Abstract
Because the transcription factor neuronal Per-Arnt-Sim-type signal-sensor protein-domain protein 2 (NPAS2) acts both as a sensor and an effector of intracellular energy balance, and because sleep is thought to correct an energy imbalance incurred during waking, we examined NPAS2's role in sleep homeostasis using npas2 knockout (npas2−/−) mice. We found that, under conditions of increased sleep need, i.e., at the end of the active period or after sleep deprivation (SD), NPAS2 allows for sleep to occur at times when mice are normally awake. Lack of npas2 affected electroencephalogram activity of thalamocortical origin; during non-rapid eye movement sleep (NREMS), activity in the spindle range (10–15 Hz) was reduced, and within the delta range (1–4 Hz), activity shifted toward faster frequencies. In addition, the increase in the cortical expression of the NPAS2 target gene period2 (per2) after SD was attenuated in npas2−/− mice. This implies that NPAS2 importantly contributes to the previously documented wake-dependent increase in cortical per2 expression. The data also revealed numerous sex differences in sleep; in females, sleep need accumulated at a slower rate, and REMS loss was not recovered after SD. In contrast, the rebound in NREMS time after SD was compromised only in npas2−/− males. We conclude that NPAS2 plays a role in sleep homeostasis, most likely at the level of the thalamus and cortex, where NPAS2 is abundantly expressed.
Keywords: circadian, clock genes, metabolism, sleep homeostasis
Neuronal Per-Arnt-Sim-type signal-sensor protein (PAS)-domain protein 2 (NPAS2) is a transcription factor that is highly expressed in the CNS (1). Many PAS-domain proteins can sense oxygen, redox, voltage, or light and are implicated in environmental and developmental signaling pathways (2). NPAS2 senses cellular energy state, in that both the dimerization of NPAS2 to its obligatory partner brain and muscle arnt-like 1 (BMAL1) and the specific DNA binding of NPAS2:BMAL1 heterodimers depend on intracellular redox potential (3). NPAS2 can also affect cellular metabolism by activating transcription of lactate dehydrogenase-1 (ldh1), which encodes the LDH subunit A (3). LDH catalyzes the reduction of pyruvate to lactate, an important neuronal energy substrate. NPAS2 thus uniquely combines sensor and effector functions and might be an important regulator of cellular metabolism in the CNS.
Sleep is governed by both circadian and homeostatic processes (4). The notion of the homeostatic regulation of sleep is based on the observation that sleep loss is compensated by an increase in sleep time and intensity that is proportional to the sleep time lost. Such observations indicate that a need for sleep accumulates during waking, although the nature of this need, i.e., the neurophysiological function of sleep, remains unknown. One prominent hypothesis states that sleep corrects a metabolic imbalance imposed upon the brain during wakefulness (5). This imbalance is thought to result in increased hyperpolarization of thalamocortical and cortical neurons during non-rapid eye movement sleep (NREMS) that, at the level of the electroencephalogram (EEG), is reflected by more prevalent delta (1- to 4-Hz) oscillations (6). EEG activity in the delta frequency range is a sensitive marker of time spent awake (4, 7) and local cortical activation (8) and is therefore widely used as an index of NREMS need and intensity.
The PAS-domain proteins, CLOCK, BMAL1, PERIOD-1 (PER1), and PER2, play crucial roles in circadian rhythm generation (9). The NPAS2 paralog CLOCK, like NPAS2, can induce the transcription of per1, per2, cryptochrome-1 (cry1), and cry2. PER and CRY proteins, in turn, inhibit CLOCK- and NPAS2-induced transcription, thereby closing a negative-feedback loop that is thought to underlie circadian rhythm generation. Although per1 and per2 expression are widely used as molecular state variables of the circadian clock, their expression in the cerebral cortex is driven by the sleep–wake distribution and can dissociate from its strict circadian expression in the suprachiasmatic nucleus (SCN; reviewed in refs. 10 and 11). Because npas2−/− mice lack rhythmic per2 expression in the cortex despite intact circadian sleep–wake rhythms (12), NPAS2 seems essential in coupling cortical per2 expression to the sleep–wake distribution. This suggests a noncircadian role of clock genes in sleep regulation (10), which is supported by several knockout (KO) studies; altered homeostatic regulation of sleep has been reported in fruit flies lacking a functional cycle gene (the fly homologue of bmal1) (13), in mice lacking both cry genes (11) or bmal1 (14), and in clock-mutant mice (15).
We propose that NPAS2 is functionally implicated in sleep homeostasis at a cellular level. We have already reported that, under baseline conditions, npas2−/− mice sleep less during the late active period (16). Here we further test this hypothesis by subjecting npas2−/− (i.e., KO) mice and their littermate controls (i.e., WT) to a SD. We also determine whether NPAS2 is involved in coupling waking to cortical per2 expression. Finally, the extensive dataset presented here gave us the opportunity to identify the sparsely studied sex effects on sleep. Apart from striking genotype and sex differences in sleep regulation, we discovered equally striking genotype–sex interactions.
Results
Sleep Time Is Reduced in Mice Lacking NPAS2.
