Abstract
Parkinson’s disease (PD) is a prevalent neurodegenerative disease in which treatment often includes an exercise regimen. Exercise is neuroprotective in animal models of PD, and, more recently, human clinical studies have verified exercise’s disease-modifying effect. Aerobic exercise and resistance training improve many of PD’s motor and non-motor symptoms, while neuromotor therapy and stretching/flexibility exercises positively contribute to the quality of life in people with PD. Therefore, understanding the role of exercise in managing this complex disorder is crucial. Exerkines are bioactive substances that are synthesized and released during exercise and have been implicated in several positive health outcomes, including neuroprotection. Exerkines protect neuronal cells in vitro and rodent PD models in vivo. Aerobic exercise and resistance training both increase exerkine levels in the blood, suggesting a role for exerkines in the neuroprotective theory. Many exerkines demonstrate the potential for protecting the brain against pathological missteps caused by PD. Every person (people) with Parkinson’s (PwP) needs a comprehensive exercise plan tailored to their unique needs and abilities. Here, we provide an exercise template to help PwP understand the importance of exercise for treating PD, describe barriers confronting many PwP in their attempt to exercise, provide suggestions for overcoming these barriers, and explore the role of exerkines in managing PD. In conclusion, exercise and exerkines together create a powerful neuroprotective system that should contribute to slowing the chronic progression of PD.
Keywords: Parkinson’s disease, exercise, exerkines, neurodegenerative disease, Alzheimer’s disease, aerobic exercise, resistance training, neuroprotective theory, myokines
1. Introduction
“Diseases fly from the presence of a person, habituated to regular physical exercise…” Susruta, a Physician in the Indus Valley civilization, 600 BCE [1,2].
1.1. Neurodegenerative Diseases and the Positive Impact of Exercise
Neurodegenerative diseases are characterized by progressive nervous system dysfunction leading to neuronal cell death. The most common neurodegenerative disorders include (given alphabetically) Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Huntington’s disease, multiple sclerosis, Parkinson’s disease (PD), spinal muscular atrophy, and spinocerebellar ataxia [3,4,5,6,7]. Neurodegenerative diseases ultimately lead to movement (motor) difficulties, decline in cognitive functioning (dementia), or a combination of both. Most neurodegenerative diseases share similar pathophysiological alterations in brain tissue: the accumulation of abnormal protein aggregates in neurons, leading to neuroinflammation and progressive neuronal cell death [4,8,9,10,11,12].
Interestingly, exercise improves the symptoms of many neurodegenerative disorders, especially PD and AD [13,14,15,16,17,18,19,20,21,22,23]. Moderate to vigorous exercise can benefit brain health and cognitive function in people with neurodegenerative diseases [24,25,26,27], in stark contrast to the adverse effects associated with physical inactivity [28,29]. In human studies, sustained exercise enhances learning, memory, and executive function and reverses age-related mental deterioration [24]. Many studies report that sustained moderate exercise improves quality of life (QoL) outcomes in people with neurodegenerative diseases [24,25,26].
1.2. Parkinson’s Disease (PD)
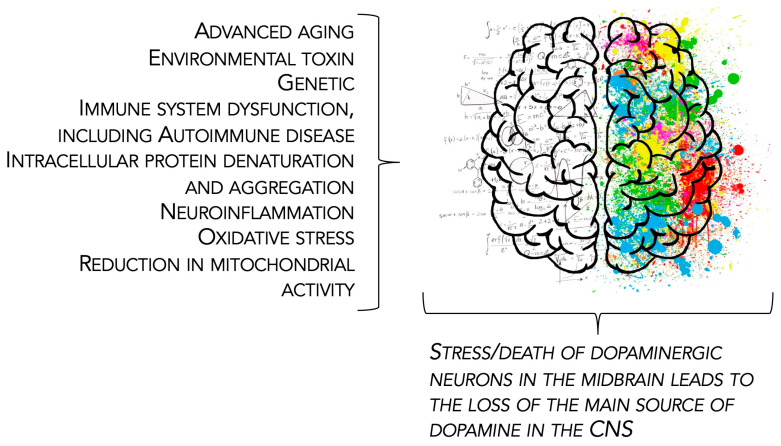
Parkinson’s disease is a neurodegenerative disorder that begins following the death of dopaminergic neurons located in the midbrain substantia nigra pars compacta [30]. The resultant degeneration of dopaminergic pathways in the basal ganglia [31,32,33,34] results in the cardinal motor signs of PD, including bradykinesia (slowness of movement), tremor (trembling in hands, arms, legs, jaw, and face), muscle rigidity (stiffness of the limbs and trunk), and impaired gait and posture [35,36,37]. Numerous non-motor symptoms of PD may include depression, psychosis, REM sleep disruption and hallucinations, difficulty swallowing and speaking, urinary problems, or constipation [38,39,40,41,42]. Most cases of PD occur sporadically and are due to a complex combination of etiologies (Figure 1).
Figure 1.
Etiology of Parkinson’s disease. The major causes of the disorder are given in alphabetical order. Abbreviation: CNS, central nervous system.
PD is both a chronic disease process and a progressive disorder [31,32,33,34]. PD occurs most commonly in people over 60 years old. Cases of PD in younger people (<40 years old) are usually linked to specific genetic mutations. Symptoms of PD occur gradually over several years, with the rate of progression and the extent to which symptoms manifest varying from person to person. PD limits movement and results in functional instability, affecting physical well-being and QoL.
At present, PD remains an incurable disease [31,32,33,34], and treatment goals revolve around symptomatic management [43,44]. The traditional first-line therapy for PD is carbidopa/levodopa or a dopamine agonist. However, the numerous pathological events that contribute to the development of PD suggest that a multi-pronged therapeutic approach is necessary to slow disease progression.
1.3. Exercise and Exerkines
One of the more important treatment strategies for PD is exercise [45,46]. Regular exercise is known to benefit the health of older adults through various physiological processes. These include improving insulin sensitivity, reducing body fat, increasing cardiovascular function, enhancing cerebral blood flow and oxygenation, and improving muscle mass and strength [47]. Physical exercise promotes the synthesis/release of signaling molecules termed exerkines [48,49,50,51,52,53,54], which exert their action through autocrine, paracrine, and endocrine processes. The composition of exerkines ranges broadly from simple organic acids (e.g., lactate) [52,55] and proteolytic enzymes (e.g., cathepsin B) [23] to more complex proteins like interleukin-6 (IL-6) [56] and micro-RNAs (e.g., miR-1192) [57].
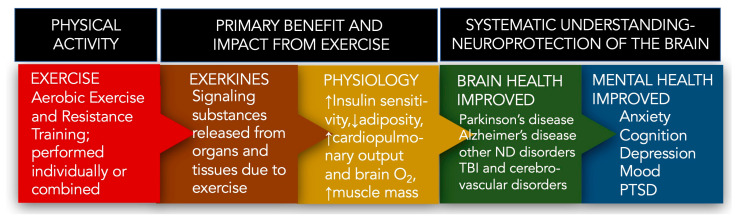
Following acute/chronic exercise, exerkines are released from numerous tissues and organs, including skeletal muscle (myokines), the heart (cardiokines), liver (hepatokines), white adipose tissue (adipokines), brown adipose tissue (baptokines), and neurons/brain (neurokines). Exerkines migrate to their target organs during and after exercise, acting on various tissues [48,49,50,51,52,53,54]. This systemic release of exerkines facilitates communication between the brain and other organs, especially the skeletal muscles that produce myokines [19,58,59,60]. Through this communication, exercise and exerkines work together to protect the brain, providing a natural strategy to prevent brain diseases and to improve mental health (Figure 2).
Figure 2.
Overview of exercise and the role of neuroprotective exerkines. The molecular mechanisms underlying the protective/regenerative effects of exerkines are under active investigation. Abbreviations: ND, neurodegenerative; TBI, traumatic brain injury; PTSD, post-traumatic stress disorder.
1.4. Parkinson’s Disease, Exercise, and Exerkines
Exercise may alleviate both the physical and neurological symptoms of PD. We completed a review of the literature to determine the relationship between exercise, exerkines, and the potential to slow the progression of PD. First, this narrative review focuses on the various forms of exercise in therapy for PD. Next, we describe several neuroprotective exerkines and the neuroprotective theory. Finally, using the neuroprotective theory, we summarize how exerkines, through interorgan crosstalk, generate a protective and restorative network in the brain, verifying the potential for exercise to serve as a healthy option for managing PD.
2. Exercise as Therapy for Parkinson’s Disease
“Movement is a medicine for creating change in a person’s physical, emotional, and mental states.” Carol Welch-Baril, Neuromuscular Therapist
This section will focus primarily on aerobic and resistance training exercises. However, it will also include an overview of neuromotor exercise programs and flexibility exercises recognized as essential to treating PD. Numerous barriers prevent some PwP from exercising. Thus, it is necessary to describe valuable ways to overcome these obstacles. This also includes sections summarizing previous systematic and meta-reviews on the use of exercise in treating PD, and overviews of its effects on the central nervous system and motor units.
2.1. Defining Physical Activity and Exercise
The U.S. Centers for Disease Control and Prevention [61] defines physical activity as “any bodily movement produced by the contraction of skeletal muscle that increases energy expenditure above a basal level”. It further defines exercise as a subcategory of physical activity to sustain or improve health and fitness [61]. Data from both animal [62,63,64,65,66] and human [45,67,68,69] models suggest that engaging in moderate-to-vigorous-intensity exercise at recommended levels shows promise in slowing the neurodegeneration associated with PD. As such, exercise has increasingly become a crucial component of a holistic approach to treatment in this population.
2.1.1. Exercise Intensity Defined
We define moderate-intensity exercise as physical activity that raises the heart rate to 50–70% above its resting rate. This level of exercise should feel somewhat challenging; breathing quickens but does not become labored. After approximately ten minutes of such activity, a light sweat develops, though conversation remains possible (from the Mayo Clinic [70,71] and the American Heart Association [72]). In contrast, vigorous-intensity exercise is distinctly challenging [70,71]. Breathing becomes deep and rapid, and sweating begins after only a few minutes of activity. It is typically challenging to speak more than a few words without pausing for breath.
2.1.2. Calculating Maximal Heart Rate during Exercise
A widely accepted format for assessing exercise intensity involves estimating the heart rate during physical activity. To calculate the maximal heart rate, one should multiply their age by 0.7 and subtract the result from 208 [70,71,72]. For instance, for a 70-year-old individual, multiplying 70 by 0.7 results in 49, which when subtracted from 208 gives 159, representing the maximal heart rate of 159 beats per minute (bpm). Using the recommendations of the American Heart Association [72], moderate-intensity exercise should correspond to 50% to approximately 70% of the maximum heart rate (% HRmax). For a 70-year-old, this translates to a range of 80 to 111 bpm, while vigorous-intensity exercise should range from 70% to about 85% of the maximum heart rate, equating to 111 to 135 bpm for someone aged 70 [70,71,72].
2.2. Aerobic Exercise
Aerobic exercise encompasses activities that increase heart rate and oxygen consumption over sustained periods, promoting cardiovascular health and overall well-being. In the context of PD, it is a cornerstone of physical activity interventions in PD management [73,74]. It has garnered considerable attention for its potential to alleviate motor symptoms and enhance cognitive function. Alberts and Rosenfeldt [75] emphasize that aerobic exercise is a universal prescription for PD management, offering benefits such as improvements in motor symptoms, balance, and overall QoL.
2.2.1. Motor Symptoms
Aerobic exercise enhances cardiovascular and respiratory function, leading to better overall fitness, improved functional capacity, and increased endurance for daily activities. The American Physical Therapy Association (APTA), in their clinical practice guideline on Parkinson’s disease, encourages physical therapists to implement moderate-to-vigorous-intensity aerobic exercise (approximately 60–85% HRmax) to improve oxygen consumption (VO2), reduce motor disease severity, and improve functional outcomes [76]. Several studies revealed reduced motor decline as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) [45,67,69,77]. Aerobic exercise as an intervention has also been shown to improve scores across various domains (gait, balance, and ADLS) [78]. It is often measured with gait-related outcomes, including the 6-Minute Walk Test [68].
Interestingly, and helpfully, similar results are seen across studies regardless of which mode of moderate-to-vigorous-intensity aerobic exercise is used, including treadmill walking [67], stationary cycling [45,79], a brisk walking and balance program [69], aquatic therapy [77,80], Nordic walking [81], and even relatively new methods such as agility exergaming [68]. It is reasonable to hypothesize that other forms of exercise performed at similar intensity levels, such as outdoor cycling, rowing, stair climbing, dance and others, might have similar effects [82].
2.2.2. Brain Structure and Cognitive Symptoms
Aerobic exercise has been shown to improve mood and cognition in otherwise healthy older adults [83,84]. A few recent studies have extended these findings, demonstrating similar benefits in individuals with PD [85,86,87,88]. Evidence also points to aerobic exercise having beneficial effects on brain structure and cognitive function over time.
Van der Kolk et al. [45] first described the Park-in-Shape study, and they reported a large improvement in off-state MDS UPDRS scores on the stationary home-trainer bicycle (30–45 min beginning with 50% to 70% HRmax and gradually increasing to 80% HRmax) compared to the control group. Johansson et al. [46], in a further assessment of the Park-in-Shape trial, concluded that aerobic exercise was associated with heightened functional connectivity between the anterior putamen and the sensorimotor cortex in its experimental groups. Compared to stretching controls, aerobic exercise mitigated global brain atrophy and contributed to enhanced cognitive control [46]. Another study suggested that aerobic exercise can change the mesolimbic dopaminergic pathway and increase evoked dopamine release in the caudate nucleus [89]. This indicated that the therapeutic benefits of exercise are perhaps related to corticostriatal plasticity and enhanced dopamine release [89]. Separately, in the Study in Parkinson Disease of Exercise (SPARX), Schenkman et al. [67] compared vigorous (80–85% Vmax) and moderate (60–65% Vmax)-intensity treadmill exercise groups to a control group. The vigorous-intensity group was found to reach targeted improvement in UPDRS scores [67].
Niemann et al. [90] found that cardiovascular interventions and motor exercises performed over six months, compared to stretching and relaxation controls, positively impacted the volume of basal ganglia nuclei and, by extension, appeared to mitigate age-related decline in cognitive functions in PwP. A more recent study by Marusiak et al. [91] revealed that eight weeks of aerobic interval training on a cycle ergometer (performed at 60–75% HRmax) demonstrated not only an improvement in executive function for their PD intervention group but also in psychomotor domains such as mood and upper-extremity bradykinesia. Furthermore, Valenzuela et al. [92] showed that walking, when combined with cognitive tasks (known as dual-tasking) in PwP, not only demonstrated improved velocity and stride length during gait but also experienced a clinically significant improvement in perceived QoL.
A proof-of-concept study by de Laat et al. [93] evaluated the dopaminergic system of thirteen patients with mild, early PD before and after a 6-month vigorous-intensity exercise program [93]. In these individuals, exercise at an average of 80% of calculated HRmax led to a notable increase in dopamine transporter availability in both the substantia nigra and putamen—this was contrary to the expected decrease. Additionally, exercise caused a significant rise in neuromelanin concentration in the substantia nigra, reversing the anticipated decline [93]. Both results suggest enhanced functionality of the remaining dopaminergic neurons post-exercise and, by extension, indicate that vigorous-intensity exercise has neuromodulator and neuroprotective effects in these PwP.
2.3. Resistance Training
Resistance training constitutes a fundamental component of exercise interventions for PwP, aiming to improve muscle strength, endurance, and functional capacity [94]. By targeting specific muscle groups affected by PD-related rigidity and weakness, resistance training contributes to mitigating motor symptoms and enhancing overall physical performance [95]. Traditional resistance training modalities, such as weightlifting, resistance band exercises, and machine-based workouts, can be adapted to accommodate the needs and abilities of individuals with PD [96]. These exercises typically involve multiple sets of controlled repetitions targeting major muscle groups, including the upper and lower extremities, core, and back. Resistance training programs are tailored to individual baseline fitness levels and progressively adjusted to challenge muscular strength and endurance over time [97].
2.3.1. Motor Symptoms, Disease Severity, and Motor Function
Corcos et al. [98] recommended progressive resistance training as part of a comprehensive exercise program for managing PD symptoms. According to the APTA, the rationale for including resistance training in a plan of care for Parkinson’s patients is to improve strength and power output, improve functional outcomes, and reduce motor disease severity as measured by the UPDRS when included in a multimodal exercise program [76].
Progressive resistance training programs offer significant benefits for PwP, improving strength and power whether implemented alone or as part of a multimodal program. Several high-quality studies [99,100,101,102] and systematic reviews with meta-analyses [94,103] consistently show that resistance training outperformed control groups (including pharmacologic treatment, education-based interventions, or low-intensity home exercises) in enhancing strength and power. Furthermore, resistance training with instability (RTI) showed greater benefits over traditional resistance training for improving strength and power of the plantarflexion and knee extension musculature, which are crucial muscle groups for maintaining functional gait patterns [104]. The RTI approach involved exercises like leg and chest presses performed on unstable devices, leading to superior results as measured by electromyography signals during isometric contractions. Silva-Batista et al. [104] found significant improvement in motor symptoms as measured by the UPDRS.
Multimodal interventions that include resistance training are generally more effective than non-exercise, education-based controls for improving strength and power in PD patients. However, these interventions are not always superior to other exercise programs or usual care in all studies. For example, a randomized controlled trial by Canning et al. [105] found that their multimodal exercise program, which targeted leg strength, balance, and freezing of gait, did not significantly reduce fall risk in their Parkinson’s patients. However, it did improve physical and psychological health.
Despite these mixed results in some comparisons, progressive resistance training has been demonstrated to address and improve functional capacity and mobility, as measured by various well-established outcome measures. Compared to pharmacological treatment, progressive resistance training was the favored intervention to improve Timed-Up-And-Go and 2-min sit-to-stand scores [45,89,103], as well as gait speed on the 10 m Walk Test [106] and 6-min walk test [107]. Specifically, resistance training with instability (RTI) had greater benefits for postural stability than traditional resistance training when measured by a digital balance system [104]. RTI also has been shown to restore gait automaticity during dual-task performance [108].
2.3.2. Non-Motor Symptoms and Quality of Life
In addition to the more tangible physical benefits of resistance exercise for PwP are potential non-motor improvements in mental health and overall QoL. Non-motor symptoms can be very disabling in patients with Parkinson’s, given that the majority do not respond efficiently to dopaminergic treatment [109]. Resistance training, when compared with non-exercise or standard pharmacological treatment control groups, led to improvements in patient-perceived QoL [81,110,111], as well as in measures of depression [99] and anxiety [104,112] in PwP. Targeted strength training for 16 weeks improved the inspiratory and expiratory muscle strength of elderly PD patients, subsequently positively impacting breathing quality and overall QoL [111]. Additionally, as part of the Progressive Resistance Exercise Training in Parkinson’s Disease, or PRET-PD trial, researchers found significant differences in the Digit Span, Stroop Test, and Brief Test of Attention after 24 months of progressive resistance training, indicating that it may improve attention and working memory in patients with mild-to-moderate Parkinson’s [86].
Though most studies examining the effects of resistance training for PwP evaluate cognitive outcomes secondarily to motor outcomes, the effects appear to be beneficial overall. In the APTA’s Clinical Practice Guideline on Parkinson’s [76], much evidence suggests that no one mode of resistance training is superior to another in improving non-motor systems.
2.4. Neuromotor Exercises to Improve Gait, Posture, Balance, and Reduce Risk of Falls
Muscle rigidity, balance, and gait disturbances are hallmark features of PD, contributing to high fall risk and other functional limitations [113,114]. Furthermore, PwP have movements that are slow (bradykinesia), hesitant (akinesia), and with reduced amplitude (hypokinesia) [115,116]. Balance and gait training with neuromotor exercises are crucial components of physical therapy for individuals with PD, aiming to improve postural control, walking ability, and fall prevention. Attempting to normalize movements is an essential goal of neuromotor exercises and contributes to QoL issues with PwP.
Dedicated, individualized balance interventions should be considered essential exercises for PwP [76]. These exercises are important in PD, especially since the motor defects associated with PD cause stiffness, impaired balance, and slow movement. However, under the guidance of their healthcare team, PwP can develop a regular exercise routine that incorporates stretching, movement, and balance, collectively termed neuromotor exercises. There are numerous exercise modalities that PwP use to improve their motor deficits, including PWR!Moves [117,118], Rock Steady Boxing [119], Nordic walking [120], Dance for PD [121], tai chi [122], golf [123,124], and yoga [125]. This variety of options offers hope for significant improvement in the QoL of PwP. Thus, this form of therapy should be utilized throughout all stages of the disorder.
Frequently, patients with PD have problems such as freezing of gait [126], limb dystonia [127], continued functional motor limitations (e.g., bradykinesia and hypokinesia), and difficulty with swallowing/speech [128]. In each case, a Movement Disorder Specialist would arrange for treatment with a qualified Physical Therapist (PT), Occupational Therapist (OT), or Speech–Language Pathologist (SLP) to develop a treatment plan for each issue. We briefly highlight three such programs. LSVT (Lee Silverman Voice Treatment)-LOUD, a very useful program, enhances the voice, increases vocal loudness (by improving articulation, vocal quality, and intonation), and positively alters PwP functional communication skills [129,130]. LSVT-BIG helps to overcome bradykinesia and hypokinesia in PwP by focusing on large amplitude movements, which give bigger, faster, and increased movement precision [131]. PWR!Moves, another highly effective program, also utilizes high-amplitude functional exercises to target deficits that affect PwP [117,118]. PWR!Moves is used by certified physical therapists and community exercise instructors specializing in treating PD and can be utilized throughout all stages of disease progression [132].
Community programs such as Dance for PD [121] and Rock Steady Boxing [119] offer additional resources for PwP to engage in group exercise. Rock Steady Boxing utilizes large amplitude movement in the form of non-contact boxing. Participants in weekly boxing classes during a 12-week observational demonstrated improved QoL, balance and gait [133]. Dance offers opportunities for social connection, use of rhythm and music, multidirectional movement, novel movement, and cognitive engagement, including observation, imagery, and sequence recall [134]. Dance has been shown to improve QoL and cognitive symptoms for PwP [135,136], as well as motor symptoms [136,137,138,139]. Several studies examining the impact of Dance for PD on UPDRS demonstrated improved participant scores [137,138,139,140]. Examples of Dance for PwP include options such as Tango lessons or Dance for Parkinson’s classes.
2.5. Stretching and Flexibility Exercises to Reduce Muscle Rigidity
Muscle stiffness (rigidity) is a prevalent motor dysfunction in PD [141,142,143]. Muscle rigidity can occur in the major muscles of the arms, legs, and trunk/neck and may affect one side of the body more frequently in the disorder’s early stages [141,142,143]. Muscle rigidity may lead to balance issues, and constant rigidity may contribute to fatigue. Over time, muscle rigidity in the back/neck may lead to a stooped posture, increasing the risk of falling [144]. Rigidity is one of the motor aspects of PD that the neurologist will assess and track over time. If muscle rigidity is prevalent, the PwP may be less inclined to perform the all-important aerobic and resistance training exercises, favoring a more sedentary lifestyle. Furthermore, PwP must understand the critical negative impact of muscle rigidity and the potential benefits of managing rigidity through stretching and flexibility exercises, which can, at minimum, improve the QoL.
Developing a personal routine for stretching to increase flexibility is typically performed with a PT, ideally with knowledge or work experience with PD issues. Moreover, physical activity in general (e.g., unloading the groceries from the car, watering the plants outside the home, straightening up a room) is movement, and any movement could help reduce muscle rigidity. Many clinical trials use stretching and flexibility exercises as their control group [145,146,147]. Alternatively, stretching exercises are included with other exercises to evaluate the impact of exercise on PD [148]. Overall, stretching and flexibility exercises should be considered part of the daily life of a PwP.
2.6. Review of Systematic and Meta-Analysis Studies of Exercise for Treating Parkinson’s Disease
Flach et al. [149] reviewed UPDRS ratings from aerobic exercise for individuals with PD. From the final list of 245 full-text articles, 53 were ultimately reviewed for this study. They found a statistically significant positive change in UPDRS scores because of aerobic exercise.
Steiger and Homann [150] reviewed 28 studies using different types of exercise (including aerobic exercise, resistance training, balance, and tai chi). PD severity was reported using the Hoehn and Yahr (H&Y) scale, where 24 studies tested PD patients from stage 1 to 3, and four studies included more severely affected PD patients at stage 4. In general, 25 of 28 studies resulted in improved gait, functional mobility, and UPDRS III scores. They recommended that PD patients with H&Y stage 1–3 perform the following exercises: two to three sessions per week of 30 to 60 min at 70–80% HRmax, two sessions of strength training with two to three sets per exercise with 10–15 repetitions, and two sessions of balance or tai chi for 15 min [150].
Choi et al. [151] performed a study using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [152] and Cochrane Handbook for Systematic Reviews of Intervention [153] guidelines. The studies evaluated walking, strength, balancing, and complex exercise. They reviewed 18 exercise studies and found that PD patients who exercised showed significantly improved scores in UPDRS, UPDRS II, and UPDRS III. Overall, they found that exercise therapies improved the overall symptoms of PD, especially motor symptoms, balance, and gait [151].
Schootemeijer et al. [154] performed a scoping and systematic review of the overall health effects of exercise for PwP. They characterized 17 randomized clinical trials, concluding that the benefits of aerobic exercise for treating PD outweigh the potential risks and hazards. They found that aerobic exercise (treadmill walking or cycling on a stationary bike) at moderate-to-vigorous intensity (60–85% HRmax) for 30 min per session and ~4 days/week, generally for H&Y stage 1–3, improved physical fitness in PwP (assessed by VO2 max) and had a beneficial effect on PD motor symptoms (assessed by MDS-UPDRS scores) (non-motor symptoms were not included as primary endpoints); however, the health-related QoL was not improved (assessed using the 39-item Parkinson’s Disease Questionnaire (PDQ-39)) [154].
