Abstract
STUDY QUESTION
What is the influence of body composition during childhood, adolescence, and adulthood, as well as metabolic parameters, on incident polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
Excess body fat, even during childhood/adolescence, and metabolic parameters, suggestive of hyperinsulinaemia/insulin resistance, significantly impact the risk of PCOS in a linear fashion.
WHAT IS KNOWN ALREADY
Observational and Mendelian randomization (MR) data have demonstrated an association between adulthood overweight/obesity and development of PCOS. However, the contribution of body composition in childhood/adolescence to incident PCOS is unclear, as is the influence of childhood overweight/obesity.
STUDY DESIGN, SIZE, DURATION
We conducted a systematic review and meta-analysis and integrated our results with a previously published systematic review. Two blinded investigators screened abstracts published between November 2010 and May 2021. Furthermore, we incorporated summary statistics from genome-wide association study (GWAS) data in subjects of European ancestry. Adult overweight was defined as BMI ≥ 25 kg/m2 and obesity as BMI ≥ 30 kg/m2; in Asian subjects, overweight was defined as BMI ≥ 23 kg/m2 and obesity as BMI ≥ 25 kg/m2.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We utilized meta-analysis and MR together to allow synthesis of genetic and observational data. For the systematic review, the search revealed 71 studies, of which 63 were included in meta-analysis by calculating odds ratios (ORs) using the random-effects model. Furthermore, we conducted a two-sample MR study of GWAS data to determine the impact of childhood and adult body size (defined categorically by BMI and childhood body size proportions), abnormal body composition and metabolic parameters (higher fasting serum insulin or lower sex hormone-binding globulin (SHBG) concentration) on the odds of incident PCOS via the inverse-variance weighted method.
MAIN RESULTS AND THE ROLE OF CHANCE
Significant associations were shown between body composition and PCOS incidence. From the systematic review/meta-analysis, women with overweight (OR 3.80, 2.87–5.03), obesity (OR 4.99, 3.74–6.67), and central obesity (OR 2.93, 2.08–4.12) had increased odds of PCOS. For adolescents with overweight and/or obesity, the PCOS odds were greater than for adults. From MR, for every standard deviation increase in BMI (4.8 kg/m2), the odds of PCOS increased by 2.76 (2.27–3.35). Childhood body size had an independent effect on PCOS odds after adjusting for adult body size (OR: 2.56, 1.57–4.20). Genetically determined body fat percentage (OR 3.05, 2.24–4.15), whole body fat mass (OR 2.53, 2.04–3.14), fasting serum insulin (OR 6.98, 2.02–24.13), and SHBG concentration (OR 0.74, 0.64–0.87) were all significantly associated with PCOS in a linear relation.
LIMITATIONS, REASONS FOR CAUTION
The meta-analysis included studies which were cross-sectional and retrospective, limiting our ability to determine causality. MR was limited by interrogating subjects only of European ancestry and including cases classified by either self-diagnosis or diagnostic criteria.
WIDER IMPLICATIONS OF THE FINDINGS
Our study demonstrates for the first time a critical role of the impact of excess childhood/adolescent adiposity on the pathophysiology of adult PCOS. Our results, driven by genetically determined childhood/adolescent body composition, higher BMI, hyperinsulinaemia, and lower SHBG, clearly favour obesity driving the metabolic, but not reproductive, PCOS phenotype. Overall, effective weight maintenance, even from the early years, is likely to reduce the risk of this reproductive endocrine disorder.
STUDY FUNDING/COMPETING INTEREST(S)
S.S.Z. was funded by a National Institute for Health and Care Research (NIHR) Academic Clinical Lectureship. U.A. is chair of the NIHR Steering Committee Trial—CASSANDRA-DN. No other authors declare any sources of funding or relevant conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relations that could be construed as a potential conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: insulin resistance, hyperinsulinaemia, polycystic ovary syndrome, obesity, Mendelian randomization, epidemiology
Introduction
Polycystic ovary syndrome (PCOS) is a highly prevalent disease, affecting 6–20% of women (Diamanti-Kandarakis et al., 1999; Asunción et al., 2000; Azziz et al., 2004b), characterized by clinical and/or biochemical androgen excess, menstrual abnormalities, and sub-/infertility (Hull, 1987; Balen et al., 1995b; Boomsma et al., 2006; Kjerulff et al., 2011; Legro et al., 2013). It has a significant impact on quality of life (Barnard et al., 2007) and is commonly associated with metabolic abnormalities (including features of the metabolic syndrome, type 2 diabetes, and non-alcoholic fatty liver disease) (Azziz et al., 2009; Escobar-Morreale, 2018; Sanchez-Garrido and Tena-Sempere, 2020; Moghetti and Tosi, 2021) and cardiovascular disease (Zhang et al., 2020). Lifestyle interventions (diet and exercise) (Palomba et al., 2015; Kim et al., 2020) represent the first-line of treatment although therapeutic approaches include targeting insulin resistance (metformin, glitazones), androgen excess (oral contraceptive pill), weight loss (glucagon-like Peptide 1 (GLP1)-receptor agonists) (Lamos et al., 2017; Han et al., 2019; Cena et al., 2020), and fertility (i.e. clomiphene citrate) (Escobar-Morreale, 2018; Pasquali, 2018).
Obesity/overweight are associated with PCOS, with observational evidence reporting a 33–88% prevalence of overweight/obesity in women with PCOS (Balen et al., 1995a; Gambineri et al., 2002; Barber et al., 2006; Lim et al., 2012; Ollila et al., 2016; Aarestrup et al., 2021). The huge variation in prevalence likely reflects differences in study settings (e.g. community, endocrine or infertility clinics) and differences in the PCOS diagnostic criteria applied. Central obesity is also crucial, given its association with hyperandrogenism and insulin resistance (Ollila et al., 2016). A previous meta-analysis in 2012 demonstrated that women with PCOS had an increased prevalence of overweight (relative risk, RR: 1.95, 95% CI: 1.52, 2.50) and obesity (RR: 2.77, 95% CI: 1.88, 4.10) compared with controls (Lim et al., 2012). This study, however, was limited by including a small number of studies of adolescents. This meant subgroup analysis was non-significant when comparing the impact of overweight on PCOS prevalence in adults and adolescents (RR, Adults 1.92, 95% CI: 1.48–2.48, Adolescents: 2.25, 95% CI: 0.42–11.98). In addition, these observational data are susceptible to confounding and, more importantly, reverse causation, with the concern that PCOS pathophysiology may in turn influence body size. Therefore, it is crucial to determine whether overweight and obesity are causal, given that BMI is a modifiable risk factor that is amenable to lifestyle (diet and exercise) (Dobbie et al., 2021; Hwalla and Jaafar, 2021), pharmacological (i.e. GLP-1 receptor agonists) (Vilsbøll et al., 2012; Wilding et al., 2021) or (metabolic) surgical intervention (Gloy et al., 2013; O’Brien et al., 2019).
Metabolic abnormalities, including insulin resistance and reduced sex hormone-binding globulin (SHBG) concentrations, have been implicated in PCOS. SHBG is generated by the liver and as the primary protein that binds sex steroids (such as oestradiol and testosterone), reduced SHBG levels results in elevated biologically active free testosterone levels, and thus hyperandrogenaemia. Low serum SHBG levels are considered a biomarker of metabolic abnormalities and are associated with insulin resistance as hyperinsulinaemia inhibits hepatic SHBG production (Qu and Donnelly, 2020). Approximately 75% of people with PCOS have insulin resistance (Tosi et al., 2017). Insulin resistance induces clinical features of PCOS; however, hyperandrogenaemia (from PCOS) also causes insulin resistance (Moghetti and Tosi, 2021). Current observational evidence lacks the ability to determine causality of insulin resistance on PCOS (Diamanti-Kandarakis and Dunaif, 2012). Genetic meta-analysis has demonstrated that eight or more polymorphisms of SHBG may predict increased PCOS risk (Li et al., 2021). However, these are a lack of evidence evaluating the effect of hyperinsulinaemia, SHBG levels, or altered body composition on PCOS risk.
Mendelian randomization (MR) is an observational approach whereby genetic variants are used as instrumental variables to estimate the causal effect of an exposure (in this case, overweight/obesity) on an outcome (development of PCOS). Since variants are randomly allocated at conception, MR is less susceptible to reverse causation and confounding than other observational designs. Prior MR studies have shown BMI to have a causal association with PCOS risk, but not vice versa (Day et al., 2015; Brower et al., 2019; Zhao et al., 2020; Yan et al., 2021). Existing studies leave several unanswered questions. First, contemporaneous BMI provides insight only into adult determinants, whereas the relative importance of childhood or adult body sizes remains unexplored. Second, recognizing the limitations of the BMI as a metric of excess body weight and as an index for adiposity, capturing both lean and fat mass, we were interested in examining the role of central obesity (Shah and Braverman, 2012; Nuttall, 2015). Finally, given the association between metabolic parameters (i.e. hyperinsulinaemia and low SHBG levels) and PCOS we wanted to examine the effect of these on incident PCOS.
