Abstract
Successful cancer control relies on overcoming resistance to cell death and on activation of host antitumor immunity. Oncolytic viruses are particularly attractive in this regard, as they lyse infected tumor cells and trigger robust immune responses during the infection. However, repeated injections of the same virus promote antiviral rather than antitumor immunity and tumors may mount innate antiviral defenses to restrict oncolytic virus replication. In this article, we have explored if alternating the therapy virus could circumvent these problems. We demonstrate in two virus-resistant animal models a substantial delay in antiviral immune- and innate cellular response induction by alternating injections of two immunologically distinct oncolytic viruses, adenovirus, and vaccinia virus. Our results are in support of clinical development of heterologous adeno-/vaccinia virus therapy of cancer.
Introduction
As conventional forms of therapy, including chemotherapy and radiation, cause damage to healthy tissue and often fail in the long-term due to acquired resistance by advanced tumors, new approaches are needed. Oncolytic viruses can be engineered to be selectively attenuated in normal cells while retaining the cytotoxic capacity of wild-type viruses in cancer cells.1 Virus infection also triggers an immune response which may expose cancer cells to recognition by the immune system.2,3 However, in most cases, effective therapy necessitates several virus injections which disproportionately boosts immune responses against the virus rather than the tumor.4–7
One strategy successfully adopted by the vaccine field to mitigate the negative impact of neutralizing antivector immune responses has been to switch the vector to another carrying the same target antigen but appearing distinct to the immune system. This approach, termed heterologous prime-boost vaccination, is showing promise both in preclinical models8–10 and in clinical trials,11,12 and it is no surprise it is garnering interest also in the oncolytic virotherapy realm.13,14 A recent study featuring sequential heterologous tumor treatment with an oncolytic adenovirus and the Lister strain of vaccinia virus demonstrated in two separate syngeneic hamsters tumor models a CD3 lymphocyte-dependent tumor clearance in up to 70% of treated animals.15 However, in these models, both viruses replicated efficiently, a scenario which is unlikely to occur in every cancer patient, and the viruses were given intratumorally, which does not reveal potential systemic virus-virus interference. Moreover, not all patients mount strong T-cell responses, particularly when immunosuppressed, and therefore, the efficacy of oncolytic virotherapy must rely on virus replication and tumor oncolysis rather than virus-induced antitumor immune responses. In this regard, oncolytic viruses display a varying infectivity range for human cancer cells, dependent on both the abundance of virus receptors and on intracellular and/or paracrine factors regulating virus replication, notably type I interferon (IFN-I).16,17 Tumor permissiveness to oncolytic viruses may be influenced also by prior chemo- or radiotherapy, which may activate IFN-I signaling.18
Considering the heterogeneous nature of human tumors and possible loss of permissiveness to infection either before or after virus injection, we explored the efficacy and safety of heterologous adeno-/vaccinia virus therapy under conditions where replication of one of the viruses (adenovirus) was basally or progressively limited. We demonstrate that while both viruses can cotransduce cancer cells in vitro, they prefer mutual exclusion and do not enhance each other’s replication. This does not, however, seem to limit coinfection of primary human tumor tissue ex vivo or human tumor xenografts in vivo, where both viruses were able to infect heterologously preinfected distant tumor nests upon intravenous virus administration. Importantly, vaccinia virus was able to increase therapeutic efficacy in a model of acquired cancer resistance to adenovirus, suggesting beneficial virus cooperation via paracrine modulation of tumor antiviral defenses. Finally, we confirm in the poorly adenovirus-permissive mouse B16.OVA melanoma model that heterologous virus treatment still affords similar efficacy as single virus treatment and also results in a significant delay in neutralizing adenovirus antibody induction. Taken together, our results highlight that heterologous virotherapy could be effective in situations where tumors have been rendered virus-resistant to at least one of the viruses.
Results
Oncolytic adenovirus and vaccinia virus preferentially occupy individual infection niches but do not prevent each other’s replication in human cancer cell cultures and primary tumor tissue
Heterologous virus infection in human cells may lead to enhancement or inhibition of replication of either virus, depending both on virus-induced paracrine factors and on heterologous interactions within the same cell, potentially affecting combination therapy efficacy and safety.19–21 We therefore assessed how oncolytic adenovirus (Ad5/3-Δ24-TK/GFP) and vaccinia virus (vvdd-tdTomato) interact in tumor cells in vitro by coinfecting several human cancer cell lines permissive for both viruses and analyzed the cells by fluorescence- and electron microscopy and viability (MTS) assay.
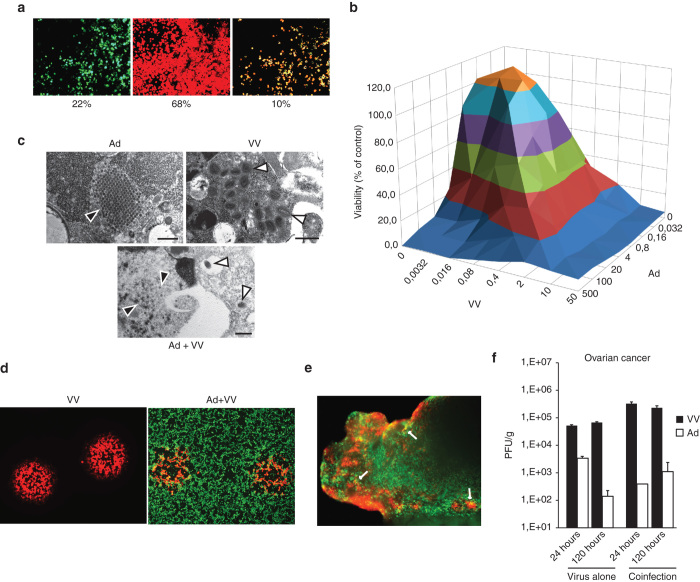
Even at saturating doses of both viruses (10 PFU per cell for vaccinia virus, 100 PFU per cell for adenovirus), double marker gene-positive cells did not exceed 10% at any time after coinfection (Figure 1a, Supplementary Figure 1), implying heterologous virus exclusion in individual cells. This was likely reflected as the slight (~10%) antagonism in combination cell killing we observed at high virus doses (Figure 1b, Supplementary Figure 2. However, cell killing was synergistic at low virus doses (Supplementary Figure 2) and all cells eventually succumbed to progressive virus replication (data not shown). Also, we saw mature virus particles even in coinfected cells on electron microscopic examination (Figure 1c), and vaccinia virus was able to produce plaques on human A549 cell monolayers preinfected for up to 12 hours at 100 PFU/cell with Ad5/3-Δ24-TK/GFP with similar efficiency as on control cell monolayers (Figure 1d). Finally, both viruses were able individually as well as in combination to establish infection in fresh surgical explant tissue from ovarian- and lung carcinoma patients (Figure 1e). While virus marker gene expression patterns in primary cancer tissue resembled those in cultured cells and suggested individual regions of infection, there was neither enhancement nor inhibition of replication of either virus when virus was quantified from tissue homogenates up to 5 days after infection (Figure 1f). Thus, we believe mutual virus exclusion in single cancer cells will not pose an obstacle for coinfection of human tumor tissue.
