Abstract
Estrogen (E2) exerts a dual function on E2-deprived breast cancer cells, with both initial proliferation and subsequent induction of stress responses to causes apoptosis. However, the mechanism by which E2 integrally regulates cell growth or apoptosis associated pathways remains to be elucidated. Here, E2 deprivation results in many alterations in stress-responsive pathways. For instance, E2-deprived breast cancer cells had higher basal levels of stress-activated protein kinase, c-Jun N-terminal kinase (JNK), compared with wild-type MCF-7 cells. E2 treatment further constitutively activated JNK after 24 hours. However, inhibition of JNK (SP600125) was unable to abolish E2-induced apoptosis, whereas SP600125 alone arrested cells at the G2-phase of the cell cycle and increased apoptosis. Further examination showed that inhibition of JNK increased gene expression of tumor necrosis alpha (TNFα) and did not effectively attenuate expression of apoptosis-related genes induced by E2. A notable finding was that E2 regulated both JNK and Akt as the downstream signals of insulin-like growth factor-1 receptor (IGF1R)/phosphoinositide 3-kinase (PI3K), but with distinctive modulation patterns: JNK was constitutively activated, whereas Akt and Akt-associated proteins, such as PTEN and mTOR, were selectively degraded. Endoplasmic reticulum-associated degradation (ERAD) was involved in the selective protein degradation. These findings highlight a novel IGF-1R/PI3K/JNK axis that plays a proliferative role during the prelude to E2-induced apoptosis and that the endoplasmic reticulum is a key regulatory site to decide cell fate after E2 treatment.
IMPLICATIONS
This study provides a new rationale for further exploration of E2-induced apoptosis to improve clinical benefit.
Keywords: estrogen-induced apoptosis, c-Jun N-terminal kinase (JNK), Akt, endoplasmic reticulum stress
Introduction
Estrogen (E2) plays a pivotal role in the development and progression of breast cancers. Blockade of E2 signaling by either aromatase inhibitors (AIs) or tamoxifen are important treatment strategies for estrogen receptor (ER) positive breast cancers (1). However, acquired resistance to anti-estrogen therapies is still a challenge in the clinic. Laboratory findings that re-transplantation of tamoxifen-stimulated tumors into successive generations of athymic mice over 5 years results in the selection of a resistant tumor cell population that is killed by physiological levels of E2 (2, 3), has resulted in the new biology of E2-induced apoptosis (4–7). Indeed, E2-induced apoptosis has been used successfully to treat breast cancer after the failure of AI therapy (8) and to explain the action of E2 replacement therapy for postmenopausal women in their 60s having a lower incidence of breast cancer and mortality (9, 10). The laboratory and clinical data describing the effects of estrogens to cause tumor cell death and tumor regression have been linked to E2-induced apoptosis in vulnerable cell populations created by selection pressure in long term E2-deprived environments (11). All of these findings encouraged us to investigate the mechanisms of E2 action in validated cellular models of long-term E2-deprived breast cancer.
Our observations show that E2-induced apoptosis through nuclear ER alpha (ERα) (12, 13) can be completely blocked by the antiestrogen 4-hydroxytamoxifen (4-OHT) or knockdown of ERα through small interfering RNA (siRNA) (13, 14). E2 activates classic ERE-regulated endogenous genes in MCF-7:5C cells (12, 13), but ERE transcriptional pathway does not directly participate in the E2-induced apoptosis in vitro (13) or in vivo (15). Our global gene array (12) data suggest that E2 signaling can occur through a non-classic transcriptional pathway involving the interaction of ER with transcription factors such as activator protein-1 (AP-1), which may regulate stress responses. The c-Jun NH2-terminus kinase (JNK) has been documented to play a major role in controlling activation of AP-1 proteins through phosphorylation (16). Furthermore, the stress-activated protein kinase JNK is a well-known stress- and inflammatory cytokine-activated kinase pathway (17, 18). One of the most extensively studied functions of JNK is its induction of apoptosis under stress conditions (19, 20). However, the precise role of JNK activation in apoptosis remains controversial (20–22). Recent studies of human tumor specimens, including breast cancer, show a correlation between elevated JNK activity and worse clinical outcome (22). Currently, there are no reports correlating alterations of JNK with functions in E2-deprived breast cancer cells, as surrogates of AI resistance.
By contrast, compelling evidence suggests that E2 induces apoptosis through accumulation of stress responses, including endoplasmic reticulum stress, oxidative stress, and inflammatory stress (12, 14, 23). Endoplasmic reticulum stress initially occurs after treatment with E2 (12). Three sensors of endoplasmic reticulum stress, protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring protein 1 alpha (IRE1α), and activating transcription factor 6 (ATF6), are activated by E2 (13). PERK attenuates protein translation which has been confirmed as an important inducer for E2-induced apoptosis (12), whereas ATF-6 and IRE1 increase endoplasmic reticulum folding capacity by up-regulating the endoplasmic reticulum chaperones and the endoplasmic reticulum-associated protein degradation (ERAD) machinery (24). IRE1 is able to modulate JNK activities (24). The initial response to E2 is proliferation in E2-deprived breast cancer cells with an increased S-phase of the cell cycle over 3 days (12, 23, 25, 26). This response differs from rapid (12 hour) chemotherapy-induced apoptosis (25). Our observations indicate that insulin-like growth factor-1 receptor (IGF-1R)/phosphoinositide 3-kinase (PI3K) is a dominant growth driver after E2 treatment in two E2-deprived breast cancer cells (23, 27), which activates Akt to promote cell growth (23, 27). Additionally, PI3K/Akt is involved in the metabolic stress and IRE1 has the capacity to regulate AKT activation (28). All of these growth or stress associated signals are tightly linked to modulate cell function under defined conditions.
