Abstract
Chimeric antigen receptor (CAR)-modified adoptive T-cell therapy (ATC) has been successfully applied to the treatment of hematologic malignancies, but faces many challenges in solid tumors. One major obstacle is the immune-suppressive effects induced in both naturally-occurring and genetically-modified tumor infiltrating lymphocytes (TILs) by inhibitory receptors (IRs), namely PD1. We hypothesized that interfering with PD1 signaling would augment CAR T cell activity against solid tumors. To address this possibility, we introduced a genetically-engineered switch receptor construct, comprising the truncated extracellular domain of PD1 and the transmembrane and cytoplasmic signaling domains of CD28, into CAR T-cells. We tested the effect of this supplement, “PD1CD28”, on human CAR T-cells targeting aggressive models of human solid tumors expressing relevant tumor antigens. Treatment of mice bearing large, established solid tumors with PD1CD28 CAR T-cells led to significant regression in tumor volume due to enhanced CAR TIL infiltrate, decreased susceptibility to tumor-induced hypofunction, and attenuation of IR expression compared to treatments with CAR T-cells alone or PD1 antibodies. Taken together, our findings suggest that the application of PD1CD28 to boost CAR T-cell activity is efficacious against solid tumors via a variety of mechanisms, prompting clinical investigation of this potentially promising treatment modality.
Introduction
Adoptive T-cell transfer (ACT) for cancer has demonstrated success in malignant melanoma and hematologic malignancies (1, 2). T-cells were originally derived from tumor-infiltrating lymphocytes (TILs). More recently, engineering T-cells with chimeric antigen receptors (CARs) or tumor-reactive T-cell receptor (TCR) clones has been used to produce tumor-reactive T-cells. TCR engineering allows for the generation of tumor-reactive T-cells that are able to process tumor-associated antigens (TAAs) but require presentation in the MHC:antigen complex (3). CARs, on the other hand, confer high-affinity, high-specificity, MHC-independent recognition of surface TAAs with potent T-cell activation via genetic engineering and the combination of various co-stimulatory domains (4). Though CAR T-cells have demonstrated significant responses in patients with treatment-refractory hematologic malignancies (5), they have resulted in, at best, only modest results in solid tumors. This is likely due to a host of hurdles encountered in the tumor microenvironment (TME) of solid tumors (6–12) including intrinsic inhibitory pathways mediated by upregulated inhibitory receptors (IRs) reacting with their cognate ligands within the tumor (12).
One of the most extensively studied T-cell IRs is programmed death-1 (PD1;CD279). PD1 is a cell surface receptor that belongs to the immunoglobulin superfamily and is expressed on T-cells and pro-B cells (13). Its expression is upregulated after antigen- and ligand-receptor engagement (14), and its currently known ligands are PDL1 (also known as B7-H1 or CD274) and PDL2 (also known as B7-DC or CD273). In the non-malignant context, PD1 is responsible for preventing T-cell-mediated autoimmunity (15). In various cancers, however, PDL1 is upregulated on the surface of solid tumors, often in response to cytokines secreted by T-cells that are tumor-reactive, and serves as a method of immune escape (10). In some studies, expression levels of PDL1 have been shown to correlate with the degree of tumor immune infiltration (16), decreased function of T-cell infiltrates (17), tumor aggressiveness (18), and overall patient prognosis (19). PD1 blockade is being tested as a novel immunotherapeutic in different cancers and has demonstrated durable clinical responses in a subpopulation of patients (20).
Our recent description of solid tumor-induced hypofunction of CAR T-cells demonstrated the contribution of PD1 upregulation on tumor-infiltrating CAR T-cells (21), and supports the strategy of combining adoptive transfer of genetically-redirected human T-cells with blockade of inhibitory signals triggered by IRs. Herein, we demonstrated that combining CAR-based ATC with IR interference is superior in tumor control than either alone.
We first demonstrated this by using anti-PD1 antibodies in combination with CAR T-cells, followed by a genetic approach described by others (22–24) in which T-cells were transduced with both a CAR and a chimeric switch-receptor containing the extracellular domain of PD1 fused to the transmembrane and cytoplasmic domain of the co-stimulatory molecule CD28. We confirmed in our own tumor targets that when the PD1 portion of this switch-receptor engages its ligand, PDL1, it will transmit an activating signal (via the CD28 cytoplasmic domain) instead of the inhibitory signal normally transduced by the PD1 cytoplasmic domain. But more importantly, we demonstrated for the first time that PD1CD28 is able to augment human CAR T-cell control of large, established solid tumors. This is done using human T-cells targeting human tumors bearing clinically relevant tumor antigens. Furthermore, we built upon prior work elucidating multiple mechanisms of PD1CD28’s function and also showed that while PD1 blockade augments the anti-tumor efficacy of CAR T-cells, the use of CAR T-cells expressing PD1CD28 was far superior in controlling tumor burden.
Materials and Methods
Cell lines and cell culture conditions
A human mesothelioma cell line derived from a patient’s tumor (March 2010) was used – EMP (parental). Since EMP did not have baseline expression of the tumor-associated antigen (TAA) mesothelin, it was lentivirally transduced to express human mesothelin (EMMESO). Green fluorescent protein (GFP) with firefly luciferase was lentivirally transduced into the lines to produce EMPffluc and EMMESOffluc.
Nalm6 is a B-cell precursor leukemia with high expression of CD19. (German DSMZ Cell Collection Cat#: ACC 128). Click beetle red (CBG) was lentivirally transduced into Nalm6 to produce Nalm6-CBG.
K562 is a chronic myelogenous leukemia (ATCC, Cat#: CCL-243). CD19 was lentivirally transduced into K562 to produce K562-CD19.
PC3 is a prostate cancer tumor line (ATCC, Cat#: CRL-1435). Prostate specific cancer antigen (PSCA) was lentivirally transduced into PC3 to produce PC3-PSCA. Click beetle green (CBG) was lentivirally transduced into PC3-PSCA to produce PC3-PSCA-CBG.
Nalm6, K562, and PC3 cell lines were purchased from ATCC and authenticated, and cultured as instructed.
All tumor cell lines used expressed low levels of PDL1 in the absence of IFNγ exposure. Thus, PDL1 was also lentivirally transduced into the aforementioned lines to produce versions that had high stable expression of PDL1.
Tumor cells and T-cells were cultured in RPMI 1640 (Gibco 11875-085) supplemented with 10% heat inactivated fetal calf serum (FCS), 100U/ml penicillin, 100ug/ml streptomycin sulfate, and 1% L-glutamine.
Generation of CAR constructs and PD1CD28 switch-receptor
CARs specific for CD19 (CD19Z with CD3ζ signaling), mesothelin (SS1BBz with CD3ζ signaling and 41BB costimulation), and PSCA (PSCA-BBz with CD3ζ signaling and 41BB costimulation) were synthesized and/or amplified by PCR, based on sequencing information provided by the relevant publications (25–28), and subcloned into pGEM.64A RNA-based vector (29), pTRPE lentiviral vectors (25), and MSGV retroviral vectors respectively. (27) (Supplementary Figure 1)
The PD1CD28 switch-receptor was constructed by fusing a truncated extracellular PD1 (AA1-155) derived from PD1-cDNA (Origene, Rockville, MD) with the transmembrane and cytoplasmic domains of CD28 (AA141-220). We also constructed a mutated version of the switch-receptor where signaling was abrogated by modifying the CD28 signaling transduction proximal YMNM motif (mutated to FFFF) and distal proline-rich motifs PRRP (mutated to ARRA) and PYAP (mutated to AYAA). We also constructed a “tailless” version of PD1 where the truncated extracellular PD1 and the PD1 transmembrane domain were included but the signaling domains (ITIM, ITSM) were excluded. (Supplementary Figure 2).
