Abstract
The olfactory system is thought to recognize odors with multiple odorant receptors (ORs) that are activated by overlapping sets of odorous molecules, ultimately generating an odor percept in the brain. We investigated how the odor percept differs between humans and Drosophila melanogaster fruit flies, species with very different OR repertoires. We devised high-throughput single fly behavior paradigms to ask how a given OR contributes to the odor percept in Drosophila. Wild-type flies showed dose- and stimulus-dependent responses to 70 of 73 odors tested, whereas mutant flies missing one OR showed subtle behavioral deficits that could not be predicted from the physiological responses of the OR. We measured human and fly judgments of odor intensity and quality and found that intensity perception is conserved between species, whereas quality judgments are species-specific. This study bridges the gap between the activation of olfactory sensory neurons and the odor percept.
Keywords: behavior, Drosophila, genetics, olfaction, psychophysics
Despite the wealth of knowledge about the molecular basis of olfaction, little is known about how the odor percept forms in the brain. The identification of hundreds of odorant receptor (OR) genes (1), each encoding a different seven-transmembrane domain protein, provided an initial mechanistic explanation for how animals can discriminate a large number of chemical stimuli. Animals are thought to be able to identify and distinguish smells because each OR is activated by a specific set of odors and each odor activates a combination of ORs, a process known as combinatorial coding (2–6). A typical OR is sensitive to a few compounds at low concentrations and to a wider range of compounds at higher concentrations (5, 6). OR repertoires differ considerably in size between species, from ≈1,200 in rodents to ≈400 in humans, and 61 in the fly (Drosophila melanogaster) (7), but it is not well understood whether or how these differences impact odor perception across species. In this study, we investigate the influence of the OR repertoire on odor perception in humans and fruit flies. Both species exhibit robust responses to odors and cohabitate in most parts of the world (8) but have very different OR repertoires.
Most of our knowledge about how an odor percept is experienced by the organism comes from experiments measuring odor perception in humans (9–12) because humans can self-report their odor experience. Sensory parameters that can be measured in human odor perception by psychophysical techniques include odor intensity, distinguishability, similarity, and sensitivity to an odor. To link OR activation and the odor percept in flies, these parameters and concepts had to be transferred to Drosophila. This was problematic because little is known about how these insects respond behaviorally to odors.
Here we report high-throughput behavioral assays that measure odor-evoked responses in single flies with great sensitivity and resolution. We used these assays to probe the sensitivity and receptive range of the Drosophila olfactory system. Genetically removing a single OR produced subtle defects in odor-evoked behaviors to a subset of the ligands that could not be predicted based on the physiological responses of the deleted OR. Finally, we carried out comparative studies of odor perception in flies and humans and show that judgment of odor intensity is conserved across these species with very different OR repertoires, whereas odor quality judgments are species-specific.
Results
Assays to Measure Fly Olfactory Behavior.
Previously described fly olfactory assays retain little temporal or spatial information about odor-induced behavior (13–18). Therefore, we designed two olfactory assays that measure responses of individual flies to an odor stimulus at high spatial and temporal resolution.
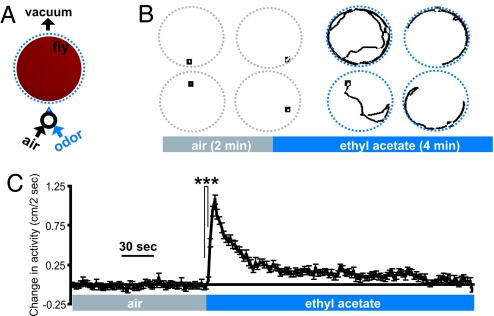
The first assay is based on previous studies that measured rapid odor-induced startle responses (19, 20). In this odor flow assay, individual flies are placed in circular arenas [Fig. 1A and supporting information (SI) Fig. 7], and videotaped for 2 min in clean air flow, followed by 4 min of uniformly distributed odor (Fig. 1B and see SI Fig. 8A and SI Movie 1). The position of the fly is recorded, and change in activity (distance moved per unit time) compared with the activity at the beginning of the experiment is calculated (Fig. 1C).
Fig. 1.
