Abstract
The rapid recall of influenza virus-specific CD8+ T cell effector function is protective, although our understanding of T cell memory remains incomplete. Recent debate has focused particularly on the CD62L lymph node homing receptor. The present analysis shows that although functional memory can be established from both CD62Lhi and CD62Llo CD8+ T cell subsets soon after initial encounter between naïve precursors and antigen, the optimal precursors are CD8+CD44hiCD25lo immune lymphocytes isolated from draining lymph nodes on day 3.5 after influenza virus infection. Analysis of primed T cells at different times after challenge indicates that the capacity to transfer memory is diminished at the peak of the primary cytotoxic T lymphocyte response, challenging speculations that the transition to memory first requires full differentiation to effector status. It seems that location rather than CD62Lhi/lo phenotype may be the more profitable focus for further dissection of the early establishment of T cell memory.
Keywords: draining lymph nodes, generation of memory
Established CD8+ T cell memory can provide substantial protection against various viral, bacterial, and parasitic infections. Although the generation of protective CD8+ T cell memory constitutes a primary goal for cell-mediated vaccines, the mechanistic basis of such memory development is still far from clear for any naturally occurring or experimental situation. What exactly are memory T cells, and how do they develop? A widely accepted idea is that the memory set is derived from the effector population, subsequent to a 10-fold or more contraction in numbers. However, recent experiments using in vitro-stimulated T cells (1), antibiotic treatment before Listeria monocytogenes infection (2) or dendritic-cell vaccination (3) suggest that the full expansion to effector status may not be a prerequisite for the generation of memory T cells.
Current fashion has it that memory T cells can be classified into two distinct populations with distinct lymph node homing properties, anatomical locations, and functions (4). The CD62Lhi “central memory” (TCM) set transits directly from blood to lymph node via the high endothelial venules, whereas the CD62Llo “effector memory” (TEM) cells access the lymph nodes only via afferent lymph and are found widely dispersed in a broad range of somatic tissues (4–8). This CD62L “gating” mechanism does not operate in the spleen. To date, there is no general agreement on the determining factors in TCM and TEM development. Most accept that a proportion of the TCM precursors can become effector and/or TEM cells after secondary challenge (7–9), whereas some experiments suggest that a TEM → TCM transition is possible in the long-term (7, 9). However, others propose that diverse TCM populations include a range of partially differentiated phenotypes that reflect a more limited and varied “signaling experience” and are a continuous source of distinct effector and TEM sets (10, 11). Another view is that the TEM and TCM populations divide into distinct lineages from the time of primary antigen exposure (12).
Established CD8+ T cell memory provides significant protection against respiratory challenge with extremely virulent influenza A viruses (13, 14). Our recent, single-cell analysis of TCR CDR3β profiles for two influenza A virus DbNP366 and DbPA224 epitopes in conventional, nontransgenic, virus-infected mice showed that both CD62Llo and CD62Lhi T cells are predominantly represented by the same large clonal expansions (15). Furthermore, clonal diversity of both CD62Llo and CD62Lhi T cell subsets is maintained from day 8 into long-term memory (longer than day 180), suggesting that stable TCM and TEM lineages are established early in the antigen-driven phase (within the first week) of influenza virus infection. The present analysis utilizes adoptive transfer protocols with TCR-transgenic T cells to ask how early and where memory T cell populations develop, and what are their key characteristics. We show that memory CD8+ T cells are preferentially established in draining lymph nodes at early (day 3.5) and late (day 28) but not acute (day 8) time-points. Furthermore, we demonstrate that establishment of memory occurs irrespective of CD62L phenotype.
Results
To detect CD8+ T cells early after influenza virus infection and to eliminate variation associated with differing TCR/epitope avidity profiles (16, 17), the present analysis utilizes adoptively transferred, CFSE-labeled, Ly5-different, TCR-transgenic (OT-I) cells specific for the KbOVA257 epitope (18). Infection of chimeric C57BL/6J (B6, H2b) mice with a recombinant influenza virus (HK-OVA) incorporating the ovalbumin SIINFEKL peptide (19) has been shown to result in substantial KbOVA257-specific clonal expansions of both endogenous, naïve Ly5.2+ and adoptively transferred naïve or memory Ly5.1+ OT-I T cells equivalent to the prominent influenza-specific CD8+DbNP366+ and CD8+DbPA224+ T cell responses (20, 21). In most experiments, OT-I T cells from unprimed mice were transferred into naïve Ly5.2+ hosts that were then challenged i.n. with the HK-OVA influenza A virus. The spleens and draining, regional mediastinal lymph nodes (MLN) of these infected mice were then sampled after an additional 3.5, 8, or 28 d. Antigen-experienced OT-I T cells that had cycled at least once and transitioned to being CD44hi were then sorted on the basis of CD62L phenotype and transferred into additional Ly5.2+-naïve recipients before secondary HK-OVA virus challenge 32–40 d later to examine persistence and recall of these cells.