Figure 1.
Interactions between oncolytic adenovirus and vaccinia virus in vitro and ex vivo. (a) Coinfection of human A549 lung adenocarcinoma cells in culture with adenovirus (Ad5/3-Δ24-TK/GFP, 100 PFU/cell, in green) and vaccinia virus (VV-tdTomato, 10 PFU/cell, in red) resulted in up to 10% double-infected cells (yellow), as assessed semi-quantitatively from fluorescence micrographs. Other tested ratios of adenovirus to vaccinia virus did not improve number of double-positive cells (Supplementary Figure 1). (b) Adeno-vaccinia coinfection of 786-O cancer cells in culture at indicated virus concentrations (PFU/cell for both viruses) resulted in roughly similar degree of cell death as with either virus alone, with slight heterologous antagonism at high multiplicity of infection, also confirmed by combination index calculation (Supplementary Figure 2). (c) Electron micrographs of single or coinfected flow-sorted SKOV3Luc cells (same infection parameters as in a—sorted for GFP+tdTomato+ cells) reveal at 24 hours after infection mature adenovirus particles (black arrows) and vaccinia virus particles (white arrows). Virus factories were not seen in coinfected cells, possibly due to more rapid dissolution of cellular architechture in these cells compared to singly infected cells. Bars = 500 nm. (d) Vaccinia virus (red) is able to replicate and spread in cells preinfected for 12 hours at 10 PFU/cell with human adenovirus (green). Plaques were visualized under fluorescence microscope 72 hours after vaccinia infection. (e) Ovarian cancer tumor slices (~2 mm3) were prepared manually by scalpel and infected immediately after processing in DMEM, 10% FCS with adenovirus (1 × 108 PFU per slice/well) or vaccinia virus (1 × 107 PFU per slice/well) and followed under fluorescence microscope up to 7 days. Shown is a representative fluorescence micrograph of a coinfected ovarian cancer tissue (vaccinia red, adenovirus green). (f) Representative quantitation of infectious virus in primary surgical cancer tissue (ovarian cancer) shows persistence of viruses in tumor tissue despite ongoing heterologous infection.
Ongoing intratumoral replication of adeno- or vaccinia virus does not interfere with entry/infection of heterologous virus from the circulation
While adeno- and vaccinia viruses were able to coinfect tumor tissue in culture, it was not clear how the viruses would interact in tumors in vivo where virus-triggered innate responses could cause vascular collapse22 and potentially hinder entry of a superinfecting virus in separate tumor nodules or metastatic tumor nests. We therefore established bilateral subcutaneous A549 human lung adenocarcinoma xenografts in the flanks of nude mice and injected the tumor on the right side with either PBS or the first virus, followed 2 days later by the second virus intravenously and 2 days after that extracted and titered both viruses from both tumors (preinjected and noninjected) as well as the livers of the animals.
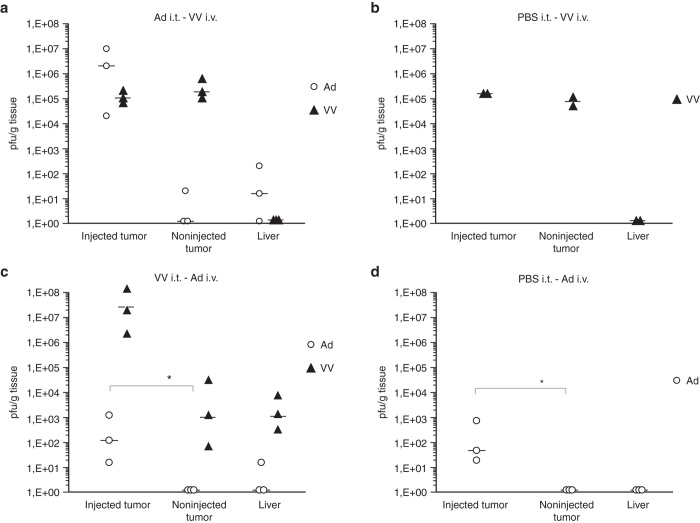
Results show that systemically delivered vaccinia virus was able to establish infection in PBS-injected-, adeno-injected– and noninjected tumors with equal efficiency, assessed both by plaque assay (Figure 2a,b) and qPCR (Supplementary Figure 3a,b). Results were similar for adenovirus when analyzed by qPCR, but infectious virus was only recovered from tumors preinjected with either vaccinia virus or PBS (Figure 2c,d, Supplementary Figure 3c,d). This was intriguing, as it implies that prior infection or physical manipulation of solid tumor tissue promotes replication of systemically delivered oncolytic adenovirus. More importantly, however, these results show that the presence of one of the viruses does not preclude tumor entry/infection by the other virus.
Figure 2.
Virus preinfection of tumors in vivo does not preclude superinfection by heterologous virus. Subcutaneous A549 human lung carcinoma xenografts were implanted in both flanks of nude mice. When tumors had formed, the graft on the right flank was injected intratumorally with either PBS or with 1 × 108 PFU Ad5/3-Δ24 or with 1 × 107 PFU VV-tdTomato. Forty-eight hours later, an i.v. injection was made with either 1 × 108 PFU Ad5/3-Δ24 or with 1 × 107 PFU VV virus. Forty-eight hours after that, tumors and liver were excised and virus was titered by plaque assay/TCID50. (a,b) Vaccinia virus (black triangles) is able upon systemic administration to enter and infect tumors preinfected with adenovirus with equal efficiency as noninfected tumors. (c,d) Infectious adenovirus (empty circles) can only be recovered from tumors preinjected by PBS or vaccinia virus (*P < 0.05, Student’s t-test on log-transformed virus titers). For quantitative genomic virus titers, see Supplementary Figure 3.
