Alzheimer’s disease], a decline in intrinsic capacities (e.g., mobility, cognition, psychological, vitality, hearing, and vision capacities) and a loss of resilience (i.e., the ability to resist or recover from adverse events) (https://population.un.org/wpp/) (45, 216, 453, 624). In fact, more than 80% of people older than 65 years have at least one chronic disease, which increases to at least three diseases by 72 years (74, 595). Consequently, the length and the severity of late-life multimorbidity leads to poor health (e.g., disabilities) requiring care and/or help with the activities of daily living. The burden of these conditions creates enormous clinical, social, and economic needs for healthcare systems on a worldwide level (32, 33, 342, 372).
Frailty is unquestionably one of the most serious worldwide challenges in the 21st century (253). Based on the aging demographics outlined above it is anticipated that the number of older adults with recognized frailty will significantly increase worldwide (402, 638). Over the past two decades, impressive scientific progress yielded great strides in the field of clinical frailty research; yet, many gaps remain including the lack of a universally accepted clinical definition of frailty. Nonetheless, the leaders in the field agree that frailty is a state of physiological vulnerability to stressors that results from age-related declines in biological systems, manifests clinically as greater risk of adverse health outcomes, and leads to a vicious cycle that results in further functional decline and disability (72, 87, 94, 106, 109, 249, 315, 399, 443, 597, 628, 630). Frailty is also considered a dynamic condition that occurs on a continuum from fit or robust to frail in which individuals transition in and out of the states of frailty (nonfrail, prefrail, frail) and in either direction over time (106, 137, 278, 478).
Up until now the reported prevalence of frailty from a worldwide perspective depends on many factors including operational definition of frailty, age, sex, socioeconomic status, race/ethnicity, environmental setting, and the approach to classify frailty (100, 122, 417, 418, 420, 493, 531). For instance, in one of the first published systematic reviews of frailty prevalence, the overall global prevalence of frailty was 11% (range 4%–59%, the year 2012) (109). Systematic reviews and meta-analysis found an overall frailty prevalence of 18% with the highest prevalence of frailty observed among hospital inpatients (~50%) or long-term care settings (>60%), 30% prevalence in primary care and out-patient settings, and a median rate of 10.8% in community-dwelling settings [ranging from 2% to 60% (142, 416, 417, 422)]. Another review evaluated the prevalence of frailty among community-dwelling older adults in low-income and middle-income countries and reported a 17.4% prevalence (531). To highlight the prevalence of frailty in the context of aging, the prevalence rate increases with advancing age, from 6.5% in those aged 60 to 69 years to 65% in those aged 90 or over with frailty occurring more frequently in women than in men (16% vs. 12%) (190).
Despite these staggering statistics on the prevalence of frailty, it is important to note that frailty is not an inevitable consequence of aging and, even at advanced ages, many people do not become frail. As a matter of fact, in the aging population, there are enormous inter-individual differences in terms of the decline in health, the onset of disabilities, and life expectancy (45, 453, 474). Indeed, some people die of age-related disease in their 60s, whereas there are active people at 100 years of age. Importantly, frailty is not limited to older people: frailty and prefrailty states can exist in individuals younger than 60 years, particularly among those with multimorbidity (coexistence of two or more diseases) (266, 431).
Evidence suggests that multimorbidity is a risk factor for frailty (151, 155, 178, 589, 592). For instance, a meta-analysis examining the relationship between frailty and multimorbidity (>14,000 community-dwelling older adults, nine studies) reveals that about three-quarters of people with frailty present with multimorbidity, and that frailty is present in 16% of people with multimorbidity (589). Consistent with these findings, a prospective analysis of approximately half a million participants shows that frailty is associated with multimorbidity, reaching a frailty prevalence of 18% among participants with four or more diseases (219). A very impactful and recently published study highlights the impact of multimorbidity on the progression of frailty such that the overall decline in health (trajectories) of people with frailty associated with multiple diseases shows an early onset of frailty, a reduced period of prefrail status, and a rapid progression to frailty compared to people classified with age-associated frailty (16). Importantly, from the perspective of this comprehensive article, these findings suggest that the underlying biological mechanisms involved in the onset of frailty related to disease are different from those involved in age-related frailty (554). Considering the current clinically based frailty conceptualization it is likely age-related frailty emerges as the physiological reserves decline beyond a threshold (declines at the cellular and molecular level across multiple systems or a specific set of critical systems) and in the presence of low resilience and resistance (612). As shown in Figure 1, the cellular and molecular components that contribute to aging biology likely contribute to the overall decline in health overtime and to the increased risk in age-related frailty. From this perspective, two individuals of the same chronological age may respond to the same stressor quite differently. Further, the observed continuum of frailty and the stages of frailty (nonfrail, prefrail, frail) reflect the amount of physiological capacities (functional, intrinsic) available to react to the health stressors.
Figure 1. Health, frailty, and aging.
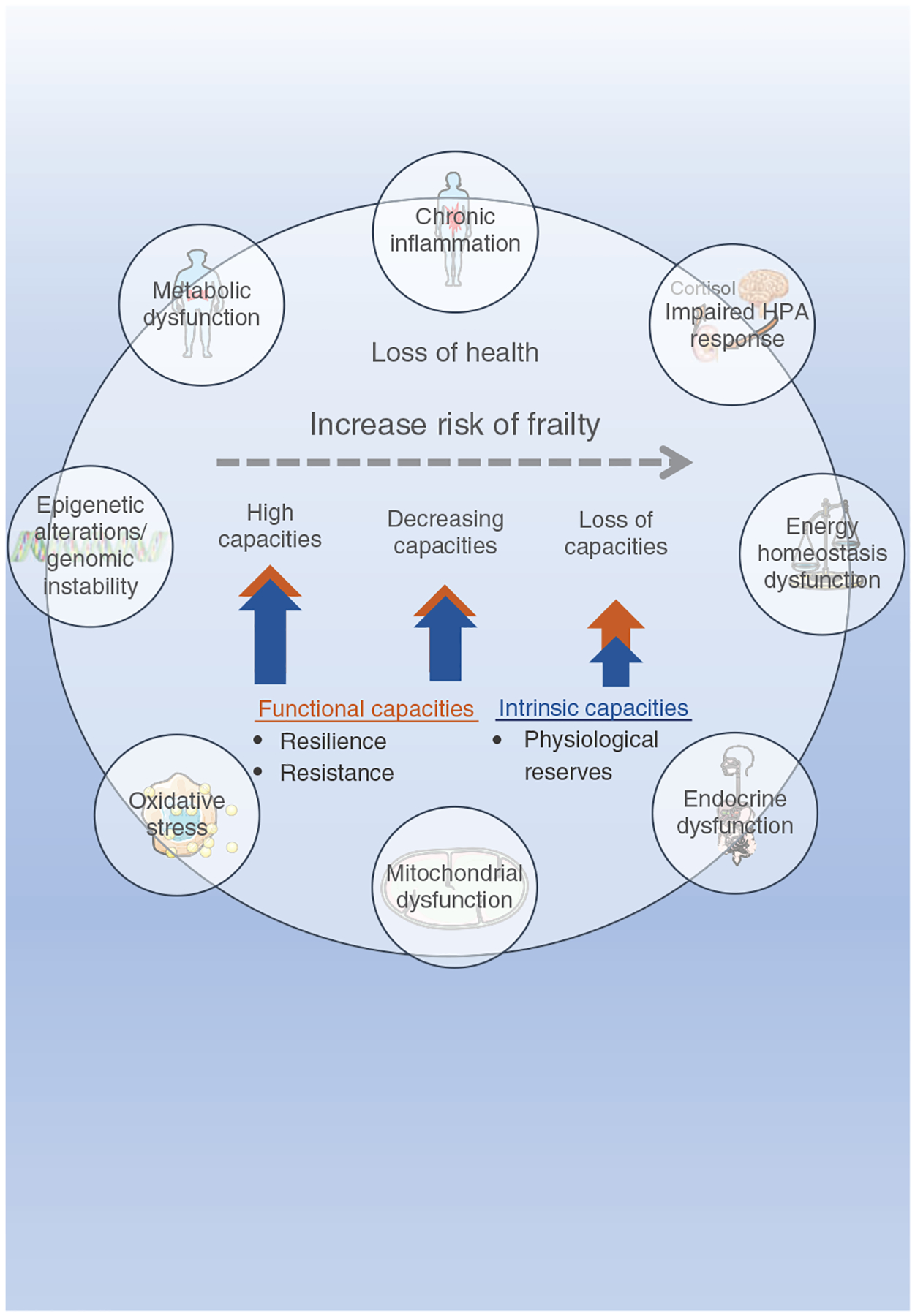
Frailty is characterized by a loss of health and is classified as an age-related medical syndrome that features the progressive reduction of health-promoting capacities. The health-promoting capacities are determined by functional capacities, when referring to both resilience and resistance abilities, and intrinsic capacities, when referring to physiological reserves. The substantial loss of these capacities increases the risk of frailty via dysregulation of multiple physiological systems. At the molecular level, epigenetic alterations, genomic instability, mitochondrial dysfunction, and oxidative stress are great contributors to impaired physiology that includes metabolic, energy homeostasis and endocrine dysfunction, chronic inflammation as well as impaired hypothalamic-pituitary-adrenal (HPA) axis response. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Impact of Geroscience
Geroscience, a relatively recent interdisciplinary field, is poised to play a critical role in defining the mechanisms underlying the continuum of frailty and the identified stages because it seeks to determine the molecular and cellular components at the intersection of the biology of aging, aging physiology, and the biology of age-related diseases (27, 311, 435, 508, 524). During the past decade, the field of Geroscience emerged due to significant advances in the understanding of the molecular and cellular pathways that drive the aging process and the ability to modify the rate of aging (27, 283, 300, 359, 368). For example, the rate of aging is modified by various interventions including behavioral, genetic, and pharmacological interventions (226, 227, 270–273, 375). These interventions also show remarkable improvements in aspects of health in older age groups, which is viewed as slowing the rate of aging in humans (357). From the efforts by the National Institute on Aging (NIA)-supported Geroscience Network the origins of the well-recognized Geroscience hypothesis, the Pillars of Aging, and the Hallmarks of Aging emerged (522). The Geroscience hypothesis states that, by reducing the rate of aging, it is possible to delay or slow down the appearance and progression of most age-related chronic diseases, in parallel (311, 435, 508, 524). Whereas, the noted Pillars of Aging and Hallmarks of Aging provide a foundation to systematically investigate and understand the multitude of pathways that drive aging (300, 359).
Historically speaking and highlighted in the Geroscience hypothesis stated above, the focus of Geroscience sought to tease out the biological underpinnings for why aging is the major risk factor for disease. However, the field soon recognized health was more than just the absence of disease (523). This major shift to or the focus on health led to the idea that aging is a main driver for the general loss of functional capacities and the development of aging phenotypes, even in the absence of overt disease. In this scenario resilience, resistance, and physiological reserves play critical roles. Resilience is an established area of investigation by researchers and clinicians in many disciplines and the definition of resilience is somewhat similar across the sciences. Resilience within the discussion of health is the ability to resist or recover from adverse events after an acute or chronic health stressor (Figure 1) (216, 573, 612, 613). In contrast to resilience, resistance is the ability to prevent or counter exogenous and endogenous stressors. Resilience is reported to decline with age when there is an increased risk of health stressors (305, 335). Physiological reserve is defined as the potential capacity of a cell, tissue, or organ system to function beyond its basal level in response to alterations in physiologic demands and is consistent with the term “intrinsic capacity” introduced by World Health Organization (WHO) (613, 624). The capability to respond, resist, or adapt to stress is dependent on multiple factors including the physiological reserves present within the collective physiological systems, the extent of the stressor, and the presence of co-existing stressors or exposure to previous stressors. Thus, in the presence of low physiological reserves across multiple physiological systems, the physiological potential to respond is greatly reduced and likely contributes to frailty. Indeed, it is possible to target the understanding of frailty by examining specific characteristics of resilient profiles (e.g., nonfrail vs. frail). For instance, at the cellular and molecular level, aberrations within the deoxyribonucleic acid (DNA) repair pathways decrease the ability to recover from DNA damaging agents (e.g., chemotherapy). Imbalances in proteostasis and increased mitochondrial damage influence stress responses, whereas interruptions in stem cells (SCs) impair tissue regeneration after injury. Importantly, many of these stress-response pathways are part of a complex integrative regulatory network that becomes dysfunctional resulting in decreased resilience.
Lastly, the field of Geroscience is still in its early days; however, the potential impact in teasing out the underlying mechanisms contributing to frailty is high. Indeed, in May 2021 the National Geroscience Initiative (people and organizations from the academic, not-for-profit, industry, and philanthropy sectors) launched a White Paper with the goal to utilize the biology of aging to optimize human performance, healthspan (defined as the portion of life that is relatively healthy and free from major deficits that impair the quality of life) and lifespan, which will yield substantial benefits to the quality of life for the aging adult (51, 250).
Considering the growing worldwide aging population, the frailty prevalence rate, the close relationships between frailty, aging and chronic disease, the field of Geroscience, and the impact of physiological reserve and physical resilience on health, there are substantial benefits to systematically evaluate the cellular and molecular factors contributing to frailty (239, 349). In this article, we provide comprehensive coverage of topics regarding what is known about factors that contribute to frailty. We base this information quite loosely on Pillars and Hallmarks of Aging; markers and processes established by leading researchers in the field of aging that are highly associated and interconnected with the aging phenotype (300, 359, 560). These factors are not necessarily causes of aging but are more so common denominators in aging phenotypes across species. The cause(s) of aging and frailty has not been identified at this time. Understanding the process of aging is the ultimate goal of the Geroscience field. A better appreciation of the elements underlying frailty is a necessity to move this goal forward. Before discussing the fundamental processes, we first review the literature whereby the clinical frailty assessment tools that classify people along the continuum of frailty were reverse-translated to preclinical animal models. With that information in mind, an assemblage of cellular and molecular evidence underlying aging biological mechanisms is then presented in terms of their potential contributions to frailty.
Frailty Assessments in Clinical Practice
Prompt identification of frailty is crucial, especially during the early stages, to maximize opportunities for intervention (475, 621). Within the past few decades, many clinically based frailty assessment tools emerged based on human performance measures, biomarkers, questionnaires, routine geriatric evaluations, or a combination [e.g., Frailty Phenotype, Frailty Index (FI), Clinical Frailty Scale, FRAIL scale, biomarker-based FI, Study of Osteoporotic Fractures frailty criteria, PRISMA-7, Tilburg Frailty Indicator, Groningen Frailty Indicator, Short Physical Performance Battery, Edmonton Frailty Scale] (137, 322, 394). To date, there are two popular, well-established approaches to assess frailty clinically that are validated in many populations and across multiple clinical and living settings: Physical Frailty Phenotype and FI of deficit accumulation (Table 1).
Table 1.
Two Common, Well-established Clinical Frailty Assessment Tools
| Physical Frailty Phenotype |
| Evaluates five clinical hallmarks (phenotypic criteria of signs and symptoms) |
Criteria
|
| Frailty states: nonfrail (0 criteria present), prefrail (1–2 criteria present), and frail (≧3 criteria present) |
| Frailty Index of accumulative deficits |
| Counts health deficits (at least 30), such as signs, symptoms, diseases, disabilities |
Health deficits should meet these criteria:
|
| Frailty score: sum of health deficits present divided by total number of deficits measured |
| Continuous score between 0 and 1, higher scores indicate higher degree of frailty, with ≧0.25 indicating frailty |
The Physical Frailty Phenotype consists of clinical hallmarks of weight loss, weakness, poor endurance/exhaustion, slowness, and low physical activity, core features hypothesized to be proxies of manifestations of dysregulation in specific physiologic domains (179). The Frailty Index hypothesizes that the accumulation of health and functional problems serves as an indicator of an individual’s aging-related health state and consists of a minimum of 30 items (the number of deficits rather than the specific type of health deficit) (395). Both frailty assessment tools are useful for identifying vulnerable adults at higher risk for adverse health outcomes.
In the Physical Frailty Phenotype approach, frailty is defined as a “biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes” (177, 179). Within this conceptualization, the biological basis of frailty is focused on altered stress response systems and energy metabolism abnormalities that drive the appearance of signs and symptoms. The Physical Frailty Phenotype consists of five clinical hallmarks (phenotypic criteria) of weight loss, weakness, poor endurance/exhaustion, slowness, and low physical activity, core features hypothesized to be proxies of manifestations of dysregulation in specific physiological domains (Table 1). Weight loss is defined as unintentional weight loss of more than 4.5 kg or 10 pounds within a year (score = 1). Weakness is identified by a grip strength test (handheld dynamometer) and is in the lowest 20% by sex and body mass index (BMI) (score = 1). Poor endurance/exhaustion is identified with self-reported positive responses to specific questions from the US Center for Epidemiologic Studies Depression Scale, 3 to 4 days/week or most of the time (score = 1) (459). Slowness is identified by a timed gait speed test (walking time/15 feet or 4.57 m) and is in the lowest 20% by sex and height (score = 1). Low physical activity is identified by the Physical Activity Scale for the elderly with energy expenditure in the lowest 20% by sex, <383 kcal/week (men) and <270 kcal/week (female) (score = 1) (605). The stages of frailty are scored across a range from 0 to 5. Frailty is then identified when 3 or more of the five phenotypic criteria are present, which indicates diminished stress response and energetics. Prefrail is identified when 1 or 2 of the five phenotypic criteria are present, which signifies a high risk of progressing to frailty. Nonfrail is identified when 0 of the phenotypic criteria are present.
In contrast, the second approach is identified as the deficit accumulation frailty and hypothesizes that the accumulation of health and functional problems serves as an indicator of an individual’s aging-related health state (395). Specifically, frailty is defined “as a continuous process characterized as a multidimensional syndrome of loss of reserves (physical ability, cognition, health, energy) that gives rise to vulnerability.” Within this conceptualization, there is an established FI, which measures a wide range of health assessments (cognition, motivation, mood, communication, mobility, balance, activities of daily living, nutrition, bowel and bladder function, comorbidities, laboratory abnormalities, as well as social resources) with more deficits conferring greater risk of mortality (Table 1). Each deficit is scored as 0 if absent and 1 if present, and a ratio is calculated by the actual number of health deficits in an individual divided by the total number of potential health deficits that were measured. The FI provides a score on a scale from 0 (no deficits) to 1 (all items exhibit deficits). Importantly, the FI is focused on the number of deficits (a minimum of 30) rather than the specific type of the health deficit (473, 536).
Both approaches to assess frailty (Physical Frailty Phenotype, FI) are useful for identifying vulnerable adults at higher risk for mortality and have been used extensively since conception (28, 601, 629). It is worth noticing that comparisons between these two clinically based frailty assessment tools show predictive validity (adverse outcomes) even in the presence of a high degree of heterogeneity with respect to the selection of tests used to meet criteria and to the inclusion of reference standards and their thresholds to determine cut-off values. In fact, these two well-established frailty assessment tools classify different groups of older adults and mice as frail, prefrail, or nonfrail indicating a discordance (104, 292, 629). It is reasonable to assume this reported discordance in specific assignment to frailty subgroups aligns with the assessment tool’s theoretical construct (physical frailty vs. health deficits). From the perspective of teasing out the underlying biology contributing to the continuum of frailty, it is now imperative to closely align theoretical construct (e.g., physical frailty) with the corresponding assessment tool (e.g., Physical Frailty Phenotype) when classifying frail, prefrail, and nonfrail individuals (20, 629).
In the 21st century to successfully prevent or treat frailty and increase healthspan, the recognition of its intrinsically complex underlying biological processes is the first step. It is not surprising to hypothesize that frailty involves a cumulative decline in physiological, cellular, and molecular functions and frailty is apparent at multiple levels of biological organization: genome, epigenome, tissues, organs, and the organism (Figure 1). Practically speaking, studying the burden of frailty in humans is challenging particularly due to the ethical (complicated, high-risk), logistical (cost, labor-intensiveness), and biological complications (genetic diversity, lifestyle) associated with working with older adults. In this regard, it is an exciting time for researchers interested in the study of the biology of frailty at the preclinical research level together with the interdisciplinary field of Geroscience.
Frailty Assessments in Preclinical Research
It is well-established that mouse models are developed (genetically), characterized, and tested to advance biomedical research in human aging and disease (71, 442, 579). The major advantages of mouse models in representing a human disease and/or aging are the investigation of the underlying biological mechanism(s), the identification of potential cellular targets for developing therapies, and the opportunity for translational bi-directional approaches. Bi-directional translational science facilitates iterative changes when additional new information is available, either preclinically or clinically, for offering the greatest opportunity for the diagnosis, prognosis, and treatment of disease or aging. The increased use of health assessments in preclinical animal aging models is an excellent example of successful translational science (3, 47, 164, 280, 470, 549).
For the study of frailty, mice are suitable because the lifespans of 1 to 3 years (strain-dependent) facilitate longitudinal lifespan research designs in both sexes (54). Mice exhibit many of the visible signs associated with humans such as hair graying, kyphosis, deafness, and baldness as well as cognitive decline and display physical performance declines such as balance, coordination, gait speed, strength, and endurance (34, 153, 183, 208–212, 280). Utilizing mice can also reduce and/or isolate factors that contribute to frailty such as lifestyle and address the possibility of detecting a frailty state before disability. As mammals, the physiology of mice resembles that of humans in many aspects. Most importantly, preclinical models enable in parallel tissue-to-tissue examination of mechanisms contributing to frailty and of the impact of genetic, pharmacological, and behavioral interventions.
Development of the mouse Frailty Phenotype
Liu et al. (351), developed a preclinical mouse Frailty Phenotype that followed the clinical criteria used by Fried et al. (179), which included measures of strength (inverted grip hang), walking speed (rotarod), physical activity (voluntary wheel running), and an endurance score (inverted grip hang plus rotarod). Each criterion was scored (score = 1) based on a selected cutoff percentile corresponding to 1.5 standard deviations below the cohort mean (i.e., the lowest seventh percentile of the group). Mice with 3 or more positive frailty markers were identified as frail, with 2 positive markers as prefrail, and with 1 or no positive frailty markers were identified nonfrail. This initial mouse Frailty Phenotype was further improved and validated in two rigorous studies that assessed cohorts of male and female mice across the lifespan (42, 321) (Table 2). In these two studies, the mouse Frailty Phenotype was redesigned to include body weight, reliable and quantitative measures of endurance/exhaustion (treadmill fatigue test) and strength (an electronic grip meter test), as well as the original walking speed (rotarod), and physical activity (voluntary wheel running) measures. Importantly, the evaluation of frailty markers in a longitudinal lifespan research design permitted the evaluation and identification of a reference group and cut-off values for each measure. Mice that fell in the bottom 20% for strength, walking speed, exhaustion, and activity were considered to be positive for the frailty measure (score = 1). In contrast, mice with the highest 20% body weight are considered positive for frailty. Designation of frail, prefrail, and nonfrail was defined with the same number of positive frailty markers as in the original Liu et al. report (179, 351). Importantly, because the measures were evaluated across the lifespan it was possible to establish that the mouse Frailty Phenotype identifies the onset of frailty, progression and prevalence of frailty, and mortality risk (41, 42, 321).
Table 2.
Reverse-translated Mouse Frailty Assessment Tools
| Mouse Physical Frailty Phenotype | ||
|---|---|---|
| Criteria | Measure | Equipment |
| Strength | Grip strength (g) | Electronic grip meter |
| Walking speed | Time (s) | Rota-rod |
| Endurance/exhaustion | Fatigue test (s) | Treadmill |
| Activity level | Daily running distance (km/day) | Running wheel |
| Bodyweight | Weight (g) | Scale |
| Mouse Clinical Frailty Index | |||
|---|---|---|---|
| Activity levels | Hemodynamic measures | Body composition | Basic metabolic status |
| Distance moved (total and maximal, cm) | Systolic pressure (mmHg) | Weight | Na (mmol/L) |
| Velocity (cm/s) | Diastolic pressure (mmHg) | BMD and BMC (g/cm2) | K (mmol/L) |
| Meander (degree/cm) | Pulse pressure (mmHg) | Body surface area (cm2) | Cl (mmol/L) |
| Duration of movement (s; % total activity) | Average BP (mmHg) | Lean and fat mass (g) | pH |
| Rearing frequency (per 5 min) | Heart rate (beats/min) | Percent body fat | Glucose (mmol/L) |
| Tail blood flow and volume (uL) | Total body tissue | Hematocrit (%) HCO3 (mmol/L) Hb (g/L) Urea (mmol/L) |
|
The mouse Physical Frailty Phenotype includes measures of strength, walking speed, endurance, physical activity, and body weight reverse-translated from the clinical Physical Frailty Phenotype (Table 1). Each criterion is scored (score = 1) based on a selected cutoff percentile corresponding to the bottom 20%, with the exception of body weight (dependent on research design). Mice with three or more positive frailty markers are identified as frail, with two positive markers as prefrail, and with one or no positive frailty marker are identified nonfrail (40–42, 321, 351). The mouse clinical Frailty Index selected 31 health-related variables to provide health information highlighting four categories: activity (distance moved, velocity of movement, rearing frequency); hemodynamic factors (systolic and diastolic blood pressures, heart rate, blood volume); body composition (body mineral content, percent body fat, percent lean tissue); and metabolic status (electrolytes, hematocrit, and urea). A graded scale is used to determine frailty, based on how many standard deviations the measured value differed from the mean reference values (adult mice) (434).
In addition to the mouse Frailty Phenotype described above, there are four other frailty assessment tools reverse-translated from the criteria within the clinical Physical Frailty Phenotype (physical frailty, Valencia Score, Comprehensive Functional Assessment Battery, Neuromuscular Healthspan Scoring System), which adapt similar criteria with modified approaches and similar cut-off values (204, 209, 212, 374, 511). Given the focus on measures of physical function within the mouse Frailty Phenotype and other assessment tools listed above, the importance of skeletal muscle arises (sarcopenia) as a major contributing factor for frailty.
Development of the mouse clinical Frailty Index
The first mouse FI selected 31 health-related variables to provide health information highlighting four categories: activity (distance moved, velocity of movement, rearing frequency); hemodynamic factors (systolic and diastolic blood pressures, heart rate, blood volume); body composition (body mineral content, percent body fat, percent lean tissue); and metabolic status (electrolytes, hematocrit, and urea (Table 2) (434). A graded scale was used to determine frailty, based on how many standard deviations the measured value differed from the mean reference values (adult mice). Because the conceptual framework of the FI is grounded on the number of deficits (a minimum of 30) rather than the specific nature of the health deficit, the mouse FI was redesigned to be noninvasive and simple to implement in the research laboratory (473, 536). The noninvasive 31-selected variables (index) provide health information across several physiological systems including the integument, musculoskeletal, vestibulocochlear/auditory, ocular, nasal, digestive, urogenital, respiratory, plus sign of discomfort, body weight, and body surface temperature measures. A severity of each deficit was rated on a simple scale of 0 = absent, 0.5 = mild, and 1 = severe. In 2017, Antoch et al. (18) defined the physiological Frailty Index (PFI) with the aim of including parameters to be (i) diverse to reflect the status of different health-related physiological systems, (ii) objective and quantitative, and (iii) minimally invasive. Using 29 variables reflective of physical fitness, the cardiovascular system, total blood cell composition, plasma concentration of chemokine C-X-C motif ligand/keratinocytes-derived chemokine (Cxcl1/Kc), triglycerides, and glucose, the PFI showed a gradual age-associated increase in frailty in a cross-sectional study with sex-specific differences (females more rapid and higher than males).
In mouse indices of frailty, the number of health-related variables and the inter-rater reliability when assessing these variables are important for experimental and data fidelity (156, 291, 611). An 8-item mouse FI shows an increase with age; however, the results lacked sensitivity to detect frailty between age groups, exhibited high variability, and there was greater test-to-test variability compared to a mouse FI with 31-items (611). Note, it is possible to achieve inter-rater reliability with careful selection and training of the raters when using the FI (156, 291). In addition to the mouse FI identified above, others are developing frailty indices in mice based on common laboratory tests (blood pressure, basic metabolic status, echocardiography, and blood-based biomarkers) (293).
Preclinical frailty research
With the development of the preclinical frailty assessment tools and the emerging interests in health and in the biology of frailty described above, more attention to assessing frailty status (phenotype) as an experimental outcome variable is taking place. To date, there are reports with the widely used C57Bl/6 mouse in both cross-sectional lifespan research (cohorts of mice at different ages) and in rigorous, prospective longitudinal lifespan research (one cohort tested across the lifespan) (41, 42, 204, 321, 351, 434, 445, 472, 611). Longitudinal lifespan studies are considered more rigorous because survival bias influences the results in studies using a cross-sectional lifespan design. In addition to the C57Bl/6 mouse, short-lived and long-lived, accelerated aging and inbred/outbred mice and mouse models of Alzheimer’s, oxidative stress, and inflammation have been assessed for frailty status (18, 25, 262, 264, 290, 295, 374, 471, 502, 609). To date, there are several studies evaluating frailty in rats, dogs, nonhuman primates, and in Caenorhabditis elegans models (30, 246, 386, 410, 537, 565, 631, 635, 646).
Given the multidimensional nature of frailty (Figure 1), it is likely the development of therapeutic interventions that target several cellular systems linked to multiple aspects of health will have the greatest beneficial effects. Indeed, several lines of evidence now point to the potential to modify frailty in preclinical animal models (mice, rats, nonhuman primates) by targeting global physiological systems (e.g., inflammation, oxidative stress) or signaling pathways [e.g., mammalian target of rapamycin complex 1 (mTORC1)] (471, 516). The well-established longevity-modulating interventions such as caloric restriction (CR), intermittent fasting, and treatment with antioxidants or mammalian target of rapamycin (mTOR) inhibitors and others reduce frailty (25, 227, 231, 270, 290, 516, 563). It is possible to reverse frailty with healthy-lifestyle interventions including defined exercise training (e.g., high-intensity interval training), physical activity, diet [e.g., reduced branched-chain amino acid (BCAA) diet], and Vitamin D supplementation (204, 210, 471, 509–512). Specific pharmacological therapies (e.g., antihypertensive agents; anti-inflammatory agents) attenuate frailty, too (299). In contrast to strategies shown to improve the status of frailty, premature or enhanced frailty is reported when testing approaches known to be detrimental to health such as polypharmacy, high-drug burden, high-fat feeding, and irradiation (18, 162, 251, 294, 366). Conceptually, these interventions converge to improve cell physiology, homeostatic functions, and boost protective cellular pathways.
Challenges
In the previous section, we describe the increasing use of the frailty assessment tools in preclinical research studies focused as an outcome variable when describing phenotypes and when testing interventions. While being informative, the evidence to support the reversal of frailty is limited, at times contradictory, inconclusive, and incomplete. For instance, in some reports, only one sex was investigated (563). Investigating both sexes is critical because there is controversy within the mouse literature indicating that either older females exhibit greater frailty than males or vice versa or no sex differences at all (18, 290, 295, 434, 611). In our work, we show sex differences in mice at a specific age within the lifespan (41). More research is definitely indicated to further expand these findings and elucidate the reasons for the varied reports (e.g., strain, cross-sectional vs. longitudinal lifespan study, frailty assessment tool). Following up on these sex-difference observations, studies evaluating therapeutics or interventions to delay frailty also show sex-specific responses whereby the sex-specific response is intervention-dependent (471). For instance, treatment with alpha-ketoglutarate reduced frailty in both sexes; whereas interventions by which there is a restriction of dietary BCAAs or supplementation with Vitamin D reduced frailty in males, but not females (471, 509, 516). With these concerns, future studies require close examination of sex-specific responses, aspects of intervention (e.g., age of initiation, dosing, toxicity testing), and comprehensive, standardized research designs to clearly understand the mechanistic details underlying frailty.
The challenges noted above bring to the forefront three important points for discussion: the multidimensional aspects of the frailty condition (domains of frailty), selection of the most appropriate frailty assessment tool, and the manner in which age is described in experimental design. To date, the assessment of frailty in preclinical models focuses on loss of physical functions (physical frailty) or as accumulation of multiple health deficits; however, in humans, there are multiple domains of frailty (cognitive, social, psychological which includes motivation and mood), that coexist, have potential to influence each other, and have specific assessment tools (215, 378). For instance, there is an association between cognitive frailty and physical frailty, and cognitive frailty is identified as a determinant of resilience to stressors (15, 215). At this time, preclinical assessments for frailty identification do not emphasize measures for cognition, depression, motivation, etc.; yet it is important to determine whether the presence of multiple frailty domains increases the risk for negative outcomes of frailty and to elucidate the biological underpinnings to develop multidomain interventions. In regards to the second point, because assessment tools to identify frailty status in preclinical animal models are in their infancy, selecting or developing a frailty assessment tool for animals requires adherence to general principles such as theoretical basis and validity of the constructs (discriminant validity, construct validity and reliability, high sensitivity and specificity), matching the assessment tool to the intended purpose (domain or domains captured), feasibility and implementation (quick and easy, testing and housing environment, time of day), past use, and degree of invasiveness, etc. Observing these principles has potential to propel frailty research in preclinical animals in a positive trajectory toward impactful discoveries. Lastly, in consideration to the third point, most studies compare organisms of the same chronological age (i.e., 18-month-old control and 18-month-old treated mice). However, our understanding of aging biology as well as clinical presentations suggest that: (i) aging rates amongst individuals differ; and (ii) various interventions can alter this rate (delay or accelerate). These observations have been verified using clock-based assessments (epigenetic, metabolomic, transcriptomic among others) that indicate the variability in biological age between individuals of the same chronological age. In other words, chronological age is a time-based description while biological age reflects differences in the rate of aging between organisms within a species as well as between cells, tissues, and organs. Thus, in this comprehensive article, when we describe the epigenetic clocks for instance, these measures are utilized as predictors of biological age and mortality and are often compared to chronological age to indicate health.