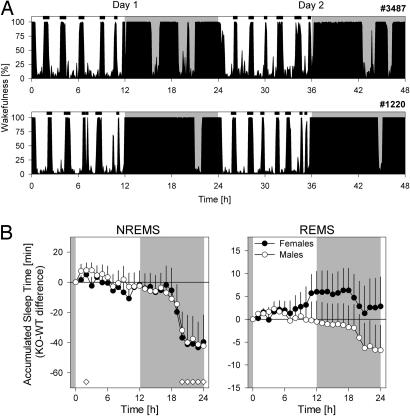
Like WT mice, npas2−/− mice were mostly asleep during the light period and mostly awake during the dark period (Fig. 1). The amount and distribution of wakefulness during the dark period, however, differed markedly from WT. The initial sustained period of wakefulness was ≈1 h longer in KO mice (7.0 ± 0.5 vs. 5.9 ± 0.3 h; P = 0.026, t test; Fig. 1), thereby delaying the occurrence of the typical night-time “nap.” Moreover, KO mice had fewer subsequent waking bouts (2.8 ± 0.2 vs. 3.6 ± 0.2; P = 0.019), whereas average bout length was increased by almost 2 h (237 ± 25 vs. 127 ± 9 min; P = 0.0003, t tests). Differences in sleep time were specific for NREMS and were restricted to the second half of the dark period (Fig. 1). The amount and consolidation of sleep during the rest period did not differ between genotypes (Table 1; Fig. 7, which is published as supporting information on the PNAS web site), whereas several sex differences were noted (Table 2). Overall, KO mice lost 41 min of NREMS each day compared with WT mice (Table 1 and Fig. 1).
Fig. 1.
Sleep–wake distribution during baseline. (A) Examples of wake distribution in baseline for two mice (no. 3487, WT female; no. 1220, KO male). Black area depicts percent wakefulness in 5-min intervals. Note the highly regular occurrence of sustained waking bouts during the rest period. Periodogram analysis revealed a significant 128-min periodicity in all individuals. Ultradian sleep–wake patterns of such periodicity have not been described previously. (B) Genotype comparison of the accumulation of sleep time in baseline. NREMS (Left) and REMS (Right) values in KO mice were expressed relative to WT. Indicated are hourly increments averaged over 2 baseline days (±1 SE of the difference) for females (filled symbols) and males (open symbols). Diamonds indicate significant genotype differences (P < 0.05, t tests). Gray areas denote dark periods.
Table 1.
Sleep time during baseline
| NREMS | REMS | |||||
|---|---|---|---|---|---|---|
| 12 h light | 12 h dark | 24 h | 12 h light | 12 h dark | 24 h | |
| WT females | 404 ± 6 | 132 ± 10 | 536 ± 13 | 68 ± 4 | 16 ± 3 | 85 ± 6 |
| WT males | 416 ± 7 | 149 ± 11 | 565 ± 12 | 68 ± 3 | 18 ± 2 | 86 ± 4 |
| KO females | 403 ± 7 | 94 ± 15* | 496 ± 12* | 74 ± 3 | 13 ± 3 | 87 ± 3 |
| KO males | 414 ± 6 | 109 ± 13* | 523 ± 10* | 67 ± 3 | 12 ± 2* | 79 ± 4 |
| Two-way ANOVA | ||||||
| Genotype | 0.77 | 0.0041 | 0.0015 | 0.41 | 0.053 | 0.64 |
| Sex | 0.10 | 0.20 | 0.022 | 0.21 | 0.85 | 0.39 |
| Interaction | 0.96 | 0.94 | 0.92 | 0.31 | 0.52 | 0.27 |
Mean (±SEM) minutes of sleep obtained over 2 baseline days. P values resulting from two-way ANOVAs with genotype and sex factors are indicated.
*Significant genotype differences (P < 0.05, t tests).
Table 2.
Listing of significant sex differences in sleep
| Sleep variable | Males | Females | P |
|---|---|---|---|
| Length of rest period, h | 11.2 ± 0.3 | 11.5 ± 0.3 | 0.0054 |
| NREMS amount, min/24 h | 544 ± 9 | 516 ± 10 | 0.042 |
| No. of short NREMS episodes, <1 min | 46 ± 2 | 54 ± 2 | 0.021 |
| No. of NREMS-to-REMS transitions | 3.6 ± 0.2 | 5.0 ± 0.3 | 0.0005 |
| NREMS delta power, 1–4 Hz | 5.46 ± 0.15 | 5.96 ± 0.08 | 0.0087 |
| NREMS sigma power, 10–15 Hz | 1.11 ± 0.03 | 0.97 ± 0.03 | 0.0058 |
| REMS delta power, 1–4 Hz | 1.25 ± 0.03 | 1.01 ± 0.04 | <0.0001 |
| REMS sigma power, 10–15 Hz | 0.48 ± 0.01 | 0.43 ± 0.01 | 0.0056 |
| Waking theta power, 5–10 Hz | 1.03 ± 0.04 | 0.86 ± 0.02 | 0.0005 |
| REMS rebound, Δ min vs. baseline | +16 ± 2 | +6 ± 3 | 0.0070 |
| Time constant of S increase, h | 8.4 ± 0.8 | 11.1 ± 0.8 | 0.0039 |
Indicated are mean (±SEM) values for males (n = 22) and females (n = 16) of both genotypes. Except for the bottom two rows, all variables are obtained during the 2 baseline days. The number of short NREMS episodes and NREM–REMS transitions is expressed per hour of NREMS. Mean EEG power in frequency bands is expressed as percentage of average total EEG power (see Methods, see also Fig. 2). P values are derived from post hoc t tests.