The PD1CD28 switch-receptor was subcloned into the viral vectors upstream of a T2A/F2A sequence which was followed by the SS1BBz or the PSCA-BBz CAR respectively. (Supplementary Figure 2A & B).
Isolation, bead activation, transduction, and expansion of primary human T lymphocytes
Isolation, bead activation, transduction, and expansion of primary human T lymphocytes were conducted as previously described. (21)
mRNA electroporation and retroviral/lentiviral transduction of human T-cells undergoing CD3/CD28 Dynabead activation have been previously described. (25, 27, 29)
In-vitro T-cell and ex-vivo TIL Effector Assays
T-cell and TIL effector assays assessing tumor lytic ability and cytokine secretion ability were conducted as previously described . (21)
Antibodies
In-Vivo Xenograft Experiments
Using different tumor cells injected subcutaneously (5 × 106 EMMESO, 1 × 106 PC3-PSCA-PDL1, or 1 × 106 PC3-PSCA per mouse) in-vivo experiments were conducted as previously described. (21)
Groups contained 10 mice each. The in-vivo experiments were repeated three times in independent fashion.
Animals
Statistical Analysis
Results
Human T-cells electroporated with mCD19Z CAR and PD1CD28 demonstrate enhanced killing and cytokine secretion
Activated human T-cells were successfully electroporated with mRNAs encoding: 1) CD19Z CAR alone, 2) CD19Z CAR plus an intact PD1 (inhibitory) construct, or 3) the CD19Z CAR plus PD1CD28 as measured by FACS (Fig. 1A).
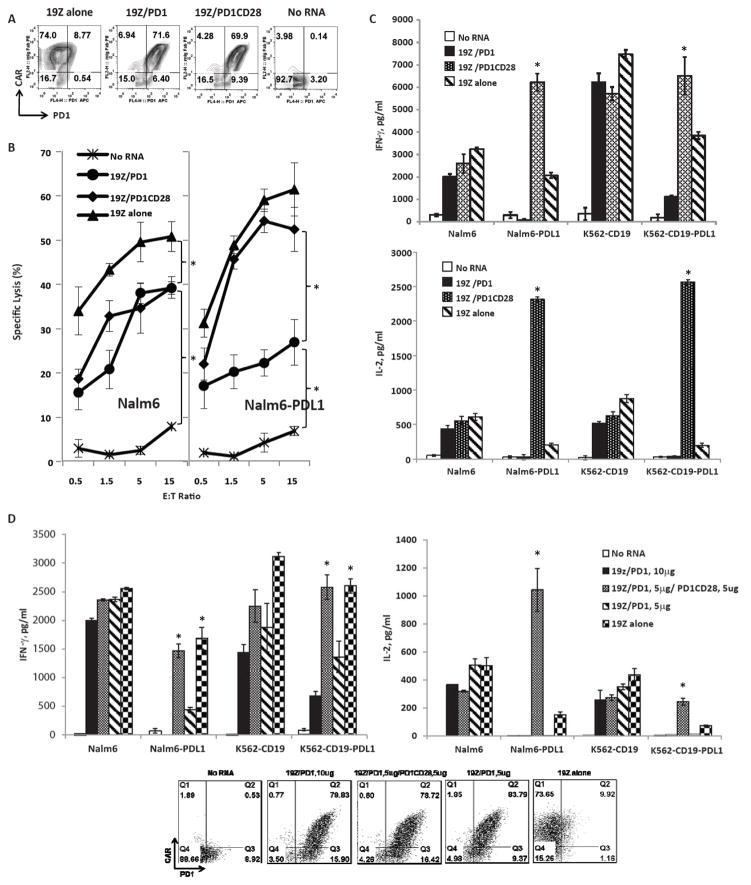
Figure 1. Increased cytokine production of T-cells co-expressing 19Z CAR and PD1CD28 switch-receptor via mRNA electroporation.
FACS analysis of T-cells one day after electroporation with no mRNA; or mRNA for CD19-zeta alone (19Z alone), co-electroporated with CD19-zeta and PD1 (19Z/PD1) or CD19-zeta and PD1CD28 switch-receptor (19Z/PD1CD28). The CAR expression was detected using an anti-mouse IgG Fab antibody, PD1 or PD1CD28 were detected with anti-PD1 antibody. (A). T-cells were tested for their cytolytic activity at indicated E:T ratios for 8hr against Nalm6 (left) or Nalm6-PDL1 (right). The results shown are the averages of three independent experiments (B). The T-cells were also co-cultured with indicated tumor cell lines for 24h for ELISA cytokine secretion measurement in culture supernatants. Bar graphs show results from a representative experiment (values represent the average ± SE of triplicates) for IFNγ (upper panel) and IL-2 (lower panel) (C). T-cells were co-electroporated with 10ug 19Z mRNA and 5ug PD1 mRNA (19Z/PD1, 5ug), with 5ug PD1CD28 mRNA (19Z/PD1, 5ug/PD1CD28) or 5ug PD1 (19Z/PD1, 10ug) as indicated. 19z alone and no RNA served as controls. One day after the electroporation, the T-cells were analyzed by FACS to confirm expression (D, dot plots) and were co-cultured with indicated tumor cell lines for 24h. Cytokine secretion was measured by ELISA analysis of culture supernatants. Bar graph show results from a representative experiment (values represent the average ± SD of triplicates) for IFNγ (left panel) and IL-2 (right panel) (D).
The T-cells demonstrated dose-dependent killing when co-cultured with Nalm6 cells at E:T ratios of 0.5:1 to 15:1 (Fig 1B, left column). Killing by 19Z/PD1 and 19Z/PD1CD28 were similar, but slightly lower at each ratio compared to 19Z T-cells. However, when the cells were co-cultured with Nalm6-PDL1 cells, the killing efficacy of the 19Z/PD1 T-cells was diminished, while the killing efficacy of the 19Z/PD1CD28 T-cells was increased (Fig 1B, right column).
The amount of cytokine produced by CAR T-cells were similar after co-culture with Nalm6 at 1:1 E:T ratio for 24 hours; however, when co-cultured with Nalm6-PDL1 cells, 19Z/PD1CD28 CAR T-cells generated significantly higher amounts of IFNγ and IL2 (Fig. 1C, left columns; p<0.01) when compared to 19Z and 19Z/PD1 T-cells. We extended our findings to K562-19 and K562-19-PDL1 cell lines (Fig. 1C, right columns).