Gamborg et al. [103] followed the PRISMA guidelines on systematic reviews of RCT studies. They identified 33 studies (18 resistance training, 14 aerobic exercise, and 1 other intensive exercise modality (OITM)) with PwP at H&Y stages ranging from 1 to 4. Resistance training involved using weight machines or free weights. Resistance training was performed at 50–80% maximum effort. Endurance training was performed using a treadmill or stationary bicycle, typically at 60–85% HRmax. The OITM study combined aerobic, strength, balance, and range of motion with stretching exercises. The analysis of UPDRS-III scores revealed that aerobic exercise could improve motor defects in PD. The review of the present work found that resistance training and OITM exercises may improve UPDRS scores. Furthermore, the meta-analysis revealed that resistance training, not aerobic exercise, positively influenced QoL [103].
Martignon et al. [155] provided guidelines for the different stages of PD. They started with 115 publications/reports and used 50 records to generate exercise prescriptions. They used 11 studies for aerobic exercise that included participants with mild to moderate disease (H&Y 1–3). Nine studies were used to measure the effect of resistance training with participants at H&Y disease stages of 1–3. The general recommendation for exercise therapy in early-stage PD was moderate-to-vigorous aerobic exercise for 45 min per session for 3 days/week at 60–89% HRmax and resistance training performed 3 days/week, with an effort of 60–80% [155]. The recommendations for exercise in moderate-stage PD were aerobic exercise for 30–40 min at moderate effort (40–59% HRmax) and resistance training at very light effort (<30%) for 2–3 days/week. In advanced-stage PD, the recommendation for aerobic exercise was daily with light effort (30–59% HRmax) for 20 min or multiple sessions of 10 min, along with 2 days/week of resistance training, again at very light effort (<30% max). Flexibility exercises for all stages of the disease were recommended at 30 min/day [155].
Hao et al. [156] performed a systematic review of ten different exercise programs, including yoga, Taiji Qigong, treadmill training, resistance training, aquatic training, virtual reality training, musical dance training, walking training, cycling training, and Baduanjin Qigong training, on their effect on motor functions in PD. From a total of >6000 documents, they investigated 60 studies. Based on changes in UPDRS scores, they found that dance, yoga, virtual reality, and resistance training significantly improved motor function [156].
Osborne et al. [76] represent the Clinical Practice Guidelines from the American Physical Therapy Association. They reviewed 16 studies (9 high-quality and 7 moderate-quality studies) on the effect of aerobic exercise on PD. Most studies used treadmill walking or stationary cycling, and the participants were at H&Y stages 1–3. The trend suggests that a reduction in motor symptoms (using UPDRS-III scores) was found more frequently in those engaged in vigorous-intensity exercise (75–85% HRmax) than those engaged in moderate-intensity exercise (60–75% HRmax) [76]. Two high-quality and two moderate-quality studies showed improvements in gait-related outcomes, while other high-quality studies showed improvements in balance and activities of daily living. They reviewed 19 high-quality and 27 moderate-quality studies on the effect of resistance training on PD. UPDRS scores were improved in several resistance studies using different exercise procedures. Interestingly, six studies (five high-quality and one moderate-quality) found no difference in disease severity when comparing resistance training to the control group. Several studies endorsed the use of resistance training to improve QoL.
Li et al. [157] systematically reviewed exercise therapy in PD focused on slowing disease progression. They utilized updated PRISMA guidelines [158] and used potential indicators of PD progression, including MDS UPDRS scores, BDNF and TNFα levels, neuroimaging of dopamine receptor binding and other brain structures, and neurophysiological test outcomes. A total of 40 trials were studied and analyzed. This systematic review showed (the evidence was low to very low) that exercise may slow the progression of PD. The evidence was the effect of exercise on lower UPDRS motor scores and improved BDNF levels. Furthermore, exercise normalized brain activation, improved dopamine receptor binding, and reduced bradykinesia. By contrast, exercise did not reduce inflammatory or oxidative stress markers or improve brain volume. They provide numerous strategies for future RCTs to further strengthen and understand the mechanism behind the ability of exercise to slow the progression of PD [157].
Ernst et al. [159] performed a Cochrane Database of Systematic Reviews on the effect of physical exercise on PwP. They studied 156 randomized clinical trials (RCTs) with 7939 participants described as mainly mild-to-moderate disease stage and without cognitive impairment. They found evidence of beneficial effects of exercise on motor signs and QoL, with no apparent preference for the type of exercise. They further comment that PD-specific exercise programs may effectively treat specific motor symptoms [159].
Langeskov-Christensen et al. [160] performed a systematic review and meta-analysis of exercise and PD. They explored whether exercise had a role in preventing the disease (primary prevention), the potential to modify the disease progression as therapy (secondary prevention), and whether exercise is effective for symptomatic treatment (tertiary prevention). Regarding primary prevention, people who participate in moderate-to-vigorous exercise have a reduced risk of developing PD. Regarding secondary prevention, exercise is effective for symptomatic treatment, and when individually developed for the PwP, exercise is very cost-effective for PD therapy. Concerning tertiary prevention, they found that exercise had a disease-modifying effect on PD.
Umbrella reviews synthesize previous systematic reviews covering the same topic [153]. Several groups have performed umbrella reviews on physical exercise and PD [161,162,163,164], which validate the conclusions of the systematic studies and meta-analyses described above. As expected, the umbrella reviews focused on motor and non-motor symptoms of PD and the influence of exercise on improving QoL. The umbrella reviews confirmed that all of the exercise categories examined showed some benefit to PwP and that PwP were encouraged to use exercise to enhance QoL and to improve both motor and non-motor symptoms of PD [161,162,163,164].
The recommended duration and frequency per week of aerobic exercise (given in the next section) are consistent with several studies described above, including the largest exercise study reported [165]. Chekroud et al. [165] examined the mental health link with exercise in a cross-sectional survey of over 1.2 million adults older than 18. They found that individuals who participated in physical exercise for 45 min and three to five days/week had significantly fewer days of poor mental health per month than those not exercising [165].
2.7. Exercise Suggestions
The four types of exercise described here represent fundamental aspects of physical activity (exercise): cardiovascular endurance (aerobic exercise), strength (resistance training), flexibility (stretching/flexibility exercises), and balance/agility (neuromotor therapy). The safety of the patient while exercising is the first and most important concern. Before beginning an exercise program, a comprehensive evaluation by a neurologist and physical therapist, considering the stage of the participant’s PD, prior history of exercising, therapeutic drug usage, and existing medical comorbidities, is crucial in determining what exercises the PwP can safely perform.
Considering several outstanding sources [75,76,97,98,103,104,154,155,160,166,167,168,169,170], we suggest that PwP arrange their weekly exercise routine to include aerobic exercise, resistance training, neuromotor rehabilitation programs, and stretching/flexibility exercises. Martignon et al. [155] recommended exercise parameters for all stages of PD, but most studies/reviews only report parameters for early-stage to moderate-stage PD. Thus, using these resources cited above and from Section 2.6, we present a scalable range of values for aerobic exercise and resistance training for all stages of PD.
From a physical therapy perspective, the focus should incorporate a participant’s most appropriate intensity range for aerobic exercise. This plan is not a one-size-fits-all but a personalized approach considering the individual’s unique needs, ability, willingness to exercise, overall health, and stage of PD. Furthermore, a given PwP may easily reach the lower range of exertion and should be encouraged to exercise at their maximal percentage effort (% HRmax and % effort for aerobic exercise and resistance training, respectively). Alternatively, a PwP (regardless of the PD stage) just beginning to exercise may be best to adhere to the lower ranges recommended until their mastery of these lower levels has been achieved.
Aerobic Exercise—A moderate-to-vigorous intensity of 60–85% individualized HRmax for at least 120–150 min/week (40–50 min a day, 3 days a week) is recommended in early-stage PD. Early-stage PwP should start at 60% HRmax and, over time, aim high to reach 85% HRmax. We encourage moderate-stage and advanced-stage PwP to strive for these moderate-to-vigorous intensity levels. However, for others, moderate intensity of 50–70% HRmax for 120–150 min/week (40 min a day, three days a week, or 30 min a day, five days a week) is advised for moderate-stage to advanced-stage PD [103,154,155].
Resistance Training—It is recommended to implement a progressive resistance training regimen, working up to 60–80% of maximal effort with 3 sets of 10 repetitions two days/week for early-stage PD and 50–69% of maximal effort with 1 to 3 sets of 10 repetitions two days/week for moderate-to-advanced-stage PD. These exercises are designed to improve muscle strength and function, which are often affected by PD [98,103,155,167].
Neuromotor Exercises—Patients with PD can significantly improve their quality of life (QoL) by incorporating the neuromotor exercise routines/programs they enjoy.
Stretching and Flexibility Exercises—It is important to include stretching and flexibility exercises in this routine. These exercises are designed to help reduce rigidity and are essential to a comprehensive exercise plan.
Figure 3 and the details above are a guide to help the PwP and Care Team (physicians and physical therapist) develop a personalized exercise plan. When followed diligently, such a plan can, over time, reduce symptoms and significantly improve the QoL for patients with PD. Please refer to these notable references for a more complete explanation of the guidelines for exercise in PD [75,76,97,98,103,104,154,155,160,166,167,168,169,170].
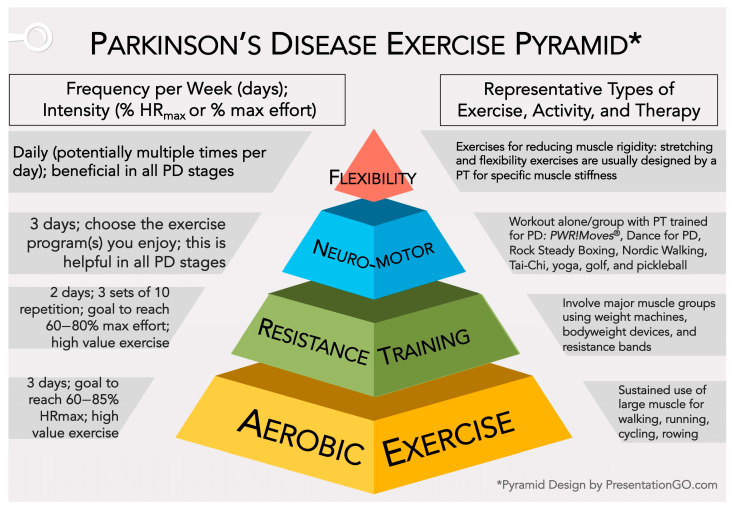
Figure 3.
Parkinson’s disease exercise pyramid. The pyramid consists of four types of exercise: aerobic exercise, resistance training, neuromotor therapy, and stretching/flexibility exercises. On the left side are the recommended days per week, a suggested % HRmax for aerobic exercise and a % max effort for resistance training (given for a participant with early-stage PD; please see the text for suggestions at other stages of PD). On the right side is a description of the kind of exercises best represented by each category.
2.8. Strategies for Overcoming Barriers to Exercise for People with Parkinson’s Disease
Rafferty et al. [171] identified a cohort in the National Parkinson Foundation Quality Improvement Initiative (NPF-QII) that showed in a two-year study that early exercisers have improved outcomes compared to sedentary individuals. Therefore, it is prudent that sedentary individuals who receive a diagnosis of PD are referred to a physical therapist who specializes in PD to initiate an exercise routine specific to the individual. However, there is always time to start. Other studies have also demonstrated that when individuals with PD who have been sedentary become habitual exercisers, their QoL declines at a slower rate as compared to peer groups who remain sedentary [89]. Furthermore, with time, their brains will make adaptations that begin to look more like the brains of habitual exercisers [89].
2.8.1. Reworking Aerobic Exercise
Research supports moderate-to-vigorous-intensity aerobic exercise as a strategy for improvement in motor and non-motor symptoms of PD, with the potential for vigorous-intensity exercise to have a more significant effect on slowing motor disease progression [67,76]. However, some individuals with PD may face barriers that limit their ability to participate in vigorous-intensity aerobic exercise at the recommended frequency and duration. These factors may include medical comorbidities, mobility, cognition, motivation, and environmental considerations. The potential for improvement in symptoms, overall health, and QoL with aerobic training warrants participation in whatever the individual can do safely [168,172].
Most studies examining the effects of aerobic exercise exclude individuals with advanced PD, atypical Parkinson’s disease, and Parkinsonism [171]. These populations may experience complicating factors including, but not limited to, mobility challenges requiring the use of an assistive device, cognitive challenges, or reliance on a caregiver. Although greater disease severity has been associated with increased inactivity, this is not the case for all individuals with advanced disease [173]. It is proposed that individuals with increased symptom progression may still benefit from cardiovascular exercise with modifications for safety [89].
For example, PwP with balance challenges can improve the safety of aerobic exercise by choosing exercise equipment with a seated option. This could include a recumbent bike, upper body ergometer, or even a NuStep with a chair that swivels to improve ease of transfers for individuals in a wheelchair.
Individuals with mild cognitive issues may benefit from using a group class, coach, or external cues such as a pace partner in which the participant tries to keep up with a certain pace (this feature is available on some, but not all, pieces of cardiovascular equipment). PwP with more significant cognitive impairments may require increased supervision from a caregiver to achieve moderate intensity levels.
PwP with comorbidities such as hypertension, congestive heart failure, atrial fibrillation, or renal disease (among others) may not be appropriate for high-intensity aerobic exercise. However, aerobic exercise at moderate intensity (50–70% HRmax) or submaximal levels may be helpful for these conditions to improve function and overall conditioning [174]. A physical therapist or medical doctor may guide individuals in this category.
When determining exercise intensity, the percentage of HRmax may not be the most appropriate measurement for all PwP. For example, heart rate response may be blunted in individuals who are taking beta blockers. Furthermore, individuals who experience autonomic dysfunction may not demonstrate an adequate rise in heart rate with exercise [175]. For these individuals, using the Borg Rate of Perceived Exertion can serve as an appropriate alternative [176,177]. A goal of 7–8 out of 10 would correlate with vigorous activity and can be utilized as a target goal for individuals in which vigorous exercise is appropriate.
Individuals diagnosed with PD are more likely to experience a decline in physical activity related to changes in physical ability, mood, or energy levels [168]. It is not uncommon for this population to experience deconditioning; therefore, it is vital to progress gradually when initiating a cardiovascular program to improve QoL, particularly for those with a sedentary background [75,178].
2.8.2. Revisiting Resistance Training
Potential barriers to resistance training include a lack of gym access, mobility challenges, and inexperience. Resistance training will benefit from modifications tailored to the individual’s needs or level of disease progression to improve participation and safety. For example, PwP with balance challenges will find seated resistance machines safer than free weights. Individuals primarily in wheelchairs may utilize ankle weights or resistance bands as part of a seated strengthening program.
PwP with inexperience will find guidance from a physical therapist or an exercise instructor with additional training in PD. Working with a personal trainer may be appropriate for individuals who may not have access to an exercise professional specializing in PD, particularly for those with earlier onset or mild presentations of PD. Individuals with moderate to advanced stages of PD will benefit from the direction of a physical therapist. For PwP who do not have access to a gym, there are various online membership programs designed for individuals with PD that one can complete from home.
As PD progresses, rigidity in the flexor groups can lead to weakness in the extensor muscle groups over time. Targeting muscles that support antigravity extension (knee extensors such as quads, hip extensors such as glutes, and trunk extension) can also improve posture.
Resistance training is not mutually exclusive to aerobic exercise. Resistance training can elicit heart rates in the target heart rate zones when performed as a high-intensity circuit. This can be achieved by choosing two or more exercises and rotating between them instead of taking a rest break between sets. By rotating exercises, one muscle group has time to rest before being rechallenged, but the body is challenged with continuous movement that may address endurance or achieve aerobic exercise components.
2.8.3. Adaption of Neuromotor Exercises: Agility and Balance
Of all the domains for exercise, agility, balance, and related activities may require the greatest consideration to ensure safety and an appropriate level of challenge. Individuals who are earlier in their progression and not at risk for falls will benefit from maintaining their current ability level. These activities will ideally include multidirectional stepping, stopping, and starting and can range from tennis, pickleball, ping pong, dancing, golf, bowling, and tai chi to PWR!Moves group exercise classes. The diversity of options ensures one can find something that excites and motivates. Li et al. [179] reported that tai chi training improved motor function, gait, and balance, as measured by the UPDRS and other parameters.
Individuals at risk for falls will benefit from a physical therapist’s guidance to establish a safe balance and agility practice for the participant. For example, individuals at mild-to-moderate risk for falls may benefit from modifications to use handheld support for multidirectional stepping. This could include TRX straps, a ballet bar, a sturdy chair, or a doorway. Instead of free dance, partner dance may offer a fun and engaging alternative. Support of an exercise professional specializing in PD can also assist in maintaining safety during supervised exercise.
PwP at moderate-to-high risk for falls require the greatest attention to safety considerations. These individuals should ensure someone is present and incorporate extra precautions to perform exercises safely. For example, they may practice weight shift, marching, or multidirectional stepping with the support of their rollator while standing in front of a sturdy chair or standing at the counter with a family member close by; both scenarios consider the potential for loss of balance.
Individuals with advanced Parkinson’s who are primarily in a wheelchair or have cognitive changes will still benefit from balance training (if it can be performed safely) with a trained caregiver present. These individuals may still have to stand and turn to transfer to a commode, chair, or bed—activities that can be susceptible to falls. An example of an appropriate balance task could be standing in front of a chair with a walker while practicing weight shift with the caregiver using a gait belt.
2.8.4. Refining Flexibility
Current guidelines recommend flexibility training 3×/week [180] or are unclear on the ideal dose [76]; however, optimal effectiveness may be achieved with daily training to address rigidity and improve range of motion. Given these guidelines and the need for further research, there are many choices for practical ways to incorporate flexibility training into daily practice. This could involve a daily mobility routine, activities such as yoga, or adding stretching to cool down for a different workout during the week.
Individuals with mobility challenges, particularly those who rely on an assistive device or wheelchair, may be challenged to incorporate flexibility training. However, mobility training is particularly important for these individuals to prevent contractures that can occur with prolonged sitting. Modifications to positioning can be utilized to improve accessibility. For example, stretches can be completed in a seated, supine, or prone position. An activity as simple as lying flat with arms stretched overhead or laying on one’s stomach can be an effective strategy to improve extension.
2.9. Examples of a Structured Exercise Strategy for Parkinson’s Disease
It is essential to consult a doctor or physical therapist before initiating a new exercise program [75], particularly for individuals new to exercise or with medical comorbidities that include, but are not limited to, cardiac disease, diabetes, or renal disease. According to the CDC, an increase in physical activity is beneficial over a sedentary lifestyle in the general population [168]. However, structured exercise beyond general activity should be encouraged for PwP due to the potential for further improvement and management of motor and non-motor symptoms. Furthermore, there is potential to modify exercise programs to tailor toward the individual’s needs, interests, and stages of disease progression. In an extensive examination of exercise testing across all stages of PD, Martignon et al. [155] recommend that PwP should engage in regular exercise and emphasize following their personalized exercise plans.
Table 1 highlights several examples of exercise programs that are unique to the individual, taking into consideration previous exercise history, current level of function, motivation, and interests. Although a future focus of this paper aims to discuss the role of exerkines specific to aerobic exercise and resistance training, Table 1 also includes examples of balance, agility, and flexibility training, widely accepted as integral components when using exercise to treat PD [180]. It is important to note that these are examples; more research is needed to determine best practice recommendations for various states of disease progression and patient-specific exercise recommendations.
Table 1.
Representative exercise programs for people with Parkinson’s disease.
| Individual Profile | Aerobic Exercise | Resistance Training | Neuromotor Rehabilitation Programs | Flexibility/Stretching Exercises |
|---|---|---|---|---|
| Eighty-year-old male with advanced PD, ambulates with a rollator and requires caregiver support. He experiences some “off times” in which his medication becomes less effective. He is highly motivated with a great family and friend network. | Rides a NuStep or recumbent bike for 40 min 3×/week. Target HR of 60–70% HRmax | Participated in a seated strengthening program with use of resistance bands for upper body and supervision of a caregiver 2×/week. Completed during “on time” of medication cycle. | Currently receiving PT once every two weeks for maintenance PT. Also receives restorative PT periodically throughout the year. Goal of working on weight shifts with his rollator alongside a trained family member 3×/week. |
Participates in daily stretches in his bed before getting up in the morning. Goal of taking a Dance for PD class online 1×/week. |
| Retired 70-year-old female with short term memory loss who enjoys staying active so she can spend time with her children and grandchildren | Walks 1–2 miles every day (when it is not raining). Target RPE for walks is 6/10. | Goes to the gym 2×/week and uses resistance machines after participation in exercise classes. | Receives restorative PT approximately 1–2×/year. Goal of taking 1–2 classes at her gym each week. (Varies between PWR!Moves, tai chi and Dance for PD) Sometimes her daughter will join her. |
Participates in 10 min of home stretches as instructed by PT 5×/week. |
| Sixty-five-year-old female who was diagnosed one year ago. She has lived a lifetime of being active and is very willing to try to explore exercise options. She is still working and will retire in 1–2 years. | Walks on a treadmill 3×-week with goal of 75% max HR and plays tennis 2×/month | Works out in her home gym using free weights 2×/week | Participates in benchmark assessments 2×/year and restorative PT as needed Participates in a mix of the following activities 1–2×/week: tennis, tai chi, golf, PWR!Moves classes |
Incorporates 15 min of stretching as a cool down after her resistance workouts, 2×/week |
| Recently diagnosed retired 76-year-old male with high blood pressure (controlled with medication) who is relatively sedentary, resistant to begin exercising but willing to give it a chance. He checks his BP daily. | Walks his family dog 3×/week for 20 min. He is working towards a goal of walking for 25 min. | Goal of participating in Rocksteady boxing 1×/week, Goal of completing body weight resistance exercises 2×/week during the commercial breaks of a television show he watches every day | Referred to PT to help establish an exercise program and address balance. PT will also instruct on modifications as needed to improve safety with exercise in individuals with high blood pressure. | Goal of incorporating 10 min of stretches after he gets up in the morning Goal of completing PWR!Moves seated mobility exercises 3×/week during the commercial breaks of a television show he watches every day |
| Retired 70-year-old male originally diagnosed at age 59 who experiences right side stiffness, right arm tremor, controlled with Carbidopa/Levodopa, NeuPro patch; follows an integrated approach to treatment. | Rides his Peloton bike for 45 min with goal of 80% HRmax 3×/week; 3–5 mile power walk 1×/week | 30–45 min weightlifting on major muscle groups 2×/week | LSVT BIG, and regularly works with a PT for any sports-related injury | Daily stretching, and 2× week aim for PWR!Moves or RockSteady Boxing. Participates in golf 2–3×/week. |
| Forty-one-year-old female who was diagnosed at age 39. She has two children ages 10 and 12 and is still working. She was previously active but not a formal exerciser. Over the past two years has worked with her healthcare team to start an exercise program that emphasizes aerobic components. | She initially used the couch to 5K program to begin running. Now participates in a running group and runs 3–5 miles 2–3×/week with target HRmax of 80%, also walks 20–30 min 2–3×/week. (She will often walk during her children’s soccer practice.) | Goes rock climbing with her son 2×/month. Goal of participating in 30 min of strength training 2×/week. She uses an online fitness membership to guide her workouts. | She worked with a PT to initiate an exercise program and participates in benchmark testing 2×/year. Returns to PT as needed to adjust exercises. Goal of participating in yoga 2×/week in addition to running. |
Goal of completing a 15 min yoga video 2×/week. Goes rock climbing with her son 2×/month. |
2.10. The Effect of Exercise on the Central Nervous System (CNS) and Motor Unit in Parkinson’s Disease
Historically, exercise has improved physical fitness. Currently, exercise is used to alter the progression of PD and other neurodegenerative diseases. As research evolves, the critical relationship between exercise, CNS health, and motor unit function becomes increasingly evident.
2.10.1. Exercise Enhances the Function of the CNS
Morgan et al. [181] reviewed the effect of exercise on CNS functions in murine models. Specifically, they examined the impact of exercise on circadian rhythm, central metabolism, cardiovascular function, stress responses in the brain stem and hypothalamic–pituitary axis, and movement. Of central importance to PD, exercise was found to induce adaptations in the basal ganglia. Moderate exercise showed changes in oxidative stress markers in the basal ganglia and increased production of striatal tyrosine hydroxylase, leading to the production of L-DOPA, the immediate precursor of dopamine. Moderate-to-vigorous intensity exercise also increased striatal BDNF (an exerkine to be discussed further in Section 3), a survival factor for dopaminergic neurons. Contrasting results were found in the basal ganglia as vigorous exercise disrupted vital signaling pathways in the striatum; however, it also increased striatal dopamine (D2) receptors. They conclude that exercise induces numerous changes in the brain stem, hypothalamus, and basal ganglia; further studies exploring exercise’s role throughout the lifespan would be critical [181].
Liu et al. [13] reviewed the brain’s beneficial responses to exercise. Exercise’s physiological impact can be found in the induction of neurotropic factors and neurotransmitters, the impact of myokines expressed from skeletal muscles, the reduction in neuroinflammation, enhanced mitochondrial health, and the ability of exosomes (small phospholipid vesicles) to facilitate the transport of exerkines across the BBB. They noted that exercise improved executive dysfunction, cognitive performance, and psychiatric problems; however, vigorous exercise was required to improve motor function in PD [13].
