Abstract
Alzheimer’s Disease (AD) affects more women than men, with women throughout the menopausal transition potentially being the most under researched and at-risk group. Sleep disruptions, which are an established risk factor for AD, increase in prevalence with normal aging and are exacerbated in women during menopause. Sex differences showing more disrupted sleep patterns and increased AD pathology in women and female animal models have been established in literature, with much emphasis placed on loss of circulating gonadal hormones with age. Interestingly, increases in gonadotropins such as follicle stimulating hormone are emerging to be a major contributor to AD pathogenesis and may also play a role in sleep disruption, perhaps in combination with other lesser studied hormones. Several sleep influencing regions of the brain appear to be affected early in AD progression and some may exhibit sexual dimorphisms that may contribute to increased sleep disruptions in women with age. Additionally, some of the most common sleep disorders, as well as multiple health conditions that impair sleep quality, are more prevalent and more severe in women. These conditions are often comorbid with AD and have bi-directional relationships that contribute synergistically to cognitive decline and neuropathology. The association during aging of increased sleep disruption and sleep disorders, dramatic hormonal changes during and after menopause and increased AD pathology may be interacting and contributing factors that lead to the increased number of women living with AD.
Keywords: Women, sleep, sex differences, menopause, hormones
INTRODUCTION
Alzheimer’s Disease (AD) and related dementias are neurodegenerative conditions common to the aging population, affecting an estimated 55 million people worldwide [1]. In the United States (US) alone, AD affects 6.5 million individuals [2], of which, 2.5 million are men and 4 million are women. This means that women account for two out of every three cases of AD in the US [3]. When broken down by age, almost every group exhibits more women than men with AD [4]. Compared to men, women are on average: diagnosed later in the disease stage [5]; have increased rates of brain atrophy [6, 7]; experience faster rates of cognitive decline [7, 8]; and have a 70% higher lifetime cost of living with the disease [9]. Moreover, women account for more than 60% of caregivers for those living with AD or related dementias [10]. In recent years, growing evidence has suggested the most at-risk group for developing AD may be the roughly 1.5 million women, aged 45 to 55, undergoing the transition through menopause each year in the US [11]. Universal symptoms experienced during menopause are repeatedly and consistently being linked to poor outcomes in women’s brain health [6, 7, 12–18], and unsurprisingly, women have a one in five lifetime risk of developing AD by age 45 as compared to a one in ten risk for age matched men [19].
While age is the largest risk factor for developing AD, there are countless others ranging from genetic and unmodifiable (APOE4 genotype, down syndrome, genetic mutations) to “modifiable” behavioral and environmental (smoking, physical/social/mental activity, diet, education, pollution, sleep, etc.) that have been linked with disease development and progression [2]. While most risk factors are prevalent in both men and women, decades of multidisciplinary research have shown that there are sex and gender differences within each risk category. According to Rocca et al. (2014), there are three categories that should be considered with regards to risk factors and disease,: 1. factors that are present at equal frequencies in both sexes but have a greater effect in one sex over the other (e.g., APOE4 genotype); 2. factors that occur more often culturally/socially in one sex, thus having a greater effect size on one sex (e.g., low education); 3. factors that are exclusive to one sex(e.g., menopause) [20]. In addition to risk contribution, differences in presentation and response to treatments and medications in men and women are well known and documented across many diseases, and as such, it is critically important to account for these differences in AD research.
SEX AND GENDER BIAS IN MEDICAL RESEARCH
To date the majority of medical research in animals and humans has been conducted on males, owing to the alleged difficulty in using female subjects with fluctuating hormones [21]. Indeed, changing hormone patterns across the female lifespan, menstrual cycles, pregnancy, and menopause can, and do, affect clinical outcomes and responses to therapies in regards to safety and efficacy [22]. The exclusion of female subjects in early to recent medical history has left an enormous gap in the healthcare system between men and women and has led to women being underdiagnosed and inappropriately treated in medicine [21]. Although almost 80% of drugs in the US are consumed by women, women are still being prescribed drug dosages derived from the average weight and metabolism of men, leading to increased risk of complications for women [23]. It was not until 1993, that Congress passed into law the National Institute of Health (NIH) Revitalization Act requiring women and minorities be included in clinical research [24], and not until 2014 that the NIH started requiring sex to be considered as a biological variable in preclinical research in humans and vertebrate animals [25].
While slow progress has been made (as of 2019, women account for about half of participants in clinical research funded by the NIH [26]), many areas, including brain research, are still behind. A 2020 study showed that in the three years prior to its publication date, only 52% of articles in leading neuroscience journals included female as well as male test subjects, and only 5% of studies used only females as compared to 26% that used only males [27]. When looking at AD and dementia research specifically, only one-third of participants in clinical trials are women; only 12% of the allocated NIH budget for AD and dementia is allotted to women-centric research; and as of 2019 the NIH Alzheimer’s budget equates to $24 per man and only $3 per woman [28].
Although both sex and gender can, and do, influence health and disease status in humans, the two are not interchangeable. In very simplistic terms, “sex” by definition is a biological trait encoded in DNA that characterizes differences in reproductive organs and hormones, while “gender” accounts for cultural, social, and psychological traits that differ between sexes through social context [29, 30]. Although multiple sex-and gender-related factors could conceivably make a compounding contribution to a person’s risk for developing AD, most research to date has focused on sex-related mechanisms at the hormonal and molecular level, and less on gender-related factors. Preclinical research in non-human animal models is also limited to sex specific exploration as non-human animals do not have gender. Owing to these limitations, this narrative review will focus primarily on sex differences in AD and the numerous impacts that biological sex has on the relationship between sleep disruptions and AD risk. We employed the use of PubMed to search more broadly for the three main topics “Alzheimer’s disease + sleep + sex”. This search was further refined by cross referencing each main topic with other subtopics including: hormones (estrogen, progesterone, follicle stimulating hormone, luteinizing hormone), age, menopause, amyloid, tau, and individual sleep disorders/health conditions (insomnia, sleep apnea, depression, chronic pain, etc.). Citations from manuscripts and other published works were also examined.
INTERRELATIONSHIPS BETWEEN SLEEP, SEX, AND AD
Decades of research have elucidated the complex and bidirectional relationship between sleep disruptions and AD, but how biological sex influences the genetic and molecular processes underlying the sleep-AD connection is largely unknown. There is considerable evidence that sleep disruptions may be one of many AD risk factors that occur at a higher frequency and have an overall greater effect in women as compared to men. The basis for this argument, as with most sex related discussions of health and disease, hinges on hormonal changes throughout the menopausal transition. Most hormone-based research regarding AD risk in women has been gonad-centric, focusing on the loss of circulating estradiol-17-β from the ovaries following natural or surgical (hysterectomy) menopause. Falling estrogen levels from perimenopause, menopause, and hysterectomy have been associated with cognitive decline [12], elevated AD pathology [13], and a rise in sleep disruptions [14] (Fig. 1). While unsurprising, due to the broad expression of estrogen receptors throughout memory and sleep-regulating centers of the brain [31, 32], alterations in circulating levels of gonadal steroids are not the only, or even the most, dramatic hormonal change that accompanies menopause.
Fig. 1. Relationship between sleep, hormones, and AD pathology in women.
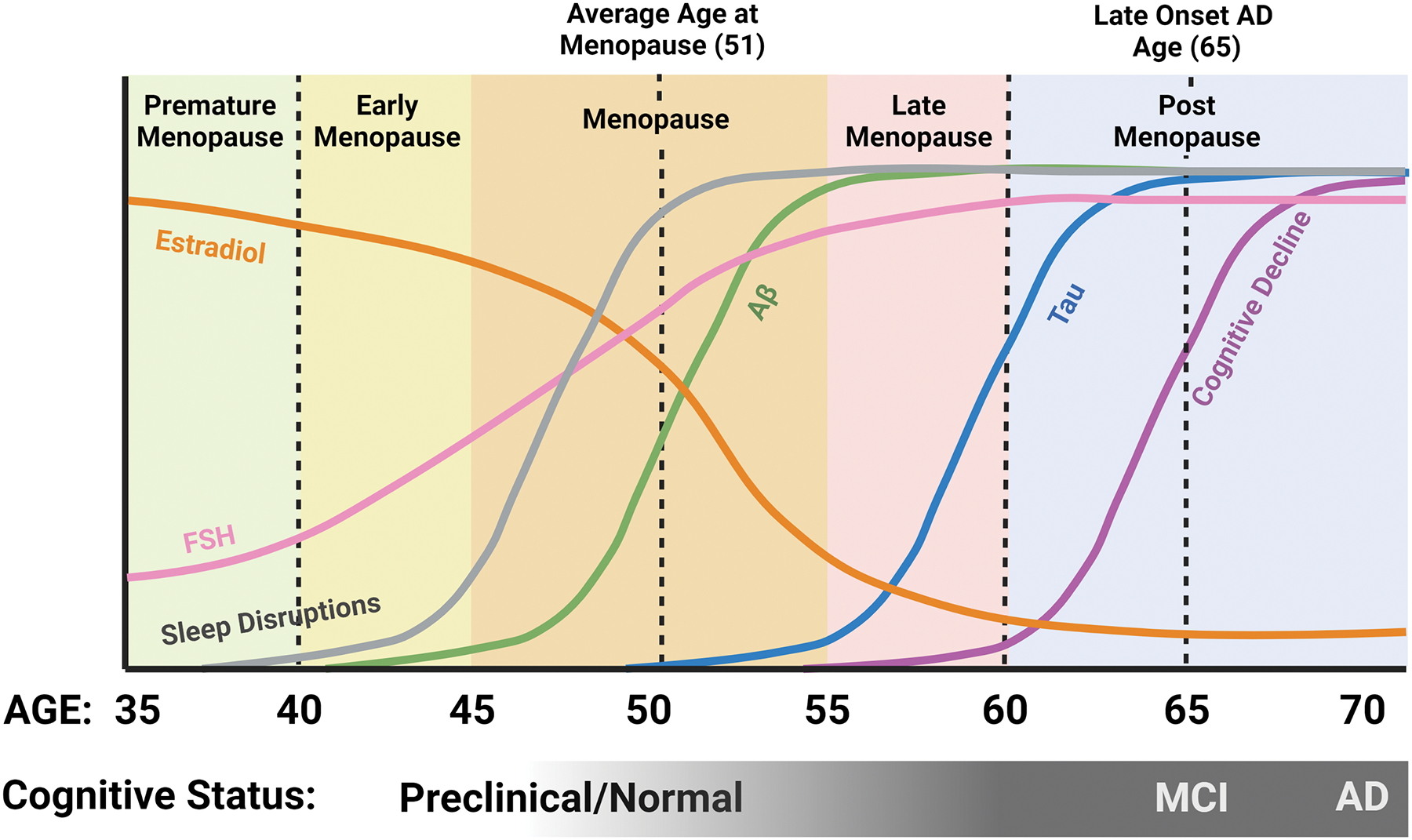
Estradiol levels begin to decline pre-menopause, while FSH levels begin to rise. Sleep disruptions become prominent during the menopausal transition, about the time when Aβ levels in the brain begin to increase, followed later by an increase in tau. Although some cognitive issues may start in late menopause, the majority of AD-related cognitive decline occurs post-menopausal. Created with BioRender.com.
Profound increases in circulating levels of pituitary gonadotropins, especially follicle stimulating hormone (FSH), are a hallmark of the menopausal transition. An increase in circulating levels of FSH is the earliest detectable hormonal alteration in the transitional period of perimenopause [33]. Throughout the transition from peri- to post-menopause, a sharp and continuous increase in FSH is inevitable, and levels of FSH remain high in the years following. Compelling studies have implicated elevated FSH levels as well as estrogen loss in menopause-related neuropathological changes including cognitive decline and AD pathology as well as sleep disruptions [34]. These neuropathological changes appear to coincide with and be exacerbated by the substantial hormonal fluctuations throughout the menopausal transition (Fig 1).
Much like hormonal fluctuations, sleep disruptions have been identified in the preclinical stages of AD and precede cognitive impairment and AD pathology by a decade or more [35]. For those who will develop late onset AD, clinical symptoms typically appear around age 65, with pathology developing up to 20 years before clinical symptoms are present [36]. When considering both this preclinical AD window and the average age of menopausal transition between 45 and 55, it becomes evident that the two overlap substantially in middle age (Fig. 1). Accordingly, a drastic increase in sleep disruptions is seen in women as compared to age matched men during this same timeframe [37, 38]. Sleep disruption commonly exemplified in AD patients includes loss of total sleep, loss of rapid eye movement (REM) sleep, loss of non-REM (NREM) sleep, loss of deep (slow wave) sleep, as well as changes in the daily sleep-wake rhythm. Increased fragmentation of the daily sleep-wake rhythm and loss of a consolidated circadian rhythm are strong AD risk factors [39–41] that may worsen as AD progresses and may contribute to AD pathology [35]. Consequently, due to its increasingly disruptive nature for both caretakers and patients, dysregulated sleep is the primary contributing factor in placing older adults (persons aged 65+ [42, 43]) into full-time care facilities [44]. Given that both AD and sleep disturbances are more prevalent in women, it is critical to unravel the complicated and bi-directional interactions between the two. Further efforts to investigate underlying biological mechanisms driving sex differences in sleep and disease will not only serve to identify potential therapeutic targets and strategies, but also to improve the quality of women’s health throughout the lifespan.
SLEEP AND ITS ROLES IN HEALTH AND AGING
To date, every studied animal species partakes in some form of sleep, a behavior which has persisted throughout millions of years of evolution. Despite its hazards and evasive critical function, humans spend about one-third of their lives asleep [45]. Research has demonstrated that sleep is necessary for survival [46] and plays widespread regulatory roles in almost every system in the body. Obtaining an appropriate amount of sleep is a proven necessity for maintaining overall health, with brain health being no exception to this rule. Sleep enhances learning and memory [47, 48]; removes waste buildup from the brain [49–51]; improves mental health and stabilizes emotions [52]; boosts the immune system and prevents infection [53]; balances insulin and glucose levels [54] and regulates appetite [55]; maintains a healthy gut microbiome [56]; facilitates healthy growth and development [57]; aids in cellular repair [58]; lowers blood pressure [59]; and more.
Failing to acquire the necessary amount of sleep can have detrimental effects on overall health and well-being. Alarmingly, lack of sleep can not only shorten life span [60], but can also lead to: disruptions in general cognitive processes [61]; increases in psychiatric conditions including depression, anxiety and suicide [62]; weakened immune function [63, 64]; and increased risk for diseases including certain cancers [65], diabetes [66], obesity [67], hypertension [68], cardiovascular disease [69], stroke [65], congestive heart failure [70], systemic inflammation and pain [53], and cognitive diseases including AD [39, 71, 72]. Currently, the CDC recommends a minimum of 7 hours of sleep per day for the average adult, however one-third of adults report getting less than 7 hours each night [73]. While a multitude of factors contributing to a chronic lack of sleep are modifiable, the general attributes of modern lifestyles (child-care, shift work, sleep disorders, noisy environments, artificial light exposure, stress, etc.) are not conducive to consistently obtaining adequate amounts of sleep. Compounding this issue, both sleep quantity and quality naturally change and decline with advancing age.
More than half of adults by the age of 65 will experience some form of sleep problem [74] in addition to having increased sensitivity to outside factors that can alter the normal sleep-wake cycle such as jet lag, shift work, stress hormones, noise, caffeine, medications, health problems, and psychiatric disorders [75–80]. Even without health issues, sleep quality in healthy individuals naturally declines with age starting as early as age 20 and is typically significant by age 60 [81, 82]. Throughout adulthood, reduced sleep duration and increased awakenings for longer periods of time are common, and often result in increased daytime drowsiness and napping [83].
With advancing age it is common for changes in sleep patterns to occur [78]. While sleeping each night, humans cycle through two phases of sleep known as NREM and REM which can be distinguished based on differences in recordings of electrical activity of brain and muscle activity. NREM sleep is further subdivided into three stages (N1-N3), with N3 sleep often referred to as deep or slow wave sleep (SWS) characterized by very slow, synchronized brain waves. In REM sleep, the electrical activity recordings are indistiguishable from those of wake, but muscle tone is absent, i.e., the brain appears awake but the body is paralyzed. During a night of sleep, an individual cycles through these stages approximately 4–6 times with occassional brief awakenings between bouts (for more detailed information on sleep stages see [84]). This pattern of progression of sleep stages throughout a sleep period is called sleep architecture. The most prominent age-related change in sleep architecture typically happens within NREM. Most older adults will experience a considerable decline in deep SWS sleep, along with a rise in the lighter sleep stages—N1 and N2 [82, 85]. Ultimately, time spent in REM decreases as well [86, 87]. All of these age related sleep changes occur in both sexes, with studies showing mixed outcomes on which sex experiences stronger age related decreases in sleep quality when measuring NREM and REM sleep [88–90].
While not a perfect model for sleep or aging, animal models have provided much insight into the basic physiological processes surrounding sleep and age (for an in depth review see [91]). Briefly, both rats and mice experience age-related changes with sleep similar to humans including an increase in fragmented sleep patterns [92–98] and changes in distribution of sleep throughout the 24 hour day [93, 95–100]. Changes in NREM and REM sleep varied by rodent strain with some showing no change with age and some showing increases or decreases with age [93–97, 100–102]. Unlike humans, most mouse strains have been shown to have increased total sleep with age [98, 102, 103].
SEX DIFFERENCES IN SLEEP
Many sex differences exist in both subjective and objective measurements of sleep. Subjectively, women report having poorer sleep quality compared to men [38, 104–108] and two out of every three adult women do not attain the CDC recommended 7 hours of sleep per night [73]. Chief complaints include increased instances of insomnia, more difficulty falling and staying asleep, increased awakenings at night, increased sleep disturbances due to noise, not feeling rested the following morning, and increased daytime sleepiness [109–116]. Women also may experience a greater sleep debt, or a greater discrepancy in the amount of sleep needed versus the amount of sleep acquired, as compared to men [117]. Overall, women have been shown to seek treatment for sleep problems and to use sleep medications more often than men [118, 119].
Contrary to subjective measures of sleep, objective sleep measures obtained with polysomnography – a multiparameter diagnostic laboratory sleep test that combines recording of sleep stages, eye movements, and muscle activity [120] — have typically shown that women obtain “better” sleep than men in young, middle, and old age [90, 107, 121–123]. Young adult women have been shown to fall asleep faster and stay asleep longer than men of similar ages [38, 81, 121, 124]. Middle-aged women spend more time asleep and exhibit higher levels of REM sleep than middle aged men [122, 124–126]. Women across all ages have be shown to have more SWS sleep [90, 127], and more restful and stable sleep than age-matched men [128, 129]. While objective measures point to women experiencing better sleep overall, the opposing outcomes of subjective sleep studies are indicative of underlying sex-related differences in how sleep is utilized in the body across the lifespan. These discrepancies could also be attributable to experimental bias and design or other sociological factors. For example: sex differences in brain wave measurements may be due to sex differences in skull thickness [123, 130, 131]; men and women may respond differently to study environment (i.e., hospital, laboratory, home) when it comes to polysomnography; and men and women report medical issues and seek medical intervention for sleep issues at different rates [118, 119]. Any additional or combination of these factors have the potential to heavily influence sleep study outcomes and warrant further investigation.
SLEEP AND THE INFLUENCE OF FLUCTUATING HORMONES ACROSS THE FEMALE LIFESPAN
From birth until old age, women experience more hormonal fluctuations and transitional periods than men, and it is throughout these regular hormonal cycles and transitory periods that sex differences in sleep are the most pronounced. There is a substantial amount of research demonstrating the role of gonadal hormones such as estrogen and progesterone on sleep, and, to a lesser extent, pituitary hormones such as FSH and luteinizing hormone (LH) on sleep. During periods of extreme hormonal fluctuations such as puberty, pregnancy, and the menopausal transition, sleep problems are reported at an increased frequency [112, 132–139]. The similar trajectories of changes in circulating hormone levels and changes in sleep architecture and sleep quality throughout the female lifespan suggest that sleep is influenced by reproductive hormones and that this phenomenon may contribute to the vast discrepancies that have been observed between sleep in both sexes. Unsurprisingly these fluctuations in ovarian hormones have yet again pushed women and non-human animals to the margins of sleep and circadian rhythm research. A review by Kuljis et al. in 2013 found that less than 20% of rhythm studies included females [140], and a review by Gervais et al. 2017 found that only about 25% of animal studies published in the previous five years included females [91]. As sleep loss over time is a compounding issue and AD develops over a decades long timespan, it is important to understand the extent of sleep disruption throughout the lifespan. It is possible that the increased rates of sleep disruption from adolescence to old age in women contributes to their increased susceptibility to AD.
Reproductive Years
During puberty, when gonadal and pituitary hormones rise to high levels inducing sexual maturity, young women are more likely to develop insomnia as compared to age matched men [137]. After menses begins and throughout reproductive years (ages 15–44 [141]), it is common for women to experience changes in sleep architecture throughout the menstrual cycle (for details on menstrual cycle and hormones see [142]). Studies have shown that most sleep problems occur during the luteal phase (characterized by decreasing estrogen, progesterone peaks, decreasing FSH and LH (Fig. 2)) and that these sleep problems include lower subjective sleep quality [143–146], longer times to fall asleep (i.e., increased sleep latency), less time asleep compared to the total amount of time spent in bed (i.e., lower sleep efficiency) [143, 144, 147], less REM sleep [134, 139], less SWS [139, 148], and more daytime sleepiness [148, 149]. These sleep problems are accompanied by, and potentially worsened by elevation of core body temperature [150, 151]. Women who experience pain from premenstrual syndrome (PMS) are more likely to experience sleep disruptions including insomnia and daytime sleepiness during the luteal phase [144, 152]. It is also of note that sleep appears to be more affected in women of late reproductive age with more reports of sleep disruption in both the early follicular and luteal phase [153], and more difficulty staying asleep plus less total time asleep during the luteal phase [154].
Fig. 2. Relationship between circulating hormone levels and sleep disruptions during reproductive years in women and female rodents.
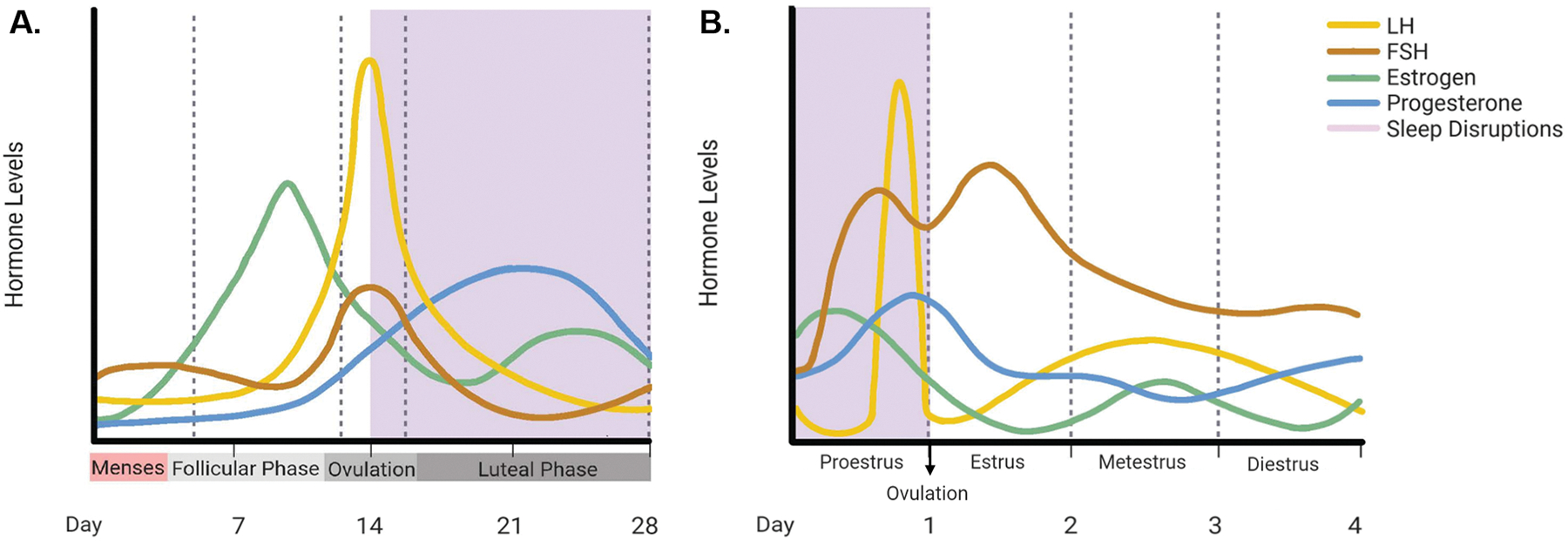
In reproductive years of women and female rodents, circulating levels of gonadal hormones (estrogen and progesterone) and gonadotropins (LH and FSH) rise and fall in accordance with the menstrual (A) and estrous (B) cycle. The majority of sleep disruptions (shown in purple shading) occur in the luteal phase for women in the menstrual cycle, and in the proestrus phase of the estrous cycle for rodents. Comparison of the two suggests that high circulating levels of LH and FSH, and rising levels of progesterone may be correlated with periods of increased sleep disruptions. Created with BioRender.com.
Although laboratory animal models such as rodents have different reproductive cycles than humans, they share many characteristics and have observable sex differences in sleep patterns. Like humans, female rodents experience changes in sleep patterns across their reproductive cycles. Unlike humans, rodents have a four to five day “estrous cycle” which consists of four phases—proestrus, estrus, metestrus, and diestrus [155] (for estrous cycle and hormones see [156] and for a summary see Fig. 2). During proestrus, when estrogen, LH, and progesterone peak and ovulation occurs, female rodents experience the least amount of sleep, and spend most of their time awake [157–160] (Fig 2). Both REM and NREM are lower in this phase than in any other phase [159, 161–166]. During the estrus phase when estrogen, LH, and FSH are declining and progesterone is low, mice sleep the most in comparison to any other phase [159].
Pregnancy
Pregnancy is another period of substantial hormonal fluctuations that can cause sleep disruption and negatively impact sleep quality in women. Throughout pregnancy, the concentrations of estrogen and progesterone increase 50 to 60-fold [167], and this is accompanied by a large increase in self-reported sleep disturbances. A clear majority (greater than two-thirds) of pregnant women report some degree of disrupted sleep [167, 168], and causes for disruptions cover a wide range of physiological symptoms including increased urge to urinate, physical discomfort, restless sleep, difficulty falling asleep, difficulty staying asleep, increased daytime sleepiness, sleep apnea, and restless legs syndrome (RLS) [168–173]. Additionally, both REM and NREM [136, 155, 174] sleep decrease throughout pregnancy. In the time immediately following childbirth, hormones typically return to normal levels within 2–5 months [175] and sleep typically returns to normal within 3–5 months [155, 176]. Sleep studies in pregnant rodents are all but non-existent, with one study showing increased total sleep and increased NREM sleep during late pregnancy [177].
