Abstract
Rationally targeted therapies have transformed cancer treatment, but many patients develop resistance through bypass signaling pathway activation. PF-07284892 (ARRY-558) is an allosteric SHP2 inhibitor designed to overcome bypass signaling mediated resistance when combined with inhibitors of various oncogenic drivers. Activity in this setting was confirmed in diverse tumor models. Patients with ALK fusion-positive lung cancer, BRAF V600E-mutant colorectal cancer, KRAS G12D-mutant ovarian cancer, and ROS1 fusion-positive pancreatic cancer, who previously developed targeted therapy resistance, were treated with PF-07284892 on the first dose level of a first-in-human clinical trial. After progression on PF-07284892 monotherapy, a novel study design allowed the addition of oncogene-directed targeted therapy that had previously failed. Combination therapy led to rapid tumor and ctDNA responses and extended the duration of overall clinical benefit.
Introduction
While single-agent targeted therapies have transformed cancer therapy for patients with oncogene-driven tumors, primary and secondary resistance can limit efficacy. Given that rationally selected combinations of targeted therapy are poised to overcome resistance, an increase in the number of combination therapy trials is an inevitable next step in precision medicine evolution. Unfortunately, traditional first-in-human (FIH) phase I clinical trials are inefficient at investigating drug combinations. FIH dose escalations are typically designed to treat patients with a single drug at a time. Drugs are only later combined after monotherapy exploration, a process that delays study completion. Furthermore, the utility of this strategy is questionable when monotherapy is unlikely to be effective. Designing combination trials that circumvent these limitations is a challenge.
This problem applies to two important circumstances. First, combination therapy is required for oncogene-driven cancers that acquire off-target resistance to targeted therapy. For example, reactivation of mitogen associated protein kinase (MAPK) pathway signaling may be induced shortly after starting targeted therapy treatment due to bypass receptor tyrosine kinase (RTK) activation (e.g., EGFR, KIT) (1–3); or after initial tumor response due to acquired genetic alterations (e.g., RAS mutations, MET amplification) (4–6). Second, targeted therapy combinations may be required for prevalent oncogenic drivers deemed intractable to single-agent targeted therapy, like de novo KRAS and PIK3CA mutations (7–10).
Both situations may be addressed by combination targeted therapies that include an inhibitor of SHP2, a protein tyrosine phosphatase. SHP2 reinforces activation of the MAPK and potentially other pathways through multiple mechanisms: dephosphorylation of inhibitory tyrosine phosphorylation on positive regulators (e.g., RTKs, Gab1, RAS) (11–13); dephosphorylation of activating tyrosine phosphorylation on negative regulators (e.g., Sprouty) (14); direct recruitment of Grb and SOS to RTKs (15,16); and stimulation of RAS GDP-GTP exchange (17). Bypass resistance can be overcome in preclinical models by combining a SHP2 inhibitor with inhibitors of diverse oncogenic drivers (8,17,18). A SHP2 inhibitor is unlikely to work on its own, however. FIH trials of the first SHP2 inhibitors to enter the clinic (e.g., RMC-4630, TNO155) clearly showed limited monotherapy activity and were not optimally designed to explore rational combinations (19,20).
In this study, we describe a bench-to-bedside approach to the characterization of combinations with a novel SHP2 inhibitor (PF-07284892/ARRY-558) that distinguishes itself from prior drug development programs by leveraging early introduction of combination therapy in a phase I trial. As opposed to treating patients with a SHP2 inhibitor alone in dose escalation, each patient with an oncogene-driven cancer was permitted the addition of matched targeted therapy after a lead-in period to characterize monotherapy safety, dose-limiting toxicity (DLT), pharmacokinetics (PK), pharmacodynamics (PD), and efficacy, prior to introduction of one of three rational combinations. This strategy resulted in rapid rescue of progressive disease on single-agent PF-07284892 and the establishment of proof-of-concept combination therapy activity.
Results
PF-07284892 Design and Preclinical Pharmacology and Monotherapy Activity
PF-07284892 (ARRY-558), an allosteric SHP2 inhibitor, was discovered by Array BioPharma (Methods). The x-ray crystal structure of PF-07284892 bound to SHP2 shows that PF-07284892 is encased in a tunnel at an interface between the N- and C-proximal SH2 and phosphatase domains (Figure 1A, Supplementary Table 1). Whereas the overall binding mode is like other SHP2 inhibitors, one difference is the fused dihydroindene phenyl of PF-07284892 which fits deeper into the tunnel and forms enhanced contacts with several residues (Supplementary Figure 1A–B).
Figure 1: PF-07284892 promotes antitumor efficacy in multiple oncogene-addicted models with upfront or acquired resistance to targeted therapies.
(A) X-ray crystal structure of PF-07284892-bound SHP2. N-SH2, C-SH2, and PTP domains are white, cyan, and tan, inhibitor is green, hydrogen bonds are yellow dashed lines, and cation: pi interactions are dark green lines. See Supplementary Table 1 for data collection and refinement statistics. (B-D) (Upper) The indicated human cancer cell lines were treated in vitro with each agent at the indicated concentrations for 4 (H3122 Lorla-06), 18 (VACO-432) or 24 (MIA PaCa-2) hours followed by preparation of cell lysates and analysis of the indicated protein by immunoblot. Quantitation of each band is shown in Supplementary Figure 3. (Lower) Immunodeficient mice (8 per group) were xenografted subcutaneously with the same human tumor cells used for in vitro signaling analysis. When tumors reached ~ 200 mm3, animals were treated orally with vehicle, PF-07284892 30 mg/kg QOD, lorlatinib, 3mg/kg QD, encorafenib 20 mg/kg QD + binimetinib 3.5 mg/kg BID, binimetinib 3.5 mg/kg BID, or with the indicated combinations (at the monotherapy doses.) Tumor size on days 22–29 were normalized to Day 1 prior to treatment. Abbreviations: BID—twice daily; C-— carboxy-proximal; CRC—colorectal cancer; kg—kilograms; mg—milligrams; mm3—cubic millimeters; N-—amino-proximal; nM—nanomolar; NSCLC—non-small cell lung cancer; every other day; p—phosphorylated; PDAC—pancreatic ductal adenocarcinoma; PTP—phosphatase; QD—daily; QOD; t—total.
In cell-free systems, PF-07284892 inhibited SHP2 biochemical activity with an IC50 of 21 nM (−/+ 5 nM, n=20) and demonstrated >~1000 -fold selectivity for SHP2 over 21 other phosphatases, including the closely related SHP1 protein (Supplementary Table 2). In cell-based assays PF-07284892 potently inhibited phosphorylated ERK (pERK) with low nM IC50s (Supplementary Table 3). PF-07284892 demonstrated favorable PK properties in animals (Supplementary Table 4). The potential for improved brain penetration compared to other SHP2 inhibitors was also observed (Supplementary Table 5). Intermittent dosing (e.g., every 2–3 days) maintained efficacy and was better tolerated (as determined by peripheral blood counts and hematocrit) than continuous daily dosing while maintaining efficacy (Supplementary Figure 2A–D).
Combination Therapy Preclinical Activity
To determine the effect of PF-07284892 treatment in combination with appropriate targeted therapies, three human tumor cell lines were investigated: NCI-H3122 lorR-06, an EML4-ALK fusion-positive lung cancer model with acquired resistance to next-generation ALK inhibitors (see Methods); VACO-432, a BRAF V600E-mutant colorectal cancer model, and MIA PaCa-2, a KRAS G12C-mutant pancreatic cancer model. Preclinically, SHP2 had been shown to contribute to primary or secondary targeted therapy resistance for each of the three driver-tumor contexts (8,17,18,21).
The following targeted therapies were chosen to combine with PF-07284892: lorlatinib (for ALK fusions), encorafenib + cetuximab or binimetinib (for BRAF V600E mutants), and binimetinib (for KRAS G12C mutants). These drugs were chosen based on clinical availability (e.g., we did not have access to a KRAS G12C inhibitor for clinical studies) and thus the potential to translate findings to the clinic. Agents were used at concentrations (in vitro) and doses (in vivo) informed by nonclinical studies (PF-07284892) or that approximated human exposures at clinically approved doses (all other combination agents).