2.10.2. Exercise Improves Motor Function
Perrey [182] provided an overview of how exercise positively altered motor system function. Restoring motor system function is a key goal for exercise and PD. The motor nervous system controls voluntary movement; it consists of the brain, spinal cord, and the nerves that connect these nervous system structures to the effector muscles. Moderate-intensity exercise, taking place over a sustained time period, was the most neuroprotective of motor systems [182].
Lavin et al. [183] evaluated a 16-week high-intensity resistance exercise program to gauge rehabilitation in PwP. The PD participants showed significant improvement in muscle mass, motor scores (from UPDRS), strength, power, motor unit activation, and several other indices of neurological function. They generated transcriptome-wide skeletal muscle libraries in PwP before and after the exercise program. They detected 304 genes related to remodeling and nervous system/muscle development that were upregulated and found downregulation of 402 genes that were primarily negative regulators of muscle development. These results imply that exercise promotes the remodeling of damaged motor units to improve motor function [183].
Palasz et al. [66] summarized that exercise in animal models of PD is protective and promotes the recovery of motor function. They found that exercise in animal models of PD could protect dopaminergic neurons, prevent further loss, and promote their restoration. The likely pathway of renewal was by neurotrophin activation, stabilization of intracellular calcium levels, upregulation of anti-oxidative substances, and the suppression of pro-inflammatory cytokines. Furthermore, exercise improved motor unit circuitry. Unfortunately, animal models of PD do not fully replicate PD since neurotoxins are the frequent mode for creating the clinical syndrome characterized in the mouse model of PD. Finally, progress in understanding PD can be advanced if common neuroprotective processes exist in human PD and animal models of PD [66].
Kelly et al. [184] studied the effect of resistance training on motor unit remodeling in PD. PD is an age-related neurodegenerative disease, and PwP have a more significant motor unit loss and disruption compared to age-matched peers without PD. They found that PwP had an unusual group of type I myofibrils and further reported that the advancement of these type I myofibrils led to disrupted motor unit recruitment. However, they describe that resistance training could reverse these dysfunctional motor units [184].
2.10.3. Long-Term Effect of Exercise on Parkinson’s Disease
Reiner et al. [185] performed a systematic review of the long-term (>5 years) relationship between physical activity and weight gain, obesity, coronary heart disease, type 2 diabetes mellitus, Alzheimer’s disease, and dementia. Their results imply that physical activity has a positive long-term influence on all selected diseases [185].
Li et al. [186] describe the global effect of exercise on PD by examining images of the brain. They found that exercise activated the cerebellum, occipital lobe, parietal lobe, and frontal lobe, suggesting that exercise improves PD due to changes in multiple regions of the brain.
Tsukita et al. [187] studied the long-term influence of exercise in early-stage PD. They found that at baseline, regular physical activity and moderate-to-vigorous intensity exercise did not affect the clinical progression of PD. Over time, continued participation in regular physical activity and moderate-to-vigorous exercise was found to slow changes in postural and gait stability, daily living activities, and processing speed. They conclude that maintenance of regular physical activity and moderate-to-vigorous intensity exercise levels provided a better clinical course of PD [187].
2.11. Summary of Exercise and Introduction to Exerkines
As presented in this section, aerobic exercise and resistance training can slow the trajectory of PD progression, while neuromotor programs and flexibility/stretching exercises contribute to an improved QoL. In the next section, we describe that physical activity reduces the risk of developing PD. Part of the successful benefit of exercise in preventing PD likely arises due to the generation and release of exerkines. Thus, the remainder of the following section describes some neuroprotective exerkines.
3. Exercise-Induced Neuroprotective Exerkines
“Natural forces within us are the true healers of disease.” Hippocrates (460–375 BCE) [2]
3.1. The Association between Cardiorespiratory Fitness and the Risk of Parkinson’s Disease
Numerous studies have measured the relationship between cardiorespiratory fitness and the risk of PD. One study reported that a higher level of physical fitness reduces the risk of PD after adjustment for age and smoking [188]. Separately, the risk of PD was reduced in the patient population of most baseline recreational activities [189]. In a test population of >40,000 people, a medium level of physical activity lowers the risk of PD [190]. Furthermore, there was a decreased risk of PD in patients with heavy leisure-time physical activity compared to no physical activity [191]. By contrast, not every study found an effect of physical activity on the risk of developing PD [192]. Although there are various ways in which physical activity and cardiorespiratory fitness are determined, most studies suggest that moderate-to-vigorous-intensity physical activity reduces the risk of PD [160].
3.2. Neuroprotective Exerkines
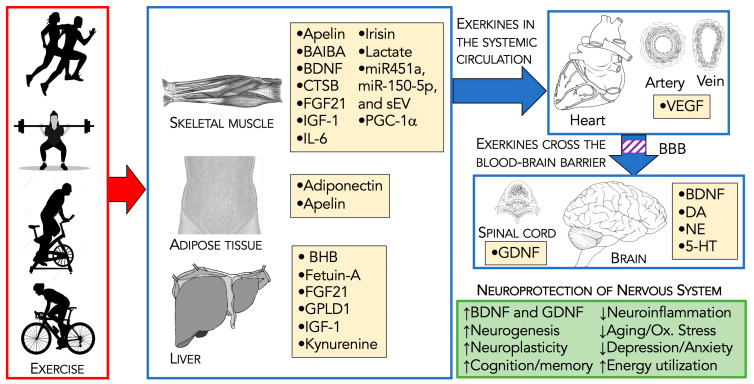
Safdar et al. [26] introduced the term exerkines to represent substances released due to exercise. The ultimate goal of exerkines is to unify endocrine, autocrine, and paracrine processes, at a cross-talk between organs, tissues, and physiological systems with the CNS/brain [193]. The function of exerkines following exercise demonstrates that exercise has the potential to be disease-modifying, which can lead to a healthier brain [13,23,194]. Table 2 and the narrative below show a partial list of neuroprotective exerkines. The exerkines are presented in terms of tissue of origin, target tissue, receptor, and signaling, and they are linked to normal brain health or are related to neurodegenerative diseases/PD.
Table 2.
Overview of some exerkines with neuroprotective properties.
| Name | Source Tissue(s) | Target Tissue(s) | Example(s) of Biological Action | Reference(s) |
|---|---|---|---|---|
| Adiponectin | WAT | Brain, heart, liver, muscle | Anti-inflammatory, glucose and fatty acid metabolism | [195,196,197,198] |
| Apelin | Skeletal muscle, WAT | Brain, bone, skeletal muscle | Anti-apoptotic | [48,199] |
| BAIBA | Skeletal muscle | Many sites | Metabolic regulator, anti-inflammatory, anti-oxidative | [200,201,202] |
| BHB | Liver | Many sites | Improves fuel utilization, anti-inflammatory | [203,204,205] |
| BDNF | Brain, skeletal muscle | Brain | Enhances synaptic plasticity, and cell growth | [206,207,208,209] |
| CTSB | Skeletal muscle | Brain | Neurogenesis, memory | [210,211] |
| Fetuin-A | Liver | Liver, brain | Neuroprotective, anti-inflammatory | [212,213,214] |
| FGF21 | Many tissues, liver, WAT | Many tissues, heart, bone, gut, brain, WAT | Improves fuel utilization (glucose, lipid) | [215,216,217] |
| GDNF | Spinal cord | Brain | Maintenance of spinal motor neurons and midbrain dopaminergic neurons | [218,219,220] |
| GPLD1 | Liver | Brain | Improves neurogenesis and cognition | [221,222] |
| IGF-1 | Liver, skeletal muscle, and other tissues | Brain, many sites | A mediator of brain health following exercise including neurogenesis and cognitive improvement | [223,224,225] |
| IL-6 | Skeletal muscle | Many sites | Energy sensor, anti-inflammatory in brain | [226,227,228] |
| Irisin (FNDC5) | Skeletal muscle | WAP, bone, β-cells, brain |
Promotes neuronal health | [229,230,231] |
| Lactate | Skeletal muscle | Many tissues, including CNS | Brain fuel source | [232,233,234] |
| Lac-Phe | Epithelial cells in gut and kidney | Many tissues, including CNS | Suppresses feeding and obesity | [235,236,237] |
| mIR451a/mIR-150-5p/sEV | Skeletal muscle and other tissues | Many tissues including CNS | Protects against depression (miR-451a) and anxiety (miR-150-5p) | [238,239,240] |
| Monoamine neurotrans-mitter (DA, NE, 5-HT) | Brain | Brain | DA—Motor control, learning, executive function; NE—stress resistance memory, cognition; 5-HT—relieve anxiety, stress protection, cognition | [241,242,243] |
| PGC-1α/ Kynurenine |
Skeletal muscle, liver, and other tissues | Skeletal muscle, and brain | PGC-1α activates kynurenine aminotransferase, switching the ratio of kynurenine (neurotoxic) to kynurenic acid (neuroprotective) | [244,245,246] |
| VEGF | Skeletal muscle and other tissues | Brain endothelium and other tissues | Promotes angiogenesis and exercise-induced neurogenesis | [247,248,249] |
3.2.1. Adiponectin
Adiponectin is mainly produced by white adipose tissue (WAT). The “gut–brain” axis interacting with adiponectin has been implicated through gut microbiota [198]. The consequences of adiponectin with its target receptors (Adipor1, AdipoR2, and T-cadherin) result in the activation of several key signal transduction pathways, most notably, AMPK, p38 MAPK, and the activation of PPARα [197]. AMPK is activated by physiological signals that indicate a negative energy balance. p38 MAPK is expressed in the brain and has functions focused on learning and memory, and it plays a vital role in synaptic regulation and function. PPARα is involved in the brain’s glutamate homeostasis and cholinergic/dopaminergic signaling. Metabolically, adiponectin is involved in glucose and fatty acid metabolism when upregulated from exercise [195]. Adiponectin has anti-inflammatory action, and there is substantial evidence that adiponectin modulates immune cell responses in both the innate and adaptive immune responses (supporting the activation of M2 anti-inflammatory macrophages) [250,251]. A dysfunction/deficiency of adiponectin with the gut–brain interface could predispose someone to a potential problem favoring the development of PD [252].
3.2.2. Apelin
WAT and skeletal muscle following exercise principally synthesize apelin [48,253]. APJ is an orphan GPCR that binds the ligand apelin [199]. Apelin/APJ interaction signals an essential set of pathways, including PI3K/AKT/mTOR, ERK 1/2, and inositol, requiring kinase 1α/XBP1/C/EBP homologous protein [254]. PI3k/AKT/mTOR activation supports the survival and development of dopaminergic neurons. In contrast, the activation of ERK 1/2 induces changes in the geometry of the nucleus in response to neuronal activity in hippocampal neurons. Apelin/APJ interaction promotes several neuroprotective events, including antioxidant and antiapoptotic action, helping preserve neuronal cell loss, reduce striatum neuroinflammation, and enhance excitotoxicity [199].
3.2.3. Beta-Aminoisobutyric Acid (BAIBA)
BAIBA is formed in skeletal muscle from an amino acid (valine) and a nucleic acid (thymine) [200,201,202]. This unusual metabolite is released during exercise, and BAIBA participates in glucose [255], lipid [256], and bone metabolism [257]. Interestingly, BAIBA helps down-regulate inflammation [258]. Furthermore, BAIBA helped reverse reactive oxygen species (ROS)-driven oxidative stress in mitochondria [259]. BAIBA levels were substantially increased in people performing an acute exercise routine compared to baseline [260]. The ability of BAIBA to protect mitochondria undergoing oxidative stress, combined with a potent leukocyte-derived anti-inflammatory reaction, suggests that BAIBA would offer considerable possibility to develop new/novel strategies to slow down the progression of PD.
3.2.4. Beta-Hydroxybutyrate (BHB)
BHB is synthesized in the liver from fatty acids [203]. Systemically, BHB is an essential energy carrier from the liver to peripheral tissues. BHB can cross the blood–brain barrier, providing energy to neurons where glucose is reduced. BHB binds to at least two cell surface G-protein-coupled receptors, HCAR2 and free fatty acid receptor 3 [204,261]. HCAR2 is also expressed in various other cell types, including immune cells, microglia, and colonic epithelial cells, in which its activation induces anti-inflammatory effects [204]. BHB’s anti-inflammatory role was shown by inhibiting the inflammasome NLRP3 in innate immunity [262]. In the CNS, BHB is partially associated with neuroprotection mediated by the induction of BDNF in cerebral cortical neurons [263]. BHB/HCAR2 activation of Ly-6CLo monocytes and/or macrophages provides a signal to the brain for neuroprotection [204].
3.2.5. Brain-Derived Neurotrophic Factor (BDNF)
BDNF is produced in the brain and skeletal muscle [48]. Moreover, from exercise, the skeletal-muscle-derived BDNF crosses the blood–brain barrier [206]. In a scenario where the degeneration of brain tissue has occurred to regrow/repair/rejuvenate neurons, BDNF fulfills a role. BDNF increases cell growth and survival, and BDNF also enhances synaptic plasticity. BDNF binds to its receptor, tropomyosin receptor kinase B (TrkB) [264]. Upon activation of TrkB, several small G proteins, including Ras, MAP kinase, PI3-kinase, and phospholipase-C-γ pathways, are upregulated, leading to neural plasticity, neurogenesis, stress resistance, and cell survival [265,266]. This suggests the comparative flexibility of TrkB in terms of pro-survival function. Not surprisingly, decreased levels of BDNF are associated with neurodegenerative diseases with neuronal loss, most notably PD, AD, multiple sclerosis, and Huntington’s disease [267]. Furthermore, reduced levels of BDNF are linked to cognitive deficits in PD [268]. By contrast, Paterno et al. [269] found that increased exercise intensity resulted in higher levels of BDNF in blood serum from PwP.
3.2.6. Cathepsin B (CTSB)
CTSB is a proteolytic enzyme secreted during exercise from skeletal muscle. Although it has a catabolic function in muscle by enabling the repair of damaged muscle, CTSB can cross the blood–brain barrier [210,211]. Moon et al. showed that CTSB led to increased levels of Bdnf mRNA and BDNF protein expression [210]; however, CTSB did not alter hippocampal cell proliferation. Furthermore, they reported that another protein is upregulated, specifically doublecortin, which has known neuroprotective activity during neuronal migration [210].
3.2.7. Fetuin-A
Fetuin-A is a hepatokine that primarily reduces inflammation in the liver to protect against liver damage [212,213,214]. Substantial research has shown that fetuin-A is a multifunctional protein with many important physiological and pathological roles. Blood-borne fetuin-A can cross the blood–brain barrier and was shown to be upregulated and neuroprotective in a mouse model of TBI [270]. In this model, fetuin-A activated Nrf2/HO-1, suppressing oxidative stress and necroptosis levels and attenuating the abnormal inflammatory response following TBI [270]. In a separate study, fetuin-A levels were reduced in human and mouse Purkinje cell PD samples [271]. Fetuin-A is a multi-tasking exercise with the primary regulator of essential cytoprotective responses in the brain, upregulating genes that can possess anti-oxidative, anti-inflammatory, and detoxifying proteins [272].
3.2.8. Fibroblast Growth Factor 21 (FGF21)
FGF21 is primarily synthesized in the liver [215]. FGF21 is a hepatic signal that induces food intake and improves several metabolic parameters, such as energy expenditure and BAT thermogenesis, mainly by its action in the hypothalamus [217]. FGF21 binds to the co-receptors FGF receptor one and β-Klotho [273]. Pre-existing diabetes is a negative risk factor for developing PD, which leads to dysregulated glucose and lipid metabolism in PD. Boosting levels of FGF21 resulted in a reduction in proinflammatory cytokines and improved fuel utilization (glucose and lipid) [274]. Although FGF21 is a liver protein, it directly influences the nervous system to help regulate energy homeostasis, glucose and lipid metabolism, and insulin sensitivity.
3.2.9. Glial Cell Line-Derived Neurotrophic Factor (GDNF)
GDNF is a crucial participant in developing and maintaining mid-brain dopaminergic and spinal motor neurons [218,219,220]. The Ret proto-oncogene is the signaling receptor for GDNF; however, receptor activation requires an accessory protein, GDNF family receptor α1 [218,219,220]. After engaging the receptor, GDNF promotes the survival of many types of neurons. Notably, GDNF is reduced in the substantia nigra of PD patients [275]. Short-term exercise increased GDNF levels in the spinal cord [218]. GDNF has both neuroprotective and neurorestorative potential in treating PD [275].
3.2.10. Glycosylphosphatidylinositol-Specific Phospholipase D1 (GPLD1)
GPLD1 is a phospholipase that cleaves GPI-anchored cell-membrane proteins. There has been much work trying to reverse or slow the effects of aging using systematic processes like exercise [276,277]. Another approach is transferring blood plasma from young to aged animals [278]. The goal was to compare exercise and young blood plasma to improve the regenerative process and cognition in the aged brains of old animals. Horowitz et al. [221] identified GPLD1 as the critical component in the blood that transfers the effects of exercise on adult neurogenesis and cognition to sedentary aged mice. Exercise increased the levels of GPLD1 detected in mouse blood, which was similarly correlated in active older humans [221]. Horowitz et al. [221] then performed in vivo transfection of GPLD1 into mice and measured increased levels of GPLD1 in plasma, which increased neurogenesis and BDNF expression in old mice. They also demonstrate the importance of this liver-to-brain axis in improving age-related regenerative and cognitive changes [221].
One system proposed to be modified by GPLD1 was the GPI-anchored uPAR of the plasminogen-urokinase protease system [221]. A physiological regulator of the plasminogen/uPA/uPAR complex is PAI-1 [279,280]. We recently proposed a detrimental link between PAI-1 and its upregulation by neuroinflammation, which would reduce the cleavage of α-synuclein (which forms Lewy Body inclusions) by plasmin as part of the pathological process of PD [281]. Exercise reduces PAI-1 levels and, when combined with exercise, increasing GPLD1, suggesting a novel pathway to potentially reduce the impact of neuroinflammation on the progress of PD. However, further in-depth studies are needed to advance this hypothesis.
3.2.11. Insulin-like Growth Factor-1 (IGF-1)
IGF-1, a neurotrophic factor, is produced in the liver, skeletal muscle, and other tissues [223,224,225]. Along with BDNF and VEGF, IGF-1 is a critical factor for brain health [224]. In this context, IGF-1 mediates the effects of exercise on brain health, protecting against brain injuries and improving memory and cognitive functions [282]. Muscle hypertrophy (i.e., resistance training) [283,284] and aerobic exercise [285] can increase levels of IGF-1 into blood following exercise. IGF-1 binds to the IGF-1 receptor on the cell surfaces of targeted tissues. Ligand binding to the α subunit of the receptor leads to a conformational change in the β subunit, activating receptor tyrosine kinase activity [286]. IGF-1 protects the nigrostriatal pathway in PD by activating essential pro-survival signaling cascades [287]. A reduction in brain insulin/IGF-1 signaling may be part of the complex pathophysiology of PD, which would support the use of anti-diabetic drugs currently being studied for their potential disease-modifying properties in PD [288].
3.2.12. Interleukin-6 (IL-6)
IL-6 is one of the most well-studied inflammatory cytokines, and some IL-6 is synthesized and released from skeletal muscle during exercise [226,227,289]. Physiologically, the role of this IL-6 is to balance lipolysis. Furthermore, and of interest to the brain, IL-6 can penetrate the blood–brain barrier and behave as an anti-inflammatory agent. The binding of IL-6 to IL-6R/gp130 promotes the signaling activity of this cytokine [290]. The production of IL-10 and IL-1 receptor antagonists primarily drives the anti-inflammation action of IL-6. Furthermore, skeletal muscle IL-6 is more closely linked to metabolism than inflammation [291], partly due to the absence of NF-kB activation. It was shown that when glycogen levels are low, contracting muscle increases IL-6 levels. Thus, from exercise, IL-6 appears to be an energy sensor that is partly regulated by muscle contraction.
3.2.13. Irisin (FNDC5)
Irisin is the proteolytic fragment of fibronectin type III domain-containing protein 5 (FNDC5), a type I transmembrane glycoprotein [229]. FNDC5 is produced in skeletal muscle from exercise, and irisin is released into the circulation. Physiologically, irisin (FNDC5) regulates energy metabolism by the transcriptional-peroxisome-proliferator-activated receptor gamma (PPARγ) coactivator 1α (PGC-1α)/FNDC5 pathway by activating thermogenic function in adipose tissue, mitochondrial biogenesis, and other gene-programming functions [229]. PGC-1α is a key regulator of mitochondrial quality control and energetic metabolism [292]. Interestingly, exercise induces both FNDC5 and BDNF expression in the brain, again dependent on PPARγ/PGC-1α [231]. Zhang et al. [293] studied a group of PD patients with a 12-week exercise program. The exercise PD group had elevated serum irisin levels and improved balance scores. They found that irisin protected mitochondria from oxidative stress [293]. Mechanistically, irisin activates Akt and ERK 1/2 signaling pathways, which reduces apoptosis in mitochondria with improved biogenesis [293]. These results with irisin are promising for further studies on treating PD.
3.2.14. Lactate
Lactate is produced during exercise in skeletal muscle. One localized effect of lactate in newly exercised muscle is a reduction in the pH of the blood to help more rapidly re-oxygenate the muscle tissue [232,233,234]. In a different role, lactate acts as a signaling molecule in the brain by stimulating the generation of new mitochondria [294]. Lactate is a “volume transmitter” in the brain, and its receptor GPR81 (also known as HCA1 or HCAR1) promotes lipid storage in the mammalian brain [295]. Lactate is a volume transmitter in brain tissue because it distributes cellular signals supporting large neuronal ensembles [296]. It is interesting to speculate that not only is lactate fueling the brain but the lactate/GPR81 interaction may pave the path of neuroprotection by then activating downstream signaling pathways (for example, PI3k/AKT/mTOR and ERK 1/2) [294,295].
3.2.15. Lac-Phe
Li et al. [235] began their search for exerkines by performing an exercise-induced metabolomics assessment of blood plasma in mice. One of the most significantly induced non-targeted metabolites was shown to be N-lactoyl-phenylalanine (Lac-Phe) [235]. They then reported that the enzyme responsible for the synthesis of Lac-Phe, CNDP2 (cytosolic non-specific dipeptidase, also known as carnosine dipeptidase 2), was found predominantly in epithelial cells and macrophages [235]. CNDP2 is found in the gut and kidney [236]. Although the mechanism is still being determined, Lac-Phe suppresses appetite and food intake [235,236]. Ultimately, the result of exercise generates Lac-Phe, which leads to a reduction in body weight. Interestingly, metformin (a drug widely used in diabetes and off-label in PD) mediates weight loss through Lac-Phe [237].
3.2.16. miR451a and miR-150-5p in Small Extracellular Vesicles (sEVs)
MicroRNAs (miRs) regulate gene expression from transcript degradation and inhibit translation. Frequently, miRs are carried through the circulation using small extracellular vesicles (sEV) [239], whereby the miRs are transported as cargo [240]. Small extracellular vesicles can penetrate the blood–brain barrier. In elegant work, D’Souza tested ten males through a rigorous acute workout and measured the release into the circulation of twenty-nine well-known miRs [297]. They found that after an acute high-intensity workout, sEVs were released following exercise that contained miR-451a and miR-250-5p [297]. Interestingly, levels of miR-451a are lower in plasma in patients with depression [298]. Likewise, in a mouse study, there was an inverse relationship between low miR-250-5p and an increase in anxiety [299]. Exercise has been shown to be effective in treating depression and anxiety [300]. These results imply that miRs, bound in sEV, can be released into the circulation following exercise, and their cargo transported to the brain may be useful tools for treating depression and anxiety, which are both common non-motor events in PD.
3.2.17. Monoamine Neurotransmitters (Dopamine (DA), Norepinephrine (NE), and Serotonin (5-HT))
Exercise leads to increased secretion of the monoamine neurotransmitters DA, NE, and 5-HT in the CNS [241,242,243]. Exercise increases striatal DA, which suggests that it improves executive function. It was also found to protect against neurotoxicity in an animal model of PD [63]. Thus, exercise may yield a partial neuroprotective effect on dopaminergic neurons [63,66,301]. Exercise enhances neuronal cell adaptation to stress in the neurons of the locus coeruleus, which is attributed to the up-regulation of galanin, leading to the suppression of NE [302]. The synthesis of 5-HT is affected both positively and negatively by exercise [302]. Exercise reduces the level of the 5-HT1B receptor in the dorsal raphe nuclei, which supports the hypothesis that exercise helps to relieve anxiety and helps mitigate the stress response. There is also a positive feedback loop that exercise would boost between 5-HT and BDNF, which assists in regulating synaptic plasticity and neuronal cell survival [303].
3.2.18. Peroxisome Proliferator-Activated Receptor-Gamma Coactivator (PGC)-1alpha (PGC-1α)/Kynurenine (Kyn) Interaction
PGC-1α is a transcription coactivator essential in regulating cellular energy metabolism [244]. Exercise activates PGC-1α in skeletal muscle and improves mitochondrial biogenesis, fatty acid oxidation, and resistance to atrophy [292]. A part of the process is the formation of the PGC-1α-PPARα-PPARδ pathway, which leads to the activation of kynurenine aminotransferase in skeletal muscle [304]. This transferase converts Kyn into kynurenic acid [305]. Why is this important? Increased amounts of Kyn, which crosses the blood–brain barrier, are associated with depression [306]. Thus, exercise leads to PGC-1α activation in skeletal muscle along with kynurenine aminotransferase and the generation of the neuroprotective kynurenic acid (which does not cross the blood–brain barrier), which reduces stress-induced depression. As mentioned, exercise induces the up-regulation of PGC-1α and irisin (FNDC5), further highlighting the importance of PGC-1α interactions favoring a neuroprotective brain microenvironment.
