Abstract
The structure and molecular signature of tumor-associated vasculature are distinct from those of the host tissue, offering an opportunity to selectively target the tumor blood vessels. To identify tumor-specific endothelial markers, we performed a microarray on tumor-associated and nonmalignant endothelium collected from patients with renal cell carcinoma (RCC), colorectal carcinoma (CRC), or colorectal liver metastasis (CRM). We identified a panel of genes consistently upregulated by tumor blood vessels of which melanoma cell adhesion molecule (MCAM) and its extracellular matrix interaction partner laminin alpha 4 (LAMA4) emerged as the most consistently expressed genes. This result was subsequently confirmed by immunohistochemical analysis of MCAM and LAMA4 expression in RCC and CRC blood vessels. Strong MCAM and LAMA4 expression was also shown to predict poor survival in RCC, but not in CRC. Notably, MCAM and LAMA4 were enhanced in locally advanced tumors as well as both the primary tumor and secondary metastases. Expression analysis in 18 different cancers and matched healthy tissues revealed vascular MCAM as highly specific in RCC, where it was induced strongly by VEGF, which is highly abundant in this disease. Lastly, MCAM monoclonal antibodies specifically localized to vessels in a murine model of RCC, offering an opportunity for endothelial-specific targeting of anticancer agents. Overall, our findings highlight MCAM and LAMA4 as prime candidates for RCC prognosis and therapeutic targeting.
Introduction
Approximately 271,000 new cases of renal cancer are diagnosed each year worldwide, 3% of all cancers, with the highest incidence rate found in North America (1). By far the most common form of kidney cancer is renal cell carcinoma (RCC), accounting for 90-95% of cases (1). Initial treatment is most commonly surgical, with this approach remaining the primary curative intervention (2). Unfortunately as RCC is often asymptomatic until the tumour is advanced or metastatic, curative surgical treatment is often not possible. Five year survival drops from 65-90% in operable cases, to less than 10% in metastatic disease (3). It is therefore clear that new prognostic and diagnostic biomarkers for RCC are urgently needed.
Inoperable or recurrent RCC is difficult to treat, with the success rate of traditional chemo or radiotherapy at 4-5% (4). Because of this, a multitude of alternative therapies have been trialled and shown efficacy in metastatic RCC (mRCC), including vascular endothelial growth factor (VEGF) targeted antiangiogenic therapies, sunitinib, bevacizumab, sorafenib and axitinib (5). RCC being a cancer characteristic for and highly dependant on excessive VEGF production, due to the loss of the tumour suppressor Von Hippel-Lindau being a common occurrence in clear cell RCC (ccRCC) (6), anti-VEGF therapies have improved the outlook for mRCC (5). Despite this, 5-year survival remains low and antiangiogenic treatment relapse is very common (5).
The endothelium is directly accessible to the blood, making it an ideal target for an emerging form of therapy, antibody-drug conjugates (ADCs). These operate by delivering antibody-guided therapeutics specifically to the tumour. The first proof of principal that this approach could be effective was provided by Burrows and Thorpe (7), when they engineered a neuroendocrine tumour to express interferon-gamma, inducing MHC-class 2 on the tumour vessels, which they then targeted with a monoclonal antibody against MHC-class 2, conjugated to ricin. This therapy rapidly induced haemorrhagic necrosis in the tumour leading to dramatic tumour regression. A key requirement for this approach is the identification of highly specific tumour vascular markers and significant effort has been spent to identify these [reviewed in (8)]
Tumour associated vessels are made distinct by many of the same pressures found in all solid tumours, such as hypoglycaemia, severe hypoxia, excessive growth factor receptor activation, infiltration of inflammatory cells and cytokine activation as well as irregular blood flow and shear stress. We therefore decided to profile the endothelium from renal cell carcinoma, colorectal carcinoma and colorectal liver metastasis to determine what common molecular changes occur in the vessels of these tumours compared to healthy tissue vessels, with the aim of identifying tumour specific endothelial markers showing robust pan-tumour expression.
Amongst the most consistently expressed vascular markers in the cancers investigated were melanoma cell adhesion molecule (MCAM), a cell surface glycoprotein first identified as a marker on the cancerous cells of malignant melanoma (9) and previously linked to tumour cell mobility (10-12) and laminin alpha 4 (LAMA4), an extracellular matrix glycoprotein and component of the laminin complex, recently identified as a ligand for MCAM (13). Strong expression of both, was shown to link negatively to renal cell carcinoma (RCC) patient survival, suggesting a utility for the markers in RCC prognostication. MCAM expression was shown to be induced by VEGF with expression particularly enhanced in ccRCC over many other tissue types. This distinct expression pattern was shown to facilitate the specific localisation of a monoclonal anti-MCAM antibody to the vascular bed of a murine RCC tumour, highlighting its potential as a ligand for specific ADC targeting in RCC.
Materials and Methods
Tissue collection and ethics
Tumour and distant healthy tissue were obtained immediately post-surgery. Full patient consent and ethical approval was granted (Queen Elizabeth Hospital Birmingham: Colorectal cancer (CRC) and Colorectal liver metastasis (CRM), South Birmingham REC, No. 2003/242. Renal Cell Carcinoma (RCC), No. 12-090).
Endothelial isolation using Ulex lectin-magnetic beads
The process is summarized in Figure 1A and based on the protocol published in (14). Briefly, tissue was minced and digested in DMEM containing 2 mg ml−1 collagenase type V (Sigma, Gillingham, UK), 7.4 mg ml−1 actinomycin D (Sigma) and 30kU ml−1 DNase I (Qiagen, Crawley, UK), shaking at 37°C for 2 hours (CRC and Colon) or 1.5 hours (RCC, Kidney, CRM and Liver). Endothelial cells were isolated from the digested single cell suspension by positive magnetic selection using 1.4×10−7 g−1 streptavidin coated Dynabeads® (Invitrogen, Paisley, UK) conjugated to biotinylated Ulex europaeus lectin (Sigma).
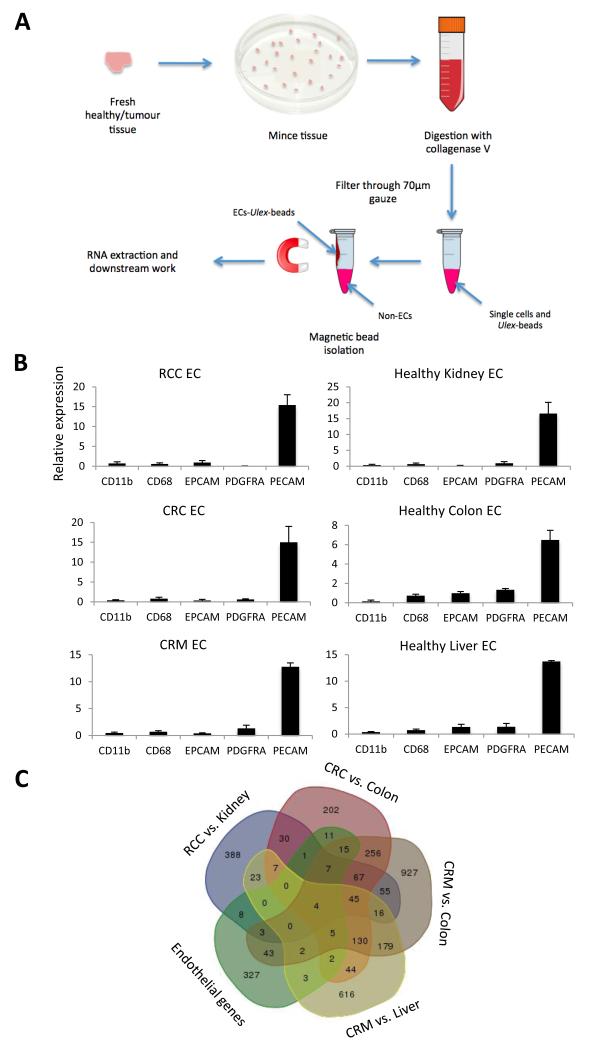
Figure 1.
