SUMMARY
Each year, the American Heart Association (AHA), in conjunction with the Centers for Disease Control and Prevention, the National Institutes of Health, and other government agencies, brings together in a single document the most up-to-date statistics related to heart disease, stroke, and the factors in the AHA’s Life’s Simple 7 (Figure1), which include core health behaviors (smoking, physical activity [PA], diet, and weight) and health factors (cholesterol, blood pressure [BP], and glucose control) that contribute to cardiovascular health. The Statistical Update represents a critical resource for the lay public, policy makers, media professionals, clinicians, healthcare administrators, researchers, health advocates, and others seeking the best available data on these factors and conditions. Cardiovascular disease (CVD) and stroke produce immense health and economic burdens in the United States and globally. The Update also presents the latest data on a range of major clinical heart and circulatory disease conditions (including stroke, congenital heart disease, rhythm disorders, subclinical atherosclerosis, coronary heart disease, heart failure (HF), valvular disease, venous disease, and peripheral arterial disease) and the associated outcomes (including quality of care, procedures, and economic costs). Since 2006, the annual versions of the Statistical Update have been cited >20 000 times in the literature. In 2015 alone, the various Statistical Updates were cited ≈4000 times.
Figure. Life’s Simple 7.

Seven approaches to staying heart healthy: be active, keep a healthy weight, learn about cholesterol, don’t smoke or use smokeless tobacco, eat a heart-healthy diet, keep blood pressure healthy, and learn about blood sugar and diabetes.
Each annual version of the Statistical Update undergoes revisions to include the newest nationally representative data, add additional relevant published scientific findings, remove older information, add new sections or chapters, and increase the number of ways to access and use the assembled information. This year-long process, which begins as soon as the previous Statistical Update is published, is performed by the AHA Statistics Committee faculty volunteers and staff and government agency partners. This year’s edition includes new data on the monitoring and benefits of cardiovascular health in the population, new metrics to assess and monitor healthy diets, a new chapter on venous disease and pulmonary hypertension (PH), new information on stroke in young adults, an enhanced focus on underserved and minority populations, a substantively expanded focus and chapter on the global burden of CVD, and further evidence-based approaches to changing behaviors, implementation strategies, and implications of the AHA’s 2020 Impact Goals. Below are a few highlights from this year’s Update.
Current State of Cardiovascular Health in the United States: What’s New? (Chapter 2)
The AHA developed a Health Campaign for Life’s Simple 7, which emphasizes that adults and young people can live healthier lives by avoiding smoking and tobacco products, engaging in daily PA, eating a healthy diet, maintaining a healthy weight, and keeping cholesterol, BP, and glucose at healthy levels. New highlights from the cardiovascular health section include the following:
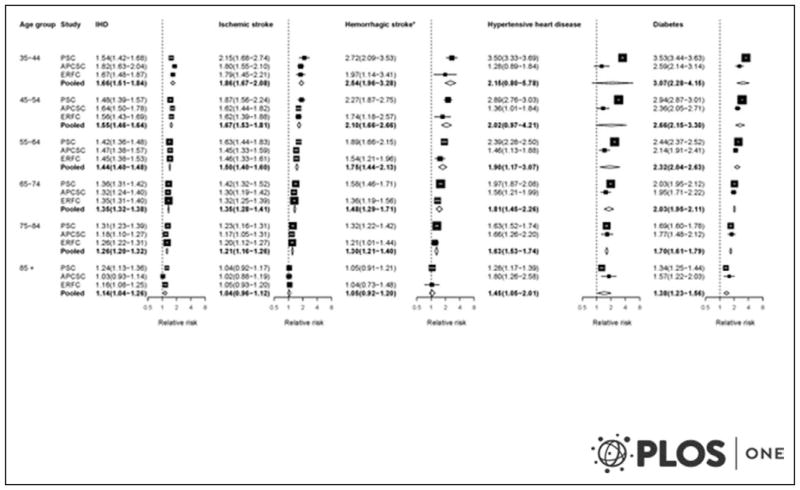
A recent meta-analysis of 9 prospective cohort studies involving 12 878 participants contributed new estimates of the importance of cardiovascular health metrics and risk for clinical events. The meta-analysis showed that achieving the greatest ideal cardiovascular health metrics was associated with a lower risk of stroke (relative risk, 0.31; 95% confidence interval [CI], 0.25–0.38), CVD (relative risk, 0.20; 95% CI, 0.11–0.37), cardiovascular mortality (relative risk, 0.25; 95% CI, 0.10–0.63), and all-cause mortality (relative risk, 0.55; 95% CI, 0.37–0.80).
The health benefits of pursuing cardiovascular health are observed across races/ethnicities and the nation. New data on measures of cardiovascular health in Hispanics find similar results as previous reports in non-Hispanic groups. Studies from non-US populations also support the importance of Life’s Simple 7 on future disease prevention.
Trends in improvements in overall cardiovascular health metrics are projected to reduce coronary heart disease deaths by 30% between 2010 and 2020.
The current evidence supports a range of complementary life course strategies to improve cardiovascular health in youth and adults as they age. Such approaches focus on both (1) improving cardiovascular health among those who currently have less than optimal levels and (2) preserving cardiovascular health among those who currently have ideal levels. The AHA and the literature support the importance of the following:
Individual-focused approaches, which target lifestyle and risk factor treatments at the individual level.
Healthcare systems approaches, which encourage, facilitate, and reward efforts by providers and patients to improve health behaviors and health factors.
Population approaches, which target lifestyle and treatments in schools, places of worship, work-places, local communities, and states, as well as throughout the nation.
Smoking and Tobacco Use (Chapter 3)
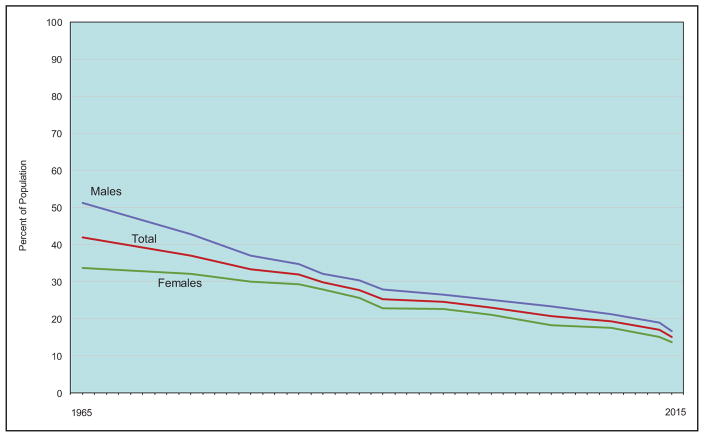
In 2015, among adults ≥18 years of age, overall rates of tobacco use were estimated to be 15.2% (16.7% of males and 13.7% of females; National Health Interview Survey).
-
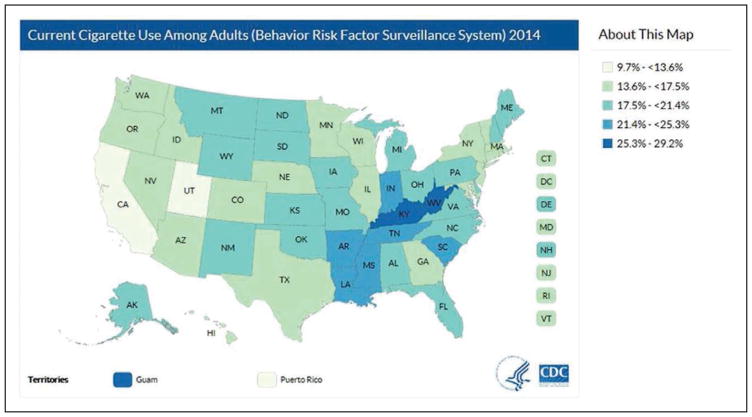
In the US, substantially higher tobacco use rates are found in low socioeconomic status, Native American, and lesbian, gay, bisexual, or transgender people reporting disability or activity limitations, as well as mentally ill populations. There also is substantial regional variation in the percentage of current smokers.
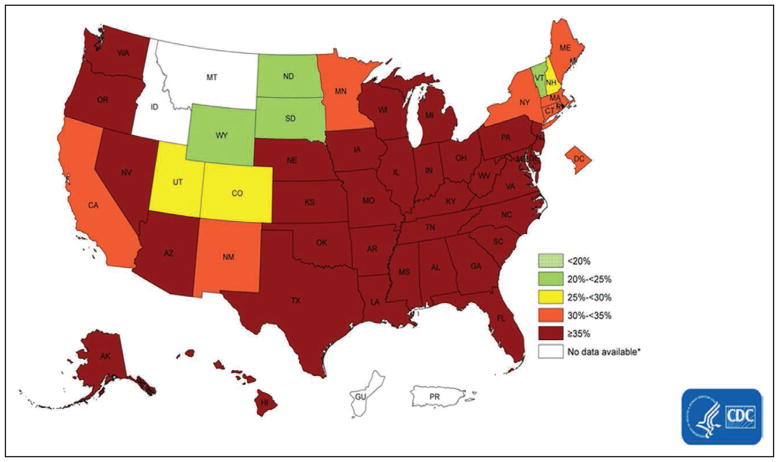
The region with the highest rates is the Midwest (20.7%), and the state with the highest percentage was West Virginia (26.7%). The lowest percentages regionally were observed in the West (13.1%), and by state in Utah (9.7%).
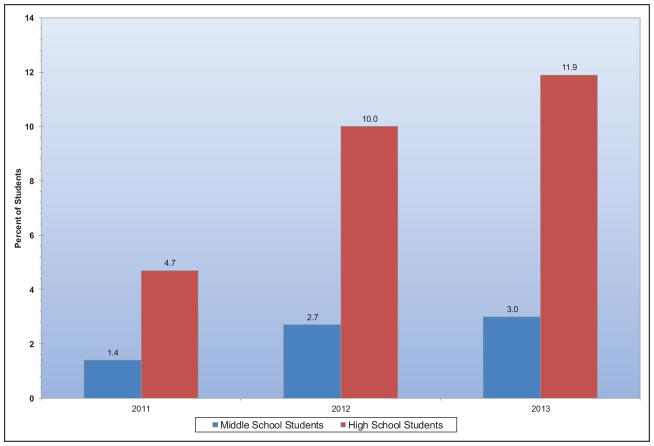
In 2015, e-cigarettes were the most commonly used tobacco product among middle school (5.3%) and high school (16.0%) students. The risks for nicotine dependence and for CVD associated with regular e-cigarette use are unknown. Use of cigarillos or other mass marketed cigars, hookahs, and water pipes has also become increasingly common in the past few years.
In May 2016, the US Food and Drug Administration placed e-cigarettes under the same regulations and restrictions as traditional combustible cigarettes. Furthermore, Tobacco 21 legislation, which mandates a minimum age of 21 years to purchase tobacco, is becoming increasingly common in the United States.
Physical Inactivity (Chapter 4)
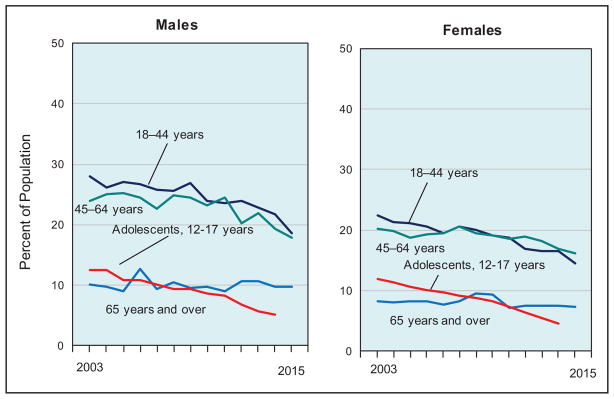
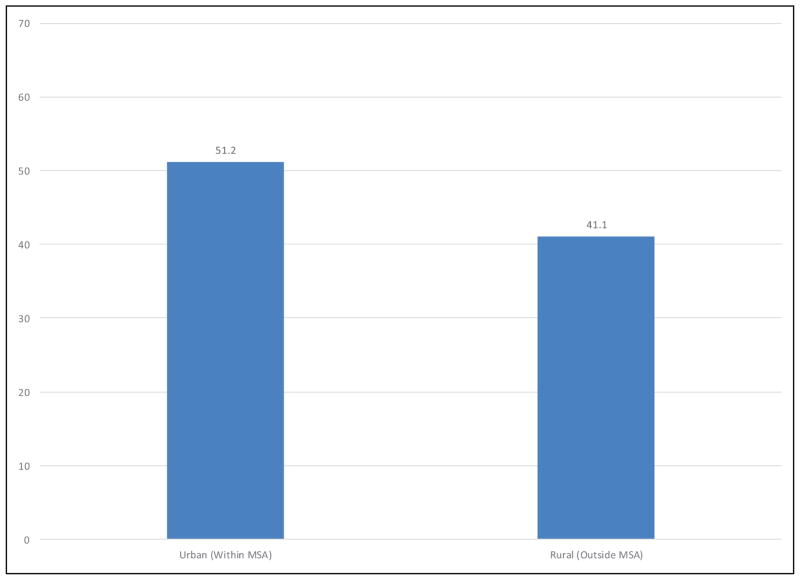
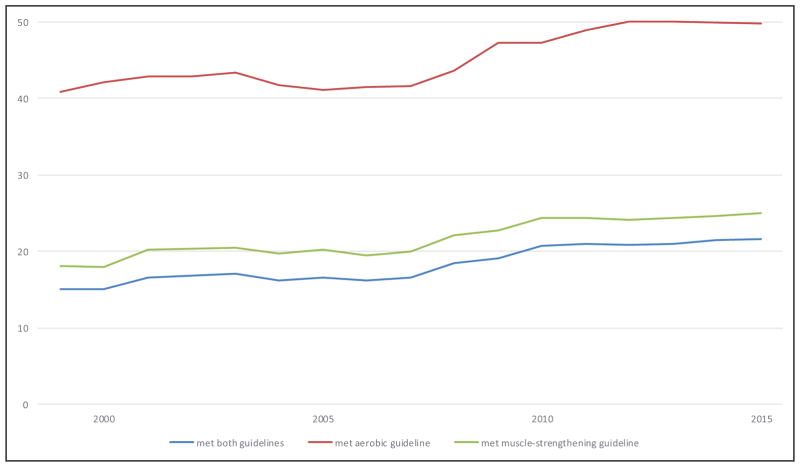
More Americans are meeting the federal PA guidelines. The age-adjusted percentage of US adults (≥18 years) who met both the muscle-strengthening and aerobic guidelines increased from 14.3% in 1998 to 21.6% in 2015. The percentage of US adults who met the aerobic guideline increased from 40.0% in 1998 to 49.8% in 2015.
In 2015, only 27.1% of high school students met activity recommendations of ≥60 minutes of PA on all 7 days of the week, and 14.3% of high school students reported that they were inactive on all of the previous 7 days.
Even low levels of leisure time PA (up to 75 minutes of brisk walking per week) were associated with reduced risk of mortality compared with participants who engaged in no PA.
A study of American adults reported that inadequate levels of aerobic PA (after adjustment for body mass index) were associated with an estimated 11.1% of aggregate healthcare expenditures.
Nutrition (Chapter 5)
The 2015 US Dietary Guidelines Advisory Committee recently concluded that a healthy dietary pattern is higher in vegetables, fruits, whole grains, low-fat or nonfat dairy, seafood, legumes, and nuts; moderate in alcohol (among adults); lower in red and processed meat; and low in sugar-sweetened foods and drinks and refined grains.
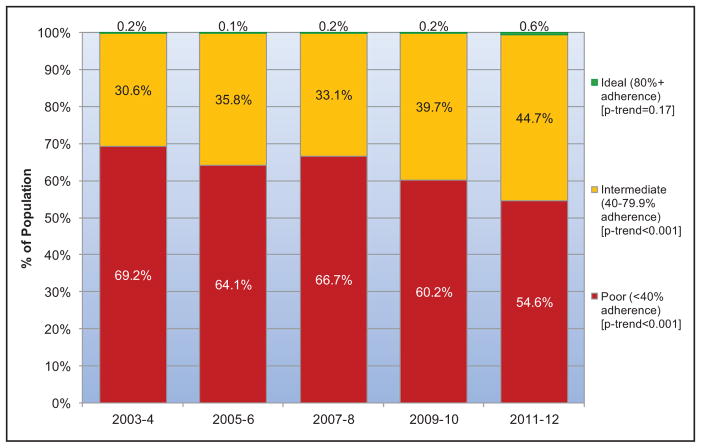
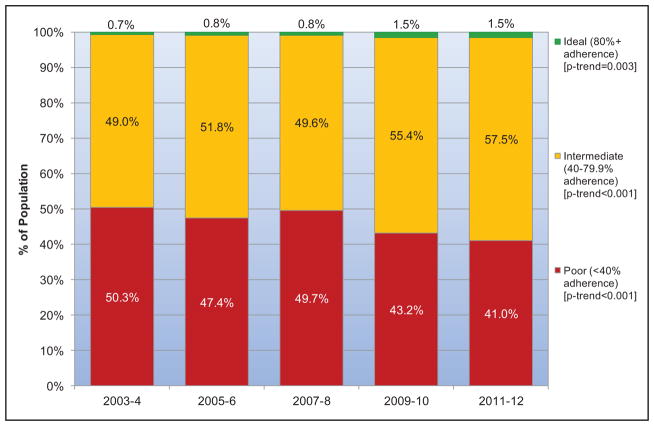
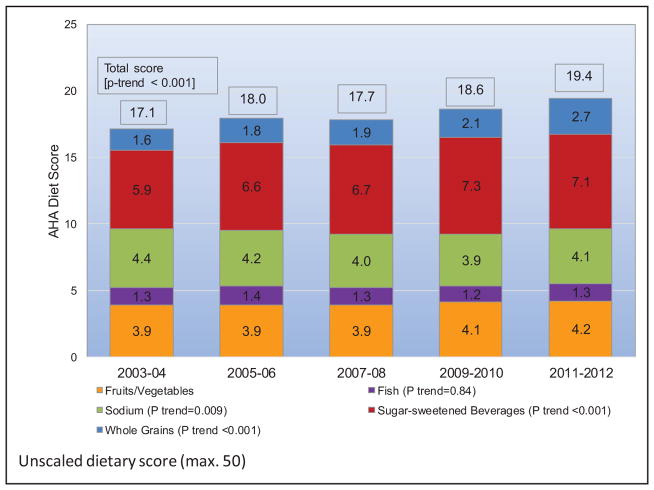
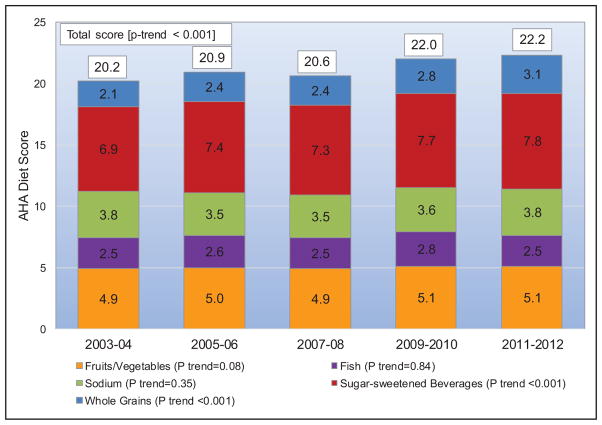
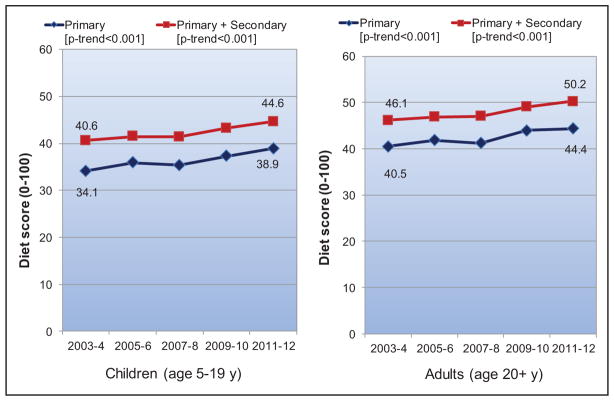
Between 2003 to 2004 and 2011 to 2012 in the United States, the mean AHA healthy diet score improved in both children and adults. The prevalence of an ideal healthy diet score (>80) increased from 0.2% to 0.6% in children and from 0.7% to 1.5% in adults. The prevalence of an intermediate healthy diet score (40–79) increased from 30.6% to 44.7% in children and from 49.0% to 57.5% in adults. These improvements were largely attributable to increased whole grain consumption and decreased sugar-sweetened beverage consumption in both children and adults.
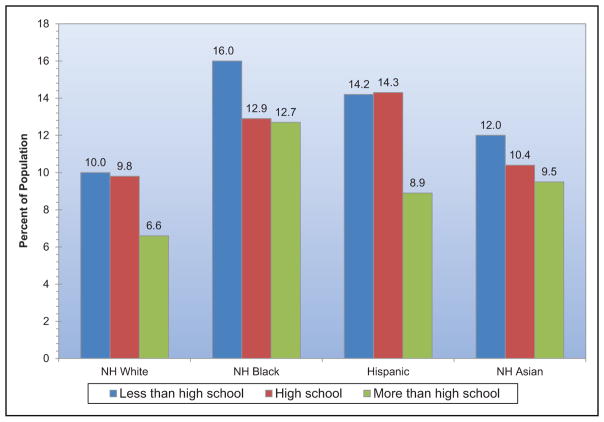
Between 1999 and 2012, although AHA healthy diet scores tended to improve in all race/ethnicity, income, and education levels, many disparities present in earlier years widened over time, with generally smaller improvements seen in minority groups and those with lower income or education.
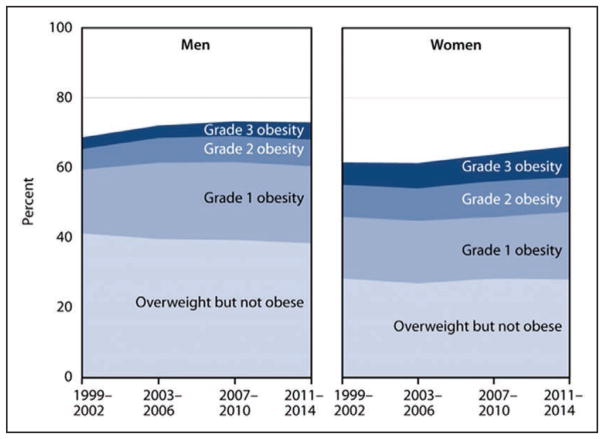
Overweight and Obesity (Chapter 6)
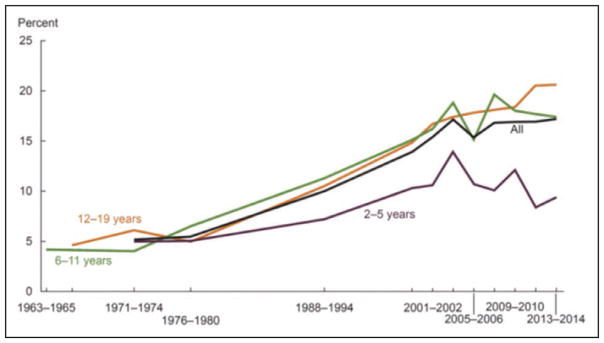
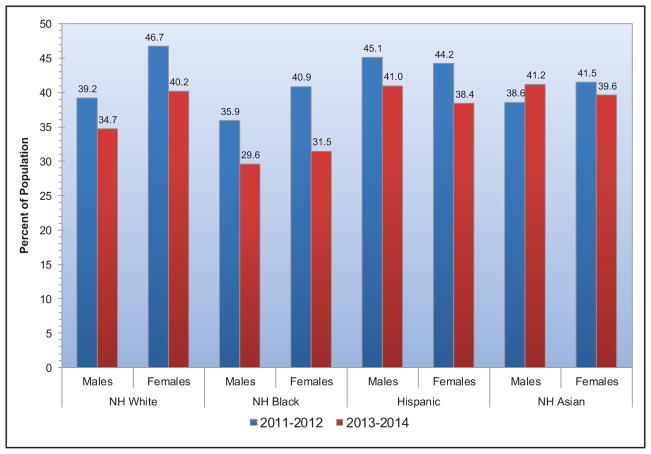
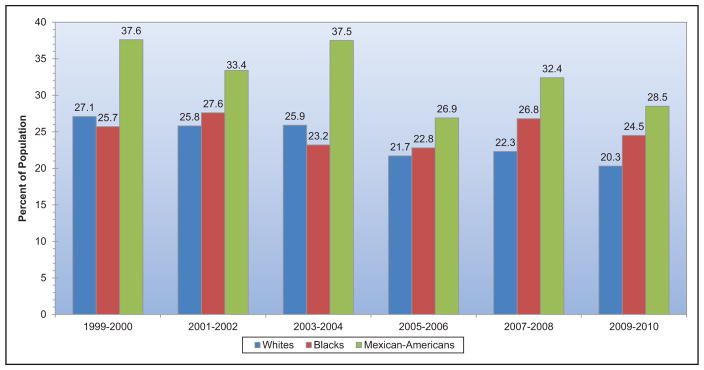
The prevalence of obesity among adults and youth in the United States increased significantly from 1999 to 2000 through 2013 to 2014. However, the increase in obesity prevalence began to level off and was not statistically significant for adults from the time period 2003 to 2004 through 2011 to 2012 and for youth from the time period 2003 to 2004 through 2013 to 2014.
Body mass index and waist circumference cut points in US guidelines underestimate obesity and CVD risk in Asian and South Asian populations.
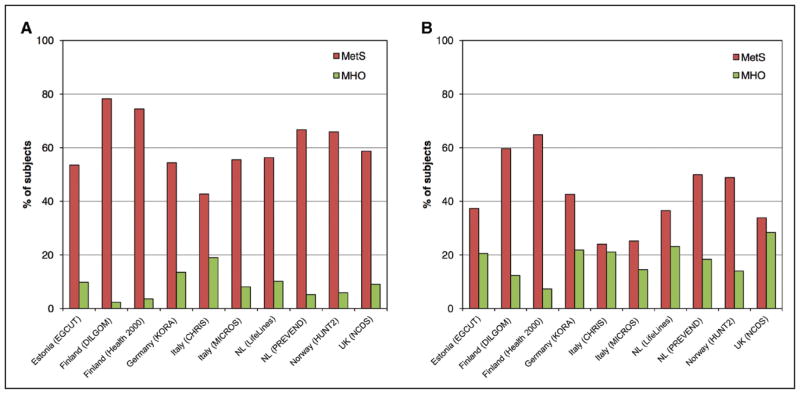
Definitions of “metabolically healthy obesity” vary, and over time, a substantial proportion of those with metabolically healthy obesity transition to metabolically unhealthy. The risk of CVD events, particularly HF, may be increased with obesity even in the absence of metabolic risk factors.
Family History and Genetics (Chapter 7)
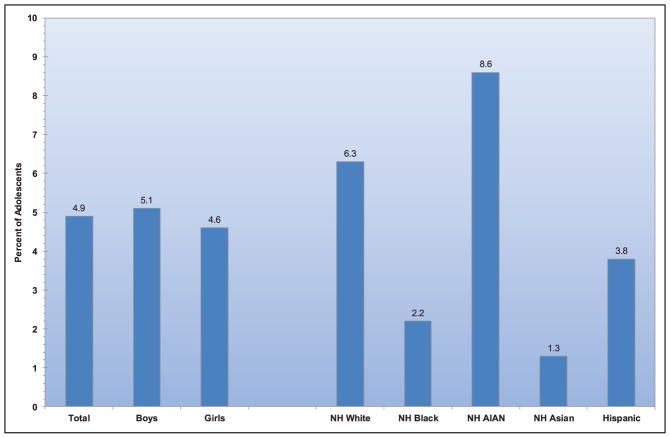
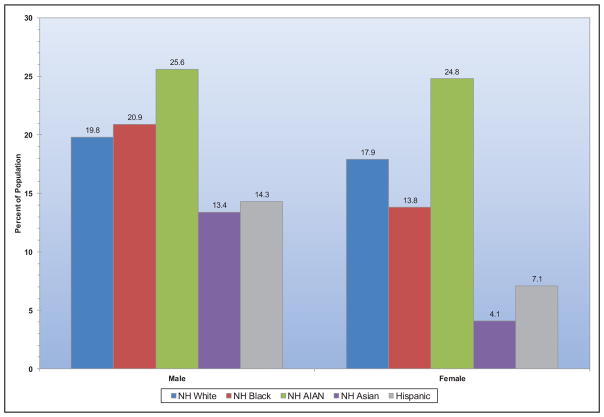
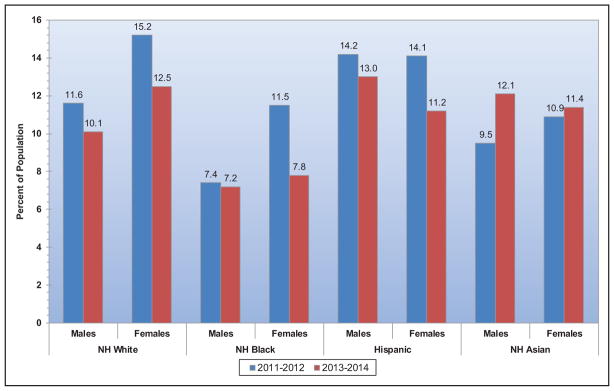
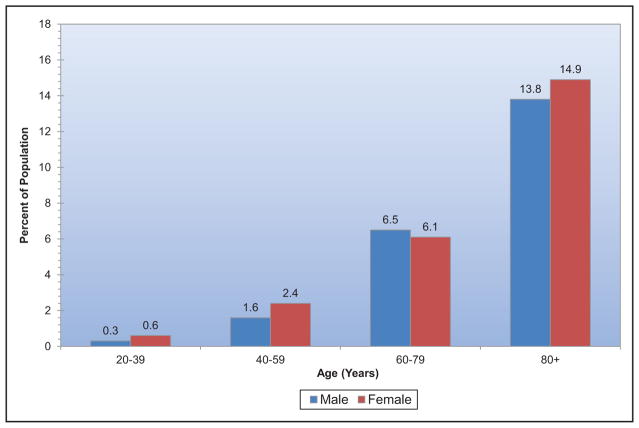
Among adults ≥20 years of age, 12.2% reported having a parent or sibling with a heart attack or angina before age 50 years, with the highest sex-specific prevalence observed among non-Hispanic white males and females.
High Blood Cholesterol and Other Lipids (Chapter 8)
Mean low-density lipoprotein cholesterol decreased from 126 mg/dL in 1999 to 2000 to 111 mg/dL in 2013 to 2014 among US adults. The age-adjusted prevalence of high low-density lipoprotein cholesterol decreased from 42.9% in 1999 to 2000 to 28.5% in 2013 to 2014.
Data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2000 to NHANES 2011 to 2012 show that the use of cholesterol-lowering treatment has increased substantially among adults, from 8% in 1999 to 2000 to 18% in 2011 to 2012. During this period, the use of statins increased from 7% to 17%.
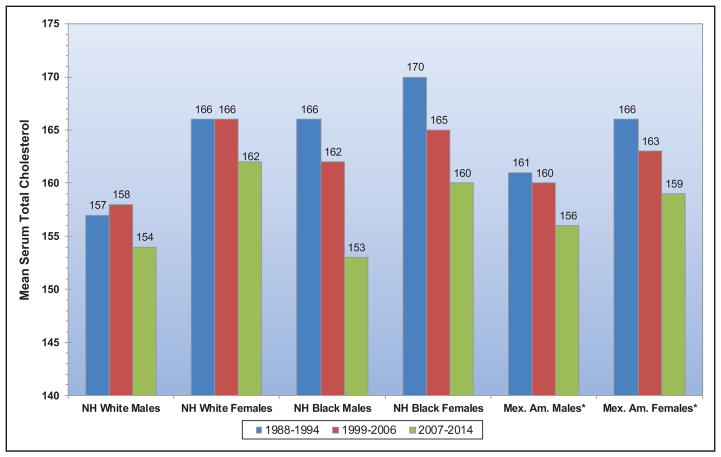
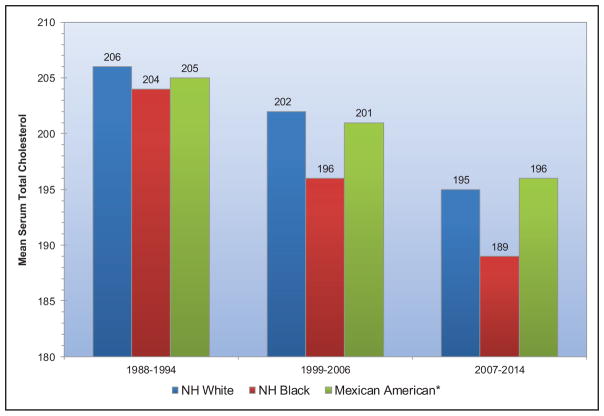
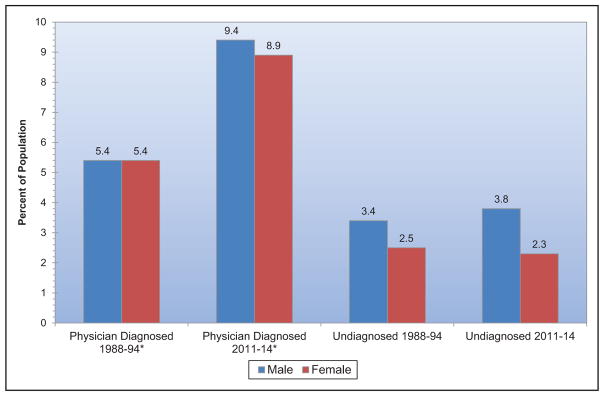
From 1988 to 1994 to 2013 to 2014, mean serum total cholesterol for adolescents 12 to 19 years of age has decreased across all subgroups of race and sex.
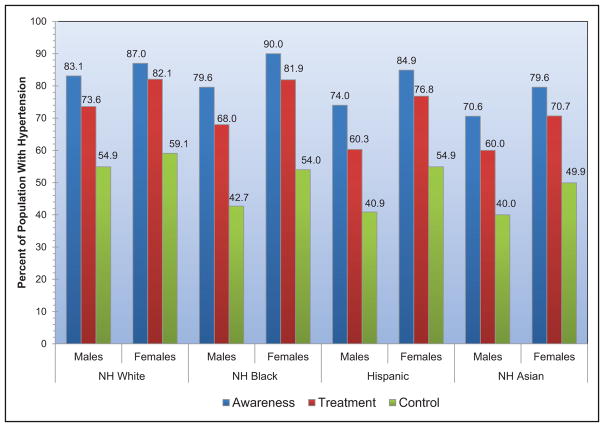
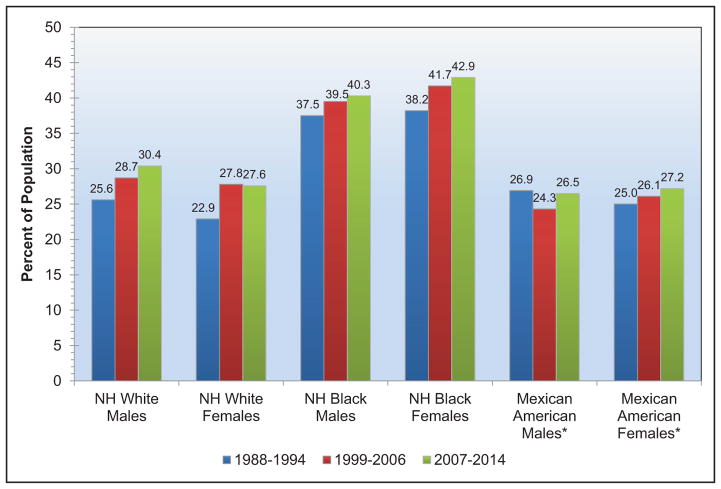
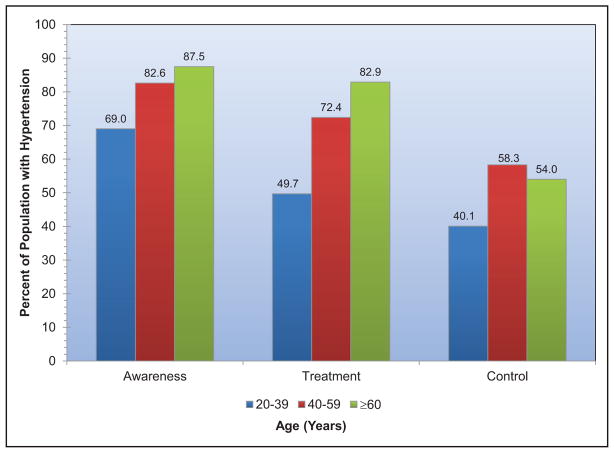
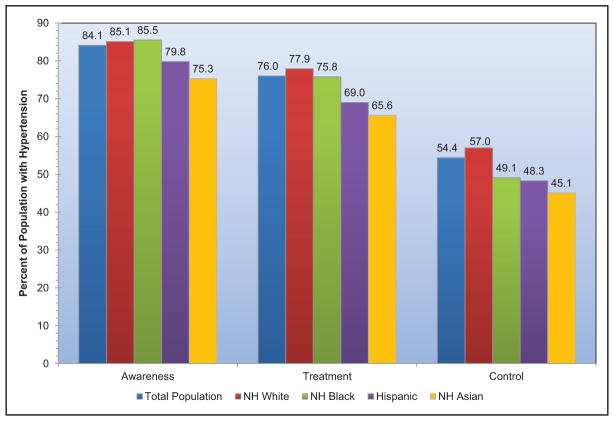
High Blood Pressure (Chapter 9)
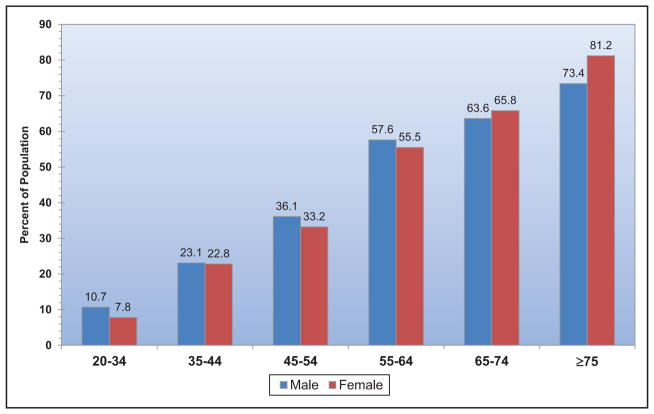
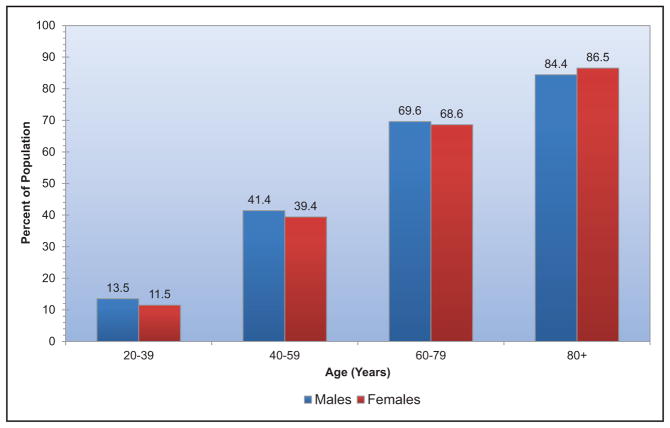
The age-adjusted prevalence of hypertension among US adults ≥20 years of age is estimated to be 34.0% in NHANES 2011 to 2014, which is equivalent to 85.7 million adults.
The prevalence of high BP or borderline high BP among US children and adolescents 8 to 17 years old is 11%.
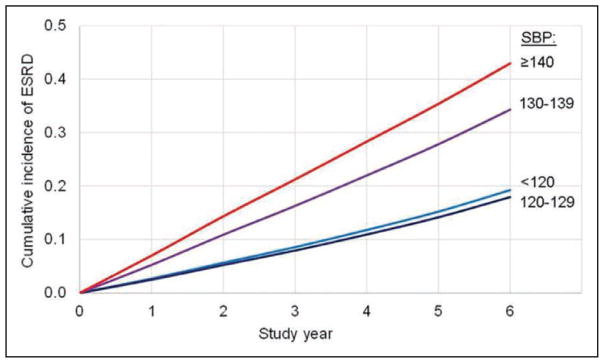
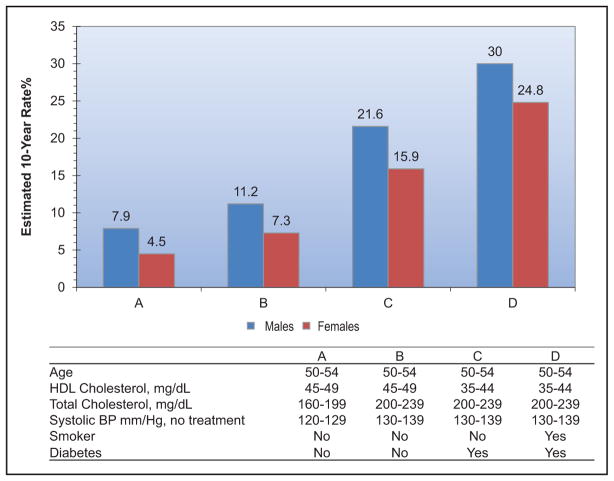
The SPRINT (Systolic Blood Pressure Intervention Trial) demonstrated lower CVD and mortality risk with a systolic BP target goal of 120 mm Hg versus 140 mm Hg. It is estimated that 16.8 million US adults meet the SPRINT eligibility criteria.
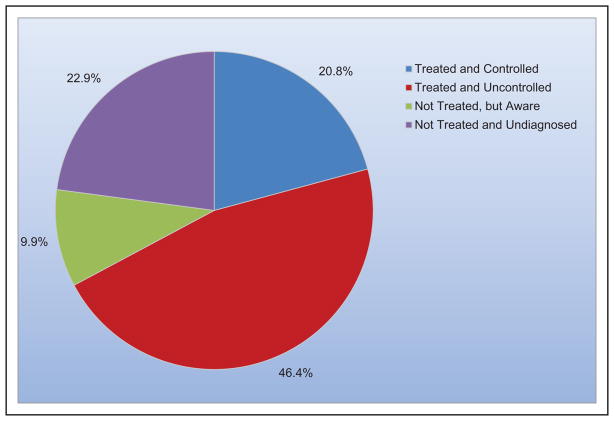
The prevalence of apparent treatment-resistant hypertension was estimated from a meta-analysis to be 13.7%.
Controlling hypertension in all patients with CVD and stage 2 hypertension could be cost-saving.
Diabetes Mellitus (Chapter 10)
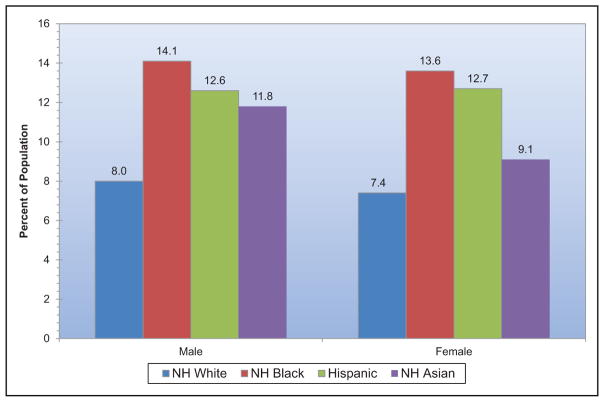
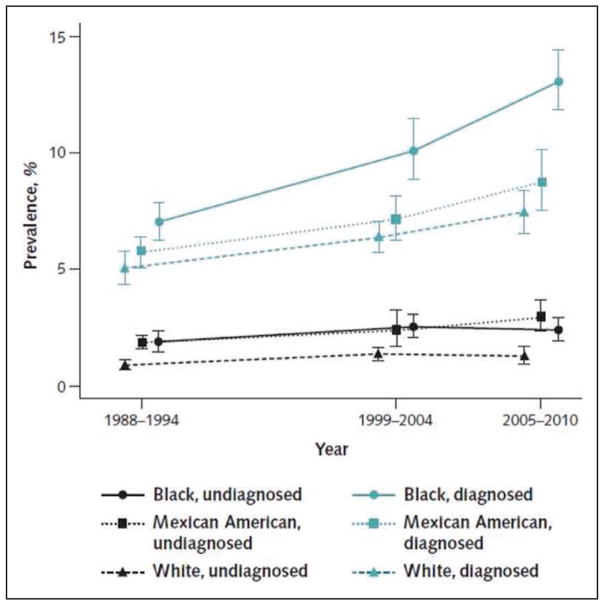
An estimated 23.4 million adults have diagnosed diabetes mellitus (DM), 7.6 million have undiagnosed DM, and 81.6 million have prediabetes.
Analyses of high school–aged blood donors in 2011 to 2012 reported that 10% had prediabetes hemoglobin A1c levels and an additional 0.6% had hemoglobin A1c ≥6.5%, the threshold endorsed to diagnose DM.
A recent large meta-analysis of randomized controlled trials showed that exercise may exert its favorable effects by significantly improving glucose tolerance and insulin resistance. The benefits of exercise were further supported by a large intervention project that showed that higher fitness was associated with a lower risk of incident DM regardless of demographic characteristics and baseline risk factors.
In 2014, there were 76 488 DM-related deaths.
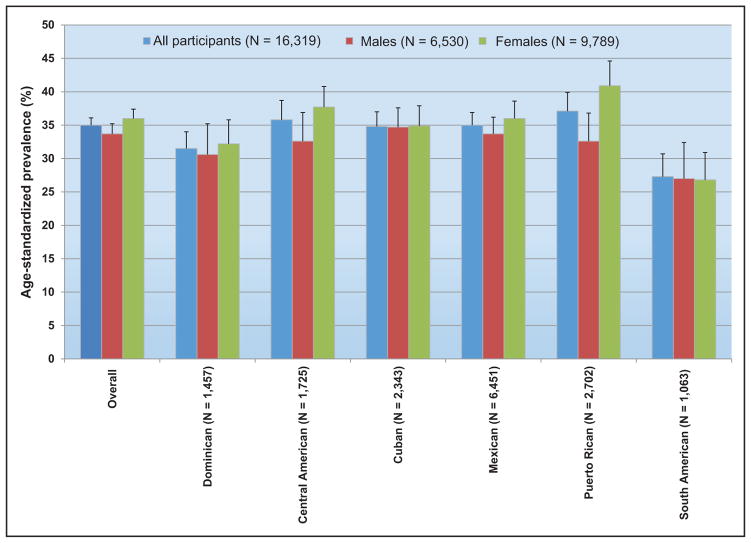
Metabolic Syndrome (Chapter 11)
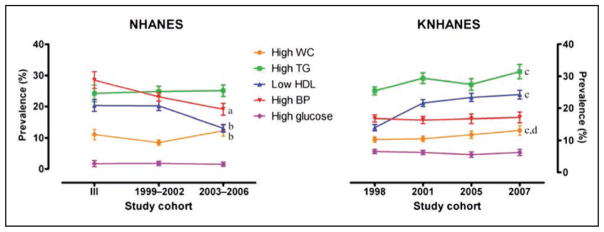
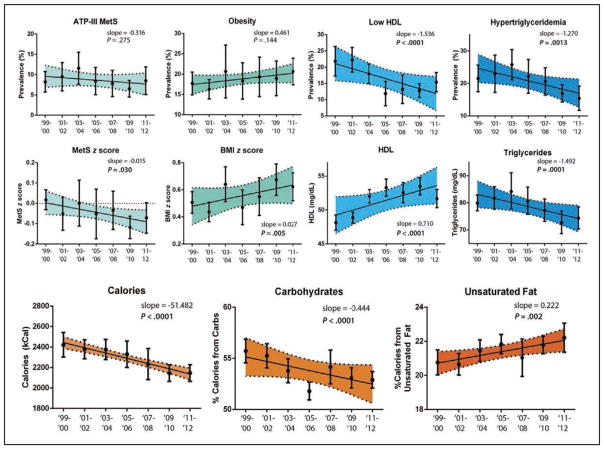
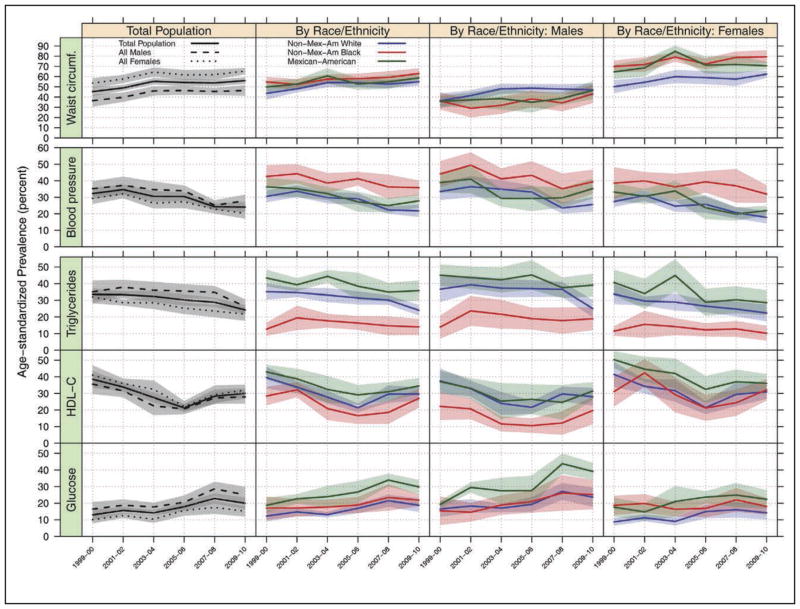
The prevalence of metabolic syndrome in youth ages 12 to 19 years old has decreased in NHANES 2009 to 2010 and 2011 to 2012. This important epidemiological statistic mirrors a previously documented plateau and decrease in the prevalence of metabolic syndrome in adults.
The decrease in metabolic syndrome in youth most closely correlates with rising high-density lipoprotein cholesterol and lowered triglyceride levels, which are potentially driven by decreased carbohydrate intake and increased unsaturated fat intake.
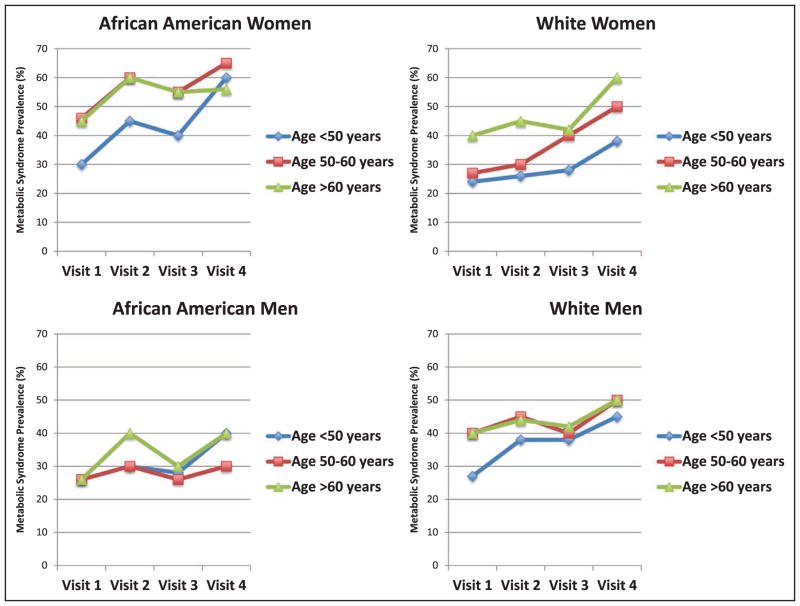
Despite these encouraging findings, recent data have confirmed that the severity of existing metabolic syndrome progresses with advancing age in approximately three quarters (76%) of adults, with faster progression of metabolic syndrome noted in women and younger people.
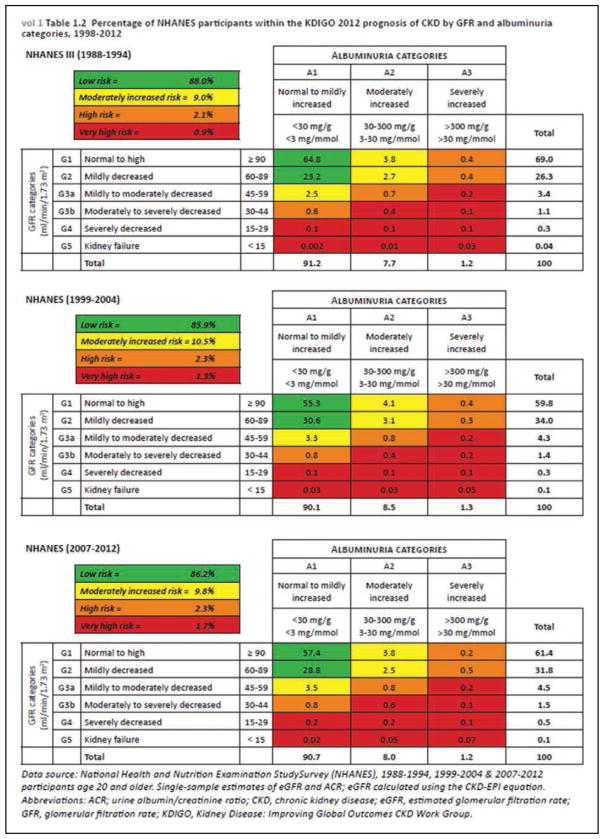
Chronic Kidney Disease (Chapter 12)
The total prevalence of chronic kidney disease is rising globally, primarily because of aging populations. The Global Burden of Disease study estimates that kidney disease is now the 19th-leading cause of death, up from the 36th-leading cause of death in 1990.
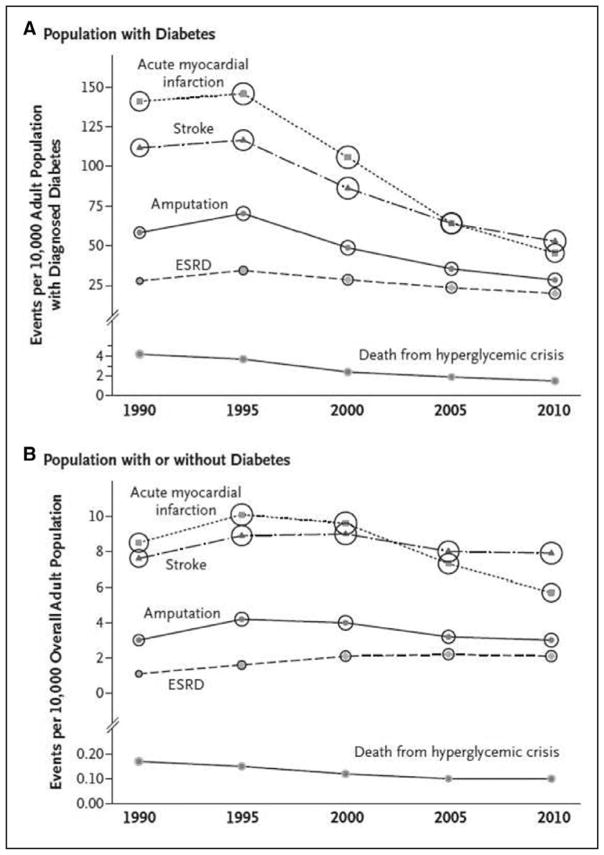
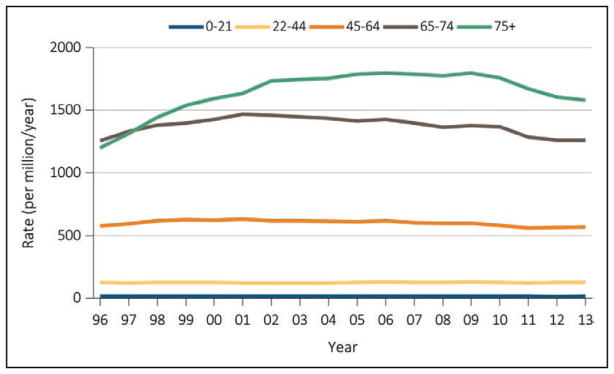
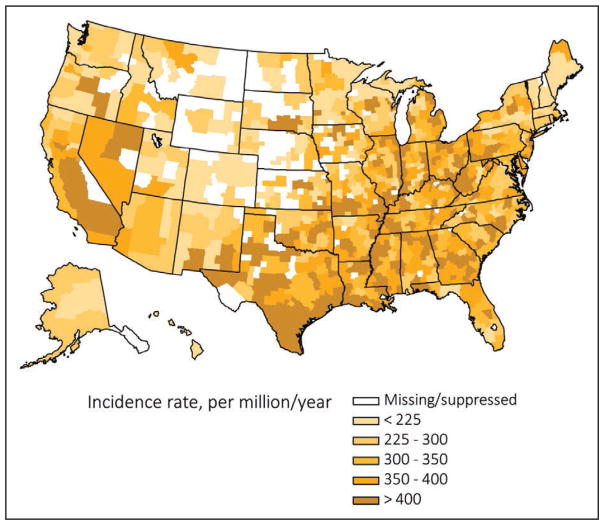
According to recent figures from the United States Renal Data System, the number of people with prevalent end-stage renal disease is increasing, with 661 648 prevalent cases as of December 31, 2013. However, the incidence rate has declined; 117 162 new cases were reported in 2013.
The prevalence of chronic kidney disease in adults ≥30 years of age is projected to increase to 14.4% in 2020 and 16.7% in 2030.
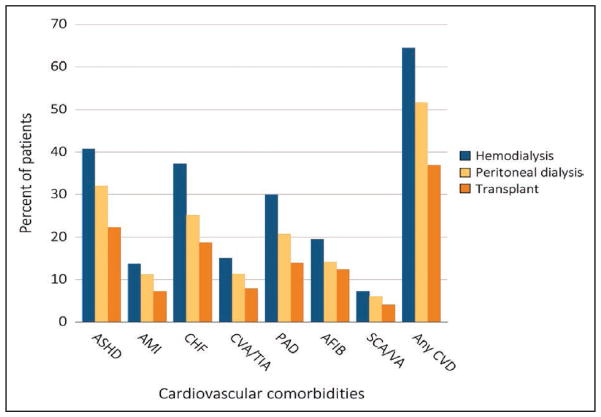
Cardiovascular risk in patients with kidney disease can now be classified as high, intermediate, and low according to estimated glomerular filtration rate and albuminuria categories defined by the Kidney Disease Improving Global Outcomes (KDIGO) working group.
Total Cardiovascular Diseases (Chapter 13)
An estimated 92.1 million US adults have at least 1 type of CVD. By 2030, 43.9% of the US adult population is projected to have some form of CVD.
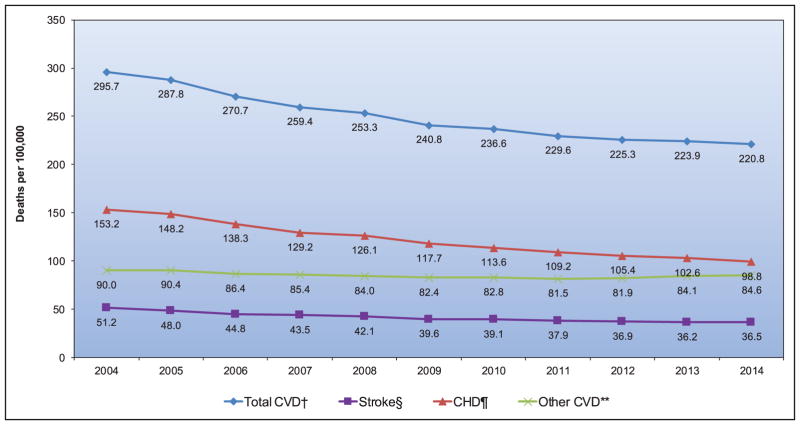
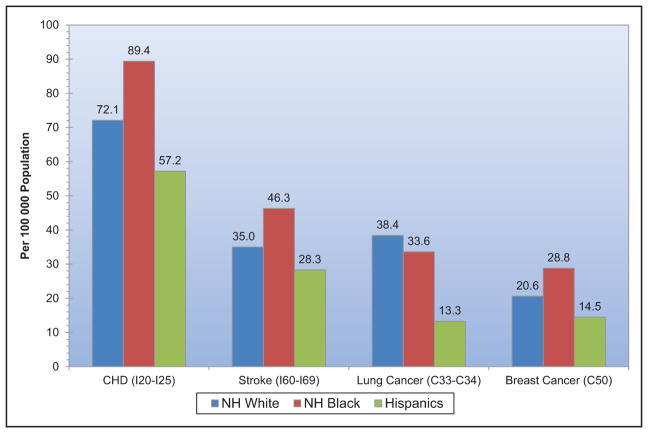
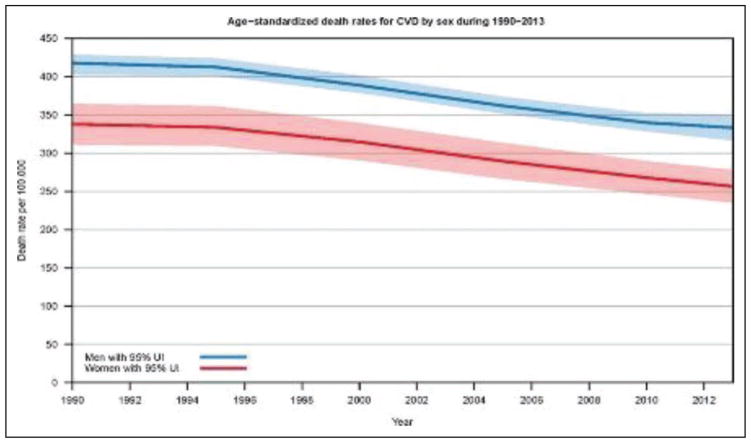
From 2004 to 2014, death rates attributable to CVD declined 25.3%. The actual number of CVD deaths decreased 6.7%.
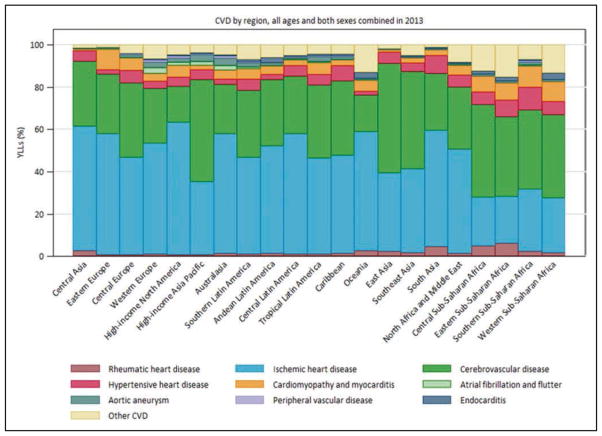
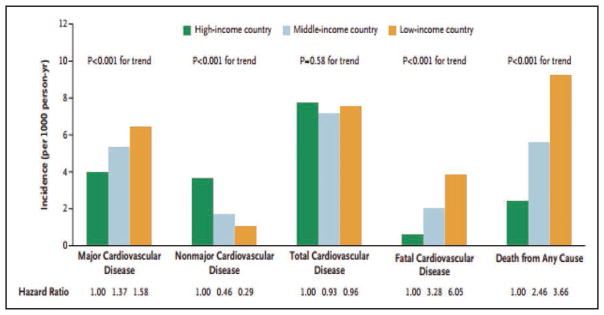
Globally, 80% of CVD deaths take place in low- and middle-income countries and occur almost equally in males and females.
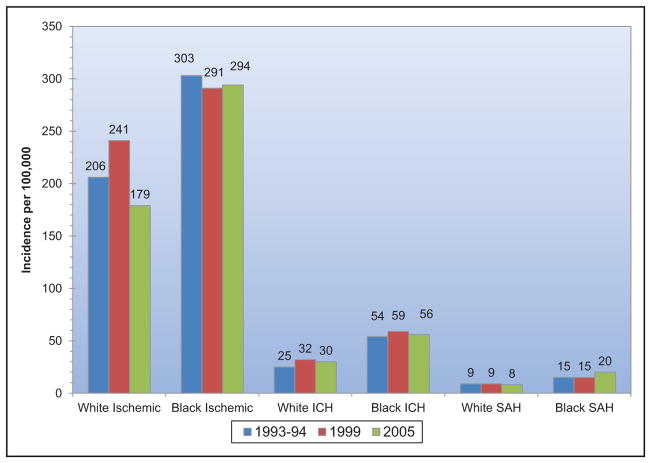
Stroke and Cerebrovascular Disease (Chapter 14)
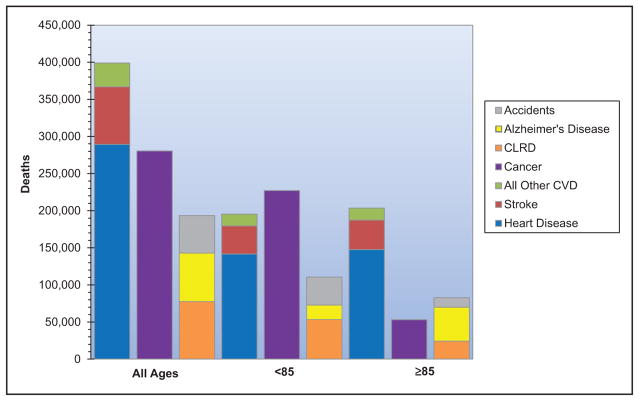
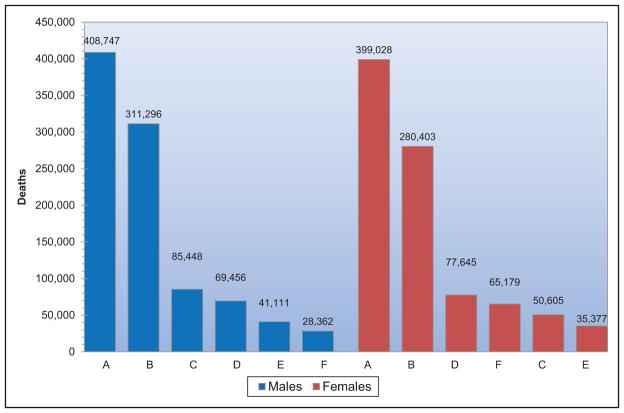
When considered separately from other CVDs, stroke ranks No. 5 among all causes of death, behind diseases of the heart, cancer, chronic lower respiratory disease, and unintentional injuries/accidents.
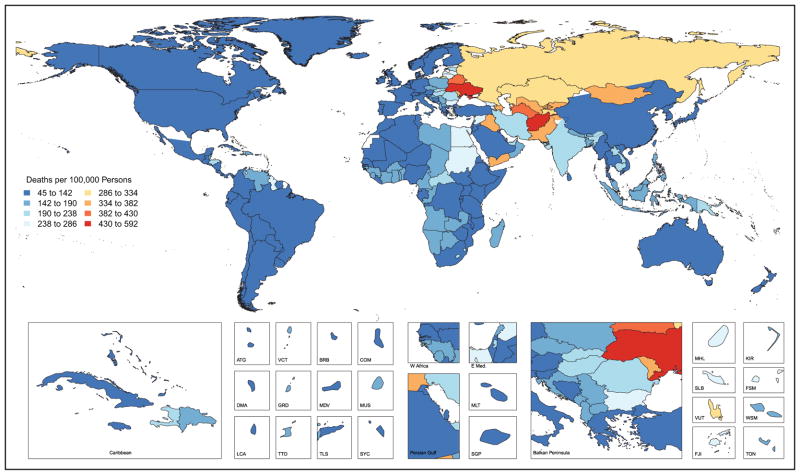
Globally, in 2013 there were 6.5 million stroke deaths, making stroke the second-leading cause of death behind ischemic heart disease.
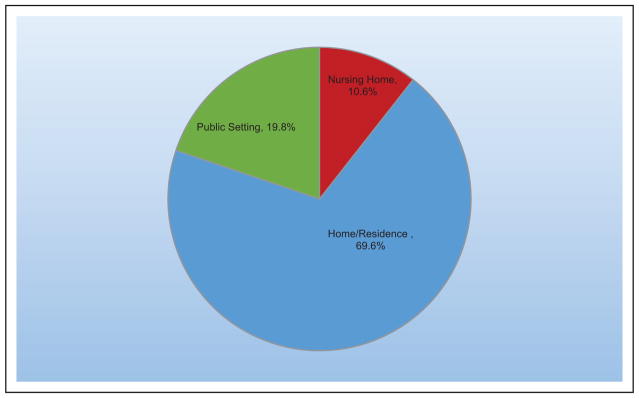
Approximately 795 000 strokes occur in the United States each year. On average, every 40 seconds, someone in the United States has a stroke, and on average, every 4 minutes, someone dies of a stroke.
Approximately 60% of stroke deaths occurred outside of an acute care hospital.
A review of recent clinical trials identified the benefit of intense BP reduction, which reduced risks of stroke outcomes.
Adherence to a Mediterranean-style diet that was higher in nuts and olive oil was associated with a reduced risk of stroke.
One year after stroke, blacks were less likely to report independence in activities of daily living and instrumental activities of daily living than whites.
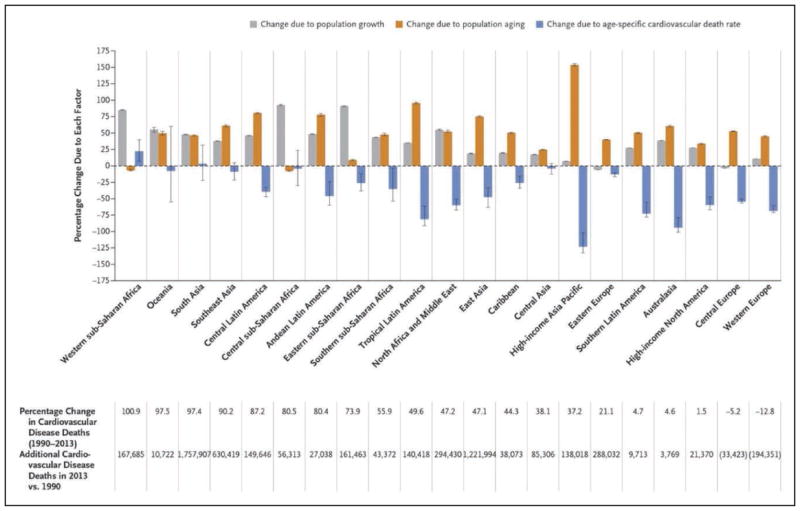
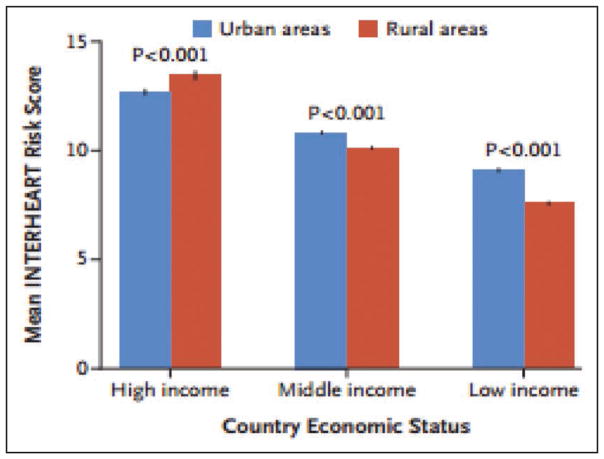
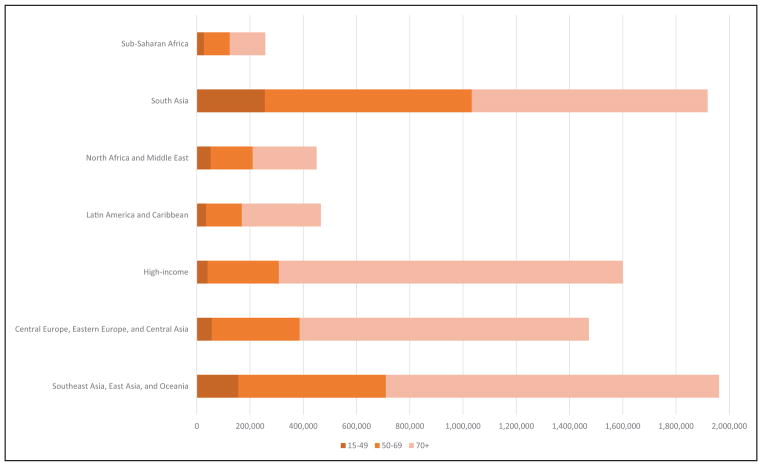
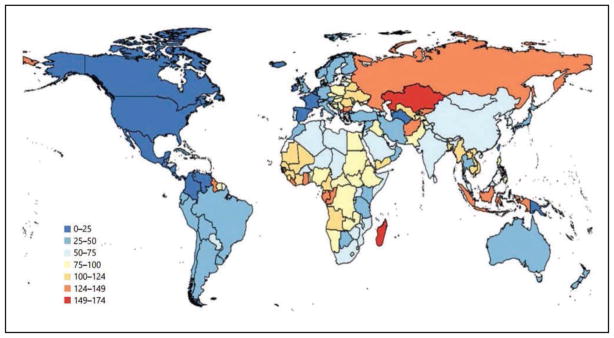
Global Cardiovascular Disease (Chapter 15)
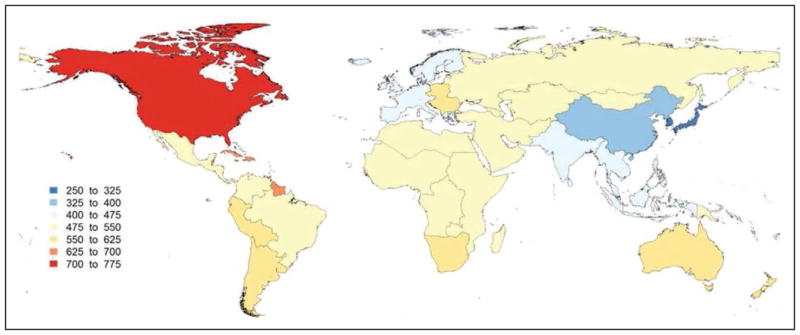
In 2013, the highest prevalence of ischemic stroke (1015 to 1184 cases per 100 000 people) was in high-income countries (particularly in the United States), with the lowest (up to 339 per 100 000) in low- and middle-income countries.
CVD was the most common underlying cause of death in the world in 2013, accounting for an estimated 17.3 million (95% uncertainty interval, 16.5–18.1 million) of 54 million total deaths, or 31.5% (95% uncertainty interval, 30.3%–32.9%) of all global deaths.
Cost-effective medications such as aspirin, statins, and BP-lowering agents remain unaffordable for much of the world. New community health worker-based strategies to improve their delivery are proving to be highly effective.
Congenital Cardiovascular Defects and Kawasaki Disease (Chapter 16)
The mortality attributed to congenital cardiovascular defects decreases with later gestational age (to 40 weeks), which suggests early delivery will not benefit most patients with congenital cardiovascular defects.
Health outcomes are improving for congenital cardiovascular defects, and survival is increasing, leading to a population shift toward adulthood.
The rising population of adults with congenital heart disease adds to management complexity and emphasizes the need for coordinated care by adult congenital cardiovascular specialists.
Disorders of Heart Rhythm (Chapter 17)
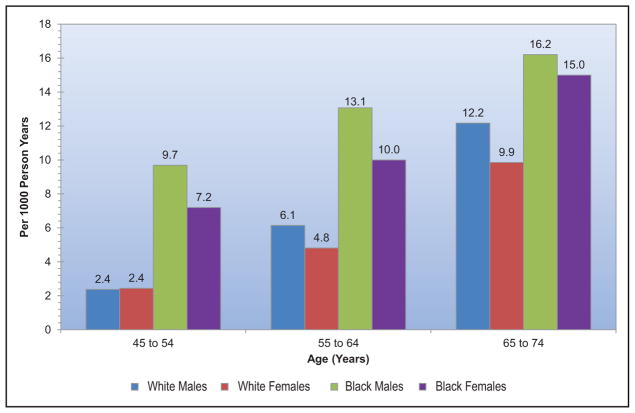
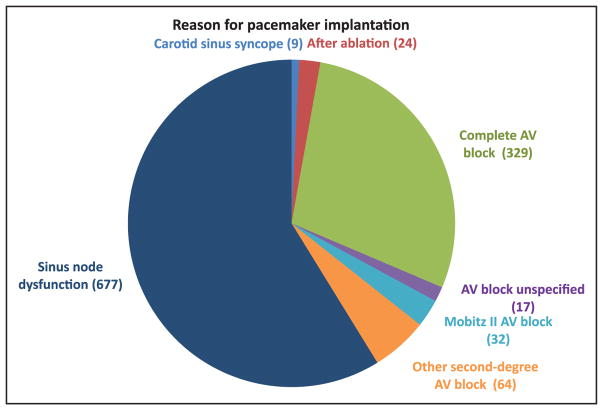
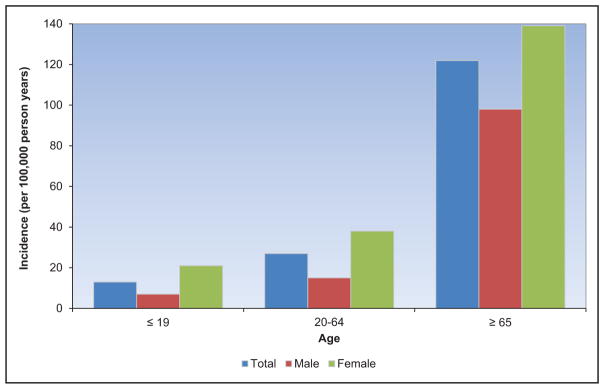
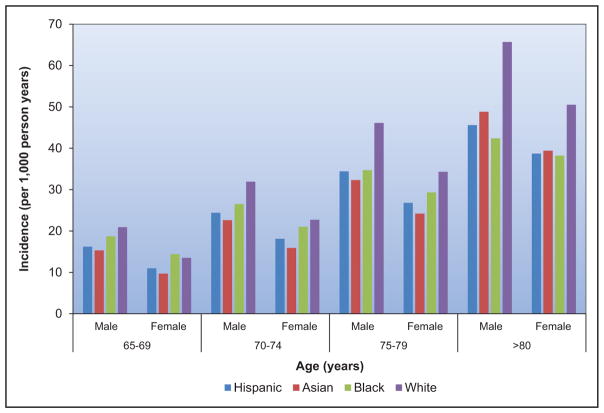
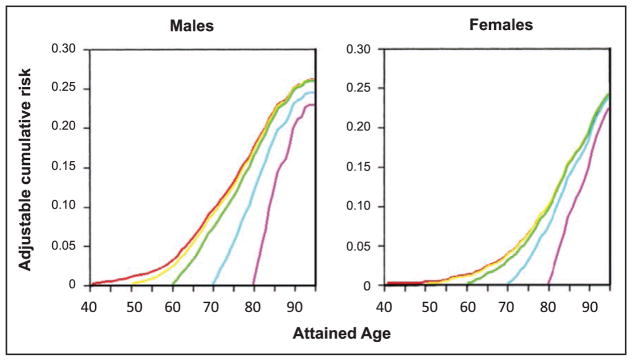
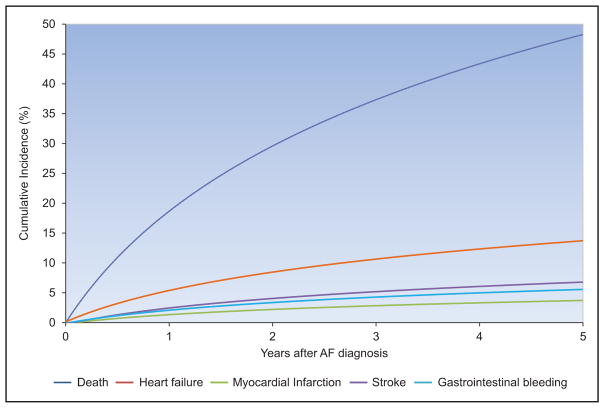
The frequency and adverse consequences of clinically unrecognized and asymptomatic atrial fibrillation (AF) are increasingly reported, particularly in older adults. For instance, in a community-based study in Sweden, >7000 people 75 to 76 years of age were monitored intermittently; 3% had newly diagnosed AF, of whom only 17% had their AF detected by a screening ECG.
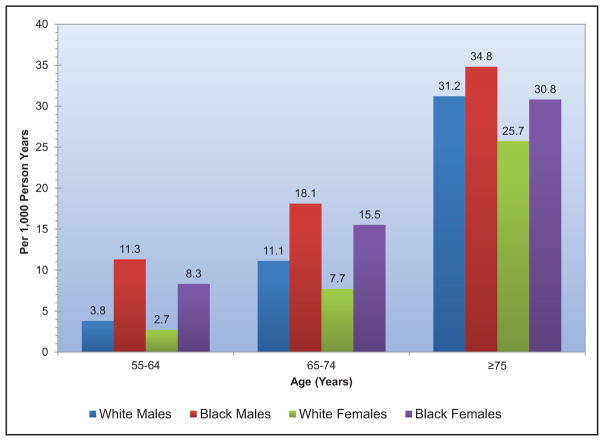
A recent meta-analysis from 4 large contemporary randomized trials revealed that AF is associated with systemic embolism, occurring at a rate of 0.24 per 100-person years compared with 1.92 for stroke per 100-person years.
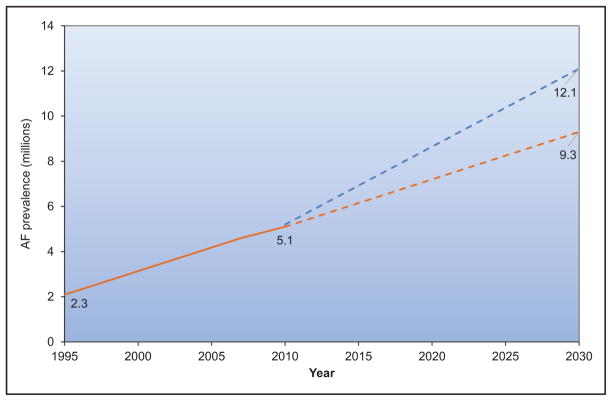
Data from the Framingham Heart Study, the Atherosclerosis Risk in Communities study, the United Kingdom, and other sites suggest that the incidence and prevalence of AF are increasing over time.
Sudden Cardiac Arrest (Chapter 18)
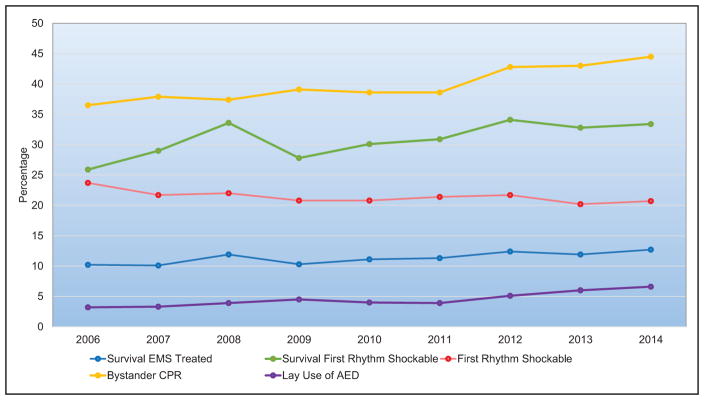
In the 2015 CARES (Cardiac Arrest Registry to Enhance Survival) National Survival Report for emergency medical services–treated nontraumatic cardiac arrest, the survival rate to hospital discharge was 10.6% for adults >18 years old, 23.5% for children 13 to 18 years old, 16.6% for children >1 to 12 years old, and 6.2% for children <1 year old.
In 2015, Get With the Guidelines–Resuscitation reported the rate of survival to hospital discharge from pulseless in-hospital cardiac arrest in adults ≥18 years old was 23.8% (95% CI, 23.2%–24.3%], whereas in children 0 to 18 years old, it was 35.9% (95% CI, 31.4%–40.6%), and in neonates (0–30 days old), it was 24.2% (95% CI, 18.2%–31.4%).
Subclinical Atherosclerosis (Chapter 19)
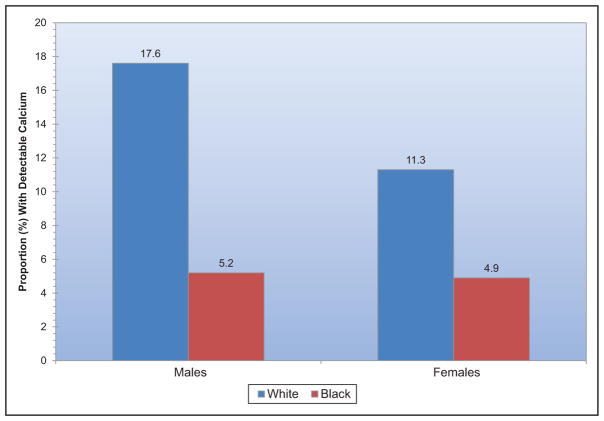
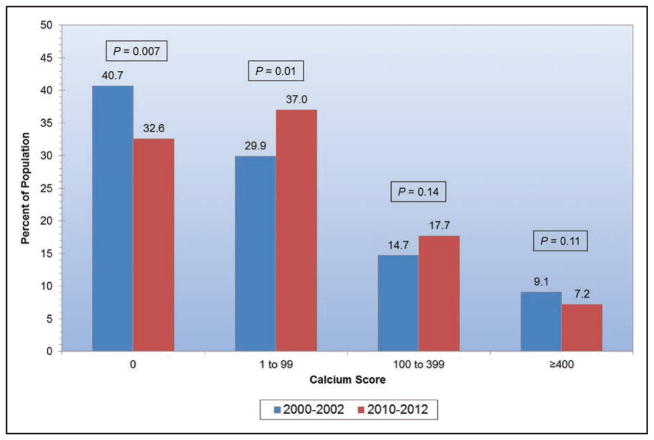
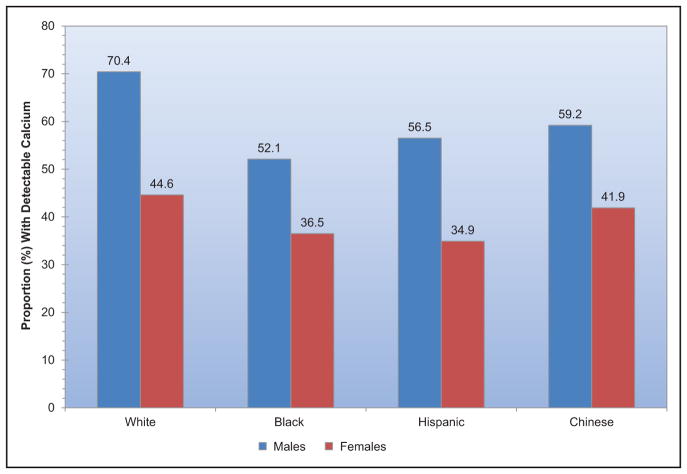
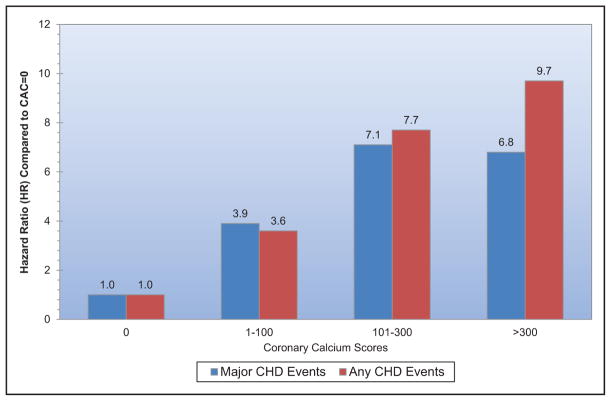
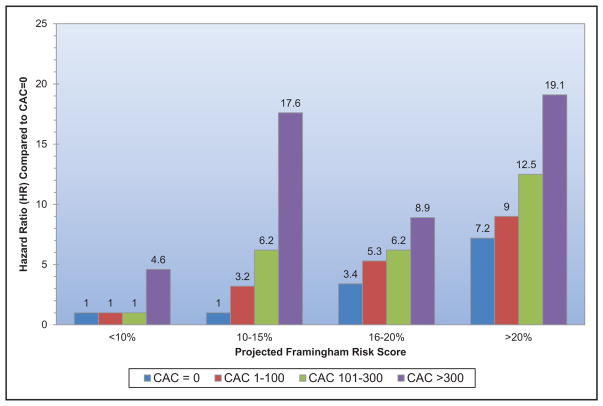
Subclinical CVD is common among US adults living in rural areas; a study from central Appalachia reported 56% of participants had coronary artery calcium scores >0.
Coronary artery calcium scores >400 versus 0 are associated with an increased risk for cancer, chronic kidney disease, pneumonia, chronic obstructive pulmonary disease, and hip fracture.
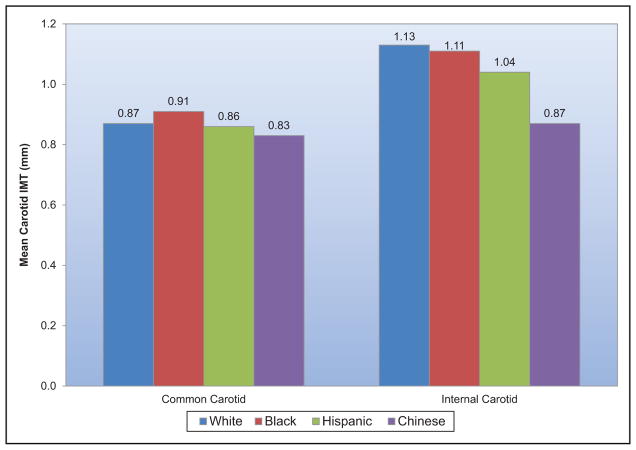
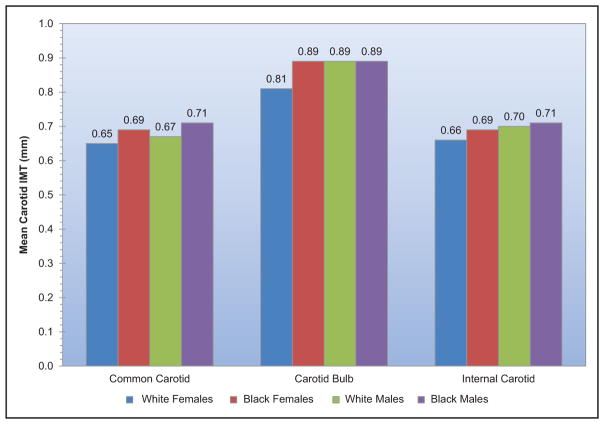
Conflicting data have been reported on the contribution of carotid intima-media thickness to risk prediction. A recent study from a consortium of 14 population-based cohorts demonstrated little additive value of common carotid intima-media thickness to Framingham Risk Score for purposes of discrimination and reclassification as far as incident myocardial infarction (MI) and stroke were concerned. However, for those at intermediate risk, the addition of mean common carotid intimamedia thickness to an existing cardiovascular risk score resulted in a small but statistically significant improvement in risk prediction.
Coronary Heart Disease, Acute Coronary Syndrome, and Angina Pectoris (Chapter 20)
A majority of MIs occur during a hospitalization for another reason, rather than being the cause of hospitalization.
Silent MIs (ie, MIs detected on ECG without a definite or probable hospitalized MI) account for almost 50% of incident MIs.
The percentage of US adults with a 10-year predicted CVD risk ≥20% decreased from 13.0% in 1999 to 2000 to 9.4% in 2011 to 2012.
Among US males and females <55 years old, coronary heart disease mortality did not decline between 1990 to 1999 and 2000 to 2011.
Between 2001 to 2003 and 2007 to 2009, age-adjusted mortality after MI decreased among white males, but no changes were present for white females or black males or females.
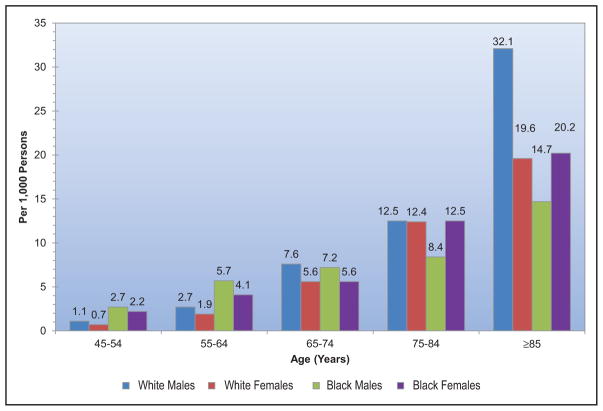
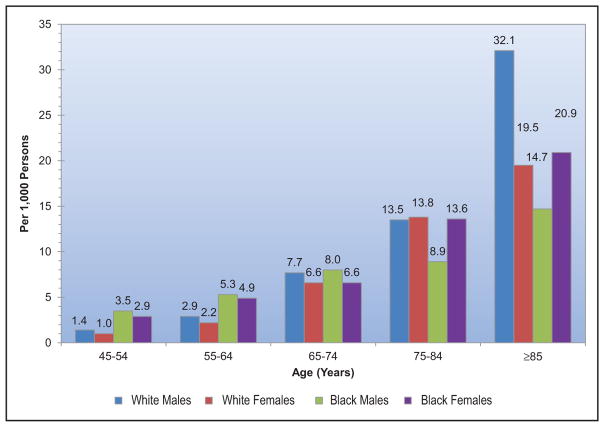
Cardiomyopathy and Heart Failure (Chapter 21)
HF prevalence has increased from 5.7 million (2009 to 2012) to 6.5 million (2011 to 2014) in Americans ≥20 years of age.
Five-year survival of HF diagnosis after an MI has also improved in 2001 to 2010 versus 1990 to 2000, from 54% to 61%.
Greater adherence to the AHA’s Life Simple 7 guidelines (better profiles in smoking, body mass index, PA, diet, cholesterol, BP, and glucose) is associated with a lower lifetime risk of HF and better cardiac structure and functional parameters by echocardiography.
Of incident hospitalized HF events, 53% had HF with reduced ejection fraction and 47% had preserved ejection fraction. Black males had the highest proportion of hospitalized HF with reduced ejection fraction (70%); white females had the highest proportion of hospitalized HF with preserved ejection fraction (59%).
Valvular Diseases (Chapter 22)
Although rheumatic heart disease is uncommon in high-income countries such as the United States, it remains an important cause of morbidity and mortality in low- and middle-income countries.
Both administrative and community-based data report that the incidence of infective endocarditis did not change after the publication of the 2007 AHA guidelines for management of infective endocarditis, 2 which restricted the indications for antibiotic prophylaxis before dental procedures.
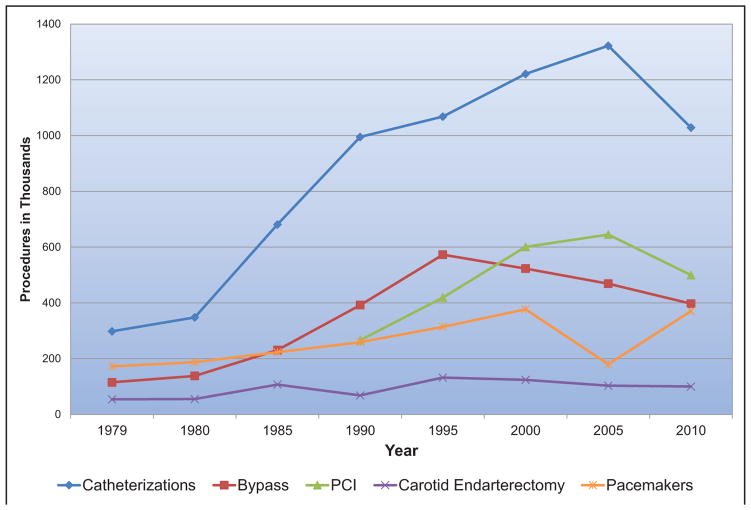
From the time of initial US Food and Drug Administration approval in late 2011 through 2014, more than 26 000 transcatheter aortic valve replacements were performed at 348 centers in 48 states in the United States. Two thirds of these patients were >80 years of age.
Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism), Chronic Venous Insufficiency, Pulmonary Hypertension (Chapter 23)
Venous Thrombosis
The main complications after venous thromboembolism are postthrombotic syndrome, which occurs in ≈40% of patients with deep vein thrombosis (DVT), and chronic thromboembolic PH, which occurs among 1.0% to 8.8% of those with pulmonary embolism.
Assuming 375 000 to 425 000 new cases of venous thromboembolism annually, the overall cost of venous thromboembolism was estimated at $7 billion to $10 billion annually.
New Section on Chronic Venous Insufficiency
Varicose veins affect 25 million US adults. More severe venous disease affects 6 million.
Venous ulcer is a substantial morbidity of chronic venous insufficiency. Estimated prevalence in adults is ≈0.3%, and incidence is ≈20% of those with chronic venous insufficiency. The estimated cost to treat venous ulcers in the United States is $1 billion annually.
Postthrombotic syndrome, a subset of chronic venous insufficiency, has risk factors that can be identified at the time of or after DVT, including recurrent ipsilateral DVT, obesity, more extensive DVT, poor quality of initial anticoagulation, ongoing symptoms or signs of DVT 1 month after diagnosis, and elevated D-dimer at 1 month.
New Section on PH
Risk factors are implicit in the World Health Organization disease classification of the 5 mechanistic subtypes of PH. The most common risk factors are left-sided heart disease and lung disease.
Mortality of PH depends on the cause and treatment. For example, an international prospective registry that included 679 patients with chronic thromboembolic PH estimated 3-year survival as 89% with pulmonary thromboendarterectomy and 70% without it.
In a study of 772 consecutive pulmonary embolism patients without major comorbidity such as cancer, the risk factors for chronic thromboembolic PH were unprovoked pulmonary embolism, hypothyroidism, symptom onset >2 weeks before pulmonary embolism diagnosis, right ventricular dysfunction on computed tomography or echocardiography, DM, and thrombolytic therapy or embolectomy. A risk prediction score that included these factors was able to predict a group with a chronic thromboembolic PH incidence of 10% (95% CI, 6.5%–15%).
Eighty percent of patients with PH live in developing countries, and the main cause of PH is heart and lung disease. Yet, schistosomiasis, rheumatic heart disease, HIV, and sickle cell disease remain prominent causes compared with high-income countries.
Peripheral Artery Disease and Aortic Diseases (Chapter 24)
From 2003 to 2011, there was a significant increase in endovascular treatment of critical limb ischemia (from 5.1% to 11.0%), which was accompanied by lower rates of in-hospital mortality and major amputation, as well as shorter hospital length of stay.
Endovascular repair may yield better outcomes in the first few years, but after 8 years of follow-up in one study, the open repair group and the endovascular repair group demonstrated similar survival. Of note, individuals in the endovascular repair group had a higher rate of eventual aneurysm rupture (5.4%) than patients who underwent open repair (1.4%).
Quality of Care (Chapter 25)
Overall, inpatient quality of care for patients with acute coronary syndromes, HF, and stroke continues to show gains, with compliance rates above 95% for some measures.
Although performance on inpatient quality-of-care measures or quality-of-care measures at discharge in patients after MI or stroke remains high (>90% for most measures), performance on outpatient quality-of-care measures, especially those that pertain to body mass index assessment and PA assessment in the outpatient setting, remains low.
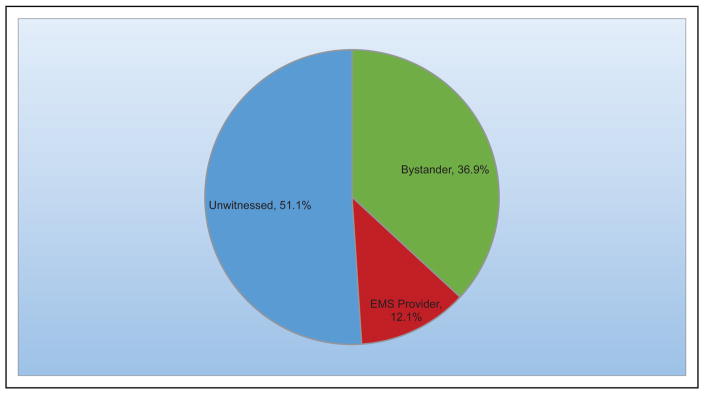
Overall rates of bystander cardiopulmonary resuscitation remain low.
Medical Procedures (Chapter 26)
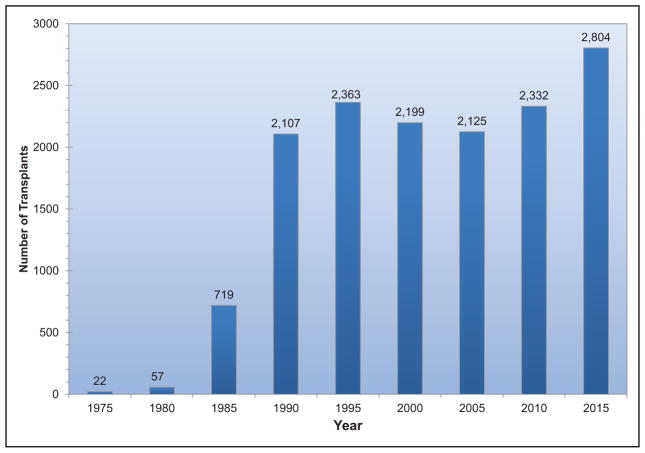
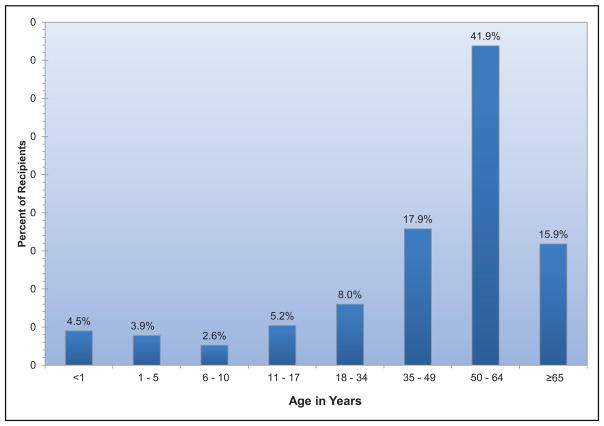
In 2015, 2804 heart transplantations were performed in the United States, the most ever.
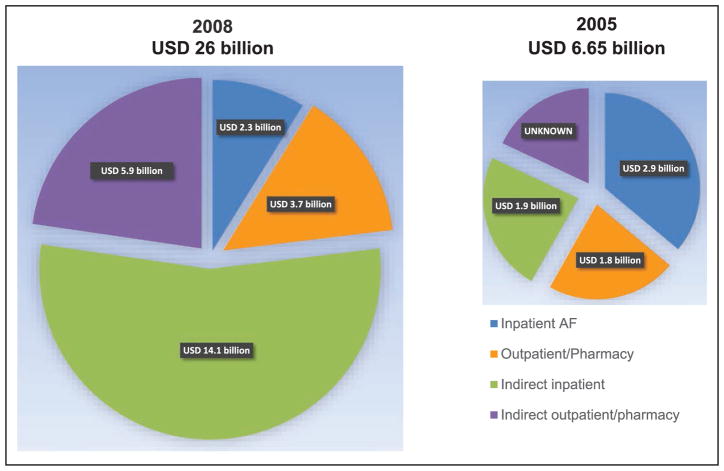
Economic Cost of Cardiovascular Disease (Chapter 27)
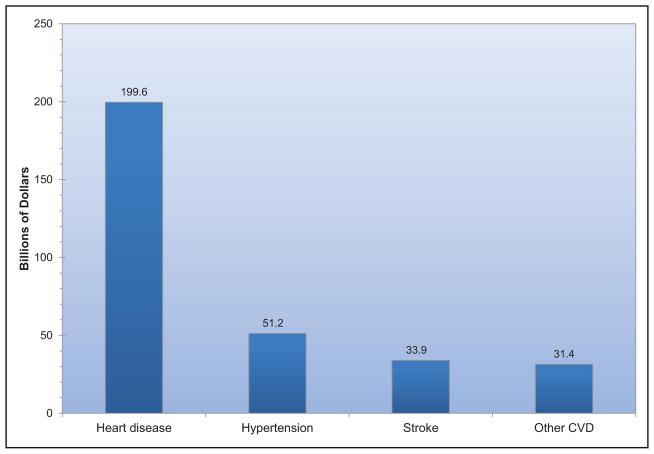
CVD and stroke accounted for 14% of total health expenditures in 2012 to 2013, more than any major diagnostic group.
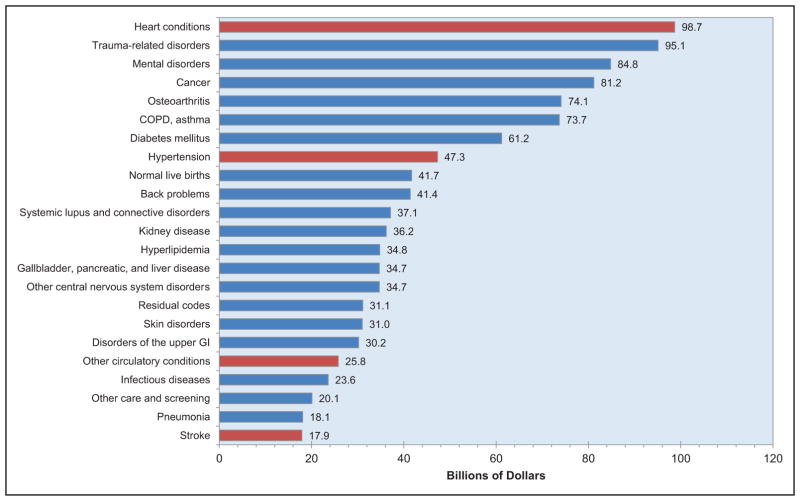
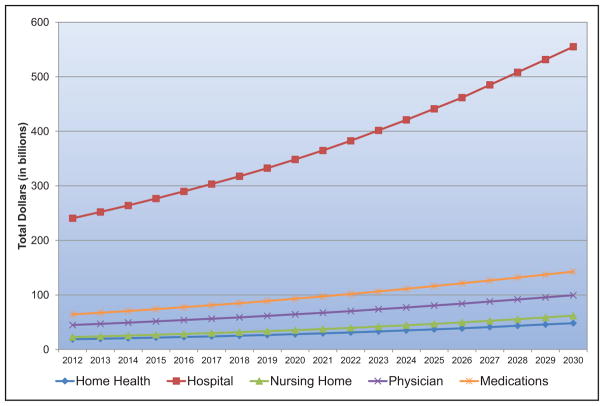
The annual direct and indirect cost of CVD and stroke in the United States was an estimated $316.1 billion in 2012 to 2013. This figure includes $189.7 billion in expenditures (direct costs, which include the cost of physicians and other professionals, hospital services, prescribed medication, and home health care, but not the cost of nursing home care) and $126.4 billion (indirect costs) in lost future productivity attributed to premature CVD and stroke mortality in 2012 to 2013.
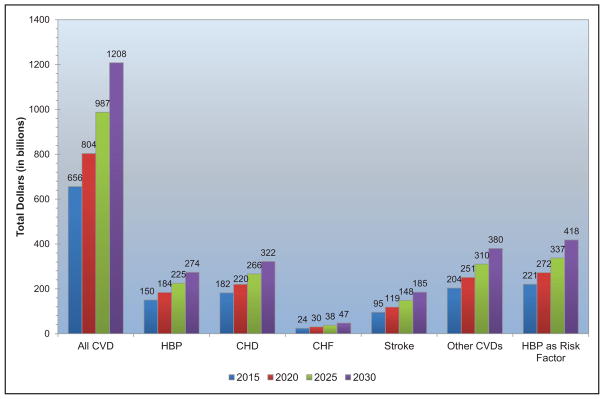
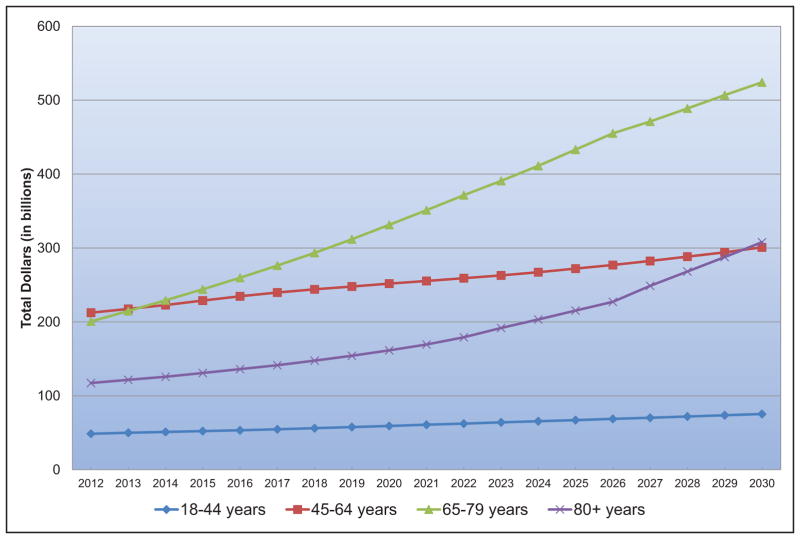
Taking into account nursing home care costs, the total direct medical costs of CVD between 2012 to 2030 are projected to increase from $396 billion to $918 billion.
Conclusions
The AHA, through its Statistics Committee, continuously monitors and evaluates sources of data on heart disease and stroke in the United States to provide the most current information available in the Statistical Update. This annual Statistical Update is the product of a full year’s worth of effort by dedicated volunteer physicians and scientists, committed government professionals, and outstanding AHA staff members, without whom publication of this valuable resource would be impossible. Their contributions are gratefully acknowledged.
-
Emelia J. Benjamin, MD, ScM, FAHA, Chair
Paul Muntner, PhD, MHSc, Vice Chair
Sally S. Wong, PhD, RD, CDN, FAHA, AHA Science & Medicine Advisor
On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee
Note: Population data used in the compilation of NHANES prevalence estimates are for the latest year of the NHANES survey being used. Extrapolations for NHANES prevalence estimates are based on the census resident population for 2014 because this is the most recent year of NHANES data used in the Statistical Update.
- 1. [Accessed November 3, 2016];Life’s Simple 7. http://www.heart.org/HEARTORG/Conditions/My-Life-Check-Lifes-Simple-7_UCM_471453_Article.jsp#.WBwQnvKQzio.
- 2.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377] Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
1. ABOUT THESE STATISTICS
The AHA works with the CDC’s NCHS, the NHLBI, the NINDS, and other government agencies to derive the annual statistics in this Heart Disease and Stroke Statistics Update. This chapter describes the most important sources and the types of data used from them. For more details, see Chapter 29 of this document, the Glossary.
Abbreviations Used in Chapter 1
| AHA | American Heart Association |
| AHRQ | Agency for Healthcare Research and Quality |
| AP | angina pectoris |
| ARIC | Atherosclerosis Risk in Communities Study |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CDC | Centers for Disease Control and Prevention |
| CHS | Cardiovascular Health Study |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| ED | emergency department |
| FHS | Framingham Heart Study |
| GCNKSS | Greater Cincinnati/Northern Kentucky Stroke Study |
| HBP | high blood pressure |
| HD | heart disease |
| HF | heart failure |
| ICD | International Classification of Diseases |
| ICD-9-CM | International Classification of Diseases, Clinical Modification, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| MEPS | Medical Expenditure Panel Survey |
| MI | myocardial infarction |
| NAMCS | National Ambulatory Medical Care Survey |
| NCHS | National Center for Health Statistics |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NINDS | National Institute of Neurological Disorders and Stroke |
| PAD | peripheral artery disease |
| WHO | World Health Organization |
| YRBSS | Youth Risk Behavior Surveillance System |
See Glossary (Chapter 29) for explanation of terms.
The surveys used are the following:
BRFSS—ongoing telephone health survey system
GCNKSS—stroke incidence rates and outcomes within a biracial population
MEPS—data on specific health services that Americans use, how frequently they use them, the cost of these services, and how the costs are paid
NHANES—disease and risk factor prevalence and nutrition statistics
NHIS—disease and risk factor prevalence
NHDS—hospital inpatient discharges and procedures (discharged alive, dead, or status unknown)
NAMCS—physician office visits
National Home and Hospice Care Survey—staff, services, and patients of home health and hospice agencies
NHAMCS—hospital outpatient and ED visits
Nationwide Inpatient Sample of the AHRQ—hospital inpatient discharges, procedures, and charges
National Nursing Home Survey—nursing home residents
National Vital Statistics System—national and state mortality data
WHO —mortality rates by country
YRBSS—health-risk behaviors in youth and young adults
Disease Prevalence
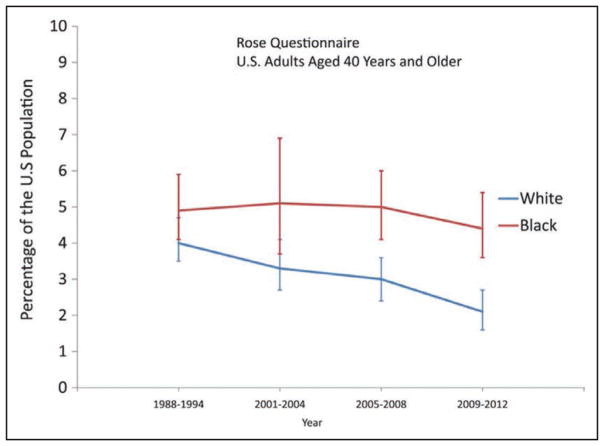
Prevalence is an estimate of how many people have a condition at a given point or period in time. The NCHS/CDC conducts health examination and health interview surveys that provide estimates of the prevalence of diseases and risk factors. In this Update, the health interview part of the NHANES is used for the prevalence of CVDs. NHANES is used more than the NHIS because in NHANES, AP is based on the Rose Questionnaire; estimates are made regularly for HF; hypertension is based on BP measurements and interviews; and an estimate can be made for total CVD, which includes MI, AP, HF, stroke, and hypertension.
A major emphasis of this Statistical Update is to present the latest estimates of the number of people in the United States who have specific conditions, to provide a realistic estimate of burden. Most estimates based on NHANES prevalence rates are based on data collected from 2011 to 2014 (in most cases, these are the latest published figures). These are applied to census population estimates for 2014. Differences in population estimates cannot be used to evaluate possible trends in prevalence because these estimates are based on extrapolations of rates beyond the data collection period by use of more recent census population estimates. Trends can only be evaluated by comparing prevalence rates estimated from surveys conducted in different years.
Risk Factor Prevalence
The NHANES 2011 to 2014 data are used in this Update to present estimates of the percentage of people with high lipid values, DM, overweight, and obesity. The NHIS is used for the prevalence of cigarette smoking and physical inactivity. Data for students in grades 9 through 12 are obtained from the YRBSS.
Incidence and Recurrent Attacks
An incidence rate refers to the number of new cases of a disease that develop in a population per unit of time. The unit of time for incidence is not necessarily 1 year, although incidence is often discussed in terms of 1 year. For some statistics, new and recurrent attacks or cases are combined. Our national incidence estimates for the various types of CVD are extrapolations to the US population from the FHS, the ARIC study, and the CHS, all conducted by the NHLBI, as well as the GCNKSS, which is funded by the NINDS. The rates change only when new data are available; they are not computed annually. Do not compare the incidence or the rates with those in past editions of the Heart Disease and Stroke Statistics Update (also known as the Heart and Stroke Statistical Update for editions before 2005). Doing so can lead to serious misinterpretation of time trends.
Mortality
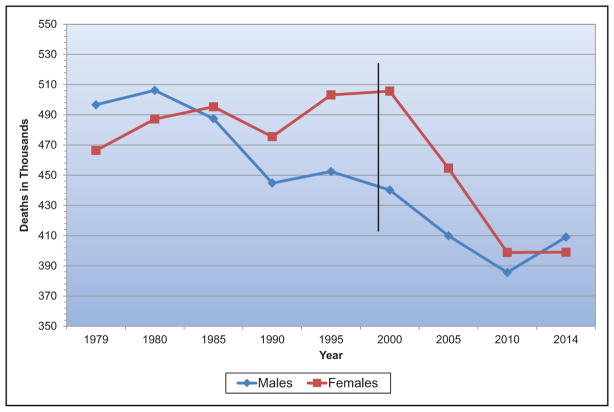
Mortality data are generally presented according to the underlying cause of death. “Any-mention” mortality means that the condition was nominally selected as the underlying cause or was otherwise mentioned on the death certificate. For many deaths classified as attributable to CVD, selection of the single most likely underlying cause can be difficult when several major comorbidities are present, as is often the case in the elderly population. It is useful, therefore, to know the extent of mortality attributable to a given cause regardless of whether it is the underlying cause or a contributing cause (ie, the “any-mention” status). The number of deaths in 2014 with any mention of specific causes of death was tabulated by the NHLBI from the NCHS public-use electronic files on mortality.
The first set of statistics for each disease in this Update includes the number of deaths for which the disease is the underlying cause. Two exceptions are Chapter 9 (High Blood Pressure) and Chapter 20 (Coronary Heart Disease, Acute Coronary Syndrome, and Angina Pectoris). HBP, or hypertension, increases the mortality risks of CVD and other diseases, and HF should be selected as an underlying cause only when the true underlying cause is not known. In this Update, hypertension and HF death rates are presented in 2 ways: (1) As nominally classified as the underlying cause and (2) as any-mention mortality.
National and state mortality data presented according to the underlying cause of death were computed from the mortality tables of the NCHS/CDC World Wide Web site or the CDC compressed mortality file. Any-mention numbers of deaths were tabulated from the electronic mortality files of the NCHS/CDC World Wide Web site.
Population Estimates
In this publication, we have used national population estimates from the US Census Bureau for 20141 in the computation of morbidity data. NCHS/CDC population estimates2 for 2014 were used in the computation of death rate data. The Census Bureau World Wide Web site contains these data, as well as information on the file layout.
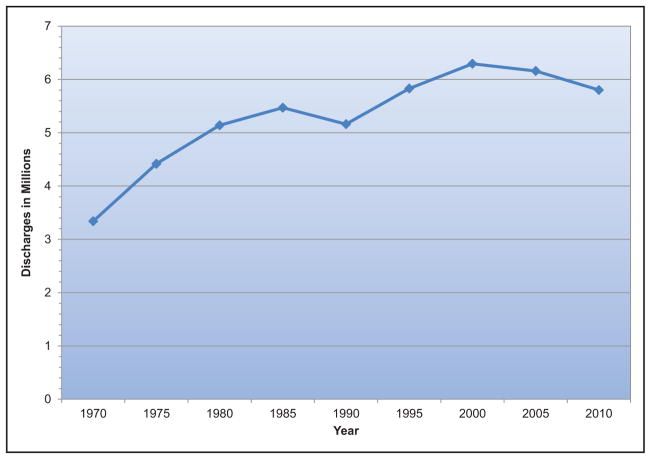
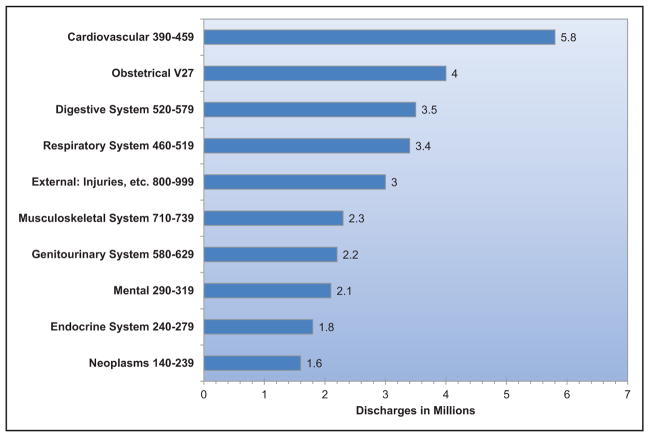
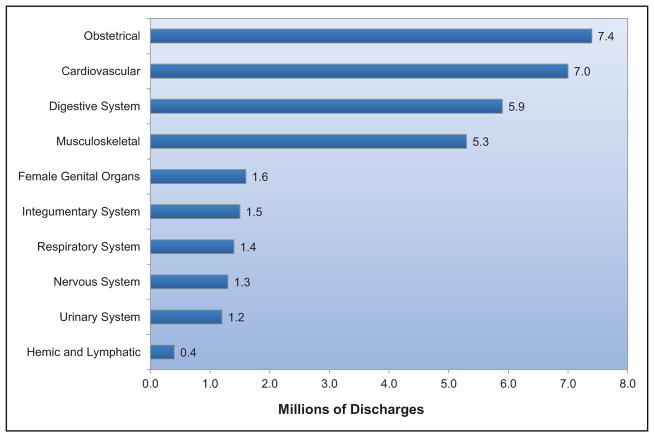
Hospital Discharges and Ambulatory Care Visits
Estimates of the numbers of hospital discharges and numbers of procedures performed are for inpatients discharged from short-stay hospitals. Discharges include those discharged alive, dead, or with unknown status. Unless otherwise specified, discharges are listed according to the first-listed (primary) diagnosis, and procedures are listed according to all listed procedures (primary plus secondary). These estimates are from the NHDS of the NCHS/CDC unless otherwise noted. Ambulatory care visit data include patient visits to physician offices and hospital outpatient departments and EDs. Ambulatory care visit data reflect the first-listed (primary) diagnosis. These estimates are from the NAMCS and NHAMCS of the NCHS/CDC. Data for community health centers, which were included in estimates in previous years, were not available for 2012 NAMCS estimates included in this Update.
International Classification of Diseases
Morbidity (illness) and mortality (death) data in the United States have a standard classification system: the ICD. Approximately every 10 to 20 years, the ICD codes are revised to reflect changes over time in medical technology, diagnosis, or terminology. Where necessary for comparability of mortality trends across the 9th and 10th ICD revisions, comparability ratios computed by the NCHS/CDC are applied as noted.3 Effective with mortality data for 1999, we are using the 10th revision (ICD-10).4 It will be a few more years before the 10th revision is systematically used for hospital discharge data and ambulatory care visit data, which are based on ICD-9-CM.5
Age Adjustment
Prevalence and mortality estimates for the United States or individual states comparing demographic groups or estimates over time are either age specific or age adjusted to the 2000 standard population by the direct method.6 International mortality data are age adjusted to the European standard.7 Unless otherwise stated, all death rates in this publication are age adjusted and are deaths per 100 000 population.
Data Years for National Estimates
In this Update, we estimate the annual number of new (incidence) and recurrent cases of a disease in the United States by extrapolating to the US population in 2013 from rates reported in a community- or hospital-based study or multiple studies. Age-adjusted incidence rates by sex and race are also given in this report as observed in the study or studies. For US mortality, most numbers and rates are for 2014. For disease and risk factor prevalence, most rates in this report are calculated from the 2011 to 2014 NHANES. Because NHANES is conducted only in the noninstitutionalized population, we extrapolated the rates to the total US population in 2014, recognizing that this probably underestimates the total prevalence, given the relatively high prevalence in the institutionalized population. The numbers and rates of hospital inpatient discharges for the United States are for 2010. Numbers of visits to physician offices and hospital EDs are for 2012, whereas hospital outpatient department visits are for 2011. Except as noted, economic cost estimates are for 2012 to 2013.
Cardiovascular Disease
For data on hospitalizations, physician office visits, and mortality, CVD is defined according to ICD codes given in Chapter 13 of the present document. This definition includes all diseases of the circulatory system, as well as congenital CVD. Unless otherwise specified, an estimate for total CVD does not include congenital CVD. Prevalence of CVD includes people with hypertension, HD, stroke, PAD, and diseases of the veins.
Race/Ethnicity
Data published by governmental agencies for some racial groups are considered unreliable because of the small sample size in the studies. Because we try to provide data for as many racial and ethnic groups as possible, we show these data for informational and comparative purposes.
Contacts
If you have questions about statistics or any points made in this Update, please contact the AHA National Center, Office of Science & Medicine. Direct all media inquiries to News Media Relations at http://www.newsroom.heart.org/newsmedia/contacts or 214-706-1173.
The AHA works diligently to ensure that this Update is error free. If we discover errors after publication, we will provide corrections at http://www.heart.org/statistics and in Circulation.
REFERENCES
- 1.US Census Bureau population estimates. Historical data: 2000s. [Accessed August 4, 2016];US Census Bureau Web site. http://www.census.gov/popest/data/historical/2000s/index.html.
- 2.National Center for Health Statistics. [Accessed August 23, 2016];Bridged-race intercensal estimates of the resident population of the United States for July 1, 2000-July 1, 2009, by year, county, single-year of age (0, 1, 2, …, 85 years and over), bridged race, Hispanic origin, and sex. http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 3.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2015. [Accessed June 15, 2016]. http://www.cdc.gov/nchs/data/hus/hus15.pdf. [PubMed] [Google Scholar]
- 4.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. 2008. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 5.National Center for Health Statistics, Centers for Medicare and Medicaid Services. [Accessed October 29, 2012];ICD-9-CM Official Guidelines for Coding and Reporting. 2011 http://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf.
- 6.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1–16. 20. [PubMed] [Google Scholar]
- 7.World Health Organization. World Health Statistics Annual. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
2. CARDIOVASCULAR HEALTH
See Tables 2-1 through 2-6 and Charts 2-1 through 2-16
Table 2-1.
Definitions of Poor, Intermediate, and Ideal Cardiovascular Health for Each Metric in the AHA 2020 Goals
| Level of Cardiovascular Health for Each Metric | |||
|---|---|---|---|
| Poor | Intermediate | Ideal | |
| Current smoking | |||
| Adults ≥20 y of age | Yes | Former ≥12 mo | Never or quit >12 mo |
| Children 12–19 y of age* | Tried during the prior 30 d | … | Never tried; never smoked whole cigarette |
| BMI† | |||
| Adults ≥20 y of age | ≥30 kg/m2 | 25–29.9 kg/m2 | <25 kg/m2 |
| Children 2–19 y of age | >95th percentile | 85th–95th percentile | <85th percentile |
| Physical activity | |||
| Adults ≥20 y of age | None | 1–149 min/wk moderate or 1–74 min/wk vigorous or 1–149 min/wk moderate + 2× vigorous | ≥150 min/wk moderate or ≥75 min/wk vigorous or ≥150 min/wk moderate + 2× vigorous |
| Children 12–19 y of age | None | >0 and <60 min of moderate or vigorous every day | ≥60 min of moderate or vigorous every day |
| Healthy diet pattern, No. of components (AHA diet score)‡ | |||
| Adults ≥20 y of age | <2 (0–39) | 2–3 (40–79) | 4–5 (80–100) |
| Children 5–19 y of age | <2 (0–39) | 2–3 (40–79) | 4–5 (80–100) |
| Total cholesterol, mg/dL | |||
| Adults ≥20 y of age | ≥240 | 200–239 or treated to goal | <200 |
| Children 6–19 y of age | ≥200 | 170–199 | <170 |
| Blood pressure | |||
| Adults ≥20 y of age | SBP ≥140 mm Hg or DBP ≥90 mm Hg | SBP 120–139 mm Hg or DBP 80–89 mm Hg or treated to goal | <120 mm Hg/<80 mm Hg |
| Children 8–19 y of age | >95th percentile | 90th–95th percentile or SBP ≥120 mm Hg or DBP ≥80 mm Hg | <90th percentile |
| Fasting plasma glucose, mg/dL | |||
| Adults ≥20 y of age | ≥126 | 100–125 or treated to goal | <100 |
| Children 12–19 y of age | ≥126 | 100–125 | <100 |
AHA indicates American Heart Association; BMI, body mass index; DBP, diastolic blood pressure; ellipses (…), data not available; and SBP, systolic blood pressure.
Age ranges in children for each metric depend on guidelines and data availability.
Represents appropriate energy balance, that is, appropriate dietary quantity and physical activity to maintain normal body weight.
In the context of a healthy dietary pattern that is consistent with a Dietary Approaches to Stop Hypertension [DASH]–type eating pattern, to consume ≥4.5 cups/d of fruits and vegetables, ≥2 servings/wk of fish, and ≥3 servings/d of whole grains and no more than 36 oz/wk of sugar-sweetened beverages and 1500 mg/d of sodium. The consistency of one’s diet with these dietary targets can be described using a continuous AHA diet score, scaled from 0 to 100 (see chapter on Nutrition).
Modified from Lloyd-Jones et al1 with permission. Copyright © 2010, American Heart Association, Inc.