The levels of endogenous PD1 on the T-cells used in the experiments above were relatively low. To determine if PD1 upregulation (as seen in hypofunctional TILs) would affect the efficacy of the switch-receptor, we electroporated T-cells with PD1 or PD1 plus PD1CD28 (Fig. 1D, dot plots), and co-cultured them with PDL1-expressing target cells. As expected, lower levels of IFNγ and IL2 were produced by 19Z/PD1 T-cells (Fig. 1D, striped and solid bars) compared to 19Z T-cells (Fig. 1D, checkered bars). However, 19Z/PD1/PD1CD28-electroporated T-cells continued to produce IFNγ and IL2 (Fig. 1D, gray bars), indicating that the PD1CD28 switch-receptor augmented T-cell cytokine production even in the presence of high levels of inhibitory PD1.
Overall, these data indicate that in the CD19Z mRNA CAR system, addition of the PD1CD28 switch-receptor can convert inhibitory signals, as induced by PDL1, into stimulatory signals, therefore resulting in increased tumor killing and cytokine production.
Human T-cells retrovirally transduced with PSCA-BBZ CAR and PD1CD28 demonstrate CD28 signaling domain-dependent enhancement of cytokine secretion
To distinguish between a dominant-negative effect on PD1 offered by the switch-receptor vs. its CD28 activating signal, PD1CD28 was compared to the mutated PD1CD28m. To generalize our findings and in anticipation of in-vivo studies, we conducted studies using T-cells retrovirally transduced with PSCA-BBz CAR (Fig. 2A) targeting PC3-PSCA with and without PDL1. (Fig. 2B)
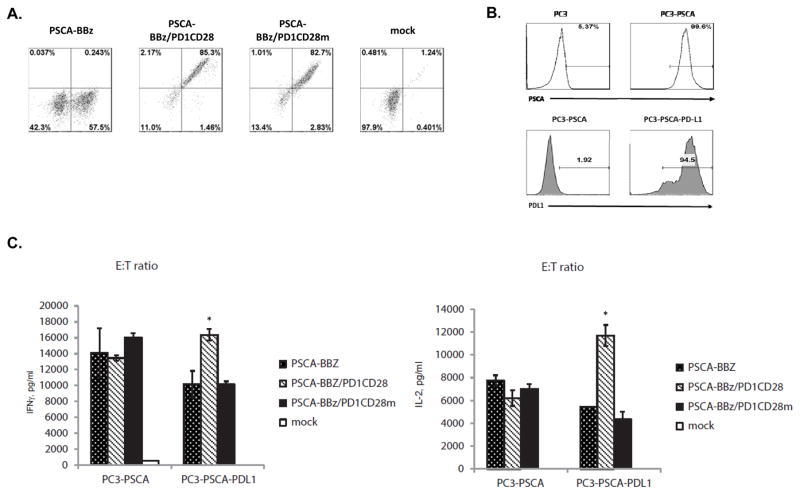
Figure 2. PD1CD28 induced enhanced cytokine secretion is dependent on CD28 signaling upon PDL1 binding.
T-cells were retrovirally transduced with either PSCA-BBz alone, PSCA-BBz with PD1CD28 switch-receptor (PSCA-BBz/PD1CD28), or PSCA-BBz with mutated PD1CD28 switch-receptor (PSCA-BBz/PD1CD28m). After confirming successful expression of the transgenes (A), T-cells were cocultured with PC3-PSCA or PC3-PSCA-PDL1 target cells (B) at an E:T ratio of 10:1 for 24h. Levels of secreted IFNγ and IL2 were measured by ELISA. (C) Mock-transduced T-cells (mock) served as control.
PSCA-BBz/PD1CD28 T-cells generated more IFNγ and IL2 upon co-culture with PC3-PSCA-PDL1 cells than PSCA-BBZ T-cells (p<0.05). However, mutated CD28 abrogated this effect, as the PSCA-BBZ/PD1CD28m T-cells produced similar levels of IFNγ and IL2 as the PSCA-BBZ cells (Fig. 2C).
Human T-cells lentivirally transduced with SS1BBZ CAR and PD1CD28 demonstrate enhanced tumor killing and cytokine secretion
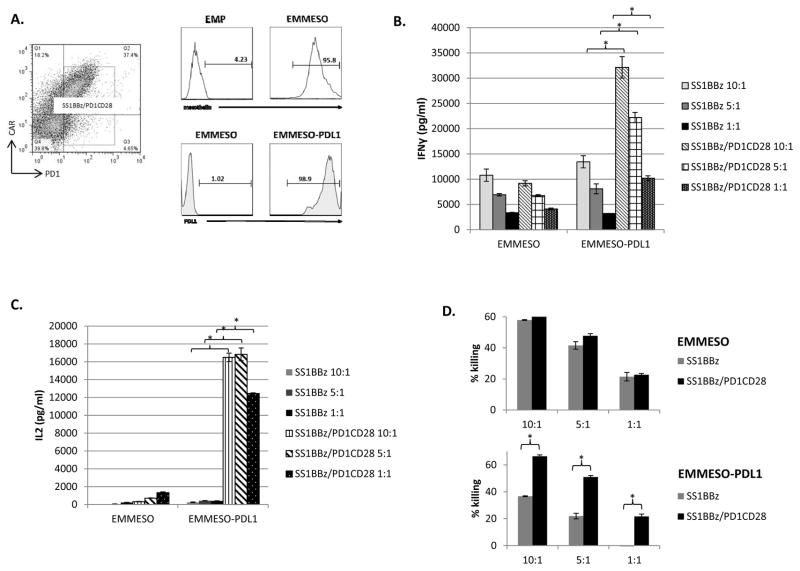
We conducted similar studies using human T-cells transduced with a second generation ant-mesothelin CAR with (SS1BBz) and without the PD1CD28 switch-receptor (SS1BBZ/PD1CD28) (Fig. 3A, dot plot) and cocultured them with mesothelin and PDL1 expressing tumor lines (Fig. 3A, histograms). Similarly as above, when exposed to EMMESO cells, both types of T-cells released equivalent amounts of cytokines (Fig. 3B & 3C, left columns). In contrast, when exposed to EMMESO-PDL1 cells, the SS1BBZ/PD1CD28 T-cells exhibited much greater secretion of IFNγ (p<0.05) (Fig. 3B, right columns) and especially IL2 (p<0.01) (Fig. 3C, right columns) at all ratios compared to SS1BBZ cells. SS1BBZ/PD1CD28 T-cells demonstrated similar 18hr lytic activity against EMMESO compared to SS1BBZ T-cells at all E:T ratios (Fig. 3D, top graph). When co-cultured with EMMESO-PDL1 target cells, SS1BBZ/PD1CD28 T-cells consistently demonstrated superior tumor killing compared to SS1BBZ T-cells at all E:T ratios (66% vs. 37% at 10:1, p<0.05; 51% vs. 22% at 5:1, p<0.05, 22% vs. 0% at 1:1, p<0.01) (Fig. 3D, bottom graph; Supp. Fig. 3).
Figure 3. Increased cytokine production by T-cells co-expressing SS1BBz CAR and PD1CD28 switch-receptor.