NPAS2 Affects Rhythmic EEG Activity.
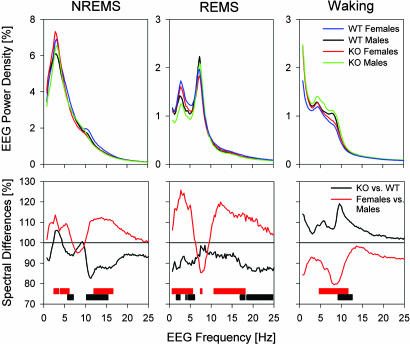
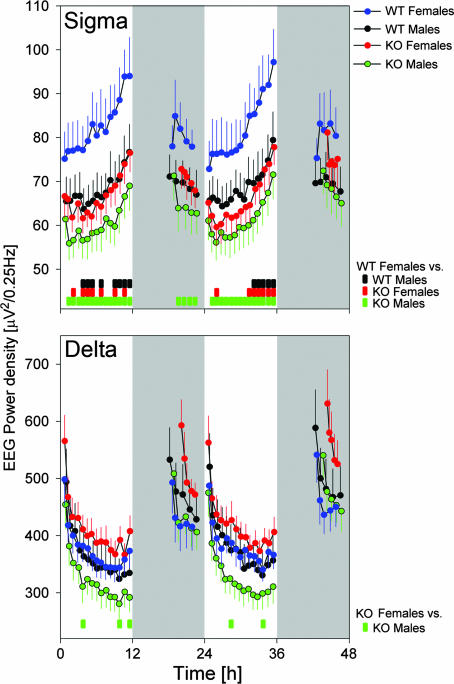
Sigma (or spindle) and delta (or slow-wave) oscillations are the hallmarks of the NREMS EEG. Activity in the sigma range (10–15 Hz), a marker of lighter stages of NREMS, was reduced in npas2−/− mice (Fig. 2). NREMS-to-REMS transitions, which are preceded by a surge in sigma power (17), might have contributed to this EEG difference. Similar to the absolute values (Fig. 3), the surge in sigma power at the transitions was smaller in KO mice (Fig. 8, which is published as supporting information on the PNAS web site). Therefore, because genotype differences in sigma activity at NREM-to-REMS transitions did not surpass the differences observed in the overall sigma activity, and because the number of these transitions did not differ among genotypes, these transitions do not seem to contribute to the overall genotype difference in sigma power. Instead, lighter stages of NREMS seemed to be less prevalent in npas2−/− mice. Female mice showed a higher number of NREM-to-REMS transitions compared with males (Table 2), which might have contributed to their increased sigma activity (Figs. 2 and 3).
Fig. 2.
EEG spectral profiles under baseline conditions averaged for all 4-s epochs scored as NREMS (Left), REMS (Middle), or waking (Right). (Upper) Average EEG spectra normalized to total EEG power. (Lower) Spectral differences as percent change for KO (black line; n = 19) versus WT (= 100%; n = 19) mice and for females (red lines; n = 16) versus males (= 100%; n = 22). Significant genotype differences are indicated by black bars; sex differences are indicated by red bars (P < 0.05, t tests).
Fig. 3.
Baseline time course of sigma (10–15 Hz; Upper) and delta (1–4 Hz; Lower) EEG activity in NREMS. Values represent mean absolute values (±1 SEM) calculated over 15- and 5-NREMS-time percentiles in the light and dark periods, respectively. Large genotype and sex effects were present for both frequency bands [three-way ANOVA (factors genotype, sex, and time), P < 0.0001; except the genotype for delta, P = 0.51), and sex affected the genotype effect (interaction P < 0.0001). The time-dependent changes in both variables did not differ among groups. Colored bars at the bottom indicate intervals in which EEG power differed among groups (P < 0.05, t tests).
Delta oscillations (1–4 Hz) are characteristic of the EEG during deeper stages of NREMS. Although not significant for any given frequency bin, power density within the delta frequency range was significantly redistributed toward faster frequencies in KO mice (Fig. 2). This shift became more pronounced after periods of prolonged wakefulness (Fig. 9, which is published as supporting information on the PNAS web site). Despite the pronounced sex and genotype differences in the absolute levels of delta and sigma power, their time courses throughout the experiment were remarkably similar among the four groups (Fig. 3).
The EEG of the other two behavioral states was also affected by genotype. During wakefulness, power in the alpha and “flutter” (18) frequency range (8–12 Hz), characteristic of quiet and nonexploratory wakefulness, was increased in KO mice (Fig. 2). During REMS, activity in the beta frequency range (18–25 Hz) and in frequencies <6 Hz were decreased. Prominent sex differences were also noted (Fig. 2) and are summarized in Table 2.
Recovery of Sleep Time Lost Is Impaired in Mice Lacking NPAS2.