For each cell line, treatment in vitro with PF-07284892 in combination with oncogene-matched targeted therapy led to maximum inhibition of pERK levels (60% to >90%) compared to PF-07284892 or each targeted therapy regimen alone (Figure 1B–D above; quantitation in Supplementary Figure 3A, B, and C, respectively).
Consistent with pERK suppression, oral treatment of mouse xenografts of each cell line with oncogene-matched targeted therapy in combination with PF-07284892 resulted in maximal tumor regression compared to either component alone (Figure 1B–D below; additional in vivo models in Supplementary Figure 4A–C). PF-07284892 at the same single oral dose (30 mg/kg) used for efficacy studies caused significant suppression of pERK in MIA PaCa-2 tumors (Supplementary Figure 4D).
Proof of Concept Clinical Activity
We designed a FIH phase I clinical trial of PF-07284892 in patients whose solid tumors demonstrated acquired or intrinsic resistance to oncogene-matched target therapy (NCT04800822). Compared to traditional phase I designs (Figure 2A), a novel dose escalation study design was employed that allowed the addition of (the same or similar) previously used oncogene-directed targeted therapy after a lead-in period of SHP2 inhibitor monotherapy (Figure 2B, Supplementary Figure 5). Twice weekly dosing of PF-07284892 was planned from the start of the trial based on nonclinical studies demonstrating improved therapeutic index (Supplementary Figure 2A–D). The sequential approach of giving monotherapy followed by combination therapy enabled assessment of safety, PK, preliminary efficacy, and contribution of effect for both PF-07284892 monotherapy and the added targeted therapies. We highlight below the first 4 patients enrolled to the first dose level and transitioned from monotherapy to combination. The disposition of all 8 patients treated at this dose level and adverse events experienced by these patients are summarized in Supplementary Table 6 and Supplementary Table 7, respectively.
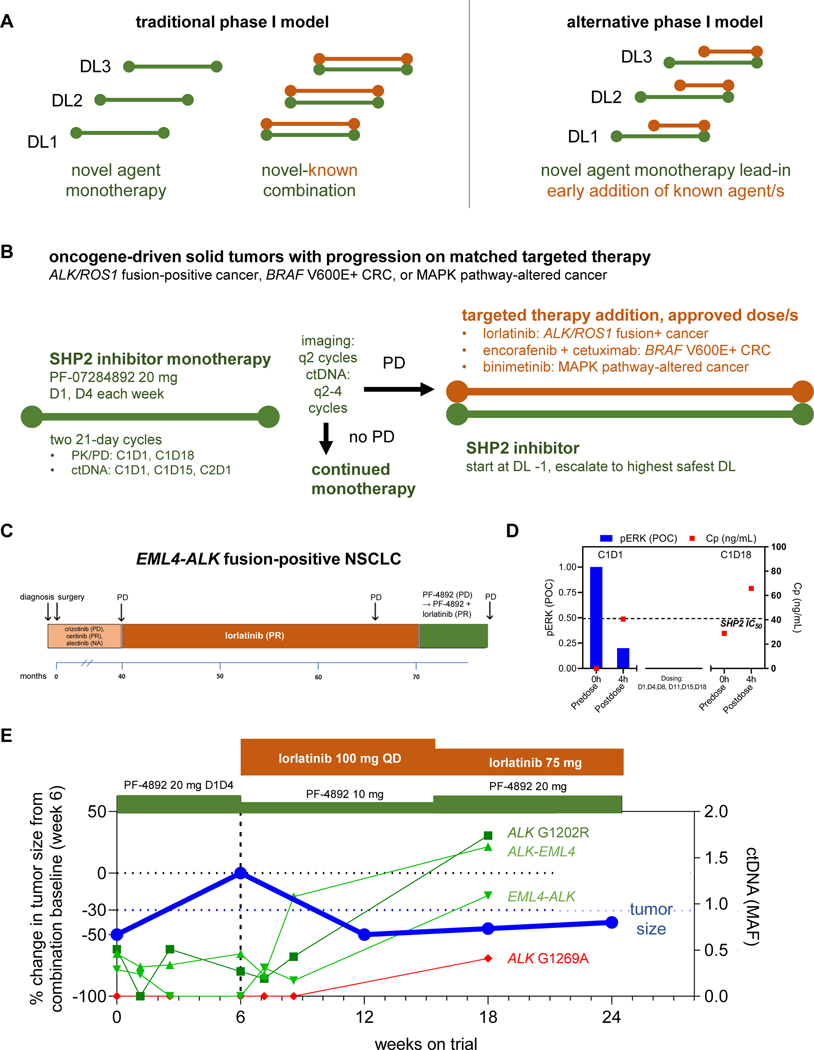
Figure 2: Proof-of-concept clinical activity in an ALK fusion-positive NSCLC patient with resistance to multiple ALK inhibitors.
(A) Overview of traditional vs alternative phase 1 combination trial design. (Left) Traditional phase 1 first-in-human trials require a new anticancer agent to be investigated as monotherapy, with the MTD/RDE identified, prior to allowing combination with a second anticancer drug. If the investigational agent is ineffective on its own, treated patients do not have the opportunity to benefit from a potentially efficacious combination. (Right) An alternative design allows patients to receive treatment in combination with a potentially effective combination after an initial period of treatment with the study drug as monotherapy. (B) Implementation of early combination testing strategy with the investigational SHP2 inhibitor PF-07284892. Prior to trial enrollment, eligible patients have experienced PD with appropriate targeted therapy. Patients begin treatment with PF-07284892 monotherapy on study. Early combination with appropriate targeted therapy (lorlatinib, encorafenib + cetuximab, or binimetinib, each at the approved dose) may be initiated after a minimum of 6 weeks of PF-07284892 monotherapy, in the absence of grade ≥3 toxicity, and after PD (tumor growth < +20% with symptoms of progressive disease was allowed). At the start of combination, the PF-07284892 dose must be lowered if the monotherapy dose level the patient was enrolled to has not yet been cleared from a safety perspective. The dose may subsequently be escalated to the highest monotherapy dose that has been cleared. (C) The patient’s previous systemic therapies included four approved ALK inhibitors. Parentheses show best overall response to each treatment. (D) Peripheral blood was isolated from the patient prior to and four hours after dosing with PF-07284892 on C1D1 and C1D18 and levels of PF-07284892 in plasma and of pERK in ex vivo CSF1-stimulated peripheral blood monocytes were analyzed (C1D18 samples for pERK were not available). The last dose of PF-07284892 prior to the C1D18 pre-dose sampling was C1D15. The horizontal dashed line indicates PF-07284892 concentration required to inhibit 50% of phosphorylated ERK in cells in vitro. (E) Changes in the sum of the longest tumor diameters of target lesions (blue, normalized to the start of combination) and in EML4-ALK, ALK-EML4, ALK G1202R (shades of green), and ALK G1269A (red) in ctDNA. Abbreviations: C—cycle; CRC—colorectal cancer; ctDNA—circulating tumor DNA; C—plasma concentration; D—day; DL—dose level; MAF—mean allele frequency; mg—milligrams; mL—milliliters; monotx—monotherapy; ng—nanograms; NA—not available; pERK—phosphorylated ERK; PK/PD—pharmacokinetics/ pharmacodynamics; POC—percent of control; PR—partial response; PD—progressive disease; PF-4892—PF-07284892; SD—stable disease.
EML4-ALK Lung Cancer.