We sought here to further understand how E2 integrally regulates proliferative growth, stress responses, and finally apoptosis in E2-deprived breast cancer cells. E2 treatment persistently activated JNK in an ER-dependent manner. However, blockade of JNK phosphorylation was unable to prevent E2-induced apoptosis. A notable finding was that E2 regulated both JNK and Akt as the downstream signals of insulin-like growth factor-1 receptor (IGF-1R)/phosphoinositide 3-kinase (PI3K), but with distinctive modulation patterns: JNK was constitutively activated, whereas Akt and Akt-associated proteins, such as PTEN and mTOR, were selectively degraded. Endoplasmic reticulum stress-associated degradation (ERAD) was responsible for the selective degradation of Akt-associated proteins after E2 treatment. All of these results provide further evidence to investigate E2-induced apoptosis in breast cancer with acquired resistance to antihormones.
Materials and Methods
Materials
Tunicamycin and the JNK inhibitor (SP600125) were purchased from Sigma-Aldrich (St. Louis, MO). The p38 inhibitor (SB203580) and the PI3K inhibitor (LY294002) were ordered from Promega (Madison, WI). The c-Src inhibitor (PP2) and the IGF-1R inhibitor (AG1024) were purchased from CalBiochem (San Diego, CA). Sources of antibodies for Western blotting are as follows: Total MAPK (#9102), phosphorylated MAPK (#9101), total Akt (#9272), phosphorylated Akt (#9271), total p38 (#9212), phosphorylated p38 (#9211), total JNK (#9252), phosphorylated JNK (#9255), total eIF2α (#9722), and phosphorylated eIF2α (#9721) antibodies were all from Cell Signaling Technology (Beverly, MA).
Cell culture conditions and cell proliferation assays
Estrogen-deprived MCF-7:5C and MCF-7:2A cells were maintained in estrogen-free RPMI 1640 medium supplemented with 10% dextran-coated charcoal-stripped fetal bovine serum as previously described (12). Our DNA fingerprinting pattern of these cell lines (27) is consistent with the report by the American Type Culture Collection (ATCC). The DNA content of the cells, a measure of proliferation, was determined as previously described (12).
Annexin V analysis of apoptosis
The FITC Annexin V Detection Kit I (BD Pharmingen, San Diego, CA) was used to quantify apoptosis by flow cytometry according to the manufacturer’s instructions as previously described (13).
Cell cycles analysis
MCF-7, MCF-7:5C, and MCF-7:2A cells were cultured in dishes. They were treated with vehicle (0.1% DMSO) or SP600125 (10−5 mol/L) for 48 hours. Cells were gradually fixed and analyzed as previously described (13).
Western blotting
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA), supplemented with Protease Inhibitor Cocktail Set I and Phosphatase Inhibitor Cocktail Set II (Calbiochem, San Diego, CA). Western blotting was performed as previously described (13).
Quantitative Real-Time PCR
Quantitative RT-PCR assays were conducted as previously described (13) using the SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA) and a 7900HT Fast Real-time PCR System (Applied Biosystems).
Gene Expression Microarrays
MCF-7, MCF-7:5C, and MCF-7:2A cells were cultured as previously described (27). Total RNA was isolated using the Qiagen RNeasy Micro kit (Qiagen, Valencia, CA). RNA integrity and purity were measured as previously described (12). Cy3 (sample) and Cy5 (reference) cRNA probes were prepared and co-hybridized to Agilent 4×44K Human 1A (V2) dual color Oligo Microarrays using manufacturer’s protocols (Agilent Technologies, Palo Alto, CA) (12). Genes significantly up- or down-regulated with a p value < 0.001 were selected for analysis. Gene expression data was deposited in Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo) with accession number GSE60079.
Statistical analysis
All reported values are the means ± SE. Statistical comparisons were determined with two-tailed Student’s t tests. Results were considered statistically significant if the P value was <0.05.
Results
Basal JNK levels are increased in two long-term E2-deprived breast cancer cells
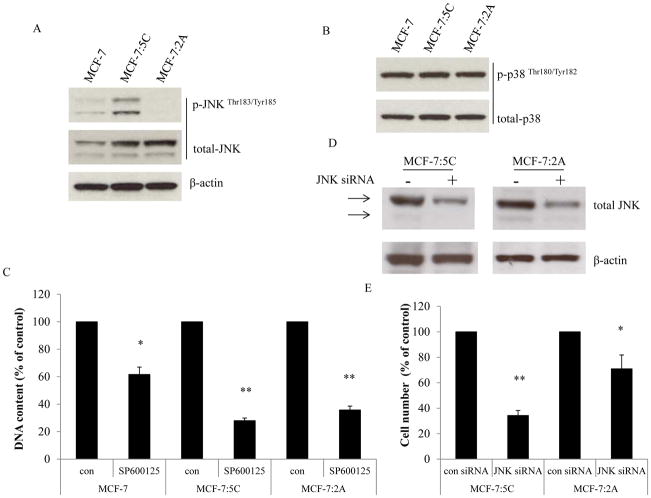
The MCF-7:5C and MCF-7:2A cells were cloned using long-term E2 deprivation of MCF-7 cells, which results in many alterations for the adaptation to nutrient deficiency (5, 12, 29). MCF-7:5C cells undergo E2-induced apoptosis within seven days of E2 treatment, whereas MCF-7:2A cells survive two weeks of E2 treatment benefitting from a stronger antioxidant defense mechanism (13, 23). Evidence has shown that stress-activated pathways, JNK and p38, are activated in endocrine-resistant breast cancer cells (30). We address the question of whether long-term E2 deprivation alters these two pathways. In the present study, increased basal levels of total JNK were observed in two long-term E2-deprived breast cancer cell lines, MCF-7:5C and MCF-7:2A (Fig. 1A). Higher phosphorylated JNK was detected in MCF-7:5C cells, but not in MCF-7:2A cells (Fig. 1A). There was no change of total and phosphorylated p38 in E2-deprived cell lines compared with wild-type MCF-7 cells (Fig. 1B). Inhibition of JNK by a specific inhibitor, SP600125, in MCF-7 cells reduced cell numbers (Fig. 1C) and arrested cells at G2-phase of the cell cycle in all three cell lines (Supplementary Fig. S1A–1C); MCF-7:5C and MCF-7:2A cells were more sensitive to the JNK inhibitor (Fig. 1C). Unexpectedly, the JNK inhibitor completely blocked the proliferation activated by E2 in MCF-7 cells (Supplementary Fig. S1D), demonstrating JNK is also a growth signal in parental cells. To further confirm the function of JNK in E2-deprived breast cancer cells, it was knocked down by specific siRNA. Our results indicate that JNK was effectively knocked down by siRNA in MCF-7:5C cells after 72 hours; however, double transfection was required for MCF-7:2A cells to get similar effect as in MCF-7:5C cells (Fig. 1D). Knockdown of JNK was more effective it inhibiting MCF-7:5C cell growth than in MCF-7:2A cells (Fig. 1E). Global gene expression profiles revealed that basal expression levels of JNK (MAPK8), but not p38 (MAPK14), were elevated in both MCF-7:5C and MCF-7:2A cells (Table S1). Furthermore, many stress-responsive genes, including inflammation (TNFRSF11B, CXCR4, TNF, etc), oxidative stress (APOE, GPX2, SOD2, etc), endoplasmic reticulum stress (EIF2AK3, ATF6, ERN1, etc), and stress-related kinases (MAPK8, MAPK14, SGK, etc) had been altered after E2 deprivation (Table S1). These results suggest that a wide range of stress-related pathways including JNK have been altered in E2-deprived breast cancer cells.