Collectively, these research studies show remarkable progress in preclinical frailty research (e.g., increase with age, predict adverse outcomes, reversible or delayed, in agreement with human populations); however, it is clear the next generation of preclinical frailty work and human frailty research can inform each other and be more integrated going forward. Very recently, a new international public-private venture emerged called the INSPIRE Research Initiative. This INSPIRE Research Initiative is dedicated to biological and healthy aging with the ultimate goal of preventing adverse health consequences of aging and delaying their onset or reducing their severity (134, 492). The INSPIRE Research Initiative is unique and has potential to be impactful for the field of frailty because it aims to create a bio-resource platform spanning from animals to humans, from cells to individuals, and from research to clinical care. INSPIRE brings together internationally recognized experts from basic and translational science, clinical gerontology, primary care, and public health with the objectives of identifying biomarkers and implementing a function-centered healthcare pathway. Importantly, INSPIRE applies the principles of Gerosciences to foster discoveries by including comprehensive phenotyping and extensive biobanking of a human translational cohort, an animal cohort (outbred Swiss mice), and the accelerated aging model Nothobranchius Furzeri (African Killifish) within the program. Considering the heterogeneity of the frailty condition, the variety of assessment tools, and experimental designs, it is fundamental to merge all potential molecular mechanisms and pathophysiological consequences into a systemic approach that facilitates advances in the field.
Investigating the Biology of Frailty
Given the multiplicity of mechanisms underlying frailty, one potential productive approach to uncover these biological mechanisms is to develop a construct composed of common pathways that become dysfunctional with time. One of the first conceptual clinical frameworks for frailty emphasized an organization of the biological connections between age-associated molecular alterations, physiological decline, and clinical signs and symptoms (Figure 2) (599). The neuro-immuno-endocrine systems formed the basis, which were theorized to be less effective in individuals with frailty, because of (or in part due to) the presence of low-grade inflammation (inflammaging) and excessive and unopposed oxidative stress. This clinical framework for frailty laid the groundwork for the current conceptualization for clinical frailty discussed below (160, 450, 597). As a significant outcome of the emerging field of Geroscience, there is potentially great overlap between the framework of aging (hallmarks/pillars) and the current, clinical conceptual framework for frailty.
Figure 2. Conceptualization of physical frailty during the first decade of the 21st century (599).
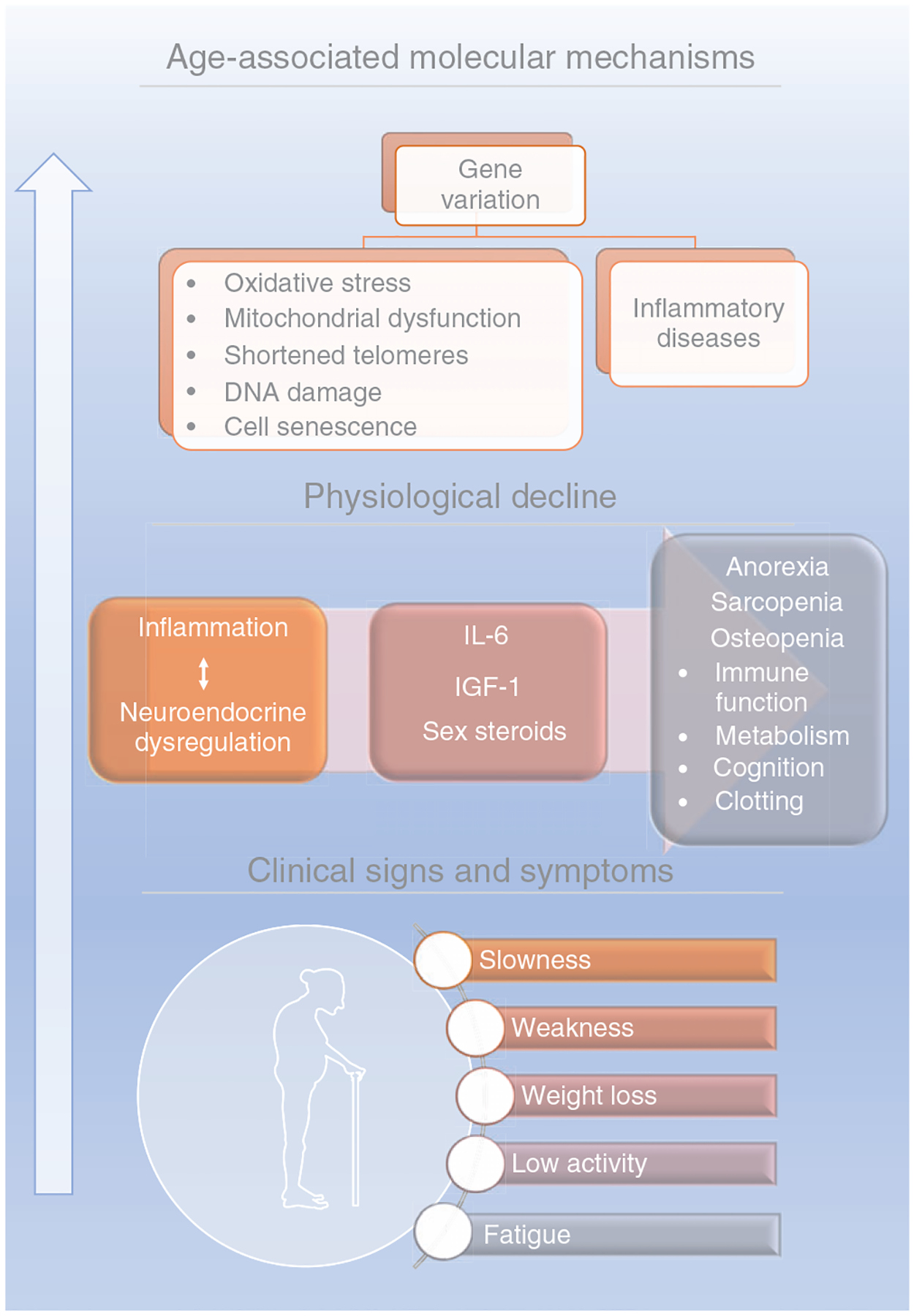
Because the clinical signs and symptoms were known to be physiologically related to one another, in theory, they provided possible connections between molecular alterations associated with aging, physiological decline, and clinical systems. These biological connections were organized conceptually. In aging, the combination of gene variation, DNA damage, and telomere shortening contribute to oxidative stress, mitochondrial dysfunction, cell senescence, and inflammation that in turn, promotes a decline in the physiological functioning of the organism. The aging-related physiological decline occurs following chronic unresolved inflammation along with neuroendocrine dysregulation triggering anorexia, sarcopenia, and osteopenia, which are conditions related to body, muscle, and bone mass loss. This systemic loss and tissue dysfunction as well as the associated cognitive decline lead to the clinical signs of frailty: slowness, weakness, weight loss, low activity, and fatigue. This conceptualization emphasized the complexity of the multiple systems and visually suggested the manifestations of frailty were a cumulative outcome of dysregulation of these multiple systems. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Hallmarks
Aiming to understand the mechanisms underlying frailty a focus on the identification and categorization of the cellular and molecular hallmarks is valuable. The concept of hallmarks is not new, and in fact, to date, there are two well-established conceptual frameworks for understanding the development and progression of human cancers and aging (Hallmarks of Cancer, Hallmarks of Aging, Pillars of Aging) (218, 300, 359). Because frailty likely arises from the failure of multiple mechanisms associated with the described Hallmarks/Pillars of Aging to sustain health, these suggested pathophysiological mechanistic pathways provide an initial scientific roadmap to drive preclinical frailty investigations. Briefly, the Hallmarks of Aging represent fundamental and interconnected biological pathways which are divided into three broad categories: primary, antagonistic, and integrative (359) (Figure 3). Genomic instability, epigenetic alterations, telomere attrition, and loss of proteostasis are described as primary hallmarks, which are the drivers or triggers of the aging process leading to damage. Thus, in the context of clinical symptoms of frailty, these make sense as underlying processes that initiate and/or propagate widespread dysfunction among multiple cell and tissue types (or organ systems). The antagonistic hallmarks include deregulated nutrient-sensing, mitochondrial dysfunction, and cellular senescence, which represent protective compensatory mechanisms. Key to the concept of compensatory mechanisms is that these mechanisms are initially protective (function to preserve homeostasis and biochemical balance); however, beyond a certain threshold and/or over prolonged time periods these compensatory mechanisms lead to severe detrimental adaptations or outcomes. Currently, it is hypothesized that these compensatory mechanisms contribute to the reported variability in survival rates and importantly, to the presence of diverse phenotypes within chronological aging (160). Because frailty is dynamic and exists on a continuum from robust (fit) to frail (or stages nonfrail, prefrail, and frail), it is logical to hypothesize the continuum of frailty is the manifestation of compensatory mechanisms within specific cells and specific tissues reaching thresholds and beyond, yielding detrimental adaptations (478). SC exhaustion and altered intercellular communication (integrative hallmarks) represent the final outcomes of the damage caused by both the primary and antagonistic hallmarks, leading to dysfunction within the various tissues and to age-related chronic diseases. The collective physiological dysfunctions potentially result in frailty, a clinical term that describes the combined deficits of many systems.
Figure 3. Biology of frailty.
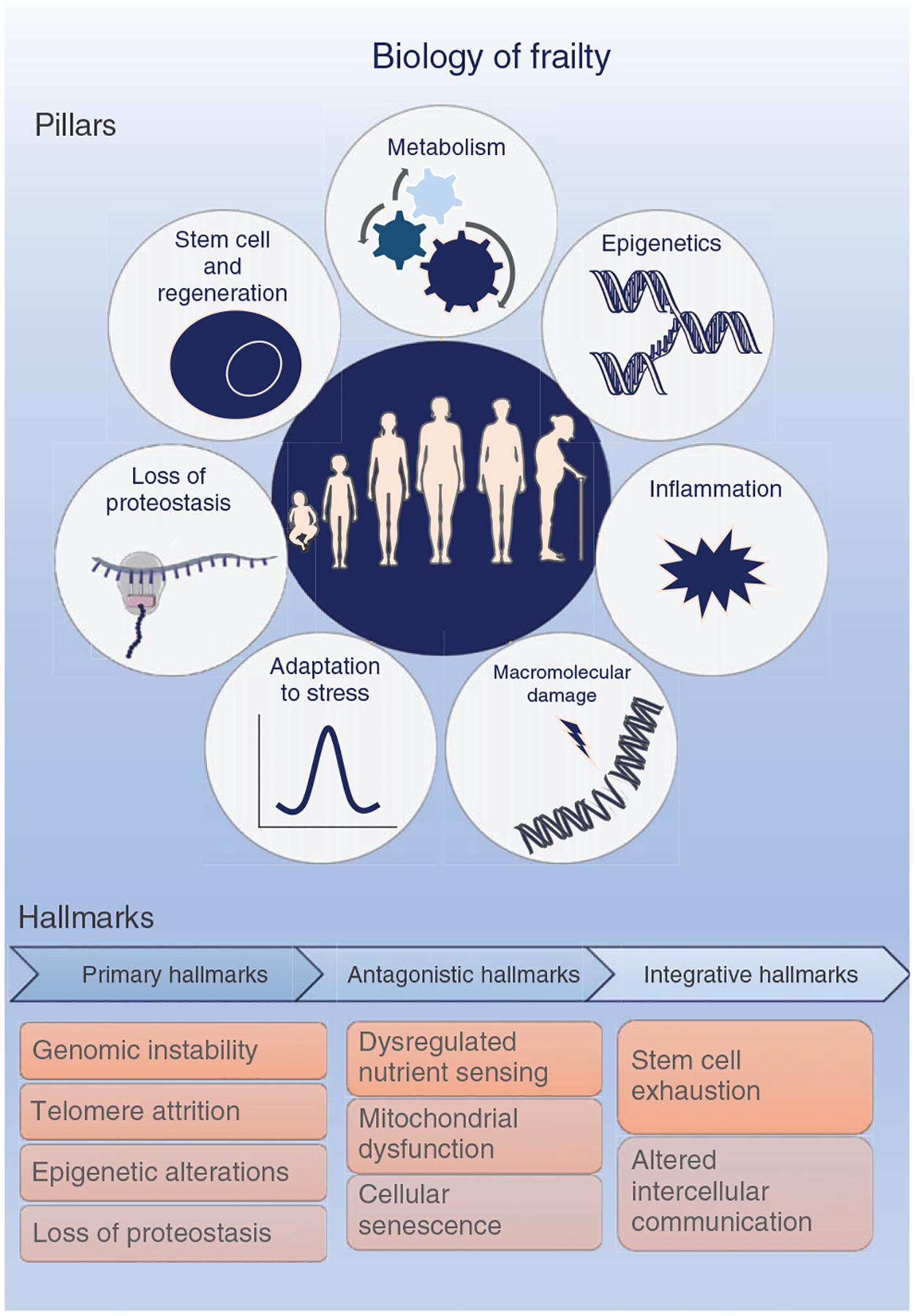
The two well-established conceptual frameworks defining the biology of aging are the Seven Pillars of Aging proposed by Kennedy et al. in (300) and the Hallmarks of Aging proposed by López-Otin et al. in (359) The Seven Pillars define the biological areas that likely contribute to the pathophysiology of aging and include metabolism, epigenetics, inflammation, macro-molecular damage, adaptation to stress, loss of proteostasis, and stem cells and regeneration. Similarly, the Hallmarks of Aging categorize the cellular and molecular processes that may lead to the aging phenotype as (i) the primary hallmarks—genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis; (ii) the antagonistic hallmarks—dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence; and (iii) the integrative hallmarks—stem cell exhaustion and altered intercellular communication. Together, these two concepts identify potential routes to be targeted to extend healthspan and prevent or reduce frailty. The pillar of metabolism defines the signal transition pathways linked to the metabolism of aging, such as impaired glucose homeostasis and dysregulated nutrient sensing, whereas the epigenetics pillar links age-related environmental pressures altering the gene function, which might trigger genomic instability. The macro-molecular damage pillar is also illustrated as a primary hallmark as genomic instability and telomere attrition, which are all considered the causes of damage that might evolve to antagonistic hallmarks that are the response to damage and includes mitochondrial dysfunction and cellular senescence. The adaptation to stress illustrates the loss of resilience and resistance or how well the organism can combat and recover from a stressor, which might be molecular (loss of proteostasis, genomic instability), cellular (macromolecular damage accumulated in stem cells, stem cell function decline) or physiological (altered intercellular communication). Once the organism reaches the integrative hallmark level, a systemic dysfunction is reached, culminating in the Frailty Phenotype. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATO-RIES SERVIER, SAS.
The seven Pillars of Aging are consistent with the Hallmarks of Aging and include adaptation to stress, epigenetics, inflammation, macromolecular damage, metabolism, proteostasis, and SCs and regeneration (Figure 3) (300). Although the contribution of each of these hallmarks or pillars towards the biology of frailty is unknown, the processes are certainly interwoven influencing physiological potential, physical resilience, and intrinsic capacity within tissues. Clinically, it is worth noting the detectable changes currently utilized to characterize frail individuals (independent of the frailty assessment tool) are only apparent when compensatory mechanisms begin to fail and results in detrimental adaptations. In this context, the nature of the drivers or triggers, the compensatory mechanisms, and their maladaptations are not well understood. It is becoming clear that the pillars and hallmarks provide guiding principles for preclinical frailty research. Arguably, targeting the compensatory mechanisms (pathways) within specific tissues and organs in lifespan longitudinal studies currently holds the strongest impact for its usefulness as a strategy to understand the underpinnings inducing frailty.
Linking the biology to the clinic
Progress in basic aging research during the last decade influenced the current clinically based frailty framework, which now states that frailty is caused by an overt age-associated dysregulation of multiple homeostatic systems or a loss of harmonic interactions between multiple domains (genetic, biological, functional, cognitive, psychological, and socioeconomic) that lead to homeostatic instability (160, 450, 597) (Figure 4). This general framework is based on a hierarchical organization of three different levels of complexity (biological mechanisms, pathophysiological mechanisms, manifestations of frailty) (159). Two of the layers (inner and intermediate) take clear advantage of the pillars and hallmarks discussed above. The inner layers focus on the biological mechanisms involved in frailty at the subcellular level [e.g., mitochondrial dysfunction, oxidative stress, DNA damage, shortening of telomere length, maladaptive DNA methylation (DNAm)]. The intermediate layers consist of potential pathophysiological mechanisms leading to frailty (chronic low-grade inflammation, energetic imbalance, anabolic deficiency, neurodegeneration). The outer layers comprise the clinical consequences and the manifestations of frailty (e.g., functional deficits, reduced mobility, cognitive impairment, loss of independence, multiple chronic diseases). At present, our understanding of the interplay of the components within each layer and between each layer is very rudimentary and remains a distant prospect. Nonetheless, these gaps in knowledge can be filled by rigorous preclinical animal research and new informatics technologies, which enable the processing and interpretation of complex constellations among interacting biological parameters.
Figure 4. The current clinically Based conceptualization of frailty (160, 450, 597).
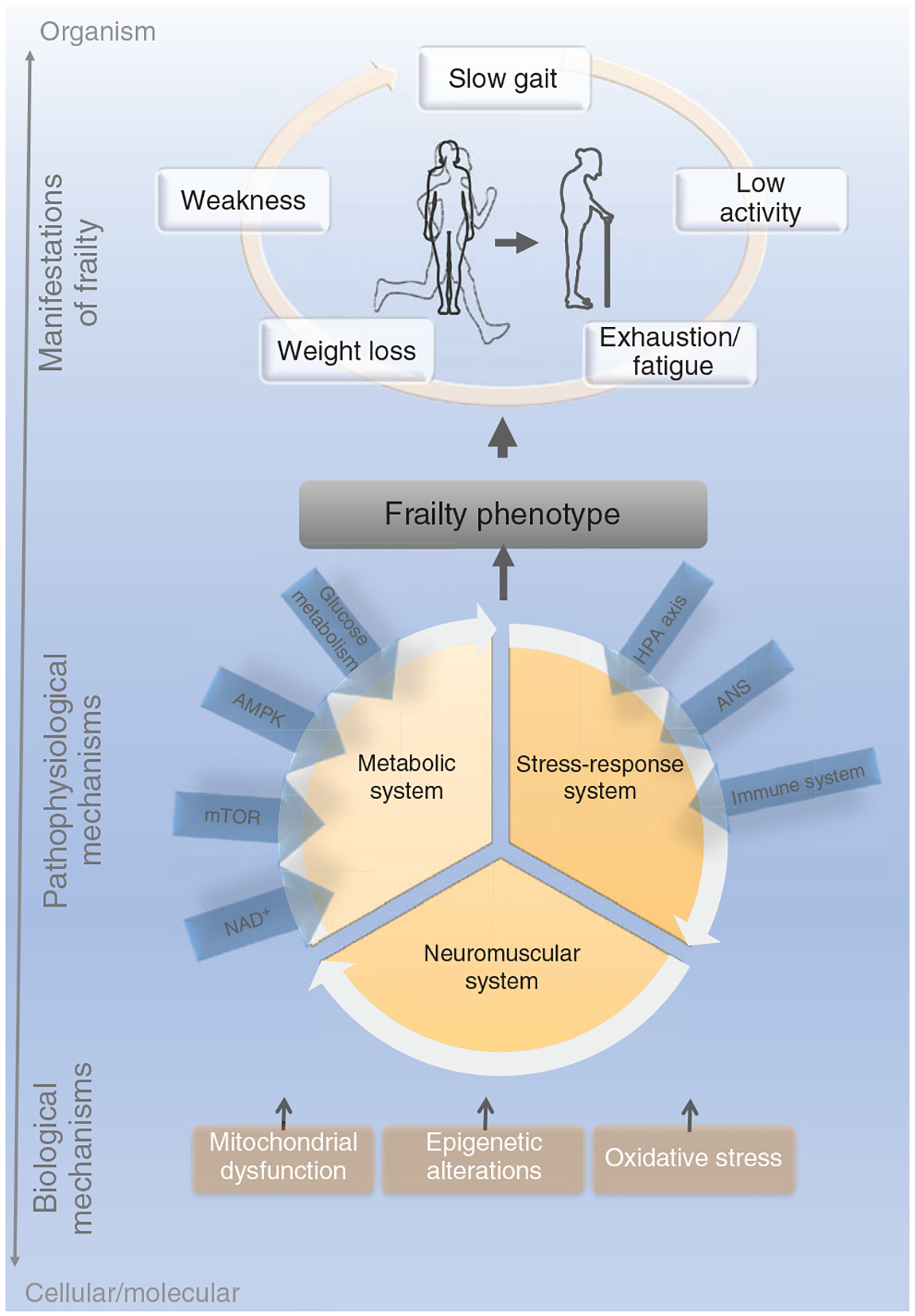
Integrating the clinical manifestations of frailty with the hallmarks/pillars of aging results in the current conceptualization. Mitochondrial dysfunction, epigenetic alterations, and oxidative stress represent cellular/molecular factors that contribute to three central physiological systems that promote the Frailty Phenotype. The mitochondrial dysfunction accounts for a reduction in the efficiency of oxidative phosphorylation and a reduction in the energy production generating long-term exhaustion/fatigue. Epigenetic alterations such as DNA methylation and histone modifications are triggered by chronological aging and environmental factors, influencing pathways of health and longevity. Lastly, oxidative stress refers to excessive production of reactive oxygen species (ROS) that leads to cell and tissue damage. The metabolic system represents pathways that are centrally mediated by nutrient-sensing mechanisms, in which the glucose metabolism, insulin signaling cascade as well as AMP-activated protein kinase (AMPK) and nicotinamide adenine dinucleotide (NAD+) are pivotal players. The stress-response system is mainly influenced by the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nervous system, and by the immune system. The cognitive and muscular declines, here illustrated by the neuromuscular category, are driven by tissue waste and dysfunction, leading to weight loss, weakness, fatigue, low activity, and slow gait at the organismal level. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Our preclinical mouse longitudinal lifespan investigations are initial steps to address the three layers within the hierarchical organization discussed above. In these studies, one of the outer layers representing manifestations of frailty (physical functional deficits) identified frailty onset, progression, and mortality risk (40–42, 321). The value of identifying the onset of frailty lies in the opportunity to tease out the factors triggering frailty. The intermediate layer focused on the metabolic demand or energetic imbalance (e.g., aerobic vs. anaerobic) is associated with the specific functional tests. For instance, the treadmill run to exhaustion yields information primarily about the cardiorespiratory system response to stress; whereas the grip meter strength test yields information about the neuromuscular system. The value of these individual functional tests within the mouse Frailty Phenotype is certainly acknowledged in providing direction in identifying mechanisms of frailty associated with muscle function. Yet arguably the biggest value lies in the observations that these physical function measures do not decline at the same rate, mice demonstrate frailty with different functional measures, and not every mouse becomes frail (40–42, 321).
Now that we introduced the general frailty framework and its development, we will expound on specific mechanisms believed to be critical to set-up or contribute to the frail phenotype described in both preclinical and clinical studies. Indeed, the current conceptual framework for frailty, distinctive and complementary, constitutes an organizing principle for rationalizing the complexities of frailty, as investigations from both preclinical and clinical research progress. With the biological and pathophysiological mechanisms of this current framework in mind, in this article (the following sections), available reports related to potential biological underpinnings of frailty are presented and include the layers, pillars, hallmarks, and other potentially related topics.
Epigenetics, Genomic Instability, and Frailty
Decades of research indicate that both genetic and environmental factors influence aging and the propensity to become frail. Appreciating that these factors also drive methylation processes suggests that epigenetics may play a role in the development or progression of frailty. We understand that frailty is strongly associated with age-related phenotypes, reduced longevity, and has been used as a measure of biological aging. Therefore, studying epigenetic alterations represents another avenue of biological research to better understand mechanisms that promote aging and potentially frailty.
Epigenetic modifications refer to chemical and structural alterations to the genome that have been shown to significantly change gene expression and phenotype without altering the underlying DNA sequence. DNAm, histone modifications as well as microRNAs (miRNAs) contribute to the epigenomic landscape with DNAm being the most common epigenetic modification studied (53, 259). It is clear that DNAm levels are modifiable, and the effects can be cumulative, thus their role in aging and age-related pathologies and disease is under intense investigation (172). In aging, a global (whole genome) decline in DNAm (172) has been observed along with an increase in variability (184, 525, 641). However, results differ as to whether lower global DNAm is associated with people considered frail compared to those that are nonfrail. These differences are due in part to a variety of challenges that face the field, including the type of frailty assessment, be it the FI or the Frailty Phenotype (described earlier in this article) as well as the type of methylation analysis performed among others (67, 110, 191). All of which takes place with the understanding that DNAm patterns likely differ in frail people.
By far, the most well-studied aspect of DNAm in its association with aging has been in the development of “clocks” which are algorithms based on DNAm status at sets of specific 5′-C-phosp-G-3″ (CpG) sites that vary with age. These DNAm clocks have been used to predict mortality and the influence of external factors by estimating biological age via predicted DNAm age (220, 241, 242). DNAm age may be considered a biomarker of aging as chronological age is not the best measure of aging processes nor mortality (as the rate of aging varies between individuals). The differences between DNAm age and chronological age are predictive of health and longevity [termed Epigenetic age acceleration; reviewed in Horvath and Raj (243), Levine et al. (343), and Lu et al. (360)]. This estimation of biological age is predicted from samples of blood (primarily) and tissue analyzed for methylation status at the sites that vary with age.
A recent report performed a meta-analysis of 61 studies (over 50,000 participants) that examined associations between chronological age and DNAm age (mostly blood-based) using either the Hannum or Horvath clocks (486). These investigators found that 56 studies showed associations with known risk factors for chronic disease and increased DNAm age. Forty-eight of these studies found a relatively strong correlation between chronological age and DNAm age. There were three frailty studies included consisting of 3092 individuals each associating frailty with increased methylation age (67, 191, 304). Two other studies supportive of the frailty and methylation age data showed decreased strength positively correlated with increased DNAm age (373, 528). Additional studies have refined the concept of the initial clocks by incorporating age-related health outcomes and training. The resulting clocks, DNAm PhenoAGE (phenotypic age) and GrimAge, act as highly predictive biomarkers of morbidity and mortality outcomes such as time to death, time to cancer, and time to coronary heart disease (343, 360). Important for the discussion of frailty, the Frailty Inferred Geriatric Health Timeline clock and a second model, the Analysis of Frailty and Death clock have been generated for use in mice and incorporate frailty indices in the prediction of chronological age and life expectancy (504, 505). Overall, these association studies predict that biologically older adults as determined by methylation age are more likely to exhibit co-morbidities and potentially to be physically frail but much more data is needed.
It is difficult to determine the cause of frailty as the biological drivers of multisystem dysregulation are many and likely to be interconnected. Few studies have focused on the biology underlying the contribution of epigenetic processes such as methylation status to the development or progression of frailty. One such report focused on understanding fatigue and muscle weakness by investigating hyperhomocysteinemia (HHcy) which has been implicated in frailty as it appears to augment the age-associated decline in physical function (584, 585). CBS+/− mice (cystathionine beta-synthase), a model of HHcy, are produced by creating a deficiency in the enzyme that metabolizes homocysteine resulting in high homocysteine. These mice are more fatigable, and exhibit reduced contraction force but their skeletal muscles exhibit no changes in muscle morphology (fiber type composition), only fewer large muscle fibers and more medium size fibers compared to wild type mice. It is known that fatigue and muscle weakness encompass both structural (decreased muscle mass, dystrophin complex assembly deficiency) as well as energy imbalances and that exercise intolerance and fatigue occur in frailty (56, 130, 163, 407, 610). Results of this study indicated that the excess fatigability was partly due to lower adenosine triphosphate (ATP) levels in skeletal muscle fibers. They also observed altered miRNAs (mir-31, mir-494) involved in dystrophin regulation, lower dystrophin levels, and decreased mitochondrial transcription factor A (MtTFA) and nuclear respiratory factor 1 (NRF-1). In contrast, no changes in enzymes regulating muscle metabolism nor changes in creatine kinase were detected, thus, an energy imbalance was not considered. Four weeks of exercise increased ATP, reversed low MtTFA, and decreased miRNAs. C2C12 myoblast cells treated with homocysteine exhibited increased mir-494, Dnmt3a-3b levels and global methylation while MtTFA and ATP decreased, supporting the animal studies. Thus, one mechanism linking epigenetics with frailty may be through enhanced DNAm. This may, in turn, change gene expression directly by downregulating MtTFA or indirectly by upregulating miRNAs resulting in epigenetic changes induced by HHcy that undermine skeletal muscle function. However, as stated above, studies directly linking epigenetic alterations to a biological outcome and then, to frailty are scarce.
Genetic outcomes have been analyzed in terms of frailty biomarkers (from blood) categorizing people into nonfrail, prefrail, and frail cohorts by investigating associations with mutagenicity, DNA repair competence, and genetic damage (572). This particular study found that genomic instability and frailty are linked but that a combination of markers would provide key information on frailty severity and assist with potential health care strategies in frail individuals.
Histone modifications represent an additional mechanism that can mediate changes in gene expression and phenotype through silencing transcription and regulating genome stability among other means (61). The patterns of histone marks have been shown to change with age at specific loci as well as globally (50). Changes in the activities of enzymes that place and remove histone marks play a large role in the outcomes of each mark and its patterning. For example, histone deacetylases have been investigated extensively in the aging field with the sirtuins capturing the most attention. In general, sirtuin activation improves skeletal muscle metabolism and protects against sarcopenia thus likely plays a role in frailty (196, 203, 608). However, a discussion of sirtuins and their modulation is beyond the focus of this article. Aging has been shown to trigger chromatin changes in skeletal muscles of mice, humans, and more recently, in killifish (84, 607). This species of fish exhibits a progressive loss of muscle function with age sometimes leading to sarcopenia that is characterized by weakening muscle strength and impaired mobility. The killifish was identified earlier in this comprehensive as a model organism to be examined in the current ongoing INSPRIRE Program. The combination of increased tri-methylation of lysine 27 on histone H3 protein (H3K27me3), heterochromatin protein 1a (HP1a), polycomb complex subunits, and senescence-associated heterochromatic foci along with reduced H3K9ac results in an accumulation of heterochromatin that is thought to contribute to the loss of muscle mass, decreased cell proliferation and mitochondrial function, and increased inflammation in old skeletal muscle. Similar findings have been reported in mice and humans (21, 607). Thus, reports regarding altered histone marks with aging are emerging but changes with frailty specifically are underexplored at this time.
Small ribonucleic acid (RNA) molecules such as miRNA impact mRNA processing and multiple processes (617). A number of miRNAs have been associated with aging as well as physiological processes in muscle and a review of miRNAs involvement in frailty is nicely presented by Rusanova and coworkers (484). Many of the studies indicate associations between specific miRNAs in older subjects and inflammation (147, 240, 426). In serum, frail individuals exhibit higher mir-21 compared to nonfrail while mir-223 and mir-483 increase in robust and frail aged participants to similar extents (483). In skeletal muscles, several laboratories have studied age-related miRNAs and found increased mir-146a, -155, -185, -206, -215, and -223 and decreased mir-148a, and -434 in mice, monkeys, and humans (141, 217, 303, 383). These miRNAs are known to modulate aspects of muscle physiology. Sarcopenic-associated miRNA changes have also been described, but results of this study rely on small numbers and warrant further examination (649).
There is an essential need to understand molecular mechanisms leading to the onset and progression of frailty. Epigenetic mechanisms are known to influence a variety of processes in aging and skeletal muscle physiology with strong associations with frailty. However, the challenges involved are many and discussed earlier in this article. The type of frailty assessment impacts the associations observed, assaying global versus specific loci, serum/plasma versus muscle tissue, direct measures versus associative studies (and use of algorithms like methylation clocks) as well as the context (patterning of histone marks and methylation, aging, frail, nonfrail) in which the studies are conducted, and the lack of animal models together impact the outcomes and determine the strength of the conclusions. Beyond the associations and presence of biomarkers lies the understanding of the biology which represents the biggest challenge to ascertain therapeutic interventions to slow, delay, reverse or prevent frailty.
Stress-response System in Frailty
The loss of physical and cognitive reserve and decreased function that often occurs with advanced age may also be accompanied by an increased vulnerability to stressors and parallel physiological dysregulation. Terminology employed within this topic includes physiological reserve, robustness, resilience (described earlier in this article), coping mechanisms, and homeostasis disruption among others. Fried and coworkers (177) describe frailty as a high-risk physical state with decreased reserves and increased vulnerability to stress and suggest that the key driver is energetic imbalance. Others suggest that this inability to generate an optimal response to stressful stimuli is the underlying mechanism that leads to frailty (68). Together, based on what is known currently, it appears that the mechanisms that contribute to frailty are multifactorial.
The pathways involved in an organisms’ response to stressors depend in part on the exposure type, strength, length, and the state of health. There is general agreement, however, that with advanced age the ability to adapt to or resist stress is lower than at younger ages resulting in heightened vulnerability (432). Another way to think about this vulnerability is that physiological systems decline in efficiency and cellular communication deteriorates with time resulting in dysregulation. This physiological dysregulation may not be apparent initially (or in the resting state) but is observed when the system is challenged. Responses to acute stress vary but can include changes in heart rate, respiratory rate, glucose availability, digestive tract activity, and muscle tension among others. In turn, chronic stress can be more detrimental and impair growth, reproduction, immune competence, bone quality, and physical functioning. Thus, the dysregulation that occurs over time that results in altered responsiveness to acute and chronic stress may contribute to the development and progression of frailty.