A patient with an EML4-ALK fusion-positive NSCLC was previously treated with ALK inhibitors crizotinib, ceritinib, alectinib, and lorlatinib (Figure 2C). Two weeks after discontinuing lorlatinib for progression, the patient started treatment with PF-07284892 monotherapy (20 mg orally twice weekly [BIW] on days 1 and 4) at dose level 1. PK analysis indicated sustained plasma levels of PF-07284892 despite intermittent dosing (29 ng/mL pre-dose cycle 1 day 18 (C1D18, 3 days after prior dose) vs. 41 ng/mL 4 hours after C1D1 dose (Figure 2D red squares; complete PK curves shown in Supplementary Figure 6). PD analysis indicated 80% decrease in the levels of pERK in peripheral blood monocytes 4 hours after dosing on C1D1 (Figure 2D blue bars). ctDNA showed clear decrease in EML4-ALK but less in ALK-EML4 or ALK G1202R (Figure 2E).
Despite this, computed tomographic (CT) imaging after 6 weeks of PF-07284892 monotherapy demonstrated progressive disease (PD, +50%; Figure 2E). In a “typical” phase 1 clinical trial, this patient would have discontinued treatment. Instead, the patient continued PF-07284892 (at an initial lower dose of 10 mg twice weekly—Figure 2E and Methods) and lorlatinib was added (100 mg daily [QD], the dose the patient previously progressed on).
A partial response (PR, −50%) was achieved after 6 weeks of combination therapy (study week 12); pre-combination therapy imaging was used as a baseline for this comparison (Figure 2E). ALK-EML4 and ALK G1202R in ctDNA showed early decreases after one week of combination, then all 3 ctDNA species increased after 3 weeks of combination (study week 9) (Figure 2E). After 9 weeks of combination (study week 15), the PF-07284892 dose was increased to 20 mg BIW while the dose of lorlatinib was reduced to 75 mg QD (for grade 2 depression related to lorlatinib); no AEs required PF-07284892 dose modification and there were no DLTs. The PR was confirmed after 12 weeks of combination therapy (study week 18), with slight increase in target lesions, further increase in ALK fusions and ALK G1202R, and new appearance of ALK G1269A, in ctDNA. The patient remained on the combination for 4.5 months (6 months on trial) until PD (Figure 2E).
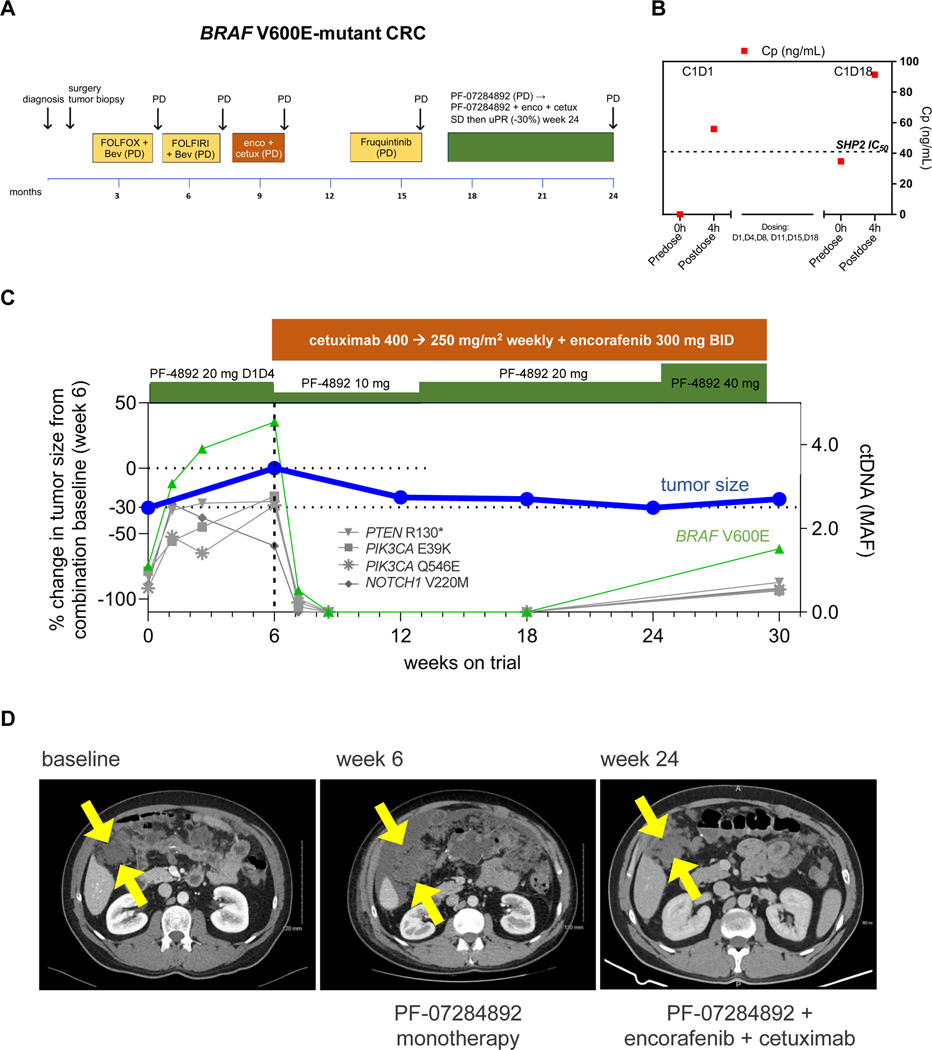
BRAF V600E Colorectal Cancer.
A patient with metastatic BRAF V600E colorectal cancer was treated with chemotherapy, encorafenib + cetuximab, and fruquintinib (VEGFR inhibitor) (Figure 3A). PF-07284892 monotherapy (same dose level as prior patient) was initiated. PK analysis indicated sustained plasma levels of PF-07284892 (35 ng/mL C1D18 vs 56 ng/mL C1D1 (Figure 3B). Blood samples were not available for pERK analysis. PD (+43%, new lesions) was observed after 6 weeks, together with increased BRAF V600E (and additional mutations) in ctDNA (Figure 3C, D).
Figure 3. PF-07284892 overcomes intrinsic resistance to encorafenib + cetuximab in a BRAF V600E-mutant CRC patient.
(A) The patient’s previous systemic therapies including chemotherapy+ bevacizumab, encorafenib + cetuximab, and fruquintinib, with best overall response PD, indicating primary progression/intrinsic resistance to each therapy. (B) Levels of PF-07284892 (red squares) in plasma as in Figure 2D; blood samples for pERK were not available. (C) Change in the sum of the longest tumor diameters of target lesions and in BRAF V600E (and other mutations) in ctDNA over time as in Figure 2E. (D) Imaging of a right sided intraabdominal target lesion mass during study treatment. The patient experienced one AE with monotherapy (grade 2 ascites not related to study treatment) and three grade 1 AEs with combination treatment (headache, fatigue, and acneiform rash [a known toxicity of cetuximab]). Abbreviations: as in Figure 2; bev—bevacizumab; cetux—cetuximab; enco – encorafenib.
PF-07284892 (initially decreased like the prior patient) was continued and encorafenib + cetuximab were added at their approved doses. After 6 weeks of the combination (study week 12), rapid and complete disappearance of BRAF V600E (and other mutations) in ctDNA occurred together with −22% decrease in the peritoneal target lesion and resolution of malignant ascites (Figure 3C). After 7 weeks of combination (study week 13), the PF-07284892 dose was increased to 20 mg BIW like the prior patient. BRAF V600E in ctDNA remained undetectable through 12 weeks of combination (study week 18). At 18 weeks of combination (study week 24), tumor reduction reached −30% (Figure 3C, D). Despite increasing the dose of PF-07284892 to 40 mg BIW, BRAF V600E was again detected in ctDNA together with a +20% increase in target lesion after 24 weeks of combination (study week 30), indicating PD (Figure 3C, D). The patient remained on combination therapy without disease progression for 6 months, three times longer than pre-study encorafenib + cetuximab. There were no DLTs and no dose modifications for AEs.