Figure 1. Basal levels of JNK and p38 in three cell lines.
(A) Expression levels of JNK in three cell lines. MCF-7 cells were cultured in E2-free medium for three days. Then, cell lysates of MCF-7, MCF-7:5C, and MCF-7:2A were harvested. Total JNK and p-JNK were examined by Western blotting. β-actin was measured as loading control. (B) Expression levels of p38 in three cell lines. Cell lysates of MCF-7, MCF-7:5C, and MCF-7:2A were the same as above. Total p38 and p-p38 were examined by Western blotting. (C) Growth response to the JNK inhibitor. MCF-7, MCF-7:5C, and MCF-7:2A cells were treated with vehicle (0.1% DMSO) or SP600125 (10−5 mol/L). Cells were harvested after 7 days of treatment and cell viability was quantitated by determination of total DNA. p<0.05, * compared with control. p<0.001, ** compared with control. (D) Knockdown of JNK through specific siRNA. MCF-7:5C cells were transfected with control siRNA or JNK siRNA for 72 hours. MCF-7:2A cells were double transfected with control siRNA or JNK siRNA for 5 days. Cell lysates were harvested for Western blotting. (E) Knockdown of JNK inhibited cell growth. MCF-7:5C and MCF-7:2A cells were double transfected with control siRNA or JNK siRNA and were grown for 5 days. Cell nuclei were counted using a Coulter counter. p<0.05, * compared with control. p<0.001, ** compared with control.
JNK functions as a growth signal in the process of E2-induced apoptosis
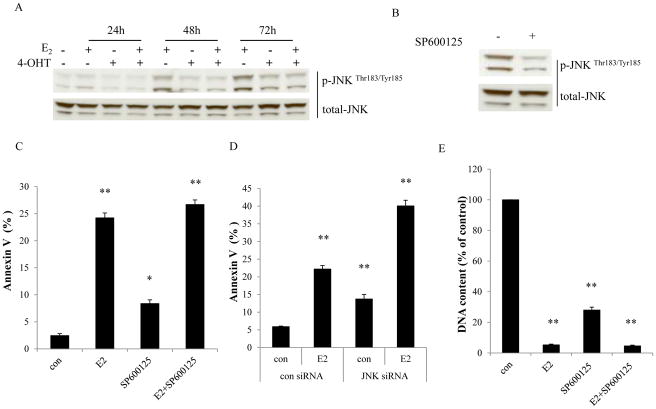
We observed that E2 started to increase levels of phosphorylated JNK after a 24 hour treatment (Fig. 2A) and constitutively activated JNK after a prolonged treatment with E2 in MCF-7:5C cells (Fig. 2A). Induction of JNK phosphorylation by E2 was effectively blocked by 4-OHT (Fig. 2A), demonstrating a dependence on the ER. Treatment with a specific inhibitor, SP600125 resulted in an effective reduction of JNK phosphorylation in MCF-7:5C cells (Fig. 2B), and similarly in MCF-7:2A cells (Supplementary Fig. S2A). To assess the function of JNK activation in the process of E2-induced apoptosis, MCF-7:5C cells were treated with E2 in the absence or presence of SP600125 for 72 hours. The JNK inhibitor alone caused apoptosis with increased percentage of Annexin V binding (Fig. 2C), and SP600125 was unable to block E2-induced apoptosis after 72 hours (Fig. 2C); similar results were found in MCF-7:2A cells after 6 days of treatment (Supplementary Fig. S2B). Consistently, knockdown of JNK through specific siRNA increased apoptosis (Fig. 2D). It was additive with E2 to cause apoptotic impairment in MCF-7:5C cells (Fig. 2D). Cell viability assays demonstrated that both E2 and the JNK inhibitor reduced cell number, and SP600125 could not prevent the reduction of cell number caused by E2 in MCF-7:5C cells (Fig. 2E). In MCF-7:2A cells, E2 did not reduce cell number after a one week treatment, but a combination of E2 with SP600125 decreased cell number to the level observed with the JNK inhibitor alone (Supplementary Fig. S2C).
Figure 2. Activation of JNK by E2 in MCF-7:5C cells.