Table 2-6.
Summary of Evidence-Based Population Approaches for Improving Diet, Increasing Physical Activity, and Reducing Tobacco Use*
| Diet | |
| Media and education | Sustained, focused media and educational campaigns, using multiple modes, for increasing consumption of specific healthful foods or reducing consumption of specific less healthful foods or beverages, either alone (Class IIa; Level of Evidence B) or as part of multicomponent strategies (Class I; Level of Evidence B)†‡§ |
| On-site supermarket and grocery store educational programs to support the purchase of healthier foods (Class IIa; Level of Evidence B)† | |
| Labeling and information | Mandated nutrition facts panels or front-of-pack labels/icons as a means to influence industry behavior and product formulations (Class IIa; Level of Evidence B)† |
| Economic incentives | Subsidy strategies to lower prices of more healthful foods and beverages (Class I; Level of Evidence A)† |
| Tax strategies to increase prices of less healthful foods and beverages (Class IIa; Level of Evidence B)† | |
| Changes in both agricultural subsidies and other related policies to create an infrastructure that facilitates production, transportation, and marketing of healthier foods, sustained over several decades (Class IIa; Level of Evidence B)† | |
| Schools | Multicomponent interventions focused on improving both diet and physical activity, including specialized educational curricula, trained teachers, supportive school policies, a formal physical education program, healthy food and beverage options, and a parental/family component (Class I; Level of Evidence A)† |
| School garden programs, including nutrition and gardening education and hands-on gardening experiences (Class IIa; Level of Evidence A)† | |
| Fresh fruit and vegetable programs that provide free fruits and vegetables to students during the school day (Class IIa; Level of Evidence A)† | |
| Workplaces | Comprehensive worksite wellness programs with nutrition, physical activity, and tobacco cessation/prevention components (Class IIa; Level of Evidence A)† |
| Increased availability of healthier food/beverage options and/or strong nutrition standards for foods and beverages served, in combination with vending machine prompts, labels, or icons to make healthier choices (Class IIa; Level of Evidence B)† | |
| Local environment | Increased availability of supermarkets near homes (Class IIa; Level of Evidence B)†‡|| |
| Restrictions and mandates | Restrictions on television advertisements for less healthful foods or beverages advertised to children (Class I; Level of Evidence B)† |
| Restrictions on advertising and marketing of less healthful foods or beverages near schools and public places frequented by youths (Class IIa; Level of Evidence B)† | |
| General nutrition standards for foods and beverages marketed and advertised to children in any fashion, including on-package promotion (Class IIa; Level of Evidence B)† | |
| Regulatory policies to reduce specific nutrients in foods (eg, trans fats, salt, certain fats) (Class I; Level of Evidence B)†§ | |
| Physical activity | |
| Labeling and information | Point-of-decision prompts to encourage use of stairs (Class IIa; Level of Evidence A)† |
| Economic incentives | Increased gasoline taxes to increase active transport/commuting (Class IIa; Level of Evidence B)† |
| Schools | Multicomponent interventions focused on improving both diet and physical activity, including specialized educational curricula, trained teachers, supportive school policies, a formal physical education program, serving of healthy food and beverage options, and a parental/family component (Class IIa; Level of Evidence A)† |
| Increased availability and types of school playground spaces and equipment (Class I; Level of Evidence B)† | |
| Increased number of physical education classes, revised physical education curricula to increase time in at least moderate activity, and trained physical education teachers at schools (Class IIa; Level of Evidence A/Class IIb; Level of Evidence A¶)† | |
| Regular classroom physical activity breaks during academic lessons (Class IIa; Level of Evidence A)†§ | |
| Workplaces | Comprehensive worksite wellness programs with nutrition, physical activity, and tobacco cessation/prevention components (Class IIa; Level of Evidence A)† |
| Structured worksite programs that encourage activity and also provide a set time for physical activity during work hours (Class IIa; Level of Evidence B)† | |
| Improving stairway access and appeal, potentially in combination with “skip-stop” elevators that skip some floors (Class IIa; Level of Evidence B)† | |
| Adding new or updating worksite fitness centers (Class IIa; Level of Evidence B)† | |
| Local environment | Improved accessibility of recreation and exercise spaces and facilities (eg, building of parks and playgrounds, increasing operating hours, use of school facilities during nonschool hours) (Class IIa; Level of Evidence B)† |
| Improved land-use design (eg, integration and interrelationships of residential, school, work, retail, and public spaces) (Class IIa; Level of Evidence B)† | |
| Improved sidewalk and street design to increase active commuting (walking or bicycling) to school by children (Class IIa; Level of Evidence B)† | |
| Improved traffic safety (Class IIa; Level of Evidence B)† | |
| Improved neighborhood aesthetics (to increase activity in adults) (Class IIa; Level of Evidence B)† | |
| Improved walkability, a composite indicator that incorporates aspects of land-use mix, street connectivity, pedestrian infrastructure, aesthetics, traffic safety, and/or crime safety (Class IIa; Level of Evidence B)† | |
| Smoking | |
| Media and education | Sustained, focused media and educational campaigns to reduce smoking, either alone (Class IIa; Level of Evidence B) or as part of larger multicomponent population-level strategies (Class I; Level of Evidence A)† |
| Labeling and information | Cigarette package warnings, especially those that are graphic and health related (Class I; Level of Evidence B)†‡§ |
| Economic incentives | Higher taxes on tobacco products to reduce use and fund tobacco control programs (Class I; Level of Evidence A)†‡§ |
| Schools and workplaces | Comprehensive worksite wellness programs with nutrition, physical activity, and tobacco cessation/prevention components (Class IIa; Level of Evidence A)† |
| Local environment | Reduced density of retail tobacco outlets around homes and schools (Class I; Level of Evidence B)† |
| Development of community telephone lines for cessation counseling and support services (Class I; Level of Evidence A)† | |
| Restrictions and mandates | Community (city, state, or federal) restrictions on smoking in public places (Class I; Level of Evidence A)† |
| Local workplace-specific restrictions on smoking (Class I; Level of Evidence A)†‡§ | |
| Stronger enforcement of local school-specific restrictions on smoking (Class IIa; Level of Evidence B)† | |
| Local residence-specific restrictions on smoking (Class IIa; Level of Evidence B)†§ | |
| Partial or complete restrictions on advertising and promotion of tobacco products (Class I; Level of Evidence B)† | |
The specific population interventions listed here are either a Class I or IIa recommendation with a Level of Evidence grade of either A or B.
At least some evidence from studies conducted in high-income Western regions and countries (eg, North America, Europe, Australia, New Zealand).
At least some evidence from studies conducted in high-income non-Western regions and countries (eg, Japan, Hong Kong, South Korea, Singapore).
At least some evidence from studies conducted in low- or middle-income regions and countries (eg, Africa, China, Pakistan, India).
Based on cross-sectional studies only; only 2 longitudinal studies have been performed, with no significant relations seen.
Class IIa; Level of Evidence A for improving physical activity; Class IIb; Level of Evidence B for reducing adiposity.
Reprinted from Mozaffarian et al34 with permission. Copyright © 2012,
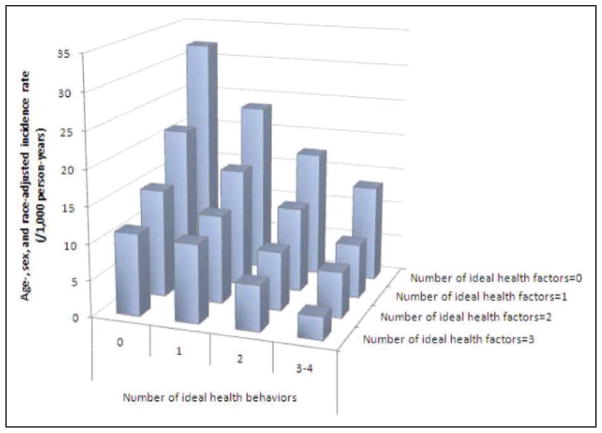
Chart 2-1. Incidence of cardiovascular disease according to the number of ideal health behaviors and health factors.
Reprinted from Folsom et al12 with permission from Elsevier. Copyright © 2011, American College of Cardiology Foundation.
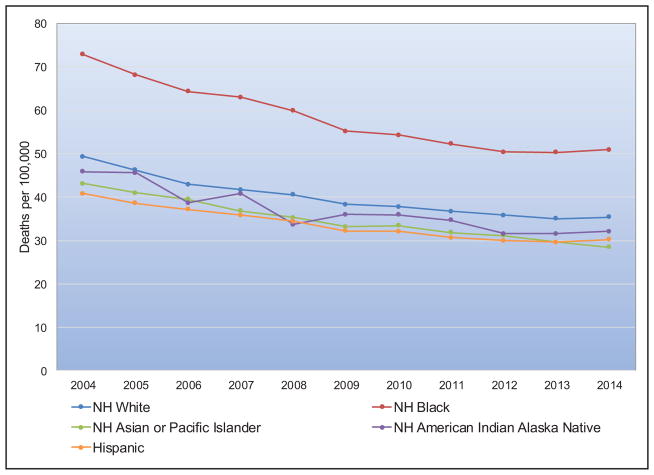
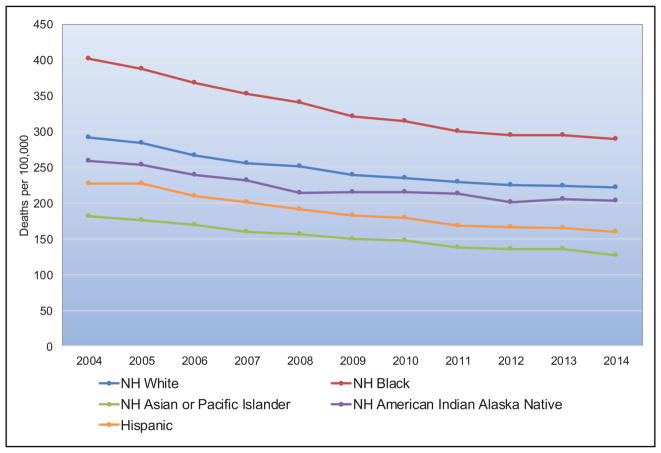
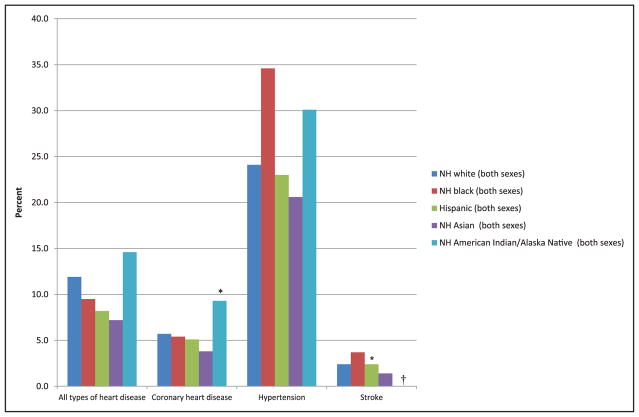
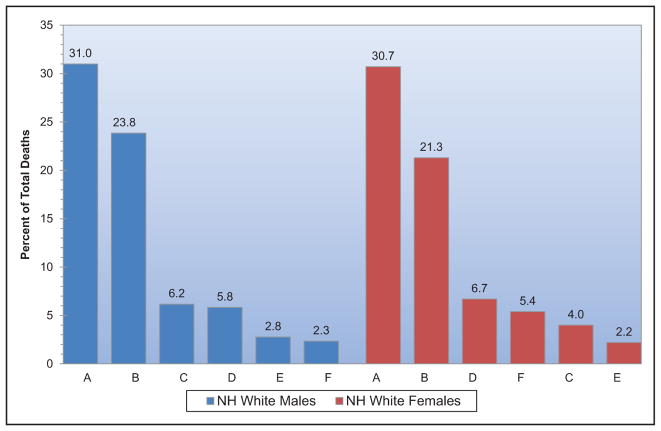
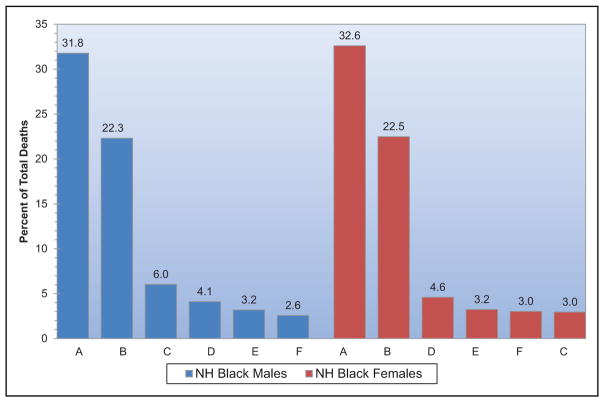
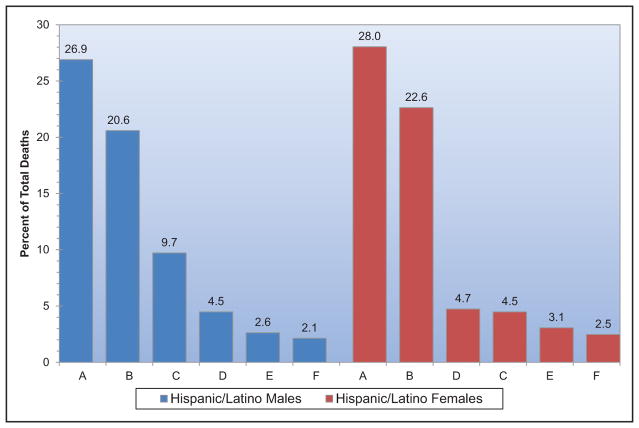
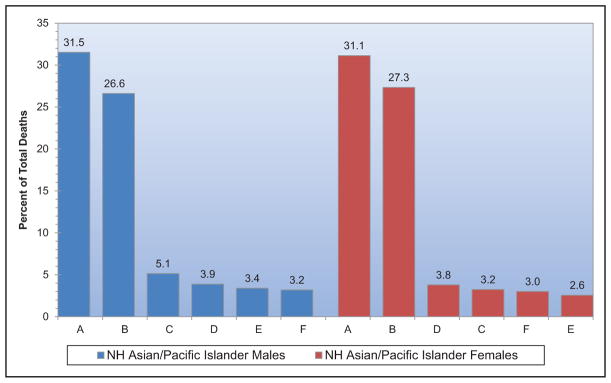
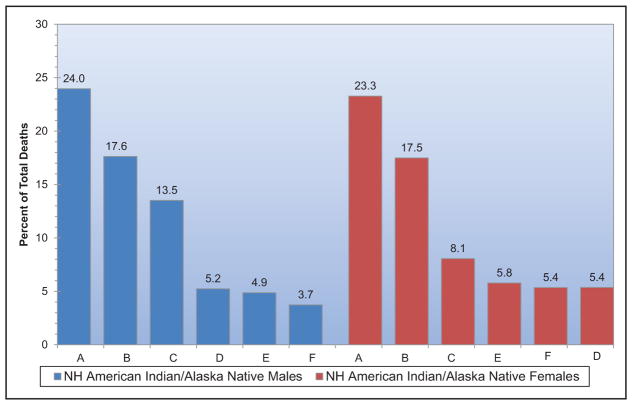
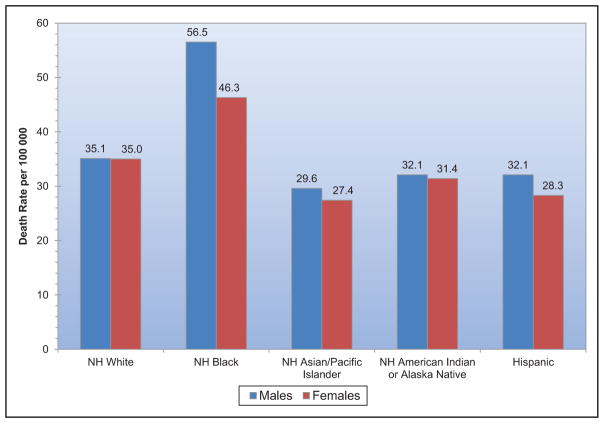
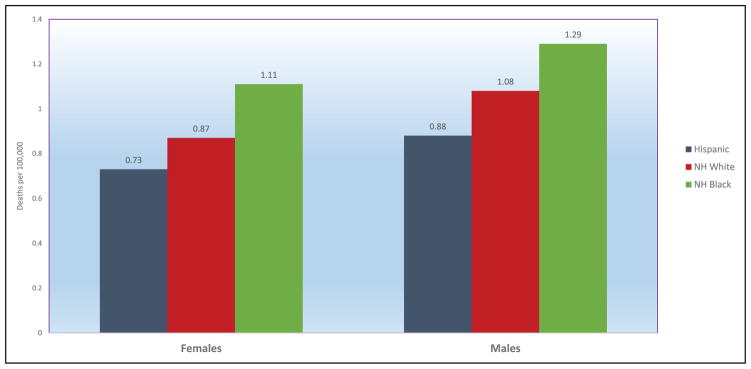
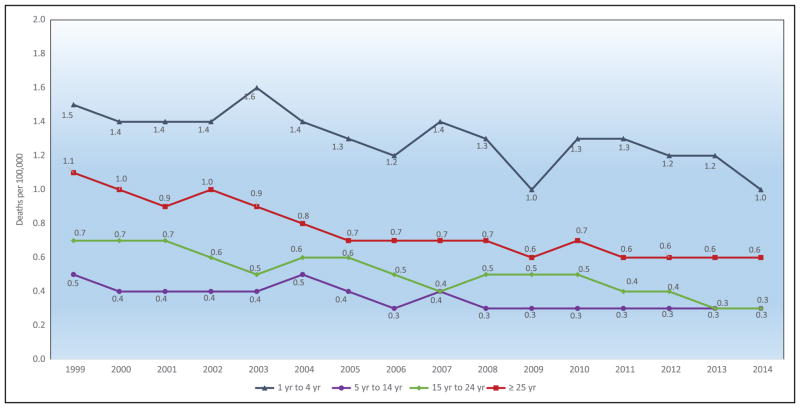
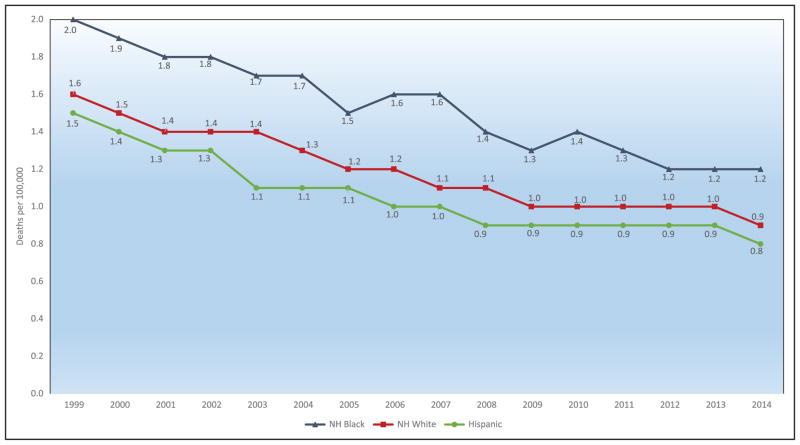
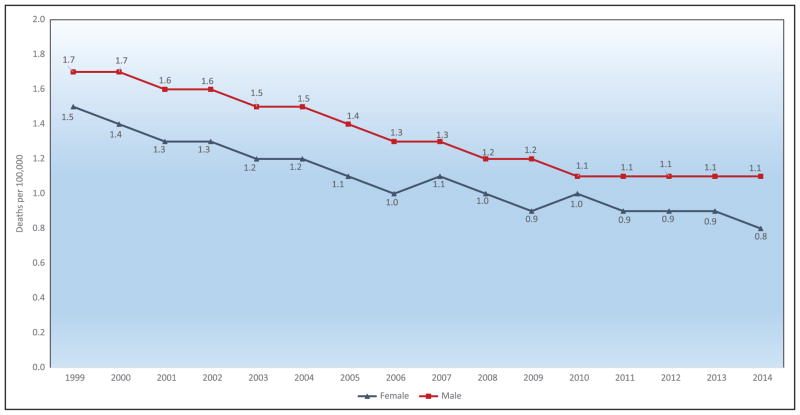
Chart 2-16. US age-standardized death rates* from stroke by race/ethnicity, 2000 to 2014.
NH indicates non-Hispanic.
*Directly standardized to the age distribution of the 2000 US standard population. Stroke (all cerebrovascular disease): International Classification of Diseases, 10th Revision (ICD-10) I60 to I69.
Source: Centers for Disease Control and Prevention, National Vital Statistics System.31
In 2011, the AHA created a new set of central Strategic Impact Goals to drive organizational priorities for the current decade:
By 2020, to improve the cardiovascular health of all Americans by 20%, while reducing deaths from CVDs and stroke by 20%.1
Abbreviations Used in Chapter 2
| AHA | American Heart Association |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CHD | coronary heart disease |
| CI | confidence interval |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| DASH | Dietary Approaches to Stop Hypertension |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| F&V | fruits and vegetables |
| FPG | fasting plasma glucose |
| HbA1c | hemoglobin A1c (glycosylated hemoglobin) |
| HBP | high blood pressure |
| HF | heart failure |
| HR | hazard ratio |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IHD | ischemic heart disease |
| IMT | intima-media thickness |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| PA | physical activity |
| REGARDS | Reasons for Geographic and Racial Differences in Stroke |
| SBP | systolic blood pressure |
| SE | standard error |
| SFat | saturated fat |
| SSB | sugar-sweetened beverage |
| svg | servings |
| TC | total cholesterol |
| WHLF | whole grain |
These goals introduce a new concept of cardiovascular health, characterized by 7 metrics (“Life’s Simple 7”2), including health behaviors (diet quality, PA, smoking, BMI) and health factors (blood cholesterol, BP, blood glucose). Ideal cardiovascular health is defined by the absence of clinically manifest CVD together with the simultaneous presence of optimal levels of all 7 metrics, including not smoking and having a healthy diet pattern, sufficient PA, normal body weight, and normal levels of TC, BP, and fasting blood glucose, in the absence of drug treatment (Table 2-1). Because a spectrum of cardiovascular health is possible and the ideal cardiovascular health profile is known to be rare in the US population, a broader spectrum of cardiovascular health can also be represented as being “ideal,” “intermediate,” or “poor” for each of the health behaviors and health factors. 1 Table 2-1 provides the specific definitions for ideal, intermediate, and poor cardiovascular health for each of the 7 metrics, both for adults and children.
This concept of cardiovascular health represents a new focus for the AHA, with 3 central and novel emphases:
An expanded focus on CVD prevention and promotion of positive “cardiovascular health,” in addition to the treatment of established CVD
Efforts to promote both healthy behaviors (healthy diet pattern, appropriate energy intake, PA, and nonsmoking) and healthy biomarker levels (optimal blood lipids, BP, glucose levels) throughout the lifespan
Population-level health promotion strategies to shift the majority of the public toward greater cardiovascular health, in addition to targeting those individuals at greatest CVD risk, because healthy lifestyles in all domains are uncommon throughout the US population
Beginning in 2011, and recognizing the time lag in the nationally representative US data sets, this chapter in the annual Statistical Update evaluates and publishes metrics and information to provide insights into both progress toward meeting the 2020 AHA goals and areas that require greater attention to meet these goals. The AHA has advocated for raising the visibility of patient-reported cardiovascular health status, which includes symptom burden, functional status, and health-related quality of life, as an indicator of cardiovascular health in future organizational goal setting.3
Relevance of Ideal Cardiovascular Health
Since the AHA announced its 2020 Impact Goals, multiple independent investigations have confirmed the importance of these metrics and the concept of cardiovascular health. Findings include strong inverse, stepwise associations in the United States of the metrics and cardiovascular health with all-cause mortality, CVD mortality, and HF; with preclinical measures of atherosclerosis such as carotid IMT arterial stiffness, and coronary artery calcium prevalence and progression; with physical functional impairment and frailty4; and with cognitive decline and depression. 5,6 Similar relationships have also been seen in non-US populations.5,7–10
A recent study in a large Hispanic/Latino cohort study in the United States found that associations of CVD and cardiovascular health metrics compared favorably with existing national estimates; however, some of the associations varied by sex and heritage, providing important information to guide targeted health promotion efforts toward achieving 2020 goals.11
Ideal health behaviors and ideal health factors are each independently associated with lower CVD risk in a stepwise fashion (Chart 2-1). In other words, across any level of health behaviors, health factors are associated with incident CVD; conversely, across any level of health factors, health behaviors are still associated with incident CVD.12
In addition, only modest intercorrelations are apparent between different cardiovascular health metrics. On the basis of NHANES 1999 to 2002, these ranged from a correlation of −0.12 between PA and HbA1c to a correlation of 0.29 between BMI and HbA1c. Thus, substantial independent variation in each cardiovascular health component exists, and each is independently related to cardiovascular outcomes.13
These findings corroborate the independent value of targeting each of these 7 metrics as separate aims.
Analyses from the US Burden of Disease Collaborators demonstrated that each of the 7 health factors and behaviors caused substantial mortality and morbidity in the United States in 2010. The top risk factor related to overall disease burden was suboptimal diet, followed by tobacco smoking, high BMI, raised BP, high fasting plasma glucose, and physical inactivity.14
A stepwise association was present between the number of ideal cardiovascular health metrics and risk of death based on NHANES 1988 to 2006 data.15 The HRs for people with 6 or 7 ideal health metrics compared with 0 ideal health metrics were 0.49 (95% CI, 0.33–0.74) for all-cause mortality, 0.24 (95% CI, 0.13–0.47) for CVD mortality, and 0.30 (95% CI, 0.13–0.68) for IHD mortality.15 Ford et al13 demonstrated similar relationships.
A recent meta-analysis of 9 prospective cohort studies involving 12 878 participants reported that achieving the most ideal cardiovascular health metrics was associated with lower risk of all-cause mortality (RR, 0.55; 95% CI, 0.37–0.80), cardiovascular mortality (RR, 0.25; 95% CI, 0.10–0.63), CVD (RR, 0.20; 95% CI, 0.11–0.37), and stroke (RR, 0.31; 95% CI, 0.25–0.38).16
-
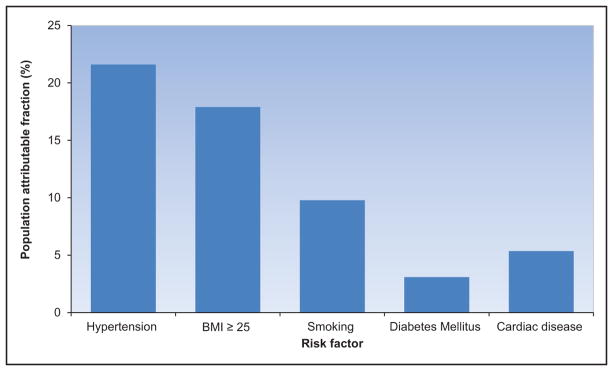
The adjusted population attributable fractions for CVD mortality were as follows15:
40.6% (95% CI, 24.5%–54.6%) for HBP
13.7% (95% CI, 4.8%–22.3%) for smoking
13.2% (95% CI, 3.5%–29.2%) for poor diet
11.9% (95% CI, 1.3%–22.3%) for insufficient PA
8.8% (95% CI, 2.1%–15.4%) for abnormal glucose levels
-
The adjusted population attributable fractions for IHD mortality were as follows15:
34.7% (95% CI, 6.6%–57.7%) for HBP
16.7% (95% CI, 6.4%–26.6%) for smoking
20.6% (95% CI, 1.2%–38.6%) for poor diet
7.8% (95% CI, 0%–22.2%) for insufficient PA
7.5% (95% CI, 3.0%–14.7%) for abnormal glucose levels
Data from the REGARDS cohort also demonstrated a stepwise association between cardiovascular health metrics and incident stroke. Using a cardiovascular health score scale ranging from 0 to 14, every unit increase in cardiovascular health was associated with an 8% lower risk of incident stroke (HR, 0.92; 95% CI, 0.88–0.95), with a similar effect size for white (HR, 0.91; 95% CI, 0.86–0.96) and black (HR, 0.93; 95% CI, 0.87–0.98) participants.17
The Cardiovascular Lifetime Risk Pooling Project showed that adults with all-optimal risk factor levels (similar to having ideal cardiovascular health factor levels of cholesterol, blood sugar, and BP, as well as nonsmoking) have substantially longer overall and CVD-free survival than those who have poor levels of ≥1 of these cardiovascular health factor metrics. For example, at an index age of 45 years, males with optimal risk factor profiles lived on average 14 years longer free of all CVD events, and 12 years longer overall, than people with ≥2 risk factors.18
Better cardiovascular health is associated with less incident HF,19 less subclinical vascular disease,20,21 better global cognitive performance and cognitive function,22,23 lower prevalence24 and incidence25 of depressive symptoms, and lower loss of physical functional status.26
The AHA’s 2020 Strategic Impact Goals are to improve cardiovascular health among all Americans. On the basis of NHANES 1999 to 2006, several social risk factors (low family income, low education level, minority race, and single-living status) were related to lower likelihood of attaining better cardiovascular health as measured by Life’s Simple 7 scores.27
Cardiovascular Health: Current Prevalence
(See Table 2-2 and Charts 2-2 through 2-10)
Table 2-2.
Prevalence of Ideal Cardiovascular Health and Its Components in the US Population in Selected Age Strata: NHANES 2011 to 2012 and 2013 to 2014
| NHANES Cycle | Age 12—19 y, % (SE) | Age ≥20 y, % (SE)* | Age 20–39 y, % (SE) | Age 40–59 y, % (SE) | Age ≥60 y, % (SE) | |
|---|---|---|---|---|---|---|
| Ideal CV health profile (7/7) | 2011–2012 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| ≥6 Ideal | 2011–2012 | 10.3 (1.7) | 4.3 (0.6) | 9.1 (1.4) | 1.8 (0.4) | 0.5 (0.3) |
| ≥5 Ideal | 2011–2012 | 41.3 (2.3) | 16.9 (1.0) | 32.7 (2.5) | 8.9 (1.0) | 3.2 (1.1) |
| Ideal health factors (4/4) | 2013–2014 | 57.7 (2.1) | 18.4 (0.9) | 32.6 (2.1) | 13.1 (1.2) | 2.9 (0.5) |
| Total cholesterol <200 mg/dL | 2013–2014 | 79.7 (0.9) | 50.1 (1.5) | 71.9 (2.2) | 41.9 (1.6) | 26.6 (1.8) |
| SBP <120/DBP <80 mm Hg | 2013–2014 | 88.7 (1.1) | 45.4 (0.9) | 68.0 (1.6) | 40.2 (2.0) | 14.1 (1.1) |
| Nonsmoker | 2013–2014 | 91.4 (1.4) | 77.1 (1.2) | 72.6 (1.4) | 74.7 (2.1) | 87.7 (1.2) |
| FPG <100 mg/dL and HbA1c <5.7% | 2013–2014 | 87.6 (1.0) | 60.8 (1.1) | 78.5 (1.3) | 57.7 (1.8) | 35.4 (1.7) |
| Ideal health behaviors (4/4) | 2011–2012 | 0.0 (0.0) | 0.1 (0.1) | 0.0 (0.0) | 0.2 (0.1) | 0.1 (0.1) |
| PA at goal | 2013–2014 | 27.7 (1.2) | 36.7 (1.1) | 45.0 (2.0) | 34.2 (1.6) | 26.7 (1.5) |
| Nonsmoker | 2013–2014 | 91.4 (1.4) | 77.1 (1.2) | 72.6 (1.4) | 74.7 (2.1) | 87.7 (1.2) |
| BMI <25 kg/m2 | 2013–2014 | 63.1 (2.4) | 29.6 (0.8) | 36.3 (1.5) | 25.4 (1.4) | 25.6 (1.1) |
| 4–5 Diet goals met† | 2011–2012 | 0.0 (0.0) | 0.4 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.8 (0.2) |
| F&V ≥4.5 C/d | 2011–2012 | 3.9 (0.7) | 12.1 (1.1) | 7.9 (1.2) | 13.9 (1.5) | 17.1 (1.5) |
| Fish ≥2 svg/wk | 2011–2012 | 10.0 (1.5) | 18.5 (1.6) | 16.3 (1.4) | 18.3 (2.4) | 22.5 (2.1) |
| Sodium <1500 mg/d | 2011–2012 | 0.0 (0.0) | 0.6 (0.2) | 0.5 (0.3) | 1.1 (0.5) | 0.4 (0.2) |
| SSB <36 oz/wk | 2011–2012 | 40.8 (2.4) | 55.9 (1.9) | 46.5 (2.5) | 56.1 (2.0) | 72.0 (2.1) |
| WHLG ≥3 1-oz svg/d | 2011–2012 | 4.1 (1.4) | 7.7 (0.6) | 5.7 (0.9) | 6.6 (1.0) | 12.3 (1.2) |
| Secondary diet metrics | ||||||
| Nuts/legumes/seeds ≥4 svg/wk | 2011–2012 | 42.1 (2.6) | 49.7 (0.9) | 47.8 (2.0) | 50.9 (1.7) | 50.9 (2.1) |
| Processed meats ≤2 svg/wk | 2011–2012 | 41.2 (2.1) | 44.6 (1.4) | 43.5 (1.9) | 44.8 (2.0) | 46.0 (2.3) |
| SFat <7% total kcal | 2011–2012 | 6.4 (0.9) | 10.4 (0.6) | 10.1 (0.8) | 10.5 (0.9) | 11.6 (1.4) |
BMI indicates body mass index; CV, cardiovascular; DBP, diastolic blood pressure; FPG, fasting plasma glucose; F&V, fruits and vegetables; HbA1c, glycosylated hemoglobin; NHANES, National Health and Nutrition Examination Survey; PA, physical activity; SBP, systolic blood pressure; SE, standard error; SFat, saturated fat; SSB, sugar-sweetened beverages; svg, servings; and WHLG, whole grains.
Standardized to the age distribution of the 2000 US standard population
Scaled to 2000 kcal/d and in the context of appropriate energy balance and a DASH (Dietary Approaches to Stop Hypertension)-type eating pattern.
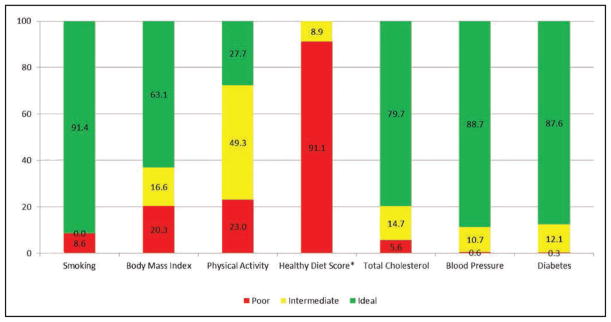
Chart 2-2. Prevalence (unadjusted) estimates of poor, intermediate, and ideal cardiovascular health for each of the 7 metrics of cardiovascular health in the American Heart Association 2020 goals, among US children aged 12 to 19 years.
*Healthy Diet Score reflects 2011 to 2012 NHANES.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2013 to 2014.
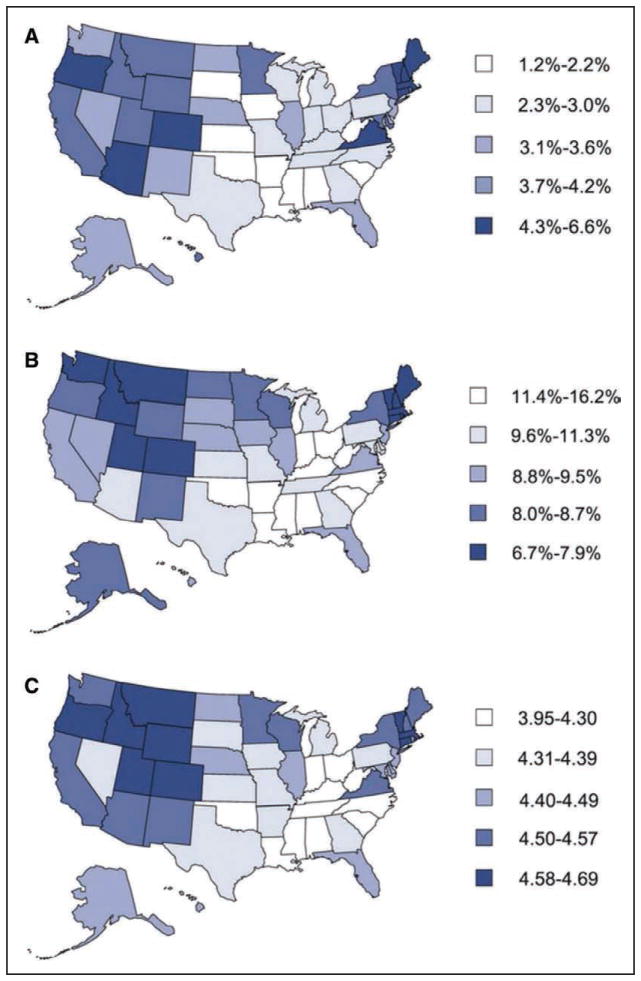
Chart 2-10. Age-standardized cardiovascular health status by US states, BRFSS, 2009.
A, Age-standardized prevalence of population with ideal cardiovascular health by states. B, Age-standardized percentage of population with 0 to 2 cardiovascular health metrics by states. C, Age-standardized mean score of cardiovascular health metrics by states.
BRFSS indicates Behavioral Risk Factor Surveillance System.
Reprinted from Fang et al28 with permission. Copyright © 2013, American Heart Association, Inc.
The most up-to-date data on national prevalence of ideal, intermediate, and poor levels of each of the 7 cardiovascular health metrics are shown for adolescents and teens (Chart 2-2) and for adults (Chart 2-3).
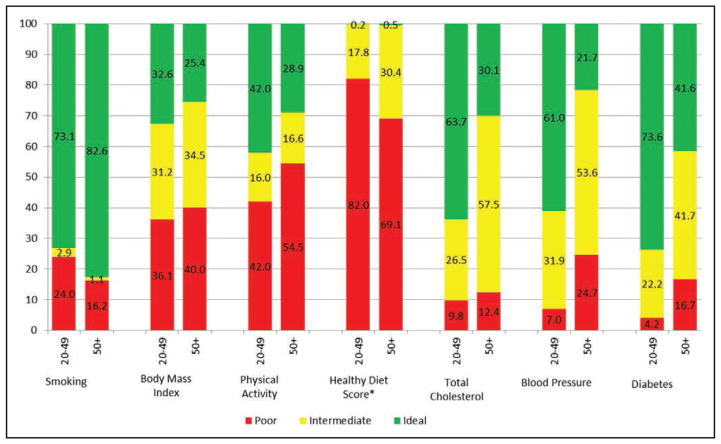
For most metrics, the prevalence of ideal levels of health behaviors and health factors is higher in US children than in US adults. The main exceptions are diet and PA, for which the prevalence of ideal levels in children are similar to (for PA) or worse (for diet) than in adults.
Among US children (Chart 2-2), the prevalence (unadjusted) of ideal levels of cardiovascular health behaviors and factors currently varies from <1% for the healthy diet pattern (ie, <1 in 100 US children meets at least 4 of the 5 dietary components or a corresponding AHA diet score of at least 80) to >80% for the smoking, BP, and fasting glucose metrics.
Among US adults (Chart 2-3), the age-standardized prevalence of ideal levels of cardiovascular health behaviors and factors currently varies from <1% for having a healthy diet pattern to up to 77% for never having smoked or being a former smoker who has quit for >12 months.
Age-standardized and age-specific prevalence estimates for ideal cardiovascular health and for ideal levels of each of its components are shown for 2011 to 2012 and 2013 to 2014 in Table 2-2. NHANES 2011 to 2012 data are used for some of the statistics that require nutritional data because 2013 to 2014 data have not been released. The prevalence of ideal levels across 7 health factors and health behaviors generally was lower with age, with much lower prevalence among older versus younger age groups. The exception was diet, for which prevalence of ideal levels was highest in older adults.
-
Chart 2-4 displays the prevalence estimates for the population of US children (12–19 years of age) meeting different numbers of criteria for ideal cardiovascular health (out of 7 possible) in 2011 to 2012.
Few US children (≈5%) meet only 0, 1, or 2 criteria for ideal cardiovascular health.
Approximately half of US children (54%) meet 3 or 4 criteria for ideal cardiovascular health, and ≈41% meet 5 or 6 criteria (mostly 5 criteria).
<1% of children meet all 7 criteria for ideal cardiovascular health.
-
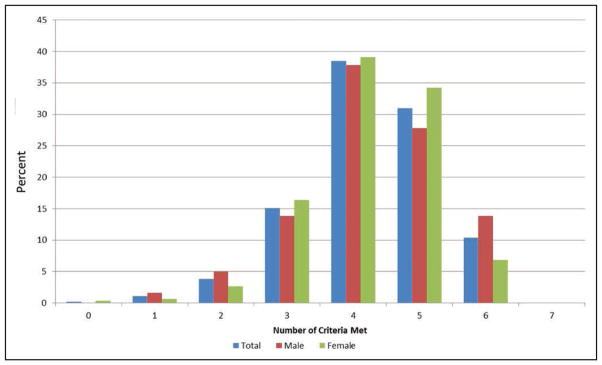
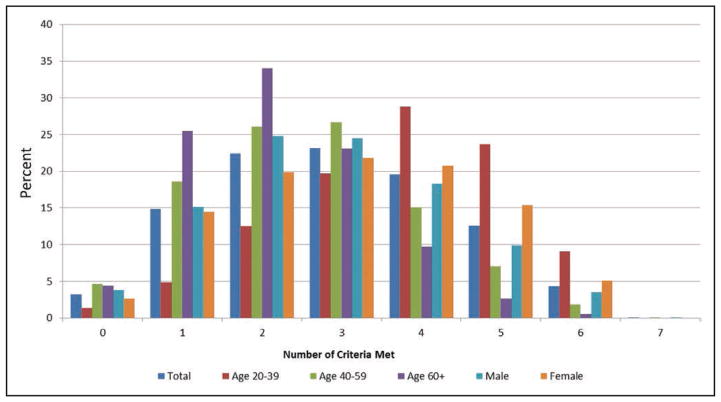
Charts 2-5 and 2-6 display the age-standardized prevalence estimates of US adults meeting different numbers of criteria for ideal cardiovascular health (out of 7 possible) in 2011 to 2012, overall and stratified by age, sex, and race.
Approximately 3% of US adults have 0 of the 7 criteria at ideal levels, and another 15% meet only 1 of 7 criteria. This is much worse than among children.
Most US adults (≈65%) have 2, 3, or 4 criteria at ideal cardiovascular health, with ≈20% adults within each of these categories.
Approximately 13% of US adults have 5 criteria, 5% have 6 criteria, and virtually 0% have 7 criteria at ideal levels.
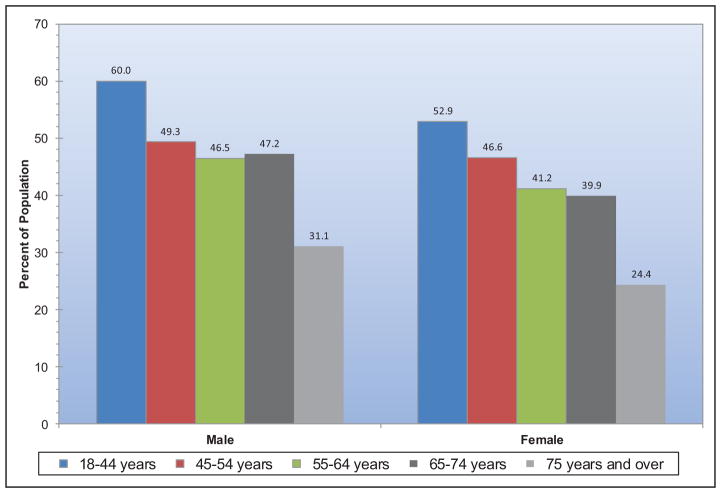
Presence of ideal cardiovascular health is both age and sex related (Chart 2-5). Younger adults are more likely to meet greater numbers of ideal metrics than are older adults. More than 60% of Americans >60 years of age have ≤2 metrics at ideal levels. At any age, females tend to have more metrics at ideal levels than do males.
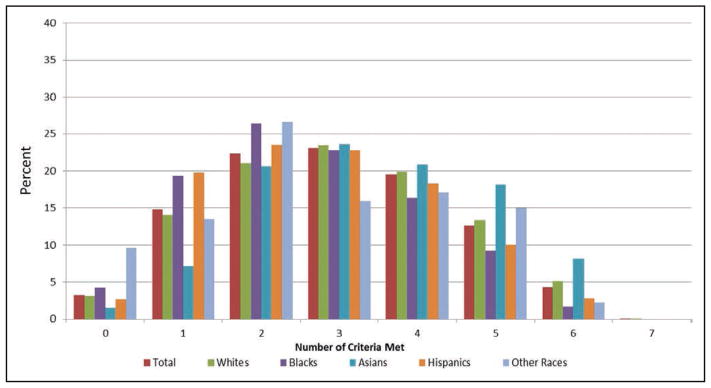
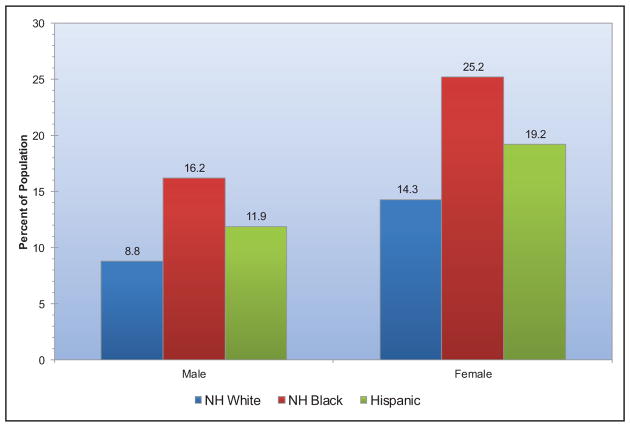
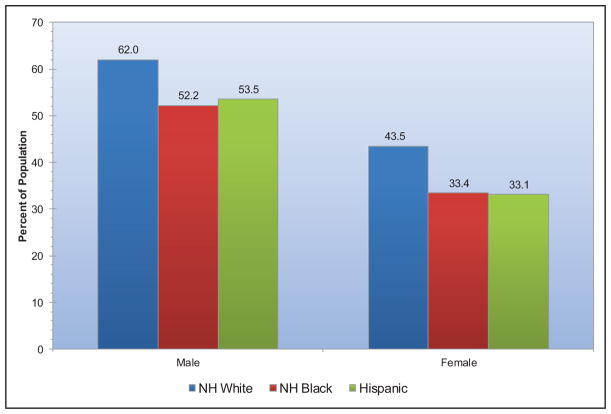
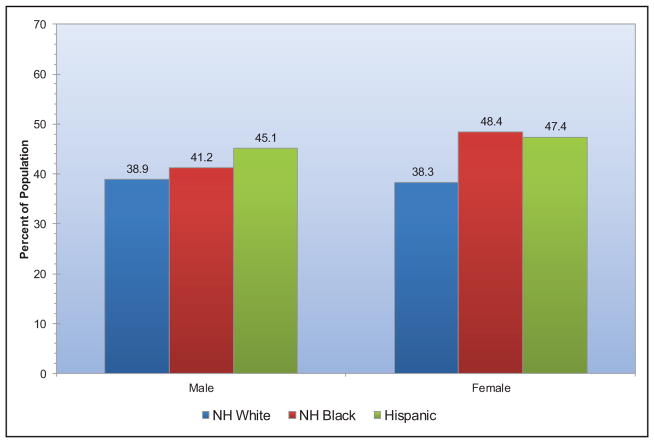
Presence of ideal cardiovascular health also varies by race (Chart 2-6). Blacks and Hispanics tend to have fewer metrics at ideal levels than whites or other races. Approximately 6 in 10 white adults and 7 in 10 black or Hispanic adults have no more than 3 of 7 metrics at ideal levels.
-
Chart 2-7 displays the age-standardized percentages of US adults and percentages of children who have ≥5 of the metrics (of 7 possible) at ideal levels.
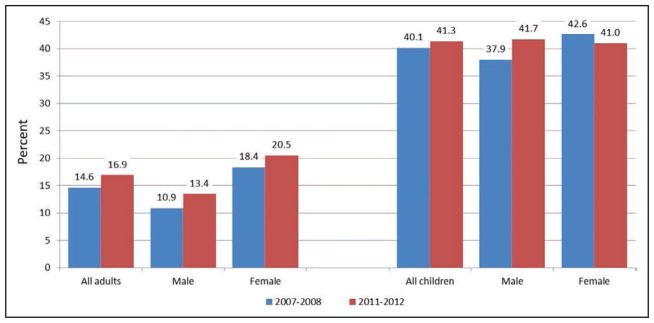
Approximately 41% of US children 12 to 19 years of age have ≥5 metrics at ideal levels, with similar prevalence in boys (42%) as in girls (41%).
In comparison, only 17% of US adults have ≥5 metrics at ideal levels, with lower prevalence in males (13%) than in females (21%).
All populations have improved since baseline year 2007 to 2008.
-
Chart 2-8 displays the age-standardized percentages of US adults and percentages of children by race/ethnicity who have ≥5 of the metrics (of 7 possible) at ideal levels.
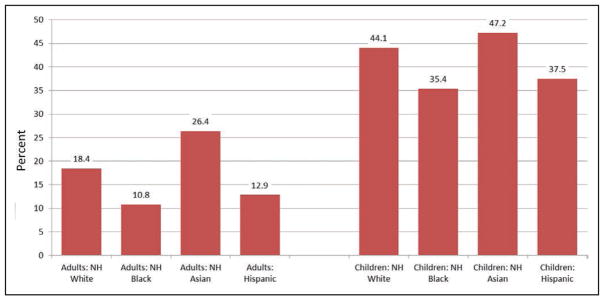
In both children and adults, non-Hispanic Asians tend to have higher prevalence of having ≥5 metrics at ideal levels than other race/ethnic groups.
Approximately 4.7 in 10 non-Hispanic Asian children, 4.4 in 10 non-Hispanic white children, 3.5 in 10 non-Hispanic black children, and 3.7 in 10 Hispanic children have ≥5 metrics at ideal levels.
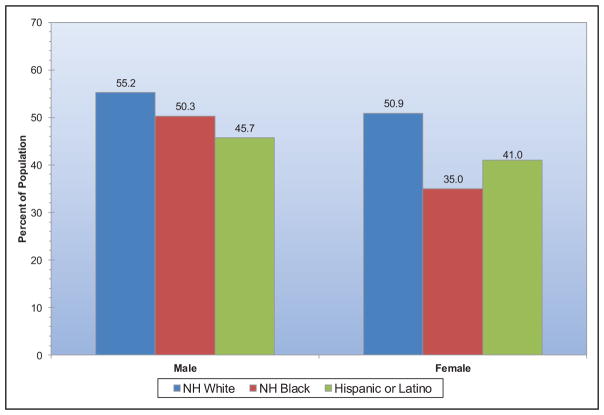
By comparison, among adults, ≈2.6 in 10 non-Hispanic Asians, 1.8 in 10 non-Hispanic whites, 1.3 in 10 Hispanics, and 1 in 10 non-Hispanic blacks have ≥5 metrics at ideal levels.
-
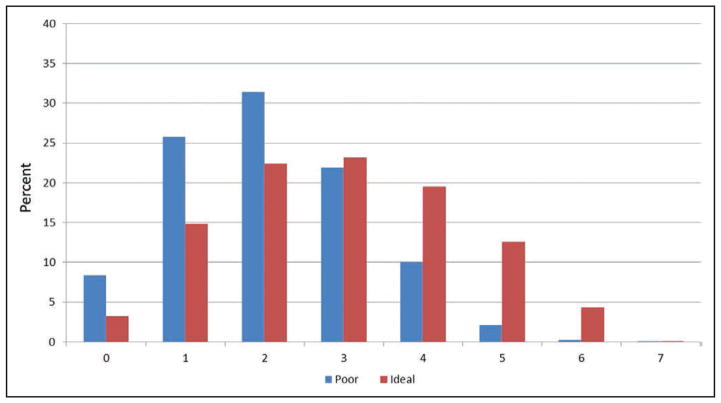
Chart 2-9 displays the age-standardized percentages of US adults who meet different numbers of criteria for both poor and ideal cardiovascular health. Meeting the AHA 2020 Strategic Impact Goals is predicated on reducing the relative percentage of those with poor levels while increasing the relative percentage of those with ideal levels for each of the 7 metrics.
Approximately 92% of US adults have ≥1 metric at poor levels.
Approximately 34% of US adults have ≥3 metrics at poor levels.
Few US adults (2.5%) have ≥5 metrics at poor levels.
More US adults have 4 to 6 ideal metrics than 4 to 6 poor metrics.
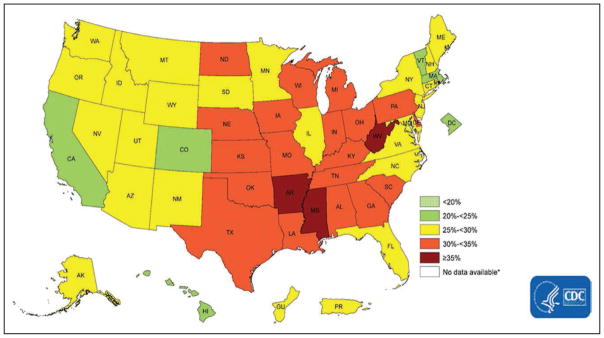
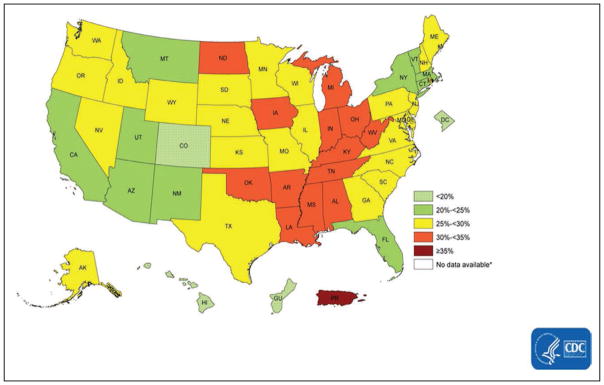
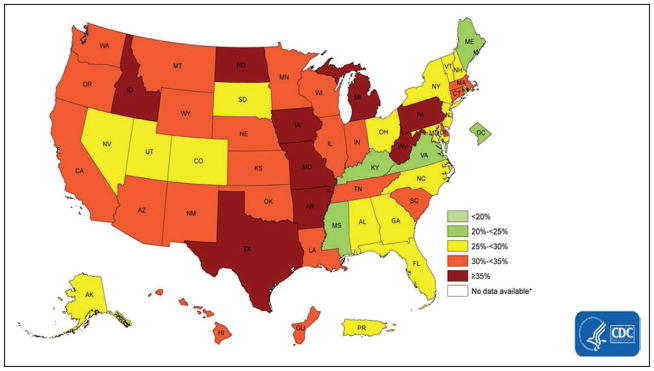
Using data from the BRFSS, Fang et al28 estimated the prevalence of ideal cardiovascular health by state (all 7 metrics at ideal level), which ranged from 1.2% (Oklahoma) to 6.9% (District of Columbia). Southern states tended to have higher percentages of poor cardiovascular health, lower percentages of ideal cardiovascular health, and lower mean cardiovascular health scores than New England and Western states (Chart 2-10).
Chart 2-3. Prevalence (unadjusted) estimates of poor, intermediate, and ideal cardiovascular health for each of the 7 metrics of cardiovascular health in the American Heart Association 2020 goals, among US adults aged 20 to 49 and ≥50 years.
*Healthy Diet Score reflects 2011 to 2012 NHANES.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2013 to 2014.
Chart 2-4. Proportion (unadjusted) of US children aged 12 to 19 years meeting different numbers of criteria for ideal cardiovascular health, overall and by sex.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2011 to 2012.
Chart 2-5. Age-standardized prevalence estimates of US adults aged ≥20 years meeting different numbers of criteria for ideal cardiovascular health, overall and by age and sex subgroups.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2011 to 2012.
Chart 2-6. Age-standardized prevalence estimates of US adults aged ≥20 years meeting different numbers of criteria for ideal cardiovascular health, overall and in selected race subgroups.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2011 to 2012.
Chart 2-7. Prevalence of meeting ≥5 criteria for ideal cardiovascular health among US adults aged ≥20 years (age-standardized) and US children aged 12 to 19 years, overall and by sex.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2007 to 2008 and 2011 to 2012.
Chart 2-8. Prevalence of meeting ≥5 criteria for ideal cardiovascular health among US adults aged ≥20 years (age standardized) and US children aged 12 to 19 years, by race/ethnicity.
NH indicates non-Hispanic.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2011 to 2012.
Chart 2-9. Age-standardized prevalence estimates of US adults meeting different numbers of criteria for ideal and poor cardiovascular health, for each of the 7 metrics of cardiovascular health in the American Heart Association 2020 goals, among US adults aged ≥20 years.
Source: National Center for Health Statistics, National Health and Nutrition Examination Survey (NHANES) 2011 to 2012.
Cardiovascular Health: Trends Over Time
(See Charts 2-11, 2-12, 2-13)
Chart 2-11. Trends in prevalence (unadjusted) of meeting criteria for ideal cardiovascular health for each of the 7 metrics among US children aged 12 to 19 years.
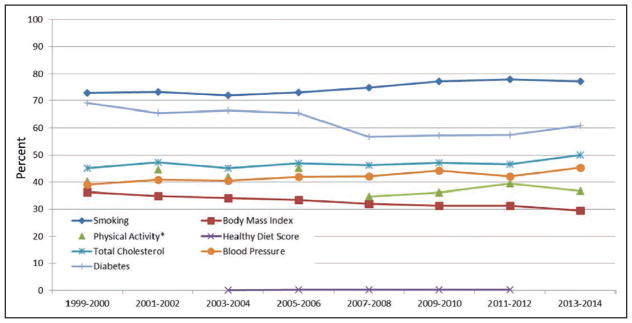
*Because of changes in the physical activity questionnaire between different cycles of the National Health and Nutrition Examination Survey (NHANES), trends over time for this indicator should be interpreted with caution, and statistical comparisons should not be attempted.
Data for the Healthy Diet Score, based on a 2-day average intake, was only available for the 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010 and 2011 to 2012 NHANES cycles at the time of this analysis.
Source: National Center for Health Statistics, NHANES 1999 to 2000 through 2013 to 2014.
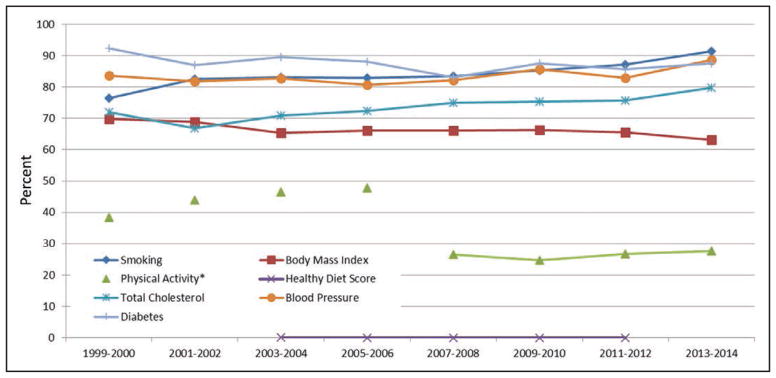
Chart 2-12. Age-standardized trends in prevalence of meeting criteria for ideal cardiovascular health for each of the 7 metrics among US adults aged ≥20 years.
*Because of changes in the physical activity questionnaire between different cycles of the National Health and Nutrition Examination Survey (NHANES), trends over time for this indicator should be interpreted with caution, and statis-tical comparisons should not be attempted.
Data for the Healthy Diet Score, based on a 2-day average intake, was only available for the 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010 and 2011 to 2012 NHANES cycles at the time of this analysis.
Source: National Center for Health Statistics, NHANES 1999 to 2000 through 2013 to 2014.
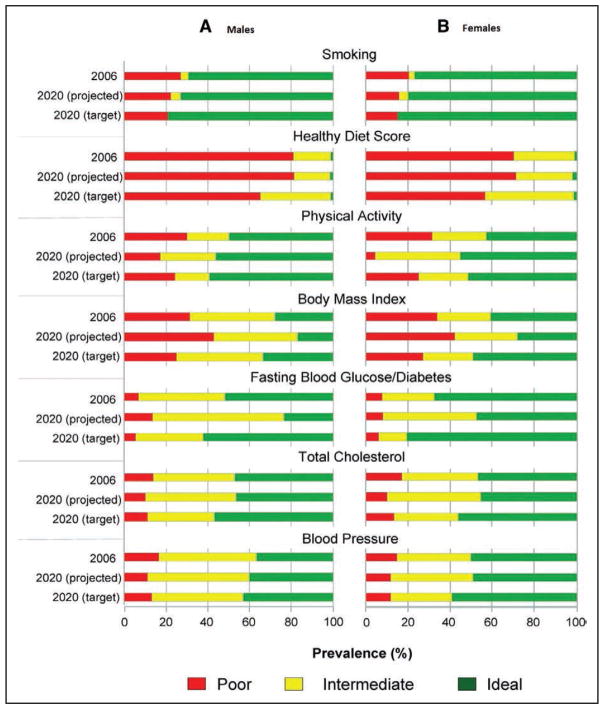
Chart 2-13. Prevalence of ideal, intermediate, and poor cardiovascular health metrics in 2006 (American Heart Association 2020 Impact Goals baseline year) and 2020 projections assuming current trends continue.
The 2020 targets for each cardiovascular health metric assume a 20% relative increase in ideal cardiovascular health prevalence metrics and a 20% relative decrease in poor cardiovascular health prevalence metrics for males and females.
Reprinted from Huffman et al29 with permission. Copyright © 2012, American Heart Association, Inc.
-
The trends over the past decade in each of the 7 cardiovascular health metrics (for diet, trends from 1999–2000 through 2013–2014) are shown in Chart 2-11 (for children 12–19 years of age) and Chart 2-12 (for adults ≥20 years of age).
The prevalence of both children and adults meeting the dietary goals improved between 2003 to 2004 and 2011 to 2012. The prevalence of ideal levels of diet (AHA diet score >80) increased from 0.2% to 0.6% in children and from 0.7% to 1.5% in adults (Chapter 5, Charts 5-2 and 5-3). The prevalence of intermediate levels of diet (AHA diet score 40–79) increased from 30.6% to 44.7% in children and from 49.0% to 57.5% in adults. These improvements were largely attributable to increased whole grain consumption and decreased sugar-sweetened beverage consumption in both children and adults, as well as small, nonsignificant trends in increased fruits and vegetables (Chapter 5, Charts 5-4 and 5-5). No major trends were evident in either children or adults meeting the target for consumption of fish or sodium.
Fewer children over time are meeting the ideal BMI metric, whereas more are meeting the ideal smoking and TC metrics. Other metrics do not show consistent trends over time in children.
More adults over time are meeting the smoking metric, whereas fewer are meeting the BMI and glucose metrics. Trends for other metrics are not evident over time in adults.
On the basis of NHANES data from 1988 to 2008, if current trends continue, estimated cardiovascular health is projected to improve by 6% between 2010 and 2020, short of the AHA’s goal of 20% improvement (Chart 2-13).29 On the basis of current trends among individual metrics, anticipated declines in prevalence of smoking, high cholesterol, and HBP (in males) would be offset by substantial increases in the prevalence of obesity and DM and smaller changes in ideal dietary patterns or PA.29
On the basis of these projections in cardiovascular health factors and behaviors, CHD deaths are projected to decrease by 30% between 2010 and 2020 because of projected improvements in TC, SBP, smoking, and PA (≈167 000 fewer deaths), offset by increases in DM and BMI (≈24 000 more deaths).30
Chart 5-2. Healthy diet targets in children (5–19 years old) by survey year: NHANES 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010, and 2011 to 2012.
Components of poor, intermediate, and ideal diet are defined in Table 5-1. Percentages based on the alternative scoring ranges described in Table 5-1.
NHANES indicates National Health and Nutrition Examination Survey.
Sources: My Life Check: Life’s Simple 71; Lloyd-Jones et al2; Rehm et al.3
Chart 5-3. Healthy diet targets in adults (≥20 years of age) by survey year: NHANES 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010, and 2011 to 2012.
Components of poor, intermediate, and ideal diet are defined in Table 5-1. Percentages based on the alternative scoring ranges described in Table 5-1.
NHANES indicates National Health and Nutrition Examination Survey.
Sources: My Life Check: Life’s Simple 71; Lloyd-Jones et al2; Rehm et al.3
Chart 5-4. Trends in AHA-defined healthy diet score components for children (5–19 years old) by survey year: NHANES 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010, and 2011 to 2012.
Unscaled dietary score (maximum=50). Mean healthy diet score components based on the alternative scoring ranges described in Table 5-1.
AHA indicates American Heart Association; max., maximum; and NHANES, National Health and Nutrition Examination Survey.
Sources: My Life Check: Life’s Simple 71; Lloyd-Jones et al2; Rehm et al.3
Chart 5-5. Trends in AHA healthy diet score components for adults (≥20 years of age) by survey year: NHANES 2003 to 2004, 2005 to 2006, 2007 to 2008, 2009 to 2010, and 2011 to 2012.
Unscaled dietary score (maximum=50). Mean healthy diet score components based on the scoring ranges described in Table 5-1.
AHA indicates American Heart Association; and NHANES, National Health and Nutrition Examination Survey.
Sources: My Life Check: Life’s Simple 71; Lloyd-Jones et al2; Rehm et al.3
CVD Mortality
(See Charts 2-14 through 2-16)
Chart 2-14. US age-standardized death rates* from cardiovascular diseases, 2000 to 2014.
CHD indicates coronary heart disease; and CVD, cardiovascular disease.
*Directly standardized to the age distribution of the 2000 US standard population. †Total CVD: International Classification of Diseases, 10th Revision (ICD-10) I00 to I99, Q20 to Q28. §Stroke (all cerebrovascular disease): ICD-10 I60 to I69. ¶CHD: ICD-10 I20 to I25. **Other CVD: ICD-10 I00 to I15, I26 to I51, I70 to I78, I80 to I89, I95 to I99.
Source: Centers for Disease Control and Prevention, National Vital Health Statistics System.31
In 2014, the age-standardized death rate attributable to all CVD in the US population was 220.8 per 100 000, down 14.9% from 259.4 per 100 000 in 2007 (baseline data for the 2020 Impact Goals on CVD and stroke mortality)31 (Chart 2-14).
The age-standardized death rate in 2014 attributable to stroke was 36.5 per 100 000, a decrease of 16.1% from 2007. The death rate attributable to CHD in 2014 was 98.8 per 100 000, a reduction of 23.5% from 2007. The rate for other CVDs was 84.6 per 100 000 in 2014, similar to the rate in 200731 (Chart 2-14).
Between 2007 and 2013, CVD and stroke death rates decreased 12.5% and 16.1% respectively in non-Hispanic whites; 16.5% and 20.2% in non-Hispanic blacks; 18.1% and 17.3% in Hispanics; 15.0% and 19.6% in non-Hispanic Asian and Pacific Islanders; and 11.3% and 22.5% in non-Hispanic American Indian or Alaska Natives31 (Charts 2-15 and 2-16).
Chart 2-15. US age-standardized death rates* from cardiovascular disease by race/ethnicity, 2000 to 2014.
NH indicates non-Hispanic.
*Directly standardized to the age distribution of the 2000 US standard population. Total CVD: International Classification of Diseases, 10th Revision (ICD-10) I00 to I99, Q20 to Q28.
Source: Centers for Disease Control and Prevention, National Vital Statistics System.31
Achieving the 2020 Impact Goals
(See Tables 2-3 through 2-6)
To achieve the AHA’s 2020 Impact Goals of reducing deaths attributable to CVD and stroke by 20%, continued emphasis is needed on the treatment of acute CVD events and secondary prevention through treatment and control of health behaviors and risk factors.
Taken together, these data continue to demonstrate both the tremendous relevance of the AHA 2020 Impact Goals for cardiovascular health and the progress that will be needed to achieve these goals over by the year 2020.
For each cardiovascular health metric, modest shifts in the population distribution toward improved health would produce appreciable increases in the proportion of Americans in both ideal and intermediate categories. For example, on the basis of NHANES 2013 to 2014, the current prevalence of ideal levels of BP among US adults is 45.4%. To achieve the 2020 goals, a 20% relative improvement would require an increase in this proportion to 54.4% by 2020 (45.4% × 1.20). On the basis of NHANES data, a reduction in population mean BP of just 5 mm Hg would result in 55.3% of US adults having ideal levels of BP, which represents a 21.8% relative improvement in this metric (Table 2-3). Larger population reductions in BP would lead to even greater numbers of people with ideal levels of BP. Such small reductions in population BP could result from small health behavior changes at a population level, such as increased PA, increased fruit and vegetable consumption, decreased sodium intake, decreased adiposity, or some combination of these and other lifestyle changes, with resulting substantial projected decreases in CVD rates in US adults.32
-
A range of complementary strategies and approaches can lead to improvements in cardiovascular health. These include the following:
Individual-focused approaches, which target lifestyle and treatments at the individual level (Table 2-4)
Healthcare systems approaches, which encourage, facilitate, and reward efforts by providers to improve health behaviors and health factors (Table 2-5)
Population approaches, which target lifestyle and treatments in schools or workplaces, local communities, and states, as well as throughout the nation (Table 2-6)
Such approaches can focus on both (1) improving cardiovascular health among those who currently have less than optimal levels and (2) preserving cardiovascular health among those who currently have ideal levels (in particular, children, adolescents, and young adults) as they age.
The metrics with the greatest potential for improvement in the United States are health behaviors, including diet quality, PA, and body weight. However, each of the 7 cardiovascular health metrics can be improved and deserves major focus.
Table 2-3.
Reduction in BP Required to Increase Prevalence of Ideal BP Among Adults ≥20 Years Old: NHANES 2013 to 2014
| % | |
|---|---|
| Percent BP ideal among adults, 2013–2014 | 45.4 |
| 20% Relative increase | 54.4 |
| Percent whose BP would be ideal if population mean BP were lowered by* | |
| 2 mm Hg | 49.5 |
| 3 mm Hg | 51.7 |
| 4 mm Hg | 52.9 |
| 5 mm Hg | 55.3 |
Reduction in BP=(observed average systolic BP–X mm Hg) and (observed average diastolic BP–X mm Hg). BP indicates blood pressure; and NHANES, National Health and Nutrition Examination Survey.
Standardized to the age distribution of the 2000 US standard population.
Table 2-4.
Evidence-Based Individual Approaches for Improving Health Behaviors and Health Factors in the Clinic Setting
|
|
|
|
|
|
|
|
Examples of approaches include mastery experiences (set a reasonable, proximal goal that the person can successfully achieve); vicarious experiences (have the person see someone with similar capabilities performing the behavior, such as walking on a treadmill or preparing a healthy meal); physiological feedback (explain to the patient when a change in their symptoms is related to worse or improved behaviors); and verbal persuasion (persuade the person that you believe in their capability to perform the behavior).
Motivational interviewing represents use of individual counseling to explore and resolve ambivalence toward changing behavior. Major principles include fostering the person’s own awareness and resolution of their ambivalence, as well as their own self-motivation to change, in a partnership with the counselor or provider.
Modified from Artinian et al.33 with permission. Copyright © 2010, American Heart Association, Inc.
Table 2-5.
Evidence-Based Healthcare Systems Approaches to Support and Facilitate Improvements in Health Behaviors and Health Factors34–38
|
|
|
|
|
|
|
BP indicates blood pressure; and PA, physical activity.
REFERENCES
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. My Life Check: Life’s Simple 7. [Accessed July 1, 2016];American Heart Association Web site. http://mylifecheck.heart.org/
- 3.Rumsfeld JS, Alexander KP, Goff DC, Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ American Heart Association Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 4.Graciani A, García-Esquinas E, López-García E, Banegas JR, Rodríguez-Artalejo F. Ideal cardiovascular health and risk of frailty in older adults. Circ Cardiovasc Qual Outcomes. 2016;9:239–245. doi: 10.1161/CIRCOUTCOMES.115.002294. [DOI] [PubMed] [Google Scholar]
- 5.Shay CM, Gooding HS, Murillo R, Foraker R. Understanding and improving cardiovascular health: an update on the American Heart Association’s concept of cardiovascular health. Prog Cardiovasc Dis. 2015;58:41–49. doi: 10.1016/j.pcad.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, Yang Y, Wang A, Cao Y, Li J, Yang Y, Zhang K, Zhang W, Wu S, Wang Z, Zhu M, Zhang Y, Wu S, Wang C, Zhao X. Association of ideal cardiovascular health metrics and cognitive functioning: the APAC study. Eur J Neurol. 2016;23:1447–1454. doi: 10.1111/ene.13056. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen TT, Pahkala K, Magnussen CG, Oikonen M, Viikari JS, Sabin MA, Daniels SR, Heinonen OJ, Taittonen L, Hartiala O, Mikkilä V, Hutri-Kähönen N, Laitinen T, Kähönen M, Raitakari OT, Juonala M. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2015;185:186–191. doi: 10.1016/j.ijcard.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, Yang H, Sun G, Sun Y. Prevalence and Metrics Distribution of Ideal Cardiovascular Health: A Population-based, Cross-sectional Study in Rural China. Heart Lung Circ. 2016;25:982–992. doi: 10.1016/j.hlc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen T. Cardiovascular health from childhood to adulthood: with special reference to early vascular changes: The Cardiovascular Risk in Young Finns Study [master’s thesis] Turku, Finland: University of Turku; 2015. [Accessed September 3, 2015]. http://www.doria.fi/bitstream/handle/10024/104430/AnnalesD%201174Laitinen.pdf?sequence=2. [Google Scholar]
- 10.Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, Virani SS, Maziak W, Agatston AS, Veledar E, Nasir K. A Systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin Proc. 2016;91:649–670. doi: 10.1016/j.mayocp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 11.González HM, Tarraf W, Rodríguez CJ, Gallo LC, Sacco RL, Talavera GA, Heiss G, Kizer JR, Hernandez R, Davis S, Schneiderman N, Daviglus ML, Kaplan RC. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J. 2016;176:134–144. doi: 10.1016/j.ahj.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol. 2016;214:279–283. doi: 10.1016/j.ijcard.2016.03.210. [DOI] [PubMed] [Google Scholar]
- 17.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple 7 and risk of incident stroke: the Reasons for Geographic and Racial Differences in Stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976.e2. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins JM, Petrone AB, Carr JJ, Pankow JS, Hunt SC, Heiss G, Arnett DK, Ellison RC, Gaziano JM, Djoussé L. Association of ideal cardiovascular health and calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2015;169:371–378.e1. doi: 10.1016/j.ahj.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleem Y, DeFina LF, Radford NB, Willis BL, Barlow CE, Gibbons LW, Khera A. Association of a favorable cardiovascular health profile with the presence of coronary artery calcification. Circ Cardiovasc Imaging. 2015;8:e001851. doi: 10.1161/CIRCIMAGING.114.001851. [DOI] [PubMed] [Google Scholar]
- 22.Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PLoS One. 2014;9:e89317. doi: 10.1371/journal.pone.0089317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life’s Simple 7 and incident cognitive impairment: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635. doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One. 2012;7:e52771. doi: 10.1371/journal.pone.0052771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.España-Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, Pate RR, Blair SN. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics. 2013;54:525–535. doi: 10.1016/j.psym.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Dhamoon MS, Dong C, Elkind MS, Sacco RL. Ideal cardiovascular health predicts functional status independently of vascular events: the Northern Manhattan Study. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.114.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caleyachetty R, Echouffo-Tcheugui JB, Muennig P, Zhu W, Muntner P, Shimbo D. Association between cumulative social risk and ideal cardiovascular health in US adults: NHANES 1999–2006. Int J Cardiol. 2015;191:296–300. doi: 10.1016/j.ijcard.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Fang J, Yang Q, Hong Y, Loustalot F. Status of cardiovascular health among adult Americans in the 50 states and the District of Columbia, 2009. J Am Heart Assoc. 2012;1:e005371. doi: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huffman MD, Lloyd-Jones DM, Ning H, Labarthe DR, Guzman Castillo M, O’Flaherty M, Ford ES, Capewell S. Quantifying options for reducing coronary heart disease mortality by 2020. Circulation. 2013;127:2477–2484. doi: 10.1161/CIRCULATIONAHA.112.000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. CDC WONDER Online Database [database online] Atlanta, GA: Centers for Disease Control and Prevention; [Accessed July 1, 2016]. Compressed mortality file: underlying cause of death 1999–2014. http://wonder.cdc.gov/mortSQl.html. [Google Scholar]
- 32.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart-Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston-Miller N, Burke LE American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR, Jr, Kraus WE, Kris-Etherton PM, Krummel DA, Popkin BM, Whitsel LP, Zakai NA American Heart Association Council on Epidemiology and Prevention, Council on Nutrition, Physical Activity and Metabolism, Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovascular Disease, Council on Peripheral Vascular Disease, and the Advocacy Coordinating Committee. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodenheimer T. Helping patients improve their health-related behaviors: what system changes do we need? Dis Manag. 2005;8:319–330. doi: 10.1089/dis.2005.8.319. [DOI] [PubMed] [Google Scholar]
- 36.Simpson LA, Cooper J. Paying for obesity: a changing landscape. Pediatrics. 2009;123(suppl 5):S301–S307. doi: 10.1542/peds.2008-2780I. [DOI] [PubMed] [Google Scholar]
- 37.Quist-Paulsen P. Cessation in the use of tobacco: pharmacologic and non-pharmacologic routines in patients. Clin Respir J. 2008;2:4–10. doi: 10.1111/j.1752-699X.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- 38.Davis D, Galbraith R American College of Chest Physicians Health and Science Policy Committee. Continuing medical education effect on practice performance: effectiveness of continuing medical education: American College of Chest Physicians Evidence-Based Educational Guidelines. Chest. 2009;135(suppl):42S–48S. doi: 10.1378/chest.08-2517. [DOI] [PubMed] [Google Scholar]
3. SMOKING/TOBACCO USE
See Table 3-1 and Charts 3-1, 3-2, 3-3, 3-4, 3-5, 3-6
Table 3-1.
Prevalence (%) of Current Cigarette Smoking for Adults ≥18 Years of Age by Sex, Race/Ethnicity, and Income Status (NHIS, 2015)
| Population Group | Prevalence, 2015* (Age ≥18 y) (%) | Cost7 |
|---|---|---|
| Both sexes | 37 662 000 (15.2) | $289 Billion per year |
| Males | 20 143 000 (16.7) | … |
| Females | 17 421 000 (13.7) | … |
| NH white males | 17.8 | … |
| NH white females | 16.8 | … |
| NH black males | 20.3 | … |
| NH black females | 13.1 | … |
| Hispanic or Latino males | 12.7 | … |
| Hispanic or Latino females | 7.0 | … |
| NH Asian-only males | 11.6 | |
| NH Asian-only females | 2.6 | … |
| NH American Indian/Alaska Native–only males | 20.9 | |
| NH American Indian/Alaska Native–only females | 22.2 | … |
| Living at <100% of poverty level† | 27.8 | … |
| Living at 100%–199% of poverty level† | 23.6 | … |
| Living at ≥200% of poverty level† | 15.0 | … |
Percentages are age adjusted. Estimates for Asian-only and American Indian/Alaska Native–only include non-Hispanic and Hispanic people. Ellipses (…) indicate data not available; and NH, non-Hispanic.
Rounded to the nearest thousand; based on total resident population.
Estimates are for 2011 to 2013.
Data derived from the National Center for Health Statistics.6
Chart 3-1. Prevalence (%) of cigarette use in the past month for adolescents aged 12 to 17 years by sex and race/ethnicity (NSDUH, 2014).
Because of methodological differences among the NSDUH, the Youth Risk Behavior Survey, the National Youth Tobacco Survey, and other surveys, percentages of cigarette smoking measured by these surveys are not directly comparable. Notably, schoolbased surveys may include students who are 18 years old, who are legally permitted to smoke and have higher rates of smoking.
AIAN indicates American Indian or Alaska Native; NH, non-Hispanic; and NSDUH, National Survey on Drug Use and Health.
Data derived from Substance Abuse and Mental Health Services Administration, NSDUH.4
Chart 3-2. Prevalence (%) of cigarette smoking for adolescents (past month) and adults (current) by sex and age (NHIS, 2003–2015; NSDUH, 2003–2014).
NSDUH indicates National Survey on Drug Use and Health; and NHIS, National Health Interview Survey.
Data derived from the Centers for Disease Control and Prevention/National Center for Health Statistics and the Substance Abuse and Mental Health Services Administration (NSDUH).4,6
Chart 3-3. Long-term trend in current cigarette smoking prevalence (%) for adults ≥18 years of age by sex (NHIS, 1965–2015, selected years).
NHIS indicates National Health Interview Survey.
Data derived from the Centers for Disease Control and Prevention/National Center for Health Statistics, Health, United States, 2015 (NHIS).71
Chart 3-4. Prevalence (%) of current cigarette smoking for adults ≥18 years of age by sex and race/ethnicity (NHIS, 2013–2015).
All percentages are age adjusted.
AIAN indicates American Indian/Alaska Native; NH, non-Hispanic; and NHIS, National Health Interview Survey.
Data derived from Centers for Disease Control and Prevention/National Center for Health Statistics, NHIS.6
Chart 3-5. Prevalence (%) of current cigarette smoking for adults, by state: United States (BRFSS, 2014).
BRFSS indicates Behavior Risk Factor Surveillance System.
Data derived from the Centers for Disease Control and Prevention.8
Chart 3-6. Percentage (%) of students who have ever tried electronic cigarettes by school level (National Youth Tobacco Survey, 2011–2013).
Data derived from the Centers for Disease Control, National Youth Tobacco Survey.56
Tobacco smoking, the most common form of tobacco use, is a major risk factor for CVD and stroke.1 The AHA has identified never having tried smoking or never having smoked a whole cigarette (for children) and never having smoked or having quit >12 months ago (for adults) as 1 of the 7 components of ideal cardiovascular health (Life’s Simple Seven).2 According to NHANES 2013 to 2014 data, 91.4% of adolescents and 77.1% of adults met these criteria. Throughout the rest of the chapter, smoking prevalence is estimated by the NSDUH for adolescents (12–17 years of age) and by the NHIS for adults (≥18 years of age).
Other forms of tobacco use are becoming increasingly common. Electronic cigarette (e-cigarette) use, which involves inhalation of a vaporized liquid that includes nicotine, solvents, and flavoring (“vaping”), has risen dramatically, particularly among young people. The variety of e-cigarette—related products has increased dramatically, giving rise to the more general term electronic nicotine delivery systems.3 Use of cigarillos and other mass market cigars, hookahs, and water pipes has also become increasingly common in recent years.
Abbreviations Used in Chapter 3
| ACS | acute coronary syndrome |
| AHA | American Heart Association |
| AIAN | American Indian or Alaska Native |
| AMI | acute myocardial infarction |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| EAGLES | Study Evaluating the Safety and Efficacy of Varenicline and Bupropion for Smoking Cessation in Subjects With and Without a History of Psychiatric Disorders |
| EVITA | Evaluation of Varenicline in Smoking Cessation for Patients Post-Acute Coronary Syndrome |
| HD | heart disease |
| HIV | human immunodeficiency virus |
| HR | hazard ratio |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| NHIS | National Health Interview Survey |
| NSDUH | National Survey on Drug Use and Health |
| PAR | population attributable risk |
| RCT | randomized controlled trial |
| RR | relative risk |
| SAH | subarachnoid hemorrhage |
| SBP | systolic blood pressure |
| WHO | World Health Organization |
Prevalence
Youth
(See Charts 3-1 through 3-2)
-
According to the NSDUH 2014 data, for adolescents aged 12 to 17 years4:
-
In the past 30 days
4.9% (1.2 million) currently smoked cigarettes.
Of adolescents who smoked, 24.1% (292 000) smoked cigarettes daily, and 11.9% smoked ≥1 pack per day in the past month.
2.1% (521 000) were current cigar smokers, 0.7% (179 000) were current pipe tobacco smokers, and 2% (490 000) used smokeless tobacco.
The percentage reporting use of tobacco products in the past month decreased from 15.2% in 2002 to 7.0% in 2014.
Male adolescents were more likely than female adolescents to report use in the past month of cigars (2.7% compared with 1.5%) and smokeless tobacco (3.3% compared with 0.6%).
18.5% had used tobacco products in their life-time, and 12.7% had done so in the past year.
The prevalence of tobacco product use was higher in male adolescents (20.2%) than female adolescents (16.6%) over their lifetimes, as well as over the past year (14.3% of male adolescents, 11.0% of female adolescents) and month (8.2% of male adolescents, 5.8% of female adolescents).
-
The lifetime use of tobacco products in adolescents also varied by
Race/ethnicity: lifetime use was highest in American Indians or Alaskan Natives (27.1%), followed by whites (21.6%), Hispanics or Latinos (16.8%), blacks or African Americans (12.7%), and Asians (6.9%).
Geographic division: The highest lifetime use was observed in the South (East South Central 19.8%), and the lowest was observed in New England (10.6%).
White adolescents (9.1%) were more likely than Hispanic (5.0%), black or African American (4.0%), or Asian (1.9%) adolescents 12 to 17 years of age to report any tobacco use, which included cigarettes, cigars, and smokeless tobacco.
Current cigarette use increases sharply when it becomes legal for adolescents at 18 years of age. In 2014, 24.0 % of adults aged 18 to 20 years were current smokers compared with 10.2% of adolescents aged 16 to 17 years. In most states, the minimum age for purchasing tobacco products is 18 years of age. However, Alabama, Alaska, New Jersey, Utah, New York City, and some other communities have set higher minimum ages. The Institute of Medicine’s review of the literature concluded that increasing the minimum age to legally purchase tobacco products would likely prevent or delay tobacco use by adolescents and young adults, especially those aged 15 to 17 years.5
-
Adults
(See Table 3-1 and Charts 3-3 through 3-5)
Since the US Surgeon General’s first report on the health dangers of smoking, age-adjusted rates of smoking among adults have declined, from 51% of males smoking in 1965 to 16.7% in 2015 and from 34% of females in 1965 to 13.7% in 2015 (Table 3-1 and Chart 3-3; NHIS).6 The decline in smoking, along with other factors (including improved treatment and reductions in the prevalence of risk factors such as uncontrolled hypertension and high cholesterol), is a contributing factor in the sharp decline in the HD death rate during this period.7
-
According to the NHIS 2013–2015 data, among adults ≥18 years of age (Chart 3-4)6:
NH Asian males (13.4%) and Hispanic males (14.3%) were less likely to be current cigarette smokers than NH American Indian or Alaska Native males (25.6%), NH white males (19.8%), and NH black males (20.9%). Similarly, in 2015, NH Asian females (4.1%) and Hispanic females (7.1%) were less likely to be current cigarette smokers than NH black females (13.8%), NH white females (17.9%), and NH American Indian or Alaska Native females (24.8%).
By region, the prevalence of current cigarette smokers was highest in the Midwest (20.7%) and lowest in the West (13.1%).8,9
In 2014, according to the BRFSS, the states with the highest percentage of current cigarette smokers were West Virginia (26.7%), Kentucky (26.2%), Arkansas (24.7%), Tennessee (24.2%), Louisiana (24.0%), Mississippi (23.0%), Indiana (22.9%), and South Carolina (21.5%), compared with the US average of 16.8%8 (Chart 3-5).
In 2014, the states with the lowest percentage of current cigarette smokers were Utah (9.7%), Puerto Rico (11.3%), California (12.8%), Hawaii (14.1%), New York (14.4%), and Texas (14.5%); all percentages were significantly lower than the US average (16.8%)8,9 (Chart 3-5).
On the basis of NHIS data, current smoking status among 18- to 24-year-old males declined 21.1%, from 23.9% in 2005 to 18.5% in 2014, and for 18- to 24-year-old females, smoking declined 28.4%, from 20.7% to 14.8%, over the same time period.9 On the basis of age-adjusted estimates in 2014, among people ≥65 years of age, 9.8% of males and 7.5% of females were current smokers.9
Smoking prevalence increases as family income declines. Among adults ≥18 years of age living below the poverty level, 26.3% were current smokers in 2014. In comparison, among adults ≥18 years of age living at or above the poverty level, 15.2% were current smokers.9
In 2014, smoking prevalence was higher among adults ≥18 years of age who reported having a disability or activity limitation (21.9%) than among those reporting no disability or limitation (16.1%).9
People with mental illness are more than twice as likely to smoke as people without mental illness.10
Likewise, lifetime tobacco use for people with psychiatric diagnoses is high, with rates of 56.1%, 55.6%, and 70.1% in patients with mood disorders, anxiety disorders, and schizophrenia, respectively.11
From 2004 to 2011, adjusted prevalence rates for tobacco use in mentally ill populations did not decline significantly; however, rates for people who are not mentally ill declined from 19.2% to 16.5%.12
In 2012 to 2013, among females 15 to 44 years of age, past-month cigarette use was lower among those who were pregnant (15.4%) than among those who were not pregnant (24.0%). Rates were higher among females 18 to 25 years of age (21.0% versus 26.2% for pregnant and nonpregnant females, respectively) than among females 26 to 44 years of age (11.8% versus 25.4%, respectively). Smoking declines by pregnancy trimester, from 19.9% of females 15 to 44 years of age in the first trimester of pregnancy to 12.8% in the third trimester (NSDUH).13
Incidence
-
According to 2014 NSDUH4:
Approximately 2.16 million people ≥12 years of age smoked cigarettes for the first time within the past 12 months, down from 2.3 million in 2012. The 2012 estimate averages out to ≈5700 new cigarette smokers every day. Half of new smokers aged ≥12 in 2013 (38.7%) were <12 to 17 years of age when they first smoked cigarettes (NSDUH); 93.3% were 12 to 25 years of age. Unfortunately, the number of new smokers did not change significantly between 2013 and 2014.
The number of new smokers 12 to 17 years of age (83 800) was down from 2002 (1.3 million); new smokers 18 to 25 years of age increased from ≈600 000 in 2002 to 1.181 million in 2014.
-
In the NHIS, between 2005 and 2014:
Among people 12 to 49 years of age who had started smoking within the past 12 months, the average age of first cigarette use was 17.8 years, the same as in 2012.
Mortality
In 2010, tobacco smoking was the second-leading risk factor for death in the United States, after dietary risks.14
Smoking was responsible for >480 000 premature deaths in the United States annually from 2005 to 2009 among those ≥35 years of age. Furthermore, almost one third of deaths of CHD are attributable to smoking and secondhand smoke exposure.7
If current smoking trends continue, 5.6 million US children will die prematurely during adulthood of smoking.7
From 2005 to 2009, smoking during pregnancy resulted in an estimated 970 infant deaths annually.7
Each year from 2005 to 2009, an estimated 41 000 US deaths were attributable to exposure to secondhand smoke among those ≥35 years of age.7
In 2009, smoking was estimated to cause 3.3 million years of potential life lost for males and 2.2 million years for females, excluding deaths attributable to smoking-attributable residential fires and adult deaths attributable to secondhand smoke.7
Recent analysis found that in addition to the known risk of death attributable to CVD and stroke that is related to smoking, the risk of mortality attributable to hypertensive HD and hypertensive renal disease is related to smoking.15
On average, male smokers die 13.2 years earlier than male nonsmokers, and female smokers die 14.5 years earlier than female nonsmokers.1
Overall mortality among US smokers is 3 times higher than that for never-smokers.16
Increased CVD mortality risks persist for older (≥60 years old) smokers as well. A recent meta-analysis comparing CVD risks in 503 905 cohort participants ≥60 years of age reported an HR for cardiovascular mortality of 2.07 (95% CI, 1.82 to 2.36) compared with never smokers and 1.37 (95% CI, 1.25 to 1.49) compared with former smokers.17
In a sample of Native Americans (Strong Heart Study), among whom the prevalence of tobacco use is highest in the United States, the PAR for total mortality was 18.4% for males and 10.9% for females.18
Since the first report on the dangers of smoking was issued by the US Surgeon General in 1964, tobacco control efforts have contributed to a reduction of 8 million premature smoking-attributable deaths.19
Cardiovascular Health Impact
A 2010 report of the US Surgeon General on how tobacco causes disease summarized an extensive body of literature on smoking and CVD and the mechanisms through which smoking is thought to cause CVD.20 There is a sharp increase in CVD risk with low levels of exposure to cigarette smoke, including secondhand smoke, and a less rapid further increase in risk as the number of cigarettes per day increases. Similar health risks for CHD events are reported with regular cigar smoking as well.21
Smoking is an independent risk factor for CHD and appears to have a multiplicative effect with the other major risk factors for CHD: high serum levels of lipids, untreated hypertension, and DM.20
Cigarette smoking and other traditional CHD risk factors may have a synergistic interaction in HIV-positive individuals.22
A meta-analysis of 75 cohort studies (≈2.4 million individuals) demonstrated a 25% greater risk for CHD in female smokers when compared with male smokers (RR, 1.25; 95% CI, 1.12 to 1.39).23
Cigarette smoking is an independent risk factor for both ischemic stroke and SAH and has a synergistic effect on other stroke risk factors such as SBP24 and oral contraceptive use.25,26
A meta-analysis comparing pooled data of ≈3.8 million smokers and nonsmokers found a similar risk of stroke associated with current smoking in females and males.27
Current smokers have a 2 to 4 times increased risk of stroke compared with nonsmokers or those who have quit for >10 years.28,29
Acute exposure to water pipe smoking is associated with a significant increase in SBP and heart rate compared with nonsmoking control subjects,30 but long-term effects remain unclear. Current use of smokeless tobacco is associated with an increased risk of CVD events in cigarette nonsmokers.31
Cost
Each year from 2005 to 2009, US smoking-attributable economic costs were between $289 billion and $333 billion, including $133 billion to $176 billion for direct medical care of adults and $151 billion for lost productivity related to premature death.7
In the United States, cigarette smoking is associated with 9% of annual aggregated healthcare spending.32
In 2008, $9.94 billion was spent on marketing cigarettes in the United States.33
Cigarette prices in the United States have increased 283% between the early 1980s and 2011, in large part because of excise taxes on tobacco products. Higher taxes have decreased cigarette consumption, which fell from ≈30 million packs sold in 1982 to ≈14 million packs sold in 2011.33
Smoking Prevention
Tobacco 21 Laws increase the minimum age of sale for tobacco products from 18 to 21 years old. Such legislation would likely reduce the rates of smoking during adolescence, a time during which the majority of smokers start smoking, by limiting access, because most people who buy cigarettes for adolescents are <21 years of age. In several towns where Tobacco 21 laws have been enacted, 47% reductions in smoking prevalence among high school students have been reported.34 Furthermore, the Institute of Medicine estimates that a nationwide Tobacco 21 law would result in 249 000 fewer premature deaths, 45 000 fewer lung cancer deaths, and 4.2 million fewer lost life-years among Americans born between 2010 and 2019.35 As of 2016, 125 localities and the states of Hawaii and California have adopted Tobacco 21 laws, and momentum is building. A federal Tobacco 21 law was introduced to the Congress in September 2015; review and a vote are pending.
Smoking Cessation
-
Smoking cessation reduces the risk of cardiovascular morbidity and mortality for smokers with and without CHD.
There is no convincing evidence to date that reducing the amount smoked by smoking fewer cigarettes per day reduces the risk of CVD, although in several studies a dose-response relationship has been seen among current smokers between the number of cigarettes smoked per day and CVD incidence.20
Quitting smoking at any age significantly lowers mortality from smoking-related diseases, and the risk declines more the longer the time since quitting smoking.36 Cessation appears to have both short-term (weeks to months) and long-term (years) benefits for lowering CVD risk. Overall, risk appears to approach that of nonsmokers after ≈10 years of cessation.
Smokers who quit smoking at 25 to 34 years of age gained 10 years of life compared with those who continued to smoke. Those aged 35 to 44 years gained 9 years and those aged 45 to 54 years gained 6 years of life, on average, compared with those who continued to smoke.16
In 2010, 48.3% of adult current smokers ≥18 years of age who had a health checkup during the preceding year reported that they had been advised to quit. Smokers between 18 and 24 (31%) and 24 to 44 (44%) years of age were less likely to be advised to quit than those at older ages (57%; NHIS).37
Cessation medications (including sustained-release bupropion, varenicline, and nicotine gum, lozenge, nasal spray, and patch) are effective for helping smokers quit.38
EVITA was an RCT that examined the efficacy of varenicline versus placebo for smoking cessation among smokers who were hospitalized for ACS. At 24 weeks, rates of smoking abstinence and reduction were significantly higher among patients randomized to varenicline. Point-prevalence abstinence rates were 47.3% in the varenicline group and 32.5% in the placebo group (P=0.012; number needed to treat=6.8). Continuous abstinence rates and reduction rates (≥50% of daily cigarette consumption) were also higher in the varenicline group.39
The recently reported EAGLES trial demonstrated efficacy and safety of 12 weeks of varenicline, bupropion, or nicotine patch in motivated-to-quit smoking patients with major depressive disorder, bipolar disorder, anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, social phobia, psychotic disorders including schizophrenia and schizoaffective disorders, and borderline personality disorder. Of note, these participants were all clinically stable from a psychiatric perspective and were believed not to be at high risk for self-injury.40
Extended use of a nicotine patch (24 weeks compared with 8 weeks) has been demonstrated to be safe and efficacious in recent randomized clinical trials.41
An RCT demonstrated the effectiveness of individual- and group-oriented financial incentives for tobacco abstinence through at least 12 months of follow-up.42
In addition to medications, smoke-free policies, increases in tobacco prices, cessation advice from healthcare professionals, and quit-lines and other counseling have contributed to smoking cessation.37
In 2010, 52.4% of adult smokers reported having tried to quit smoking in the past year; 6.2% reported they had recently quit smoking. Of those who tried to quit smoking, 30.0% used cessation medications.37
The majority of ex-smokers report that they quit without any formal assistance.43
Mass media antismoking campaigns, such as the CDC’s Tips campaign (Tips From Former Smokers), have been shown to reduce smoking-attributable morbidity and mortality and are cost-effective.44
Despite states having collected $25.6 billion in 2012 from the 1998 Tobacco Master Settlement Agreement and tobacco taxes, <2% of those funds are spent on tobacco prevention and cessation programs. In addition, progress in passing and raising tobacco taxes and enacting smoke-free laws has slowed.45
In 2014, a major drug store chain, CVS Caremark Corporation, stopped selling tobacco products, a step that could reduce access to tobacco products and therefore encourage some smokers to quit.46
Electronic Cigarettes
(See Chart 3-6)
Electronic nicotine delivery systems, more commonly called electronic cigarettes or e-cigarettes, are battery-operated devices that deliver nicotine, flavors, and other chemicals to the user in an aerosol. Although e-cigarettes were introduced less than a decade ago, there are currently >450 e-cigarette brands on the market, and sales in the United States were projected to be $2 billion in 2014.47
Because these products have not been well studied, their risks and benefits are not fully understood, although they are thought to have a lower risk of harmful effects than conventional cigarettes. 48,49 Specifically, the health risks from the inhaled nicotine, solvents, heavy metals, flavorings, and other chemicals in e-cigarettes are not entirely known.7,50,51 However, a recent cross-sectional study compared the effects of electronic cigarettes versus traditional cigarettes on markers of oxidative stress and endothelial function in healthy smokers and nonsmoker adults and found that e-cigarettes appeared to have a lesser impact.52 Until prospective cohort studies evaluating hard clinical outcomes become available, future studies are required to examine the association of e-cigarettes and markers reflecting long-term cardiovascular damage, such as coronary artery calcium.
In addition to uncertainty about the harmful effects to users, the risks associated with secondhand exposure to e-cigarettes have not been well studied. 48,49,53 Moreover, there is also the potential for thirdhand exposure with e-cigarettes, which results from tobacco toxins remaining on the surfaces in areas where people have smoked. A study found that thirdhand exposure levels differed depending on the surface and the e-cigarette brand.54
E-cigarettes could play a beneficial role in helping smokers reduce or eliminate their conventional cigarette smoking; however, there are concerns that e-cigarettes could be a gateway to nicotine addiction and tobacco use by nonsmokers, especially teenagers, or could promote relapse among former smokers.7,50,51
Teenagers are increasingly trying e-cigarettes. In 2015, e-cigarettes were the most commonly used tobacco product among middle school (5.3%) and high school (16.0%) students.55 In 2013, 3.0% of middle school students had ever tried e-cigarettes, up from 1.4% in 2011. In 2013, 11.9% of high school students had ever tried e-cigarettes, up from 4.7% in 201156 (Chart 3-6).
In 2014, 18.3 million US middle and high school students were exposed to e-cigarette advertising. 55 Among US adults during 2010 to 2013, awareness and use of e-cigarettes increased considerably, and more than one third of current cigarette smokers reported ever having used e-cigarettes.57
As of June 30, 2016, 46 states, Guam, and the US Virgin Islands had banned e-cigarettes sales to minors, and 8 states prohibited e-cigarette use in private worksites, restaurants, and bars.58
Many public health advocates are worried that e-cigarettes will reverse decades of efforts to denormalize smoking, which contributed to the decline in smoking.7,50,51,53
The answers to some of these questions may become clearer as the regulatory oversight of e-cigarettes becomes more defined.7 Currently, only e-cigarettes that are marketed for therapeutic purposes are regulated by the US Food and Drug Administration, but in April 2014, the US Food and Drug Administration proposed extending its tobacco product authorities to include e-cigarettes.59
In May 2016, the US Food and Drug Administration released its long-awaited final “deeming” regulations on noncigarette tobacco products, including e-cigarettes, cigars, little cigars, pipe tobacco, hookah pipes, and other tobacco products. Under this rule, e-cigarettes will be subjected to the same regulatory restriction as traditional cigarettes, including manufacture, marketing, and distribution. As a result, all e-cigarette products will need to undergo federal review to remain on the market, with the exception of products that entered the market before February 15, 2007. This action will also ban the sale of e-cigarettes to minors and require photo identification to purchase these products.60
Secondhand Smoke
-
Data from the US Surgeon General on the consequences of secondhand smoke indicate the following:
Nonsmokers who are exposed to secondhand smoke at home or at work increase their risk of developing CHD by 25% to 30%.20
Short exposures to secondhand smoke can cause blood platelets to become stickier, damage the lining of blood vessels, and decrease coronary flow velocity reserves, potentially increasing the risk of an AMI.20
Exposure to secondhand smoke increases the risk of stroke by 20% to 30%.7
Nearly 34 000 premature deaths of HD occur each year in the United States among nonsmokers.7
These data are supported by a recent meta-analysis of 23 prospective and 17 case-control studies of cardiovascular risks associated with second-hand smoke exposure demonstrated 18%, 23%, 23%, and 29% increased risks for total mortality, total CVD, CHD, and stroke, respectively, in those exposed to secondhand smoke.61
Data from the 2008 BRFSS from 11 states showed that the majority of people surveyed in each state reported having smoke-free home rules, ranging from 68.8% in West Virginia to 85.6% in Arizona.62
As of June 2016, 27 states and the District of Columbia had laws that prohibited smoking in indoor areas of worksites, restaurants, and bars; no states had such laws in 2000. As of June 2016, 9 states had no laws banning or restricting areas for smoking in private workplaces.63
In 2012, 30 of the 50 largest US cities prohibited indoor smoking in private workplaces, through either state or local ordinances.64
Pooled data from 17 studies in North America, Europe, and Australasia suggest that smoke-free legislation can reduce the incidence of acute coronary events by 10%.65
The percentage of the US nonsmoking population with serum cotinine ≥0.05 ng/mL (which indicates exposure to secondhand smoke) declined from 52.5% in 1999 to 2000 to 25.3% in 2011 to 2012, with declines occurring for both children and adults. During 2011 to 2012, the percentage of nonsmokers with detectable serum cotinine was 40.6% for those 3 to 11 years of age, 33.8% for those 12 to 19 years of age, and 21.3% for those ≥20 years of age. The percentage was also higher for non-Hispanic blacks (46.8%) than for non-Hispanic whites (21.8%) and Mexican Americans (23.9%). People living below the poverty level (43.2%) and those living in rental housing (36.8%) had higher rates of secondhand smoke exposure than their counterparts (21.1% of those living above the poverty level and 19.0% of those who owned their homes; NHANES).66
Global Burden of Smoking
Although tobacco use in the United States has been declining, tobacco use worldwide has climbed steeply and is currently responsible for 5 million deaths annually.45
The number of smokers was estimated to have grown from 721 million in 1980 to 967 million in 2012.67
Worldwide, tobacco smoking (including second-hand smoke) was 1 of the top 3 leading risk factors for disease and contributed to an estimated 6.2 million deaths in 2010.68
In 2004, 40% of children, 33% of male nonsmokers, and 35% of female nonsmokers were exposed to secondhand smoke, which was estimated to have caused 630 000 premature deaths.69
To help combat the global problem of tobacco exposure, in 2003 the WHO adopted the Framework Convention on Tobacco Control treaty. The WHO Framework Convention on Tobacco Control contains a set of universal standards to limit tobacco supply and demand worldwide. These standards include the use of tax policies to reduce tobacco consumption, a ban on the indoor use of tobacco products, implementation of educational programs about the dangers of tobacco use, and restrictions of the sale of tobacco products to international travelers. Since it came into force in 2005, 180 countries have ratified the WHO Framework Convention on Tobacco Control. 70
REFERENCES
- 1.The 2004 United States Surgeon General’s Report: The Health Consequences of Smoking. N S W Public Health Bull. 2004;15:107. [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) [Accessed June 8, 2016];Electronic Nicotine Delivery Systems Key Facts. 2014 https://chronicdata.cdc.gov/Policy/Electronic-Nicotine-Delivery-Systems-Key-Facts-Inf/nwhw-m4ki.
- 4.Center for Behavioral Health Statistics and Quality. [Accessed August 25, 2016];Behavioral Health Trends in the United States: Results From the 2014 National Survey on Drug Use and Health. 2015 http://www.samhsa.gov/data/
- 5.Institute of Medicine. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. Washington, DC: National Academy of Sciences; 2015. [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. [Accessed July 18, 2016];National Health Interview Survey. 2015 Public-use data file and documentation: NCHS tabulations http://www.cdc.gov/nchs/nhis/nhis_2015_data_release.htm.
- 7.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Accessed July 18, 2016];State Tobacco Activities Tracking and Evaluation (STATE) System. http://www.cdc.gov/statesystem/cigaretteuseadult.html.
- 9.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, King BA. Current cigarette smoking among adults: United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64:1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 10.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 11.Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug Alcohol Depend. 2011;119:28–36. doi: 10.1016/j.drugalcdep.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311:172–182. doi: 10.1001/jama.2013.284985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings [and detailed tables] Rockville, MD: Substance Abuse and Mental health Services Administration; 2014. [Accessed June 8, 2016]. http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. [Google Scholar]
- 14.Murray CJ, Abraham J, Ali MK, et al. The state of US Health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, Jacobs EJ. Smoking and mortality: beyond established causes. N Engl J Med. 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 16.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 17.Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O’Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H CHANCES Consortium. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, An Q, Yeh F, Zhang Y, Howard BV, Lee ET, Zhao J. Smoking-attributable mortality in American Indians: findings from the Strong Heart Study. Eur J Epidemiol. 2015;30:553–561. doi: 10.1007/s10654-015-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, Levy DT. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311:164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 21.Chang CM, Corey CG, Rostron BL, Apelberg BJ. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health. 2015;15:390. doi: 10.1186/s12889-015-1617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60:1415–1423. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 23.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Barzi F, Lam TH, Huxley R, Feigin VL, Ueshima H, Woo J, Gu D, Ohkubo T, Lawes CM, Suh I, Woodward M Asia Pacific Cohort Studies Collaboration. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke. 2008;39:1694–1702. doi: 10.1161/STROKEAHA.107.496752. [DOI] [PubMed] [Google Scholar]
- 25.Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:498–505. [PubMed] [Google Scholar]
- 26.Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348:505–510. [PubMed] [Google Scholar]
- 27.Peters SA, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke. 2013;44:2821–2828. doi: 10.1161/STROKEAHA.113.002342. [DOI] [PubMed] [Google Scholar]
- 28.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azar RR, Frangieh AH, Mroué J, Bassila L, Kasty M, Hage G, Kadri Z. Acute effects of waterpipe smoking on blood pressure and heart rate: a real-life trial. Inhal Toxicol. 2016;28:339–342. doi: 10.3109/08958378.2016.1171934. [DOI] [PubMed] [Google Scholar]
- 31.Yatsuya H, Folsom AR ARIC Investigators. Risk of incident cardiovascular disease among users of smokeless tobacco in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2010;172:600–605. doi: 10.1093/aje/kwq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48:326–333. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General, 2012. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Accessed May 30, 2012]. http://www.surgeongeneral.gov/library/reports/preventing-youth-tobacco-use/prevent_youth_by_section.html. [Google Scholar]
- 34.Kessel Schneider S, Buka SL, Dash K, Winickoff JP, O’Donnell L. Community reductions in youth smoking after raising the minimum tobacco sales age to 21. Tob Control. 2016;25:355–359. doi: 10.1136/tobaccocontrol-2014-052207. [DOI] [PubMed] [Google Scholar]
- 35.Morain SR, Winickoff JP, Mello MM. Have Tobacco 21 laws come of age? N Engl J Med. 2016;374:1601–1604. doi: 10.1056/NEJMp1603294. [DOI] [PubMed] [Google Scholar]
- 36.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-Year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults: United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 38.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, Cassavar D, Dion D, Haught H, Mehta SR, Baril JF, Lambert C, Madan M, Abramson BL, Dehghani P EVITA Investigators. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133:21–30. doi: 10.1161/CIRCULATIONAHA.115.019634. [DOI] [PubMed] [Google Scholar]
- 40.Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EA-GLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 41.Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Wileyto EP, Hitsman B. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504–511. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AL, Chapman S. Quitting smoking unassisted: the 50-year research neglect of a major public health phenomenon. JAMA. 2014;311:137–138. doi: 10.1001/jama.2013.282618. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Alexander RL, Jr, Simpson SA, Goates S, Nonnemaker JM, Davis KC, McAfee T. A cost-effectiveness analysis of the first federally funded antismoking campaign. Am J Prev Med. 2015;48:318–325. doi: 10.1016/j.amepre.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antman E, Arnett D, Jessup M, Sherwin C. The 50th anniversary of the US Surgeon General’s report on tobacco: what we’ve accomplished and where we go from here. J Am Heart Assoc. 2014;3:e000740. doi: 10.1161/JAHA.113.000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frieden T, Yox S. CDC Reacts to CVS ending tobacco sales: An interview with CDC Director Tom Frieden, MD, MPH. Medscape; Feb 6, 2014. [Accessed June 9, 2016]. http://www.medscape.com/viewarticle/820269. [Google Scholar]
- 47.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310:685–686. doi: 10.1001/jama.2013.109501. [DOI] [PubMed] [Google Scholar]
- 51.Fairchild AL, Bayer R, Colgrove J. The renormalization of smoking? E-cigarettes and the tobacco “endgame” [published correction appears in N Engl J Med. 2014;370:2354] N Engl J Med. 2014;370:293–295. doi: 10.1056/NEJMp1313940. [DOI] [PubMed] [Google Scholar]
- 52.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goniewicz ML, Lee L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res. 2015;17:256–258. doi: 10.1093/ntr/ntu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students–United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. [Accessed July 18, 2016];National Youth Tobacco Survey (NYTS) http://www.cdc.gov/tobacco/data_statistics/surveys/nyts/
- 57.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. [Accessed August 30, 2016];STATE System E-Cigarette Fact Sheet. https://chronicdata.cdc.gov/Legislation/STATE-System-E-Cigarette-Fact-Sheet/qte6-7jwd.
- 59.FDA proposes to extend its tobacco authority to additional tobacco products, including e-cigarettes [news release] Silver Spring, MD: US Food and Drug Administration; Apr 24, 2014. [Accessed July 18, 2016]. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394667.htm. [Google Scholar]
- 60.Food and Drug Administration, HHS. Deeming tobacco products to be subject to the federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products: final rule. Fed Regist. 2016;81:28973–29106. [PubMed] [Google Scholar]
- 61.Lv X, Sun J, Bi Y, Xu M, Lu J, Zhao L, Xu Y. Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int J Cardiol. 2015;199:106–115. doi: 10.1016/j.ijcard.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) State-specific secondhand smoke exposure and current cigarette smoking among adults: United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1232–1235. [PubMed] [Google Scholar]
- 63.Tynan MA, Holmes CB, Promoff G, Hallett C, Hopkins M, Frick B. State and local comprehensive smoke-free laws for worksites, restaurants, and bars: United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:623–626. doi: 10.15585/mmwr.mm6524a4. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention (CDC) Comprehensive smoke-free laws: 50 largest U.S. cities, 2000 and 2012. MMWR Morb Mortal Wkly Rep. 2012;61:914–917. [PubMed] [Google Scholar]
- 65.Mackay DF, Irfan MO, Haw S, Pell JP. Meta-analysis of the effect of comprehensive smoke-free legislation on acute coronary events. Heart. 2010;96:1525–1530. doi: 10.1136/hrt.2010.199026. [DOI] [PubMed] [Google Scholar]
- 66.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, Garrett BE, Sosnoff CS, Wang L Centers for Disease Control and Prevention (CDC) Vital signs: disparities in nonsmokers’ exposure to secondhand smoke: United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64:103–108. [PMC free article] [PubMed] [Google Scholar]
- 67.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 68.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. [Accessed July 18, 2016];The WHO Framework Convention on Tobacco Control: An Overview. 2003 http://www.who.int/fctc/about/en/index.html.
- 71.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2015. [Accessed August 24, 2016]. http://www.cdc.gov/nchs/data/hus/hus15.pdf. [PubMed] [Google Scholar]
4. PHYSICAL INACTIVITY
(See Table 4-1 and Charts 4-1, 4-2, 4-3, 4-4, 4-5, 4-6, 4-7, 4-8, 4-9, 4-10, 4-11)
Table 4-1.
Met 2008 Federal Aerobic and Strengthening PA Guidelines for Adults
| Population Group | Prevalence, 2015 (Age ≥18 y), % |
|---|---|
| Age 18 y and over, age adjusted | 21.5 |
| Age 18 y and over, crude | 20.9 |
| Both sexes | 21.6 |
| Males | 25.3 |
| Females | 17.9 |
| NH white | 23.4 |
| NH black | 19.8 |
| Hispanic or Latino | 16.8 |
| Asian | 19.1 |
| American Indian/Alaska Native | 18.9 |
“Met 2008 Federal Aerobic and Strengthening PA Guidelines for Adults” is defined as engaging in ≥150 min of moderate or 75 min of vigorous aerobic leisure-time physical activity per week (or an equivalent combination) and engaging in leisure-time strengthening physical activity at least twice a week. Data are age adjusted for adults ≥18 years of age.
NH indicates non-Hispanic; and PA, physical activity.
Source: National Health Interview Survey 2015 (National Center for Health Statistics).11
Chart 4-1. Prevalence of students in grades 9 to 12 who did not participate in ≥60 minutes of physical activity on any day in the past 7 days by race/ethnicity and sex.
NH indicates non-Hispanic.
Source: Youth Risk Behavior Surveillance Survey 2015.8
Chart 4-2. Prevalence of students in grades 9 to 12 who met currently recommended levels of physical activity during the past 7 days by race/ethnicity and sex.
“Currently recommended levels” was defined as activity that increased their heart rate and made them breathe hard some of the time for a total of ≥60 min/d on 5 of the 7 days preceding the survey.
NH indicates non-Hispanic.
Source: Youth Risk Behavior Surveillance Survey 2015.8
Chart 4-3. Percentage of students in grades 9 to 12 who used a computer for ≥3 hours on an average school day by race/ethnicity and sex.
NH indicates non-Hispanic.
Source: Youth Risk Behavior Surveillance Survey 2015.8
Chart 4-4. Prevalence of meeting the aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by sex and age (NHIS: 2015).
The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisuretime physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
NHIS indicates National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-5. Prevalence of meeting the aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by race/ethnicity and sex (NHIS: 2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
NH indicates non-Hispanic; and NHIS, National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-6. Prevalence of meeting the aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥25 years of age by educational attainment (NHIS: 2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
GED indicates General Educational Development; and NHIS, National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-7. Prevalence of meeting the aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by location of residence (NHIS: 2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
MSA indicates metropolitan statistical area; and NHIS, National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-8. Prevalence of meeting the aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by disability status (NHIS: 2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
NHIS indicates National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-9. Percent distribution of meeting the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by poverty level and type of activity (NHIS: 2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination and perform muscle-strengthening activities at least 2 d/wk.
NHIS indicates National Health Interview Survey.
Source: NHIS 2015 (National Center for Health Statistics).11
Chart 4-10. Prevalence of children 12 to 15 years of age who had adequate levels of cardiorespiratory fitness, by sex and age (NHANES, National Youth Fitness Survey: 2012).
NHANES indicates National Health and Nutrition Examination Survey.
Source: NHANES, National Youth Fitness Survey 2012.12
Chart 4-11. Trends in meeting the physical activity guidelines of the 2008 Federal Physical Activity Guidelines for Americans among adults ≥18 years of age by type of activity (NHIS: 1998–2015).
Percentages are age adjusted. The aerobic guidelines of the 2008 Federal Physical Activity Guidelines for Americans recommend engaging in moderate leisure-time physical activity for ≥150 min/wk or vigorous activity ≥75 min/wk or an equivalent combination.
NH indicates non-Hispanic; and NHIS, National Health Interview Survey.
Source: NHIS 1998 to 2015 (National Center for Health Statistics).11
Physical inactivity is a major risk factor for CVD and stroke.1 Meeting the guidelines for PA is one of the AHA’s 7 components of ideal cardiovascular health for both children and adults.2 The AHA and 2008 federal guidelines on PA recommend that children get at least 60 minutes of PA daily (including aerobic and muscle-and bone-strengthening activity). The guidelines recommend that adults get at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic activity (or an equivalent combination) per week and perform muscle strengthening activities at least 2 days per week.3 In 2011 to 2012, on the basis of survey interviews, 36.5% of children and 44.0% of adults met these criteria (NHANES).
Abbreviations Used in Chapter 4
| AHA | American Heart Association |
| BMI | body mass index |
| BNP | B-type natriuretic peptide |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CHD | coronary heart disease |
| CI | confidence interval |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| ED | emergency department |
| EF | ejection fraction |
| EPIC-Norfolk | European Prospective Investigation Into Cancer and Nutrition—Norfolk Cohort |
| FMD | flow-mediated dilation |
| GED | General Educational Development |
| HbA1c | hemoglobin A1c |
| HBP | high blood pressure |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HR | hazard ratio |
| IHD | ischemic heart disease |
| LDL-C | low-density lipoprotein cholesterol |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| MI | myocardial infarction |
| MSA | metropolitan statistical area |
| NAVIGATOR | A Multinational, Randomized, Double-Blind, Placebo-Controlled, Forced-Titration, 2 × 2 Factorial Design Study of the Efficacy and Safety of Longterm Administration of Nateglinide and Valsartan in the Prevention of Diabetes and Cardiovascular Outcomes in Subjects With Impaired Glucose Tolerance (IGT) |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| NHIS | National Health Interview Survey |
| PA | physical activity |
| PAD | peripheral artery disease |
| PAR | population attributable risk |
| QALY | quality-adjusted life-year |
| RCT | randomized controlled trial |
| RR | relative risk |
| SBP | systolic blood pressure |
| SD | standard deviation |
| TC | total cholesterol |
| WHI | Women’s Health Initiative |
| WHO | World Health Organization |
| YRBSS | Youth Risk Behavior Surveillance System |
Being physically active improves health, and being inactive is unhealthy.3 PA reduces premature mortality. In addition, PA improves risk factors for CVD (such as HBP and high cholesterol) and reduces the likelihood of diseases related to CVD, including CHD, stroke, type 2 DM, and sudden heart attacks.3 Benefits from PA are seen for all ages and groups, including older adults, pregnant females, and people with disabilities and chronic conditions. Therefore, the federal guidelines recommend being as physically active as abilities and conditions allow and, if not currently meeting the recommendations, increasing PA gradually.
Defining and Measuring PA
There are 4 dimensions of PA (mode or type, frequency, duration, and intensity) and 4 common domains (occupational, domestic, transportation, and leisure time). The federal guidelines specify the suggested frequency, duration, and intensity of activity. Historically, recommendations on PA for health purposes have focused on leisure-time activity. However, because all domains of PA could have an impact on health, and because an increase in 1 domain may sometimes be compensated for by a decrease in another domain, ideally data will be collected on all dimensions and domains of PA.
There are 2 broad categories of methods to assess PA: (1) subjective methods that use questionnaires and diaries/logs and (2) objective methods that use wearable monitors (pedometers, accelerometers, etc). Studies that compare the findings between subjective methods (respondent reported) and objective methods (such as wearable monitors, like pedometers or accelerometers) have found that there is marked discordance between self-reported and measured PA, with respondents often overstating their PA, especially the intensity.4,5 The majority of studies linking inactivity/PA to cardiovascular outcomes obtain PA data from respondent reports, which may overestimate the amount or intensity of activity.
Another consideration in the measurement of PA is that surveys often ask only about leisure-time PA. PA also may come from occupational, domestic, and transportation responsibilities. People who get a lot of PA from these other responsibilities may be less like to engage in leisure-time PA, and yet they may meet the federal PA guidelines, but they may be less likely to be identified as meeting the guidelines.
Chronic physical inactivity contributes to poor levels of cardiorespiratory (or aerobic) fitness, which is a stronger predictor of adverse cardiometabolic and cardiovascular outcomes than traditional risk factors. Although both PA and cardiorespiratory fitness are inversely related to the risk of CVD and other clinical outcomes, they are distinct measures in the assessment of CVD risk.6 PA is a behavior that can potentially improve cardiorespiratory fitness. Although many studies have shown that increasing the amount and quality of PA can improve cardiorespiratory fitness, other factors can contribute, such as a genetic predisposition to perform aerobic exercise.7 Because cardiorespiratory fitness is directly measured and reflects both participation in PA and the state of physiological systems affecting performance, the relationship between cardiorespiratory fitness and clinical outcomes is stronger than the relationship of PA to a series of clinical outcomes.6 Unlike health behaviors such as PA and risk factors that are tracked by federally funded programs, there are no national data on cardiorespiratory fitness; the development of a national cardiorespiratory fitness registry has been proposed.6 Such additional data on the cardiorespiratory fitness levels of Americans may give a fuller and more accurate picture of physical fitness levels.6
Prevalence
Youth
Inactivity
(See Chart 4-1)
In 2015 (YRBSS)8:
Nationwide, 14.3% of adolescents reported that they were inactive on all of the previous 7 days (that is, they did not participate in ≥60 minutes of any kind of PA that increased their heart rate and made them breathe hard on any 1 of the previous 7 days).
Girls were more likely than boys to report physical inactivity (17.5% versus 11.1%).
The prevalence of inactivity was highest among non-Hispanic black (25.2%) and Hispanic (19.2%) girls, followed by non-Hispanic black boys (16.2%), non-Hispanic white girls (14.3%), Hispanic boys (11.9%), and non-Hispanic white boys (8.8%). (Chart 4-1)
There was substantial variation by state. The state reporting the highest prevalence of inactivity was Mississippi (22.9%), whereas the lowest was Montana (10.7%).
Activity Recommendations
(See Chart 4-2)
-
In 2015 (YRBSS)8:
The prevalence of high school students who met activity recommendations of ≥60 minutes of PA on all 7 days of the week was 27.1% nationwide and declined from 9th (31.0%) to 12th (25.3%) grades. At each grade level, the prevalence was higher in boys than in girls.
More than double the percentage of high school boys (36.0%) than girls (17.7%) reported having been physically active ≥60 minutes per day on all 7 days.
The prevalence of students meeting currently recommended levels of physical activity (defined as having been physically active at least 60 minutes per day on at least 5 of the 7 days) was higher among non-Hispanic white boys (62.0%), non-Hispanic black boys (52.2%), and Hispanic boys (53.5%) than non-Hispanic white girls (43.5%), non-Hispanic black girls (33.4%), and Hispanic girls (33.1%) (Chart 4-2).
The proportion of students who participated in muscle strengthening activities on ≥3 days of the week was 53.4% nationwide and declined from 9th (males 67.3%, females 48.2%) to 12th (males 59.9%, females 39.9%) grades.
More high school boys (63.7%) than girls (42.7%) reported having participated in muscle-strengthening activities on ≥3 days of the week. At each grade level, the proportion was higher in boys than in girls.
On the basis of accelerometer counts per minute >2020, 42% of 6- to 11-year-olds accumulated ≥260 minutes of moderate to vigorous PA on ≥5 days per week, whereas only 8% of 12- to 15-year-olds and 7.6% of 16- to 19-year-olds achieved similar activity levels.4
More boys than girls met PA recommendations (≥60 minutes of moderate to vigorous activity on most days of the week) as measured by accelerometry.4
Television/Video/Computers
(See Chart 4-3)
Research suggests that screen time (watching television or using a computer) may lead to less PA among children. 9 In addition, television viewing time is associated with poor nutritional choices, overeating, and weight gain (refer to Chapter 5, Nutrition).
-
In 2015 (YRBSS)8:
Nationwide, 41.7% of adolescents used a computer for activities other than school work (eg, videogames or other computer games) for ≥3 hours per day on an average school day. From 2003 to 2009, the computer usage rate increased significantly from 22.1% to 24.9%. The rate of computer use increased much more dramatically from 2009 to 2015 (24.9% to 41.7%).
The prevalence of using computers ≥3 hours per day (for activities other than school work) was highest among non-Hispanic black girls (48.4%), followed by Hispanic girls (47.4%), Hispanic boys (45.1%), non-Hispanic black boys (41.2%), non-Hispanic white boys (38.9%), and non-Hispanic white girls (38.3%) (Chart 4-3).
The prevalence of watching television ≥3 hours per day was highest among non-Hispanic black girls (41.5%) and boys (37.0%), followed by Hispanic girls (29.2%) and boys (27.4%) and non-Hispanic white boys (21.4%) and girls (18.8%).
Structured Activity Participation
Despite recommendations from the National Association for Sport and Physical Education that schools should require daily physical education for students in kindergarten through 12th grade,10 in 2015 only 29.8% of students attended physical education classes in school daily (33.8% of boys and 29.6% of girls) (YRBSS).8
Daily physical education class participation declined from the 9th grade (44.6% for boys, 39.5% for girls) through the 12th grade (27.9% for boys, 16.0% for girls) (YRBSS).8
Just over half (57.6%) of high school students played on at least 1 school or community sports team in the previous year: 53.0% of girls and 62.2% of boys (YRBSS).8
Adults
Inactivity
-
According to 2015 data from the NHIS, of adults ≥18 years of age11:
30.4% do not engage in leisure-time PA (“no leisure time PA/inactivity” refers to no sessions of light/moderate or vigorous PA of ≥10 minutes’ duration).
Inactivity was higher among females than males (31.7% versus 29.9%, age adjusted) and increased with age from 24.7% (ages 18–44 years) to 32.6% (ages 45–64 years), 36.3% (ages 65–74 years), and 53.0% (≥75 years of age).
Hispanic and non-Hispanic black adults were more likely to be inactive (38.8% and 39.0%, respectively) than were non-Hispanic white adults (27.0%) on the basis of age-adjusted estimates.
Activity Recommendations
(See Table 4-1 and Charts 4-4, 4-5, 4-6, 4-7, 4-8, 4-9)
-
According to 2015 data from the NHIS, of adults ≥18 years of age11:
21.6% met the 2008 federal PA guidelines for both aerobic and strengthening activity, an important component of overall physical fitness, based on leisure-time activity (Table 4-1).
The age-adjusted proportion who reported meeting the 2008 aerobic PA guidelines for Americans (≥150 minutes of moderate PA or 75 minutes of vigorous PA or an equivalent combination each week) was 49.8%, with 53.0% of males and 46.9% of females meeting the aerobic guidelines.