Fig. 1. Odor-evoked activity in the odor flow assay. (A) Schematic showing one arena with odor distribution visualized 40 sec after odor onset by using pH-sensitive paper and hydrogen chloride gas (see SI Fig. 8A). (B) Example tracks of four animals exposed to air for 2 min (Left) and subsequently to ethyl acetate [18% saturated vapor (SV)] for 4 min (Right). See also SI Movie 1. (C) Change in activity compared with the start of the experiment (n – 484; ***, P < 0.0001, unpaired t test). See also SI Fig. 9.
The properties of this assay are illustrated here with ethyl acetate. The response to ethyl acetate was rapid, showing a statistically significant increase in the first 2 sec and peaking after 8 sec (Fig. 1C). This response was dependent on the activity of the fly at odor onset but not on gender or circadian time (SI Fig. 9).
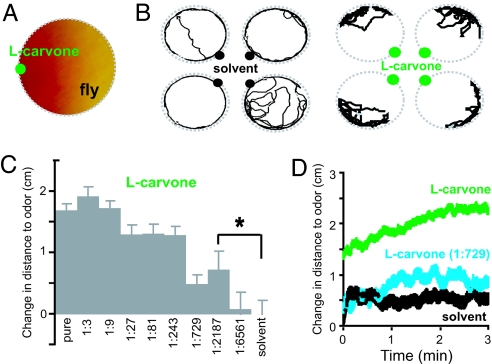
To study the behavior of flies in odor gradients, we developed a second assay, the stationary odor source assay, which is similar to the chemotaxis assay for Drosophila larvae (18, 21). Individual flies are placed in a Petri dish after an odor or solvent is applied to a filter paper at the wall of the dish. In time, the odor forms a steep gradient in the dish (Fig. 2A and SI Fig. 8). The position of the fly is videotaped for 3 min (Fig. 2B and SI Movies 2 and 3), and the mean change in distance to odor is calculated (Fig. 2C). For the representative odor l-carvone the distance to the odor source decreased with decreasing odor concentration (Fig. 2 C and D).
Fig. 2.
Odor avoidance in the stationary odor source assay. (A) Schematic of the assay with pH-sensitive paper showing odor distribution 40 sec into the experiment (see SI Fig. 8B). (B) Example tracks of four animals (3 min) for paraffin oil solvent (Left) and l-carvone (Right) (see also SI Movies 2 and 3). (C) Concentration dependence of responses to l-carvone. Distance to odor is normalized to zero for the behavior produced by solvent (mean ± SEM; n = 37–208 per odor; total n = 703). Distance to odor differs from solvent at the 1/2,187 dilution (∗, P < 0.05, unpaired t test). (D) Temporal profile of distance to odor was plotted by using data from C with solvent (black), pure l-carvone (green), and l-carvone diluted 1:729 (cyan). The change in distance to odor compared with the start of the experiment is shown.
Using these two assays, we measured the responses to 73 odors. The odors selected for this study comprise nine different functional groups and diverse odor qualities (SI Table 2). Included are alcohols and esters found in fruits that are food sources for the fly, as well as terpinenes and aromatics found in plant material not eaten by Drosophila (SI Table 3). For the majority of the odors tested here in behavioral experiments, complementing physiological data are available (4, 6, 22, 23). Throughout this paper, odor-evoked changes in activity were measured in the odor flow assay, whereas changes in the distance to the odor were measured in the stationary odor assay.
Odors Inducing Behaviors in Flies.
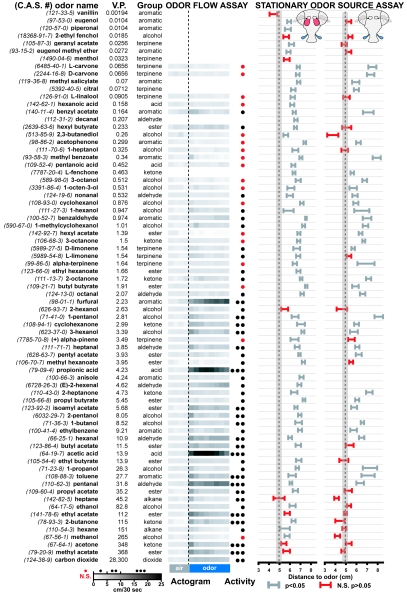
Previous studies of olfactory behavior in Drosophila focused on a limited number of odorants (14–19). We were interested in the behavioral receptive range of the fly olfactory system and therefore tested responses to a large number of odorants, including many odorants often found in fruit (SI Tables 2 and 3), ligands that activate known subsets of glomeruli (4), and stimuli used for olfactory conditioning (14). In total, flies responded to 70 of 73 odors tested (Fig. 3 and SI Figs. 10–12).