The key biological systems that respond to stress and impact daily activities include the nervous (sympathetic), endocrine, and immune systems, leading to downstream physiological/metabolic adaptations to short- or long-term conditions (Figure 4). What dictates a “stressor” or stressful situation is beyond the scope of this article, but in general terms, the physiological response to a stressor involves coordination of events in both the brain and periphery. Physiological systems activated by a stressor are many and range from molecular to organismal. Activation of the hypothalamic-pituitary-adrenal (HPA) axis is one component of the systemic response assisting the organism in coping with stress. The HPA axis is primarily involved in energy mobilization but has evolved in the literature as a biomarker of stress (a discussion that is also beyond the scope of this article and in many cases is truly integral to the overall systemic response) (365).
HPA axis and frailty
We think of the HPA axis as a primary coordinator generating behavioral responses but also the adaptive responsiveness in intermediary metabolism and immunity as well as reproduction and feeding (102, 103). As such, upon a stressful event activation of the HPA axis includes corticotropin-releasing hormone (CRH) release from the hypothalamus and subsequent stimulation of adrenocorticotropic hormone (ACTH) release from the anterior pituitary. ACTH propagates the signal by stimulating the adrenal gland to release glucocorticoids (cortisol in humans, corticosterone in rodents). In acute situations, glucocorticoids rise within minutes to hours and impact neuronal activities, glucose stores, and immune cell distribution, among other events (glucocorticoids impact thousands of genes). As with other endocrine factors, negative feedback is in place to maintain homeostasis with glucocorticoids downregulating the release of CRH and ACTH. Thus, in the context of physiological dysregulation and the development and progression of frailty, maladaptive or unrestrained responses of the HPA axis may be considered one of the main drivers of a physically frail state interacting with metabolism and the musculoskeletal system (Figure 4) (177, 187, 432).
Accordingly, an increased vulnerability to stressors is documented as neurons age that in turn, impacts HPA hormone production and release (180). Plasma cortisol levels vary with time of day (diurnal variation) but are typically high in the morning and lower in the evening. Although differences in morning cortisol levels in prefrail, frail, and nonfrail individuals varied between studies, all the studies found that physical frailty was associated with higher evening cortisol levels and an overall blunted diurnal variation in cortisol. This blunted or loss of a dynamic cortisol response (via altered negative feedback of ACTH) results in prolonged exposure to higher overall cortisol levels in these older adults and likely contributes to vulnerability and the clinical presentation of frailty (195, 238, 267, 334, 415, 581). This low reactivity of the HPA axis has been previously correlated with negative health outcomes (447). Furthermore, the physical characteristics of gait speed and grip strength, which are two tests within the Frailty Phenotype assessment tool, are correlated with morning to evening cortisol ratios (194, 195, 267, 546). Walking speed and chair rise time (measure of strength) are also associated with impaired diurnal cortisol (194). These findings suggest a link between disrupted cortisol and muscle atrophy underlying physical frailty. Consistent with HPA axis dysregulation and prolonged exposure to cortisol there is evidence that these changes also contribute to altered stress responsiveness and deterioration including neurodegeneration and cognitive decline (157, 494, 495, 553).
There are several examples of stimulus-response experiments that have been conducted to characterize responses to stress that strongly support the hypothesis that this dysregulation in community-dwelling older adults contributes to frailty [as reviewed in Fried et al. (177)]. Within the HPA axis, an ACTH challenge elicited exaggerated dehydroepiandrosterone (DHEA) responses associated with increasing frailty from nonfrail to prefrail and frail suggesting inappropriate negative feedback (334). In women challenged with lower extremity isometric exercise, skeletal muscle phosphocreatine recovery was slower in frail when compared to prefrail and nonfrail individuals (580). Consistent with the previous study, Lewsey and coworkers (345) also showed that exercised frail persons exhibited significant declines in skeletal muscle energetics compared to nonfrail older adults. When considering glucose metabolism, in nondiabetic older women subjected to an oral glucose tolerance test (stress challenge), those categorized as physically frail exhibited an exaggerated and prolonged increase in mean insulin and glucose levels compared to nonfrail and prefrail women (285–287). In addition, the frail women displayed dysregulated ghrelin following this glucose tolerance test (614). Though glucose dysregulation was not uncommon in these individuals, it was remarkably dysregulated in the frail women. Finally, when considering responses within the cardiovascular system orthostatic hypotension was significantly more prevalent in community-dwelling older adults considered frail compared to nonfrail when challenged by an orthostatic blood pressure test (lying to standing) (614). In individuals categorized as frail, each of these altered responses to a stressful event provides evidence of increased susceptibility to stress that is tied to physiological dysregulation across many physiological systems.
In view of the discussion of stress adaptation and frailty, it is important to recognize the role glucocorticoids play in skeletal muscle glucose and protein metabolism. Indeed, glucocorticoids inhibit insulin-stimulated glucose uptake and glycolysis as well as by decreasing protein synthesis and enhancing proteolysis (320). In the presence of chronic glucocorticoid-mediated protein degradation by the ubiquitin-proteasome system and autophagy-lysosome system, there is significant skeletal muscle atrophy and weakness (66, 143, 320, 497). We also know that dehydroepiandrosterone sulfate (DHEAS), another adrenal-derived hormone, exerts anabolic functions in muscle and is decreased with aging. Moreover, the serum cortisol/DHEAS ratio (≥0.2) from older adult patients aged ≥65 years with type 2 diabetes (T2D) was identified as the strongest risk factor for sarcopenia and was associated with increased odds of frailty in a 10-year longitudinal study (44, 632). Thus, the concomitant increase in cortisol levels and decrease in DHEA likely contribute to physical frailty and sarcopenia (PF&S) (632).
Taken together, while the evidence is still incomplete and with the contributions of the many cellular mechanisms that regulate glucocorticoid levels unknown, the inability to maintain homeostatic control and the resulting “persistent high cortisol levels” are likely playing a role in triggering frailty onset and frailty progression within multiple tissues (105).
Somatotropic axis in frailty
Other components of the hypothalamic-pituitary (HP) axis have also been linked to aging and frailty. The somatotropic axis, in particular, has been investigated for its anabolic role in muscle and as a major player in longevity (69, 70, 586). The somatotropic axis consists of growth hormone (GH), upstream hypothalamic hormones, the insulin-like growth factors (IGFs), and downstream signaling molecules. The balance of two hypothalamic factors, growth hormone-releasing hormone (GHRH) and somatostatin (SS) determines the rate of GH secretion from the anterior pituitary. Plasma GH directly stimulates IGF-1 production and secretion by the liver in addition to exerting direct effects on other tissues. Local tissue production of GH or IGF-1 also occurs, suggesting the importance of autocrine and paracrine actions of these hormones. GH and IGF-1 have both somatic effects stimulating the growth of tissues and metabolic effects that play a role in protein, carbohydrate, and lipid metabolism. Alterations in these interrelated pathways can thus lead to both growth retardation or tissue proliferation and a variety of metabolic disturbances.
In mammals, there is a natural age-related decline in plasma GH levels and a concomitant decrease in IGF-1 that likely act as protective mechanisms to decrease metabolic activity and cellular division (255). Many studies have shown that GH secretion patterns, GH receptor deletions, IGF-1 receptor (IGF1R) mutations, and low circulating IGF-1 levels are associated with longevity and survival in nonagenarians and centenarians (49, 58, 390, 548, 575, 576). Yet, the role of the IGF-1 pathway in relation to aging and longevity in mammals is inconclusive, which may be related to ethnicity, sex, age, and dietary differences among cohorts and the fact that GH is driving much of the IGF-1 expression (95, 391, 461, 645). Briefly, the field began to focus on IGF-1 instead of GH because invertebrate longevity data pointed to the insulin-IGF signaling pathway as integral to lifespan determination. Invertebrates (nematodes, flies) do not have GH or an upstream master regulator and function associated with GH driving insulin/IGF activities (at least not identified at this point in time). Thus, the translation from invertebrate systems to mammalian signaling is not direct, misinterpreted, and perhaps misguided (35). That said, while GH/IGF-1 pathway declines with aging in mammals, intriguingly, from a frailty biomarker perspective, low IGF-1 levels increase the odds of frailty and symptoms of frailty (strength, physical performance) (139, 338, 578). These reported associations involving the GH/IGF-1 signaling pathway are likely due to its role as a major player in metabolism, whereby its decline leads to a multitude of physiological consequences (e.g., frailty). For instance, IGF-1 promotes a major role in regulating skeletal and cardiac muscle growth by increasing myocyte number, activating muscle cell hypertrophy, anti-apoptotic properties, or inhibiting muscle protein breakdown (36, 446, 567). Similar to the insulin pathway, the IGF-1 signaling cascade is centrally regulated by Akt (protein kinase B), that controls protein synthesis via the kinases mTOR and glycogen synthase kinase 3β (GSK3β), while protein degradation is mediated by forkhead box protein O (FoxO) transcription factors (503). Indeed, variants in AKT1 and FOXO3A genes were identified in 567 nonagenarians/centenarians as important to the aging phenotype (229, 616). Overall, the somatotropic system plays a role in the maintenance of muscle and its function as well as in aging and longevity and therefore, is key to our understanding the hormonal contributions to frailty.
The physiological response to stress changes with age. With advanced age, key biological systems such as the HPA axis and the somatotropic signaling axis respond less optimally resulting in a decreased ability to adapt to stress and heightened vulnerability compared to younger organisms. It is this loss of resilience that may lead to prefrail and frail states in older organisms.
Inflammation and Frailty
An area under current intense investigation focuses on the role of the immune system as an underlying cause of aging processes, age-related disease, and frailty. Chronic nonre-solved inflammation is a shared clinical condition among many immunometabolic disorders, including age-related diseases and frailty (186, 515). The contribution of chronic inflammation to the pathogenesis of age-related disorders has been termed inflammaging. Conceptually, when the resolution phase of inflammatory process is delayed or fails, other severe detrimental conditions ensue such as chronic secretion of proinflammatory cytokines and glucocorticoids, inappropriate initiation of inflammatory stimuli, misplaced molecules, misfolded molecules, and oxidized proteins which may promote inflammaging (119, 173, 174, 577). The overproduction and/or extended exposure to high levels of proinflammatory cytokines may lead to a loss of homeostasis and an exacerbated catabolic state within tissues that are especially vulnerable [e.g., muscle, (4, 186, 518)]. Figure 5 highlights potential triggers of inflammation, mediators, and the consequences of inflammaging.
Figure 5. Inflammaging.
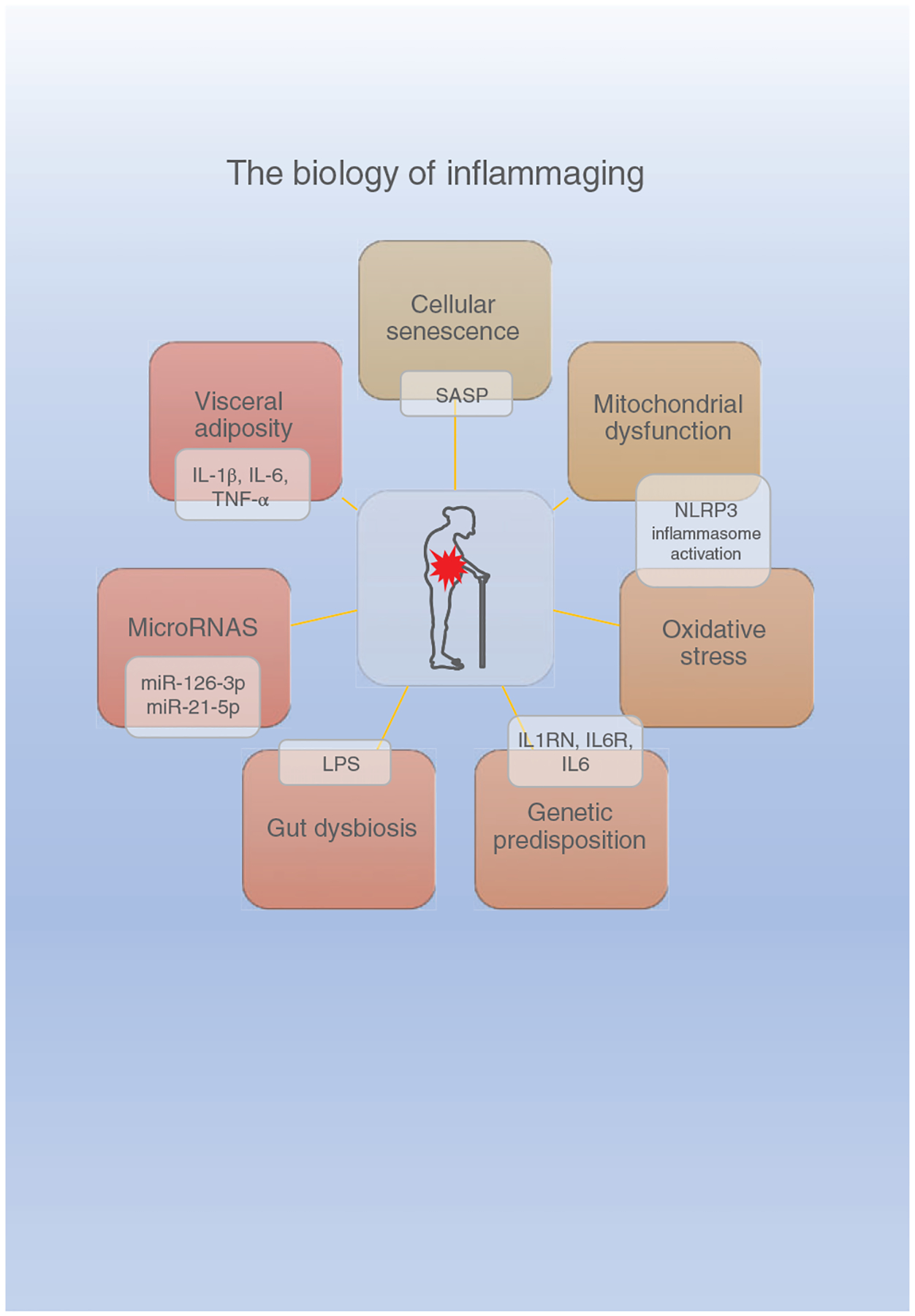
The biology underlying inflammaging is multifactorial. The mechanisms that contribute to inappropriate inflammatory responses and ultimately low-level chronic inflammaging include cellular senescence, mitochondrial dysfunction, oxidative stress, visceral adiposity, gut dysbiosis, genetic predisposition, and epigenetics factors such as microRNAs. Potential mediators contributing to the chronic inflammation have both local and systemic impacts that likely promote physical decline. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Over the last decade, there is an abundance of analyses investigating inflammatory markers associated with aging and frailty from a biomarker perspective to identify populations at risk for poor outcomes (80, 281). Briefly, the most commonly studied inflammatory markers are C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, and interferon-gamma (IFN-γ) with CRP, IL-1-receptor antagonist, IL-6, IL-18, and TNF-α receptor-1 [nuclear factor kappa B (NF-κB)-mediated pathway markers of inflammation] associated with adverse health outcomes and mortality in older adults (22, 119, 173, 455, 582).
There is a preponderance of cross-sectional studies reporting that high levels of IL-6 and CRP are associated with frailty, frailty severity, poor physical performance (gait speed, strength, physical activity), and poor cognitive performance (23, 277, 337, 449, 455, 498, 539, 600). Prefrail and frail older adults also show elevation in white blood cells and fibrinogen compared to robust adults (111, 339, 340, 539). Importantly, many of the clinical frailty-associated inflammatory markers identified above and other biomarkers (e.g., transferrin) are also confirmed with advanced technologies such as proteomics-based screening (128). In contrast, the analyses from four large prospective studies (longitudinal design) failed to confirm these findings and other studies do not always report elevation of these classical inflammatory biomarkers (328, 633). Differences between studies are due, in part, to a variety of challenges that face the field, including study design, and conditions that increase inflammatory markers such as medical conditions and presence of obesity (328, 533, 534, 539, 587). Collectively, although evidence is emerging that greater inflammatory activity is associated with frailty, it is not true of all inflammatory markers. These studies are promising but much more research is needed to identify best practices (e.g., inclusion criteria of participants, selection and analyses of biomarkers) to yield the most useful information. We foresee longitudinal research studies further delineating the role of inflammation at the onset of frailty and transitions between frailty severity, delineating inflammatory sources and their targets, and development of targeted interventions.
Interestingly, only a few of these inflammatory biomarker studies are designed to report sex differences. For instance, high concentrations of CRP and fibrinogen are more strongly predictive of incident frailty in women than in men (188). In older institutionalized men with multiple comorbidities, a higher IL-6 level is positively associated with the Frailty Phenotype, while no significant correlations are noted for TNF-α and CRP levels (324). Recently, while investigating the immunological aspects of frailty higher numbers of myeloid-derived neutrophils and monocytes, but not lymphoid-derived T-, B-, or NK(natural killer)-cell numbers, were associated with frailty in both women and men (491).
Although much of the research is focused on inflammatory biomarkers, this approach has identified associations between frailty and with distinct metabolic/hormonal pathways (IGF-1, triiodothyronine, CRP, erythrocyte sedimentation rate, white blood cell, and lymphocyte counts), between frailty and reactive oxygen metabolites and between slower gait speeds (a frailty symptom) and isoprostanes, lipoprotein phospholipase A2 and osteoprotegerin (350, 498). Collectively, these associations demonstrate the loss of homeostasis across many cellular systems and tissues (169, 539). However, the underlying biological explanations for these changes in terms of initiation have yet to be uncovered.
The biomarker approach is useful for demonstrating that inflammatory activity is related to frailty and frailty risk, but there is a dearth of studies focused on mechanistic information needed to combat frailty or focused on the anti-inflammatory regulatory pathways. One example of a mechanistic study comparing sixteen pairs of frail and age-, race-, and sex-matched nonfrail participants, found that pro-inflammatory C-X-C motif chemokine ligand 10 (CXCL10) expression as well as serum IL-6 levels positively correlated with frailty status, suggesting CXCL10 as a possible biological target in preventing frailty (456). Future research in the field of frailty and inflammation will take advantage of molecular, transcriptional, and proteomic biomarkers as well as analyses to integrate information of inflammatory activity and immune regulation and dysregulation (131).
Given that preclinical animal investigations focused on frailty and inflammation are in their infancy, there are a few studies implicating chronic inflammation as an underlying mechanism to frailty. For example, treating frail mice with Enalapril, an angiotensin-converting enzyme (ACE) inhibitor, alleviated symptoms of physical function while reducing proinflammatory cytokines IL-1α, monocyte-recruiting chemokine monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1a and up-regulating the anti-inflammatory IL-10 cytokine (299). Paralleling the development of preclinical assessment tools to classify frailty, the use of frailty as an outcome variable, and the studies focused on frailty intervention, there’s a growing body of evidence using preclinical rodent models that mimic specific aspects of immune dysfunction that are proposed to contribute to frailty.
IL-10KO mouse and IL-10/6DKO mouse:
The IL-10KO (Il10tm1Cgn/J) mouse develops a chronic inflammatory bowel disease and is a model proposed to study the biological basis of frailty (598). Briefly, IL-10 is a cytokine with anti-inflammatory properties and maintains the balance of the immune response by allowing the clearance of infection while minimizing damage (496). Using the deletion of the IL10 gene as a comparable model to human frailty, skeletal muscle weakness paralleled enhanced serum levels of proinflammatory cytokines such as IL-6, IL-1β, TNF-α, IFN-γ, CXCL 1 (308). Because of these findings a series of studies determined whether the IL-10KO mice display a Frailty Phenotype and further sought to examine cellular pathways closely associated with the Frailty Phenotype. In this regard, the IL-10KO mice exhibit phenotypic frailty characteristics in onset of muscle weakness, fat mass and resting metabolic rate, and activation of low-grade inflammatory pathways (526). Other effects associated with IL-10 KO mice include dysregulated adipokine and hormone levels (leptin, adiponectin), differential expression of skeletal muscle genes related to mitochondrial function and apoptosis, and altered mitophagy pathways suggesting failure to clear abnormal mitochondria (8, 307, 308, 598, 609). With respect to mitochondrial dysfunction, the IL-10KO mice present with low rates of ATP synthesis, reduced energy release from ATP hydrolysis and mitochondrial death signaling, and high levels of damaged mitochondria (8, 307). The disruption in mitochondrial homeostasis likely contributes, in part, to increased oxidative stress damage and further triggers apoptosis, and the observed performance impairment and phenotype (strength and exhaustion). Lastly, these mice show cardiovascular changes such as stiffer blood vessels, impaired vascular relaxation, cardiac hypertrophy, and contractile dysfunction consistent with widespread metabolic changes. Indeed, these extensive properties support the hypothesis of inflammation and tissue dysfunction; however, this mouse model also has limitations that are associated with IL-10 deficiency and require attention when interpreting results (e.g., altered lymphocyte and myeloid profiles, increased cancers, altered responses to inflammatory stimuli).
IL-10tm/tm/IL-6tm/tm mouse:
To identify the precise role of IL-6 on chronic inflammation and mitochondrial impairment, a double knockout (DKO) mouse deficient in both IL-10 and IL-6 was created, IL-10tm/tm/IL-6tm/tm (364). Briefly, IL-6 is a pleiotropic cytokine with a central role in the integrated immune defense network in response to tissue damage and infections (480, 556). The biological consequences of IL-6 production are associated with both pro- and anti-inflammatory effects, highlighting IL-6’s pivotal role in the activation and regulation of the immune response (501). Phenotypic characteristics, serum measurements (cytokine, lipid metabolite, and mitochondrial energetics), cardiac oxidative metabolism and mitochondrial energetics, treadmill testing, and survival were determined in the DKO mice and compared to age- and gender-matched IL-10 KO and WT mice. The overall findings demonstrate that selective knock-down of IL-6 in a frail mouse with chronic inflammation results in the reversal of some of the chronic inflammation-related alterations. The DKO mice had increased protective mitochondrial-associated lipid metabolites (serum), improved myocardial oxidative metabolism, and a transitory improvement in functional performance. However, these mice also had higher mortality.
Inducible IL-6 expression (IL-6TET-ON/+) mouse:
The Inducible IL-6 expression (IL-6TET-ON/+) mouse was developed to determine to what extent a single cytokine in isolation, recapitulates features of frailty in mice (264). IL-6 was selected because serum IL-6 is consistently found to be elevated in frail individuals and was suggested to be a causal driver. In this model, IL-6 induction was doxycycline dose-dependent and increased independently of other inflammatory cytokines and to levels observed in old mice. Importantly, increased IL-6 levels lead to increased frailty and disrupted muscle mitochondrial homeostasis. These results suggest a direct causal relationship between IL-6 and frailty.
Lastly, to provide a more complete picture of the inflammatory state during frailty and to the connectedness of the pillars, cellular senescence will be discussed briefly. Cellular senescence is a complex process, which is characterized by the inability of cells to proliferate, leading to over-production of proinflammatory factors (cytokines, chemokines, and other pro-inflammatory molecules) by senescent cells (senescence-associated secretory phenotype, SASP) (117). This process is favored by aging, was reported in multiple tissues, has a wide range of effects, and can promote chronic health diseases including insulin resistance, CVD, chronic obstructive pulmonary disease (COPD), neurological disorders, cancer, and osteoarthritis (302, 651). Recently SASP proteins [a panel of seven SASP factors composed of growth differentiation factor 15 (GDF15), TNF receptor superfamily member 6, osteopontin, tumor necrosis factor receptor 1 (TNFR1), Activin A, chemokine (C-C motif) ligand 3 (CCL3), and IL-15] were positively associated with age, frailty, and adverse postsurgery outcomes (500). In addition to these seven SASP proteins which are closely associated with inflammation; increased cell senescence due to enhanced autocrine and paracrine feedback mediated by NF-κB, cycloxygenase-2 (COX-2), and reactive oxygen species (ROS), results in enhanced telomere dysfunction (279). Evidence suggests it is possible to suppress the elevated SASP by targeting the Janus kinase (JAK) pathway and observing a wide range of effects. In fact, JAK inhibition in frail mice alleviated both adipose tissue and systemic inflammation while enhancing physical function and improving many symptoms of frailty (627). Equally interesting is the fact that targeting cellular senescence using multiple approaches to reduce inflammation (e.g., senolytics: Dasatinib and Quercetin) in old mice and mouse models of accelerated aging improved physical function and reversed aspects of frailty, suggesting cellular senescence is a driver of the diminished skeletal muscle function (626, 652). It is important to note that the field identifies and provides evidence for many potential causes of SASP including DNA damage, dysfunctional telomeres, epigenomic disruption, mitogenic signals and oxidative stress, infections, lifestyle, and environment; hence, interventions to prevent or attenuate cellular senescence will likely be multifactorial (78, 144, 545, 639).
Complex and deeply integrated physiological systems work together to maintain homeostasis and function. Thus, an imbalance or disruption in one system has multiple downstream effects. So much that, the aging-related chronic physiological stimulation of the innate immune system may arise from metabolic regulation at the same time that immune molecules and cells may also impact metabolism in turn. Dysfunctional immunometabolism increases susceptibility to age-related disorders and physical frailty.
Metabolism and Frailty
The major contributor to aging and mechanism by which biological changes are induced with age is metabolism (Figure 3). There is compelling evidence that a multitude of metabolic-associated genes and major signaling pathways compress the period of morbidity including target of rapamycin (TOR), adenosine monophosphate-activated protein kinase (AMPK), and the nicotinamide adenine dinucleotide (NAD+)-dependent sirtuin deacylases among others. It is believed that these pathways sense and respond to the nutritional environment and promote cellular defense mechanisms in the face of stress with the overall goal of maintaining homeostasis. Collectively, metabolic processes have been shown to change over time in multiple organs and represent an underlying cause of aging that likely contributes to the onset and progression of frailty.
Many theories describe the metabolic features that drive aging and potentially frailty. Two of them are complementary, associating energy expenditure and oxidative stress to physiological and physical decline. The Rate of Living Theory postulates that the metabolic rate is inversely related to lifespan (i.e., lower metabolic rate associates with longer lifespan). Similarly, the Free Radical Theory links the excessive mitochondrial ATP production from high metabolic rate to oxidative stress damage and lifespan shortening (165, 468). In this sense, limiting energy consumption could potentially cause metabolic rate to decline, delaying frailty. In fact, since 1935, evidence consistently highlights CR as the only intervention able to extend lifespan (Figure 6) in many species, including humans and rhesus monkeys (113, 114, 532). It is worth noting that the rhesus monkey provides important insights on the health benefits of CR to humans given that the rhesus monkey shares nearly 93% of sequence identity with the human genome and that, similarly as in humans, CR increases survival and lowers age-related morbidity (113, 114, 379, 393).
Figure 6. Caloric restriction.
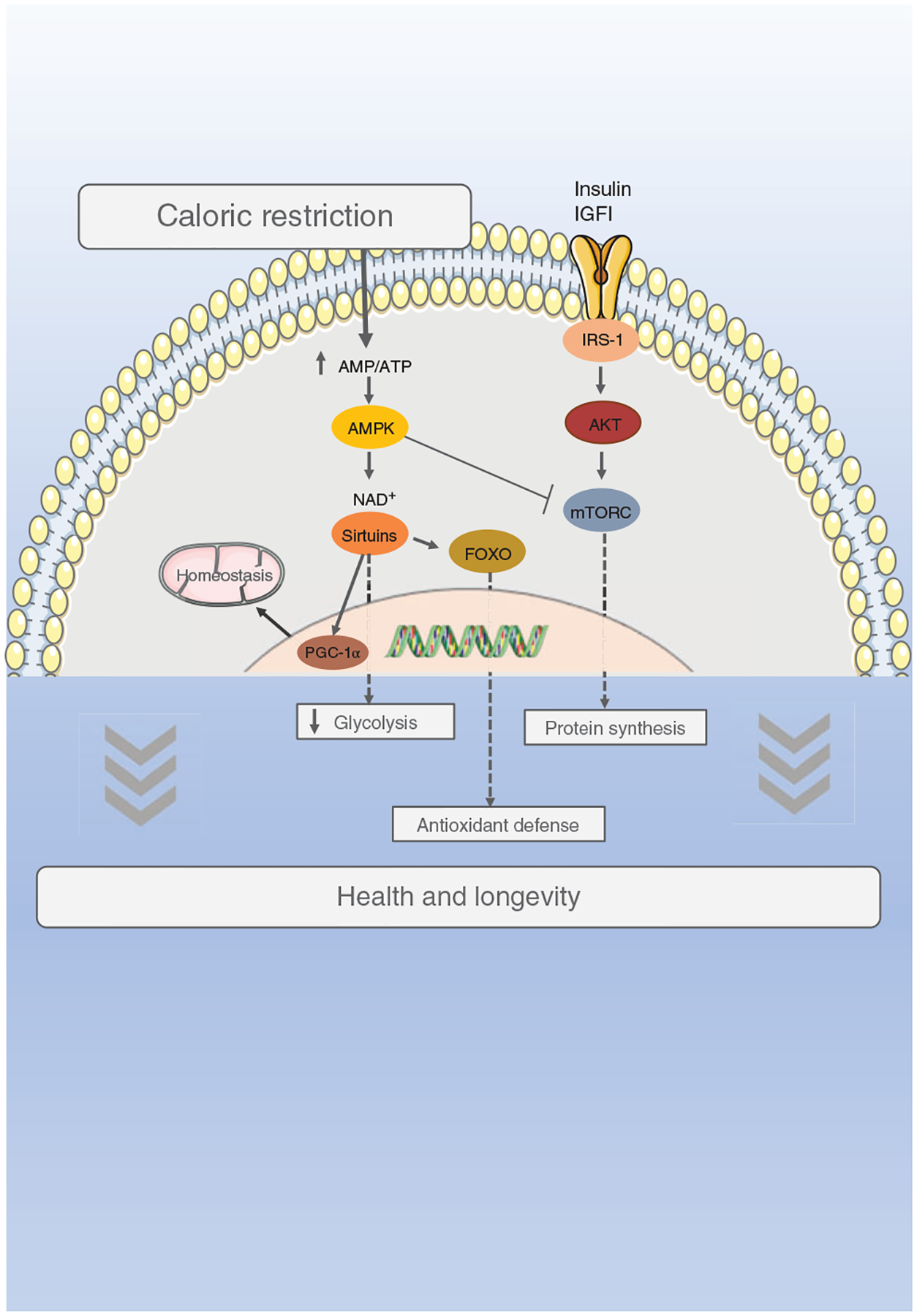
Caloric restriction is the most well-established longevity-modulating intervention. Importantly, dietary restriction whether caloric (protein, carbohydrates, fat), intermittent feeding, or fasting improves health by decreasing morbidities that are associated with aging including frailty. It does so through alterations in energy restriction pathways. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Population-based studies, randomized controlled trials, and intervention studies clearly provide evidence that CR (15%–30%) for extended periods of time (1–2 years) in healthy individuals decreases mortality rate, slows metabolic rate, and reduces oxidative stress (48, 235, 468). Results of these studies support both the rate of living and free radical theories discussed above. Other physiological adaptations associated with CR include reductions in body weight, central obesity (visceral fat mass), daily energy expenditure, inflammation, and cardiometabolic risk, as well as increases in insulin sensitivity (230, 261, 269, 384, 401, 458, 467). Similarly, these physiological adaptations to CR are reported in individuals with obesity following CR regimens (269, 331).
Next, the question arises as to whether CR reduces frailty. Although there is an emerging number of small studies the findings are very promising. One such example is a study in mice by Kane and colleagues (290) whereby reduced calorie intake led to an improvement in frailty status as determined by the FI. In another rodent model, dietary restriction in rats led to a decrease in frailty incidence, improvement in animal activity, and spatial memory (563). Likewise, genetically long-lived hypopituitary Ames dwarf mice subject to 30% CR show a protection in some of the clinical features of the frailty syndrome (i.e., grip strength and fatigue test) (24). Consistent with the observed improved behavioral performances, CR delays sarcopenia by favoring protein synthesis, regulating mitochondrial function, and promoting SC regeneration in several animal models [reviewed in Xie et al. (625)].
To date, it is unknown whether intermittent fasting reduces frailty per se; however, there is evidence that there are beneficial effects in frailty features [physical activity and survival in older mice (24-month age)] (485). It is also important to point out that there is evidence that the gut microbiome shows a shift in metabolic and taxonomic properties with increasing frailty, demonstrating changes in availability of specific nutrients (330). In nonhuman primates, a long-term study clearly indicates that CR reduces the incidence of frailty and increases healthy lifespan (631). In a close view of these limited number of studies, the results suggest that interventions of longer duration and/or initiated earlier in the lifespan have potential to be most effective for frailty (563).
With the noted benefits of CR, reduced levels of certain macronutrients primarily in human studies raise questions of whether longevity and more recently healthspan are affected by the calories per se, or if they are related to proteins specifically (22, 107, 108, 433, 535). There are numerous studies highlighting important concepts related to diet (e.g., single essential amino acid, methionine, specific BCAAs, ratio of macronutrients, sources of proteins and timeframes) supporting longevity, multiple metabolic benefits, and healthspan (170, 310, 344, 428, 471, 636). For example, we understand that restricting dietary proteins, such as branch-chain amino acids (important mTOR regulators) extends lifespan and promotes metabolic health in mice (471). It is also logical to see the potential impact to prevent, delay or reverse physical frailty given the importance of amino acid availability to alleviate sarcopenia through skeletal muscle protein synthesis (333). However, the literature in the field of macronutrients reveals great complexity. Clinically, in patients with cachexia, protein supplementation is not always successful on preserving lean mass as fat mass is the tissue depot that often prevails [reviewed in Evans et al. (152)]. Moreover, the effectiveness of dietary protein supplementation against muscle mass depletion and poor physical function might be dependent upon many factors such as physical stimulation (85, 233, 555). While being promising and exciting at this time more research is indicated to evaluate the cellular mechanisms with preclinical frailty models based on findings relating restricted protein intake to sarcopenia and frailty (108). Regardless, to date, the role of CR in human longevity remains to be elucidated.