Our aim was to evaluate the evidence regarding the association of overweight, obesity, and central obesity with incident PCOS, updating the previous meta-analysis conducted by Lim et al. (2012). Subsequently, we sought to evaluate the causal impact of BMI on the development of PCOS by performing a series of two-sample MR studies to address the unresolved issues of the role of childhood and adult body size, metabolic parameters and the influence specifically of abnormal body composition.
Materials and methods
Systematic review and meta-analysis
We conducted a systematic review and meta-analysis interrogating how body composition during childhood, adolescence, and adulthood impacts PCOS risk. We utilized the systematic review methodology previously published by Lim et al. (2012). In total, 101 of the studies from this review concerned the prevalence of obesity in women with PCOS; these were excluded as they did not include a control group. Thirty-five of the studies from the previous systematic review (Lim et al., 2012) were carried through to our meta-analysis.
Search strategy
We conducted a literature search based on the previous systematic review strategy (Supplementary Table SI). All articles published after and including November 2010 were screened up until May 2021 (Lim et al., 2012). In addition to this, review articles were screened.
Selection criteria
To allow meta-analysis, we extracted the most consistently reported outcome(s).
Inclusion criteria
This systematic review considered the odds of PCOS in adolescents and adults with/without overweight, obesity, and central obesity. Studies in which women with PCOS were consecutively recruited or randomly sampled were included. For study inclusion, PCOS was defined according to the National Institutes of Health (NIH), ESHRE/American Society for Reproductive Medicine (ASRM) criteria (also termed Rotterdam criteria) or Androgen Excess-PCOS (AE-PCOS criteria) (Supplementary Table SII) (Wild et al., 2010; Fauser et al., 2012; Conway et al., 2014). Studies comparing odds of PCOS in women with and without overweight/obesity were included if control subjects were not matched based on weight or BMI. Studies assessing odds of PCOS in women with/without central obesity were included if control subjects were not matched based on waist circumference (WC) or waist–hip ratio (WHR).
Exclusion criteria
We excluded studies where participants were selected by body weight, BMI, WC, or WHR.
Definitions
Body weight was analysed as a binary outcome (overweight/non-overweight; obesity/no obesity; central obesity/no central obesity). In addition, BMI cut-offs vary between different ethnicities (e.g. White Europeans versus South-East Asians), meaning meta-analysis of continuous BMI data would be challenging. Adult overweight was defined as BMI ≥ 25 kg/m2 and obesity as BMI ≥ 30 kg/m2 as per World Health Organization (WHO) criteria (World Health Organization, 2000). In studies of Asian subjects, overweight was BMI ≥ 23 kg/m2 and obesity was BMI ≥ 25 kg/m2 according to the International Obesity Task Force (Goda and Masuyama, 2016). For adolescents, age-gender-specific percentile BMI distributions were used to define overweight as the 85–95th percentile and obesity as the ≥95 percentile (Barlow, 2007). Central obesity was defined as a WC ≥80 cm (IDF, 2020), WHR >0.85 (World Health Organization, 2000), or WC >88 cm according to the Adult Treatment Panel III (Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults, 2001). The central obesity definition was applied (Supplementary Table SIII) depending on that utilized in the included paper, as per the previous systematic review (Lim et al., 2012).
Record screening
Two reviewers screened half of the studies identified (L.J.D. and B.P.) with each independently validating 10% of the others work. Discrepancies were resolved through discussion with co-authors.
Data extraction
General study characteristics (author, publication year, study location, study period, study design, number of women with and without PCOS), characteristics of the study population (recruitment source, sampling method, age, ethnicity), the definition of PCOS (NIH, ESHRE/ASRM, AE-PCOS), pre-existing medication use, physical activity and diet history, the definition of overweight/obesity (WHO, International Obesity Task Force (IOTF) and central obesity (International Diabetes Federation (IDF) WHO, Adult Treatment Panel III (ATP III)), measurements of height, weight, and WC, and the proportion of women with overweight, obesity, or central obesity were extracted from all included studies. Data were extracted in duplicate (L.J.D. and B.P.).
Quality assessment
Two reviewers (L.J.D., and D.J.C.) assessed risk of bias using the Newcastle-Ottawa Scale for all included studies.
Outcomes of interest
The primary endpoints were odds of PCOS in adolescent and adult women with/without overweight, obesity, and central obesity.
Meta-analysis
Data collected here was integrated with the meta-analysis by Lim et al. (2012). Our analytical technique differed, as we calculated odds ratio (OR) rather than RR. Random effects models were used to estimate the pooled prevalence, using the inverse variance weighting method. Heterogeneity of meta-analysis estimates was presented using the I2 statistic. Funnel plots were used to assess risk of publication bias. Sensitivity analysis assessed the impact of age (adult versus adolescence) and study type (Cross-sectional, Retrospective, Case-control, Cohort). Analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) and the ‘meta’ and ‘metaphor’ packages.
Mendelian randomization
Data sources
Data for BMI associated traits were obtained from genome-wide associations studies (GWAS), as summarized in Table I. We instrumented BMI using GWAS of both males and females, since preliminary analyses showed that sex-specific instruments did not provide meaningful difference to results. By contrast, SHBG instruments did provide different result; therefore, we chose variants from GWAS of female SHBG. PCOS data were obtained from a GWAS meta-analysis of 10 074 cases and 103 164 controls of European ancestry. PCOS was diagnosed according to NIH, Rotterdam criteria or self-diagnosis (Day et al., 2018) (Supplementary Figs S1 and S2).
Table 1.
Summary data of genetic instruments used for Mendelian randomization.
| Unit | Sample size (n=) | Number of SNPs | R2 | F | |
|---|---|---|---|---|---|
| Childhood body size | Thinner, About Average, Plumper | 453 169 | 313 | 2.0% | 30 |
| Adult body size | BMI categorized into childhood body size proportions | 453 169 | 580 | 2.9% | 23 |
| BMI (kg/m2) | 4.8 kg/m2 | 806 834 | 543 | 5.7% | 89 |
| Whole body fat mass (kg) | 9.6 kg | 454 137 | 435 | 6.6% | 58 |
| Body fat percentage (%) | 8.5% | 454 633 | 395 | 5.3% | 55 |
| Appendicular lean mass (kg) | 5.6 kg | 450 243 | 690 | 10.4% | 97 |
| Fasting insulin | 0.79 pmol/l | 108 557 | 14 | NA | NA |
| Glycated haemoglobin | 6.7 mmol/mol | 344 182 | 320 | 11.9% | 146 |
| SHBG | 27.7 nmol/l | 312 215 | 264 | 10.1% | 46 |
Summary data for BMI and glycaemic associated traits obtained from genome-wide association studies (GWAS), which were utilized for Mendelian randomization. Childhood body size was categorized as either ‘thinner’, ‘about average’, or ‘plumper’, as per the GWAS data. Adult body size was determined as BMI categorized into childhood body size proportions as per the GWAS data. F statistic >10 suggests adequate instrument strength. SHBG: sex-hormone binding globulin; SNP: single-nucleotide polymorphism; %: percentage; R2: variance explained; F: F statistic; NA: not applicable. Data sources: Pulit et al. (2019) and Richardson et al. (2020).
Instrument identification and data harmonization
We selected independent (linkage disequilibrium (LD) threshold of r2 < 0.001 using PLINK and phase 3 version 5 of the 1000 genomes project as reference panel) genome-wide significant (P < 5 × 10−8) single-nucleotide polymorphisms (SNPs). The number of variants for each exposure is summarized in Table I. For multivariable MR, we repeated LD clumping for the combined set of SNPs from all exposures included in the model. All effect alleles were checked to be on the forward strand; ambiguous palindromes were not excluded. Where possible, SNPs absent in one of the exposure-outcome sets were proxied using variants in LD (r2 > 0.8).
Statistical analysis
Variance explained (r2) was calculated using 2EAF(1 − EAF)β2, where EAF is the effect allele frequency. F statistic was derived using (r2/K)/[(1 − r2)(N − K − 1)], where K is the number of SNPs and N the sample size. Conditional F statistics was derived using the R MVMR package (R Foundation for Statistical Computing, Vienna, Austria) for multivariable MR. F statistics >10 is considered suggestive of adequate instrument strength. The inverse variance weighted (IVW) method was used for the primary analysis, which provides a weighted average of variant effects analogous to random-effect meta-analysis (Burgess et al., 2013). Effect sizes are interpreted as per unit increase in the exposure, the definition of which is given in Table I. We used the weighted median, weighed mode and MR Egger methods to evaluate the robustness of IVW estimates to horizontal pleiotropy (Bowden et al., 2015). Horizontal pleiotropy is a main source of bias in MR, whereby genetic variants influence the exposure and outcome via two separate biological pathways (Verbanck et al., 2018). For MVMR, we used the IVW method, with MR-Egger as sensitivity analysis (Bowden et al., 2015). The pairwise covariance between SNP associations was assumed to be zero in the primary analysis. All analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) using the TwoSampleMR and MVMR packages.