The proportion of respondents who met the federal aerobic PA guidelines declined with age for both males and females. Among males, 60.0% of 18- to 44-year-olds met the aerobic activity guidelines compared with 31.1% of noninstitutionalized males ≥75 years of age. Among females, 52.9% of 18- to 44-year-olds met the aerobic activity guidelines compared with 24.4% of noninstitutionalized females ≥75 years of age11 (Chart 4-4).
Age-adjusted prevalence was 53.1% for non-Hispanic whites, 42.1% for non-Hispanic blacks, and 43.3% for Hispanics. Among both males and females, non-Hispanic whites were more likely to meet the PA aerobic guidelines with leisure-time activity than non-Hispanic blacks and Hispanics (Chart 4-5).11
Education was positively associated with meeting the federal guidelines. Among adults ≥25 years of age, 66.8% of participants with no high school diploma, 57.2% of those with a high school diploma or a General Educational Development (GED) high school equivalency credential, 46.9% of those with some college, and 35.0% of those with a bachelor’s degree or higher did not meet the full (aerobic and muscle-strengthening) federal PA guidelines.
For aerobic PA alone, among adults ≥25 years of age, 30.0% of participants with no high school diploma, 38.9% of those with a high school diploma or General Educational Development (GED) high school equivalency credential, 46.8% of those with some college, and 62.8% of those with a bachelor’s degree or higher met the federal guidelines11 (Chart 4-6).
Adults residing in urban (metropolitan statistical areas) are more likely to meet the federal aerobic PA guidelines than those residing in rural areas (51.2% versus 41.1%)11 (Chart 4-7).
The federal guidelines do not set different PA standards by age, disability status, or other factors. Instead, the federal guidelines recommend being as physically active as abilities and conditions permit. Adults with a disability (defined by the constructs any basic actions difficulty or complex activity limitation) were less likely to meet the aerobic PA guidelines than those without a disability (36.9% compared with 56.9%)3,11 (Chart 4-10).
Adults living at below 200% of the poverty level are less likely to meet the federal PA guidelines than adults living at >200% above the poverty level11 (Chart 4-9).
For adolescents ages 12 to 15 years, boys in all age groups were more likely to have adequate levels of cardiorespiratory fitness than girls12 (Chart 4-10).
-
Using the PA recommendations in effect at the time of the survey, adherence to PA recommendations was much lower when based on PA measured by accelerometer in NHANES 2003 to 20044:
Among adults 20 to 59 years of age, 3.8% of males and 3.2% of females met recommendations to engage in moderate to vigorous PA (accelerometer counts >2020/min) for 30 minutes (in sessions of ≥10 minutes) on ≥5 of 7 days.
Among those ≥60 years of age, adherence was 2.5% in males and 2.3% in females.
Accelerometry data from NHANES 2003 to 2006 showed that males engaged in 35 minutes and females in 21 minutes of moderate activity per day. More than 75% of moderate activity was accumulated in 1-minute bouts. Levels of activity declined sharply after the age of 50 years in all groups.13
In a review examining self-reported versus actual measured PA (eg, accelerometers, pedometers, indirect calorimetry, doubly labeled water, heart rate monitor), 60% of respondents self-reported higher values of activity than what was measured by use of direct methods.14
Among males, self-reported PA was 44% greater than actual measured values; among females, self-reported activity was 138% greater than actual measured PA.14
Mortality
According to the WHO, physical inactivity is the fourth-leading risk factor for global death, responsible for 3.2 million deaths annually, including an estimated 670 000 premature deaths (people aged <60 years) and 30% of IHD burden.15
The Global Burden of Disease investigators estimated that worldwide, physical inactivity contributed to 2.2 million deaths in 2013.16
Adults ≥40 years of age who participated in moderate to vigorous PA once a week or more had a lower mortality risk than those who were inactive. Furthermore, older adults who engaged in moderate to vigorous PA ≥5 times per week had a significantly lower mortality risk than those who participated in a lower frequency of PA.17
A meta-analysis of 9 cohort studies, representing 122 417 patients, found that as little as 15 minutes of daily moderate to vigorous PA reduced all-cause mortality in adults ≥60 years of age. This protective effect of PA was dose dependent; the most rapid reduction in mortality per minute of added PA was for those at the lowest levels of PA. These findings suggest that older adults can benefit from PA time far below the amount recommended by the federal guidelines.18
A study of US adults that linked a large, nationally representative sample to death records found that meeting the aerobic PA guidelines reduced all-cause mortality, with an HR of 0.64, after adjustment for potential confounding factors.19
A pooled study of >600 000 participants found that even low levels of leisure-time PA (up to 75 minutes of brisk walking per week) were associated with reduced risk of mortality compared with participants with no PA. If this is a causal relationship, this low level of PA would confer a 1.8-year gain in life expectancy after age 40 years compared with no PA. For participants meeting the PA guidelines, the gain in life expectancy was 3.4 years over those with no PA.20
With television watching as a sedentary activity, 2 hours of television per day is associated with an RR for type 2 DM of 1.20 (95% CI, 1.14–1.27), an RR for fatal or nonfatal CVD of 1.15 (95% CI, 1.06–1.23), and an RR for all-cause mortality of 1.13 (95% CI, 1.07–1.18). The risk for all-cause mortality further increases with >3 hours of television daily.21
Adherence to PA guidelines for both aerobic and muscle-strengthening activities is associated with 27% lower all-cause mortality among adults without existing chronic conditions such as DM, cancer, MI, angina, CVD, stroke, or respiratory diseases and with 46% lower mortality among people with chronic comorbidities.22
In a 20-year study of older male veterans, an inverse, graded, and independent association between impaired exercise capacity and all-cause mortality risk was found. For each increase of 1 metabolic equivalent task in exercise capacity, mortality risk was 12% lower (HR, 0.88; 95% CI, 0.86–0.90). Unfit individuals who improved their fitness status had a 35% lower mortality risk (HR, 0.65; 95% CI, 0.46–0.93) than those who remained unfit.23
In the Cooper Center Longitudinal Study, an analysis conducted on 16 533 participants revealed that across all risk factor strata, the presence of low cardiorespiratory fitness was associated with a greater risk of CVD death over a mean follow-up of 28 years.24
In the Southern Community Cohort Study of 63 308 individuals (with a large proportion of black adults) followed up for >6.4 years, more time spent being sedentary (>12 h/d versus <5.76 h/d) was associated with a 20% to 25% increased risk of all-cause mortality in blacks and whites. Both PA (beneficial) and sedentary time (detrimental) were associated with mortality risk.25
In a study involving 55 137 adults followed up over an average of 15 years, running even 5 to 10 min/d and at slow speeds (<6 mph) was associated with a markedly reduced risk of death attributable to all causes and CVD.26
A population-based cohort in New South Wales, Australia, of 204 542 adults followed up for an average of 6.5 years evaluated the relationship of PA to mortality risk. It found that compared with those who reported no moderate to vigorous PA, the adjusted HRs for all-cause mortality were 0.66 for those reporting 10–149 min/wk, 0.53 for those reporting 150–299 min/wk, and 0.46 for those reporting ≥300 min/wk of activity.27
An analysis of pooled data from 6 studies in the National Cancer Institute Cohort Consortium involving 661 137 males and females followed up for an average of 14.2 years revealed that compared with individuals reporting no leisure-time PA, an inverse dose-response relationship was observed between level of leisure-time PA (HR=0.80 for less than the recommended minimum of the PA guidelines, HR=0.69 for 1 to 2 times the recommended minimum, and HR=0.63 for 2 to 3 times the minimum) and mortality, with the upper threshold for mortality benefit occurring at 3 to 5 times the PA recommendations (HR, 0.61). Furthermore, there was no evidence of harm associated with performing ≥10 times the recommended minimum (HR, 0.69). Thus, meeting the 2008 minimum PA guidelines for Americans through either moderate- or vigorous-intensity activities was essentially associated with a nearly optimal reduction in mortality risk. This study supports the view that although healthcare professionals should encourage inactive people to become physically active, they should not discourage adults who are already very active at levels far above those recommended by the guidelines.28
A study that prospectively assessed the association of continuous inactivity and of changes in sitting time for 2 years with subsequent long-term all-cause mortality found that compared with people who remained consistently sedentary, the HRs for mortality were 0.91 in those who were newly sedentary, 0.86 in formerly sedentary individuals, and 0.75 in those who remained consistently non-sedentary. Thus, participants who reduced their sitting time over 2 years experienced a reduction in mortality.29
A meta-analysis of 17 eligible studies on PA in patients with DM revealed that the highest PA category in each study was associated with a lower RR (0.61) for all-cause mortality and CVD (0.71) than the lowest PA category. Although more PA was associated with larger reductions in future all-cause mortality and CVD, in patients with DM, any amount of habitual PA was better than inactivity.30
Costs
The economic consequences of physical inactivity are substantial. Using data derived primarily from WHO publications and data warehouses, one study estimated the economic costs of physical inactivity account for 1.5% to 3.0% of total direct healthcare expenditures in developed countries such as the United States.31
A study of Australian females aged 50 to 55 years found that healthcare costs were 26% higher in sedentary females than in moderately active females. Costs included visits to general practitioners, medical specialists, and outpatient pathology and radiology services.32
A study of American adults reported that inadequate levels of aerobic PA (after adjusting for BMI) were associated with an estimated 11.1% of aggregate healthcare expenditures (including expenditures for inpatient, outpatient, ED, office-based, dental, vision, home health, prescription drug, and other services).33
An evaluation of healthcare costs based on the cardiovascular risk factor profile (including ≥30 minutes of moderate to vigorous PA ≥5 times per week) found that among adults aged ≥40 years with CVD, the highest marginal expenditures ($2853) were for those not meeting the PA guidelines. Healthcare costs included hospitalizations, prescribed medications, outpatient visits (hospital outpatient visits and office-based visits), ED visits, and other expenditures (dental visits, vision aid, home health care, and other medical supplies).34
Secular Trends
Youth
-
In 2015 (YRBSS)8:
During the 2013 to 2015 time period, the percentage of adolescents not participating in ≥60 minutes of any kind of physical activity on at least 1 day did not substantively change, at 15.2% in 2013 and 14.3% in 2015.
Among students nationwide, there was a significant increase in the prevalence of having participated in muscle-strengthening activities on ≥3 days per week, from 47.8% in 1991 to 53.4% in 2015; however, the prevalence did not change substantively from 2013 (51.7%) to 2015 (53.4%).
A significant increase occurred in the prevalence of having used computers not for school work for ≥3 hours per day compared with 2003 (22.1% versus 41.7% in 2015). The prevalence increased from 2003 to 2009 (22.1% versus 24.9%) and then increased more rapidly from 2009 to 2015 (24.9% versus 41.7%).
Among adolescents nationwide, the prevalence of attending physical education classes at least once per week did not change substantively between 2013 (48.0%) and 2015 (51.6%).
Among high school students, the prevalence of attendance in daily physical education classes changed in nonlinear ways over time. Attendance initially decreased from 1991 to 1995 (from 41.6% to 25.4%) and was not substantively changed between 1995, 2013, and 2015 (25.4%, 29.4%, and 29.8%, respectively).
The prevalence of adolescents playing ≥1 team sport in the past year did not substantively change between 2013 (54.0%) and 2015 (57.6%).
In 2012, the prevalence of adolescents aged 12 to 15 years with adequate levels of cardiorespiratory fitness (based on age- and sex-specific standards) was 42.2% in 2012, down from 52.4% in 1999 to 2000.12 (Chart 4-10)
Adults
(See Chart 4-11)
Between 1988 to 1994 and 2001 to 2006 (NHANES), the percentage of adults who reported engaging in >12 bouts of PA per month declined from 57.0% to 43.3% in males and from 49.0% to 43.3% in females (crude percentages).35
The age-adjusted percentage of US adults who met both the muscle-strengthening and aerobic guidelines increased from 14.3% in 1998 to 21.6% in 2015 (Chart 4-11). The percentage of US adults who met the aerobic guideline increased from 40.0% in 1998 to 49.8% in 2015, and the percentage meeting the muscle-strengthening guideline increased from 17.7% in 1998 to 25.0% in 2015.11,36
A 2.3% decline in physical inactivity between 1980 and 2000 was estimated to have prevented or postponed ≈17 445 deaths (≈5%) attributable to CHD in the United States.37
Cardiovascular Health Impact
Youth
Total and vigorous PA are inversely correlated with body fat and the prevalence of obesity.38
Among children 4 to 18 years of age, increased time spent engaging in moderate to vigorous PA was associated with improvements in waist circumference, SBP, fasting triglycerides, HDL-C, and insulin. These findings were significant regardless of the amount of the children’s sedentary time.39
Among children aged 4 to 18 years, both higher activity levels and lower sedentary time measured by accelerometry were associated with more favorable metabolic risk factor profiles.39
Adults
Cardiovascular and Metabolic Risk
Participants in the Diabetes Prevention Program randomized trial who met the goal of 150 minutes of PA per week were 44% less likely to develop DM after 3.2 years of follow-up, even if they did not meet the weight-loss target.40
A review of the US Preventive Services Task Force recommendations examined the evidence on whether relevant counseling interventions for a healthful diet and PA in primary care modify self-reported behaviors, intermediate physiological outcomes, DM incidence, and cardiovascular morbidity or mortality in adults with CVD risk factors. It was concluded that after 12 to 24 months, intensive lifestyle counseling for individuals selected because of risk factors reduced TC levels by an average of 0.12 mmol/L, LDL-C levels by 0.09 mmol/L, SBP by 2.03 mm Hg, DBP by 1.38 mm Hg, fasting glucose by 0.12 mmol/L, DM incidence by an RR of 0.58, and weight outcomes by a standardized difference of 0.25.41
Weight loss from increased physical exercise, without dietary interventions, was associated with significant reductions in DBP (−2 mm Hg; 95% CI, −4 to −1 mm Hg), triglycerides (−0.2 mmol/L; 95% CI, −0.3 to −0.1 mmol/L), and fasting glucose (−0.2 mmol/L; 95% CI, −0.3 to −0.1 mmol/L).42
A total of 120 to 150 min/wk of moderate-intensity activity, compared with none, can reduce the risk of developing metabolic syndrome.43
In CARDIA, females who maintained high PA through young adulthood gained 6.1 fewer kilograms of weight and 3.8 fewer centimeters in waist circumference in middle age than those with lower activity. Highly active males gained 2.6 fewer kilograms and 3.1 fewer centimeters than their lower-activity counterparts.44
In 3 US cohort studies, males and females who increased their PA over time gained less weight in the long term, whereas those who decreased their PA over time gained more weight and those who maintained their current PA had intermediate weight gain.45
Among US males and females, every hour per day of increased television watching was associated with 0.3 lb of greater weight gain every 4 years, whereas every hour per day of decreased television watching was associated with a similar amount of relative weight loss.45
In a sample of 466 605 participants in the China Kadoorie Biobank study, a 1-SD (1.5 h/d) increase in sedentary time was associated with a 0.19-unit higher BMI, a 0.57-cm larger waist circumference, and 0.44% more body fat. Both sedentary leisure time and lower PA were independently associated with an increased BMI.46
Cardiovascular Events
The PA guidelines for adults cite evidence that ≈150 min/wk of moderate-intensity aerobic activity, compared with none, can reduce the risk of CVD.22
In the WHI observational study (n=71 018), sitting for ≥10 h/d compared with ≤5 h/d was associated with increased CVD risk (HR, 1.18) in multivariable models that included PA. Low PA was also associated with higher CVD risk. It was concluded that both low PA and prolonged sitting augment CVD risk.47
Longitudinal studies commonly report a graded, inverse association of PA amount and duration (ie, dosage) with incident CHD and stroke.48
In a meta-analysis of longitudinal studies among females, RRs of incident CHD were 0.83 (95% CI, 0.69–0.99), 0.77 (95% CI, 0.64–0.92), 0.72 (95% CI, 0.59–0.87), and 0.57 (95% CI, 0.41–0.79) across increasing quintiles of PA compared with the lowest quintile.49
A study of the factors related to declining CVD among Norwegian adults ≥25 years of age found that increased PA (≥1 hour of strenuous PA per week) accounted for 9% of the decline in hospitalized and nonhospitalized fatal and nonfatal CHD events.50
In the Health Professionals Follow-Up Study, for every 3-hour-per-week increase in vigorous-intensity activity, the multivariate RR of MI was 0.78 (95% CI, 0.61–0.98) for males. This 22% reduction of risk can be explained in part by beneficial effects of PA on HDL-C, vitamin D, apolipoprotein B, and HbA1c.51
A 2003 meta-analysis of 23 studies on the association of PA with stroke indicated that compared with low levels of activity, high (RR, 0.79; 95% CI, 0.69–0.91) and moderate (RR, 0.91; 95% CI, 0.80–1.05) levels of activity were inversely associated with the likelihood of developing total stroke (ischemic and hemorrhagic).52
In a study involving 1.1 million females without prior vascular disease and followed up over an average of 9 years, those who reported moderate activity were found to be at lower risk of CHD, a cardiovascular event, or a first thrombotic event. However, strenuous PA was not found to be as beneficial as moderate PA.53
In a large clinical trial (NAVIGATOR) involving 9306 people with impaired glucose tolerance, ambulatory activity assessed by pedometer at baseline and 12 months was found to be inversely associated with risk of a cardiovascular event.54
In the EPIC-Norfolk study, males and females with abdominal obesity with features of the metabolic syndrome who reported themselves to be physically very active were characterized by a lower (≈50%) risk of CHD than sedentary abdominally obese subjects with the metabolic syndrome.55
Self-reported low lifetime recreational activity has been associated with increased PAD.56
Primordial and Primary Prevention
Community-level interventions have been shown to be effective in promoting increased PA.
-
The US Surgeon General has introduced Step It Up!, a Call to Action to Promote Walking and Walkable Communities in recognition of the importance of PA.57 There are roles for the following:
Communities: Communities can encourage walking with street design that includes side-walks, improved street lighting, and landscaping design that reduces traffic speed to improve pedestrian safety.
Schools: Schools can provide opportunities for PA through physical education, recess, after-school activity programs, and PA breaks. Despite these recommendations, only one-quarter of students participate in daily PA classes (see Structured Activity Participation).
Worksites: Worksites can offer access to onsite exercise facilities or employer-subsidized off-site exercise facilities to encourage PA among employees.
Community-wide campaigns: Such campaigns include a variety of strategies, such as media coverage, risk factor screening and education, community events, and policy or environmental changes.
A systematic review of population-based interventions to encourage PA found that improving biking trails, distributing pedometers, and school-based PA were most cost-effective.58
Educating the public on the recommended PA guidelines may increase adherence. In a study examining awareness of current US PA guidelines, only 33% of respondents had direct knowledge of the recommended dosage of PA (ie, frequency/duration).59
-
Interventions and community strategies to increase PA have been shown to be cost-effective in terms of reducing medical costs60:
Nearly $3 in medical cost savings is realized for every $1 invested in building bike and walking trails.
Incremental cost and incremental effectiveness ratios range from $14 000 to $69 000 per QALY gained from interventions such as pedometer or walking programs compared with no intervention, especially in high-risk groups.
Secondary Prevention
In a retrospective cohort study of 2086 patients (39% females, 56% white) who underwent clinical treadmill testing and had a first MI during follow-up (mean of 11 years), a higher baseline level of cardiorespiratory fitness was independently associated with a decreased risk of short-term mortality (28 days after a first MI).61
PA improves inflammatory markers in people with existing stable CHD. After a 6-week training session, CRP levels declined by 23.7% (P<0.001), and plasma vascular cell adhesion molecule-1 levels declined by 10.23% (P<0.05); there was no difference in leukocyte count or levels of intercellular adhesion molecule-1.62
In a randomized trial of patients with PAD, supervised treadmill exercise training and lower-extremity resistance training were each associated with significant improvements in functional performance and quality of life compared with a usual-care control group. Exercise training also improved brachial artery FMD, whereas resistance training was associated with better stair-climbing ability than control.63
On the basis of a meta-analysis of 34 RCTs, exercise-based cardiac rehabilitation after MI was associated with lower rates of reinfarction, cardiac mortality, and overall mortality.64
The benefit of intense exercise training for cardiac rehabilitation in people with HF was tested in a trial of 27 patients with stable, medically treated HF. Intense activity (an aerobic interval-training program 3 times per week for 12 weeks) was associated with a significant 35% improvement in LVEF and decreases in pro-BNP (40%), LV end-diastolic volume (18%), and LV end-systolic volume (25%) compared with control and endurance-training groups.65
Exercise training in patients with HF with preserved EF was associated with improved exercise capacity and favorable changes in diastolic function.66
Global Burden
Physical inactivity is responsible for 12.2% of the global burden of MI after accounting for other CVD risk factors such as cigarette smoking, DM, hypertension, abdominal obesity, lipid profile, excessive alcohol intake, and psychosocial factors.67
Worldwide, the prevalence of physical inactivity (35%) is now greater than the prevalence of smoking (26%). On the basis of the HRs associated with these 2 behaviors (1.57 for smoking and 1.28 for inactivity), it was concluded that the PAR was greater for inactivity (9%) than for smoking (8.7%). Inactivity was estimated to be responsible for 5.3 million deaths compared with 5.1 million deaths for smoking.68
REFERENCES
- 1.Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart-Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston-Miller N, Burke LE American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. [Accessed July 11, 2014];Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/pdf/paguide.pdf.
- 4.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 5.Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, Richardson CR, Smith DT, Swartz AM on behalf of the American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health and Cardiovascular, Exercise, Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 6.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Piña IL, Weintraub WS, Williams MA American Heart Association Advocacy Coordinating Committee, Council on Clinical Cardiology, and Council on Nutrition, Physical Activity and Metabolism. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 7.DeFina LF, Haskell WL, Willis BL, Barlow CE, Finley CE, Levine BD, Cooper KH. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015;57:324–329. doi: 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, Queen B, Lowry R, Olsen EO, Chyen D, Whittle L, Thornton J, Lim C, Yamakawa Y, Brener N, Zaza S. Youth Risk Behavior Surveillance: United States, 2015. MMWR Surveill Summ. 2016;65:1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman DA, Chamberlin B, Medina E, Jr, Franklin BA, Sanner BM, Vafiadis DK Power of Play: Innovations in Getting Active Summit Planning Committee. The power of play: Innovations in Getting Active Summit 2011: a science panel proceedings report from the American Heart Association. Circulation. 2011;123:2507–2516. doi: 10.1161/CIR.0b013e318219661d. [DOI] [PubMed] [Google Scholar]
- 10.National Association for Sport and Physical Education. Moving Into the Future: National Standards for Physical Education. 2. Reston, VA: National Association for Sport and Physical Education; 2004. [Google Scholar]
- 11.National Center for Health Statistics. [Accessed July 18, 2016];National Health Interview Survey. 2015 Public-use data file and documentation: NCHS tabulations. http://www.cdc.gov/nchs/nhis/nhis_2015_data_release.htm.
- 12.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY, Fulton JE. Cardiorespiratory fitness levels among U.S. youth aged 12–15 years: United States, 1999–2004 and 2012. NCHS Data Brief. 2014;(153):1–8. [PubMed] [Google Scholar]
- 13.Luke A, Dugas LR, Durazo-Arvizu RA, Cao G, Cooper RS. Assessing physical activity and its relationship to cardiovascular risk factors: NHANES 2003–2006. BMC Public Health. 2011;11:387. doi: 10.1186/1471-2458-11-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 16.GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RE, Riddell MC, Macpherson AK, Canning KL, Kuk JL. The association between frequency of physical activity and mortality risk across the adult age span. J Aging Health. 2013;25:803–814. doi: 10.1177/0898264313492823. [DOI] [PubMed] [Google Scholar]
- 18.Hupin D, Roche F, Gremeaux V, Chatard JC, Oriol M, Gaspoz JM, Barthélémy JC, Edouard P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1262–1267. doi: 10.1136/bjsports-2014-094306. [DOI] [PubMed] [Google Scholar]
- 19.Zhao G, Li C, Ford ES, Fulton JE, Carlson SA, Okoro CA, Wen XJ, Balluz LS. Leisure-time aerobic physical activity, muscle-strengthening activity and mortality risks among US adults: the NHANES linked mortality study. Br J Sports Med. 2014;48:244–249. doi: 10.1136/bjsports-2013-092731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9:e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Prev Med. 2011;40:514–521. doi: 10.1016/j.amepre.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, Papademetriou V, Fletcher R. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122:790–797. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 24.Wickramasinghe CD, Ayers CR, Das S, de Lemos JA, Willis BL, Berry JD. Prediction of 30-year risk for cardiovascular mortality by fitness and risk factor levels: the Cooper Center Longitudinal Study. Circ Cardiovasc Qual Outcomes. 2014;7:597–602. doi: 10.1161/CIRCOUTCOMES.113.000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews CE, Cohen SS, Fowke JH, Han X, Xiao Q, Buchowski MS, Hargreaves MK, Signorello LB, Blot WJ. Physical activity, sedentary behavior, and cause-specific mortality in black and white adults in the Southern Community Cohort Study. Am J Epidemiol. 2014;180:394–405. doi: 10.1093/aje/kwu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk [published correction appears in J Am Coll Cardiol. 2014;64:1537] J Am Coll Cardiol. 2014;64:472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians [published correction appears in JAMA Intern Med. 2015;175:1248] JAMA Intern Med. 2015;175:970–977. doi: 10.1001/jamainternmed.2015.0541. [DOI] [PubMed] [Google Scholar]
- 28.Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.León-Muñoz LM, Martínez-Gómez D, Balboa-Castillo T, López-García E, Guallar-Castillón P, Rodríguez-Artalejo F. Continued sedentariness, change in sitting time, and mortality in older adults. Med Sci Sports Exerc. 2013;45:1501–1507. doi: 10.1249/MSS.0b013e3182897e87. [DOI] [PubMed] [Google Scholar]
- 30.Kodama S, Tanaka S, Heianza Y, Fujihara K, Horikawa C, Shimano H, Saito K, Yamada N, Ohashi Y, Sone H. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Care. 2013;36:471–479. doi: 10.2337/dc12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldridge NB. Economic burden of physical inactivity: healthcare costs associated with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2008;15:130–139. doi: 10.1097/HJR.0b013e3282f19d42. [DOI] [PubMed] [Google Scholar]
- 32.Brown WJ, Hockey R, Dobson AJ. Physical activity, body mass index and health care costs in mid-age Australian women. Aust N Z J Public Health. 2008;32:150–155. doi: 10.1111/j.1753-6405.2008.00192.x. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57:315–323. doi: 10.1016/j.pcad.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valero-Elizondo J, Salami JA, Ogunmoroti O, Osondu CU, Aneni EC, Malik R, Spatz ES, Rana JS, Virani SS, Blankstein R, Blaha MJ, Veledar E, Nasir K. Favorable cardiovascular risk profile is associated with lower healthcare costs and resource utilization: the 2012 Medical Expenditure Panel Survey. Circ Cardiovasc Qual Outcomes. 2016;9:143–153. doi: 10.1161/CIRCOUTCOMES.115.002616. [DOI] [PubMed] [Google Scholar]
- 35.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988–2006. Am J Med. 2009;122:528–534. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2015. [Accessed June 15, 2016]. http://www.cdc.gov/nchs/data/hus/hus15.pdf. [PubMed] [Google Scholar]
- 37.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y, Lee S. Physical activity and abdominal obesity in youth. Appl Physiol Nutr Metab. 2009;34:571–581. doi: 10.1139/H09-066. [DOI] [PubMed] [Google Scholar]
- 39.Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A International Children’s Accelerometry Database (ICAD) Collaborators. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents [published correction appears in JAMA. 2012;307:1915] JAMA. 2012;307:704–712. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeFevre ML US Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;161:587–593. doi: 10.7326/M14-1796. [DOI] [PubMed] [Google Scholar]
- 42.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Department of Health and Human Services, Centers for Disease Control and Prevention. [Accessed August 1, 2011];Physical activity and health: the benefits of physical activity. http://www.cdc.gov/physicalactivity/everyone/health/index.html#ReduceCardiovascularDisease.
- 44.Hankinson AL, Daviglus ML, Bouchard C, Carnethon M, Lewis CE, Schreiner PJ, Liu K, Sidney S. Maintaining a high physical activity level over 20 years and weight gain [published correction appears in JAMA. 2011;305:150] JAMA. 2010;304:2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, Chen J, Bian Z, Hong LS, Feng S, Chen X, Chen L, Zhou R, Mao E, Peto R, Chen Z China Kadoorie Biobank Collaborative Group. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97:487–496. doi: 10.3945/ajcn.112.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol. 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carnethon MR. Physical activity and cardiovascular disease: how much is enough? Am J Lifestyle Med. 2009;3(suppl):44S–49S. doi: 10.1177/1559827609332737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Mannsverk J, Wilsgaard T, Mathiesen EB, Løchen ML, Rasmussen K, Thelle DS, Njølstad I, Hopstock LA, Bønaa KH. Trends in modifiable risk factors are associated with declining incidence of hospitalized and nonhospitalized acute coronary heart disease in a population. Circulation. 2016;133:74–81. doi: 10.1161/CIRCULATIONAHA.115.016960. [DOI] [PubMed] [Google Scholar]
- 51.Chomistek AK, Chiuve SE, Jensen MK, Cook NR, Rimm EB. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc. 2011;43:1884–1890. doi: 10.1249/MSS.0b013e31821b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ Million Women Study Collaborators. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 54.Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, Califf RM, Holman RR, McMurray JJ, Bethel MA, Tuomilehto J, Davies MJ, Kraus WE. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059–1066. doi: 10.1016/S0140-6736(13)62061-9. [DOI] [PubMed] [Google Scholar]
- 55.Broekhuizen LN, Boekholdt SM, Arsenault BJ, Despres JP, Stroes ES, Kastelein JJ, Khaw KT, Wareham NJ. Physical activity, metabolic syndrome, and coronary risk: the EPIC-Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil. 2011;18:209–217. doi: 10.1177/1741826710389397. [DOI] [PubMed] [Google Scholar]
- 56.Wilson AM, Sadrzadeh-Rafie AH, Myers J, Assimes T, Nead KT, Higgins M, Gabriel A, Olin J, Cooke JP. Low lifetime recreational activity is a risk factor for peripheral arterial disease. J Vasc Surg. 2011;54:427–432. 432.e1–4. doi: 10.1016/j.jvs.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Department of Health and Human Services. Step It Up! The Surgeon General’s Call to Action to Promote Walking and Walkable Communities. Washington, DC: US Department of Health and Human Services, Office of the Surgeon General; 2015. [Google Scholar]
- 58.Laine J, Kuvaja-Köllner V, Pietilä E, Koivuneva M, Valtonen H, Kankaanpää E. Cost-effectiveness of population-level physical activity interventions: a systematic review. Am J Health Promot. 2014;29:71–80. doi: 10.4278/ajhp.131210-LIT-622. [DOI] [PubMed] [Google Scholar]
- 59.Bennett GG, Wolin KY, Puleo EM, Mâsse LC, Atienza AA. Awareness of national physical activity recommendations for health promotion among US adults. Med Sci Sports Exerc. 2009;41:1849–1855. doi: 10.1249/MSS.0b013e3181a52100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC, Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular Disease in the Young; Council on the Kidney in Cardiovascular Disease; Council on Epidemiology and Prevention; Council on Cardiovascular Nursing; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Clinical Cardiology, and Stroke Council. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 61.Shaya GE, Hung RK, Nasir K, Blumenthal RS, Keteyian SJ, Brawner CA, Qureshi W, Al-Mallah M, Blaha MJ. High cardiorespiratory fitness attenuates risk of short-term mortality after first myocardial infarction: the Henry Ford Hospital Exercise Testing (FIT) Project. Circulation. 2014;130(suppl 2):A13463. Abstract. [Google Scholar]
- 62.Ranković G, Milicić B, Savić T, Dindić B, Mancev Z, Pesić G. Effects of physical exercise on inflammatory parameters and risk for repeated acute coronary syndrome in patients with ischemic heart disease. Vojnosanit Pregl. 2009;66:44–48. doi: 10.2298/vsp0901044r. [DOI] [PubMed] [Google Scholar]
- 63.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui MH. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial [published correction appears in JAMA. 2012; 307:1694] JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571–584.e2. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 66.Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 67.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, Mc-Queen M, Budaj A, Pais P, Varigos J, Lisheng L INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 68.Wen CP, Wu X. Stressing harms of physical inactivity to promote exercise. Lancet. 2012;380:192–193. doi: 10.1016/S0140-6736(12)60954-4. [DOI] [PubMed] [Google Scholar]
5. NUTRITION
See Tables 5-1, 5-2, 5-3 and Charts 5-1 5-2 5-3 5-4 5-5 5-6 5-7
Table 5-1.
AHA Dietary Targets and Healthy Diet Score for Defining Cardiovascular Health
| AHA Target | Consumption Range for Alternative Healthy Diet Score* | Alternative Scoring Range* | |
|---|---|---|---|
| Primary dietary metrics† | |||
| Fruits and vegetables | ≥4.5 cups/d‡ | 0 to ≥4.5 cups/d‡ | 0–10 |
| Fish and shellfish | 2 or more 3.5-oz servings/wk (≥200 g/wk) | 0 to ≥7 oz/wk | 0–10 |
| Sodium | ≤1500 mg/d | ≤1500 to >4500 mg/d | 10–0 |
| SSBs | ≤36 fl oz/wk | ≤36 to >210 fl oz/wk | 10–0 |
| Whole grains | 3 or more 1-oz-equivalent servings/d | 0 to ≥3 oz/d | 0–10 |
| Secondary dietary metrics† | |||
| Nuts, seeds, and legumes | ≥4 servings/wk (nuts/seeds: 1 oz; legumes: ½ cup) | 0 to ≥4 servings/d | 0–10 |
| Processed meats | 2 or fewer 1.75-oz servings/wk (≤100 g/wk) | ≤3.5 to >17.5 oz/wk | 10–0 |
| Saturated fat | ≤7% energy | ≤7 to >15 (% energy) | 10–0 |
| AHA Diet Score (primary) | Ideal: 4 or 5 dietary targets
(≥80%) Intermediate: 2 or 3 dietary targets (40%–79%) Poor: <2 dietary targets (<40%) |
Sum of scores for primary metrics | 0 (worst) to 100 (best)§ Ideal: 80–100 Intermediate: 40–79 Poor: <40 |
| AHA Diet Score (secondary) | Ideal: 4 or 5 dietary targets
(≥80%) Intermediate: 2 or 3 dietary targets (40%–79%) Poor: <2 dietary targets (<40%) |
Sum of scores for primary and secondary metrics | 0 (worst) to 100 (best)§ Ideal:
80–100 Intermediate: 40–79 Poor: <40 |
AHA indicates American Heart Association; and SSBs, sugar-sweetened beverages.
Consistent with other dietary pattern scores, the highest score (10) was given for meeting or exceeding the AHA target (eg, at least 4.5 cups of fruits and vegetables per day; no more than 1500 mg/d of sodium), and the lowest score (0) was given for zero intake (protective factors) or for very high intake (harmful factors). The score for each metric was scaled continuously within this range. For harmful factors, the level of high intake that corresponded to a zero score was identified as approximately the 90th percentile distribution of US population intake.
Selected by the AHA based on evidence for likely causal effects on cardiovascular events, diabetes mellitus, or obesity; a general prioritization of food rather than nutrient metrics; consistency with US and AHA dietary guidelines; ability to measure and track these metrics in the US population; and parsimony, that is, the inclusion of as few components as possible that had minimal overlap with each other while at the same time having some overlap with the many other relevant dietary factors that were not included.2 The AHA dietary metrics should be targeted in the context of a healthy diet pattern that is appropriate in energy balance and consistent with a DASH (Dietary Approaches to Stop Hypertension)-type eating plan, including but not limited to these metrics.
Including up to one 8-oz serving per day of 100% fruit juice and up to 0.42 cups/d (3 cups/wk) of starchy vegetables such as potatoes or corn.
The natural range of the primary AHA Diet Score is 0 to 50 (5 components), and the natural range of the secondary AHA Diet Score is 0 to 80 (8 components). Both scores are then rescaled to a range of 0 to 100 for comparison purposes. The ideal range of the primary AHA Diet Score corresponds to the AHA scoring system of meeting at least 4 of 5 binary dietary targets (≥80%), the intermediate range corresponds to meeting 2 or 3 dietary targets (40%–79%), and the poor range corresponds to meeting <2 dietary targets (<40%). The same ranges are used for the secondary AHA Diet Score for consistency and comparison.
Table 5-2.
Dietary Consumption of Selected Foods and Nutrients Related to Cardiometabolic Health Among US Adults ≥20 Years of Age in 2011 to 2012
| NH White Males | NH White Females | NH Black Males | NH Black Females | Mexican American Males | Mexican American Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | |
| Foods | ||||||||||||
| Whole grains, servings/d | 1.1±1.0 | 9.0 | 1.1±0.8 | 7.3 | 0.9±1.2 | 7.7 | 0.8±0.7 | 4.8 | 0.6±0.5 | 3.9 | 0.8±0.7 | 6.1 |
| Total fruit, servings/d | 1.5±0.8 | 9.2 | 1.6±1.6 | 9.2 | 1±1.3 | 6.9 | 1.2±1.3 | 6.8 | 1.4±1.4 | 6.6 | 1.4±1.0 | 5.7 |
| Total fruits including 100% juices, servings/d | 2.0±1.7 | 15.3 | 2.2±1.9 | 14 | 2±1.8 | 15.8 | 2±1.3 | 14 | 1.9±1.5 | 16.4 | 2.1±1.5 | 14.5 |
| Nonstarchy vegetables, servings/d | 2.3±2.0 | 7.9 | 2.7±1.6 | 12.4 | 1.7±1.0 | 4.3 | 2.0±1.3 | 5.9 | 2.0±0.7 | 3.2 | 2.5±0.5 | 7.7 |
| Starchy vegetables, servings/d† | 0.9±0.3 | NA | 0.8±0.3 | NA | 0.9±0.5 | NA | 0.9±0.4 | NA | 0.7±0.2 | NA | 0.7±0.3 | NA |
| Legumes, servings/wk | 1.6±1.4 | 22.1 | 1.1±0.7 | 14.4 | 1.1±1.1 | 12.8 | 1.1±1.2 | 13.3 | 4.7±4.0 | 47.4 | 2.3±2.6 | 24.8 |
| Fish and shellfish, servings/wk | 1.1±1.1 | 16.7 | 1.0±0.9 | 16.5 | 1.7±2.7 | 23.3 | 1.9±0.9 | 26.7 | 1.7±1.7 | 19.7 | 0.8±0.1 | 14.6 |
| Nuts and seeds, servings/wk | 3.5±5.9 | 23.7 | 3.6±3.3 | 24.0 | 2.2±3.4 | 16.2 | 2.8±8.6 | 16.0 | 2.4±1.6 | 12.4 | 2.6±3.8 | 11.8 |
| Unprocessed red meat, servings/wk | 3.6±1.6 | NA | 2.5±1.3 | NA | 3.1±0.8 | NA | 2.6±1.3 | NA | 4.8±0.6 | NA | 3.2±1.5 | NA |
| Processed meats, servings/wk | 2.4±1.4 | 57.4 | 1.6±1.0 | 69.5 | 2.5±1.5 | 55 | 1.8±0.6 | 65.6 | 1.7±1.1 | 68.9 | 1.1±0.5 | 78.9 |
| Sugar-sweetened beverages, servings/wk | 9.1±11.0 | 50.9 | 6.8±8.3 | 65.4 | 11.1±8.9 | 32.3 | 11±8.0 | 33.9 | 11.7±7.7 | 32.7 | 8.4±6.6 | 42.7 |
| Sweets and bakery desserts, servings/wk | 6.4±3.6 | 33.1 | 7.3±4.1 | 31.8 | 5.8±4.0 | 42.7 | 6.4±3.0 | 37.4 | 3.9±2.5 | 54.0 | 5.8±0.6 | 38.5 |
| Nutrients | ||||||||||||
| Total calories, kcal/d | 2482±551 | NA | 1789±421 | NA | 2354±682 | NA | 1853±514 | NA | 2576±506 | NA | 1848±467 | NA |
| EPA/DHA, g/d | 0.083±0.053 | 7.4 | 0.074±0.054 | 6.4 | 0.101±0.08 | 9.9 | 0.110±0.10 | 13.7 | 0.117±0.075 | 10.7 | 0.058±0.037 | 4.0 |
| ALA, g/d | 1.58±0.35 | 41.9 | 1.75±0.38 | 85.0 | 1.51±0.37 | 35.5 | 1.72±0.43 | 83.1 | 1.57±0.46 | 41.2 | 1.66±0.22 | 81.9 |
| n-6 PUFA, % energy | 7.0±1.4 | NA | 7.3±1.5 | NA | 7.2±1.2 | NA | 7.8±1.6 | NA | 6.8±1.4 | NA | 7.0±0.5 | NA |
| Saturated fat, % energy | 11.0±1.8 | 36.3 | 10.7±1.8 | 43.1 | 10.2±1.8 | 47.1 | 10.3±1.5 | 46.3 | 10.7±1.4 | 42.0 | 10.9±2.4 | 46.9 |
| Dietary cholesterol, mg/d | 281±177 | 67.7 | 256±180 | 71.4 | 322±193 | 58.9 | 299±176 | 63.1 | 343±216 | 51.6 | 290±221 | 68.7 |
| Total fat, % energy | 33.7±5.3 | 52.7 | 33.2±4.6 | 58.8 | 33.2±4.8 | 58.3 | 33.9±4.0 | 51.1 | 33.1±3.8 | 63.9 | 33.5±3.6 | 56.6 |
| Carbohydrate, % energy | 47.9±7.7 | NA | 49.8±7.2 | NA | 48.1±7.2 | NA | 50±4.9 | NA | 49.1±4.4 | NA | 50.5±5.7 | NA |
| Dietary fiber, g/d | 17.2±6.3 | 7.3 | 18.4±6.2 | 10.3 | 14.3±4.6 | 3.7 | 15.2±4.6 | 5.3 | 19.3±5.9 | 13.4 | 18.9±5.8 | 14.2 |
| Sodium, g/d | 3.4±0.49 | 6.7 | 3.4±0.62 | 6.4 | 3.4±0.43 | 6.4 | 3.5±0.63 | 8.4 | 3.4±0.48 | 8.4 | 3.5±0.63 | 8.4 |
Values for average consumption are mean±SD. Data are from the National Health and Nutrition Examination Survey 2011 to 2012, derived from two 24-hour dietary recalls per person, with population standard deviations adjusted for within-person vs between-person variation (analyses courtesy of Dr Colin Rehm, Tufts University). All values are energy adjusted by individual regressions or percent energy, and for comparability, means and proportions are reported for a 2000-kcal/d diet. To obtain actual mean consumption levels, the group means for each food or nutrient can be multiplied by the group-specific total calories (kcal/d) divided by 2000 kcal/d. Compared with 2014 and earlier American Heart Association Statistical Updates, the calculations for foods now use the US Department of Agriculture’s (USDA) Food Patterns Equivalent Database on composition of various mixed dishes, which incorporates partial amounts of various foods (eg, vegetables, nuts, processed meats) in mixed dishes; in addition, the characterization of whole grains is now derived from the USDA database instead of the ratio of total carbohydrate to fiber. ALA indicates α-linoleic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; NA, not available; NH, non-Hispanic; and n-6-PUFA, ω-6-polyunsaturated fatty acid.
All intakes and guidelines adjusted to a 2000 kcal/d diet. Servings are defined as follows: whole grains, 1-oz equivalents; fruits and vegetables, ½-cup equivalents; legumes, ½ cup; fish/shellfish, 3.5 oz or 100 g; nuts and seeds, 1 oz; unprocessed red or processed meat, 3.5 oz or 100 g; sugar-sweetened beverages, 8 fl oz; sweets and bakery desserts, 50 g. Guidelines defined as follows: whole grains, 3 or more 1-oz equivalent (eg, 21 g whole wheat bread, 82 g cooked brown rice, 31 g Cheerios) servings/d54; fruits, 2 or more cups/d54; nonstarchy vegetables, 2½ or more cups/d; legumes, 1.5 or more cups/wk54; fish or shellfish, 2 or more 100-g (3.5-oz) servings/wk54; nuts and seeds, 4 or more 1-oz servings/wk54; processed meats (bacon, hot dogs, sausage, processed deli meats), 2 or fewer 100-g (3.5-oz) servings/wk (¼ of discretionary calories); sugar-sweetened beverages (defined as ≥50 cal/8 oz, excluding 100% fruit juices), ≤36 oz/wk (≈¼ of discretionary calories); sweets and bakery desserts, 2.5 or fewer 50-g servings/wk (≈¼ of discretionary calories); EPA/DHA, ≥0.250 g/d54; ALA, ≥1.6/1.1 g/d (males/females)54; saturated fat, <10% energy; dietary cholesterol, <300 mg/d54; total fat, 20% to 35% energy; dietary fiber, ≥28 g/d54; and sodium, <2.3 g/d.54 No dietary targets are listed for starchy vegetables and unprocessed red meats because of their positive association with long-term weight gain and their positive or uncertain relation with diabetes mellitus and cardiovascular disease.
Including white potatoes (chips, fries, mashed, baked, roasted, mixed dishes), corn, plantain, green peas, etc. Sweet potatoes, pumpkin, and squash are considered red-orange vegetables by the USDA and are included in nonstarchy vegetables.
Table 5-3.
Dietary Consumption of Selected Foods and Nutrients Related to Cardiometabolic Health Among US Children and Teenagers in 2011 to 2012
| Boys (5–9 y) | Girls (5–9 y) | Boys (10–14 y) | Girls (10–14 y) | Boys (15–19 y) | Girls (15–19 y) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | Average Consumption | % Meeting Guidelines* | |
| Foods | ||||||||||||
| Whole grains, servings/d | 0.9±0.4 | 2.2 | 0.9±0.5 | 2.8 | 0.8±0.4 | 3.8 | 0.7±0.4 | 2.5 | 0.9±0.8 | 4.7 | 0.9±0.3 | 4.1 |
| Fruits, servings/d | 1.7±1.1 | 8.3 | 1.9±1.4 | 13.7 | 1.4±0.9 | 8.3 | 1.4±0.8 | 3.3 | 1.3±1.1 | 6.1 | 0.9±0.8 | 4.7 |
| Fruits including 100% fruit juice, servings/d | 2.7±1.3 | 22.3 | 2.7±1.5 | 23.1 | 2.1±1.2 | 16.3 | 2.0±1.1 | 16.5 | 2.0±0.4 | 14.3 | 1.6±1.2 | 11.2 |
| Nonstarchy vegetables, servings/d | 1.1±0.8 | 0.9 | 1.1±0.7 | 0.0 | 1.2±0.6 | 1.3 | 1.2±0.7 | 1.1 | 1.4±0.4 | 0.5 | 1.5±0.1 | 1.2 |
| Starchy vegetables, servings/d † | 0.6±0.2 | NA | 0.7±0.2 | NA | 0.7±0.3 | NA | 0.7±0.3 | NA | 0.8±0.6 | NA | 0.7±0.3 | NA |
| Legumes, servings/wk | 1.2±1.7 | 14.2 | 0.7±0.8 | 9.2 | 1.0±1.0 | 13.9 | 1.2±1.5 | 16.6 | 1.3±1.4 | 15.4 | 0.9±0.4 | 9.6 |
| Fish/shellfish, servings/wk | 1.0±1.0 | 14.3 | 0.5±0.7 | 7.2 | 0.3±0.3 | 6.7 | 0.4±0.3 | 7.7 | 0.6±1.3 | 10.8 | 0.9±0.6 | 11.9 |
| Nuts and seeds, servings/wk | 2.3±4.6 | 15.4 | 2.1±1.5 | 13.3 | 1.1±0.8 | 6.4 | 1.3±0.2 | 10.9 | 1.8±1.3 | 17.4 | 2.7±2.0 | 12.9 |
| Unprocessed red meats, servings/wk | 1.7±0.7 | NA | 1.6±1.1 | NA | 2.3±1.5 | NA | 1.9±1.1 | NA | 3.6±1.8 | NA | 2.5±1.5 | |
| Processed meats, servings/wk | 2.0±0.5 | 54.1 | 1.6±1.0 | 63.9 | 2.0±0.5 | 64.4 | 1.6±0.2 | 70.3 | 2.3±1.5 | 57.7 | 1.4±1.0 | 75.4 |
| Sugar-sweetened beverages, servings/wk | 7.7±6.2 | 44.2 | 6.0±3.8 | 52.5 | 11.6±5.3 | 20.7 | 9.7±7.9 | 37.8 | 12.4±5.8 | 24.5 | 14±6.0 | 32.8 |
| Sweets and bakery desserts, servings/wk | 8.0±1.8 | 27.0 | 7.7±2.8 | 15.5 | 8.3±4.5 | 29.1 | 6.6±2.5 | 31.2 | 4.7±3.5 | 42.0 | 6.0±3.5 | 38.7 |
| Nutrients | ||||||||||||
| Total calories, kcal/d | 2048±457 | NA | 1767±238 | NA | 2145±497 | NA | 1745±343 | NA | 2444±664 | NA | 1838±287 | NA |
| EPA/DHA, g/d | 0.063±0.052 | 5.7 | 0.041±0.024 | 2.1 | 0.034±0.028 | 1.8 | 0.034±0.025 | 2.1 | 0.065±0.092 | 6.3 | 0.055±0.045 | 5.2 |
| ALA, g/d | 1.39±0.21 | 26.4 | 1.41±0.17 | 76.7 | 1.36±0.11 | 29.0 | 1.45±0.16 | 72.7 | 1.45±0.16 | 33.9 | 1.54±0.17 | 76.5 |
| n-6 PUFA, % energy | 6.4±1.2 | NA | 6.7±1.2 | NA | 6.5±1.0 | NA | 6.7±1.2 | NA | 6.8±1.3 | NA | 7.1±0.8 | NA |
| Saturated fat, % energy | 11.7±1.6 | 23.7 | 11.3±0.8 | 30.4 | 11.0±1.6 | 31.0 | 11.4±2.2 | 30.0 | 10.8±1.3 | 40.3 | 10.9±0.6 | 39.9 |
| Dietary cholesterol, mg/d | 215±144 | 80.1 | 212±142 | 79.8 | 242±175 | 77.7 | 226±149 | 78.9 | 269±152 | 67.9 | 269±200 | 74.1 |
| Total fat, % energy | 33±4.1 | 66.3 | 33±2.7 | 63.5 | 31.9±3.1 | 69.0 | 32.6±0.6 | 60.6 | 32.5±3.8 | 57.8 | 32.6±2.9 | 66.1 |
| Carbohydrate, % energy | 53.9±2.7 | NA | 53.9±1.5 | NA | 54.7±4.0 | NA | 54±3.8 | NA | 51.8±3.0 | NA | 53.2±3.3 | NA |
| Dietary fiber, g/d | 15.1±2.8 | 1.8 | 15.9±2.9 | 1.0 | 14.9±3.3 | 2.0 | 15.4±2.8 | 1.2 | 15.7±3.9 | 2.8 | 14.4±3.4 | 1.0 |
| Sodium, g/d | 3.2±0.41 | 7.2 | 3.1±0.40 | 11.1 | 3.3±0.28 | 4.4 | 3.4±0.49 | 7.9 | 3.5±0.32 | 3.0 | 3.5±0.52 | 2.4 |
Values for average consumption are mean±SD. Data are from the National Health and Nutrition Examination Survey 2011 to 2012, derived from two 24-hour dietary recalls per person, with population standard deviations adjusted for within-person vs between-person variation (analyses courtesy of Dr Colin Rehm, Tufts University). All values are energy adjusted by individual regressions or percent energy, and for comparability, means and proportions are reported for a 2000-kcal/d diet. To obtain actual mean consumption levels, the group means for each food or nutrient can be multiplied by the group-specific total calories (kcal/d) divided by 2000 kcal/d. Compared with 2014 and earlier American Heart Association Statistical Updates, the calculations for foods now use the US Department of Agriculture’s (USDA) Food Patterns Equivalent Database on composition of various mixed dishes, which incorporates partial amounts of various foods (eg, vegetables, nuts, processed meats) in mixed dishes; in addition, the characterization of whole grains is now derived from the USDA database instead of the ratio of total carbohydrate to fiber. ALA indicates α-linoleic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; NA, not available; and n-6-PUFA, ω-6-polyunsaturated fatty acid.
All intakes and guidelines adjusted to a 2000 kcal/d diet. Servings defined as follows: whole grains, 1-oz equivalents; fruits and vegetables, ½-cup equivalents; legumes, ½ cup; fish/shellfish, 3.5 oz or 100 g; nuts and seeds, 1 oz; unprocessed red or processed meat, 3.5 oz or 100 g; sugar-sweetened beverages, 8 fl oz; sweets and bakery desserts, 50 g. Guidelines defined as follows: whole grains, 3 or more 1-oz equivalent (eg, 21 g whole wheat bread, 82 g cooked brown rice, 31 g Cheerios) servings/d54; fruits, 2 or more cups/d54; non-starchy vegetables, 2½ or more cups/d54; legumes, 1.5 or more cups/wk54; fish or shellfish, 2 or more 100-g (3.5-oz) servings/wk54; nuts and seeds, 4 or more 1-oz servings/wk54; processed meats (bacon, hot dogs, sausage, processed deli meats), 2 or fewer 100-g (3.5-oz) servings/wk (¼ of discretionary calories)54; sugar-sweetened beverages (defined as ≥50 cal/8 oz, excluding 100% fruit juices), ≤36 oz/wk (≈¼ of discretionary calories)14,54; sweets and bakery desserts, 2.5 or fewer 50-g servings/wk (≈¼ of discretionary calories)14,54; EPA/DHA, ≥0.250 g/d54; ALA, ≥1.6/1.1 g/d (males/females)54; saturated fat, <10% energy54; dietary cholesterol, <300 mg/d54; total fat, 20% to 35% energy54; dietary fiber, ≥28/d54; and sodium, <2.3 g/d.54 No dietary targets are listed for starchy vegetables and unprocessed red meats because of their positive association with long-term weight gain and their positive or uncertain relation with diabetes mellitus and cardiovascular disease.
Including white potatoes (chips, fries, mashed, baked, roasted, mixed dishes), corn, plantain, green peas, etc. Sweet potatoes, pumpkin, and squash are considered red-orange vegetables by the USDA and are included in nonstarchy vegetables.
Chart 5-1. Trends in mean healthy diet scores for children and adults, NHANES 2003 to 2004 through 2011 to 2012.
Primary metrics include fruits/vegetables, whole grains, fish, sugar-sweetened beverages, and sodium. Secondary metrics include nuts, seeds, and legumes; processed meats; and saturated fats. Components of poor, intermediate, and ideal diet are defined in Table 5-1. Mean healthy diet scores based on the alternative scoring ranges described in Table 5-1.
NHANES indicates National Health and Nutrition Examination Survey.
Sources: My Life Check: Life’s Simple 71; Lloyd-Jones et al2; Rehm et al.3
Chart 5-6. Percentage of sodium from dietary sources in the United States, 2005 to 2006.
Source: Applied Research Program, National Cancer Institute.4
Chart 5-7. Total US food expenditures away from home and at home, 1977 and 2007.
Data derived from Davis et al.10
This chapter of the Update highlights national dietary habits, focusing on key foods, nutrients, dietary patterns, and other dietary factors related to cardiometabolic health. It is intended to examine current intakes, trends and changes in intakes, and estimated effects on disease to support and further stimulate efforts to monitor and improve dietary habits in relation to cardiovascular health.
Abbreviations Used in Chapter 5
| AHA | American Heart Association |
| ALA | α-linoleic acid |
| ARIC | Atherosclerosis Risk in Communities Study |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CHD | coronary heart disease |
| CI | confidence interval |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DASH | Dietary Approaches to Stop Hypertension |
| DBP | diastolic blood pressure |
| DHA | docosahexaenoic acid |
| DM | diabetes mellitus |
| EPA | eicosapentaenoic acid |
| GFR | glomerular filtration rate |
| GISSI | Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico |
| HbA1c | hemoglobin A1c (glycosylated hemoglobin) |
| HD | heart disease |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| LDL-C | low-density lipoprotein cholesterol |
| max. | maximum |
| MI | myocardial infarction |
| n-6-PUFA | ω-6-polyunsaturated fatty acid |
| NA | not available |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| OR | odds ratio |
| PA | physical activity |
| PREDIMED | Prevención con Dieta Mediterránea |
| RCT | randomized controlled trial |
| RR | relative risk |
| SBP | systolic blood pressure |
| SD | standard deviation |
| SSB | sugar-sweetened beverage |
| TC | total cholesterol |
| USDA | US Department of Agriculture |
| WHI | Women’s Health Initiative |
Prevalence and Trends in the AHA 2020 Healthy Diet Metrics
(See Table 5-1 and Charts 5-1 through 5-5)
The AHA’s 2020 Impact Goals include a new priority of improving cardiovascular health.1 The definition of cardiovascular health includes a healthy diet pattern, characterized by 5 primary and 3 secondary metrics (Table 5-1), that should be consumed within the context of a healthy dietary pattern that is appropriate in energy balance and consistent with a DASH-type eating plan.1
The AHA scoring system for ideal, intermediate, and poor diet patterns uses a binary-based scoring system, which awards 1 point for meeting the ideal target for each metric and 0 points otherwise.2 For better consistency with other dietary pattern scores such as DASH, an alternative continuous scoring system has been developed to measure small improvements over time towards the AHA ideal target levels (Table 5-1). The dietary targets remain the same, and progress toward each of these targets is assessed by use of a more granular range of 1 to 10 (rather than 0 to 1).
-
On the basis of the alternative scoring system, between 2003 to 2004 and 2011 to 2012 in the United States, the mean AHA healthy diet score improved in both children and adults (Chart 5-1). The prevalence of an ideal healthy diet score (>80) increased from 0.2% to 0.6% in children (Chart 5-2) and from 0.7% to 1.5% in adults (Chart 5-3). The prevalence of an intermediate healthy diet score (40–79) increased from 30.6% to 44.7% in children and from 49.0% to 57.5% in adults.3
These improvements were largely attributable to increased whole grain consumption and decreased sugar-sweetened beverage consumption in both children and adults (Charts 5-4 and 5-5).3 No major trends were evident in adults in progress toward the targets for consumption of fish or sodium.
Other significant changes between 1999 and 2012 included national increases in nuts, seeds, and legumes (0.26 servings/d) and whole fruit (0.15 servings/d) and decreases in 100% fruit juice (0.11 servings/d) and white potatoes (0.07 servings/d (95% CI).3
Although AHA healthy diet scores tended to improve in all race/ethnicity, income, and education levels, many disparities present in earlier years widened over time, with generally smaller improvements seen in minority groups and those with lower income or education.3 For example, among Americans with the highest family income (income-to-poverty ratio ≥3.0), the proportion with a poor diet decreased from 50.5% to 35.7%, whereas among those with lowest family income (income-to-poverty ratio <1.3), the proportion with a poor diet decreased from 67.8% to only 60.6%.
Dietary Habits in the United States: Current Intakes
Foods and Nutrients
Adults
The dietary consumption by US adults of selected foods and nutrients related to cardiometabolic health is detailed in Table 5-2 according to sex and race or ethnic subgroups.
Average daily energy intake among US adults was ≈2500 calories in males and 1800 calories in females.
Average consumption of whole grains was 1.1 servings per day by non-Hispanic white males and females, 0.8 to 0.9 servings per day by non-Hispanic black males and females, and 0.6 to 0.8 servings by Mexican American males and females. For each of these groups, fewer than 10% of adults met guidelines of ≥3 servings per day.
Average fruit consumption ranged from 1.0 to 1.6 servings per day in these sex and race or ethnic subgroups: ≈9% of non-Hispanic whites, 7% of non-Hispanic blacks, and 6% of Mexican Americans met guidelines of ≥2 cups per day. When 100% fruit juices were included, the number of servings increased and the proportions of adults consuming ≥2 cups per day nearly doubled in non-Hispanic whites, doubled in non-Hispanic blacks, and more than doubled in Mexican Americans.
Average nonstarchy vegetable consumption ranged from 1.7 to 2.7 servings per day. Across all race/ethnic subgroups, non-Hispanic white females were the only group meeting the target of consuming ≥2.5 cups per day.
Average consumption of fish and shellfish was lowest among Mexican American females and white females (0.8 and 1.0 servings per week, respectively) and highest among non-Hispanic black females and non-Hispanic black and Mexican American males (1.9 and 1.7 servings per week, respectively). Generally, only 15% to 27% of adults in each sex and race or ethnic subgroup consumed at least 2 servings per week.
Average weekly consumption of nuts and seeds was ≈3.5 servings among non-Hispanic whites and 2.5 servings among non-Hispanic blacks and Mexican Americans. Approximately 1 in 4 whites, 1 in 6 non-Hispanic blacks, and 1 in 8 Mexican Americans met guidelines of ≥4 servings per week.
Average consumption of unprocessed red meats was higher in males than in females, up to 4.8 servings per week in Mexican American males.
Average consumption of processed meats was lowest among Mexican American females (1.1 servings per week) and highest among non-Hispanic black and non-Hispanic white males (≈2.5 servings per week). Between 57% (non-Hispanic white males) and 79% (Mexican American females) of adults consumed 2 or fewer servings per week.
Average consumption of sugar-sweetened beverages ranged from 6.8 servings per week among non-Hispanic white females to nearly 12 servings per week among Mexican American males. Females generally consumed less than males. Some adults, 33% (Mexican American males) to 65% (non-Hispanic white females), consumed <36 oz/wk.
Average consumption of sweets and bakery desserts ranged from 3.9 servings per week (Mexican American males) to >7 servings per week (white females). Approximately 1 in 3 adults (1 in 2 Mexican American males) consumed no more than 2.5 servings per week.
Average consumption of eicosapentaenoic acid and docosahexaenoic acid ranged from 0.058 to 0.117 g/d in each sex and race or ethnic subgroup. Fewer than 8% of non-Hispanic whites, 14% of non-Hispanic blacks, and 11% of Mexican Americans consumed ≥0.250 g/d.
One third to one half of adults in each sex and race or ethnic subgroup consumed <10% of total calories from saturated fat, and approximately one half to two thirds consumed <300 mg of dietary cholesterol per day.
Only ≈7% to 10% of non-Hispanic whites, 4% to 5% of blacks, and 13% to 14% of Mexican Americans consumed ≥28 g of dietary fiber per day.
Only approximately 6% to 8% of adults in each age and race or ethnic subgroup consumed less than the recommended 2.3 g of sodium per day. Sodium is widespread in the US food supply, with diverse sources (Chart 5-6).4
Foods and Nutrients
Children and Teenagers
(See Table 5-3)
The dietary consumption by US children and teenagers of selected foods and nutrients related to cardiometabolic health is detailed in Table 5-3:
Average whole grain consumption was low, <1 serving per day in all age and sex groups, with <5% of all children in different age and sex subgroups meeting guidelines of ≥3 servings per day.
Average fruit consumption was low and decreased with age: 1.7 to 1.9 servings per day in younger boys and girls (5–9 years of age), 1.4 servings per day in adolescent boys and girls (10–14 years of age), and 0.9 to 1.3 servings per day in teenage boys and girls (15–19 years of age). The proportion meeting guidelines of ≥2 cups per day was also low and decreased with age: ≈8% to 14% in those 5 to 9 years of age, 3% to 8% in those 10 to 14 years of age, and 5% to 6% in those 15 to 19 years of age. When 100% fruit juices were included, the number of servings consumed increased by ≈50%, and proportions consuming ≥2 cups per day increased to nearly 25% of those 5 to 9 years of age, 20% of those 10 to 14 years, and 15% of those 15 to 19 years of age.
Average nonstarchy vegetable consumption was low, ranging from 1.1 to 1.5 servings per day, with <1.5% of children in different age and sex subgroups meeting guidelines of ≥2.5 cups per day.
Average consumption of fish and shellfish was low, ranging between 0.3 and 1.0 servings per week in all age and sex groups. Among all ages, only 7% to 14% of youths consumed ≥2 servings per week.
Average consumption of nuts, seeds, and beans ranged from 1.1 to 2.7 servings per week among different age and sex groups, and generally fewer than 15% of children in different age and sex subgroups consumed ≥4 servings per week.
Average consumption of unprocessed red meats was higher in boys than in girls and increased with age, up to 3.6 and 2.5 servings per week in 15- to 19-year-old boys and girls, respectively.
Average consumption of processed meats ranged from 1.4 to 2.3 servings per week, and the majority of children consumed no more than 2 servings per week of processed meats.
Average consumption of sugar-sweetened beverages was higher in boys than in girls in the 5- to 9-year-old (7.7±6.2 versus 6.0±3.8 servings per week) and 10- to 14-year-old (11.6±5.3 versus 9.7±7.9 servings per week) groups, but higher in girls than in boys in the 15- to 19-year-old group (14±6.0 versus 12.4±5.8 servings per week). Only about half of children 5 to 9 years of age and one quarter of boys 15 to 19 years of age consumed <4.5 servings per week.
Average consumption of sweets and bakery desserts was higher among 5- to 9-year-old and 10- to 14-year-old boys and girls and modestly lower (4.7 to 6 servings per week) among 15- to 19-year-olds. A minority of children in all age and sex subgroups consumed no more than 2.5 servings per week.
Average consumption of eicosapentaenoic acid and docosahexaenoic acid was low, ranging from 0.034 to 0.065 g/d in boys and girls in all age groups. Fewer than 7% of children and teenagers at any age consumed ≥250 mg/d.
Average consumption of saturated fat was ≈11% of calories in boys and girls in all age groups, and average consumption of dietary cholesterol ranged from ≈210 to 270 mg/d, increasing with age. Approximately 25% to 40% of youths consumed <10% energy from saturated fat, and ≈70% to 80% consumed <300 mg of dietary cholesterol per day.
Average consumption of dietary fiber ranged from ≈14 to 16 g/d. Less than 3% of children in all age and sex subgroups consumed ≥28 g/d.
Average consumption of sodium ranged from 3.1 to 3.5 g/d. Only between 2% and 11% of children in different age and sex subgroups consumed <2.3 g/d.
Among children and teenagers, average daily caloric intake is higher in boys than in girls and increases with age in boys.
Impact on US Mortality
One report used consistent and comparable risk assessment methods and nationally representative data to estimate the impact of all major modifiable risk factors on mortality and morbidity in the United States in 1990 and in 2010.5 Among 17 leading risk factors in the United States in 2010, suboptimal dietary habits were the leading cause of both mortality and DALYs lost. In 2010, a total of 678 000 deaths of all causes were attributable to suboptimal diet.
A previous investigation reported the estimated mortality effects of several specific dietary risk factors in 2005 in the United States. High dietary salt consumption was estimated to be responsible for 102 000 annual deaths, low dietary omega-3 fatty acids for 84 000 annual deaths, high dietary trans fatty acids for 82 000 annual deaths, and low consumption of fruits and vegetables for 55 000 annual deaths.6
Cost
(See Chart 5-7)
The US Department of Agriculture forecast that the Consumer Price Index for all food would increase 3.0% to 4.0% in 2013 as retailers continued to pass on higher commodity and energy costs to consumers in the form of higher retail prices. The Consumer Price Index for food increased 3.7% in 2011. Prices for foods eaten at home increased 4.8% in 2011, whereas prices for foods eaten away from home increased by 1.9%.7
A meta-analysis of price comparisons of healthier versus unhealthier diet patterns found that the healthiest diet patterns cost, on average, approximately $1.50 more per person per day to consume.8
In an assessment of snacks served at YMCA afterschool programs from 2006 to 2008, healthful snacks were ≈50% more expensive ($0.26 per snack) than less healthful snacks.9 Higher snack costs were driven by serving fruit juice compared with water; serving refined grains without trans fat compared with refined grains with trans fats; and serving fruit and canned or frozen vegetables. Serving fresh vegetables (mostly carrots or celery) or whole grains did not alter price.
As a proportion of income, food has become less expensive over time in the United States. As a share of personal disposable income, average (mean) total food expenditures by families and individuals have decreased from 22.3% (1949) to 18.1% (1961) to 14.9% (1981) to 11.3% (2011). For any given year, the share of disposable income spent on food is inversely proportional to absolute income. The share increases as absolute income levels decline.7
The proportion of total US food expenditures for meals outside the home, as a share of total food dollars, increased from 27% in 1961 to 40% in 1981 to 49% in 2011.10
The proportion of sales of meals and snacks from fast-food restaurants, compared with total meals and snacks away from home, increased from 5% in 1958 to 29% in 1982 to 36% in 2011.7
Among 153 forms of fruits and vegetables priced with 2008 Nielsen Homescan data, price and calorie per portion of 20 fruits and vegetables were compared with 20 common snack foods, such as cookies, chips, pastries, and crackers. Average price per portion of fruits and vegetables was 31 cents, with an average of 57 calories per portion, compared with 33 cents and 183 calories per portion for snack foods.7
An overview of the costs of various strategies for primary prevention of CVD determined that the estimated costs per year of life gained were between $9800 and $18 000 for statin therapy, $1500 for nurse screening and lifestyle advice, $500 to $1250 for smoking cessation, and $20 to $900 for population-based healthy eating.11
Each year, more than $33 billion in medical costs and $9 billion in lost productivity resulting from HD, cancer, stroke, and DM are attributed to poor nutrition.12–15
Two separate cost-effectiveness analyses estimated that population reductions in dietary salt would not only be cost-effective, but actually cost-saving. 16,17 In 1 analysis, a 1.2-g/d reduction in dietary sodium was projected to reduce US annual cases of incident CHD by 60 000 to 120 000, stroke by 32 000 to 66 000, and total mortality by 44 000 to 92 000.17 If accomplished through a regulatory intervention, estimated savings in healthcare costs would be $10 to $24 billion annually. 17 Such an intervention would be more cost-effective than using medications to lower BP in all people with hypertension.
Secular Trends
Trends in Dietary Patterns
In addition to individual foods and nutrients, overall dietary patterns can be very useful to assess diet quality.18 Different dietary patterns have been defined, such as Mediterranean, DASH-type, Healthy Eating Index–2010, Alternate Healthy Eating Index, Western, prudent, and vegetarian patterns. The original DASH diet was low fat; a higher-monounsaturated-fat DASH-type diet is even more healthful and similar to a traditional Mediterranean dietary pattern.19,20 The Healthy Eating Index–2010, which reflects compliance with the 2010 US Dietary Guidelines, exhibits a wide distribution among the US population, with a 5th percentile score of 31.7 and a 95th percentile score of 70.4 based on NHANES 2003 to 2004 data (theoretical maximum=100).21 Average diet quality is worse in males (score=49.8) than in females (52.7), in younger adults (45.4) than in older adults (56.1), and in smokers (45.7) than in nonsmokers (53.3).
Between 1999 and 2010, the average Alternate Healthy Eating Index–2010 score of US adults improved from 39.9 to 46.8.22 This was related to reduced intake of trans fat (accounting for more than half of the improvement), sugar-sweetened beverages, and fruit juice, as well as an increased intake of whole fruit, whole grains, polyunsaturated fatty acids, and nuts and legumes. Adults with greater family income and education had higher scores, and the gap between low and high socioeconomic status widened over time, from 3.9 points in 1999 to 2000 to 7.8 points in 2009 to 2010.
Worldwide, 2 separate, relatively uncorrelated dietary patterns can be characterized, 1 by greater intakes of health-promoting foods (eg, fruits, vegetables, nuts, fish), and 1 by lower intakes of less optimal foods (eg, processed meats, sugar-sweetened beverages).23 In 2010, compared with low-income nations, high-income nations had better diet patterns based on healthful foods, but substantially worse diet patterns based on unhealthful foods. Between 1990 and 2010, both types of dietary patterns improved in high-income Western countries but worsened or did not improve in low-income countries in Africa and Asia. Middle-income countries showed the largest improvements in dietary patterns based on healthful foods, but the largest deteriorations in dietary patterns based on unhealthful foods. Overall, global consumption of healthy foods improved, but was outpaced by increased intake of unhealthy foods in most world regions.
Trends in Dietary Supplements
Use of dietary supplements is common in the United States among both adults and children:
Approximately half of US adults in 2007 to 2010 used ≥1 dietary supplement, with the most common supplement being multivitamin-multimineral products (32% of males and females reporting such use).24 It has been shown that most supplements are taken daily and for ≥2 years.25 Supplement use is associated with older age, higher education, greater PA, moderate alcohol consumption, lower BMI, abstinence from smoking, having health insurance, and white race.24,25 Previous research also suggests that supplement users have higher intakes of most vitamins and minerals from their food choices alone than non-users. 26,27 The primary reasons US adults in 2007 to 2010 reported for using dietary supplements were to “improve overall health” (45%) and to “maintain health” (33%).24
One third (32%) of US children (birth to 18 years of age) used dietary supplements in 1999 to 2002, with the highest use (48.5%) occurring among 4- to 8-year-olds. The most common supplements were multivitamins and multiminerals (58% of supplement users). The primary nutrients supplemented (either by multivitamins or individual vitamins) included vitamin C (29% of US children), vitamin A (26%), vitamin D (26%), calcium (21%), and iron (19%). Supplement use was associated with higher family income, a smoke-free home environment, lower child BMI, and less screen time (television, video games, or computers).24,28 In a 2005 to 2006 telephone survey of US adults, 41.3% were making or had made in the past a serious weight-loss attempt. Of these, one third (33.9%) had used a dietary supplement for weight loss, with such use being more common in females (44.9%) than in males (19.8%) and in blacks (48.7%) or Hispanics (41.6%) than in whites (31.2%); in those with high school education or less (38.4%) than in those with some college or more (31.1%); and in those with household income <$40 000 per year (41.8%) than in those with higher incomes (30.3%).29
A multicenter randomized trial in patients with diabetic nephropathy found that B vitamin supplementation (folic acid 2.5 mg/d, vitamin B6 25 mg/d, and vitamin B12 1 mg/d) decreased GFR and increased risk of MI and stroke compared with placebo.30
Fish oil supplements at doses of 1 to 2 g/d have shown CVD benefits in 2 large randomized, open-label trials and 1 large randomized, placebo-controlled trial (GISSI-Prevenzione, Japan Eicosapentaenoic Acid Lipid Intervention Study, and GISSI-HF),31–33 but several other trials of fish oil have not shown significant effects on CVD risk.34 A meta-analysis of all RCTs demonstrated a significant reduction for cardiac mortality but no statistically significant effects on other CVD end points.35
Trends in Energy Balance and Adiposity
Energy balance, or consumption of total calories appropriate for needs, is determined by the balance of average calories consumed versus expended. This balance depends on multiple factors, including calories consumed, PA, body size, age, sex, and underlying basal metabolic rate. Thus, one person could consume relatively high calories but have negative energy balance (as a result of even greater calories expended), whereas another might consume relatively few calories but have positive energy balance (because of low calories expended). Given such variation, the most practical and reasonable method to assess energy balance in populations is to assess changes in weight over time. Growing evidence indicates that calorie for calorie, certain foods may be more highly obesogenic; others, modestly obesogenic; others, relatively neutral; and still others, actually protective against weight gain when their consumption is increased. These varying effects appear to relate to divergent influences on complex physiological pathways of long-term weight regulation, including those related to hunger, satiety, brain reward, hepatic de novo lipogenesis, adipocyte function, visceral adiposity, interactions with the gut inflammasome and microbiome, and energy expenditure. This evidence is detailed below.
The US overweight and obesity epidemic continues to be a public health concern. The ageadjusted prevalence of obesity in 2013 to 2014 was 35% among males and 40.4% among females. For females, the prevalence of overall obesity and of class 3 obesity showed significant linear trends for increase between 2005 and 2014, whereas there were no significant trends for males.36
In a nationally representative study between 1988 and 2014 of US children and adolescents aged 2 to 19 years, the prevalence of obesity in 2011 to 2014 was 17.0% and that of extreme obesity was 5.8%. By age group, the prevalence varied significantly. For children 2 to 5 years old, obesity prevalence was ≈9%; for children 6 to 11 years old, it was 17.5%; and for adolescents 12 to 19 years of age, it was 20.5%. For children 6 to 11 years old, the prevalence rose but leveled off in 2007 to 2008, but for adolescents (12 to 19 years old), the upward trajectory has continued.37
Until 1980, total energy intake remained relatively constant.38,39 However, data from NHANES indicate that between 1971 and 2004, average total energy intake among US adults increased by 22% in females (from 1542 to 1886 kcal/d) and by 10% in males (from 2450 to 2693 kcal/d).40 These increases are supported by data from 2 older surveys, the Nationwide Food Consumption Survey (1977–1978) and the Continuing Surveys of Food Intake (1989–1998).41 More recent data show that energy intake appears to have relatively stabilized among US adults between 1999 and 2008.42
Another analysis of national data estimated that increases in energy intake between 1980 and 1997 were primarily attributable to increases in dietary carbohydrates.43 Specifically, nearly 80% of the increase in total energy came from carbohydrates, 12% from protein, and only 8% from fat. These increases in calories were primarily attributable to greater refined carbohydrate intake, particularly of starches, refined grains, and sugars.
Other specific changes related to increased caloric intake in the United States since 1980 include larger portion sizes, greater food quantity and calories per meal, and increased consumption of sugar-sweetened beverages, snacks, and commercially prepared (especially fast-food) meals.41,44–49 In more recent years, intakes of sugar-sweetened beverages have been decreasing nationally.22,50
Between 1977 and 1996, the average portion sizes for many foods increased at fast-food outlets, other restaurants, and home. On the basis of 1 study, these included a 33% increase in the average portion of Mexican food (from 408 to 541 calories), a 34% increase in the average portion of cheeseburgers (from 397 to 533 calories), a 36% increase in the average portion of french fries (from 188 to 256 calories), and a 70% increase in the average portion of salty snacks such as crackers, potato chips, pretzels, puffed rice cakes, and popcorn (from 132 to 225 calories).41
In 1 analysis, among U.S. children 2 to 7 years of age, an estimated energy imbalance of only 110 to 165 kcal/d (the equivalent of one 12- to 16-oz bottle of soda/cola) was sufficient to account for the excess weight gain between 1988 to 1994 and 1999 to 2002.51
In a quantitative analysis using various US surveys between 1977 and 2010, the relations of national changes in energy density, portion sizes, and number of daily eating/drinking occasions to changes in total energy intake were assessed.38,39 Changes in energy density were not consistently linked to energy intake over time, whereas increases in both portion size and number of eating occasions were linked to greater energy intake.
Among US children 2 to 18 years of age, increases in energy intake between 1977 and 2006 (179 kcal/d) were entirely attributable to substantial increases in energy eaten away from home (255 kcal/d).52 The percentage of energy eaten away from home increased from 23.4% to 33.9% during this time, with a shift toward energy from fast food as the largest contributor to foods eaten away from home for all age groups.
A county-level investigation based on BRFSS and NHANES data found that prevalence of sufficient PA in the United States actually increased from 2001 to 2009, but that this was matched by increases in obesity in almost all counties during the same time period, with low correlation between level of PA and obesity in US counties.53
Trends in Specific Dietary Habits
Several changes in foods and nutrients have occurred over time. Selected changes are highlighted below.
Macronutrients
Starting in 1977 and continuing until the most recent dietary guidelines revision in 2015, a major focus of US dietary guidelines was reduction of dietary fats.54 Between 1977 and 1999, average total fat consumption declined as a percentage of calories from 36.9% to 33.4% in males and from 36.1% to 33.8% in females.55 After this significant decline, total fat consumption remained relatively stable among US adults from 1999 to 2008.42
Dietary guidelines during this time also emphasized eating a variety of foods with plenty of whole grain products, fruits, and vegetables while limiting sugar, fat, and other selected nutrients.10,54 Consistent with this message, from 1971 to 2004, total carbohydrate intake increased from 42.4% to 48.2% of calories in males and from 45.4% to 50.6% of calories in females.55 Evaluated as absolute intakes, the increase in total calories consumed during this period was attributable primarily to the greater consumption of carbohydrates, both as foods (starches and grains) and as beverages.55,56 In more recent years, these trends have stabilized, with relatively stable intakes to slight declines in carbohydrate intake (expressed as percentage of energy) among US children and adults from 1999 to 2010, with corresponding slight increases in protein intake.57 However, intakes of whole grains, fruits, and vegetables have remained consistently stable and below levels recommended by the US Dietary Guidelines.
Sugar-Sweetened Beverages
(See Charts 5-4 and 5-6)
Between 1965 and 2002, the average percentage of total calories consumed from beverages in the United States increased from 11.8% to 21.0% of energy, which represents an overall absolute increase of 222 kcal/d per person.48 This increase was largely caused by increased consumption of sugar-sweetened beverages and alcohol: Average consumption of fruit juices went from 20 to 39 kcal/d; of milk, from 125 to 94 kcal/d; of alcohol, from 26 to 99 kcal/d; of sweetened fruit drinks, from 13 to 38 kcal/d; and of soda/cola, from 35 to 143 kcal/d.38
In addition to increased overall consumption, the average portion size of a single sugar-sweetened beverage increased by >50% between 1977 and 1996, from 13.1 to 19.9 fl oz.41
Among children and teenagers (2–19 years of age), the largest increases in consumption of sugar-sweetened beverages between 1988 to 1994 and 1999 to 2004 were seen among black and Mexican American youth compared with white youth.49
Between 2003 to 2004 and 2011 to 2012, there was significant progress toward success for both US adults and children in achieving the AHA 2020 dietary target of no more than 36 fl oz of sugar-sweetened beverages per week (Charts 5-4 and 5-5).
In contrast, between 1999 and 2010, sugar-sweetened beverage intake decreased among both youth and adults in the United States, consistent with increased attention to their importance as a cause of obesity. In 2009 to 2010, youth and adults consumed a daily average of 155 and 151 kcal from sugar-sweetened beverages, respectively, a decrease from 1999 to 2000 of 68 and 45 kcal/d, respectively.58 This reduction parallels the plateau of the obesity epidemic in US youth.59
Globally, between 1999 and 2010, sugar-sweetened beverage intake increased in several countries. 60 Among adults, mean global intake was highest in males aged 20 to 39 years, at 1.04 8-oz servings per day. In comparison, globally, females >60 years of age had the lowest mean consumption at 0.34 servings per day. Sugar-sweetened beverage consumption was highest in the Caribbean, with adults consuming on average 2 servings per day, and lowest in East Asia, at 0.20 servings per day. Adults in the United States had the 26th-highest consumption of 187 countries.
Selected Foods
(See Charts 5-4 through 5-5)
Between 1994 and 2005, the average consumption of fruits and vegetables declined slightly, from a total of 3.4 to 3.2 times per day. The proportions of males and females consuming combined fruits and vegetables ≥5 times per day were low (≈20% and 29%, respectively) and did not change during this period.61
Between 2003 to 2004 and 2011 to 2012, there was no major change in the success of US adults or children in achieving the AHA 2020 dietary targets of 4.5 cups of total fruits and vegetables per day or 2 servings of fish per week. During this same period, there was significant progress toward success of both US adults and children in achieving the AHA 2020 dietary target of at least three 1-oz servings of whole grains per day (Charts 5-4 and 5-5).3
Sodium
(See Charts 5-4 through 5-5)
Although inconsistent methodology over time limits the ability to make strong conclusions, the current available data suggest that US sodium intake has remained relatively stable between 1957 and 2003.62
Worldwide in 2010, mean sodium intake among adults was 3950 mg/d, which corresponds to salt intake of ≈10 g/d.63 Across world regions, mean sodium intakes were highest in Central Asia (5510 mg/d) and lowest in Eastern sub-Saharan Africa (2180 mg/d). Across countries, the lowest observed mean national intakes were ≈1500 mg/d. Between 1990 and 2010, global mean sodium intake appeared to remain relatively stable, although data on trends in many world regions were suboptimal.63
Cardiovascular Health Impact
Dietary habits affect multiple cardiovascular risk factors, including both established risk factors (SBP, DBP, LDL-C levels, HDL-C levels, glucose levels, and obesity/weight gain) and novel risk factors (eg, inflammation, cardiac arrhythmias, endothelial cell function, triglyceride levels, lipoprotein[a] levels, and heart rate).
Cardiovascular and Metabolic Risk
Overweight and Obesity
For short-term (up to 1–2 years) weight loss among overweight and obese individuals, the macronutrient composition of the diet has much less influence than compliance with the selected diet.64
In ad libitum (not energy restricted) diets, a low-carbohydrate (high fat) diet demonstrated better weight loss and reduced fat mass than a low-fat (high carbohydrate) diet at 1 year.65
In ad libitum (not energy restricted) diets, intake of dietary sugars was positively linked to weight gain.66 However, isoenergetic exchange of dietary sugars with other carbohydrates had no relationship with body weight,66 which suggests that all refined carbohydrates (complex starches and simple sugars) might be similarly obesogenic.
In pooled analyses across 3 prospective cohort studies of US males and females, increased glycemic index and glycemic load were independently associated with greater weight gain over time.67
Across types of foods, energy density (total calories per gram of food) is not consistently linked with weight gain or obesity. For example, nuts have relatively high energy density and are inversely linked to weight gain, and cheese has high energy density and appears relatively neutral, whereas sugar-sweetened beverages have low energy density and increase obesity.67 National changes in energy density over time are not consistently linked to changes in energy intake.38
In analyses of >120 000 US males and females in 3 separate US cohorts followed up for up to 20 years, changes in intakes of different foods and beverages were linked to long-term weight gain in different ways.67,68 Foods and beverages most positively linked to weight gain included high-glycemic carbohydrates such as potatoes, white bread, white rice, low-fiber breakfast cereals, sweets/desserts, and sugar-sweetened beverages, as well as red and processed meats. In contrast, increased consumption of several other foods, including nuts, whole grains, fruits, vegetables, legumes, fish, and yogurt, was linked to relative weight loss over time. These findings suggest that attention to food-based dietary quality, not simply counting total calories, is crucial for long-term weight homeostasis.68
In both adults and children, intake of sugar-sweetened beverages has been linked to weight gain and obesity.69 Randomized trials in children demonstrate reductions in obesity when sugar-sweetened beverages are replaced with noncaloric beverages.69
Diet quality influences activation of brain reward centers, such as the nucleus accumbens. Isocaloric meals richer in rapidly digestible carbohydrate increased hunger and stimulated brain regions associated with reward and craving compared with isocaloric meals that had identical macronutrient content, palatability, and sweetness but were lower in rapidly digestible carbohydrate.70
Dietary factors that stimulate hepatic de novo lipogenesis, such as rapidly digestible grains, starches, and sugars, as well as trans fat, appear more strongly related to weight gain.67,68,71
In animal experiments, probiotics in yogurt alter gut immune responses and protect against obesity and nonalcoholic fatty acid liver disease.72–74
Diet quality might also influence energy expenditure. After intentional weight loss, isocaloric diets higher in fat and lower in rapidly digestible carbohydrates produced significantly smaller declines in total energy expenditure than low-fat, high-carbohydrate diets, with a mean difference of >300 kcal/d.64
Other possible nutritional determinants of positive energy balance (more calories consumed than expended), as determined by adiposity or weight gain, include larger portion sizes, skipping breakfast, consumption of fast food, and eating foods prepared outside the home, although the evidence for long-term relevance of these factors has been inconsistent.75–78
Lower average sleep duration is consistently linked to greater adiposity in both children and adults, and short-term trials demonstrate effects of insufficient sleep on hunger, food choices, and leptin/ghrelin concentrations.79
Societal and environmental factors independently associated with diet quality, adiposity, or weight gain include education, income, race/ethnicity, and (at least cross-sectionally) neighborhood availability of supermarkets.80–82
Other local food-environment characteristics, such as availability of grocery stores (ie, smaller stores than supermarkets), convenience stores, and fast-food restaurants, are not consistently associated with diet quality or adiposity and could be linked to social determinants of health for CVD.83
BP and Blood Cholesterol
Sodium linearly raises BP in a dose-dependent fashion, with stronger effects among older people, hypertensive people, and blacks,84 and induces additional BP-independent damage to renal and vascular tissues.85,86
Compared with a usual Western diet, a DASH-type dietary pattern with low sodium reduced SBP by 7.1 mm Hg in adults without hypertension and by 11.5 mm Hg in adults with hypertension.87
Compared with the low-fat DASH diet, DASH-type diets that increased consumption of either protein or unsaturated fat had similar or greater beneficial effects on CVD risk factors. Compared with a baseline usual diet, each of the DASH-type diets, which included various percentages (27%–37%) of total fat and focused on whole foods such as fruits, vegetables, whole grains, and fish, as well as potassium and other minerals and low sodium, reduced SBP by 8 to 10 mm Hg, DBP by 4 to 5 mm Hg, and LDL-C by 12 to 14 mg/dL. The diets that had higher levels of protein and unsaturated fat also lowered triglyceride levels by 16 and 9 mg/dL, respectively.88 The DASH-type diet higher in unsaturated fat also improved glucose-insulin homeostasis compared with the low-fat/high-carbohydrate DASH diet.89
In a meta-analysis of 60 randomized controlled feeding trials, consumption of 1% of calories from saturated fat in place of carbohydrate raised LDL-C concentrations but also raised HDL-C and lowered triglycerides, with no significant effects on apolipoprotein B concentrations.90
In a meta-analysis of RCTs, consumption of 1% of calories from trans fat in place of saturated fat, monounsaturated fat, or polyunsaturated fat, respectively, increased the ratio of TC to HDL-C by 0.031, 0.054, and 0.67; increased apolipoprotein B levels by 3, 10, and 11 mg/L; decreased apolipoprotein A-1 levels by 7, 5, and 3 mg/L; and increased lipoprotein(a) levels by 3.8, 1.4, and 1.1 mg/L.91
In meta-analyses of RCTs, consumption of eicosapentaenoic acid and docosahexaenoic acid for 212 weeks lowered SBP by 2.1 mm Hg92 and lowered resting heart rate by 2.5 beats per minute.93
In a pooled analysis of 25 randomized trials totaling 583 males and females both with and without hypercholesterolemia, nut consumption significantly improved blood lipid levels.61,94 For a mean consumption of 67 g of nuts per day, TC was reduced by 10.9 mg/dL (5.1%), LDL-C by 10.2 mg/dL (7.4%), and the ratio of TC to HDL-C by 0.24 (5.6% change; P<0.001 for each). Triglyceride levels were also reduced by 20.6 mg/dL (10.2%) in subjects with high triglycerides (2150 mg/dL). Different types of nuts had similar effects.94
In an RCT, compared with a low-fat diet, 2 Mediterranean dietary patterns that included either virgin olive oil or mixed nuts lowered SBP by 5.9 and 7.1 mm Hg, plasma glucose by 7.0 and 5.4 mg/dL, fasting insulin by 16.7 and 20.4 pmol/L, the homeostasis model assessment index by 0.9 and 1.1, and the ratio of TC to HDL-C by 0.38 and 0.26 and raised HDL-C by 2.9 and 1.6 mg/dL, respectively. The Mediterranean dietary patterns also lowered levels of CRP, interleukin-6, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1.95
Blood Glucose
A review of cross-sectional and prospective cohort studies suggests that higher intake of sugar-sweetened beverages is associated with greater visceral fat and higher risk of type 2 DM.96
Among 24 prospective cohort studies, greater consumption of refined carbohydrates and sugars, as measured by higher glycemic load, was positively associated with risk of type 2 DM: For each 100-g increment in glycemic load, 45% higher risk was seen (95% CI, 1.31–1.61; P<0.001; n=24 studies, 7.5 million person-years of follow-up).97
In one meta-analysis of observational studies and trials, greater consumption of nuts was linked to lower incidence of type 2 DM (RR per 4 weekly 1-oz servings, 0.87; 95% CI, 0.81–0.94).98
A meta-analysis of 102 randomized controlled feeding trials that included 239 diet arms and 4220 adults evaluated the effects of exchanging different dietary fats and carbohydrate on markers of glucose-insulin homeostasis.99 Although replacing 5% energy from carbohydrate with saturated fat generally had no significant effects, replacing carbohydrate with unsaturated fats lowered both HbA1c and insulin. Replacing saturated fat with polyunsaturated fat significantly lowered glucose, HbA1c, C-peptide, and the homeostatic model assessment of insulin resistance. On the basis of “gold standard” acute insulin response in 10 trials, polyunsaturated fat also significantly improved insulin secretion capacity (+0.5 pmol/L/min; 0.2, 0.8) whether it replaced carbohydrate, saturated fat, or even monounsaturated fat.
Cardiovascular Events
Because dietary habits affect a broad range of established and novel risk factors, estimation of the impact of nutritional factors on cardiovascular health by considering only a limited number of pathways (eg, only effects on lipids, BP, and obesity) will systematically underestimate or even misconstrue the actual total impact on cardiovascular health. RCTs and prospective observational studies have been used to quantify the total effects of dietary habits on clinical outcomes.
Fats and Carbohydrates
In the WHI randomized clinical trial (n=48 835), reduction of total fat consumption from 37.8% energy (baseline) to 24.3% energy (at 1 year) and 28.8% energy (at 6 years) had no effect on incidence of CHD (RR, 0.98; 95% CI, 0.88–1.09), stroke (RR, 1.02; 95% CI, 0.90–1.15), or total CVD (RR, 0.98; 95% CI, 0.92–1.05) over a mean of 8.1 years.100 This was consistent with null results of 4 prior randomized clinical trials and multiple large prospective cohort studies that indicated little effect of total fat consumption on CVD risk.101
In 3 separate meta-analyses of prospective cohort studies, the largest of which included 21 studies with up to 2 decades of follow-up, saturated fat consumption overall had no significant association with incidence of CHD, stroke, or total CVD.102–104 In comparison, in a pooled individual-level analysis of 11 prospective cohort studies, the specific exchange of polyunsaturated fat consumption in place of saturated fat was associated with lower CHD risk, with 13% lower risk for each 5% energy exchange (RR, 0.87; 95% CI, 0.70–0.97).105 These findings are consistent with a meta-analysis of RCTs in which increased poly-unsaturated fat consumption in place of saturated fat reduced CHD events, with 10% lower risk for each 5% energy exchange (RR, 0.90; 95% CI, 0.83–0.97).106
In a pooled analysis of individual-level data from 11 prospective cohort studies in the United States, Europe, and Israel that included 344 696 participants, each 5% higher energy consumption of carbohydrate in place of saturated fat was associated with a 7% higher risk of CHD (RR, 1.07; 95% CI, 1.01–1.14).105 Each 5% higher energy consumption of monounsaturated fat in place of saturated fat was not significantly associated with CHD risk.105 A more recent meta-analysis of prospective cohort studies found that increased intake of polyunsaturated fats was associated with lower risk of CHD, whether replacing saturated fat or carbohydrate.107 These findings suggest that reducing saturated fat without specifying the replacement might have minimal effects on CHD risk, whereas increasing polyunsaturated fats from vegetable oils will reduce CHD, whether replacing saturated fat or carbohydrate.20
In a meta-analysis of prospective cohort studies, each 2% of calories from trans fat was associated with a 23% higher risk of CHD (RR, 1.23; 95% CI, 1.11–1.37).108
In meta-analyses of prospective cohort studies, greater consumption of refined complex carbohydrates, starches, and sugars, as assessed by glycemic index or load, was associated with significantly higher risk of CHD and DM. When the highest category was compared with the lowest category, risk of CHD was 36% greater (glycemic load: RR, 1.36; 95% CI, 1.13–1.63), and risk of DM was 40% greater (glycemic index: RR, 1.40; 95% CI, 1.23–1.59).109,110
Foods and Beverages
In meta-analyses of prospective cohort studies, each daily serving of fruits or vegetables was associated with a 4% lower risk of CHD (RR, 0.96; 95% CI, 0.93–0.99) and a 5% lower risk of stroke (RR, 0.95; 95% CI, 0.92–0.97).111,112
In a meta-analysis of prospective cohort studies, greater whole grain intake (2.5 compared with 0.2 servings per day) was associated with a 21% lower risk of CVD events (RR, 0.79; 95% CI, 0.73–0.85), with similar estimates in males and females and for various outcomes (CHD, stroke, and fatal CVD). In contrast, refined grain intake was not associated with lower risk of CVD (RR, 1.07; 95% CI, 0.94–1.22).113
In a meta-analysis of 16 prospective cohort studies that included 326 572 generally healthy individuals in Europe, the United States, China, and Japan, fish consumption was associated with significantly lower risk of CHD mortality.114 Compared with no consumption, an estimated 250 mg of long-chain omega-3 fatty acids per day was associated with 35% lower risk of CHD death (P<0.001).
In a meta-analysis of prospective cohort and case-control studies from multiple countries, consumption of unprocessed red meat was not significantly associated with incidence of CHD. In contrast, each 50-g serving per day of processed meats (eg, sausage, bacon, hot dogs, deli meats) was associated with a higher incidence of CHD (RR, 1.42; 95% CI, 1.07–1.89).115
In a meta-analysis of prospective cohort studies that included 442 101 participants and 28 228 DM cases, unprocessed red meat consumption was associated with a higher risk of DM (RR, 1.19; 95% CI, 1.04–1.37, per 100 g/d). On a per g/d basis, risk of DM was nearly 7-fold higher for processed meat consumption (RR, 1.51; 95% CI, 1.25–1.83, per 50 g/d).116
In a meta-analysis of 6 prospective observational studies, nut consumption was associated with lower incidence of fatal CHD (RR per 4 weekly 1-oz servings, 0.76; 95% CI, 0.69–0.84) and nonfatal CHD (RR, 0.78; 95% CI, 0.67–0.92).98 Nut consumption was not significantly associated with stroke risk based on 4 studies.98
In a meta-analysis of 6 prospective observational studies, consumption of legumes (beans) was associated with lower incidence of CHD (RR per 4 weekly 100-g servings, 0.86; 95% CI, 0.78–0.94).98
Higher consumption of dairy or milk products is associated with lower incidence of DM and trends toward lower risk of stroke.94,109,110 The inverse associations with DM appear strongest for both yogurt and cheese.117
Dairy consumption is not significantly associated with higher or lower risk of CHD.103,118
Among 88 520 generally healthy females in the Nurses’ Health Study who were 34 to 59 years of age in 1980 and were followed up from 1980 to 2004, regular consumption of sugar-sweetened beverages was independently associated with higher incidence of CHD, with 23% and 35% higher risk with 1 and ≥2 servings per day, respectively, compared with <1 per month.119 Among the 15 745 participants in the ARIC study, the OR for developing CHD was 2.59 for participants who had a serum uric acid level >9.0 mg/dL and who drank >1 sugar-sweetened soda per day.120
In a meta-analysis of 15 country-specific observational cohorts, which together included 636 151 unique participants, 6.5 million person-years of follow-up, and 28 271 total deaths, 9783 cases of incident CVD, and 23 954 cases of incident type 2 DM, consumption of butter had small or neutral overall associations with mortality, CVDs, and DM.121 For example, butter consumption was weakly associated with all-cause mortality (per 14 g [1 tbsp] per day: RR, 1.01; 95% CI, 1.00–1.03); was not associated with CVD (RR, 1.00; 95% CI, 0.98–1.02), CHD (RR, 0.99, 95% CI, 0.96–1.03), or stroke (RR, 1.01, 95% CI, 0.98–1.03); and was associated with lower risk of DM (RR, 0.96, 95% CI, 0.93–0.99).
Potassium and Sodium
Major dietary sources of potassium include vegetables, fruits, whole grains, legumes, nuts, and dairy. In randomized trials, potassium lowers BP, with stronger effects among hypertensive people and when dietary sodium intake is high.122 BP lowering is related to both increased urinary potassium excretion and a lower urine sodium-to-potassium ratio. Consistent with these benefits, potassium-rich diets are associated with lower risk of CVD, especially stroke.123
Nearly all observational studies demonstrate a positive association between higher estimated sodium intakes (eg, >4000 mg/d) and CVD events, in particular stroke.124,125 Some studies have also observed higher CVD risk at estimated low intakes (eg, <3000 g/d), which suggests a potential J-shaped relationship with risk.126–128 Unique limitations in estimating sodium intake in observational studies, whether by urine collection or diet questionnaires, could explain the J shape seen in certain studies.129
During extended surveillance in a large sodium study that excluded sick people at baseline and collected multiple 24-hour urine samples per subject, individuals with sodium intake <2300 mg/d experienced 32% lower CVD risk than those who consumed 3600 to 4800 mg/d, with evidence for linearly decreasing risk.130
In ecological studies, the lowest mean intake level associated with both lower SBP and a lower age-BP slope was 614 mg/d.131
In well-controlled randomized feeding trials, the lowest tested intake for which BP reductions were clearly documented was 1500 mg/d.87
In meta-analyses of prospective observational studies, the lowest mean intakes associated with lower risk of CVD events ranged from 1787 to 2391 mg/d.124,125
In a post hoc analysis of the Trials of Hypertension Prevention, participants randomized to low-sodium interventions had a 25% lower risk of CVD (RR, 0.75; 95% CI, 0.57–0.99) after 10 to 15 years of follow-up after the original trials.132
Dietary Patterns
The 2015 US Dietary Guidelines Advisory Committee recently summarized the evidence for benefits of healthful diet patterns on a range of cardiometabolic and other disease outcomes.18 They concluded that a healthy dietary pattern is higher in vegetables, fruits, whole grains, low-fat or nonfat dairy, seafood, legumes, and nuts; moderate in alcohol (among adults); lower in red and processed meat; and low in sugar-sweetened foods and drinks and refined grains.
In a cohort of 380 296 US males and females, greater versus lower adherence to a Mediterranean dietary pattern, characterized by higher intakes of vegetables, legumes, nuts, fruits, whole grains, fish, and unsaturated fat and lower intakes of red and processed meat, was associated with a 22% lower cardiovascular mortality (RR, 0.78; 95% CI, 0.69–0.87).133 Similar findings have been seen for the Mediterranean dietary pattern and risk of incident CHD and stroke134 and for the DASH-type dietary pattern.135
In a cohort of 72 113 US female nurses, a dietary pattern characterized by higher intakes of vegetables, fruits, legumes, fish, poultry, and whole grains was associated with a 28% lower cardiovascular mortality (RR, 0.72; 95% CI, 0.60–0.87), whereas a dietary pattern characterized by higher intakes of processed meat, red meat, refined grains, french fries, and sweets/desserts was associated with a 22% higher cardiovascular mortality (RR, 1.22; 95% CI, 1.01–1.48).136 Similar findings have been seen in other cohorts and for other outcomes, including development of DM and metabolic syndrome.137–143
The observational findings for benefits of a healthy food–based dietary pattern have been confirmed in 2 randomized clinical trials, including a small secondary prevention trial in France among patients with recent MI144 and a large primary prevention trial in Spain among patients with CVD risk factors.145 The latter trial, PREDIMED, demonstrated a 30% reduction in the risk of stroke, MI, and death attributable to cardiovascular causes in those patients randomized to Mediterranean-style diets rich in extra-virgin olive oil or mixed nuts.
REFERENCES
- 1.My Life Check: Life’s Simple 7. [Accessed June 13, 2016];American Heart Association Web site. http://mylifecheck.heart.org/
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary Intake Among US Adults, 1999–2012. JAMA. 2016;315:2542–2553. doi: 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Sources of sodium among the US population, 2005–06. [Accessed June 14, 2016];Applied Research Program Web site. http://appliedresearch.cancer.gov/diet/foodsources/sodium/
- 5.US Burden of Disease Collaborators. The state of US Health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors [published correction appears in PLoS Med. 2011;8] PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Department of Agriculture Economic Research Service. [Accessed June 14, 2016];Food price outlook. 2016 http://www.ers.usda.gov/dataproducts/food-price-outlook.aspx.
- 8.Rao M, Afshin A, Singh G, Mozaffarian D. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open. 2013;3:e004277. doi: 10.1136/bmjopen-2013-004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian RS, Andry A, Lee RM, Wiecha JL, Gortmaker SL. Price and healthfulness of snacks in 32 YMCA after-school programs in 4 US metropolitan areas, 2006–2008. Prev Chronic Dis. 2012;9:E38. doi: 10.5888/pcd9.110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis C, Saltos E. Dietary recommendations and how they have changed over time. In: Frazao E, editor. America’s Eating Habits: Changes and Consequences. Washington, DC: US Department of Agriculture; 1999. pp. 33–50. Agriculture Information Bulletin No. 750. [Google Scholar]
- 11.Brunner E, Cohen D, Toon L. Cost effectiveness of cardiovascular disease prevention strategies: a perspective on EU food based dietary guidelines. Public Health Nutr. 2001;4:711–715. doi: 10.1079/phn2001161. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services, Centers for Disease Control and Prevention. [Accessed June 14, 2016];Preventing chronic diseases: investing wisely in health preventing obesity and chronic diseases through good nutrition and physical activity. 2008 http://atfiles.org/files/pdf/CDC-HHS.pdf.
- 13.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. [published corrections appear in Circulation. 2006;114:e629 and Circulation. 2006;114:e27] Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 14.International Society for the Study of Fatty Acids and Lipids. Recommendations for intake of polyunsaturated fatty acids in healthy adults. Devon, United Kingdom: International Society for the Study of Fatty Acids and Lipids; 2004. [Accessed June 14, 2016]. http://www.issfal.org/statements/pufa-recommendations/statement-3. [Google Scholar]
- 15.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: Institute of Medicine, National Academies Press; 2005. [Accessed June 14, 2016]. https://fnic.nal.usda.gov/sites/fnic.nal.usda.gov/files/uploads/energy_full_report.pdf. [Google Scholar]
- 16.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med. 2010;152:481–487. W170–W173. doi: 10.7326/0003-4819-152-8-201004200-00212. [DOI] [PubMed] [Google Scholar]
- 17.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2015 Dietary Guidelines Advisory Committee. [Accessed June 13, 2016];Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015 doi: 10.3945/an.116.012120. http://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [DOI] [PMC free article] [PubMed]
- 19.Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39:99–110. doi: 10.1016/j.diabet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144:399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, Mozaffarian D Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health. 2015;3:e132–e142. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 25.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 26.Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr. 2011;94:1376–1381. doi: 10.3945/ajcn.111.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet. 2012;112:657–663.e4. doi: 10.1016/j.jand.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picciano MF, Dwyer JT, Radimer KL, Wilson DH, Fisher KD, Thomas PR, Yetley EA, Moshfegh AJ, Levy PS, Nielsen SJ, Marriott BM. Dietary supplement use among infants, children, and adolescents in the United States, 1999–2002. Arch Pediatr Adolesc Med. 2007;161:978–985. doi: 10.1001/archpedi.161.10.978. [DOI] [PubMed] [Google Scholar]
- 29.Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring) 2008;16:790–796. doi: 10.1038/oby.2007.136. [DOI] [PubMed] [Google Scholar]
- 30.House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, Dresser GK, Spence JD. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303:1603–1609. doi: 10.1001/jama.2010.490. [DOI] [PubMed] [Google Scholar]
- 31.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial: Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico [published corrections appear in Lancet. 2001;357:642 and Lancet. 2007;369:106] Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 32.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA Lipid Intervention Study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis [published correction appears in Lancet. 2007;370:220] Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 33.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 35.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977–2006. PLoS Med. 2011;8:e1001050. doi: 10.1371/journal.pmed.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffey KJ, Popkin BM. Causes of increased energy intake among children in the U.S., 1977–2010. Am J Prev Med. 2013;44:e1–e8. doi: 10.1016/j.amepre.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Center for Health Statistics. [Accessed August, 24, 2016];National Health Interview Survey. 2015 Public-use data file and documentation. http://www.cdc.gov/nchs/nhis/nhis_2015_data_release.htm. NCHS tabulations.
- 41.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. JAMA. 2003;289:450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 42.Wright JD, Wang CY. Trends in intake of energy and macronutrients in adults from 1999–2000 through 2007–2008. NCHS Data Brief. 2010;49:1–8. [PubMed] [Google Scholar]
- 43.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 44.Kant AK, Graubard BI. Eating out in America, 1987–2000: trends and nutritional correlates. Prev Med. 2004;38:243–249. doi: 10.1016/j.ypmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 46.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84:1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States [published correction appears in Am J Clin Nutr. 2007;86:525] Am J Clin Nutr. 2006;83:529–542. doi: 10.1093/ajcn.83.3.529. [DOI] [PubMed] [Google Scholar]
- 48.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15:2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- 49.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121:e1604–e1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 50.Mesirow MS, Welsh JA. Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: National Health and Nutrition Examination Survey 2001–2010. J Acad Nutr Diet. 2015;115:559–566.e4. doi: 10.1016/j.jand.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Wang YC, Gortmaker SL, Sobol AM, Kuntz KM. Estimating the energy gap among US children: a counterfactual approach. Pediatrics. 2006;118:e1721–e1733. doi: 10.1542/peds.2006-0682. [DOI] [PubMed] [Google Scholar]
- 52.Poti JM, Popkin BM. Trends in energy intake among US children by eating location and food source, 1977–2006. J Am Diet Assoc. 2011;111:1156–1164. doi: 10.1016/j.jada.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dwyer-Lindgren L, Freedman G, Engell RE, Fleming TD, Lim SS, Murray CJ, Mokdad AH. Prevalence of physical activity and obesity in US counties, 2001–2011: a road map for action. Popul Health Metr. 2013;11:7. doi: 10.1186/1478-7954-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.US Department of Health and Human Services. [Accessed June 14, 2016];Dietary Guidelines for Americans. 2010 https://health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf.
- 55.Centers for Disease Control and Prevention (CDC) Trends in intake of energy and macronutrients: United States, 1971–2000. MMWR Morb Mortal Wkly Rep. 2004;53:80–82. [PubMed] [Google Scholar]
- 56.Egan SK, Bolger PM, Carrington CD. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Environ Epidemiol. 2007;17:573–582. doi: 10.1038/sj.jes.7500554. [DOI] [PubMed] [Google Scholar]
- 57.Ervin RB, Ogden CL. Trends in intake of energy and macronutrients in children and adolescents from 1999–2000 through 2009–2010. NCHS Data Brief. 2013;(113):1–8. [PubMed] [Google Scholar]
- 58.Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr. 2013;98:180–188. doi: 10.3945/ajcn.112.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh GM, Micha R, Khatibzadeh S, Shi S, Lim S, Andrews KG, Engell RE, Ezzati M, Mozaffarian D on behalf of the Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS One. 2015;10:e0124845. doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis. 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 62.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr. 2010;92:1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, Chen CS, Klag MJ, Whelton PK, He J. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161:309–318. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 67.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101:1216–1224. doi: 10.3945/ajcn.114.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, Goldstein JM, Ludwig DS. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr. 2013;98:641–647. doi: 10.3945/ajcn.113.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15:1675–1684. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 72.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against nonalcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steenhuis IH, Vermeer WM. Portion size: review and framework for interventions. Int J Behav Nutr Phys Act. 2009;6:58. doi: 10.1186/1479-5868-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bezerra IN, Curioni C, Sichieri R. Association between eating out of home and body weight. Nutr Rev. 2012;70:65–79. doi: 10.1111/j.1753-4887.2011.00459.x. [DOI] [PubMed] [Google Scholar]
- 77.Lachat C, Nago E, Verstraeten R, Roberfroid D, Van Camp J, Kolsteren P. Eating out of home and its association with dietary intake: a systematic review of the evidence. Obes Rev. 2012;13:329–346. doi: 10.1111/j.1467-789X.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- 78.Mesas AE, Muñoz-Pareja M, López-García E, Rodríguez-Artalejo F. Selected eating behaviours and excess body weight: a systematic review. Obes Rev. 2012;13:106–135. doi: 10.1111/j.1467-789X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 79.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review [published correction appears in Sleep Med Rev. 2012;16:491] Sleep Med Rev. 2012;16:231–241. doi: 10.1016/j.smrv.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li F, Harmer PA, Cardinal BJ, Bosworth M, Acock A, Johnson-Shelton D, Moore JM. Built environment, adiposity, and physical activity in adults aged 50–75. Am J Prev Med. 2008;35:38–46. doi: 10.1016/j.amepre.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumanyika S, Grier S. Targeting interventions for ethnic minority and low-income populations. Future Child. 2006;16:187–207. doi: 10.1353/foc.2006.0005. [DOI] [PubMed] [Google Scholar]
- 82.Sallis JF, Glanz K. The role of built environments in physical activity, eating, and obesity in childhood. Future Child. 2006;16:89–108. doi: 10.1353/foc.2006.0009. [DOI] [PubMed] [Google Scholar]
- 83.Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR, Jr, Kraus WE, Kris-Etherton PM, Krummel DA, Popkin BM, Whitsel LP, Zakai NA American Heart Association Council on Epidemiology and Prevention, Council on Nutrition, Physical Activity and Metabolism, Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovascular Disease, Council on Peripheral Vascular Disease, and the American Heart Association Advocacy Coordinating Committee. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 85.Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med. 2010;362:2102–2112. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 86.Susic D, Frohlich ED. Salt consumption and cardiovascular, renal, and hypertensive diseases: clinical and mechanistic aspects. Curr Opin Lipidol. 2012;23:11–16. doi: 10.1097/MOL.0b013e32834d9c52. [DOI] [PubMed] [Google Scholar]
- 87.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 88.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 89.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER., 3rd The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the Omni-Heart trial. Diabetes Care. 2013;36:1132–1137. doi: 10.2337/dc12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 91.Uauy R, Aro A, Clarke R, Ghafoorunissa, L’Abbe MR, Mozaffarian D, Skeaff CM, Stender S, Tavella M. WHO Scientific Update on trans fatty acids: summary and conclusions. Eur J Clin Nutr. 2009;63:S68–S75. [Google Scholar]
- 92.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 94.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 95.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E PREDIMED Study Investigators. Effects of a Mediterraneanstyle diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 96.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr. 2013;97:584–596. doi: 10.3945/ajcn.112.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:278–288. doi: 10.3945/ajcn.113.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13:e1002087. doi: 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 101.Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation; Geneva. 28 January – 1 February 2002; 2003. [Accessed June 14, 2016]. http://www.who.int/dietphysicalactivity/publications/trs916/en/gsfao_introduction.pdf. [Google Scholar]
- 102.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab. 2009;55:173–201. doi: 10.1159/000229002. [DOI] [PubMed] [Google Scholar]
- 103.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 104.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 109.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk: a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 110.Dong JY, Zhang YH, Wang P, Qin LQ. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am J Cardiol. 2012;109:1608–1613. doi: 10.1016/j.amjcard.2012.01.385. [DOI] [PubMed] [Google Scholar]
- 111.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology. 2005;65:1193–1197. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- 112.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 113.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 114.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillain B, Drogan D, Franks PW, Gavrila D, Gonzalez C, Halkjaer J, Kaaks R, Moskal A, Nilsson P, Overvad K, Palli D, Panico S, Quirós JR, Ricceri F, Rinaldi S, Rolandsson O, Sacerdote C, Sánchez MJ, Slimani N, Spijkerman AM, Teucher B, Tjonneland A, Tormo MJ, Tumino R, van der ADL, Sharp SJ, Langenberg C, Feskens EJ, Riboli E, Wareham NJ InterAct Consortium. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr. 2012;96:382–390. doi: 10.3945/ajcn.111.021907. [DOI] [PubMed] [Google Scholar]
- 118.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158–171. doi: 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–616. doi: 10.1038/ki.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pimpin L, Wu JH, Haskelberg H, Del Gobbo L, Mozaffarian D. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PLoS One. 2016;11:e0158118. doi: 10.1371/journal.pone.0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2015;33:1509–1520. doi: 10.1097/HJH.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 123.D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 124.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poggio R, Gutierrez L, Matta MG, Elorriaga N, Irazola V, Rubinstein A. Daily sodium consumption and CVD mortality in the general population: systematic review and meta-analysis of prospective studies. Public Health Nutr. 2015;18:695–704. doi: 10.1017/S1368980014000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;71:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 127.Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175:410–419. doi: 10.1001/jamainternmed.2014.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations [published correction appears in Circulation. 2013;27:e263] Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 129.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 130.Centers for Disease Control and Prevention. [Accessed August 13, 2016];2015 Cardiac Arrest Registry to Enhance Survival (CARES) National Summary Report. http://mycares.net/sitepages/uploads/2016/2015%20Non-Traumatic%20National%20Summary%20Report.pdf.
- 131.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure: results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitrou PN, Kipnis V, Thiébaut AC, Reedy J, Subar AF, Wirfält E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 134.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women [published correction appears in Circulation. 2009;119:e379] Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 136.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Osler M, Heitmann BL, Gerdes LU, Jørgensen LM, Schroll M. Dietary patterns and mortality in Danish men and women: a prospective observational study. Br J Nutr. 2001;85:219–225. doi: 10.1079/bjn2000240. [DOI] [PubMed] [Google Scholar]
- 138.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136:201–209. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 139.Heidemann C, Hoffmann K, Spranger J, Klipstein-Grobusch K, Möhlig M, Pfeiffer AF, Boeing H European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study Cohort. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study cohort. Diabetologia. 2005;48:1126–1134. doi: 10.1007/s00125-005-1743-1. [DOI] [PubMed] [Google Scholar]
- 140.Brunner EJ, Mosdøl A, Witte DR, Martikainen P, Stafford M, Shipley MJ, Marmot MG. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–1421. doi: 10.1093/ajcn/87.5.1414. [DOI] [PubMed] [Google Scholar]
- 141.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 142.Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost. 2012;10:189–198. doi: 10.1111/j.1538-7836.2011.04588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joosten MM, Grobbee DE, van der ADL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010;91:1777–1783. doi: 10.3945/ajcn.2010.29170. [DOI] [PubMed] [Google Scholar]
- 144.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 145.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
6. OVERWEIGHT AND OBESITY
See Table 6-1 and Charts 6-1 through 6-11
Table 6-1.
Overweight and Obesity
| Prevalence of Overweight and Obesity, 2011–2014, Age ≥20 y | Prevalence of Obesity, 2011–2014, Age ≥20 y | Prevalence of Overweight and Obesity 2011–2014, Ages 2–19 y | Prevalence of Obesity, 2011–2014, Ages 2–19 y | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | Cost, 2008* | |
| Total | 157 232 115 | 69.4 | 82 241 005 | 36.3 | 24 036 573 | 32.1 | 12 339 701 | 16.5 | $147 Billion |
| Males | 78 854 444 | 72.5 | 37 306 309 | 34.3 | 12 326 869 | 32.3 | 6 231 683 | 16.3 | … |
| Females | 78 215 543 | 66.4 | 45 115 291 | 38.3 | 11 709 947 | 32.0 | 6 107 613 | 16.7 | … |
| NH white | |||||||||
| Males | 53 310 267 | 73.0 | 24 537 328 | 33.6 | 5 962 553 | 29.3 | 2 848 504 | 14.0 | … |
| Females | 49 632 907 | 63.7 | 27 660 411 | 35.5 | 5 419 620 | 28.0 | 2 847 989 | 14.7 | … |
| NH black | |||||||||
| Males | 7 968 039 | 69.1 | 4 324 189 | 37.5 | 1 734 453 | 32.8 | 924 000 | 17.5 | … |
| Females | 11 782 661 | 82.2 | 8 156 124 | 56.9 | 1 929 861 | 37.6 | 1 026 805 | 20.0 | … |
| NH Asian | |||||||||
| Males | 2 504 566 | 46.6 | 601 956 | 11.2 | 416 430 | 24.9 | 190 805 | 11.4 | |
| Females | 2 165 586 | 34.6 | 744 811 | 11.9 | 245 206 | 15.0 | 86 088 | 5.3 | |
| Hispanic | |||||||||
| Males | 13 015 852 | 79.6 | 6 377 113 | 39.0 | 3 601 223 | 40.4 | 1 939 812 | 21.7 | … |
| Females | 12 721 527 | 77.1 | 7 540 516 | 45.7 | 3 400 898 | 39.8 | 1 798 951 | 21.0 | … |
Overweight and obesity in adults is defined as body mass index (BMI) ≥25 kg/m2. Obesity in adults is defined as BMI ≥30 kg/m2. In children, overweight and obesity are based on BMI-for-age values at or above the 85th percentile of the 2000 Centers for Disease Control and Prevention (CDC) growth charts. In children, obesity is based on BMI-for-age values at or above the 95th percentile of the CDC growth charts. In January 2007, the American Medical Association’s Expert Task Force on Childhood Obesity recommended new definitions for overweight and obesity in children and adolescents112; however, statistics based on this new definition are not yet available. Estimates for the total include those of “other” racial-ethnic groups.
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Data from Finkelstein et al.72
Sources: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014 (adults), unpublished CDC tabulation; NHANES 2011 to 2014 (ages 2–19 years) from Ogden et al.7 Population count extrapolations calculated using the average of the 2011 and 2013 American Community Survey Summary File data.113
Chart 6-1. US children and adolescents with obesity by race/ethnicity, 2011 to 2014.
Obesity is body mass index (BMI) at or above the sex-and age-specific 95th percentile BMI cutoff points from the 2000 CDC growth charts.
CDC indicates Centers for Disease Control and Prevention.
Source: CDC/National Center for Health Statistics, Health, United States, 2015, Figure 22. Data from the National Health and Nutrition Examination Survey (NHANES), Table 59.18
Chart 6-11. Trends in obesity prevalence among adults aged ≥20 years (age adjusted) and youth aged 2 to 19 years, United States, 1999 to 2000 through 2013 to 2014.
Data from the National Center for Health Statistics 2015 Data Brief.7
Overweight and obesity are major risk factors for CVD, including CHD, stroke,1,2 AF,3 VTE,4 and CHF. The AHA has identified BMI <85th percentile (ages 2–19 years) and <25 kg/m2 (ages ≥20 years) as 1 of the 7 components of ideal cardiovascular health.5 In 2013 to 2014, 63.1% of children and 29.6% of adults met these criteria (Chapter 2, Cardiovascular Health). According to NHANES 2013 to 2014, 37.7% % of US adults were obese, and 7.7% had class III obesity.6,7
Abbreviations Used in Chapter 6
| AF | atrial fibrillation |
| AFFIRM | Atrial Fibrillation Follow-up Investigation of Rhythm Management |
| AHA | American Heart Association |
| AMI | acute myocardial infarction |
| APCSC | Asia-Pacific Cohort Studies Collaboration |
| ARIC | Atherosclerosis Risk in Communities Study |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CHF | congestive heart failure |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DM | diabetes mellitus |
| ERFC | Emerging Risk Factor Collaboration |
| HbA1c | hemoglobin A1c |
| HD | heart disease |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HR | hazard ratio |
| HUNT 2 | Nord-Trøndelag Health Study |
| IHD | ischemic heart disease |
| IMT | intima-media thickness |
| LABS | Longitudinal Assessment of Bariatric Surgery |
| Look AHEAD | Look: Action for Health in Diabetes |
| MEPS | Medical Expenditure Panel Survey |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MHO | metabolically healthy obese |
| MI | myocardial infarction |
| NCDR | National Cardiovascular Data Registry |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| OR | odds ratio |
| PSC | Prospective Studies Collaboration |
| QALY | quality-adjusted life-year |
| RCT | randomized controlled trial |
| RR | relative risk |
| RYGB | Roux-en-Y gastric bypass |
| SBP | systolic blood pressure |
| SCD | sudden cardiac death |
| SD | standard deviation |
| SOS | Swedish Obese Subjects |
| STEMI | ST-segment–elevation myocardial infarction |
| TC | total cholesterol |
| UI | uncertainty interval |
| VTE | venous thromboembolism |
| WHI | Women’s Health Initiative |
| YRBS | Youth Risk Behavior Surveillance |
Classification of Overweight and Obesity
For adults, NHLBI weight categories are as follows: overweight (25.0≤ BMI ≤29.9 kg/m2), and obese class I (BMI 30–35 kg/m2), class II (BMI >35 to 39.9 kg/m2), and class III (BMI ≥40 kg/m2). BMI cutoffs often misclassify obesity, for instance, overestimating obesity in very muscular individuals and underestimating obesity in older adults with reduced muscle mass. BMI categories also vary in prognostic impact by race/ethnicity; they appear to overestimate risk in African Americans and underestimate risk in Asians.8 For this reason, lower BMI categories have been recommended for Asian and South Asian populations.9
For children, when sex-specific BMI-for-age 2000 CDC growth charts for the United States are used,10 overweight is defined as 85th to <95th percentile, and obese is defined as ≥95th percentile. These categories were previously called “at risk for overweight” and “overweight.” The change in terminology reflects the labels used by organizations such as the Health and Medicine Division and the American Academy of Pediatrics. More information is available elsewhere.11
A recent AHA scientific statement recommended that the definition of severe obesity for children ≥2 years old and adolescents be changed to BMI ≥120% of the 95th percentile for age and sex, or an absolute BMI ≥35 kg/m, whichever is lower.12 This definition of severe obesity among children could better identify this small but important group compared with the other common definition of BMI ≥99th percentile for age and sex.12
Current obesity guidelines define waist circumference ≥40 inches (102 cm) for males and ≥35 inches (88 cm) for females as associated with increased cardiovascular risk13; however, lower cutoffs have been recommended for various racial/ethnic groups, for example, ≥80 cm for Asian females and ≥90 cm for Asian males.8,14 Waist circumference measurement is recommended for those with BMI of 25 to 34.9 kg/m2, to provide additional information on CVD risk, but may be unnecessary among those with BMI ≥35 kg/m2, who are unlikely to have waist circumference less than these cutoffs.15
Prevalence
Youth
According to 2013 to 2014 data from NHANES (NCHS/CDC), the overall prevalence of overweight, including obesity, in children and adolescents aged 2 to 19 years was 33.4% based on a BMI-for-age value ≥85th percentile of the 2000 CDC growth charts: 16.2% were overweight, and 17.2% were obese (≥95th percentile). By age group, the prevalence of obesity for children aged 2 to 5 years was 9.4%; for children aged 6 to 11 years, prevalence was 17.4%; and for adolescents aged 12 to 19 years, prevalence was 20.6%.16 There were no significant differences in overweight (including obesity) prevalence for boys and girls.17
According to 2011 to 2014 data from NHANES (NCHS/CDC), the overall prevalence of obesity by age group was as follows: 8.9% for children aged 2 to 5 years; 17.5% for children aged 6 to 11 years; and 20.5% for adolescents aged 12 to 19 years. In this period, there were no significant differences in obesity prevalence for boys and girls.7,18
According to 2011 to 2014 data from NHANES (NCHS/CDC), among children and adolescents aged 2 to 19 years, the overall prevalence of obesity (≥95th percentile of the 2000 CDC growth charts) was 16.5%, but it was lower for non-Hispanic Asian and non-Hispanic white children than for non-Hispanic black and Hispanic children, without significant differences between non-Hispanic black and Hispanic children (Chart 6-1).7,18
According to 2013 to 2014 NHANES data, among all children aged 2 to 19 years, the prevalence of obesity was lower for non-Hispanic Asian boys (12.1%) and girls (5.0%) than for non-Hispanic white (15.9%, 14.6%), non-Hispanic black (16.8%, 20.9%), and Hispanic (20.6%, 22.1%) boys and girls.16,17
The prevalence of childhood obesity varies by socioeconomic status. According to 2011 to 2014 NHANES data, for children 12 to 19 years old, the prevalence of obesity by percentage of poverty level was 22.4% for those below 100%, 25.7% for 100% to 199%, 19.7% for 200% to 399%, and 13.7% for ≥400% of poverty level.18
In addition, obesity prevalence among adolescents was higher for those whose parents had a high school degree or less education than for adolescents whose parents had a bachelor’s degree or higher.19
According to NHANES 2011 to 2014 data, 5.8% of youth aged 2 to 19 years had extreme obesity, defined as BMI ≥120% of the 95th percentile for age and sex, which was similar for boys (5.7%) and girls (5.9%) but was higher among Hispanic and non-Hispanic black youth than among non-Hispanic white youth.17
According to self-reported height and weight data from the YRBS 2015,20 13.9% of US high school students were obese and 16.0% were overweight. The percentages of obesity were higher in boys (16.8%) than girls (10.8%) and in blacks (16.8%) and Hispanics (16.4%) than in whites (12.4%). Obesity rates varied by states: The highest rates of obesity in females were observed in Kentucky and Mississippi (16.2%), and in males, West Virginia (23.4%); the lowest rates in females were observed in Nevada (6.3%), whereas for males, the lowest rates were seen in Montana (13.0%).
Adults
(See Table 6-1 and Charts 6-2, 6-3, 6-4, 6-5, 6-6, 6-7, 6-8)
Chart 6-2. Age-adjusted prevalence of obesity in adults ≥20 years of age by sex and age group (NHANES 2011–2014).