3.2.19. Vascular Endothelial Growth Factor (VEGF)
VEGF is a potent angiogenic and vascular permeability factor. Interestingly, VEGF also has neurotrophic, neuroprotective, and angiogenic properties through the inhibition of apoptosis and stimulation of neurogenesis [247,248,249]. VEGF also mediates multiple processes, including angiogenesis, blood–brain barrier permeability for glucose, and antioxidant activation, indirectly resulting in neuroprotection [247,248,249]. Importantly, VEGF prevents neurons from death under critical conditions such as hypoxia and glucose deprivation by binding to specific receptors, which include VEGFR-2/flk-1/KDR receptors. VEGFR-2 is the major receptor transmitting mitogenic and trophic signals in neurons [307]. Flk-1 participates in neurogenesis, hematopoiesis, vasculogenesis, and angiogenesis [308]. KDR is the main mediator of VEGF-induced endothelial proliferation, survival, migration, tubular morphogenesis, and sprouting [309]. VEGF is decreased in the substantia nigra in experimental models of PD; however, the therapeutic potential to replace the VEGF is plausible [310].
3.3. Exerkines and the Ability to Cross the Blood–Brain Barrier
The majority of exerkines listed in Table 2 can pass through the blood–brain barrier (BBB) into the brain, including Apelin [311], BHB [312], BDNF [313], CTSB [210,211], Fetuin-A [314], FGF21 [315], IGF-1 [316], IL-6 [317], Irisin [318], Lactate [319], mIR451a in sEV [320], mIR-150-5p in sEV [321], and VEGF [322]. PGC-1α may not be needed to cross the BBB because PGC-1α is up-regulated in neuronal inflammatory cells [244]. It was first reported that Adiponectin could not penetrate the BBB, although more recent work has shown that it does [323,324,325]. Since the brain is a target for Lac-Phe to suppress the appetite, it is logical to presume that the exerkine can navigate the BBB [235,236,237]. GDNF is already in the CNS, so it is likely not critical that it cannot pass through the BBB [326]. Dopamine cannot pass through the BBB; however, its precursor, levodopa, can enter [327]. Likewise, serotonin is not allowed free access into the brain, but its immediate precursor, 5-hydroxy-tryptophan, can pass through [328]. The BBB prevents entry of norepinephrine [328,329]. This has not been studied for BAIBA and the BBB.
3.4. Summary of Exerkines
Exercise-induced exerkines support several targeted pathways for the brain that allow it to renew, rejuvenate, and repair damaged/dysfunctional neuronal tissue. The following section compares aerobic (endurance-based) and resistance training (strength-based), age, sedentary lifestyle, and muscle mass on exerkine production.
4. Factors That Modulate the Production of Exerkines
“Look to the nervous system as the key to maximum health.” Galen (130 AD–210 AD) [2]
4.1. Aerobic Exercise (Endurance-Based) versus Resistance Training (Strength-Based)
Aerobic exercise (or endurance-based) and resistance training (or strength-based) activities give the two extremes of the spectrum for exercise [330]. Both exercise modalities provide substantial health benefits, but there are differences; aerobic exercise is better for reducing cardiovascular risk factors, and resistance training is better for maintaining primary metabolism and muscle mass and function, especially in older adults (see Table 2 [330]). Importantly, combining aerobic exercise with resistance training is more effective in reducing insulin issues in the obese population with metabolic syndrome and improving health in many other disorders [330,331,332]. Exercise substantially and quickly alters the physiological background since ~9800 molecular analytes (proteins, transcripts, metabolites, and lipids) changed expression levels from a single round of aerobic exercise [333]. A similar comparative metabolomics study reported that 666 and 708 metabolites were altered in blood plasma samples following aerobic and resistance exercises, respectively [334].
Since skeletal muscle is the largest tissue in the human body, it releases many myokines into the peripheral circulation following exercise [335,336]. Numerous studies have compared the effects of aerobic exercise and resistance training on myokine/exerkine production. A partial list of myokines detected in blood following an episode of exercise is provided in Table 3. Interestingly, some myokines respond preferentially to a specific type of exercise, while others respond similarly regardless of the exercise type. Many of the exerkines included in Table 3 were described as putative neuroprotective exerkines, suggesting that both forms of exercise can promote exerkine release, promoting a neuroprotective effect.
Table 3.
Exercise-induced myokines detected in blood following aerobic exercise or resistance training. Arrows describe an increase (↑) or decrease (↓) in exerkine levels from exercise.
| Aerobic Exercise [Reference(s)] |
Resistance Training [Reference(s)] |
|---|---|
| Apelin ↑ [253,337] BAIBA ↑ [338] BDNF ↑ [339,340] CTSB ↑ [341] FGF21 ↑ [342] Fractalkine ↑ [343] IGF-1 ↑ [285,344] IL-6 ↑ [345,346] IL-15 ↑ [347] Irisin ↑ [229] Lactate ↑ [348] LIF ↑ [349] MCP-1 ↑ [343] Musclin ↑ [350] Myonectin ↑ [351] Myostatin ↓ [352] PGC-1α ↑ [353,354] SDF1 ↑ [355] SPARC ↑ [356] RANTES ↑ [216] VEGF ↑ [357] |
Angiopoietin-like 4 ↑ [358] BDNF ↑ [359] BMP7 ↑ [360] CTSB ↑ [361] Decorin ↑ [362] FGF21 ↑ [363,364] IGF-1 ↑ [283,365] IL-6 ↑ [346] IL-15 ↑ [366] Irisin ↑ [364,367] Lactate ↑ [368] Myostatin ↓ [352] PGC-1α ↑ [369] VEGF ↑ [370] |
García-Hermoso et al. [371] conducted a comprehensive review of the effect of exercise on type 2 diabetes, involving more than 2000 patients and control groups. The exercise routines in these studies encompassed a wide range, including aerobic exercise, high-intensity interval training, resistance training, and exercise with two modalities. The duration of these routines varied from 8 weeks to 96 weeks, with training sessions held from one to five times per week [371]. The meta-analysis from this study showed that any form of regular exercise alters the blood levels of many exerkines, including adiponectin, fetuin-A, FGF-21, IL-6, IL-10, leptin, resistin, and TNF-α, in patients with type 2 diabetes compared to control groups. Specifically, aerobic exercise, resistance training, or high-intensity interval protocols, performed at moderate-to-vigorous intensity for more than 24 weeks with three sessions per week and lasting more than 60 min per session, yielded the most promising results for type 2 diabetes patients [371].
The impact of resistance training on neuroprotective factors has yet to be studied as thoroughly as aerobic exercise. Rodríguez-Gutiérrez et al. [372] performed a systematic review and meta-analysis of resistance training and changes in levels of IGF-1, BDNF, and VEGF. Thirty randomized clinical trials were included in their study [372]. They found a significant effect of resistance training on increased levels of IGF-1 but not for BDNF (VEGF was not determined due to the lack of publications). Their study supports a neuroprotective effect of resistance training in middle to late adulthood primarily due to the increased release of IGF-1 [372].
4.2. Age
Advanced age is the most significant risk factor for developing PD [373]. Mild cognitive impairment (MCI) is linked to an irregular aging pattern that can lead to dementia, with a risk of developing further into Alzheimer’s disease (AD) [374]. Cognitive changes can also occur in PD; thus, finding treatment for MCI is essential for both AD and PD. To better understand the mechanism of exercise in treating MCI, Tsai et al. [375] compared aerobic exercise and resistance training in neurocognitive performance in older adults with MCI. They also measured changes in several exerkines in both forms of exercise. Aerobic exercise significantly increased BDNF and IGF-1 and a minor increase in VEGF, while resistance training only increased IGF-1 [375]. Both aerobic exercise and resistance training improved neurocognitive performance. They concluded that neuroprotective exerkines were operational in older adults with MCI, which suggests that exercise-dependent plasticity is possible [375].
In a unique approach, Kettinen et al. [376] investigated cognitive decline in aging populations by employing age-appropriate cognitive demanding aerobic exercises. Healthy older golfers performed these exercises, and the study was particularly interested in cognitive changes in three groups: 18 holes of golf, 6 km of Nordic walking, or 6 km of regular walking [376]. The researchers also tested for changes in BDNF and CTSB. Interestingly, despite not detecting any changes in the exerkines measured, all three exercise groups showed improved cognitive functions. Furthermore, the study revealed that Nordic and regular walking enhanced executive functions. This research addresses a crucial question: can we design age-appropriate exercises to enable an aging population to improve their cognitive function [376]? Moreover, this study further underscores the importance of exercise, suggesting that it may be more beneficial than doing nothing.
4.3. Muscle Wasting
Frequently, with advanced aging, adults can suffer a significant loss of skeletal muscle mass, which results in a disorder called sarcopenia [377,378]. Sarcopenia is found to be increased in PD when compared to other aged-match non-PD patients. Since many exerkines are generated in skeletal muscle, Kwon et al. [379] reported that the following myokines, regulated by exercise, were altered by aging: apelin, BAIBA, BMP-7, decorin, IGF-1, IL-15, irisin, SDF-1, sestrin, SPARC, and VEGF were decreased, while IL-6 and myostatin were increased. Aerobic exercise increased apelin, BAIBA, IL-15, IL-6, irisin, SDF-1, sestrin, SPARC, and VEGF expression, while resistance training enhanced BMP-7, decorin, IGF-1, IL-15, IL-6, irisin, and VEGF expression [379]. Both forms of exercise downregulated myostatin. They concluded that it is critical to develop combined aerobic exercise and resistance training programs for aging adults [379]. Likewise, Piccirillo [380] and Graham et al. [381] provide further mechanistic insight into the role of exercise and myokines in preventing muscle wasting.
4.4. Sedentary Lifestyle
PD can promote a sedentary lifestyle in older adults [382]. To address the sedentary lifestyle (this study did not pertain to PD), MacNeil et al. [383] studied 68 healthy younger and older volunteers and measured 44 low-abundance growth factors, cytokines, and chemokines from their blood. They found that long-term physically active people had a broader induction of factors post-exercise, irrespective of age [383]. They concluded that sedentary older adults have a lower aerobic capacity and muscle function, which implies that the lack of change in myokines released into the bloodstream was likely due to the reduced force of muscle contractions [383]. This study reinforces the notion that well-planned exercise programs can potentially help manage a sedentary lifestyle.
Sustained moderate-to-vigorous exercise leads to enhanced plasticity in the motor cortex [384]. McDonnell et al. [385] wanted to know the effect of a single exercise session. They found that a single session of light exercise promoted a neuroplastic response, which could enhance the effectiveness of motor learning [385]. Furthermore, Steib et al. [386] demonstrated that a single session of moderately intense cycling led to rapid improvement in motor skill consolidation in patients with PD. These studies suggest that introducing exercise to a PD patient who has been sedentary can lead to rapid improvements in motor skills and learning deficits, providing a strong sense of encouragement and motivation.
In the next section, we start with a description of the neuroprotective theory. From there, we provide a road map of exerkines and how they potentially enable the brain to protect itself. In the case of PD, exercise and exerkines may unite to join an offensive attack, promoting regeneration (or neuroplasticity) in the brain.
5. The Neuroprotective Theory to Slow the Progression of Parkinson’s Disease
“Your age is the sum total of your physical condition, the condition of your mind, and how you feel.” Jack LaLanne (1914–2011; LaLanne hosted the first and longest-running syndicated fitness television program in the U.S., from 1951 to 1985)
5.1. Assessing the Neuroprotective Theory
Neuroprotection is the strategy for safeguarding the CNS from neuronal cell damage caused by acute injury (e.g., stroke or trauma) or chronic neurodegenerative diseases [387]. Therefore, the neuroprotective theory includes the process and components derived from exercise, which creates the physiological setting for the formation and release of exerkines into the blood circulatory system [388,389]. Following this is the subsequent crosstalk between the brain and multiple organs/tissues, instructing the various exerkines to migrate to their target cells in the CNS [50,390], thus beginning the ligand (exerkines)/receptor-binding site interaction, which leads to the activation of numerous cell-surface receptors, changing the properties of the neurons, and then producing/secreting their own bioactive molecules. The neuroprotective theory can promote either neuroprotection or neuro-restoration of damaged/dead neurons [391].
Increasing evidence indicates both preventive and therapeutic functions for exerkines [392,393]. What is intriguing about the neuroprotective theory is that most exerkines do not originate in the brain; by contrast, skeletal muscle, liver, and white adipose tissue likely account for the influx of exerkines to the brain [394]. The neuroprotective theory functions well for many individuals who will never develop PD or another neurodegenerative disorder. However, for some, factors include exposure to neurotoxins, alterations in immunological or inflammatory systems, genetic misprinting, mitochondria dysfunction, or numerous other possibilities that shift the equilibrium to favor disease over health [387]. Although a neurodegenerative disorder presents a life-or-death scenario, the exercise/exerkines response is less robust than the full burst impact of the polymorphonuclear leukocytes in acute inflammation attacking/phagocytosing an extracellular microbial invader [395], the mononuclear leukocytes of chronic inflammation again matched up against a foreign invading substance [396] or the ultimate firepower generated by a T-cell/B-cell-directed immune attack of an invading organism [397]. Perhaps the protective shield of neuroprotection is more of a gentle salve soothing a vital tissue/organ, not the sheer extreme firepower found in our immunological/inflammatory machinery. However, the equilibrium shifts when one chooses a more intense and sustained exercise program, creating an in vivo scenario of increased neuroprotection and, over time, the potential to repair and regenerate neuronal tissue by neuroplasticity.
The neuroprotective theory involves exerkines with many different biological roles. However, for this physiologic process to succeed, it is essential to appreciate what it is up against when encountering the development and progression of PD [30,398]. As shown in Figure 1, several environmental and genetic missteps contribute to the genesis of PD. Furthermore, it is likely a combination of missteps in these molecular processes that provide for the development of PD, therefore creating a complicated target for the neuroprotective machinery to manage. One assumes that the neuroprotective factors provided by exercise and exerkines participate in maintaining neuronal health, circuitry, architecture, and normal function.
5.2. Initiation Pathways for Aerobic Exercise and Resistance Training
Aerobic exercise aims to enhance skeletal muscle capacity for aerobic metabolism and to resist fatigue [330,399,400]. This is achieved through an increase in the number of mitochondria and types I and II muscle fibers in skeletal muscle [401]. The process of aerobic exercise triggers PGC1-α synthesis [402], which in turn promotes the expression of irisin (derived from FNDC5) in skeletal muscle [229]. As described by Wrann et al. [231], aerobic exercise could have the same effect on the brain (hippocampus), specifically through the PGC-1α/FNDC5/BDNF pathway. This novel finding suggests that aerobic exercise could enhance the transcription of Pgc1a and Erra, activating gene expression of Fndc5 [231]. From there, FNDC5 (irisin) acts as a positive regulator of BDNF expression, potentially offering a new avenue for neuroprotection in PD.
In contrast to aerobic exercise, resistance training stimulates skeletal muscle growth by promoting myofibrillar protein synthesis [330,403,404,405]. This process leads to muscle hypertrophy, the increase in myofibrillar volume in type II skeletal muscle fibers [406,407,408]. To achieve hypertrophy, skeletal muscle overexpresses IGF-1 and activates the PI3/Akt signaling pathway [336,380]. Similarly, in the brain, IGF-1 binds to its receptor, IGF-1R, along with other growth factors like BDNF (which binds to TRkB) and VEGF (which binds to VEGFR) [409,410]. Together, they trigger the PI3/Akt pathway [410]. Downstream of PI3/Akt pathway are several targets in the brain, and mTOR activation promotes neurogenesis [410].
Combining aerobic exercise with resistance training offers neuroprotection for the brain [27,73,150,411,412,413,414] and benefits the entire body and other tissues/organs [415,416,417]. This powerful synergy of both types of exercise underscores the potential for comprehensive health improvement, whether in the absence or presence of PD.
5.3. Neuroprotective Action from Myokines in Model Systems of Parkinson’s Disease
Exercise and exerkines have been well studied in some neurodegenerative diseases (e.g., AD) and neurocognitive disorders (e.g., MCI), but not as much in PD. The following in vitro system was used to study the neuroprotective role of myokines in model systems of PD: cultured neuronal cells (SH-ST5Y cells or PC12 cells), adding a neurotoxin to initiate a PD-like scenario (6-OHDA, MPTP, or MPP+), and then adding myokines (apelin, FGF21, or IGF-1) exogenously (Table 4). For each myokine tested, the studies found that myokines reduced cell death (apelin, FGF21, and IGF-1), increased α-synuclein clearance (apelin, FGF21), and promoted an anti-inflammatory response (apelin, FGF21, IGF-1). Furthermore, similar results were obtained when using mice and rats, though not directly involving exercise, which supports the hypothesis that myokines are neuroprotective in PD.
Table 4.
Neuroprotective properties of myokines using neuronal cells and animal models of PD *.
| Myokine | Experimental Model |
Mechanism of Action |
Physiological Function |
Reference |
|---|---|---|---|---|
| Apelin-13 | SH-ST5Y cells + MPP+ + apelin-13 | ↑ ERK1/2 phosphorylation ↓ ER stress |
↓ Cell death | [418] |
| Apelin-36 | SH-ST5Y cells + MPP+ + apelin-36 | ↑ PI3K/Akt/mTOR autophagy | ↑ α-Synuclein clearance | [419] |
| Apelin-36 | Mice + MPTP + apelin-36 | ↑ GSH and SOD ↓ Caspase-3 |
↓ Anti-oxidative stress | [420] |
| Apelin-13 | SH-ST5Y cells + 6-OHDA + apelin-13 | ↑ PI3K ↓ Caspase 3 ↓ Cytochrome c |
↓ Cell death | [421] |
| FGF21 | Mice + MPTP + FGF21 SH-ST5Y cells + MPTP + FGF21 |
↑ SIRT1-autophagy | ↑ α-Synuclein clearance | [422] |
| FGF21 | Mice injected with MPTP + FGF21 SH-ST5Y cells + MPTP + FGF21 |
↑ BCL2/Bax ↓ Caspase 3 cleavage and JNK phosphorylation |
↓ Cell death | [423] |
| FGF21 | Primary dopaminergic neurons + MPP+ + FGF21 | ↓ IL-1, IL-12, IFN-γ, and TNF-α ↓ Astrocyte and microglia activation |
↑ Anti-inflammation | [423] |
| IGF-1 | PC12 cells + 6-OHDA + IGF-1 | ↓ Caspase-3 activation, and PARP cleavage | ↓ Cell death | [424] |
| IGF-1 | Rats + 6-OHDA + IGF-1 | ↑ PI3K/Akt | ↓ Cell death | [425] |
| IGF-1 | Rats + 6-OHDA + IGF-1 | ↑ ERK 1/2/CREB ↑ Akt/GSK3α/β/ β-catenin |
↓ Cell death | [287] |
| IGF-1 | PC12 cells + 6-OHDA + IGF-1 | ↑ HO-1 | ↓ Anti-oxidative stress | [424] |
* The table is partly based on Lee et al. [19]. Apelin-13 and -36 are modified forms of apelin. Arrows describe an increase (↑) or decrease (↓) in the biological activity measured from the experiment.
5.4. Neuroprotective Components Provided by Exerkines
What the brain senses due to exercise still needs to be fully understood. A part of it is an endocrine communication system (“crosstalk”) between the brain and other organs with exerkines, and another aspect must be the physiological changes in the body because of exercise (e.g., oxidative changes or the changes induced early on by exerkines interacting with their target receptors). Figure 4 provides a composite view of the exercise-induced exerkines described in Section 3 and the collective neuroprotective process in PD. Skeletal muscle provides the most significant number of exerkines to the neuroprotective process, followed by the liver and white adipose tissue [426]. The role of exerkines in the neuroprotective theory provides several powerful physiological reactions to manage the numerous PD symptoms. We describe some of the potential intervention points exerkines supply during this process.
Figure 4.
Potential role of neuroprotective exerkines to slow the progression of Parkinson’s disease.
Anti-Aging and Reduction in Oxidative Stress—Irisin is a protein that activates another protein called Nrf-2 in liver, heart, and endothelial cells [427]. Nrf-2 helps regulate nitric oxide signaling in blood vessel cells [428], which reduces the amount of reactive oxygen species in the body. This helps to increase the amount of superoxide dismutase in the brain, which protects against oxidative damage. In older mice, GPLD1 has been found to promote the growth of new brain cells and the expression of BDNF, which is essential for learning and memory [221]. Additionally, BAIBA activates two different signaling pathways in the body, including the AMPK and PI3K/Akt pathways. This activation helps to protect cells against oxidative stress [202,255].
Depression and Anxiety—In a study conducted on mice with symptoms like chronic depression, aerobic exercise increased the production of PGC-1α, which, in turn, up-regulated AMPK (a critical component of energy balance) [429]. This led to significant improvement in depression-like behavior. Additionally, kynurenine aminotransferase was also upregulated, and the ratio shifted in favor of kynurenic acid over Kyn, resulting in a reduction in depression [306].
Energy Utilization in Mitochondria—Mitochondria play a crucial role in producing ATP, which supports the maintenance of neuronal cells and facilitates neurotransmitter communication [430]. Heo et al. [431], on reviewing exerkines and brain mitochondria, suggest that BDNF, IL-6, BHB, lactate, adiponectin, irisin, and FGF21 all impact mitochondrial bioenergetics and energy production.
Neurogenesis—Generating neurons in the adult brain requires activating quiescent neuronal stem cells, which BDNF can achieve in the hippocampus [432,433]. IGF-1 can promote neurogenesis and help coordinate the impact of BDNF and VEGF in the brain [247,434].
Neuroinflammation—Neuroinflammation refers to the host defense system components that play a crucial role in Parkinson’s disease (PD) pathogenesis [435]. Adiponectin helps to control microglia function by promoting anti-inflammatory responses through PPAR-γ signaling [436,437]. Apelin-13, a derivative of apelin, has been found to inhibit microglia and astrocyte activation, reduce IL-1β and TNF-α expression, and cause hippocampal BDNF/TrkB expression deficit in rats with Alzheimer’s disease [438]. BHB effectively blocks inflammasome activation in PD [439]. IL-6 is a potent anti-neuroinflammatory agent as it upregulates key anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonists [227].
Neuroplasticity—Adult neuroplasticity is described as changes in the strength of excitatory and inhibitory synapses, while various attempts to regenerate connections have met with limited success [440]. In a comprehensive review of neuroprotective exerkines, Vints et al. [441] provided evidence for long-term synaptic potentiation, a component of the signaling pathways that promote neuroplasticity for BDNF, IGF-1, irisin, CTSB, apelin, adiponectin, kyn, lactate, and BHB.
Tissue Regeneration—Exercise and exercise-induced exerkines aim to promote a scenario that can protect, repair, or regenerate the brain of someone with PD. Exercise exerts a physiological response [47] while exerkines support, in part, biochemical and molecular signaling responses (two examples are given) [48]. One such example is the activation of the PI3K/Akt signaling pathway [410], a crucial factor in response to exerkines, notably IGF-1 [224], VEGF [247,248,249], and BDNF [265,266], which promotes neuronal survival, proliferation, and maturation. The expression of PGC-1α leads to activation of the AMPK/SIRT1/PGC-1α signaling pathway [410]. The role of PGC-1α is essential to mitochondrial biosynthesis and is also involved in cell proliferation and differentiation, which are critical processes in exercise-induced neural regeneration [442]. PGC-1α also forms heterodimer complexes with PPARs and estrogen-related receptors, thus widening the impact of PGC-1α exercise-induced neural regeneration [443].
Gut Microbiome–Brain Interaction—There is evidence for a gut microbiome–brain conduit in the pathogenesis of PD [444,445,446,447]. The peripheral nervous system, notably the vagus nerve, is the communication link between the gut and brain. Alterations in the gut microbiome produce toxins that help form α-synuclein aggregates. Then, presumably by a prion-like process, the α-synuclein aggregates are transferred into the peripheral nervous system. Thus, pathological changes to the gut microbiome may contribute to PD [444,445,446,447]. Likewise, exercise can positively influence the microbiome, promoting an anti-inflammatory response [448,449,450]. A part of the neuroprotective theory may be the positive impact of exercise in generating an anti-inflammatory gut microbiome that signals through the peripheral and central nervous systems to augment the neuroprotective exerkines in slowing the progression of PD.
Summary—Combined with exercise, the following exerkines (listed alphabetically) demonstrate potential for protecting the brain against the pathological missteps caused by PD: apelin, BHB, BDNF, FGF21, GDNF, IGF-1, IL-6, irisin (FNDC5), lactate, PGC-1α, and VEGF.
6. Strengths, Limitations, and Challenges
“We must turn to nature itself, to the observations of the body in health and in disease to learn the truth.” Hippocrates (460–375 BCE) [2]
Strengths—One of the goals of this review was to use previous work on PD as a foundation to help guide the narrative topics. From these studies/reviews, we determined that there were some critical unanswered questions about PD, therapy, exercise, and exerkines. Can exercise target the complex pathophysiology of PD [451,452]? Alternatively, is exercise promoting a healthier total self physiologically to allow for a more robust reaction against PD [453]? Exercise is now considered a primary component in treating PD; however, a better appreciation of specific molecular and cellular pathways is needed [53,454]. Can exercise further improve cognition or executive function deficits [455]? Is aerobic exercise or resistance training better for treating the symptoms of PD [45,154,411,456,457,458,459,460,461]? Can exercise be personalized by healthcare providers to match the variable presentation of PD from person to person [451,462,463]? Another goal of this review was to unite the complex field of assessing exercise for treating PD with the critical role of exercise-induced exerkines to promote the benefit of exercise.