Results
Characteristics of included studies
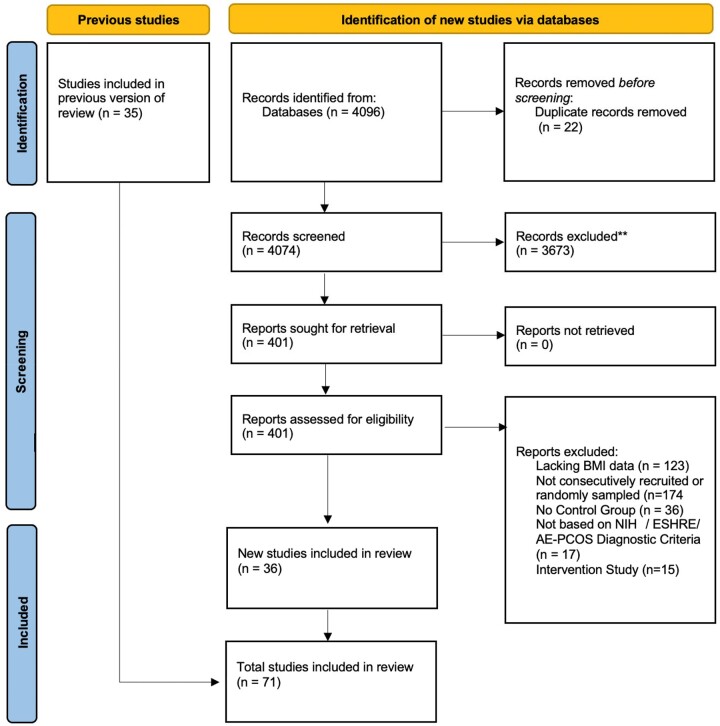
The search yielded 4074 citations from which (based on our selection criteria) 36 studies were included (Fig. 1). These studies were combined with 35 studies from the prior systematic review by Lim et al. (2012), providing 71 studies in total (Supplementary Tables SIV, SV, and SVI).
Figure 1.
Systematic review PRISMA flow diagram. PRISMA flow diagram created from a template by Page et al. (2021). Previous studies included are from Lim et al. (2012). AE-PCOS: androgen excess PCOS; NIH: National Institutes of Health.
Thirty-five studies were included for meta-analysis of overweight (BMI ≥ 25 or ≥23 kg/m2 for Asian subjects) versus non-overweight in women with/without PCOS (n = 29 adult, n = 6 adolescent). Thirty studies were included for meta-analysis of obesity (BMI ≥ 30 or BMI ≥25 kg/m2 for Asian subjects) versus non-obese in women with/without PCOS (n = 25 adult, n = 5 adolescent). Sixteen studies were included for meta-analysis of central obesity versus non-central obesity in women with/without PCOS (n = 12 adult, n = 4 adolescent).
In terms of study design, 47.9% (34/71) were cross-sectional, 16.9% (12/71) retrospective, 22.5% (16/71) case-control, and 12.7% (9/71) prospective cohort studies. In terms of geographic distribution, 36.6% (26/71) were conducted in Europe (29.6% (21/71) Asia, 28.2% (20/71) Americas, 5.6% (4/71) Oceania) (Supplementary Tables SIV and SV). For quality assessment, 60.6% (43/71) were high-quality (≥5/9) and 39.4% (28/71) were low-quality (range 2/9 to 8/9) (Supplementary Table SVI). Funnel plots for overweight, obesity, and central obesity meta-analyses (Supplementary Figs S3, S4, and S5) reported no statistical evidence of heterogeneity. However, the Funnel plot of the overweight meta-analysis trended towards significance (P = 0.0624, Supplementary Fig S3).
Meta-analyses of odds of PCOS in women of each weight category
Overweight
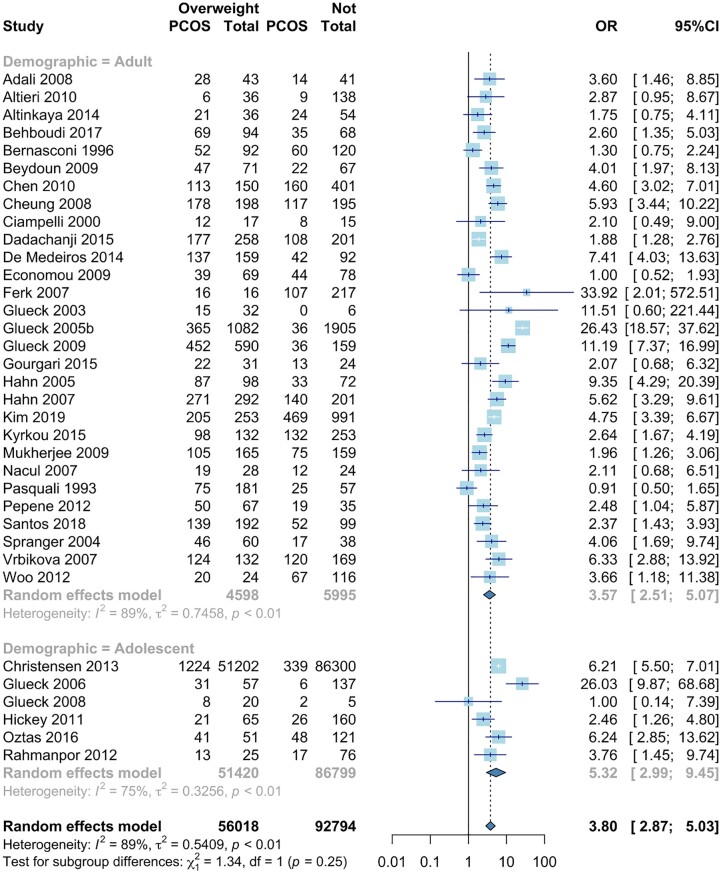
In the 35 studies providing overweight data, odds of PCOS were significantly higher in women with overweight (OR 3.80, 95% CI: 2.87, 5.03) (Pasquali et al., 1993; Bernasconi et al., 1996; Ciampelli et al., 2000; Glueck et al., 2003, 2005b, 2006, 2008, 2009; Spranger et al., 2004; Hahn et al., 2005, 2007; Ferk et al., 2007; Nácul et al., 2007; Vrbikova et al., 2007; Adali et al., 2008; Cheung et al., 2008; Beydoun et al., 2009; Economou et al., 2009; Mukherjee et al., 2009; Altieri et al., 2010; Chen et al., 2010; Hickey et al., 2011; Pepene, 2012; Rahmanpour et al., 2012; Woo et al., 2012; Christensen et al., 2013; Altinkaya et al., 2014; de Medeiros et al., 2014; Dadachanji et al., 2015; Gourgari et al., 2015; Kyrkou et al., 2016; Oztas et al., 2016; Behboudi-Gandevani et al., 2017; Santos et al., 2018; Kim et al., 2019). Odds of PCOS were higher for adolescents with overweight (adult: OR: 3.57, 95% CI 2.51, 5.07, versus adolescent: OR 5.32, 95% CI 2.99, 9.45) (lower part, Fig. 2). Five of the six childhood/adolescence studies showed that overweight increased odds of PCOS (Glueck et al., 2006; Hickey et al., 2011; Rahmanpour et al., 2012; Christensen et al., 2013; Oztas et al., 2016). One study found that overweight does not increase odds of PCOS; however, this was the smallest study of this sub-group (Glueck et al., 2008). Women with overweight had increased odds of PCOS across all included study types (Supplementary Fig. S6). There was significant statistical heterogeneity (I2 = 89%, P < 0.0001).
Figure 2.
Meta-analysis of odds of PCOS in women with overweight versus non-overweight. Forest plot for odds ratio of PCOS in women with overweight versus non-overweight. Quantifying heterogeneity: τ2 = 0.5409, I2 = 89%, Test of heterogeneity: P-value <0.0001. OR: odds ratio.