Adeno-poxvirus combination virotherapy delays IFN-I–associated acquired antiviral resistance
Having established that adeno- and vaccinia virus do not preclude each other from human cancer tissue in vitro or in vivo, we assessed the efficacy of combination therapy in a model of acquired antiviral resistance. Disseminated intraperitoneal SKOV3Luc human ovarian carcinoma nodules in SCID mice are initially sensitive to adenovirus but then become refractory to the virus over repeated intraperitoneal virus injections in association with upregulation of several IFN-stimulated genes.23 Vaccinia virus, on the other hand, potently antagonizes IFN-I signaling through both intracellular and secreted molecules and may thereby facilitate replication of IFN-sensitive viruses.24,25
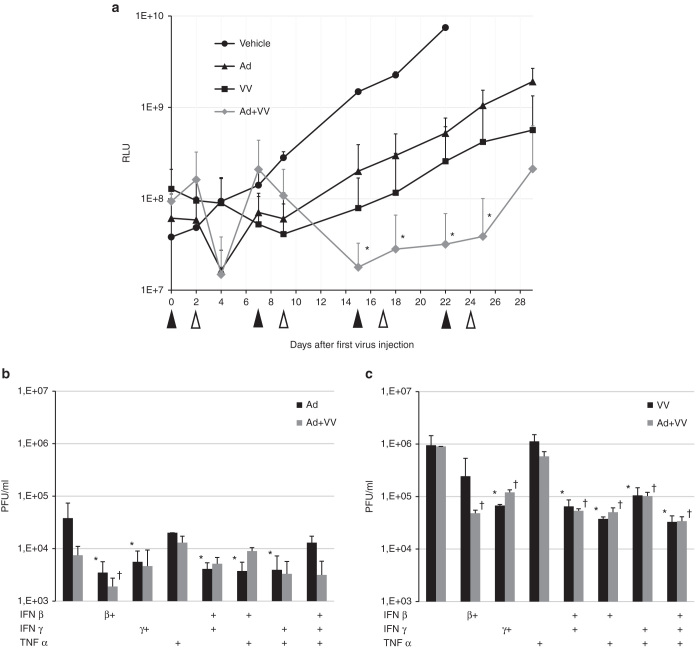
We found that addition of vaccinia virus injections into the weekly adenovirus regimen caused a significant retardation of tumor growth compared to adenovirus alone (Figure 3a). Interestingly, the SKOV3Luc tumors seemed to eventually generate resistance also to vaccinia virus, which has not been reported before. To gain clues about the mechanisms of tumor antiviral resistance, we treated SKOV3Luc cells in culture with cytokines with known antiviral properties—IFN-β and -γ, tumor necrosis factor (TNF)-α, as well as combinations thereof—before virus infection. Results show that both adenovirus and vaccinia virus are able to productively replicate in tumor cells pretreated with IFN-β and -γ, albeit with reduced kinetics compared to untreated control cells (Figure 3b,c). In SKOV3Luc cells, TNF-α did not appear to inhibit either virus. However, all infected cells ultimately succumbed (data not shown), arguing that additional factors contribute to virus resistance in SKOV3Luc cells in vivo.
Figure 3.
Combination with vaccinia virus is able to control growth of disseminated intraperitoneal virus-resistant ovarian cancer. (a) 5- to 7-week-old SCID mice (groups of five mice each) were injected intraperitoneally with 3 × 106 SKOV3Luc cells in 100 µl DMEM. Three days later, mice received an i.p. injection of either PBS (vehicle) or 1 × 109 VP adenovirus (Ad5/3-Δ24) (black arrows). Two days after, mice received i.p. either PBS or 1 × 108 PFU vaccinia virus (open arrows). The schedule was maintained weekly until study termination. Tumor burden was quantitated by IVIS; shown are means + SD of group tumors. This is a model where resistance to adenovirus develops rapidly.23 A similar result emerged for vaccinia virus alone, but when the two viruses were combined, a significant (P < 0.05, area under curve) additive reduction in overall tumor burden was observed. (b,c) Adenovirus and vaccinia virus titers (PFU/well) 72 hours after infection, respectively, in SKOV3Luc cells pretreated or not with combinations of IFN-I (human recombinant IFN β, 5,000 IU/ml final conc.), IFN-II (human recombinant IFN-γ, 500 ng/ml final conc.) or TNF-α (500 ng/ml final conc.) for 4 hours prior to infection with adenovirus or vaccinia virus or both (Ad5/3-Δ24-TK/GFP, 10 PFU/cell, VV-tdTomato, 0.1 PFU/cell). Results show a statistically significant reduction (*) of both adenovirus and vaccinia virus by any regimen containing IFN-β or -γ but not TNF-α.
Adeno-vaccinia combination therapy yields antitumor effects in a poorly adenovirus-permissive immunocompetent tumor model
Whereas adeno-vaccinia combination therapy was effective in immunocompetent hamsters where both viruses replicate,15 we wanted to assess whether heterologous virotherapy would work in immunocompetent animals harboring tumors intrinsically semi-permissive to at least one of the viruses.17,26 We proceeded with subcutaneous B16.OVA mouse melanoma tumors, which are infectable by human adenovirus but which do not display significant oncolysis in vitro in our hands (Supplementary Figure 4) and treated them with intratumoral virus or PBS injections according to the schedule shown in Supplementary Figure 5, which included vaccinia virus at 1 × 108 PFUs per injection and two consecutive high-dose injections of 1 × 1010 viral particles (VPs, roughly equivalent of 2 × 108 PFUs in human cells) of adenovirus to evoke an inflammatory response even in the absence of virus replication.