Limitations—This paper was written as a narrative review, not as a systematic review with a meta-analysis study. Thus, some studies cited here are only sometimes consistent about a specific type of exercise linked to improved motor function or changing levels of exerkines. These differences are likely due to testing design, sample detection limits, and whether the study was large enough to gain statistical significance. In many cases, the exerkine studies we described were based on cardiovascular diseases or neurocognitive disorders, not PD. While much evidence supports the role of exercise and exerkines in providing neurological-based protection, there are only a few papers on exerkines in PD (most often without exercise) [252,268,269,275,287,293,301,303,310,418,419,420,421,422,423,424,425]. Interestingly, a clinical trial is underway with GDNF therapy in PD [464].
Challenges—As asked by Wrann et al. [231], how does the brain sense exercise? The answer is still pending. The difficulty in answering this all-important question is likely because exercise changes the body. Identifying the signals that lead to the cross-talk between multiple tissues will help provide a molecular exercise map [465]. Exercise studies today use many different types of exercise, and some include aerobic exercise [466], resistance training [460], exercise alone and in groups [414], and even high-intensity interval training [93]. Many studies have a variable time of study with different intensities. These variabilities further complicate the best-targeted exercise for treating a disorder [467]. PD is a complex disorder, started by a multitude of factors, with numerous symptoms and a variable degree of progression, which means that patients enrolling in exercise studies may require different levels of exercise training depending upon their disorder, and their baseline fitness may be different. However, performing these kinds of studies will provide us with knowledge and a spectrum of information about how to use exercise to manage the complexity of PD.
7. Conclusions
“The use of exercise has had an important share in the treatment of disease since Hippocrates used it at the sanitarium at Cos, and Galen advocated it in words that are as true now as they were eighteen hundred years ago…” R. Tait McKenzie, M.D. (1909) in “Exercise in Education and Medicine” [468].
The concept of exercise as medicine is ancient [1,2,468]. We are currently unraveling the molecular processes and pathways to how exercise provides a protective shield to our tissues/organs. In this narrative review, we described the importance of exercise and various exercise programs to best support each PwP. We presented the ability of exercise-induced exerkines to provide neuroprotection, reducing the risk of PD and offering neuroprotection and possibly neuro-restoration for those afflicted with PD.
The diverse nature of PD and the varying progression rates found in PwP necessitate tailored management strategies. Additionally, PwP may present with different levels of physical fitness, further complicating their physical and mental health management. We wrote this review to assist Care Teams (both professional and personal) in understanding the role and application of exercise (aerobic exercise, resistance training, neuromotor exercise programs, and stretching/flexibility exercises) and in developing comprehensive and personalized management plans for PwP. Furthermore, by highlighting the molecular and physiological properties of exerkines, the Care Teams and PwP can gain knowledge and appreciate this neuroprotective process empowered by exercise. Finally, as our understanding of the role of exerkines expands and evolves, this will provide scientific, physiological, and pharmacological platforms to improve the health of PwP further.
Acknowledgments
F.C.C. gratefully acknowledges Russell R. Broaddus in the Department of Pathology and Laboratory Medicine at the UNC School of Medicine for supporting his Parkinson’s disease scholarship. We thank Chantelle Rein-Smith for helpful editorial advice on the manuscript. F.C.C. dedicates this review to the following physical therapists (listed in alphabetical order) who have supported his journey over the past decade: Jennifer Bazan-Wigle; Rebecca R. Bliss; Korinne Dodd; Becky Farley; Alicia Malinsky; Claire McLean; and Diane Meyer.
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| AMPK | AMP-activated protein kinase |
| BAIBA | beta-aminoisobutyric acid |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BHB | beta-hydroxybutyrate |
| Bpm | beats per minute |
| CNS | central nervous system |
| CTSB | cathepsin B |
| DA | dopamine |
| ERK 1/2 | extracellular signal-regulated kinase 1/2 |
| FGF21 | fibroblast growth factor 21 |
| FNDC5 | fibronectin type III domain-containing Protein 5 |
| GDNF | glial cell line-derived neurotropic factor |
| GPLD1 | glycosylphosphatidylinositol-specific phospholipase D1 |
| GPCR | G protein-coupled receptor |
| % HRmax | % maximal heart rate |
| HCAR2 | hydroxycarboxylic acid receptor 2 |
| H&Y | Hoehn and Yahr scale of PD stage |
| 6-OHDA | 6-hydroxydopamine |
| IGF-1 | insulin-like growth factor 1 |
| IL-1, -6, -10 | interleukin-1, -6, -10 |
| Kyn | Kynurenine |
| Lac-Phe | Lactoylphenylalanine |
| miR | microRNA |
| MPTP, MPP+ | 1-methyl-4-phenylpyridinium (Inactive/active forms) |
| MCI | mild cognitive impairment |
| p38 MAPK | p38 mitogen-activated protein kinase |
| ND | neurodegenerative |
| NE | norepinephrine |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OT | occupational therapist |
| PD | Parkinson’s disease |
| PwP | people (person)-with-Parkinson’s |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARα/δ/γ | peroxisome proliferator-activated receptor alpha/delta/gamma |
| PI3K/AKT/mTOR | phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin |
| PT | physical therapist |
| PTSD | Post traumatic stress disorder |
| Irisin | proteolyzed fragment of FNDC5 |
| PAI-1 | plasminogen activator inhibitor-1 |
| 5-HT | serotonin |
| SIRT1 | sirtuin 1 |
| SLP | speech–language pathologist |
| sEV | small extracellular vesicles |
| TBI | Traumatic brain injury |
| TrkB | tropomyosin receptor kinase B |
| TNF-α | Tumor necrosis factor-alpha |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VEGF(R) | vascular endothelial growth factor (receptor) |
| WAT | white-adipose tissue |
Author Contributions
Conceptualization, F.C.C., A.K.M. and R.R.B.; writing—original draft preparation, A.K.M., R.R.B. and F.C.C.; writing—review and editing, A.K.M., R.R.B. and F.C.C.; visualization, F.C.C., A.K.M. and R.R.B.; project administration, F.C.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tipton C.M. Susruta of India, an unrecognized contributor to the history of exercise physiology. J. Appl. Physiol. 2008;104:1553–1556. doi: 10.1152/japplphysiol.00925.2007. [DOI] [PubMed] [Google Scholar]
- 2.Tipton C.M. The history of “Exercise Is Medicine” in ancient civilizations. Adv. Physiol. Educ. 2014;38:109–117. doi: 10.1152/advan.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skovronsky D.M., Lee V.M.-Y., Trojanowski J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2006;1:151–170. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- 4.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021;27:954–963. doi: 10.1038/s41591-021-01382-x. [DOI] [PubMed] [Google Scholar]
- 6.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.Amor S., Puentes F., Baker D., Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brettschneider J., Tredici K.D., Lee V.M.-Y., Trojanowski J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015;16:109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spires-Jones T.L., Attems J., Thal D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134:187–205. doi: 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spillantini M.G., Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. doi: 10.1016/S0166-2236(98)01337-X. [DOI] [PubMed] [Google Scholar]
- 11.Ross C.A., Poirier M.A. What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. 2005;6:891–898. doi: 10.1038/nrm1742. [DOI] [PubMed] [Google Scholar]
- 12.Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Yan T., Chu J.M.-T., Chen Y., Dunnett S., Ho Y.-S., Wong G.T.-C., Chang R.C.-C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019;99:943–957. doi: 10.1038/s41374-019-0232-y. [DOI] [PubMed] [Google Scholar]
- 14.Marques-Aleixo I., Beleza J., Sampaio A., Stevanović J., Coxito P., Gonçalves I., Ascensão A., Magalhães J. Preventive and therapeutic potential of physical exercise in neurodegenerative diseases. Antioxid. Redox Signal. 2021;34:674–693. doi: 10.1089/ars.2020.8075. [DOI] [PubMed] [Google Scholar]
- 15.Quan H., Koltai E., Suzuki K., Aguiar A.S., Jr., Pinho R., Boldogh I., Berkes I., Radak Z. Exercise, redox system and neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020;1866:165778. doi: 10.1016/j.bbadis.2020.165778. [DOI] [PubMed] [Google Scholar]
- 16.Memon A.A., Coleman J.J., Amara A.W. Effects of exercise on sleep in neurodegenerative disease. Neurobiol. Dis. 2020;140:104859. doi: 10.1016/j.nbd.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farì G., Lunetti P., Pignatelli G., Raele M.V., Cera A., Mintrone G., Ranieri M., Megna M., Capobianco L. The effect of physical exercise on cognitive impairment in neurodegenerative disease: From pathophysiology to clinical and rehabilitative aspects. Int. J. Mol. Sci. 2021;22:11632. doi: 10.3390/ijms222111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M., Barker R., Williams G., Carr J., Gunnarsson R. The effect of exercise on high-level mobility in individuals with neurodegenerative disease: A systematic literature review. Physiotherapy. 2020;106:174–193. doi: 10.1016/j.physio.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee B., Shin M., Park Y., Won S.-Y., Cho K.S. Physical exercise-induced myokines in neurodegenerative diseases. Int. J. Mol. Sci. 2021;22:5795. doi: 10.3390/ijms22115795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenzuela P.L., Castillo-Garcia A., Morales J.S., de la Villa P., Hampel H., Emanuele E., Lista S., Lucia A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020;62:101108. doi: 10.1016/j.arr.2020.101108. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R., Khan R., Cortes C.J. Forgot to Exercise? Exercise Derived Circulating Myokines in Alzheimer’s Disease: A Perspective. Front. Neurol. 2021;12:649452. doi: 10.3389/fneur.2021.649452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M., Zhang H., Liang J., Huang J., Chen N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J. Neuroinflammation. 2023;20:76. doi: 10.1186/s12974-023-02753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rody T., De Amorim J.A., De Felice F.G. The emerging neuroprotective roles of exerkines in Alzheimer’s disease. Front. Aging Neurosci. 2022;14:965190. doi: 10.3389/fnagi.2022.965190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotman C.W., Berchtold N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 26.Safdar A., Saleem A., Tarnopolsky M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016;12:504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 27.Loprinzi P.D., Moore D., Loenneke J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020;10:913. doi: 10.3390/brainsci10120913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth F.W., Roberts C.K., Thyfault J.P., Ruegsegger G.N., Toedebusch R.G. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017;97:1351–1402. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth F.W., Roberts C.K., Laye M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012;2:1143. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D.-C., Chen Y., Xu Y., ShenTu C.-Y., Peng L.-H. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023;8:73. doi: 10.1038/s41392-023-01353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalia L., Lang A. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 32.Ahlskog J.E. The New Parkinson’s Disease Treatment Book: Partnering with Your Doctor to Get the Most from Your Medications. Oxford University Press; New York, NY, USA: 2015. [Google Scholar]
- 33.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.-E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005;20:S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- 36.Santens P., Boon P., Van Roost D., Caemaert J. The pathophysiology of motor symptoms in Parkinson’s disease. Acta Neurol. Belg. 2003;103:129–134. [PubMed] [Google Scholar]
- 37.Ferrazzoli D., Ortelli P., Cucca A., Bakdounes L., Canesi M., Volpe D. Motor-cognitive approach and aerobic training: A synergism for rehabilitative intervention in Parkinson’s disease. Neurodegener. Dis. Manag. 2020;10:41–55. doi: 10.2217/nmt-2019-0025. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhuri K.R., Schapira A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 39.Berganzo K., Tijero B., Gonzalez-Eizaguirre A., Somme J., Lezcano E., Gabilondo I., Fernandez M., Zarranz J., Gómez-Esteban J. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurología. 2016;31:585–591. doi: 10.1016/j.nrl.2014.10.010. (In English) [DOI] [PubMed] [Google Scholar]
- 40.Vuletić V. Mind and Brain. Springer Nature; Cham, Switzerland: 2020. Non-motor Symptoms in Parkinson’s Disease; pp. 109–118. [DOI] [Google Scholar]
- 41.Schapira A.H., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18:435. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016;22:S119–S122. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Church F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules. 2021;11:612. doi: 10.3390/biom11040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: A review. JAMA. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 45.van der Kolk N.M., de Vries N.M., Kessels R.P., Joosten H., Zwinderman A.H., Post B., Bloem B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019;18:998–1008. doi: 10.1016/S1474-4422(19)30285-6. [DOI] [PubMed] [Google Scholar]
- 46.Johansson M.E., Cameron I.G., Van der Kolk N.M., de Vries N.M., Klimars E., Toni I., Bloem B.R., Helmich R.C. Aerobic Exercise Alters Brain Function and Structure in Parkinson’s Disease: A Randomized Controlled Trial. Ann. Neurol. 2022;91:203–216. doi: 10.1002/ana.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel T., Brechat P.H., Leprêtre P.M., Kaltenbach G., Berthel M., Lonsdorfer J. Health benefits of physical activity in older patients: A review. Int. J. Clin. Pract. 2009;63:303–320. doi: 10.1111/j.1742-1241.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 48.Chow L.S., Gerszten R.E., Taylor J.M., Pedersen B.K., Van Praag H., Trappe S., Febbraio M.A., Galis Z.S., Gao Y., Haus J.M. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022;18:273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giudice J., Taylor J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017;34:49–55. doi: 10.1016/j.coph.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Türkel İ., Özerkliğ B., Atakan M.M., Aktitiz S., Koşar Ş.N., Yazgan B. Exercise and metabolic health: The emerging roles of novel exerkines. Curr. Protein Pept. Sci. 2022;23:437–455. doi: 10.2174/1389203723666220629163524. [DOI] [PubMed] [Google Scholar]
- 51.Robbins J.M., Gerszten R.E. Exercise, exerkines, and cardiometabolic health: From individual players to a team sport. J. Clin. Investig. 2023;133:e168121. doi: 10.1172/JCI168121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooks G.A., Osmond A.D., Arevalo J.A., Duong J.J., Curl C.C., Moreno-Santillan D.D., Leija R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023;134:529–548. doi: 10.1152/japplphysiol.00497.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westphal A. New insights into the molecular basis of how physical activity contributes to human health. Acta Physiol. 2023;239:e14047. doi: 10.1111/apha.14047. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen B.K. The physiology of optimizing health with a focus on exercise as medicine. Annu. Rev. Physiol. 2019;81:607–627. doi: 10.1146/annurev-physiol-020518-114339. [DOI] [PubMed] [Google Scholar]
- 55.Brooks G.A., Osmond A.D., Arevalo J.A., Curl C.C., Duong J.J., Horning M.A., Moreno Santillan D.D., Leija R.G. Lactate as a major myokine and exerkine. Nat. Rev. Endocrinol. 2022;18:712. doi: 10.1038/s41574-022-00724-0. [DOI] [PubMed] [Google Scholar]
- 56.Górecka M., Krzemiński K., Mikulski T., Ziemba A.W. ANGPTL4, IL-6 and TNF-α as regulators of lipid metabolism during a marathon run. Sci. Rep. 2022;12:19940. doi: 10.1038/s41598-022-17439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva G.J., Bye A., El Azzouzi H., Wisløff U. MicroRNAs as important regulators of exercise adaptation. Prog. Cardiovasc. Dis. 2017;60:130–151. doi: 10.1016/j.pcad.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Isaac A.R., Lima-Filho R.A., Lourenco M.V. How does the skeletal muscle communicate with the brain in health and disease? Neuropharmacology. 2021;197:108744. doi: 10.1016/j.neuropharm.2021.108744. [DOI] [PubMed] [Google Scholar]
- 59.Townsend L.K., MacPherson R.E., Wright D.C. New horizon: Exercise and a focus on tissue-brain crosstalk. J. Clin. Endocrinol. Metab. 2021;106:2147–2163. doi: 10.1210/clinem/dgab333. [DOI] [PubMed] [Google Scholar]
- 60.Benarroch E. What Muscle Signals Mediate the Beneficial Effects of Exercise on Cognition? Neurology. 2022;99:298–304. doi: 10.1212/WNL.0000000000201049. [DOI] [PubMed] [Google Scholar]
- 61.NHIS Adult Physical Activity—Glossary. [(accessed on 17 April 2024)]; Available online: https://www.cdc.gov/nchs/nhis/physical_activity/pa_glossary.htm.
- 62.Tolwani R.J., Jakowec M.W., Petzinger G.M., Green S., Waggie K. Experimental models of Parkinson’s disease: Insights from many models. Comp. Med. 1999;49:363–371. [PubMed] [Google Scholar]
- 63.Lau Y.S., Patki G., Das-Panja K., Le W.D., Ahmad S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goes A., Souza L., Del Fabbro L., De Gomes M., Boeira S., Jesse C. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience. 2014;256:61–71. doi: 10.1016/j.neuroscience.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 65.Petzinger G.M., Walsh J.P., Akopian G., Hogg E., Abernathy A., Arevalo P., Turnquist P., Vučković M., Fisher B.E., Togasaki D.M. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palasz E., Niewiadomski W., Gasiorowska A., Wysocka A., Stepniewska A., Niewiadomska G. Exercise-induced neuroprotection and recovery of motor function in animal models of Parkinson’s disease. Front. Neurol. 2019;10:1143. doi: 10.3389/fneur.2019.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schenkman M., Moore C.G., Kohrt W.M., Hall D.A., Delitto A., Comella C.L., Josbeno D.A., Christiansen C.L., Berman B.D., Kluger B.M. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018;75:219–226. doi: 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tollár J., Nagy F., Hortobágyi T. Vastly different exercise programs similarly improve parkinsonian symptoms: A randomized clinical trial. Gerontology. 2019;65:120–127. doi: 10.1159/000493127. [DOI] [PubMed] [Google Scholar]
- 69.Mak M.K., Wong-Yu I.S. Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: A randomized controlled trial. J. Park. Dis. 2021;11:1431–1441. doi: 10.3233/JPD-202503. [DOI] [PubMed] [Google Scholar]
- 70.How Much Should the Average Adult Exercise Every Day? [(accessed on 1 August 2024)]. Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/expert-answers/exercise/faq-20057916.
- 71.Exercise Intensity: How to Measure, It. [(accessed on 1 August 2024)]. Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/in-depth/exercise-intensity/art-20046887.
- 72.How Much Physical Activity Do You Need? [(accessed on 1 August 2024)]. Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-infographic.
- 73.Zhen K., Zhang S., Tao X., Li G., Lv Y., Yu L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. npj Park. Dis. 2022;8:146. doi: 10.1038/s41531-022-00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schootemeijer S., Darweesh S.K.L., de Vries N.M. Clinical Trial Highlights—Aerobic Exercise for Parkinson’s Disease. J. Park. Dis. 2022;12:2297–2306. doi: 10.3233/JPD-229006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alberts J.L., Rosenfeldt A.B. The universal prescription for Parkinson’s disease: Exercise. J. Park. Dis. 2020;10:S21–S27. doi: 10.3233/JPD-202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osborne J.A., Botkin R., Colon-Semenza C., DeAngelis T.R., Gallardo O.G., Kosakowski H., Martello J., Pradhan S., Rafferty M., Readinger J.L. Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys. Ther. 2022;102:pzab302. doi: 10.1093/ptj/pzab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carroll L.M., Volpe D., Morris M.E., Saunders J., Clifford A.M. Aquatic exercise therapy for people with Parkinson disease: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2017;98:631–638. doi: 10.1016/j.apmr.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Landers M.R., Navalta J.W., Murtishaw A.S., Kinney J.W., Richardson S.P. A high-intensity exercise boot camp for persons with Parkinson disease: A phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J. Neurol. Phys. Ther. 2019;43:12–25. doi: 10.1097/NPT.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 79.Penko A.L., Zimmerman N.M., Crawford M., Linder S.M., Alberts J.L. Effect of aerobic exercise on cardiopulmonary responses and predictors of change in individuals with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2021;102:925–931. doi: 10.1016/j.apmr.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Cugusi L., Manca A., Bergamin M., Di Blasio A., Monticone M., Deriu F., Mercuro G. Aquatic exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: A systematic review. J. Physiother. 2019;65:65–74. doi: 10.1016/j.jphys.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Cugusi L., Solla P., Serpe R., Carzedda T., Piras L., Oggianu M., Gabba S., Di Blasio A., Bergamin M., Cannas A. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation. 2015;37:245–254. doi: 10.3233/NRE-151257. [DOI] [PubMed] [Google Scholar]
- 82.de Almeida F.O., Santana V., Corcos D.M., Ugrinowitsch C., Silva-Batista C. Effects of endurance training on motor signs of Parkinson’s disease: A systematic review and meta-analysis. Sports Med. 2022;52:1789–1815. doi: 10.1007/s40279-022-01650-x. [DOI] [PubMed] [Google Scholar]
- 83.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blumenthal J.A., Babyak M.A., Moore K.A., Craighead W.E., Herman S., Khatri P., Waugh R., Napolitano M.A., Forman L.M., Appelbaum M. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 85.Altmann L.J., Stegemöller E., Hazamy A.A., Wilson J.P., Bowers D., Okun M.S., Hass C.J. Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: Results of a controlled study. J. Int. Neuropsychol. Soc. 2016;22:878–889. doi: 10.1017/S135561771600076X. [DOI] [PubMed] [Google Scholar]
- 86.David F.J., Robichaud J.A., Leurgans S.E., Poon C., Kohrt W.M., Goldman J.G., Comella C.L., Vaillancourt D.E., Corcos D.M. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov. Disord. 2015;30:1657–1663. doi: 10.1002/mds.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duchesne C., Lungu O., Nadeau A., Robillard M., Boré A., Bobeuf F., Lafontaine A., Gheysen F., Bherer L., Doyon J. Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka K., de Quadros A.C., Jr., Santos R.F., Stella F., Gobbi L.T.B., Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69:435–441. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Sacheli M.A., Neva J.L., Lakhani B., Murray D.K., Vafai N., Shahinfard E., English C., McCormick S., Dinelle K., Neilson N. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. 2019;34:1891–1900. doi: 10.1002/mds.27865. [DOI] [PubMed] [Google Scholar]
- 90.Niemann C., Godde B., Staudinger U.M., Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014;281:147–163. doi: 10.1016/j.neuroscience.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 91.Marusiak J., Fisher B.E., Jaskólska A., Słotwiński K., Budrewicz S., Koszewicz M., Kisiel-Sajewicz K., Kamiński B., Jaskólski A. Eight weeks of aerobic interval training improves psychomotor function in patients with Parkinson’s disease—Randomized controlled trial. Int. J. Environ. Res. Public Health. 2019;16:880. doi: 10.3390/ijerph16050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valenzuela C.S.M., Moscardó L.D., López-Pascual J., Serra-Añó P., Tomás J.M. Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: Results of randomized controlled DUALGAIT trial. Arch. Phys. Med. Rehabil. 2020;101:1849–1856.e1. doi: 10.1016/j.apmr.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 93.de Laat B., Hoye J., Stanley G., Hespeler M., Ligi J., Mohan V., Wooten D.W., Zhang X., Nguyen T.D., Key J. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson’s disease. npj Park. Dis. 2024;10:34. doi: 10.1038/s41531-024-00641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung C.L.H., Thilarajah S., Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2016;30:11–23. doi: 10.1177/0269215515570381. [DOI] [PubMed] [Google Scholar]
- 95.Hirsch M.A., Toole T., Maitland C.G., Rider R.A. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch. Phys. Med. Rehabil. 2003;84:1109–1117. doi: 10.1016/S0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 96.Stone M.H., Stone M., Sands W.A. Principles and Practice of Resistance Training. Human Kinetics; Houston, TX, USA: 2007. [Google Scholar]
- 97.Hunter G.R., McCarthy J.P., Bamman M.M. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 98.Corcos D.M., Lamotte G., Luthra N.S., McKee K.E. Advice to People with Parkinson’s in My Clinic: Exercise. J. Park. Dis. 2024;14:609–617. doi: 10.3233/JPD-230277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Lima T.A., Ferreira-Moraes R., Alves W.M.G.d.C., Alves T.G.G., Pimentel C.P., Sousa E.C., Abrahin O., Cortinhas-Alves E.A. Resistance training reduces depressive symptoms in elderly people with Parkinson disease: A controlled randomized study. Scand. J. Med. Sci. Sports. 2019;29:1957–1967. doi: 10.1111/sms.13528. [DOI] [PubMed] [Google Scholar]
- 100.Leal L.C., Abrahin O., Rodrigues R.P., da Silva M.C., Araújo A.P., de Sousa E.C., Pimentel C.P., Cortinhas-Alves E.A. Low-volume resistance training improves the functional capacity of older individuals with Parkinson’s disease. Geriatr. Gerontol. Int. 2019;19:635–640. doi: 10.1111/ggi.13682. [DOI] [PubMed] [Google Scholar]
- 101.Silva-Batista C., Corcos D.M., Kanegusuku H., Piemonte M.E.P., Gobbi L.T.B., de Lima-Pardini A.C., de Mello M.T., Forjaz C.L., Ugrinowitsch C. Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait Posture. 2018;61:90–97. doi: 10.1016/j.gaitpost.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 102.Cherup N.P., Buskard A.N., Strand K.L., Roberson K.B., Michiels E.R., Kuhn J.E., Lopez F.A., Signorile J.F. Power vs strength training to improve muscular strength, power, balance and functional movement in individuals diagnosed with Parkinson’s disease. Exp. Gerontol. 2019;128:110740. doi: 10.1016/j.exger.2019.110740. [DOI] [PubMed] [Google Scholar]
- 103.Gamborg M., Hvid L.G., Dalgas U., Langeskov-Christensen M. Parkinson’s disease and intensive exercise therapy—An updated systematic review and meta-analysis. Acta Neurol. Scand. 2022;145:504–528. doi: 10.1111/ane.13579. [DOI] [PubMed] [Google Scholar]
- 104.Silva-Batista C., Corcos D.M., Barroso R., David F.J., Kanegusuku H., Forjaz C., MT D.E.M., Roschel H., Tricoli V., Ugrinowitsch C. Instability Resistance Training Improves Neuromuscular Outcome in Parkinson’s Disease. Med. Sci. Sports Exerc. 2017;49:652–660. doi: 10.1249/MSS.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 105.Canning C.G., Sherrington C., Lord S.R., Close J.C., Heritier S., Heller G.Z., Howard K., Allen N.E., Latt M.D., Murray S.M., et al. Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology. 2015;84:304–312. doi: 10.1212/WNL.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bloem B.R., Marinus J., Almeida Q., Dibble L., Nieuwboer A., Post B., Ruzicka E., Goetz C., Stebbins G., Martinez-Martin P., et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2016;31:1342–1355. doi: 10.1002/mds.26572. [DOI] [PubMed] [Google Scholar]
- 107.Shulman L.M., Katzel L.I., Ivey F.M., Sorkin J.D., Favors K., Anderson K.E., Smith B.A., Reich S.G., Weiner W.J., Macko R.F. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70:183–190. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vieira-Yano B., Martini D.N., Horak F.B., de Lima-Pardini A., Almeida F., Santana V.P., Lima D., Batista A.X., Marquesini R., Lira J., et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Mov. Disord. 2021;36:152–163. doi: 10.1002/mds.28298. [DOI] [PubMed] [Google Scholar]
- 109.Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 110.Santos L., Fernandez-Rio J., Winge K., Barragán-Pérez B., González-Gómez L., Rodríguez-Pérez V., González-Díez V., Lucía A., Iglesias-Soler E., Dopico-Calvo X., et al. Effects of progressive resistance exercise in akinetic-rigid Parkinson’s disease patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017;53:651–663. doi: 10.23736/S1973-9087.17.04572-5. [DOI] [PubMed] [Google Scholar]
- 111.Alves W.M., Alves T.G., Ferreira R.M., Lima T.A., Pimentel C.P., Sousa E.C., Abrahin O., Alves E.A. Strength training improves the respiratory muscle strength and quality of life of elderly with Parkinson disease. J. Sports Med. Phys. Fit. 2019;59:1756–1762. doi: 10.23736/S0022-4707.19.09509-4. [DOI] [PubMed] [Google Scholar]
- 112.Ferreira R.M., Alves W., de Lima T.A., Alves T.G.G., Alves Filho P.A.M., Pimentel C.P., Sousa E.C., Cortinhas-Alves E.A. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson’s disease: A randomized controlled trial. Arq. Neuropsiquiatr. 2018;76:499–506. doi: 10.1590/0004-282x20180071. [DOI] [PubMed] [Google Scholar]
- 113.Park J.-H., Kang Y.-J., Horak F.B. What is wrong with balance in Parkinson’s disease? J. Mov. Disord. 2015;8:109. doi: 10.14802/jmd.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grabli D., Karachi C., Welter M.-L., Lau B., Hirsch E.C., Vidailhet M., François C. Normal and pathological gait: What we learn from Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2012;83:979–985. doi: 10.1136/jnnp-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morris M.E., Iansek R. Characteristics of motor disturbance in Parkinson’s disease and strategies for movement rehabilitation. Hum. Mov. Sci. 1996;15:649–669. doi: 10.1016/0167-9457(96)00020-6. [DOI] [Google Scholar]
- 116.Angel R.W., Alston W., Higgins J.R. Control of movement in Parkinson’s disease. Brain. 1970;93:1–14. doi: 10.1093/brain/93.1.1. [DOI] [PubMed] [Google Scholar]
- 117.Farley B.G., Fox C.M., Ramig L.O., McFarland D.H. Intensive amplitude-specific therapeutic approaches for Parkinson’s disease: Toward a neuroplasticity-principled rehabilitation model. Top. Geriatr. Rehabil. 2008;24:99–114. doi: 10.1097/01.TGR.0000318898.87690.0d. [DOI] [Google Scholar]
- 118.Hall M.-F.E., Church F.C. Integrative medicine and health therapy for Parkinson disease. Top. Geriatr. Rehabil. 2020;36:176–186. doi: 10.1097/TGR.0000000000000278. [DOI] [Google Scholar]
- 119.Larson D., Yeh C., Rafferty M., Bega D. High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey. Disabil. Rehabil. 2022;44:6034–6041. doi: 10.1080/09638288.2021.1963854. [DOI] [PubMed] [Google Scholar]
- 120.van Eijkeren F.J., Reijmers R.S., Kleinveld M.J., Minten A., Bruggen J.P.t., Bloem B.R. Nordic walking improves mobility in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008;23:2239–2243. doi: 10.1002/mds.22293. [DOI] [PubMed] [Google Scholar]
- 121.Westheimer O. Why dance for Parkinson’s disease. Top. Geriatr. Rehabil. 2008;24:127–140. doi: 10.1097/01.TGR.0000318900.95313.af. [DOI] [Google Scholar]
- 122.Gao Q., Leung A., Yang Y., Wei Q., Guan M., Jia C., He C. Effects of Tai Chi on balance and fall prevention in Parkinson’s disease: A randomized controlled trial. Clin. Rehabil. 2014;28:748–753. doi: 10.1177/0269215514521044. [DOI] [PubMed] [Google Scholar]
- 123.Cash M.F., Ulanowski E., Danzl M. Development of a community-based golf and exercise program for people with Parkinson’s disease. Complement. Ther. Clin. Pract. 2018;33:149–155. doi: 10.1016/j.ctcp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 124.Bliss R.R., Church F.C. Golf as a Physical Activity to Potentially Reduce the Risk of Falls in Older Adults with Parkinson’s Disease. Sports. 2021;9:72. doi: 10.3390/sports9060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Puymbroeck M., Walter A., Hawkins B.L., Sharp J.L., Woschkolup K., Urrea-Mendoza E., Revilla F., Adams E.V., Schmid A.A. Functional improvements in Parkinson’s disease following a randomized trial of yoga. Evid.-Based Complement. Altern. Med. 2018;2018:8516351. doi: 10.1155/2018/8516351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bloem B.R., Hausdorff J.M., Visser J.E., Giladi N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 127.Tolosa E., Compta Y. Dystonia in Parkinson’s disease. J. Neurol. 2006;253:vii7–vii13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- 128.Robbins J.A., Logemann J.A., Kirshner H.S. Swallowing and speech production in Parkinson’s disease. Ann. Neurol. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 129.Fox C.M., Ramig L.O., Ciucci M.R., Sapir S., McFarland D.H., Farley B.G. The science and practice of LSVT/LOUD: Neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin. Speech Lang. 2006;27:283–299. doi: 10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- 130.Sapir S., Ramig L.O., Hoyt P., Countryman S., O’Brien C., Hoehn M. Speech loudness and quality 12 months after intensive voice treatment (LSVT®) for Parkinson’s disease: A comparison with an alternative speech treatment. Folia Phoniatr. Logop. 2002;54:296–303. doi: 10.1159/000066148. [DOI] [PubMed] [Google Scholar]
- 131.Fox C., Ebersbach G., Ramig L., Sapir S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Park. Dis. 2012;2012:391946. doi: 10.1155/2012/391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kraus A., Joyner N., Simpson H., Farley B. The Application of PD Specific Functional Training in Group Rehabilitative Sessions-Physical Performance Outcomes. 2024. [(accessed on 7 September 2024)]. Available online: https://files.constantcontact.com/fb612c26101/0ee251fe-1346-4e34-8e13-eff9a368c859.pdf.
