INTRODUCTION
Palliative care is defined as multidisciplinary, specialized medical care that addresses the physical, spiritual, and psychosocial needs of patients with serious illness and their caregivers.[1,2] The benefits of palliative care are increasingly recognized across disease states and for patients with decompensated cirrhosis (DC).[3–5] This American Association for the Study of Liver Diseases (AASLD) guidance to providing palliative care for patients with cirrhosis was developed with the support and oversight of the AASLD Practice Guidelines Committee. The AASLD Practice Guidelines Committee chose to commission a guidance, rather than a guideline, because of the paucity of randomized controlled trials (RCTs) on this topic. AASLD guidelines are supported by systematic reviews of the literature, formal ratings of evidence quality and strength of recommendations, and, if appropriate, meta-analysis of results using the Grading of Recommendations Assessment Development and Evaluation system. In contrast, this document was developed by consensus of an expert panel and provides guidance statements based on formal review and analysis of the literature on the topics and questions related to the palliative care needs of patients with cirrhosis and their caregivers. Although palliative care can be considered regardless of the stage of cirrhosis, this guidance document predominantly addresses issues pertinent to adult patients with DC because this group bears considerable physical, psychosocial, and financial burden. We specifically focus on topics that are not covered in existing AASLD practice guidelines/guidance documents and thus refer the readers to the AASLD practice guidelines for specific recommendations for the diagnosis and management of ascites, hepatic encephalopathy (HE), hepatocellular carcinoma (HCC), and portal hypertension.[6–9] In addition, the complex palliative care needs for patients with HCC are not specifically addressed by this guidance, but are addressed by other guidelines.[10,11]
PALLIATIVE CARE DEFINITIONS
Palliative care can be provided at any stage of a serious illness and concurrently with disease-directed and curative treatments (including organ transplants). Over the past decade, the body of evidence supporting early palliative care has expanded to include persons with nonmalignant conditions, such as cirrhosis.[3,4] Palliative care takes a comprehensive, person-centered approach to care, focusing on the aspects of care most important to patients and their families/informal caregivers. Because of this comprehensive scope, management typically relies on a team, including physicians, nurses, chaplains, social workers, and other providers.
Table 1 illustrates the conceptual distinctions between primary and specialty palliative care. Specialty palliative care refers to care delivered by specialists with advanced palliative care skills such as board-certified palliative care physicians or palliative-certified nurses, social workers, pharmacists, and chaplains.[12] However, primary palliative care describes care aligned with the principles of palliative care (e.g., person-centered, communication-focused symptom management) that can be delivered by any medical professional.[12] The National Consensus Project for Quality Palliative Care defined eight core domains of high-quality palliative care around which this guidance is framed (Figure 1).[13,14] Although all eight domains can apply to patients with DC, their relative importance may vary across the illness trajectory.[5,15]
TABLE 1.
Key similarities and differences between primary palliative care, specialty palliative care, hospice, and advance care planning
| Primary palliative care | Specialty palliative care | Hospice | Advance care planning | |
|---|---|---|---|---|
| Primary focus | Quality of life, symptoms, psychosocial and spiritual support | Quality of life, symptoms, psychosocial and spiritual support | Quality of life, symptoms, psychosocial and spiritual support | Longitudinal process of discussing and documenting patient values and preferences around their care (e.g., end of life); identifying surrogate decision makers |
| Delivered by | Primary or specialist treating teams | Palliative care clinicians/teams, as consultants or embedded within practices | Usually private hospice agencies (or within Veterans Administration system for veterans) | Any clinician; persons can also complete some documents on their own. |
| Timing | Any time a need is identified | Any time a need is identified | Prognosis ≤6 months | Can be addressed early in the illness course and revisited on a regular basis and when there are major clinical changes |
| Location | Anywhere under the care of treating team | Inpatient, outpatient, community (home, nursing home) | Home, nursing home, inpatient (limited time for uncontrolled symptoms) | Anywhere |
| Reimbursement | Routine CMS billing | Routine CMS billing | Capitated payment model through Medicare Part A | Can be reimbursed with ACP billing codes: 99497 (first 30 min) 99498 (additional 30 min) |
FIGURE 1.
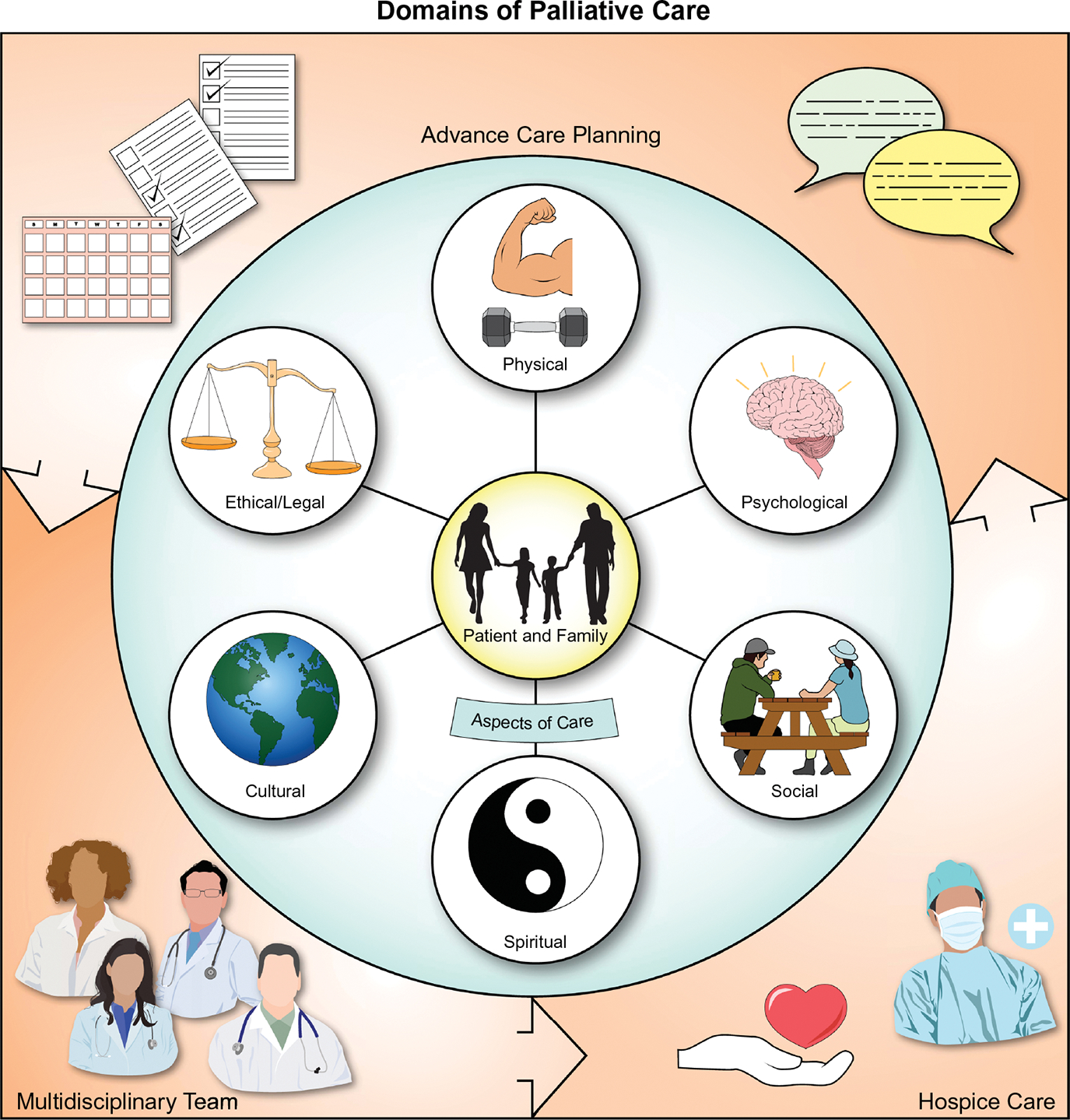
Domains of palliative care. The eight domains of palliative care, as defined by the National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. Reference: Ferrell et al. 2018 include spiritual, cultural, social, psychological, ethical, physical, care at the end of life, and processes of care (e.g., ACP).[14] This figure also includes patients, families, and the multidisciplinary team as central to palliative care
Advance care planning (ACP) is a component of palliative care that involves the iterative and longitudinal process of medical decision making for patients and their families over the course of their illness trajectory.[16,17] ACP includes identifying surrogate decision makers, illness education and prognostic disclosure, and formal documentation of goals for medical and end-of-life care (EoLC) through advance directives that center on the goals, values, and preferences of patients and their families.[17]
Hospice is EoLC focused on allowing people in the last phases of incurable disease to live as fully and comfortably as possible.[14] Hospice is different than palliative care in that it focuses exclusively on comfort, rather than disease-directed curative treatment, and includes only persons with a life expectancy measured in months. In the USA, the hospice benefit is provided under Medicare Part A, and patients must have a defined, time-limited prognosis (certified by one or more physicians as ≤6 months).[18] DC is in many cases incurable, and life expectancy, which can be predicted with models such as the Model for End-Stage Liver Disease (MELD) and Child-Pugh-Turcotte scores, may be well below 6 months.[6,19–21] Because of the poor prognosis of DC, many patients with DC qualify for hospice benefits, which can help support patients and their caregivers.
Guidance statements
-
1
Palliative care can be provided to patients with DC at any stage of their illness.
-
2
Palliative care can be delivered by any member of the care team ( primary palliative care) as well as teams with subspecialty training (specialty palliative care) for more-complex cases.
-
3
Palliative care does not preclude the delivery of disease-directed or even curative treatments.
-
4
Hospice is different than palliative care in that it focuses exclusively on comfort, rather than disease-directed curative treatment, and includes only persons with life expectancy measured in months.
THE ROLE OF CAREGIVERS IN PALLIATIVE CARE
Attention to patients’ families and support persons, herein called caregivers, is a core value and component of palliative care (Figure 1). Caregivers are discussed in The National Consensus Project under the heading of clinical implications. Throughout this document, we address the role of caregivers in each aspect of palliative care. Caregivers of patients with DC often face under-recognized psychological, physical, and financial burdens.[22–27] Their symptoms include stress, anxiety, depression, insomnia, decreased health-related quality of life (HRQoL), and worse physical health.[23,25–27] Psychological symptom burden among caregivers is associated with patients’ alcohol use, encephalopathy, ascites, liver disease (LD) severity, repeated hospitalizations, prognostic uncertainty, and lack of information.[25–29] Caregivers of patients with DC report feeling unprepared to provide physical care, medication management, and transportation to their loved ones and often experience significant financial burdens.[23–25,27,30–32] Importantly, the financial, social, and psychological burdens of cirrhosis experienced by caregivers can continue to impact caregivers after patients die. As such, support for caregivers of patients with cirrhosis should start early and extend through the bereavement period. This support can include inquiring about how they are doing during a medical visit, acknowledging the challenges of caregiving, and referring to services for support. Hepatology providers should assess caregivers’ goals and consider their needs. Being knowledgeable about and providing a list of ancillary services to caregivers is something that can occur in hepatology. Services of interest to caregivers may include pastoral care or spiritual support, mental health resources, bereavement support, grief counseling, caregiver support websites, and peer support groups.
Guidance statements
-
5
Caring for caregivers is a central component of providing palliative and hospice care.
-
6
Caregiver support should be provided across the trajectory of liver disease and is critically important in the context of DC, EoLC, and after the patient’s death.
PALLIATIVE CARE IN PATIENTS WITH DC: CURRENT PRACTICES AND BARRIERS
Cirrhosis is associated with poor HRQoL attributable to multiple physical, cognitive, psychological, and social stressors that are challenging to manage.[30,33,34] Although the prognosis for patients with DC is variable, with a 5-year mortality ranging from 20% to 80% across studies, the overall disease trajectory is progressive, with declining health and increasing symptom burden and frequent hospitalizations at the end of life.[19,35–37] Though liver transplantation (LT) can be curative, few patients are ever waitlisted for or receive LT.[38]
Palliative care needs of patients with cirrhosis and their caregivers are frequently underaddressed.[39] Only 11% of patients with cirrhosis receive specialty palliative care or hospice care referrals, and consultation often occurs very late in the disease course.[40–44] Consequently, patients with cirrhosis receive EoLC that is more resource intensive and invasive than that of patients with other serious, life-limiting illnesses.[27,44,45]
There are several barriers to palliative care implementation that have been identified specifically in hepatology. These barriers include a shortage of specialty palliative care providers, absence of evidence-based referral criteria, lack of role clarity between specialists, stigma that palliative care is synonymous with “giving up” on curative treatments, lack of provider training, competing demands on providers’ time, and prognostic uncertainty.[22,27,41,46–50] In addition, many of the symptoms experienced by persons with DC are highly liver specific and managed longitudinally by liver teams. This is in contrast to oncology practice models in which palliative care teams frequently have a key role managing malignant pain syndromes throughout the disease course.[51] Transplant evaluation and listing may also present a unique barrier to palliative care for patients with cirrhosis, related to the perceived incompatibility of transplantation and palliative care. One study found that patients with cirrhosis undergoing transplant evaluation received lower-quality EoLC.[52] Similarly, a qualitative study of 42 patients and 46 clinicians found that transplant teams avoided discussing nonaggressive treatment options with patients, leading caregivers to feel unprepared to support their loved ones at the end of life.[53] Finally, hepatology training does not routinely include palliative care training, and palliative care competencies for hepatologists have not been developed.
Despite these barriers, hepatology clinicians, especially those with a longitudinal relationship with patients and families, can play a key role in delivering primary palliative care to address patients’ and caregivers’ needs. Some elements of primary palliative care that could be provided as part of routine hepatology care include evaluating and managing symptoms, identifying and documenting surrogate decision makers, eliciting patient preferences about treatment and aligning care plans with these preferences, providing counseling about what to expect in the future, and referring patients for social services to increase support in the community.
Guidance statements
-
7
Patients with DC and their caregivers often have significant unmet, unrecognized palliative care needs that include psychological, physical, social, financial, and spiritual health burdens.
-
8
Evaluation for unmet palliative care needs and specialty palliative care consultation should be considered for all patients with DC and their caregivers.
-
9
In patients with DC, disease-directed care, such as transplantation evaluation and listing, does not preclude palliative care delivery or specialty palliative care consultation.
-
10
Given shortages of specialty palliative care providers, hepatology clinicians should play a central role in offering primary palliative care services to patients with cirrhosis, including symptom assessment and management, basic ACP (e.g., identifying surrogate decision makers), counseling, and referral for additional support when feasible and necessary.
EFFECTIVENESS OF PALLIATIVE CARE INTERVENTIONS FOR PATIENTS WITH CIRRHOSIS
A growing body of evidence supports the integration of curative and palliative care approaches for patients with DC (Table 2).[54–57] Observational studies have demonstrated reduced resource use, decreased symptoms, and improved HRQoL for patients with cirrhosis who receive palliative care services.[50,58,59] A single-arm, single-center study found that outpatient specialty palliative care referral for all patients undergoing LT evaluation resulted in improved physical and psychological symptom scores.[54] Likewise, an outpatient primary palliative care intervention led by hepatology nurses with palliative care and communication training was feasible and acceptable to recently discharged patients and their health care teams.[57] The intervention was associated with improved HRQoL, care coordination, coping, and anticipatory planning.
TABLE 2.
Palliative care interventions in patients with cirrhosis and their caregivers
| Author, year | Setting and population | Intervention | Comparator arm (study design) | Outcomes | Results | Palliative care domain addressed |
|---|---|---|---|---|---|---|
| Baumann etal. 2015[54] | Outpatients being evaluated for LT | One-time nurse practitioner and board-certified PC physician performing comprehensive physical and psychological symptom assessment; ACP | None (pre-/post-single-arm quality improvement study) | Physical and psychological symptom burden; ACP | Improved pruritus, fatigue, well-being, appetite; decreased depression; increased ACP | 1, 2, 3, 8 |
| Kimbellet al.2018[57] | Outpatients with DC | Nurse specialists assisted in care coordination, illness education, financial and psychosocial support, and ACP and provided a summary of this discussion to the patient’s primary care physician and hepatologist | None (single-arm feasibility study) | HRQoL; perceived care coordination, coping, anticipatory planning (qualitative) | Improved HRQoL and secondary outcomes | 1,4, 8 |
| Lamba et al. 2012[55] | Surgical ICU admission for patients pre-LT and post-LT | Two-part communication-based intervention involving palliative care team (APRN, family support counseling, chaplain): initial physical and psychological symptom assessment with ACP, followed by interdisciplinary family meeting within 72 h | None (prestudy/poststudy design) | Length of stay in ICU, mortality, goals of care consensus (qualitative) | Decreased ICU length of stay, better consensus in goals of care, lower receipt of life-sustaining treatment, and earlier provision of comfort-focused care; no difference in mortality | 7, 8 |
| Shinall et al. 2019[56] | Inpatients with DC | Board-certified PC physician or nurse practitioner, during which patients were provided with an informational packet containing education on LD and PC | Control group of inpatients with DC receiving usual care (RCT) | Time until first readmission; days alive outside the hospital, referral to hospice care, death, readmissions, patient quality of life, depression, anxiety, and quality of EoLC over 6 months | Increased time to readmission; no change in other outcomes; poor enrollment | 1,3 |
| Bailey et al.2017[60] | Outpatient dyads of patients awaiting LT and their caregivers | Six-week telephonic intervention of a self-management intervention (n = 56 dyads) vs. LD education (n = 59 dyads); self-management intervention included coping skills and uncertainty management strategies. | Attention control group of patient-caregiver dyads receiving LD education alone (RCT) | Illness uncertainty, uncertainty management, depression, anxiety, self-efficacy, and quality of life at 10–12 weeks postintervention | No significant differences between groups and in most measures pre- to post-, though there was a numerical improvement in self-efficacy in both patients and caregivers. | 3, 4, CG |
| Bajaj etal. 2017[61] | Outpatients with cirrhosis (n = 20) and depressive symptoms (Beck Depression Inventory >14) and their caregivers | Mindfulness-based stress reduction intervention with four weekly hour-long group sessions and audio-guided home practice | None (pre-/post-single-arm study) | Depressive symptoms, sleep, anxiety, encephalopathy, HRQoL, perceived caregiver burden, and caregiver depression, sleep quality | Significant reduction in depressive symptoms, improvement in sleep and overall HRQoL but not in anxiety or encephalopathy rates in patients; improved caregiver burden, depression, sleep | 2, 3, CG |
| Ufere et al.2021[86] | Transplant-ineligible inpatients and outpatients with advanced LD (n = 50) | Five-minute ACP video decision-support tool depicting three levels of goals of care: life-prolonging care (CPR and intubation); life-limiting care (hospitalization, no CPR/ intubation); and comfort care | Control group listened to verbal narrative of the three levels of goals of care (RCT). | Feasibility; acceptability, knowledge of EoLC options, postintervention goals of care and CPR/intubation preferences | High enrollment rate; video highly acceptable to patients in video arm, who had higher EoLC knowledge scores and were less likely to prefer to receive CPR | 1,7 |
Note: Domain 1: Structures and Processes of Care. Domain 2: Physical Aspects of Care. Domain 3: Psychological and Psychiatric Aspects of Care. Domain 4: Social Aspects of Care. Domain 5: Spiritual, Religious, and Existential Aspects of Care. Domain 6: Cultural Aspects of Care. Domain 7: Care of the Patient Nearing the End of Life. Domain 8: Ethical and Legal Aspects of Care. CG: Caregiver Support (included under Clinical Implications in The National Consensus Project for Quality Palliative Care).
Abbreviations: APRN, advanced practice registered nurse; CPR, cardiopulmonary resuscitation; ICU, intensive care unit; PC, palliative care.
Two studies that examined inpatient palliative care also had favorable findings. A specialty palliative care intervention in a single academic surgical intensive care was associated with earlier consensus around goals of care, reduced length of stay, and earlier provision of comfort-focused care without any change in mortality.[55] Caregivers also reported having more time to say goodbye.[55] This intervention included early consultation with an interdisciplinary palliative care team, who provided a comprehensive evaluation within 24 h of admission, including discussion of prognosis, caregiver support, ACP, and symptom assessment. The palliative care team then participated in daily rounds and conducted a caregiver meeting within 72 h of admission.