Menopause
Menopause is a universal physiological event experienced by aging women who live to midlife and beyond. On average, menopause occurs at age 51 meaning that women will spend about one-third of their lives in a post-menopausal state [178]. Throughout the transition from peri- to post-menopause, menstruation ceases permanently as the ovaries stop producing estrogen and progesterone. Without inhibition from estrogen and progesterone, the anterior pituitary produces higher circulating levels of FSH and LH, which remain high post-menopause [178] (Fig. 1). Sleep disruptions and changes in sleep architecture accompany these dramatic hormonal shifts, such that the percentage of women reporting sleep problems nearly doubles in the post-menopausal state to 61% [15, 179]. In fact, post-menopausal women report the highest rate of insomnia in the US [15]. During menopause the most common sleep complaints include trouble falling and staying asleep, frequent nighttime awakenings, daytime sleepiness, and less satisfaction with sleep quality [133, 180–182].
One of the main drivers of sleep loss throughout menopause is thought to be the loss of estrogen. Decreasing levels of estrogen are associated with increased nighttime awakenings and difficulty falling asleep [181]. Higher FSH levels in association with decreasing estrogen levels have been associated with increased nighttime awakenings in peri- and post-menopausal women [183], and poorer sleep quality and trouble sleeping in peri-menopausal women [181, 184–186] (Fig 1). Interestingly, women who undergo surgical menopause by full or partial hysterectomy have more subjective sleep complaints than those who experience natural menopause [187]. Menopausal symptoms can last for ten or more years [188], and in view of these findings, it is not surprising that women have a higher risk than men for experiencing age-related changes in sleep [37, 38]. These findings point to an intriguing but understudied topic—the potential role of endocrine changes during menopause in driving sex differences in age-related sleep disruption and AD risk.
Sex differences in sleep and their dependence on gonadal steroids has been demonstrated in laboratory rodents. In general, female rodents sleep less and have reduced NREM and REM sleep during the dark phase, as compared to males [91, 139, 189–194]. Ovariectomy of mice and rats eliminates sex differences in their sleep patterns, making females virtually indistinguishable from males in total and REM sleep amounts. Treatment of ovariectomized females with estrogen to restore their circulating levels to physiological range recreates the expected sex differences in total sleep and REM sleep [162, 163, 190, 195–197]. Furthermore, various approaches to restoring physiologically appropriate hormone levels after ovariectomy, including treatment with estrogen, or a combination of estrogen and progesterone, induced wakefulness and reduced dark phase sleep in females [159, 165, 195], and decreased both REM and NREM sleep in females [161, 162, 190, 195, 198, 199]. Concerning fragmentation versus consolidation of sleep, studies in mice reveal that males generally have more fragmented sleep than females [189, 200, 201], and that gonadectomy removes this sex difference [189]. In rats, the results are more variable and largely dependent on estrous cycle phase with males having more fragmented sleep patterns unless compared to female rats in proestrus [194, 202].
SLEEP-RELATED CHANGES IN AD
It is widely accepted that sleep impacts memory and cognition, and as such it has been postulated that disturbances in sleep are a risk factor for cognitive decline and neurodegenerative diseases such as AD [203–207]. Individuals with Alzheimer’s disease frequently have disrupted sleep-wake patterns compared to cognitively normal older adults [208–210]. It is estimated that up to half of those individuals with mild cognitive impairment (MCI)—a state between normal aging and early dementia [211]— and two-thirds of those with AD suffer from dysregulated sleep [203–205, 212–214]. Common sleep problems in AD patients range from increased napping, excessive daytime sleepiness, sundowning, insomnia, sleep disordered breathing, less time spent asleep while in bed (decreased sleep efficiency), decreased total sleep, decreased SWS during NREM, and increased latency to REM sleep [215, 216]. Previously, it was thought that sleep disruptions were side effects caused by the AD process, however mounting evidence in recent years has shown that sleep disruptions occur early in the course of the disease and probably contribute to the progression of pathological changes (Fig 1). It is likely that a bi-directional relationship exists between the two phenomena [217].
Long before cognitive symptoms are readily noticeable, in a preclinical stage of AD that can last as long as 15–20 years, amyloid-beta (Aβ) begins to build up in the brain [36, 218]. The accumulation of Aβ is believed to be a key driver of the disease (Fig 3). It is during this time frame that sleep problems begin to manifest and to progress alongside AD pathology [208] (Fig. 1). This hypothesis is supported by several studies. In cognitively normal individuals a decrease in time spent asleep and an increase in the number of daytime naps has been correlated with amyloid status [219]; shorter fragments of sleep duration and overall poor sleep quality have been associated with increased amyloid pathology in specific brain regions [219–222] (Fig 3); and individuals with increased sleep fragmentation have been shown to have a 1.5 fold increased risk of developing AD [39, 41, 72, 223]. Both amyloid and tau must be accounted for when considering the relationship between AD pathological changes and sleep disturbances. Tau is a well-known correlate of cognitive decline in AD [224, 225], and has also been noted as the earliest observable AD-related pathology in sleep-wake regulating brain regions [226] (Fig 3). Changes to sleep architecture have been observed both in cognitively normal older adults and those with MCI. For example, both amyloid and tau deposition are associated with decreased SWS [227–230] and increased CSF levels of Aβ42 are seen in patients with more frequent arousals during SWS [231]. It has been proposed that poor sleep quality in middle-age may be predictive of amyloid and tau burden in the brain, and thus directly tied to AD risk [232].
Fig 3. Sleep-wake influencing brain regions and AD pathology progression.
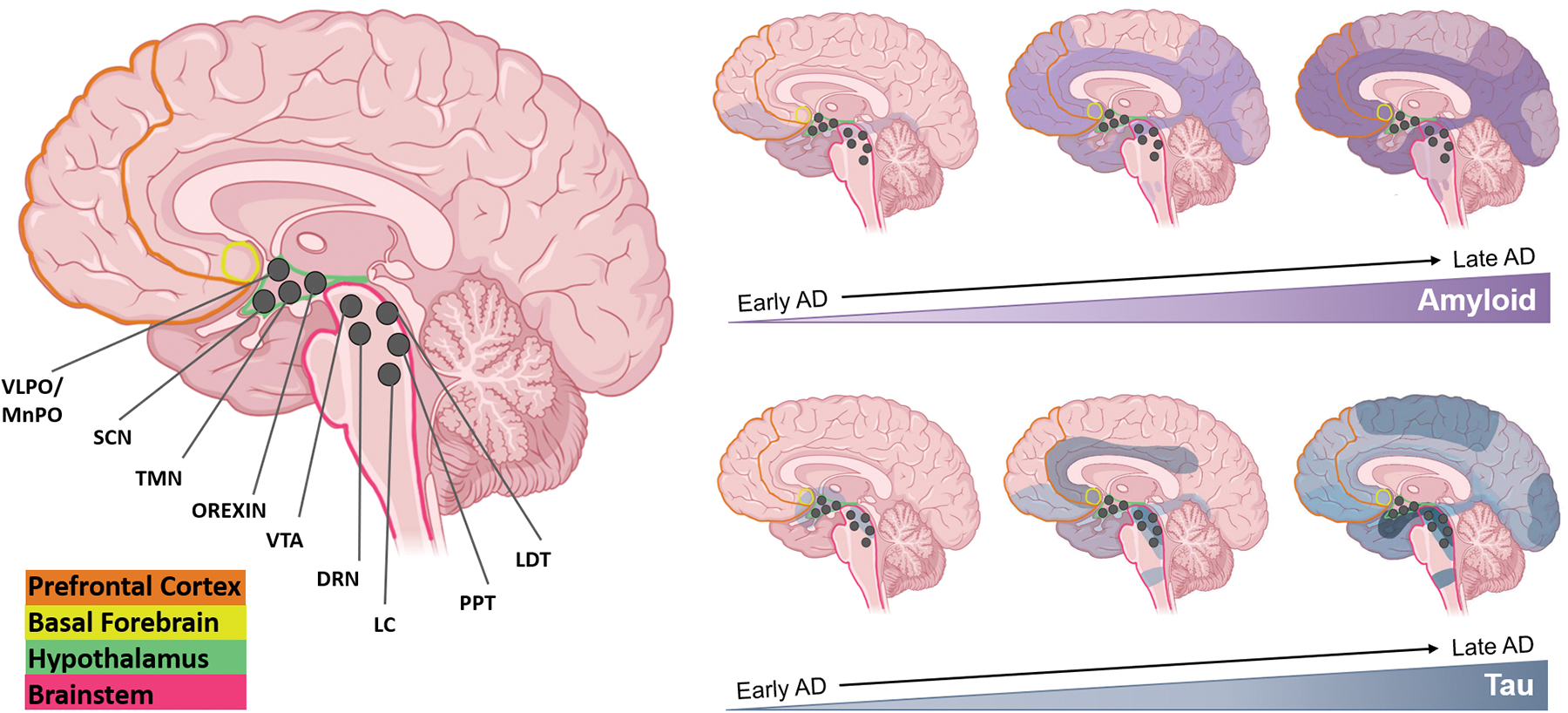
Several major brain regions are involved in maintaining states of sleep and wake—PFC (orange), BF (yellow), hypothalamus (green), and the BS (pink). Within the hypothalamus and BS, many sleep-wake influencing nuclei have been identified and are represented as dark grey circles—VLPO/MnPO, SCN, TMN, Orexin neurons, VTA, DRN, LC, PPT, and LDT. Visualizing the progression of both Aβ and tau pathology may be useful in understanding how sleep is affected throughout the course of AD. Many of the areas/nuclei involved in maintaining states of sleep and wake are affected by both Aβ and tau pathology early on in the disease, and worsening pathology with time is often correlated with more dysregulated sleep patterns. Created with Biorender.com.
The specific biological mechanisms that cause changes in sleep architecture with AD are unknown, and much research has explored the relationship between amyloid and tau burden and sleep. Sleep regulates diurnal fluctuations in Aβ levels in the hippocampal interstitial fluid (ISF) of mice and the cerebrospinal fluid (CSF) of humans—soluble amyloid is high during wakefulness, and much lower during sleep [233, 234]. When healthy young adults are deprived of sleep, even for just one night, levels of soluble Aβ38, Aβ40 and Aβ42 are significantly increased by 25–30% in the CSF, potentially due to increased overnight amyloid production when compared to control subjects [235, 236]. In contrast to the body’s lymphatic system which aids in removal of metabolic waste products, the brain has its own unique system dubbed the glymphatic system that is especially active during sleep [49–51]. During wakefulness, waste products like the AD-related proteins— amyloid and tau— accumulate in the interstitial space around brain cells [237, 238]. During sleep, pulses of CSF continuously enter the interstitial space and interchange with ISF [239]. This constant flux of CSF drives waste build-up out of the interstitial space and towards the lymphatic system to be removed from the brain and body [240, 241]. Rodent studies have shown that the interstitial space increases by more than 60% during sleep, and during this time both amyloid and tau are cleared more efficiently than during wake [49, 242].
Sleep studies in AD mouse models, despite their limitations, have proven informative in elucidating how sleep dysregulation relates to AD. For example, many mouse lines have shown that increasing AD pathology is associated with increased sleep disruption. Mouse lines with increased amyloid deposition (APP/PS1, 5xFAD, PDAPP TgCRND8 and Tg2576) have more fragmented sleep wake cycles, increased wake time, and decreased levels of both NREM sleep and REM sleep [243–248]. Conversely, APP/PS1 mice that were actively immunized against amyloid regained a normal sleep-wake cycle [243]. Mouse lines with increased tau levels have also shown disrupted sleep patterns including increased wake bout duration and decreases in both NREM and REM sleep [96, 249, 250]. Alternatively, many studies have attempted to demonstrate the opposite by measuring the effect of sleep disruption on AD pathology. Common models of sleep disruption or deprivation in mice include: 1. the “flowerpot technique” (a method in which mice are placed on a small platform over water such that loss in muscle tone during REM sleep causes the mouse to fall into the water and wake up) [234, 251], 2. intermittent tactile stimulation [234], or 3. light-dark cycle changes that disrupt circadian rhythm [252]. These techniques are not reflective of the sleep fragmentation into shorter bouts that are seen in AD patients, and only a few studies to date have attempted to model more intermittent sleep fragmentation with either presentation of wheel running or toys and gentle tactile stimulation [253, 254]. In AD mouse models, acute sleep deprivation increases Aβ levels in the hippocampal ISF, while chronic sleep restriction accelerates amyloid plaque deposition in many brain regions [234, 255]. Studies comparing sleep fragmentation versus sleep deprivation have found enhanced amyloid deposition with the sleep fragmentation approach [253, 254]. Sleep deprivation in mice has also been found to increase tau in ISF and to accelerate tau spreading in specific brain regions [256].
AGE-RELATED SEX DIFFERENCES IN AMYLOID AND TAU
Many studies have investigated sex and gender differences in amyloid and tau pathology in individuals who are cognitively normal, and individuals with MCI or AD. Overall, women have been shown to have more global AD pathology than men [257, 258]. When looking at cognitively normal individuals ranging from middle-age to advanced age, outcomes have been mixed with some showing no difference between men and women [259–263], and others showing a greater amyloid burden in women [257, 264]. Tau pathology, including neurofibrillary tangles, as well as total and phosphorylated tau in CSF, has consistently been shown to be higher in women than in men [257, 258, 264–269], especially in the rostral middle frontal, inferior parietal, temporal, and lateral occipital lobes [270–272]. Additionally, among cognitively normal women, tau pathology has been shown to be higher in those with greater amyloid pathology [259, 271, 273, 274]. In those with MCI and AD, several studies have shown higher tau in women as compared to men [258, 261, 265, 275, 276]. Sex also influences the effect of APOE4 on AD pathology. Among cognitively normal older adults as well as older adults with MCI, women who carry the APOE4 allele have higher levels of phosphorylated and total tau in CSF than men, and one study found increased phosphorylated tau in women who carry the APOE4 allele in addition to higher levels of amyloid [268, 277].
Despite the mixed outcomes of measuring amyloid pathology over a wide age range or in very old adults post-mortem, earlier and more dramatic changes in amyloid have been exemplified in multiple studies involving women undergoing the menopausal transition [6, 7, 16–18]. The same trends are seen with tau. Menopause has long been hypothesized to increase the risk of AD in women [34, 278, 279]. Symptoms during menopause are largely neurological and include disruptions in many processes regulated by estrogen, including thermoregulation, sleep and circadian rhythms, mood disorders, and cognitive decline [278]. Throughout menopause, women have increased levels of amyloid and tau, show a loss of white matter integrity and reduced grey matter volume, and exhibit decreased glucose metabolism [6, 7, 16–18]. In all of these studies, comparisons of pre-, peri-, and post-menopausal women to age-matched men found that these pathological changes were significantly higher in post-menopausal women than in men or pre-menopausal women, while the latter two groups were not significantly different [6, 7, 16–18]. Women who experienced menopause before age 50 had increased levels of tau [280] compared to those who underwent menopause later. Additionally, women who experienced hysterectomy before 50 years of age experienced an increased rate of cognitive decline with advancing age and exhibited greater amyloid pathology post-mortem [281, 282]. Overall, the changes in these neuropathological features in menopausal women precede any clinical AD symptoms, and are independent of other risk factors such as age, APOE4 status, and other comorbidities [283].
In animal models of AD, loss of circulating estradiol after ovariectomy is associated with increased levels of amyloid and decreased glucose metabolism in the brain [284] [285]. Furthermore, the concomitant increase of FSH accelerates amyloid and tau pathology and impairs cognition, while blocking FSH receptors ameliorates these disease phenotypes [34]. These findings suggest that the AD process begins earlier in women than in men, and that the menopausal transition is a critical time window when sex differences in AD may emerge, potentially putting women at an elevated risk for progression to AD [261, 283].
AD NEUROPATHOLOGY IN SLEEP/WAKE REGULATING AREAS OF THE BRAIN
Many studies have looked to pathological damage by Aβ or tau in centers of the brain that regulate the sleep-wake cycle to explain the connection between AD pathology and sleep disruption and most, if not all, of these regions show some sort of AD pathology or neuronal loss [226, 286]. Multiple regions and nuclei in the brain are responsible for maintaining states of sleep or wake including: the hypothalamus containing the suprachiasmatic nucleus (SCN), tuberomammillary nucleus (TMN), ventrolateral preoptic area (VLPO)/median preoptic area (MNPO), and orexin/hypocretin neurons; the brain stem containing the dorsal raphe nuclei (DRN), locus coeruleus (LC), pedunculopontine nucleus (PPT), and laterodorsal tegmental nucleus (LDT); the midbrain which contains the ventral tegmental area (VTA) ; and the basal forebrain (BF) [287] (Fig 3). The interrelationship between these areas is complex and while each region may be involved in more than one process, the LDT, PPT, TMN, DRN, and LC are generally classified as the major “wake promoting” regions [288, 289]; and the VLPO is classified as the major “sleep promoting” region. While many other regions are involved in both sleep and wake promotion, the following section will focus on sexually dimorphic areas involved in sleep promotion (for a review on all sleep/wake promoting areas see [287]).
The VLPO in rodents and its human homolog, the intermediate nucleus, is a major sleep promoting neural substrate that stimulates sleep and inhibits wake by releasing neurotransmitters γ-aminobutyric acid (GABA) and galanin [290]. GABA and galanin bind to receptors in the hypothalamus and brainstem and serve to dampen or inhibit the wake promoting neural circuits of the brain while promoting sleep [291]. Amyloid pathology has been found in this region of AD patients, while tau pathology has been found infrequently [292]. In older adult humans, AD was associated with decreased expression of galanin-immunoreactive neurons in the intermediate nucleus [293]. Furthermore, sleep fragmentation in subjects with or without AD was inversely associated with the number of galanin-immunoreactive neurons in the area [293]. It is interesting to note that the VLPO has been proposed to be sexually dimorphic [294]. In addition to being influenced by estrogen [158, 295] the VLPO in females also has a smaller neuronal volume, and more dramatic cell loss throughout the lifespan as compared to males [294, 296].
The hypothalamic SCN, dubbed the “master clock” of the body, synchronizes circadian rhythms with the external light-dark cycle and influences the propensity to fall asleep [297]. With normal aging, circadian rhythms are altered, often showing a reduction in amplitude, meaning the difference between the peak and trough of the 24-hour rhythm is reduced. In AD these changes are exacerbated and sleep may become fragmented; with some patients suffering a complete loss of sleep-wake rhythms [298–300]. The SCN undergoes functional and morphological changes with age, and these changes are exacerbated in AD, where degeneration of SCN neurons may also occur [301–305]. Additionally, the SCN has been shown to be sexually dimorphic in shape (longer and thinner) in women as compared to men [304, 306, 307]; and women with AD have been shown to have a greater loss of vasoactive intestinal peptide (VIP) expressing neurons which make up the core of the SCN [308].
In addition to circadian regulation, propensity to sleep is strongly regulated by the amount of time spent awake, often referred to as “sleep pressure” or the homeostatic sleep drive [309]. Sleep pressure is mediated by the neuromodulator adenosine, a product of adenosine triphosphate degradation, which accumulates throughout the day. The more adenosine is accumulated, the greater sleep pressure will be. Throughout wake, adenosine is hypothesized to be produced and accumulate at higher levels in specific brain regions, one of which is the BF [310, 311]. In AD, the BF exhibits degeneration of cholinergic neurons, which appears to be one of the earliest pathological events of the disease [312, 313] (Fig 3). In studies of cognitively normal, MCI and early AD patients, increases in tau pathology in the BF correlate with degree of dementia [314–316]. Additionally, loss of cholinergic neurons in this region has been associated with loss of sleep-wake regulation in AD, specifically loss of REM sleep [231, 316, 317]. The BF is also sexually dimorphic, at least in rats, as a larger neuronal volume is found in this area in male rats compared to females, and it is influenced by estradiol [318–320].
The prefrontal cortex (PFC) plays an important role in sleep as it is one of the most common sites of origin of slow-wave activity that predominates in the deepest stage of NREM sleep[321]. With normal aging, the density of slow waves declines predominantly in frontal brain regions [322–324] and, interestingly, loss of slow wave sleep occurs early in the course of AD and contributes to its neuropathological progression [325, 326]. Amyloid aggregates in cortical regions in both cognitively normal adults and those with AD [322, 327] and its increase correlates not only with memory impairment, but with decreased slow wave activity in cognitively normal older adults [227] (Fig 3). As the glymphatic system functions predominately during sleep, and Aβ42 levels have been shown to increase with decreasing SWS, it is probable that increasing Aβ and decreasing SWS exacerbate one another in a cyclic fashion, increasing neuronal degradation in AD [328]. It is of interest to note that the PFC is sexually dimorphic during early development with larger volumes present in females than in males [329, 330].
While sexual dimorphism exists in many sleep-wake regulating areas of the brain, it is possible that sex differences in the major sleep regulating region (VLPO) in combination with sex differences in multiple sleep influencing areas (SCN, BF, and PFC) have an additive effect on sleep changes in women with age. Smaller neuronal volume, more dramatic cell loss with age and AD, influence of circulating estrogen, and variance with development all have the potential to alter biological processes including sleep as well as AD progression in women.
SEX DIFFERENCES IN APOE4 AND SLEEP
One area that is still under investigation is the relationship of apolipoprotein E (APOE) and sleep. Sex differences have been reported regarding APOE4, demonstrating that females carrying this allele are at greater risk of developing AD earlier in life, have more rapid disease progression, and have more severe cognitive decline compared to males [261, 331]. Sleep may also be influenced by APOE4 genotype, with some studies reporting a relationship between APOE4 and sleep apnea [332]. For example, studies have shown that individuals with APOE4 alleles can be at increased risk for sleep apnea [333]; individuals with both APOE4 alleles and sleep apnea may have higher rates of impaired spatial working memory [334]; and individuals with APOE4 alleles may have significantly worse sleep quality than those without [335]. These studies suggest that women may be uniquely vulnerable to the combination of sleep disorders and APOE4 genotype as a risk factor for AD.
HORMONES AND THEIR EFFECTS ON SLEEP AND AD IN WOMEN
Gonadal Steroids
The scientific literature regarding sex differences in disease and aging commonly discuss sex steroid hormones—estrogen and progesterone—with most research focusing on 17β-estradiol, the predominant form of estrogen. Most studies demonstrate an increased risk of AD and related dementia with loss of circulating 17β-estradiol, citing menopause or the surgical removal of ovaries prior to natural menopause as the main driver [281, 336, 337]. Interventions and treatment strategies have logically followed a hormone replacement therapy (HRT) approach to mitigate symptoms from hormone loss as well as risk of certain diseases (it is important to note that while hormonal contraceptives have had both positive and negative associations with many diseases and health conditions throughout the female lifespan it is incredibly complex and beyond the scope of this review. See [338–340] for an overview of HRT and perimenopause). These therapies have employed either estrogen alone or a combinatorial approach using estrogen and progesterone. Results for these studies have been mixed depending on a multitude of factors such as age at administration, menopausal status, dosing strength, duration of administration, hormone formulation, and more [341]. Most studies have shown that AD risk is lower in those who used some form of HRT, with decreasing risk associated with increased dose and duration [13, 341–346]. Overall, these findings suggest that there may be a critical window, route of administration, and treatment plan in which HRT should be administered to be maximally effective in protecting the brain during aging from negative effects of reproductive hormone loss, including sleep disruption and neurodegeneration [347].
Estrogen
While estrogen is most commonly associated with female reproduction, it is also the most studied hormone in regards to AD. Women produce three different forms of estrogen – estradiol, estrone, and estriol [348]. Estradiol is the primary form of estrogen in circulation throughout the reproductive years, however it has many functions extending beyond reproduction, including roles in sleep. There are two subtypes of estrogen receptors, ERα and ERβ, and they are distributed widely throughout the brain in areas associated with sleep and/or AD, including but not limited to, the hippocampus, cortex, amygdala, SCN, VTA, LC, VLPO, and pineal gland [349–351]. Estrogen replacement therapy is often utilized throughout menopause to combat unwanted symptoms.
The effects of estrogen on sleep is complex because it regulates many systems in the body. In a one month study in women of reproductive age, those with low variation in sleep duration (i.e. routinely obtaining 7–8 hours of sleep per day) were shown to have 60% lower levels of estradiol as compared to those with higher variations in sleep durations [352]. Another study showed a correlation between amount of sleep and estrogen levels with estradiol increasing by almost 4% with every one additional hour of sleep obtained by participants [353]. Throughout the menopausal transition women using HRT report fewer sleep complaints including: fewer hot flash and movement-related arousals [354]; reduced insomnia symptoms and improved sleep quality [355]; increased REM sleep [356], reduced time to fall asleep, reduced nighttime awakenings, increased total sleep time, and increased SWS [357].
In women with AD, plasma levels of estrogen are lower when compared to age matched controls [358]. Animal models of AD have shown that loss of circulating 17β-estradiol after ovariectomy is associated with increased AD-like neuropathological changes including amyloid [359, 360] as well as decreased cognitive function [361, 362]. Treatment with 17β-estradiol after ovariectomy mitigated these effects to varying degrees [359–361]. Changes in the expression of estrogen receptors in the brain has also been implicated in AD. Declines in ERβ in female rats have been associated with a decline in cognitive function [363]. Cell and animal models have been beneficial in demonstrating the neuroprotective effects of estrogen as it has been shown in these models to: protect against neuronal cell loss from amyloid toxicity [364, 365]; prevent apoptosis [366]; reduce excitotoxic cell death [367, 368]; prevent oxidative damage [369]; suppress inflammation [370]; promote synaptogenesis [371]; reduce tau hyperphosphorylation [372, 373]; reduce amyloid production [362, 374, 375]; induce APP processing via the non-amyloidogenic pathway [376]; inhibit APP processing via the amyloidogenic pathway [377, 378]; promote amyloid clearance [379]; increase neurogenesis [380]; and enhance learning and memory by increasing dendritic spine density [381]. While estrogen has been widely studied and shown to have protective effects on the brain, many gaps in research still remain, especially pertaining to estrogen in both men and women living with AD in general, and more specifically how it pertains to sleep and AD.
Progesterone
Progesterone, the ovarian steroid that often works in conjunction with estrogen and supports pregnancy, has been much less studied than its counterpart. Past studies have yielded mixed results, likely due to the complexities of several interacting factors. First, progesterone often works in combination with estrogen, and the combined effects are often difficult to tease apart. Second, there are multiple forms of synthetic progesterone, or progestin, rather than one standard progestin used across all scientific studies. Currently, there are three different groups of synthetic progestins divided by their structural properties, and within each of these groups there are 2–4 subtypes [382, 383]. Clinical availability of progestin as a therapeutic is dependent on country, thus making the form used or prescribed highly variable, which can affect preclinical and clinical outcomes. Progesterone and its metabolites are thought to regulate sleep and produce changes in sleep architecture through agonistic modulation of GABA receptors, much like benzodiazepines [384–386]. Additionally, progesterone is a primary regulator of LH pulse frequency in women. As LH has been shown to affect sleep patterns, changes in progesterone levels with age may have cascading effects [387].
The majority of studies involving progesterone and sleep have shown that progesterone has sedative and sleep promoting qualities similar to benzodiazepines [388]. Treatment with progesterone increases NREM and REM sleep, decreases the amount of time to fall asleep, decreases awakenings during sleep, and increases total sleep time [386, 388–391]. In one study of women, increasing levels of progesterone during the luteal phase were associated with increased levels of sleep, with each hour increase in sleep corresponding with increased levels of progesterone [353]. It has been hypothesized that increased levels of progesterone at the beginning of pregnancy, which are much higher than normal physiological range, may be the culprit behind the increased levels of tiredness, decreased concentration, poor coordination, and memory complaints that are often experienced [386, 392]. Alternatively, decreased levels of progesterone throughout menopause, which are much lower than normal physiological range, are associated with sleep disruption, while administration of intranasal progesterone during the same time period has been shown to induce sleep [393, 394].
