Abstract
The ability to adapt to altered availability of free water is a fundamental property of living cells. The principles underlying osmoadaptation are well conserved. The yeast Saccharomyces cerevisiae is an excellent model system with which to study the molecular biology and physiology of osmoadaptation. Upon a shift to high osmolarity, yeast cells rapidly stimulate a mitogen-activated protein (MAP) kinase cascade, the high-osmolarity glycerol (HOG) pathway, which orchestrates part of the transcriptional response. The dynamic operation of the HOG pathway has been well studied, and similar osmosensing pathways exist in other eukaryotes. Protein kinase A, which seems to mediate a response to diverse stress conditions, is also involved in the transcriptional response program. Expression changes after a shift to high osmolarity aim at adjusting metabolism and the production of cellular protectants. Accumulation of the osmolyte glycerol, which is also controlled by altering transmembrane glycerol transport, is of central importance. Upon a shift from high to low osmolarity, yeast cells stimulate a different MAP kinase cascade, the cell integrity pathway. The transcriptional program upon hypo-osmotic shock seems to aim at adjusting cell surface properties. Rapid export of glycerol is an important event in adaptation to low osmolarity. Osmoadaptation, adjustment of cell surface properties, and the control of cell morphogenesis, growth, and proliferation are highly coordinated processes. The Skn7p response regulator may be involved in coordinating these events. An integrated understanding of osmoadaptation requires not only knowledge of the function of many uncharacterized genes but also further insight into the time line of events, their interdependence, their dynamics, and their spatial organization as well as the importance of subtle effects.
INTRODUCTION
Yeasts Live in a Variable Environment
Yeasts are ubiquitous unicellular fungi and hence eukaryotic microorganisms (306). They live as saprophytes on plant or animal material, where they catabolize preferentially sugars but also polyols, alcohols, organic acids, and amino acids as sources for carbon and energy (570). To better decompose their substrates, many yeasts take an active role by forming filaments or pseudohyphae and producing hydrolytic enzymes, properties that make those species potentially pathogenic for plants and animals, including humans (329, 525).
On substrates such as fruits and flowers, yeasts are exposed to a highly variable environment with respect to the availability and quality of nutrients, temperature, pH, radiation, access to oxygen, and especially water activity (230). Water activity is defined as the chemical potential of free water in solution. Low water activity limits yeast growth, a fact that has been used for centuries for the preservation of fruits in dry form or with very high sugar levels, such as in marmalades (486). In the yeast's natural environment, the water activity can range widely and rapidly, due to both external influences and the activity of the yeast itself. In order to maintain an appropriate cell volume and a ratio of free to bound water favorable for biochemical reactions, the water activity of the cytosol and its organelles has to be lower than that of the surrounding medium. In this way, a constant force is maintained, driving water into the cell along its concentration gradient. This force is counteracted by turgor pressure, which is established by the limited ability for expansion of the plasma membrane and especially the cell wall (48, 653).
From the yeast's point of view, (at least) two different aspects need to be considered: survival of sudden changes in the water activity and the acquisition of tolerance to low water activity, i.e., to high external osmolarity. For instance, yeast cells in a water droplet on a grape berry may suddenly be exposed to high sugar levels when the berry breaks open due to animal or fungal activity. Then yeast cells experience a hyperosmotic shock (or osmotic upshift), accompanied by rapid water outflow and cell shrinking. On the other hand, cells adapted to high sugar levels on drying fruits or flowers may be washed away in a rain shower into essentially distilled water. Such a hypo-osmotic shock (or osmotic downshift) increases the water concentration gradient and leads to rapid influx of water, cell swelling, and hence increased turgor pressure. Within wide limits, the yeast cell wall prevents cell bursting (565).
The ability to survive a sudden change in water activity must be an intrinsic property of the cell, which means that the appropriate survival systems are in place under all conditions. Survival mechanisms need to operate within the first seconds after a sudden osmotic shift because passive water loss or uptake occurs very fast (reviewed in references 48, 65, and 66). While relatively little is known about the mechanisms ensuring survival of a hyperosmotic shock, we have insight into the cell's strategy to survive a hypo-osmotic shock, and those will be discussed in this review.
Yeast cells may also be exposed to slowly decreasing water activity, for instance, when their substrate is drying in the sun. Cellular water follows its concentration gradient by passive diffusion, so that the cells lose water and the concentration of biomolecules and ions in the cell increases, eventually resulting in an arrest of cellular activity: the cell suffers high osmolarity or hyperosmotic stress (in the literature, often synonymous with osmotic stress).
Yeast cells have developed mechanisms to adjust, within certain limits, to high external osmolarity and maintain or reestablish an inside-directed driving force for water (although accurate measurements quantifying this force do not exist). Adaptation to altered osmolarity is an active process based on sensing of osmotic changes and appropriate cellular responses aimed at maintaining cellular activity. Adaptation after a hyperosmotic shock may well take several hours (reviewed in references 48, 228, and 360). As will be discussed in detail, the accumulation of chemically inert osmolytes, such as glycerol, plays a central role in osmoadaptation (63, 568, 661). Therefore, yeast cells can be metabolically active and proliferate over a range of external water activities. This range is species specific (48, 64). Beyond those limits of cellular activity, yeasts have the ability to survive almost complete dehydration, a property that is used for the production of dry yeast known to every home baker (112). Dry yeast contains less than 10% residual water.
The underlying molecular mechanisms for survival of a hyperosmotic shock and adaptation to high osmolarity are probably distinct but overlapping: cells adapted to moderately high osmolarity survive a severe osmotic shock better than nonadapted cells (49, 533, 612, 627). The main body of this article will address mechanisms involved in the adaptation to high osmolarity. In the laboratory, yeast cells are usually exposed to a hyperosmotic shock and then their responses are studied. Alternatively, yeast cells growing in media of low and high osmolarity, i.e., fully adapted cells, are compared.
Initial interest in the molecular mechanisms of yeast osmoadaptation originated from the need to improve the performance of yeast strains under industrial conditions, which are often associated with rapid alterations in water activity and especially with high osmolarity (112, 478, 491). Additional practical aims are the improvement of food preservation methods, which require a better understanding of the impact of low water activity on yeast cells, especially in combination with other stress factors such as heat, cold, acidity, and chemical food preservatives (168). These aspects have motivated early research into the control of the cellular content of glycerol and trehalose, low-molecular-weight compounds serving as compatible solutes to adjust intracellular water activity and to protect biomolecules from denaturation (48, 63, 67, 173, 415, 429, 593).
The field of yeast osmoadaptation has received much wider scientific interest with the discovery in 1993 of the involvement in osmoadaptation of a mitogen-activated protein kinase (MAP kinase) cascade, a conserved eukaryotic signal transduction module (61, 205). Present knowledge confirms that many principles of osmoadaptation are conserved across eukaryotes, and therefore yeasts are ideal model systems with which to study the underlying mechanisms. Osmoadaptation is part of cellular osmoregulation, which plays an important yet not fully appreciated role in cell growth and morphogenesis.
This review deals with the responses and mechanisms of adaptation to changes in the relative water concentration between the inside and the outside of cells which affect cell volume and turgor pressure. The water activity in the cytosol can also change due to the presence of substances that cross the plasma membrane readily. The most prominent of such substances is ethanol, the product of sugar fermentation by yeasts. In certain wine fermentations, the ethanol concentration can reach 15 to 20% per volume. Ethanol causes water stress because it affects hydration of biomolecules (reviewed in references 208 and 465). The underlying response mechanisms are different from, though overlapping with, those for volume or turgor changes. I will also not discuss in any detail the mechanisms with which yeast cells manage to survive two extreme forms of osmotic stress, desiccation and freezing; relatively little is known about the underlying molecular mechanisms. This review also does not discuss the interesting question of why certain yeasts can tolerate or even prefer much lower water activities than others. The molecular bases for the species-specific differences in osmotolerance are not well understood (48). Finally, this review will discuss to only a very limited extent yeast responses to salt stress. NaCl is very commonly used in the laboratory to increase medium osmolarity. NaCl stimulates osmotic responses in essentially the same way as sugars or sugar alcohols at concentrations causing similar water activity (86, 500, 501). Na+, however, is toxic, because it replaces K+ in biomolecules (537, 538). Therefore, Na+ stimulates additional detoxification responses.
Yeasts as Model Systems
Osmoadaptation mechanisms have been studied in different yeast species. Because of the industrial interest, the large number of experimental tools, and the many laboratories worldwide studying it, baker's yeast (budding yeast), Saccharomyces cerevisiae, is the most commonly used system. In fission yeast, Schizosaccharomyces pombe, focus is mainly on signal transduction pathways in osmoadaptation. Studies on fission yeast signaling very well complement those on budding yeast, because the underlying mechanisms are similar though distinct and often claimed to be more related to those of higher animals (205, 383). Osmoregulation in Candida albicans is studied because of the relevance of this pathogenic yeast to human health. Zygosaccharomyces rouxii is an important osmophilic food spoilage yeast. Finally, Debaryomyces hansenii has attracted some interest because this marine yeast is highly sodium tolerant (48). Studies on a number of other yeasts focus on either very specific aspects, such as transport phenomena, or comparison with mechanisms discovered in S. cerevisiae.
Striving for an Integrative View
Since osmotic changes can be controlled very well experimentally, many groups have chosen osmoadaptation to study principles of cell biology and molecular physiology. This is illustrated, for instance, by the fact that five independent studies on global gene expression after osmotic upshift have been published recently, resulting in an explosion of data that provide insight into the impact of osmotic changes on cellular physiology (86, 191, 471, 501, 656). The control of transmembrane transport, the sensing of osmotic changes, the mechanisms, dynamics, and spatial organization of signal transmission, metabolic adjustments, the effects on the cytoskeleton, cell cycle progression, translation, and cell wall dynamics are being analyzed experimentally. These diverse studies on yeast osmoregulation could possibly allow in the foreseeable future a comprehensive view on the time line, spatial dynamics, interaction, and mutual dependency of the underlying cellular events.
SENSING OSMOTIC CHANGES
Numerous proteins have been classified as osmosensors, often based on “guilt by association.” The mere fact that a protein is needed for mediating responses to an osmotic shock plus its predicted location in the plasma membrane is used as an argument for a role in osmosensing. However, the molecular mechanism(s) by which osmosensors detect osmotic changes remains a matter of intensive research. Ultimate proof that a protein functions as an osmosensor requires defined in vitro studies (518, 653). In light of the difficulties associated with expression, purification, and reconstitution of transmembrane proteins (38), such in vitro data are still rare. Moreover, in vitro studies on osmosensors that do not at the same time transport substances require suitable monitoring and reporter systems.
A genuine osmosensor does not, per definitionem, function as a receptor for a certain (range of) compound, distinguishing it from chemosensors. Rather, an osmosensor might detect changes in the physicochemical properties of the solvent due to altered water concentration or water structure. Alternatively, it may sense mechanical stimuli that may occur as a consequence of the changes in water activity (205, 653). It is usually anticipated that osmosensors operate at the cell surface as integral membrane proteins. However, an osmosensor could also be a soluble protein. I will return to the possible mechanisms of sensing after discussing the known yeast proteins that are (probably) involved in this process. An excellent recent review discusses the physicochemical bases and possible mechanisms of osmosensing in detail, with an emphasis on bacterial systems, but the principles apply to any cell (653).
Two different types of proteins have been most intensely studied with regard to their control by osmotic changes. On the one hand, there are transmembrane transport proteins whose function is controlled by mechanical stimulation or changes in medium osmolarity. It is generally assumed that these mechanosensitive channels sense osmotic changes solely to control their own transport activity, but they could of course also connect to signaling pathways. I will discuss the mechanisms controlling them in more detail together with the osmoregulated yeast osmolyte exporter Fps1p. The second category of proteins are bona fide sensors that control signaling pathways leading to osmoadaptive responses.
Osmosensors
Proteins that control signaling pathways in cellular responses to osmotic changes have been identified and studied at the molecular level in bacteria and fungi. In S. cerevisiae, Sln1p and Sho1p have been described as sensors of the two upstream branches controlling the high osmolarity glycerol (HOG) MAP kinase pathway (see below). Evidence for their role as such sensors is indeed based on guilt by association: the HOG pathway is stimulated by osmotic upshift (61), genetic evidence places Sho1p (473) and Sln1p (357) upstream of all other HOG pathway components, mutations in SHO1 (473) and SLN1 (357) affect the activity of the HOG pathway, and both Sho1p (498) and Sln1p (440) are located in the plasma membrane.
Sho1p.
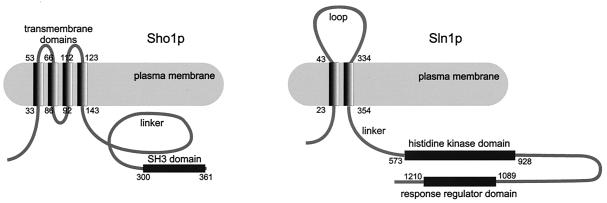
Sho1p is a protein of 367 amino acids consisting of four predicted transmembrane domains within the N-terminal part, a linker domain, and an SH3 domain for protein-protein interaction (Fig. 1) (473, 488). Functional homologs of Sho1p have been isolated from the yeasts Candida utilis and Kluyveromyces lactis by complementation of the S. cerevisiae sho1Δ mutant (554). As expected, sequence conservation is highest in the transmembrane region (the C. utilis and K. lactis homologs show 56 and 54% identity to the S. cerevisiae protein, respectively) and the SH3 domain (62 and 73% identity). The sequence of the linker domain is only poorly conserved except for the 20 to 25 amino acids immediately flanking the transmembrane and SH3 domains (in the case of the K. lactis homolog) as well as a 10-amino-acid peptide in the center of the linker, whose function is not known. Homologs from higher eukaryotes have not been reported.
FIG. 1.
Topology of Sho1p and Sln1p. Numbers indicate amino acid positions.
Elegant deletion and domain-swapping analyses have provided compelling evidence that Sho1p functions to recruit to the cell surface via its SH3 domain another component of the HOG pathway, the Pbs2p kinase (488). The Sho1p membrane-spanning parts could be replaced by those of the mating pheromone receptor or even a myristoylation site, resulting in a membrane anchor lacking any transmembrane sections. Also, the linker between the transmembrane regions and the SH3 domain could be replaced by an unrelated sequence without notably affecting function. Finally, even the SH3 domain was not needed for function provided Pbs2p was covalently linked to the rest of Sho1p (488). These observations suggest that Sho1p is not an osmosensor itself. Since, however, the Sho1p branch certainly mediates HOG pathway activation upon an osmotic upshock, an as yet unidentified osmosensor should exist. For instance, Wsc proteins that span the membrane once and extend into the cell wall (629, 678) could sense mechanical stress and hence are candidate osmosensors.
The role of Sho1p as an anchor protein rather than a genuine osmosensor makes previous observations suggesting a role of Sho1p in the pseudohyphal development pathway more comprehensible (439). As outlined below, the Sho1p branch of the osmosensing HOG pathway and the pseudohyphal development pathway share several components, apparently including Sho1p. Sho1p is located at places on the cell surface where growth and cell expansion occur, such as the bud neck, the growing bud, and mating projections, as well as in internal structures, possibly the vacuole (488, 498). It is plausible that the cell has to monitor osmotic changes very closely as well as plasma membrane and cell wall remodeling in expanding areas of its surface to ensure coordinated cell morphogenesis. Sho1p could function as a protein that directs signal transduction complexes to such areas.
It is not known how Sho1p itself achieves its specific localization at areas of cell growth. It has, however, been reported that latrunculin A disturbs the location of Sho1p within the bud but not at the bud neck or growing bud (498). Latrunculin A disrupts the actin cytoskeleton (394). This observation could hint at the involvement of components of the actin skeleton in locating Sho1p to certain areas of the cell surface. Consistent with this idea, Sho1p-dependent signaling requires the G-protein Cdc42p (488, 498), which in turn is involved in actin nucleation and in localization of signaling components to places of active cell growth (reviewed in references 264 and 287). However, Cdc42p is not needed for the specific localization of Sho1p (488, 498).
It is known that an osmotic shock disrupts the actin cytoskeleton, which is reorganized during recovery and adaptation (62). If Sho1p interacts with the actin cytoskeleton, directly or via other proteins, osmotic changes may affect this interaction, thereby enabling Sho1p to recruit the Pbs2p kinase and other pathway components to the cell surface. This scenario, though speculative at this point, illustrates that osmosensing could well occur within the cell rather than within the plasma membrane. It should be noted that there is also evidence arguing against an involvement of the actin cytoskeleton in controlling the Sho1p branch: latrunculin A does not seem to affect osmosensing (498). However, the effects of such compounds are difficult to control and quantitate.
It should also be noted that although anchoring of Sho1p to the cell surface is necessary for signaling, the specific localization of Sho1p at the cell surface does not seem to be needed for sensing of an osmotic shock. As outlined above, derivatives of Sho1p lacking any of its three apparent structural domains can perform the function, as long as they recruit Pbs2p to the surface (488). It is unlikely that all these different constructs attain the same specific cell surface localization, although this has not been tested. In any case, the osmotic shock treatments performed in laboratory experiments may not reflect the true physiological role for Sho1p and associated proteins, which might have specific functions in monitoring subtle osmotic changes during cell growth and surface remodeling. Certainly more work is needed to understand the precise role of Sho1p in osmoregulated signaling.
Sln1p.
Sln1p is a protein of 1,220 amino acids (Fig. 1) (442). As discussed in detail later, Sln1p is a negative regulator of the HOG signaling pathway, and deletion of SLN1 is lethal because of pathway overactivation (357). The protein is organized into four distinct regions: (i) an N-terminal section containing two predicted transmembrane domains separated by a loop, probably facing the periplasmic space; (ii) a linker region; (iii) a histidine kinase domain; and (iv) a receiver domain (442). Histidine kinases and receiver domains form so-called two-component systems, which are the prototype sensing and signaling units of prokaryotes (521, 579). Up to 80 such systems have been predicted from the genome sequences of certain species (387). Eukaryotic organisms employ histidine kinase signaling systems much less frequently.
Sln1p is the only sensor histidine kinase in the S. cerevisiae proteome, while other fungi may in fact have several: C. albicans has at least three (12, 79, 403, 573, 657), S. pombe also has at least three (72), and one each have so far been reported for Neurospora crassa (11), Aspergillus nidulans (634), and Aspergillus fumigatus (477). The slime mold Dictyostelium discoideum has at least 11 (579). At least eight histidine kinase systems have been found in plants (240, 521, 620), but so far none has been reported from animals (579).
The Sln1p histidine kinase domain, which is highly homologous to that of histidine kinases from bacteria and other eukaryotes, contains a phosphorylated histidine in position 576. The receiver domain of typical prokaryotic two-component systems is usually located within the second protein component, the response regulator, often a transcription factor (579). Sln1p, like other known eukaryotic systems, is a hybrid histidine kinase containing a receiver domain within the same polypeptide. This receiver domain is also well conserved; the phosphate-receiving aspartate residue is located at position 1144.
Only three of the known eukaryotic histidine kinases may be true Sln1p homologs, based on functional data or sequence comparison. The closest homolog is C. albicans Sln1 (CaSln1). The protein has a similar size (1,377 amino acids versus 1,220) and structural organization (403). It also shows significant sequence similarity with Sln1p not only in its histidine kinase (69% identity over 162 amino acids) and receiver domains (61% over 122 amino acids) but also in the putative sensor domain (31% over 297 amino acids). Within this sensor domain, the two transmembrane domains and the sequences immediately surrounding them are especially well conserved (49 and 45% identity), while the external loop shows only 28% identity spread over the entire domain. These data are in line with deletion analysis of Sln1p, which indicates that the first transmembrane domain is needed for the sensitivity of the histidine kinase activity to osmotic upshift (440). CaSln1 can suppress the lethality caused by an sln1Δ mutation in S. cerevisiae, but the receiver domain is not needed for suppression, suggesting that phosphotransfer bypasses two steps in the phosphorelay system (see below), although this has not been tested experimentally (403). The sln1 deletion mutant of C. albicans is viable and shows only some slight growth retardation in high-osmolarity medium (403). Hence, the precise role of CaSln1 in signaling or that of the downstream pathway may be different.
A protein from the filamentous fungus Aspergillus nidulans (1,070 amino acids; NCBI accession number BAB07814) has a similar architecture. Its external loop is somewhat longer (350 amino acids, versus about 300 in the two yeasts), but it exhibits weak homology to S. cerevisiae Sln1p even in the sensor domain (27% identity over the first 167 amino acids). The histidine kinase domain (52% over 91 amino acids) and the receiver domain (52% over 128 amino acids) are well conserved. Functional data for the A. nidulans protein have not been reported.
The Arabidopsis thaliana histidine kinase ATHK1 (1,207 amino acids) also has a domain structure similar to that of Sln1p, although the first transmembrane domain is preceded by an approximately 60-amino-acid extension. ATHK1 has been shown to complement the lethality of the S. cerevisiae sln1Δ mutation (618-620). When comparing the structurally related putative sensor domains of S. cerevisiae and C. albicans Sln1p with that of the A. nidulans protein and further with the A. thaliana homolog, significant sequence similarity disappears. The ATHK1 histidine kinase and receiver domains are significantly similar to those of Sln1p (49% identity over 76 and 78 amino acids, respectively).
All available data suggest that the Sho1p and Sln1p osmosensing systems function independently and upstream of two different branches of the HOG pathway (see below). However, a possible interaction, in physical or regulatory terms, between the two systems has so far not been studied directly. Also, the precise localization of Sln1p on the cell surface has not been reported so far.
What Are Osmosensors Sensing?
As indicated above, there is strong evidence that Sln1p directly senses osmotic changes. Further support comes from the observation that the phosphorylation state of Ypd1p, which receives its phosphate from the Sln1p receiver domain, decreases upon an osmotic shift in vivo (476). This suggests, together with other data, that the default state of the histidine kinase under low osmolarity is “on,” initiating a phosphorelay that prevents signaling beyond the response regulator Ssk1p (472, 476). Upon a hyperosmotic shock, the histidine kinase activity drops transiently, eventually leading to activation of the downstream kinase Ssk2p/Ssk22p by dephosphorylated Ssk1p. Hence, Sln1p is a sensor activated by hypo-osmolarity, i.e., cell swelling. As will be outlined in more detail, Sln1p activation by cell swelling not only prevents activation of the HOG MAP kinase cascade but also seems to stimulate a different pathway.
Significant work on the mechanisms of osmosensing has been done especially on bacterial sensors such as the KdpD-KpdE system, which controls expression of the components of the high-affinity K+ transporter Kpd and the EnvZ-OmpR system, which controls expression of outer membrane porins. The histidine kinase KdpD has been proposed to be a turgor sensor, but the actual mechanism of stimulation is not understood (653). The protein has been purified and reconstituted in proteoliposomes (268). In this in vitro system, KdpD is activated by increased ionic strength and K+ ions attenuate the activity, suggesting that osmolarity sensing by KdpD is overlapped by some solute specificity (269). Interestingly, KdpD function in vitro seems to require negatively charged phospholipids, indicating that interaction with the surrounding lipids might be important for regulation and signal transmission (575). Although KdpD-KdpE is a two-component system, the domain organization of the sensor histidine kinase is different from that of Sln1p: KdpD has four transmembrane domains located in the central part of the protein.
EnvZ, however, has a domain structure that is very similar to that of Sln1p, with two transmembrane domains in the N-terminal part separated by an external loop; however, apart from the histidine kinase domain, there is no apparent sequence similarity to Sln1p. EnvZ has been studied intensively, with a focus on the function of the histidine kinase domain and its intrinsic phosphatase activity (147). Distinct domains have been purified and their structures have been analyzed (590, 607), but EnvZ has not been purified as a complete protein, nor has it been reported to be functionally reconstituted in proteoliposomes. Mutational analysis suggests that the transmembrane regions are required for sensing (603-606). This observation again suggests that sensing occurs in the membrane, for instance, in response to membrane stretching, but it does not exclude the possibility that the transmembrane domains play their role by positioning, for instance, the external loop in an osmoresponsive way. In such a scenario, the transmembrane domains would be required for intramolecular signaling through the membrane without being directly involved in sensing.
Since a range of different substances that alter water activity can stimulate the HOG pathway, it seems unlikely that binding of a ligand is the primary event. As for KdpD and EnvZ, it is rather anticipated that Sln1p is affected by a physical stimulus. The role of the sensor domain of Sln1p has been studied by a low-density deletion analysis (440). The data suggest that the first transmembrane domain is required for the control of histidine kinase activity by osmotic changes, since deletion of this domain renders the kinase constitutively active and unresponsive to an osmotic shock. This observation, however, does not reveal if the first transmembrane domain has any particular features important for osmosensing. Replacing it with a different transmembrane domain, as was done with Sho1p, could possibly provide such information. The external loop region seems to contain a dimerization domain, which can be replaced by an unrelated leucine zipper element. Dimerization is necessary for hybrid kinases because histidine autophosphorylation occurs in trans (579).
Truncation of Sln1p so that it lacks both transmembrane segments and the loop strongly diminishes its activity, resulting in hyperactivation of the downstream pathway (440). Clearly, more detailed functional analyses of Sln1p are needed to better understand its mechanism in osmosensing. In particular, it would be most revealing if Sln1p histidine kinase activity could be monitored in yeast secretory vesicles (406) or even reconstituted in proteoliposomes; such systems could allow more detailed studies on the biophysical mechanisms controlling Sln1p.
As indicated earlier, it is intuitively anticipated that osmosensing occurs at the cell surface, although this is by no means mechanistically necessary (653). In fact, the osmosensing histidine kinase of the cell wall-less slime mold Dictyostelium discoideum appears to be a cytosolic protein, since it is lacking any apparent transmembrane domains (535). The following scenarios, although all speculative, illustrate some possibilities for osmosensing beyond physical impact on the plasma membrane.
As mentioned above, an osmotic upshock causes the actin cytoskeleton to dissociate, and it is subsequently reorganized during adaptation. Possible sites of osmosensing are cortical actin patches, which consist of helical bundles of actin wrapped around plasma membrane invaginations (58, 400). Although this has not been studied in detail, it is likely that the size and shape of the invaginations change upon osmotic shock, affecting the conformation of actin bundles (205). In fact, the dynamic reestablishment of the cytoskeleton during adaptation (62, 97) illustrates that the cell senses that its actin network is disturbed. Therefore, the actin cytoskeleton may serve as a genuine osmosensor, at least for its own reorganization. This dynamic process would also make an excellent system for termination of the response. Although it has been known for many years that certain actin alleles (428, 644) as well as mutations in different components of the cytoskeleton cause osmosensitivity, there is no direct experimental evidence for an involvement of actin or its associated proteins in the control of signaling pathways or any other osmoadaptive processes in the cell. The notable and important exception is the G-protein Cdc42p, which plays an important role in the control of assembly of the actin cytoskeleton (198, 264) and is involved in the Sho1 branch of the HOG pathway (488, 498), as well as in other signaling pathways (264, 287).
An osmotic upshock causes water to flow out of the cell. One consequence of this is an increase in the concentration of all cellular components. In particular, the higher concentration of certain ions could serve as a signal. Recently, a higher internal concentration of K+ has been proposed to control the osmoregulated BetP channel in Corynebacterium glutamicum as a kind of concentration-dependent second messenger (518). A sensor protein could undergo a conformational change on binding of such an ion and thereby initiate a response. Since many proteins and especially enzymes are well known to respond to low-molecular-weight effectors, the cell is rich in putative osmosensors in this sense. Posttranslational effects, such as changes in metabolism (see below), could be controlled via altered concentration of low-molecular-weight compounds in the shrunken cell.
SIGNALING OSMOTIC CHANGES
Overview of Signaling Pathways Involved in Osmoadaptation
An osmotic upshift causes a impressive transcriptional response, affecting expression of about 10% of the yeast genes (86, 191, 471, 501, 656). Increased and decreased expression of genes is controlled by signaling pathways that sense osmotic changes and transmit the signal to the transcriptional machinery. Changes in medium osmolarity have been shown to affect different signaling pathways in yeasts. By far the best-characterized system is the HOG pathway, which is activated within less than 1 min by osmotic upshift (61). The inability of mutants with an inactive HOG pathway to adapt properly to high-osmolarity medium and the known function of genes whose expression is stimulated via the HOG pathway confirm that the cellular role of the HOG pathway is indeed to orchestrate a significant part of the transcriptional response of yeast cells to high osmolarity. The HOG pathway also mediates posttranscriptional effects.
In addition to the HOG pathway, protein kinase A (cyclic AMP [cAMP]-dependent protein kinase) has been shown to affect expression of genes upon an osmotic upshift (423). Protein kinase A mediates a general stress response that is observed under essentially all stress conditions, such as heat shock, nutrient starvation, high ethanol levels, oxidative stress, and osmotic stress (362, 519, 555). Hence, protein kinase A most probably does not respond directly to osmotic changes. It is not well understood how the activity of protein kinase A is controlled by stress.
Other signaling pathways have recently been associated with responses to an osmotic upshift. It has been observed that an osmotic shock stimulates production of phosphatidylinositol-3,5-bisphosphate, which could serve as a second messenger in an osmotic signaling system (145). In addition, evidence has been provided that the Snf1p AMP-dependent kinase, which controls the general glucose repression system in S. cerevisiae, is involved in transcriptional responses to osmotic shock (613; M. Krantz and S. Hohmann, unpublished data).
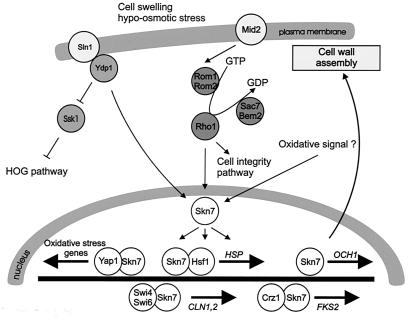
Less is known about responses to osmotic downshifts. Rapid, posttranslational mechanisms seem to play an important role in survival, such as glycerol export through the Fps1p channel. In addition, it has been observed that a hypo-osmotic shock stimulates influx of calcium into yeast cells (30), but the physiological significance is unclear. It has also been shown that a hypo-osmotic shock rapidly stimulated the cell integrity pathway (125), but again the physiological significance is unclear. Finally, recent data indicate that Sln1p, Ypd1p, and Skn7p form a phosphorelay system that could activate gene expression upon cell swelling (338, 591), although this has not been tested directly and the overall impact on cellular physiology also remains to be elucidated. Global gene expression analysis has not yet revealed a characteristic gene expression pattern caused by an osmotic downshift but rather a reversal of the expression pattern observed in cells growing at high osmolarity (191), although more thorough analyses of hypo-osmotic shock responses may be performed in the near future.
HOG MAP Kinase Pathway in S. cerevisiae
The HOG pathway is the best-understood osmoresponsive system in eukaryotes and hence serves, together with the Sty1 pathway of S. pombe, as a prototype osmoregulating signaling pathway. In addition, the HOG pathway is one of the best-understood MAP kinase pathways.
MAP kinase pathways.
MAP kinase pathways (for nomenclature of proteins, see Fig. 2) are highly conserved signaling units apparently occurring in all eukaryotes, where they play essential roles in the response to environmental signals or hormones, growth factors, and cytokines. MAP kinase pathways control cell growth, morphogenesis, proliferation, and stress responses, and they are involved in many disease processes. Excellent reviews on MAP kinase pathways are published frequently (for instance, see references 28, 88, 205, 304, 308, and 339), and therefore I summarize only essential principles relevant to the further discussion.
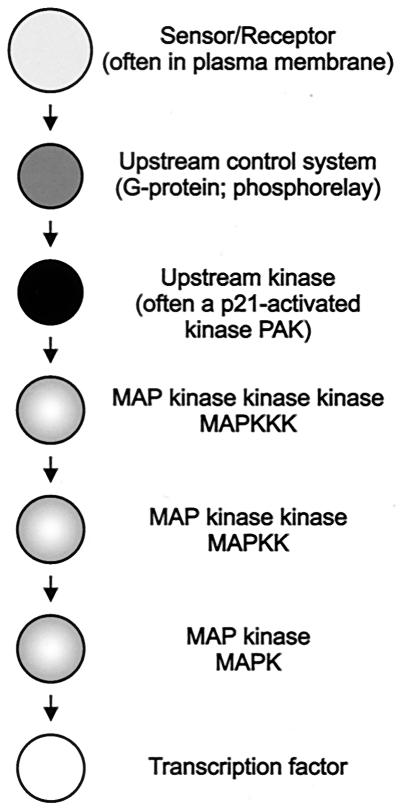
FIG. 2.
Nomenclature of proteins in MAP kinase pathways. Arrows only indicate the flow of information; pathway-specific protein complexes are common and are required for signal transmission.
Central to each MAP kinase pathway are three tiers of protein kinases, a MAP kinase, a MAP kinase kinase (MAPKK) or MEK, and a MAPKKK (MAPKKK) or MEKK. The MAPKKK phosphorylates and thereby activates the MAPKK on serine and threonine within a conserved part at the N-terminal lobe of the kinase domain. Subsequently, the MAPKK phosphorylates the MAP kinase on a threonine (sometimes serine) and tyrosine residue, which are located adjacent to each other separated by a single amino acid (Thr/Ser-X-Tyr). This phosphorylation site is located in the activation loop of the catalytic domain; dual phosphorylation on threonine and tyrosine is needed for activation of the MAP kinase. Typically, phosphorylation stimulates transfer of the MAP kinase from the cytosol to the nucleus, where it phosphorylates targets on serine/threonine followed by a proline. However, a portion of activated MAP kinase is apparently also present in the cytoplasm to mediate posttranslational effects.
MAPKKKs consist of an N-terminal regulatory and a C-terminal catalytic kinase domain. The regulatory domain locks the C-terminal kinase domain in the inactive state. Activation may occur by phosphorylation through an upstream protein kinase or through interaction with other proteins, a process that often involves small G-proteins. The activation mechanisms and sensor systems upstream of MAP kinase pathways are diverse and include receptor-tyrosine kinase (in animal systems), G-protein-coupled receptors, phosphorelay systems, and others.
Different MAP kinase pathways form interacting signaling systems. For instance, one MAPKK may control several different MAP kinases, as is observed even in the relatively simple yeast system. Different pathways within the same organism often share kinases. Especially in higher eukaryotes but even in S. cerevisiae, this situation results in highly complex network systems of signaling pathways. Pathway specificity is commonly but probably not exclusively achieved by scaffold proteins. Scaffolds may be proteins apparently dedicated to this purpose, such as S. cerevisiae Ste5p, or may be part of active components of the MAP kinase cascade, such as the yeast MAPKK Pbs2p. Hence, presentation of MAP kinase systems as linear pathways, as shown in Fig. 2 to explain nomenclature, is actually an oversimplification.
MAP kinase pathways are negatively controlled by protein phosphatases acting on both the MAPKK and the MAP kinase (serine-threonine phosphatases) or only on the MAP kinase (tyrosine phosphatases) (283).
S. cerevisiae MAP kinase pathways.
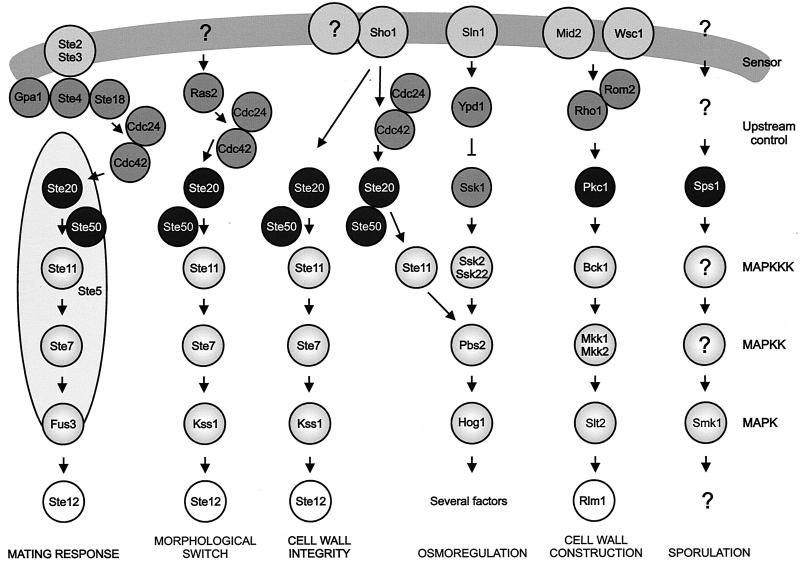
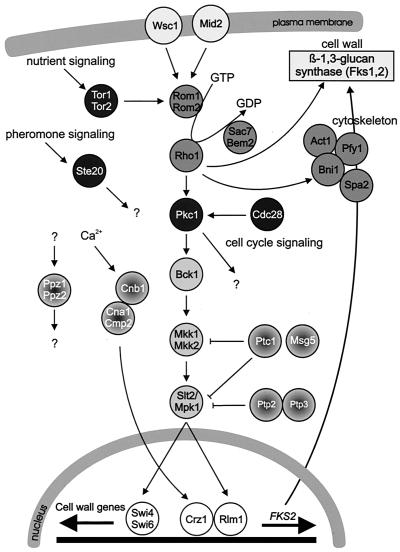
Since components of the MAP kinase cascade can be recognized by sequence similarity, the set of relevant kinases in the yeast proteome is known. S. cerevisiae has five MAP kinases. Based on genetic analyses as well as studies on the transcriptional readout upon physiological, pharmacological and genetic stimulation, the five MAP kinases are allocated to six distinct MAP kinase pathways (Fig. 3): (i) the mating pheromone response pathway (MAP kinase Fus3p), (ii) the pseudohyphal development pathway (Kss1p), (iii) the HOG pathway (Hog1p), (iv) the protein kinase C (PKC) or cell integrity pathway (Slt2/Mpk1p), and (v) the spore wall assembly pathway (Smk1p) (149, 205, 222, 474).
FIG. 3.
Outline of the yeast MAP kinase pathways, illustrating similarities and difference in architecture. Gpa1p, Ste4p, and Ste18p form a heterotrimeric G-protein; Ras2p is one of two yeast Ras proteins. Other proteins are explained in the text.
Recently, two independent genetic screens led to the conclusion that components of the pheromone response pathway plus Sho1p are part of a distinct MAP kinase pathway, termed the STE vegetative growth pathway (Fig. 3). This pathway appears to be involved in the control of cell wall integrity (114, 149, 323, 324). The five MAP kinases are controlled by four MAPKKs, Ste7p, Pbs2p, and the apparently redundant Mkk1p and Mkk2p, and four MAPKKKs, Ste11p, Bck1p, and the apparently redundant Ssk2p and Ssk22p. Hence, as is the case with MAP kinase pathways in higher eukaryotes, certain MAPKKKs and MAPKKs control more than one MAP kinase. Ste7p controls the mating pheromone response pathway (Fus3p), the pseudohyphal development pathway (Kss1p), and the STE vegetative growth pathway (Kss1p). The MAPKKK Ste11p, which activates Ste7p in three pathways, has been shown to also be required for the activation of Pbs2p in the Sho1 branch of the HOG pathway.
The specificity of signal transmission is ensured by scaffold proteins (457), which are specific to their respective pathway: Ste5p is needed for the mating pheromone response pathway (74), and Pbs2p also functions as the MAPKK of the HOG pathway (473). Additional mechanisms ensuring pathway specificity and involving the MAP kinases Fus3p and Hog1p have been discussed; deletion of these two kinases results in inappropriate cross talk (354, 439). For the spore wall assembly pathway, only a MAP kinase and an upstream protein kinase have been found; the MAPKK and MAPKKK are missing. Hence, Smk1p is activated either by an unusual mechanism or by any of the known MAPKKs and MAPKKKs.
The sensing input systems differ between pathways, and it appears that yeast MAP kinase pathways represent a range of possible input devices. The mating pheromone response pathway is controlled by the pheromone receptors Ste2p and Ste3p (for the mating pheromones α and a, respectively), which are G-protein-coupled receptors. For signal transmission, Ste50p, whose molecular function is not well understood, and the upstream kinase Ste20p, a member of the PAK (p21-activated protein kinase) family (122), are required. Ste20p serves as upstream kinase not only in the mating pheromone response pathway but apparently in all Ste11p-dependent pathways (321, 439, 508); at least in some instances, Cla4p (35) and perhaps Sps1p (368), yeast kinases related to Ste20p, may perform related or redundant functions. Localization of Ste20p to sites of cell growth and activation of Ste20p as well as of Cla4p require the G-protein Cdc42p and its exchange factor, Cdc24p. As mentioned above, Cdc42p also interacts with the actin cytoskeleton (reviewed in references 89, 149, 154, and 287).
The sensor of the pseudohyphal development pathway has not been unambiguously identified, although evidence is accumulating that Mep2p is needed for pathway activation through nitrogen-dependent signals (348). Mep2, which has strong similarity to ammonium transporters, is a member of a new class of sensor proteins derived from transporters (171). The two branches of the HOG pathway are controlled by apparently completely different input systems. As outlined above, the sensor for the Sho1 branch is presently not known; the formation upon stimulation of a protein complex between Sho1p and Pbs2p, probably also involving Ste50p, the upstream kinase Ste20p, the G-protein Cdc42p, and the MAPKKK Ste11, is needed for the activation of this system (488, 498). The Sln1 branch of the HOG pathway is controlled by a phosphorelay system. The upstream sensing system of the STE vegetative growth pathway seems to involve proteins from the Sho1 branch of the HOG pathway, including Sho1p itself (149). Multiple putative sensors have been described for the cell integrity pathway, as discussed below (216). The MAP kinase cascade is controlled by the upstream kinase Pkc1p, the single yeast homolog of protein kinase C, which also has additional functions. For the spore wall formation pathway, the upstream kinase Sps1p has been identified, but the mechanisms that control this protein are not known.
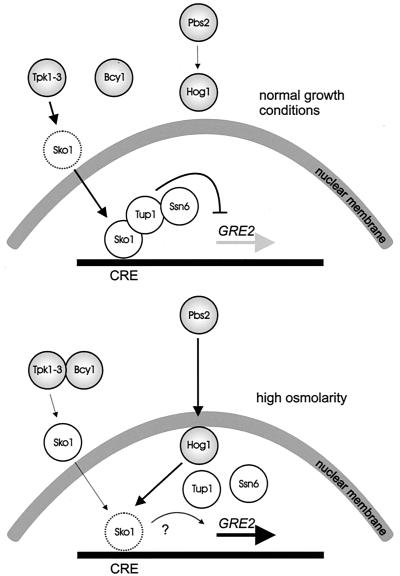
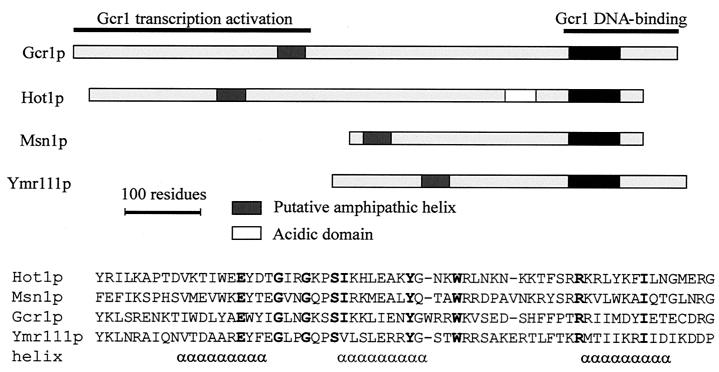
Upon activation, a portion of the MAP kinase moves to the nucleus, where it controls transcriptional regulatory proteins. Transcription factors have been associated with most of the yeast pathways, and the control of these factors by the MAP kinase is known to different degrees of molecular detail. The type of transcription factor and the way it is controlled are apparently not conserved between MAP kinase pathways. Fus3p of the mating pheromone response pathway activates the zinc finger protein Ste12p, which binds to pheromone response elements and activates a set of genes whose products play roles in mating and cell fusion (205, 499, 507). Ste12p activation requires inactivation of two negative regulators, Dig1p and Dig2p. Ste12p cooperates on target promoters with Mcm1p, which is a factor binding to numerous promoters. Ste12p is also the transcription factor needed for the transcriptional output of the pseudohyphal development pathway, where it cooperates with Tec1p on target promoters or controls expression of TEC1 (329). The Hog1p kinase mediates its effects through at least five different transcription factors: the possibly redundant zinc finger proteins Msn2p and Msn4p (533), Hot1p (which does not belong to a known family of factors) (503), the bZIP protein Sko1/Acr1p (480), and the MADS box protein Smp1p (F. Posas, 2001, personal communication). The cell integrity pathway mediates transcriptional responses through Rlm1p, which controls genes encoding proteins required for cell wall assembly, as well as SBF (Swi4/Swi6 cell cycle box-binding factor) (216). The transcription factor(s) controlled by Smk1p has not been identified.
Present knowledge of the architecture, sensors, and outputs of the yeast MAP kinase leads to an emerging picture where these pathways, in concert, orchestrate morphogenesis, directed cell growth, and remodeling of the cell surface. While the primary decisions for cell polarity seem to be controlled by other mechanisms (89), the MAP kinase pathways are required for directed cell growth (bud formation, mating projections, and pseudohyphal growth), the necessary remodeling of the cell surface associated with growth (cell wall integrity, cell integrity, and HOG pathways), and maintenance of the appropriate turgor pressure (HOG and cell integrity pathways). Spore formation is also accompanied by remodeling of the surface. While cellular morphogenesis in response to developmental and external stimuli seems to be the primary role of the yeast MAP kinase pathways, several of those (the mating pheromone response, cell integrity, and HOG pathways) have demonstrated roles in also coordinating cell proliferation (cell cycle control) with morphogenesis.
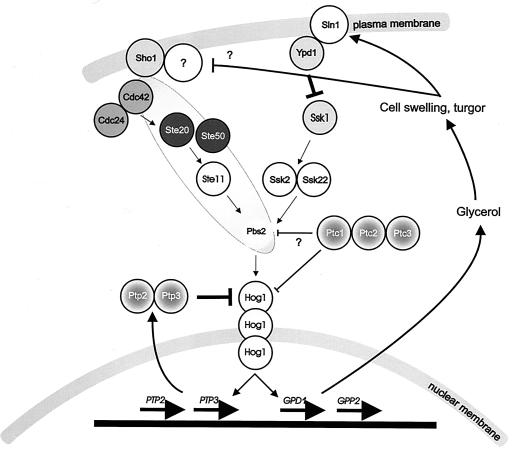
HOG pathway architecture.
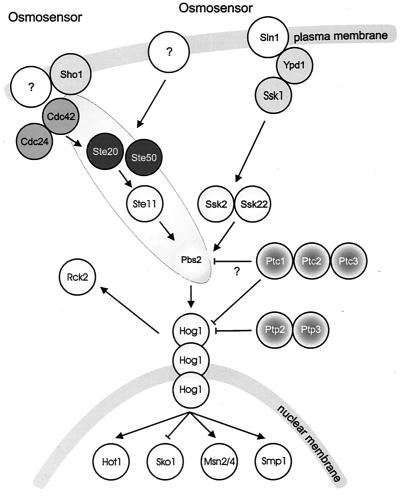
The architecture of the HOG pathway (Fig. 4) has been elucidated through several clever genetic screens and epistasis analysis, the latter partly based on expected analogy to other pathways. In the following I summarize the genetic evidence for the architecture of the HOG pathway, while in the next section I discuss in more detail the dynamic operation of the pathway as elucidated by tools of biochemistry and cell biology. Phenotypes of key HOG pathway mutants are summarized in Table 1.
FIG. 4.
Outline of the HOG pathway. Pbs2p functions both as a scaffold and as a MAPKKK; it is not known exactly with which components of the Sho1 branch it interacts.
TABLE 1.
Key mutations affecting the HOG pathway and their phenotypes
| Allele(s) | Mutation | Effect | Phenotype (reference) |
|---|---|---|---|
| sln1Δ | Deletion | Pathway overactivation | Lethal (357) |
| sln1∗ | Different point mutations | Diminished pathway activation, activation of Skn7p | Sensitivity to high osmolarity (164) |
| ypd1Δ | Deletion | Pathway overactivation | Lethal (476) |
| ssk1Δ | Deletion | Block of Sln1 branch | Sensitivity to high osmolarity in sho1Δ (355) |
| ssk2ΔN or ssk22ΔN | N-terminal truncation | Pathway overactivation | Lethal (355) |
| ssk2Δ ssk22Δ | Double deletion | Block of Sln1 branch | Sensitivity to high osmolarity in sho1Δ (355) |
| pbs2Δ | Deletion | Pathway block | Sensitivity to high osmolarity (61) |
| PBS2(EE) | S514E and T518E | Pathway activation | Lethal (40) |
| pbs2-389 | K389M, catalytically inactive | Pathway block | Sensitivity to high osmolarity (498) |
| pbs2-96 | P96S, no interaction with Sho1p | Pathway block | Sensitivity to high osmolarity in ssk2Δ ssk22Δ (355) |
| hog1Δ | Deletion | Pathway block | Sensitivity to high osmolarity (61) |
| hog1 | Various point mutations | Constitutive | Osmoresistant in a pbs2Δ background (33) |
| ptc1Δ ptp2Δ | Double deletion | Pathway overactivation | Lethal (254) |
| sho1Δ | Deletion | Block of Sho1 branch | Sensitivity to high osmolarity in an ssk2Δ ssk22Δ mutant (355) |
| ste11Δ | Deletion | Block of Sho1 branch | Sensitivity to high osmolarity in an ssk2Δ ssk22Δ mutant (355) |
| ste50Δ | Deletion | Block of Sho1 branch | Sensitivity to high osmolarity in an ssk2Δ ssk22Δ mutant (475) |
| ste20Δ | Deletion | Partial block of Sho1 branch | Slight sensitivity to high osmolarity in an ssk2Δ ssk22Δ mutant (439) |
Two genes encoding HOG pathway components, the MAP kinase Hog1p and the MAPKK Pbs2p, were found within a set of osmosensitive mutants (61). PBS2 had been identified earlier in a screen for genes that upon overexpression confer resistance to polymyxin B, which affects the plasma membrane (50, 51); the molecular basis for this finding is not understood. Deletion of PBS2 or HOG1 causes inability to grow at elevated osmolarity; hog1Δ and pbs2Δ mutants typically fail to proliferate on medium with more than 0.5 M NaCl or 1 M sorbitol. Instead, such mutants acquire an unusual morphology, resembling mating projections or pseudohyphae (50, 61, 62, 439). This phenotype is due to inappropriate activation of the pheromone response pathway and the pseudohyphal development pathway in such mutants (124, 439). Both hog1Δ and pbs2Δ mutants accumulate only half as much of the osmolyte glycerol as the wild type under osmotic stress (7, 61). Since both single mutations and the hog1Δ pbs2Δ double mutation caused identical defects in growth, proliferation, and glycerol accumulation at high osmolarity, it was concluded that the proteins are part of the same pathway. This was confirmed by the observation that an osmotic upshift caused rapid phosphorylation of Hog1p in a Pbs2p-dependent manner (61), placing Pbs2p upstream of Hog1p.
This pioneering work of Gustin and colleagues marked the realization that osmotic responses are mediated by specific and potentially conserved signaling pathways (61). Saito and colleagues discovered further pathway components. They were searching for mutations that caused synthetic lethality in combination with deletion of PTP2, which encodes a protein tyrosine phosphatase. This screen identified the genes SLN1 and YPD1 (357, 476). It subsequently turned out that deletion of SLN1 and YPD1 by themselves causes lethality. This lethality, however, was suppressed by overexpression of PTP2, confirming a genetic link (357, 476). The SLN1 gene had initially been identified in a screen for mutations causing synthetic lethality with a mutation affecting protein degradation by the ubiquitin-dependent pathway (441, 442). In light of the presently known role of Sln1p, this early finding remains mysterious. Perhaps presently unknown ubiquitin-dependent proteolytic mechanisms are part of osmotic responses. The sln1Δ lethality seems to depend on the genetic background: sln1Δ mutants of some strains grow poorly, especially on defined (SC) medium (442).
Mutations that suppressed the lethality of the sln1Δ mutation identified the already known genes HOG1 and PBS2 (357), linking Sln1p to the HOG pathway. Since cellular depletion of Sln1p, as achieved by expression of SLN1 from the repressible GAL1 promoter, caused heavy tyrosine phosphorylation of Hog1p, and because of the similarity of Sln1p to sensor histidine kinases, it was concluded that Sln1p is an upstream sensor of the HOG pathway. The same suppressor search identified further components of this pathway, the MAPKKs Ssk2p and Ssk1p, which contain a response regulator domain (357). A search for genes homologous to SSK2 revealed SSK22 (355). These two proteins are 69% identical in the C-terminal kinase domain and 47% identical in the N-terminal regulatory domain. At least within the HOG pathway, both proteins seem to perform the same function (355).
Epistasis analysis confirmed the pathway organization indicated by the similarity of the protein kinases to components of MAP kinase pathways. For instance, inappropriate activation of the HOG pathway by N-terminal truncation of the Ssk2p and Ssk22p kinases causes lethality, as does deletion of Sln1p. This lethality is suppressed by deletion of PBS2 and HOG1, but not by deletion of SSK1 (355), placing Ssk2p and Ssk22p upstream of Pbs2p and Hog1p but downstream of Ssk1p. Multicopy suppression of the sln1Δ lethality also generated a rich harvest: the genes encoding the protein phosphatases Ptp2p and Ptp3p, which encode phosphotyrosine phosphatases, and Ptc1p and Ptc3p, which encode serine/threonine protein phosphatases (356, 357, 441). Interestingly, none of the suppressor screens revealed any candidate downstream targets of the pathway, such as transcription factors or genes whose expression is controlled by the pathway. This is due to the fact that the HOG pathway controls the expression of many genes via different transcriptional regulators, and individual deletion of such genes causes only moderate suppression, if any (503).
Several observations suggested that the pathway had not yet been completely uncovered. Even the double ssk2Δ ssk22Δ mutant still showed Hog1p phosphorylation upon osmotic shock, and the mutant did not display an osmosensitive phenotype, indicating alternative routes to activate the downstream MAPKK Pbs2p (355). Several components of this route were identified in an exhaustive search for mutations that caused osmosensitivity in an ssk2Δ ssk22Δ background. This screen identified an allele of Pbs2p as well as Sho1p (355), and direct interaction between Pbs2p and Sho1p via the SH3 domain of Sho1p was demonstrated. The same screen also identified the Ste11p MAPKKK. Triple ssk2Δ ssk22Δ sho1Δ and ssk2Δ ssk22Δ ste11Δ mutants are as osmosensitive as pbs2Δ and hog1Δ mutants and fail to phosphorylate Hog1p upon osmotic shock (355, 473), indicating that the three MAPKKKs Ssk2p, Ssk22, and Ste11p represent the activators of Pbs2p. Only when more than 1.4 M NaCl is applied do such triple mutants show Hog1p phosphorylation; it is not known if this is a truly physiological response (622), and it may be caused by Hog1p autophosphorylation (33). Finally, the same screen also identified another component of the pheromone response pathway, Ste50p (475). Ste50p is needed for activation of Hog1p via the Sho1 branch, and an ssk2Δ ssk22Δ ste50Δ triple mutant is osmosensitive (439, 475).
The fact that Ste11p is involved in both the pheromone response pathway and the HOG pathway prompted a screen for mutations that allowed cross talk, i.e., activation of the pheromone response pathway by osmotic shock (439). It was found that mutations in the gene for Hog1p caused such cross talk and that stimulation of the pheromone response pathway by osmotic shock in a hog1Δ mutant required Sho1p, Ste50p, and also Ste20p. In fact, a triple ssk2Δ ssk22Δ ste20Δ mutant is osmosensitive and defective in Hog1p phosphorylation, identifying Ste20p as a pathway component. However, the osmosensitivity is not as strong as, e.g., in an ssk2Δ ssk22Δ ste50Δ mutant (439, 488). This is probably due to the Ste20p homolog, Cla4p (117); overexpression of CLA4 rescues the osmosensitivity of the ssk2Δ ssk22Δ ste20Δ triple mutant (488), indicating that the proteins have overlapping functions. Finally, the essential G-protein Cdc42p, which binds Ste20p as well as Cla4p (35, 117), is involved in signal transduction through the Sho1p branch according to several lines of evidence: the Ste20p domain for interaction with Cdc42p is required for signaling through the HOG pathway, a dominant negative allele of Cdc42p reduces Hog1p phosphorylation in an ssk2Δ ssk22Δ background, a partially defective allele of CDC42 reduces Hog1p phosphorylation in an ssk1Δ background, and Cdc42p is required for the osmoshock-induced localization of Ste20p to the cell surface (see below) (488, 498).
At this point, genetic analyses leave a couple of open questions. Which protein functions as the osmosensor in the Sho1 branch of the pathway? Recent analysis indicates that Sho1p itself probably only functions as a recruiting factor (488). The Saito group has done an exhaustive search for mutations conferring osmosensitivity on an ssk2Δ ssk22Δ double mutant (475), but since no candidate sensor was found, it (they) may be encoded by redundant genes or one of the known components might actually function as the sensor. Integrin receptor-like type I transmembrane proteins, such as the Wsc proteins (629), are possible sensor candidates. Alternatively, the actin cytoskeleton may be involved in the sensing process, as discussed above.