Fig. 3.
Receptive range of Drosophila olfactory behavior in response to 73 odors. The left three columns show odor name and Chemical Abstracts Service registry number (C.A.S.#), vapor pressure (V.P.) (in torr), and odor group. The fourth column depicts odor flow assay responses shown as binned actograms with each square representing 30 sec of activity (scale at bottom) or overall activity (mean ± SEM; n = 39–170 per odor; total n = 5031). A red dot indicates no significant response when comparing aggregate activity 60 sec before odor onset with either the first 60 sec after odor onset or the last 60 sec (P < 0.01; paired t test); black dots indicate significant responses of 0–5, 5–10, or >10 cm/30 sec (see scale at bottom). The fifth and sixth columns show responses in the stationary odor source assay of intact (mean ± SEM; n = 10–32 per odor; total n = 1,524) and antenna-less flies (mean ± SEM; n = 7–32 per odor; total n = 931). Gray data points differ from behavior evoked by solvent (P < 0.05); red values are not significantly different (N.S.; P > 0.05). The dotted line and shaded area represent the behavior of an animal in the absence of odor (mean distance ± SEM: 4.83 ± 0.31 cm). Odors were diluted 1:10 in paraffin oil, except for vanillin, piperonal, and menthol, which were used as a saturated dilution in dipropylene glycol.
We found that 44 of the 62 odors tested in the odor flow assay induced significant responses (Fig. 3). The level of odor-elicited activity correlates with the vapor pressure (V.P.) of the odor [correlation coefficient (excluding carbon dioxide) = 0.32]. Methanol is the only odor with a V.P. of >5 torr that did not elicit a response.
In the second behavioral assay, the stationary odor source assay, 61 of 69 tested odors induced significant responses. Different odors elicited responses in the two assays, demonstrating that behavioral responses to an odor are assay-dependent. More odors elicited responses in the stationary odor source assay (88%) than in the odor flow assay (71%) (Fig. 3). Flies detect many odors, including those not found in fruit and not perceived as smelling fruity (SI Tables 2 and 3).
The adult fly has two olfactory organs, the third antennal segment and the maxillary palp. The antenna has ≈1,200 olfactory sensory neurons (OSNs) expressing 37 ORs (24), and the maxillary palp has 120 OSNs expressing 7 ORs (25). To determine the contribution of these 7 ORs to the odor percept, flies with antennae surgically removed were tested in the stationary odor source assay. The behavioral receptive range of flies that rely on the maxillary palp for odor perception is reduced, in that such flies only responded to 61% of the 59 odors tested (Fig. 3).
In contrast, flies lacking maxillary palps but retaining antennae responded to 90% of the 10 odors tested (SI Fig. 13). In these palp-less flies, only the response to propyl acetate was reduced (P < 0.05; total n = 301; SI Fig. 13A). Flies missing both antennae and maxillary palps still responded to α-terpinene, but not to the other 15 odors tested (P < 0.01; total n = 348; SI Fig. 13B). α-Terpinene may activate nonolfactory neurons as we have shown previously for benzaldehyde (26).
Temporal Dynamics and Sensitivity of Odor Responses.