Cell Division and CD62L Expression Through the Primary Response.
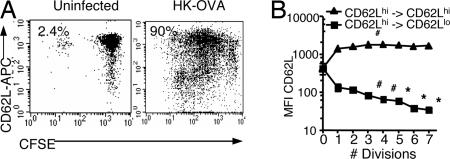
Naïve Ly5.2+ mice were given CFSE-labeled Ly5.1+ TCR-transgenic T cells and the HK-OVA influenza virus to probe the correlation between cell cycling and CD62L phenotype early (day 3.5) in the course of infection (Fig. 1). The CFSE-labeled Ly5.1+CD8+OT-I T cells were 90% CD62Lhi at time of spleen cell transfer into uninfected Ly5.2+ B6 recipients. Some of these mice were challenged i.n. with the HK-OVA virus 24 h later, whereas others were left uninfected and spleens analyzed after another 3.5 d. A few of the CD62LhiLy5.1+CD8+ T cells recovered from the uninfected controls had divided (2.4%), but most remained CFSEhiCD62Lhi (Fig. 1A). Similar levels (<3%) of spontaneous OT-I division were found in mice infected with control HK virus (M.R.J., unpublished work). By contrast, the majority of the Ly5.1+CD8+OT-I T cells in the HK-OVA-infected mice (90%) had divided at least once, and many more were now CD62Llo (Fig. 1A). Although the CD62Lhi T cells still predominated at day 3.5 after infection, the percentage of CD62Lhi diminished with continuing division. The intensity of CD62L expression on the CD62Lhi T cells did not change with continued dividing, but the residual CD62L detected on the surface of the “minimal expressors” classified as CD62Llo was at a significantly higher level for cells in division 1 when compared with divisions 3 to 7 (Fig. 1B). Staining for the IL-7R, however, dropped immediately from division 1 for both the CD62Llo and CD62Lhi Ly5.1+CD8+OT-I T cells, whereas the pattern of CD44 increase was also consistent for the two subsets, with the levels being lower for cells that had cycled only once (data not shown). Thus, although the acquisition of differentiated phenotypes, such as the capacity to make various cytokines, is considered to progress with ongoing cell division (22), the antigen-driven cycling of these OT-I T cells seems to result in two distinct profiles of CD62L (but not IL-7R or CD44) expression. It seems clear that at least some CD62Lhi T cells transit to being CD62Llo, although there is another subset that divides but maintains a consistent CD62Lhi phenotype for at least 3.5 d after antigen challenge.
Fig. 1.
Correlation of cell division with CD62L profiles at day 3.5 after influenza virus infection. Splenocytes from the donor OT-I-Ly5.1 mice were labeled with CFSE at 5 μM for 10 min at 37°C, then injected i.v. into naïve B6-Ly5.2 recipients at 5 × 106 to 1 × 107 splenocytes per mouse. Most were then infected i.n. with HK-OVA virus 24 h later. Lymphocytes taken on day 3.5 after infection were analyzed for CFSE, CD62L (APC), CD8 (PerCP-Cy5.5 or APC-Cy7), and Ly5.1 (PE or PE-Cy7). (A) Typical patterns of CFSE and CD62L staining are shown for Ly5.1+CD8+ OT-I T cells from an uninfected recipient and from a mouse challenged i.n. with the HK-OVA virus. (B) Mean fluorescence intensity (MFI) of CD62L staining for dividing Ly5.1+CD8+ OT-I T cells. Statistical significance was assessed between cells in the first and subsequent divisions (∗, P < 0.01; #, P < 0.05). Groups of three mice were used in each of three experiments.
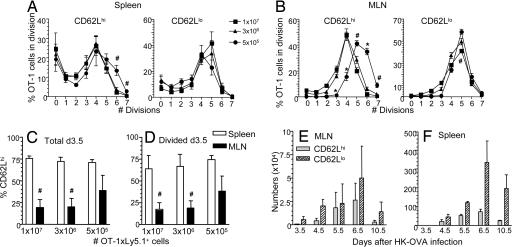
This experiment used large numbers of enriched spleen cells (5 × 106 to 1 × 107; ≈20% CD8+ T cells), which might be thought to reduce the antigen-driven “pressure” on any individual naïve CD62Lhi precursor to divide extensively and switch to a CD62Llo phenotype (12). Dropping the cell dose three times and 20 times (Fig. 2 A–F) did not, however, cause any significant decrease in CD62Lhi frequency for either the total (Fig. 2C) or dividing (Fig. 2 A, B, and D) Ly5.1+OT-I T cell populations measured at day 3.5 after challenge. In both cases, the relative prevalence of CD62Lhi versus CD62Llo OT-I T cells was substantially lower in the regional, MLN than in the spleen, reflecting earlier evidence that influenza virus-specific CD8+ T cells are first detected in the draining nodes (Fig. 2 C and D). Interestingly, more CD62Lhi Ly5.1+OT-I T cells went through five to seven divisions when smaller numbers of naïve cells were transferred (Fig. 2 A–D). This argues against the possibility that a proportion of cycling Ly5.1+OT-I precursors maintain CD62L expression as a consequence of competition for “optimal” inductive signals.