Interestingly, there is little to no research on the sole effects or levels of progesterone in the brain of AD patients. Despite this, progesterone has been shown to have positive effects on cognition. In post-menopausal women, progesterone administration has been associated with better concentration, verbal memory, and executive function [395, 396]. All forms of progesterone are often prescribed for HRT during menopause in conjunction with estrogen. Combination HRT has shown some negative effects on short and long-term verbal memory [397], but have had positive effects on sleep [398]. Multiple studies show that progesterone is an important neuromodulator in the central nervous system and has a neuroprotective effect. In vitro and in vivo, progesterone has been shown to exert multiple neuroprotective effects in several animal models of brain injury [399, 400], improve learning and memory [401], increase neuronal survival rate [402, 403], decrease neuronal apoptosis [404, 405], improve glucose metabolism of neurons [406], decrease oxidative stress [407, 408], increase synaptogenesis [409], protect against amyloid-induced toxicity [364], and reduce tau hyperphosphorylation [361]. An extensive amount of research is still needed on progesterone in regards to men and women with AD, as well as progesterone and its involvement in sleep for both men and women throughout the lifespan and in those with AD.
Gonadotropins
Until recently, the impact of increasing levels of gonadotropins during reproductive aging on age-related changes in the brain was virtually ignored. However, mounting studies in cells, rodents, and humans demonstrate that elevated levels of circulating gonadotropins throughout normal aging may have important roles in cognition and neuronal disease. Gonadotropin levels are regulated through the hypothalamic-pituitary-gonadal (HPG) axis through a feedback loop. The hypothalamus releases gonadotropin-releasing hormone (GnRH) which stimulates secretion of the gonadotropins LH and FSH, which act sequentially to stimulate follicular growth and trigger ovulation, respectively, during the menstrual cycle. Release of 17β-estradiol from the growing ovarian follicles at first inhibits LH and FSH, but very high levels then trigger a surge release of LH, which induces ovulation and the formation of the corpus luteum that then secretes progesterone [410]. During menopause, ovarian follicles are depleted, leading to the cessation of menstrual cycles and the loss of circulating levels of estrogen and progesterone. This causes disinhibition of LH and FSH secretion such that circulating gonadotropin levels increase greatly [411, 412]. The menopausal loss of ovarian steroids causes a 3–4 fold increase in LH and a 4–18 fold increase in FSH levels [413] (Fig. 1). In contrast to women, age-related changes in reproductive hormones in men are more gradual and moderate, as decreases in androgens with age are associated with a 2–3 fold increase in LH and FSH [414]. Several studies have shown that AD patients have higher levels of FSH and LH compared to cognitively normal age matched controls [415–417]. Interestingly, as both LH and FSH receptors have been localized in the brain, age-related increases in circulating gonadotropin levels may have roles in neurological symptoms connecting menopause, sleep, and AD [34, 418, 419].
Luteinizing Hormone
The effects of sleep on LH have been somewhat characterized, but the converse, the effects of LH on sleep, have been understudied. Receptors for LH have been found in the brain in areas involved in memory and sleep such as the hippocampus, hypothalamus, cortex, and brainstem [420–425]. Throughout puberty, in both males and females, pulsatile LH secretion is increased dramatically only during sleep [426–428] with studies showing that the increase occurs during SWS and is accompanied by brief episodes of wakefulness [429]. During the early follicular phase of the menstrual cycle, LH pulses decrease in frequency but increase in amplitude which has been linked to nighttime awakenings [430]. Additionally, menopausal hot flashes which disrupt sleep are associated with increasing levels of LH [431, 432].
There is some evidence that LH may influence neurodegenerative disease processes. Several studies associate increasing levels of LH with a decline in cognition and poor memory recall in cognitively normal individuals [433–435]. A small study of aged men showed that increasing plasma LH levels were correlated with increasing amyloid levels [435, 436]. When looking at AD brains compared to control brains, LH has been found to accumulate intracellularly in pyramidal neurons in those with AD [437]. When looking at individuals with Down’s syndrome, who are already at a higher risk for developing AD, levels of LH were shown to be elevated [438]. More extensive research is needed to determine whether the correlations observed in these studies are indicative of causation.
In rats, studies have shown that LH can cross the blood brain barrier [439], and that LH receptors are found at high levels in the hippocampus [425]. Neuronal cells treated with LH were shown to increase secretion of Aβ; similarly, animal models have shown that LH increases levels of amyloid in the brain, increases AβPP expression, and impairs hippocampal associated behavior [440–443]. Additionally, when rats are chronically treated with leuprolide acetate, a gonadotropin-releasing hormone agonist that lowers LH levels, Aβ in the frontal cortex and hippocampus decreases, and cognitive performance increases [440, 444]. In view of these findings, gonadotropins have been proposed as a potential target for treating AD [445]. Overall, clinical research is extremely sparse in regards to LH in AD in both men and women, as well as in LH and its involvement in sleep throughout the lifespan of men and women.
Follicle Stimulating Hormone
Similarly to LH, studies on FSH are limited and inconsistent when it comes to the effects of FSH on sleep. Multiple studies show no influences of increased circulating FSH levels on sleep architecture in menopausal women [153, 446, 447] or positive influences of FSH on sleep duration and SWS [186, 448]. One study has shown that women who sleep for shorter periods of time have 20% less urinary FSH than those who are long sleepers [449]. Alternatively, other studies have shown that rising circulating FSH levels during the menopausal transition are associated with increased wakefulness after initially falling asleep, increased numbers of awakenings, and decreased sleep quality [153, 181, 183, 450]. Women with a more rapid increase in FSH throughout menopause reported lower overall sleep quality [186]. Additionally, hot flashes which are a prevalent feature of the menopausal transition and a source of frequent awakenings [451] have been shown to increase in prevalence and severity with increasing FSH levels [183, 431, 432].
Typically, circulating FSH levels increase substantially about 2 years before the final menstrual period, but can begin rising up to 6 years beforehand [452]. A few studies have shown that increasing FSH levels are associated with AD. In women an increased FSH/estradiol ratio during menopause has been associated with cognitive impairment [453], may have a negative effect on verbal memory [454], and has been associated with a reduction in amygdala volume and working memory [455]. Interestingly, specific variants of the FSH receptor gene have a protective effect while other variants are associated with an earlier age at menopause [456].
In one study using several mouse models of AD, FSH was shown to cross the blood brain barrier, increase amyloid and tau levels in the hippocampus and cortex, and to reduce cognitive function and spatial memory [34]. The same study showed that blocking FSH receptors ameliorates AD like pathology and restores cognition [34]. This study, as well as the studies blocking LH with leuprolide acetate (described above), support the concept that attenuating the dramatic post-menopausal increases in circulating gonadotropins may be a promising therapeutic strategy for AD. Future research should aim to confirm the role of FSH in AD in women and in men, as well as extend upon the knowledge of FSH and its role in sleep throughout the lifespan in both men and women.
SEX DIFFERENCES IN SLEEP DISORDERS AND THEIR ASSOCIATION WITH AD
When it comes to sleep disruption and disorders, studies have shown that there appears to be a sex bias, with women experiencing sleep disorders at a higher frequency. As sleep disorders negatively impact overall health over time, the increased prevalence of sleep disorders in women could lead to a substantial negative impact on women’s overall health - especially brain health - with age. While most sleep disorders as well as comorbidities that impair sleep occur in both sexes, women experience higher rates of insomnia [37, 457–460], depression and anxiety [461, 462], restless legs syndrome (RLS) [173, 463, 464], chronic pain [465], and thyroid disorders [466], all of which have been shown to worsen with age. Obstructive sleep apnea has traditionally been regarded as a male dominated disease, however recent research has shown that this disease presents differently in women and as such may be underdiagnosed [467]. Additionally, sleep apnea rates rise dramatically in women after menopause, and both sleep apnea and menopause increase AD risk [393, 468]. Other than menopause, female specific diseases such as polycystic ovary syndrome (PCOS) that are highly prevalent yet often overlooked, have been heavily linked to sleep disorders due to their comorbidities that disrupt sleep [469]. As all of these sleep disorders and associated comorbidities are indirect drivers of AD risk, their increased prevalence in women may be a contributing factor to the increased rate of AD in women (Fig 4).
Fig. 4. Shared characteristics between common sleep disorders and health conditions with increased prevalence in women.
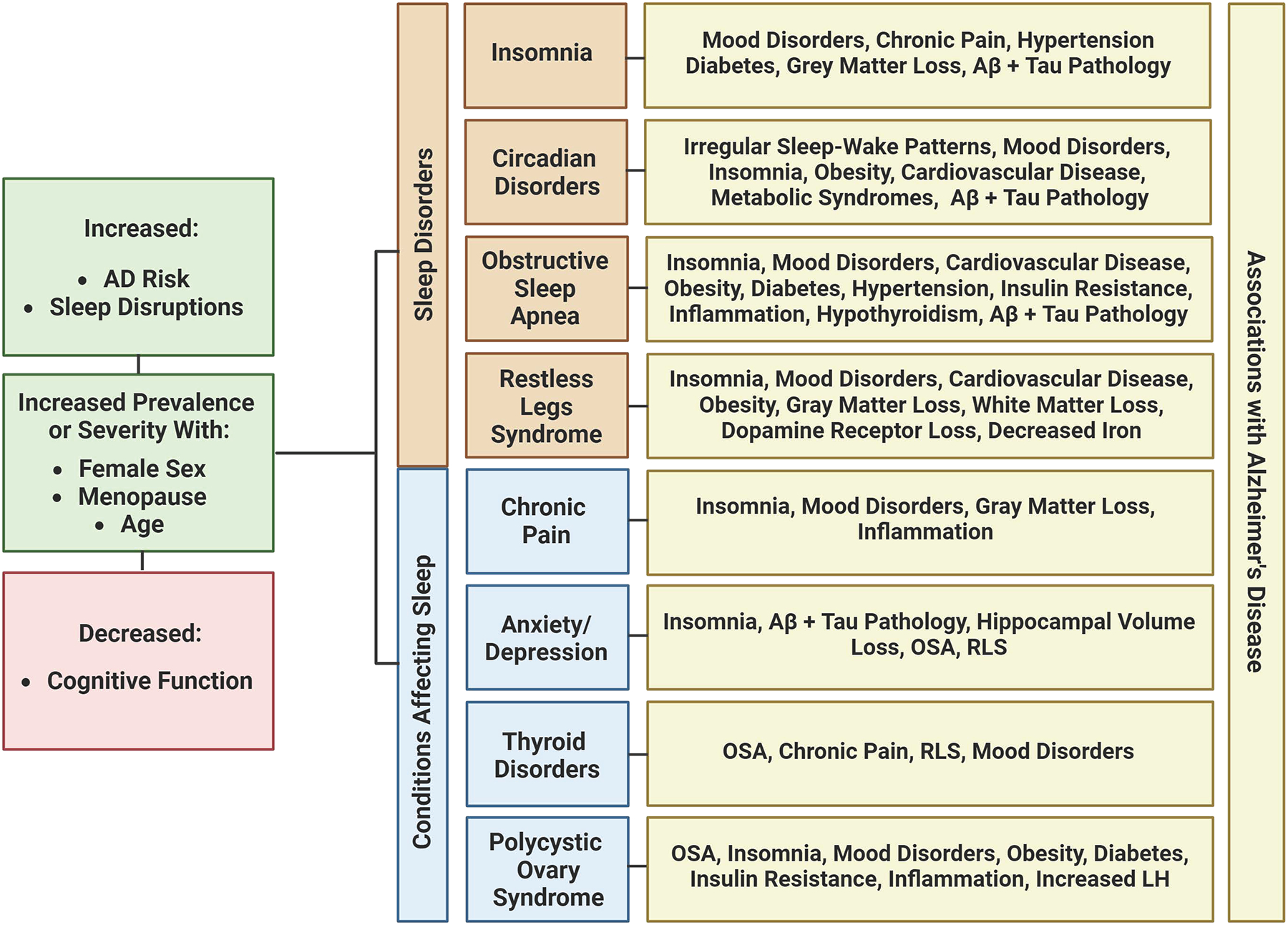
All conditions have been associated with an increased AD risk, increased sleep disruptions, and decreased cognitive functions. They are all increased in prevalence or severity with age, especially throughout the menopausal condition. Additionally, each condition shares many overlapping symptoms, comorbidities, and pathology with AD. Created with BioRender.com.
Insomnia
Insomnia is a common sleep disorder that has been linked to AD and has known sex differences. People with insomnia struggle with falling asleep, staying asleep, or achieving quality sleep. The buildup of these deficits night after night results in impaired daytime functioning [470]. It is the most commonly occurring sleep problem [471] in the US and it typically occurs in conjunction with a variety of physical and mental health diseases [471, 472] such as depression and anxiety [473, 474], pain disorders [475], and other sleep disorders [476–479]. Additionally, it increases risk of hypertension, type 2 diabetes, neurocognitive disorders, and mortality [480, 481]. Insomnia prevalence increases with age with 30–40% of older adults reporting symptoms [482, 483]. It occurs more often in women than in men [37, 457–460], and as a result, women are more likely to report impaired daytime functioning [484, 485] and unsatisfactory sleep [486–488].
Like many other diseases, insomnia may be affected by fluctuating gonadal hormones. In girls who are transitioning from a pre-menses to post-menses state at age 10, insomnia risk was found to have a 2.75-fold increase as compared to girls who had not yet experienced menses. When compared to boys of the same age, the risk of insomnia for post-menses girls was found to be 2.5 fold [137], and this risk remains higher in women throughout middle age [482]. Continuing into old age and throughout menopause, insomnia rates only continue to increase [489, 490].
Insomnia increases the risk of developing dementia and occurs in up to one-third of dementia patients [491, 492]. In older adults, insomnia impairs cognitive function [493, 494] and has also been shown to reduce grey matter volume in areas related to AD including the hippocampus and cortex [495, 496]. Insomnia induced experimentally by sleep deprivation and/or chronic sleep restriction have been shown to promote AD neuropathology in both humans and rodents. In rodents, sleep deprivation has been shown to impair cognition, and increase levels of amyloid and phosphorylated tau in the brain [251, 252, 497, 498] (Fig 4). Interestingly, in a study of cognitively normal middle-aged individuals, those with insomnia showed an increase in Aβ42 in CSF [499], possibly indicating that changes in Aβ metabolism related to sleep disruption might precede AD by several decades.
Restless Legs Syndrome
Restless legs syndrome (RLS) is a common sensorimotor sleep disorder thought to be exacerbated by low iron, and characterized by prickling sensations, involuntary limb movements, and the urge to move the legs while at rest [500–502]. Oftentimes symptoms last for hours and can be partially or completely relieved by movement such as walking [503]. RLS symptoms occur predominantly at nighttime, making sleep disruption a serious consequence of this disease as it ultimately results in decreased daytime functioning and decreased overall health [173, 503–508].
RLS typically worsens with time and increases in prevalence with age [509, 510] and is twice as common in women as in men [173, 463, 464]. During reproductive years, RLS increases in severity during menstruation, and may be linked to low iron levels [511–513]. RLS is also experienced at an increased rate by many women during pregnancy [172, 173, 514, 515], has a higher rate of prevalence in older adult women [510], and increases throughout menopause [508].
Studies have shown that risk of dementia may be 1.74 times higher in those with RLS [516], and that RLS is associated with several indirect drivers of AD including sleep disturbances, mood disorders, obesity, and cardiovascular disease [517–523]. Similarly to AD, RLS can cause patients to have trouble with concentrating on tasks, short term memory, and learning [524]. Several studies have shown that patients with AD and RLS share some common pathophysiology such as decreased white and gray matter volumes [525, 526], and decreased dopamine receptors [527, 528]. It is also thought that iron deficiency, which is a core contributing factor to RLS, could induce cognitive impairment or dementia [516, 529] (Fig 4).
Chronic Obstructive Sleep Apnea
Chronic obstructive sleep apnea (OSA) is the most frequently occurring form of sleep disordered breathing in the US [530], occurring in 26% of the population between ages 30 and 70 [531]. During sleep, individuals who experience OSA have repetitive collapses or near collapses of the upper airway which cause momentary cessation of breathing and episodes of hypoxia [532]. Eventually, these nighttime symptoms become so disruptive that neurological impairments arise during the daytime [532–534]. As with many other diseases, OSA presents differently in women versus men and can vary with age and hormonal status including menopause and pregnancy. These differences in clinical presentation contribute to underdiagnosis and undertreatment in women [467] in a “traditionally male” disease. Early studies suggested that OSA might be much more prevalent in men (i.e., 3–5 times more [535]), however more recent studies suggest that women: 1. are nearly as likely as men to have OSA (current averages are 27% in men and 22% in women) [536, 537]; and 2. represent 40–50% of the current OSA patients in sleep clinics [538].
Women with OSA tend to be more symptomatic and [539] have increased rates of: insomnia, daytime fatigue, headaches in the morning, mood related symptoms and nightmares; and lower energy levels the following day [467, 540–543]. Women also have more comorbidities associated with OSA [544] including hypothyroidism, sleep disorders and AD [542]. When looking at OSA and menopause, there is a lack of research as well as a lack of defined clinical definitions and presentations for the disease. OSA doubles in prevalence in women after menopause, and peaks at age 65, which is on average, 10 years later than the peak age of onset in males [545–547]. Additionally, women who undergo surgical menopause as compared to natural menopause, have an increased rate of OSA [548].
Not only is OSA more prevalent in older adults, it has also been associated with decreased cognitive function and AD [549–551]. OSA has been shown to increase risk of developing AD by three times, and may lead to earlier onset of MCI [551–553]. Almost half of AD patients experience OSA [554] and the severity of OSA is often indicative of severity of AD symptoms [555]. Clinical studies have shown that cognitively normal individuals with OSA have increased levels of Aβ42 in CSF, and those with cognitive impairment with untreated OSA had higher total tau/Aβ42 ratio and lower levels of Aβ42 in CSF than those with treated OSA [556]. Similarly, in mouse models of OSA, chronic intermittent hypoxia increased brain amyloid and phosphorylated tau levels [557] (Fig 4). When OSA is treated: executive function in cognitively normal individuals is improved; and in those with mild to moderate AD, cognitive decline is slowed and memory, executive function, sleep and mood are improved [558–561].
CONDITIONS WITH INCREASED PREVALENCE IN WOMEN AND THEIR RELATIONSHIP TO SLEEP AND AD
Chronic Pain
It has been well documented that sleep disorders are prevalent in those suffering from chronic pain, which are defined as pain lasting more than 3 months [562]. Chronic pain is associated with negative effects on many aspects of cognitive and behavioral health [563]. Up to 88% of people living with chronic pain experience sleep disorders [564, 565] and the two conditions have been shown to have a bi-directional relationship [566, 567]. Furthermore, chronic pain, sleep disturbances, and AD share a wide array of comorbidities including obesity [568–570], type 2 diabetes [569–573], depression [574–580], and cardiovascular disease [581, 582]. In large population-based research it is consistently estimated that women have a higher prevalence of pain than men due to increased rates of chronic conditions (e.g., fibromyalgia, chronic tension type headache, irritable bowel syndrome, etc.) [465], self-report pain more often, and have a greater sensitivity to pain [583–585]. Additionally, women who are experiencing menopausal symptoms are more likely to experience chronic pain [586].
Insomnia is the most common sleep disorder associated with pain occurring at a rate of 50% [587]. Additionally, insomnia has been shown to increase risk of developing chronic pain [588], and increase pain in those already living with chronic pain [567, 588–591] . Alternatively, improvement in sleep quality is associated with a remission in chronic pain [565, 567, 588–592].
In older adults, chronic pain is common and has been associated with cognitive deficits [593]. Estimates show that up to 50% of those living with chronic pain experience cognitive issues [594, 595] and with increasing pain comes increasing cognitive impairment [596–598]. Unfortunately, it is common for chronic pain to be underdiagnosed and undertreated in patients with AD due to difficulties in communication/expression of pain with decreased cognitive abilities, and difficulties in the perception of pain by providers [599, 600]. What is more disturbing is that those with both AD and chronic pain may actually be having worse chronic pain [601]. Those experiencing chronic pain have declines in grey matter volume [563, 602–606] in several brain areas, most commonly the PFC, an area also involved in sleep and affected in AD [607–609]. Furthermore, chronic pain has been found to be comorbid with AD in approximately half of patients [610], with those having more prevalent and severe pain having more severe dementia [611–614]. Recent research has shown that some of the underlying neurological changes associated with chronic pain or AD pathology may overlap. Neurological changes can include deficits in noradrenergic neurons in the LC [615–621], as well as increased inflammation [617, 622, 623] and microglial activation [623–626] in brain areas affected by AD (Fig 4).
Thyroid Disorders
Thyroid disorders are common in the general population [627] and can have expansive effects in the body. The two most common forms of thyroid dysfunction, which are hypothyroidism (underactive thyroid) and hyperthyroidism (overactive thyroid) [628], are up to ten times more common in women than in men [466, 629–632].
Both too much and too little thyroid activity have been associated with sleep disruptions. Some studies have shown an association between hypothyroidism and poor sleep quality, longer times to fall asleep, and shorter duration of sleep [633]. Hypothyroidism may contribute to OSA [634–637] as it may cause an increase in thyroid size, which could cause or worsen upper airway obstruction and alter respiratory muscle function [638, 639]. Additionally, low thyroid hormone levels have been associated with musculoskeletal pain, cold intolerance, RLS, and increased anxiety, all of which have been linked to sleep disturbances and insomnia [640], and several of which show increased prevalence in women. In a study of patients with hyperthyroidism, two-thirds of patients had difficulties falling asleep [641] and additional studies have associated hyperthyroidism with prolonged times to fall asleep, difficulty staying asleep, increased daytime sleepiness, tremor which caused difficulty falling and staying asleep [642], RLS [643], and insomnia [644]. Hyperthyroidism can also cause or exacerbate other health conditions that have been known to cause sleep disruptions and insomnia, such as depression and anxiety [645, 646],.
Thyroid diseases are not only a risk for sleep disorders but have also been linked to AD risk. During routine testing of older adults for dementia, screening for thyroid function is conducted since both short term hypo- and hyperthyroidism can cause reversible cognitive impairment [647–650], while long-term hypo- and hyperthyroidism can cause irreversible dementia [651–655]. One longitudinal study showed that either abnormally low or high circulating levels of thyroid stimulating hormone (TSH) were linked to increased AD risk, but only in women [656]. Studies have also shown an association between the APOE4 allele in older people with Down syndrome such that women with hypothyroidism and APOE4 had increased AD risk [657]. Another study found that higher levels of TSH were associated with increased atrophy of the hippocampus and amygdala [658]. Several studies have shown that low TSH levels may contribute to AD pathology such as increased amyloid levels and increases tau phosphorylation [659, 660] (Fig 4).
Anxiety and Depression
Sleep disturbances are the most commonly reported symptoms experienced by those with anxiety and depression [661–663] and there is much evidence for a bidirectional relationship between sleep disturbances and these disorders [664]. High prevalence of sleep disturbances is a strong predictor of future anxiety and depression, which in turn increase the risk for future insomnia [661, 665–668]. Additionally, when sleep disturbances are treated, it has been shown that individuals are more likely to experience a decrease in anxiety and depression [669, 670]. For both anxiety and depression, sleep disruption has been associated with more severe symptoms, higher rates of relapse, and lower rates of remission [666, 671–677]. The most commonly seen sleep disturbances in those with anxiety and depression, either independently or in combination, are insomnia, hypersomnia, OSA, RLS and evening circadian disorders [678–685].
Women are twice as likely as men to suffer from depression and most anxiety disorders [461, 462]. Anxiety and depression occur at increased rates in women in every age group, with anxiety being twice as likely in girls as young as age 6 [686], depression being twice as likely in girls beginning at puberty [687], and both remain higher in women throughout menopause and beyond [462, 688]. Women appear to be more susceptible to anxiety and depression during periods of extreme hormonal fluctuation from monthly menstrual cycles, pregnancy, and menopause [689–691], and increased levels of FSH and LH throughout menopause have been linked with depression [692].
Although the rates of anxiety and depression do not increase substantially with age, [693–697] almost all AD patients exhibit some sort of behavioral and psychological symptoms of dementia, with anxiety and depression being present in up to 75% of cases [698]. Oftentimes, they are early symptoms of AD and have been associated with more severe cognitive impairment and faster rates of cognitive decline [699–706]. In addition to behavioral aspects of AD, patients with anxiety and depression display increased amyloid and tau pathology, along with atrophy in certain brain regions [707–717] (Fig 4). As depression, anxiety and AD are all correlated with increased sleep disruptions, the three could have a synergistic effect in patients with more than one comorbidity [718] and could potentially have a more detrimental effect in women due to their increased prevalence. One potential contributing factor to this complex relationship could be orexins, which are neuropeptides integral in regulating wakefulness. Orexins have also been implicated in anxiety and depression [719, 720] as individuals with depression display a disrupted 24-hour orexin rhythm [721]. Sex-based orexin differences have been observed as orexin immunoreactivity in the PFC is higher in women with depression [722], and CSF levels of orexin are reported to be higher in women with AD [723].
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) has recently had many documented interactions with sleep disruptions and other AD risk factors [469]. PCOS is a lifelong endocrine disorder with unknown causes that affects not only reproduction, but also metabolism and behavioral health. It affects 5–10% of women of reproductive age [724, 725] and is driven by increased male sex hormone production, or hyperandrogenism, in the ovaries [726]. Patient symptoms and comorbidities vary, ranging from hormonal imbalance, insulin resistance, obesity, inflammation, irregular menstruation, ovarian cysts, fertility problems, high blood pressure, cardiovascular diseases, worsened cognition, as well as sleep disorders [469, 726–729]. The varied presentation of the disease makes it difficult to treat and diagnose [730], and most physicians managing PCOS are trained very little in the management of the accompanying sleep disorders [731, 732].
Sleep problems occur up to twice as often in those with PCOS compared to age matched controls and include poor sleep quality, trouble falling asleep, trouble staying asleep, excessive daytime sleepiness, insomnia and sleep disordered breathing [733–742]. The most common sleep disorder in those with PCOS is OSA, which affects up to three-quarters of those with the condition [730, 733, 734, 739, 743, 744], followed by insomnia [742]. Behavioral disorders like anxiety and depression are also elevated in women with PCOS [745–748], and one study of women with PCOS and depression showed a three-fold increase in sleep disturbances [736]. Similar to other diseases, chronic poor sleep is thought to have a bidirectional relationship with PCOS and both may exacerbate many of the symptoms and comorbidities of the other.
Many of the comorbidities of PCOS that play a role in sleep disturbances are known risk factors for AD; furthermore, many of them interact with each other in a circular fashion. For example, insulin resistance, which is present in the majority of PCOS patients [749], is strongly associated with excessive daytime sleepiness [750], and is the strongest risk factor for sleep apnea [739]. Both insulin resistance and OSA increase the risk of AD [751–753], OSA is higher in women after menopause [468, 545–547], and menopause increases risk of insulin resistance [754]. Lastly, women with PCOS often have a high LH/FSH ratio with overproduction of LH [755–758] and as previously mentioned, a correlation between increased LH, a decline in memory, an increase in amyloid levels, and an increase in AD risk may exist [433–435, 440–443, 759] (Fig 4). Further confirmatory research is needed to determine whether there is a direct link between PCOS and AD risk in women.