Isolation of endothelial cells using Ulex lectin coated magnetic beads, for microarray analysis. A, the workflow of the main steps involved in the endothelial isolation procedure. B, confirmation of endothelial isolation efficiency by RTqPCR for markers of leukocytes (CD11b), macrophages (CD68), epithelium (EPCAM), smooth muscle (PDGFRA) and endothelium (PECAM) in the endothelial isolates (EC) from renal cell carcinoma (RCC, n = 8), colorectal carcinoma (CRC, n = 8), colorectal liver metastases (CRM, n = 7) and associated healthy tissues (n = 8,8,7), standardised to flotillin 2 (a house keeping gene) and normalised for marker expression in patient matched bulk tissue. The fold change of marker expression between the endothelial and bulk fraction is shown. Confidence limits are ± standard error of the mean (SEM). C, Venn diagram showing the commonality of genes at least 2 fold upregulated in 4 tumour vs. healthy tissue analyses and present in a list of known endothelial restricted genes.
Microarray and analysis
RNA was isolated from the Ulex lectin-bead isolated endothelium, reverse transcribed to cDNA, transcribed, amplified and labelled with Cy3 in accordance with manufacturers’ protocols (Low input quick amp labelling kit, Agilent technologies, Wokingham, UK). Labelled cRNA samples were then hybridized to 8 × 66k whole human genome expression microarrays (Agilent).
The R programming language (Lucent Technologies), marray (15) and the Limma (Bioconductor) plug-in were used to subtract background, quantile normalize probe signal intensities and perform differential gene expression analyses on the microarray data. Raw and processed data from this analysis is deposited in the GEO repository, accession number: GSE77199.
Quantitative real-time PCR
RNA isolation was performed using the miRNeasy mini kit (Qiagen) and complementary DNA generated using the High Capacity cDNA Reverse Transcription kit (Invitrogen) in accordance with manufacturers’ protocols. Quantitative real time PCR was performed using the Exiqon universal probe system (Roche, Burgess Hill, UK) as previously described (16). Primer sequences are provided in Supplementary Table 1. The Delta-Delta Ct method was used to compare the expression levels between samples and Flotillin-2 was used to standardise expression.
Immunohistochemistry
Immunohistochemistry of human tissues and arrays were performed using 2 μg/ml mouse polyclonal antisera to CD31 (clone JC70, Dako, Ely, UK) and rabbit polyclonal antisera to MCAM (HPA008848, Atlas Antibodies, Sigma, 0.3-0.6 μg/ml) and LAMA4 (HPA015693, Atlas Antibodies, Sigma, 0.5 μg/ml) and stained and visualized using the ImmPRESS universal antibody kit and ImmPACT NovaRed chromagen (Vector labs, Burlingame, CA, USA). The sections were then counterstained with Mayer’s hematoxylin (Sigma), dehydrated and mounted in distyrene–plasticizer–xylene resin (Sigma). All images were acquired using a Leica DM6000 light microscope (Leica, Milton Keynes, UK). The following tissue arrays were used: MA2, MAN2, MB4, MBN4, MC4, MCN4, CD4, CDN4, CDA3, CL2 (Superbiochip, Seoul, Korea), Hkid-CRC180Sur-01, KD951a, BC07001, KD807 (US biomax, Rockville, USA). Human tissue cohort scoring was performed independently by three observers (JWW, HJMF and JAA) blinded to the patient data.
MCAM induction
Human umbilical cord vein endothelial cells (HUVEC) were isolated from human umbilical cords as previously described (17) and used at passage 2. Human dermal microvascular endothelial cells (HDMEC) were purchased from PromoCell, Heidelberg, Germany and used within two passages. HUVEC and human dermal microvascular endothelial cells (HDMEC) isolates were plated at a density of 14,000 cells cm−2, grown in M199 (Sigma), L-glutamine (Gibco) and 1% FCS (Gibco) for 16 hours, then cultured ± 100 ng/ml recombinant human VEGF (Peprotech) for 24 hours.
Western blot
VEGF treated HUVEC were harvested by scraping, lysed, and subjected to 8% SDS-polyacrylamide gel electrophoresis. The protein was blotted onto nitrocellulose, stained with 0.3 μg/ml rabbit polyclonal antisera to MCAM (HPA008848, Atlas Antibodies, Sigma), visualized with ECL peroxidase linked donkey anti–rabbit IgG (NA9340V, GE Healthcare) and ECL detection reagent (GE Healthcare) and used to develop Amersham Hyperfilm™ ECL (GE Healthcare).
Murine tumour and antibody localization experiments
Mice were handled and treated in accordance with home office requirements (Licence number, PPL. 40/3339). The RENCA cell line was originally obtained from the American Type Culture Collection (ATCC), resuscitated from early passage liquid nitrogen stocks, treated as described in (18) and used in this experiment less than 1 months after the re-initiation of culture. Cells were tested negative for mycoplasma contamination. ATCC uses morphology, karyotyping, and PCR based approaches to confirm the identity of human cell lines. The RENCA murine renal cell carcinoma cell line was used to develop subcutaneous tumours. 1.25×105 RENCA cells were injected into the flank of healthy male Balb/c mice. Tumours were permitted to grow to 1 cm3. Mice were then intravenously inoculated with 20 ug monoclonal rat anti-mouse MCAM antibodies (clone 733216, R&D systems) one hour prior to cull. The tumours and several other organs were then collected snap frozen in liquid nitrogen and stored at −80°C for immunofluorescent staining.
Immunofluorescent staining
Immunofluorescent staining of frozen mouse tissue was performed using 4 μg/ml goat polyclonal antisera to rat IgGs conjugated to alexafluor 546 (A11081, Invitrogen) to visualise localised anti-MCAM antibodies. The tissue was also stained with 75 ng/ml rabbit polyclonal antisera to PECAM-1 (ab28364, Abcam) and 4 μg/ml donkey polyclonal antisera to rabbit IgGs conjugated to alexafluor 488 (A21206, Invitrogen) and mounted in ProLong Gold mounting media containing DAPI (Invitrogen). Quantification of fluorescence was conducted using the ImageJ software package (19).
Statistical analysis
Mann-Whitney, Kaplan-Meier, Log-ranks, Chi-square and Cox-regression statistical analyses were performed using the SPSS statistics suite (IBM, USA).
Results
Identification of tumour vascular associated genes
In order to identify the difference in expression profile between tumour associated and healthy vasculature, fresh matched healthy and tumour tissues were collected from resections of colorectal carcinoma (CRC, n=8, Supplementary Table 2), colorectal liver metastasis (CRM, n=7, Supplementary Table 3) and renal cell carcinoma (RCC, n=8, Supplementary Table 4), which had not received any neoadjuvant therapy, and were processed within three hours of surgery. The endothelium was isolated from these tissues, according to the workflow shown in Figure 1A, using magnetic beads conjugated to Ulex agglutinin I, a lectin isolated from Ulex europaeus, which binds specifically to the L-fucose residues restricted to glycoproteins on the surface of human endothelial cells. Endothelial specific isolation was confirmed by RTqPCR for markers of common cell types, leukocytes (CD11b), macrophages (CD68), epithelium (EPCAM), smooth muscle (PDGFRA) and endothelium (PECAM) in the magnetic bead isolates and normalised to the expression levels in the bulk tissue (Figure 1B). RTqPCR analysis determined that endothelium was enriched by between 7- and 17- fold by this procedure.