Which proteins are involved in a possible third input system into the HOG pathway? The existence of a third sensing system is based on the observations that even in sho1Δ ssk1Δ and sho1Δ ssk2Δ ssk22Δ mutants, deletion of HOG1 leads to osmo-induced activation of the pheromone response pathway. In addition, it was observed that a sho1Δ ssk1Δ strain is less osmosensitive than an ste11Δ ssk1Δ or ste50Δ ssk1Δ strain, suggesting that Ste11p can also be activated by osmotic shock independently of Sho1p (439).
What is the mechanism that stimulates Hog1p phosphorylation by severe osmotic stress in an sho1Δ ssk2Δ ssk22Δ mutant (622)? Principally, the second and third questions might lead to the same answer, although Hog1p autophosphorylation may also be a simple possibility. More work is needed to clarify the possible existence of alternative input systems into the HOG pathway.
Activation of HOG pathway function: subcellular localization and dynamics. (i) Role of the two branches of the HOG pathway.
The genetic evidence outlined above suggests that the upstream branches of the HOG pathway operate independently of each other; blocking one branch of the pathway still allows rapid Hog1p phosphorylation upon an osmotic shock, and such cells are apparently fully resistant to high osmolarity. Although these observations suggest redundant functions of the two branches, it is unlikely that the cell maintains two different complex pathways to activate Pbs2p.
It has been proposed that different sensitivities of the two branches may allow the cell to respond over a wide range of osmolarity changes (355). In an ssk2Δ ssk22Δ double mutant, which completely relies on the Sho1 branch, stimulation of Hog1p tyrosine phosphorylation requires at least 300 mM NaCl, becomes visible after about 2 min, and reaches a maximum at 5 min. In contrast, in an sho1Δ ssk22Δ mutant, which relies on the Sln1 branch only, Hog1p phosphorylation is already apparent with 100 mM NaCl and is maximal after 1 min with 300 mM NaCl. These data suggest that Sln1p is more sensitive than the sensor of the Sho1 branch. It also appears from the same set of experiments that the Sho1 branch operates in an on-off fashion, while the Sln1 branch shows an approximately linear dose response up to about 600 mM NaCl. Different sensitivities seem to be a plausible though not fully satisfactory explanation for the existence of the two branches.
At an osmolarity where only one of the two branches is activated, i.e., below 300 mM NaCl, hog1Δ mutants still grow, indicating that the pathway is not absolutely necessary under these conditions. In addition, in nature yeast cells are commonly exposed to far more dramatic osmolarity changes. At high osmolarity, when Hog1p phosphorylation is apparently saturated, the period of Hog1p phosphorylation becomes progressively longer the more osmoticum is used to shock the cells. In other words, the cell seems to modulate the response by the amplitude only at lower osmolarity and by the period of activation at higher osmolarity (622; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). Taken together, the observed different sensitivities and responsiveness of the two branches may rather reflect different mechanisms of stimulation, i.e., the two branches may interpret osmotic changes via different physical stimuli, such as membrane stretch, cell wall stress, or impact on the cytoskeleton. If so, it should be possible to find conditions under which only one of the two branches is activated.
The recent finding that components of the Sho1 branch are localized or recruited to places of active cell growth (488, 498) indicates that it fulfils a specific localized role in osmosensing. The precise subcellular localization of components of the Sln1 branch has not been reported, but on the basis of our present understanding, one might speculate that the Sho1 branch monitors (mainly) osmotic changes during cell growth and expansion, while the Sln1p branch (mainly) senses osmotic changes in the environment.
Such an idea is further supported by several observations. If we assume that (one branch of) the HOG pathway monitors osmotic changes during cell growth, we would expect the pathway to perform functions even at normal, constant external osmolarity. Simultaneous deletion of the genes PTC1 and PTP2, which encode protein phosphatases, is lethal under normal growth conditions, and this lethality is suppressed by additional deletion of HOG1 (254, 356). This suggests that Hog1p is activated even at low osmolarity but that this activation is counteracted by the phosphatases; the upstream branch involved in this homeostatic activation has not been determined.
Furthermore, evidence has been reported for a role of the HOG pathway in controlling cell surface composition under ambient osmolarity: Hog1p is required for the proper localization of Mnn1p, a Golgi glycosyltransferase (505). In addition, mutations in the HOG pathway, in fact in both branches, increase tolerance to the antifungal drug calcofluor, which targets chitin-containing fungal cell walls (188). Moreover, a shift to low pH was shown to cause Hog1p-dependent changes in the expression of genes encoding cell wall proteins and a concomitant change in cell wall composition (276). Low pH is not known to stimulate the HOG pathway, and hence these effects may be secondary. Which upstream branches of the HOG pathway were required for the effect was not studied. Taking the results together, it is apparent that the HOG pathway confers activity even without osmotic stress.
This function could be a reflection of its role in monitoring turgor during cell growth, and this in turn could be a specific role of the Sho1 branch. Sho1p together with Ste20p, Ste11p, Ste7p, Kss1p, and Ste12p appears to form an independent MAP kinase pathway, the STE vegetative growth pathway, which seems to be part of the systems that control cell wall integrity (114, 324). That pathway is required for growth at normal osmolarity. In addition, a genetic screen for multicopy suppressors of the growth defect of an ste11Δ ssk2Δ ssk22Δ triple mutant at moderately high osmolarity and elevated temperature, which is probably due to simultaneous inactivation of the HOG and STE vegetative growth pathways, revealed the genes LRE1 and HLR1 (16). The products of these two genes are thought to affect cell wall composition. Overexpression of LRE1 and HLR1 also partially suppressed the osmosensitivity of a hog1Δ and a pbs2Δ mutant, suggesting that the HOG pathway, directly or via interaction with other pathways, affects cell wall composition (16).
Together, both branches of the HOG pathway may then orchestrate osmotic responses and integrate the need for cell expansion in response to osmotic signals generated by growth and by the environment.
(ii) Activation via the Sln1 branch.
While the physical signal(s) that controls the Sln1p histidine kinase is not known, the further events in signaling through the phosphorelay system have been studied in detail. The Sln1p histidine kinase is activated by hypo-osmolarity (cell swelling) and inhibited by hyperosmolarity (cell shrinking). Sln1p and Ypd1p function as negative regulators of the HOG pathway, as indicated by the fact that deletion of SLN1 and YPD1 causes lethality due to HOG pathway overactivation (357). Moreover, activated SLN1 alleles cause diminished responsiveness of the HOG pathway and sensitivity to high osmolarity (164). In addition, Ypd1p is phosphorylated under normal growth conditions, but it is phosphorylated to a lesser extent after a hyperosmotic shock (476).
Therefore, under low osmolarity, Sln1p constantly autophosphorylates itself on His576. This phosphate is then transferred to Asp1144, within the receiver domain of Sln1p. Phosphotransfer can occur (or may occur obligatorily) between different Sln1p molecules: Sln1p mediates inhibition of the HOG pathway when the N-terminal section containing the histidine kinase and the C-terminal response regulator domain are expressed separately in an sln1Δ mutant (476). Subsequently, the phosphate group is transferred to His64 on Ypd1p and further to Asp554 on Ssk1p. This sequence of events has been demonstrated convincingly by analysis of truncated and mutated proteins both in vivo and in vitro (256-258, 476). These studies also demonstrate that in vivo the histidine kinase cannot bypass the Sln1p response regulator. Hence, Ypd1 does not normally become phosphorylated directly by the Sln1p histidine kinase (476), although this reaction can occur in vitro (256).
Several very interesting questions concerning the function of the Sln1p-Ypd1p-Ssk1p phosphorelay system remain to be answered. First of all, why does S. cerevisiae (eukaryote) employ a phosphorelay system instead of a two-component system for osmosensing, as bacteria do? One reason may be to establish additional steps for modulation and control of the system. In particular, phosphate groups from alternative donors, such as phosphorylated metabolites, could provide possibilities to downregulate the system. Posas et al. (476) suggested the possibility of feedback control if glycerol-3-phosphate, an intermediate of glycerol production, served as a phosphate donor. There is no experimental evidence for such a mechanism at the moment. It has, however, been demonstrated that Ssk1p can autophosphorylate itself in vitro using acetylphosphate as a substrate, indicating that the potential for accepting phosphate from metabolites does exist (257). Another reason for the apparent preference for phosphorelay rather than two-component systems may be the need to transmit signals to the nucleus. For instance, the Arabidopsis cytokinin system employs a plasma membrane sensor, a nuclear response regulator that controls transcription, and a phosphotransfer protein that shuttles between cytosol and nucleus (240). Such a mechanism may be relevant for the Sln1p-Ypd1p-Skn7p system (see below).
Transfer of phosphate between different steps in the phosphorelay system is directed downward. Transfer from Sln1p His576 to Asp1144 was reported to be unidirectional, while that from Sln1p Asp1144 to Ypd1p His64 was reversible. The entire reaction is, however, driven towards phosphorylation of Ssk1p, since the equilibrium of the reaction from Ypd1p His64 to Ssk1p Asp554 was on the side of the latter (256-258). It was observed that Ypd1p has a significant stabilizing effect on phospho-Ssk1p; this effect is much less pronounced with a mutant version of Ypd1p that cannot be phosphorylated (256). In vitro, the half-life of phospho-Ssk1p was about 13 min in the absence of Ypd1p, and this period increased 200-fold in the presence of Ypd1p. This effect was apparently specific, since the half-life of phospho-Sln1p was 13 min irrespective of the presence or absence of Ypd1p in the reaction mix.
Why does the phosphorelay system function as a negative regulator of the HOG pathway? One reason may be that the system has evolved from the Sln1p-Ypd1p-Skn7p pathway, which seems to activate gene expression upon cell swelling. In addition, it appears that this particular design ensures that the HOG pathway is activated until the cell that suffers from low turgor or cell shrinking starts to regain turgor and swells again. As such, the phosphorelay in itself operates as an effective feedback system for the HOG pathway. The fact that inappropriate pathway activation prevents proliferation apparently makes effective systems for downregulation necessary, and this could be another reason why the sensor is designed as a shut-off mechanism. The observation that Ypd1p stabilizes phospho-Ssk1 is certainly a possibility to further tightly prevent pathway activation at low osmolarity. On the other hand, the unusually long half-life of phospho-Ssk1p raises the question of how the downstream pathway can be activated within about 1 min upon hyperosmotic shock, since this activation requires dephospho-Ssk1p (472). Either a specific, as yet unidentified phosphatase mediates rapid dephosphorylation, or under these conditions phosphate is transferred backwards in the phosphorelay system (521).
Histidine kinases in bacterial two-component systems often also have phosphatase activity (579); this has not been studied systematically for Sln1p. While numerous interesting details on the function of the Sln1p-Ypd1p-Ssk1p phosphorelay system are now available, more work needs to be done to understand its function. Computer simulations combined with experimentation in vivo using sensitive and rapid monitoring systems are appropriate means to address such questions.
Dephosphorylated Ssk1p activates the MAPKKKs Ssk2p and Ssk22p (355, 357, 472, 474, 476). Ssk1p-dependent activation of Ssk2p has been dissected into a two-step process, binding of Ssk1p to the N-terminal, regulatory domain of Ssk2p and autophosphorylation of Ssk2p. Interaction between the two proteins has been demonstrated by two-hybrid analysis as well as coimmunoprecipitation (355, 472), and the site of interaction between Ssk1p and Ssk2p was mapped to amino acids 294 to 413 within the N terminus of Ssk2p and amino acids 475 to 670 of Ssk1p, which essentially cover the receiver domain (472). Upon osmotic shock, Ssk2p becomes phosphorylated, and this phosphorylation requires the presence of Ssk1p as well as the Ssk1p binding domain of Ssk2p. In addition, phosphorylation of Ssk2p was not observed with a catalytically inactive allele of Ssk2p, demonstrating that Ssk2p phosphorylates itself, probably on Thr1460, rather than being phosphorylated by Ssk1p. Using a truncated version of Ssk2p, it was shown that autophosphorylation is an intramolecular event. The MAPKK Pbs2p has been shown to be a direct substrate for phosphorylated Ssk2p in vitro (472), confirming the genetic evidence that places Pbs2p downstream of Ssk2p within the same pathway (355, 357).
Relatively little is known about the precise subcellular localization of components of the Sln1 branch and any possible changes occurring to the localization upon activation or deactivation of the pathway. This question became of interest after the recent report on the impressive dynamics of the signaling system of the Sho1 branch.
(iii) Activation via the Sho1 branch.
Activation of the Sho1 branch of the HOG pathway involves rapid and transient formation of a protein complex at the cell surface, specifically at places of cell growth (488, 498). The complex formed appears to consist of at least Sho1p and Pbs2p. These two proteins interact via a proline-rich region around position 96 in the N terminus of Pbs2p and an SH3 domain located in the hydrophilic C terminus of Sho1p (355, 473). Furthermore, both proteins colocalize transiently after an osmotic shock (498). In addition, based on the known involvement in signaling from Sho1p to Pbs2p, the complex probably also contains, not necessarily at the same time, the PAK Ste20p (or, at least in an ste20 mutant, the homolog Cla4p) (488), the rho-like G-protein Cdc42p (488, 498), and the MAPKKK Ste11p (439, 473), as well as Ste50p, which is required for Ste11p function (260, 439, 475).
Reiser et al. (498) suggest a model for the series of events that, together with additional information and data from Raitt et al. (488), may be interpreted as follows. Sho1p is located at places of polarized cell growth (488, 498). The G-protein Cdc42p, which is known to mark places where new cell material is deposited (677; reviewed in references 89, 154, 264, and 287), is also located in such areas. However, Cdc42p does not seem to be needed for the specific localization of Sho1p (498). Rather, Cdc42p is required for signaling because it recruits and activates Ste20p (322, 460, 488). However, a more direct involvement of Cdc42p/Ste20p in initial complex formation cannot be excluded. Analysis of the precise role of Ste20p-Cdc42p is somewhat complicated by the fact that Ste20p can be replaced by Cla4p (488), while an ste20Δ cla4Δ double mutant is inviable (117), as is a cdc42 null mutant. In any case, the domain of Ste20p that mediates interaction with Cdc42p is required for Ste20p function in the HOG pathway (488), demonstrating the importance of Cdc42p-Ste20p interaction.
The initial signaling event may be demasking of the Sho1p SH3 domain by an osmotic shock, although it is unknown how this may occur. Since Sho1p does not seem to function as a sensor itself (488), additional proteins are probably required for this event. Sho1p then binds Pbs2p and thereby recruits it to the cell surface. This event may mark the generation of the signaling-competent complex that recruits (and somehow activates) Cdc42p plus the interacting PAK kinase(s) Ste20p (and Cla4p) and the MAPKKK Ste11p. Rho-like G-proteins such as Cdc42p are known to monitor protein complex formation in different contexts (264, 287), and the assembly of the appropriate signaling complex may then lead to activation of the PAK Ste20p (Cla4p), phosphorylation of Ste11p, and subsequently phosphorylation of Pbs2p. Somehow, the successful execution of the signaling program, i.e., phosphorylation and activation of Pbs2p, then leads to dissociation of the complex. These events occur rapidly: in wild-type cells, it has not yet been possible to monitor complex formation microscopically. This was only possible by using a mutant with a catalytically inactive allele of Pbs2p, in which complex dissociation apparently does not occur so fast (498).
The fact that the catalytic activity of Pbs2p is required while the presence of Hog1p is not needed for rapid complex dissociation suggests that successful execution of the signaling program is monitored at a step between phosphorylation of Pbs2p and phosphorylation of Hog1p, perhaps by a conformational change upon ATP binding of Pbs2p (498). This also means that indeed all signaling events between Sho1p and Pbs2p must occur between the initial binding of Pbs2p to Sho1p and the dissociation of phosphorylated, ATP-loaded Pbs2p, i.e., on the Sho1p-Pbs2p complex. It is unlikely that phosphorylation of Hog1p also occurs at the complex, since Pbs2p dissociates from Sho1p even in a hog1Δ mutant (498). Clearly, many of the events in this dynamic scenario are based on “static” data from genetic and biochemical analyses and therefore are assumptions. Hence, genetic and biochemical analyses alone will eventually not be sufficient to resolve the dynamics of the events; in addition, advanced microscopic techniques and especially the use of green fluorescent protein (GFP) alleles with different emission wavelengths together with the possibility of monitoring fluorescence resonance energy transfer as an indicator of protein interaction will be needed to observe complex formation and dissociation in the living cell.
(iv) Events downstream of the MAPKKKs.
Pbs2p is activated by phosphorylation on Ser514 and Thr518 by any of the three MAPKKKs Ssk2p/Ssk22p and Ste11p. Pbs2p is a cytoplasmic protein and appears to be specifically excluded from the nucleus (166, 497). Therefore, phosphorylation of the substrate of Pbs2p, the Hog1p MAP kinase, occurs in the cytosol. Dual phosphorylation on the conserved Thr174 and Tyr176 activates the MAP kinase Hog1p (61, 533). Phosphorylation causes a rapid and marked concentration of Hog1p in the nucleus, while under normal conditions Hog1p appears to be evenly distributed between the cytosol and the nucleus (166, 497). Nuclear concentration of Hog1p-GFP can be observed within less than 1 min after a hyperosmotic shock. This effect is specific, since a range of other stress conditions do not cause Hog1p phosphorylation and do not mediate nuclear translocation (166, 497, 533).
Phosphorylation on both Thr174 and Tyr176 of Hog1p by Pbs2p is necessary and sufficient for nuclear concentration, since mutation of one or both of these sites makes the subcellular localization of Hog1p unresponsive to osmotic shock (166, 497). The catalytic activity of Hog1p, however, is not required for transfer to the nucleus, since a catalytically inactive mutant of Hog1p is transferred to the nucleus very much like wild-type Hog1p (166, 497). Concentration of Hog1p in the nucleus requires Gsp1p (166), a Ran G-protein needed for nuclear import of proteins containing nuclear localization signals (436). Nuclear import of Hog1p also requires the karyopherin-beta Nmd5p, while several other known nuclear import factors do not seem to be required (166). Although the rapid nuclear concentration of Hog1p is impressive to observe under the microscope, it is clear that activated Hog1p also mediates regulatory effects outside the nucleus. The best documented of such effects is activation of the protein kinase Rck2p (40, 592), which controls translation efficiency (592). Therefore, either a portion of Hog1p sufficient to mediate these effects remains in the cytosol or rapid shuttling between nucleus and cytosol ensures that a portion of activated Hog1p is always present in the cytosol.
Interestingly, the more severe the osmotic shock, the longer it takes until phosphorylated, active Hog1p is translocated into the nucleus, an observation at odds with the apparent need to respond even more rapidly to severe stress (622). Also, the HOG-dependent transcriptional response is delayed under such conditions, as apparent from time courses of mRNA levels of HOG-dependent genes after osmotic shock (500, 622). When cells initially treated with 1.4 M NaCl within that lag phase are shifted back to 0.5 M NaCl, Hog1p is rapidly phosphorylated and translocated immediately to the nucleus, and transcriptional responses are observed (622). This suggests that some adaptation must occur before Hog1p can be activated and transferred to the nucleus and that translocation is specifically blocked in an unknown way until this process is completed. More work is needed to better understand this interesting phenomenon.
Both phosphorylation of Hog1p and nuclear localization are transient effects. Depending on the severity of the osmotic shock, Hog1p remains phosphorylated and located in the nucleus for several minutes or even up to a few hours (61, 357, 372, 589, 622). There is good correlation between the period of Hog1p phosphorylation and its apparent nuclear localization (166, 372, 497), which could indicate a causal relationship between nuclear export and dephosphorylation. However, recent data suggest that both events can possibly be separated from each other. Mutants defective in the tyrosine phosphatases Ptp2p and Ptp3p show much-prolonged Hog1p tyrosine phosphorylation after an osmotic shock, but the kinetics of Hog1p nuclear entry and exit seem to be largely unchanged. Also, a ptc1Δ ptp2Δ mutant, which grows very poorly due to overphosphorylation of Hog1p on both Thr174 and Tyr176 (254, 356), did not show a significantly altered period of nuclear residence (372).
Several aspects complicate interpretation of these data. First of all, the same study (372) observed that the levels of Ptp2p and Ptp3p affect nuclear residence, leading to the idea that Ptp2p could serve as a nuclear tether and Ptp3p as a cytosolic anchor. Hence, deletion of PTP2 and PTC1 both removes the putative nuclear anchor and diminishes phosphatase activity, effects that could possibly balance each other. In addition, while it is known that the phosphatases Ptp2p, Ptp3p, Ptc1p, Ptc2p, and Ptp3p affect the level or period of Hog1p phosphorylation and/or interact with HOG1 genetically, the spectrum of physiologically relevant phospho-Hog1p phosphatases is not well established (254, 357, 372, 640, 654). Finally, to monitor the phosphorylation state of Hog1p, an antibody specific for tyrosine-phosphorylated MAP kinase is commonly used, while activation of Hog1p requires phosphorylation on both Thr174 and Tyr176 (533). Hence, measurements with the phosphotyrosine-specific antibody only allow the conclusion that the kinase is inactive (i.e., when not detected), while Hog1p detected with this antibody may also be inactive. The use of an antibody specifically detecting dually phosphorylated Hog1p as a more direct measure for the active kinase is now becoming more common (372, 589, 622). It remains to be settled how the phosphorylation state of Hog1p affects nuclear export, but since dual phosphorylation of Hog1p is apparently sufficient to mediate import, dephosphorylation is required to achieve an even distribution of the protein during adaptation.
Several proteins affect residence of Hog1p in the nucleus. As mentioned above, deletion of PTP2 diminishes and overexpression prolongs the period of nuclear residence, while the opposite was observed for PTP3 (372). In addition, deletion of the genes encoding the transcription factors Msn2p, Msn4p (497), Hot1p, and Msn1p (503) has been shown to reduce the period of Hog1p nuclear localization, suggesting that these proteins bind Hog1p in the nucleus. In the related Sty1 pathway in S. pombe (see below), the transcription factor Atf1 is needed for nuclear accumulation of the Sty1 kinase (180). These observations indicate that interaction with the substrate and perhaps successful execution of a signaling program may determine nuclear export.
Recently, the isolation of HOG1 alleles that encode partially activated Hog1p has been reported (33). These mutations, which confer some degree of osmotolerance even in the absence of Pbs2p, will be highly instrumental in studying the function and dynamic control of the HOG pathway further.
Modulation and feedback control of the HOG pathway.
The appearance of phosphorylated Hog1p is a transient event (254, 357, 589, 622). The same transient effect is also observed for the rise of the mRNA levels of genes induced after osmotic shock (500, 503). The timing and the period of the response depend on the severity of the shock. When an osmotic shock is given with a lower dose of osmoticum, such as 0.4 M NaCl, Hog1p phosphorylation peaks within 1 min and disappears within about 30 min. With a more severe osmotic shock, for instance, 1.4 M NaCl, Hog1p phosphorylation peaks at about 30 min and remains high for several hours before it declines (622; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). These observations illustrate that the pathway is controlled by specific feedback mechanisms.
As indicated above, two phosphotyrosine phosphatases (Ptp2p and Ptp3p) as well as three phosphoserine/threonine phosphatases (Ptc1p to Ptc3p) genetically interact with the HOG pathway; overexpression of any of these phosphatases suppresses the lethality caused by inappropriate activation of the HOG pathway (254, 357, 372, 441, 640, 654). This observation alone does not of course establish that these phosphatases are truly involved in controlling the pathway, since overexpression may cause them to dephosphorylate substrates that are not normally their physiological targets. In the case of Ptp2p, Ptp3p, and Ptc1p, however, there is ample direct evidence that they affect the HOG pathway and act upon Hog1p.
Overexpression of PTP2 and PTP3 suppresses inappropriate activation of the HOG pathway conferred by deletion of SLN1 or constitutive activation of Sln1p, Ssk2p, or Pbs2p, suggesting that they indeed target the MAP kinase (254, 654). As expected, overexpression of PTP2 and PTP3 diminishes the amount of tyrosine-phosphorylated Hog1p. Ptp2p and Ptp3p interact directly with Hog1p, as demonstrated by coimmunoprecipitation from extracts of cells expressing catalytically inactive Ptp2p and Ptp3p (254, 654). The fact that interaction was not observed with active phosphatases suggests that binding occurs specifically to phosphorylated Hog1p and that the phosphatases dissociate rapidly from the kinase after dephosphorylation.
Remarkably, overexpression of catalytically inactive Ptp2p also suppressed the lethality caused by overactivation of the HOG pathway but without mediating dephosphorylation, suggesting that binding of the phosphatase blocks active Hog1p in different ways (654). It is not known if this effect has any physiological relevance. In ptp2Δ mutants, and even more so in ptp2Δ ptp3Δ double mutants, tyrosine phosphorylation of Hog1p upon osmotic shock is stronger and more prolonged and in the double mutant is also observed without osmotic shock (254, 654). Since even in the ptp2Δ ptp3Δ double mutant the level of tyrosine-phosphorylated Hog1p is still responsive to osmotic shock, additional dephosphorylation mechanisms must exist (254, 654).
Ptp2p seems to be more important for Hog1p dephosphorylation than Ptp3p, possibly because Ptp2p is predominantly nuclear, as is activated Hog1p, while Ptp3p is located in both the cytosol and the nucleus (373). Other data instead suggest that the two phosphatases have different substrate specificities determined by the noncatalytic N-terminal domain. This domain seems to be responsible for targeting Ptp2p preferentially to Hog1p and Ptp3p preferentially to the MAP kinase of the pheromone response pathway, Fus3p (675). Further work is needed to clarify where these phosphatases perform their function on Hog1p, which is important to better understand deactivation and the control of subcellular localization.
Among the serine/threonine phosphatases, Ptc1p seems to be the one that truly functions in the deactivation of the HOG pathway. In mutants lacking both Ptc1p and Ptp2p, the inappropriate HOG pathway overactivation causes a growth defect. No other combination of deletion mutations between the two Ptps and the three Ptcs causes similar effects (640). The suppressive effect of overexpression of PTC1 requires its phosphatase activity and diminishes Hog1p kinase activity without affecting the level of tyrosine phosphorylation (640). The latter observation was taken as indirect evidence that Ptc1p does not affect Pbs2p, because in that case one would have expected Pbs2p-mediated tyrosine phosphorylation of Hog1p to be diminished. However, to confirm this notion will require more direct experiments on the phosphorylation state and activity of Pbs2p. Deletion of PTC1 causes constitutive dual phosphorylation of Hog1p which is hardly responsive to osmotic shock (640), a finding that is somewhat difficult to explain in light of the still present phosphotyrosine-specific phosphatases. Perhaps Ptp2p preferentially dephosphorylates Hog1p that already is dephosphorylated on Thr174, a possibility that could be addressed experimentally. In any case, Ptc1p inactivates Hog1p and also dephosphorylates phosphothreonine in vitro (640). Taken together, these data demonstrate that Ptc1p is indeed a, perhaps the main, phosphatase that acts on the phosphothreonine in Hog1p.
Two different observations have been claimed as arguments for a scenario in which the phosphatases Ptp2p and Ptp3p are part of a feedback loop inactivating Hog1p during adaptation. First, when a catalytically inactive Hog1p is expressed in a hog1Δ mutant, it is heavily and constantly tyrosine phosphorylated, even without an osmotic shock. If the same catalytically inactive Hog1p is coexpressed with active Hog1p, it becomes dephosphorylated normally (654). Hence, dephosphorylation of Hog1p requires its catalytic activity. This has been interpreted to mean that Hog1p activates the phosphatases to stimulate its own deactivation. However, in cells expressing only an inactive Hog1p allele, all processes controlled by Hog1p are blocked. Hence, this experiment does not distinguish between a direct effect of the Hog1p kinase activity on feedback control and its requirement for mediating adaptive responses, which then may be needed for downregulation of the pathway. For instance, it has been observed that mutants unable to produce any glycerol (556) or to properly accumulate glycerol (589; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations) show strong and sustained Hog1p phosphorylation after an osmotic shock. Control of glycerol production is an essential event in osmoadaptation and partially controlled by the HOG pathway (7, 19, 61, 426). Therefore, it appears that downregulation of the HOG pathway is mediated by the successful execution of an adaptation program, which of course requires the catalytic activity of Hog1p.
The observation that expression of PTP2 and PTP3 is stimulated after osmotic shock in a HOG-dependent manner was taken as evidence that the phosphatases are part of a feedback loop (254, 654). Hence, upon osmotic shock, the cell apparently increases its capacity to downregulate the pathway. This is actually a very common phenomenon observed in cellular adaptation. However, this transcriptional effect cannot be responsible for the observed rapid decline of the phosphorylation state of Hog1p, simply because the increase in phosphatase activity due to increased production of the enzyme occurs after the level of Hog1p phosphorylation has started to decline (654).
Instead, several arguments and observations suggest that the phosphatases might not perform as specific regulators. First of all, the phosphotyrosine phosphatases Ptp2p and Ptp3p are well known to control more than Hog1p. Fus3p (mating pheromone response), Kss1p (pseudohyphal development pathway) (674), and Slt2/Mpk1p (cell integrity pathway) (373) have been shown to be substrates for both phosphatases. In fact, expression of PTP2 and PTP3 has been reported to be heat stress induced in an Slt2/Mpk1p-dependent manner (373), which was used as an argument for a transcriptional feedback loop in this pathway as well. The lack of pathway specificity, however, makes it difficult to imagine how the phosphatases could confer a specific, modulating function. For instance, the pheromone response pathway and the cell integrity pathway are both stimulated during the mating process at different time points (73, 216), illustrating the difficulty in explaining pathway-specific feedback control by the phosphatases.
A negative feedback loop via Hog1p-dependent stimulation of PTP2 and PTP3 expression would mean that the pathway inactivates itself in an autoregulatory mode. This does not seem to make sense for an osmosensing and osmoregulating pathway. First of all, the pathway must remain activatable at any time, because even after an initial osmotic shock a second shock or continuous further reduction of the water activity may well occur. We have observed that after an initial osmotic shock with 0.5 M NaCl, dual Hog1p phosphorylation can be further stimulated or restimulated with a second addition of 0.5 M NaCl at different time points during or after the initial peak. Restimulation displays amplitude and kinetics very similar to those of the initial stimulation (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). This indicates that the pathway is not desensitized or autonomously inactivated. In addition, an autonomous negative feedback loop would essentially ignore the success of the response, i.e., to readjust the osmotic balance.
As mentioned above, mutants that cannot produce or retain the osmolyte glycerol show strongly enhanced and sustained Hog1p phosphorylation (556; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). It was also suggested, based on data obtained with different mutants, that the intracellular glycerol level, via its effects on turgor pressure, stimulates activity of the Sln1p osmosensor, leading to deactivation of the HOG pathway (591). Finally, there seems to be good correlation in different mutants between the time points at which Hog1p phosphorylation starts to decline and the onset of glycerol accumulation (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations).
Taken together, these observations suggest that the cell integrates information on the success of signaling through the HOG pathway, i.e., reestablishment of turgor pressure, to downregulate the pathway (Fig. 5). Specifically, deactivation of the HOG pathway might take place at the level of the sensor. Since Sln1p functions as a sensor for hypo-osmolarity-induced cell expansion, the onset of glycerol accumulation and hence cell expansion would lead to activation of the sensor and phosphorylation of Ssk1p, which in turn cannot activate the MAPKKKs Ssk2p and Ssk2p any further, thereby blocking further signaling. At this point the phosphatases are needed to shut down the pathway by dephosphorylation. Unless the phosphorylated form of these kinases is itself highly unstable, it would make sense if phosphatases also acted at the level of the MAPKK and MAPKKK. Together, the phosphatases could, in a constitutive way, downregulate the pathway to ensure that as soon as stimulation of the sensor systems shuts off further signaling, the entire pathway is rapidly shut off. Their enhanced production may support this effect.
FIG. 5.
Possible mechanism of feedback control of the HOG pathway, indicating protein phosphatases whose production is stimulated after osmotic shock. The production of glycerol leads to restoration of turgor and hence stops further activation of the pathway.
In this scenario, the role of Sln1p as an inhibitor of the pathway at lower osmolarity makes sense, since it ensures continuous pathway activation until cells start to reexpand. The fact that pathway activation is deleterious when inappropriate, as for any of the yeast MAP kinase pathways, makes strong inhibition as performed by the phosphatases necessary and may explain why several phosphatases with overlapping function and localization are employed by the cell. Certainly further data are needed to better understand the importance of different events in adaptive downregulation of the pathway.
The above discussion has focused on the HOG pathway from Sln1p to Hog1p. Essentially no information is available on feedback regulation in the Sho1p branch of the pathway. Interestingly, however, a shock with 0.4 M NaCl appears to give different Hog1p phosphorylation profiles when wild-type cells as well as sho1Δ (only Sln1 branch active) and ssk2Δ ssk22Δ (only Sho1 branch active) mutants are compared. While Hog1p dual phosphorylation peaks after 1 min in all three strains, in wild-type cells the level is down to prestress levels within 30 to 45 min and in ssk2Δ ssk22Δ cells already after 10 to 30 min, and in sho1Δ cells phosphorylated Hog1p is still detectable after 60 min (622). This observation suggests that both branches in concert determine the Hog1p phosphorylation profile in the wild type. More importantly, this observation also confirms that feedback control and adaptation are determined by upstream components, probably the sensors, and not only the downstream part on which the known phosphatases act. More work is needed to decipher how the HOG pathway is inactivated after its initial stimulation.
Cross talk between HOG pathway and other MAP kinase pathways.
Since the HOG pathway shares protein kinases and phosphatases with other MAP kinase pathways, there are numerous nodes for interaction between these pathways. Indeed, it has been demonstrated that mutations of PBS2 or HOG1 lead to osmostress-induced stimulation of the pseudohyphal development pathway and the pheromone response pathway (124, 207, 439). In a hog1Δ mutant, osmotic stress leads to a stimulation of Fus3p phosphorylation and activation of a reporter gene (FUS1-lacZ) that is normally responsive to pheromone through the pheromone response pathway (207). A dedicated screen for mutations that activate the pheromone response pathway by osmotic stress also yielded a hog1Δ allele, as described above (439). Activation of the FUS1-lacZ reporter by osmotic shock in a hog1Δ or pbs2Δ mutant requires the Sho1p branch of the HOG pathway: Ste20p, Ste50p, Ste11p, Ste7p, and Fus3p or Kss1p (439). Transcriptional profiling in a hog1Δ mutant after osmotic shock shows that probably all known target genes of the pheromone response pathway become highly expressed (501). Indeed, hog1Δ and pbs2Δ mutants display a morphological abnormality under osmotic stress that resembles mating projections. Moreover, deletion of hog1Δ partially suppresses the mating deficiency of ste4Δ and ste5Δ mutants in the presence of 1 M sorbitol (439). While these data convincingly demonstrate that cross talk occurs in a hog1Δ mutant, there also seems to be some cross talk upon osmotic shock even in wild-type cells (207). Whether this has any physiological relevance is not known.
Gustin and coworkers searched for multicopy suppressors of the growth defect of a leaky pbs2-3 mutant and identified MSG5 (124). Msg5 is a dual-specific phosphatase acting on Slt2/Mpk1p and Fus3p (144, 369). In this case, the suppressing effect conferred by overexpression of MSG5 was due to inhibition of Kss1p. Kss1p, the MAP kinase of the pseudohyphal development pathway, becomes activated by osmotic stress in the pbs2-3 mutant. Interestingly, deletion of KSS1 but not of FUS3 or SLT2/MPK1 partially suppressed the osmosensitivity of the pbs2-3 mutant. It also prevented the formation of projections that are formed by pbs2 mutants under osmotic stress. This suggests that projection formation in a HOG pathway mutant under osmotic stress is due to a combination of activation of both Fus3p and Kss1p and that this morphological effect contributes to the growth defect of HOG pathway mutants on high-osmolarity medium (124). Consistent with the above observations, overexpression of HOG1 inhibits formation of pseudohyphae (15).
From the observations described above it was concluded that the MAP kinase Hog1p somehow prevents cross talk, probably via a feedback loop acting at the level of Sho1p (439). However, a gpd1Δ gpd2Δ double mutant, which is unable to make glycerol under osmotic stress, also partially activates at least some target genes of the pheromone response pathway under osmotic stress (656), an interesting observation that will require further confirmation. This mutant does not lack Hog1p and rather shows a stronger and more sustained activation of Hog1p (556). Hence, it appears that in mutants in which the successful execution of the adaptation program is prevented, such as those lacking PBS2, HOG1, or GPD1 and GPD2, sustained HOG pathway overactivation at the level of the sensor system of the Sho1 branch leads to signal overflow to pathways that share protein kinases.
Transcriptional Regulators of the HOG Pathway
Until recently, very little was known about proteins that mediate the transcriptional responses downstream of the HOG pathway. It is now clear that several transcriptional regulators mediate downstream transcriptional responses. As an additional complication, there are only very few genes for which stimulation by osmotic stress is controlled exclusively by the HOG pathway; instead, target promoters, such as those of the well-known genes GPD1 and ENA1, seem to be fairly complex (155, 481, 500). Systems with apparently simpler expression patterns have now been identified by global gene expression analyses (191, 471, 501).
Presently, Sko1/Acr1p, Hot1p, and the probably redundant Msn2p and Msn4p, as well as Smp1p, are implicated in HOG-dependent transcription (Table 2). All these factors belong to different families of transcriptional regulators, documenting that sequence or structural conservation cannot be used to identify such factors. Present knowledge also suggests that the mechanisms with which Hog1p controls transcription may differ among these factors. For instance, Msn2p and Msn4p are activators (158, 160, 370); they mediate a general stress response (reviewed in references 158, 519, and 555). It is well established that upon osmotic shock Msn2p/Msn4p-dependent genes require the HOG pathway for induction, but it is not known if this is due to direct control of Msn2p/Msn4p by Hog1p (533). Sko1/Acr1p, on the other hand, is a repressor (455, 481) or both repressor and activator (502), whose repressive function is controlled by Hog1p directly (480). Hot1p is a protein that recruits the Hog1p kinase to target promoters (9, 503). Smp1p is a recently identified factor, which is phosphorylated by Hog1p and contributes to expression of some genes (F. Posas, 2001, personal communication); details remain to be published.
TABLE 2.
Transcriptional regulators of the HOG pathway
| Protein | Family | Function | Evidence | Reference(s) |
|---|---|---|---|---|
| Sko1/Acr1p | bZIP, CREB | Repressor; also needed for activation from CREs (target genes in Table 3) | Hog1p target in vitro and in vivo; genetic evidence, promoter association | 481 |
| Msn2p/Msn4p | Zinc finger | Activator; binds to STREs (CCCCT) and mediates protein kinase A-dependent gene expression | Target genes depend on Hog1p under osmotic stress; STRE-dependent reporter depends on Msn2p/Msn4p and HOG pathway | 501, 533 |
| Hot1p | Novel helix-loop-helix | Activator; present together with Hog1p on some target promoters; needed for normal expression of some genes (Table 4) | Interaction with Hog1p, Hog1p-dependent phosphorylation, promoter association | 9, 503 |
| Smp1 | MADS box | Activator | Hog1p target | F. Posas, personal communication |
| Msn1p | Novel helix-loop-helix | Activator; also involved in pseudohyphal growth and many more processes | Required for full expression of some Hog1p targets | 503 |
| Sgd1p | Zinc finger | Putative activator | Overexpression suppresses hog1Δ and pbs2Δ osmosensitivity | 4 |
| Gen4p | bZIP | Activator; required for stimulated expression of genes under general amino acid control | Required for Hog1p-dependent activation of HAL1 expression from CRE | 455 |
| Skn7p | Heat shock factor | Activator; involved in numerous cellular processes, such as oxidative stress response and cell wall metabolism | Part of Sln1p-Ypd1p phosphorelay, activates hypo-osmotic genes | 69 |
Genetic evidence and gene expression data link additional transcription factors to HOG pathway function: Msn1p (503), Sgd1p (4), and Gcn4p (455). In addition, the Sln1p-Ypd1p sensor system controls a second response regulator, Skn7p. This protein is apparently involved in multiple cellular processes and genetically interacts with both the HOG and the cell integrity pathways. Recent data suggest that it functions as a mediator of hypo-osmotic signals.
Sko1/Acr1p.
Sko1/Acr1p is a protein of 647 amino acids belonging to the ATF/CREB family of AP1-related transcription factors (ATF) (412, 632), which in mammalian cells are known as cAMP response element (CRE)-binding (CREB) proteins (reviewed in reference 126). Such factors possess a bZIP domain, i.e., a leucine zipper for dimerization, and an adjacent basic transcription activation domain. Many ATF/CREBs have the ability to form dimers not only with themselves but also with other members of the same family, depending on how the binding site is organized.
Sko1/Acr1p was initially identified in two different genetic screens before the HOG pathway was known. Vincent and Struhl found that CREs mediate reporter gene repression in S. cerevisiae and mutations in the gene ACR1 lifted this repression (632). Nehlin and Ronne attempted to identify proteins that downregulate protein kinase A. For this screen, they overexpressed TPK2, which encodes a catalytic subunit of protein kinase A, from the GAL1 promoter. This causes lethality on galactose medium. The screen identified transcription factors that apparently repress the GAL1 promoter, such as Mig1p and Sko1/Acr1p (412, 413). While the role of Mig1p in repressing the GAL1 promoter has now been fully elucidated (513), the function of Sko1/Acr1p in this system is not understood. Recent data indicate that Sko1/Acr1p is, in fact, a target of protein kinase A (454, 480), and hence at least part of the suppressing effect of Sko1/Acr1p overexpression could be due to titration of excess protein kinase A.
The cellular role of Sko1/Acr1p remained elusive until three independent studies recently arrived at the conclusion that Sko1/Acr1p mediates HOG pathway-dependent control of CREs. Proft and Serrano found a functional CRE when studying the organization of the ENA1 promoter (481). ENA1/PMR2 encodes a plasma membrane Na+ export pump required for growth in the presence of Na+ or Li+ (213, 645; reviewed in references 537 and 538). The expression of ENA1 is controlled in a complex way by sodium stress via the calcineurin phosphatase, by glucose repression via the repressors Mig1p and Mig2p, and by osmotic stress via Hog1p (364, 365, 481). An upstream element (−502 to −525) containing the CRE (around −506) is necessary and sufficient to confer Sko1/Acr1p-dependent repression on a truncated CYC1 promoter, which is constitutively active, under normal conditions and derepression after osmotic shock. Sko1/Acr1p binds to this site in vitro (481).
Sko1/Acr1p also controls expression of HAL1, which was isolated in a screen for genes that confer enhanced salt tolerance when overexpressed. The function of Hal1p in salt tolerance is not known (455, 506). An upstream element (−231 to −209), which contains the HAL1 CRE around position −220, also confers Sko1/Acr1p-dependent osmotic regulation on the CYC1 promoter fragment. Sko1/Acr1p binds to this site in vitro (455).
Rep and colleagues were searching for S. cerevisiae transcription factors that resembled Atf1, a bZIP controlling transcription downstream of the S. pombe Sty1 pathway, which is related to the HOG pathway (see below). Three factors that resemble Atf1 exist in S. cerevisiae: Aca1p, Aca2p, and Sko1/Acr1p (187, 502). Studies on the GRE2 promoter confirmed the important role of Sko1/Acr1p in mediating HOG-dependent responses (502). GRE2, which was initially identified in a search for osmo-induced genes, encodes a protein of unknown function with similarity to plant dihydroflavonol-4-reductases (186). The gene subsequently attracted interest because it appeared in global gene expression analyses as strongly induced by osmotic and oxidative stress (195, 502). The upstream sequence of GRE2 contains two CREs at positions −224 and −192, which both mediate Sko1/Acr1p-dependent regulation, as shown by mutational analysis (502). The promoters of HAL1 and GRE2 seem to be simpler than that of ENA1 in that stimulated expression under salt stress is mediated exclusively via the CRE site(s).
In parallel it was found that Sko1/Acr1p, Aca1p, and Aca2p are the only three Atf1-related proteins of the ATF/CREB family in S. cerevisiae that mediate transcriptional responses from CREs (187). This study made use of a derivative of the HIS3 promoter containing a CRE as the upstream controlling sequence. This construct is controlled by Sko1/Acr1p-dependent repression, which is modulated by osmotic stress in a Hog1p-dependent manner (187).
In sko1/acr1Δ mutants, expression of the genes ENA1, HAL1, and GRE2 as well as the activity of CRE-dependent CYC1- or HIS3-based reporter genes is elevated and hardly or not at all responsive to osmotic stress (187, 455, 481, 502). Taken together, these data demonstrate that Sko1/Acr1p represses gene expression from CRE sites, thereby mediating osmotic regulation.
Derepression upon osmotic shock of Sko1p-dependent genes or the different CRE-containing reporter genes absolutely requires Hog1p (187, 455, 481, 502). Hence, in a hog1 mutant, expression of ENA1, GRE2, or HAL1 or the CRE-dependent reporters is low or undetectable and unresponsive to osmotic shock. The hog1Δ sko1Δ double mutant shows essentially the same phenotype as the single sko1Δ mutant, i.e., a high level of expression and unresponsiveness to osmotic stress (455, 481, 502), confirming that Sko1/Acr1p operates downstream of Hog1p. Moreover, deletion of SKO1/ACR1 partially suppresses the osmosensitivity of the hog1Δ mutant, and the sko1/acr1Δ single mutant grows better on high-osmolarity plates than the wild type, consistent with Sko1/Acr1p's being a repressor inactivated by Hog1p (481). This genetic evidence has received direct molecular confirmation. Sko1/Acr1p and Hog1p interact in coimmunoprecipitation experiments (480). Sko1/Acr1p is phosphorylated by Hog1p on Ser108, Thr113, and Ser126, and mutation of these three sites makes Sko1/Acr1p a more effective repressor, as judged from reduced expression of GRE2 and HAL1. Notably, however, regulation by osmotic shock of these two genes in the triple Sko1/Acr1p mutant lacking the Hog1p-dependent phosphorylation sites still partly maintains HOG-dependent regulation, suggesting that Hog1p also controls Sko1/Acr1p function in other ways.
Interestingly, protein kinase A also contributes to the control of Sko1/Acr1p at very high salt concentrations (454, 480). Under normal conditions, Sko1/Acr1p is localized to the nucleus, while it also appears in the cytosol under severe stress. Protein kinase A is required for nuclear localization under normal conditions, since mutants with low protein kinase A activity show distributed Sko1/Acr1p and partial derepression of a Sko1p-dependent CRE reporter gene. On the other hand, high protein kinase A activity, as in a bcy1Δ mutant, does not prevent redistribution upon stress.
Protein kinase A seems to control Sko1/Acr1p phosphorylation through three serines at positions 380, 393 and 399. However, a Sko1/Acr1p triple serine-to-alanine mutation, making these sites unphosphorylatable, confers normal GRE2 and HAL1 regulation in a wild-type background; subcellular distribution was not tested in these mutants. When the triple mutant lacking the three Hog1p-dependent phosphorylation sites was expressed in a bcy1Δ background, expression of GRE2 was essentially abolished and unregulated by osmotic stress. Further evidence for an involvement of protein kinase A was obtained by generating a Sko1/Acr1p mutant lacking all six phosphorylation sites. This construct further diminished responsiveness of GRE2 and HAL1 expression to osmotic stress, although it still did not completely abolish it (480). It appears that protein kinase A and Hog1p collaborate in the control of Sko1/Acr1p, where protein kinase A supports nuclear localization and Hog1p controls its activity. The mechanism that mediates stress-induced nuclear export remains to be identified.
The repressive effect of Sko1/Acr1p on the activity of the artificial CRE-CYC1 or CRE-HIS3 promoter systems or on the complete ENA1 promoter, as well as on the mRNA level of target genes, requires Ssn6p and Tup1p (364, 481, 502). Ssn6p and Tup1p form a complex that binds to a number of yeast sequence-specific DNA-binding proteins. In this way, Ssn6p and Tup1p repress gene expression without themselves binding to DNA. The repressive function of Ssn6p and Tup1p is mediated by reorganization of the chromatin structure (reviewed in reference 564). Sko1/Acr1p, fused to the DNA-binding domain of GAL4, confers osmotic regulation on a Gal4p-dependent GAL1 promoter, which is normally not responsive to osmotic stress, in an Ssn6p- and Tup1p-dependent manner (481). Ssn6p and Tup1p can be coimmunoprecipitated together with Sko1/Acr1p, confirming that these proteins interact (480).
It appears that Tup1p is the subunit directly contacting Sko1/Acr1p (454). This interaction seems to be perturbed when the Sko1/Acr1p allele, which cannot be phosphorylated by Hog1p, is used in the coprecipitation experiments, suggesting that Hog1p-dependent phosphorylation of Sko1/Acr1p might control binding of the corepressor complex (480). Although further studies are needed to corroborate this notion, these data suggest a straightforward picture (Fig. 6), where Sko1/Acr1p, together with Ssn6p and Tup1p, represses expression of target genes under normal growth conditions. Upon osmotic stress, Hog1p phosphorylates Sko1/Acr1p, causing Ssn6p and Tup1p to dissociate, and expression of target genes is derepressed. Protein kinase A supports repression by mediating Sko1/Acr1p nuclear localization.
FIG. 6.
Model for the mechanisms with which the HOG pathway controls gene expression via Sko1p. Protein kinase A activity stimulates nuclear localization; some redistribution of Sko1/Acr1p to the cytosol is only observed under severe osmotic stress. Sko1/Acr1p may also have an activating function in addition to its demonstrated role as a repressor.
It should be noted that the general role of the Ssn6p-Tup1p corepressor complex suggested by Marquez et al. (364) so far has not been confirmed. On the contrary, expression of GPD1 was found not to be affected at all by deletion of SSN6 (500), and enhanced expression of most osmoresponsive genes seems to depend on transcriptional activators.
Genetic and biochemical evidence firmly established Sko1/Acr1p as a repressor blocking gene expression; hence, Sko1/Acr1p target genes must have mechanisms that ensure gene activation under derepressing conditions. This could be achieved by constitutive AT-rich promoter elements, such as those that drive expression of the HIS3-based reporter gene used to study CRE function (187). However, the situation appears to be more complex. CREs are known to be binding sites for both repressors (Sko1/Acr1p) and activators (Aca1p and Aca2p) (187). The high basal activity in a sko1Δ mutant of the GRE2 and CRE-HIS3 promoters is indeed largely due to the Aca1p and Aca2p activators (187, 502). Hence, in a triple sko1/acr1Δ aca1Δ aca2Δ mutant, expression of these two genes is very low. On the other hand, in the presence of Sko1/Acr1p, deletion of ACA1 and/or ACA2 has no effect on GRE2 expression (502).
In the CRE-HIS3 system, Aca1p and Aca2p only seem to significantly affect expression in an sko1/acr1Δ mutant. This suggests that Aca1p and Aca2p gain access to these CREs only when SKO1/ACR1 has been deleted. This also appears to be the case under osmotic stress, suggesting that Sko1/Acr1p binds to the GRE2 promoter even under these conditions. This observation seems to contradict the observed redistribution of Sko1/Acr1p under stress (454). However, redistribution is only observed under severe stress, and in addition the protein does not seem to be excluded from the nucleus and rather may have a higher rate of shuttling between the two compartments. Whether (a portion of) Sko1/Acr1p remains bound to target promoters under stress conditions needs to be investigated, for instance, by chromatin immunoprecipitation.
The cellular role of Aca1p and Aca2p remains to be determined. Several phenotypes have been described for deletion of the two genes, including caffeine resistance and poor growth on nonfermentable carbon sources (187, 502). Several of the phenotypes are counteracted by deletion of SKO1/ACR1, further indicating that these factors interact at target promoters, although the physiological relevance of this observation is unknown. ACA2 seems to be the more strongly expressed gene, while deletion of ACA1 causes effects only in an aca2Δ background (187, 502). Phenotypes related to osmotic stress were not observed. However, deletion of ACA2 confers a synthetic growth defect in a hog1Δ background even under normal growth conditions, suggesting that Aca2p functions in a pathway parallel to Hog1p (502). The involvement of Aca1p and Aca2p in expression of GRE2 and the CRE-HIS3 promoter seems to be fortuitous. Interestingly, in the triple sko1/acr1Δ aca1Δ aca2Δ mutant, both promoters regain the ability to respond to osmotic stress in a HOG-dependent manner (187), although with much slower induction kinetics (502). This suggests that other proteins can bind in the absence of the three factors or that the Hog1p kinase can slowly activate promoters directly (see under Hot1p).
The observation that the basal promoter activity of GRE2 and CRE-HIS3 is very low in an sko1/acr1Δ aca1Δ aca2Δ triple mutant indicates that the CREs do not function as upstream repressing but rather upstream activating sequences (UAS). Indeed, mutation or deletion of the CREs in the GRE2 and HAL1 promoters causes low expression that is largely or completely unresponsive to osmotic stress (455, 502). Hence, the binding site for the Sko1/Acr1p repressor at the same time is needed for the activation of transcription. These data as well as similar observations on the CRE-HIS3 promoter (187) suggest that an activator binds to the CRE to activate expression. In the case of the HAL1 promoter, this activator may be Gcn4p (455). Gcn4p is the transcriptional regulator of the general amino acid control system (224, 225). Gcn4p is known to bind to sequences related to CREs but not to the CREs tested by the Struhl laboratory (187).
The surprising finding that Gcn4p activates transcription in response to osmotic stress appears to be due to inhibition of amino acid uptake systems under salt and osmotic stress (422, 455). Gcn4p and Sko1/Acr1p seem to compete for binding to the CRE in the HAL1 promoter (455). Certainly more work is needed to better understand how Sko1/Acr1p and Gcn4p interact at the promoter level. In this context, it will also be interesting to identify the function of HAL1 in order to understand the physiological basis of this control. The time course of Gcn4p-dependent induction will also be interesting to study, since amino acid starvation should probably not appear as a rapid response after osmotic shock. Moreover, global gene expression analysis has so far not revealed a general stimulation of expression of genes encoding enzymes for amino acid biosynthesis, although it should be noted that all these experiments have been done shortly after osmotic shock. In any case, this initial finding points to interesting connections between stress responses and metabolic regulation.
Data on GRE2 and CRE-HIS3 are consistent with Sko1/Acr1p's itself being involved in activation of CRE-dependent gene expression under osmotic stress (Fig. 6) (187, 502). As indicated above, there is indirect evidence that Sko1/Acr1p binds to the GRE2 and CRE-HIS3 promoters even under osmotic stress. Moreover, in these two promoters, deletion of SSN6 or TUP1 causes a different phenotype than deletion of SKO1 (187, 502), while in the HAL1 and CRE-CYC1 systems deletion of SKO1, SSN6, and TUP1 causes the same complete derepression (455, 481). Specifically, deletion of TUP1 or SSN6 appears to cause only slightly increased basal expression of GRE2 or CRE-HIS3 (187, 502), and expression of GRE2 was strongly regulated by osmotic stress in these mutants (502).
Together with the complete loss of osmotic regulation in the sko1Δ mutant, these data indicate that Sko1/Acr1p is also needed for activated expression of at least these genes. How Sko1/Acr1p can function as both activator and repressor, if this is truly the case, remains to be studied. An attractive speculation, based on recent findings with Hot1p as the recruiting factor for Hog1p and the ability of Hog1p to activate transcription when targeted to a promoter (9) (see below), would be that Sko1/Acr1p and Hog1p form a complex that activates transcription. Interestingly, it was suggested that Atf1, the closest Sko1/Acr1p homolog in S. pombe and well known to be a Sty1-dependent transcriptional activator, may also function as a repressor (129).
The list of genes now known to be controlled by Sko1/Acr1p is given in Table 3.
TABLE 3.
Known genes controlled by Sko1p
| Gene | Function | Reference |
|---|---|---|
| ENA1 (YDR040C) | P-type ATPase involved in Na+ and Li+ efflux, required for Na+ tolerance | 481 |
| GRE2 (YOL151w) | Protein of unknown function with similarity to plant dihydroflavonol-4-reductases | 502 |
| AHP1 (YLR109w) | One of five general hydroperoxide peroxidases, antioxidant function is independent of glutathione | 502 |
| GLR1 (YPL091w) | Glutathione reductase, required for recycling of oxidized glutathione (GSSG) to its reduced form (GSH) | 502 |
| SFA1 (YDL168w) | Long-chain alcohol dehydrogenase, oxidizes fatty acid in presence of glutathione | 502 |
| HAL1 (YPR005C) | Protein involved in ion homeostasis | 455 |
| YML131w | Putative NAD-dependent oxidoreductase | 502 |
Hot1p.
Hot1p is a protein of 719 amino acids. The gene was identified in a two-hybrid search for proteins interacting with Hog1p (503). Hot1p shows similarity to the yeast proteins Gcr1p, Msn1p, and Ymr111p, but no homologs from higher organisms have been reported (503; M. Rep, J. M. Thevelein, and S. Hohmann, unpublished results). The similarity of these four proteins is largely restricted to a region close to the C terminus that covers the DNA-binding domain of Gcr1p (Fig. 7) (616, 617). This domain is predicted to fold into a helix-loop-helix structure, although the exact locations of the helices differ somewhat among predictions (617; M. Rep, J. M. Thevelein, and S. Hohmann, unpublished results). An additional structural feature common to all four proteins is a predicted amphipathic helix within the N-terminal half of the protein, which was implicated in dimerization of Gcr1p (135). Gcr1p is the best-characterized factor of this small family; it controls expression of genes encoding glycolytic enzymes and ribosomal proteins (25, 347). Msn1p, the closest homolog to Hot1p (see below), has been implicated in different cellular processes. Ymr111p is uncharacterized.