- 133.Combs S.A., Diehl M.D., Staples W.H., Conn L., Davis K., Lewis N., Schaneman K. Boxing training for patients with Parkinson disease: A case series. Phys. Ther. 2011;91:132–142. doi: 10.2522/ptj.20100142. [DOI] [PubMed] [Google Scholar]
- 134.Bek J., Arakaki A.I., Lawrence A., Sullivan M., Ganapathy G., Poliakoff E. Dance and Parkinson’s: A review and exploration of the role of cognitive representations of action. Neurosci. Biobehav. Rev. 2020;109:16–28. doi: 10.1016/j.neubiorev.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 135.Kalyani H., Sullivan K., Moyle G., Brauer S., Jeffrey E.R., Kerr G. Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson’s disease. NeuroRehabilitation. 2019;45:273–283. doi: 10.3233/NRE-192788. [DOI] [PubMed] [Google Scholar]
- 136.de Natale E.R., Paulus K.S., Aiello E., Sanna B., Manca A., Sotgiu G., Leali P.T., Deriu F. Dance therapy improves motor and cognitive functions in patients with Parkinson’s disease. NeuroRehabilitation. 2017;40:141–144. doi: 10.3233/NRE-161399. [DOI] [PubMed] [Google Scholar]
- 137.Bearss K.A., DeSouza J.F. Parkinson’s disease motor symptom progression slowed with multisensory dance learning over 3-years: A preliminary longitudinal investigation. Brain Sci. 2021;11:895. doi: 10.3390/brainsci11070895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hashimoto H., Takabatake S., Miyaguchi H., Nakanishi H., Naitou Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: A quasi-randomized pilot trial. Complement. Ther. Med. 2015;23:210–219. doi: 10.1016/j.ctim.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 139.Heiberger L., Maurer C., Amtage F., Mendez-Balbuena I., Schulte-Mönting J., Hepp-Reymond M.-C., Kristeva R. Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson’s disease. Front. Aging Neurosci. 2011;3:14. doi: 10.3389/fnagi.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hackney M.E., Kantorovich S., Levin R., Earhart G.M. Effects of tango on functional mobility in Parkinson’s disease: A preliminary study. J. Neurol. Phys. Ther. 2007;31:173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 141.Berardelli A., Sabra A., Hallett M. Physiological mechanisms of rigidity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1983;46:45–53. doi: 10.1136/jnnp.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andrews C.J., Burke D., Lance J.W. The response to muscle stretch and shortening in Parkinsonian rigidity. Brain. 1972;95:795–812. doi: 10.1093/brain/95.4.795. [DOI] [PubMed] [Google Scholar]
- 143.Ferreira-Sánchez M.d.R., Moreno-Verdú M., Cano-de-La-Cuerda R. Quantitative measurement of rigidity in Parkinson’s disease: A systematic review. Sensors. 2020;20:880. doi: 10.3390/s20030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benatru I., Vaugoyeau M., Azulay J.-P. Postural disorders in Parkinson’s disease. Neurophysiol. Clin./Clin. Neurophysiol. 2008;38:459–465. doi: 10.1016/j.neucli.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 145.Mak M.K., Wong-Yu I.S. Exercise for Parkinson’s disease. Int. Rev. Neurobiol. 2019;147:1–44. doi: 10.1016/bs.irn.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 146.Palmer S.S., Mortimer J.A., Webster D.D., Bistevins R., Dickinson G.L. Exercise therapy for Parkinson’s disease. Arch. Phys. Med. Rehabil. 1986;67:741–745. doi: 10.1016/0003-9993(86)90007-9. [DOI] [PubMed] [Google Scholar]
- 147.Emig M., George T., Zhang J.K., Soudagar-Turkey M. The role of exercise in Parkinson’s disease. J. Geriatr. Psychiatry Neurol. 2021;34:321–330. doi: 10.1177/08919887211018273. [DOI] [PubMed] [Google Scholar]
- 148.Gobbi L.T., Oliveira-Ferreira M.D., Caetano M.J.D., Lirani-Silva E., Barbieri F.A., Stella F., Gobbi S. Exercise programs improve mobility and balance in people with Parkinson’s disease. Park. Relat. Disord. 2009;15:S49–S52. doi: 10.1016/S1353-8020(09)70780-1. [DOI] [PubMed] [Google Scholar]
- 149.Flach A., Jaegers L., Krieger M., Bixler E., Kelly P., Weiss E.P., Ahmad S.O. Endurance exercise improves function in individuals with Parkinson’s disease: A meta-analysis. Neurosci. Lett. 2017;659:115–119. doi: 10.1016/j.neulet.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 150.Steiger L., Homann C.N. Exercise therapy in Parkinson’s disease–An overview of current interventional studies. Physiother. Res. Rep. 2019;1:1–10. doi: 10.15761/PRR.1000117. [DOI] [Google Scholar]
- 151.Choi H., Cho K.-H., Jin C., Lee J., Kim T.-H., Jung W.-S., Moon S.-K., Ko C.-N., Cho S.-Y., Jeon C.-Y. Exercise Therapies for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Park. Dis. 2020;2020:2565320. doi: 10.1155/2020/2565320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.PRISMA Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) [(accessed on 1 August 2024)]. Available online: http://www.prismastatement.org.
- 153.Higgins J. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration and John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2008. [Google Scholar]
- 154.Schootemeijer S., van der Kolk N.M., Bloem B.R., de Vries N.M. Current perspectives on aerobic exercise in people with Parkinson’s disease. Neurotherapeutics. 2020;17:1418–1433. doi: 10.1007/s13311-020-00904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martignon C., Pedrinolla A., Ruzzante F., Giuriato G., Laginestra F.G., Bouca-Machado R., Ferreira J.J., Tinazzi M., Schena F., Venturelli M. Guidelines on exercise testing and prescription for patients at different stages of Parkinson’s disease. Aging Clin. Exp. Res. 2021;33:221–246. doi: 10.1007/s40520-020-01612-1. [DOI] [PubMed] [Google Scholar]
- 156.Hao Z., Zhang X., Chen P. Effects of ten different exercise interventions on motor function in Parkinson’s disease patients—A Network meta-analysis of randomized controlled trials. Brain Sci. 2022;12:698. doi: 10.3390/brainsci12060698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li J.A., Loevaas M.B., Guan C., Goh L., Allen N.E., Mak M.K., Lv J., Paul S.S. Does exercise attenuate disease progression in people with Parkinson’s disease? A systematic review with meta-analyses. Neurorehabilit. Neural Repair. 2023;37:328–352. doi: 10.1177/15459683231172752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 159.Ernst M., Folkerts A.-K., Gollan R., Lieker E., Caro-Valenzuela J., Adams A., Cryns N., Monsef I., Dresen A., Roheger M. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2024;1:CD013856. doi: 10.1002/14651858.CD013856.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Langeskov-Christensen M., Franzén E., Hvid L.G., Dalgas U. Exercise as medicine in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2024 doi: 10.1136/jnnp-2023-332974. ahead of print . [DOI] [PubMed] [Google Scholar]
- 161.Padilha C., Souza R., Grossl F.S., Gauer A.P.M., de Sá C.A., Rodrigues-Junior S.A. Physical exercise and its effects on people with Parkinson’s disease: Umbrella review. PLoS ONE. 2023;18:e0293826. doi: 10.1371/journal.pone.0293826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moratelli J.A., Lima A.G., Alexandre K.H., Fausto D.Y., Haas A.N., de Azevedo Guimarães A.C. Evidence of physical activity interventions on non-motor symptoms of people with Parkinson’s disease: An umbrella review. Sport Sci. Health. 2024;20:321–336. doi: 10.1007/s11332-024-01197-6. [DOI] [Google Scholar]
- 163.Yu J., Wu J., Lu J., Wei X., Zheng K., Liu B., Xiao W., Shi Q., Xiong L., Ren Z. Efficacy of virtual reality training on motor performance, activity of daily living, and quality of life in patients with Parkinson’s disease: An umbrella review comprising meta-analyses of randomized controlled trials. J. Neuro Eng. Rehabil. 2023;20:133. doi: 10.1186/s12984-023-01256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodrigues-Juniora S.A., Padilhaa C., Souzaa R., de Sáa C.A. Physical exercise interventions for people with Parkinson’s disease: A bibliometric review of systematic reviews. Geriatr. Gerontol. Aging. 2023;18:e0230035. doi: 10.53886/gga.e0230035. [DOI] [Google Scholar]
- 165.Chekroud S.R., Gueorguieva R., Zheutlin A.B., Paulus M., Krumholz H.M., Krystal J.H., Chekroud A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 166.Cordani C., Mosconi B. What type of physical exercise works best to improve movement and quality of life for people with Parkinson’s disease?-A Cochrane Review summary with commentary. NeuroRehabilitation. 2024;54:699–702. doi: 10.3233/NRE-246004. [DOI] [PubMed] [Google Scholar]
- 167.Chodzko-Zajko W., Proctor D., Fiatarone Singh M., Minson C., Nigg C., Salem G., Skinner J. Exercise and physical activity for older adults. American College of Sports Medicine position stand. Med. Sci. Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 168.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., George S.M., Olson R.D. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goldman J.G., Volpe D., Ellis T.D., Hirsch M.A., Johnson J., Wood J., Aragon A., Biundo R., Di Rocco A., Kasman G.S. Delivering multidisciplinary rehabilitation Care in Parkinson’s disease: An international consensus statement. J. Park. Dis. 2024;14:135–166. doi: 10.3233/JPD-230117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Domingos J., Keus S.H.J., Dean J., de Vries N.M., Ferreira J.J., Bloem B.R. The European Physiotherapy Guideline for Parkinson’s Disease: Implications for Neurologists. J. Park. Dis. 2018;8:499–502. doi: 10.3233/jpd-181383. [DOI] [PubMed] [Google Scholar]
- 171.Rafferty M.R., Schmidt P.N., Luo S.T., Li K., Marras C., Davis T.L., Guttman M., Cubillos F., Simuni T., all NPF-QII Investigators Regular exercise, quality of life, and mobility in Parkinson’s disease: A longitudinal analysis of national Parkinson foundation quality improvement initiative data. J. Park. Dis. 2017;7:193–202. doi: 10.3233/JPD-160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Speelman A.D., Van De Warrenburg B.P., Van Nimwegen M., Petzinger G.M., Munneke M., Bloem B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 173.van Nimwegen M., Speelman A.D., Hofman-van Rossum E.J., Overeem S., Deeg D.J., Borm G.F., van der Horst M.H., Bloem B.R., Munneke M. Physical inactivity in Parkinson’s disease. J. Neurol. 2011;258:2214–2221. doi: 10.1007/s00415-011-6097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ghadieh A.S., Saab B. Evidence for exercise training in the management of hypertension in adults. Can. Fam. Physician. 2015;61:233–239. [PMC free article] [PubMed] [Google Scholar]
- 175.Speelman A.D., Groothuis J.T., van Nimwegen M., van der Scheer E.S., Borm G.F., Bloem B.R., Hopman M.T., Munneke M. Cardiovascular responses during a submaximal exercise test in patients with Parkinson’s disease. J. Park. Dis. 2012;2:241–247. doi: 10.3233/JPD-2012-012111. [DOI] [PubMed] [Google Scholar]
- 176.Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 177.Chen M.J., Fan X., Moe S.T. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002;20:873–899. doi: 10.1080/026404102320761787. [DOI] [PubMed] [Google Scholar]
- 178.Cook D., McRae C., Kumar R. Impact of self-efficacy education on physical and psychosocial functioning in newly-diagnosed Parkinson patients. Mov. Disord. 2016;31:S170. [Google Scholar]
- 179.Li G., Huang P., Cui S.-S., Tan Y.-Y., He Y.-C., Shen X., Jiang Q.-Y., Huang P., He G.-Y., Li B.-Y. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022;11:6. doi: 10.1186/s40035-022-00280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Foundation P.s. Exercise Convening Summary Report. [(accessed on 22 July 2024)]. Available online: https://cdn.flipsnack.com/widget/v2/widget.html?hash=vp3ycpz2gx.