(A) Activation of JNK by E2. MCF-7:5C cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and E2 (10−9 mol/L) plus 4-OHT (10−6 mol/L) for the time points indicated. p-JNK was examined by Western blotting. Total JNK was measured as loading control. (B) The JNK inhibitor effectively blocked phosphorylation of JNK. MCF-7:5C cells were treated with vehicle (0.1% DMSO) or SP600125 (10−5 mol/L) for 24 hours. p-JNK was examined by Western blotting. Total JNK was measured as loading control. (C) The JNK inhibitor could not block E2-induced apoptosis. MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), SP600125 (10−5 mol/L), and E2 (10−9 mol/L) plus SP600125 (10−5 mol/L) for 72 hours. Annexin V binding assay was used to detect apoptosis. p<0.05, * compared with control. p<0.001, ** compared with control. (D) Knockdown of JNK could not block E2-induced apoptosis. MCF-7:5C cells were transfected with control siRNA or JNK siRNA for 72 hours. Then, cells were treated with vehicle (0.1% EtOH) or E2 (10−9 mol/L) for 72 hours. Annexin V binding assay was used to detect apoptosis. p<0.001, ** compared with control. (E) Growth response to the JNK inhibitor in the presence or absence of E2. MCF-7:5C cells were treated with the same compound as in (C). Cells were harvested after 7 days treatment and cell viability was quantitated by determination of total DNA. p<0.001, ** compared with control.
Inhibition of JNK activity does not attenuate apoptosis-related genes activated by E2
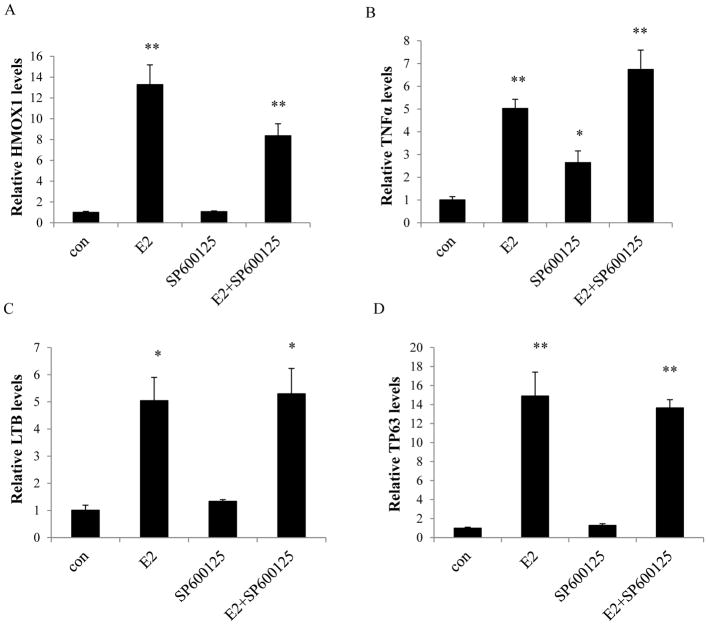
Our observations have shown that E2 activates multiple apoptosis-related genes in MCF-7:5C cells (13), which are involved in the oxidative stress, endoplasmic reticulum stress, and inflammatory pathways (13). To further identify the function of JNK activation in the regulation of these apoptosis-related genes, real-time RT-PCR was performed to measure gene expression levels. The indicator of oxidative stress, heme oxygenase 1 gene (HMOX1), was dramatically up-regulated by E2 (Fig. 3A). Inhibition of JNK alone did not increase the expression level of HMOX1 (Fig. 3A), but SP600125 could not completely block the up-regulation of HMOX1 by E2 (Fig. 3A). It is known that TNFα induces apoptosis in MCF-7:5C cells (13). Levels of mRNA from members of TNF family (i.e. TNF and LTB) were increased by E2, but the JNK inhibitor was unable to prevent this induction (Fig. 3B and 3C). Furthermore, elevated expression level of TNF was detected by the JNK inhibitor alone (Fig. 3B). Other stress-related genes, such as TP63, PMAIP1, and PPP1R15A, were up-regulated by E2 (Fig. 3D and Supplementary Fig. S3), but could not be blocked by the JNK inhibitor. These results indicate that inhibition of JNK does not effectively block E2-induced apoptosis-related genes. It also suggests that JNK is not an apoptotic signal in the process of E2-induced apoptosis.
Figure 3. Regulation of apoptosis-related genes by the JNK inhibitor.
MCF-7:5C cells were treated with the same compounds as in Fig. 2C for 72 hours. Cells were harvested in TRIzol. Gene expression levels were quantitated by RT-PCR. (A) HMOX1, (B) TNF, (C) LTB, (D) TP63. p<0.05, * compared with control. p<0.001, ** compared with control.