FIG. 7.
Domain organization of Hot1p and related yeast transcription factors. The putative DNA-binding domains are aligned, highlighting possible α-helices.
Deletion of HOT1 partially suppresses the lethality caused by overactivation of the HOG pathway, as expected for a factor mediating HOG-dependent responses (503). Hot1p affects expression of a small subset of Hog1p target genes (Table 4) (501, 503). Those include the genes encoding the two key enzymes in glycerol biosynthesis, GPD1 and GPP2. Deletion of HOT1 reduces the mRNA level of these two genes under osmotic stress by about 50%, without affecting the time course of induction. STL1, which encodes a homolog of hexose transporters of unknown function, is an interesting Hot1p target gene because it appeared in global expression analyses as the most strongly osmostress-induced yeast gene (86, 191, 471, 501, 656). Its induction is completely absent in hot1Δ as well as hog1Δ mutants (501), but it appears that Msn1p (9; M. Krantz and S. Hohmann, unpublished data) and Smp1p (F. Posas, 2001, personal communication) also affect its expression.
TABLE 4.
Known Hot1p target genes
| Gene | Function | Reference |
|---|---|---|
| GPD1 (YDL022W) | Glycerol-3-phosphate dehydrogenase | 503 |
| GPP2 (YER062C) | Glycerol-3-phosphatase | 503 |
| STL1 (YDR536W) | Member of the hexose transporter family of the major facilitator superfamily | 501 |
| CHA1 (YCL064C) | Catabolic l-serine/l-threonine dehydratase | 501 |
| PHO84 (YML123C) | High-affinity inorganic phosphate/H+ symporter | 501 |
| YGR043C | Protein of unknown function, strong similarity to Tal1p | 501 |
| YGR052W | Serine/threonine protein kinase of unknown function | 501 |
| YHR087W | Protein of unknown function | 501 |
Hot1p is a nuclear protein both under normal growth conditions and under osmotic stress (503). As revealed by chromatin immunoprecipitation, the protein is associated with the GPD1 promoter under all conditions, although the level of Hot1p on the promoter increases under stress. This association is independent of Hog1p (9). The situation seems to be different in the STL1 promoter, where Hot1p binds only under osmotic stress and needs Hog1p to become associated with the promoter (9). Hot1p also appears to be associated with the promoters of CTT1 and HSP12 (9), although its contribution to osmotic induction of these genes is minor (503). The binding site for Hot1p on target promoters is not known.
Most remarkably, the Hog1p kinase itself becomes associated with the GPD1, STL1, HSP12, and CTT1 promoters, and this association requires Hot1p, suggesting that Hot1p and Hog1p form a complex on target promoters (9). The time course of Hog1p association is very similar to the kinetics of Hog1p phosphorylation, Hog1p nuclear residence, and the stimulation of mRNA levels of these target genes. Hog1p's catalytic activity supports its association with the GPD1 promoter, while it is needed for Hog1p association with the promoters of HSP12, CTT1, and STL1. It is not known if other proteins are part of this complex. While, in a more traditional scenario, a MAP kinase controls the activity of a transcriptional regulator, the appearance of Hog1p on target promoters indicates that Hog1p itself might take part in the activation process. Such a scenario was recently also proposed for the Snf1p kinase in the release from glucose repression (298).
Indirect support for a role of Hog1p in transcriptional activation comes from two observations. Hog1p, when fused to the DNA-binding domain of LexA, constitutively stimulates expression of a reporter containing the LexA binding site, showing that Hog1p can activate transcription (9). In addition, epistasis analysis of hot1Δ and hog1Δ mutations, as studied on the profile of mRNA levels of GPD1 and GPP2 after osmotic shock, revealed that the hog1Δ single mutation conferred a more severe reduction of the mRNA level and a delay in the response. The hot1Δ hog1Δ double mutant showed consistently in various experiments a somewhat lower target gene mRNA level than either single mutant, but the time course was identical to that of the hog1Δ mutant. Hence, genetic evidence is inconsistent with the kinase's acting upstream of the transcription factor, placing it downstream instead (503).
Chromatin immunoprecipitation with different promoters did not give a clear picture of whether binding of Hot1p to target promoters is the critical step controlled by the Hog1p kinase. When Hot1p is recruited to an artificial promoter system via the LexA DNA-binding domain, it retains its ability to stimulate gene expression after osmotic shock in a Hog1p-dependent way. This is also observed with the Hot1p derivative lacking the three Hog1p-dependent phosphorylation sites. While these data do not exclude the possibility that Hog1p at least affects Hot1p promoter recruitment, they clearly show that Hog1p mediates control of Hot1p-regulated promoters by mechanisms different from that of Hot1p DNA-binding (9). Since Hot1p and Hog1p seem to be part of a complex on target promoters, a most important questions concerns the protein(s) in this complex that activates transcription.
Hot1p is phosphorylated in both Hog1p-dependent and -independent ways. A triple Hot1p mutant lacking putative phosphorylation sites at positions 153, 360, and 410 does not show Hog1p-dependent phosphorylation upon osmotic shock but otherwise seems to function like the wild-type protein. Hence, although Hot1p may be a direct phosphorylation target of Hog1p, its phosphorylation does not seem to be a crucial step in transcriptional activation (9).
Msn1p.
Msn1p, a protein of 382 amino acids, is the closest homolog of Hot1p, with an overall identity of 25%, and 42% identity within the putative DNA-binding domain (Fig. 7). Msn1p has been isolated in a number of different genetic screens, suggesting that it is involved, directly or indirectly, in many cellular processes. The first screen that identified MSN1 was multicopy suppression of the inability of the snf1Δ mutant to grow on carbon sources other than glucose (159). Deletion of MSN1 causes diminished expression of the SUC2 gene, encoding invertase, a known target of Snf1p-dependent regulation. MSN1 (as MSS10) was also found as a multicopy activator of the STA genes, encoding glucoamylase, which in some strains confer the ability to catabolize starch (313). The STA genes are also under Snf1p control.
While these data suggested a role for Msn1p in mediating effects downstream of the Snf1p kinase, no further evidence for such a link has been provided so far. Most interestingly, the promoter of the STA2 gene is essentially identical to that of FLO11/MUC1, a surface mucin-like protein required for cell-cell attachment and pseudohyphal growth (312, 342), and consequently it was found that MSN1 is needed for pseudohyphal development (178, 179). Overexpression of MSN1 also suppresses the inability in haploid cells for invasive growth of snf1 mutants under glucose starvation (115). Overexpression also suppresses the defect in pseudohyphal growth observed in homozygous mep1Δ mep2Δ diploids, which have reduced ammonium uptake and lack the ammonium sensor Mep2p (349).
The gene was also identified in a screen for mutations that cause a defect in pseudohyphal growth (PHD2) (194). How Msn1p is involved in pseudohyphal development is unclear, especially since epistasis analysis places MSN1 upstream of MSS11, a gene for another transcriptional regulator (179), indicating that the involvement of Msn1p may be indirect. Msn1p has been reported to affect cell wall carbohydrate composition (350). Finally, MSN1 was found as FUP1 in a screen for genes whose overexpression enhanced growth in iron-limited raffinose medium (148).
Deletion of MSN1 diminishes expression of GPD1, GPP2, CTT1, and STL1. The effects on CTT1 and STL1 were strongest, with a reduction of the mRNA level of 50% (503; M. Krantz and S. Hohmann, unpublished data). Msn1p can be detected by chromatin immunoprecipitation on the promoter of GPD1, where its presence requires Hot1p (9). While these data suggest that Msn1p contributes to expression of some HOG target genes, deletion of MSN1 did not suppress the lethality caused by overactivation of the HOG pathway (503). Hence, either Msn1p is not a HOG target or its contribution to HOG-mediated effects is too minor to suppress the lethality caused by HOG overactivation. Also, Msn1p does not contribute significantly, if at all, to the recruitment of Hog1p to target promoters (9).
Consistent with this notion, global gene expression analysis of an msn1Δ mutant after osmotic shock did not reveal any genes whose osmotic induction was fully dependent on Msn1p (M. Krantz and S. Hohmann, unpublished data). Thus, although Msn1p seems to perform some function related to expression of osmostress-induced genes, a direct involvement with the MAP kinase cascade could not be demonstrated. While some of the effects on stress-induced gene expression may be direct, as evidenced by the presence of Msn1p on a target promoter like GPD1, many of the effects that relate to carbon metabolism and starvation may be indirect. For instance, it appears that deletion of MSN1 diminishes the expression of those HXT genes that encode the metabolically relevant hexose transporters (M. Krantz and S. Hohmann, unpublished data). Reduced sugar uptake capacity and affinity would be a plausible explanation for many of the phenotypes of msn1 mutants. More work is required to identify the primary targets of Msn1p and the interesting possible role of Msn1p in coordination of stress responses and carbon and energy metabolism.
Sgd1p.
Sgd1p is a nuclear protein of 899 amino acids which does not show similarity to any previously characterized proteins. The SGD1 gene is essential (4). The gene was isolated as a suppressor of an osmosensitive mutation, and subsequently it was shown that overexpression partially suppressed the osmosensitivity of hog1Δ and pbs2Δ mutants. Furthermore, consistent with a possible role downstream of the HOG pathway, overexpression in a hog1Δ mutant also enhanced expression of GPD1 and to some extent glycerol production (4). Apart from these data, no molecular evidence is available that links Sgd1p to the HOG pathway.
General Stress Response: Msn2p, Msn4p, and Protein Kinase A
It has been known for many years that exposure of yeast cells to a mild form of one type of stress strongly improves their ability to cope with a more severe shock with a different type of stress (335, 451, 612). Moreover, cells growing under limiting nutrient availability, or starved cells, are known to acquire tolerance to a range of stress conditions, a property that is exploited in sugar-limited culture regimens for the production of baker's yeast (reviewed in references 158, 173, 358, 519, 555, 595, and 596). These observations indicated that S. cerevisiae has a global mechanism for stress tolerance acquisition that protects cells from a number of different stress conditions. This system has been termed the general stress response (reviewed in references 358, 519, and 555).
Global gene expression analysis has now shown that the expression of probably more than 10% of yeast genes is jointly affected by several seemingly unrelated stress conditions, such as nutrient starvation, oxidative stress, heat shock, and hyperosmotic shock. The exact gene number differs between 216 and 300 induced genes and 283 and 600 repressed genes (86, 191). In these reports, the general stress responses have been termed environmental stress response and common environmental response, respectively, also encompassing genes whose expression is diminished.
One way in which the cell achieves induction under several different stress conditions is to control expression of individual genes by different specific stress-signaling pathways that converge at the promoter level. For instance, a number of genes (Table 3) whose expression is induced by osmotic shock and oxidative stress are controlled by Sko1/Acr1p via the HOG pathway and by Yap1p, which specifically mediates oxidative stress responses (reviewed in reference 255). Sko1/Acr1p and Yap1p control target promoters via distinct promoter elements (502). Certain heat shock protein genes are induced by heat shock through the activity of the heat shock transcription factor and require Skn7p for induction by oxidative stress; both factors appear to operate via the same promoter site, the heat shock element (487). Another example is genes apparently induced by osmotic stress and amino acid depletion; HAL1 expression appears to be controlled by Hog1p-Sko1/Acr1p and Gcn4p through the same promoter site, a CRE (455). Future analyses will certainly uncover many such examples of complex stress-responsive promoters.
On the other hand, a large number of general stress-responsive genes are controlled by so-called stress response elements (STREs) via the transcriptional regulators Msn2p and Msn4p (370, 532). STREs have been found by computer analysis in many yeast promoters (398, 610), and about 150 genes show altered expression in a mutant lacking both MSN2 and MSN4 (86, 191, 501). Notably, not all genes whose expression under stress is strongly affected in an msn2Δ msn4Δ mutant contain an obvious STRE in their promoter. Hence, Msn2p and Msn4p might control many genes indirectly; alternatively, the two factors may be able to bind to different promoter elements, perhaps in conjunction with other factors. Most genes that have been demonstrated to be directly controlled by Msn2p and Msn4p contain several STREs in close proximity (398). Certainly more work is needed to better understand the organization of promoters controlled by Msn2p and Msn4p.
STRE control of a promoter can also be combined with specific stress signaling. Some data suggest that the fairly complex control of the glycerol-3-phosphate dehydrogenase gene, GPD1, may be such an example. This promoter contains three STREs (155). However, induction of GPD1 by osmotic stress is essentially normal in an msn2Δ msn4Δ mutant, while induction by heat shock is diminished (191, 503). Control by osmotic stress is mediated partly by Hog1p and Hot1p, influenced by Msn1p (as is induction by heat shock), and also by at least one additional pathway (155, 500, 503).
Finally there is a version of STRE, the postdiauxic shift element (55), which seems to control expression of genes after glucose has been consumed from the medium. This element is controlled by a different transcription factor, Gis1p (458).
STREs and the transcription factors Msn2p and Msn4p.
Stress response elements were characterized during studies on the promoters of CTT1, which encodes cytosolic catalase, and DDR2, which encodes a protein of still unknown function (32, 290, 291). Initially, it was realized that these genes were induced by heat shock in a manner independent of Hsf1p and heat shock elements (290, 291, 646). STREs, with the core sequence CCCCT, were then shown to be necessary and sufficient for stress-induced gene expression in suitable reporter systems (291, 362).
Eventually, the hunt for the transcriptional regulator that binds to these elements identified Msn2p and Msn4p (370, 532). MSN2 and MSN4 were originally isolated in a screen for multicopy suppressors of the inability of the Snf1p mutant to grow on carbon sources other than glucose (160), the same screen that also identified Msn1p (159). The two factors are only 32% identical, but their zinc finger DNA-binding domains are very similar and are predicted to bind to the same sequence (370). Msn2p and Msn4p seem to perform redundant functions, but Msn2p generally makes a much stronger contribution (370, 532). Msn2p and Msn4p together are essential for stress-induced expression of STRE-dependent reporter genes (370, 532). Even before global expression analysis was employed, the multistress-induced expression of a large number of genes was shown to be dependent on STREs and Msn2p and Msn4p (see references 158, 398, and 555 and references therein). Importantly for this discussion, the stimulation of expression by hyperosmotic shock of an STRE-dependent reporter gene depends on Msn2p and Msn4p as well as on the HOG pathway (533).
The analysis of the mechanisms that control STREs and Msn2p and Msn4p is complicated by the fact that essentially all culture conditions that diminish the maximal capacity of the yeast cell to proliferate affect STRE-dependent expression. This also makes genetic screens for altered STRE activity very difficult and hinders study of plasmid-carrying transformants; transformants need to be cultured in synthetic medium, which is sufficient to at least partially stimulate STRE-dependent gene expression. Therefore, studies on the function of Msn2p and Msn4p have largely focused on the proteins themselves. An important level of control seems to be exerted by shuttling Msn2p and Msn4p between the cytosol and the nucleus (199). Under optimal growth conditions, Msn2p and Msn4p are cytosolic proteins, and upon imposition of different stress conditions Msn2p and Msn4p are translocated to the nucleus (199). Translocation is rapidly reversible and independent of protein synthesis, and mutations that eliminate several protein kinase A-dependent phosphorylation sites cause the protein to be nuclear even under optimal growth conditions (199).
Control of Msn2p and Msn4p localization.
It has been known for many years that the expression of STRE-dependent genes or reporters is sensitive to altered activity of protein kinase A (32, 41, 362). Yeast protein kinase A consists of three catalytic subunits of overlapping but distinct functions, Tpk1p to Tpk3p, as well as the regulatory, cAMP-binding subunit Bcy1p (reviewed in reference 595). Low activity of protein kinase A causes higher basal expression of STRE-dependent genes, and high protein kinase A activity causes lower expression of these genes under both basal and stress condition (32, 41, 290). Direct genetic evidence for control of Msn2p and Msn4p by protein kinase A comes from the observation that the lethality at the restrictive temperature of a mutant lacking protein kinase activity (tpk1 tpk2ts tpk3) is suppressed by deletion of MSN2 and MSN4, while the growth defect of the same mutant at the permissive temperature is aggravated by overexpression of MSN2 (563). This suggests that an essential role of protein kinase A is to inactivate Msn2p and Msn4p, probably by mediating export of Msn2p and Msn4p from the nucleus.
Low, unregulated protein kinase A activity, as in a bcy1Δ mutant carrying deletions of TPK2 and TPK3 and a leaky mutation of TPK1, renders the protein nuclear even under optimal conditions. On the other hand, addition of cAMP to a strain whose protein kinase A activity can be manipulated by external cAMP causes Msn2p to be mainly cytosolic even upon stress (199). In addition, mutations of protein kinase A target serine residues cause Msn2p to localize to the nucleus even in the presence of cAMP. These data are consistent with a model in which protein kinase A mediates translocation of Msn2p and Msn4p from the nucleus to the cytosol and hence counteracts stress-induced nuclear localization (199). Export of Msn2p from the nucleus requires the nuclear export receptor protein Msn5p (94).
Whether Msn2p and Msn4p are indeed direct targets of protein kinase A remains to be determined; at least, these observations have motivated research into the localization of the regulatory and different catalytic subunits of protein kinase A (202, 203). Another interesting question concerns the mechanism that mediates rapid nuclear import upon stress, since protein kinase A stimulates export of Msn2p and Msn4p from the nucleus. If phosphorylation by protein kinase A were the only modification that controls localization, a stress-controlled phosphatase should dephosphorylate Msn2p and Msn4p in the cytosol to mediate nuclear import, unless phospho-Msn2p/Msn4p is notoriously unstable. Alternatively, a different cytosolic protein kinase(s) could phosphorylate Msn2p and Msn4p upon stress on different sites and thereby mediate nuclear import. The latter idea has received support from some observations. Heat shock and diauxic shift are associated with hyperphosphorylation of Msn2p, which itself is negatively controlled by cAMP (189). Hence, under optimal conditions nuclear localization could be inhibited by protein kinase A by mediating nuclear export and by inhibiting a cytosolic kinase that mediates nuclear import upon stress.
Additional protein kinases have indeed been reported to affect the subcellular localization of Msn2p and Msn4p. Tor1p and Tor2p are the targets of the immunosuppressant rapamycin and control cell growth in response to nutrient availability. Note that a distinction is made between cell growth, controlled by the TOR pathway, and cell proliferation, controlled by protein kinase A (529, 598). Nutrient limitation activates responses that are similar to those observed when the TOR kinases are blocked by rapamycin. TOR kinases apparently control many processes in the cell, such as translation, and they are conserved from S. cerevisiae to humans (529). One aspect of this system seems to be the control of the subcellular localization of certain transcriptional regulators, such as Gln3p (involved in nitrogen metabolism) and Msn2p (31). Rapamycin causes the nuclear accumulation of Msn2p, an effect not seen in a TOR1-1 mutant insensitive to the drug, suggesting that the response is specific.
TOR-dependent control of Msn2p localization involves the 14-3-3 protein Bmh2p. Bmh2p may be a cytosolic anchor of Msn2p. These observations indicate that Msn2p localization in response to carbon source depletion involves the TOR pathway. It has not been investigated if Bmh2p (and/or its homolog Bmh1p) and the TOR proteins also mediate the localization of Msn2p and Msn4p under osmotic stress. It has also not been investigated so far if other nutrient-controlled pathways, such as the Snf1p pathway, are also involved in controlling Msn2p and Msn4p localization. Nutrient starvation could be a secondary effect of hyperosmotic stress due to inhibition of nutrient transport systems (422, 455).
Another protein kinase that affects Msn2p localization is Ssn3p/Srb10p. Srb10p is a subunit of the mediator complex of RNA polymerase II and functions as a cyclin-dependent protein kinase (CDK) (340). Srb10p phosphorylates Msn2p in vitro and in vivo. Srb10p-dependent phosphorylation of Msn2p is required for nuclear export, and Msn2p is predominantly nuclear in an srb10 mutant even under nonstress conditions (94). This observation is consistent with the elevated expression of Msn2p/Msn4p target genes in an srb10 mutant, as determined by microarray analysis (235). The physiological role of this control is unclear. Phosphorylation by Srb10p makes Msn2p a target for ubiquitination by the SCF (Skp, CDC53/cullin, F-box receptor) ubiquitin ligase system. In contrast to Gcn4p, which is also phosphorylated by Srb10p and ubiquitinated by SCF, Msn2p does not seem to be degraded upon ubiquitination (94).
Thus, Msn2p and Msn4p are probably targets for several different signaling-control systems.
What mechanisms control protein kinase A under stress?
The observation that Msn2p/Msn4p localization is controlled both by protein kinase A and by stress implies that the stress signal is transmitted through protein kinase A. Additional observations link protein kinase A to stress responses. It is well known that mutants with high protein kinase A activity display a low tolerance to stress, while mutants with low protein kinase activity have high stress tolerance. As mentioned above, optimal growth conditions are associated with low stress tolerance and suboptimal growth conditions with high stress tolerance. This high stress tolerance can be overruled by high protein kinase activity and vice versa (reviewed in references 173 and 595). Together with the transient glucose-induced stimulation of cAMP levels as well as the requirement for protein kinase A activity to pass the Start point of the cell cycle, these observations have led to the generally accepted view that cAMP and protein kinase A activities are high under optimal and low under suboptimal growth conditions. It should, however, be stated that direct evidence for different physiological conditions truly affecting steady-state protein kinase A activity has so far not been presented.
The data on Msn2p/Msn4p localization under stress would be consistent with a scenario in which a cytoplasmic protein kinase(s), such as the Tor proteins, regulates nuclear import, which is counteracted by a constitutive protein kinase A activity. The observation that stress mediates stimulated expression of genes encoding components of the Ras-cAMP-protein kinase A pathway in an MSN2/MSN4-dependent manner (191) indicates that the cell increases its capacity to counteract the stress response once conditions return to optimal. However, this observation does not demonstrate that protein kinase A truly mediates the stress signal. As long as the mechanisms are not understood and reliable means to directly monitor in vivo protein kinase A activity are not available, stress control of protein kinase activity remains an assumption.
Protein kinase A is obviously controlled by the cellular level of cAMP. cAMP is produced by adenylate cyclase (Cdc35p) and degraded by the high-affinity (Pde2p) and low-affinity (Pde1p) phosphodiesterases. Principally, only two conditions have been well documented to cause a rapid, significant, and transient cAMP increase: intracellular acidification and addition of glucose to cells grown in the presence of a poor carbon source (reviewed in reference 596). Glucose mediates transient cAMP synthesis and hence adenylate cyclase activation via the G-protein-coupled receptor Gpr1p and its Gα protein Gpa1p (the β and γ subunits have not been identified). Acidification, on the other hand, seems to activate adenylate cyclase via the Ras proteins Ras1p and Ras2p. Their activity in turn is controlled by the exchange factors Cdc25p and Sdc25 as well as the GTPase-activating proteins (GAP) Ira1p and Ira2p. These two pathways for control of cAMP production seem to interact. Phosphodiesterase activity is controlled at the transcriptional level: PDE2 expression is stimulated by stress in an Msn2p/Msn4-dependent manner (611). There is evidence that protein kinase A is controlled via cAMP-independent mechanisms (reviewed in references 595 and 596).
Some recent observations may provide hints to possible mechanisms of stress-dependent control of protein kinase A. Ssa1p, a heat shock protein and chaperone, has been reported to interact with Cdc25p. Stress could engage Ssa1p with denatured proteins and allow Cdc25p to activate the Ras proteins and eventually protein kinase A (193). This remains to be studied in more detail. In a different report, evidence has been provided that compounds that alter the membrane potential affect STRE-dependent gene expression (397). The molecular mechanisms and the relevance to osmoadaptation remain to be studied. A more detailed discussion of the mechanisms that control protein kinase A activity is beyond the scope of this article and has appeared elsewhere (139, 173, 555, 594-596).
Control of Msn2p/Msn4p-dependent genes by the HOG pathway.
Several observations indicate that under osmotic stress Msn2p and Msn4p translocation to the nucleus is necessary but not sufficient for stimulation of gene expression. First of all, global expression analysis reveals a remarkable correlation between Msn2p/Msn4p- and Hog1p-dependent genes (501). Essentially all genes whose expression after osmotic shock is diminished by more than 75% in an msn2Δ msn4Δ mutant are also strongly reduced in a hog1Δ mutant. On the other hand, there are many genes whose expression is strongly diminished in a hog1Δ mutant but is largely unaffected by deletion of MSN2 and MSN4 (501), consistent with the fact that Hog1p mediates transcriptional responses through several factors. While this observation is essentially based on correlation, it has been known for several years that the osmotic induction of an STRE-driven reporter gene requires the HOG pathway (370, 533).
The localization of Msn2p/Msn4p is unaffected in a hog1Δ mutant (199). These observations may indicate that Hog1p controls the activity of nuclear Msn2p and Msn4p after osmotic shock. However, it was also observed that although deletion of MSN2 and MSN4 drastically reduces expression of the STRE-driven reporter, induction specifically by osmotic stress but not by other stress conditions was still intact, albeit at a lower basal level (533). Additional deletion of PBS2 in the msn2Δ msn4Δ mutant completely eliminated induction (370). This suggests that under osmotic stress, presently uncharacterized proteins mediate a Hog1p-dependent induction of STRE-driven genes; whether those come into action only in the absence of Msn2p and Msn4p remains to be studied. In conclusion, more work is needed to better understand the HOG-dependent control of STRE-driven genes and whether Hog1p controls Msn2p/Msn4p directly.
Nutrient-Controlled Signaling and Stress Responses
Cellular adaptations to nutrient availability and stress responses, including the response to osmotic shock, are related phenomena. Nutrient deprivation itself is stressful, and cells mount the general stress response discussed above when grown under limited nutrient availability (86, 191). Nutrient limitation (such as very low glucose levels) or availability of only poor nutrient sources (such as ethanol instead of glucose as a carbon and energy source or proline instead of ammonium as a nitrogen source) stimulates responses that lead to the adaptation or reprogramming of cellular metabolism, which involves several signaling systems with partially overlapping function (for a review, see reference 139). For instance, adjustment of cellular metabolism at the transcriptional and posttranscriptional levels in response to the availability or quality of carbon sources is controlled by the Snf3/Rgt2 sugar-sensing pathway (52), the Gpr1-cAMP-protein kinase A pathway (329, 596), the Snf1 pathway (84, 185), and the TOR pathway (512, 529). Availability and quality of nitrogen sources and amino acids are monitored by the Ssy1 sensing system (171), the Gcn4p transcriptional regulator (225), a pathway that involves the ammonium sensor Mep2p's mediating nitrogen catabolite repression (329), and again the TOR pathway (512, 529). While some pathways appear to monitor nutrient availability by sensing external nutrients through plasma membrane-located sensors, such as Snf3p and Rgt2p, Gpr1p, Mep2p, and Ssy1p, other systems appear to check nutrient utilization inside the cell, such as the Snf1p kinase (probably by monitoring the AMP:ATP ratio), Gcn4p (controlled by translation efficiency), and perhaps the Tor1p and Tor2p kinases.
Osmotic stress demands metabolic adjustments in several different ways. Stress in general requires the cell to invest energy to cope with the consequences of cell damage or the production of protective proteins or metabolites; hence, the cell has to compromise between its desire to grow and proliferate at a maximal rate to repair or prevent cellular damage. The production of glycerol from phosphorylated precursors under osmotic stress constitutes a significant energy investment (437). Glycerol production is also an investment of carbon, although glycerol that is not needed for osmotic adjustment anymore may be further catabolized (184). Glycerol production also affects redox metabolism, since glycerol is more reduced than the substrate glucose; hence, redox metabolism needs to be adjusted accordingly (19, 42, 48). These aspects are discussed further below.
An entirely different effect imposed by osmotic stress is inhibition of transport processes. As mentioned above, osmotic stress strongly diminishes the uptake of several amino acids (422, 455), and expression analysis of cells fully adapted to and actively growing under osmotic/salt stress might reveal an amino acid starvation response. Several observations suggest that amino acid starvation is a significant aspect under osmotic stress. As outlined above, the transcriptional regulator Gcn4p takes part in the osmo-induced expression of HAL1 so that the HOG pathway mediates relief from Sko1/Acr1p-dependent repression, while Gcn4p induces transcription. Mutants lacking GCN4 are sensitive to salt and osmotic stress (455), suggesting that the role of Gcn4p under such conditions is not restricted to HAL1 and that a response to amino acid starvation is essential to cope with high osmolarity. This observation also indicates that yeast strains that carry auxotrophic markers blocking amino acid biosynthetic pathways may have diminished osmotolerance compared to prototrophic strains and that such markers may account, in part, for the well-known strain differences in osmotolerance.
Amino acid starvation also affects the TOR pathway (83, 212, 512, 542). The TOR kinases are large proteins (in S. cerevisiae, 2,470 and 2,473 amino acids) showing homology to phosphatidylinositol kinases within their protein kinase domains (for recent reviews, see references 492, 512, and 529). TOR stands for target of rapamycin; the Tor kinases are inhibited by a complex formed between the FKBP12 prolyl isomerase (in S. cerevisiae, Fpr1p) and rapamycin, a drug that is used as an immunosuppressant because it inhibits the proliferation of T cells. The essential TOR kinases are well conserved from S. cerevisiae to humans not only in terms of sequence but it appears also in function and physiological role.
The yeast proteins Tor1p and Tor2p are 68% identical and probably perform similar though not identical functions (217, 220). The Tor kinases directly control translation in response to amino acid availability. Rapamycin affects cell growth because it inhibits the Tor kinases, thereby blocking translation and arresting the cell cycle in the G1 phase in both S. cerevisiae and mammalian cells (29, 631). In addition, the Tor kinases have broad impact on transcription in response to poor nitrogen and carbon sources (83, 212, 542). The Tor kinases seem to confer at least part of those responses by mediating under optimal growth conditions the cytosolic localization of transcriptional regulators such as Gln3p, Gat1p, and Msn2p/Msn4p via the 14-3-3 proteins Bmh1p and Bmh2p (see above) (31, 512).
Recently it has been reported that the TOR pathway controls expression of the ENA1 gene, which encodes the main Li+ and Na+ export pump (109). Inhibition of TOR by rapamycin causes enhanced expression of ENA1, suggesting that the activity of the Tor kinases leads to inhibition of expression of ENA1. This effect seems to be conferred by the GATA transcription factors Gln3p and Gat1p, since deletion of GLN3 reduced and double deletion of GLN3 and GAT1 abolished ENA1 expression under basal and salt stress conditions. There are several possible binding sites for those factors in the ENA1 upstream region. In addition, Gln3p and Gat1p are required for growth under salt stress, although apparently not under osmotic stress. Deletion of TOR1 also causes salt sensitivity, an observation in apparent contradiction to the observed stimulated expression of ENA1 upon TOR inhibition (109). In S. pombe, Tor1 is needed for growth under osmotic stress as well as heat stress (279). Taken together, these observations indicate that part of the response to osmotic stress is governed by internal nutrient-sensing systems that activate Gcn4p and the TOR pathway. However, more work is needed to better understand how these nutrient signaling systems function under osmotic stress and how they cooperate with pathways specifically mediating osmotic responses.
Snf1p/Cat1p was first isolated in screens for genes that affect catabolite repression (Cat) (153) and cause inability to ferment sucrose (Snf) (87). The protein kinase of 633 amino acids is structurally and functionally homologous to AMP-dependent protein kinase from mammals and plants (reviewed in references 209 to 211). Snf1p kinases seem to monitor the energy status of the cell; they are activated by AMP, which is high when the energy level of the cell is low (649). However, in S. cerevisiae the signal that controls Snf1p is not clearly defined; the long-known fact that the hexokinases affect catabolite repression keeps the discussion open if they somehow control Snf1p directly (84, 185, 233).
Snf1p is known as the master regulator in S. cerevisiae glucose repression because it is needed for the derepression of genes encoding enzymes required to catabolize carbon sources such as galactose, sucrose, raffinose, and maltose (84, 185). Snf1p is also needed for the activation of expression of gluconeogenic and respiratory genes. Mutants lacking Snf1p are therefore strongly impaired or unable to grow on substrates other than glucose. Snf1p controls transcriptional regulators such as Mig1p and Mig2p, which mediate glucose repression, and Cat8p and Sip4p, which mediate activation of gluconeogenic genes (84, 185). In addition, Snf1p is involved in numerous systems that relate to sugar metabolism, such as pseudohyphal development, accumulation of glycogen, sporulation, life span, and heat shock sensitivity in stationary phase.
The Snf1p kinase complex consists of three different subunits: the catalytic α subunit, Snf1p, variable β subunits (Sip1, Sip2p, and Gal83p), and the regulatory γ subunit, Snf4p (Cat3p), which is needed to activate Snf1p. The β subunits are involved in substrate recognition (531) and target the complex to different cellular locations when cells are grown with glycerol instead of glucose as the carbon source: Sip1p is located in or at the vacuole, Sip2p is in the cytosol and excluded from the nucleus, and Gal83p targets the complex to the nucleus (633).
Being apparently a master regulator of energy metabolism responding to internal signals, Snf1p is likely to adjust cellular energy metabolism under stress (209). In S. cerevisiae, however, this aspect has not been studied systematically so far. The expression profiles obtained shortly after shifting cells to stress conditions do not reveal a characteristic pattern for cells that activate Snf1p, although this again may be due to the fact that all experiments have so far monitored expression only shortly after the shock.
Interestingly, as indicated above, several genes that play crucial roles in stress responses have been isolated as suppressors of snf1: MSN1 (159), MSN2 and MSN4 (160), transcription factors involved in stress responses; MSN5 (10), encoding a protein needed for nuclear export of Msn2p/Msn4p; SSN3 (299), a CDK of the mediator complex that phosphorylates Msn2p and Msn4p; and SSN6 (534), a transcriptional corepressor that affects not only stress genes (102). This may be a coincidence but might also hint of a role for Snf1p in stress responses. Mutants lacking Snf1p do not show a significant stress phenotype, but they have been reported to be heat shock sensitive, at least in certain genetic backgrounds (8, 599). Snf1p also controls expression of the ENA1 Li+ and Na+ exporter through the Mig1p repressor, and snf1Δ mutants are salt sensitive (8; T. Ye, M. J. Tamás, and S. Hohmann, unpublished data). Recently, it has been reported that osmotic induction of the DOG2 gene, which encodes an enzyme with 2-deoxyglucose-5-phosphatase activity, requires both Hog1p and Snf1p; in single hog1Δ and snf1Δ mutants, expression of the genes was diminished, but in an snf1Δ hog1Δ double mutant, induction by osmotic shock was completely abolished (613). Hence, at least two genes, ENA1 and DOG2, are apparently controlled by both the HOG and the Snf1 pathways, and more examples may be found. These observations indicate that a more detailed analysis of the role of Snf1p in adjusting metabolism under osmotic stress may be rewarding.
Cell Integrity Pathway
The cell integrity or protein kinase C pathway (Fig. 8) orchestrates changes in cellular morphology by controlling the expression of genes encoding enzymes involved in cell wall metabolism, by directly controlling at least one such enzyme, and by taking part in reorganizing the actin cytoskeleton (205, 216, 270). The cell integrity pathway is not a single straight cascade but rather a network of interacting signaling routes that diverge from or converge at protein kinase C (Pkc1p) and the G-protein Rho1p as well as proteins controlling those. Pathways that interact with these central components of the cell integrity pathway, physically and/or genetically, are the Slt2/Mpk1 MAP kinase cascade (327), the calcineurin pathway (190), the TOR pathway (530), the HOG pathway (124, 219), a phosphatidylinositol pathway (665), Cdc28p-dependent control of the cell cycle (201, 363, 671), and probably additional pathways (214, 216, 418, 470).
FIG. 8.
Outline of the cell integrity pathway. Two pathways acting in parallel, the calcineurin and the Ppz pathways, are indicated.
Mutants in which signaling through the upstream components or through the MAP kinase cascade of the pathway is blocked display, with different degrees of severity, cell wall defects (332, 511). Such cells require an osmotic stabilizer, for instance, 1 M sorbitol, for growth and proliferation, especially at elevated temperature (247, 327, 331, 366). At the restrictive temperature, mutants terminate growth with a small bud and lyse, indicating that cells pass the Start point of the cell cycle but fail to progress beyond an early point in bud formation (214, 333, 377, 609, 659). Bud emergence and development are a manifestation of polarized cell growth (for reviews, see references 89, 482, and 483). The actin cytoskeleton, composed of patches and cables, directs vesicular transport and hence cell surface building blocks, metabolic enzymes relevant for cell surface assembly as well as regulatory proteins to places of cell growth. Such directed growth also occurs during development of projections towards a mating partner (89, 143) and during pseudohyphal development (89, 329).
Mutants defective in the cell integrity pathway lyse when exposed to pheromone, during early stages of the development of mating projections (157). Caffeine, which for unknown reasons specifically affects mutants with a defective cell wall (133, 288), seems to promote lysis of cell integrity pathway mutants (367), while they are more resistant to calcofluor white (132, 133, 282), which affects the assembly of chitin fibrils (151). Mutants defective in the pathway display additional though unexplained phenotypes, such as inability to grow on nonfermentable carbon sources and loss of viability under nitrogen starvation (106, 641). Consistent with several of the phenotypes listed above, the pathway is activated, with different timing and kinetics, by hypo-osmotic shock (125), by heat shock (272), during bud emergence (671), upon exposure to mating pheromone (73, 671), and upon various treatments leading to cell wall perturbation (36, 132, 282). Common to those stimuli is that they cause, or are thought to cause, cell wall expansion due to environmental or developmental signals.
Control of gene expression by the cell integrity pathway is mediated by a MAP kinase cascade, whose components are, as far as we know, not used in any other yeast MAP kinase pathway (205). Most of the characterized genes that are controlled by this cascade encode proteins involved in cell wall metabolism and bud development (270). Pkc1p and Rho1p control additional pathways, which also mediate transcriptional responses and/or directly control the activity or localization of proteins that are involved in polarized cell growth. In fact, it appears that one of the roles of Rho1p is to recruit pathway components to sites of polarized cell growth (18, 75, 134, 146, 361). Rho1p colocalizes with actin patches and places of cell growth, and the localization of Pkc1p to those sites depends on Rho1p (18). These and several additional observations (100, 461, 637) highlight the importance of the upstream components of the pathway in polarized cell growth and morphogenesis, although it is difficult to establish within this network which of the components primarily determine localization or are recruited (89). Rho1p also interacts directly with (245, 375, 485) and perhaps recruits (134) the enzyme β-1,3-glucan synthase, which synthesizes the main cell wall polysaccharide. Several upstream sensors have been identified that all span the membrane once and probably extend into the cell wall, where they might sense alterations in wall elasticity (252, 282, 462, 629).
Taken together, all properties of the cell integrity pathway are consistent with the view that it controls cell wall metabolism during growth and development and upon external impact leading to cell expansion or cell wall damage. In this way, the cell integrity pathway is involved in controlling cellular turgor and is discussed here for this reason. At first sight it appears that the cell integrity pathway and the HOG pathway have opposing functions. The HOG pathway is stimulated upon hyperosmotic shock (61) and the cell integrity pathway upon hypo-osmotic shock (125). While responses mediated by the cell integrity pathway appear to aim at diminished turgor pressure on the cell wall or at a cell wall's sustaining pressure more effectively, the HOG pathway seems to mediate build-up of such pressure.
Another view on the role of the two pathways suggests, however, that they collaborate in the same process. First of all it appears that the HOG pathway monitors cell swelling or shrinking at the plasma membrane level, while the cell integrity pathway seems to do the same at the level of the cell wall. Responses mediated by the HOG pathway lead to increased turgor pressure, which is interpreted by the cell integrity pathway to coordinate cell wall strength and cell expansion and thereby diminish turgor pressure. Together, the two pathways set the osmotic conditions and appropriate turgor pressure for cell morphogenesis. The MAP kinase cascades of the two pathways seem to operate so that only one is active at any time, as also indicated by the effects of genetic alterations (125), but during cell growth both pathways may well be activated and deactivated within short intervals to balance between cell expansion and cell wall development. Skn7p might be a link between the two pathways, as discussed later.
Pathway architecture: MAP kinase cascade.
The first pathway component identified was Pkc1p, the only yeast homolog of mammalian protein kinase C isoforms (333). The 1,151-amino-acid PKC1 gene product shows significant similarity to mammalian counterparts, containing essentially all domains found in different mammalian protein kinase C isoforms. Therefore, Pkc1p is regarded as an archetypal protein kinase C (reviewed in reference 379). Mutant cells lacking PKC1 arrest growth uniformly with small buds, indicating that the cell cycle arrests at a specific point early in S-phase. The growth defect can be rescued by supplementing the growth medium with 1 M sorbitol as an osmotic stabilizer at 30°C or lower (331); hence, cells might employ a morphology checkpoint to monitor appropriate turgor pressure before undergoing further bud growth and cytokinesis (214).
The osmoremedial growth defect of pkc1 mutants is shared with mutants lacking components of the downstream MAP kinase cascade (247) and certain alleles of the essential RHO1 gene (273, 421, 484). A PKC1 allele was also isolated in a genetic screen for mutants unable to grow at 37°C without osmotic stabilizer (449). Studies on purified Pkc1p indicated that the enzyme does not respond to allosteric effectors such as phospholipids, diacylglycerol, and Ca2+, known to activate mammalian protein kinase C isoenzymes (20). Whether this is also true in vivo, especially with regard to activation by diacylglycerol, remains a matter of debate; mutation of the putative diacylglycerol binding site in Pkc1p diminishes its activity (216, 253). PKC1 has been found in several unrelated genetic screens and hence has numerous names, such as STT1, for staurosporine- and temperature-sensitive mutant (664), and HPO2, for hypo-osmolarity-sensitive mutant (547).
Genetic analyses revealed components operating downstream of Pkc1p (reviewed in references 205 and 216). BCK1 (bypass of C kinase) encodes a MAPKKK and was first identified via a dominantly activated mutation (BCK1-50) that suppressed the growth defect of a pkc1Δ mutant (328). The same gene was isolated simultaneously as SLK1 (for synthetic lethal kinase) in a screen for mutations that cause synthetic lethality in combination with a spa2 mutation, which causes defects in morphogenesis of mating projections (104, 177, 544). Deletion of BCK1 causes a phenotype that is similar to that of a pkc1 mutant but less severe, i.e., growth is rescued by 1 M sorbitol even at 37°C (328). The genes MKK1 and MKK2 as well as SLT2/MPK1 were isolated as multicopy suppressors of the pkc1Δ mutation (247, 327). Mkk1p and Mkk2p are 59% identical and highly similar to MAPKKs. Mutation of either of the two genes does not cause an obvious phenotype, but an mkk1 mkk2 double mutant also requires an osmotic stabilizer for growth. SLT2/MPK1, a gene encoding a MAP kinase, was originally isolated by complementation of the lysis-sensitive lyt2 mutant (609), which seems to contain two independent mutations (327). The slt2/mpk1Δ mutant also requires an osmotic stabilizer (247, 327, 366). Epistasis analysis further confirmed the order of pathway components; Pkc1p apparently controls the MAP kinase cascade (247). The genetic interactions were recently summarized (216).
A genuine scaffold protein has not been identified for the cell integrity MAP kinase cascade and may not be required, since pathway components do not seem to function in different pathways. Interestingly, however, two-hybrid analysis indicates that Pkc1p interacts with Mkk1p but not with the immediate downstream kinase, Bck1p. Mkk1p also interacts with Bck1p and Mkk2p, and Mkk2p interacts with Slt2/Mpk1p (450, 567). Taken together, these observations indicate that, as in the HOG pathway, the MAPKK could play an important role in mediating the formation of a complex between pathway components. Given the dynamics of subcellular localization of pathway components observed, for instance, for the HOG pathway (488, 498), it will be interesting to perform such studies on the kinases involved in the cell integrity pathway.
Pathway control: the Rho1p G-protein.
Rho1p is a small (209 amino acids) GTP-binding protein. In S. cerevisiae, there are four additional related GTP-binding proteins: Rho2p (53% identity to Rho1p), Cdc42p (50%), Rho3p (44%), and Rho4p (38%). As discussed above, Cdc42p is involved in signaling through several MAP kinase pathways in S. cerevisiae (143, 154, 287). Generally, Rho proteins are involved in cellular morphogenesis and polarity (reviewed in references 75 and 89). Rho2p may share some functions with Rho1p (218, 530). The roles of Rho3p and Rho4p are less well understood. Rho1p is localized to places of cell growth, such as in small buds, and colocalizes there with actin patches (24, 485, 659).
Rho1p is essential for viability but can partially be replaced by mammalian RhoA (352, 484). Several strong lines of evidence show that Rho1p controls Pkc1p. The temperature sensitivity of the yeast strain expressing RhoA instead of Rho1p is suppressed by dominant mutations in the pseudosubstrate site within Pkc1p (421). In addition, overexpression of PKC1 suppresses temperature-sensitive alleles of RHO1 (273). Rho1p and Pkc1p physically interact in the two-hybrid system and coimmunoprecipitate (273, 421). Finally, like pkc1Δ mutations, certain conditional rho1 alleles cause cells to arrest and lyse with a small bud (444).
Rho1p itself is controlled by the GDP/GTP exchange factors Rom1p and Rom2p. ROM1 and ROM2 were isolated as multicopy suppressors of a dominant negative rho1 mutation. Deletion of both ROM1 and ROM2 together is lethal, and cells arrest with small buds and lyse, like rho1 mutants (444). Rom2p has been shown to have GTP/GDP exchange function on Rho1p (36). Rom2p seems to have a stronger contribution, and deletion of ROM2 itself causes a number of phenotypes (444). Sac7p (530) and Bem2p (461) function as GTPase-activating proteins and hence as negative regulators of Rho1p. Mutants lacking SAC7 or BEM2 show increased Slt2/Mpk1p phosphorylation (369). sac7 and rom2 mutations suppress each other, consistent with the notion that they perform opposite functions on Rho1p (530). Rho1p seems to be the entry point for several morphogenesis signals into the cell integrity pathway, and it mediates responses by several different effectors.
Cell surface sensors of the cell integrity pathway.
Several proteins have been implicated in the control of the cell integrity pathway upstream of Rom2p-Rho1p. Those putative sensors are Wsc1p (Slg1p, Hcs77p) (134, 201, 252, 629), Mid2p (282, 462, 489), Mtl1p (489), and Wsc2p-4p (629).
All these proteins are type I transmembrane proteins, i.e., they have in common the presence of a single transmembrane domain. In addition, all these proteins share the presence of an N-terminal signal sequence as well as a highly serine/threonine-rich domain in front of the transmembrane domain. This domain is extracellular and heavily glycosylated (282, 344, 462, 489). Apart from these similarities, the six proteins listed above can be divided into two groups. Wsc1p to Ws4p show significant sequence similarity to each other (344, 629), as do Mid2p and Mtl1p (282, 489). However, the two groups, while structurally similar, do not display significant sequence similarity. In addition, Mid2p and Mtl1p do not posses a cysteine-rich region that is found between the signal sequence and the serine/threonine-rich domain in the Wsc proteins. The N-terminal, extracellular domain probably interacts with or extends into the cell wall; glycosylation of the putative sensors seems to be required for signaling, perhaps because it is needed for interaction with the glucan layers of the cell wall (462).
Wsc1p was simultaneously identified in three different genetic screens as a multicopy suppressor of the growth and budding defects of the swi4 mutant, which is defective in the SBF transcriptional activator complex (see below) (201); as a mutant that requires the activated allele of the MAPKKK gene BCK1-20 for growth (252); and in a screen for genes that suppress the heat shock sensitivity of a strain expressing a hyperactivated allele of the G-protein Ras2p (629). Genetic interaction between WSC1 and RAS2 was further confirmed by the observation that deletion of RAS2 suppresses the heat sensitivity of the wsc1 mutant (629). This unexpected link between Wsc1p and Ras2p has so far not been explained, but mutants defective in the cell integrity pathway and those with hyperactive Ras proteins share some phenotypes, such as a defect in the acquisition of heat shock tolerance (272) and an inability to adjust to nutrient limitation (106, 595, 596).
Mid2p has also appeared in a number of different genetic screens: as a mutation that causes pheromone-induced cell death (438); as a gene that enhances in multicopy the transcriptional activity of the Skn7p transcription factor (see below) (282); and as a multicopy suppressor of the heat sensitivity of the wsc1 mutant (489). Both MID2 and WSC1 appeared as a multicopy suppressor of an spc100 mutant. SPC100 encodes a component of the spindle pole body, and the mutant protein displays reduced calcium-binding ability and is unable to form a mitotic spindle (578). Wsc2p to Wsc4p and Mtl1p seem to make minor contributions to pathway activation and have been identified because of their similarity to Wsc1p and Mid2p, respectively (282, 489, 629). For instance, wsc2Δ and wsc3Δ mutations do not show phenotypes on their own or in combination, but they somewhat enhance phenotypes conferred by the wsc1Δ mutation (629). One possible interpretation of this observation is that these proteins function under specific conditions or also control other pathways.
Single wsc1Δ and mid2Δ mutants display several phenotypes in common with mutants defective in downstream components of the cell integrity pathway. mid2Δ mutants are not heat sensitive but are caffeine sensitive, sensitive to α-factor, and resistant to calcofluor white (282, 438, 489, 578). wsc1Δ mutants are also caffeine and α-factor sensitive and in addition are sensitive to both high and low temperatures (249, 252, 578, 629). Double wsc1Δ mid2Δ mutants have a severe growth defect that can be rescued by osmotic stabilizers (249, 282, 489). Excellent evidence links both Mid2p and Wsc1p to the cell integrity pathway: both are required for GTP loading of Rho1p (134, 462) and for activation of Slt2/Mpk1p (134, 201, 282, 462, 489, 629). Several phenotypes of wsc1Δ and mid2Δ mutants are suppressed by overexpression of RHO1 and PKC1 and genes encoding components of the MAP kinase cascade (134, 201, 252, 282, 462, 489, 629). The C-terminal, cytoplasmic domains of Wsc1p and Mid2p interact in the two-hybrid system with Rom2p but not with Rho1p, suggesting that the putative sensors transmit a signal via Rom2p-Rho1p-Pkc1p to the MAP kinase cascade and other target pathways branching off fromRho1p and Pkc1p (462). Thus, it appears that Wsc1p and Mid2p probably directly sense alterations of certain cell wall properties to mediate activation of the cell integrity pathway via the GTP exchange factor Rom2p (and probably Rom1p).
Several lines of evidence suggest that Wsc1p and Mid2p have overlapping but not identical functions. For instance, Mid2p is required for stimulated Slt2/Mpk1p phosphorylation under heat stress, while apparently Wsc1p is not (369). Note that this observation is at odds with the phenotype of the wsc1Δ (heat shock sensitive) and mid2Δ (not sensitive) mutations (see above)! Depolarization of the actin cytoskeleton upon cell wall stress requires Wsc1p but not Mid2p. As mentioned above, Rho1p is localized to places of cell growth, such as in small buds, and colocalizes there with actin patches (24, 485, 659). Upon cell wall stress, such as after heat shock, the actin cytoskeleton becomes depolarized and actin patches become evenly distributed over the cell surface. After a certain adaptation period, polarization of the actin cytoskeleton is reestablished. The cellular distribution of Rho1p and of Fks1p, a subunit of 1,3-β-glucan synthase (Rho1p is also a subunit of 1,3-β-glucan synthase; see below), follows the same pattern (134). Depolarization of the actin system largely requires Rom2p and Wsc1p and therefore seems to depend on sensor-mediated activation of Rho1p. Deletion mutants lacking components of the MAP kinase cascade were unaffected.
Expression of activated alleles of Rho1p and Pkc1p but not of Bck1p or Mkk1p is sufficient to stimulate depolarization. On the other hand, repolarization after recovery from heat shock does require the MAP kinase cascade (134). These data suggest that Wsc1p-Rom2p-Rho1p and Pkc1p stimulate a rapid delocalization of the actin cytoskeleton and of 1,3-β-glucan synthase in order to allow repair of cell wall damage over the entire cell surface; however, for successful repair of damage and/or for monitoring successful repair, which then leads to repolarization of actin, the MAP kinase cascade is needed.
Thus, Wsc1p and Mid2p appear to be cell surface sensors of related though probably not identical structure and function that are required for activation of the cell integrity pathway by cell wall stress. As with genuine membrane-localized osmosensors, such as Sln1p, the mechanism of sensing is not well understood. A deletion analysis of Wsc1p has shown that essentially all distinct domains of the protein are needed for function (344). It has been pointed out that the Wsc1p- and Mid2p-like proteins have properties and structural features in common with mammalian integrin receptors (36, 134). Although mammalian cells do not have a cell wall, they have an extracellular polysaccharide matrix that could be compared with the fungal cell wall. Integrin receptors have a single transmembrane domain and extend to the outside of the cell into the extracellular matrix, where they are thought to receive mechanical stimuli. Like Mid2p and Wsc1p in yeast cells, integrins seem to link such external mechanical stimuli to the cytoskeleton and protein kinase C as well as MAP kinase pathways (for recent reviews, see references 95, 197, 336, and 515). Hence, the structural and functional organizations of mammalian integrin signaling and the yeast cell integrity pathway seem to show similarities. It might be interesting to test whether mammalian integrin receptors or chimeras between them and the yeast proteins could replace Mid2p and Wsc1p function.
Other systems controlling the cell integrity pathway.
In addition to control by cell surface sensors, the cell integrity pathway is also regulated by the TOR pathway, the cell cycle machinery, and the mating pheromone response pathway. The TOR pathway (see also above) controls cellular growth in response to nutrient availability and has been shown to have a role in the reorganization of the actin cytoskeleton (529). Since Rho1p colocalizes with actin patches, a link to the TOR pathway is not surprising. Analysis of TOR2 has identified mutations within this gene that were placed into three different classes with respect to their effects (218). Class A mutations specifically affected actin cytoskeleton organization and resulted in growth arrest at the restrictive temperature as cells with small buds. This class of mutations was suppressed by overexpression of Rom2p, Rho2p, and Pkc1p (218). Additional direct evidence demonstrates that Tor2p functions through Rom2p, probably directly, and the effects are mediated via Pkc1p (36, 218, 219, 530). Thus, Tor2p seems to link nutrient sensing with cell growth and morphogenesis in several different ways: by controlling translational efficiency, protein turnover, actin cytoskeleton organization, and perhaps more (512, 529). Cell wall damage induced either by sodium dodecyl sulfate (SDS) or by various different mutations suppresses the lethality of a temperature-sensitive tor2 allele. This observations suggests that activation of the essential Rho1p protein either by a nutritional TOR-dependent signal or by cell wall stress is required for growth and that Rho1p may be the target protein that integrates these different stimuli (36).
The TOR kinases are related to phosphatidylinositol kinases. STT4 encodes a phosphatidylinositol-4-kinase, which was identified in a screen for mutations that confer tolerance to staurosporine, which also identified PKC1 (STT1) (665, 666). STT4 deletion mutants share a couple of phenotypes with pkc1 mutants, and some of those are suppressed by overexpression of PKC1. However, another multicopy suppressor of stt4, MSS4, does not seem to function in the same pathway as Pkc1p. In fact, MSS4 is also a suppressor of tor2 and seems to suppress more than one class of tor2 mutants (218). Yeast cells also possess a phosphoinositide-specific phospholipase C encoded by PLC1, which hydrolyzes phosphatidylinositols to generate diacylglycerol and inosites, which are second messengers. Overexpression of PLC1 also suppresses certain TOR2 alleles (218). However, mutations in PLC1 cause phenotypes clearly distinct from those of cell integrity pathway mutations, such as sensitivity to hyperosmotic stress (169). The possible link between phosphatidylinositol signaling and the TOR and cell integrity pathways will require further studies.
Cdc28p is a cyclin-dependent protein kinase and the master regulator of the budding yeast cell cycle (380). Double mutants with temperature-sensitive alleles in CDC28 and PKC1 show enhanced temperature sensitivity (363), and overactivation of Cdc28p partially suppresses the temperature sensitivity of a pkc1 allele (399). Activation of Cdc28p stimulates Slt2/Mpk1p (363). The activation of the cell integrity pathway coincides with a Cdc28p-dependent hydrolysis of phosphatidylcholine to generate diacylglycerol, which is known to activate mammalian protein kinase C but whose effect on yeast Pkc1p is controversial (20, 253). In any case, this report suggests that upon the Start of the cell cycle, Cdc28p activates Pkc1p and hence the cell integrity pathway, which then mediates expression of genes encoding enzymes important for cell wall reorganization during bud emergence (363). The link to cell cycle control goes further, since one downstream target of the Slt2/Mpk1p protein kinase is the cell cycle-dependent transcriptional complex SBF with its components Swi4p and Swi6p (353) (see below). SBF, in contrast to the related factor MBF, is specifically involved in controlling expression of genes encoding functions important for cell wall and cell membrane biosynthesis and bud formation (251).