Totals were age-adjusted by the direct method to the 2000 US census population using the age groups 20 to 39, 40 to 59, and ≥60 years old. Crude estimates are 36.5% for all, 34.5% for males, and 38.5% for females.
NHANES indicates National Health and Nutrition Examination Survey.
1Significantly different from those aged 20 to 39 years. 2Significantly different from females of the same age group.
Source: Centers for Disease Control and Prevention/National Center for Health Statistics, NHANES, 2011 to 2014.7
Chart 6-3. Age-adjusted prevalence of obesity in adults ≥20 years of age by sex and race-ethnicity (NHANES 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey.
1Significantly different from non-Hispanic Asian people. 2Significantly different from non-Hispanic white people. 3Significantly different from females of the same race and Hispanic origin. 4Significantly different from non-Hispanic black people.
Source: Centers for Disease Control and Prevention/National Center for Health Statistics, NHANES, 2011 to 2014.7
Chart 6-4. Trends in overweight and obesity between 1999 to 2002 and 2011 to 2014 among US adults aged ≥20 years, by sex.
Overweight but not obese (25≤ body mass index (BMI) <30 kg/m2); grade 1 obesity (30≤ BMI <35 kg/m2); grade 2 obesity (35≤ BMI <40 kg/m2); grade 3 obesity (BMI ≥40 kg/m2).
Source: Centers for Disease Control and Prevention/National Center for Health Statistics, Health, United States, 2015, Figure 9 and Table 58. Data from National Health and Nutrition Examination Survey (NHANES).18
Chart 6-5. Prevalence† of self-reported obesity among US adults aged ≥20 years by state and territory, BRFSS, 2014.
RFSS indicates Behavioral Risk Factor Surveillance System.
* Sample size <50 or the relative standard error (dividing the standard error by the prevalence) ≥30%.
†Prevalence estimates reflect BRFSS methodological changes started in 2011. These estimates should not be compared to prevalence estimates before 2011.
Source: Centers for Disease Control and Prevention, Obesity Prevalence Map, 2012–2014.21
Chart 6-6. Prevalence of self-reported obesity among non-Hispanic white adults aged ≥20 years, by state and territory, BRFSS, 2012 to 2014.
BRFSS indicates Behavioral Risk Factor Surveillance System.
* Sample size <50 or the relative standard error (dividing the standard error by the prevalence) ≥30%.
Source: Centers for Disease Control and Prevention, Obesity Prevalence Map, 2012–2014.21
Chart 6-7. Prevalence of self-reported obesity among Hispanic adults aged ≥20 years, by state and territory, BRFSS, 2012 to 2014.
BRFSS indicates Behavioral Risk Factor Surveillance System.
*Sample size <50 or the relative standard error (dividing the standard error by the prevalence) ≥30%.
Source: Centers for Disease Control and Prevention, Obesity Prevalence Map, 2012–2014.21
Chart 6-8. Prevalence of self-reported obesity among non-Hispanic black adults aged ≥20 years, by state and territory, BRFSS, 2012 to 2014.
BRFSS indicates Behavioral Risk Factor Surveillance System.
*Sample size <50 or the relative standard error (dividing the standard error by the prevalence) ≥30%.
Source: Centers for Disease Control and Prevention, Obesity Prevalence Map, 2012–2014.21
-
According to NHANES 2013 to 2014, among US adults aged ≥20 years6:
37.7% of US adults were obese (35.0% of males and 40.4% of females), and 7.7% had class III obesity (5.5% of males and 9.9% of females).
Among males, the prevalence of obesity and class III obesity was not significantly different for non-Hispanic blacks (38.0% and 7.2%), non-Hispanic Asians (12.6% and not available for class III obesity), Hispanics (37.9% and 5.4%), and non-Hispanic whites (34.7% and 5.6%). The prevalence of obesity among non-Hispanic Asians was 12.6%.
Among females, the prevalence of obesity and class III obesity, respectively, was greater in non-Hispanic blacks (57.2% and 16.8%), lower in non-Hispanic Asians (12.4%, not available for class III obesity), and similar in Hispanics (46.9% and 8.7%) compared with non-Hispanic whites (38.2% and 9.7%).
According to NHANES 2011 to 2014, the age-adjusted prevalence of obesity was higher among middle-aged (40.2%) and older (37.0%) adults than younger (32.3%) adults. This pattern (lower prevalence of obesity among younger adults) was similar for males and females, although the prevalence of obesity was higher among females7 (Chart 6-2).
Using NHANES 2011 to 2014, obesity prevalence was higher in females than males when stratified by race-ethnicity (Table 6-1; Chart 6-3). By sex, the only significant differences were higher prevalence of obesity among non-Hispanic black females than among non-Hispanic black males and among Hispanic females than among Hispanic males7 (Table 6-1 and Chart 6-3).
According to NHANES data from 1999 to 2002 through 2011 to 2014, females have had a higher prevalence of class III obesity and a lower prevalence of overweight than males18 (Chart 6-4).
In the United States, the prevalence of obesity, as estimated from self-reported height and weight in the BRFSS/CDC (2014), varies by region and state. Self-reported estimates usually underestimate BMI and obesity.21 In 2013, by region, the prevalence of obesity was higher in the Midwest (30.7%) and the South (30.6%) and lower in the Northeast (27.3%) and West (25.7%). By state, the prevalence of obesity ranged from 20% to 25% in 5 states and the District of Columbia to ≥35% in Arkansas, Mississippi, and West Virginia21 (Chart 6-5). Combining BRFSS data from 2012 to 2014, the prevalence of obesity by state was higher for Hispanic adults and substantially higher for non-Hispanic black adults than for white adults (Charts 6-6 through 6-8).
Among adults aged ≥20 years, the prevalence of obesity varies by socioeconomic status, sex, and race-ethnicity. Using data from NHANES 2005 to 2008, among adult males, obesity prevalence was similar across income levels, except that for non-Hispanic blacks and Mexican Americans, obesity prevalence was higher for higher-income than lower-income males. Among adult females, the prevalence of obesity was slightly lower in higher-income females (29.0%) than middle-income (39.0%) or lower-income females (42.0%).22
Complications
Youth
According to the National Longitudinal Study of Adolescent Health, compared with those with normal weight or those who were overweight, adolescents who were obese had a 16-fold increased risk of having severe obesity (BMI ≥40 kg/m2) as adults, and 70.5% of adolescents with severe obesity maintained this weight status into adulthood.23
-
Children and adolescents who are overweight and obese are at increased risk for future adverse health effects, including the following24:
Increased prevalence of traditional cardiovascular risk factors such as hypertension, hyperlipidemia, and DM. Despite these risks, a recent article examined 2 decades of data from 1974 to 1993 and found that although the prevalence of obesity among children increased during this time period, hypertension did not.25
Poor school performance, tobacco use, alcohol use, premature sexual behavior, and poor diet.
Other associated health conditions, such as asthma, hepatic steatosis, sleep apnea, stroke, some cancers (breast, colon, and kidney), renal insufficiency, musculoskeletal disorders, and gallbladder disease.
Data from 4 Finnish cohort studies examining childhood and adult BMI with a mean follow-up of 23 years found that overweight or obese children who were obese in adulthood had increased risks of type 2 DM, hypertension, dyslipidemia, and carotid atherosclerosis, whereas those who achieved normal weight by adulthood had risks comparable to individuals who were never obese.26
The CARDIA study showed that young adults who were overweight or obese had lower health-related quality of life than normal-weight participants 20 years later.27
Adults
Obesity is associated with increased prevalence of type 2 DM, hypertension, dyslipidemia, sleep-disordered breathing, subclinical atherosclerosis, CHD, stroke VTE, AF, and dementia.
Using data from NHANES 2007 to 2010, type 2 DM prevalence was 18.5% for adults who were obese, 8.2% for those who were overweight, and 5.4% for normal-weight adults; hypertension prevalence was 35.7%, 26.4%, and 19.8%, respectively; and dyslipidemia prevalence was 49.7%, 44.2%, and 28.6%, respectively.28
Analyses of continuous BMI show the risk of type 2 DM increases with increasing BMI.29
Among 68 070 adult participants across multiple NHANES surveys, the decline in BP in recent birth cohorts slowed, mediated by BMI.30
Another systematic review and meta-analysis of 37 studies showed that high childhood BMI was associated with an increased incidence of adult DM (OR, 1.70; 95% CI, 1.30–2.22), CHD (OR, 1.20; 95% CI, 1.10–1.31), and a range of cancers, but not stroke or breast cancer. However, the accuracy of childhood BMI when predicting any adult morbidity was low. Only 31% of future DM and 22% of future hypertension and CHD occurred in those who as youth aged ≥12 years had been classified as being overweight or obese. Only 20% of future adult cancers occurred in children classified as being overweight or obese.31
A meta-analysis of 123 cohorts with 1.4 million adults and 52 000 CVD events found that associations of BMI with IHD, hypertensive HD, stroke, and DM declined with age (Chart 6-9) but were similar by sex and by region, with RRs for 5-kg/m2 higher BMI for ages 55 to 64 years ranging from 1.36 (1.32–1.38) for IHD to 2.03 (1.95–2.11) for DM. On the basis of their data, the authors suggested that the theoretical minimum-risk exposure distribution for BMI is 21 to 23 kg/m2 ± 1.1 to 1.8 kg/m2.32
Cardiovascular risks may be even higher with class III obesity than with class I or class II obesity. 33 Among 156 775 postmenopausal females in the WHI, for severe obesity versus normal BMI, HRs (95% CIs) for mortality were 1.97 (1.77–2.20) in white females, 1.55 (1.20–2.00) in African American females, and 2.59 (1.55–4.31) in Hispanic females; for CHD, HRs were 2.05 (1.80–2.35), 2.24 (1.57–3.19), and 2.95 (1.60–5.41), respectively; and for CHF, HRs were 5.01 (4.33–5.80), 3.60 (2.30–5.62), and 6.05 (2.49–14.69), respectively. However, CHD risk was strongly related to CVD risk factors across BMI categories, even in severe obesity, and CHD incidence was similar by race/ethnicity when adjusted for differences in BMI and CVD risk factors.33
In a meta-analysis from 58 cohorts representing 221 934 people in 17 developed countries with 14 297 incident CVD outcomes, BMI, waist circumference, and waist-to-hip ratio were strongly associated with intermediate risk factors of DM, higher SBP and TC, and lower HDL-C. The strong associations of adiposity measures (BMI, waist circumference, waist-to-hip ratio) with CVD outcomes were almost completely attenuated after adjustment for intermediate risk factors (DM, SBP, TC, and HDL-C), along with age, sex, and smoking status. Measures of adiposity also did not improve risk discrimination or reclassification when data on intermediate risk factors were included.34
Obesity was cross-sectionally associated with subclinical atherosclerosis, including CAC and carotid IMT, among adults in MESA, and this association persisted after adjustment for CVD risk factors.35
Obesity is also a strong predictor of sleep-disordered breathing, as well as numerous cancers, nonalcoholic fatty liver disease, gallbladder disease, musculoskeletal disorders, and reproductive abnormalities.36
A systematic review of prospective studies examining overweight and obesity as predictors of major stroke subtypes in >2 million participants over ≥4 years found an adjusted RR for ischemic stroke of 1.22 (95% CI, 1.05–1.41) in overweight individuals and an RR of 1.64 (95% CI, 1.36–1.99) for obese individuals relative to normal-weight individuals. RRs for hemorrhagic stroke were 1.01 (95% CI, 0.88–1.17) and 1.24 (95% CI, 0.99–1.54) for overweight and obese individuals, respectively. These risks were graded with increasing BMI and were independent of age, lifestyle, and other cardiovascular risk factors.37
A recent report from ARIC showed that VTE risk over 15.5 years (237 375 person-years) was associated with higher BMI (and current smoking) but not with other CVD risk factors.4
A recent meta-analysis of 15 prospective studies demonstrated the increased risk for Alzheimer disease or vascular dementia and any dementia was 1.35 and 1.26 for overweight, respectively, and 2.04 and 1.64 for obesity, respectively.38 The inclusion of obesity in dementia forecast models increased the estimated prevalence of dementia through 2050 by 9% in the United States and 19% in China.39
A BMI paradox is often reported, with higher-BMI patients demonstrating favorable outcomes in CHF, hypertension, peripheral vascular disease, and CAD; similar findings have been seen for percent body fat. In AFFIRM, a multicenter trial of AF, obese patients had lower all-cause mortality (HR, 0.77; P=0.01) than normal-weight patients after multivariable adjustment over a 3-year follow-up period.40 In another study of 2625 participants with new-onset DM, rates of total, CVD, and non-CVD mortality were higher among normal-weight people than among overweight/obese participants, with adjusted HRs of 2.08 (95% CI, 1.52–2.85), 1.52 (95% CI, 0.89–2.58), and 2.32 (95% CI, 1.55–3.48), respectively.41
Recent studies have evaluated risks for MHO versus “metabolically unhealthy” or “metabolically abnormal” obesity. The definition of MHO has varied across studies, but it has often been comprising 0 or 1 metabolic abnormality by metabolic syndrome criteria, sometimes excluding waist circumference. Using strict criteria of 0 metabolic syndrome components and no previous CVD diagnosis, a recent report of 10 European cohort studies (n=163 517 people) reported that the prevalence of MHO varied from 7% to 28% in females and from 2% to 19% in males.42 MHO appears to be unstable over time, with 1 study showing that 44.5% of MHO individuals transitioned to metabolically unhealthy obesity over 8 years of follow-up.43 Among younger adults in the CARDIA study, after 20 years of follow-up, 47% of people were defined as being metabolically healthy overweight (presence of 0 or 1 metabolic risk factor).44 Recent meta-analyses suggest that CVD risk is higher in MHO than metabolically healthy normal-weight participants, but one showed that MHO people had lower CVD risk than metabolically unhealthy normal weight participants.45 Other reports suggest that obesity, especially long-lasting or severe obesity, without metabolic abnormalities may not increase risk for AMI but does increase risk for HF.46,47
Chart 6-9. Relative risks for diseases associated with body mass index by age group.
APCSC indicates Asia-Pacific Cohort Studies Collaboration; ERFC, Emerging Risk Factor Collaboration; IHD, ischemic heart disease; and PSC, Prospective Studies Collaboration.
Reprinted from Singh et al.32 Copyright © 2013, The Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mortality
Childhood BMIs in the highest quartile were associated with premature death as an adult in a cohort of 4857 American Indian children during a median follow-up of 23.9 years.48
According to NHIS-linked mortality data, among young adults aged 18 to 39 years, the HR for all-cause mortality was 1.07 (95% CI, 0.91–1.26) for self-reported overweight (not including obese) people, 1.41 (95% CI, 1.16–1.73) for obese people, and 2.46 (95% CI, 1.91–3.16) for extremely obese people. 49
Among adults, obesity was associated with nearly 112 000 excess deaths (95% CI, 53 754–170 064) relative to normal weight in 2000. Class I obesity (BMI 30–35 kg/m2) was associated with almost 30 000 of these excess deaths (95% CI, 8534–68 220) and classes II to III obesity (BMI ≥35 kg/ m2) with >82 000 (95% CI, 44 843–119 289). Underweight was associated with nearly 34 000 excess deaths (95% CI, 15 726–51 766). As other studies have found,50 being overweight but not obese (BMI 25 to <30 kg/m2) was not associated with excess deaths.51
A recent systematic review (2.88 million people and >270 000 deaths) showed that relative to normal BMI (18.5 to <25 kg/m2), all-cause mortality was lower for overweight but not obese individuals (HR, 0.94; 95% CI, 0.91–0.96) and was not elevated for class I obesity (HR, 0.95; 95% CI, 0.88–1.01). All-cause mortality was higher for obesity overall (HR, 1.18; 95% CI, 1.12–1.25) and for the subset of class II and III obesity (HR, 1.29; 95% CI, 1.18–1.41).52
Recent meta-analysis of 3.74 million deaths among 30.3 million participants found that overweight and obesity were associated with higher risk of all-cause mortality, with lowest risks at BMI 22 to 23 kg/m2 in healthy never-smokers and 20 to 22 kg/ m2 in never-smokers with ≥20 years of follow-up.53
In a collaborative analysis of data from almost 900 000 adults in 57 prospective studies, mostly in western Europe and North America, overall mortality was lowest at a BMI of ≈22.5 to 25 kg/m2 in both sexes and at all ages, after exclusion of early follow-up and adjustment for smoking status. Above this range, each 5-kg/m2-higher BMI was associated with ≈30% higher all-cause mortality, and no specific cause of death was inversely associated with BMI. Below 22.5 to 25 kg/m2, the overall inverse association with BMI was predominantly related to strong inverse associations for smoking-related respiratory disease, and the only clearly positive association was for IHD.54
In a meta-analysis of 1.46 million white adults, over a mean follow-up period of 10 years, all-cause mortality was lowest at BMI levels of 20.0 to 24.9 kg/ m2. Among females, compared with a BMI of 22.5 to 24.9 kg/m2, the HRs for death were as follows: BMI 15.0 to 18.4 kg/m2, 1.47; 18.5 to 19.9 kg/ m2, 1.14; 20.0 to 22.4 kg/m2, 1.0; 25.0 to 29.9 kg/m2, 1.13; 30.0 to 34.9 kg/m2, 1.44; 35.0 to 39.9 kg/m2, 1.88; and 40.0 to 49.9 kg/m2, 2.51. Similar estimates were observed in males.55
According to data from the NCDR, among patients presenting with STEMI and a BMI ≥40 kg/m2, in-hospital mortality rates were higher for patients with class III obesity (OR, 1.64; 95% CI, 1.32–2.03) when class I obesity was used as the referent.56
Overweight was associated with significantly increased mortality resulting from DM or kidney disease and was not associated with increased mortality resulting from cancer or CVD in an analysis of 2004 data from NHANES. Obesity was associated with significantly increased mortality caused by CVD, some cancers, and DM or kidney disease. Obesity was associated with 13% of CVD deaths in 2004.57
On the basis of a comparison of data from 1980 and 2000, reductions in smoking, cholesterol, BP, and physical inactivity levels resulted in estimated gains of 2 770 500 life-years but with a loss of 715 000 life-years caused by the increased prevalence of obesity and DM.58
In a study of 22 203 females and males from England and Scotland, metabolically unhealthy obese individuals were at an increased risk of all-cause mortality compared with MHO individuals (HR, 1.72; 95% CI, 1.23–2.41).59
In a comparison of 5 different anthropometric variables (BMI, waist circumference, hip circumference, waist-to-hip ratio, and waist-to-height ratio) in 62 223 individuals from Norway with 12 years of follow-up from the HUNT 2 study, the risk of death per SD increase in each measure was 1.02 (95% CI, 0.99–1.06) for BMI, 1.10 (95% CI, 1.06–1.14) for waist circumference, 1.01 (95% CI, 0.97–1.05) for hip circumference, 1.15 (95% CI, 1.11–1.19) for waist-to-hip ratio, and 1.12 (95% CI, 1.08–1.16) for waist-to-height ratio. For CVD mortality, the risk of death per SD increase was 1.12 (95% CI, 1.06–1.20) for BMI, 1.19 (95% CI, 1.12–1.26) for waist circumference, 1.06 (95% CI, 1.00–1.13) for hip circumference, 1.23 (95% CI, 1.16–1.30) for waist-to-hip ratio, and 1.24 (95% CI, 1.16–1.31) for waist-to-height ratio.60 However, because BMI and waist circumference are strongly correlated, large samples are needed to evaluate their independent contributions to risk.13,61 A recent pooled analysis of waist circumference and mortality in 650 386 adults followed up for a median of 9 years revealed that a 5-cm increment in waist circumference was associated with an increase in all-cause mortality at all BMI categories examined from 20 to 50 kg/m2.62 Similarly, in an analysis of postmenopausal females in the WHI limited to those with BMI ≥40 kg/m2, mortality, CHD, and CHF incidence all increased with waist circumference >115 and >122 cm compared with ≤108.4 cm.33 Finally, among 14 941 males and females in ARIC, the risk of SCD was associated with higher BMI and waist circumference, with traditional risk factors mediating the association with BMI but not with waist circumference.63
Cost
Obesity costs the healthcare system, healthcare payers, and obese individuals themselves.
In the United States, the estimated annual medical cost of obesity in 2008 was $147 billion; the annual medical costs for those who were obese were $1429 higher than for those at normal weight.64 A more recent study estimated mean annual per capita healthcare expenses of obesity were $1160 for males and $1525 for females.65
According to NHANES I data linked to Medicare and mortality records, 45-year-olds who were obese had lifetime Medicare costs of $163 000 compared with $117 000 for those who were normal weight at 65 years of age.66
In the absence of obesity, annual medical expenditures would be 6.7% (based on 2006 MEPS data) to 10.7% (based on 2006 BRFSS data) lower.67
According to data from the Medicare Current Beneficiary Survey from 1997 to 2006, in 1997, expenditures for Part A and Part B services per beneficiary were $6832 for a normal-weight person, which was more than for overweight ($5473) or obese ($5790) people; however, over time, expenses increased more rapidly for overweight and obese people.68
The costs of obesity are high: People with obesity pay on average $1429 (42%) more for healthcare costs than normal-weight people. For beneficiaries who are obese, Medicare pays $1723 more, Medicaid pays $1021 more, and private insurers pay $1140 more than for beneficiaries who are at normal weight. Similarly, people who are obese have 46% higher inpatient costs and 27% more outpatient visits and spend 80% more on prescription drugs.64
The total excess cost related to the current prevalence of adolescent overweight and obesity is estimated to be $254 billion ($208 billion in lost productivity secondary to premature morbidity and mortality and $46 billion in direct medical costs).69
A recent study recommended the use of $19 000 as the incremental lifetime medical cost of a child with obesity relative to a normal-weight child who maintains normal weight throughout adulthood.70
According to the 2006 NHDS, the incidence of bariatric surgery was estimated at 113 000 cases per year, with costs of nearly $1.5 billion annually.71
A recent cost-effectiveness study of laparoscopic adjustable gastric banding showed that after 5 years, $4970 was saved in medical expenses; if indirect costs were included (absenteeism and presenteeism), savings increased to $6180 and $10 960, respectively.72 However, when expressed per QALY, only $6600 was gained for laparoscopic gastric bypass, $6200 for laparoscopic adjustable gastric band, and $17 300 for open RYGB, none of which exceeded the standard $50 000 per QALY gained.73 Two other recent large studies failed to demonstrate a cost benefit for bariatric surgery versus matched patients over 6 years of follow-up74,75; however, another 2 studies showed cost savings for bariatric surgery among patients with DM at baseline.76,77
Secular Trends
Youth
Among infants and children from birth to >2 years old, the prevalence of high weight for recumbent length (ie, ≥95th percentile of sex-specific CDC 2000 growth charts) was 9.5% in 2003 to 2004 and 8.1% in 2011 to 2014. The decrease of 1.4% was not statistically significant.78
According to NCHS/CDC and NHANES surveys, the prevalence of obesity among children and adolescents increased substantially from 1963 to 1965 through 2013 to 2014, but this increase has slowed and has begun to reverse among children aged 2 to 5 years (Chart 6-10).
Specifically, according to NHANES data, from 1988 to 1994 to 2003 to 2006 to 2011 to 2014, the percentage of children 12 to 19 years of age classified as obese increased from 10.5% to 17.6% to 20.5%, respectively18; however, during the same time periods, among children aged 2 to 5 years, the prevalence of obesity went from 7.2% in 1988 to 1994 to 12.5% in 2003 to 2006 to 8.9% in 2011 to 2014.17,18 Another analysis of NHANES data showed that between 1988 to 1994 and 2013 to 2014, obesity increased among adolescents aged 12 to 19 years (from 10.5% to 20.6%), and extreme obesity increased among children aged 6 to 11 years (from 3.6% to 4.3%) and adolescents aged 12 to 19 years (from 2.6% to 9.1%). However, between 2005 to 2006 and 2013 to 2014, no significant increasing trends were observed.17
According to the YRBS, among US high school students between 1999 and 2015, there was a significant linear increase in the prevalence of obesity (from 10.6% to 13.9%) and in the prevalence of overweight (from 14.1% to 16.0%). Between 1991 to 2015, there was a corresponding significant linear increase of students reporting trying to lose weight, from 41.8% to 45.6%.20
Chart 6-10. US children and adolescents with obesity, 1963 to 2014.
Obesity is body mass index (BMI) at or above the sex- and age-specific 95th percentile BMI cutoff points from the 2000 CDC growth charts.
CDC indicates Centers for Disease Control and Prevention.
Source: CDC/National Center for Health Statistics, Health, United States, 2015, Figure 8 and Table 59. Data from the National Health and Nutrition Examination Survey (NHANES).18
Adults
In the United States, the prevalence of obesity among adults, estimated using NHANES data, increased from 1999 to 2000 through 2013 to 2014 from 30.5% to 37.7%7 (Chart 6-11); however, from 2005 to 2006 through 2013 to 2014, there was a significant linear trend for the increase in obesity and class III obesity for females (from 35.6% to 41.1% and from 7.5% to 10.0%, respectively) but not males (from 33.4% to 35.1% and from 7.5% to 10.0%, respectively).6
From NHANES 1999 to 2002 to NHANES 2007 to 2010, the prevalence of total and undiagnosed DM, total hypertension, total dyslipidemia, and smoking did not change significantly within any of the BMI categories, but there was a lower prevalence of dyslipidemia (−3.4; −6.3 to −0.5%) among overweight adults. However, the prevalence of untreated hypertension decreased among overweight and obese adults and that of untreated dyslipidemia decreased for all BMI categories (normal, overweight, obese, and BMI ≥35 kg/m2).28
Another study reported that for females, but not males, the increase in waist circumference from NHANES 1999 to 2000 to NHANES 2010 to 2011 was greater than expected based on the increase in BMI.79
In northern Sweden, across 6 population surveys from 1986 to 2009, the prevalence of metabolic health (no DM, hypertension, and total cholesterol <5.0 mmol/L) increased for normal weight, overweight, and obese adults.80
Prevention
A systematic review and meta-analysis of 15 prospective cohort studies with 200 777 participants showed that children and adolescents who were obese were ≈5 times more likely to be obese in adulthood than those who were not obese. Approximately 55% of children who are obese go on to be obese in adolescence, 80% of obese adolescents will still be obese in adulthood, and 70% will be obese over age 30. However, 70% of obese adults were not obese in childhood or adolescence, so reducing the overall burden of adult obesity might require interventions beyond targeting obesity reduction solely at obese or overweight children.81
The CDC Prevention Status Reports highlight the status of public health policies and practices to address public health problems including obesity, by state. Reports rate the extent to which the state has implemented the policies or practices identified from systemic reviews, national strategies or action plans, or expert bodies.82 Obesity reduction policies and programs implemented by country are also provided online.83
Awareness
According to NHANES 2003 to 2006 data, ≈23% of overweight and obese adults misperceived themselves to be at a healthier weight status, and those people were less likely to have tried to lose weight in the prior year.84
Recent studies show that parents’ perceptions of overweight and obesity differ according to the child’s race and sex. Boys 6 to 15 years of age with obesity were more likely than girls to be misperceived as being “about the right weight” by their parents (OR, 1.40; 95% CI, 1.12–1.76, P=0.004). Obesity was significantly less likely to be misperceived among girls 11 to 15 years of age than among girls 6 to 10 years of age (OR, 0.46; 95% CI, 0.29–0.74; P=0.002) and among Hispanic males than among white males (OR, 0.58; 95% CI, 0.36–0.93, P=0.02).85 Notification of a child’s unhealthy weight by healthcare practitioners increased from 22% in 1999 to 34% in 2014.86
Treatment and Control
The randomized trial Look AHEAD showed that among adults who were overweight and obese and had type 2 DM, an intensive lifestyle intervention produced a greater percentage of weight loss at 4 years than DM support education.87 After 8 years of intervention, the percentage of weight loss ≥5% and ≥10% was greater in the intensive lifestyle intervention than in DM support education groups (50.3% and 26.9% versus 35.7% and 17.2%, respectively).88 Intensive lifestyle interventions produce greater weight loss than education alone among those with class III obesity89 and childhood obesity.90
Look AHEAD was stopped early with a median 9.6 years of follow-up for failure to show a significant difference in CVD events between the intensive lifestyle intervention and control group.91
Ten-year follow-up data from the nonrandomized SOS bariatric intervention study (see Bariatric Surgery) suggested that to maintain a favorable effect on cardiovascular risk factors, more than the short-term goal of 5% weight loss is needed to overcome secular trends and aging effects.92 Long-term follow-up may be necessary to show reductions in CVD risk.
Bariatric Surgery
Lifestyle interventions often do not provide sustained significant weight loss for people who are obese. Among adults who are obese, bariatric surgery produces greater weight loss and maintenance of lost weight than lifestyle intervention, with some variations depending on the type of procedure and the patient’s initial weight.29 Gastric bypass surgery is typically performed as an RYGB, vertical sleeve gastrectomy, adjustable gastric banding, or biliopancreatic diversion with duodenal switch. Outcomes vary by bariatric surgery technique.93 Adult candidates for bariatric surgery include those with BMI >40 kg/m2, or BMI>35 kg/m2 with an obesity-related comorbidity, and bariatric surgery can be considered for those with BMI 30 to 34.9 kg/m2 with DM or metabolic syndrome.94 A recent DM consensus statement recommended bariatric surgery to treat type 2 DM among adults with class III obesity and recommended it be considered to treat type 2 DM among adults with class I obesity.95
Benefits reported for bariatric surgery include substantial weight loss; remission of DM, hypertension, and dyslipidemia; reduced incidence of mortality; and fewer CVD events. Reported risks with bariatric surgery include not only perioperative mortality and adverse events but also weight regain, DM recurrence (particularly for those with longer DM duration before surgery), bone loss, increases in substance use disorders, suicide, and nutritional deficiencies. Outcomes must be assessed cautiously, because most bariatric surgery data come from nonrandomized observational studies, with only a few randomized clinical trials comparing bariatric surgery to medical treatment for patients with DM. Furthermore, studies have not always reported their definition of “remission” or “partial remission” for comorbidities such as DM, hypertension, and dyslipidemia, and many have not reported laboratory values or medication use.93,96
In a large bariatric surgery cohort, the prevalence of high 10-year predicted CVD risk was 36.5%,97 but 76% of those with low 10-year risk had high lifetime predicted CVD risk. The corresponding prevalence in US adults is 18% and 56%, respectively.98
A recent meta-analysis reported postsurgery percent remission separately for randomized clinical trials and observational studies as follows: for DM remission, ≈92% (randomized clinical trials) and 86% (observational studies); for hypertension remission, 75% (randomized clinical trials) and 74% (observational studies); and for dyslipidemia remission, 76% (randomized clinical trials) and 68% (observational studies). Overall percentage of excess weight loss was ≈60% (randomized clinical trials) and 46% (observational studies) at 1 year and ≈42% (randomized clinical trials) and 62% (observational studies) at 5 years. However, the percentage of excess weight loss was greater with gastric bypass (63%–72%) than with sleeve gastrectomy (51%–79%) or adjustable gastric banding (33%–34%) at 1 year but more similar for observational data at 3 years: 76% for gastric bypass versus ≈59% for adjustable gastric banding or sleeve gastrectomy. Postsurgery mortality was low, but complication rates were ≈17% (randomized clinical trials) and 10% (observational studies) higher for gastric bypass. Reoperation rates were ≈7% (randomized clinical trials) and 6% (observational studies) higher for adjustable gastric banding than for gastric bypass or sleeve gastrectomy.99
A meta-analysis of RCTs also showed substantially higher weight loss and DM remission for bariatric surgery than for conventional medical therapy, with follow-up of ≤2 years.100
With 3-year follow-up, a randomized clinical trial among obese patients with uncontrolled type 2 DM reported 38%, 24%, and 5% of patients in the gastric bypass, sleeve gastrectomy, and medical therapy group, respectively, had HbA1c ≤6.0%.65 At 3-year follow-up in the LABS study, 67.5% (RYGB) and 28.6% (laparoscopic adjustable gastric banding) of patients had partial remission of DM. Dyslipidemia resolved in 62% (RYGB) and 27% (laparoscopic adjustable gastric banding); remission of hypertension occurred in 38% (RYGB) and 17.4% (laparoscopic adjustable gastric banding).101
Long-term follow-up data are scarce, but in the nonrandomized SOS study, remission of DM at 2 years was 72% for the surgical group and 16% in the matched control group but decreased in both groups at 10 and 15 years, although it remained higher in the surgical group (38% at 10 years and 30% at 15 years for the surgical group).102
Another meta-analysis of observational cohort studies showed a reduced risk of mortality (OR, 0.48; 95% CI, 0.35–0.64), CVD (OR, 0.54; 95% CI, 0.41–0.70), MI (OR, 0.46; 95% CI, 0.30–0.69), and stroke (OR, 0.49; 95% CI, 0.32–0.75), but RCT data are lacking.103
According to retrospective data from the United States, among 9949 patients who underwent gastric bypass surgery, after a mean of 7 years, long-term mortality was 40% lower among the surgically treated patients than among obese control subjects. Specifically, cancer mortality was reduced by 60%, DM mortality by 92%, and CAD mortality by 56%. Nondisease death rates (eg, accidents, suicide) were 58% higher in the surgery group.104
In a propensity-matched retrospective cohort study from the Veterans Affairs medical system, bariatric surgery was not associated with reduced mortality compared with obese control subjects (time-adjusted HR, 0.94; 95% CI, 0.64–1.39) over a mean 6.7 years of follow-up,105 but mortality was reduced during 5 to 14 years of follow-up.106
Global Burden
Maps of the prevalence of obesity worldwide are available online.107
Although there is considerable variability in overweight and obesity data methodology and quality worldwide, cross-country comparisons can help reveal different patterns. Worldwide, from 1975 to 2014, the prevalence of obesity increased from 3.2% in 1975 to 10.8% in 2014 in males and from 6.4% to 14.9% in females, and mean age-standardized BMI increased from 21.7 to 24.2 kg/m2 in males and from 22.1 to 24.4 kg/m2 in females.108 Worldwide, between 1980 and 2013, the proportion of overweight or obese adults increased from 28.8% (95% UI, 28.4%–29.3%) to 36.9% (95% UI, 36.3%–37.4%) among males and from 29.8% (95% UI, 29.3%–30.2%) to 38.0% (95% UI, 37.5%–38.5%) among females. Since 2006, the increase in adult obesity in developed countries has slowed. The estimated prevalence of adult obesity exceeded 50% of males in Tonga and females in Kuwait, Kiribati, the Federated States of Micronesia, Libya, Qatar, Tonga, and Samoa. As of 2013, around the world, obesity rates are higher for females than males and in developed countries than in developing countries. Higher obesity rates for females than for males occur for age ≥45 years in developed countries but at age ≥25 years in developing countries.109
Between 1980 and 2008, mean BMI increased worldwide by 0.4 kg/m2 per decade for males and 0.5 kg/m2 per decade for females, with trends varying between nations. In 2008, an estimated 1.46 billion adults were overweight or obese. The prevalence of obesity was estimated at 205 million males and 297 million females. The highest prevalence of male obesity is in the United States, Southern and Central Latin America, Australasia, and Central and Western Europe, and the lowest prevalence is in South and Southeast Asia and East, Central, and West Africa. For females, the highest prevalence of obesity is in Southern and North Africa, the Middle East, Central and Southern Latin America, and the United States, and the lowest is in South, East, and Southeast Asia; the high-income Asia-Pacific subregion; and East, Central, and West Africa.110
Between 1990 and 2010, estimated worldwide deaths attributable to high BMI increased 1.7-fold, from 1 963 549 to 3 371 232, and DALYs lost because of high BMI rose 1.8-fold, from 51 565 to 93 609. Therefore, between 1990 and 2010, high BMI went from tenth to sixth in ranking of contribution to the global burden of disease and was among the top 5 risk factors for global burden of disease in all regions except high-income Asia-Pacific; East, Southeast, and South Asia; and East, Central, and West sub-Saharan Africa.111
REFERENCES
- 1.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation in the WHS (Women’s Health Study) J Am Coll Cardiol. 2010;55:2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wattanakit K, Lutsey PL, Bell EJ, Gornik H, Cushman M, Heckbert SR, Rosamond WD, Folsom AR. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism: a time-dependent analysis. Thromb Haemost. 2012;108:508–515. doi: 10.1160/TH11-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 8.Rao G, Powell-Wiley TM, Ancheta I, Hairston K, Kirley K, Lear SA, North KE, Palaniappan L, Rosal MC American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association [published correction appears in Circulation. 2015;132:e130] Circulation. 2015;132:457–472. doi: 10.1161/CIR.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies [published correction appears in Lancet. 2004;363:902] Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.CDC growth charts. [Accessed July 13, 2016];Centers for Disease Control and Prevention Web site. http://www.cdc.gov/growthcharts/cdc_charts.htm.
- 11.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;(25):1–5. [PubMed] [Google Scholar]
- 12.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, and Council on Clinical Cardiology. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 13.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Waist circumference and waist–hip ratio: report of a WHO expert consultation; Geneva. 8–11 December 2008; Geneva, Switzerland: WHO Document Production Services; [Accessed June 13, 2016]. 2008. [Google Scholar]
- 15.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society [published correction appears in Circulation. 2014;129(suppl 2): S139–S140] Circulation. 2014;129(suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryar CD, Carroll MD, Ogden C. Prevalence of Overweight and Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2013–2014. Health E-Stats. 2016 Jul; [Google Scholar]
- 17.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2016. [Accessed August 24, 2016]. http://www.cdc.gov/nchs/data/hus/hus15.pdf. [PubMed] [Google Scholar]
- 19.Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci U S A. 2014;111:1338–1342. doi: 10.1073/pnas.1321355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, Queen B, Lowry R, Olsen EO, Chyen D, Whittle L, Thornton J, Lim C, Yamakawa Y, Brener N, Zaza S. Youth Risk Behavior Surveillance: United States, 2015. MMWR Surveill Summ. 2016;65:1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) [Accessed May 15, 2016];Obesity prevalence maps. 2012–2014 https://www.cdc.gov/obesity/data/prevalence-maps.html.
- 22.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States, 2005–2008. NCHS Data Brief. 2010;(50):1–8. [PubMed] [Google Scholar]
- 23.The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association Childhood Obesity Research Summit: executive summary. Circulation. 2009;119:2114–2123. doi: 10.1161/CIRCULATIONAHA.109.192215. [DOI] [PubMed] [Google Scholar]
- 25.Freedman DS, Goodman A, Contreras OA, DasMahapatra P, Srinivasan SR, Berenson GS. Secular trends in BMI and blood pressure among children and adolescents: the Bogalusa Heart Study. Pediatrics. 2012;130:e159–e166. doi: 10.1542/peds.2011-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 27.Kozak AT, Daviglus ML, Chan C, Kiefe CI, Jacobs DR, Jr, Liu K. Relationship of body mass index in young adulthood and health-related quality of life two decades later: the Coronary Artery Risk Development in Young Adults study. Int J Obes (Lond) 2011;35:134–141. doi: 10.1038/ijo.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saydah S, Bullard KM, Cheng Y, Ali MK, Gregg EW, Geiss L, Imperatore G. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring) 2014;22:1888–1895. doi: 10.1002/oby.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidelines (2013) for Managing Overweight and Obesity in Adults. Preface to the Expert Panel Report (comprehensive version which includes systematic evidence review, evidence statements, and recommendations) Obesity (Silver Spring) 2014;22(suppl 2):S40. doi: 10.1002/oby.20822. [DOI] [PubMed] [Google Scholar]
- 30.Goff DC, Jr, Gillespie C, Howard G, Labarthe DR. Is the obesity epidemic reversing favorable trends in blood pressure? Evidence from cohorts born between 1890 and 1990 in the United States. Ann Epidemiol. 2012;22:554–561. doi: 10.1016/j.annepidem.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. 2016;17:56–67. doi: 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 32.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group; Asia-Pacific Cohort Studies Collaboration (APCSC); Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe (DECODE); Emerging Risk Factor Collaboration (ERFC); Prospective Studies Collaboration (PSC) The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McTigue KM, Chang YF, Eaton C, Garcia L, Johnson KC, Lewis CE, Liu S, Mackey RH, Robinson J, Rosal MC, Snetselaar L, Valoski A, Kuller LH. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity (Silver Spring) 2014;22:801–810. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 34.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, Chung H, Carnethon MR. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? Am J Med. 2009;122(suppl 1):S4–11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–e426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 38.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 39.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring) 2013;21:E51–E55. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 40.Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, Jacob S. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123:646–651. doi: 10.1016/j.amjmed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes [published correction appears in JAMA. 2012;308:2085] JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T, Joensuu A, Newby C, Pang C, Partinen E, Reischl E, Schwienbacher C, Tammesoo ML, Swertz MA, Burton P, Ferretti V, Fortier I, Giepmans L, Harris JR, Hillege HL, Holmen J, Jula A, Kootstra-Ros JE, Kvaløy K, Holmen TL, Männistö S, Metspalu A, Midthjell K, Murtagh MJ, Peters A, Pramstaller PP, Saaristo T, Salomaa V, Stolk RP, Uusitupa M, van der Harst P, van der Klauw MM, Waldenberger M, Perola M, Wolffenbuttel BH. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamer M, Bell JA, Sabia S, Batty GD, Kivimäki M. Stability of metabolically healthy obesity over 8 years: the English Longitudinal Study of Ageing. Eur J Endocrinol. 2015;173:703–708. doi: 10.1530/EJE-15-0449. [DOI] [PubMed] [Google Scholar]
- 44.Fung MD, Canning KL, Mirdamadi P, Ardern CI, Kuk JL. Lifestyle and weight predictors of a healthy overweight profile over a 20-year follow-up. Obesity (Silver Spring) 2015;23:1320–1325. doi: 10.1002/oby.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:956–966. doi: 10.1177/2047487315623884. [DOI] [PubMed] [Google Scholar]
- 46.Mørkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trøndelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 47.Janszky I, Romundstad P, Laugsand LE, Vatten LJ, Mukamal KJ, Mørkedal B. Weight and weight change and risk of acute myocardial infarction and heart failure: the HUNT Study. J Intern Med. 2016;280:312–322. doi: 10.1111/joim.12494. [DOI] [PubMed] [Google Scholar]
- 48.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma J, Flanders WD, Ward EM, Jemal A. Body mass index in young adulthood and premature death: analyses of the US National Health Interview Survey linked mortality files. Am J Epidemiol. 2011;174:934–944. doi: 10.1093/aje/kwr169. [DOI] [PubMed] [Google Scholar]
- 50.McGee DL Diverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 52.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults [published correction appears in N Engl J Med. 2011;365:869] N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction: results from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2011;58:2642–2650. doi: 10.1016/j.jacc.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 58.Capewell S, Hayes DK, Ford ES, Critchley JA, Croft JB, Greenlund KJ, Labarthe DR. Life-years gained among US adults from modern treatments and changes in the prevalence of 6 coronary heart disease risk factors between 1980 and 2000. Am J Epidemiol. 2009;170:229–236. doi: 10.1093/aje/kwp150. [DOI] [PubMed] [Google Scholar]
- 59.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97:2482–2488. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petursson H, Sigurdsson JA, Bengtsson C, Nilsen TI, Getz L. Body configuration as a predictor of mortality: comparison of five anthropometric measures in a 12 year follow-up of the Norwegian HUNT 2 study. PLoS One. 2011;6:e26621. doi: 10.1371/journal.pone.0026621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Després JP. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol. 2011;57:1887–1889. doi: 10.1016/j.jacc.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 62.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, Ebbert JO, English DR, Gapstur SM, Giles GG, Horn-Ross PL, Park Y, Patel AV, Robien K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hartge P, Bernstein L, Berrington de Gonzalez A. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adabag S, Huxley RR, Lopez FL, Chen LY, Sotoodehnia N, Siscovick D, Deo R, Konety S, Alonso A, Folsom AR. Obesity related risk of sudden cardiac death in the Atherosclerosis Risk in Communities Study. Heart. 2015;101:215–221. doi: 10.1136/heartjnl-2014-306238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 65.An R. Health care expenses in relation to obesity and smoking among U.S. adults by gender, race/ethnicity, and age group: 1998–2011. Public Health. 2015;129:29–36. doi: 10.1016/j.puhe.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Cai L, Lubitz J, Flegal KM, Pamuk ER. The predicted effects of chronic obesity in middle age on Medicare costs and mortality. Med Care. 2010;48:510–517. doi: 10.1097/MLR.0b013e3181dbdb20. [DOI] [PubMed] [Google Scholar]
- 67.Trogdon JG, Finkelstein EA, Feagan CW, Cohen JW. State- and payer-specific estimates of annual medical expenditures attributable to obesity. Obesity (Silver Spring) 2012;20:214–220. doi: 10.1038/oby.2011.169. [DOI] [PubMed] [Google Scholar]
- 68.Alley D, Lloyd J, Shaffer T, Stuart B. Changes in the association between body mass index and Medicare costs, 1997–2006. Arch Intern Med. 2012;172:277–278. doi: 10.1001/archinternmed.2011.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lightwood J, Bibbins-Domingo K, Coxson P, Wang YC, Williams L, Goldman L. Forecasting the future economic burden of current adolescent overweight: an estimate of the coronary heart disease policy model. Am J Public Health. 2009;99:2230–2237. doi: 10.2105/AJPH.2008.152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics. 2014;133:854–862. doi: 10.1542/peds.2014-0063. [DOI] [PubMed] [Google Scholar]
- 71.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200:378–385. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkelstein EA, Allaire BT, Dibonaventura MD, Burgess SM. Incorporating indirect costs into a cost-benefit analysis of laparoscopic adjustable gastric banding. Value Health. 2012;15:299–304. doi: 10.1016/j.jval.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Wang BC, Wong ES, Alfonso-Cristancho R, He H, Flum DR, Arterburn DE, Garrison LP, Sullivan SD. Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur J Health Econ. 2014;15:253–263. doi: 10.1007/s10198-013-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maciejewski ML, Livingston EH, Smith VA, Kahwati LC, Henderson WG, Arterburn DE. Health expenditures among high-risk patients after gastric bypass and matched controls. Arch Surg. 2012;147:633–640. doi: 10.1001/archsurg.2012.818. [DOI] [PubMed] [Google Scholar]
- 75.Weiner JP, Goodwin SM, Chang HY, Bolen SD, Richards TM, Johns RA, Momin SR, Clark JM. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148:555–562. doi: 10.1001/jamasurg.2013.1504. [DOI] [PubMed] [Google Scholar]
- 76.Makary MA, Clark JM, Shore AD, Magnuson TH, Richards T, Bass EB, Dominici F, Weiner JP, Wu AW, Segal JB. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery [published correction appears in Arch Surg. 2011;146:659] Arch Surg. 2010;145:726–731. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 77.Keating C, Neovius M, Sjöholm K, Peltonen M, Narbro K, Eriksson JK, Sjöström L, Carlsson LM. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study [published correction appears in Lancet Diabetes Endocrinol. 2015;3:e11] Lancet Diabetes Endocrinol. 2015;3:855–865. doi: 10.1016/S2213-8587(15)00290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freedman DS, Ford ES. Are the recent secular increases in the waist circumference of adults independent of changes in BMI? Am J Clin Nutr. 2015;101:425–431. doi: 10.3945/ajcn.114.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benckert M, Lilja M, Söderberg S, Eliasson M. Improved metabolic health among the obese in six population surveys 1986 to 2009: the Northern Sweden MONICA study. BMC Obes. 2015;2:7. doi: 10.1186/s40608-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17:95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention. [Accessed June 8, 2016];Prevention Status Reports. 2016 http://www.cdc.gov/psr/
- 83.World Obesity Federation. [Accessed June 8, 2016];Policies and Interventions. 2015 http://www.worldobesity.org/resources/policies-and-interventions/
- 84.Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nutr Phys Act. 2011;8:20. doi: 10.1186/1479-5868-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Twarog JP, Politis MD, Woods EL, Daniel LM, Sonneville KR. Is obesity becoming the new normal? Age, gender and racial/ethnic differences in parental misperception of obesity as being “about the right weight”. Int J Obes (Lond) 2016;40:1051–1055. doi: 10.1038/ijo.2016.40. [DOI] [PubMed] [Google Scholar]
- 86.Hansen AR, Duncan DT, Baidal JA, Hill A, Turner SC, Zhang J. An increasing trend in health care professionals notifying children of unhealthy weight status: NHANES 1999–2014. Int J Obes (Lond) 2016;40:1480–1485. doi: 10.1038/ijo.2016.85. [DOI] [PubMed] [Google Scholar]
- 87.Wing RR Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring) 2014;22(1):5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wagenknecht LE, Wing RR Look AHEAD Research Group. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126:236–242. 242.e1–2. doi: 10.1016/j.amjmed.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, Collins C. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130:e1647–e1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- 91.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes [published correction appears in N Engl J Med. 2014;370:1866] N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sjöström CD, Lystig T, Lindroos AK. Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int J Obes (Lond) 2011;35:1413–1420. doi: 10.1038/ijo.2010.282. [DOI] [PubMed] [Google Scholar]
- 93.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: co-sponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract. 2013;19:337–372. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 96.Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackey RH, Belle SH, Courcoulas AP, Dakin GF, Deveney CW, Flum DR, Garcia L, King WC, Kuller LH, Mitchell JE, Pomp A, Pories WJ, Wolfe BM Longitudinal Assessment of Bariatric Surgery Consortium Writing Group. Distribution of 10-year and lifetime predicted risk for cardiovascular disease prior to surgery in the longitudinal assessment of bariatric surgery-2 study. Am J Cardiol. 2012;110:1130–1137. doi: 10.1016/j.amjcard.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–2304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 103.Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, Mamas MA, Loke YK. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173:20–28. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 104.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 105.Maciejewski ML, Livingston EH, Smith VA, Kavee AL, Kahwati LC, Henderson WG, Arterburn DE. Survival among high-risk patients after bariatric surgery. JAMA. 2011;305:2419–2426. doi: 10.1001/jama.2011.817. [DOI] [PubMed] [Google Scholar]
- 106.Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS, JR, Eid G, Weidenbacher H, Maciejewski ML. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 107.World Obesity Federation. [Accessed June 13, 2016];World map of obesity. 2015 http://www.worldobesity.org/resources/world-map-obesity/
- 108.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013 [published correction appears in Lancet. 2014;384:746] Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim S, Jang HC, Park KS, Cho SI, Lee MG, Joung H, Mozumdar A, Liguori G. Changes in metabolic syndrome in American and Korean youth, 1997–2008. Pediatrics. 2013;131:e214–e222. doi: 10.1542/peds.2012-0761. [DOI] [PubMed] [Google Scholar]
- 112.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 113.United States Census Bureau. [Accessed August 22, 2016];American Community Survey Summary File. https://www.census.gov/programs-surveys/acs/data/summary-file.html.
7. FAMILY HISTORY AND GENETICS
See Tables 7-1 through 7-3
Table 7-1.
OR for Combinations of Parental Heart Attack History
| OR (95% CI) | |
|---|---|
| No family history | 1.00 |
| One parent with heart attack ≥50 y of age | 1.67 (1.55–1.81) |
| One parent with heart attack <50 y of age | 2.36 (1.89–2.95) |
| Both parents with heart attack ≥50 y of age | 2.90 (2.30–3.66) |
| Both parents with heart attack, one <50 y of age | 3.26 (1.72–6.18) |
| Both parents with heart attack, both <50 y of age | 6.56 (1.39–30.95) |
CI indicates confidence interval; and OR, odds ratio.
Data derived from Chow et al.6
Biologically related first-degree relatives (siblings, offspring, and parents) share roughly 50% of their genetic variation with one another. This constitutes much greater sharing of genetic variation than with a randomly selected person from the population, and thus, familial aggregation of traits lends support for a genetic basis for the trait. Similarly, racial/ethnic minorities are more likely to share their genetic variation within their demographic than with other demographics. Familial aggregation of CVD may be related to aggregation of specific behaviors (eg, smoking, alcohol use) or risk factors (eg, hypertension, DM, obesity) that may themselves have environmental and genetic contributors. Unlike classic mendelian genetic risk factors, whereby usually 1 mutation directly causes 1 disease, a complex trait’s genetic contributors can increase risk without necessarily always causing the condition. The effect size of any specific contributor to risk may be small but widespread throughout a population, or it may be large but affect only a small population, or it may have an enhanced risk when an environmental contributor is present. We present a summary of evidence that a genetic risk for CVD is likely, as well as a summary of evidence on the most consistently replicated genetic markers for CHD and stroke identified to date. A comprehensive scientific statement on the role of genetics and genomics for the prevention and treatment of CVD is available elsewhere.1
Abbreviations Used in Chapter 7
| AAA | abdominal aortic aneurysm |
| ABI | ankle-brachial index |
| ACS | acute coronary syndrome |
| AF | atrial fibrillation |
| ASCVD | atherosclerotic cardiovascular disease |
| BMI | body mass index |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| CARDIoGRAMplusC4D | Coronary Artery Disease Genome-wide Replication and Meta-Analysis (CARDIOGRAM) plus the Coronary Artery Disease (C4D) Genetics Consortium |
| CHD | coronary heart disease |
| CI | confidence interval |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| FHS | Framingham Heart Study |
| GFR | glomerular filtration rate |
| HbA1c | hemoglobin A1c (glycosylated hemoglobin) |
| HD | heart disease |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HR | hazard ratio |
| LDL-C | low-density lipoprotein cholesterol |
| MI | myocardial infarction |
| NHANES | National Health and Nutrition Examination Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| OR | odds ratio |
| PAD | peripheral artery disease |
| SBP | systolic blood pressure |
| SE | standard error |
| SNP | single-nucleotide polymorphism |
| VTE | venous thromboembolism |
Family History
Prevalence
-
Among adults ≥20 years of age, 12.2% (SE 0.5%) reported having a parent or sibling with a heart attack or angina before the age of 50 years. The racial/ethnic breakdown from NHANES 2011 to 2014 is as follows (unpublished NHLBI tabulation):
For non-Hispanic whites, 12.2% (SE 0.9%) for males, 14.7% (SE 0.9%) for females
For non-Hispanic blacks, 8.1% (SE 0.9%) for males, 12.0% (SE 0.9%) for females
For Hispanics, 7.4% (SE 0.7%) for males, 10.1% (SE 0.9%) for females
For non-Hispanic Asians, 5.7% (SE 0.9%) for males, 5.3% (SE 0.8%) for females
-
HD occurs as people age, so the prevalence of family history will vary depending on the age at which it is assessed. The breakdown of reported family history of heart attack by age of survey respondent in the US population as measured by NHANES 2011 to 2014 is as follows (unpublished NHLBI tabulation):
Age 20 to 39 years, 8.7% (SE 0.8%) for males, 9.7% (SE 0.8%) for females
Age 40 to 59 years, 12.3% (SE 1.3%) for males, 16.0% (SE 1.5%) for females
Age 60 to 79 years, 13.6% (SE 1.8%) for males, 15.4% (SE 1.3%) for females
Age ≥80 years, 8.1% (SE 2.1%) for males, 13.7% (SE 2.7%) for females
In the multigenerational FHS, only 75% of participants with a documented parental history of a heart attack before age 55 years reported that history when asked.2
Impact of Family History
Coronary Heart Disease
(See Table 7-1)
Paternal history of premature heart attack has been shown to approximately double the risk of a heart attack in males and increase the risk in females by ≈70%.3,4 Among people with a family history, coronary artery calcium is a robust marker of ASCVD risk.5
History of a heart attack in both parents increases the risk of heart attack, especially when 1 parent had a premature heart attack before 50 years of age.6
Sibling history of CVD has been shown to increase the odds of CVD in males and females by 45% (OR, 1.45; 95% CI, 1.19–1.91) in models accounting for CVD risk factors.7
Family history of premature angina, MI, angioplasty, or bypass surgery increased the lifetime risk by ≈50% for both HD (from 8.9% to 13.7%) and CVD (from 14.1% to 21%) mortality.8
In a recent international study of individuals with premature ACS (age ≤55 years), more females (28%) than males (20%) had a family history of CAD (P=0.008). However, compared with patients without a family history, patients with a family history of CAD had a higher prevalence of traditional CVD risk factors, including dyslipidemia and obesity. Females with a family history had a higher prevalence of each traditional risk factor (obesity, DM, dyslipidemia, and hypertension) except smoking.9
Other CVDs
A parental history of AF was associated with ≈80% increased odds of AF in males and females.10 The risk of AF was increased the younger the age of onset and the more family members affected.11 In a Swedish study,12 the odds of AF associated with familial AF (OR, 5.04; 95% CI, 4.26–5.82) were higher in people with a history of premature AF (diagnosed AF at age <50 years). Interestingly, there was modest spousal aggregation of AF, consistent with a contribution of shared environment to AF risk; the spousal OR for AF was 1.16 (95% CI, 1.13–1.19).12
A history of stroke in a first-degree relative increases the odds of stroke in males and females by ≈50%.13
A parental history of HF also is associated with an increased odds of offspring HF (multivariable-adjusted HR, 1.7; 95% CI, 1.11–2.60).14
In a Swedish population-based case-control study, the risk of thoracic aortic disease increased the greater the number of affected relatives and the younger the individual affected. The OR was 5.8 (95% CI, 4.3–7.7) with 1 affected relative versus 20 (95% CI, 2.2–179) with ≥2 affected relatives.15
Similarly, the odds of having PAD were elevated (OR, 1.83; 95% CI, 1.03–3.26) in individuals with a family history of PAD.16
A family history of VTE is associated with 2- to 3-fold odds of VTE, irrespective of identified known predisposing genetic factors.17,18
Genetics
Heart Disease
(See Table 7-2)
Table 7-2.
Validated SNPs for CAD, the Nearest Gene, and the OR From the CARDIoGRAMplusC4D Consortium
| SNP | Chromosome | Gene | Effect Size (OR) | Effect Allele Frequency |
|---|---|---|---|---|
| rs602633 | 1 | SORT1 | 1.12 | 0.77 |
| rs17464857 | 1 | MIA3 | 1.05 | 0.87 |
| rs17114036 | 1 | PPAP2B | 1.11 | 0.91 |
| rs11206510 | 1 | PCSK9 | 1.06 | 0.84 |
| rs4845625 | 1 | IL6R | 1.04 | 0.47 |
| rs6725887 | 2 | WDR12 | 1.12 | 0.11 |
| rs515135 | 2 | APOB | 1.08 | 0.82 |
| rs2252641 | 2 | ZEB2-AC074093.1 | 1.04 | 0.46 |
| rs1561198 | 2 | VAMP5-VAMP8-GGCX | 1.05 | 0.45 |
| rs6544713 | 2 | ABCG5-ABCG8 | 1.06 | 0.30 |
| rs9818870 | 3 | MRAS | 1.07 | 0.14 |
| rs7692387 | 4 | GUCY1A3 | 1.06 | 0.81 |
| rs1878406 | 4 | EDNRA | 1.06 | 0.15 |
| rs273909 | 5 | SLC22A4-SLC22A5 | 1.09 | 0.14 |
| rs12205331 | 6 | ANKS1A | 1.04 | 0.81 |
| rs9369640 | 6 | PHACTR1 | 1.09 | 0.65 |
| rs12190287 | 6 | TCF21 | 1.07 | 0.59 |
| rs3798220 | 6 | LPA | 1.28 | 0.01 |
| rs10947789 | 6 | KCNK5 | 1.06 | 0.76 |
| rs4252120 | 6 | PLG | 1.06 | 0.73 |
| rs11556924 | 7 | ZC3HC1 | 1.08 | 0.65 |
| rs12539895 | 7 | 7q22 | 1.08 | 0.19 |
| rs2023938 | 7 | HDAC9 | 1.07 | 0.10 |
| rs264 | 8 | LPL | 1.05 | 0.86 |
| rs2954029 | 8 | TRIB1 | 1.04 | 0.55 |
| rs1333049 | 9 | CDKN2A, CDKN2B | 1.23 | 0.47 |
| rs579459 | 9 | ABO | 1.07 | 0.21 |
| rs2505083 | 10 | KIAA1462 | 1.06 | 0.42 |
| rs501120 | 10 | CXCL12 | 1.07 | 0.83 |
| rs12413409 | 10 | CYP17A1-CNNM2-NT5C2 | 1.10 | 0.89 |
| rs2246833 | 10 | LIPA | 1.06 | 0.38 |
| rs9326246 | 11 | ZNF259-APOA5-A4-C3-A1 | 1.09 | 0.10 |
| rs974819 | 11 | PDGFD | 1.07 | 0.29 |
| rs3184504 | 12 | SH2B3 | 1.07 | 0.40 |
| rs4773144 | 13 | COL4A1-COL4A2 | 1.07 | 0.42 |
| rs9319428 | 13 | FLT1 | 1.05 | 0.32 |
| rs2895811 | 14 | HHIPL1 | 1.06 | 0.43 |
| rs7173743 | 15 | ADAMTS7 | 1.07 | 0.58 |
| rs17514846 | 15 | FURIN-FES | 1.05 | 0.44 |
| rs2281727 | 17 | SMG6-SRR | 1.05 | 0.36 |
| rs12936587 | 17 | RASD1-SMCR3-PEMT | 1.06 | 0.59 |
| rs15563 | 17 | UBE2Z-GIP-ATP5G1-SNF8 | 1.04 | 0.52 |
| rs1122608 | 19 | LDLR | 1.10 | 0.76 |
| rs2075650 | 19 | ApoE-ApoC1 | 1.11 | 0.14 |
| rs9982601 | 21 | KCNE2 | 1.13 | 0.13 |
CAD indicates coronary artery disease; CARDIoGRAMplusC4D, Coronary Artery Disease Genome-wide Replication and Meta-analysis (CARDIOGRAM) plus the Coronary Artery Disease (C4D) Genetics Consortium; OR, odds ratio; and SNP, single-nucleotide polymorphism.
Data derived from Deloukas et al.19
Genome-wide association is a robust technique to identify associations between genotypes and phenotypes. Table 7-2 presents results from the CARDIoGRAMplusC4D Consortium, which represents the largest genetic study of CAD to date. Although the ORs are modest, ranging from 1.06 to 1.51 per copy of the risk allele (individuals can harbor up to 2 copies of a risk allele), these are common alleles, which suggests that the attributable risk could be substantial. Additional analysis suggested that loci associated with CAD were involved in lipid metabolism and inflammation pathways.19
The relationship between genetic variants associated with CHD and measured CHD risk factors is complex, with some genetic markers associated with multiple risk factors and other markers showing no association with risk factors.20
Genetic markers discovered thus far have not been shown to add to cardiovascular risk prediction tools beyond current models that incorporate family history.21 Genetic markers also have not been shown to improve prediction of subclinical atherosclerosis beyond traditional risk factors.22 However, an association between genetic markers and CAC has been seen.23
The most consistently replicated genetic marker for HD in European-derived populations is located at 9p21.3. At this single-nucleotide polymorphism, ≈27% of the white population is estimated to have 0 risk alleles, 50% is estimated to have 1 risk allele, and the remaining 23% is estimated to have 2 risk alleles.24 In meta-analyses of individuals of East Asian ancestry, variants at 9p21.3 have also been reported to be associated with CHD (OR per risk allele, 1.3; 95% CI, 1.25–1.35).25
The 10-year HD risk for a 65-year-old man with 2 risk alleles at 9p21.3 and no other traditional risk factors is ≈13.2%, whereas a similar man with 0 alleles would have a 10-year risk of ≈9.2%. The 10-year HD risk for a 40-year-old woman with 2 alleles and no other traditional risk factors is ≈2.4%, whereas a similar woman with 0 alleles would have a 10-year risk of ≈1.7%.24
Variation at the 9p21.3 region also is associated with an increased risk of HF26 and sudden death.27 Associations have also been observed between the 9p21.3 region and CAC.28,29 Additionally, stronger associations have been found between variation at 9p21.3 and earlier28,29 and more severe30 heart attacks. Paradoxically, a recent meta-analysis reported that variants at 9p21.3 were associated with incident (HR, 1.19; 95% CI, 1.17–1.22) but not recurrent (HR, 1.01; 95% CI, 0.97–1.06) CHD events,31 which supports the genetic complexity of CHD. The biological mechanisms under-pinning the association of genetic variation in the 9p21 region with disease outcomes are still under investigation.
A recent study using pathway and network analysis methodologies identified multiple mechanistic pathways and key driver genes for the development of CVD and type 2 DM.32
Stroke
The 9p21.3 region has also been associated with intracranial aneurysm,33 AAA,34 and ischemic stroke.35
For large-vessel ischemic stroke, an association for large-vessel stroke with histone deacetylase 9 on chromosome 7p21.1 has been identified (>9000 subjects) and replicated (>12 000 subjects).35,36
CVD Risk Factors
(See Table 7-3)
Table 7-3.
Heritability of CVD Risk Factors From the FHS
| Trait | Heritability |
|---|---|
| ABI | 0.2137 |
| SBP | 0.4238 |
| DBP | 0.3938 |
| Left ventricular mass | 0.24–0.3239 |
| BMI | 0.37 (mean age, 40 y)–0.52 (mean age, 60 y)40 |
| Waist circumference | 0.4141 |
| Visceral abdominal fat | 0.3642 |
| Subcutaneous abdominal fat | 0.5742 |
| Fasting glucose | 0.3443 |
| CRP | 0.3044 |
| HbA1c | 0.2743 |
| Triglycerides | 0.4845 |
| HDL-C | 0.5245 |
| Total cholesterol | 0.5745 |
| LDL-C | 0.5945 |
| Estimated GFR | 0.3346 |
ABI indicates ankle-brachial index; BMI, body mass index; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; FHS, Framingham Heart Study; GFR, glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; and SBP, systolic blood pressure.
Heritability is the ratio of genetically caused variation to the total variation of a trait or measure. Table 7-3 presents heritability estimates for standard CVD risk factors using data generated from the FHS. These data suggest that most CVD risk factors have at least moderate heritability.
REFERENCES
- 1.Ganesh SK, Arnett DK, Assimes TL, Basson CT, Chakravarti A, Ellinor PT, Engler MB, Goldmuntz E, Herrington DM, Hershberger RE, Hong Y, Johnson JA, Kittner SJ, McDermott DA, Meschia JF, Mestroni L, O’Donnell CJ, Psaty BM, Vasan RS, Ruel M, Shen WK, Terzic A, Waldman SA American Heart Association Council on Functional Genomics and Translational Biology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Basic Cardiovascular Sciences; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular and Stroke Nursing; American Heart Association Stroke Council. Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association [published correction appears in Circulation. 2014;129:e398] Circulation. 2013;128:2813–2851. doi: 10.1161/01.cir.0000437913.98912.1d. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, Nam BH, D’Agostino RB, Sr, Lloyd-Jones DM, O’Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 5.Patel J, Al Rifai M, Blaha MJ, Budoff MJ, Post WS, Polak JF, Bluemke DA, Scheuner MT, Kronmal RA, Blumenthal RS, Nasir K, McEvoy JW. Coronary artery calcium improves risk assessment in adults with a family history of premature coronary heart disease: results from Multiethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e003186. doi: 10.1161/CIRCIMAGING.115.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow CK, Islam S, Bautista L, Rumboldt Z, Yusufali A, Xie C, Anand SS, Engert JC, Rangarajan S, Yusuf S. Parental history and myocardial infarction risk across the world: the INTERHEART Study. J Am Coll Cardiol. 2011;57:619–627. doi: 10.1016/j.jacc.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 7.Murabito JM, Pencina MJ, Nam BH, D’Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O’Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann JM, Willis BL, Ayers CR, Khera A, Berry JD. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center Longitudinal Study. Circulation. 2012;125:3092–3098. doi: 10.1161/CIRCULATIONAHA.111.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Daskalopoulou SS, Thanassoulis G, Karp I, Pelletier R, Behlouli H, Pilote L GENESIS-PRAXY Investigators. Sex- and gender-related risk factor burden in patients with premature acute coronary syndrome. Can J Cardiol. 2014;30:109–117. doi: 10.1016/j.cjca.2013.07.674. [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 11.Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, Larson MG, Ellinor PT, Benjamin EJ. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zöller B, Ohlsson H, Sundquist J, Sundquist K. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. J Am Heart Assoc. 2013;2:e003384. doi: 10.1161/JAHA.112.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao D, Myers R, Hunt S, Shahar E, Paton C, Burke G, Province M, Heiss G. Familial history of stroke and stroke risk: the Family Heart Study. Stroke. 1997;28:1908–1912. doi: 10.1161/01.str.28.10.1908. [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O’Donnell CJ, Nam BH, Larson MG, D’Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 15.Olsson C, Granath F, Ståhle E. Family history, comorbidity and risk of thoracic aortic disease: a population-based case-control study. Heart. 2013;99:1030–1033. doi: 10.1136/heartjnl-2013-303654. [DOI] [PubMed] [Google Scholar]
- 16.Wassel CL, Loomba R, Ix JH, Allison MA, Denenberg JO, Criqui MH. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: the San Diego Population Study. J Am Coll Cardiol. 2011;58:1386–1392. doi: 10.1016/j.jacc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009;169:610–615. doi: 10.1001/archinternmed.2008.589. [DOI] [PubMed] [Google Scholar]
- 18.Noboa S, Le Gal G, Lacut K, Mercier B, Leroyer C, Nowak E, Mottier D, Oger E EDITH Collaborative Study Group. Family history as a risk factor for venous thromboembolism. Thromb Res. 2008;122:624–629. doi: 10.1016/j.thromres.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 19.CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelakopoulou A, Shah T, Sofat R, Shah S, Berry DJ, Cooper J, Palmen J, Tzoulaki I, Wong A, Jefferis BJ, Maniatis N, Drenos F, Gigante B, Hardy R, Laxton RC, Leander K, Motterle A, Simpson IA, Smeeth L, Thomson A, Verzilli C, Kuh D, Ireland H, Deanfield J, Caulfield M, Wallace C, Samani N, Munroe PB, Lathrop M, Fowkes FG, Marmot M, Whincup PH, Whittaker JC, de Faire U, Kivimaki M, Kumari M, Hypponen E, Power C, Humphries SE, Talmud PJ, Price J, Morris RW, Ye S, Casas JP, Hingorani AD. Comparative analysis of genome-wide association studies signals for lipids, diabetes, and coronary heart disease: Cardiovascular Biomarker Genetics Collaboration. Eur Heart J. 2012;33:393–407. doi: 10.1093/eurheartj/ehr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes MV, Harrison S, Talmud PJ, Hingorani AD, Humphries SE. Utility of genetic determinants of lipids and cardiovascular events in assessing risk. Nat Rev Cardiol. 2011;8:207–221. doi: 10.1038/nrcardio.2011.6. [DOI] [PubMed] [Google Scholar]
- 22.Hernesniemi JA, Seppälä I, Lyytikäinen LP, Mononen N, Oksala N, Hutri-Kähönen N, Juonala M, Taittonen L, Smith EN, Schork NJ, Chen W, Srinivasan SR, Berenson GS, Murray SS, Laitinen T, Jula A, Kettunen J, Ripatti S, Laaksonen R, Viikari J, Kähönen M, Raitakari OT, Lehtimäki T. Genetic profiling using genome-wide significant coronary artery disease risk variants does not improve the prediction of subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study and the Health 2000 Survey: a meta-analysis of three independent studies. PLoS One. 2012;7:e28931. doi: 10.1371/journal.pone.0028931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thanassoulis G, Peloso GM, Pencina MJ, Hoffmann U, Fox CS, Cupples LA, Levy D, D’Agostino RB, Hwang SJ, O’Donnell CJ. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: the Framingham Heart Study. Circ Cardiovasc Genet. 2012;5:113–121. doi: 10.1161/CIRCGENETICS.111.961342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palomaki GE, Melillo S, Bradley LA. Association between 9p21 genomic markers and heart disease: a meta-analysis. JAMA. 2010;303:648–656. doi: 10.1001/jama.2010.118. [DOI] [PubMed] [Google Scholar]
- 25.Dong L, Wang H, Wang DW, Ding H. Association of chromosome 9p21 genetic variants with risk of coronary heart disease in the East Asian population: a meta-analysis. Ann Hum Genet. 2013;77:183–190. doi: 10.1111/ahg.12010. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi K, Folsom AR, Rosamond WD, Boerwinkle E ARIC Investigators. A genetic variant on chromosome 9p21 and incident. doi: 10.1093/eurheartj/ehp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton-Cheh C, Cook NR, VanDenburgh M, Rimm EB, Ridker PM, Albert CM. A common variant at 9p21 is associated with sudden and arrhythmic cardiac death. Circulation. 2009;120:2062–2068. doi: 10.1161/CIRCULATIONAHA.109.879049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assimes TL, Knowles JW, Basu A, Iribarren C, Southwick A, Tang H, Absher D, Li J, Fair JM, Rubin GD, Sidney S, Fortmann SP, Go AS, Hlatky MA, Myers RM, Risch N, Quertermous T. Susceptibility locus for clinical and subclinical coronary artery disease at chromosome 9p21 in the multi-ethnic ADVANCE study. Hum Mol Genet. 2008;17:2320–2328. doi: 10.1093/hmg/ddn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell CJ, Cupples LA, D’Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, Wolf PA, Demissie S. Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI’s Framingham Heart Study. BMC Med Genet. 2007;8(suppl 1):S4. doi: 10.1186/1471-2350-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandona S, Stewart AF, Chen L, Williams K, So D, O’Brien E, Glover C, Lemay M, Assogba O, Vo L, Wang YQ, Labinaz M, Wells GA, McPherson R, Roberts R. Gene dosage of the common variant 9p21 predicts severity of coronary artery disease. J Am Coll Cardiol. 2010;56:479–486. doi: 10.1016/j.jacc.2009.10.092. [DOI] [PubMed] [Google Scholar]
- 31.Patel RS, Asselbergs FW, Quyyumi AA, Palmer TM, Finan CI, Tragante V, Deanfield J, Hemingway H, Hingorani AD, Holmes MV. Genetic variants at chromosome 9p21 and risk of first versus subsequent coronary heart disease events: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2234–2245. doi: 10.1016/j.jacc.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KH, Huang YT, Meng Q, Wu C, Reiner A, Sobel EM, Tinker L, Lusis AJ, Yang X, Liu S. Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circ Cardiovasc Genet. 2014;7:911–919. doi: 10.1161/CIRCGENETICS.114.000676. [DOI] [PubMed] [Google Scholar]
- 33.Foroud T, Koller DL, Lai D, Sauerbeck L, Anderson C, Ko N, Deka R, Mosley TH, Fornage M, Woo D, Moomaw CJ, Hornung R, Huston J, Meissner I, Bailey-Wilson JE, Langefeld C, Rouleau G, Connolly ES, Worrall BB, Kleindorfer D, Flaherty ML, Martini S, Mackey J, De Los Rios La Rosa F, Brown RD, Jr, Broderick JP FIA Study Investigators. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke. 2012;43:2846–2852. doi: 10.1161/STROKEAHA.112.656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jääskeläinen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsäter A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemelä M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 35.International Stroke Genetics Consortium (ISGC), Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, Fornage M, Ikram MA, Malik R, Bevan S, Thorsteinsdottir U, Nalls MA, Longstreth W, Wiggins KL, Yadav S, Parati EA, Destefano AL, Worrall BB, Kittner SJ, Khan MS, Reiner AP, Helgadottir A, Achterberg S, Fernandez-Cadenas I, Abboud S, Schmidt R, Walters M, Chen WM, Ringelstein EB, O’Donnell M, Ho WK, Pera J, Lemmens R, Norrving B, Higgins P, Benn M, Sale M, Kuhlenbäumer G, Doney AS, Vicente AM, Delavaran H, Algra A, Davies G, Oliveira SA, Palmer CN, Deary I, Schmidt H, Pandolfo M, Montaner J, Carty C, de Bakker PI, Kostulas K, Ferro JM, van Zuydam NR, Valdimarsson E, Nordestgaard BG, Lindgren A, Thijs V, Slowik A, Saleheen D, Paré G, Berger K, Thorleifsson G, Hofman A, Mosley TH, Mitchell BD, Furie K, Clarke R, Levi C, Seshadri S, Gschwendtner A, Boncoraglio GB, Sharma P, Bis JC, Gretarsdottir S, Psaty BM, Rothwell PM, Rosand J, Meschia JF, Stefansson K, Dichgans M, Markus HS Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies [published correction appears in Lancet Neurol. 2015;14:788] Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murabito JM, Guo CY, Fox CS, D’Agostino RB. Heritability of the ankle-brachial index: the Framingham Offspring study. Am J Epidemiol. 2006;164:963–968. doi: 10.1093/aje/kwj295. [DOI] [PubMed] [Google Scholar]
- 38.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17: genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham Heart Study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 39.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham Heart Study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 40.Atwood LD, Heard-Costa NL, Cupples LA, Jaquish CE, Wilson PW, D’Agostino RB. Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am J Hum Genet. 2002;71:1044–1050. doi: 10.1086/343822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox CS, Heard-Costa NL, Wilson PW, Levy D, D’Agostino RB, Sr, Atwood LD. Genome-wide linkage to chromosome 6 for waist circumference in the Framingham Heart Study. Diabetes. 2004;53:1399–1402. doi: 10.2337/diabetes.53.5.1399. [DOI] [PubMed] [Google Scholar]
- 42.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 43.Meigs JB, Panhuysen CI, Myers RH, Wilson PW, Cupples LA. A genome-wide scan for loci linked to plasma levels of glucose and HbA(1c) in a community-based sample of Caucasian pedigrees: the Framingham Offspring Study. Diabetes. 2002;51:833–840. doi: 10.2337/diabetes.51.3.833. [DOI] [PubMed] [Google Scholar]
- 44.Schnabel RB, Lunetta KL, Larson MG, Dupuis J, Lipinska I, Rong J, Chen MH, Zhao Z, Yamamoto JF, Meigs JB, Nicaud V, Perret C, Zeller T, Blankenberg S, Tiret L, Keaney JF, Jr, Vasan RS, Benjamin EJ. The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ Cardiovasc Genet. 2009;2:229–237. doi: 10.1161/CIRCGENETICS.108.804245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kathiresan S, Manning AK, Demissie S, D’Agostino RB, Surti A, Guiducci C, Gianniny L, Burtt NP, Melander O, Orho-Melander M, Arnett DK, Peloso GM, Ordovas JM, Cupples LA. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, Wilson PW, Levy D. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15:2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
8. HIGH BLOOD CHOLESTEROL AND OTHER LIPIDS
See Table 8-1 and Charts 8-1 through 8-4
Table 8-1.
High TC and LDL-C and Low HDL-C
| Population Group | Prevalence of TC ≥200 mg/dL, 2011–2014 Age ≥20 y |
Prevalence of TC ≥240 mg/dL, 2011–2014 Age ≥20 y |
Prevalence of LDL-C ≥130 mg/ dL, 2011–2014 Age ≥20 y |
Prevalence of HDL-C <40 mg/dL, 2011–2014 Age ≥20 y |
|---|---|---|---|---|
| Both sexes, n (%)* | 94 600 000 (39.7) | 28 500 000 (11.9) | 71 300 000 (30.3) | 44 000 000 (18.7) |
| Males, n (%)* | 42 300 000 (37.0) | 12 100 000 (10.6) | 34 000 000 (30.0) | 32 100 000 (27.9) |
| Females, n (%)* | 52 300 000 (42.0) | 16 400 000 (13.0) | 37 300 000 (30.4) | 11 900 000 (10.0) |
| NH white males, % | 37.0 | 10.8 | 29.3 | 28.4 |
| NH white females, % | 43.4 | 13.8 | 32.1 | 10.3 |
| NH black males, % | 32.6 | 7.3 | 29.9 | 20.7 |
| NH black females, % | 36.1 | 9.6 | 27.9 | 8.0 |
| Hispanic males, % | 43.1 | 13.6 | 36.6 | 30.7 |
| Hispanic females, % | 41.2 | 12.5 | 28.7 | 11.8 |
| NH Asian males, % | 39.9 | 10.8 | 29.2 | 25.0 |
| NH Asian females, % | 40.5 | 11.2 | 25.0 | 6.7 |
Prevalence of TC ≥200 mg/dL includes people with TC ≥240 mg/dL. In adults, levels of 200 to 239 mg/dL are considered borderline high. Levels of ≥240 mg/dL are considered high. HDL-C indicates high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NH, non-Hispanic; and TC, total cholesterol.
Total data for TC are for Americans ≥20 years of age. Data for LDL-C, HDL-C, and all racial/ethnic groups are age adjusted for age ≥20 years.
Source for TC ≥200 mg/dL, ≥240 mg/dL, LDL-C, and HDL-C: National Health and Nutrition Examination Survey (2011–2014), National Center for Health Statistics, and National Heart, Lung, and Blood Institute. Estimates from National Health and Nutrition Examination Survey 2011 to 2014 (National Center for Health Statistics) were applied to 2014 population estimates.
Chart 8-1. Trends in mean serum total cholesterol among adolescents 12 to 19 years of age by race, sex, and survey year (NHANES 1988–1994, 1999–2006, and 2007–2014).
Values are in mg/dL.
Mex. Am. indicates Mexican American; NH, non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
*The category of Mexican Americans was consistently collected in all NHANES years, but the combined category of Hispanics was only used starting in 2007. Consequently, for long-term trend data, the category Mexican American is used.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 8-4. Age-adjusted trends in the prevalence of serum total cholesterol ≥240 mg/dL in adults ≥20 years of age by race/ethnicity, sex, and survey year (NHANES 2011–2012 and 2013–2014).
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
For information on dietary cholesterol, total fat, saturated fat, and other factors that affect blood cholesterol levels, see Chapter 5 (Nutrition).
Abbreviations Used in Chapter 8
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| ASCVD | atherosclerotic cardiovascular disease |
| ATP III | Adult Treatment Panel III |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DM | diabetes mellitus |
| HDL-C | high-density lipoprotein cholesterol |
| HIC | high-income countries |
| IHD | ischemic heart disease |
| LDL-C | low-density lipoprotein cholesterol |
| Mex. Am. | Mexican American |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| TC | total cholesterol |
| UI | uncertainty interval |
| WHO | World Health Organization |
Prevalence of High TC
Youth
(See Chart 8-1)
-
Among children 6 to 11 years of age, the mean TC level is 158.9 mg/dL. For boys, it is 158.5 mg/ dL; for girls, it is 159.3 mg/dL. The racial/ethnic breakdown in NHANES 2011 to 2014 is as follows (unpublished NHLBI tabulation):
For non-Hispanic whites, 156.5 mg/dL for boys and 159.6 mg/dL for girls
For non-Hispanic blacks, 162.1 mg/dL for boys and 162.2 mg/dL for girls
For Hispanics, 159.5 mg/dL for boys and 156.9 mg/dL for girls
For non-Hispanic Asians, 161.9 mg/dL for boys and 167.6 mg/dL for girls
From 1988 to 2014, mean serum TC for adolescents 12 to 19 years of age decreased across all subgroups of race and sex (Chart 8-1).
-
Among adolescents 12 to 19 years of age in NHANES 2011 to 2014, the mean TC level was 156.7 mg/dL. For boys, it was 152.3 mg/dL; for girls, it was 161.3 mg/dL. The racial/ethnic breakdown was as follows (unpublished NHLBI tabulation):
For non-Hispanic whites, 151.7 mg/dL for boys and 162.0 mg/dL for girls
For non-Hispanic blacks, 152.3 mg/dL for boys and 159.5 mg/dL for girls
For Hispanics, 154.7 mg/dL for boys and 160.5 mg/dL for girls
For non-Hispanic Asians, 158.1 mg/dL for boys and 166.7 mg/dL for girls
The prevalence of abnormal lipid levels among youths 12 to 19 years of age is 20.3%; 14.2% of normal-weight youths, 22.3% of overweight youths, and 42.9% of obese youths have ≥1 abnormal lipid level (NHANES 1999–2006, NCHS).1
Approximately 8.2% of adolescents 12 to 19 years of age in NHANES 2011 to 2014 have TC levels ≥200 mg/dL (unpublished NHLBI tabulation).
Twenty percent of male adolescents and 27% of female adolescents have TC levels of 170 to 199 mg/dL.2
Among youths aged 6 to 19 years, there was a decrease in mean TC from 165 to 160 mg/dL and a decrease in the prevalence of elevated TC from 11.3% to 8.1% from 1988 through 1994 to 2007 through 2010.3
Mean non-HDL–C (111.7 mg/dL) and prevalence of elevated non–HDL-C both decreased significantly from the periods 1988 through 1994 to 2007 through 2010. In 2007 to 2010, 22% of youths had either a low HDL-C level or a high non–HDL-C level, which was lower than the 27.2% in 1988 to 1994.3
Among adolescents (aged 12–19 years) between 1988 to 1994 and 2007 to 2010, there was a decrease in mean LDL-C from 95 to 90 mg/dL and a decrease in geometric mean triglycerides from 82 to 73 mg/dL. The prevalence of elevated LDL-C and triglycerides also decreased significantly between 1988 to 1994 and 2007 to 2010.3
Fewer than 1% of adolescents are potentially eligible for pharmacological treatment on the basis of guidelines from the American Academy of Pediatrics.1,4
Adults (Aged ≥20 Years)
(See Table 8-1 and Charts 8-2 through 8-4)
Chart 8-2. Age-adjusted trends in mean serum total cholesterol among adults ≥20 years old by race and survey year (NHANES 1988–1994, 1999–2006, and 2007–2014).
Values are in mg/dL.
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
*The category of Mexican Americans was consistently collected in all NHANES years, but the combined category of Hispanics was only used starting in 2007. Consequently, for long-term trend data, the category Mexican American is used.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
An estimated 28.5 million adults ≥20 years of age have serum TC levels ≥240 mg/dL (extrapolated for 2014 by use of NCHS/NHANES 2011–2014 data), with a prevalence of 11.9%. From 1988 to 2014, mean serum TC for adults ≥20 years of age decreased across all subgroups of race (Chart 8-2).
-
During the period from 2011 to 2014
The percentage of adults with high TC was lower for non-Hispanic black (8.6%) than for non-Hispanic white (12.5%) and Hispanic (13.1%) adults, and the same patterns were seen in males and females.5
Overall and for males, the prevalence of high TC was lower in non-Hispanic black than in non-Hispanic Asian subgroups, but this difference was not seen in females (Chart 8-3).5
Non-Hispanic black males ≥20 years of age had the lowest age-adjusted prevalence of serum TC ≥240 mg/dL (Chart 8-4).
Women had higher prevalence of high TC (13.0%) than males (10.6%).5
The prevalence of high TC has decreased over time, from 18.3% of adults in 1999 to 2000 to 11.0% of adults in 2013 to 2014.5
New research suggests that long-term exposure to even modestly elevated cholesterol levels can lead to CHD later in life.6
In NHANES 2011 to 2014, ≈5.8% of adults ≥20 years of age had undiagnosed hypercholesterolemia, defined as a TC level ≥240 mg/dL and the participant having responded “no” to ever having been told by a doctor or other healthcare professional that the participant’s blood cholesterol level was high (unpublished NHLBI tabulation). Adults with lower education levels (<12 years of education) are more likely to be undiagnosed (6.6%) than adults with higher education (>12 years of education; 5.8%).
The age-adjusted mean TC level for adults ≥20 years of age declined linearly from 206 mg/dL (95% CI, 205–207 mg/dL) in 1988 to 1994 to 203 mg/dL (95% CI, 201–205 mg/dL) in 1999 to 2002 and to 196 mg/dL (95% CI, 195–198 mg/dL) in 2007 to 2010 (P<0.001 for linear trend).7
Data from NHANES 2007 to 2010 (NCHS/CDC) showed the serum total crude mean cholesterol level in adults to be 194 mg/dL for males and 198 mg/dL for females.7 Statistically significant declining trends in age-adjusted mean TC levels from 1988 through 2014 were observed in all race/ ethnicity subgroups (Chart 8-2).
The Healthy People 2010 guideline8 of an age-adjusted mean TC level of ≤200 mg/dL has been achieved in adults, in males, in females, and in all race/ethnicity and sex subgroups. The Healthy People 2020 target is a mean total blood cholesterol of 177.9 mg/dL for adults, which has not yet been achieved for any group9 (an improvement of 10%).10
Overall, the decline in cholesterol levels in recent years appears to reflect greater uptake of cholesterol-lowering medications rather than changes in dietary patterns.11
The declining TC level appears to reflect a worldwide trend; a report on trends in TC in 199 countries and territories indicated that TC declined in high-income regions of the world (Australasia, North America, and Western Europe).12 During the period from 1999 to 2006, 26.0% of adults had hypercholesterolemia, 9% of adults had both hypercholesterolemia and hypertension, 1.5% of adults had DM and hypercholesterolemia, and 3% of adults had all 3 conditions.13
Chart 8-3. Age-adjusted trends in the prevalence of serum total cholesterol ≥200 mg/dL in adults ≥20 years of age by race/ethnicity, sex, and survey year (NHANES 2011–2012 and 2013–2014).
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
Screening
The percentage of adults who reported having had a cholesterol check increased from 68.6% during 1999 to 2000 to 74.8% during 2005 to 200614 and declined to 69.4% in 2011 to 2012.15
-
Nearly 70% of adults (67% of males and nearly 72% of females) had been screened for cholesterol (defined as being told by a doctor their cholesterol was high and indicating they had their blood cholesterol checked <5 years ago) according to data from NHANES 2011 to 2012, which was unchanged since 2009 to 2010.15
Among non-Hispanic whites, 71.8% were screened (70.6% of males and 72.9% of females).
Among non-Hispanic blacks, 71.9% were screened (66.8% of males and 75.9% of females).
Among non-Hispanic Asians, 70.8% were screened (70.6% of males and 70.9% of females).
Among Hispanic adults, 59.3% were screened (54.6% of males and 64.2% of females).
The percentage of adults screened for cholesterol in the past 5 years was lower for Hispanic adults than for non-Hispanic white, non-Hispanic black, and non-Hispanic Asian adults.
The Healthy People 2020 target is 82% of adults having their blood cholesterol checked within the past 5 years.9
Awareness
Data from the 2013 BRFSS study of the CDC showed that among adults screened for high cholesterol, the percentage who had reported they had high cholesterol ranged from 33.4% in Utah to 44.4% in Alabama. The median percentage among states was 38.4%.16
Among adults with hypercholesterolemia, 42% were told they had high TC in 1999 to 2000 compared with 50.4% during 2005 to 2006.14
Awareness of high LDL-C increased from 48.9% in 1999 to 2000 to 62.8% in 2003 to 2004; however, awareness did not increase further through 2009 to 2010 (61.5%). Treatment among those aware of having high LDL-C increased from 41.3% in 1999 to 2000 to 72.6% in 2007 to 2008. In 2009 to 2010, it was 70.0%.17
Treatment
In 2013, the ACC/AHA released a revised recommendation for statin treatment.18 Unlike ATP III and other previous recommendations, which had LDL-C and non–HDL-C goals based on patient’s risk category, the 2013 ACC/AHA guideline recommended lipid measurement at baseline, at 1 to 3 months after statin initiation, and then annually to check for the expected percentage decrease of LDL-C levels (30% to <50% with a moderate-intensity statin and ≥50% with a high-intensity statin). They also recommended a discussion regarding statin therapy in 4 identified groups in whom it has been clearly shown to reduce ASCVD risk. The 4 statin benefit groups are (1) people with clinical ASCVD, (2) those with primary elevations of LDL-C >190 mg/dL, (3) people aged 40 to 75 years who have DM with LDL-C 70 to 189 mg/dL and without clinical ASCVD, and (4) those without clinical ASCVD or DM with LDL-C 70 to 189 mg/dL and estimated 10-year ASCVD risk >7.5%. Approximately 31.9% of the ASCVD-free, nonpregnant US population between 40 and 79 years of age has a 10-year risk of a first hard CHD event of ≥10% or has DM.19
According to a recent analysis of NHANES data from 2005 to 2010, the number of people eligible for statin therapy would rise from 43.2 million US adults (37.5%) to 56.0 million (48.6%) based on the new ACC/AHA guidelines for the management of blood cholesterol.18 Most of the increase comes from adults 60 to 75 years old without CVD who have a 10-year ASCVD risk ≥7.5%; the net number of new statin prescriptions could potentially increase by 12.8 million, including 10.4 million for primary prevention.20 Individuals eligible for treatment under ATP III but not ACC/AHA guidelines had higher LDL-C levels but were otherwise at lower risk than individuals eligible under both guidelines or only under ACC/AHA guidelines.21
Data from NHANES 1999 to 2012 show that the use of cholesterol-lowering treatment has increased substantially among adults, from 8% in 1999 to 2000 to 18% in 2011 to 2012.22 During this period, the use of statins increased from 7% to 17%.22
Adherence
Youth
The American Academy of Pediatrics recommends screening for dyslipidemia in children and adolescents who have a family history of dyslipidemia or premature CVD, those whose family history is unknown, and those youths with risk factors for CVD, such as being overweight or obese, having hypertension or DM, or being a smoker.1 In 2011, the NHBLI Expert Panel recommended universal dyslipidemia screening for all children between 9 and 11 years of age and again between 17 and 21 years of age.23
Analysis of data from NHANES 1999 to 2006 showed that the overall prevalence of abnormal lipid levels among youths 12 to 19 years of age was 20.3%.1
Adults
New criteria from the “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults”18 could result in >45 million middle-aged Americans who do not have CVD being recommended for consideration of statin therapy: 33.0 million are at ≥7.5% 10-year risk, and 12.8 million are at >5.0% to 7.4% 10-year risk. This is ≈1 in every 3 American adults, many of whom are already undergoing statin treatment under the previous US guidelines.24
From 2005 to 2010, among adults with high LDL-C, age-adjusted control of LDL-C increased from 22.3% to 29.5%.25 The prevalence of LDL-C control was lowest among people who reported receiving medical care less than twice in the previous year (11.7%), being uninsured (13.5%), being Mexican American (20.3%), or having income below the poverty level (21.9%).26
Global Burden of Hypercholesterolemia
Between 1980 and 2008, the mean age-adjusted TC declined from 4.72 to 4.64 mmol/L (95% UI, 4.51–4.76) for males and from 4.83 to 4.76 mmol/L (95% UI, 4.62–4.91) for females. Globally, mean TC changed little between 1980 and 2008, falling by <0.1 mmol/L per decade in males and females.27
TC went from being the 14th leading risk factor in 1990 for the global burden of disease, as quantified by DALYs, to the number 15 risk factor in 2010.28
Raised cholesterol, defined as ≥190 mg/dL or ≥5.0 mmol/L, is estimated to cause 2.6 million deaths (4.5% of total deaths) and 29.7 million DALYs (2.0% of total DALYs).29
The prevalence of elevated TC was highest in the WHO European Region (54% for both sexes), followed by the WHO Region of the Americas (48% for both sexes). The WHO African Region and the WHO South-East Asia Region showed the lowest percentages (23% and 30%, respectively).29
In low-income countries, approximately one fourth of adults had raised TC. In lower-middle-income countries, this rose to approximately one third of the population for both sexes. In HIC, >50% of adults had raised TC, more than double the level of the low-income countries.29
Twenty-nine percent of IHD DALYs can be attributed to high TC, the second-leading physiological risk factor.28
Lipid Levels
LDL (Bad) Cholesterol
Youth
There are limited data available on LDL-C for children 6 to 11 years of age.
-
Among adolescents 12 to 19 years of age in NHANES 2011 to 2014, the mean LDL-C level was 87.7 mg/dL (boys, 85.7 mg/dL; girls, 89.8 mg/ dL). The racial/ethnic breakdown was as follows (unpublished NHLBI tabulation):
For non-Hispanic whites, 86.5 mg/dL for boys and 89.9 mg/dL for girls
For non-Hispanic blacks, 86.6 mg/dL for boys and 90.9 mg/dL for girls
For Hispanic Americans, 85.9 mg/dL for boys and 87.8 mg/dL for girls
For non-Hispanic Asians, 84.5 mg/dL for boys and 96.9 mg/dL for girls
High levels of LDL-C occurred in 5.5% of male adolescents and 7.5% of female adolescents during 2011 to 2014 (unpublished NHLBI tabulation).
Adults
The mean level of LDL-C for American adults ≥20 years of age was 113.4 mg/dL in 2011 to 2014 (unpublished NHLBI tabulation).
-
According to NHANES 2011 to 2014 (unpublished NHLBI tabulation)
Among non-Hispanic whites, mean LDL-C levels were 112.1 mg/dL for males and 114.9 mg/dL for females.
Among non-Hispanic blacks, mean LDL-C levels were 110.4 mg/dL for males and 111.4 mg/dL for females.
Among Hispanics, mean LDL-C levels were 119.2 mg/dL for males and 112.6 mg/dL for females.
Among non-Hispanic Asians, mean LDL-C levels were 112.4 mg/dL for males and 110.3 mg/dL for females.
The prevalence of high LDL-C decreased from 59% in 1976 to 1980 to 42% in 1988 to 1994 and to 33% in 2001 to 2004, reaching 27% in 2007 to 2010. Between 1976 to 1980 and 2007 to 2010, the prevalence of high LDL-C decreased significantly for males (from 65% to 31%), females (54% to 24%), and adults aged 40 to 64 years (56% to 27%) and 65 to 74 years (72% to 30%).30
Mean levels of LDL-C decreased from 126.2 mg/ dL during 1999 to 2000 to 111.3 mg/dL during 2013 to 2014. The age-adjusted prevalence of high LDL-C decreased from 42.9% during 1999 to 2000 to 28.5% during 2013 to 2014. (unpublished NHLBI tabulation)
HDL (Good) Cholesterol
Youth
-
Among children 6 to 11 years of age in NHANES 2011 to 2014, the mean HDL-C level was 54.3 mg/dL. For boys, it was 55.6 mg/dL, and for girls, it was 52.9 mg/dL. The racial/ethnic breakdown was as follows (unpublished NHLBI tabulation):
For non-Hispanic whites, 55.1 mg/dL for boys and 52.8 mg/dL for girls
For non-Hispanic blacks, 60.0 mg/dL for boys and 56.3 mg/dL for girls
For Hispanics, 54.3mg/dL for boys and 51.3 mg/dL for girls
For non-Hispanic Asians, 55.8 mg/dL for boys and 54.5 mg/dL for girls
-
Among adolescents 12 to 19 years of age, the mean HDL-C level was 51.0 mg/dL. For boys, it was 49.1 mg/dL, and for girls, it was 52.9 mg/dL. The racial/ethnic breakdown was as follows (NHANES 2011–2014, unpublished NHLBI tabulation):
For non-Hispanic whites, 48.3 mg/dL for boys and 52.0 mg/dL for girls
For non-Hispanic blacks, 52.4 mg/dL for boys and 55.7 mg/dL for girls
For Hispanics, 48.8 mg/dL for boys and 52.8 mg/dL for girls
For non-Hispanic Asians, 52.0 mg/dL for boys and 57.1 mg/dL for girls
Low levels of HDL-C occurred in 19.2% of male adolescents and 12.5% of female adolescents in NHANES 2011 to 2014 (unpublished NHLBI tabulation).
Adults
-
According to NHANES 2011 to 2014 (unpublished NHLBI tabulation)
The mean level of HDL-C for American adults ≥20 years of age is 52.9 mg/dL.
Among non-Hispanic whites, mean HDL-C levels were 47.6 mg/dL for males and 58.6 mg/ dL for females.
Among non-Hispanic blacks, mean HDL-C levels were 51.3 mg/dL for males and 58.1 mg/ dL for females.
Among Hispanics, mean HDL-C levels were 45.7 mg/dL for males and 53.9 mg/dL for females.
Among non-Hispanic Asians, mean HDL-C levels were 47.9 mg/dL for males and 58.8 mg/ dL for females.
-
The prevalence of low HDL-C (<40 mg/dL) was higher (23%) in those with lower education (<12 years) than in those with higher education (>12 years; 17%). Approximately 17% of adults (just over one quarter of males and <10% of females) had low HDL-C during 2011 to 2012. The percentage of adults with low HDL-C has decreased 20% since 2009 to 2010.15
Among non-Hispanic whites, 17.1% (25.4% of males and 9.3% of females) had low HDL-C.
Among non-Hispanic blacks, 12.7% (19.1% of males and 7.8% of females) had low HDL-C. The percentage of adults with low HDL-C was lower among non-Hispanic blacks than non-Hispanic whites. These racial and ethnic differences were also observed in males but not in females.
Among non-Hispanic Asians, 14.3% (24.5% of males and 5.1% of females) had low HDL-C. The prevalence of low HDL-C was 5 times greater among non-Hispanic Asian males than females. Non-Hispanic Asian adults had consistently lower percentages of low HDL-C than Hispanic adults.
The prevalence of low HDL-C was 5 times higher in non-Hispanic Asian males (24.5%) than in non-Hispanic Asian females (5.1%).31
Among Hispanic adults, 21.8% (32.6% of males and 11.3% of females) had low HDL-C. The percentage of adults with low HDL-C was higher in Hispanic adults than in non-Hispanic black or non-Hispanic white adults. These racial and ethnic differences were also observed in males but not in females.
Triglycerides
Youth
There are limited data available on triglycerides for children 6 to 11 years of age.
-
Among adolescents 12 to 19 years of age in NHANES 2011 to 2014, the geometric mean triglyceride level was 79.4 mg/dL. For boys, it was 81.9 mg/dL, and for girls, it was 76.8 mg/dL. The racial/ethnic breakdown was as follows (unpublished NHLBI tabulation):
Among non-Hispanic whites, 82.3 mg/dL for boys and 77.3 mg/dL for girls
Among non-Hispanic blacks, 62.8 mg/dL for boys and 62.7 mg/dL for girls
Among Hispanics, 89.0 mg/dL for boys and 85.2 mg/dL for girls
Among non-Hispanic Asians, 78.3 mg/dL for boys and 88.0 mg/dL for girls
High levels of triglycerides occurred in 8.7% of male adolescents and 6.3% of female adolescents during 2011 to 2014.
Adults
The geometric mean level of triglycerides for American adults ≥20 years of age was 103.5 mg/ dL in NHANES 2011 to 2014 (unpublished NHLBI tabulation).
Approximately 24.2% of adults had high triglyceride levels (≥150 mg/dL) in NHANES 2011 to 2014 (unpublished NHLBI tabulation).
-
Among males, the age-adjusted geometric mean triglyceride level was 111.6 mg/dL in NHANES 2011 to 2014 (unpublished NHLBI tabulation), with the following racial/ethnic breakdown:
113.2 mg/dL for non-Hispanic white males
86.7 mg/dL for non-Hispanic black males
124.1 mg/dL for Hispanic males
115.3 mg/dL for non-Hispanic Asian males
-
Among females, the age-adjusted geometric mean triglyceride level was 96.4 mg/dL in NHANES 2011 to 2014 (unpublished NHLBI tabulation), with the following racial/ethnic breakdown:
99.8 mg/dL for non-Hispanic white females
75.1 mg/dL for non-Hispanic black females
105.3 mg/dL for Hispanic females
91.5 mg/dL for non-Hispanic Asian females
The prevalence of high triglycerides (≥150 mg/ dL) was higher (27%) in those with lower education (<12 years) than in those with higher education (>12 years; 23%) (unpublished NHLBI tabulation).
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of abnormal lipid levels among youths: United States, 1999–2006 [published correction appears in MMWR Morb Mortal Wkly Rep. 2010;59:78] MMWR Morb Mortal Wkly Rep. 2010;59:29–33. [PubMed] [Google Scholar]
- 2.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–1376. doi: 10.1161/CIRCULATIONAHA.113.001559. [DOI] [PubMed] [Google Scholar]
- 3.Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C. Trends in serum lipids among US youths aged 6 to 19 years, 1988–2010. JAMA. 2012;308:591–600. doi: 10.1001/jama.2012.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Li C, Zhao G, Mokdad AH. Concentrations of low-density lipoprotein cholesterol and total cholesterol among children and adolescents in the United States. Circulation. 2009;119:1108–1115. doi: 10.1161/CIRCULATIONAHA.108.816769. [DOI] [PubMed] [Google Scholar]
- 5.Carroll MD, Fryar CD, Kit BK. Total and high-density lipoprotein cholesterol in adults: United States, 2011–2014. NCHS Data Brief. 2015;(226):1–8. [PubMed] [Google Scholar]
- 6.Navar-Boggan AM, Peterson ED, D’Agostino RB, Sr, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131:451–458. doi: 10.1161/CIRCULATIONAHA.114.012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 9.US Department of Health and Human Services. Healthy People 2020. [Accessed July 13, 2016];2020 Topics and Objectives: Heart Disease and Stroke. https://www.healthypeople.gov/2020/topics-objectives/topic/heart-disease-and-stroke/objectives.
- 10.US Department of Health and Human Services. [Accessed July 13, 2016];Healthy People 2020: HDS-8: Reduce the mean total blood cholesterol levels among adults. https://www.healthypeople.gov/2020/topics-objectives/objective/hds-8.
- 11.Ford ES, Capewell S. Trends in total and low-density lipoprotein cholesterol among U.S. adults: contributions of changes in dietary fat intake and use of cholesterol-lowering medications. PLoS One. 2013;8:e65228. doi: 10.1371/journal.pone.0065228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, high serum total cholesterol, and diabetes: racial and ethnic prevalence differences in U.S. adults, 1999–2006. NCHS Data Brief. 2010;(36):1–8. [PubMed] [Google Scholar]
- 14.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140:226–235. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Carroll MD, Kit BK, Lacher DA, Yoon SSS. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2011–2012. NCHS data brief. 2013;(132):1–8. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services; 2013. [Accessed December 15, 2016]. http://www.cdc.gov/brfss/brfssprevalence/index.html. [Google Scholar]
- 17.Muntner P, Levitan EB, Brown TM, Sharma P, Zhao H, Bittner V, Glasser S, Kilgore M, Yun H, Woolley JM, Farkouh ME, Rosenson RS. Trends in the prevalence, awareness, treatment and control of high low density lipoprotein-cholesterol among United States adults from 1999–2000 through 2009–2010. Am J Cardiol. 2013;112:664–670. doi: 10.1016/j.amjcard.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2015;132:e396 and Circulation. 2014;129(suppl 2):S46–S48) Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/ American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75] Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 21.Bittner V. New ACC-AHA cholesterol guidelines significantly increase potential eligibility for statin treatment. Evid Based Med. 2014;19:198. doi: 10.1136/ebmed-2014-110029. [DOI] [PubMed] [Google Scholar]
- 22.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–United States, 2005–2013. MMWR Suppl. 2014;63:3–27. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol: United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:109–114. [PubMed] [Google Scholar]
- 27.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, Singh GM, Lin JK, Stevens GA, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Cholesterol) National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 28.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 [published corrections appear in Lancet. 2013;381:628 and Lancet. 2013;381:1276] Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alwan A. Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 30.Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302:2104–2110. doi: 10.1001/jama.2009.1672. [DOI] [PubMed] [Google Scholar]
- 31.Aoki Y, Yoon SS, Chong Y, Carroll MD. Hypertension, abnormal cholesterol, and high body mass index among non-Hispanic Asian adults: United States, 2011–2012. NCHS Data Brief. 2014;(140):1–8. [PubMed] [Google Scholar]
9. HIGH BLOOD PRESSURE
ICD-9 401 to 404, ICD-10 I10 to I15. See Tables 9-1 and 9-2 and Charts 9-1 through 9-5
Table 9-1.
High Blood Pressure
| Population Group | Prevalence, 2011–2014, Age ≥20 y | Mortality,* 2014, All Ages | Hospital Discharges, 2010, All Ages | Estimated Cost, 2012–2013 |
|---|---|---|---|---|
| Both sexes | 85 700 000 (34.0%) | 73 345 | 488 000 | $51.2 Billion |
| Males | 40 800 000 (34.5%) | 34 688 (47.3%)† | 216 000 | … |
| Females | 44 900 000 (33.4%) | 38 657 (52.7%)† | 272 000 | … |
| NH white males | 34.5% | 23 232 | … | … |
| NH white females | 32.3% | 27 618 | … | … |
| NH black males | 45.0% | 7448 | … | … |
| NH black females | 46.3% | 7276 | … | … |
| Hispanic males | 28.9% | 2627 | … | … |
| Hispanic females | 30.7% | 2406 | … | … |
| NH Asian males | 28.8% | 916‡ | … | … |
| NH Asian females | 25.7% | 1025‡ | ||
| NH American Indian/Alaska Native | 26.4§ | 440 | … | … |
Hypertension is defined in terms of National Health and Nutrition Examination Survey blood pressure measurements and health interviews. A subject was considered hypertensive if systolic blood pressure was ≥140 mm Hg or diastolic blood pressure was ≥90 mm Hg, if the subject said “yes” to taking antihypertensive medication, or if the subject was told on 2 occasions that he or she had hypertension. Prevalence in American Indian or Alaska Natives is based on self-report data from the National Health Interview Survey, with hypertension defined as subjects having been told on ≥2 different visits that they had hypertension or high blood pressure. Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for Hispanic, American Indian or Alaska Native, and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total high blood pressure mortality that is for males vs females.
Includes Chinese, Filipino, Hawaiian, Japanese, and other Asian or Pacific Islander.
National Health Interview Survey (2014), National Center for Health Statistics; data are weighted percentages for Americans ≥18 years of age. Individuals had to have been told on ≥2 different visits that they had hypertension or high blood pressure to be classified as hypertensive.31
Sources: Prevalence: National Health and Nutrition Examination Survey (2011–2014), National Center for Health Statistics, and National Heart, Lung, and Blood Institute. Percentages for racial/ethnic groups are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolated to the 2014 US population estimates. Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death–United States. These data represent underlying cause of death only. Mortality for NH Asians includes Pacific Islanders. Hospital discharges: National Hospital Discharge Survey, National Center for Health Statistics; data include those discharged alive, dead, or status unknown. Cost: Medical Expenditure Panel Survey data include estimated direct costs for 2012 to 2013 (annual average); indirect costs calculated by National Heart, Lung, and Blood Institute for 2012 to 2013 (annual average).
Table 9-2.
Hypertension Awareness, Treatment, and Control: NHANES 1999 to 2006 and 2007 to 2014, by Race/Ethnicity and Sex
| Awareness | Treatment | Control | ||||
|---|---|---|---|---|---|---|
| 1999–2006 | 2007–2014 | 1999–2006 | 2007–2014 | 1999–2006 | 2007–2014 | |
| NH white males | 71.8 | 81.3 | 61.8 | 73.0 | 41.9 | 53.9 |
| NH white females | 76.9 | 85.5 | 68.1 | 80.6 | 40.0 | 58.0 |
| NH black male | 70.1 | 79.8 | 59.6 | 68.4 | 34.1 | 41.6 |
| NH black females | 85.3 | 88.9 | 76.6 | 81.2 | 43.8 | 53.4 |
| Mexican American males | 57.7 | 68.5 | 41.8 | 57.7 | 25.6 | 37.0 |
| Mexican American females | 69.9 | 80.8 | 57.9 | 73.1 | 31.9 | 49.2 |
Values are percentages. Hypertension is defined in terms of NHANES blood pressure measurements and health interviews. A subject was considered hypertensive if systolic blood pressure was ≥140 mm Hg or diastolic blood pressure was ≥90 mm Hg, or if the subject said “yes” to taking antihypertensive medication. NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
The category of Mexican Americans was consistently collected in all NHANES years, but the combined category of Hispanics was only used starting in 2007. Consequently, for long-term trend data, the category Mexican American is used.
Sources: NHANES (1999–2006, 2007–2014) and National Heart, Lung, and Blood Institute.
Chart 9-1. Prevalence of high blood pressure in adults ≥20 years of age by sex and age (NHANES 2011–2014).
Hypertension is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, if the subject said “yes” to taking antihypertensive medication, or if the subject was told on 2 occasions that he or she had hypertension.
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 9-5. Extent of awareness, treatment, and control of high blood pressure by race/ethnicity and sex (NHANES 2011–2014).
Hypertension is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or if the subject said “yes” to taking antihypertensive medication.
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
HBP is a major risk factor for CVD and stroke.1 The AHA has identified untreated BP <90th percentile (for children) and <120/<80 mm Hg (for adults aged ≥20 years) as 1 of the 7 components of ideal cardiovascular health.2 In 2013 to 2014, 88.7% of children and 45.4% of adults met these criteria (Chapter 2, Cardiovascular Health).
Abbreviations Used in Chapter 9
| ACEI | angiotensin-converting enzyme inhibitor |
| AHA | American Heart Association |
| ARB | angiotensin receptor blocker |
| ASCVD | atherosclerotic cardiovascular disease |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CDC | Centers for Disease Control and Prevention |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| ED | emergency department |
| ESRD | end-stage renal disease |
| FHS | Framingham Heart Study |
| HBP | high blood pressure |
| HCHS/SOL | Hispanic Community Health Study/Study of Latinos |
| HD | heart disease |
| HF | heart failure |
| HIC | high-income countries |
| HR | hazard ratio |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-9-CM | International Classification of Diseases, Clinical Modification, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| JNC | Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure |
| MEPS | Medical Expenditure Panel Survey |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| NAMCS | National Ambulatory Medical Care Survey |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHES | National Health Examination Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| OR | odds ratio |
| PA | physical activity |
| RCT | randomized controlled trial |
| REACH US | Racial and Ethnic Approaches to Community Health Across the US |
| SBP | systolic blood pressure |
| SPRINT | Systolic Blood Pressure Intervention Trial |
Prevalence
(See Table 9-1, Chart 9-1 and Chart 9-2)
Chart 9-2. Age-adjusted prevalence trends for high blood pressure in adults ≥20 years of age by race/ethnicity, sex, and survey year (NHANES 1988–1994, 1999–2006, and 2007–2014).
Hypertension is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, if the subject said “yes” to taking antihypertensive medication, or if the subject was told on 2 occasions that he or she had hypertension.
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
*The category of Mexican Americans was consistently collected in all NHANES years, but the combined category of Hispanics was only used starting in 2007. Consequently, for long-term trend data, the category Mexican American is used.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Surveillance definitions vary widely in the published literature.3
-
For surveillance purposes, the following definition of HBP has been proposed3:
SBP ≥140 mm Hg or DBP ≥90 mm Hg or selfreported antihypertensive medicine use, or
Having been told previously, at least twice, by a physician or other health professional that one has HBP.
With this definition, the age-adjusted prevalence of hypertension among US adults ≥20 years of age was estimated to be 34.0% in NHANES in 2011 to 2014 (34.5% for males and 33.4% for females). This equates to an estimated 85.7 million adults ≥20 years of age who have HBP (40.8 million males and 44.9 million females), extrapolated to 2014 data (Table 9-1; unpublished NHLBI tabulation).
The prevalence of HBP in adults ≥20 years of age is presented by both age and sex in Chart 9-1.
In 2011 to 2014, the prevalence of HBP was 11.6% among those 20 to 39 years of age, 37.3% among those 40 to 59 years of age, and 67.2% among those ≥60 years of age (unpublished NHLBI tabulation).
In 2011 to 2014, a higher percentage of males than females had hypertension up to 64 years of age. For those ≥65 years of age, the percentage of females with hypertension was higher than for males (unpublished NHLBI tabulation).
Data from NHANES 2013 to 2014 indicate that 15.9% of US adults with hypertension are not aware they have it (unpublished NHLBI tabulation).
The prevalence of hypertension increased between 1988 to 1994, 1999 to 2006, and 2007 to 2014 among non-Hispanic black males (37.5%, 39.5%, and 40.3%, respectively) and females (38.2%, 41.7%, and 42.9%, respectively), non-Hispanic white males (25.6%, 28.7%, and 30.4%, respectively) and females (22.9%, 27.8%, and 27.6%, respectively), and Mexican American females (25.0%, 26.1%, and 27.2%, respectively) but not Mexican American males (26.9%, 24.3%, and 26.5%, respectively). The category of Mexican Americans was consistently collected in all NHANES years, but the combined category of Hispanics was only used starting in 2007. Consequently, for long-term trend data, the category Mexican American is used (Chart 9-2).
Data from the 2013 BRFSS/CDC indicate that the percentage of adults ≥18 years of age who had been told that they had HBP ranged from 24.2% in Utah to 41.0% in West Virginia. The mean percentage for the United States was 32.5%.4
According to 2005 to 2008 NHANES data, among US adults with hypertension, 11.8% met the criteria for resistant hypertension (SBP/DBP ≥140/90 mm Hg and reported use of antihypertensive medications from 3 different drug classes or drugs from ≥4 antihypertensive drug classes regardless of BP). This represents an increase from 5.5% in 1998 to 1994 and 8.5% in 1999 to 2004.5
Among US adults not taking antihypertensive medication, the prevalence of isolated systolic hypertension (SBP ≥ 140 mm Hg with DBP < 90 mm Hg) decreased from 10.8% in 1999 to 2004 to 8.5% in 2005 to 2010.6
A meta-analysis of 24 studies (n=961 035) estimated the prevalence of apparent treatment-resistant hypertension to be 13.7% (95% CI, 11.2%–16.2%).7
SPRINT demonstrated an SBP goal of <120 mm Hg resulted in fewer CVD events and a reduction in mortality than an SBP goal of <140 mm Hg among people with SBP ≥130 mm Hg and increased cardiovascular risk.8 Using NHANES 2007 to 2012 data, it was estimated that 7.6% (95% CI, 7.0%–8.3%) or 16.8 million (95% CI, 15.7–17.8 million) US adults meet the SPRINT inclusion/exclusion criteria.9
Projections show that by 2030, ≈41.4% of US adults will have hypertension, an increase of 8.4% from 2012 estimates (unpublished AHA computation, based on methodology described by Heidenreich et al10).
Older Adults
In the FHS, the incidence of hypertension at 4 years of follow-up was higher among adults 65 to 94 years of age than among those 35 to 64 years of age. The percentage of those 65 to 94 years old versus 35 to 64 years old developing hypertension was 16.0% versus 5.3% with baseline SBP/DBP <120/80 mm Hg, 25.5% versus 17.6% with SBP/DBP of 120 to 129/80 to 84 mm Hg, and 49.5% and 37.3% with SBP/DBP of 130 to 130/85 to 89 mm Hg.11
In 2011 to 2014, the prevalence of hypertension was 67.2% among US adults ≥60 years of age. Of those with hypertension, 54.0% had controlled BP (SBP/DBP <140/90 mm Hg; unpublished NHLBI tabulation).
Data from NHANES 2005 to 2010 found that 76.5% of US adults ≥80 years of age had hypertension, representing an increase from 69.2% in 1988 to 1994.12
In 2005 to 2010, 30.9% of US adults ≥80 years of age were taking ≥3 classes of antihypertensive medication. This represents an increase from 7.0% and 19.2% in 1988 to 1994 and 1999 to 2004, respectively.12
Children and Adolescents
In 2011 to 2012, 11.0% (95% CI, 8.8%–13.4%) of children and adolescents aged 8 to 17 years had either HBP or borderline HBP. No change occurred in the prevalence of borderline HBP (7.6% [95% CI, 5.8%–9.8%] versus 9.4% [95% CI, 7.2%–11.9%]; P=0.90) or either HBP or borderline HBP (10.6% [8.4%–13.1%] versus 11.0% [95% CI, 8.8%–13.4%]; P=0.26) between 1999 to 2000 and 2011 to 2012.13 In this age group, HBP declined from 3.0% to 1.6% between 1999 to 2000 and 2011 to 2012.13
In 2011 to 2012, HBP was more common among boys (1.8%) than girls (1.4%) and among Hispanics (2.4%) than among non-Hispanic blacks (1.9%), non-Hispanic whites (1.1%), and non-Hispanic Asians (1.7%). Although having either HBP or borderline HBP was more common among boys than girls, non-Hispanic blacks were more likely to have either HBP or borderline HBP than Hispanic, non-Hispanic white, or non-Hispanic Asian boys or girls.13
Data from participants aged 12 to 19 years in the 2005 to 2010 NHANES found ideal BP (<90th percentile) to be present in 78% of males and 90% of females; poor BP (≥95th percentile) was found in 2.9% of male and 3.7% of female participants.14
In 2003 to 2010, for girls 8 to 11 years of age, 3.5%, 5.0%, and 91.5% had BP in the poor, intermediate, and ideal levels according to the AHA 2020 Strategic Impact Goals. For boys 8 to 11 years of age, 2.8%, 4.8%, and 92.5% had BP in the poor, intermediate, and ideal levels according to Life’s Simple 7.15
Among children and adolescents 3 to 19 years of age in NHANES 1999 to 2012, compared with normal-weight children and adolescents, mean SBP was 108.5, 111.0, 112.6, and 116.2 mm Hg among those who were overweight with class I, II, and III obesity, respectively. Mean DBP was 57.0, 58.8, 58.7, and 64.5 mm Hg for those who were overweight with class I, II, and III obesity, respectively.16
Analysis of data for children and adolescents aged 8 to 17 years from NHANES 1999 to 2002 through NHANES 2009 to 2012 found that mean SBP decreased from 105.6 to 104.9 mm Hg and DBP decreased from 60.3 to 56.1 mm Hg.13,17
Analysis of the NHES, the Hispanic Health and Nutrition Examination Survey, and the NHANES/ NCHS surveys of the NCHS (1963–2002) found that the BP, pre-HBP, and HBP trends in children and adolescents 8 to 17 years of age moved downward from 1963 through 1970 to 1988 through 1994 and upward thereafter. Pre-HBP and HBP increased from 7.7% to 10% and from 2.7% to 3.7%, respectively, between 1988 to 1994 and 1999 to 2000. Increased obesity (abdominal obesity more so than general obesity) partially explained the HBP and pre-HBP rise from 1988 through 1994 to 1999 through 2002. BP and HBP reversed their downward trends 10 years after the increase in the prevalence of obesity. In addition, an ethnic and sex gap appeared in 1988 to 1994 for pre-HBP and in 1999 to 2002 for HBP: non-Hispanic blacks and Mexican Americans had a higher prevalence of HBP and pre-HBP than non-Hispanic whites, and the prevalence was greater in boys than in girls. In that study, HBP in children and adolescents was defined as SBP or DBP that was, on repeated measurement, ≥95th percentile.18
Longitudinal BP outcomes from the National Childhood Blood Pressure database (ages 13–15 years) were examined after a single BP measurement. Among those determined to have prehypertension, 14% of boys and 12% of girls had hypertension 2 years later; the overall rate of progression from prehypertension to hypertension was ≈7%.19
The AHA has outlined conditions in which ambulatory BP monitoring may be helpful in children and adolescents. These include secondary hypertension, CKD, type 1 and type 2 DM, obesity, sleep apnea, genetic syndromes, treated patients with hypertension, and for research.20 In a retrospective study of 500 children screened for potential hypertension with ambulatory BP monitoring, 12% had white coat hypertension and 10% had masked hypertension.21
Race/Ethnicity and HBP
The prevalence of hypertension in blacks in the United States is among the highest in the world. In 2011 to 2014, the age-adjusted prevalence of hypertension was 45.0% and 46.3% among non-Hispanic black males and females; 34.5% and 32.3% among non-Hispanic white males and females; 28.8% and 25.7% among non-Hispanic Asian males and females; and 28.9% and 30.7% among Hispanic males and females, respectively (unpublished NHLBI tabulation).
From 1999 to 2000 through 2009 to 2010, the prevalence of hypertension did not increase among non-Hispanic black males (38.0% and 39.6% in 1999–2000 and 2009–2010, respectively) or females (40.8% and 43.1% in 1999–2000 and 2009–2010, respectively).22
Compared with whites, blacks develop HBP earlier in life, and their average BP is much higher.23,24
The incidence of hypertension is higher for blacks than whites through 75 years of age; for a 45-year-old without hypertension, the 40-year cumulative incidence of hypertension is 92.7% among blacks, 92.4% among Hispanics, 86.0% among whites, and 84.1% among Asians.25
African Americans are more likely than whites to have nondipping BP (ie, BP that declines >10% from daytime to nighttime) and nighttime hypertension on ambulatory BP monitoring.26 These phenotypes are associated with increased CVD risk, although there are few published data on the prognostic importance of these phenotypes in African Americans.27
In a study of 18 865 adults in the southeastern United States, blacks were more likely to transition from prehypertension to hypertension than whites (adjusted HR, 1.35; 95% CI, 1.30–1.40).28
The same increment in SBP is associated with a higher stroke risk for blacks than for whites.29
Higher SBP explains ≈50% of the excess stroke risk among blacks compared with whites.30
Data from the 2014 NHIS showed that black adults 18 years of age were more likely (33.0%) to have been told on ≥2 occasions that they had hypertension than American Indian/Alaska Native adults (26.4%), white adults (23.5%), Hispanic or Latino adults (22.9%), or Asian adults (19.5%).31
In the HCHS/SOL, for US Hispanic or Latino males, the age-standardized prevalence of hypertension ranged from a low of 19.9% among those from South America to 32.6% among their counterparts from the Dominican Republic. For US Hispanic or Latino females, the age-standardized prevalence of hypertension was lowest for those of South American descent (15.9%) and highest for their counterparts from Puerto Rico (29.1%).32
Also in the HCHS/SOL, there was substantial heterogeneity in awareness, treatment, and control of hypertension, with Central Americans having the lowest prevalence and Cubans having the highest prevalence among males. Among females, South Americans had the lowest prevalence of awareness and treatment, whereas hypertension control was lowest among Central American females. Only Hispanic females reporting mixed/other origin had a hypertension control rate that exceeded 50%.33
Among NHIS 1997 to 2005 respondents, hypertension prevalence was higher among US-born adults than among foreign-born adults (adjusted OR, 1.28; 95% CI, 1.21–1.36). Hypertension prevalence was higher among US-born non-Hispanic blacks than either foreign-born non-Hispanic blacks (adjusted OR, 1.24; 95% CI, 1.02–1.50) or all African-born immigrants of any race/ethnicity (adjusted OR, 1.45; 95% CI, 1.07–1.97).34
Between 2009 and 2012, activities to control BP, including taking medication, changing eating habits, cutting down on sodium consumption, and reducing alcohol intake, improved among people with self-reported hypertension in 6 Hispanic communities engaged in the REACH US study. No changes in PA were present.35
Data from MESA found that being born outside the United States, speaking a language other than English at home, and living fewer years in the United States were each associated with a decreased prevalence of hypertension.36
Among adults 60 to 79 years with hypertension in NHANES 2005 to 2012, blacks were more likely than whites to be current smokers (18.5% versus 11.%) and have DM (47.0% versus 27.7%). Additionally, mean 10-year predicted ASCVD risk was higher for blacks than whites, at 23.9% versus 21.1%, respectively.37
Among adults with hypertension, blacks are more likely to have resistant hypertension than whites and Hispanics (19.0% versus 13.5% and 11.2%, respectively).38
Among US adults with hypertension, compared with non-Hispanic whites, non-Hispanic blacks and Hispanics are more likely to not have health insurance and to not have a personal doctor or healthcare provider and more likely are unable to visit a doctor because of the cost. Non-Hispanic Asians/ Pacific Islanders were less likely than whites to have these barriers.39
Mortality
(See Table 9-1)
Using data from the National Vital Statistics System, in 2014, there were 73 345 deaths attributable to HBP. In 2014, there were 410 624 deaths with any mention of HBP. The 2014 age-adjusted death rate attributable to HBP was 19.9 per 100 000. Age-adjusted death rates attributable to HBP (per 100 000) in 2014 were 19.3 for non-Hispanic white males, 50.1 for non-Hispanic black males, 19.1 for Hispanic males, 14.0 for non-Hispanic Asian/Pacific Islander males, 22.9 for non-Hispanic American Indian/Alaska Native males, 15.8 for non-Hispanic white females, 35.6 for non-Hispanic black females, 14.6 for Hispanic females, 11.9 for non-Hispanic Asian/Pacific Islander females, and 18.9 for non-Hispanic American Indian/Alaska Native females.40
From 2004 to 2014, the death rate attributable to HBP increased 7.6%, and the actual number of deaths attributable to HBP rose 34.1%. During this 10-year period, in non-Hispanic whites, the HBP death rate increased 13.5%, whereas the actual number of deaths attributable to HBP increased 33.5%. In non-Hispanic blacks, the HBP death rate increased 11.0%, whereas the actual number of deaths attributable to HBP increased 19.7%. In Hispanics, the HBP death rate increased 1.8% and the actual number of deaths attributable to HBP increased 79.9% (unpublished NHLBI tabulation).