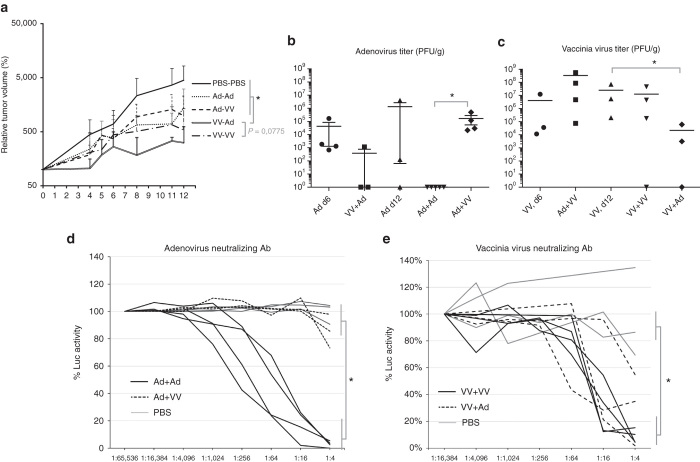
Animals receiving an intratumoral “prime” of vaccinia virus showed a statistically significant retardation of tumor growth compared to mice in which vaccinia virus was given as a booster, where the VV+Ad regimen was more effective than the reciprocal Ad+VV treatment as well as the homologous Ad+Ad regimen (Figure 4a). Homologous adenovirus prime-boost resulted in loss of adenovirus from all mice at the end of the experiment (Figure 4b), associated with greater induction of neutralizing antibodies than in the group receiving heterologous Ad+VV prime-boost (Figure 4d). Compared to a single injection of adenovirus alone (Ad, d12), a booster injection of vaccinia virus (Ad+VV) seemed to allow adenovirus to persist in the tumors (Figure 4b), although variation within the groups did not permit statistical confirmation. Reciprocally, however, boosting with adenovirus (VV+Ad) resulted in a statistically significant reduction of vaccinia virus replication compared to vaccinia virus alone (VV, d12) (Figure 4c).
Figure 4.
Combination virotherapy reduces tumor burden in immunocompetent mice and delays virus clearance. (a) A summary of two independent experiments (n = 10 per group) performed according to the scheme in Supplementary Figure 5 revealed antitumor effects of all treatment regimens compared to PBS, and statistically significant difference of VV-primed groups compared to the other groups and a trend toward VV+Ad being the best group compared to the next best group, VV+VV (P = 0.0775, AUC). (b) Titration of tumors at study endpoint, 6 days after the last injection, shows that vaccinia virus injection into a tumor previously injected with adenovirus (Ad+VV) does not reduce adenovirus titers compared to adenovirus alone (Ad d12). This is in stark contrast to the complete elimination of infectious adenovirus in tumors in the homologous Ad+Ad prime-boost group (*P < 0.05, Student’s t-test on log-transformed virus titers). (c) Vaccinia virus is able to persist for up to 12 days in B16.OVA tumors irrespective of homologous prime-boost injection or of the presence of adenovirus but that adenovirus injection into tumors preinfected with vaccinia virus reduces VV titers (*) compared to PBS-injection (VV d12). (d) Adenovirus serum neutralizing antibody assay shows that the homologous Ad+Ad regimen significantly accelerates neutralizing antibody induction compared to heterologous Ad+VV regimen. Each animal is shown as an individual line. (e) Injection of adenovirus into VV-preinfected tumors (VV+Ad) does not alter the magnitude of anti-VV antibody induction compared to VV itself (VV+VV).
To confirm that vaccinia virus retains its oncolytic capacity in B16.OVA tumors whereas adenovirus is not able to lyse these cells, we explanted tumor tissue from singly-treated tumors day 6 after infection and monitored for cell outgrowth for up to 9 days. Results showed cells quickly populated the culture dishes from adenovirus-treated and PBS-injected tumors, whereas vaccinia virus, confirmed by tdTomato expression, destroyed any attaching cells and prevented cell outgrowth (Supplementary Figure 6).
Taken together, our results show that heterologous vaccinia-adenovirus treatment is effective even in adenovirus-resistant tumors and delays induction of neutralizing adenovirus-antibodies.
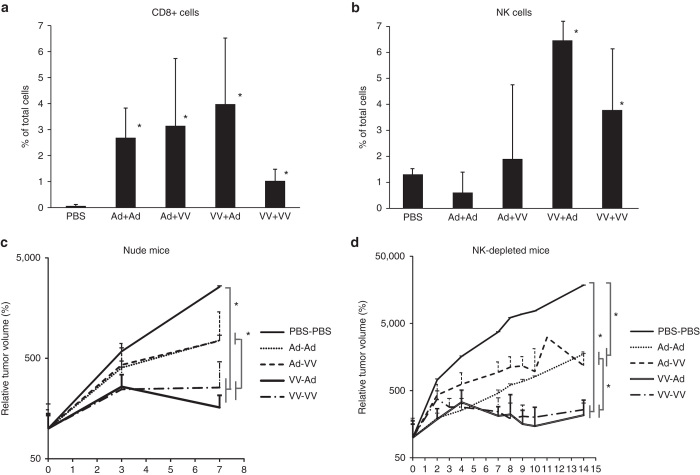
Lack of T/B cells or NK cells does not affect heterologous virotherapy under single-virus–restricted settings
To understand the role of virus-induced immune responses in the vaccinia-biased B16.OVA tumor model during heterologous virotherapy, we first characterized the tumor immune infiltrate after heterologous virotherapy. We saw a statistically significant increase in CD3+CD8+ cells in all virus-treated groups compared to PBS-injected tumors and a statistically significant increase in NK cells in vaccinia primed tumors compared to all other groups (Figure 5a,b). To test the functional significance of these immune cell subsets, we treated both nude mice, which display a wide range of immune dysfunction including mature T and B cells, and NK-cell–depleted C57 mice (Supplementary Figure 7) harboring B16.OVA tumors with the virus combinations. Vaccinia prime was again more effective than adenovirus prime both in nude mice and in NK-depleted animals (Figure 5c,d), showing that mature T and B cells or NK cells are dispensable for vaccinia virus oncolytic potency in the B16.OVA mouse model, as has been observed also in other tumor models,27,28 and highlighting that heterologous vaccinia-adenovirus therapy will be effective even under selectively immunocompromised conditions. Compared to the recent findings in hamster tumor models where both viruses were shown to replicate and where priming with adenovirus gave the strongest antitumor efficacy,15 we show better efficacy when priming with the more replicating virus in tumors resistant to one of the viruses.