CONCLUSION
Over the past several decades it has become clear that women face increased disease risk and burden owing to inadequate attention in healthcare. This imbalance stems not only from intrinsic biological factors related to female sex, but is also a result of exclusion from research, underfunding of woman-centric research, and underdiagnosis and less than optimal treatment in medicine. With the increasing aging population, gaps in knowledge of women’s brain health are a glaring example of this shortcoming as women with AD far outnumber the men. The same hormonal fluctuations that caused women and female animals to be excluded from clinical and scientific research are proving to be highly relevant and critical in the treatment and prevention of age-related diseases.
Throughout the lifespan, girls and women experience significantly more hormonal fluctuation compared to boys and men with the onset of puberty, pregnancy, and menopause. While it is possible that hormonal fluctuation from these life events has an additive effect on disease risk for women over time, it is certain that the menopausal transition is contributing to an overall decrease in brain health for most, if not all, middle-aged women. Numerous studies have linked menopause with increased AD risk, which may help to partially explain the increased number of women with AD.
Coinciding with menopause is a drastic increase in sleep disturbances which is a known risk factor for AD. Both menopause and sleep disturbances have been shown to increase AD pathology in the brain, and both pathology and sleep disturbances may be worsened by the loss of gonadal steroids and the increase of gonadotropins during menopause. Studies have shown that women have more global, amyloid, and tau pathology than men and increased brain atrophy in almost all age groups, and the interaction between sex, hormones, and sleep disruption may be a contributing factor.
What’s more, women are also prone to having more subjective sleep complaints throughout the lifespan, and are either more likely to suffer from sleep disorders and related conditions throughout the lifespan, or to face a drastic increase in both throughout menopause. Women are at increased risk for insomnia, RLS, thyroid disorders, chronic pain, anxiety and depression from adolescence to late adulthood, and the risk for all of these plus OSA increases throughout menopause and persists into late life. All of these health conditions have in turn been shown to have a negative impact on brain health and may influence AD pathology and neurodegeneration in a bidirectional manner. What is lacking is a better understanding of what the downstream mechanisms are for these interactions at the molecular level. Partially, this is due to the complex nature of the problem, although it is also at least somewhat reflective of the lack of attention to sex differences in the biomedical literature until relatively recently. A deeper examination of these understudied processes could lead to sex and gender-based therapeutics that might bridge the gap between men and women living with AD and related dementias.
ACKNOWLEDGEMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported in part by AG068215 (MJD and MPM); C.E. Johnson is supported by T32 AG078110.
TERMINOLOGY
- Older adult
Persons aged 65+ according to the NIH and CDC (variations exist among studies)
- AD
Alzheimer’s Disease
- NREM
Non-rapid Eye Movement
- REM
Rapid Eye Movement
- SWS
Slow-wave Sleep
- FSH
Follicle Stimulating Hormone
- LH
Luteinizing Hormone
- MCI
Mild Cognitive Impairment
- ISF
Interstitial Fluid
- CSF
Cerebrospinal Fluid
- Aβ
Amyloid-beta
- SCN
Suprachiasmatic Nucleus
- TMN
Tuberomammillary Nucleus
- VLPO
Ventrolateral Preoptic Area
- MNPO
Median Preoptic Area
- DRN
Dorsal Raphe Nuclei
- LC
Locus Coeruleus
- PPT
Pedunculopontine Nucleus
- LDT
Laterodorsal Tegmental Nucleus
- VTA
Ventral Tegmental Area
- BF
Basal Forebrain
- PFC
Prefrontal Cortex
- GABA
γ-aminobutyric Acid
- HPG-Axis
Hypothalamic-Pituitary-Gonadal Axis
- GnRH
Gonadotropin Releasing Hormone
- HRT
Hormone Replacement Therapy
- TSH
Thyroid Stimulating Hormone
Footnotes
CONFLICT OF INTEREST
M. PAUL MURPHY, M.A., PH.D. is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
REFERENCES
- [1].Organization WH, Dementia https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Currently%20more%20than%2055%20million,million%20new%20cases%20every%20year, 20 September 2022,
- [2].(2022) 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 18, 700–789. [DOI] [PubMed] [Google Scholar]
- [3].Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA (2021) Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement 17, 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vina J, Lloret A (2010) Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis 20 Suppl 2, S527–533. [DOI] [PubMed] [Google Scholar]
- [5].Sundermann EE, Biegon A, Rubin LH, Lipton RB, Landau S, Maki PM, Alzheimer’s Disease Neuroimaging I (2017) Does the Female Advantage in Verbal Memory Contribute to Underestimating Alzheimer’s Disease Pathology in Women versus Men? J Alzheimers Dis 56, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD (2018) Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLoS One 13, e0207885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, Jett S, Jackson H, Lee A, Sarva H, Pahlajani S, Matthews D, Dyke J, de Leon MJ, Isaacson RS, Brinton RD, Mosconi L (2020) Sex-driven modifiers of Alzheimer risk: A multimodality brain imaging study. Neurology 95, e166–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, Alzheimer’s Disease Neuroimaging I (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM (2017) Societal and Family Lifetime Cost of Dementia: Implications for Policy. J Am Geriatr Soc 65, 2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rabarison KM, Bouldin ED, Bish CL, McGuire LC, Taylor CA, Greenlund KJ (2018) The Economic Value of Informal Caregiving for Persons With Dementia: Results From 38 States, the District of Columbia, and Puerto Rico, 2015 and 2016 BRFSS. Am J Public Health 108, 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Valdes A, Bajaj T (2023) Estrogen Therapy In StatPearls, Treasure Island (FL). [Google Scholar]
- [12].Gurvich C, Hoy K, Thomas N, Kulkarni J (2018) Sex Differences and the Influence of Sex Hormones on Cognition through Adulthood and the Aging Process. Brain Sci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paganini-Hill A, Henderson VW (1994) Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 140, 256–261. [DOI] [PubMed] [Google Scholar]
- [14].(2005) NIH State-of-the-Science Conference Statement on management of menopause-related symptoms. NIH Consens State Sci Statements 22, 1–38. [PubMed] [Google Scholar]
- [15].Kripke DF, Brunner R, Freeman R, Hendrix SL, Jackson RD, Masaki K, Carter RA (2001) Sleep Complaints of Postmenopausal Women. Clin J Womens Health 1, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mosconi L, Berti V, Dyke J, Schelbaum E, Jett S, Loughlin L, Jang G, Rahman A, Hristov H, Pahlajani S, Andrews R, Matthews D, Etingin O, Ganzer C, de Leon M, Isaacson R, Brinton RD (2021) Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci Rep 11, 10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schelbaum E, Loughlin L, Jett S, Zhang C, Jang G, Malviya N, Hristov H, Pahlajani S, Isaacson R, Dyke JP, Kamel H, Brinton RD, Mosconi L (2021) Association of Reproductive History With Brain MRI Biomarkers of Dementia Risk in Midlife. Neurology 97, e2328–e2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD (2017) Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology 89, 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chene G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S (2015) Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 11, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rocca WA, Mielke MM, Vemuri P, Miller VM (2014) Sex and gender differences in the causes of dementia: a narrative review. Maturitas 79, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Davidson K, Women have been overlooked in medical research for years,Northwell Health, https://www.northwell.edu/katz-institute-for-womens-health/articles/women-overlooked-in-medical-research,
- [22].FDA, Introduction: Women’s Health Research Roadmap,US Food and Drug Administration, https://www.fda.gov/science-research/womens-health-research/introduction-womens-health-research-roadmap,
- [23].Schiebinger L (2003) Women’s health and clinical trials. J Clin Invest 112, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hohmann AA, Parron DL (1996) How the new NIH Guidelines on Inclusion of Women and Minorities apply: efficacy trials, effectiveness trials, and validity. J Consult Clin Psychol 64, 851–855. [DOI] [PubMed] [Google Scholar]
- [25].Health NIo (2023), ed. Health OoRoWs NIH, Bethesda, MD. [Google Scholar]
- [26].Health OoRoWs (2021), ed. Health OoRoWsHaNSfRoWs U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Bethesda, MD. [Google Scholar]
- [27].Mamlouk GM, Dorris DM, Barrett LR, Meitzen J (2020) Sex bias and omission in neuroscience research is influenced by research model and journal, but not reported NIH funding. Front Neuroendocrinol 57, 100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baird MD, al. MAZe (2023) Women’s Health Access Matters, Rand Corporation. [Google Scholar]
- [29].Rodin J, Ickovics JR (1990) Women’s health. Review and research agenda as we approach the 21st century. Am Psychol 45, 1018–1034. [DOI] [PubMed] [Google Scholar]
- [30].(2010) In Women’s Health Research: Progress, Pitfalls, and Promise, Washington (DC). [Google Scholar]
- [31].Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I (1997) The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology 138, 5649–5652. [DOI] [PubMed] [Google Scholar]
- [32].Foster TC (2012) Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22, 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee SJ, Lenton EA, Sexton L, Cooke ID (1988) The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod 3, 851–855. [DOI] [PubMed] [Google Scholar]
- [34].Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, Padilla A, Miyashita S, Chan P, Zhang Z, Katsel P, Burgess J, Gumerova A, Ievleva K, Sant D, Yu SP, Muradova V, Frolinger T, Lizneva D, Iqbal J, Goosens KA, Gera S, Rosen CJ, Haroutunian V, Ryu V, Yuen T, Zaidi M, Ye K (2022) FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 603, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vitiello MV, Borson S (2001) Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS Drugs 15, 777–796. [DOI] [PubMed] [Google Scholar]
- [36].Holtzman DM, Morris JC, Goate AM (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3, 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang B, Wing YK (2006) Sex differences in insomnia: a meta-analysis. Sleep 29, 85–93. [DOI] [PubMed] [Google Scholar]
- [38].Reyner LA, Horne JA, Reyner A (1995) Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep 18, 127–134. [PubMed] [Google Scholar]
- [39].Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA (2013) Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].The Lancet Healthy L (2020) The Lancet Healthy Longevity: Health For All, For Longer. Lancet Healthy Longev 1, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K, Group SOFR (2011) Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Prevention CfDCa (2015), ed. Promotion NCfCDPaH.
- [43].Health NIo (2022), ed. Services HaH.
- [44].Pollak CP, Perlick D (1991) Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol 4, 204–210. [DOI] [PubMed] [Google Scholar]
- [45].Aminoff MJ, Boller F, Swaab DF (2011) We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol 98, vii. [DOI] [PubMed] [Google Scholar]
- [46].Gilliland MA, Bergmann BM, Rechtschaffen A (1989) Sleep deprivation in the rat: VIII. High EEG amplitude sleep deprivation. Sleep 12, 53–59. [DOI] [PubMed] [Google Scholar]
- [47].Diekelmann S, Born J (2010) The memory function of sleep. Nat Rev Neurosci 11, 114–126. [DOI] [PubMed] [Google Scholar]
- [48].Maquet P (2001) The role of sleep in learning and memory. Science 294, 1048–1052. [DOI] [PubMed] [Google Scholar]
- [49].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The Glymphatic System: A Beginner’s Guide. Neurochem Res 40, 2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aukland K, Reed RK (1993) Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73, 1–78. [DOI] [PubMed] [Google Scholar]
- [52].Freeman D, Sheaves B, Goodwin GM, Yu LM, Nickless A, Harrison PJ, Emsley R, Luik AI, Foster RG, Wadekar V, Hinds C, Gumley A, Jones R, Lightman S, Jones S, Bentall R, Kinderman P, Rowse G, Brugha T, Blagrove M, Gregory AM, Fleming L, Walklet E, Glazebrook C, Davies EB, Hollis C, Haddock G, John B, Coulson M, Fowler D, Pugh K, Cape J, Moseley P, Brown G, Hughes C, Obonsawin M, Coker S, Watkins E, Schwannauer M, MacMahon K, Siriwardena AN, Espie CA (2017) The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry 4, 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Besedovsky L, Lange T, Born J (2012) Sleep and immune function. Pflugers Arch 463, 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schmid SM, Hallschmid M, Schultes B (2015) The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 3, 52–62. [DOI] [PubMed] [Google Scholar]
- [55].Grandner MA, Jackson N, Gerstner JR, Knutson KL (2014) Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res 23, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E, Lopez JV, Tartar JL (2019) Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14, e0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhou Y, Aris IM, Tan SS, Cai S, Tint MT, Krishnaswamy G, Meaney MJ, Godfrey KM, Kwek K, Gluckman PD, Chong YS, Yap F, Lek N, Gooley JJ, Lee YS (2015) Sleep duration and growth outcomes across the first two years of life in the GUSTO study. Sleep Med 16, 1281–1286. [DOI] [PubMed] [Google Scholar]
- [58].Zada D, Bronshtein I, Lerer-Goldshtein T, Garini Y, Appelbaum L (2019) Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun 10, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Calhoun DA, Harding SM (2010) Sleep and hypertension. Chest 138, 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mazzotti DR, Guindalini C, Moraes WA, Andersen ML, Cendoroglo MS, Ramos LR, Tufik S (2014) Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front Aging Neurosci 6, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Drummond SP, Brown GG (2001) The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 25, S68–73. [DOI] [PubMed] [Google Scholar]
- [62].Paulsen VM, Shaver JL (1991) Stress, support, psychological states and sleep. Soc Sci Med 32, 1237–1243. [DOI] [PubMed] [Google Scholar]
- [63].Spiegel K, Sheridan JF, Van Cauter E (2002) Effect of sleep deprivation on response to immunization. JAMA 288, 1471–1472. [DOI] [PubMed] [Google Scholar]
- [64].Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB (2009) Sleep habits and susceptibility to the common cold. Arch Intern Med 169, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].von Ruesten A, Weikert C, Fietze I, Boeing H (2012) Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One 7, e30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yaggi HK, Araujo AB, McKinlay JB (2006) Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 29, 657–661. [DOI] [PubMed] [Google Scholar]
- [67].Chen X, Beydoun MA, Wang Y (2008) Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 16, 265–274. [DOI] [PubMed] [Google Scholar]
- [68].Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D (2006) Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47, 833–839. [DOI] [PubMed] [Google Scholar]
- [69].Redline S, Foody J (2011) Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation 124, 2049–2051. [DOI] [PubMed] [Google Scholar]
- [70].Javaheri S, Dempsey JA (2013) Central sleep apnea. Compr Physiol 3, 141–163. [DOI] [PubMed] [Google Scholar]
- [71].Matthew Walker P (2017) Why We Sleep : Unlocking the Power of Sleep and Dreams, Scribner, New York. [Google Scholar]
- [72].Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS (2018) Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol 75, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Prevention CfDCa, Data and Statistics: Short Sleep Duration Among US Adults,National Center for Chronic Disease Prevention and Health Promotion, https://www.cdc.gov/sleep/data_statistics.html#:~:text=Adults%20need%207%20or%20more,the%20best%20health%20and%20wellbeing.&text=Short%20sleep%20duration%20is%20defined,sleep%20per%2024%2Dhour%20period., May 2, 2017,
- [74].Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG (1995) Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18, 425–432. [DOI] [PubMed] [Google Scholar]
- [75].Moline ML, Pollak CP, Monk TH, Lester LS, Wagner DR, Zendell SM, Graeber RC, Salter CA, Hirsch E (1992) Age-related differences in recovery from simulated jet lag. Sleep 15, 28–40. [DOI] [PubMed] [Google Scholar]
- [76].Robillard R, Bouchard M, Cartier A, Nicolau L, Carrier J (2015) Sleep is more sensitive to high doses of caffeine in the middle years of life. J Psychopharmacol 29, 688–697. [DOI] [PubMed] [Google Scholar]
- [77].Vgontzas AN, Bixler EO, Wittman AM, Zachman K, Lin HM, Vela-Bueno A, Kales A, Chrousos GP (2001) Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J Clin Endocrinol Metab 86, 1489–1495. [DOI] [PubMed] [Google Scholar]
- [78].Webb WB (1982) The measurement and characteristics of sleep in older persons. Neurobiol Aging 3, 311–319. [DOI] [PubMed] [Google Scholar]
- [79].Zepelin H, McDonald CS, Zammit GK (1984) Effects of age on auditory awakening thresholds. J Gerontol 39, 294–300. [DOI] [PubMed] [Google Scholar]
- [80].Ancoli-Israel S, Ayalon L, Salzman C (2008) Sleep in the elderly: normal variations and common sleep disorders. Harv Rev Psychiatry 16, 279–286. [DOI] [PubMed] [Google Scholar]
- [81].Carrier J, Monk TH, Buysse DJ, Kupfer DJ (1997) Sleep and morningness-eveningness in the ‘middle’ years of life (20–59 y). J Sleep Res 6, 230–237. [DOI] [PubMed] [Google Scholar]
- [82].Landolt HP, Borbely AA (2001) Age-dependent changes in sleep EEG topography. Clin Neurophysiol 112, 369–377. [DOI] [PubMed] [Google Scholar]
- [83].Feinsilver SH, Hernandez AB (2017) Sleep in the Elderly: Unanswered Questions. Clin Geriatr Med 33, 579–596. [DOI] [PubMed] [Google Scholar]
- [84].Patel AK, Reddy V, Shumway KR, Araujo JF (2023) Physiology, Sleep Stages In StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- [85].Klerman EB, Wang W, Duffy JF, Dijk DJ, Czeisler CA, Kronauer RE (2013) Survival analysis indicates that age-related decline in sleep continuity occurs exclusively during NREM sleep. Neurobiol Aging 34, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC (1982) Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging 3, 299–309. [DOI] [PubMed] [Google Scholar]
- [87].Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV (2004) Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273. [DOI] [PubMed] [Google Scholar]
- [88].Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH (2001) The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38, 232–242. [PubMed] [Google Scholar]
- [89].Luca G, Haba Rubio J, Andries D, Tobback N, Vollenweider P, Waeber G, Marques Vidal P, Preisig M, Heinzer R, Tafti M (2015) Age and gender variations of sleep in subjects without sleep disorders. Ann Med 47, 482–491. [DOI] [PubMed] [Google Scholar]
- [90].Hume KI, Van F, Watson A (1998) A field study of age and gender differences in habitual adult sleep. J Sleep Res 7, 85–94. [DOI] [PubMed] [Google Scholar]
- [91].Dib R, Gervais NJ, Mongrain V (2021) A review of the current state of knowledge on sex differences in sleep and circadian phenotypes in rodents. Neurobiol Sleep Circadian Rhythms 11, 100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mendelson WB, Bergmann BM (1999) EEG delta power during sleep in young and old rats. Neurobiol Aging 20, 669–673. [DOI] [PubMed] [Google Scholar]
- [93].Rosenberg RS, Zepelin H, Rechtschaffen A (1979) Sleep in young and old rats. J Gerontol 34, 525–532. [DOI] [PubMed] [Google Scholar]
- [94].Zepelin H, Whitehead WE, Rechtschaffen A (1972) Aging and sleep in the albino rat. Behav Biol 7, 65–74. [DOI] [PubMed] [Google Scholar]
- [95].Hasan S, Dauvilliers Y, Mongrain V, Franken P, Tafti M (2012) Age-related changes in sleep in inbred mice are genotype dependent. Neurobiol Aging 33, 195 e113–126. [DOI] [PubMed] [Google Scholar]
- [96].Jyoti A, Plano A, Riedel G, Platt B (2015) Progressive age-related changes in sleep and EEG profiles in the PLB1Triple mouse model of Alzheimer’s disease. Neurobiol Aging 36, 2768–2784. [DOI] [PubMed] [Google Scholar]
- [97].Welsh DK, Richardson GS, Dement WC (1986) Effect of age on the circadian pattern of sleep and wakefulness in the mouse. J Gerontol 41, 579–586. [DOI] [PubMed] [Google Scholar]
- [98].Wimmer ME, Rising J, Galante RJ, Wyner A, Pack AI, Abel T (2013) Aging in mice reduces the ability to sustain sleep/wake states. PLoS One 8, e81880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Li H, Satinoff E (1995) Changes in circadian rhythms of body temperature and sleep in old rats. Am J Physiol 269, R208–214. [DOI] [PubMed] [Google Scholar]
- [100].Van Gool WA, Mirmiran M (1983) Age-related changes in the sleep pattern of male adult rats. Brain Res 279, 394–398. [DOI] [PubMed] [Google Scholar]
- [101].Colas D, Cespuglio R, Sarda N (2005) Sleep wake profile and EEG spectral power in young or old senescence accelerated mice. Neurobiol Aging 26, 265–273. [DOI] [PubMed] [Google Scholar]
- [102].Soltani S, Chauvette S, Bukhtiyarova O, Lina JM, Dube J, Seigneur J, Carrier J, Timofeev I (2019) Sleep-Wake Cycle in Young and Older Mice. Front Syst Neurosci 13, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].McKillop LE, Fisher SP, Cui N, Peirson SN, Foster RG, Wafford KA, Vyazovskiy VV (2018) Effects of Aging on Cortical Neural Dynamics and Local Sleep Homeostasis in Mice. J Neurosci 38, 3911–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P (1989) Gender differences in the circadian temperature rhythms of healthy elderly subjects: relationships to sleep quality. Sleep 12, 529–536. [PubMed] [Google Scholar]
- [105].Kische H, Ewert R, Fietze I, Gross S, Wallaschofski H, Volzke H, Dorr M, Nauck M, Obst A, Stubbe B, Penzel T, Haring R (2016) Sex Hormones and Sleep in Men and Women From the General Population: A Cross-Sectional Observational Study. J Clin Endocrinol Metab 101, 3968–3977. [DOI] [PubMed] [Google Scholar]
- [106].Madrid-Valero JJ, Martinez-Selva JM, Ribeiro do Couto B, Sanchez-Romera JF, Ordonana JR (2017) Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac Sanit 31, 18–22. [DOI] [PubMed] [Google Scholar]
- [107].van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H (2009) Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep 32, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Carrier J, Semba K, Deurveilher S, Drogos L, Cyr-Cronier J, Lord C, Sekerovick Z (2017) Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol 47, 66–85. [DOI] [PubMed] [Google Scholar]
- [109].Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G (1997) Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep 20, 381–387. [DOI] [PubMed] [Google Scholar]
- [110].Husby R, Lingjaerde O (1990) Prevalence of reported sleeplessness in northern Norway in relation to sex, age and season. Acta Psychiatr Scand 81, 542–547. [DOI] [PubMed] [Google Scholar]
- [111].Li RH, Wing YK, Ho SC, Fong SY (2002) Gender differences in insomnia--a study in the Hong Kong Chinese population. J Psychosom Res 53, 601–609. [DOI] [PubMed] [Google Scholar]
- [112].Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R (2011) Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci 31, 16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mniszek DH (1988) Brighton sleep survey: a study of sleep in 20–45-year olds. J Int Med Res 16, 61–65. [DOI] [PubMed] [Google Scholar]
- [114].Janson C, Gislason T, De Backer W, Plaschke P, Bjornsson E, Hetta J, Kristbjarnason H, Vermeire P, Boman G (1995) Prevalence of sleep disturbances among young adults in three European countries. Sleep 18, 589–597. [PubMed] [Google Scholar]
- [115].Tsai LL, Li SP (2004) Sleep patterns in college students: gender and grade differences. J Psychosom Res 56, 231–237. [DOI] [PubMed] [Google Scholar]
- [116].Rediehs MH, Reis JS, Creason NS (1990) Sleep in old age: focus on gender differences. Sleep 13, 410–424. [PubMed] [Google Scholar]
- [117].Fox EC, Wang K, Aquino M, Grandner MA, Xie D, Branas CC, Gooneratne NS (2018) Sleep debt at the community level: impact of age, sex, race/ethnicity and health. Sleep Health 4, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Neutel CI, Patten SB (2009) Sleep medication use in Canadian seniors. Can J Clin Pharmacol 16, e443–452. [PubMed] [Google Scholar]
- [119].Sandlund C, Westman J, Hetta J (2016) Factors associated with self-reported need for treatment of sleeping difficulties: a survey of the general Swedish population. Sleep Med 22, 65–74. [DOI] [PubMed] [Google Scholar]
- [120].Rundo JV, Downey R 3rd (2019) Polysomnography. Handb Clin Neurol 160, 381–392. [DOI] [PubMed] [Google Scholar]
- [121].Goel N, Kim H, Lao RP (2005) Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int 22, 905–915. [DOI] [PubMed] [Google Scholar]
- [122].Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, Vela-Bueno A, Chrousos GP (2009) Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res 18, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dijk DJ, Beersma DG, Bloem GM (1989) Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep 12, 500–507. [DOI] [PubMed] [Google Scholar]
- [124].Ursin R, Bjorvatn B, Holsten F (2005) Sleep duration, subjective sleep need, and sleep habits of 40- to 45-year-olds in the Hordaland Health Study. Sleep 28, 1260–1269. [DOI] [PubMed] [Google Scholar]
- [125].Kobayashi R, Kohsaka M, Fukuda N, Honma H, Sakakibara S, Koyama T (1998) Gender differences in the sleep of middle-aged individuals. Psychiatry Clin Neurosci 52, 186–187. [DOI] [PubMed] [Google Scholar]
- [126].Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A (2004) The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 164, 406–418. [DOI] [PubMed] [Google Scholar]
- [127].Fukuda N, Honma H, Kohsaka M, Kobayashi R, Sakakibara S, Kohsaka S, Koyama T (1999) Gender difference of slow wave sleep in middle aged and elderly subjects. Psychiatry Clin Neurosci 53, 151–153. [DOI] [PubMed] [Google Scholar]
- [128].Gaillard JM, Blois R (1981) Spindle density in sleep of normal subjects. Sleep 4, 385–391. [DOI] [PubMed] [Google Scholar]
- [129].Markovic A, Kaess M, Tarokh L (2020) Gender differences in adolescent sleep neurophysiology: a high-density sleep EEG study. Sci Rep 10, 15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ma J, Dijk DJ, Svetnik V, Tymofyeyev Y, Ray S, Walsh JK, Deacon S (2011) EEG power spectra response to a 4-h phase advance and gaboxadol treatment in 822 men and women. J Clin Sleep Med 7, 493–501A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mourtazaev MS, Kemp B, Zwinderman AH, Kamphuisen HA (1995) Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep 18, 557–564. [DOI] [PubMed] [Google Scholar]
- [132].Mallampalli MP, Carter CL (2014) Exploring sex and gender differences in sleep health: a Society for Women’s Health Research Report. J Womens Health (Larchmt) 23, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Baker A, Simpson S, Dawson D (1997) Sleep disruption and mood changes associated with menopause. J Psychosom Res 43, 359–369. [DOI] [PubMed] [Google Scholar]
- [134].Baker FC, Driver HS, Paiker J, Rogers GG, Mitchell D (2002) Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J Appl Physiol (1985) 92, 1684–1691. [DOI] [PubMed] [Google Scholar]
- [135].Brown AMC, Gervais NJ (2020) Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan. Endocrinology 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Brunner DP, Munch M, Biedermann K, Huch R, Huch A, Borbely AA (1994) Changes in sleep and sleep electroencephalogram during pregnancy. Sleep 17, 576–582. [DOI] [PubMed] [Google Scholar]
- [137].Johnson EO, Roth T, Schultz L, Breslau N (2006) Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics 117, e247–256. [DOI] [PubMed] [Google Scholar]
- [138].Erkkola R, Holma P, Jarvi T, Nummi S, Punnonen R, Raudaskoski T, Rehn K, Ryynanen M, Sipila P, Tunkelo E, et al. (1991) Transdermal oestrogen replacement therapy in a Finnish population. Maturitas 13, 275–281. [DOI] [PubMed] [Google Scholar]
- [139].Gervais NJ, Mong JA, Lacreuse A (2017) Ovarian hormones, sleep and cognition across the adult female lifespan: An integrated perspective. Front Neuroendocrinol 47, 134–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS (2013) Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154, 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gemmill A, Berger BO, Crane MA, Margerison CE (2022) Mortality Rates Among U.S. Women of Reproductive Age, 1999–2019. Am J Prev Med 62, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Messinis IE, Messini CI, Dafopoulos K (2014) Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online 28, 714–722. [DOI] [PubMed] [Google Scholar]