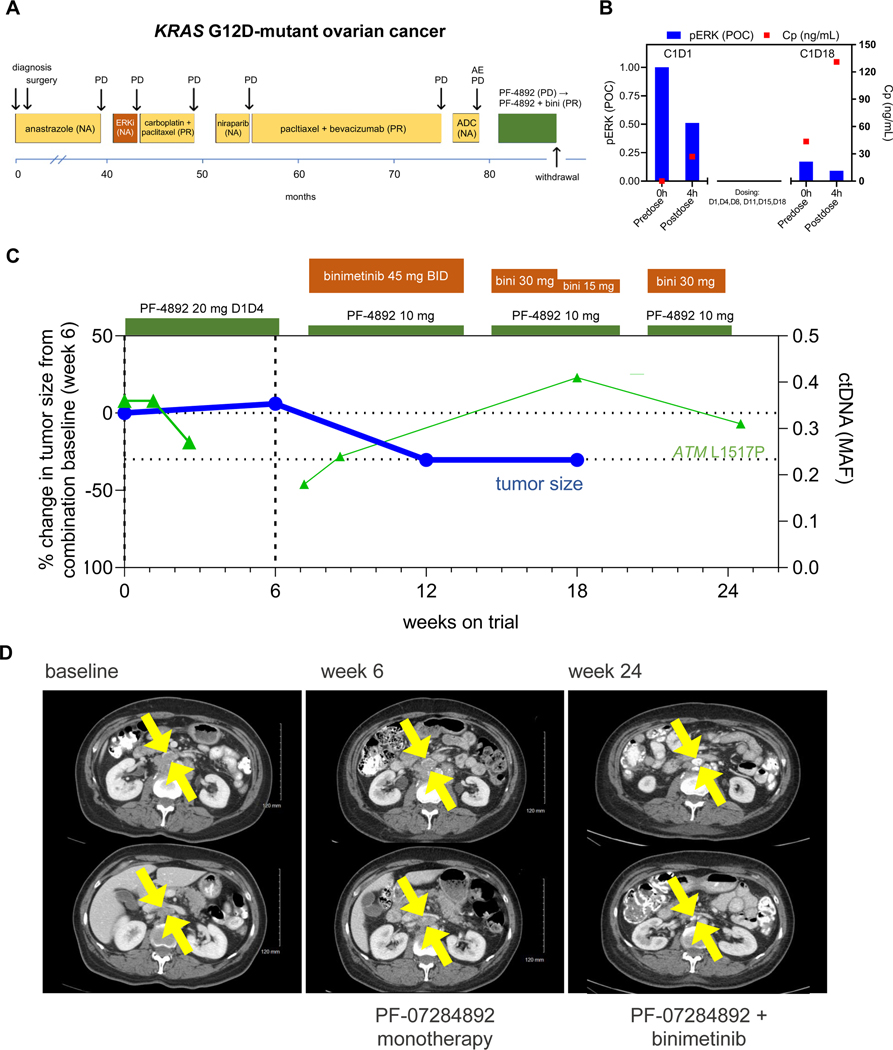
KRAS G12D Mutant Ovarian Cancer.
A patient with KRAS G12D mutant low-grade serous ovarian cancer underwent surgical resection and adjuvant anastrozole. For recurrent/metastatic disease, she received ASN-007 (investigational ERK inhibitor), 2 lines of chemotherapy, niraparib, and SGN-STNV (antibody-drug conjugate) (Figure 4A). PF-07284892 monotherapy was initiated at the same dose as prior patients. PK/PD analysis demonstrated sustained PF-07284892 and inhibition of pERK in peripheral blood monocytes, including on C1D18, 3 days after the last prior dose (Figure 4B). Nevertheless, after 6 weeks of monotherapy, imaging demonstrated +6% tumor growth in two target lesion mediastinal lymph nodes, together with worsening disease-related abdominal pain and distension (Figure 4C, D).
Figure 4. PF-07284892 sensitizes a patient with KRAS G12D-mutant ovarian cancer to the MAPK pathway inhibitor binimetinib.
(A) Prior treatments for metastatic disease included ASN-007 (investigational ERK inhibitor), chemotherapy, niraparib, and SGN-STV (investigational ADC). (B) Levels of PF-07284892 in plasma (red squares) and pERK in ex vivo CSF1-stimulated peripheral blood monocytes (blue bars) as in Figure 2D. (C) Change in the sum of the longest tumor diameters of target lesions and of ATM L1517P in ctDNA over time as in Figure 2E. (D) Imaging of two abdominal target lesions during study treatment. All treatment-related AEs were grade 1 except for edema (Grade 1–2, starting on monotherapy, worsening on combination, and leading to dose modification), fatigue (grade 2–3), weight gain, diarrhea, and eczema (each grade 2 and resolved by the end of treatment). Abbreviations: as in Figure 2; bini—binimetinib.
The patient continued PF-07284892 (decreased like the prior patients) and binimetinib was added at the approved dose. A PR (−34%) was achieved after 6 weeks of combination therapy (study week 12) (Figure 4C, D). Despite binimetinib dose reduction for edema, the patient continued combination treatment with PR for 5 months, at which time treatment was discontinued for persistent edema. There were no PF-07284892 dose modifications and no DLTs.
Although the founder KRAS G12D mutation was not detected in ctDNA, ATM L1517P appeared to decrease early during monotherapy and combination (study week 6 sample was not available) and increased at later time points (Figure 4C).
GOPC-ROS1 Fusion-Positive Pancreatic Cancer.
A patient with a GOPC-ROS1 fusion-positive pancreatic adenocarcinoma was previously treated with gemcitabine + abraxane + nivolumab followed by repotrectinib (best response stable disease, progressed after ~10 months, Supplementary Figure 7A). PF-07284892 monotherapy was initiated at the same dose as prior patients. PK analysis indicated sustained plasma levels of PF-07284892 despite intermittent dosing (Supplementary Figure 7B). After 6 weeks of monotherapy, imaging demonstrated PD in two target lesion liver metastases (+26% overall), together with an increase in the GOPC-ROS1 and CTNNB1 S45F in ctDNA (Supplementary Figure 7C, D).
The patient continued PF-07284892 and lorlatinib was added at 100 mg QD. After 2 weeks of combination (study week 8), both mutations in ctDNA decreased by 95% compared to baseline before starting any study treatment (Supplementary Figure 7C). After 6 weeks of combination (study week 12), target liver lesions decreased by −35% compared to pre-combination imaging consistent with PR, confirmed after 12 weeks of combination (study week 18) Supplementary Figure 7C, D). She remained on combination for a total of 7.5 months until PD.
Discussion
This study underscores the utility of early combination therapy exploration in drug development programs that include a novel agent. PF-07284892 is a highly potent, selective, allosteric SHP2 inhibitor. Compared to other SHP2 inhibitors, PF-07284892 has a long half-life, causing sustained pERK inhibition in preclinical models and peripheral blood monocytes from patients despite intermittent dosing, enhanced target binding, and potential intracranial coverage. Most importantly, significant preclinical antitumor activity was only observed in oncogene-driven cancers when administered with matched targeted therapy.
Traditional phase I trials would have explored PF-07284892 with a dose escalation design that only featured monotherapy, or only allowed combination after full monotherapy dose exploration. This strategy raises ethical concerns as patients risk exposure not only to subtherapeutic doses in early dose levels, but also ineffective treatment even at later doses. All patients in this series unsurprisingly had primary progression on PF-07284892 monotherapy. These findings are consistent with trials of other SHP2 inhibitors where little single-agent activity was observed (20,22). Furthermore, patients with florid disease progression may clinically deteriorate and miss the opportunity to receive subsequent cancer-directed therapies altogether.
Proof-of-concept data presented here demonstrate that early addition of combination therapy in dose escalation is safe and feasible. Primary progression on SHP2 inhibitor monotherapy was rescued with the addition of matched targeted therapy in patients with cancers driven by BRAF V600E, KRAS G12D, and ALK/ROS1 fusions, each of whom had progressed on the same/similar targeted therapy prior to study entry. Benefit correlated with molecular response in founder or companion alterations in ctDNA after combination therapy initiation. These outcomes were enabled by the trial’s contemporary study design approved by regulatory authorities. This design is scientifically informed and patient-centric and should be considered by other drug development stakeholders for future trials.
