Abstract
Background
Opisthorchis felineus, O. viverrini, and Clonorchis sinensis (family Opisthorchiidae) are parasitic flatworms that pose a serious threat to humans in some countries and cause opisthorchiasis/clonorchiasis. Chronic disease may lead to a risk of carcinogenesis in the biliary ducts. MicroRNAs (miRNAs) are small noncoding RNAs that control gene expression at post-transcriptional level and are implicated in the regulation of various cellular processes during the parasite- host interplay. However, to date, the miRNAs of opisthorchiid flukes, in particular those essential for maintaining their complex biology and parasitic mode of existence, have not been satisfactorily described.
Methodology/Principal Findings
Using a SOLiD deep sequencing-bioinformatic approach, we identified 43 novel and 18 conserved miRNAs for O. felineus (miracidia, metacercariae and adult worms), 20 novel and 16 conserved miRNAs for O. viverrini (adult worms), and 33 novel and 18 conserved miRNAs for C. sinensis (adult worms). The analysis of the data revealed differences in the expression level of conserved miRNAs among the three species and among three the developmental stages of O. felineus. Analysis of miRNA genes revealed two gene clusters, one cluster-like region and one intronic miRNA in the genome. The presence and structure of the two gene clusters were validated using a PCR-based approach in the three flukes.
Conclusions
This study represents a comprehensive description of miRNAs in three members of the family Opistorchiidae, significantly expands our knowledge of miRNAs in multicellular parasites and provides a basis for understanding the structural and functional evolution of miRNAs in these metazoan parasites. Results of this study also provides novel resources for deeper understanding the complex parasite biology, for further research on the pathogenesis and molecular events of disease induced by the liver flukes. The present data may also facilitate the development of novel approaches for the prevention and treatment of opisthorchiasis/clonorchiasis.
Author Summary
Liver flukes of the family Opisthorchiidae cause diseases of the hepatobiliary system, known as opisthorchiasis/clonorchiasis. The chronic forms of these diseases greatly increase the risk of cancer developing in the biliary ducts. Much has been elucidated regarding the developmental biology of opisthorchiid flukes and the molecular pathological effects on the definitive host; however, the role of microRNAs (short non-coding RNAs) capable of influencing the pathogenic process and host-parasite interactions have not yet been comprehensively studied. The aim of the present work was to identify the miRNA genes of the liver flukes and provide a basis for further investigating the roles of these miRNAs in the complex opisthorchiidae life cycle and the pathogenesis of disease.
Introduction
Opisthorchis felineus, O. viverrini, and Clonorchis sinensis (class Trematoda; order Plagiorchiida; family Opisthorchiidae) are parasitic flatworms with complex life cycles, which include three hosts, with human and piscivorous mammals as definitive hosts [1]. These three flukes cause diseases of the hepatobiliary system, referred to as opisthorchiasis/clonorchiasis. These diseases are characterized by chronicity and severe consequences, some of which are cancers of the biliary tract and liver [2–5]. C. sinensis is endemic in China, Taiwan, Vietnam, Korea, Japan, the Lao People's Democratic Republic and the Russian Far East; O. viverrini is found in Cambodia, the Lao People's Democratic Republic, Thailand, and Vietnam; and O. felineus is spread in the former Soviet Union (Ukraine, Belarus, Kazakhstan, the Baltic Republics and Russia, particularly Western Siberia) and some European countries [6,7].
Recently, many studies focusing on the developmental biology of the opisthorchiid flukes and the molecular mechanism of their pathological effects on host organisms were conducted using advanced genomic and transcriptomic techniques. For example, protein-coding transcriptomes have been well characterized for O. felineus [8], O. viverrini [9,10] and C. sinensis [9,11,12], allowing investigations of diverse issues of the host-parasite interaction at the molecular and cellular levels as well as indicating the diagnostic potential of particular proteins from the excretory secretory products (ESP) of the flukes. However, the microRNA-containing transcriptomes, which are known to dramatically influence many protein patterns, have not been comprehensively studied to date in opisthorchiid flukes.
It is well known that microRNAs (18–22 nucleotide, non-coding RNAs) are able to down-regulate target mRNA expression at the post-transcriptional level in multicellular animals and thus play important roles in many biological processes including development, differentiation, viral defense and apoptosis [13]. A miRNA becomes mature after processing of its stem-loop precursors by RNase III enzymes with short miRNA duplex generation. In addition, miRNA becomes functionally active upon detachment from its complement (miRNA*) in the duplex during integration into RNA-induced silencing complexes (RISC) [13,14]. Both the miRNA and the miRNA* are potentially functional in the RISC [15–18]; however, only one miRNA remains functional, and the other degrades [19–21]. The RISC-containing miRNA induces translational repression or the degradation of the target mRNA by binding to its 3’-UTR [14,22].
Increasing evidence shows that the action of miRNAs has great importance and broad roles in pathogen-host interactions and the regulation of immunity against infectious agents [23]. Recently, miRNAs have been detected circulating outside of cells in the serum within exosomes or in association with specific proteins [24]. These extracellular RNAs are stable in bodily fluids [24] and are involved in cell-to-cell communication [25,26]. Therefore, they have attracted attention as biomarkers of disease [26,27]. Moreover, parasite-derived miRNAs have recently been identified in the serum of hosts infected with Schistosoma mansoni [28] and in exosome-like vesicles in the ESP (Dicrocoelium dendriticum) [29]. MiRNA manipulation in parasites has been also proposed as a new strategy for controlling schistosomiasis and cystic echinococcosis [23]. Parasite miRNA studies have thus become promising for elucidating the molecular mechanisms of parasitic diseases and for the development of more specific diagnostic tools [30].
In the last decade, numerous miRNAs have been discovered in several flatworms species, such as Schmidtea mediterranea [31–33], Dugesia japonica [17,34], Orientobilharzia turkestanicum [35], S. mansoni [36,37], S. japonicum [38–40], C. sinensis [41], Eurytrema pancreaticum [42], Echinococcus granulosus, E. multilocularis [43], Fasciola gigantica, F. hepatica [44], D. dendriticum [29], Hymenolepis microstoma [45], Taenia saginata [46] and Gyrodactylus salaris [47]. Most of the miRNAs of E. granulosus, E. multilocularis, S. japonicum, S. mansoni and S. mediterranea have been described and are well annotated in miRBase (Release 21: June 2014). All proteins necessary for miRNA maturation and miRNA-induced silencing were identified in several flatworms species, for example, in S. mansoni [48]. The set of orthologous proteins were also found in opisthorchiid species [10,12,49]. So the description of miRNA transcriptomes of opisthorchiids is necessary for understanding gene expression and function in these parasites.
The aims of the present study were to identify the miRNAs of O. felineus, O. viverrini and C. sinensis, describe respective miRNA genes and provide a basis for further investigations of the roles of miRNAs in the regulation of gene expression in liver flukes.
Materials and Methods
Adult worms of C. sinensis, O. felineus, and O. viverrini, as well as O. felineus metacercariae were taken for five RNA sample preparations. The first sample was prepared from adults of C. sinensis (14 flukes) that had been grown in rats (Rattus norvegicus) from metacercariae harvested from naturally infected Amur bitterling (Rhodeus sericeus) from the Bolshaya Ussurka river (Primorsky Krai, Russian Far East). The second sample was prepared from adults of O. viverrini (20 flukes) that were grown in golden hamsters (Mesocricetus auratus) from metacercariae extracted from naturally infected cyprinoid fish captured in Khon Kaen province (Thailand). The third and fourth samples were prepared from adults of O. felineus (20 flukes) that were grown in golden hamsters from metacercariae harvested from naturally infected ides (Leuciscus idus) from the Ob’ river (Novosibirsk city). The two O. felineus samples were Adult+Eggs—the manually dissected body portion with distal branches of the uterus filled with the eggs containing embryos (miracidia), and AdultNoEggs—the remaining body portion. The fifth sample (further as metacercariae) was prepared from 5000 O. felineus metacercariae from the same source.
The territories where sample collection (fishing) took place were neither conservation areas nor private or otherwise protected areas; hence, no fishing permits were required. The fish species collected are not considered endangered or rare, and fishing methods were in full compliance with the Federal Law N166-F3 of 20.12.2004 (ed. 18.07.2011) "Fishing and conservation of water bio-resources”. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the animal ethics committee of the Institute of Cytology and Genetics (Permit Number: 7 of 19.11.2011). Euthanasia was performed by decapitation, and all efforts were made to minimize suffering.
RNA preparation
For the detection of small RNAs of the three opisthorchiids, an enrichment technique consisting of the selective fractionation of RNA (18–200 nt) in polyethylene glycol solutions of various concentrations was used as described by Wang et al. [50]. The size distribution of the RNA molecules was analyzed by micro-electrophoresis with a BioAnalyzer (Agilent).
SOLiD sequencing
The miRNA libraries were constructed using an Ambion® SOLiD Small RNA Expression Kit. For each sample, three libraries (technical replicates) were sequenced: two with Adaptor Mix A (yields the template for SOLiD sequencing from the 5' end of the sense strand) and one with Adaptor Mix B (yields the reverse complement sequence).