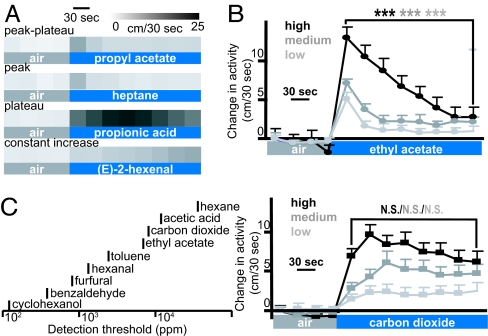
The odor flow assay data allowed us to examine the temporal dynamics of behavioral responses to odors. Odors induced responses with different temporal dynamics, which we divide into four classes (Fig. 4A). Most odorants (55%) elicited a response similar to that of ethyl acetate (Fig. 1C) where activity peaked in the first 30 sec after odor onset and then plateaued to a level above the pre-odor activity. For 9% of the odors, activity peaked in the first 30 sec but then returned to baseline, whereas for 27% of the odors, including carbon dioxide, activity remained near the peak throughout odor exposure. Finally, another 9% of the odors induced a constant increase in activity throughout the experiment. All four odors in this last class are aldehydes, but not all aldehydes elicited this constantly increasing activity (SI Table 1). The temporal dynamics were odor-specific and independent of concentration (Fig. 4B).
Fig. 4.
Temporal dynamics and sensitivity. (A) Examples of four response types: peak-plateau (highest activity 0–30 sec after odor onset, no return to baseline), peak (highest activity 0–30 sec, return to baseline), plateau (highest activity 30–150 sec), and constant increase (highest activity after 150 sec). Actograms (see scale at top) of one representative odor in each response class are shown (odors, 18% SV; CO2, 10%; n = 52, 109, 118, and 50, respectively). (B) Responses to three different concentrations of ethyl acetate or CO2 (high, 36% SV and 20%; medium, 18% SV and 10%; low, 9% SV and 5%; mean ± SEM; n = 97–167 per odor; total n = 720). The change in activity compared with the start of the experiment is shown. There is no significant difference (N.S.) between activity in the first 30 sec of odor exposure and the last 30 sec for CO2 (Lower), but the same comparison is highly significant for ethyl acetate (Upper; ∗∗∗, P < 0.001). (C) Odor thresholds in parts per million (ppm) measured in the odor flow assay (black bars, total n for measurements of responses to a variety of concentrations of the nine odors = 2,441). The values shown are the lowest concentrations to which a statistically significant response was measured (P < 0.05).
Fly detection thresholds for nine representative odors were determined by measuring the response in the odor flow assay at different concentrations (Fig. 4C). The lowest tested concentration in parts per million (ppm) that induced a statistically significant response is plotted.
Consequences of Losing Sensory Input from One OR.
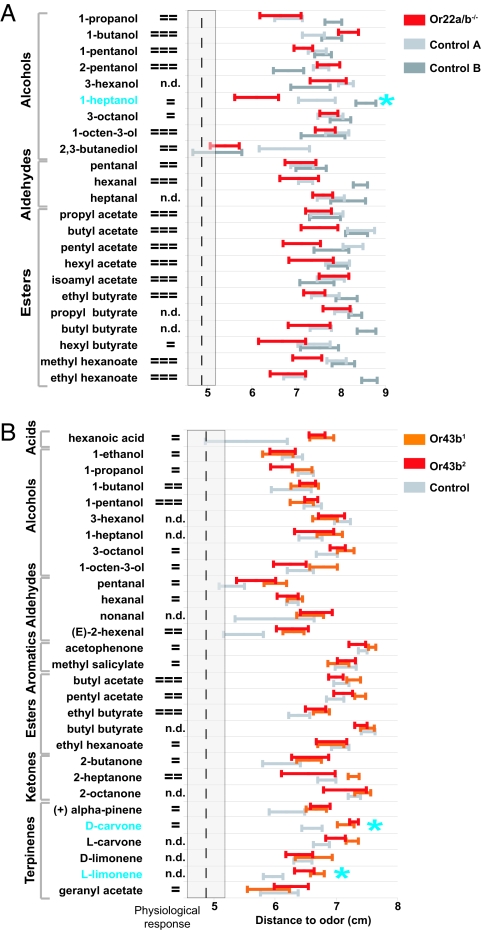
We next used the stationary odor source assay to ask whether small perturbations of the OR repertoire affect the odor percept. We first examined the Or22a receptor, which is sensitive to esters and alcohols (6, 27). Responses of flies carrying a deletion that includes the Or22a gene and genetically matched controls (22, 28) were tested to 23 odors. Of the odors tested, only the response to 1-heptanol, a weak agonist (27), was significantly reduced (Fig. 5A).
Fig. 5.