Obesity
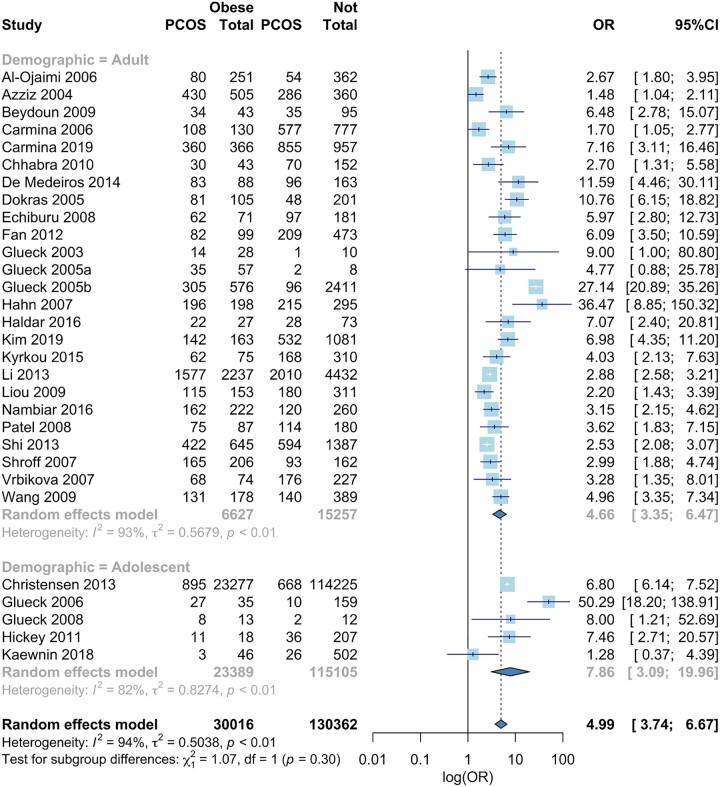
In the 30 studies providing obesity data, the odds of PCOS were significantly higher in women with obesity (OR 4.99, 95% CI 3.74, 6.67) (Glueck et al., 2003, 2005b, 2005a, 2006, 2009; Azziz et al., 2004a; Dokras et al., 2005; Al-Ojaimi, 2006; Carmina et al., 2006, 2019; Hahn et al., 2007; Shroff et al., 2007; Vrbikova et al., 2007; Echiburú et al., 2008; Patel et al., 2008; Beydoun et al., 2009; Liou et al., 2009; Wang et al., 2009; Chhabra and Venkatraman, 2010; Hickey et al., 2011; Fan et al., 2012; Christensen et al., 2013; Li et al., 2013; Shi et al., 2013; de Medeiros et al., 2014; Kyrkou et al., 2016; Nambiar et al., 2016; Haldar et al., 2018; Kaewnin et al., 2018; Kim et al., 2019) (Fig. 3). Odds of PCOS were higher in adolescents with obesity (Adult: OR 4.66, 95% CI 3.35, 6.47, Adolescent: OR 7.86, 95% CI 3.09, 19.96) (lower part, Fig. 3). Four out of five of the childhood/adolescence studies showed that obesity increased odds of PCOS (Glueck et al., 2006, 2008; Hickey et al., 2011; Christensen et al., 2013). One study showed no association between adolescent obesity and odd of PCOS; however, the OR trended towards significance (Kaewnin et al., 2018). Women with obesity had increased odds of PCOS across all included study types (Supplementary Fig. S7). There was significant heterogeneity (I2 = 94%, P < 0.0001).
Figure 3.
Meta-analysis of odds of PCOS in women with obesity versus non-obesity. Forest plot for odds ratio of PCOS in women with obesity vs non-obesity, Quantifying heterogeneity: τ2 = 0.5038, I2 = 94.0%, Test of heterogeneity: P-value <0.0001. OR: odds ratio.
Central obesity
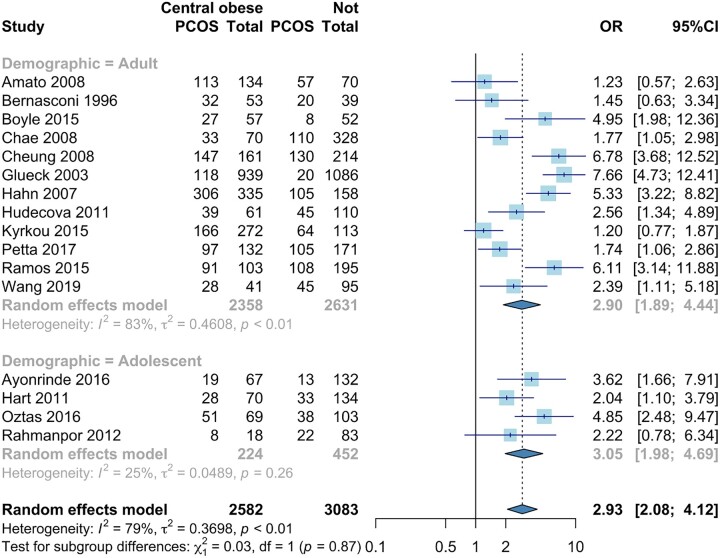
In the 16 studies providing central obesity data, the odds of PCOS were significantly higher in women with central obesity (OR 2.93, 95% CI 2.08, 4.12) (Bernasconi et al., 1996; Glueck et al., 2003; Hahn et al., 2007; Amato et al., 2008; Chae et al., 2008; Cheung et al., 2008; Hart et al., 2011; Hudecova et al., 2011; Rahmanpour et al., 2012; Boyle et al., 2015; Ramos and Spritzer, 2015; Ayonrinde et al., 2016; Kyrkou et al., 2016; Oztas et al., 2016; Petta et al., 2017; Wang et al., 2019) (Fig. 4). When analysed by age, the odds of PCOS were similar for both adolescents and adults (Adult: OR 2.90, 95% CI 1.89, 4.44, Adolescent: OR 3.05, 95% CI 1.98, 4.69) (lower part, Fig. 4). Three of the four childhood/adolescent studies showed that central obesity increased odds of PCOS (Hart et al., 2011; Ayonrinde et al., 2016; Oztas et al., 2016). One study found no significant association but trended towards significance (Rahmanpour et al., 2012). Women with central obesity had increased odds of PCOS across all included study types (Supplementary Fig. S8). There was significant statistical heterogeneity (I2 = 79%, P < 0.0001).
Figure 4.
Meta-analysis of odds of PCOS in women with central obesity versus non-central obesity. Forest plot for odds ratio of PCOS in women with central obesity versus non-central obesity, Quantifying heterogeneity: τ2 = 0.3698, I2 = 79%, Test of heterogeneity: P-value <0.0001. OR: odds ratio.
Systematic review of childhood/adolescent body composition on odds of PCOS
Our review highlighted 16 studies investigating the role of childhood/adolescent body composition in PCOS risk; 9 of these were included for systematic review only and 7 were included for both systematic review and meta-analysis (Hart et al., 2011; Rahmanpour et al., 2012; Christensen et al., 2013; Roe et al., 2013; Gümüş et al., 2015; Ayonrinde et al., 2016; Çinar et al., 2016; Oztas et al., 2016; de Zegher et al., 2017; Li et al., 2017; Ates et al., 2018; Bedaiwy et al., 2018; Kaewnin et al., 2018; Wu et al., 2018; Koivuaho et al., 2019; Aarestrup et al., 2021). Of note, a large study of adolescent girls demonstrated that PCOS was preceded by a marked z-score increase between birthweight and BMI at diagnosis (de Zegher et al., 2017). Similarly, in a Danish study (n = 65 665), girls age 7–13 years with overweight were at higher risk (Age 7 years: hazard ratio (HR): 2.83, Age 13 years: HR 2.99) of PCOS than those without overweight, with overweight at both time points further increasing the risk (Aarestrup et al., 2021). Christensen et al. (2013) (n = 138 502 adolescents age 15–19) demonstrated that higher BMI category increased the PCOS risk further (OR compared to normal weight, overweight: OR 3.85, obese: OR 10.25, extreme obesity: OR 23.1). Furthermore, a Northern Finland longitudinal study demonstrated that an earlier age of fat gain and higher BMI in childhood increased PCOS risk (Koivuaho et al., 2019).
Mendelian randomization
The number of variants used to instrument each exposure, and their respective F statistics, are summarized in Table I.
BMI
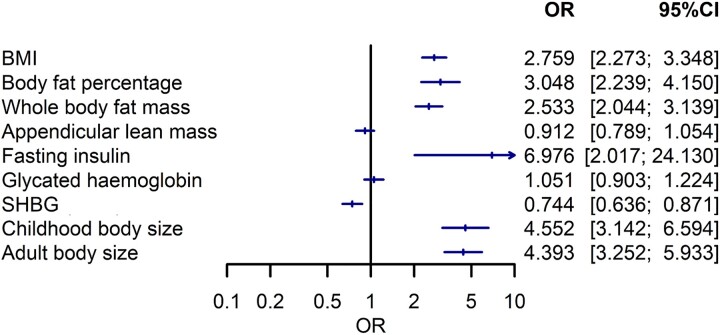
Each standard deviation (4.8 kg/m2) increase in BMI was associated with 2.8-fold higher odds of PCOS (OR: 2.76, 95% CI: 2.27–3.35) (Fig. 5).