Microarray analysis was performed on 4 selected biological replicates of endothelium isolated from each of RCC, CRC, CRM, as well as that isolated from patient-matched healthy (non-malignant) tissues. The microarray analysis was performed using the R (64bit) software package with the Limma and marray plugins and separated into 4 comparison matrices; RCC vs. Kidney, CRC vs. Colon, CRM vs. Colon and CRM vs. Liver. The CRM data was compared to the colon as well as the liver from which its vessels are derived, in order to set up a direct comparison between the markers induced by the colorectal primary tumour and the colorectal metastasis. This analysis identified multiple matrix metallopeptidases consistently upregulated within the three tumour types studied, particularly MMP9, MMP12 and MMP14, whilst MMP7 and 11 were consistently upregulated in the vessels of tumours derived from colorectal malignancy alone (Supplementary Table 5). Many collagens were also modulated by exposure to the tumour environment (Supplementary Table 6). Collagen type IV family members in particular were altered in their expression levels, whilst alpha 1 and 2 were upregulated in the tumours, alpha 3, 4 and 5 were downregulated (Supplementary Table 6).
In order to generate a shortlist of consistently upregulated tumour endothelial markers (TEMs), comparative Venn analysis was performed on genes 2 fold upregulated in the cancer with a p-value < 0.001, from each of the four comparison matrices, together with a list of known endothelial genes identified previously by in silico analysis (20) (Figure 1C). From this analysis a shortlist of key genes of interest was generated (Table 1). This list included a number of well-known pan-tumour endothelial markers such as angiopoietin 2, lysyl oxidase, apelin and neuropilin, validating the approach as a method of identifying tumour endothelial markers. One of the most consistently upregulated genes in this analysis was Laminin-alpha-4 (LAMA4) a component of the laminin complex, a major noncollagenous constituent of the basal lamina. The recently identified endothelial receptor for LAMA4, melanoma cell adhesion molecule (MCAM) (13) was also identified by this analysis as consistently upregulated in cancer. We therefore decided to investigate these two interacting proteins further.
Table 1.
Consistently upregulated vascular genes across cancer types (gene expression comparison shown as Log2 fold change).
| Gene ID | Gene symbol | GenBank accession No. | RCC vs Kidney | CRC vs Colon | CRM vs Colon | CRM vs Liver | Gene ontology |
|---|---|---|---|---|---|---|---|
| Lysyl oxidase | LOX | NM_002317 | 2.80 | 1.20 | 1.00 | 1.86 | EX and IC |
| Angiopoietin 2 | ANGPT2 | NM_001147 | 2.50 | 3.40 | 2.48 | 1.22 | EX |
| Laminin, alpha 4 | LAMA4 | NM_001105206 | 2.41 | 1.50 | 1.53 | 1.75 | EX |
| Melanoma cell adhesion molecule | MCAM | NM_006500 | 2.38 | 1.70 | 1.43 | −0.51 | PM |
| Regulator of G-protein signaling 5 | RGS5 | NM_003617 | 2.24 | 2.79 | 1.22 | −1.40 | PM |
| Matrix metallopeptidase 1 | MMP1 | NM_002421 | 0.16 | 4.49 | −0.57 | 2.12 | EX |
| Endothelial cell-specific molecule 1 | ESM1 | NM_007036 | 0.91 | 2.63 | 1.37 | −0.17 | EX |
| Transglutaminase 2 | TGM2 | NM_198951 | −0.32 | 2.60 | 2.36 | 1.54 | IC and PM |
| Serpin peptidase inhibitor H1 | SERPINH1 | NM_001207014 | −0.04 | 2.09 | 2.06 | 1.04 | EX and IC |
| Biglycan | BGN | NM_001711 | 0.92 | 1.29 | 3.75 | 0.03 | EX |
| Neuropilin 1 | NRP1 | NM_001024629 | 0.66 | 1.23 | 2.37 | 0.25 | PM |
| Coagulation factor II (thrombin) receptor | F2R | NM_001992 | 1.18 | 1.42 | 2.12 | 1.07 | PM |
| Troponin C type 2 (fast) | TNNC2 | NM_003279 | −0.26 | 1.28 | 1.11 | 3.38 | IC |
| Serpin peptidase inhibitor B5 | SERPINB5 | NM_002639 | −0.33 | 1.12 | 0.35 | 2.82 | EX |
| Apelin | APLN | NM_017413 | 1.34 | 1.98 | 1.20 | 0.30 | EX |
| Protease, serine, 23 | PRSS23 | NM_007173 | 1.02 | 1.53 | 0.65 | 0.68 | IC |
| Serpin peptidase inhibitor E1 | SERPINE1 | NM_000602 | 1.09 | 0.85 | 1.81 | 0.71 | EX and PM |
| Secreted protein, acidic, cysteine-rich | SPARC | NM_003118 | 0.47 | 1.39 | 1.64 | −0.10 | EX |
| Sushi-repeat containing protein, X-linked 2 | SRPX2 | NM_014467 | −0.62 | 1.22 | 1.11 | 1.92 | EX and IC |
| Peroxidasin homolog (Drosophila) | PXDN | NM_012293 | 1.36 | 1.20 | 1.44 | 0.68 | EX |
| Calcitonin receptor-like | CALCRL | NM_005795 | 1.26 | 1.03 | 1.42 | −0.08 | PM |
| Chemokine (C-C motif) ligand 20 | CCL20 | NM_004591 | 1.25 | 1.04 | 1.29 | 0.08 | EX |
| Follistatin-like 1 | FSTL1 | NM_007085 | 0.69 | 1.48 | 1.44 | 1.03 | EX |
| Transcription factor 4 | TCF4 | NM_003199 | 1.05 | 1.41 | 1.15 | −1.02 | IC |
| Lectin, galactoside-binding, soluble, 1 | LGALS1 | NM_002305 | 1.10 | 1.25 | 1.35 | 0.66 | EX and IC |
| Vimentin | VIM | NM_003380 | 1.01 | 0.50 | 1.01 | −0.89 | IC and PM |
Validation of MCAM and LAMA4 as tumour endothelial markers
In order to confirm the findings of the microarray analysis, RTqPCR was performed to assess mRNA expression levels of MCAM and LAMA4 in endothelial isolates from RCC (n=8), CRC (n=8) and CRM (n=6) and associated healthy tissues (n=8, 8, 6). This analysis showed a pronounced and consistent upregulation of both genes in the endothelium of the cancers when compared to the matched healthy tissues (Figure 2A). The identity of these genes as markers of tumour endothelium was further confirmed by semi-quantitative analysis of their protein expression level by immunohistochemisty (IHC), compared to that of the pan endothelial marker PECAM-1 (Figure 2B). Vessels of both RCC and CRC strongly stained for MCAM and LAMA4 while the vessels of the associated healthy kidney and colon did not stain or stained only weakly at equivalent antibody concentrations. This was particularly apparent in healthy kidney tissue where PECAM was strongly stained within the glomerulus, a highly vascular structure, whereas neither MCAM nor LAMA4 staining was detected in glomeruli. Immunohistochemical staining for both targets was absent in a small cohort of colorectal liver metastases probed (n=6, data not shown).
Figure 2.