Mating pheromone stimulates the cell integrity pathway, and Slt2p/Mpk1 phosphorylation is observed at a time when mating projections start to develop (73, 671). Mutants defective in the cell integrity pathway lyse during shmoo formation or are defective in cell fusion (104, 106, 463). Pheromone-dependent stimulation of the cell integrity pathway seems to involve Ste20p, the PAK of the pheromone response pathway, which could indicate a direct link between these pathways (671). On the other hand, pheromone-induced activation of Slt2/Mpk1p phosphorylation occurs late after the first stimulation of the pheromone response pathway and seems to depend on the cell surface sensor Mid2p (282, 489). Together with the observation that the caffeine sensitivity of the wsc1Δ mutant is suppressed by overexpression of STE20 (252), this suggests a more complex scenario. Perhaps pheromone-induced reorganization of cell polarity and the initiation of polarized growth towards the mating partner generate a signal that stimulates the sensors of the cell integrity pathway. A possible direct connection between the mating pheromone response pathway and the cell integrity pathway may further adjust the response.
It has been suggested that the cell surface sensors of the cell integrity pathway behave like a signal transducer or a stress-specific actin landmark that both controls and responds to the actin cytoskeleton, similar to the bidirectional signaling between integrin receptors and the actin cytoskeleton in mammalian cells (134). More work is needed to better understand the underlying mechanisms.
Pathways controlled by Rho1p and Pkc1p.
Mutations blocking the MAP kinase cascade confer a more moderate phenotype (osmoremedial at 37°C) than pkc1Δ mutations (osmoremedial at 30°C), which confer a more moderate phenotype then rho1 mutations (deletion is lethal). Hence, Pkc1p has a function(s) in addition to activation of the MAP kinase cascade, and Rho1p has a function(s) in addition to activating Pkc1p.
Four effectors of Rho1p are known to date: Pkc1p (as described above), 1,3-β-glucanase, Bni1p, and Skn7p. Rho1p is a component of 1,3-β-glucanase. Rho1p and the Fks1p subunit of 1,3-β-glucanase copurify and colocalize within the cells at places of active cell growth (75, 375, 485). Rho1p activates the enzyme in a GTP-dependent manner, and Rho1p is required for 1,3-β-glucanase activity (146, 375, 485). Hence, Rho1p probably controls 1,3-β-glucanase in three different ways: by mediating its proper localization, by activating the enzyme, and, via the MAP kinase cascade, by controlling expression of the genes for the catalytic subunits of the enzyme, FKS1 and FKS2 (132, 270, 676).
BNI1 was identified in screens for genes that interact with CDC12 (163), for mutants affected in bud site selection (668), for mutations affecting control of mother cell-specific expression of the HO gene (261), for genes causing lethality when overexpressed (3), and for genes required for pseudohyphal growth (395). Subsequently, the same gene was identified in a two-hybrid screen for proteins that interact with Rho1p (292). Bni1p also interacts with another small G-protein, Cdc42p (161). Bni1p localizes to the tip of growing buds and mating projections (161, 174, 177), and the dynamics of its movements in growing buds have recently been reported (445). Bni1p operates in the same pathway as Spa2p, which was previously known to be involved in determination of cell polarity and to localize to places of cell growth (192, 566).
Screening for mutations that show synthetic lethality with spa2 was one approach that led to the identification of BCK1 (SLK1) (106). In fact, both spa1 and bni1 mutants show synthetic lethality in combination with pkc1 mutants and mutants affected in the MAP kinase cascade (177), suggesting that Rho1p, Spa2p, and Bin1p are part of a pathway that performs a function in parallel to the MAP kinase cascade. rho1 and spa2 mutants are affected in the polarized localization of Bin1p (177). Together with the fact that Rho1p is needed for localization of Pkc1p (18) as well as other observations, this suggests that these proteins form a network of colocalizing proteins. Bin1p shows physical or genetic interaction with numerous other proteins involved in bud formation, cell polarity, and the actin cytoskeleton, including Act1p itself (161). Bni1p is part of a conserved family of proteins that contain so-called FH1 and FH2 (formin homology) domains, which interact with profilin. Indeed, Bni1p interacts with profilin, Pfy1p, an actin-binding protein (161, 244). Hence, the Rho1p-Spa1p-Bni1-Pfy1p connection links cell integrity signaling with the actin cytoskeleton.
Finally, the Sln1p-dependent response regulator Skn7p appears to link the cell integrity pathway to the HOG pathway. Skn7p interacts directly with Rho1p (5). The role of Skn7p will be discussed in more detail below.
Transcriptional targets of PKC signaling.
The MAP kinase cascade of the cell integrity pathway appears to mediate transcriptional responses via two regulators, Rlm1p and the SBF complex (216).
(i) Rlm1p.
The RLM1 gene was identified as a mutation that suppressed the lethality caused by strong overexpression from the GAL1 promoter of a constitutive activated allele of MKK1, Mkk1S386P (641). Rlm1p is a protein of 676 amino acids with three distinct domains. The N-terminal end of the protein contains the putative DNA-binding domain, which is highly similar to the MADS (Mcm1-Agamous-Deficiens-serum response factor) box (142, 641). The C-terminal part of the protein contains the transcriptional activation domain, and a central part of the protein is the target for Slt2/Mpk1p-dependent phosphorylation (642). These two sections of the protein fused to the DNA-binding domain of LexA are necessary and sufficient to mediate Slt2/Mpk1p-dependent transcriptional activation (642).
S. cerevisiae has a protein that is highly similar to Rlm1p, Smp1p. The two proteins are 89% identical within their DNA-binding domains and display very similar although not identical DNA-binding specificities (142). The DNA-binding domains of Rlm1p and Smp1p can form heterodimers in vitro (142), but whether this has any significance in vivo is not known. Smp1p was reported not to perform a redundant function with Rlm1p (142). Interestingly, it was discovered recently that Smp1p controls transcriptional regulation downstream of the HOG pathway. Smp1p appears to be a direct target of Hog1p (F. Posas, 2001, personal communication). If Rlm1p and Smp1p interact in vivo, this could open an interesting possibility for cross talk between the HOG and the cell integrity pathways.
Several lines of evidence demonstrate that Rlm1p is a target for Slt2/Mpk1p. As indicated above, deletion of RLM1 suppresses the growth defect conferred by inappropriate activation of the MAP kinase cascade (641). Moreover, an Rlm1p hybrid protein carrying the transcriptional activation domain of Gal4p, and therefore mediating transcriptional activation independent of Slt2/Mpk1p, suppresses the temperature and caffeine sensitivity of bck1Δ und slt2/mpk1Δ mutants (641). Deletion of RLM1 does not confer the same osmoremedial temperature sensitivity as mutations blocking the MAP kinase cascade, an observation consistent with the idea that several transcription factors mediate Slt2/Mpk1p-dependent responses. However, the mutant is also sensitive to caffeine (641). Remarkably, the rlm1Δ mutant is more resistant to calcofluor white and zymolyase (142), indicative of cell wall alterations. This phenotype is just the opposite of those of mutants affected in the MAP kinase cascade; the reasons are not well understood. However, the mutant used in these studies was also reported to grow to much higher cell densities (142), suggesting that phenotypic analysis was blurred by the presence of the URA3 marker, which is known to affect growth even in rich medium (96). Rlm1p and Slt2/Mpk1p interact in the two-hybrid system (641), and Rlm1p is phosphorylated in an Slt2/Mpk1p-dependent manner (642). Finally, expression of reporter genes driven by either an Rlm1p binding site (142) or a LexA binding site in cells expressing a LexA-Rlm1p fusion protein (642) is fully dependent on Slt2/Mpk1p.
Global gene expression analysis further confirmed that Rlm1p mediates transcriptional responses downstream of the cell integrity MAP kinase cascade (270). Stimulation of the MAP kinase cascade by expression of MKK1S386P from the GAL1 promoter resulted in enhanced expression of 20 genes. Northern blot analysis demonstrated that stimulated expression of all these genes by expression of MKK1S386P required Rlm1p. These genes also show enhanced expression upon overexpression of PKC1 and RHO1 from the GAL1 promoter (507). All genes have sequences resembling the Rlm1p-binding site CTA(A/T)4TAG in their promoter; comparison of these sequence elements revealed possible deviations in this consensus sequence, initially established in vitro (142). The C in the first position in particular shows variability (270). Expression of all 20 genes is also induced by heat shock, which is known to stimulate the cell integrity MAP kinase cascade (272), and again, this stimulation completely requires Rlm1p (270). Several of these genes are also moderately induced by hypo-osmotic shock, as is the gene SLT2/MPK1 for the MAP kinase itself (191). Most interestingly, all 20 genes controlled by the cell integrity MAP kinase cascade and Rlm1p encode proteins either known or predicted to play a role in cell wall metabolism, thereby confirming that the cell integrity MAP kinase pathway plays a crucial role in controlling cell wall assembly.
Interestingly, Rlm1p also interacts with a different protein kinase, Mlp1. This protein is the closest homolog to Slt2/Mpk1p, with 53% identity. However, Mlp1p is not classified as a MAP kinase due to divergence in the activation and the catalytic domains (642). Deletion of MLP1 does not cause any obvious phenotypes, but it enhances the caffeine sensitivity of an slt2/mpk1Δ mutant and overexpression diminishes the caffeine sensitivity of a bck1Δ mutant (642). The role of Mlp1p is unknown.
(ii) SBF (Swi4p and Swi6p).
Another transcription factor apparently targeted by Slt2p/Mpk2p is SBF (205), which is composed of the DNA-binding subunit Swi4p and the regulatory subunit Swi6p (479). SBF is required in the G1 phase of the cell cycle and stimulates the periodic expression of the cyclin genes CLN1, CLN2, PCL1, and PCL2 as well as a large number of genes required for bud emergence, including genes encoding enzymes required for cell wall metabolism (241, 251). The expression of genes encoding proteins important for cell wall maintenance via SBF appears to be controlled by the cell integrity pathway, while expression of the cyclin genes is not (201, 241, 353). Swi4p and Swi6p interact with Slt2/Mpk1p, and they are both phosphorylated by the kinase (353).
Lack of SWI4 causes phenotypes similar to those of mutants defective in the cell integrity pathway, such as an osmo-remedial cell lysis defect (353). SBF components and the cell integrity pathway interact genetically; swi4Δ mutations are synthetically lethal with pkc1 and slt2/mpk1Δ, and swi4Δ mutations are suppressed by overexpression of PKC1 (201, 241, 242, 353). These observations suggest that SBF and the cell integrity pathway control the same process in concert but also via mechanisms independent of each other.
As indicated above, the cell cycle machinery via Cdc28p activates Pkc1p (363). By periodic activation of the cell integrity pathway, Cdc28p appears to mediate enhanced expression of cell wall genes in the G1 phase of the cell cycle via SBF. Hence, it appears that the cell integrity MAP kinase cascade controls cell wall remodeling at the transcriptional level in response to both external stimuli (mediated via cell wall sensors and Rho1p) and cell cycle-dependent stimulation. Both signals seem to converge at Pkc1p. The pathway may then activate transcription via Rlm1p upon cell wall damage and via SBF upon cell cycle stimulation. Indeed, the cell wall genes controlled by Rlm1p and SBF appear to overlap, although they are clearly distinct (241, 251, 270). A plausible explanation for this observation would be that Rlm1p and SBF interact with other proteins to confer signal specificity. Candidates for such proteins are another MADS box factor for Rlm1p and G1-specific cyclins for SBF. The division of tasks between Rlm1p and SBF in controlling cell wall maintenance and remodeling has not yet been studied systematically, but the availability of global expression analysis should certainly help to address this interesting issue.
The genes NHP6A and NHP6B have been identified as multicopy suppressors of an slt2/mpk1 mutant (105, 205), indicating that these HMG (high mobility group) nonhistone proteins could be targets for the MAP kinase cascade. In addition, deletion of both NHP6A and NHP6B causes cell lysis phenotypes similar to those observed in mutants defective in the cell integrity MAP kinase cascade. Deletion of both NHP6A and NHP6B does not further enhance such phenotypes in an slt2/mpk1Δ mutant, consistent with a role of these two proteins downstream of the MAP kinase. However, the Nhp proteins affect expression of many genes that are unrelated to cell integrity and hence are unlikely to be specific targets of the cell integrity pathway.
Calcium-Dependent Signaling: Calcium Pulse and Calcineurin
Calcineurin is a calcium- and calmodulin-dependent serine-threonine protein phosphatase conserved throughout eukaryotic organisms, in which it mediates numerous calcium-dependent signaling events (for a review, see references 22 and 520). Yeast CNA1 and CNA2 encode apparently redundant catalytic subunits that are more than 50% identical to their human counterparts (118, 341). CNB1 encodes the regulatory subunit (119, 305). To study calcineurin function, it can be inactivated by deleting either both CNA1 and CNA2 or CNB1 or by the addition of the anti-inflammatory drug FK506 (341). In S. cerevisiae, calcineurin-dependent signaling is activated by a rise in intracellular Ca2+, which in turn is stimulated by a shift to high temperature (676), hypo-osmotic shock (30), sustained exposure to mating pheromone (119, 170), or an increase in the level of extracellular ions such as Na+, Li+, and Ca2+ (22, 381, 407, 520).
Typical phenotypes caused by inactivation of calcineurin are sensitivity to Na+, Li+, and other ions (381, 407), but not to hyperosmotic stress, as well as inability to recover from pheromone exposure (119, 170). The latter phenotype, as well as several of the conditions known to activate calcineurin, is shared with the cell integrity pathway. Indeed, a block of both the cell integrity pathway (by deletion of PKC1 or SLT2/MPK1) and calcineurin is lethal (190), suggesting that both pathways together are involved in the control of some essential function. In contrast to cell integrity pathway mutants, however, a block in calcineurin function does not appear to cause sensitivity to low osmolarity. The salt sensitivity of mutants lacking calcineurin is suppressed by deletion of the genes PPZ1 and PPZ2, which encode members of the PP family of protein phosphatases, related to mammalian PP1 phosphatases (469). ppz1Δ ppz2Δ mutants themselves share phenotypes with mutants defective in the cell integrity pathway, such as osmoremedial cell lysis (239, 470). In fact, ppz1Δ ppz2Δ and slt2/mpk1Δ mutations are synthetically lethal (635), and overexpression of PPZ1 or PPZ2 can suppress mutations that cause defects in the cell integrity pathway (326). Hence, genetic evidence suggests that calcineurin, the PPZ phosphatases, and the cell integrity pathway perform related and overlapping functions, although the molecular basis of this connection is poorly understood.
The calcineurin phosphatase exerts its effect by dephosphorylating and thereby activating nuclear translocation of the Crz1p zinc finger-containing transcription factor (371, 576). Crz1p binds to a promoter element called the calcineurin-dependent response element (576). The role of calcineurin in ion homeostasis is effected by controlling PMC1, PMR1, and PMR2, which encode P-type ATPases that mediate transport of calcium ions between different cellular compartments (116), ENA1 (213, 365, 381), and TRK1 and TRK2, which encode K+ uptake systems. Control of TRK1 and -2 appears to be independent of Crz1p (539). The role of calcineurin in cell wall metabolism is mediated by the Crz1p-dependent control of expression of FKS2 (376, 576, 676). The latter observation provides an explanation for the synthetic lethality caused by blocking both the calcineurin and the cell integrity pathways. Expression of FKS1 requires an active cell integrity pathway (241), while expression of FKS2 is controlled in a complex way by both the calcineurin and the cell integrity pathways (376, 676). Fks1p and Fks2p are 88% identical, and either of the two β-1,3-glucan synthases is required for enzyme activity and hence for growth (376).
Thus, although defects in calcineurin signaling do not cause an apparent phenotype directly related to osmotic stress, this pathway contributes to recovery from NaCl-induced osmotic stress and interacts with pathways that mediate cell surface remodeling in response to external signals, such as turgor increase and mating pheromone.
Skn7p: Integrating Input from Different Osmosensing Pathways?
Skn7p (70), also identified as Pos9p (297) and Bry1p (392), is the only S. cerevisiae protein apart from Sln1p and Ssk1p that contains a response regulator domain. Skn7p (622 amino acids) shows extensive structural and also sequence similarity to the heat shock transcription factor Hsf1p, especially within the N-terminal DNA-binding domain (amino acids 87 to 151) and a coiled-coil homology region in the center of the protein (222 to 303); the response regulator domain is located between 378 and 497. As in Hsf1p, a transcriptional activation domain is located at the C-terminal end (497 to 622) (59, 69, 487).
Skn7p seems to be predominantly localized in the nucleus (69, 487). The protein has been shown to bind to the promoters of TRX2 (encoding thioredoxin) (390), TSA1 (a thiol-specific antioxidant) (324), and OCH1 (encoding a mannosyltransferase) (338). Different DNA-binding sites have been reported for Skn7p, which may reflect its interaction with other DNA-binding proteins affecting its DNA-binding specificity. Such cooperation may occur with the heat shock transcription factor Hsf1p (487), the calcineurin-dependent transcription factor Crz1p (648), the cell cycle transcription factor Mbp1p (59), and the oxidative stress transcription factor Yap1p (91, 324, 390). In the TRX2 promoter, the Skn7p site of 23 bp contains an HSE (heat shock element)-like element, and Skn7p binds this site in vitro, suggesting that it might have a DNA-binding specificity similar to that of Hsf1p (390). Careful analysis of the OCH1 promoter suggests that neither SCB-like nor HSE-like sequences determine Skn7p binding. Instead it appears that a repeat of the sequence ATTTGGCC(T)GGG(C)CC is necessary and sufficient to mediate Skn7p-dependent gene regulation (338). The exact mode of DNA interaction of Skn7p remains to be determined.
Skn7p functions as a transcriptional activator in corresponding reporter systems when fused to the DNA-binding domain of LexA or Gal4p (69, 297). For this reason, because of the presence of a response regulator domain and because of its involvement in a number of important but seemingly unrelated cellular processes, the protein has attracted considerable interest. Skn7p has been implicated in cell cycle control (59, 392), the oxidative stress response (90, 91, 267, 296, 297, 324, 325, 337, 390, 516), the heat shock response (487), cell wall metabolism (70, 282, 338), and nitrogen starvation-induced diploid filamentous growth (349). Genetic and physical interactions link Skn7p not only to the HOG pathway (164, 281, 337, 591) but also to cell cycle-dependent transcription (59, 391, 392), the cell integrity pathway (5, 69), the heat shock transcription factor (487), the Yap1p oxidative stress transcription factor (91, 324, 390, 516), and calcineurin-dependent signaling (648).
What could be the common denominator behind the different phenomena that are affected by Skn7p, and what is the molecular mechanism with which Skn7p affects expression of genes controlled by numerous other transcription factors? As discussed below, several observations indicate that Skn7p controls cellular responses to cell swelling and hypo-osmotic signals leading to adjustments in cell wall metabolism, control of cell proliferation, and stress responses (59, 337, 338, 591); recent data suggest that at least some effects on transcriptional regulation could be due to stabilization of other transcriptional regulators from protein degradation (648). Despite the involvement in so many different cellular processes, Skn7p is not an essential protein, suggesting that it participates in modulating and fine tuning responses and/or that it is involved in partially redundant systems (59, 69, 648). Strong overexpression of SKN7 from a GAL1 promoter, however, is lethal (5, 59, 392), resulting in the accumulation of swollen unbudded cells (59), consistent with defects in osmoregulation and cell wall metabolism.
Skn7p is controlled by the Sln1p-Ypd1p osmosensing phosphorelay system and osmotic signals.
The response regulator domain contains the conserved, phosphorylatable aspartate residue in position 427. Using fusion proteins of Skn7p with the DNA-binding domain of Gal4p or LexA and suitable reporter genes driven by Gal4p- and LexA-binding sites, the importance of this residue in modulating Skn7p activity was investigated. Indeed, mutation of the putative phosphorylation site Asp427 to alanine, asparagine, or arginine diminished promoter activity, while replacement of Asp427 with glutamic acid, mimicking phosphorylation, enhanced it (69, 281, 297).
There is some uncertainty about the importance of Skn7p phosphorylation with respect to its role in the oxidative stress response. Entian and colleagues found that mutants with alanine or arginine in position 427 did not complement the sensitivity to oxidative stress of an skn7Δ mutant, while the mutant carrying glutamic acid in this position did (297). Johnston and colleagues, on the other hand, reported that Skn7p carrying asparagine in position 427 behaves like the wild-type protein in a very similar test (338, 390). These variable interpretations may be due to different strain backgrounds or the specific experimental conditions or constructs used; the view that the involvement of Skn7p in oxidative stress responses is independent of the receiver domain appears more widely accepted at this point (338). Control via the receiver domain might have a modulating rather than an essential function for some of the roles of Skn7p. In any case, Skn7p is certainly controlled by more than one mechanism, as suggested for instance by its genetic link to both the HOG and the cell integrity pathways (see below) and by the fact that Skn7p is phosphorylated at multiple sites (69).
Several lines of evidence confirm that the Skn7p response regulator domain is controlled by the only yeast histidine kinase, Sln1p, as well as by the phosphotransfer protein Ypd1p. Direct evidence that Skn7p is a substrate for the Sln1p-Ypd1p system was obtained in vitro; Skn7p is phosphorylated in a Sln1p- and Ypd1p-dependent way (256, 337). In vivo, the activity of a reporter gene controlled by a LexA-Skn7p construct is diminished in sln1Δ and ypd1Δ mutants, which were made viable by additional deletion of SSK1 (281). In this system, reporter gene activity was reduced to the same level as in an sln1Δ or ypd1Δ mutant when Asp427 was replaced by asparagine, and it was enhanced to wild-type levels even in sln1Δ and ypd1Δ mutants when Asp427 was replaced by glutamic acid. The data also show that the fusion protein has significant basal activity even in its unphosphorylatable form. Moreover, reporter constructs driven by the TRX2 or OCH1 promoter respond to the presence of activated alleles of Sln1p (sln1*, Table 1) in an Skn7p- and Asp427-dependent way (337, 338).
To study Sln1p function, Fassler and colleagues have been using artificial reporter genes that contain Mcm1p binding sites as activating promoter elements. The use of an Mcm1p-dependent reporter goes back to the isolation of sln1 mutations that affect reporter activity (667). The precise way in which Skn7p affects this promoter system is not understood, and Skn7p-dependent activation is observed even when the Mcm1p binding site is mutationally altered (164). But the system has proven useful for studying the control of Skn7p by Sln1p and several aspects of Sln1p function (164, 337, 591).
Activity of the Mcm1p-dependent promoter is strongly diminished in an sln1Δ mutant and elevated in cells carrying activated sln1* alleles. These activated alleles, some of which map to the Sln1p receiver domain, apparently shift the equilibrium between unphosphorylated and phosphorylated Sln1p to the latter (164). As one would expect for such mutants, they mediate strong inhibition of the HOG MAP kinase cascade and cause sensitivity to high osmolarity and strongly diminished Hog1p phosphorylation after osmotic shock (164). Activation of the Mcm1p-dependent promoter system by the activated Sln1p alleles requires Skn7p. No sln1*-dependent reporter gene activation was observed when Asp427 was mutated to asparagine, and reporter gene activity was increased to intermediate levels that did not respond to activated Sln1p when Asp427 was replaced by glutamic acid (337). Very similar observations were recently made with the authentic Skn7p-dependent promoter of the OCH1 gene, further strengthening the notion that Sln1p-Ypd1p modulate Skn7p activity via Asp427 (338).
Hence, the Sln1p-Ypd1p phosphorelay system controls two response regulator proteins, Ssk1p and Skn7p. How are those related to each other? The phenotypes conferred by deletion of SSK1 and SKN7 are fundamentally different, suggesting that the two proteins are not part of the same pathway. Instead, the HOG pathway branches below Sln1p-Ypd1p: one branch controls the HOG pathway MAP kinase cascade via the response regulator Ssk1p, and the other branch confers different functions via the response regulator Skn7p (Fig. 9). On the other hand, SKN7 shows genetic interaction with the HOG pathway. Deletion of SKN7 causes a synthetic growth defect with deletion of PTC1, which encodes the most important serine/threonine phosphatase inhibiting Hog1p activity (281). The synthetic growth defect is suppressed by deletion of HOG1 or PBS2 and by overexpression of PTP2 or PTC2 (281). This observation suggests that overactivation of the HOG pathway in combination with deletion of SKN7 is detrimental and has been interpreted as evidence that Skn7p modulates HOG pathway activity (281). However, this observation could equally well indicate that Skn7p functions in a different signaling system with opposite function to the HOG pathway.
FIG. 9.
Model for the role of Skn7p. How oxidative stress or Rho1p activates Skn7p is not known. It is also not known where in the cell interaction between Skn7p and the Sln1p-Ypd1p system or the Rho1p GTP-binding protein occurs.
As outlined above, Sln1p-Ypd1p is activated by low osmolarity, leading to inhibition of the HOG pathway and activation of Skn7p. While in this way the MAP kinase cascade is activated by high osmolarity (cell shrinking), Skn7p has the potential to mediate responses to cell swelling. Another pathway known to respond to cell swelling is the cell integrity pathway (125, 205).
Skn7p interacts with the cell integrity pathway.
Indeed, several lines of genetic and molecular evidence link Skn7p to the cell integrity pathway. Skn7p interacts directly with Rho1p, the G-protein that functions upstream of protein kinase C (5). Where in the cell this interaction occurs is not known; given the fact that Skn7p is a nuclear protein (69) and Rho1p is located at the cell surface (24, 485, 659), this is certainly an interesting question. Furthermore, moderate SKN7 overexpression from a multicopy plasmid suppresses the inability of a pkc1Δ mutant to grow without osmotic support, while an skn7Δ pkc1Δ double mutation is lethal, even in the presence of osmotic support (69). In addition, a screen for multicopy activators of a LexA-Skn7p-dependent reporter gene identified MID2, which encodes a type I transmembrane protein. Genetic analysis places this protein upstream of the cell integrity pathway as a possible sensor (see above). In addition to the evidence that links Skn7p to cell wall metabolism (70, 282), a key aspect controlled by the cell integrity pathway, these data suggest that Skn7p functions in parallel to the cell integrity pathway, probably branching off this pathway downstream from Rho1p but upstream of Pkc1p (69, 392).
Could Skn7p be a master regulator of responses to hypo-osmolarity?
Skn7p seems to function opposite to the pathway responding to high osmolarity and in parallel to a pathway responding to low osmolarity. Hence, is its function controlled by an osmotic downshift? This interesting question has so far not been addressed directly; however, mutations that cause increased intracellular glycerol levels and hence probably mimic a hypo-osmotic situation enhance the activity of the Mcm1p-dependent reporter and of OCH1 expression in an Skn7p- and Asp427-dependent manner (164, 338, 591). It appears that the Sln1p-Ypd1p-Skn7p system is a genuine two-component (or phosphorelay) system mediating osmotic responses to a transcriptional regulator.
This view on Skn7p function leads to a number of predictions and interesting questions. Does Skn7p actually mediate transcriptional responses to low osmolarity? Apparently, there is no pronounced transcriptional response when shifting cells from high to low osmolarity, as studied by global expression analysis (191). However, the hypo-osmotic shock employed in this study was rather mild, and the use of different growth conditions as well as of the fps1Δ mutant, which fails to export glycerol upon hypo-osmotic shock (see below), may reveal a more pronounced response.
Genetic evidence links Skn7p to the upstream part of the cell integrity pathway. Hence, is Skn7p activity modulated by mechanisms in addition to phosphorylation at Asp427? Indeed, the protein seems to be phosphorylated on various sides; the overall phosphorylation state of Skn7p does not seem to depend on Pkc1p, but this is not so surprising because genetic evidence places Skn7p parallel to Pkc1p (69). Research has so far focused on the modulation of Skn7p activity by Sln1p-Ypd1p and via the response regulator domain. Other proteins that apparently affect Skn7p function have been identified in two genetic screens. fab mutants are defective in Gal4p-Skn7p-dependent activation of a reporter gene. Only FAB7, an essential gene, has been characterized to some extent (267). Another screen for activation of a reporter gene driven by the LexA-Skn7p fusion by multicopy plasmids has revealed a number of ASK genes; so far only MID2 (282), encoding a possible sensor of the cell integrity pathway (see above), and ASK10 (446), a transcription factor, have been characterized to some extent. Apparently the screens for Skn7p modulators lead to a fairly rich harvest, and the challenge is to find those genes whose products directly affect Skn7p function.
Why is the Sln1p-Ypd1p-Skn7p system designed as a phosphorelay system? Skn7p seems to be located in the nucleus under all conditions (390, 487). Possibly, Ypd1p serves to transfer the signal from the plasma membrane to the nucleus, as was recently observed for phosphotransfer proteins of a cytokinin-responsive phosphorelay system from Arabidopsis thaliana (240). Possible dynamics of Ypd1p localization have so far not been reported. Finally, considering the evidence that Skn7p is a regulator controlled by hypo-osmotic signals, how could we explain the role of the protein in cell wall metabolism, heat shock response, calcineurin signaling, cell cycle control, and oxidative stress response?
Could coordination of cell wall biogenesis be the common denominator of Skn7p function?
Although many of the available studies on Skn7p have focused on other phenomena, SKN7 was originally isolated as a suppressor of a mutation causing defects in cell wall assembly (70). KRE9 encodes a cell surface glycoprotein which is involved in glucan biosynthesis. kre9 mutants have drastically reduced levels of cell wall β-1,6-glucan and grow very poorly, form multiple buds, and fail to generate mating projections (68, 541). SKN7 did not suppress other mutations in the pathway leading to β-1,6-glucan, indicating a rather specific effect (70). Mutants lacking SKN7 are sensitive to hygromycin, a drug to which many cell wall-defective mutants are sensitive (648).
The lethality caused by strong overexpression of SKN7 is probably due to perturbations of cell wall assembly and is partially rescued by overexpression of genes encoding components of the cell integrity pathway (392). Titration of Rho1p could be the basis for this effect of Skn7p overproduction. As outlined above, Skn7p interacts with Rho1p, which in turn is an essential subunit of 1,3-β-d-glucan synthase (375, 485). Ample evidence shows that perturbations of the cell wall affect Rho1p activity and that Rho1p controls cell wall assembly by Pkc1p-dependent and -independent mechanisms (36, 100, 132, 134, 282, 369, 462; reviewed in references 75, 133, and 205). The fact that Skn7p interacts with Rho1p genetically and physically (although according to present knowledge both proteins appear to localize to different compartments) is additional strong evidence for a role of Skn7p in cell wall biogenesis.
The HOG pathway also seems to affect cell wall biogenesis, as reported recently (16). The cell wall plays a crucial role in maintaining turgor pressure, and hence cell wall biogenesis and osmoregulation, specifically turgor control, must be closely coordinated processes. The evidence that Skn7p is apparently controlled by sensors of both the HOG pathway and the cell integrity pathway makes Skn7p an excellent candidate for a regulator that coordinates osmoregulation and cell wall biogenesis. Assuming that coordination of turgor-controlled cell wall assembly is the primary role of Skn7p, can the other effects that involve Skn7p by related to this primary function?
Recently it has been shown that Skn7p interacts with Hsf1p and binds to HSE. Skn7p participates in the expression of genes encoding heat shock proteins such as SSA1, HSP12, HSP26, and HSP104. Skn7p is required for the stimulated expression of these genes under oxidative stress, and in the case of SSA1 it is also needed for normal expression both under basal conditions and after heat shock. The skn7Δ mutant is sensitive to acute heat stress (487). Although the role of Skn7p in the expression of heat shock protein genes seems to be more related to mediating oxidative stress responses, the link to heat stress is potentially interesting. Heat stress has been shown to activate the cell integrity pathway (272), probably because high temperature affects the cell wall and hence turgor control, linking Skn7p to these processes.
Skn7p controls calcineurin signaling by binding to both the calcineurin phosphatase and the calcineurin-dependent transcriptional regulator Crz1p (648). SKN7 interacts genetically with CNB1, which encodes the regulatory subunit required for the activity of calcineurin. Both mutants are sensitive to hygromycin, and the double mutant is even more sensitive. Both mutants are insensitive to low levels of hydrogen peroxide, while the double mutant is sensitive. Hence, Skn7p and calcineurin appear to have parallel functions. Furthermore, deletion of SKN7 diminishes expression of genes controlled by calcineurin and its transcription factor Crz1p, such as PMC1, FKS2, and a reporter driven by a calcineurin-dependent response element (648). Apparently, Skn7p mediates its effects by stabilizing Crz1p. Calcineurin and Skn7p have very similar genetic interactions with the cell integrity pathway, suggesting that both operate in parallel to the cell integrity pathway. The observation that Skn7p, together with Crz1p, participates in expression of cell wall biogenesis genes provides further evidence for the role of Skn7p in coordinating events in cell surface assembly.
Mbp1p is the DNA-binding component of the MluI cell cycle box (MCB) element binding factor complex (MBF), also called DSC1 (DNA synthesis control). Together with SBF, which binds to SCB, MBF controls expression of genes that are required for the execution of Start at the G1 to S transition of the cell cycle. Target genes controlled by SCB and MCB include the G1 cyclins CLN1 and CLN2 as well as a large number of genes encoding functions that are required right after Start to initiate DNA replication, bud emergence, and cell wall biogenesis (reviewed in reference 380). The DNA-binding components of SBF and MBF, Swi4p and Mbp1p, respectively, are related in sequence, and both complexes have Swi6p as a regulatory component. SBF and MBF seem to have partially redundant functions, but elimination of both complexes in double swi4Δ swi6Δ and swi4Δ mbp1Δ mutants is lethal.
The observation that, although Swi6p is part of both complexes, it is not an essential gene prompted Johnston and colleagues to screen for multicopy suppressors that overcome the requirement for SBF and MBF. This screen identified BRY1/SKN7. Overexpression of SKN7 restores CLN1 and CLN2 expression, suggesting that Skn7p participates in controlling expression of these genes (391, 392). Mbp1p and Skn7p interact genetically; deletion of the one suppresses the lethality conferred by strong overexpression of the other (59). In addition, Skn7p is required for overexpression of MBP1 to suppress lethality and restore expression of CLN1 and CLN2 of an swi4ts swi6Δ double mutant. Mbp1p and Skn7p interact physically in vitro and in the two-hybrid system, although Skn7p does not appear to be part of the core MBF complex (59). Deletion of MBP1 also shows a synthetic defect, although not lethality, in combination with pkc1Δ, indicating a link to the cell integrity pathway. Moreover, separate studies have shown that the cell integrity pathway controls Swi4p- and Swi6p-dependent gene expression (201, 216, 241, 353), further linking Mbp1p and Skn7p to the same genetic system.
The role of Skn7p in the transition from G1 to S was further defined by genetic analysis (59). Deletion of SKN7 as well as MBP1 suppresses the growth defect conferred by overexpression of a dominant negative allele of CDC42, which encodes a G-protein involved in different pathways (see above) (264, 287) and is required for actin polarization and cell polarity. Moreover, high-copy expression of SKN7 aggravates the growth defect (reduces the restrictive temperature) of a cdc42-1 mutant as well as a bem1Δ mutant, which is also defective in cell polarity.
Thus, Skn7p and Mbp1p seem to form a functional complex for transcriptional regulation and as such may be involved in cell polarization and bud emergence at the G1 to S transition. Obviously, bud emergence requires active cell wall breakdown and biosynthesis. During polarized growth and hence active cell wall metabolism, such as during budding, pseudohyphal development, and growth of mating projections, cells are likely to be exquisitely sensitive to osmotic changes and have to monitor turgor pressure constantly. This is illustrated, for instance, by the fact that the cell integrity pathway is stimulated during polarized growth (377) or that the Fps1p glycerol export channel, which is necessary for adjustment to hypo-osmotic shock (see below), is required for cell fusion during mating (463). Skn7p appears to be part of this monitoring system.
The link between the role of Skn7p in osmoregulation/cell wall assembly and oxidative stress is less apparent. Note, again, that it appears that the role of Skn7p in oxidative stress responses is independent of the receiver domain, and hence Sln1p-Ypd1p. Skn7p has been isolated as POS9 in a screen for mutants sensitive to oxidative stress (297). As a fusion to Gal4p, Skn7p has been shown to confer oxygen-dependent gene expression on a GAL1 promoter (91). Skn7p binds to promoters that are controlled by oxidative stress, such as TRX1 (390) and TSA1 (324). In addition, Skn7p is required for normal expression of a large set of proteins whose production is stimulated under oxidative stress (324). Thus, Skn7p plays an important and undisputed role in defense from oxidative damage.
The oxidative stress response is also controlled by another transcription factor, Yap1p (133, 200, 301, 577; reviewed in references 85 and 255), which controls an even larger set of genes (140, 324) and is involved in responses to a variety of compounds that cause or are thought to cause the generation of reactive oxygen species within the cell. The role of Skn7p, however, can apparently be defined more narrowly. First of all, skn7 mutants are apparently specifically sensitive to peroxides and not to, for instance, diamide or cadmium (59, 297, 324, 325, 390, 630), suggesting that Skn7p might play a more specific role in the defense from peroxides imposed from the environment rather than reactive oxygen species generated through cellular metabolism. Consistent with this notion, Toledano and colleagues could grossly classify proteins whose production was induced by hydrogen peroxide into two categories, Yap1p- and Skn7p-dependent proteins, which included proteins for the detoxification of peroxides, and Yap1p-dependent but Skn7p-independent proteins, which included metabolic enzymes involved in redox metabolism, probably important for the generation of NADPH for metabolism (324). Hence, Skn7p seems to be involved in the immediate defense from oxidative damage rather than a corresponding metabolic adjustment.
These observations may then provide a link to cell wall metabolism, because peroxides, exposed to the cell, may damage the cell wall (suggested in reference 59). Interestingly, Skn7p seems to have a role in supporting Yap1p in expression of its target genes. Deletion of SKN7 generally causes a more moderate sensitivity to peroxides than deletion of YAP1, and additional deletion of SKN7 does not aggravate the sensitivity of a yap1 mutant (324). In addition, while some 30 proteins were found to depend on Yap1p, only about half of those required Skn7p for full expression and only two proteins were reported to be Skn7p dependent but Yap1p independent. These observations could fit into a picture where Skn7p assists Yap1p in defense from oxidative damage, for instance, when damage of the cell wall occurs. This aspect has so far not been addressed directly. It is noteworthy to mention in this context that there is substantial overlap between the responses to osmotic and oxidative stress in S. cerevisiae (45, 191, 195, 501, 502). The HOG pathway has even been reported to be stimulated by hydrogen peroxide and diamide (562), while other data have shown that this is not the case (533).
More work is clearly needed to understand the role of Skn7p. Critical issues concern elucidation of the relevance of both genetic and physical interactions and the places in the cell where those occur, as well as the precise mechanisms that control Skn7p function.
Is there a common mechanism by which Skn7p mediates its effects?
Recently, Williams and Cyert (648) showed that the role of Skn7p in calcineurin-dependent signaling can probably be explained by Skn7p-dependent stabilization of Crz1p, the calcineurin-dependent transcription factor. It is tempting to speculate that Skn7p may operate in such a way in other systems, too. It is noteworthy that in all systems where Skn7p has been shown to play a role, it cooperates with or essentially “assists” a different transcription factor, Crz1p, Yap1p, Mbp1p, or Hsf1p. Clearly more work is needed to better understand the role of Skn7p in transcriptional regulation and its possible role in coordinating different aspects of turgor control, growth, and cell surface assembly.
Stress-Inducible Sty1 MAP Kinase Pathway in S. pombe
The fission yeast S. pombe possesses a signaling pathway whose known components display strong similarity to the S. cerevisiae HOG pathway (reviewed in reference 205). The Sty1 pathway (Table 5, Fig. 10), like the HOG pathway, is stimulated by osmotic shock (130, 384, 548) and controls expression of genes important for osmoadaptation, such as the gpd1+ gene (2), whose product is involved in glycerol biosynthesis. However, with respect to pathway control and architecture, there are a number of important and very interesting differences. Most significantly, while the HOG pathway is apparently specifically responsive to osmotic shock (533), Sty1 phosphorylation and Sty1-dependent responses can be stimulated by a whole range of stress conditions, including osmotic upshift (384, 548, 553), heat shock (130, 522, 545, 553), exposure to hydrogen peroxide or other oxidative stress agents (130, 522, 545, 553), UV light and alkylating agents (183), nitrogen starvation (550), carbon starvation (553), and the protein biosynthesis inhibitor anisomycin (545). Mutants defective in signaling via Sty1 also show sensitivity to the conditions that stimulate the pathway. These observations suggest that the Sty1 pathway has an apparently different role in the physiology of fission yeast than the HOG pathway in budding yeast. The multiple-stress-responsive character of the pathway further suggests that both the sensing system(s) and the transcriptional output of the Sty1 and HOG pathways differ. As will be discussed below, this seems to be the case.
TABLE 5.
Corresponding regulators in the fission yeast Sty1 and the budding yeast HOG pathways
| Budding yeast protein | Fission yeast homolog | Function | % Identity/ % similarity | Reference(s) |
|---|---|---|---|---|
| Hog1p | Sty1/Spc1/Phh1 | MAP kinase | 82/91 | 278, 384, 548 |
| Pbs2p | Wis1 | MAPKK | 44/58 | 639 |
| Ssk2p and Ssk22p | Wis4/Wik1/Wak1 and Win1 | MAPKKK | 30/50 | 162, 522, 523, 553, 639 |
| Ssk1p | Mcs4 | Response regulator | 42/58 | 107, 545, 553 |
| Ypd1p | Mpr1/Spy1/Sph1 | Phosphorelay protein | 48/75 | 21, 416 |
| Ptp2p | Pyp1 | Phosphotyrosine phosphatase | 27/41 | 385, 443 |
| Ptp3p | Pyp2 | Phosphotyrosine phosphatase | 21/37 | 385, 443 |
| Ptc1p | Ptc1 | Phosphothreonine phosphatase | 46/66 | 549 |
| Ptc2p | Ptc3 | Phosphothreonine phosphatase | 50/68 | 549 |
| Ptc3p | Ptc2 | Phosphothreonine phosphatase | 48/65 | 549 |
| Yap1p | Pap1 | Transcriptional activator, oxidative stress | 26/41 | 600, 601 |
| Sko1/Acr1p | Atf1 | Transcriptional regulator, osmotic stress | 25/37 | 550, 586, 647 |
| Skn7p | Prr1 | Response regulator, transcriptional regulator | 32/49 | 434 |
FIG. 10.
Model for the fission yeast Sty1 pathway. Mak2 and Mak3 seem to be sensors for oxidative stress and may form a phosphorelay system together with Mpr1 and Mcs4. Sensors for other stress conditions have not been identified yet.
Another interesting difference between the HOG and the Sty1 pathways concerns a demonstrated role of the latter in fission yeast cell cycle control and in developmental decisions. Mutants with a block in the Sty1 pathway are characterized by a defect in passing through the G2-mitosis transition of the cell cycle, and the delayed onset of mitosis results in cells twice as long as wild-type cells (384, 548, 639). On the other hand, mutants with an overactive Sty1 pathway or in which the pathway has been activated, for instance, by high osmolarity, appear to enter mitosis prematurely and hence are much shorter than wild-type cells (384, 385, 551, 639). Therefore, the Sty1 pathway is an activator of mitosis. No such role in affecting cell size has been reported for the budding yeast HOG pathway. However, it should be noted that effects on cell size can be monitored much more easily in fission yeast, which grows in length. Moreover, the initial identification of Sty1 pathway components emerged from genetic analysis of the cell cycle and cell size control (639) and hence with a totally different emphasis than studies on the HOG pathway.
Some observations do link the budding yeast HOG pathway to cell cycle control, and here specifically to the transition from G2 to mitosis. Osmotic shock has been shown to result in a temporary arrest in G2; this arrest requires Hog1p (13, 62). Moreover, the Rck2p protein kinase, a cytosolic target of Hog1p, has initially been identified in both fission and budding yeasts as part of a G2 checkpoint control system (40, 121, 490). How these observations relate to each other remains to be investigated.
The fission yeast Sty1 pathway is also required for mating of cells of opposite mating type. Fission yeast mates upon nitrogen starvation; cells arrest in G1 when nitrogen limits growth and activate expression of the gene encoding the Ste11 transcription factor. This factor then initiates production of pheromone and components required for pheromone response in order to undergo mating (658). The Sty1 pathway is required for starvation-induced G1 arrest and expression of the ste11+ gene, and hence the pathway is needed to initiate this developmental program (278, 550). Mating of budding yeast is apparently unrelated to specific medium conditions; rather, meiosis is initiated by nitrogen starvation, but the HOG pathway has not been reported to affect any of those processes (99, 572).
Several features, especially the responsiveness to multiple stress conditions, seem to make the Sty1 pathway more similar to mammalian stress-activated pathways than is the case for the budding yeast HOG pathway (130).
Known components and architecture of the Sty1 pathway.
The stress-activated MAP kinase Sty1 is 82% identical to Hog1p. The sty1+ gene (also called spc1+) was identified as a suppressor of the pyp1 pyp2 double mutant lacking two phosphotyrosine phosphatases, the apparent counterparts of budding yeast Ptp2p and Ptp3p (384, 548). Single pyp1 and pyp2 mutants display diminished cell size due to premature entry into mitosis, while the double pyp1 pyp2 mutant is inviable and arrests growth as swollen, spherical cells (384, 385). Mutation of sty1+ suppresses this lethality and causes cells to divide at twice their normal length in both a pyp1+ pyp2+ wild-type and mutant background. This finding is consistent with the notion that lack of these two phosphatases causes drastic overactivation of the Sty1 kinase (384). Note that in budding yeast deletion of the genes encoding the phosphotyrosine phosphatases, PTP2 and PTP3, does not confer a lethal phenotype and does confer only mild Hog1p activation, while deletion of PTP2 plus PTC1 (which encodes a phosphothreonine phosphatase) causes lethality (254). This suggests qualitative differences in the control of the activity of the MAP kinases in the two yeasts.
The sty1+ gene was also identified as phh1+ (S. pombe homolog of Hog1p) (278). As mentioned above, deletion of the sty1+ gene causes multiple stress phenotypes, i.e., inability to grow in high-osmolarity medium, sensitivity to heat shock, sensitivity to oxidative stress, sensitivity to UV light, and an inability to arrest in G1 under nitrogen limitation, leading to sensitivity to starvation and an inability to mate (129, 130, 278, 384, 548, 550), to list the most relevant phenotypes.
These phenotypes are shared with wis1 mutants. wis genes were identified in a screen for multicopy suppressors of the conditional lethal phenotype of a win1-1 wee1ts cdc25ts triple mutant, affected in cell cycle progression (162, 638, 639). Subsequently, wis1+ was isolated in the same screen for suppressors of the pyp1 pyp2 mutant that identified sty1+. wis1+ encodes a MAPKK. The phenotypes conferred by wis1 and sty1 single mutations and by the wis sty1 double mutations are essentially identical, and phosphorylation of Sty1 under various stress conditions requires Wis1 (384, 548). Moreover, deletion of sty1+ suppresses the growth defect conferred by overexpression of wis1+ (522, 546, 548), confirming that both kinases operate within the same pathway and that Wis1 functions upstream of Sty1.
The closest homolog of Wis1 is budding yeast Pbs2p. The sequence conservation is, however, largely restricted to the C-terminal kinase domain. Wis1 does not seem to possess an SH3-binding domain, with which Pbs2p interacts with the transmembrane protein Sho1p. Components of a pathway branch that could resemble the Sho1-Ste11-Pbs2 signaling module of the budding yeast HOG pathway have not been reported in fission yeast, but, as will be discussed below, there is ample evidence that different branches control the Sty1 pathway.
As in the Sln1 branch of the budding yeast HOG pathway, two MAPKKKs appear to operate upstream of the Wis1 MAPKK: Wis4 (also called Wik1 and Wak1) (545, 553) and Win1 (546). Wis4 and Win1 are 38% identical and most closely related to budding yeast Ssk2p and Ssk22p. Like budding yeast Ssk2p/Ssk22p, Wis4 and Win1 also appear to perform redundant functions, but in contrast to the situation in budding yeast, deletion of wis4+ or win1+ individually confers moderate stress sensitivity as well as slight cell elongation. A double wis4 win1 mutant is as heat sensitive and osmosensitive as a wis1 or sty1 mutant and fails to show stimulation of expression of Sty1 target genes under different stress conditions (546), suggesting that they are the only MAPKKKs controlling wis1 under these conditions.
mcs4+ was initially identified as one of six suppressor mutations that rescued the mitotic catastrophe of a cdc2-3w wee1-50 mutant (388). mcs4+ encodes a response regulator that is structurally and functionally related to budding yeast Ssk1p (107, 545, 553). mcs4+ expressed in budding yeast can replace SSK1 and vice versa (545). Loss of Mcs4 affects the cell cycle in a similar way as loss of Sty1, Wis1, or Wis4 plus Win1, causing enlarged cells. The mcs4 deletion mutant also shares most phenotypes with mutants affected in the MAP kinase cascade although generally the phenotypes are less pronounced (107, 416, 545, 553), suggesting that additional proteins control the MAP kinase cascade. The observation that wis4 win1 and mcs4 mutants of fission yeast have clear phenotypes while the corresponding ssk2Δ ssk22Δ and ssk1Δ mutants from budding yeast do not suggests that fission yeast does not have a partially redundant branch like the budding yeast Sho1 branch for activation of the MAPKK or, if it exists, that it performs a somewhat different function.
Signaling to the Sty1 pathway.
The fact that a range of different stress conditions can stimulate the Sty1 pathway makes elucidation of the underlying mechanisms most exciting. How can a single pathway be activated by multiple stress conditions? For the general stress response in budding yeast, it is speculated that a variety of stress conditions may cause a common internal signal. The main alternative to such a scenario is a number of different sensors dedicated to each type of stress that control the activity of the same pathway. Although far from solved, some recent findings indicate that indeed the Sty1 pathway is controlled by different specific input systems.
Two sensor histidine kinases, Mak2 and Mak3, appear to specifically monitor oxidative stress (72). Mak2 and Mak3 are only 25% identical to each other but share an overall structural organization. Both proteins are very large, more than 2,300 amino acids. Most importantly, they are apparently not functionally identical to Sln1p and they lack transmembrane domains or membrane anchors and hence appear to be cytosolic proteins. A third protein, Mak1, shares some features with Mak2 and Mak3 but is about 700 amino acids shorter and lacks a putative serine/threonine protein kinase domain present in the N-terminal portion of Mak2 and Mak3. The function of this domain is unknown. All three proteins possess, in the vicinity of the histidine kinase domain, PAS/PAC motifs, which are found in diverse proteins involved in sensing changes in the redox state of cells from evolutionarily unrelated organisms (110). Mak2 and Mak3 but not Mak1 also have a GAF domain that is found, for instance, in redox-regulated transcription factors (23). Both Mak2 and Mak3, but not Mak1, are required for hydrogen peroxide-induced phosphorylation of Sty1 and the Sty1-dependent transcription factor Atf1 (72). Consequently, deletion of Mak2 and Mak3 also strongly diminishes expression of Atf1 target genes upon treatment with hydrogen peroxide. Interestingly, while Mak1 has only a minor effect on Atf1 targets, expression of a target gene of Pap1 (see below) is more affected by deletion of Mak1 (72).
Mak2 and Mak3 appear to mediate their effects through Mpr1 (also called Spy1) (21, 72, 416) and Mcs4. Mpr1 is a phosphorelay protein similar to budding yeast Ypd1p. Also, Mpr1 is needed for the hydrogen peroxide-induced phosphorylation of Sty1. Interestingly, Mcs4 is also specifically required for signaling upon oxidative stress: deletion of mcs4+ and specific mutation of the phosphate-accepting Asp412 to asparagine cause different phenotypes. Perhaps Mcs4 is part of a multiprotein complex and therefore deletion has a far more pleiotropic effect than the specific point mutation blocking phosphorelay signaling (72). It thus appears that Mak2 and Mak3 as well as Mpr1 and Mcs4 form a phosphorelay system that monitors oxidative stress in the fission yeast cytosol. Why both Mak2 and Mak3 individually are required for signaling is not understood, but this observation indicates that the two proteins do not have redundant functions. Physical interaction between them could not be demonstrated (72).
To further stress the dissimilarity to the yeast Sln1p-Ypd1p-Ssk1p system, it should be emphasized that deletion of neither Mak2/Mak3 nor Mpr1 causes lethality, while deletion of SLN1 and YPD1 is lethal due to pathway overactivation. Hence, the fission yeast Mak2/3-Mpr1-Mcs4 system apparently activates rather than inhibits the downstream MAP kinase. It will be interesting to see the detailed functional analysis of this system and the mechanisms with which it controls the MAP kinase cascade. The most recent studies demonstrate that oxidative stress is indeed signaled through the kinase cascade (417, 522, 552). As will be discussed below, activation of Sty1 is not the only mechanism by which oxidative stress mediates transcriptional responses in fission yeast.
How do other stress conditions control Sty1 activity? Stimulation by osmotic stress seems to require the MAPKKKs Wis4 and Win1 as well as Wis1 (384, 546, 548, 552), but the sensor system(s) is not known. The complete fission yeast genome sequence may reveal such sensors. Nitrogen starvation-induced mating seems to require Wis1, but the MAPKKKs Win1 and Wis4 seem to be at least partially dispensable (546); this could indicate the existence of an alternative pathway that controls the Wis1 MAP kinase under nitrogen starvation. Such a pathway could be related to the budding yeast Sho1p branch, since all its components are in fact also involved in the pathway that controls pseudohyphal growth under nitrogen starvation (329).
Heat shock seems to modulate Sty1 kinase activity in an unusual way. It appears that the Wis1 kinase is required, but its activation via the MAPKKK and hence through phosphorylation of Ser469 and Thr473 is not needed: mutants in which the two phosphorylatable Wis1 residues are changed to alanine or aspartic acid do not show enhanced Sty1 phosphorylation on mutational activation of the MAPKKK or upon osmotic or oxidative stress. However, heat shock still stimulates Sty1 phosphorylation, suggesting that a basal activity of Wis1 but not its MAPKKK-dependent activation is needed (552). Instead it appears that heat shock inhibits the phosphotyrosine phosphatases Pyp1 and Pyp2, but not the phosphothreonine phosphatases Ptc1 and Ptc3. These observations suggest that upon heat shock, the basal Wis1 activity or Sty1 autophosphorylation, together with inhibition of the phosphotyrosine phosphatases, causes transient activation of Sty1, which is rapidly attenuated by the phosphothreonine phosphatases (417). More work will be needed to better understand the role of the different phosphatases in activation and downregulation of Sty1 and if these proteins play a role in feedback control (see discussion on the HOG pathway). The mechanism with which UV activates Sty1 may be related to generation of reactive oxygen species upon UV treatment and subsequent response to oxidative stress (129).
Transcriptional responses mediated by the Sty1 pathway.
Three transcription factors mediate responses via the Sty1 pathway or affect expression of Sty1 target genes: Atf1 (586, 647), a bZIP factor closely related to mammalian ATF2; Pap1 (600, 601), which is related to budding yeast Yap1p; and Prr1 (434, 435), a response regulator transcription factor related to budding yeast Skn7p.
Atf1 was found during systematic sequencing of the S. pombe genome (586). The protein is between 40 and 50% identical to mammalian ATFs and hence more closely related to those than to any S. cerevisiae bZIP (187, 502). Atf1 binds to CREs (such as S. cerevisiae Sko1p) (274, 586), interacts with Sty1 in vitro and in the two-hybrid system, and is a direct target in vitro and in vivo for stress-induced phosphorylation by Sty1 (129, 550, 647).
As with Hog1p and other MAP kinases, phosphorylated Sty1 dissociates from Wis1 and concentrates in the nucleus upon activation. Wis1 does not function as a cytoplasmic anchor for Sty1 because Sty1 remains mainly cytosolic in a wis1 deletion mutant. Phosphorylation by Wis1 is required for nuclear translocation of Sty1. Remarkably, in mutants lacking Atf1, Sty1 does not accumulate in the nucleus after stress treatment, suggesting that Atf1 functions as a nuclear anchor for Sty1 (180, 181). Atf1 is nuclear under all conditions but requires another bZIP, Pcr1, with which it forms heterodimers, for nuclear localization (180). Export of Sty1 from the nucleus requires Crm1 (181). Transcription factors that mediate Hog1p-dependent responses in budding yeast also seem to affect nuclear retention of the Hog1p kinase, but those only diminish the period of nuclear residence (9, 496, 497, 503), while fission yeast Atf1 seems to be needed for any visible nuclear accumulation of the kinase (180).
Atf1 is required for the Sty1/Wis1-dependent transcriptional activation of gpd1+ (glycerol-3-phosphate dehydrogenase), ctt1+ (catalase; but see oxidative stress response below), fbp1+ (fructose-1,6.-bisphosphatase), pyp2+ (phosphotyrosine phosphatase), ste11+ (transcription factor required for mating competence), and atf1+ itself in response to osmotic as well as other types of stress (129, 176, 404, 405, 411, 550, 647). Mutants lacking Atf1 are also sensitive to different stress conditions, very much like mutants lacking Sty1 or Wis1, except for oxidative stress (129, 550, 647). This is also in contrast to the situation in budding yeast; S. cerevisiae mutants lacking transcription factors downstream of the HOG pathway do not seem to show apparent stress phenotypes (503), with the exception of Sko1/Acr1p (481). This suggests less pronounced redundancy (and perhaps less flexibility) in stress signaling in fission yeast. Consistently, mutants lacking Atf1 or Sty1 appear to be completely devoid of any response to stress of all target genes studied so far, while in budding yeast expression of most HOG target genes is only partially affected by lack of Hog1p or its transcription factors (501). Once global expression studies become available for fission yeast, further analyses to compare the stress-induced transcriptional responses in the two yeasts can be performed.