The cDNA libraries were produced using 200 ng of the small RNA fraction, following the protocol supplied with the kit, and amplified using barcoded primers and 17 PCR cycles for Mix A libraries and 15 PCR cycles for Mix B libraries. Amplified products were concentrated using the Fermentas® GeneJET PCR Purification Kit and gel purified using 6% acrylamide gels. Gel pieces containing PCR products of ~105–150 bp were excised, libraries were eluted by 5M ammonium acetate and cleaned by ethanol precipitation. Each library was diluted to a concentration of 0.5 pM for full-scale template bead preparation. Approximately 40 million beads for each sample were deposited on ¼ slide of the SOLiD 3.5 System and sequenced in 35-base runs. Sequencing was performed at the Siberian Branch of Russian Academy of Science (SB RAS) Genomics Core Facility. The library designations with corresponding GenBank database accession numbers are:
C. sinensis—A1 (SRX817942), rA1 (SRX817990), B1 (SRX817989)
O. viverrini—A2 (SRX817991), rA2 (SRX817993), B2 (SRX817992)
- O. felineus
- AdultNoEggs—A3 (SRX817994), rA3 (SRX817996), B3 (SRX817995)
- Metacercaria—A4 (SRX817997), rA4 (SRX817999), B4 (SRX817998)
- Adult+Eggs—A5 (SRX818000), rA5 (SRX818002), B5 (SRX818001)
Computational analysis
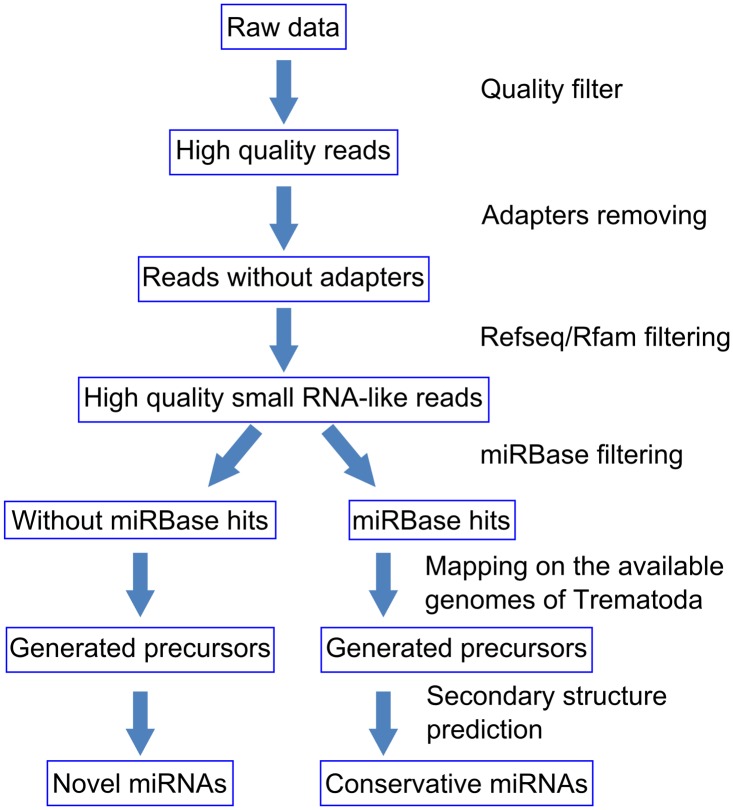
The pipeline of the computational search for conserved and novel miRNAs in the opisthorchiid species is presented in Fig 1. First, quality filtering of the sequences was performed using the SOLiD preprocess filter [51] using the following parameters: Min count for Polyclonal Analysis—1, Min QV for Polyclonal Analysis—25, Max count permitted errors—100, Max QV to consider an error—10, Removal of reads with negative QV score—y, and Truncation—off. The adapter fragments were removed by cutadapt v. 0.9.5 [52] with a maximum error rate of 12.0% and a minimum read length of 18 bp.
Fig 1. Computational pipeline for analyzing small RNA sequencing data.
To remove possible fragments of messenger and non-microRNA sequences, we mapped the reads to mRNA sequences in Refseq (rel. 106) [53], mRNA sequences of plathyhelmints and nematode taxa from the GenBank database (December, 2011) [54], and sequences from Rfam (rel. 10), [55] excluding miRNAs using BFAST [56]. The BFAST program was chosen, since it allows the mapping of short reads and uses the Smith-Waterman method, with gaps to support the detection of small indels at its final processing stage. This step improves the sensitivity of alignment, which, in our case, is important for mapping reads to genomes from different species. A significant advantage of this approach is that the alignment of sequences in the SOLiD 2-base color coding reduces the influence of sequencing errors. The following BFAST parameters were used: editing distance (the number of substitutions/insertions/deletions allowed in read alignment) ≤ 2, multiple mapping of reads was allowed, and other parameters were set as default. All reads mapped to these databases were removed from further analysis.
To identify conserved miRNAs, the remaining reads were mapped to animal pre-miRNA sequences in miRBase (Release 21: June 2014) [57] using BFAST with the following parameters: editing distance ≤ 4, multiple mapping of read was allowed, and other parameters were set as default. To identify genome-specific sequences of known miRNAs, we performed additional mapping of reads similar to miRBase sequences onto C. sinensis [58], S. mansoni (rel. 4) [59] and S. japonicum (rel. 2) [60] genomes with editing distances ≤ 2. To verify that these sequences can form pre-miRNA hairpins within their genomic context, the secondary structures of these candidate pre-miRNAs were reconstructed using the UNAFold program [61]. Two variants of the candidate pre-miRNA sequences were selected. The first variant spans from 50 bp upstream to 10 bp downstream of the miRNA region. The second variant spans from 10 bp upstream to 50 bp downstream of the miRNA region. We inferred miRNA sequences that met the following criteria: (1) ΔG ≤ -20 kcal / mol; (2) the fraction of paired nucleotides in the hairpin corresponding to the mature miRNA is > 70%; (3) no branching interactions for the hairpin forming nucleotides are allowed; (4) the sequence of miRNA is not in the terminal loop; and (5) the difference in the side lengths of internal loops and bulge size is not more than two nucleotides [62,63].
To identify novel miRNAs, the small RNA-like reads without similarity to sequences in miRBase were mapped to the genomic sequences of C. sinensis, S. mansoni, and S. japonicum. RepeatMasker (http://www.repeatmasker.org/) was used to mask repeats and regions with low complexity in the genomes. We used BFAST with an editing distance of ≤ 2, filtered out multiple mapped reads, and other parameters were as default. Genomic regions with lengths of ≤ 25 bp that were covered by at least three reads were considered as candidates for novel species-specific miRNAs. To verify the stem-loop pre-miRNA secondary structure of these sequences, we applied UNAFold analysis for their two extended sequence variants. The sequences meeting the above mentioned secondary structure criteria were considered as novel miRNA candidates.
To estimate reproducibility of technical replicates, the Spearman's rank correlation coefficients of normalized (RPKM) expression level of several conserved miRNAs (that are common for three flukes) were established using Past3 [64] (S1 Table). Conserved miRNAs were used in reproducibility analysis because new miRNAs have low non-normalized expression levels (around three reads were mapped to the genome for each new miRNA; therefore, the novel miRNAs were not detected in all technical replicates).
Additional similarity searches were performed using the BLAST [65]. To detect violations of one of the criteria of the conservative cluster definition (cluster of miRNAs should be a group of miRNA precursors expressed as a polycistronic unit [66]) we applied the protein coding gene-finding procedure using the Fgenesh program [67].
The alignments of some miRNAs (two miR-71/ miR-2 clusters, miR-1, miR-133, and miR-190) with sequences of these miRNAs orthologs (obtained from S. mediterranea, G. salaris, S. mansoni, S. japonicum, E. granulosus, E. multilocularis, H. microstoma and T. solium genomes) were performed using the program CLUSTALW [68]; miRNA sequences of T. solium, namely miR-1, miR-2b, miR-2c, miR-71, miR-133, miR-190, were obtained by homology search of these miRNAs in T. solium genome (http://www.genedb.org/Homepage/Tsolium) using the BLAST [65]. All time-consuming computations were performed using a high-throughput computing system at the Joint Access Center for Bioinformatics and a computational cluster at the Novosibirsk State University.
Genomic region PCR amplification and sequencing
The following primers were used for the amplification of genomic regions hosting the miRNA genes of the three opisthorchiid species: clust1-for1 (5'-CACAGCCAGTATTGATGAAC-3'), clust1-for2 (5'-ACAGCCCTGCTTGGGACAC-3'), clust1-rev (5'-CCAAAGCTTGGACTGTGAT-3'), clust2-for (5'-AAAGACTTGAGTAGTGAGACGCT-3'), clust2-rev (5'-TCGTCACCTAAGCAGGACT-3'), Cl1-F (5'-CGCAAGTGATCAATGTTTTCCTC-3') and Cl1-R (5'-GCGCACCAACGGCCTAA-3'). The amplification was conducted using a DNA thermal cycler (Mastercycler gradient Eppendorf) as follows: initial denaturation at 95°C for 2 min, followed by 35 amplification cycles (95°C for 25 s, 56°C for reactions with clust1-rev, clust1-for1, clust1-for2, Cl1-F and Cl2-R and 53°C for reactions with clust2-for and clust2-rev for 30 s, 72°C for 30 s) and a final extension cycle (72°C for 5 min). PCR products were analyzed by agarose gel (2%) electrophoresis. Purification of PCR products was performed by the method of Exo-TsAP. To 20 μl of PCR product were added 1 μl of Exonuclease I and 1 μl of Thermosensitive Alkaline Phosphatase, followed by an incubation for 15 min at 37°C and then 15 min at 80°C. Sequencing reactions were performed using the BigDye ® Terminator v3.1 Cycle Sequencing Kit according to the manufacturer's instructions and analyzed at the SB RAS Genomics Core Facility.