MCAM and its extracellular matrix binding partner LAMA4 are specific markers of endothelium in both renal and colorectal cancers. A, confirmation of cancer specific enrichment of MCAM and LAMA4 by RTqPCR on endothelial isolates from RCC, (n = 8), CRC, (n = 8), CRM, (n = 6) and associated healthy tissues (n = 8,8,6). Expression standardised to flotillin 2, confidence limits ± SEM, statistical analysis: Mann-Whitney U test (*** p < 0.001, ** p < 0.01, * p < 0.05). B, confirmation of cancer specific enrichment of MCAM and LAMA4 by immunohistochemistry (IHC). Representative images of kidney (arrows show glomeruli), RCC, colon and CRC stained for PECAM (endothelial marker), MCAM and LAMA4. Scale bar = 50 μm. C, analysis of marker expression in 18 common cancers and associated healthy tissues by IHC. The proportion of tissue of each type scored as exhibiting strong staining is shown. D, VEGF significantly induced MCAM expression in endothelial cells. HUVEC isolates (n=6) and HDMEC isolates (n=2 mixed isolates each in triplicate) were serum and growth factor starved for 12 hours, then cultured with serum depleted media ± 100 ng/ml recombinant human VEGF (hVEGF), MCAM expression was determined by western blot and RTqPCR. Confidence limits ± SEM, statistical analysis: Mann-Whitney U test, ** p < 0.01.
In order to identify the tissue specific expression profile of MCAM and LAMA4, 10 samples each of 18 common cancers and associated healthy tissues were stained and scored for strength of staining (Figure 2C). Both MCAM and LAMA4 demonstrated markedly specific vascular expression profiles, with vessels the primary source of staining in all tissues other than melanoma, where MCAM expression was primarily found on the tumour cells (data not shown). Of note, 90% of kidney tumours showed strong vascular staining for MCAM, in excess of any healthy tissue and most cancerous tissues examined, highlighting MCAM as a promising vascular target in RCC. LAMA4 on the other hand was shown to be highly expressed in a broad range of both tumour and healthy tissues (Figure 2C).
MCAM expression is induced by vascular endothelial growth factor (VEGF)
MCAM expression on tumour vessels appears to be highly RCC specific. High VEGF expression is common in cases of clear cell RCC (6), due to the loss of the tumour suppressor Von-Hippel-Lindau (VHL), as previously discussed. In order to investigate whether enhanced VEGF production within the tumour could be the cause of high MCAM expression, isolated human umbilical cord vein endothelium (HUVEC) and commercially purchased human dermal microvascular endothelial cells (HDMEC) were exposed to recombinant VEGF. Six HUVEC isolates and two multisource HDMEC isolates, each used in triplicate, were serum starved for 16 hours and then cultured with or without 100 ng ml−1 recombinant VEGF for 24 hours before being harvested. RTqPCR and western blot analysis for both cell types showed that MCAM was significantly upregulated by VEGF (Mann-Whitney U test, p<0.01) (Figure 2D).
Clear cell RCC alone is characteristic for high VEGF expression and all the RCC tumours in the multiorgan expression analysis were clear cell. In order to investigate whether MCAM expression is specific for ccRCC, tissues from five different types of renal malignancy were stained by IHC. This analysis revealed that a significantly greater proportion of clear cell tumours stained strongly for MCAM than non-clear cell tumours (61%, n=64 vs. 30.5%, n= 36; chi-square, p<0.005). A break down of results for individual renal malignancy histology types is shown in Supplementary Table 7. This result supports the suggestion that VEGF is playing a part in enhancing MCAM expression in ccRCC. Additionally this result suggested that ccRCC should be the focus of investigation.
Identification of strong MCAM and LAMA4 expression as potent adverse prognostic indicators in clear cell RCC
In order to investigate whether the strong expression of MCAM and LAMA4 in the vessels of many RCC and CRC tumours could have some prognostic value, excised tissue from cohorts of ccRCC (n=81[cohort 1], 48 [cohort 2], 47 [cohort 3]) and CRC (n=90) were stained by IHC for each marker and semi-quantitatively scored. Demographic information is shown for each cohort in Supplementary Tables 8 (RCC cohort 1), 9 (RCC cohort 2), 10 (RCC cohort 3) and 11 (CRC cohort).
For effective investigation of prognostic linkage to marker expression, the analytical tools must be sensitive to the full range of marker expression within the cohort. As the multi-organ tissue array staining for MCAM resulted in 90% of renal cell carcinoma samples staining strongly, the antibody concentration was titrated down from 0.6 to 0.3 μg/ml, to a level where a range of MCAM staining in renal cell carcinoma could be observed. 0.6 μg/ml was used for the results listed in Figure 2, 0.3 μg/ml was used for all other IHC analyses.
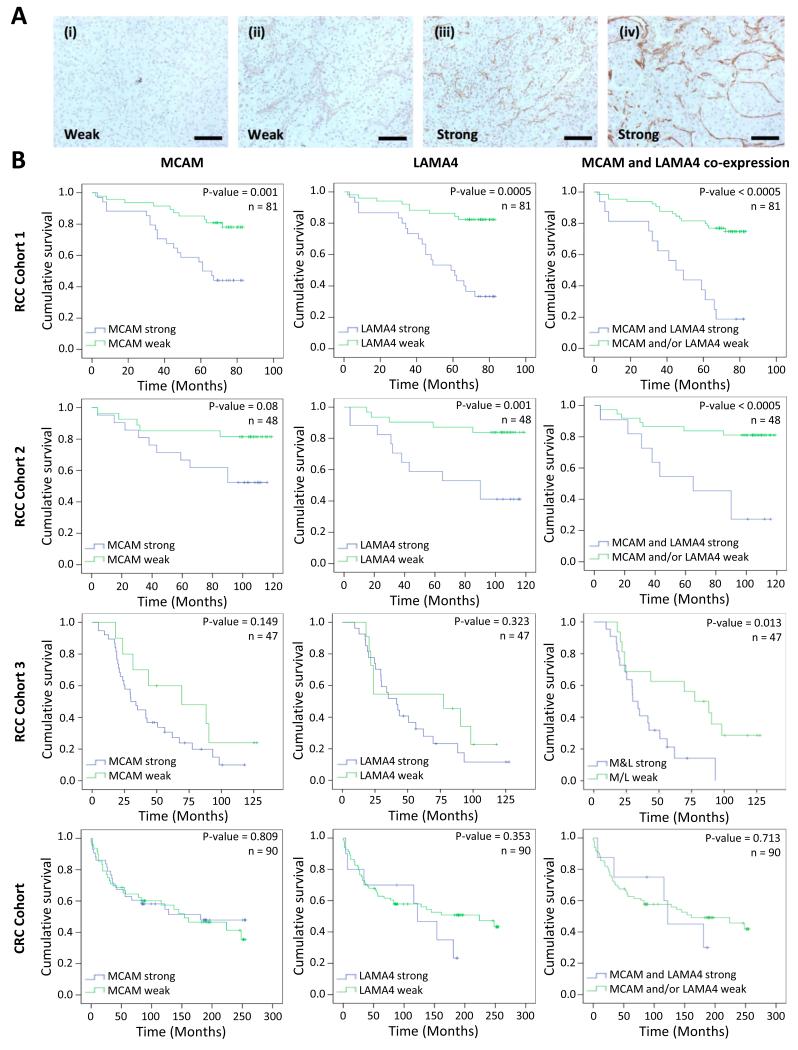
Each cohort was split into tumours exhibiting strong or weak marker staining, as judged by three independent scorers, JWW, JAA and HJMF (representative images of each group are shown in Figure 3A, Supplementary Figure 1). Tumour marker staining was correlated with patient survival by Kaplan-Meier analysis (Figure 3B). This analysis identified a significant decrease in survival in patients whose tumours exhibited strong MCAM and LAMA4 staining in both RCC cohort 1 (log-ranks, p=0.001 & 0.0005 respectively) and cohort 2 (p=0.08 & 0.001 respectively) (Figure 3B). In patients with tumours exhibiting strong staining for both MCAM and LAMA4 together this effect was more pronounced, with only 18% surviving to date versus 75% in tumours that were not strongly stained for either marker in RCC cohort 1 and 27% vs. 81% in RCC cohort 2 (p<0.0005, both cohorts) (Figure 3B). In the CRC cohort there was no significant association between survival and strong MCAM (p=0.809) or LAMA4 (p=0.353) expression alone or when co-expressed (p=0.713) (Figure 3B). This suggests that MCAM and LAMA4 play a unique, tumour-specific role in ccRCC patient survival.