Contribution of Or22a and Or43b to Drosophila olfactory behavior. (A) Responses to 23 odors in the stationary odor source assay of Or22a/b−/− (delta-halo) flies compared with genetic background controls [Control A, Df(2L)frtz25; Control B, Df(2L)frtz14; ref. 28] (mean distance to odor ± SEM; n = 15–16 per odor; total n = 1,101). Responses of the mutant are compared with both parental controls. (B) Responses to 29 odors in Or43b1, Or43b2, and isogenic w1118 control flies (23) measured as in A (n = 8–60 per odor; total n = 1,829 flies). Physiological responses are taken from published studies (4, 6, 27) as follows: =, weak, defined as <50 spikes per sec or <20% ΔF/F at 40% SV); = =, moderate, defined as 50–100 spikes per sec or ≥20% ΔF/F at 20% SV; = = =, strong, defined as ≥100 spikes per sec or ≥20% ΔF/F at 2–10% SV; n.d., not done. To control for false positives, significance at the P < 0.05 level is required for both comparisons [delta-halo to Df(2L)frtz25 and delta-halo to Df(2L)frtz14 in A; Or43b1 to isogenic w1118 and Or43b2 to isogenic w1118 in B]. Odors for which both comparisons are significant (P < 0.05) are marked with a cyan asterisk.
To confirm that the results with Or22a reflect a general principle in the utilization of weak ligands by the olfactory system, we examined two independent null mutants for Or43b (23). Or43b1 and Or43b2 mutant flies were previously tested in trap assays to a large number of odor stimuli, including those that strongly activate Or43b, but no behavioral defects were observed (23). We measured responses to 29 odors (Fig. 5B) and compared the results from Or43b1 and Or43b2 mutants to a genetically matched control strain to identify behavioral phenotypes. For two odors, d-carvone, a weak agonist of Or43b-expressing neurons (4), and l-limonene, responses increased in both Or43b mutant alleles (Fig. 5B). The increase in the response was stereoselective, with responses to l-carvone and d-limonene unaffected. As seen for Or22a, responses to strong Or43b agonists were unchanged in the Or43b mutants.
Judgment of Odor Intensity in Humans and Fruit Flies.
We extended this analysis to ask how olfactory perception varies between fruit flies and humans, animals with olfactory systems of very different sizes and complexity. In flies, the concentration-dependent magnitude of responses in the odor flow assay was used as a measure of perceived odor intensity. Human odor intensity judgments were obtained by psychophysical methods (see SI Methods). Pairwise comparisons of the odor intensity judgments of two odorants within the same functional group showed that flies and humans agree in 72% of the cases on which odor is stronger (Fig. 6 A–C; χ2 test, P < 0.0001). Humans and flies disagreed considerably on the intensity of only two odors: 1-heptanol, which was rated as very intense by humans and evoked no responses in flies, and methyl acetate, which flies responded to strongly and humans ranked as a weak odor.
Fig. 6.
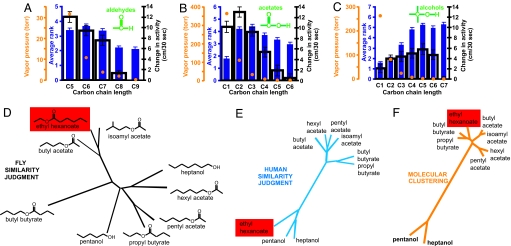
Comparative analysis of odor perception in Drosophila and humans. (A–C) Odor intensity judgments for a homologous series of aldehydes (A), acetates (B), and alcohols (C) in flies (open black bars plot odor flow activity data from Fig. 3) and humans (solid blue bars show rankings of homologous series according to odor intensity). V.P. (torr) is shown by the orange circles, and the carbon chain length of each odor is indicated under the graph. (D) Fly odor similarity tree for nine odors constructed from cross-adaptation experiments (n = 19–24 per odor pair; total n = 1,281; see SI Fig. 14A). Only the nodes connecting the three banana odors (see SI Table 2), ethyl hexanoate, butyl acetate, and isoamyl acetate, have statistically significant stability (see SI Fig. 15A). (E) Human odor similarity tree for the same odors constructed from odor similarity judgments (n = 27). All of the nodes except those connecting isoamyl acetate, pentyl acetate, and hexyl acetate have statistically significant stability (see SI Fig. 15B). (F) Molecular clustering of these odors by structural similarity as determined by the Tanimoto distances of fingerprint descriptors with equal weights of 2D fingerprints and atom pair distances (see SI Methods).