The preclinical and clinical combination therapy activity presented here support the role of SHP2 as an “Achilles’ heel” whose inhibition may sensitize or re-sensitize diverse tumors to targeted therapy. Of note, while responses to upfront combination of KRAS G12C and SHP2 inhibitors (e.g., JDQ443 and TNO155 or adagrasib and RMC-4630) have previously been described in KRAS G12C-mutant cancers, the patients had not first received either agent as monotherapy, challenging ascertaining the need for combination treatment (20,23). Our data support combination therapy in other oncogene-driven cancers. We are not aware of previous reports of responses to SHP2 + fusion kinase inhibitor therapy in ALK/ROS1 fusion-positive cancer patients.
Early combination therapy exploration likewise enables early safety data readouts. Other SHP2 inhibitors had narrow therapeutic indexes (TI), requiring intermittent dosing to mitigate on-target toxicity. PF-07284892 preclinical data demonstrated consistent activity with wider TI with intermittent dosing and led us to pursue intermittent dosing in patients from the start (Supplementary Figure 2A–D). Although two of four patients required dose decreases of the combination agent due to drug-related depression (lorlatinib) or edema (binimetinib), none required dose reduction of PF-07284892 for AEs, and mostly low-grade toxicities were observed with combination therapy in the rest. Although earlier in development, PF-07284892’s overall safety profile appears like other investigational SHP2 inhibitors (compared in Supplementary Table 8). We note the potential for consistent target inhibition by PF-07284892 (as measured by inhibition of pERK in patient monocytes) despite intermittent dosing.
There are limitations to this study. As summarized in Supplemental Table 6, some patients were not able to start or receive sufficient combination treatment due to clinical deterioration, disease progression, and/or toxicity during monotherapy. The number of patients treated with combination therapy should be increased to firmly establish the contribution of individual components to observed clinical effects. Higher doses of PF-07284892 should be explored to determine if even greater pERK inhibition and efficacy in patients is achieved.
Two patients had previously been treated with targeted therapy that differed from the actual combination agent provided on study: an investigational ERK inhibitor for the ovarian cancer patient, and repotrectinib for the pancreatic cancer patient. Although it is possible that the efficacy observed in these two patients was driven primarily by the study combination agent patients were not previously exposed to (binimetinib or lorlatinib, respectively), this seems less likely. The ERK inhibitor inhibits the MAPK pathway downstream of binimetinib and the absence of acquired resistance mutations in GOPC-ROS1 may be consistent with occult bypass resistance to repotrectinib that is not generally sensitive to single-agent targeted therapy. Furthermore, lorlatinib is not clearly active after reprotrectinib (24).
Regarding long-term combination therapy toxicities, clearly attributing side effects to individual drug components or their combination may be challenging, although in a traditional phase 1 design, if monotherapy is ineffective, most patients will discontinue treatment early and long-term side effects cannot be interrogated. Finally, although knowing the clinical AE profile of similar drugs is helpful, it is not necessary for this type of trial design if robust nonclinical data are available on potential efficacy, toxicities, and drug-drug interactions that support drug combination.
In summary, we describe the discovery and early clinical development of the investigational SHP2 inhibitor PF-07284892 using a novel FIH phase 1 clinical trial design that allowed each patient to receive one of three rational combinations of targeted therapy and PF-07284892. This approach led to tumor and ctDNA reductions and extended clinical benefit for four patients treated at the first dose level. We are not aware of another trial where the first patients in dose escalation had access to rational combination treatments instituted immediately upon monotherapy progression. This phase 1 study is ongoing and will more fully evaluate upfront combination therapy safety and efficacy at higher doses in patients with prior progression on appropriate targeted treatment.
Methods
PF-07282892 structure and design.
See US Patent 11,634,417, Example No. 6.
SHP2 protein expression and purification.
N-terminally poly-His tagged SHP2(2–257) for enzyme assay and SHP2(1–525) for crystallography were expressed in E. coli BL21(DE3) and purified by standard methods including metal affinity, anionic ion-exchange, followed by size-exclusion column chromatography. The poly-His tag was removed from SHP2(1–525) by the TEV protease that recognizes the TEV cleavage sequence engineered between the poly-His tag and the SHP2(1–525) amino sequence. The SH2(1–525) was concentrated to 8mg/mL in a buffer of 25mM Tris, pH8.0, 150mM NaCl and 1mM tris(2-carboxyethyl)phosphine (TCEP) for crystallization.
Co-crystallization, X-ray data collection, data processing, and structure solution of SHP2 with PF-07282892.
The inhibitor bound SHP2 protein complex was prepared by mixing purified SHP2(1–525) with a five-fold molar excess of PF-07282892 (in DMSO) and incubated on ice for at least 30 minutes. Equal volume of SHP2: PF-07282892 complex and the reservoir solution, which consists of 9% PEG3350, 0.1M Bicine, pH9.2, 30mM ammonium acetate and 5% Tacsimate were mixed with additional micro-crystal seeds to promote the crystallization process. Crystallization was performed by the hanging-drop vapor diffusion method at 14 °C for ~ 7 days before harvesting. Rectangular plate crystals with sizes of 200–300 micros in two dimensions were collected and cryoprotected in the reservoir solution containing 25% (v/v) glycerol and flash-frozen in a 100K nitrogen gas stream. X-ray diffraction data were collected on a Rigaku FRE SuperBright X-ray generator equipped with Confocal VariMax optics and EIGER 1M detector. Diffraction data were processed using Mosflm at the CCP4 software package (25). Structures were solved by molecular replacement using a published SHP structure (PDB ID: 2SHP) as a search model. Itinerate rounds of structure refinement (26) and manual model rebuilding were performed with Refmac5 (27) and COOT (28). Data collection and refinement statistics are shown in Supplementary Table 1. The image in Figure 1A was generated using the Maestro graphical interface (version 13.3.121; SchrÖdinger LLC).
SHP2 Enzyme assays.
C-terminal, Histidinex6 (His6) tagged full-length SHP2 (amino acids 2–527) was recombinantly expressed in and purified from E. Coli using standard methods. Fluorescence intensity kinetic assays were configured for full-length SHP2 to monitor the amount of DiFMU (6,8-difluoro-7-hydroxy-4-methylcoumarin) formed upon hydrolysis of DiFMUP (6,8-difluoro-4-methylumbelliferyl phosphate). The assay mixture consisted of 25 mM K+HEPES, pH 7.4, 0.01% Triton X-100, 1 mM DTT, 50 mM KCl, 100 μg/mL bovine γ-globulin, 50 μM DiFMUP, 1 μM SHP2 activating peptide (LN(pY)IDLDLV(dPEG8)LST(pY)-ASINFQK-amide), 1 nM full-length SHP2 (His6-tagged SHP2(2–527), and 2% (v/v) DMSO (from compound). Compounds were typically diluted in DMSO across a 10-point dosing range created using a 3-fold serial dilution protocol at a top dose of 20 μM. The assays were run in 384-well, polystyrene, low-volume, non-treated, black microtiter plates (Costar 4511) in a final volume of 20 μL. Low control wells lacked enzyme. The assays were initiated by the addition of a mixture of SHP2 and the activating peptide and, following a 15-s mix on an orbital shaker, were read in kinetic mode for 15 min (30 s/cycle) at ambient temperature on a PerkinElmer EnVision microplate reader (, ). Initial velocities (slopes of the tangents at t = 0) were estimated from exponential fits to the slightly nonlinear progress curves and then were converted to percent of control (POC) using the following equation:
where is the average of the uninhibited controls and is the average of the background samples. A 4-parameter logistic model was the fit to the POC data for each compound. From that fit, the IC50 was estimated and is defined as the concentration of compound at which the curve crosses 50 POC.
Phosphatase profiling.