Figure 5.
Mature T/B cells or NK cells triggered by heterologous virus injections do not explain antitumor efficacy. Tumors in Figure 4a were minced and passed through a 40 µm nylon mesh to create a single cell suspension. After overnight rest at 37 °C, cells were stained and analyzed by flow cytometry. (a) Intratumoral adeno- or vaccinia virus injections in the B16.OVA model resulted in a statistically greater influx of cytotoxic T cells (CD3+CD8+) compared to PBS. These were not specific for chicken ovalbumin expressed in the B16.OVA cells or endogenous melanocyte antigen TRP2 by pentamer staining (data not shown). (b) NK cells (NKp46+) were found more numerous in tumors treated with vaccinia virus as the first injection. Heterologous virotherapy was as effective (c) in NK-depleted mice as (d) in nude mice, which do not harbor mature T or B cells.
Discussion
While the capacity of vaccine vectors to replicate has been proposed to be critical for proper CD8 T-cell responses in immunocompetent hosts,29 the issue of disparate host cell permissiveness or potential interference by host innate responses has not been addressed with multivirus therapy. Most human cancer cell lines are readily infectable and killed by oncolytic viruses in vitro and mathematical models are useful to guide curative virus dosing in experimental tumors in vivo.30–32 Yet, complete tumor responses in human cancer patients are rare and lack of transgene expression in the majority of patients following repeated intratumoral or intravesical virus injections argues that human tumors rapidly mount antiviral defenses to halt virus replication.33,34 To address this issue with two promising clinical oncolytic virus candidates, adenovirus and vaccinia virus, we first we confirmed that the two viruses did not preclude each other from human tumor cells in culture/ex vivo or from tumor nests in vivo. This information was, on one hand, a prerequisite for testing how a superinfecting virus would affect acquired innate virus resistance. On the other hand, coinjection heterologous virotherapy is also being developed for clinical applications,35,36 necessitating understanding of how viruses interact locally in tumors, where both heterologous antagonism and virus enhancement could impact safety and efficacy.21
Despite a preference for one virus dominating each cell, possibly because of differences in entry and replication kinetics, some double-infection did occur with adenovirus and vaccinia virus, even in primary human cancer tissue where replication of either virus was not affected by coinfection to a significant degree (Figure 1f). This was in contrast to a study combining modified vaccinia virus Ankara (MVA) with adenovirus, where coinfection resulted in shutdown of adenovirus promoter activity.37 It is likely that innate cell responses elicited by MVA, which are known to be greater than those elicited by vaccinia virus in many cell types38,39 were responsible for interference with adenovirus replication during MVA+Ad coinfection in that study. Reciprocally, in our own study in B16.OVA tumors, we noted that adenovirus injection into tumors primed with VV resulted in reduced VV titers compared to PBS-injected control tumors (Figure 4c), indicating that in these mouse tumors adenovirus triggered responses capable of interfering with VV replication. Such responses may involve NK cells, which are antiviral against another oncolytic virus, HSV-1,40 and which are induced also in the absence of adenovirus replication,41 and antiviral cytokines, such as TNF-α and IFN-I and -II to which vaccinia virus in sensitive in mice.27,42
As heterologous virus interference was minimal in human tumor tissue ex vivo, we tested and found that both vaccinia virus and adenovirus were able to enter and infect tumor tissue in vivo already preinfected with the other virus (Figure 2), allowing us to assess the impact of heterologous virotherapy on innate paracrine tumor defenses. Interestingly, while vaccinia virus entered both injected and noninjected tumors with similar efficacy (Figure 2a,b), intravenous injection of adenovirus resulted in recovery of infectious virus only from PBS- or VV-preinjected tumors (Figure 2c,d). It is possible, that physical manipulation of the tumors caused pressure-induced changes in cell surface receptor expression43 that may have facilitated adenovirus entry. Another possibility is that the degree of intratumoral blood-clotting increased upon PBS/VV-injection, sequestering the intravenously injected adenovirus.44 While interesting, we considered these mechanisms unrelated to heterologous virus interactions and therefore beyond the scope of this article.
Subsequently, in the intraperitoneal SCID mouse human ovarian carcinoma SKOV3Luc model, known to acquire resistance to adenovirus,23 addition of vaccinia virus resulted in significant tumor control over either virus alone (Figure 3a). However, even if IFN-I or IFN-γ were able to slow down virus replication in SKOV3Luc cells in vitro (Figure 3b,c), these cytokines were unlikely the sole factors responsible for the tumor antiviral defense in vivo, as tumors in the mice ultimately progressed despite continued injections, whereas IFN-pretreated SKOV3Luc cells in culture succumbed. A recent study showed that pretreatment of mice with a replication defective human adenovirus vector expressing murine IFN-α generated a systemic protective antiviral response against lethal wild-type vaccinia virus challenge,45 demonstrating that innate responses are able to control even vaccinia virus, despite that such inhibition is not apparent in cultured cancer cells. Therefore, our conclusion is that the in vivo microenvironment and/or mouse stroma protects the SKOV3Luc cells from the viruses through additional mechanisms not apparent in vitro, but that such resistance can still be reduced or delayed by reciprocal virus complementation.
From a therapeutic point of view, it is not clear how innate responses from virus-exposed normal cells would affect overall outcome; while innate responses may slow virus replication/spread in tumors, it is even possible that overall efficacy would be increased, as IFNs have antitumor activity per se and—possibly even more importantly—are critical in bridging the innate and adaptive arms of the immune system.27 However, in the B16.OVA model in which adenovirus replication is significantly poorer than vaccinia virus (Supplementary Figures 5 and 6), but which is prone to adenovirus particle-triggered inflammatory responses, treatment efficacy was greater in vaccinia- than in adenovirus-primed mice (Figure 4a), arguing that the more replicative virus must compensate for lack of replication of the other. We assessed the role of select immune cells that were induced by the combinations (Figure 5a,b) in antitumor efficacy but could not see a clear difference in the pattern of tumor control in NK-depleted or nude mice compared to normal C57 mice (Figure 5c,d versus Figure 4a); again vaccinia virus prime was better than adenovirus prime, and may even have produced stronger tumor control than in C57 mice, arguing that the analyzed immune cell subsets are antiviral toward vaccinia virus, although variation between individual mice did not permit firm conclusions. Interestingly, when instead adenovirus was used as prime, booster injection of vaccinia virus stabilized adenovirus titers in the tumors up until the end of the experiment compared to the homologous Ad+Ad group (Figure 4b). Although differences to adenovirus alone were not significant, we speculate that vaccinia virus may have antagonized innate antiviral responses to adenovirus, similarly to VV-mediated facilitation of other oncolytic viruses in tumors.26
While vaccinia virus exerted significant tumor control on its own in the B16.OVA model, the synergy that is observed upon sequential or prime-boost type treatment of tumors with different viruses in different types of tumors10,13,14 warrants further scrutiny to optimize heterologous prime-boost-type virotherapy for human use in the future. Our data shows that acquired tumor resistance or unequal permissiveness is not an obstacle for such development and that host antiviral responses may be delayed by switching viruses without losing treatment efficacy.