Results
Computational identification of miRNAs
A three-step mapping and filtering procedure was applied to the reads (Fig 1) generated from the 15 libraries to obtain the pool of small RNA-like sequences for the three opisthorchiid species. The results of filtering are given in S2 Table. For O. felineus, the sequencing of nine libraries generated 446 million reads that were distributed as follows: 131 millions for three Adult+Eggs libraries, 152 millions for three AdultNoEggs libraries, and 162 millions for three Metacercaria libraries. For C.sinensis, three libraries were sequenced and 126 million reads were obtained. For O. viverrini, three libraries were sequenced with 150 million reads obtained. After filtering low quality tags, including 5′ and 3′ adaptors and adaptor-adaptor ligation products, a total of 279 million reads with high quality were retained for O. felineus (Adult+Eggs (84 million reads), AdultNoEggs (108 million reads), Metacercaria (87 million reads)), 75 million reads for C. sinensis, and 83 million for O. viverrini. Among the clean reads, an average of 13.6% were found to be rRNA, tRNA, snRNA, and snoRNA, when searched against the Refseq/Rfam databases. The percentage of the remaining reads mapping to miRBase sequences averaged 2.85%. Spearman's rank correlation coefficient analysis showed high reproducibility between A and rA libraries (~ 0.9) and somewhat less reproducibility between B and either A or rA libraries (~ 0.8), which might be explained by the fact that rA libraries were exact technical replicates of A libraries whereas B libraries were created using another adaptor.
The miRNA was regarded as conserved if it had an ortholog in another animal species. The ortholog search for the miRNAs of the three opisthorchiids yielded 19 conserved miRNAs belonging to 13 families (bantam, let-7, miR-1, miR-2, mir-7, miR-10, miR-36, miR-46, miR-71, miR-124, miR-125, miR-133, and miR-190) (Fig 2A, Table 1, S3 Table). Most families included one miRNA variant, but the miR-71 family consisted of two variants and the miR-2 family comprised five variants. Interestingly, the expression of miRNA* from the duplex carrier strands for two miRNAs (let-7 and miR-10) was also found (S3 Table).
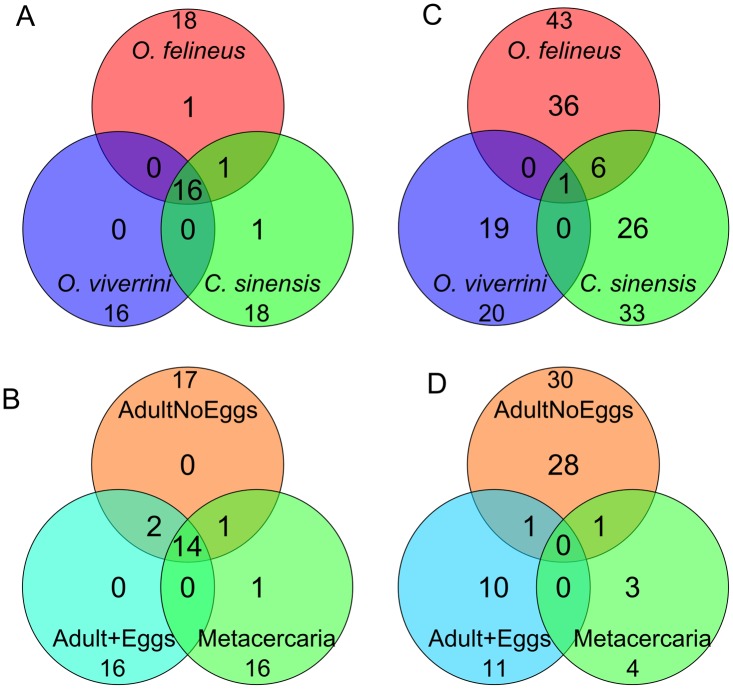
Fig 2. Venn diagrams of the miRNAs sets.
(A) conserved miRNAs in three opisthorchiid species, (B) O. felineus conserved miRNAs at different developmental stages, (C) novel miRNAs in the three opisthorchiid species, (D) O. felineus novel miRNAs at different developmental stages.
Table 1. List of conserved miRNAs identified in three Opisthorchiidae species.
| Species | miRNAs |
|---|---|
| O. felineus, C. sinensis and O. viverrini | bantam, let-7, miR-1, miR-2(a,b,c,d,e), miR-7, miR-36(a), miR-71(a,b), miR-124, miR-125, miR-133, miR-190 |
| O. felineus and C. sinensis | miR-281 (miR-46 family) |
| O. felineus | miR-10 |
| C. sinensis | miR-36b |
Sixteen conserved miRNAs were identified as common in all three opisthorchiids. Additionally, miR-281 (miR-46 family) was found in two species—O. felineus and C. sinensis. There were also conserved miRNAs either in O. felineus only (miR-10) or in C. sinensis only (miR-36b) (Fig 2A; Table 1).
Eighteen conserved miRNAs were identified for O. felineus when combining the Adult+Eggs, AdultNoEggs, and Metacercaria samples. Individual analyses of the O. felineus samples (Adult+Eggs, AdultNoEggs, Metacercaria) revealed differences in miRNA composition between the samples. Fourteen of the eighteen O. felineus miRNAs were identified in all three samples. Two miRNAs (bantam and miR-281) were identified in AdultNoEggs and Adult+Eggs samples only, but not in the Metacercaria sample. miR-7 was detected in AdultNoEggs and Metacercaria samples but not in the Adult+Eggs sample, and miR-10 was found in the Metacercaria samples only (Fig 2B, Table 2). The mapping results demonstrated that most of the conserved miRNA sequences identified in the present study are common among opisthorchiid and schistosome species, which was expected.
Table 2. List of conserved miRNAs identified in different samples of O. felineus.
| Developmental stage of O. felineus | miRNAs |
|---|---|
| AdultNoEggs & Adult+Eggs & Metacercaria | let-7, miR-1, miR-2(a,b,c,d,e), miR-36, miR-71(a,b), miR-124, miR-125, miR-133, miR-190 |
| AdultNoEggs & Adult+Eggs | bantam, miR-281(miR-46 family) |
| AdultNoEggs & Metacercaria | miR-7 |
| Metacercaria | miR-10 |
Candidate sequences for novel miRNAs (S4 Table) were selected from reads without matches to miRBase sequences after mapping them to the C. sinensis genome and processing the genomic fragments encompassing the resultant hits through the secondary structure filter (see Materials and Methods). We identified 43 such miRNAs for O. felineus, 20 for O. viverrini and 33 for C. sinensis. The occurrence of novel common and species-specific miRNAs in the samples from the three Opisthorchiidae species is presented in Fig 2C and S4 Table.
Interestingly, most of these novel miRNAs were species-specific. Only one miRNA (new_miR-001) had orthologs in all three species. The greatest number of novel specific miRNA candidates was identified for O. felineus (83%); however, the fraction of unique species-specific miRNAs was highest for O. viverrini (95%).
Forty-three novel miRNAs were obtained for O. felineus when combining the Adult+Eggs, AdultNoEggs and Metacercaria samples. The distribution of stage-specific and stage-nonspecific novel miRNA candidates in O. felineus demonstrated that no common miRNAs were identified in all three sample types, and only two of the 43 novel miRNAs were identified in more than one stage/body part (Fig 2D).
Genomic organization of opisthorchiid miRNA genes
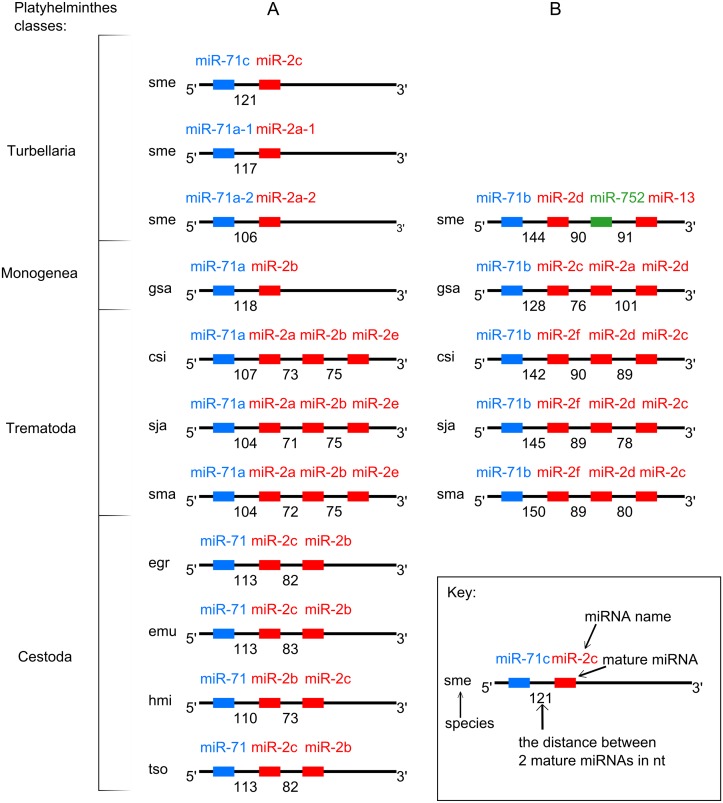
Mapping the conserved miRNAs onto the C. sinensis genome provided evidence supporting the presence of two miRNA clusters: miR-71a/miR-2a/miR-2b/miR-2e (miR-71a/2) and miR-71b/miR-2d/miR-2c (miR-71b/2). Homologous clusters have been previously described for seven flatworms (E. granulosus, E. multilocularis, G. salaris, H. microstoma, S. mediterranea, S. japonicum and S. mansoni) [45,47], and in the current study, were also found in the T. solium genomic sequences (Fig 3, S1 Appendix). We compared the structures of these miRNA clusters from the C. sinensis genome with homologous sequences from the flatworm genomes mentioned (Fig 3).