PF-07284892 was evaluated by Eurofins, Inc. with Phosphatase Profiler™ for 22 phosphatases (20 human phosphatases, YoPH (bacterial) and lambda (phage). Compounds were run at 10,000, 1,000, and 100 nM final compound concentration according to Eurofin’s specifications. IC50 values were estimated from the percent of control (POC) of the three concentrations evaluated.
Cell lines and xenografts.
NCI-H3122, HT29, MIAPaCa-2, and NCI-H1975 cells were obtained from ATCC, KYSE-520 from DSMZ, RT 112/84 from ECACC, and EBC-1 from JCRB. All cell lines were authenticated by STR profiling, and regularly evaluated for Mycoplasma (MycoAlert™, Lonza, Inc.). CR5087 PDX was obtained from Crown Biosciences. MGH915–4Y10 PDX was provided by Dr. Aaron Hata, MGH Cancer Center. Lorla-06 (EML4-ALK) fusion-positive NSCLC cell lines were derived from parental NCI-H3122 cells by long-term in vitro culture in the presence of lorlatinib. See Supplementary Methods for additional molecular validation of cell lines and PDXs.
Quantitative phospho-ERK cell analysis.
Cells were seeded at 5e4/well into clear, black-bottom 96-well plates, incubated overnight at 37°C, incubated for 1 hour with a 9-point dilution series of each inhibitor, followed by followed by 3.7% formaldehyde fixation, costaining with pERK and GAPDH antibodies, costaining with goat secondary antibodies conjugated to IrDye 800CW (for pERK) or IRDye 680RD (for GAPDH), and analysis of staining intensity by InCell Western (Li-Cor, Inc.). Signal intensity for pERK was normalized to GAPDH and the DMSO-treated control samples to generate percent of control (POC) data, which were then plotted versus compound concentration using GraphPad Prism 5 software to generate IC50 data using a 3-parameter curve fit. See Supplementary Table 9 for sources of primary antibodies.
Immunoblotting.
Each cell line was seeded in 12-well plates at 2.5e5 cells/well (1ml total volume), incubated overnight at 37°C, and incubated for the indicated times with the indicated concentrations of each inhibitor dissolved in DMSO (DMSO used for vehicle control). Cells were lysed with 100μL Cell Lysis Buffer (Cell Signaling Technologies), processed for SDS-PAGE, and transferred to nitrocellulose membrane for Immunoblot analyses with the indicated primary and appropriate antibodies, followed imaging analysis (Bio-Rad ChemiDoc XR/ECL for Figure 1B; LiCor Odyssey Scanner/fluorescent imaging for Figure 1C–D). See Supplementary Table 9 for sources antibodies.
Animal care, xenograft preparation, and treatment.
All procedures performed on animals were in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee. Tissue samples were collected in accordance with regulations and established guidelines for humane treatment of research animals and were reviewed and approved by an Institutional Animal Care and Use Committee. All animals were obtained at 6–8 weeks of age (Envigo), housed in groups of 5 and allowed a one-week acclimation period before cancer cell injection. Food, water, temperature, and humidity were prepared per Pharmacology Testing Facility performance standards (SOP’s) which are in accordance with the 2011 Guide for the Care and Use of Laboratory Animals (NRC) and AAALAC-International.
Each cell line (5 × 106 cells) or PDX (cell suspension prepared with Miltenyi gentleMACS) was injected subcutaneously into the right flank of female Foxn1nu mice and allowed to grow to approximately 200 mm3 (efficacy) or approximately 500 mm3 (pharmacokinetics/ pharmacodynamics) prior to randomization by tumor size intro dosing groups of 8 (for efficacy) or 3 (for PK-PD) analysis. Animals were dosed by oral gavage with vehicle (1% carboxymethylcellulose/ 0.5% Tween-80), PF-07284892 (20% hydroxypropyl-β-cyclodextrin in 50 mM citric acid, pH 4), lorlatinib (water for injection pH 3), encorafenib plus binimetinib (1% carboxymethylcellulose/ 0.5% Tween-80), binimetinib (1% carboxymethylcellulose/ 0.5% Tween-80), or their combination with PF-07284892.
Animal Efficacy studies.
Animals were dosed with vehicle, PF-07284892 (30 mg/kg QOD), appropriate targeted therapy (lorlatinib, 3mg/kg QD, encorafenib 20 mg/kg QD + binimetinib 3.5 mg/kg BID, binimetinib 3.5 mg/kg BID), or the combination of each targeted therapy with PF-07284892 at the doses used for monotherapy. Tumor size was determined by the formula (length x width x width)/2. For each animal, tumor size on Days 22–29 of treatment was normalized to tumor size on Day 0 immediately prior to first treatment and displayed as % Tumor Volume Change.
Animal PK assessment.
Concentrations of PF-07284892 in mouse, rat, dog, and monkey plasma and mouse brain were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS) following protein precipitation. Oral and intravenous mouse, rat, dog, and monkey pharmacokinetic parameters were calculated using non-compartmental analysis (NCA) of total PF-07284892 concentration in plasma, and mouse brain-to-plasma ratios were calculated following adjustment for the unbound fraction of PF-07284892 in both brain homogenate and plasma.
For the quantitation of PF-07284892 concentrations in plasma, blood samples were collected into tubes containing K2EDTA as anticoagulant and processed to the plasma component via centrifugation. A 12-point calibration curve, ranging from 0.282 to 50,000 ng/mL, was prepared in duplicate. A solution of 400 μg/mL PF-07284892 in DMSO was serially diluted 3-fold, and 2.5 μL of each standard solution was added to 20 μL of naïve plasma. To mimic extraction of the standard curve, 2.5 μL of DMSO was added to 20 μL aliquots of test plasma. Both calibration and test plasma samples were spiked with 2.5 μL of internal standard in DMSO.
For the quantitation of PF-07284892 in brain, whole brains were harvested from mice administered an oral dose of PF-07284892, weighed, and homogenized in matrix D lysing tubes (MP Biomedicals, Inc.; Santa Ana, CA) following the addition of 500 μL of 4:1 water: methanol. A 10-point calibration curve, ranging from 0.508 to 10,000 ng/mL, was prepared in duplicate. A solution of 400 μg/mL PF-07284892 in DMSO was serially diluted 3-fold in DMSO, and then 2.5 μL of each standard solution was added to 100 μL of naïve mouse brain homogenate. To mimic extraction of the standard curve, 2.5 μL of DMSO was added to 100 μL aliquots of test brain homogenate. Both calibration and test brain homogenate samples were spiked with 2.5 μL of internal standard in DMSO.
Proteins in plasma and brain preparations were precipitated by the addition of 300 μL of acetonitrile. Samples were vortex-mixed, and precipitated proteins were removed via centrifugation. A 75 μL aliquot of supernatant was transferred to a 96-well plate and diluted 1:1 with water for analysis.
Animal PK/PD studies.
Each animal was administered a single dose of 30 mg/kg PF-07284892 by oral gavage. The mice were euthanized at 1, 8, 24, and 48 hr post-dose by CO2 inhalation and tumors were excised, flash-frozen in liquid nitrogen, and stored at −80°C. Blood was collected into tubes containing 10% v/v EDTA, processed to the plasma component via centrifugation, and stored at −80°C for bioanalysis. To determine the levels of pERK in tumors, tumor tissue was homogenized in lysis buffer (1% NP-40, 20 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, 2mM EDTA, protease and phosphatase inhibitors) and centrifuged twice to separate insoluble material. Protein levels in each lysate were determined using Comassie protein reagent (Pierce, Inc.). pERK levels were determined in 250 μg each sample by immunoblot and normalized to GAPDH in the same lysate and vehicle-treated controls, and results were expressed as POC pERK levels in tumors. To determine the levels of PF-07284892 in plasma, see Animal PK assessment above.
Clinical studies
Trial design.