Materials and Methods
Cells and viruses
Human renal carcinoma 786-O and lung carcinoma A549 cells were from American Type Culture Collection. SKOV3Luc human ovarian carcinoma cells have been described.23 Mouse melanoma B16.OVA cells were a kind gift of Prof. Richard Vile, Mayo Clinic, MN. Cells were propagated at 37 °C and 5% CO2 in high glucose Dulbecco’s modified Eagle medium (HyClone) supplemented with 10% fetal calf serum (FCS) for human cells or RPMI, 10% FCS and 10% G418 for B16.OVA. Ad5/3-Δ24 and Ad5/3-Δ24-TK/GFP have been described.46 Vaccinia virus strain Western Reserve deleted described.47 Vaccinia virus infectious units were determined by standard plaque assay on Vero cells where coinfecting adenovirus did not form plaques in the 48-hour assay time window. Adenovirus infectious units were determined by standard 50% tissue culture infective dose (TCID50) assay in A549 cells, where coinfecting vaccinia virus was first removed by 0.2 um sterile filtration. Adenovirus infectious units are expressed as plaque-forming units (PFU), estimated using the formula 1 TCID50 = 0.7 PFU.
Cytotoxicity assays
Cell viability was measured by MTS assay according to manufacturer’s instructions (CellTiter 96 AQueous One, Promega, Madison, WI). For testing synergistic cell killing, 786-O cells in 96-well plates (50,000 cells per well) were infected in sixtuplicate with serial dilutions of vaccinia virus and adenovirus, and 72 hours later viability was assessed by MTS and combination index calculated using CalcuSyn software (Biosoft) according to the method of Chou and Talalay.
Electron microscopy
Cells were gently scraped off and immediately fixed with glutaraldehyde (2% final conc) and stored at 4 °C. The next day, cells were dehydrated, embedded in LX-112 resin, sectioned and mounted on EM grids and analyzed under JEOL 1400 Transmission Electron Microscope as described.48
Patients tumor explant tissues
Primary tumor tissue was obtained fresh from the surgical theater under Ethics committee permission and informed consent and placed in chilled DMEM supplemented with 10% FCS and 2× Penicillin/streptomycin (Life Technologies, Gibco, USA). Tumor tissue was promptly cut into small pieces manually using scalpel blades and one piece placed per well in a 24-well plate with 0.5 ml standard DMEM. Tumor bits in triplicate were infected with viruses (Ad5/3-Δ24-TK/GFP, 1 × 108 PFU per slice or vaccinia virus, 1 × 107 PFU per slice), and followed under fluorescence microscope over 7 days.
Animal experiments
Nude mouse A549 model.
3 × 106 A549 cells were implanted subcutaneously in nude mice. When palpable tumors had formed, grafts on the right flank were injected intratumorally with either PBS or with 1 × 108 PFU Ad5/3-Δ24 or with 1 × 107 PFU VV-tdTomato (n = 3 per group). Forty-eight hours later, an i.v. injection was made with either 1 × 108 PFU Ad5/3-Δ24 or with 1 × 107 PFU VV virus. Forty-eight hours after that, tumors and liver were excised and virus was titered by qPCR.
SCID mouse model.
3 × 106 SKOV3Luc cells were injected intraperitoneally into 5- to 6-week-old female SCID mice. Three days later, mice were divided into groups (n = 5) and received either 100 µl PBS or 1 × 109 VP Ad5/3-Δ24 virus in 100 µl PBS i.p. Two days later, mice received either 100 µl PBS or 1 × 108 PFU vaccinia virus in 100 µl PBS. The adeno-vaccinia regimen was repeated every week for 4 weeks total so that adenovirus injections were always 7–9 days apart. Mice were imaged twice a week for the duration of the experiment by IVIS (Xenogen); in brief, each mouse received an i.p. injection of 3 mg D-luciferin (MBP Bio) in 100 µl PBS and was imaged 8 minutes later for 10 seconds under isoflurane anesthesia. Experiment was repeated twice.
B16.OVA model.
Female mice (nude or C57BL/6) aged 5–6 weeks received 2.5 × 105 B16.OVA cells subcutaneously in the right flank in 50 µl RPMI. When palpable tumors had formed (10 days later), mice were divided into groups (n = 5) and received either 50 µl PBS or 1 × 1010 VP Ad5/3-Δ24 virus or 1 × 108 PFU vaccinia virus in 50 µl PBS i.t. Mice receiving adenovirus received another similar injection the day after. Six days after the first virus injection, a separate set of mice (four each) were euthanized for virus titration and another set of mice received another intratumoral injection of either adenovirus or vaccinia virus, forming the indicated treatment groups (five to six mice each) as depicted in Supplementary Figure 3. Twelve days after the first virus injection remaining mice were euthanized and organs and tumor extracted for analysis. Experiment was repeated twice.
NK depletion.
Anti-asialo GM1 (Wako Chemicals, cat no 986–10001, Neuss, Germany) was diluted 1.4 times with distilled water, then 50 µl per mouse (35.7 µl undiluted antibody) was given i.p. on days −3, 0, 7, and 15 after tumor implantation. NK-cell depletion was confirmed by FACS (Supplementary Figure 7).
Nude mice.
Because of accelerated B16.OVA tumor growth rate in nude mice, regimen was altered so that booster was given 3 days after prime and animals were euthanized 4 days after that.