Within the discussion of CR, it is unclear whether CR-related weight loss might be detrimental to overweight older individuals [reviewed in Locher et al. (355)]. Epidemiological data indicate that overweight older adults have lower risk of mortality than normal-weight older adults (97, 166). There are potential detrimental effects to muscle and bone health with CR-induced weight loss because the weight loss parallels reduced lean mass and bone mineral density in humans [reviewed in Locher et al. (355)].
Taken together, metabolism is significantly impacted by diet (amount, composition, feeding regimen, etc.) which in turn, modulates aging processes likely resulting in changes in susceptibility to the development and progression of frailty. Yet, given the heterogeneity of frailty, the efficacy of dietary interventions might depend on age, research model, interventional strategy (e.g., % of calories, % of macronutrients, length of intervention), stage of frailty, presence of comorbidities, etc. In the following paragraphs, some of the key factors involved in metabolism will be introduced and discussed as they relate to aging. However, these descriptions are not meant to be all encompassing but are focused on their relationship to frailty.
mTOR
At the center of aging and metabolism is the mTOR pathway. The mTOR signaling network is a major nutrient-sensing system which ultimately through downstream effects regulates metabolism, mRNA translation, and protein turnover (Figure 6) (499). Within the aging research community, there is extensive effort to understand this signaling network because disruption is reported in many diseases, including cancer, T2D, and frailty. From an aging perspective, mTOR inhibition (e.g., Rapamycin) is a well-established mechanism that is subject to genetic and drug manipulation to influence healthspan and longevity (55). Of note, rapamycin is an Food and Drug Administration (FDA)-approved immunosuppressive drug to which clinical relevance is applied to organ allograft rejection and more recently in clinical practice for immunological, physical performance, and cognitive outcomes among older adults (317, 368, 369).
Indeed, in a range of animal models, mTOR is a key modulator of lifespan and healthspan acting through various mechanisms (227, 270, 387, 388). These mechanisms include extension of lifespan while promoting gut homeostasis via SC function (inhibiting mTORC-1 activation) (361), microbiome remodeling, as well as via inhibition of senescence and SASP by suppressing translation of IL-1α and suppression of carcinogenesis (17, 323). Moreover, feeding mice rapamycin reduces resting metabolic rate, delayed death, and development of pathological lesions while improving motor function in both sexes (647).
Loss of skeletal muscle mass and strength is central to the phenotypic criteria or clinical hallmarks used to classify physical frailty (Figure 8). Regulation of muscle mass is thus a critical biological step in triggering frailty onset and frailty progression. To date, animal studies are the main contributors to the current knowledge on the physiological aspects leading to muscle atrophy. These studies demonstrate the activation of the mTOR network by IGF-1 and phosphoinositide-3-kinase (PI3K)/Akt promotes protein synthesis via S6 kinase phosphorylation (488, 499, 503). The importance of S6 kinase is further reported in studies whereby mice lacking S6 kinase show extension of lifespan along with a resistance to age-related pathologies (513). Important to this comprehensive article, with aging the chronic activation of mTORC1 stimulates increased numbers of abnormal skeletal muscle mitochondria leading to oxidative stress, fiber damage, and fiber loss over the lifespan (557). The pro-oxidative mitochondrial effect is likely associated with alterations in expression of GDFs, including GDF-15 (557). Whereas, inhibiting mTORC1 (rapamycin) alleviates oxidative stress and reduces muscle fiber loss in old mice (557). The preservation of muscle fiber size and muscle mass is also associated with decreasing gene expression of cellular senescence markers (276). In principle, in the presence of chronic activation or by the inhibition of mTORC1 in skeletal muscle it is logical to predict a significant impact on muscle function leading to changes in physical performance.
Figure 8. Physiological systems promoting frailty.
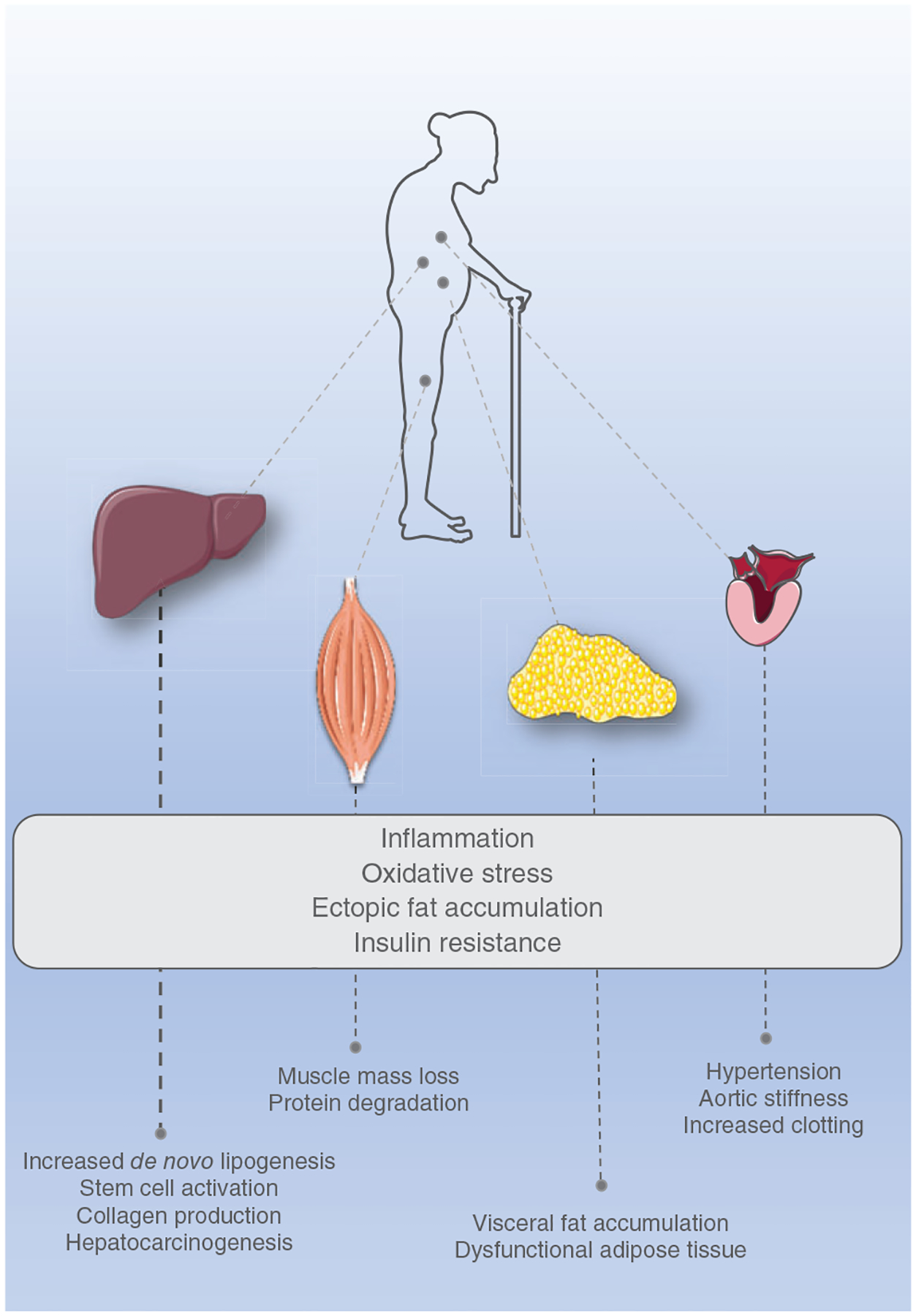
Frailty involves a multiple organ network that deteriorates with age and features a decline in functional reserves of many physiological systems. There are common impaired responses observed in many organs of the individual with frailty including inflammation, oxidative stress, ectopic fat accumulation, and insulin resistance. The liver is a central player in metabolism and has thus a key role in the aging process. In frailty, there is an increase in de novo lipogenesis that refers to the biochemical synthesis of fatty acids from the carbohydrate catabolism, boosting ectopic fat accumulation. The fatty liver, combined with inflammation and oxidative stress, promotes hepatocyte injury, facilitating fibrosis (collagen production), stem cell activation, and even cancer development. Muscle is also central to the biology of frailty and is the main organ system contributing to the Frailty Phenotype as muscle mass loss and protein degradation trigger weakness, slowness, and weight loss. As compared to subcutaneous adiposity, visceral adiposity is the most detrimental to health due to its pro-inflammatory profile. The increased inflammation, ectopic fat accumulation, and oxidative stress are all risk factors to cardiovascular events by facilitating endothelial dysfunction, aortic stiffness, and clotting. On top of that, increased visceral adiposity and hepatic de novo lipogenesis promote dyslipidemia, which also contributes to cardiovascular dysfunction. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
In a pilot study with a focus on frailty, rapamycin administration did not improve frailty status even in the presence of reduced inflammation (530). In contrast, in an accelerated aging mouse model [genetically enhanced NF-κB activity (nfκb1−/−)], rapamycin reduces frailty and improves long-term memory, as well as neuromuscular coordination and tissue architecture (118). Additional positive outcomes indicative of a protective role against frailty are reported in IL-10 homozygous knockout mice treated with rapamycin and/or maraviroc—[the only specific C-C chemokine receptor type 5 (CCR5) antagonist approved for clinical use] (438). Overall, the evidence supporting the role of inhibiting mTOR to combat frailty is promising; but at this time the identification of a specific target tissue is lacking which is critical for a significant impact. Taken together, in the next generation of preclinical frailty research, understanding the mTOR signaling pathway and its downstream effects may be critical to preserve muscle function and prevent the onset of frailty.
AMPK
AMPK represents an additional promising metabolic target in the quest to prevent, delay or reverse frailty (Figure 6). The rationale to target this molecule is not based on substantial evidence assessing frailty but is based on investigations in the fields of aging (longevity) and pharmacological strategies to manage chronic diseases (e.g., diabetes T2D). The beneficial effects of AMPK are via metabolic modulation through a multitude of pathways in many tissues. Briefly, AMPK is a highly conserved molecule, acting as an energy sensor such that upon low intracellular ATP levels, AMPK stimulates catabolic pathways while regulating mitochondrial homeostasis through complex processes that aim to switch off anabolic pathways (AMPK-dependent transcriptional reprogramming) (232). In fact, in skeletal muscle, AMPK regulates energy metabolism in a NAD+- and SIRT1(NAD-dependent deacetylase sirtuin-1)-dependent manner, leading to activation of the peroxisome proliferator-activated receptor (PPAR)-gamma and the FOXO1 and FOXO3 transcription factors discussed earlier in this article. Energy metabolism is also influenced as AMPK modulates metabolic enzymes, which are part of the fatty-acid and sterol synthesis network [e.g., acetyl-CoA carboxylase (ACC) and β-hydroxy β-methylglutaryl-coenzyme A (HMG-CoA) reductase] (185).
Critical to the discussion of metabolism, aging, and the development of frailty interventions, AMPK regulates glucose uptake in muscle and adipose tissues by stimulating glucose transporter type 4 (GLUT4) trafficking (182). To date, there are well-established means to regulate glucose uptake through AMPK activation such as physical exercise and insulin sensitizers (thiazolidinediones); however, other classical glucose uptake regulators (e.g., anti-T2D interventions) are also emerging as exciting global therapeutics for health (31, 116, 171, 643). Since optimal glucose and insulin levels are integral to health and disease prevention, past and present studies utilizing metformin, in particular, are gaining attention. Indeed, as a key regulator of many metabolic pathways that are also involved in age-related diseases, metformin has also emerged as a potential anti-age player with effects that mimics those observed in CR. It is well-established that metformin attenuates microvascular and macrovascular complications in T2D in addition to its antihyperglycemic actions (via SIRT1/LKB1/AMPK pathway) (98, 519, 650). Metformin treatment also results in decreased hepatic gluconeogenesis and mitochondrial redox state, inhibition of mTORC signaling and Akt phosphorylation, and down-regulation of lipogenic pathways (356, 408, 439). Collectively, these metformin-associated changes have potential to influence the homeostasis within tissues. A compelling link to understanding frailty and potential cellular mechanisms is the increasing evidence supporting the role of AMPK to combat inflammation (demonstrated as NF-kB dependent via both AMPK dependent and independent pathways) (371, 489, 490). Understandably, metformin has been studied by many investigators as an intervention to delay aging in part because it is already FDA-approved for use in humans. Regarding AMPK as a target of metformin, the field of Geroscience and others are convinced that metformin has a geroprotective effect because it reduces all-cause mortality as well as age-related diseases (77, 347). The mortality benefits of metformin are more easily observed when comparing patients with T2D to individuals without T2D. Indeed, these two groups have similar mortality rates, even though they are more obese and exhibit co-morbidities (29).
To date, there are a small number of studies investigating frailty with metformin treatment as an intervention. In one study, two clinical features of frailty are differentially affected in healthy older adults, gait speed performance improved with metformin treatment whereas there was no change in grip strength (325). The protective effect of metformin against frailty and symptoms of frailty is also observed in older diabetic patients (decreased odds of frailty; improved muscle strength and body balance) (550, 603). Yet, the efficacy of metformin against frailty is inconclusive. For instance, one recent study indicates no correlation between metformin consumers and frailty prevalence (228). Because there are a small number of studies with differing outcomes, at this time the efficacy of metformin as a therapeutic strategy against frailty is unknown. We will await corroboration, as ongoing clinical trials (e.g., preventive nature of metformin against frailty in prediabetic adults aged more than 65 years old; TAME study) are completed (150).
There is a plethora of studies investigating metformin treatment in preclinical animal studies (e.g., C. elegans, rodents) that are beyond the focus of this article. Collectively, however, metformin-induced benefits, acting through AMPK activation, encompass extension of lifespan, improvement in physical performance and insulin sensitivity, and reductions in oxidative stress and inflammatory damage (73, 375, 506). These findings suggest a role for AMPK in triggering the onset of physical frailty, which is consistent with reports indicating an attenuation or suppression of AMPK activation in muscles from older rats with contractile dysfunction (222). Overall, there is promising evidence to pursue AMPK as a target to alter the onset and progression of physical features of frailty in both aging individuals and preclinical animal studies.
NAD+
A reduction in NAD+, a cofactor of key enzymatic reactions in many metabolic pathways and plays a pivotal role in maintaining the integrity of the mitochondrial electron transport chain, is likely involved in frailty. Yet there is a dearth of specific investigations focused in this area (Figures 4, 6, 7). Many studies in the aging field suggest that an increase in NAD(+) catabolism (down-regulation of NAD+) due to DNA oxidative damage occurs through SIRT3 pathways and impairs normal cellular function (76, 377). Because of the multiple cellular roles of NAD(+) a reduction has a widespread impact including disruption of the peroxisome proliferative activated receptor, gamma, coactivator (PGC)-1α/β-independent nuclear-mitochondrial communication (203). Hence, in view of the importance of energetics for cellular metabolism, there are substantial efforts to design therapeutics that target this pathway with the goal to increase, regulate or maintain NAD+ at youthful levels. For instance, elevating NAD+ with nicotinamide riboside (NAD+ precursor) in a placebo-controlled, randomized, double-blind, crossover trial, elevates muscle NAD+-related factors as determined in the metabolome while reducing systemic inflammation. Transcriptional expression via RNA sequencing further revealed downregulation of energy metabolism and mitochondrial pathways in muscle in these aged men (146). Promising results are also reported in patients with T2D (treatment with nicotinic acid derivative acipimox, an NAD+ precursor) revealing reduced skeletal muscle lipid content, increased insulin sensitivity, and further enhanced ex vivo mitochondrial respiration likely through activation of the mitochondrial unfolded protein response (UPR) in skeletal muscle (574).
Figure 7. Sarcopenia.
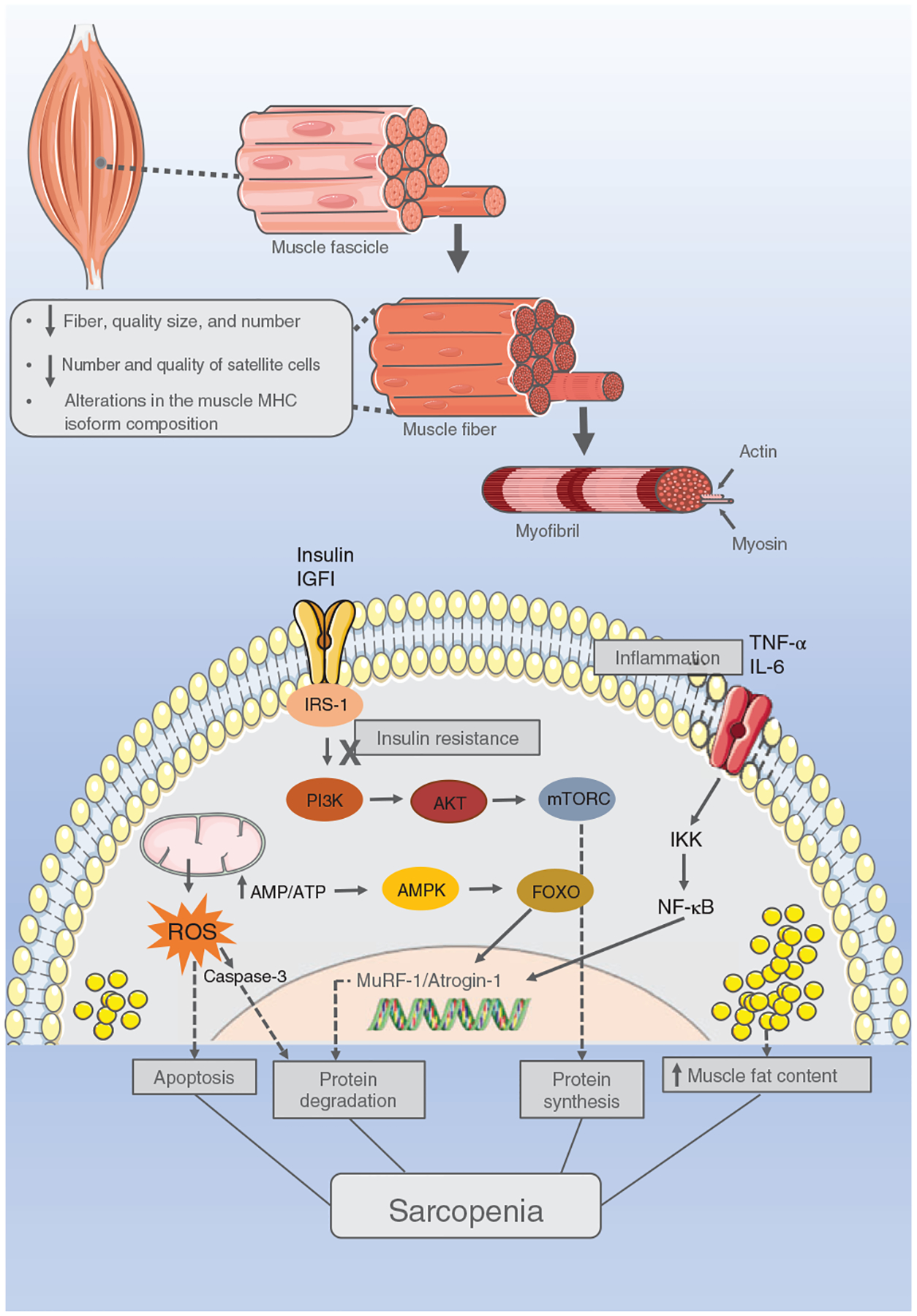
Sarcopenia is the natural event that is characterized by muscle loss and function. At the muscle fiber level, it is observed as a reduction in the fiber quality, size, and number. There is also a reduction in the number and quality of satellite cells, which are stem cells that promote skeletal muscle homeostasis and repair. In the muscle cell, the sarcopenic process is not only driven by increased protein degradation and decreased synthesis, but also by oxidative stress, insulin resistance, ectopic fat accumulation, and inflammation. Multiple signaling pathways provide avenues for therapeutic intervention. Illustrations were obtained on https://smart.servier.com, Published by LES LABORATORIES SERVIER, SAS.
Investigations in preclinical animal models (naturally aging, accelerated aging, mitochondrial and diabetic disease models, genetically engineered models to overexpress SIRT1 or SIRT6) and encompassing a broad range of outcome measures provide very promising support for targeting this pathway, too (60, 86, 133, 389, 558, 637). Foremost, investigations specifically focused on NAD+ therapeutic intervention and frailty are limited; however, the improvements associated with frailty symptoms in the following studies suggest regulating NAD+ levels would have positive outcomes to prevent, delay, or reverse frailty. Pharmacological prevention of age-related NAD+ decline (using 78c, a thiazoloquin(az)olin(on)e CD38 inhibitor) improves glucose tolerance, muscle function, exercise capacity, cardiac function along with enhanced expression of pro-longevity factors such as AMPK (558). Age-associated physiological decline is mitigated with nicotinamide mononucleotide (NMN) administration, a key NAD+ intermediate, as revealed by enhanced energy metabolism, physical activity, along with improved insulin sensitivity and plasma lipid profile (389). In old mice, NMN supplementation reverses the age-derived decline in endothelial function while upregulating NAD+, restoring vascular SIRT1 activity and increasing manganese superoxide dismutase (MnSOD) levels (133). Lastly, muscle SCs respond to nicotinamide riboside by inducing the mitochondrial UPR and synthesis of prohibitin proteins, causing the SCs to rejuvenate (644). Indeed, collectively these experiments uncover not only the multitude of effects of this pathway but demonstrate the wide range of benefits.
From a muscle perspective and its importance in physical frailty, depleting skeletal muscle of an essential enzyme in the NAD+ pathway results in fiber degeneration and progressive loss of two of the frailty symptoms, strength, and treadmill endurance in mice (175). In view of the positive outcomes of targeting this pathway in aging studies noted above, it is not surprising these functional deficits and muscle morphological changes are reversed with nicotinamide riboside supplementation (175). As evidenced in muscle disease studies, the Duchenne’s muscular dystrophy mouse model exhibits reduced NAD+ levels, decreased mitochondrial function, and impairment of tissue energetics. Targeting this pathway to replenish NAD+ levels results in significant improvements in mitochondrial function and structural protein expression as well as significant reductions in general poly (ADP)-ribosylation, inflammation, and fibrosis. These morphological changes are associated with improvements in skeletal muscle function and heart pathology (487). This cofactor plays a significant role in metabolism and specifically in metabolic pathways integral to aging and disease processes. Looking forward the NAD+ pathway will be pivotal in future studies identifying targets to prevent onset and progression of physical frailty.
Metabolites and frailty
Metabolites are substrates and products of metabolism or “proxies of metabolism” which have far-reaching cellular effects. Several of the cellular functions closely associated with this comprehensive review include regulation of epigenetic and SC mechanisms; cellular responses and signal transduction; and metabolism. Importantly, given that metabolites have effects within the local environment in which they are produced, they also have potential to impact and/or control homeostasis (268, 296). Notably, the homeostatic controls are likely compromised with age, leading to a failure to return to steady state and ultimately to a functional decline.
With the opportunities to profile metabolites in biofluids, cells, and tissues and to the advances in bioinformatics and analytical technologies, understanding tissue- and system-level effects of metabolites is emerging (268, 296). Due to the accuracy, specificity, and sensitivity of metabolomics, there is the possibility of detecting subtle alterations in biological pathways to provide insight into the multiple mechanisms underlying frailty and the progression of frailty and then integrate this knowledge with functional and mechanistic biological studies (112, 348). Although investigations focused on metabolites in their infancy in the field of frailty, evidence is emerging of differential expression of metabolites in individuals with frailty and in individuals at risk of becoming frail (prefrail) (161, 288, 316, 454, 466). As expected, these initial metabolomic studies identify diminished antioxidative defenses (e.g., carnitine shuttle, peroxisomal degradation, kynurenine pathway, vitamin E metabolism), decreases in radical scavengers (methionine, proline, tryptophan), disruptions in protein-amino acid, lipid and nitrogen metabolism, aminoacyl-transfer RNA biosynthesis, and citric acid cycle, and in the metabolome of frail individuals (288, 466). Equally interesting, there is a discriminating profile for prefrailty, which is sex-dependent (changes in 2,4-diaminobutyric acid for both genders, dimethyloxazole for men, and threonine, phenylalanine, and fructose for women). Thus, these metabolites form molecular signatures of frail and prefrail phenotypes, suggest the involvement of underlying biological mechanisms, and importantly have potential to tease out mechanisms that trigger frailty onset (354, 454).
Many of the metabolites identified above are consistent with the metabolome of skeletal muscle in frail individuals, correlate with physical performance (e.g., gait speed), and suggest dysregulation or decline in skeletal muscle mass and quality (sarcopenia) (75, 154, 363, 370, 396, 405). Furthermore, several of the metabolites overlap with metabolites that decrease with aging and cognition, demonstrating the connectedness of the physiological systems (288, 316, 454). Not only do these findings align well with the hallmarks and pillars (e.g., overwhelmed compensatory mechanisms: oxidative stress resulting from diminished antioxidant levels) the overlapping metabolic profiles support the idea that frailty is a dynamic condition involving multiple and integrated physiological systems.
The overall goal of maintaining homeostasis involves multiple metabolic processes and pathways that have been shown to change over time and represent an underlying cause of aging that likely contributes to the onset and progression of frailty. Interpreting the responses of multiple pathways suggests metabolic and neuroendocrine changes occur to conserve metabolic energy. The imbalance or dysregulation in overall energy metabolism likely influences physiological reserve within tissues. As noted earlier in this article, physiological reserve and resilience are key players in clinical frailty.
Mitochondrial Function and Frailty
Mitochondrial oxidative phosphorylation is the major source of energy production for cellular functions (423, 424). There is substantial evidence supporting the involvement of impaired mitochondrial function in the development of diseases, including manifestations of aging (65, 123–125, 214). Briefly, mitochondrial health is dependent on many fundamental mitochondrial processes such as biogenesis, fission, fusion, autophagy/mitophagy, proteostasis, and pathways associated with the regulation of quality control, metabolism, and oxidative stress as well as the crosstalk between tissues and organs that influence inflammation, the senescence of distant tissues, and the whole-body metabolic homeostasis. Indeed, it is well-known that dysfunctional mitochondria produce an excessive amount of ROS, which can trigger inflammation (341, 451, 552). There is evidence that mitochondrial dysfunction is associated with chronic inflammation likely leading to a loss in cellular homeostasis in many tissues (358).
The maintenance of mitochondrial health or a functional mitochondrial network is paramount for preserving skeletal muscle homeostasis, whereby a disruption in the pathways controlling mitochondrial quality is a mechanism triggering sarcopenia and has potential to impact physical frailty (158, 319). Prevailing evidence supports the association between a reduction in mitochondrial oxidative capacity and physical performance such as walking speed, strength, and physical activity, symptoms of frailty and between oxidative protein damage and low grip strength (5, 101, 244, 527, 642). In principle, the loss of a functional mitochondrial network has far-reaching results such as alterations in ATP production, proteostasis, calcium handling, oxidative stress, and inflammation, all with the potential to impact frailty. An example of this complex cascade is supported by the decline in ATP production which is accompanied by enhanced ROS production, leading to further mitochondrial DNA (mtDNA) damage and electron transport chain dysfunction that amplifies the energetic deficit (89, 521). Moreover, a transcriptional signature of mitochondrial bioenergetic dysfunction in skeletal muscle is defined with (205) low PGC-1α/estrogen-related receptor (ERR) signaling, as well as downregulation of oxidative phosphorylation (385). In the functional perspective, such transcriptional modulations are translated into fewer mitochondria, reduced mitochondrial respiratory complex activity, and perturbed NAD+ biosynthesis resulting in low NAD+ levels in sarcopenic muscle (385). With alterations in the mitochondrial quality control mechanisms, there is abnormal organelle accumulation reducing the mitochondrial ability to adapt (or compensate) to challenging conditions (stress, increased vulnerability).
A growing body of evidence supports the contribution of mitochondrial dysfunction as an early biological mechanism triggering the onset of frailty (prefrail status) and a biological mechanism in the progression of frailty (Figure 3). Evidence of impaired function (reduced phosphocreatine recovery along with declined mitochondrial respiratory complex protein and activity) and down-regulation of mitochondrial genes in prefrail individuals, highlights the key role of mitochondrial function in frailty development (14). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that mediates antioxidant responses contributing to the regulation of mitochondrial function (257). While investigating the role of Nrf2 in frailty and sarcopenia, Huang et al. (247) demonstrate the contribution of Nrf2 as a regulator of mitochondrial biogenesis and dynamics in promoting muscle mass and maintaining physical function, where Nrf2 deficiency exacerbates frailty in a time-dependent manner. There is evidence of lower mtDNA copy number associated with frail individuals indicating processes such as mitochondrial depletion, energy reserves, and oxidative stress are playing a role in the progression or continuum of frailty (26).
Mimicking mitochondrial dysfunction by boosting oxidative stress in preclinical rodent models of frailty allows important advances for investigating the mechanisms underlying this syndrome. The SOD1 knockout (SOD1KO) mice, which lack the antioxidant enzyme copper- and zinc-containing superoxide dismutase (Cu/ZnSOD), is an animal model proposed to study frailty. Briefly, Cu/ZnSOD is the major antioxidant enzyme responsible for removing superoxide anions (conversion of superoxide anions to hydrogen peroxide) in the cytosol and intermembrane space of the mitochondria and protecting cells from oxidative stress/damage (425). The Sod1KO mice display signs of accelerated aging such as hearing loss, cataracts, skin thinning and impaired wound healing, muscle atrophy, and a 30% reduction in lifespan (145, 258, 298, 403, 427). The Sod1KO mice exhibit characteristics of the Frailty Phenotype: weight loss, weakness, low physical activity, and exhaustion, and the skeletal muscle of Sod1KO mice show a dramatic increase in oxidative damage (135, 403). Frailty in Sod1KO mice is attenuated by dietary restriction. Sixty percent of ad libitum fed dietary restriction reversed the loss of muscle mass and function, improved mitochondrial function, and attenuated the increase in oxidative damage, cell senescence, and circulating levels of IL-6 (262, 648). As such, it is suggested that the Sod1KO mouse model may assist in investigating the biology of frailty and therapeutic strategies, specifically focused on oxidative stress, mitochondrial dysfunction, inflammation, and cell senescence.
A transgenic mouse model, with overexpression of the antioxidant glucose-6-phosphate dehydrogenase (G6PDH), the rate-limiting enzyme responsible for nicotinamide adenine dinucleotide phosphate (NADPH) protection against oxidative damage, was developed to evaluate if improved ROS detoxification improved healthspan (414). This mouse model exhibits increased resilience in response to age-associated decline of muscular and brain function suggesting that a lower accumulation of oxidative damage is beneficial for healthspan (414). Because these transgenic mice show partial protection from age-related functional declines that depend on several metabolic processes (e.g., glucose tolerance, insulin sensitivity, neuromuscular function) they may be a model for future frailty investigations targeted at the role of metabolism. In this respect, previously generated geroprotected animal models are available and could readily be utilized to study metabolic or other physiological aspects that contribute to prevented/delayed frailty.
In summary, most studies concur that mitochondrial dysfunction is a major player in frailty through multiple mechanisms. The beginning or early stages of frailty include unresolved inflammation and increased oxidative stress triggering a myriad of metabolic changes that involve many tissues, especially skeletal muscle. Initially, the compensatory mechanisms within cells, tissues, and organs are activated to maintain homeostasis and structure/function. However, at some point, these compensatory mechanisms become overwhelmed resulting in metabolic imbalance and frailty progression.
Stem Cells and Frailty
SCs are characterized by their multipotency and capacity to self-renew, providing progeny with important SC properties to ensure the SC pool and progeny that differentiate to repair injured tissues. Advancements in the study of SCs over the past two decades provide novel paradigms for the development of therapeutic strategies aimed at addressing multiple diseases. Importantly, there is an abundance of research highlighting the detrimental effects of aging on all types of SCs [e.g., hematopoietic SCs, bone-marrow mesenchymal stem cells (MSCs), umbilical cord SCs, adipose-derived SCs, skeletal SCs, muscle satellite cells] and the subsequent influence of these effects to further accelerate tissue deterioration (392, 463, 464, 594). Prevailing evidence suggests that the negative impact of aging is all encompassing with every biological characteristic of the SC being affected including capacity for self-renewal, proliferative activity, differentiation potential, regenerative and repair capacity, immunomodulatory potential, anti-inflammatory capacity, stimulatory capacity, interaction potential with the microenvironment (paracrine action), and others (79, 297). Whether these biological changes are driven by SC-intrinsic and/or extrinsic alterations, these molecular, functional, and phenotypical changes collectively contribute to a loss of tissue homeostasis, to physiological systems failure, and to a decline in overall health, including frailty.