When any mention of HBP was present, the overall age-adjusted death rate in 2014 was 111.7 per 100 000 population. Death rates were 120.5 for non-Hispanic white males, 213.5 for non-Hispanic black males, 84.6 for non-Hispanic Asian or Pacific Islander males, 148.5 for non-Hispanic American Indian or Alaska Native males (underestimated because of underreporting), and 113.8 for Hispanic males. In females, rates were 92.0 for non-Hispanic white females, 153.5 for non-Hispanic black females, 66.7 for non-Hispanic Asian or Pacific Islander females, 111.5 for non-Hispanic American Indian or Alaska Native females (underestimated because of underreporting), and 85.7 for Hispanic females.40
The proportion of all decedents aged ≥45 years with any mention of hypertension on their death certificate increased from 11.1% in 2000 to 16.4% in 2013.41
The age-adjusted hypertension-related death rate increased 23.1% from 2000 through 2013. The rate for all other causes of death combined decreased 21.0% over this time period.41
The hypertension-related death rate increased 6.8% from 523.8 per 100 000 population in 2000 to 559.3 in 2005 for non-Hispanic blacks, and then it decreased 8.8% to 509.9 in 2013. Among Hispanics, the rate increased 21.9% from 233.7 in 2000 to 284.8 in 2013. For the non-Hispanic white population, the rate increased 29.8% from 228.5 in 2000 to 296.5 in 2013.41
HD, stroke, cancer, and DM accounted for 65% of all deaths with any mention of hypertension in 2000 and for 54% in 2013.41
Among US adults with hypertension from 1971 to 1975 followed up through 1992 (NHANES I), age-adjusted mortality was 18.8 per 1000 person-years. This declined to 14.3 per 1000 person-years for adults with hypertension between 1988 and 1994 followed up through 2006 (NHANES III). The reduction was higher in males than in females but was similar for blacks and whites. This rate of decline was also observed for individuals without hypertension, but US adults with hypertension had higher mortality than their counterparts without hypertension.42
The elimination of hypertension could reduce CVD mortality by 30.4% among males and 38.0% among females.43 The elimination of hypertension is projected to have a larger impact on CVD mortality than the elimination of all other risk factors among females and all except smoking among males.
Assessment of 30-year follow-up of the Hypertension Detection and Follow-up Program identified the long-term benefit of stepped care (ie, initial treatment followed by additional antihypertensive medications in a stepwise approach until BP goal was achieved), as well as increased survival for hypertensive African Americans, although disparities in death rates did persist.41,44
Risk Factors
Numerous risk factors and markers for development of hypertension have been identified, including age, race/ethnicity, family history of hypertension and genetic factors, lower education and socioeconomic status, greater weight, lower PA, tobacco use, psychosocial stressors, sleep apnea, and dietary factors (including dietary fats, higher sodium intake, lower potassium intake, and excessive alcohol intake).
Data from the Nurses’ Health Study suggest that a large proportion of incident hypertension in females can be prevented by controlling dietary and lifestyle risk factors.45
Among 31 146 females without hypertension at baseline in the Women’s Health Initiative, randomization to a low-fat diet versus usual diet resulted in a 4% lower risk for incident hypertension and 0.66 mm Hg lower SBP at 1 year of follow-up.46
The consumption of sugar-sweetened beverages was associated with a risk ratio for hypertension of 1.12 (95% CI, 1.06–1.17) in a meta-analysis of 240 508 people.47 This equated to an 8.2% increased risk for hypertension for each additional sugar-sweetened beverage consumed per day.
Bisphenol A, a synthetic monomer used in the manufacture of polycarbonate plastics and epoxy resins, has been associated with hypertension in 3 cross-sectional studies.48
Risk prediction models for developing hypertension have been developed and validated. A commonly used risk prediction model was developed in the FHS that includes age, sex, SBP, DBP, BMI, smoking, and parental history of hypertension.49,50 In young adults, this model was better able to identify those who developed hypertension over 25 years of follow-up than prehypertension; however, this model systematically underestimated the risk for hypertension.51
A systematic review identified 15 different hypertension risk prediction models. Two models (the FHS and Hopkins models) have been validated externally with acceptable discrimination (c-statistic 0.7–0.8).52
Aftermath
The risk for stroke and HD mortality increases in a log-linear fashion from SBP levels <115 mm Hg to >180 mm Hg and from DBP levels <75 mm Hg to >105 mm Hg. Each 20 mm Hg higher SBP and 10 mm Hg higher DBP is associated with a doubling in the risk of death caused by stroke, HD, or other vascular disease.53
In a study of >1 million adults with hypertension, the lifetime risk of cardiovascular disease at age 30 years was 63.3% compared with 46.1% for those with normal BP. Those with hypertension developed cardiovascular disease 5.0 years earlier than their counterparts without hypertension.54 The largest lifetime risk differences between people with versus without hypertension was for angina, myocardial infarction and stroke. At age 60 years, the lifetime risk for CVD is 60.2% for those with hypertension and 44.6% for their counterparts without hypertension.
Data from the FHS/NHLBI indicate that recent (within the past 10 years) and remote antecedent BP levels could be an important determinant of CVD and HF risk over and above the current BP level.55,56
-
Data from the FHS/NHLBI indicate that hypertension is associated with shorter overall life expectancy, shorter life expectancy free of CVD, and more years lived with CVD.57
Total life expectancy was 5.1 years longer for normotensive males and 4.9 years longer for normotensive females than for hypertensive people of the same sex at 50 years of age.
Compared with hypertensive males at 50 years of age, males with untreated BP <140/90 mm Hg survived on average 7.2 years longer without CVD and spent 2.1 fewer years of life with CVD. Similar results were observed for females.
Overall, the prevalence of healthy lifestyle behaviors varied widely among those with self-reported hypertension: 20.5% had a normal weight, 82.3% did not smoke, 94.1% reported no or limited alcohol intake, 14.1% consumed the recommended amounts of fruits or vegetables, and 46.6% engaged in the recommended amount of PA.4
Hospital Discharges/Ambulatory Care Visits
(See Table 9-1)
From 2000 to 2010, the number of inpatient discharges from short-stay hospitals with HBP as the first-listed diagnosis increased from 457 000 to 488 000 (no significant difference; NCHS, NHDS). The number of discharges with any listing of HBP increased from 8 034 000 to 11 282 000 (NHLBI, unpublished data from the NHDS, 2010; diagnoses in 2010 were truncated at 7 diagnoses for comparability with earlier year).
Data from the NIS from the years 2000 to 2011 found the frequency of hospitalizations for malignant hypertension and hypertensive encephalopathy increased while hospitalizations for essential hospitalization decreased, which coincided with the introduction of medical severity diagnosis-related group billing. Overall, the annual incidence of hypertension-related hospitalizations increased over the time period from ≈87 000 in 2000 to ≈120 000 in 2011.58
In 2006 to 2010, 7.1% of patients with hypertension attending outpatient visits had treatment-resistant hypertension. The use of thiazide diuretics and chlorthalidone was low (56.4% and 1.2%, respectively).59
In 2012, there were 34 016 000 physician office visits for a primary diagnosis of essential hypertension (ICD-9-CM 401) (NCHS, NAMCS, NHLBI tabulation). In 2012, there were 909 000 ED visits and in 2011, there were 3 743 000 hospital outpatient visits for essential hypertension (NCHS, NHAMCS, NHLBI tabulation).
Awareness, Treatment, and Control
(See Table 9-2 and Charts 9-3 through 9-5)
Chart 9-3. Extent of awareness, treatment, and control of high blood pressure by age (NHANES 2007–2012).
Hypertension is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or if the subject said “yes” to taking antihypertensive medication.
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Data from NHANES 2011 to 2014 showed that of those with hypertension who were ≥20 years of age, 84.1% were aware of their condition, 76.0% were under current treatment, 54.4% had their hypertension under control, and 45.6% did not have it controlled. Awareness and treatment of hypertension were higher at older ages. Hypertension control was higher in US adults 40 to 59 years of age (58.3%) and those ≥60 years of age (54.0%) than in their counterparts 20 to 39 years of age (40.1%; Chart 9-3). A higher percentage of non-Hispanic white and non-Hispanic black adults were aware of their hypertension (85.1% and 85.5%, respectively) than Hispanic and non-Hispanic Asian adults (79.8% and 75.3%, respectively; Chart 9-4; unpublished NHLBI tabulation). Overall, females were more likely than males in all race/ethnicity groups to be aware of their condition, under treatment, or in control of their hypertension (Chart 9-5).
Data from NHANES 1999 to 2008 and BRFSS 1997 to 2009 showed awareness, treatment, and control of hypertension varied across the country and was highest in the southeastern United States.60
Analysis of NHANES 1999 to 2006 and 2009 to 2012 found the proportion of adults aware of their hypertension increased within each race/ethnicity and sex subgroup. Similarly, large increases in hypertension treatment and control (≈10%) occurred in each of these groups (Table 9-2).
According to data from NHANES 2003 to 2004 through 2011 to 2012, HBP control rates improved from 39.4% to 51.8%. Awareness increased from 75.2% to 82.1%, and treatment improved from 65.0% to 74.5%.61
Among US adults taking prescription antihypertensive medication, the age-adjusted percentage with BP control increased from 61.9% to 70.4%.61
In 2011 to 2012, medication use to lower BP among those with hypertension was lowest for those aged 18 to 39 years (44.5%) compared with those aged 40 to 59 years (73.7%) and those aged ≥60 years (82.2%). Non-Hispanic black adults were more likely to take antihypertensive medication than non-Hispanic white, Hispanic, or Asian adults (77.4%, 76.7%, 73.5%, and 65.2% respectively).62
Data from NHANES 2005 to 2010 show that among those ≥80 years of age, 79.4% of those with hypertension were aware of this condition, 57.4% were treated, and 39.8% had controlled their BP to JNC 7 targets.12
Data from NHANES 1999 to 2012 show that the use of various classes of antihypertensive treatment has increased substantially among people ≥20 years of age. During this period, the use of ACEIs increased from 6.3% of the US population to 12%, ARBs from 2.1% to 5.8%, β-blockers from 6.0% to 11%, and thiazide diuretic drugs from 5.6% to 9.4%. The use of calcium channel blockers remained the same, at 6%.63
Among 1509 NHANES 2005 to 2006 participants aged ≥30 years with hypertension, 24% were categorized as low risk, 21% as intermediate risk, and 23% as high risk according to Framingham global risk. Treatment for hypertension varied by risk category and ranged from 58% to 75%; hypertension control was 80% for those in the low-risk category and <50% for those in the high-risk category.64
Chart 9-4. Extent of awareness, treatment, and control of high blood pressure by race/ethnicity (NHANES 2011–2014).
Hypertension is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or if the subject said “yes” to taking antihypertensive medication.
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Cost
(See Table 9-1)
The estimated direct and indirect cost of HBP for 2012 to 2013 (annual average) was $51.2 billion (MEPS, NHLBI tabulation).
Controlling hypertension in all patients with CVD or stage 2 hypertension (SBP ≥160 mm Hg or DBP ≥100 mm Hg) could be effective and cost-saving. Treating stage 1 hypertension could be cost-effective for males and for females 45 to 74 years of age.65
The median amount of US states’ costs attributable to hypertension was $1.62 million in 2004 to 2008, with 35% of these costs borne by Medicare or Medicaid.66
Projections show that by 2030, the total direct costs of HBP could increase to an estimated $200 billion (unpublished AHA computation, based on methodology described in Heidenreich et al10).
Global Burden of Hypertension
In 2000, it was estimated that 972 million adults worldwide had hypertension.67
-
Between 1980 and 200868:
The global mean age-adjusted SBP declined from 130.5 mm Hg in 1980 to 128.1 mm Hg in males and from 127.2 to 124.4 mm Hg in females.
The global age-adjusted prevalence of uncontrolled hypertension decreased from 33% to 29% among males and from 29% to 25% among females.
Because of population growth and aging, the number of people worldwide with uncontrolled hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) increased from 605 million to 978 million between 1980 and 2008.68
HBP went from being the fourth-leading risk factor in 1990, as quantified by DALYs, to being the number 1 risk factor in 2010.69
A meta-analysis of 242 studies (n=1.5 million adults) reported 32.3% (95% CI, 29.4%–35.3%) of adults have hypertension, with the highest prevalence in the Latin America and Caribbean region. Hypertension prevalence estimates were highest across upper-middle-income countries (37.8%, 95% CI 35.0%–40.6%) and lowest across low-income countries (23.1%, 95% CI 20.1%–26.2%).70
In a cross-sectional study of 628 communities (3 HIC, 10 upper-middle income and low-middle income countries, and 4 low-income countries), awareness, treatment, and control of hypertension were lowest in low-income countries.71
In 2010, HBP was 1 of the 5 leading risk factors in all regions with the exception of Oceania, Eastern sub-Saharan Africa, and Western sub-Saharan Africa.69
Prehypertension
Among adults without hypertension, prehypertension is defined by an untreated SBP of 120 to 139 mm Hg or untreated DBP of 80 to 89 mm Hg.
Among US adults ≥20 years of age, the prevalence of prehypertension or hypertension did not change substantially between 1999 to 2000 (55.8%) and 2009 to 2010 (52.6%); non-Hispanic black females and males were more likely to have prehypertension or hypertension than non-Hispanic whites or Hispanics.22
Among participants without a history of CVD or cancer in NHANES 1999 to 2006, the prevalence of prehypertension was 36.3%. The prevalence was higher in males than in females. Furthermore, prehypertension was correlated with an adverse cardiometabolic risk profile.72
Follow-up of 9845 males and females in the FHS/ NHLBI who attended examinations from 1978 to 1994 revealed that at 35 to 64 years of age, the 4-year incidence of hypertension was 5.3% for those with baseline BP <120/80 mm Hg, 17.6% for those with SBP of 120 to 129 mm Hg or DBP of 80 to 84 mm Hg, and 37.3% for those with SBP of 130 to 139 mm Hg or DBP of 85 to 89 mm Hg. At 65 to 94 years of age, the 4-year incidences of hypertension were 16.0%, 25.5%, and 49.5% for these BP categories, respectively.11
Among participants with and without prehypertension in MESA, 23.6% and 5.3%, respectively, developed hypertension over 4.8 years of follow-up.49
Among young adults (18 to 30 years old at baseline) with and without prehypertension in CARDIA, 23.1% and 3.8%, respectively, developed hypertension over 5 years of follow-up.51
Multiple meta-analyses have demonstrated prehypertension to be associated with an increased risk for CVD, ESRD, and mortality. These risks are greater for people in the upper (130–139/85–89 mm Hg) versus lower (120–129/80–84 mm Hg) range of prehypertension.73–82
Two RCTs have reported that pharmacological treatment of hypertension is associated with a lower incidence of hypertension.83,84
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Crim MT, Yoon SS, Ortiz E, Wall HK, Schober S, Gillespie C, Sorlie P, Keenan N, Labarthe D, Hong Y. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes. 2012;5:343–351. doi: 10.1161/CIRCOUTCOMES.111.963439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J, Moore L, Loustalot F, Yang Q, Ayala C. Reporting of adherence to healthy lifestyle behaviors among hypertensive adults in the 50 states and the District of Columbia, 2013. J Am Soc Hypertens. 2016;10:252–262.e3. doi: 10.1016/j.jash.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9:197–205. doi: 10.1016/j.jash.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28:355–361. doi: 10.1093/ajh/hpu151. [DOI] [PubMed] [Google Scholar]
- 8.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol. 2016;67:463–472. doi: 10.1016/j.jacc.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 12.Bromfield SG, Bowling CB, Tanner RM, Peralta CA, Odden MC, Oparil S, Muntner P. Trends in hypertension prevalence, awareness, treatment, and control among US adults 80 years and older, 1988–2010. J Clin Hypertens (Greenwich) 2014;16:270–276. doi: 10.1111/jch.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169:272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–1376. doi: 10.1161/CIRCULATIONAHA.113.001559. [DOI] [PubMed] [Google Scholar]
- 15.Ning H, Labarthe DR, Shay CM, Daniels SR, Hou L, Van Horn L, Lloyd-Jones DM. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. 2015;8:164–171. doi: 10.1161/CIRCOUTCOMES.114.001274. [DOI] [PubMed] [Google Scholar]
- 16.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. 2015;373:1307–1317. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 17.Xi B, Zhang T, Zhang M, Liu F, Zong X, Zhao M, Wang Y. Trends in elevated blood pressure among US children and adolescents: 1999–2012. Am J Hypertens. 2016;29:217–225. doi: 10.1093/ajh/hpv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 19.Falkner B, Gidding SS, Portman R, Rosner B. Blood pressure variability and classification of prehypertension and hypertension in adolescence. Pediatrics. 2008;122:238–242. doi: 10.1542/peds.2007-2776. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubrano R, Paoli S, Spiga S, Falsaperla R, Vitaliti G, Gentile I, Elli M. Impact of ambulatory blood pressure monitoring on the diagnosis of hypertension in children. J Am Soc Hypertens. 2015;9:780–784. doi: 10.1016/j.jash.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Voors AW, Webber LS, Berenson GS. Time course studies of blood pressure in children: the Bogalusa Heart Study. Am J Epidemiol. 1979;109:320–334. doi: 10.1093/oxfordjournals.aje.a112685. [DOI] [PubMed] [Google Scholar]
- 24.Voors AW, Webber LS, Berenson GS. Time course study of blood pressure in children over a three-year period: Bogalusa Heart Study. Hypertension. 1980;2(pt 2):102–108. [PubMed] [Google Scholar]
- 25.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28:640–648. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, O’Brien E, Roush GC ABC-H Investigators. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) meta-analysis. Hypertension. 2016;67:693–700. doi: 10.1161/HYPERTENSIONAHA.115.06981. [DOI] [PubMed] [Google Scholar]
- 28.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–587. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez CJ, Swett K, Agarwal SK, Folsom AR, Fox ER, Loehr LR, Ni H, Rosamond WD, Chang PP. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the Atherosclerosis Risk in Communities study [published correction appears in JAMA Intern Med. 2014;174:1419] JAMA Intern Med. 2014;174:1252–1261. doi: 10.1001/jamainternmed.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. [Accessed July 10, 2015];National Health Interview Survey. 2014 Public-use data file and documentation: NCHS tabulations. http://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm.
- 32.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/ Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorlie PD, Allison MA, Avilés-Santa ML, Cai J, Daviglus ML, Howard AG, Kaplan R, Lavange LM, Raij L, Schneiderman N, Wassertheil-Smoller S, Talavera GA. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/ Study of Latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang J, Ayala C, Loustalot F. Association of birthplace and self-reported hypertension by racial/ethnic groups among US adults: National Health Interview Survey, 2006–2010. J Hypertens. 2012;30:2285–2292. doi: 10.1097/HJH.0b013e3283599b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y, Siegel PZ, White S, Dulin R, Taylor A. Improving actions to control high blood pressure in Hispanic communities: Racial and Ethnic Approaches to Community Health Across the U.S. Project, 2009–2012. Prev Med. 2016;83:11–15. doi: 10.1016/j.ypmed.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran A, Diez Roux AV, Jackson SA, Kramer H, Manolio TA, Shrager S, Shea S. Acculturation is associated with hypertension in a multiethnic sample. Am J Hypertens. 2007;20:354–363. doi: 10.1016/j.amjhyper.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Egan BM, Bland VJ, Brown AL, Ferdinand KC, Hernandez GT, Jamerson KA, Johnson WR, Kountz DS, Li J, Osei K, Reed JW, Saunders E. Hypertension in African Americans aged 60 to 79 years: statement from the International Society of Hypertension in Blacks. J Clin Hypertens (Greenwich) 2015;17:252–259. doi: 10.1111/jch.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, Kalantar-Zadeh K, Jacobsen SJ. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–1107. doi: 10.1016/j.mayocp.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang J, Yang Q, Ayala C, Loustalot F. Disparities in access to care among US adults with self-reported hypertension. Am J Hypertens. 2014;27:1377–1386. doi: 10.1093/ajh/hpu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed July 15, 2016];Mortality multiple cause micro-data files, 2014: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 41.Kung HC, Xu J. Hypertension-related mortality in the United States, 2000–2013. NCHS Data Brief. 2015;(193):1–8. [PubMed] [Google Scholar]
- 42.Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123:1737–1744. doi: 10.1161/CIRCULATIONAHA.110.005645. [DOI] [PubMed] [Google Scholar]
- 43.Patel SA, Winkel M, Ali MK, Narayan KM, Mehta NK. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med. 2015;163:245–253. doi: 10.7326/M14-1753. [DOI] [PubMed] [Google Scholar]
- 44.Lackland DT, Egan BM, Mountford WK, Boan AD, Evans DA, Gilbert G, McGee DL. Thirty-year survival for black and white hypertensive individuals in the Evans County Heart Study and the Hypertension Detection and Follow-up Program. J Am Soc Hypertens. 2008;2:448–454. doi: 10.1016/j.jash.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allison MA, Aragaki AK, Ray RM, Margolis KL, Beresford SA, Kuller L, Jo O’Sullivan M, Wassertheil-Smoller S, Van Horn L. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: tertiary analysis of the WHI Dietary Modification Trial. Am J Hypertens. 2016;29:959–968. doi: 10.1093/ajh/hpv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayalath VH, de Souza RJ, Ha V, Mirrahimi A, Blanco-Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ, Sievenpiper JL. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr. 2015;102:914–921. doi: 10.3945/ajcn.115.107243. [DOI] [PubMed] [Google Scholar]
- 48.Rancière F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T, Shaw JE, Magliano DJ. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health. 2015;14:46. doi: 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muntner P, Woodward M, Mann DM, Shimbo D, Michos ED, Blumenthal RS, Carson AP, Chen H, Arnett DK. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1339–1345. doi: 10.1161/HYPERTENSIONAHA.109.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D’Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 51.Carson AP, Lewis CE, Jacobs DR, Jr, Peralta CA, Steffen LM, Bower JK, Person SD, Muntner P. Evaluating the Framingham hypertension risk prediction model in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2013;62:1015–1020. doi: 10.1161/HYPERTENSIONAHA.113.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Echouffo-Tcheugui JB, Batty GD, Kivimäki M, Kengne AP. Risk models to predict hypertension: a systematic review. PLoS One. 2013;8:e67370. doi: 10.1371/journal.pone.0067370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies [published correction appears in Lancet. 2003;361:1060] Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 54.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonifonte A, Ayer T, Veledar E, Clark A, Wilson PW. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015;9:690–696.e1. doi: 10.1016/j.jash.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, Levy D, D’Agostino RB, Sr, Vasan RS. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 57.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 58.Polgreen LA, Suneja M, Tang F, Carter BL, Polgreen PM. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension. 2015;65:1002–1007. doi: 10.1161/HYPERTENSIONAHA.115.05241. [DOI] [PubMed] [Google Scholar]
- 59.Fontil V, Pletcher MJ, Khanna R, Guzman D, Victor R, Bibbins-Domingo K. Physician underutilization of effective medications for resistant hypertension at office visits in the United States: NAMCS 2006–2010. J Gen Intern Med. 2014;29:468–476. doi: 10.1007/s11606-013-2683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olives C, Myerson R, Mokdad AH, Murray CJ, Lim SS. Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001–2009. PLoS One. 2013;8:e60308. doi: 10.1371/journal.pone.0060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65:54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;(133):1–8. [PubMed] [Google Scholar]
- 63.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong ND, Dede J, Chow VH, Wong KS, Franklin SS. Global cardiovascular risk associated with hypertension and extent of treatment and control according to risk group. Am J Hypertens. 2012;25:561–567. doi: 10.1038/ajh.2012.2. [DOI] [PubMed] [Google Scholar]
- 65.Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins-Domingo K, Coxson PG, Goldman L. Cost-effectiveness of hypertension therapy according to 2014 guidelines [published correction appears in N Engl J. Med. 2015;372:1677] N Engl J Med. 2015;372:447–455. doi: 10.1056/NEJMsa1406751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trogdon JG, Murphy LB, Khavjou OA, Li R, Maylahn CM, Tangka FK, Nurmagambetov TA, Ekwueme DU, Nwaise I, Chapman DP, Orenstein D. Costs of chronic diseases at the state level: the Chronic Disease Cost Calculator. Prev Chronic Dis. 2015;12:E140. doi: 10.5888/pcd12.150131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 68.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure) National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 69.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 70.Sarki AM, Nduka CU, Stranges S, Kandala NB, Uthman OA. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1959. doi: 10.1097/MD.0000000000001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S PURE (Prospective Urban Rural Epidemiology) Study Investigators. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 72.Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease-free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010;33:905–910. doi: 10.1038/hr.2010.91. [DOI] [PubMed] [Google Scholar]
- 73.Guo X, Zhang X, Guo L, Li Z, Zheng L, Yu S, Yang H, Zhou X, Zhang X, Sun Z, Li J, Sun Y. Association between pre-hypertension and cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Curr Hypertens Rep. 2013;15:703–716. doi: 10.1007/s11906-013-0403-y. [DOI] [PubMed] [Google Scholar]
- 74.Guo X, Zhang X, Zheng L, Guo L, Li Z, Yu S, Yang H, Zhou X, Zou L, Zhang X, Sun Z, Li J, Sun Y. Prehypertension is not associated with all-cause mortality: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8:e61796. doi: 10.1371/journal.pone.0061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y, Cai X, Li Y, Su L, Mai W, Wang S, Hu Y, Wu Y, Xu D. Prehypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153–1161. doi: 10.1212/WNL.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y, Cai X, Liu C, Zhu D, Hua J, Hu Y, Peng J, Xu D. Prehypertension and the risk of coronary heart disease in Asian and Western populations: a meta-analysis. J Am Heart Assoc. 2015;4:e001519. doi: 10.1161/JAHA.114.001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Y, Cai X, Zhang J, Mai W, Wang S, Hu Y, Ren H, Xu D. Prehypertension and incidence of ESRD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:76–83. doi: 10.1053/j.ajkd.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 78.Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, Wu Y, Tang H, Xu D. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J. 2014;167:160–168.e1. doi: 10.1016/j.ahj.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med. 2013;11:177. doi: 10.1186/1741-7015-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee M, Saver JL, Chang B, Chang KH, Hao Q, Ovbiagele B. Presence of baseline prehypertension and risk of incident stroke: a meta-analysis. Neurology. 2011;77:1330–1337. doi: 10.1212/WNL.0b013e3182315234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen L, Ma H, Xiang MX, Wang JA. Meta-analysis of cohort studies of baseline prehypertension and risk of coronary heart disease. Am J Cardiol. 2013;112:266–271. doi: 10.1016/j.amjcard.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Wu H, Zhang Q, Xu J, Fan Y. Impact of baseline prehypertension on cardiovascular events and all-cause mortality in the general population: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168:4857–4860. doi: 10.1016/j.ijcard.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 83.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr, Messerli FH, Oparil S, Schork MA Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 84.Lüders S, Schrader J, Berger J, Unger T, Zidek W, Böhm M, Middeke M, Motz W, Lübcke C, Gansz A, Brokamp L, Schmieder RE, Trenkwalder P, Haller H, Dominiak P PHARAO Study Group. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487–1496. doi: 10.1097/HJH.0b013e3282ff8864. [DOI] [PubMed] [Google Scholar]
10. DIABETES MELLITUS
ICD-9 250; ICD-10 E10 to E14. See Table 10-1 and Charts 10-1 through 10-6
Table 10-1.
Diabetes Mellitus
| Population Group | Prevalence of Physician- Diagnosed DM, 2011–2014: Age ≥20 y | Prevalence of Undiagnosed DM, 2011–2014: Age ≥20 y | Prevalence of Prediabetes, 2011–2014: Age ≥20 y | Incidence of Diagnosed DM: Age ≥20 y* | Mortality, 2014: All Ages† | Hospital Discharges, 2010: All Ages | Cost, 2012‡ |
|---|---|---|---|---|---|---|---|
| Both sexes | 23 400 000 (9.1%) | 7 600 000 (3.1%) | 81 600 000 (33.9%) | 1 700 000 | 76 488 | 630 000 | $245 Billion |
| Males | 11 400 000 (9.4%) | 4 500 000 (3.8%) | 46 200 000 (40.2%) | … | 41 111 (53.7%)§ | 311 000 | … |
| Females | 12 000 000 (8.9%) | 3 100 000 (2.3%) | 35 400 000 (27.8%) | … | 35 377 (46.3%)§ | 319 000 | … |
| NH white males | 8.0% | 3.6% | 39.6% | … | 28 743 | … | … |
| NH white females | 7.4% | 1.5% | 29.2% | … | 23 201 | … | … |
| NH black males | 14.1% | 2.8% | 32.8% | … | 6369 | … | … |
| NH black females | 13.6% | 3.5% | 24.1% | … | 6895 | … | … |
| Hispanic males | 12.6% | 6.3% | 45.9% | … | 4149 | … | … |
| Hispanic females | 12.7% | 4.4% | 25.0% | … | 3646 | … | … |
| NH Asian males | 11.8% | 5.7% | 42.0% | … | 1208 | … | … |
| NH Asian females | 9.1% | 4.3% | 25.5% | … | 1115 | … | … |
| NH American Indian or Alaska Native | … | … | … | … | 908 | … | … |
Undiagnosed DM is defined as those whose fasting glucose is ≥126 mg/dL but who did not report being told by a healthcare provider that they had DM. Prediabetes is a fasting blood glucose of 100 to <126 mg/dL (impaired fasting glucose); prediabetes includes impaired glucose tolerance. DM indicates diabetes mellitus; ellipses (…), data not available; and NH, non-Hispanic.
Centers for Disease Control and Prevention, National Diabetes Statistics Report, 2014.3
Mortality for Hispanic, American Indian or Alaska Native, and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
American Diabetes Association.70
These percentages represent the portion of total DM mortality that is for males vs females.
Sources: Prevalence: Prevalence of diagnosed and undiagnosed DM: National Health and Nutrition Examination Survey 2011 to 2014, National Center for Health Statistics (NCHS), and National Heart, Lung, and Blood Institute. Percentages for racial/ethnic groups are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolations to the 2014 US population estimates. Mortality: Centers for Disease Control and Prevention/NCHS, 2014 Mortality Multiple Cause-of-Death–United States. These data represent underlying cause of death only. Mortality for NH Asians includes Pacific Islanders. Hospital discharges: National Hospital Discharge Survey, NCHS; data include those inpatients discharged alive, dead, or status unknown.
Chart 10-1. Age-adjusted prevalence of physician-diagnosed diabetes mellitus in adults ≥20 years of age by race/ethnicity and sex (NHANES 2011–2014).
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination
Survey. Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 10-6. Trends in age-standardized rates of diabetes mellitus–related complications among US adults with and without diagnosed diabetes.
ESRD indicates end-stage renal disease. Reprinted From Gregg et al37 with permission from Massachusetts Medical Society. Copyright © 2014, Massachusetts Medical Society.
DM is a major risk factor for CVD, such as CHD, stroke, PAD, HF, and AF.1 The AHA has identified untreated fasting blood glucose levels of <100 mg/dL for children and adults as 1 of the 7 components of ideal cardiovascular health.2 In 2011 to 2012, 85.3% of children and 56.5% of adults met this criterion.
Abbreviations Used in Chapter 10
| ACC | American College of Cardiology |
| ACS | acute coronary syndrome |
| ADVANCE | Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| AHRQ | Agency for Healthcare Research and Quality |
| AMI | acute myocardial infarction |
| ARIC | Atherosclerosis Risk in Communities |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CARRS | Center for Cardiometabolic Risk Reduction in South Asia |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DCCT | Diabetes Control and Complications Trial |
| DM | diabetes mellitus |
| ED | emergency department |
| EDIC | Epidemiology of Diabetes Interventions and Complications Study |
| ESRD | end-stage renal disease |
| EVEREST | Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan |
| FHS | Framingham Heart Study |
| HbA1c | hemoglobin A1c |
| HCHS/SOL | Hispanic Community Health Study/Study of Latinos |
| HD | heart disease |
| HF | heart failure |
| HR | hazard ratio |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IDDM | insulin-dependent diabetes mellitus |
| LDL-C | low-density lipoprotein cholesterol |
| MASALA | Mediators of Atherosclerosis in South Asians Living in America |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| NRMI | National Registry of Myocardial Infarction |
| NSTEMI | non–ST-segment–elevation myocardial infarction |
| OR | odds ratio |
| PA | physical activity |
| PAD | peripheral artery disease |
| PAR | population attributable risk |
| RR | relative risk |
| SBP | systolic blood pressure |
| SEARCH | Search for Diabetes in Youth Study |
| STEMI | ST-segment–elevation myocardial infarction |
| TC | total cholesterol |
| TIMI | Thrombolysis in Myocardial Infarction |
| TODAY | Treatment Options for Type 2 Diabetes in Adolescents and Youth |
| TRIUMPH | Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status |
| UA | unstable angina |
| USRDS | US Renal Data System |
Prevalence
Youth
Approximately 186 000 people <20 years of age have DM. During 2008 to 2009, >18 000 people <20 years of age were diagnosed with type 1 DM each year.3 Healthcare providers are diagnosing people <20 years of age with type 2 DM, a disease usually diagnosed in adults ≥40 years of age. Children who develop type 2 DM are typically overweight or obese and have a family history of the disease, with incidence rates among American Indian, black, Asian, and Hispanic/Latino children 3- to 8-fold higher than in non-Hispanic whites.4
Between 2001 and 2009, the prevalence of type 2 DM in youths increased by 30.5%.5
Among adolescents 10 to 19 years of age diagnosed with DM, 57.8% of blacks were diagnosed with type 2 versus type 1 DM compared with 46.1% of Hispanic youths and 14.9% of white youths.4
According to the Bogalusa Heart Study, a long-term follow-up study of youths aging into adulthood, youths who were prediabetic or who had DM were more likely to have a constellation of metabolic disorders in young adulthood (19–44 years of age), including obesity, hypertension, dyslipidemia, and metabolic syndrome, all of which predispose to CHD.6
Among youths with type 2 DM, 10.4% are overweight and 79.4% are obese.7
According to NHANES data from 1999 to 2008, among US adolescents aged 12 to 19 years, the prevalence of prediabetes and DM increased from 9% to 23%.8
Analyses of a cohort of consecutive high school blood donors in north Texas from September 2011 to March 2012 comprising 14 850 adolescents showed that 10.0% had HbA1c values in the prediabetes range (HbA1c 5.7%–6.4%), and an additional 0.6% had HbA1c ≥6.5%, the threshold endorsed to diagnose DM.9
The results of the TODAY study demonstrated that less than half of the children in the study (41.1% Hispanic and 31.5% non-Hispanic black) maintained durable glycemic control with mono-therapy,10 a higher rate of treatment failure than observed in adult cohorts. Youths who had type 2 DM were sedentary >56 minutes longer per day (via accelerometry) than obese youths from NHANES.11
Adults
(See Table 10-1 and Charts 10-1 through 10-4)
Chart 10-4. Trends in the prevalence of diagnosed and undiagnosed diabetes mellitus (calibrated hemoglobin A1c levels >6.5%), by race/ethnic group.
Data from US adults aged 20 years in NHANES 1988 to 1994, 1999 to 2004, and 2005 to 2010.
NHANES indicates National Health and Nutrition Examination Survey.
Reprinted from Selvin et al13 with the permission of American College of Physicians, Inc. Copyright © 2014, American College of Physicians. All Rights Reserved.
On the basis of data from NHANES 2011 to 2014 (unpublished NHLBI tabulation), an estimated 23.4 million adults have diagnosed DM, 7.6 million adults have undiagnosed DM, and 81.6 million adults (33.9%) have prediabetes (eg, fasting blood glucose of 100 to <126 mg/dL; Table 10-1).
The prevalence of prediabetes and DM differs by sex and race/ethnicity (Table 10-1; unpublished NHLBI tabulation).
According to NHANES data from 1988 to 1994 compared with 2005 to 2010, the prevalence of DM (diagnosed and undiagnosed) increased from 8.4% to 12.1%. This increase was most pronounced among those ≥65 years of age (increase in prevalence from 18.6% to 28.5% when adjusted for sex, race/ethnicity, and education).12
Type 2 DM accounts for 90% to 95% of all diagnosed cases of DM in adults.3
Analysis of NHANES/NCHS data from 1988 to 1994 and from 2005 to 2010 in adults ≥20 years of age showed that the prevalence of DM (diagnosed DM or HbA1c ≥6.5%) among adults ≥20 years of age increased from 6.2% in 1988 to 1994 to 9.9% (21 million adults) in 2005 to 2010.13
Minority groups remain disproportionately affected by DM.13 The prevalence of total DM (diagnosed DM or HbA1c ≥6.5%) in non-Hispanic blacks is almost twice as high as in whites (15.4% versus 8.6%), and Mexican Americans had a 35% higher prevalence of DM than whites (11.6% versus 8.6%, respectively).13
The prevalence of diagnosed DM in adults ≥65 years of age was 26.9% in 2010, and an additional 50% (>20 million) had prediabetes based on fasting glucose, oral glucose tolerance testing, or HbA1c. In addition, data from NHANES 2005 to 2006 show that 46% of DM cases remain undiagnosed in this group aged ≥65 years.14
According to the 2014 National Diabetes Statistics Report, males >20 years of age have a slightly higher prevalence of DM (13.6%) than females (11.2%).3
After adjustment for population age differences, 2011 to 2014 NHANES national survey data for people ≥20 years of age indicate that 8.0% of non-Hispanic white males and 7.4% of non-Hispanic white females, 11.8% of non-Hispanic Asian males and 9.1% of non-Hispanic Asian females, 12.6% of Hispanic males and 12.7% of Hispanic females, and 14.1% of non-Hispanic black males and 13.6% of non-Hispanic black females had physician-diagnosed DM (Chart 10-1; unpublished NCHS/NHLBI tabulation).
On the basis of NHANES 2011 to 2014 data, the age-adjusted prevalence of physician-diagnosed DM in adults ≥20 years of age varies by race/ethnicity and years of education. Non-Hispanic white adults with more than a high school education had the lowest prevalence (6.6%), and non-Hispanic black adults with less than a high school education had the highest prevalence (16.0%; Chart 10-2; unpublished NCHS/NHLBI tabulation).
The prevalence of physician-diagnosed DM in adults was higher for both males and females in the 2011 to 2014 NHANES data than in the 1988 to 1994 NHANES data. Prevalence of undiagnosed DM differed only slightly for both males and females between studies (Chart 10-3; unpublished NCHS/NHLBI tabulation). In 1997, the threshold definition for physician-diagnosed DM was lowered from ≥140 mg/dL to ≥126 mg/dL.15
The prevalence of physician-diagnosed DM in adults was higher for non-Hispanic black, non-Hispanic white, and Hispanic adults in NHANES 1988 to 2010 than in NHANES 1988 to 1994. Prevalence of undiagnosed DM increased slightly between studies (Chart 10-4; unpublished NCHS/NHLBI tabulation).
Compared with non-Hispanic white adults, the risk of diagnosed DM was 18% higher among Asian Americans, 66% higher among Hispanics/Latinos, and 77% higher among non-Hispanic blacks.16
In the prospective, multicenter, population-based HCHS/SOL, 16 415 adults of Hispanic/Latino descent aged 18 to 74 years were enrolled from 4 US metropolitan areas from 2008 to 2011. The prevalence of DM was considerably diverse across adults with different Hispanic backgrounds. DM prevalence ranged from 10.2% in South Americans to 13.4% in Cubans, 17.7% in Central Americans, 18.0% in Dominicans and Puerto Ricans, and 18.3% in Mexicans.17
On the basis of 2014 BRFSS (CDC) data, the age-adjusted prevalence of adults in the United States who reported ever having been told by a physician that they had DM ranged from 6.9% in Vermont to 12.0% in West Virginia. The mean percentage among all states was 9.1%.18,19
On the basis of projections from NHANES studies between 1984 and 2004, the total prevalence of DM in the United States is expected to more than double from 2005 to 2050 (from 5.6% to 12.0%) in all age, sex, and race/ethnicity groups. Increases are projected to be largest for the oldest age groups (for instance, projected to increase by 220% among those 65–74 years of age and by 449% among those ≥75 years of age). DM prevalence is projected to increase by 99% among non-Hispanic whites, by 107% among non-Hispanic blacks, and by 127% among Hispanics. The age/race/ethnicity group with the largest increase is expected to be blacks ≥75 years of age (projected increase of 606%).20
Chart 10-2. Age-adjusted prevalence of physician-diagnosed diabetes mellitus in adults ≥20 years of age by race/ethnicity and years of education (NHANES 2011–2014).
NH indicates non-Hispanic; and NHANES, National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 10-3. Trends in diabetes mellitus prevalence in adults ≥20 years of age by sex (NHANES 1988–1994 and 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey.
*The definition of diabetes changed in 1997 (from glucose ≥140 mg/dL to ≥126 mg/dL).15
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Incidence
Youth
During 2008 to 2009, an estimated 18 436 people <20 years of age in the United States were newly diagnosed with type 1 DM annually, and 5089 individuals <20 years old were newly diagnosed with type 2 DM annually.3
In the SEARCH study, the incidence of DM in youths overall was 24.3 per 100 000 person-years. Of 2291 individuals <20 years of age with newly diagnosed DM, slightly more than half (54.5%) had autoimmune insulin-sensitive DM, and 15.9% had nonautoimmune insulin-resistant DM.21 The highest rates of incident type 1 DM were observed in non-Hispanic white youths (18.6, 28.1, and 32.9 per 100 000 person-years for age groups of 0–4, 5–9, and 10–14 years, respectively). Overall, type 2 DM was relatively infrequent among youths, with the highest rates (17.0–49.4 per 100 000 person-years) seen among 15- to 19-year-old minority groups.4
Projecting disease burden for the US population <20 years of age by 2050, the number of youths with type 1 DM will conservatively increase from 166 018 to 203 382, and the number with type 2 DM will increase from 20 203 to 30 111. Less conservative modeling projects the number of youths with type 1 DM at 587 488 and those with type 2 DM at 84 131 by 2050.22
Adults
(See Table 10-1)
A total of 1.7 million new cases of DM (type 1 or type 2) were diagnosed in US adults ≥20 years of age in 20123 (Table 10-1).
Data from the FHS indicate a doubling in the incidence of DM over the past 30 years, most dramatically during the 1990s. Among adults 40 to 55 years of age in each decade of the 1970s, 1980s, and 1990s, the age-adjusted 8-year incidence rates of DM were 2.0%, 3.0%, and 3.7% among females and 2.7%, 3.6%, and 5.8% among males, respectively. Compared with the 1970s, the age- and sex-adjusted OR for DM was 1.40 in the 1980s and 2.05 in the 1990s (P for trend=0.0006). Most of the increase in absolute incidence of DM occurred in individuals with a BMI ≥30 kg/m2 (P for trend=0.03).23
DM incidence in adults also varies markedly by race. Over 5 years of follow-up in 45- to 84-year-olds in MESA, 8.2% of the cohort developed DM. The cumulative incidence was highest in Hispanics (11.3%), followed by black (9.5%), Chinese (7.7%), and white (6.3%) participants.24
Of 15.4 million people being treated with glucose-lowering medication (86.6% of the diagnosed diabetic population), 8.5 million (55.2%) had their hyperglycemia under control (ie, had calibrated HbA1c <7%), and 6.9 million (44.8%) were being treated but had HbA1c ≥7%. An estimated 2.4 million individuals with diagnosed DM are not treated with glucose-lowering therapy.13
According to data from NHANES and BRFSS, up to 48.7% of individuals with self-reported DM did not meet glycemic, BP, and lipid targets, and only 14.3% met all 3 targets and did not smoke.25
Gestational DM complicates 2% to 10% of pregnancies and increases the risk of developing type 2 DM by 35% to 60%.16
Mortality
(See Table 10-1)
DM was listed as the underlying cause of mortality for 76 488 people in the United States in 2014 (Table 10-1). There were 245 016 deaths with DM as the primary or secondary cause of death in 2014.26
The 2014 overall underlying-cause death rate attributable to DM was 20.9 per 100 000. Death rates per 100 000 population were 23.4 for non-Hispanic white males, 43.9 for non-Hispanic black males, 30.0 for Hispanic males, 18.0 for non-Hispanic Asian/Pacific Islander males, 48.2 for non-Hispanic American Indian/Alaska Native males, 14.6 for non-Hispanic white females, 34.0 for non-Hispanic black females, 21.3 for Hispanic females, 12.8 for non-Hispanic Asian/Pacific Islander females, and 35.4 for non-Hispanic American Indian/Pacific Islander females.26
-
According to data from the CDC, the National Diabetes Information Clearinghouse, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institutes of Health:
At least 68% of people >65 years of age with DM die of some form of HD; 16% die of stroke.
HD death rates among adults with DM are 2 to 4 times higher than the rates for adults without DM.16
In a collaborative meta-analysis of 820 900 individuals from 97 prospective studies, DM was associated with the following risks: all-cause mortality (HR, 1.80; 95% CI, 1.71–1.90), cancer death (HR, 1.25; 95% CI, 1.19–1.31), and vascular death (HR, 2.32; 95% CI, 2.11–2.56). In particular, DM was associated with death attributable to the following cancers: liver, pancreas, ovary, colorectal, lung, bladder, and breast. A 50-year-old with DM died on average 6 years earlier than an individual without DM.27
FHS/NHLBI data showed that having DM significantly increased the risk of developing CVD (HR 2.5 for females and 2.4 for males) and of dying when CVD was present (HR 2.2 for females and 1.7 for males). Males and females ≥50 years of age with DM lived an average of 7.5 and 8.2 years less than their counterparts without DM. The differences in life expectancy free of CVD were 7.8 and 8.4 years, respectively.28
Analysis of data from the FHS from 1950 to 2005 found reductions in all-cause and CVD mortality among males and females with and without DM; however, all-cause and CVD mortality rates among individuals with DM remain ≈2-fold higher than for individuals without DM.29
According to NHIS linked data from 1997 to 2006, the rate of CVD death among adults with DM decreased by 40% (95% CI, 23%–54%). Similarly, all-cause mortality decreased by 23% (95% CI, 10%–35%). In contrast, over this same period among adults without DM, the CVD mortality rate decreased by 60%, and the all-cause mortality rate decreased by 44%.30
Awareness
(See Chart 10-5)
Chart 10-5. Diabetes mellitus awareness, treatment, and control in adults ≥20 years of age (NHANES 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Heart, Lung, and Blood Institute.
On the basis of NHANES 2011 to 2014 data for adults with DM, 20.8% had their DM treated and controlled, 46.4% had their DM treated but uncontrolled, 9.9% were aware they had DM but were not treated, and 22.9% were undiagnosed and not treated (Chart 10-5; unpublished NHLBI tabulations).
Although the prevalence of diagnosed DM has increased significantly over the past decade, the numbers of adults with undiagnosed DM and impaired fasting glucose has remained relatively stable. Of the estimated 21 million adults with DM, 84.8% were told they had DM or were undergoing treatment, and 11% (2.3 million) of those with confirmed DM (calibrated HbA1c level ≥6.5% and fasting plasma glucose level ≥126 mg/dL) were unaware of the diagnosis.13 In the HCHS/SOL population-based study of adults of Hispanic/Latino descent, only 58.7% of participants with DM were aware of their diagnosis.17
The prevalence of undiagnosed DM among patients with MI was assessed with data from the TRIUMPH US multicenter AMI registry with data collection from 2005 to 2008. This study revealed that DM that had not been previously diagnosed affected 1 in 10 patients based on research core laboratory testing of HbA1c, yet DM was diagnosed by the care team only one third of the time.
Aftermath
(See Chart 10-6)
Although the exact date of DM onset can be difficult to determine, increasing duration of DM diagnosis is associated with increasing CVD risk. Longitudinal data from the FHS suggest that the risk factor–adjusted RR of CHD is 1.38 (95% CI, 0.99–1.92) times higher and the risk for CHD death is 1.86 (95% CI, 1.17–2.93) times higher for each 10-year increase in duration of DM.31
-
On the basis of data from the NCHS/NHIS, 1997 to 200532:
The estimated number of people ≥35 years of age with DM with a self-reported cardiovascular condition increased 36%, from 4.2 million in 1997 to 5.7 million in 2005; however, the respective age-adjusted prevalence decreased 11.2%, from 36.6% in 1997 to 32.5% in 2005, which reflects an increase in the number of patients diagnosed with DM that exceeded the increase in CVD prevalence.
Age-adjusted CVD prevalence was higher among males than among females, among whites than among blacks, and among non-Hispanic than among Hispanics. Among females, the age-adjusted prevalence decreased by 11.2%; among males, it did not decrease significantly. Among blacks, the age-adjusted prevalence of self-reported CVD decreased by 25.3%; among whites, no significant decrease occurred; among non-Hispanic, the rate decreased by 12%. No clear trends were detected among Hispanics.
Because the total number of people with DM and self-reported CVD increased over this period but proportions with self-reported CVD declined, the data suggest that the mean age at which people are diagnosed with DM is decreasing, or the higher CVD mortality rate among older individuals with DM is removing them from the ability to self-report CVD. These and other data show a consistent increase over time in the United States of the number of people with DM and CVD.
-
Data from the FHS show that despite improvements in CVD morbidity and mortality over >4 decades of observation, DM continues to be associated with incremental CVD risk. Participants 45 to 64 years of age from the FHS original and offspring cohorts who attended examinations in 1950 to 1966 (“earlier” time period) and in 1977 to 1995 (“later” time period) were followed up for incident MI, CHD death, and stroke. Among participants with DM, the age- and sex-adjusted CVD incidence rate was 286.4 per 10 000 person-years in the earlier period and 146.9 per 10 000 person-years in the later period, a 35.4% decline. HRs for DM as a predictor of incident CVD were not significantly different in the earlier (risk factor–adjusted HR, 2.68; 95% CI, 1.88–3.82) versus later (HR, 1.96; 95% CI, 1.44–2.66) period. Thus, although there was a 50% reduction in the rate of incident CVD events among adults with DM, the absolute risk of CVD remained 2-fold greater than among people without DM.33
Data from these earlier and later time periods in the FHS also suggest that the increasing prevalence of DM is leading to an increasing rate of CVD, resulting in part from CVD risk factors that commonly accompany DM. The age- and sex-adjusted HR for DM as a CVD risk factor was 3.0 in the earlier time period and 2.5 in the later time period. Because the prevalence of DM has increased over time, the PAR for DM as a CVD risk factor increased from 5.4% in the earlier time period to 8.7% in the later time period (attributable risk ratio, 1.62; P=0.04). Adjustment for CVD risk factors (age, sex, hypertension, current smoking, high cholesterol, and obesity) weakened this attributable risk ratio to 1.5 (P=0.12).34
Other data from the FHS show that over a 30-year period, CVD among females with DM was 54.8% among normal-weight females but 78.8% among obese females. Among normal-weight males with DM, the lifetime risk of CVD was 78.6%, whereas it was 86.9% among obese males.35
In analyses from the NRMI comprising data registered on 1 734 431 patients admitted with AMI to 1964 participating US hospitals, the incremental adjusted OR for hospital mortality associated with DM declined from 1.24 (95% CI, 1.16–1.32) in 1994 to 1.08 (95% CI, 0.99–1.19) in 2006, which demonstrates a closing of the acute hospital mortality gap associated with DM.36
On the basis of analyses of data from the NHIS, the NHDS, the USRDS, and the US National Vital Statistics System, between 1990 and 2010, the rate of incident MI among patients with DM declined 67.8% (Chart 10-6).37
A subgroup analysis was conducted of patients with DM enrolled in randomized clinical trials that evaluated ACS therapies. The data included 62 036 patients from TIMI studies (46 577 with STEMI and 15 459 with UA/NSTEMI). Of these, 17.1% had DM. Modeling showed that mortality at 30 days was significantly higher among patients with DM than among those without DM who presented with UA/NSTEMI (2.1% versus 1.1%; P≤0.001) and STEMI (8.5% versus 5.4%; P=0.001), with adjusted risks for 30-day mortality in DM versus no DM of 1.78 for UA/NSTEMI (95% CI, 1.24–2.56) and 1.40 (95% CI, 1.24–1.57) for STEMI. DM was also associated with significantly higher mortality 1 year after UA/NSTEMI or STEMI. By 1 year after ACS, patients with DM who presented with UA/NSTEMI had a risk of death that approached that of patients without DM who presented with STEMI (7.2% versus 8.1%).38
-
DM increases the risk of HF and adversely affects outcomes among patients with HF.
DM alone qualifies for the most recent ACC/AHA diagnostic criteria for stages A and B HF, a classification of patients without HF but at notably high risk for its development.39
In MESA, DM was associated with a 2-fold increased adjusted risk of incident HF among 6814 individuals free of CVD at baseline over a mean follow-up of 4 years (HR, 1.99; 95% CI, 1.08–3.68).40
Post hoc analysis of data from the EVEREST randomized trial of patients hospitalized with decompensated systolic HF stratified by DM status, which evaluated cardiovascular outcomes over a follow-up period of 9.9 months, demonstrated an increased adjusted HR for the composite of cardiovascular mortality and HF rehospitalization associated with DM (HR, 1.17; 95% CI, 1.04–1.31).41
DM increases the risk of AF. On the basis of a meta-analysis of published observational data comprising 11 studies and >1.6 million participants, DM was associated with a 39% increased risk for AF (RR, 1.39; 95% CI, 1.10–1.75), with the association remaining statistically significant after multivariable adjustment (adjusted RR, 1.24; 95% CI, 1.06–1.44), yielding an estimate of the population attributable fraction of AF attributable to DM of 2.5%.42
-
DM increases the risk of stroke, with the RR ranging from 1.8- to 6-fold increased risk.31,43
DM is associated with increased ischemic stroke incidence at all ages, with the incremental risk associated with DM being most prominent before 55 years of age in blacks and before 65 years of age in whites.43
Ischemic stroke patients with DM are younger, more likely to be black, and more likely to have hypertension, prior MI, and high cholesterol than patients without DM.43
-
On the basis of analyses of data from the NHIS, the NHDS, the USRDS, and the US National Vital Statistics System, between 1990 and 2010
The age-standardized rate of incident stroke among patients with DM declined 52.7% (Chart 10-6).37
The age-standardized rate of incident ESRD among patients with DM declined 28.3% (Chart 10-6).37
The relative decline in the age-standardized rate is 51.4% for amputation and 64.4% for death due to hyperglycemic crisis (Chart 10-6).37
DM accounted for 44% of the new cases of ESRD in 2011.44
In 2012, the incidence rate of ESRD attributed to DM in adults ≥20 years in the Veterans Affairs health system increased with age, from 4.44 per 100 000 in those aged 20 to 29 years to 110.35 per 100 000 in those ≥70 years old, compared with rates of 2.40 and 81.88, respectively, in those without DM.45
HbA1c levels ≥6.5% can be used to diagnose DM.46 In the population-based ARIC study, over a 14-year follow-up period that preceded the endorsement of HbA1c as a diagnostic criterion, HbA1c levels ≥6.5% at study entry were associated with a multivariable-adjusted HR of 16.5 (95% CI, 14.2–19.1) for diagnosed DM based on contemporaneous diagnostic criteria and 1.95 (95% CI, 1.53–2.48) for CHD relative to those with HbA1c <5.0%.47
Risk Factors for Developing DM
Risk for developing type 2 DM is higher in males than in females even after accounting for other risk factors.48–51
Analysis of data from the NHLBI-funded CHS found that lifestyle risk factors assessed late in life, including PA level, dietary habits, smoking habits, alcohol use, and adiposity measures, were each independently associated with risk of new-onset DM. Participants whose PA level and dietary, smoking, and alcohol habits were all in the low-risk group had an 82% lower incidence of DM than all other participants. When absence of adiposity was added to the other 4 low-risk lifestyle factors, incidence of DM was 89% lower.52
A recent large meta-analysis suggests that exercise interventions significantly improved lipid profile, glucose tolerance, and insulin sensitivity among healthy adults.53 In a study of 69 885 patients referred for treadmill testing in a single US healthcare system, higher fitness was associated with a lower risk of incident DM regardless of demographic characteristics and baseline risk factors.54 However, according to 2007 data from the BRFSS, only 25% of adults with DM achieved recommended levels of total PA based on the 2007 American Diabetes Association guidelines.55
On the basis of meta-analyses of 4 longitudinal cohort studies comprising 175 938 individuals and 1.1 million person-years of follow-up, a statistically significant adjusted association was observed between net duration of television viewing and risk for incident type 2 DM, with a 20% increased risk per each 2-hour daily increment of exposure (adjusted RR, 1.20; 95% CI, 1.14–1.27).56
A recent study, which used mediation analysis methodologies, found that the effects of low birth weight on type 2 DM risk appears to be mediated mainly by insulin resistance, which is further explained by circulating levels of sex hormone–binding globulin and E-selectin, as well as SBP.57
Treatment of CVD Among Patients With DM
DM, especially type 2 DM, is associated with clustered risk factors for CHD, with a prevalence of 75% to 85% for hypertension among adults with DM, 70% to 80% for elevated LDL-C, and 60% to 70% for obesity.13,58
Aggressive treatment of hypertension is recommended for adults with DM to prevent cardiovascular complications.59 Between NHANES III (1984–1992) and NHANES 1999 to 2004, the proportion of patients with DM whose BP was treated increased from 76.5% to 87.8%, and the proportion whose BP was controlled nearly doubled (from 15.9% to 29.6%).60
Aggressive treatment of hypercholesterolemia is recommended for adults with DM, with the cornerstone of treatment being statin therapy, which is recommended for all patients with DM >40 years of age independent of baseline cholesterol, with at least a moderate dose of statin therapy.61
CHD risk factors among patients with DM remain suboptimally treated, although improvements have been observed over the past decade. Between 1999 to 2000 and 2007 to 2008, in up to 2623 adult participants with DM, data from NHANES showed that improvements were observed for the achieved targets for control of HbA1c (from 37.0% to 55.2%), BP (from 35.2% to 51.0%), and LDL-C (from 32.5% to 52.9%).62
Data from the 2012 National Healthcare Disparities Report (AHRQ, US Department of Health and Human Services) found that only ≈23% of adults >40 years of age with DM received all 4 interventions (≥2 HbA1c tests, foot examination, dilated eye examination, and flu shot) to reduce risk factors recommended for comprehensive DM care in 2009. The proportion receiving all 4 interventions was lower among blacks and Hispanics than whites.63
In multivariable models, among those aged 40 to 64 years, only ≈65% had BP <140/80 mm Hg, with blacks less likely than whites to achieve this BP level.63
In 1 large academic medical center, outpatients with type 2 DM were observed during an 18-month period for proportions of patients who had HbA1c levels, BP, or TC levels measured; who had been prescribed any drug therapy if HbA1c levels, SBP, or LDL-C levels exceeded recommended treatment goals; and who had been prescribed greater-than-starting-dose therapy if these values were above treatment goals. Patients were less likely to have cholesterol levels measured (76%) than HbA1c levels (92%) or BP (99%; P<0.0001 for either comparison). The proportion of patients who received any drug therapy was greater for above-goal HbA1c (92%) than for above-goal SBP (78%) or LDL-C (38%; P<0.0001 for each comparison). Similarly, patients whose HbA1c levels were above the treatment goal (80%) were more likely to receive greater-than-starting-dose therapy than were those who had above-goal SBP (62%) and LDL-C levels (13%; P<0.0001).64
CVD risk factors among females with DM were managed less aggressively than among males with DM. Females were less likely than males to have HbA1c <7% (without CHD: adjusted OR for females versus males 0.84, P=0.005; with CHD: 0.63, P<0.0001). Females without CHD were less likely than males to be treated with lipid-lowering medication (0.82; P=0.01) or, when treated, to have LDL-C levels <100 mg/dL (0.75; P=0.004), and they were less likely than males to be prescribed aspirin (0.63; P<0.0001). Females with DM and CHD were less likely than males to be prescribed aspirin (0.70; P<0.0001) and when treated for hypertension or hyperlipidemia were less likely to have BP levels <130/80 mm Hg (0.75; P<0.0001) or LDL-C levels <100 mg/dL (0.80; P=0.006).65
Hospitalizations
(See Table 10-1)
Youth
NIS data from 1993 to 2004 were analyzed for individuals 0 to 29 years of age with a diagnosis of DM. Rates of hospitalizations increased by 38%. Hospitalization rates were higher for females (42%) than for males (29%). Inflation-adjusted total charges for DM hospitalizations increased 130%, from $1.05 billion in 1993 to $2.42 billion in 2004.66
Adults
According to NHDS data reported by the CDC in an analysis of data from 2010, DM was a listed diagnosis in 16% of US adult hospital discharges. Of the 5.1 million discharges with DM listed in the 2010 data, circulatory diseases were the most common first-listed diagnosis (24.1%; 1.3 million discharges) and DM the second most common (11.5%; 610 000 discharges).67
Hypoglycemia
Hypoglycemia is a common side effect of DM treatment, typically defined as a blood glucose level <50 mg/dL; severe hypoglycemia is additionally defined as patients who need assistance to treat themselves. In the ADVANCE trial, 2.1% of the DM patients had an episode of severe hypoglycemia.
Severe hypoglycemia was associated with an increased risk of major macrovascular events (HR, 2.88; 95% CI, 2.01–4.12), cardiovascular death (HR, 2.68; 95% CI, 1.72–4.19), and all-cause death (HR, 2.69; 95% CI, 1.97–3.67), including nonvascular outcomes. The lack of specificity of hypoglycemia with vascular outcomes suggests that it might be a marker for susceptibility. In the ADVANCE trial, risk factors for hypoglycemia included older age, DM duration, worse renal function, lower BMI, lower cognitive function, use of multiple glucose-lowering medications, and randomization to the intensive glucose control arm.68
According to data from the 2004 to 2008 MarketScan database, which included 536 581 individuals with type 2 DM, the incidence rate of hypoglycemia was 153.8 per 10 000 person-years and was highest in adults aged 18 to 34 years (218.8 per 10 000 person-years).69
Cost
(See Table 10-1)
In 2012, the cost of DM was estimated at $245 billion (Table 10-1), up from $174 billion in 2007, accounting for 1 in 5 healthcare dollars.70 Of these costs, $176 billion were direct medical costs and $69 billion resulted from reduced productivity. Inpatient care accounted for 43% of these costs, 18% were attributable to prescription costs to treat DM complications, and 12% were related to anti-DM agents and supplies.70
After adjustment for age and sex, medical costs for patients with DM were 2.3 times higher than for people without DM.16
According to the insurance claims and MarketScan data from 7556 youth <19 years of age with insulin-treated DM, costs for youths with hypoglycemia were $12 850 compared with $8970 for youths without hypoglycemia. For diabetic ketoacidosis, costs were $14 236 for youths with versus $8398 for youths without diabetic ketoacidosis.71
The cost of hypoglycemia, according to data from 536 581 individuals with type 2 DM from the 2004 to 2008 MarketScan database, was $52 223 675, which accounted for 1.0% of inpatient costs, 2.7% of ED costs, and 0.3% of outpatient costs. This resulted in a mean cost of $17 564 for an inpatient admission, $1387 for an ED visit, and $394 for an outpatient visit.69
Type 1 DM
Type 1 DM constitutes 5% to 10% of DM in the United States.72
The Colorado IDDM Study Registry and SEARCH for Diabetes in Youth registry demonstrated an increasing incidence of type 1 DM among Colorado youths ≤17 years of age, with an increase in the incidence of 2.3% (95% CI, 1.6%–3.1%) per year over the past 26 years.73
Between 1996 and 2010, the number of youths with type 1 DM increased by 5.7% per year.74
Among youths with type 1 DM, the prevalence of overweight is 22.1% and the prevalence of obesity is 12.6%.7
A long-term study of patients with type 1 DM that began in 1966 showed that over 30 years of follow-up, overall risk of mortality associated with type 1 DM was 7 times greater than that of the general population. Females had a 13.2-fold incremental mortality risk compared with a 5.0-fold increased risk in males. During the course of study, the incremental mortality risk associated with type 1 DM declined from 9.3 to 5.6 times that of nondiabetic control subjects.75
According to 30-year mortality data from Allegheny County, PA, those with type 1 DM have a mortality rate 5.6 times higher than the general population.76
The leading cause of death among patients with type 1 DM is CVD, which accounted for 22% of deaths among those in the Allegheny County, PA, type 1 DM registry, followed by renal (20%) and infectious (18%) causes.77
Long-term follow-up data from the DCCT/EDIC Study Research Group showed that intensive versus conventional treatment in the DCCT was associated with a 42% reduced risk of CVD (P=0.02) and a 57% reduced risk of the composite end point (P=0.02; included nonfatal MI, stroke, and CVD death).78
Among 3610 older patients (>60 years of age) with type 1 DM, the risk of severe hypoglycemia was twice as high as for those <60 years of age (40.1 versus 24.3 per 100 patient-years).79
Global Burden of DM
The prevalence of DM for adults worldwide was estimated to be 6.4% in 2010 and is projected to be 7.7% in 2030. The total number of people with DM is projected to rise from 285 million in 2010 to 439 million in 2030.80
According to international survey and epidemiological data from 2.7 million participants, the prevalence of DM in adults increased from 8.3% in males and 7.5% in females in 1980 to 9.8% in males and 9.2% in females in 2008. The number of individuals affected with DM increased from 153 million in 1980 to 347 million in 2008.81
The prevalence of prediabetes is high among Indians living in the United States and might be higher than the prevalence of prediabetes among Indians living in India. In a comparison of the MASALA and CARRS studies, the prevalence of prediabetes was 33% in the United States sample and 24% in the Chennai, India, sample.82 A low amount of exercise was most strongly associated with prediabetes in MASALA.83
In 2010, DM and other endocrine disorders caused >2.7 million deaths worldwide, accounting for 5.2% of all deaths.84
REFERENCES
- 1.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research, and the American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691–718. doi: 10.1161/CIR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 4.Writing Group for the SEARCH for Diabetes in Youth Study Group. Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States [published correction appears in JAMA. 2007;298:627] JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 5.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care. 2008;31:2044–2049. doi: 10.2337/dc08-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, Dabelea D, Hamman R, Waitzfelder B, Kahn HS SEARCH for Diabetes in Youth Study Group. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 8.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 9.Gore MO, Eason SJ, Ayers CR, Turer A, Khera A, de Lemos JA, McGuire DK, Sayers M. Glycated hemoglobin in 14,850 adolescent blood donors: a pilot screening program. Diabetes Care. 2014;37:e3–e4. doi: 10.2337/dc13-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriska A, Delahanty L, Edelstein S, Amodei N, Chadwick J, Copeland K, Galvin B, El ghormli L, Haymond M, Kelsey M, Lassiter C, Mayer-Davis E, Milaszewski K, Syme A. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131:e850–e856. doi: 10.1542/peds.2012-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng YJ, Imperatore G, Geiss LS, Wang J, Saydah SH, Cowie CC, Gregg EW. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988–2010. Diabetes Care. 2013;36:2690–2696. doi: 10.2337/dc12-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006 [published correction appears in Diabetes Care. 2011;34:2338] Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 17.Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello AL, Heiss G, Kaplan RC, LaVange LM, Teng Y, Villa-Caballero L, Avilés-Santa ML. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2014;37:2233–2239. doi: 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Web site. [Accessed September 1, 2014];Behavioral Risk Factor Surveillance System: annual survey data. 2013 http://www.cdc.gov/brfss/annual_data/annual_2013.html.
- 19.Diagnosed Diabetes. United States Diabetes Surveillance System, Division of Diabetes Translation. [Accessed August 24, 2016];Centers for Disease Control and Prevention Web site. 2016 http://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html.
- 20.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 21.Dabelea D, Pihoker C, Talton JW, D’Agostino RB, Jr, Fujimoto W, Klingensmith GJ, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Imperatore G, Dolan LM SEARCH for Diabetes in Youth Study. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515–2520. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 24.Nettleton JA, Steffen LM, Ni H, Liu K, Jacobs DR., Jr Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31:1777–1782. doi: 10.2337/dc08-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010 [published correction appears in N Engl J Med. 2013;369:587] N Engl J Med. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. [Accessed May 19, 2015];Mortality multiple cause micro-data files, 2013: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 27.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death [published correction appears in N Engl J Med. 2011;364:1281] N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 29.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULA-TIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, Barker L. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35:1252–1257. doi: 10.2337/dc11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research, Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2011;42:e26] Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Prevalence of self-reported cardiovascular disease among persons aged > or =35 years with diabetes: United States, 1997–2005. MMWR Morb Mortal Wkly Rep. 2007;56:1129–1132. [PubMed] [Google Scholar]
- 33.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D’Agostino RB, Sr, Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 34.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 35.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB., Sr Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31:1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booth GL, Kapral MK, Fung K, Tu JV. Recent trends in cardiovascular complications among men and women with and without diabetes. Diabetes Care. 2006;29:32–37. doi: 10.2337/diacare.29.01.06.dc05-0776. [DOI] [PubMed] [Google Scholar]
- 37.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 38.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 39.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2010;121:e258] Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 40.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 41.Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M EVEREST Investigators. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15:194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 44.United States Renal Data System. [Accessed September 18, 2014];2013 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease. 2013 http://www.usrds.org/atlas.aspx.
- 45.Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System: United States. Atlanta, GA: US Department of Health and Human Services; 2012. [Accessed July 11, 2014]. http://nccd.cdc.gov/CKD/detail.aspx?QNum=Q86. [Google Scholar]
- 46.American Diabetes Association. Diagnosis and classification of diabetes mellitus [published correction appears in Diabetes Care. 2010;33:e57] Diabetes Care. 2010;33(suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 49.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, Kerrison N, Ekelund U, Barroso I, Panico S, Tormo MJ, Spranger J, Griffin S, van der Schouw YT, Amiano P, Ardanaz E, Arriola L, Balkau B, Barricarte A, Beulens JW, Boeing H, Bueno-de-Mesquita HB, Buijsse B, Chirlaque Lopez MD, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillan B, Deloukas P, Dorronsoro M, Drogan D, Froguel P, Gonzalez C, Grioni S, Groop L, Groves C, Hainaut P, Halkjaer J, Hallmans G, Hansen T, Huerta Castaño JM, Kaaks R, Key TJ, Khaw KT, Koulman A, Mattiello A, Navarro C, Nilsson P, Norat T, Overvad K, Palla L, Palli D, Pedersen O, Peeters PH, Quirós JR, Ramachandran A, Rodriguez-Suarez L, Rolandsson O, Romaguera D, Romieu I, Sacerdote C, Sánchez MJ, Sandbaek A, Slimani N, Sluijs I, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, van der ADL, Verschuren WM, Tuomilehto J, Feskens E, McCarthy M, Riboli E, Wareham NJ InterAct Consortium. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54:2272–2282. doi: 10.1007/s00125-011-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 52.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002014. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juraschek SP, Blaha MJ, Blumenthal RS, Brawner C, Qureshi W, Keteyian SJ, Schairer J, Ehrman JK, Al-Mallah MH. Cardiorespiratory fitness and incident diabetes: the FIT (Henry Ford ExercIse Testing) project. Diabetes Care. 2015;38:1075–1081. doi: 10.2337/dc14-2714. [DOI] [PubMed] [Google Scholar]
- 55.Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc. 2011;59:132–137. doi: 10.1111/j.1532-5415.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- 56.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Y, Huang YT, Song Y, Hevener AL, Ryckman KK, Qi L, LeBlanc ES, Kazlauskaite R, Brennan KM, Liu S. Birthweight, mediating biomarkers and the development of type 2 diabetes later in life: a prospective study of multi-ethnic women. Diabetologia. 2015;58:1220–1230. doi: 10.1007/s00125-014-3479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) [published correction appears in JAMA. 2014;311:1809] JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 60.Suh DC, Kim CM, Choi IS, Plauschinat CA, Barone JA. Trends in blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988–2004. J Hypertens. 2009;27:1908–1916. doi: 10.1097/HJH.0b013e32832d4aee. [DOI] [PubMed] [Google Scholar]
- 61.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2015;132:e396 and Circulation. 2014;129:S46–S48] Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 62.Ford ES. Trends in the control of risk factors for cardiovascular disease among adults with diagnosed diabetes: findings from the National Health and Nutrition Examination Survey 1999–2008. J Diabetes. 2011;3:337–347. doi: 10.1111/j.1753-0407.2011.00148.x. [DOI] [PubMed] [Google Scholar]
- 63.US Department of Health and Human Services, Agency for Healthcare Research and Quality. National Healthcare Disparities Report, 2012. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [Accessed December 19, 2016]. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr12/2012nhdr.pdf. [Google Scholar]
- 64.Grant RW, Cagliero E, Murphy-Sheehy P, Singer DE, Nathan DM, Meigs JB. Comparison of hyperglycemia, hypertension, and hypercholesterolemia management in patients with type 2 diabetes. Am J Med. 2002;112:603–609. doi: 10.1016/s0002-9343(02)01103-8. [DOI] [PubMed] [Google Scholar]
- 65.Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28:514–520. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 66.Lee JM, Okumura MJ, Freed GL, Menon RK, Davis MM. Trends in hospitalizations for diabetes among children and young adults: United States, 1993–2004. Diabetes Care. 2007;30:3035–3039. doi: 10.2337/dc07-0769. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, Division of Health Care Statistics. [Accessed July 22, 2013];Distribution of first-listed diagnoses among hospital discharges with diabetes as any listed diagnosis, adults aged 18 years and older, United States. 2010 http://www.cdc.gov/diabetes/statistics/hosp/adulttable1.htm.
- 68.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 69.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care. 2011;17:673–680. [PubMed] [Google Scholar]
- 70.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shrestha SS, Zhang P, Barker L, Imperatore G. Medical expenditures associated with diabetes acute complications in privately insured U.S. youth. Diabetes Care. 2010;33:2617–2622. doi: 10.2337/dc10-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Redberg RF, Greenland P, Fuster V, Pyörälä K, Blair SN, Folsom AR, Newman AB, O’Leary DH, Orchard TJ, Psaty B, Schwartz JS, Starke R, Wilson PW. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group III: risk assessment in persons with diabetes. Circulation. 2002;105:e144–e152. doi: 10.1161/01.cir.0000013955.34262.af. [DOI] [PubMed] [Google Scholar]
- 73.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 74.Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr. 2012;161:652–7.e1. doi: 10.1016/j.jpeds.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 75.Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study: mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 76.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33:2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216–3222. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schütt M, Fach EM, Seufert J, Kerner W, Lang W, Zeyfang A, Welp R, Holl RW DPV Initiative and the German BMBF Competence Network Diabetes Mellitus. Multiple complications and frequent severe hypoglycaemia in “elderly” and “old” patients with type 1 diabetes. Diabet Med. 2012;29:e176–e179. doi: 10.1111/j.1464-5491.2012.03681.x. [DOI] [PubMed] [Google Scholar]
- 80.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 82.Gujral UP, Narayan KM, Pradeepa RG, Deepa M, Ali MK, Anjana RM, Kandula NR, Mohan V, Kanaya AM. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: the CARRS and MASALA studies. Diabetes Care. 2015;38:1312–1318. doi: 10.2337/dc15-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah AD, Vittinghoff E, Kandula NR, Srivastava S, Kanaya AM. Correlates of prediabetes and type II diabetes in US South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Ann Epidemiol. 2015;25:77–83. doi: 10.1016/j.annepidem.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.University of Washington, Institute for Health Metrics and Evaluation. Global Burden of Disease Data Visualizations. GBD Compare. [Accessed July 31, 2014];Global, deaths, both sexes, all ages. 2010 http://vizhub.healthdata.org/gbd-compare/
11. METABOLIC SYNDROME
See Charts 11-1 through 11-10
Chart 11-1. Secular trend of metabolic syndrome components in youth in the NHANES and KNHANES cohorts.
BP indicates blood pressure; HDL, high-density lipoprotein cholesterol; KNHANES, Korean National Health and Nutrition Examination Survey; NHANES, National Health and Nutrition Examination Survey; TG, triglycerides; and WC, waist circumference.
*Significant difference between NHANES 2003 to 2006 and NHANES III. †Significant difference between NHANES 2003 to 2006 and NHANES 1999 to 2002.
‡Significant difference between KNHANES 2007 and KNHANES 1998. §Significant difference between KNHANES 2007 and KNHANES 2001.
Reproduced with permission from from Lim et al.15 Copyright © 2013, by the American Academy of Pediatrics.
Chart 11-10. Ten-year progression of metabolic syndrome in the ARIC study, stratified by age, sex, and race/ethnicity.
ARIC indicates Atherosclerosis Risk in Communities.
Reprinted from Vishnu et al72 with permission from Elsevier. Copyright © 2015, Elsevier Ireland Ltd.
Abbreviations Used in Chapter 11
| AF | atrial fibrillation |
| AHA | American Heart Association |
| ARIC | Atherosclerosis Risk in Communities |
| ATP III | Adult Treatment Panel III |
| BIOSHARE-EU | Biobank Standardization and Harmonization for Research Excellence in the European Union |
| BMI | body mass index |
| BP | blood pressure |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| CARRS | Center for Cardiometabolic Risk Reduction in South Asia |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CHRIS | Collaborative Health Research in South Tyrol Study |
| CI | confidence interval |
| COURAGE | Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation |
| CRP | C-reactive protein |
| CT | computed tomography |
| CVD | cardiovascular disease |
| DESIR | Data From an Epidemiological Study on the Insulin Resistance Syndrome |
| DILGOM | Dietary, Lifestyle, and Genetics Determinants of Obesity and Metabolic Syndrome |
| DM | diabetes mellitus |
| ECG | electrocardiogram |
| EGCUT | Estonian Genome Center of the University of Tartu |
| GFR | glomerular filtration rate |
| HCHS/SOL | Hispanic Community Health Study/Study of Latinos |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HIV | human immunodeficiency virus |
| HR | hazard ratio |
| HUNT2 | Nord-Trøndelag Health Study |
| IMT | intima-media thickness |
| KNHANES | Korean National Health and Nutrition Examination Survey |
| KORA | Cooperative Health Research in the Region of Augsburg |
| LDL-C | low-density lipoprotein cholesterol |
| LV | left ventricular |
| MASALA | Mediators of Atherosclerosis in South Asians Living in America |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MetS | metabolic syndrome |
| Mex.-Am. | Mexican American |
| MHO | metabolically healthy obesity |
| MI | myocardial infarction |
| MICROS | Microisolates in South Tyrol Study |
| MORGAM | MONICA [Monitoring Trends and Determinants in Cardiovascular Disease], Risk, Genetics, Archiving and Monograph Project |
| MRI | magnetic resonance imaging |
| NCDS | National Child Development Study |
| NHANES | National Health and Nutrition Examination Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIPPON DATA | National Integrated Project for Prospective Observation of Noncommunicable Disease and Its Trends in Aged |
| NL | The Netherlands |
| OR | odds ratio |
| PA | physical activity |
| PAD | peripheral artery disease |
| PAR | population attributable risk |
| PREVEND | Prevention of Renal and Vascular End-Stage Disease |
| RCT | randomized controlled trial |
| RR | relative risk |
| RV | right ventricular |
| TG | triglycerides |
| VTE | venous thromboembolism |
| Waist circumf. | waist circumference |
| WC | waist circumference |
| WHO | World Health Organization |
-
Metabolic syndrome is a multicomponent risk factor for CVD and type 2 DM that reflects the clustering of individual cardiometabolic risk factors related to abdominal obesity and insulin resistance. Clinically, metabolic syndrome is a useful entity for communicating the nature of lifestyle-related cardiometabolic risk to both patients and other clinicians. Although several different clinical definitions for metabolic syndrome have been proposed, the International Diabetes Federation, NHLBI, AHA, and others recently proposed a harmonized definition for metabolic syndrome.1 By this definition, metabolic syndrome is diagnosed when any 3 of the following 5 risk factors are present:
Fasting plasma glucose ≥100 mg/dL or undergoing drug treatment for elevated glucose
HDL-C <40 mg/dL in males or <50 mg/dL in females or undergoing drug treatment for reduced HDL-C
Triglycerides ≥150 mg/dL or undergoing drug treatment for elevated triglycerides
Waist circumference >102 cm in males or >88 cm in females for people of most ancestries living in the United States. Ethnicity and country-specific thresholds can be used for diagnosis in other groups, particularly Asians and individuals of non-European ancestry who have predominantly resided outside the United States.
BP ≥130 mm Hg systolic or ≥85 mm Hg diastolic or undergoing drug treatment for hypertension, or antihypertensive drug treatment in a patient with a history of hypertension.
The new harmonized metabolic syndrome definition identifies a similar risk group and predicts CVD risk similarly to the prior metabolic syndrome definitions.2
There are many adverse health conditions that are related to metabolic syndrome but are not part of its clinical definition. These include nonalcoholic fatty liver disease, sexual/reproductive dysfunction (erectile dysfunction in males and polycystic ovarian syndrome in females), obstructive sleep apnea, certain forms of cancer, and possibly osteoarthritis, as well as a general proinflammatory and prothrombotic state.3
Those with a fasting glucose level ≥126 mg/dL or a casual glucose value ≥200 mg/dL or taking hypoglycemic medication will normally be classified separately as having DM; many of these people will also have metabolic syndrome because of the presence of ≥2 of the additional risk factors noted above. For treatment purposes, many will prefer to separate those with DM into a separate group.
Identification and treatment of metabolic syndrome fits closely with the current AHA 2020 Impact Goals, including emphasis on PA, healthy diet, and healthy weight for attainment of ideal BP, serum cholesterol, and fasting blood glucose. Metabolic syndrome should be considered largely a disease of unhealthy lifestyle. Prevalence of metabolic syndrome is a secondary metric in the 2020 Impact Goals. Identification of metabolic syndrome represents a call to action for the healthcare provider and patient to address the underlying lifestyle-related risk factors. A multidisciplinary team of healthcare professionals is desirable to adequately address these multiple issues in patients with metabolic syndrome.4
Despite its prevalence (see below), the public’s recognition of metabolic syndrome is limited.5 A diagnosis of metabolic syndrome may increase risk perception and motivation toward a healthier behavior.6
Prevalence
Youth
(See Charts 11-1 and 11-2)
Chart 11-2. Prevalence of metabolic syndrome in youth.
The prevalence of metabolic syndrome in youth has decreased in the most recent NHANES follow-up (2009–2010 and 2011–2012). This is most evident when considering a metabolic syndrome severity score. Despite increasing obesity, decreased metabolic syndrome has been driven by increased HDL and decreased triglycerides. There has been concomitant decrease in calorie intake and carbohydrate intake as well as an increase in unsaturated fat.16
ATP indicates Adult Treatment Panel; BMI, body mass index; HDL, high-density lipoprotein; MetS, metabolic syndrome; and NHANES, National Health and Nutrition Examination Survey.
According to the 2009 AHA scientific statement about metabolic syndrome in children and adolescents, metabolic syndrome should be diagnosed with caution in this age group, because metabolic syndrome categorization in adolescents is not stable.7 Approximately half of the 1098 adolescent participants in the Princeton School District Study diagnosed with pediatric ATP III metabolic syndrome lost the diagnosis over 3 years of follow-up.8 Despite this, mathematical research in the form of confirmatory factor analysis strongly suggests the existence of a single grouping of cardio-metabolic risk factors shared in common across the spectrum from children to adults.9
Additional evidence of the instability of the diagnosis of metabolic syndrome in children exists. In children 6 to 17 years of age participating in research studies in a single clinical research hospital, the diagnosis of metabolic syndrome was unstable in 46% of cases after a mean of 5.6 years of follow-up.10
Uncertainty remains concerning the definition of the obesity component of metabolic syndrome in the pediatric population because it is age dependent. Therefore, use of BMI percentiles11 and waist-height ratio12 has been recommended. Using standard CDC and FitnessGram standards for pediatric obesity, the prevalence of metabolic syndrome in obese youth ranges from 19% to 35%.11 On the basis of NHANES 1999 to 2002 data, the prevalence of metabolic syndrome in adolescents 12 to 19 years of age ranged from 0% to 9.4%; variation in the estimate was a result of differing methods to define metabolic syndrome.13
In 1999 to 2004, ≈4.5% of US adolescents 12 to 17 years of age had metabolic syndrome according to the definition developed by the International Diabetes Federation.14 In 2006, this prevalence would have represented ≈1.1 million adolescents 12 to 17 years of age with metabolic syndrome. It increased from 1.2% among those 12 to 13 years of age to 7.1% among those 14 to 15 years of age and was higher among boys (6.7%) than girls (2.1%). Furthermore, 4.5% of white adolescents, 3.0% of black adolescents, and 7.1% of Mexican American adolescents had metabolic syndrome.
Using NHANES data from 1988 through 2006, authors suggested that the age-adjusted prevalence of metabolic syndrome in those aged 12 to 19 years appeared to be decreasing. In that report, the age-adjusted prevalence from 1988 to 1994 was 7.3%, dropping to 6.7% from 1999 to 2002 and to 6.5% from 2003 to 2006. This is in contrast to the Korean NHANES, in which the prevalence of metabolic syndrome in those aged 12 to 19 years increased from 4.0% to 7.8%. In the United States, improvements in HDL-C and BP led to the decreased prevalence, whereas increases in dyslipidemia and abdominal obesity contributed to the increasing prevalence in Korea15 (Chart 11-1).
Data from NHANES 2009 to 2010 and 2011 to 2012 suggest that the prevalence of metabolic syndrome is decreasing in 12- to 19-year-olds. This appears to be correlated with decreases in low HDL-C and decreases in triglycerides despite a persistently increasing level of obesity. The lifestyle factors that correlate with decreasing metabolic syndrome are decreasing carbohydrate intake and increasing unsaturated fat intake (Chart 11-2).16
Of 31 participants in the NHLBI Lipid Research Clinics Princeton Prevalence Study and the Princeton Follow-Up Study who had metabolic syndrome at baseline, 21 (68%) had metabolic syndrome 25 years later.17 After adjustment for age, sex, and race, the baseline status of metabolic syndrome was significantly associated with an increased risk of having metabolic syndrome during adulthood (OR, 6.2; 95% CI, 2.8–13.8).
In the Bogalusa Heart Study, 4 variables (BMI, homeostasis model assessment of insulin resistance, ratio of triglycerides to HDL-C, and mean arterial pressure) considered to be part of metabolic syndrome clustered together in blacks and whites and in both children and adults18; however the degree of clustering was stronger among adults than among children. As in adults, preclinical cardiovascular abnormalities, such as elevated carotid IMT, are closely associated with metabolic syndrome in children and adolescents.19,20
Adults
(See Charts 11-3 through 11-8)
Chart 11-3. Age-adjusted prevalence of metabolic syndrome in the United States, NHANES 1999 to 2010.
NHANES indicates National Health and Nutrition Examination Survey.
Data derived from Beltrán-Sánchez et al.25
Chart 11-8. Age-standardized prevalence of metabolic syndrome by age and sex in Hispanics/Latinos, 2008 to 2011.
Values were weighted for survey design and nonresponse and were age standardized to the population described by the 2010 US census.
Source: Hispanic Community Health Study/Study of Latinos.28
The following estimates include many who also have DM, in addition to those with metabolic syndrome without DM:
Prevalence of metabolic syndrome varies by the definition used, with definitions such as that from the International Diabetes Federation and the harmonized definition suggesting lower thresholds for defining central obesity in European whites, Asians (in particular, South Asians), Middle Easterners, sub-Saharan Africans, and Hispanics, which results in higher prevalence estimates.21
The phenotypic expression of metabolic syndrome also varies by race/ethnicity22 and is likely influenced by genetic factors. For example, in community-based US data from the 2000 to 2002 MESA study participants, nonalcoholic fatty liver disease was present in 17% of African Americans with metabolic syndrome but was present in 39% of Hispanics with metabolic syndrome.23 The phenotypic expression of metabolic syndrome also varies by country and culture, particularly in Europe.24
-
On the basis of data from NHANES 1999 to 2010, the age-adjusted prevalence of metabolic syndrome in the United States peaked in the 2001 to 2002 cycle and declined in the 2009 to 2010 NHANES cycle25 (Chart 11-3).
In the 1999 to 2000 cycle, the age-adjusted prevalence of metabolic syndrome was 25.5%. In 2001 to 2002, the age-adjusted prevalence peaked at 27.4%. In 2009 to 2010, the age-adjusted prevalence was 22.9% (Chart 11-3).
In 1999 to 2000, the age-adjusted prevalence was 23.3% in males and 27.5% in females. In 2009 to 2010, the age adjusted prevalence was 23.7% in males and 21.8% in females (Chart 11-3). A more recent NHANES report using data from 2003 to 2013 suggests declining overall rates in females.26
Prevalence of metabolic syndrome was lower in non-Hispanic black males than white males and Mexican-American males in the NHANES cycle 1999 to 2010 (Chart 11-4).
Prevalence of metabolic syndrome was higher in Mexican-American females than white and black females in the NHANES cycle 1999 to 2010 (Chart 11-5).
The changing trends in age-adjusted metabolic syndrome prevalence are attributable to changes in the prevalence of its individual components. From NHANES data cycles 1999 through 2010, hypertriglyceridemia and elevated BP were lower in the total population, whereas hyperglycemia and elevated waist circumference were higher in the total population; however, these trends varied significantly by sex and race/ethnicity (Chart 11-6).
Using different modeling strategies, other reports using NHANES 2003 to 2006 data and National Cholesterol Education Program/ATP III definitions reported an age-adjusted prevalence of ≈34% for adults ≥20 years of age.27 Differences in the prevalence statistics are the result of different handling of age adjustment as the prevalence of metabolic syndrome increases with age and handling of medication therapy for its component conditions.
-
Additionally, on the basis of NHANES 2003 to 2006 data27
Among males, the age-specific prevalence of metabolic syndrome ranged from 20.3% among people 20 to 39 years of age to 40.8% for people 40 to 59 years of age and 51.5% for people ≥60 years of age. Among females, the age-specific prevalence ranged from 15.6% among people 20 to 39 years of age to 37.2% for people 40 to 59 years of age and 54.4% for those ≥60 years of age.
The prevalence of metabolic syndrome is high among Hispanics/Latinos of diverse backgrounds living in the United States. Using data from the population-representative HCHS/SOL study 2008 to 2011, the overall prevalence of metabolic syndrome among Hispanics/Latinos was 34% among males and 36% among females (Chart 11-7); it increased with age, with the highest prevalence in females 70 to 74 years of age (Chart 11-8). In males and females, the lowest prevalence of metabolic syndrome was observed among South Americans (27%). In males, the highest prevalence was observed in Cubans (35%), and in females, the highest prevalence was observed among Puerto Ricans (41%). Some differences in individual components exist by specific Hispanic/Latino background. See Chart 11-7 for complete details.28
The prevalence of metabolic syndrome is similarly high in the African American population from the South (Mississippi). Using data from the Jackson Heart Study, the overall prevalence of metabolic syndrome was 34%, and it was higher in females than in males (40% versus 27%, respectively).29
The prevalence of prediabetes is high among Indians living in the United States and might be higher than the prevalence of prediabetes among Indians living in India. In a comparison of the MASALA and CARRS studies, the prevalence of prediabetes was 33% in the United States sample and 24% in the Chennai, India, sample.30 A low amount of exercise was most strongly associated with prediabetes in MASALA.31 The overall prevalence of metabolic syndrome in the MASALA study was 34.5% (Alka Kanaya, MD, unpublished data, 2015). Similarly, Filipinos in the United States are at high risk for metabolic syndrome at lower BMI levels.32
Other studies have confirmed that the prevalence of metabolic syndrome is high among immigrant Asian Indians, ranging between 26.8% and 38.2% depending on the definition used.33
Among American Indian and Alaska Native people living in the southwestern United States, the prevalence of metabolic syndrome was reported to be 43.2% in males and 47.3% in females; among Alaska Native people, the prevalence was 26.5% and 31.2%, respectively.34
The prevalence of metabolic syndrome among pregnant females increased to 26.5% during 1999 to 2004 from 17.8% during 1988 to 1994.35
The prevalence of metabolic syndrome has been noted to be high among select special populations, including those with schizophrenia spectrum disorders,36 those taking atypical antipsychotic drugs,37 those receiving prior organ transplants,38 HIV-infected individuals,39 those previously treated for blood cancers,40 those with systemic inflammatory disorders such as psoriasis,41 individuals with well-treated type 1 DM,42 those with hypopi-tuitarism,43 those with prior gestational DM,44 and individuals in select professions, including law enforcement45 and firefighters.46
There is a bidirectional relationship between metabolic syndrome and depression. In prospective studies, the presence of depression increases the risk of metabolic syndrome (OR, 1.49; 95% CI, 1.19–1.87), whereas metabolic syndrome increases the risk of depression (OR, 1.52; 95% CI, 1.20–1.91).47
Perhaps most important with respect to meeting the 2020 goals, the prevalence of metabolic syndrome increases with greater cumulative life-course exposure to sedentary behavior and physical inactivity48; screen time, including television viewing49; fast food intake50; short sleep duration51; and intake of sugar-sweetened beverages.52,53 Each of these risk factors is reversible with lifestyle change.
Chart 11-4. Age-adjusted prevalence of metabolic syndrome among adult males by race, NHANES 1999 to 2010.
NHANES indicates National Health and Nutrition Examination Survey.
Data derived from Beltrán-Sánchez et al.25
Chart 11-5. Age-adjusted prevalence of metabolic syndrome among adult females by race, NHANES 1999 to 2010.
NHANES indicates National Health and Nutrition Examination Survey.
Data derived from Beltrán-Sánchez et al.25
Chart 11-6. Prevalence and trends of the 5 components of metabolic syndrome in the adult US population (≥20 years old), 1999 to 2010, by sex (first column), race/ethnicity (second column), and race/ethnicity and sex (third and fourth columns).
Shaded areas represent 95% confidence intervals.
HDL-C indicates high-density lipoprotein cholesterol; Mex-Am, Mexican American; and Waist circumf., waist circumference.
Reprinted from Beltrán-Sánchez et al25 with permission from the American College of Cardiology Foundation. Copyright © 2013, by the American College of Cardiology Foundation.
Chart 11-7. Age-standardized prevalence of metabolic syndrome by sex and Hispanic/Latino background, 2008 to 2011.
Values were weighted for survey design and nonresponse and were age standardized to the population described by the 2010 US census.
Source: Hispanic Community Health Study/Study of Latinos.28
Global Burden of Metabolic Syndrome
(See Chart 11-9)
Chart 11-9. Age-standardized prevalence of metabolic syndrome (MetS) and metabolically healthy obesity (MHO) among obese (body mass index ≥30 kg/m2) males (A) and females (B) in different cohorts.
CHRIS indicates Collaborative Health Research in South Tyrol Study; DILGOM, Dietary, Lifestyle, and Genetics Determinants of Obesity and Metabolic Syndrome; EGCUT, Estonian Genome Center of the University of Tartu; HUNT2, Nord-Trøndelag Health Study; KORA, Cooperative Health Research in the Region of Augsburg; MICROS, Microisolates in South Tyrol Study; NCDS, National Child Development Study; NL, the Netherlands; and PREVEND, Prevention of Renal and Vascular End-Stage Disease. Reprinted from van Vliet-Ostaptchouketet al.67 Copyright © 2014, van Vliet-Ostaptchouk et al.; licensee BioMed Central Ltd.
Metabolic syndrome is becoming hyperendemic around the world. Recent evidence has described the prevalence of metabolic syndrome in Canada,54 Latin America,55 India,56,57 Bangladesh,58 Iran,59 Nigeria,60 South Africa,61 and Vietnam,62 as well as many other countries. On the basis of data from NIPPON DATA, the age-adjusted prevalence of metabolic syndrome in a Japanese population was 19.3%.63 In a partially representative Chinese population, the age-adjusted prevalence of metabolic syndrome in China was 21.3%,64 whereas in northwest China, the prevalence was 15.1%.65
In the INTERHEART case-control study of MI in 26 903 subjects from 52 countries, metabolic syndrome was present in 29.1% of case subjects and just 16.8% of control subjects. The age- and obesity-adjusted prevalence of metabolic syndrome was highest among females (32.1%), South Asians (29.8%), and other Asians (28.7%).66
In a report from BIOSHARE-EU, which harmonizes modern data from 10 different population-based cohorts in 7 European countries, the age-adjusted prevalence of metabolic syndrome in obese subjects ranged from 24% to 65% in females and from ≈43% to ≈78% in males. In the obese population, the prevalence of metabolic syndrome far exceeded the prevalence of metabolically healthy obesity, which had a prevalence of 7% to 28% in females and 2% to 19% in males. The prevalence of metabolic syndrome varied considerably by European country in the BIOSHARE-EU consortium67 (Chart 11-9).
The prevalence of metabolic syndrome has been reported to be low (14.6%) in a population-representative study in France compared with other industrialized countries.68
In a recent systematic review of 10 Brazilian studies, the weighted mean prevalence of metabolic syndrome in Brazil was 29.6%.69
In a report from a representative survey of the northern state of Nuevo León, Mexico, the prevalence of metabolic syndrome in adult individuals (defined as ≥16 years old) was 54.8%. In obese adults, the prevalence reached 73.8%. The prevalence in adult North Mexican females (60.4%) was higher than in adult North Mexican males (48.9%).70
Metabolic syndrome is highly prevalent in modern indigenous populations, notably in Brazil and Australia. The prevalence of metabolic syndrome was estimated to be 41.5% in indigenous groups in Brazil,69 33.0% in Australian Aborigines, and 50.3% in Torres Strait Islanders.71
Natural History and Progression of Metabolic Syndrome
(See Chart 11-10)
Preclinical forms of metabolic syndrome are commonly progressive and precede the development of overt metabolic syndrome. In the ARIC study, a sex and race/ethnicity-specific metabolic syndrome severity score increased in 76% of participants, with faster progression observed in younger participants and in females. The metabolic syndrome severity score predicted time to development of incident metabolic syndrome over mean 10-year follow-up (1987–1989 to 1996–1998). In ARIC, prevalence of metabolic syndrome increased from 33% to 50% over the mean 10-year follow-up, with differences by age and sex. The prevalence of metabolic syndrome was lower in African American males than white males at all time points and for all ages across the study. African American females had higher prevalence of metabolic syndrome than white females at baseline and subsequent steps for all ages except those >60 years of age (Chart 11-10).72
Isolated metabolic syndrome, which could be considered an earlier form of metabolic syndrome, has been defined as those with ≥3 metabolic syndrome components but without overt hypertension and DM. In a population-based random sample of 2042 Olmsted County, MN, residents, those with isolated metabolic syndrome were found to be at increased risk of incident hypertension, DM, diastolic dysfunction, and reduced renal function (GFR <60 mL/min) compared with healthy controls (P<0.05). However, isolated metabolic syndrome was not significantly associated with higher rates of mortality (P=0.12) or development of HF (P=0.64) over the 8-year follow-up.73
Risk
Youth
Few prospective pediatric studies have examined the future risk for CVD or DM according to baseline metabolic syndrome status. Data from 771 participants 6 to 19 years of age from the NHLBI’s Lipid Research Clinics Princeton Prevalence Study and the Princeton Follow-up Study showed that the risk of developing CVD was substantially higher among those with metabolic syndrome than among those without this syndrome (OR, 14.6; 95% CI, 4.8–45.3) who were followed up for 25 years.17
Another analysis of 814 participants in this cohort showed that those 5 to 19 years of age who had metabolic syndrome at baseline had an increased risk of having DM 25 to 30 years later compared with those who did not have the syndrome at baseline (OR, 11.5; 95% CI, 2.1–63.7).74
Additional data from the Princeton Follow-Up Study, the Fels Longitudinal Study, and the Muscatine Study suggest that the absence of components of metabolic syndrome in childhood has a high negative predictive value for the development of metabolic syndrome or DM in adulthood.75
In a study of 6328 subjects from 4 prospective studies, compared with people with normal BMI as children and as adults, those with consistently high adiposity from childhood to adulthood had an increased risk of the following metabolic syndrome components: hypertension (RR, 2.7; 95% CI, 2.2–3.3), low HDL-C (RR, 2.1; 95% CI, 1.8–2.5), elevated triglycerides (RR, 3.0; 95% CI, 2.4–3.8), type 2 DM (RR, 5.4; 95% CI, 3.4–8.5), and increased carotid IMT (RR, 1.7; 95% CI, 1.4–2.2). Those who were overweight or obese during childhood but were not obese as adults had no increased risk compared with those with consistently normal BMI.76
In 1757 youths from the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study, those with metabolic syndrome in youth and adulthood were at 3.4 times increased risk of high carotid IMT and 12.2 times increased risk of type 2 DM in adulthood as those without metabolic syndrome at either time. Adults whose metabolic syndrome had resolved after their youth were at no increased risk of having high IMT or type 2 DM.77
In the Princeton Lipid Research Cohort Study, metabolic syndrome severity scores during childhood were lowest among those who never developed CVD and were proportionally higher progressing from those who developed early CVD (mean 38 years old) to those who developed CVD later in life (mean 50 years old).78 Metabolic syndrome severity score was also strongly associated with early onset of DM.79 Similarly, a metabolic syndrome score, based on the number of components of metabolic syndrome, was associated with biomarkers of inflammation, endothelial damage and CVD risk in a separate cohort of 677 prepubertal children.80
Adults
Clinical CVD
Consistent with 2 previous meta-analyses, a recent meta-analysis of prospective studies concluded that metabolic syndrome increased the risk of developing CVD (summary RR, 1.78; 95% CI, 1.58–2.00).81 The RR of CVD tended to be higher in females (summary RR, 2.63) than in males (summary RR, 1.98; P=0.09). On the basis of results from 3 studies, metabolic syndrome remained a predictor of cardiovascular events after adjustment for the individual components of the syndrome (summary RR, 1.54; 95% CI, 1.32–1.79). Metabolic syndrome is also associated with incident CVD independent of the baseline subclinical CVD.82 A more recent meta-analysis among 87 studies comprising 951 083 subjects showed an even higher risk of CVD associated with metabolic syndrome (summary RR, 2.35; 95% CI, 2.02–2.73), with significant increased risks (RRs ranging from 1.6 to 2.9) for all-cause mortality, CVD mortality, MI, and stroke, as well as for those with metabolic syndrome without DM.83
In one of the earlier studies among US adults, mortality follow-up of the second NHANES showed a stepwise increase in risk of CHD, CVD, and total mortality across the spectrum of no disease, metabolic syndrome (without DM), DM, prior CVD, and those with CVD and DM, with an HR for CHD mortality of 2.02 (95% CI, 1.42–2.89) associated with metabolic syndrome. Increased risk was seen with increased numbers of metabolic syndrome risk factors.84
Estimates of RR for CVD generally increase as the number of components of metabolic syndrome increases.85 Compared with males without an abnormal component in the Framingham Offspring Study, the HRs for CVD were 1.48 (95% CI, 0.69–3.16) for males with 1 or 2 components and 3.99 (95% CI, 1.89–8.41) for males with ≥3 components.86 Among females, the HRs were 3.39 (95% CI, 1.31–8.81) for 1 or 2 components and 5.95 (95% CI, 2.20–16.11) for ≥3 components. Compared with males without a metabolic abnormality in the British Regional Heart Study, the HRs were 1.74 (95% CI, 1.22–2.39) for 1 component, 2.34 (95% CI, 1.65–3.32) for 2 components, 2.88 (95% CI, 2.02–4.11) for 3 components, and 3.44 (95% CI, 2.35–5.03) for 4 or 5 components.85
The cardiovascular risk associated with metabolic syndrome varies on the basis of the combination of metabolic syndrome components present. Of all possible ways to have 3 metabolic syndrome components, the combination of central obesity, elevated BP, and hyperglycemia conferred the greatest risk for CVD (HR, 2.36; 95% CI, 1.54–3.61) and mortality (HR, 3.09; 95% CI, 1.93–4.94) in the Framingham Offspring Study.87
Data from the Aerobics Center Longitudinal Study indicate that risk for CVD mortality is increased in males without DM who have metabolic syndrome (HR, 1.8; 95% CI, 1.5–2.0); however, among those with metabolic syndrome, the presence of DM is associated with even greater risk for CVD mortality (HR, 2.1; 95% CI, 1.7–2.6).88
Among patients with stable CAD in the COURAGE trial, the presence of metabolic syndrome was associated with an increased risk of death or MI (unadjusted HR, 1.41; 95% CI, 1.15–1.73; P=0.001); however, after adjustment for its individual components, metabolic syndrome was no longer significantly associated with outcome (HR, 1.15; 95% CI, 0.79–1.68; P=0.46).89
In the INTERHEART case-control study of 26 903 subjects from 52 countries, metabolic syndrome was associated with an increased risk of MI, both according to the WHO (OR, 2.69; 95% CI, 2.45–2.95) and the International Diabetes Federation (OR, 2.20; 95% CI, 2.03–2.38) definitions, with a PAR of 14.5% (95% CI, 12.7%–16.3%) and 16.8% (95% CI, 14.8%–18.8%), respectively, and associations that were similar across all regions and ethnic groups. In addition, the presence of ≥3 risk factors with subthreshold values was associated with increased risk of MI (OR, 1.50; 95% CI, 1.24–1.81) compared with having “normal” values. Similar results were observed when the International Diabetes Federation definition was used.66
In the Three-City Study, among 7612 participants aged ≥65 years who were followed up for 5.2 years, metabolic syndrome was associated with increased total CHD (HR, 1.78; 95% CI, 1.39–2.28) and fatal CHD (HR, 2.40; 95% CI, 1.41–4.09); however, metabolic syndrome was not associated with CHD beyond its individual risk components.90
The United States has a higher prevalence of metabolic syndrome and a higher CVD mortality rate than Japan. It is estimated that 13.3% to 44% of the excess CVD mortality in the Unites States is explained by metabolic syndrome or metabolic syndrome–related existing CVD.63
In addition to CVD, metabolic syndrome has been associated with incident AF,91 recurrent AF after ablation,92 HF,93 PAD,94 erectile dysfunction,95 and cognitive decline.96 Data from case-control studies, but not prospective studies, support an association with VTE.97 There may be an association with increased incident asthma.98 In MESA, the prevalence of erectile dysfunction among participants aged 55 to 65 years with metabolic syndrome was 16% compared with 10% without metabolic syndrome (P<0.001).
Using the 36 cohorts represented in the MORGAM Project, the prognosis associated with metabolic syndrome has been shown to vary substantially by age and sex.99
Subclinical CVD
In MESA, among 6603 people aged 45 to 84 years (1686 [25%] with metabolic syndrome without DM and 881 [13%] with DM), subclinical atherosclerosis assessed by CAC was more severe in people with metabolic syndrome and DM than in those without these conditions, and the extent of CAC was a strong predictor of CHD and CVD events in these groups.100 There appears to be a synergistic relationship between metabolic syndrome, nonalcoholic fatty liver disease, and prevalence of CAC,101,102 as well as a synergistic relationship with smoking.103 Furthermore, the progression of CAC was greater in people with metabolic syndrome and DM than in those without, and progression of CAC predicted future CVD event risk both in those with metabolic syndrome and in those with DM.104,105 In MESA, the prevalence of thoracic calcification was 33% for people with metabolic syndrome compared with 38% for those with DM (with and without metabolic syndrome) and 24% of those with neither DM nor metabolic syndrome.106
In the DESIR cohort, metabolic syndrome was associated with an unfavorable hemodynamic profile, including increased brachial central pulse pressure and increase pulse pressure amplification, compared with similar individuals with isolated hypertension but without metabolic syndrome.107 In MESA, metabolic syndrome was associated with major and minor ECG abnormalities, although this varied by sex.108 Metabolic syndrome is associated with reduced heart rate variability and altered cardiac autonomic modulation in adolescents.109
Individuals with metabolic syndrome have a higher degree of endothelial dysfunction than individuals with a similar burden of traditional cardiovascular risk factors.110 Furthermore, individuals with both metabolic syndrome and DM have demonstrated increased microvascular and macrovascular dysfunction.111 Metabolic syndrome is associated with increased thrombosis, including increased resistance to aspirin112 and clopidogrel loading.113
In modern imaging studies using echocardiography, MRI, cardiac CT, and positron emission tomography, metabolic syndrome has been shown to be closely related to increased epicardial adipose tissues,114 regional neck fat distribution,115 increased visceral fat in other locations,116 high-risk coronary plaque features including increased necrotic core,117 impaired coronary flow reserve,118 abnormal indices of LV strain,119 LV diastolic dysfunction,120 LV dyssynchrony,121 and subclinical RV dysfunction.122
Metabolic syndrome is associated with increased healthcare use and healthcare-related costs among individuals with and without DM. Overall, healthcare costs increase by ≈24% for each additional metabolic syndrome component present.123
Risk Factors
Risk of metabolic syndrome probably begins before birth. The Prediction of Metabolic Syndrome in Adolescence Study showed that the coexistence of low birth weight, small head circumference, and parental history of overweight or obesity places children at the highest risk for metabolic syndrome in adolescence. Other risk factors identified included parental history of DM, gestational hypertension in the mother, and lack of breastfeeding.124 However, a recent RCT testing a breastfeeding promotion intervention did not lead to reduced childhood metabolic syndrome among healthy term infants.125
In NHANES, adolescents 12 to 19 years old were at greater risk of metabolic syndrome if they had concurrent exposure to secondhand smoke and low exposure to certain nutrients (vitamin E and omega-3 polyunsaturated fatty acids)126 and if they consumed more sugar in their diet.127
In prospective or retrospective cohort studies, the following factors have been reported as being directly associated with incident metabolic syndrome, defined by 1 of the major definitions: age,25 low educational attainment,128,129 low socioeconomic status,130 not being able to understand or read food labels,131 urbanization,132 smoking,129,130,133,134 parental smoking,135 low levels of PA,129,130,133,134 low levels of physical fitness,136–138 intake of soft drinks,139 intake of diet soda,140 fructose intake,141 magnesium intake,142,143 energy intake,144 carbohydrate intake,128,134,145 total fat intake,74,146 Western dietary pattern, meat intake, (red but not white meat147), intake of fried foods,140 skipping breakfast,148 heavy alcohol consumption,149 abstention from alcohol use,128 parental history of DM,74 long-term stress at work,150 pediatric metabolic syndrome,74 obesity or BMI,77,88,100,146,151 childhood obesity,152 intra-abdominal fat,153 gain in weight or BMI,135,146 weight fluctuation,154 heart rate,155 homeostasis model assessment,156,157 fasting insulin,157 2-hour insulin,157 proinsulin,157 oxidized LDL-C,156 lipoprotein-associated phospholipase A2,158 uric acid,159,160 γ-glutamyltransf erase,159,161,162 alanine transaminase,159,161,163,164 plasminogen activator inhibitor-1,165 aldosterone,165 leptin,166 ferritin,167 CRP,168,169 adipocyte–fatty acid binding protein,170 testosterone and sex hormone–binding globulin,171,172 matrix metalloproteinase 9,173 active periodontitis,174 and urinary bisphenol A levels.175 In cross-sectional studies, a high-salt diet,176 stress,177 and obstructive sleep apnea178 were significant predictors of metabolic syndrome.
The following factors have been reported as being inversely associated with incident metabolic syndrome, defined by 1 of the major definitions, in prospective or retrospective cohort studies: muscular strength,179 increased PA or physical fitness,134,180 aerobic training,181 moderate alcohol intake,86,100 fiber intake,182 fruits and vegetables,183 white fish intake,184 Mediterranean diet,185 dairy consumption140 (particularly yogurt and low-fat dairy products186), consumption of fermented milk with Lactobacillus plantarum,187 animal or fat protein,188 hot tea consumption (but not sugar-sweetened iced tea),189 coffee consumption,190 vitamin D intake,191,192 intake of tree nuts,193 avocado intake,194 long-chain omega-3 polyunsaturated fatty acid,195 potassium intake,196 ability to interpret nutrition labels,131 insulin sensitivity,157 ratio of aspartate aminotransferase to ala-nine transaminase,163 total testosterone,153,157,197 serum 25-hydroxyvitamin D,198 sex hormone–binding globulin,153,157,197 and Δ5-desaturase activity.199 In cross-sectional studies, increased standing,200 a vegetarian diet,201 subclinical hypothyroidism in males,202 and marijuana use203 were inversely associated with metabolic syndrome.