To the best of our knowledge, Shinall et al. conducted the only RCT of a palliative care intervention in inpatients with cirrhosis. The intervention included patients with a life expectancy <12 months and involved an inpatient specialty palliative care consultation and monthly postdischarge follow-up with the palliative care team, who reviewed medications, symptoms, and goals of care.[56] The comparison group received usual care that included palliative care at the discretion of the hepatologist (19% received palliative care), and results were analyzed on an intention-to-treat basis. Although this trial was prematurely terminated because of low enrollment, patients in the intervention group had significantly reduced readmission rates and more days alive outside of the hospital as compared with patients in the control group.[56] The researchers cited barriers to enrollment including patients and family feeling overwhelmed at the time of index hospitalization, concerns about whether insurance would cover palliative care, challenges with identifying caregivers, and a narrow screen-in to enrollment window.[56]
Few interventions have included caregivers of patients with cirrhosis. A randomized trial of a cognitive behavior-based coping intervention delivered by trained nurses or social workers by telephone (as compared with LD education as an attention control) did not significantly impact mental health or HRQoL for patients or caregivers.[60] However, a mindfulness-based stress reduction and supportive group therapy intervention was associated with improved patient and caregiver mental health symptoms and HRQoL.[61] More trials are needed to understand how to best help caregivers of patients with cirrhosis.
Although these early data generally support the effectiveness of palliative care interventions for patients with cirrhosis and their caregivers, larger trials are needed to further characterize the optimal timing and content of these interventions. Conducting palliative care trials in this population is especially challenging for several reasons. Such trials are resource and time intensive; the population is heterogeneous (in terms of illness severity, trajectory, and treatments) with considerable prognostic uncertainty; trials require rigorous, potentially intrusive study monitoring; and there is limited interest in the topic from commercial sources, among others. The ongoing PAL LIVER trial, funded by the Patient-Centered Outcomes Research Institute, is recruiting patients with advanced liver disease and their caregivers from 18 clinical centers in the United States, comparing the effectiveness of palliative care delivered by specialists versus trained hepatologists for improving patients’ HRQoL, symptoms, caregiver burden, and health care use.[62] PAL LIVER and other studies are needed to establish which palliative outcomes matter most to patients and their caregivers.
Guidance statements
-
11
In patients with DC, outpatient palliative care may be associated with improved symptoms, improved care coordination, and better anticipatory planning.
-
12
Inpatient specialty PC consultations with postdischarge follow-up may be associated with greater consensus between patients and clinicians about the goals of care, reduced life-sustaining treatment use, earlier provision of comfort-focused care, and reduced readmission.
EFFECTIVENESS OF PALLIATIVE CARE INTERVENTIONS IN GENERAL POPULATIONS
As cirrhosis-specific data accumulate, trials of palliative care in other similar populations may help to inform care. Over the past decade, several landmark studies have assessed the impact of multicomponent, interdisciplinary palliative care interventions in patients with advanced heart, lung, renal, and hematological diseases across multiple domains of palliative care (Table S1).[13,14] Palliative care interventions have improved physical (e.g., fatigue, pain), psychological (e.g., depression, anxiety, and mood), social, spiritual, EoLC, ACP, caregivers’ quality of life, and cost-effectiveness of care.[63–77] However, evidence for improvement in religious, existential, and cultural aspects of care remains limited.[14] In general, interventions with a comprehensive evaluation by palliative care specialists, including nurses, social workers, or physicians, were associated with improved quality of care and symptom management.
Guidance statements
-
13
A wide range of studies have demonstrated the effectiveness of palliative care interventions in patients with other chronic illnesses, with benefits including` reduced symptom burden, improved mental health, better quality of life, and decreased health care use.
-
14
Palliative care interventions can positively impact caregivers’ symptoms.
ADVANCE CARE PLANNING (ACP)
ACP is a proactive, ongoing, collaborative process of decision making about health care preferences, goals, and values in the context of a life-limiting illness.[16,17] Decisions around life-sustaining treatments, completing advance directives, and identifying surrogate decision makers are all part of ACP, which is based on continuous assessment and documentation of patients’ personal values, preferences, and caregiver input.[14,17] Written documentation (such as advance directives) can help ensure that these values and preferences are respected across clinical teams and health care settings. ACP interventions are also associated with greater concordance between patient preferences and care delivery, completion of advance directives, and improved end-of-life management.[78] Thus, facilitating ACP is an important component of caring for persons with DC.
Table 3 outlines key ACP definitions, and Figure 2 illustrates key components of ACP, including assessing patients’ capacity and willingness to engage in ACP, identifying surrogate decision makers who should be present for conversations, and eliciting and documenting preferences.[14,17,79] It is critical to document this information early in the trajectory of cirrhosis, before the onset of encephalopathy, for example. It is also notable that a person who is present at an outpatient appointment or at the hospital bedside may not be the person that local surrogacy laws identify as a default surrogate or the person that the patient wants to be making decisions on their behalf.
TABLE 3.
| Term | Definition |
|---|---|
| Advance directive | Legal documents that can guide care when the patient is unable to engage in decision making |
| Living will | Advance directives that specify the types of medical care acceptable vs. unacceptable to the patient if they cannot communicate at the end of life |
| Health care proxy (also referred to as durable power of attorney for health care) | Identification and documentation of a health care agent by the patient |
| Physician orders for life-sustaining treatment/medical orders for life-sustaining treatment | Orders signed by physicians (or advance practice providers in some states) that document patient preferences around specific treatments (e.g., code status, hospitalization, artificial nutrition/hydration) |
FIGURE 2.
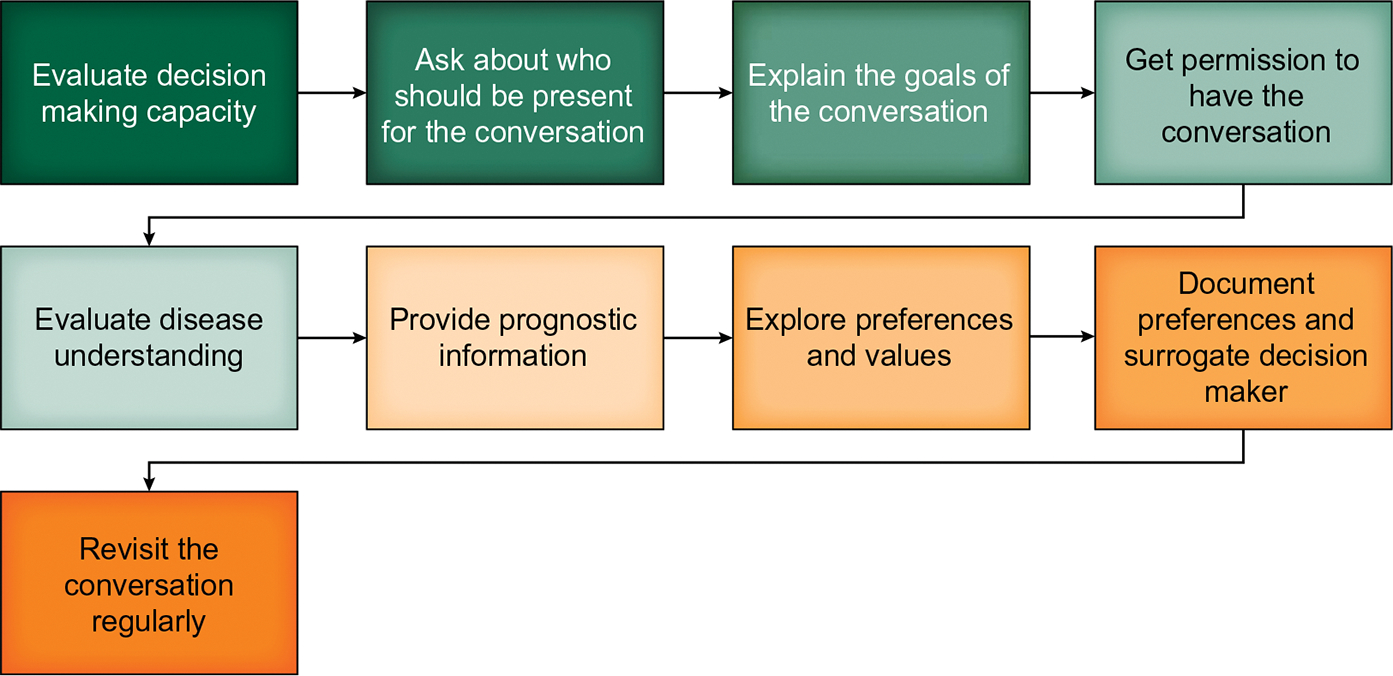
Steps in Advance Care Planning (ACP). ACP is an iterative process that involves working with patients and their families to identify and document their preferences for care. This graphic provides a roadmap for engaging in ACP
Ideally, clinicians with a continuity relationship with a patient and an understanding of their prognosis should regularly engage in conversations regarding prognosis and goals of care.[14] Unfortunately, these conversations often do not occur until the end of life for patients with DC.[36,43,48,53,59,80–83] Insufficient and delayed discussions likely contribute to receipt of high-intensity EoLC.[84] Conversely, early palliative care referral has been associated with higher rates of goals-of-care discussions in retrospective cohort studies.[58,59] One pilot trial found that provider education and standardized documentation were associated with a 23% increase in advance directive completion and a 51% increase in goals-of-care conversations.[85] Similarly, specialty palliative care consultation with transplant evaluation led to early identification of surrogate decision-maker and ACP documentation.[54] In a single-site pilot RCT, a 5-min ACP video decision support tool for transplant-ineligible patients with DC significantly improved their knowledge about EoLC, informed their preferences for resuscitation and intubation, and was highly acceptable to patients (Table 2).[86]
An expert panel, supported by modified Delphi methods, provided guidance on the timing of ACP in patients with DC.[87] They recommended that advance directives should be completed as early in the course of cirrhosis as possible and preferably before hepatic decompensation and potentially loss of decision-making capacity. Similarly, goals-of-care discussions, with or without the support of specialty palliative care services, should be prioritized when LT is being considered or if death is anticipated within 6 months.[87,88] Resources that can support hepatologists in leading ACP include patient-facing visual aids, communication training, and goals-of-care communication training workshops and webinars (Table S2).[16,89–95]
Guidance statement
-
15
ACP is an iterative process that should start with a diagnosis of cirrhosis and preferably occur before hepatic decompensation and loss of decision-making capacity.
STRUCTURED COMMUNICATION FRAMEWORKS TO SUPPORT COMPLEX CONVERSATIONS AROUND PROGNOSIS AND GOALS OF CARE
Communication skills are a key component of ACP and palliative care. Uncertainty of the illness trajectory in cirrhosis complicates communication about prognosis. Furthermore, care preferences can fluctuate with a patient’s changing clinical status. Clinicians may struggle to effectively communicate the complexity and uncertainty associated with the fluctuating and unpredictable clinical course—indeed, 80% of respondents in a large survey of hepatologists felt that the communication training they had received (in this study, around end-of-life communication) was inadequate.[48] Patients and families struggle to get the information they need or desire, particularly in the setting of critical illness.[84] Several published communication frameworks may be well suited to address the unpredictability associated with cirrhosis (Figure 3).[89–95] Although communication training is associated with improved person-centered outcomes, more work is needed to tailor approaches to training and communication in hepatology settings.[90] The medical team should also ensure that communication occurs in the language preferred by patients and families using a professional medical interpreter, if the preferred language is different than that of the provider.[96]
FIGURE 3.
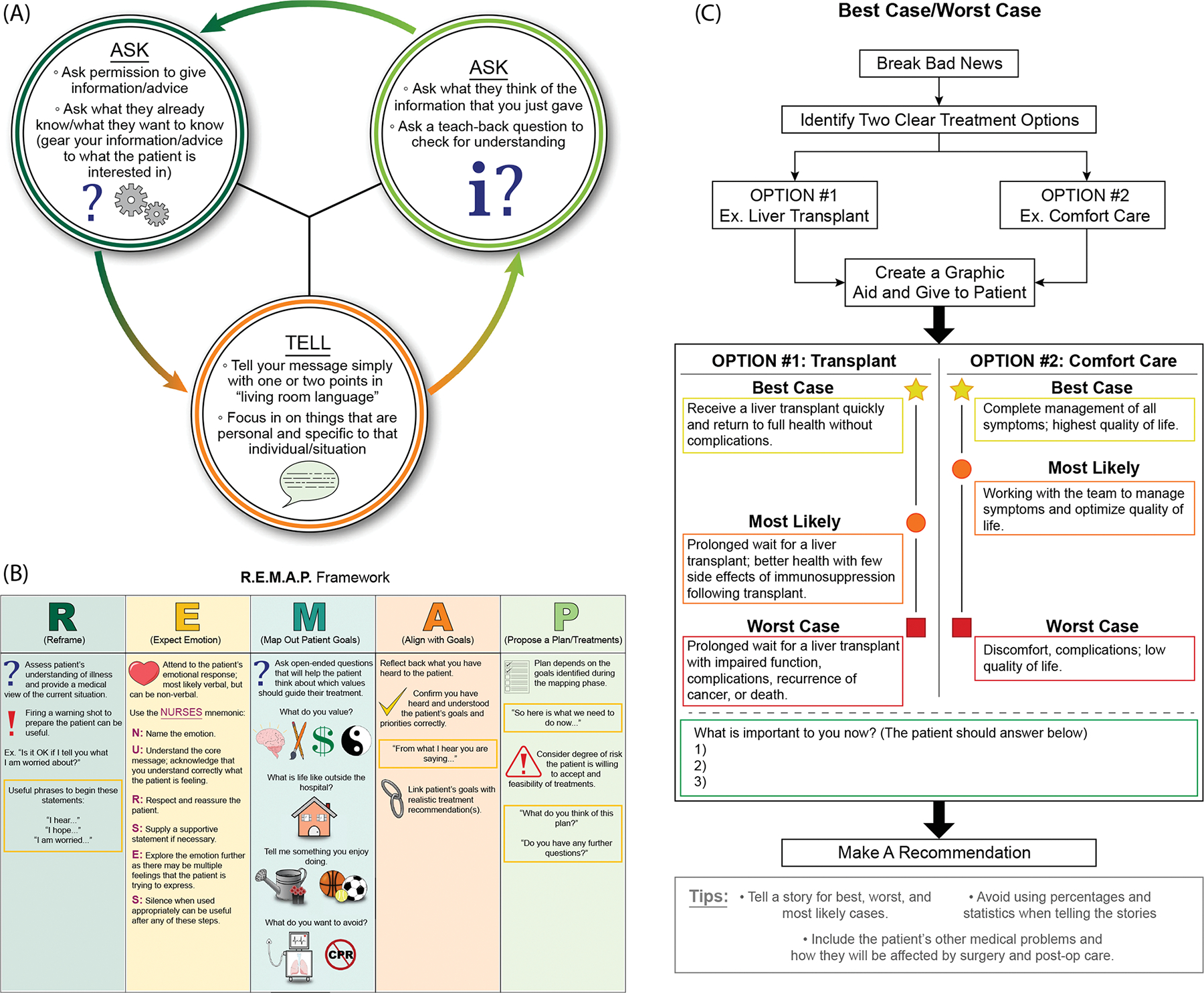
Structured frameworks for goals-of-care conversations. (A) Ask-Tell-Ask is a simple approach that can be used to deliver bad news. (B) The R.E.M.A.P. Framework is a step-wise approach to engaging patients in more-complex decision making. (C) Best-case worst-case provides a way to frame difficult choices, such as the choice to pursue a transplant. References: ©2021 VitalTalk. All rights reserved. VitalTalk content is for informational, educational, and noncommercial use only.[89] Zimmermann et al. 2020; Schwarze et al. 2020; Back et al. 2005; and Childers et al. 2017.[92–95] Readers can learn more at https://www.vitaltalk.org/
Guidance statements
-
16
Structured communication frameworks can be used to communicate uncertainty, discuss serious news, and establish a plan of care that is aligned with patient values.
-
17
Serious illness conversations should occur in the language preferred by the patient and their family. Medical teams should use a professional medical interpreter to facilitate these conversations.
PSYCHOSOCIAL, SPIRITUAL, AND CULTURAL ASPECTS OF PALLIATIVE CARE
Psychosocial, spiritual, and cultural aspects of care are understudied in all patient populations, despite being important to patients.[44] In general, an interdisciplinary, person-centered approach is recommended when addressing these potentially sensitive topics, and it is important to be aware of the local supportive care resources through ongoing conversations with social work, pharmacy, patient navigation, chaplaincy, spiritual care, and pastoral care, where available.[14]
A key component of person-centered palliative care is assessing patient and caregiver psychosocial needs across social determinants of health, including financial stability, employment, food insecurity, housing, transportation, and access for necessary equipment/supplies.[14] Assessing financial burden is also important for patients with DC, who often report high rates of cost-related nonadherence to medications and food insecurity.[31,97,98] The question “Are you having difficulty paying for your medical care?” is an effective early screen for financial burden and engaging social work and pharmacy services to help support patients and their families in mitigating financial stress.[31,97,98] Cirrhosis carries a significant financial burden for patients and families. Patients with DC require a high number of informal caregiving hours each week compared with persons with other illnesses, which can lead to difficulty maintaining employment for family caregivers.[31]
Patients differ in their beliefs and approaches to ACP, palliative care, and death and dying related to their spiritual and cultural frames of reference.[20] The limited relevant spirituality research in cirrhosis includes two qualitative studies that identified spirituality as a key determinant of HRQoL and perceptions of medical care.[99,100] General palliative care guidelines recommend routinely evaluating the spiritual and cultural needs of patients and their families through understanding their preferred language, communication preferences, values, traditions, customs, sources of spiritual strength, existential concerns, fears, and approaches to death and dying.[14] Involving medical interpreters, chaplaincy, and spiritual care providers as appropriate in the care of patients and families may aid in providing culturally sensitive palliative care that also respects their spiritual beliefs and practices and can also help to support caregivers during the time of bereavement.[96] Although chaplains and spiritual care providers are not universally available across health care settings, inviting patients and their families to include religious or spiritual leaders from the community to participate in their care can be helpful in certain scenarios.
Past work has demonstrated racial and ethnic disparities in palliative care and pain management.[101–104] More work is needed to examine the extent to which these differences reflect disparities in health care access versus cultural or religious differences in preferences for care. However, a broader understanding of patients’ spiritual and cultural needs may improve ACP and palliative care delivery to patients with DC from diverse backgrounds.
Guidance statements
-
18
Financial considerations should be assessed when providing palliative care, given that these can contribute to the burden on patients and caregivers.
-
19
Social work referral should be considered for persons with unmet psychosocial needs, whenever available.
-
20
Involvement of chaplaincy, spiritual, or pastoral care from the hospital or community can help to address spiritual or existential distress.
OVERVIEW OF THE APPROACH TO SYMPTOM ASSESSMENT, TRIAGE, AND MANAGEMENT
Symptom management is a core component of caring for patients with DC, who suffer from several, often inter-related, symptoms. Based on a recent systematic review, the most frequently reported symptoms are pain (prevalence range, 30%–79%), breathlessness (20%–88%), muscle cramps (56%–68%), sleep disturbance (insomnia, 26%–77%; daytime sleepiness, 29.5%–71.0%), psychological symptoms (depression, 4.5%–64.0%; anxiety, 14%–45%), and sexual dysfunction (53%–93%).[105] Because often not all symptoms can be addressed in a single clinical encounter, it is important to develop an approach to track and prioritize symptoms. Although a full review of patient-reported outcomes and measurement tools is out of scope for this Guidance, the approach to addressing symptoms may include unstructured to structured questions asking about generic or disease-specific symptoms. Several such instruments have been used in palliative care, including the Functional Assessment of Chronic Illness Therapy-Palliative Care and the Edmonton Symptom Assessment scales.[106,107]
Clinicians should assess symptom severity, exacerbating and relieving factors, and the functional impact of symptom(s) to the patient.[108] Symptoms are experienced through the cognitive and emotional lens of the patient and must be evaluated in the context of the whole patient.[109,110] Therefore, symptom ratings should be interpreted relative to past ratings from the same person over time.[108] Because clinicians may be unfamiliar with the management of certain symptoms, particularly at the end of life, a team approach and early referrals to spcialty palliative care, social work, and other services are important in providing comprehensive palliative care to patients and caregivers.[14]
In the following section, we discuss the most common patient-reported symptoms and their management.[105] Nonpharmacological approaches, such as physical therapy and cognitive behavioral therapy, should be tried when possible, given that these can address multiple symptoms with minimal risk. Pharmacotherapeutics can be challenging in patients with cirrhosis, so a conservative, “start low, go slow” approach is generally recommended. Table 4 highlights common side effects of common classes and types of medications used for palliative care of patients with cirrhosis.[111]
TABLE 4.