Similar to the various discussions presented earlier in this article specific changes occur in the genomic and epigenomic landscapes of aged SCs with respect to DNA modifications (e.g., methylation), specific histone posttranslational modifications, chromatin architecture, and epi-polarity (46, 167, 551). There is evidence of transcriptional changes, reductions in the DNA damage response and repair and dysregulation of quiescence (429, 520, 538, 544). Many of these intrinsic SC changes in the genomic and epigenomic landscapes lead to permanent dysfunction (591). SC dysfunction is also driven by dysregulation of metabolic pathways (421). With aging, the basal metabolism of SCs transitions to oxidative metabolism, which increases the production of ROS leading to SC metabolic related-stress and loss of SC mitochondrial homeostasis. Indeed, these consequences contribute to many of the age-associated SC phenotypes such as abnormal SC proliferation, compromised SC self-renewal, disruption of quiescence, and SC apoptosis (52, 92, 421, 566, 588). The specialized microenvironments, SC niches, promote SC maintenance and regulate many of the SC functions. Most studies suggest that there is breakdown of the interactions between SCs, their niches, the molecular feedback loops, and signaling pathways with aging [e.g., extracellular matrix (ECM) components, fibronectin, Notch signaling; TGF1-β, (FGF)-extracellular-signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK), NF-κB or wingless-related integration site (Wnt)] (57, 64, 90, 91, 167, 168, 329, 362, 457). Just as the SC niche is critical for SC function, maintenance of the SC proteome is equally important to prevent further SC cellular damage and dysfunction (381). Loss of autophagy, lower levels of proteasomal degradation, and inactive UPR endoplasmic reticulum (ER) in SCs lead to protein and metabolic stress, which impair self-renewal activity and regenerative potential (237, 400, 604).
Chronic inflammation also creates a detrimental environment for SCs (460). Foremost, SCs adopt an immunoregulatory phenotype in response to inflammatory factors such as IFN-γ and TNF-α through paracrine effects (e.g., metabolic regulation) and exosomes, and these immunomodulatory properties are reduced in aged SCs (306, 318). Skeletal muscle studies provide evidence indicating upon injury a “temporally-regulated, acute and transient immune response” is necessary for regeneration (562). With aging, chronic accumulation of pro-inflammatory mediators disrupts this tightly regulated immune response resulting in altered cytokine composition in the SC niche (62). Accordingly, the exposure of the SCs to this altered niche environment results in many detrimental consequences to the delicate balance of regulatory networks necessary to regulate tissue remodeling such as miscommunication between immune cells and SCs, SC exhaustion, impaired regeneration, and favored adipogenic differentiation (538). Taken together, these studies on age-related changes in SCs culminate in challenges to maintain cellular homeostasis, to preserve healthy tissue function, and to prevent frailty.
Frailty and stem cells
During the last decade, it was suggested that alterations in the production (numbers) and differentiation capacity of MSC were contributing to physical frailty in older adults (198, 199, 201, 202, 223–225). Because the physical performance measures described in the Physical Frailty Phenotype involve tissues (e.g., muscle, bone) from mesenchymal origin they share the same precursor or progenitor cell, the MSC (136, 179). Thus, in principle, it is logical to hypothesize that alterations in MSC function may play a potential role in frailty by aggravating muscle contractility (e.g., strength, endurance) as well as contributing to degeneration of other critical components required for optimal muscle contractility (e.g., neuromuscular junctions) (352). Yet, to date, there is a dearth of published investigations to support this hypothesis. The lack of investigations is likely due to the many challenges in evaluating the contributions of MSC to the development and progression of frailty.
One challenge identified in the frailty literature is a reliable source of MSC even though they can be obtained from almost any tissue within the human body. With the goal to investigate the role of SCs and frailty, circulating osteogenic progenitor (COP) cells were proposed and evaluated as a surrogate marker of the MSC population within the bone marrow. Using this approach, significant associations between COP cells and frailty and disability were reported with a confirmatory study that included a role for lamin A (a factor critical for muscle and bone function) (9). Although these initial studies incorporating this novel methodology are encouraging, there is still a vast amount of missing information surrounding SCs and frailty. A recent pilot study noted that circulating hematopoietic stem cells (cHSC) from frail seniors show the highest total DNA damage, compared to fit seniors and young controls providing initial evidence linking SCs with frailty (380).
Regenerative medicine and frailty
Currently, there are multiple clinical trials using MSCs to test therapeutic interventions in a large number of clinical conditions in almost every organ system (192, 223–225, 476, 541, 568). Many of these clinical conditions are associated with frailty and provide a rationale to consider SCs as a therapeutic to fight frailty. These organ systems include cardiac (dilated cardiomyopathy, heart failure); bone (nonunion bone fracture); eye (glaucoma, macular degeneration, retinitis pigmentosa); kidney (acute kidney injury); lung (COPD, pulmonary fibrosis); immune (rheumatoid arthritis); nervous (multiple sclerosis); and endocrine systems (diabetes type 1) (192, 568, 606). Collectively and important for this comprehensive article, the results from multiple clinical trials show that allogeneic human mesenchymal stem cells (hMSCs) are safe, irrespective of age (200). Equally interesting is the fact that for older adults allogeneic SCs harvested from younger donors are preferable because age-related changes in SCs make them less efficient for transplantation (559).
In 2017, the first human clinical trials were designed to determine the effects of allogeneic MSCs as an intervention to fight frailty. Results of these early-phase trials (www.clinicaltrials.gov: #NCT02065245) identified as CRATUS, allogeneiC human mesenchymal stem cells (allo-hMSCs) in Patients With Aging FRAilTy Via IntravenoUS delivery were encouraging demonstrating significant improvements in physical performance measures closely associated with the clinical manifestations of frailty. Outcomes of a Phase II randomized, double-blinded, placebo-controlled trial of allo-hMSC (www.clinicaltrials.gov: #NCT02065245) were consistent with the phase I safety study indicating that infusion of allo-hMSCs was safe in older individuals with frailty and produced benefits in multiple outcome measures of physical performance as well inflammatory biomarkers (564). Together, these early studies suggest that allo-hMSCs may be an effective biological modifier of frailty and support ongoing investigation of allo-hMSCs. At this time, a review of ClinicalTrials.gov for clinical trials focused on key words “frailty and stem cells” highlights 14 registered trials from around the world indicating the excitement and evidence supporting a role for SCs in treating aspects of frailty in older adults. Importantly, these clinical trials include assessments of frailty with well-established assessment tools such as the Physical Frailty Phenotype (179).
Most recent efforts in preclinical animal studies demonstrate the utility of SCs to fight physical dysfunction boosting the evidence for both the understanding of molecular underpinnings and treatment potential. A series of studies tested whether transplanting adipose-derived mesenchymal stem cells (ADSCs) from young and aged donors caused detrimental physical effects in middle-aged mice (301, 353, 602, 634). Intriguingly, ADSCs from old donors significantly impairs walking speed, grip strength, endurance, and daily activity in the middle-aged mice posttransplantation. Whereas the middle-aged mice transplanted with the same number of ADSCs from the young donors do not show these impairments. Overall, these findings suggest that ADSCs from old donors can induce physical frailty, which is highly associated with morbidity and loss of independence (148). Thus, regenerative approaches entailing transplantation of ADSCs from aged donors might generate previously unrecognized risks.
The transcriptomes of the ADSC isolated from the young and old donors show that several SASP-related genes are up-regulated in the ADSC isolated from old donors. However, it is also worth noting that the study identifies p21high cells (identified as an expression level greater than 97% of cells from young donors) with transcriptomic signatures similar to in vitro-generated senescent cells, which have altered signaling pathways closely associated with muscle dysfunction; hence, these specific cells (p21high cells) may be a culprit contributing to the physical dysfunction (626).
In principle, the future is bright in the use of SCs as a strategy to combat frailty. However, the field faces several hurdles from the understanding of the SCs precise molecular underpinnings to specific clinical protocols that will improve clinical outcomes. For instance, if chronic inflammation within SCs is one of the main contributors to the progression of frailty, repeated SC administration over time will be required to maintain low levels of inflammation. Important considerations in repeated SC administration are cell dose, time intervals between administration, and route of delivery.
Age-related Frailty and Disease
Knowing that multiple physiological systems contribute to the susceptibility to frailty, it would be remiss not to present known associations of chronic disease with frailty. Thus, we present some of the major morbidities that are strongly associated with tissue level dysfunction and states of frailty. Importantly and noted earlier in this comprehensive article the underlying biological mechanisms involved in the onset and progression of frailty related to disease are different from those involved in age-related frailty (16, 554).
Sarcopenia
The definition of sarcopenia is evolving with the increased emphasis on aging research. “Sarcopenia” was first described as the decline in muscle mass caused by aging in 1989 (481, 482). In the following decade, the definition of sarcopenia was changed to include low muscle mass as identified as lean appendicular mass/height2 with specific cut-off points (being <2 SDs below the sex-specific mean of a young reference group) (43). In 2010, the definition of sarcopenia was further modified by the European Working Group on Sarcopenia for Older Persons (EWGSOP) (121). The EWGSOP defined sarcopenia as generalized loss of skeletal muscle mass together with low muscle function (a measure of strength or performance) and also recommended sex-specific cut-off points for sarcopenia diagnosis. Recently, the EWGSOP2 refined the definition of sarcopenia (120). The refined sarcopenia definition includes documentation of both low muscle strength and low muscle mass and includes recommendations of new cut-off points for sarcopenia diagnosis. In this refined definition, physical performance is used to categorize the severity of sarcopenia (120). The evolvement of the definitions for sarcopenia reflects the high-quality research over the past three decades and the complex nature of skeletal muscle health—the combination of muscle tissue (quantity and quality) and neuromuscular function translating into muscle strength and physical performance.
Sarcopenia and frailty are distinct entities. One of the most compelling and impactful observation contributing to our understanding of these two entities emerges from the Toledo Study of Healthy Aging (≥65 years) whereby sarcopenia correlates with frailty; yet, the results clearly establish these two terms cannot be used interchangeably. The major take-home message for the field is sarcopenia is not a useful clinical biomarker of frailty, but an individual’s sarcopenia status (specifical absence of sarcopenia) is useful to exclude the presence of frailty or a frailty diagnosis (129). Nonetheless, both are associated with similar adverse health outcomes and most likely share pathophysiological similarities including inflammation, oxidative stress, and hormonal and energy imbalances (120, 179, 327). Conceptually, in this article focused on frailty, sarcopenia contributes to the decline of physical function (Figure 7) through pathophysiological processes when frailty is identified with the Physical Frailty Phenotype assessment tool. Indeed, physical performance or function is defined as an objectively measured whole-body function related to locomotion, involving muscles and nervous functions (central and peripheral) (120). Within this conceptualization, sarcopenia may precede frailty or predispose an individual to physical frailty due to multisystemic dysfunctions (120). Because a comprehensive review of sarcopenia is beyond the scope of this article, here we briefly describe the evidence for the relationship between sarcopenia and frailty and an overview of the established mechanisms underlying sarcopenia that perturb cellular homeostasis potentially leading to the onset and progression of frailty.
There are several cross-sectional observational studies describing positive correlations between sarcopenia and frailty, and as expected, there are studies demonstrating relationships between sarcopenia and individual symptoms of frailty (99, 129, 152, 176, 181, 376, 542). For instance, analysis with older adults from the Berlin Aging Study II shows that lower appendicular lean mass related to BMI has higher odds (1.4–2.8 times) of difficulties in physical activity (540). Importantly, because of the decades of research aimed at sarcopenia, longitudinal studies are now concluding that sarcopenia is associated with increased risk of incident disability, institutionalization, and mortality (236).
In the last decade, PF&S and the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force (internationally recognized scientists and clinicians) emerged to recognize the strong relationship between PF&S and to accelerate discoveries in treatment and prevention to combat sarcopenia and frailty (88, 477). One initiative of the ICFSR was the identification of promising biomarkers of frailty and sarcopenia resulting in one study identifying an association between GDF-15 and slower gait speed, increased walking time for 400 m (increased exhaustion/poor endurance), and lower physical performance in older adults (Baltimore Longitudinal Study of Aging) (514). The importance of GDF-15 in frailty, a member of the transforming growth factor-β (TGF-β)/bone morphogenetic protein (BMP) superfamily, is first based in its involvement with sarcopenia-related outcomes (muscle wasting and cachexia) and second in the consequences associated with dysregulated metabolism (mitochondrial dysfunction and energy imbalance) (115, 436, 569). Inflammation and oxidative stress activate transcription factors (e.g., p53, hypoxia-inducible factor-1α, nuclear factor-κB) to upregulate GDF15 expression (569) increasing skeletal muscle’s vulnerability to metabolic changes within the microenvironment. Although this report is promising, caution is advised because of the complex pathophysiology of frailty and other skeletal muscle markers, such as protein quality control markers, that are associated with frailty (140). Moreover, it is unlikely that there will be a single biomarker identifying frailty.
Along similar lines altered energy metabolism is present within skeletal muscle of individuals with prefrailty and frailty, and this altered energy metabolism is related to the level of physical performance. For instance, in older adults, the postrecovery rate of phosphocreatine is lower and is correlated with poorer performance in fast and long walking tasks (101). The energetic cost of muscle contraction (measured during maximal intermittent and maximal contractions in the quadriceps) reveals a higher ATP cost potentially contributing to the observed age-related decline in muscle efficiency (332). These energy-related alterations or changes in bioenergetics are further exposed under conditions of stress such that frail adults have faster reductions in skeletal muscle energetics during fatigue tests compared to nonfrail adults (e.g., fatigue, postexercise) (14, 101, 275, 345). Based on the altered energy metabolism present in the pre-frail state, an interpretation of the findings it that skeletal muscle mitochondrial impairment is a hallmark of prefrailty development and the onset of decline in muscle function (14). Indeed, research incorporating the preclinical assessment tools to classify frailty or physical function supports this conclusion. Mice exhibiting frail phenotypes exhibit reductions in skeletal muscle ATP kinetics, high-energy phosphate levels, and energy release from ATP hydrolysis (8). The decreases of physical function and muscle mass are associated with reduced expression levels of genes involved in mitochondrial biogenesis and dynamics, as well as reductions in mitochondrial number and content, mtDNA copy number, and abnormal mitochondria morphology (247). These reported aberrations in skeletal muscle metabolism and energetics are also influenced by the reduction in glucose metabolism and cell membrane phospholipids and an increase in small extracellular vesicles (448). Overall, the rapid decline, delayed recovery, altered kinetics and energy cost, and aberrations in mitochondrial structure suggest that changes in skeletal muscle metabolism (including microenvironment) disrupts the delicate balance between muscle structure and function, increasing vulnerability which may lead to frailty.
Much work in the field of sarcopenia shows evidence of increased muscle fat infiltration/content (a.k.a. myosteatosis), decreased protein synthesis, and enhanced proteolysis as causal factors contributing to aberrations within the microenvironment, loss of muscle quality (structure and composition), and muscle atrophy (fiber size and number) (Figure 7) (13, 121, 409, 640). The triggers for these events arise from many sources including inflammation, oxidative stress, lipid stress, and senescence (82, 409, 413).
Considering fat infiltration and frailty, fat accumulation within skeletal muscles is consistently reported in frail/prefrail individuals and in individuals where performance measures are used as proxy for frailty (12, 234, 382, 441, 570, 571). The consequences of fat accumulation within skeletal muscle are certainly detrimental, as observed in metabolic syndromes (MetSs) and high-fat diets. Indeed, frail individuals show increased fat infiltration (intermuscular adipose tissue) and inflammation (IL-6) and low functioning individuals show an abundance of senescent-like cells and intermuscular adipose tissue compared to nonfrail or highly functional individuals (4, 282). The consequences of these changes within the skeletal muscle microenvironment impact other components such as motor units and the structure of neuromuscular junctions, leading to impaired innervation and altered physical performance (81, 583).
There is evidence of deficits in protein quality control (e.g., increased levels of heat shock proteins, protein modifications, lipofuscin, misfolded proteins, aggregation, impaired mitophagy/autophagy), which contribute to reductions in muscle quantity and quality with aging and MetSs (Figure 7) (1, 11, 206, 430). To date the number of studies investigating both skeletal muscle protein quality control in the presence of frailty is limited; however, the results point toward impairment with the protein quality control network and the involvement of several physiological systems (1, 140, 254, 367). For instance, autophagy and mitophagy gene expression is downregulated in inactive frail older adults suggesting some degree of mitochondrial dysfunction; the presence of autophagy markers denotes the processes of autophagosome formation and autophagosome-lysosome fusion are affected in frail adults; and the low expression of E3 ubiquitin ligases suggests impaired proteolytic systems in frail adults (1, 140, 254). At this time the role of these changes in protein quality control per se in sarcopenia, frailty, and even in health is not fully understood; however, changes in the protein quality control network are now recognized in the pathophysiology of neurodegenerative disease and afford potential directions for further investigations in skeletal muscle (274, 618, 619). Thus, loss in proteostasis likely contributes to frailty through protein quality control disruptions in skeletal muscle.
Equally interesting is the impairment in skeletal muscle’s anabolic response to stimuli (e.g., exercise, diet), defined as anabolic resistance, observed in older adults and in frail individuals (7, 127). It is suggested that the presence of anabolic resistance to stimuli predisposes protein synthesis to decrease, while protein degradation is facilitated (615). Indeed, older men require a higher dose of dietary proteins than younger men to stimulate similar postprandial activation of muscle protein synthesis (398). Likewise, postprandial muscle protein synthesis rates are reduced in older individuals (596). Anabolic resistance is observed in individuals with frailty and in those in the earlier stages of frailty following various exercise interventions programs (7, 561). Several cell-signaling pathways contribute to this impaired response such as inhibition of IGF-1/PI3K/Akt1/mTORC1 signaling pathway in the presence of inflammation, oxidative stress, and others [reviewed in Bonaldo and coworkers (59, 207, 406)].
Together these results suggest there is potential overlap in the pathophysiological mechanisms underlying sarcopenia and frailty. Although mechanistic details are still not understood, it is becoming clear that cellular homeostasis is disrupted in aging skeletal muscle through the interactions of metabolism, inflammation, adaptation to stress, and proteostasis that lead to physical frailty. Even though remarkable progress is noted in interplay between sarcopenia and frailty, formidable challenges lie ahead in the understanding of the critical mechanisms triggering the onset of frailty.
Metabolic syndrome
Here, we discuss the features of MetS because their pathophysiology such as reduced insulin secretion, insulin resistance, poor glucose homeostasis, chronic inflammation, oxidative stress, mitochondrial dysfunction, and dyslipidemia within many tissues, potentially play a role in triggering frailty and its progression (Figure 8) (96, 193). Foremost, MetS is a cluster of risk factors for diabetes and CVD featured by increased visceral obesity, insulin resistance, sustained hyperglycemia, and hypertension (10). To date, the studies investigating associations between MetS and frailty produce varied results. MetS is associated with frailty risk and prefrailty; whereas, there are reports indicating no association between MetS and frailty (37, 221, 289). These mixed findings bring to the forefront the impact of study designs, including definition of MetS and selection of frailty assessment tools and populations, etc. Even so, individuals with MetS are more likely to have frailty (593).
Visceral obesity
Several aspects of body composition, in particular the distribution and amount of body fat, and lean body mass (muscle) play an important role in overall health and likely frailty (Figure 8). In fact, abdominal obesity is identified as a driver of muscle dysfunction, supported by population-based investigations (265). Given the importance of this topic, the concept of sarcopenic obesity emerged whereby the accumulation of intramuscular lipid leads to an enhanced catabolic state (309, 312, 507). The negative metabolic consequences of visceral obesity together with skeletal muscle dysfunctions contribute to the development and progression of frailty in this population (2, 138, 252). In particular, accumulation of adipose tissue or the presence of adipocyte-infiltrating macrophages leads to increased secretion of pro-inflammatory cytokines, and production of pro-inflammatory adipokines promoting lipotoxicity in skeletal muscle, thereby contributing to further pathophysiology of muscle and impaired function (e.g., loss of homeostasis, defects in protein quality control) (39, 256, 326). Indeed, in large population-based studies, abdominal obesity predicts frailty incidence or is associated with frailty (193, 248, 346, 462, 469, 543).
Insulin resistance
Insulin resistance has a myriad of consequences because insulin action is involved in various functions in a multitude of tissues. In general, there is evidence that insulin resistance (in the presence or absence of diabetes) is a risk factor for, is associated with, and predicts frailty, demonstrating the importance of regulating insulin and glucose homeostasis (Figure 8) (149, 284, 286, 287, 437, 440, 529). In skeletal muscle, insulin resistance leads to autophagy, protein degradation, and mitochondrial dysfunction resulting in muscle atrophy and weakness (Figure 7). The loss of muscle mass subsequently impacts glucose transport and further exacerbates insulin resistance (336, 622). Within the cellular signaling pathways, insulin resistance triggers the downregulation of the PI3K/Akt pathway, decreasing protein synthesis as well as FoxO phosphorylation. There is stimulation of atrogin-1 and muscle RING-finger protein-1 (MuRF1), both E3 enzymes, and the ubiquitin-proteasome proteolytic pathway is enhanced (Figure 7). Kalyani and collaborators report that the enhanced expression of E3 enzymes in insulin-resistant individuals is the mechanistic link contributing to skeletal muscle protein degradation (284). Importantly, this reduction in muscle mass further impacts blood glucose homeostasis through lower peripheral glucose uptake causing hyperinsulinemia and insulin resistance, a vicious cycle with detrimental outcomes (126, 132, 517). Further, metabolic changes in lipid metabolism associated with fat accumulation also affect skeletal muscle synergizing within this vicious cycle (444).
Overall, individuals with MetS including visceral obesity and insulin resistance experience considerable alterations in metabolism in key organs including adipose tissue, liver, and skeletal muscle. Because of the tightly coordinated cellular processes within these tissues and the crosstalk between these tissues, the loss in cellular homeostasis facilitates a dangerous cycle. These dysregulated systems create an imbalance between anabolism-catabolism, which will overwhelm the compensatory mechanisms and decrease physiological reserve, potentially leading to the onset and progression of frailty. Moving forward, more research is needed to rigorously differentiate frailty from metabolic disorders. Even though there is noted considerable overlap in pathological mechanisms (e.g., systemic inflammation, oxidative stress) between these, there may be subtle differences in the ability to mitigate frailty in these groups with interventions. It will be important to determine under which conditions metabolic disorders precede frailty and when the presence of frailty induces these diseases.
Cardiovascular disease
CVD and frailty may share several common underlying pathophysiologies (e.g., endocrine and immunologic systems) such as elevated levels of CRP, IL-6 (6, 106, 600). These pathophysiological manifestations underlie the increased systemic arteriolopathy (e.g., arterial stiffness) that may be found in both CVD and sarcopenia (260, 313, 419). Thus, it is not surprising that there are observational studies and systematic reviews (cross-sectional and longitudinal) reporting associations of CVD risk factors with frailty (Figure 8) (63, 93, 189, 197, 213, 412, 547, 620).
Individuals with hypertension are highly heterogeneous, with variability in their physiological capacity, physiological reserve, and vulnerability to stress (404). Yet, it is suggested that long-term hypertension and poor control of blood pressure contribute to the observed systemic arteriolopathy noted above, causing ischemia, tissue damage, and dysfunction. These outcomes could potentially impair physiological reserve, increase vulnerability, and trigger frailty in this clinical population (452). Indeed, frailty is associated with hypertension (38, 83, 314, 411). In particular, a recent meta-analysis based on six cohort studies and one cross-sectional study demonstrates a significant association between frailty status (frail > prefrail > robust) and risk of falls and all-cause hospitalization among patients with hypertension (245). Whereas it is important to point out, there are reports that do not support a relationship between frailty and hypertension (19, 465, 479, 590). Vetrano and coworkers (590) investigated the prevalence of frailty in hypertensive individuals by reviewing 27 articles from longitudinal and cross-sectional studies with mixed results. The reasons underlying the inconsistencies within the reports are many including the status of the interrelationships between the physiological systems within individuals and the varied components with the designs of the studies (e.g., presence, types, and duration of co-morbidities). to pinpoint the role of hypertension and frailty, the dissection of the multiple pathways contributing to the metabolic perturbations may provide insights. Currently, the limited number of preclinical animal models investigating cardiac physiology and frailty provide evidence of associations between frailty and atrial dysfunction (electrophysiology, fibrosis, myocyte morphology) (263, 397, 434).
Collectively, we understand that molecular, cellular, and tissue level pathophysiology contribute to the susceptibility to frailty. The consideration of multiple co-morbid states increases the complexity of potential therapeutic development (Figure 8).
Conclusion
Considering the remarkably complex nature of the biological processes that underlie frailty, it is not surprising that the biological/clinical framework for frailty is stated in broad terms and many of the proposed biological processes lack sound, rigorous scientific evidence. Thus, it is challenging to unify all the relevant aspects. Yet, significant progress is obvious due to the synergy between the fields of Geroscience and clinical frailty, the reverse-translation of the two-well established frailty assessment tools into preclinical animal studies, and the development of advanced “omic” technologies providing a window into molecular and cellular processes and critical transition events in the presence or absence of frailty. Although reviewing the literature clearly demonstrates phenotypic parameters are available in both human and preclinical animal studies and cellular homeostasis is disrupted through a multitude of mechanisms within many tissues, the limited knowledge about the compensatory mechanisms and when they can no longer compensate hampers forward progress to fully understand frailty development. As recent findings from preclinical animal studies increase the palette of possibilities for mechanisms (and potential therapeutics), making almost all of the “pillars or hallmarks of aging” targets as mechanisms, growing interest is expected. Consequently, more and more attention will be given to preclinical models in the field of frailty. It is important to emphasize that pinpointing the molecular and cellular pathways along the frailty continuum is crucial (frailty onset, transitions between robust, prefrail, frail) and will not only answer biological scientific questions but will also impact healthspan and lead to improvements in quality of life. The multitude of “omics” studies is important in identifying affected pathways but remains descriptive of the Frailty Phenotype versus understanding the biology of risk and onset. It will be critical to continue to identify genetic, lifestyle and environmental risk factors for frailty knowing that frailty is not an inevitable consequence of aging. Equally important is the development of therapeutic targets to lower risk, prevent frailty onset, and slow progression of ongoing disease. In turn, accomplishing these aims will assist in removing the stigma of advanced age, create opportunities and allow us to continue productive lives.
Table 3.
List of Abbreviations
| ACC | acetyl-CoA carboxylase |
| ACE | angiotensin-converting enzyme |
| ACTH | adrenocorticotropic hormone |
| ADSCs | adipose-derived mesenchymal stem cells |
| Akt | protein kinase B |
| AMPK | adenosine monophosphate-activated protein kinase |
| ATP | adenosine triphosphate |
| BCAA | branched-chain amino acid |
| BMI | body mass index |
| BMP | bone morphogenetic protein |
| CBS | cystathionine beta-synthase |
| CCR5 | C-C chemokine receptor type 5 |
| CD | cluster of differentiation |
| cHSC | circulating hematopoietic stem cells |
| COP | circulating osteogenic progenitor |
| COPD | chronic obstructive pulmonary disease |
| COX-2 | cycloxygenase-2 |
| CpG | 5′-C-phosp-G-3″ |
| CR | caloric restriction |
| CRH | corticotropin-releasing hormone |
| CRP | C-reactive protein |
| Cu/ZnSOD | copper- and zinc-containing superoxide dismutase |
| CVD | cardiovascular disease |
| CXCL | chemokine C-X-C motif ligand |
| Cxcl1/Kc | chemokine C-X-C motif ligand/keratinocytes-derived chemokine |
| DHEA | dehydroepiandrosterone |
| DHEAS | dehydroepiandrosterone sulfate |
| DKO | double knockout |
| DNA | deoxyribonucleic acid |
| DNAm | DNA methylation |
| ECM | extracellular matrix |
| ER | endoplasmic reticulum |
| ERK | extracellular-signal-regulated kinase |
| ERR | estrogen-related receptor |
| FDA | Food and Drug Administration |
| FI | Frailty Index |
| FoxO | forkhead box protein O |
| G6PDH | glucose-6-phosphate dehydrogenase |
| GDF | growth differentiation factor |
| GH | growth hormone |
| GHRH | growth hormone-releasing hormone |
| GLUT4 | glucose transporter type 4 |
| GSK3β | glycogen synthase kinase 3β |
| H3K27me3 | tri-methylation of lysine 27 on histone H3 protein |
| HMG-CoA | β-hydroxy β-methylglutaryl-coenzyme A |
| HP | hypothalamic-pituitary |
| HPA | hypothalamic-pituitary-adrenal |
| HP1a | heterochromatin protein 1a |
| HHcy | hyperhomocysteinemia |
| ICFSR | International Conference on Frailty and Sarcopenia Research |
| IFN-γ | interferon-gamma |
| IGF | insulin-like growth factor |
| IGF1R | IGF-1 receptor |
| IL | interleukin |
| JAK | Janus kinase |
| LKB1 | liver kinase B1 |
| LMNA | lamin A/C |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MetS | metabolic syndrome |
| miRNA | microRNA |
| MnSOD | manganese superoxide dismutase |
| MSC | mesenchymal stem cell |
| mtDNA | mitochondrial DNA |
| MtTFA | mitochondrial transcription factor A |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MuRF1 | muscle RING-finger protein-1 |
| NAD+ | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa B |
| NIA | National Institute on Aging |
| NK | natural killer |
| NMN | nicotinamide mononucleotide |
| NRF-1 | nuclear respiratory factor 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PFI | Physiological Frailty Index |
| PGC | peroxisome proliferative activated receptor, gamma, coactivator |
| PhenoAGE | phenotypic age |
| PI3K | phosphoinositide-3-kinase |
| PF&S | physical frailty and sarcopenia |
| PPAR | peroxisome proliferator-activated receptor |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| SIRT | NAD-dependent deacetylase sirtuin |
| SOD | superoxide dismutase |
| Sod1KO | SOD knockout |
| SS | somatostatin |
| SC | stem cell |
| T2D | type 2 diabetes mellitus |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor-alpha |
| TNFR1 | tumor necrosis factor receptor 1 |
| UPR | unfolded protein response |
| WHO | World Health Organization |
| Wnt | wingless-related integration site |
Didactic Synopsis.
Major teaching points
Frailty is emerging as a serious global public health challenge.
Two well-established clinical frailty assessment tools, the Frailty Phenotype and Frailty Index, have been reverse-translated successfully in preclinical animal studies.
The conceptual frameworks, Hallmarks/Pillars of Aging, provide a roadmap of the biological areas contributing to the aging phenotype and to the pathophysiology of frailty. Key biological areas include genetics/epigenetics, adaptation to stress, inflammation, metabolism, proteostasis, and stem cells and regeneration.
There is a growing body of evidence indicating that frailty occurs when compensatory mechanisms can no longer maintain homeostasis and is characterized by physiological dysregulation and increased vulnerability to stressors.
There is overlap in the pathophysiological mechanisms underlying sarcopenia and physical frailty.
There is a need for design standards, multiomics approaches coupled with physiological methods, and development of preclinical animal models that closely mimic frailty in humans to test specific mechanisms that contribute to this complex syndrome.
Acknowledgements
This work is supported, in part, by the Travis Roy Endowed Professorship (to L.V. Thompson) and the National Institute on Aging (R56 AG-067724 to L.V. Thompson and H. Brown-Borg) and (K07 AG-072124 to L.V. Thompson).