In >6 years of follow-up in the ARIC study, 1970 individuals (25%) developed metabolic syndrome, and compared with the normal-weight group (BMI <25 kg/m2), the ORs of developing metabolic syndrome were 2.81 (95% CI, 2.50–3.17) and 5.24 (95% CI, 4.50–6.12) for the overweight (BMI 25–30 kg/m2) and obese (BMI γ30 kg/m2) groups, respectively. Compared with the lowest quartile of leisure-time PA, the ORs of developing metabolic syndrome were 0.80 (95% CI, 0.71–0.91) and 0.92 (95% CI, 0.81–1.04) for people in the highest and middle quartiles, respectively.204
REFERENCES
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIR-CULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Hari P, Nerusu K, Veeranna V, Sudhakar R, Zalawadiya S, Ramesh K, Afonso L. A gender-stratified comparative analysis of various definitions of metabolic syndrome and cardiovascular risk in a multiethnic U.S. population. Metab Syndr Relat Disord. 2012;10:47–55. doi: 10.1089/met.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tota-Maharaj R, Defilippis AP, Blumenthal RS, Blaha MJ. A practical approach to the metabolic syndrome: review of current concepts and management. Curr Opin Cardiol. 2010;25:502–512. doi: 10.1097/HCO.0b013e32833cd474. [DOI] [PubMed] [Google Scholar]
- 4.Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, Defilippis AP. A practical “ABCDE” approach to the metabolic syndrome. Mayo Clin Proc. 2008;83:932–941. doi: 10.4065/83.8.932. [DOI] [PubMed] [Google Scholar]
- 5.Lewis SJ, Rodbard HW, Fox KM, Grandy S SHIELD Study Group. Self-reported prevalence and awareness of metabolic syndrome: findings from SHIELD. Int J Clin Pract. 2008;62:1168–1176. doi: 10.1111/j.1742-1241.2008.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jumean MF, Korenfeld Y, Somers VK, Vickers KS, Thomas RJ, Lopez-Jimenez F. Impact of diagnosing metabolic syndrome on risk perception. Am J Health Behav. 2012;36:522–532. doi: 10.5993/AJHB.36.4.9. [DOI] [PubMed] [Google Scholar]
- 7.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus-Snyder ML American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULA-TIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 8.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–2322. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viitasalo A, Lakka TA, Laaksonen DE, Savonen K, Lakka HM, Hassinen M, Komulainen P, Tompuri T, Kurl S, Laukkanen JA, Rauramaa R. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia. 2014;57:940–949. doi: 10.1007/s00125-014-3172-5. [DOI] [PubMed] [Google Scholar]
- 10.Gustafson JK, Yanoff LB, Easter BD, Brady SM, Keil MF, Roberts MD, Sebring NG, Han JC, Yanovski SZ, Hubbard VS, Yanovski JA. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94:4828–4834. doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurson KR, Welk GJ, Eisenmann JC. Diagnostic performance of BMI percentiles to identify adolescents with metabolic syndrome. Pediatrics. 2014;133:e330–e338. doi: 10.1542/peds.2013-1308. [DOI] [PubMed] [Google Scholar]
- 12.Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62:742–751. doi: 10.1016/j.jacc.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 15.Lim S, Jang HC, Park KS, Cho SI, Lee MG, Joung H, Mozumdar A, Liguori G. Changes in metabolic syndrome in American and Korean youth, 1997–2008. Pediatrics. 2013;131:e214–e222. doi: 10.1542/peds.2012-0761. [DOI] [PubMed] [Google Scholar]
- 16.Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents [published correction appears in Pediatrics. 2016;137] Pediatrics. 2016;137:e20153177. doi: 10.1542/peds.2015-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am J Epidemiol. 2007;166:527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 19.Chinali M, de Simone G, Roman MJ, Best LG, Lee ET, Russell M, Howard BV, Devereux RB. Cardiac markers of pre-clinical disease in adolescents with the metabolic syndrome: the strong heart study. J Am Coll Cardiol. 2008;52:932–938. doi: 10.1016/j.jacc.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toledo-Corral CM, Ventura EE, Hodis HN, Weigensberg MJ, Lane CJ, Li Y, Goran MI. Persistence of the metabolic syndrome and its influence on carotid artery intima media thickness in overweight Latino children. Atherosclerosis. 2009;206:594–598. doi: 10.1016/j.atherosclerosis.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown TM, Voeks JH, Bittner V, Safford MM. Variations in prevalent cardiovascular disease and future risk by metabolic syndrome classification in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am Heart J. 2010;159:385–391. doi: 10.1016/j.ahj.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick SL, Lai BS, Brancati FL, Golden SH, Hill-Briggs F. Metabolic syndrome risk profiles among African American adolescents: national health and nutrition examination survey, 2003–2010. Diabetes Care. 2013;36:436–442. doi: 10.2337/dc12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tota-Maharaj R, Blaha MJ, Zeb I, Katz R, Blankstein R, Blumenthal RS, Budoff MJ, Nasir K. Ethnic and sex differences in fatty liver on cardiac computed tomography: the Multi-Ethnic Study of Atherosclerosis. Mayo Clin Proc. 2014;89:493–503. doi: 10.1016/j.mayocp.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, Mattace Raso FU, Muiesan ML, Ryliškyte˙ L, Rietzschel E, Strait J, Vlachopoulos C, Völzke H, Lakatta EG, Nilsson PM Metabolic Syndrome and Arteries Research (MARE) Consortium. Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol. 2014;22:486–491. doi: 10.1177/2047487314525529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 27.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 28.Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie C, Carnethon M, Kaplan R, Giachello A, Gallo L, Loehr L, Avilés-Santa L. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37:2391–2399. doi: 10.2337/dc13-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan RJ, Gebreab SY, Sims M, Riestra P, Xu R, Davis SK. Prevalence, associated factors and heritabilities of metabolic syndrome and its individual components in African Americans: the Jackson Heart Study. BMJ Open. 2015;5:e008675. doi: 10.1136/bmjo-pen-2015-008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gujral UP, Narayan KM, Pradeepa RG, Deepa M, Ali MK, Anjana RM, Kandula NR, Mohan V, Kanaya AM. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: the CARRS and MASALA studies. Diabetes Care. 2015;38:1312–1318. doi: 10.2337/dc15-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah AD, Vittinghoff E, Kandula NR, Srivastava S, Kanaya AM. Correlates of prediabetes and type II diabetes in US South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Ann Epidemiol. 2015;25:77–83. doi: 10.1016/j.annepidem.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abesamis CJ, Fruh S, Hall H, Lemley T, Zlomke KR. Cardiovascular health of Filipinos in the United States: a review of the literature. J Transcult Nurs. 2016;27:518–528. doi: 10.1177/1043659615597040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, Shah V, Vijay K, Mudaliar S, Iyer D, Balasubramanyam A. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications. 2010;24:145–153. doi: 10.1016/j.jdiacomp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher C, Ferucci ED, Lanier AP, Slattery ML, Schraer CD, Raymer TW, Dillard D, Murtaugh MA, Tom-Orme L. Metabolic syndrome: prevalence among American Indian and Alaska native people living in the southwestern United States and in Alaska. Metab Syndr Relat Disord. 2008;6:267–273. doi: 10.1089/met.2008.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos RG, Olden K. The prevalence of metabolic syndrome among US women of childbearing age. Am J Public Health. 2008;98:1122–1127. doi: 10.2105/AJPH.2007.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350–1363. doi: 10.1001/jamapsy-chiatry.2014.1314. [DOI] [PubMed] [Google Scholar]
- 37.Pramyothin P, Khaodhiar L. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes. 2010;17:460–466. doi: 10.1097/MED.0b013e32833de61c. [DOI] [PubMed] [Google Scholar]
- 38.Sorice GP, Di Pizio L, Sun VA, Schirò T, Muscogiuri G, Mezza T, Cefalo CM, Prioletta A, Pontecorvi A, Giaccari A. Metabolic syndrome in transplant patients: an updating point of view. Minerva Endocrinol. 2012;37:211–220. [PubMed] [Google Scholar]
- 39.van Wijk JP, Cabezas MC. Hypertriglyceridemia, metabolic syndrome, and cardiovascular disease in HIV-infected patients: effects of antiretroviral therapy and adipose tissue distribution. Int J Vasc Med. 2012;2012:201027. doi: 10.1155/2012/201027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia: from the St. Jude Lifetime Cohort. Br J Haematol. 2014;165:364–374. doi: 10.1111/bjh.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol. 2011;147:419–424. doi: 10.1001/archderma-tol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63:181–187. doi: 10.1016/j.metabol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Verhelst J, Mattsson AF, Luger A, Thunander M, Góth MI, Koltowska-Häggström M, Abs R. Prevalence and characteristics of the metabolic syndrome in 2479 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. Eur J Endocrinol. 2011;165:881–889. doi: 10.1530/EJE-11-0599. [DOI] [PubMed] [Google Scholar]
- 44.Noctor E, Crowe C, Carmody LA, Kirwan B, O’Dea A, Glynn LG, McGuire BE, O’Shea PM, Dunne FP. ATLANTIC-DIP: prevalence of metabolic syndrome and insulin resistance in women with previous gestational diabetes mellitus by International Association of Diabetes in Pregnancy Study Groups criteria. Acta Diabetol. 2015;52:153–160. doi: 10.1007/s00592-014-0621-z. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman FH. Cardiovascular disease and risk factors in law enforcement personnel: a comprehensive review. Cardiol Rev. 2012;20:159–166. doi: 10.1097/CRD.0b013e318248d631. [DOI] [PubMed] [Google Scholar]
- 46.Donovan R, Nelson T, Peel J, Lipsey T, Voyles W, Israel RG. Cardiorespiratory fitness and the metabolic syndrome in firefighters. Occup Med (Lond) 2009;59:487–492. doi: 10.1093/occmed/kqp095. [DOI] [PubMed] [Google Scholar]
- 47.Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, Berrigan D, Troiano RP, Koster A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wennberg P, Gustafsson PE, Howard B, Wennberg M, Hammarström A. Television viewing over the life course and the metabolic syndrome in mid-adulthood: a longitudinal population-based study. J Epidemiol Community Health. 2014;68:928–933. doi: 10.1136/jech-2013-203504. [DOI] [PubMed] [Google Scholar]
- 50.Bahadoran Z, Mirmiran P, Hosseini-Esfahani F, Azizi F. Fast food consumption and the risk of metabolic syndrome after 3-years of follow-up: Tehran Lipid and Glucose Study. Eur J Clin Nutr. 2013;67:1303–1309. doi: 10.1038/ejcn.2013.217. [DOI] [PubMed] [Google Scholar]
- 51.Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2014;18:293–297. doi: 10.1016/j.smrv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Barrio-Lopez MT, Martinez-Gonzalez MA, Fernandez-Montero A, Beunza JJ, Zazpe I, Bes-Rastrollo M. Prospective study of changes in sugar-sweetened beverage consumption and the incidence of the metabolic syndrome and its components: the SUN cohort. Br J Nutr. 2013;110:1722–1731. doi: 10.1017/S0007114513000822. [DOI] [PubMed] [Google Scholar]
- 53.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GB, McFarlane PA, Ross R, Teoh H, Verma S, Anand S, Camelon K, Chow CM, Cox JL, Després JP, Genest J, Harris SB, Lau DC, Lewanczuk R, Liu PP, Lonn EM, McPherson R, Poirier P, Qaadri S, Rabasa-Lhoret R, Rabkin SW, Sharma AM, Steele AW, Stone JA, Tardif JC, Tobe S, Ur E Cardiometabolic Risk Working Group: Executive Committee. Cardiometabolic risk in Canada: a detailed analysis and position paper by the Cardiometabolic Risk Working Group. Can J Cardiol. 2011;27:e1–e33. doi: 10.1016/j.cjca.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 55.López-Jaramillo P, Sánchez RA, Diaz M, Cobos L, Bryce A, Parra Carrillo JZ, Lizcano F, Lanas F, Sinay I, Sierra ID, Peñaherrera E, Bendersky M, Schmid H, Botero R, Urina M, Lara J, Foss MC, Márquez G, Harrap S, Ramírez AJ, Zanchetti A Latin America Expert Group. Latin American consensus on hypertension in patients with diabetes type 2 and metabolic syndrome. J Hypertens. 2013;31:223–238. doi: 10.1097/HJH.0b013e32835c5444. [DOI] [PubMed] [Google Scholar]
- 56.Sawant A, Mankeshwar R, Shah S, Raghavan R, Dhongde G, Raje H, D’souza S, Subramanium A, Dhairyawan P, Todur S, Ashavaid TF. Prevalence of metabolic syndrome in urban India. Cholesterol. 2011;2011:920983. doi: 10.1155/2011/920983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadav D, Mahajan S, Subramanian SK, Bisen PS, Chung CH, Prasad GB. Prevalence of metabolic syndrome in type 2 diabetes mellitus using NCEP-ATPIII, IDF and WHO definition and its agreement in Gwalior Chambal region of Central India. Glob J Health Sci. 2013;5:142–155. doi: 10.5539/gjhs.v5n6p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanam MA, Qiu C, Lindeboom W, Streatfield PK, Kabir ZN, Wahlin Å. The metabolic syndrome: prevalence, associated factors, and impact on survival among older persons in rural Bangladesh. PLoS One. 2011;6:e20259. doi: 10.1371/journal.pone.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amirkalali B, Fakhrzadeh H, Sharifi F, Kelishadi R, Zamani F, Asayesh H, Safiri S, Samavat T, Qorbani M. Prevalence of metabolic syndrome and its components in the Iranian adult population: a systematic review and meta-analysis. Iran Red Crescent Med J. 2015;17:e24723. doi: 10.5812/ircmj.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oguoma VM, Nwose EU, Richards RS. Prevalence of cardio-metabolic syndrome in Nigeria: a systematic review. Public Health. 2015;129:413–423. doi: 10.1016/j.puhe.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Peer N, Lombard C, Steyn K, Levitt N. High prevalence of metabolic syndrome in the black population of Cape Town: the Cardiovascular Risk in Black South Africans (CRIBSA) study. Eur J Prev Cardiol. 2015;22:1036–1042. doi: 10.1177/2047487314549744. [DOI] [PubMed] [Google Scholar]
- 62.Binh TQ, Phuong PT, Nhung BT, Tung do D. Metabolic syndrome among a middle-aged population in the Red River Delta region of Vietnam. BMC Endocr Disord. 2014;14:77. doi: 10.1186/1472-6823-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Miura K, Fujiyoshi A, Kadota A, Miyagawa N, Nakamura Y, Ohkubo T, Okayama A, Okamura T, Ueshima H. Impact of metabolic syndrome on the risk of cardiovascular disease mortality in the United States and in Japan. Am J Cardiol. 2014;113:84–89. doi: 10.1016/j.amjcard.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 64.Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013;57:867–871. doi: 10.1016/j.ypmed.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, Yan H, Yang R, Li Q, Dang S, Wang Y. Prevalence and determinants of metabolic syndrome among adults in a rural area of Northwest China. PLoS One. 2014;9:e91578. doi: 10.1371/journal.pone.0091578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, Rangarajan S, Gerstein HC, Anand SS INTERHEART Investigators. Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55:2390–2398. doi: 10.1016/j.jacc.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 67.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T, Joensuu A, Newby C, Pang C, Partinen E, Reischl E, Schwienbacher C, Tammesoo ML, Swertz MA, Burton P, Ferretti V, Fortier I, Giepmans L, Harris JR, Hillege HL, Holmen J, Jula A, Kootstra-Ros JE, Kvaløy K, Holmen TL, Männistö S, Metspalu A, Midthjell K, Murtagh MJ, Peters A, Pramstaller PP, Saaristo T, Salomaa V, Stolk RP, Uusitupa M, van der Harst P, van der Klauw MM, Waldenberger M, Perola M, Wolffenbuttel BH. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vernay M, Salanave B, de Peretti C, Druet C, Malon A, Deschamps V, Hercberg S, Castetbon K. Metabolic syndrome and socioeconomic status in France: the French Nutrition and Health Survey (ENNS, 2006–2007) Int J Public Health. 2013;58:855–864. doi: 10.1007/s00038-013-0501-2. [DOI] [PubMed] [Google Scholar]
- 69.de Carvalho Vidigal F, Bressan J, Babio N, Salas-Salvadó J. Prevalence of metabolic syndrome in Brazilian adults: a systematic review. BMC Public Health. 2013;13:1198. doi: 10.1186/1471-2458-13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salas R, del Bibiloni MM, Ramos E, Villarreal JZ, Pons A, Tur JA, Sureda A. Metabolic syndrome prevalence among Northern Mexican adult population. PLoS One. 2014;9:e105581. doi: 10.1371/journal.pone.0105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M, McCulloch B, McDermott R. Metabolic syndrome and incident coronary heart disease in Australian indigenous populations. Obesity (Silver Spring) 2012;20:1308–1312. doi: 10.1038/oby.2011.156. [DOI] [PubMed] [Google Scholar]
- 72.Vishnu A, Gurka MJ, DeBoer MD. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: the Atherosclerosis Risk in Communities Study. Atherosclerosis. 2015;243:278–285. doi: 10.1016/j.atherosclerosis.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel PA, Scott CG, Rodeheffer RJ, Chen HH. The natural history of patients with isolated metabolic syndrome. Mayo Clin Proc. 2016;91:623–633. doi: 10.1016/j.mayocp.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Schubert CM, Sun SS, Burns TL, Morrison JA, Huang TT. Predictive ability of childhood metabolic components for adult metabolic syndrome and type 2 diabetes. J Pediatr. 2009;155:S6. e1–S6. e7. doi: 10.1016/j.jpeds.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 77.Magnussen CG, Koskinen J, Juonala M, Chen W, Srinivasan SR, Sabin MA, Thomson R, Schmidt MD, Nguyen QM, Xu JH, Skilton MR, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Rönnemaa T, Viikari JS, Berenson GS, Raitakari OT. A diagnosis of the metabolic syndrome in youth that resolves by adult life is associated with a normalization of high carotid intima-media thickness and type 2 diabetes mellitus risk: the Bogalusa Heart and Cardiovascular Risk in Young Finns studies. J Am Coll Cardiol. 2012;60:1631–1639. doi: 10.1016/j.jacc.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 78.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of metabolic syndrome as a predictor of cardiovascular disease between childhood and adulthood: the Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Valle M, Cañete R, Tojo R, Moreno LA, Gil Á. A continuous metabolic syndrome score is associated with specific biomarkers of inflammation and CVD risk in prepubertal children. Ann Nutr Metab. 2015;66:72–79. doi: 10.1159/000369981. [DOI] [PubMed] [Google Scholar]
- 81.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 82.Xanthakis V, Sung JH, Samdarshi TE, Hill AN, Musani SK, Sims M, Ghraibeh KA, Liebson PR, Taylor HA, Vasan RS, Fox ER. Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson Heart Study. Diabetes Care. 2015;38:1082–1088. doi: 10.2337/dc14-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 84.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 85.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 86.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 87.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB., Sr Trajectories of entering the metabolic syndrome: the Framingham Heart Study. Circulation. 2009;120:1943–1950. doi: 10.1161/CIRCULATIONAHA.109.855817. [DOI] [PubMed] [Google Scholar]
- 88.Church TS, Thompson AM, Katzmarzyk PT, Sui X, Johannsen N, Earnest CP, Blair SN. Metabolic syndrome and diabetes, alone and in combination, as predictors of cardiovascular disease mortality among men. Diabetes Care. 2009;32:1289–1294. doi: 10.2337/dc08-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maron DJ, Boden WE, Spertus JA, Hartigan PM, Mancini GB, Sedlis SP, Kostuk WJ, Chaitman BR, Shaw LJ, Berman DS, Dada M, Teo KK, Weintraub WS, O’Rourke RA COURAGE Trial Research Group. Impact of metabolic syndrome and diabetes on prognosis and outcomes with early percutaneous coronary intervention in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol. 2011;58:131–137. doi: 10.1016/j.jacc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 90.Rachas A, Raffaitin C, Barberger-Gateau P, Helmer C, Ritchie K, Tzourio C, Amouyel P, Ducimetière P, Empana JP. Clinical usefulness of the metabolic syndrome for the risk of coronary heart disease does not exceed the sum of its individual components in older men and women: the Three-City (3C) Study. Heart. 2012;98:650–655. doi: 10.1136/heartjnl-2011-301185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159:850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin KJ, Cho SI, Tiwari N, Bergman M, Kizer JR, Palma EC, Taub CC. Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;39:211–223. doi: 10.1007/s10840-013-9863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vidula H, Liu K, Criqui MH, Szklo M, Allison M, Sibley C, Ouyang P, Tracy RP, Chan C, McDermott MM. Metabolic syndrome and incident peripheral artery disease: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;243:198–203. doi: 10.1016/j.atherosclerosis.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Besiroglu H, Otunctemur A, Ozbek E. The relationship between metabolic syndrome, its components, and erectile dysfunction: a systematic review and a meta-analysis of observational studies. J Sex Med. 2015;12:1309–1318. doi: 10.1111/jsm.12885. [DOI] [PubMed] [Google Scholar]
- 96.Raffaitin C, Féart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 97.Ageno W, Di Minno MN, Ay C, Jang MJ, Hansen JB, Steffen LM, Vayà A, Rattazzi M, Pabinger I, Oh D, Di Minno G, Braekkan SK, Cushman M, Bonet E, Pauletto P, Squizzato A, Dentali F. Association between the metabolic syndrome, its individual components, and unprovoked venous thromboembolism: results of a patient-level meta-analysis. Arterioscler Thromb Vasc Biol. 2014;34:2478–2485. doi: 10.1161/ATVBAHA.114.304085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brumpton BM, Camargo CA, Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42:1495–1502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 99.Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T, Palmieri L, Giampaoli S, Donfrancesco C, Kee F, Mancia G, Cesana G, Kuulasmaa K, Salomaa V, Sans S, Ferrieres J, Dallongeville J, Söderberg S, Arveiler D, Wagner A, Tunstall-Pedoe H, Drygas W, Olsen MH MORGAM Project. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans: the MORGAM Prospective Cohort Project [published correction appears in PLoS One. 2015;10:e0128848] PLoS One. 2014;9:e107294. doi: 10.1371/journal.pone.0107294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malik S, Budoff MJ, Katz R, Blumental RS, Bertoni AG, Nasir K, Szklo M, Barr RG, Wong ND. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–2290. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hong HC, Hwang SY, Ryu JY, et al. The synergistic impact of non-alcoholic fatty liver disease and metabolic syndrome on subclinical atherosclerosis [published online ahead of print September 5, 2015] [Accessed September 6, 2016];Clin Endocrinol (Oxf) 2015 doi: 10.1111/cen.12940. [DOI] [PubMed] [Google Scholar]
- 102.Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, Katz R, Blumenthal RS, Blaha MJ. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239:629–633. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YA, Kang SG, Song SW, Rho JS, Kim EK. Association between metabolic syndrome, smoking status and coronary artery calcification. PLoS One. 2015;10:e0122430. doi: 10.1371/journal.pone.0122430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, Szklo M, Lakoski SG, Bertoni AG, Kronmal RA, Blumenthal RS, Nasir K. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, Guerci A, Jacobs DR, Jr, Kronmal R, Liu K, Saad M, Selvin E, Tracy R, Detrano R. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5:358–366. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katz R, Budoff MJ, O’Brien KD, Wong ND, Nasir K. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the Multi-Ethnic Study of Atherosclerosis. Diabet Med. 2015;33:912–919. doi: 10.1111/dme.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Safar ME, Balkau B, Lange C, Protogerou AD, Czernichow S, Blacher J, Levy BI, Smulyan H. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61:12–19. doi: 10.1016/j.jacc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 108.Ebong IA, Bertoni AG, Soliman EZ, Guo M, Sibley CT, Chen YD, Rotter JI, Chen YC, Goff DC., Jr Electrocardiographic abnormalities associated with the metabolic syndrome and its components: the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2012;10:92–97. doi: 10.1089/met.2011.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodríguez-Colón SM, He F, Bixler EO, Fernandez-Mendoza J, Vgontzas AN, Calhoun S, Zheng ZJ, Liao D. Metabolic syndrome burden in apparently healthy adolescents is adversely associated with cardiac autonomic modulation: Penn State Children Cohort. Metabolism. 2015;64:626–632. doi: 10.1016/j.metabol.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Hua Q, Lerman LO, Lerman A. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968–975. doi: 10.1016/j.mayocp.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol. 2015;35:1022–1029. doi: 10.1161/ATVBAHA.114.304591. [DOI] [PubMed] [Google Scholar]
- 112.Smith JP, Haddad EV, Taylor MB, Oram D, Blakemore D, Chen Q, Boutaud O, Oates JA. Suboptimal inhibition of platelet cyclooxygenase-1 by aspirin in metabolic syndrome. Hypertension. 2012;59:719–725. doi: 10.1161/HYPERTENSIONAHA.111.181404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feldman L, Tubach F, Juliard JM, Himbert D, Ducrocq G, Sorbets E, Triantafyllou K, Kerner A, Abergel H, Huisse MG, Roussel R, Esposito-Farèse M, Steg PG, Ajzenberg N. Impact of diabetes mellitus and metabolic syndrome on acute and chronic on-clopidogrel platelet reactivity in patients with stable coronary artery disease undergoing drug-eluting stent placement. Am Heart J. 2014;168:940–947.e5. doi: 10.1016/j.ahj.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111:73–78. doi: 10.1016/j.amjcard.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 115.Torriani M, Gill CM, Daley S, Oliveira AL, Azevedo DC, Bredella MA. Compartmental neck fat accumulation and its relation to cardiovascular risk and metabolic syndrome. Am J Clin Nutr. 2014;100:1244–1251. doi: 10.3945/ajcn.114.088450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van der Meer RW, Lamb HJ, Smit JW, de Roos A. MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology. 2012;264:21–37. doi: 10.1148/radiol.12110772. [DOI] [PubMed] [Google Scholar]
- 117.Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, McPherson J, Schiele F, Dudek D, Fahy M, Xu K, Lansky A, Templin B, Zhang Z, de Bruyne B, Serruys PW, Stone GW. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. 2012;5(suppl):S42–S52. doi: 10.1016/j.jcmg.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. 2011;52:1369–1377. doi: 10.2967/jnumed.110.082883. [DOI] [PubMed] [Google Scholar]
- 119.Almeida AL, Teixido-Tura G, Choi EY, Opdahl A, Fernandes VR, Wu CO, Bluemke DA, Lima JA. Metabolic syndrome, strain, and reduced myocardial function: multi-ethnic study of atherosclerosis. Arq Bras Cardiol. 2014;102:327–335. doi: 10.5935/abc.20140040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dinh W, Lankisch M, Nickl W, Gies M, Scheyer D, Kramer F, Scheffold T, Krahns T, Sause A, Füth R. Metabolic syndrome with or without diabetes contributes to left ventricular diastolic dysfunction. Acta Cardiol. 2011;66:167–174. doi: 10.2143/AC.66.2.2071247. [DOI] [PubMed] [Google Scholar]
- 121.Crendal E, Walther G, Dutheil F, Courteix D, Lesourd B, Chapier R, Naughton G, Vinet A, Obert P. Left ventricular myocardial dyssynchrony is already present in nondiabetic patients with metabolic syndrome. Can J Cardiol. 2014;30:320–324. doi: 10.1016/j.cjca.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 122.Tadic M, Cuspidi C, Sljivic A, Andric A, Ivanovic B, Scepanovic R, Ilic I, Jozika L, Marjanovic T, Celic V. Effects of the metabolic syndrome on right heart mechanics and function. Can J Cardiol. 2014;30:325–331. doi: 10.1016/j.cjca.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 123.Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ, Okamoto LJ. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 124.Efstathiou SP, Skeva II, Zorbala E, Georgiou E, Mountokalakis TD. Metabolic syndrome in adolescence: can it be predicted from natal and parental profile? The Prediction of Metabolic Syndrome in Adolescence (PREMA) study. Circulation. 2012;125:902–910. doi: 10.1161/CIRCULATIONAHA.111.034546. [DOI] [PubMed] [Google Scholar]
- 125.Martin RM, Patel R, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T, Thompson J, Gillman MW, Smith GD, Oken E. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation. 2014;129:321–329. doi: 10.1161/CIRCULATIONAHA.113.005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore BF, Clark ML, Bachand A, Reynolds SJ, Nelson TL, Peel JL. Interactions between diet and exposure to secondhand smoke on metabolic syndrome among children: NHANES 2007–2010. J Clin Endocrinol Metab. 2016;101:52–58. doi: 10.1210/jc.2015-2477. [DOI] [PubMed] [Google Scholar]
- 127.Rodriguez LA, Madsen KA, Cotterman C, Lustig RH. Added sugar intake and metabolic syndrome in US adolescents: cross-sectional analysis of the National Health and Nutrition Examination Survey 2005–2012. Public Health Nutr. 2016:1–11. doi: 10.1017/S1368980016000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carnethon MR, Loria CM, Hill JO, Sidney S, Savage PJ, Liu K Coronary Artery Risk Development in Young Adults study. Risk factors for the metabolic syndrome: the Coronary Artery Risk Development in Young Adults (CARDIA) study, 1985–2001. Diabetes Care. 2004;27:2707–2715. doi: 10.2337/diacare.27.11.2707. [DOI] [PubMed] [Google Scholar]
- 129.Wilsgaard T, Jacobsen BK. Lifestyle factors and incident metabolic syndrome: the Tromsø Study 1979–2001. Diabetes Res Clin Pract. 2007;78:217–224. doi: 10.1016/j.diabres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 130.Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, Heiss G. Life course socioeconomic conditions and metabolic syndrome in adults: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2009;19:875–883. doi: 10.1016/j.annepidem.2009.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang HT, Shim JY, Lee YJ, Linton JA, Park BJ, Lee HR. Reading nutrition labels is associated with a lower risk of metabolic syndrome in Korean adults: the 2007–2008 Korean NHANES. Nutr Metab Cardiovasc Dis. 2013;23:876–882. doi: 10.1016/j.numecd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 132.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, Whelton PK, He J InterASIA Collaborative Group. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 133.Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL. Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health. 2007;7:154. doi: 10.1186/1471-2458-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: effects of lifestyle changes. J Am Geriatr Soc. 2006;54:1909–1914. doi: 10.1111/j.1532-5415.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 135.Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Jokinen E, Sun C, Viikari JS, Dwyer T, Raitakari OT. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Arterioscler Thromb Vasc Biol. 2012;32:1024–1031. doi: 10.1161/ATVBAHA.111.243261. [DOI] [PubMed] [Google Scholar]
- 136.Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, Yates T, Biddle SJ. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7:e34916. doi: 10.1371/journal.pone.0034916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferreira I, Henry RM, Twisk JW, van Mechelen W, Kemper HC, Stehouwer CD Amsterdam Growth and Health Longitudinal Study. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med. 2005;165:875–882. doi: 10.1001/archinte.165.8.875. [DOI] [PubMed] [Google Scholar]
- 138.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505–512. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 139.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 140.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 141.Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30:503–510. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 142.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, Jacobs DR, Jr, Savage PJ. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- 143.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:1438–1444. doi: 10.2337/diacare.28.6.1438. [DOI] [PubMed] [Google Scholar]
- 144.Ferreira I, Twisk JW, van Mechelen W, Kemper HC, Stehouwer CD. Development of fatness, fitness, and lifestyle from adolescence to the age of 36 years: determinants of the metabolic syndrome in young adults: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med. 2005;165:42–48. doi: 10.1001/archinte.165.1.42. [DOI] [PubMed] [Google Scholar]
- 145.Mirmiran P, Noori N, Azizi F. A prospective study of determinants of the metabolic syndrome in adults. Nutr Metab Cardiovasc Dis. 2008;18:567–573. doi: 10.1016/j.numecd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 147.Cocate PG, Natali AJ, de Oliveira A, de Alfenas RC, do Peluzio MC, Longo GZ, dos Santos EC, Buthers JM, de Oliveira LL, Hermsdorff HH. Red but not white meat consumption is associated with metabolic syndrome, insulin resistance and lipid peroxidation in Brazilian middle-aged men. Eur J Prev Cardiol. 2015;22:223–230. doi: 10.1177/2047487313507684. [DOI] [PubMed] [Google Scholar]
- 148.Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O’Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults: the National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2012:1–10. doi: 10.1017/S1368980012004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baik I, Shin C. Prospective study of alcohol consumption and metabolic syndrome. Am J Clin Nutr. 2008;87:1455–1463. doi: 10.1093/ajcn/87.5.1455. [DOI] [PubMed] [Google Scholar]
- 150.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 152.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, Grave GD, Siervogel RM. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tong J, Boyko EJ, Utzschneider KM, McNeely MJ, Hayashi T, Carr DB, Wallace TM, Zraika S, Gerchman F, Leonetti DL, Fujimoto WY, Kahn SE. Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia. 2007;50:1156–1160. doi: 10.1007/s00125-007-0651-y. [DOI] [PubMed] [Google Scholar]
- 154.Vergnaud AC, Bertrais S, Oppert JM, Maillard-Teyssier L, Galan P, Hercberg S, Czernichow S. Weight fluctuations and risk for metabolic syndrome in an adult cohort. Int J Obes (Lond) 2008;32:315–321. doi: 10.1038/sj.ijo.0803739. [DOI] [PubMed] [Google Scholar]
- 155.Tomiyama H, Yamada J, Koji Y, Yambe M, Motobe K, Shiina K, Yamamoto Y, Yamashina A. Heart rate elevation precedes the development of metabolic syndrome in Japanese men: a prospective study. Hypertens Res. 2007;30:417–426. doi: 10.1291/hypres.30.417. [DOI] [PubMed] [Google Scholar]
- 156.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palaniappan L, Carnethon MR, Wang Y, Hanley AJ, Fortmann SP, Haffner SM, Wagenknecht L Insulin Resistance Atherosclerosis Study. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- 158.Acevedo M, Varleta P, Kramer V, Valentino G, Quiroga T, Prieto C, Parada J, Adasme M, Briones L, Navarrete C. Comparison of lipoprotein-associated phospholipase A2 and high sensitive C-reactive protein as determinants of metabolic syndrome in subjects without coronary heart disease: in search of the best predictor. Int J Endocrinol. 2015;2015:934681. doi: 10.1155/2015/934681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ryu S, Song J, Choi BY, Lee SJ, Kim WS, Chang Y, Kim DI, Suh BS, Sung KC. Incidence and risk factors for metabolic syndrome in Korean male workers, ages 30 to 39. Ann Epidemiol. 2007;17:245–252. doi: 10.1016/j.annepidem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 160.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–852. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.André P, Balkau B, Vol S, Charles MA, Eschwège E DESIR Study Group. Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care. 2007;30:2355–2361. doi: 10.2337/dc07-0440. [DOI] [PubMed] [Google Scholar]
- 162.Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D’Agostino RB, Vasan RS. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 163.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 164.Schindhelm RK, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ, Diamant M. Alanine aminotransferase and the 6-year risk of the metabolic syndrome in Caucasian men and women: the Hoorn Study. Diabet Med. 2007;24:430–435. doi: 10.1111/j.1464-5491.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 165.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D’Agostino RB, Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 166.Galletti F, Barbato A, Versiero M, Iacone R, Russo O, Barba G, Siani A, Cappuccio FP, Farinaro E, della Valle E, Strazzullo P. Circulating leptin levels predict the development of metabolic syndrome in middle-aged men: an 8-year follow-up study. J Hypertens. 2007;25:1671–1677. doi: 10.1097/HJH.0b013e3281afa09e. [DOI] [PubMed] [Google Scholar]
- 167.Iwanaga S, Sakano N, Taketa K, Takahashi N, Wang DH, Takahashi H, Kubo M, Miyatake N, Ogino K. Comparison of serum ferritin and oxidative stress biomarkers between Japanese workers with and without metabolic syndrome. Obes Res Clin Pract. 2014;8:e201–e298. doi: 10.1016/j.orcp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 168.Hassinen M, Lakka TA, Komulainen P, Gylling H, Nissinen A, Rauramaa R. C-reactive protein and metabolic syndrome in elderly women: a 12-year follow-up study. Diabetes Care. 2006;29:931–932. doi: 10.2337/diacare.29.04.06.dc05-2508. [DOI] [PubMed] [Google Scholar]
- 169.Laaksonen DE, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403–1410. doi: 10.1007/s00125-004-1472-x. [DOI] [PubMed] [Google Scholar]
- 170.Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, Yeung DC, Janus ED, Sham PC, Lam KS. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115:1537–1543. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 171.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–1624. doi: 10.2337/dc09-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yadav SS, Mandal RK, Singh MK, Verma A, Dwivedi P, Sethi R, Usman K, Khattri S. High serum level of matrix metalloproteinase 9 and promoter polymorphism –1562 C:T as a new risk factor for metabolic syndrome. DNA Cell Biol. 2014;33:816–822. doi: 10.1089/dna.2014.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nibali L, Tatarakis N, Needleman I, Tu YK, D’Aiuto F, Rizzo M, Donos N. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 175.Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol. 2012;2012:598180. doi: 10.1155/2012/598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oh SW, Han KH, Han SY, Koo HS, Kim S, Chin HJ. Association of sodium excretion with metabolic syndrome, insulin resistance, and body fat. Medicine (Baltimore) 2015;94:e1650. doi: 10.1097/MD.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Janczura M, Bochenek G, Nowobilski R, Dropinski J, Kotula-Horowitz K, Laskowicz B, Stanisz A, Lelakowski J, Domagala T. The relationship of metabolic syndrome with stress, coronary heart disease and pulmonary function: an occupational cohort-based study. PLoS One. 2015;10:e0133750. doi: 10.1371/journal.pone.0133750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, Ji Q. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15:105. doi: 10.1186/s12890-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–1855. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 180.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 181.Bateman LA, Slentz CA, Willis LH, Shields AT, Piner LW, Bales CW, Houmard JA, Kraus WE. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise –- STRRIDE-AT/RT) Am J Cardiol. 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grooms KN, Ommerborn MJ, Pham DQ, Djousse L, Clark CR. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999–2010. Am J Med. 2013;126:1059–1067. e1–4. doi: 10.1016/j.amjmed.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shin JY, Kim JY, Kang HT, Han KH, Shim JY. Effect of fruits and vegetables on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Int J Food Sci Nutr. 2015;66:416–425. doi: 10.3109/09637486.2015.1025716. [DOI] [PubMed] [Google Scholar]
- 184.Vázquez C, Botella-Carretero JI, Corella D, Fiol M, Lage M, Lurbe E, Richart C, Fernández-Real JM, Fuentes F, Ordóñez A, de Cos AI, Salas-Salvadó J, Burguera B, Estruch R, Ros E, Pastor O, Casanueva FF WISH-CARE Study Investigators. White fish reduces cardiovascular risk factors in patients with metabolic syndrome: the WISH-CARE study, a multicenter randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24:328–335. doi: 10.1016/j.numecd.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 185.Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nuñez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care. 2007;30:2957–2959. doi: 10.2337/dc07-1231. [DOI] [PubMed] [Google Scholar]
- 186.Babio N, Becerra-Tomás N, Martínez-González MÁ, Corella D, Estruch R, Ros E, Sayón-Orea C, Fitó M, Serra-Majem L, Arós F, Lamuela-Raventós RM, Lapetra J, Gómez-Gracia E, Fiol M, Díaz-López A, Sorlí JV, Martínez JA, Salas-Salvadó J PREDIMED Investigators. Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J Nutr. 2015;145:2308–2316. doi: 10.3945/jn.115.214593. [DOI] [PubMed] [Google Scholar]
- 187.Barreto FM, Colado Simão AN, Morimoto HK, Batisti Lozovoy MA, Dichi I, Helena da Silva Miglioranza L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition. 2014;30:939–942. doi: 10.1016/j.nut.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 188.Hill AM, Harris Jackson KA, Roussell MA, West SG, Kris-Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2015;102:757–770. doi: 10.3945/ajcn.114.104026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vernarelli JA, Lambert JD. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur J Nutr. 2013;52:1039–1048. doi: 10.1007/s00394-012-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shang F, Li X, Jiang X. Coffee consumption and risk of the metabolic syndrome: a meta-analysis. Diabetes Metab. 2016;42:80–87. doi: 10.1016/j.diabet.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 191.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 192.Maki KC, Fulgoni VL, 3rd, Keast DR, Rains TM, Park KM, Rubin MR. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab Syndr Relat Disord. 2012;10:363–372. doi: 10.1089/met.2012.0020. [DOI] [PubMed] [Google Scholar]
- 193.O’Neil CE, Keast DR, Nicklas TA, Fulgoni VL., 3rd Nut consumption is associated with decreased health risk factors for cardiovascular disease and metabolic syndrome in U.S. adults: NHANES 1999–2004. J Am Coll Nutr. 2011;30:502–510. doi: 10.1080/07315724.2011.10719996. [DOI] [PubMed] [Google Scholar]
- 194.Fulgoni VL, 3rd, Dreher M, Davenport AJ. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Nutr J. 2013;12:1. doi: 10.1186/1475-2891-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim YS, Xun P, He K. Fish consumption, long-chain omega-3 poly-unsaturated fatty acid intake and risk of metabolic syndrome: a meta-analysis. Nutrients. 2015;7:2085–2100. doi: 10.3390/nu7042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shin D, Joh HK, Kim KH, Park SM. Benefits of potassium intake on metabolic syndrome: the fourth Korean National Health and Nutrition Examination Survey (KNHANES IV) Atherosclerosis. 2013;230:80–85. doi: 10.1016/j.atherosclerosis.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 197.Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Can G. Determinants and definition of abdominal obesity as related to risk of diabetes, metabolic syndrome and coronary disease in Turkish men: a prospective cohort study. Atherosclerosis. 2007;191:182–190. doi: 10.1016/j.atherosclerosis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 198.Chacko SA, Song Y, Manson JE, Van Horn L, Eaton C, Martin LW, McTiernan A, Curb JD, Wylie-Rosett J, Phillips LS, Plodkowski RA, Liu S. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in post-menopausal women. Am J Clin Nutr. 2011;94:209–217. doi: 10.3945/ajcn.110.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Warensjö E, Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 200.Shuval K, Barlow CE, Finley CE, Gabriel KP, Schmidt MD, DeFina LF. Standing, obesity, and metabolic syndrome: findings from the Cooper Center Longitudinal Study. Mayo Clin Proc. 2015;90:1524–1532. doi: 10.1016/j.mayocp.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 201.Sabaté J, Wien M. A perspective on vegetarian dietary patterns and risk of metabolic syndrome. Br J Nutr. 2015;113(suppl 2):S136–S143. doi: 10.1017/S0007114514004139. [DOI] [PubMed] [Google Scholar]
- 202.Benseñor IM, Goulart AC, del Molina MC, de Miranda ÉJ, Santos IS, Lotufo PA. Thyrotropin levels, insulin resistance, and metabolic syndrome: a cross-sectional analysis in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Metab Syndr Relat Disord. 2015;13:362–369. doi: 10.1089/met.2015.0045. [DOI] [PubMed] [Google Scholar]
- 203.Vidot DC, Prado G, Hlaing WM, Florez HJ, Arheart KL, Messiah SE. Metabolic syndrome among marijuana users in the United States: an analysis of National Health and Nutrition Examination Survey Data. Am J Med. 2016;129:173–179. doi: 10.1016/j.amjmed.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cheriyath P, Duan Y, Qian Z, Nambiar L, Liao D. Obesity, physical activity and the development of metabolic syndrome: the Atherosclerosis Risk in Communities study. Eur J Cardiovasc Prev Rehabil. 2010;17:309–313. doi: 10.1097/HJR.0b013e32833189b8. [DOI] [PubMed] [Google Scholar]
12. CHRONIC KIDNEY DISEASE
ICD-10 N18.0. See Charts 12-1 through 12-8
Chart 12-1. Trends in adjusted* end-stage renal disease incidence rate by age group in the United States (2015 USRDS Annual Data Report, volume 2, Figure 1.4b).
USRDS indicates US Renal Data System.
*Adjusted for sex and race.
The standard population was the US population in 2011.
Source: Special analyses, USRDS end-stage renal disease database.
Chart 12-8. Prevalence of CVD in patients with end-stage renal disease (ESRD) by treatment modality, 2013 (2015 USRDS Annual Data Report, volume 2, Figure 9.2).
Point prevalent hemodialysis, peritoneal dialysis, and transplant patients at all ages, with Medicare as primary payer on January 1, 2011, who were continuously enrolled in Medicare Parts A and B from July, 1, 2010 to December 31, 2010; ESRD service date was at least 90 days before January 1, 2011; and survived past 2012.
AFIB indicates atrial fibrillation; AMI, acute myocardial infarction; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; PAD, peripheral arterial disease; SCA/VA, sudden cardiac arrest and ventricular arrhythmias; and USRDS, US Renal Data System.
Source: Special analyses, USRDS ESRD database.
Abbreviations Used in Chapter 12
| ACC | American College of Cardiology |
| ACR | albumin-to-creatinine ratio |
| ACTION | Acute Coronary Treatment and Intervention Outcomes Network |
| AF | atrial fibrillation |
| AFIB | atrial fibrillation |
| AHA | American Heart Association |
| AMI | acute myocardial infarction |
| ASHD | atherosclerotic heart disease |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| CHF | congestive heart failure |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CRIC | Chronic Renal Insufficiency Cohort |
| CVA/TIA | cerebrovascular accident/transient ischemic attack |
| CVD | cardiovascular disease |
| DES | drug-eluting stent |
| DM | diabetes mellitus |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| GBD | Global Burden of Disease |
| GFR | glomerular filtration rate |
| HBP | high blood pressure |
| HF | heart failure |
| HR | hazard ratio |
| HTN | hypertension |
| ICD-10 | International Classification of Diseases, 10th Revision |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| LMIC | low- and middle-income countries |
| MDRD | Modification of Diet in Renal Disease |
| MI | myocardial infarction |
| NHANES | National Health and Nutrition Examination Survey |
| OR | odds ratio |
| PAD | peripheral arterial disease |
| PCI | percutaneous coronary intervention |
| RR | relative risk |
| SBP | systolic blood pressure |
| SCA/VA | sudden cardiac arrest and ventricular arrhythmias |
| SCD | sudden cardiac death |
| SR | self-report |
| TAVR | transcatheter aortic valve replacement |
| USRDS | United States Renal Data System |
| VHD | valvular heart disease |
End-Stage Renal Disease
Prevalence, Incidence, and Risk Factors
(See Charts 12-1 through 12-3)
Chart 12-3. Estimated cumulative incidence of ESRD across categories of time-updated SBP among participants in the CRIC Study.
CRIC indicates Chronic Renal Insufficiency Cohort; ESRD, end-stage renal disease; and SBP, systolic blood pressure.
Reprinted from Anderson et al.2
Copyright © 2015, American College of Physicians. All Rights Reserved.
Reprinted with the permission of American College of Physicians, Inc.
ESRD is a condition that is most commonly associated with DM or HBP, occurs when the kidneys are functioning at a very low level, and is currently defined as the receipt of chronic renal replacement treatment such as hemodialysis, peritoneal dialysis, or kidney transplantation. The ESRD population increased more than 4-fold since the 1970s, when Medicare began providing reimbursement for ESRD treatment.
The number of people with prevalent ESRD continues to increase in the United States.1 This is primarily because of improved survival, because the incidence rate appears to be stabilizing or decreasing slightly.
Data from the 2015 annual report of the USRDS showed that on December 31, 2013, there were 661 648 prevalent cases of ESRD in the United States. Of these, 70% were being treated with dialysis and 29% had a functioning kidney transplant.1
Since 2006, the incidence rate of new cases of ESRD has been declining in the United States, and declines are greatest among historically high-risk groups such as adults >75 years old and Native Americans1 (Chart 12-1).
In 2013, 117 162 new cases of ESRD were reported. Using a 2011 US population standard, the age-, sex-, and race/ethnicity-adjusted incidence rate of 352 per million/year is the lowest since 1997.1
The incidence rate (per million/year) of ESRD differs regionally across the United States (Chart 12-2).
Older age, DM, and hypertension are the most prevalent risk factors for advanced kidney disease in high-income countries.
SBP maintains a strong and graded association with increased ESRD risk among the general population and among individuals with CKD2,3 (Chart 12-3). BP-associated risks were greater in males than in females and in blacks than in whites.4
Results from a large community-based population showed that higher BMI was associated with an increased risk of ESRD.5 In a separate study, the association between BMI and ESRD was reported to be modified by metabolic risk factors, and a strong association was present between metabolic syndrome and ESRD risk independent of BMI.6
Chart 12-2. Map of the adjusted* incidence rate (per million/year) of end-stage renal disease by health service area in the US population, 2013 (2015 USRDS Annual Data Report, volume 2, Figure 1.3).
USRDS indicates US Renal Data System.
*Adjusted for age, sex, and race. The standard population was the US population in 2011.
Source: Special analyses, USRDS end-stage renal disease database.
Demographics
In 2013, the mean age of new ESRD cases was 62 years; 58% were male, 26% were African American, and 15% were Hispanic.1
Treatment of ESRD is more common in males than in females.1
Blacks, Hispanics, Asian Americans, and Native Americans have significantly higher rates of ESRD than do whites/Europeans. Blacks represent nearly one third of treated patients with ESRD.1
Compared with white patients with similar levels of kidney function, black patients are much more likely to progress to ESRD and are on average 10 years younger when they reach ESRD.7,8
The higher incidence of ESRD among blacks than whites is explained in part by the higher prevalence of albuminuria in this population; however, even after controlling for major ESRD risk factors, blacks have a higher risk of ESRD than whites.9
The geographic distribution of incident cases mirrors that of traditional risk factors such as DM and HBP.1
Chronic Kidney Disease
Definition, Staging, and Estimating GFR
(See Chart 12-4)
Chart 12-4. Percentage of NHANES participants within the KDIGO 2012 prognosis of chronic kidney disease by GFR and albuminuria categories, 1998 to 2012 (2014 USRDS Annual Report, volume 1, Table 1.2).
GFR indicates glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; NHANES, National Health and Nutrition Examination Survey; and USRDS, US Renal Data System.
CKD is a serious health condition and a worldwide public health problem. The incidence and prevalence of CKD are increasing in the United States and are associated with poor outcomes and a high cost to the US healthcare system.
CKD is usually defined as reduced GFR (<60 mL·min−1·1.73 m−2), excess urinary albumin excretion (≥30 mg/d or mg/gCr), or both.
In 2002, the National Kidney Foundation Kidney Disease Outcome Quality Initiative defined stages of CKD according to level of eGFR and whether there was other evidence of kidney damage (eg, presence of albuminuria).10
The KDIGO working group released a 2012 update recommending classification of CKD by cause, GFR, and albuminuria category11 (Chart 12-4).
The CKD-EPI Collaboration has developed equations to more accurately estimate GFR from serum creatinine compared with the previously established MDRD Study equation.12
Serum cystatin C, another marker of kidney function, has been proposed to be a more sensitive indicator of kidney function than serum creatinine and creatinine-based estimating formulas at higher levels of GFR. It is a low-molecular-weight protein produced at a constant rate by all nucleated cells and appears not to be affected significantly across ages, sexes, and levels of muscle mass. Cystatin C is excreted by the kidneys, filtered through the glomerulus, and nearly completely reabsorbed by proximal tubular cells.13 The CKD-EPI collaboration has proposed equations to estimate kidney function using cystatin C alone or in combination with serum creatinine.14
Prevalence
(See Chart 12-4)
-
According to the USRDS 2015 annual data report,1 based on NHANES data in 2007 to 2012
The overall prevalence of CKD (stages 1–5) was 13.6%.
The prevalence of stage 1 CKD (eGFR ≥90 mL·min−1·1.73 m−2 with kidney damage) was 4.2%.
The prevalence of stage 2 CKD (eGFR 60–89 mL·min−1·1.73 m−2 with kidney damage) was 3.0%.
The prevalence of stage 3 CKD (eGFR 30–59 mL·min−1·1.73 m−2) was 5.9%.
The prevalence of stages 4 and 5 CKD (eGFR <30 mL·min−1·1.73 m−2) was 0.6%.
Chart 12-4 shows the 2007 to 2012 NHANES prevalence data by 2012 KDIGO category. More than 85% of the participants across NHANES studies from 1988 through 2012 would be considered low risk for CKD diagnosis within the KDIGO 2012 prognosis of CKD.15
For US adults aged 30 to 49, 50 to 64, and ≥65 years with no CKD at baseline, the residual lifetime incidences of CKD are 54%, 52%, and 42%, respectively. The prevalence of CKD in adults ≥30 years of age is projected to increase to 14.4% in 2020 and 16.7% in 2030.16
More than 80% of individuals with CKD are unaware of their diagnosis.17,18 Additionally, providers might not regularly screen for CKD. For example, only 12% of Medicare beneficiaries without a diagnosis of CKD had a urine albumin test in 2013.1
Demographics
-
The prevalence of CKD was higher with older age, as follows1:
5.7% for those 20 to 39 years of age
8.9% for those 40 to 59 years of age
33.2% for those ≥60 years of age
Among the very old (>80 years), the prevalence of an eGFR <60 mL·min−1·1.73 m−2 increased from 40.5% in 1988 to 1994 to 49.9% and 51.2% in 1999 to 2004 and 2005 to 2010, respectively. The prevalence of albuminuria (albumin-to-creatinine ratio ≥30 mg/g) was 30.9%, 33.0%, and 30.6% in 1988 to 1994, 1999 to 2004, and 2005 to 2010, respectively.19,20
In 2007 to 2012, the prevalence of CKD was higher at older age and among women. By race/ethnicity category, the prevalence of CKD was highest among non-Hispanic blacks.1
Risk Factors
(See Chart 12-5)
Chart 12-5. Adjusted odds ratios of chronic kidney disease (CKD) in NHANES by risk factor, 1998 to 2012 (2015 USRDS Annual Data Report, volume 1, Figure 1.9).
CKD defined as presence of estimated glomerular filtration rate (eGFR) <60 mL·min−1·1.73 m−2, urine albumin-to-creatinine ratio (ACR) ≥30 mg/g, and either eGFR <60 mL·min−1·1.73 m−2 or ACR ≥30 mg/g for each of the comorbid conditions. Adjusted for age, sex, and race; single-sample estimates of eGFR and ACR; eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation. Whisker lines indicate 95% confidence intervals.
BMI indicates body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension; NHANES, National Health and Nutrition Examination Survey; SR, self-report; and USRDS, US Renal Data System. Source: NHANES 1988 to 1994, 1999 to 2004, and 2007 to 2012 participants aged ≥20 years.
Many traditional CVD risk factors are also risk factors for CKD, including older age, male sex, hypertension, DM, smoking, and family history of CVD (Chart 12-5). Among those with CKD in NHANES, ≈30% have HBP and ≈40% have DM. Nearly 17% are obese (BMI >30 kg/m2). Adjusted (age, sex, race) ORs for CKD were 3.8 for HBP, 3.1 for DM, and 1.47 for obesity.1 Time-updated BP is associated with CKD progression.2
Recent evidence suggests that BMI is associated with worsening CKD. In a cohort of 652 African American individuals with hypertensive nephrosclerosis, BMI was independently associated with urine total protein and albumin excretion.21 In a nationally representative sample of >3 million veterans, obstructive sleep apnea was associated with CKD and CKD progression independent of BMI and other traditional risk factors.22
Importantly, cardiovascular fitness and healthy lifestyles are associated with decreased risk of CKD and CKD progression.23,24
Both the degree of CKD (ie, eGFR) and urine albumin are strongly associated with the progression from CKD to ESRD. Furthermore, urine albumin level is associated with progression to CKD across all levels of reduced eGFR.25
Other risk factors include systemic conditions such as autoimmune diseases, systemic infections, and drug exposure, as well as anatomically local conditions such as urinary tract infections, urinary stones, lower urinary tract obstruction, and neoplasia. Even after adjustment for these risk factors, excess CVD risk remains.26
Kidney Disease and CVD
(See Charts 12-6, 12-7, and 12-8)
Chart 12-6. Adjusted relative risk of (A) all-cause mortality and (B) cardiovascular mortality in the general population categorized by KDIGO 2012 categories of chronic kidney disease.
Data are derived from categorical meta-analysis of population cohorts. Pooled relative risks are expressed relative to the reference (Ref) cell. Colors represent the ranking of the adjusted relative risks (green=low risk; yellow=moderate risk; orange=high risk; red=very high risk).
ACR indicates urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; and KDIGO, Kidney Disease: Improving Global Outcomes.
Modified from Levey et al80 with permission from International Society of Nephrology. Copyright © 2011, International Society of Nephrology.
Chart 12-7. CVD in patients with or without CKD, 2013 (2015 USRDS Annual Data Report, volume 1, Figure 4.1).
Patients aged ≥66, alive, without end-stage renal disease, and residing in the United States on December 31, 2013 with fee-for-service coverage for the entire calendar year. Totals of patients for the study cohort: N=1 238 888; with CKD=132 840; without CKD=1 106 048.
AFIB indicates atrial fibrillation; AMI, acute myocardial infarction; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; PAD, peripheral artery disease; SCA/VA, sudden cardiac arrest and ventricular arrhythmias; USRDS, US Renal Data System; and VHD, valvular heart disease.
Source: Special analyses, Medicare 5% sample.
The adjusted RR of all-cause mortality and cardiovascular mortality is highest in those with eGFR 15 to 30 mL·min−1·1.73 m−2 and those with a urine albumin-to-creatinine ratio >300 mg/g (Chart 12-6).
Controversy exists about whether CKD itself independently causes incident CVD, but it is clear that people with CKD, as well as those with ESRD, represent a population at very high risk for CVD events (Chart 12-7.) In fact, individuals with CKD are 6 times more likely to die of CVD than to transition to ESRD.27
-
CVD is a leading cause of death among those with kidney disease.
CVD mortality is 5 to 30 times higher in dialysis patients than in subjects from the general population of the same age, sex, and race.28,29
Individuals with earlier-stage kidney disease are also at significantly increased CVD risk independent of typical CVD risk factors.30
CKD is also a risk factor for recurrent CVD events,31,32 stroke,33 HF,34 and AF.35
Cardiovascular prognosis depends not only on eGFR30,36–41 but also on category of albuminuria.11,34,42–47 Both eGFR and albuminuria appear to be more strongly associated with cardiovascular mortality and HF than CHD or stroke.34
In a study of 1.3 million people, the risk for MI was 18.5 per 1000 person-years for those with a history of MI, 6.9 per 1000 person-years for those with CKD but no history of MI or DM, and 5.4 per 1000 person-years among those without MI or CKD but with DM.48
The association of CKD with CVD risk has been reported to be similar across age, race, and sex subgroups.49
GFR appears to predict stroke risk but might not be as strongly associated as albuminuria. In 4 community-based cohorts, lower eGFR (45 versus 95 mL·min−1·1.73 m−2) was associated with an increased risk for ischemic stroke (HR, 1.30; 95% CI, 1.01–1.68) but not hemorrhagic stroke (HR, 0.92; 95% CI, 0.47–1.81). Albuminuria (albumin-to-creatinine ratio of 300 versus 5 mg/g) was associated with both ischemic and hemorrhagic stroke (HR, 1.62 [95% CI, 1.27–2.07] and 2.57 [95% CI, 1.37–4.83], respectively).50 In a meta-analysis of 83 studies of >30 000 strokes, there were linear relationships of both eGFR and albuminuria with stroke regardless of stroke subtype.33 Among patients with CKD, proteinuria but not eGFR independently predicted stroke risk.51
-
Elevated levels of the filtration marker cystatin C have been associated with increased risk for all-cause mortality in studies from a broad range of cohorts.52–54 In addition to eGFR and urine albumin-to-creatinine ratio, cystatin C provides incremental information for the prediction of clinical outcomes.
The addition of cystatin C to the combination of creatinine and albumin-to-creatinine ratio significantly improves the prediction of all-cause mortality, cardiovascular death, and development of ESRD.55,56
Cystatin C is independently associated with a range of cardiovascular outcomes including stroke, MI,57 HF,58,59 PAD events,60 subclinical measures of CVD,61 recurrent cardiovascular events,52,62 and SCD.63
This strengthened associations with outcomes might be explained in part by non-GFR determinants of cystatin C such as inflammation, obesity, and DM.55,64,65
On the basis of data from the USRDS ESRD database, the prevalence of CVD in ESRD patients differs by treatment modality. Approximately 65% of ESRD patients on hemodialysis have any CVD, whereas ≈38% of ESRD transplant patients have any CVD (Chart 12-8).
One potential explanation for the higher CVD event rate in patients with CKD is the low uptake of standard therapies for patients presenting with MI. In a recent analysis from the ACTION registry, patients presenting with CKD had a substantially higher mortality rate. In addition, patients with CKD were less likely to receive standard therapies for the treatment of MI.66
In a nationwide US cohort that included 4726 participants with CKD, 2366 (50%) were taking statins, and 1984 participants (42%) met recommendations for statin treatment according to the ACC/AHA guidelines but were not using statins.67 The Pooled Cohort risk equations were well calibrated (Hosmer-Lemeshow χ2=2.7, P=0.45), with moderately good discrimination (C index, 0.71; 95% CI, 0.65–0.77).67
-
People with advanced CKD and ESRD are often excluded from clinical trials of cardiovascular drugs and devices, although recent observational data from large registries can provide insight into the risks and benefits in this population.
For CKD and ESRD patients with multivessel CAD, coronary bypass surgery may be associated with improved outcomes compared with PCI.68 Similar findings were seen in a Northern California Kaiser Permanente cohort.69
In a study of >12 000 patients undergoing hemodialysis in the USRDS who had AF, only 15% initiated warfarin therapy within 30 days, and 70% discontinued use within 1 year.70 Warfarin was marginally associated with reductions in ischemic stroke and mortality.
In 4880 veterans with CKD who had PCI, dual-antiplatelet therapy use beyond 12 months decreased risk of death or MI for those receiving first-generation DES.71
Patients with moderate kidney disease (eGFR <60 mL·min−1·1.73 m−2) and left bundle-branch block (but not other morphologies) appear to derive greater absolute reductions in death and HF from cardiac resynchronization with a defibrillator than patients with higher eGFR72; however, in a smaller propensity-matched cohort study, primary prevention implantation of an implantable cardioverter-defibrillator was not associated with improved 3-year mortality among ESRD patients with systolic HF.73
People with CKD are at higher risk of complications after PCI, and accurate estimation of renal function is required to dose antiplatelet and antithrombotic medications. Compared with older equations, the CKD-EPI eGFR equation more accurately predicted kidney outcomes and appropriate drug dosing in a large sample of nearly 130 000 patients undergoing PCI in Michigan.74
For patients undergoing TAVR in the United Kingdom, CKD (eGFR <45 mL·min−1·1.73 m−2) is associated with higher odds of in-hospital (adjusted OR, 1.45; 95% CI; 1.03–2.05) and longer-term (median, 543 days; adjusted OR, 1.36; 95% CI, 1.17–1.58) mortality.75
Costs
The total annual cost of treating ESRD in the United States was $30.9 billion in 2013, which represents >7% of total Medicare claims paid.1
Among US Medicare beneficiaries, average yearly costs per person with any CKD were $21 909 in 2013, although costs were higher for those with comorbid DM ($24 916) and HF ($34 715).1 Yearly per person costs attributable specifically to stage 1, 2, 3, and 4 CKD, respectively, compared with no CKD were $1600 (95% CI, −$900 to $3870), $1700 (95% CI, $530–$2840), $3500 (95% CI, $1780–$4620), and $12 700 (95% CI, $6000–$49 650).76
In the Study of Heart and Renal Protection of patients in Europe, North America, and Australasia, nonfatal major cardiovascular events were associated with £6133 (95% CI, £5608–£6658) higher costs for ESRD patients on dialysis and £4350 (95% CI, £3819–£4880) for other CKD patients in the year of the event.77
Global Burden
In 2013, the incidence rate of treated ESRD in the United States was one of the highest in the world, surpassed only by Taiwan and Jalisco State in Mexico among countries for which data were available.1
-
The total prevalence of CKD is rising globally, primarily because of aging populations. The GBD study has estimated the global prevalence of CKD by category and the percentage change from 1990 to 201378:
The prevalence of CKD attributable to DM rose 82% during this time period to 89 million (95% CI, 71–111 million) in 2013, and age-standardized prevalence rose 12%.
CKD attributable to HBP rose 27% to 101 million (95% CI, 81–130 million) in 2013, but age-standardized prevalence decreased 11%.
CKD attributable to glomerulonephritis rose 33% to 109 million (95% CI, 88–135 million) in 2013, but age standardized prevalence decreased 14%.
CKD attributable to other causes rose 54% to 173 million (95% CI, 142–213 million) in 2013, and age-standardized prevalence rose 3%.
Globally, total years lived with disability attributable to CKD were 12.3 million (95% CI, 9.1–15.8 million) in 2013.78
CKD rose from the 36th leading cause of death in 1990 to the 19th leading cause of death in 2013.79
In LMIC, the burden of CKD is high, but data on the magnitude of the association between CKD and various cardiovascular outcomes are lacking. These data are necessary to properly model the public health and economic burden of CKD in these countries.
REFERENCES
- 1.United States Renal Data System. 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2015. [Accessed June 8, 2016]. https://www.usrds.org/2015/view/Default.aspx. [Google Scholar]
- 2.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER, 3rd, Rahman M, Steigerwalt S, Weir M, Wright JT, Jr, Feldman HI Chronic Renal Insufficiency Cohort Study Investigators. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–265. doi: 10.7326/M14-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell EK, Gao L, Judd S, Glasser SP, McClellan W, Gutiérrez OM, Safford M, Lackland DT, Warnock DG, Muntner P. Blood pressure indexes and end-stage renal disease risk in adults with chronic kidney disease. Am J Hypertens. 2012;25:789–796. doi: 10.1038/ajh.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Panwar B, Hanks LJ, Tanner RM, Muntner P, Kramer H, McClellan WM, Warnock DG, Judd SE, Gutiérrez OM. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int. 2015;87:1216–1222. doi: 10.1038/ki.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 8.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122:672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJ, Solomon SD, Levey AS, Ivanovich P, Eckardt KU, Kewalramani R, Toto R, Pfeffer MA. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. Am Heart J. 2015;170:322–329. doi: 10.1016/j.ahj.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 11.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR: history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clin-biochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Renal Data System. 2014 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2014. [Google Scholar]
- 16.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65:403–411. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuot DS, Plantinga LC, Hsu CY, Jordan R, Burrows NR, Hedgeman E, Yee J, Saran R, Powe NR Centers for Disease Control Chronic Kidney Disease Surveillance Team. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17:225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowling CB, Sharma P, Fox CS, O’Hare AM, Muntner P. Prevalence of reduced estimated glomerular filtration rate among the oldest old from 1988–1994 through 2005–2010. JAMA. 2013;310:1284–1286. doi: 10.1001/jama.2013.252441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowling CB, Sharma P, Muntner P. Prevalence, trends and functional impairment associated with reduced estimated glomerular filtration rate and albuminuria among the oldest-old U.S. adults. Am J Med Sci. 2014;348:115–120. doi: 10.1097/MAJ.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toto RD, Greene T, Hebert LA, Hiremath L, Lea JP, Lewis JB, Pogue V, Sika M, Wang X AASK Collaborative Research Group. Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis. 2010;56:896–906. doi: 10.1053/j.ajkd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Mucsi I, Novak M, Szabo Z, Freire AX, Huch KM, Arah OA, Ma JZ, Lu JL, Sim JJ, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70:888–895. doi: 10.1136/thoraxjnl-2015-206970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, Fink JC, Frydrych A, Jensvold NG, Lustigova E, Nessel LC, Porter AC, Rahman M, Wright Nunes JA, Daviglus ML, Lash JP CRIC Study Investigators. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokkinos P, Faselis C, Myers J, Sui X, Zhang J, Tsimploulis A, Chawla L, Palant C. Exercise capacity and risk of chronic kidney disease in US veterans: a cohort study. Mayo Clin Proc. 2015;90:461–468. doi: 10.1016/j.mayocp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR Alberta Kidney Disease Network. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Coresh J, Astor B, Sarnak MJ. Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2004;13:73–81. doi: 10.1097/00041552-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarnak MJ, Coronado BE, Greene T, Wang SR, Kusek JW, Beck GJ, Levey AS. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002;57:327–335. doi: 10.5414/cnp57327. [DOI] [PubMed] [Google Scholar]
- 29.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS, Sarnak MJ. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 31.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Baber U, Gutierrez OM, Levitan EB, Warnock DG, Farkouh ME, Tonelli M, Safford MM, Muntner P. Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am Heart J. 2013;166:373–380.e2. doi: 10.1016/j.ahj.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:1162–1169. doi: 10.1093/ndt/gfv009. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 37.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 38.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 39.Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A. Renal function and intensive lowering of blood pressure in hypertensive participants of the Hypertension Optimal Treatment (HOT) study. J Am Soc Nephrol. 2001;12:218–225. doi: 10.1681/ASN.V122218. [DOI] [PubMed] [Google Scholar]
- 40.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 41.Hailpern SM, Cohen HW, Alderman MH. Renal dysfunction and ischemic heart disease mortality in a hypertensive population. J Hypertens. 2005;23:1809–1816. doi: 10.1097/01.hjh.0000183120.92455.2a. [DOI] [PubMed] [Google Scholar]
- 42.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULA-TIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 43.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 44.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 45.Yuyun MF, Adler AI, Wareham NJ. What is the evidence that micro-albuminuria is a predictor of cardiovascular disease events? Curr Opin Nephrol Hypertens. 2005;14:271–276. doi: 10.1097/01.mnh.0000165895.90748.3b. [DOI] [PubMed] [Google Scholar]
- 46.Wattanakit K, Folsom AR, Criqui MH, Kramer HJ, Cushman M, Shea S, Hirsch AT. Albuminuria and peripheral arterial disease: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2008;201:212–216. doi: 10.1016/j.atherosclerosis.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT PREVEND Study Group. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23:3851–3858. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- 48.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380:807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 49.Hui X, Matsushita K, Sang Y, Ballew SH, Fülöp T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. Am J Kidney Dis. 2013;62:691–702. doi: 10.1053/j.ajkd.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, Woodward M, Longstreth WT, Jr, Psaty BM, Shlipak MG, Folsom AR, Gansevoort RT, Coresh J. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke. 2014;45:1925–1931. doi: 10.1161/STROKEAHA.114.004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandsmark DK, Messé SR, Zhang X, Roy J, Nessel L, Lee Hamm L, He J, Horwitz EJ, Jaar BG, Kallem RR, Kusek JW, Mohler ER, 3rd, Porter A, Seliger SL, Sozio SM, Townsend RR, Feldman HI, Kasner SE CRIC Study Investigators. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: Chronic Renal Insufficiency Cohort Study. Stroke. 2015;46:2075–2080. doi: 10.1161/STROKEAHA.115.009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 54.Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF. Cystatin C and mortality risk in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 55.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muntner P, Mann D, Winston J, Bansilal S, Farkouh ME. Serum cystatin C and increased coronary heart disease prevalence in US adults without chronic kidney disease. Am J Cardiol. 2008;102:54–57. doi: 10.1016/j.amjcard.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 58.Djoussé L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians’ Health Study (PHS) Am Heart J. 2008;155:82–86. doi: 10.1016/j.ahj.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG Cardiovascular Health Study. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 60.O’Hare AM, Newman AB, Katz R, Fried LF, Stehman-Breen CO, Seliger SL, Siscovick DS, Shlipak MG. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005;165:2666–2670. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- 61.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis. 2009;204:298–303. doi: 10.1016/j.atherosclerosis.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 63.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUT-COMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muntner P, Winston J, Uribarri J, Mann D, Fox CS. Overweight, obesity, and elevated serum cystatin C levels in adults in the United States. Am J Med. 2008;121:341–348. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schei J, Stefansson VT, Mathisen UD, Eriksen BO, Solbu MD, Jenssen TG, Melsom T. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol. 2016;11:280–286. doi: 10.2215/CJN.07360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD Acute Coronary Treatment and Intervention Outcomes Network registry. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colantonio LD, Baber U, Banach M, Tanner RM, Warnock DG, Gutiérrez OM, Safford MM, Wanner C, Howard G, Muntner P. Contrasting cholesterol management guidelines for adults with CKD. J Am Soc Nephrol. 2015;26:1173–1180. doi: 10.1681/ASN.2014040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Revascularization in patients with multivessel coronary artery disease and chronic kidney disease: everolimus-eluting stents versus coronary artery bypass graft surgery. J Am Coll Cardiol. 2015;66:1209–1220. doi: 10.1016/j.jacc.2015.06.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnaswami A, McCulloch CE, Tawadrous M, Jang JJ, Lee H, Melikian V, Yee G, Leong TK, Go AS. Coronary artery bypass grafting and percutaneous coronary intervention in patients with end-stage renal disease. Eur J Cardiothorac Surg. 2015;47:e193–e198. doi: 10.1093/ejcts/ezv104. [DOI] [PubMed] [Google Scholar]
- 70.Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–688. doi: 10.1053/j.ajkd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siddiqi OK, Smoot KJ, Dufour AB, Cho K, Young M, Gagnon DR, Ly S, Temiyasathit S, Faxon DP, Gaziano JM, Kinlay S. Outcomes with prolonged clopidogrel therapy after coronary stenting in patients with chronic kidney disease. Heart. 2015;101:1569–1576. doi: 10.1136/heartjnl-2014-307168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daimee UA, Moss AJ, Biton Y, Solomon SD, Klein HU, McNitt S, Polonsky B, Zareba W, Goldenberg I, Kutyifa V. Long-term outcomes with cardiac resynchronization therapy in patients with mild heart failure with moderate renal dysfunction. Circ Heart Fail. 2015;8:725–732. doi: 10.1161/CIRCHEARTFAILURE.115.002082. [DOI] [PubMed] [Google Scholar]
- 73.Pun PH, Hellkamp AS, Sanders GD, Middleton JP, Hammill SC, Al-Khalidi HR, Curtis LH, Fonarow GC, Al-Khatib SM. Primary prevention implantable cardioverter defibrillators in end-stage kidney disease patients on dialysis: a matched cohort study. Nephrol Dial Transplant. 2015;30:829–835. doi: 10.1093/ndt/gfu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parsh J, Seth M, Aronow H, Dixon S, Heung M, Mehran R, Gurm HS. Choice of estimated glomerular filtration rate equation impacts drug-dosing recommendations and risk stratification in patients with chronic kidney disease undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2015;65:2714–2723. doi: 10.1016/j.jacc.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 75.Ferro CJ, Chue CD, de Belder MA, Moat N, Wendler O, Trivedi U, Ludman P, Townend JN UK TAVI Steering Group; National Institute for Cardiovascular Outcomes Research. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): an analysis of the UK TAVI registry. Heart. 2015;101:546–552. doi: 10.1136/heartjnl-2014-307041. [DOI] [PubMed] [Google Scholar]
- 76.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24:1478–1483. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kent S, Schlackow I, Lozano-Kühne J, Reith C, Emberson J, Haynes R, Gray A, Cass A, Baigent C, Landray MJ, Herrington W, Mihaylova B SHARP Collaborative Group. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65. doi: 10.1186/s12882-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report [published correction appears in Kidney Int. 2011;80:1000] Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
13. TOTAL CARDIOVASCULAR DISEASES
ICD-9 390 to 459, ICD-10 I00 to I99. See Tables 13-1 through 13-3 and Charts 13-1 through 13-23
Table 13-1.
Cardiovascular Diseases
| Population Group | Prevalence, 2011–2014: Age ≥20 y | Mortality, 2014: All Ages* | Hospital Discharge, 2010: All Ages | Cost, 2012–2013 |
|---|---|---|---|---|
| Both sexes | 92 100 000 (36.6%) | 807 775 | 5 802 000 | $316.1 Billion |
| Males | 44 300 000 (37.4%) | 408 747 (50.6%)† | 3 021 000 | … |
| Females | 47 800 000 (35.9%) | 399 028 (49.4%)† | 2 781 000 | … |
| NH white males | 37.7% | 320 859 | … | … |
| NH white females | 35.1% | 316 843 | … | … |
| NH black males | 46.0% | 49 210 | … | … |
| NH black females | 47.7% | 48 573 | … | … |
| Hispanic males | 31.3% | 24 875 | … | … |
| Hispanic females | 33.3% | 21 571 | … | … |
| NH Asian males | 31.0% | 9784‡ | … | … |
| NH Asian females | 27.0% | 9147‡ | … | … |
| NH American Indian/Alaska Native | … | 4054 | … | … |
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for Hispanic, American Indian or Alaska Native, and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total cardiovascular disease mortality that is attributable to males vs females.
Includes Chinese, Filipino, Hawaiian, Japanese, and other Asian or Pacific Islander.
Sources: Prevalence: National Health and Nutrition Examination Survey 2011 to 2014, National Center for Health Statistics (NCHS) and National Heart, Lung, and Blood Institute (NHLBI). Percentages for racial/ethnic groups are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolated to the 2014 US population estimates. Mortality: Centers for Disease Control and Prevention/NCHS, 2014 Mortality Multiple Cause-of-Death–United States. These data represent underlying cause of death only for International Classification of Diseases, 10th Revision codes I00 to I99 (diseases of the circulatory system). Mortality for NH Asians includes Pacific Islanders. Hospital discharges: National Hospital Discharge Survey, NCHS. Data include those inpatients discharged alive, dead, or of unknown status. Cost: NHLBI. Data include estimated direct and indirect costs for 2012 to 2013 (annual average).
Table 13-3.
Death Rates for Cardiovascular Diseases and All Causes in Selected Countries
| Sorted Alphabetically by Country | CVD | CHD | Stroke | Total | Sorted by Descending CVD Death Rate | CVD | CHD | Stroke | Total |
|---|---|---|---|---|---|---|---|---|---|
| Males aged 35–74 y | |||||||||
| Australia (11) | 126.1 | 76.9 | 18.7 | 530.3 | Belarus (11) | 1178.0 | 826.0 | 232.0 | 2375.9 |
| Austria (14) | 169.4 | 98.6 | 23.1 | 671.6 | Russian Federation (11) | 1087.0 | 610.2 | 268.2 | 2254.4 |
| Belarus (11) | 1178.0 | 826.0 | 232.0 | 2375.9 | Ukraine (12) | 1067.2 | 718.1 | 216.6 | 2069.3 |
| Belgium (12) | 151.8 | 64.4 | 26.1 | 734.9 | Bulgaria (12) | 740.1 | 184.2 | 187.4 | 1426.4 |
| Bulgaria (12) | 740.1 | 184.2 | 187.4 | 1426.4 | Romania (12) | 594.6 | 242.9 | 167.0 | 1427.8 |
| Croatia (13) | 356.7 | 181.3 | 93.6 | 1050.8 | Hungary (13) | 499.7 | 268.1 | 95.4 | 1378.5 |
| Czech Republic (13) | 340.8 | 191.9 | 52.0 | 979.1 | Croatia (13) | 356.7 | 181.3 | 93.6 | 1050.8 |
| Denmark (12) | 135.8 | 57.3 | 29.1 | 694.9 | Czech Republic (13) | 340.8 | 191.9 | 52.0 | 979.1 |
| Finland (13) | 230.0 | 128.2 | 37.9 | 727.4 | United States (14) | 233.0 | 125.3 | 27.1 | 810.7 |
| France (11) | 121.2 | 46.4 | 22.6 | 718.4 | Finland (13) | 230.0 | 128.2 | 37.9 | 727.4 |
| Germany (13) | 197.9 | 96.2 | 27.4 | 740.3 | Germany (13) | 197.9 | 96.2 | 27.4 | 740.3 |
| Hungary (13) | 499.7 | 268.1 | 95.4 | 1378.5 | Ireland (12) | 180.2 | 117.4 | 20.3 | 614.2 |
| Ireland (12) | 180.2 | 117.4 | 20.3 | 614.2 | Austria (14) | 169.4 | 98.6 | 23.1 | 671.6 |
| Israel (13) | 106.2 | 48.7 | 23.7 | 548.1 | Taiwan (13) | 169.4 | 48.4 | 54.1 | 841.6 |
| Italy (12) | 141.8 | 64.9 | 25.5 | 579.1 | United Kingdom (13) | 166.5 | 103.3 | 24.3 | 620.3 |
| Japan (13) | 132.4 | 43.8 | 43.5 | 563.8 | New Zealand (11) | 164.8 | 106.3 | 25.9 | 576.1 |
| Korea, South (13) | 112.4 | 32.4 | 48.7 | 672.4 | Belgium (12) | 151.8 | 64.4 | 26.1 | 734.9 |
| Netherlands (13) | 127.0 | 47.0 | 23.5 | 573.4 | Sweden (13) | 151.1 | 83.5 | 22.3 | 519.3 |
| New Zealand (11) | 164.8 | 106.3 | 25.9 | 576.1 | Italy (12) | 141.8 | 64.9 | 25.5 | 579.1 |
| Norway (13) | 123.9 | 66.8 | 22.2 | 550.5 | Portugal (13) | 141.5 | 52.4 | 48.9 | 764.5 |
| Portugal (13) | 141.5 | 52.4 | 48.9 | 764.5 | Denmark (12) | 135.8 | 57.3 | 29.1 | 694.9 |
| Romania (12) | 594.6 | 242.9 | 167.0 | 1427.8 | Spain (13) | 132.7 | 62.2 | 22.9 | 605.9 |
| Russian Federation (11) | 1087.0 | 610.2 | 268.2 | 2254.4 | Japan (13) | 132.4 | 43.8 | 43.5 | 563.8 |
| Spain (13) | 132.7 | 62.2 | 22.9 | 605.9 | Netherlands (13) | 127.0 | 47.0 | 23.5 | 573.4 |
| Sweden (13) | 151.1 | 83.5 | 22.3 | 519.3 | Australia (11) | 126.1 | 76.9 | 18.7 | 530.3 |
| Switzerland (13) | 112.1 | 54.3 | 13.8 | 506.0 | Norway (13) | 123.9 | 66.8 | 22.2 | 550.5 |
| Taiwan (13) | 169.4 | 48.4 | 54.1 | 841.6 | France (11) | 121.2 | 46.4 | 22.6 | 718.4 |
| Ukraine (12) | 1067.2 | 718.1 | 216.6 | 2069.3 | Korea, South (13) | 112.4 | 32.4 | 48.7 | 672.4 |
| United Kingdom (13) | 166.5 | 103.3 | 24.3 | 620.3 | Switzerland (13) | 112.1 | 54.3 | 13.8 | 506.0 |
| United States (14) | 233.0 | 125.3 | 27.1 | 810.7 | Israel (13) | 106.2 | 48.7 | 23.7 | 548.1 |
| Females aged 35–74 y | |||||||||
| Australia (11) | 52.3 | 21.6 | 13.5 | 318.3 | Ukraine (12) | 454.2 | 294.7 | 115.8 | 833.7 |
| Austria (14) | 66.8 | 28.9 | 14.5 | 359.3 | Russian Federation (11) | 431.5 | 212.5 | 137.8 | 864.4 |
| Belarus (11) | 431.1 | 271.3 | 118.7 | 821.2 | Belarus (11) | 431.1 | 271.3 | 118.7 | 821.2 |
| Belgium (12) | 69.4 | 18.8 | 18.9 | 408.9 | Bulgaria (12) | 330.1 | 60.8 | 95.7 | 645.1 |
| Bulgaria (12) | 330.1 | 60.8 | 95.7 | 645.1 | Romania (12) | 279.9 | 93.9 | 97.3 | 641.8 |
| Croatia (13) | 141.9 | 60.3 | 49.6 | 465.9 | Hungary (13) | 205.2 | 97.5 | 45.5 | 649.2 |
| Czech Republic (13) | 138.2 | 63.1 | 28.6 | 472.9 | Croatia (13) | 141.9 | 60.3 | 49.6 | 465.9 |
| Denmark (12) | 58.9 | 18.9 | 17.5 | 451.1 | Czech Republic (13) | 138.2 | 63.1 | 28.6 | 472.9 |
| Finland (13) | 68.6 | 27.5 | 19.4 | 345.3 | United States (14) | 114.9 | 48.5 | 20.0 | 514.0 |
| France (11) | 42.9 | 10.0 | 12.3 | 330.7 | Germany (13) | 81.4 | 28.4 | 16.6 | 398.0 |
| Germany (13) | 81.4 | 28.4 | 16.6 | 398.0 | New Zealand (11) | 77.9 | 34.3 | 22.3 | 400.6 |
| Hungary (13) | 205.2 | 97.5 | 45.5 | 649.2 | United Kingdom (13) | 72.3 | 31.2 | 17.9 | 404.6 |
| Ireland (12) | 70.7 | 33.7 | 17.7 | 382.5 | Ireland (12) | 70.7 | 33.7 | 17.7 | 382.5 |
| Israel (13) | 47.6 | 13.5 | 13.3 | 326.9 | Belgium(12) | 69.4 | 18.8 | 18.9 | 408.9 |
| Italy (12) | 59.4 | 18.2 | 16.4 | 313.8 | Finland (13) | 68.6 | 27.5 | 19.4 | 345.3 |
| Japan (13) | 48.4 | 11.5 | 18.7 | 257.3 | Austria (14) | 66.8 | 28.9 | 14.5 | 359.3 |
| Korea, South (13) | 47.7 | 10.1 | 24.1 | 269.4 | Taiwan (13) | 65.6 | 15.6 | 21.9 | 394.4 |
| Netherlands (13) | 59.5 | 15.6 | 16.4 | 390.0 | Sweden (13) | 65.1 | 25.4 | 16.0 | 344.6 |
| New Zealand (11) | 77.9 | 34.3 | 22.3 | 400.6 | Portugal (13) | 60.0 | 14.4 | 24.7 | 329.0 |
| Norway (13) | 49.7 | 17.7 | 15.1 | 352.7 | Netherlands (13) | 59.5 | 15.6 | 16.4 | 390.0 |
| Portugal (13) | 60.0 | 14.4 | 24.7 | 329.0 | Italy (12) | 59.4 | 18.2 | 16.4 | 313.8 |
| Romania (12) | 279.9 | 93.9 | 97.3 | 641.8 | Denmark (12) | 58.9 | 18.9 | 17.5 | 451.1 |
| Russian Federation (11) | 431.5 | 212.5 | 137.8 | 864.4 | Australia (11) | 52.3 | 21.6 | 13.5 | 318.3 |
| Spain (13) | 47.3 | 13.9 | 13.0 | 270.8 | Norway (13) | 49.7 | 17.7 | 15.1 | 352.7 |
| Sweden (13) | 65.1 | 25.4 | 16.0 | 344.6 | Japan (13) | 48.4 | 11.5 | 18.7 | 257.3 |
| Switzerland (13) | 44.7 | 14.2 | 10.7 | 295.0 | Korea, South (13) | 47.7 | 10.1 | 24.1 | 269.4 |
| Taiwan (13) | 65.6 | 15.6 | 21.9 | 394.4 | Israel (13) | 47.6 | 13.5 | 13.3 | 326.9 |
| Ukraine (12) | 454.2 | 294.7 | 115.8 | 833.7 | Spain (13) | 47.3 | 13.9 | 13.0 | 270.8 |
| United Kingdom (13) | 72.3 | 31.2 | 17.9 | 404.6 | Switzerland (13) | 44.7 | 14.2 | 10.7 | 295.0 |
| United States (14) | 114.9 | 48.5 | 20.0 | 514.0 | France (11) | 42.9 | 10.0 | 12.3 | 330.7 |
Rates are for the most recent year available (shown in parentheses); most current data available as of April 2016. Rates are per 100 000 people, adjusted to the European Standard population. International Classification of Diseases, 10th Revision codes used were I00 to I99 for cardiovascular disease, I20 to I25 for coronary heart disease, and I60 to I69 for stroke. CHD indicates coronary heart disease; and CVD, cardiovascular disease.
Sources: The World Health Organization, National Center for Health Statistics, and National Heart, Lung, and Blood Institute.
Chart 13-1. Prevalence of cardiovascular disease in adults ≥20 years of age by age and sex (NHANES 2011–2014).
These data include coronary heart disease, heart failure, stroke, and hypertension.
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-23. Estimated average 10-year cardiovascular disease risk in adults 50 to 54 years of age according to levels of various risk factors (FHS).
BP indicates blood pressure; FHS, Framingham Heart Study; and HDL, high-density lipoprotein.
Data derived from D’Agostino et al.50
Abbreviations Used in Chapter 13
| AHA | American Heart Association |
| AP | angina pectoris |
| ARIC | Atherosclerosis Risk in Communities |
| BMI | body mass index |
| BP | blood pressure |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CI | confidence interval |
| CLRD | chronic lower respiratory disease |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| ED | emergency department |
| FHS | Framingham Heart Study |
| GED | Tests of General Educational Development |
| HBP | high blood pressure |
| HD | heart disease |
| HDL | high-density lipoprotein |
| HF | heart failure |
| HIV | human immunodeficiency virus |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IHD | ischemic heart disease |
| LDL-C | low-density lipoprotein cholesterol |
| LMIC | low- and middle-income countries |
| MEPS | Medical Expenditure Panel Survey |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| NAMCS | National Ambulatory Medical Care Survey |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHHCS | National Home and Hospice Care Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| PA | physical activity |
| RR | relative risk |
| SBP | systolic blood pressure |
| TC | total cholesterol |
Prevalence
(See Table 13-1 and Chart 13-1 through 13-4)
Chart 13-4. Age-adjusted percentages of selected circulatory diseases among American adults by race and ethnicity using data from Summary Health Statistics: NHIS, 2014.
NH indicates non-Hispanic; and NHIS, National Health Interview Survey.
*Data may not be reliable. †Data are not available.
Source: Summary Health Statistics: NHIS, 2014, Centers for Disease Control and Prevention National Center for Health Statistics.51
On the basis of NHANES 2011 to 2014 data, an estimated 92.1 million American adults (>1 in 3) have ≥1 type of CVD (Table 13-1). Of these, 46.7 million are estimated to be ≥60 years of age. A total of 11.5% of American adults (27.6 million) have been diagnosed with HD.1 By 2030, 43.9% of the US population is projected to have some form of CVD (unpublished AHA tabulation based on methodology described by Heidenreich et al2). Total CVD includes diseases listed in the bullet points below. Because of overlap across conditions, it is not possible to add these conditions to arrive at a total (unpublished NHLBI tabulation).
The prevalence of CVD in adults increases with age in both males and females (Chart 13-1).
The prevalence of all types of HD (including CHD, hypertension, and stroke) is greater in individuals with lower education (Chart 13-2) and individuals who are unemployed (Chart 13-3).
The percentage of adults with selected circulatory diseases varies by race/ethnicity. Although the percentage of adults with CHD is similar for non-Hispanic white, non-Hispanic black, and Hispanic adults, the percentage of adults with hypertension is higher for non-Hispanic black adults (Chart 13-4).
The number of adults with HBP is 85.7 million (defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg, use of antihypertensive medication, or being told at least twice by a physician or other health professional that one has HBP).
-
The number of adults with CHD is 16.5 million.
MI (heart attack)—7.9 million
AP (chest pain)—8.7 million
The number of adults with HF is 6.5 million.
The number of adults with stroke (all types) is 7.2 million.
-
The following age-adjusted race/ethnicity prevalence estimates from the NHIS, NCHS/CDC are for diagnosed conditions for people ≥18 years of age in 20143:
Among non-Hispanic whites, 11.1% have HD (includes CHD, AP, MI, or any other heart condition or disease), 5.6% have CHD (includes CHD, AP, or MI), 23.5% have hypertension, and 2.3% have had a stroke.
Among blacks or African Americans, 10.3% have HD, 5.5% have CHD, 33.0% have hypertension, and 4.0% have had a stroke.
Among Hispanics or Latinos (predominately Mexican Americans in this sample), 7.8% have HD, 4.9% have CHD, 23.0% have hypertension, and 2.4% have had a stroke.
Among Asians, 6.0% have HD, 3.3% have CHD, 19.5% have hypertension, and 1.5% have had a stroke.
Among American Indians or Alaska Natives, 13.7% have HD, 6.0% have CHD, 26.4% have hypertension, and 3.0% have had a stroke.
Among Native Hawaiians or other Pacific Islanders, 19.1% have HD, 6.9% have CHD, and 36.4% have hypertension.
It is important to bear in mind that within these aggregated groups, there are significant variations. For example, Asian Indian adults (9%) are ≈2-fold more likely than Korean adults (4%) to have ever been told they have HD, based on data for 2004 to 2006.4
The AHA’s 2020 Impact Goals are to improve the cardiovascular health of all Americans by 20% while reducing deaths attributable to CVDs and stroke by 20%.5
Chart 13-2. Age-adjusted percentage of selected circulatory diseases among American adults by education level using data from Summary Health Statistics: NHIS, 2014.
GED indicates Tests of General Educational Development; and NHIS, National Health Interview Survey.
Source: Summary Health Statistics: NHIS, 2014, Centers for Disease Control and Prevention National Center for Health Statistics.51
Chart 13-3. Age-adjusted percentages of selected circulatory diseases among American adults by employment status using data from Summary Health Statistics: NHIS, 2014.
NHIS indicates National Health Interview Survey.
Source: Summary Health Statistics: NHIS, 2014, Centers for Disease Control and Prevention National Center for Health Statistics.51
Mortality
(See Tables 13-1 through 13-3 and Charts 13-5 through 13-20)
Chart 13-5. Deaths attributable to diseases of the heart (United States: 1900–2014).
See Glossary (Chapter 29) for an explanation of “diseases of the heart.” In the years 1900 to 1920, the International Classification of Diseases codes were 77 to 80; for 1925, 87 to 90; for 1930 to 1945, 90 to 95; for 1950 to 1960, 402 to 404 and 410 to 443; for 1965, 402 to 404 and 410 to 443; for 1970 to 1975, 390 to 398 and 404 to 429; for 1980 to 1995, 390 to 398, 402, and 404 to 429; for 2000 to 2014, I00 to I09, I11, I13, and I20 to I51. Before 1933, data are for a death registration area and not the entire United States. In 1900, only 10 states were included in the death registration area, and this increased over the years, so part of the increase in numbers of deaths is attributable to an increase in the number of states.
Source: National Center for Health Statistics.
Chart 13-20.
US maps corresponding to state death rates (including the District of Columbia), 2014.
ICD-10 I00 to I99 for CVD; C00 to C97 for cancer; C33 to C34 for lung cancer; C50 for breast cancer; J40 to J47 for CLRD; G30 for Alzheimer disease; E10 to E14 for DM; and V01 to X59 and Y85 to Y86 for accidents.
In every year since 1919, CVD accounted for more deaths than any other major cause of death in the United States.6,7
Deaths attributable to diseases of the heart and CVD in the United States increased steadily during the 1900s to the 1980s and declined into the 2010s (Charts 13-5 and 13-6).
CHD (45.1%) is the leading cause of deaths attributable to CVD in the United States, followed by stroke (16.5%), HF (8.5%), HBP (9.1%), diseases of the arteries (3.2%), and other CVDs (Chart 13-7).
-
On the basis of 2014 mortality data6:
CVD, listed as the underlying cause of death, accounted for 30.8% (807 775) of all 2 626 418 deaths, or ≈1 of every 3 deaths in the United States (Table 13-1). CVD any-mentions (1 413 654 deaths in 2014) constituted 53.8% of all deaths that year (NHLBI; NCHS public-use data files).
On average, ≈2200 Americans die of CVD each day, an average of 1 death every 40 seconds.
CVD currently claims more lives each year than cancer and CLRD combined (Charts 13-8, 13-9, 13-10, 13-11, 13-12, 13-13, 13-14, 13-15, 13-16, 13-17, 13-18).
The death rate attributable to CVD was 219.9 per 100 000.
More than 360 000 people die every year of CHD, the most common type of HD.8
The age-adjusted death rates were 266.1 for males and 182.1 for females. The rates were 267.8 for non-Hispanic white males, 352.4 for non-Hispanic black males, 192.4 for Hispanic males, 182.1 for non-Hispanic white females, 241.3 for non-Hispanic black females, and 131.7 for Hispanic females.
The mortality trends for males and females in the United States have declined from 1979 to 2014 (Chart 13-19).
From 2004 to 2014, age-adjusted death rates attributable to CVD declined 25.3%. In the same period, the actual number of CVD deaths per year declined by 6.7% (NHLBI tabulation).
Among other causes of death, cancer caused 591 699 deaths; CLRD, 147 101; accidents, 136 053; and Alzheimer disease, 93 541.
The leading causes of death in females ≥65 years of age were diseases of the heart (No. 1), cancer (No. 2), stroke (No. 3), and CLRD (No. 4). In older males, they were diseases of the heart (No. 1), cancer (No. 2), CLRD (No. 3), and stroke (No. 4).
CVD caused ≈1 death every 1 minute 19 seconds among females, or 399 028 deaths. That represents approximately the same number of female lives that were claimed by cancer, CLRD, and DM combined (unpublished NHLBI tabulation). There were 41 213 deaths attributable to breast cancer in females; lung cancer claimed 70 700 females. Age-adjusted death rates for females were 20.6 for breast cancer and 34.7 for lung cancer. One in 4.6 females died of cancer, whereas 1 in 3.3 died of CVD. For comparison of specific types of cancer and CVD, 1 in 31.5 deaths of females was attributable to breast cancer, whereas 1 in 8.3 was attributable to CHD.
Approximately 155 000 Americans who were <65 years of age died of CVD, and 36% of deaths attributed to CVD occurred before the age of 75 years, which is well below the average life expectancy of 78.8 years.
If all forms of major CVD were eliminated, life expectancy could rise by almost 7 years. If all forms of cancer were eliminated, the estimated gain could be 3 years. According to the same study, the probability at birth of eventually dying of major CVD (ICD-10 I00–I78) is 47%, and the chance of dying of cancer is 22%. Additional probabilities are 3% for accidents, 2% for DM (unrelated to CVD), and 0.7% for HIV.9
The age-adjusted death rates per 100 000 population for CVD, CHD, and stroke differ by US state (Chart 13-20; Table 13-2) and globally (Table 13-3).
Chart 13-6. Deaths attributable to cardiovascular disease (United States: 1900–2014).
Cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99) does not include congenital heart disease. Before 1933, data are for a death registration area and not the entire United States.
Source: National Center for Health Statistics.
Chart 13-7. Percentage breakdown of deaths attributable to cardiovascular disease (United States: 2014).
Total may not add to 100 because of rounding. Coronary heart disease includes International Classification of Diseases, 10th Revision (ICD-10) codes I20 to I25; stroke, I60 to I69; heart failure, I50; high blood pressure, I10 to I15; diseases of the arteries, I70 to I78; and other, all remaining ICD-I0 I categories.
*Not a true underlying cause. With any-mention deaths, heart failure accounts for 36% of cardiovascular disease deaths.
Source: National Heart, Lung, and Blood Institute from National Center for Health Statistics reports and data sets.
Chart 13-8. Cardiovascular disease (CVD) deaths versus cancer deaths by age (United States: 2014).
CVD includes International Classification of Diseases, 10th Revision codes I00 to I99; cancer, C00 to C97.
Source: National Center for Health Statistics.
Chart 13-9. Cardiovascular disease (CVD) and other major causes of death: total, <85 years of age, and ≥85 years of age.
Deaths among both sexes, United States, 2014. Heart disease includes International Classification of Diseases, 10th Revision codes I00 to I09, I11, I13, and I20 to I51; stroke, I60 to I69; all other CVD, I10, I12, I15, and I70 to I99; cancer, C00 to C97; chronic lower respiratory disease (CLRD), J40 to J47; Alzheimer disease, G30; and accidents, V01 to X59 and Y85 and Y86.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-10. Cardiovascular disease (CVD) and other major causes of death in males: total, <85 years of age, and ≥85 years of age.
Deaths among males, United States, 2014. Heart disease includes International Classification of Diseases, 10th Revision codes I00 to I09, I11, I13, and I20 to I51; stroke, I60 to I69; all other CVD, I10, I12, I15, and I70 to I99; cancer, C00 to C97; chronic lower respiratory disease (CLRD), J40 to J47; and accidents, V01 to X59 and Y85 and Y86.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-11. Cardiovascular disease (CVD) and other major causes of death in females: total, <85 years of age, and ≥85 years of age.
Deaths among females, United States, 2014. Heart disease includes International Classification of Diseases, 10th Revision codes I00 to I09, I11, I13, and I20 to I51; stroke, I60 to I69; all other CVD, I10, I12, I15, and I70 to I99; cancer, C00 to C97; chronic lower respiratory disease (CLRD), J40 to J47; and Alzheimer disease, G30.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-12. Cardiovascular disease and other major causes of death for all males and females (United States: 2014).
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 and Y85–Y86); D, chronic lower respiratory disease (J40–J47); E, diabetes mellitus (E10–E14); and F, Alzheimer disease (G30).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-13. Cardiovascular disease and other major causes of death for non-Hispanic (NH) white males and females (United States: 2014).
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 andY85–Y86); D, chronic lower respiratory disease (J40–J47); E, diabetes mellitus (E10–E14); and F, Alzheimer disease (G30).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-14. Cardiovascular disease and other major causes of death for non-Hispanic (NH) black males and females (United States: 2014).
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 and Y85–Y86); D, diabetes mellitus (E10–E14); E, chronic lower respiratory disease (J40–J47); and F, nephritis (N00–N07, N17–N19, and N25–N27).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-15. Cardiovascular disease and other major causes of death for Hispanic or Latino males and females (United States: 2014).
Number of deaths shown may be lower than actual because of underreporting in this population.
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 and Y85–Y86); D, diabetes mellitus (E10–E14); E, chronic lower respiratory disease (J40–J47); and F, Alzheimer disease (G30).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-16. Cardiovascular disease and other major causes of death for non-Hispanic (NH) Asian or Pacific Islander males and females (United States: 2014).
“Asian or Pacific Islander” is a heterogeneous category that includes people at high cardiovascular disease risk (eg, South Asian) and people at low cardiovascular disease risk (eg, Japanese). More specific data on these groups are not available. Number of deaths shown may be lower than actual because of underreporting in this population.
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 and Y85–Y86); D, diabetes mellitus (E10–E14); E, chronic lower respiratory disease (J40–J47); and F, influenza and pneumonia (J09–J18).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-17. Cardiovascular disease and other major causes of death for non-Hispanic (NH) American Indian or Alaska Native males and females (United States: 2014).
Number of deaths shown may be lower than actual because of underreporting in this population.
A indicates cardiovascular disease (International Classification of Diseases, 10th Revision codes I00–I99); B, cancer (C00–C97); C, accidents (V01–X59 and Y85–Y86); D, diabetes mellitus (E10–E14); E, chronic liver disease (K70 and K73–K74); and F, chronic lower respiratory disease (J40–J47).
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-18. Age-adjusted death rates for coronary heart disease (CHD), stroke, and lung and breast cancer for white and black females (United States: 2014).
CHD includes International Classification of Diseases, 10th Revision codes I20 to I25; stroke, I60 to I69; lung cancer, C33 to C34; and breast cancer, C50.
NH indicates non-Hispanic.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute. NH indicates non-Hispanic.
Chart 13-19. Cardiovascular disease (CVD) mortality trends for males and females (United States: 1979–2014).
CVD excludes congenital cardiovascular defects (International Classification of Diseases, 10th Revision [ICD-10] codes I00–I99). The overall comparability for cardiovascular disease between the International Classification of Diseases, 9th Revision (1979–1998) and ICD-10 (1999–2013) is 0.9962. No comparability ratios were applied.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Table 13-2.
Age-Adjusted Death Rates per 100 000 Population for CVD, CHD, and Stroke by State, 2012 to 2014
| State | CVD* | CHD† | Stroke‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank§ | Death Rate | % Change, 2002–2004 to 2012–2014 | Rank§ | Death Rate | % Change, 2002–2004 to 2012–2014 | Rank§ | Death Rate | % Change, 2002–2004 to 2012–2014 | |
| Alabama | 51 | 294.8 | −22.0 | 23 | 95.9 | −33.3 | 52 | 48.6 | −28.4 |
| Alaska | 10 | 190.9 | −23.8 | 7 | 75.9 | −30.2 | 35 | 38.4 | −32.7 |
| Arizona | 7 | 188.3 | −29.2 | 22 | 95.6 | −36.4 | 6 | 28.9 | −37.5 |
| Arkansas | 49 | 280.7 | −21.6 | 49 | 130.3 | −29.1 | 50 | 47.4 | −32.9 |
| California | 20 | 202.5 | −32.7 | 26 | 97.5 | −41.5 | 19 | 34.7 | −39.5 |
| Colorado | 3 | 176.1 | −31.1 | 6 | 71.8 | −38.7 | 12 | 32.7 | −35.4 |
| Connecticut | 11 | 191.6 | −28.1 | 10 | 80.5 | −41.8 | 2 | 27.2 | −36.8 |
| Delaware | 29 | 219.1 | −29.3 | 38 | 109.4 | −37.1 | 33 | 38.0 | −20.2 |
| District of Columbia | 44 | 263.5 | −25.8 | 50 | 133.5 | −33.8 | 11 | 32.5 | −27.8 |
| Florida | 14 | 198.1 | −29.9 | 29 | 98.7 | −40.1 | 7 | 31.4 | −29.7 |
| Georgia | 37 | 241.1 | −30.3 | 11 | 80.8 | −42.8 | 42 | 42.0 | −34.8 |
| Hawaii | 5 | 183.7 | −28.3 | 3 | 68.9 | −31.5 | 21 | 34.7 | −38.1 |
| Idaho | 19 | 202.3 | −26.7 | 12 | 82.6 | −37.9 | 26 | 36.5 | −37.8 |
| Illinois | 33 | 224.1 | −28.7 | 28 | 98.1 | −40.5 | 30 | 37.3 | −32.2 |
| Indiana | 40 | 245.6 | −25.0 | 37 | 108.0 | −33.6 | 41 | 41.7 | −28.5 |
| Iowa | 28 | 217.0 | −24.0 | 42 | 114.5 | −29.6 | 18 | 34.0 | −37.6 |
| Kansas | 30 | 220.7 | −25.3 | 17 | 88.3 | −34.1 | 37 | 38.8 | −31.6 |
| Kentucky | 45 | 263.6 | −26.5 | 44 | 114.8 | −35.0 | 43 | 42.6 | −30.7 |
| Louisiana | 48 | 276.1 | −22.3 | 39 | 109.8 | −34.7 | 46 | 44.5 | −27.9 |
| Maine | 13 | 196.0 | −28.4 | 14 | 85.6 | −37.8 | 16 | 33.6 | −36.3 |
| Maryland | 32 | 223.4 | −27.8 | 34 | 104.9 | −38.1 | 27 | 36.9 | −33.0 |
| Massachusetts | 4 | 181.5 | −31.3 | 9 | 79.8 | −38.2 | 4 | 28.3 | −39.3 |
| Michigan | 43 | 255.0 | −24.0 | 47 | 128.6 | −30.0 | 29 | 37.2 | −32.5 |
| Minnesota | 2 | 166.8 | −26.4 | 1 | 64.3 | −35.2 | 13 | 33.0 | −32.5 |
| Mississippi | 52 | 304.9 | −24.9 | 41 | 114.3 | −36.1 | 51 | 48.1 | −25.8 |
| Missouri | 42 | 251.0 | −26.9 | 45 | 118.8 | −34.0 | 40 | 41.3 | −30.0 |
| Montana | 18 | 201.3 | −23.2 | 13 | 84.8 | −25.5 | 25 | 35.8 | −35.0 |
| Nebraska | 17 | 200.6 | −27.6 | 8 | 76.0 | −34.4 | 23 | 35.3 | −33.2 |
| Nevada | 39 | 244.6 | −25.1 | 31 | 100.7 | −26.7 | 17 | 33.8 | −40.4 |
| New Hampshire | 9 | 189.3 | −33.1 | 18 | 89.5 | −44.1 | 5 | 28.6 | −38.7 |
| New Jersey | 27 | 216.8 | −27.4 | 33 | 104.9 | −39.2 | 10 | 32.1 | −24.9 |
| New Mexico | 12 | 192.3 | −24.0 | 24 | 97.2 | −28.2 | 8 | 31.7 | −25.6 |
| New York | 34 | 225.9 | −30.2 | 48 | 129.7 | −39.8 | 1 | 26.3 | −26.5 |
| North Carolina | 31 | 222.6 | −30.0 | 25 | 97.4 | −37.4 | 44 | 42.7 | −34.8 |
| North Dakota | 22 | 204.5 | −24.7 | 21 | 95.3 | −35.9 | 22 | 35.1 | −37.2 |
| Ohio | 41 | 246.4 | −25.5 | 40 | 113.7 | −35.6 | 38 | 40.4 | −28.5 |
| Oklahoma | 50 | 287.7 | −26.3 | 52 | 146.7 | −33.9 | 45 | 44.4 | −32.3 |
| Oregon | 6 | 188.1 | −31.3 | 5 | 69.7 | −41.9 | 31 | 37.4 | −43.0 |
| Pennsylvania | 36 | 229.5 | −27.8 | 35 | 105.4 | −37.1 | 28 | 37.1 | −29.4 |
| Puerto Rico | 1 | 166.2 | −28.6 | 4 | 69.6 | −40.3 | 9 | 31.8 | −30.1 |
| Rhode Island | 21 | 203.4 | −30.7 | 43 | 114.6 | −41.7 | 3 | 27.9 | −35.2 |
| South Carolina | 38 | 243.0 | −26.8 | 27 | 97.8 | −34.4 | 49 | 45.8 | −35.1 |
| South Dakota | 23 | 207.1 | −24.3 | 36 | 107.7 | −27.5 | 34 | 38.0 | −25.9 |
| Tennessee | 47 | 267.8 | −27.5 | 51 | 136.2 | −33.1 | 48 | 45.0 | −34.2 |
| Texas | 35 | 226.9 | −29.7 | 32 | 100.7 | −40.2 | 39 | 41.2 | −31.8 |
| Utah | 15 | 198.8 | −23.0 | 2 | 68.7 | −30.3 | 32 | 38.0 | −29.6 |
| Vermont | 16 | 200.4 | −25.5 | 30 | 99.1 | −30.9 | 14 | 33.1 | −30.6 |
| Virginia | 26 | 211.2 | −30.2 | 16 | 86.4 | −37.7 | 36 | 38.7 | −35.2 |
| Washington | 8 | 188.9 | −30.8 | 15 | 85.7 | −38.5 | 20 | 34.7 | −43.3 |
| West Virginia | 46 | 264.7 | −27.9 | 46 | 124.6 | −36.1 | 47 | 44.6 | −23.6 |
| Wisconsin | 25 | 209.4 | −25.7 | 20 | 94.1 | −31.6 | 24 | 35.5 | −34.0 |
| Wyoming | 24 | 207.7 | −23.9 | 19 | 90.3 | −30.1 | 15 | 33.3 | −33.8 |
| Total United States | 222.3 | −28.4 | 102.2 | −37.7 | 36.5 | −32.8 | |||
CHD indicates coronary heart disease; and CVD, cardiovascular disease.
CVD is defined here as International Classification of Diseases, 10th Revision (ICD-10) codes I00 to I99.
CHD is defined here as ICD-10 codes I20 to I25.
Stroke is defined here as ICD-10 codes I60 to I69.
Rank is lowest to highest.
Source: Centers for Disease Control and Prevention (CDC) Wide-Ranging Online Data for Epidemiologic Research (WONDER), 2002 to 2014. Data provided by personal communication with the National Heart, Lung, and Blood Institute.
Additional resources for state-level mortality data: The Agency for Healthcare Research and Quality has released state-level data for heart disease for all 50 states and the District of Columbia; the data are taken from the congressionally mandated National Healthcare Quality Report.44 In addition, the Women’s Health and Mortality Chartbook of the National Center for Health Statistics has state-related data for women.45 Metropolitan/micropolitan area risk data are available for 500 such areas nationwide.46 Behavioral Risk Factor Surveillance System data are also collected within each state.47 The CDC has the Geographic Information Systems, which provides mortality rates down to the county level, by sex and ethnicity.48 The 2008 Atlas of Stroke Hospitalizations Among Medicare Beneficiaries is a resource that provides data down to the county level, by sex and race.49
Hospital Discharges, Ambulatory Care Visits, Home Healthcare Patients, Nursing Home Residents, and Hospice Care Discharges
(See Table 13-1 and Charts 13-21 and 13-22)
Chart 13-21. Hospital discharges for cardiovascular disease (United States: 1970–2010).
Hospital discharges include people discharged alive, dead, and “status unknown.”
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 13-22. Hospital discharges (International Classification of Diseases, 9th Revision) for the 10 leading diagnostic groups (United States: 2010).
Source: National Hospital Discharge Survey/National Center for Health Statistics and National Heart, Lung, and Blood Institute.
From 2000 to 2010, the number of inpatient discharges from short-stay hospitals with CVD as the first-listed diagnosis decreased from 6 294 000 to 5 802 000 (NHDS, NCHS, and NHLBI) (Table 13-1). In 2010, CVD ranked highest among all disease categories in hospital discharges (NHDS, NCHS, and NHLBI).
From 1970 to 2010, the number of hospital discharges for CVD increased in the United States (Chart 13-21).
In 2010, cardiovascular causes were the leading diagnostic group of hospital discharges in the United States (Chart 13-22).
In 2012, there were 69 184 000 physician office visits with a primary diagnosis of CVD (NCHS, NAMCS, NHLBI tabulation). In 2012, there were 4 357 000 ED visits, and in 2011, there were 8 505 000 hospital outpatient department visits (no overnight stay) with a primary diagnosis of CVD (NCHS, NHAMCS, NHLBI tabulation).
Among the 1 459 900 home healthcare patients each day in 2007, CVD was the leading primary diagnosis; almost one fifth of home healthcare patients had a primary diagnosis of CVD at admission into home health care (18.3% or 267 300 residents) or at the time of interview (18.9% or 275 700 residents) (NCHS, NHHCS). The majority (62.9%, or 918 900 patients) of home healthcare patients each day in 2007 had some diagnosis of CVD at the time of interview.10
Among the 1 045 100 patients discharged from hospice in 2007, CVD was the primary diagnosis for 15.8% (or 165 100 discharges) at admission and 15.9% (or 165 700 discharges) at discharge. Half (50%, or 523 000) of all hospice discharges had any diagnosis of CVD at the time of discharge.10
Operations and Procedures
(See Chapter 26 for detailed information)
In 2010, an estimated 7 588 000 inpatient cardiovascular operations and procedures were performed in the United States; 4.4 million were performed on males, and 3.2 million were performed on females (NHLBI tabulation of NHDS, NCHS).
Cost
(See Chapter 27 for detailed information)
Among the estimated 45 million people with functional disabilities in the United States, HD, stroke, and hypertension are among the 15 leading conditions that caused those disabilities. Disabilities were defined as difficulty with activities of daily living or instrumental activities of daily living, specific functional limitations (except vision, hearing, or speech), and limitation in ability to do housework or work at a job or business.11
The estimated direct and indirect cost of CVD for 2012 to 2013 is $316.1 billion (MEPS, NHLBI tabulation).
In 2011, the AHA estimated that by 2030, 40.5% of the US population would have some form of CVD. Direct medical costs in 2030 were projected to reach $818 billion. Indirect costs for 2030 were estimated to reach $276 billion.2
Risk Factors
In a study based on the Second NHANES with 17 years of follow-up, 9 of 10 adults who died of CHD had at least 1 of the 3 well-established risk factors: hypertension, elevated TC, or cigarette smoking.12
Analysis of data from the NCHS/CDC was used to determine the number of disease-specific deaths attributable to all nonoptimal levels of each risk factor exposure, by age and sex. In 2005, HBP was the single largest risk factor for cardiovascular mortality in the United States and was responsible for an estimated 395 000 (95% CI, 372 000–414 000) cardiovascular deaths (45% of all cardiovascular deaths). Additional risk factors for cardiovascular mortality were overweight/obesity, physical inactivity, high LDL-C, smoking, high dietary salt, high dietary trans fatty acids, and low dietary omega-3 fatty acids.9
Neighborhood-level socioeconomic deprivation was associated with greater risk of CVD mortality in older males in Britain, independent of individual social class or risk factors.13 In the United States, there are significant state-level variations in poor cardiovascular health explained by individual and state-level factors such as policies, food, and PA environments.14
Impact of Healthy Lifestyle and Low Risk Factor Levels
(See Chapter 2 for more detailed statistics regarding healthy lifestyles and low risk factor levels.)
A number of studies suggest that prevention of risk factor development at younger ages could be the key to “successful aging,” and they highlight the need for evaluation of the potential benefits of intensive prevention efforts at younger and middle ages once risk factors develop to increase the likelihood of healthy longevity.
A study of the decrease in US deaths attributable to CHD from 1980 to 2000 suggests that ≈47% of the decrease was attributable to increased use of evidence-based medical therapies for secondary prevention and 44% to changes in risk factors in the population attributable to lifestyle and environmental changes.7
Approximately 80% of CVDs can be prevented through not smoking, eating a healthy diet, engaging in PA, maintaining a healthy weight, and controlling HBP, DM, and elevated lipid levels. The presence of a greater number of optimal cardiovascular health metrics is associated with a graded and significantly lower risk of total and CVD mortality.15
Data from the Cardiovascular Lifetime Risk Pooling Project, which involved 18 cohort studies and combined data on 257 384 black males and females and white males and females, indicate that at 45 years of age, participants with optimal risk factor profiles had a substantially lower lifetime risk of CVD events than those with 1 major risk factor (1.4% versus 39.6% among males; 4.1% versus 20.2% among females). Having ≥2 major risk factors further increased lifetime risk to 49.5% in males and 30.7% in females.16
In another study, FHS investigators followed up 2531 males and females who were examined between the ages of 40 and 50 years and observed their overall rates of survival and survival free of CVD to 85 years of age and beyond. Low levels of the major risk factors in middle age were associated with overall survival and morbidity-free survival to ≥85 years of age.17
-
Data from the Chicago Heart Association Detection Project (1967–1973, with an average follow-up of 31 years) showed the following:
In younger females (18–39 years of age) with favorable levels for all 5 major risk factors (BP, serum cholesterol, BMI, DM, and smoking), future incidence of CHD and CVD is rare, and long-term and all-cause mortality are much lower than for those who have unfavorable or elevated risk factor levels at young ages. Similar findings applied to males in this study.18
Participants (18–64 years of age at baseline) without a history of MI were investigated to determine whether traditional CVD risk factors were similarly associated with CVD mortality in black and white males and females. In general, the magnitude and direction of associations were similar by race. Most traditional risk factors demonstrated similar associations with mortality in black and white adults of the same sex. Small differences were primarily in the strength and not the direction of the association.19
Data from NHANES 2005 to 2010 showed that only 8.8% of adults complied with ≥6 heart-healthy behaviors. Of the 7 factors studied, healthy diet was the least likely to be achieved (only 22% of adults with a healthy diet).15
In the United States, higher whole grain consumption was associated with lower CVD mortality, independent of other dietary and lifestyle factors. Every serving (28 g/d) of whole grain consumption was associated with a 9% (95% CI, 4%–13%) lower CVD mortality.20
Seventeen-year mortality data from the NHANES II Mortality Follow-Up Study indicated that the RR for fatal CHD was 51% lower for males and 71% lower for females with none of the 3 major risk factors (hypertension, current smoking, and elevated TC [≥240 mg/dL]) than for those with ≥1 risk factor. Had all 3 major risk factors not occurred, it is hypothesized that 64% of all CHD deaths among females and 45% of CHD deaths in males could have been avoided.12
Disparities in CVD Risk Factors
(See Chart 13-23)
Although traditional cardiovascular risk factors are generally similar for males and females, there are several female-specific risk factors such as disorders of pregnancy, adverse pregnancy outcomes, and menopause.21
Analysis of >14 000 middle-aged participants in the ARIC study sponsored by the NHLBI showed that ≈90% of CVD events in black participants, compared with ≈65% in white participants, appeared to be explained by elevated or borderline risk factors. Furthermore, the prevalence of participants with elevated risk factors was higher in black participants; after accounting for education and known CVD risk factors, the incidence of CVD was identical in black and white participants. Although organizational and social barriers to primary prevention do exist, the primary prevention of elevated risk factors might substantially impact the future incidence of CVD, and these beneficial effects would likely be applicable not only for white but also for black participants.22
Mortality data from the National Vital Statistics System from 2001 to 2010 show that the avoidable death rate among blacks was nearly twice that of whites.23
-
Data from the MEPS 2004 Full-Year Data File showed that nearly 26 million US adults ≥18 years of age were told by a doctor that they had HD, stroke, or any other heart-related disease24:
Among those told that they had HD, 33.9% had a healthy weight compared with 39.3% who had never been told they had HD.
Among those ever told that they had indicators of HD, 18.3% continued to smoke.
More than 93% engaged in at least 1 recommended behavior for prevention of HD (not smoking, engaging in physical exercise regularly, and maintaining healthy weight): 75.5% engaged in 1 or 2; 18% engaged in all 3; and 6.5% did not engage in any of the recommended behaviors.
-
Age-based variations:
Moderate to vigorous PA ≥3 times per week varied according to age. Younger people (18–44 years of age) were more likely (59.9%) than those who were older (45–64 and ≥65 years of age, 55.3% and 48.5%, respectively) to engage in regular PA.
A greater percentage of those 18 to 44 years of age had a healthy weight (43.7%) than did those 45 to 64 years of age and ≥65 years of age (31.4% and 37.3%, respectively).
People ≥65 years of age were more likely to be nonsmokers (89.7%) than were people 18 to 44 years of age and 45 to 64 years of age (76.1% and 77.7%, respectively).
-
Race/ethnicity-based variations:
Non-Hispanic whites were more likely than Hispanics or non-Hispanic blacks to engage in moderate to vigorous PA (58.5% versus 51.4% and 52.5%, respectively).
Non-Hispanic whites were more likely to have maintained a healthy weight than were Hispanics or non-Hispanic blacks (39.8% versus 32.1% and 29.7%, respectively).
Hispanics were more likely to be nonsmokers (84.2%) than were non-Hispanic whites and non-Hispanic blacks (77.8% and 76.3%, respectively).
-
Sex-based variations:
Males were more likely to have engaged in moderate to vigorous PA ≥3 times per week than females (60.3% versus 53.1%, respectively).
Females were more likely than males to have maintained a healthy weight (45.1% versus 31.7%, respectively).
Data from the CDC’s Vital and Health Statistics 2008 to 2010 showed that 82% of females did not currently smoke compared with 77.6% of males.25
-
Variations based on education level:
A greater percentage of adults with at least some college education engaged in moderate to vigorous PA ≥3 times per week (60.8%) than did those with a high school education or less than a high school education (55.3% and 48.3%, respectively).
A greater percentage of adults with at least some college education had a healthy weight (41.2%) than did those with a high school or less than high school education (36.2% and 36.1%, respectively).
There was a greater percentage of non-smokers among those with a college education (85.5%) than among those with a high school or less than high school education (73.8% and 69.9%, respectively).
Data from the CDC’s Vital and Health Statistics 2008 to 2010 showed that smokers with family incomes below the poverty level were more than twice as likely as adults in the highest family income group to be current smokers (29.2% versus 13.9%, respectively) (NCHS/CDC, 2013).25
A study of nearly 1500 participants in MESA found that Hispanics with hypertension, hypercholesterolemia, or DM who spoke Spanish at home (as a proxy of lower levels of acculturation) or had spent less than half a year in the United States had higher SBP, LDL-C, and fasting blood glucose, respectively, than Hispanics who were preferential English speakers and who had lived a longer period of time in the United States.26
Recent findings from >15 000 Hispanics of diverse backgrounds demonstrated that a sizeable proportion of both males and females had major CVD risk factors, with higher prevalence among Puerto Rican subgroups and those with lower socioeconomic status and a higher level of acculturation.27
Family History of CVD
(See Chapter 7 for more detailed information.)
A family history of CVD increases risk of CVD, with the largest increase in risk if the family member’s CVD was premature.28
There is consistent evidence from multiple large-scale prospective epidemiology studies for a strong and significant association of a reported family history of premature parental CHD with incident MI or CHD in offspring. In the FHS, the occurrence of a validated premature atherosclerotic CVD event in either a parent29 or a sibling30 was associated with an ≈2-fold elevated risk for CVD, independent of other traditional risk factors. Addition of a family history of premature CVD to a model that contained traditional risk factors provided improved prognostic value in the FHS.29
Parental history of premature CHD is associated with increased burden of subclinical atherosclerosis in the coronary arteries and the abdominal aorta.31,32
In the FHS, a parental history of validated HF was associated with a 1.7-fold higher risk of HF in offspring after multivariable adjustment.33
Despite the importance of family history, several barriers impede first-degree relatives of people with CVD from engaging in risk-reducing behaviors, such as few being aware of the specific health information from relatives necessary to develop a family history; in addition, there is an inappropriate risk perception or an underestimation of one’s own vulnerability.34
Awareness of Warning Signs and Risk Factors for CVD
Surveys conducted every 3 years since 1997 by the AHA to evaluate trends in females’ awareness, knowledge, and perceptions related to CVD found most recently (in 2012) that awareness of HD as the leading cause of death among females was 56%, compared with 30% in 1997 (P<0.05). Awareness among black and Hispanic females in 2012 was similar to that of white females in 1997; however, awareness rates in 2012 among black and Hispanic females remained well below that of white females. Awareness of heart attack signs remained low for all racial/ethnic and age groups surveyed during the same time.35
Global Burden of CVD
(See Table 13-3)
The death rates for all causes and CVD in selected countries are presented in Table 13-3. In these data, Belarus had the highest CVD death rate, whereas Israel had the lowest.
CVD is the leading global cause of death, accounting for >17.3 million deaths per year in 2013,36 a number that is expected to grow to >23.6 million by 2030.37 Deaths attributable to IHD increased by an estimated 41.7% from 1990 to 2013.38
In 2013, CVD deaths represented 31% of all global deaths.39
In 2011, data from the World Economic Forum found that CVD represented 50% of noncommunicable disease deaths.38 CVD represents 37% of deaths of individuals under the age of 70 years that are attributable to noncommunicable diseases.40
In 2013, ≈70% of CVD deaths occurred in LMIC.41 CVD deaths occur almost equally in males and females.37
In May 2012, during the World Health Assembly, ministers of health agreed to adopt a global target to reduce premature (age 30–70 years) noncommunicable disease mortality by 25% by 2025.42 Targets for 6 risk factors (tobacco and alcohol use, salt intake, obesity, and raised BP and glucose) were also agreed on to address this goal. It is projected that if the targets are met, premature death attributable to CVDs in 2025 will be reduced by 34%, with 11.4 million and 15.9 million deaths delayed or prevented in those aged 30 to 69 years and ≥70 years, respectively.43
In 2010, the estimated global cost of CVD was $863 billion, and it is estimated to rise to $1044 billion by 2030.38
REFERENCES
- 1.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. [Accessed August, 24, 2016];National Health Interview Survey. 2015 Public-use data file and documentation: NCHS tabulations. http://www.cdc.gov/nchs/nhis/nhis_2015_data_release.htm. NCHS tabulations.
- 4.Barnes PM, Adams PF, Powell-Griner E. Advance Data From Vital and Health Statistics. 394. Hyattsville, MD: National Center for Health Statistics; 2008. Health characteristics of the Asian adult population: United States, 2004–2006. [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. [Accessed July 15, 2016];Mortality multiple cause micro-data files, 2014: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 7.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. CDC WONDER Online Database [database online] Atlanta, GA: Centers for Disease Control and Prevention; [Accessed August 24, 2016]. Underlying cause of death 1999–2014. http://wonder.cdc.gov/ucd-icd10.html. [Google Scholar]
- 9.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caffrey C, Sengupta M, Moss A, Harris-Kojetin L, Valverde R. Home health care and discharged hospice care patients: United States, 2000 and 2007. Natl Health Stat Report. 2011;(38):1–27. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults: United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 12.Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II) Am J Prev Med. 2005;29(suppl 1):68–74. doi: 10.1016/j.amepre.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay SE, Morris RW, Whincup PH, Subramanian SV, Papacosta AO, Lennon LT, Wannamethee SG. The influence of neighbourhood-level socioeconomic deprivation on cardiovascular disease mortality in older age: longitudinal multilevel analyses from a cohort of older British men. J Epidemiol Community Health. 2015;69:1224–1231. doi: 10.1136/jech-2015-205542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebreab SY, Davis SK, Symanzik J, Mensah GA, Gibbons GH, Diez-Roux AV. Geographic variations in cardiovascular health in the United States: contributions of state- and individual-level factors. J Am Heart Assoc. 2015;4:e001673. doi: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry DF, Pencina MJ, Vasan RS, Murabito JM, Wolf PA, Hayes MK, Levy D, D’Agostino RB, Benjamin EJ. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;53:1944–1950. doi: 10.1111/j.1532-5415.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 18.Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd-Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292:1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 19.Carnethon MR, Lynch EB, Dyer AR, Lloyd-Jones DM, Wang R, Garside DB, Greenland P. Comparison of risk factors for cardiovascular mortality in black and white adults. Arch Intern Med. 2006;166:1196–1202. doi: 10.1001/archinte.166.11.1196. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med. 2015;175:373–384. doi: 10.1001/jamainternmed.2014.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects: Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Vital signs: avoidable deaths from heart disease, stroke, and hypertensive disease: United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2013;62:721–727. [PMC free article] [PubMed] [Google Scholar]
- 24.Soni A. Personal Health Behaviors for Heart Disease Prevention Among the U.S. Adult Civilian Noninstitutionalized Population, 2004. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2007. [Accessed August 3, 2011]. MEPS Statistical Brief No. 165. http://meps.ahrq.gov/mepsweb/data_files/publications/st165/stat165.pdf. [Google Scholar]
- 25.Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. Vital Health Stat. 2013;10(257):1–184. [PubMed] [Google Scholar]
- 26.Eamranond PP, Legedza AT, Diez-Roux AV, Kandula NR, Palmas W, Siscovick DS, Mukamal KJ. Association between language and risk factor levels among Hispanic adults with hypertension, hypercholesterolemia, or diabetes. Am Heart J. 2009;157:53–59. doi: 10.1016/j.ahj.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow CK, Islam S, Bautista L, Rumboldt Z, Yusufali A, Xie C, Anand SS, Engert JC, Rangarajan S, Yusuf S. Parental history and myocardial infarction risk across the world: the INTERHEART Study. J Am Coll Cardiol. 2011;57:619–627. doi: 10.1016/j.jacc.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 30.Murabito JM, Pencina MJ, Nam BH, D’Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O’Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 31.Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 32.Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, Murabito JM, Massaro JM, Hoffmann U, O’Donnell CJ. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 33.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O’Donnell CJ, Nam BH, Larson MG, D’Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 34.Ton TG, Fogg TT, Fong CT, John C, Li SX, Marshall JA, Peters K, Neal W, Pearson TA. Knowledge, perception, and behaviors of relatives of people with premature heart disease: a systematic literature review. Circulation. 2011;124:958–964. doi: 10.1161/CIRCULATIONAHA.110.940593. [DOI] [PubMed] [Google Scholar]
- 35.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on High Blood Pressure Research, and Council on Nutrition, Physical Activity and Metabolism. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127:1254–1263. doi: 10.1161/CIR.0b013e318287cf2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: World Health Organization; 2014. [Accessed September 2, 2016]. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf. [Google Scholar]
- 38.Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Non-communicable Diseases. Geneva, Switzerland: World Economic Forum; 2011. [Google Scholar]
- 39.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization (WHO) [Accessed April 10, 2015];Cardiovascular diseases (CVDs) 2015 Fact sheet No. 317. http://www.who.int/mediacentre/fact-sheets/fs317/en/
- 41.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 42.Smith SC, Jr, Collins A, Ferrari R, Holmes DR, Jr, Logstrup S, McGhie DV, Ralston J, Sacco RL, Stam H, Taubert K, Wood DA, Zoghbi WA. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke) Circulation. 2012;126:2769–2775. doi: 10.1161/CIR.0b013e318267e99f. [DOI] [PubMed] [Google Scholar]
- 43.Kontis V, Mathers CD, Rehm J, Stevens GA, Shield KD, Bonita R, Riley LM, Poznyak V, Beaglehole R, Ezzati M. Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet. 2014;384:427–437. doi: 10.1016/S0140-6736(14)60616-4. [DOI] [PubMed] [Google Scholar]
- 44.2007 State snapshots. [Accessed October 21, 2013];Agency for Healthcare Research and Quality Web site. https://archive.ahrq.gov/qual/kt/statesnap2007.htm.
- 45.Brett KM, Hayes SG. Women’s Health and Mortality Chartbook. Washington, DC: DHHS Office on Women’s Health; 2004. [Accessed October 21, 2013]. DHHS publication No. 04-1032. http://www.cdc.gov/nchs/data/healthy-women/womenschartbook_aug2004.pdf. [Google Scholar]
- 46.SMART: BRFSS city and county data: selected metropolitan/micropolitan area risk trends. Centers for Disease Control and Prevention; [Accessed December 19, 2016]. https://www.cdc.gov/brfss/smart/smart_data.htm. [Google Scholar]
- 47.Centers for Disease Control and Prevention Web site. [Accessed December 19, 2016];Behavioral Risk Factor Surveillance System: prevalence and trends data. https://www.cdc.gov/brfss/brfssprevalence/
- 48.Geographic Information Systems (GIS) and Public Health at CDC. [Accessed October 21, 2013];Centers for Disease Control and Prevention Web site. http://www.cdc.gov/gis/
- 49.Casper ML, Nwaise IA, Croft JB, Nilasena DS. Atlas of Stroke Hospitalizations Among Medicare Beneficiaries. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 50.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 51.National Center for Health Statistics. [Accessed December 19, 2016];Summary Health Statistics: National Health Interview Survey. 2014 Table A-1. https://www.cdc.gov/nchs/nhis/released201412.htm.
14. STROKE (CEREBROVASCULAR DISEASE)
ICD-9 430 to 438; ICD-10 I60 to I69. See Table 14-1 and Charts 14-1 through 14-12
Table 14-1.
Stroke
| Population Group | Prevalence, 2014: Age ≥20 y | New and Recurrent Attacks, All Ages | Mortality, 2014: All Ages* | Hospital Discharges, 2010: All Ages | Cost, 2012–2013 |
|---|---|---|---|---|---|
| Both sexes | 7 200 000 (2.7%) | 795 000 | 133 103 | 1 015 000 | $33.9 Billion |
| Males | 3 100 000 (2.4%) | 370 000 (46.5%)† | 55 471 (41.7%)† | 485 000 | … |
| Females | 4 100 000 (2.9%) | 425 000 (53.5%)† | 77 632 (58.3%)† | 530 000 | … |
| NH white males | 2.2% | 325 000‡ | 41 410 | … | … |
| NH white females | 2.8% | 365 000‡ | 60 916 | … | … |
| NH black males | 3.9% | 45 000‡ | 7650 | … | … |
| NH black females | 4.0% | 60 000‡ | 9233 | … | … |
| Hispanic males | 2.0% | … | 4092 | … | … |
| Hispanic females | 2.6% | … | 4621 | … | … |
| NH Asian males | 1.0% | … | 1890§ | … | … |
| NH Asian females | 2.5% | 2382§ | |||
| NH American Indian or Alaska Native | 3.0%||¶ | … | 616 | … | … |
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for Hispanic, American Indian or Alaska Native, and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total stroke incidence or mortality that applies to males vs females.
Estimates include Hispanics and non-Hispanics. Estimates for whites include other nonblack races.
Includes Chinese, Filipino, Hawaiian, Japanese, and other Asian or Pacific Islander.
National Health Interview Survey (2014), National Center for Health Statistics; data are weighted percentages for Americans ≥18 years of age.51
Estimate considered unreliable or does not meet standards of reliability or precision.
Sources: Prevalence: National Health and Nutrition Examination Survey 2011 to 2014, National Center for Health Statistics (NCHS) and National Heart, Lung, and Blood Institute (NHLBI). Percentages for racial/ethnic groups are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolated to the 2014 US population. Incidence: Greater Cincinnati/Northern Kentucky Stroke Study/National Institutes of Neurological Disorders and Stroke data for 1999 provided on August 1, 2007. US estimates compiled by NHLBI. See also Kissela et al.336 Data include children. Mortality: Centers for Disease Control and Prevention/NCHS, 2014 Mortality Multiple Cause-of-Death–United States. These data represent underlying cause of death only. Mortality for NH Asians includes Pacific Islanders. Hospital discharges: National Hospital Discharge Survey, NCHS. Data include those inpatients discharged alive, dead, or status unknown. Cost: NHLBI. Data include estimated direct and indirect costs for 2012 to 2013 (average annual).
Chart 14-1. Prevalence of stroke by age and sex (NHANES 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 14-2. Annual age-adjusted incidence of first-ever stroke by race.
Hospital plus out-of-hospital ascertainment, 1993 to 1994, 1999, and 2005. ICH indicates intracerebral hemorrhage; and SAH, subarachnoid hemorrhage. Data derived from Kleindorfer et al.12
Abbreviations Used in Chapter 14
| ACCORD | Action to Control Cardiovascular Risk in Diabetes |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| AHI | apnea-hypopnea index |
| ARIC | Atherosclerosis Risk in Communities study |
| AHRQ | Agency for Healthcare Research and Quality |
| BASIC | Brain Attack Surveillance in Corpus Christi |
| BNP | B-type natriuretic peptide |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CDC | Centers for Disease Control and Prevention |
| CHD | coronary heart disease |
| CHF | congestive heart failure |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| CLRD | chronic lower respiratory disease |
| CREST | Carotid Revascularization Endarterectomy Versus Stenting Trial |
| CT | computed tomography |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DM | diabetes mellitus |
| ED | emergency department |
| eGFR | estimated glomerular filtration rate |
| EPIC | European Prospective Investigation Into Cancer and Nutrition |
| ESCAPE | Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times |
| EXTEND-IA | Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial |
| FHS | Framingham Heart Study |
| FRS | Framingham Risk Score |
| FUTURE | Follow-up of TIA and Stroke Patients and Unelucidated Risk Factor Evaluation |
| GCNKSS | Greater Cincinnati/Northern Kentucky Stroke Study |
| GFR | glomerular filtration rate |
| GWAS | genome-wide association studies |
| GWTG | Get With The Guidelines |
| HBP | high blood pressure |
| HCUP | Healthcare Cost and Utilization Project |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HIC | high-income countries |
| HR | hazard ratio |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| ICH | intracerebral hemorrhage |
| IHD | ischemic heart disease |
| IPSYS | Italian Project on Stroke in Young Adults |
| IQ | intelligence quotient |
| LDLC | low-density lipoprotein cholesterol |
| LIMC | low- and middle-income countries |
| MEPS | Medical Expenditure Panel Survey |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| MRI | magnetic resonance imaging |
| NAMCS | National Ambulatory Medical Care Survey |
| NDPSS | North Dublin Population Stroke Study |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NINDS | National Institutes of Neurological Disorders and Stroke |
| NIS | Nationwide Inpatient Sample |
| NOMAS | Northern Manhattan Study |
| ONTARGET | Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial |
| OR | odds ratio |
| PA | physical activity |
| PAR | population attributable risk |
| PREVEND | Prevention of Renal and Vascular End-Stage Disease |
| RCT | randomized controlled trial |
| REGARDS | Reasons for Geographic and Racial Differences in Stroke |
| REVASCAT | Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke due to Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours of Symptom Onset |
| RR | relative risk |
| SAH | subarachnoid hemorrhage |
| SBP | systolic blood pressure |
| SHS | Strong Heart Study |
| SPRINT | Systolic Blood Pressure Intervention Trial |
| SPS3 | Secondary Prevention of Small Subcortical Strokes |
| STOP | Stroke Prevention Trial in Sickle Cell Anemia |
| SWIFTPRIME | Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment |
| TC | total cholesterol |
| TIA | transient ischemic attack |
| tPA | tissue-type plasminogen activator |
| VIPS | Vascular Effects of Infection in Pediatric Stroke |
Stroke Prevalence
(See Table 14-1 and Chart 14-1)
Stroke prevalence estimates may differ slightly between studies because each study selects and recruits a sample of participants to represent the target study population (eg, state, region, or country).
An estimated 7.2 million Americans ≥20 years of age self-report having had a stroke (extrapolated to 2014 by use of NHANES 2011–2014 data). Overall stroke prevalence during this period was an estimated 2.7% (NHANES, NHLBI; Table 14-1).
Prevalence of stroke in the United States increases with age in both men and women (Chart 14-1).
According to data from the 2014 BRFSS (CDC), 2.8% of men and 2.8% of women ≥18 years of age had a history of stroke; 2.5% of non-Hispanic whites, 4.5% of non-Hispanic blacks, 1.8% of Asian/Pacific Islanders, 2.4% of Hispanics (of any race), 5.4% of American Indian/Alaska Natives, and 4.7% of other races or multiracial people had a history of stroke.1
Over the time period 2006 to 2010, data from BRFSS show that the overall self-reported stroke prevalence did not change. Older adults, blacks, people with lower levels of education, and people living in the southeastern United States had higher stroke prevalence.2
Analysis of temporal trends in age-, sex-, and race/ethnicity-specific stroke prevalence rates from 2006 to 2010 according to the BRFSS revealed that stroke prevalence remained stable in individuals aged 18 to 44 and 45 to 64 years but had a declining trend among individuals ≥65 years old across the study period (−1.2%, P=0.09). From 2006 to 2010, stroke prevalence declined in men (−3.6%, P<0.01) while remaining stable in women across the study period, such that in more recent years, stroke prevalence was similar in both men and women. From 2006 to 2010, there were no statistically significant temporal trends in stroke prevalence by race/ethnicity.
In studies conducted from 1993 to 2005, the prevalence of silent cerebral infarction was estimated to range from 6% to 28%, with higher prevalence with increasing age.3–5
The prevalence of stroke-related symptoms was found to be relatively high in a general population free of a prior diagnosis of stroke or TIA. On the basis of data from 18 462 participants enrolled in a national cohort study, 17.8% of the population >45 years of age reported at least 1 symptom. Stroke symptoms were more likely among blacks than whites, among those with lower income and lower educational attainment, and among those with fair to poor perceived health status. Symptoms also were more likely in participants with higher Framingham stroke risk score (REGARDS, NINDS).6
Projections show that by 2030, an additional 3.4 million US adults aged ≥18 years will have had a stroke, a 20.5% increase in prevalence from 2012. The highest increase (29%) is projected to be in Hispanic men.7
With the increase in the aging population, prevalence of stroke survivors is projected to increase, especially among elderly women.8
Stroke Incidence
(See Table 14-1 and Charts 14-2 through 14-5)
Chart 14-5. Age-adjusted incidence of stroke/transient ischemic attack by race and sex, ages 45 to 74 years, ARIC study cohort, 1987 to 2001.
ARIC indicates Atherosclerosis Risk in Communities.
Data derived from the National Heart, Lung, and Blood Institute’s 2006 Chart Book on Cardiovascular and Lung Diseases.337
Each year, ≈795 000 people experience a new or recurrent stroke (Table 14-1). Approximately 610 000 of these are first attacks, and 185 000 are recurrent attacks (GCNKSS, NINDS, and NHLBI; GCNKSS and NINDS data for 1999 provided July 9, 2008; estimates compiled by NHLBI).
Of all strokes, 87% are ischemic and 10% are ICH strokes, whereas 3% are SAH strokes (GCNKSS, NINDS, 1999).
On average, every 40 seconds, someone in the United States has a stroke (AHA computation based on the latest available data).
Temporal trend data from the BASIC Project for the time period 2000 through 2010 demonstrated that ischemic stroke rates declined significantly in people aged ≥60 years but remained largely unchanged over time in those aged 45 to 59 years. Rates of stroke decline did not differ significantly for non-Hispanic whites and Mexican Americans overall in any age group, but ethnic disparities between Mexican Americans and non-Hispanic whites in stroke rates in the 45- to 59-year-old and 60- to 74-year-old age groups persist.9
Analysis of data from the FHS suggests that stroke incidence is declining over time in this largely white cohort. Data from 1950 to 1977, 1978 to 1989, and 1990 to 2004 showed that the age-adjusted incidence of first stroke per 1000 person-years in each of the 3 periods was 7.6, 6.2, and 5.3 in men and 6.2, 5.8, and 5.1 in women, respectively. Lifetime risk for incident stroke at 65 years of age decreased significantly in the latest data period compared with the first, from 19.5% to 14.5% in men and from 18.0% to 16.1% in women.10
In a similar fashion, data from a 20% sample of hospitalized Medicare beneficiaries showed that the rate of first stroke among patients aged >65 years decreased by ≈40% over the past 2 decades (1988–2008), a decline driven primarily by marked reductions in the incidence of ischemic stroke.11
Annual age-adjusted incidence for first-ever stroke was higher in black individuals than white individuals in data collected in 1993 to 1994, 1999, and 2005 for each of the following stroke types: ischemic, ICH, and SAH (Chart 14-2).