Summary of pharmacotherapies for and side effects of the symptomatic treatment of patients with DC[105]
| Medication | Side effects, cautions |
|---|---|
| Nociceptive pain | |
| acetaminophen | Generally safe at low dose (2 gram daily maximum), can cause hepatic failure at high dose |
| Topical NSAIDs | Not tested in patients with cirrhosis (note that systemic NSAIDs are generally avoided in patients with cirrhosis) |
| Lidocaine patch | Site reactions (erythema), petechia, edema, pruritus, nausea, vomiting |
| Capsaicin cream | Site reactions (burning, pain, erythema), limb pain, hypertension |
| Opioids | HE, habit forming, respiratory depression, constipation/obstipation, overdose; preferred are oxycodone and hydromorphone |
| Neuropathic pain | |
| Gabapentinoids | Ataxia, sedation, myoclonus/asterixis, dose adjust in renal impairment, withdrawal syndrome, possible increased viral infections |
| SNRIs | Discontinuation syndrome, nausea, vomiting, sexual dysfunction |
| Tricyclic antidepressant medications | Anticholinergic, orthostasis, drowsiness, weight gain, sexual dysfunction |
| Muscle cramps | |
| Baclofen | HE, confusion, dizziness, sedation, nausea, vomiting, rare neurotoxicity in patients with renal failure, discontinuation syndrome |
| Zinc | Gastric irritation, rare neurological side effects |
| Methocarbamol | Hypotension, bradycardia, dyspepsia, pruritis, confusion, ataxia, HE, headache, sedation, changes in taste, seizure, vertigo, leukopenia, jaundice, changes in vision (dose reduced in cirrhosis) |
| Orphenadrine | Palpitations, tachycardia, confusion, sedation, pruritis, constipation, nausea, vomiting, tremor, urinary retention, blurred vision, central nervous system depression |
| L-carnitine | Side effects common with intravenous formulation; oral formulation generally tolerated well at normal doses |
| Vitamin E | Nausea, diarrhea |
| Taurine | Nausea, dizziness, headache |
| BCAAs | Possible nausea |
| Depression/anxiety | |
| SNRIs | Discontinuation syndrome, nausea, vomiting, sexual dysfunction, rare hepatitis |
| SSRIs | QTc prolongation and seizure risk with citalopram, sedation with mirtazapine, nausea, vomiting, weight gain, sexual dysfunction, insomnia, bleeding risk |
| Benzodiazepines | Physical dependence, sedation, HE, only for short-term use at the end of life in cirrhosis |
| Dyspnea | |
| Opioids | HE, habit forming, respiratory depression, constipation/obstipation |
| Benzodiazepines | Physical dependence, sedation, HE, only for short-term use at the end of life in cirrhosis |
| HE a | |
| Zinc | Gastric irritation, rare neurological side effects |
| l-carnitine | Side effects common with i.v. formulation; p.o generally tolerated well at normal doses |
| Insomnia | |
| Melatonin | Headache, fragmented sleep, confusion |
| Zolpidem | Headache, drowsiness, dizziness, palpitations, anxiety, disorientation, hallucination, use with caution and only in low doses for short time periods in patients with cirrhosis, particularly in the presence of HE |
| Fatigue | |
| Modafinil | Headache, abdominal pain, decreased appetite, chest pain, tachycardia, anxiety, insomnia, confusion, diarrhea, exacerbation of psychiatric symptoms, dose reduction is generally recommended; evidence is poor |
| Methylphenidate | Not studied in cirrhosis, insomnia, headache, irritability, weight loss, anorexia, xerostomia, nausea, tachycardia, hypertension, emotional lability, dizziness, depression, anxiety, nausea, vomiting, diarrhea, abdominal pain, possible increased infection risk; evidence is poor |
| Pruritus | |
| Cholestyramine | Edema, syncope, abdominal pain, anorexia, arthralgia, headache (caution in renal impairment) |
| Antihistamines | Sedation, dizziness, HE, rare QT prolongation, hallucination, headache |
| Nausea, vomiting, dyspepsia | |
| Ondansetron | QTc prolongation, headache, constipation |
| Metoclopramide | QTc prolongation, drowsiness, fatigue, restlessness, dystonic reaction (age related, but can be severe), arrhythmia, hypotension, caution in renal impairment |
| Haloperidol | Increased risk of death in older adults with dementia, extrapyramidal symptoms (e.g., dystonia, akathisia, and tardive dyskinesia), aspiration risk, cytopenias, hyperprolactinemia, neuroleptic malignant syndrome, metabolic derangements, QTc prolongation, seizures, sexual dysfunction |
| Medical cannabinoids | Psychosis, encephalopathy, ascites, hyperemesis |
| Antihistamines | Sedation, dizziness, HE, rare QTc prolongation, hallucination, headache |
| Proton pump inhibitors | Increased infection risk in cirrhosis, abdominal pain, diarrhea, nausea, dizziness, headache, rash |
| H2 antagonists | Dizziness, delirium, confusion, agitation, headache, change in bowel habits |
| Erectile dysfunction | |
| Tadalafil | Dyspepsia, headache, caution if encephalopathy or low blood pressure |
Note: Lexicomp.com used for drug information.
Alternatives to standard treatments.
Guidance statements
-
21
A wide range of symptoms co-occur in patients with DC, and addressing these symptoms is a key component of high-quality cirrhosis care.
-
22
For patients with DC, following general palliative care principles, we recommend systematically evaluating the presence and severity of a wide range of symptoms and addressing the symptoms most important to patients.
-
23
Often, the first approach to symptom management may be nonpharmacological, such as behavioral intervention, physical therapy, or other modalities that address multiple symptoms.
-
24
Underlying causes of symptoms should be identified and managed first.
-
25
Symptom management should consider best practices, disease stage, and patient goals and preferences.
-
26
Evaluation and management of symptoms should be interdisciplinary, when possible, including nursing, social work, and chaplaincy.
Pain
The etiology of pain in persons with cirrhosis can be divided into liver-associated mechanical pain, inflammatory pain, and non-liver-associated pain. Cirrhosis can directly lead to somatic or nociceptive pain through splenomegaly, ascites, and hepatic capsular stretch or indirectly because of elevation of proinflammatory cytokines.[112–117] Nonhepatic etiologies of pain can range widely, but the most common are neuropathic (e.g., diabetic neuropathy) and musculoskeletal (e.g., osteoarthritis).[113]
Table 5 summarizes the general approach to chronic pain management for patients with cirrhosis, which can be divided into pharmacological and nonpharmacological approaches. The first step in addressing pain is to assess and treat reversible causes (e.g., tense ascites, local infection, and musculoskeletal injury). The chronicity of pain also determines the approach. For example, acute pain (≤12 weeks) is more responsive to short-term opioid therapy than chronic pain.[118] Optimal chronic pain management often involves multimodal, nonpharmacological approaches, including behavioral management, physical therapy, and procedural approaches.[119,120] As such, more-complex, chronic pain (e.g., pain refractory to conservative management, of unclear etiology, or with associated symptoms) may require consultation with palliative care specialists or experts in procedural pain management.
TABLE 5.
Palliative management of pain for patients with DC
| The management of pain is complex and requires treatment of other contributing symptoms (e.g., sleep disorders, depression). Multidisciplinary approaches are often beneficial |
| Nonpharmacological options |
| Hot/cold |
| Physical therapy |
| Mindfulness/meditation |
| Other behavioral pain self-management strategies (e.g., cognitive behavioral therapy) |
| Acupuncture (caution if platelets <50,000) |
| Other complementary options based on preferences (e.g., transcutaneous nerve stimulation) |
| Pharmacological options Topical/injection treatments |
| Lidocaine patches Capsaicin cream or patch |
| Topical nonsteroidal anti-inflammatory medications (e.g., diclofenac sodium 1% gel) |
| Injections by pain specialists (e.g., osteoarthritis of knee) |
| Systemic therapies |
| APAP 500 mg q6h for a maximum of 2 g/d is safe in most patients with. |
| Gabapentin 300 mg daily (starting dose) or pregabalin 50 mg b.i.d. (starting dose)a (for neuropathic pain) |
| Fentanyl patch 12-μg starting dose (typically not recommended as the initial agent; avoid in patients with sarcopenia/cachexia or fever) |
| Hydromorphone 1-mg q6h prn starting dose |
| Oxycodone 2.5-mg p.o q6–8h prn starting dose |
Note: Once the goals of care are focused on comfort, opioid medications should be titrated up to meet the patient’s needs without concerns for long-term impacts.
Abbreviations: b.i.d., twice a day; d, day; h, hour; q, every; g, grams; prn, as needed.
Renal dosing adjustments needed; cannot be stopped without tapering; can cause nausea, sedation, and ataxia.
Behavioral approaches to pain management have not been specifically evaluated in patients with cirrhosis, but pain self-management programs, cognitive behavioral therapy, and physical and occupational therapy are safe and efficacious in other populations for the management of chronic pain.[121–123] Procedural approaches have been largely untested in cirrhosis, but acupuncture is efficacious for chronic musculoskeletal pain and headache in general populations.[124,125] Although there are no direct data about the safety of acupuncture in patients with cirrhosis, observational studies of drug-related thrombocytopenia indicate that acupuncture is likely safe in patients with platelets >50,000/ml, and a recent review evaluated the potential benefits of acupuncture for cirrhosis more generally.[126,127] Similarly, injections can be used for limited indications, such as knee osteoarthritis, though physical therapy remains first line.[128]
Because of impaired hepatic metabolism and the risk of precipitating encephalopathy, lower doses and less systemic therapies are generally preferred for pharmacological management of chronic pain in patients with cirrhosis.[112,120,129,130] Local pharmacotherapies are generally first line for localized pain. These can include injections, as discussed previously, or topical creams or patches. Although there are no data supporting the use of topical analgesics in patients with cirrhosis, they are generally considered safe and have demonstrated efficacy in other populations. Lidocaine patches are applied directly to a site of discomfort, and topical creams for localized neuropathic (e.g., capsaicin) and musculoskeletal pain (e.g., topical nonsteroidal anti-inflammatory drugs [NSAIDs]) are effective and have limited systemic absorption, rendering them likely safe (though untested) in persons with cirrhosis.[131–135]
Acetaminophen (APAP) up to 2 g daily (typically prescribed as 500 mg, every 6 hours, as needed) is generally safe and should be the first-line systemic pharmacological therapy for pain in this population.[112,120,136] However, patients should be informed that other over-the-counter and prescription formulations may include APAP, which counts toward the daily limit.[137] NSAIDs are among the most commonly inappropriately used medications in patients with cirrhosis.[138,139] Multiple studies demonstrate the deleterious impact of NSAIDs in patients with cirrhosis attributable to risk of renal injury, bleeding, and ascites.[112,120,136,140] One trial of 23 patients with cirrhosis demonstrated that receiving five doses of 500 mg of naproxen (vs. placebo or celecoxib) was associated with significantly worsened renal function, suppression of furosemide responsiveness, and inhibition of platelet aggregation and thromboxane production.[141] Thus, systemic NSAIDs should be avoided in patients with cirrhosis.
Although much has been written regarding analgesic side effects in cirrhosis, a more nuanced approach to analgesia may be warranted, particularly when comfort is prioritized over disease-directed care.[120,130,136] Although opioids are associated with increased pain-related disability, encephalopathy, ascites, post-transplant mortality, and readmission in patients with cirrhosis, patients at the end of life may accept these risks and prioritize short-term analgesia over cognition.[113,116,142–144] That said, chronic opioid medications are not effective for the management of chronic pain, and patients with LD are among the group with the highest risk of opioid-related adverse events.[145–147]
When opioids are required, there are several notable safety considerations. First, prophylactic medications should be considered proactively to prevent constipation and encephalopathy (e.g., lactulose).[112,120] Opioids have unique pharmacodynamics, such that codeine, morphine, and tramadol should generally be avoided in patients with cirrhosis.[129] Tramadol is often inappropriately selected for first-line use in patients with cirrhosis because of the perception that it is not an opioid.[116] However, tramadol is an opioid that requires first-pass hepatic metabolism, has variable pharmacokinetics across persons with cirrhosis, is challenging to titrate, and has notably unpredictable side effects, including hypoglycemia.[148] Although i.v. fentanyl is a preferred opioid analgesic in persons with cirrhosis because of its favorable metabolism profile, outpatient use is limited by its delivery in this population. The patch comes in set doses with the lowest outpatient dose of a 12-μg/h patch, which may be too high for patients with cirrhosis. Furthermore, cachexia is a relative contraindication to transdermal fentanyl, rendering it impractical to use in many patients with cirrhosis.[149] Because of a long and variable half-life and multiple drug interactions, methadone should only be used in consultation with a specialist.[129] First-line opioids for patients with cirrhosis generally include low-dose hydromorphone (e.g., 1 mg p.o or 0.4 mg i.v.) or oxycodone (e.g., 2.5 mg p.o.) with extended dosing intervals.[129]
Prescribing clinicians should adhere to best practices for risk assessment, setting patient expectations, and monitoring when starting opioids.[118] For example, first-line treatment should be a 7-day supply of low-dose, short-acting opioids. Patients require close follow-up, and risk factors for adverse opioid-related events should be assessed (e.g., past opioid use disorder, concurrent benzodiazepine use, or other opioid prescriptions in the prescription drug monitoring program data).[118] Conversations with patients and caregivers should include information about risks and benefits and setting of expectations (e.g., taking medications as prescribed, proper disposal).
Other analgesics have potential benefits as low-dose adjuvants, but have notable risks in persons with cirrhosis. Gabapentinoids are effective in treating neuropathic pain, though they are increasingly recognized as potentially habit forming and are associated with somnolence and mental status changes.[150] For patients with cirrhosis, very low starting doses of gabapentin (e.g., ≤300 mg/d) are recommended. Serotonin-norepinephrine reuptake inhibitors (SNRIs) can provide good adjuvant pain medication at low doses, though there is a low risk of hepatotoxicity.[111,151] Although tricyclic antidepressants are often used as adjuvant analgesia, their anticholinergic effects and associated mental status impairments generally preclude their use in patients with cirrhosis.[111,152]
Medical cannabinoids, which have notable central nervous system and gastrointestinal side effects, are U.S. Food and Drug Administration (FDA) approved for the treatment of nausea, vomiting, and anorexia.[153] Cannabis (marijuana), which is often used for pain management, is not FDA approved for any indication at this time. Although early data suggest that cannabinoids may be effective as short-term adjuvant treatment for chronic neuropathic pain, there is not strong enough evidence to recommend cannabis for the treatment of chronic noncancer pain, particularly in patients with DC.[154–157] Existing studies of cannabinoids for analgesia have excluded patients with comorbid illnesses (e.g., cirrhosis) or a history of substance use disorders and have not included optimal outcome measures.[153] Thus, significantly more data are needed before recommending cannabinoid/cannabis as an analgesic for patients with DC.
Guidance statements
-
27
Multimodal pain management approaches are ideal and include a person-centered holistic, multidisciplinary approach, engaging a combination of expertise from across a number of specialties (e.g., palliative care, psychiatry, pain management, pharmacy, physical and occupational therapy, or social work).
-
28
Pain in patients with DC requires a systematic approach that starts with assessing and treating reversible causes of pain (e.g., ascites, local infection, or musculoskeletal injury).
-
29
Localized pain (e.g., knee osteoarthritis) should first be addressed with local, rather than systemic, therapies.
-
30
Acetaminophen, 500 mg every 6 h, up to a maximum dose of 2 g/d, is the preferred first-line pharmacotherapy for the management of pain in patients with cirrhosis.
-
31
Systemic NSAIDs should be avoided in patients with cirrhosis.
-
32
We recommend avoiding opioids, when possible, for chronic pain. However, when necessary, opioid use should be approached with caution and with careful discussion with patients and caregivers. Low-dose oxycodone or hydromorphone can be started in select cases on an as-needed basis and titrated to effect, often in consultation with pain management experts.
ABDOMINAL DISTENSION ATTRIBUTABLE TO REFRACTORY ASCITES
Ascites, the most common complication of cirrhosis, results from renal sodium retention, and specific recommendations in this population, including a large volume paracentesis (LVP), are covered in the relevant AASLD Practice Guidance.[6] LVP requires multiple visits to the hospital and can be burdensome, especially toward the end of life. TIPS is an option, but many patients with refractory ascites are not candidates for the placement of TIPS because of encephalopathy, cardiac contraindications, or high MELD scores.[6] Recent studies have evaluated the feasibility and safety of longer-term abdominal drains and automated low-flow ascites pumps in patients with refractory ascites.
Indwelling peritoneal drains or catheters have been evaluated for the palliative management of refractory ascites. A recent review of 18 studies including 176 patients summarized the evidence for long-term ascites drains.[158] Most catheters were placed for palliation in patients who were not candidates for LT. Patients’ MELD scores ranged from 10 to 22 in these studies, and most patients received prophylactic antibiotics either at the time of insertion or during follow-up. In these studies, technical success was 100% and rates of noninfectious complications were generally low (<12%). Spontaneous bacterial peritonitis (SBP) occurred in 0%–42% of patients across studies, with an overall combined rate of 17%. Most episodes of SBP were treated with antibiotics with the catheter left in situ. Other complications included cellulitis at the catheter insertion site (6%), transient hyponatremia (11%), and increased serum creatinine (8%).[158] A 12-week, small feasibility, nonblinded RCT compared LVP to long-term abdominal drains in 36 transplant-ineligible patients with refractory ascites.[159] Outpatient drain insertion was performed using ultrasound guidance, and participants were maintained on chronic antibiotics. There were no drain-related serious adverse events or drain removals attributable to complications. Patients in the LVP group had more than double the ascites-related hospital time than the drain group. Self-limiting cellulitis/leakage occurred in 41% in the drain group versus 11% in the LVP group; peritonitis incidence was 6% versus 11%, respectively. Overall attrition was high (42%), mostly attributable to death (7 of 16 in the abdominal drain group and 5 of 18 in the LVP group). At the end of the study, all surviving drain participants elected to retain the drains and reported benefits, including improved symptom control and increased time at home, whereas the LVP group reported frequent trips to the hospital with lengthy wait times and a complex care path.[160] However, these differences were not borne out in quality-of-life measures in this small study.[160] The study demonstrated feasibility, but was not powered to assess clinical or patient-reported outcomes. Thus, although drains can be considered in TIPS-ineligible patients with comfort-focused goals, more study is still required.
Automated low-flow ascites pumps (alfapumps), which are not yet available in the USA or Canada, use a an s.c.-implanted, battery-powered pump to divert ascites into the bladder for urinary elimination. A recent systematic review of nine studies included 206 patients treated with this device.[161] After pump insertion, 48% of patients continued to require LVP, and adverse events occurred in >75% of patients. Acute kidney injury (AKI), urinary tract infection, and SBP occurred in 30%, 20%, and 27% of patients, respectively. An RCT comparing LVP to low-flow pumps found that abdominal symptoms and activity scores improved significantly only in the low-flow pump group. However, there were also significantly increased risks of adverse events (96.3% vs. 77.4%) and serious adverse events (85.2% vs. 45.2%) in the pump group.[162] Another study of low-flow pumps in 30 TIPS-ineligible patients identified improvements in HRQoL and nutritional status with no AKI episodes in the first week postinsertion.[163] However, in this study, patients were maintained on prophylactic antibiotics and had relatively preserved hepatic function (average MELD = 11). Finally, a case series of 21 patients who received low-flow pumps reported that 71% of patients had pump complications and only 3 patients (14%) remained enrolled at a median of 153 days because of attrition from death.[164] Thus, there are insufficient data to recommend the use of low-flow ascites pumps in patients with DC at the end of life.
Guidance statements
-
33
Abdominal drains may be an alternative to serial LVP for patients with refractory ascites who are transplant and TIPS ineligible and whose goals are comfort focused. However, more comparative effectiveness research is needed before recommending this approach.
Dyspnea
Dyspnea, or the subjective experience of breathlessness, is experienced by 47%–88% of persons with cirrhosis and can be attributed to multiple causes, including ascites, volume overload, hepatopulmonary syndrome, portopulmonary syndrome, and infection.[35,165,166] Patients close to the end of life may experience dyspnea associated with diuretic-refractory fluid accumulation, worsening acidosis, progressive renal failure, and anxiety.[166–169] Dyspnea can be both distressing and debilitating and thus requires a multidisciplinary management approach.[170] Patients experience dyspnea as difficulty with air movement, effort, rapidity, or overall distress, but clinicians may need to use surrogate markers, such as hypoxia, tachypnea, and use of accessory respiratory muscles, in patients who are unable to report their symptoms.[171]
Within palliative care literature, the focus of dyspnea management centers around pulmonary, cardiac, and malignant conditions; in contrast, there is a lack of direct evidence in patients with cirrhosis.[172] General principles suggest that dyspnea should be approached by first assessing and addressing underlying causes, balanced with patient preferences about their care. For example, some patients with a shorter prognosis may nonetheless prefer more-intensive therapies whereas others may prefer to avoid hospitalizations, where possible, and focus on comfort. Disease-directed therapies may be used as appropriate, including diuresis, bronchodilators, phosphodiesterase inhibitors, and steroids.[172,173] Procedural interventions may include paracentesis, thoracentesis, and TIPS.[173,174] Indwelling abdominal drains may be used in some cases to address volume-related dyspnea when goals are comfort focused (see the section above, Abdominal Distension Attributable to Refractory Ascites).[159,160]
Given the general principle of minimizing sedating medications in patients with cirrhosis, the approach to the palliative management of dyspnea (Table 6) begins with nonpharmacological treatments, such as supplemental oxygen (even in the absence of hypoxia) or a bedside fan.[175,176] In the USA, patients must meet specific clinical criteria to have insurance coverage for home oxygen; however, those on hospice have more liberalized coverage of oxygen in the home. Self-care interventions like relaxation, meditation, and guided imagery may decrease dyspnea and result in better symptom-related quality of life.[177]
TABLE 6.