Fig. 2.
Anatomical distribution of CD62Lhi and CD62Llo Ly5.1+CD8+ OT-I immune T cells related to cell dose and time after infection. (A and B) Correlation of cell division with CD62L expression at 3.5 d after HK-OVA infection of recipient mice that were given graded numbers of enriched Ly5.1+CD8+ cells. Cells from spleens (A) or MLNs (B) were analyzed for the prevalence of CD62Lhi and CD62Llo T cells at each cell division, i.e., the fraction of cells that have divided x number of times. (C and D) The percentage of CD62Lhi is shown for the total (C) and divided (D) Ly5.1+CD8+ OT-I sets from MLN (black bars) or spleen (white bars) at 3.5 d after the infection of recipient mice transferred with graded numbers of enriched Ly5.1+CD8+ cells. (E and F) The number of divided (CFSE loss) CD62Lhi and CD62Llo Ly5.1+CD8+ OT-I T cells from MLN (E) or spleen (F) at intervals after HK-OVA infection of chimeric B6 mice (5 × 105 Ly5.1+ T cells). Experiments were performed as in Fig. 1A.
Transfer of 5 × 105 enriched splenocytes (≈20% CD8+ T cells, i.e., ≈1 × 105 CD8+ T cells) was at the level of detection for recall responses on day 3.5 after influenza virus infection (cell numbers shown in Fig. 2 E and F). A recent report (12) found an increased proportion of CD62Llo OT-I cells after VSV-OVA infection when 500 cells were transferred in comparison with 1 × 105 or 1 × 107 cells, although the numbers of recovered cells were not discussed. However, because our analysis found no difference between CD62Llo and CD62Lhi expression for any of the precursor frequencies giving us detectable cell numbers on day 3.5, subsequent experiments used between 5 × 105 and 1 × 107 enriched splenocytes for the initial transfer experiments. Similarly, no difference between the precursor frequency of transferred populations and CD62L phenotype was found in a recent study (23).
A time course (Fig. 2 E and F) using the lowest cell dose (5 × 105 enriched splenocytes, ≈1 × 105 CD8+ T cells) then effectively reproduced the CD62Lhi/CD62Llo profiles seen previously for the endogenous CD8+DbNP366+ and CD8+DbPA224+ responses (15). Although the CD62Llo T cells clearly outgrow the CD62Lhi set, a small population of divided (more than seven times) CD62Lhi T cells is still apparent (day 10.5, Fig. 2 E and F) when the endogenous expansion is at maximum size or just beginning to decline. Thus, both CD62Llo and CD62Lhi CD8+ T cell populations are present in both MLN and spleen at any time-point during the primary response.
Survival of CD62Llo and CD62Lhi Ly5.1+ OT-I T Cells in “Resting” Memory.
Dividing CD62Llo and CD62Lhi CD44hiLy5.1+OT-I T cells were isolated from spleen or MLN at days 3.5, 8, or 28 after HK-OVA infection [supporting information (SI) Fig. 5], transferred (1 × 104 cells) into naïve B6 Ly5.2+ recipients, then left for 32–40 d. Between days 32 and 40, the numbers of CD62Lhi and CD62Llo Ly5.1+OT-I T cells in the spleen were very low for all time points (SI Fig. 6). However, because the frequencies shown in SI Fig. 6C were generated from FACS data at the limits of detection, it is possible that the values might be slightly elevated because of nonspecific detection. Therefore, it is fair to say that the 1 × 104 adoptively transferred days 3.5, 8, or 28 Ly5.1+OT-I immune T cells gave rise to counts that were no greater than 0.2–1 × 103 T cells in spleen 32–40 d later. Interestingly, the size of the resident spleen OT-I CD8+ set closely resemble estimates of precursor frequency for naïve antigen-specific CD8+ T cells (24) and thus resembles a physiological situation. The majority of the recovered memory Ly5.1+ OT-I T cells was CD62Lhi (data not shown), irrespective of whether the transferred population was CD62Lhi or CD62Llo. In addition, there were no indications of “homeostatic” OT-I memory T cell proliferation after transfer into normal, uninfected mice. No OT-I cells could be detected in any of the lymph nodes tested (MLN, axillary, cervical, and inguinal; data not shown).
Recall of CD8+ T Cell Memory.