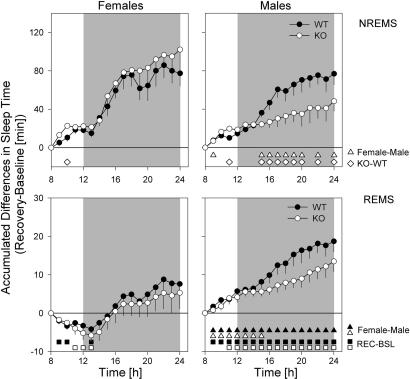
Sleep deprivation (SD) profoundly impacted subsequent sleep in genotype- and sex-specific manner. To summarize these findings, relative differences from corresponding baseline hours were accumulated over the recovery period (Fig. 4). Although for all four groups, NREMS values were significantly elevated compared to baseline, this relative increase was smaller in KO male mice. Especially early in the dark period [Zeitgeber time (ZT)13–17], when the other three groups achieved their largest increase in NREMS (i.e., 61% of the overall increase), KO males lagged behind (Fig. 4). As a result, KO male mice gained significantly less extra NREMS (+48 ± 10 min, n = 11 vs. +85 ± 9 min, n = 27, P = 0.025, t test) compared with the other three groups by the end of the recovery period. This deficit in recuperated NREMS time aggravated the daily loss of NREMS time during baseline (Fig. 1).
Fig. 4.
Accumulation of recovery-baseline (REC-BSL) differences in sleep time. Differences in NREMS time (Upper) and REMS (Lower) are calculated at 1-h increments for 18 h, starting from the end of the SD (ZT8). Indicated are mean differences (±1 SE of the difference) for females (Left) and males (Right). For NREMS, accumulated values were significantly above baseline from the first interval onward (P < 0.05, t tests) for all four groups (WT, filled symbols; KO, open symbols). For REMS-significant REC-BSL, differences are indicated with open (KO) and filled (WT) squares. Significant genotype differences within each sex are indicated by diamonds; significant sex differences are indicated by open (KO) and filled (WT) triangles (P < 0.05, t tests).
The SD-incurred changes in REMS were not affected by genotype, but prominent sex differences were observed. In males, the extra REMS obtained (compared with baseline) accumulated steadily (Fig. 4), and 16 extra minutes of REMS were accrued (n = 22, P < 0.0001, paired t test). In contrast, in females, REMS time was below baseline for the initial 5 h of recovery, thus further increasing the SD-induced REMS loss (Fig. 4). Only in subsequent hours was some REMS time recovered, resulting in a nonsignificant increase of 6 min over baseline (Table 2).
NPAS2 Modulates the Rebound in EEG Delta Power After SD.
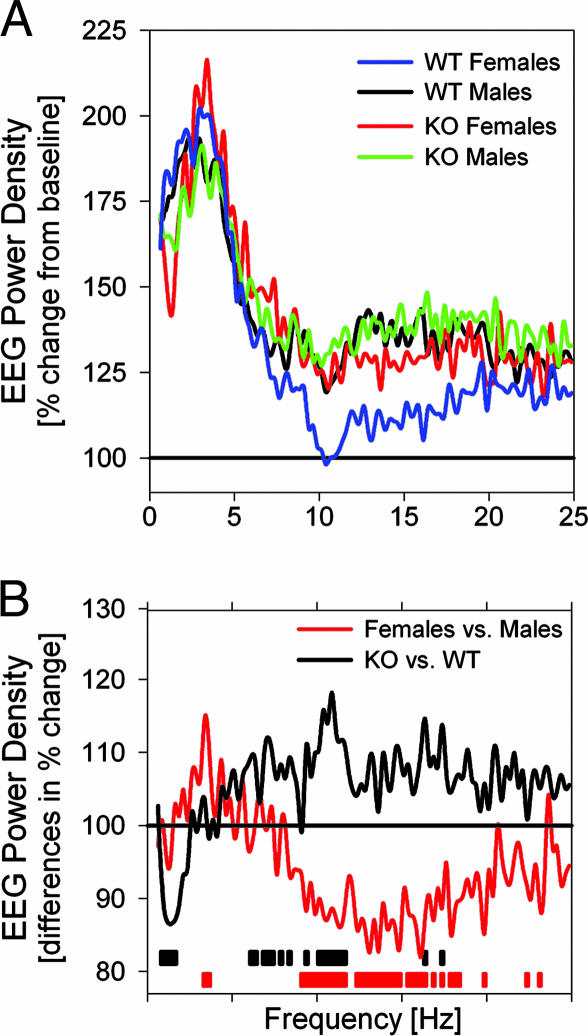
The immediate effects of SD on the NREMS EEG were assessed in the first 30 min of recovery sleep. EEG power increased over the entire frequency range analyzed, with the exception of the spindle frequencies in WT females (Fig. 5). Changes in the delta frequency range were most pronounced, and a 2-fold increase over baseline was reached. This relative increase differed among genotypes and in the low delta frequency range (1–2 Hz) was smaller in npas2−/− mice (Fig. 5). An analysis of the ratio of EEG power in fast (2.5- to 4.0-Hz) and slow (1.0- to 2.25-Hz) delta frequencies revealed that the relative contribution of slow delta power was generally smaller (and the ratio higher) in npas2−/− mice (Fig. 9). This ratio was increased during NREMS immediately after prolonged periods of wakefulness during baseline and after SD; this transient increase was more pronounced in npas2−/− mice, thereby deviating even further from WT mice.
Fig. 5.
Spectral changes in the NREMS EEG immediately after SD. (A) In the first 30 min of recovery, prominent increases in EEG power were observed in all groups except in the 9.5- to 17.75-Hz range for WT females (statistics not indicated). Values are expressed as a percentage of corresponding baseline values. (B) Group comparisons revealed different responses to SD: percent change in KO vs. WT (black line) and females vs. males (red line). Frequency bins with significant genotype and sex differences are indicated by black and red bars, respectively (P < 0.05, t tests).