It is presently thought that fission yeast Atf1 functions as a transcriptional activator whose function is stimulated by Sty1-dependent phosphorylation. The situation may, however, not be that simple. When analyzing the UV-induced expression of ctt1+, Degols and Russell (129) observed that deletion of sty1+ causes very low, unregulated ctt1+ expression, while deletion of atf1+, individually and in combination with sty1+, caused a higher basal expression level that was unresponsive to stress. It should be noted that other studies did not find any effect of deletion of atf1+ on expression of ctt1+ upon treatment with hydrogen peroxide, perhaps due to strain differences or different stress treatments (608). In any case, this finding was interpreted to mean that Atf1 functions as a repressor under normal growth conditions and as an activator under stress conditions, similar to recent suggestions for budding yeast Sko1/Acr1p, based on studies on the budding yeast GRE2 promoter (187, 481, 502).
The function of Sko1/Acr1p as a repressor is based on recruitment of the Tup1p-Ssn6p corepressor complex (480, 481). Recently it was reported that the Atf1-dependent fbp1+ promoter in fission yeast is controlled by Tup1p-like proteins that repress the promoter (259). Further work will show if the functions of Atf1 and Sko1/Acr1p are perhaps more similar than is presently assumed. Another interesting aspect concerns the apparently tight interaction between Atf1 and Sty1, documented by the requirement of Atf1 for nuclear localization of Sty1 (180). Recently it was reported that Hog1p is part of a complex also containing Hot1p on target promoters, opening the possibility that the kinase itself participates in transcriptional activation (9). It will be crucial to determine if Sko1/Acr1p and Atf1 also have the ability to recruit their cognate MAP kinases to target promoters and then if the transcription factors, the kinase, or both together mediate transcriptional activation.
As indicated above, Atf1 is required for the activation of transcription of atf1+ and pyp2+. This suggests both a positive and a negative feedback loop (550, 647). The observation that Sty1 phosphorylation is strongly enhanced and prolonged upon stress in an atf1 mutant was taken as further evidence that the transcriptional activation of pyp2+ is needed for downregulation of the pathway (647). However, as with similar arguments on HOG pathway control, this observation cannot distinguish between effects of Atf1 on phosphatase activity and the requirement for feedback control of successful execution of the transcriptional program in stress adaptation. Hence, if transcriptional induction of atf1+ and pyp2+ truly contributes to pathway regulation or if this response leads to an adjustment of pathway capacity to respond more rapidly to subsequent environmental changes has not yet been addressed (see also discussion on feedback control in the budding yeast HOG pathway).
As pointed out above, deletion of atf1+ causes many of the phenotypes also observed for deletion of genes encoding components of the MAP kinase cascade, with two important exceptions: deletion of atf1+ does not cause longer cells and hence does not affect G2 control of the cell cycle. Thus, Sty1 affects the cell cycle independently of Atf1 (550, 647). Another phenotype that is not shared between atf1 and sty1 mutants is that the former mutation does not confer sensitivity to oxidative stress (129).
Sty1-dependent responses to oxidative stress require Pap1. Pap1 is related to budding yeast Yap1p (600, 601). Deletion of atf1+ does not affect or only moderately affects hydrogen peroxide-induced expression of a set of genes, including ctt1+, important for combating oxidative stress (416, 608). Deletion of pap1+, however, abolishes induction completely and diminishes basal expression of ctt1+ (608). Other studies found that deletion of pap1+ diminishes ctt1+ expression but that only simultaneous deletion of both pap1+ and atf1+ completely abolishes expression (416). Those differences may be due to the strains or the specific conditions employed. Deletion of sty1+ does not confer the same effect but strongly diminishes induction, suggesting that expression is also controlled by Sty1-independent mechanisms (see below) (608). Consistent with this observation, both sty1 and pap1 mutants but not atf1 mutants are sensitive to a number of compounds that are known or thought to cause generation of oxygen radicals.
Pap1, like budding yeast Yap1p (302, 660), is translocated to the nucleus upon oxidative stress. Interestingly, this process requires Sty1 and its activation by Wis1 (608). On the other hand, like budding yeast Yap1p (302, 660), a C-terminal, cysteine-rich domain is thought to sense the redox state of the cell and enhance nuclear localization upon oxidative stress (300). Interestingly, it appears that Pap1 is not phosphorylated by Sty1 (647), suggesting that Sty1 affects the subcellular localization of Pap1 by a different mechanism, perhaps by direct protein interaction and cotransport into the nucleus. Pap1 seems to actively shuttle between cytosol and nucleus, since it was observed to be nuclear under all conditions in mutants defective in the nuclear export factor Crm1 (608), similar to the situation in budding yeast (660). Furthermore, Pap1 seems to be controlled by the Mak1 histidine kinase via an uncharacterized mechanism that does not involve the Sty1 kinase cascade (72, 416).
Prr1 is a response regulator protein with homology to the DNA-binding domain of heat shock transcription factor (434, 435), being most closely related to budding yeast Skn7p. Although Prr1 has so far not been studied as extensively, it appears that the two proteins may have similar functions. Mutants lacking Prr1 are sensitive to hydrogen peroxide but not to diamide (435). Prr1 does not seem to be involved in signaling through the Sty1 pathway (416), but it appears to be required for normal expression of some genes induced by oxidative stress in an Atf1- and Pap1-dependent way (434). Hence, as with Skn7p, Prr1 may support the AP1-like factor Pap1 in gene expression. Mak1 could perhaps be a cognate histidine kinase (72). In addition, Prr1 seems to be involved in the control of the Atf1 target ste11+ in response to nitrogen starvation. Prr1 mutants fail to induce ste11+ expression and hence are defective in mating (435). It will certainly be most interesting to study the function of Prr1 in fission yeast in more detail and to compare it with that of budding yeast Skn7p.
Interestingly, vacuolar morphology seems to change in fission yeast upon osmotic shock treatment in a manner dependent on two different MAP kinases (53).
Osmosensing Signaling Pathways in Other Yeasts
Components of the HOG pathway have been identified in other yeasts. Functional homologues of SLN1 (139, 403) and HOG1 (15, 525) have been described in the pathogenic yeast C. albicans. The C. albicans hog1 mutant displays sensitivity to high osmolarity and diminished glycerol accumulation, as well as morphological alterations, cell wall defects, and diminished virulence (15, 525). In addition, genes encoding homologs of YPD1 (80) and SSK1 (78, 81) have been described in C. albicans. Furthermore, HOG1 genes have been isolated from the osmotolerant yeast Zygosaccharomyces rouxii (250, 285) and the salt-tolerant yeast Debaryomyces hansenii (26). SHO1 homologs have been reported from Candida utilis and Kluyveromyces lactis (554).
CELLULAR SYSTEMS INVOLVED IN RESPONSE TO HYPEROSMOTIC SHOCK
Genome-Wide Expression Analyses Reveal Genes and Proteins Responsive to Osmotic Shock
Global gene expression analyses employing microarray technology have the potential to reveal the complete set of genes that are expressed under a given growth condition. Within less than 1 year, five independent studies have been published in which S. cerevisiae cells were exposed to osmotic stress and the expression of essentially all annotated yeast genes was analyzed (86, 191, 471, 501, 656). The data sets generated in these different studies are, however, not redundant because each study used a different experimental setup as well as different microarray technology (Table 6). Most significantly, the studies differed with respect to the S. cerevisiae strain used, the concentrations as well as the nature of the osmoticum (salt versus sorbitol), and the extent to which mRNA expression was followed over time. It should be noted, however, that all the studies focused on the early phase of the response to an osmotic shock; none of these studies explored the expression pattern of cells actively growing in high-osmolarity medium. All the studies also investigated certain mutants, providing information on the fraction of the response that can be allocated to a certain regulatory system. Finally, two of the studies explored the response not only to osmotic stress but also to other types of stress, allowing the identification of the set of genes whose expression is affected by essentially any type of environmental challenge (86, 191). These two data sets can be explored in different ways on the World Wide Web (Table 6).
TABLE 6.
Microarray studies of osmotic shock
| Study | NaCl concn (M) | Sorbitol concn (M) | Time points tested | Other stresses studied | Technology useda | Strain used | Mutation(s) | Website |
|---|---|---|---|---|---|---|---|---|
| Rep et al. (501) | 0.7 | 0.95 | 45 min (NaCl) and 30 min (sorbitol) | None | Gene filters (Research Genetics/Invitrogen) | W303-1A | hog1Δ, hot1Δ, msn2Δ msn4Δ | http://www.gmm.gu.se/groups/hohmann/arrays.html |
| Posas et al. (471) | 0.4, 0.8 | None | 10 and 20 min | None | Glass slides with complete ORFs from Research Genetics | TM141 | hog1Δ | http://evalga.uv.es/scsie-docs/chipsdna/chips_arino.html |
| Gasch et al. (191) | None | 1 | 5, 15, 30, 45, 60, 90, 120 min | Temperature shifts, oxidative stresses, dithiothreitol, hypo-osmotic shock, starvation, stationary phase | Glass slides with complete ORFs (Stanford technology) | DBY7286 | msn2Δ msn4Δ | http://genome-www.stanford.edu/yeast_stress/ |
| Causton et al. (86) | 1 | 1.5 | 15, 30, 45, 60, 90, 120 min | Heat shock, acidity, alkali, H2O2 | Glass slides with oligonucleotides (Affymetrix) | S288C | msn2Δ msn4Δ (in W303-1A) | http://web.wi.mit.edu/young/environment/ |
| Yale and Bohnert (656) | 1 | None | 10, 30, 90 min | None | Gene filters (Research Genetics/Invitrogen) | S150-2B | gpd1Δ gpd2Δ (in W303-1A) | http://transcriptome.ens.fr/ymgv/ |
ORFs, open reading frames.
A couple of general conclusions can be drawn from these studies. (i) The set of genes whose expression responds specifically to hyperosmotic shock but not to any other type of stress is rather small, and within this group most expression changes are not very strong; the majority of these genes are uncharacterized. (ii) Both stimulation of expression and diminished expression of different genes seem to be important aspects of the response of S. cerevisiae to stress. The number of genes whose expression drops seems to be higher than that of those whose expression increases. Genes whose mRNA level is diminished upon stress are often referred to as repressed genes, while those whose mRNA level increases upon stress are referred to as induced genes. It should be noted that the terms repressed and induced indicate that effects occur at the transcriptional level, and they even point to specific regulatory mechanisms. This is obviously misleading, as the mRNA level is the combined result of production and degradation rates. Moreover, enhanced transcription can be due to transcriptional activation or derepression.
(iii) Global expression analyses have both confirmed the cellular systems that seem to be part of the (osmotic) stress response and extended the set of genes encoding functions within a certain system or pathway. In addition, global expression analyses have revealed a number of additional cellular systems that seem to be adjusted during osmotic adaptation, although in each case experimental evidence is needed to verify the relevance. (iv) A significant realization was that, to a very large extent, the transcriptional response is transient and follows a distinct temporal pattern. This phenomenon was pointed out in studies analyzing expression of specific genes (500, 503) but is now known to be generally the case (191). Many genes are induced strongly, often 50-fold to several hundred fold, within minutes after a shift to different growth conditions, and then the mRNA level falls back to the prestress level within less than 1 h. For many genes, such as STL1, upon osmotic shock, this peak can be so sharp that it is completely missed if measured 15 min earlier or later (501).
Both increased and diminished mRNA levels follow essentially the same transient pattern. The precise time course and the amplitude of the increase or drop depend on the severity of the stress (191, 500, 503); more severe stress causes a delay in the response, which then is usually stronger and more sustained. It will be important to follow this expression pattern further, up to exponential growth under high-osmolarity conditions, in order to reveal the complete temporal program of osmotic adaptation (or any other stress condition). The transient nature of the transcriptional response is certainly a source for contradictory reports in the literature concerning the responsiveness of certain genes to different stress conditions.
(v) There is clearly a large set of genes that are induced or repressed under all conditions that challenge cell survival or proliferation. This general stress response (358, 519), variously termed the environmental stress response (191) or common environmental response (86), seems to aim mainly at the production of cellular protectants and the adjustment of energy metabolism. As pointed out above, there are genes whose expression appears to be affected only by a subset of stress conditions, such as osmotic and oxidative stress, but those stress-specific gene sets are surprisingly small (86). (vi) The extent of the cellular response to altered growth conditions is impressive. Causton et al. (86) point out that more than half of all yeast genes display altered expression by at least a factor of 2 under at least one of the conditions they studied (Table 6). While this number provides a reflection of the impact of altered growth conditions on a microbial cell, the number of genes whose products truly contribute to adaptation is certainly much lower, though still remarkable. About 10% of all yeast genes significantly alter expression upon a given type of stress and can probably be expected to contribute to adaptation.
(vii) Following this observation, an interesting question concerns the true importance of those genes, or their altered expression, for survival or adaptation to stress. Only a very small fraction of the genes whose expression changes under, for instance, osmotic stress have previously been shown by deletion or overexpression to affect growth or survival under osmotic stress under laboratory conditions. Dedicated searches for mutants that fail to grow in high-osmolarity medium have revealed some 10 genes (61, 71, 318), and transposon mutagenesis has identified about 30 additional genes that are needed for growth in high-salt medium (165, 517), while expression of more than 600 genes is affected by a shift to high osmolarity. Lack of an obvious phenotype is usually explained to mean that those genes may reveal their importance only in nature and over many generations. This argument calls for more sophisticated ways to monitor phenotypes in the laboratory, such as cocultivation of mutants and wild types over many generations and subsequent determination of the relative proportion of strains in the mixture (651). In any case, altered expression under a certain growth condition is in itself an important phenotype pointing to a certain function of a gene product.
(viii) An exciting finding concerns isogenes. The budding yeast genome is known to contain different isoforms for many cellular functions, probably due to a duplication of the genome and subsequent loss of parts of the duplication, rearrangements, and subsequent gene amplifications (536, 652). Global expression analyses now suggest that isozymes may have properties that make them more suitable under certain conditions (86, 191, 501). To confirm this notion will require more detailed analyses, especially of the biochemical properties of the isoenzymes, but expression data already indicate that the extensive isoform pattern of S. cerevisiae may reflect an adaptation to a highly variable environment. (ix) Expression changes may not necessarily reflect physiological changes. For instance, expression of genes encoding enzymes needed for production of glycerol or trehalose is stimulated under any type of environmental challenge, but net production of trehalose and glycerol is observed only under specific conditions. This calls to mind trivial aspects of molecular biology that are often ignored when global gene expression data are interpreted. First of all, mRNA has first to be translated into protein. Hence, it is of utmost importance to correlate the global gene expression data to protein expression, which can be monitored on a global scale (with important limitations concerning low-abundance proteins and membrane proteins) by two-dimensional gel electrophoresis (47, 195). But protein function and localization as well as enzyme activity are also regulated in many ways, and those can at present only be analyzed to a limited extent by global or high-throughput methods. Global gene expression data are so impressive that we can hardly withstand the temptation to draw conclusions, for instance, with respect to the occurrence or even the timing of metabolic events, although we are aware of the fact that without any detailed biochemical or physiological analyses, such conclusions are at best speculation.
(x) Although stress responses have been studied for many years, the number of functionally uncharacterized genes among those whose expression is affected by altered growth conditions is remarkable, about 50 to 60%. This fraction is even higher than for the entire genome! There is a higher proportion of characterized genes among those that are highly expressed and whose expression is strongly enhanced or diminished, while among those that are poorly expressed and weakly affected, the proportion of uncharacterized genes is higher. Whether this is a relevant observation with respect to function and physiological importance remains to be seen.
In the following sections I will summarize some hallmarks of the general response to altered growth conditions. I also refer to relevant discussions in the papers reporting the global expression data and their websites (Table 6) (86, 191). I will also discuss the pathways leading to trehalose and glycerol production and aspects of the isozyme pattern. Subsequently I will briefly discuss our limited knowledge on sets of genes whose expression specifically responds to hyper- or hypo-osmotic shock.
Hallmarks of a General Response to Environmental Challenges
The number of genes whose expression responds to a whole range of altered growth conditions differs somewhat between studies; Gasch et al. (191) mention increased mRNA levels for 300 genes and decreased mRNA levels for 600 genes, while Causton et al. (86) focus on 216 and 283 genes, respectively.
Why have a general stress response?
The term general stress response has often been interpreted to mean that the cell expresses a whole battery of stress protection mechanisms under a given stress condition because one type of stress may, in nature, increase the likelihood that other stresses will occur (519, 555). While this may be a plausible interpretation, I argue that the general stress response exactly reflects the needs of the cell under any environmental conditions.
The genes whose expression is adjusted under stress belong largely to three classes: (i) gene expression and protein production, (ii) cell protection from oxidative damage and protein denaturation, and (iii) enzymes in redox and carbohydrate metabolism (86, 191, 501). This interpretation may be oversimplified and also biased, because the presently characterized genes may not be representative of the entire set of stress-regulated genes. In any case, slowdown in the proliferation rate or even a temporary arrest makes it necessary to diminish protein production and to adjust metabolism to an overall diminished biosynthetic demand. Damage of proteins is likely to occur under a wide variety of stress conditions, and hence it makes sense that cells produce a set of chaperones under stressful conditions.
Significant expression changes occur in carbon metabolism, altering the set of isoenzymes and enhancing the capacity to produce glycerol and trehalose for protection purposes and glycogen as a reserve. Metabolic adjustments seem to include stimulation of respiration and accordingly alterations in redox metabolism as well as protection from reactive oxygen species. In nature the most common source for reactive oxygen species is cellular metabolism, especially the mitochondrial electron transport chain. Taken together, the metabolic adjustments seem to have very specific aims, which may indeed apply to any stressful condition: optimization of the use of the available resources and accumulation of reserves and stress protectants.
As discussed above, stimulated expression under a range of stress conditions depends largely on Msn2p and Msn4p. Despite the wide impact of Msn2p/Msn4p on the stress-induced gene expression program, the two genes are not essential. In fact, an msn2Δ msn4Δ double mutant is not obviously sensitive to different mild stress conditions. However, carbon starvation or strong osmotic or heat stress causes enhanced killing of the mutant (370). Even in the double msn2Δ msn4Δ mutant, treatment with a mild form of stress still results in significant, though diminished, acquisition of stress tolerance (370). While in nature these differences may have dramatic consequences, it is clear that Msn2p and Msn4p do not control essential functions, rather adjustments, and that essential functions are controlled in addition by other, stress-specific systems. As discussed above, the signal that stimulates the Msn2p/Msn4p-dependent part of the general stress response is not known, but given the apparent link of the response to cellular metabolism, the signal may be generated within the cell.
Adjustments of protein production.
Two large-scale analyses comparing responses to different stress treatments came to similar conclusions with respect to “repressed” genes (86, 191). There appear to be two or possibly three clusters that differ mainly with respect to the time point at which repression becomes apparent as well as the type of stress under which the drop in the mRNA level is more pronounced. Genes whose expression drops rapidly, especially under alkali, oxidative, and osmotic (both salt and sorbitol) stress, are classified under amino acid metabolism, cell wall maintenance, nucleosome structure, DNA synthesis, and nucleotide metabolism. These processes are in less demand when cells do not proliferate, and hence the drop in expression may reflect a temporary proliferation arrest upon sudden stress or a slowdown of the proliferation rate under mild stress. A number of genes encoding proteins involved in RNA processing and modification as well as about 100 uncharacterized genes show a very similar expression pattern, with a rapid and pronounced drop in the mRNA level, but in addition they also show a somewhat sharper drop under heat, acid, and alkali stress.
Gasch et al. (191) pointed out the existence of two different sequence motifs that are found upstream of many of the characterized genes in these two groups, GCGATGAGCTG and GAAAA/TTTTTC; the function of these motifs is not known. With a delay of a few minutes, the mRNA levels of about 45 genes encoding ribosomal proteins drop as well. The transient drop in expression of genes encoding proteins required for gene expression and protein production is also likely due to a temporary proliferation arrest. Expression of ribosomal protein genes has been known for many years to be diminished upon stress treatment (221) and to be a sensitive reporter of the cellular proliferation potential (359). For instance, expression of ribosomal protein genes is high in cells growing in glucose medium, where proliferation is fast, and low in glycerol-grown cells, where the growth rate is much lower (196, 204, 293). Expression of ribosomal protein genes requires the transcription factors Rap1p and Abf1p, which are involved in the control of many genes (466). Protein kinase A appears to stimulate expression of ribosomal protein genes in cAMP-dependent and -independent ways (459, 595). The expression pattern of ribosomal protein genes appears to be the inverse of that of stress response genes controlled by Msn2p and Msn4p. Interestingly, the two studies focusing on salt-induced gene expression (471, 656) found that very early after salt addition, expression of ribosomal protein genes first increases and then drops. The relevance of this observation is unknown.
In addition to the drop in expression of translation functions, there is evidence that under osmotic stress, translation elongation is directly downregulated through a linear signaling pathway (592); the incorporation of radiolabeled leucine drops upon osmotic challenge by a factor of at least 3. Rck2p is a member of the calmodulin protein kinase family (378) that phosphorylates and thereby inhibits translation elongation factor EF-2 (40, 592). Rck2p is a direct target of Hog1p (40), and both Hog1p and Rck2p are needed for osmostress-induced reduction of the incorporation of radiolabeled leucine into protein (592). Inhibition of protein synthesis contributes to the lethality conferred by HOG pathway overactivation, since deletion of RCK2 or expression of catalytically inactive Rck2p suppresses the proliferation defect caused by overexpression of N-terminally truncated Ssk2p (592). The inhibition of EF-2 by phosphorylation appears to be a mechanism that is conserved from S. cerevisiae to humans (592).
While an overall reduction of protein synthesis may well be compatible with a transient inhibition of cell growth and proliferation, the expression of genes encoding functions important for stress adaptation is stimulated, and their translation has to be ensured. Using DNA microarrays, Kuhn et al. (303) studied transcripts that displayed altered association with polysomes after cells were shifted from glucose- to glycerol-containing medium. Despite an overall reduction in translation initiation following the shift, most of the 600 transcripts that were increased became associated with polyribosomes, while 100 transcripts whose level dropped (i.e., only a fraction, mainly those encoding ribosomal proteins) became less associated with polysomes. These results show a strong correlation between gene induction and transcript association with polysomes under the conditions studied (303). These observations suggest that mechanisms must exist that allow the preferential translation of subsets of mRNAs under certain conditions. Little is known about the molecular bases.
Studies on global protein expression under salt stress by two-dimensional gel electrophoresis concluded that the response is characterized mainly by an increase in the production rate of certain proteins, while the production rate of only a very few proteins was diminished (46). This appears to contradict the notion that the genes that show lower mRNA levels upon stress outnumber those displaying enhanced levels. However, the amount of protein applied to gels is kept constant, and the majority of cellular proteins are ribosomal; this may balance the diminished production rate of most proteins. Only those proteins that are actively degraded under stress or whose production rate is strongly enhanced may become apparent. In microarray studies, mRNA samples taken from different conditions compete for the probe, and hence changes can be assessed directly.
Enhanced gene expression under different stress conditions.
The mRNA level of about 220 to 300 genes (depending on the study [86, 191]) increases under a range of different stress conditions. Upon a shift to a certain condition, such as high osmolarity, the time courses of the mRNA level changes are very similar for most of these genes. Global expression analyses investigated the role of Msn2p and Msn4p by monitoring expression at lower pH (86), heat shock and peroxide stress (191) as well as salt stress (501) in an msn2Δ msn4Δ mutant. Stimulated expression upon acidity treatment of 93% of the general stress-responsive genes was abolished in an msn2Δ msn4Δ mutant (86). Under salt stress, expression of about 25% of the induced genes was diminished by more than 75% in an msn2Δ msn4Δ mutant; this analysis did not distinguish between general stress-responsive genes and salt stress genes. Therefore, the proportion of the general stress genes that are Msn2p/Msn4p dependent under salt stress is higher (501). Altogether, 180 of the approximately 300 genes whose expression increases upon different stress treatments were affected by deletion of MSN2 and MSN4 after heat shock or under oxidative stress (191). More than 90% of those genes, plus many others, showed enhanced expression when MSN2 or MSN4 was overexpressed, confirming that these two factors directly or indirectly control expression of this set of genes.
Interestingly, a fraction of these genes were dependent on Msn2p and Msn4p only under heat stress, but under oxidative stress stimulated expression required Yap1p (191). Hence, genes whose expression is enhanced under various stress conditions do not necessarily employ Msn2p and Msn4p for stimulated expression under all these conditions. Rather, in addition, stress-specific signals may also impinge on target promoters. This fits with observations on GPD1 expression, whose induction by heat shock is strongly diminished in an msn2Δ msn4Δ mutant (191, 501) while induction by osmotic stress is governed mainly by Hot1p and Msn1p (9, 500, 501, 503). Control of expression of GRE2 provides another example: stimulated expression by osmotic stress is achieved through the Hog1p-Sko1/Acr1psystem (480, 502), induction by oxidative stress is mediated by Yap1p (502), and enhanced expression by heat stress seems to depend on Msn2p and Msn4p (191). It therefore appears that even the general stress response differentiates between different types of stress by employing complex promoters with binding sites for different stress-controlled transcription factors.
It is not understood how Msn2p and Msn4p can mediate only a subset of stress responses on particular promoters while they mediate responses to a wider range of conditions on other promoters. Certainly, Msn2p and Msn4p collaborate with stress-specific factors such as, for instance, Hsf1p on the HSP26 promoter (17). To elucidate the interaction between general and specific stress responses will require more detailed analysis on promoter architecture and function of promoter elements, individually and in concert. One way that Msn2p/Msn4p can acquire stress specificity is interaction with stress-specific factors of signaling pathways. This is illustrated by the fact that Msn2p/Msn4p-dependent osmostress-induced gene expression requires Hog1p (see above).
Systems potentially upregulated by different stress conditions.
More than half of the 220 to 300 genes whose expression is stimulated under various stress conditions have not been characterized and general stress induction is the first functional information for many of those (86, 191). Some of the uncharacterized genes are among the most strongly stress-induced genes. Expression of YBR116C, YDL202W, YDL222C, and YDR070W, to name a few, is stimulated more than 50- to 100-fold under different environmental conditions, but no specific data addressing their function are available and the gene products do not show significant sequence similarity to other proteins.
Among the genes whose expression is most strongly stimulated under a wide range of stress conditions are several heat shock proteins, many of which have been shown to or may function as chaperones in protein folding (Hsp26p, Hsp42p, Hsp78p, Hsp104p, Ssa4p, and Sse2p [389]). This suggests that protein damage is a threat common to many environmental conditions. The function of the product of one of the most strongly induced heat shock proteins, HSP12, is not known. Recently, Hsp12p has been classified as a hydrophilin together with several other poorly characterized, osmostress-induced genes (186a). It is speculated that such proteins may protect biomolecules under water limitation, but detailed mechanisms remain to be studied (186a).
A large number of expression changes hint at adjustment of carbohydrate and energy metabolism. Under suboptimal conditions, the yeast cell has to invest energy to cope with stress, to repair damage, and to maintain cell homeostasis. Examples of energy-demanding processes include maintenance of intracellular pH by pumping H+ out of the cell via the plasma membrane H+-ATPase, which is thought to be the most important ATP consumer in yeast cells (393). Furthermore, the function of chaperones requires ATP, as does production of solutes and cell protectants such as glycerol and trehalose. It has been estimated, based on chemostat studies with glucose-limited, fully aerobic cultures, that 0.9 M NaCl increases by about 60% the specific energy demand to build up biomass (437). On the other hand, under such conditions cells proliferate more slowly and hence require, per unit of time, less energy for biomass production. Consequently, under salt stress cells produce more heat, indicative of a partial uncoupling between energy production and consumption by biosynthesis (316). A possible interpretation of these seemingly contradictory observations is that cells are in energy excess shortly after the shock, before proliferation resumes. Then, in fact, cells will have a higher than normal energy demand for proliferation and biomass production.
Both global gene and protein expression studies have confirmed and extended earlier findings that the enzyme composition in the glycolytic pathway as well as the pathways connected to it changes dramatically under stress conditions (14, 46, 60, 86, 191, 195, 424, 425, 471, 501, 656). This is particularly apparent for genes encoding sugar transporters and kinases as well as glyceraldehyde-3-phosphate dehydrogenase (see below). Fairly little is known about how much these expression changes truly affect flux through metabolic pathways. Altered expression appears to aim at providing building blocks for the production of stress protectants and reserves such as glycerol, trehalose, and glycogen, at adjusting energy metabolism, and at adjusting redox metabolism. Hence, just as glycolysis is the central metabolic pathway in S. cerevisiae, stress-induced adjustments of glycolysis appear to be central to controlling the adaptation of metabolism to stress.
As pointed out above, the S. cerevisiae genome is rich in gene duplications (536, 652). Causton et al. (86) list a total of 74 genes encoding 37 pairs of highly homologous proteins that are differentially expressed under stress. Commonly, expression of one of the genes is stimulated while that of the other remains unchanged or is even diminished. Twelve of those isoform pairs are enzymes in sugar or energy metabolism. In addition to isoform pairs, there are examples of functions that are encoded by multiple, differentially expressed isoforms.
Hexose transporters and sugar kinases illustrate the adjustment of the isoform pattern in sugar catabolism. S. cerevisiae has 17 HXT genes, encoding highly homologous sugar uptake systems, and six of those (HXT1, HXT2, HXT3, HXT4, HXT6, and HXT7) appear to contribute to sugar uptake during batch culture with 2% glucose as the sole carbon source (52, 493, 494). The expression of HXT5, which is not among those six, is highly induced under several stress conditions, especially under osmotic stress. In addition, it appears that expression of HXT6 and HXT7, which encode high-affinity sugar transporters, is also stimulated by different stress conditions, and expression of HXT1 seems to be slightly enhanced specifically under osmotic stress. Expression of HXT2 and -3 is diminished under various stress conditions. Thus, it appears that the cell uses a different set of sugar transporters under stress than it does under normal conditions.
Expression of GLK1 and HXK1, encoding, respectively, a glucokinase and a hexokinase, and of YDR516C, which encodes an uncharacterized homolog of GLK1, is strongly enhanced under stress. At the same time, expression of HXK2, the main hexokinase under optimal conditions, is diminished under stress. A summary of the expression changes of genes encoding enzymes in central metabolism is presented in Fig. 11. To better understand the physiological importance of the altered isoform pattern will require a combination of genetic engineering, biochemical studies, physiological analyses, and theoretical approaches. Recently, it was reported that under cadmium stress, expression of isoforms depleted of sulfur-containing amino acids allows sulfur to be channeled to glutathione for detoxification (630). This observation confirms that altered isoform patterns serve specific metabolic purposes, many of which remain to be discovered.
FIG. 11.
Upper part of the glycolytic pathway and pathways for production of glycerol, trehalose, and glycogen, with all proteins and their isoforms indicated. Expression of the genes encoding the proteins that are underlined is stimulated after osmotic shock, and expression of those that are overlined is diminished.
A hint of changes in energy generation can be derived from data on genes encoding mitochondrial functions (86, 191). Expression of genes encoding enzymes of the tricarboxylic acid cycle as well as the respiratory chain is diminished during growth on glucose and becomes derepressed when cells pass the diauxic shift and switch from glucose fermentation to aerobic catabolism of ethanol. A cluster of genes showing an expression pattern similar to that of HAP4 encompasses about 50 genes encoding almost exclusively mitochondrial functions. Expression of most of these genes is also stimulated by heat shock and to a lesser and quite variable extent by other types of stress. Hap4p is part of the HAP complex, which activates expression of genes encoding tricarboxylic acid cycle and respiratory enzymes (138). Ectopic overexpression of HAP4 has been shown to stimulate oxidative catabolism of glucose and increases the biomass yield, indicative of more effective energy generation (44). Therefore, enhanced expression of HAP4 and genes encoding mitochondrial enzymes may result in the stimulation of oxidative metabolism in order to meet the energy demand during growth under these conditions.
Redox metabolism and overlap with oxidative stress responses.
The expression of remarkably many genes encoding proteins and enzymes involved in defense from oxidative damage and in redox metabolism is stimulated under a range of stress conditions. Examples of such genes include CTT1 (catalase T), TRX2 (thioredoxin), TTR1 (glutaredoxin), and several genes with similarity to TTR1, genes encoding enzymes possibly involved in detoxification such as GLO1 (glyoxylase) and DAK1 (dihydroxyacetone kinase), and numerous oxidoreductases that may be involved in metabolism of oxidized biomolecules or in adjusting redox metabolism to provide sufficient NADPH for detoxification. Diminished expression of genes encoding enzymes in methionine and cysteine biosynthesis can be interpreted as a response aimed at securing sulfur for production of proteins involved in reductive detoxification, such as thioredoxin and glutathione.
Genes within this category do not necessarily cluster together when expression data are compared, indicating that the underlying control mechanisms differ. This can be illustrated with some examples. As pointed out above, expression of a subset of the general stress response genes depends on Msn2p and Msn4p under heat shock but at least partially on Yap1p under oxidative stress (191). This group of genes includes TRX2, TTR1, two genes encoding proteins resembling thiol peroxidases, and several uncharacterized genes. Expression of these genes is stimulated via the global Msn2p/Msn4p-dependent response system, probably to prepare the cell to combat reactive oxygen species. This may also apply to a group of genes (Table 3) controlled in a similar way under osmotic and oxidative stress (501). Expression of GRE2, which is the best studied, is stimulated by osmotic stress via Hog1p-Sko1/Acr1p, by oxidative stress via Yap1p (501), and by heat stress via Msn2p and Msn4p (191). Hence, its expression pattern is a composite of different mechanisms. On the other hand, expression of the catalase T gene CTT1, one of the prime examples of STRE-controlled genes, depends under essentially all stress conditions on Msn2p and Msn4p (362, 370, 533, 646). Thus, it appears that part of the response to oxidative stress is mounted upon various stress conditions, while certain functions are then (further) induced once oxidative stress truly occurs.
As outlined above, it appears that under stress, oxidative metabolism is stimulated, leading to enhanced intracellular production of reactive oxygen species. In addition, stressful conditions, especially heat and osmotic/salt stress, may also interfere with electron transport chains, further enhancing the production of reactive oxygen species. Imposing heat or osmotic stress under anaerobic conditions should be one simple way to test if the stimulated expression of oxidative defense genes is a response to the actual generation of reactive oxygen species or rather a response if oxidative damage is expected to occur. Since the nuclear accumulation of Yap1p seems to be mediated directly by a lowered reductive potential of the cytosol (133, 248, 302), one would expect that the Yap1p-dependent part of this response would not occur under anaerobic conditions. The experiment is being performed in my laboratory.
Metabolism and Transport of Glycerol
The pathways for production of glycerol, trehalose, and glycogen all start with intermediates of glycolysis (Fig. 11). The three compounds have different physiological roles: glycerol serves as an osmolyte (7, 48, 63, 228), trehalose might be a more general stress protectant and assists chaperones in controlling protein denaturation and renaturation (173, 560, 561), and glycogen is a storage carbohydrate (173). Expression of the genes encoding the enzymes in production and breakdown of these compounds is remarkably coregulated and enhanced under essentially all stress conditions (86, 191). Msn2p and Msn4p play an important role in controlling expression of these genes, but under osmotic stress the HOG pathway and Hot1p are needed for high-level expression of genes encoding enzymes of glycerol biosynthesis. This may account for the observed superinduction of glycerol biosynthesis genes under osmotic stress.
While expression of the genes important for metabolism of glycerol, trehalose, and glycogen is induced by multiple stress conditions, net production of these compounds is only observed under specific conditions. The pathways for glycerol and trehalose metabolism not only serve to produce the corresponding compounds but also have important demonstrated roles in controlling and balancing cellular metabolism.
Glycerol metabolic pathway.
Glycerol is produced in two steps from the glycolytic intermediate dihydroxyacetonephosphate. These two steps are catalyzed by NAD-dependent glycerol-3-phosphate dehydrogenase (Gpd) and glycerol-3-phosphatase (Gpp), respectively. For both enzymes S. cerevisiae possesses two differentially expressed isoforms, GPD1 and GPD2 (7, 19, 156, 318) and GPP1 (RHR2) and GPP2 (HOR2) (226, 426, 447). S. cerevisiae also possesses genes that might encode the enzymes glycerol dehydrogenase (GCY1 and YPR1) as well as dihydroxyacetone kinase (DAK1 and DAK2). These two enzymes could form a pathway for glycerol degradation (46, 424). Expression of the genes DAK1 and GCY1 (and to a lesser extent also YPR1) is stimulated under a range of stress conditions (86, 191, 424). Whether such a Gcy-Dak pathway for glycerol degradation exists, as it does in other yeasts, remains to be clearly demonstrated. A role for Gcy1p and Dak1p in redox regulation has been suggested (103).
The pathway for glycerol production via Gpd and Gpp converts NADH to NAD, while the conversion of glycerol to dihydroxyacetone phosphate via Gcy1p and Dak1p reduces NADP to NADPH. Hence, a glycerol-dihydroxyacetonephosphate cycle could essentially function as a transhydrogenase for interconversion of NADH to NADPH (46, 424). This pathway could be driven by energy consumption during the Dak1p reaction. S. cerevisiae does not possess a transhydrogenase for performing the NADH-NADPH conversion directly (420), and therefore such cycles might serve this role, with several possible levels of control. Since stress conditions confer a higher demand for NADPH for combating reactive oxygen species (see above), such pathways could be important specifically under stress. The capacity for other NADPH-generating reactions, such as those of the pentosephosphate pathway, is also increased under stress.
An additional transhydrogenase cycle could be formed by NAD (Gdh2p) and NADP-dependent (Gdh1p and Gdh3p) glutamate dehydrogenases; however, while expression of GDH2 is stimulated under stress, that of GDH1 and GDH3 is rather diminished. Dihydroxyacetone kinase may also be involved in detoxification: dihydroxyacetone is a highly reactive molecule that might be generated as a by-product during glycerol biosynthesis. Another by-product of glycerol biosynthesis is methylglyoxal, which is toxic. Methylglyoxal has been proposed to have a regulatory role in triosephosphate metabolism (1). Expression of the genes encoding enzymes in methylglyoxal metabolism, GLO1 (246) and GRE3 (1, 186), is also induced under stress in a Hog1p-dependent (for osmotic stress) and Msn2p/Msn4p-dependent (1, 246, 501) way.
Glycerol metabolic pathways are also important for phospholipid biosynthesis, where glycerol-3-phosphate and dihydroxyacetone phosphate are required as precursors (123). However, it is not known if the systems that control phospholipid biosynthesis impinge on glycerol metabolism directly.
In addition, glycerol metabolic pathways serve as cellular redox valves. A shuttle of dihydroxyacetone phosphate and glycerol-3-phosphate employing two entirely different glycerol-3-phosphate dehydrogenases, one NAD dependent (Gpd1p and Gpd2p) and one flavin adenine dinucleotide (FAD) dependent (Gut2p), provide a means to pass electrons from NADH to FADH and then into the mitochondrial respiratory chain (317). Under anaerobic conditions, when the respiratory chain cannot be employed for the consumption of reducing equivalents, glycerol production is used and is essential for the reoxidation of excess NADH (19, 42, 103). In addition to its role in this shuttle system, Gut2p is also involved in a pathway that channels glycerol into glycolysis (514).
Glycerol can be used by S. cerevisiae as a source for carbon and energy; it is taken up perhaps by Gup1p and Gup2p (234), which encode transmembrane proteins not belonging to any known family of transporter, and it is phosphorylated by the glycerol kinase Gut1p (456, 571) and then oxidized by Gut2p (514, 571) to the glycolytic intermediate dihydroxyacetone phosphate. None of these genes seems to play a direct role in osmoregulation, and their expression is not controlled by osmotic cues; however, the putative glycerol uptake proteins Gup1p and Gup2p were isolated by virtue of their ability to rescue under osmotic stress mutants unable to produce any glycerol in the presence of small amounts of glycerol in the medium, indicating that they may play a role in uptake of glycerol from the surrounding medium (234). Osmotolerant yeasts employ active uptake to accumulate glycerol against a large concentration gradient (310); however, S. cerevisiae has not been reported to have this ability.
As mentioned above, the genes encoding the isoforms of NAD-dependent glycerol-3-phosphate dehydrogenase and glycerol 3-phosphate are differentially expressed and therefore have different physiological roles. This is best illustrated in the case of GPD1 and GPD2. Mutants lacking both genes are unable to produce glycerol (19), although various reports indicate that gpd1Δ gpd2Δ double mutants produce glycerol upon prolonged exposure to high osmolarity (543). The source of this glycerol is not known. Expression of GPD1 is stimulated under various stress conditions, most prominently under osmotic stress (7, 500). However, expression of GPD2 is not upregulated under stress, and its mRNA level drops under high osmolarity (19). Instead, expression of GPD2 is stimulated under anaerobic or microaerobic conditions, indicating that Gpd2p is employed for the reoxidation of NADH under these conditions (19, 103). The different physiological importance of the two isoforms is confirmed by mutant phenotypes. gpd1Δ mutants are sensitive to high osmolarity (1.0 M NaCl [7, 318]), while the gpd2Δ mutant grew somewhat more slowly under anaerobic conditions (19). gpd1Δ gpd2Δ double mutants are highly sensitive to even moderate osmotic stress (such as 0.5 M NaCl) and fail to grow under anaerobic conditions, and the growth arrest is accompanied by accumulation of NADH (19).
The situation is slightly different for gpp mutants. Single gpp1Δ and gpp2Δ mutants are not osmosensitive, while the double mutant is as sensitive as a gpd1Δ gpd2Δ mutant (447). Hence, as is the case for Gpd, the two isoforms can at least partially substitute for each other. Expression of both genes is stimulated under osmotic stress, but this effect is far more pronounced for the normally more weakly expressed GPP2 (226, 426, 447, 501). The mRNA level of GPP2 increases transiently about 50-fold, while that of GPP1 is stimulated only about 4-fold, and both isogenes appear to be expressed to similar levels under osmotic stress. Under anaerobic conditions, the single gpp1Δ mutant shows a slight growth defect, and double mutants fail to grow under these conditions (103, 447). Expression of GPP1 is stimulated under anaerobic or microaerobic conditions (103, 447). The single gpp1Δ mutant accumulates glycerol-3-phosphate but the double mutant accumulates even more, indicating that other phosphatases cannot dephosphorylate this compound. Thus, the pair Gpd1p-Gpp2p seems to form the most important pathway for glycerol production under osmotic stress, while Gpd2p-Gpp1p are more prominent in redox balancing. Whether these isoform pairs have specific biochemical properties, such as different cofactor affinities that make them specifically suitable for these different roles, remains to be investigated.
Control of glycerol production under osmotic stress.
The expression pattern of GPD1 and GPP2 is very similar in the wild type and several mutant strains, suggesting that it is controlled by the same regulatory elements (7, 500, 503). Expression is rapidly and transiently stimulated by an osmotic upshift, up to about 50-fold. In adapted cells growing in high-osmolarity medium, i.e., in adapted cells, the mRNA level is enhanced only between two- and fivefold, depending on the level of osmoticum (7, 19, 155, 318, 503). The Gpd1p and Gpp2p protein levels as well as the specific enzyme activity for both Gdp and Gpp increase under osmotic stress about 3- to 10-fold, again depending on the severity of the stress (7, 45, 318, 424, 425, 447).
The role of the rapid and dramatic overshoot of the mRNA levels in stimulating the production of gene product is not completely understood. The spike in mRNA is not translated into a protein peak; rather, the protein and specific enzyme activity levels increase steadily (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). Perhaps the rapidly increased mRNA level supports initiation of production of the corresponding protein by providing a means to effectively compete for ribosomes. The mRNA profile of GPD1 and GPP2 depends greatly on the severity of the stress. Mild stress, such as 0.5 M NaCl, causes a response within minutes that lasts for 30 to 60 min and has moderate amplitude. More severe stress (1.0 M NaCl) causes a delayed response (30 to 60 min), which is more sustained (120 to 180 min) and with a higher amplitude. When cells are exposed to even more severe stress (1.4 M NaCl), there is no initial transient peak and only after several hours is a three- to fivefold-higher mRNA level observed (500). As discussed earlier, the inability to mount a rapid transcriptional response under severe osmotic stress is accompanied by a delay in phosphorylation and nuclear translocation of Hog1p (622). The molecular basis and physiological relevance of the signaling delay under severe stress are not known; apparently cells undergo an adaptation before HOG signaling initiates the transcriptional response.
The transient character of the transcriptional response, which is paralleled by the phosphorylation state as well as the nuclear concentration of Hog1p, also suggests that it is controlled by a feedback mechanism. This aspect has been discussed above. During growth of adapted cells in high-osmolarity medium, an increased level of GPD1 and GPP2 mRNAs as well as of the corresponding gene products is maintained; the mechanisms controlling the high steady-state levels have not been studied in the same detail.
The rapid and transient transcriptional response to osmotic stress of GPD1 and GPP2 expression is highly dependent on the HOG pathway. The transcript level of both genes is diminished in a hog1Δ mutant under both normal and stress conditions, and the fold stimulation is also strongly diminished. However, after a moderate osmotic shock, there is still a substantial residual, somewhat delayed stimulation of expression. Protein kinase A and Msn2p and Msn4p do not seem to be responsible for this effect (500, 503). Altered protein kinase A activity also does not seem to affect the Gpd1p and Gpp1p protein levels in cells adapted to high osmolarity (423); given the fact that protein kinase A affects most stress-controlled genes, this observation is remarkable. In any case, the signaling pathways and the transcription factors responsible for the HOG-independent part of GPD1 and GPP2 regulation remain to be identified.
The transcription factors important for osmoinduced expression of GPD1 and GPP2 seem to be Hot1p and Msn1p. Deletion of HOT1 strongly diminishes the mRNA level upon osmotic shock. The effect of deletion of MSN1 is minor but significant, and the double hot1Δ msn1Δ mutant has an even lower mRNA level (503). As discussed above, both Hot1p and Msn1p are present on the GPD1 promoter, although their binding site has not been determined. There is strong evidence that Hot1p and Msn1p control glycerol production directly at the transcriptional level. Mutants lacking Hot1p display a clearly diminished glycerol production rate after osmotic upshift and require longer to accumulate the same amount of glycerol as wild-type cells. However, this delay does not translate to a visible growth defect of the hot1Δ mutant on plates with up to 1.2 M NaCl. The msn1Δ mutant, on the other hand, seems to be salt sensitive, but since no sensitivity to high sorbitol levels was observed, this effect is probably unrelated to the role of Msn1p in controlling glycerol production (501; M. Krantz and S. Hohmann, unpublished data).
The promoter of GPD1 has been analyzed to a certain extent (155). This analysis depicted a region from −478 to −324 relative to the transcriptional start which is sufficient to confer osmotic regulation on a reporter system. This region contains three binding sites for the general transcription factor Rap1p, and Rap1p binding to the promoter seems to be essential for its function. The GPP2 promoter does not seem to have Rap1p binding sites. The binding sites for factors that mediate the HOG-dependent and -independent osmoinduction of the promoter have not yet been described.
Following in parallel cell proliferation, glycerol levels, glycerol production rate, GPD1/GPP2 mRNA levels, the phosphorylation state of Hog1p, and the level of Gpd1p allows some interesting conclusions (503; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). After a shift to 0.7 M NaCl, the GPD1 and GPP2 mRNA levels peak within 45 min and fall back to preshock levels within 90 min. Gpd1p protein levels and Gpd enzyme activity increase steadily and reach a plateau after about 90 min. The glycerol production rate also increases steadily up to about 90 min, and then it drops again to a steady-state level that is about threefold higher than the basal level. The internal glycerol concentration starts to increase after about 30 min and reaches its plateau after about 90 min. Hence, there seems to be good correlation between stimulated gene expression, the increased capacity of the glycerol production pathway, and the glycerol production rate. At about the same time point (90 min) at which the mRNA level has dropped to the basal level, the protein level, the glycerol production rate, and the glycerol level reach their maximums, and subsequently the production rate diminishes slowly and the glycerol content levels off. Hence, at this time point it appears that the glycerol production rate is uncoupled from the enzyme level, indicating that glycerol production is now controlled by different mechanisms. How the glycerol production rate is controlled in adapted cells is not known. Cell proliferation resumes after about 90 to 120 min, when cells have reached the final internal glycerol level. More work is needed to monitor different events in osmoadaptation in parallel over time to better understand how those depend on each other.
As discussed above, the expression of the genes GPD1 and GPP2 is stimulated upon different stress treatments, indicative of a role for glycerol production under conditions other than osmotic stress. Recently, it has been reported that the osmosensitivity of the hog1Δ mutant is thermoremedial at 37°C (556). Glycerol levels were found to be stimulated at elevated temperature, suggesting that enhanced glycerol levels may protect the hog1Δ mutant. Consistently, a hog1Δ mutant that was also unable to effectively accumulate glycerol due to a constitutive glycerol export channel (see below) lost the thermoremedial character. Moreover, the gpd1Δ gpd2Δ and gpp1Δ gpp2Δ double mutants were both found to be heat sensitive, and this sensitivity could be partially relieved by adding glycerol to the growth medium. These findings are the first reports that link glycerol to heat tolerance, and the molecular basis for this effect is not understood. Given the pleiotropic effects conferred by the hog1Δ, gpdΔ, and gppΔ mutations as well as heat treatment, conclusions should be made with some caution (556). The gpp1Δ gpp2Δ double mutant was also reported to be sensitive to paraquat, which causes generation of reactive oxygen species. Whether this reflects a role of glycerol in protection from oxidative damage or the need for glycerol-3-phosphatase in the glycerol-dihydroxyacetone phosphate cycle in cellular redox regulation under oxidative stress remains to be determined (447).
Transmembrane flux of glycerol.
The cellular glycerol content is additionally controlled at the level of export. This was unexpected because glycerol can pass the lipid bilayer readily. However, it appears that the yeast plasma membrane is well adapted to the use of glycerol as an osmolyte. Simple diffusion of glycerol is measurable but low, and it seems to be even lower in cells growing in high-osmolarity medium (585, 588). In addition to direct evidence based on glycerol transport studies, there is ample indirect evidence for a low membrane glycerol permeability in S. cerevisiae; mutants either lacking the glycerol export channel Fps1p or expressing constitutively active Fps1p or the Escherichia coli glycerol facilitator GlpF show altered glycerol transport rates and display phenotypes consistent with altered glycerol transmembrane flux (351, 498a, 588, 589, 602).
The specific characteristics of the yeast plasma membrane that cause this low glycerol permeability are not fully understood but may be related to membrane lipid composition and here especially to the sterol content. Upon osmoshock, expression changes of several genes encoding enzymes in lipid metabolism have been observed by global gene expression analysis (501). Enhanced expression of genes such as PSD2 (phosphatidylserine decarboxylase), CKI1 (choline kinase), and OPI3 (phospholipid-N-methyltransferase) may hint at an increased demand for phosphatidylethanolamine and phosphatidylcholine. Perhaps more importantly, strongly and rapidly diminished expression of several ERG genes (86, 501), which encode enzymes involved in synthesis of the yeast membrane sterol ergosterol, could hint at a diminished demand for sterols. Lower levels of ergosterol could make the membrane more compact and less flexible and hence lead to diminished transmembrane flux of glycerol. Recently it was shown that the addition of ergosterol to yeast cells enhances glycerol efflux, especially in mutants lacking the export channel Fps1p or mutants that were unable to produce high levels of ergosterol due to mutation of the ERG1 gene. Ergosterol also increased survival after a hypo-osmotic shock, which requires glycerol export from yeast cells (602). Although these observations require further studies in in vitro systems of defined lipid composition, they hint at the possibility that the yeast cell can actively adjust the flow of glycerol, and perhaps also water, through the lipid bilayer.
Glycerol transport via Fps1p.
The apparently low permeability of the yeast plasma membrane for glycerol allows control of transmembrane flux using a specific transport protein. Fps1p, which was initially isolated as a multicopy suppressor of a tps1 (trehalose-6-phosphate synthase) allele (fdp1 suppressor), causing inability to grow on glucose medium (621), is an important determinant of intracellular glycerol levels (351, 585, 588). Mutants lacking Fps1p show highly diminished flux of glycerol both into and out of the cell and display a number of phenotypes consistent with an inability to rapidly export glycerol from the cell. Most prominently, the rapid export upon a hypo-osmotic shock of about 80% of the glycerol accumulated under hyperosmotic conditions depends almost completely on Fps1p (351, 588). Consequently, fps1Δ mutants die in 100-fold-higher numbers than the wild type upon a hypo-osmotic shock, and those that survive resume growth more slowly. In combination with an slt2/mpk1Δ mutation, which causes sensitivity to hypo-osmotic shock due to a weaker cell wall, deletion of FPS1 causes lethality upon a hypo-osmotic shock and visible cell bursting (463, 588). fps1Δ is so far the only known mutation that causes sensitivity specifically to a hypo-osmotic shock while not notably affecting proliferation in low-osmolarity medium, illustrating the importance of rapid glycerol export upon a shift to low osmolarity.
In addition, deletion of FPS1 also causes an inability to grow normally under anaerobic conditions, when glycerol is produced for redox regulation (588). fps1Δ mutants accumulate high levels of glycerol under these conditions and only grow after a lag phase of more than 1 day. The growth observed then may be due to the expression of a different transport protein that could mediate glycerol export or to altered membrane composition, leading to increased passive diffusion.
The FPS1 gene has been isolated in two additional genetic screens. A search for mutations that cause elevated expression of reporter genes dependent on Sln1p and Skn7p identified alleles of FPS1 that seem to behave like deletion mutations (338, 591). The higher internal glycerol level in fps1 mutants even under normal growth conditions apparently causes cell swelling and hypo-osmotic stress, resulting in enhanced activity of the Sln1p osmosensor system and activation of the Skn7p response regulator transcription factor and Skn7p-dependent reporters, as described above (338, 591). The enhanced reporter gene activity in an fps1Δ mutant is diminished when cells are exposed to high osmolarity, confirming that the fps1Δ mutant has a hypo-osmotic feeling under normal growth conditions.
That inability to export glycerol via Fps1p affects osmotic homeostasis even in the absence of osmotic stress has been confirmed by the isolation of alleles of FPS1 in a screen for mutants that are defective in cell fusion during mating of haploid cells (463). Like the cell integrity pathway, Fps1p is apparently needed to prevent cell bursting during cell fusion. Cell fusion requires highly coordinated cell wall degradation and reestablishment as well as appropriate adjustments of turgor pressure. The cell fusion defect caused by lack of Fps1p can be suppressed by enhanced medium osmolarity or by deletion of GPD1, in line with the idea that Fps1p functions as a turgor valve during cell fusion (463).
Since Fps1p is a glycerol channel required under specific conditions, its functions should be tightly regulated. Indeed, it appears that the Fps1p channel can close upon a hyperosmotic shock and open upon a hypo-osmotic shock within less than 1 min, probably much faster (351, 588). Mutants expressing an FPS1 allele which has lost its ability to close display sensitivity to high osmolarity, a dramatic delay in their ability to accumulate glycerol, while at the same time overproducing glycerol. Such mutants also show strongly enhanced and sustained phosphorylation of Hog1p and expression of Hog1p-dependent genes (589; B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations).
The mechanisms that control Fps1p transport function are not fully understood. However, other proteins that participate in this control have so far not been identified, despite attempts to link known osmosensing signaling pathways to Fps1p control or to specifically search for such mutants (165, 588). Together with the very rapid control of Fps1p, this could mean that Fps1p senses osmotic changes directly. Details on structural aspects of Fps1p control will be discussed below.
Studies on stimulation of the HOG pathway by external glycerol in the wild type and FPS1 mutants further confirm the notion that the yeast plasma membrane is not easily permeable to glycerol: when 1 M glycerol is added to yeast cells, a rapid and transient Hog1p phosphorylation is observed and expression of GPD1 is stimulated (589). This effect is not observed in cells expressing a constitutive allele of Fps1p. Hence, addition of glycerol is perceived by yeast cells as a hyperosmotic shock. Fps1p apparently closes, and cells mount a response aimed at adjusting to high external glycerol (which probably never occurs in nature) by increasing intracellular glycerol. When Fps1p cannot close due to mutation, glycerol appears to equilibrate rapidly between inside and outside, and the initial concentration gradient collapses rapidly. Hence, in this situation only very brief turgor stress is imposed on the cell. This experiment not only illustrates the apparent low glycerol permeability of the yeast plasma membrane, it also suggests that the HOG pathway is indeed stimulated by turgor changes, i.e., by substances that maintain a concentration gradient across the plasma membrane (589).
Interestingly, deletion of FPS1 affects membrane lipid composition. Specifically, such mutants display a lower ergosterol level than wild-type cells. The sensitivity to hypo-osmotic shock of the fps1Δ mutant can be partially suppressed by addition of ergosterol to the medium. The observation could hint at a regulatory role of Fps1p in sterol metabolism, but this could also be an indirect effect due to a response to higher internal glycerol levels and hence increased turgor. More work is required to better understand this phenomenon (602).