FACS analysis of CD4 and CD8 bulk T-cells activated via anti-CD3/CD28 microbeads at a ratio of 3:1 bead:T-cell and transduced with lentivirus led to successful co-expression of both SS1BBz CAR and PD1CD28 switch-receptor (~40%). The CAR expression was detected using an anti-mouse IgG Fab antibody and PD1CD28 was detected with anti-PD1 antibody (A, dot plot). FACS analysis of EMP, EMMESO, and EMMESO-PDL1 tumor cells was performed to confirm high expression of mesothelin and PDL1. (A, histograms) T-cells co-expressing SS1BBz and PD1CD28 (SS1BBz/PD1CD28) or SS1BBz alone (SS1BBz) were co-cultured with EMMESO or EMMESO-PDL1, at different E:T ratios x 18hrs. ELISA was performed to measure the levels of IFNγ (B) and IL-2 (C) present in the supernatants of the cocultures. % specific lysis of both EMMESO and EMMESO-PDL1 by SS1BBz and SS1BBz/PD1CD28 was also calculated after 18hrs of coculture (D). Bar charts show results from a representative experiment (values represent the average ± SE of triplicates)
PD1CD28 augments CAR T-cell anti-tumor activity beyond PD1 antibody blockade in animal models of solid tumor growth
We evaluated the effect of PD1CD28 in two independent in-vivo model systems involving CAR T-cells which are either currently in clinical trials or soon will be – the EMMESO mesothelioma tumor using SS1BBZ CAR T-cells and the PC3 prostate cancer model using PSCA-BBZ CAR T-cells. To further explore the mechanism, we also studied the effect of an anti-PD1 antibody (pembrolizumab) in the EMMESO model, and PD1CD28m in the PC3 model.
EMMESO flank tumor-bearing NSG mice were treated with a single dose of 1×107 mock-transduced (mock) or SS1BBZ CAR T-cells intravenously (IV) +/- intraperitoneal (IP) administration of pembrolizumab (10mg/kg every 5 days) (Fig. 4A). Mice injected with mock T-cells or with pembrolizumab alone grew at similar rates. Treatment with pembrolizumab + mock T-cells did not result in significant reduction of tumor volume. Treatment with SS1BBZ CAR resulted in a marked and significant slowing of tumor growth (1340 mm3 in mock vs. 562 mm3 in SS1BBZ at day 34, p<0.01).
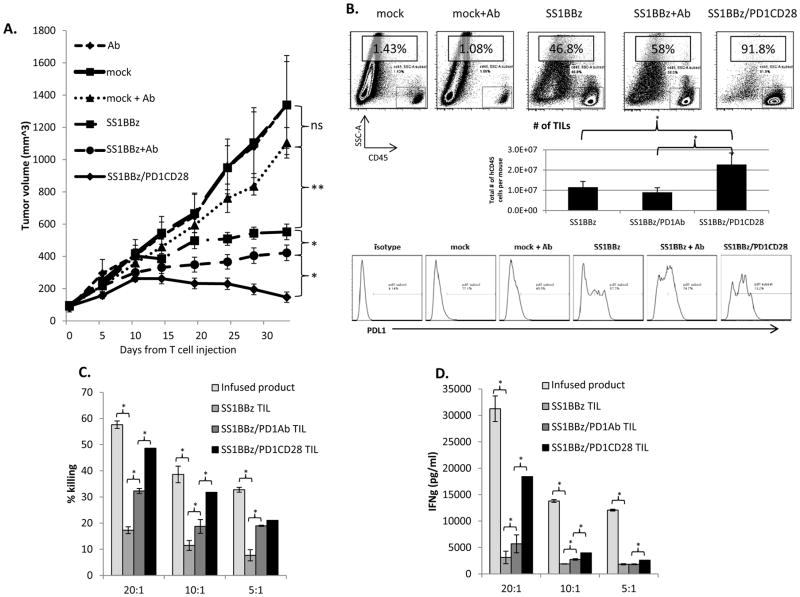
Figure 4. In-vivo and ex-vivo anti-tumor function of SS1BBz T-cells augmented by anti-PD1 antibody blockade and PD1CD28 modification.
When EMMESO tumors injected in the flank of mice (n=10/group; 2×106 cells/mouse, s.c.) reached an average volume of approximately 100mm3, mice were randomly assigned to six groups and were injected with either anti-PD1 Ab alone (Ab), 1×107 mock transduced T-cells (mock), 1×107 mock T-cells and anti-PD1 Ab (mock + Ab), 1×107 SS1BBz T-cells (SS1BBz), 1×107 SS1BBz T-cells and anti-PD1 Ab (SS1BBz + Ab), or 1×107 SS1BBz T-cells modified with PD1CD28 switch-receptor (SS1BBz/PD1CD28). T-cells were injected once intravenously and antibody was injected at a dose of 10mg/kg/mouse every five days intraperitoneally. Flank tumors were measured by calipers every 5 days. Values represent the average flank tumor volume ± SE of measurements of ten mice/group. (A) Approximately 35 days after treatment initiation, flank tumors were harvested, digested, and processed into single cell suspension. A portion of the cells was stained with CD45 antibody to assess T-cell infiltration via FACS analysis. Dot-plots are FACS analysis from a representative experiment. Bar graphs represent average absolute number of CD45+ cells in tumors per mouse ± SE. Histograms represent PDL1 staining on the live, CD45− events in the tumor digest from a representative experiment. (B) The rest of the cells in each group were pooled and were subjected to CD45 positive isolation via magnetic beads and were cocultured at 20:1, 10:1, and 5:1 E:T ratios with EMMESO tumor targets x 18hrs. Specific lysis (C) and IFNγ measurement by ELISA (D) of TILs from each group was measured in comparison to uninjected cryopreserved control SS1BBz T-cells (infused product). Bar graph values represent the average values ± SE of measurements from triplicates.
The addition of pembrolizumab treatment to the SS1BBZ CAR group resulted in a modest tumor inhibition (422 vs. 552mm3, p<0.05). Notably, the strongest anti-tumor effect was seen in the SS1BBZ/PD1CD28-treated group (552 mm3 in SS1BBz vs. 147mm3 in SS1BBZ/PD1CD28, p<0.05) where some tumors actually regressed.