Summary of therapies for palliative management of dyspnea, muscle cramps, pruritus, and nausea
| Symptom | Nonpharmacotherapies | Pharmacotherapies |
|---|---|---|
| Dyspnea | • Manage reversible causes (e.g., volume overload, asthma, sleep apnea) • Bedside fans • Supplemental oxygen therapy • Mindfulness, meditation, guided imagery • Paracentesis • Thoracentesis • Placement of drains (usually in the setting of hospice care) |
• Opioids can be used cautiously in select cases, typically at the end of life (example: starting dose i.v. hydromorphone 0.2 mg every 3 h as needed, titrated to symptom relief) • Anxiolytics can be considered for dyspnea-associated anxiety (typically at the end of life when focus of care is comfort) |
| Muscle cramps | • Correct electrolytes | • Taurine (2–3 g daily) • Vitamin E (300 mg three times a day) • Baclofen (5–10 mg three times a day) |
| Pruritis | • Moisturizing creams • Avoid hot baths and harsh soaps and detergents • Use loose-fitting clothing • Cool humidified air |
• Cholestyramine (4 g/d, titrated to 16 g/d if needed) • Sertraline (25 mg/d, titrated to 75–100 mg if needed) • RIF and naltrexone may improve pruritus, but their use is limited in palliative treatment of patients with DC. • Antihistamines (e.g., diphenhydramine and hydroxyzine) may help with pruritis-associated sleep disturbance given their sedating properties |
| Nausea and vomiting | • Correct electrolytes • Evaluate and treat adrenal insufficiency • Manage constipation • Review medications and eliminate potential triggers (e.g., lactulose, opioids) • Ginger • Mindfulness, relaxation • Acupuncture (use caution if platelets <50,000) |
• Antacids (if contributing reflux) • Ondansetron, up to 8 mg/d is preferred • Metoclopramide up to 60 mg/d (very preliminary safety data; potential adverse reactions) • May consider alternatives (e.g., prochlorperazine, haloperidol) depending on goals of care |
The mainstays of pharmacological management of dyspnea at the end of life are low-dose opioids and benzodiazepines.[178,179] Given the risk of potentiating encephalopathy, opioids or benzodiazepines should be used with caution, with careful discussion of risks and benefits.[111] Because morphine, which is often used for breathlessness, is relatively contraindicated for patients with advanced cirrhosis, clinicians should first consider hydromorphone or oxycodone, as detailed in the Pain section above.[120,129]
Guidance statements
-
34
Patients should routinely be assessed for the presence of dyspnea. The impact of dyspnea on patient quality of life and function as well as on caregivers should be evaluated.
-
35
Nonpharmacological therapies should be used to manage dyspnea when possible and include the use of a fan, supplemental oxygen (even for non-hypoxic patients), and mindfulness exercises.
-
36
Pharmacological interventions for dyspnea may include opioids and anxiolytics, which may be used with careful consideration of risks, patient goals, and prognosis.
HE
HE is a common symptom in patients with cirrhosis, with neuropsychiatric manifestations including disturbances in sleep, mood, wakefulness, and cognition.[7] Patients with encephalopathy and their caregivers face profound psychological, physical, and financial burdens. Because the medical management of encephalopathy is covered in detail in AASLD guidelines, we limit this discussion to the unique palliative aspects of encephalopathy management.[7]
Studies have found that patients with HE and their caregivers experience burdens that can be alleviated by education about medication administration, dose titration, and the waxing and waning course of encephalopathy.[25,180] The development of encephalopathy may be an opportunity for hepatology clinicians to evaluate the needs of a patient/caregiver as a unit and augment supportive care services with consideration of referral to social work, additional care coordination, or home health support. The cognitive impairment associated with encephalopathy underscores the importance of ensuring that a surrogate decision maker is identified and documented before the onset of encephalopathy, as discussed under ACP.
Although rifaximin (RIF) and lactulose are standard for encephalopathy management, as persons approach the end of life, patient preferences and acceptability of treatment side effects may change. For example, lactulose is associated with bloating, abdominal pain, and diarrhea, and some patients or caregivers may prefer using polyethylene glycol to prevent constipation or discontinuing treatment to avoid incontinence at the end of life. Because RIF may not be available in some hospice settings because of its high cost and the capitated payment model of hospice, off-label neomycin or metronidazole may be considered as an alternative to RIF.[7] Also, zinc repletion, branched-chain amino acids (BCAAs), probiotics, and, possibly, carnitine can be offered as adjuvant therapies.[7,181–183]
Guidance statements
-
37
Evaluating reversible causes and addressing HE can benefit both patient and caregiver quality of life.
-
38
Onset of encephalopathy can be an opportunity to provide education, elicit preferences, and discuss the overall trajectory of LD with a focus on ACP.
-
39
Approaches to the treatment of encephalopathy may depart from standard care at the end of life to align with patient goals and values.
Muscle cramps
Muscle cramps are common in cirrhosis and negatively impact HRQoL.[105,184] Although the precise etiology of muscle cramps is unclear, alterations in nerve function, energy metabolism, plasma volume, and electrolytes may contribute.[184–186] Muscle cramping associated with cirrhosis is often spontaneous, intermittent, and nocturnal.[187] In patients with new onset of persistent pain, other disorders, such as rhabdomyolysis, myositis, or kidney injury, should be considered.[187] Although there are mixed data regarding associations between hypokalemia, hypomagnesemia, and zinc deficiency with muscle cramps, correcting such deficiencies is recommended, given the low risk of repleting electrolytes.[186,187]
BCAAs (one sachet three times a day), taurine (2–3 g daily), and vitamin E (200 mg three times a day) have been evaluated in patients with cirrhosis. A multicenter RCT of daytime versus nocturnal BCAA supplementation in 37 patients with cirrhosis found that there was a significant decrease in cramping in both groups without adverse effects of treatment, though this agent is not yet widely used in practice.[188] Several small, open-label studies have evaluated taurine as a treatment for muscle cramps in cirrhosis, with resolution of cramps in 30%–40% of patients over 4 weeks.[189–191] These findings were confirmed in a small RCT, in which patients treated with 2 g of taurine daily experienced a reduction in cramp frequency, duration, and severity compared with placebo.[192] Vitamin E has had mixed effectiveness in treating cramps in small studies of patients with cirrhosis. Although a small, open-label study found significant improvement in cramping with vitamin E at 200 mg three times a day used over 4 weeks, a subsequent pilot randomized, crossover study of 9 patients with cirrhosis found no significant improvement over placebo.[193,194] In the absence of larger efficacy trials, these agents can be considered, given their favorable safety profiles (Table 4).
Muscle relaxants, such as baclofen, methocarbamol, and orphenadrine, have all demonstrated efficacy in short-term trials of patients with cirrhosis. Baclofen (30 mg/d) led to at least partial resolution in 92% of users after 3 months.[195] Baclofen can be started at 10 mg daily (typically given at night) with a weekly increase of 10 mg/d up to a maximum daily dose of 30 mg (10 mg three times a day). Methocarbamol was associated with significantly decreased frequency and duration of cramps in 100 patients with hepatitis C–related cirrhosis, who experienced minor side effects of dry mouth and drowsiness.[196] Orphenadrine improved muscle spasms in patients with cirrhosis after 4 weeks of treatment.[197] However, the long-term effectiveness of these agents requires further evaluation.
Small studies have demonstrated preliminary effectiveness for albumin infusion, L-carnitine, and zinc in managing muscle cramps. Intravenous albumin (100 ml of 25% albumin) used once-weekly for 4 weeks significantly decreased cramp frequency and improved mean arterial pressure in patients with cirrhosis in a small crossover study, though this is a less-feasible solution for most patients.[184] L-carnitine at the dose of 300 mg, three to four times a day for 8 weeks, was associated with improvement in symptoms in a recent study.[198] Although zinc levels are lower in patients with cirrhosis versus healthy controls, zinc level is not directly correlated with muscle cramps in this population.[199] However, oral zinc sulfate 220 mg twice-daily for 12 weeks significantly decreased cramp frequency.[200] Recommendations for the management of muscle cramps are summarized in Table 6.
Guidance statements
-
40
Checking serum electrolyte levels and repleting potassium, magnesium, and zinc is a first step in the management of muscle cramps in patients with DC.
-
41
Taurine (2–3 g daily), vitamin E (200 mg three times a day), and baclofen (5–10 mg three times a day) have preliminary supportive data and can be considered in patients with cirrhosis and significant muscle cramps.
Sleep disturbances
Sleep disturbances, which include insomnia (poor sleep quality), excessive daytime sleepiness, and sleep-wake inversion, affect 50%–80% of patients with cirrhosis.[105,201–203] Although encephalopathy is associated with sleep-wake inversion and daytime sleepiness, up to half of patients with cirrhosis without encephalopathy experience sleep disturbances, associated with melatonin metabolism changes, immune mechanisms, and impaired thermoregulation, which may disturb circadian rhythms.[202,204–208] Patients with cirrhosis also have a higher prevalence of sleep-related movement disorders, such as restless leg syndrome, compared with the general population.[202] Complications of LD, such as ascites and edema, and disease-specific symptoms (e.g., pruritus in primary biliary cholangitis, obstructive sleep apnea in patients with metabolic-associated fatty liver disease) may also contribute.[209,210] Sleep disturbances are associated with fatigue, depression, anxiety, and poor quality of life in patients with cirrhosis.[201,203,204]
The evaluation of sleep disturbances in cirrhosis should include an assessment of sleep-wake timing, nighttime sleep quality, and daytime sleepiness.[204] Clinicians should perform a thorough assessment of the timing of physical activity, meals, and medications to promote good sleep hygiene; for example, late administration of diuretics and lactulose during the evening hours may lead to nocturnal awakenings and contribute to poor sleep.[204] Encephalopathy, sleep apnea, restless leg syndrome, and pruritus should be treated, if present, because these conditions can impair sleep.[204,211,212]
There are several potential treatments for patients with cirrhosis who suffer from sleep disorders (Table 6). Evidence-based behavioral treatments for primary sleep disorders in general populations include cognitive behavioral therapy for insomnia, which has little downside.[213] In patients with cirrhosis, a 4-week trial of mindfulness-based stress reduction led to significantly improved sleep quality.[61] Light therapy has also been evaluated. Among 12 inpatients with cirrhosis and significantly impaired sleep, light therapy did not impact sleep quality; however, because light therapy is low risk and helpful in other populations, some researchers still favor a trial of light therapy in outpatients with cirrhosis.[202,214,215]
There are few pharmacological options for sleep disturbance in patients with cirrhosis, especially those with DC, beyond agents that treat contributing encephalopathy. Melatonin 3 mg nightly for 2 weeks and zolpidem 5 mg nightly for 4 weeks have each been tested in randomized trials of patients with Child-Pugh A and B cirrhosis and found to be effective for improving sleep quality, but these medications have not been trialed in more-advanced disease or for longer duration.[216,217] Despite these preliminary data, zolpidem should generally be avoided in patients with DC, because of impaired hepatic clearance, minimal data, and potentiation of encephalopathy.[111] Hydroxyzine 25 mg at bedtime was tested (vs. placebo) in a trial of 35 patients with cirrhosis and minimal encephalopathy, with significantly improved subjective and objective sleep measures in the hydroxyzine group.[218] Benzodiazepines should generally not be used for sleep in this population given the risk for sedation, respiratory depression, cognitive impairment, and falls.[139,219] However, there are potential, limited indications for benzodiazepines in this population at the end of life, as discussed in the Dyspnea section above.
Guidance statements
-
42
Clinicians should first evaluate and treat underlying causes for insomnia such as HE, pruritus, obstructive sleep apnea, and restless leg syndrome.
-
43
Clinicians should perform a thorough assessment of the timing of physical activity, meals, and medications to promote good sleep hygiene.
-
44
Mindfulness-based stress reduction therapy and cognitive behavioral therapy approaches can be considered in patients with cirrhosis and disordered sleep.
-
45
Short-term use of melatonin 3 mg or hydroxyzine 25 mg nightly can improve sleep quality in patients with Child-Pugh A and B cirrhosis, but data on long-term use of these medications are limited.
-
46
Although chronic use of benzodiazepines should be generally avoided in patients with DC, specific clinical circumstances may warrant their use, such as anxiety at the end of life when comfort is the stated priority.
Fatigue
Fatigue is among the most frequent, debilitating, and distressing symptoms in patients with cirrhosis, with a prevalence of 50%–86%.[105,220] Fatigue is a complex, subjective sensation, with complex physiological, psychological, behavioral, and social underpinnings.[220] LD is associated with disturbances in homeostasis, inflammation, and metabolism that can contribute to central and peripheral fatigue.[221] Central fatigue develops as a result of changes in neurotransmission, whereas peripheral fatigue is associated with neuromuscular dysfunction that results in muscle wasting and weakness.[222] Fatigue has been associated with disease severity, ascites, encephalopathy, level of physical activity, poor sleep quality, decreased quality of life, mood disturbance, anxiety, depression, and job performance in cirrhosis and with age, sex, diet, mental status, and personality type in general populations.[220,223–228] The evaluation of fatigue should include a comprehensive assessment of other potential contributing factors, such as hypothyroidism, depression, adrenal insufficiency, anemia, vitamin deficiency, and medication side effects (e.g., beta-blockers).[222,226,229,230]
Nonpharmacological management of fatigue includes education, recommendations about energy-conserving and -restoring activities, and referral to physical and occupational therapy. A structured four-component behavioral approach, called Treat, Ameliorate, Cope, and Empathize, focuses on treating what is treatable, addressing ameliorating factors (e.g., sleep disturbances), providing coping skills, and empathizing with patients.[230,231] Several studies in patients with Child Class A and B cirrhosis have found that physical exercise can reduce fatigue.[227,232,233] Stimulants, including modafinil and methylphenidate, have been successfully used for fatigue in other populations.[234,235] However, although modafinil was successfully used in patients with HIV and HCV coinfection and initial open-label trials in primary biliary cholangitis were promising, an RCT of modafinil in patients with primary biliary cholangitis found no significant impact on fatigue.[229,236,237] Thus, there are not data to support the use of stimulants in patients with DC.
Guidance statements
-
47
A multidisciplinary approach to addressing fatigue includes evaluation and treatment of contributing factors (e.g., encephalopathy, hypothyroidism, adrenal insufficiency, depression, and medications), providing behavioral education, and recommending physical activity.
-
48
There are insufficient data to support the use of stimulants to treat fatigue in patients with cirrhosis.
Pruritus
Pruritus is a common symptom in patients with cirrhosis of all etiologies, although it disproportionately affects patients with cholestatic LDs. As such, most of the existing studies have been conducted in patients with primary biliary cholangitis and other cholestatic LD.[238,239] Although the pathophysiology of pruritus is incompletely understood, the presentation of pruritus varies in distribution, frequency, and correlation with the severity of LD. Initial treatment includes moisturizing creams, avoidance of hot baths and rubbing of the skin, minimizing use of harsh soaps and detergents, and using loose-fitting clothing and cool humidified air (Table 6).[240] Antihistamines, which are often used for pruritus, may have sedating effects or exacerbate encephalopathy and are generally not efficacious for pruritus attributable to cholestasis.[238] However, the sedating properties of antihistamines may render them helpful for addressing pruritus-mediated sleep disturbances, and antihistamines are efficacious for certain skin conditions (e.g., urticaria).[238,241]
Pharmacological treatments for cholestasis-associated pruritus include cholestyramine (or colestipol), RIF, naltrexone, and selective serotonin reuptake inhibitors (SSRIs), as presented in Table 6. Cholestyramine, a bile acid sequestrant, was shown to be effective in randomized studies with small numbers of patients (n = 8 and n = 10).[242] It is generally considered as the first-line treatment for pruritus; however, given the potential for drug interactions, consultation with a pharmacist and thoughtful spacing from other medication administration are often required. The recommended starting dose for cholestyramine is 4 g/d, with titration up to 16 g/d as needed and tolerated.[238] An alternative to cholestyramine use is colestipol, which some patients may find more palatable.
A meta-analysis of five trials showed that RIF was effective in treating chronic pruritus.[243] Although safe as short-term treatment, longer-term use of RIF can be associated with hepatotoxicity.[152] Therefore, RIF should be initiated at a quarter dose and titrated slowly to 150–300 mg twice-daily with careful monitoring of liver biochemistries. RIF should not be used in patients with bilirubin levels >2.5 mg/dl, making it impractical for many patients with DC.[238,244]
Naltrexone, an opioid antagonist, was effective in treating cholestatic pruritus in prospective placebo-controlled trials.[244–248] Naltrexone for this indication requires a low dose, starting with 12.5 mg daily and up-titrating every 3–7 days to reach the maximum dose of 50 mg/d.[246,247] Although uncommon, naltrexone-related hepatotoxicity can occur and necessitates close follow-up of liver biochemistries.[152] Naltrexone use is also often inappropriate in persons receiving opioids for pain because it can block opioid binding, thus reducing analgesia and potentially causing opioid withdrawal.[249]
Sertraline, an SSRI, has been shown to reduce cholestatic pruritus symptoms at 75-to 100-mg doses.[250–252] Sertraline was well tolerated and showed moderate antipruritic effects in a randomized trial with a small number of patients with liver-associated pruritus.[250] Notably, the antipruritic effect was independent from an improvement in depression.[250] Because sertraline is largely metabolized in the liver, it requires careful administration (e.g., a starting dose of 25 mg with titration) in patients with DC. Paroxetine, another SSRI, may also be beneficial for pruritus, and other agents, such as ileal bile acid transporters, are undergoing investigation.[249,253]
Guidance statements
-
49
Our suggested approach to pruritus in patients with DC includes starting with nonpharmacological options, including using moisturizing creams, avoiding hot baths and harsh soaps, and using loose-fitting clothes and cool humidified air.
-
50
Cholestyramine (4 g/d with titration to 16 g/d) is first-line treatment for pruritus.
-
51
Alternative agents include low-dose naltrexone, RIF (in anicteric patients), and sertraline, but these agents require careful titration in the context of DC.
Sexual dysfunction
Recent AASLD guidelines address reproductive concerns for women with cirrhosis, so we have focused on the palliative aspects of sexual dysfunction management for patients with DC.[254] Approximately two thirds of men and 80% of women with cirrhosis suffer from sexual dysfunction, which leads to impaired HRQoL.[255–257]
Sexual function is one of the least evaluated symptoms in medical encounters and is often under-reported because of cultural norms about discussing sexuality.[258] Thus, asking patients is the most important first step toward addressing sexual symptoms (e.g., “Are you sexually satisfied?” and “Are you able to achieve orgasm?”). Evaluation should include first a search for reversible contributors, such as underlying medical comorbidities, including diabetes or hypertension, medications (such as beta-blockers), alcohol, and psychiatric conditions, such as depression.[255,259] Sexual dysfunction can result from cirrhosis because of associated hypogonadism, low testosterone, and altered circulation.[260]
Treatment of sexual dysfunction includes pharmacological and nonpharmacological approaches. First, medications (e.g., beta-blockers) and substances (e.g., alcohol, tobacco) that can cause sexual dysfunction should be removed, where appropriate.[261] Second, underlying conditions that contribute to sexual dysfunction, such as depression and diabetes, should be medically managed.[261,262] Third, one can consider using phosphodiesterase inhibitors. One small study of tadalafil in 25 men with cirrhosis, erectile dysfunction, and a mean MELD of 13 ± 4 found that 10 mg over a 4-week period was a safe and efficacious approach.[263] Although 55% of the men studied had decompensated disease, the trial excluded those with encephalopathy. Three patients had adverse events, including dyspepsia (n = 2) and headache (n = 1), but these were not dose limiting. Studies of portopulmonary hypertension treatment using these agents have been successful, though in the setting of hypotension such medications are contraindicated.[264–267] There is an ongoing trial of tadalafil for patients with cirrhosis that should add to this literature.[268] Nonpharmacological treatments, including sex therapy or vacuum devices, have not been tried in patients with cirrhosis, but may be a safe alternative.[269] Furthermore, a small study found that sexual satisfaction and erectile dysfunction improved after LT.[257,260] More research is needed to determine how to manage sexual dysfunction in this population, particularly among women.