The number of molecules emitted per time by an odor source depends on the V.P. of the odor: the higher the V.P., the more molecules are emitted. Thus, we anticipated that the perceived intensity of an odor would increase with an increase in V.P. This was the case for aldehydes and acetates in both flies and humans. In these two chemical classes, the odor with the higher V.P. was perceived as being stronger in 84% of the comparisons. Intriguingly, the opposite was true in both species for alcohols, where in 72% of all comparisons the odor with the higher V.P. was perceived as weaker (Fig. 6 A–C; χ2 test, P < 0.0001).
Judgment of Odor Quality by Humans and Fruit Flies.
We next asked whether flies and humans agree in their judgment of perceived odor quality. Odor similarity between two odorants was investigated because this feature is the only judgment of odor quality accessible in animals. There are constraints in how similarity judgments can be obtained from humans and flies, because only the former are able to follow verbal instructions. We therefore used the most reliable methods for each species, semantic-free scaling of odor quality in humans (10) and cross-adaptation experiments, in which the change of the behavior in response to an odor after adaptation to another odor is measured, in flies (29).
Perceived similarity between a set of nine odors in flies was measured (see Methods, Fig. 6D, and SI Fig. 12A) and contrasted both with experiments in which human subjects categorized odorants based on perceptual similarity (Fig. 6E and SI Fig. 12B) and with a computational analysis of chemical similarity (Fig. 6F). Untrained human subjects grouped odor similarity in striking agreement with clustering based on molecular structure. The only exception was ethyl hexanoate, which humans did not place in one category with the other esters. Instead, subjects placed this odor into its own group in 43% of all cases. Fly similarity judgments differed considerably from the human similarity judgment and the clustering based on molecular structure (Fig. 6D). We conclude that odor quality judgments differ between humans and flies.
Discussion
Olfactory Perception in Drosophila Is Not Constrained by a Simple Olfactory System.
In this study we provide a comprehensive quantitative description of odor-guided behavior of fruit flies. Drosophila responded behaviorally to all 73 odors tested except vanillin, 2-ethylfenchone, and menthol. The first two odors have in common a low V.P. (<0.035 torr), so the failure to elicit a behavioral response may reflect low odor concentration. The fruit fly olfactory system, like the olfactory system of humans, may be capable of being activated by a very large number of structurally and perceptually different chemical ligands. A specialization to odors associated with fruits is not apparent from these data (SI Tables 2 and 3). However, little is known about ecologically relevant odors and the natural habitat of D. melanogaster (8).
Another important conclusion of this work is that the perceived attractiveness or repulsiveness of an odor to a fly is strongly dependent on the assay used to measure the behavior. For instance, ethyl acetate was attractive for starved flies in a trap assay (15) and arousing in the odor flow assay (Fig. 3), but produced no response in the stationary odor source assay (Fig. 3). We argue that perceived odor quality is not a fixed property of the odor but shows a strong dependence on the assay being used, the odor concentration, and the motivational state of the fly. Motivational state can be altered by starving flies before the experiment or by olfactory conditioning, in which an odor is paired with electric shock and thereafter avoided (14). Thus, thinking about odors as inherently attractive or repulsive is unlikely to be meaningful.
Genetic Perturbation of Drosophila Olfactory Behavior.
Our data confirm previous findings that disrupting a single OR does not alter responses to the strongest ligands of the OR (23). Instead, we find that disrupting an OR causes behavioral responses that cannot be predicted from knowing the physiological responses of the OR. For instance, at the odor concentration tested in our assay, deletion of Or22a decreased responses to only a single odor, 1-heptanol, a weak agonist of Or22a (27), but not to esters that are stronger ligands for this OR (6). The role of Or22a in mediating responses to esters, but not to 1-heptanol, apparently can be compensated by other ORs. The consequences of mutating a single OR may be more or less pronounced at other concentrations.