Figure 3.
High MCAM and LAMA4 tumour vessel expression has a significant detrimental effect on the survival of RCC patients but not CRC patients. A, representative images demonstrative of scoring for staining intensity. Images are of MCAM staining in RCC at a weak (i-ii) or strong (iii-iv) level. Scale bar = 50 μm. B, Kaplan-Meier survival analysis of patients, from RCC cohort 1,2 and 3 and the CRC cohort, with strong vs. weak staining for MCAM, LAMA4 and marker co-expression. Statistical analysis: Log-ranks test, p and n for each test are shown. Crosses mark censored cases.
No significant survival effect was observed In RCC cohort 3, with the exception of where the markers were co-expressed (p=0.013). RCC cohorts 1 and 2 are primarily made up of nonmetastatic tumours, with only five with metastasis across the two cohorts and survival to last check up is 64% and 66% respectively (Supplementary Tables 8 and 9). These types of cohort are ideal for looking at survival effects, as the potential for a separation in survival between two groups is great. RCC cohort 3 is entirely made up of metastatic tumours and survival to last check up is 21% (Supplementary Table 10). It is reasonable to suggest therefore that a single marker survival effect is not seen as too few patients survive. This does however suggest that prognostication using MCAM and LAMA4 marker expression will find its greatest utility in early-stage disease.
Multivariate cox-regression analysis on the largest RCC cohort, cohort 1, identified MCAM (p=0.006), LAMA4 (p=007) individually and in combination (p=0.002) as independent risk factors for reduced survival in patients with tumours exhibiting strong staining, independent of gender, age, histopathological tumour grade or T-stage (Table 2). This analysis additionally identified a considerably greater risk of death in patients exhibiting strong MCAM and LAMA4 expression: MCAM, odds ratio 3.4, confidence interval 1.4-8.1; LAMA4, OR 3.3, CI 1.4-7.9; Co-expression, OR 4.1, CI 1.7-10 (Table 2).
Table 2.
Multivariate analysis (Cox regression) of prognostic markers in RCC cohort 1 (n = 81), censored cases n = 52 (64.2%). p < 0.05 are in bold
| Prognostic factor | Relative Risk Exp(B) | 95% Confidence Interval | P-value M and L | P-value MCAM | P-value LAMA4 |
|---|---|---|---|---|---|
| M and L co-expression (high vs. low) | 4.102 | 1.690-9.954 | 0.002 | ||
| MCAM (high vs. low) | 3.402 | 1.419-8.157 | 0.006 | ||
| LAMA4 (high vs. low) | 3.297 | 1.379-7.884 | 0.007 | ||
| Sex (female vs. male) | 0.624 | 0.270-1.442 | 0.27 | 0.164 | 0.835 |
| Age (over 60 vs. under 60) | 1.811 | 0.780-3.862 | 0.076 | 0.058 | 0.246 |
| Grade (G2 and above vs. G1) | 2.233 | 0.881-5.660 | 0.091 | 0.319 | 0.223 |
| T-stage (T2 and above vs. T1) | 2.363 | 0.923-6.049 | 0.073 | 0.002 | 0.041 |
MCAM and LAMA4 expression is enhanced in locally invasive and metastatic disease
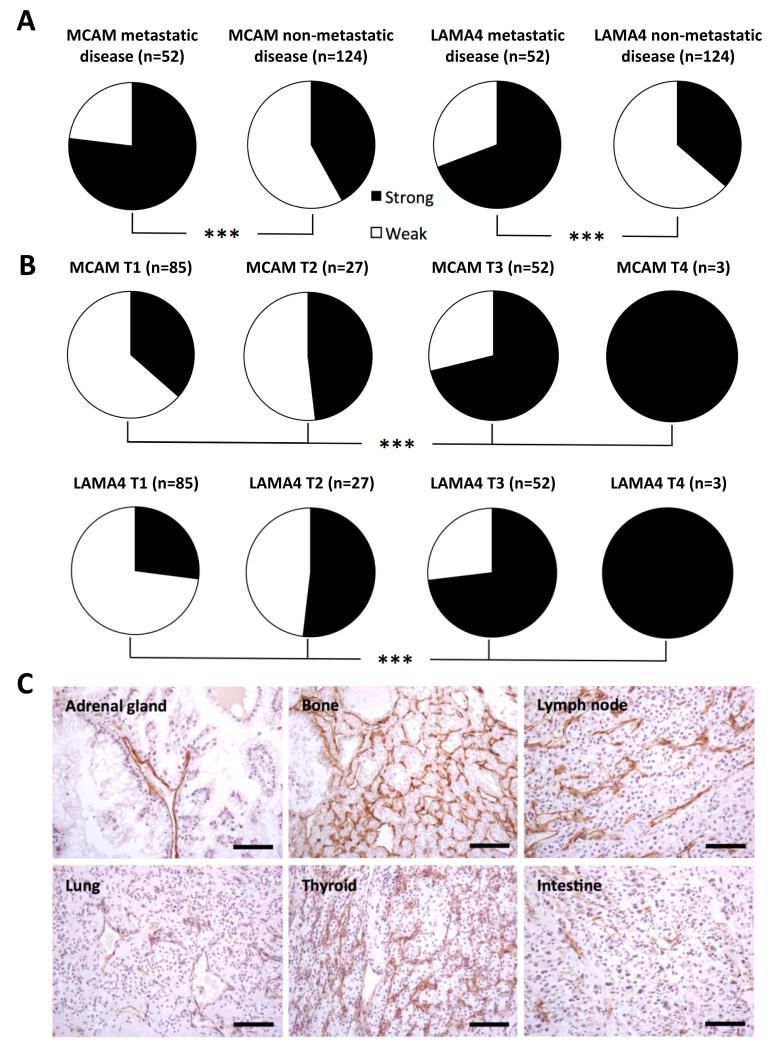
Metastatic disease is the area of most therapeutic need for renal cell carcinoma, survival is lowest and there is the greatest need for systemic therapies. A tumour vascular target would ideally have utility in this setting. In order to investigate the expression of MCAM and LAMA4 in metastatic disease, tumours from ccRCC cohorts 1, 2 and 3 were grouped based on their metastatic status. This revealed that the proportion of metastatic tumours exhibiting strong staining is significantly greater than the in tumours with no known metastases (76% vs. 41%-MCAM, 68% vs. 36%-LAMA4, chi-square, p<0.001) (Figure 4A). It should be borne in mind however that the majority metastatic tumours are from one cohort and non-metastatic from another and possible differences in tissue preparation may have impacted the result. An additional analysis grouping the tumours based on T-stage found that both markers are significantly enriched in tumours exhibiting greater local invasion (chi-square, p<0.001) (Figure 4B). In the case of metastatic disease it is not just the primary tumour that must be treated, but also the metastases, therefore MCAM staining in metastases from clear cell RCC tumours was investigated (Figure 4C). This analysis revealed that 73% of clear cell RCC metastases (n=15) exhibit strong MCAM staining across seven different metastasis locations (Supplementary Table 7). Sample numbers were too low, however, to dissect whether MCAM expression is greater in certain metastasis locations over others. MCAM expression was additionally observed in metastases from papillary and squamous cell RCC, again however sample numbers (n=2,2) were too low to make any significant conclusions (Supplementary Table 7). This data collectively identifies MCAM and LAMA4 as markers of advanced disease and MCAM as an ideal target for the treatment of metastatic renal cell carcinoma.