Quantifying the Sleep Homeostatic Process (Process S).
The dependence of EEG delta power on the sleep–wake distribution was quantified by using a simulation analysis (7, 19). The simulation accurately predicted delta power (Fig. 10, which is published as supporting information on the PNAS web site), and the correlation between empirical and simulated values indicates that >80% of the variation in delta power could be accounted for by the sleep–wake distribution (Table 3). The time constants describing Process S obtained in the WT males were strikingly similar to those previously reported for this inbred strain (7). The estimated rate at which delta power increases during wakefulness tended to be slower in npas2−/− mice compared with WT (−12%) and was significantly slower in females compared with males (−33%; Tables 2 and 3). These effects persisted even when different model assumptions were used (Table 4, which is published as supporting information on the PNAS web site; see also Table 3 legend).
Table 3.
Parameters describing the time course of Process S
| τi, h | τd, h | UA, % | LA, % | So, % | r | |
|---|---|---|---|---|---|---|
| WT females | 10.2 ± 0.8 | 2.1 ± 0.2 | 251 ± 6 | 54 ± 1 | 145 ± 3 | 0.91 |
| WT males | 7.9 ± 0.6 | 1.9 ± 0.2 | 282 ± 8 | 55 ± 1 | 148 ± 8 | 0.91 |
| KO females | 12.0 ± 1.3 | 2.1 ± 0.2 | 259 ± 8 | 50 ± 1 | 153 ± 5 | 0.90 |
| KO males | 8.8 ± 0.9 | 2.0 ± 0.1 | 282 ± 9 | 53 ± 2 | 164 ± 8 | 0.92 |
| Two-way ANOVA | ||||||
| Genotype | 0.13 | 0.72 | 0.60 | 0.11 | 0.08 | 0.87 |
| Sex | 0.0039 | 0.23 | 0.0029 | 0.31 | 0.36 | 0.47 |
| Interaction | 0.61 | 0.78 | 0.64 | 0.50 | 0.56 | 0.47 |
Mean time constants (±SEM) with which the best fit between empirical and simulated (Process S) values of EEG delta power was obtained. Process S was assumed to increase during wakefulness and REMS and to decrease during NREMS, according to exponential saturating functions with time constants τi and τd determining the rate of increase and decrease, respectively. Upper asymptotes (UA) and lower asymptotes (LA) and level of S at time 0 were derived from the data (see Supporting Text) and expressed as a percentage of the delta power reached in the last 4 h of the baseline light periods. Genotype and sex effects were assessed by two-way ANOVA (P values are indicated). r values represent correlation coefficients between empirical and simulated data. The UA was found to be lower in females, but the slower increase rate in this sex did not depend on this lower UA (see Table 4).
NPAS2 Mediates the SD-Induced Increase in per2 Expression.
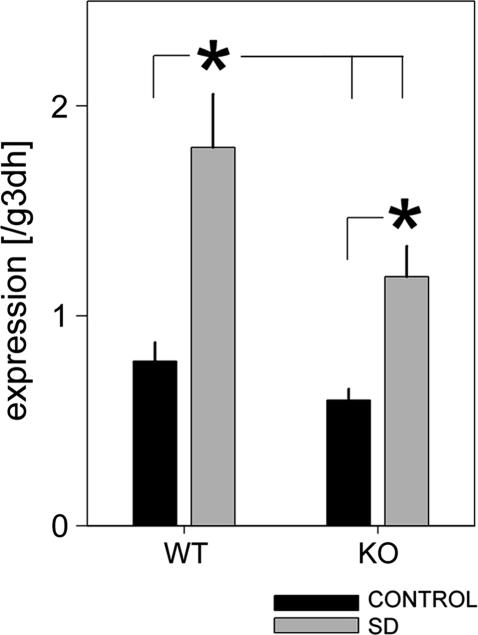
In WT mice, we found a 2.3-fold increase in forebrain per2 expression after 6 h of SD (Fig. 6), confirming our earlier observations (11). In sleep-deprived npas2−/− mice, lower per2 levels were reached compared with sleep-deprived WT mice, and values did not significantly differ from WT mice that had been sleeping ad lib (Fig. 6). Nevertheless, per2 expression in npas2−/− mice did significantly increase with SD when compared with levels in npas2−/− mice that could sleep. It thus appears that NPAS2 mediates an important part (56% in this study), but not all, of the wake-related increase in forebrain per2 expression. In situ analysis of coronal sections taken at the level of the SCN revealed that per2 expression was increased with SD in layers II-III and V-VI of the cerebral cortex (Fig. 11, which is published as supporting information on the PNAS web site). SD-related changes in per2 expression were not evident in the SCN and, although the genotypic differences determined by RT-PCR were not easily discerned by eye, optical density values did confirm a smaller increase in per2 with SD in the npas2−/− mice.
Fig. 6.
Mean (±1 SEM) forebrain per2 mRNA levels during baseline and after 6 h of SD determined by real-time RT-PCR. SD increased per2 expression, but lower values were reached in npas2−/− (KO) compared with WT mice (∗, P < 0.05, post hoc Tukey's test, n = 8 per experimental group; two-way ANOVA, factor SD, P < 0.0001; genotype, P = 0.011; interaction, P = 0.15).