Guidance statements
-
52
Sexual dysfunction is often important for quality of life of patients with DC and should be assessed.
-
53
There are sparse data about management of erectile dysfunction treatment in this population, though tadalafil may be a safe short-term option in select patients and is undergoing further evaluation.
-
54
There is a notable lack of research regarding the assessment or management of sexual dysfunction in women with cirrhosis.
Depression and anxiety
Depression and anxiety are common among patients with cirrhosis, with a prevalence of 30%–60%.[105] Untreated depressive symptoms are associated with pain, disability, worse HRQoL, increased health care use, and increased mortality.[25,109,113,270–273] A recent report found that patients with new cirrhosis diagnoses were at increased risk of suicide, highlighting the importance of identifying and treating mental health symptoms in this population.[274] Mental health symptoms can have organic causes (e.g., vitamin deficiency) and can mimic encephalopathy and dementia, such that a high index of suspicion is required to distinguish mental health symptoms from other related disorders. Evaluation can include validated screeners. For example, the Patient Health Questionnaire-2 is a two-question screening tool that asks patients to rate (1) interest in activities and (2) feeling down, depressed, or hopeless over the past 2 weeks on a 4-point scale, where a score of 3 triggers further evaluation.[275]
A thorough exploration of evidence-based mental health treatments is beyond the scope of this Guidance. However, having a low threshold for referral to mental health specialists is recommended, and efforts should be made to combat stigma about seeking mental health care. Treating contributors, such as vitamin B12, iron, or folate deficiency, sleep disorders, and encephalopathy, is an important aspect of managing mental health symptoms. The backbone of primary management of mental health symptoms should be cognitive behavioral therapy with or without pharmacotherapy.[276] Although SSRIs are generally considered to be efficacious and safe, selection requires consideration of other symptoms, and presence of sexual dysfunction, somnolence, and/or weight loss should guide the selection of specific SSRIs.[277] These nuanced treatment considerations are often best managed by a mental health professional, who can also provide evidence-based behavioral therapies. Regardless of the type of treatment, psychosocial support is part of a key component of primary palliative and hepatology care.[14]
Guidance statements
-
55
Depression is associated with worse quality of life and increased mortality in patients with cirrhosis, and clinicians should routinely assess mood for patients with cirrhosis.
-
56
Clinicians should investigate medical contributors to depression, including vitamin deficiencies, sleep disorders, and HE.
-
57
Although the evaluation of mental health symptoms is within the scope of hepatology care, providers caring for patients with cirrhosis should have a low threshold for referral to allied mental health professionals, particularly when considering pharmacotherapy.
Nausea and vomiting
Nausea is experienced by over half of patients with cirrhosis attributable to a number of potential etiologies, including medications (e.g., lactulose, opioids), adrenal insufficiency, electrolyte imbalance (e.g., hyponatremia, hypercalcemia), uremia, reflux, and constipation.[35,43] LD can also be associated with gastroparesis and increased intra-abdominal pressure (e.g., with tense ascites). Thus, nonpharmacological approaches to the management of nausea and vomiting can include behavioral avoidance (e.g., avoiding triggering smells and tastes), removal of triggering medications, treatment of underlying physiological causes, use of ginger, guided imagery or relaxation techniques, and acupuncture (Table 6).[278,279]
First-line pharmacotherapy for nausea and vomiting in patients with cirrhosis may include a trial of a histamine H2 antagonist or proton pump inhibitor in patients with suspected reflux-associated nausea. Ondansetron (a serotonin 5-HT3 antagonist) can be used in patients with liver impairment at a dose of up to 8 mg/d, though patients should be monitored for constipation and QTc prolongation.[111] A small, randomized trial of 8 patients with cirrhosis and mild encephalopathy were treated with metoclopramide (up to 60 mg daily) versus placebo for nausea over a period of 2 weeks; all patients also received neomycin.[280] Although metoclopramide was associated with reduced nausea and heartburn in this small study, the risk of extrapyramidal side effects limits its long-term use, and a 50% dose reduction is recommended for patients with hepatic impairment.[280,281] Other medications for the palliative treatment of nausea may include olanzapine, haloperidol, or prochlorperazine; however, all have significant side-effect profiles (Table 4); prochlorperazine should be avoided in the setting of jaundice given case reports of cholestatic liver injury.[282]
Cannabinoids are another potential treatment for nausea and vomiting. Because of media coverage and conflicting data, clinicians should be prepared to discuss the benefits and potential harms of medical marijuana and other cannabinoids with patients with cirrhosis and their caregivers. The strongest supportive data for the use of medical marijuana are for nausea and vomiting. However, although one meta-analysis found that cannabinoids were associated with a reduction of nausea and vomiting of >50%, the data, particularly in cirrhosis, are limited.[283] Adverse effects of cannabinoids in the general population include dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination.[283] Some observational studies identified associations between cannabis and accelerated hepatic fibrosis, encephalopathy, and ascites in patients with chronic LD attributable to HCV and fatty liver disease.[284–287] However, other observational studies, including two meta-analyses of marijuana use and HCV, did not find a significant association between cannabis and hepatic fibrosis.[288–291] Trials of medical cannabinoids/marijuana have generally excluded patients with cirrhosis, given their vulnerability to neuropsychiatric symptoms. Thus, cannabinoids should be used with caution and with attention to institutional protocols for its use. When cannabinoids are used, edible formulations are favored over inhaled because of the potential infectious and respiratory effects of smoking.
Guidance statements
-
58
For patients with cirrhosis who suffer with nausea and/or vomiting, an evaluation should include assessment of electrolytes, adrenal insufficiency, and pharmaceutical causes as well as assessment for and treatment of gastroesophageal reflux disease.
-
59
First-line pharmacotherapy for nausea and vomiting is ondansetron (maximum 8 mg/d), using caution given constipating effects; most antiemetics require monitoring for QTc prolongation.
-
60
Medical marijuana is not first-line management for any symptom for patients with DC. However, providers should be able to engage in an educated conversation about its risks and benefits.
END-OF-LIFE CARE
The burden of symptoms and distress that patients with DC experience at the end of life is comparable to that of patients with advanced colorectal and lung cancer.[30] Symptoms commonly include pain, lack of energy, drowsiness, insomnia, and difficulty concentrating.[35] Eliciting treatment preferences for EoLC and addressing palliative needs based on priority are critical to ensuring that care at the end of life is patient and caregiver centered.
Patients and caregivers often want to know what to expect nearing the end of life; thus, health care teams should provide education about prognosis and help them develop plans for addressing infection, hemorrhage, and encephalopathy, which are the most common scenarios leading to death.[30] Care plans developed based on these discussions may often require balancing goals that are simultaneously aggressive and nonaggressive. This can be a particular challenge for patients and caregivers who wish to pursue EoLC at home or in the community, though the majority of patients with cirrhosis die in a health care facility.[292,293] There are a number of scenarios that are important to discuss and difficult to address. For example, patients with variceal hemorrhage may be managed with endoscopic treatment to preserve the time left or may elect to manage bleeding at home with dark towels and sedation if dying at home is prioritized. Another common scenario is managing encephalopathy when patients are unable to swallow and decisions regarding nasogastric tube placement are being considered. Conversely, in some cases, patients may continue LD-focused care even on hospice if it is for palliation (e.g., paracentesis for tense ascites), in collaboration with the hospice agency. Thus, developing plans that address preferences around hospital readmission are crucial.
Clinical decision making may shift when death is imminent, as discussed throughout this document. For example, whereas past opioid use disorder is a relative contraindication for chronic opioid analgesia, EoLC should prioritize analgesia, comfort, and compassionate care over concerns about long-term comorbidities or adverse medication effects. Consequently, medications that may be generally contraindicated for patients with DC (e.g., benzodiazepines) can be considered in the context of EoLC. Because prognostic information may impact caregivers’ decision making around difficult issues, it is critical that hepatology team members engage in frequent prognostic conversations and shared decision making with families.
Hospice is comfort-focused EoLC that, in the USA, is covered by Medicare Part A.[18,294] It is typically provided in the home and is focused on symptom management, psychosocial support, and counseling by an interdisciplinary team. Generally, the hospice team continues to follow bereaved family members for up to a year after death. Bereavement services are an important part of the hospice benefit that are not consistently provided by routine medical care and should be considered as a potential benefit in the decision-making process. Hospice is generally appropriate for patients who prioritize comfort-focused care and are no longer pursuing disease-directed therapies and whose prognosis is <6 months. Hospice has been associated with improved control of physical and psychological symptoms, quality of life, and caregiver bereavement.[13,14] Evidence shows that hospice is provided infrequently and, when provided, is very close to the end of life for patients with DC.[48,292,293,295,296] Likely factors responsible for this observation include infrequent and delayed discussions about EoLC, defaulting to aggressive, disease-directed care, the unpredictability of decompensating events, and clinician discomfort with end-of-life conversations.[48] Centers for Medicare & Medicaid Services (CMS) eligibility criteria for hospice in the USA are shown in Table 7; MELD score >21 or Child-Pugh score ≥12 are also associated with an ~6-month life expectancy, which can be useful prognostic information for patients and caregivers.[297]
TABLE 7.
Hospice criteria for patients with DC[288]
| Patients will be considered to be in the terminal stage of LD (life expectancy of ≤6 months) if they meet the following criteria (criteria 1 and 2 should be present; factors from criterion 3will lend supporting documentation): |
| 1. The patient should show both a and b: |
| a. Prothrombin time prolonged >5 s over control or international normalized ratio >1.5; |
| b. Serum albumin <2.5 g/dl. |
| 2. End-stage liver disease is present and the patient shows at least one of the following: |
| a. Ascites, refractory to treatment or patient noncompliant; |
| b. SBP; |
| c. Hepatorenal syndrome (elevated creatinine and blood urea nitrogen with oliguria); |
| d. HE, refractory to treatment or patient noncompliant; |
| e. Recurrent variceal bleeding, despite intensive therapy. |
| 3. Documentation of the following factors will support eligibility for hospice care: |
| a. Progressive malnutrition; |
| b. Muscle wasting with reduced strength and endurance; |
| c. Continued active alcoholism (>80 g of ethanol per day); |
| d. HCC; |
| e. HBsAg positive; |
| f. Hepatitis C refractory to interferon treatment.Patients awaiting LT who otherwise fit these criteria may be certified for the Medicare hospice benefit; but if a donor organ is procured, the patient should be discharged from hospice. |
| Reference: Hospice determining terminal status. CMS. https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=34538. Last accessed January 7, 2022 |
A significant policy-level barrier to hospice for patients with cirrhosis is that the Medicare hospice benefit does not financially cover disease-directed care. That said, it is possible (though uncommon) for patients to remain on the transplant list and receive hospice benefits.[294] There are alternative models that have been piloted for patients with LD at the end of life. A feasibility study provided hospice care to 157 patients who were being evaluated or listed for LT; 6 study patients ultimately received transplantation.[298] To date, such models have not been widely implemented in the USA, owing, in part, to the complexities of payment models that would be needed to support a hybridized hospice/transplant approach. In general, when people elect to enroll in hospice, the agency generally assumes management of their medical care. Consultants may follow and bill for services; however, this is best arranged with the hospice agency on a case-by-case basis. Evidence from the oncology literature suggests that patients and families may feel a sense of abandonment by their treating teams at the time of hospice transition; however, no such studies have been conducted among patients with cirrhosis.[299] Nonetheless, hospice remains the standard of high-quality EoLC. More information about how to best tailor hospice services to the needs of this population, and to better understand contributors to complicated grief among caregiver members, is needed.
Guidance statements
-
61
Addressing palliative needs at the end of life involves eliciting end-of-life treatment preferences, creating care plans, and providing education about possible scenarios, including infection, bleeding, and HE.
-
62
Hospice is a patient-and caregiver-centered option for EoLC that is underused by patients dying with cirrhosis.
-
63
In addition to current criteria, hepatology teams may consider using MELD >21 and Child-Pugh >12 to determine whether patients are prognostically appropriate for hospice (i.e., have estimated survival of ≤6 months).
-
64
Providing caregivers with information about imminent death may help support decisions aligned with the patient’s prognosis (e.g., providing pain medications despite past substance use disorder at the end of life).
FUTURE CLINICAL AND POLICY IMPLICATIONS
To successfully weave the principles of palliative care into hepatology care requires substantial research, clinical innovation, culture change, and health policy advocacy. A critical first step is workforce training and programmatic development to support palliative care. Although not every hepatology trainee or team member requires expert-level palliative care skills, there is a growing consensus that clinicians across all medical and surgical specialties need to develop core competencies in providing PPC and then engage with specialists as needed. This is even more critical in light of palliative care workforce shortages.[46] Palliative care is a growing field, but its penetrance is uneven across the USA, with the largest gaps existing in ambulatory and community palliative care services.[300] Several societies have developed key competencies in PPC for nonspecialists, including communication, ACP, and symptoms assessment and management skills.[301–303] For example, competencies for cardiologists include, but are not limited to, communication and shared decision-making skills, pain and symptom management, caring for caregivers, understanding hospice indications, end-of-life decision making and care, and working with an interdisciplinary team to manage psychosocial, spiritual, and cultural aspects of care.[301,302] Similarly, some skills that are important for hepatology clinicians include identifying and documenting surrogate decision maker, communicating prognosis, eliciting and documenting goals and values, and delivering care concordant with these goals and values. Offering trainees in hepatology the opportunity to serve on palliative care consultative services and in hospice settings may provide hands-on experience and training that can diffuse into current hepatology practitioners.[5] Resources for further skill development are offered in Table S2.
In addition to training opportunities, there are other potential opportunities to increase palliative care implementation in clinical hepatology settings. For example, specialty palliative care providers can round on inpatient hepatology services or have colocated clinics with hepatology.[5] Alternatively, palliative care consultation can be automated based on trigger criteria (e.g., intensive care unit admission, removal from the transplant list).[88] Simply offering information about palliative care in hepatology settings can help caregivers and patients become more aware of their options.[22] Routine symptom assessment in hepatology can increase providers’ expertise with triaging and managing symptoms that are often underaddressed for patients with cirrhosis.
Policy and infrastructure changes can also facilitate palliative care implementation in hepatology. Advocating for payers and hospice agencies to innovate to best meet the needs of patients with cirrhosis will ensure that the specific issues facing our patients are addressed. For example, policies supporting reimbursement for specialty palliative services should be considered. Providing palliative care training to social workers, psychologists, and other members of multidisciplinary liver teams or seeking out palliative care–trained specialists in these disciplines can extend the reach of the existing workforce. Developing reimbursement infrastructure around the various aspects of palliative care can also facilitate its implementation. Similarly, formal communication and collaboration between palliative care and hepatology societies could facilitate palliative care implementation in patients with DC. Furthermore, dissemination of information about available supports and benefits is important. For example, many are unaware that patients on the transplant list can receive hospice benefits until a donor organ becomes available.[294] Finally, incorporating measures of hospice and palliative care into cirrhosis quality metrics can demonstrate the importance of these topics to providers and payors.
Clinical research gaps have been highlighted throughout this guidance. These include developing evidence around which interventions work at what times for patients with cirrhosis, delineating the optimal ways to measure and report patient-reported outcomes for patients with cirrhosis, and addressing the high symptom burden of patients with cirrhosis, particularly using nonpharmacological approaches. Thus, fully implementing palliative care for patients with DC requires a multipronged approach to address ongoing training, practice, policy, and research gaps.
Guidance statements
-
65
Future workforce training is needed, and developing hepatology competencies in primary palliative care is a first step toward this goal.
-
66
Policy changes, such as funding for multidisciplinary palliative care teams and extended reimbursement for specialty palliative care services, can help to support patients with cirrhosis and their caregivers at the end of life.
CONCLUSIONS
Palliative care is a multidisciplinary approach to addressing the psychosocial, spiritual, and physical needs of patients and their caregivers that is associated with improved health outcomes in the context of cirrhosis and other chronic diseases, but is highly underused in patients with DC. Although palliative care can be provided by specialists, we encourage all members of the hepatology care team to consider providing palliative care to address the symptomatic, spiritual, and psychosocial needs of their patients and their caregivers. The core principles of palliative care are clearly aligned with high-quality, value-based hepatology care: communication; coordinated interdisciplinary care; comprehensive symptom assessment and management; and care plans that align with patients’ values and preferences. Future research is needed to identify the optimal approaches to train medical staff in primary palliative care skills, symptom management, engagement of specialty palliative care, and innovative models of care that successfully blend these currently distinct disciplines. Palliative care should be offered earlier and to more patients with cirrhosis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the valuable contributions of the AASLD Practice Guideline Committee (PGC), particularly Cynthia Levy. Members of the PGC include George Ioannou (chair), Rabab Ali, Scott W. Biggins, Roniel Cabrera, Henry Chang, Michael F. Chang, David S. Goldberg, W. Ray Kim (board liaison), Cynthia Levy, Jeff McIntyre, Jessica L. Mellinger, Mindie H. Nguyen, Nadia Ovchinsky, Anjana A. Pillai, Daniel S. Pratt, Hugo R. Rosen, Matthew J. Stotts, Christopher J. Sonnenday, Lisa B. VanWagner, and Elizabeth C. Verna.
Funding information
Funding for the development of this Practice Guidance was provided by the American Association for the Study of Liver Diseases. M.V. was supported by aAQ19 Patient-Centered Outcomes Research Institute (PCORI) Award (PLC-1609–36714). S.R.’s time was supported, in part, by K23DA048182. C.W.’s time was supported by R03 AG067992.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ACP
advance care planning
- APAP
acetaminophen
- BCAAs
branched-chain amino acids
- CMS
Centers for Medicare & Medicaid Services
- DC
decompensated cirrhosis
- EoLC
end-of-life care
- HRQoL
health-related quality of life
- LD
liver disease
- LT
liver transplantation
- LVP
large volume paracentesis
- MELD
Model for End-Stage Liver Disease
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PC
palliative care
- RCTs
randomized controlled trials
- RIF
rifampicin
- SBP
spontaneous bacterial peritonitis
- SNRIs
serotonin-norepinephrine reuptake inhibitors
- SSRIs
selective serotonin reuptake inhibitors
Footnotes
CONFLICT OF INTEREST
The opinions expressed in this article are the authors’ own and do not reflect the views of the National Institutes of Health or the United States government.
ETHICS STATEMENT
This guidance document does not qualify as human subjects research.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
Data are available in the guidance document or the cited references.