Figure 5.
Two sample Mendelian randomization analysis of body composition and metabolic parameters on odds of PCOS. Forest plot of impact of exposure (body composition, metabolic parameters) on PCOS odds. SHBG: sex hormone-binding globulin; OR: odds ratio.
Fat and fat-free mass
Each standard deviation increase in body fat percentage (OR 3.05 per 8.5% increase; 95% CI: 2.24–4.15) and whole-body fat mass (OR 2.53 per 9.6 kg increase; 95% CI: 2.04–3.14) were significantly associated with odds of PCOS. By contrast, appendicular lean mass was not associated with PCOS (OR 0.91 per 5.6 kg increase; 95% CI: 0.79–1.05) (Fig. 5).
Childhood and adult body size
Each increase in category of genetically predicted childhood (OR: 4.55, 95% CI: 3.14–6.59) and adult body sizes (OR: 4.39, 95% CI: 3.25–5.93) were associated with odds of PCOS in univariable MR models (Fig. 5). In MVMR, conditional F statistics were 11 and 13 for childhood and adult body sizes, respectively, suggesting adequate instrument strength. Childhood body size had an independent effect on PCOS after accounting for adult body size (OR 2.56; 95% CI: 1.57, 4.20).
Effect of SHBG and fasting insulin on PCOS
Fasting insulin (OR: 6.98 per 0.79 pmol/l increase; 95% CI: 2.02–24.13) and SHBG levels (OR: 0.74 per 28 nmol/l increase, 95% CI: 0.64–0.87) were associated with odds of PCOS. Glycated haemoglobin was not associated with PCOS odds (OR: 1.05 per 6.7 mmol/mol increase, 95% CI: 0.90–1.22) (Fig. 5).
Discussion
Using two complementary methodologies (systematic review/meta-analysis and MR), we provide robust evidence in support of a strong causal association between first, overweight/obesity, and specifically central fat accumulation, and second, metabolic parameters suggestive of insulin resistance (including hyperinsulinaemia and low SHBG), with incident PCOS. Importantly, our study demonstrates for the first time a critical role for the impact of excess childhood/adolescent adiposity in the pathophysiology of adult PCOS. This novel finding starkly reminds us of the adult implications of living with overweight and obesity in childhood and adolescence, besides its effects in adulthood, on the risk of incident PCOS.
Studies selected via systematic review suggest an association between abnormal childhood/adolescent body composition and odds of PCOS. A two-cohort study from the systematic review demonstrated an increased risk of PCOS when there was a marked Z score increase in birthweight (de Zegher et al., 2017). Additionally, Christensen et al.’s cross-sectional study (n = 138 502) reported that a longer duration of living with overweight increased PCOS risk in a linear relation (Christensen et al., 2013). In fact, adolescents with severe obesity (BMI ≥ 40 kg/m2) had a 23.1-fold increased odds of PCOS (overweight OR 3.85, obese OR 10.25). Notably, the study design limits determination of causality between exposure (BMI) and outcome (Christensen et al., 2013). Our MR data implicates early life body size in PCOS risk, independent of adult body size. Importantly, this suggests that young females with overweight and obesity, even following reversion to a normal adult weight, still have an increased PCOS risk. Overall, there is a clear causal relation between aspects of abnormal body composition during childhood, adolescence and adulthood, and incident PCOS.
In general, BMI increases rapidly during the first year of life, then subsequently decreases and reaches a nadir at ∼6 years. Thereafter, BMI increases again throughout childhood, and this second rise is referred to as the adiposity rebound. An earlier adiposity rebound is implicated as a predictive marker of obesity in later childhood, adolescence, and adulthood (Kang, 2018). Age at adiposity rebound is also associated with PCOS diagnosis: in the Northern Finland cohort study of 280 women with PCOS and 1573 controls, women with PCOS had lower birthweight, earlier adiposity rebound and higher subsequent BMI versus control. One proposed underlying mechanism linking low-birthweight individuals with subsequent metabolic disease relates to a reduced capacity for subcutaneous fat storage. This means during subsequent weight gain and accretion of fat mass, especially occurring at an early age, fat is partitioned to the ectopic sites, including the liver and visceral adipose tissue, driving systemic insulin resistance and contributing to PCOS development (Koivuaho et al., 2019). This mechanism in children of low birthweight suggests that targeting metabolic abnormalities and weight maintenance is particularly important in this subgroup. This has been shown with metformin therapy in low-birthweight girls, and with weight loss in adults/adolescents with PCOS and obesity, with reduced adiposity and insulin sensitizing addressing hyperandrogenism, insulin resistance and restoring normal ovulatory function (Gambineri et al., 2002; Kuchenbecker et al., 2011; Lass et al., 2011). The contribution of lifestyle factors, diet, physical activity, and sedentary behaviour to BMI in women with and without PCOS was convincingly shown in the Australian Longitudinal Study of Women’s Health (Moran et al., 2013).
SHBG is an important surrogate marker of insulin resistance, which mediates the hyperandrogenism in PCOS. Our MR data reports that lower genetically determined SHBG increases PCOS odds. Similarly, a MR study reported that a 1 standard deviation higher genetically determined testosterone increased PCOS odds (OR: 1.51, 1.33–1.72) (Ruth et al., 2020). Two cross-sectional studies also associate peripubertal obesity with hyperandrogenaemia and hyperinsulinaemia in early puberty; this supports excess adiposity as predisposing to hyperandrogenism and metabolic dysfunction (Laitinen et al., 2003; McCartney et al., 2006, 2007). Furthermore, in the Northern Finland birth cohort, weight gain and hyperandrogenism during early adulthood (age 14–31 years) were associated with PCOS diagnosis and symptoms (Ollila et al., 2016). These data add to the strength of evidence suggesting adolescent hyperandrogenism is a biochemical precursor of PCOS, occurring with weight gain. This is important given the exponentially increasing obesity epidemic, particularly in younger people.
Phenotypic clustering analysis has afforded new mechanistic insights into the pathophysiology of PCOS and the existence of two potential PCOS phenotypes: metabolic and reproductive phenotypes (Dapas et al., 2020). While the reproductive phenotype is characterized by lesser adiposity, a relatively low BMI, normal insulin levels and increased LH and SHBG concentrations, the metabolic phenotype is characterized by higher BMI and hyperinsulinaemia but low LH and SHBG (Dapas et al., 2020; Yan et al., 2021). Our results, driven by genetically determined childhood/adolescent body composition, higher BMI, hyperinsulinaemia, and lower SHBG, clearly favour obesity driving the metabolic, but not reproductive, PCOS phenotype. Further research should investigate the observational and genetic epidemiology of the potential PCOS phenotypes.
Strengths and limitations
There are several strengths of this study. First, we combine two different methodological approaches, namely MR and meta-analysis, facilitating determination of the mediating effect of excess adiposity on PCOS risk. Second, our systematic review screening criteria were based on a previously published high-quality systematic review/meta-analysis, updating it c.10 years later. Finally, we used a two-sample MR approach, which is more robust against confounding and reverse causation compared to traditional observational designs.
The study has several limitations. First, the optimal MA would have been of prospective studies only. Given that these studies were limited, we also included cross-sectional and retrospective case-control studies and, as such, this limits our ability to determine causality between exposure (body composition) and outcome (PCOS). However, sensitivity analysis demonstrated the meta-analysis findings persisted across all study types (including prospective cohort studies). Second, ideally all patients would have been recruited from the general population to minimize the selection biases. In our study, a large proportion of studies recruited patients from hospital clinics and specific demographics. There is a risk that the recruited population does not reflect the population’s overall exposure rates. Third, there was a smaller number of studies of adolescents than adults, such that for adolescents the MA measure of PCOS odds is less precise (with wider CIs). In terms of MR, the analyses were based on PCOS classified by both diagnostic criteria and self-diagnosis, which may have potentially biased findings, including individuals not fulfilling the diagnostic criteria utilized in the systematic review. Furthermore, the current analysis estimates the effect of various exposure on PCOS susceptibility, which may not generalize to PCOS progression. Adiposity is likely to have a non-linear association with PCOS, which should be the focus of future studies. MR was analysed on a continuous scale (per unit increase in standard deviation), whereas meta-analysis was analysed using categorical data. This may limit the integration of results. The study population included mainly participants of European ancestry; future study among other ethnic populations may be helpful in confirming the generalizability of the current findings. Finally, GWAS of BMI did not exclude PCOS from the analyses. However, PCOS prevalence would be low in the total cohort, which includes males and females, and there is greater causality for BMI causing PCOS given the observational data Therefore, risk of bias due to not excluding individuals with PCOS from analyses is unlikely.