Discussion
A Role for NPAS2 in Sleep Homeostasis.
Consistent with our hypothesis that NPAS2 plays a role in sleep homeostasis, we observed that the regulation of NREMS time was compromised in npas2−/− mice. These mice were found to sleep less in the latter half of the baseline dark period, a time of day when sleep need is high, and WT mice showed a consolidated period of sleep (i.e., nap), conceivably to discharge sleep pressure accumulated during the preceding period of wakefulness. After experimentally further raising sleep need by means of SD, npas2−/− mice were once more incapable of initiating the appropriate compensatory behavior (i.e., sleep) during the circadian phase when mice are usually awake. This is reminiscent of the inability of npas2−/− mice to adapt to a new food regime in which food was offered at a circadian phase mice normally fast and extends our previous interpretation that NPAS2 allows for the behavioral adaptability necessary to overcome the strict times set for these behaviors by the SCN when challenged (16). Consistent with our conjecture, mice mutant for CLOCK, an essential component of the circadian pacemaker in the SCN, show increased behavioral adaptability under conditions of food restriction (20) and no deficits in NREMS time recovery after SD (15) and, like WT mice, nap during the dark period (15).
CRY proteins are powerful repressors of NPAS2 transcriptional activation. Mice lacking cry1 and -2 (cry1,2−/−) can thus be seen as a model of increased NPAS2 activity, which is supported by the increased cortical expression of the NPAS2 target gene per2 in cry1,2−/− mice compared with WT (11). If this model holds, sleep changes found in cry1,2−/− mice should be opposite to those seen in npas2−/− mice. The increased NREMS time during the dark period observed in cry1,2−/− mice is consistent with this. In addition, cry1,2−/− mice showed increased EEG delta power and a faster increase rate of Process S (11). In npas2−/− mice, the increase rate of sleep need during waking appeared slower and delta power lower, although these aspects of NREMS regulation did not reach significance levels.
In cry1,2−/− mice as well as in sleep-deprived WT mice, increased per2 expression in the cerebral cortex specifically seems to be associated with elevated sleep need (see ref. 11; reviewed in ref. 10). The present study demonstrates that NPAS2 importantly contributed to the SD-dependent changes in forebrain per2 expression. This suggests that the coupling between wakefulness and per2 expression in the forebrain is weakened in npas2−/− mice and could, in part, explain the lack of a circadian modulation of forebrain per2 expression in the presence of a circadian activity rhythm in these mice (12). Other factors related to SD, such as stress, might have contributed to the increase in per2, because several studies indicate a relationship between glucocorticoid signaling and per expression (see, e.g., refs. 21 and 22).
NPAS2 Affects EEG Oscillations During NREMS.
The two most conspicuous EEG features of NREMS are delta and spindle oscillations, both generated in thalamus and cerebral cortex (23). NPAS2, which is abundantly expressed in these areas (1), affected both these features. npas2−/− mice displayed an overall reduction in sigma power, whereas the characteristic daily time course of sigma power, which is determined by both circadian and sleep–wake-dependent factors (24), was not affected. It seems therefore unlikely that the reduction in sigma power is related to NPAS2's role in sleep–wake-dependent clock-gene expression. Alternatively, NPAS2 might play a role in postnatal thalamocortical developmental; npas2 expression first appears after postnatal week 1 (1), immediately preceding the time at which, in rats, the first slow waves and spindles appear (25). Various lines of evidence show that sleep spindles play a role in cortical plasticity and memory processes (26–28). The reduction in sigma power in npas2−/− mice, which correlates well with sleep spindle prevalence (29, 30), might be functionally linked to the poorer performance on specific complex memory tasks observed in these mice (31).
The SD-induced increase in the “slow” delta frequency range (1–2 Hz) in NREMS EEG power was significantly smaller in npas2−/− mice. Similar frequency-specific genotype differences were observed in NREMS immediately after long periods of wakefulness under baseline conditions. Other studies also reported that slow and fast delta oscillations were differentially modulated by prolonged waking (32, 33). One type of oscillation that contributes to the activity in the delta frequencies originates from thalamocortical neurons (6). When the membrane potential of these neurons reaches levels of hyperpolarization characteristic of deep NREMS (stage 4 in humans), their frequency becomes faster, and their contribution to delta activity at the level of the EEG is greater (6, 34). This could underlie the transient shift to faster delta frequencies immediately after long periods of wakefulness, because NREMS is then deepest, and hyperpolarization is greatest (34). Following this conjecture, the higher fast-to-slow delta power ratio in npas2−/− mice suggests that membrane potential of thalamocortical neurons during NREMS, on average, is more hyperpolarized. This is consistent with the reduction in sleep spindles that predominantly occur at intermediate levels of membrane hyperpolarization (23) and suggests a role for NPAS2 in the generation of EEG rhythms of thalamocortical origin. The mechanisms through which this transcription factor affects thalamocortical and cortical activity deserve further investigation. These analyses also underscore that activity in the delta frequencies does not uniformly respond to prior wake duration. The fast-to-slow delta ratio defined here could provide an additional EEG measure to gauge sleep homeostasis.
Genotype–Sex Interactions.
Although numerous sex differences in sleep and sleep EEG have been described in humans (35), sex studies on spontaneous sleep in mice are lacking. We identified a large number of sleep aspects that differed between male and female mice, all of which warrant further investigation. It must be pointed out that the females were studied without knowledge of estrus cycle phase. Such cyclicity, however, does not seem to have an important effect on sleep in mice (36).