PD1CD28 potentiates CAR T-cell expansion in EMMESO tumors
At the end of our in-vivo study, TIL analysis revealed that of the tumor digests mock T-cells made up <5%; SS1BBZ T-cells, 47%; and SS1BBz+Ab T-cells, 58%. SS1BBZ/PD1CD28-treated mice exhibited the greatest degree of T-cell infiltration in the tumor (92%) (Fig. 4B, dot plots). To address the potential confounding variable of different tumor sizes, absolute numbers of TILs were calculated and confirmed a greater number of TILs was present in the SS1BBZ/PD1CD28-treated mice. (Fig. 4B, bar graph). Analysis of PDL1 expression on tumor cells revealed upregulation of PDL1 expression with treatment. (Fig. 4B, histograms)
PD1CD28 preserves tumor lytic activity and cytokine secretion of CAR TILs
TILs and T-cells frozen at time of injection (infused product) were exposed to freshly cultured tumor cells ex-vivo at varying E:T ratios to assess killing and cytokine release. Freshly isolated TILs show marked decrements in tumor lytic activity and IFNγ release (Fig. 4C and Fig 4D, infused product vs. SS1BBZ TIL bars). However, compared to the SS1BBz TILs, the SS1BBz+Ab TILs showed significantly enhanced TIL function (p<0.05). Importantly, SS1BBZ/PD1CD28 TILs exhibited significantly (p<0.01) greater lytic and cytokine-producing ability than either of the other types of TILs. (Fig. 4C & 4D, Supp. Fig. 4)
PD1CD28 attenuates upregulation of inhibitory receptors (IRs) on EMMESO-infiltrated TILs
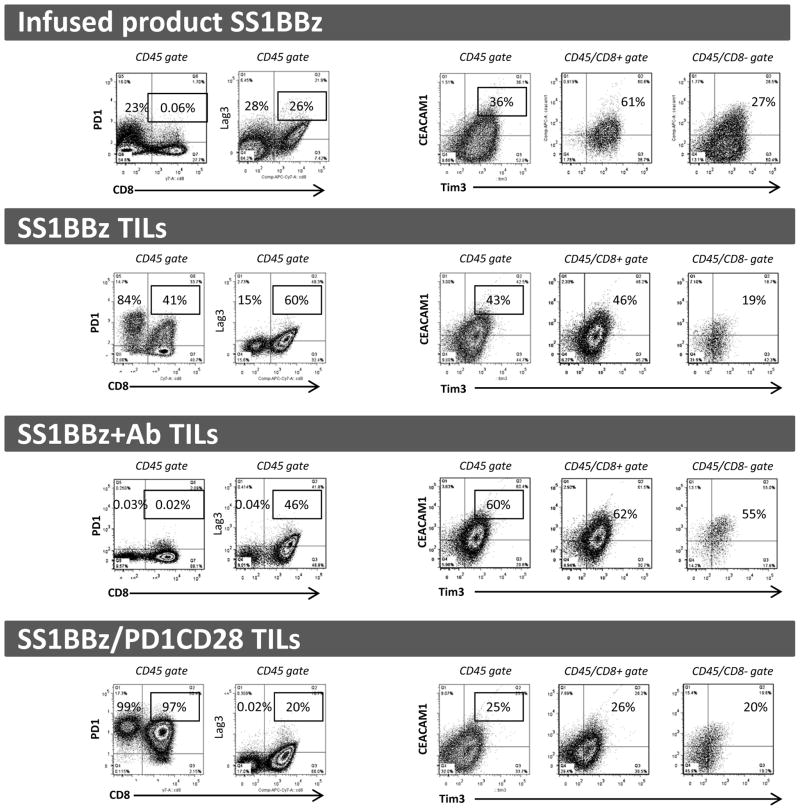
Compared to infused T-cells, we observed significant upregulation of PD1 and LAG3 expression on SS1BBZ TILs. The percentage of CD8 T-cells expressing PD1 increased from 0.06% to 41% (Fig. 5, 1st row/1st dot-plot versus 2nd row/1st dot-plot). SS1BBZ+Ab TILs had no detectable PD1 staining, confirming adequate exposure of T-cells to the pembrolizumab throughout the experiment (Fig. 5, 3rd row/1st dot-plot). Almost all SS1BBZ/PD1CD28 TILs expressed PD1, reflecting both PD1 upregulation and enrichment of gene-modified T-cells (Fig. 5, 4th row/1st dot-plot). The percentage of CD8 T-cells expressing LAG3 increased from 26% to 60% (Fig. 5, 1st row/2nd dot-plot vs. 2nd row/2nd dot-plot), but decreased to 46% when SS1BBZ TILs were combined with pembrolizumab (Fig. 5, 3rd row/2nd dot-plot), and decreased even further to 20% in the SS1BBZ/PD1CD28 TILs (Fig. 5, 4th row/2nd dot-plot). The percentage of infused product SS1BBZ T-cells co-expressing TIM3 and CEACAM1 was 36% but increased to 43% in the SS1BBZ TILs (Fig. 5, 1st row/3rd dot-plot vs. 2nd row/3rd dot-plot). It was further increased to 60% in SS1BBZ+Ab TILs (Fig. 5, 3rd row/3rd dot-plot). SS1BBZ/PD1CD28 TILs had the lowest TIM3/CEACAM1 expression, at 25% (Fig. 5, 4th row/3rd dot-plot).
Figure 5. PD1CD28 leads to reduced upregulation of IRs on SS1BBz TILS.
Single cell suspension from digested tumors were subjected to FACS analysis to measure expression of PD1, Lag3, Tim3, and CEACAM1. The first three dot plots of each row represent analyses of cells in the CD45+ gate. The fourth and fifth dot plot of each row represents analyses of cells in the CD45+/CD8+ and CD45+/CD8− gates respectively. The highlighted percentages represent frequency of PD1+ events among CD8+ events (the first two dot plots) and the frequency of Tim3/CEACAM1 double-positive events (the last three dot plots.) Each row demonstrates representative analysis of TILs from three independent animal experiments. Infused product SS1BBz T-cells were used as control comparisons for baseline levels of IRs prior to adoptive transfer into mice.
PD1CD28 augments in-vivo tumor control of PC3 flank tumors
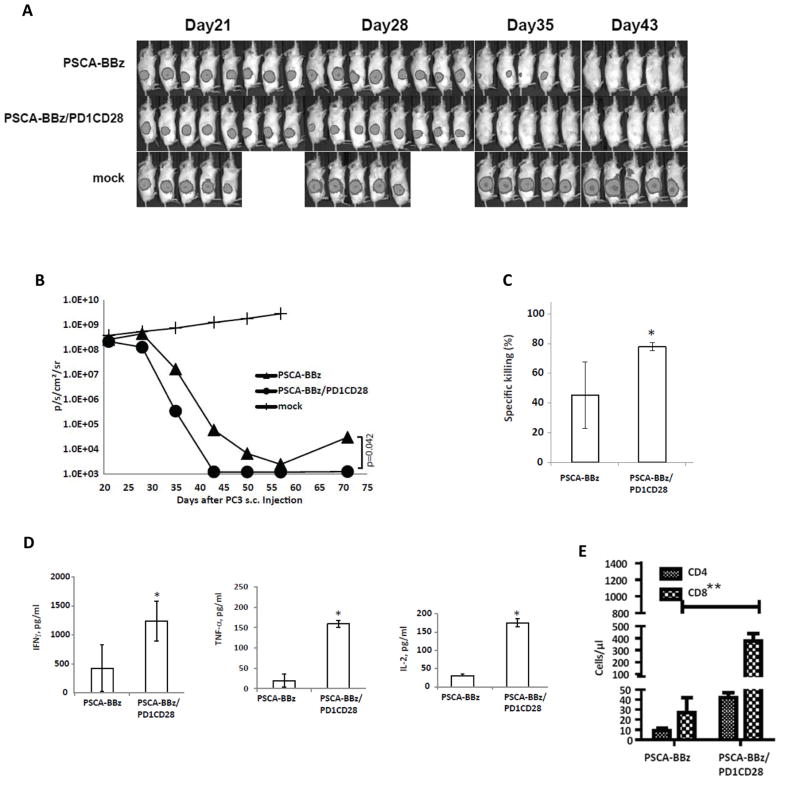
To ascertain the generalizability of these findings, we also assessed PD1CD28 efficacy in mice bearing PSCA-expressing PC3 tumors. Tumor-bearing NSG mice were treated IV with 2×106 PSCA-BBZ±PD1CD28 CAR T-cells or mock T-cells. Tumors were tracked with bioluminescence imaging (Fig. 6A and 6B). The greatest anti-tumor effects were seen with PSCA-BBZ/PD1CD28 T-cell administration (Day 35, p=0.05, Day 43, p=0.05; Day 70, p=0.042) (Fig. 6B). While a few tumors escaped by Day 70 in the PSCA-BBZ group, all remaining animals in the PSCA-BBZ/PD1CD28 group remained cured (p<0.05) (Fig. 6B).