Conclusion
In conclusion, from several sources of evidence, childhood, adolescent, and adulthood overweight and obesity drive a distinct metabolic phenotype of PCOS, with implications for early weight maintenance to minimize the risk of subsequently developing PCOS and later cardiometabolic complications.
Supplementary Material
Contributor Information
Laurence J Dobbie, Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK; University Hospital Aintree, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Bradley Pittam, Manchester University Hospital NHS Foundation Trust, Manchester, Greater Manchester, UK.
Sizheng Steven Zhao, Centre for Epidemiology Versus Arthritis, Division of Musculoskeletal and Dermatological Science, School of Biological Sciences, Faculty of Biological Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Uazman Alam, Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK; University Hospital Aintree, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Theresa J Hydes, Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK; University Hospital Aintree, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Thomas M Barber, Warwickshire Institute for the Study of Diabetes, Endocrinology and Metabolism, University Hospitals Coventry and Warwickshire, Coventry, UK; Warwick Medical School, University of Warwick, Coventry, UK.
Daniel J Cuthbertson, Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, UK; University Hospital Aintree, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
Data for the systematic review and Mendelian randomization are available upon reasonable request to the corresponding author.
Authors’ roles
All authors contributed to the study conception and design. Data preparation was performed by L.J.D., S.S.Z., and B.P. Data analysis was performed by L.J.D. and S.S.Z. Data interpretation was performed by all authors. L.J.D., S.S.Z., and D.J.C. drafted the first version of the article with all authors providing input and approving the finalized version.
Funding
S.S.Z. was funded by a National Institute for Health Research (NIHR) Academic Clinical Lectureship. No other authors declare any sources of funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aarestrup J, Pedersen DC, Thomas PE, Glintborg D, Holm JC, Bjerregaard LG, Baker JL.. Birthweight, childhood body mass index, height and growth, and risk of polycystic ovary syndrome. Obes Facts 2021;14:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adali E, Yildizhan R, Kurdoglu M, Kolusari A, Edirne T, Sahin HG, Yildizhan B, Kamaci M.. The relationship between clinico-biochemical characteristics and psychiatric distress in young women with polycystic ovary syndrome. J Int Med Res 2008;36:1188–1196. [DOI] [PubMed] [Google Scholar]
- Al-Ojaimi EH. Pregnancy outcomes after laparoscopic ovarian drilling in women with polycystic ovarian syndrome. Saudi Med J 2006;27:519–525. [PubMed] [Google Scholar]
- Altieri P, Gambineri A, Prontera O, Cionci G, Franchina M, Pasquali R.. Maternal polycystic ovary syndrome may be associated with adverse pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol 2010;149:31–36. [DOI] [PubMed] [Google Scholar]
- Altinkaya SÖ, Nergiz S, Küçük M, Yüksel H.. Apelin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2014;176:168–172. [DOI] [PubMed] [Google Scholar]
- Amato MC, Galluzzo A, Finocchiaro S, Criscimanna A, Giordano C.. The evaluation of metabolic parameters and insulin sensitivity for a more robust diagnosis of the polycystic ovary syndrome. Clin Endocrinol (Oxf) 2008;69:52–60. [DOI] [PubMed] [Google Scholar]
- Asunción M, Calvo RM, San Millá NJ, Sancho J, Avila S, Escobar-Morreale HF.. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000;85:2434–2438. [DOI] [PubMed] [Google Scholar]
- Ates S, Aydın S, Ozcan P, Soyman Z, Gokmen Karasu AF, Sevket O.. Clinical and metabolic characteristics of Turkish adolescents with polycystic ovary syndrome. J Obstet Gynaecol J Inst Obstet Gynaecol 2018;38:236–240. [DOI] [PubMed] [Google Scholar]
- Ayonrinde OT, Adams LA, Doherty DA, Mori TA, Beilin LJ, Oddy WH, Hickey M, Sloboda DM, Olynyk JK, Hart R.. Adverse metabolic phenotype of adolescent girls with non-alcoholic fatty liver disease plus polycystic ovary syndrome compared with other girls and boys. J Gastroenterol Hepatol 2016;31:980–987. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE. et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009;91:456–488. [DOI] [PubMed] [Google Scholar]
- Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR.. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004a;89:453–462. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO.. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004b;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS.. Andrology: polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995a;10:2107–2111. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS.. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995b;10:2107–2111. [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Wass JAH, Franks S.. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006;65:137–145. [DOI] [PubMed] [Google Scholar]
- Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120(Suppl):5164–5192. [DOI] [PubMed] [Google Scholar]
- Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L.. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 2007;22:2279–2286. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Abdel-Rahman MY, Tan J, AbdelHafez FF, Abdelkareem AO, Henry D, Lisonkova S, Hurd WW, Liu JH.. Clinical, hormonal, and metabolic parameters in women with subclinical hypothyroidism and polycystic ovary syndrome: a cross-sectional study. J Womens Health (Larchmt) 2018;27:659–664. [DOI] [PubMed] [Google Scholar]
- Behboudi-Gandevani S, R, Tehrani F, B, Yarandi R, Noroozzadeh M, Hedayati M, Azizi F.. The association between polycystic ovary syndrome, obesity, and the serum concentration of adipokines. J Endocrinol Invest 2017;40:859–866. [DOI] [PubMed] [Google Scholar]
- Bernasconi D, Del Monte P, Meozzi M, Randazzo M, Marugo A, Badaracco B, Marugo M.. The impact of obesity on hormonal parameters in hirsute and Nonhirsute women. Metabolism 1996;45:72–75. [DOI] [PubMed] [Google Scholar]
- Beydoun HA, Stadtmauer L, Beydoun MA, Russell H, Zhao Y, Oehninger S.. Polycystic ovary syndrome, body mass index and outcomes of assisted reproductive technologies. Reprod Biomed Online 2009;18:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS.. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673–683. [DOI] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S.. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JA, Cunningham J, Norman RJ, Dunbar T, O’Dea K.. Polycystic ovary syndrome and metabolic syndrome in Indigenous Australian women. Intern Med J 2015;45:1247–1254. [DOI] [PubMed] [Google Scholar]
- Brower MA, Hai Y, Jones MR, Guo X, Chen Y-DI, Rotter JI, Krauss RM, Legro RS, Azziz R, Goodarzi MO.. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod 2019;34:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Thompson SG.. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmina E, Nasrallah MP, Guastella E, Lobo RA.. Characterization of metabolic changes in the phenotypes of women with polycystic ovary syndrome in a large Mediterranean population from Sicily. Clin Endocrinol (Oxf) 2019;91:553–560. [DOI] [PubMed] [Google Scholar]
- Carmina E, Rosato F, Jannì A, Rizzo M, Longo RA.. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab 2006;91:2–6. [DOI] [PubMed] [Google Scholar]
- Cena H, Chiovato L, Nappi RE.. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab 2020;105:e2695–e2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae SJ, Kim JJ, Choi YM, Hwang KR, Jee BC, Ku SY, Suh CS, Kim SH, Kim JG, Moon SY.. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod 2008;23:1924–1931. [DOI] [PubMed] [Google Scholar]
- Chen M-J, Chiu H-M, Chen C-L, Yang W-S, Yang Y-S, Ho H-N.. Hyperandrogenemia is independently associated with elevated alanine aminotransferase activity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2010;95:3332–3341. [DOI] [PubMed] [Google Scholar]
- Cheung LP, Ma RCW, Lam PM, Lok IH, Haines CJ, So WY, Tong PCY, Cockram CS, Chow CC, Goggins WB.. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod 2008;23:1431–1438. [DOI] [PubMed] [Google Scholar]
- Chhabra S, Venkatraman S.. Menstrual dysfunction in rural young women and the presence of polycystic ovarian syndrome. J Obstet Gynaecol J Inst Obstet Gynaecol 2010;30:41–45. [DOI] [PubMed] [Google Scholar]
- Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C.. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampelli M, Guido M, Cucinelli F, Cinque B, Barini A, Lanzone A.. Hypothalamic-pituitary-adrenal axis sensitivity to opioids in women with polycystic ovary syndrome. Fertil Steril 2000;73:712–717. [DOI] [PubMed] [Google Scholar]
- Çinar M, Aksoy RT, Güzel AI, Tokmak A, Çandar T, Taşçi Y.. The predictive role of serum cystatin C levels in polycystic ovary syndrome in adolescents. J Pediatr Adolesc Gynecol 2016;29:353–356. [DOI] [PubMed] [Google Scholar]
- Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R. et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 2014;171:P1–P29. [DOI] [PubMed] [Google Scholar]
- Dadachanji R, Shaikh N, Khavale S, Patil A, Shah N, Mukherjee S.. PON1 polymorphisms are associated with polycystic ovary syndrome susceptibility, related traits, and PON1 activity in Indian women with the syndrome. Fertil Steril 2015;104:207–216. [DOI] [PubMed] [Google Scholar]