On the basis of data from the 1999 GCNKSS, the annual rate of first cerebral infarction (Chart 14-3) and all first-ever stroke (Chart 14-4) was lower for black and white women than for black and white men for ages 45 to 74 years. Black women had higher annual rates of first cerebral infarction and first-ever stroke than black men for ages ≥75 years. White women had similar rates of first cerebral infarction and first-ever stroke as men in the 75- to 84-year-old age group; however the rates were lower for white women than white men at ages ≥85 years (Charts 14-3 and 14-4).
The age-adjusted incidence of stroke/TIA was lower for black women than for black men at all ages in the ARIC study cohort (1987–2001). White men and women had a similar incidence of stroke/TIA at ages 45 to 54 years in this study; however, white women had a lower incidence at ages 55 to 74 years (Chart 14-5).
Regarding trends in incidence by race, the most recent GCNKSS data show that compared with the 1990s, when incidence rates of stroke were stable, stroke incidence in 2005 had decreased for whites. A similar decline was not seen in blacks. These changes for whites were driven by a decline in ischemic strokes. There were no changes in incidence of ischemic stroke for blacks or of hemorrhagic strokes in blacks or whites.12
In contrast, in the multicenter ARIC study of black and white adults, stroke incidence and mortality rates decreased from 1987 to 2011. The decreases varied across age groups but were similar across sex and race.13
Data from the BASIC Project showed that the age-, sex-, and ethnicity-adjusted incidence of ICH decreased from 2000 to 2010 (from an annual incidence rate of 5.21 per 10 000 [95% CI, 4.36–6.24] to 4.30 per 10 000 [95% CI, 3.21–5.76]).14
Each year, ≈55 000 more women than men have a stroke (GCNKSS, NINDS).12
Women have a higher lifetime risk of stroke than men. In the FHS, lifetime risk of stroke among those 55 to 75 years of age was 1 in 5 for women (95% CI, 20%–21%) and ≈1 in 6 for men (95% CI, 14%–17%).15
Age-specific incidence rates are substantially lower in women than men in younger and middle-age groups, but these differences narrow so that in the oldest age groups, incidence rates in women are approximately equal to or even higher than in men.8,16–20
In the national REGARDS cohort, in 27 744 participants followed up for 4.4 years (2003–2007), the overall age- and sex-adjusted black/white incidence rate ratio was 1.51, but for ages 45 to 54 years, it was 4.02, whereas for those ≥85 years of age, it was 0.86.21 Similar trends for decreasing black/white incidence rate ratio with older age were seen in the GCNKSS.22
The BASIC Project (NINDS) demonstrated an increased incidence of stroke among Mexican Americans compared with non-Hispanic whites in a community in southeast Texas. The crude 3-year cumulative incidence (2000–2002) was 16.8 per 1000 in Mexican Americans and 13.6 per 1000 in non-Hispanic whites. Specifically, Mexican Americans had a higher cumulative incidence of ischemic stroke than non-Hispanic whites at younger ages (45–59 years of age: RR, 2.04; 95% CI, 1.55–2.69; 60–74 years of age: RR, 1.58; 95% CI, 1.31–1.91) but not at older ages (≥75 years of age: RR, 1.12; 95% CI, 0.94–1.32). Mexican Americans also had a higher incidence of ICH and SAH than non-Hispanic whites, after adjustment for age.23
The age-adjusted incidence of first ischemic stroke per 1000 was 0.88 in whites, 1.91 in blacks, and 1.49 in Hispanics according to data from NOMAS (NINDS) for 1993 to 1997. Among blacks, compared with whites, the relative rate of intracranial atherosclerotic stroke was 5.85; of extracranial atherosclerotic stroke, 3.18; of lacunar stroke, 3.09; and of cardioembolic stroke, 1.58. Among Hispanics (primarily Cuban and Puerto Rican), compared with whites, the relative rate of intracranial atherosclerotic stroke was 5.00; of extracranial atherosclerotic stroke, 1.71; of lacunar stroke, 2.32; and of cardioembolic stroke, 1.42.24
Among 4507 American Indian participants without a prior stroke in the SHS in 1989 to 1992, the age- and sex-adjusted incidence of stroke through 2004 was 6.79 per 100 person-years, with 86% of incident strokes being ischemic.25
In the REGARDS study, the increased risk of ICH with age differed between blacks and whites: There was a 2.25-fold (95% CI, 1.63–3.12) increase per decade older age in whites but no age association with ICH risk in blacks (HR, 1.09; 95% CI, 0.70–1.68 per decade older age).26
Chart 14-3. Annual rate of first cerebral infarction by age, sex, and race (GCNKSS 1999).
Rates for black men and women 45 to 54 years of age and for black men ≥75 years of age are considered unreliable.
GCNKSS indicates Greater Cincinnati/Northern Kentucky Stroke Study.
Source: Unpublished data from the GCNKSS.
Chart 14-4. Annual rate of all first-ever strokes by age, sex, and race (GCNKSS 1999).
Rates for black men and women 45 to 54 years of age and for black men ≥75 years of age are considered unreliable.
GCNKSS indicates Greater Cincinnati/Northern Kentucky Stroke Study.
Source: Unpublished data from the GCNKSS.
TIA: Prevalence, Incidence, and Prognosis
In a nationwide survey of US adults, the estimated prevalence of self-reported physician-diagnosed TIA increased with age and was 2.3% overall, which translates to ≈5 million people. The true prevalence of TIA is likely to be greater, because many patients who experience neurological symptoms consistent with a TIA fail to report it to their healthcare provider.27
In the GCNKSS, according to data from 1993 and 1994, the age-, sex-, and race-adjusted incidence rate for TIA was 0.83 per 10 000.28 The age- and sex-adjusted incidence rate for TIA in Rochester, MN, was estimated at 0.68 per 1000 for the years 1985 through 1989.29 In a more recent Italian community-based registry conducted in 2007 to 2009, the crude TIA incidence rate was 0.52 per 1000.30
Incidence of TIA increases with age and varies by sex and race/ethnicity. Men, blacks, and Mexican Americans have higher rates of TIA than their female and non-Hispanic white counterparts.23,28
Approximately 15% of all strokes are heralded by a TIA.31
TIAs confer a substantial short-term risk of stroke, hospitalization for CVD events, and death. Of 1707 TIA patients evaluated in the ED of Kaiser Permanente Northern California, 180 (11%) experienced a stroke within 90 days, and 91 (5%) had a stroke within 2 days. Predictors of stroke included age >60 years, DM, focal symptoms of weakness or speech impairment, and symptoms that lasted >10 minutes.32
Meta-analyses of cohorts of patients with TIA have shown the short-term risk of stroke after TIA to be ≈3% to 10% at 2 days and 9% to 17% at 90 days.33,34
Individuals who have a TIA and survive the initial high-risk period have a 10-year stroke risk of roughly 19% and a combined 10-year stroke, MI, or vascular death risk of 43% (4% per year).35
In the GCNKSS, the 1-year mortality rate after a TIA was 12%.28
In the population-based Oxford Vascular Study, among patients with TIA, disability levels increased from 14% (modified Rankin scale >2) before the TIA to 23% at 5 years after the TIA (P=0.002). In this same study, the 5-year risk of institutionalization after TIA was 11%.36
In a meta-analysis of 47 studies,37 it was estimated that approximately one third of TIA patients have an acute lesion present on diffusion-weighted MRI and thus would be classified as having had a stroke under a tissue-based case definition38,39; however, substantial between-study heterogeneity was noted.
Recurrent Stroke
In a cohort of 10 399 patients discharged with a primary diagnosis of stroke in the state of South Carolina in 2002, recurrent stroke rates were 1.8% at 1 month, 5% at 6 months, 8% at 1 year, and 18.1% at 4 years.40
Among 600 Scandinavian stroke patients followed up for 2 years, 55 (9.2%) had a recurrent stroke, 15 (2.5%) had a TIA, 4 (0.7%) had a coronary event, and 24 (4.0%) had died. Recurrent stroke occurred in 19.2% of patients with index stroke caused by large-artery disease, 4.9% with small-vessel disease, 8.2% with cardioembolic cause, 5.6% with cryptogenic cause, and 12.8% with other and undetermined causes combined.41
Recurrent stroke is associated with a greater number of risk factors and a higher incidence of large-artery atherosclerosis than the first stroke.42
Among 1626 first-ever stroke patients in the South London Stroke Register,43 first stroke recurrence rates (95% CI) during the first, second, third, fourth, and fifth years were 8% (6.5%–9.8%), 3.3% (2.2%–4.9%), 3.5% (2.1%–5.8%), 1.2% (0.4%–3.7%), and 1.8% (0.4%–7.4%), respectively. Cumulative risks of first stroke recurrence (95% CI) were 2.6% (1.9%–3.7%) at 3 months, 8.0% (6.5%–9.8%) at 1 year, 14.1% (11.8%–16.7%) at 3 years, and 16.6% (13.5%–20.4%) at 5 years.43
During a median 5.3 years of follow-up among 987 ARIC participants with first-ever strokes, there were 183 recurrent strokes among 147 participants. Approximately 70% of recurrent strokes were of the same subtype; however, 28% were the same when the index stroke was lacunar. One-year stroke recurrence rates by index subtype were 7.9% for thrombotic, 6.5% for cardioembolic, and 6.5% for lacunar events.44
In a long-term follow-up study of recurrent vascular events among 724 first-ever TIA, stroke, or ICH patients aged 18 to 50 years in the Netherlands, cumulative 20-year risk of recurrent stroke was 17.3% (95% CI, 9.5%–25.1%) after TIA, 19.4% (95% CI, 14.6%–24.3%) after ischemic stroke, and 9.8% (95% CI, 1.0%–18.7%) after ICH.45
Among 1867 stroke patients aged 18 to 45 years in IPSYS, the 10-year cumulative risk of brain ischemia was 14.0% (95% CI, 11.4%–17.1%).46
In the NDPSS, the cumulative 2-year stroke recurrence rate was 10.8%, and case fatality was 38.6%.47
Children with arterial ischemic stroke, particularly those with arteriopathy, remain at high risk for recurrent arterial ischemic stroke despite increased use of antithrombotic agents. In the VIPS, the cumulative stroke recurrence rate was 6.8% (95% CI, 4.6%–10%) at 1 month and 12% (95% CI, 8.5%–15%) at 1 year. The 1-year recurrence rate was 32% (95% CI, 18%–51%) for moyamoya, 25% (95% CI, 12%–48%) for transient cerebral arteriopathy, and 19% (95% CI, 8.5%–40%) for arterial dissection.48
Annual recurrent stroke rates in control arms of stroke prevention trials fell from 8.71% in trials launched in the 1960s to 6.10% in the 1970s, 5.41% in the 1980s, 4.04% in the 1990s, and were 4.98% in the 2000s. If one assumes a continued linear decline, the annual recurrent stroke rate in trial control arms in the coming decade is projected to be 2.25%.49
From 1994 to 2002, 1-year recurrent ischemic stroke rates declined by almost 5% among elderly Medicare beneficiaries, but declines were heterogeneous across geographic regions of the United States.50
Stroke Mortality
(See Table 14-1 and Charts 14-6 and 14-7)
Chart 14-6. Age-adjusted death rates for stroke by sex and race/ethnicity, 2014.
Death rates for the American Indian or Alaska Native and Asian or Pacific Islander populations are known to be underestimated. Stroke includes International Classification of Diseases, 10th Revision codes I60 through I69 (cerebrovascular disease). Mortality for non-Hispanic (NH) Asians includes Pacific Islanders.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 14-7. Stroke death rates, 2011 through 2013.
All ages, by county. Rates are spatially smoothed to enhance the stability of rates in counties with small populations. International Classification of Diseases, 10th Revision codes for stroke: I60 through I69.
Data source: National Vital Statistics System and the US Census Bureau.
See “Factors Influencing the Decline in Stroke Mortality: a Statement From the American Heart Association/American Stroke Association”46 for more in-depth coverage of factors contributing to the decline in stroke mortality over the past several decades.
-
In 201451
On average, every 4 minutes, someone died of a stroke.
Stroke accounted for ≈1 of every 20 deaths in the United States.
When considered separately from other CVDs, stroke ranks No. 5 among all causes of death, behind diseases of the heart, cancer, CLRD, and unintentional injuries/accidents.
The number of deaths with stroke as an underlying cause was 133 103 (Table 14-1); the age-adjusted death rate for stroke as an underlying cause of death was 36.5 per 100 000, whereas the age-adjusted rate for any-mention of stroke as a cause of death was 61.5 per 100 000.
Approximately 60% of stroke deaths occurred outside of an acute care hospital.
Non-Hispanic black men and women had higher age-adjusted death rates for stroke than non-Hispanic white, non-Hispanic Asian, non-Hispanic Indian or Alaska Native, and Hispanic men and women in the United States (Chart 14-6).
More women than men die of stroke each year because of a larger number of elderly women than men. Women accounted for 58% of US stroke deaths in 2014.
From 2004 to 2014, the age-adjusted stroke death rate decreased 28.7% (from 51.2 per 100 000 to 36.5 per 100 000), and the actual number of stroke deaths declined 11.3% (from 150 074 deaths to 133 103 deaths).
-
Conclusions about changes in stroke death rates from 1981 to 2014 are as follows46:
There was a slightly greater decline in age-adjusted stroke death rates in men (−61.2%) than in women (−58.4%) aged ≥18 years.
Stroke death rates declined more among people aged ≥65 years (−54.1%; from 534.1 to 245.0 per 100 000) than those aged 45 to 64 years (−53.1%; from 43.5 to 20.4 per 100 000) or those aged 18 to 44 years (−43.2%; from 3.7 to 2.1 per 100 000).
Age-adjusted stroke death rates for adults aged ≥18 years declined by ≈50% or more among all racial groups; however, in 2014, rates remained higher among non-Hispanic black (66.8 per 100 000) than other races, including non-Hispanic whites (47.4 per 100 000), Asians/Pacific Islanders (38.0 per 100 000), and American Indians/Alaska Natives (34.1 per 100 000). In 2014, Hispanics had an age-adjusted stroke death rate of 40.6 per 100 000. Data from 1981 are not available for Hispanics.
There are substantial geographic disparities in stroke mortality, with higher rates in the southeastern United States, known as the “stroke belt” (Chart 14-7). This area is usually defined to include the 8 southern states of North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas. These geographic differences have existed since at least 1940,52 and despite some minor shifts,53 they persist.54–56 Within the stroke belt, a “buckle” region along the coastal plain of North Carolina, South Carolina, and Georgia has been identified with an even higher stroke mortality rate than the remainder of the stroke belt. Historically, the overall average stroke mortality has been ≈30% higher in the stroke belt than in the rest of the nation and ≈40% higher in the stroke buckle.57
In examining trends in stroke mortality by US census divisions from 1999 to 2007 for people ≥45 years of age, the rate of decline varied by geographic region and race/ethnic group. Among black and white women and white men, rates declined by ≥2% annually in every census division, but among black men, rates declined little in the East and West South Central divisions.58
On the basis of national death statistics for the time period 1990 to 2009, stroke mortality rates among American Indians and Alaska Natives were higher than among whites for both men and women in contract health services delivery area counties in the United States and were highest in the youngest age groups (35–44 years old). Stroke mortality rates and the rate ratios for American Indians/Alaska Natives to whites varied by region, with the lowest in the Southwest and the highest in Alaska. Starting in 2001, rates among American Indian/Alaska Native people decreased in all regions.59
Data from the ARIC study (1987–2011; 4 US cities) showed that the cumulative all-cause mortality rate after a stroke was 10.5% at 30 days, 21.2% at 1 year, 39.8% at 5 years, and 58.4% at the end of 24 years of follow-up. Mortality rates were higher after an incident hemorrhagic stroke (67.9%) than an ischemic stroke (57.4%). Age-adjusted mortality after an incident stroke decreased over time (absolute decrease of 8.1 deaths per 100 strokes after 10 years), which was mainly attributed to the decrease in mortality among those aged ≤65 years (absolute decrease of 14.2 deaths per 100 strokes after 10 years).13
Data from the BASIC Project showed there was no change in ICH case fatality or long-term mortality from 2000 to 2010 in a South Texas community. Yearly age-, sex-, and ethnicity-adjusted 30-day case fatality ranged from a low of 28.3% (95% CI, 19.9%–40.3%) in 2006 to 46.5% (95% CI, 35.5%–60.8%) in 2008.14
A report released by the CDC in collaboration with the Centers for Medicare & Medicaid Services, the Atlas of Stroke Hospitalizations Among Medicare Beneficiaries, found that in Medicare beneficiaries over the time period 1995 to 2002, the 30-day mortality rate varied by age: 9% in patients 65 to 74 years of age, 13.1% in those 74 to 84 years of age, and 23% in those ≥85 years of age.54
Projections of stroke mortality from 2012 to 2030 differ based on what factors are included in the forecasting.60 Conventional projections that only incorporate expected population growth and aging reveal that the number of stroke deaths in 2030 could increase by ≈50% compared with the number of stroke deaths in 2012. However, if previous stroke mortality trends are also incorporated into the forecasting, the number of stroke deaths among the entire population is projected to remain stable through 2030, with potential increases among the population aged ≥65 years. Moreover, the trend-based projection method reveals that the disparity in stroke deaths among non-Hispanic blacks compared with non-Hispanic whites could increase from a relative risk of 1.10 (95% CI, 1.08–1.13) in 2012 to 1.30 (95% CI, 0.45–2.44) in 2030.7
Stroke Risk Factors
(See Chart 14-8)
Chart 14-8. Estimated 10-year stroke risk in adults 55 years of age according to levels of various risk factors (FHS).
AF indicates atrial fibrillation; CVD, cardiovascular disease; and FHS, Framingham Risk Study.
Data derived from Wolf et al.338
For prevalence and other information on any of these specific risk factors, refer to the specific risk factor chapters. The estimated 10-year stroke risk in adults 55 years of age differs by sex and the increasing presence of multiple risk factors (eg, HBP, DM, cigarette smoking, history of AF, and history of CVD) (Chart 14-8).
High BP
(See Chapter 9 for more information.)
Median SBP declined 16 mm Hg between 1959 and 2010 for different age groups in association with large accelerated reductions in stroke mortality. In clinical trials, antihypertensive therapy has been associated with reductions in stroke incidence, with an average 41% reduction in stroke risk with SBP reductions of 10 mm Hg.46
BP is a powerful determinant of risk for both ischemic stroke and intracranial hemorrhage.
-
Risk prediction models identify elevated BP as a key parameter in the assessment of cardiovascular and stroke risk.61
Diabetic subjects with BP <120/80 mm Hg have approximately half the lifetime risk of stroke of subjects with hypertension. The treatment and lowering of BP among diabetic hypertensive individuals was associated with a significant reduction in stroke risk.62
A review identified the benefit of intense BP reduction and reduced stroke outcome risks in recent clinical trials.63 Combined results from the SPRINT and ACCORD trials demonstrated that intensive BP control (<120 mm Hg) compared with standard treatment (<140 mm Hg) resulted in a significantly lower risk of stroke (RR, 0.75; 95% CI, 0.58–0.97).64 SPRINT further enhanced findings showing that treating high-risk hypertensive adults >50 years of age to a target of 120 mm Hg significantly reduced cardiovascular events by 30% and reduced all-cause mortality by nearly 25% compared with patients treated to a target of 140 mm Hg.
In the REGARDS study (NINDS), between the ages of 45 and 64 years (an age group in which African Americans are at 2 to 3 times the risk of stroke as whites), ≈40% of the excess stroke risk in African Americans was attributable to traditional stroke risk factors, with levels of SBP accounting for approximately one half of this impact.65 For each 10-mm Hg increase in levels of SBP, the increased stroke risk in whites was ≈8%66; however, a similar 10-mm Hg increase in SBP in African Americans was associated with a 24% increase in stroke risk, an impact 3 times greater than in whites.67
Cross-sectional baseline data from the SPS3 trial showed that more than half of all patients with symptomatic lacunar stroke had uncontrolled hypertension at 2.5 months after stroke.68
A meta-analysis of 19 prospective cohort studies (including 762 393 participants) found that prehypertension is associated with incident stroke. The risk is particularly noted in those with BP values in the higher prehypertension range.69
In cross-sectional analysis from the REGARDS study (NINDS), blacks with hypertension were more likely to be aware of their HBP and more frequently received treatment for it than whites but were less likely than whites to have their BP controlled.67 In the SPS3 trial, black participants were more likely to have SBP ≥150 mm Hg at both study entry (40%) and end-study visit (17%; mean follow-up, 3.7 years) than whites (9%) and Hispanics (11%) at end-study visit.66
The higher stroke risk for the stroke belt compared with other regions does not appear to be attributable to hypertension management, because treatment and control rates were similar for the 2 geographic areas.70
Several studies have shown significantly lower rates of recurrent stroke with lower BPs. Most recently, the BP-reduction component of the SPS3 trial showed that targeting an SBP <130 mm Hg was likely to reduce recurrent stroke by ≈20% (P=0.08) and significantly reduced ICH by two-thirds compared with an SBP goal of 130 to 149 mm Hg.66
Diabetes Mellitus
(See Chapter 10 for more information.)
DM increases ischemic stroke incidence at all ages, but this risk is most prominent (risk ratio for ischemic stroke conferred by DM >5) before 65 years of age in both blacks and whites. Overall, ischemic stroke patients with DM are younger, more likely to be black, and more likely to have HBP, MI, and high cholesterol than nondiabetic patients.71
The association between DM and stroke risk differs between sexes. A systematic review of 64 cohort studies representing 775 385 individuals and 12 539 strokes revealed that the pooled fully adjusted RR of stroke associated with DM was 2.28 (95% CI 1.93–2.69) in women and 1.83 (1.60–2.08) in men. Compared with men with DM, women with DM had a 27% greater RR for stroke when baseline differences in other major cardiovascular risk factors were taken into account (pooled RR, 1.27; 95% CI, 1.10–1.46; I2=0%).72
Prediabetes, defined as impaired glucose tolerance or a combination of impaired fasting glucose and impaired glucose tolerance, may be associated with a higher future risk of stroke, but the RRs are modest. A meta-analysis of 15 prospective cohort studies including 760 925 participants revealed that when prediabetes was defined as fasting glucose 110 to 125 mg/dL (5 studies), the adjusted RR for stroke was 1.21 (95% CI, 1.02–1.44; P=0.03).73
DM is an independent risk factor for stroke recurrence; a meta-analysis of 18 studies involving 43 899 participants with prior stroke revealed higher stroke recurrence in patients with DM than in those without (HR, 1.45; 95% CI, 1.32–1.59).74
Data from the US NIS revealed that from 1997 to 2006, the absolute number of acute ischemic stroke hospitalizations declined by 17%; however, the absolute number of acute ischemic stroke hospitalizations with comorbid DM rose by 27% (from 97 577 [20%] to 124 244 [30%]). The rise in comorbid DM was more pronounced in individuals who were younger, black or “other” race, on Medicaid, or admitted to hospitals located in the South. Factors independently associated with higher odds of DM in acute ischemic stroke patients were black or “other” (versus white) race, CHF, peripheral vascular disease, and history of MI, renal disease, or hypertension.75
A population-based study of 1375 first-ever stroke patients 25 to 74 years old who were followed up to 23 years found that diabetic patients had a higher risk of death than nondiabetic patients (adjusted HR, 1.67; 95% CI, 1.58–1.76). The reduced survival of diabetic stroke patients was more pronounced in women (P=0.02) and younger individuals (P<0.001).76
A meta-analysis of prospective RCTs of interventions that targeted people with prediabetes revealed a 24% RR reduction in fatal and nonfatal strokes (HR, 0.76; 95% CI, 0.58–0.99).77
A meta-analysis of 4 RCTs including 27 544 patients revealed that those randomized to intensive glucose control did not have a reduction in stroke risk compared with those with conventional glucose control; however, there was a 14% reduction in nonfatal MI (incidence rate ratio, 0.86; 95% CI, 0.77–0.97).78
A retrospective analysis of diabetic patients with acute ischemic stroke revealed that those who had been taking and continued taking sulfonylureas were less likely to experience symptomatic hemorrhagic transformation than those who did not take sulfonylureas (P=0.016).79
A meta-analysis of 40 RCTs of BP lowering among 100 354 participants with DM revealed a lower risk of stroke (combined RR, 0.73; 95% CI, 0.64–0.83; absolute risk reduction, 4.06; 95% CI, 2.53–5.40).80
A subsequent meta-analysis of 28 RCTs involving 96 765 participants with DM revealed that a decrease in SBP by 10 mm Hg was associated with lower risk of stroke (RR, 0.74; 95% CI, 0.66–0.83). Significant interactions were observed, with lower RRs (RR, 0.71; 95% CI, 0.63–0.80) observed among trials with mean baseline SBP ≥140 mm Hg and no significant associations among trials with baseline SBP <140 mm Hg. The associations between BP lowering and stroke risk reduction were present for both the achieved SBP of <130 mm Hg or ≥130-mm Hg stratum.81
The ACCORD study showed that in patients with type 2 DM, targeting SBP to <120 mm Hg did not reduce the rate of cardiovascular events compared with subjects in whom the SBP target was <140 mm Hg, except for the end point of stroke, for which intensive therapy reduced the risk of any stroke (HR, 0.59; 95% CI, 0.39–0.89) and nonfatal stroke (HR, 0.63; 95% CI, 0.41–0.96).62
ONTARGET revealed that in both patients with and without DM, the adjusted risk of stroke continued to decrease down to achieved SBP values of 115 mm Hg, whereas there was no benefit for other fatal or nonfatal cardiovascular outcomes below an SBP of 130 mm Hg.82
Disorders of Heart Rhythm
(See Chapter 17 for more information.)
AF is a powerful risk factor for stroke, independently increasing risk ≈5-fold throughout all ages. The percentage of strokes attributable to AF increases steeply from 1.5% at 50 to 59 years of age to 23.5% at 80 to 89 years of age.83,84
Because AF is often asymptomatic85,86 and likely frequently undetected clinically,87 the stroke risk attributed to AF may be substantially underestimated.88 Screening for AF in patients with cryptogenic stroke or TIA by use of outpatient telemetry for 21 to 30 days has resulted in an AF detection rate of 12% to 23%.87–89
Among 2580 participants ≥65 years of age with hypertension in whom a cardiac rhythm device that included an atrial lead was implanted, 35% developed subclinical tachyarrhythmias (defined as an atrial rate ≥190 beats per minute that lasted ≥6 minutes). These subclinical events were independently associated with a 2.5-fold increased risk of ischemic stroke or systemic embolism.90
An analysis of patients from the Veterans Administration showed that among patients with device-documented AF, the presence of relatively brief amounts of AF raised the short-term risk of stroke 4- to 5-fold. This risk was highest in the initial 5 to 10 days after the episode of AF and declined rapidly after longer periods.91
Important risk factors for stroke in the setting of AF include advancing age, hypertension, HF, DM, previous stroke or TIA, vascular disease, and female sex.92–94 Additional biomarkers, including high levels of troponin and BNP, increase the risk of stroke in the setting of AF independent of those well-established clinical characteristics.95
Left atrial enlargement is associated with AF, causing the 2 conditions to often coexist. A systematic review of 9 cohort studies including 67 875 participants revealed that those with left atrial enlargement in the setting of sinus rhythm had stroke rates ranging from 0.64 to 2.07 per 100 person-years. Two studies found indications of modification by sex, with only positive associations observed in women.96
High Blood Cholesterol and Other Lipids
(See Chapter 8 for more information.)
For clarity, different types of cholesterol (TC, subfractions) are described here and are bolded in each bullet point. Overall, the association of each cholesterol subfraction with total stroke has shown inconsistent results, and the data are limited on associations with specific ischemic stroke subtypes. Further research is needed to identify the association of cholesterol with ischemic stroke subtypes, as well as the association of lobar versus deep intracerebral hemorrhage.97–102
An association between TC and ischemic stroke has been found in some prospective studies103–105 but not others.97–99 In the Women’s Pooling Project, including those <55 years of age without CVD, TC was associated with an increased risk of stroke at the highest quintile (mean cholesterol 7.6 mmol/L).100 An association of elevated TC with risk of stroke was noted to be present in those 40 to 49 years old and 50 to 59 years old but not in other age groups in the Prospective Studies Collaboration.101 Elevated TC is inversely associated in multiple studies with hemorrhagic stroke.102
Data from the Honolulu Heart Program//NHLBI found that in Japanese men 71 to 93 years of age, low concentrations of HDL-C were more likely to be associated with a future risk of thromboembolic stroke than were high concentrations.102 However, a meta-analysis of 23 studies performed in the Asia-Pacific Region showed no significant association between low HDL-C and stroke risk,106 although another meta-analysis without geographic restriction demonstrated a protective association of HDL-C with stroke.98 A Finnish study of 27 703 men and 30 532 women followed up for >20 years for ischemic stroke found an independent inverse association of HDL-C with the risks of total and ischemic stroke in women.99 In the CHS, higher HDL-C was associated with a lower risk of ischemic stroke in men but not in women.107
In an analysis by the Emerging Risk Factors Collaboration of individual records on 302 430 people without initial vascular disease from 68 long-term prospective studies, the HR for ischemic stroke was 1.12 (95% CI, 1.04–1.20) with quintiles of non-HDL-C.108 In the Women’s Health Study, LDL-C was associated with an increased risk of stroke,103 and LDL-C may have a stronger association for large-artery atherosclerotic subtype.109 In a pooled analysis of CHS and ARIC, low LDL-C (<158.8 mg/dL) was associated with an increased risk of ICH.110
Among 13 951 patients in the Copenhagen Heart Study followed up for 33 years for ischemic stroke, increasing stepwise levels of nonfasting triglycerides were associated with increased risk of ischemic stroke in both men and women,111 although in ARIC and the Physician’s Health Study, there was no association.112,113 In the Rotterdam study (n=9068), increasing quartiles of serum triglycerides were associated with a reduced risk of ICH.114
Smoking/Tobacco Use
(See Chapter 3 for more information.)
Current smokers have a 2 to 4 times increased risk of stroke compared with nonsmokers or those who have quit for >10 years.115,116
Cigarette smoking is a risk factor for ischemic stroke and SAH.115–117
Smoking is perhaps the most important modifiable risk factor in preventing SAH, with the highest PAR of any SAH risk factor.118
In a large Danish cohort study, among people with AF, smoking was associated with a higher risk of ischemic stroke/arterial thromboembolism or death, even after adjustment for other traditional risk factors.119
Data support a dose-response relationship between smoking and risk of stroke across old and young age groups.117,120
A meta-analysis comparing pooled data of ≈3.8 million smokers and nonsmokers found a similar risk of stroke associated with current smoking in women and men.121
Discontinuation of smoking has been shown to reduce stroke risk across sex, race, and age groups.120
Smoking may impact the effect of other stroke risk factors on stroke risk. For example, a synergistic effect appears to exist between SBP122 and oral contraceptives123,124 and the risk of stroke.
Exposure to second-hand smoke, also termed passive smoking or environmental tobacco smoke, is a risk factor for stroke. Meta-analyses have estimated a pooled RR of 1.25 for exposure to spousal smoking (or nearest equivalent) and risk of stroke. A dose-response relationship between exposure to secondhand smoke and stroke risk was also reported.125,126 Data from REGARDS support these findings; after adjustment for other stroke risk factors, the risk of overall stroke was increased 30% among non-smokers who had secondhand smoke exposure during adulthood (95% CI, 2%–67%).127 Data from another large-scale prospective cohort study of women in Japan showed that environmental tobacco smoke exposure at home during adulthood was associated with an increased risk of stroke mortality in those aged ≥80 years (HR, 1.24; 95% CI, 1.05–1.46). Overall, the increased risk was most evident for SAH (HR, 1.66; 95% CI, 1.02–2.70) in all age groups.128
Physical Inactivity
(See Chapter 4 for more information.)
Results from REGARDS found that participants reporting PA <4 times per week had a 20% increased risk of incident stroke over a mean of 5.7 years compared with those exercising ≥4 times per week. This relationship, which was more pronounced in men than in women, could be explained in large part by the effect of PA on reducing traditional risk factors, such as obesity and DM.129
Over a mean follow-up of 17 years, the ARIC study found a significant trend among African-Americans toward reduced incidence of stroke with increasing level of PA; a similar trend was observed for whites in the study, although it was not statistically significant. Data from this study showed that although the highest levels of activity were most protective, even modest levels of PA appeared to be beneficial.130
In NOMAS, a prospective cohort that included white, black, and Hispanic adults in an urban setting followed up for a median of 9 years, moderate to vigorous leisure-time PA was associated with an overall 35% reduction in risk of ischemic stroke.131
In the Aerobics Center Longitudinal Study of participants who underwent evaluation at the Cooper Clinic in Dallas, TX (46 405 men and 15 282 women), investigators found that cardiorespiratory fitness as measured by exercise treadmill testing was associated with a reduced risk of fatal and nonfatal stroke. Investigators noted that the effect was mainly notable for a higher intensity level of fitness achieved (7 to 8 maximum metabolic equivalents).132 A prospective cohort study of 22 841 men and 24 880 women in Finland found a similar dose-response–independent protective effect from vigorous leisure-time PA on ischemic stroke, ICH, and SAH. The effect was more modest for commuting-time PA and was no longer present after adjustment for leisure-time PA.133
Timing of PA in relation to stroke onset has also been examined in several studies. In a hospital-based case-control study from Heidelberg, Germany, recent activity (within the prior months) was associated with reduced odds of having a stroke or TIA, whereas sports activity during young adulthood that was not continued into adulthood showed no benefit.134 In a Danish case-control study, ischemic stroke patients were less physically active in the week preceding the stroke than age- and sex-matched control subjects, with the highest activity scores associated with the greatest reduction in odds of stroke.135
Several recent prospective studies found associations of PA and stroke risk in women. In the Million Women Study, a prospective cohort study among women in England and Scotland, over an average follow-up of 9 years, self-report of any PA at baseline was associated with reduced risk of any stroke, as well as stroke subtypes; however, more frequent or strenuous activity was not associated with increased protection against stroke.136 Similarly, a low level of leisure-time PA was associated with a 1.5 times higher risk of stroke and a nearly 2.5 times higher risk of fatal stroke than intermediate to high levels of activity in a cohort of ≈1500 women followed up for up to 32 years.137 The EPIC-Heidelberg cohort included 25 000 men and women and identified stroke outcomes over a mean of 13 years of follow-up. Among women, participation in any level of PA was associated with a nearly 50% reduction in stroke risk compared with inactivity; no similar pattern was seen for men.138
A dose-response effect was seen for total number of hours spent walking per week, and increased walking time was associated with reduced risk of incident stroke among 4000 men in the British Regional Heart Study. Those reporting ≥22 hours of walking per week had one third the risk of incident stroke as those who walked <4 hours per week. No clear association between walking speed or distance walked was seen in this study.139
Nutrition
(See Chapter 5 for more information.)
Adherence to a Mediterranean-style diet that was higher in nuts and olive oil was associated with a reduced risk of stroke (HR, 0.54; 95% CI, 0.35–0.84) in a randomized clinical trial conducted in Spain. The protective benefit of the Mediterranean diet observed was greater for strokes than for MI, but stroke subtype was not available.140
In the Nurses Health and Health Professionals Follow-up Studies, each 1-serving increase in sugar-sweetened soda beverage was associated with a 13% increased risk of ischemic stroke but not hemorrhagic stroke. Conversely, each 1-serving increase in low-calorie or diet soda was associated with a 7% increased risk of ischemic stroke and 27% increased risk of hemorrhagic stroke.141
A meta-analysis of >94 000 people with 34 817 stroke events demonstrated that eating ≥5 servings of fish per week versus eating <1 serving per week was associated with a 12% reduction in stroke risk; however, these results were not consistent across all cohort studies.142
According to registry data from Sweden, people eating ≥7 servings of fruits and vegetables per day had a 19% reduced risk of stroke compared with those only eating 1 serving per day. This effect was only seen in people who did not have hypertension.143
A meta-analysis of case-control, prospective cohort studies and an RCT investigating the association between olive oil consumption and the risk of stroke (38 673 participants) revealed a reduction in stroke risk (RR, 0.74; 95% CI, 0.60–0.92).144
A meta-analysis of 10 prospective cohort studies including 314 511 nonoverlapping individuals revealed that higher monounsaturated fatty acid intake was not associated with risk of overall stroke (RR, 0.86; 95% CI, 0.74–1.00) and risk of ischemic stroke (RR, 0.92; 95% CI, 0.79–1.08) but was associated with a reduced risk of hemorrhagic stroke (RR, 0.68; 95% CI, 0.49–0.96).145
A meta-analysis of prospective cohort studies evaluating the impact of dairy intake on CVD noted that total dairy intake and calcium from dairy were associated with an inverse summary RR estimate for stroke (0.91 [95% CI, 0.83–0.99] and 0.69 [95% CI, 0.60–0.81]).146
A meta-analysis of 20 prospective cohort studies of the association between nut consumption and cardiovascular outcomes (n=467 389) revealed no association between nut consumption and stroke (2 studies; RR, 1.05; 95% CI, 0.69–1.61) but did find an association with stroke mortality (3 studies; RR, 0.83; 95% CI, 0.69–1.00).147
A meta-analysis of 8 prospective studies (n=410 921) revealed no significant association between consumption of refined grains and risk of stroke.148 A second meta-analysis of 8 prospective studies (n=468 887) revealed that a diet containing greater amounts of legumes was not associated with a lower risk of stroke; however, a diet with greater amounts of nuts was associated with lower risk of stroke (summary RR, 0.90; 95% CI, 0.81–0.99). Sex significantly modified the effects of nut consumption on stroke risk, and high nut intake was associated with reduced risk of stroke in women (SRR, 0.85; 95% CI, 0.75–0.97) but not in men (SRR, 0.95; 95% CI, 0.82–1.11).149
A meta-analysis of 21 studies (n=13 033) evaluating the effect of vitamin D on cardiovascular outcomes revealed that vitamin D supplementation was not associated with a lower risk of stroke (HR, 1.07; 95% CI, 0.91–1.29).150
A meta-analysis of 14 cohorts (n=333 250) revealed that potassium intake is associated with a lower risk of stroke (RR, 0.80; 95% CI, 0.72–0.90). In addition, the dose-response analysis showed that for every 1 g/d (25.6 mmol/d) increase in vitamin K intake, there was a 10% reduction in stroke risk (RR, 0.90; 95% CI, 0.84–0.96).151
A meta-analysis of 8 studies (n=280 174) indicated an inverse association between flavonol intake and stroke (summary RR, 0.86; 95% CI, 0.75–0.99). An increase in flavonol intake of 20 mg/d was associated with a 14% decrease in the risk for developing stroke (summary RR, 0.86; 95% CI, 0.77–0.96). Subgroup analyses suggested an inverse association between highest flavonol intake and stroke risk among men (summary RR, 0.74; 95% CI, 0.56–0.97) but not women (summary RR, 0.99; 95% CI, 0.85–1.16).152
In a population of Chinese adults, folate therapy combined with enalapril was associated with a significant reduction in ischemic stroke risk (HR, 0.76; 95% CI, 0.64–0.91). Although the US population is not as likely to be at risk of folate deficiency because of folate fortification of grains, this study demonstrated the importance of adequate folate levels for stroke prevention.153
Family History and Genetics
(See Chapter 7 for more information.)
In the FHS, a documented parental ischemic stroke by the age of 65 years was associated with a 3-fold increase in ischemic stroke risk in offspring, even after adjustment for other known stroke risk factors. The absolute magnitude of the increased risk was greatest in those in the highest quintile of the FRS. By age 65 years, people in the highest FRS quintile with an early parental ischemic stroke had a 25% risk of stroke compared with a 7.5% risk of ischemic stroke for those without such a history.154
Recent heritability studies using common genome-wide genotype data have confirmed that genetic susceptibility to ischemic stroke differs by age and by sex, with a trend toward higher heritabilities in younger cases and in women. Heritability of ischemic stroke also varies by stroke subtype, with higher estimated heritabilities for large-vessel disease (40.3%) and cardioembolic stroke (32.6%) than for small-vessel disease (16.1%).155–157
Gene regions associated at genome-wide levels of significance with large-vessel ischemic stroke and replicated in independent samples include HDAC,158,159 ABO,160,161 and TSPAN2.162
Gene regions associated at genome-wide levels of significance with cardioembolic stroke and replicated in independent samples include PTX233,157 and ZFHX3.163,164 These regions were also identified in GWAS of AF.163,165
Gene regions associated with small-vessel disease stroke identified by GWAS include ALDH2/SH2B3162,166,167 and FOXF2.168 Follow-up experimental studies in mouse and zebrafish models of small-vessel disease demonstrated a role of FOXF2 consistent with a small-vessel disease pathogenesis.168
The PMF1/BGLAP region has been associated at a genome-wide level with nonlobar ICH, and this has been replicated in an independent sample.169
Apolipoprotein E alleles have been associated at a genome-wide level with lobar ICH, and this has been replicated in an independent sample.170
Chronic Kidney Disease
(See Chapter 12 for more information.)
A meta-analysis of >280 000 patients showed a 43% increased incident stroke risk among patients with a GFR <60 mL·min−1·1.73 m−2.171
In a study of 539 287 Swedish men and women followed up for 12 years,172 HRs for ICH were as follows: for GFR 60 to 90 mL·min−1·1.73 m−2 (mild), 1.04 (95% CI, 0.93–1.15); for GFR 30 to 60 mL·min−1·1.73 m−2 (moderate), 1.26 (95% CI, 0.96–1.64); and for GFR 15 to 30 mL·min−1·1.73 m−2 (severe impairment), 2.31 (95% CI, 1.10–4.87). Among 128 patients with ICH, the presence of GFR <45 mL·min−1·1.73 m−2 was associated with larger, lobar hematomas and poor outcome.173
A pooled analysis of 4 prospective community-based cohorts (ARIC, MESA, CHS, and PREVEND) including 29 595 participants showed that low eGFR (45 mL·min−1·1.73 m−2) was significantly associated with increased risk of ischemic stroke (HR, 1.30; 95% CI, 1.01–1.68) but not hemorrhagic stroke (HR, 0.92; 95% CI, 0.47–1.81) compared with normal GFR (95 mL·min−1·1.73 m−2). A high albumin-to-creatinine ratio of 300 mg/g was associated with both ischemic stroke (HR, 1.62; 95% CI, 1.27–2.07) and hemorrhagic stroke (HR, 2.57; 95% CI, 1.37–4.83) compared with 5 mg/g.174
Among patients registered in the Scottish Stroke Care Audit, 32% of the 2520 stroke patients admitted to 2 teaching hospitals over 3 years had renal dysfunction (eGFR <45 mL·min−1·1.73 m−2). Stroke patients admitted with renal dysfunction were more likely to die in the hospital (OR, 1.59; 95% CI, 1.26–2.00).175
Proteinuria and albuminuria are better predictors of stroke risks than eGFR in patients with kidney disease.176
Risk Factor Issues Specific to Women
See the “Guidelines for the Prevention of Stroke in Women: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association” for more in-depth coverage of stroke risk factors unique to women.177
On average, women are ≈4 years older at stroke onset than men (≈75 years compared with 71 years).178
In the setting of AF, women have a significantly higher risk of stroke than men.179–183
Analysis of data from the FHS found that women with natural menopause before 42 years of age had twice the ischemic stroke risk of women with natural menopause after 42 years of age; however, no association was found between age at natural menopause and risk of ischemic or hemorrhagic stroke in the Nurse’s Health Study.184
Overall, randomized clinical trial data indicate that the use of estrogen plus progestin, as well as estrogen alone, increases stroke risk in postmenopausal, generally healthy women and provides no protection for postmenopausal women with established CHD185–188 and recent stroke or TIA.189
In a nested case-control study of the United Kingdom’s General Practice Research Database, stroke risk was not increased for users of low-dose (≤50 μg) estrogen patches (RR, 0.81; 95% CI, 0.62–1.05) but was increased for users of high-dose (>50 μg) patches (RR, 1.89; 95% CI, 1.15–3.11) compared with nonusers.190
Low-estrogen-dose oral contraceptives are associated with a 93% increased risk of ischemic stroke, but the absolute increased risk is small (4.1 ischemic strokes per 100 000 nonsmoking, normotensive women).191–193
Migraine with aura is associated with ischemic stroke in younger women, particularly if they smoke or use oral contraceptives. The combination of all 3 factors increases the risk ≈9-fold compared with women without any of these factors.194,195
In the Baltimore-Washington Cooperative Young Stroke Study, the risk of ischemic stroke or ICH during pregnancy and the first 6 weeks after giving birth was 2.4 times greater than for nonpregnant women of similar age and race. The excess risk of stroke (all types except SAH) attributable to the combined pregnancy/postpregnancy period was 8.1 per 100 000 pregnancies.196
Analyses of the US NIS from 1994 to 1995 and from 2006 to 2007 show a temporal increase in the proportion of pregnancy hospitalizations that were associated with a stroke, with a 47% increase for antenatal hospitalizations and an 83% increase for postpartum hospitalizations. Increases in the prevalence of HD and hypertensive disorders accounted for almost all the increase in postpartum stroke hospitalizations but not the antenatal stroke hospitalizations.103
Preeclampsia is a risk factor for ischemic stroke remote from pregnancy.196 The increase in stroke risk related to preeclampsia may be mediated by later risk of hypertension and DM.197
Sleep Apnea
In the Wisconsin Sleep Cohort Study, for the time period 2001 to 2010, the prevalence of sleep-disordered breathing, defined as an AHI ≥5, was estimated to be 34% for men and 17% for women aged 30 to 70 years.198
In the MESA Sleep Cohort, the prevalence of mild (AHI 5–14), moderate (AHI 15–29), and severe (AHI ≥30) sleep-disordered breathing, respectively, was estimated to be 32.6%, 17.9%, and 12.4% in whites; 31.1%, 17.5%, and 14.9% in blacks; 33.3%, 20.5%, and 17.7% in Hispanics; and 27.0%, 21.6%, and 17.8% in Chinese. After accounting for sex, age, and study site, blacks had a greater odds of sleep apnea syndrome (AHI ≥5 and Epworth Sleepiness Scale score >10) than whites, and Hispanics had a greater odds of mild, moderate, and severe sleep-disordered breathing than whites.199
Obstructive sleep apnea is common after stroke, with prevalence well in excess of 50%.200–203
In the BASIC Project, Mexican Americans had a higher prevalence of poststroke sleep-disordered breathing, defined as an AHI ≥10, than non-Hispanic whites after adjustments for confounders (prevalence ratio, 1.21; 95% CI, 1.01–1.46).203
In the Sleep Heart Health Study, obstructive sleep apnea measured by the obstructive AHI was associated with risk of incident ischemic stroke in men after adjustment for confounders (P=0.016 for linear trend associated with quartiles of AHI) but not in women. Compared with men in the lowest quartile of AHI (0 to <4.1), men in the highest quartile (AHI >19) had an adjusted HR of 2.9 (95% CI, 1.1–7.4).204
In a prospective analysis of nationwide databases of the entire Danish population from 2000 to 2011, risk of ischemic stroke was significantly higher in those with sleep apnea than in the general population (RR, 1.50; 95% CI, 1.35–1.66).202
In a meta-analysis of 5 studies, obstructive sleep apnea was associated with incident stroke, with an OR of 2.2 (95% CI, 1.6–3.2). Similar results were found in 2 subsequent meta-analyses that included additional studies (OR, 2.1 [95% CI, 1.5–2.9] and OR, 2.0 [95% CI, 1.4–2.9]).205–207
In the BASIC Project, acute infarction involving the brainstem (versus no brainstem involvement) was associated with the odds of sleep-disordered breathing, defined as an AHI ≥10, with an OR of 3.76 (95% CI, 1.44–9.81) after adjustment for demographics, risk factors, and stroke severity.179 In this same study, ischemic stroke subtype was not found to be associated with the presence or severity of sleep-disordered breathing.208
Obstructive sleep apnea is associated with higher poststroke mortality209–211 and worse functional outcome.212
No definitive study has been conducted to determine whether treatment with continuous positive airway pressure prevents stroke or improves post-stroke outcomes.
Psychosocial Factors
Among 6019 adults followed up for a mean of 16.3 years from the first NHANES, higher levels of anxiety symptoms were associated with increased risk of incident stroke after adjustment for demographic, cardiovascular, and behavioral risk factors (HR, 1.14; 95% CI, 1.03–1.25). This association remained significant with further adjustment for depressive symptoms.213
In the Chicago Health and Aging Project, higher psychological distress was associated with higher stroke mortality (HR, 1.29; 95% CI, 1.10–1.52) and incident hemorrhagic strokes (HR, 1.70; 95% CI, 1.28–2.25) among 4120 adults after risk adjustment for age, sex, race, and stroke risk factors.214
Depression was associated with a nearly 2-fold increased odds of stroke after adjustment for age, socioeconomic status, lifestyle, and physiological risk factors (OR, 1.94; 95% CI, 1.37–2.74) in a cohort of 10 547 women aged 47 to 52 years who were followed up for 12 years as part of the Australian Longitudinal Study on Women’s Health.215
In a meta-analysis of 17 community-based or population-based prospective studies published between 1994 and 2010 involving 206 641 participants, people with a history of depression experienced a 34% higher risk for the development of subsequent stroke after adjustment for potential confounding factors (pooled RR, 1.34; 95% CI, 1.17–1.54); however, substantial between-study heterogeneity was noted. Associations were similar for men and women.216
A meta-analysis of 28 prospective cohort studies comprising 317 540 participants with a follow-up period that ranged from 2 to 29 years found that depression was associated with an increased risk of total stroke (pooled HR, 1.45; 95% CI, 1.29–1.63), fatal stroke (pooled HR, 1.55; 95% CI, 1.25–1.93), and ischemic stroke (pooled HR, 1.25; 95% CI, 1.11–1.40).217
Several meta-analyses have revealed that approximately 1 of every 3 stroke survivors develops poststroke depression. The most recent meta-analysis involving 61 studies (n=25 488) revealed similar results, with depression being present in 33% (95% CI, 26%–39%) of patients at 1 year after stroke, with a decline beyond 1 year: 25% (95% CI, 16%–33%) up to 5 years and 23% (95% CI, 14%–31%) at 5 years.218
Poststroke depression is associated with higher mortality. A meta-analysis of 13 studies involving 59 598 people revealed a pooled OR for mortality at follow-up of 1.22 (95% CI, 1.02–1.47).219
Twelve RCTs (n=1121) suggested that antidepressant medications could be effective in treating poststroke depression, with a beneficial effect of antidepressants on remission (pooled OR for meeting criteria for depression, 0.47; 95% CI, 0.22–0.98) and response, measured as a >50% reduction in mood scores (pooled OR, 0.22; 95% CI, 0.09–0.52).220
Six trials (n=675) suggested that brief psychosocial interventions could be useful and effective in treatment of poststroke depression.220–223
A meta-analysis of 8 RCTs assessing the efficacy of preventive pharmacological interventions among 776 initially nondepressed stroke patients revealed that the likelihood of developing post-stroke depression was reduced among subjects receiving active pharmacological treatment (OR, 0.34; 95% CI, 0.22–0.53), especially after a 1-year treatment (OR, 0.31; 95% CI, 0.18–0.56), and with the use of a selective serotonin reuptake inhibitor (OR, 0.37; 95% CI, 0.22–0.61). All studies excluded those with aphasia or significant cognitive impairment, which limits the generalizability.224
Five RCTs (n=1078) suggested that psychosocial therapies could prevent the development of poststroke depression; however, the studies were limited by heterogeneity in design, analysis, inclusion and exclusion criteria, inadequate concealment of randomization, and high numbers of dropouts.220,225
Awareness of Stroke Warning Signs and Risk Factors
In the 2009 NHIS, 51.2% of subjects were aware of 5 stroke warning symptoms and would first call 9-1-1 if they thought that someone was having a stroke. Awareness of all 5 stroke warning symptoms and calling 9-1-1 was higher among whites than blacks and Hispanics (55.9%, 47.1%, and 36.5%, respectively), women than men (53.6% versus 48.6%), and people with higher versus lower educational attainment (59.0% for people with a bachelor’s degree or more compared with 51.4% for people with a high school diploma or some college and 36.7% for those who had not received a high school diploma; unpublished NHLBI tabulation).
In the BRFSS from 2005 (n=71 994), 43.6% of respondents were aware of the 5 principal stroke symptoms, but only 18.6% responded correctly when they were also asked to identify that chest pain was not a stroke symptom. Respondents who were white and college educated were more likely to identify stroke-related symptoms correctly, and there was significant geographic variability (highest proportion of correct responses in Minnesota, Virginia, and Iowa; lowest in Louisiana, Oklahoma, and Tennessee).226
A study was conducted of patients admitted to an ED with possible stroke to determine their knowledge of the signs, symptoms, and risk factors of stroke. Of the 163 patients able to respond, 39% did not know a single sign or symptom. Patients ≥65 years of age were less likely than those <65 years old to know a sign or symptom of stroke (28% versus 47%), and 43% did not know a single risk factor. Overall, almost 40% of patients did not know the signs, symptoms, and risk factors for stroke.227
A study of patients who had experienced a stroke found that only 60.5% were able to accurately identify 1 stroke risk factor and that 55.3% were able to identify 1 stroke symptom. Patients’ median delay time from onset of symptoms to admission in the ED was 16 hours, and only 31.6% accessed the ED in <2 hours. Analysis showed that the appearance of nonmotor symptoms as the primary symptom and nonuse of the 9-1-1 system were significant predictors of delay >2 hours. Someone other than the patient made the decision to seek treatment in 66% of the cases.228
Spanish-speaking Hispanics are less likely to know all stroke symptoms than English-speaking Hispanics, non-Hispanic blacks, and non-Hispanic whites. Lack of English proficiency is strongly associated with lack of stroke knowledge among Hispanics.229 Hispanics have been noted to more frequently misidentify chest pain as a stroke symptom, and both Hispanics and African-Americans were less likely to identify the brain as the organ involved in stroke and to identify stroke symptoms.230
Overall, several studies have demonstrated low knowledge regarding risk factors for stroke in African-Americans, particularly in relation to hypertension, whereas stress is often identified as a major risk factor for stroke instead.231,232 Although data are more limited, knowledge of stroke risk factors and symptoms is also limited in children.233
A study of CVD awareness performed by the AHA among women in the United States who were >75 years old (n=1205) showed that low proportions of women identified severe headache (23%), dizziness (20%), and vision loss/changes (18%) as stroke warning symptoms.234
Further research is required in identifying interventions aimed at improving stroke literacy, as well as whether these interventions translate to improved risk factor control and earlier arrival at the hospital for acute stroke care.
Complications and Recovery
(See Charts 14-9 through 14-11)
Chart 14-9. Probability of death within 1 year after first stroke.
Source: Pooled data from the Framingham Heart Study, Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Multi-Ethnic Study of Atherosclerosis, Coronary Artery Risk Development in Young Adults, and Jackson Heart Study of the National Heart, Lung, and Blood Institute.
Chart 14-11. Probability of death with recurrent stroke in 5 years after first stroke.
Source: Pooled data from the Framingham Heart Study, Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Multi-Ethnic Study of Atherosclerosis, Coronary Artery Risk Development in Young Adults, and Jackson Heart Study of the National Heart, Lung, and Blood Institute.
Stroke is a leading cause of serious long-term disability in the United States (Survey of Income and Program Participation, a survey of the US Census Bureau).235 Approximately 3% of men and 2% of women reported that they were disabled because of stroke.
On the basis of pooled data from several large studies, the probability of death within 1 year or 5 years after a stroke was highest in individuals ≥75 years of age (Charts 14-9 and 14-10). The probability of death within 1 year of a stroke was lowest in black men aged 45 to 64 years (Chart 14-9). The probability of death within 5 years of a stroke was lowest for white men aged 45 to 64 years (Chart 14-10).
On the basis of pooled data from several large studies, the probability of death with recurrent stroke in 5 years after a stroke was lowest for black men 45 to 64 years of age and highest for black women 65 to 74 years of age (Chart 14-11).
Stroke was among the top 18 diseases contributing to years lived with disability in 2010; of these 18 causes, only the age-standardized rates for stroke increased significantly between 1990 and 2010 (P<0.05).236
In 2003, among Medicare patients discharged from the hospital after stroke, ≈45% returned directly home, 24% were discharged to inpatient rehabilitation facilities, and 31% were discharged to skilled nursing facilities. Of stroke patients returning directly home, 32% used home healthcare services.237 In data from 2011, 19% of Medicare patients were discharged to inpatient rehabilitation facilities, 25% were discharged to skilled nursing facilities, and 12% received home health care.238
The 30-day readmission rate for Medicare fee-for-service beneficiaries with ischemic stroke in 2006 was 14.4%.239
The 30-day hospital readmission rate after discharge from postacute rehabilitation for stroke was 12.7% among fee-for-service Medicare patients. The mean rehabilitation length of stay for stroke was 14.6 days.240
Data from the BRFSS (CDC) 2005 survey on stroke survivors in 21 states and the District of Columbia found that 30.7% of stroke survivors received outpatient rehabilitation. The findings indicated that the prevalence of stroke survivors receiving outpatient stroke rehabilitation was lower than would be expected if clinical practice guideline recommendations for all stroke patients had been followed.241
After stroke, women often have greater disability than men. For example, an analysis of community-living adults (>65 years of age) found that women were half as likely to be independent in activities of daily living after stroke, even after controlling for age, race, education, and marital status.242
A meta-analysis of >25 studies examining sex differences in long-term outcomes among stroke survivors found that women tended to have worse functional recovery and hence greater long-term disability and handicap; however, confidence in these conclusions was limited by the quality of the studies and variability in the statistical approach to confounding.243
A national study of inpatient rehabilitation after first stroke found that blacks were younger, had a higher proportion of hemorrhagic stroke, and were more disabled on admission. Compared with non-Hispanic whites, blacks and Hispanics also had a poorer functional status at discharge but were more likely to be discharged to home rather than to another institution, even after adjustment for age and stroke subtype. After adjustment for the same covariates, compared with non-Hispanic whites, blacks also had less improvement in functional status per inpatient day.244
Blacks were less likely to report independence in activities of daily living and instrumental activities of daily living than whites 1 year after stroke after controlling for stroke severity and comparable rehabilitation use.245
In a study of 90-day poststroke outcomes among ischemic stroke patients in the BASIC Project, Mexican Americans scored worse on neurological, functional, and cognitive outcomes than non-Hispanic whites after multivariable adjustment.246
Chart 14-10. Probability of death within 5 years after first stroke.
Source: Pooled data from the Framingham Heart Study, Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Multi-Ethnic Study of Atherosclerosis, Coronary Artery Risk Development in Young Adults, and Jackson Heart Study of the National Heart, Lung, and Blood Institute.
Stroke in Children
On the basis of pathogenic differences, pediatric strokes are typically classified as either perinatal (occurring at ≤28 days of life and including in utero strokes) or (later) childhood.
Estimates of the overall annual incidence of stroke in US children are 6.4 per 100 000 children (0 to 15 years) in 1999 in the GCNKSS247 and 4.6 per 100 000 children (0 to 19 years) in 1997 to 2003 in a northern California population.209 Approximately half of all incident childhood strokes are hemorrhagic.247–249
The prevalence of perinatal strokes is 29 per 100 000 live births, or 1 per 3500 live births in the 1997 to 2003 Kaiser Permanente of Northern California population.248
A history of infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis are independent maternal risk factors for perinatal arterial ischemic stroke.250 However, maternal health and pregnancies are normal in most cases.251
Diagnostic delays are more common in ischemic than hemorrhagic stroke in children, with a median time from symptom onset to diagnostic neuroim-aging of 3 hours for hemorrhagic and 24 hours for ischemic stroke in a population-based study from the south of England.252
The most common cause of arterial ischemic stroke in children is a cerebral arteriopathy, found in more than half of all cases.253,254 Childhood arteriopathies are heterogeneous and can be difficult to distinguish from a partially thrombosed artery in the setting of a cardioembolic stroke; incorporation of clinical data and serial vascular imaging is important for diagnosis.255
In a retrospective population-based study in northern California, 7% of childhood ischemic strokes and 2% of childhood hemorrhagic strokes were attributable to congenital heart defects. Congenital heart defects increased a child’s risk of stroke 19-fold (OR, 19; 95% CI, 4.2–83). The majority of children with stroke related to congenital heart defects were outpatients at the time of the stroke.256 In a single-center Australian study, infants with cyanotic congenital heart defects undergoing palliative surgery were the highest-risk group to be affected by arterial ischemic stroke during the periprocedural period; stroke occurred in 22 per 2256 cardiac surgeries (1%).257
In another study of the same northern Californian population, adolescents with migraine had a 3-fold increased odds of ischemic stroke compared with those without migraine (OR, 3.4; 95% CI, 1.2–9.5); younger children with migraine had no significant difference in stroke risk.258
Head or neck trauma in the prior week is a strong risk factor for childhood arterial ischemic stroke (adjusted OR, 36; 95% CI, 5–281), present in 10% of cases. Exposure to minor infection in the prior month is another independent risk factor, present in one third of cases (adjusted OR, 3.9; 95% CI, 2.0–7.4).259 The effect of infection on pediatric stroke risk is short-lived, lasting for days; 80% of infections preceding childhood stroke are respiratory.260 A prospective study of 326 children with acute stroke and serological evidence of acute herpesvirus infection showed that herpesvirus infection doubled the odds of childhood arterial ischemic stroke, even after adjustment for age, race, and socioeconomic status (OR, 2.2; 95% CI, 1.2–4.0; P=0.007).261 Among the 187 cases with both acute and covalescent blood samples (considered the gold standard for defining positive acute herpesvirus serologies among cases), 45% showed evidence of acute herpesvirus infection. Most infections were asymptomatic, and herpes simplex virus 1 was most common.
Thrombophilias (genetic and acquired) are risk factors for childhood stroke, with summary ORs ranging from 1.6 to 8.8 in a meta-analysis.262
In a prospective Swiss registry,263 atherosclerotic risk factors were less common in children with arterial ischemic stroke than in young adults; the most common of these factors in children was hyperlipidemia (15%). However, an analysis of the NIS suggests a low but rising prevalence of these factors among US adolescents and young adults hospitalized for ischemic stroke (1995 versus 2008).264
Compared with girls, US boys have a 25% increased risk of ischemic stroke and a 34% increased risk of ICH, whereas a study in the United Kingdom found no sex difference in childhood ischemic stroke.265 Compared with white children, black children in both the United States and United Kingdom have a >2-fold risk of stroke.266 The increased risk among blacks is not fully explained by the presence of sickle cell disease, nor is the excess risk among boys fully explained by trauma.266
The excess ischemic stroke mortality in US black children compared with white children has diminished since 1998 when the STOP trial was published, which established a method for primary stroke prevention in children with sickle cell disease.267
Among young adult survivors of childhood stroke, 37% had a normal modified Rankin score, 42% had mild deficits, 8% had moderate deficits, and 15% had severe deficits.268 Concomitant involvement of the basal ganglia, cerebral cortex, and posterior limb of the internal capsule predicts a persistent hemiparesis.269
Survivors of childhood arterial ischemic stroke have, on average, low normal cognitive performance,270,271 with poorest performance in visual-constructive skills, short-term memory, and processing speed. Younger age at stroke and seizures, but not laterality of stroke (left versus right), predict worse cognitive outcome.271
Compared with control children with asthma, childhood stroke survivors have greater impairments in adaptive behaviors, social adjustment, and social participation, even if their IQ is normal.272 Severity of disability after perinatal stroke correlates with maternal psychosocial outcomes such as depression and quality of life.273
Despite current treatment, at least 1 of 10 children with ischemic or hemorrhagic stroke will have a recurrence within 5 years.274,275
Cerebral arteriopathies increase risk of recurrence,276 with a 5-year recurrence risk as high as 60% among children with abnormal arteries on vascular imaging.277 The recurrence risk after perinatal stroke, however, is negligible.277
Among 59 long-term survivors of pediatric brain aneurysms, 41% developed new or recurrent aneurysm during a median follow-up of 34 years; of those, one third developed multiple aneurysms.278
More than 25% of survivors of perinatal ischemic strokes develop delayed seizures within 3 years; babies with larger strokes are at higher risk.279 The cumulative risk of delayed seizures after later childhood stroke is 13% at 5 years and 30% at 10 years.280 Children with acute seizures (within 7 days of their stroke) have the highest risk for delayed seizures, >70% by 5 years after the stroke.281 In survivors of ICH in childhood, 13% developed delayed seizures and epilepsy with 2 years.282 Elevated intracranial pressure requiring acute intervention at the time of acute ICH is a risk factor for delayed seizures and epilepsy.
Pediatric stroke teams and stroke centers283 are developing worldwide. In a study of 124 children presenting to a children’s hospital ED with stroke symptoms in which a “stroke alert” was paged, 24% had a final diagnosis of stroke, 2% were TIA, and 14% were other neurological emergencies, which underscores the need for prompt evaluation of children with “brain attacks.”284
In a study of 111 pediatric stroke cases admitted to a single US children’s hospital, the median 1-year direct cost of a childhood stroke (inpatient and outpatient) was ≈$50 000, with a maximum approaching $1 000 000. More severe neurological impairment after a childhood stroke correlated with higher direct costs of a stroke at 1 year and poorer quality of life in all domains.285
A prospective study at 4 centers in the United States and Canada found that the median 1-year out-of-pocket cost incurred by the family of a child with a stroke was $4354 (maximum $38 666), which exceeded the median American household cash savings of $3650 at the time of the study and represented 6.8% of the family’s annual income.286
Stroke in the Young
In the NIS, hospitalizations for ischemic stroke increased among adolescents and young adults (aged 5–44 years) between 1995 and 2010, whereas SAH hospitalizations decreased during that same period.264,287
Approximately 10% of all strokes occur in individuals 18 to 50 years of age.288
In the 2005 GCNKSS study period, the sex-adjusted incidence rate of first-ever stroke was 48 per 100 000 (95% CI, 42–53) among whites aged 20 to 54 years compared with 128 per 100 000 among blacks of the same age. Both races had a significant increase in the incidence rate from 1993 to 1994.178
Among 20- to 54-year-olds in the 2005 GCNKSS, ischemic stroke was the most common stroke type, constituting 68.6% of all strokes, followed by ICH (16.9%), SAH (9.8%), and unknown pathogenesis (4.7%).178
Vascular risk factors are common among stroke patients aged 20 to 54 years. During 2005, in the biracial GCNKSS, hypertension prevalence was estimated at 52%, hyperlipidemia at 18%, DM at 20%, CHD at 12%, and current smoking at 46% among stroke patients 20 to 54 years of age.178
In the FUTURE study, the 30-day case fatality rate among stroke patients 18 to 50 years of age was 4.5%. One-year mortality among 30-day survivors was 1.2% (95% CI, 0.0%–2.5%) for TIA, 2.4% (95% CI, 1.2%–3.7%) for ischemic stroke, and 2.9% (95% CI, 0.0%–6.8%) for ICH.289
In the FUTURE study, after a mean follow-up of 13.9 years, 44.7% of young stroke patients had poor functional outcome, defined as a modified Rankin score >2. The strongest baseline predictors of poor outcome were female sex (OR, 2.7; 95% CI, 1.5–5.0) and baseline NIHSS (OR, 1.1, 95% CI, 1.1–1.2 per point increase).290
Stroke in the Very Elderly
Stroke patients >85 years of age make up 17% of all stroke patients, and in this age group, stroke is more prevalent in women than men.291,292
Very elderly patients have a higher risk-adjusted mortality,293 have greater disability,293 have longer hospitalizations,294 receive less evidenced-based care,249,250 and are less likely to be discharged to their original place of residence.294,295
According to analyses from the US NIS, over the past decade, in-hospital mortality rates after stroke have declined for every age/sex group except men aged >84 years.296
Over the next 40 years (2010–2050), the number of incident strokes is expected to more than double, with the majority of the increase among the elderly (aged ≥75 years) and minority groups.297
A Danish stroke registry reported on 39 centenarians (age range, 100–107 years) hospitalized with acute stroke. Although they had more favorable risk profiles than other age groups (lower prevalence of previous MI, stroke, and DM), their strokes were more severe and were associated with high 1-month mortality (38.5%).298
Organization of Stroke Care
Among 30 947 patients hospitalized with acute ischemic stroke in the state of New York between 2005 and 2006, admission to a designated stroke center was associated with lower 30-day mortality (10.1% versus 12.5%; adjusted mortality difference, −2.5%; 95% CI, −3.6% to −1.4%) and greater use of thrombolytic therapy (4.8% versus 1.7%; adjusted difference, 2.2%; 95% CI, 1.6%–2.8%), but there was no difference in 30-day all-cause readmission or discharge to a skilled nursing facility.299
A study using Medicare data found that among 6197 SAH and 31 272 ICH stroke discharges in 2006, patients treated at Joint Commission–certified primary stroke centers had lower 30-day risk-adjusted mortality than patients treated at noncertified centers (SAH OR, 0.66 [95% CI, 0.58–0.76]; ICH OR, 0.86 [95% CI, 0.80–0.92]), but no difference was seen for 30-day all-cause readmission.300
A Cochrane review of 28 trials involving 5855 participants concluded that stroke patients who receive organized inpatient care in a stroke unit had better outcomes, including a decreased odds of mortality (median of 1 year; OR, 0.87; 95% CI, 0.69–0.94), death or institutionalized care (0.78; 95% CI, 0.68–0.89), and death or dependency (OR, 0.79; 95% CI, 0.68–0.90) than patients treated in an alternative form of inpatient care. The findings were independent of patient age, sex, initial stroke severity, or stroke type.301
Data have shown a steady increase in the proportion of ischemic stroke patients who are treated with tPA therapy. For example, administrative data in 2009 found that between 3.4% and 5.2% of acute ischemic strokes were treated with tPA, which was approximately double the treatment rate observed in 2005.302 Similarly, analysis of data from the GWTG-Stroke program demonstrated substantial increases in tPA treatment rates over the period from 2003 to 2011.303
Analysis of tPA-treated patients in the GWTG-Stroke program between 2003 and 2009 found that the majority were not treated within the guideline-recommended interval of 60 minutes from hospital arrival and that this proportion had increased only modestly during this period (from 19% in 2003 to 29% in 2009).260 Paradoxically, door-to-needle times were found to be inversely related to onset to arrival times; thus, tPA-treated patients who arrived earlier were less likely to receive treatment within 60 minutes of arrival.304
Implementation of Target Stroke, a national quality improvement initiative to improve the timeliness of tPA administration, found that among 71 169 patients with acute ischemic stroke treated with tPA at 1030 GWTG-Stroke participating hospitals, participation in the program was associated with a decreased door-to-needle time, lower inhospital mortality (OR, 0.89; 95% CI, 0.83–0.94) and intracranial hemorrhage (OR, 0.83; 95% CI, 0.76–0.91), and an increase in the percentage of patients discharged home (OR, 1.14; 95% CI, 1.09–1.19).305
Approximately 70% of Medicare beneficiaries who are discharged with acute stroke use Medicare-covered postacute care,306 with most receiving care from >1 type of setting.307,308 The majority of stroke patients receive rehabilitation care in a skilled nursing facility after discharge (32%), followed by an inpatient rehabilitation facility (22%), and then home health care (15%).306
The proportion of stroke patients not referred to any postacute care has increased in recent years,306 with an analysis of 2006 Medicare data finding that proportion to be as high as 42%.309
Hospital Discharges/Ambulatory Care Visits
(See Table 14-1)
From 2000 to 2010, the number of inpatient discharges from short-stay hospitals with stroke as the first-listed diagnosis increased, from 981 000 to 1,015 000, respectively (NHDS, NHLBI tabulation).310
In 2012, there were 717 000 ED visits with stroke as the first-listed diagnosis, and in 2011, there were 209 000 outpatient visits with stroke as the first-listed diagnosis (NHAMCS, unpublished NHLBI tabulation). In 2012, physician office visits for a first-listed diagnosis of stroke totaled 2 381 000 (NAMCS, unpublished NHLBI tabulation).
In 2010, men and women accounted for roughly the same number of hospital stays for stroke in the 18- to 44-year-old age group. Among people 45 to 64 years of age, 57.1% of stroke patients were men. After 65 years of age, women were the majority. Among people 65 to 84 years of age, 53.4% of stroke patients were women, whereas among those ≥85 years of age, women constituted 66.2% of all stroke patients.311
A first-ever county-level Atlas of Stroke Hospitalizations Among Medicare Beneficiaries was released in 2008 by the CDC in collaboration with the Centers for Medicare & Medicaid Services. It found that the stroke hospitalization rate for blacks was 27% higher than for the US population in general, 30% higher than for whites, and 36% higher than for Hispanics. In contrast to whites and Hispanics, the highest percentage of strokes in blacks (42.3%) occurred in the youngest Medicare age group (65–74 years of age).312
Operations and Procedures
(See Chart 14-12)
Chart 14-12. Trends in carotid endarterectomy and carotid stenting procedures (United States: 1993–2013).
Source: Nationwide Inpatient Sample, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.
In 2010, an estimated 100 000 inpatient endarterectomy procedures were performed in the United States. Carotid endarterectomy is the most frequently performed surgical procedure to prevent stroke (NHDS, NHLBI tabulation).
Although rates of carotid endarterectomy decreased between 1997 and 2013, the use of carotid stenting increased dramatically (NIS, HCUP, AHRQ) (Chart 14-12).
The practice of carotid stenting in the United States is expanding, from <3% of all carotid artery revascularization procedures in 1998 to 13% in 2008.313
The randomized CREST study compared carotid endarterectomy and stenting for symptomatic and asymptomatic carotid stenosis. There was no overall difference in the primary end point of stroke, MI, or death; however, carotid endarterectomy showed superiority with increasing age, with the crossover point at approximately age 70 years, and was associated with fewer strokes, which had a greater impact on quality of life than MI.314,315
In-hospital mortality for carotid endarterectomy has decreased steadily from 1993 to 2013 (NIS, HCUP, AHRQ).
In the Medicare population, in-hospital stroke rates and mortality are similar for carotid endarterectomy and carotid stenting.316
Carotid stenting is associated with significantly higher costs than carotid endarterectomy in asymptomatic patients317 and may be less cost-effective in general.318
The percentage of patients undergoing carotid end-arterectomy within 2 weeks of the onset of stroke increased from 13% in 2007 to 47% in 2010.319
Several recent clinical trials reported improved functional outcome at 90 days among patients receiving endovascular treatment in conjunction with intravenous tPA for acute ischemic stroke caused by occlusions in the proximal anterior intracranial circulation versus tPA alone. In the SWIFT PRIME trial, thrombectomy with a stent retriever plus intravenous tPA reduced disability at 90 days over the entire range of scores on the modified Rankin scale (P<0.001). The rate of functional independence (modified Rankin scale score 0–2) was higher in the intervention group than in the control group (60% versus 35%, P<0.001).320
In patients with ischemic stroke with a proximal cerebral arterial occlusion and salvageable tissue on CT perfusion imaging in the EXTEND-IA trial, early thrombectomy with the Solitaire FR stent retriever, compared with alteplase alone, improved reperfusion, early neurological recovery, and functional outcome. Endovascular therapy, initiated at a median of 210 minutes after the onset of stroke, increased early neurological improvement at 3 days (80% versus 37%, P=0.002) and improved functional outcome at 90 days, with more patients achieving functional independence (score of 0–2 on the modified Rankin scale, 71% versus 40%; P=0.01).321
Among patients with acute ischemic stroke with a proximal vessel occlusion in the ESCAPE trial, rapid endovascular treatment improved functional outcomes and reduced mortality. The rate of functional independence (90-day modified Rankin score of 0–2) was increased with the intervention (53.0% versus 29.3% in the control group, P<0.001).322
Among patients with anterior circulation stroke in the REVASCAT trial, stent retriever thrombectomy reduced the severity of disability over the range of the modified Rankin scale (adjusted OR for improvement of 1 point, 1.7; 95% CI, 1.05–2.8) and led to higher rates of functional independence (a score of 0–2) at 90 days (43.7% versus 28.2%; adjusted OR, 2.1; 95% CI, 1.1–4.0).323
Cost
(See Table 14-1)
-
In 2012 to 2013 (average annual)324:
The direct and indirect cost of stroke was $33.9 billion (MEPS, NHLBI tabulation; Table 14-1).
The estimated direct medical cost of stroke was $17.9 billion. This includes hospital out-patient or office-based provider visits, hospital inpatient stays, ED visits, prescribed medicines, and home health care.
The mean expense per patient for direct care for any type of service (including hospital inpatient stays, outpatient and office-based visits, ED visits, prescribed medicines, and home health care) in the United States was estimated at $5232.324
Between 2012 and 2030, total direct medical stroke-related costs are projected to triple, from $71.6 billion to $184.1 billion, with the majority of the projected increase in costs arising from those 65 to 79 years of age.7
The total cost of stroke from 2005 to 2050, in 2005 dollars, is projected to be $1.52 trillion for non-Hispanic whites, $313 billion for Hispanics, and $379 billion for blacks. The per capita estimated cost of stroke is highest in blacks ($25 782), followed by Hispanics ($17 201) and non-Hispanic whites ($15 597). Loss of earnings is expected to be the highest cost contributor in each race/ethnic group.325
During 2001 to 2005, the average cost for outpatient stroke rehabilitation services and medications the first year after inpatient rehabilitation discharge was $11 145. The corresponding average yearly cost of medication was $3376, whereas the average cost of yearly rehabilitation service utilization was $7318.326
Patients with recurrent stroke had 38% higher costs per patient 1 year after discharge from index hospitalization than new stroke patients.327
In adjusted models that controlled for relevant covariates, the attributable 1-year cost of post-stroke aphasia was estimated at $1703 in 2004 dollars.328
Data from Sweden show that healthcare costs associated with stroke survivors with spasticity are 4-fold higher than for stroke survivors without spasticity.329
The estimated cost of acute pediatric stroke in the United States was $42 million in 2003. The mean cost of short-term hospital care was $20 927 per discharge.330
After adjustment for routine healthcare costs, the average 5-year cost of a neonatal stroke was $51 719 and that of a childhood stroke was $135 161. Costs among children with stroke continued to exceed those in age-matched control children even in the fifth year by an average of $2016.331
Global Burden of Stroke
See Chapter 15 for more information.
Although global age-adjusted mortality rates for ischemic and hemorrhagic stroke decreased between 1990 and 2013, the absolute number of people who have strokes annually, as well as related deaths and DALYs lost, increased. The majority of global stroke burden is in LIMC.166,332
Prevalence and Incidence
Incidence
In 2010, there were an estimated 11.6 million events of incident ischemic stroke and 5.3 million events of incident hemorrhagic stroke, 63% and 80%, respectively, in LIMC.334
-
Between 1990 and 2010334:
Incidence of ischemic stroke was significantly reduced by 13% (95% CI, 6%–18%) in HIC. No significant change was seen in LIMC.
Incidence of hemorrhagic stroke decreased by 19% in HIC. Rates increased by 22% in LIMC, with a 19% increase in those aged <75 years.
Mortality
-
In 2013166:
There were 6.5 million stroke deaths worldwide, making stroke the second-leading global cause of death behind IHD.
Stroke deaths accounted for 11.8% of total deaths worldwide.
The absolute number of stroke deaths increased 40.2% between 1990 and 2013; however, the age-standardized death rate decreased 22.5%.
A total of 3.3 million individuals died of ischemic stroke and 3.2 million of hemorrhagic stroke.
Age-standardized death rates decreased 19.6% and 25.9% for ischemic and hemorrhagic stroke, respectively, since 1990.
In 2010, 39.4 million DALYs were lost because of ischemic stroke and 62.8 million because of hemorrhagic stroke (64% and 85%, respectively, in LIMC).334
In 2010, the mean age of stroke-related death in HIC was 80.4 years compared with 72.1 years in LIMC.335
Between 1990 and 2010, ischemic stroke mortality decreased 37% in HIC and 14% in LIMC. Hemorrhagic stroke mortality decreased 38% in HIC and 23% in LIMC.334
REFERENCES
- 1.Behavioral Risk Factor Surveillance System: annual survey data. [Accessed September 1, 2014];Centers for Disease Control and Prevention Web site. 2013 http://www.cdc.gov/brfss/annual_data/annual_2013.html.
- 2.Centers for Disease Control and Prevention (CDC) Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61:379–382. [PubMed] [Google Scholar]
- 3.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, Yoshita M, D’Agostino RB, Sr, DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard VJ, McClure LA, Meschia JF, Pulley L, Orr SC, Friday GH. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischemic attack in a general population: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Arch Intern Med. 2006;166:1952–1958. doi: 10.1001/archinte.166.18.1952. [DOI] [PubMed] [Google Scholar]
- 7.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG American Heart Association Advocacy Coordinating Committee and Stroke Council. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association [published correction appears in Stroke. 2015;46:e179] Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 8.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgenstern LB, Smith MA, Sánchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Baek J, Lisabeth LD. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. doi: 10.1002/ana.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 11.Fang MC, Coca Perraillon M, Ghosh K, Cutler DM, Rosen AB. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. Am J Med. 2014;127:608–615. doi: 10.1016/j.amjmed.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 14.Zahuranec DB, Lisabeth LD, Sánchez BN, Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Morgenstern LB. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82:2180–2186. doi: 10.1212/WNL.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z Oxford Vascular Study. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 17.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population: the Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega T, Zurriaga O, Ramos JM, Gil M, Alamo R, Lozano JE, López A, Miralles MT, Vaca P, del Alvarez MM Group of Research for the RECENT Project. Stroke in Spain: epidemiologic incidence and patterns: a health sentinel network study. J Stroke Cerebrovasc Dis. 2009;18:11–16. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Sealy-Jefferson S, Wing JJ, Sánchez BN, Brown DL, Meurer WJ, Smith MA, Morgenstern LB, Lisabeth LD. Age- and ethnic-specific sex differences in stroke risk. Gend Med. 2012;9:121–128. doi: 10.1016/j.genm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewsey JD, Gillies M, Jhund PS, Chalmers JW, Redpath A, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, Macintyre K. Sex differences in incidence, mortality, and survival in individuals with stroke in Scotland, 1986 to 2005. Stroke. 2009;40:1038–1043. doi: 10.1161/STROKEAHA.108.542787. [DOI] [PubMed] [Google Scholar]
- 21.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, Moomaw CJ, Schneider A, Miller R, Shukla R, Kissela B. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37:2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 23.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al-Wabil A, Al-Senani F, Brown DL, Moyé LA. Excess stroke in Mexican Americans compared with non-Hispanic whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577–1584. doi: 10.1161/CIRCULATIONAHA.108.772285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard G, Cushman M, Howard VJ, Kissela BM, Kleindorfer DO, Moy CS, Switzer J, Woo D. Risk factors for intracerebral hemorrhage: the REasons for geographic and racial differences in stroke (REGARDS) study [published correction appears in Stroke. 2013;44:e63] Stroke. 2013;44:1282–1287. doi: 10.1161/STROKEAHA.111.000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston SC, Fayad PB, Gorelick PB, Hanley DF, Shwayder P, van Husen D, Weiskopf T. Prevalence and knowledge of transient ischemic attack among US adults. Neurology. 2003;60:1429–1434. doi: 10.1212/01.wnl.0000063309.41867.0f. [DOI] [PubMed] [Google Scholar]
- 28.Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 29.Brown RD, Jr, Petty GW, O’Fallon WM, Wiebers DO, Whisnant JP. Incidence of transient ischemic attack in Rochester, Minnesota, 1985–1989. Stroke. 1998;29:2109–2113. doi: 10.1161/01.str.29.10.2109. [DOI] [PubMed] [Google Scholar]
- 30.Cancelli I, Janes F, Gigli GL, Perelli A, Zanchettin B, Canal G, D’Anna L, Russo V, Barbone F, Valente M. Incidence of transient ischemic attack and early stroke risk: validation of the ABCD2 score in an Italian population-based study. Stroke. 2011;42:2751–2757. doi: 10.1161/STROKEAHA.110.612705. [DOI] [PubMed] [Google Scholar]
- 31.Hankey G. Impact of treatment of people with transient ischaemic attacks on stroke incidence and public health. Cerebrovasc Dis. 1996;6:26–33. doi: 10.1159/000108068. [DOI] [Google Scholar]
- 32.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 33.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417–2422. doi: 10.1001/archinte.167.22.2417. [DOI] [PubMed] [Google Scholar]
- 34.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 35.Clark TG, Murphy MF, Rothwell PM. Long term risks of stroke, myocardial infarction, and vascular death in “low risk” patients with a non-recent transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2003;74:577–580. doi: 10.1136/jnnp.74.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, Cuthbertson FC, Rothwell PM Oxford Vascular Study. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44:2854–2861. doi: 10.1161/STROKEAHA.113.001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PA, Muir K, Wardlaw JM. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. 2014;75:67–76. doi: 10.1002/ana.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 39.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng W, Hendry RM, Adams RJ. Risk of recurrent stroke, myocardial infarction, or death in hospitalized stroke patients. Neurology. 2010;74:588–593. doi: 10.1212/WNL.0b013e3181cff776. [DOI] [PubMed] [Google Scholar]
- 41.Redfors P, Jood K, Holmegaard L, Rosengren A, Blomstrand C, Jern C. Stroke subtype predicts outcome in young and middle-aged stroke sufferers. Acta Neurol Scand. 2012;126:329–335. doi: 10.1111/j.1600-0404.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee BI, Nam HS, Heo JH, Kim DI Yonsei Stroke Team. Yonsei Stroke Registry. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001;12:145–151. doi: 10.1159/000047697. 47697. [DOI] [PubMed] [Google Scholar]
- 43.Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD South London Stroke Register. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003;34:1457–1463. doi: 10.1161/01.STR.0000072985.24967.7F. [DOI] [PubMed] [Google Scholar]
- 44.Jones SB, Sen S, Lakshminarayan K, Rosamond WD. Poststroke outcomes vary by pathogenic stroke subtype in the Atherosclerosis Risk in Communities Study. Stroke. 2013;44:2307–2310. doi: 10.1161/STROKEAHA.113.000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, van der Vlugt MJ, van Dijk EJ, de Leeuw FE. Long-term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74:592–601. doi: 10.1002/ana.23953. [DOI] [PubMed] [Google Scholar]
- 46.Pezzini A, Grassi M, Lodigiani C, Patella R, Gandolfo C, Zini A, DeLodovici ML, Paciaroni M, Del Sette M, Toriello A, Musolino R, Calabrò RS, Bovi P, Adami A, Silvestrelli G, Sessa M, Cavallini A, Marcheselli S, Bonifati DM, Checcarelli N, Tancredi L, Chiti A, Del Zotto E, Spalloni A, Giossi Al, Volonghi I, Costa P, Giacalone G, Ferrazzi P, Poli L, Morotti A, Rasura M, Simone AM, Gamba M, Cerrato P, Micieli G, Melis M, Massucco D, De Giuli V, Iacoviello L, Padovani A on behalf of the Italian Project on Stroke in Young Adults (IPSYS) Investigators. Predictors of long-term recurrent vascular events after ischemic stroke at young age: the Italian Project on Stroke in Young Adults. Circulation. 2014;129:1668–1676. doi: 10.1161/CIRCULATIONAHA.113.005663. [DOI] [PubMed] [Google Scholar]
- 47.Callaly E, Ni Chroinin D, Hannon N, Marnane M, Akijian L, Sheehan O, Merwick A, Hayden D, Horgan G, Duggan J, Murphy S, O’Rourke K, Dolan E, Williams D, Kyne L, Kelly PJ. Rates, predictors, and outcomes of early and late recurrence after stroke: the North Dublin Population Stroke Study. Stroke. 2016;47:244–246. doi: 10.1161/STROKEAHA.115.011248. [DOI] [PubMed] [Google Scholar]
- 48.Fullerton HJ, Wintermark M, Hills NK, Dowling MM, Tan M, Rafay MF, Elkind MS, Barkovich AJ, deVeber GA VIPS Investigators. Risk of recurrent arterial ischemic stroke in childhood: a prospective international study. Stroke. 2016;47:53–59. doi: 10.1161/STROKEAHA.115.011173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation. 2011;123:2111–2119. doi: 10.1161/CIRCULATIONAHA.109.934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, Lichtman JH. Trends in one-year recurrent ischemic stroke among the elderly in the USA: 1994–2002. Cerebrovasc Dis. 2010;30:525–532. doi: 10.1159/000319028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Center for Health Statistics. National Health Interview Survey. 2014 Public-use data file and documentation: NCHS tabulations. http://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm. NCHS tabulations. Accessed xxx.
- 52.Lanska DJ. Geographic distribution of stroke mortality in the United States: 1939–1941 to 1979–1981. Neurology. 1993;43:1839–1851. doi: 10.1212/wnl.43.9.1839. [DOI] [PubMed] [Google Scholar]
- 53.Casper ML, Wing S, Anda RF, Knowles M, Pollard RA. The shifting stroke belt: changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke. 1995;26:755–760. doi: 10.1161/01.str.26.5.755. [DOI] [PubMed] [Google Scholar]
- 54.Casper ML, Nwaise IA, Croft JB, Nilasena DS. Atlas of Stroke Hospitalizations Among Medicare Beneficiaries. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 55.Howard G, Evans GW, Pearce K, Howard VJ, Bell RA, Mayer EJ, Burke GL. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke. 1995;26:1153–1158. doi: 10.1161/01.str.26.7.1153. [DOI] [PubMed] [Google Scholar]
- 56.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 57.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- 58.Gillum RF, Kwagyan J, Obisesan TO. Ethnic and geographic variation in stroke mortality trends. Stroke. 2011;42:3294–3296. doi: 10.1161/STROKEAHA.111.625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schieb LJ, Ayala C, Valderrama AL, Veazie MA. Trends and disparities in stroke mortality by region for American Indians and Alaska Natives. Am J Public Health. 2014;104(suppl 3):S368–S376. doi: 10.2105/AJPH.2013.301698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, Rehm CD, Afshin A, Danaei G, Kypridemos C, Gaziano T, Mozaffarian D, Capewell S, O’Flaherty M. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation. 2016;133:967–978. doi: 10.1161/CIRCULATIONAHA.115.019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75] Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 62.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lackland DT, Voeks JH, Boan AD. Hypertension and stroke: an appraisal of the evidence and implications for clinical management. Expert Rev Cardiovasc Ther. 2016;14:609–616. doi: 10.1586/14779072.2016.1143359. [DOI] [PubMed] [Google Scholar]
- 64.Perkovic V, Rodgers A. Redefining blood-pressure targets: SPRINT starts the marathon. N Engl J Med. 2015;373:2175–2178. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 65.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, Howard VJ REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–3375. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.SPS3 Study Group. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial [published correction appears in Lancet. 2013;382:506] Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White CL, Pergola PE, Szychowski JM, Talbert R, Cervantes-Arriaga A, Clark HD, Del Brutto OH, Godoy IE, Hill MD, Pelegrí A, Sussman CR, Taylor AA, Valdivia J, Anderson DC, Conwit R, Benavente OR SPS3 Investigators. Blood pressure after recent stroke: baseline findings from the Secondary Prevention of Small Subcortical Strokes Trial. Am J Hypertens. 2013;26:1114–1122. doi: 10.1093/ajh/hpt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y, Cai X, Li Y, Su L, Mai W, Wang S, Hu Y, Wu Y, Xu D. Pre-hypertension and the risk of stroke: a meta-analysis. Neurology. 2014;82:1153–1161. doi: 10.1212/WNL.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 70.Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, Graham A, Howard V. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 71.Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, Broderick JP, Kissela BM. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke. 2013;44:1500–1504. doi: 10.1161/STROKEAHA.113.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 73.Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564. doi: 10.1136/bmj.e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shou J, Zhou L, Zhu S, Zhang X. Diabetes is an independent risk factor for stroke recurrence in stroke patients: a meta-analysis. J Stroke Cerebrovasc Dis. 2015;24:1961–1968. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Towfighi A, Markovic D, Ovbiagele B. Current national patterns of comorbid diabetes among acute ischemic stroke patients. Cerebrovasc Dis. 2012;33:411–418. doi: 10.1159/000334192. [DOI] [PubMed] [Google Scholar]
- 76.Eriksson M, Carlberg B, Eliasson M. The disparity in long-term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis. 2012;34:153–160. doi: 10.1159/000339763. [DOI] [PubMed] [Google Scholar]
- 77.Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil. 2011;18:813–823. doi: 10.1177/1741826711421687. [DOI] [PubMed] [Google Scholar]
- 78.Marso SP, Kennedy KF, House JA, McGuire DK. The effect of intensive glucose control on all-cause and cardiovascular mortality, myocardial infarction and stroke in persons with type 2 diabetes mellitus: a systematic review and meta-analysis. Diab Vasc Dis Res. 2010;7:119–130. doi: 10.1177/1479164109353367. [DOI] [PubMed] [Google Scholar]
- 79.Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, Castellani RJ, Mandava P, Kent TA, Simard JM. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol. 2012;72:799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 81.Xie XX, Liu P, Wan FY, Lin SG, Zhong WL, Yuan ZK, Zou JJ, Liu LB. Blood pressure lowering and stroke events in type 2 diabetes: A network meta-analysis of randomized controlled trials. Int J Cardiol. 2016;208:141–146. doi: 10.1016/j.ijcard.2016.01.197. [DOI] [PubMed] [Google Scholar]
- 82.Redon J, Mancia G, Sleight P, Schumacher H, Gao P, Pogue J, Fagard R, Verdecchia P, Weber M, Böhm M, Williams B, Yusoff K, Teo K, Yusuf S ONTARGET Investigators. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) J Am Coll Cardiol. 2012;59:74–83. doi: 10.1016/j.jacc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 83.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 84.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 85.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 86.Strickberger SA, Ip J, Saksena S, Curry K, Bahnson TD, Ziegler PD. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2:125–131. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 87.Tayal AH, Tian M, Kelly KM, Jones SC, Wright DG, Singh D, Jarouse J, Brillman J, Murali S, Gupta R. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71:1696–1701. doi: 10.1212/01.wnl.0000325059.86313.31. [DOI] [PubMed] [Google Scholar]
- 88.Elijovich L, Josephson SA, Fung GL, Smith WS. Intermittent atrial fibrillation may account for a large proportion of otherwise cryptogenic stroke: a study of 30-day cardiac event monitors. J Stroke Cerebrovasc Dis. 2009;18:185–189. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke. 2012;43:2788–2790. doi: 10.1161/STROKEAHA.112.665844. [DOI] [PubMed] [Google Scholar]
- 90.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke [published correction appears in N Engl J Med. 2016;374:998] N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 91.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. doi: 10.1161/CIRCEP.114.003057. [DOI] [PubMed] [Google Scholar]
- 92.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 93.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 94.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, Wallentin L. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) sub-study. Circulation. 2012;125:1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 96.Overvad TF, Nielsen PB, Larsen TB, Søgaard P. Left atrial size and risk of stroke in patients in sinus rhythm: a systematic review. Thromb Haemost. 2016;116:206–219. doi: 10.1160/TH15-12-0923. [DOI] [PubMed] [Google Scholar]
- 97.Prospective Studies Collaboration. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Lancet. 1995;346:1647–1653. [PubMed] [Google Scholar]
- 98.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196:489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke. 2012;43:1768–1774. doi: 10.1161/STROKEAHA.111.646778. [DOI] [PubMed] [Google Scholar]
- 100.Horenstein RB, Smith DE, Mosca L. Cholesterol predicts stroke mortality in the Women’s Pooling Project. Stroke. 2002;33:1863–1868. doi: 10.1161/01.str.0000020093.67593.0b. [DOI] [PubMed] [Google Scholar]
- 101.Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths [published correction appears in Lancet. 2008;372:292] Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. 2013;44:1833–1839. doi: 10.1161/STROKEAHA.113.001326. [DOI] [PubMed] [Google Scholar]
- 103.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Blood pressure, cholesterol, and stroke in eastern Asia. Lancet. 1998;352:1801–1807. [PubMed] [Google Scholar]
- 105.Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 106.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, Shaw J, Ueshima H, Zimmet P, Jee SH, Patel JV, Caterson I, Perkovic V, Woodward M Asia Pacific Cohort Studies Collaboration and the Obesity in Asia Collaboration. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124:2056–2064. doi: 10.1161/CIRCULATIONAHA.111.028373. [DOI] [PubMed] [Google Scholar]
- 107.Psaty BM, Anderson M, Kronmal RA, Tracy RP, Orchard T, Fried LP, Lumley T, Robbins J, Burke G, Newman AB, Furberg CD. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:1639–1647. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 108.Emerging Risk Factors Collaboration. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke. 2009;40:382–388. doi: 10.1161/STROKEAHA.108.529537. [DOI] [PubMed] [Google Scholar]
- 110.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–2725. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 111.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 112.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR Atherosclerosis Risk in Communities Study. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 113.Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, Gaziano JM. Cholesterol and the risk of ischemic stroke. Stroke. 2003;34:2930–2934. doi: 10.1161/01.STR.0000102171.91292.DC. [DOI] [PubMed] [Google Scholar]
- 114.Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2011;31:2982–2989. doi: 10.1161/ATVBAHA.111.234948. [DOI] [PubMed] [Google Scholar]
- 115.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research, Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2011;42:e26] Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 118.Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, Jauch E, Moomaw CJ, Shukla R, Gebel J, Fontaine R, Broderick J. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. 2002;33:1321–1326. doi: 10.1161/01.str.0000014773.57733.3e. [DOI] [PubMed] [Google Scholar]
- 119.Albertsen IE, Rasmussen LH, Lane DA, Overvad TF, Skjøth F, Overvad K, Lip GY, Larsen TB. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation. Chest. 2014;145:559–566. doi: 10.1378/chest.13-1740. [DOI] [PubMed] [Google Scholar]
- 120.Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH, Stern BJ, Kittner SJ. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439–2443. doi: 10.1161/STROKEAHA.107.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peters SA, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke. 2013;44:2821–2828. doi: 10.1161/STROKEAHA.113.002342. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura K, Barzi F, Lam TH, Huxley R, Feigin VL, Ueshima H, Woo J, Gu D, Ohkubo T, Lawes CM, Suh I, Woodward M Asia Pacific Cohort Studies Collaboration. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke. 2008;39:1694–1702. doi: 10.1161/STROKEAHA.107.496752. [DOI] [PubMed] [Google Scholar]
- 123.WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet (London, England) 1996;348:498–505. [PubMed] [Google Scholar]
- 124.WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:505–510. [PubMed] [Google Scholar]
- 125.Lee PN, Forey BA. Environmental tobacco smoke exposure and risk of stroke in nonsmokers: a review with meta-analysis. J Stroke Cerebrovasc Dis. 2006;15:190–201. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 126.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011;33:496–502. doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 127.Malek AM, Cushman M, Lackland DT, Howard G, McClure LA. Secondhand smoke exposure and stroke: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Prev Med. 2015;49:e89–e97. doi: 10.1016/j.amepre.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nishino Y, Tsuji I, Tanaka H, Nakayama T, Nakatsuka H, Ito H, Suzuki T, Katanoda K, Sobue T, Tominaga S Three-Prefecture Cohort Study Group. Stroke mortality associated with environmental tobacco smoke among never-smoking Japanese women: a prospective cohort study. Prev Med. 2014;67:41–45. doi: 10.1016/j.ypmed.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 129.McDonnell MN, Hillier SL, Hooker SP, Le A, Judd SE, Howard VJ. Physical activity frequency and risk of incident stroke in a national US study of blacks and whites. Stroke. 2013;44:2519–2524. doi: 10.1161/STROKEAHA.113.001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–907. doi: 10.1249/MSS.0b013e31827d87ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Willey JZ, Moon YP, Paik MC, Boden-Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology. 2009;73:1774–1779. doi: 10.1212/WNL.0b013e3181c34b58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hooker SP, Sui X, Colabianchi N, Vena J, Laditka J, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of fatal and nonfatal stroke in asymptomatic women and men. Stroke. 2008;39:2950–2957. doi: 10.1161/STROKEAHA.107.495275. [DOI] [PubMed] [Google Scholar]
- 133.Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36:1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- 134.Grau AJ, Barth C, Geletneky B, Ling P, Palm F, Lichy C, Becher H, Buggle F. Association between recent sports activity, sports activity in young adulthood, and stroke. Stroke. 2009;40:426–431. doi: 10.1161/STROKEAHA.108.527978. [DOI] [PubMed] [Google Scholar]
- 135.Krarup LH, Truelsen T, Pedersen A, Lerke H, Lindahl M, Hansen L, Schnohr P, Boysen G. Level of physical activity in the week preceding an ischemic stroke. Cerebrovasc Dis. 2007;24:296–300. doi: 10.1159/000105683. [DOI] [PubMed] [Google Scholar]
- 136.Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ Million Women Study Collaborators. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 137.Blomstrand A, Blomstrand C, Ariai N, Bengtsson C, Björkelund C. Stroke incidence and association with risk factors in women: a 32-year follow-up of the Prospective Population Study of Women in Gothenburg. BMJ Open. 2014;4:e005173. doi: 10.1136/bmjopen-2014-005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tikk K, Sookthai D, Monni S, Gross ML, Lichy C, Kloss M, Kaaks R. Primary preventive potential for stroke by avoidance of major lifestyle risk factors: the European Prospective Investigation into Cancer and Nutrition-Heidelberg cohort. Stroke. 2014;45:2041–2046. doi: 10.1161/STROKEAHA.114.005025. [DOI] [PubMed] [Google Scholar]
- 139.Jefferis BJ, Whincup PH, Papacosta O, Wannamethee SG. Protective effect of time spent walking on risk of stroke in older men. Stroke. 2014;45:194–199. doi: 10.1161/STROKEAHA.113.002246. [DOI] [PubMed] [Google Scholar]
- 140.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet [published correction appears in N Engl J Med. 2014;370:886] N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 141.Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–1199. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, Franco OH. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. doi: 10.1136/bmj.e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larsson SC, Virtamo J, Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: a prospective study. Atherosclerosis. 2013;227:147–152. doi: 10.1016/j.atherosclerosis.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 144.Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M. Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case-control, cohort and intervention studies. Br J Nutr. 2014;112:248–259. doi: 10.1017/S0007114514000713. [DOI] [PubMed] [Google Scholar]
- 145.Cheng P, Wang J, Shao W. Monounsaturated fatty acid intake and stroke risk: a meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2016;25:1326–1334. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 146.Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Fryzek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr. 2016;115:737–750. doi: 10.1017/S0007114515005000. [DOI] [PubMed] [Google Scholar]
- 147.Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr. 2016;115:212–225. doi: 10.1017/S0007114515004316. [DOI] [PubMed] [Google Scholar]
- 148.Wu D, Guan Y, Lv S, Wang H, Li J. No evidence of increased risk of stroke with consumption of refined grains: a meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2015;24:2738–2746. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 149.Shi ZQ, Tang JJ, Wu H, Xie CY, He ZZ. Consumption of nuts and legumes and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:1262–1271. doi: 10.1016/j.numecd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 150.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 151.D’Elia L, Iannotta C, Sabino P, Ippolito R. Potassium-rich diet and risk of stroke: updated meta-analysis. Nutr Metab Cardiovasc Dis. 2014;24:585–587. doi: 10.1016/j.numecd.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 152.Wang ZM, Zhao D, Nie ZL, Zhao H, Zhou B, Gao W, Wang LS, Yang ZJ. Flavonol intake and stroke risk: a meta-analysis of cohort studies. Nutrition. 2014;30:518–523. doi: 10.1016/j.nut.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 153.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF CSPPT Investigators. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 154.Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly-Hayes M, Debette S, DeStefano AL, Romero JR, Kase CS, Wolf PA. Parental occurrence of stroke and risk of stroke in their children: the Framingham study. Circulation. 2010;121:1304–1312. doi: 10.1161/CIRCULATIONAHA.109.854240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bluher A, Devan WJ, Holliday EG, Nalls M, Parolo S, Bione S, Giese AK, Boncoraglio GB, Maguire JM, Müller-Nurasyid M, Gieger C, Meschia JF, Rosand J, Rolfs A, Kittner SJ, Mitchell BD, O’Connell JR, Cheng YC. Heritability of young- and old-onset ischaemic stroke. Eur J Neurol. 2015;22:1488–1491. doi: 10.1111/ene.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Traylor M, Rutten-Jacobs LC, Holliday EG, Malik R, Sudlow C, Rothwell PM, Maguire JM, Koblar SA, Bevan S, Boncoraglio G, Dichgans M, Levi C, Lewis CM, Markus HS. Differences in common genetic predisposition to ischemic stroke by age and sex. Stroke. 2015;46:3042–3047. doi: 10.1161/STROKEAHA.115.009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, Farrall M, Rothwell PM, Sudlow C, Dichgans M, Markus HS. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genome-wide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 158.International Stroke Genetics Consortium (ISGC) and Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, Fornage M, Ikram MA, Malik R, Bevan S, Thorsteinsdottir U, Nalls MA, Longstreth W, Wiggins KL, Yadav S, Parati EA, Destefano AL, Worrall BB, Kittner SJ, Khan MS, Reiner AP, Helgadottir A, Achterberg S, Fernandez-Cadenas I, Abboud S, Schmidt R, Walters M, Chen WM, Ringelstein EB, O’Donnell M, Ho WK, Pera J, Lemmens R, Norrving B, Higgins P, Benn M, Sale M, Kuhlenbäumer G, Doney AS, Vicente AM, Delavaran H, Algra A, Davies G, Oliveira SA, Palmer CN, Deary I, Schmidt H, Pandolfo M, Montaner J, Carty C, de Bakker PI, Kostulas K, Ferro JM, van Zuydam NR, Valdimarsson E, Nordestgaard BG, Lindgren A, Thijs V, Slowik A, Saleheen D, Paré G, Berger K, Thorleifsson G, Hofman A, Mosley TH, Mitchell BD, Furie K, Clarke R, Levi C, Seshadri S, Gschwendtner A, Boncoraglio GB, Sharma P, Bis JC, Gretarsdottir S, Psaty BM, Rothwell PM, Rosand J, Meschia JF, Stefansson K, Dichgans M, Markus HS Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies [published correction appears in Lancet Neurol. 2015;14:788] Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, Zhao W, Abrantes P, Amouyel P, Attia JR, Battey TW, Berger K, Boncoraglio GB, Chauhan G, Cheng YC, Chen WM, Clarke R, Cotlarciuc I, Debette S, Falcone GJ, Ferro JM, Gamble DM, Ilinca A, Kittner SJ, Kourkoulis CE, Lemmens R, Levi CR, Lichtner P, Lindgren A, Liu J, Meschia JF, Mitchell BD, Oliveira SA, Pera J, Reiner AP, Rothwell PM, Sharma P, Slowik A, Sudlow CL, Tatlisumak T, Thijs V, Vicente AM, Woo D, Seshadri S, Saleheen D, Rosand J, Markus HS, Worrall BB, Dichgans M. ISGC Analysis Group; METASTROKE collaboration; Wellcome Trust Case Control Consortium 2 (WTCCC2); NINDS Stroke Genetics Network (SiGN)Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration [published correction appears in Neurology. 2016;87:1306] Neurology. 2016;86:1217–1226. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, Traylor M, Bevan S, Dichgans M, Rothwell PM, Sudlow C, Farrall M, Silander K, Kaunisto M, Wagner P, Saarela O, Kuulasmaa K, Virtamo J, Salomaa V, Amouyel P, Arveiler D, Ferrieres J, Wiklund PG, Ikram MA, Hofman A, Boncoraglio GB, Parati EA, Helgadottir A, Gretarsdottir S, Thorsteinsdottir U, Thorleifsson G, Stefansson K, Seshadri S, DeStefano A, Gschwendtner A, Psaty B, Longstreth W, Mitchell BD, Cheng YC, Clarke R, Ferrario M, Bis JC, Levi C, Attia J, Holliday EG, Scott RJ, Fornage M, Sharma P, Furie KL, Rosand J, Nalls M, Meschia J, Mosely TH, Evans A, Palotie A, Markus HS, Grant PJ, Spector TD EuroCLOT Investigators; Wellcome Trust Case Control Consortium 2; MOnica Risk, Genetics, Archiving and Monograph; MetaStroke; International Stroke Genetics Consortium. Ischemic stroke is associated with the ABO locus: the EuroCLOT study [published correction appears in Ann Neurol. 2014;75:166–167] Ann Neurol. 2013;73:16–31. doi: 10.1002/ana.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.NINDS Stroke Genetics Network (SiGN); International Stroke Genetics Consortium (ISGC) Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study [published correction appears in Lancet Neurol. 2016;15:241] Lancet Neurol. 2016;15:174–184. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, Gulcher J, Mathiesen EB, Njølstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Kucera G, Stubblefield T, Carter S, Roden D, Ng MC, Baum L, So WY, Wong KS, Chan JC, Gieger C, Wichmann HE, Gschwendtner A, Dichgans M, Kuhlenbäumer G, Berger K, Ringelstein EB, Bevan S, Markus HS, Kostulas K, Hillert J, Sveinbjörnsdóttir S, Valdimarsson EM, Løchen ML, Ma RC, Darbar D, Kong A, Arnar DO, Thorsteinsdottir U, Stefansson K. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Traylor M, Mäkelä KM, Kilarski LL, Holliday EG, Devan WJ, Nalls MA, Wiggins KL, Zhao W, Cheng YC, Achterberg S, Malik R, Sudlow C, Bevan S, Raitoharju E, Oksala N, Thijs V, Lemmens R, Lindgren A, Slowik A, Maguire JM, Walters M, Algra A, Sharma P, Attia JR, Boncoraglio GB, Rothwell PM, de Bakker PI, Bis JC, Saleheen D, Kittner SJ, Mitchell BD, Rosand J, Meschia JF, Levi C, Dichgans M, Lehtimäki T, Lewis CM, Markus HS METASTROKE, International Stroke Genetics Consortium, Wellcome Trust Case Consortium 2 (WTCCC2) A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genet. 2014;10:e1004469. doi: 10.1371/journal.pgen.1004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, Kostulas K, Kuhlenbäumer G, Bevan S, Jonsdottir T, Bjarnason H, Saemundsdottir J, Palsson S, Arnar DO, Holm H, Thorgeirsson G, Valdimarsson EM, Sveinbjörnsdottir S, Gieger C, Berger K, Wichmann HE, Hillert J, Markus H, Gulcher JR, Ringelstein EB, Kong A, Dichgans M, Gudbjartsson DF, Thorsteinsdottir U, Stefansson K. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- 166.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kilarski LL, Achterberg S, Devan WJ, Traylor M, Malik R, Lindgren A, Pare G, Sharma P, Slowik A, Thijs V, Walters M, Worrall BB, Sale MM, Algra A, Kappelle LJ, Wijmenga C, Norrving B, Sandling JK, Rönnblom L, Goris A, Franke A, Sudlow C, Rothwell PM, Levi C, Holliday EG, Fornage M, Psaty B, Gretarsdottir S, Thorsteinsdottir U, Seshadri S, Mitchell BD, Kittner S, Clarke R, Hopewell JC, Bis JC, Boncoraglio GB, Meschia J, Ikram MA, Hansen BM, Montaner J, Thorleifsson G, Stefanson K, Rosand J, de Bakker PI, Farrall M, Dichgans M, Markus HS, Bevan S GARNET Collaborative Research Group, Wellcome Trust Case Control Consortium 2, Australian Stroke Genetic Collaborative, the METASTROKE Consortium, and the International Stroke Genetics Consortium. Meta-analysis in more than 17,900 cases of ischemic stroke reveals a novel association at 12q24.12. Neurology. 2014;83:678–685. doi: 10.1212/WNL.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neurology Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium the Stroke Genetics Network (SiGN) and the International Stroke Genetics Consortium (ISGC) Identification of additional risk loci for stroke and small vessel disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2016;15:695–707. doi: 10.1016/S1474-4422(16)00102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, Anderson CD, Brouwers HB, Valant V, Battey TW, Radmanesh F, Raffeld MR, Baedorf-Kassis S, Deka R, Woo JG, Martin LJ, Haverbusch M, Moomaw CJ, Sun G, Broderick JP, Flaherty ML, Martini SR, Kleindorfer DO, Kissela B, Comeau ME, Jagiella JM, Schmidt H, Freudenberger P, Pichler A, Enzinger C, Hansen BM, Norrving B, Jimenez-Conde J, Giralt-Steinhauer E, Elosua R, Cuadrado-Godia E, Soriano C, Roquer J, Kraft P, Ayres AM, Schwab K, McCauley JL, Pera J, Urbanik A, Rost NS, Goldstein JN, Viswanathan A, Stögerer EM, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Montaner J, Fernandez-Cadenas I, Delgado P, Malik R, Dichgans M, Greenberg SM, Rothwell PM, Lindgren A, Slowik A, Schmidt R, Langefeld CD, Rosand J International Stroke Genetics Consortium. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–521. doi: 10.1016/j.ajhg.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, Jimenez-Conde J, Hansen BM, Fernandez-Cadenas I, Cortellini L, Ayres A, Schwab K, Juchniewicz K, Urbanik A, Rost NS, Viswanathan A, Seifert-Held T, Stoegerer EM, Tomás M, Rabionet R, Estivill X, Brown DL, Silliman SL, Selim M, Worrall BB, Meschia JF, Montaner J, Lindgren A, Roquer J, Schmidt R, Greenberg SM, Slowik A, Broderick JP, Woo D, Rosand J International Stroke Genetics Consortium. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Holzmann MJ, Aastveit A, Hammar N, Jungner I, Walldius G, Holme I. Renal dysfunction increases the risk of ischemic and hemorrhagic stroke in the general population. Ann Med. 2012;44:607–615. doi: 10.3109/07853890.2011.582136. [DOI] [PubMed] [Google Scholar]
- 173.Molshatzki N, Orion D, Tsabari R, Schwammenthal Y, Merzeliak O, Toashi M, Tanne D. Chronic kidney disease in patients with acute intracerebral hemorrhage: association with large hematoma volume and poor outcome. Cerebrovasc Dis. 2011;31:271–277. doi: 10.1159/000322155. [DOI] [PubMed] [Google Scholar]
- 174.Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, Woodward M, Longstreth WT, Jr, Psaty BM, Shlipak MG, Folsom AR, Gansevoort RT, Coresh J. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke. 2014;45:1925–1931. doi: 10.1161/STROKEAHA.114.004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rowat A, Graham C, Dennis M. Renal dysfunction in stroke patients: a hospital-based cohort study and systematic review. Int J Stroke. 2014;9:633–639. doi: 10.1111/ijs.12264. [DOI] [PubMed] [Google Scholar]
- 176.Sandsmark DK, Messé SR, Zhang X, Roy J, Nessel L, Lee Hamm L, He J, Horwitz EJ, Jaar BG, Kallem RR, Kusek JW, Mohler ER, 3rd, Porter A, Seliger SL, Sozio SM, Townsend RR, Feldman HI, Kasner SE CRIC Study Investigators. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: Chronic Renal Insufficiency Cohort Study. Stroke. 2015;46:2075–2080. doi: 10.1161/STROKEAHA.115.009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association [published corrections appear in Stroke. 2014;45:e95 and Stroke. 2014;45:e214] Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Friberg J, Scharling H, Gadsbøll N, Truelsen T, Jensen GB Copenhagen City Heart Study. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (the Copenhagen City Heart Study) Am J Cardiol. 2004;94:889–894. doi: 10.1016/j.amjcard.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 180.Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, Go AS. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dagres N, Nieuwlaat R, Vardas PE, Andresen D, Lévy S, Cobbe S, Kremastinos DT, Breithardt G, Cokkinos DV, Crijns HJ. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49:572–577. doi: 10.1016/j.jacc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 182.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb Haemost. 2009;101:938–942. [PubMed] [Google Scholar]
- 183.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. doi: 10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 184.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham Heart Study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 186.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 187.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 188.Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen-progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 189.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 190.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 191.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 192.Gillum LA, Johnston SC. Oral contraceptives and stroke risk: the debate continues. Lancet Neurol. 2004;3:453–454. doi: 10.1016/S1474-4422(04)00819-1. [DOI] [PubMed] [Google Scholar]
- 193.Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;(8):CD011054. doi: 10.1002/14651858.CD011054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.MacClellan LR, Giles W, Cole J, Wozniak M, Stern B, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: the Stroke Prevention in Young Women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 195.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 198.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnson DA, Lisabeth L, Lewis TT, Sims M, Hickson DA, Samdarshi T, Taylor H, Diez Roux AV. The contribution of psychosocial stressors to sleep among African Americans in the Jackson Heart Study. Sleep. 2016;39:1411–1419. doi: 10.5665/sleep.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Broadley SA, Jørgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, Antic R. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 202.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–137. [PMC free article] [PubMed] [Google Scholar]
- 203.Lisabeth LD, Sánchez BN, Chervin RD, Morgenstern LB, Zahuranec DB, Tower SD, Brown DL. High prevalence of post-stroke sleep disordered breathing in Mexican Americans. Sleep Med. doi: 10.1016/j.sleep.2016.01.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229:489–495. doi: 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 206.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 207.Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol. 2014;172:466–469. doi: 10.1016/j.ijcard.2013.12.230. [DOI] [PubMed] [Google Scholar]
- 208.Brown DL, Mowla A, McDermott M, Morgenstern LB, Hegeman G, 3rd, Smith MA, Garcia NM, Chervin RD, Lisabeth LD. Ischemic stroke subtype and presence of sleep-disordered breathing: the BASIC sleep apnea study. J Stroke Cerebrovasc Dis. 2015;24:388–393. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, Soriano Y, Román-Sánchez P, Illa FB, Canal JM, Durán-Cantolla J. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 210.Parra O, Arboix A, Montserrat JM, Quintó L, Bechich S, García-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:267–272. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 211.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, Franklin KA. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 212.Turkington PM, Bamford J, Wanklyn P, Elliott MW. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke. 2002;33:2037–2042. doi: 10.1161/01.str.0000023576.94311.27. [DOI] [PubMed] [Google Scholar]
- 213.Lambiase MJ, Kubzansky LD, Thurston RC. Prospective study of anxiety and incident stroke. Stroke. 2014;45:438–443. doi: 10.1161/STROKEAHA.113.003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Henderson KM, Clark CJ, Lewis TT, Aggarwal NT, Beck T, Guo H, Lunos S, Brearley A, Mendes de Leon CF, Evans DA, Everson-Rose SA. Psychosocial distress and stroke risk in older adults. Stroke. 2013;44:367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. 2013;44:1555–1560. doi: 10.1161/STROKEAHA.113.001147. [DOI] [PubMed] [Google Scholar]
- 216.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43:32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- 217.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review [published correction appears in JAMA. 2011;306:2565] JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 219.Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carrà G, Agostoni E, Clerici M. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat. 2013;2013:862978. doi: 10.1155/2013/862978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev. 2008;(4):CD003437. doi: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- 221.Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, Tirschwell D, Teri L. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke. 2009;40:3073–3078. doi: 10.1161/STROKEAHA.109.549808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Thomas SA, Walker MF, Macniven JA, Haworth H, Lincoln NB. Communication and Low Mood (CALM): a randomized controlled trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil. 2013;27:398–408. doi: 10.1177/0269215512462227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alexopoulos GS, Wilkins VM, Marino P, Kanellopoulos D, Reding M, Sirey JA, Raue PJ, Ghosh S, O’Dell MW, Kiosses DN. Ecosystem focused therapy in poststroke depression: a preliminary study. Int J Geriatr Psychiatry. 2012;27:1053–1060. doi: 10.1002/gps.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Salter KL, Foley NC, Zhu L, Jutai JW, Teasell RW. Prevention of poststroke depression: does prophylactic pharmacotherapy work? J Stroke Cerebrovasc Dis. 2013;22:1243–1251. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 225.Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial [published correction appears in JAMA. 2009;301:1024] JAMA. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Centers for Disease Control and Prevention (CDC) Awareness of stroke warning symptoms: 13 states and the District of Columbia, 2005. MMWR Morb Mortal Wkly Rep. 2008;57:481–485. [PubMed] [Google Scholar]
- 227.Kothari R, Sauerbeck L, Jauch E, Broderick J, Brott T, Khoury J, Liu T. Patients’ awareness of stroke signs, symptoms, and risk factors. Stroke. 1997;28:1871–1875. doi: 10.1161/01.str.28.10.1871. [DOI] [PubMed] [Google Scholar]
- 228.Zerwic J, Hwang SY, Tucco L. Interpretation of symptoms and delay in seeking treatment by patients who have had a stroke: exploratory study. Heart Lung. 2007;36:25–34. doi: 10.1016/j.hrtlng.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 229.DuBard CA, Garrett J, Gizlice Z. Effect of language on heart attack and stroke awareness among U.S. Hispanics. Am J Prev Med. 2006;30:189–196. doi: 10.1016/j.amepre.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 230.Willey JZ, Williams O, Boden-Albala B. Stroke literacy in Central Harlem: a high-risk stroke population. Neurology. 2009;73:1950–1956. doi: 10.1212/WNL.0b013e3181c51a7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pratt CA, Ha L, Levine SR, Pratt CB. Stroke knowledge and barriers to stroke prevention among African Americans: implications for health communication. J Health Commun. 2003;8:369–381. doi: 10.1080/10810730305725. [DOI] [PubMed] [Google Scholar]
- 232.Sallar AM, Williams PB, Omishakin AM, Lloyd DP. Stroke prevention: awareness of risk factors for stroke among African American residents in the Mississippi delta region. J Natl Med Assoc. 2010;102:84–94. doi: 10.1016/s0027-9684(15)30495-8. [DOI] [PubMed] [Google Scholar]
- 233.Williams O, Noble JM. “Hip-hop” stroke: a stroke educational program for elementary school children living in a high-risk community. Stroke. 2008;39:2809–2816. doi: 10.1161/STROKEAHA.107.513143. [DOI] [PubMed] [Google Scholar]
- 234.Mochari-Greenberger H, Towfighi A, Mosca L. National women’s knowledge of stroke warning signs, overall and by race/ethnic group. Stroke. 2014;45:1180–1182. doi: 10.1161/STROKEAHA.113.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults: United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 236.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Buntin MB, Colla CH, Deb P, Sood N, Escarce JJ. Medicare spending and outcomes after postacute care for stroke and hip fracture. Med Care. 2010;48:776–784. doi: 10.1097/MLR.0b013e3181e359df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Medicare Payment Advisory Commission (MedPAC) [Accessed August 23, 2016];Report to the Congress: Medicare payment policy. 2013 http://www.medpac.gov/docs/default-source/reports/mar13_entirereport.pdf?sfvrsn=0.