- [143].Baker FC, Driver HS (2004) Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res 56, 239–243. [DOI] [PubMed] [Google Scholar]
- [144].Manber R, Bootzin RR (1997) Sleep and the menstrual cycle. Health Psychol 16, 209–214. [DOI] [PubMed] [Google Scholar]
- [145].Chaturvedi SK, Chandra PS, Issac MK, Sudarshan CY, Beena MB, Sarmukkadam SB, Rao S, Kaliaperumal VG (1993) Premenstrual experiences: the four profiles and factorial patterns. J Psychosom Obstet Gynaecol 14, 223–235. [DOI] [PubMed] [Google Scholar]
- [146].Patkai P, Johannson G, Post B (1974) Mood, alertness and sympathetic-adrenal medullary activity during the menstrual cycle. Psychosom Med 36, 503–512. [DOI] [PubMed] [Google Scholar]
- [147].Sharkey KM, Crawford SL, Kim S, Joffe H (2014) Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med 15, 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lamarche LJ, Driver HS, Wiebe S, Crawford L, JM DEK (2007) Nocturnal sleep, daytime sleepiness, and napping among women with significant emotional/behavioral premenstrual symptoms. J Sleep Res 16, 262–268. [DOI] [PubMed] [Google Scholar]
- [149].Shibui K, Uchiyama M, Okawa M, Kudo Y, Kim K, Kamei Y, Hayakawa T, Akamatsu T, Ohta K, Ishibashi K (1999) Diurnal fluctuation of sleep propensity across the menstrual cycle. Psychiatry Clin Neurosci 53, 207–209. [DOI] [PubMed] [Google Scholar]
- [150].Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D (2001) Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol 530, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA (1996) Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab 81, 728–735. [DOI] [PubMed] [Google Scholar]
- [152].Sheldrake P, Cormack M (1976) Variations in menstrual cycle symptom reporting. J Psychosom Res 20, 169–177. [DOI] [PubMed] [Google Scholar]
- [153].Kravitz HM, Janssen I, Santoro N, Bromberger JT, Schocken M, Everson-Rose SA, Karavolos K, Powell LH (2005) Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med 165, 2370–2376. [DOI] [PubMed] [Google Scholar]
- [154].Zheng H, Harlow SD, Kravitz HM, Bromberger J, Buysse DJ, Matthews KA, Gold EB, Owens JF, Hall M (2015) Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: Study of Women’s Health Across the Nation Sleep Study. Menopause 22, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM (1992) Sleep in normal late pregnancy. Sleep 15, 246–251. [DOI] [PubMed] [Google Scholar]
- [156].Ajayi AF, Akhigbe RE (2020) Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Levine JE (2015) Chapter 26 Neuroendocrine Control of the Ovarian Cycle of the Rat In Knobil and Neill’s Physiology of Reproduction, pp. 1199–1257. [Google Scholar]
- [158].Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA (2008) Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci 27, 1780–1792. [DOI] [PubMed] [Google Scholar]
- [159].Schwierin B, Borbely AA, Tobler I (1998) Sleep homeostasis in the female rat during the estrous cycle. Brain Res 811, 96–104. [DOI] [PubMed] [Google Scholar]
- [160].Fessler DM (2003) No time to eat: an adaptationist account of periovulatory behavioral changes. Q Rev Biol 78, 3–21. [DOI] [PubMed] [Google Scholar]
- [161].Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH (1968) Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res 7, 173–181. [DOI] [PubMed] [Google Scholar]
- [162].Colvin GB, Whitmoyer DI, Sawyer CH (1969) Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol 25, 616–625. [DOI] [PubMed] [Google Scholar]
- [163].Fang J, Fishbein W (1996) Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res 734, 275–285. [PubMed] [Google Scholar]
- [164].Kleinlogel H (1983) The female rat’s sleep during oestrous cycle. Neuropsychobiology 10, 228–237. [DOI] [PubMed] [Google Scholar]
- [165].Schwartz MD, Mong JA (2013) Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol 305, R271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Yamaoka S (1978) Participation of limbic-hypothalamic structures in circadian rhythm of slow wave sleep and paradoxical sleep in the rat. Brain Res 151, 255–268. [DOI] [PubMed] [Google Scholar]
- [167].Hardie L, Trayhurn P, Abramovich D, Fowler P (1997) Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf) 47, 101–106. [DOI] [PubMed] [Google Scholar]
- [168].Mindell JA, Jacobson BJ (2000) Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs 29, 590–597. [DOI] [PubMed] [Google Scholar]
- [169].Schweiger MS (1972) Sleep disturbance in pregnancy. A subjective survey. Am J Obstet Gynecol 114, 879–882. [DOI] [PubMed] [Google Scholar]
- [170].Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV (2002) Effects of pregnancy on mothers’ sleep. Sleep Med 3, 37–42. [DOI] [PubMed] [Google Scholar]
- [171].Bourjeily G, Ankner G, Mohsenin V (2011) Sleep-disordered breathing in pregnancy. Clin Chest Med 32, 175–189. [DOI] [PubMed] [Google Scholar]
- [172].Manconi M, Ulfberg J, Berger K, Ghorayeb I, Wesstrom J, Fulda S, Allen RP, Pollmacher T (2012) When gender matters: restless legs syndrome. Report of the “RLS and woman” workshop endorsed by the European RLS Study Group. Sleep Med Rev 16, 297–307. [DOI] [PubMed] [Google Scholar]
- [173].Berger K, Luedemann J, Trenkwalder C, John U, Kessler C (2004) Sex and the risk of restless legs syndrome in the general population. Arch Intern Med 164, 196–202. [DOI] [PubMed] [Google Scholar]
- [174].Driver HS, Shapiro CM (1992) A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep 15, 449–453. [DOI] [PubMed] [Google Scholar]
- [175].Chauhan G, Tadi P (2023) Physiology, Postpartum Changes In StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- [176].Lee KA, Zaffke ME, McEnany G (2000) Parity and sleep patterns during and after pregnancy. Obstet Gynecol 95, 14–18. [DOI] [PubMed] [Google Scholar]
- [177].Komiya H, Miyoshi C, Iwasaki K, Hotta-Hirashima N, Ikkyu A, Kanno S, Honda T, Gosho M, Hamada H, Satoh T, Fukamizu A, Funato H, Yanagisawa M (2018) Sleep/Wake Behaviors in Mice During Pregnancy and Pregnancy-Associated Hypertensive Mice. Sleep 41. [DOI] [PubMed] [Google Scholar]
- [178].Peacock K, Ketvertis KM (2023) Menopause In StatPearls, Treasure Island (FL). [Google Scholar]
- [179].Nelson HD (2008) Menopause. Lancet 371, 760–770. [DOI] [PubMed] [Google Scholar]
- [180].Ballinger CB (1976) Subjective sleep disturbance at the menopause. J Psychosom Res 20, 509–513. [DOI] [PubMed] [Google Scholar]
- [181].Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR (2008) Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 31, 979–990. [PMC free article] [PubMed] [Google Scholar]
- [182].Young T, Rabago D, Zgierska A, Austin D, Laurel F (2003) Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 26, 667–672. [DOI] [PubMed] [Google Scholar]
- [183].Woods NF, Mitchell ES (2010) Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep 33, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Dennerstein L, Lehert P, Burger HG, Guthrie JR (2007) New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update 13, 551–557. [DOI] [PubMed] [Google Scholar]
- [185].Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M (2001) Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol 98, 391–397. [DOI] [PubMed] [Google Scholar]
- [186].Sowers MF, Zheng H, Kravitz HM, Matthews K, Bromberger JT, Gold EB, Owens J, Consens F, Hall M (2008) Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep 31, 1339–1349. [PMC free article] [PubMed] [Google Scholar]
- [187].Xu Q, Lang CP (2014) Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause 21, 1301–1318. [DOI] [PubMed] [Google Scholar]
- [188].Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, Thurston RC, Study of Women’s Health Across the N (2015) Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 175, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Paul KN, Dugovic C, Turek FW, Laposky AD (2006) Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 29, 1211–1223. [DOI] [PubMed] [Google Scholar]
- [190].Paul KN, Laposky AD, Turek FW (2009) Reproductive hormone replacement alters sleep in mice. Neurosci Lett 463, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ehlen JC, Hesse S, Pinckney L, Paul KN (2013) Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One 8, e62205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Koehl M, Battle S, Meerlo P (2006) Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29, 1224–1231. [DOI] [PubMed] [Google Scholar]
- [193].Sare RM, Lemons A, Song A, Smith CB (2020) Sleep Duration in Mouse Models of Neurodevelopmental Disorders. Brain Sci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Swift KM, Keus K, Echeverria CG, Cabrera Y, Jimenez J, Holloway J, Clawson BC, Poe GR (2020) Sex differences within sleep in gonadally intact rats. Sleep 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Cusmano DM, Hadjimarkou MM, Mong JA (2014) Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology 155, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW (2003) Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology 144, 230–239. [DOI] [PubMed] [Google Scholar]
- [197].Yamaoka S (1980) Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res 185, 385–398. [DOI] [PubMed] [Google Scholar]
- [198].Branchey M, Branchey L, Nadler RD (1971) Effects of estrogen and progesterone on sleep patterns of female rats. Physiol Behav 6, 743–746. [DOI] [PubMed] [Google Scholar]
- [199].Deurveilher S, Rusak B, Semba K (2011) Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep 34, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Huitron-Resendiz S, Nadav T, Krause S, Cates-Gatto C, Polis I, Roberts AJ (2018) Effects of Withdrawal from Chronic Intermittent Ethanol Exposure on Sleep Characteristics of Female and Male Mice. Alcohol Clin Exp Res 42, 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].India N, Scott V, September H, Ehlen JC, Allison B, Ketema P (2020) Sleep Deprivation alters the influence of biological sex on active-phase sleep behavior. bioRxiv, 2020.2002.2021.958231. [Google Scholar]
- [202].Kostin A, Alam MA, Siegel JM, McGinty D, Alam MN (2020) Sex- and Age-dependent Differences in Sleep-wake Characteristics of Fisher-344 Rats. Neuroscience 427, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Bombois S, Derambure P, Pasquier F, Monaca C (2010) Sleep disorders in aging and dementia. J Nutr Health Aging 14, 212–217. [DOI] [PubMed] [Google Scholar]
- [204].Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, Trabucchi M (1995) Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia 6, 108–112. [DOI] [PubMed] [Google Scholar]
- [205].Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino FI, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili FM, Passero S, Perri R, Rocchi R, Sucapane P, Tognoni G, Zabberoni S, Sorbi S (2012) Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Rongve A, Boeve BF, Aarsland D (2010) Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc 58, 480–486. [DOI] [PubMed] [Google Scholar]
- [207].Wennberg AMV, Wu MN, Rosenberg PB, Spira AP (2017) Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin Neurol 37, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Ju YE, Lucey BP, Holtzman DM (2014) Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol 10, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA (2005) Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med 6, 347–352. [DOI] [PubMed] [Google Scholar]
- [210].Tractenberg RE, Singer CM, Kaye JA (2005) Symptoms of sleep disturbance in persons with Alzheimer’s disease and normal elderly. J Sleep Res 14, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [212].Anderson KN, Bradley AJ (2013) Sleep disturbance in mental health problems and neurodegenerative disease. Nat Sci Sleep 5, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288, 1475–1483. [DOI] [PubMed] [Google Scholar]
- [214].Muangpaisan W, Intalapaporn S, Assantachai P (2008) Neuropsychiatric symptoms in the community-based patients with mild cognitive impairment and the influence of demographic factors. Int J Geriatr Psychiatry 23, 699–703. [DOI] [PubMed] [Google Scholar]
- [215].Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, Widrow L, Guilleminault C, Zarcone VP, Dement WC (1989) REM latency in Alzheimer’s disease. Biol Psychiatry 25, 320–328. [DOI] [PubMed] [Google Scholar]
- [216].Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK (1990) Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol 45, M131–138. [DOI] [PubMed] [Google Scholar]
- [217].Musiek ES, Xiong DD, Holtzman DM (2015) Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 47, e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- [219].Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM (2013) Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM (2013) Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, Laws SM, Taddei K, Macaulay SL, Ames D, Fowler C, Maruff P, Masters CL, Rowe CC, Martins RN, Group AR (2016) The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep 39, 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM (2015) Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36, 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Li P, Gao L, Gaba A, Yu L, Cui L, Fan W, Lim ASP, Bennett DA, Buchman AS, Hu K (2020) Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longev 1, e96–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64, 343–349. [DOI] [PubMed] [Google Scholar]
- [225].Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosen E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttila T, Wallin A, Jonhagen ME, Minthon L, Winblad B, Blennow K (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393. [DOI] [PubMed] [Google Scholar]
- [226].Holth J, Patel T, Holtzman DM (2017) Sleep in Alzheimer’s Disease - Beyond Amyloid. Neurobiol Sleep Circadian Rhythms 2, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP (2015) beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP (2013) Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 16, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, Holtzman DM (2019) Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Kastanenka KV, Calvo-Rodriguez M, Hou SS, Zhou H, Takeda S, Arbel-Ornath M, Lariviere A, Lee YF, Kim A, Hawkes JM, Logan R, Feng D, Chen X, Gomperts SN, Bacskai BJ (2019) Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci Rep 9, 8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Sanchez-Espinosa MP, Atienza M, Cantero JL (2014) Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-beta and cortical thinning. Neuroimage 98, 395–404. [DOI] [PubMed] [Google Scholar]
- [232].Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, Knight RT, Jagust WJ, Walker MP (2019) Sleep as a Potential Biomarker of Tau and beta-Amyloid Burden in the Human Brain. J Neurosci 39, 6315–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ (2012) Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol 69, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, Patterson BW, Baty J, Morris JC, Ovod V, Mawuenyega KG, Bateman RJ (2018) Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Ann Neurol 83, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA (2014) Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 71, 971–977. [DOI] [PubMed] [Google Scholar]
- [237].Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922. [DOI] [PubMed] [Google Scholar]
- [238].Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, Holtzman DM (2011) In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci 31, 13110–13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD (2014) Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Ishida K, Yamada K, Nishiyama R, Hashimoto T, Nishida I, Abe Y, Yasui M, Iwatsubo T (2022) Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM (2012) Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 4, 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Colby-Milley J, Cavanagh C, Jego S, Breitner JC, Quirion R, Adamantidis A (2015) Sleep-Wake Cycle Dysfunction in the TgCRND8 Mouse Model of Alzheimer’s Disease: From Early to Advanced Pathological Stages. PLoS One 10, e0130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Huitron-Resendiz S, Sanchez-Alavez M, Gallegos R, Berg G, Crawford E, Giacchino JL, Games D, Henriksen SJ, Criado JR (2002) Age-independent and age-related deficits in visuospatial learning, sleep-wake states, thermoregulation and motor activity in PDAPP mice. Brain Res 928, 126–137. [DOI] [PubMed] [Google Scholar]
- [246].Schneider F, Baldauf K, Wetzel W, Reymann KG (2014) Behavioral and EEG changes in male 5xFAD mice. Physiol Behav 135, 25–33. [DOI] [PubMed] [Google Scholar]
- [247].Sethi M, Joshi SS, Webb RL, Beckett TL, Donohue KD, Murphy MP, O’Hara BF, Duncan MJ (2015) Increased fragmentation of sleep-wake cycles in the 5XFAD mouse model of Alzheimer’s disease. Neuroscience 290, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Zhang B, Veasey SC, Wood MA, Leng LZ, Kaminski C, Leight S, Abel T, Lee VM, Trojanowski JQ (2005) Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer’s disease with injury to pedunculopontine cholinergic neurons. Am J Pathol 167, 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Holth JK, Mahan TE, Robinson GO, Rocha A, Holtzman DM (2017) Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann Clin Transl Neurol 4, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, Utan A, Merrick G, Ryan D, Melis V, Wan H, Mingarelli M, Porcu E, Scrocchi L, Welch A, Riedel G (2011) Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS One 6, e27068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP (2013) Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res 1529, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Di Meco A, Joshi YB, Pratico D (2014) Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging 35, 1813–1820. [DOI] [PubMed] [Google Scholar]
- [253].Minakawa EN, Miyazaki K, Maruo K, Yagihara H, Fujita H, Wada K, Nagai Y (2017) Chronic sleep fragmentation exacerbates amyloid beta deposition in Alzheimer’s disease model mice. Neurosci Lett 653, 362–369. [DOI] [PubMed] [Google Scholar]
- [254].Duncan MJ, Guerriero LE, Kohler K, Beechem LE, Gillis BD, Salisbury F, Wessel C, Wang J, Sunderam S, Bachstetter AD, O’Hara BF, Murphy MP (2022) Chronic Fragmentation of the Daily Sleep-Wake Rhythm Increases Amyloid-beta Levels and Neuroinflammation in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Neuroscience 481, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, Snider BJ, Li M, Yanagisawa M, de Lecea L, Holtzman DM (2014) Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med 211, 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62, 685–691. [DOI] [PubMed] [Google Scholar]
- [258].Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA (2018) Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol 136, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA (2019) Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol 76, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Jack CR Jr., Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, Lowe V, Senjem ML, Gunter JL, Machulda MM, Gregg BE, Pankratz VS, Rocca WA, Petersen RC (2015) Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurol 72, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative I (2014) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM, Boeve BF, Senjem ML, Vemuri P, Petersen RC, Jack CR Jr. (2012) Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 79, 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S, Hinkle KM, DiLello SK, Johnson MF, Aziz A, Ertekin-Taner N, Ross OA, Dickson DW, Murray ME (2018) Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol 136, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Edwards L, La Joie R, Iaccarino L, Strom A, Baker SL, Casaletto KB, Cobigo Y, Grant H, Kim M, Kramer JH, Mellinger TJ, Pham J, Possin KL, Rosen HJ, Soleimani-Meigooni DN, Wolf A, Miller BL, Rabinovici GD (2021) Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: greater tau-PET retention in females. Neurobiol Aging 105, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H (2004) The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci 1019, 24–28. [DOI] [PubMed] [Google Scholar]
- [267].Filon JR, Intorcia AJ, Sue LI, Vazquez Arreola E, Wilson J, Davis KJ, Sabbagh MN, Belden CM, Caselli RJ, Adler CH, Woodruff BK, Rapscak SZ, Ahern GL, Burke AD, Jacobson S, Shill HA, Driver-Dunckley E, Chen K, Reiman EM, Beach TG, Serrano GE (2016) Gender Differences in Alzheimer Disease: Brain Atrophy, Histopathology Burden, and Cognition. J Neuropathol Exp Neurol 75, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL, Alzheimer’s Disease Genetics C, the Alzheimer’s Disease Neuroimaging I (2018) Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol 75, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, Hepker M, Hwang JH, Miller ZA, Huang EJ, Karydas AM, Grant H, Boxer AL, Gorno-Tempini ML, Rosen HJ, Kramer JH, Miller BL, Seeley WW, Rabinovici GD, Grinberg LT (2021) Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain 144, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Pereira JB, Harrison TM, La Joie R, Baker SL, Jagust WJ (2020) Spatial patterns of tau deposition are associated with amyloid, ApoE, sex, and cognitive decline in older adults. Eur J Nucl Med Mol Imaging 47, 2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Wisch JK, Meeker KL, Gordon BA, Flores S, Dincer A, Grant EA, Benzinger TL, Morris JC, Ances BM (2021) Sex-related Differences in Tau Positron Emission Tomography (PET) and the Effects of Hormone Therapy (HT). Alzheimer Dis Assoc Disord 35, 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Buckley RF, Scott MR, Jacobs HIL, Schultz AP, Properzi MJ, Amariglio RE, Hohman TJ, Mayblyum DV, Rubinstein ZB, Manning L, Hanseeuw BJ, Mormino EC, Rentz DM, Johnson KA, Sperling RA (2020) Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann Neurol 88, 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Luchsinger JA, Palta P, Rippon B, Soto L, Ceballos F, Pardo M, Laing K, Igwe K, Johnson A, Tomljanovic Z, He H, Reitz C, Kreisl W, Razlighi Q, Teresi J, Moreno H, Brickman AM (2020) Sex Differences in in vivo Alzheimer’s Disease Neuropathology in Late Middle-Aged Hispanics. J Alzheimers Dis 74, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Palta P, Rippon B, Tahmi M, Pardo M, Johnson A, Tomljanovic Z, He H, Laing KK, Razlighi QR, Teresi JA, Moreno H, Brickman AM, Kreisl WC, Luchsinger JA (2021) Sex differences in in vivo tau neuropathology in a multiethnic sample of late middle-aged adults. Neurobiol Aging 103, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ, Alzheimer’s Disease Neuroimaging I (2020) Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun 2, fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Ossenkoppele R, Lyoo CH, Jester-Broms J, Sudre CH, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S, Kramer J, Boxer AL, Gorno-Tempini ML, Miller BL, La Joie R, Rabinovici GD, Hansson O (2020) Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol 77, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD, Alzheimer’s Disease Neuroimaging I (2012) Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci 32, 8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E (2015) Perimenopause as a neurological transition state. Nat Rev Endocrinol 11, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H, Women’s Brain P, the Alzheimer Precision Medicine I (2018) Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol 14, 457–469. [DOI] [PubMed] [Google Scholar]
- [280].Buckley RF, O’Donnell A, McGrath ER, Jacobs HIL, Lois C, Satizabal CL, Ghosh S, Rubinstein ZB, Murabito JM, Sperling RA, Johnson KA, Seshadri S, Beiser AS (2022) Menopause Status Moderates Sex Differences in Tau Burden: A Framingham PET Study. Ann Neurol 92, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL (2014) Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 82, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ 3rd (2007) Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083. [DOI] [PubMed] [Google Scholar]
- [283].Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O’Brien R, Zonderman AB (2012) Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging 33, 720–731 e724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R (2005) Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A 102, 19198–19203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Ding F, Yao J, Rettberg JR, Chen S, Brinton RD (2013) Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One 8, e79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Wang C, Holtzman DM (2019) Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Scammell TE, Arrigoni E, Lipton JO (2017) Neural Circuitry of Wakefulness and Sleep. Neuron 93, 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Van Erum J, Van Dam D, De Deyn PP (2019) Alzheimer’s disease: Neurotransmitters of the sleep-wake cycle. Neurosci Biobehav Rev 105, 72–80. [DOI] [PubMed] [Google Scholar]
- [289].Saper CB, Chou TC, Scammell TE (2001) The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24, 726–731. [DOI] [PubMed] [Google Scholar]
- [290].Peplow M (2013) Structure: the anatomy of sleep. Nature 497, S2–3. [DOI] [PubMed] [Google Scholar]
- [291].Frahm C, Haupt C, Weinandy F, Siegel G, Bruehl C, Witte OW (2004) Regulation of GABA transporter mRNA and protein after photothrombotic infarct in rat brain. J Comp Neurol 478, 176–188. [DOI] [PubMed] [Google Scholar]
- [292].Standaert DG, Lee VM, Greenberg BD, Lowery DE, Trojanowski JQ (1991) Molecular features of hypothalamic plaques in Alzheimer’s disease. Am J Pathol 139, 681–691. [PMC free article] [PubMed] [Google Scholar]
- [293].Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB (2014) Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Hofman MA, Swaab DF (1989) The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat 164, 55–72. [PMC free article] [PubMed] [Google Scholar]
- [295].Deurveilher S, Cumyn EM, Peers T, Rusak B, Semba K (2008) Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 295, R1328–1340. [DOI] [PubMed] [Google Scholar]
- [296].Swaab DF, Hofman MA (1988) Sexual differentiation of the human hypothalamus: ontogeny of the sexually dimorphic nucleus of the preoptic area. Brain Res Dev Brain Res 44, 314–318. [DOI] [PubMed] [Google Scholar]
- [297].Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35, 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH (2004) Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 127, 1061–1074. [DOI] [PubMed] [Google Scholar]
- [299].Hofman MA, Swaab DF (2006) Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 5, 33–51. [DOI] [PubMed] [Google Scholar]
- [300].Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF (1990) Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry 27, 563–572. [DOI] [PubMed] [Google Scholar]
- [301].Duncan MJ (2020) Interacting influences of aging and Alzheimer’s disease on circadian rhythms. Eur J Neurosci 51, 310–325. [DOI] [PubMed] [Google Scholar]
- [302].Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, Satlin A (1999) Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol 58, 29–39. [DOI] [PubMed] [Google Scholar]
- [303].Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB (2015) Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol 78, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Swaab DF, Fliers E, Partiman TS (1985) The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res 342, 37–44. [DOI] [PubMed] [Google Scholar]
- [305].Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES (2018) Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med 215, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Brown-Grant K, Raisman G (1977) Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 198, 279–296. [DOI] [PubMed] [Google Scholar]
- [307].Wiegand SJ, Terasawa E, Bridson WE, Goy RW (1980) Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31, 147–157. [DOI] [PubMed] [Google Scholar]
- [308].Zhou JN, Hofman MA, Swaab DF (1995) VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 16, 571–576. [DOI] [PubMed] [Google Scholar]
- [309].Borbely AA, Daan S, Wirz-Justice A, Deboer T (2016) The two-process model of sleep regulation: a reappraisal. J Sleep Res 25, 131–143. [DOI] [PubMed] [Google Scholar]
- [310].Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW (2000) Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res 115, 183–204. [DOI] [PubMed] [Google Scholar]
- [311].Porkka-Heiskanen T, Strecker RE, McCarley RW (2000) Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. [DOI] [PubMed] [Google Scholar]
- [312].Bowen DM, Smith CB, White P, Davison AN (1976) Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99, 459–496. [DOI] [PubMed] [Google Scholar]