The apparent importance of Fps1p in S. cerevisiae osmoregulation suggests that similar proteins also exist in other organisms. Moreover, controlled and specific export of different polyol osmolytes has been observed in Pichia sorbitophila and Zygosaccharomyces rouxii (279a). Recent genome sequencing reveals Fps1p homologs at least in several ascomycetous yeasts as well as other fungi. However, no functional homolog has so far been characterized. Similar functions for osmolyte export appear to be performed by the bacterial proteins MscL and MscS, although their transport function appears to be far more unspecific than that of Fps1p (56, 334). In addition, the process mediated by Fps1p upon hypo-osmotic shock resembles the regulated volume decrease known from mammalian cells. The proteins mediating osmolyte export in these systems are still unknown (307).
Metabolism of Trehalose and Glycogen
Yeast cells produce and accumulate trehalose and glycogen. Trehalose is a nonreducing disaccharide composed of two glucose moieties with α(1,1) linkage, while glycogen is a branched polysaccharide with both α(1,4) and α(1,6) bonds. Both production and degradation of these compounds are regulated by several overlapping mechanisms, not only at the level of gene expression, but also by control of enzyme activity and availability of substrates. The posttranscriptional control mechanisms of glycogen metabolism appear to be well conserved from S. cerevisiae to humans.
Trehalose and glycogen appear to have overlapping though distinct roles in yeast physiology. On the basis of the characteristics of its production and degradation, glycogen appears to have all the features of a typical storage carbohydrate. Trehalose, on the other hand, appears to have in addition, or perhaps mainly, a role as a stress protectant. It is, however, still unclear if trehalose can serve the role of an osmolyte in S. cerevisiae, as it does in bacteria (280, 580, 581). The role as a protectant may rather be specifically relevant for heat stress and extreme forms of osmotic stress, such as desiccation and freezing (236, 527, 561, 580). Hence, it appears that glycerol serves as an osmolyte for proliferating cells, while trehalose accumulation may rather aim at survival when proliferation has ceased. In addition, it appears that metabolism of trehalose plays important roles for the control of glycolysis and potentially as a system fine-tuning the ATP/ADP ratio of the cell under stress (46, 173, 597). For these reasons, I discuss the pathways for trehalose and glycogen metabolism in this context. Recent reviews discuss the roles and the metabolism of trehalose and glycogen in more detail (173, 429, 561, 597).
Trehalose occurs in diverse organisms, such as insects (as blood sugar), fungi, and, as recently discovered, also in plants (401, 561). Especially in fungal spores, trehalose is accumulated to very high levels (593). The trehalose content of commercial baker's yeast preparations may be as high as 20% of the dry mass (622). This high trehalose content, which is achieved by growing cells under constantly low sugar concentrations in a fed-batch system, is believed to protect cells during drying and rehydration as well as serving as an important source for carbon dioxide production during the initial phases of dough leavening. For commercial applications, empirical studies suggest that 10% trehalose (dry mass) is needed for a stable baker's yeast product (622). On the other hand, it has been shown that as little as 3% trehalose (dry mass) is sufficient to protect yeast cells during drying (527). This effect seems to be due to a diminished amount of bound water and the apparent ability of trehalose to replace water around biomolecules, where trehalose appears to form a glass-like structure (111, 112, 330, 527). Numerous studies demonstrate a correlation between the cellular trehalose content and the ability to survive diverse stress conditions, ranging from especially heat shock and desiccation to starvation, oxidative stress, ethanol stress, cold stress, freezing, hydrostatic pressure, and osmotic stress (references 139, 158, 173, 429, 560, and 595 and references therein).
The cellular levels of both trehalose and glycogen are very low in the early phases of proliferation in batch culture with glucose as the carbon source. Glycogen accumulation starts in late logarithmic phase, before glucose is consumed, as expected for a storage carbohydrate. Trehalose is produced after glucose has been consumed almost completely, making use of the residual glucose and the ethanol produced during sugar fermentation. In batch culture, there is an excellent correlation between the expression of genes encoding enzymes of trehalose and glycogen metabolism and the onset of the accumulation of these compounds. When all carbon is depleted, the glycogen stores are consumed first, before the cellular trehalose content is also diminished, consistent with a role of glycogen as a storage compound and for trehalose as a stress protectant. When yeast cells that have accumulated glycogen and trehalose are shifted to a rich medium, glycogen and trehalose are rapidly mobilized before cells start to proliferate (summarized in reference 173).
Studies using partially synchronized continuous cultures with different dilution rates (proliferation rates) suggest that the accumulation of glycogen and trehalose is cell cycle regulated and occurs mainly during the G1 phase. Upon transition through Start, glycogen and trehalose are mobilized, perhaps to provide the cell with a balanced supply of energy during the subsequent S-phase (173, 558, 559, 622, 625). Mutants unable to synthesize trehalose or glycogen do not show a cell cycle defect but appear to compensate by an enhanced ATP production rate (559). Since yeast cultures that proliferate rapidly have few cells in G1 at any given time while slow-growing cultures have many, the much higher trehalose level in the latter may be explained by the apparent cell cycle control of trehalose and glycogen metabolism. Again, there is a good correlation with stress tolerance, since slowly proliferating cells are known to be generally more tolerant than fast-growing cells (139, 595)
The production of both trehalose and glycogen (Fig. 11) starts with the conversion of glucose-6-phosphate to glucose-1-phosphate by phosphoglucomutase (Pgm1p and Pgm2p) and then to UDP glucose by UDP-glucose pyrophosphorylase (Ugp1p). Expression of both PGM2 and UGP1 is stimulated by multiple stresses (86, 191). Glycogen production is nucleated by glycogenin, encoded by GLG1 and GLG2, which produce short α(1,4) chains attached to it. Subsequently, the chains are elongated by glycogen synthase (Gsy1p and Gsy2p), and the branching enzyme, Glc3p, performs branching. The breakdown of glycogen is catalyzed by a debranching enzyme (Gdp1p) and glycogen phosphorylase (Gph1p), which produces glucose-1-phosphate. This pathway is essentially identical to that in mammalian cells (summarized in reference 173). Expression of the genes GLG1, GSY1, GSY2, GLC3, and GPH1, as well as that of some genes encoding regulatory enzymes, is stimulated by multiple stress conditions (86, 191).
For trehalose production, UDP-glucose and glucose-6-phosphate are converted first to trehalose-6-phosphate by trehalose-6-phosphate synthase (Tps1p) and then to trehalose by trehalose-6-phosphatase (Tps2p). Tps1p and Tps2p are part of a complex that also contains Tsl1p and Tps3p. Those are probably alternative, regulatory, or stabilizing subunits (34, 345, 346, 495, 636, 650). Interestingly, all four subunits show about 35% sequence identity over the entire 495 amino acids of Tps1p, although only the latter confers trehalose-6-phosphate activity (34, 495). Possibly, the conserved sequences serve as an interface for complex formation. Tps1p and the unique C terminus of Tps2p are similar to the corresponding E. coli enzymes, which do not form a complex (271). Yeast Tps1p is indeed active even without the other subunits in a tps2Δ tsl1Δ tps3Δ triple mutant (34). Expression of TPS1, TPS2, and TSL1 is strongly stimulated by essentially all stress conditions, while TPS3 expression is only weakly stimulated by heat shock (86, 191, 650).
Trehalose is hydrolyzed by trehalase to two glucose molecules. S. cerevisiae has two entirely different trehalases. Acid trehalase (Ath1p), which has a low pH optimum, seems to be involved in growth with trehalose as the carbon source (432); a report suggesting a role in culture density and stress tolerance (284) suffers from the use of an ath1Δ allele constructed with the URA3 marker, whose complementation causes several of the phenotypes reported (96). Uptake of trehalose is probably mediated by the Agt1p hexose transporter-like protein (467). The mechanism of catabolism of external trehalose is uncertain and may involve endocytosis of trehalose bound to Ath1p, since Ath1p is reported to be a vacuolar enzyme (173, 429). The cytosolic neutral trehalase, Nth1p, is responsible for the breakdown of trehalose accumulated in yeast cells (429-431); the conditions under which Nth2p, which is 78% identical to Nth1p, plays a role are not known. Expression of the NTH1 gene is also stress regulated (669). All the stress-regulated genes whose products are involved in trehalose or glycogen metabolism are controlled by STREs via Msn2p and Msn4p (86, 173, 191, 452, 453, 650, 669, 670).
Despite the apparent correlation between trehalose content and stress tolerance and the long-known ability of trehalose to stabilize proteins and membranes (111, 237, 330), study of the function of endogenously produced trehalose as a stress protectant in vivo was complicated in two ways. As pointed out above, all conditions that enhance trehalose accumulation in yeast cells are essentially the same as those that stimulate a general stress response. Hence, in wild-type cells, it is not possible to distinguish between the effect of trehalose and, for instance, enhanced production of heat shock proteins or the many other effects caused by stress (464). In addition, tps1Δ mutants unable to produce trehalose have a growth defect on fermentable carbon sources. This is due to an unusual control mechanism of glycolysis: the hexokinase step seems to be inhibited by trehalose-6-phosphate and perhaps other, Tps1p-dependent mechanisms (597). This makes it necessary to use a tps1Δ hxk2Δ double mutant, in which the gene for the main hexokinase has been deleted. The double mutant can grow on glucose but, due to the lack of Hxk2p, displays other pleiotropic effects, for instance, in glucose repression (231, 233). The use of a tps2Δ mutant to block trehalose biosynthesis is also problematic, since this mutation has pleiotropic effects due to trehalose-6-phosphate accumulation (597).
In any case, recent elegant studies have demonstrated directly that trehalose participates, together with the chaperone Hsp104p (150, 524), in protection of proteins (560, 561). More specifically, trehalose has the ability to prevent protein denaturation, and it can also prevent the aggregation of denatured proteins, thereby maintaining them in a partially unfolded state from which chaperones can catalyze renaturation to functional proteins. Unexpectedly, however, trehalose also prevents protein renaturation. This observation has resolved a long-standing puzzle in establishing the role of trehalose in stress tolerance; trehalose has to be degraded once stress has been relieved, and for this reason nth1Δ mutants recover poorly from heat stress (431, 643). In addition, by using mutants of the fungus Aspergillus fumigatus defective in trehalose production, in which trehalose-6-phosphate synthase does not seem to be required for growth on glucose, a role of trehalose in the acquisition of tolerance to different stresses has been confirmed (167).
Trehalose reportedly has a role as osmolyte in E. coli, where it is produced during certain stages of osmoadaptation. E. coli uses in addition K+ as well as proline, glycine betaine, and taurine as compatible solutes (280, 315, 580). A possible role of trehalose in yeast osmoadaptation is supported by the Msn2p/Msn4p- and Hog1p-dependent stimulation of expression of the genes encoding all enzymes in trehalose metabolism (501), the reported sensitivity to high osmolarity of the tps2Δ mutant (61, 238), and the diminished survival after severe osmotic stress of mutants unable to produce trehalose (238). However, yeast cells do not actually accumulate trehalose to appreciable levels after an osmotic shock (238, 453). Apparently, under these conditions the simultaneously increased capacity for trehalose production and breakdown results in a futile cycle of trehalose production and hydrolysis. Evidence that such a cycle truly exists comes from the increased trehalose level in an nth1Δ mutant during exposure to high osmolarity as well as under oxidative stress (453, 669). The apparent flux through trehalose metabolism may also explain the osmosensitivity of the tps2Δ mutant, which is not shared by a tps1Δ mutant and hence may be due to trehalose-6-phosphate accumulation.
Apparently, transcriptional activation alone cannot explain the pattern of trehalose accumulation. This leads to two questions. How are trehalose accumulation and breakdown controlled by temperature and nutrient shifts, i.e., the two conditions in which net trehalose accumulation is observed? Why do yeast cells run a futile trehalose cycle under osmotic stress conditions?
Trehalose and glycogen metabolism is controlled at the posttranscriptional level. This has been particularly well studied for glycogen metabolism; it appears that especially protein kinase A but also the Snf1p pathway, the TOR pathway, and the Pho85 cyclin-dependent kinase affect the enzymes for production and breakdown of glycogen (reviewed in reference 173). Protein kinase A appears to control trehalose metabolism at the posttranslational level by phosphorylating both Nth1p (activating it) and Tps1p (inactivating it) (reviewed in references 173, 266, and 429). Recently, it has been shown that protein phosphatase 2A, encoded by PPH22, controls Nth1p in a nutrient-dependent way (582). Under heat stress, the highly different temperature optima of trehalose-producing and trehalose-degrading enzymes may account for the observed rapid accumulation of trehalose upon a shift to high temperature and trehalose hydrolysis upon a shift to ambient conditions. Trehalose synthase operates optimally above 40°C, while trehalase has a temperature optimum of about 25°C (172, 346, 414, 453).
The apparent futile cycles observed under, for instance, osmotic and oxidative stress have stimulated some speculations (46, 173). There is no direct evidence that these cycles play any particular role, but since they use energy, one would intuitively expect them to perform some useful function. The stress-induced proliferation slowdown or arrest in an otherwise rich environment causes an imbalance in cellular metabolism between energy generation and consumption. In order to maintain cellular activity, it may be necessary for the cell to dispose of the energy surplus and a futile, ATP-consuming cycle of trehalose, glycogen, or even glycerol metabolism would be an option. At the same time, trehalose-6-phosphate as an intermediate of the pathway may reduce sugar influx into glycolysis by inhibiting the hexokinases. On the other hand, given the fact that the flux through the trehalose and glycogen pathways is about 100-fold lower than that through the glycolytic pathway (173), it can be questioned if such futile cycles provide an efficient system for maintaining the energy balance. As mentioned above, cells also seem to have a higher energy demand for biomass production under stress, suggesting that futile cycles may only be of relevance during adaptation, before growth resumes. More work is needed to better understand the phenomenon.
Other osmolytes.
Apparently S. cerevisiae employs glycerol exclusively as an osmolyte in growing cells. However, mannitol, sorbitol, and xylitol can perform this function too, provided yeast cells are engineered to produce those compounds (92, 294, 543). Using a test system making use of constitutive Fps1p, which allows diffusion into the cell of a range of polyols, I and a colleague have recently shown that essentially all polyols transported by the system are compatible with yeast growth, indicating that high intracellular levels of many polyols are indeed tolerated (S. Karlgren and S. Hohmann, unpublished data). Other yeasts and fungi are known to produce and/or accumulate from the environment different polyols as compatible solutes, although genetic studies that confirm the importance of these compounds for growth under osmotic stress have not been reported. Reported examples of such compounds include erythritol, ribitol, arabinitol, xylitol, sorbitol, mannitol, galacticol, and others (reviewed in reference 48).
Genes Whose Expression Responds Specifically to Hyperosmotic Shock
Causton et al. list 179 genes whose expression responds to sorbitol or salt stress but not (or in a reverse way) to heat shock, peroxide, acidity, or alkali (86). A subset of about 40 of those genes display altered expression specifically to salt but not to sorbitol. From the approximately 140 specifically sorbitol-affected genes, 33 show enhanced expression and the rest show diminished expression after hyperosmotic shock.
Generally, the effects on mRNA levels of this set of genes were highly short-lived and rather weak, with only a few exceptions. Such short-lived effects may be due to the observed osmoshock-induced perturbation of the nucleus, which is apparent by the transient relocation of many nuclear and nucleolar proteins to the cytoplasm after hyperosmotic shock (407a). Only two genes showed a more than 10-fold-stimulated expression: YLR042C, which encodes a putative glycosylphosphatidylinositol (GPI)-anchored protein of unknown function, and HAP2, which encodes a component of the transcription factor complex required for expression of genes encoding mitochondrial functions discussed above. The other genes, whose mRNA level was stimulated less than 10-fold, encode diverse functions or are uncharacterized, making it difficult to predict any physiological significance. None of these genes is known to be controlled by the HOG pathway. The significance of the observed transcriptional response of these genes for cellular physiology may be questionable, and certainly its assessment requires much more work.
An interesting example of an (almost) osmospecific gene is STL1, which encodes a sugar transporter homolog of unknown function. As mentioned above, this gene appears to be the most strongly osmoinduced yeast gene, and stimulation by osmotic stress depends on the HOG pathway and Hot1p (501). In addition to osmotic stress, only nutrient starvation seems to stimulate STL1 expression (191).
For the subset of genes whose expression level is diminished specifically after hyperosmotic shock but not by other stress treatments, the significance of the observed effect may be doubtful (86). In most instances, diminished expression was only observed in the first sample (after 15 min), while subsequently the mRNA level recovered to (almost) that before stress. Very few genes show a strongly reduced mRNA level upon sorbitol shock, such as BNR1 (a protein related to Bni1p, which in turn is a target of Rho1p [Fig. 8]), RSC1 (encoding a component of the RSC (remodeling the structure of chromatin) complex involved in chromatin remodeling), or RPN1 (encoding a component of the 19S regulatory cap of the 26S proteasome complex). Once again, elucidation of the significance of these transcriptional effects awaits further studies.
Genes Whose Expression Responds to Hypo-Osmotic Shock
Gasch et al. (191) studied the response after shifting cells from 1 M sorbitol to medium without sorbitol. This is a fairly mild hypo-osmotic shock, and therefore the response is not very pronounced. Generally a rapid reversion of the hyperosmotic response was observed, i.e., the mRNA levels of the genes that were increased under hyperosmotic conditions dropped rapidly after hypo-osmotic shock. In addition, however, it appears that expression of some genes becomes stimulated under these conditions, although in almost all instances the effect was highly short-lived (within the first 5 to 10 min). Often, such genes are also induced by heat shock. Interestingly, SLT2/MPK1, which encodes the MAP kinase of the cell integrity pathway, is one of these genes. As discussed above, Slt2/Mpk1p is phosphorylated and activated under these conditions (125, 272). The Slt2 cluster encompasses about 20 to 30 genes. Among those are genes whose function may be relevant for hypo-osmotic signaling such as ASK10, which is a suppressor of skn7Δ (see above) (446), RCK2, which encodes a protein kinase controlled by Hog1p (see above) (40), and CMP2, which encodes a catalytic subunit of calcineurin. Since a hypo-osmotic shock causes a calcium pulse (30), enhanced expression of calcineurin may have some significance. It is noteworthy that many genes in the Slt2 cluster encode mitochondrial functions.
Certainly more work is needed to elucidate the transcriptional response of yeast cells to hypo-osmotic shock. This will require experiments with harsher shock conditions (such as from 2 M sorbitol to medium without sorbitol) and the use of an fps1Δ mutant, which is expected to show a more pronounced and sustained response because of its inability to rapidly export glycerol and hence to diminish excessive turgor (351, 588). Subsequently, mutations in known regulators mediating hypo-osmotic signals, such as SLT2/MPK1, RLM1, and SKN7, may prove useful to characterize the response further.
TRANSPORT SYSTEMS INVOLVED IN OSMOADAPTATION
MIP Channels
The Fps1p glycerol channel discussed above belongs to the MIP (major intrinsic protein) family of channels, proteins that have been identified in organisms ranging from archaea to humans (232). MIP channels encompass water-transporting aquaporins and glycerol facilitators (57), but the substrate spectrum of certain family members appears to be broader and encompasses many uncharged molecules (175) and even ions (655, 662). MIP channels have six transmembrane domains (TMDs), and intramolecular sequence and structural similarity indicates that they have developed by direct duplication of a protein with three TMDs (223). In addition to the six TMDs, the loops B and E form two half-TMDs that seem to meet within the lipid bilayer to form essentially a seventh TMD that participates in the formation of the pore. The crystal structure of E. coli GlpF has been determined (175), and structural information at approximately 3.5 Å resolution, consistent with this domain arrangement, is available for mammalian AQP1 (133, 152, 402).
Both half-TMDs carry the MIP channel signature motif asparagine-proline-alanine (NPA), which is almost perfectly conserved among the more than 200 MIP channels found to date (223, 229, 263). The NPA motifs are part of the channel constriction. All MIP channels reconstituted so far are homotetramers, with each subunit forming an individual pore (574). Transport specificity has attracted a lot of interest because GlpF, for instance, was reported to transport glycerol in preference to water, making a simple size exclusion mechanism unlikely. Moreover, a water-transporting channel must exclude protons (in the form of H3O+) to prevent collapse of the electrochemical gradient across membranes. Structural information as well as biophysical studies suggests the formation of a successive series of hydrogen bonds between the substrate and titratable sites within the channel, and the specific orientation of such sites in addition to size parameters determines transport specificity (175, 402, 672, 673).
Most bacterial organisms have either no, one, or commonly two (rarely more) MIP channels (229). In most bacteria that have two MIP channels, such as E. coli, one is a glycerol facilitator (GlpF), involved in glycerol uptake for its catabolism as a source for carbon and energy, and the second one is an aquaporin (AqpZ in E. coli). The physiological role of E. coli AqpZ is not fully understood, but it is believed to facilitate water efflux during rapid cell proliferation and hypo-osmotic conditions (76, 77, 132). MIP channels are highly common in plants; Arabidopsis has 36 MIP channels in four subfamilies (98, 263, 528). Those occur both in the plasma membrane and in the tonoplast (the vacuolar membrane), and they are expressed differentially in response to environmental and developmental signals. Plant MIPs are channels of different specificity and together form a network for the transcellular transport of water and solutes. Finally, humans appear to have 10 MIP channels expressed in different tissues having different, partially overlapping roles in cellular as well as whole-body water homeostasis (57, 419, 628). Best studied are the kidney aquaporins, which mediate transcellular water flux between the bloodstream and urine. AQP2, which is regulated by vasopressin, is needed for the concentration of primary urine in the mammalian kidney (127, 128).
Given the importance of MIP channels in diverse organisms, MIP channels in S. cerevisiae have attracted some attention. Genome sequencing has revealed the existence of four genes encoding such proteins, the glycerol facilitator-like Fps1p and Yfl054p and the water channels Aqy1p and Aqy2p.
Fps1p.
The role of Fps1p as a regulated glycerol export channel has been discussed above. Fps1p is an unusual member of the family for different reasons. First of all, the canonical NPA motifs in loops B and E are not conserved in Fps1p but instead replaced by NPS and NLA. Remarkably, introducing these changes into E. coli GlpF renders that protein nonfunctional (39). This suggests additional structural differences between these otherwise homologous proteins. Indeed, structural modeling suggests that the pore of Fps1p may be more flexible, which could be a reflection of its specific role in export rather than uptake and the fact that its transport function is regulated.
In addition to divergent NPA motifs, Fps1p is characterized by large N- and C-terminal extensions (about 250 and 150 amino acids, respectively), which make the entire protein 669 amino acids long, compared to 281 amino acids in the related GlpF. Partial sequence information indicates that Fps1p homologs from other yeasts (569) also possess such long extensions, but sequence conservation seems to be poor. A marked exception is a well-conserved short sequence element located in the N terminus between amino acids 222 to 238, just 20 residues in front of the first TMD. Deletion of this sequence or mutation of specific amino acids within this domain renders Fps1p constitutive, leading to an inability to accumulate glycerol under osmotic stress (see above) and hence osmosensitivity (351, 588; M. J. Tamás, S. Karlgren, J. M. Thevelein, and S. Hohmann, unpublished data). The sequence of this domain (PKTLYQNPQTPTVLPST) does not reveal information on its functions. Remarkably, the 216 amino acids upstream of this element can be deleted without affecting regulation of Fps1p. Recent data indicate that elements within the C-terminal extension may also be required for the closing of Fps1p upon osmotic upshock (K. Hedfalk, R. Bill, J. Rydström, and S. Hohmann, unpublished data).
The molecular function of these regulatory domains is not understood. Other proteins that might be involved in regulation have not been found. This fact, together with the apparent speed with which closing seems to occur, could indicate that Fps1p functions as an osmosensor for its own sake, perhaps similar to volume-regulated or stretch-activated channels, which have been known for many years based on biophysical and physiological studies on mammalian and bacterial cells. They are thought to play a role in cell volume (osmo)regulation by controlling the export of solutes and ions from the cell, like Fps1p (227, 584). The molecular identity of such channels is still elusive in mammalian cells. By far the best studied system is bacterial MscL. This protein mediates the nonselective export of solutes from bacterial cells upon a drastic hypo-osmotic shock (584). Recently the crystal structure of the channel has been reported (37, 583). Modeling based on this structure as well as a series of cross-linking and mutagenesis experiments supports a two-stage model of channel opening, which leads to an increase in pore diameter of from 1 to 13 Å. In this model, membrane tension leads to a conformational change from a closed to closed-expanded and eventually open conformation, where certain transmembrane helices twist dramatically within the membrane (37, 583). These pioneering studies will certainly stimulate further research in the function of stretch-activated channels.
There are several possibilities for how Fps1p functions. The regulatory domain may fold so that it closes the channel, similar to the situation with gated K+ channels in animals (265). Alternatively, the extensions may help to orient the Fps1p TMDs in the membrane so that they are specifically sensitive to membrane stretching, in analogy to MscL (37, 583). It can also not be excluded that these domains interact with other proteins, such as the actin cytoskeleton, leading to conformational changes upon osmotic shock-induced disturbances. However, attempts to identify proteins interacting with Fps1p have failed so far. Further studies in this direction will require defined in vitro systems and purified protein. The power of such systems was demonstrated recently; BetP, an osmoregulated betaine carrier of Corynebacterium glutamicum, was shown to be regulated by the internal K+ concentration rather than by mechanical stimuli. Hence, this protein functions as a chemosensor, using an ion as a concentration-dependent second messenger for cell shrinkage (518). A similar mode of regulation has been proposed for the KdpD two-component osmosensor of E. coli (269).
In addition to Fps1p, S. cerevisiae possesses a second gene whose predicted product groups with the glycerol facilitator subfamily (229). Yfl054p is also unusual because it is predicted to have an even longer, 300-amino-acid N-terminal extension, which does not show sequence similarity to that of Fps1p. Homologs of Yfl0564p exist in other fungi. Attempts to allocate a function to Yfl054p have failed so far.
Yeast aquaporins.
Genome sequencing revealed the existence of two genes, AQY1 and AQY2, which are predicted to encode aquaporin water channels, most closely related to plant plasma membrane water channels (229). Remarkably, both genes appear to be inactivated by mutations in most laboratory strains (54, 311). AQY1 has two point mutations that contribute to inactivation of the gene product, while AQY2 is interrupted by an 11-bp deletion. However, the somewhat unusual strain Σ1278b, which is also used to study pseudohyphal development, appears to have functional AQY1 and AQY2 genes (82, 311). While the water transport function of Aqy1p has been demonstrated in the heterologous oocyte system, water transport via Aqy2p could not be shown in that system (54, 82, 382).
The physiological role of the yeast aquaporins is not well understood, but gene and protein expression data suggest that the two 83% identical gene products may have different roles. AQY1 is expressed in both haploids and diploids after the diauxic shift, and the mRNA level increases most strongly in diploids entering meiosis upon nitrogen depletion (F. Sidoux-Walter, V. Laizé, and S. Hohmann, unpublished data). The gene product, however, could not be detected in vegetative cells (382; F. Sidoux-Walter, V. Laizé, and S. Hohmann, unpublished data). Rather, when monitored by using an Aqy1p- GFP fusion, it becomes visible in yeast spores, where it appears to be expressed abundantly (F. Sidoux-Walter, V. Laizé, and S. Hohmann, unpublished data). In a heterozygous diploid expressing a genomic AQY1-GFP fusion, only two spores contained the fusion protein, suggesting that only mRNA produced after spore separation is used. Aqy1p may be involved in spore maturation (water outflow) and/or spore germination (water inflow).
AQY2, on the other hand, is expressed exclusively in proliferating cells, and the mRNA diminishes after nutrient depletion (382; F. Sidoux-Walter, V. Laizé, and S. Hohmann, unpublished data). Interestingly, expression of AQY2 is diminished upon a hyperosmotic shock in an apparently HOG-dependent manner. Subsequent hypo-osmotic shock reactivates gene expression. In addition, expression of AQY2 is activated by protein kinase A and here specifically by the Tpk2p isoform of the catalytic subunit (509). These observations could indicate that Aqy2p plays a role in water efflux in turgor control during rapid growth and under low-osmolarity conditions, as suggested for E. coli AqpZ (76).
Ion Transport
Patch clamp studies have provided evidence for the existence of stretch-activated ion channels in yeast cells (206), which could potentially be involved in osmoregulated transport of ions. Recently it was shown that expression of S. cerevisiae Mid1p in a hamster ovary system results in the appearance of a stretch-activated nonselective cation channel (275). Mid1p is an integral protein of the plasma membrane; it has 458 amino acids with four predicted transmembrane domains (243). The channel observed in ovary cells seems to have properties similar to those described by Gustin and coworkers (206), except for the fact that Mid1p mediates calcium conductance in ovary cells. The significance of the finding of Kanzaki and colleagues remains, however, unclear, since in yeast cells Mid1p appears to require a second protein, Cch1p, for calcium transport into the cell (448). Cch1p is a large protein of 2,039 amino acids with 24 predicted transmembrane domains, which shows homology to voltage-gated calcium channels from higher eukaryotes (343, 448). Hence, Cch1p could be the actual calcium pore, while Mid1p might be a regulatory subunit. In this case, Mid1p might activate a channel in ovary cells, and the properties monitored may be those of a hamster ion channel (275).
Simultaneous deletion in S. cerevisiae of both CCH1 and MID1 causes the same defects in calcium uptake as in either single mutant, suggesting that both proteins function in the same system (343, 448). Mid1p- and Cch1p-mediated calcium uptake is necessary for the recovery of yeast cells from arrest caused by mating pheromone. The mechanisms by which calcium, calmodulin, and the calcium-dependent calcineurin protein phosphatase relieve this growth arrest (396), as well as the mechanisms that activate calcium uptake under these conditions, are not well understood.
In yeast cells, a hypo-osmotic shock stimulates an increase in the cytosolic concentration of calcium ions (30). This increase is rapid and transient and is eliminated in the presence of gadolinium, a blocker of stretch-activated channels. The initial source for calcium ions seems to be intracellular stores, probably the endoplasmic reticulum. Subsequently, calcium is apparently taken up from the medium, because calcium chelators inhibit later stages of the calcium pulse (30). It has not been reported if the Cch1p/Mid1p system is responsible for this calcium uptake. If so, this would indeed indicate that Cch1p and Mid1p form a stretch-activated calcium channel in yeast cells. The mechanisms controlling the calcium pulse and its physiological significance are unclear.
The uptake or export of ions from the cell plays important roles in osmoadaptation in essentially all types of organisms. For instance, bacteria such as E. coli activate uptake systems for K+ ions under high osmolarity in order to accumulate the ion as an osmolyte (653). In mammalian cells, regulated volume decrease upon hypo-osmotic shock-induced cell swelling has been known for a long time to be mediated by stretch-sensitive channels that cause efflux of osmolytes, including different ions (314). Yeast cells specifically control the intracellular proportion of Na+ and K+ ions, actively exporting the former and accumulating the latter. K+ and Na+ transport is mediated either by ATP-driven active transport or via H+ antiport, the latter making use of the proton gradients across both the cytoplasmic and vacuolar membranes, which are generated by highly activated ATPases (for reviews, see references 510, 539, and 540). While these mechanisms are specifically important for ion homeostasis under salt stress, several of the transporters have also been implicated in osmotic adaptation and/or are regulated by osmotic signals.
Ena1p is a P-type ATPase that mediates the active efflux of Na+ ions from the cytosol to the exterior (213). ENA1 is part of a gene cluster whose number of repeats is strain specific (645). Expression of the ENA1 gene is controlled in a highly complex manner by glucose repression, calcineurin, and the HOG pathway via Sko1/Acr1p (365, 481). Mutants lacking the ENA genes are highly sensitive to even low concentrations of Na+ and Li+, especially at neutral pH, but they do not display osmosensitivity, and therefore it is unclear why the HOG pathway controls the gene. At low pH, which is prevalent in the natural environment of S. cerevisiae, proton gradient-driven transport processes play an important role: Trk1p and Trk2p mediate high-affinity uptake of K+ (289), while Nha1p mediates the export of Na+ and K+ from the cell. In this way, Nha1p contributes to ion homeostasis, and an nha1Δ mutant is sensitive to both high Na+ and high K+ levels. Nha1p is part of a family of fungal Na+/H+ exchange proteins and has an unusual C-terminal extensions that might have a regulatory role (27, 286).
Nhx1p is related to intracellular mammalian Na+/H+ exchange proteins and has an endosomal location (408-410). The protein is thought to be involved in the sequestration of Na+ into the vacuole, thereby contributing to adaptation to high-salt medium (408). In addition, it has been reported that Nhx1p also contributes to growth in high-sorbitol medium, and this effect requires a low external pH, consistent with transport driven by the proton gradient (410). More specifically, Nhx1p seems to contribute to the early response to high osmolarity, an effect that correlated with an apparent role of Nhx1p in the recovery of vacuolar morphology after a hyperosmotic shock (410). This may indicate that Nhx1p contributes to osmoregulation of the vacuole by importing ions (see below).
Osmolyte Uptake
Bacteria accumulate osmolytes, especially amino acids and their derivates, from the environment (113, 653). Yeasts in general are known to employ active uptake of polyols from their environment (310). Although the ability of yeasts to accumulate external glycerol seems to correlate with halotolerance, little is known about the actual importance of uptake for osmoregulation due to the lack of specific mutants. S. cerevisiae has been reported to perform glycerol/H+ symport under specific growth conditions (309, 585). Although glycerol accumulation from the environment has not been reported, gpd1Δ gpd2Δ mutants can be rescued on high-salt medium by addition of as little as 5 mM glycerol (234), suggesting a capacity to accumulate externally provided glycerol. The gene encoding Gup1p (and subsequently its homolog Gup2p), a protein with 8 to 10 predicted transmembrane domains unrelated to known transport protein families, was isolated in a screen for gpd1Δ gpd2Δ mutants that had lost the ability to recover from salt stress in the presence of glycerol. Both proteins seem to be involved in glycerol/H+ symport, and also for the utilization of glycerol as a carbon source, but it is not known if they mediate transport themselves (234). Gup1p has also been linked to triglyceride biosynthesis (433).
Global expression analysis revealed that expression of PUT4, which encodes high-affinity proline uptake (622), is strongly and specifically stimulated under hyperosmotic conditions. Since proline is an osmolyte in plants, yeast cells may find abundant proline in their environment and hence could potentially use it as an osmolyte as well. However, this has not been studied systematically.
Possible Roles of the Vacuole in Osmoadaptation
In plants, the vacuole appears to play an important role in osmoregulation. This organelle takes up a volume exceeding that of the cytosol, often by more than 10-fold, giving it the potential to serve as a water reservoir for the cytosol and its highly osmosensitive chloroplasts. In addition, plant vacuolar membranes (the tonoplasts) are exquisitely permeable to water, due to the presence of abundant water channels (98, 374, 614). A. thaliana has 10 different vacuolar water channels (TIPs) (262), and maize has even more (93). However, the much smaller yeast vacuole does not seem to perform a similar function, and its membrane does not seem to be specifically water permeable (108).
On the other hand, the vacuole itself has to adjust to altered osmolarity. In order to maintain its shape, it is likely to have a slightly higher turgor pressure than the surrounding cytosol. Interestingly, it appears that the vacuole shrinks after an osmotic shock (410). Subsequently, it appears that the vacuole recovers, indicating an active vacuolar osmoregulation. The recovery requires Nhx1p, an endosomal Na+/H+ exchange protein (see above); perhaps Nhx1p contributes to vacuolar osmoregulation by increasing the concentration of Na+ or other ions in the vacuolar lumen (410). In fission yeast, a dynamic behavior of the vacuole under osmotic changes has also been observed. Upon a hypo-osmotic shock, smaller vacuole fragments seem to fuse to a larger structure, while upon hyperosmotic shock, the vacuole tends to fragment. In this way, the same amount of vacuolar membrane may accommodate different vacuolar volumes. Hyperosmotic shock-induced fragmentation requires both Sty1 and Pmk1, the fission yeast counterparts of budding yeast Hog1p and Slt2/Mpk1p, respectively. Vacuolar fusion upon hypo-osmotic shock requires Pmk1, upstream components of the Sty1 pathway, and Sty1, but not its hyperphosphorylation. The physiological relevance and the mechanism of this phenomenon are not understood (53, 182).
Several additional observations relate to vacuolar osmoregulation without providing any clear picture. Many years ago a range of salt-sensitive mutants with defects in vacuolar biogenesis were isolated, but only 2 of 17 complementation groups have been allocated to known genes (319, 320). Those mutations, doa4 and vps27, confer sensitivity to a number of conditions, making the relevance of salt sensitivity doubtful. Another link between osmoregulation and vacuolar function comes from the observation that osmotic stress results in production of phosphatidylinositol-3,5-bisphosphate from phosphatidylinositol-3 phosphate. A candidate protein for performing this reaction is, among others, Fab1p (145), which is required for normal vacuolar morphology and function (101). Again, the relevance to osmoadaptation is not understood.
STRIVING FOR AN INTEGRATED VIEW OF OSMOADAPTATION
As becomes clear from this overview, studies on yeast osmoadaptation easily turn into a journey through many aspects of cellular biology and physiology. Osmoadaptation encompasses adjustment of cell proliferation, conserved signaling pathways, impressive dynamics of subcellular protein localization, adjustments at the level of the cytoskeleton, control of morphogenesis at the cell and organelle level, an astonishing resetting of the gene expression program at the level of transcription and translation, and wide-ranging adjustments of cellular metabolism. Changes of the osmolarity of the growth medium can be well controlled in the laboratory, which also contributes to the interest in studying the responses. In the era of global expression analysis, we see for the first time the possibility of obtaining a comprehensive picture of certain cellular systems or even the whole cell. For the reasons mentioned above, osmoadaptation has the potential to become a cellular system for which global, integrative insight might become feasible in the foreseeable future; some conditions to achieve this goal are summarized below.
Apparently we know the function of only about 40% of the genes whose expression increases or decreases upon osmotic shock. We might not necessarily need to know the function of all those genes or proteins in detail. First of all, the significance of altered gene expression, especially when only moderate, may be questionable. Apart from a detailed gene-by-gene analysis, straightforward and simple approaches to testing such significance are not available. Responsiveness of the mRNA level to genetic changes of known signaling pathways may at least strengthen relevance. Moreover, for many of the proteins it may, in a first approximation, be sufficient to allocate them to molecular systems or pathways. Studies on the now available comprehensive yeast knockout mutant collection as well as clustering of different microarray data may help in this regard. On the other hand, even “known” genes or proteins involved in stress responses are in fact poorly characterized, such as the genes encoding certain heat shock proteins. At a further advanced level, we also lack an understanding of the specific properties of alternatively expressed isogenes, while we often know very well the biochemical function of their products. Functional analysis of the genes and proteins involved in osmoadaptation is certainly needed when we strive for a deeper understanding.
For an integrated picture, we need not only qualitative information, such as knowledge of the proteins, pathways, and events during adaptation; we also need to address questions such as what proportion of Hog1p is in the nucleus during the first 10 min after an osmotic shock? Hence, we are in need of quantitative data, data on spatial organization, and data addressing the time line of events in order to obtain a comprehensive view of the dynamics of the underlying adaptation process. These types of questions are of course not restricted to osmoadaptation, but in fact are central to the objectives of systems biology in general. Microarray analysis provides us with quantitative data, since we can assess expression levels and fold changes. We can also design microarray analyses to provide data on the time course with which expression levels change, and with certain limitations we can also conclude rates of changes. Similar data can be obtained from global protein expression. We also can measure, though presently only with large limitations at a global scale, the levels of many metabolites and the rates of change during adaptation. To measure the rate of a futile cycle is already less simple, as is the determination of the in vivo characteristics of each enzyme and its isoforms. Even more difficult to assess, for instance, is the exact proportion of the Hog1p kinase in the nucleus and the cytosol at a given time point and the rate with which the protein shuttles between the two compartments.
Advanced microscopic techniques allow us to address such questions, and they also start to allow the observation of more than one protein at a time and protein-protein interactions. Still, the resolution of these techniques is a limitation. In any case, these types of quantitative data are also needed to generate realistic computer models of the adaptation process, which in turn will be helpful to test the dynamics, flexibility, and robustness of the underlying cellular mechanisms, as illustrated for cell cycle control (reviewed in reference 615).
In addition, we lack an understanding of the ultimate interdependence of different events in osmoadaptation. The underlying problem is illustrated by trying to understand the feedback control of signaling through the HOG pathway or its cross talk with the pheromone response pathway. As discussed in detail above, active Hog1p kinase is needed to shut down the HOG pathway during adaptation, and it is needed to minimize cross talk. This simply means that the Hog1p kinase itself is involved in these two control systems or any other event whose execution depends on Hog1p. This may often not be so simple to distinguish. In this particular case, mutants that fail to produce glycerol show effects similar to those of a hog1Δ mutant, i.e., sustained signaling though the HOG pathway and also some cross talk. This leads to a different interpretation of the interdependence of events in signaling. Therefore, to understand interdependence, it is necessary to combine genetic analysis with the use of well-defined genetic changes and growth conditions for the generation of quantitative and preferably global data.
Another aspect of interdependence is the time line of events. It is trivial that event A (such as shutdown of the HOG pathway) can depend only on event B (glycerol production) when B starts before A. In the following, I try to summarize the time line of events after osmotic shock in yeast cells as interpreted from observations from different studies performed under different growth conditions. To advance the resolution and precision of this time line is a major goal of future studies.
Time Course of Events in Adaptation to Hyperosmotic Shock
The time course of events depends on the severity of the shock, i.e., the time window is smaller after a mild shock and progressively longer after a more severe shock.
Within seconds after a shift to high osmolarity, the cell loses water and shrinks to a fraction of its initial volume, which can be less than 50% upon severe shock, such as with 1.2 M NaCl (6). Also within seconds, osmosensors such as those of the HOG pathway are stimulated. Osmoresponsive channels also respond immediately; the transport capacity of Fps1p drops within less than 15 s after the shift (351, 588). Signaling through the HOG pathway, i.e., until phosphorylated Hog1p becomes detectable, takes about 1 min (61, 357) (if measurements are reliable at this time scale) and also within approximately the first 3 min nuclear accumulation of Hog1p and Msn2p/Msn4p can be observed (166, 199, 497). Upon more severe stress, there is a delay in the occurrence of Hog1p phosphorylation and nuclear accumulation, which suggests that certain adaptation events have to be completed before signaling progresses (622). At this early point the cell also arrests proliferation and begins to adjust protein biosynthesis by posttranslational control mechanisms. The increase or decrease in the mRNA level of osmoresponsive genes starts remarkably simultaneously and is observed within less than 10 min after a mild shock (191) and more than 1 h after a severe shock (500, 622), approximately in phase with the occurrence of phosphorylated Hog1p.
The onset of alterations of the mRNA levels seems to be followed with some delay by an increase or decrease in the corresponding protein levels (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). The cell certainly adjusts its basic gene expression machinery to the new conditions, although the details are not understood. Metabolic adjustments, most importantly glycerol accumulation, are usually not observed before 1 h after the shock. The onset of glycerol accumulation marks the decline of the level of phosphorylated Hog1p, and mRNA levels now start to return to prestress levels (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations) within about the same time period it took initially to reach peak or bottom levels, respectively, typically about 30 min for a mild shock and 60 min for a more severe shock (86, 191, 500). Glycerol accumulation proceeds for 2 h or more, depending on the severity of the shock, and proliferation resumes at about the same point that maximal glycerol levels are achieved (B. Nordlander, M. Rep, M. J. Tamás, and S. Hohmann, unpublished observations). Subsequently, it appears that the cell fine tunes the adaptive response; for instance, it can be observed that the mRNA level of osmoresponsive genes peaks again at a later time point, although reaching much lower levels (M. Krantz and S. Hohmann, unpublished data). The steady-state mRNA level of osmoresponsive genes such as GPD1 and GPP2 in adapted cells growing at high osmolarity depends on the severity of the stress but commonly is only a fraction of that of the peak level shortly after the shock. Fully adapted cells resume proliferation, are characterized by an adjusted carbon, energy, and redox metabolism, maintain a higher glycerol level, and display altered cell surface properties.
Time Course of Events in Adaptation to Hypo-Osmotic Shock
When cells are shifted from high to low osmolarity, they take up water and swell within seconds, leading probably immediately to the stimulation of sensors and osmoresponsive channels. Within the first minute after the shock, this leads to an increase in the cytoplasmic calcium level (30), opening of Fps1p (351, 588), and increase in the level of phosphorylated Slt2/Mpk1p (125). Accumulated glycerol leaves the cell within the first 3 min after the shock (125), and this event seems to determine the feedback control of Slt2/Mpk1p phosphorylation, since it is more sustained in a mutant lacking Fps1p. Gene expression responses after an osmotic downshift from 1 M sorbitol, a mild shock, are observed within about 5 to 10 min, and the level declines rapidly (191). It is not known if this short response leads to altered protein levels. The mRNA level of genes whose expression is enhanced in high-osmolarity medium starts to drop within 10 to 15 min after the hypo-osmotic shock (191). It has not been determined when cells resume proliferation or if they actually arrest proliferation after the shock. Cells that have adapted to low osmolarity are characterized by low glycerol levels, and they have adjusted their cell surface composition.
Acknowledgments
I thank the many colleagues, especially L. Adler, G. Ammerer, A. Blomberg, J. Fassler, J. Labarre, M. Proft, F. Posas, B. A. Prior, and P. Sunnerhagen, who shared data and discussed their interpretation. Special thanks go to A. Gasch for help with the interpretation of microarray data and the members of my group for many discussions and patience during preparation of the manuscript. I apologize to those who might feel that their work was not adequately taken into account.
I am a special researcher (särkild forskare) supported by the Swedish Research Council. Work in my laboratory is supported by grants from the Swedish Research Council, Formas (the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning), several contracts with the European Commission, and the Human Frontier Science Organization.
REFERENCES
- 1.Aguilera, J., and J. A. Prieto. 2001. The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr. Genet. 39:273-283. [DOI] [PubMed] [Google Scholar]
- 2.Aiba, H., H. Yamada, R. Ohmiya, and T. Mizuno. 1995. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 376:199-201. [DOI] [PubMed] [Google Scholar]
- 3.Akada, R., J. Yamamoto, and I. Yamashita. 1997. Screening and identification of yeast sequences that cause growth inhibition when overexpressed. Mol. Gen. Genet. 254:267-274. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar, N., A. K. Pahlman, K. Larsson, A. H. Corbett, and L. Adler. 2000. SGD1 encodes an essential nuclear protein of Saccharomyces cerevisiae that affects expression of the GPD1 gene for glycerol 3-phosphate dehydrogenase. FEBS Lett. 483:87-92. [DOI] [PubMed] [Google Scholar]
- 5.Alberts, A. S., N. Bouquin, L. H. Johnston, and R. Treisman. 1998. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273:8616-8622. [DOI] [PubMed] [Google Scholar]
- 6.Albertyn, J., S. Hohmann, and B. A. Prior. 1994. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr. Genet. 25:12-18. [DOI] [PubMed] [Google Scholar]
- 7.Albertyn, J., S. Hohmann, J. M. Thevelein, and B. A. Prior. 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14:4135-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alepuz, P. M., K. W. Cunningham, and F. Estruch. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol. Microbiol. 26:91-98. [DOI] [PubMed] [Google Scholar]
- 9.Alepuz, P. M., A. Jovanovic, V. Reiser, and G. Ammerer. 2001. Stress-induced MAP kinase Hog1 is part of transcription activation complexes. Mol. Cell 7:767-777. [DOI] [PubMed] [Google Scholar]
- 10.Alepuz, P. M., D. Matheos, K. W. Cunningham, and F. Estruch. 1999. The Saccharomyces cerevisiae RanGTP-binding protein Msn5p is involved in different signal transduction pathways. Genetics 153:1219-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alex, L. A., K. A. Borkovich, and M. I. Simon. 1996. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc. Natl. Acad. Sci. USA 93:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alex, L. A., C. Korch, C. P. Selitrennikoff, and M. I. Simon. 1998. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc. Natl. Acad. Sci. USA 95:7069-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander, M. R., M. Tyers, M. Perret, B. M. Craig, K. S. Fang, and M. C. Gustin. 2001. Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress. Mol. Biol. Cell 12:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexandre, H., V. Ansanay-Galeote, S. Dequin, and B. Blondin. 2001. Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae. FEBS Lett. 498:98-103. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Monge, R., F. Navarro-Garcia, G. Molero, R. Diez-Orejas, M. Gustin, J. Pla, M. Sanchez, and C. Nombela. 1999. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181:3058-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso-Monge, R., E. Real, I. Wojda, J. P. Bebelman, W. H. Mager, and M. Siderius. 2001. Hyperosmotic stress response and regulation of cell wall integrity in Saccharomyces cerevisiae share common functional aspects. Mol. Microbiol. 41:717-730. [DOI] [PubMed] [Google Scholar]
- 17.Amoros, M., and F. Estruch. 2001. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 39:1523-1532. [DOI] [PubMed] [Google Scholar]
- 18.Andrews, P. D., and M. J. Stark. 2000. Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J. Cell Sci. 113:2685-2693. [DOI] [PubMed] [Google Scholar]
- 19.Ansell, R., K. Granath, S. Hohmann, J. M. Thevelein, and L. Adler. 1997. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 16:2179-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonsson, B., S. Montessuit, L. Friedli, M. A. Payton, and G. Paravicini. 1994. Protein kinase C in yeast. Characteristics of the Saccharomyces cerevisiae PKC1 gene product. J. Biol. Chem. 269:16821-16828. [PubMed] [Google Scholar]
- 21.Aoyama, K., Y. Mitsubayashi, H. Aiba, and T. Mizuno. 2000. Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator. J. Bacteriol. 182:4868-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 23.Aravind, L., and C. P. Ponting. 1997. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 22:458-459. [DOI] [PubMed] [Google Scholar]
- 24.Ayscough, K. R., and D. G. Drubin. 1998. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 8:927-930. [DOI] [PubMed] [Google Scholar]
- 25.Baker, H. 1986. Glycolytic gene expression in Saccharomyces cerevisiae: nucleotide sequence of GCR1, null mutants and evidence for expression. Mol. Cell. Biol. 6:3774-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal, P. K., and A. K. Mondal. 2000. Isolation and sequence of the HOG1 homologue from Debaryomyces hansenii by complementation of the hog1Δ strain of Saccharomyces cerevisiae. Yeast 16:81-88. [DOI] [PubMed] [Google Scholar]
- 27.Banuelos, M. A., H. Sychrova, C. Bleykasten-Grosshans, J. L. Souciet, and S. Potier. 1998. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 144:2749-2758. [DOI] [PubMed] [Google Scholar]
- 28.Banuett, F. 1998. Signalling in yeast: an informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbet, N. C., U. Schneider, S. B. Helliwell, I. Stansfield, M. F. Tuite, and M. N. Hall. 1996. TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 7:25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batiza, A. F., T. Schulz, and P. H. Masson. 1996. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271:23357-23362. [DOI] [PubMed] [Google Scholar]
- 31.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689-692. [DOI] [PubMed] [Google Scholar]
- 32.Belazzi, T., A. Wagner, R. Wieser, M. Schanz, G. Adam, A. Hartig, and H. Ruis. 1991. Negative regulation of transcription of the Saccharomyces cerevisiae catalase T (CTT1) gene by cAMP is mediated by a positive control element. EMBO J. 10:585-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell, M., R. Capone, I. Pashtan, A. Levitzki, and D. Engelberg. 2001. Isolation of hyperactive mutants of the MAP kinase p38/Hog1 that are independent of MAPKK activation. J. Biol. Chem. 276:25351-25358. [DOI] [PubMed] [Google Scholar]
- 34.Bell, W., W. Sun, S. Hohmann, S. Wera, A. Reinders, C. De Virgilio, A. Wiemken, and J. M. Thevelein. 1998. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J. Biol. Chem. 273:33311-33319. [DOI] [PubMed] [Google Scholar]
- 35.Benton, B. K., A. Tinkelenberg, I. Gonzalez, and F. R. Cross. 1997. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol. Cell. Biol. 17:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bickle, M., P. A. Delley, A. Schmidt, and M. N. Hall. 1998. Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO J. 17:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biggin, P. C., and M. S. Sansom. 2001. Channel gating: twist to open. Curr. Biol. 11:R364-366. [DOI] [PubMed] [Google Scholar]
- 38.Bill, R. M. 2001. Yeast—a panacea for the structure-function analysis of membrane proteins? Curr. Genet. 40:157-171. [DOI] [PubMed] [Google Scholar]
- 39.Bill, R. M., K. Hedfalk, S. Karlgren, J. G. Mullins, J. Rydstrom, and S. Hohmann. 2001. Analysis of the pore of the unusual MIP channel, yeast Fps1p. J. Biol. Chem. 276:36543-36549. [DOI] [PubMed] [Google Scholar]
- 40.Bilsland-Marchesan, E., J. Arino, H. Saito, P. Sunnerhagen, and F. Posas. 2000. Rck2 kinase is a substrate for the osmotic stress-activated mitogen- activated protein kinase Hog1. Mol. Cell. Biol. 20:3887-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bissinger, P. H., R. Wieser, B. Hamilton, and H. Ruis. 1989. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol. Cell. Biol. 9:1309-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Björkqvist, S., R. Ansell, L. Adler, and G. Liden. 1997. Physiological response to anaerobicity of glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 63:128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Blom, J., M. J. De Mattos, and L. A. Grivell. 2000. Redirection of the respiro-fermentative flux distribution in Saccharomyces cerevisiae by overexpression of the transcription factor Hap4p. Appl. Environ. Microbiol. 66:1970-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blomberg, A. 1995. Global changes in protein synthesis during adaptation of the yeast Saccharomyces cerevisiae to 0.7 M NaCl. J. Bacteriol. 177:3563-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blomberg, A. 2000. Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions: questions, some answers and a model. FEMS Microbiol. Lett. 182:1-8. [DOI] [PubMed] [Google Scholar]
- 47.Blomberg, A. 1997. Osmoresponsive proteins and functional assessment strategies in Saccharomyces cerevisiae. Electrophoresis 18:1429-1440. [DOI] [PubMed] [Google Scholar]
- 48.Blomberg, A., and L. Adler. 1992. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 33:145-212. [DOI] [PubMed] [Google Scholar]
- 49.Blomberg, A., and L. Adler. 1989. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 171:1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boguslawski, G. 1992. PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J. Gen. Microbiol. 138:2425-2432. [DOI] [PubMed] [Google Scholar]
- 51.Boguslawski, G., and J. O. Polazzi. 1987. Complete nucleotide sequence of a gene conferring polymyxin B resistance on yeast: similarity of the predicted polypeptide to protein kinases. Proc. Natl. Acad. Sci. USA 84:5848-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boles, E., and C. P. Hollenberg. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 21:85-111. [DOI] [PubMed] [Google Scholar]
- 53.Bone, N., J. B. Millar, T. Toda, and J. Armstrong. 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8:135-144. [DOI] [PubMed] [Google Scholar]
- 54.Bonhivers, M., J. M. Carbrey, S. J. Gould, and P. Agre. 1998. Aquaporins in Saccharomyces. Genetic and functional distinctions between laboratory and wild-type strains. J. Biol. Chem. 273:27565-27572. [DOI] [PubMed] [Google Scholar]
- 55.Boorstein, W. R., and E. A. Craig. 1990. Regulation of yeast HSP70 gene by a cAMP responsive transcription control element. EMBO J. 9:2543-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Booth, I. R., and P. Louis. 1999. Managing hypoosmotic stress: aquaporins and mechanosensitive channels in Escherichia coli. Curr. Opin. Microbiol. 2:166-169. [DOI] [PubMed] [Google Scholar]
- 57.Borgnia, M., S. Nielsen, A. Engel, and P. Agre. 1999. Cellular and molecular biology of aquaporin water channels. Annu. Rev. Biochem. 68:425-458. [DOI] [PubMed] [Google Scholar]
- 58.Botstein, D., D. Amberg, J. Mulholland, T. Huffaker, A. Adams, D. Drubin, and T. Stearns. 1997. The yeast cytoskeleton, p. 1-90. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 59.Bouquin, N., A. L. Johnson, B. A. Morgan, and L. H. Johnston. 1999. Association of the cell cycle transcription factor Mbp1 with the Skn7 response regulator in budding yeast. Mol. Biol. Cell 10:3389-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boy-Marcotte, E., G. Lagniel, M. Perrot, F. Bussereau, A. Boudsocq, M. Jacquet, and J. Labarre. 1999. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 33:274-283. [DOI] [PubMed] [Google Scholar]
- 61.Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 259:1760-1763. [DOI] [PubMed] [Google Scholar]
- 62.Brewster, J. L., and M. C. Gustin. 1994. Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast 10:425-439. [DOI] [PubMed] [Google Scholar]
- 63.Brown, A. D. 1978. Compatible solute and extreme water stress in eukaryotic micro-organisms. Adv. Microb. Physiol. 17:181-242. [DOI] [PubMed] [Google Scholar]
- 64.Brown, A. D. 1974. Microbial water relations: features of the intracellular composition of sugar-tolerant yeasts. J. Bacteriol. 118:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown, A. D. 1990. Microbial water stress physiology: principles and perspectives. J. Wiley and Sons Ltd., Chichester, England.