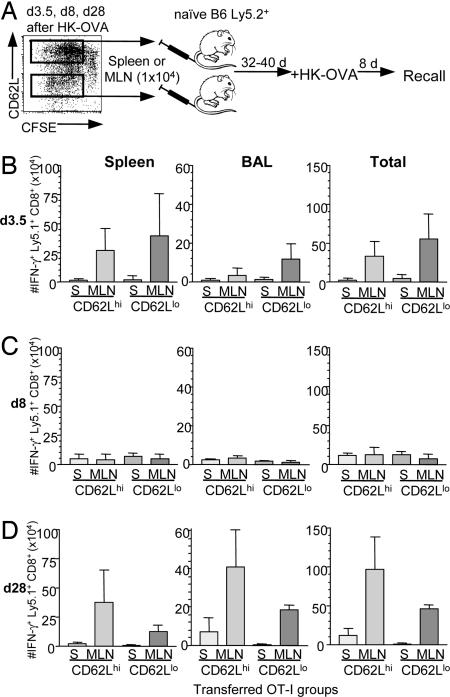
“Good” memory T cells are characterized by persistence in the absence of antigen and by the capacity to mount a vigorous recall response to reinfection (25). After secondary challenge at days 32–40 after transfer of day 3.5-primed CD62Lhi and CD62Llo Ly5.1+ OT-I T cells (Fig. 3A), memory precursors from all four groups (CD62Llo and CD62Lhi derived from either MLNs or spleens) proliferated further and localized to the infected lung (Fig. 3B and SI Fig. 7A). However, although both the CD8+CD44hi CD62Lhi and CD62Llo Ly5.1+ OT-I populations that were taken on day 3.5 after priming contributed to the memory T cell pool, the Ly5.1+OT-I T cells sorted from the MLNs on day 3.5 proliferated more than those from the spleen (Fig. 3B). Thus, T cell memory is established as early as day 3.5 after the initial antigen exposure of naïve, OT-I T precursors. The enhanced proliferation response from the MLN could not be attributed to differences in CD62L expression, because both CD62Lhi and CD62Llo populations from the MLN gave better recall responses than the comparable spleen sets (Fig. 3B). Furthermore, 10 times more Ly5.1+OT-I CD44hi T cells were recovered from MLN compared with spleen at day 3.5 (per organ per mouse; SI Fig. 5B), suggesting an even larger contribution of MLN-derived cells to the total memory pool at this time point.
Fig. 3.
Recall of secondarily stimulated IFN-γ+CD8+Ly5.1+OT-I donor T cells derived from spleen or MLN at different phases after primary HK-OVA infection. (A) On days 3.5, 8, and 28 after HK-OVA infection, CD62Llo or CD62Lhi CD44hiCD8+Ly5.1+OT-I cells that had divided at least twice (by CFSE-labeling) were isolated separately from MLNs and spleens (S). The sorted CD62Llo or CD62Lhi T cell subsets were then transferred (1 × 104 per mouse) into naïve Ly5.2+ B6 recipients, left for a further 32–40 days, and then infected with HK-OVA. Memory CD8+Ly5.1+OT-I cells generated at 3.5 (B), 8 (C), or 28 d (D) from spleen or MLN were assessed for their capacity to mediate recall responses on day 8 after HK-OVA challenge. Lymphocytes from spleen and BAL (data shown), lung, and MLN (data not shown) were assessed by ICS for IFN-γ production. The counts in the “Total” column were calculated by adding the numbers obtained from spleen, BAL, lung, and MLN. Although the y axis of the different histograms all relates to a 1 × 104 baseline, the scales are different, reflecting the numbers recovered from the particular anatomical site. Recall responses by transferred memory CD8+ T cells derived from MLNs at 3.5 and 28 d after primary exposure were significantly higher than those mediated by the comparable sets in spleen (P < 0.05).
To determine whether the superior proliferative capacity of these MLN (vs. spleen) memory T cells (Fig. 3B) is true only for lymphocytes recovered very early (day 3.5) in the primary response, the same comparison was made for T cells sampled on day 8 (acute phase) or day 28 (established memory). The recall response (Fig. 3C) by OT-I T cells from day-8 spleen and MLN populations was remarkably diminished for all four groups (compare Fig. 3 B and C and SI Fig. 7 A and B), suggesting that the memory sets available for further clonal expansion have been greatly diluted by large numbers of terminally differentiated effectors. Conversely, the day-28 CD62Lhi and CD62Llo CD44hi OT-I MLN T cells again (as in Fig. 3B) gave a superior response (Fig. 3D). Both proliferative capacity (SI Fig. 7C) and IFN-γ production (Fig. 3D) were greater for the MLN-derived memory sets, with the numerical hierarchy being: MLN-CD62Lhi > MLN-CD62Llo > spleen-CD62Lhi > spleen-CD62Llo. Most of the fully differentiated CTL effectors would have been edited out by day 28, but it is intriguing that the “before” (day 3.5) and “after” (day 28) virus-elimination phases look so similar when it comes to providing optimal CD8+ T cell memory (Fig. 3 and SI Fig. 7). Thus, memory precursors are clearly enriched in the MLN vs. the spleen, irrespective of CD62L phenotype.