The same discontinuity between the sensitivity of an OR to an odor and its role in mediating a behavioral response was found for flies lacking Or43b, another OR with known ligands (23). Responses to diverse odorants that were shown to activate Or43b are not altered in Or43b mutants, confirming earlier findings (23). Instead, we found that responses to d-carvone and l-limonene were affected. Responses to l-carvone and s-limonene were unaltered, which is consistent with the finding that the Or43b glomerulus responds differentially to l-carvone and d-carvone (4). Intriguingly, flies that lack Or43b showed increased responses to the affected odors, whereas flies lacking Or22a showed decreased responses. It is plausible that this change in the OR repertoire produces a new odor percept that induces novel behavioral responses.
The effect of deleting a single OR was small, probably due to high redundancy between ORs. This redundancy also may account for the somewhat unexpected finding that flies without antennae still responded to 61% of the odorants. Drosophila larvae expressing only a single OR that is also expressed in the maxillary palps (Or42a) can smell 43% of the tested odors (21). Thus, Or42a or other ORs that are activated by many odors may be responsible for the ability of antenna-less flies to respond to most of the tested odors at the relatively high odor concentrations used here.
Comparative Analysis of Odor Perception in Humans and Fruit Flies.
The perceived similarity between the quality of two stimuli depends not only on the properties of the stimuli but also on the properties of the sensory system perceiving them. This is probably clearest in the case of ethyl hexanoate, which was grouped with isoamyl acetate and butyl acetate by flies, but not by human subjects. Other differences such as the categorization of the two alcohols in one group in humans, but not in flies, are also interesting to note. However, the stability of the nodes involved in categorizing the alcohols in the fly odor similarity tree is not statistically significant (SI Fig. 15).
How do the differences in odor similarity judgment between humans and flies arise? There are likely to be many ways of discriminating a large number of odors with different combinations of ORs. We propose that humans and flies achieve this in different fashions, with OR gene families subject to different evolutionary pressures. The olfactory systems of the two species may have a different level of resolution in parts of the olfactory space, which in turn may cause these organisms to differ in how they categorize odors. Therefore, odors that smell similar to the human observer do not necessarily smell similar to the experimental animal. This is in striking contrast to the agreement in experienced odor intensity between humans and fruit flies.
In summary, our experiments provide insights into how flies experience odors, how these experienced odor percepts relate to the activation pattern of OSNs, and how their experiences relate to our own subjective experience of odor stimuli.
Methods
Drosophila Stocks.
Flies were maintained on cornmeal-agar-molasses medium under a 12-h light:12-h dark cycle. See SI Methods for genotypes and sources of flies used.
Olfactory Assays.
Odor flow assay.
Single flies were placed into each of 16 circular arenas (10 cm diameter, 1 cm high, tilted walls) in a custom-built apparatus outfitted with individual odor intakes and outlets and a Plexiglas lid to isolate flies in each arena (see SI Fig. 7). Flies were acclimated to a constant flow of pure air (590 ml/min) for 5 min. After acclimation, flies were videotaped for the 6-min experiment, which consisted of 2 min of exposure to flow of pure air and 4 min of subsequent exposure to air containing 18% SV concentration of odor. See SI Methods and SI Fig. 8A for further information.
Stationary odor source assay.
Odorants (5 μl undiluted or diluted in paraffin oil) were pipetted onto a piece of filter paper placed vertically at the wall in each of four Petri dishes (8.5 cm diameter, 1.3 cm high). Immediately afterward, a single fly was introduced into each dish, and its x–y coordinate was videotaped and tracked with Ethovision software (Noldus, Wageningen, The Netherlands) for 3 min at 6 Hz. Avoidance (distance to odor source) was calculated. See SI Methods and SI Fig. 8B.
Odor cross-adaptation.
Responses to nine odorants (ethyl hexanoate, hexyl acetate, isoamyl acetate, 1-pentanol, 1-heptanol, butyl acetate, pentyl acetate, butyl butyrate, and propyl butyrate) were measured after preexposure in a Petri dish for 30 min to 5 μl of a 1/10 dilution of one of six reference odors (ethyl hexanoate, hexyl acetate, isoamyl acetate, 1-pentanol, pentyl acetate, and propyl butyrate). See SI Methods and SI Fig. 14A for details.