- 67.Brown, A. D., and J. R. Simpson. 1972. Water relation of sugar-tolerant yeasts: the role of intracellular polyols. J. Gen. Microbiol. 72:589-591. [DOI] [PubMed] [Google Scholar]
- 68.Brown, J. L., and H. Bussey. 1993. The yeast KRE9 gene encodes an O glycoprotein involved in cell surface beta-glucan assembly. Mol. Cell. Biol. 13:6346-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown, J. L., H. Bussey, and R. C. Stewart. 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J. 13:5186-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown, J. L., S. North, and H. Bussey. 1993. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J. Bacteriol. 175:6908-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruning, A. R., J. Bauer, B. Krems, K. D. Entian, and B. A. Prior. 1998. Physiological and genetic characterisation of osmosensitive mutants of Saccharomyes cerevisiae. Arch. Microbiol. 170:99-105. [DOI] [PubMed] [Google Scholar]
- 72.Buck, V., J. Quinn, T. Soto Pino, H. Martin, J. Saldanha, K. Makino, B. A. Morgan, and J. B. Millar. 2001. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12:407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buehrer, B. M., and B. Errede. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burns, N., B. Grimwade, P. B. Ross-Macdonald, E. Y. Choi, K. Finberg, G. S. Roeder, and M. Snyder. 1994. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 8:1087-1105. [DOI] [PubMed] [Google Scholar]
- 75.Cabib, E., J. Drgonova, and T. Drgon. 1998. Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem. 67:307-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calamita, G. 2000. The Escherichia coli aquaporin-Z water channel. Mol. Microbiol. 37:254-262. [DOI] [PubMed] [Google Scholar]
- 77.Calamita, G., B. Kempf, M. Bonhivers, W. R. Bishai, E. Bremer, and P. Agre. 1998. Regulation of the Escherichia coli water channel gene aqpZ. Proc. Natl. Acad. Sci. USA 95:3627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calera, J. A., and R. A. Calderone. 1999. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast 15:1243-1254. [DOI] [PubMed] [Google Scholar]
- 79.Calera, J. A., G. H. Choi, and R. A. Calderone. 1998. Identification of a putative histidine kinase two-component phosphorelay gene (CaHK1) in Candida albicans. Yeast 14:665-674. [DOI] [PubMed] [Google Scholar]
- 80.Calera, J. A., D. Herman, and R. Calderone. 2000. Identification of YPD1, a gene of Candida albicans which encodes a two-component phosphohistidine intermediate protein. Yeast 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 81.Calera, J. A., X. J. Zhao, and R. Calderone. 2000. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun 68:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carbrey, J. M., M. Bonhivers, J. D. Boeke, and P. Agre. 2001. Aquaporins in Saccharomyces: characterization of a second functional water channel protein. Proc. Natl. Acad. Sci. USA 98:1000-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardenas, M. E., N. S. Cutler, M. C. Lorenz, C. J. Di Como, and J. Heitman. 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13:3271-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlson, M. 1999. Glucose repression in yeast. Curr. Opin. Microbiol. 2:202-207. [DOI] [PubMed] [Google Scholar]
- 85.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. [DOI] [PubMed] [Google Scholar]
- 86.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Celenza, J. L., and M. Carlson. 1984. Cloning and genetic mapping of SNF1, a gene required for expression of glucose-repressible genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 4:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 89.Chant, J. 1999. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15:365-391. [DOI] [PubMed] [Google Scholar]
- 90.Charizanis, C., H. Juhnke, B. Krems, and K. D. Entian. 1999. The mitochondrial cytochrome c peroxidase Ccp1 of Saccharomyces cerevisiae is involved in conveying an oxidative stress signal to the transcription factor Pos9 (Skn7). Mol. Gen. Genet. 262:437-447. [DOI] [PubMed] [Google Scholar]
- 91.Charizanis, C., H. Juhnke, B. Krems, and K. D. Entian. 1999. The oxidative stress response mediated via Pos9/Skn7 is negatively regulated by the Ras/PKA pathway in Saccharomyces cerevisiae. Mol. Gen. Genet. 261:740-752. [DOI] [PubMed] [Google Scholar]
- 92.Chaturvedi, V., A. Bartiss, and B. Wong. 1997. Expression of bacterial mtlD in Saccharomyces cerevisiae results in mannitol synthesis and protects a glycerol-defective mutant from high salt and oxidative stress. J. Bacteriol. 179:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaumont, F., F. Barrieu, E. Wojcik, M. J. Chrispeels, and R. Jung. 2001. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 125:1206-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chi, Y., M. J. Huddleston, X. Zhang, R. A. Young, R. S. Annan, S. A. Carr, and R. J. Deshaies. 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15:1078-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiquet, M. 1999. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 18:417-426. [DOI] [PubMed] [Google Scholar]
- 96.Chopra, R., V. M. Sharma, and K. Ganesan. 1999. Elevated growth of Saccharomyces cerevisiae ATH1 null mutants on glucose is an artifact of nonmatching auxotrophies of mutant and reference strains. Appl. Environ. Microbiol 65:2267-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury, S., K. W. Smith, and M. C. Gustin. 1992. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 118:561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chrispeels, M. J., R. Morillon, C. Maurel, P. Gerbeau, P. Kjellbom, and I. Johansson. 2001. Aquaporins in plants: structure, function, regulation, and role in plant water relations, p. 277-334. In S. Hohmann, S. Nielsen, and P. Agre (ed.), Aquaporins, vol. 51. Academic Press, San Diego, Calif. [Google Scholar]
- 99.Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. [DOI] [PubMed] [Google Scholar]
- 100.Cid, V. J., R. Cenamor, M. Sanchez, and C. Nombela. 1998. A mutation in the Rho1-GAP-encoding gene BEM2 of Saccharomyces cerevisiae affects morphogenesis and cell wall functionality. Microbiology 1:25-36. [DOI] [PubMed] [Google Scholar]
- 101.Cooke, F. T., S. K. Dove, R. K. McEwen, G. Painter, A. B. Holmes, M. N. Hall, R. H. Michell, and P. J. Parker. 1998. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr. Biol. 8:1219-1222. [DOI] [PubMed] [Google Scholar]
- 102.Cooper, J. P., S. Y. Roth, and R. T. Simpson. 1994. The global transcriptional regulators, SSN6 and TUP1, play distinct roles in the establishment of a repressive chromatin structure. Genes Dev. 8:1400-1410. [DOI] [PubMed] [Google Scholar]
- 103.Costenoble, R., H. Valadi, L. Gustafsson, C. Niklasson, and C. J. Franzen. 2000. Microaerobic glycerol formation in Saccharomyces cerevisiae. Yeast 16:1483-1495. [DOI] [PubMed] [Google Scholar]
- 104.Costigan, C., S. Gehrung, and M. Snyder. 1992. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol. Cell. Biol. 12:1162-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costigan, C., D. Kolodrubetz, and M. Snyder. 1994. NHP6A and NHP6B, which encode HMG1-like proteins, are candidates for downstream components of the yeast SLT2 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 14:2391-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costigan, C., and M. Snyder. 1994. SLK1, a yeast homolog of MAP kinase activators, has a RAS/cAMP-independent role in nutrient sensing. Mol. Gen. Genet. 243:286-296. [DOI] [PubMed] [Google Scholar]
- 107.Cottarel, G. 1997. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics 147:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coury, L. A., M. Hiller, J. C. Mathai, E. W. Jones, M. L. Zeidel, and J. L. Brodsky. 1999. Water transport across yeast vacuolar and plasma membrane-targeted secretory vesicles occurs by passive diffusion. J. Bacteriol. 181:4437-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crespo, J. L., K. Daicho, T. Ushimaru, and M. N. Hall. 2001. The GATA transcription factors GLN3 and GAT1 link TOR to salt stress in Saccharomyces cerevisiae. J. Biol. Chem. 276:34441-34444. [DOI] [PubMed] [Google Scholar]
- 110.Crews, S. T., and C. M. Fan. 1999. Remembrance of things PAS: regulation of development by bHLH-PAS proteins. Curr. Opin. Genet. Dev. 9:580-587. [DOI] [PubMed] [Google Scholar]
- 111.Crowe, J. H., L. M. Crowe, and D. Chapman. 1984. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223:701-703. [DOI] [PubMed] [Google Scholar]
- 112.Crowe, J. H., F. A. Hoekstra, and L. M. Crowe. 1992. Anhydrobiosis. Annu. Rev. Physiol. 54:579-599. [DOI] [PubMed] [Google Scholar]
- 113.Csonka, L. N., and A. D. Hansen. 1991. Prokaryotic osmoregulation. Annu. Rev. Microbiol. 45:569-606. [DOI] [PubMed] [Google Scholar]
- 114.Cullen, P. J., J. Schultz, J. Horecka, B. J. Stevenson, Y. Jigami, and G. F. Sprague, Jr. 2000. Defects in protein glycosylation cause SHO1-dependent activation of a STE12 signaling pathway in yeast. Genetics 155:1005-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cullen, P. J., and G. F. Sprague, Jr. 2000. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 97:13619-13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cunningham, K. W., and G. R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cvrckova, F., C. De Virgilio, E. Manser, J. R. Pringle, and K. Nasmyth. 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9:1817-1830. [DOI] [PubMed] [Google Scholar]
- 118.Cyert, M. S., R. Kunisawa, D. Kaim, and J. Thorner. 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cyert, M. S., and J. Thorner. 1992. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol. 12:3460-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reference deleted.
- 121.Dahlkvist, A., G. Kanter-Smoler, and P. Sunnerhagen. 1995. The RCK1 and RCK2 protein kinase genes from Saccharomyces cerevisiae suppress cell cycle checkpoint mutations in Schizosaccharomyces pombe. Mol. Gen. Genet. 246:316-326. [DOI] [PubMed] [Google Scholar]
- 122.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 123.Daum, G., N. D. Lees, M. Bard, and R. Dickson. 1998. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14:1471-1510. [DOI] [PubMed] [Google Scholar]
- 124.Davenport, K. D., K. E. Williams, B. D. Ullmann, and M. C. Gustin. 1999. Activation of the Saccharomyces cerevisiae filamentation/invasion pathway by osmotic stress in high-osmolarity glycogen pathway mutants. Genetics 153:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 126.De Cesare, D., and P. Sassone-Corsi. 2000. Transcriptional regulation by cyclic AMP-responsive factors. Prog. Nucleic Acid Res. Mol. Biol. 64:343-369. [DOI] [PubMed] [Google Scholar]
- 127.Deen, P. M., N. Marr, E. J. Kamsteeg, and B. W. van Balkom. 2000. Nephrogenic diabetes insipidus. Curr. Opin. Nephrol. Hypertens. 9:591-595. [DOI] [PubMed] [Google Scholar]
- 128.Deen, P. M. T., and D. Brown. 2001. Trafficking of native and mutant mammalian MIP proteins, p. 235-277. In S. Hohmann, S. Nielsen, and P. Agre (ed.), Aquaporins, vol. 51. Academic Press, San Diego, Calif. [Google Scholar]
- 129.Degols, G., and P. Russell. 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Groot, B. L., A. Engel, and H. Grubmuller. 2001. A refined structure of human aquaporin-1. FEBS Lett. 504:206-211. [DOI] [PubMed] [Google Scholar]
- 132.Delamarche, C., D. Thomas, J. P. Rolland, A. Froger, J. Gouranton, M. Svelto, P. Agre, and G. Calamita. 1999. Visualization of AqpZ-mediated water permeability in Escherichia coli by cryoelectron microscopy. J. Bacteriol. 181:4193-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Delaunay, A., A. D. Isnard, and M. B. Toledano. 2000. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Delley, P. A., and M. N. Hall. 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deminoff, S. J., J. Tornow, and G. M. Santangelo. 1995. Unigenic evolution: a novel genetic method localizes a putative leucine zipper that mediates dimerization of the Saccharomyces cerevisiae regulator Gcr1p. Genetics 141:1263-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 137.de Nobel, H., H. van Den Ende, and F. M. Klis. 2000. Cell wall maintenance in fungi. Trends Microbiol. 8:344-345. [DOI] [PubMed] [Google Scholar]
- 138.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 139.Deschenes, R. J., H. Lin, A. D. Ault, and J. S. Fassler. 1999. Antifungal properties and target evaluation of three putative bacterial histidine kinase inhibitors. Antimicrob. Agents Chemother. 43:1700-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Winde, J. H., and L. A. Grivell. 1995. Regulation of mitochondrial biogenesis in Saccharomyces cerevisiae. Intricate interplay between general and specific transcription factors in the promoter of the QCR8 gene. Eur. J. Biochem. 233:200-208. [DOI] [PubMed] [Google Scholar]
- 141.de Winde, J. H., J. M. Thevelein, and J. Winderickx. 1997. From feast to famine: adaptation to nutrient depletion in yeast, p. 7-52. In S. Hohmann and W. H. Mager (ed.), Yeast stress responses. R. G. Landes, Austin, Tex.
- 142.Dodou, E., and R. Treisman. 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dohlman, H. G., and J. W. Thorner. 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70:703-754. [DOI] [PubMed] [Google Scholar]
- 144.Doi, K., A. Gartner, G. Ammerer, B. Errede, H. Shinkawa, K. Sugimoto, and K. Matsumoto. 1994. MSG5, a novel protein phosphatase promotes adaptation to pheromone response in S. cerevisiae. EMBO J. 13:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dove, S. K., F. T. Cooke, M. R. Douglas, L. G. Sayers, P. J. Parker, and R. H. Michell. 1997. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390:187-192. [DOI] [PubMed] [Google Scholar]
- 146.Drgonova, J., T. Drgon, K. Tanaka, R. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 147.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 148.Eide, D., and L. Guarente. 1992. Increased dosage of a transcriptional activator gene enhances iron-limited growth of Saccharomyces cerevisiae. J. Gen. Microbiol. 138:347-354. [DOI] [PubMed] [Google Scholar]
- 149.Elion, E. A. 2000. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 3:573-581. [DOI] [PubMed] [Google Scholar]
- 150.Elliott, B., R. S. Haltiwanger, and B. Futcher. 1996. Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics 144:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elorza, M. V., H. Rico, and R. Sentandreu. 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129:1577-1582. [DOI] [PubMed] [Google Scholar]
- 152.Engel, A., Y. Fujiyoshi, and P. Agre. 2000. The importance of aquaporin water channel protein structures. EMBO J. 19:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Entian, K. D., and F. K. Zimmermann. 1982. New genes involved in carbon catabolite repression and derepression in the yeast Saccharomyces cerevisiae. J. Bacteriol. 151:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erickson, J. W., and R. A. Cerione. 2001. Multiple roles for Cdc42 in cell regulation. Curr. Opin. Cell Biol. 13:153-157. [DOI] [PubMed] [Google Scholar]
- 155.Eriksson, P., H. Alipour, L. Adler, and A. Blomberg. 2000. Rap1p-binding sites in the Saccharomyces cerevisiae GPD1 promoter are involved in its response to NaCl. J. Biol. Chem. 275:29368-29376. [DOI] [PubMed] [Google Scholar]
- 156.Eriksson, P., L. Andre, R. Ansell, A. Blomberg, and L. Adler. 1995. Molecular cloning of GPD2, a second gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) in Saccharomyces cerevisiae, and its comparison to GPD1. Mol. Microbiol. 17:95-107. [DOI] [PubMed] [Google Scholar]
- 157.Errede, B., R. M. Cade, B. M. Yashar, Y. Kamada, D. E. Levin, K. Irie, and K. Matsumoto. 1995. Dynamics and organization of MAP kinase signal pathways. Mol. Reprod. Dev. 42:477-485. [DOI] [PubMed] [Google Scholar]
- 158.Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 24:469-486. [DOI] [PubMed] [Google Scholar]
- 159.Estruch, F., and M. Carlson. 1990. Increased dosage of the MSN1 gene restores invertase expression in yeast mutants defective in the SNF1 protein kinase. Nucleic Acids Res. 11:6959-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Estruch, F., and M. Carlson. 1993. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Evangelista, M., K. Blundell, M. S. Longtine, C. J. Chow, N. Adames, J. R. Pringle, M. Peter, and C. Boone. 1997. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276:118-122. [DOI] [PubMed] [Google Scholar]
- 162.Fantes, P. A., E. Warbrick, D. A. Hughes, and S. A. MacNeill. 1991. New elements in the mitotic control of the fission yeast Schizosaccharomyces pombe. Cold Spring Harbor Symp. Quant. Biol. 56:605-611. [DOI] [PubMed] [Google Scholar]
- 163.Fares, H., L. Goetsch, and J. R. Pringle. 1996. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J. Cell Biol. 132:399-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fassler, J. S., W. M. Gray, C. L. Malone, W. Tao, H. Lin, and R. J. Deschenes. 1997. Activated alleles of yeast SLN1 increase Mcm1-dependent reporter gene expression and diminish signaling through the Hog1 osmosensing pathway. J. Biol. Chem. 272:13365-13371. [DOI] [PubMed] [Google Scholar]
- 165.Ferreira, M., X.-M. Bao, V. Laizé, and S. Hohmann. 2001. Transposon mutagenesis reveals novel loci affecting tolerance to salt stress and growth at low temperature. Curr. Genet. 40:27-39. [DOI] [PubMed] [Google Scholar]
- 166.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAP kinase requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fillinger, S., M. K. Chaveroche, P. van Dijck, R. de Vries, G. Ruijter, J. Thevelein, and C. d'Enfert. 2001. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147:1851-1862. [DOI] [PubMed] [Google Scholar]
- 168.Fleet, G. 1992. Spoilage yeasts. Crit. Rev. Biotechnol. 12:1-44. [DOI] [PubMed] [Google Scholar]
- 169.Flick, J. S., and J. Thorner. 1993. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:5861-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Foor, F., S. A. Parent, N. Morin, A. M. Dahl, N. Ramadan, G. Chrebet, K. A. Bostian, and J. B. Nielsen. 1992. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature 360:682-684. [DOI] [PubMed] [Google Scholar]
- 171.Forsberg, H., and P. O. Ljungdahl. 2001. Sensors of extracellular nutrients in Saccharomyces cerevisiae. Curr. Genet. 40:91-109. [DOI] [PubMed] [Google Scholar]
- 172.Francois, J., M. J. Neves, and H. G. Hers. 1991. The control of trehalose biosynthesis in Saccharomyces cerevisiae: evidence for a catabolite inactivation and repression of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Yeast 7:575-587. [DOI] [PubMed] [Google Scholar]
- 173.Francois, J., and J. L. Parrou. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:125-145. [DOI] [PubMed] [Google Scholar]
- 174.Frazier, J. A., and C. M. Field. 1997. Actin cytoskeleton: are FH proteins local organizers? Curr. Biol. 7:R414-417. [DOI] [PubMed] [Google Scholar]
- 175.Fu, D., A. Libson, L. J. Miercke, C. Weitzman, P. Nollert, J. Krucinski, and R. M. Stroud. 2000. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481-486. [DOI] [PubMed] [Google Scholar]
- 176.Fujiwara, D., O. Kobayashi, H. Yoshimoto, S. Harashima, and Y. Tamai. 1999. Molecular mechanism of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast 15:1183-1197. [DOI] [PubMed] [Google Scholar]
- 177.Fujiwara, T., K. Tanaka, A. Mino, M. Kikyo, K. Takahashi, K. Shimizu, and Y. Takai. 1998. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9:1221-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gagiano, M., D. Van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Divergent regulation of the evolutionarily closely related promoters of the Saccharomyces cerevisiae STA2 and MUC1 genes. J. Bacteriol. 181:6497-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gagiano, M., D. van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Msn1p/Mss10p, Mss11p and Muc1p/Flo11p are part of a signal transduction pathway downstream of Mep2p regulating invasive growth and pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Microbiol. 31:103-116. [DOI] [PubMed] [Google Scholar]
- 180.Gaits, F., G. Degols, K. Shiozaki, and P. Russell. 1998. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12:1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gaits, F., and P. Russell. 1999. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/Sty1 in fission yeast. Mol. Biol. Cell 10:1395-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gaits, F., and P. Russell. 1999. Vacuole fusion regulated by protein phosphatase 2C in fission yeast. Mol. Biol. Cell 10:2647-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gaits, F., K. Shiozaki, and P. Russell. 1997. Protein phosphatase 2C acts independently of stress-activated kinase cascade to regulate the stress response in fission yeast. J. Biol. Chem. 272:17873-17879. [DOI] [PubMed] [Google Scholar]
- 184.Gancedo, C., J. M. Gancedo, and A. Sols. 1968. Glycerol metabolism in yeasts. Pathways of utilization and production. Eur. J. Biochem. 5:165-172. [DOI] [PubMed] [Google Scholar]
- 185.Gancedo, J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Garay-Arroyo, A., and A. A. Covarrubias. 1999. Three genes whose expression is induced by stress in Saccharomyces cerevisiae. Yeast 15:879-892. [DOI] [PubMed] [Google Scholar]
- 186a.Garay-Arroyo, A., J. M. Colmenero-Flores, A. Garciarrubio, and A. A. Covarrubias. 2000. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 275:5668-5674. [DOI] [PubMed] [Google Scholar]
- 187.Garcia-Gimeno, M. A., and K. Struhl. 2000. Aca1 and Aca2, ATF/CREB activators in Saccharomyces cerevisiae, are important for carbon source utilization but not the response to stress. Mol. Cell. Biol. 20:4340-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Garcia-Rodriguez, L. J., A. Duran, and C. Roncero. 2000. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 182:2428-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garreau, H., R. N. Hasan, G. Renault, F. Estruch, E. Boy-Marcotte, and M. Jacquet. 2000. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology 146:2113-2120. [DOI] [PubMed] [Google Scholar]
- 190.Garrett-Engele, P., B. Moilanen, and M. S. Cyert. 1995. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol. Cell. Biol. 15:4103-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gehrung, S., and M. Snyder. 1990. The SPA2 gene of Saccharomyces cerevisiae is important for pheromone-induced morphogenesis and efficient mating. J. Cell Biol. 111:1451-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Geymonat, M., L. Wang, H. Garreau, and M. Jacquet. 1998. Ssa1p chaperone interacts with the guanine nucleotide exchange factor of ras Cdc25p and controls the cAMP pathway in Saccharomyces cerevisiae. Mol. Microbiol. 30:855-864. [DOI] [PubMed] [Google Scholar]
- 194.Gimeno, C. J., and G. R. Fink. 1994. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol. Cell. Biol. 14:2100-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Godon, C., G. Lagniel, J. Lee, J. M. Buhler, S. Kieffer, M. Perrot, H. Boucherie, M. B. Toledano, and J. Labarre. 1998. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273:22480-22489. [DOI] [PubMed] [Google Scholar]
- 196.Goncalves, P. M., G. Griffioen, R. Minnee, M. Bosma, L. S. Kraakman, W. H. Mager, and R. J. Planta. 1995. Transcription activation of yeast ribosomal protein genes requires additional elements apart from binding sites for Abf1p or Rap1p. Nucleic Acids Res. 23:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gonzalez-Amaro, R., and F. Sanchez-Madrid. 1999. Cell adhesion molecules: selectins and integrins. Crit. Rev. Immunol. 19:389-429. [PubMed] [Google Scholar]
- 198.Goode, B. L., and A. A. Rodal. 2001. Modular complexes that regulate actin assembly in budding yeast. Curr. Opin. Microbiol. 4:703-712. [DOI] [PubMed] [Google Scholar]
- 199.Görner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schüller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gounalaki, N., and G. Thireos. 1994. Yap1p, a yeast transcriptional activator that mediates multidrug resistance, regulates the metabolic stress response. EMBO J. 13:4036-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Griffioen, G., P. Anghileri, E. Imre, M. D. Baroni, and H. Ruis. 2000. Nutritional control of nucleocytoplasmic localization of cAMP-dependent protein kinase catalytic and regulatory subunits in Saccharomyces cerevisiae. J. Biol. Chem. 275:1449-1456. [DOI] [PubMed] [Google Scholar]
- 203.Griffioen, G., P. Branduardi, A. Ballarini, P. Anghileri, J. Norbeck, M. D. Baroni, and H. Ruis. 2001. Nucleocytoplasmic distribution of budding yeast protein kinase A regulatory subunit Bcy1 requires Zds1 and is regulated by Yak1-dependent phosphorylation of its targeting domain. Mol. Cell. Biol. 21:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Griffioen, G., W. H. Mager, and R. J. Planta. 1994. Nutritional upshift response of ribosomal protein gene transcription in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 123:137-144. [DOI] [PubMed] [Google Scholar]
- 205.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gustin, M. C., X.-L. Zhou, B. Martinac, and C. Kung. 1988. A mechanosensitive ion channel in the yeast plasma membrane. Science 242:762-765. [DOI] [PubMed] [Google Scholar]
- 207.Hall, J. P., V. Cherkasova, E. Elion, M. C. Gustin, and E. Winter. 1996. The osmoregulatory pathway represses mating pathway activity in Saccharomyces cerevisiae: isolation of a FUS3 mutant that is insensitive to the repression mechanism. Mol. Cell. Biol. 16:6715-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hallsworth, J. E. 1998. Ethanol-induced water stress in yeast. J. Ferment. Bioeng. 85:125-137. [Google Scholar]
- 209.Hardie, D. G. 1999. Roles of the AMP-activated/SNF1 protein kinase family in the response to cellular stress. Biochem. Soc. Symp. 64:13-27. [PubMed] [Google Scholar]
- 210.Hardie, D. G., and D. Carling. 1997. The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur. J. Biochem. 246:259-273. [DOI] [PubMed] [Google Scholar]
- 211.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67:821-855. [DOI] [PubMed] [Google Scholar]
- 212.Hardwick, J. S., F. G. Kuruvilla, J. K. Tong, A. F. Shamji, and S. L. Schreiber. 1999. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl. Acad. Sci. USA 96:14866-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Haro, R., B. Garciadeblas, and A. Rodriguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 214.Harrison, J. C., E. S. Bardes, Y. Ohya, and D. J. Lew. 2001. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3:417-420. [DOI] [PubMed] [Google Scholar]
- 215.Reference deleted.
- 216.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 217.Heitman, J., N. R. Movva, and M. N. Hall. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905-909. [DOI] [PubMed] [Google Scholar]
- 218.Helliwell, S. B., I. Howald, N. Barbet, and M. N. Hall. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Helliwell, S. B., A. Schmidt, Y. Ohya, and M. N. Hall. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211-1214. [DOI] [PubMed] [Google Scholar]
- 220.Helliwell, S. B., P. Wagner, J. Kunz, M. Deuter-Reinhard, R. Henriquez, and M. N. Hall. 1994. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol. Biol. Cell 5:105-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Herruer, M. H., W. H. Mager, H. A. Raue, P. Vreken, E. Wilms, and R. J. Planta. 1988. Mild temperature shock affects transcription of yeast ribosomal protein genes as well as the stability of their mRNAs. Nucleic Acids Res. 16:7917-7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Herskowitz, I. 1995. MAP kinase pathways in yeast: for mating and more. Cell 80:187-197. [DOI] [PubMed] [Google Scholar]
- 223.Heymann, J. B., and A. Engel. 2000. Structural clues in the sequences of the aquaporins. J. Mol. Biol. 295:1039-1053. [DOI] [PubMed] [Google Scholar]
- 224.Hinnebusch, A. G. 1986. The general control of amino acid biosynthetic genes in the yeast Saccharomyces cerevisiae. Crit. Rev. Biochem. 21:277-317. [DOI] [PubMed] [Google Scholar]
- 225.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 226.Hirayama, T., T. Maeda, H. Saito, and K. Shinozaki. 1995. Cloning and characterization of seven cDNAs for hyperosmolarity-responsive (HOR) genes of Saccharomyces cerevisiae. Mol. Gen. Genet. 249:127-138. [DOI] [PubMed] [Google Scholar]
- 227.Hoffmann, E. K., and P. B. Dunham. 1995. Membrane mechanisms and intracellular signalling in cell volume regulation. Int. Rev. Cytol. 161:173-262. [DOI] [PubMed] [Google Scholar]
- 228.Hohmann, S. 1997. Shaping up: the response of yeast to osmotic stress, p. 101-145. In S. Hohmann and W. H. Mager (ed.), Yeast stress responses. R. G. Landes, Austin, Tex.
- 229.Hohmann, S., G. Kayingo, R. M. Bill, and B. A. Prior. 2000. Microbial MIP channels. Trends Microbiol. 8:33-38. [DOI] [PubMed] [Google Scholar]
- 230.Hohmann, S., and W. H. Mager. 1997. Yeast stress responses. R. G. Landes Company, Austin, Tex.
- 231.Hohmann, S., M. J. Neves, W. de Koning, R. Alijo, J. Ramos, and J. M. Thevelein. 1993. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr. Genet. 23:281-289. [DOI] [PubMed] [Google Scholar]
- 232.Hohmann, S., S. Nielsen, and P. Agre. 2001. Aquaporins, vol. 51. Academic Press, San Diego, Calif.
- 233.Hohmann, S., J. Winderickx, J. H. de Winde, D. Valckx, P. Cobbaert, K. Luyten, C. de Meirsman, J. Ramos, and J. M. Thevelein. 1999. Novel alleles of yeast hexokinase PII with distinct effects on catalytic activity and catabolite repression of SUC2. Microbiology 145:703-714. [DOI] [PubMed] [Google Scholar]
- 234.Holst, B., C. Lunde, F. Lages, R. Oliveira, C. Lucas, and M. C. Kielland-Brandt. 2000. GUP1 and its close homologue GUP2, encoding multi-membrane-spanning proteins involved in active glycerol uptake in Saccharomyces cerevisiae. Mol. Microbiol. 37:108-124. [DOI] [PubMed] [Google Scholar]
- 235.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 236.Hottiger, T., T. Boller, and A. Wiemken. 1987. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 220:113-115. [DOI] [PubMed] [Google Scholar]
- 237.Hottiger, T., C. de Virgilio, M. N. Hall, T. Boller, and A. Wiemken. 1994. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 219:187-193. [DOI] [PubMed] [Google Scholar]
- 238.Hounsa, C. G., E. V. Brandt, J. Thevelein, S. Hohmann, and B. A. Prior. 1998. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 3:671-680. [DOI] [PubMed] [Google Scholar]
- 239.Hughes, V., A. Muller, M. J. Stark, and P. T. Cohen. 1993. Both isoforms of protein phosphatase Z are essential for the maintenance of cell size and integrity in Saccharomyces cerevisiae in response to osmotic stress. Eur. J. Biochem. 216:269-279. [DOI] [PubMed] [Google Scholar]
- 240.Hwang, I., and J. Sheen. 2001. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413:383-389. [DOI] [PubMed] [Google Scholar]
- 241.Igual, J. C., A. L. Johnson, and L. H. Johnston. 1996. Coordinated regulation of gene expression by the cell cycle transcription factor SWI4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15:5001-5013. [PMC free article] [PubMed] [Google Scholar]
- 242.Igual, J. C., W. M. Toone, and L. H. Johnston. 1997. A genetic screen reveals a role for the late G1-specific transcription factor Swi4p in diverse cellular functions including cytokinesis. J. Cell Sci. 110:1647-1654. [DOI] [PubMed] [Google Scholar]
- 243.Iida, H., H. Nakamura, T. Ono, M. S. Okumura, and Y. Anraku. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14:8259-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Imamura, H., K. Tanaka, T. Hihara, M. Umikawa, T. Kamei, K. Takahashi, T. Sasaki, and Y. Takai. 1997. Bni1p and Bnr1p: downstream targets of the rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16:2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Inoue, S. B., H. Qadota, M. Arisawa, T. Watanabe, and Y. Ohya. 1999. Prenylation of Rho1p is required for activation of yeast 1,3-beta-glucan synthase. J. Biol. Chem. 274:38119-38124. [DOI] [PubMed] [Google Scholar]
- 246.Inoue, Y., Y. Tsujimoto, and A. Kimura. 1998. Expression of the glyoxalase I gene of Saccharomyces cerevisiae is regulated by high osmolarity glycerol mitogen-activated protein kinase pathway in osmotic stress response. J. Biol. Chem. 273:2977-2983. [DOI] [PubMed] [Google Scholar]
- 247.Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki, K. Matsumoto, and Y. Oshima. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Isoyama, T., A. Murayama, A. Nomoto, and S. Kuge. 2001. Nuclear import of the yeast AP-1-like transcription factor Yap1p is mediated by transport receptor Pse1p, and this import step is not affected by oxidative stress. J. Biol. Chem. 276:21863-21869. [DOI] [PubMed] [Google Scholar]
- 249.Ivanovska, I., and M. D. Rose. 2000. SLG1 plays a role during G1 in the decision to enter or exit the cell cycle. Mol. Gen. Genet. 262:1147-1156. [DOI] [PubMed] [Google Scholar]
- 250.Iwaki, T., Y. Tamai, and Y. Watanabe. 1999. Two putative MAP kinase genes, ZrHOG1 and ZrHOG2, cloned from the salt-tolerant yeast Zygosaccharomyces rouxii are functionally homologous to the Saccharomyces cerevisiae HOG1 gene. Microbiology 145:241-248. [DOI] [PubMed] [Google Scholar]
- 251.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell cycle transcription factors SBF and MBF. Nature 409:533-538. [DOI] [PubMed] [Google Scholar]
- 252.Jacoby, J. J., S. M. Nilius, and J. J. Heinisch. 1998. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol. Gen. Genet. 258:148-155. [DOI] [PubMed] [Google Scholar]
- 253.Jacoby, J. J., H. P. Schmitz, and J. J. Heinisch. 1997. Mutants affected in the putative diacylglycerol binding site of yeast protein kinase C. FEBS Lett. 417:219-222. [DOI] [PubMed] [Google Scholar]
- 254.Jacoby, T., H. Flanagan, A. Faykin, A. G. Seto, C. Mattison, and I. Ota. 1997. Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J. Biol. Chem. 272:17749-17755. [DOI] [PubMed] [Google Scholar]
- 255.Jamieson, D. J. 1998. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 14:1511-1527. [DOI] [PubMed] [Google Scholar]
- 256.Janiak-Spens, F., D. P. Sparling, and A. H. West. 2000. Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J. Bacteriol. 182:6673-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Janiak-Spens, F., J. M. Sparling, M. Gurfinkel, and A. H. West. 1999. Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory SLN1 and SSK1 proteins. J. Bacteriol. 181:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Janiak-Spens, F., and A. H. West. 2000. Functional roles of conserved amino acid residues surrounding the phosphorylatable histidine of the yeast phosphorelay protein YPD1. Mol. Microbiol. 37:136-144. [DOI] [PubMed] [Google Scholar]
- 259.Janoo, R. T., L. A. Neely, B. R. Braun, S. K. Whitehall, and C. S. Hoffman. 2001. Transcriptional regulators of the Schizosaccharomyces pombe fbp1+ gene include two redundant Tup1p-like corepressors and the CCAAT binding factor activation complex. Genetics 157:1205-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jansen, G., F. Buhring, C. P. Hollenberg, and M. Ramezani Rad. 2001. Mutations in the SAM domain of STE50 differentially influence the MAP kinase-mediated pathways for mating, filamentous growth and osmotolerance in Saccharomyces cerevisiae. Mol. Genet. Genomics 265:102-117. [DOI] [PubMed] [Google Scholar]
- 261.Jansen, R. P., C. Dowzer, C. Michaelis, M. Galova, and K. Nasmyth. 1996. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell 84:687-697. [DOI] [PubMed] [Google Scholar]
- 262.Johanson, U., M. Karlsson, I. Johansson, S. Gustavsson, S. Sjovall, L. Fraysse, A. R. Weig, and P. Kjellbom. 2001. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 126:1358-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Johansson, I., M. Karlsson, U. Johanson, C. Larsson, and P. Kjellbom. 2000. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta 1465:324-342. [DOI] [PubMed] [Google Scholar]
- 264.Johnson, D. I. 1999. Cdc42: an essential rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jordt, S.-E., and T. J. Jentsch. 1997. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 16:1582-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jorge, J. A., M. L. Polizeli, J. M. Thevelein, and H. F. Terenzi. 1997. Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol. Lett. 154:165-171. [DOI] [PubMed] [Google Scholar]
- 267.Juhnke, H., C. Charizanis, F. Latifi, B. Krems, and K. D. Entian. 2000. The essential protein fap7 is involved in the oxidative stress response of Saccharomyces cerevisiae. Mol. Microbiol. 35:936-948. [DOI] [PubMed] [Google Scholar]
- 268.Jung, K., B. Tjaden, and K. Altendorf. 1997. Purification, reconstitution, and characterization of KdpD, the turgor sensor of Escherichia coli. J. Biol. Chem. 272:10847-10852. [DOI] [PubMed] [Google Scholar]
- 269.Jung, K., M. Veen, and K. Altendorf. 2000. K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J. Biol. Chem. 275:40142-40147. [DOI] [PubMed] [Google Scholar]
- 270.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 271.Kaasen, I., J. McDougall, and A. R. Strom. 1994. Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase/phosphatase complex. Gene 145:9-15. [DOI] [PubMed] [Google Scholar]
- 272.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 273.Kamada, Y., H. Qadota, C. P. Python, Y. Anraku, Y. Ohya, and D. E. Levin. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193-9196. [DOI] [PubMed] [Google Scholar]
- 274.Kanoh, J., Y. Watanabe, M. Ohsugi, Y. Iino, and M. Yamamoto. 1996. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells 1:391-408. [DOI] [PubMed] [Google Scholar]
- 275.Kanzaki, M., M. Nagasawa, I. Kojima, C. Sato, K. Naruse, M. Sokabe, and H. Iida. 1999. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285:882-886. [DOI] [PubMed] [Google Scholar]
- 276.Kapteyn, J. C., B. Ter Riet, E. Vink, S. Blad, H. De Nobel, H. Van Den Ende, and F. M. Klis. 2001. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 39:469-480. [DOI] [PubMed] [Google Scholar]
- 277.Reference deleted.
- 278.Kato, T., Jr., K. Okazaki, H. Murakami, S. Stettler, P. A. Fantes, and H. Okayama. 1996. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 378:207-212. [DOI] [PubMed] [Google Scholar]
- 279.Kawai, M., A. Nakashima, M. Ueno, T. Ushimaru, K. Aiba, H. Doi, and M. Uritani. 2001. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr. Genet. 39:166-174. [DOI] [PubMed] [Google Scholar]
- 279a.Kayingo, G., S. G. Kilian, and B. A. Prior. 2001. Conservation and release of osmolytes by yeasts during hypo-osmotic stress. Arch. Microbiol. 177:29-35. [DOI] [PubMed] [Google Scholar]
- 280.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 281.Ketela, T., J. L. Brown, R. C. Stewart, and H. Bussey. 1998. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol. Gen. Genet. 259:372-378. [DOI] [PubMed] [Google Scholar]
- 282.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Keyse, S. M. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 284.Kim, J., P. Alizadeh, T. Harding, A. Hefner-Gravink, and D. J. Klionsky. 1996. Disruption of the yeast ATH1 gene confers better survival after dehydration, freezing, and ethanol shock: potential commercial applications. Appl. Environ. Microbiol. 62:1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kinclova, O., S. Potier, and H. Sychrova. 2001. The Zygosaccharomyces rouxii strain CBS732 contains only one copy of the HOG1 and the SOD2 genes. J. Biotechnol. 88:151-158. [DOI] [PubMed] [Google Scholar]
- 286.Kinclova, O., J. Ramos, S. Potier, and H. Sychrova. 2001. Functional study of the Saccharomyces cerevisiae Nha1p C terminus. Mol. Microbiol. 40:656-668. [DOI] [PubMed] [Google Scholar]
- 287.Kjoller, L., and A. Hall. 1999. Signaling to rho GTPases. Exp. Cell Res. 253:166-179. [DOI] [PubMed] [Google Scholar]
- 288.Klis, F. M. 1994. Review: cell wall assembly in yeast. Yeast 10:851-869. [DOI] [PubMed] [Google Scholar]
- 289.Ko, C. H., and R. F. Gaber. 1991. TRK1 and TRK2 encodes structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kobayashi, N., and K. McEntee. 1990. Evidence for a heat shock transcription factor-independent mechanism for heat shock induction of transcription in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 87:6550-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kobayashi, N., and K. McEntee. 1993. Identification of cis and trans components of a novel heat shock stress regulatory pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kohno, H., K. Tanaka, A. Mino, M. Umikawa, H. Imamura, T. Fujiwara, Y. Fujita, K. Hotta, H. Qadota, T. Watanabe, Y. Ohya, and Y. Takai. 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:6060-6068. [PMC free article] [PubMed] [Google Scholar]
- 293.Kraakman, L. S., G. Griffioen, S. Zerp, P. Groeneveld, J. M. Thevelein, W. H. Mager, and R. J. Planta. 1993. Growth-related expression of ribosomal protein genes in Saccharomyces cerevisiae. Mol. Gen. Genet. 239:196-204. [DOI] [PubMed] [Google Scholar]
- 294.Krallish, I., H. Jeppsson, A. Rapoport, and B. Hahn-Hagerdal. 1997. Effect of xylitol and trehalose on dry resistance of yeasts. Appl. Microbiol. Biotechnol. 47:447-451. [DOI] [PubMed] [Google Scholar]
- 295.Reference deleted.
- 296.Krems, B., C. Charizanis, and K. D. Entian. 1995. Mutants of Saccharomyces cerevisiae sensitive to oxidative and osmotic stress. Curr. Genet. 27:427-434. [DOI] [PubMed] [Google Scholar]
- 297.Krems, B., C. Charizanis, and K. D. Entian. 1996. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr. Genet. 29:327-334. [DOI] [PubMed] [Google Scholar]
- 298.Kuchin, S., I. Treich, and M. Carlson. 2000. A regulatory shortcut between the Snf1 protein kinase and RNA polymerase II holoenzyme. Proc. Natl. Acad. Sci. USA 97:7916-7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kuchin, S., P. Yeghiayan, and M. Carlson. 1995. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 92:4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kudo, N., H. Taoka, T. Toda, M. Yoshida, and S. Horinouchi. 1999. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J. Biol. Chem. 274:15151-15158. [DOI] [PubMed] [Google Scholar]
- 301.Kuge, S., and N. Jones. 1994. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 13:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kuge, S., N. Jones, and A. Nomoto. 1997. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 16:1710-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kuhn, K. M., J. L. DeRisi, P. O. Brown, and P. Sarnow. 2001. Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol. Cell. Biol. 21:916-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kultz, D., and M. Burg. 1998. Evolution of osmotic stress signaling via MAP kinase cascades. J. Exp. Biol. 201:3015-3021. [DOI] [PubMed] [Google Scholar]
- 305.Kuno, T., H. Tanaka, H. Mukai, C. D. Chang, K. Hiraga, T. Miyakawa, and C. Tanaka. 1991. cDNA cloning of a calcineurin B homolog in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 180:1159-1163. [DOI] [PubMed] [Google Scholar]
- 306.Kurtzman, C. P., and J. W. Fell. 1998. The yeasts—a taxonomic study, 4th ed. Elsevier Science, Amsterdam, The Netherlands.
- 307.Kwon, H. M., and J. S. Handler. 1995. Cell volume regulated transporters of compatible solutes. Curr. Opin. Cell Biol. 7:465-471. [DOI] [PubMed] [Google Scholar]
- 308.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 309.Lages, F., and C. Lucas. 1997. Contribution to the physiological characterization of glycerol active uptake in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1322:8-18. [DOI] [PubMed] [Google Scholar]
- 310.Lages, F., M. Silva-Graca, and C. Lucas. 1999. Active glycerol uptake is a mechanism underlying halotolerance in yeasts: a study of 42 species. Microbiology 145:2577-2585. [DOI] [PubMed] [Google Scholar]
- 311.Laizé, V., F. Tacnet, P. Ripoche, and S. Hohmann. 2000. Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast 16:897-903. [DOI] [PubMed] [Google Scholar]
- 312.Lambrechts, M. G., F. F. Bauer, J. Marmur, and I. S. Pretorius. 1996. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA 93:8419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lambrechts, M. G., P. Sollitti, J. Marmur, and I. S. Pretorius. 1996. A multicopy suppressor gene, MSS10, restores STA2 expression in Saccharomyces cerevisiae strains containing the STA10 repressor gene. Curr. Genet. 29:523-529. [PubMed] [Google Scholar]
- 314.Lang, F., G. L. Busch, M. Ritter, H. Volkl, S. Waldegger, E. Gulbins, and D. Haussinger. 1998. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78:247-306. [DOI] [PubMed] [Google Scholar]
- 315.Larsen, P. I., L. K. Sydnes, B. Landfald, and A. R. Strom. 1987. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch. Microbiol. 147:1-7. [DOI] [PubMed] [Google Scholar]
- 316.Larsson, C., and L. Gustafsson. 1987. Glycerol production in relation to the ATP pool and heat production rate of the yeast Debaryomyces hansenii and Saccharomyces cerevisiae during salt stress. Arch. Microbiol. 147:358-363. [DOI] [PubMed] [Google Scholar]
- 317.Larsson, C., I. L. Påhlman, R. Ansell, M. Rigoulet, L. Adler, and L. Gustafsson. 1998. The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast 14:347-357. [DOI] [PubMed] [Google Scholar]
- 318.Larsson, K., P. Eriksson, R. Ansell, and L. Adler. 1993. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 10:1101-1111. [DOI] [PubMed] [Google Scholar]
- 319.Latterich, M., and M. D. Watson. 1993. Evidence for a dual osmoregulatory mechanism in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 191:1111-1117. [DOI] [PubMed] [Google Scholar]
- 320.Latterich, M., and M. D. Watson. 1991. Isolation and characterization of osmosensitive vacuolar mutants of Saccharomyces cerevisiae. Mol. Microbiol. 5:2417-2426. [DOI] [PubMed] [Google Scholar]
- 321.Leberer, E., D. Dignard, D. Harcus, D. Y. Thomas, and M. Whiteway. 1992. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 11:4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Leberer, E., C. Wu, T. Leeuw, A. Fourest-Lieuvin, J. E. Segall, and D. Y. Thomas. 1997. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 16:83-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lee, B. N., and E. A. Elion. 1999. The MAPKKK Ste11 regulates vegetative growth through a kinase cascade of shared signaling components. Proc. Natl. Acad. Sci. USA 96:12679-12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lee, J., C. Godon, G. Lagniel, D. Spector, J. Garin, J. Labarre, and M. B. Toledano. 1999. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 274:16040-16046. [DOI] [PubMed] [Google Scholar]
- 325.Lee, J., D. Spector, C. Godon, J. Labarre, and M. B. Toledano. 1999. A new antioxidant with alkyl hydroperoxide defense properties in yeast. J. Biol. Chem. 274:4537-4544. [DOI] [PubMed] [Google Scholar]
- 326.Lee, K. S., L. K. Hines, and D. E. Levin. 1993. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell. Biol. 13:5843-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lee, K. S., K. Irie, Y. Gotoh, Y. Watanabe, H. Araki, E. Nishida, K. Matsumoto, and D. E. Levin. 1993. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signaling by protein kinase C. Mol. Cell. Biol. 13:3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Leslie, S. B., S. A. Teter, L. M. Crowe, and J. H. Crowe. 1994. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim. Biophys. Acta 1192:7-13. [DOI] [PubMed] [Google Scholar]
- 331.Levin, D. E., and E. Bartlett-Heubusch. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116:1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Levin, D. E., B. Bowers, C. Y. Chen, Y. Kamada, and M. Watanabe. 1994. Dissecting the protein kinase C/MAP kinase signalling pathway of Saccharomyces cerevisiae. Cell. Mol. Biol. Res. 40:229-239. [PubMed] [Google Scholar]
- 333.Levin, D. E., F. O. Fields, R. Kunisawa, J. M. Bishop, and J. Thorner. 1990. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell 62:213-224. [DOI] [PubMed] [Google Scholar]
- 334.Levina, N., S. Totemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lewis, J. G., R. P. Learmonth, and K. Watson. 1995. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology 141:687-694. [DOI] [PubMed] [Google Scholar]
- 336.Li, C., and Q. Xu. 2000. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 12:435-445. [DOI] [PubMed] [Google Scholar]
- 337.Li, S., A. Ault, C. L. Malone, D. Raitt, S. Dean, L. H. Johnston, R. J. Deschenes, and J. S. Fassler. 1998. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17:6952-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li, S., S. Dean, Z. Li, J. Horecka, R. J. Deschenes, and J. J. Fassler. 2002. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13:412-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ligterink, W., and H. Hirt. 2001. Mitogen-activated protein [MAP] kinase pathways in plants: versatile signaling tools. Int. Rev. Cytol. 201:209-275. [DOI] [PubMed] [Google Scholar]
- 340.Liu, J., and E. T. Kipreos. 2000. Evolution of cyclin-dependent kinases (CDKs) and CDK-activating kinases (CAKs): differential conservation of CAKs in yeast and metazoa. Mol. Biol. Evol. 17:1061-1074. [DOI] [PubMed] [Google Scholar]
- 341.Liu, Y., S. Ishii, M. Tokai, H. Tsutsumi, O. Ohki, R. Akada, K. Tanaka, E. Tsuchiya, S. Fukui, and T. Miyakawa. 1991. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol. Gen. Genet. 227:52-59. [DOI] [PubMed] [Google Scholar]
- 342.Lo, W. S., and A. M. Dranginis. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Locke, E. G., M. Bonilla, L. Liang, Y. Takita, and K. W. Cunningham. 2000. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lodder, A. L., T. K. Lee, and R. Ballester. 1999. Characterization of the Wsc1 protein, a putative receptor in the stress response of Saccharomyces cerevisiae. Genetics 152:1487-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Londesborough, J., and O. Vuorio. 1991. Trehalose-6-phosphate synthase/phosphatase complex from bakers' yeast: purification of a proteolytically activated form. J. Gen. Microbiol. 137:323-330. [DOI] [PubMed] [Google Scholar]
- 346.Londesborough, J., and O. E. Vuorio. 1993. Purification of trehalose synthase from baker's yeast. Its temperature-dependent activation by fructose 6-phosphate and inhibition by phosphate. Eur. J. Biochem. 216:841-848. [DOI] [PubMed] [Google Scholar]
- 347.Lopez, M. C., and H. V. Baker. 2000. Understanding the growth phenotype of the yeast gcr1 mutant in terms of global genomic expression patterns. J. Bacteriol. 182:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lorenz, M. C., and J. Heitman. 1998. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lorenz, M. C., and J. Heitman. 1998. Regulators of pseudohyphal differentiation in Saccharomyces cerevisiae identified through multicopy suppressor analysis in ammonium permease mutant strains. Genetics 150:1443-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lussier, M., A. M. White, J. Sheraton, T. di Paolo, J. Treadwell, S. B. Southard, C. I. Horenstein, J. Chen-Weiner, A. F. Ram, J. C. Kapteyn, T. W. Roemer, D. H. Vo, D. C. Bondoc, J. Hall, W. W. Zhong, A. M. Sdicu, J. Davies, F. M. Klis, P. W. Robbins, and H. Bussey. 1997. Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics 147:435-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Luyten, K., J. Albertyn, W. F. Skibbe, B. A. Prior, J. Ramos, J. M. Thevelein, and S. Hohmann. 1995. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 14:1360-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Madaule, P., R. Axel, and A. M. Myers. 1987. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 84:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 354.Madhani, H. D., C. A. Styles, and G. R. Fink. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673-684. [DOI] [PubMed] [Google Scholar]
- 355.Maeda, T., M. Takekawa, and H. Saito. 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269:554-558. [DOI] [PubMed] [Google Scholar]
- 356.Maeda, T., A. Y. M. Tsai, and H. Saito. 1993. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:5408-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Maeda, T., S. M. Wurgler-Murphy, and H. Saito. 1994. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369:242-245. [DOI] [PubMed] [Google Scholar]
- 358.Mager, W. H., and A. J. De Kruijff. 1995. Stress-induced transcriptional activation. Microbiol. Rev. 59:506-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mager, W. H., and R. J. Planta. 1991. Coordinate expression of ribosomal protein genes in yeast as a function of cellular growth rate. Mol. Cell. Biochem. 104:181-187. [DOI] [PubMed] [Google Scholar]
- 360.Mager, W. H., and J. C. Varela. 1993. Osmostress response of the yeast Saccharomyces. Mol. Microbiol. 10:253-258. [PubMed] [Google Scholar]
- 361.Manning, B. D., R. Padmanabha, and M. Snyder. 1997. The rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell 8:1829-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Marchler, G., C. Schüller, G. Adam, and H. Ruis. 1993. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 12:1997-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Marini, N. J., E. Meldrum, B. Buehrer, A. V. Hubberstey, D. E. Stone, A. Traynor-Kaplan, and S. I. Reed. 1996. A pathway in the yeast cell division cycle linking protein kinase C (Pkc1) to activation of Cdc28 at START. EMBO J. 15:3040-3052. [PMC free article] [PubMed] [Google Scholar]
- 364.Marquez, J. A., A. Pascual-Ahuir, M. Proft, and R. Serrano. 1998. The Ssn6-Tup1 repressor complex of Saccharomyces cerevisiae is involved in the osmotic induction of HOG-dependent and -independent genes. EMBO J. 17:2543-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Marquez, J. A., and R. Serrano. 1996. Multiple transduction pathways regulate the sodium-extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 382:89-92. [DOI] [PubMed] [Google Scholar]
- 366.Martin, H., J. Arroyo, M. Sanchez, M. Molina, and C. Nombela. 1993. Activity of the yeast MAP kinase homologue Slt2 is critically required for cell integrity at 37 degrees C. Mol. Gen. Genet. 241:177-184. [DOI] [PubMed] [Google Scholar]
- 367.Martin, H., M. C. Castellanos, R. Cenamor, M. Sanchez, M. Molina, and C. Nombela. 1996. Molecular and functional characterization of a mutant allele of the mitogen-activated protein-kinase gene SLT2 (MPK1) rescued from yeast autolytic mutants. Curr. Genet. 29:516-522. [DOI] [PubMed] [Google Scholar]
- 368.Martin, H., A. Mendoza, J. M. Rodriguez-Pachon, M. Molina, and C. Nombela. 1997. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol. Microbiol. 23:431-444. [DOI] [PubMed] [Google Scholar]
- 369.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 370.Martinez-Pastor, M. T., G. Marchler, C. Schüller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE). EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 371.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Mattison, C. P., and I. M. Ota. 2000. Two protein tyrosine phosphatases, Ptp2 and Ptp3, modulate the subcellular localization of the Hog1 MAP kinase in yeast. Genes Dev. 14:1229-1235. [PMC free article] [PubMed] [Google Scholar]
- 373.Mattison, C. P., S. S. Spencer, K. A. Kresge, J. Lee, and I. M. Ota. 1999. Differential regulation of the cell wall integrity mitogen-activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol. Cell. Biol. 19:7651-7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Maurel, C., and M. J. Chrispeels. 2001. Aquaporins. A molecular entry into plant water relations. Plant Physiol. 125:135-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Mazur, P., and W. Baginsky. 1996. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604-14609. [DOI] [PubMed] [Google Scholar]
- 376.Mazur, P., N. Morin, W. Baginsky, M. el-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Mazzoni, C., P. Zarov, A. Rambourg, and C. Mann. 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Melcher, M. L., and J. Thorner. 1996. Identification and characterization of the CLK1 gene product, a novel CaM kinase-like protein kinase from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271:29958-29968. [DOI] [PubMed] [Google Scholar]
- 379.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C superfamily. Biochem. J. 332:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Mendenhall, M. D., and A. E. Hodge. 1998. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1191-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mendoza, I., F. Rubio, A. Rodriguez-Navarro, and J. M. Pardo. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792-8796. [PubMed] [Google Scholar]
- 382.Meyrial, V., V. Laize, R. Gobin, P. Ripoche, S. Hohmann, and F. Tacnet. 2001. Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur. J. Biochem. 268:334-343. [DOI] [PubMed] [Google Scholar]
- 383.Millar, J. B. 1999. Stress-activated MAP kinase (mitogen-activated protein kinase) pathways of budding and fission yeasts. Biochem. Soc. Symp. 64:49-62. [PubMed] [Google Scholar]
- 384.Millar, J. B., V. Buck, and M. G. Wilkinson. 1995. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9:2117-2130. [DOI] [PubMed] [Google Scholar]
- 385.Millar, J. B., P. Russell, J. E. Dixon, and K. L. Guan. 1992. Negative regulation of mitosis by two functionally overlapping PTPases in fission yeast. EMBO J. 11:4943-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Reference deleted.