- [313].Davies P, Maloney AJ (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2, 1403. [DOI] [PubMed] [Google Scholar]
- [314].Mesulam M, Shaw P, Mash D, Weintraub S (2004) Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 55, 815–828. [DOI] [PubMed] [Google Scholar]
- [315].Stratmann K, Heinsen H, Korf HW, Del Turco D, Ghebremedhin E, Seidel K, Bouzrou M, Grinberg LT, Bohl J, Wharton SB, den Dunnen W, Rub U (2016) Precortical Phase of Alzheimer’s Disease (AD)-Related Tau Cytoskeletal Pathology. Brain Pathol 26, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Iraizoz I, Guijarro JL, Gonzalo LM, de Lacalle S (1999) Neuropathological changes in the nucleus basalis correlate with clinical measures of dementia. Acta Neuropathol 98, 186–196. [DOI] [PubMed] [Google Scholar]
- [317].Rogers JD, Brogan D, Mirra SS (1985) The nucleus basalis of Meynert in neurological disease: a quantitative morphological study. Ann Neurol 17, 163–170. [DOI] [PubMed] [Google Scholar]
- [318].Veng LM, Granholm AC, Rose GM (2003) Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav 80, 27–36. [DOI] [PubMed] [Google Scholar]
- [319].Henderson VW, Watt L, Buckwalter JG (1996) Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology 21, 421–430. [DOI] [PubMed] [Google Scholar]
- [320].Abraham IM, Koszegi Z, Tolod-Kemp E, Szego EM (2009) Action of estrogen on survival of basal forebrain cholinergic neurons: promoting amelioration. Psychoneuroendocrinology 34 Suppl 1, S104–112. [DOI] [PubMed] [Google Scholar]
- [321].Steriade M (2006) Grouping of brain rhythms in corticothalamic systems. Neuroscience 137, 1087–1106. [DOI] [PubMed] [Google Scholar]
- [322].Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G (2009) Source modeling sleep slow waves. Proc Natl Acad Sci U S A 106, 1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G (2004) The sleep slow oscillation as a traveling wave. J Neurosci 24, 6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D (2011) Sleep slow wave changes during the middle years of life. Eur J Neurosci 33, 758–766. [DOI] [PubMed] [Google Scholar]
- [325].Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA (2012) Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc 18, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C (1982) Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging 3, 361–370. [DOI] [PubMed] [Google Scholar]
- [327].Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Varga AW, Wohlleber ME, Gimenez S, Romero S, Alonso JF, Ducca EL, Kam K, Lewis C, Tanzi EB, Tweardy S, Kishi A, Parekh A, Fischer E, Gumb T, Alcolea D, Fortea J, Lleo A, Blennow K, Zetterberg H, Mosconi L, Glodzik L, Pirraglia E, Burschtin OE, de Leon MJ, Rapoport DM, Lu SE, Ayappa I, Osorio RS (2016) Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Abeta42 Levels in Cognitively Normal Elderly. Sleep 39, 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A (2020) Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci U S A 117, 18788–18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, Hakonarson H, Gur RE, Gur RC (2017) Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood. J Neurosci 37, 5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW (2017) Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol 74, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Kadotani H, Kadotani T, Young T, Peppard PE, Finn L, Colrain IM, Murphy GM Jr., Mignot E (2001) Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA 285, 2888–2890. [DOI] [PubMed] [Google Scholar]
- [333].Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, Young T (2004) APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology 63, 664–668. [DOI] [PubMed] [Google Scholar]
- [334].Cosentino FI, Bosco P, Drago V, Prestianni G, Lanuzza B, Iero I, Tripodi M, Spada RS, Toscano G, Caraci F, Ferri R (2008) The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med 9, 831–839. [DOI] [PubMed] [Google Scholar]
- [335].Drogos LL, Gill SJ, Tyndall AV, Raneri JK, Parboosingh JS, Naef A, Guild KD, Eskes G, Hanly PJ, Poulin MJ (2016) Evidence of association between sleep quality and APOE epsilon4 in healthy older adults: A pilot study. Neurology 87, 1836–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G (2010) Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 30, 43–50. [DOI] [PubMed] [Google Scholar]
- [337].Rocca WA, Grossardt BR, Shuster LT (2011) Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 1379, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Inayama Y, Mizuno K, Yamaguchi K, Hamanishi J, Takeuchi M, Egawa M, Mandai M, Kawakami K (2023) Hormone replacement therapy and cancer risks in perimenopausal women: A retrospective cohort study using a Japanese claims database. J Obstet Gynaecol Res 49, 1805–1814. [DOI] [PubMed] [Google Scholar]
- [339].Health NIo (2018), ed. Institute NC. [Google Scholar]
- [340].Cho MK (2018) Use of Combined Oral Contraceptives in Perimenopausal Women. Chonnam Med J 54, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Henderson VW (2006) Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience 138, 1031–1039. [DOI] [PubMed] [Google Scholar]
- [342].Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC, Cache County Study G (2001) Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology 57, 2210–2216. [DOI] [PubMed] [Google Scholar]
- [343].Hogervorst E, Boshuisen M, Riedel W, Willeken C, Jolles J (1999) 1998 Curt P. Richter Award. The effect of hormone replacement therapy on cognitive function in elderly women. Psychoneuroendocrinology 24, 43–68. [DOI] [PubMed] [Google Scholar]
- [344].Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E (1997) A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48, 1517–1521. [DOI] [PubMed] [Google Scholar]
- [345].LeBlanc ES, Janowsky J, Chan BK, Nelson HD (2001) Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA 285, 1489–1499. [DOI] [PubMed] [Google Scholar]
- [346].Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R (1996) Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348, 429–432. [DOI] [PubMed] [Google Scholar]
- [347].Craig MC, Maki PM, Murphy DG (2005) The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 4, 190–194. [DOI] [PubMed] [Google Scholar]
- [348].Cui J, Shen Y, Li R (2013) Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med 19, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Shughrue PJ, Lane MV, Merchenthaler I (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388, 507–525. [DOI] [PubMed] [Google Scholar]
- [350].Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I (1998) Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids 63, 498–504. [DOI] [PubMed] [Google Scholar]
- [351].Shughrue PJ, Merchenthaler I (2000) Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol 21, 95–101. [DOI] [PubMed] [Google Scholar]
- [352].Merklinger-Gruchala A, Ellison PT, Lipson SF, Thune I, Jasienska G (2008) Low estradiol levels in women of reproductive age having low sleep variation. Eur J Cancer Prev 17, 467–472. [DOI] [PubMed] [Google Scholar]
- [353].Michels KA, Mendola P, Schliep KC, Yeung EH, Ye A, Dunietz GL, Wactawski-Wende J, Kim K, Freeman JR, Schisterman EF, Mumford SL (2020) The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol Int 37, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Polo-Kantola P, Erkkola R, Irjala K, Pullinen S, Virtanen I, Polo O (1999) Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril 71, 873–880. [DOI] [PubMed] [Google Scholar]
- [355].Ensrud KE, Guthrie KA, Hohensee C, Caan B, Carpenter JS, Freeman EW, LaCroix AZ, Landis CA, Manson J, Newton KM, Otte J, Reed SD, Shifren JL, Sternfeld B, Woods NF, Joffe H (2015) Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 38, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Thomson J, Oswald I (1977) Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br Med J 2, 1317–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Moe KE, Larsen LH, Vitiello MV, Prinz PN (2001) Estrogen replacement therapy moderates the sleep disruption associated with nocturnal blood sampling. Sleep 24, 886–894. [DOI] [PubMed] [Google Scholar]
- [358].Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R (2000) Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology 54, 833–837. [DOI] [PubMed] [Google Scholar]
- [359].Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, Murphy MP, Beckett TL, Finch CE, Brinton RD, Pike CJ (2012) 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology 153, 5467–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Petanceska SS, Nagy V, Frail D, Gandy S (2000) Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp Gerontol 35, 1317–1325. [DOI] [PubMed] [Google Scholar]
- [361].Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27, 13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Levin-Allerhand JA, Lominska CE, Wang J, Smith JD (2002) 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis 4, 449–457. [DOI] [PubMed] [Google Scholar]
- [363].Frick KM (2009) Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav 55, 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Goodman Y, Bruce AJ, Cheng B, Mattson MP (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem 66, 1836–1844. [DOI] [PubMed] [Google Scholar]
- [365].Green PS, Gridley KE, Simpkins JW (1996) Estradiol protects against beta-amyloid (25–35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett 218, 165–168. [DOI] [PubMed] [Google Scholar]
- [366].Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem 72, 1552–1563. [DOI] [PubMed] [Google Scholar]
- [367].Azcoitia I, Sierra A, Garcia-Segura LM (1998) Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. Neuroreport 9, 3075–3079. [DOI] [PubMed] [Google Scholar]
- [368].Nilsen J, Chen S, Brinton RD (2002) Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res 930, 216–234. [DOI] [PubMed] [Google Scholar]
- [369].Vedder H, Anthes N, Stumm G, Wurz C, Behl C, Krieg JC (1999) Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem 72, 2531–2538. [DOI] [PubMed] [Google Scholar]
- [370].Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM (1993) Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res 628, 271–278. [DOI] [PubMed] [Google Scholar]
- [371].Del Cerro S, Garcia-Estrada J, Garcia-Segura LM (1995) Neuroactive steroids regulate astroglia morphology in hippocampal cultures from adult rats. Glia 14, 65–71. [DOI] [PubMed] [Google Scholar]
- [372].Liu XA, Zhu LQ, Zhang Q, Shi HR, Wang SH, Wang Q, Wang JZ (2008) Estradiol attenuates tau hyperphosphorylation induced by upregulation of protein kinase-A. Neurochem Res 33, 1811–1820. [DOI] [PubMed] [Google Scholar]
- [373].Zhang QG, Wang R, Khan M, Mahesh V, Brann DW (2008) Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci 28, 8430–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Chang D, Kwan J, Timiras PS (1997) Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv Exp Med Biol 429, 261–271. [DOI] [PubMed] [Google Scholar]
- [375].Harris-White ME, Chu T, Miller SA, Simmons M, Teter B, Nash D, Cole GM, Frautschy SA (2001) Estrogen (E2) and glucocorticoid (Gc) effects on microglia and A beta clearance in vitro and in vivo. Neurochem Int 39, 435–448. [DOI] [PubMed] [Google Scholar]
- [376].Gandy S, Petanceska S (2001) Regulation of alzheimer beta-amyloid precursor trafficking and metabolism. Adv Exp Med Biol 487, 85–100. [DOI] [PubMed] [Google Scholar]
- [377].Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD (2011) 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging 32, 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Qin Y, An D, Xu W, Qi X, Wang X, Chen L, Chen L, Sha S (2020) Estradiol Replacement at the Critical Period Protects Hippocampal Neural Stem Cells to Improve Cognition in APP/PS1 Mice. Front Aging Neurosci 12, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Toran-Allerand CD, Singh M, Setalo G Jr. (1999) Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol 20, 97–121. [DOI] [PubMed] [Google Scholar]
- [380].Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. [DOI] [PubMed] [Google Scholar]
- [381].Lamprecht R, LeDoux J (2004) Structural plasticity and memory. Nat Rev Neurosci 5, 45–54. [DOI] [PubMed] [Google Scholar]
- [382].Cooper DB, Patel P, Mahdy H (2023) Oral Contraceptive Pills In StatPearls, Treasure Island (FL). [Google Scholar]
- [383].Edwards M, Can AS (2023) Progestin In StatPearls, Treasure Island (FL). [Google Scholar]
- [384].Lancel M, Faulhaber J, Holsboer F, Rupprecht R (1996) Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol 271, E763–772. [DOI] [PubMed] [Google Scholar]
- [385].Lancel M, Faulhaber J, Holsboer F, Rupprecht R (1999) The GABA(A) receptor antagonist picrotoxin attenuates most sleep changes induced by progesterone. Psychopharmacology (Berl) 141, 213–219. [DOI] [PubMed] [Google Scholar]
- [386].Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R (1997) Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther 282, 1213–1218. [PubMed] [Google Scholar]
- [387].Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ (1984) Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 58, 378–383. [DOI] [PubMed] [Google Scholar]
- [388].Schussler P, Kluge M, Yassouridis A, Dresler M, Held K, Zihl J, Steiger A (2008) Progesterone reduces wakefulness in sleep EEG and has no effect on cognition in healthy postmenopausal women. Psychoneuroendocrinology 33, 1124–1131. [DOI] [PubMed] [Google Scholar]
- [389].Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R (1997) Progesterone-induced changes in sleep in male subjects. Am J Physiol 272, E885–891. [DOI] [PubMed] [Google Scholar]
- [390].Heuser G, Ling GM, Kluver M (1967) Sleep induction by progesterone in the pre-optic area in cats. Electroencephalogr Clin Neurophysiol 22, 122–127. [DOI] [PubMed] [Google Scholar]
- [391].Caufriez A, Leproult R, L’Hermite-Baleriaux M, Kerkhofs M, Copinschi G (2011) Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women. J Clin Endocrinol Metab 96, E614–623. [DOI] [PubMed] [Google Scholar]
- [392].Driver HS, Baker FC (1998) Menstrual factors in sleep. Sleep Med Rev 2, 213–229. [DOI] [PubMed] [Google Scholar]
- [393].Jehan S, Masters-Isarilov A, Salifu I, Zizi F, Jean-Louis G, Pandi-Perumal SR, Gupta R, Brzezinski A, McFarlane SI (2015) Sleep Disorders in Postmenopausal Women. J Sleep Disord Ther 4. [PMC free article] [PubMed] [Google Scholar]
- [394].Schussler P, Kluge M, Adamczyk M, Beitinger ME, Beitinger P, Bleifuss A, Cordeiro S, Mattern C, Uhr M, Wetter TC, Yassouridis A, Rupprecht R, Friess E, Steiger A (2018) Sleep after intranasal progesterone vs. zolpidem and placebo in postmenopausal women - A randomized, double-blind cross over study. Psychoneuroendocrinology 92, 81–86. [DOI] [PubMed] [Google Scholar]
- [395].Gron G, Friess E, Herpers M, Rupprecht R (1997) Assessment of cognitive performance after progesterone administration in healthy male volunteers. Neuropsychobiology 35, 147–151. [DOI] [PubMed] [Google Scholar]
- [396].Hogervorst E, Yaffe K, Richards M, Huppert FA (2009) Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst Rev 2009, CD003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K (2007) Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology 69, 1322–1330. [DOI] [PubMed] [Google Scholar]
- [398].Cintron D, Lipford M, Larrea-Mantilla L, Spencer-Bonilla G, Lloyd R, Gionfriddo MR, Gunjal S, Farrell AM, Miller VM, Murad MH (2017) Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 55, 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Pettus EH, Wright DW, Stein DG, Hoffman SW (2005) Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res 1049, 112–119. [DOI] [PubMed] [Google Scholar]
- [400].Roof RL, Hall ED (2000) Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 17, 367–388. [DOI] [PubMed] [Google Scholar]
- [401].Foy MR, Akopian G, Thompson RF (2008) Progesterone regulation of synaptic transmission and plasticity in rodent hippocampus. Learn Mem 15, 820–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Gonzalez Deniselle MC, Garay L, Gonzalez S, Saravia F, Labombarda F, Guennoun R, Schumacher M, De Nicola AF (2007) Progesterone modulates brain-derived neurotrophic factor and choline acetyltransferase in degenerating Wobbler motoneurons. Exp Neurol 203, 406–414. [DOI] [PubMed] [Google Scholar]
- [403].Schumacher M, Guennoun R, Stein DG, De Nicola AF (2007) Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther 116, 77–106. [DOI] [PubMed] [Google Scholar]
- [404].Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M (1999) Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine (Phila Pa 1976) 24, 2134–2138. [DOI] [PubMed] [Google Scholar]
- [405].Qin Y, Chen Z, Han X, Wu H, Yu Y, Wu J, Liu S, Hou Y (2015) Progesterone attenuates Abeta(25–35)-induced neuronal toxicity via JNK inactivation and progesterone receptor membrane component 1-dependent inhibition of mitochondrial apoptotic pathway. J Steroid Biochem Mol Biol 154, 302–311. [DOI] [PubMed] [Google Scholar]
- [406].Wu H, Wu ZG, Shi WJ, Gao H, Wu HH, Bian F, Jia PP, Hou YN (2019) Effects of progesterone on glucose uptake in neurons of Alzheimer’s disease animals and cell models. Life Sci 238, 116979. [DOI] [PubMed] [Google Scholar]
- [407].Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M (2007) Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res 85, 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Nilsen J, Brinton RD (2003) Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A 100, 10506–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Zhao Y, Wang J, Liu C, Jiang C, Zhao C, Zhu Z (2011) Progesterone influences postischemic synaptogenesis in the CA1 region of the hippocampus in rats. Synapse 65, 880–891. [DOI] [PubMed] [Google Scholar]
- [410].Janes L (2016) Hypothalamic-Pituitary-Gonadal (HPG) Axis In Encyclopedia of Personality and Individual Differences. [Google Scholar]
- [411].Couzinet B, Schaison G (1993) The control of gonadotrophin secretion by ovarian steroids. Hum Reprod 8 Suppl 2, 97–101. [DOI] [PubMed] [Google Scholar]
- [412].McEwen BS, Alves SE, Bulloch K, Weiland NG (1998) Clinically relevant basic science studies of gender differences and sex hormone effects. Psychopharmacol Bull 34, 251–259. [PubMed] [Google Scholar]
- [413].Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JW (1976) Hormonal profiles after the menopause. Br Med J 2, 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Neaves WB, Johnson L, Porter JC, Parker CR Jr., Petty CS (1984) Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 59, 756–763. [DOI] [PubMed] [Google Scholar]
- [415].Bowen RL, Isley JP, Atkinson RL (2000) An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol 12, 351–354. [DOI] [PubMed] [Google Scholar]
- [416].Short RA, Bowen RL, O’Brien PC, Graff-Radford NR (2001) Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 76, 906–909. [DOI] [PubMed] [Google Scholar]
- [417].Butchart J, Birch B, Bassily R, Wolfe L, Holmes C (2013) Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer Dis Assoc Disord 27, 153–156. [DOI] [PubMed] [Google Scholar]
- [418].Chu C, Gao G, Huang W (2008) A study on co-localization of FSH and its receptor in rat hippocampus. J Mol Histol 39, 49–55. [DOI] [PubMed] [Google Scholar]
- [419].Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES (1993) Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 132, 2262–2270. [DOI] [PubMed] [Google Scholar]
- [420].Rao CV, Li X, Toth P, Lei ZM, Cook VD (1993) Novel expression of functional human chorionic gonadotropin/luteinizing hormone receptor gene in human umbilical cords. J Clin Endocrinol Metab 77, 1706–1714. [DOI] [PubMed] [Google Scholar]
- [421].Badr M, Pelletier G (1987) Characterization and autoradiographic localization of LHRH receptors in the rat brain. Synapse 1, 567–571. [DOI] [PubMed] [Google Scholar]
- [422].AA AL-H, Lei ZM, Rao CV (1997) Novel expression of functional luteinizing hormone/chorionic gonadotropin receptors in cultured glial cells from neonatal rat brains. Biol Reprod 56, 501–507. [DOI] [PubMed] [Google Scholar]
- [423].Badr M, Marchetti B, Pelletier G (1988) Modulation of hippocampal LHRH receptors by sex steroids in the rat. Peptides 9, 441–442. [DOI] [PubMed] [Google Scholar]
- [424].Reubi JC, Maurer R (1984) Visualization of LHRH receptors in the rat brain. Eur J Pharmacol 106, 453–454. [DOI] [PubMed] [Google Scholar]
- [425].Badr M, Marchetti B, Pelletier G (1989) Changes in hippocampal LH-RH receptor density during maturation and aging in the rat. Brain Res Dev Brain Res 45, 179–184. [DOI] [PubMed] [Google Scholar]
- [426].Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L (1972) Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287, 582–586. [DOI] [PubMed] [Google Scholar]
- [427].Apter D, Butzow TL, Laughlin GA, Yen SS (1993) Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 76, 940–949. [DOI] [PubMed] [Google Scholar]
- [428].Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K (1992) Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab 74, 890–897. [DOI] [PubMed] [Google Scholar]
- [429].Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE (2012) Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab 97, E2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Hall JE, Sullivan JP, Richardson GS (2005) Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 90, 2050–2055. [DOI] [PubMed] [Google Scholar]
- [431].Casper RF, Yen SS, Wilkes MM (1979) Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science 205, 823–825. [DOI] [PubMed] [Google Scholar]
- [432].Randolph JF Jr., Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, Brockwell SE, Matthews KA (2005) The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 90, 6106–6112. [DOI] [PubMed] [Google Scholar]
- [433].Hyde Z, Flicker L, Almeida OP, McCaul KA, Jamrozik K, Hankey GJ, Chubb SA, Yeap BB (2010) Higher luteinizing hormone is associated with poor memory recall: the health in men study. J Alzheimers Dis 19, 943–951. [DOI] [PubMed] [Google Scholar]
- [434].Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E, Prince R, Devine A, Mehta P, Beilby J, Atwood CS, Martins RN (2008) Gonadotropins and cognition in older women. J Alzheimers Dis 13, 267–274. [DOI] [PubMed] [Google Scholar]
- [435].Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, Martins RN, Group AR (2014) Associations between gonadotropins, testosterone and beta amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry 19, 69–75. [DOI] [PubMed] [Google Scholar]
- [436].Verdile G, Yeap BB, Clarnette RM, Dhaliwal S, Burkhardt MS, Chubb SA, De Ruyck K, Rodrigues M, Mehta PD, Foster JK, Bruce DG, Martins RN (2008) Luteinizing hormone levels are positively correlated with plasma amyloid-beta protein levels in elderly men. J Alzheimers Dis 14, 201–208. [DOI] [PubMed] [Google Scholar]
- [437].Bowen RL, Smith MA, Harris PL, Kubat Z, Martins RN, Castellani RJ, Perry G, Atwood CS (2002) Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. J Neurosci Res 70, 514–518. [DOI] [PubMed] [Google Scholar]
- [438].Hestnes A, Stovner LJ, Husoy O, Folling I, Fougner KJ, Sjaastad O (1991) Hormonal and biochemical disturbances in Down’s syndrome. J Ment Defic Res 35 ( Pt 3), 179–193. [DOI] [PubMed] [Google Scholar]
- [439].Lukacs H, Hiatt ES, Lei ZM, Rao CV (1995) Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav 29, 42–58. [DOI] [PubMed] [Google Scholar]
- [440].Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS (2004) Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem 279, 20539–20545. [DOI] [PubMed] [Google Scholar]
- [441].Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA (2007) Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol 269, 107–111. [DOI] [PubMed] [Google Scholar]
- [442].Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS (2007) Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. Biochem Biophys Res Commun 364, 522–527. [DOI] [PubMed] [Google Scholar]
- [443].Wahjoepramono EJ, Wijaya LK, Taddei K, Bates KA, Howard M, Martins G, deRuyck K, Matthews PM, Verdile G, Martins RN (2011) Direct exposure of guinea pig CNS to human luteinizing hormone increases cerebrospinal fluid and cerebral beta amyloid levels. Neuroendocrinology 94, 313–322. [DOI] [PubMed] [Google Scholar]
- [444].Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762, 447–452. [DOI] [PubMed] [Google Scholar]
- [445].Casadesus G, Puig ER, Webber KM, Atwood CS, Escuer MC, Bowen RL, Perry G, Smith MA (2006) Targeting gonadotropins: an alternative option for Alzheimer disease treatment. J Biomed Biotechnol 2006, 39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Polo-Kantola P, Erkkola R, Irjala K, Helenius H, Pullinen S, Polo O (1999) Climacteric symptoms and sleep quality. Obstet Gynecol 94, 219–224. [DOI] [PubMed] [Google Scholar]
- [447].Antonijevic IA, Murck H, Frieboes RM, Uhr M, Steiger A (2003) On the role of menopause for sleep-endocrine alterations associated with major depression. Psychoneuroendocrinology 28, 401–418. [DOI] [PubMed] [Google Scholar]
- [448].Lampio L, Polo-Kantola P, Himanen SL, Kurki S, Huupponen E, Engblom J, Heinonen OJ, Polo O, Saaresranta T (2017) Sleep During Menopausal Transition: A 6-Year Follow-Up. Sleep 40. [DOI] [PubMed] [Google Scholar]
- [449].Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R (2002) Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril 77, 738–744. [DOI] [PubMed] [Google Scholar]
- [450].de Zambotti M, Colrain IM, Baker FC (2015) Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab 100, 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M (2015) Insomnia in women approaching menopause: Beyond perception. Psychoneuroendocrinology 60, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Randolph JF Jr., Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M (2011) Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 96, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [453].Hestiantoro A, Wiwie M, Shadrina A, Ibrahim N, Purba JS (2017) FSH to estradiol ratio can be used as screening method for mild cognitive impairment in postmenopausal women. Climacteric 20, 577–582. [DOI] [PubMed] [Google Scholar]
- [454].Berent-Spillson A, Persad CC, Love T, Sowers M, Randolph JF, Zubieta JK, Smith YR (2012) Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinol Metab 97, E1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [455].Zhang S, Fan W, Hu H, Wen L, Gong M, Liu B, Hu J, Li G, Zhang D (2021) Subcortical Volume Changes in Early Menopausal Women and Correlation With Neuropsychological Tests. Front Aging Neurosci 13, 738679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [456].Corbo RM, Gambina G, Broggio E, Scacchi R (2011) Influence of variation in the follicle-stimulating hormone receptor gene (FSHR) and age at menopause on the development of Alzheimer’s disease in women. Dement Geriatr Cogn Disord 32, 63–69. [DOI] [PubMed] [Google Scholar]
- [457].Zeng LN, Zong QQ, Yang Y, Zhang L, Xiang YF, Ng CH, Chen LG, Xiang YT (2020) Gender Difference in the Prevalence of Insomnia: A Meta-Analysis of Observational Studies. Front Psychiatry 11, 577429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [458].Chen LJ, Steptoe A, Chen YH, Ku PW, Lin CH (2017) Physical activity, smoking, and the incidence of clinically diagnosed insomnia. Sleep Med 30, 189–194. [DOI] [PubMed] [Google Scholar]
- [459].Chiou JH, Chen HC, Chen KH, Chou P (2016) Correlates of self-report chronic insomnia disorders with 1–6 month and 6-month durations in home-dwelling urban older adults - the Shih-Pai Sleep Study in Taiwan: a cross-sectional community study. BMC Geriatr 16, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [460].Kim KW, Kang SH, Yoon IY, Lee SD, Ju G, Han JW, Kim TH, Lee CS, Kim T (2017) Prevalence and clinical characteristics of insomnia and its subtypes in the Korean elderly. Arch Gerontol Geriatr 68, 68–75. [DOI] [PubMed] [Google Scholar]
- [461].Salk RH, Hyde JS, Abramson LY (2017) Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [462].Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51, 8–19. [DOI] [PubMed] [Google Scholar]
- [463].Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L (2004) Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med 5, 237–246. [DOI] [PubMed] [Google Scholar]
- [464].Hogl B, Kiechl S, Willeit J, Saletu M, Frauscher B, Seppi K, Muller J, Rungger G, Gasperi A, Wenning G, Poewe W (2005) Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology 64, 1920–1924. [DOI] [PubMed] [Google Scholar]