E2-activated JNK is regulated by IGF-1R/PI3K pathways in MCF-7:5C cells
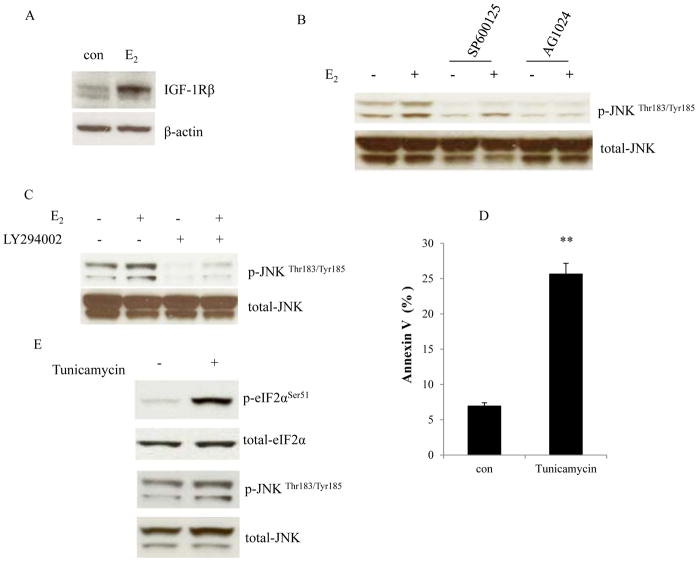
The insulin-like growth factor-1 receptor (IGF-1R) has been confirmed as an important growth driver in the two E2-deprived breast cancer cells used here (23, 27). E2 up-regulated IGF-1R protein and mRNA levels in MCF-7:5C cells (Fig. 4A and Supplementary Fig. S4A). To verify the functional regulation between JNK and IGF-1R, MCF-7:5C cells were treated with E2 in the absence or presence of tyrosine kinase inhibitor of IGF-1R, AG1024, which effectively blocked phosphorylation of IGF-1R (Supplementary Fig.S4B) (31, 32). The IGF-1R inhibitor abolished JNK phosphorylation and its activation by E2 (Fig. 4B). This suggests that IGF-1R is one of the upstream signals of JNK in MCF-7:5C cells. Our observations have shown that PI3K is a downstream growth signal of IGF-1R in E2-deprived cells (23, 27). Interestingly, blockade of PI3K by a specific inhibitor, LY294002, was able to reduce basal levels of phosphorylated JNK and completely inhibit the activation of JNK by E2 (Fig. 4C). Predictably, inhibition of IGF-1R and PI3K suppressed phosphorylation of Akt (Supplementary Fig. S5A). In addition to activating growth pathways, E2 activates endoplasmic reticulum stress (12) and it widely elicits stress-related genes and inflammatory response genes after E2 treatment in MCF-7:5C cells (12). JNK is considered a molecular link between stress and apoptosis (18). To gain further insights into the regulatory relationship between JNK activation and endoplasmic reticulum stress, an inducer of endoplasmic reticulum stress, Tunicamycin (33) was used to treat MCF-7:5C cells. As expected, it caused apoptosis in MCF-7:5C cells (Fig. 4D) and activated eukaryotic translation initiation factor-2α (eIF2α) (Fig. 4E), which is activated by PERK (13). However, Tunicamycin treatment did not activate JNK (Fig. 4E). These data confirm that E2-activated JNK is mainly regulated by IGF-1R/PI3K in MCF-7:5C and functions as a growth signal in E2-deprived cells.
Figure 4. Regulation of the JNK activation.
(A) E2 elevated IGF-1R. MCF-7:5C cells were treated with vehicle (0.1% EtOH) or E2 (10−9 mol/L) for 72 hours. IGF-1R was examined by Western blotting. β-actin was measured as loading control. (B) Regulation of JNK activation by IGF-1R. MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), SP600125 (10−5 mol/L), E2 (10−9 mol/L) plus SP600125 (10−5 mol/L), AG1024 (5×10−6 mol/L), E2 (10−9 mol/L) plus AG1024 (5×10−6 mol/L) for 48 hours. p-JNK was examined by Western blotting. Total JNK was measured as loading control. (C) Regulation of JNK activation by PI3K. MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), LY294002 (10−6 mol/L), and E2 (10−9 mol/L) plus LY294002 (10−6 mol/L) for 48 hours. p-JNK was examined by Western blotting. Total JNK was measured as loading control. (D) Induction of apoptosis by the endoplasmic reticulum stress inducer, Tunicamycin. MCF-7:5C cells were treated with vehicle (0.1% DMSO) or Tunicamycin (10−5 mol/L) for 24 hours. Annexin V binding assay was used to detect apoptosis. p<0.001, ** compared with control. (E) Effects of Tunicamycin on the activation of JNK. MCF-7:5C cells were treated with the same compound as in (D). p-eIF2α and p-JNK were examined by Western blotting. Total eIF2α and JNK were measured as loading controls.
E2 selectively disrupts Akt-associated proteins in MCF-7:5C cells
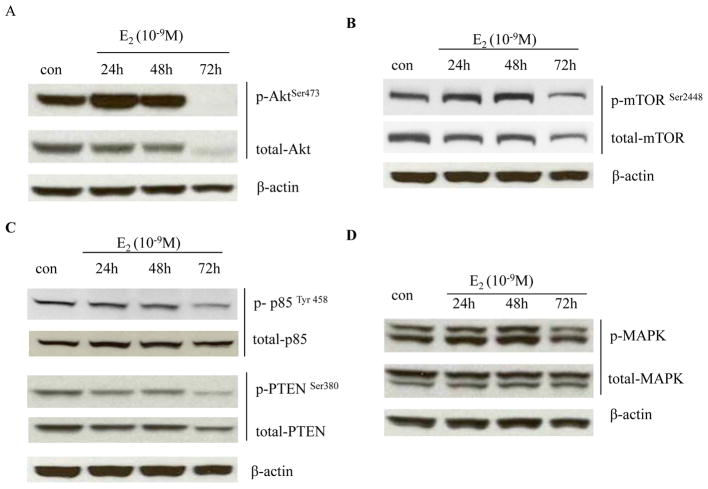
It is well known that Akt transduces the downstream signal of PI3K and plays an important role in protecting cell from apoptosis (34). Intriguingly, results shown above indicate that activation of JNK is regulated by IGF-1R/PI3K and has a proliferative role in MCF-7:5C cells. This encouraged us to investigate the alterations of two basal growth pathways: PI3K/Akt and ERK/MAPK after E2 treatment. Notably, E2 significantly activated Akt between 24–48 hours, but resulted in a reduction in levels of both total and phosphorylated Akt after 72 hours of exposure (Fig. 5A). Similar effects were noted in the expression of total and phosphorylated mTOR (Fig. 5B), a well-described Akt downstream target (34). The activation of the PI3K/Akt pathway is tightly regulated by phosphatases, especially the reversion of PIP3 back to PIP2 by phosphatase and tensin homolog (PTEN) (35). However, PTEN and Akt upstream protein p85 (regulatory subunit of PI3K) were also decreased by E2 at this time point (Fig. 5C). In contrast, ERK/MAPK was moderately activated by E2 between 24–48 hours, and went down to the same level as control at 72 hours (Fig. 5D). All of these findings suggest that E2 selectively disrupts Akt-related pathways.
Figure 5. E2 degraded Akt-associated proteins.