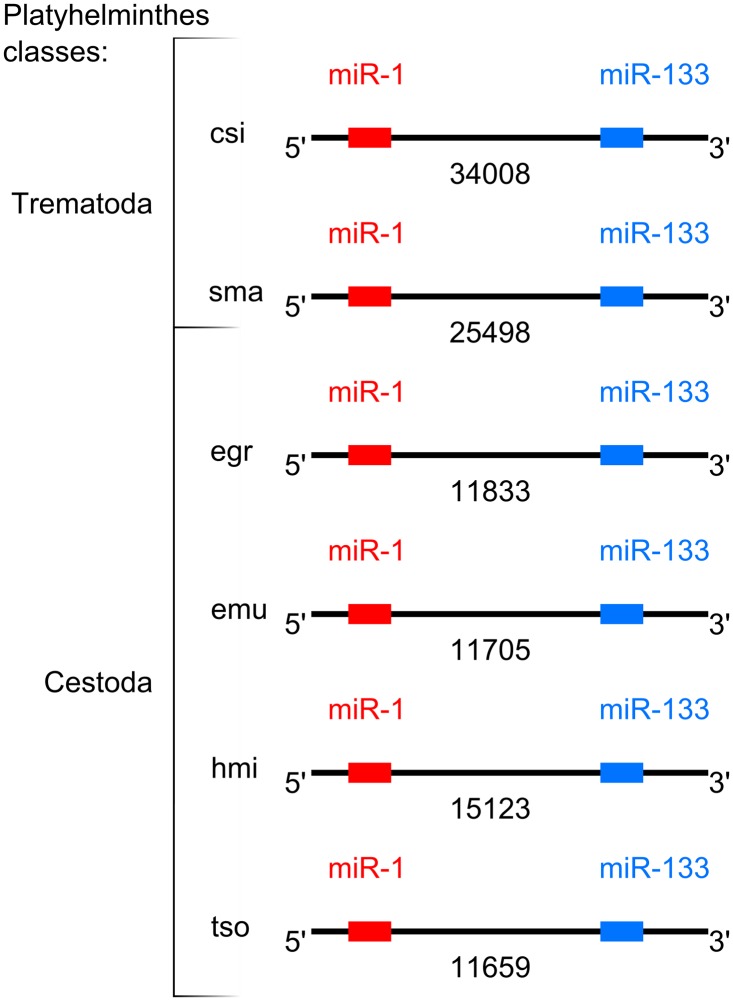
Fig 3. Scheme of miRNA gene clusters in Platyhelminthes.
(A) miR-71a/2 cluster group, (B) miR-71b/2 cluster group. Species designations: csi—C. sinensis, sma—S. mansoni, sja—S. japonicum, sme—S. mediterranea, hmi—H. microstoma, egr—E. granulosus, emu—E. multilocularis, gsa- G. salaris.
All mature miRNA sequences of miR-71 family were located in the 5’ arm of their precursors, while all mature miRNAs of the miR-2 family were located in the 3’ arm [69]. In the miR-71a/2 cluster group, the distance between the mature miR-71 and the nearest miR-2 varied from 104 to 121 bp; in the miR-71b/2 cluster group, this distance ranged from 121 to 150 bp. The minimal distance between the two mature sequences of miR-2 family was found in the miR-71a/2 group (71 bp between miR-2a and miR-2b in S. japonicum); the maximal distance was found in the miR-71b/2 group (101 bp between miR-2a and miR-2d in G. salaris) [45,47].
The miR-71a/2 cluster mapped to C. sinensis contig 2339 and spanned 441 nucleotides (from 103849 to 104290), with the miRNA order the same as in both Schistosoma genomes. A comparative analysis of the miR-71a/2 cluster genomic organization among the flatworms revealed three distinct types (Fig 3A). The first type, comprising the precursors for miR-71a and the three miR-2 isoforms, was exemplified by clusters from the genomes of C. sinensis, S. japonicum, and S. mansoni. The second type, consisting of the precursors for miR-71 and the two miR-2 isoforms, was represented in the genomes of the cestodes H. microstoma, E. granulosus and E. multilocularis. The third type, with the precursors for miR-71 and only one miR-2 isoform, was observed in the monogenean G. salaris and the planarian S. meditteranea (in three genomic copies) [45,47].
The miR-71b/2 cluster mapped to C. sinensis contig 2957 and spans 416 nucleotides (from 323569 to 323984). Detailed analysis of the cluster sequences in three trematode, one monogenean and one turbellarian genome resulted in the discovery of the precursor for miR-2f in C. sinensis (Fig 3B, S5 Table). This miRNA was previously described for two schistosomes [70,71]. The miRNA order in these orthologous clusters was also well conserved in the C. sinensis, S. japonicum and S. mansoni genomes (Fig 3B). It should be noted that sme-miR-752, although not formally assigned to the mir-2 family, is recognized as having evolved from miR-2 [47].
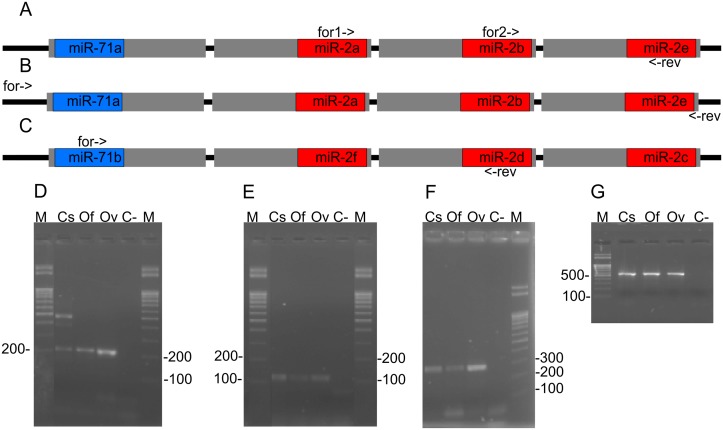
Experimental verification of the miRNA clusters miR-71a/miR-2a/miR-2b/miR-2e and miR-71b/miR-2f/miR-2d/miR-2c
Because the mature miRNA sequences of the two clusters were identical among the three opisthorchiid species, we designed two primer sets to experimentally prove the presence of the clusters and partially structure the clusters using PCR amplification of corresponding regions in the three genomes. To amplify a fragment of cluster miR-71a/2, the primer set clust1-for1, clust1-for2, clust1-rev was used (Fig 4A, S2 Appendix). For the miR-71b/2 cluster, the primer set clust2-for, clust2-rev was employed (Fig 4C, S2 Appendix). The electropherogram presented in Fig 4D, 4E and 4F) show the PCR products generated using these primer sets with DNA templates prepared from C. sinensis, O. felineus and O. viverrini.
Fig 4. Upper: Scheme of primer target site positions in clusters: (A and B) miR-71a/2, (C) miR-71b/2.
Grey rectangles mean miRNA precursors, blue, purple and read rectangles—mature miRNAs; for->, for1->, for2-> and <-rev indicate primers and their directions. Lower: electrophoretogram of PCR products generated out of the cluster genomic regions. Designations: C-—negative control, M—lengths marker. DNA templates: Cs—C. sinensis, Of—O. felineus, Ov—O. viverrini. Primers used: (D) clust1-for1 and clust1-rev; (E) clust1-for2 and clust1-rev; (F) clust2-for and clust2-rev; G) Cl1-F and Cl2-r. The original electrophoretogram photographs are presented in S1 Fig.
The sequence alignments of the corresponding genomic regions of the three opisthorchid species revealed specific variable positions: 8 per 387 nucleotides in cluster miR-71a/2 and 11 per 299 nucleotides in cluster miR-71b/2 (S3 Appendix). These variable positions were located mainly in the regions corresponding to the ends of the pre-miRNA, the terminal loops of the pre-miRNA and the spacers between miRNA precursors.
It is worth noting that miR-2f from cluster miR-71b/2 of C. sinensis, S. japonicum and S. mansoni (Fig 3) was also discovered in the respective clusters of O. felineus and O. viverrini. Furthermore, the alignment of this genomic region demonstrated high conservation among the three opisthorchiid species: only four variable positions (which were located closer to the precursor of miR-2d) per 154 nucleotides were found.