Human Olfactory Psychophysics.
All procedures were approved by the Rockefeller University Hospital Institutional Review Board. Normal human subjects (n = 29; 18 female, ages 21–40) were asked to rank odors according to intensity by arranging odor vials in a line with the weakest odor on the left and the strongest odor on the right. In the same session, subjects (n = 27; 18 female; ages 21–40) were asked to rate the similarity of nine odorants (butyl acetate, pentyl acetate, hexyl acetate, isoamyl acetate, propyl butyrate, butyl butyrate, ethyl hexanoate, 1-pentanol, and 1-heptanol) by arranging the vials in groups according to similarity. Subjects were instructed to make as many or as few groups as desired. See SI Methods for details.
Supplementary Material
Acknowledgments
We thank Dean Smith (UT Southwestern Medical Center, Dallas, TX) and Michael Welte (Brandeis University, Waltham, MA) for fly strains; Lei Shi for performing the molecular clustering; Lylyan Salas, Daniel Baez, and Heike Wagner for expert technical assistance; Avery Gilbert, Emil Gotschlich, Barry Coller, and the staff of the Rockefeller University Hospital for assistance with the human psychophysical study; the Aspen Center for Physics for office space; and Austen Gess and members of the L.B.V. laboratory for comments on the manuscript. This work was supported by National Institutes of Health Grant R01 DC005036 (to L.B.V.). A.K. received support from a Marco S. Stoffel Fellowship in Mind, Brain, and Behavior.
Abbreviations
- OR
odorant receptor
- OSN
olfactory sensory neuron
- V.P.
vapor pressure
- SV
saturated vapor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605321104/DC1.
References
- 1.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Malnic B, Hirono J, Sato T, Buck LB. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 3.Araneda RC, Kini AD, Firestein S. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 4.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 5.Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallem EA, Carlson JR. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P. Science. 1999;286:707–711. doi: 10.1126/science.286.5440.707. [DOI] [PubMed] [Google Scholar]
- 8.Keller A. Curr Biol. 2007;17:R77–81. doi: 10.1016/j.cub.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Wise P, Olsson M, Cain W. Chem Senses. 2000;25:429–443. doi: 10.1093/chemse/25.4.429. [DOI] [PubMed] [Google Scholar]
- 10.Stevens D, O'Connell R. Physiol Behav. 1996;60:211–215. doi: 10.1016/0031-9384(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 11.Todrank J, Wysocki CJ, Beauchamp GK. Chem Senses. 1991;16:467–482. [Google Scholar]
- 12.Cain WS, Polak EH. Chem Senses. 1992;17:481–491. [Google Scholar]
- 13.Barrows WM. J Exp Zool. 1907;4:515–537. [Google Scholar]
- 14.Quinn WG, Harris WA, Benzer S. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodard C, Huang T, Sun H, Helfand SL, Carlson J. Genetics. 1989;123:315–326. doi: 10.1093/genetics/123.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayyub C, Paranjape J, Rodrigues V, Siddiqi O. J Neurogenet. 1990;6:243–262. doi: 10.3109/01677069009107114. [DOI] [PubMed] [Google Scholar]
- 17.Anholt RR, Lyman RF, Mackay TF. Genetics. 1996;143:293–301. doi: 10.1093/genetics/143.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 19.McKenna M, Monte P, Helfand SL, Woodard C, Carlson J. Proc Natl Acad Sci USA. 1989;86:8118–8122. doi: 10.1073/pnas.86.20.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf FW, Rodan AR, Tsai LT, Heberlein U. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 23.Elmore T, Ignell R, Carlson JR, Smith DP. J Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couto A, Alenius M, Dickson BJ. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Pelz D, Roeske T, Syed Z, de Bruyne M, Galizia CG. J Neurobiol. 2006;66:1544–1563. doi: 10.1002/neu.20333. [DOI] [PubMed] [Google Scholar]
- 28.Gross SP, Guo Y, Martinez JE, Welte MA. Curr Biol. 2003;13:1660–1668. doi: 10.1016/j.cub.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Boyle J, Cobb M. J Exp Biol. 2005;208:3483–3491. doi: 10.1242/jeb.01810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.