- [465].Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [466].Meng Z, Liu M, Zhang Q, Liu L, Song K, Tan J, Jia Q, Zhang G, Wang R, He Y, Ren X, Zhu M, He Q, Wang S, Li X, Hu T, Liu N, Upadhyaya A, Zhou P, Zhang J (2015) Gender and Age Impacts on the Association Between Thyroid Function and Metabolic Syndrome in Chinese. Medicine (Baltimore) 94, e2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [467].Bonsignore MR, Saaresranta T, Riha RL (2019) Sex differences in obstructive sleep apnoea. Eur Respir Rev 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Hall MH, Kline CE, Nowakowski S (2015) Insomnia and sleep apnea in midlife women: prevalence and consequences to health and functioning. F1000Prime Rep 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [469].Sarahian N, Sarvazad H, Sajadi E, Rahnejat N, Eskandari Roozbahani N (2021) Investigation of common risk factors between polycystic ovary syndrome and Alzheimer’s disease: a narrative review. Reprod Health 18, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [470].Sateia MJ (2014) International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394. [DOI] [PubMed] [Google Scholar]
- [471].Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, Malhotra A, American Thoracic Society ad hoc Committee on Healthy S (2015) An Official American Thoracic Society Statement: The Importance of Healthy Sleep. Recommendations and Future Priorities. Am J Respir Crit Care Med 191, 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Abraham O, Pu J, Schleiden LJ, Albert SM (2017) Factors contributing to poor satisfaction with sleep and healthcare seeking behavior in older adults. Sleep Health 3, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [473].Emamian F, Khazaie H, Okun ML, Tahmasian M, Sepehry AA (2019) Link between insomnia and perinatal depressive symptoms: A meta-analysis. J Sleep Res 28, e12858. [DOI] [PubMed] [Google Scholar]
- [474].Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Frojmark M, Palagini L, Rucker G, Riemann D, Baglioni C (2019) Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med Rev 43, 96–105. [DOI] [PubMed] [Google Scholar]
- [475].Morin CM, Benca R (2012) Chronic insomnia. Lancet 379, 1129–1141. [DOI] [PubMed] [Google Scholar]
- [476].Walsh JK, Coulouvrat C, Hajak G, Lakoma MD, Petukhova M, Roth T, Sampson NA, Shahly V, Shillington A, Stephenson JJ, Kessler RC (2011) Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep 34, 997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [477].Appleton SL, Gill TK, Lang CJ, Taylor AW, McEvoy RD, Stocks NP, Gonzalez-Chica DA, Adams RJ (2018) Prevalence and comorbidity of sleep conditions in Australian adults: 2016 Sleep Health Foundation national survey. Sleep Health 4, 13–19. [DOI] [PubMed] [Google Scholar]
- [478].Nesbitt AD (2018) Delayed sleep-wake phase disorder. J Thorac Dis 10, S103–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [479].Zhu L, Zee PC (2012) Circadian rhythm sleep disorders. Neurol Clin 30, 1167–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [480].Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD (2016) Objective but Not Subjective Short Sleep Duration Associated with Increased Risk for Hypertension in Individuals with Insomnia. Sleep 39, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO (2013) Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 17, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [482].Ohayon MM (2002) Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6, 97–111. [DOI] [PubMed] [Google Scholar]
- [483].Foley D, Ancoli-Israel S, Britz P, Walsh J (2004) Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res 56, 497–502. [DOI] [PubMed] [Google Scholar]
- [484].Hoffmann G (1999) Evaluation of severe insomnia in the general population--implications for the management of insomnia: focus on results from Belgium. J Psychopharmacol 13, S31–32. [DOI] [PubMed] [Google Scholar]
- [485].Hetta J, Broman JE, Mallon L (1999) Evaluation of severe insomnia in the general population--implications for the management of insomnia: insomnia, quality of life and healthcare consumption in Sweden. J Psychopharmacol 13, S35–36. [DOI] [PubMed] [Google Scholar]
- [486].Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M (2000) Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res 9, 35–42. [DOI] [PubMed] [Google Scholar]
- [487].Ohayon M (1996) Epidemiological study on insomnia in the general population. Sleep 19, S7–15. [DOI] [PubMed] [Google Scholar]
- [488].Yeo BK, Perera IS, Kok LP, Tsoi WF (1996) Insomnia in the community. Singapore Med J 37, 282–284. [PubMed] [Google Scholar]
- [489].Owens JF, Matthews KA (1998) Sleep disturbance in healthy middle-aged women. Maturitas 30, 41–50. [DOI] [PubMed] [Google Scholar]
- [490].Punyahotra S, Dennerstein L, Lehert P (1997) Menopausal experiences of Thai women. Part 1: Symptoms and their correlates. Maturitas 26, 1–7. [DOI] [PubMed] [Google Scholar]
- [491].Mayer G, Jennum P, Riemann D, Dauvilliers Y (2011) Insomnia in central neurologic diseases--occurrence and management. Sleep Med Rev 15, 369–378. [DOI] [PubMed] [Google Scholar]
- [492].Sindi S, Kareholt I, Johansson L, Skoog J, Sjoberg L, Wang HX, Johansson B, Fratiglioni L, Soininen H, Solomon A, Skoog I, Kivipelto M (2018) Sleep disturbances and dementia risk: A multicenter study. Alzheimers Dement 14, 1235–1242. [DOI] [PubMed] [Google Scholar]
- [493].Ancoli-Israel S, Cooke JR (2005) Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc 53, S264–271. [DOI] [PubMed] [Google Scholar]
- [494].Isaia G, Corsinovi L, Bo M, Santos-Pereira P, Michelis G, Aimonino N, Zanocchi M (2011) Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr 52, 133–137. [DOI] [PubMed] [Google Scholar]
- [495].Alperin N, Wiltshire J, Lee SH, Ramos AR, Hernandez-Cardenache R, Rundek T, Curiel Cid R, Loewenstein D (2019) Effect of sleep quality on amnestic mild cognitive impairment vulnerable brain regions in cognitively normal elderly individuals. Sleep 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [496].Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM (2014) Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology 83, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [497].Chen L, Huang J, Yang L, Zeng XA, Zhang Y, Wang X, Chen M, Li X, Zhang Y, Zhang M (2017) Sleep deprivation accelerates the progression of alzheimer’s disease by influencing Abeta-related metabolism. Neurosci Lett 650, 146–152. [DOI] [PubMed] [Google Scholar]
- [498].Qiu H, Zhong R, Liu H, Zhang F, Li S, Le W (2016) Chronic Sleep Deprivation Exacerbates Learning-Memory Disability and Alzheimer’s Disease-Like Pathologies in AbetaPP(swe)/PS1(DeltaE9) Mice. J Alzheimers Dis 50, 669–685. [DOI] [PubMed] [Google Scholar]
- [499].Chen DW, Wang J, Zhang LL, Wang YJ, Gao CY (2018) Cerebrospinal Fluid Amyloid-beta Levels are Increased in Patients with Insomnia. J Alzheimers Dis 61, 645–651. [DOI] [PubMed] [Google Scholar]
- [500].Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ (2013) The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol 88, 261–264. [DOI] [PubMed] [Google Scholar]
- [501].Scofield H, Roth T, Drake C (2008) Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep 31, 1221–1227. [PMC free article] [PubMed] [Google Scholar]
- [502].Gosselin N, Lanfranchi P, Michaud M, Fantini L, Carrier J, Lavigne G, Montplaisir J (2003) Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol 114, 2188–2195. [DOI] [PubMed] [Google Scholar]
- [503].Broman JE, Mallon L, Hetta J (2008) Restless legs syndrome and its relationship with insomnia symptoms and daytime distress: epidemiological survey in Sweden. Psychiatry Clin Neurosci 62, 472–475. [DOI] [PubMed] [Google Scholar]
- [504].Ekbom K, Ulfberg J (2009) Restless legs syndrome. J Intern Med 266, 419–431. [DOI] [PubMed] [Google Scholar]
- [505].Klingelhoefer L, Bhattacharya K, Reichmann H (2016) Restless legs syndrome. Clin Med (Lond) 16, 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [506].Ulfberg J, Nystrom B, Carter N, Edling C (2001) Restless Legs Syndrome among working-aged women. Eur Neurol 46, 17–19. [DOI] [PubMed] [Google Scholar]
- [507].Ulfberg J, Nystrom B, Carter N, Edling C (2001) Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord 16, 1159–1163. [DOI] [PubMed] [Google Scholar]
- [508].Wesstrom J, Nilsson S, Sundstrom-Poromaa I, Ulfberg J (2008) Restless legs syndrome among women: prevalence, co-morbidity and possible relationship to menopause. Climacteric 11, 422–428. [DOI] [PubMed] [Google Scholar]
- [509].Milligan SA, Chesson AL (2002) Restless legs syndrome in the older adult: diagnosis and management. Drugs Aging 19, 741–751. [DOI] [PubMed] [Google Scholar]
- [510].Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K (2000) Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology 54, 1064–1068. [DOI] [PubMed] [Google Scholar]
- [511].Fernandez-Jimenez MC, Moreno G, Wright I, Shih PC, Vaquero MP, Remacha AF (2020) Iron Deficiency in Menstruating Adult Women: Much More than Anemia. Womens Health Rep (New Rochelle) 1, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [512].Ghorayeb I, Bioulac B, Scribans C, Tison F (2008) Perceived severity of restless legs syndrome across the female life cycle. Sleep Med 9, 799–802. [DOI] [PubMed] [Google Scholar]
- [513].Hwang S, Shin Y-W, Jung K-Y (2019) Symptom Aggravation in Restless Legs Syndrome during Menstrual Cycle. J Sleep Med 16, 109–112. [Google Scholar]
- [514].Ekbom K (1960) [A croparesthesia and restless legs in pregnancy]. Sven Lakartidn 57, 2597–2603. [PubMed] [Google Scholar]
- [515].Manconi M, Govoni V, De Vito A, Economou NT, Cesnik E, Casetta I, Mollica G, Ferini-Strambi L, Granieri E (2004) Restless legs syndrome and pregnancy. Neurology 63, 1065–1069. [DOI] [PubMed] [Google Scholar]
- [516].Kim KY, Kim EH, Lee M, Ha J, Jung I, Kim E (2023) Restless leg syndrome and risk of all-cause dementia: a nationwide retrospective cohort study. Alzheimers Res Ther 15, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [517].Liampas I, Siokas V, Kyrozis A, Sakoutis G, Yannakoulia M, Kosmidis MH, Sakka P, Sakkas GK, Giannaki CD, Stefanidis I, Scarmeas N, Dardiotis E, Hadjigeorgiou GM (2022) Prevalence and Determinants of Restless Legs Syndrome (Willis-Ekbom Disease) in an Older Greek Population. Behav Sleep Med, 1–13. [DOI] [PubMed] [Google Scholar]
- [518].Gao X, Schwarzschild MA, Wang H, Ascherio A (2009) Obesity and restless legs syndrome in men and women. Neurology 72, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [519].Szentkiralyi A, Volzke H, Hoffmann W, Trenkwalder C, Berger K (2014) Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology 82, 2026–2033. [DOI] [PubMed] [Google Scholar]
- [520].Xu W, Tan CC, Zou JJ, Cao XP, Tan L (2020) Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 91, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [521].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimaki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [522].Walters AS, Rye DB (2009) Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 32, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [523].Trenkwalder C, Allen R, Hogl B, Paulus W, Winkelmann J (2016) Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 86, 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [524].Banno K, Delaive K, Walld R, Kryger MH (2000) Restless legs syndrome in 218 patients: associated disorders. Sleep Med 1, 221–229. [DOI] [PubMed] [Google Scholar]
- [525].Unrath A, Juengling FD, Schork M, Kassubek J (2007) Cortical grey matter alterations in idiopathic restless legs syndrome: An optimized voxel-based morphometry study. Mov Disord 22, 1751–1756. [DOI] [PubMed] [Google Scholar]
- [526].Stefani A, Mitterling T, Heidbreder A, Steiger R, Kremser C, Frauscher B, Gizewski ER, Poewe W, Hogl B, Scherfler C (2019) Multimodal Magnetic Resonance Imaging reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep 42. [DOI] [PubMed] [Google Scholar]
- [527].Martorana A, Koch G (2014) “Is dopamine involved in Alzheimer’s disease?”. Front Aging Neurosci 6, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [528].Mitchell UH, Obray JD, Hunsaker E, Garcia BT, Clarke TJ, Hope S, Steffensen SC (2018) Peripheral Dopamine in Restless Legs Syndrome. Front Neurol 9, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [529].Hong CH, Falvey C, Harris TB, Simonsick EM, Satterfield S, Ferrucci L, Metti AL, Patel KV, Yaffe K (2013) Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 81, 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [530].MD PS, Obstructive Sleep Apnea,Mayo Clinic, https://www.mayoclinic.org/diseases-conditions/obstructive-sleep-apnea/symptoms-causes/syc-20352090#:~:text=Obstructive%20sleep%20apnea%20is%20the,common%20is%20obstructive%20sleep%20apnea.,
- [531].Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R (2018) Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath 22, 555–568. [DOI] [PubMed] [Google Scholar]
- [532].Jennum P, Riha RL (2009) Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J 33, 907–914. [DOI] [PubMed] [Google Scholar]
- [533].Ito E, Inoue Y (2015) [The International Classification of Sleep Disorders, third edition. American Academy of Sleep Medicine. Includes bibliographies and index]. Nihon Rinsho 73, 916–923. [PubMed] [Google Scholar]
- [534].Riha RL (2015) Diagnostic approaches to respiratory sleep disorders. J Thorac Dis 7, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [535].Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J (2016) Obstructive Sleep Apnea in Women: Specific Issues and Interventions. Biomed Res Int 2016, 1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [536].Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [537].Theorell-Haglow J, Miller CB, Bartlett DJ, Yee BJ, Openshaw HD, Grunstein RR (2018) Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults - What do we know? A clinical update. Sleep Med Rev 38, 28–38. [DOI] [PubMed] [Google Scholar]
- [538].Franklin KA, Sahlin C, Stenlund H, Lindberg E (2013) Sleep apnoea is a common occurrence in females. Eur Respir J 41, 610–615. [DOI] [PubMed] [Google Scholar]
- [539].Kulkas A, Duce B, Leppanen T, Hukins C, Toyras J (2017) Gender differences in severity of desaturation events following hypopnea and obstructive apnea events in adults during sleep. Physiol Meas 38, 1490–1502. [DOI] [PubMed] [Google Scholar]
- [540].Kapsimalis F, Kryger MH (2002) Gender and obstructive sleep apnea syndrome, part 1: Clinical features. Sleep 25, 412–419. [PubMed] [Google Scholar]
- [541].Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F (2011) Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J 37, 1137–1143. [DOI] [PubMed] [Google Scholar]
- [542].Anttalainen U, Grote L, Fietze I, Riha RL, Ryan S, Staats R, Hedner J, Saaresranta T, Collaborators ES (2019) Insomnia symptoms combined with nocturnal hypoxia associate with cardiovascular comorbidity in the European sleep apnea cohort (ESADA). Sleep Breath 23, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [543].Young T, Hutton R, Finn L, Badr S, Palta M (1996) The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med 156, 2445–2451. [PubMed] [Google Scholar]
- [544].Saaresranta T, Hedner J, Bonsignore MR, Riha RL, McNicholas WT, Penzel T, Anttalainen U, Kvamme JA, Pretl M, Sliwinski P, Verbraecken J, Grote L, Group ES (2016) Clinical Phenotypes and Comorbidity in European Sleep Apnoea Patients. PLoS One 11, e0163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [545].Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163, 608–613. [DOI] [PubMed] [Google Scholar]
- [546].Young T, Finn L, Austin D, Peterson A (2003) Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 167, 1181–1185. [DOI] [PubMed] [Google Scholar]
- [547].Anttalainen U, Saaresranta T, Aittokallio J, Kalleinen N, Vahlberg T, Virtanen I, Polo O (2006) Impact of menopause on the manifestation and severity of sleep-disordered breathing. Acta Obstet Gynecol Scand 85, 1381–1388. [DOI] [PubMed] [Google Scholar]
- [548].Huang T, Lin BM, Redline S, Curhan GC, Hu FB, Tworoger SS (2018) Type of Menopause, Age at Menopause, and Risk of Developing Obstructive Sleep Apnea in Postmenopausal Women. Am J Epidemiol 187, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [549].Daulatzai MA (2015) Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 93, 1778–1794. [DOI] [PubMed] [Google Scholar]
- [550].Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D (2011) Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 6, e19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [551].Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ, Alzheimer’s Disease Neuroimaging I (2015) Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84, 1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [552].Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, Anderson WM (2017) Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 40. [DOI] [PubMed] [Google Scholar]
- [553].Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL (2011) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [554].Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, Rosenzweig I, Sepehry AA (2016) The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front Aging Neurosci 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [555].Ancoli-Israel S, Klauber MR, Butters N, Parker L, Kripke DF (1991) Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc 39, 258–263. [DOI] [PubMed] [Google Scholar]
- [556].Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, Placidi F (2017) Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 40. [DOI] [PubMed] [Google Scholar]
- [557].Shiota S, Takekawa H, Matsumoto SE, Takeda K, Nurwidya F, Yoshioka Y, Takahashi F, Hattori N, Tabira T, Mochizuki H, Takahashi K (2013) Chronic intermittent hypoxia/reoxygenation facilitate amyloid-beta generation in mice. J Alzheimers Dis 37, 325–333. [DOI] [PubMed] [Google Scholar]
- [558].Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS (2008) Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc 56, 2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [559].Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S (2009) Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 5, 305–309. [PMC free article] [PubMed] [Google Scholar]
- [560].Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, Simon RD Jr., Guilleminault C, White DP, Goodwin JL, Schweitzer PK, Leary EB, Hyde PR, Hirshkowitz M, Green S, McEvoy LK, Chan C, Gevins A, Kay GG, Bloch DA, Crabtree T, Dement WC (2012) Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 35, 1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [561].Troussiere AC, Charley CM, Salleron J, Richard F, Delbeuck X, Derambure P, Pasquier F, Bombois S (2014) Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 85, 1405–1408. [DOI] [PubMed] [Google Scholar]
- [562].Cao S, Fisher DW, Yu T, Dong H (2019) The link between chronic pain and Alzheimer’s disease. J Neuroinflammation 16, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [563].Malfliet A, Coppieters I, Van Wilgen P, Kregel J, De Pauw R, Dolphens M, Ickmans K (2017) Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur J Pain 21, 769–786. [DOI] [PubMed] [Google Scholar]
- [564].Morin CM, Jarrin DC (2022) Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med Clin 17, 173–191. [DOI] [PubMed] [Google Scholar]
- [565].Smith MT, Haythornthwaite JA (2004) How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 8, 119–132. [DOI] [PubMed] [Google Scholar]
- [566].Doufas AG, Panagiotou OA, Ioannidis JP (2012) Concordance of sleep and pain outcomes of diverse interventions: an umbrella review. PLoS One 7, e40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [567].Nitter AK, Pripp AH, Forseth KO (2012) Are sleep problems and non-specific health complaints risk factors for chronic pain? A prospective population-based study with 17 year follow-up. Scand J Pain 3, 210–217. [DOI] [PubMed] [Google Scholar]
- [568].Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR (2003) Obesity and quality of life: mediating effects of pain and comorbidities. Obes Res 11, 209–216. [DOI] [PubMed] [Google Scholar]
- [569].Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1863, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [570].Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67, 505–512. [DOI] [PubMed] [Google Scholar]
- [571].Davies M, Brophy S, Williams R, Taylor A (2006) The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 29, 1518–1522. [DOI] [PubMed] [Google Scholar]
- [572].Knutson KL, Ryden AM, Mander BA, Van Cauter E (2006) Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 166, 1768–1774. [DOI] [PubMed] [Google Scholar]
- [573].Williams JW, Plassman BL, Burke J, Benjamin S (2010) Preventing Alzheimer’s disease and cognitive decline. Evid Rep Technol Assess (Full Rep), 1–727. [PMC free article] [PubMed] [Google Scholar]
- [574].Finan PH, Smith MT (2013) The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev 17, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [575].Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC (2002) Major depression and insomnia in chronic pain. Clin J Pain 18, 77–83. [DOI] [PubMed] [Google Scholar]
- [576].Saiz-Vazquez O, Gracia-Garcia P, Ubillos-Landa S, Puente-Martinez A, Casado-Yusta S, Olaya B, Santabarbara J (2021) Depression as a Risk Factor for Alzheimer’s Disease: A Systematic Review of Longitudinal Meta-Analyses. J Clin Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [577].Drevets WC, Rubin EH (1989) Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry 25, 39–48. [DOI] [PubMed] [Google Scholar]
- [578].Lyketsos CG, Tune LE, Pearlson G, Steele C (1996) Major depression in Alzheimer’s disease. An interaction between gender and family history. Psychosomatics 37, 380–384. [DOI] [PubMed] [Google Scholar]
- [579].de Oliveira FF, Bertolucci PH, Chen ES, Smith MC (2014) Assessment of risk factors for earlier onset of sporadic Alzheimer’s disease dementia. Neurol India 62, 625–630. [DOI] [PubMed] [Google Scholar]
- [580].Canton-Habas V, Rich-Ruiz M, Romero-Saldana M, Carrera-Gonzalez MDP (2020) Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [581].van Hecke O, Hocking LJ, Torrance N, Campbell A, Padmanabhan S, Porteous DJ, McIntosh AM, Burri AV, Tanaka H, Williams FM, Smith BH (2017) Chronic pain, depression and cardiovascular disease linked through a shared genetic predisposition: Analysis of a family-based cohort and twin study. PLoS One 12, e0170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [582].de Bruijn RF, Ikram MA (2014) Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med 12, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [583].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10, 447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [584].Gerdle B, Bjork J, Coster L, Henriksson K, Henriksson C, Bengtsson A (2008) Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord 9, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [585].Mogil JS (2012) Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13, 859–866. [DOI] [PubMed] [Google Scholar]
- [586].Gibson CJ, Li Y, Bertenthal D, Huang AJ, Seal KH (2019) Menopause symptoms and chronic pain in a national sample of midlife women veterans. Menopause 26, 708–713. [DOI] [PubMed] [Google Scholar]
- [587].Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ (2007) Comorbidity of chronic insomnia with medical problems. Sleep 30, 213–218. [DOI] [PubMed] [Google Scholar]
- [588].Odegard SS, Sand T, Engstrom M, Stovner LJ, Zwart JA, Hagen K (2011) The long-term effect of insomnia on primary headaches: a prospective population-based cohort study (HUNT-2 and HUNT-3). Headache 51, 570–580. [DOI] [PubMed] [Google Scholar]
- [589].Boardman HF, Thomas E, Millson DS, Croft PR (2006) The natural history of headache: predictors of onset and recovery. Cephalalgia 26, 1080–1088. [DOI] [PubMed] [Google Scholar]
- [590].Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R (2005) Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. Eur J Epidemiol 20, 243–249. [DOI] [PubMed] [Google Scholar]
- [591].Mork PJ, Nilsen TI (2012) Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheum 64, 281–284. [DOI] [PubMed] [Google Scholar]
- [592].Davies KA, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, McBeth J (2008) Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 47, 1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [593].Larsson C, Hansson EE, Sundquist K, Jakobsson U (2017) Chronic pain in older adults: prevalence, incidence, and risk factors. Scand J Rheumatol 46, 317–325. [DOI] [PubMed] [Google Scholar]
- [594].Baker KS, Gibson S, Georgiou-Karistianis N, Roth RM, Giummarra MJ (2016) Everyday Executive Functioning in Chronic Pain: Specific Deficits in Working Memory and Emotion Control, Predicted by Mood, Medications, and Pain Interference. Clin J Pain 32, 673–680. [DOI] [PubMed] [Google Scholar]
- [595].Dick B, Eccleston C, Crombez G (2002) Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum 47, 639–644. [DOI] [PubMed] [Google Scholar]
- [596].Brown SC, Glass JM, Park DC (2002) The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain 96, 279–284. [DOI] [PubMed] [Google Scholar]
- [597].Grilli M (2017) Chronic pain and adult hippocampal neurogenesis: translational implications from preclinical studies. J Pain Res 10, 2281–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [598].Moriarty O, McGuire BE, Finn DP (2011) The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 93, 385–404. [DOI] [PubMed] [Google Scholar]
- [599].Achterberg W, Lautenbacher S, Husebo B, Erdal A, Herr K (2020) Pain in dementia. Pain Rep 5, e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [600].Achterberg WP, Pieper MJ, van Dalen-Kok AH, de Waal MW, Husebo BS, Lautenbacher S, Kunz M, Scherder EJ, Corbett A (2013) Pain management in patients with dementia. Clin Interv Aging 8, 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [601].Monroe TB, Gibson SJ, Bruehl SP, Gore JC, Dietrich MS, Newhouse P, Atalla S, Cowan RL (2016) Contact heat sensitivity and reports of unpleasantness in communicative people with mild to moderate cognitive impairment in Alzheimer’s disease: a cross-sectional study. BMC Med 14, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [602].Busatto GF, Diniz BS, Zanetti MV (2008) Voxel-based morphometry in Alzheimer’s disease. Expert Rev Neurother 8, 1691–1702. [DOI] [PubMed] [Google Scholar]
- [603].Kang D, McAuley JH, Kassem MS, Gatt JM, Gustin SM (2019) What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain? Eur J Pain 23, 203–219. [DOI] [PubMed] [Google Scholar]
- [604].Zhang Y, Yu T, Qin B, Li Y, Song G, Yu B (2016) Microstructural Abnormalities in Gray Matter of Patients with Postherpetic Neuralgia: A Diffusional Kurtosis Imaging Study. Pain Physician 19, E601–611. [PubMed] [Google Scholar]
- [605].Phillips JS, Da Re F, Dratch L, Xie SX, Irwin DJ, McMillan CT, Vaishnavi SN, Ferrarese C, Lee EB, Shaw LM, Trojanowski JQ, Wolk DA, Grossman M (2018) Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer’s disease. Neurobiol Aging 63, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [606].Schmidt-Wilcke T, Poljansky S, Hierlmeier S, Hausner J, Ibach B (2009) Memory performance correlates with gray matter density in the ento-/perirhinal cortex and posterior hippocampus in patients with mild cognitive impairment and healthy controls--a voxel based morphometry study. Neuroimage 47, 1914–1920. [DOI] [PubMed] [Google Scholar]
- [607].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM (2014) Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuroimage Clin 4, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [608].May A (2008) Chronic pain may change the structure of the brain. Pain 137, 7–15. [DOI] [PubMed] [Google Scholar]
- [609].Yuan C, Shi H, Pan P, Dai Z, Zhong J, Ma H, Sheng L (2017) Gray Matter Abnormalities Associated With Chronic Back Pain: A Meta-Analysis of Voxel-based Morphometric Studies. Clin J Pain 33, 983–990. [DOI] [PubMed] [Google Scholar]
- [610].van Kooten J, Binnekade TT, van der Wouden JC, Stek ML, Scherder EJ, Husebo BS, Smalbrugge M, Hertogh CM (2016) A Review of Pain Prevalence in Alzheimer’s, Vascular, Frontotemporal and Lewy Body Dementias. Dement Geriatr Cogn Disord 41, 220–232. [DOI] [PubMed] [Google Scholar]
- [611].van Kooten J, Smalbrugge M, van der Wouden JC, Stek ML, Hertogh C (2017) Prevalence of Pain in Nursing Home Residents: The Role of Dementia Stage and Dementia Subtypes. J Am Med Dir Assoc 18, 522–527. [DOI] [PubMed] [Google Scholar]
- [612].Rajkumar AP, Ballard C, Fossey J, Orrell M, Moniz-Cook E, Woods RT, Murray J, Whitaker R, Stafford J, Knapp M, Romeo R, Woodward-Carlton B, Khan Z, Testad I, Corbett A (2017) Epidemiology of Pain in People With Dementia Living in Care Homes: Longitudinal Course, Prevalence, and Treatment Implications. J Am Med Dir Assoc 18, 453 e451–453 e456. [DOI] [PubMed] [Google Scholar]
- [613].Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK (2017) Association Between Persistent Pain and Memory Decline and Dementia in a Longitudinal Cohort of Elders. JAMA Intern Med 177, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [614].Scherder EJ, Eggermont L, Plooij B, Oudshoorn J, Vuijk PJ, Pickering G, Lautenbacher S, Achterberg W, Oosterman J (2008) Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer’s disease. The need to control for mood. Gerontology 54, 50–58. [DOI] [PubMed] [Google Scholar]