- Dapas M, Lin FTJ, Nadkarni GN, Sisk R, Legro RS, Urbanek M, Hayes MG, Dunaif A.. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med 2020;17:e1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV. et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L. et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet 2018;14:e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros SF, Barbosa JS, de Medeiros MAS, da Silva EB, de Souza ACMC, Yamamoto MMW.. Is glycated hemoglobin related to other dysmetabolic variables implicated in the increase of cardiovascular risk in polycystic ovary syndrome? A comparative study. Exp Clin Endocrinol Diabetes 2014;122:553–557. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Reinehr T, Malpique R, Darendeliler F, López-Bermejo A, Ibáñez L.. Reduced prenatal weight gain and/or augmented postnatal weight gain precedes polycystic ovary syndrome in adolescent girls. Obesity 2017;25:1486–1489. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A.. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI.. A survey of the polycystic ovary syndrome in the Greek Island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab 1999;84:4006–4011. [DOI] [PubMed] [Google Scholar]
- Dobbie LJ, Tahrani A, Alam U, James J, Wilding J, Cuthbertson DJ.. Exercise in obesity—the role of technology in health services: can this approach work? Curr Obes Rep 2021;11:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokras A, Bochner M, Hollinrake E, Markham S, VanVoorhis B, Jagasia DH.. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol 2005;106:131–137. [DOI] [PubMed] [Google Scholar]
- Echiburú B, Pérez-Bravo F, Maliqueo M, Sánchez F, Crisosto N, Sir-Petermann T.. Polymorphism T → C (-34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: a preliminary study. Metabolism 2008;57:1765–1771. [DOI] [PubMed] [Google Scholar]
- Economou F, Xyrafis X, Livadas S, Androulakis II, Argyrakopoulou G, Christakou CD, Kandaraki E, Palioura E, Diamanti-Kandarakis E.. In overweight/obese but not in normal-weight women, polycystic ovary syndrome is associated with elevated liver enzymes compared to controls. Hormones (Athens) 2009;8:199–206. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- Fan P, Liu H, Wang Y, Zhang F, Bai H.. Apolipoprotein E-containing HDL-associated platelet-activating factor acetylhydrolase activities and malondialdehyde concentrations in patients with PCOS. Reprod Biomed Online 2012;24:197–205. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE.. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012;97:28–38.e25. [DOI] [PubMed] [Google Scholar]
- Ferk P, Teran N, Gersak K.. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod 2007;22:1031–1036. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R.. Obesity and the polycystic ovary syndrome. Int J Obes 2002;26:883–896. [DOI] [PubMed] [Google Scholar]
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ.. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P.. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med 2005a;145:72–82. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, Sieve L.. Obesity and extreme obesity, manifest by ages 20-24 years, continuing through 32-41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 2005b;122:206–212. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Morrison JA, Friedman LA, Goldenberg N, Stroop DM, Wang P.. Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism 2006;55:508–514. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Morrison JA, Goldenberg N, Wang P.. Coronary heart disease risk factors in adult premenopausal white women with polycystic ovary syndrome compared with a healthy female population. Metabolism 2009;58:714–721. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Morrison JA, Wang P.. Insulin resistance, obesity, hypofibrinolysis, hyperandrogenism, and coronary heart disease risk factors in 25 pre-perimenarchal girls age < or =14 years, 13 with precocious puberty, 23 with a first-degree relative with polycystic ovary syndrome. J Pediatr Endocrinol Metab 2008;21:973–984. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L.. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism 2003;52:908–915. [DOI] [PubMed] [Google Scholar]
- Goda A, Masuyama T.. Obesity and overweight in Asian people. Circ J 2016;80:2425–2426. [DOI] [PubMed] [Google Scholar]
- Gourgari E, Lodish M, Shamburek R, Keil M, Wesley R, Walter M, Sampson M, Bernstein S, Khurana D, Lyssikatos C. et al. Lipoprotein particles in adolescents and young women with PCOS provide insights into their cardiovascular risk. J Clin Endocrinol Metab 2015;100:4291–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gümüş Ü, Güzel AI, Topcu HO, Timur H, Yilmaz N, Danişman N.. Plasma visfatin levels in adolescents with polycystic ovary syndrome: a prospective case-control study. J Pediatr Adolesc Gynecol 2015;28:249–253. [DOI] [PubMed] [Google Scholar]
- Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, Kimmig R, Benson S, Balamitsa E, Elsenbruch S.. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol 2005;153:853–860. [DOI] [PubMed] [Google Scholar]
- Hahn S, Tan S, Sack S, Kimmig R, Quadbeck B, Mann K, Janssen OE.. Prevalence of the metabolic syndrome in German women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 2007;115:130–135. [DOI] [PubMed] [Google Scholar]
- Haldar D, Agrawal N, Patel S, Kambale PR, Arora K, Sharma A, Tripathi M, Batra A, Kabi BC.. Association of VDBP and CYP2R1 gene polymorphisms with vitamin D status in women with polycystic ovarian syndrome: a North Indian Study. Eur J Nutr 2018;57:703–711. [DOI] [PubMed] [Google Scholar]
- Han Y, Li Y, He B.. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online 2019;39:332–342. [DOI] [PubMed] [Google Scholar]
- Hart R, Doherty DA, Mori T, Huang RC, Norman RJ, Franks S, Sloboda D, Beilin L, Hickey M.. Extent of metabolic risk in adolescent girls with features of polycystic ovary syndrome. Fertil Steril 2011;95:2347–2353.e1. [DOI] [PubMed] [Google Scholar]
- Hickey M, Doherty DA, Atkinson H, Sloboda DM, Franks S, Norman RJ, Hart R.. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Hum Reprod 2011;26:1469–1477. [DOI] [PubMed] [Google Scholar]
- Hudecova M, Holte J, Olovsson M, Larsson A, Berne C, Sundstrom-Poromaa I.. Prevalence of the metabolic syndrome in women with a previous diagnosis of polycystic ovary syndrome: long-term follow-up. Fertil Steril 2011;96:1271–1274. [DOI] [PubMed] [Google Scholar]
- Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1987;1:235–245. [DOI] [PubMed] [Google Scholar]
- Hwalla N, Jaafar Z.. Dietary management of obesity: a review of the evidence. Diagnostics 2021;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF. The IDF consensus worldwide definition of the metabolic syndrome. Int Diabetes Fed 2020;28:186–191. [Google Scholar]
- Kaewnin J, Vallibhakara O, Arj-Ong Vallibhakara S, Wattanakrai P, Butsripoom B, Somsook E, Hongsanguansri S, Sophonsritsuk A.. Prevalence of polycystic ovary syndrome in Thai University adolescents. Gynecol Endocrinol 2018;34:476–480. [DOI] [PubMed] [Google Scholar]
- Kang MJ. The adiposity rebound in the 21st century children: meaning for what? Korean J Pediatr 2018;61:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chon SJ, Lee SH.. Effects of lifestyle modification in polycystic ovary syndrome compared to metformin only or metformin addition: a systematic review and meta-analysis. Sci Rep 2020;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Hwang KR, Oh SH, Chae SJ, Yoon SH, Choi YM.. Prevalence of insulin resistance in Korean women with polycystic ovary syndrome according to various homeostasis model assessment for insulin resistance cutoff values. Fertil Steril 2019;112:959–966.e1. [DOI] [PubMed] [Google Scholar]
- Kjerulff LE, Sanchez-Ramos L, Duffy D.. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol 2011;204:558.e1–6. [DOI] [PubMed] [Google Scholar]
- Koivuaho E, Laru J, Ojaniemi M, Puukka K, Kettunen J, Tapanainen JS, Franks S, Järvelin M-R, Morin-Papunen L, Sebert S. et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes (Lond) 2019;43:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchenbecker WKH, Groen H, van Asselt SJ, Bolster JHT, Zwerver J, Slart RHJ, Vd Jagt EJ, Muller Kobold AC, Wolffenbuttel BHR, Land JA. et al. In women with polycystic ovary syndrome and obesity, loss of intra-abdominal fat is associated with resumption of ovulation. Hum Reprod 2011;26:2505–2512. [DOI] [PubMed] [Google Scholar]
- Kyrkou G, Trakakis E, Attilakos A, Panagopoulos P, Chrelias C, Papadimitriou A, Vaggopoulos V, Alexiou E, Mastorakos G, Lykeridou A. et al. Metabolic syndrome in Greek women with polycystic ovary syndrome: prevalence, characteristics and associations with body mass index. A prospective controlled study. Arch Gynecol Obstet 2016;293:915–923. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S. et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes 2003;27:710–715. [DOI] [PubMed] [Google Scholar]
- Lamos EM, Malek R, Davis SN.. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol 2017;10:401–408. [DOI] [PubMed] [Google Scholar]
- Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T.. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab 2011;96:3533–3540. [DOI] [PubMed] [Google Scholar]