Figure 4.
MCAM and LAMA4 expression is enhanced in metastatic and locally advanced clear cell RCC. A, pie-chart proportional representation of strong (black) vs. weak (white) marker expression in metastatic and non-metastatic RCC, determined by IHC on RCC cohorts 1,2 and 3. Statistical analysis: chi-square, *** p<0.001. B, pie-chart proportional representation of strong (black) vs. weak (white) marker expression at different RCC T-stages, determined by IHC on RCC cohorts 1,2 and 3. Statistical analysis: chi-Square, *** p<0.001. C, representative images of MCAM staining in clear cell RCC metastases to various organs, generated by IHC. Scale bar = 50 μm.
Monoclonal anti-MCAM antibodies specifically localise to murine RCC tumour vessels
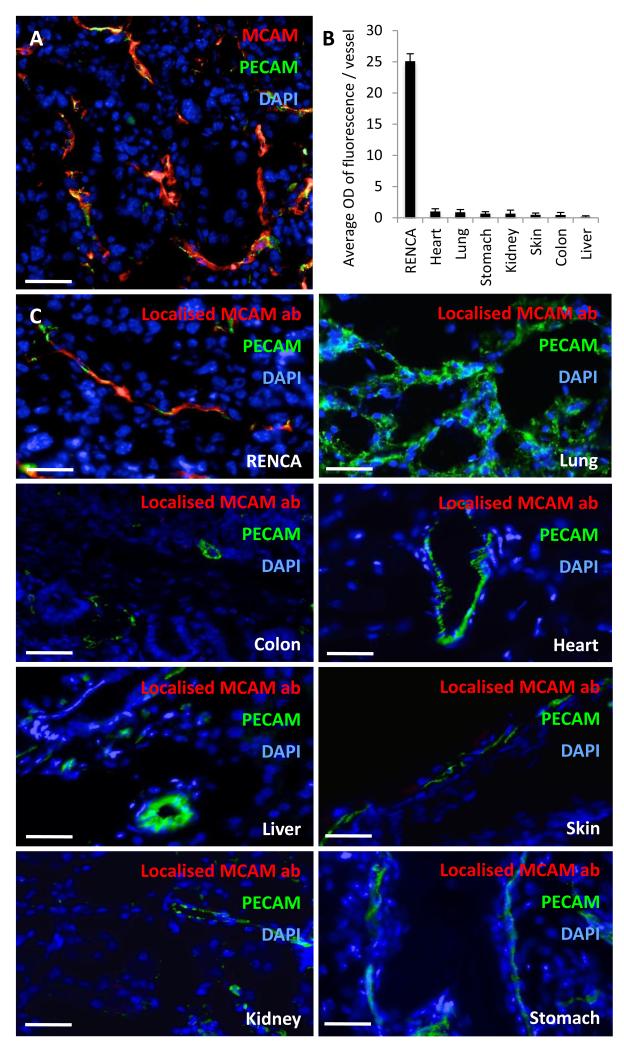
The abundance and specificity of MCAM expression in renal tumours opens the possibility of using it as a targeting ligand for therapeutic agents. In order to investigate this potential utility, the localisation of a monoclonal rat anti-MCAM antibody was determined, following intravenous injection into murine renal cell carcinoma (RENCA) tumour bearing mice. The RENCA model was chosen as the tumours have previously been shown to express VEGF at a high level (21), closely modelling the majority of human renal cell cancers. Additionally subcutaneous RENCA tumours strongly express MCAM on their vessels (Figure 5A) identifying it as an ideal model for use in this situation. An hour after antibody infusion, the mice were culled, the organs harvested and then processed for frozen sectioning. Localised antibody was detected by fluorescently labelled anti-Rat IgGs. Tissues from the RENCA tumour, stomach, heart, liver, colon, kidney, skin and lung were probed. The average optical density of fluorescence emanating from localised anti-MCAM antibody within the vessels of each tissue in two mice and 12 regions of interest, was quantified and demonstrated an at least 25 fold greater localisation of antibody in the vessels of the RENCA tumours than any other tissue probed (Figures 5B and 5C). This finding suggests that renal cell carcinoma therapies could potentially use anti-MCAM antibodies to localise therapeutics to the tumour vasculature specifically, permitting a functional anticancer effect.
Figure 5.
A monoclonal anti-MCAM antibody specifically localises to murine RCC tumour vessels. A, triple immunofluorescent staining of a murine RCC (RENCA) tumour for; PECAM-1 (green), MCAM (red) and DAPI (blue). Scale bar = 25 μm. B & C, 20 μg of MCAM monoclonal antibody was intravenously injected into RENCA tumour bearing mice 1 hour prior to cull. The tumour and selected organs were collected. Frozen sections were stained with anti-rat IgGs (red), PECAM-1 (green) and DAPI (blue). B, the average optical density of fluorescence detected in the anti-rat IgG (red) channel, within regions of vascular (PECAM-1) staining was quantified. The tissue from two mice was assessed, with six regions of interest selected for each organ and mice (n=12), confidence limits ± SEM. C, representative images of MCAM monoclonal antibody localisation. Scale bar = 12.5 μm.
Discussion
The aim of this study was to identify vascular markers with pan-tumour expression and demonstrate their utility as specific ligands against which to target immunological therapies. This study identified MCAM and LAMA4 as promising markers with specific overexpression in endothelial isolates from both colorectal and renal malignancies. A significant link between high expression of these markers and poor patient survival, invasive local disease and metastasis, was demonstrated in clear cell RCC, but not CRC. MCAM expression was found to be highly enriched in the vessels of ccRCC, in excess of other tumour and healthy tissues, possibly due to VEGF induction demonstrated in this paper. This data highlighted MCAM as a potential specific target in renal cell carcinoma and this utility was demonstrated by specific localisation of MCAM monoclonal antibodies to the tumour vessels in a model of RCC.
Comparative analysis of vessels derived from colorectal carcinoma (CRC), colorectal liver metastasis (CRM) and renal cell carcinoma (RCC) with patient matched healthy tissues, identified a small group of endothelial genes consistently upregulated in these tumours. Many of these genes are stimulatory to angiogenesis and tumour invasion, such as LOX (22), MCAM (23), LAMA4 (24), NRP1 (25), MMP1 (26), APLN (27) and SPARC (28) suggesting a signature characterised by active angiogenesis.
One of the most consistent pan-tumour endothelial markers in the analysis was laminin alpha 4 (LAMA4) an extracellular matrix glycoprotein and component of the laminin complex. Laminins are made up of three chains, alpha, beta and gamma and have been implicated in a wide variety of cellular processes, from cell attachment and differentiation, to influences on cell shape and movement, maintenance of tissue phenotype, and promotion of tissue survival (29). The function of individual laminin chains is poorly understood, however LAMA4, a constituent of laminin-8, 9 and 14 (30), has been shown to have an endothelial specific expression pattern, and also to promote angiogenesis (24). LAMA4 has been shown to co-distribute and interact with integrins αvβ3, α3β1, and together with α6β1 mediate endothelial cell-LAMA4 interactions and blood vessel formation (24).