To experimentally prove the overall structure of the miR-71a/2 clusters in the three species, we designed primers Cl1-F and Cl1-r, which are capable of amplifying the genomic regions encompassing the clusters, using the only available sequences for C. sinensis (Fig 4B, S1 Appendix). The results are presented in the electrophoretogram (Fig 4G).
The sequencing of the three species-specific amplicons (S4 Appendix) allowed us to determine the four pre-miRNA sequences for each of the three flukes. The secondary structures of these pre-miRNAs were estimated by UNAFold (S2 Fig). The results of UNAFold demonstrated that the nucleotide substitutions discriminating the pre-miRNA sequences of each of the opisthorchiid species exerted minor or no effects on the pre-miRNA secondary structures.
Cluster-like regions miR-1/miR-133
Upon analysis by Jin et al. [45], the genomic regions with matches for miR-1 and miR-133 were designated as orthologous miRNA gene clusters in three flatworms, namely the cestodes E. granulosus, E. multilocularis and H. microstoma.
We extended this list of flatworm species by demonstrating that C. sinensis and S. mansoni also have similar genomic regions. It should be mentioned that miR-133 were not annotated for S. mansoni in previous reports [36,37,71]. However, we found sequences highly similar to this miRNA in read archives (ERR278825, ERR278826, ERR278827, ERR278828) using a BLAST search. The UNAFold secondary structure prediction for the precursors of the conserved miRNAs showed no canonical structure for the putative S. mansoni pre-miR-133, which could possibly explain the delay in sma-miR-133 annotation (S6 Table).
Our alignment analysis did not show complete conservation over these regions of the five genomes. Remarkably, large spacers were detected between the sites matching the miRNAs, ranging from 11705 bp in E. multilocularis to 34008 bp in C. sinensis (Fig 5, S5 Appendix). Hence, we referred to the regions as “cluster-like regions miR-1/miR-133”.
Fig 5. Genomic organization scheme of cluster-like regions miR-1/miR-133 in five flatworms.
Designations are the same as in Fig 3.
To elucidate the content of the spacers in genomes of five parasitic flatworms, we employed the gene prediction program Fgenesh [67] using S. mansoni-specific gene-finding parameters and found few unannotated ORFs without significant similarity among the species (S6 Appendix). We then explored the genomic context beyond the cluster-like regions miR-1/miR-133 in the five flatworm species using information from the C. sinensis database (http://fluke.sysu.edu.cn/CsinGeno/home.php), NCBI (http://www.ncbi.nlm.nih.gov) (for S. mansoni) and Genedb (http://www.genedb.org/Homepage) (for E. granulosus, E. multilocularis and H. microstoma). We found that miR-133 is located near a gene encoding one of several Mind bomb proteins in all five genomes. We also found that miR-1 mapped near a gene encoding another Mind bomb protein in the genomes of S. mansoni, E. granulosus and E. multilocularis (S7 Table).
Although these miRNA sites were conservatively linked (forming a putative synteny group), the inter-microRNA distances exceeded 10 kb and contained putative genes. Altogether, the features suggested that the expression of these two miRNAs was unlikely as a single transcriptional unit in either genome. Therefore, we concluded that the case under consideration did not adhere to the conservative definition for a miRNA gene cluster [66].
miR-190 is an intronic miRNA
The mapping of miR-190, which was also identified in the three opisthorchiid species, to the available flatworm genomic sequences showed that this miRNA is located in an intron of the gene encoding the talin protein. Therefore, we could classify this miR-190 as intronic [72]. It is noteworthy that, despite some variability in the nucleotide content of the talin exons surrounding the intronic miRNA (S7 Appendix), the overall protein structure was conserved enough (S8 Appendix) to ensure a reliable comparative analysis of the gene structure (Table 3).
Table 3. Structures of Trematoda and Cestoda talin genes with intronic miR-190.
| Species | Gene id | Gene length in bp | Exons counts | Number of intron containing miRNA | Intron length in bp |
|---|---|---|---|---|---|
| Trematoda | |||||
| C. sinensis | csin001953 | 70200 | 37 | 32nd | 2574 |
| S. japonicum | Sjp_0006570 | 139915 | 43 | 40th | 685 |
| S. mansoni | Smp_037860 | 12434 | 10 | 7th | 1237 |
| Cestoda | |||||
| E. granulosus | EgrG_000736000 | 26856 | 48 | 44th | 225 |
| E. multilocularis | EmuJ_000736000 | 26634 | 43 | 39th | 224 |
| H. microstoma | HmN_000220000 | 43376 | 41 | 37th | 356 |
| T. solium | TsM_000902500 | 27324 | 44 | 40th | 225 |
The intronic miRNA showed the motives corresponding to both mature miR-190 and miR-190*(S9 Appendix). The alignment depicted the sites with high conservation in either the flatworm class and those with evident inter-class variations, which, nevertheless, likely did not hamper the intronic miRNA’s ability to form the necessary secondary structure and effectively undergo maturation.
Discussion
Using deep sequencing with SOLiD technology, we have identified 88 novel and 19 conserved miRNAs in three liver flukes of the family Opisthorchiidae—C. sinensis, O. felineus, and O. viverrini. The discovery of the novel opisthorchid-specific miRNAs is interesting, since they could be responsible for some opisthorchid-specific features of their parasitic life style including some pathogenicity features in definitive hosts. Interestingly, the number of the novel species-specific candidate miRNAs identified in the opisthorchiid flukes was larger than that of conserved miRNAs. This may relate to a low coverage of individual novel miRNAs (three reads were mapped to the genome for each new miRNA). Nevertheless, it is worth noting that similar species-specific/conserved miRNA families ratios (miRBase Release 21: June 2014) are also observed for other trematodes—S. japonicum (28/22) and S. mansoni (82/22). The same is seen also for free living planarian S. mediterranea (45/44). The addition of our data on the miRNAs of opisthorchiid flukes likely raises the question as to whether an excess of novel (species-specific) miRNAs compared with conserved (family-, class-, and phylum-specific) miRNAs could relate to a difference in life style (free vs. parasitic), as proposed previously [36]. It seems that even a less significant difference in parasitic style between schistosomes or opisthorchiids is associated with generation of numerous family-, genus-, and species-specific miRNAs. More additional data for flatworms’ miRNAs are needed to elucidate the evolutionary and biological significance of species-specific miRNAs of parasitic flatworms.
The identification of 19 conserved miRNAs in three liver flukes of the family Opisthorchiidae has strengthened the results of previous attempts to explore miRNAs of liver flukes. It should be noted that, in a previous study of C. sinensis, numerous miRNA-like sequences were found among reads generated with high-throughput sequencing using Solexa/Illumina technology [41]. However, the authors had no opportunity to carry out mapping the miRNA-like sequences on to the C. sinensis genome to achieve a confident assignment of their miRNA-like sequence sets to miRNA families annotated in miRBase. Therefore, we now provide the results of miRNA-like sequence mapping on to the C. sinensis genome, thus improving the reliability of miRNA identification for members of the Opisthorchiidae. Furthermore, we provide the results of the miRNA family classification. The occurrence of the 19 conserved miRNAs in organisms of various taxa (including 10 miRNAs out of 34 ones arisen after “bilaterian expansion” [72]) is presented (S8 Table).
We should mention two curious C. sinensis miRNAs: the reads corresponding to csi-miR-36b were found in our study but were not found by Xu et al. [41], and the reads corresponding to miR-10 as indicated by Xu et al. [41] were readily mapped in the C. sinensis genome, but were detected in our study for O. felineus only. Perhaps these cases need further investigation.
The mapping of 19 conserved miRNAs on to the three genomes available for Trematoda (C. sinensis, S. mansoni and S. japonicum) are presented in Table 4. Interestingly, there was some shortfall in hits for few miRNAs after the mapping of sequencing data. This could be due to the incompleteness of either genome assembly (miR-125 was not found in C. sinensis genome) or indeed by the species specificity of miRNA genes (we did not find the opisthorchid miR-1 in S. japonicum genome, we also did not locate opisthorchid miR-36b in either schistosome genome).
Table 4. Results of conservative miRNAs mapping onto Trematoda genomes.
| miRNA | Genomes | ||
|---|---|---|---|
| C. sinensis | S. mansoni | S. japonicum | |
| bantam | + | + | + |
| let-7 | + | + | + |
| miR-1 | + | + | − |
| miR-2a | + | + | + |
| miR-2b | + | + | + |
| miR-2c | + | + | + |
| miR-2d | + | + | + |
| miR-2e | + | + | + |
| miR-7 | + | + | + |
| miR-10 | + | + | + |
| miR-36a | + | + | + |
| miR-36b | + | − | − |
| miR-281 | + | + | + |
| miR-71a | + | + | + |
| miR-71b | + | + | + |
| miR-124 | + | + | + |
| miR-125 | − | + | + |
| miR-133 | + | + | + |
| miR-190 | + | + | + |
Mapped miRNA is designated by plus; unmapped—by minus.