Figure 6. PD1CD28 improves the therapeutic effect of PSCA-BBz T-cells against advanced vascularized PC3-PSCA tumors in mice.
T-cells modified with PSCA-BBz alone or PSCA-BBz with PD1-CD28 (PSCA-BBz/PD1CD28) by retroviral transduction were tested in PC3-PSCA-CBG engrafted NSG mice. Mice (n=5–8) were implanted with PC3-PSCA-CBG tumor cells (1×106 cells/mouse, s.c.) on the right flank on day 0. The mice were treated with 2×106 T-cells (IV) at day 23 after tumor inoculation. Mock transduced T-cells (mock) served as control. Animals were imaged at the indicated times post-tumor inoculation (A&B). 34 days post tumor inoculation, three mice from each treatment group were sacrificed and tumor infiltrating lymphocytes (TILs) were isolated. An aliquot of freshly purified TILs was used in a killing assay using PC3-PSCA-CBG as target cells at E:T ratio of 5:1 (C). Levels of secreted IFNγ, TNFα, and IL2 were measured in the supernatants by ELISA (D). 35 days post tumor inoculation, blood drawn from the remaining mice (5 mice/group) were subjected to FACS True-Count staining to detect human CD4 and CD8 T-cells (E).
PSCA-BBZ/PD1CD28 TILs demonstrated greater infiltration; ex-vivo killing ability and cytokine secretion than PSCA-BBZ CAR TILs
When TILs were harvested from flank tumors at the end of the experiment, and co-cultured with fresh PC3-PSCA tumors cells at a 5:1 E:T ratio, PSCA-BBZ/PD1CD28 TILs demonstrated greater tumor lysis than PSCA CAR TILs (p=0.05; Fig. 6C).
Compared with PSCA-BBZ TILs, PSCA-BBZ/PD1CD28 TILs showed significantly greater (p<0.05) secretion of IL2, TNFα, and IFNγ after overnight TIL co-culture with PC3-PSCA cells (Fig. 6D).
Analysis of blood from mice at 35 days post-tumor inoculation using flow cytometry Tru-Count staining revealed greater number of CD8 T-cells per μl in the PSCA-BBZ/PD1CD28 group compared to the PSCA-BBZ group (p<0.01; Fig. 6E).
The signaling motif of PD1CD28 is critical to augment PSCA-BBZ CAR T-cell control of PC3-PSCA tumor growth
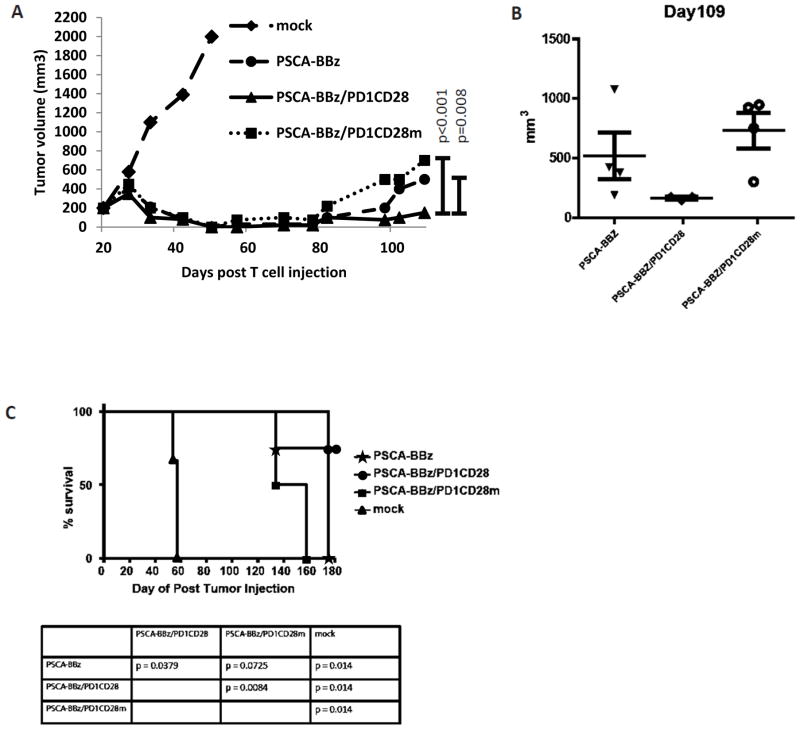
After demonstrating the abrogation of PD1CD28-induced cytokine secretion by mutating the CD28 signaling motif (as described above), we compared the effects of PSCA-BBZ, PSCA-BBZ/PD1CD28, and PSCA-BBZ/PD1CD28m T-cells at a dose of 2×106 T-cells/mouse in PSCA-PC3-PDL1 flank tumor-bearing NSG mice. All T-cell types induced marked tumor regressions. However, at about 80 days after treatment, the tumors treated with the PSCA-BBZ T-cells began to recur, and by Day 109 reached an average size of ~600 mm3 (Fig. 7A & 7B). The size of the tumors treated with PSCA-BBZ/PD1CD28 T-cells was significantly smaller (180mm3, p<0.008) than both other groups at Day 109 (Fig. 7B). Interestingly, though the PSCA-BBZ/PD1CD28m-treated tumors initially regressed early in the study, they eventually rebounded and were just as large as the PSCA-BBZ-treated tumors by Day 109 (Fig. 7A & 7B); we observed similar trends in terms of mortality in both groups (Fig. 7C).
Figure 7. The enhanced anti-tumor effect offered by PD1CD28 is dependent on CD28 signaling induced by PDL1 binding.
T-cells retrovirally transduced with either PSCA-BBz alone, PSCA-BBz with PD1CD28 switch-receptor (PSCA-BBz/PD1CD28), or PSCA-BBz together with mutated PD1CD28 switch-receptor in which the CD28 signaling transduction proximal YMNM motif and distal proline-rich motifs PRRP and PYAP were mutated to FFFF, ARRA and AYAA respectively (PSCA-BBz/PD1CD28m) were tested in PC3-PSCA-PDL1 tumor engrafted NSG mice. Mice (n=5) were implanted in the flanks with PC3-PSCA-PD-L1 tumor cells (1×106 cells/mouse, s.c.) and were treated with T-cells (IV) at day 23 after tumor inoculation. T-cells were given as a single injection of 2×106/mouse. Injection of mock T-cells served as control. Tumor sizes were measured and the tumor volume was calculated and plotted (A & B). The percentage survival per group was determined on a daily basis and is represented in a Kaplan-Meier survival curve (C).