(A) Regulation of Akt by E2. MCF-7:5C cells were treated with vehicle (0.1% EtOH) or E2 (10−9 mol/L) for different time points indicated. Cell lysates were harvested. p-Akt and total Akt were examined by Western blotting. β-actin was measured as loading control. (B) Regulation of mTOR by E2. Cells were treated the same as in (A). p-mTOR and total mTOR were examined by Western blotting. β-actin was measured as loading control. (C) Regulation of PTEN and p85 by E2. Cell lysates were the same as above. p-PTEN, total PTEN, p-p85, and total p85 were examined by Western blotting. β-actin was measured as loading control. (D) Regulation of MAPK by E2. MCF-7:5C cells were treated with vehicle (0.1% EtOH) or E2 (10−9 mol/L) for different time points indicated. Cell lysates were harvested. p-MAPK and total MAPK were examined by Western blotting. β-actin was measured as loading control.
Degradation of Akt by E2 is mediated by endoplasmic reticulum stress in MCF-7:5C cells
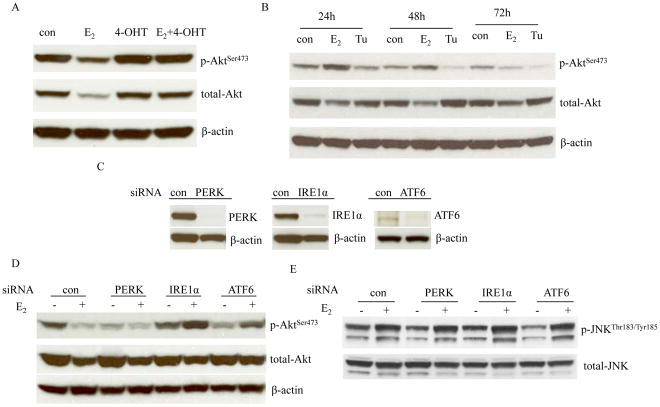
The ER is the initial site for E2 to induce all signals related with either proliferation or apoptosis (13). Thus, targeting ER by 4-OHT can prevent the degradation of total and phosphorylated Akt induced by E2 (Fig. 6A). Although c-Src is an important adapter protein to mediate ER signaling pathways in MCF-7:5C cells (13), blockade of c-Src was insufficient to abolish the degradation of Akt by E2 (Supplementary Fig. S5B). Evidence suggests that endoplasmic reticulum stress causes down-regulation of Akt (36). To better understand the regulation of Akt by endoplasmic reticulum stress, MCF-7:5C cells were treated with an endoplasmic reticulum stress inducer, Tunicamycin (33). The levels of phosphorylated Akt decreased, but not for total Akt after a 24 hours treatment (Fig. 6B). To further investigate the function of three sensors of endoplasmic reticulum stress in the regulation of Akt, each one was knocked down by specific siRNAs (Fig. 6C) which partially prevented E2-induced apoptosis (Supplementary Fig. S6A–6C). Interestingly, knockdown of IRE1α could effectively reverse the degradation of p-Akt by E2 (Fig. 6D). Knockdown of ATF6 partially increased the levels of Akt (Fig. 6D). However, knockdown of PERK alone reduced p-Akt and had no effects on preventing degradation of p-Akt by E2 (Fig. 6D). In contrast, JNK phosphorylation activated by E2 was not affected by knockdown of three sensors (Fig. 6E). Expression levels of IGF-1R were not altered after the knockdown of each of the three sensors (Supplementary Fig. S6D). These results indicate that endoplasmic reticulum stress participates in the deregulation of Akt by E2 in MCF-7:5C cells (Fig. 7), despite the three sensors having differential functions.
Figure 6. Regulation of Akt by endoplasmic reticulum stress.
(A) 4-OHT prevented the degradation of Akt by E2. MCF-7:5C cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and 4-OHT (10−6 mol/L) plus E2 (10−9 mol/L) for 72 hours. Cell lysates were harvested. p-Akt and total Akt were examined by Western blotting. β-actin was measured as loading control. (B) Effects of Tunicamycin on Akt phosphorylation. MCF-7:5C cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), and Tunicamycin (10−5 mol/L) for different time points indicated. p-Akt and total-Akt were examined by Western blotting. β-actin was measured as loading control. (C) Knockdown of three sensors of endoplasmic reticulum stress by siRNAs. MCF-7:5C cells were transfected with scrambled, PERK, IRE1α, and ATF6 siRNA for 72 hours. Respective proteins were examined by Western blotting. β-actin was measured as loading control. (D) Effects on Akt after knockdown of three sensors. MCF-7:5C cells were transfected with specific siRNAs as above for 72 hours. Then, cells were treated with or without E2 for 72 hours. p-Akt and total-Akt were examined by Western blotting. β-actin was measured as loading control. (E) Effects on JNK phosphorylation after knockdown of three sensors. Cell lysates were the same as in (D). p-JNK and total-JNK were examined by Western blotting.
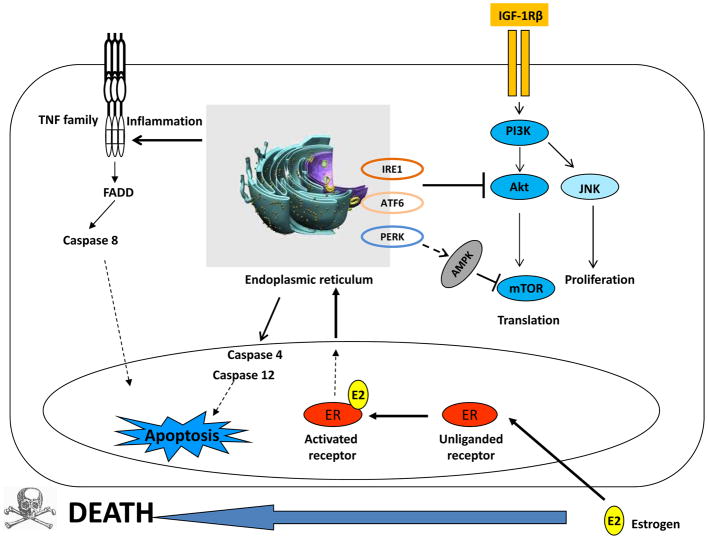
Figure 7. Endoplasmic reticulum is a joint regulatory site to integrally modulate growth or apoptosis associated pathways.