Patients were treated on the FIH phase 1 clinical trial of PF-07284892 (NCT04800822). The FDA and Institutional Review Boards from each site approved the trial, the studies were conducted in accordance with the Declaration of Helsinki and in compliance with all International Council for Harmonization Good Clinical Practice guidelines, and each patient (or legal guardians/representatives) provided written informed consent. Early combination was initiated after 2 cycles/6 weeks of PF-07284892 monotherapy, absent grade ≥3 toxicity, after radiographic evidence of disease progression (tumor growth < +20% with symptoms of progression was allowed) and starting at one dose level lower than the highest safe monotherapy dose of PF-07284892 based on an acceptable rate of first treatment cycle DLTs. Intra-patient dose escalation of PF-07284892 to previously determined safe dose(s) was permitted after 1 cycle and absent grade ≥3 toxicity.
Treatment and response assessment.
PF-07284892 was administered to patients as a powder-in-capsule. Lorlatinib, encorafenib, cetuximab, and binimetinib were administered at their labeled doses. Dose modifications and interruptions followed a prescribed algorithm. Adverse events were graded using CTCAE version 5.0. Response was evaluated by computed tomographic (CT) imaging using RECIST version 1.1 every 6 weeks.
Pharmacokinetic assessment.
Plasma samples were collected prior to treatment and at defined intervals after dosing on day 1 and day 18 of cycle 1 at the starting PF-07284892 monotherapy dose. PF-07284892 plasma concentrations were determined using a validated LC-MS/MS assay.
Patient pharmacodynamic assessment.
Whole blood was collected from patients in 4 mL collection tubes and shipped on the same day with refrigerated gel packs to the Pfizer Early Clinical Development Precision Medicine Flow Cytometry Lab in Groton, CT. Samples received within the 24 h stability window were processed and analyzed. Whole blood samples were stimulated with 9 ng/mL recombinant human M-CSF (R&D Systems) for 12 minutes at room temperature. Unstimulated samples were used as a control sample. Whole blood was stained by incubating with a mixture of antibodies to anti-CD45-APC-H7 (clone 2D1, Becton Dickinson), anti-CD14-BV421 (clone M5E2, Becton Dickinson), Erk1/2-AF-647 (clone 137F5, Cell Signaling), and pERK1/2 T202/Y204-PE (clone 20A, Becton Dickinson) for 30 min at room temperature in the dark. Samples were analyzed on Becton Dickinson FACSCanto Flow Cytometer, using BD FACSDiva software v8.0,1, Flow Jo V10. Whole blood samples were gated to exclude doublets by using FSC-A vs FSC-H. Cells were gated for CD45+ and CD14+ cells. Median fluorescent intensity (MFI) of ERK1/2 in CD14+ monocytes and MFI of pERK1/2 in CD14+ monocytes is reported. MFI of pERK1/2 in CD14+ monocytes at 9 ng/mL is normalized to the unstimulated sample to account for a change in cell numbers.
Patient plasma cfDNA extraction and analyses.
Whole blood was collected from patients in 2×10 ml collection tubes containing K2EDTA as anti-coagulant. Within 30 minutes, plasma was processed by centrifugation at approximately 1,600 X g for 10 min using a pre-chilled centrifuge set to 4°C. Plasma was then transferred to a 15mL centrifuge tube, centrifuged as above at approximately 3,000 X g for 25 min, aliquoted, and frozen at - 80°C until shipment to Guardant Health. Extraction of cfDNA and next generation sequencing was performed at Guardant Health using the G360 73-gene panel (Panel v 2.10, bioinformatics pipeline v 3.5.2). MAF of each mutation was plotted.
Supplementary Material
Significance.
PF-07284892-targeted therapy combinations overcame bypass signaling-mediated resistance in a clinical setting where neither component was active on its own. This provides proof-of-concept of the utility of SHP2 inhibitors in overcoming resistance to diverse targeted therapies and provides a paradigm for accelerated testing of novel drug combinations early in clinical development.
Acknowledgements
Pfizer Boulder R&D and Array BioPharma colleagues, including Shannon L. Winski, James F. Blake, Mark Chicarelli, and Lauren Hanson.
Funding
The study was funded by Pfizer. Dr. Drilon was supported in part by the National Cancer Institute of the National Institutes of Health P30 CA008748 and 1R01CA273224-01 grants.
Conflict of Interest Statement
Alexander Drilon. HONORARIA/ADVISORY BOARDS: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, ,MORE Health, Abbvie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical, Remedica Ltd, mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen, Entos, Treeline Bio, Prelude, Applied Pharmaceutical Science, Inc, AiCME, I3 Health, MonteRosa, Innocare, Boundless Bio; Equity: Treeline Bio; ASSOCIATED RESEARCH PAID TO INSTITUTION: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; COPYRIGHT: Selpercatinib-Osimertinib (filed/pending); RESEARCH: Foundation Medicine; ROYALTIES: Wolters Kluwer; OTHER (Food/Beverage): Merck, Puma, Merus, Boehringer Ingelheim; CME HONORARIA: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, Med Learning, Imedex, Answers in CME, Clinical Care Options, EPG Health, JNCC/Harborside, Liberum, Remedica Ltd., Lungevity.
Manish R. Sharma. STOCK OR OTHER OWNERSHIP: Pfizer
Melissa L. Johnson. CONSULTING/ADVISORY ROLE PAID TO INSTIUTION: AbbVie, Amgen, Arcus Biosciences, Arrivent, Astellas, AstraZeneca, Axelia Oncology, Black Diamond, Calithera Biosciences, Checkpoint Therapeutics, CytomX Therapeutics, Daiichi Sankyo, EcoR1, Editas Medicine, Eisai, Genentech/Roche, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Ideaya Biosciences, Immunocore, iTeos, Janssen, Jazz Pharmaceuticals, Lilly, Merck, Mirati Therapeutics, Molecular Axiom, Novartis, Oncorus, Pyramid Biosciences, Regeneron Pharmaceuticals, Revolution Medicines, Ribon Therapeutics, Sanofi-Aventis, SeaGen, Takeda Pharmaceuticals, Turning Point Therapeutics, VBL Therapeutics; RESEARCH FUNDING PAID TO INSTITUTION: AbbVie, Acerta, Adaptimmune, Amgen, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, AstraZeneca, Atreca, BeiGene, BerGenBio, BioAtla, Black Diamond, Boehringer Ingelheim, Calithera Biosciences, Carisma Therapeutics, Corvus Pharmaceuticals, Curis, CytomX, Daiichi Sankyo, Dracen Pharmaceuticals, Dynavax,Lilly, Elicio Therapeutics, EMD Serono, EQRx, Erasca, Exelixis, Fate Therapeutics, Genentech/Roche, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Harpoon, Helsinn Healthcare SA, Hengrui Therapeutics, Hutchison MediPharma, IDEAYA Biosciences, IGM Biosciences, Immunitas Therapeutics, Immunocore, Incyte, Janssen, Kadmon Pharmaceuticals, Kartos Therapeutics, Loxo Oncology, Lycera, Memorial Sloan-Kettering, Merck, Merus, Mirati Therapeutics, NeoImmune Tech, Neovia Oncology, Novartis, Numab Therapeutics, Nuvalent, OncoMed Pharmaceuticals, Palleon Pharmaceuticals, Pfizer, PMV Pharmaceuticals, Rain Therapeutics, Regeneron Pharmaceuticals, Relay Therapeutics, Revolution Medicines, Ribon Therapeutics, Rubius Therapeutics, Sanofi, Seven and Eight Biopharmaceuticals / Birdie Biopharmaceuticals, Shattuck Labs, Silicon Therapeutics, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, Turning Point Therapeutics, University of Michigan, Vyriad, Y-mAbs Therapeutics.