References
- 1.Aas SN, Hamarsland H, Cumming KT, Rognlien SH, Aase OJ, Nordseth M, Karsrud S, Godager S, Tømmerbakke D, Handegard V, Raastad T. The impact of age and frailty on skeletal muscle autophagy markers and specific strength: A cross-sectional comparison. Exp Gerontol 125: 110687, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 40: 937–943, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, Peters LL. Aging research using mouse models. Curr Protoc Mouse Biol 5: 95–133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J Nutr Health Aging 18: 532–538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelnia F, Urbanek J, Osawa Y, Shardell M, Brennan NA, Fishbein KW, Spencer RG, Simonsick EM, Schrack JA, Ferrucci L. Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J Am Geriatr Soc 67: 1695–1699, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 63: 747–762, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre LE, Villareal DT. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser 83: 83–92, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akki A, Yang H, Gupta A, Chacko VP, Yano T, Leppo MK, Steenbergen C, Walston J, Weiss RG. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr) 36: 21–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Saedi A, Gunawardene P, Bermeo S, Vogrin S, Boersma D, Phu S, Singh L, Suriyaarachchi P, Duque G. Lamin A expression in circulating osteoprogenitors as a potential biomarker for frailty: The Nepean Osteoporosis and Frailty (NOF) Study. Exp Gerontol 102: 69–75, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469–480, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Alway SE, Mohamed JS, Myers MJ. Mitochondria initiate and regulate sarcopenia. Exerc Sport Sci Rev 45: 58–69, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, Magaziner J, Hannan MT, Bouxsein ML, Kiel DP. Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci 71: 811–816, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade TA, Evangelista AF, Campos AH, Poles WA, Borges NM, Camillo CM, Soares FA, Vassallo J, Paes RP, Zerbini MC, Scapulatempo C, Alves AC, Young KH, Colleoni GW. A microRNA signature profile in EBV+ diffuse large B-cell lymphoma of the elderly. Oncotarget 5: 11813–11826, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreux PA, van Diemen MPJ, Heezen MR, Auwerx J, Rinsch C, Groeneveld GJ, Singh A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep 8: 8548, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 3: e2232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angioni D, Macaron T, Takeda C, Sourdet S, Cesari M, Virecoulon Giudici K, Raffin J, Lu WH, Delrieu J, Touchon J, Rolland Y, de Souto Barreto P, Vellas B. Can we distinguish age-related frailty from frailty related to diseases: Data from the MAPT study. J Nutr Health Aging 24: 1144–1151, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 176: 2092–2097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoch MP, Wrobel M, Kuropatwinski KK, Gitlin I, Leonova KI, Toshkov I, Gleiberman AS, Hutson AD, Chernova OB, Gudkov AV. Physiological Frailty Index (PFI): Quantitative in-life estimate of individual biological age in mice. Aging (Albany NY) 9: 615–626, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM. Impact of the treatment of isolated systolic hypertension on behavioral variables. Results from the systolic hypertension in the elderly program. Arch Intern Med 154: 2154–2160, 1994. [PubMed] [Google Scholar]
- 20.Aprahamian I, Xue QL. Shaping the next steps of research on frailty: Challenges and opportunities. BMC Geriatr 21: 432, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aramillo Irizar P, Schäuble S, Esser D, Groth M, Frahm C, Priebe S, Baumgart M, Hartmann N, Marthandan S, Menzel U, Müller J, Schmidt S, Ast V, Caliebe A, König R, Krawczak M, Ristow M, Schuster S, Cellerino A, Diekmann S, Englert C, Hemmerich P, Sühnel J, Guthke R, Witte OW, Platzer M, Ruppin E, Kaleta C. Transcriptomic alterations during ageing reflect the shift from cancer to degenerative diseases in the elderly. Nat Commun 9: 327, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artaza-Artabe I, Sáez-López P, Sánchez-Hernández N, Fernández-Gutierrez N, Malafarina V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 93: 89–99, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Arts MH, Collard RM, Comijs HC, Naudé PJ, Risselada R, Naarding P, Oude Voshaar RC. Relationship between physical frailty and low-grade inflammation in late-life depression. J Am Geriatr Soc 63: 1652–1657, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Arum O, Rasche ZA, Rickman DJ, Bartke A. Prevention of neuromusculoskeletal frailty in slow-aging ames dwarf mice: Longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS One 8: e72255, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arum O, Rickman DJ, Kopchick JJ, Bartke A. The slow-aging growth hormone receptor/binding protein gene-disrupted (GHR-KO) mouse is protected from aging-resultant neuromusculoskeletal frailty. Age (Dordr) 36: 117–127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashar FN, Moes A, Moore AZ, Grove ML, Chaves PHM, Coresh J, Newman AB, Matteini AM, Bandeen-Roche K, Boerwinkle E, Walston JD, Arking DE. Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med (Berl) 93: 177–186, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austad SN, Allison DB. Perspectives in aging: Nutritional and energetic interventions. Exp Gerontol 86: 1–3, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, Xue QL, Walston JD, Kasper JD. Frailty in older adults: A nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 70: 1427–1434, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16: 1165–1173, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Banzato T, Franzo G, Di Maggio R, Nicoletto E, Burti S, Cesari M, Canevelli M. A Frailty Index based on clinical data to quantify mortality risk in dogs. Sci Rep 9: 16749, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes BR, Long YC, Steiler TL, Leng Y, Galuska D, Wojtaszewski JF, Andersson L, Zierath JR. Changes in exercise-induced gene expression in 5′-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. Diabetes 54: 3484–3489, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ, Bonini S, Seeger W, Belvisi MG, Ward B, Holmes A. Barriers to new drug development in respiratory disease. Eur Respir J 45: 1197–1207, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 380: 37–43, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Barreto G, Huang TT, Giffard RG. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. J Neurosurg Anesthesiol 22: 214–219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartke A, Brown-Borg H. Mutations affecting mammalian aging: GH and GHR vs IGF-1 and insulin. Front Genet 12: 667355, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol (1985) 108: 1069, 2010–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med 167: 635–641, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Bastos-Barbosa RG, Ferriolli E, Coelho EB, Moriguti JC, Nobre F, Lima NK, da Costa Lima NK. Association of frailty syndrome in the elderly with higher blood pressure and other cardiovascular risk factors. Am J Hypertens 25: 1156–1161, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14: 513–537, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann C, Kwak D, Thompson L. Phenotypic frailty assessment in mice: Development, discoveries, and experimental considerations. Physiology 35: 405–414, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumann CW, Kwak D, Thompson L. Sex-specific components of frailty in C57BL/6 mice. Aging (Albany NY) 11: 5206–5214, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumann CW, Kwak D, Thompson LV. Assessing onset, prevalence and survival in mice using a frailty phenotype. Aging (Albany NY) 10: 4042–4053, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA. Immune-endocrine biomarkers as predictors of frailty and mortality: A 10-year longitudinal study in community-dwelling older people. Age (Dordr) 35: 963–971, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, Lloyd-Sherlock P, Epping-Jordan JE, Peeters GMEE, Mahanani WR, Thiyagarajan JA, Chatterji S. The World report on ageing and health: A policy framework for healthy ageing. Lancet 387: 2145–2154, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, Rossi DJ. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 12: 413–425, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Bellantuono I, de Cabo R, Ehninger D, Di Germanio C, Lawrie A, Miller J, Mitchell SJ, Navas-Enamorado I, Potter PK, Tchkonia T, Trejo JL, Lamming DW. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc 15: 540–574, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE. Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J Gerontol A Biol Sci Med Sci 73: 4–10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Avraham D, Govindaraju DR, Budagov T, Fradin D, Durda P, Liu B, Ott S, Gutman D, Sharvit L, Kaplan R, Bougnères P, Reiner A, Shuldiner AR, Cohen P, Barzilai N, Atzmon G. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci Adv 3: e1602025, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: Linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol 16: 593–610, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett C, de Cabo R, Pasquina PF, Smith WK. White paper on a national investment in Geroscience. 2021. https://www.geroscience.health.
- 52.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development 141: 4206–4218, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bird A Perceptions of epigenetics. Nature 447: 396–398, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol 23: 570–582, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Blagosklonny MV. An anti-aging drug today: From senescence-promoting genes to anti-aging pill. Drug Discov Today 12: 218–224, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 21: 854–862, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonafe L, Dermitzakis ET, Unger S, Greenberg CR, Campos-Xavier BA, Zankl A, Ucla C, Antonarakis SE, Superti-Furga A, Reymond A. Evolutionary comparison provides evidence for pathogenicity of RMRP mutations. PLoS Genet 1: e47, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6: 25–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonkowski MS, Sinclair DA. Slowing ageing by design: The rise of NAD. Nat Rev Mol Cell Biol 17: 679–690, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Booth LN, Brunet A. The aging epigenome. Mol Cell 62: 728–744, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouché M, Muñoz-Cánoves P, Rossi F, Coletti D. Inflammation in muscle repair, aging, and myopathies. Biomed Res Int 2014: 821950, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouillon K, Batty GD, Hamer M, Sabia S, Shipley MJ, Britton A, Singh-Manoux A, Kivimäki M. Cardiovascular disease risk scores in identifying future frailty: The Whitehall II Prospective Cohort Study. Heart 99: 737–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest 123: 951–957, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol 6: 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breitling LP, Saum KU, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics 8: 21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brivio P, Paladini MS, Racagni G, Riva MA, Calabrese F, Molteni R. From healthy aging to frailty: In search of the underlying mechanisms. Curr Med Chem 26: 3685–3701, 2019. [DOI] [PubMed] [Google Scholar]
- 69.Brown-Borg HM. Hormonal control of aging in rodents: The somatotropic axis. Mol Cell Endocrinol 299: 64–71, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: Potential mechanisms of altered stress resistance. Exp Gerontol 44: 10–19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bryda EC. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo Med 110: 207–211, 2013. [PMC free article] [PubMed] [Google Scholar]
- 72.Buckinx F, Rolland Y, Reginster JY, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: Perspectives for a public health challenge. Arch Public Health 73: 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calderón-Larrañaga A, Vetrano DL, Onder G, Gimeno-Feliu LA, Coscollar-Santaliestra C, Carfí A, Pisciotta MS, Angleman S, Melis RJF, Santoni G, Mangialasche F, Rizzuto D, Welmer AK, Bernabei R, Prados-Torres A, Marengoni A, Fratiglioni L. Assessing and measuring chronic multimorbidity in the older population: A proposal for its operationalization. J Gerontol A Biol Sci Med Sci 72: 1417–1423, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calvani R, Picca A, Marini F, Biancolillo A, Cesari M, Pesce V, Lezza AMS, Bossola M, Leeuwenburgh C, Bernabei R, Landi F, Marzetti E. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study: Rationale, design and methods. Eur J Intern Med 56: 19–25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab 23: 1127–1139, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev 40: 31–44, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Campisi J Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 9: 11–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, Ortolano S, Pani G, Athanasopoulou S, Gonos ES, Schosserer M, Grillari J, Peterson P, Tuna BG, Dogan S, Meyer A, van Os R, Trendelenburg AU. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev 47: 214–277, 2018. [DOI] [PubMed] [Google Scholar]
- 81.Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8: 1509–1521, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carter CS, Justice JN, Thompson L. Lipotoxicity, aging, and muscle contractility: Does fiber type matter? Geroscience 41: 297–308, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castrejón-Pérez RC, Gutiérrez-Robledo LM, Cesari M, Pérez-Zepeda MU. Diabetes mellitus, hypertension and frailty: A population-based, cross-sectional study of Mexican older adults. Geriatr Gerontol Int 17: 925–930, 2017. [DOI] [PubMed] [Google Scholar]
- 84.Cencioni C, Heid J, Krepelova A, Rasa SMM, Kuenne C, Guenther S, Baumgart M, Cellerino A, Neri F, Spallotta F, Gaetano C. Aging triggers H3K27 trimethylation hoarding in the chromatin of Nothobranchius furzeri skeletal muscle. Cells 8: 1169, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, Viscomi C, Zeviani M. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19: 1042–1049, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 43: 10–12, 2014. [DOI] [PubMed] [Google Scholar]
- 88.Cesari M, Landi F, Calvani R, Cherubini A, Di Bari M, Kortebein P, Del Signore S, Le Lain R, Vellas B, Pahor M, Roubenoff R, Bernabei R, Marzetti E, SPRINTT Consortium. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res 29: 81–88, 2017. [DOI] [PubMed] [Google Scholar]
- 89.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol 5: e201, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chandel NS, Jasper H, Ho TT, Passegué E. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol 18: 823–832, 2016. [DOI] [PubMed] [Google Scholar]
- 93.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: The Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci 60: 729–735, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: Evidence from a meta-analysis. J Nurs Scholarsh 49: 529–536, 2017. [DOI] [PubMed] [Google Scholar]
- 95.Chew J, Tay L, Lim JP, Leung BP, Yeo A, Yew S, Ding YY, Lim WS. Serum myostatin and IGF-1 as gender-specific biomarkers of frailty and low muscle mass in community-dwelling older adults. J Nutr Health Aging 23: 979–986, 2019. [DOI] [PubMed] [Google Scholar]
- 96.Chhetri JK, Zheng Z, Xu X, Ma C, Chan P. The prevalence and incidence of frailty in pre-diabetic and diabetic community-dwelling older population: Results from Beijing Longitudinal Study of Aging II (BLSA-II). BMC Geriatr 17: 47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Childers DK, Allison DB. The ‘obesity paradox’: A parsimonious explanation for relations among obesity, mortality rate and aging? Int J Obes (Lond) 34: 1231–1238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, Yu KS, Cho JY. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRα/POMC pathway. Sci Rep 5: 8145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choe YR, Jeong JR, Kim YP. Grip strength mediates the relationship between muscle mass and frailty. J Cachexia Sarcopenia Muscle 11: 441–451, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi J, Ahn A, Kim S, Won CW. Global prevalence of physical frailty by Fried’s criteria in community-dwelling elderly with national population-based surveys. J Am Med Dir Assoc 16: 548–550, 2015. [DOI] [PubMed] [Google Scholar]
- 101.Choi S, Reiter DA, Shardell M, Simonsick EM, Studenski S, Spencer RG, Fishbein KW, Ferrucci L. 31p magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 71: 1638–1645, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332: 1351–1362, 1995. [DOI] [PubMed] [Google Scholar]
- 103.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381, 2009. [DOI] [PubMed] [Google Scholar]
- 104.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: The Health and Retirement Study. J Am Geriatr Soc 57: 830–839, 2009. [DOI] [PubMed] [Google Scholar]
- 105.Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol 6: 743–752, 2018. [DOI] [PubMed] [Google Scholar]
- 106.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 381: 752–762, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coelho-Júnior HJ, Calvani R, Picca A, Gonçalves IO, Landi F, Bernabei R, Cesari M, Uchida MC, Marzetti E. Protein-related dietary parameters and frailty status in older community-dwellers across different frailty instruments. Nutrients 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coelho-Junior HJ, Marzetti E, Picca A, Cesari M, Uchida MC, Calvani R. Protein intake and frailty: A matter of quantity, quality, and timing. Nutrients 12: 2915, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 60: 1487–1492, 2012. [DOI] [PubMed] [Google Scholar]
- 110.Collerton J, Gautrey HE, van Otterdijk SD, Davies K, Martin-Ruiz C, von Zglinicki T, Kirkwood TB, Jagger C, Mathers JC, Strathdee G. Acquisition of aberrant DNA methylation is associated with frailty in the very old: Findings from the Newcastle 85+ Study. Biogerontology 15: 317–328, 2014. [DOI] [PubMed] [Google Scholar]
- 111.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev 133: 456–466, 2012. [DOI] [PubMed] [Google Scholar]
- 112.Collino S, Martin FP, Karagounis LG, Horcajada MN, Moco S, Franceschi C, Kussmann M, Offord E. Reprint of: Musculoskeletal system in the old age and the demand for healthy ageing biomarkers. Mech Ageing Dev 136–137: 94–100, 2014. [DOI] [PubMed] [Google Scholar]
- 113.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5: 3557, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conte M, Martucci M, Mosconi G, Chiariello A, Cappuccilli M, Totti V, Santoro A, Franceschi C, Salvioli S. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol 11: 915, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3: 403–416, 2006. [DOI] [PubMed] [Google Scholar]
- 117.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Correia-Melo C, Birch J, Fielder E, Rahmatika D, Taylor J, Chapman J, Lagnado A, Carroll BM, Miwa S, Richardson G, Jurk D, Oakley F, Mann J, Mann DA, Korolchuk VI, Passos JF. Rapamycin improves healthspan but not inflammaging in nfκb1. Aging Cell 18: e12882, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Costamagna D, Costelli P, Sampaolesi M, Penna F. Role of inflammation in muscle homeostasis and myogenesis. Mediators Inflamm 2015: 805172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cruz-Jentoft A, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer A, Schneider S, Sieber C, Topinkova E, Vandewoude M, Visser M, Zamboni M. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48: 16–31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Da Mata FA, Pereira PP, Andrade KR, Figueiredo AC, Silva MT, Pereira MG. Prevalence of frailty in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS One 11: e0160019, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: From molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 16: 1492–1526, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc Res 93: 79–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal state hyperinsulinemia in healthy normoglycemic adults heralds dysglycemia after more than two decades of follow up. Diabetes Metab Res Rev 28: 618–624, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Dardevet D, Rémond D, Peyron MA, Papet I, Savary-Auzeloux I, Mosoni L. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. ScientificWorldJournal 2012: 269531, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Darvin K, Randolph A, Ovalles S, Halade D, Breeding L, Richardson A, Espinoza SE. Plasma protein biomarkers of the geriatric syndrome of frailty. J Gerontol A Biol Sci Med Sci 69: 182–186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davies B, García F, Ara I, Artalejo FR, Rodriguez-Mañas L, Walter S. Relationship between sarcopenia and frailty in the Toledo Study of Healthy Aging: A population based cross-sectional study. J Am Med Dir Assoc 19: 282–286, 2018. [DOI] [PubMed] [Google Scholar]
- 130.Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: Old and new players. Nat Rev Mol Cell Biol 7: 762–773, 2006. [DOI] [PubMed] [Google Scholar]
- 131.Davis MM, Tato CM, Furman D. Systems immunology: Just getting started. Nat Immunol 18: 725–732, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 80: 219–227, 2006. [DOI] [PubMed] [Google Scholar]
- 133.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Souto, Barreto P, Guyonnet S, Ader I, Andrieu S, Casteilla L, Davezac N, Dray C, Fazilleau N, Gourdy P, Liblau R, Parini A, Payoux P, Pénicaud L, Rampon C, Rolland Y, Valet P, Vergnolle N, Vellas B. The INSPIRE Research Initiative: A program for GeroScience and Healthy Aging Research going from animal models to humans and the healthcare system. J Frailty Aging 10: 86–93, 2021. [DOI] [PubMed] [Google Scholar]
- 135.Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: The Cu/Zn superoxide dismutase knockout mouse. Geroscience 39: 187–198, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Demontiero O, Gunawardene P, Duque G. Postoperative prevention of falls in older adults with fragility fractures. Clin Geriatr Med 30: 333–347, 2014. [DOI] [PubMed] [Google Scholar]
- 137.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: A review. Eur J Intern Med 31: 3–10, 2016. [DOI] [PubMed] [Google Scholar]
- 138.Després JP, Lemieux I, Alméras N. Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int J Obes (Lond) 30 Suppl 1: S44–S52, 2006. [DOI] [PubMed] [Google Scholar]
- 139.Doi T, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Makino K, Suzuki T, Shimada H. Association between insulin-like growth factor and frailty among older adults. J Nutr Health Aging 22: 68–72, 2018. [DOI] [PubMed] [Google Scholar]
- 140.Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: A cross-sectional comparison. J Gerontol A Biol Sci Med Sci 69: 1040–1048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab 295: E1333–E1340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duarte N, Teixeira L, Ribeiro O, Paúl C. Frailty phenotype criteria in centenarians: Findings from the Oporto Centenarian Study. Eur Geriatr Med 5: 371–376, 2014. [Google Scholar]
- 143.Dunlap KR, Steiner JL, Rossetti ML, Kimball SR, Gordon BS. A clinically relevant decrease in contractile force differentially regulates control of glucocorticoid receptor translocation in mouse skeletal muscle. J Appl Physiol (1985) 130: 1052–1063, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev 158: 46–52, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24: 367–380, 2005. [DOI] [PubMed] [Google Scholar]
- 146.Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N, Wilson M, Lucas SJE, Akerman I, Seabright A, Lai YC, Tennant DA, Nightingale P, Wallis GA, Manolopoulos KN, Brenner C, Philp A, Lavery GG. Nicotinamide riboside augments the aged human skeletal muscle NAD. Cell Rep 28: 1717–1728.e1716, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N, Brefort T, Leidinger P, Backes C, Meese E, Schreiber S, Rosenstiel P, Franke A, Nebel A. Genome-wide miRNA signatures of human longevity. Aging Cell 11: 607–616, 2012. [DOI] [PubMed] [Google Scholar]
- 148.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR, Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 57: 492–498, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 60: 652–660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Espinoza SE, Musi N, Wang CP, Michalek J, Orsak B, Romo T, Powers B, Conde A, Moris M, Bair-Kelps D, Li Y, Ganapathy V, Jergensen TE, Kelly LC, Jiwani R. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with prediabetes. J Gerontol A Biol Sci Med Sci 75: 102–109, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Espinoza SE, Quiben M, Hazuda HP. Distinguishing comorbidity, disability, and frailty. Curr Geriatr Rep 7: 201–209, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, Lattanzio F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 11: 527–536, 2010. [DOI] [PubMed] [Google Scholar]
- 153.Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging 32: 1868–1880, 2011. [DOI] [PubMed] [Google Scholar]
- 154.Fazelzadeh P, Hangelbroek RW, Tieland M, de Groot LC, Verdijk LB, van Loon LJ, Smilde AK, Alves RD, Vervoort J, Müller M, van Duynhoven JP, Boekschoten MV. The muscle metabolome differs between healthy and frail older adults. J Proteome Res 15: 499–509, 2016. [DOI] [PubMed] [Google Scholar]
- 155.Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, Raat H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS One 12: e0178383, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Feridooni HA, Sun MH, Rockwood K, Howlett SE. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci 70: 686–693, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferrari E, Magri F. Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev 7: 225–233, 2008. [DOI] [PubMed] [Google Scholar]
- 158.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15: 505–522, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferrucci L, Fabbri E, Walston JD. Frailty. In: Halter JB, Ouslander JG, Studenski S, High KP, Asthana S, Supiano MA, Ritchie, editors. Hazzard’s Geriatric Medicine and Gerontology (7th ed). New York, NY: McGraw-Hill Education, 2017. [Google Scholar]
- 160.Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, Salimi S, Sierra F, de Cabo R. Measuring biological aging in humans: A quest. Aging Cell 19: e13080, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ferrucci L, Zampino M. A mitochondrial root to accelerated ageing and frailty. Nat Rev Endocrinol 16: 133–134, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fielder E, Weigand M, Agneessens J, Griffin B, Parker C, Miwa S, von Zglinicki T. Sublethal whole-body irradiation causes progressive premature frailty in mice. Mech Ageing Dev 180: 63–69, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, Saligan LN. Association of mitochondrial dysfunction and fatigue: A review of the literature. BBA Clin 1: 12–23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fischer KE, Hoffman JM, Sloane LB, Gelfond JA, Soto VY, Richardson AG, Austad SN. A cross-sectional study of male and female C57BL/6Nia mice suggests lifespan and healthspan are not necessarily correlated. Aging (Albany NY) 8: 2370–2391, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Flanagan EW, Most J, Mey JT, Redman LM. Calorie restriction and aging in humans. Annu Rev Nutr 40: 105–133, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Flegal KM, Kalantar-Zadeh K. Overweight, mortality and survival. Obesity (Silver Spring) 21: 1744–1745, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Florian MC, Klose M, Sacma M, Jablanovic J, Knudson L, Nattamai KJ, Marka G, Vollmer A, Soller K, Sakk V, Cabezas-Wallscheid N, Zheng Y, Mulaw MA, Glauche I, Geiger H. Aging alters the epigenetic asymmetry of HSC division. PLoS Biol 16: e2003389, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA, Scharffetter-Kochanek K, Kestler HA, Zheng Y, Geiger H. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503: 392–396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fontana L, Addante F, Copetti M, Paroni G, Fontana A, Sancarlo D, Pellegrini F, Ferrucci L, Pilotto A. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 12: 459–466, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, Lamming DW. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 16: 520–530, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 102: 10604–10609, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69 Suppl 1: S4–S9, 2014. [DOI] [PubMed] [Google Scholar]
- 174.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14: 576–590, 2018. [DOI] [PubMed] [Google Scholar]
- 175.Frederick DW, Loro E, Liu L, Davila A, Chellappa K, Silverman IM, Quinn WJ, Gosai SJ, Tichy ED, Davis JG, Mourkioti F, Gregory BD, Dellinger RW, Redpath P, Migaud ME, Nakamaru-Ogiso E, Rabinowitz JD, Khurana TS, Baur JA. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab 24: 269–282, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: Cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol 16: 554–562, 2006. [DOI] [PubMed] [Google Scholar]
- 177.Fried L, Cohen A, Xue Q-L, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging 1: 36–46, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59: 255–263, 2004. [DOI] [PubMed] [Google Scholar]
- 179.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001. [DOI] [PubMed] [Google Scholar]
- 180.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol 72: 67–73, 2009. [DOI] [PubMed] [Google Scholar]
- 181.Frisoli A, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: Results from the Women’s Health and Aging Study (WHAS) II. Bone 48: 952–957, 2011. [DOI] [PubMed] [Google Scholar]
- 182.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006. [DOI] [PubMed] [Google Scholar]
- 183.Fujita T, Yamashita D, Uehara N, Inokuchi G, Hasegawa S, Otsuki N, Nibu K. A high-fat diet delays age-related hearing loss progression in C57BL/6J mice. PLoS One 10: e0117547, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, Miyazaki T, Ogura C, Okazaki Y, Jinno Y. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: An HPLC-based study. Ann Hum Genet 68: 196–204, 2004. [DOI] [PubMed] [Google Scholar]
- 185.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19: 1649–1654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med 25: 1822–1832, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neurosci Biobehav Rev 68: 928–945, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: The English Longitudinal Study of Ageing. Age (Dordr) 35: 2493–2501, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gale CR, Cooper C, Sayer AA. Framingham cardiovascular disease risk scores and incident frailty: The English Longitudinal Study of Ageing. Age (Dordr) 36: 9692, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: Findings from the English Longitudinal Study of Ageing. Age Ageing 44: 162–165, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gale CR, Marioni RE, Harris SE, Starr JM, Deary IJ. DNA methylation and the epigenetic clock in relation to physical frailty in older people: The Lothian Birth Cohort 1936. Clin Epigenetics 10: 101, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Galipeau J, Sensébé L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 22: 824–833, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.García-Esquinas E, Graciani A, Guallar-Castillón P, López-García E, Rodríguez-Mañas L, Rodríguez-Artalejo F. Diabetes and risk of frailty and its potential mechanisms: A prospective cohort study of older adults. J Am Med Dir Assoc 16: 748–754, 2015. [DOI] [PubMed] [Google Scholar]
- 194.Gardner MP, Lightman S, Sayer AA, Cooper C, Cooper R, Deeg D, Ebrahim S, Gallacher J, Kivimaki M, Kumari M, Kuh D, Martin RM, Peeters G, Ben-Shlomo Y. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: An individual participant meta-analysis. Psychoneuroendocrinology 38: 40–49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gardner MP, Lightman SL, Gallacher J, Hardy R, Kuh D, Ebrahim S, Bayer A, Ben-Shlomo Y, team Hs. Diurnal cortisol patterns are associated with physical performance in the Caerphilly Prospective Study. Int J Epidemiol 40: 1693–1702, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gharacholou SM, Roger VL, Lennon RJ, Rihal CS, Sloan JA, Spertus JA, Singh M. Comparison of frail patients versus nonfrail patients ≥65 years of age undergoing percutaneous coronary intervention. Am J Cardiol 109: 1569–1575, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, Heldman AW, Miki R, Goldstein BJ, Mushtaq M, Levis-Dusseau S, Byrnes JJ, Lowery M, Natsumeda M, Delgado C, Saltzman R, Vidro-Casiano M, Pujol MV, Da Fonseca M, Oliva AA, Green G, Premer C, Medina A, Valasaki K, Florea V, Anderson E, El-Khorazaty J, Mendizabal A, Goldschmidt-Clermont PJ, Hare JM. Allogeneic human mesenchymal stem cell infusions for aging frailty. J Gerontol A Biol Sci Med Sci 72: 1505–1512, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Golpanian S, DiFede DL, Pujol MV, Lowery MH, Levis-Dusseau S, Goldstein BJ, Schulman IH, Longsomboon B, Wolf A, Khan A, Heldman AW, Goldschmidt-Clermont PJ, Hare JM. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 7: 11899–11912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol 65: 125–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moyé L, Simari RD, Wolf A, Hare JM. Concise review: Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med 5: 186–191, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: Mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev 96: 1127–1168, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, Garcia-Garcia FJ, Olaso-Gonzalez G, Salvador-Pascual A, Tarazona-Santabalbina FJ, Viña J. A new frailty score for experimental animals based on the clinical phenotype: Inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci 72: 885–891, 2017. [DOI] [PubMed] [Google Scholar]
- 205.Gonzalez-Freire M, Scalzo P, D’Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, Zane A, Chen B, Becker KG, Lehrmann E, Zukley L, Chia CW, Tanaka T, Coen PM, Bernier M, de Cabo R, Ferrucci L. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 17: e12725, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 207.Gorissen SHM, Witard OC. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc Nutr Soc 77: 20–31, 2018. [DOI] [PubMed] [Google Scholar]
- 208.Graber TG, Fandrey KR, Thompson LV. Novel individualized power training protocol preserves physical function in adult and older mice. Geroscience 41: 165–183, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci 68: 1326–1336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci 70: 1045–1058, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Graber TG, Kim JH, Grange RW, McLoon LK, Thompson LV. C57BL/6 life span study: Age-related declines in muscle power production and contractile velocity. Age (Dordr) 37: 9773, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Graber TG, Maroto R, Fry CS, Brightwell CR, Rasmussen BB. Measuring exercise capacity and physical function in adult and older mice. J Gerontol A Biol Sci Med Sci 76: 819–824, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Graciani A, García-Esquinas E, López-García E, Banegas JR, Rodríguez-Artalejo F. Ideal cardiovascular health and risk of frailty in older adults. Circ Cardiovasc Qual Outcomes 9: 239–245, 2016. [DOI] [PubMed] [Google Scholar]
- 214.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333: 1109–1112, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gross AL, Xue QL, Bandeen-Roche K, Fried LP, Varadhan R, McAdams-DeMarco MA, Walston J, Carlson MC. Declines and impairment in executive function predict onset of physical frailty. J Gerontol A Biol Sci Med Sci 71: 1624–1630, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hadley EC, Kuchel GA, Newman AB. Report: NIA Workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci 72: 980–990, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, Zhou L, Isales CM, Mi QS. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun 400: 379–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. [DOI] [PubMed] [Google Scholar]
- 219.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493,737 UK Biobank participants. Lancet Public Health 3: e323–e332, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49: 359–367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hao Q, Song X, Yang M, Dong B, Rockwood K. Understanding risk in the oldest old: Frailty and the metabolic syndrome in a Chinese community sample aged 90+ years. J Nutr Health Aging 20: 82–88, 2016. [DOI] [PubMed] [Google Scholar]
- 222.Hardman SE, Hall DE, Cabrera AJ, Hancock CR, Thomson DM. The effects of age and muscle contraction on AMPK activity and heterotrimer composition. Exp Gerontol 55: 120–128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol 69: 526–537, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 308: 2369–2379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277–2286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13: 273–282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hazuda HP, Pan Q, Florez H, Luchsinger JA, Crandall JP, Venditti EM, Golden SH, Kriska AM, Bray GA. Association of intensive lifestyle and metformin interventions with frailty in the Diabetes Prevention Program Outcomes Study. J Gerontol A Biol Sci Med Sci 76: 929–936, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.He YH, Lu X, Yang LQ, Xu LY, Kong QP. Association of the insulin-like growth factor binding protein 3 (IGFBP-3) polymorphism with longevity in Chinese nonagenarians and centenarians. Aging (Albany NY) 6: 944–956, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 295: 1539–1548, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Henderson YO, Bithi N, Link C, Yang J, Schugar R, Llarena N, Brown JM, Hine C. Late-life intermittent fasting decreases aging-related frailty and increases renal hydrogen sulfide production in a sexually dimorphic manner. Geroscience 43: 1527–1554, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Herzig S, Shaw RJ. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19: 121–135, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hidayat K, Chen GC, Wang Y, Zhang Z, Dai X, Szeto IMY, Qin LQ. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J Nutr Health Aging 22: 237–245, 2018. [DOI] [PubMed] [Google Scholar]