E2 activates IGF-1R/PI3K and its downstream signals, Akt and JNK to promote cell growth. Simultaneously, E2 activates endoplasmic reticulum stress which activates a set of signaling pathways including three sensors (PERK, IRE1α, and ATF6), inflammatory responses, caspase 4/12, and adenosine monophosphate (AMP)-activated protein kinase (AMPK). The AMPK and Akt converge on mTOR with opposing regulatory effects to coordinate bioenergetics and cell viability. IRE1α and ATF6 are involved in the degradation of Akt.
Discussion
Although the potential limitation of translational research to address the treatment of hormone responsive breast cancer is that only a limited number of ER positive breast cancer cell lines are available for use routinely (37), a characteristic of the MCF-7 E2-deprived breast cancer cell lines is vulnerability to apoptosis induced by E2 (4, 12). This new biology of E2-induced apoptosis has been successfully used in clinical trials (7, 8). In the past, we have focused on the investigation of apoptosis-related signal pathways, genes, and cytoplasm organelles’ stress induced by E2 in long-term E2-deprived breast cancer cells (5, 12, 13, 14, 23). However, it needs to be emphasized that E2 exerts a dual function on MCF-7:5C cells, with both initial proliferation and subsequent apoptosis (12, 25, 26). This is very different from the immediate actions of paclitaxel (25). Even though the characteristic E2-induced apoptosis occurs after 72-hour treatment, cell numbers are initially increased by E2 with a high percentage in S-phase (12, 25, 26). These observations suggest that the cell growth pathways are simultaneously activated by E2 in the process of apoptosis. How E2 integrally regulates these growth or apoptosis associated pathways is now addressed.
One of the important growth drivers up-regulated by E2 in E2-deprived breast cancer cells is IGF-1R, which is the upstream of PI3K/Akt to mediate a mechanism of growth and create an anti-apoptotic advantage (23, 27). IGF-1R is regulated by E2 in an ER-dependent manner, but not through IGFs autophosphorylation (27). The present study demonstrates that constitutive activation of JNK by E2 is regulated by the IGF-1R/PI3K pathway. In agreement with our findings, clinical studies show that levels of phosphorylated JNK correlate with breast cancer metastasis and decreased overall survival (22). Although compelling evidence has implicated JNK to be an essential component of a signal transduction pathway which leads to programmed cell death (38, 39), activation of JNK does not participate in E2-induced apoptosis (Fig. 2C). Inhibition of JNK cannot prevent E2-induced apoptosis in MCF-7:5C cells (Fig. 2C and 2D), whereas blockade of JNK is associated with increased cell death (Fig. 2E). Despite being another stress-responsive kinase, p38 (17) is activated by E2 in MCF-7:5C cells, and does not play a central role in E2-induced apoptosis (Supplementary Fig. S7A). All of these observations suggest that stress-activated kinases can function as pro- or anti-apoptotic factors, depending on the signaling network and cell context (40). The data, overall, again illustrate the unique aspects of E2-induced apoptosis in cancer cell biology.
A notable finding is that E2 regulates both JNK and Akt as downstream signals of IGF-1R/PI3K, but with distinct modulation patterns: JNK is constitutively activated (Fig. 2A), whereas Akt is first activated and then degraded (Fig. 5A). It is well known that the PI3K/Akt pathway controls fundamental aspects of metabolism and cell growth (41, 42). However, the total Akt and Akt-related proteins are reduced by E2 through a poorly understood mechanism. The c-Src inhibitor which abrogates E2-induced apoptosis (13, 27) fails to prevent E2-induced Akt disruption (Supplementary Fig. S5B). This finding indicates that inactivation of Akt by E2 does not simply represent a secondary, caspase-dependent event. Our results suggest that endoplasmic reticulum stress is responsible for the disruption of Akt. However, three sensors of endoplasmic reticulum stress exert differential functions on the regulation of Akt in MCF-7:5C cells (Fig. 6D). Knockdown of IRE1α is the most effective to prevent degradation of Akt by E2 (Fig. 6D). Consistent with our observation, IRE1α has been reported to promote plasma lipid protein metabolism (43), which is also a major sensor in the mediation of endoplasmic reticulum-associated degradation (ERAD) (44). Additionally, E2 simultaneously degrades Akt-associated proteins, PTEN, p85, and mTOR in MCF-7:5C cells (Fig. 5B and 5C), which also suggests the existence of an additional or alternative mechanism for the protein degradation process, such as the ubiquitin-proteasome system and the autophago-lysosomal pathway that may be activated by E2 to facilitate the selective degradation of plasma membrane proteins (45–49). Three protein degradation processes are tightly linked to regulate cellular metabolism, not only for the stress response, but also as part of an overall regulatory system that balances anabolic and catabolic pathways (48–50). This is the focus of our future studies.
In summary, E2 widely activates growth or apoptosis associated signaling pathways to integrally regulate cellular responses in E2-deprived breast cancer cells (13, 23). To maintain cellular homeostasis, the initial proliferative signal activated by E2 induces endoplasmic reticulum stress (12, 13) to properly fold proteins or remove unfolded proteins (51). Endoplasmic reticulum is also a focal point to activate a set of signaling pathways which might promote apoptotic cell death if stress is not alleviated (51). For instance, it can activate inflammatory responses including the TNF family members to cause cell death (Fig. 7, refs. 12 and 13). Here, we identify that endoplasmic reticulum stress participates in the selective disruption of Akt-associated proteins, which results in differential regulation patterns between JNK and Akt, even though they are common downstream targets of IGF-1R/PI3K axis in E2-deprived breast cancer cells (Fig. 7). This work offers a mechanistic rationale for better understanding E2 decision making to promote growth or apoptosis and provides a new insight into the vulnerability or security of survival networks to be applied to the treatment of endocrine-resistant breast cancer (7, 11).