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK.. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Eriksson M, He W, Hall P, Czene K.. Associations between childhood body size and seventeen adverse outcomes: analysis of 65,057 European women. Sci Rep 2017;7:16917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fang L, Yan Y, Wang Z, Wu Z, Jia Q, Cheng JC, Sun YP.. Association between human SHBG gene polymorphisms and risk of PCOS: a meta-analysis. Reprod Biomed Online 2021;42:227–236. [DOI] [PubMed] [Google Scholar]
- Lim SS, Davies MJ, Norman RJ, Moran LJ.. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012;18:618–637. [DOI] [PubMed] [Google Scholar]
- Liou TH, Yang JH, Hsieh CH, Lee CY, Sen Hsu C, Hsu MI.. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril 2009;92:1960–1965. [DOI] [PubMed] [Google Scholar]
- Li T, Wu K, You L, Xing X, Wang P, Cui L, Liu H, Cui Y, Bian Y, Ning Y. et al. Common variant rs9939609 in gene FTO confers risk to polycystic ovary syndrome. PLoS One 2013;8:e66250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S. et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007;92:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC.. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714–1722. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Tosi F.. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest 2021;44:233–244. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ.. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod 2013;28:2276–2283. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Shaikh N, Khavale S, Shinde G, Meherji P, Shah N, Maitra A.. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol 2009;160:855–862. [DOI] [PubMed] [Google Scholar]
- Nácul AP, Andrade CD, Schwarz P, de Bittencourt PIH, Spritzer PM.. Nitric oxide and fibrinogen in polycystic ovary syndrome: associations with insulin resistance and obesity. Eur J Obstet Gynecol Reprod Biol 2007;133:191–196. [DOI] [PubMed] [Google Scholar]
- Nambiar V, Vijesh VV, Lakshmanan P, Sukumaran S, Suganthi R.. Association of adiponectin and resistin gene polymorphisms in South Indian women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2016;200:82–88. [DOI] [PubMed] [Google Scholar]
- Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today 2015;50:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, Crosthwaite G, Brown W.. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 2019;29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollila M-ME, Piltonen T, Puukka K, Ruokonen A, Järvelin M-R, Tapanainen JS, Franks S, Morin-Papunen L.. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab 2016;101:739–747. [DOI] [PubMed] [Google Scholar]
- Oztas E, Ozler S, Tokmak A, Yilmaz N, Celik HT, Kazancı FH, Danisman N, Ergin M, Yakut HI.. Increased levels of serum granzyme-B is associated with insulin resistance and increased cardiovascular risk in adolescent polycystic ovary syndrome patients. Eur J Obstet Gynecol Reprod Biol 2016;198:89–93. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Santagni S, Falbo A, La Sala GB.. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health 2015;7:745–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R. Contemporary approaches to the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2018;9:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Casimirri F, Cantobelli S, Labate AMM, Venturoli S, Paradisi R, Zannarini L.. Insulin and androgen relationships with abdominal body fat distribution in women with and without hyperandrogenism. Horm Res Paediatr 1993;39:179–187. [DOI] [PubMed] [Google Scholar]
- Patel AA, Bloomgarden ZT, Futterweit W.. Premicroalbuminuria in women with polycystic ovary syndrome: a metabolic risk marker. Endocr Pract 2008;14:193–200. [DOI] [PubMed] [Google Scholar]
- Pepene CE. Evidence for visfatin as an independent predictor of endothelial dysfunction in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;76:119–125. [DOI] [PubMed] [Google Scholar]
- Petta S, Ciresi A, Bianco J, Geraci V, Boemi R, Galvano L, Magliozzo F, Merlino G, Craxì A, Giordano C.. Insulin resistance and hyperandrogenism drive steatosis and fibrosis risk in young females with PCOS. PLoS One 2017;12:e0186136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, Yengo L, Ferreira T, Marouli E, Ji Y. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet 2019;28:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Donnelly R.. Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. Int J Mol Sci 2020;21:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanpour H, Jamal L, Mousavinasab SN, Esmailzadeh A, Azarkhish K.. Association between polycystic ovarian syndrome, overweight, and metabolic syndrome in adolescents. J Pediatr Adolesc Gynecol 2012;25:208–212. [DOI] [PubMed] [Google Scholar]
- Ramos RB, Spritzer PM.. FTO gene variants are not associated with polycystic ovary syndrome in women from Southern Brazil. Gene 2015;560:25–29. [DOI] [PubMed] [Google Scholar]
- Richardson TG, Sanderson E, Elsworth B, Tilling K, Smith GD.. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ 2020;369:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AH, Prochaska E, Smith M, Sammel M, Dokras A.. Using the androgen excess-PCOS society criteria to diagnose polycystic ovary syndrome and the risk of metabolic syndrome in adolescents. J Pediatr 2013;162:937–941. [DOI] [PubMed] [Google Scholar]
- Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 2020;26:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Garrido MA, Tena-Sempere M.. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos BR, Lecke SB, Spritzer PM.. Apa-I polymorphism in VDR gene is related to metabolic syndrome in polycystic ovary syndrome: a cross-sectional study. Reprod Biol Endocrinol 2018;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NR, Braverman ER.. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012;7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Han T, Cui L, Wu G, Zheng R, Xia M, Chen ZJ.. White blood cell differential counts in patients with polycystic ovary syndrome: a pilot study on Chinese women. Eur J Obstet Gynecol Reprod Biol 2013;170:162–164. [DOI] [PubMed] [Google Scholar]
- Shroff R, Syrop CH, Davis W, Voorhis BJ, Van, Dokras A.. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril 2007;88:1389–1395. [DOI] [PubMed] [Google Scholar]
- Spranger J, Möhlig M, Wegewitz U, Ristow M, Pfeiffer AFH, Schill T, Schlösser HW, Brabant G, Schöfl C.. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;61:738–746. [DOI] [PubMed] [Google Scholar]
- Spritzer PM. Polycystic ovary syndrome: reviewing diagnosis and management of metabolic disturbances. Arq Bras Endocrinol Metabol 2014;58:182–187. [DOI] [PubMed] [Google Scholar]
- Tosi F, Bonora E, Moghetti P.. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod 2017;32:2515–2521. [DOI] [PubMed] [Google Scholar]
- Verbanck M, Chen C-Y, Neale B, Do R.. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018;50:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL.. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrbikova J, Dvorakova K, Grimmichova T, Hill M, Stanicka S, Cibula D, Bendlova B, Starka L, Vondra K.. Prevalence of insulin resistance and prediction of glucose intolerance and type 2 diabetes mellitus in women with polycystic ovary syndrome. 2007;45:639–644. [DOI] [PubMed] [Google Scholar]
- Wang T, Han S, Tian W, Zhao M, Zhang H.. Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS). Gynecol Endocrinol 2019;35:807–810. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang L, Zhao Y, Shi Y, Wang L, Chen ZJ.. No association of the Arg51Gln and Leu72Met polymorphisms of the ghrelin gene and polycystic ovary syndrome. Hum Reprod 2009;24:485–490. [DOI] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA.. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010;95:2038–2049. [DOI] [PubMed] [Google Scholar]
- Wilding JPH, Batterham RL, Calanna S, Davies M, Gaal LF, Van Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA. et al. ; STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002. [DOI] [PubMed] [Google Scholar]
- Woo H-Y, Kim K-H, Rhee E-J, Park H, Lee M-K.. Differences of the association of anti-Müllerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J 2012;59:781–790. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity Task Force. International Association for the Study of Obesity. The Asia—Pacific Perspective: Redefining Obesity and Its Treatment. Western Pacific Region: World Health Organisation 2000;8–45.
- Wu W, Fan X, Yu Y, Wang Z, Wang Y.. Alteration of ghrelin/obestatin ratio in adolescence with polycystic ovarian syndrome. Gynecol Endocrinol 2018;34:36–39. [DOI] [PubMed] [Google Scholar]
- Yan YS, Qu Z, Lv P, Ping, Huang HF.. Pediatric and adult obesity concerns in female health: a Mendelian randomization study. Endocrine 2021;75:400–408. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu J-H, Qu Q-Q, Zhong G-Q.. Risk of cardiovascular and cerebrovascular events in polycystic ovarian syndrome women: a meta-analysis of cohort studies. Front Cardiovasc Med 2020;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xu Y, Wang X, Xu L, Chen J, Gao C, Wu C, Pan D, Zhang Q, Zhou J. et al. Body mass index and polycystic ovary syndrome: a 2-sample bidirectional Mendelian randomization study. J Clin Endocrinol Metab 2020;105:1778–1784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the systematic review and Mendelian randomization are available upon reasonable request to the corresponding author.