Our analysis of the genomic organization of the opisthorchid miRNA genes confirmed the presence of gene clusters and intronic miRNAs. It is known that the miR-71/miR-2 cluster, which we experimentally proved to be in two copies in opisthorchiids (like in other parasitic trematodes studied) is present as one copy in parasitic cestodes, and five copies in the free-living planarian S. mediterranea [45]. This variation in the number of miR-71/miR-2 clusters in the genomes of representative flatworms of different classes could not be explained by the biology of the organism or by the reduction of targets for these miRNAs. The parasitic nematode Ascaris suum and Brugia malayi display one miR-71/miR-2 cluster, while the freeliving Caenorhabditis species have either one or no such cluster (miRBase Release 21: June 2014). Therefore, it seems that the miR-71/miR-2 cluster evolution proceeded differently in the Nematoda compared with the Platyhelminthes, and the details of the evolution remains to clarify in further studies.
Both clusters miR-71a/miR-2a/miR-2b/miR-2e and miR-71b/miR-2f/miR-2d/miR-2c were conserved, suggesting their functional importance in all three opisthorchiid species (Fig 3). To date, some miRNAs belonging to miR-71 and miR-2 families are known to have female-biased expression in S. mansoni [71] and to play an important role in regenerative processes in planarian [73]. Also the miR-2 family miRNAs are probably involved in neural development and maintenance in Drosophila melanogaster and C. elegans [69]. Their detection in exosome-like vesicles in the ESP of the liver fluke D. dendriticum leads to a speculation about the possible implications of trematode miRNAs in the modulation of parasite-host interactions by a new means of regulating host gene expression [29].
The fact that expressed sequences (reads) corresponding to miR-2f have not been detected in opisthorchiids requires further studies to explain why the expression pattern of this putative miRNA is so strikingly different from that of its neighbors.
In previous papers, the combination of the miR-1/miR-133 miRNA genes was described also as a miRNA cluster for many animal species (see data in miRBase) [74] including flatworms [45]. However, this combination in Drosophila genomes has been shown to escape the conserved cluster definition [66]. Hence, due to the distance between the sites corresponding to the miRNAs in flatworms, as well as the capability to predict protein-coding genes in between these sites, we suggest referring to these regions as “cluster-like regions miR-1/miR-133”, which form a putative synteny group.
The next miRNA cluster that should be discussed is let-7/miR-100/miR-125. Its main characters are conserved in almost all Deuterostomia taxa. However, in Protostomia, many variations of its structure have been discovered, while in some animals (Annelida, Trichinella, Arthropoda), its general structure is conserved. Important is that the cluster was shown to be disintegrated in flatworms with a complete loss of miR-100 [75]. We can support this conclusion for opisthorchiids also. First, mir-100-like sequences were not detected in the three opisthorchiid species. Second, the combination of let-7/ miR-125 genes is unlikely to exist as a synteny group, as the two miRNA genes map to different chromosomes in S. mansoni (S9 Table).
The present analysis corroborates the classification of the miR-190 gene as intronic within the talin gene. The intronic nature of the miR-190 gene has been described for many animals [36,45,76]. High conservation of the structural (and maybe functional) association between miR-190 and the talin protein in platyhelminths appears very interesting and is worthy of further elucidation.
Just prior to submission of this manuscript, an article on the O. viverrini genome was published [49]. In the article, the authors predicted in silico 178 conserved miRNA genes. These data will give us the opportunity for a more detailed analysis of O. viverrini miRNA genes, in particular for a comparison of our data based on miRNA real expression with the results of in silico prediction based on genomic sequence analysis.
In conclusion, the present study presents the results of large-scale identification and characterization of miRNAs sets encoded in the genomes of O. felineus, O. viverrini and C. sinensis. This first comprehensive comparative analysis of the miRNA genes of these species allowed us to reveal the conserved and species-specific miRNAs in these sets. For several conserved opisthorchiid miRNAs, the genomic organization was analyzed by comparison with orthologous genes in other platyhelminths. The structures of two miRNA gene clusters were experimentally validated for the three opisthorchiid species. The differences in expression level found for some conserved miRNA among the three species and among the three stages of O. felineus stimulate studies to more precisely profile the expression of miRNAs. Finally, the present data provide a sound basis for further studies of the molecular mechanisms of host interactions of opisthorchiids and for development of novel methods to control these neglected parasites.
Supporting Information
- 297 (gi|124128508|gb|AAWT01093310.1|) (- strand)
- 1403 (gi|124163860|gb|AA WT01057958.1|) (+ strand) (locus 1)
- 5146 (gi|124196975|gb|AA WT01024843.1|) (+ strand) (locus 2)
- 2151 (gi|124194162|gb|AA WT01027656.1|) (+ strand)
Cluster of C. sinensis was obtained from contig 2339 (gi|353340623|dbj|BADR02002339.1|) (- strand). Cluster of S. mansoni was obtained from chromosome W (gi|360043576|emb|HE601631.1|) (+ strand). Cluster of S. japonicum was obtained from contig S000054 (gi|227129020|emb|FN331028.1|) (- strand). Cluster of H. microstoma was obtained from contig 370001 (gi|528309775|emb|CBLW010002354.1|) (+ strand). Cluster of E. granulosus was obtained from contig 0003137 (gi|528307253|emb|CBLN010000400.1|) (- strand). Cluster of E. multilocularis was obtained from contig 007728 (gi|528775369|emb|CBLO010002090.1|) (- strand). Cluster of T. solium was obtained from contig 01703 (pathogen_TSM_contig_01703) (+ strand). Cluster of G. salaris was obtained from scaffold 7180006951238_l_32618_C_75.085_gc_34.438 (+ strand)
(PDF)
csi—C. sinensis. Secondary structure of miRNA precursors is indicated by a parentheses and dots. Mature miRNA sequences including miR-2f are in bold type and underlined.
(PDF)
sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined.
(PDF)
sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined.
(PDF)
csi—C. sinensis, sma—S. mansoni, hmi—H. microstoma, egr—E. granulosus, emu—E. multilocularis, tso—T. solium, sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined. Mature miRNA sequences are in bold type and underlined. Cluster of C. sinensis was obtained from scaffold 198 (gi|353342189|dbj|BADR02000773.1|) (- strand). Cluster of S. mansoni was obtained from chromosome W (gi|360043576|emb|HE601631.1|) (- strand). Cluster of H. microstoma was obtained from contig 880003 (gi|528308588|emb|CBLW010003541.1|) (- strand). Cluster of E. granulosus was obtained from contig 00137 (gi|528306514|emb|CBLN010001139.1|) (+ strand). Cluster of E. multilocularis was obtained from contig 007760 (gi|528775363|emb|CBLO010002096.1|) (+ strand). Cluster of T. solium was obtained from contig 00015 (pathogen_TSM_contig_00015) (+ strand).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Mature miRNAs are red. Diferensis in sequences of miRNA precursors in red circles.
(PDF)
(XLSX)
(XLS)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to Dr. V. Besprosvannykh for samples of C. sinensis larvae. We thank B. Fromm and C. Hahn for providing G. salaris genome sequences. We are grateful to Dr. E. Berezikov and Dr. L. Daniel for critical reading of the manuscript and very valuable comments and to Dr. I. Malykh for valuable advises. We are grateful to the Joint Access Center for Bioinformatics and a computational cluster at the Novosibirsk State University for providing access to high-throughput computing system to perform all time-consuming computations. We thank also the SB RAS Genomics Core Facility for sequencing processing.
Data Availability
Raw sequencinq data are available from the NCBI (accession numbers SRX817942, SRX817990, SRX817989, SRX817991, SRX817993, SRX817992, SRX817994, SRX817996, SRX817995, SRX817997, SRX817999, SRX817998, SRX818000, SRX818002, SRX81800. All other relevant data are in the manuscript and Supporting Information files.