Timothy A. Yap. Employment: University of Texas MD Anderson Cancer Center, where I am Medical Director of the Institute for Applied Cancer Science, which has a commercial interest in DDR and other inhibitors (IACS30380/ART0380 was licensed to Artios). Grant/Research support (to Institution): Acrivon, Artios, AstraZeneca, Bayer, Beigene, BioNTech, Blueprint, BMS, Boundless bio, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, Haihe, Ideaya ImmuneSensor, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Merck, Mirati, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, Vivace and Zenith. Consultancies: AbbVie, AstraZeneca, Acrivon, Adagene, Almac, Aduro, Amphista, Artios, Athena, Atrin, Avoro, Axiom, Baptist Health Systems, Bayer, Beigene, Blueprint Medicines, Boxer, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Circle Pharma, Clovis, CUHK Committee, Cybrexa, Dark Blue Therapeutics, Diffusion, Ellipses.Life, EMD Serono, F-Star, Genentech, Genmab, Gerson and Lehrman Group, Glenmark, GLG, Globe Life Sciences, GSK, Guidepoint, Idience, Ignyta, I-Mab, ImmuneSensor, Institut Gustave Roussy, Intellisphere, Jansen, Kyn, LRG1, MEI pharma, Mereo, Merck, Natera, Nexys, Novocure, OHSU, OncoSec, Ono Pharma, Panangium, Pegascy, PER, Pfizer, Piper-Sandler, Pliant Therapeutics, Prolynx, Radiopharm Theranostics, Repare, resTORbio, Roche, Sanofi, Schrodinger, Seagen, Synthis Therapeutics, Terremoto Biosciences, Tessellate Bio, TD2 Theragnostics, Tome Biosciences, Varian, Versant, Vibliome, Xinthera, Zai Labs, Zentalis and ZielBio. Stockholder in: Seagen.
Allison S. Harney, Mohammed Elsayed, Adam W. Cook, Christina E. Wong, Yutong Jiang, Ellen R. Laird, Wen-I Wu, Anurag Singh, Ping Wei, John J. Gaudino, Patrice A. Lee. EMPLOYMENT: Pfizer.
Dale Nepert, Gang Feng, Micaela B. Reddy, Eric N Brown, Nickolas A. Neitzel, Keith A. Ching. EMPLOYMENT AND STOCK OR OTHER OWNERSHIP: Pfizer.
Ronald J. Hincklin. EMPLOYMENT AND STOCK OR OTHER OWNERSHIP: Pfizer; PATENTS, ROYALTIES, OTHER INTELLECTUAL PROPERTY: ARRY-558 patent co-inventor.
Dylan P. Hartley, S. Michael Rothenberg. EMPLOYMENT, LEADERSHIP, AND STOCK OR OTHER OWNERSHIP: Pfizer.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2(3):227–35 doi 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Science Translational Medicine 2012;4(120):120ra17-ra17 doi doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483(7387):100–3 doi 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. science 2007;316(5827):1039–43. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4(1):80–93 doi 10.1158/2159-8290.Cd-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagogo-Jack I, Yoda S, Lennerz JK, Langenbucher A, Lin JJ, Rooney MM, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clinical Cancer Research 2020;26(11):2535–45 doi 10.1158/1078-0432.Ccr-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015;7(283):283ra51 doi 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol 2018;20(9):1064–73 doi 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019;380(20):1929–40 doi 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Liu C, Velazquez R, Wang H, Dunkl LM, Kazic-Legueux M, et al. SHP2 Inhibition Overcomes RTK-Mediated Pathway Reactivation in KRAS-Mutant Tumors Treated with MEK Inhibitors. Mol Cancer Ther 2019;18(7):1323–34 doi 10.1158/1535-7163.Mct-18-0852. [DOI] [PubMed] [Google Scholar]
- 11.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol 2003;23(21):7875–86 doi 10.1128/mcb.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagner A, Yart A, Dance M, Perret B, Salles J-P, Raynal P. A Novel Role for Gab1 and SHP2 in Epidermal Growth Factor-induced Ras Activation*. Journal of Biological Chemistry 2005;280(7):5350–60 doi 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- 13.Liotti F, Kumar N, Prevete N, Marotta M, Sorriento D, Ieranò C, et al. PD-1 blockade delays tumor growth by inhibiting an intrinsic SHP2/Ras/MAPK signalling in thyroid cancer cells. Journal of Experimental & Clinical Cancer Research 2021;40(1):22 doi 10.1186/s13046-020-01818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanafusa H, Torii S, Yasunaga T, Matsumoto K, Nishida E. Shp2, an SH2-containing Protein-tyrosine Phosphatase, Positively Regulates Receptor Tyrosine Kinase Signaling by Dephosphorylating and Inactivating the Inhibitor Sprouty*. Journal of Biological Chemistry 2004;279(22):22992–5 doi 10.1074/jbc.M312498200. [DOI] [PubMed] [Google Scholar]
- 15.Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci U S A 1994;91(15):7335–9 doi 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Nishimura R, Kashishian A, Batzer AG, Kim WJ, Cooper JA, et al. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol 1994;14(1):509–17 doi 10.1128/mcb.14.1.509-517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedele C, Ran H, Diskin B, Wei W, Jen J, Geer MJ, et al. SHP2 Inhibition Prevents Adaptive Resistance to MEK Inhibitors in Multiple Cancer Models. Cancer Discov 2018;8(10):1237–49 doi 10.1158/2159-8290.Cd-18-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dardaei L, Wang HQ, Singh M, Fordjour P, Shaw KX, Yoda S, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med 2018;24(4):512–7 doi 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou S- HI, Koczywas M, Ulahannan S, Janne PA, Pacheco JM, Burris HA, et al. The SHP2 Inhibitor RMC-4630 in Patients with KRAS-mutant Non-Small Cell Lung Cancer: Preliminary Evaluation of a First-in-man Phase 1 Clinical Trial AACR-AASLC Conference 2020. [cited 2020 Jan 12] Available from: https://wwwrevmedcom/media/shp2-inhibitor-rmc-4630-patients-kras-mutant-non-small-cell-lung-cancer-preliminary-02020.
- 20.Brana I, Shapiro G, Johnson ML, Yu HA, Robbrecht D, Tan DS- W, et al. Initial results from a dose finding study of TNO155, a SHP2 inhibitor, in adults with advanced solid tumors. Journal of Clinical Oncology 2021;39(15_suppl):3005- doi 10.1200/JCO.2021.39.15_suppl.3005. [DOI] [Google Scholar]
- 21.Ahmed TA, Adamopoulos C, Karoulia Z, Wu X, Sachidanandam R, Aaronson SA, et al. SHP2 Drives Adaptive Resistance to ERK Signaling Inhibition in Molecularly Defined Subsets of ERK-Dependent Tumors. Cell Rep 2019;26(1):65–78.e5 doi 10.1016/j.celrep.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koczywas M, Haura EB, Janne PA, Pacheco JM, Ulahannan S, Wang JS, et al. Anti-tumor Activity and Tolerability of the SHP2 Inhibitor RMC-4630 as a Single Agent in Patients with RAS-Addicted Solid Cancers. AACR Annual Meeting 2021. [cited 2021 Apr 10] Available from: https://wwwrevmedcom/media/anti-tumor-activity-and-tolerability-shp2-inhibitor-rmc-4630-single-agent-patients-ras2021. [Google Scholar]
- 23.GS F, BT L, KR M. Sotorasib in combination with RMC-4630, a SHP2 inhibitor, in KRAS p.G12C-mutated NSCLC and other solid tumors. . IASLC 2022 World Conference on Lung Cancer 2022;Abstract OA03. [Google Scholar]
- 24.Drilon A, Ou S- HI, Cho BC, Kim D-W, Lee J, Lin JJ, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discovery 2018;8(10):1227–36 doi 10.1158/2159-8290.Cd-18-0484. [DOI] [PubMed] [Google Scholar]
- 25.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 2011;67(Pt 4):235–42 doi 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannu NS, Murshudov GN, Dodson EJ, Read RJ. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr D Biol Crystallogr 1998;54(Pt 6 Pt 2):1285–94 doi 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 27.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 2011;67(Pt 4):355–67 doi 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004;60(Pt 12 Pt 1):2126–32 doi 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.