- [615].Hayashida KI, Obata H (2019) Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [616].Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E (2016) Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 338, 93–113. [DOI] [PubMed] [Google Scholar]
- [617].Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sanchez-Blazquez P, Meana JJ, Berrocoso E (2013) Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry 73, 54–62. [DOI] [PubMed] [Google Scholar]
- [618].Simic G, Babic Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milosevic N, Bazadona D, Buee L, de Silva R, Di Giovanni G, Wischik CM, Hof PR (2017) Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol 151, 101–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [619].Matthews KL, Chen CP, Esiri MM, Keene J, Minger SL, Francis PT (2002) Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry 51, 407–416. [DOI] [PubMed] [Google Scholar]
- [620].German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM (1992) Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 32, 667–676. [DOI] [PubMed] [Google Scholar]
- [621].Itoi K, Ohara S, Kobayashi K (2013) Selective ablation of dopamine beta-hydroxylase neurons in the brain by immunotoxin-mediated neuronal targeting: new insights into brain catecholaminergic circuitry and catecholamine-related diseases. Adv Pharmacol 68, 155–166. [DOI] [PubMed] [Google Scholar]
- [622].Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, Wei X, Xu T, Xin WJ, Pang RP, Li YY, Qin ZH, Murugan M, Mattson MP, Wu LJ, Liu XG (2017) TNF-alpha Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J Neurosci 37, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [623].Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- [624].Hains BC, Waxman SG (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26, 4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [625].Mosher KI, Wyss-Coray T (2014) Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol 88, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [626].Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19, 610–621. [DOI] [PubMed] [Google Scholar]
- [627].Clinic C, Thyroid Disease, https://my.clevelandclinic.org/health/diseases/8541-thyroid-disease#:~:text=It%20can%20be%20present%20at,some%20type%20of%20thyroid%20disorder.,
- [628].Green ME, Bernet V, Cheung J (2021) Thyroid Dysfunction and Sleep Disorders. Front Endocrinol (Lausanne) 12, 725829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [629].Vanderpump MP (2011) The epidemiology of thyroid disease. Br Med Bull 99, 39–51. [DOI] [PubMed] [Google Scholar]
- [630].Cooper DS (2003) Antithyroid drugs in the management of patients with Graves’ disease: an evidence-based approach to therapeutic controversies. J Clin Endocrinol Metab 88, 3474–3481. [DOI] [PubMed] [Google Scholar]
- [631].Dasgupta S, Savage MW (2005) Evaluation of management of Graves’ disease in District General Hospital: achievement of consensus guidelines. Int J Clin Pract 59, 1097–1100. [DOI] [PubMed] [Google Scholar]
- [632].Fumarola A, Di Fiore A, Dainelli M, Grani G, Calvanese A (2010) Medical treatment of hyperthyroidism: state of the art. Exp Clin Endocrinol Diabetes 118, 678–684. [DOI] [PubMed] [Google Scholar]
- [633].Song L, Lei J, Jiang K, Lei Y, Tang Y, Zhu J, Li Z, Tang H (2019) The Association Between Subclinical Hypothyroidism and Sleep Quality: A Population-Based Study. Risk Manag Healthc Policy 12, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [634].Resta O, Pannacciulli N, Di Gioia G, Stefano A, Barbaro MP, De Pergola G (2004) High prevalence of previously unknown subclinical hypothyroidism in obese patients referred to a sleep clinic for sleep disordered breathing. Nutr Metab Cardiovasc Dis 14, 248–253. [DOI] [PubMed] [Google Scholar]
- [635].Lin CC, Tsan KW, Chen PJ (1992) The relationship between sleep apnea syndrome and hypothyroidism. Chest 102, 1663–1667. [DOI] [PubMed] [Google Scholar]
- [636].Kittle WM, Chaudhary BA (1988) Sleep apnea and hypothyroidism. South Med J 81, 1421–1425. [DOI] [PubMed] [Google Scholar]
- [637].Bielicki P, Przybylowski T, Kumor M, Barnas M, Wiercioch M, Chazan R (2016) Thyroid Hormone Levels and TSH Activity in Patients with Obstructive Sleep Apnea Syndrome. Adv Exp Med Biol 878, 67–71. [DOI] [PubMed] [Google Scholar]
- [638].Thavaraputta S, Dennis JA, Laoveeravat P, Nugent K, Rivas AM (2019) Hypothyroidism and Its Association With Sleep Apnea Among Adults in the United States: NHANES 2007–2008. J Clin Endocrinol Metab 104, 4990–4997. [DOI] [PubMed] [Google Scholar]
- [639].Mete T, Yalcin Y, Berker D, Ciftci B, Guven Firat S, Topaloglu O, Cinar Yavuz H, Guler S (2013) Relationship between obstructive sleep apnea syndrome and thyroid diseases. Endocrine 44, 723–728. [DOI] [PubMed] [Google Scholar]
- [640].Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL (2011) Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep 34, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [641].Stern RA, Robinson B, Thorner AR, Arruda JE, Prohaska ML, Prange AJ Jr. (1996) A survey study of neuropsychiatric complaints in patients with Graves’ disease. J Neuropsychiatry Clin Neurosci 8, 181–185. [DOI] [PubMed] [Google Scholar]
- [642].Sridhar GR, Putcha V, Lakshmi G (2011) Sleep in thyrotoxicosis. Indian J Endocrinol Metab 15, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [643].Ahmed N, Kandil M, Elfil M, Jamal A, Koo BB (2021) Hypothyroidism in restless legs syndrome. J Sleep Res 30, e13091. [DOI] [PubMed] [Google Scholar]
- [644].Xia L, Chen GH, Li ZH, Jiang S, Shen J (2013) Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research. PLoS One 8, e71065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [645].Trzepacz PT, McCue M, Klein I, Levey GS, Greenhouse J (1988) A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 10, 49–55. [DOI] [PubMed] [Google Scholar]
- [646].Chattopadhyay C, Chakrabarti N, Ghosh S (2012) An assessment of psychiatric disturbances in Graves disease in a medical college in eastern India. Niger J Clin Pract 15, 276–279. [DOI] [PubMed] [Google Scholar]
- [647].Smith JS, Kiloh LG (1981) The investigation of dementia: results in 200 consecutive admissions. Lancet 1, 824–827. [DOI] [PubMed] [Google Scholar]
- [648].Cummings J, Benson DF, LoVerme S Jr. (1980) Reversible dementia. Illustrative cases, definition, and review. JAMA 243, 2434–2439. [DOI] [PubMed] [Google Scholar]
- [649].van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J (2004) Thyroid function, depressed mood, and cognitive performance in older individuals: the Maastricht Aging Study. Psychoneuroendocrinology 29, 891–898. [DOI] [PubMed] [Google Scholar]
- [650].Wahlin A, Bunce D, Wahlin TB (2005) Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology 30, 625–637. [DOI] [PubMed] [Google Scholar]
- [651].van Osch LA, Hogervorst E, Combrinck M, Smith AD (2004) Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology 62, 1967–1971. [DOI] [PubMed] [Google Scholar]
- [652].Ganguli M, Burmeister LA, Seaberg EC, Belle S, DeKosky ST (1996) Association between dementia and elevated TSH: a community-based study. Biol Psychiatry 40, 714–725. [DOI] [PubMed] [Google Scholar]
- [653].Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM (2000) Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol (Oxf) 53, 733–737. [DOI] [PubMed] [Google Scholar]
- [654].Wieland DR, Wieland JR, Wang H, Chen YH, Lin CH, Wang JJ, Weng CH (2022) Thyroid Disorders and Dementia Risk: A Nationwide Population-Based Case-Control Study. Neurology 99, e679–e687. [DOI] [PubMed] [Google Scholar]
- [655].Kim JH, Lee HS, Kim YH, Kwon MJ, Kim JH, Min CY, Yoo DM, Choi HG (2022) The Association Between Thyroid Diseases and Alzheimer’s Disease in a National Health Screening Cohort in Korea. Front Endocrinol (Lausanne) 13, 815063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [656].Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S (2008) Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 168, 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [657].Percy ME, Potyomkina Z, Dalton AJ, Fedor B, Mehta P, Andrews DF, Mazzulli T, Murk L, Warren AC, Wallace RA, Chau H, Jeng W, Moalem S, O’Brien L, Schellenberger S, Tran H, Wu L (2003) Relation between apolipoprotein E genotype, hepatitis B virus status, and thyroid status in a sample of older persons with Down syndrome. Am J Med Genet A 120A, 191–198. [DOI] [PubMed] [Google Scholar]
- [658].de Jong FJ, den Heijer T, Visser TJ, de Rijke YB, Drexhage HA, Hofman A, Breteler MM (2006) Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 91, 2569–2573. [DOI] [PubMed] [Google Scholar]
- [659].O’Barr SA, Oh JS, Ma C, Brent GA, Schultz JJ (2006) Thyroid hormone regulates endogenous amyloid-beta precursor protein gene expression and processing in both in vitro and in vivo models. Thyroid 16, 1207–1213. [DOI] [PubMed] [Google Scholar]
- [660].Luo L, Yano N, Mao Q, Jackson IM, Stopa EG (2002) Thyrotropin releasing hormone (TRH) in the hippocampus of Alzheimer patients. J Alzheimers Dis 4, 97–103. [DOI] [PubMed] [Google Scholar]
- [661].Weyerer S, Dilling H (1991) Prevalence and treatment of insomnia in the community: results from the Upper Bavarian Field Study. Sleep 14, 392–398. [PubMed] [Google Scholar]
- [662].Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, Singleton N, Meltzer H (2006) Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep 29, 1391–1397. [DOI] [PubMed] [Google Scholar]
- [663].Tsuno N, Besset A, Ritchie K (2005) Sleep and depression. J Clin Psychiatry 66, 1254–1269. [DOI] [PubMed] [Google Scholar]
- [664].Jansson-Frojmark M, Lindblom K (2008) A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res 64, 443–449. [DOI] [PubMed] [Google Scholar]
- [665].Taylor DJ, Lichstein KL, Durrence HH (2003) Insomnia as a health risk factor. Behav Sleep Med 1, 227–247. [DOI] [PubMed] [Google Scholar]
- [666].Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W (2008) Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep 31, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [667].Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D (2011) Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 135, 10–19. [DOI] [PubMed] [Google Scholar]
- [668].Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, Nordhus IH, Overland S (2012) The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med 74, 758–765. [DOI] [PubMed] [Google Scholar]
- [669].Staner L (2003) Sleep and anxiety disorders. Dialogues Clin Neurosci 5, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [670].Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, Trockel M, Kraemer HC, Thase ME (2016) Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined With Antidepressant Pharmacotherapy in Patients With Comorbid Depression and Insomnia: A Randomized Controlled Trial. J Clin Psychiatry 77, e1316–e1323. [DOI] [PubMed] [Google Scholar]
- [671].Baglioni C, Spiegelhalder K, Lombardo C, Riemann D (2010) Sleep and emotions: a focus on insomnia. Sleep Med Rev 14, 227–238. [DOI] [PubMed] [Google Scholar]
- [672].Ohayon MM, Roth T (2003) Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res 37, 9–15. [DOI] [PubMed] [Google Scholar]
- [673].Alvaro PK, Roberts RM, Harris JK (2013) A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep 36, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [674].Chan JW, Lam SP, Li SX, Yu MW, Chan NY, Zhang J, Wing YK (2014) Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep 37, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [675].Kearns JC, Coppersmith DDL, Santee AC, Insel C, Pigeon WR, Glenn CR (2020) Sleep problems and suicide risk in youth: A systematic review, developmental framework, and implications for hospital treatment. Gen Hosp Psychiatry 63, 141–151. [DOI] [PubMed] [Google Scholar]
- [676].Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ (2005) Epidemiology of insomnia, depression, and anxiety. Sleep 28, 1457–1464. [DOI] [PubMed] [Google Scholar]
- [677].Batterham PJ, Werner-Seidler A, Calear AL, McCallum S, Gulliver A (2021) Specific aspects of sleep disturbance associated with suicidal thoughts and attempts. J Affect Disord 282, 574–579. [DOI] [PubMed] [Google Scholar]
- [678].Winkelmann J, Prager M, Lieb R, Pfister H, Spiegel B, Wittchen HU, Holsboer F, Trenkwalder C, Strohle A (2005) “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J Neurol 252, 67–71. [DOI] [PubMed] [Google Scholar]
- [679].Andrews JG, Oei TP (2004) The roles of depression and anxiety in the understanding and treatment of Obstructive Sleep Apnea Syndrome. Clin Psychol Rev 24, 1031–1049. [DOI] [PubMed] [Google Scholar]
- [680].Gillin JC (1998) Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr Scand Suppl 393, 39–43. [DOI] [PubMed] [Google Scholar]
- [681].Difrancesco S, Lamers F, Riese H, Merikangas KR, Beekman ATF, van Hemert AM, Schoevers RA, Penninx B (2019) Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study. Depress Anxiety 36, 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [682].Roeser K, Schlarb AA, Kübler A (2013) The Chronotype-Academic Performance Model (CAM): Daytime sleepiness and learning motivation link chronotype and school performance in adolescents. Personality and Individual Differences 54, 836–840. [Google Scholar]
- [683].Bauducco S, Richardson C, Gradisar M (2020) Chronotype, circadian rhythms and mood. Curr Opin Psychol 34, 77–83. [DOI] [PubMed] [Google Scholar]
- [684].Haraden DA, Mullin BC, Hankin BL (2017) The relationship between depression and chronotype: A longitudinal assessment during childhood and adolescence. Depress Anxiety 34, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [685].Soehner AM, Kaplan KA, Harvey AG (2014) Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord 167, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [686].Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB (1998) Gender differences in anxiety disorders and anxiety symptoms in adolescents. J Abnorm Psychol 107, 109–117. [DOI] [PubMed] [Google Scholar]
- [687].Altemus M, Sarvaiya N, Neill Epperson C (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [688].Huang S, Wang Z, Zheng D, Liu L (2023) Anxiety disorder in menopausal women and the intervention efficacy of mindfulness-based stress reduction. Am J Transl Res 15, 2016–2024. [PMC free article] [PubMed] [Google Scholar]
- [689].Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR (1998) Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 338, 209–216. [DOI] [PubMed] [Google Scholar]
- [690].Rubinow DR, Schmidt PJ, Roca CA (1998) Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry 44, 839–850. [DOI] [PubMed] [Google Scholar]
- [691].Schmidt PJ, Rubinow DR (2009) Sex hormones and mood in the perimenopause. Ann N Y Acad Sci 1179, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [692].Freeman EW, Sammel MD, Lin H, Nelson DB (2006) Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 63, 375–382. [DOI] [PubMed] [Google Scholar]
- [693].Muller-Spahn F (2003) Behavioral disturbances in dementia. Dialogues Clin Neurosci 5, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [694].Wetherell JL, Reynolds CA, Gatz M, Pedersen NL (2002) Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci 57, P246–255. [DOI] [PubMed] [Google Scholar]
- [695].Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C (2013) Gender differences in depression and anxiety: the role of age. Psychiatry Res 210, 1301–1303. [DOI] [PubMed] [Google Scholar]
- [696].Blazer DG (2003) Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 58, 249–265. [DOI] [PubMed] [Google Scholar]
- [697].Hasin DS, Goodwin RD, Stinson FS, Grant BF (2005) Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry 62, 1097–1106. [DOI] [PubMed] [Google Scholar]
- [698].Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP (2013) Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci U S A 110, 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [699].Somme J, Fernandez-Martinez M, Molano A, Zarranz JJ (2013) Neuropsychiatric symptoms in amnestic mild cognitive impairment: increased risk and faster progression to dementia. Curr Alzheimer Res 10, 86–94. [DOI] [PubMed] [Google Scholar]
- [700].Kaiser NC, Liang LJ, Melrose RJ, Wilkins SS, Sultzer DL, Mendez MF (2014) Differences in anxiety among patients with early- versus late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 26, 73–80. [DOI] [PubMed] [Google Scholar]
- [701].Lyketsos CG, Olin J (2002) Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry 52, 243–252. [DOI] [PubMed] [Google Scholar]
- [702].Cannon-Spoor HE, Levy JA, Zubenko GS, Zubenko WW, Cohen RM, Mirza N, Putnam K, Sunderland T (2005) Effects of previous major depressive illness on cognition in Alzheimer disease patients. Am J Geriatr Psychiatry 13, 312–318. [DOI] [PubMed] [Google Scholar]
- [703].Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, Smith GE, Geda YE, Cummings JL, Petersen RC, Sunderland T (2003) A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry 160, 857–866. [DOI] [PubMed] [Google Scholar]
- [704].Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FR (2005) The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry 20, 523–530. [DOI] [PubMed] [Google Scholar]
- [705].Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, Breitner JC, Steffens DC, Tschanz JT, Cache County I (2008) Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 23, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [706].Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Xu W, Li JQ, Wang J, Lai TJ, Yu JT (2016) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 190, 264–271. [DOI] [PubMed] [Google Scholar]
- [707].Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P, Australian Imaging B, Lifestyle Research G (2014) Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. Br J Psychiatry 204, 400–401. [DOI] [PubMed] [Google Scholar]
- [708].Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, Johnson KA, Sperling RA, Harvard Aging Brain S (2018) Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am J Psychiatry 175, 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [709].Scaricamazza E, Colonna I, Sancesario GM, Assogna F, Orfei MD, Franchini F, Sancesario G, Mercuri NB, Liguori C (2019) Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer’s disease patients: a retrospective observational study. Neurol Sci 40, 1377–1382. [DOI] [PubMed] [Google Scholar]
- [710].Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD, Ferretti-Rebustini REL, You M, Oh J, Nitrini R, Pasqualucci CA, Jacob-Filho W, Kramer JH, Gatchel JR, Grinberg LT (2018) Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 66, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [711].Mah L, Binns MA, Steffens DC, Alzheimer’s Disease Neuroimaging I (2015) Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am J Geriatr Psychiatry 23, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [712].Boccia M, Acierno M, Piccardi L (2015) Neuroanatomy of Alzheimer’s Disease and Late-Life Depression: A Coordinate-Based Meta-Analysis of MRI Studies. J Alzheimers Dis 46, 963–970. [DOI] [PubMed] [Google Scholar]
- [713].Banning LCP, Ramakers I, Rosenberg PB, Lyketsos CG, Leoutsakos JS, Alzheimer’s Disease Neuroimaging I (2021) Alzheimer’s disease biomarkers as predictors of trajectories of depression and apathy in cognitively normal individuals, mild cognitive impairment, and Alzheimer’s disease dementia. Int J Geriatr Psychiatry 36, 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [714].Banning LCP, Ramakers I, Kohler S, Bron EE, Verhey FRJ, de Deyn PP, Claassen J, Koek HL, Middelkoop HAM, van der Flier WM, van der Lugt A, Aalten P, Alzheimer’s Disease Neuroimaging I, Parelsnoer Institute Neurodegenerative Diseases study g (2020) The Association Between Biomarkers and Neuropsychiatric Symptoms Across the Alzheimer’s Disease Spectrum. Am J Geriatr Psychiatry 28, 735–744. [DOI] [PubMed] [Google Scholar]
- [715].Forstl H, Burns A, Luthert P, Cairns N, Lantos P, Levy R (1992) Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med 22, 877–884. [DOI] [PubMed] [Google Scholar]
- [716].Lebedeva A, Westman E, Lebedev AV, Li X, Winblad B, Simmons A, Wahlund LO, Aarsland D, Alzheimer’s Disease Neuroimaging I (2014) Structural brain changes associated with depressive symptoms in the elderly with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 85, 930–935. [DOI] [PubMed] [Google Scholar]
- [717].Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL (1989) Neuropathology of aminergic nuclei in Alzheimer’s disease. Prog Clin Biol Res 317, 353–365. [PubMed] [Google Scholar]
- [718].Burke SL, Cadet T, Alcide A, O’Driscoll J, Maramaldi P (2018) Psychosocial risk factors and Alzheimer’s disease: the associative effect of depression, sleep disturbance, and anxiety. Aging Ment Health 22, 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [719].Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L (2007) Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol 17, 573–579. [DOI] [PubMed] [Google Scholar]
- [720].Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A (2010) A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [721].Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E (2003) Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry 54, 96–104. [DOI] [PubMed] [Google Scholar]
- [722].Lu J, Zhao J, Balesar R, Fronczek R, Zhu QB, Wu XY, Hu SH, Bao AM, Swaab DF (2017) Sexually Dimorphic Changes of Hypocretin (Orexin) in Depression. EBioMedicine 18, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [723].Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, Kaisers UX, Thiery J, Hegerl U, Schonknecht P (2013) Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. PLoS One 8, e63136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [724].Spinedi E, Cardinali DP (2018) The Polycystic Ovary Syndrome and the Metabolic Syndrome: A Possible Chronobiotic-Cytoprotective Adjuvant Therapy. Int J Endocrinol 2018, 1349868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [725].Khan MJ, Ullah A, Basit S (2019) Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl Clin Genet 12, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [726].Wang J, Wu D, Guo H, Li M (2019) Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci 236, 116940. [DOI] [PubMed] [Google Scholar]
- [727].Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS (2006) A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12, 673–683. [DOI] [PubMed] [Google Scholar]
- [728].Giudice LC (2006) Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 20, 235–244. [DOI] [PubMed] [Google Scholar]
- [729].Perovic M, Wugalter K, Einstein G (2022) Review of the effects of polycystic ovary syndrome on Cognition: Looking beyond the androgen hypothesis. Front Neuroendocrinol 67, 101038. [DOI] [PubMed] [Google Scholar]
- [730].Fernandez RC, Moore VM, Van Ryswyk EM, Varcoe TJ, Rodgers RJ, March WA, Moran LJ, Avery JC, McEvoy RD, Davies MJ (2018) Sleep disturbances in women with polycystic ovary syndrome: prevalence, pathophysiology, impact and management strategies. Nat Sci Sleep 10, 45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [731].Tasali E, Van Cauter E, Ehrmann DA (2008) Polycystic Ovary Syndrome and Obstructive Sleep Apnea. Sleep Med Clin 3, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [732].Bahman M, Hajimehdipoor H, Afrakhteh M, Bioos S, Hashem-Dabaghian F, Tansaz M (2018) The Importance of Sleep Hygiene in Polycystic Ovary Syndrome from the View of Iranian Traditional Medicine and Modern Medicine. Int J Prev Med 9, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [733].Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP (2001) Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 86, 1175–1180. [DOI] [PubMed] [Google Scholar]
- [734].Lin TY, Lin PY, Su TP, Li CT, Lin WC, Chang WH, Chen TJ, Bai YM, Chen MH (2017) Risk of developing obstructive sleep apnea among women with polycystic ovarian syndrome: a nationwide longitudinal follow-up study. Sleep Med 36, 165–169. [DOI] [PubMed] [Google Scholar]
- [735].El-Sharkawy AA, Abdelmotaleb GS, Aly MK, Kabel AM (2014) Effect of metformin on sleep disorders in adolescent girls with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol 27, 347–352. [DOI] [PubMed] [Google Scholar]
- [736].Naqvi SH, Moore A, Bevilacqua K, Lathief S, Williams J, Naqvi N, Pal L (2015) Predictors of depression in women with polycystic ovary syndrome. Arch Womens Ment Health 18, 95–101. [DOI] [PubMed] [Google Scholar]
- [737].Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM (2015) Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod 30, 466–472. [DOI] [PubMed] [Google Scholar]
- [738].Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill CM, Brook N, Macklon N, Cheong Y (2013) Poor sleep in PCOS; is melatonin the culprit? Hum Reprod 28, 1348–1353. [DOI] [PubMed] [Google Scholar]
- [739].Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP (2001) Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 86, 517–520. [DOI] [PubMed] [Google Scholar]
- [740].Tasali E, Chapotot F, Leproult R, Whitmore H, Ehrmann DA (2011) Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 96, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [741].de Sousa G, Schluter B, Menke T, Trowitzsch E, Andler W, Reinehr T (2011) A comparison of polysomnographic variables between adolescents with polycystic ovarian syndrome with and without the metabolic syndrome. Metab Syndr Relat Disord 9, 191–196. [DOI] [PubMed] [Google Scholar]
- [742].Franik G, Krysta K, Madej P, Gimlewicz-Pieta B, Oslizlo B, Trukawka J, Olszanecka-Glinianowicz M (2016) Sleep disturbances in women with polycystic ovary syndrome. Gynecol Endocrinol 32, 1014–1017. [DOI] [PubMed] [Google Scholar]
- [743].Suri J, Suri JC, Chatterjee B, Mittal P, Adhikari T (2016) Obesity may be the common pathway for sleep-disordered breathing in women with polycystic ovary syndrome. Sleep Med 24, 32–39. [DOI] [PubMed] [Google Scholar]
- [744].Tasali E, Van Cauter E, Ehrmann DA (2006) Relationships between sleep disordered breathing and glucose metabolism in polycystic ovary syndrome. J Clin Endocrinol Metab 91, 36–42. [DOI] [PubMed] [Google Scholar]
- [745].Barry JA, Kuczmierczyk AR, Hardiman PJ (2011) Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 26, 2442–2451. [DOI] [PubMed] [Google Scholar]
- [746].Blay SL, Aguiar JV, Passos IC (2016) Polycystic ovary syndrome and mental disorders: a systematic review and exploratory meta-analysis. Neuropsychiatr Dis Treat 12, 2895–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [747].Dokras A, Clifton S, Futterweit W, Wild R (2011) Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol 117, 145–152. [DOI] [PubMed] [Google Scholar]
- [748].Dokras A, Clifton S, Futterweit W, Wild R (2012) Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 97, 225–230 e222. [DOI] [PubMed] [Google Scholar]
- [749].Legro RS, Castracane VD, Kauffman RP (2004) Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59, 141–154. [DOI] [PubMed] [Google Scholar]
- [750].Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A (2005) Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90, 4510–4515. [DOI] [PubMed] [Google Scholar]
- [751].Gomez JM (2008) Growth hormone and insulin-like growth factor-I as an endocrine axis in Alzheimer’s disease. Endocr Metab Immune Disord Drug Targets 8, 143–151. [DOI] [PubMed] [Google Scholar]
- [752].Sabayan B, Foroughinia F, Borhani Haghighi A, Mowla A (2007) Are women with polycystic ovary syndrome (PCOS) at higher risk for development of Alzheimer disease? Alzheimer Dis Assoc Disord 21, 265. [DOI] [PubMed] [Google Scholar]
- [753].Andrade AG, Bubu OM, Varga AW, Osorio RS (2018) The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J Alzheimers Dis 64, S255–S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [754].Park SK, Harlow SD, Zheng H, Karvonen-Gutierrez C, Thurston RC, Ruppert K, Janssen I, Randolph JF Jr. (2017) Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet Med 34, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [755].McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC (2009) Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 94, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [756].Qiao J, Feng HL (2011) Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 17, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [757].Kalro BN, Loucks TL, Berga SL (2001) Neuromodulation in polycystic ovary syndrome. Obstet Gynecol Clin North Am 28, 35–62. [DOI] [PubMed] [Google Scholar]
- [758].Malini NA, Roy George K (2018) Evaluation of different ranges of LH:FSH ratios in polycystic ovarian syndrome (PCOS) - Clinical based case control study. Gen Comp Endocrinol 260, 51–57. [DOI] [PubMed] [Google Scholar]
- [759].Ahmad MH, Fatima M, Mondal AC (2019) Role of Hypothalamic-Pituitary-Adrenal Axis, Hypothalamic-Pituitary-Gonadal Axis and Insulin Signaling in the Pathophysiology of Alzheimer’s Disease. Neuropsychobiology 77, 197–205. [DOI] [PubMed] [Google Scholar]