Supplementary Material
Acknowledgments
VCJ is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under a Stand Up To Cancer Dream Team Translational Research Grant, Grant number SU2C-AACR-DT0409 (Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research); the Susan G Komen for the Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975), the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008 and the National Institutes of Health MD Anderson’s Cancer Center Support Grant, CA016672. We thank Karen Creswell for helping with the apoptosis analysis at the Flow Cytometry Shared Resource at Georgetown University.
Footnotes
There are no conflicts to disclose.
References
- 1.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- 3.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 4.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 6.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan VC. The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr Relat Cancer. 2015;22:R1–31. doi: 10.1530/ERC-14-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney EE, Fan P, Jordan VC. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the women’s health initiative study. Cancer Res. 2014;74:7060–8. doi: 10.1158/0008-5472.CAN-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan VC. Linking estrogen-induced apoptosis with decreases in mortality following long term adjuvant tamoxifen therapy. JNCI. 2014;106 doi: 10.1093/jnci/dju296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108:18879–86. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan P, Griffith OL, Agboke FA, Anur P, Zou X, McDaniel RE, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510–20. doi: 10.1158/0008-5472.CAN-12-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obiorah IE, Fan P, Jordan VC. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen deprived state. Cancer Prev Res (Phila) 2014;7:939–49. doi: 10.1158/1940-6207.CAPR-14-0061. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhao H, Asztalos S, Chisamore M, Sitabkhan Y, Tonetti DA. Estradiol-induced regression in T47D:A18/PKCalpha tumors requires the estrogen receptor and interaction with the extracellular matrix. Mol Cancer Res. 2009;7:498–510. doi: 10.1158/1541-7786.MCR-08-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–36. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 17.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 18.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 19.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An J, Liu H, Magyar CE, Guo Y, Veena MS, Srivatsan ES, et al. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013;73:1374–85. doi: 10.1158/0008-5472.CAN-12-2362. [DOI] [PubMed] [Google Scholar]
- 21.Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res. 2003;9:391–401. [PubMed] [Google Scholar]
- 22.Yeh YT, Hou MF, Chung YF, Chen YJ, Yang SF, Chen DC, et al. Decreased expression of phosphorylated JNK in breast infiltrating ductal carcinoma is associated with a better overall survival. Int J Cancer. 2006;118:2678–84. doi: 10.1002/ijc.21707. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney EE, Fan P, Jordan VC. Mechanisms underlying differential response to estrogen-induced apoptosis in long-term estrogen-deprived breast cancer cells. Int J Oncol. 2014;44:1529–38. doi: 10.3892/ijo.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 25.Obiorah I, Sengupta S, Fan P, Jordan VC. Delayed triggering of oestrogen induced apoptosis that contrasts with rapid paclitaxel-induced breast cancer cell death. Br J Cancer. 2014;110:1488–96. doi: 10.1038/bjc.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obiorah IE, Jordan VC. Differences in the rate of oestrogen-induced apoptosis in breast cancer by oestradiol and the triphenylethylene bisphenol. Br J Pharmacol. 2014;171:4062–72. doi: 10.1111/bph.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan P, Agboke FA, McDaniel RE, Sweeney EE, Zou X, Creswell K, et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2014;50:457–68. doi: 10.1016/j.ejca.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha DA, Gurzov EN, Naamane N, Ortis F, Cardozo AK, Bugliani M, et al. JunB protects β-cells from lipotoxicity via the XBP1-AKT pathway. Cell Death Differ. 2014;21:1313–24. doi: 10.1038/cdd.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pink JJ, Jiang SY, Fritsch M, Jordan VC. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995 May;55:2583–90. [PubMed] [Google Scholar]
- 30.Aesoy R, Sanchez BC, Norum JH, Lewensohn R, Viktorsson K, Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008;6:1630–8. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 31.Fan P, Agboke FA, Cunliffe HE, Ramos P, Jordan VC. A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. Eur J Cancer. 2014;50:2866–76. doi: 10.1016/j.ejca.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–81. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–72. doi: 10.1038/nature13228. [DOI] [PubMed] [Google Scholar]
- 34.Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–97. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 35.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosoi T, Hyoda K, Okuma Y, Nomura Y, Ozawa K. Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 2007;1152:27–31. doi: 10.1016/j.brainres.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 37.Sweeney EE, McDaniel RE, Maximov PY, Fan P, Jordan VC. Models and Mechanisms of Acquired Antihormone Resistance in Breast Cancer: Significant Clinical Progress Despite Limitations. Horm Mol Biol Clin Investig. 2012;9:143–63. doi: 10.1515/hmbci-2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–9. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 39.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 40.Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1:112–6. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 41.Hait WN, Versele M, Yang JM. Surviving metabolic stress: of mice (squirrels) and men. Cancer Discov. 2014;4:646–9. doi: 10.1158/2159-8290.CD-14-0114. [DOI] [PubMed] [Google Scholar]
- 42.Dillion RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–45. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 43.So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012;16:487–99. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schröder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- 45.Lassot I, Robbins I, Kristiansen M, Rahmeh R, Jaudon F, Magiera MM, et al. Trim17, a novel E3 ubiquitin-ligase, initiates neuronal apoptosis. Cell Death Differ. 2010;17:1928–41. doi: 10.1038/cdd.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65:341–57. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn R, Klos DA, Adler AS, Hicke L. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J Cell Biol. 2004;165:135–44. doi: 10.1083/jcb.200309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemus L, Goder V. Regulation of endoplasmic reticulum-associated protein degradation (ERAD) by ubiquitin. Cell. 2014;3:824–47. doi: 10.3390/cells3030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garyali P, Segvich DM, DePaoli-Roach AA, Roach PJ. Protein degradation and quality control in cells from laforin and malin knockout mice. J Biol Chem. 2014;289:20606–14. doi: 10.1074/jbc.M114.580167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babst M, Odorizzi G. The balance of protein expression and degradation: an ESCRTs point of view. Curr Opin Cell Biol. 2013;25:489–94. doi: 10.1016/j.ceb.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.