- 387.Mizuno, T. 1998. His-Asp phosphotransfer signal transduction. J. Biochem. (Tokyo) 123:555-563. [DOI] [PubMed] [Google Scholar]
- 388.Molz, L., R. Booher, P. Young, and D. Beach. 1989. cdc2 and the regulation of mitosis: six interacting mcs genes. Genetics 122:773-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Morano, K. A., P. C. Liu, and D. J. Thiele. 1998. Protein chaperones and the heat shock response in Saccharomyces cerevisiae. Curr. Opin. Microbiol. 1:197-203. [DOI] [PubMed] [Google Scholar]
- 390.Morgan, B. A., G. R. Banks, W. M. Toone, D. Raitt, S. Kuge, and L. H. Johnston. 1997. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Morgan, B. A., N. Bouquin, and L. H. Johnston. 1995. Two-component signal-transduction systems in budding yeast MAP a different pathway? Trends Cell Biol. 5:453-457. [DOI] [PubMed] [Google Scholar]
- 392.Morgan, B. A., N. Bouquin, G. F. Merrill, and L. H. Johnston. 1995. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 14:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Morsomme, P., C. W. Slayman, and A. Goffeau. 2000. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H+-ATPase. Biochim. Biophys. Acta 1469:133-157. [DOI] [PubMed] [Google Scholar]
- 394.Morton, W. M., K. R. Ayscough, and P. J. McLaughlin. 2000. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2:376-378. [DOI] [PubMed] [Google Scholar]
- 395.Mösch, H. U., and G. R. Fink. 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145:671-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Moser, M. J., J. R. Geiser, and T. N. Davis. 1996. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol. Cell. Biol. 16:4824-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Moskvina, E., E. M. Imre, and H. Ruis. 1999. Stress factors acting at the level of the plasma membrane induce transcription via the stress response element (STRE) of the yeast Saccharomyces cerevisiae. Mol. Microbiol. 32:1263-1272. [DOI] [PubMed] [Google Scholar]
- 398.Moskvina, E., C. Schüller, C. T. C. Maurer, W. H. Mager, and H. Ruis. 1998. A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast 14:1041-1050. [DOI] [PubMed] [Google Scholar]
- 399.Muchardt, C., J. C. Reyes, B. Bourachot, E. Leguoy, and M. Yaniv. 1996. The hbrm and BRG-1 proteins, components of the human SNF/SWI complex, are phosphorylated and excluded from the condensed chromosomes during mitosis. EMBO J. 15:3394-3402. [PMC free article] [PubMed] [Google Scholar]
- 400.Mulholland, J., D. Preuss, A. Moon, A. Wong, D. Drubin, and D. Botstein. 1994. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 125:381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Muller, J., R. A. Aeschbacher, A. Wingler, T. Boller, and A. Wiemken. 2001. Trehalose and trehalase in Arabidopsis. Plant Physiol. 125:1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Murata, K., K. Mitsuoka, T. Hirai, T. Walz, P. Agre, J. B. Heymann, A. Engel, and Y. Fujiyoshi. 2000. Structural determinants of water permeation through aquaporin-1. Nature 407:599-605. [DOI] [PubMed] [Google Scholar]
- 403.Nagahashi, S., T. Mio, N. Ono, T. Yamada-Okabe, M. Arisawa, H. Bussey, and H. Yamada-Okabe. 1998. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144:425-432. [DOI] [PubMed] [Google Scholar]
- 404.Nakagawa, C. W., K. Yamada, and N. Mutoh. 1999. Identification of the catalase gene promoter region involved in superinduction in Schizosaccharomyces pombe caused by cycloheximide and hydrogen peroxide. FEMS Microbiol. Lett. 173:373-378. [DOI] [PubMed] [Google Scholar]
- 405.Nakagawa, C. W., K. Yamada, and N. Mutoh. 2000. Role of Atf1 and Pap1 in the induction of the catalase gene of fission yeast Schizosaccharomyces pombe. J. Biochem. (Tokyo) 127:233-238. [DOI] [PubMed] [Google Scholar]
- 406.Nakamoto, R. K., R. Rao, and C. W. Slayman. 1991. Expression of the yeast plasma membrane [H+]ATPase in secretory vesicles. A new strategy for directed mutagenesis. J. Biol. Chem. 266:7940-7949. [PubMed] [Google Scholar]
- 407.Nakamura, T., Y. Liu, D. Hirata, H. Namba, S. Harada, T. Hirokawa, and T. Miyakawa. 1993. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 12:4063-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407a.Nanduri, J., and A. M. Tartakoff. 2001. Perturbation of the nucleus: a novel Hog1p-independent, Pkc1p-dependent consequence of hypertonic shock in yeast. Mol. Biol. Cell 12:1835-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nass, R., K. W. Cunningham, and R. Rao. 1997. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem. 272:26145-26152. [DOI] [PubMed] [Google Scholar]
- 409.Nass, R., and R. Rao. 1998. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 273:21054-21060. [DOI] [PubMed] [Google Scholar]
- 410.Nass, R., and R. Rao. 1999. The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology 145:3221-3228. [DOI] [PubMed] [Google Scholar]
- 411.Neely, L. A., and C. S. Hoffman. 2000. Protein kinase A and mitogen-activated protein kinase pathways antagonistically regulate fission yeast fbp1 transcription by employing different modes of action at two upstream activation sites. Mol. Cell. Biol. 20:6426-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Nehlin, J. O., M. Carlberg, and H. Ronne. 1992. Yeast SKO1 gene encodes a bZIP protein that binds to the CRE motif and acts as a repressor of transcription. Nucleic Acids Res. 20:5271-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Nehlin, J. O., and H. Ronne. 1990. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 9:2891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Neves, M. J., and J. Francois. 1992. On the mechanism by which a heat shock induces trehalose accumulation in Saccharomyces cerevisiae. Biochem. J. 288:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Nevoigt, E., and U. Stahl. 1997. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:231-241. [DOI] [PubMed] [Google Scholar]
- 416.Nguyen, A. N., A. Lee, W. Place, and K. Shiozaki. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Nguyen, A. N., and K. Shiozaki. 1999. Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 13:1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Nickas, M. E., and M. P. Yaffe. 1996. BRO1, a novel gene that interacts with components of the Pkc1p-mitogen-activated protein kinase pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Nielsen, S., T. H. Kwon, B. M. Christensen, D. Promeneur, J. Frokiaer, and D. Marples. 1999. Physiology and pathophysiology of renal aquaporins. J. Am. Soc. Nephrol. 10:647-663. [DOI] [PubMed] [Google Scholar]
- 420.Nissen, T. L., M. Anderlund, J. Nielsen, J. Villadsen, and M. C. Kielland-Brandt. 2001. Expression of a cytoplasmic transhydrogenase in Saccharomyces cerevisiae results in formation of 2-oxoglutarate due to depletion of the NADPH pool. Yeast 18:19-32. [DOI] [PubMed] [Google Scholar]
- 421.Nonaka, H., K. Tanaka, H. Hirano, T. Fujiwara, H. Kohno, M. Umikawa, A. Mino, and Y. Takai. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Norbeck, J., and A. Blomberg. 1998. Amino acid uptake is strongly affected during exponential growth of Saccharomyces cerevisiae in 0.7 M NaCl medium. FEMS Microbiol. Lett. 158:121-126. [DOI] [PubMed] [Google Scholar]
- 423.Norbeck, J., and A. Blomberg. 2000. The level of cAMP-dependent protein kinase A activity strongly affects osmotolerance and osmo-instigated gene expression changes in Saccharomyces cerevisiae. Yeast 16:121-137. [DOI] [PubMed] [Google Scholar]
- 424.Norbeck, J., and A. Blomberg. 1997. Metabolic and regulatory changes associated with growth of Saccharomyces cerevisiae in 1.4 M NaCl. Evidence for osmotic induction of glycerol dissimilation via the dihydroxyacetone pathway. J. Biol. Chem. 272:5544-5554. [DOI] [PubMed] [Google Scholar]
- 425.Norbeck, J., and A. Blomberg. 1996. Protein expression during exponential growth in 0.7 M NaCl medium of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 137:1-8. [DOI] [PubMed] [Google Scholar]
- 426.Norbeck, J., A. K. Påhlman, N. Akhtar, A. Blomberg, and L. Adler. 1996. Purification and characterization of two isoenzymes of dl-glycerol-3-phosphatase from Saccharomyces cerevisiae. Identification of the corresponding GPP1 and GPP2 genes and evidence for osmotic regulation of Gpp2p expression by the osmosensing mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 271:13875-13881. [DOI] [PubMed] [Google Scholar]
- 427.Reference deleted.
- 428.Novick, P., and D. Botstein. 1985. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell 40:415-426. [DOI] [PubMed] [Google Scholar]
- 429.Nwaka, S., and H. Holzer. 1998. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 58:197-237. [DOI] [PubMed] [Google Scholar]
- 430.Nwaka, S., M. Kopp, and H. Holzer. 1995. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J. Biol. Chem. 270:10193-10198. [DOI] [PubMed] [Google Scholar]
- 431.Nwaka, S., B. Mechler, M. Destruelle, and H. Holzer. 1995. Phenotypic features of trehalase mutants in Saccharomyces cerevisiae. FEBS Lett. 360:286-290. [DOI] [PubMed] [Google Scholar]
- 432.Nwaka, S., B. Mechler, and H. Holzer. 1996. Deletion of the ATH1 gene in Saccharomyces cerevisiae prevents growth on trehalose. FEBS Lett. 386:235-238. [DOI] [PubMed] [Google Scholar]
- 433.Oelkers, P., A. Tinkelenberg, N. Erdeniz, D. Cromley, J. T. Billheimer, and S. L. Sturley. 2000. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275:15609-15612. [DOI] [PubMed] [Google Scholar]
- 434.Ohmiya, R., C. Kato, H. Yamada, H. Aiba, and T. Mizuno. 1999. A fission yeast gene (prr1+) that encodes a response regulator implicated in oxidative stress response. J. Biochem. (Tokyo) 125:1061-1066. [DOI] [PubMed] [Google Scholar]
- 435.Ohmiya, R., H. Yamada, C. Kato, H. Aiba, and T. Mizuno. 2000. The Prr1 response regulator is essential for transcription of ste11+ and for sexual development in fission yeast. Mol. Gen. Genet. 264:441-451. [DOI] [PubMed] [Google Scholar]
- 436.Oki, M., E. Noguchi, N. Hayashi, and T. Nishimoto. 1998. Nuclear protein import, but not mRNA export, is defective in all Saccharomyces cerevisiae mutants that produce temperature-sensitive forms of the Ran GTPase homologue Gsp1p. Mol. Gen. Genet. 257:624-634. [DOI] [PubMed] [Google Scholar]
- 437.Ölz, R., K. Larsson, L. Adler, and L. Gustafsson. 1993. Energy flow and osmoregulation of Saccharomyces cerevisiae grown in a chemostat under NaCl stress. J. Bacteriol. 175:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ono, T., T. Suzuki, Y. Anraku, and H. Iida. 1994. The MID2 gene encodes a putative integral membrane protein with a Ca2+-binding domain and shows mating pheromone-stimulated expression in Saccharomyces cerevisiae. Gene 151:203-208. [DOI] [PubMed] [Google Scholar]
- 439.O'Rourke, S. M., and I. Herskowitz. 1998. The Hog1 MAP kinase prevents cross talk between the HOG and pheromone response MAP kinase pathways in Saccharomyces cerevisiae. Genes Dev. 12:2874-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Ostrander, D. B., and J. A. Gorman. 1999. The extracellular domain of the Saccharomyces cerevisiae Sln1p membrane osmolarity sensor is necessary for kinase activity. J. Bacteriol. 181:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Ota, I. M., and A. Varshavsky. 1992. A gene encoding a putative tyrosine phosphatase suppresses lethality of an N-end rule-dependent mutant. Proc. Natl. Acad. Sci. USA 89:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Ota, I. M., and A. Varshavsky. 1993. A yeast protein similar to bacterial two-component regulators. Science 262:566-569. [DOI] [PubMed] [Google Scholar]
- 443.Ottilie, S., J. Chernoff, G. Hannig, C. S. Hoffman, and R. L. Erikson. 1992. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol. Cell. Biol. 12:5571-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196-2207. [PMC free article] [PubMed] [Google Scholar]
- 445.Ozaki-Kuroda, K., Y. Yamamoto, H. Nohara, M. Kinoshita, T. Fujiwara, K. Irie, and Y. Takai. 2001. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Page, N., J. Sheraton, J. L. Brown, R. C. Stewart, and H. Bussey. 1996. Identification of ASK10 as a multicopy activator of Skn7p-dependent transcription of a HIS3 reporter gene. Yeast 12:267-272. [DOI] [PubMed] [Google Scholar]
- 447.Påhlman, A. K., K. Granath, R. Ansell, S. Hohmann, and L. Adler. 2001. The yeast glycerol 3-phosphatases Gpp1p and Gpp2p are required for glycerol biosynthesis and differentially involved in the cellular responses to osmotic, anaerobic, and oxidative stress. J. Biol. Chem. 276:3555-3563. [DOI] [PubMed] [Google Scholar]
- 448.Paidhungat, M., and S. Garrett. 1997. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 17:6339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Paravicini, G., M. Cooper, L. Friedli, D. J. Smith, J. L. Carpentier, L. S. Klig, and M. A. Payton. 1992. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol. Cell. Biol. 12:4896-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Paravicini, G., and L. Friedli. 1996. Protein-protein interactions in the yeast PKC1 pathway: Pkc1p interacts with a component of the MAP kinase cascade. Mol. Gen. Genet. 251:682-691. [DOI] [PubMed] [Google Scholar]
- 451.Park, J. I., C. M. Grant, P. V. Attfield, and I. W. Dawes. 1997. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal transduction pathway. Appl. Environ. Microbiol. 63:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Parrou, J. L., B. Enjalbert, and J. Francois. 1999. STRE- and cAMP-independent transcriptional induction of Saccharomyces cerevisiae GSY2 encoding glycogen synthase during diauxic growth on glucose. Yeast 15:1471-1484. [DOI] [PubMed] [Google Scholar]
- 453.Parrou, J. L., M. A. Teste, and J. Francois. 1997. Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology 143:1891-1900. [DOI] [PubMed] [Google Scholar]
- 454.Pascual-Ahuir, A., F. Posas, R. Serrano, and M. Proft. 2001. Multiple levels of control regulate the yeast cAMP-response element-binding protein repressor Sko1p in response to stress. J. Biol. Chem. 276:37373-37378. [DOI] [PubMed] [Google Scholar]
- 455.Pascual-Ahuir, A., R. Serrano, and M. Proft. 2001. The Sko1p repressor and Gcn4p activator antagonistically modulate stress-regulated transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Pavlik, P., M. Simon, T. Schuster, and H. Ruis. 1993. The glycerol kinase (GUT1) gene of Saccharomyces cerevisiae: cloning and characterization. Curr. Genet. 24:21-25. [DOI] [PubMed] [Google Scholar]
- 457.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 458.Pedruzzi, I., N. Burckert, P. Egger, and C. De Virgilio. 2000. Saccharomyces cerevisiae Ras/cAMP pathway controls postdiauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 19:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pernambuco, M. B., J. Winderickx, M. Crauwels, G. Griffioen, W. H. Mager, and J. M. Thevelein. 1996. Glucose-triggered signalling in Saccharomyces cerevisiae: different requirements for sugar phosphorylation between cells grown on glucose and those grown on non-fermentable carbon sources. Microbiology 142:1775-1782. [DOI] [PubMed] [Google Scholar]
- 460.Peter, M., A. M. Neiman, H. O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 461.Peterson, J., Y. Zheng, L. Bender, A. Myers, R. Cerione, and A. Bender. 1994. Interactions between the bud emergence proteins Bem1p and Bem2p and rho-type GTPases in yeast. J. Cell Biol. 127:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Philips, J., and I. Herskowitz. 1997. Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J. Cell Biol. 138:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Piper, P. 1998. Differential role of Hsps and trehalose in stress tolerance. Trends Microbiol 6:43-44. [DOI] [PubMed] [Google Scholar]
- 465.Piper, P. W. 1995. The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol. Lett. 134:121-127. [DOI] [PubMed] [Google Scholar]
- 466.Planta, R. J., P. M. Goncalves, and W. H. Mager. 1995. Global regulators of ribosome biosynthesis in yeast. Biochem. Cell Biol. 73:825-834. [DOI] [PubMed] [Google Scholar]
- 467.Plourde-Owobi, L., S. Durner, J. L. Parrou, R. Wieczorke, G. Goma, and J. Francois. 1999. AGT1, encoding an alpha-glucoside transporter involved in uptake and intracellular accumulation of trehalose in Saccharomyces cerevisiae. J. Bacteriol. 181:3830-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Reference deleted.
- 469.Posas, F., M. Camps, and J. Arino. 1995. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem. 270:13036-13041. [DOI] [PubMed] [Google Scholar]
- 470.Posas, F., A. Casamayor, and J. Arino. 1993. The PPZ protein phosphatases are involved in the maintenance of osmotic stability of yeast cells. FEBS Lett. 318:282-286. [DOI] [PubMed] [Google Scholar]
- 471.Posas, F., J. R. Chambers, J. A. Heyman, J. P. Hoeffler, E. de Nadal, and J. Arino. 2000. The transcriptional response of yeast to saline stress. J. Biol. Chem. 275:17249-17255. [DOI] [PubMed] [Google Scholar]
- 472.Posas, F., and H. Saito. 1998. Activation of the yeast SSK2 MAPKKK by the SSK1 two-component response regulator. EMBO J. 17:1385-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Posas, F., and H. Saito. 1997. Osmotic activation of the HOG MAP kinase pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science 276:1702-1705. [DOI] [PubMed] [Google Scholar]
- 474.Posas, F., M. Takekawa, and H. Saito. 1998. Signal transduction by MAP kinase cascades in budding yeast. Curr. Opin. Microbiol. 1:175-182. [DOI] [PubMed] [Google Scholar]
- 475.Posas, F., E. A. Witten, and H. Saito. 1998. Requirement of STE50 for osmostress-induced activation of the STE11 mitogen-activated protein kinase kinase kinase in the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 18:5788-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 477.Pott, G. B., T. K. Miller, J. A. Bartlett, J. S. Palas, and C. P. Selitrennikoff. 2000. The isolation of FOS-1, a gene encoding a putative two-component histidine kinase from Aspergillus fumigatus. Fungal Genet. Biol. 31:55-67. [DOI] [PubMed] [Google Scholar]
- 478.Pretorius, I. S. 2000. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675-729. [DOI] [PubMed] [Google Scholar]
- 479.Primig, M., S. Sockanathan, H. Auer, and K. Nasmyth. 1992. Anatomy of a transcription factor important for the start of the cell cycle in Saccharomyces cerevisiae. Nature 358:593-597. [DOI] [PubMed] [Google Scholar]
- 480.Proft, M., A. Pascual-Ahuir, E. de Nadal, J. Arino, R. Serrano, and F. Posas. 2001. Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 20:1123-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Proft, M., and R. Serrano. 1999. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell. Biol. 19:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:365-375. [DOI] [PubMed] [Google Scholar]
- 483.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 484.Qadota, H., Y. Anraku, D. Botstein, and Y. Ohya. 1994. Conditional lethality of a yeast strain expressing human RHOA in place of RHO1. Proc. Natl. Acad. Sci. USA 91:9317-9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 486.Rahman, M. S. 1999. Handbook of food preservation. Marcel Dekker Inc., New York, N.Y.
- 487.Raitt, D. C., A. L. Johnson, A. M. Erkine, K. Makino, B. Morgan, D. S. Gross, and L. H. Johnston. 2000. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 11:2335-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Raitt, D. C., F. Posas, and H. Saito. 2000. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAP kinase pathway. EMBO J. 19:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Rajavel, M., B. Philip, B. M. Buehrer, B. Errede, and D. E. Levin. 1999. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Ramne, A., E. Bilsland-Marchesan, S. Erickson, and P. Sunnerhagen. 2000. The protein kinases Rck1 and Rck2 inhibit meiosis in budding yeast. Mol. Gen. Genet. 263:253-261. [DOI] [PubMed] [Google Scholar]
- 491.Randez-Gil, F., P. Sanz, and J. A. Prieto. 1999. Engineering baker's yeast: room for improvement. Trends Biotechnol. 17:237-244. [DOI] [PubMed] [Google Scholar]
- 492.Raught, B., A. C. Gingras, and N. Sonenberg. 2001. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA 98:7037-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Reifenberger, E., E. Boles, and M. Ciriacy. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245:324-333. [DOI] [PubMed] [Google Scholar]
- 494.Reifenberger, E., K. Freidel, and M. Ciriacy. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16:157-167. [DOI] [PubMed] [Google Scholar]
- 495.Reinders, A., N. Burckert, S. Hohmann, J. M. Thevelein, T. Boller, A. Wiemken, and C. De Virgilio. 1997. Structural analysis of the subunits of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae and their function during heat shock. Mol. Microbiol. 24:687-695. [DOI] [PubMed] [Google Scholar]
- 496.Reiser, V., G. Ammerer, and H. Ruis. 1999. Nucleocytoplasmic traffic of MAP kinases. Gene Expr. 7:247-254. [PMC free article] [PubMed] [Google Scholar]
- 497.Reiser, V., H. Ruis, and G. Ammerer. 1999. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 10:1147-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Reiser, V., S. M. Salah, and G. Ammerer. 2000. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2:620-627. [DOI] [PubMed] [Google Scholar]
- 498a.Remize, F., L. Barnavon, and S. Dequin. 2001. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatases, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab. Eng. 3:301-312. [DOI] [PubMed] [Google Scholar]
- 499.Ren, B., F. Robert, J. J. Wyrick, O. Aparicio, E. G. Jennings, I. Simon, J. Zeitlinger, J. Schreiber, N. Hannett, E. Kanin, T. L. Volkert, C. J. Wilson, S. P. Bell, and R. A. Young. 2000. Genome-wide location and function of DNA-binding proteins. Science 290:2306-2309. [DOI] [PubMed] [Google Scholar]
- 500.Rep, M., J. Albertyn, J. M. Thevelein, B. A. Prior, and S. Hohmann. 1999. Different signalling pathways contribute to the control of GPD1 expression by osmotic stress in Saccharomyces cerevisiae. Microbiology 145:715-727. [DOI] [PubMed] [Google Scholar]
- 501.Rep, M., M. Krantz, J. M. Thevelein, and S. Hohmann. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275:8290-8300. [DOI] [PubMed] [Google Scholar]
- 502.Rep, M., M. Proft, F. Remize, M. Tamas, R. Serrano, J. M. Thevelein, and S. Hohmann. 2001. The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol. Microbiol. 40:1067-1083. [DOI] [PubMed] [Google Scholar]
- 503.Rep, M., V. Reiser, U. Holzmüller, J. M. Thevelein, S. Hohmann, G. Ammerer, and H. Ruis. 1999. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 19:5474-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Reference deleted.
- 505.Reynolds, T. B., B. D. Hopkins, M. R. Lyons, and T. R. Graham. 1998. The high osmolarity glycerol response (HOG) MAP kinase pathway controls localization of a yeast Golgi glycosyltransferase. J. Cell Biol. 143:935-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Rios, G., A. Ferrando, and R. Serrano. 1997. Mechanisms of salt tolerance conferred by overexpression of the HAL1 gene in Saccharomyces cerevisiae. Yeast 13:515-528. [DOI] [PubMed] [Google Scholar]
- 507.Roberts, C. J., B. Nelson, M. J. Marton, R. Stoughton, M. R. Meyer, H. A. Bennett, Y. D. He, H. Dai, W. L. Walker, T. R. Hughes, M. Tyers, C. Boone, and S. H. Friend. 2000. Signaling and circuitry of multiple MAP kinase pathways revealed by a matrix of global gene expression profiles. Science 287:873-880. [DOI] [PubMed] [Google Scholar]
- 508.Roberts, R. L., and G. R. Fink. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8:2974-2985. [DOI] [PubMed] [Google Scholar]
- 509.Robertson, L. S., H. C. Causton, R. A. Young, and G. R. Fink. 2000. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. USA 97:5984-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Rodriguez-Navarro, A. 2000. Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469:1-30. [DOI] [PubMed] [Google Scholar]
- 511.Roemer, T., G. Paravicini, M. A. Payton, and H. Bussey. 1994. Characterization of the yeast (1→6)-beta-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J. Cell Biol. 127:567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Rohde, J., J. Heitman, and M. E. Cardenas. 2001. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276:9583-9586. [DOI] [PubMed] [Google Scholar]
- 513.Ronne, H. 1995. Glucose repression in fungi. Trends Genet. 11:12-17. [DOI] [PubMed] [Google Scholar]
- 514.Ronnow, B., and M. Kielland-Brandt. 1993. GUT2, a gene for mitochondrial glycerol 3-phosphate dehydrogenase of Saccharomyces cerevisiae. Yeast 9:1121-1130. [DOI] [PubMed] [Google Scholar]
- 515.Roovers, K., and R. K. Assoian. 2000. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22:818-826. [DOI] [PubMed] [Google Scholar]
- 516.Ross, S. J., V. J. Findlay, P. Malakasi, and B. A. Morgan. 2000. Thioredoxin peroxidase is required for the transcriptional response to oxidative stress in budding yeast. Mol. Biol. Cell 11:2631-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Ross-Macdonald, P., P. S. Coelho, T. Roemer, S. Agarwal, A. Kumar, R. Jansen, K. H. Cheung, A. Sheehan, D. Symoniatis, L. Umansky, M. Heidtman, F. K. Nelson, H. Iwasaki, K. Hager, M. Gerstein, P. Miller, G. S. Roeder, and M. Snyder. 1999. Large-scale analysis of the yeast genome by transposon tagging and gene disruption. Nature 402:413-418. [DOI] [PubMed] [Google Scholar]
- 518.Rübenhagen, R., S. Morbach, and R. Krämer. 2001. The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for cytoplasmic K+. EMBO J. 20:5412-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ruis, H., and C. Schüller. 1995. Stress signaling in yeast. Bioessays 17:959-965. [DOI] [PubMed] [Google Scholar]
- 520.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 521.Saito, H. 2001. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev. 101:2497-2509. [DOI] [PubMed] [Google Scholar]
- 522.Samejima, I., S. Mackie, and P. A. Fantes. 1997. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 16:6162-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Samejima, I., S. Mackie, E. Warbrick, R. Weisman, and P. A. Fantes. 1998. The fission yeast mitotic regulator win1+ encodes an MAPKKK that phosphorylates and activates Wis1 MAPKK in response to high osmolarity. Mol. Biol. Cell 9:2325-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Sanchez, Y., J. Taulien, K. A. Borkovich, and S. Lindquist. 1992. Hsp104 is required for tolerance to many forms of stress. EMBO J. 11:2357-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Sanchez-Martinez, C., and J. Perez-Martin. 2001. Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis—similar inputs, different outputs. Curr. Opin. Microbiol. 4:214-221. [DOI] [PubMed] [Google Scholar]
- 526.San Jose, C., R. A. Monge, R. Perez-Diaz, J. Pla, and C. Nombela. 1996. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178:5850-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Sano, F., N. Asakawa, Y. Inoue, and M. Sakurai. 1999. A dual role for intracellular trehalose in the resistance of yeast cells to water stress. Cryobiology 39:80-87. [DOI] [PubMed] [Google Scholar]
- 528.Santoni, V., P. Gerbeau, H. Javot, and C. Maurel. 2000. The high diversity of aquaporins reveals novel facets of plant membrane functions. Curr. Opin. Plant Biol. 3:476-481. [DOI] [PubMed] [Google Scholar]
- 529.Schmelzle, T., and M. N. Hall. 2000. TOR, a central controller of cell growth. Cell 103:253-262. [DOI] [PubMed] [Google Scholar]
- 530.Schmidt, A., M. Bickle, T. Beck, and M. N. Hall. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531-542. [DOI] [PubMed] [Google Scholar]
- 531.Schmidt, M. C., and R. R. McCartney. 2000. Beta-subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 19:4936-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Schmitt, A. P., and K. McEntee. 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Schüller, G., J. L. Brewster, M. R. Alexander, M. C. Gustin, and H. Ruis. 1994. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 13:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Schultz, J., and M. Carlson. 1987. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:3637-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Schuster, S. C., A. A. Noegel, F. Oehme, G. Gerisch, and M. I. Simon. 1996. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 15:3880-3889. [PMC free article] [PubMed] [Google Scholar]
- 536.Seoighe, C., and K. H. Wolfe. 1999. Yeast genome evolution in the post-genome era. Curr. Opin. Microbiol. 2:548-554. [DOI] [PubMed] [Google Scholar]
- 537.Serrano, R. 1996. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int. Rev. Cytol. 165:1-52. [DOI] [PubMed] [Google Scholar]
- 538.Serrano, R., J. A. Márquez, and G. Ríos. 1997. Crucial factors in salt tolerance., p. 147-169. In S. Hohmann and W. H. Mager (ed.), Yeast stress responses. R. G. Landes, Austin, Tex.
- 539.Serrano, R., J. M. Mulet, G. Rios, J. A. Marquez, L. I. F. de, M. P. Leube, I. Mendizabal, A. A. Pascual, M. Proft, R. Ros, and C. Montesinos. 1999. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 50:1023-1036. [Google Scholar]
- 540.Serrano, R., and A. Rodriguez-Navarro. 2001. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 13:399-404. [DOI] [PubMed] [Google Scholar]
- 541.Shahinian, S., and H. Bussey. 2000. β-1,6-Glucan synthesis in Saccharomyces cerevisiae. Mol. Microbiol. 35:477-489. [DOI] [PubMed] [Google Scholar]
- 542.Shamji, A. F., F. G. Kuruvilla, and S. L. Schreiber. 2000. Partitioning the transcriptional program induced by rapamycin among the effectors of the Tor proteins. Curr. Biol. 10:1574-1581. [DOI] [PubMed] [Google Scholar]
- 543.Shen, B., S. Hohmann, R. G. Jensen, and H. Bohnert. 1999. Roles of sugar alcohols in osmotic stress adaptation. Replacement of glycerol by mannitol and sorbitol in yeast. Plant Physiol. 121:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Sheu, Y. J., B. Santos, N. Fortin, C. Costigan, and M. Snyder. 1998. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18:4053-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Shieh, J. C., M. G. Wilkinson, V. Buck, B. A. Morgan, K. Makino, and J. B. Millar. 1997. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 11:1008-1022. [DOI] [PubMed] [Google Scholar]
- 546.Shieh, J. C., M. G. Wilkinson, and J. B. Millar. 1998. The Win1 mitotic regulator is a component of the fission yeast stress-activated Sty1 MAP kinase pathway. Mol. Biol. Cell 9:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Shimizu, J., K. Yoda, and M. Yamasaki. 1994. The hypo-osmolarity-sensitive phenotype of the Saccharomyces cerevisiae hpo2 mutant is due to a mutation in PKC1, which regulates expression of β-glucanase. Mol. Gen. Genet. 242:641-648. [DOI] [PubMed] [Google Scholar]
- 548.Shiozaki, K., and P. Russell. 1995. Cell cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378:739-743. [DOI] [PubMed] [Google Scholar]
- 549.Shiozaki, K., and P. Russell. 1994. Cellular function of protein phosphatase 2C in yeast. Cell. Mol. Biol. Res. 40:241-243. [PubMed] [Google Scholar]
- 550.Shiozaki, K., and P. Russell. 1996. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10:2276-2288. [DOI] [PubMed] [Google Scholar]
- 551.Shiozaki, K., and P. Russell. 1995. Counteractive roles of protein phosphatase 2C (PP2C) and a MAPKK homolog in the osmoregulation of fission yeast. EMBO J. 14:492-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Shiozaki, K., M. Shiozaki, and P. Russell. 1998. Heat stress activates fission yeast Spc1/Sty1 MAP kinase by a MEKK-independent mechanism. Mol. Biol. Cell 9:1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Shiozaki, K., M. Shiozaki, and P. Russell. 1997. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol. Biol. Cell 8:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Siderius, M., C. P. Kolen, H. van Heerikhuizen, and W. H. Mager. 2000. Candidate osmosensors from Candida utilis and Kluyveromyces lactis: structural and functional homology to the Sho1p putative osmosensor from Saccharomyces cerevisiae. Biochim. Biophys. Acta 1517:143-147. [DOI] [PubMed] [Google Scholar]
- 555.Siderius, M., and W. H. Mager. 1997. The general stress response in search for a common denominator, p. 213-230. In S. Hohmann and W. H. Mager (ed.), Yeast stress responses. R. G. Landes Company, Austin, Tex.
- 556.Siderius, M., O. Van Wuytswinkel, K. A. Reijenga, M. Kelders, and W. H. Mager. 2000. The control of intracellular glycerol in Saccharomyces cerevisiae influences osmotic stress response and resistance to increased temperature. Mol. Microbiol. 36:1381-1390. [DOI] [PubMed] [Google Scholar]
- 557.Reference deleted.
- 558.Sillje, H. H., E. G. ter Schure, A. J. Rommens, P. G. Huls, C. L. Woldringh, A. J. Verkleij, J. Boonstra, and C. T. Verrips. 1997. Effects of different carbon fluxes on G1 phase duration, cyclin expression, and reserve carbohydrate metabolism in Saccharomyces cerevisiae. J. Bacteriol. 179:6560-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Sillje, H. H. W., J. W. G. Paalman, E. G. ter Schure, S. Q. B. Olsthoorn, A. J. Verkleij, J. Boonstra, and C. T. Verrips. 1999. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J. Bacteriol. 181:396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Singer, M. A., and S. Lindquist. 1998. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1:639-648. [DOI] [PubMed] [Google Scholar]
- 561.Singer, M. A., and S. Lindquist. 1998. Thermotolerance in Saccharomyces cerevisiae: the yin and yang of trehalose. Trends Biotechnol. 16:460-468. [DOI] [PubMed] [Google Scholar]
- 562.Singh, K. K. 2000. The Saccharomyces cerevisiae Sln1p-Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic. Biol. Med. 29:1043-1050. [DOI] [PubMed] [Google Scholar]
- 563.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Smith, R. L., and A. D. Johnson. 2000. Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem. Sci. 25:325-330. [DOI] [PubMed] [Google Scholar]
- 565.Smits, G. J., J. C. Kapteyn, H. van den Ende, and F. M. Klis. 1999. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2:348-352. [DOI] [PubMed] [Google Scholar]
- 566.Snyder, M. 1989. The SPA2 protein of yeast localizes to sites of cell growth. J. Cell Biol. 108:1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Soler, M., A. Plovins, H. Martin, M. Molina, and C. Nombela. 1995. Characterization of domains in the yeast MAP kinase Slt2 (Mpk1) required for functional activity and in vivo interaction with protein kinases Mkk1 and Mkk2. Mol. Microbiol. 17:833-842. [DOI] [PubMed] [Google Scholar]
- 568.Somero, G. N., and P. H. Yancey. 1997. Osmolytes and cell volume regulation: physiological and evolutionary principles, p. 441-484. In J. F. Hoffmann and J. D. Jamieson (ed.), Handbook of physiology, section 14: cell physiology. Oxford University Press, New York, N.Y.
- 569.Souciet, J., M. Aigle, F. Artiguenave, G. Blandin, M. Bolotin-Fukuhara, E. Bon, P. Brottier, S. Casaregola, J. de Montigny, B. Dujon, P. Durrens, C. Gaillardin, A. Lepingle, B. Llorente, A. Malpertuy, C. Neuveglise, O. Ozier-Kalogeropoulos, S. Potier, W. Saurin, F. Tekaia, C. Toffano-Nioche, M. Wesolowski-Louvel, P. Wincker, and J. Weissenbach. 2000. Genomic exploration of the hemiascomycetous yeasts. 1. A set of yeast species for molecular evolution studies. FEBS Lett. 487:3-12. [DOI] [PubMed] [Google Scholar]
- 570.Spencer, J. F. T., and D. M. Spencer. 1997. Yeasts in natural and artificial habitats. Springer-Verlag, Heidelberg, Germany.
- 571.Sprague, G. F., and J. E. Cronan. 1977. Isolation and characterization of Saccharomyces cerevisiae mutants defective in glycerol catabolism. J. Bacteriol. 129:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Sprague, G. F. J., and J. W. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae, p. 657-744. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces: gene expression, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 573.Srikantha, T., L. Tsai, K. Daniels, L. Enger, K. Highley, and D. R. Soll. 1998. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology 144:2715-2729. [DOI] [PubMed] [Google Scholar]
- 574.Stahlberg, H., B. Heymann, K. Mitsuoka, Y. Fuyijoshi, and A. Engel. 2001. The aquaporin superfamily: structure and function, p. 39-119. In S. Hohmann, S. Nielsen, and P. Agre (ed.), Aquaporins, vol. 51. Academic Press, San Diego, Calif. [Google Scholar]
- 575.Stallkamp, I., W. Dowhan, K. Altendorf, and K. Jung. 1999. Negatively charged phospholipids influence the activity of the sensor kinase KdpD of Escherichia coli. Arch. Microbiol. 172:295-302. [DOI] [PubMed] [Google Scholar]
- 576.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Stephen, D. W., S. L. Rivers, and D. J. Jamieson. 1995. The role of the YAP1 and YAP2 genes in the regulation of the adaptive oxidative stress responses of Saccharomyces cerevisiae. Mol. Microbiol. 16:415-423. [DOI] [PubMed] [Google Scholar]
- 578.Stirling, D. A., and M. J. Stark. 2000. Mutations in SPC110, encoding the yeast spindle pole body calmodulin-binding protein, cause defects in cell integrity as well as spindle formation. Biochim. Biophys. Acta 1499:85-100. [DOI] [PubMed] [Google Scholar]
- 579.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 580.Strom, A. R. 1998. Osmoregulation in the model organism Escherichia coli: genes governing the synthesis of glycine betaine and trehalose and their use in metabolic engineering of stress tolerance. J. Biosci. 23:437-445. [Google Scholar]
- 581.Strom, A. R., and I. Kaasen. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205-210. [DOI] [PubMed] [Google Scholar]
- 582.Sugajska, E., W. Swiatek, P. Zabrocki, I. Geyskens, J. M. Thevelein, S. Zolnierowicz, and S. Wera. 2001. Multiple effects of protein phosphatase 2A on nutrient-induced signalling in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 40:1020-1026. [DOI] [PubMed] [Google Scholar]
- 583.Sukharev, S., M. Betanzos, C. S. Chiang, and H. R. Guy. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature 409:720-724. [DOI] [PubMed] [Google Scholar]
- 584.Sukharev, S. I., P. Blount, B. Martinac, and C. Kung. 1997. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59:633-657. [DOI] [PubMed] [Google Scholar]
- 585.Sutherland, F. C. W., F. Lages, C. Lucas, K. Luyten, J. Albertyn, S. Hohmann, B. A. Prior, and S. G. Kilian. 1997. Characteristics of Fps1-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J. Bacteriol. 179:7790-7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Takeda, T., T. Toda, K. Kominami, A. Kohnosu, M. Yanagida, and N. Jones. 1995. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 14:6193-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Reference deleted.
- 588.Tamás, M. J., K. Luyten, F. C. W. Sutherland, A. Hernandez, J. Albertyn, H. Valadi, H. Li, B. A. Prior, S. G. Kilian, J. Ramos, L. Gustafsson, J. M. Thevelein, and S. Hohmann. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087-1104. [DOI] [PubMed] [Google Scholar]
- 589.Tamás, M. J., M. Rep, J. M. Thevelein, and S. Hohmann. 2000. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 472:159-165. [DOI] [PubMed] [Google Scholar]
- 590.Tanaka, T., S. K. Saha, C. Tomomori, R. Ishima, D. Liu, K. I. Tong, H. Park, R. Dutta, L. Qin, M. B. Swindells, T. Yamazaki, A. M. Ono, M. Kainosho, M. Inouye, and M. Ikura. 1998. NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature 396:88-92. [DOI] [PubMed] [Google Scholar]
- 591.Tao, W., R. J. Deschenes, and J. S. Fassler. 1999. Intracellular glycerol levels modulate the activity of Sln1p, a Saccharomyces cerevisiae two-component regulator. J. Biol. Chem. 274:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Teige, M., E. Scheikl, V. Reiser, H. Ruis, and G. Ammerer. 2001. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc. Natl. Acad. Sc.i USA 98:5625-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Thevelein, J. M. 1984. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 48:42-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Thevelein, J. M. 1994. Signal transduction in yeast. Yeast 10:1753-1790. [DOI] [PubMed] [Google Scholar]
- 595.Thevelein, J. M., L. Cauwenberg, S. Colombo, J. H. De Winde, M. Donation, F. Dumortier, L. Kraakman, K. Lemaire, P. Ma, D. Nauwelaers, F. Rolland, A. Teunissen, P. Van Dijck, M. Versele, S. Wera, and J. Winderickx. 2000. Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance, and growth in yeast. Enzyme Microb. Technol. 26:819-825. [DOI] [PubMed] [Google Scholar]
- 596.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 597.Thevelein, J. M., and S. Hohmann. 1995. Trehalose synthase, guard to the gate of glycolysis in yeast? Trends Biochem. Sci. 20:3-10. [DOI] [PubMed] [Google Scholar]
- 598.Thomas, G., and M. N. Hall. 1997. TOR signalling and control of cell growth. Curr. Opin. Cell Biol. 9:782-787. [DOI] [PubMed] [Google Scholar]
- 599.Thompson-Jaeger, S., J. Francois, J. P. Gaughran, and K. Tatchell. 1991. Deletion of SNF1 affects the nutrient response of yeast and resembles mutations which activate the adenylate cyclase pathway. Genetics 129:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Toda, T., M. Shimanuki, Y. Saka, H. Yamano, Y. Adachi, M. Shirakawa, Y. Kyogoku, and M. Yanagida. 1992. Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol. Cell. Biol. 12:5474-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Toda, T., M. Shimanuki, and M. Yanagida. 1991. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 5:60-73. [DOI] [PubMed] [Google Scholar]
- 602.Toh, T.-H., G. Kayingo, M. J. van der Merwe, S. G. Kilian, J. E. Hallsworth, S. Hohmann, and B. A. Prior. 2001. Implications of FPS1 deletion and membrane ergosterol content for glycerol efflux from Saccharomyces cerevisiae. FEMS Yeast Res. 1::205-211. [DOI] [PubMed] [Google Scholar]
- 603.Tokishita, S., A. Kojima, H. Aiba, and T. Mizuno. 1991. Transmembrane signal transduction and osmoregulation in Escherichia coli. Functional importance of the periplasmic domain of the membrane-located protein kinase EnvZ. J. Biol. Chem. 266:6780-6785. [PubMed] [Google Scholar]
- 604.Tokishita, S., A. Kojima, and T. Mizuno. 1992. Transmembrane signal transduction and osmoregulation in Escherichia coli: functional importance of the transmembrane regions of membrane-located protein kinase EnvZ. J. Biochem. (Tokyo) 111:707-713. [DOI] [PubMed] [Google Scholar]
- 605.Tokishita, S., and T. Mizuno. 1994. Transmembrane signal transduction by the Escherichia coli osmotic sensor, EnvZ: intermolecular complementation of transmembrane signalling. Mol. Microbiol. 13:435-444. [DOI] [PubMed] [Google Scholar]
- 606.Tokishita, S., H. Yamada, H. Aiba, and T. Mizuno. 1990. Transmembrane signal transduction and osmoregulation in Escherichia coli: II. The osmotic sensor, EnvZ, located in the isolated cytoplasmic membrane displays its phosphorylation and dephosphorylation abilities as to the activator protein, OmpR. J. Biochem. (Tokyo) 108:488-493. [DOI] [PubMed] [Google Scholar]
- 607.Tomomori, C., T. Tanaka, R. Dutta, H. Park, S. K. Saha, Y. Zhu, R. Ishima, D. Liu, K. I. Tong, H. Kurokawa, H. Qian, M. Inouye, and M. Ikura. 1999. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat. Struct. Biol. 6:729-734. [DOI] [PubMed] [Google Scholar]
- 608.Toone, W. M., S. Kuge, M. Samuels, B. A. Morgan, T. Toda, and N. Jones. 1998. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 12:1453-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Torres, L., H. Martin, M. I. Garcia-Saez, J. Arroyo, M. Molina, M. Sanchez, and C. Nombela. 1991. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol. Microbiol. 5:2845-2854. [DOI] [PubMed] [Google Scholar]
- 610.Treger, J. M., T. R. Magee, and K. McEntee. 1998. Functional analysis of the stress response element and its role in the multistress response of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 243:13-19. [DOI] [PubMed] [Google Scholar]
- 611.Treger, J. M., A. P. Schmitt, J. R. Simon, and K. McEntee. 1998. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J. Biol. Chem. 273:26875-26879. [DOI] [PubMed] [Google Scholar]
- 612.Trollmo, C., L. André, A. Blomberg, and L. Adler. 1988. Physiological overlap between osmotolerance and thermotolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 56:321-326. [Google Scholar]
- 613.Tsujimoto, Y., S. Izawa, and Y. Inoue. 2000. Cooperative regulation of DOG2, encoding 2-deoxyglucose-6-phosphate phosphatase, by Snf1 kinase and the high-osmolarity glycerol-mitogen-activated protein kinase cascade in stress responses of Saccharomyces cerevisiae. J. Bacteriol. 182:5121-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Tyerman, S. D., H. J. Bohnert, C. Maurel, E. Steudle, and J. A. C. Smith. 1999. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J. Exp. Bot. 50:1055-1071. [Google Scholar]
- 615.Tyson, J. J., K. Chen, and B. Novak. 2001. Network dynamics and cell physiology. Nat. Rev. Mol. Cell Biol. 2:908-916. [DOI] [PubMed] [Google Scholar]
- 616.Uemura, H., and Y. Jigami. 1995. Mutations in GCR1, a transcriptional activator of Saccharomyces cerevisiae glycolytic genes, function as suppressors of gcr2 mutations. Genetics 139:511-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Uemura, H., and M. Suzuki. 1995. Multidomainial structure of the yeast transcription factor, Gcr1, and its possible mode of DNA-binding. Proc. Jpn. Acad. 71:269-273. [Google Scholar]
- 618.Urao, T., S. Miyata, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2000. Possible His to Asp phosphorelay signaling in an Arabidopsis two-component system. FEBS Lett. 478:227-232. [DOI] [PubMed] [Google Scholar]
- 619.Urao, T., B. Yakubov, R. Satoh, K. Yamaguchi-Shinozaki, M. Seki, T. Hirayama, and K. Shinozaki. 1999. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11:1743-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Urao, T., K. Yamaguchi-Shinozaki, and K. Shinozaki. 2000. Two-component systems in plant signal transduction. Trends Plant Sci. 5:67-74. [DOI] [PubMed] [Google Scholar]
- 621.Van Aelst, L., S. Hohmann, F. K. Zimmermann, A. W. Jans, and J. M. Thevelein. 1991. A yeast homologue of the bovine lens fibre MIP gene family complements the growth defect of a Saccharomyces cerevisiae mutant on fermentable sugars but not its defect in glucose-induced RAS-mediated cAMP signalling. EMBO J. 10:2095-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Vandenbol, M., J. C. Jauniaux, and M. Grenson. 1989. Nucleotide sequence of the Saccharomyces cerevisiae PUT4 proline-permease-encoding gene: similarities between CAN1, HIP1 and PUT4 permeases. Gene 83:153-159. [DOI] [PubMed] [Google Scholar]
- 623.Van Dijck, P., D. Colavizza, P. Smet, and J. M. Thevelein. 1995. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 61:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Van Doorn, J., M. E. Scholte, P. W. Postma, R. Van Driel, and K. Van Dam. 1988. Regulation of trehalase activity during the cell cycle of Saccharomyces cerevisiae. J. Gen. Microbiol. 134:785-790. [DOI] [PubMed] [Google Scholar]
- 625.Van Doorn, J., J. A. Valkenburg, M. E. Scholte, L. J. Oehlen, R. Van Driel, P. W. Postma, N. Nanninga, and K. Van Dam. 1988. Changes in activities of several enzymes involved in carbohydrate metabolism during the cell cycle of Saccharomyces cerevisiae. J. Bacteriol. 170:4808-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Van Wuytswinkel, O., V. Reiser, M. Siderius, M. C. Kelders, G. Ammerer, H. Ruis, and W. H. Mager. 2000. Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol. Microbiol. 37:382-397. [DOI] [PubMed] [Google Scholar]
- 627.Varela, J. C., C. van Beekvelt, R. J. Planta, and W. H. Mager. 1992. Osmostress-induced changes in yeast gene expression. Mol. Microbiol. 6:2183-2190. [DOI] [PubMed] [Google Scholar]
- 628.Verkman, A. S., and A. K. Mitra. 2000. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 278:F13-28. [DOI] [PubMed] [Google Scholar]
- 629.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Vido, K., D. Spector, G. Lagniel, S. Lopez, M. B. Toledano, and J. Labarre. 2001. A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 276:8469-8474. [DOI] [PubMed] [Google Scholar]
- 631.Vilella-Bach, M., P. Nuzzi, Y. Fang, and J. Chen. 1999. The FKBP12-rapamycin-binding domain is required for FKBP12-rapamycin-associated protein kinase activity and G1 progression. J. Biol. Chem. 274:4266-4272. [DOI] [PubMed] [Google Scholar]
- 632.Vincent, A. C., and K. Struhl. 1992. ACR1, a yeast ATF/CREB repressor. Mol. Cell. Biol. 12:5394-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Vincent, O., R. Townley, S. Kuchin, and M. Carlson. 2001. Subcellular localization of the Snf1 kinase is regulated by specific beta subunits and a novel glucose signaling mechanism. Genes Dev. 15:1104-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Virginia, M., C. L. Appleyard, W. L. McPheat, and M. J. Stark. 2000. A novel ‘two-component’ protein containing histidine kinase and response regulator domains required for sporulation in Aspergillus nidulans. Curr. Genet. 37:364-372. [DOI] [PubMed] [Google Scholar]
- 635.Vissi, E., J. Clotet, E. de Nadal, A. Barcelo, E. E. Bako, P. Gergely, V. V. Dombradi, and J. Arino. 2001. Functional analysis of the Neurospora crassa PZL-1 protein phosphatase by expression in budding and fission yeast. Yeast 18:115-124. [DOI] [PubMed] [Google Scholar]
- 636.Vuorio, O. E., N. Kalkkinen, and J. Londesborough. 1993. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 216:849-861. [DOI] [PubMed] [Google Scholar]
- 637.Wang, T., and A. Bretscher. 1995. The rho-GAP encoded by BEM2 regulates cytoskeletal structure in budding yeast. Mol. Biol. Cell 6:1011-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Warbrick, E., and P. A. Fantes. 1992. Five novel elements involved in the regulation of mitosis in fission yeast. Mol. Gen. Genet. 232:440-446. [DOI] [PubMed] [Google Scholar]
- 639.Warbrick, E., and P. A. Fantes. 1991. The wis1 protein kinase is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 10:4291-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Warmka, J., J. Hanneman, J. Lee, D. Amin, and I. Ota. 2001. Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 21:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Watanabe, Y., G. Takaesu, M. Hagiwara, K. Irie, and K. Matsumoto. 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Wera, S., E. De Schrijver, I. Geyskens, S. Nwaka, and J. M. Thevelein. 1999. Opposite roles of trehalase activity in heat-shock recovery and heat-shock survival in Saccharomyces cerevisiae. Biochem J. 343:621-626. [PMC free article] [PubMed] [Google Scholar]
- 644.Wertman, K. F., D. G. Drubin, and D. Botstein. 1992. Systematic mutational analysis of the yeast ACT1 gene. Genetics 132:337-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Wieland, J., A. M. Nitsche, J. Strayle, H. Steiner, and H. K. Rudolph. 1995. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 14:3870-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Wieser, R., G. Adam, A. Wagner, C. Schüller, G. Marchler, H. Ruis, Z. Krawiec, and T. Bilinski. 1991. Heat shock factor-independent heat control of transcription of the CTT1 gene encoding the cytosolic catalase T of Saccharomyces cerevisiae. J. Biol. Chem. 266:12406-12411. [PubMed] [Google Scholar]
- 647.Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J. C. Shieh, T. Toda, J. B. Millar, and N. Jones. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289-2301. [DOI] [PubMed] [Google Scholar]
- 648.Williams, K. E., and M. S. Cyert. 2001. The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. EMBO J. 20:3473-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Wilson, W. A., S. A. Hawley, and D. G. Hardie. 1996. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr. Biol. 6:1426-1434. [DOI] [PubMed] [Google Scholar]
- 650.Winderickx, J., J. H. de Winde, M. Crauwels, A. Hino, S. Hohmann, P. Van Dijck, and J. M. Thevelein. 1996. Regulation of genes encoding subunits of the trehalose synthase complex in Saccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol. Gen. Genet. 252:470-482. [DOI] [PubMed] [Google Scholar]
- 651.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, H. Bussey, A. M. Chu, C. Connelly, K. Davis, F. Dietrich, S. W. Dow, M. El Bakkoury, F. Foury, S. H. Friend, E. Gentalen, G. Giaever, J. H. Hegemann, T. Jones, M. Laub, H. Liao, R. W. Davis, et al. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 652.Wolfe, K. H., and D. C. Shields. 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387:708-713. [DOI] [PubMed] [Google Scholar]
- 653.Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Wurgler-Murphy, S. M., T. Maeda, E. A. Witten, and H. Saito. 1997. Regulation of the Saccharomyces cerevisiae Hog1 mitogen-activated protein kinase by the Ptp2 and Ptp3 protein tyrosine phosphatases. Mol. Cell. Biol. 17:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Wysocki, R., C. C. Chery, D. Wawrzycka, M. Van Hulle, R. Cornelis, J. M. Thevelein, and M. J. Tamas. 2001. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 40:1391-1401. [DOI] [PubMed] [Google Scholar]
- 656.Yale, J., and H. J. Bohnert. 2001. Transcript expression in Saccharomyces cerevisiae at high salinity. J. Biol. Chem. 276:15996-16007. [DOI] [PubMed] [Google Scholar]
- 657.Yamada-Okabe, T., T. Mio, N. Ono, Y. Kashima, M. Matsui, M. Arisawa, and H. Yamada-Okabe. 1999. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Yamamoto, M. 1996. Regulation of meiosis in fission yeast. Cell Struct. Funct. 21:431-436. [DOI] [PubMed] [Google Scholar]
- 659.Yamochi, W., K. Tanaka, H. Nonaka, A. Maeda, T. Musha, and Y. Takai. 1994. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125:1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Yan, C., L. H. Lee, and L. I. Davis. 1998. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 17:7416-7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]
- 662.Yasui, M., A. Hazama, T. H. Kwon, S. Nielsen, W. B. Guggino, and P. Agre. 1999. Rapid gating and anion permeability of an intracellular aquaporin. Nature 402:184-187. [DOI] [PubMed] [Google Scholar]
- 663.Reference deleted.
- 664.Yoshida, S., E. Ikeda, I. Uno, and H. Mitsuzawa. 1992. Characterization of a staurosporine-sensitive and temperature-sensitive mutant, stt1, of Saccharomyces cerevisiae—STT1 is allelic to PKC1. Mol. Gen. Genet. 231:337-344. [DOI] [PubMed] [Google Scholar]
- 665.Yoshida, S., Y. Ohya, M. Goebl, A. Nakano, and Y. Anraku. 1994. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 269:1166-1172. [PubMed] [Google Scholar]
- 666.Yoshida, S., Y. Ohya, A. Nakano, and Y. Anraku. 1994. Genetic interactions among genes involved in the STT4-PKC1 pathway of Saccharomyces cerevisiae. Mol. Gen. Genet. 242:631-640. [DOI] [PubMed] [Google Scholar]
- 667.Yu, G., R. J. Deschenes, and J. S. Fassler. 1995. The essential transcription factor, Mcm1, is a downstream target of Sln1, a yeast “two-component” regulator. J. Biol. Chem. 270:8739-8743. [DOI] [PubMed] [Google Scholar]
- 668.Zahner, J. E., H. A. Harkins, and J. R. Pringle. 1996. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 16:1857-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Zähringer, H., M. Burgert, H. Holzer, and S. Nwaka. 1997. Neutral trehalase Nth1p of Saccharomyces cerevisiae encoded by the NTH1 gene is a multiple stress responsive protein. FEBS Lett. 412:615-620. [DOI] [PubMed] [Google Scholar]
- 670.Zähringer, H., J. M. Thevelein, and S. Nwaka. 2000. Induction of neutral trehalase Nth1 by heat and osmotic stress is controlled by STREs and Msn2/Msn4 transcription factors: variations of PKA effect during stress and growth. Mol. Microbiol. 35:397-406. [DOI] [PubMed] [Google Scholar]
- 671.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]
- 672.Zeuthen, T. 2001. How water molecules pass through aquaporins. Trends Biochem. Sci. 26:77-79. [DOI] [PubMed] [Google Scholar]
- 673.Zeuthen, T., and D. A. Klaerke. 1999. Transport of water and glycerol in aquaporin 3 is gated by H+. J. Biol. Chem. 274:21631-21636. [DOI] [PubMed] [Google Scholar]
- 674.Zhan, X. L., R. J. Deschenes, and K. L. Guan. 1997. Differential regulation of FUS3 MAP kinase by tyrosine-specific phosphatases PTP2/PTP3 and dual-specificity phosphatase MSG5 in Saccharomyces cerevisiae. Genes Dev. 11:1690-1702. [DOI] [PubMed] [Google Scholar]
- 675.Zhan, X. L., and K. L. Guan. 1999. A specific protein-protein interaction accounts for the in vivo substrate selectivity of Ptp3 towards the Fus3 MAP kinase. Genes Dev. 13:2811-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Ziman, M., D. Preuss, J. Mulholland, J. M. O'Brien, D. Botstein, and D. I. Johnson. 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Zu, T., J. Verna, and R. Ballester. 2001. Mutations in WSC genes for putative stress receptors result in sensitivity to multiple stress conditions and impairment of Rlm1-dependent gene expression in Saccharomyces cerevisiae. Mol. Genet. Genomics 266:142-155. [DOI] [PubMed] [Google Scholar]