Discussion
The potential efficacy of tumor immunotherapy utilizing the adoptive transfer of T-cells has changed dramatically with the introduction of chimeric antigen receptors (CARs) (30). However, the responses seen in hematologic malignancies have not yet been reflected in efforts against solid tumors (31). One primary reason is the tumor-induced hypofunction of tumor infiltrating lymphocytes (TILs) that has been described by multiple research groups in both humans and murine models (32–39). This hypofunction seems, in large part, due to the upregulation of inhibitory receptors (IRs). (40)
PDL1:PD1 interaction contributes to the suppression of effector T-cell function and clonal deletion with the goal of maintaining immune tolerance (41). However, tumors appear to take advantage of this pathway and express PDL1, presenting a substantial hurdle for adoptive T-cell immunotherapeutic strategies. (42)
Using a unique in-vivo model of adoptively transferred human CAR T-cells targeting human solid tumors, we have recently demonstrated tumor-induced CAR TIL hypofunction associated with PD1 upregulation similar to that described in naturally occurring TILs (21). Based on that observation, we set out to interrupt PD1 signaling in combination with adoptive CAR T-cell therapy. PD1 blockade using antibodies has already demonstrated promising responses in early clinical trials of melanoma, lung cancer and other malignancies (20). The utility of checkpoint blockade in tumors which lack sufficient infiltration of immune cells that bear tumor-reactivity at baseline is questionable. Thus, we and others have hypothesized that the combination of adoptively transferring genetically-augmented tumor-reactive T-cells with checkpoint interference could be a promising immunotherapeutic strategy for solid tumors. Specifically, it would provide PDL1-resistant, tumor-reactive T-cells in cases where tumors express high levels of ligand but lack sufficient immune infiltration.
Prosser et al. initially introduced the PD1CD28 switch-receptor. Upon binding of PDL1, T-cells showed increased levels of ERK phosphorylation, cytokine secretion, proliferation, and granzyme B expression (24). Subsequently, Ankri et al. were able to demonstrate enhanced anti-tumor function using PD1CD28 T-cells in two somewhat artificial in-vivo models of very early melanoma formation – a chicken embryo chorioallantoic membrane model (CAM), and a WINN assay where T-cells and tumors cells were mixed together and then injected into athymic nude-Foxn1nu mice. (22) A primary goal of our study was to test the effects of PD1CD28 on CAR-engineered T-cells injected to treat large, established, solid tumors that are more clinically relevant. We also wanted to compare the effects of PD1CD28 to PD1 antibody blockade (an immunotherapy strategy already in the clinic) and to dominant-negative receptor strategies.
In the context of different tumor cell lines, PD1CD28 expression enhanced cytokine secretion (particularly IL2) by T-cells modified with CARs targeting mesothelin-expressing targets and CD19-expressing targets in a PDL1-dependent manner. SS1BBZ/PD1CD28 T-cells secreted >30-fold more IL2 than SS1BBZ when co-cultured with EMMESO-PDL1 cells. 19Z/PD1CD28 T-cells secreted >10-fold more IL2 than CD19Z T-cells when co-cultured with Nalm6-PDL1 or K562-CD19-PDL1 cells.
PD1CD28 also demonstrated modestly increased killing of PDL1-transduced tumor targets in short-term in vitro killing assays. More importantly, PD1CD28 led to significantly enhanced tumor control by CAR T-cells (as measured by both bioluminescent imaging and caliper assessment) in two different xenograft models of established, large tumors (EMMESO, a human pleural mesothelioma cell line, and PC3-PSCA, a human prostate cancer cell line). By bioluminescence measurements, a faster onset of tumor regression and greater long-term tumor control were seen in mice treated with PSCA-BBZ/PD1CD28 T-cells compared to those treated with of PSCA-BBZ T-cells (Fig. 6). When PC3-PSCA flank tumors expressing high levels of PDL1 were targeted, PSCA-BBZ/PD1CD28 T-cells demonstrated statistically significant augmentation of tumor control over PSCA-BBZ T-cells (Fig. 7).
There are a number of likely mechanisms for this enhanced effect, many of which we are demonstrating for the first time. First, the PD1CD28 switch-receptor led to a significant increase in frequency of viable TILs as was observed in both the peripheral blood of mice bearing PC3-PSCA flank tumors 15 days after T-cell transfer, and in the tumor digests from mice bearing EMMESO flank tumors 34 days after T-cell transfer. Although not known for certain, we speculate this was due to a combination of enhanced survival and enhanced proliferation due to the higher levels of IL2 that were likely present at the tumor site. This enhanced anti-tumor effect was likely not seen to the same degree in in vitro testing due to the relatively short period of time of assessment (18–24 hours). Second, the CAR/PD1CD28 TILs were able to retain more anti-tumor function and cytokine secretion ability compared to CAR TIL counterparts as measured by ex-vivo killing of freshly plated tumor targets. PD1CD28-expressing CAR TILs demonstrated greater killing of tumor cells and enhanced ability to secrete cytokines in response to both PC3-PSCA cells and EMMESO cells compared to non-expressing CAR TILs upon fresh isolation from flank tumors. Furthermore, SS1BBZ/PD1CD28 TILs had greater ex-vivo anti-tumor function than SS1BBZ TILs from mice in the SS1BBZ+Ab group.
We hypothesized that there were at least two ways in which our switch-receptor is able to exert its effects: 1) the receptor functions as a dominant negative receptor, engaging the PDL1 present on tumor and myeloid cells and sequestering it from the intact inhibitory PD1 receptor on the T-cells; and 2) the switch-receptor was actively signaling through the CD28 cytoplasmic domain after engagement with PDL1.
A number of pieces of data suggest that active signaling played the more important role. First, PD1CD28 augmented CAR T-cell anti-tumor function to a greater degree than pembrolizumab. IP injections of pembrolizumab every 5 days was able to augment tumor control by SS1BBZ CAR T-cells by ~24% reduction in tumor size, while modification with PD1CD28 demonstrated a much greater augmentation in efficacy (~72% reduction in tumor size.) Second, to more carefully look at the role of signaling, we constructed a mutated, )—essentially a dominant-negative receptor. Intravenous injection of a single low dose (2×106 T-cells/mouse) of PSCA-BBZ/PD1CD28m CAR T-cells resulted in the same anti-tumor activity (as measured by tumor volume, bioluminescence, and survival) as the PSCA-BBZ CAR T-cells when tested in NSG mice bearing PC3-PSCA-PDL1 flank tumors. This was in contrast to the significantly greater tumor control seen in mice injected with active PSCA-BBZ/PD1CD28 CAR T-cells. This supports the conclusion that PD1CD28 is primarily exerting its enhancing effect through the CD28 costimulatory signal. When T-cells were injected at a higher dose (10×106 T-cells/mouse), we actually saw a decrease in the anti-tumor activity of PSCA-BBZ CAR T-cells expressing PD1CD28m as measured by tumor volume and survival (data not shown). One explanation for this observation is that PD1 binding to PDL1 interferes with the anti-tumor activity of CAR T-cells independently of PD1 signaling via its cytoplasmic motifs, which is supported by data recently published(43). Yokosuka et al. demonstrated that PD1 can form microclusters that interfere with TCR synapse formation, independent of PD1’s signaling via tyrosine motifs. Consistent with this mechanism, we have preliminary data showing that SS1BBZ T-cells expressing a truncated, signal-dead PD1 (PD1tailless) injected into NSG mice bearing EMMESO flank tumors demonstrated significantly worse tumor control compared to SS1BBZ T-cells (Supp. Fig. 5). Studies are currently underway to test whether the effect demonstrated by Yokosuka et al. also takes place in CAR synapse formation.