REFERENCES
- 1.Sepúlveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organization’s global perspective. J Pain Symptom Manage. 2002;24(2):91–6. 10.1016/s0885-3924(02)00440-2 [DOI] [PubMed] [Google Scholar]
- 2.Radbruch L, De Lima L, Knaul F, Wenk R, Ali Z, Bhatnaghar S, et al. Redefining palliative care—a new consensus-based definition. J Pain Symptom Manage. 2020;60(4):754–64. 10.1016/j.jpainsymman.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma M, Tapper EB, Singal AG, Navarro V. Nonhospice palliative care within the treatment of end-stage liver disease. Hepatology. 2020;71(6):2149–59. 10.1002/hep.31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon P, Walling A, Patton H, Taddei T. AGA clinical practice update on palliative care management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2021;19(4):646–56.e3. 10.1016/j.cgh.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 5.Verma M, Bakitas MA. Creating effective models for delivering palliative care in advanced liver disease. Curr Hepatol Rep. 2021;20(2):43–53. 10.1007/s11901-021-00562-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;72(2):1014–48. 10.1002/hep.31884 [DOI] [PubMed] [Google Scholar]
- 7.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35. 10.1002/hep.27210 [DOI] [PubMed] [Google Scholar]
- 8.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017;65(1):310–35. 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 10.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–45. 10.1200/JCO.20.02672 [DOI] [PubMed] [Google Scholar]
- 11.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96–112. 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 12.Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173–5. 10.1056/NEJMp1215620 [DOI] [PubMed] [Google Scholar]
- 13.Ahluwalia SC, Chen C, Raaen L, Motala A, Walling AM, Chamberlin M, et al. A systematic review in support of the National Consensus Project clinical practice guidelines for quality palliative care, fourth edition. J Pain Symptom Manage. 2018;56(6):831–70. 10.1016/j.jpainsymman.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 14.Ferrell BR, Twaddle ML, Melnick A, Meier DE. National Consensus project clinical practice guidelines for quality palliative care guidelines. J Palliat Med. 2018;21(12):1684–9. 10.1089/jpm.2018.0431 [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 16.Myers J, Cosby R, Gzik D, Harle I, Harrold D, Incardona N, et al. Provider tools for advance care planning and goals of care discussions: a systematic review. Am J Hosp Palliat Care. 2018;35(8):1123–32. 10.1177/1049909118760303 [DOI] [PubMed] [Google Scholar]
- 17.Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821–32.e1. 10.1016/j.jpainsymman.2016.12.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Medicare hospice benefits; 2020. [cited 2021 Jun 28]. Available from: https://www.medicare.gov/Pubs/pdf/02154-medicare-hospice-benefits.pdf
- 19.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–31. 10.1016/j.jhep.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Edwards A, Pang N, Shiu V, Chan C. Review: The understanding of spirituality and the potential role of spiritual care in end-of-life and palliative care: a meta-study of qualitative research. Palliat Med. 2010;24(8):753–70. 10.1177/0269216310375860 [DOI] [PubMed] [Google Scholar]
- 21.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–60. 10.1053/j.gastro.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 22.Donlan J, Ufere NN, Indriolo T, Jackson V, Chung RT, El-Jawahri A, et al. Patient and caregiver perspectives on palliative care in end-stage liver disease. J Palliat Med. 2021;24(5):719–24. 10.1089/jpm.2020.0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hareendran A, Devadas K, Sreesh S, Tom Oommen T, Varghese J, Lubina S, et al. Quality of life, caregiver burden and mental health disorders in primary caregivers of patients with cirrhosis. Liver Int. 2020;40(12):2939–49. 10.1111/liv.14614 [DOI] [PubMed] [Google Scholar]
- 24.Yanny B, Pham NV, Saleh H, Saab S. Approaches to assessing burden in caregivers of patients with cirrhosis. J Clin Transl Hepatol. 2020;8(2):127–34. 10.14218/JCTH.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646–53. 10.1038/ajg.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki ET, dos Santos R, Miyazaki MC, Domingos NM, Felicio HC, Rocha MF, et al. Patients on the waiting list for liver transplantation: caregiver burden and stress. Liver Transpl. 2010;16(10):1164–8. 10.1002/lt.22130 [DOI] [PubMed] [Google Scholar]
- 27.Hudson B, Hunt V, Waylen A, McCune CA, Verne J, Forbes K. The incompatibility of healthcare services and end-of-life needs in advanced liver disease: a qualitative interview study of patients and bereaved carers. Palliat Med. 2018;32(5):908–18. 10.1177/0269216318756222 [DOI] [PubMed] [Google Scholar]
- 28.Kimbell B, Boyd K, Kendall M, Iredale J, Murray SA. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open. 2015;5(11):e009241. 10.1136/bmjopen-2015-009241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen L, Leo MC, Chang MF, Zucker BL, Sasaki A. Pain and self-care behaviours in adult patients with end-stage liver disease: a longitudinal description. J Palliat Care. 2014;30(1):32–40. [PMC free article] [PubMed] [Google Scholar]
- 30.Roth K, Lynn J, Zhong Z, Borum M, Dawson NV. Dying with end stage liver disease with cirrhosis: insights from SUPPORT. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc. 2000;48(S1):S12 2–30. [PubMed] [Google Scholar]
- 31.Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology. 2012;55(1):184–91. 10.1002/hep.24616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ufere NN, Donlan J, Indriolo T, Richter J, Thompson R, Jackson V, et al. Burdensome transitions of care for patients with end-stage liver disease and their caregivers. Dig Dis Sci. 2021;66(9):2942–55. 10.1007/s10620-020-06617-4 [DOI] [PubMed] [Google Scholar]
- 33.Loria A, Escheik C, Gerber NL, Younossi ZM. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2013;15(1):301. 10.1007/s11894-012-0301-5 [DOI] [PubMed] [Google Scholar]
- 34.Rogal SS, Yakovchenko V, Gonzalez R, Park A, Lamorte C, Gibson SP, et al. Characterizing patient-reported outcomes in veterans with cirrhosis. PLoS One. 2020;15(9):e0238712. 10.1371/journal.pone.0238712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen L, Leo MC, Chang MF, Zaman A, Naugler W, Schwartz J. Symptom distress in patients with end-stage liver disease toward the end of life. Gastroenterol Nurs. 2015;38(3):201–10. 10.1097/SGA.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low J, Davis S, Vickerstaff V, Greenslade L, Hopkins K, Langford A, et al. Advanced chronic liver disease in the last year of life: a mixed methods study to understand how care in a specialist liver unit could be improved. BMJ Open. 2017;7(8):e016887. 10.1136/bmjopen-2017-016887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–52. 10.1038/ajg.2011.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of population-based data to demonstrate how waitlist-based metrics overestimate geographic disparities in access to liver transplant care. Am J Transplant. 2016;16(10):2903–11. 10.1111/ajt.13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langberg KM, Kapo JM, Taddei TH. Palliative care in decompensated cirrhosis: a review. Liver Int. 2018;38(5):768–75. 10.1111/liv.13620 [DOI] [PubMed] [Google Scholar]
- 40.Ufere NN, Halford JL, Caldwell J, Jang MY, Bhatt S, Donlan J, et al. Health care utilization and end-of-life care outcomes for patients with decompensated cirrhosis based on transplant candidacy. J Pain Symptom Manage. 2020;59(3):590–8. 10.1016/j.jpainsymman.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteban JPG, Rein L, Szabo A, Saeian K, Rhodes M, Marks S. Attitudes of liver and palliative care clinicians toward specialist palliative care consultation for patients with end-stage liver disease. J Palliat Med. 2019;22(7):804–13. 10.1089/jpm.2018.0553 [DOI] [PubMed] [Google Scholar]
- 42.Beck KR, Pantilat SZ, O’Riordan DL, Peters MG. Use of palliative care consultation for patients with end-stage liver disease: survey of liver transplant service providers. J Palliat Med. 2016;19(8):836–41. 10.1089/jpm.2016.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poonja Z, Brisebois A, Veldhuyzen van Zanten S, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol. 2014;12(4):692–8. 10.1016/j.cgh.2013.08.027 [DOI] [PubMed] [Google Scholar]
- 44.Kelly SG, Campbell TC, Hillman L, Said A, Lucey MR, Agarwal PD. The utilization of palliative care services in patients with cirrhosis who have been denied liver transplantation: a single center retrospective review. Ann Hepatol. 2017;16(3):395–401. [DOI] [PubMed] [Google Scholar]
- 45.Ufere NN, Brahmania M, Sey M, Teriaky A, El-Jawahri A, Walley KR, et al. Outcomes of in-hospital cardiopulmonary resuscitation for patients with end-stage liver disease. Liver Int. 2019;39(7):1256–62. 10.1111/liv.14079 [DOI] [PubMed] [Google Scholar]
- 46.Lupu D, Quigley L, Mehfoud N, Salsberg ES. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55(4):1216–23. 10.1016/j.jpainsymman.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 47.Standing H, Jarvis H, Orr J, Exley C, Hudson M, Kaner E, et al. How can primary care enhance end-of-life care for liver disease? Qualitative study of general practitioners’ perceptions and experiences. BMJ Open. 2017;7(8):e017106. 10.1136/bmjopen-2017-017106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ufere NN, Donlan J, Waldman L, Dienstag JL, Friedman LS, Corey KE, et al. Barriers to use of palliative care and advance care planning discussions for patients with end-stage liver disease. Clin Gastroenterol Hepatol. 2019;17(12):2592–9. 10.1016/j.cgh.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J.; for the SUPPORT Investigators. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA. 1999;282(17):1638–45. 10.1001/jama.282.17.1638 [DOI] [PubMed] [Google Scholar]
- 50.Woodland H, Hudson B, Forbes K, McCune A, Wright M; on behalf of the British Association for the Study of the Liver (BASL) End of Life Special Interest Group. Palliative care in liver disease: what does good look like? Frontline Gastroenterol. 2020;11(3):218–27. 10.1136/flgastro-2019-101180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol. 2018;19(11):e588–653. 10.1016/S1470-2045(18)30415-7 [DOI] [PubMed] [Google Scholar]
- 52.Walling AM, Asch SM, Lorenz KA, Wenger NS. Impact of consideration of transplantation on end-of-life care for patients during a terminal hospitalization. Transplantation. 2013;95(4):641–6. 10.1097/TP.0b013e318277f238 [DOI] [PubMed] [Google Scholar]
- 53.Patel AA, Ryan GW, Tisnado D, Chuang E, Walling AM, Saab S, et al. Deficits in advance care planning for patients with decompensated cirrhosis at liver transplant centers. JAMA Intern Med. 2021;181(5):652–60. 10.1001/jamainternmed.2021.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumann AJ, Wheeler DS, James M, Turner R, Siegel A, Navarro VJ. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage. 2015;50(6):882–6.e2. 10.1016/j.jpainsymman.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 55.Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012;44(4):508–19. 10.1016/j.jpainsymman.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 56.Shinall MC, Karlekar M, Martin S, Gatto CL, Misra S, Chung CY, et al. COMPASS: a pilot trial of an early palliative care intervention for patients with end-stage liver disease. J Pain Symptom Manage. 2019;58(4):614–22.e3. 10.1016/j.jpainsymman.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimbell B, Murray SA, Byrne H, Baird A, Hayes PC, MacGilchrist A, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med. 2018;32(5):919–29. 10.1177/0269216318760441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes A, Woodman RJ, Kleinig P, Briffa M, To T, Wigg AJ. Early palliative care referral in patients with end stage liver disease is associated with reduced resource utilisation. J Gastroenterol Hepatol. 2019;35(3):840–5. 10.1111/jgh.14877 [DOI] [PubMed] [Google Scholar]
- 59.Ufere NN, O’Riordan DL, Bischoff KE, Marks AK, Eneanya N, Chung RT, et al. Outcomes of palliative care consultations for hospitalized patients with liver disease. J Pain Symptom Manage. 2019;58(5):766–73. 10.1016/j.jpainsymman.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey DE, Hendrix CC, Steinhauser KE, Stechuchak KM, Porter LS, Hudson J, et al. Randomized trial of an uncertainty self-management telephone intervention for patients awaiting liver transplant. Patient Educ Couns. 2017;100(3):509–17. 10.1016/j.pec.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajaj JS, Ellwood M, Ainger T, Burroughs T, Fagan A, Gavis EA, et al. Mindfulness-based stress reduction therapy improves patient and caregiver-reported outcomes in cirrhosis. Clin Transl Gastroenterol. 2017;8(7):e108. 10.1038/ctg.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma M, Kosinski AS, Volk ML, Taddei T, Ramchandran K, Bakitas M, et al. Introducing palliative care within the treatment of end-stage liver disease: the study protocol of a cluster randomized controlled trial. J Palliat Med. 2019;22(S1):S-34–43. 10.1089/jpm.2019.0121 [DOI] [PubMed] [Google Scholar]
- 63.Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017;70(3):331–41. 10.1016/j.jacc.2017.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med. 2018;178(4):511–9. 10.1001/jamainternmed.2017.8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dionne-Odom JN, Ejem DB, Wells R, Azuero A, Stockdill ML, Keebler K, et al. Effects of a telehealth early palliative care intervention for family caregivers of persons with advanced heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Netw Open. 2020;3(4):e202583. 10.1001/jamanetworkopen.2020.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bakitas M, Dionne-Odom JN, Pamboukian SV, Tallaj J, Kvale E, Swetz KM, et al. Engaging patients and families to create a feasible clinical trial integrating palliative and heart failure care: results of the ENABLE CHF-PC pilot clinical trial. BMC Palliat Care. 2017;16(1):45. 10.1186/s12904-017-0226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bakitas MA, Dionne-Odom JN, Ejem DB, Wells R, Azuero A, Stockdill ML, et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med. 2020;180(9):1203–13. 10.1001/jamainternmed.2020.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Jawahri A, Nelson AM, Gray TF, Lee SJ, LeBlanc TW. Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol. 2020;38(9):944–53. 10.1200/JCO.18.02386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, et al. Social worker-aided palliative care intervention in high-risk patients with heart failure (SWAP-HF): a pilot randomized clinical trial. JAMA Cardiol. 2018;3(6):516–9. 10.1001/jamacardio.2018.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong FKY, Ng AYM, Lee PH, Lam P-T, Ng JSC, Ng NHY, et al. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart. 2016;102(14):1100–8. 10.1136/heartjnl-2015-308638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kavalieratos D, Corbelli J, Zhang DI, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104–14. 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative care interventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20(1):84–92. 10.1089/jpm.2016.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–9. 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parikh RB, Temel JS. Early specialty palliative care. N Engl J Med. 2014;370(11):1075–6. 10.1056/NEJMc1400243 [DOI] [PubMed] [Google Scholar]
- 75.Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, et al. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2020;9:CD012780. 10.1002/14651858.CD012780.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans CJ, Bone AE, Yi D, Gao W, Morgan M, Taherzadeh S, et al. Community-based short-term integrated palliative and supportive care reduces symptom distress for older people with chronic noncancer conditions compared with usual care: a randomised controlled single-blind mixed method trial. Int J Nurs Stud. 2021;120:103978. 10.1016/j.ijnurstu.2021.103978 [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Jaramillo V, Fuhrer V, Gonzalez-Jaramillo N, Kopp-Heim D, Eychmüller S, Maessen M. Impact of home-based palliative care on health care costs and hospital use: a systematic review. Palliat Support Care. 2021;19(4):474–87. 10.1017/S1478951520001315 [DOI] [PubMed] [Google Scholar]
- 78.Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–89. 10.1016/j.jamda.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 79.National Institute on Aging. Advance care planning: health care directives. Available from: https://www.nia.nih.gov/health/advance-care-planning-health-care-directives#proxy
- 80.Valery PC, Clark PJ, McPhail SM, Rahman T, Hayward K, Martin J, et al. Exploratory study into the unmet supportive needs of people diagnosed with cirrhosis in Queensland. Australia. Intern Med J. 2017;47(4):429–35. 10.1111/imj.13380 [DOI] [PubMed] [Google Scholar]
- 81.Carbonneau M, Davyduke T, Spiers J, Brisebois A, Ismond K, Tandon P. Patient views on advance care planning in cirrhosis: a qualitative analysis. Can J Gastroenterol Hepatol. 2018;2018:4040518. 10.1155/2018/4040518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sprange A, Ismond KP, Hjartarson E, Chavda S, Carbonneau M, Kowalczewski J, et al. Advance care planning preferences and readiness in cirrhosis: a prospective assessment of patient perceptions and knowledge. J Palliat Med. 2020;23(4):552–7. 10.1089/jpm.2019.0244 [DOI] [PubMed] [Google Scholar]
- 83.Wang CW, Lebsack A, Sudore RL, Lai JC. Low rates of advance care planning (ACP) discussions despite readiness to engage in ACP among liver transplant candidates. Dig Dis Sci. 2020;66(5):1446–51. 10.1007/s10620-020-06369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen L, Press N, Rosenkranz SJ, Baggs JG, Kendall J, Kerber A, et al. Life-sustaining treatment decisions in the ICU for patients with ESLD: a prospective investigation. Res Nurs Health. 2012;35(5):518–32. 10.1002/nur.21488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel A, Kogekar N, Agarwal R, Cohen C, Esteban JP, Pourmand K, et al. Improving advance care planning in outpatients with decompensated cirrhosis: a pilot study. J Pain Symptom Manage. 2020;59(4):864–70. 10.1016/j.jpainsymman.2019.12.355 [DOI] [PubMed] [Google Scholar]
- 86.Ufere NN, Robinson B, Donlan J, Indriolo T, Bloom J, Scherrer A, et al. Pilot randomized controlled trial of an advance care planning video decision tool for patients with advanced liver disease. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.10.027 [DOI] [PubMed] [Google Scholar]
- 87.Walling AM, Ahluwalia SC, Wenger NS, Booth M, Roth CP, Lorenz K, et al. Palliative care quality indicators for patients with end-stage liver disease due to cirrhosis. Dig Dis Sci. 2017;62(1):84–92. 10.1007/s10620-016-4339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verma M, Taddei T, Volk M, Navarro V. Advance care planning: a convincing argument to make it part of liver transplant evaluation. Liver Transpl. 2021;27(3):461–2. 10.1002/lt.25901 [DOI] [PubMed] [Google Scholar]
- 89.VitalTalk. [cited 2021 Aug 24]. Available from: https://www.vitaltalk.org
- 90.Back AL, Fromme EK, Meier DE. Training clinicians with communication skills needed to match medical treatments to patient values. J Am Geriatr Soc. 2019;67(S2):S435–41. 10.1111/jgs.15709 [DOI] [PubMed] [Google Scholar]
- 91.Taylor LJ, Nabozny MJ, Steffens NM, Tucholka JL, Brasel KJ, Johnson SK, et al. A framework to improve surgeon communication in high-stakes surgical decisions: best case/worst case. JAMA Surg. 2017;152(6):531–8. 10.1001/jamasurg.2016.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmermann CJ, Jhagroo RA, Wakeen M, Schueller K, Zelenski A, Tucholka JL, et al. Opportunities to improve shared decision making in dialysis decisions for older adults with life-limiting kidney disease: a pilot study. J Palliat Med. 2020;23(5):627–34. 10.1089/jpm.2019.0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarze ML, Zelenski A, Baggett ND, Kalbfell E, Ljumani F, Silverman E, et al. Best case/worst case: ICU (COVID-19)-a tool to communicate with families of critically ill patients with COVID-19. Palliat Med Rep. 2020;1(1):3–4. 10.1089/pmr.2020.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Back AL, Arnold RM, Baile WF, Tulsky JA, Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55(3):164–77. 10.3322/canjclin.55.3.164 [DOI] [PubMed] [Google Scholar]
- 95.Childers JW, Back AL, Tulsky JA, Arnold RM. REMAP: a framework for goals of care conversations. J Oncol Pract. 2017;13(10):e844–50. 10.1200/JOP.2016.018796 [DOI] [PubMed] [Google Scholar]
- 96.Silva MD, Genoff M, Zaballa A, Jewell S, Stabler S, Gany FM, et al. Interpreting at the end of life: a systematic review of the impact of interpreters on the delivery of palliative care services to cancer patients with limited English proficiency. J Pain Symptom Manage. 2016;51(3):569–80. 10.1016/j.jpainsymman.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lago-Hernandez C, Nguyen NH, Khera R, Loomba R, Asrani SK, Singh S. Financial hardship from medical bills among adults with chronic liver diseases: national estimates from the United States. Hepatology. 2021;74(3):1509–22. 10.1002/hep.31835 [DOI] [PubMed] [Google Scholar]
- 98.Lago-Hernandez C, Nguyen NH, Khera R, Loomba R, Asrani SK, Singh S. Cost-related nonadherence to medications among US adults with chronic liver diseases. Mayo Clin Proc. 2021;96(10):2639–50. 10.1016/j.mayocp.2021.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shabanloei R, Ebrahimi H, Ahmadi F, Mohammadi E, Dolatkhah R. Despair of treatment: a qualitative study of cirrhotic patients’ perception of treatment. Gastroenterol Nurs. 2017;40(1):26–37. 10.1097/SGA.0000000000000162 [DOI] [PubMed] [Google Scholar]