- 181.Morgan J.A., Corrigan F., Baune B.T. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry. 2015;3:3. doi: 10.1186/s40303-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Perrey S. Promoting motor function by exercising the brain. Brain Sci. 2013;3:101–122. doi: 10.3390/brainsci3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lavin K.M., Ge Y., Sealfon S.C., Nair V.D., Wilk K., McAdam J.S., Windham S.T., Kumar P.L., McDonald M.-L.N., Bamman M.M. Rehabilitative impact of exercise training on human skeletal muscle transcriptional programs in Parkinson’s disease. Front. Physiol. 2020;11:653. doi: 10.3389/fphys.2020.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kelly N.A., Hammond K.G., Bickel C.S., Windham S.T., Tuggle S.C., Bamman M.M. Effects of aging and Parkinson’s disease on motor unit remodeling: Influence of resistance exercise training. J. Appl. Physiol. 2018;124:888–898. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reiner M., Niermann C., Jekauc D., Woll A. Long-term health benefits of physical activity—A systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li J., Guo J., Sun W., Mei J., Wang Y., Zhang L., Zhang J., Gao J., Su K., Lv Z. Effects of exercise on Parkinson’s disease: A meta-analysis of brain imaging studies. Front. Hum. Neurosci. 2022;16:796712. doi: 10.3389/fnhum.2022.796712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tsukita K., Sakamaki-Tsukita H., Takahashi R. Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology. 2022;98:e859–e871. doi: 10.1212/WNL.0000000000013218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Müller J., Myers J. Association between physical fitness, cardiovascular risk factors, and Parkinson’s disease. Eur. J. Prev. Cardiol. 2018;25:1409–1415. doi: 10.1177/2047487318771168. [DOI] [PubMed] [Google Scholar]
- 189.Thacker E.L., Chen H., Patel A.V., McCullough M.L., Calle E.E., Thun M.J., Schwarzschild M.A., Ascherio A. Recreational physical activity and risk of Parkinson’s disease. Mov. Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang F., Trolle Lagerros Y., Bellocco R., Adami H.-O., Fang F., Pedersen N.L., Wirdefeldt K. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2015;138:269–275. doi: 10.1093/brain/awu323. [DOI] [PubMed] [Google Scholar]
- 191.Sääksjärvi K., Knekt P., Männistö S., Lyytinen J., Jääskeläinen T., Kanerva N., Heliövaara M. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur. J. Epidemiol. 2014;29:285–292. doi: 10.1007/s10654-014-9887-2. [DOI] [PubMed] [Google Scholar]
- 192.Logroscino G., Sesso H., Paffenbarger R., Lee I.-M. Physical activity and risk of Parkinson’s disease: A prospective cohort study. J. Neurol. Neurosurg. Psychiatry. 2006:1318–1322. doi: 10.1136/jnnp.2006.097170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Castillo-Armengol J., Fajas L., Lopez-Mejia I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019;20:e47903. doi: 10.15252/embr.201947903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou N., Gong L., Zhang E., Wang X. Exploring exercise-driven exerkines: Unraveling the regulation of metabolism and inflammation. PeerJ. 2024;12:e17267. doi: 10.7717/peerj.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamauchi T., Kamon J., Minokoshi Y.a., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 196.Bouassida A., Chamari K., Zaouali M., Feki Y., Zbidi A., Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010;44:620–630. doi: 10.1136/bjsm.2008.046151. [DOI] [PubMed] [Google Scholar]
- 197.Fang H., Judd R.L. Adiponectin regulation and function. Compr. Physiol. 2011;8:1031–1063. doi: 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 198.Polito R., Di Meo I., Barbieri M., Daniele A., Paolisso G., Rizzo M.R. Adiponectin role in neurodegenerative diseases: Focus on nutrition review. Int. J. Mol. Sci. 2020;21:9255. doi: 10.3390/ijms21239255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou J.-X., Shuai N.-N., Wang B., Jin X., Kuang X., Tian S.-W. Neuroprotective gain of Apelin/APJ system. Neuropeptides. 2021;87:102131. doi: 10.1016/j.npep.2021.102131. [DOI] [PubMed] [Google Scholar]
- 200.Kammoun H.L., Febbraio M.A. Come on BAIBA light my fire. Cell Metab. 2014;19:1–2. doi: 10.1016/j.cmet.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 201.Tanianskii D.A., Jarzebska N., Birkenfeld A.L., O’Sullivan J.F., Rodionov R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients. 2019;11:524. doi: 10.3390/nu11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yi X., Yang Y., Li T., Li M., Yao T., Hu G., Wan G., Chang B. Signaling metabolite beta-aminoisobutyric acid as a metabolic regulator, biomarker, and potential exercise pill. Front. Endocrinol. 2023;14:1192458. doi: 10.3389/fendo.2023.1192458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thickbroom G.W. The therapeutic potential of ketone bodies in Parkinson’s disease. Expert Rev. Neurother. 2021;21:255–257. doi: 10.1080/14737175.2021.1881483. [DOI] [PubMed] [Google Scholar]
- 204.Rahman M., Muhammad S., Khan M.A., Chen H., Ridder D.A., Müller-Fielitz H., Pokorná B., Vollbrandt T., Stölting I., Nadrowitz R. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 205.Newman J.C., Verdin E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neeper S.A., Góauctemez-Pinilla F., Choi J., Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 207.Matthews V.B., Åström M.-B., Chan M., Bruce C.R., Krabbe K., Prelovsek O., Åkerström T., Yfanti C., Broholm C., Mortensen O.H. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 208.Koliatsos V.E., Clatterbuck R.E., Winslow J.W., Cayouette M.H., Prices D.L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-M. [DOI] [PubMed] [Google Scholar]
- 209.Nicolini C., Michalski B., Toepp S.L., Turco C.V., D’Hoine T., Harasym D., Gibala M.J., Fahnestock M., Nelson A.J. A single bout of high-intensity interval exercise increases corticospinal excitability, brain-derived neurotrophic factor, and uncarboxylated osteolcalcin in sedentary, healthy males. Neuroscience. 2020;437:242–255. doi: 10.1016/j.neuroscience.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 210.Moon H.Y., Becke A., Berron D., Becker B., Sah N., Benoni G., Janke E., Lubejko S.T., Greig N.H., Mattison J.A. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016;24:332–340. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hummel R.P., James J.H., Warner B.W., Hasselgren P.-O., Fischer J.E. Evidence that cathepsin B contributes to skeletal muscle protein breakdown during sepsis. Arch. Surg. 1988;123:221–224. doi: 10.1001/archsurg.1988.01400260105013. [DOI] [PubMed] [Google Scholar]
- 212.Watt M.J., Miotto P.M., De Nardo W., Montgomery M.K. The liver as an endocrine organ—Linking NAFLD and insulin resistance. Endocr. Rev. 2019;40:1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 213.Sargeant J.A., Aithal G.P., Takamura T., Misu H., Takayama H., Douglas J.A., Turner M.C., Stensel D.J., Nimmo M.A., Webb D.R. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 2018;43:482–490. doi: 10.1139/apnm-2017-0639. [DOI] [PubMed] [Google Scholar]
- 214.Keihanian A., Arazi H., Kargarfard M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol. Int. 2019;106:70–80. doi: 10.1556/2060.106.2019.01. [DOI] [PubMed] [Google Scholar]
- 215.Kim K.H., Kim S.H., Min Y.-K., Yang H.-M., Lee J.-B., Lee M.-S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Izumiya Y., Bina H.A., Ouchi N., Akasaki Y., Kharitonenkov A., Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hondares E., Iglesias R., Giralt A., Gonzalez F.J., Giralt M., Mampel T., Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McCullough M.J., Gyorkos A.M., Spitsbergen J.M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience. 2013;240:258–268. doi: 10.1016/j.neuroscience.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gyorkos A.M., McCullough M.J., Spitsbergen J.M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience. 2014;257:111–118. doi: 10.1016/j.neuroscience.2013.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Treanor J.J., Goodman L., de Sauvage F., Stone D.M., Poulsen K.T., Beck C.D., Gray C., Armanini M.P., Pollock R.A., Hefti F., et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 221.Horowitz A.M., Fan X., Bieri G., Smith L.K., Sanchez-Diaz C.I., Schroer A.B., Gontier G., Casaletto K.B., Kramer J.H., Williams K.E. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abdolmaleki F., Heidarianpour A. Endurance exercise training restores diabetes-induced alteration in circulating Glycosylphosphatidylinositol-specific phospholipase D levels in rats. Diabetol. Metab. Syndr. 2020;12:43. doi: 10.1186/s13098-020-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Voss M.W., Nagamatsu L.S., Liu-Ambrose T., Kramer A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stein A.M., Silva T.M.V., Coelho F.G.D.M., Arantes F.J., Costa J.L.R., Teodoro E., Santos-Galduróz R.F. Physical exercise, IGF-1 and cognition A systematic review of experimental studies in the elderly. Dement. Neuropsychol. 2018;12:114–122. doi: 10.1590/1980-57642018dn12-020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 226.Steensberg A., van Hall G., Osada T., Sacchetti M., Saltin B., Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. Pt 1J. Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Spooren A., Kolmus K., Laureys G., Clinckers R., De Keyser J., Haegeman G., Gerlo S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 228.Juttler E., Tarabin V., Schwaninger M. Interleukin-6 (IL-6): A possible neuromodulator induced by neuronal activity. Neuroscientist. 2002;8:268–275. doi: 10.1177/1073858402008003012. [DOI] [PubMed] [Google Scholar]
- 229.Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Bostrom E.A., Choi J.H., Long J.Z., et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hashemi M.S., Ghaedi K., Salamian A., Karbalaie K., Emadi-Baygi M., Tanhaei S., Nasr-Esfahani M.H., Baharvand H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 231.Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nalbandian M., Takeda M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology. 2016;5:38. doi: 10.3390/biology5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Willkomm L., Schubert S., Jung R., Elsen M., Borde J., Gehlert S., Suhr F., Bloch W. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 2014;12:742–753. doi: 10.1016/j.scr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 234.Takimoto M., Hamada T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J. Appl. Physiol. (1985) 2014;116:1238–1250. doi: 10.1152/japplphysiol.01288.2013. [DOI] [PubMed] [Google Scholar]
- 235.Li V.L., He Y., Contrepois K., Liu H., Kim J.T., Wiggenhorn A.L., Tanzo J.T., Tung A.S.-H., Lyu X., Zushin P.-J.H. An exercise-inducible metabolite that suppresses feeding and obesity. Nature. 2022;606:785–790. doi: 10.1038/s41586-022-04828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xiao S., Li V.L., Long J.Z. Lac-Phe (N-lactoyl-phenylalanine) Trends Endocrinol. Metab. 2024;35:758–759. doi: 10.1016/j.tem.2024.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xiao S., Li V.L., Lyu X., Chen X., Wei W., Abbasi F., Knowles J.W., Tung A.S.-H., Deng S., Tiwari G. Lac-Phe mediates the effects of metformin on food intake and body weight. Nat. Metab. 2024;6:659–669. doi: 10.1038/s42255-024-00999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fuller O.K., Whitham M., Mathivanan S., Febbraio M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells. 2020;9:2182. doi: 10.3390/cells9102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fischetti F., Poli L., De Tommaso M., Paolicelli D., Greco G., Cataldi S. The role of exercise parameters on small extracellular vesicles and microRNAs cargo in preventing neurodegenerative diseases. Front. Physiol. 2023;14:1241010. doi: 10.3389/fphys.2023.1241010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khoury R., Nagy C. Running from stress: A perspective on the potential benefits of exercise-induced small extracellular vesicles for individuals with major depressive disorder. Front. Mol. Biosci. 2023;10:1154872. doi: 10.3389/fmolb.2023.1154872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lin T.W., Kuo Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013;3:39–53. doi: 10.3390/brainsci3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.van Praag H. Exercise and the brain: Something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chaouloff F. Physical exercise and brain monoamines: A review. Acta Physiol. Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 244.Panes J.D., Wendt A., Ramirez-Molina O., Castro P.A., Fuentealba J. Deciphering the role of PGC-1alpha in neurological disorders: From mitochondrial dysfunction to synaptic failure. Neural Regen. Res. 2022;17:237–245. doi: 10.4103/1673-5374.317957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M., Kelly D.P., Holloszy J.O. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 246.Wright D.C., Han D.H., Garcia-Roves P.M., Geiger P.C., Jones T.E., Holloszy J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 247.Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 248.Richardson R.S., Wagner H., Mudaliar S.R., Saucedo E., Henry R., Wagner P.D. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- 249.Rosenstein J.M., Krum J.M., Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ohashi K., Parker J.L., Ouchi N., Higuchi A., Vita J.A., Gokce N., Pedersen A.A., Kalthoff C., Tullin S., Sams A. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lovren F., Pan Y., Quan A., Szmitko P.E., Singh K.K., Shukla P.C., Gupta M., Chan L., Al-Omran M., Teoh H. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am. J. Physiol.-Heart Circ. Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kataoka H., Sugie K. Serum adiponectin levels between patients with Parkinson’s disease and those with PSP. Neurol. Sci. 2020;41:1125–1131. doi: 10.1007/s10072-019-04216-4. [DOI] [PubMed] [Google Scholar]
- 253.Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C., Daviaud D., Mir L., Marques M., Thalamas C. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int. J. Obes. 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 254.Yang Y., Zhang X., Cui H., Zhang C., Zhu C., Li L. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci. Lett. 2014;568:44–49. doi: 10.1016/j.neulet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 255.Jung T.W., Hwang H.J., Hong H.C., Yoo H.J., Baik S.H., Choi K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia. 2015;58:2096–2105. doi: 10.1007/s00125-015-3663-z. [DOI] [PubMed] [Google Scholar]
- 256.Roberts L.D., Bostrom P., O’Sullivan J.F., Schinzel R.T., Lewis G.D., Dejam A., Lee Y.K., Palma M.J., Calhoun S., Georgiadi A., et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kitase Y., Vallejo J.A., Gutheil W., Vemula H., Jahn K., Yi J., Zhou J., Brotto M., Bonewald L.F. beta-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018;22:1531–1544. doi: 10.1016/j.celrep.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jung T.W., Park H.S., Choi G.H., Kim D., Lee T. beta-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018;25:27. doi: 10.1186/s12929-018-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Minato T., Nakamura N., Saiki T., Miyabe M., Ito M., Matsubara T., Naruse K. beta-Aminoisobutyric acid, L-BAIBA, protects PC12 cells from hydrogen peroxide-induced oxidative stress and apoptosis via activation of the AMPK and PI3K/Akt pathway. IBRO Neurosci. Rep. 2022;12:65–72. doi: 10.1016/j.ibneur.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Stautemas J., Van Kuilenburg A.B.P., Stroomer L., Vaz F., Blancquaert L., Lefevere F.B.D., Everaert I., Derave W. Acute Aerobic Exercise Leads to Increased Plasma Levels of R- and S-beta-Aminoisobutyric Acid in Humans. Front. Physiol. 2019;10:1240. doi: 10.3389/fphys.2019.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Priyadarshini M., Layden B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets. 2015;7:e1045182. doi: 10.1080/19382014.2015.1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Neudorf H., Little J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2023;47:100677. doi: 10.1016/j.bj.2023.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jensen N.J., Wodschow H.Z., Nilsson M., Rungby J. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci. 2020;21:8767. doi: 10.3390/ijms21228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nagappan G., Lu B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 265.Numakawa T., Odaka H., Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018;19:3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Colucci-D’Amato L., Speranza L., Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020;21:7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zuccato C., Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 268.Wang Y., Liu H., Zhang B.-S., Soares J.C., Zhang X.Y. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Park. Relat. Disord. 2016;29:66–71. doi: 10.1016/j.parkreldis.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 269.Paterno A., Polsinelli G., Federico B. Changes of brain-derived neurotrophic factor (BDNF) levels after different exercise protocols: A systematic review of clinical studies in Parkinson’s disease. Front. Physiol. 2024;15:1352305. doi: 10.3389/fphys.2024.1352305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhao P., Wei Y., Sun G., Xu L., Wang T., Tian Y., Chao H., Tu Y., Ji J. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J. Neuroinflammation. 2022;19:269. doi: 10.1186/s12974-022-02633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yoon S., Boonpraman N., Kim C.Y., Moon J.-S., Yi S.S. Reduction of fetuin-A levels contributes to impairment of Purkinje cells in cerebella of patients with Parkinson’s disease. BMB Rep. 2023;56:308. doi: 10.5483/BMBRep.2022-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chung H.S., Choi K.M. Organokines in disease. Adv. Clin. Chem. 2020;94:261–321. doi: 10.1016/bs.acc.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 273.Hill C.M., Laeger T., Dehner M., Albarado D.C., Clarke B., Wanders D., Burke S.J., Collier J.J., Qualls-Creekmore E., Solon-Biet S.M., et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep. 2019;27:2934–2947.e3. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Liang Q., Zhong L., Zhang J., Wang Y., Bornstein S.R., Triggle C.R., Ding H., Lam K.S., Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 275.Kazmerova Z., Zilka N., Cente M., Neradil P., Zilkova M., Novak M. Can we teach old dogs new tricks? Neuroprotective cell therapy in Alzheimer’s and Parkinson’s disease. J. Alzheimer’s Dis. 2013;37:251–272. doi: 10.3233/JAD-130572. [DOI] [PubMed] [Google Scholar]
- 276.Distefano G., Goodpaster B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018;8:a029785. doi: 10.1101/cshperspect.a029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brown B., Peiffer J., Martins R. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry. 2013;18:864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 278.Horowitz A.M., Villeda S.A. Therapeutic potential of systemic brain rejuvenation strategies for neurodegenerative disease. F1000Research. 2017;6:1291. doi: 10.12688/f1000research.11437.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rau J., Beaulieu L., Huntington J., Church F. Serpins in thrombosis, hemostasis and fibrinolysis. J. Thromb. Haemost. 2007;5:102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gramling M.W., Church F.C. Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb. Res. 2010;125:377–381. doi: 10.1016/j.thromres.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Reuland C.J., Church F.C. Synergy between plasminogen activator inhibitor-1, α-synuclein, and neuroinflammation in Parkinson’s disease. Med. Hypotheses. 2020;138:109602. doi: 10.1016/j.mehy.2020.109602. [DOI] [PubMed] [Google Scholar]
- 282.Trejo J.L., Llorens-Martín M.V., Torres-Alemán I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 283.McCall G.E., Allen D.L., Haddad F., Baldwin K.M. Transcriptional regulation of IGF-I expression in skeletal muscle. Am. J. Physiol.-Cell Physiol. 2003;285:C831–C839. doi: 10.1152/ajpcell.00047.2003. [DOI] [PubMed] [Google Scholar]
- 284.Tsai C.L., Wang C.-H., Pan C.Y., Chen F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015;9:23. doi: 10.3389/fnbeh.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Voss M.W., Erickson K.I., Prakash R.S., Chaddock L., Kim J.S., Alves H., Szabo A., Phillips S.M., Wójcicki T.R., Mailey E.L., et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kappeler L., Filho C.D.M., Dupont J., Leneuve P., Cervera P., Périn L., Loudes C., Blaise A., Klein R., Epelbaum J., et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ayadi A.E., Zigmond M.J., Smith A.D. IGF-1 protects dopamine neurons against oxidative stress: Association with changes in phosphokinases. Exp. Brain Res. 2016;234:1863–1873. doi: 10.1007/s00221-016-4572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bassil F., Delamarre A., Canron M.H., Dutheil N., Vital A., Négrier-Leibreich M.L., Bezard E., Fernagut P.O., Meissner W.G. Impaired brain insulin signalling in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2022;48:e12760. doi: 10.1111/nan.12760. [DOI] [PubMed] [Google Scholar]
- 289.Meex R.C., Watt M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017;13:509–520. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 290.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 291.Sawada M., Imamura K., Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006;70:373–381. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- 292.Halling J.F., Pilegaard H. PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020;45:927–936. doi: 10.1139/apnm-2020-0005. [DOI] [PubMed] [Google Scholar]
- 293.Zhang X., Xu S., Hu Y., Liu Q., Liu C., Chai H., Luo Y., Jin L., Li S. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. npj Park. Dis. 2023;9:13. doi: 10.1038/s41531-023-00453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cai M., Wang H., Song H., Yang R., Wang L., Xue X., Sun W., Hu J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front. Nutr. 2022;9:800901. doi: 10.3389/fnut.2022.800901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J., Sutton S.W., Li X., Yun S.J., Mirzadegan T., et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 296.Bergersen L.H., Gjedde A. Is lactate a volume transmitter of metabolic states of the brain? Front. Neuroenergetics. 2012;4:5. doi: 10.3389/fnene.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.D’Souza R.F., Woodhead J.S., Zeng N., Blenkiron C., Merry T.L., Cameron-Smith D., Mitchell C.J. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol.-Endocrinol. Metab. 2018;315:E723–E733. doi: 10.1152/ajpendo.00138.2018. [DOI] [PubMed] [Google Scholar]
- 298.Kuang W.-H., Dong Z.-Q., Tian L.-T., Li J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz. J. Med. Biol. Res. 2018;51:e7212. doi: 10.1590/1414-431x20187212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang W.-J., Cao W.-Y., Huang Y.-Q., Cui Y.-H., Tu B.-X., Wang L.-F., Zou G.-J., Liu Y., Hu Z.-L., Hu R. The role of miR-150 in stress-induced anxiety-like behavior in mice. Neurotox. Res. 2019;35:160–172. doi: 10.1007/s12640-018-9943-x. [DOI] [PubMed] [Google Scholar]
- 300.Carek P.J., Laibstain S.E., Carek S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 301.Hou L., Chen W., Liu X., Qiao D., Zhou F.-M. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front. Aging Neurosci. 2017;9:358. doi: 10.3389/fnagi.2017.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.da Costa Daniele T.M., de Bruin P.F.C., de Matos R.S., de Bruin G.S., Junior C.M.C., de Bruin V.M.S. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model—A systematic review and meta-analysis. Behav. Brain Res. 2020;383:112488. doi: 10.1016/j.bbr.2020.112488. [DOI] [PubMed] [Google Scholar]
- 303.Pietrelli A., Matković L., Vacotto M., Lopez-Costa J., Basso N., Brusco A. Aerobic exercise upregulates the BDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol. Learn. Mem. 2018;155:528–542. doi: 10.1016/j.nlm.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 304.Agudelo L.Z., Femenía T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., Correia J.C., Izadi M., Bhat M., Schuppe-Koistinen I. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 305.Han Q., Cai T., Tagle D.A., Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Myint A.-M., Kim Y.K., Verkerk R., Scharpé S., Steinbusch H., Leonard B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 307.Wang X., Bove A.M., Simone G., Ma B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front. Cell Dev. Biol. 2020;8:599281. doi: 10.3389/fcell.2020.599281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N.P.H., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 309.Kanno S., Oda N., Abe M., Terai Y., Ito M., Shitara K., Tabayashi K., Shibuya M., Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 310.Ceci C., Lacal P.M., Barbaccia M.L., Mercuri N.B., Graziani G., Ledonne A. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: Pathophysiological roles and therapeutic implications. Pharmacol. Res. 2024;201:107101. doi: 10.1016/j.phrs.2024.107101. [DOI] [PubMed] [Google Scholar]
- 311.Higuchi K., Masaki T., Gotoh K., Chiba S., Katsuragi I., Tanaka K., Kakuma T., Yoshimatsu H. Apelin, an APJ Receptor Ligand, Regulates Body Adiposity and Favors the Messenger Ribonucleic Acid Expression of Uncoupling Proteins in Mice. Endocrinology. 2007;148:2690–2697. doi: 10.1210/en.2006-1270. [DOI] [PubMed] [Google Scholar]
- 312.Wang B.L., Wu J.F., Xiao D., Wu B., Wei D.X. 3-hydroxybutyrate in the brain: Biosynthesis, function, and disease therapy. Brain-X. 2023;1:e6. doi: 10.1002/brx2.6. [DOI] [Google Scholar]
- 313.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 314.Wang H., Li W., Zhu S., Li J., D’amore J., Ward M.F., Yang H., Wu R., Jahnen-Dechent W., Tracey K.J. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J. Cereb. Blood Flow Metab. 2010;30:493–504. doi: 10.1038/jcbfm.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hsuchou H., Pan W., Kastin A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pan W., Kastin A.J. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 317.Banks W.A., Kastin A.J., Broadwell R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 318.Qi J., Yang L., Wang X., Wang M., Li X., Feng B., Wu Y., Liu S., Zhang K. Mechanism of CNS regulation by irisin, a multifunctional protein. Brain Res. Bull. 2022;188:11–20. doi: 10.1016/j.brainresbull.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 319.Van Hall G., Stømstad M., Rasmussen P., Jans Ø., Zaar M., Gam C., Quistorff B., Secher N.H., Nielsen H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 320.Koopaei N.N., Chowdhury E.A., Jiang J., Noorani B., da Silva L., Bulut G., Hakimjavadi H., Chamala S., Bickel U., Schmittgen T.D. Enrichment of the erythrocyte miR-451a in brain extracellular vesicles following impairment of the blood-brain barrier. Neurosci. Lett. 2021;751:135829. doi: 10.1016/j.neulet.2021.135829. [DOI] [PubMed] [Google Scholar]
- 321.Rani A., O’Shea A., Ianov L., Cohen R.A., Woods A.J., Foster T.C. miRNA in circulating microvesicles as biomarkers for age-related cognitive decline. Front. Aging Neurosci. 2017;9:323. doi: 10.3389/fnagi.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhang Z.G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Van Bruggen N., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Spranger J., Verma S., Göhring I., Bobbert T., Seifert J., Sindler A.L., Pfeiffer A., Hileman S.M., Tschöp M., Banks W.A. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. doi: 10.2337/diabetes.55.01.06.db05-1077. [DOI] [PubMed] [Google Scholar]
- 324.Yau S.Y., Li A., Hoo R.L., Ching Y.P., Christie B.R., Lee T.M., Xu A., So K.-F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA. 2014;111:15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bloemer J., Pinky P.D., Govindarajulu M., Hong H., Judd R., Amin R.H., Moore T., Dhanasekaran M., Reed M.N., Suppiramaniam V. Role of Adiponectin in Central Nervous System Disorders. Neural Plast. 2018;2018:4593530. doi: 10.1155/2018/4593530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Boado R.J., Zhang Y., Zhang Y., Wang Y., Pardridge W.M. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotechnol. Bioeng. 2008;100:387–396. doi: 10.1002/bit.21764. [DOI] [PubMed] [Google Scholar]
- 327.Bertler A., Falck B., Owman C., Rosengrenn E.B. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol. Rev. 1966;18:369–385. [PubMed] [Google Scholar]
- 328.Hardebo J.E., Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann. Neurol. 1980;8:1–31. doi: 10.1002/ana.410080102. [DOI] [PubMed] [Google Scholar]
- 329.Lindvall M., Hardebo J.E., Owman C. Barrier mechanisms for neurotransmitter monoamines in the choroid plexus. Acta Physiol. Scand. 1980;108:215–221. doi: 10.1111/j.1748-1716.1980.tb06525.x. [DOI] [PubMed] [Google Scholar]
- 330.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 331.Mann S., Beedie C., Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Herrero F., San Juan A., Fleck S., Balmer J., Perez M., Canete S., Earnest C.P., Foster C., Lucia A. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int. J. Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 333.Contrepois K., Wu S., Moneghetti K.J., Hornburg D., Ahadi S., Tsai M.S., Metwally A.A., Wei E., Lee-McMullen B., Quijada J.V., et al. Molecular choreography of acute exercise. Cell. 2020;181:1112–1130. doi: 10.1016/j.cell.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Morville T., Sahl R.E., Moritz T., Helge J.W., Clemmensen C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 2020;33:108554. doi: 10.1016/j.celrep.2020.108554. [DOI] [PubMed] [Google Scholar]
- 335.Huh J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018;41:14–29. doi: 10.1007/s12272-017-0994-y. [DOI] [PubMed] [Google Scholar]