The regulation of both REMS time and NREMS need differed greatly between sexes. The slower increase of sleep need in females might be related to differences in wake quality that impact subsequent sleep (7, 37). Support for this comes from the waking EEG, showing reduced theta power in females, indicative of reduced explorative activity (38). The reduced NREMS time during baseline might also reflect a reduced sleep need. We made similar observations among male mice of various inbred strains; the strain with the lowest theta power during wakefulness and lowest NREMS amount displayed the slowest increase rate of Process S, whereas the strain with the fastest increase rate slept the most and displayed the highest theta power (7, 17, 39).
Of the NREMS EEG features that differed with sex, differences in spindle activity were most pronounced. Also in humans, a larger number of sleep spindles in females has been reported (40). The higher level of sigma activity during baseline in WT females might underlie the absence of its further increase immediately after SD (the “ceiling” effect). This lack constituted the main sex difference in the EEG response to SD. Also the higher delta power in females is consistent with observations in humans (41). The sex differences that interacted with genotype are of particular interest. Specifically, the reduced compensation of NREMS time after SD was observed only in npas2−/− males. In mice mutant for CLOCK, the regulation of sex hormones, which can be assumed to play a role in the sex differences observed here, is disrupted (42). Given that CLOCK and NPAS2 have overlapping target genes, NPAS2 might also affect these hormones.
Conclusions
NPAS2 affected the homeostatic regulation of NREMS time but not the dynamics of the sleep homeostatic process. In terms of homeostatic control circuitry, this would place NPAS2 downstream from the “error” signal. Assuming this sleep-need signal represents a cellular metabolic deficiency (5), and given NPAS2's ability to both sense and alter cellular energy state (3), NPAS2 could act as a sensor and actuator in a sleep-regulatory feedback loop. Our findings also stress the necessity to study both sexes, because gene effects can go unnoticed if only one sex or not enough individuals of both sexes are included.
Methods
Generation of NPAS2-Deficient Mice.
An NPAS2-lacZ line was generated in 129S6/SvEvTac-derived embryonic stem cells, as described (31). Chimeric males were bred to WT (npas2+/+) C57BL/6J females, producing F1 progeny. F1 males heterozygous for the NPAS2-lacZ allele were backcrossed to WT C57BL/6J females to create N2 progeny. Heterozygous N2 males were again backcrossed to generation N9 to ensure that the 129SvEvTac contribution was <0.2%. The WT mice and mice homozygous for the NPAS2-lacZ allele (KO or npas2−/−) used in this experiment were littermates.
EEG/EMG Monitoring and Analyses.
Male (n = 11 per genotype) and female (n = 8 per genotype) mice, 12 weeks of age [weights: males, 28.6 ± 3.6 g, n = 22; females, 20.4 ± 1.0 g, n = 16), were prepared for chronic monitoring of EEG/EMG signals (see ref. 16 for details). After surgery, mice were housed individually under a 12-h:12-h light–dark cycle at 23°C ambient temperature. After 7–10 days of recovery, they were connected to a commutator by using counterbalanced recording leads and allowed to adapt for 10–14 days before the experiment. The experiment consisted of 3 consecutive days, beginning at light onset, i.e., ZT0. After 2 baseline days, animals were sleep-deprived by gentle handling for 8 h (ZT0–ZT8). Recovery was defined as ZT8–ZT24 after SD. Data were recorded in separate sessions for males and females. Throughout the experiments, EEG/EMG signals were continuously recorded [Grass amplifier models 15A94 and -54 for EEG and EMG, respectively (Grass Instruments, Quincy, MA)], digitized (250 Hz), and stored on a personal computer. Off line, EEG/EMG records were visually scored in 4-s epochs as awake, REMS, or NREMS. For a detailed account of sleep and EEG analysis, see Supporting Text, which is published as supporting information on the PNAS web site.
Brain Expression of period-2 (per2).
SD-induced changes in forebrain per2 expression were compared between WT and KO male mice (n = 40; 20 per genotype). Animals were kept in the same conditions as for the EEG recordings. Ten animals per genotype were sleep-deprived for 6 h (ZT0–ZT6). The remaining 10 per genotype served as controls. Between ZT6 and ZT7, all animals were killed, and brains were rapidly removed. For the quantification of per2 expression, real-time RT-PCR analysis was used in 32 mice (n = 8 per condition per genotype). Brains were dissected into forebrain, brainstem, and cerebellum. Only forebrain tissue is analyzed here. Relative per2 abundance was normalized to gapd. The remaining eight mice (n = 2 per genotype per condition) were used for in situ hybridization to identify brain regions that contributed to the overall increase in forebrain per2 expression after SD. Brains were rapidly removed, frozen on dry ice, stored at −70°C, and sectioned coronally at 15 μm through the entire rostrocaudal SCN region. Further detailed information concerning the SYBR green RT-PCR analysis and the in situ hybridization is provided in Supporting Text.
Supplementary Material
Acknowledgments
We thank Vinh Cao for technical assistance and Grace Hagiwara and Mehdi Tafti for comments on the manuscript. This work was supported by National Institute of Mental Health Grants MH67752 (to P.F.) and 5R37 MH059388-07 (to S.L.M.) and by the McKnight Foundation for Neurosciences, the Morton Meyerson Family Tzedakah Fund, and unrestricted funds from an anonymous donor.