- 100.Abdi F, Daryani NE, Khorvash F, Yousefi Z. Experiences of individuals with liver cirrhosis: a qualitative study. Gastroenterol Nurs. 2015;38(4):252–7. 10.1097/SGA.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 101.Wen Y, Jiang C, Koncicki HM, Horowitz CR, Cooper RS, Saha A, et al. Trends and racial disparities of palliative care use among hospitalized patients with ESKD on dialysis. J Am Soc Nephrol. 2019;30(9):1687–96. 10.1681/ASN.2018121256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187–204. 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 103.Griggs JJ. Disparities in palliative care in patients with cancer. J Clin Oncol. 2020;38(9):974–9. 10.1200/JCO.19.02108 [DOI] [PubMed] [Google Scholar]
- 104.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between Blacks and Whites. Proc Natl Acad Sci U S A. 2016;113(16):4296–301. 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peng JK, Hepgul N, Higginson IJ, Gao W. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med. 2019;33(1):24–36. 10.1177/0269216318807051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41(2):456–68. 10.1016/j.jpainsymman.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 107.Lyons KD, Bakitas M, Hegel MT, Hanscom B, Hull J, Ahles TA. Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative care (FACIT-Pal) scale. J Pain Symptom Manage. 2009;37(1):23–32. 10.1016/j.jpainsymman.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain. 2016;17(Suppl 9):T10–20. 10.1016/j.jpain.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pérez-San-Gregorio MA, Martín-Rodríguez A, Domínguez-Cabello E, Fernández-Jimenez E, Pérez-Bernal J. Biopsychosocial functioning in liver patients of alcoholic etiology as a function of self-perceived pain level. Transplant Proc. 2012;44(9):2612–5. 10.1016/j.transproceed.2012.09.055 [DOI] [PubMed] [Google Scholar]
- 110.Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72(6):1133–59. 10.1111/j.1467-6494.2004.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lexi-Drugs. Subscription required to view. [cited 2021 Jun 2]. http://online.lexi.com
- 112.Chandok N, Watt KDS. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–8. 10.4065/mcp.2009.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogal SS, Bielefeldt K, Wasan AD, Lotrich FE, Zickmund S, Szigethy E, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(5):1009–16. 10.1016/j.cgh.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rogal SS, Bielefeldt K, Wasan AD, Szigethy E, Lotrich F, DiMartini AF. Fibromyalgia symptoms and cirrhosis. Dig Dis Sci. 2015;60(5):1482–9. 10.1007/s10620-014-3453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madan A, Barth KS, Balliet WE, Hernandez-Tejada MA, Borckardt JJ, Malcolm R, et al. Chronic pain among liver transplant candidates. Prog Transplant. 2012;22(4):379–84. 10.7182/pit2012535 [DOI] [PubMed] [Google Scholar]
- 116.Rogal SS, Beste LA, Youk A, Fine MJ, Ketterer B, Zhang H, et al. Characteristics of opioid prescriptions to veterans with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(6):1165–74.e3. 10.1016/j.cgh.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rogal SS, Winger D, Bielefeldt K, Rollman BL, Szigethy E. Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver Int. 2013;33(10):1497–503. 10.1111/liv.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15):1624–45. 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeffery MM, Butler M, Stark A, Kane RL. Multidisciplinary pain programs for chronic noncancer pain. Comparative Effectiveness Technical Briefs, No. 8. Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 120.Klinge M, Coppler T, Liebschutz JM, Dugum M, Wassan A, DiMartini A, et al. The assessment and management of pain in cirrhosis. Curr Hepatol Rep. 2018;17(1):42–51. 10.1007/s11901-018-0389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099–110. 10.1001/jama.2009.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Merlin JS, Westfall AO, Long D, Davies S, Saag M, Demonte W, et al. A randomized pilot trial of a novel behavioral intervention for chronic pain tailored to individuals with HIV. AIDS Behav. 2018;22(8):2733–42. 10.1007/s10461-018-2028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson M, Finlay M, Orr M, Barbosa-Leiker C, Sherazi N, Roberts MLA, et al. Engagement in online pain self-management improves pain in adults on medication-assisted behavioral treatment for opioid use disorders. Addict Behav. 2018;86:130–7. 10.1016/j.addbeh.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 124.Yuan Q-L, Wang P, Liu L, Sun FU, Cai Y-S, Wu W-T, et al. Acupuncture for musculoskeletal pain: a meta-analysis and meta-regression of sham-controlled randomized clinical trials. Sci Rep. 2016;6(1):30675. 10.1038/srep30675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vickers AJ, Vertosick EA, Lewith G, MacPherson H, Foster NE, Sherman KJ, et al. ; on behalf of the Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. 2018;19(5):455–74. 10.1016/j.jpain.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cybularz PA, Brothers K, Singh GM, Feingold JL, Lewis ME, Niesley ML. The safety of acupuncture in patients with cancer therapy-related thrombocytopenia. Med Acupunct. 2015;27(3):224–9. 10.1089/acu.2015.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qi LU, Li S, Xu J, Xu J, Lou W, Cheng L, et al. Acupuncture for the treatment of liver cirrhosis: a meta-analysis. Gastroenterol Res Pract. 2020;2020:4054781. 10.1155/2020/4054781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382(15):1420–9. 10.1056/NEJMoa1905877 [DOI] [PubMed] [Google Scholar]
- 129.Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014;29(7):1356–60. 10.1111/jgh.12560 [DOI] [PubMed] [Google Scholar]
- 130.Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs. 2012;72(12):1645–69. 10.2165/11635500-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 131.Rainsford KD, Kean WF, Ehrlich GE. Review of the pharmaceutical properties and clinical effects of the topical NSAID formulation, diclofenac epolamine. Curr Med Res Opin. 2008;24(10):2967–92. 10.1185/03007990802381364 [DOI] [PubMed] [Google Scholar]
- 132.Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4:CD007400. 10.1002/14651858.CD007400.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;7:CD010958. 10.1002/14651858.CD010958.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Derry S, Rice AS, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1:CD007393. 10.1002/14651858.CD007393.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maloney J, Pew S, Wie C, Gupta R, Freeman J, Strand N. Comprehensive review of topical analgesics for chronic pain. Curr Pain Headache Rep. 2021;25(2):7. 10.1007/s11916-020-00923-2 [DOI] [PubMed] [Google Scholar]
- 136.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132–56. 10.1111/apt.12324 [DOI] [PubMed] [Google Scholar]
- 137.Serper M, Wolf MS, Parikh NA, Tillman H, Lee WM, Ganger DR. Risk factors, clinical presentation, and outcomes in overdose with acetaminophen alone or with combination products: results from the Acute Liver Failure Study Group. J Clin Gastroenterol. 2016;50(1):85–91. 10.1097/MCG.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weersink RA, Taxis K, Drenth JPH, Houben E, Metselaar HJ, Borgsteede SD. Prevalence of drug prescriptions and potential safety in patients with cirrhosis: a retrospective real-world study. Drug Saf. 2019;42(4):539–46. 10.1007/s40264-018-0744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomson MJ, Lok ASF, Tapper EB. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology. 2021;73(6):2429–40. 10.1002/hep.31548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hong YM, Yoon KT, Heo J, Woo HY, Lim W, An DS, et al. The prescription pattern of acetaminophen and non-steroidal anti-inflammatory drugs in patients with liver cirrhosis. J Korean Med Sci. 2016;31(10):1604–10. 10.3346/jkms.2016.31.10.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clària J, Kent JD, López-Parra M, Escolar G, Ruiz-del-Arbol L, Ginès P, et al. Effects of celecoxib and naproxen on renal function in nonazotemic patients with cirrhosis and ascites. Hepatology. 2005;41(3):579–87. 10.1002/hep.20595 [DOI] [PubMed] [Google Scholar]
- 142.Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(2):319–31. 10.1111/apt.13858 [DOI] [PubMed] [Google Scholar]
- 143.Rogal SS, Winger D, Bielefeldt K, Szigethy E. Pain and opioid use in chronic liver disease. Dig Dis Sci. 2013;58(10):2976–85. 10.1007/s10620-013-2638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moon AM, Jiang Y, Rogal SS, Tapper EB, Lieber SR, Barritt AS IV. Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther. 2020;51(6):652–60. 10.1111/apt.15639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–21. 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 146.Zedler B, Xie L, Wang LI, Joyce A, Vick C, Kariburyo F, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911–29. 10.1111/pme.12480 [DOI] [PubMed] [Google Scholar]
- 147.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872–82. 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Juba KM, van Manen RP, Fellows SE. A review of the Food and Drug Administration adverse event reporting system for tramadol-related hypoglycemia. Ann Pharmacother. 2020;54(3):247–53. 10.1177/1060028019885643 [DOI] [PubMed] [Google Scholar]
- 149.Suno M, Endo Y, Nishie H, Kajizono M, Sendo T, Matsuoka J. Refractory cachexia is associated with increased plasma concentrations of fentanyl in cancer patients. Ther Clin Risk Manag. 2015;11:751–7. 10.2147/TCRM.S79374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Evoy KE, Sadrameli S, Contreras J, Covvey JR, Peckham AM, Morrison MD. Abuse and misuse of pregabalin and gabapentin: a systematic review update. Drugs. 2021;81(1):125–56. 10.1007/s40265-020-01432-7 [DOI] [PubMed] [Google Scholar]
- 151.Ferreira GE, McLachlan AJ, Lin C-W, Zadro JR, Abdel-Shaheed C, O’Keeffe M, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ. 2021;372:m4825. 10.1136/bmj.m4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Antidepressant agents. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Updated April 27, 2018. [cited 2021 Jun 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548584/ [PubMed] [Google Scholar]
- 153.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–83. 10.1001/jama.2015.6199 [DOI] [PubMed] [Google Scholar]
- 154.Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755–96. [PubMed] [Google Scholar]
- 155.Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: systematic review of randomized controlled trials. Can Fam Physician. 2015;61(8):e372–81. [PMC free article] [PubMed] [Google Scholar]
- 156.Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182. 10.1002/14651858.CD012182.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campbell G, Stockings E, Nielsen S. Understanding the evidence for medical cannabis and cannabis-based medicines for the treatment of chronic non-cancer pain. Eur Arch Psychiatry Clin Neurosci. 2019;269(1):135–44. 10.1007/s00406-018-0960-9 [DOI] [PubMed] [Google Scholar]
- 158.Macken L, Hashim A, Mason L, Verma S. Permanent indwelling peritoneal catheters for palliation of refractory ascites in end-stage liver disease: a systematic review. Liver Int. 2019;39(9):1594–607. 10.1111/liv.14162 [DOI] [PubMed] [Google Scholar]
- 159.Macken L, Bremner S, Gage H, Touray M, Williams P, Crook D, et al. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther. 2020;52(1):107–22. 10.1111/apt.15802 [DOI] [PubMed] [Google Scholar]
- 160.Cooper M, Pollard A, Pandey A, Bremner S, Macken L, Evans CJ, et al. Palliative long-term abdominal drains versus large volume paracentesis in refractory ascites due to cirrhosis (REDUCe Study): qualitative outcomes. J Pain Symptom Manage. 2021;62(2):312–25.e2. 10.1016/j.jpainsymman.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 161.Lepida A, Marot A, Trépo E, Degré D, Moreno C, Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther. 2019;50(9):978–87. 10.1111/apt.15502 [DOI] [PubMed] [Google Scholar]
- 162.Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, Peck-Radosavljevic M, et al. Alfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. J Hepatol. 2017;67(5):940–9. 10.1016/j.jhep.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 163.Wong F, Bendel E, Sniderman K, Frederick T, Haskal ZJ, Sanyal A, et al. Improvement in quality of life and decrease in large-volume paracentesis requirements with the automated low-flow ascites pump. Liver Transpl. 2020;26(5):651–61. 10.1002/lt.25724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Solbach P, Höner zu Siederdissen C, Wellhöner F, Richter N, Heidrich B, Lenzen H, et al. Automated low-flow ascites pump in a real-world setting: complications and outcomes. Eur J Gastroenterol Hepatol. 2018;30(9):1082–9. 10.1097/MEG.0000000000001149 [DOI] [PubMed] [Google Scholar]
- 165.Kaltsakas G, Antoniou E, Palamidas AF, Gennimata S-A, Paraskeva P, Smyrnis A, et al. Dyspnea and respiratory muscle strength in end-stage liver disease. World J Hepatol. 2013;5(2):56–63. 10.4254/wjh.v5.i2.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MAE, et al. International Liver Transplant Society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100(7):1440–52. 10.1097/TP.0000000000001229 [DOI] [PubMed] [Google Scholar]
- 167.American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159(1):321–40. 10.1164/ajrccm.159.1.ats898 [DOI] [PubMed] [Google Scholar]
- 168.Lv Y, Han G, Fan D. Hepatic hydrothorax. Ann Hepatol. 2018;17(1):33–46. 10.5604/01.3001.0010.7533 [DOI] [PubMed] [Google Scholar]
- 169.Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680–90. 10.1016/j.jpainsymman.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 170.Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: assessment, burdens, and etiologies. J Palliat Med. 2011;14(10):1167–72. 10.1089/jpm.2011.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tulaimat A, Patel A, Wisniewski M, Gueret R. The validity and reliability of the clinical assessment of increased work of breathing in acutely ill patients. J Crit Care. 2016;34:111–5. 10.1016/j.jcrc.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 172.Goldstein NE, Morrison RS. Evidence-based practice of palliative medicine, 1st edn. Elsevier Saunders; 2013. [Google Scholar]
- 173.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–3. 10.1002/hep.26359 [DOI] [PubMed] [Google Scholar]
- 174.Young S, Bermudez J, Zhang L, Rostambeigi N, Golzarian J. Transjugular intrahepatic portosystemic shunt (TIPS) placement: a comparison of outcomes between patients with hepatic hydrothorax and patients with refractory ascites. Diagn Interv Imaging. 2019;100(5):303–8. 10.1016/j.diii.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 175.Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429. 10.1002/14651858.CD006429.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qian Y, Wu Y, Rozman de Moraes A, Yi X, Geng Y, Dibaj S, et al. Fan therapy for the treatment of dyspnea in adults: a systematic review. J Pain Symptom Manage. 2019;58(3):481–6. 10.1016/j.jpainsymman.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 177.Kwekkeboom KL, Bratzke LC. A systematic review of relaxation, meditation, and guided imagery strategies for symptom management in heart failure. J Cardiovasc Nurs. 2016;31(5):457–68. 10.1097/JCN.0000000000000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feliciano JL, Waldfogel JM, Sharma R, Zhang A, Gupta A, Sedhom R, et al. Pharmacologic interventions for breathlessness in patients with advanced cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(2):e2037632. 10.1001/jamanetworkopen.2020.37632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, Hameleers N, Wouters EFM, Janssen DJA. Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med. 2020;180(10):1306–14. 10.1001/jamainternmed.2020.3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Montagnese S, Bajaj JS. Impact of hepatic encephalopathy in cirrhosis on quality-of-life issues. Drugs. 2019;79(1):11–6. 10.1007/s40265-018-1019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shen YC, Chang YH, Fang CJ, Lin YS. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J. 2019;18(1):34. 10.1186/s12937-019-0461-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzales AD, Reinert JP. Zinc and probiotic therapy for management of hepatic encephalopathy. Sr Care Pharm. 2020;35(4):171–5. 10.4140/TCP.n.2020.171 [DOI] [PubMed] [Google Scholar]
- 183.Hanai T, Shiraki M, Imai K, Suetugu A, Takai K, Shimizu M. Usefulness of carnitine supplementation for the complications of liver cirrhosis. Nutrients. 2020;12(7):1915. 10.3390/nu12071915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Angeli P, Albino G, Carraro P, Pria MD, Merkel C, Caregaro L, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology. 1996;23(2):264–73. 10.1002/hep.510230211 [DOI] [PubMed] [Google Scholar]
- 185.Marotta PJ, Graziadei IW, Ghent CN. Muscle cramps: a ‘complication’ of cirrhosis. Can J Gastroenterol. 2000;14:214916. 10.1155/2000/214916. [DOI] [PubMed] [Google Scholar]
- 186.Chatrath H, Liangpunsakul S, Ghabril M, Otte J, Chalasani N, Vuppalanchi R. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125(10):1019–25. 10.1016/j.amjmed.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vidot H, Carey S, Allman-Farinelli M, Shackel N. Systematic review: the treatment of muscle cramps in patients with cirrhosis. Aliment Pharmacol Ther. 2014;40(3):221–32. 10.1111/apt.12827 [DOI] [PubMed] [Google Scholar]
- 188.Hidaka H, Nakazawa T, Kutsukake S, Yamazaki Y, Aoki I, Nakano S, et al. The efficacy of nocturnal administration of branched-chain amino acid granules to improve quality of life in patients with cirrhosis. J Gastroenterol. 2013;48(2):269–76. 10.1007/s00535-012-0632-x [DOI] [PubMed] [Google Scholar]
- 189.Yamamoto S, Ohmoto K, Ideguchi S, Yamamoto R, Mitsui Y, Shimabara M, et al. Painful muscle cramps in liver cirrhosis and effects of oral taurine administration. Article in Japanese. Nihon Shokakibyo Gakkai Zasshi. 1994;91(7):1205–9. [PubMed] [Google Scholar]
- 190.Matsuzaki Y, Tanaka N, Osuga T. Is taurine effective for treatment of painful muscle cramps in liver cirrhosis? Am J Gastroenterol. 1993;88(9):1466–7. [PubMed] [Google Scholar]
- 191.Jang ES, Hwang SH, Kim JW, Jeong SH. Effectiveness of 4-week oral taurine treatment for muscle cramps in patients with liver cirrhosis: a single-arm pilot study. Yonsei Med J. 2021;62(1):21–8. 10.3349/ymj.2021.62.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vidot H, Cvejic E, Carey S, Strasser SI, McCaughan GW, Allman-Farinelli M, et al. Randomised clinical trial: oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment Pharmacol Ther. 2018;48(7):704–12. 10.1111/apt.14950 [DOI] [PubMed] [Google Scholar]
- 193.Konikoff F, Ben-Amitay G, Halpern Z, Weisman Y, Fishel B, Theodor E, et al. Vitamin E and cirrhotic muscle cramps. Isr J Med Sci. 1991;27(4):221–3. [PubMed] [Google Scholar]
- 194.Chandok N, Tan P, Uhanova J, Shankar N, Marotta P. A pilot study of vitamin E for the treatment of cirrhotic muscle cramps. Liver Int. 2011;31(4):586–7. 10.1111/j.1478-3231.2011.02464.x [DOI] [PubMed] [Google Scholar]
- 195.Elfert AA, Abo Ali L, Soliman S, Zakaria S, Shehab El-Din I, Elkhalawany W, et al. Randomized placebo-controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(11):1280–4. 10.1097/MEG.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 196.Abd-Elsalam S, Arafa M, Elkadeem M, Elfert A, Soliman S, Elkhalawany W, et al. Randomized-controlled trial of methocarbamol as a novel treatment for muscle cramps in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31(4):499–502. 10.1097/MEG.0000000000001310 [DOI] [PubMed] [Google Scholar]
- 197.Abd-Elsalam S, Ebrahim S, Soliman S, Alkhalawany W, Elfert A, Hawash N, et al. Orphenadrine in treatment of muscle cramps in cirrhotic patients: a randomized study. Eur J Gastroenterol Hepatol. 2020;32(8):1042–5. 10.1097/MEG.0000000000001622 [DOI] [PubMed] [Google Scholar]
- 198.Nakanishi H, Kurosaki M, Tsuchiya K, Nakakuki N, Takada H, Matsuda S, et al. l-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(8):1540–3. 10.1016/j.cgh.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 199.Baskol M, Ozbakir O, Coşkun R, Baskol G, Saraymen R, Yucesoy M. The role of serum zinc and other factors on the prevalence of muscle cramps in non-alcoholic cirrhotic patients. J Clin Gastroenterol. 2004;38(6):524–9. 10.1097/01.mcg.0000129059.69524.d9 [DOI] [PubMed] [Google Scholar]
- 200.Kugelmas M. Preliminary observation: oral zinc sulfate replacement is effective in treating muscle cramps in cirrhotic patients. J Am Coll Nutr. 2000;19(1):13–15. 10.1080/07315724.2000.10718908 [DOI] [PubMed] [Google Scholar]
- 201.Ghabril M, Jackson M, Gotur R, Weber R, Orman E, Vuppalanchi R, et al. Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatol. 2017;15(8):1271–8. e6. 10.1016/j.cgh.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Plotogea OM, Ilie M, Bungau S, Chiotoroiu AL, Stanescu AMA, Diaconu CC. Comprehensive overview of sleep disorders in patients with chronic liver disease. Brain Sci. 2021;11(2):142. 10.3390/brainsci11020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27(2):339–45. 10.1002/hep.510270204 [DOI] [PubMed] [Google Scholar]
- 204.Formentin C, Garrido M, Montagnese S. Assessment and management of sleep disturbance in cirrhosis. Curr Hepatol Rep. 2018;17(1):52–69. 10.1007/s11901-018-0390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Montagnese S, De Pittà C, De Rui M, Corrias M, Turco M, Merkel C, et al. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2014;59(2):705–12. 10.1002/hep.26555 [DOI] [PubMed] [Google Scholar]