Quantitative virus PCR
qPCR for oncolytic adenovirus using E4-specific primers and probe has been described.49 Using the same methodology, qPCR for vaccinia virus was performed using primers/probe specific for the terminal VGF regions: FWD 5′-gatgatgcaactctatcatgta-3′, REV 5′-gtataattatcaaaatacaagacgtc-3′, probe 5′-FAM-agtgcttggtataaggag-3′.
Immunological assays
Tumors from the B16.OVA experiment were minced in RPMI, 10% FCS, supplemented with 2× penicillin/streptomycin (Gibco) through a 40 um nylon mesh to create single-cell suspensions. Cells pooled from five mice were incubated in 20 cm diameter culture dishes in RPMI at standard culture conditions for 24 hours, following which they were stained with the following rat antimouse antibody cocktail (antibodies from BD): NKp46-V450, CD19-PE or CD19-FITC, CD3-PerCP-Cy5.5, CD8-PE or CD8-FITC. Additionally, cells were stained with pentamers against TCRs specific for MHC-I expressed chicken ovalbumin or endogenous melanocyte antigen TRP2 (Proimmune, cat no. 093 and 185). Labeled cells were washed twice in FACS buffer (2% FBS, PBS) and analyzed by flow cytometry (BD FACSAria cytometer, FACSDiva software), counting at least 100,000 events per sample.
Statistics
Kaplan–Meier survival data was analyzed by log rank test. Group virus titers were log-transformed and compared using two-tailed Student’s t-test. Serial measurements (area-under-curve) analysis for IVIS and tumor volume data was performed using MedCalc software. Viability data was compared by unpaired two-tailed Student’s t-test.
Acknowledgments
This study has been supported by Biocentrum Helsinki, Biocenter Finland, European Research Council, ASCO Foundation, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Academy of Finland, Finnish Cancer Organizations.
Footnotes
A.H. is an employee and a shareholder in TILT Biotherapeutics Inc and in Oncos Therapeutics, Ltd. The other authors declare no conflict of interest.
References
- Naik, S and Russell, SJ (2009). Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther 9: 1163–1176. [DOI] [PubMed] [Google Scholar]
- Tong, AW, Senzer, N, Cerullo, V, Templeton, NS, Hemminki, A and Nemunaitis, J (2012). Oncolytic viruses for induction of anti-tumor immunity. Curr Pharm Biotechnol 13: 1750–1760. [DOI] [PubMed] [Google Scholar]
- Kanerva, A, Nokisalmi, P, Diaconu, I, Koski, A, Cerullo, V, Liikanen, I et al. (2013). Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 19: 2734–2744. [DOI] [PubMed] [Google Scholar]
- Pesonen, S, Kangasniemi, L and Hemminki, A (2011). Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm 8: 12–28. [DOI] [PubMed] [Google Scholar]
- Crompton, AM and Kirn, DH (2007). From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets 7: 133–139. [DOI] [PubMed] [Google Scholar]
- Diaconu, I, Cerullo, V, Hirvinen, ML, Escutenaire, S, Ugolini, M, Pesonen, SK et al. (2012). Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 72: 2327–2338. [DOI] [PubMed] [Google Scholar]
- Cerullo, V, Pesonen, S, Diaconu, I, Escutenaire, S, Arstila, PT, Ugolini, M et al. (2010). Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 70: 4297–4309. [DOI] [PubMed] [Google Scholar]
- Paris, RM, Kim, JH, Robb, ML and Michael, NL (2010). Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines 9: 1055–1069. [DOI] [PubMed] [Google Scholar]
- Barefoot, B, Thornburg, NJ, Barouch, DH, Yu, JS, Sample, C, Johnston, RE et al. (2008). Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine 26: 6108–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näslund, TI, Uyttenhove, C, Nordström, EK, Colau, D, Warnier, G, Jondal, M et al. (2007). Comparative prime-boost vaccinations using Semliki Forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory CTL response against the P815 tumor. J Immunol 178: 6761–6769. [DOI] [PubMed] [Google Scholar]
- Watkins, DI (2010). HIV vaccine development. Top HIV Med 18: 35–36. [PubMed] [Google Scholar]
- O’Connell, RJ, Kim, JH, Corey, L and Michael, NL (2012). Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med 2: a007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle, BW, Clouthier, D, Zhang, L, Pol, J, Chen, L, Lichty, BD et al. (2013). Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8(+) T-cell responses to anticancer vaccines. Oncoimmunology 2: e26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, JG, Zhang, L, Bridle, BW, Stephenson, KB, Rességuier, J, Hanson, S et al. (2014). Maraba virus as a potent oncolytic vaccine vector. Mol Ther 22: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysome, JR, Li, X, Wang, S, Wang, P, Gao, D, Du, P et al. (2012). A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin Cancer Res 18: 6679–6689. [DOI] [PubMed] [Google Scholar]
- Hall, K, Blair Zajdel, ME and Blair, GE (2010). Unity and diversity in the human adenoviruses: exploiting alternative entry pathways for gene therapy. Biochem J 431: 321–336. [DOI] [PubMed] [Google Scholar]
- Monsurrò, V, Beghelli, S, Wang, R, Barbi, S, Coin, S, Di Pasquale, G et al. (2010). Anti-viral state segregates two molecular phenotypes of pancreatic adenocarcinoma: potential relevance for adenoviral gene therapy. J Transl Med 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarev, NN, Roizman, B and Weichselbaum, RR (2012). Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin Cancer Res 18: 3015–3021. [DOI] [PubMed] [Google Scholar]
- Khoobyarian, N (1964). Interference induced against vaccinia in an adenovirus-RHF-1 system. J Bacteriol 87: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkassar, M, Gärtner, B, Roemer, K, Graesser, F, Rommelaere, J, Kaestner, L et al. (2011). The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo. J Neurooncol 104: 715–727. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela, MJ, Le Boeuf, F, Lemay, C, De Silva, N, Diallo, JS, Cox, J et al. (2013). Resistance to two heterologous neurotropic oncolytic viruses, Semliki Forest virus and vaccinia virus, in experimental glioma. J Virol 87: 2363–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach, CJ, Arulanandam, R, De Silva, N, Thorne, SH, Patt, R, Daneshmand, M et al. (2013). Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res 73: 1265–1275. [DOI] [PubMed] [Google Scholar]