Funding Statement
Financial support for this study was provided in part by Siberian Branch of the Russian Academy of Science Integration Project №19 (URLs of Siberian Branch of the Russian Academy of Science are http://www.sbras.ru and http://www.nsc.ru/en), State Project of ICG SB RAS VI.60.1.1, the Russian Foundation for Basic Research (RFBR # 15-04-03551a), and Project №14.B25.31.0033, Resolution No. 220 of the Government of the Russian Federation of April 9, 2010. The NGS data analysis was supported by Russian Scientific Foundation (Project No 14-24-00123). EVK, AVK, GVV, VYO, VAM and DAA received funding from State Project of ICG SB RAS VI.60.1.1. VAM and VYO received funding from Siberian Branch of the Russian Academy of Science Integration Project №19. VAM received funding from the Russian Foundation for Basic Research (RFBR # 15-04-03551a). DAA received funding from the Russian Scientific Foundation (Project No 14-24-00123). VAM, DAA, VYO, EVK and GVV received funding from Project №14.B25.31.0033, Resolution No.220 of the Government of the Russian Federation of April 9, 2010. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scholz T. Family Opisthorchiidae Looss, 1899 In: Bray RA, Gibson DI, Jones A, editors. Keys to Trematoda. Vol. 3 1st ed London: CAB International and Natural History Museum; 2008. p. 9–51. [Google Scholar]
- 2. Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008. November;24: 497–501. 10.1016/j.pt.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hong S-T, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol. Int. Elsevier Ireland Ltd; 2012. March;61: 17–24. [DOI] [PubMed] [Google Scholar]
- 4. Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007. July;4: e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sripa B, Nawa Y, Sithithaworn P, Andrews R, Brindley PJ. Discovery of human opisthorchiasis: a mysterious history. Parasitol. Int. Elsevier Ireland Ltd; 2012. March;61: 3–4. [DOI] [PubMed] [Google Scholar]
- 6. King S, Scholz T. Trematodes of the family Opisthorchiidae: a minireview. Korean J. Parasitol. 2001. September;39: 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mordvinov VA, Furman DP. The Digenea parasite Opisthorchis felineus: a target for the discovery and development of novel drugs. Infect. Disord. Drug Targets. 2010. October;10: 385–401. [DOI] [PubMed] [Google Scholar]
- 8. Pomaznoy M, Tatkov S, Katokhin A, Afonnikov D, Babenko V, Furman D, et al. Adult Opisthorchis felineus major protein fractions deduced from transcripts: comparison with liver flukes Opisthorchis viverrini and Clonorchis sinensis. Exp. Parasitol. Elsevier Inc.; 2013. October;135: 297–306. [DOI] [PubMed] [Google Scholar]
- 9. Young ND, Campbell BE, Hall RS, Jex AR, Cantacessi C, Laha T, et al. Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2010. January;4: e719 10.1371/journal.pntd.0000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jex AR, Young ND, Sripa J, Hall RS, Scheerlinck J-P, Laha T, et al. Molecular changes in Opisthorchis viverrini (Southeast Asian liver fluke) during the transition from the juvenile to the adult stage. PLoS Negl. Trop. Dis. 2012. January;6: e1916 10.1371/journal.pntd.0001916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang S-Y, Zhao G-H, Fu B-Q, Xu M-J, Wang C-R, Wu S-M, et al. Genomics and molecular genetics of Clonorchis sinensis: current status and perspectives. Parasitol. Int. Elsevier Ireland Ltd; 2012. March;61: 71–6. [DOI] [PubMed] [Google Scholar]
- 12. Huang Y, Chen W, Wang X, Liu H, Chen Y, Guo L, et al. The carcinogenic liver fluke, Clonorchis sinensis: new assembly, reannotation and analysis of the genome and characterization of tissue transcriptomes. PLoS One. 2013. January;8: e54732 10.1371/journal.pone.0054732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J. Physiol. Biochem. 2011. March;67: 129–39. 10.1007/s13105-010-0050-6 [DOI] [PubMed] [Google Scholar]
- 14. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. Nature Publishing Group; 2010. September;11: 597–610. [DOI] [PubMed] [Google Scholar]
- 15. Ghildiyal M, Xu J, Seitz H, Weng Z, Zamore PD. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA. 2010. January;16: 43–56. 10.1261/rna.1972910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu HY, Yan Z, Xu Y, Hu H, Menzel C, Zhou YH, et al. Sequence features associated with microRNA strand selection in humans and flies. BMC Genomics. 2009. January;10: 413 10.1186/1471-2164-10-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin Y-F, Zhao J-M, Bao Z-X, Zhu Z-Y, Mai J, Huang Y-B, et al. Identification of small non-coding RNAs in the planarian Dugesia japonica via deep sequencing. Genomics. 2012. May;99: 315–21. 10.1016/j.ygeno.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 18. Okamura K, Phillips MD, Tyler DM, Duan H, Chou Y, Lai EC. The regulatory activity of microRNA* species has substantial influence on microRNA and 3’ UTR evolution. Nat. Struct. Mol. Biol. 2008. April;15: 354–63. 10.1038/nsmb.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005. August 4;436: 740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawamata T, Tomari Y. Making RISC. Trends Biochem. Sci. Elsevier Ltd; 2010. July;35: 368–76. [DOI] [PubMed] [Google Scholar]
- 21. Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, et al. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002. March 15;16: 720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morozova N, Zinovyev A, Nonne N, Pritchard L, Gorban AN, Harel-bellan A. Kinetic signatures of microRNA modes of action. RNA. 2012;18: 1635–55. 10.1261/rna.032284.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manzano-Román R, Siles-Lucas M. MicroRNAs in parasitic diseases: potential for diagnosis and targeting. Mol. Biochem. Parasitol. Elsevier B.V.; 2012. December;186: 81–6. [DOI] [PubMed] [Google Scholar]
- 24. Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717: 85–90. 10.1016/j.mrfmmm.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu L, Yang B-F, Ai J. MicroRNA transport: a new way in cell communication. J. Cell. Physiol. 2013. August;228: 1713–9. 10.1002/jcp.24344 [DOI] [PubMed] [Google Scholar]
- 26. Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012. February 3;110: 483–95. 10.1161/CIRCRESAHA.111.247452 [DOI] [PubMed] [Google Scholar]
- 27. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 2008. July 29;105: 10513–8. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoy AM, Lundie RJ, Ivens A, Quintana JF, Nausch N, Forster T, et al. Parasite-derived microRNAs in host serum as novel biomarkers of helminth infection. PLoS Negl. Trop. Dis. 2014. February;8: e2701 10.1371/journal.pntd.0002701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernal D, Trelis M, Montaner S, Cantalapiedra F, Galiano A, Hackenberg M, et al. Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. J. Proteomics. Elsevier B.V.; 2014. June 13;105: 232–41. [DOI] [PubMed] [Google Scholar]
- 30. Zheng Y, Cai X, Bradley JE. microRNAs in parasites and parasite infection. RNA Biol. 2013. March;10: 371–9. 10.4161/rna.23716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedländer MR, Adamidi C, Han T, Lebedeva S, Isenbarger TA, Hirst M, et al. High-resolution profiling and discovery of planarian small RNAs. Proc. Natl. Acad. Sci. U. S. A. 2009. July 14;106: 11546–51. 10.1073/pnas.0905222106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palakodeti D, Smielewska M, Graveley BR. MicroRNAs from the Planarian Schmidtea mediterranea: a model system for stem cell biology. RNA. 2006. September;12: 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu Y-C, Smielewska M, Palakodeti D, Lovci MT, Aigner S, Yeo GW, et al. Deep sequencing identifies new and regulated microRNAs in Schmidtea mediterranea. RNA. 2009. August;15: 1483–91. 10.1261/rna.1702009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu Z, Chen M, Ren Z, Zhang N, Xu H, Liu X, et al. Deep sequencing identifies regulated small RNAs in Dugesia japonica. Mol. Biol. Rep. 2013. June;40: 4075–81. 10.1007/s11033-012-2485-z [DOI] [PubMed] [Google Scholar]
- 35. Wang C-R, Xu M-J, Fu J-H, Nisbet AJ, Chang Q-C, Zhou D-H, et al. Characterization of microRNAs from Orientobilharzia turkestanicum, a neglected blood fluke of human and animal health significance. PLoS One. 2012. January;7: e47001 10.1371/journal.pone.0047001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Souza Gomes M, Muniyappa MK, Carvalho SG, Guerra-Sá R, Spillane C. Genome-wide identification of novel microRNAs and their target genes in the human parasite Schistosoma mansoni. Genomics. Elsevier B.V.; 2011. August;98: 96–111. [DOI] [PubMed] [Google Scholar]
- 37. Simões MC, Lee J, Djikeng A, Cerqueira GC, Zerlotini A, da Silva-Pereira RA, et al. Identification of Schistosoma mansoni microRNAs. BMC Genomics. BioMed Central Ltd; 2011. January;12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hao L, Cai P, Jiang N, Wang H, Chen Q. Identification and characterization of microRNAs and endogenous siRNAs in Schistosoma japonicum. BMC Genomics. 2010. January;11: 55 10.1186/1471-2164-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang J, Hao P, Chen H, Hu W, Yan Q, Liu F, et al. Genome-wide identification of Schistosoma japonicum microRNAs using a deep-sequencing approach. PLoS One. 2009. January;4: e8206 10.1371/journal.pone.0008206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xue X, Sun J, Zhang Q, Wang Z, Huang Y, Pan W. Identification and characterization of novel microRNAs from Schistosoma japonicum. PLoS One. 2008. January;3: e4034 10.1371/journal.pone.0004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu M-J, Liu Q, Nisbet AJ, Cai X-Q, Yan C, Lin R-Q, et al. Identification and characterization of microRNAs in Clonorchis sinensis of human health significance. BMC Genomics. 2010. January;11: 521 10.1186/1471-2164-11-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu M-J, Wang C-R, Huang S-Y, Fu J-H, Zhou D-H, Chang Q-C, et al. Identification and characterization of microRNAs in the pancreatic fluke Eurytrema pancreaticum. Parasit. Vectors. Parasites & Vectors; 2013. January;6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cucher M, Prada L, Mourglia-Ettlin G, Dematteis S, Camicia F, Asurmendi S, et al. Identification of Echinococcus granulosus microRNAs and their expression in different life cycle stages and parasite genotypes. Int. J. Parasitol. Australian Society for Parasitology Inc.; 2011. March;41: 439–48. [DOI] [PubMed] [Google Scholar]