Two-Way CD62L Transition.
As might be expected, the adoptively transferred days 3.5, 8, and 28 CD62Lhi and CD62Llo CD8+CD44hi populations from both MLN and spleen all gave rise to CD62Llo progeny upon further exposure to antigen (SI Fig. 8 A–C). Furthermore, it was apparent that some of the CD62Llo precursors transitioned to a CD62Lhi phenotype by day 8 after challenge. This effect was most obvious for transferred “day-3.5 spleen” T cells that were detected in the spleen and MLN of the challenged mice and for the “day-28 spleen” in the responding, recipient MLN and spleen.
Optimal Transfer by Primed CD25lo Precursors.
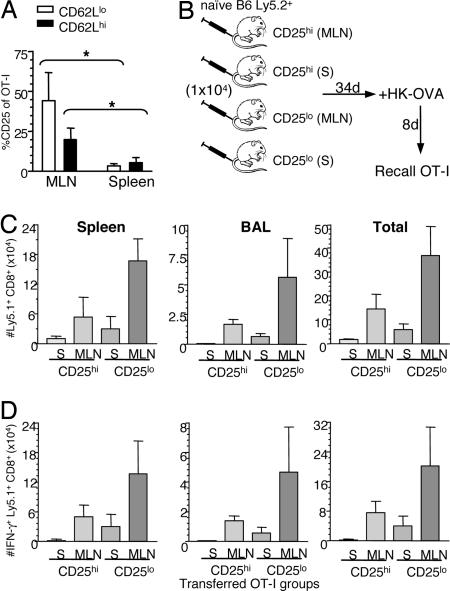
Given that the CD8+ T cells from the day-3.5 draining lymph node give better recall responses than those from spleen (Fig. 3B), can differences in cell-surface phenotype be identified for precursors in these two sites? Analysis of CD127 (IL-7Rα), CD25 (IL-2Rα), and NKG2a FACS profiles for the CD62Lhi and CD62Llo OT-I T cells showed many more positive cells and high levels of CD25 staining on both the CD62Llo and CD62Lhi subsets from MLN vs. spleen (Fig. 4A). Otherwise, there were no significant, site-related differences for CD127 or for NKG2a staining on OT-I CD8+ T cells (SI Fig. 9). Because both the optimal mediators of long-term memory in the adoptively transferred day-3.5 population (Fig. 3B) are found in the regional MLN, and many more of the MLN CD44hi CD62Llo and CD62Lhi OT-I cells are CD25hi (IL-2Rα+) (Fig. 4A), we asked whether the CD25hi sets constituted the key precursor populations. The recall responses at day 34 after cell transfer (Fig. 4B) showed the hierarchy: MLN CD25lo > MLN CD25hi > spleen CD25lo > spleen CD25hi (Fig. 4 C and D). Clearly, the MLN cells in both the CD25lo and CD25hi subsets are superior to the spleen precursors, so CD25 expression cannot be the only key determinant. A likely possibility is that the CD25hi cells are at an IL-2-dependent phase of differentiation and may tend to activation-induced cell death when removed from the IL-2-rich environment of the responding lymphoid tissue.
Fig. 4.
Optimal transfer by primed CD25lo precursors derived from MLN. Divided CD62Llo or CD62Lhi CD8+Ly5.1+OT-I cells from spleens or MLNs at 3.5 d after HK-OVA infection were analyzed for expression of CD25. (A) Shows the mean ± SD (n = 6) prevalence of staining for the CD62Llo and CD62Lhi CD8+Ly5.1+OT-I T cells from MLN or spleen; ∗, P < 0.01 for the comparisons shown. (B–D) Memory CD8+Ly5.1+OT-I cells generated at 3.5 d after HK-OVA infection from dividing CD25lo or CD25hi CD44hiCD8+Ly5.1+OT-I cells isolated from either spleens or MLNs were assessed for their capacity to mediate recall responses as described (B). On day 8 after HK-OVA challenge, cells from spleen and BAL (data shown), lung, and MLN (data not shown) were assessed for total CD8+Ly5.1+OT-I numbers (C) or by ICS for IFN-γ production (D).