- 234.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys Ther 88: 1336–1344, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hindhede M The effect of food restriction during war on mortality in Copenhagen. JAMA 74: 381–382, 1920. [Google Scholar]
- 236.Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc 16: 607–613, 2015. [DOI] [PubMed] [Google Scholar]
- 237.Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543: 205–210, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Holanda CM, Guerra RO, Nóbrega PV, Costa HF, Piuvezam MR, Maciel Á. Salivary cortisol and frailty syndrome in elderly residents of long-stay institutions: A cross-sectional study. Arch Gerontol Geriatr 54: e146–e151, 2012. [DOI] [PubMed] [Google Scholar]
- 239.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: Implications for clinical practice and public health. Lancet 394: 1365–1375, 2019. [DOI] [PubMed] [Google Scholar]
- 240.Hooten N, Fitzpatrick M, Wood WH, De S, Ejiogu N, Zhang Y, Mattison JA, Becker KG, Zonderman AB, Evans MK. Age-related changes in microRNA levels in serum. Aging (Albany NY) 5: 725–740, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Horvath S DNA methylation age of human tissues and cell types. Genome Biol 14: R115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Horvath S Erratum to: DNA methylation age of human tissues and cell types. Genome Biol 16: 96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet 19: 371–384, 2018. [DOI] [PubMed] [Google Scholar]
- 244.Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Guralnik JM, Semba RD. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol (1985) 103: 17–20, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hu K, Zhou Q, Jiang Y, Shang Z, Mei F, Gao Q, Chen F, Zhao L, Jiang M, Ma B. Association between frailty and mortality, falls, and hospitalization among patients with hypertension: A systematic review and meta-analysis. BioMed Research International 2021: 2690296, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hua J, Hoummady S, Muller C, Pouchelon JL, Blondot M, Gilbert C, Desquilbet L. Assessment of frailty in aged dogs. Am J Vet Res 77: 1357–1365, 2016. [DOI] [PubMed] [Google Scholar]
- 247.Huang DD, Fan SD, Chen XY, Yan XL, Zhang XZ, Ma BW, Yu DY, Xiao WY, Zhuang CL, Yu Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp Gerontol 119: 61–73, 2019. [DOI] [PubMed] [Google Scholar]
- 248.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 65: 377–381, 2010. [DOI] [PubMed] [Google Scholar]
- 249.Hubbard RE, O’Mahony MS, Woodhouse KW. Characterising frailty in the clinical setting – a comparison of different approaches. Age Ageing 38: 115–119, 2009. [DOI] [PubMed] [Google Scholar]
- 250.Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating health span in preclinical models of aging and disease: Guidelines, challenges, and opportunities for Geroscience. J Gerontol A Biol Sci Med Sci 71: 1395–1406, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huizer-Pajkos A, Kane AE, Howlett SE, Mach J, Mitchell SJ, de Cabo R, Le Couteur DG, Hilmer SN. Adverse geriatric outcomes secondary to polypharmacy in a mouse model: The influence of aging. J Gerontol A Biol Sci Med Sci 71: 571–577, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes Rev 11: 11–18, 2010. [DOI] [PubMed] [Google Scholar]
- 253.Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: Frailty and its implications for health systems. Health Serv Res 50: 305–320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Inglés M, Mas-Bargues C, Gimeno-Mallench L, Cruz-Guerrero R, García-García FJ, Gambini J, Borrás C, Rodríguez-Mañas L, Viña J. Relation between genetic factors and frailty in older adults. J Am Med Dir Assoc 20: 1451–1457, 2019. [DOI] [PubMed] [Google Scholar]
- 255.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73: 1081–1088, 1991. [DOI] [PubMed] [Google Scholar]
- 256.Ishii S, Tanaka T, Akishita M, Ouchi Y, Tuji T, Iijima K. Metabolic syndrome, sarcopenia and role of sex and age: Cross-sectional analysis of Kashiwa cohort study. PLoS One 9: e112718, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Itoh K, Ye P, Matsumiya T, Tanji K, Ozaki T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr 56: 91–97, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Iuchi Y, Roy D, Okada F, Kibe N, Tsunoda S, Suzuki S, Takahashi M, Yokoyama H, Yoshitake J, Kondo S, Fujii J. Spontaneous skin damage and delayed wound healing in SOD1-deficient mice. Mol Cell Biochem 341: 181–194, 2010. [DOI] [PubMed] [Google Scholar]
- 259.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet 33 Supp 1: 245–254, 2003. [DOI] [PubMed] [Google Scholar]
- 260.Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis 237: 381–390, 2014. [DOI] [PubMed] [Google Scholar]
- 261.Jakobsdottir S, van Nieuwpoort IC, van Bunderen CC, de Ruiter MB, Twisk JW, Deijen JB, Veltman DJ, Drent ML. Acute and short-term effects of caloric restriction on metabolic profile and brain activation in obese, postmenopausal women. Int J Obes (Lond) 40: 1671–1678, 2016. [DOI] [PubMed] [Google Scholar]
- 262.Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, Chaudhuri A, Qi W, Li Y, Huang JY, Verdin E, Richardson A, Van Remmen H. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell 11: 770–782, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jansen HJ, Moghtadaei M, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA. Atrial structure, function and arrhyth-mogenesis in aged and frail mice. Sci Rep 7: 44336, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jergović M, Thompson HL, Bradshaw CM, Sonar SA, Ashgar A, Mohty N, Joseph B, Fain MJ, Cleveland K, Schnellman RG, Nikolich-Žugich J. IL-6 can singlehandedly drive many features of frailty in mice. Geroscience 43: 539–549, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ji C, Xia Y, Tong S, Wu Q, Zhao Y. Association of handgrip strength with the prevalence of metabolic syndrome in US adults: The National Health and Nutrition Examination Survey. Aging (Albany NY) 12: 7818–7829, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jin X, Ren Y, Shao L, Guo Z, Wang C, He Y, Zhou L, Cong M, Ma H, Wang W, Zhou C, Feng Y, Ba Y, Gao J, Lu M, Zhang M, Gu X, Song C, Xu H, Shi H. Prevalence of frailty and prediction of mortality in Chinese cancer patients using a frailty index-based clinical algorithm—A multicentre study. Cancer Med 10: 6207–6217, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Johar H, Emeny RT, Bidlingmaier M, Reincke M, Thorand B, Peters A, Heier M, Ladwig KH. Blunted diurnal cortisol pattern is associated with frailty: A cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab 99: E464–E468, 2014. [DOI] [PubMed] [Google Scholar]
- 268.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17: 451–459, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes 65: 74–84, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 493: 338–345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Johnson TE. Caenorhabditis elegans 2007: The premier model for the study of aging. Exp Gerontol 43: 1–4, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Johnson TE. 25 years after age-1: Genes, interventions and the revolution in aging research. Exp Gerontol 48: 640–643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Johnson TE. Cell biology. Rapid aging rescue? Science 340: 1299–1300, 2013. [DOI] [PubMed] [Google Scholar]
- 274.Johnston HE, Samant RS. Alternative systems for misfolded protein clearance: Life beyond the proteasome. FEBS J 288: 4464–4448, 2020. [DOI] [PubMed] [Google Scholar]
- 275.Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11: 801–809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, Eash JK, Shavlakadze T, Glass DJ. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol 39: e00141–19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Junior G, Perez D, Tonet-Furioso A, Gomes L, Vilaça K, Alves V, Moraes C, Nóbrega O. Circulating interleukin-6 (but not other immune mediators) associates with criteria for Fried’s Frailty among very old adults. J Aging Res 2020: 6831791, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Junius-Walker U, Onder G, Soleymani D, Wiese B, Albaina O, Bernabei R, Marzetti E. The essence of frailty: A systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med 56: 3–10, 2018. [DOI] [PubMed] [Google Scholar]
- 279.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2: 4172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 36: 583–592, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, Barzilai N, Kuchel GA. A framework for selection of blood-based biomarkers for Geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. Geroscience 40: 419–436, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci 73: 939–945, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40: 554–563, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2: 819–829, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kalyani RR, Tian J, Xue QL, Walston J, Cappola AR, Fried LP, Brancati FL, Blaum CS. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc 60: 1701–1707, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging 16: 679–686, 2012. [DOI] [PubMed] [Google Scholar]
- 287.Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci 67: 1300–1306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kameda M, Teruya T, Yanagida M, Kondoh H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci U S A 117: 9483–9489, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kane AE, Gregson E, Theou O, Rockwood K, Howlett SE. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience 39: 221–229, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kane AE, Hilmer SN, Boyer D, Gavin K, Nines D, Howlett SE, de Cabo R, Mitchell SJ. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci 71: 333–339, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kane AE, Hilmer SN, Huizer-Pajkos A, Mach J, Nines D, Boyer D, Gavin K, Mitchell SJ, de Cabo R. Factors that impact on interrater reliability of the mouse clinical frailty index. J Gerontol A Biol Sci Med Sci 70: 694–695, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kane AE, Huizer-Pajkos A, Mach J, Mitchell SJ, de Cabo R, Le Couteur DG, Howlett SE, Hilmer SN. A comparison of two mouse frailty assessment tools. J Gerontol A Biol Sci Med Sci 72: 904–909, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kane AE, Keller KM, Heinze-Milne S, Grandy SA, Howlett SE. A murine frailty index based on clinical and laboratory measurements: Links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J Gerontol A Biol Sci Med Sci 74: 275–282, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kane AE, Mitchell SJ, Mach J, Huizer-Pajkos A, McKenzie C, Jones B, Cogger V, Le Couteur DG, de Cabo R, Hilmer SN. Acetaminophen hepatotoxicity in mice: Effect of age, frailty and exposure type. Exp Gerontol 73: 95–106, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kane AE, Shin S, Wong AA, Fertan E, Faustova NS, Howlett SE, Brown RE. Sex differences in healthspan predict lifespan in the 3xTg-AD mouse model of Alzheimer’s Disease. Front Aging Neurosci 10: 172, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Karve TM, Cheema AK. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids 2011: 207691, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Keating A Mesenchymal stromal cells: New directions. Cell Stem Cell 10: 709–716, 2012. [DOI] [PubMed] [Google Scholar]
- 298.Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res 209: 76–85, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Keller K, Kane A, Heinze-Milne S, Grandy SA, Howlett SE. Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro-and anti-inflammatory cytokines in aging male and female C57BL/6 mice. J Gerontol A Biol Sci Med Sci 74: 1149–1157, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: Linking aging to chronic disease. Cell 159: 709–713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Khong SML, Lee M, Kosaric N, Khong DM, Dong Y, Hopfner U, Aitzetmüller MM, Duscher D, Schäfer R, Gurtner GC. Single-cell transcriptomics of human mesenchymal stem cells reveal age-related cellular subpopulation depletion and impaired regenerative function. Stem Cells 37: 240–246, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 16: 263–275, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kim JY, Park YK, Lee KP, Lee SM, Kang TW, Kim HJ, Dho SH, Kim SY, Kwon KS. Genome-wide profiling of the microRNA-mRNA regulatory network in skeletal muscle with aging. Aging (Albany NY) 6: 524–544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 39: 83–92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci 71: 1407–1414, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kizilay Mancini O, Shum-Tim D, Stochaj U, Correa JA, Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res Ther 6: 140, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ko F, Abadir P, Marx R, Westbrook R, Cooke C, Yang H, Walston J. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp Gerontol 73: 23–27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ko F, Yu Q, Xue QL, Yao W, Brayton C, Yang H, Fedarko N, Walston J. Inflammation and mortality in a frail mouse model. Age (Dordr) 34: 705–715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kob R, Bollheimer LC, Bertsch T, Fellner C, Djukic M, Sieber CC, Fischer BE. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 16: 15–29, 2015. [DOI] [PubMed] [Google Scholar]
- 310.Kobayashi S, Asakura K, Suga H, Sasaki S. High protein intake is associated with low prevalence of frailty among old Japanese women: A multicenter cross-sectional study. Nutr J 12: 164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kohanski RA, Deeks SG, Gravekamp C, Halter JB, High K, Hurria A, Fuldner R, Green P, Huebner R, Macchiarini F, Sierra F. Reverse Geroscience: How does exposure to early diseases accelerate the age-related decline in health? Ann N Y Acad Sci 1386: 30–44, 2016. [DOI] [PubMed] [Google Scholar]
- 312.Kohara K Sarcopenic obesity in aging population: Current status and future directions for research. Endocrine 45: 15–25, 2014. [DOI] [PubMed] [Google Scholar]
- 313.Kohara K, Okada Y, Ochi M, Ohara M, Nagai T, Tabara Y, Igase M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program Study. J Cachexia Sarcopenia Muscle 8: 557–566, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Koizumi Y, Hamazaki Y, Okuro M, Iritani O, Yano H, Higashikawa T, Iwai K, Morimoto S. Association between hypertension status and the screening test for frailty in elderly community-dwelling Japanese. Hypertens Res 36: 639–644, 2013. [DOI] [PubMed] [Google Scholar]
- 315.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 47: 193–200, 2018. [DOI] [PubMed] [Google Scholar]
- 316.Kondoh H, Kameda M, Yanagida M. Whole blood metabolomics in aging research. Int J Mol Sci 22: 175, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kraig E, Linehan LA, Liang H, Romo TQ, Liu Q, Wu Y, Benavides AD, Curiel TJ, Javors MA, Musi N, Chiodo L, Koek W, Gelfond JAL, Kellogg DL. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol 105: 53–69, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24: 386–398, 2006. [DOI] [PubMed] [Google Scholar]
- 319.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. [DOI] [PubMed] [Google Scholar]
- 320.Kuo T, Harris CA, Wang JC. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380: 79–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kwak D, Baumann CW, Thompson LV. Identifying characteristics of frailty in female mice using a phenotype assessment tool. J Gerontol A Biol Sci Med Sci 75: 640–646, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kwak D, Thompson LV. Frailty: Past, present, and future? Sports Med Health Sci 3: 1–10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17: 1049–1061, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lai HY, Chang HT, Lee YL, Hwang SJ. Association between inflammatory markers and frailty in institutionalized older men. Maturitas 79: 329–333, 2014. [DOI] [PubMed] [Google Scholar]
- 325.Laksmi PW, Setiati S, Tamin TZ, Soewondo P, Rochmah W, Nafrialdi N, Prihartono J. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: A double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med Indones 49: 118–127, 2017. [PubMed] [Google Scholar]
- 326.Lana A, Valdés-Bécares A, Buño A, Rodríguez-Artalejo F, Lopez-Garcia E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis 8: 240–249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, Onder G, Marzetti E. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med 31: 367–374, 2015. [DOI] [PubMed] [Google Scholar]
- 328.Landino K, Tanaka T, Fantoni G, Candia J, Bandinelli S, Ferrucci L. Characterization of the plasma proteomic profile of frailty phenotype. Geroscience 43: 1029–1037, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 32: 795–803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE, Beiko RG. Microbial shifts in the aging mouse gut. Microbiome 2: 50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK, Richardson RS. Skeletal muscle work efficiency with age: The role of non-contractile processes. Clin Sci (Lond) 128: 213–223, 2015. [DOI] [PubMed] [Google Scholar]
- 333.Le Couteur DG, Solon-Biet SM, Cogger VC, Ribeiro R, de Cabo R, Raubenheimer D, Cooney GJ, Simpson SJ. Branched chain amino acids, aging and age-related health. Ageing Res Rev 64: 101198, 2020. [DOI] [PubMed] [Google Scholar]
- 334.Le NP, Varadhan R, Fried LP, Cappola AR. Cortisol and dehydroepiandrosterone response to adrenocorticotropic hormone and frailty in older women. J Gerontol A Biol Sci Med Sci 76: 901–905, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.LeBrasseur NK. Physical resilience: Opportunities and challenges in translation. J Gerontol A Biol Sci Med Sci 72: 978–979, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, Everson-Rose SA, Barrett-Connor E, Orwoll ES, Osteoporotic Fractures in Men Study Research Group. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc 59: 1217–1224, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J Am Geriatr Soc 50: 1268–1271, 2002. [DOI] [PubMed] [Google Scholar]
- 338.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 16: 153–157, 2004. [DOI] [PubMed] [Google Scholar]
- 339.Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: Results from the Women’s Health and Aging Studies I. Exp Gerontol 44: 511–516, 2009. [DOI] [PubMed] [Google Scholar]
- 340.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc 55: 864–871, 2007. [DOI] [PubMed] [Google Scholar]
- 341.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Leroy L, Bayliss E, Domino M, Miller BF, Rust G, Gerteis J, Miller T. The agency for healthcare research and quality multiple chronic conditions research network: Overview of research contributions and future priorities. Med Care 52 Suppl 3: S15–S22, 2014. [DOI] [PubMed] [Google Scholar]
- 343.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10: 573–591, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19: 407–417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lewsey SC, Weiss K, Schär M, Zhang Y, Bottomley PA, Samuel TJ, Xue QL, Steinberg A, Walston JD, Gerstenblith G, Weiss RG. Exercise intolerance and rapid skeletal muscle energetic decline in human age-associated frailty. JCI Insight 5: e141246, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res 30: 1319–1325, 2018. [DOI] [PubMed] [Google Scholar]
- 347.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 32: 1620–1625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lindon JC, Nicholson JK. The emergent role of metabolic phenotyping in dynamic patient stratification. Expert Opin Drug Metab Toxicol 10: 915–919, 2014. [DOI] [PubMed] [Google Scholar]
- 349.Liotta G, Ussai S, Illario M, O’Caoimh R, Cano A, Holland C, Roller-Wirnsberger R, Capanna A, Grecuccio C, Ferraro M, Paradiso F, Ambrosone C, Morucci L, Scarcella P, De Luca V, Palombi L. Frailty as the future core business of public health: Report of the activities of the A3 Action Group of the European Innovation Partnership on active and healthy ageing (EIP on AHA). Int J Environ Res Public Health 15: 2843, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Liu CK, Lyass A, Larson MG, Massaro JM, Wang N, D’Agostino RB, Benjamin EJ, Murabito JM. Biomarkers of oxidative stress are associated with frailty: The Framingham Offspring Study. Age (Dordr) 38: 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci 69: 1485–1491, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 6: e26464, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Liu Y, Conboy MJ, Mehdipour M, Tran TP, Blotnick A, Rajan P, Santos TC, Conboy IM. Application of bio-orthogonal proteome labeling to cell transplantation and heterochronic parabiosis. Nat Commun 8: 643, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Livshits G, Malkin I, Bowyer RCE, Verdi S, Bell JT, Menni C, Williams FMK, Steves CJ. Multi-OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain 159: 2565–2572, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Exp Gerontol 86: 4–13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Loh K, Tam S, Murray-Segal L, Huynh K, Meikle PJ, Scott JW, van Denderen B, Chen Z, Steel R, LeBlond ND, Burkovsky LA, O’Dwyer C, Nunes JRC, Steinberg GR, Fullerton MD, Galic S, Kemp BE. Inhibition of adenosine monophosphate-activated protein kinase-3-hydroxy-3-methylglutaryl coenzyme a reductase signaling leads to hypercholesterolemia and promotes hepatic steatosis and insulin resistance. Hepatol Commun 3: 84–98, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: Are we ready? Aging Cell 14: 497–510, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13: 106–118, 2013. [DOI] [PubMed] [Google Scholar]
- 359.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 11: 303–327, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lu J, Temp U, Müller-Hartmann A, Esser J, Grönke S, Partridge L. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nature Aging 1: 60–72, 2021. [DOI] [PubMed] [Google Scholar]
- 362.Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, Karaz S, Jacot G, Schmidt M, Li L, Metairon S, Raymond F, Lee U, Sizzano F, Wilson DH, Dumont NA, Palini A, Fässler R, Steiner P, Descombes P, Rudnicki MA, Fan CM, von Maltzahn J, Feige JN, Bentzinger CF. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med 22: 897–905, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Lustgarten MS, Price LL, Chale A, Phillips EM, Fielding RA. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A Biol Sci Med Sci 69: 717–724, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ma L, Nidadavolu LS, Yang H, Langdon J, Westbrook R, Tsui BMW, Lee TS, Hinson J, Ling S, Marx-Rattner R, Wu Y, Nguyen T, Tan J, Khadeer M, Moaddel R, Le A, Walston JD, Abadir PM. Targeted deletion of interleukin-6 in a mouse model of chronic inflammation demonstrates opposing roles in aging: Benefit and harm. J Gerontol A Biol Sci Med Sci 76: 211–215, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.MacDougall-Shackleton SA, Bonier F, Romero LM, Moore IT. Glucocorticoids and “stress” are not synonymous. Integr Org Biol 1: obz017, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Mach J, Gemikonakli G, Logan C, Vander Wyk B, Allore H, Ekambareshwar S, Kane AE, Howlett SE, de Cabo R, Le Couteur DG, Hilmer SN. Chronic polypharmacy with increasing drug burden index exacerbates frailty and impairs physical function, with effects attenuated by deprescribing, in aged mice. J Gerontol A Biol Sci Med Sci 76: 1010–1018, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mahmoudi R, Jaisson S, Badr S, Jaidi Y, Bertholon LA, Novella JL, Gillery P. Post-translational modification-derived products are associated with frailty status in elderly subjects. Clin Chem Lab Med 57: 1153–1161, 2019. [DOI] [PubMed] [Google Scholar]
- 368.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med 6: 268ra179, 2014. [DOI] [PubMed] [Google Scholar]
- 369.Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, Glass DJ, Klickstein LB. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med 10: eaaq1564, 2018. [DOI] [PubMed] [Google Scholar]
- 370.Marcos-Pérez D, Sánchez-Flores M, Maseda A, Lorenzo-López L, Millán-Calenti JC, Gostner JM, Fuchs D, Pásaro E, Laffon B, Valdiglesias V. Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front Immunol 9: 1056, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Marcucci F, Romeo E, Caserta CA, Rumio C, Lefoulon F. Context-dependent pharmacological effects of metformin on the immune system. Trends Pharmacol Sci 41: 162–171, 2020. [DOI] [PubMed] [Google Scholar]
- 372.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev 10: 430–439, 2011. [DOI] [PubMed] [Google Scholar]
- 373.Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, Corley J, Taylor A, Murphy L, Starr JM, Horvath S, Visscher PM, Wray NR, Deary IJ. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol 44: 1388–1396, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Martinez de Toda I, Garrido A, Vida C, Gomez-Cabrera MC, Viña J, De la Fuente M. Frailty quantified by the “Valencia Score” as a potential predictor of lifespan in mice. J Gerontol A Biol Sci Med Sci 73: 1323–1329, 2018. [DOI] [PubMed] [Google Scholar]
- 375.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun 4: 2192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, Broccatelli M, Savera G, D’Elia M, Pahor M, Bernabei R, Landi F, SPRINTT Consortium. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 29: 35–42, 2017. [DOI] [PubMed] [Google Scholar]
- 377.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7: e42357, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H, Kasai T, Nagamatsu H, Ozawa T, Izawa K, Yamamoto S, Aizawa N, Yonezawa R, Oka K, Momomura SI, Kagiyama N. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur J Heart Fail 22: 2112–2119, 2020. [DOI] [PubMed] [Google Scholar]
- 379.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Mazzola P, Bombelli S, Grasselli C, Bolognesi M, Antolini L, Cattoretti G, Annoni G, Perego R. Correlation between frailty and DNA damage in hematopoietic stem cells: A pilot study. Innov Aging 3: S87, 2019. [Google Scholar]
- 381.Mejia-Ramirez E, Geiger H, Florian MC. Loss of epigenetic polarity is a hallmark of hematopoietic stem cell aging. Hum Mol Genet 29: R248–R254, 2020. [DOI] [PubMed] [Google Scholar]
- 382.Melville DM, Mohler J, Fain M, Muchna AE, Krupinski E, Sharma P, Taljanovic MS. Multi-parametric MR imaging of quadriceps musculature in the setting of clinical frailty syndrome. Skeletal Radiol 45: 583–589, 2016. [DOI] [PubMed] [Google Scholar]
- 383.Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, Mattison J, Cookson MR, Gorospe M, de Cabo R, Abdelmohsen K. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 5: 692–703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: A randomized controlled trial in non-obese humans. Aging (Albany NY) 8: 1416–1431, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, Forrester T, Barton SJ, Leow MK, Antoun E, Charpagne A, Seng Chong Y, Descombes P, Feng L, Francis-Emmanuel P, Garratt ES, Giner MP, Green CO, Karaz S, Kothandaraman N, Marquis J, Metairon S, Moco S, Nelson G, Ngo S, Pleasants T, Raymond F, Sayer AA, Ming Sim C, Slater-Jefferies J, Syddall HE, Fang Tan P, Titcombe P, Vaz C, Westbury LD, Wong G, Yonghui W, Cooper C, Sheppard A, Godfrey KM, Lillycrop KA, Karnani N, Feige JN. Mitochondrial oxidative capacity and NAD. Nat Commun 10: 5808, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Miller MG, Thangthaeng N, Shukitt-Hale B. A clinically relevant frailty index for aging rats. J Gerontol A Biol Sci Med Sci 72: 892–896, 2017. [DOI] [PubMed] [Google Scholar]
- 387.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13: 468–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab 24: 795–806, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 13: 769–771, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Milman S, Huffman DM, Barzilai N. The somatotropic axis in human aging: Framework for the current state of knowledge and future research. Cell Metab 23: 980–989, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Mistriotis P, Bajpai VK, Wang X, Rong N, Shahini A, Asmani M, Liang MS, Wang J, Lei P, Liu S, Zhao R, Andreadis ST. NANOG reverses the myogenic differentiation potential of senescent stem cells by restoring actin filamentous organization and SRF-dependent gene expression. Stem Cells 35: 207–221, 2017. [DOI] [PubMed] [Google Scholar]
- 393.Mitchell SJ, Mitchell GJ, Mitchell JR. Modulation of frailty syndrome by diet: A review of evidence from mouse studies. Mech Ageing Dev 180: 82–88, 2019. [DOI] [PubMed] [Google Scholar]
- 394.Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB. Age-related frailty and its association with biological markers of ageing. BMC Med 13: 161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1: 323–336, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Moaddel R, Fabbri E, Khadeer MA, Carlson OD, Gonzalez-Freire M, Zhang P, Semba RD, Ferrucci L. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 71: 1266–1272, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Moghtadaei M, Jansen HJ, Mackasey M, Rafferty SA, Bogachev O, Sapp JL, Howlett SE, Rose RA. The impacts of age and frailty on heart rate and sinoatrial node function. J Physiol 594: 7105–7126, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. [DOI] [PubMed] [Google Scholar]
- 399.Morley JE. Frailty: Diagnosis and management. J Nutr Health Aging 15: 667–670, 2011. [DOI] [PubMed] [Google Scholar]
- 400.Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, Stranks AJ, Glanville J, Knight S, Jacobsen SE, Kranc KR, Simon AK. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 208: 455–467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev 39: 36–45, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mousa A, Savva GM, Mitnitski A, Rockwood K, Jagger C, Brayne C, Matthews FE. Is frailty a stable predictor of mortality across time? Evidence from the cognitive function and ageing studies. Age Ageing 47: 721–727, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med 40: 1993–2004, 2006. [DOI] [PubMed] [Google Scholar]
- 404.Muller M, Smulders YM, de Leeuw PW, Stehouwer CD. Treatment of hypertension in the oldest old: A critical role for frailty? Hypertension 63: 433–441, 2014. [DOI] [PubMed] [Google Scholar]
- 405.Murphy RA, Moore S, Playdon M, Kritchevsky S, Newman AB, Satterfield S, Ayonayon H, Clish C, Gerszten R, Harris TB. Metabolites associated with risk of developing mobility disability in the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 74: 73–80, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Murton AJ. Muscle protein turnover in the elderly and its potential contribution to the development of sarcopenia. Proc Nutr Soc 74: 387–396, 2015. [DOI] [PubMed] [Google Scholar]
- 407.Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med 2: 1–16, 2009. [PMC free article] [PubMed] [Google Scholar]
- 408.Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: Role of specificity protein (Sp) transcription factors. J Biol Chem 289: 27692–27701, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Narici MV, Maffulli N. Sarcopenia: Characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010. [DOI] [PubMed] [Google Scholar]
- 410.Newell Stamper BL, Cypser JR, Kechris K, Kitzenberg DA, Tedesco PM, Johnson TE. Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway. Aging Cell 17: e12704, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 56: M158–M166, 2001. [DOI] [PubMed] [Google Scholar]
- 412.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295: 2018–2026, 2006. [DOI] [PubMed] [Google Scholar]
- 413.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. [DOI] [PubMed] [Google Scholar]
- 414.Nóbrega-Pereira S, Fernandez-Marcos PJ, Brioche T, Gomez-Cabrera MC, Salvador-Pascual A, Flores JM, Viña J, Serrano M. G6PD protects from oxidative damage and improves healthspan in mice. Nat Commun 7: 10894, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Noordam R, Jansen SW, Akintola AA, Oei NY, Maier AB, Pijl H, Slagboom PE, Westendorp RG, van der Grond J, de Craen AJ, van Heemst D. Familial longevity is marked by lower diurnal salivary cortisol levels: The Leiden Longevity Study. PLoS One 7: e31166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.O’Caoimh R, Galluzzo L, Rodríguez-Laso Á, Van der Heyden J, Ranhoff AH, Carcaillon-Bentata L, Beltzer N, Kennelly S, Liew A. Transitions and trajectories in frailty states over time: A systematic review of the European Joint Action ADVANTAGE. Ann Ist Super Sanita 54: 246–252, 2018. [DOI] [PubMed] [Google Scholar]
- 417.O’Caoimh R, Galluzzo L, Rodríguez-Laso Á, Van der Heyden J, Ranhoff AH, Lamprini-Koula M, Ciutan M, López-Samaniego L, Carcaillon-Bentata L, Kennelly S, Liew A. Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: A systematic review and meta-analysis. Ann Ist Super Sanita 54: 226–238, 2018. [DOI] [PubMed] [Google Scholar]
- 418.O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, Liew A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 50: 96–104, 2021. [DOI] [PubMed] [Google Scholar]
- 419.Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 212: 327–332, 2010. [DOI] [PubMed] [Google Scholar]
- 420.Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, Gasevic D, Ademi Z, Korhonen MJ, LoGiudice D, Bell JS, Liew D. Global incidence of frailty and prefrailty among community-dwelling older adults: A systematic review and meta-analysis. JAMA Netw Open 2: e198398, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Oh J, Lee YD, Wagers AJ. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat Med 20: 870–880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.O’Halloran AM, Finucane C, Savva GM, Robertson IH, Kenny RA. Sustained attention and frailty in the older adult population. J Gerontol B Psychol Sci Soc Sci 69: 147–156, 2014. [DOI] [PubMed] [Google Scholar]
- 423.Ohlendieck K Proteomics of skeletal muscle differentiation, neuromuscular disorders and fiber aging. Expert Rev Proteomics 7: 283–296, 2010. [DOI] [PubMed] [Google Scholar]
- 424.Ohlendieck K Proteomics of skeletal muscle glycolysis. Biochim Biophys Acta 1804: 2089–2101, 2010. [DOI] [PubMed] [Google Scholar]
- 425.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001. [DOI] [PubMed] [Google Scholar]
- 426.Olivieri F, Spazzafumo L, Santini G, Lazzarini R, Albertini M, Rippo M, Galeazzi R, Abbatecola A, Marcheselli F, Daniela Monti D, Rita Ostan R, Cevenini E, Antonicelli R, Franceschi C, Procopio A. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech Ageing Dev 133: 675–685, 2012. [DOI] [PubMed] [Google Scholar]
- 427.Olofsson EM, Marklund SL, Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: An effect of nitric oxide? Free Radic Biol Med 42: 1098–1105, 2007. [DOI] [PubMed] [Google Scholar]
- 428.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 123: 269–274, 1993. [DOI] [PubMed] [Google Scholar]
- 429.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat Rev Genet 9: 115–128, 2008. [DOI] [PubMed] [Google Scholar]
- 430.Palikaras K, Lionaki E, Tavernarakis N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ 22: 1399–1401, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Panchangam C, White DA, Goudar S, Birnbaum B, Malloy-Walton L, Gross-Toalson J, Reid KJ, Shirali G, Parthiban A. Translation of the frailty paradigm from older adults to children with cardiac disease. Pediatr Cardiol 41: 1031–1041, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Pardon MC. Stress and ageing interactions: A paradox in the context of shared etiological and physiopathological processes. Brain Res Rev 54: 251–273, 2007. [DOI] [PubMed] [Google Scholar]
- 433.Park Y, Choi JE, Hwang HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 108: 1026–1033, 2018. [DOI] [PubMed] [Google Scholar]
- 434.Parks RJ, Fares E, Macdonald JK, Ernst MC, Sinal CJ, Rockwood K, Howlett SE. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci 67: 217–227, 2012. [DOI] [PubMed] [Google Scholar]
- 435.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature 561: 45–56, 2018. [DOI] [PubMed] [Google Scholar]
- 436.Patel MS, Lee J, Baz M, Wells CE, Bloch S, Lewis A, Donaldson AV, Garfield BE, Hopkinson NS, Natanek A, Man WD, Wells DJ, Baker EH, Polkey MI, Kemp PR. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle 7: 436–448, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Peng PS, Kao TW, Chang PK, Chen WL, Peng PJ, Wu LW. Association between HOMA-IR and frailty among U.S. middle-aged and elderly population. Sci Rep 9: 4238, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Pérez-Martínez L, Romero L, Muñoz-Galván S, Verdugo-Sivianes EM, Rubio-Mediavilla S, Oteo JA, Carnero A, Blanco JR. Implications of maraviroc and/or rapamycin in a mouse model of fragility. Aging (Albany NY) 12: 8565–8582, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Pérez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, Di Monte DA. Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis 5: e1209, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Pérez-Tasigchana RF, León-Muñoz LM, Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M, Rodríguez-Artalejo F, Guallar-Castillón P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 46: 807–812, 2017. [DOI] [PubMed] [Google Scholar]
- 441.Perkisas S, De Cock AM, Verhoeven V, Vandewoude M. Intramuscular adipose tissue and the functional components of sarcopenia in hospitalized geriatric patients. Geriatrics (Basel) 2: 11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Perrin S Preclinical research: Make mouse studies work. Nature 507: 423–425, 2014. [DOI] [PubMed] [Google Scholar]
- 443.Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, Annoni G, Bellelli G. Frailty and delirium in older adults: A systematic review and meta-analysis of the literature. J Am Geriatr Soc 66: 2022–2030, 2018. [DOI] [PubMed] [Google Scholar]