- Liikanen, I, Monsurrò, V, Ahtiainen, L, Raki, M, Hakkarainen, T, Diaconu, I et al. (2011). Induction of interferon pathways mediates in vivo resistance to oncolytic adenovirus. Mol Ther 19: 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf, F, Diallo, JS, McCart, JA, Thorne, S, Falls, T, Stanford, M et al. (2010). Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther 18: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parato, KA, Breitbach, CJ, Le Boeuf, F, Wang, J, Storbeck, C, Ilkow, C et al. (2012). The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther 20: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning, P, Andersson, KM, Frykholm, K, Ali, A, Magnusson, MK, Nygren, PA et al. (2005). Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Ther 12: 211–224. [DOI] [PubMed] [Google Scholar]
- Wang, LC, Lynn, RC, Cheng, G, Alexander, E, Kapoor, V, Moon, EK et al. (2012). Treating tumors with a vaccinia virus expressing IFNß illustrates the complex relationships between oncolytic ability and immunogenicity. Mol Ther 20: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel, S, Raab, V, Yu, YA, Worschech, A, Wang, E, Marincola, FM et al. (2011). Viral-mediated oncolysis is the most critical factor in the late-phase of the tumor regression process upon vaccinia virus infection. BMC Cancer 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster, P, Teigler, JE, Obeng, RC, Kang, ZH, Provine, NM, Parenteau, L et al. (2014). Augmented replicative capacity of the boosting antigen improves the protective efficacy of heterologous prime-boost vaccine regimens. J Virol 88: 6243–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, K, Kirk, A, Naik, S, Nace, R, Steele, MB, Suksanpaisan, L et al. (2013). Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS ONE 8: e73759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto, ML, Worschech, A, Yu, Z, Adams, S, Reinboth, J, Chen, NG et al. (2011). Permissivity of the NCI-60 cancer cell lines to oncolytic Vaccinia Virus GLV-1h68. BMC Cancer 11: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne, SH, Hwang, TH, O’Gorman, WE, Bartlett, DL, Sei, S, Kanji, F et al. (2007). Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 117: 3350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer, R, Rochlitz, C, Velu, T, Acres, B, Limacher, JM, Bleuzen, P et al. (2008). Intralesional adenovirus-mediated interleukin-2 gene transfer for advanced solid cancers and melanoma. Mol Ther 16: 985–994. [DOI] [PubMed] [Google Scholar]
- Burke, JM, Lamm, DL, Meng, MV, Nemunaitis, JJ, Stephenson, JJ, Arseneau, JC et al. (2012). A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 188: 2391–2397. [DOI] [PubMed] [Google Scholar]
- Betts, G, Poyntz, H, Stylianou, E, Reyes-Sandoval, A, Cottingham, M, Hill, A et al. (2012). Optimising immunogenicity with viral vectors: mixing MVA and HAdV-5 expressing the mycobacterial antigen Ag85A in a single injection. PLoS ONE 7: e50447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval, A, Rollier, CS, Milicic, A, Bauza, K, Cottingham, MG, Tang, CK et al. (2012). Mixed vector immunization with recombinant adenovirus and MVA can improve vaccine efficacy while decreasing antivector immunity. Mol Ther 20: 1633–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashima, S, Yoshizaki, S, Shinoda, K, Yoshida, A, Kondo, A, Mizuguchi, H et al. (2010). Co-administration of viral vector-based vaccines suppresses antigen-specific effector CD8 T cells. Vaccine 28: 3257–3264. [DOI] [PubMed] [Google Scholar]
- Waibler, Z, Anzaghe, M, Ludwig, H, Akira, S, Weiss, S, Sutter, G et al. (2007). Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. J Virol 81: 12102–12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibler, Z, Anzaghe, M, Frenz, T, Schwantes, A, Pöhlmann, C, Ludwig, H et al. (2009). Vaccinia virus-mediated inhibition of type I interferon responses is a multifactorial process involving the soluble type I interferon receptor B18 and intracellular components. J Virol 83: 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Breckenridge, CA, Yu, J, Price, R, Wojton, J, Pradarelli, J, Mao, H et al. (2012). NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med 18: 1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzek, MC, Kavanagh, BF, Scaria, A, Richards, SM and Garman, RD (2002). Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol Ther 5: 115–124. [DOI] [PubMed] [Google Scholar]
- Sambhi, SK, Kohonen-Corish, MR and Ramshaw, IA (1991). Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci USA 88: 4025–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson, MD, Yu, CF, Herden-Kirchoff, O, Ellermeier, M, Sanders, MA, Merrell, RC et al. (2000). Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem 78: 47–61. [DOI] [PubMed] [Google Scholar]
- Koski, A, Rajecki, M, Guse, K, Kanerva, A, Ristimäki, A, Pesonen, S et al. (2009). Systemic adenoviral gene delivery to orthotopic murine breast tumors with ablation of coagulation factors, thrombocytes and Kupffer cells. J Gene Med 11: 966–977. [DOI] [PubMed] [Google Scholar]
- Smee, DF, Wong, MH, Hurst, BL, Ennis, J and Turner, JD (2013). Effects of nasal or pulmonary delivered treatments with an adenovirus vectored interferon (mDEF201) on respiratory and systemic infections in mice caused by cowpox and vaccinia viruses. PLoS ONE 8: e68685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raki, M, Hakkarainen, T, Bauerschmitz, GJ, Särkioja, M, Desmond, RA, Kanerva, A et al. (2007). Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther 14: 1380–1388. [DOI] [PubMed] [Google Scholar]
- Parviainen, S, Ahonen, M, Diaconu, I, Hirvinen, M, Karttunen, Å, Vähä-Koskela, M et al. (2014). CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther 21: 195–204. [DOI] [PubMed] [Google Scholar]
- Engelhardt, P, Meriläinen, J, Zhao, F, Uchiyama, S, Fukui, K and Lehto, VP (2007). Whole-mount immunoelectron tomography of chromosomes and cells. Methods Mol Biol 369: 387–405. [DOI] [PubMed] [Google Scholar]
- Kanerva, A, Wang, M, Bauerschmitz, GJ, Lam, JT, Desmond, RA, Bhoola, SM et al. (2002). Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 5: 695–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.