Discussion
Recent debate has focused particularly on the importance of the CD62L lymph node homing receptor as a marker for T cell memory, although how, when, and where CD62LhiCD8+ and CD62LloCD8+ memory T cell precursors (4, 7, 8, 26) subset during the antigen-driven phase of the response is far from clear. The present analysis dissects the capacity of CD62Llo or CD62Lhi CD8+ T cell subsets recovered from distinct anatomical sites at different times after influenza virus infection to give rise to memory populations after transfer into naïve hosts. The comparison used CD8+ T cells sampled early (day 3.5), at the peak of acute infection (day 8), or during the established memory phase (day 28). Three outcomes seemed possible. The first is that CD8+ T cells obtained from any site on day 3.5 after infection will die after transfer into a naïve host, and only a small proportion of those recovered on day 8 will survive, although the majority of the day-28 CD8+ T cells persist and are recalled after secondary challenge. This result would support the paradigm that memory T cells are derived from the effector population. A second alternative is that CD8+ T cells transferred on day 3.5 after infection will maintain in the “normal” environment of the “naïve” recipients, suggesting that memory precursors are established early after challenge (before the effector phase) and that they do not require continued exposure to the cytokine/chemokine milieu associate with infection to become established in the long term. Finally, whatever the timing or location, CD62L phenotype may indeed be the critical factor for the establishment of memory.
This analysis establishes that memory T cell precursors are generated preferentially in the draining lymph node early (by day 3.5) after influenza virus infection. The least efficient population when it comes to the transfer of memory is the highly activated day-8 population, an observation tending against the idea that memory T cells are optimally derived from fully functional “effector” T lymphocytes. As with many issues in this complex field, there is some semantic confusion when it comes to the use of the word “effector.” Claims that effectors give rise to memory T cells (7, 27) can, in fact, be supported only if the differentiation (or dedifferentiation) pathway is followed from a single precursor or if it is established beyond reasonable doubt that all of the T cells in a population are maximally activated and that there are no “less-stimulated” precursors in the available lymphocyte pool. As shown here, primed CD8+ T cells proliferate very rapidly, and a minimal minority set can rapidly swamp what was initially a majority constituency.
Prior evidence that T cell memory can be generated very early in the course of an immune response is available from in vitro stimulation experiments showing that naïve TCR-transgenic T cells pulsed with antigen for 24 h in culture can then establish memory after transfer into mice (1). This analysis indeed establishes that the memory T cell program can be triggered very rapidly, but the situation is very different from what happens in a whole animal. Pulsed antigen-presenting cells (APCs) are available to T cells immediately upon in vitro stimulation, whereas in vivo priming involves a whole cascade of events related to the kinetics of infection, replication of the pathogen, antigen processing, DC migrationm, and time of antigen presentation to the T cells.
An informative set of experiments used mice that were challenged with Listeria monocytogenes 24 h before or after treatment with an antibiotic that substantially controls this bacterial infection (2). The response after the antibiotic treatment after infection looked substantially normal and resulted in comparable effector and memory populations with those observed in untreated mice. This reflects the fact that L. monocytogenes causes a rapid, high-level systemic infection that may well promote the presence of antigen-presenting dendritic cells that persist long after the pathogen is cleared. The finding that antibiotic treatment before infection dramatically reduced both the bacterial load and the extent of T cell effector expansion, but not the magnitude of memory T cell population, indicated that (as shown here) a major contraction phase is not essential for the establishment of memory. However, the memory T cell precursors were not removed from possible further encounter with APCs in these experiments, and our adoptive transfers into naïve hosts provide a much more definitive demonstration that early, dividing CD62Llo and CD62Lhi T cell populations can indeed provide antigen-independent long-term memory.
Influenza A viruses cause localized (to the respiratory tract), not systemic, infections in mice. During the course of influenza pneumonia, viral antigen is thought to be carried to the regional lymph nodes by dendritic cells that have either been nonproductively infected (make viral proteins but no progeny virus) or have taken up antigen in the lung during the first 36 h after virus challenge (28). The net consequence is, then, that the APC “environment” that determines the nature of the primary response is likely to be both established early and limited in extent. Infectious virus is cleared from lung epithelium within 10 d after primary virus challenge, and most evidence suggests that all viral antigen is eliminated within 14–21 d of infection (29) (J. Mintern, S.J.T., and P.C.D., unpublished work). In our study, we transferred CD8+ T cells sorted under stringent conditions into naïve hosts to assure an antigen-free environment.
Although striking differences were found in the memory potential of antigen-stimulated T cells from two different anatomical sites (spleen and MLN), further sorting into CD62Llo and CD62Lhi CD8+ T cell populations on days 3.5 and 28 did not support the view that there is a clear division in functional capacity for these two subsets. Given that we knew years ago that CD62L is the lymph node homing receptor and that fully functional effector T cells are likely to be CD62Llo, it seems appropriate to ask whether it is useful to focus further analysis of memory establishment around the TCM/TEM classification. We did find that the CD62Lhi set contains more T cells that look to have suboptimal TCRs (15) but, because these contribute little to recall responses (26), they can hardly be regarded as optimal “central memory” precursors.