- 444.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 300: 1140–1142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Petr MA, Alfaras I, Krawcyzk M, Bair WN, Mitchell SJ, Morrell CH, Studenski SA, Price NL, Fishbein KW, Spencer RG, Scheibye-Knudsen M, Lakatta EG, Ferrucci L, Aon MA, Bernier M, de Cabo R. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. Elife 10: e62952, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Interact 7: 208–218, 2007. [PubMed] [Google Scholar]
- 447.Phillips AC, Ginty AT, Hughes BM. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol 90: 1–7, 2013. [DOI] [PubMed] [Google Scholar]
- 448.Picca A, Beli R, Calvani R, Coelho-Júnior HJ, Landi F, Bernabei R, Bucci C, Guerra F, Marzetti E. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells 9: 973, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, Leng SX, Kirk GD. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 70: 1542–1547, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Pilotto A, Custodero C, Maggi S, Polidori MC, Veronese N, Ferrucci L. A multidimensional approach to frailty in older people. Ageing Res Rev 60: 101047, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Popov LD. Mitochondrial biogenesis: An update. J Cell Mol Med 24: 4892–4899, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 386: 801–812, 2015. [DOI] [PubMed] [Google Scholar]
- 453.Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet 385: 549–562, 2015. [DOI] [PubMed] [Google Scholar]
- 454.Pujos-Guillot E, Pétéra M, Jacquemin J, Centeno D, Lyan B, Montoliu I, Madej D, Pietruszka B, Fabbri C, Santoro A, Brzozowska A, Franceschi C, Comte B. Identification of pre-frailty sub-phenotypes in elderly using metabolomics. Front Physiol 9: 1903, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, Skalska A, Jonas M, Franek E, Mossakowska M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun Ageing 13: 21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine 46: 319–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Quéré R, Saint-Paul L, Carmignac V, Martin RZ, Chrétien ML, Largeot A, Hammann A, Pais de Barros JP, Bastie JN, Delva L. Tif1γ regulates the TGF-β1 receptor and promotes physiological aging of hematopoietic stem cells. Proc Natl Acad Sci U S A 111: 10592–10597, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: Feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc 20: 149–166, 1991. [DOI] [PubMed] [Google Scholar]
- 460.Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J 2: 239–242, 2012. [PMC free article] [PubMed] [Google Scholar]
- 461.Ramakrishnan P, Alyousefi N, Abdul-Rahman P, Kamaruzzaman S, Chin A, Tan M. A systematic review of studies comparing potential biochemical biomarkers of frailty with frailty assessments. Eur Geriatric Med 8: 397–407, 2017. [Google Scholar]
- 462.Ramsay SE, Arianayagam DS, Whincup PH, Lennon LT, Cryer J, Papacosta AO, Iliffe S, Wannamethee SG. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart 101: 616–622, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Rando TA, Wyss-Coray T. Stem cells as vehicles for youthful regeneration of aged tissues. J Gerontol A Biol Sci Med Sci 69 Suppl 1: S39–S42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Rao MS, Mattson MP. Stem cells and aging: Expanding the possibilities. Mech Ageing Dev 122: 713–734, 2001. [DOI] [PubMed] [Google Scholar]
- 465.Rastas S, Pirttilä T, Viramo P, Verkkoniemi A, Halonen P, Juva K, Niinistö L, Mattila K, Länsimies E, Sulkava R. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc 54: 912–918, 2006. [DOI] [PubMed] [Google Scholar]
- 466.Rattray NJW, Trivedi DK, Xu Y, Chandola T, Johnson CH, Marshall AD, Mekli K, Rattray Z, Tampubolon G, Vanhoutte B, White IR, Wu FCW, Pendleton N, Nazroo J, Goodacre R. Metabolic dysregulation in vitamin E and carnitine shuttle energy mechanisms associate with human frailty. Nat Commun 10: 5027, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB. A 2-year randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 70: 1097–1104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab 27: 805–815.e804, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Reinders I, Visser M, Schaap L. Body weight and body composition in old age and their relationship with frailty. Curr Opin Clin Nutr Metab Care 20: 11–15, 2017. [DOI] [PubMed] [Google Scholar]
- 470.Richardson A, Fischer KE, Speakman JR, de Cabo R, Mitchell SJ, Peterson CA, Rabinovitch P, Chiao YA, Taffet G, Miller RA, Rentería RC, Bower J, Ingram DK, Ladiges WC, Ikeno Y, Sierra F, Austad SN. Measures of healthspan as indices of aging in mice – A recommendation. J Gerontol A Biol Sci Med Sci 71: 427–430, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, Finke M, Haider LR, Yu D, Flores V, Pak HH, Ahmad S, Ahmed S, Radcliff A, Wu J, Williams EM, Abdi L, Sherman DS, Hacker T, Lamming DW. Lifelong restriction of dietary branchedchain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging 1: 73–86, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Rockwood K, Blodgett JM, Theou O, Sun MH, Feridooni HA, Mitnitski A, Rose RA, Godin J, Gregson E, Howlett SE. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep 7: 43068, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 27: 17–26, 2011. [DOI] [PubMed] [Google Scholar]
- 474.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 54: 975–979, 2006. [DOI] [PubMed] [Google Scholar]
- 475.Rodríguez Mañas L, García-Sánchez I, Hendry A, Bernabei R, Roller-Wirnsberger R, Gabrovec B, Liew A, Carriazo AM, Redon J, Galluzzo L, Viña J, Antoniadou E, Targowski T, Di Furia L, Lattanzio F, Bozdog E, Telo M. Key messages for a frailty prevention and management policy in Europe from the ADVANTAGE JOINT ACTION Consortium. J Nutr Health Aging 22: 892–897, 2018. [DOI] [PubMed] [Google Scholar]
- 476.Rodríguez-Fuentes D, Fernández-Garza L, Samia-Meza J, Barrera-Barrera S, Caplan A, Barrera-Saldaña H. Mesenchymal stem cells current clinical applications: A systematic review. Arch of Med Res 52: 93–101, 2021. [DOI] [PubMed] [Google Scholar]
- 477.Rodriguez-Mañas L, Araujo de Carvalho I, Bhasin S, Bischoff-Ferrari HA, Cesari M, Evans W, Hare JM, Pahor M, Parini A, Rolland Y, Fielding RA, Walston J, Vellas B. ICFSR Task Force perspective on biomarkers for sarcopenia and frailty. J Frailty Aging 9: 4–8, 2020. [DOI] [PubMed] [Google Scholar]
- 478.Romero-Ortuno R, O’Shea D. Fitness and frailty: Opposite ends of a challenging continuum! Will the end of age discrimination make frailty assessments an imperative? Age Ageing 42: 279–280, 2013. [DOI] [PubMed] [Google Scholar]
- 479.Rosano C, Longstreth WT, Boudreau R, Taylor CA, Du Y, Kuller LH, Newman AB. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J Am Geriatr Soc 59: 390–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat Rev Rheumatol 13: 399–409, 2017. [DOI] [PubMed] [Google Scholar]
- 481.Rosenberg H Summary comments: Epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 50: 1231S–1233S, 1989. [PubMed] [Google Scholar]
- 482.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr 127: 990S–991S, 1997. [DOI] [PubMed] [Google Scholar]
- 483.Rusanova I, Diaz-Casado ME, Fernández-Ortiz M, Aranda-Martínez P, Guerra-Librero A, García-García FJ, Escames G, Mañas L, Acuña-Castroviejo D. Analysis of plasma microRNAs as predictors and biomarkers of aging and frailty in humans. Oxid Med Cell Longev 2018: 7671850, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Rusanova I, Fernández-Martínez J, Fernández-Ortiz M, Aranda-Martínez P, Escames G, García-García FJ, Mañas L, Acuña-Castroviejo D. Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp Gerontol 124: 110637, 2019. [DOI] [PubMed] [Google Scholar]
- 485.Rusli F, Lute C, Boekschoten MV, van Dijk M, van Norren K, Menke AL, Müller M, Steegenga WT. Intermittent calorie restriction largely counteracts the adverse health effects of a moderate-fat diet in aging C57BL/6J mice. Mol Nutr Food Res 61: 1600677, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Ryan J, Wrigglesworth J, Loong J, Fransquet PD, Woods RL. A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. J Gerontol A Biol Sci Med Sci 75: 481–494, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mázala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, Bellemin S, Iyer SR, Wang X, Gariani K, Sauve AA, Cantó C, Conley KE, Walter L, Lovering RM, Chin ER, Jasmin BJ, Marcinek DJ, Menzies KJ, Auwerx J. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med 8: 361ra139, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Sabatini DM. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A 114: 11818–11825, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Saisho Y Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 15: 196–205, 2015. [DOI] [PubMed] [Google Scholar]
- 490.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24: 835–845, 2012. [DOI] [PubMed] [Google Scholar]
- 491.Samson LD, Boots AMH, Verschuren WMM, Picavet HSJ, Engelfriet P, Buisman AM. Frailty is associated with elevated CRP trajectories and higher numbers of neutrophils and monocytes. Exp Gerontol 125: 110674, 2019. [DOI] [PubMed] [Google Scholar]
- 492.Santin Y, Lopez S, Ader I, Andrieu S, Blanchard N, Carrière A, Casteilla L, Cousin B, Davezac N, De Souto, Barreto P, Dray C, Fazilleau N, Gonzalez-Dunia D, Gourdy P, Guyonnet S, Jabrane-Ferrat N, Kunduzova O, Lezoualc’h F, Liblau R, Martinez LO, Moro C, Payoux P, Pénicaud L, Planat-Bénard V, Rampon C, Rolland Y, Schanstra JP, Sierra F, Valet P, Varin A, Vergnolle N, Vellas B, Viña J, Guiard BP, Parini A. Towards a large-scale assessment of the relationship between biological and chronological aging: The INSPIRE Mouse Cohort. J Frailty Aging 10: 121–131, 2021. [DOI] [PubMed] [Google Scholar]
- 493.Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 64: 675–681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Exp Gerontol 34: 721–732, 1999. [DOI] [PubMed] [Google Scholar]
- 495.Sapolsky RM, Altmann J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol Psychiatry 30: 1008–1016, 1991. [DOI] [PubMed] [Google Scholar]
- 496.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181, 2010. [DOI] [PubMed] [Google Scholar]
- 497.Sato AY, Richardson D, Cregor M, Davis HM, Au ED, McAndrews K, Zimmers TA, Organ JM, Peacock M, Plotkin LI, Bellido T. Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology 158: 664–677, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Saum KU, Dieffenbach AK, Jansen EH, Schottker B, Holleczek B, Hauer K, Brenner H. Association between oxidative stress and frailty in an elderly German population: Results from the ESTHER Cohort Study. Gerontology 61: 407–415, 2015. [DOI] [PubMed] [Google Scholar]
- 499.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 169: 361–371, 2017. [DOI] [PubMed] [Google Scholar]
- 500.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, Mazula DL, Brown AK, Berning M, Aversa Z, Kotajarvi B, Bruce CJ, Greason KL, Suri RM, Tracy RP, Cummings SR, White TA, LeBrasseur NK. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 5: e133668, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888, 2011. [DOI] [PubMed] [Google Scholar]
- 502.Scheuren AC, D’Hulst G, Kuhn GA, Masschelein E, Wehrle E, De Bock K, Müller R. Hallmarks of frailty and osteosarcopenia in prematurely aged PolgA. J Cachexia Sarcopenia Muscle 11: 1121–1140, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet Muscle 1: 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Schultz MB, Kane AE, Mitchell SJ, MacArthur MR, Warner E, Vogel DS, Mitchell JR, Howlett SE, Bonkowski MS, Sinclair DA. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun 11: 4618, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Schultz MB, Kane AE, Mitchell SJ, MacArthur MR, Warner E, Vogel DS, Mitchell JR, Howlett SE, Bonkowski MS, Sinclair DA. Publisher Correction: Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun 11: 5143, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6: 280–293, 2007. [DOI] [PubMed] [Google Scholar]
- 507.Scott D, Sanders KM, Aitken D, Hayes A, Ebeling PR, Jones G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity (Silver Spring) 22: 1568–1574, 2014. [DOI] [PubMed] [Google Scholar]
- 508.Seals DR, Justice JN, LaRocca TJ. Physiological Geroscience: Targeting function to increase healthspan and achieve optimal longevity. J Physiol 594: 2001–2024, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Seldeen KL, Berman RN, Pang M, Lasky G, Weiss C, MacDonald BA, Thiyagarajan R, Redae Y, Troen BR. Vitamin D insufficiency reduces grip strength, grip endurance and increases frailty in aged C57Bl/6J mice. Nutrients 12: 3005, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Seldeen KL, Lasky G, Leiker MM, Pang M, Personius KE, Troen BR. High intensity interval training improves physical performance and frailty in aged mice. J Gerontol A Biol Sci Med Sci 73: 429–437, 2018. [DOI] [PubMed] [Google Scholar]
- 511.Seldeen KL, Redae YZ, Thiyagarajan R, Berman RN, Leiker MM, Troen BR. High intensity interval training improves physical performance in aged female mice: A comparison of mouse frailty assessment tools. Mech Ageing Dev 180: 49–62, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Seldeen KL, Redae YZ, Thiyagarajan R, Berman RN, Leiker MM, Troen BR. Short session high intensity interval training and treadmill assessment in aged mice. J Vis Exp, 2019. DOI: 10.3791/59138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Semba RD, Gonzalez-Freire M, Tanaka T, Biancotto A, Zhang P, Shardell M, Moaddel R, Ferrucci L. Elevated plasma growth and differentiation factor 15 is associated with slower gait speed and lower physical performance in healthy community-dwelling adults. J Gerontol A Biol Sci Med Sci 75: 175–180, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Sendama W The effect of ageing on the resolution of inflammation. Ageing Res Rev 57: 101000, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Shahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, Wiley CD, Gan G, Kim DE, Kasler HG, Kuehnemann C, Kaplowitz B, Bhaumik D, Riley RR, Kennedy BK, Lithgow GJ. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab 32: 447–456.e446, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 31 Suppl 2: S262–S268, 2008. [DOI] [PubMed] [Google Scholar]
- 518.Sharma B, Dabur R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: A systematic review. Curr Med Chem 27: 2161–2188, 2020. [DOI] [PubMed] [Google Scholar]
- 519.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Shen J, Tsai YT, Dimarco NM, Long MA, Sun X, Tang L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci Rep 1: 67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102: 5618–5623, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Sierra F Moving Geroscience into uncharted waters. J Gerontol A Biol Sci Med Sci 71: 1385–1387, 2016. [DOI] [PubMed] [Google Scholar]
- 523.Sierra F The emergence of Geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med 6: a025163, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience 39: 1–5, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Sierra MI, Fernández AF, Fraga MF. Epigenetics of aging. Curr Genomics 16: 435–440, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD, Ellis C, Ross D, Vandegaer K, Bedja D, Gabrielson K, Walston JD, Berkowitz DE, Barouch LA. Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp Gerontol 48: 128–135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: Perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc 64: 1287–1292, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Simpkin AJ, Cooper R, Howe LD, Relton CL, Davey Smith G, Teschendorff A, Widschwendter M, Wong A, Kuh D, Hardy R. Are objective measures of physical capability related to accelerated epigenetic age? Findings from a British birth cohort. BMJ Open 7: e016708, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Sinclair DA, Dunning T, Rodriguez-Mañas L. Diabetes in older people: New insights and remaining challenges. Lancet Diabetes Endocrinol 3: 275–285, 2015. [DOI] [PubMed] [Google Scholar]
- 530.Singh M, Jensen MD, Lerman A, Kushwaha S, Rihal CS, Gersh BJ, Behfar A, Tchkonia T, Thomas RJ, Lennon RJ, Keenan LR, Moore AG, Kirkland JL. Effect of low-dose rapamycin on senescence markers and physical functioning in older adults with coronary artery disease: Results of a pilot study. J Frailty Aging 5: 204–207, 2016. [DOI] [PubMed] [Google Scholar]
- 531.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 8: e018195, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 7: 478–490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Solmi M, Veronese N, Luchini C, Manzato E, Sergi G, Favaro A, Santonastaso P, Correll CU. Oxidative stress and antioxidant levels in patients with anorexia nervosa after oral re-alimentation: A systematic review and exploratory meta-analysis. Eur Eat Disord Rev 24: 101–105, 2016. [DOI] [PubMed] [Google Scholar]
- 534.Solmi M, Veronese N, Manzato E, Sergi G, Favaro A, Santonastaso P, Correll CU. Oxidative stress and antioxidant levels in patients with anorexia nervosa: A systematic review and exploratory meta-analysis. Int J Eat Disord 48: 826–841, 2015. [DOI] [PubMed] [Google Scholar]
- 535.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19: 418–430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 58: 681–687, 2010. [DOI] [PubMed] [Google Scholar]
- 537.Sonowal R, Swimm A, Sahoo A, Luo L, Matsunaga Y, Wu Z, Bhingarde JA, Ejzak EA, Ranawade A, Qadota H, Powell DN, Capaldo CT, Flacker JM, Jones RM, Benian GM, Kalman D. Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A 114: E7506–E7515, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506: 316–321, 2014. [DOI] [PubMed] [Google Scholar]
- 539.Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 31: 1–8, 2016. [DOI] [PubMed] [Google Scholar]
- 540.Spira D, Buchmann N, Nikolov J, Demuth I, Steinhagen-Thiessen E, Eckardt R, Norman K. Association of low lean mass with frailty and physical performance: A comparison between two operational definitions of sarcopenia-data from the Berlin Aging Study II (BASE-II). J Gerontol A Biol Sci Med Sci 70: 779–784, 2015. [DOI] [PubMed] [Google Scholar]
- 541.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant 25: 829–848, 2016. [DOI] [PubMed] [Google Scholar]
- 542.Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin Interv Aging 12: 835–845, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Stenholm S, Strandberg TE, Pitkälä K, Sainio P, Heliövaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: The Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci 69: 73–78, 2014. [DOI] [PubMed] [Google Scholar]
- 544.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev 129: 163–173, 2008. [DOI] [PubMed] [Google Scholar]
- 545.Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL. 17alpha-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci 72: 3–15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Strandberg TE, Pitkälä KH. Frailty in elderly people. Lancet 369: 1328–1329, 2007. [DOI] [PubMed] [Google Scholar]
- 547.Strandberg TE, Sirola J, Pitkälä KH, Tilvis RS, Strandberg AY, Stenholm S. Association of midlife obesity and cardiovascular risk with old age frailty: A 26-year follow-up of initially healthy men. Int J Obes (Lond) 36: 1153–1157, 2012. [DOI] [PubMed] [Google Scholar]
- 548.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A 105: 3438–3442, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Sukoff Rizzo SJ, Anderson LC, Green TL, McGarr T, Wells G, Winter SS. Assessing healthspan and lifespan measures in aging mice: Optimization of testing protocols, replicability, and rater reliability. Curr Protoc Mouse Biol 8: e45, 2018. [DOI] [PubMed] [Google Scholar]
- 550.Sumantri S, Setiati S, Purnamasari D, Dewiasty E. Relationship between metformin and frailty syndrome in elderly people with Type 2 diabetes. Acta Med Indonesia 46: 183–188, 2014. [PubMed] [Google Scholar]
- 551.Sun B, Jeong YH, Jung JW, Seo K, Lee YS, Kang KS. Regulation of human umbilical cord blood-derived multi-potent stem cells by autogenic osteoclast-based niche-like structure. Biochem Biophys Res Commun 357: 92–98, 2007. [DOI] [PubMed] [Google Scholar]
- 552.Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell 61: 654–666, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4: 141–194, 2005. [DOI] [PubMed] [Google Scholar]
- 554.Takeda C, Angioni D, Setphan E, Macaron T, De Souto Barreto P, Sourdet S, Sierra F, Vellas B. Age-related frailty: A clinical model for Geroscience? J Nutr Health Aging 24: 1140–1143, 2020. [DOI] [PubMed] [Google Scholar]
- 555.Takeuchi I, Yoshimura Y, Shimazu S, Jeong S, Yamaga M, Koga H. Effects of branched-chain amino acids and vitamin D supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: A multicenter randomized controlled trial. Geriatr Gerontol Int 19: 12–17, 2019. [DOI] [PubMed] [Google Scholar]
- 556.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6: a016295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Tang H, Inoki K, Brooks SV, Okazawa H, Lee M, Wang J, Kim M, Kennedy CL, Macpherson PCD, Ji X, Van Roekel S, Fraga DA, Wang K, Zhu J, Wang Y, Sharp ZD, Miller RA, Rando TA, Goldman D, Guan KL, Shrager JB. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 18: e12943, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ, Chini EN. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD. Cell Metab 27: 1081–1095.e1010, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Tashiro J, Elliot SJ, Gerth DJ, Xia X, Pereira-Simon S, Choi R, Catanuto P, Shahzeidi S, Toonkel RL, Shah RH, El Salem F, Glassberg MK. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl Res 166: 554–567, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Tchkonia T, Palmer A, Kirkland J. New horizons: Novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J Clin Endocrinol Metab 106: E1481–E1487, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vander-voort AA, Jones GR. The effectiveness of exercise interventions for the management of frailty: A systematic review. J Aging Res 2011: 569194, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 17: 165–178, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Todorovic ST, Smiljanic KR, Ruzdijic SD, Djordjevic ANM, Kanazir SD. Effects of different dietary protocols on general activity and frailty of male wistar rats during aging. J Gerontol A Biol Sci Med Sci 73: 1036–1044, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV, Heldman AW, Miki R, Goldschmidt-Clermont PJ, Goldstein BJ, Mushtaq M, Levis-Dusseau S, Byrnes JJ, Lowery M, Natsumeda M, Delgado C, Saltzman R, Vidro-Casiano M, Da Fonseca M, Golpanian S, Premer C, Medina A, Valasaki K, Florea V, Anderson E, El-Khorazaty J, Mendizabal A, Green G, Oliva AA, Hare JM. Allogeneic mesenchymal stem cells ameliorate aging frailty: A phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci 72: 1513–1522, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Torgovnick A, Schiavi A, Maglioni S, Ventura N. Healthy aging: What can we learn from Caenorhabditis elegans? Z Gerontol Geriatr 46: 623–628, 2013. [DOI] [PubMed] [Google Scholar]
- 566.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007. [DOI] [PubMed] [Google Scholar]
- 567.Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, Lavandero S. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab 25: 128–137, 2014. [DOI] [PubMed] [Google Scholar]
- 568.Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 17: 11–22, 2015. [DOI] [PubMed] [Google Scholar]
- 569.Tsai VW, Lin S, Brown DA, Salis A, Breit SN. Anorexia-cachexia and obesity treatment may be two sides of the same coin: Role of the TGF-b superfamily cytokine MIC-1/GDF15. Int J Obes (Lond) 40: 193–197, 2016. [DOI] [PubMed] [Google Scholar]
- 570.Tuttle LJ, Hastings MK, Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther 92: 133–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther 91: 923–930, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Valdiglesias V, Sánchez-Flores M, Marcos-Pérez D, Lorenzo-López L, Maseda A, Millán-Calenti JC, Pásaro E, Laffon B. Exploring genetic outcomes as frailty biomarkers. J Gerontol A Biol Sci Med Sci 74: 168–175, 2019. [DOI] [PubMed] [Google Scholar]
- 573.van Abbema R, Bielderman A, De Greef M, Hobbelen H, Krijnen W, van der Schans C. Building from a conceptual model of the resilience process during ageing, towards the Groningen Aging Resilience Inventory. J Adv Nurs 71: 2208–2219, 2015. [DOI] [PubMed] [Google Scholar]
- 574.van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, Nowotny P, Sparks LM, Paglialunga S, Szendroedi J, Havekes B, Moullan N, Pirinen E, Hwang JH, Schrauwen-Hinderling VB, Hesselink MK, Auwerx J, Roden M, Schrauwen P. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 64: 1193–1201, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.van der Spoel E, Jansen SW, Akintola AA, Ballieux BE, Cobbaert CM, Slagboom PE, Blauw GJ, Westendorp RGJ, Pijl H, Roelfsema F, van Heemst D. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell 15: 1126–1131, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.van der Spoel E, Rozing MP, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJ, Westendorp RG, van Heemst D. Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden Longevity Study. Aging (Albany NY) 7: 956–963, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.van Niekerk G, du Toit A, Loos B, Engelbrecht AM. Nutrient excess and autophagic deficiency: Explaining metabolic diseases in obesity. Metabolism 82: 14–21, 2018. [DOI] [PubMed] [Google Scholar]
- 578.van Nieuwpoort IC, Vlot MC, Schaap LA, Lips P, Drent ML. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur J Endocrinol 179: 73–84, 2018. [DOI] [PubMed] [Google Scholar]
- 579.Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci 6: 2–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Varadhan R, Russ DW, Gabr RE, Huang J, Kalyani RR, Xue QL, Cappola AR, Bandeen-Roche K, Fried LP. Relationship of physical frailty to phosphocreatine recovery in muscle after mild exercise stress in the oldest-old women. J Frailty Aging 8: 162–168, 2019. [DOI] [PubMed] [Google Scholar]
- 581.Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci 63: 190–195, 2008. [DOI] [PubMed] [Google Scholar]
- 582.Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N, Fallin MD, Newman A, Bandeen-Roche K, Tracy R, Ferrucci L, Walston J. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 69: 165–173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Vasilaki A, Richardson A, Van Remmen H, Brooks SV, Larkin L, McArdle A, Jackson MJ. Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J Physiol 595: 6409–6415, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Veeranki S, Tyagi SC. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int J Mol Sci 14: 15074–15091, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Veeranki S, Winchester LJ, Tyagi SC. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim Biophys Acta: 732, 2015–741, 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Veronese N, Cereda E, Solmi M, Fowler SA, Manzato E, Maggi S, Manu P, Abe E, Hayashi K, Allard JP, Arendt BM, Beck A, Chan M, Audrey YJ, Lin WY, Hsu HS, Lin CC, Diekmann R, Kimyagarov S, Miller M, Cameron ID, Pitkälä KH, Lee J, Woo J, Nakamura K, Smiley D, Umpierrez G, Rondanelli M, Sund-Levander M, Valentini L, Schindler K, Törmä J, Volpato S, Zuliani G, Wong M, Lok K, Kane JM, Sergi G, Correll CU. Inverse relationship between body mass index and mortality in older nursing home residents: A meta-analysis of 19,538 elderly subjects. Obes Rev 16: 1001–1015, 2015. [DOI] [PubMed] [Google Scholar]
- 588.Verovskaya EV, Dellorusso PV, Passegué E. Losing sense of self and surroundings: Hematopoietic stem cell aging and leukemic transformation. Trends Mol Med 25: 494–515, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, Lopez Samaniego L, Rodríguez-Mañas L, Bernabei R, Onder G. Frailty and multimorbidity: A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 74: 659–666, 2019. [DOI] [PubMed] [Google Scholar]
- 590.Vetrano DL, Palmer KM, Galluzzo L, Giampaoli S, Marengoni A, Bernabei R, Onder G. Hypertension and frailty: A systematic review and meta-analysis. BMJ Open 8: e024406, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol 75: 645–668, 2013. [DOI] [PubMed] [Google Scholar]
- 592.Villacampa-Fernández P, Navarro-Pardo E, Tarín JJ, Cano A. Frailty and multimorbidity: Two related yet different concepts. Maturitas 95: 31–35, 2017. [DOI] [PubMed] [Google Scholar]
- 593.Viscogliosi G The metabolic syndrome: A risk factor for the frailty syndrome? J Am Med Dir Assoc 17: 364–366, 2016. [DOI] [PubMed] [Google Scholar]
- 594.Vizoso FJ, Eiro N, Costa L, Esparza P, Landin M, Diaz-Rodriguez P, Schneider J, Perez-Fernandez R. Mesenchymal stem cells in homeostasis and systemic diseases: Hypothesis, evidences, and therapeutic opportunities. Int J Mol Sci 20: 3738, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med 22 Suppl 3: 391–395, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, van Loon LJ. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 10: e0140903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE, Fried LP, Robinson TN, Afilalo J, Newman AB, López-Otín C, De Cabo R, Theou O, Studenski S, Cohen HJ, Ferrucci L. Moving frailty toward clinical practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc 67: 1559–1564, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci 63: 391–398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54: 991–1001, 2006. [DOI] [PubMed] [Google Scholar]
- 600.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med 162: 2333–2341, 2002. [DOI] [PubMed] [Google Scholar]
- 601.Walston JD, Bandeen-Roche K. Frailty: A tale of two concepts. BMC Med 13: 185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Wang B, Liu Z, Chen VP, Wang L, Inman CL, Zhou Y, Guo C, Tchkonia T, Rowe DW, Kuchel GA, Robson P, Kirkland JL, Xu M. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell 19: e13106, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Wang CP, Lorenzo C, Espinoza SE. Frailty attenuates the impact of metformin on reducing mortality in older adults with type 2 diabetes. J Endocrinol Diabetes Obes 2: 1031, 2014. [PMC free article] [PubMed] [Google Scholar]
- 604.Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegué E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 494: 323–327, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol 46: 153–162, 1993. [DOI] [PubMed] [Google Scholar]
- 606.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol Sin 34: 747–754, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Weidner CI, Wagner W. The epigenetic tracks of aging. Biol Chem 395: 1307–1314, 2014. [DOI] [PubMed] [Google Scholar]
- 608.Wenz T PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB Life 61: 1051–1062, 2009. [DOI] [PubMed] [Google Scholar]
- 609.Westbrook RM, Yang HL, Langdon JM, Roy CN, Kim JA, Choudhury PP, Xue QL, di Francesco A, de Cabo R, Walston J. Aged interleukin-10tm1Cgn chronically inflamed mice have substantially reduced fat mass, metabolic rate, and adipokines. PLoS One 12: e0186811, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Westerblad H, Bruton JD, Katz A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res 316: 3093–3099, 2010. [DOI] [PubMed] [Google Scholar]
- 611.Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE. A clinical frailty index in aging mice: Comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 69: 621–632, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Whitson HE, Cohen HJ, Schmader KE, Morey MC, Kuchel G, Colon-Emeric CS. Physical resilience: Not simply the opposite of frailty. J Am Geriatr Soc 66: 1459–1461, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: Systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci 71: 489–495, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: Review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. [DOI] [PubMed] [Google Scholar]
- 615.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 47: 123–132, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: The diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 1114: 434–455, 2007. [DOI] [PubMed] [Google Scholar]
- 617.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234, 2009. [DOI] [PubMed] [Google Scholar]
- 618.Wong SQ, Kumar AV, Mills J, Lapierre LR. Autophagy in aging and longevity. Hum Genet 139: 277–290, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Wong SQ, Kumar AV, Mills J, Lapierre LR. C. elegans to model autophagy-related human disorders. Prog Mol Biol Transl Sci 172: 325–373, 2020. [DOI] [PubMed] [Google Scholar]
- 620.Wong TY, Massa MS, O’Halloran AM, Kenny RA, Clarke R. Cardiovascular risk factors and frailty in a cross-sectional study of older people: Implications for prevention. Age Ageing 47: 714–720, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Woolford SJ, Sohan O, Dennison EM, Cooper C, Patel HP. Approaches to the diagnosis and prevention of frailty. Aging Clin Exp Res 32: 1629–1637, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Workeneh B, Bajaj M. The regulation of muscle protein turnover in diabetes. Int J Biochem Cell Biol 45: 2239–2244, 2013. [DOI] [PubMed] [Google Scholar]
- 623.World Health Organization. World Report on Ageing and Health. Geneva: World Health Organization, 2015. [Google Scholar]
- 624.World Health Organization. Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity. Geneva: World Health Organization, Licence: CC BY-NC-SA 3.0 IGO, 2017. [PubMed] [Google Scholar]
- 625.Xie WQ, Xiao WF, Tang K, Wu YX, Hu PW, Li YS, Duan Y, Lv S. Caloric restriction: Implications for sarcopenia and potential mechanisms. Aging (Albany NY) 12: 24441–24452, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 112: E6301–E6310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med 27: 1–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Xue QL, Tian J, Walston JD, Chaves PHM, Newman AB, Bandeen-Roche K. Discrepancy in frailty identification: Move beyond predictive validity. J Gerontol A Biol Sci Med Sci 75: 387–393, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Xue QL, Walston JD, Fried LP, Beamer BA. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The Women’s Health and Aging Study. Arch Intern Med 171: 1119–1121, 2011. [DOI] [PubMed] [Google Scholar]
- 631.Yamada Y, Kemnitz JW, Weindruch R, Anderson RM, Schoeller DA, Colman RJ. Caloric restriction and healthy life span: Frail phenotype of nonhuman primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J Gerontol A Biol Sci Med Sci 73: 273–278, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Yanagita I, Fujihara Y, Kitajima Y, Tajima M, Honda M, Kawajiri T, Eda T, Yonemura K, Yamaguchi N, Asakawa H, Nei Y, Kayashima Y, Yoshimoto M, Harada M, Araki Y, Yoshimoto S, Aida E, Yanase T, Nawata H, Muta K. A high serum cortisol/DHEA-S ratio is a risk factor for sarcopenia in elderly diabetic patients. J Endocr Soc 3: 801–813, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, Tian J, Yeh SH, Resnick B, Xu X, Walston J, Fried LP, Leng SX. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 29: 5015–5021, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Ye X, Liao C, Liu G, Xu Y, Tan J, Song Z. Age-related changes in the regenerative potential of adipose-derived stem cells isolated from the prominent fat pads in human lower eyelids. PLoS One 11: e0166590, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Yorke A, Kane AE, Hancock Friesen CL, Howlett SE, O’Blenes S. Development of a rat clinical frailty index. J Gerontol A Biol Sci Med Sci 72: 897–903, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Yoshida S, Yamahara K, Kume S, Koya D, Yasuda-Yamahara M, Takeda N, Osawa N, Chin-Kanasaki M, Adachi Y, Nagao K, Maegawa H, Araki SI. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell 17: e12796, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Yu R, Wong M, Chong KC, Chang B, Lum CM, Auyeung TW, Lee J, Lee R, Woo J. Trajectories of frailty among Chinese older people in Hong Kong between 2001 and 2012: An age-period-cohort analysis. Age Ageing 47: 254–261, 2018. [DOI] [PubMed] [Google Scholar]
- 639.Yuan J, Liu Y, Wang J, Zhao Y, Li K, Jing Y, Zhang X, Liu Q, Geng X, Li G, Wang F. Long-term persistent organic pollutants exposure induced telomere dysfunction and senescence-associated secretary phenotype. J Gerontol A Biol Sci Med Sci 73: 1027–1035, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: A relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med 10: 5–6, 2019. [DOI] [PubMed] [Google Scholar]
- 641.Zampieri M, Ciccarone F, Calabrese R, Franceschi C, Bürkle A, Caiafa P. Reconfiguration of DNA methylation in aging. Mech Ageing Dev 151: 60–70, 2015. [DOI] [PubMed] [Google Scholar]
- 642.Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, Studenski SA, Spencer RG, Ferrucci L. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 16: 461–468, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Zhang BB, Zhou G, Li C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9: 407–416, 2009. [DOI] [PubMed] [Google Scholar]
- 644.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352: 1436–1443, 2016. [DOI] [PubMed] [Google Scholar]
- 645.Zhang WB, Aleksic S, Gao T, Weiss EF, Demetriou E, Verghese J, Holtzer R, Barzilai N, Milman S. Insulin-like Growth Factor-1 and IGF binding proteins predict all-cause mortality and morbidity in older adults. Cells 9: 1368, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Zhang WB, Sinha DB, Pittman WE, Hvatum E, Stroustrup N, Pincus Z. Extended twilight among isogenic C. elegans causes a disproportionate scaling between lifespan and health. Cell Syst 3: 333–345.e334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 69: 119–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1. Redox Biol 11: 30–37, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Zheng Y, Kong J, Li Q, Wang Y, Li J. Role of miRNAs in skeletal muscle aging. Clin Interv Aging 13: 2407–2419, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y, Jin H, He Y, Gu Q, Xu X. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61: 217–228, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17: 324–328, 2014. [DOI] [PubMed] [Google Scholar]
- 652.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15: 428–435, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