- 44. Xu M-J, Ai L, Fu J-H, Nisbet AJ, Liu Q-Y, Chen M-X, et al. Comparative characterization of microRNAs from the liver flukes Fasciola gigantica and F. hepatica. PLoS One. 2012. January;7: e53387 10.1371/journal.pone.0053387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin X, Lu L, Su H, Lou Z, Wang F, Zheng Y, et al. Comparative analysis of known miRNAs across platyhelminths. FEBS J. 2013. August;280: 3944–51. 10.1111/febs.12395 [DOI] [PubMed] [Google Scholar]
- 46. Ai L, Xu MJ, Chen MX, Zhang YN, Chen SH, Guo J, et al. Characterization of microRNAs in Taenia saginata of zoonotic significance by Solexa deep sequencing and bioinformatics analysis. Parasitol. Res. 2012. June 28;110: 2373–8. 10.1007/s00436-011-2773-x [DOI] [PubMed] [Google Scholar]
- 47. Fromm B, Worren MM, Hahn C, Hovig E, Bachmann L. Substantial loss of conserved and gain of novel MicroRNA families in flatworms. Mol. Biol. Evol. 2013. December;30: 2619–28. 10.1093/molbev/mst155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomes MS, Cabral FJ, Jannotti-Passos LK, Carvalho O, Rodrigues V, Baba EH, et al. Preliminary analysis of miRNA pathway in Schistosoma mansoni. Parasitol. Int. Elsevier B.V.; 2009. March;58: 61–8. [DOI] [PubMed] [Google Scholar]
- 49. Young ND, Nagarajan N, Lin SJ, Korhonen PK, Jex AR, Hall RS, et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. Nature Publishing Group; 2014. January;5: 4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X-W, Xiong A-S, Yao Q-H, Zhang Z, Qiao Y-S. Direct isolation of high-quality low molecular weight RNA of pear peel from the extraction mixture containing nucleic acid. Mol. Biotechnol. 2010. January;44: 61–5. 10.1007/s12033-009-9204-6 [DOI] [PubMed] [Google Scholar]
- 51. Sasson A, Michael TP. Filtering error from SOLiD Output. Bioinformatics. 2010. March 15;26: 849–50. 10.1093/bioinformatics/btq045 [DOI] [PubMed] [Google Scholar]
- 52. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2012;17: 10–2. [Google Scholar]
- 53. Pruitt KD, Tatusova T, Brown GR, Maglott DR. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 2012. January;40: D130–5. 10.1093/nar/gkr1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Benson D a, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2011. January;39: D32–7. 10.1093/nar/gkq1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gardner PP, Daub J, Tate JG, Nawrocki EP, Kolbe DL, Lindgreen S, et al. Rfam: updates to the RNA families database. Nucleic Acids Res. 2009. January;37: D136–40. 10.1093/nar/gkn766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Homer N, Merriman B, Nelson SF. BFAST: an alignment tool for large scale genome resequencing. PLoS One. 2009. January;4: e7767 10.1371/journal.pone.0007767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011. January;39: D152–7. 10.1093/nar/gkq1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang X, Chen W, Huang Y, Sun J, Men J, Liu H, et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. BioMed Central Ltd; 2011. January;12: R107 10.1186/gb-2011-12-10-r107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460: 352–8. 10.1038/nature08160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou Y, Zheng H, Chen X, Zhang L, Wang K, Guo J, et al. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009. July 16;460: 345–51. 10.1038/nature08140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Markham NR, Zuker M. UNAFold In: Keith JM, editor. Bioinformatics. Vol. II Totowa, NJ: Humana Press; 2008. [cited 2015 Mar 9]. p. 3–31. [Google Scholar]
- 62. Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, et al. A uniform system for microRNA annotation. RNA. 2003;9: 277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Z, Xue X, Sun J, Luo R, Xu X, Jiang Y, et al. An “in-depth” description of the small non-coding RNA population of Schistosoma japonicum schistosomulum. PLoS Negl. Trop. Dis. 2010. January;4: e596 10.1371/journal.pntd.0000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hammer Ø, Harper DAT, Ryan PD. PAST: PALEONTOLOGICAL STATISTICS SOFTWARE PACKAGE FOR EDUCATION AND DATA ANALYSIS. Palaeontol. Electron. 2001;4: 1–9. [Google Scholar]
- 65. Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009. January;10: 421 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marco A, Ninova M, Ronshaugen M, Griffiths-Jones S. Clusters of microRNAs emerge by new hairpins in existing transcripts. Nucleic Acids Res. 2013. September;41: 7745–52. 10.1093/nar/gkt534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Solovyev V, Kosarev P, Seledsov I, Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006. January;7 Suppl 1: S10.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics. 2002. August;Chapter 2: Unit 2.3. [DOI] [PubMed] [Google Scholar]
- 69. Marco A, Hooks KB, Griffiths-Jones S. Evolution and function of the extended miR-2 microRNA family. RNA Biol. 2012;9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Souza Gomes M, Donoghue MT a, Muniyappa M, Pereira RV, Guerra-Sá R, Spillane C. Computational identification and evolutionary relationships of the microRNA gene cluster miR-71/2 in protostomes. J. Mol. Evol. 2013. June;76: 353–8. 10.1007/s00239-013-9563-2 [DOI] [PubMed] [Google Scholar]
- 71. Marco A, Kozomara A, Hui JHL, Emery AM, Rollinson D, Griffiths-Jones S, et al. Sex-biased expression of microRNAs in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2013. January;7: e2402 10.1371/journal.pntd.0002402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. Nature Publishing Group; 2011. December;12: 846–60. [DOI] [PubMed] [Google Scholar]
- 73. Sasidharan V, Lu Y-C, Bansal D, Dasari P, Poduval D, Seshasayee A, et al. Identification of neoblast- and regeneration-specific miRNAs in the planarian Schmidtea mediterranea. RNA. 2013. October;19: 1394–404. 10.1261/rna.038653.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wystub K, Besser J, Bachmann A, Boettger T, Braun T. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet. 2013. January;9: e1003793 10.1371/journal.pgen.1003793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hertel J, Bartschat S, Wintsche A, Otto C, 2011 TS of the B computer L, Stadler PF. Evolution of the let-7 microRNA Family. RNA Biol. 2012;9: 231–41. 10.4161/rna.18974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Campo-Paysaa F, Sémon M, Cameron RA, Peterson KJ, Schubert M. microRNA complements in deuterostomes: origin and evolution of microRNAs. Evol. Dev. 2011;13: 15–27. 10.1111/j.1525-142X.2010.00452.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- 297 (gi|124128508|gb|AAWT01093310.1|) (- strand)
- 1403 (gi|124163860|gb|AA WT01057958.1|) (+ strand) (locus 1)
- 5146 (gi|124196975|gb|AA WT01024843.1|) (+ strand) (locus 2)
- 2151 (gi|124194162|gb|AA WT01027656.1|) (+ strand)
Cluster of C. sinensis was obtained from contig 2339 (gi|353340623|dbj|BADR02002339.1|) (- strand). Cluster of S. mansoni was obtained from chromosome W (gi|360043576|emb|HE601631.1|) (+ strand). Cluster of S. japonicum was obtained from contig S000054 (gi|227129020|emb|FN331028.1|) (- strand). Cluster of H. microstoma was obtained from contig 370001 (gi|528309775|emb|CBLW010002354.1|) (+ strand). Cluster of E. granulosus was obtained from contig 0003137 (gi|528307253|emb|CBLN010000400.1|) (- strand). Cluster of E. multilocularis was obtained from contig 007728 (gi|528775369|emb|CBLO010002090.1|) (- strand). Cluster of T. solium was obtained from contig 01703 (pathogen_TSM_contig_01703) (+ strand). Cluster of G. salaris was obtained from scaffold 7180006951238_l_32618_C_75.085_gc_34.438 (+ strand)
(PDF)
csi—C. sinensis. Secondary structure of miRNA precursors is indicated by a parentheses and dots. Mature miRNA sequences including miR-2f are in bold type and underlined.
(PDF)
sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined.
(PDF)
sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined.
(PDF)
csi—C. sinensis, sma—S. mansoni, hmi—H. microstoma, egr—E. granulosus, emu—E. multilocularis, tso—T. solium, sec. struc.—secondary structure. Mature miRNA sequences are in bold type and underlined. Mature miRNA sequences are in bold type and underlined. Cluster of C. sinensis was obtained from scaffold 198 (gi|353342189|dbj|BADR02000773.1|) (- strand). Cluster of S. mansoni was obtained from chromosome W (gi|360043576|emb|HE601631.1|) (- strand). Cluster of H. microstoma was obtained from contig 880003 (gi|528308588|emb|CBLW010003541.1|) (- strand). Cluster of E. granulosus was obtained from contig 00137 (gi|528306514|emb|CBLN010001139.1|) (+ strand). Cluster of E. multilocularis was obtained from contig 007760 (gi|528775363|emb|CBLO010002096.1|) (+ strand). Cluster of T. solium was obtained from contig 00015 (pathogen_TSM_contig_00015) (+ strand).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Mature miRNAs are red. Diferensis in sequences of miRNA precursors in red circles.
(PDF)
(XLSX)
(XLS)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
Raw sequencinq data are available from the NCBI (accession numbers SRX817942, SRX817990, SRX817989, SRX817991, SRX817993, SRX817992, SRX817994, SRX817996, SRX817995, SRX817997, SRX817999, SRX817998, SRX818000, SRX818002, SRX81800. All other relevant data are in the manuscript and Supporting Information files.