Establishment of memory T cell precursors preferentially in the draining LN rather than spleens suggests that CD8+ T cell derived from these two sites are potentially distinct populations that could be characterized by differential expression of cell-surface activation/differentiation markers, key marker(s) have yet to be identified. The differential expression of IL-2Rα in CD8+ T cells derived from MLN and spleen on day 3.5 after influenza virus infection indicates a potential role for IL-2 in the early establishment of T cell memory. However, IL-2 may only be part of the answer, with the development of T cell memory dependent on both the initial internal stimulus through the TCR as well as external signals mediated by various lymphokines that have been implicated in the development and maintenance of memory (30, 31).
Overall, the results indicate that “optimal” memory T cells can emerge early in the antigen-driven stage of the response, that they are less apparent at the peak effector phase, and that they then reemerge with time. One possibility is that the memory set is diluted for a time by maximally expanded, highly activated cytotoxic T lymphocytes (CTLs) but is again at enhanced relative frequency after antigen is cleared, and the majority of these effectors die off. The problem with this interpretation is, although, that we would expect terminally differentiated CTLs to all be CD62Llo, and, in these experiments, the d8 CD62Lhi set was also a poor source of T cells for the recall response. It is thus possible that the “high cytokine”/inflammatory environment in acutely responding lymphoid tissue make memory T cell precursors vulnerable in some way when they are removed for adoptive transfer.
Our recent, single-cell analysis of CDR3β sequences in the native response to influenza virus epitopes (15) showed that the progeny of the largest clonotypes can be CD62Lhi or CD62Llo and that both sets are maintained into long-term memory. The present results for day 28 establish that CD62Lhi and CD62Llo memory T cells contribute to the recall response. Evidence of subsetting within clonotypes (15), however, supports the conclusion that the CD62Lhi/lo dichotomy is characteristic of early expansions from a single precursor stimulated by perhaps one, or a few, dendritic cells that are in close proximity. The CD62L/CD25 fate decision may thus reflect the consequences of local cytokine/chemokine gradients or the extent of contact with accessory molecules on DCs or stromal cells in lymph node microenvironments.
There are, of course, other formal possibilities to explain some of these findings, but both the present and previous (1–3) studies suggest that further analysis of the establishment of memory is best directed at the onset of the response in the draining lymph nodes rather than at CD62Lhi/lo subsetting. Do, for instance, CD62LhiCD25lo and CD62LloCD25lo memory T cells go through a very early IL-2Rα+ iteration in the MLN? Are they taken out of the effector pathway by being IL-2-independent through the initial, antigen driven stage? If IL-2 exposure during the acute phase of primary infection is essential for the establishment CD8+ T cell memory (23), is it possible that the optimal day-3.5 CD8+CD44hi CD25lo memory population found in our study has already differentiated beyond IL-2 dependence? Our study provides insights into the location and early generation of memory T cells. We propose a refined paradigm in which CD8+ memory T cell precursors are generated early after infection (at least by day 3) in draining lymph nodes. This memory set is then diluted by the large number of fully differentiated effector cells when lymphoid tissues are sampled at the peak of the infectious process. After virus control and the consequent contraction of the CTL pool, these established memory T cells then persist at a frequency comparable with that observed early during the infection.
Methods
Animals.
C57BL/6J (B6) and congenic OT-I-Ly5.1 mice were bred at the University of Melbourne. The OT-IxLy5.1 mice provided TCR transgenic cells for transfer to naïve Ly5.2+ B6 recipients. All experiments followed guidelines of the University of Melbourne Animal Ethics Experimentation Committee.
Tissue Sampling and Cell Preparation.
Spleen, BAL, lung, or MLN samples were recovered from mice at various time points after infection or transfer as described below. Lymphocytes were recovered from the infected lung (BAL) were incubated on Petri dishes for 1 h at 37°C to remove macrophages. Spleens and MLNs were disrupted; spleens were enriched for CD8+ T cells by panning on plates coated with goat anti-mouse IgG and IgM antibodies to remove B cells for 1 h at 37°C (Jackson ImmunoResearch, West Grove, PA).
Adoptive Transfer and Analysis of CFSE-Labeled OT-I+Ly5.1+ Cells.
Naïve OT-I cells were taken from the spleens of OT-I/Ly5.1 B6 mice (18). Lymphocytes were resuspended at 1 × 107 per milliliter and stained with CFSE. The CFSE-labeled cells were washed twice with PBS, resuspended at 2.5 × 106 to 5 × 107 cells per milliliter and injected (200 μl) i.v. into B6 Ly5.2 recipients. These chimeric mice were then anesthetized by isofluorane inhalation and infected i.n. 24 h later with 104 plaque-forming units (pfu) of an engineered HKx31 virus expressing the OVA257–264 peptide within the NA stalk (HKx31-OVA) of influenza virus (19). Spleens and MLNs were obtained after another 3.5, 8, or 28 d for either phenotypic analysis or isolation of CD62Llo and CD62Lhi CD44hiCD8+ T cell subsets for transfer studies.