The exact role LAMA4 plays in cancer is unclear. In this study it is shown to be strongly upregulated on tumour blood vessels in colorectal and renal malignancies, when compared to surrounding non-malignant tissue. Its tissue distribution appears to be diverse when more broadly investigated however, appearing in both healthy and tumour tissues. This analysis suggests that using LAMA4 as a target for cancer therapy could be problematic. This study does however demonstrate a highly significant link between LAMA4 expression in RCC and poor patient survival, which is not shared in CRC. A strong association between LAMA4 expression and both metastasis and local invasion is also shown. LAMA4 has previously been associated with increased tumour invasion and metastasis in hepatocellular carcinoma (31), the transition from premalignant to malignant breast carcinomas and reduced relapse-free survival in estrogen receptor negative breast cancer patients (32), marking LAMA4 as a useful prognostic marker in certain cancers, not least renal cell carcinoma. A recombinant form of the LAMA4 chain containing Laminin-411 (Laminin-8) has been reported to have an anti-adhesive effect on RCC cells grown on fibronectin (33). This report, combined with our observations suggests a potential mechanism in which heightened vascular LAMA4 might impair RCC tumour adhesion and thereby increase metastasis, negatively impacting patient survival. The functional relevance of this mechanism warrants further investigation.
This study also identified MCAM as a potent vascular marker in clear cell renal cell carcinoma. The role of MCAM on the vascular endothelium is poorly understood, but it is thought to promote angiogenesis (23) and act as a co-receptor for VEGFR-2, thus enhancing endothelial migration and microvessel formation (34). Endothelial conditional knock out of MCAM in mice, results in impaired vessel formation in VEGF-dependant angiogenesis assays (34). MCAM is also thought to play a role in cell to cell junctions and vascular permeability (35). Besides this, MCAM over expression has been associated with pro-survival signaling including protein kinase B (PKB) phosphorylation and down-regulation of BCL2-Associated Agonist Of Cell Death (BAD) expression (36). It is therefore plausible that upregulation of MCAM in tumor vessels could act as a survival mechanism, as well as impacting on angiogenesis and vascular integrity.
MCAM was first identified as a marker on the carcinoma cells of malignant melanoma, emerging as a potential prognostic indicator of cancer progression (9). MCAM expression has also been reported on the carcinoma cells of prostate (10), breast (11) and ovarian cancer (12), suggesting that MCAM could be a widely expressed tumour antigen. However, in this study, MCAM expression was only occasionally observed on the tumour cells of tissue examined, with MCAM expression being almost exclusively reserved to tumour vessels in all malignancies aside from melanoma, calling into question the relative importance of tumour and endothelial cell MCAM expression in these malignancies.
A significant association between high vascular MCAM expression and poor RCC patient survival and increased metastasis and local invasion, was demonstrated in this study. Heightened MCAM mRNA expression has previously been reported in bulk tumour tissue from patients with RCC, with the highest levels observed in metastatic disease, indicating a direct correlation between increasing MCAM expression and disease progression (37), partially corroborating our observations. We further this finding by highlighting vascular MCAM as key to this process and demonstrating a direct survival impact. High MCAM expression has additionally been associated with poor survival in patients with non-small cell lung adenocarcinoma (but not squamous cell carcinoma) (38). This data highlights MCAM as an important prognostic marker in cancer, in particular RCC, where co-expression with its recently identified extracellular ligand LAMA4 (13), was shown to be highly predictive of very poor patient survival. This observation suggests that the expression and interaction of MCAM and LAMA4 could be highly significant in RCC progression and should be further investigated. MCAM interaction with the LAMA4 containing laminin-9 complex has been shown to promote migration of tumour cells, when associated with α6β1 integrin (39), but the importance of this interaction for endothelial cell or cancer biology is unknown.
Multi-tissue analysis identified MCAM as highly specific to RCC in its expression. Whilst it is present in other tissues, its expression in ccRCC vessels is greatly in excess. This study identified VEGF-mediated induction of MCAM in endothelial cells as a potential explanation for this. This is the first study to demonstrate that VEGF, a growth factor highly expressed in RCC (6), will induce MCAM expression in endothelial cells. The regulation of MCAM on vessels in poorly understood. The expression of MCAM in HUVEC was found to be upregulated by culture with media conditioned with a hepatoma cell line (23), however the exact mechanism was not determined. Tumour necrosis factor has been reported to induce the formation of a soluble form of MCAM in various cell types including endothelium (40,41). Of note, insulin-like growth factor-binding protein 4 (IGFBP-4) has been reported to induce MCAM in renal cell carcinoma. IGFBP-4 transfected renal tumour cells were found to exhibit enhanced cell growth, invasion and motility, as well as enhancing MCAM expression (42). However as has been discussed, in this study MCAM expression was primarily observed on tumour vasculature, with no discernable RCC tumour cell expression, so the significance of IGFBP-4 induced MCAM expression on RCC tumour cells in human cancer, is unclear.
The finding that MCAM, a cell surface glycoprotein, was highly specific to RCC vessels, marked it out as an ideal target for anti-cancer immunological therapies. This study demonstrates this utility by showing a monoclonal anti-MCAM antibody specifically localise to the vessels of a murine model of RCC, accumulating in the tumour at at least 25 fold greater density. This constitutes a striking level of antibody specificity to cancer, in excess of other successful tumour localisation studies (43,44). A number of successes in anti-cancer cell targeting using antibodies have been achieved, including FDA approved ADCs Brentuximab Vedotin in Hodgkins lymphoma (45) and Transtuzumab Emtansine in breast cancer (46). However as mentioned in the introduction the targeting of tumour blood vessels does offer a number of advantages; the blood vessels are easily accessible for ligand targeting; up to 100 tumour cells are dependent on a single endothelial cell (47) for survival, making the vessels an extremely efficient target; the vasculature is thought to be more genetically stable and so more homogenous in terms of marker expression (48) and finally therapeutics disrupting the vessels have been shown to cause preferential lysis in the core of large tumours (49), a region poorly treated by antiangiogenics and traditional chemotherapeutics (50). This study therefore identifies and validates MCAM as a new ligand with which to specifically target therapeutics against renal cell carcinoma vessels, potentially improving treatment of this malignancy.
Supplementary Material
Acknowledgements
We are grateful to Dr Andy Reynolds for his provision of the RENCA cell line and Prof. Nick James for funding the RCC tissue collection.
Financial support:
J.W. Wragg, J.P. Finnity, V.L. Heath and R. Bicknell received funds from the University of Birmingham CRUK Cancer Center. J.A. Anderson, H.J.M Ferguson, E. Porfiri, R.I. Bhatt and P.G. Murray received funds from the UHB NHS Foundation Trust.