- 336.Zunner B.E., Wachsmuth N.B., Eckstein M.L., Scherl L., Schierbauer J.R., Haupt S., Stumpf C., Reusch L., Moser O. Myokines and resistance training: A narrative review. Int. J. Mol. Sci. 2022;23:3501. doi: 10.3390/ijms23073501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Luo J., Liu W., Feng F., Chen L. Apelin/APJ system: A novel therapeutic target for locomotor system diseases. Eur. J. Pharmacol. 2021;906:174286. doi: 10.1016/j.ejphar.2021.174286. [DOI] [PubMed] [Google Scholar]
- 338.Arefi Shirvan R., Ghorbanian B., Ghorbanzadeh B. The effect of 12 weeks of aerobic exercise on the serum levels of angiopoietin-like protein 4 (ANGPTL4) and beta-aminoisobutyric acid (BAIBA) in men with metabolic syndrome. J. Sport Biosci. 2022;14:19–32. [Google Scholar]
- 339.Griffin É.W., Mullally S., Foley C., Warmington S.A., O’Mara S.M., Kelly Á.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 340.Ribeiro D., Petrigna L., Pereira F.C., Muscella A., Bianco A., Tavares P. The impact of physical exercise on the circulating levels of BDNF and NT 4/5: A review. Int. J. Mol. Sci. 2021;22:8814. doi: 10.3390/ijms22168814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mazo C.E., Miranda E.R., Shadiow J., Vesia M., Haus J.M. High intensity acute aerobic exercise elicits alterations in circulating and skeletal muscle tissue expression of neuroprotective exerkines. Brain Plast. 2022;8:5–18. doi: 10.3233/BPL-220137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tanimura Y., Aoi W., Takanami Y., Kawai Y., Mizushima K., Naito Y., Yoshikawa T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol. Rep. 2016;4:e12828. doi: 10.14814/phy2.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Catoire M., Mensink M., Kalkhoven E., Schrauwen P., Kersten S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014;46:256–267. doi: 10.1152/physiolgenomics.00174.2013. [DOI] [PubMed] [Google Scholar]
- 344.He Y., Wang Q., Jiang N. The role of IGF-1 in exercise to improve obesity-related cognitive dysfunction. Front. Neurosci. 2023;17:1229165. doi: 10.3389/fnins.2023.1229165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ostrowski K., Schjerling P., Pedersen B.K. Physical activity and plasma interleukin-6 in humans–effect of intensity of exercise. Eur. J. Appl. Physiol. 2000;83:512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 346.Mitchell J.B., Phillips M.D., Yellott R.C., Currie L.M. Resistance and aerobic exercise: The influence of mode on the relationship between IL-6 and glucose tolerance in young men who are obese. J. Strength Cond. Res. 2011;25:1529–1537. doi: 10.1519/JSC.0b013e3182176638. [DOI] [PubMed] [Google Scholar]
- 347.Shin K.O., Bae J.Y., Woo J., Jang K.S., Kim K.S., Park J.S., Kim I.K., Kang S. The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J. Exerc. Nutr. Biochem. 2015;19:91. doi: 10.5717/jenb.2015.15061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Spriet L.L., Howlett R.A., Heigenhauser G.J. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000;32:756–763. doi: 10.1097/00005768-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 349.Broholm C., Mortensen O.H., Nielsen S., Akerstrom T., Zankari A., Dahl B., Pedersen B.K. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J. Physiol. 2008;586:2195–2201. doi: 10.1113/jphysiol.2007.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Subbotina E., Sierra A., Zhu Z., Gao Z., Koganti S.R.K., Reyes S., Stepniak E., Walsh S.A., Acevedo M.R., Perez-Terzic C.M. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA. 2015;112:16042–16047. doi: 10.1073/pnas.1514250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Seldin M.M., Peterson J.M., Byerly M.S., Wei Z., Wong G.W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J. Biol. Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hittel D.S., Axelson M., Sarna N., Shearer J., Huffman K.M., Kraus W.E. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med. Sci. Sports Exerc. 2010;42:2023. doi: 10.1249/MSS.0b013e3181e0b9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Kang C., Chung E., Diffee G., Ji L.L. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: Role of PGC-1α. Exp. Gerontol. 2013;48:1343–1350. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 354.Wang G. Aerobic exercise ameliorates myocardial ischemia/reperfusion injury and thrombosis of diabetic rats via activation of AMPK/Sirt1/PGC-1α pathway. Gen. Physiol. Biophys. 2022;41:319–328. doi: 10.4149/gpb_2022010. [DOI] [PubMed] [Google Scholar]
- 355.Puchert M., Adams V., Linke A., Engele J. Evidence for the involvement of the CXCL12 system in the adaptation of skeletal muscles to physical exercise. Cell. Signal. 2016;28:1205–1215. doi: 10.1016/j.cellsig.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 356.Aoi W., Naito Y., Takagi T., Tanimura Y., Takanami Y., Kawai Y., Sakuma K., Hang L.P., Mizushima K., Hirai Y. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 357.Arany Z., Foo S.-Y., Ma Y., Ruas J.L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S.M. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 358.Laker R., Garde C., Camera D., Smiles W., Zierath J., Hawley J., Barrès R. Transcriptomic and epigenetic responses to short-term nutrient-exercise stress in humans. Sci. Rep. 2017;7:15134. doi: 10.1038/s41598-017-15420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Church D.D., Hoffman J.R., Mangine G.T., Jajtner A.R., Townsend J.R., Beyer K.S., Wang R., La Monica M.B., Fukuda D.H., Stout J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016;121:123–128. doi: 10.1152/japplphysiol.00233.2016. [DOI] [PubMed] [Google Scholar]
- 360.Kim J.S., Lee Y.H., Yi H.K. Gradual downhill running improves age-related skeletal muscle and bone weakness: Implication of autophagy and bone morphogenetic proteins. Exp. Physiol. 2016;101:1528–1540. doi: 10.1113/EP085852. [DOI] [PubMed] [Google Scholar]
- 361.Kim J., McKenna C.F., Askow A.T., Salvador A.F., Scaroni S.E., Cerna J., Cannavale C.N., Paluska S.A., De Lisio M., Petruzzello S.J. Higher Protein Intake does not Modulate Resistance Training–Induced Changes in Myokines and Cognitive Function in Middle-Aged Adults. J. Cogn. Enhanc. 2024;8:76–94. doi: 10.1007/s41465-024-00285-2. [DOI] [Google Scholar]
- 362.Kanzleiter T., Rath M., Görgens S.W., Jensen J., Tangen D.S., Kolnes A.J., Kolnes K.J., Lee S., Eckel J., Schürmann A. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014;450:1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 363.He Z., Tian Y., Valenzuela P.L., Huang C., Zhao J., Yin S., Lucia A. Myokine response to high-intensity interval vs. resistance exercise: An individual approach. Front. Physiol. 2018;9:416035. doi: 10.3389/fphys.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Haghighi A.H., Hajinia M., Askari R., Abbasian S., Goldfied G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Can. J. Physiol. Pharmacol. 2022;100:937–944. doi: 10.1139/cjpp-2021-0712. [DOI] [PubMed] [Google Scholar]
- 365.Vega S.R., Knicker A., Hollmann W., Bloch W., Strüder H.K. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- 366.Bazgir B., Salesi M., Koushki M., Amirghofran Z. Effects of eccentric and concentric emphasized resistance exercise on IL-15 serum levels and its relation to inflammatory markers in athletes and non-athletes. Asian J. Sports Med. 2015;6:e27980. doi: 10.5812/asjsm.27980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kim H.-J., So B., Choi M., Kang D., Song W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015;70:11–17. doi: 10.1016/j.exger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 368.Lantis D.J., Farrell III J.W., Cantrell G.S., Larson R.D. Eight weeks of high-volume resistance training improves onset of blood lactate in trained individuals. J. Strength Cond. Res. 2017;31:2176–2182. doi: 10.1519/JSC.0000000000001686. [DOI] [PubMed] [Google Scholar]
- 369.Deng H., Zhang W., Ruan D., Chen D., Xu X., He X., Chen C., Li L. The Combination of Aerobic and Resistance Exercise Induces Weight Loss via the PGC-1α/Irisin/UCP-1 Pathway. J. Biomater. Tissue Eng. 2019;9:1388–1394. doi: 10.1166/jbt.2019.2123. [DOI] [Google Scholar]
- 370.Gavin T., Drew J., Kubik C., Pofahl W., Hickner R. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 2007;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 371.García-Hermoso A., Ramírez-Vélez R., Díez J., González A., Izquierdo M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport Health Sci. 2023;12:147–157. doi: 10.1016/j.jshs.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rodríguez-Gutiérrez E., Torres-Costoso A., Pascual-Morena C., Pozuelo-Carrascosa D.P., Garrido-Miguel M., Martínez-Vizcaíno V. Effects of resistance exercise on neuroprotective factors in middle and late life: A systematic review and meta-analysis. Aging Dis. 2023;14:1264. doi: 10.14336/AD.2022.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gauthier S., Reisberg B., Zaudig M., Petersen R.C., Ritchie K., Broich K., Belleville S., Brodaty H., Bennett D., Chertkow H. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 375.Tsai C.L., Ukropec J., Ukropcová B., Pai M.C. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. NeuroImage Clin. 2018;17:272–284. doi: 10.1016/j.nicl.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kettinen J., Tikkanen H., Hiltunen M., Murray A., Horn N., Taylor W.R., Venojärvi M. Cognitive and biomarker responses in healthy older adults to a 18-hole golf round and different walking types: A randomised cross-over study. BMJ Open Sport Exerc. Med. 2023;9:e001629. doi: 10.1136/bmjsem-2023-001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 378.Peball M., Mahlknecht P., Werkmann M., Marini K., Murr F., Herzmann H., Stockner H., de Marzi R., Heim B., Djamshidian A. Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: A cross-sectional study. Gerontology. 2019;65:216–228. doi: 10.1159/000492572. [DOI] [PubMed] [Google Scholar]
- 379.Kwon J.H., Moon K.M., Min K.-W. Exercise-induced myokines can explain the importance of physical activity in the elderly: An overview. Healthcare. 2020;8:378. doi: 10.3390/healthcare8040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019;10:444796. doi: 10.3389/fphys.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Graham Z.A., Lavin K.M., O’Bryan S.M., Thalacker-Mercer A.E., Buford T.W., Ford K.M., Broderick T.J., Bamman M.M. Mechanisms of exercise as a preventative measure to muscle wasting. Am. J. Physiol.-Cell Physiol. 2021;321:C40–C57. doi: 10.1152/ajpcell.00056.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ellingson L.D., Zaman A., Stegemöller E.L. Sedentary Behavior and Quality of Life in Individuals With Parkinson’s Disease. Neurorehabil. Neural Repair. 2019;33:595–601. doi: 10.1177/1545968319856893. [DOI] [PubMed] [Google Scholar]
- 383.MacNeil L.G., Tarnopolsky M.A., Crane J.D. Acute, exercise-induced alterations in cytokines and chemokines in the blood distinguish physically active and sedentary aging. J. Gerontol. Ser. A. 2021;76:811–818. doi: 10.1093/gerona/glaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Cirillo J., Lavender A.P., Ridding M.C., Semmler J.G. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J. Physiol. 2009;587:5831–5842. doi: 10.1113/jphysiol.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.McDonnell M.N., Buckley J.D., Opie G.M., Ridding M.C., Semmler J.G. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J. Appl. Physiol. (1985) 2013;114:1174–1182. doi: 10.1152/japplphysiol.01378.2012. [DOI] [PubMed] [Google Scholar]
- 386.Steib S., Wanner P., Adler W., Winkler J., Klucken J., Pfeifer K. A Single Bout of Aerobic Exercise Improves Motor Skill Consolidation in Parkinson’s Disease. Front. Aging Neurosci. 2018;10:328. doi: 10.3389/fnagi.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Sarkar S., Raymick J., Imam S. Neuroprotective and therapeutic strategies against Parkinson’s disease: Recent perspectives. Int. J. Mol. Sci. 2016;17:904. doi: 10.3390/ijms17060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Faden A.I., Stoica B. Neuroprotection: Challenges and opportunities. Arch. Neurol. 2007;64:794–800. doi: 10.1001/archneur.64.6.794. [DOI] [PubMed] [Google Scholar]
- 389.Nay K., Smiles W.J., Kaiser J., McAloon L.M., Loh K., Galic S., Oakhill J.S., Gundlach A.L., Scott J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021;22:4052. doi: 10.3390/ijms22084052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 391.Vajda F.J. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 2002;9:4–8. doi: 10.1054/jocn.2001.1027. [DOI] [PubMed] [Google Scholar]
- 392.Murphy R.M., Watt M.J., Febbraio M.A. Metabolic communication during exercise. Nat. Metab. 2020;2:805–816. doi: 10.1038/s42255-020-0258-x. [DOI] [PubMed] [Google Scholar]
- 393.Walzik D., Wences Chirino T.Y., Zimmer P., Joisten N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct. Target. Ther. 2024;9:138. doi: 10.1038/s41392-024-01841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Reddy I., Yadav Y., Dey C.S. Cellular and molecular regulation of exercise—A neuronal perspective. Cell. Mol. Neurobiol. 2023;43:1551–1571. doi: 10.1007/s10571-022-01272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Ryan G.B., Majno G. Acute inflammation. A review. Am. J. Pathol. 1977;86:183. [PMC free article] [PubMed] [Google Scholar]
- 396.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Abbas A.K., Lichtman A.H., Pillai S. Basic Immunology E-Book: Functions and Disorders of the Immune System. Elsevier Health Sciences; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 398.Müller-Nedebock A.C., Dekker M.C., Farrer M.J., Hattori N., Lim S.-Y., Mellick G.D., Rektorová I., Salama M., Schuh A.F., Stoessl A.J. Different pieces of the same puzzle: A multifaceted perspective on the complex biological basis of Parkinson’s disease. npj Park. Dis. 2023;9:110. doi: 10.1038/s41531-023-00535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 400.Neufer P.D., Bamman M.M., Muoio D.M., Bouchard C., Cooper D.M., Goodpaster B.H., Booth F.W., Kohrt W.M., Gerszten R.E., Mattson M.P. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 401.Hood D.A., Memme J.M., Oliveira A.N., Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 2019;81:19–41. doi: 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- 402.Finck B.N., Kelly D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Spiering B.A., Kraemer W.J., Anderson J.M., Armstrong L.E., Nindl B.C., Volek J.S., Maresh C.M. Resistance exercise biology: Manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008;38:527–540. doi: 10.2165/00007256-200838070-00001. [DOI] [PubMed] [Google Scholar]
- 404.Schoenfeld B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 405.Damas F., Libardi C.A., Ugrinowitsch C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018;118:485–500. doi: 10.1007/s00421-017-3792-9. [DOI] [PubMed] [Google Scholar]
- 406.McGee S.L., Hargreaves M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020;16:495–505. doi: 10.1038/s41574-020-0377-1. [DOI] [PubMed] [Google Scholar]
- 407.Farup J., Sørensen H., Kjølhede T. Similar changes in muscle fiber phenotype with differentiated consequences for rate of force development: Endurance versus resistance training. Hum. Mov. Sci. 2014;34:109–119. doi: 10.1016/j.humov.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 408.Hoppeler H., Baum O., Lurman G., Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr. Physiol. 2011;1:1383–1412. doi: 10.1002/cphy.c100042. [DOI] [PubMed] [Google Scholar]
- 409.Dyer A.H., Vahdatpour C., Sanfeliu A., Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 410.Chen J., Zhou R., Feng Y., Cheng L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 2022;7:383. doi: 10.1038/s41392-022-01233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Hortobágyi T., Vetrovsky T., Balbim G.M., Silva N.C.B.S., Manca A., Deriu F., Kolmos M., Kruuse C., Liu-Ambrose T., Radák Z. The impact of aerobic and resistance training intensity on markers of neuroplasticity in health and disease. Ageing Res. Rev. 2022;80:101698. doi: 10.1016/j.arr.2022.101698. [DOI] [PubMed] [Google Scholar]
- 412.Grazioli E., Tranchita E., Borriello G., Cerulli C., Minganti C., Parisi A. The effects of concurrent resistance and aerobic exercise training on functional status in patients with multiple sclerosis. Curr. Sports Med. Rep. 2019;18:452–457. doi: 10.1249/JSR.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 413.Lee J. Effects of aerobic and resistance exercise interventions on cognitive and physiologic adaptations for older adults with mild cognitive impairment: A systematic review and meta-analysis of randomized control trials. Int. J. Environ. Res. Public Health. 2020;17:9216. doi: 10.3390/ijerph17249216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Krumpolec P., Vallova S., Slobodova L., Tirpakova V., Vajda M., Schon M., Klepochova R., Janakova Z., Straka I., Sutovsky S. Aerobic-strength exercise improves metabolism and clinical state in Parkinson’s disease patients. Front. Neurol. 2017;8:698. doi: 10.3389/fneur.2017.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Caminiti G., Iellamo F., Mancuso A., Cerrito A., Montano M., Manzi V., Volterrani M. Effects of 12 weeks of aerobic versus combined aerobic plus resistance exercise training on short-term blood pressure variability in patients with hypertension. J. Appl. Physiol. 2021;130:1085–1092. doi: 10.1152/japplphysiol.00910.2020. [DOI] [PubMed] [Google Scholar]
- 416.D’hooge R., Hellinckx T., Van Laethem C., Stegen S., De Schepper J., Van Aken S., Dewolf D., Calders P. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: A randomized controlled trial. Clin. Rehabil. 2011;25:349–359. doi: 10.1177/0269215510386254. [DOI] [PubMed] [Google Scholar]
- 417.Villareal D.T., Aguirre L., Gurney A.B., Waters D.L., Sinacore D.R., Colombo E., Armamento-Villareal R., Qualls C. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Pouresmaeili-Babaki E., Esmaeili-Mahani S., Abbasnejad M., Ravan H. Protective effect of neuropeptide apelin-13 on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y dopaminergic cells: Involvement of its antioxidant and antiapoptotic properties. Rejuvenation Res. 2018;21:162–167. doi: 10.1089/rej.2017.1951. [DOI] [PubMed] [Google Scholar]
- 419.Zhu J., Dou S., Jiang Y., Bai B., Chen J., Wang C., Cheng B. Apelin-36 exerts the cytoprotective effect against MPP+-induced cytotoxicity in SH-SY5Y cells through PI3K/Akt/mTOR autophagy pathway. Life Sci. 2019;224:95–108. doi: 10.1016/j.lfs.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 420.Zhu J., Gao W., Shan X., Wang C., Wang H., Shao Z., Dou S., Jiang Y., Wang C., Cheng B. Apelin-36 mediates neuroprotective effects by regulating oxidative stress, autophagy and apoptosis in MPTP-induced Parkinson’s disease model mice. Brain Res. 2020;1726:146493. doi: 10.1016/j.brainres.2019.146493. [DOI] [PubMed] [Google Scholar]
- 421.Chen P., Wang Y., Chen L., Song N., Xie J. Apelin-13 protects dopaminergic neurons against rotenone—Induced neurotoxicity through the AMPK/mTOR/ULK-1 mediated autophagy activation. Int. J. Mol. Sci. 2020;21:8376. doi: 10.3390/ijms21218376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Chen Y., Shen J., Qi G., Zha Q., Zhang C., Yao W., Gao X., Chen S. Potential therapeutic role of fibroblast growth factor 21 in neurodegeneration: Evidence for ameliorating parkinsonism via silent information regulator 2 homolog 1 and implication for gene therapy. Neuropharmacology. 2020;181:108335. doi: 10.1016/j.neuropharm.2020.108335. [DOI] [PubMed] [Google Scholar]
- 423.Fang X., Ma J., Mu D., Li B., Lian B., Sun C. FGF21 protects dopaminergic neurons in Parkinson’s disease models via repression of neuroinflammation. Neurotox. Res. 2020;37:616–627. doi: 10.1007/s12640-019-00151-6. [DOI] [PubMed] [Google Scholar]
- 424.Kim Y., Li E., Park S. Insulin-like growth factor-1 inhibits 6-hydroxydopamine-mediated endoplasmic reticulum stress-induced apoptosis via regulation of heme oxygenase-1 and Nrf2 expression in PC12 cells. Int. J. Neurosci. 2012;122:641–649. doi: 10.3109/00207454.2012.702821. [DOI] [PubMed] [Google Scholar]
- 425.Quesada A., Lee B.Y., Micevych P.E. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev. Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Novelli G., Calcaterra G., Casciani F., Pecorelli S., Mehta J.L. ‘Exerkines’: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines. 2024;12:1975. doi: 10.3390/biomedicines12091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Mazur-Bialy A.I., Pocheć E. The time-course of antioxidant irisin activity: Role of the Nrf2/HO-1/HMGB1 axis. Antioxidants. 2021;10:88. doi: 10.3390/antiox10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Wang Y., Dong Z., Zhang Z., Wang Y., Yang K., Li X. Postconditioning with irisin attenuates lung ischemia/reperfusion injury by suppressing ferroptosis via induction of the Nrf2/HO-1 signal axis. Oxidative Med. Cell. Longev. 2022;2022:9911167. doi: 10.1155/2022/9911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Luo J., Tang C., Chen X., Ren Z., Qu H., Chen R., Tong Z. Impacts of aerobic exercise on depression-like behaviors in chronic unpredictable mild stress mice and related factors in the AMPK/PGC-1α pathway. Int. J. Environ. Res. Public Health. 2020;17:2042. doi: 10.3390/ijerph17062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Burtscher J., Millet G.P., Place N., Kayser B., Zanou N. The muscle-brain axis and neurodegenerative diseases: The key role of mitochondria in exercise-induced neuroprotection. Int. J. Mol. Sci. 2021;22:6479. doi: 10.3390/ijms22126479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Heo J., Noble E.E., Call J.A. The role of exerkines on brain mitochondria: A mini-review. J. Appl. Physiol. 2023;134:28–35. doi: 10.1152/japplphysiol.00565.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Cefis M., Chaney R., Wirtz J., Méloux A., Quirié A., Leger C., Prigent-Tessier A., Garnier P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023;16:1275924. doi: 10.3389/fnmol.2023.1275924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Palasz E., Wysocka A., Gasiorowska A., Chalimoniuk M., Niewiadomski W., Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020;21:1170. doi: 10.3390/ijms21031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Maass A., Düzel S., Brigadski T., Goerke M., Becke A., Sobieray U., Neumann K., Lövdén M., Lindenberger U., Bäckman L. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–154. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 435.Jurcau A., Andronie-Cioara F.L., Nistor-Cseppento D.C., Pascalau N., Rus M., Vasca E., Jurcau M.C. The involvement of neuroinflammation in the onset and progression of Parkinson’s disease. Int. J. Mol. Sci. 2023;24:14582. doi: 10.3390/ijms241914582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Choi H.M., Doss H.M., Kim K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020;21:1219. doi: 10.3390/ijms21041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Tilg H., Moschen A.R. Role of adiponectin and PBEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clin. Sci. 2008;114:275–288. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 438.Luo H., Xiang Y., Qu X., Liu H., Liu C., Li G., Han L., Qin X. Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat model of Alzheimer’s disease through activation of BDNF-TrkB signaling pathway. Front. Pharmacol. 2019;10:395. doi: 10.3389/fphar.2019.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Shippy D.C., Wilhelm C., Viharkumar P.A., Raife T.J., Ulland T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation. 2020;17:280. doi: 10.1186/s12974-020-01948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Quartarone A., Ghilardi M., Boller F. Neuroplasticity: From Bench to Bedside. Volume 3 Elsevier Science; Amsterdam, The Netherlands: 2022. Defining neuroplasticity. [Google Scholar]
- 441.Vints W.A., Levin O., Fujiyama H., Verbunt J., Masiulis N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocrinol. 2022;66:100993. doi: 10.1016/j.yfrne.2022.100993. [DOI] [PubMed] [Google Scholar]
- 442.Wang Y., Xu E., Musich P.R., Lin F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019;25:816–824. doi: 10.1111/cns.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cheng C.-F., Ku H.-C., Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Santos S.F., De Oliveira H.L., Yamada E.S., Neves B.C., Pereira Jr A. The gut and Parkinson’s disease—A bidirectional pathway. Front. Neurol. 2019;10:574. doi: 10.3389/fneur.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Dos Santos J.C.C., Oliveira L.F., Noleto F.M., Gusmão C.T.P., de Castro Brito G.A., de Barros Viana G.S. Gut-microbiome-brain axis: The crosstalk between the vagus nerve, alpha-synuclein and the brain in Parkinson’s disease. Neural Regen. Res. 2023;18:2611–2614. doi: 10.4103/1673-5374.373673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Liddle R.A. Parkinson’s disease from the gut. Brain Res. 2018;1693:201–206. doi: 10.1016/j.brainres.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Elfil M., Kamel S., Kandil M., Koo B.B., Schaefer S.M. Implications of the gut microbiome in Parkinson’s disease. Mov. Disord. 2020;35:921–933. doi: 10.1002/mds.28004. [DOI] [PubMed] [Google Scholar]
- 448.Imdad S., Lim W., Kim J.-H., Kang C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int. J. Mol. Sci. 2022;23:2679. doi: 10.3390/ijms23052679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Blume G.R., Royes L.F.F. Peripheral to brain and hippocampus crosstalk induced by exercise mediates cognitive and structural hippocampal adaptations. Life Sci. 2024;352:122799. doi: 10.1016/j.lfs.2024.122799. [DOI] [PubMed] [Google Scholar]
- 450.Rojas-Valverde D., Bonilla D.A., Gómez-Miranda L.M., Calleja-Núñez J.J., Arias N., Martínez-Guardado I. Examining the Interaction between Exercise, Gut Microbiota, and neurodegeneration: Future research directions. Biomedicines. 2023;11:2267. doi: 10.3390/biomedicines11082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ellis T., Rochester L. Mobilizing Parkinson’s Disease: The Future of Exercise. J. Park. Dis. 2018;8:S95–S100. doi: 10.3233/JPD-181489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Petzinger G.M., Fisher B.E., Van Leeuwen J.E., Vukovic M., Akopian G., Meshul C.K., Holschneider D.P., Nacca A., Walsh J.P., Jakowec M.W. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov. Disord. 2010;25:S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Pedersen B.K. Which type of exercise keeps you young? Curr. Opin. Clin. Nutr. Metab. Care. 2019;22:167–173. doi: 10.1097/MCO.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 454.Campos C., Rocha N.B.F., Lattari E., Paes F., Nardi A.E., Machado S. Exercise-induced neuroprotective effects on neurodegenerative diseases: The key role of trophic factors. Expert Rev. Neurother. 2016;16:723–734. doi: 10.1080/14737175.2016.1179582. [DOI] [PubMed] [Google Scholar]
- 455.Cruise K., Bucks R., Loftus A., Newton R., Pegoraro R., Thomas M. Exercise and Parkinson’s: Benefits for cognition and quality of life. Acta Neurol. Scand. 2011;123:13–19. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 456.Carvalho A., Barbirato D., Araujo N., Martins J.V., Cavalcanti J.L.S., Santos T.M., Coutinho E.S., Laks J., Deslandes A.C. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson’s disease: Pilot study. Clin. Interv. Aging. 2015;10:183–191. doi: 10.2147/CIA.S68779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zhou X., Zhao P., Guo X., Wang J., Wang R. Effectiveness of aerobic and resistance training on the motor symptoms in Parkinson’s disease: Systematic review and network meta-analysis. Front. Aging Neurosci. 2022;14:935176. doi: 10.3389/fnagi.2022.935176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Fernández del Olmo M.Á., Sánchez J.A., Morenill L., Gómez Varela J., Fernández-Lago H., Bello O., Santos García D. Aerobic and resistance exercises in Parkinson’s disease: A narrative review. Eur. J. Hum. Mov. 2018;41:149–174. [Google Scholar]
- 459.Roeder L., Costello J.T., Smith S.S., Stewart I.B., Kerr G.K. Effects of resistance training on measures of muscular strength in people with Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0132135. doi: 10.1371/journal.pone.0132135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Brienesse L.A., Emerson M.N. Effects of resistance training for people with Parkinson’s disease: A systematic review. J. Am. Med. Dir. Assoc. 2013;14:236–241. doi: 10.1016/j.jamda.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 461.Pinho R.A., Aguiar A.S., Jr., Radak Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants. 2019;8:529. doi: 10.3390/antiox8110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Hirsch M.A., Farley B.G. Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur. J. Phys. Rehabil. Med. 2009;45:215–229. [PubMed] [Google Scholar]
- 463.Crotty G.F., Schwarzschild M.A. Chasing protection in Parkinson’s disease: Does exercise reduce risk and progression? Front. Aging Neurosci. 2020;12:186. doi: 10.3389/fnagi.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.McFarthing K., Buff S., Rafaloff G., Fiske B., Mursaleen L., Fuest R., Wyse R.K., Stott S.R. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J. Park. Dis. 2023;13:427–439. doi: 10.3233/JPD-239901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Sanford J.A., Nogiec C.D., Lindholm M.E., Adkins J.N., Amar D., Dasari S., Drugan J.K., Fernández F.M., Radom-Aizik S., Schenk S. Molecular transducers of physical activity consortium (MoTrPAC): Mapping the dynamic responses to exercise. Cell. 2020;181:1464–1474. doi: 10.1016/j.cell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Ahlskog J.E. Aerobic exercise: Evidence for a direct brain effect to slow parkinson disease progression. Mayo Clin. Proc. 2018;93:360–372. doi: 10.1016/j.mayocp.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 467.Lam F.M., Huang M.-Z., Liao L.-R., Chung R.C., Kwok T.C., Pang M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018;64:4–15. doi: 10.1016/j.jphys.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 468.McKenzie R.T. Exercise in Education and Medicine. Saunders; Philadelphia, PA, USA: 1909. 604p [Google Scholar]