Phenotypic Characterization of CD62Lhi and CD62Llo OT-I+CD8+ T Cells on Day 3.5 After HKx31 Infection.
Cells from spleens and MLN were stained with conjugated mAbs to murine CD62L (APC), CD8α (PerCP-Cy5.5) and Ly5.1/CD45.1 (PE) for 30 min at 4°C, washed, and analyzed on a FACScalibur. Other combinations were analyzed on LSRII after staining for CD62L-APC, CD8α-APC-Cy7, IL-7R-PE (CD45.1-PE or CD25-PE) and biotinylated CD45.1 (CD44 or NKG2a), followed by two washes and SA-PE-Cy7.
Isolation of CD62Llo and CD62Lhi CD8+ T Cell Subsets, Adoptive Transfer, and Recall Responses.
On days 3.5, 8, and 28 after HK-OVA infection, enriched lymphocyte populations were stained with CD62L-APC, CD8α-APC-Cy7, CD45.1-PE and biotinylated CD44 (Pharmingen, San Diego, CA) mAbs for 30 min on ice in sort buffer (0.1% BSA in PBS). After two washes, cells were stained with SA-PE-Cy7 (Pharmingen) for 30 min on ice, washed twice, and transferred to polypropylene FACS tubes (BD Labware, Franklin Lakes, NJ) for sorting. Lymphocytes were sorted into dividing CD62Llo or dividing CD62Lhi CD44hiCD8+ OT-I cells from spleens and MLN separately. Sorted CD62Llo or CD62Lhi T cell subsets were adoptively transferred into naïve B6 mice at 1 × 104 cells per mouse and left for 32–40 d. At this time, mice were either killed and analyzed for survival of OT-I CD8+ T cells into memory or infected with HK-OVA. Recall responses were assessed on day 8 after infection by ICS (19) or staining with CD45.1-PE, CD62L-APC, CD8-PerCP-Cy5.5 mAbs. In selected experiments, cells were sorted for dividing CD25hi or CD25lo CD44hiCD8+ OT-I cells.
Supplementary Material
Acknowledgments
We thank Nicole La Gruta, Paul Thomas, Justine Mintern, and Carole Guillonneau for review of the manuscript; Ken Field for FACS sorting; Dina Stockwell for technical assistance; and Frank Carbone (Department of Microbiology and Immunology, University of Melbourne) for OT-I mice. This work was funded by a Burnet Award of the Australian National Health and Medical Research Council (NHMRC) (to P.C.D.). K.K. is an NHMRC R. D. Wright Fellow and S.J.T. is a Pfizer Senior Research Fellow. M.R.J. was a recipient of an Australian Postgraduate Award.
Abbreviations
- H
viral hemagglutinin molecule
- N or NA
viral neuraminidase
- i.n.
intranasally
- HKx31-OVA257
HKx31 virus expressing OVA257–264 peptide within the NA stalk
- BAL
bronchoalveolar lavage.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703699104/DC1.
References
- 1.Kaech SM, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac VP, Porter BB, Harty JT. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature. 1999;401:708–712. [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Marzo AL, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 6.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 7.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, Von Andrian UH, Ahmed R. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 8.Roberts AD, Ely KH, Woodland DL. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanzavecchia A, Sallusto F. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia A, Sallusto F. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 12.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas P, Keating R, Hulse-Post D, Doherty PC. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty PC, Turner SJ, Webby RG, Thomas PG. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 15.Kedzierska K, Venturi V, Field K, Davenport MP, Turner SJ, Doherty PC. Proc Natl Acad Sci USA. 2006;103:9184–9189. doi: 10.1073/pnas.0603289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Gruta NL, Turner SJ, Doherty PC. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 17.Kedzierska K, La Gruta NL, Davenport MP, Turner SJ, Doherty PC. Proc Natl Acad Sci USA. 2005;102:11432–11437. doi: 10.1073/pnas.0504851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MR, Webby R, Doherty PC, Turner SJ. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- 20.Turner SJ, Diaz G, Cross R, Doherty PC. Immunity. 2003;18:549–559. doi: 10.1016/s1074-7613(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 21.Kedzierska K, Turner SJ, Doherty PC. Proc Natl Acad Sci USA. 2004;101:4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gett AV, Hodgkin PD. Proc Natl Acad Sci USA. 1998;95:9488–9493. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams MA, Tyznik AJ, Bevan MJ. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. J Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- 25.Badovinac VP, Harty JT. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 26.Kedzierska K, La Gruta NL, Turner SJ, Doherty PC. Immunol Rev. 2006;211:133–145. doi: 10.1111/j.0105-2896.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 28.Legge KL, Braciale TJ. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 29.Flynn KJ, Riberdy JM, Christensen JP, Altman JD, Doherty PC. Proc Natl Acad Sci USA. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prlic M, Lefrancois L, Jameson SC. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.