- 206.Chojnacki C, Wachowska-Kelly P, Blasiak J, Reiter RJ, Chojnacki J. Melatonin secretion and metabolism in patients with hepatic encephalopathy. J Gastroenterol Hepatol. 2013;28(2):342–7. 10.1111/jgh.12055 [DOI] [PubMed] [Google Scholar]
- 207.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14(32):3408–16. 10.2174/138161208786549281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shah NM, Malhotra AM, Kaltsakas G. Sleep disorder in patients with chronic liver disease: a narrative review. J Thorac Dis. 2020;12(Suppl 2):S248–60. 10.21037/jtd-cus-2020-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Montagnese S, Nsemi LM, Cazzagon N, Facchini S, Costa L, Bergasa NV, et al. Sleep-wake profiles in patients with primary biliary cirrhosis. Liver Int. 2013;33(2):203–9. 10.1111/liv.12026 [DOI] [PubMed] [Google Scholar]
- 210.Bajaj JS, Thacker LR, Leszczyszyn D, Taylor SA, Heuman DM, Raman S, et al. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(2):390–7.e1. 10.1016/j.cgh.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.De Rui M, Schiff S, Aprile D, Angeli P, Bombonato G, Bolognesi M, et al. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis. 2013;28(2):245–8. 10.1007/s11011-012-9360-4 [DOI] [PubMed] [Google Scholar]
- 212.Bruyneel M, Sersté T, Libert W, van den Broecke S, Ameye L, Dachy B, et al. Improvement of sleep architecture parameters in cirrhotic patients with recurrent hepatic encephalopathy with the use of rifaximin. Eur J Gastroenterol Hepatol. 2017;29(3):302–8. 10.1097/MEG.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 213.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- 214.De Rui M, Middleton B, Sticca A, Gatta A, Amodio P, Skene DJ, et al. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: effect of light therapy. Neurochem Res. 2015;40(2):284–92. 10.1007/s11064-014-1414-z [DOI] [PubMed] [Google Scholar]
- 215.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. 10.1016/j.smrv.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 216.Sharma MK, Kainth S, Kumar S, Bhardwaj A, Agarwal HK, Maiwall R, et al. Effects of zolpidem on sleep parameters in patients with cirrhosis and sleep disturbances: a randomized, placebo-controlled trial. Clin Mol Hepatol. 2019;25(2):199–209. 10.3350/cmh.2018.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.De Silva AP, Niriella MA, Ediriweera DS, De Alwis JP, Liyanage IK, Ettickan U, et al. Low-dose melatonin for sleep disturbances in early-stage cirrhosis: a randomized, placebo-controlled, cross-over trial. JGH Open. 2020;4(4):749–56. 10.1002/jgh3.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol. 2007;102(4):744–53. [DOI] [PubMed] [Google Scholar]
- 219.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(9):1670–5. 10.1016/j.cgh.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 220.Gerber LH, Weinstein AA, Mehta R, Younossi ZM. Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol. 2019;25(28):3669–83. 10.3748/wjg.v25.i28.3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Austin PW, Gerber L, Karrar AK. Fatigue in chronic liver disease: exploring the role of the autonomic nervous system. Liver Int. 2015;35(5):1489–91. 10.1111/liv.12784 [DOI] [PubMed] [Google Scholar]
- 222.Swain MG. Fatigue in liver disease: pathophysiology and clinical management. Can J Gastroenterol. 2006;20(3):624832. 10.1155/2006/624832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014;31(5):562–75. 10.1177/1049909113494748 [DOI] [PubMed] [Google Scholar]
- 224.Younossi ZM, Wong V-S, Anstee QM, Romero-Gomez M, Trauner MH, Harrison SA, et al. Fatigue and pruritus in patients with advanced fibrosis due to nonalcoholic steatohepatitis: the impact on patient-reported outcomes. Hepatol Commun. 2020;4(11):1637–50. 10.1002/hep4.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ekerfors U, Sunnerhagen KS, Westin J, Jakobsson Ung E, Marschall H-U, Josefsson A, et al. Muscle performance and fatigue in compensated chronic liver disease. Scand J Gastroenterol. 2019;54(7):925–33. 10.1080/00365521.2019.1635638 [DOI] [PubMed] [Google Scholar]
- 226.Kalaitzakis E, Josefsson A, Castedal M, Henfridsson P, Bengtsson M, Hugosson I, et al. Factors related to fatigue in patients with cirrhosis before and after liver transplantation. Clin Gastroenterol Hepatol. 2012;10(2):174–81.e1. 10.1016/j.cgh.2011.07.029 [DOI] [PubMed] [Google Scholar]
- 227.Wu LJ, Wu MS, Lien GS, Chen FC, Tsai JC. Fatigue and physical activity levels in patients with liver cirrhosis. J Clin Nurs. 2012;21(1–2):129–38. 10.1111/j.1365-2702.2011.03900.x [DOI] [PubMed] [Google Scholar]
- 228.Rossi D, Galant LH, Marroni CA. Psychometric property of fatigue severity scale and correlation with depression and quality of life in cirrhotics. Arq Gastroenterol. 2017;54(4):344–8. 10.1590/s0004-2803.201700000-85 [DOI] [PubMed] [Google Scholar]
- 229.Lee JY, Danford CJ, Trivedi HD, Tapper EB, Patwardhan VR, Bonder A. Treatment of fatigue in primary biliary cholangitis: a systematic review and meta-analysis. Dig Dis Sci. 2019;64(8):2338–50. 10.1007/s10620-019-5457-5 [DOI] [PubMed] [Google Scholar]
- 230.Swain MG, Jones DEJ. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int. 2019;39(1):6–19. 10.1111/liv.13919 [DOI] [PubMed] [Google Scholar]
- 231.Newton JL, Jones DE. Managing systemic symptoms in chronic liver disease. J Hepatol. 2012;56(Suppl 1):S46–55. 10.1016/S0168-8278(12)60006-3 [DOI] [PubMed] [Google Scholar]
- 232.Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69(5):1164–77. 10.1016/j.jhep.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 233.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1920–6.e2. 10.1016/j.cgh.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 234.Mücke M, Mochamat M, Cuhls H, Peuckmann-Post V, Minton O, Stone P, et al. Pharmacological treatments for fatigue associated with palliative care: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2016;7(1):23–7. 10.1002/jcsm.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mücke M, Mochamat M, Cuhls H, Peuckmann-Post V, Minton O, Stone P, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015;5:CD006788. 10.1002/14651858.CD006788.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rabkin JG, McElhiney MC, Rabkin R. Modafinil and armodafinil treatment for fatigue for HIV-positive patients with and without chronic hepatitis C. Int J STD AIDS. 2011;22(2):95–101. 10.1258/ijsa.2010.010326 [DOI] [PubMed] [Google Scholar]
- 237.Silveira MG, Gossard AA, Stahler AC, Jorgensen RA, Petz JL, Ali AH, et al. A randomized, placebo-controlled clinical trial of efficacy and safety: modafinil in the treatment of fatigue in patients with primary biliary cirrhosis. Am J Ther. 2017;24(2):e167–76. 10.1097/MJT.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 238.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. 10.1002/hep.30145 [DOI] [PubMed] [Google Scholar]
- 239.Hegade VS, Kendrick SF, Jones DE. Drug treatment of pruritus in liver diseases. Clin Med (Lond). 2015;15(4):351–7. 10.7861/clinmedicine.15-4-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bhalerao A, Mannu GS. Management of pruritus in chronic liver disease. Dermatol Res Pract. 2015;2015:295891. 10.1155/2015/295891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Thurmond RL, Kazerouni K, Chaplan SR, Greenspan AJ. Antihistamines and itch. In: Cowan AEE, Yosipovitch G, eds. Pharmacology of Itch. Springer; 2015:257–90. 10.1007/978-3-662-44605-8_15 [DOI] [PubMed] [Google Scholar]
- 242.Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra- and extra-hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6(12):773–6. [PubMed] [Google Scholar]
- 243.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26(8):943–8. 10.1111/j.1478-3231.2006.01326.x [DOI] [PubMed] [Google Scholar]
- 244.Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007;102(7):1528–36. [DOI] [PubMed] [Google Scholar]
- 245.Bergasa NV, Alling DW, Talbot TL, Swain MG, Yurdaydin C, Turner ML, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis. a double-blind, randomized, controlled trial. Ann Intern Med. 1995;123(3):161–7. 10.7326/0003-4819-123-3-199508010-00001 [DOI] [PubMed] [Google Scholar]
- 246.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113(4):1264–9. 10.1053/gast.1997.v113.pm9322521 [DOI] [PubMed] [Google Scholar]
- 247.Terg R, Coronel E, Sordá J, Muñoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717–22. 10.1016/s0168-8278(02)00318-5 [DOI] [PubMed] [Google Scholar]
- 248.Mansour-Ghanaei F, Taheri A, Froutan H, Ghofrani H, Nasiri-Toosi M, Bagheradeh A-H, et al. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12(7):1125–8. 10.3748/wjg.v12.i7.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Siemens W, Xander C, Meerpohl JJ, Buroh S, Antes G, Schwarzer G, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database Syst Rev. 2016;11:CD008320. 10.1002/14651858.CD008320.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45(3):666–74. 10.1002/hep.21553 [DOI] [PubMed] [Google Scholar]
- 251.Mazzetti M, Marconi G, Mancinelli M, Benedetti A, Marzioni M, Maroni L. The management of cholestatic liver diseases: current therapies and emerging new possibilities. J Clin Med. 2021;10(8):1763. 10.3390/jcm10081763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Khanna A, Jones DE. Novel strategies and therapeutic options for the management of primary biliary cholangitis. Therap Adv Gastroenterol. 2017;10(10):791–803. 10.1177/1756283X17728669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Feldman AG, Sokol RJ. Recent developments in diagnostics and treatment of neonatal cholestasis. Semin Pediatr Surg. 2020;29(4):150945. 10.1016/j.sempedsurg.2020.150945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sarkar M, Brady CW, Fleckenstein J, Forde KA, Khungar V, Molleston JP, et al. Reproductive health and liver disease: practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):318–65. 10.1002/hep.31559 [DOI] [PubMed] [Google Scholar]
- 255.Paternostro R, Heinisch BB, Reiberger T, Mandorfer M, Schwarzer R, Seeland B, et al. Erectile dysfunction in cirrhosis is impacted by liver dysfunction, portal hypertension, diabetes and arterial hypertension. Liver Int. 2018;38(8):1427–36. 10.1111/liv.13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huyghe E, Kamar N, Wagner F, Capietto A-H, El-Kahwaji L, Muscari F, et al. Erectile dysfunction in end-stage liver disease men. J Sex Med. 2009;6(5):1395–401. 10.1111/j.1743-6109.2008.01169.x [DOI] [PubMed] [Google Scholar]
- 257.Klein J, Tran S-N, Mentha-Dugerdil A, Giostra E, Majno P, Morard I, et al. Assessment of sexual function and conjugal satisfaction prior to and after liver transplantation. Ann Transplant. 2013;18:136–45. 10.12659/AOT.883860 [DOI] [PubMed] [Google Scholar]
- 258.Palaiodimos L, Herman HS, Wood E, Karamanis D, Martinez-Rodriguez C, Sanchez-Lopez A, et al. Practices and barriers in sexual history taking: a cross-sectional study in a public adult primary care clinic. J Sex Med. 2020;17(8):1509–19. 10.1016/j.jsxm.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 259.Ma BO, Shim SG, Yang HJ. Association of erectile dysfunction with depression in patients with chronic viral hepatitis. World J Gastroenterol. 2015;21(18):5641–6. 10.3748/wjg.v21.i18.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chiang HC, Chien YC, Lin PY, Lee HL, Chen YL. Assessing men with erectile dysfunction before and after living donor liver transplantation in real-world practice: integrating laboratories into clinical settings. PLoS One. 2018;13(11):e0206438. 10.1371/journal.pone.0206438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Heidelbaugh JJ. Management of erectile dysfunction. Am Fam Physician. 2010;81(3):305–12. [PubMed] [Google Scholar]
- 262.Neong SF, Billington EO, Congly SE. Sexual dysfunction and sex hormone abnormalities in patients with cirrhosis: review of pathogenesis and management. Hepatology. 2019;69(6):2683–95. 10.1002/hep.30359 [DOI] [PubMed] [Google Scholar]
- 263.Thakur J, Rathi S, Grover S, Chopra M, Agrawal S, Taneja S, et al. Tadalafil, a phosphodiesterase-5 inhibitor, improves erectile dysfunction in patients with liver cirrhosis. J Clin Exp Hepatol. 2019;9(3):312–7. 10.1016/j.jceh.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cheng CH, Wang YC, Wu TH, Lee C-F, Wu T-J, Chou H-S, et al. Sildenafil monotherapy to treat portopulmonary hypertension before liver transplant. Transplant Proc. 2019;51(5):1435–8. 10.1016/j.transproceed.2019.01.139 [DOI] [PubMed] [Google Scholar]
- 265.Mancuso L, Scordato F, Pieri M, Valerio E, Mancuso A. Management of portopulmonary hypertension: new perspectives. World J Gastroenterol. 2013;19(45):8252–7. 10.3748/wjg.v19.i45.8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vionnet J, Yerly P, Aubert J-D, Pascual M, Aldenkortt F, Berney T, et al. Management of severe portopulmonary hypertension with dual oral therapy before liver transplantation. Transplantation. 2018;102(5):e194. 10.1097/TP.0000000000002142 [DOI] [PubMed] [Google Scholar]
- 267.Mindikoglu AL, Dowling TC, Schaub DJ, Hutson WR, Potosky DR, Christenson RH, et al. Pharmacokinetics and tolerability of intravenous sildenafil in two subjects with Child-Turcotte-Pugh Class C cirrhosis and renal dysfunction. Dig Dis Sci. 2015;60(11):3491–4. 10.1007/s10620-015-3771-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tadalafil for erectile dysfunction in patients with cirrhosis. Clinical Trials. June 23, 2021. [cited 2022 Jan 7]. https://clinicaltrials.gov/ct2/show/NCT03566914 [Google Scholar]
- 269.Beaudreau SA, Van Moorleghem K, Dodd SM, Liou-Johnson V, Suresh M, Gould CE. Satisfaction with a vacuum constriction device for erectile dysfunction among middle-aged and older veterans. Clin Gerontol. 2021;44(3):307–15. 10.1080/07317115.2020.1823922 [DOI] [PubMed] [Google Scholar]
- 270.Buganza-Torio E, Mitchell N, Abraldes JG, Thomas L, Ma M, Bailey RJ, et al. Depression in cirrhosis—a prospective evaluation of the prevalence, predictors and development of a screening nomogram. Aliment Pharmacol Ther. 2019;49(2):194–201. 10.1111/apt.15068 [DOI] [PubMed] [Google Scholar]
- 271.Nardelli S, Pentassuglio I, Pasquale C, Ridola L, Moscucci F, Merli M, et al. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metab Brain Dis. 2013;28(2):239–43. 10.1007/s11011-012-9364-0 [DOI] [PubMed] [Google Scholar]
- 272.Patidar KR, Thacker LR, Wade JB, White MB, Gavis EA, Fagan A, et al. Symptom domain groups of the patient-reported outcomes measurement information system tools independently predict hospitalizations and re-hospitalizations in cirrhosis. Dig Dis Sci. 2017;62(5):1173–9. 10.1007/s10620-017-4509-y [DOI] [PubMed] [Google Scholar]
- 273.Rogal SS, Udawatta V, Akpan I, Moghe A, Chidi A, Shetty A, et al. Risk factors for hospitalizations among patients with cirrhosis: a prospective cohort study. PLoS One. 2017;12(11):e0187176. 10.1371/journal.pone.0187176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jang SY, Rou WS, Kim SH, Lee BS, Eun HS. Association between new-onset liver cirrhosis and suicide risk in South Korea: a nationwide cohort study. Clin Mol Hepatol. 2021;27(2):283–94. 10.3350/cmh.2020.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levis B, Sun Y, He C, Wu Y, Krishnan A, Bhandari PM, et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA. 2020;323(22):2290–300. 10.1001/jama.2020.6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Ann Intern Med. 2019;170(6):369–79. 10.7326/M18-2229 [DOI] [PubMed] [Google Scholar]
- 277.Ghaffari Darab M, Hedayati A, Khorasani E, Bayati M, Keshavarz K. Selective serotonin reuptake inhibitors in major depression disorder treatment: an umbrella review on systematic reviews. Int J Psychiatry Clin Pract. 2020;24(4):357–70. 10.1080/13651501.2020.1782433 [DOI] [PubMed] [Google Scholar]
- 278.Naemi AR, Kashanitabar V, Kamali A, Shiva A. Comparison of the effects of haloperidol, metoclopramide, dexmedetomidine and ginger on postoperative nausea and vomiting after laparoscopic cholecystectomy. J Med Life. 2020;13(2):206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dossett ML, Cohen EM, Cohen J. Integrative medicine for gastrointestinal disease. Prim Care. 2017;44(2):265–80. 10.1016/j.pop.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uribe M, Ballesteros A, Strauss R, Rosales J, Garza J, Villalobos A, et al. Successful administration of metoclopramide for the treatment of nausea in patients with advanced liver disease. A double-blind controlled trial. Gastroenterology. 1985;88(3):757–62. 10.1016/0016-5085(85)90147-7 [DOI] [PubMed] [Google Scholar]
- 281.Magueur E, Hagege H, Attali P, Singlas E, Etienne JP, Taburet AM. Pharmacokinetics of metoclopramide in patients with liver cirrhosis. Br J Clin Pharmacol. 1991;31(2):185–7. 10.1111/j.1365-2125.1991.tb05511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lok AS, Ng IO. Prochlorperazine-induced chronic cholestasis. J Hepatol. 1988;6(3):369–73. 10.1016/s0168-8278(88)80056-4 [DOI] [PubMed] [Google Scholar]
- 283.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 284.Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6(1):69–75. 10.1016/j.cgh.2007.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hézode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani E-S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63–71. 10.1002/hep.20733 [DOI] [PubMed] [Google Scholar]
- 286.Rashid W, Patel V, Ravat V, Madireddy S, Jaladi PR, Tahir M, et al. Problematic cannabis use and risk of complications in patients with chronic hepatitis C. Cureus. 2019;11(8):e5373. 10.7759/cureus.5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Choi CJ, Weiss SH, Nasir UM, Pyrsopoulos NT. Cannabis use history is associated with increased prevalence of ascites among patients with nonalcoholic fatty liver disease: a nationwide analysis. World J Hepatol. 2020;12(11):993–1003. 10.4254/wjh.v12.i11.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Farooqui MT, Khan MA, Cholankeril G, Khan Z, Mohammed Abdul MK, Li AA, et al. Marijuana is not associated with progression of hepatic fibrosis in liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31(2):149–56. 10.1097/MEG.0000000000001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brunet L, Moodie EEM, Rollet K, Cooper C, Walmsley S, Potter M, et al. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis. 2013;57(5):663–70. 10.1093/cid/cit378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28(7):804969. 10.1155/2014/804969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wijarnpreecha K, Panjawatanan P, Ungprasert P. Use of cannabis and risk of advanced liver fibrosis in patients with chronic hepatitis C virus infection: a systematic review and meta-analysis. J Evid Based Med. 2018;11(4):272–7. 10.1111/jebm.12317 [DOI] [PubMed] [Google Scholar]
- 292.Altaii H, Al-Kindi SG, Yaqoob Z, Al-Khazaraji A, Romero-Marrero C. Place of death and hospice utilization among patients who die from cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):142–3. 10.1016/j.cgh.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 293.Myers RP, Quan H, Hubbard JN, Shaheen AA, Kaplan GG. Predicting in-hospital mortality in patients with cirrhosis: results differ across risk adjustment methods. Hepatology. 2009;49(2):568–77. 10.1002/hep.22676 [DOI] [PubMed] [Google Scholar]
- 294.Local Coverage Determination (LCD): Hospice Determining Terminal Status (L34538). Centers for Medicare & Medicaid Services. [cited 2021 Aug 5]. Available from: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=34538 [Google Scholar]
- 295.Fukui N, Golabi P, Otgonsuren M, Mishra A, Venkatesan C, Younossi ZM. Demographics, resource utilization, and outcomes of elderly patients with chronic liver disease receiving hospice care in the United States. Am J Gastroenterol. 2017;112(11):1700–8. 10.1038/ajg.2017.290 [DOI] [PubMed] [Google Scholar]
- 296.Brown CL, Hammill BG, Qualls LG, Curtis LH, Muir AJ. Significant morbidity and mortality among hospitalized end-stage liver disease patients in Medicare. J Pain Symptom Manage. 2016;52(3):412–9.e1. 10.1016/j.jpainsymman.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Salpeter SR, Luo EJ, Malter DS, Stuart B. Systematic review of noncancer presentations with a median survival of 6 months or less. Am J Med. 2012;125(5):512.e1–6. 10.1016/j.amjmed.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 298.Medici V, Rossaro L, Wegelin JA, Kamboj A, Nakai J, Fisher K, et al. The utility of the model for end-stage liver disease score: a reliable guide for liver transplant candidacy and for select patients, simultaneous hospice referral. Liver Transpl. 2008;14(8):1100–6. 10.1002/lt.21398 [DOI] [PubMed] [Google Scholar]
- 299.Odagiri T, Morita T, Aoyama M, Kizawa Y, Tsuneto S, Shima Y, et al. Families’ sense of abandonment when patients are referred to hospice. Oncologist. 2018;23(9):1109–15. 10.1634/theoncologist.2017-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Morrison RS, Augustin R, Souvanna P, Meier DE. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. J Palliat Med. 2011;14(10):1094–6. 10.1089/jpm.2011.9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Crousillat DR, Keeley BR, Buss MK, Zheng H, Polk DM, Schaefer KG. Palliative care education in cardiology. J Am Coll Cardiol. 2018;71(12):1391–4. 10.1016/j.jacc.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 302.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, et al. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11(5):433–43. 10.1093/eurjhf/hfp041 [DOI] [PubMed] [Google Scholar]
- 303.Bradley CT, Brasel KJ. Core competencies in palliative care for surgeons: interpersonal and communication skills. Am J Hosp Palliat Care. 2007;24(6):499–507. 10.1177/1049909107310063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the guidance document or the cited references.