Abbreviations
- REMS
rapid eye movement sleep
- NREMS
non-REMS
- EEG
electroencephalogram
- EMG
electromyogram
- SD
sleep deprivation
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
- KO
knockout.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Zhou Y. D., Barnard M., Tian H., Li X., Ring H. Z., Francke U., Shelton J., Richardson J., Russell D. W., McKnight S. L. Proc. Natl. Acad. Sci. USA. 1997;94:713–718. doi: 10.1073/pnas.94.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu Y. Z., Hogenesch J. B., Bradfield C. A. Annu. Rev. Pharmacol. Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Rutter J., Reick M., Wu L. C., McKnight S. L. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 4.Borbely A. A. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 5.Benington J. H., Heller H. C. Prog. Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 6.Amzica F., Steriade M. Electroencephalogr. Clin. Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 7.Franken P., Chollet D., Tafti M. J. Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber R., Ghilardi M. F., Massimini M., Tononi G. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 9.Lowrey P. L., Takahashi J. S. Annu. Rev. Genom. Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw P. J., Franken P. J. Neurobiol. 2003;54:179–202. doi: 10.1002/neu.10167. [DOI] [PubMed] [Google Scholar]
- 11.Wisor J., O'Hara B., Terao A., Selby C., Kilduff T., Sancar A., Edgar D., Franken P. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reick M., Garcia J. A., Dudley C., McKnight S. L. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 13.Shaw P. J., Tononi G., Greenspan R. J., Robinson D. F. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 14.Laposky A., Easton A., Dugovic C., Walisser J., Bradfield C., Turek F. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 15.Naylor E., Bergmann B. M., Krauski K., Zee P. C., Takahashi J. S., Vitaterna M. H., Turek F. W. J. Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley C. A., Erbel-Sieler C., Estill S. J., Reick M., Franken P., Pitts S., McKnight S. L. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 17.Franken P., Malafosse A., Tafti M. Am. J. Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 18.Nerad L., Bilkey D. K. J. Neurophysiol. 2005;93:1246–1254. doi: 10.1152/jn.00199.2004. [DOI] [PubMed] [Google Scholar]
- 19.Huber R., Deboer T., Tobler I. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 20.Pitts S., Perone E., Silver R. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2003;285:R57–R67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schutz G., Schibler U. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S., Yokota S., Hara R., Kobayashi T., Akiyama M., Moriya T., Shibata S. Endocrinology. 2001;142:4910–4917. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- 23.Steriade M., McCormick D. A., Sejnowski T. J. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 24.Dijk D. J., Czeisler C. A. J. Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis F. C., Frank M. G., Heller H. C. In: Regulation of Sleep and Circadian Rhythms. Turek F. W., Zee P., editors. New York: Dekker; 1999. pp. 19–79. [Google Scholar]
- 26.Siapas A. G., Wilson M. A. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 27.Gais S., Molle M., Helms K., Born J. J. Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timofeev I., Grenier F., Bazhenov M., Houweling A. R., Sejnowski T. J., Steriade M. J. Physiol. (London) 2002;542:583–598. doi: 10.1113/jphysiol.2001.013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dijk D. J., Hayes B., Czeisler C. A. Brain Res. 1993;626:190–199. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 30.Vyazovskiy V. V., Achermann P., Borbely A. A., Tobler I. Arch. Ital. Biol. 2004;142:511–523. [PubMed] [Google Scholar]
- 31.Garcia J. A., Zhang D., Estill S. J., Michnoff C., Rutter J., Reick M., Scott K., Diaz-Arrastia R., McKnight S. L. Science. 2000;288:2226–2230. doi: 10.1126/science.288.5474.2226. [DOI] [PubMed] [Google Scholar]
- 32.Huber R., Deboer T., Tobler I. J. Neurophysiol. 2000;84:1888–1893. doi: 10.1152/jn.2000.84.4.1888. [DOI] [PubMed] [Google Scholar]
- 33.Deboer T., Fontana A., Tobler I. J. Neurophysiol. 2002;88:839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- 34.Dossi R. C., Nunez A., Steriade M. J. Physiol. (London) 1992;447:215–234. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manber R., Armitage R. Sleep. 1999;22:540–555. [PubMed] [Google Scholar]
- 36.Koehl M., Battle S. E., Turek F. W. Sleep. 2003;26:267–272. doi: 10.1093/sleep/26.3.267. [DOI] [PubMed] [Google Scholar]
- 37.Meerlo P., Turek F. W. Brain Res. 2001;907:84–92. doi: 10.1016/s0006-8993(01)02603-8. [DOI] [PubMed] [Google Scholar]
- 38.Huber R., Deboer T., Tobler I. J. Sleep Res. 1999;8:30–36. doi: 10.1046/j.1365-2869.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 39.Franken P., Malafosse A., Tafti M. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- 40.Huupponen E., Himanen S. L., Varri A., Hasan J., Lehtokangas M., Saarinen J. Neuropsychobiology. 2002;45:99–105. doi: 10.1159/000048684. [DOI] [PubMed] [Google Scholar]
- 41.Dijk D. J., Beersma D. G., Bloem G. M. Sleep. 1989;12:500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 42.Miller B. H., Olson S. L., Turek F. W., Levine J. E., Horton T. H., Takahashi J. S. Curr. Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.