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Jemal A, Bray F, Center Melissa M., Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Rathmell WK, Godley P. Renal cell carcinoma. Current Opinion in Oncology. 2008;20:300–6. doi: 10.1097/CCO.0b013e3282f9782b. [DOI] [PubMed] [Google Scholar]
- 3.Patil S, Manola J, Elson P, Negrier S, Escudier B, Eisen T, et al. Improvement in overall survival of patients with advanced renal cell carcinoma: prognostic factor trend analysis from an international data set of clinical trials. The Journal of Urology. 2012;188:2095–100. doi: 10.1016/j.juro.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Cohen HT, McGovern FJ. Renal-Cell Carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 5.Singer EA, Gupta GN, Marchalik D, Srinivasan R. Evolving therapeutic targets in renal cell carcinoma. Current Opinion in Oncology. 2013;25:1–280. doi: 10.1097/CCO.0b013e32835fc857. [DOI] [PubMed] [Google Scholar]
- 6.Cowey CL, Rathmell WK. VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr Oncol Rep. 2009;11:94–101. doi: 10.1007/s11912-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrows FJ, Thorpe PE. Eradication of large solid tumors in mice with an immunotoxin directed against tumor vasculature. Proc Natl Acad Sci USA. 1993;90:8996–9000. doi: 10.1073/pnas.90.19.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wragg JW, Bicknell R. Vascular Targeting Approaches to Treat Cancer. Cancer Targeted Drug Delivery. 2013:59–95. New York, NY: Springer New York. [Google Scholar]
- 9.Lehmann JM, Riethmüller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA. 1989;86:9891–5. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu GJ, Peng Q, Fu P, Wang SW, Chiang CF, Dillehay DL, et al. Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene. 2004;327:201–13. doi: 10.1016/j.gene.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Zabouo G, Imbert A-M, Jacquemier J, Finetti P, Moreau T, Esterni B, et al. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 2009;11:R1. doi: 10.1186/bcr2215. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Wu Z, Li J, Yang X, Wang Y, Yu Y, et al. MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumor Biol. 2012;33:1619–28. doi: 10.1007/s13277-012-0417-0. Springer Netherlands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, et al. Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS ONE. 2012;7:e40443. doi: 10.1371/journal.pone.0040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuang X, Herbert JMJ, Lodhia P, Bradford J, Turner AM, Newby PM, et al. Identification of novel vascular targets in lung cancer. Br J Cancer. 2014;112:485–94. doi: 10.1038/bjc.2014.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YH, Paquet A, Dudoit S. [cited 2015 Apr 13];marray: Exploratory analysis for two-color spotted microarray data [Internet] 2009 R package version 1.44.0. bioconductor.org. http://www.maths.usyd.edu.au/u/jeany/ Available from: http://www.bioconductor.org/packages/release/bioc/html/marray.html.
- 16.Armstrong L-J, Heath VL, Sanderson S, Kaur S, Beesley JFJ, Herbert JMJ, et al. ECSM2, an endothelial specific filamin a binding protein that mediates chemotaxis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1640–6. doi: 10.1161/ATVBAHA.108.162511. Lippincott Williams & Wilkins. [DOI] [PubMed] [Google Scholar]
- 17.Crampton SP, Davis J, Hughes CCW. Isolation of Human Umbilical Vein Endothelial Cells (HUVEC) J Vis Exp. 2007:e183–3. doi: 10.3791/183. MyJoVE Corporation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verheul HMW, Hammers H, van Erp K, Wei Y, Sanni T, Salumbides B, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–8. doi: 10.1158/1078-0432.CCR-06-2553. American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert JM, Stekel D, Sanderson S, Heath VL, Bicknell R. A novel method of differential gene expression analysis using multiple cDNA libraries applied to the identification of tumour endothelial genes. BMC Genomics. 2008;9:153. doi: 10.1186/1471-2164-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando NT, Koch M, Rothrock C, Gollogly LK, D'Amore PA, Ryeom S, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14:1529–39. doi: 10.1158/1078-0432.CCR-07-4126. American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 22.Baker A-M, Bird D, Welti JC, Gourlaouen M, Lang G, Murray GI, et al. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 2013;73:583–94. doi: 10.1158/0008-5472.CAN-12-2447. American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan X, Lin Y, Yang D, Shen Y, Yuan M, Zhang Z, et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102:184–91. doi: 10.1182/blood-2002-04-1004. American Society of Hematology. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, et al. Complex interactions between the laminin α4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci USA. 2002;99:16075–80. doi: 10.1073/pnas.252649399. National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 Is Expressed by Endothelial and Tumor Cells as an Isoform-Specific Receptor for Vascular Endothelial Growth Factor. Cell. 1998;92:735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 26.Fisher C, Gilbertson-Beadling S, Powers EA, Petzold G, Poorman R, Mitchell MA. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994;162:499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- 27.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, et al. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochemical and Biophysical Research Communications. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 28.Jendraschak E, Sage EH. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol. 1996;7:139–46. doi: 10.1006/scbi.1996.0019. [DOI] [PubMed] [Google Scholar]
- 29.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. John Wiley & Sons, Inc. [DOI] [PubMed] [Google Scholar]
- 30.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Ji G, Wu Y, Wan B, Yu L. LAMA4, highly expressed in human hepatocellular carcinoma from Chinese patients, is a novel marker of tumor invasion and metastasis. J Cancer Res Clin Oncol. 2007;134:705–14. doi: 10.1007/s00432-007-0342-6. Springer-Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross JB, Huh D, Noble LB, Tavazoie SF. Identification of molecular determinants of primary and metastatic tumour re-initiation in breast cancer. Nat Cell Biol. 2015;17:651–64. doi: 10.1038/ncb3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vainionpää N, Lehto V-P, Tryggvason K, Virtanen I. Alpha4 chain laminins are widely expressed in renal cell carcinomas and have a de-adhesive function. Laboratory Investigation. 2007;87:780–91. doi: 10.1038/labinvest.3700592. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 34.Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120:2330–9. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- 35.Bardin N, Anfosso F, Massé JM, Cramer E, Sabatier F, Le Bivic A, et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–84. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 36.Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1795:130–6. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Feng G, Fang F, Liu C, Zhang F, Huang H, Pu C. CD146 gene expression in clear cell renal cell carcinoma: a potential marker for prediction of early recurrence after nephrectomy. Int Urol Nephrol. 2012;44:1663–9. doi: 10.1007/s11255-012-0255-4. Springer Netherlands. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Wang Z, Kang Y, Li X, Ma X, Ma L. MCAM expression is associated with poor prognosis in non-small cell lung cancer. Clin Transl Oncol. 2013;16:178–83. doi: 10.1007/s12094-013-1057-6. Springer Milan. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa T, Wondimu Z, Oikawa Y, Gentilcore G, Kiessling R, Egyhazi Brage S, et al. Laminins 411 and 421 differentially promote tumor cell migration via α6β1 integrin and MCAM (CD146) Matrix Biology. 2014;38:69–83. doi: 10.1016/j.matbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka S, Fujiwara H, Higuchi T, Yamada S, Maeda M, Fujii S. Melanoma cell adhesion molecule (MCAM/CD146) is expressed on human luteinizing granulosa cells: enhancement of its expression by hCG, interleukin-1 and tumour necrosis factor-alpha. Mol Hum Reprod. 2003;9:311–9. doi: 10.1093/molehr/gag042. [DOI] [PubMed] [Google Scholar]
- 41.Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto J-P, et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:746–53. doi: 10.1161/ATVBAHA.108.183251. Lippincott Williams & Wilkins. [DOI] [PubMed] [Google Scholar]
- 42.Ueno K, Hirata H, Majid S, Tabatabai ZL, Hinoda Y, Dahiya R. IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int J Cancer. 2011;129:2360–9. doi: 10.1002/ijc.25899. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 43.Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12:3200–8. doi: 10.1158/1078-0432.CCR-05-2804. American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa M, Mukai Y, Okada Y, Tsumori Y, Tsunoda SI, Tsutsumi Y, et al. Robo4 is an effective tumor endothelial marker for antibody-drug conjugates based on the rapid isolation of the anti-Robo4 cell-internalizing antibody. Blood. 2013;121:2804–13. doi: 10.1182/blood-2012-12-468363. [DOI] [PubMed] [Google Scholar]
- 45.Younes A, Forero-Torres A, Bartlett NL, Leonard JP, Lynch C, Kennedy DA, et al. Multiple complete responses in a phase 1 dose-Multiple complete responses in a phase 1 dose-escalation study of the antibody-drug conjugate SGN-35 in patients with relapsed or refractory CD30 positive lymphomas. Blood. 2008;112:1006. [Google Scholar]
- 46.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajitou A, Pasqualini R, Arap W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16:80–8. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 49.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–9. doi: 10.1002/cncr.20299. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 50.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–27. doi: 10.1158/1078-0432.ccr-0642-03. American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.