We also identified another potential mechanism for enhanced T-cell function in PD1CD28-expressing cells. We conducted detailed FACS analysis to assess whether interference of PD1 signaling affected the expression pattern of other known IRs. This effort was in light of a growing body of literature describing the co-expression of multiple IRs in hypofunctional T-cells (44–48), as well as our own published data demonstrating upregulation of PD1, TIM3, and LAG3 on human CAR TILs in human solid tumor (21). Analysis of TILs from our SS1BBZ/EMMESO experiment revealed upregulation of PD1, TIM3, and LAG3 on SS1BBZ TILs compared to the infused SS1BBZ cryopreserved T-cells. We also found that CEACAM1, a cell adhesion molecule shown to endow TIM3 with inhibitory function (49), was co-expressed with TIM3 to a much greater extent on TILs than infused T-cells. Pembrolizumab decreased the percentage of LAG3+ CD8 TILs; however, there was a compensatory upregulation of TIM3+/CEACAM1+ TILs with antibody blockade. In contrast, modifying SS1BBZ CAR T-cells with PD1CD28 led to reduction in both LAG3 expression and TIM3/CEACAM1 co-expression. This phenomenon, i.e., PD1CD28 allowing adoptively transferred T-cells to circumvent inhibition by IRs other than PD1, was also demonstrated in a murine study looking at CTLA-4 (50). Further investigations to understand the underlying mechanisms are planned, but one leading hypothesis is that the PD1CD28 modified TILs that are exposed to significantly higher levels of IL2 represent “younger” T-cells whose chronicity of activation and exposure to the TME is substantially less than their unmodified counterparts.
An additional theoretical advantage of PD1CD28 is the ability to introduce 3rd generation CAR signaling in a more targeted and safe fashion. In lieu of reports of toxicity using T-cells bearing 3rd generation CARs with multiple costimulatory domains (i.e. CD3ζ, 4-1BB, and CD28) (51), the majority of clinical trials testing adoptively transferred CAR T-cells are using 2nd generation chimeric constructs that signal via CD3ζ plus 4-1BB or CD28, not both. However, thus far, the transfer of T-cells bearing 2nd generation CARs has led to mixed results in trials involving solid tumor (52). Utilizing T-cells modified with both CARs engineered with 2nd generation signaling and chimeric switch-receptors that provide an additional costimulatory signal, but only when triggered by checkpoint ligands expressed in the tumor microenvironment (EMMESO upregulates PDL1 in response to T-cell activity in-vivo), would offer the maximum T-cell activation signal but only in the locale of the tumor, potentially avoiding systemic toxicity.
One deliberate decision we made in our study was to exclusively study human T-cells. We chose this approach due to the large differences that we and others have observed in the behavior of murine versus human adoptively transferred T-cells. Compared to transduced human T-cells, transduced murine T-cells: 1) are much more sensitive to activation-induced cell death, 2) have a much lower in-vitro and in-vivo proliferative potential, 3) often require IL-2 in-vivo, and 4) have much shorter persistence after injection into mice (in our experience only 7–10 days). Given the translational intent of our study, it seemed critical to study the behavior of the chimeric switch-receptor in a model where we have shown that T-cells proliferate, persist, and undergo hypofunction like naturally occurring TILs, thus requiring us to use human T-cells. As a result of this approach, we acknowledge that a potential criticism of our study is that our immunodeficient mice cannot take into account the contributions of other potentially important cell types. As examples, endogenous MDSC’s might have high levels of PDL1 that could interact with T-cells (53) or the increased levels of IL2 secretion by cells expressing the switch-receptor might increase the frequency of T-regulatory cells. (54) These questions have been addressed to some extent in a recent study in which a murine version of similar switch-receptor was transduced into mouse OT1 cells and showed increased efficacy. (23) The actual effects of MDSC and Tregs on the switch-receptor transduced human T-cells in patients will need to await clinical trials.
In summary, this study demonstrates the ability to augment CAR T-cells targeting advanced solid tumors by co-expressing a chimeric PD1CD28 switch-receptor. More so, we have significantly built on prior PD1CD28 studies by 1) extending studies to human T-cells, 2) evaluating human T-cells in large, established human tumors bearing clinically relevant tumor antigens, 3) elucidating multiple new mechanistic pathways through which the switch-receptor augments human CAR T-cells, and 4) demonstrating a more potent effect of PD1CD28 on CAR T-cells than currently available antibody-based PD1 blockade. Finally, the PD1CD28 switch-receptor offers a potential way to deliver 2nd generation CAR T-cells with more potent 3rd generation activation turned on specifically within the immunosuppressive TME.
Supplementary Material
Acknowledgments
Financial Support: The research was supported by funding from K08 (CA163941-04; E.K. Moon), P01 (CA66726; X. Liu, S. Jiang, Y. Zhao, C. June), and RO1 (2R01CA120409; Y. Zhao, C. June) grants from the National Cancer Institute (NIH), and the Penn-Novartis Alliance (X. Liu, S. Jiang, Y. Zhao, C. June, S. Kim, E.K. Moon).
Footnotes
Conflict of Interest Statement: X.L., C.H.J. and Y.Z. have financial interests due to intellectual property and patents in the field of cell and gene therapy. Conflicts of interest are managed in accordance with University of Pennsylvania policy and oversight. The remaining authors have declared that no conflict of interest exists.
References
- 1.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govers C, Sebestyen Z, Coccoris M, Willemsen RA, Debets R. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol Med. 2010;16:77–87. doi: 10.1016/j.molmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Chmielewski M, Hombach AA, Abken H. Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells. Front Immunol. 2013;4:371. doi: 10.3389/fimmu.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zoughbi W, Huang J, Paramasivan GS, Till H, Pichler M, Guertl-Lackner B, et al. Tumor macroenvironment and metabolism. Semin Oncol. 2014;41:281–95. doi: 10.1053/j.seminoncol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9:50–5. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 13.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25(3):381–8. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 15.Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72–9. doi: 10.1016/j.cellimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 20.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–73. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol. 2013;191:4121–9. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- 23.Kobold S, Grassmann S, Chaloupka M, Lampert C, Wenk S, Kraus F, et al. Impact of a New Fusion Receptor on PD-1-Mediated Immunosuppression in Adoptive T Cell Therapy. J Natl Cancer Inst. 2015;107:1–10. doi: 10.1093/jnci/djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012;51:263–72. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high-affinity human monoclonal antibody to mesothelin. Int J Cancer. 2011;128:2020–30. doi: 10.1002/ijc.25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate-Daga D, Lagisetty KH, Tran E, Zheng Z, Gattinoni L, Yu Z, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25:1003–12. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–5. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174:1830–40. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 34.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–54. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med. 2000;6:465–79. [PMC free article] [PubMed] [Google Scholar]
- 36.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlosser HA, Theurich S, Shimabukuro-Vornhagen A, Holtick U, Stippel DL, von Bergwelt-Baildon M. Overcoming tumor-mediated immunosuppression. Immunotherapy. 2014;6:973–88. doi: 10.2217/imt.14.58. [DOI] [PubMed] [Google Scholar]
- 38.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–40. doi: 10.1007/s10555-011-9280-5. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–40. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–56. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 42.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010 Sep;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–90. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Condomines M, Arnason J, Benjamin R, Gunset G, Plotkin J, Sadelain M. Tumor-Targeted Human T Cells Expressing CD28-Based Chimeric Antigen Receptors Circumvent CTLA-4 Inhibition. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 54.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.