- 239.Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. Preventable readmissions within 30 days of ischemic stroke among Medicare beneficiaries. Stroke. 2013;44:3429–3435. doi: 10.1161/STROKEAHA.113.003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ottenbacher KJ, Karmarkar A, Graham JE, Kuo YF, Deutsch A, Reistetter TA, Al Snih S, Granger CV. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA. 2014;311:604–614. doi: 10.1001/jama.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ali M, Hazelton C, Lyden P, Pollock A, Brady M VISTA Collaboration. Recovery from poststroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair. 2013;27:133–141. doi: 10.1177/1545968312454683. [DOI] [PubMed] [Google Scholar]
- 242.Whitson HE, Landerman LR, Newman AB, Fried LP, Pieper CF, Cohen HJ. Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:1325–1331. doi: 10.1093/gerona/glq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke. 2012;43:1982–1987. doi: 10.1161/STROKEAHA.111.632547. [DOI] [PubMed] [Google Scholar]
- 244.Ottenbacher KJ, Campbell J, Kuo YF, Deutsch A, Ostir GV, Granger CV. Racial and ethnic differences in postacute rehabilitation outcomes after stroke in the United States. Stroke. 2008;39:1514–1519. doi: 10.1161/STROKEAHA.107.501254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ellis C, Boan AD, Turan TN, Ozark S, Bachman D, Lackland DT. Racial differences in poststroke rehabilitation utilization and functional outcomes. Arch Phys Med Rehabil. 2015;96:84–90. doi: 10.1016/j.apmr.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 246.Lisabeth LD, Sánchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, Brown DL, Morgenstern LB. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke. 2014;45:1096–1101. doi: 10.1161/STROKEAHA.113.003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kleindorfer D, Khoury J, Kissela B, Alwell K, Woo D, Miller R, Schneider A, Moomaw C, Broderick JP. Temporal trends in the incidence and case fatality of stroke in children and adolescents. J Child Neurol. 2006;21:415–418. doi: 10.1177/08830738060210050301. [DOI] [PubMed] [Google Scholar]
- 248.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 250.Lee J, Croen LA, Backstrand KH, Yoshida CK, Henning LH, Lindan C, Ferriero DM, Fullerton HJ, Barkovich AJ, Wu YW. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–729. doi: 10.1001/jama.293.6.723. [DOI] [PubMed] [Google Scholar]
- 251.Kirton A, Armstrong-Wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, Carpenter J, Yager JY, Lynch JK, Ferriero DM International Pediatric Stroke Study Investigators. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128:e1402–e1410. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 252.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, Parker AP, Wassmer E, Wraige E, Amin S, Edwards HB, O’Callaghan FJ. Diagnostic delays in paediatric stroke. J Neurol Neurosurg Psychiatry. 2015;86:917–921. doi: 10.1136/jnnp-2014-309188. [DOI] [PubMed] [Google Scholar]
- 253.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, Deveber GA, Ganesan V International Pediatric Stroke Study Group. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 254.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 255.Wintermark M, Hills NK, deVeber GA, Barkovich AJ, Elkind MS, Sear K, Zhu G, Leiva-Salinas C, Hou Q, Dowling MM, Bernard TJ, Friedman NR, Ichord RN, Fullerton HJ VIPS Investigators. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke. 2014;45:3597–3605. doi: 10.1161/STROKEAHA.114.007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fox CK, Sidney S, Fullerton HJ. Community-based case-control study of childhood stroke risk associated with congenital heart disease. Stroke. 2015;46:336–340. doi: 10.1161/STROKEAHA.114.007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Asakai H, Cardamone M, Hutchinson D, Stojanovski B, Galati JC, Cheung MM, Mackay MT. Arterial ischemic stroke in children with cardiac disease. Neurology. 2015;85:2053–2059. doi: 10.1212/WNL.0000000000002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gelfand AA, Fullerton HJ, Jacobson A, Sidney S, Goadsby PJ, Kurth T, Pressman A. Is migraine a risk factor for pediatric stroke? Cephalalgia. 2015;35:1252–1260. doi: 10.1177/0333102415576222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hills NK, Johnston SC, Sidney S, Zielinski BA, Fullerton HJ. Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke. Ann Neurol. 2012;72:850–858. doi: 10.1002/ana.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 261.Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, deVeber GA, Fullerton HJ VIPS Investigators. Herpesvirus infections and childhood arterial ischemic stroke: results of the VIPS Study. Circulation. 2016;133:732–741. doi: 10.1161/CIRCULATIONAHA.115.018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kenet G, Lütkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, Chabrier S, Chan A, deVeber G, Fiedler B, Fullerton HJ, Goldenberg NA, Grabowski E, Günther G, Heller C, Holzhauer S, Iorio A, Journeycake J, Junker R, Kirkham FJ, Kurnik K, Lynch JK, Male C, Manco-Johnson M, Mesters R, Monagle P, van Ommen CH, Raffini L, Rostásy K, Simioni P, Sträter RD, Young G, Nowak-Göttl U. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121:1838–1847. doi: 10.1161/CIRCULATIONAHA.109.913673. [DOI] [PubMed] [Google Scholar]
- 263.Bigi S, Fischer U, Wehrli E, Mattle HP, Boltshauser E, Bürki S, Jeannet PY, Fluss J, Weber P, Nedeltchev K, El-Koussy M, Steinlin M, Arnold M. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70:245–254. doi: 10.1002/ana.22427. [DOI] [PubMed] [Google Scholar]
- 264.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 265.Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, Parker AP, Wassmer E, Wraige E, Amin S, Edwards HB, Tilling K, O’Callaghan FJ. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014;13:35–43. doi: 10.1016/S1474-4422(13)70290-4. [DOI] [PubMed] [Google Scholar]
- 266.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 267.Lehman LL, Fullerton HJ. Changing ethnic disparity in ischemic stroke mortality in US children after the STOP trial. JAMA Pediatr. 2013;167:754–758. doi: 10.1001/jamapediatrics.2013.89. [DOI] [PubMed] [Google Scholar]
- 268.Elbers J, deVeber G, Pontigon AM, Moharir M. Long-term outcomes of pediatric ischemic stroke in adulthood. J Child Neurol. 2014;29:782–788. doi: 10.1177/0883073813484358. [DOI] [PubMed] [Google Scholar]
- 269.Boardman JP, Ganesan V, Rutherford MA, Saunders DE, Mercuri E, Cowan F. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics. 2005;115:321–326. doi: 10.1542/peds.2004-0427. [DOI] [PubMed] [Google Scholar]
- 270.Hajek CA, Yeates KO, Anderson V, Mackay M, Greenham M, Gomes A, Lo W. Cognitive outcomes following arterial ischemic stroke in infants and children. J Child Neurol. 2014;29:887–894. doi: 10.1177/0883073813491828. [DOI] [PubMed] [Google Scholar]
- 271.Studer M, Boltshauser E, Capone Mori A, Datta A, Fluss J, Mercati D, Hackenberg A, Keller E, Maier O, Marcoz JP, Ramelli GP, Poloni C, Schmid R, Schmitt-Mechelke T, Wehrli E, Heinks T, Steinlin M. Factors affecting cognitive outcome in early pediatric stroke. Neurology. 2014;82:784–792. doi: 10.1212/WNL.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 272.Lo W, Gordon A, Hajek C, Gomes A, Greenham M, Perkins E, Zumberge N, Anderson V, Yeates KO, Mackay MT. Social competence following neonatal and childhood stroke. Int J Stroke. 2014;9:1037–1044. doi: 10.1111/ijs.12222. [DOI] [PubMed] [Google Scholar]
- 273.Bemister TB, Brooks BL, Dyck RH, Kirton A. Parent and family impact of raising a child with perinatal stroke. BMC Pediatr. 2014;14:182. doi: 10.1186/1471-2431-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 275.Tuppin P, Samson S, Woimant F, Chabrier S. Management and 2-year follow-up of children aged 29 days to 17 years hospitalized for a first stroke in France (2009–2010) Arch Pediatr. 2014;21:1305–1315. doi: 10.1016/j.arcped.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 276.Sultan SM, Beslow LA, Vossough A, Elkind MS, Kasner SE, Mirsky DM, Licht DJ, Ichord RN. Predictive validity of severity grading for cerebral steno-occlusive arteriopathy in recurrent childhood ischemic stroke. Int J Stroke. 2015;10:213–218. doi: 10.1111/ijs.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 278.Koroknay-Pál P, Niemelä M, Lehto H, Kivisaari R, Numminen J, Laakso A, Hernesniemi J. De novo and recurrent aneurysms in pediatric patients with cerebral aneurysms. Stroke. 2013;44:1436–1439. doi: 10.1161/STROKEAHA.111.676601. [DOI] [PubMed] [Google Scholar]
- 279.Wusthoff CJ, Kessler SK, Vossough A, Ichord R, Zelonis S, Halperin A, Gordon D, Vargas G, Licht DJ, Smith SE. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics. 2011;127:e1550–e1557. doi: 10.1542/peds.2010-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fox CK, Glass HC, Sidney S, Lowenstein DH, Fullerton HJ. Acute seizures predict epilepsy after childhood stroke. Ann Neurol. 2013;74:249–256. doi: 10.1002/ana.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hsu CJ, Weng WC, Peng SS, Lee WT. Early-onset seizures are correlated with late-onset seizures in children with arterial ischemic stroke. Stroke. 2014;45:1161–1163. doi: 10.1161/STROKEAHA.113.004015. [DOI] [PubMed] [Google Scholar]
- 282.Beslow LA, Abend NS, Gindville MC, Bastian RA, Licht DJ, Smith SE, Hillis AE, Ichord RN, Jordan LC. Pediatric intracerebral hemorrhage: acute symptomatic seizures and epilepsy. JAMA Neurol. 2013;70:448–454. doi: 10.1001/jamaneurol.2013.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bernard TJ, Rivkin MJ, Scholz K, deVeber G, Kirton A, Gill JC, Chan AK, Hovinga CA, Ichord RN, Grotta JC, Jordan LC, Benedict S, Friedman NR, Dowling MM, Elbers J, Torres M, Sultan S, Cummings DD, Grabowski EF, McMillan HJ, Beslow LA, Amlie-Lefond C Thrombolysis in Pediatric Stroke Study. Emergence of the primary pediatric stroke center: impact of the thrombolysis in pediatric stroke trial. Stroke. 2014;45:2018–2023. doi: 10.1161/STROKEAHA.114.004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ladner TR, Mahdi J, Gindville MC, Gordon A, Harris ZL, Crossman K, Pruthi S, Abramo TJ, Jordan LC. Pediatric acute stroke protocol activation in a children’s hospital emergency department. Stroke. 2015;46:2328–2331. doi: 10.1161/STROKEAHA.115.009961. [DOI] [PubMed] [Google Scholar]
- 285.Hamilton W, Huang H, Seiber E, Lo W. Cost and outcome in pediatric ischemic stroke. J Child Neurol. 2015;30:1483–1488. doi: 10.1177/0883073815570673. [DOI] [PubMed] [Google Scholar]
- 286.Plumb P, Seiber E, Dowling MM, Lee J, Bernard TJ, deVeber G, Ichord RN, Bastian R, Lo WD. Out-of-pocket costs for childhood stroke: the impact of chronic illness on parents’ pocketbooks. Pediatr Neurol. 2015;52:73–6.e2. doi: 10.1016/j.pediatrneurol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ramirez L, Kim-Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ, Towfighi A. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc. 2016;5:e003233. doi: 10.1161/JAHA.116.003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, Schroth G, Remonda L, Sturzenegger M, Fischer U, Baumgartner RW. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76:191–195. doi: 10.1136/jnnp.2004.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw FE. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309:1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- 290.Synhaeve NE, Arntz RM, van Alebeek ME, van Pamelen J, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, de Kort PL, van Dijk EJ, de Leeuw FE. Women have a poorer very long-term functional outcome after stroke among adults aged 18–50 years: the FUTURE study. J Neurol. 2016;263:1099–1105. doi: 10.1007/s00415-016-8042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4:e001967. doi: 10.1161/JAHA.115.001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Russo T, Felzani G, Marini C. Stroke in the very old: a systematic review of studies on incidence, outcome, and resource use. J Aging Res. 2011;2011:108785. doi: 10.4061/2011/108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Forti P, Maioli F, Procaccianti G, Nativio V, Lega MV, Coveri M, Zoli M, Sacquegna T. Independent predictors of ischemic stroke in the elderly: prospective data from a stroke unit [published correction appears in Neurology. 2013;81:1882] Neurology. 2013;80:29–38. doi: 10.1212/WNL.0b013e31827b1a41. [DOI] [PubMed] [Google Scholar]
- 294.Saposnik G, Black S Stroke Outcome Research Canada (SORCan) Working Group. Stroke in the very elderly: hospital care, case fatality and disposition. Cerebrovasc Dis. 2009;27:537–543. doi: 10.1159/000214216. [DOI] [PubMed] [Google Scholar]
- 295.Kammersgaard LP, Jørgensen HS, Reith J, Nakayama H, Pedersen PM, Olsen TS Copenhagen Stroke Study. Short- and long-term prognosis for very old stroke patients: the Copenhagen Stroke Study. Age Ageing. 2004;33:149–154. doi: 10.1093/ageing/afh052. [DOI] [PubMed] [Google Scholar]
- 296.Ovbiagele B, Markovic D, Towfighi A. Recent age- and gender-specific trends in mortality during stroke hospitalization in the United States. Int J Stroke. 2011;6:379–387. doi: 10.1111/j.1747-4949.2011.00590.x. [DOI] [PubMed] [Google Scholar]
- 297.Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20. doi: 10.1111/j.1749-6632.2012.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Olsen TS, Andersen KK. Stroke in centenarians. Geriatr Gerontol Int. 2014;14:84–88. doi: 10.1111/ggi.12058. [DOI] [PubMed] [Google Scholar]
- 299.Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, Chappel AR, Peterson ED, Friedman B. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lichtman JH, Jones SB, Leifheit-Limson EC, Wang Y, Goldstein LB. 30-day mortality and readmission after hemorrhagic stroke among Medicare beneficiaries in Joint Commission primary stroke center-certified and noncertified hospitals. Stroke. 2011;42:3387–3391. doi: 10.1161/STROKEAHA.111.622613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;(9):CD000197. doi: 10.1002/14651858.CD000197.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau-Sepulveda MV, Peterson ED, Fonarow GC. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 304.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, Schwamm LH GWTG-Stroke Steering Committee and Investigators. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431–1439. doi: 10.1161/STROKEAHA.110.583815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Xian Y, Hernandez AF, Peterson ED, Schwamm LH. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 306.Buntin MB, Colla CH, Escarce JJ. Effects of payment changes on trends in post-acute care. Health Serv Res. 2009;44:1188–1210. doi: 10.1111/j.1475-6773.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kramer A, Holthaus D, Goodrish G, Epstein A. A Study of Stroke Post-Acute Care Costs and Outcomes: Final Report. Washington, DC: US Department of Health and Human Services; Dec 28, 2006. [Google Scholar]
- 308.Berg K, Intrator O. Postacute care following stroke or hip fracture: single services and combinations used by Medicare beneficiaries (1987–1992) J Aging Health. 1999;11:27–48. doi: 10.1177/089826439901100102. [DOI] [PubMed] [Google Scholar]
- 309.Gage B, Morley M, Spain P, Ingber M. Examining Post Acute Care Relationships in an Integrated Hospital System. Waltham, PA: US Department of Health and Human Services; Feb 1, 2009. [Google Scholar]
- 310.Centers for Disease Control and Prevention, National Center for Health Statistics. 2010 National Ambulatory Medical Care Survey and 2010 National Hospital Ambulatory Medical Care Survey. [Accessed July 17, 2013];Ambulatory health care data: questionnaires, datasets, and related documentation. For methodology, see National Center for Health Statistics, Public Use Data File Documentation: 2010 National Ambulatory Medical Care Survey and Public Use Data File Documentation: 2010 National Hospital Ambulatory Medical Care Survey. http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm.
- 311.Elixhauser A, Jiang H. Hospitalizations for Women With Circulatory Disease, 2003. Rockville, MD: Agency for Healthcare Research and Quality; May, 2006. [Accessed August 3, 2011]. HCUP Statistical Brief No. 5. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb5.pdf. [PubMed] [Google Scholar]
- 312.Casper M, Barnett E, Williams GI, Jr, Halverson JA, Braham VE, Greenlund KJ. Atlas of Stroke Mortality: Racial, Ethnic, and Geographic Disparities in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 313.Dumont TM, Rughani AI. National trends in carotid artery revas-cularization surgery. J Neurosurg. 2012;116:1251–1257. doi: 10.3171/2012.3.JNS111320. [DOI] [PubMed] [Google Scholar]
- 314.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis [published corrections appear in N Engl J Med. 2010;363:198 and N Engl J Med. 2010;363:498] N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC, 3rd, Aronow HD, Eskandari MK, Sheffet AJ, Lal BK, Meschia JF, Brott TG CREST Investigators. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. Stroke. 2011;42:3484–3490. doi: 10.1161/STROKEAHA.111.624155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang FW, Esterbrooks D, Kuo YF, Mooss A, Mohiuddin SM, Uretsky BF. Outcomes after carotid artery stenting and endarterectomy in the Medicare population. Stroke. 2011;42:2019–2025. doi: 10.1161/STROKEAHA.110.608992. [DOI] [PubMed] [Google Scholar]
- 317.McDonald RJ, Kallmes DF, Cloft HJ. Comparison of hospitalization costs and Medicare payments for carotid endarterectomy and carotid stenting in asymptomatic patients. AJNR Am J Neuroradiol. 2012;33:420–425. doi: 10.3174/ajnr.A2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sternbergh WC, 3rd, Crenshaw GD, Bazan HA, Smith TA. Carotid endarterectomy is more cost-effective than carotid artery stenting. J Vasc Surg. 2012;55:1623–1628. doi: 10.1016/j.jvs.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 319.Witt AH, Johnsen SP, Jensen LP, Hansen AK, Hundborg HH, Andersen G. Reducing delay of carotid endarterectomy in acute ischemic stroke patients: a nationwide initiative. Stroke. 2013;44:686–690. doi: 10.1161/STROKEAHA.111.678565. [DOI] [PubMed] [Google Scholar]
- 320.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 321.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 322.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 323.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 324.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: household component summary tables. Table 7: Total Expenses and Percent Distribution for Selected Conditions by Type of Service: United States, Average Annual 2011–2012. [Accessed April 28, 2015];Agency for Healthcare Research and Quality Web site. http://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HC2Y2012&Table=HC2Y2012%5FCNDXP%5FC&_Debug=
- 325.Brown DL, Boden-Albala B, Langa KM, Lisabeth LD, Fair M, Smith MA, Sacco RL, Morgenstern LB. Projected costs of ischemic stroke in the United States. Neurology. 2006;67:1390–1395. doi: 10.1212/01.wnl.0000237024.16438.20. [DOI] [PubMed] [Google Scholar]
- 326.Godwin KM, Wasserman J, Ostwald SK. Cost associated with stroke: outpatient rehabilitative services and medication. Top Stroke Rehabil. 2011;18(Suppl 1):676–684. doi: 10.1310/tsr18s01-676. [DOI] [PubMed] [Google Scholar]
- 327.Engel-Nitz NM, Sander SD, Harley C, Rey GG, Shah H. Costs and outcomes of noncardioembolic ischemic stroke in a managed care population. Vasc Health Risk Manag. 2010;6:905–913. doi: 10.2147/VHRM.S10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ellis C, Simpson AN, Bonilha H, Mauldin PD, Simpson KN. The one-year attributable cost of poststroke aphasia. Stroke. 2012;43:1429–1431. doi: 10.1161/STROKEAHA.111.647339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lundström E, Smits A, Borg J, Terént A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke. 2010;41:319–324. doi: 10.1161/STROKEAHA.109.558619. [DOI] [PubMed] [Google Scholar]
- 330.Perkins E, Stephens J, Xiang H, Lo W. The cost of pediatric stroke acute care in the United States [published correction appears in Stroke. 2010;41:e600] Stroke. 2009;40:2820–2827. doi: 10.1161/STROKEAHA.109.548156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Gardner MA, Hills NK, Sidney S, Johnston SC, Fullerton HJ. The 5-year direct medical cost of neonatal and childhood stroke in a population-based cohort. Neurology. 2010;74:372–378. doi: 10.1212/WNL.0b013e3181cbcd48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2014;383:218] Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran AE, Sacco RL, Truelsen T, Davis S, Pandian JD, Naghavi M, Forouzanfar MH, Nguyen G, Johnson CO, Vos T, Meretoja A, Murray CJ, Roth GA GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 Study. Neuroepidemiology. 2015;45:161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381:628] Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the Greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 337.Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 338.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
15. GLOBAL CVD AND STROKE
See Table 15-1 and Charts 15-1 through 15-11
Table 15-1.
Top Causes of Cardiovascular Disease Estimated for the Global Burden of Disease 2013 Study
| Cause | Deaths in 2013 | 95% UI |
|---|---|---|
| Ischemic heart disease | 8 139 852 | 7 322 942–8 758 490 |
| Ischemic stroke | 3 272 924 | 2 812 654–3 592 562 |
| Hemorrhagic and other nonischemic stroke | 3 173 951 | 2 885 717–3 719 684 |
| Hypertensive heart disease | 1 068 585 | 849 758–1 242 160 |
| Other cardiovascular and circulatory diseases | 554 588 | 499 143–654 152 |
| Cardiomyopathy and myocarditis | 443 297 | 370 111–511 997 |
| Rheumatic heart disease | 275 054 | 222 622–353 938 |
| Aortic aneurysm | 151 493 | 124 201–179 954 |
| Atrial fibrillation and flutter | 112 209 | 97 716–126 677 |
| Endocarditis | 65 036 | 48 593–79 435 |
| Peripheral vascular disease | 40 492 | 35 487–44 883 |
UI indicates uncertainty interval.
Reprinted from Roth et al.4 Copyright © 2015, American Heart Association, Inc.
Chart 15-1. Incidence of ischemic and hemorrhagic or other stroke type by age and sex.
Reprinted from Barker-Collo et al7 with permission of the publisher. Copyright © 2015, S. Karger AG, Basel.
Chart 15-11. Contribution of changes in population growth, population aging, and rates of age-specific cardiovascular death to changes in cardiovascular mortality, 1990 to 2013.
Reprinted from Roth et al.11 Copyright © 2015, Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Cardiovascular risks and diseases are a major determinant of global health. This new chapter of the AHA Statistical Update is intended to provide an overview of global trends in CVDs overall and IHD and stroke in particular. A global perspective on other CVD risks and conditions can be found in the relevant chapter of the Update.
Global Burden of CVD
Incidence of IHD and Stroke
(See Chart 15-1)
In 2013, there were an estimated 8.56 million (95% UI, 8.20–8.92) cases of AMI.1
In 2013, there were an estimated 10.3 million new strokes, two thirds (67%) of which were ischemic strokes. Incidence for both ischemic and hemorrhagic stroke is higher among males than females (132 versus 99 per 100 000 [95% UI] and 65 versus 56 per 100 000 [95% UI] for ischemic and hemorrhagic respectively)2 (Chart 15-1).
Prevalence of IHD and Stroke
The highest prevalence of ischemic stroke (1015–1184 cases per 100 000 people) was in developed countries (particularly in the United States), with the lowest (up to 339 per 100 000) in developing countries in 2013.3
Abbreviations Used in Chapter 15
| ACEI | angiotensin-converting enzyme inhibitor |
| AHA | American Heart Association |
| AMI | acute myocardial infarction |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DM | diabetes mellitus |
| HBP | high blood pressure |
| HF | heart failure |
| HIC | high-income countries |
| IHD | ischemic heart disease |
| LMIC | low-and middle-income countries |
| MI | myocardial infarction |
| PURE | Prospective Urban Rural Epidemiology study |
| SBP | systolic blood pressure |
| UI | uncertainty interval |
| WHO | World Health Organization |
| YLL | years of life lost |
Mortality
(See Table 15-1 and Charts 15-2 through 15-8)
Chart 15-2. Age-standardized global death rates for CVD stratified by sex, 1990 to 2013.
CVD indicates cardiovascular disease; and UI, uncertainty interval.
Reprinted from Roth et al.4 Copyright © 2015, American Heart Association, Inc.
Chart 15-8. Risk factor burden, by rural and urban regions, in selected high-, middle-and low-income countries.
Reprinted from Yusuf et al.8 Copyright © 2014, Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
CVD was the most common underlying cause of death in the world in 2013, accounting for an estimated 17. 3 million (95% UI, 16.5–18.1 million) of 54 million total deaths, or 31.5% (95% UI, 30.3%–32.9%) of all global deaths4 (Table 15-1).
An estimated 12 million CVD deaths (95% UI, 11.3–12.6 million), or ≈70% of all CVD deaths, in 2013 occurred in LMIC.4
CVD was the cause of an estimated 29.4% (95% UI, 28.7%–30.2%) of deaths among males and 34.1% (95% UI, 31.4%–36.8%) of deaths among females in 2013. Since 1990, there has been a persistent disparity in the age-standardized risk of dying of CVD for males versus females of ≈70 deaths per 100 000. The estimated global age-standardized CVD death rate among males in 2013 fell to the level observed among females in 1990 (333 deaths per 100 000 people)4 (Chart 15-2).
An estimated 329 700 000 DALYs (95% UI, 311 188 000–348 200 000) were lost because of CVD in 2013. There were 308.5 million YLL (95% UI, 290.8–325.5) prematurely to CVD in 2013, measured from the longest observed average global life expectancy of 86 years. DALYs are a measure of health lost because of both premature death and disability. This accounted for 13.5% of all health lost globally in 2013 (95% UI, 12.5%–14.6%).5
The leading cause of CVD-related deaths in 2013 was IHD. IHD caused an estimated 8.2 million deaths in 2013 (95% UI, 7.3–8.7 million), with 3.8 million of these among females. This accounted for 144.4 million (95% UI) YLL prematurely in 20135 (Chart 15-3).
The second most common cause of CVD-related deaths in 2013 was cerebrovascular disease, accounting for more than 8 million deaths (95% UI, 7.3–8.7 million). Ischemic and hemorrhagic strokes were each the cause of more than 3 million deaths in 2013 (95% UI, 2 812 654–3 592 562 for ischemic stroke and 95% UI, 2 885 717–3 719 684 for hemorrhagic stroke).5
CVD was the leading cause of total deaths in all regions of the world except sub-Saharan Africa. Stroke dominated over IHD as the leading cause of CVD death in sub-Saharan Africa, East Asia, and Southeast Asia4 (Chart 15-4).
CVD age-standardized death rates were lowest in Japan (110 per 100 000; 95% UI, 101–125), along with Taiwan, France, Israel, and Canada. The geographic region of the former Soviet Union had the highest rate, with age-standardized CVD death rates >600 per 100 0006 (Chart 15-5).
The highest ischemic stroke mortality rates (124 to 174 per 100 000 person-years) were observed in Russia and Kazakhstan, with the lowest (at or below 25 per 100 000 person-years) were seen in Western Europe, North and Central America, Turkmenistan, and Papua New Guinea7 (Chart 15-6).
Major CVD event rates, CVD death rates, and all-cause death rates were lowest in HIC and highest in low-income countries, according to the PURE study of 628 urban and rural communities in 17 countries. Major CVD event rates were 3.99, 5.38, and 6.43 events per 1000 person-years in high-, middle-, and low-income countries, respectively. This was true although CVD risk, as measured by the INTERHEART summary cardiovascular risk score, was higher in higher-income countries. The increasing event rate in lower-income countries was attributable to MI, with no pattern seen for stroke or HF8 (Charts 15-7 and 15-8).
Chart 15-3. Number of ischemic heart disease deaths by age, 2013.
Source: Institute for Health Metrics and Evaluation.5
Chart 15-4. Proportion of YLLs because of CVD stratified by global region, 2013.
YLL is a measure of premature mortality calculated by using a normative goal for survival computed from the lowest observed death rate across countries.
CVD indicates cardiovascular disease; and YLL, years of life lost.
Reprinted from Roth et al.4 Copyright © 2015, American Heart Association, Inc.
Chart 15-5. Map of age-standardized ischemic heart disease mortality rate per 100 000 people in 2013.
Source: Institute for Health Metrics and Evaluation.5
Chart 15-6. Age-standardized annual mortality rates (per 100 000) of ischemic stroke in 2013.
Reprinted from Feigin et al15 with permission of the publisher. Copyright © 2015, S. Karger AG, Basel.
Chart 15-7. Cardiovascular disease event rates in selected high-, middle-, and low-income countries.
Reprinted from Yusuf et al.8 Copyright © 2014, Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Economic Burden
(See Chart 15-9)
Chart 15-9. Proportion of survey respondents who experienced catastrophic health spending (out-of-pocket health spending >40% nonfood expenditures) and distress financing after cardiovascular disease–related hospitalization, divided by income strata.
Differences across income strata were considered statistically significant (P<0.05) for China (catastrophic health spending and distress financing), India (catastrophic health spending), and Tanzania (catastrophic health spending and distress financing).
Reprinted from Huffman et al.9 Copyright © 2011, Huffman et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
In 2008 to 2009, out-of-pocket spending over the year after CVD hospitalization in select LMIC ranged widely, from international $374 in Tanzania to international $2917 in India. Distressed and catastrophic health spending was common9 (Chart 15-9).
In 2010, the global cost of CVD was estimated at US $863 billion.10 This estimate, performed at the level of WHO regions, took into account productivity loss because of premature CVD deaths; productivity loss because of disability from stroke, HF, and angina; regional unemployment rate; and lost work time because of medical care. The costs of managing HBP and cholesterol were included, but DM management and tobacco cessation costs were not.
Secular Trends
(See Charts 15-10 and 15-11)
Chart 15-10. Change in age-standardized CVD death rate and total number of CVD deaths, 1990 to 2013.
CVD indicates cardiovascular disease; and UI, uncertainty interval.
Reprinted from Roth et al.4 Copyright © 2015, American Heart Association, Inc.
The age-standardized global death rate attributable to CVD has declined from 375 in 1990 (95% UI, 361–389) to 293 in 2013 (95% UI, 280–306) per 100 000 people, a decline of 22%. The largest decline was observed between the years 2000 and 20054 (Chart 15-10).
Despite declines in the age-standardized global death rate for CVD, the total number of CVD deaths increased from 12.3 million (95% UI, 11.8–12.8 million) to 17.3 million (95% UI, 16.5–18.1 million) between 1990 and 2013, an increase of 41%, mostly attributable to population growth and aging.11 However, the effect of population growth and aging on the number of cardiovascular deaths varies widely across regions (Charts 15-11).
Contrary to trends in high-income regions, the age-adjusted mortality rate for CVD in sub-Saharan Africa has not declined. The age-standardized CVD mortality rate in 1990 was 327.6 per 100 000 (95% CI, 306.2–351.7) and 330.2 per 100 000 (95% CI, 312.9–360.0) in 2013, representing little change over more than 2 decades.12
The age-standardized global IHD death rate declined from an estimated 177 deaths per 100 000 in 1990 (95% UI, 162–190) to 138 (95% UI, 124–148) per 100 000 in 2013. The highest age-standardized rates of IHD death in the world were found in Eastern Europe (320 per 100 000) and Central Asia (368 per 100 000). The largest increase in IHD death rates between 1990 and 2013 were found in Bangladesh (4.7%).5
Prevention, Awareness, Treatment, and Control
In a survey of 598 communities in 18 countries, the availability of aspirin, β-blockers, ACEIs, and statins varied widely. In the low-income countries, only 1 of 30 rural and only 25 of 32 urban communities had all 4 medications available. Availability rose to 37% (rural) and 62% (urban) in lower middle-income countries and 73% (rural) and 80% (urban) in upper middle-income countries. The 4 medications were potentially unaffordable for 60% of households in low-income countries, 33% of households in lower middle-income countries, and 25% of households in upper middle-income countries, compared with only 0.14% of households in HIC.13
Effective strategies for delivering cardiovascular medications are being developed for LMIC where health system infrastructure is underdeveloped. An important example is the SimCard study, a cluster-randomized controlled trial of 47 villages, 27 in China and 20 in India, in which community health workers screened all household members for HBP and provided a fixed-dose medication guided by a computer tablet–based decision aid. Communities receiving the intervention significantly increased the proportion of patients using BP-lowering medications and lowered SBP by an average 2.7 mm Hg (P = 0.04).14
Future Research
There are large gaps in our knowledge on the global burden of CVD. The research agenda should focus on improving surveillance using verbal autopsy, household examination surveys, and when available, electronic record data linked to vital records. Further investment is needed to expand high-quality prospective cohort studies in LMIC to track emerging CVD risks, including air pollution and poor diet. A concerted effort is needed to measure access to and quality of effective health interventions for acute and chronic CVD, including emergency care for cardiac arrest, MI, and stroke. Both LMIC governments working to develop their health systems and HIC governments providing development assistance will need to increase expenditures related to CVD and improve coordination and sharing of best practices related to noncommunicable diseases.
REFERENCES
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Accessed August 30, 2016];Sex Differences in Stroke Incidence, Prevalence, Mortality and DALYs: Results from the Global Burden of Disease Study. 2013 doi: 10.1159/000441103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632242/ [DOI] [PMC free article] [PubMed]
- 3.Institute for Health Metrics and Evaluation (IHME) GBD Compare Data Visualization. Seattle, WA: University of Washington; 2013. [Accessed July 19, 2016]. Global Burden of Disease Data Visualizations. GBD Compare. Global, deaths, both sexes, all ages, 2013. http://vizhub.healthdata.org/gbd-compare/ [Google Scholar]
- 4.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 5.Institute for Health Metrics and Evaluation (IHME) GBD Compare Data Visualization. Seattle, WA: University of Washington; 2015. [Accessed April 29, 2016]. Global Burden of Disease Data Visualizations. GBD Compare. Global, deaths, both sexes, all ages, 2015. http://vizhub.healthdata.org/gbd-compare. [Google Scholar]
- 6.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker-Collo S, Bennett DA, Krishnamurthi RV, Parmar P, Feigin VL, Naghavi M, Forouzanfar MH, Johnson CO, Nguyen G, Mensah GA, Vos T, Murray CJ, Roth GA GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group. Sex differences in stroke incidence, prevalence, mortality and disability-adjusted life years: results from the Global Burden of Disease Study 2013. Neuroepidemiology. 2015;45:203–214. doi: 10.1159/000441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 9.Huffman MD, Rao KD, Pichon-Riviere A, Zhao D, Harikrishnan S, Ramaiya K, Ajay VS, Goenka S, Calcagno JI, Caporale JE, Niu S, Li Y, Liu J, Thankappan KR, Daivadanam M, van Esch J, Murphy A, Moran AE, Gaziano TA, Suhrcke M, Reddy KS, Leeder S, Prabhakaran D. A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. PLoS One. 2011;6:e20821. doi: 10.1371/journal.pone.0020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C The Global Economic Burden of Non-communicable Diseases. Geneva, Switzerland: World Economic Forum; 2011. [Accessed April 29, 2016]. http://www.weforum.org/reports/global-economic-burden-non-communicable-diseases. [Google Scholar]
- 11.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mensah GA, Roth GA, Sampson UK, Moran AE, Feigin VL, Forouzanfar MH, Naghavi M, Murray CJ GBD 2013 Mortality and Causes of Death Collaborators. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Africa. 2015;26(suppl 1):S6–10. doi: 10.5830/CVJA-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatib R, McKee M, Shannon H, Chow C, Rangarajan S, Teo K, Wei L, Mony P, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear SA, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Kazmi K, Rahman O, Rosengren A, Monsef N, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Temizhan A, Dagenais G, Gafni A, Yusuf S PURE Study Investigators. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387:61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 14.Tian M, Ajay VS, Dunzhu D, Hameed SS, Li X, Liu Z, Li C, Chen H, Cho K, Li R, Zhao X, Jindal D, Rawal I, Ali MK, Peterson ED, Ji J, Amarchand R, Krishnan A, Tandon N, Xu LQ, Wu Y, Prabhakaran D, Yan LL. A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard Trial) in Rural Tibet, China, and Haryana, India. Circulation. 2015;132:815–824. doi: 10.1161/CIRCULATIONAHA.115.015373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feigin VL, Mensah GA, Norrving B, Murray CJ, Roth GA GBD 2013 Stroke Panel Experts Group. Atlas of the Global Burden of Stroke (1990–2013): the GBD 2013 Study. Neuroepidemiology. 2015;45:230–236. doi: 10.1159/000441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
16. CONGENITAL CARDIOVASCULAR DEFECTS AND KAWASAKI DISEASE
ICD-9 745 to 747, ICD-10 Q20 to Q28. See Tables 16-1 through 16-4 and Charts 16-1 through 16-5
Table 16-1.
Congenital Cardiovascular Defects
| Population Group | Estimated Prevalence, 2002, All Ages | Mortality, 2014, All Ages* | Hospital Discharges, 2010, All Ages |
|---|---|---|---|
| Both sexes | 650 000 to 1.3 million15 | 2921 | 62 000 |
| Males | … | 1603 (54.9%)† | 38 000 |
| Females | … | 1318 (45.1%)† | 24 000 |
| NH white males | … | 989 | … |
| NH white females | … | 783 | … |
| NH black males | … | 259 | … |
| NH black females | … | 230 | … |
| Hispanic males | … | 277 | … |
| Hispanic females | … | 233 | … |
| NH Asian or Pacific Islander males | … | 51 | … |
| NH Asian or Pacific Islander females | … | 48 | … |
| NH American Indian or Alaska Native | … | 36 | … |
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for Hispanic, NH American Indian or Alaska Native, and NH Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total congenital cardiovascular mortality that is for males vs females.
Sources: Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death—United States. These data represent underlying cause of death only. Hospital discharges: National Hospital Discharge Survey, National Center for Health Statistics; data include those inpatients discharged alive, dead, or status unknown.
Table 16-4.
Surgery for Congenital Heart Disease
| Sample | Population, Weighted | |
|---|---|---|
| Surgery for congenital heart disease, n | 14 888 | 25 831 |
| Deaths, n | 736 | 1253 |
| Mortality rate, % | 4.9 | 4.8 |
| By sex (81 missing in sample) | ||
| Male, n | 8127 | 14 109 |
| Deaths, n | 420 | 714 |
| Mortality rate, % | 5.2 | 5.1 |
| Female, n | 6680 | 11 592 |
| Deaths, n | 315 | 539 |
| Mortality rate, % | 4.7 | 4.6 |
| By type of surgery | ||
| ASD secundum surgery, n | 834 | 1448 |
| Deaths, n | 3 | 6 |
| Mortality rate, % | 0.4 | 0.4 |
| Norwood procedure for HLHS, n | 161 | 286 |
| Deaths, n | 42 | 72 |
| Mortality rate, % | 26.1 | 25.2 |
In 2003, 25 000 cardiovascular operations for congenital cardiovascular defects were performed on children <20 years of age. Inpatient mortality rate after all types of cardiac surgery was 4.8%. Nevertheless, mortality risk varies substantially for different defect types, from 0.4% for ASD repair to 25.2% for first-stage palliation for HLHS. Fifty-five percent of operations were performed in males. In unadjusted analysis, mortality after cardiac surgery was somewhat higher for males than for females (5.1% vs 4.6%). ASD indicates atrial septal defect; and HLHS, hypoplastic left heart syndrome.
Source: Data derived from Ma et al.118
Chart 16-1. Trends in age-adjusted death rates attributable to congenital heart defects, 1999 to 2014.
Source: National Center for Health Statistics, National Vital Statistics System.
Chart 16-5. Age-adjusted death rates attributable to congenital cardiovascular defects, by sex and race/ethnicity, 2014.
NH indicates non-Hispanic.
Source: National Center for Health Statistics, National Vital Statistics System.
Congenital cardiovascular defects, also known as congenital heart defects, are structural problems that arise from abnormal formation of the heart or major blood vessels. ICD-9 lists 25 congenital heart defect codes, of which 21 designate specific anatomic or hemodynamic lesions; however, there are many more lesions that are not well described by ICD-9 or ICD-10 codes because of the wide diversity of congenital heart malformations. Defects range in severity from tiny pinholes between chambers that can resolve spontaneously to major malformations that can require multiple surgical procedures before school age and can result in death in utero, in infancy, or in childhood. As such, congenital heart defects are serious and common conditions that have a significant impact on morbidity, mortality, and healthcare costs in children and in adults.1 Some types of congenital heart defects are associated with diminished quality of life,2 on par with what is seen in other chronic pediatric health conditions,3 as well as deficits in cognitive functioning4 and neurodevelopmental outcomes.5 Health outcomes are improving for congenital cardiovascular defects and survival is increasing, leading to a population shift toward adulthood, which means there are many more adults with both congenital heart defects and adult medical diagnoses,6 adding to the complexity of their management7,8 and emphasizing the need for coordinated care by an adult congenital heart defects specialist.9
Abbreviations Used in Chapter 16
| AHA | American Heart Association |
| ASD | atrial septal defect |
| AV | atrioventricular |
| CABG | coronary artery bypass graft |
| CDC | Centers for Disease Control and Prevention |
| CI | confidence interval |
| DM | diabetes mellitus |
| HCUP | Healthcare Cost and Utilization Project |
| HD | heart disease |
| HLHS | hypoplastic left heart syndrome |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| KD | Kawasaki disease |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHIS | National Health Interview Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| OR | odds ratio |
| RR | relative risk |
| RV | right ventricle |
| STS | Society of Thoracic Surgeons |
| TGA | transposition of the great arteries |
| TOF | tetralogy of Fallot |
| UI | uncertainty interval |
| VSD | ventricular septal defect |
Prevalence
(See Tables 16-1 through 16-3)
Table 16-3.
Estimated Prevalence of Congenital Cardiovascular Defects and Percent Distribution by Type, United States, 2002* (in Thousands)
| Type | Prevalence, n | Percent of Total | ||||
|---|---|---|---|---|---|---|
| Total | Children | Adults | Total | Children | Adults | |
| Total | 994 | 463 | 526 | 100 | 100 | 100 |
| VSD† | 199 | 93 | 106 | 20.1 | 20.1 | 20.1 |
| ASD | 187 | 78 | 109 | 18.8 | 16.8 | 20.6 |
| Patent ductus arteriosus | 144 | 58 | 86 | 14.2 | 12.4 | 16.3 |
| Valvular pulmonic stenosis | 134 | 58 | 76 | 13.5 | 12.6 | 14.4 |
| Coarctation of aorta | 76 | 31 | 44 | 7.6 | 6.8 | 8.4 |
| Valvular aortic stenosis | 54 | 25 | 28 | 5.4 | 5.5 | 5.2 |
| TOF | 61 | 32 | 28 | 6.1 | 7 | 5.4 |
| AV septal defect | 31 | 18 | 13 | 3.1 | 3.9 | 2.5 |
| TGA | 26 | 17 | 9 | 2.6 | 3.6 | 1.8 |
| Hypoplastic right heart syndrome | 22 | 12 | 10 | 2.2 | 2.5 | 1.9 |
| Double-outlet RV | 9 | 9 | 0 | 0.9 | 1.9 | 0.1 |
| Single ventricle | 8 | 6 | 2 | 0.8 | 1.4 | 0.3 |
| Anomalous pulmonary venous | 9 | 5 | 3 | 0.9 | 1.2 | 0.6 |
| connection | ||||||
| Truncus arteriosus | 9 | 6 | 2 | 0.7 | 1.3 | 0.5 |
| HLHS | 3 | 3 | 0 | 0.3 | 0.7 | 0 |
| Other | 22 | 12 | 10 | 2.1 | 2.6 | 1.9 |
Average of the low and high estimates, two thirds from low estimate.15 ASD indicates atrial septal defect; AV, atrioventricular; HLHS, hypoplastic left heart syndrome; RV, right ventricle; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; and VSD, ventricular septal defect.
Excludes an estimated 3 million bicuspid aortic valve prevalence (2 million in adults and 1 million in children).
Small VSD, 117 000 (65 000 adults and 52 000 children); large VSD, 82 000 (41 000 adults and 41 000 children).
Source: Data derived from Hoffman et al.15
The population with congenital heart defects has grown substantially over the past several decades, which is related to better surgical outcomes and improved medical management; this has led to an aging of the congenital heart defects population.10 The 32nd Bethesda Conference estimated that the total number of adults living with congenital heart defects in the United States in 2000 was 800 000.1,11 In 2002, the estimated prevalence of congenital cardiovascular defects was 650 000 to 1.3 million in all age groups (Table 16-1). The annual birth prevalence of congenital cardiovascular defects range from 2.4 to 13.7 per 1000 live births (Table 16-2). In the United States, 1 in 150 adults is expected to have some form of congenital heart defect, including mild phenotypes such as bicuspid aortic valve as well as more severe disease.7 The estimated prevalence of congenital heart defects ranges from 2.5% for hypoplastic right heart syndrome to 20.1% for VSD in children and from 1.8% for TGA to 20.1% for VSD in adults (Table 16-3). In population data from Canada, the measured prevalence of congenital heart defects in the general population was 11.89 per 1000 children and 4.09 per 1000 adults in the year 2000.12 Extrapolated to the US population in the same year, this yields published estimates of 859 000 children and 850 000 adults for the year 2000.13 The expected growth rates of the congenital heart defects population vary from 1% to 5% per year depending on age and the distribution of lesions.14
Table 16-2.
| Type of Presentation | Rate per 1000 Live Births | Estimated Number (Variable With Yearly Birth Rate) |
|---|---|---|
| Fetal loss | Unknown | Unknown |
| Invasive procedure during the first year | 2.4 | 9200 |
| Detected during first year* | 8 | 36 000 |
| Bicuspid aortic valve | 13.7 | 54 800 |
Includes stillbirths and pregnancy termination at <20 weeks’ gestation; includes some defects that resolve spontaneously or do not require treatment.
Estimates of the distribution of lesions in the congenital heart defects population using available data vary with assumptions made. If all those born with congenital heart defects between 1940 and 2002 were treated, there would be 750 000 survivors with simple lesions, 400 000 with moderate lesions, and 180 000 with complex lesions; in addition, there would be 3.0 million people alive with bicuspid aortic valves.15 Without treatment, the number of survivors in each group would be 400 000, 220 000, and 30 000, respectively. The actual numbers surviving were projected to be between these 2 sets of estimates as of 1 decade ago.15 According to measurements from population data in Canada, the prevalence of severe forms of congenital heart defects increased 85% in adults and 22% in children from 1985 to 2000.12 The most common types of defects in children are VSD, 620 000 people; ASD, 235 000 people; valvar pulmonary stenosis, 185 000 people; and patent ductus arteriosus, 173 000 people.15 The most common lesions seen in adults are ASD and TOF.11
Limited information is available about the prevalence of congenital heart defects outside of North America and Western Europe.16 A study of 4 Chinese provinces found the prevalence of congenital HD among those from birth to 18 years of age was 1.7%.17 A population-based study in Himachal, India, showed the population prevalence was 0.6% of people.18
Incidence
The incidence of congenital heart defects in the United States is commonly reported as being between 4 and 10 per 1000, clustering around 8 per 1000 live births.19 Using “recalled” diagnoses of congenital heart defects from the NHIS combined with other national databases (US Census, National Vital Statistics System, Human Mortality Database), the birth prevalence is 3.3 and 3.2 per 1000 for males and females, respectively.20 Incidence (birth prevalence) in Europe is reported as 6.9 per 1000 births; birth prevalence in Asia is reported as 9.3 per 1000.16,20 The overall birth prevalence of congenital heart defects at the Bhabha Atomic Research Centre Hospital in Mumbai from 2006 through 2011 was 13.28 per 1000 live births.21
Variations in incidence rates could be related to the age at detection; major defects can be apparent in the prenatal or neonatal period, but minor defects might not be detected until adulthood, which makes it challenging to estimate incidence and prevalence. To distinguish more serious defects, some studies report the number of new cases of sufficient severity to result in death or an invasive procedure within the first year of life, in addition to overall birth prevalence. Incidence rates are likely to increase over time because of better detection by fetal cardiac ultrasound,22 screening pulse oximetry,23 and echocardiography during infancy.
Overall Incidence
(See Table 16-2)
According to population-based data from the Metropolitan Atlanta Congenital Defects Program (Atlanta, GA), congenital heart defects occurred in 1 of every 111 to 125 births (live, still, or >20 weeks’ gestation) from 1995 to 1997 and from 1998 to 2005. Some defects showed variations by sex and racial distribution.24
According to population-based data from Alberta, Canada, there was a total prevalence of 12.42 per 1000 total births (live, still, or >20 weeks’ gestation).25
An estimated minimum of 40 000 infants are expected to be affected by congenital heart defects each year in the United States. Of these, ≈25%, or 2.4 per 1000 live births, require invasive treatment in the first year of life (Table 16-2).
Incidence of Specific Defects
The National Birth Defects Prevention Network for 13 states in the United States from 2004 to 2006 showed the average prevalence of 21 selected major birth defects. These data indicated that there are >6100 estimated annual cases of 5 cardiovascular defects: truncus arteriosus (0.07/1000 births), TGA (0.3/1000 births), TOF (0.4/1000 births), AV septal defect (0.47/1000 births), and HLHS (0.23/1000 births).27
Metropolitan Atlanta Congenital Defects Program data for specific defects at birth showed the following: VSD, 4.2/1000 births; ASD, 1.3/1000 births; valvar pulmonic stenosis, 0.6/1000 births; TOF, 0.5/1000 births; aortic coarctation, 0.4/1000 births; AV septal defect, 0.4/1000 births; and TGA (0.2/1000 births).13,24
Bicuspid aortic valve occurs in 13.7 per 1000 people; these defects might not require treatment in infancy but can cause problems later in adulthood.14
Mortality
(See Tables 16-1 and 16-4 and Charts 16-1 through 16-5)
-
Overall mortality attributable to congenital heart defects:
-
In 201428:
Mortality related to congenital cardiovascular defects was 2921 deaths (Table 16-1). Any-mention mortality related to congenital cardiovascular defects was 4674 deaths.
Congenital cardiovascular defects (ICD-10 Q20–Q28) were the most common cause of infant deaths resulting from birth defects (ICD-10 Q00–Q99); 23.5% of infants who died of a birth defect had a heart defect (ICD-10 Q20-Q24).
The age-adjusted death rate (deaths per 100 000 people) attributable to congenital cardiovascular defects was 0.9 (Chart 16-1).
-
-
Congenital heart defect–related mortality varies substantially by age, with infants showing the highest mortality rates from 1999 to 2014 (Chart 16-4).
According to a review of Norwegian national mortality data in live-born children with congenital HD from 1994 to 2009, the all-cause mortality rate was 17.4% for children with severe congenital heart defects and 3.0% for children with nonsevere congenital heart defects, with declining mortality rates over the analysis period related to declining operative mortality and more frequent pregnancy terminations.29
Death rates attributed to congenital heart defects decrease as gestational age advances toward 40 weeks.30 In-hospital mortality of infants with major congenital heart defects is independently associated with late-preterm birth (OR, 2.70; 95% CI, 1.69–4.33) compared with delivery at later gestational ages.31 Similarly, postoperative mortality of infants with congenital heart defects born near term (37 weeks) is 1.34 (1.05–1.71; P=0.02) higher than those born full term,32,33 with higher complication rates and longer length of stay. The presence of congenital heart defects substantially increases mortality of very low-birth-weight infants; in a study of very low-birth-weight infants, the mortality rate with serious congenital heart defects was 44% compared with 12.7% in very low-birth-weight infants without serious congenital heart defects.34
Analysis of the STS Congenital Heart Surgery Database, a voluntary registry with self-reported data for a 4-year cycle (2011–2014) from 116 centers performing congenital heart defects surgery (112 based in 40 US states, 3 in Canada, and 1 in Turkey),35 showed that of 97 996 total patients who underwent an operation, the aggregate hospital discharge mortality rate was 3.3%.36 The mortality rate was 9.2% for neonates (0–30 days of age),37 2.9% for infants (31 days to 1 year of age),38 1.1% for children (>1 year to 18 years of age),39 and 1.9% for adults (>18 years of age).40
The Japan Congenital Cardiovascular Surgery Database reported similar surgical outcomes for congenital HD from 28 810 patients operated on between 2008 and 2012, with 2.3% and 3.5% mortality at 30 and 90 days, respectively.41
-
Among adults with congenital HD, mortality rates are greater than the general population.
In population-based data from Canada, 8123 deaths occurred among 71 686 patients with congenital heart defects followed up for nearly 1 million patient-years.7
Among 12 644 adults with congenital HD followed up at a single Canadian center from 1980 to 2009, 308 patients in the study cohorts (19%) died.42
In 2007, 189 000 life-years were lost before 55 years of age because of deaths attributable to congenital cardiovascular defects. This is almost as many life-years as were lost from leukemia and asthma combined (NHLBI tabulation of NCHS mortality data).
-
Congenital cardiovascular defect mortality varies by race/ethnicity and sex (Charts 16-2 and 16-3).
From 1999 to 2014, there was a downward decline in the age-adjusted death rates attributable to congenital heart defects in black, white, and Hispanic people (Chart 16-2), in both males and females (Chart 16-3), and in age groups 1 to 4 years, 5 to 14 years, 5 to 24 years, and ≥25 years (Chart 16-4) in the United States.
The US 2014 age-adjusted death rate (deaths per 100 000 people) attributable to congenital cardiovascular defects was 1.1 for non-His-panic white males, 1.3 for non-Hispanic black males, 0.8 for Hispanic males, 0.8 for non-Hispanic white females, 1.1 for non-Hispanic black females, and 0.7 for Hispanic females. Infant (<1 year of age) mortality rates were 30.1 for non-Hispanic white infants, 39.6 for non-Hispanic black infants, and 32.2 for His-panic infants28 (Chart 16-5).
Mortality after congenital heart surgery also differs between races/ethnicity, even after adjustment for access to care. The risk of in-hospital mortality for minority patients compared with white patients is 1.22 (95% CI, 1.05–1.41) for Hispanics, 1.27 (95% CI, 1.09–1.47) for non-Hispanic blacks, and 1.56 (95% CI, 1.37–1.78) for other non-Hispanic people.43 Similarly, another study found that a higher risk of in-hospital mortality was associated with nonwhite race (OR, 1.36; 95% CI, 1.19–1.54) and Medicaid insurance (OR, 1.26; 95% CI, 1.09–1.46).44 One center’s experience suggested race was independently associated with neonatal surgical outcomes only in patients with less complex congenital heart defects.45
The population-weighted mortality rate for surgery for congenital HD is slightly higher in males (5.1%) than females (4.6%) <20 years old (Table 16-4).
Data from the HCUP’s Kids’ Inpatient Database from 2000, 2003, and 2006 show male children had more congenital heart defects surgeries in infancy, more high-risk surgeries, and more procedures to correct multiple cardiac defects. Female infants with high-risk congenital heart defects had a 39% higher adjusted mortality than males.38,46 According to CDC multiple-cause death data from 1999 to 2006, sex differences in mortality over time varied with age. Between the ages of 18 and 34 years, mortality over time decreased significantly in females but not in males.47
-
Congenital heart defect mortality is declining.
In studies that examined trends since 1979, age-adjusted death rates declined 22% for critical congenital heart defects48 and 39% for all congenital heart defects,49 and deaths tended to occur at progressively older ages. CDC mortality data from 1979 to 2005 showed all-age death rates had declined by 60% for VSD and 40% for TOF.50 Population-based data from Canada showed overall mortality decreased by 31% and the median age of death increased from 2 to 23 years between 1987 and 2005.7
Further analysis of the Kids’ Inpatient Database from 2000 to 2009 showed a decrease in HLHS stage 3 mortality by 14% and a decrease in stage 1 mortality by 6%.51 Surgical interventions are the primary treatment for reducing mortality. A Pediatric Heart Network study of 15 North American centers revealed that even in lesions associated with the highest mortality, such as HLHS, aggressive palliation can lead to an increase in the 12-month survival rate, from 64% to 74%.52 Surgical interventions are common in adults with congenital heart defects. Mortality rates for 12 congenital heart defect procedures were examined with data from 1988 to 2003 reported in the NIS. A total of 30 250 operations were identified, which yielded a national estimate of 152 277±7875 operations. Of these, 27% were performed in patients ≥18 years of age. The overall inhospital mortality rate for adult patients with congenital heart defects was 4.71% (95% CI, 4.19%–5.23%), with a significant reduction in mortality observed when surgery was performed on such adult patients by pediatric versus nonpediatric heart surgeons (1.87% versus 4.84%; P<0.0001).53 For adults with congenital heart defects, specialist care is a key determinant of mortality and morbidity. In a single-center report of 4461 adult patients with congenital heart defects with 48 828 patient-years of follow-up, missed appointments and delay in care were predictors of mortality.54
Chart 16-4. Trends in age-adjusted death rates attributable to congenital cardiovascular defects by age at death, 1999 to 2014.
Source: National Center for Health Statistics, National Vital Statistics System.
Chart 16-2. Trends in age-adjusted death rates attributable to congenital cardiovascular defects by race/ethnicity, 1999 to 2014.
NH indicates non-Hispanic.
Source: National Center for Health Statistics, National Vital Statistics System.
Chart 16-3. Trends in age-adjusted death rates attributable to congenital cardiovascular defects by sex, 1999 to 2014.
Source: National Center for Health Statistics, National Vital Statistics System.
Hospitalizations
(See Table 16-1)
In 2004, birth defects accounted for >139 000 hospitalizations, representing 47.4 stays per 100 000 people. Cardiac and circulatory congenital anomalies accounted for 34% of all hospital stays for birth defects. Between 1997 and 2004, hospitalization rates increased by 28.5% for cardiac and circulatory congenital anomalies.55
In 2010, the total number of hospital discharges for congenital heart defects for all ages was 62 000 (Table 16-1).
Hospitalization of infants with congenital heart defects is common; one third of patients with congenital heart defects require hospitalization during infancy,56,57 often in an intensive care unit.
Although the most common congenital heart defect lesions were shunts, including patent ductus arteriosus, VSDs, and ASDs, TOF accounted for a higher proportion of in-hospital death than any other birth defect.
Cost
-
Among pediatric hospitalizations (age 0–20 years) in the HCUP 2012 Kids’ Inpatient Database58:
Pediatric hospitalizations with congenital heart defects (4.4% of total pediatric hospitalizations) accounted for $6.6 billion in hospitalization spending (23% of total pediatric hospitalization costs).
26.7% of all congenital heart defect costs were attributed to critical congenital heart defects, with the highest costs attributable to HLHS, coarctation of the aorta, and TOF.
Median hospital cost was $51 302 ($32 088–$100 058) in children who underwent cardiac surgery, $21 920 ($13 068–$51 609) in children who underwent cardiac catheterization, $4134 ($1771–$10 253) in children who underwent noncardiac surgery, and $23 062 ($5529–$71 887) in children admitted for medical treatments. These data are presented as median and interquartile range.
The mean cost of congenital heart defects was higher in infancy ($36 601) than in older ages and in those with critical congenital heart defects ($52 899).
Other studies confirm the high cost of HLHS. An analysis of 1941 neonates with HLHS showed a median cost of $99 070 for stage 1 palliation (Norwood or Sano procedure), $35 674 for stage 2 palliation (Glenn procedure), $36 928 for stage 3 palliation (Fontan procedure), and $289 292 for transplantation.59
Other congenital heart defect lesions are less costly. In 2124 patients undergoing congenital heart operations between 2001 and 2007, total costs for the surgeries were $12 761 (ASD repair), $18 834 (VSD repair), $28 223 (TOF repair), and $55 430 (arterial switch operation).60
Risk Factors
Numerous intrinsic and extrinsic nongenetic risk factors contribute to congenital heart defects.61
Intrinsic risk factors for congenital heart defects include various genetic syndromes. Twins are at higher risk for congenital heart defects62; one report from Kaiser Permanente data showed monochorionic twins were at particular risk (RR, 11.6; CI, 9.2–14.5).63 Known risks generally focus on maternal exposures, but a study of paternal occupational exposure documented a higher incidence of congenital heart defects with paternal exposure to phthalates.64
Other paternal exposures that increase risk for congenital heart defects include paternal anesthesia, which has been implicated in TOF (3.6%); sympathomimetic medication and coarctation of the aorta (5.8%); pesticides and VSDs (5.5%); and solvents and HLHS (4.6%).65
Known maternal risks include maternal smoking66,67 during the first trimester of pregnancy, which has also been associated with a ≥30% increased risk of the following lesions in the fetus: ASD, pulmonary valvar stenosis, truncus arteriosus, TGA,68 and septal defects (particularly for heavy smokers [≥25 cigarettes daily]).69 Maternal smoking may account for 1.4% of all congenital heart defects.
Exposure to secondhand smoke has also been implicated as a risk factor.70
Air pollutants might also increase the risk of congenital HD. In a retrospective review of singleton infants born in Florida from 2000 to 2009, maternal exposure during pregnancy to the air pollutant benzene was associated with an increased risk in the fetus of critical and noncritical congenital heart defects (1.33; 95% CI, 1.07–1.65), including conotruncal defects.71
Maternal binge drinking72 is also associated with an increased risk of congenital cardiac defects, and the combination of binge drinking and smoking may be particularly dangerous: Mothers who smoke and report any binge drinking in the 3 months before pregnancy are at an increased risk of giving birth to a child with congenital heart defects (adjusted OR, 12.65).72
Maternal obesity is also associated with congenital heart defects. A meta-analysis of 14 studies of females without gestational DM showed infants born to mothers who were moderately and severely obese, respectively, had 1.1 and 1.4 times greater risk of congenital heart defects than infants born to normal-weight mothers.73–75 The risk of TOF was 1.9 times higher among infants born to mothers with severe obesity than among infants born to normal-weight mothers.74
Maternal DM, including gestational DM, has also been associated with cardiac defects, both isolated and multiple.76,77 Pre-DM is also associated with congenital heart defects, specifically TOF.78
Preeclampsia is also a risk factor for congenital heart defects, although not critical defects.79
Folate deficiency is a well-accepted risk for congenital defects, including congenital heart defects, and folic acid supplementation is recommended during pregnancy.61 An observational study of folic acid supplementation in Hungarian females showed a decrease in the incidence of congenital heart defects including VSD (OR, 0.57; 95% CI; 0.45–0.73), TOF (OR, 0.53; 95% CI, 0.17–0.94), d-TGA (OR, 0.47; 95% CI, 0.26–0.86), and ASD secundum (OR, 0.63; 95% CI, 0.40–0.98).80 A US population-based case-control study showed an inverse relationship between folic acid use and the risk of TGA (Baltimore-Washington Infant Study, 1981–1989).81
An observational study from Quebec, Canada, of 1.3 million births from 1990 to 2005 found a 6% per year reduction in severe congenital heart defects using a time-trend analysis before and after public health measures were instituted that mandated folic acid fortification of grain and flour products in Canada.82
Maternal infections, including rubella and chlamydia, have been associated with congenital heart defects.83,84
High altitude has also been described as a risk factor for congenital heart defects; Tibetan children living at 4200 to 4900 m had a higher prevalence of congenital heart defects (12.09 per 1000) than those living at lower altitudes of 3500 to 4100 m; patent ductus arteriosus and ASD contributed to the increased prevalence.85
Screening
Pulse oximetry screening for critical congenital heart defects, a group of defects that cause severe and life-threatening symptoms and require intervention within the first days or first year of life, was recommended by the US Department of Health and Human Services on October 15, 2010,86 was incorporated as part of the US recommended uniform screening panel for newborns in 2011, and has been endorsed by the AHA and the American Academy of Pediatrics.87 The recommendation has been controversial, yet several studies demonstrated benefit.88–90
Several key factors contribute to effective screening, including probe placement (postductal), oximetry cutoff (<95%), timing (>24 hours of life), and altitude (<2643 ft, 806 m).
If fully implemented, screening would identify 1189 additional infants with critical congenital heart defects and would yield 1975 false-positive results.91
A simulation model estimates that screening the entire United States for congenital heart defects with pulse oximetry would uncover 875 infants (95% UI, 705–1060) who truly have nonsyndromic congenital heart defects versus 880 (UI, 700–1080) false-negative screenings (no HD).92
It has been estimated that 29.5% (95% CI, 28.1%–31.0%) of nonsyndromic children with congenital heart defects are diagnosed after 3 days and thus might benefit from pulse oximetry screening.93
A meta-analysis of 13 studies that included 229 421 newborns found pulse oximetry had a sensitivity of 76.5% (95% CI, 67.7%–83.5%) for detection of critical congenital heart defects and a specificity of 99.9% (95% CI, 99.7%–99.9%) with a false-positive rate of 0.14% (95% CI, 0.06%–0.33%).94
The cost of identifying a newborn with critical congenital heart defects has been estimated at $20 862 per newborn detected and $40 385 per life-year gained (2011 US dollars).92
Reports outside of the United States show similar performance of pulse oximetry screening in identifying critical congenital HD,95 with a sensitivity and specificity of pulse oximetry screening for critical congenital heart defects of 100% and 99.7%, respectively.
Kawasaki Disease
ICD-9 446.1; ICD-10 M30.3.
Mortality—3. Any-mention mortality—5.
KD is an acute inflammatory illness characterized by fever, rash, nonexudative limbal sparing conjunctivitis, extremity changes, red lips and strawberry tongue, and a swollen lymph node. The most feared consequence of this vasculitis is coronary artery aneurysms, which may result in coronary ischemic events in the acute period or years later.96 The cause of KD is unknown, but it may be an immune response to an acute infectious illness based in part on genetic susceptibilities.97,98 This is supported by variation in incidence related to geography, race/ethnicity, sex, age, and season.99
The incidence of KD is highest in Japan, at 239.6 cases per 100 000 children aged <4 years,100 followed by Taiwan at 164.6/100 000 in children <5 years old101 and Korea, where the rate reached 113.1/100 000 children <5 years old in 2008.102 Measured over 4 years, 2008 through 2012, the incidence of KD in Shanghai was lower, 30.3 to 71.9 per 100 000 children aged 0 to 4 years.103
KD is much less common in the United States, with an incidence of 20.8/100 000 children aged <5 years in 2006.104 The incidence of KD is even lower in Germany, affecting 7.2 of 100 000 children <5 years of age, although this low rate might be affected by lack of recognition.105
The incidence of KD is rising worldwide, including in the United States. US hospitalizations for KD rose from 17.5/100 000 children aged <5 years in 2000 to 19/100 000 children <5 years of age in 2009.106,107 Japan experienced its highest-ever incidence rate in 2010.100 In addition to geographic variation in the incidence of KD, the age of children affected can also differ. In northern Europe (Finland, Sweden, and Norway), 67.8% of patients with KD were <5 years of age, compared with 86.4% of patients in Japan (P<0.001).108
Race-specific incidence rates indicate that KD is most common among Americans of Asian and Pacific Island descent (30.3/100 000 children <5 years of age), occurs with intermediate frequency in non-Hispanic blacks (17.5/100 000 children <5 years of age) and Hispanics (15.7/100 000 children <5 years of age), and is least common in whites (12.0/100 000 children <5 years of age).104 US states with higher Asian American populations have higher rates of KD; for example, rates are 2.5-fold higher in Hawaii than in the continental United States.107
Boys have a 1.5-fold higher incidence of KD than girls.107 Although KD can be seen as late as adolescence, 76.8% of children with KD are <5 years of age.104,106,107 There are seasonal variations in KD: KD is more common during the winter and early spring months, except in Hawaii, where no clear seasonal trend is seen.109 KD can recur in 2% to 4% of children who have already experienced KD.110
Treatment of KD rests on diminishing the inflammatory response with intravenous immunoglobulin infusion, which reduces the incidence of coronary artery aneurysms from ≈25% to ≈2%. Addition of prednisolone to the standard regimen of intravenous immunoglobulin for patients with severe KD appears to result in further reductions in the incidence of coronary artery anomalies (RR, 0.20; 95% CI, 0.12–0.28),111 a result supported by a meta-analysis of steroid treatment in 9 trials that included 1011 patients with KD.112
Risk factors for coronary artery abnormalities include late diagnosis, young age (<6 months), male sex, and Asian background.96 Long-term prognosis in children with coronary artery aneurysms is predicted in part by coronary artery size at 1 month of illness, with the 10-year ischemia event-free and aneurysm persistence probability being 87.5% and 20.6%, respectively.113 Successful surgical treatment (eg, CABG) of late sequelae of symptomatic coronary artery stenoses has been described.114
REFERENCES
- 1.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–e833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 2.Fteropoulli T, Stygall J, Cullen S, Deanfield J, Newman SP. Quality of life of adult congenital heart disease patients: a systematic review of the literature. Cardiol Young. 2013;23:473–485. doi: 10.1017/S1047951112002351. [DOI] [PubMed] [Google Scholar]
- 3.Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen M, Limbers C, Marino BS Pediatric Cardiac Quality of Life Inventory Testing Study Consortium. Health-related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. 2014;164:781–788.e1. doi: 10.1016/j.jpeds.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- 5.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Jr, Li J, Smith SE, Bellinger DC, Mahle WT American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 6.Roche SL, Silversides CK. Hypertension, obesity, and coronary artery disease in the survivors of congenital heart disease. Can J Cardiol. 2013;29:841–848. doi: 10.1016/j.cjca.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 8.Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, Graham TP, Gurvitz MZ, Kovacs A, Meadows AK, Reid GJ, Reiss JG, Rosenbaum KN, Sagerman PJ, Saidi A, Schonberg R, Shah S, Tong E, Williams RG American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454–1485. doi: 10.1161/CIR.0b013e3182107c56. [DOI] [PubMed] [Google Scholar]
- 9.Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M Alliance for Adult Research in Congenital Cardiology (AARCC) and Adult Congenital Heart Association. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial) J Am Coll Cardiol. 2013;61:2180–2184. doi: 10.1016/j.jacc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ávila P, Mercier LA, Dore A, Marcotte F, Mongeon FP, Ibrahim R, Asgar A, Miro J, Andelfinger G, Mondésert B, de Guise P, Poirier N, Khairy P. Adult congenital heart disease: a growing epidemic. Can J Cardiol. 2014;30(suppl):S410–S419. doi: 10.1016/j.cjca.2014.07.749. [DOI] [PubMed] [Google Scholar]
- 11.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 12.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 13.Marelli AJ, Therrien J, Mackie AS, Ionescu-Ittu R, Pilote L. Planning the specialized care of adult congenital heart disease patients: from numbers to guidelines: an epidemiologic approach. Am Heart J. 2009;157:1–8. doi: 10.1016/j.ahj.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 16.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Yang YN, Xie X, Li XM, Ma X, Fu ZY, Chen BD, Huang Y, Shan CF, Ma YT, Gao XM. Prevalence of congenital heart disease in Xinjiang multi-ethnic region of China. PLoS One. 2015;10:e0133961. doi: 10.1371/journal.pone.0133961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhardwaj R, Kandoria A, Marwah R, Vaidya P, Singh B, Dhiman P, Sood A, Sharma A. Prevalence of congenital heart disease in rural population of Himachal: a population-based study. Indian Heart J. 2016;68:48–51. doi: 10.1016/j.ihj.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuler CO, Black GB, Jerrell JM. Population-based treated prevalence of congenital heart disease in a pediatric cohort. Pediatr Cardiol. 2013;34:606–611. doi: 10.1007/s00246-012-0505-3. [DOI] [PubMed] [Google Scholar]
- 20.Benziger CP, Stout K, Zaragoza-Macias E, Bertozzi-Villa A, Flaxman AD. Projected growth of the adult congenital heart disease population in the United States to 2050: an integrative systems modeling approach. Popul Health Metr. 2015;13:29. doi: 10.1186/s12963-015-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawant SP, Amin AS, Bhat M. Prevalence, pattern and outcome of congenital heart disease in Bhabha Atomic Research Centre Hospital, Mumbai. Indian J Pediatr. 2013;80:286–291. doi: 10.1007/s12098-012-0910-x. [DOI] [PubMed] [Google Scholar]
- 22.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 23.Koppel RI, Druschel CM, Carter T, Goldberg BE, Mehta PN, Talwar R, Bierman FZ. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111:451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 24.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedard T, Lowry RB, Sibbald B, Harder JR, Trevenen C, Horobec V, Dyck JD. Congenital heart defect case ascertainment by the Alberta Congenital Anomalies Surveillance System. Birth Defects Res A Clin Mol Teratol. 2012;94:449–458. doi: 10.1002/bdra.23007. [DOI] [PubMed] [Google Scholar]
- 26.Deleted in proof.
- 27.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A National Birth Defects Prevention Network. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed May 19, 2015];Mortality multiple cause micro-data files. 2013 public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 29.Jortveit J, Øyen N, Leirgul E, Fomina T, Tell GS, Vollset SE, Eskedal L, Døhlen G, Birkeland S, Holmstrøm H. Trends in mortality of congenital heart defects. Congenit Heart Dis. 2016;11:160–168. doi: 10.1111/chd.12307. [DOI] [PubMed] [Google Scholar]
- 30.Cnota JF, Gupta R, Michelfelder EC, Ittenbach RF. Congenital heart disease infant death rates decrease as gestational age advances from 34 to 40 weeks. J Pediatr. 2011;159:761–765. doi: 10.1016/j.jpeds.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Swenson AW, Dechert RE, Schumacher RE, Attar MA. The effect of late preterm birth on mortality of infants with major congenital heart defects. J Perinatol. 2012;32:51–54. doi: 10.1038/jp.2011.50. [DOI] [PubMed] [Google Scholar]
- 32.Costello JM, Pasquali SK, Jacobs JP, He X, Hill KD, Cooper DS, Backer CL, Jacobs ML. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129:2511–2517. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, Bacha EA, Allan CK, Cohen JN, Laussen PC. Birth before 39 weeks’ gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:277–284. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 34.Archer JM, Yeager SB, Kenny MJ, Soll RF, Horbar JD. Distribution of and mortality from serious congenital heart disease in very low birth weight infants. Pediatrics. 2011;127:293–299. doi: 10.1542/peds.2010-0418. [DOI] [PubMed] [Google Scholar]
- 35.Society of Thoracic Surgeons. The Society of Thoracic Surgeons (STS) national database: Congenital Heart Surgery Database participants. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/congenitalMap_2.pdf.
- 36.Society of Thoracic Surgeons. STS Congenital Heart Surgery Executive Summary: all patients: STS period ending 12/31/2014. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_AllPatients_0.pdf.
- 37.Society of Thoracic Surgeons. STS Congenital Heart Surgery Executive Summary: neonates: STS period ending 12/31/201. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_Neonates_0.pdf.
- 38.Society of Thoracic Surgeons. STS Congenital Heart Surgery Executive Summary: Infants: STS period ending 12/31/2014. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_Infants_0.pdf.
- 39.Society of Thoracic Surgeons. STS Congenital Heart Surgery Executive Summary: Children: STS period ending 12/31/2014. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_Children_0.pdf.
- 40.Society of Thoracic Surgeons. STS Congenital Heart Surgery Executive Summary: Adults: STS period ending 12/31/2014. [Accessed July 8, 2015];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_Adults_0.pdf.
- 41.Hoashi T, Miyata H, Murakami A, Hirata Y, Hirose K, Matsumura G, Ichikawa H, Sawa Y, Takamoto S. The current trends of mortality following congenital heart surgery: the Japan Congenital Cardiovascular Surgery Database. Interact Cardiovasc Thorac Surg. 2015;21:151–156. doi: 10.1093/icvts/ivv109. [DOI] [PubMed] [Google Scholar]
- 42.Greutmann M, Tobler D, Kovacs AH, Greutmann-Yantiri M, Haile SR, Held L, Ivanov J, Williams WG, Oechslin EN, Silversides CK, Colman JM. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117–127. doi: 10.1111/chd.12201. [DOI] [PubMed] [Google Scholar]
- 43.Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post-operative mortality following congenital heart surgery. J Pediatr. 2011;159:222–226. doi: 10.1016/j.jpeds.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 44.Chan T, Pinto NM, Bratton SL. Racial and insurance disparities in hospital mortality for children undergoing congenital heart surgery. Pediatr Cardiol. 2012;33:1026–1039. doi: 10.1007/s00246-012-0221-z. [DOI] [PubMed] [Google Scholar]
- 45.Lasa JJ, Cohen MS, Wernovsky G, Pinto NM. Is race associated with morbidity and mortality after hospital discharge among neonates undergoing heart surgery? Pediatr Cardiol. 2013;34:415–423. doi: 10.1007/s00246-012-0475-5. [DOI] [PubMed] [Google Scholar]
- 46.Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population-based study. Circulation. 2010;122(suppl):S234–S240. doi: 10.1161/CIRCULA-TIONAHA.109.928325. [DOI] [PubMed] [Google Scholar]
- 47.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 50.Pillutla P, Shetty KD, Foster E. Mortality associated with adult congenital heart disease: trends in the US population from 1979 to 2005. Am Heart J. 2009;158:874–879. doi: 10.1016/j.ahj.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Czosek RJ, Anderson JB, Heaton PC, Cassedy A, Schnell B, Cnota JF. Staged palliation of hypoplastic left heart syndrome: trends in mortality, cost, and length of stay using a national database from 2000 through 2009. Am J Cardiol. 2013;111:1792–1799. doi: 10.1016/j.amjcard.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 52.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW Pediatric Heart Network Investigators. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karamlou T, Diggs BS, Person T, Ungerleider RM, Welke KF. National practice patterns for management of adult congenital heart disease: operation by pediatric heart surgeons decreases in-hospital death. Circulation. 2008;118:2345–2352. doi: 10.1161/CIRCULATIONAHA.108.776963. [DOI] [PubMed] [Google Scholar]
- 54.Kempny A, Diller GP, Dimopoulos K, Alonso-Gonzalez R, Uebing A, Li W, Babu-Narayan S, Swan L, Wort SJ, Gatzoulis MA. Determinants of outpatient clinic attendance amongst adults with congenital heart disease and outcome. Int J Cardiol. 2016;203:245–250. doi: 10.1016/j.ijcard.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 55.Russo C, Elixhauser A. Hospitalizations for Birth Defects, 2004. Rockville, MD: US Agency for Healthcare Research and Quality; Jan, 2007. [Accessed July 18, 2011]. HCUP Statistical Brief No. 24. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb24.pdf. [PubMed] [Google Scholar]
- 56.Dorfman AT, Marino BS, Wernovsky G, Tabbutt S, Ravishankar C, Godinez RI, Priestley M, Dodds KM, Rychik J, Gruber PJ, Gaynor JW, Levy RJ, Nicolson SC, Montenegro LM, Spray TL, Dominguez TE. Critical heart disease in the neonate: presentation and outcome at a tertiary care center. Pediatr Crit Care Med. 2008;9:193–202. doi: 10.1097/PCC.0b013e318166eda5. [DOI] [PubMed] [Google Scholar]
- 57.Marino BS, Bird GL, Wernovsky G. Diagnosis and management of the newborn with suspected congenital heart disease. Clin Perinatol. 2001;28:91–136. doi: 10.1016/s0095-5108(05)70071-3. [DOI] [PubMed] [Google Scholar]
- 58.Faraoni D, Nasr VG, DiNardo JA. Overall hospital cost estimates in children with congenital heart disease: analysis of the 2012 Kid’s Inpatient Database. Pediatr Cardiol. 2016;37:37–43. doi: 10.1007/s00246-015-1235-0. [DOI] [PubMed] [Google Scholar]
- 59.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatrics. 2011;128:e1181–e1186. doi: 10.1542/peds.2010-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasquali SK, Sun JL, d’Almada P, Jaquiss RD, Lodge AJ, Miller N, Kemper AR, Lannon CM, Li JS. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;4:306–312. doi: 10.1161/CIRCOUTCOMES.110.958959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL American Heart Association Council on Cardiovascular Disease in the Young. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULA-TIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 62.Herskind AM, Almind Pedersen D, Christensen K. Increased prevalence of congenital heart defects in monozygotic and dizygotic twins. Circulation. 2013;128:1182–1188. doi: 10.1161/CIRCU-LATIONAHA.113.002453. [DOI] [PubMed] [Google Scholar]
- 63.Pettit KE, Merchant M, Machin GA, Tacy TA, Norton ME. Congenital heart defects in a large, unselected cohort of monochorionic twins. J Perinatol. 2013;33:457–461. doi: 10.1038/jp.2012.145. [DOI] [PubMed] [Google Scholar]
- 64.Snijder CA, Vlot IJ, Burdorf A, Obermann-Borst SA, Helbing WA, Wildhagen MF, Steegers EA, Steegers-Theunissen RP. Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod. 2012;27:1510–1517. doi: 10.1093/humrep/des043. [DOI] [PubMed] [Google Scholar]
- 65.Wilson PD, Loffredo CA, Correa-Villaseñor A, Ferencz C. Attributable fraction for cardiac malformations. Am J Epidemiol. 1998;148:414–423. doi: 10.1093/oxfordjournals.aje.a009666. [DOI] [PubMed] [Google Scholar]
- 66.Lee LJ, Lupo PJ. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: a systematic review and metaanalysis. Pediatr Cardiol. 2013;34:398–407. doi: 10.1007/s00246-012-0470-x. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan PM, Dervan LA, Reiger S, Buddhe S, Schwartz SM. Risk of congenital heart defects in the offspring of smoking mothers: a population-based study. J Pediatr. 2015;166:978–984.e2. doi: 10.1016/j.jpeds.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 68.Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011;127:e647–e653. doi: 10.1542/peds.2010-1399. [DOI] [PubMed] [Google Scholar]
- 69.Malik S, Cleves MA, Honein MA, Romitti PA, Botto LD, Yang S, Hobbs CA National Birth Defects Prevention Study. Maternal smoking and congenital heart defects. Pediatrics. 2008;121:e810–e816. doi: 10.1542/peds.2007-1519. [DOI] [PubMed] [Google Scholar]
- 70.Patel SS, Burns TL, Botto LD, Riehle-Colarusso TJ, Lin AE, Shaw GM, Romitti PA National Birth Defects Prevention Study. Analysis of selected maternal exposures and non-syndromic atrioventricular septal defects in the National Birth Defects Prevention Study, 1997–2005. Am J Med Genet A. 2012;158A:2447–2455. doi: 10.1002/ajmg.a.35555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanner JP, Salemi JL, Stuart AL, Yu H, Jordan MM, DuClos C, Cavicchia P, Correia JA, Watkins SM, Kirby RS. Associations between exposure to ambient benzene and PM(2.5) during pregnancy and the risk of selected birth defects in offspring. Environ Res. 2015;142:345–353. doi: 10.1016/j.envres.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Mateja WA, Nelson DB, Kroelinger CD, Ruzek S, Segal J. The association between maternal alcohol use and smoking in early pregnancy and congenital cardiac defects. J Womens Health (Larchmt) 2012;21:26–34. doi: 10.1089/jwh.2010.2582. [DOI] [PubMed] [Google Scholar]
- 73.Baardman ME, Kerstjens-Frederikse WS, Corpeleijn E, de Walle HE, Hofstra RM, Berger RM, Bakker MK. Combined adverse effects of maternal smoking and high body mass index on heart development in offspring: evidence for interaction? Heart. 2012;98:474–479. doi: 10.1136/heartjnl-2011-300822. [DOI] [PubMed] [Google Scholar]
- 74.Cai GJ, Sun XX, Zhang L, Hong Q. Association between maternal body mass index and congenital heart defects in offspring: a systematic review. Am J Obstet Gynecol. 2014;211:91–117. doi: 10.1016/j.ajog.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 75.Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, Gallaway MS, Correa A National Birth Defects Prevention Study. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- 76.Øyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. doi: 10.1161/CIRCULATIONAHA.115.017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simeone RM, Devine OJ, Marcinkevage JA, Gilboa SM, Razzaghi H, Bardenheier BH, Sharma AJ, Honein MA. Diabetes and congenital heart defects: a systematic review, meta-analysis, and modeling project. Am J Prev Med. 2015;48:195–204. doi: 10.1016/j.amepre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Priest JR, Yang W, Reaven G, Knowles JW, Shaw GM. Maternal midpregnancy glucose levels and risk of congenital heart disease in offspring. JAMA Pediatr. 2015;169:1112–1116. doi: 10.1001/jamapediatrics.2015.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Auger N, Fraser WD, Healy-Profitós J, Arbour L. Association between preeclampsia and congenital heart defects. JAMA. 2015;314:1588–1598. doi: 10.1001/jama.2015.12505. [DOI] [PubMed] [Google Scholar]
- 80.Czeizel AE, Vereczkey A, Szabó I. Folic acid in pregnant women associated with reduced prevalence of severe congenital heart defects in their children: a national population-based case-control study. Eur J Obstet Gynecol Reprod Biol. 2015;193:34–39. doi: 10.1016/j.ejogrb.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 81.Scanlon KS, Ferencz C, Loffredo CA, Wilson PD, Correa-Villaseñor A, Khoury MJ, Willett WC. Preconceptional folate intake and malformations of the cardiac outflow tract: Baltimore-Washington Infant Study Group. Epidemiology. 1998;9:95–98. [PubMed] [Google Scholar]
- 82.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ. 2009;338:b1673. doi: 10.1136/bmj.b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong DY, Binongo JN, Kancherla V. Maternal chlamydia infection during pregnancy and risk of cyanotic congenital heart defects in the offspring. Matern Child Health J. 2016;20:66–76. doi: 10.1007/s10995-015-1804-0. [DOI] [PubMed] [Google Scholar]
- 84.Oster ME, Riehle-Colarusso T, Correa A. An update on cardiovascular malformations in congenital rubella syndrome. Birth Defects Res A Clin Mol Teratol. 2010;88:1–8. doi: 10.1002/bdra.20621. [DOI] [PubMed] [Google Scholar]
- 85.Zheng JY, Tian HT, Zhu ZM, Li B, Han L, Jiang SL, Chen Y, Li DT, He JC, Zhao Z, Cao Y, Qiu YG, Li TC. Prevalence of symptomatic congenital heart disease in Tibetan school children. Am J Cardiol. 2013;112:1468–1470. doi: 10.1016/j.amjcard.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 86.US Department of Health and Human Services, Secretary’s Advisory Committee on Heritable Disorders. [Accessed July 31, 2014];Letter to the Secretary of Health and Human Services re: the addition of critical congenital cyanotic heart disease to the Committee’s Recommended Uniform Screening Panel. 2010 Oct 15; http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/criticalcongenital.pdf.
- 87.Mahle WT, Martin GR, Beekman RH, 3rd, Morrow WR Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 88.de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegårdh J, Verdicchio M, Ostman-Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meberg A, Brügmann-Pieper S, Due R, Jr, Eskedal L, Fagerli I, Farstad T, Frøisland DH, Sannes CH, Johansen OJ, Keljalic J, Markestad T, Nygaard EA, Røsvik A, Silberg IE. First day of life pulse oximetry screening to detect congenital heart defects [published correction appears in J Pediatr. 2009;154:629] J Pediatr. 2008;152:761–765. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 90.Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine–results from a prospective multicenter study. Eur J Pediatr. 2010;169:975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132:e595–e603. doi: 10.1542/peds.2013-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ailes EC, Gilboa SM, Honein MA, Oster ME. Estimated number of infants detected and missed by critical congenital heart defect screening. Pediatrics. 2015;135:1000–1008. doi: 10.1542/peds.2014-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson C, Ailes E, Riehle-Colarusso T, Oster ME, Olney RS, Cassell CH, Fixler DE, Carmichael SL, Shaw GM, Gilboa SM. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168:361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379:2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 95.Jawin V, Ang HL, Omar A, Thong MK. Beyond critical congenital heart disease: newborn screening using pulse oximetry for neonatal sepsis and respiratory diseases in a middle-income country. PLoS One. 2015;10:e0137580. doi: 10.1371/journal.pone.0137580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 97.Falcini F, Rigante D, Masi L, Covino M, Franceschelli F, Leoncini G, Tarantino G, Matucci Cerinic M, Brandi ML. Fibroblast growth factor 23 (FGF23) gene polymorphism in children with Kawasaki syndrome (KS) and susceptibility to cardiac abnormalities. Ital J Pediatr. 2013;39:69. doi: 10.1186/1824-7288-39-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan Y, Ma Y, Liu Y, Hu H, Shen Y, Zhang S, Ma Y, Tao D, Wu Q, Peng Q, Yang Y. Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet. 2013;132:669–680. doi: 10.1007/s00439-013-1279-2. [DOI] [PubMed] [Google Scholar]
- 99.Son MB, Newburger JW. Kawasaki disease. Pediatr Rev. 2013;34:151–162. doi: 10.1542/pir.34-4-151. [DOI] [PubMed] [Google Scholar]
- 100.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, Kotani K, Tsogzolbaatar EO, Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results of the 2009–2010 nationwide survey. J Epidemiol. 2012;22:216–221. doi: 10.2188/jea.JE20110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu MH, Chen HC, Yeh SJ, Lin MT, Huang SC, Huang SK. Prevalence and the long-term coronary risks of patients with Kawasaki disease in a general population <40 years: a national database study. Circ Cardiovasc Qual Outcomes. 2012;5:566–570. doi: 10.1161/CIRCOUTCOMES.112.965194. [DOI] [PubMed] [Google Scholar]
- 102.Park YW, Han JW, Hong YM, Ma JS, Cha SH, Kwon TC, Lee SB, Kim CH, Lee JS, Kim CH. Epidemiological features of Kawasaki disease in Korea, 2006–2008. Pediatr Int. 2011;53:36–39. doi: 10.1111/j.1442-200X.2010.03178.x. [DOI] [PubMed] [Google Scholar]
- 103.Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, Huang GY Shanghai Kawasaki Disease Research Group. Epidemiologic features of Kawasaki Disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. 2016;35:7–12. doi: 10.1097/INF.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 104.Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997–2007. Pediatr Infect Dis J. 2010;29:483–488. doi: 10.1097/INF.0b013e3181cf8705. [DOI] [PubMed] [Google Scholar]
- 105.Jakob A, Whelan J, Kordecki M, Berner R, Stiller B, Arnold R, von Kries R, Neumann E, Roubinis N, Robert M, Grohmann J, Höhn R, Hufnagel M. Kawasaki disease in Germany: a prospective, population-based study adjusted for underreporting. Pediatr Infect Dis J. 2016;35:129–134. doi: 10.1097/INF.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 106.Luca NJ, Yeung RS. Epidemiology and management of Kawasaki disease. Drugs. 2012;72:1029–1038. doi: 10.2165/11631440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 107.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salo E, Griffiths EP, Farstad T, Schiller B, Nakamura Y, Yashiro M, Uehara R, Best BM, Burns JC. Incidence of Kawasaki disease in northern European countries. Pediatr Int. 2012;54:770–772. doi: 10.1111/j.1442-200X.2012.03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holman RC, Christensen KY, Belay ED, Steiner CA, Effler PV, Miyamura J, Forbes S, Schonberger LB, Melish M. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawaii. Hawaii Med J. 2010;69:194–197. [PMC free article] [PubMed] [Google Scholar]
- 110.Maddox RA, Holman RC, Uehara R, Callinan LS, Guest JL, Schonberger LB, Nakamura Y, Yashiro M, Belay ED. Recurrent Kawasaki disease: USA and Japan. Pediatr Int. 2015;57:1116–1120. doi: 10.1111/ped.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 112.Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99:76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 113.Lin MT, Sun LC, Wu ET, Wang JK, Lue HC, Wu MH. Acute and late coronary outcomes in 1073 patients with Kawasaki disease with and without intravenous γ-immunoglobulin therapy. Arch Dis Child. 2015;100:542–547. doi: 10.1136/archdischild-2014-306427. [DOI] [PubMed] [Google Scholar]
- 114.Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S, Kudo Y, Itoh S, Ishii H, Ueno T, Tashiro T, Nobuyoshi M, Kato H, Matsuishi T. Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation. 2011;123:1836–1842. doi: 10.1161/CIRCULATIONAHA.110.978213. [DOI] [PubMed] [Google Scholar]
- 115.Roguin N, Du ZD, Barak M, Nasser N, Hershkowitz S, Milgram E. High prevalence of muscular ventricular septal defect in neonates. J Am Coll Cardiol. 1995;26:1545–1548. doi: 10.1016/0735-1097(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 116.Sands AJ, Casey FA, Craig BG, Dornan JC, Rogers J, Mulholland HC. Incidence and risk factors for ventricular septal defect in “low risk” neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81:F61–F63. doi: 10.1136/fn.81.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–855. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 118.Ma M, Gauvreau K, Allan CK, Mayer JE, Jr, Jenkins KJ. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83:1438–1445. doi: 10.1016/j.athoracsur.2006.10.073. [DOI] [PubMed] [Google Scholar]
17. DISORDERS OF HEART RHYTHM
See Table 17-1 and Charts 17-1 through 17-11
Table 17-1.
Cumulative Incidence Rate (%) Over 5 Years After AF Diagnosis by Age*
| Age Group, y | Mortality | Heart Failure | Myocardial Infarction | Stroke | Gastrointestinal Bleeding |
|---|---|---|---|---|---|
| 67–69 | 28.8 | 11.0 | 3.3 | 5.0 | 4.4 |
| 70–74 | 32.3 | 12.1 | 3.6 | 5.7 | 4.9 |
| 75–79 | 40.1 | 13.3 | 3.9 | 6.9 | 5.9 |
| 80–84 | 52.1 | 15.1 | 4.3 | 8.1 | 6.4 |
| 85–89 | 67.0 | 15.8 | 4.4 | 8.9 | 6.6 |
| ≥90 | 84.3 | 13.7 | 3.6 | 6.9 | 5.4 |
Chart 17-1. Long-term outcomes in individuals with prolonged PR interval (>200 ms; first-degree atrioventricular block) compared with individuals with normal PR interval in the FHS.
FHS indicates Framingham Heart Study.
Data derived from Cheng et al.14
Chart 17-11. Global age-adjusted atrial fibrillation prevalence rates (per 100 000 population) in the 2010 GBD.
GBD indicates Global Burden of Diseases, Injuries, and Risk Factors Study.
Reprinted from Chugh et al.190 Copyright © 2014, American Heart Association, Inc.
Bradyarrhythmias
ICD-9 426.0, 426.1, 427.81; ICD-10 I44.0 to I44.3, I49.5.
Mortality—974. Any-mention mortality—5553. Hospital discharges—110 000.
Abbreviations Used in Chapter 17
| ACCORD | Action to Control Cardiovascular Risk in Diabetes |
| AF | atrial fibrillation |
| AMI | acute myocardial infarction |
| ARIC | Atherosclerosis Risk in Communities study |
| ASSERT | Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial |
| AV | atrioventricular |
| BMI | body mass index |
| BNP | B-type natriuretic peptide |
| BP | blood pressure |
| CABG | coronary artery bypass graft |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CHA2DS2-VASc | Clinical prediction rule for estimating the risk of stroke based on congestive heart failure, hypertension, diabetes mellitus, and sex (1 point each); age ≥75 y and stroke/transient ischemic attack/thromboembolism (2 points each); plus history of vascular disease, age 65 to 74 y, and (female) sex category |
| CHADS2 | Clinical prediction rule for estimating the risk of stroke based on congestive heart failure, hypertension, age ≥75 y, diabetes mellitus (1 point each), and prior stroke/transient ischemic attack/thromboembolism (2 points) |
| CHARGE-AF | Cohorts for Heart and Aging Research in Genomic Epidemiology–Atrial Fibrillation |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| DALY | disability-adjusted life-year |
| DM | diabetes mellitus |
| ECG | electrocardiogram |
| ED | emergency department |
| EF | ejection fraction |
| EMPHASIS-HF | Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure |
| EPIC-Norfolk | European Prospective Investigation Into Cancer and Nutrition—Norfolk Cohort |
| ESRD | end-stage renal disease |
| FHS | Framingham Heart Study |
| FIT | Henry Ford Exercise Testing |
| GBD | Global Burden of Diseases, Injuries, and Risk Factors Study |
| GWAS | genome-wide association studies |
| HD | heart disease |
| HF | heart failure |
| HR | hazard ratio |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IHD | ischemic heart disease |
| Look AHEAD | Look: Action for Health in Diabetes |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| METs | metabolic equivalents |
| MI | myocardial infarction |
| NAMCS | National Ambulatory Medical Care Survey |
| NCHS | National Center for Health Statistics |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHDS | National Hospital Discharge Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NSTEMI | non–ST-segment–elevation myocardial infarction |
| OHCA | out-of-hospital cardiac arrest |
| OPERA | Oulu Project Elucidating Risk of Atherosclerosis |
| OR | odds ratio |
| ORBIT-AF | Outcomes Registry for Better Informed Treatment of Atrial Fibrillation |
| PAR | population attributable risk |
| PREVEND | Prevention of Renal and Vascular End-Stage Disease |
| PVC | premature ventricular contraction |
| PVT | polymorphic ventricular tachycardia |
| QALY | quality-adjusted life-year |
| REGARDS | Reasons for Geographic and Racial Differences in Stroke |
| RE-LY | Randomized Evaluation of Long-term Anticoagulant Therapy |
| RR | relative risk |
| RV | right ventricular |
| SBP | systolic blood pressure |
| SCD | sudden cardiac death |
| STEMI | ST-segment–elevation myocardial infarction |
| STROKESTOP | Systematic ECG Screening for Atrial Fibrillation Among75-Year-Old Subjects in the Region of Stockholm and Halland, Sweden |
| SVT | supraventricular tachycardia |
| TdP | torsade de pointes |
| UI | uncertainty interval |
| USD | US dollars |
| VF | ventricular fibrillation |
| VT | ventricular tachycardia |
| WPW | Wolff-Parkinson-White |
AV Block
Prevalence and Incidence
In a healthy sample of participants from the ARIC study (mean age 53 years), the prevalence of first-degree AV block was 7.8% in black males, 3.0% in black females, 2.1% in white males, and 1.3% in white females.1 Lower prevalence estimates were noted in the relatively younger population (mean age 45 years) of the CARDIA study at its year 20 follow-up examination: 2.6% in black males, 1.9% in black females, 1.2% in white males, and 0.1% in white females.2
The prevalence of PR interval prolongation was observed to be 2.1% in Finnish middle-aged people, but the authors noted that the PR interval normalized in follow-up in 30% of these people.3
Mobitz II second-degree AV block is rare in healthy individuals (≈0.003%), whereas Mobitz I (Wenckebach) is observed in 1% to 2% of healthy young people, especially during sleep.4,5
The prevalence of third-degree AV block in the general adult population is ≈0.02% to 0.04%.6,7
Third-degree AV block is very rare in apparently healthy people. Johnson et al8 found only 1 case among >67 000 symptom-free US Air Force males; Rose et al,9 in their study of >18 000 civil servants, did not find any cases. On the other hand, among 293 124 patients with DM and 552 624 with hypertension enrolled with Veterans Health Administration hospitals, third-degree AV block was present in 1.1% and 0.6%, respectively.10
Congenital complete AV block is estimated to occur in 1 of 15 000 to 20 000 live births,11 An English register study estimated the incidence of infant complete AV block as 2.1 per 100 000 live births.12 Congenital complete heart block could be attributable to transplacental transfer of maternal anti-SSA/Ro or SSB/La antibodies.11
Complications
(See Chart 17-1)
In the FHS, PR interval prolongation (>200 ms) was associated with an increased risk of AF (HR, 2.06; 95% CI, 1.36–3.12),13,14 pacemaker implantation (HR, 2.89; 95% CI, 1.83–4.57),14 and all-cause mortality (HR, 1.44; 95% CI, 1.09–1.91).14 Compared with people with a PR ≤200 ms, those with a PR interval >200 ms had an absolute increased risk per year of 1.04% for AF, 0.53% for pacemaker implantation, and 2.05% for death (Chart 17-1).
Patients with abnormalities of AV conduction may be asymptomatic or may experience serious symptoms related to bradycardia, ventricular arrhythmias, or both.
Decisions about the need for a pacemaker are influenced by the presence or absence of symptoms directly attributable to bradycardia. Permanent pacing improves survival in patients with third-degree AV block, especially if syncope has occurred.15 Nevertheless, the overall prognosis depends to a large extent on the underlying HD.
Although there is little evidence to suggest that pacemakers improve survival in patients with isolated first-degree AV block,16 it is recognized that marked first-degree AV block (PR >300 ms) can lead to symptoms even in the absence of higher degrees of AV block.17
Prognosis
Investigators at Northwestern University compared older adult outpatients (>60 years old) with (n=470) and without (n=2090) asymptomatic bradycardia. Over a mean follow-up of 7.2 years, patients with asymptomatic bradycardia had a higher adjusted incidence of pacemaker insertion (HR, 2.14; 95% CI, 1.30–3.51; P=0.003), which appeared after a lag time of 4 years. However, the absolute rate of pacemaker implantation was low (<1% per year), and asymptomatic bradycardia was not associated with a higher risk of death.18
Risk Factors
In healthy individuals without CVD or its risk factors from MESA, PR interval was longer with advancing age, in males compared with females, and in blacks compared with whites.19
Although first-degree AV block and Mobitz type I second-degree AV block can occur in apparently healthy people, presence of Mobitz II second- or third-degree AV block usually indicates underlying HD, including CHD and HF.4
Reversible causes of AV block include electrolyte abnormalities, drug-induced AV block, perioperative AV block attributable to hypothermia, or inflammation near the AV conduction system after surgery in this region. Some conditions might warrant pacemaker implantation because of the possibility of disease progression even if the AV block reverses transiently (eg, sarcoidosis, amyloidosis, and neuromuscular diseases).15
Long sinus pauses and AV block can occur during sleep apnea. In the absence of symptoms, these abnormalities are reversible and do not require pacing.20
Prevention
Sinus Node Dysfunction
Prevalence and Incidence
The prevalence of sinus node dysfunction has been estimated to be between 403 and 666 per million, with an incidence rate of 63 per million per year requiring pacemaker therapy.22
Sinus node dysfunction occurs in 1 of every 600 cardiac patients >65 years of age and accounts for ≈50% of implantations of pacemakers in the United States.23,24
Sinus node dysfunction is commonly present with other causes of bradyarrhythmias (carotid sinus hypersensitivity in 33% of patients and advanced AV conduction abnormalities in 17%).25,26
The incidence rate of sick sinus syndrome was 0.8 per 1000 person-years of follow-up in 2 biracial US cohorts, ARIC and CHS.27 The incidence increased with advancing age (HR, 1.73; 95% CI, 1.47–2.05 per 5-year increment), and blacks were at 41% lower risk of sick sinus syndrome than their white counterparts (HR, 0.59; 95% CI, 0.37–0.98). Investigators projected that in the United States, the number of new cases of sick sinus syndrome per year would rise from 78 000 in 2012 to 172 000 in 2060.
Complications
(See Chart 17-2)
Chart 17-2. Primary indications (in thousands) for pacemaker placement between 1990 and 2002 from the NHDS, NCHS.
AV indicates atrioventricular; NCHS, National Center for Health Statistics; and NHDS, National Hospital Discharge Survey.
Data derived from Birnie et al.35
In a small prospective study of 35 patients at least 45 years of age with sinus node dysfunction, 57% experienced symptoms over a 4-year follow-up period if untreated; 31% experienced syncope over the 4 years.28
Approximately 50% of patients with sinus node dysfunction develop tachy-brady syndrome over a lifetime; such patients have a higher risk of stroke and death. The survival of patients with sinus node dysfunction appears to depend primarily on the severity of underlying cardiac disease and is not significantly changed by pacemaker therapy.29–31
In a retrospective study,32 patients with sinus node dysfunction who had pacemaker therapy were followed up for 12 years; at 8 years, mortality among those with ventricular pacing was 59% compared with 23% among those with atrial pacing. This discrepancy can be attributed to selection bias. For instance, the physiological or anatomic disorder (eg, fibrosis of conductive tissue) that led to the requirement for the particular pacemaker might have influenced prognosis, rather than the type of pacemaker used.
In a multicenter study from the Netherlands of people with bradycardia treated with pacemaker implantation, the actuarial 1-, 3-, 5-, and 7-year survival rates were 93%, 81%, 69%, and 61%, respectively. Individuals without CVD at baseline had similar survival rates as age- and sex-matched control subjects.33
With sinus node dysfunction, the incidence of sudden death is extremely low, and pacemaker implantation does not appear to alter longevity.15,34
SVT including AF was prevalent in 53% of patients with sinus node dysfunction.31
On the basis of records from the NHDS, age-adjusted pacemaker implantation rates increased progressively from 370 per million in 1990 to 612 per million in 2002. This escalating implantation rate is attributable to increasing implantation for isolated sinus node dysfunction; implantation for sinus node dysfunction increased by 102%, whereas implantation for all other indications did not increase35 (Chart 17-2).
In patients paced for sick sinus syndrome, the CHA2DS2-VASc score is associated with an increased risk of stroke and death, even in those patients without AF at baseline.36
Risk Factors
The causes of sinus node dysfunction can be classified as intrinsic (secondary to pathological conditions involving the sinus node) or extrinsic (caused by depression of sinus node function by external factors such as drugs or autonomic influences).37
Idiopathic degenerative disease is probably the most common cause of sinus node dysfunction.38
Collected data from 28 different studies on atrial pacing for sinus node dysfunction showed a median annual incidence of second- and third-degree AV block of 0.6% (range, 0%–4.5%) and an overall prevalence of 2.1% (range, 0%–11.9%). This suggests that the degenerative process also affects the specialized conduction system, although the rate of progression is slow and does not dominate the clinical course of disease.39
IHD may be responsible for one third of sinus node dysfunction cases. Transient sinus node dysfunction can complicate MI; it is common during inferior MI and is caused by autonomic influences. Cardiomyopathy, long-standing hypertension, infiltrative disorders (eg, amyloidosis and sarcoidosis), collagen vascular disease, and surgical trauma can also result in sinus node dysfunction.40,41
In the CHS and ARIC studies, factors associated with incident sick sinus syndrome included white (versus black) race, higher mean BMI, height, prevalent hypertension, lower heart rate, right bundle-branch block, N-terminal pro-BNP, cystatin C, and history of a major cardiovascular event.27
SVT (Excluding AF and Atrial Flutter)
ICD-9 427.0; ICD-10 I47.1.
Mortality—159. Any-mention mortality—1329. Hospital discharges—23 000.
Prevalence and Incidence
(See Chart 17-3)
Chart 17-3. Incidence rate of paroxysmal supraventricular tachycardia per 100 000 person-years by age and sex.
Data derived from Orejarena et al.42
Data from the Marshfield Epidemiologic Study Area in Wisconsin suggested the incidence of documented paroxysmal SVT was 35 per 100 000 person-years. The mean age at SVT onset was 57 years, and both female sex and age >65 years were significant risk factors42 (Chart 17-3).
A review of ED visits from 1993 to 2003 revealed that an estimated 550 000 visits were for SVT (0.05% of all visits; 95% CI, 0.04%–0.06%), or ≈50 000 visits per year. Of these patients, 24% (95% CI, 15%–34%) were admitted to the hospital, and 44% (95% CI, 32%–56%) were discharged without specific follow-up.43
The prevalence of SVT that is clinically undetected is likely much greater than the estimates from ED visits and electrophysiology procedures would suggest. For example, among a random sample of 604 middle-aged individuals in the Finnish OPERA study, 7 (1.2%) fulfilled the diagnostic criteria for inappropriate sinus tachycardia.44
Of 1383 participants in the Baltimore Longitudinal Study of Aging undergoing maximal exercise testing, 6% exhibited SVT during the test; increasing age was a significant risk factor. Only 16% exhibited >10 beats of SVT, and only 4% were symptomatic. Over an average of 6 years of follow-up, people with exercise-induced SVT were more likely to develop SVT or AF.45
In a study of males applying for a pilot’s license, the surface ECG revealed that the prevalence of ectopic atrial tachycardia was estimated to be 0.34% in asymptomatic patients and 0.46% in symptomatic patients.46
Complications
Rare cases of incessant SVT can lead to a tachycardia-induced cardiomyopathy,47 and rare cases of sudden death attributed to SVT as a trigger have been described.48
A California administrative database study suggested that after the exclusion of people with diagnosed AF, SVT was associated with an adjusted doubling of the risk of stroke in follow-up (HR, 2.10; 95% CI, 1.69–2.62). The absolute stroke rate was low, however. The cumulative stroke rate was 0.94% (95% CI, 0.76%–1.16%) over 1 year in patients with SVT versus 0.21% (95% CI, 0.21%–0.22%; P<0.001, log-rank test) in those without SVT.49
Specific Types
Among those presenting for invasive electrophysiological study and ablation, AV nodal reentrant tachycardia (a circuit that requires 2 AV nodal pathways) is the most common mechanism of SVT50,51 and usually represents the majority of cases (56% of 1 series of 1754 cases from Loyola University Medical Center).51
AV reentrant tachycardia (an arrhythmia that requires the presence of an extranodal connection between the atria and ventricles or specialized conduction tissue) is the second most common52,53 type of SVT (27% in the Loyola series),51 and atrial tachycardia is the third most common (17% in the Loyola series).51
In the pediatric population, AV reentrant tachycardia is the most common SVT mechanism, followed by AV nodal reentrant tachycardia and then atrial tachycardia.54
AV reentrant tachycardia prevalence decreases with age, whereas AV nodal reentrant tachycardia and atrial tachycardia prevalence increase with advancing age.51
The majority of AV reentrant tachycardia patients in the Loyola series were males (55%), whereas the majority of patients with AV nodal reentrant tachycardia (70%) or atrial tachycardia (62%) were females.51
Multifocal atrial tachycardia is an arrhythmia that is commonly confused with AF and is characterized by 3 distinct P-wave morphologies, irregular R-R intervals, and a rate >100 beats per minute. It is uncommon in both children52 and adults,53 with a prevalence in hospitalized adults estimated at 0.05% to 0.32%.55,56 The average age in adults is 70 to 72 years. Adults with multifocal atrial tachycardia have a high mortality rate, with estimates around 45%, but this is generally ascribed to the underlying condition(s).53,57
WPW Syndrome
Prevalence
WPW syndrome was observed in 0.11% of males and 0.04% of females among 47 358 ECGs from adults participating in 4 large Belgian epidemiological studies.55 In a study of 32 837 Japanese students who were required by law to receive ECGs before entering school, WPW was reported in 0.073%, 0.070%, and 0.174% of elementary, junior high, and high school students, respectively.56
Complications
WPW syndrome, a diagnosis reserved for those with both ventricular preexcitation (evidence of an anterograde conducting AV accessory pathway on a 12-lead ECG) and tachyarrhythmias,58 deserves special attention because of the associated risk of sudden death. Sudden death is generally attributed to rapid heart rates in AF conducting down an accessory pathway and leading to VF.59,60 Of note, AF is common in WPW patients, and surgical or catheter ablation of the accessory pathway often results in elimination of the AF.61
Asymptomatic adults with ventricular preexcitation appear to be at no increased risk of sudden death compared with the general population,59,60,62,63 although certain characteristics found during invasive electrophysiological study (including inducibility of AV reentrant tachycardia or AF, accessory pathway refractory period, and the shortest R-R interval during AF) can help risk stratify these patients.60,64
In a single-center prospective registry study of 2169 patients who agreed to undergo an electro-physiology study for WPW syndrome from 2005 to 2010, 1168 (206 asymptomatic) underwent radio-frequency ablation, none of whom had malignant arrhythmias or VF in up to 8 years of follow-up. Of those who did not receive radiofrequency ablation (n=1001; 550 asymptomatic) in follow-up, 1.5% had VF, most of whom (13 of 15) were children. The authors noted that poor prognosis was related to accessory pathway electrophysiological properties rather than patient symptoms.65
In a meta-analysis of 20 studies involving 1869 asymptomatic patients with a WPW ECG pattern followed up for a total of 11 722 person-years, the risk of sudden death in a random effects model that was used because of heterogeneity across studies was estimated to be 1.25 (95% CI, 0.57–2.19) per 1000 person-years. Risk factors for sudden death included male sex, inclusion in a study of children (<18 years of age), and inclusion in an Italian study.66
Although some studies in asymptomatic children with ventricular preexcitation suggest a benign prognosis,62,67 others suggest that electrophysiological testing can identify a group of asymptomatic children with a risk of sudden death or VF as high as 11% over 19 months of follow-up.68 In a pediatric hospital retrospective review of 444 children with WPW syndrome, 64% were symptomatic at presentation, and 20% had onset of symptoms during follow-up. The incidence of sudden death was 1.1 per 1000 person-years in patients without structural HD.69
Subclinical Atrial Tachyarrhythmias, Unrecognized AF, Screening for AF
Device-Detected AF
Pacemakers and defibrillators have increased clinician awareness of the frequency of subclinical AF and atrial high-rate episodes in people without a documented history of AF. Several studies have suggested that device-detected high-rate atrial tachyarrhythmias are surprisingly frequent and are associated with an increased risk of AF,64 thrombo-embolism,64,70 and total mortality.64
-
Investigators in the ASSERT study prospectively enrolled 2580 patients with a recent pacemaker or defibrillator implantation who were ≥65 years of age, had a history of hypertension, and had no history of AF. They classified individuals by presence versus absence of subclinical atrial tachyarrhythmias (defined as atrial rate >190 beats per minute for >6 minutes in the first 3 months) and conducted follow-up for 2.5 years.71 Subclinical atrial tachyarrhythmias in the first 3 months occurred in 10.1% of the patients and were associated with the following:
An almost 6-fold higher risk of clinical AF (HR, 5.56; 95% CI, 3.78–8.17; P<0.001)
A more than doubling in the adjusted risk of the primary end point, ischemic stroke or systemic embolism (HR, 2.50; 95% CI, 1.28–4.89; P<0.008)
An annual ischemic stroke or systemic embolism rate of 1.69% (versus 0.69% in those without)
A 13% PAR for ischemic stroke or systemic embolism
Over the subsequent 2.5 years of follow-up, an additional 34.7% of the patients had subclinical atrial tachyarrhythmias, which were 8-fold more frequent than clinical AF episodes.71
A pooled analysis of 5 prospective studies in patients without permanent AF revealed that over 2 years of follow-up, cardiac implanted electronic devices detected ≥5 minutes of AF in 43% of the patients (total n=10 016). Adjustment for CHADS2 score and anticoagulation revealed that AF burden was associated with an increased risk of stroke.72
Community Screening
In a community-based study in Sweden (STROKESTOP Study), half of the population 75 to 76 years of age were invited to a stepwise screening program for AF, and 7173 participated in the screening, of whom 218 had newly diagnosed AF (3%; 95% CI, 2.7%–3.5%) and an additional 666 (9.3%; 95% CI, 8.6%–10.0%) had previously diagnosed AF. Of the 218 newly diagnosed AF cases, only 37 were diagnosed by screening ECG, whereas intermittent monitoring detected 4 times as many cases. Of those individuals with newly diagnosed AF, 93% initiated treatment with oral anticoagulant drugs.73
-
There have been 2 recent systematic reviews regarding the effectiveness of screening to detect unknown AF.
Lowres et al74 identified 30 separate studies that included outpatient clinics or community screening. In individuals without a prior diagnosis of AF, they observed that 1.0% (95% CI, 0.89%–1.04%) of those screened had AF (14 studies, n=67 772), whereas among those individuals ≥65 years of age, 1.4% (95% CI, 1.2%–1.6%; 8 studies, n=18 189) had AF.
Another systematic review by Moran et al75 observed that in individuals >65 years of age, systematic screening (OR, 1.57; 95% CI, 1.08–2.26) and opportunistic screening (OR, 1.58; 95% CI, 1.10–2.29) were associated with enhanced detection of AF. The number needed to screen by either method was ≈170 individuals.
There has been increasing interest in the use of smart phone technology to aid in community screening.76,77
AF and Atrial Flutter
ICD-9 427.3; ICD-10 I48.
Prevalence
(See Chart 17-4)
Chart 17-4. Current and future US prevalence projections for AF.
Projections assume no increase (red dashed line) or logarithmic growth (blue dashed line) in incidence of AF from 2007.
AF indicates atrial fibrillation.
Data derived from Go et al78 and modified from Colilla et al80 with permission from Elsevier. Copyright © 2013, Elsevier Inc.
Estimates of the prevalence of AF in the United States ranged from ≈2.7 million to 6.1 million in 2010,78,79 and AF prevalence is estimated to rise to 12.1 million in 2030.80
In the European Union, the prevalence of AF in adults >55 years of age was estimated to be 8.8 million (95% CI, 6.5–12.3 million) in 2010 and was projected to rise to 17.9 million in 2060 (95% CI, 13.6–23.7 million).81
Data from a California health plan suggest that compared with whites, blacks (OR, 0.49; 95% CI, 0.47–0.52), Asians (OR, 0.68; 95% CI, 0.64–0.72), and Hispanics (OR, 0.58; 95% CI, 0.55–0.61) have a significantly lower adjusted prevalence of AF.82
-
Data from the NHDS/NCHS (1996–2001) on cases that included AF as a primary discharge diagnosis found the following:
Approximately 44.8% of patients were males.
The mean age for males was 66.8 years versus 74.6 years for females.
The racial breakdown for admissions was 71.2% white, 5.6% black, and 2.0% other races (20.8% were not specified).
Black patients were much younger than patients of other races.
Among Medicare patients aged ≥65 years, diagnosed from 1993 to 2007, the prevalence of AF increased ≈5% per year, from ≈41.1 per 1000 beneficiaries to 85.5 per 1000 beneficiaries.83
Incidence
(See Table 17-1 and Chart 17-5)
Chart 17-5. Atrial fibrillation incidence by race.
Incidence increases with advancing age among different races and sexes in the United States.
Data derived from Dewland et al.84
-
Data from the NHDS/NCHS (1996–2001) on cases that included AF as a primary discharge diagnosis found the following:
The incidence in males ranged from 20.6 per 100 000 people per year for patients between 15 and 44 years of age to 1077.4 per 100 000 people per year for patients ≥85 years of age.
In females, the incidence ranged from 6.6 per 100 000 people per year for patients between 15 and 44 years of age to 1203.7 per 100 000 people per year for those ≥85 years of age.
Five years after diagnosis with AF, the cumulative incidence rate of mortality, HF, MI, stroke, and gastrointestinal bleeding was higher in older age groups (80–84, 85–89, and ≥90 years old) than younger age groups (67–69, 70–74, and 75–79 years of age) (Table 17-1).
Data from California administrative databases were analyzed with regard to racial variation in incidence of AF. After adjustment for AF risk factors, compared with their white counterparts, lower incidence rates were found in blacks (HR, 0.84; 95% CI, 0.82–0.85; P<0.001), Hispanics (HR, 0.78; 95% CI, 0.77–0.79; P<0.001), and Asians (HR, 0.78; 95% CI, 0.77–0.79; P<0.001)84 (Chart 17-5).
In a Medicare sample, the incidence of AF was ≈28 per 1000 person-years and did not change substantively between 1993 and 2007. Of individuals with incident AF in 2007, ≈55% were females, 91% were white, 84% had hypertension, 36% had HF, and 30% had cerebrovascular disease.83
Using data from a health insurance claims database covering 5% of the United States, the incidence of AF was estimated at 1.6 million cases in 2010 and was projected to increase to 2.6 million cases in 2030.80
Lifetime Risk and Cumulative Risk
(See Chart 17-6)
Chart 17-6. Lifetime cumulative risk for atrial fibrillation (AF) at different ages (through age 94 years) by sex.
With increasing incidence of AF with aging, lifetime risk is unchanged.
Reprinted from Lloyd-Jones et al.85 Copyright © 2004, American Heart Association, Inc.
Participants of largely European ancestry in the NHLBI-sponsored FHS were followed up from 1968 to 1999. At 40 years of age, remaining lifetime risks for AF were 26.0% for males and 23.0% for females. At 80 years of age, lifetime risks for AF were 22.7% for males and 21.6% for females (Chart 17-6). In further analysis, counting only those who had development of AF without prior or concurrent HF or MI, lifetime risk for AF was ≈16%.85 Estimates of lifetime risks of AF were similar in the Rotterdam Study.86
In a medical insurance database study from the Yunnan Province in China, the estimated lifetime risk of AF at age 55 years was 21.1% (19.3%–23.0%) for females and 16.7% (15.4%–18.0%) for males.87
Investigators from the NHLBI-sponsored ARIC study observed that the cumulative risk of AF was 21% in white males, 17% in white females, and 11% in African Americans of both sexes by 80 years of age.88
Mortality
(See Chart 17-7)
Chart 17-7. Cumulative incidence of events in the 5 years after diagnosis of incident AF in Medicare patients.
AF indicates atrial fibrillation.
Reprinted from Piccini et al129 by permission of the European Society of Cardiology. Copyright © 2013, The Authors.
In 2014, AF was mentioned on 137 435 US death certificates and was the underlying cause in 21 713 of those deaths (NCHS, NHLBI).89
In adjusted analyses from the FHS, AF was associated with an increased risk of death in both males (OR, 1.5; 95% CI, 1.2–1.8) and females (OR, 1.9; 95% CI, 1.5–2.2).90 Furthermore, there was an interaction with sex, such that AF appeared to diminish the survival advantage typically observed in females.
Although there was significant heterogeneity in the studies (P<0.001), a meta-analysis confirmed that the adjusted risk of death was significantly stronger in females than in males with AF (RR, 1.12; 95% CI, 1.07–1.17).91
In Medicare beneficiaries ≥65 years of age with new-onset AF, mortality decreased modestly but significantly between 1993 and 2007. In 2007, the age- and sex-adjusted mortality at 30 days was 11%, and at 1 year, it was 25%.83
An observational study from Rochester County, MN, of >4600 patients diagnosed with first AF showed that risk of death within the first 4 months after the AF diagnosis was high. The most common causes of CVD death were CAD, HF, and ischemic stroke, which accounted for 22%, 14%, and 10%, respectively, of the early deaths (within the first 4 months) and 15%, 16%, and 7%, respectively, of the late deaths.92
Although stroke is the most feared complication of AF, a recent clinical trial (RE-LY) reported that stroke accounted for only ≈7.0% of deaths in AF, with SCD (22.25%), progressive HF (15.1%), and noncardiovascular death (35.8%) accounting for the majority of deaths.93
AF is also associated with increased mortality in individuals with other cardiovascular conditions and procedures, including HF,94,95 HF with preserved EF96,97 and reduced EF96 (with a meta-analysis suggesting a worse prognosis in preserved versus reduced EF96), MI,98,99 CABG100,101 (both short-term and long-term101), and stroke.102 In non-cardiovascular conditions, AF also is associated with an increased risk of death, including in DM,103 ESRD,104 sepsis,105,106 and noncardiac surgery.107
In Medicare unadjusted analysis, blacks and Hispanics had a higher risk of death than their white counterparts with AF. However, after adjustment for comorbidities, blacks (HR, 0.95; 95% CI, 0.93–0.96; P<0.001) and Hispanics (HR, 0.82; 95% CI, 0.80–0.84; P<0.001) had a lower risk of death than whites with AF.108
The increased risk of death associated with incident AF has also been observed in a nationwide administrative database in Taiwan.109
Complications
-
Thromboembolism excluding stroke
In a Danish population-based registry of individuals 50 to 89 years of age discharged from the hospital, individuals with new-onset AF had an elevated risk of thromboembolic events to the aorta, renal mesenteric, pelvic, and peripheral arteries. The event rate was 2 to 10 per 1000 person-years. Compared with referents in the Danish population, the RR of diagnosed extracranial embolism was 4.0 (95% CI, 3.5–4.6) in males and 5.7 (95% CI, 5.1–6.3) in females.110
-
Extracranial systemic embolic events
Investigators pooled data from 4 large contemporary randomized anticoagulation trials and observed 221 systemic emboli in 91 746 person-years of follow-up. Systemic embolic rate was 0.24 versus a stroke rate of 1.92 per 100 person-years. Compared with individuals experiencing stroke, patients experiencing systemic emboli were more likely to be females (56% versus 47%; P=0.01) but had similar mean age and CHADS2 score as those with stroke. Both stroke (RR, 6.79; 95% CI, 6.22–7.41) and systemic emboli (RR, 4.33; 95% CI, 3.29–5.70) were associated with an increased risk of death compared with patients with neither event.111
-
Stroke (see Chart 17-7)
Stroke rates per 1000 patient-years declined in AF patients taking anticoagulant drugs, from 46.7 in 1992 to 19.5 in 2002, for ischemic stroke but remained fairly steady for hemorrhagic stroke (1.6–2.9).112
Before the widespread use of anticoagulant drugs, after accounting for standard stroke risk factors, AF was associated with a 4- to 5-fold increased risk of ischemic stroke.113 Although the RR of stroke associated with AF did not vary (≈3- to 5-fold increased risk) substantively with advancing age, the proportion of strokes attributable to AF increased significantly. In the FHS, AF accounted for ≈1.5% of strokes in individuals 50 to 59 years of age and ≈23.5% in those 80 to 89 years of age.113
AF was also an independent risk factor for ischemic stroke severity, recurrence, and mortality.102 In an observational study, at 5 years only 39.2% (95% CI, 31.5%–46.8%) of ischemic stroke patients with AF were alive, and 21.5% (95% CI, 14.5%–31.3%) had experienced recurrent stroke.114 In one study, individuals who had AF and were not treated with anticoagulant drugs had a 2.1-fold increase in risk for recurrent stroke and a 2.4-fold increase in risk for recurrent severe stroke.115
Studies have demonstrated an underutilization of warfarin therapy. In a recent meta-analysis, males and individuals with prior stroke were more likely to receive warfarin, whereas factors associated with lower use included alcohol and drug abuse, noncompliance, warfarin contraindications, dementia, falls, both gastrointestinal and intracranial hemorrhage, renal impairment, and advancing age.116 The underutilization of anticoagulation in AF has been demonstrated to be a global problem.117
In Medicare analyses that were adjusted for comorbidities, blacks (HR, 1.46; 95% CI, 1.38–1.55; P<0.001) and Hispanics (HR, 1.11; 95% CI, 1.03–1.18; P<0.001) had a higher risk of stroke than whites with AF.108 The increased risk persisted in analyses adjusted for anticoagulant status.
A meta-analysis that examined stroke risk by sex and presence of AF reported that AF conferred a multivariable-adjusted 2-fold stroke risk in females compared with males (RR, 1.99; 95% CI, 1.46–2.71); however, the studies were noted to be significantly heterogeneous.91
-
Cognition
Individuals with AF have an adjusted 2-fold increased risk of dementia.118
A meta-analysis of 21 studies indicated that AF was associated with an increased risk of cognitive impairment in patients after stroke (RR, 2.70; 95% CI, 1.82–4.00) and in patients without a history of stroke (RR 1.37; 95% CI, 1.08–1.73). The risk of dementia was similarly increased (RR, 1.38; 95% CI, 1.22–1.56]).119
In individuals with AF in Olmsted County, MN, the cumulative rate of dementia at 1 and 5 years was 2.7% and 10.5%, respectively.120
-
Physical disability and subjective health
-
Falls
In the REGARDS study, AF was significantly associated with an adjusted higher risk of falls (10%) than among those without AF (6.6%; OR, 1.22; 95% CI, 1.04–1.44). The presence of a history of both AF and falls was associated with a significantly higher risk of mortality (per 1000 person-years: AF plus falls, 51.2; AF and no falls, 34.4; no AF and falls, 29.8; no AF and no falls, 15.6). Compared with those with neither AF nor falls, those with both conditions had an adjusted 2-fold increased risk of death (HR, 2.12; 95% CI, 1.64–2.74).125
A systematic review and Markov decision analytic modeling report focused on people with AF ≥65 years of age noted that warfarin treatment was associated with 12.9 QALYs per patient with typical risks of stroke and falls versus 10.15 QALYs for those treated with neither warfarin or aspirin. Of interest, sensitivity analyses of the probability of falls or stroke did not substantively influence the results.126
A Medicare study noted that patients at high risk for falls with a CHADS2 score of at least 2 who had been prescribed warfarin had a 25% lower risk (HR, 0.75; 95% CI, 0.61–0.91; P=0.004) of a composite cardiovascular outcome (out-of-hospital death or hospitalization for stroke, MI, or hemorrhage).127
HF (see Chart 17-7)
AF and HF share many antecedent risk factors, and ≈40% of people with either AF or HF will develop the other condition.94
In the community, estimates of the incidence of HF in individuals with AF ranged from 3.394 to 4.4128 per 100 person-years of follow-up.
Among older adults with AF in Medicare, the 5-year event rate was high, with rates of death and HF exceeding those for stroke (see Chart 17-7). Higher event rates after new-onset AF were associated with older age and higher mean CHADS2 score.129
Investigators examined the incidence rate of HF in individuals with systolic dysfunction versus preserved LVEF (<40% versus >50%, respectively) in a Netherlands community-based cohort study (PREVEND). Per 1000 person-years, the incidence rate of systolic HF was 12.75 versus 1.99 for those with versus without AF, with a multivariable-adjusted HR of AF of 5.79 (95% CI, 2.40–13.98). Corresponding numbers for preserved EF were 4.90 versus 0.85 with and without AF, with a multivariable-adjusted HR of AF of 4.80 (95% CI, 1.30–17.70).130
-
MI (see Chart 17-7)
In the REGARDS study, in models that adjusted for standard risk factors, AF was associated with a 70% increased risk of incident MI (HR, 1.96; 95% CI, 1.52–2.52); the risk was higher in females and blacks. In individuals with AF, the age-adjusted incidence rate per 1000 person-years was 12.0 (95% CI, 9.6–14.9) in those with AF compared with 6.0 (95% CI, 5.6–6.6) in those without AF.131
In ARIC, AF was associated with an adjusted increased risk of NSTEMI (HR, 1.80; 95% CI, 1.39–2.31) but not STEMI (P for comparison HR=0.004).132 Furthermore, the adjusted association between AF and NSTEMI was stronger in females (HR, 2.72; 95% CI, 1.98–3.74) than in males (HR, 1.21; 95% CI, 0.82–1.78; Pinteraction<0.0002).
The CHS also observed a higher risk of incident MI in individuals with AF who were black (HR, 3.1; 95% CI, 1.7–5.6) than in whites (HR, 1.6; 95% CI, 1.2–2.1; Pinteraction=0.03).133
CKD
In a Japanese community-based study, individuals with AF had approximately a doubling in increased risk of developing kidney dysfunction or proteinuria, even in those without baseline DM or hypertension. Per 1000 person-years of follow-up, the incidence of kidney dysfunction was 6.8 in those without and 18.2 in those with AF at baseline.134
In a Kaiser Permanente study of people with CKD, new-onset AF was associated with an adjusted 1.67-fold increased risk of developing ESRD compared with those without AF (74 versus 64 per 1000 person-years of follow-up).135
-
SCD and VF
In a study that examined data from 2 population-based studies, AF was associated with a doubling in the risk of SCD after accounting for baseline and time-varying confounders. In ARIC, the unadjusted incidence rate was 1.30 (95% CI, 1.14–1.47) in those without AF and 2.89 (95% CI, 2.00–4.05) in those with AF; corresponding rates in CHS were 3.82 (95% CI, 3.35–4.35) and 12.00 (95% CI, 9.45–15.25), respectively. The multivariable-adjusted HR associated with AF for sudden death was 2.47 (95% CI, 1.95–3.13).136
An increased risk of VF was observed in a community-based case-control study from the Netherlands. Individuals with ECG-documented VF during OHCA were matched with non-VF community control subjects. The prevalence of AF in the 1397 VF cases was 15.4% versus 2.6% in the community control subjects. Individuals with AF had an overall adjusted 3-fold increased risk of VF (adjusted OR, 3.1; 95% CI, 2.1–4.5). The association was similar across age and sex categories and was observed in analyses of individuals without comorbidities, without AMI, and not using antiarrhythmic or QT-prolonging drugs.137
-
AF type and complications
A meta-analysis of 12 studies reported that compared with paroxysmal AF, nonparoxysmal AF was associated with a multivariable-adjusted increased risk of thromboembolism (HR, 1.355; 95% CI, 1.151–2.480; P<0.001) and death (HR, 1.217; 95% CI, 1.085–1.365; P<0.001).138
In the FHS, atrial flutter had a much lower incidence rate (36 per 100 000 person-years) than AF (578 per 100 000 person-years). Although based on only 112 individuals, in age- and sex-adjusted analyses, incident atrial flutter was associated with a 5-fold hazard of AF (HR, 5.0; 95% CI, 3.1–8.0). Compared with AF, atrial flutter was associated with a similar age- and sex-adjusted increased risk of HF, stroke, and death; however, the risk of MI was higher in people with atrial flutter.139
Hospitalizations and Ambulatory Care Visits
-
Data from the NHDS/NCHS in 2010 on cases that included AF as a primary discharge diagnosis found the following140:
Hospital discharges—479 000.
Approximately 50.8% of patients were males.
The mean age for males was 65.5 years versus 74.1 years for females.
The rate of AF hospitalization in males ranged from 32.6 per 100 000 people per year for patients between 15 and 44 years of age to 1275.8 per 100 000 people per year for patients ≥85 years of age.
The rate of AF hospitalization in females ranged from 5.4 per 100 000 people per year for patients between 15 and 44 years of age to 1323.4 per 100 000 people per year for those ≥85 years of age.
From 1996 to 2001, hospitalizations with AF as the first-listed diagnosis increased by 34%.141
On the basis of Medicare and MarketScan databases, annually, people with AF (37.5%) are approximately twice as likely to be hospitalized as age- and sex-matched control subjects (17.5%).142
In 2012, there were 4 296 000 physician office visits for AF (NAMCS, NHLBI tabulation). In 2012, there were 360 000 ED visits for AF, and in 2011, there were 784 000 outpatient visits for AF (NHAMCS, NHLBI tabulation).
Cost
(See Chart 17-8)
Chart 17-8. AF cost estimates, where AF is diagnosed in inpatient and outpatient encounters.
Indirect costs are incremental costs of inpatient and outpatient visits.
AF indicates atrial fibrillation; and USD, US dollars.
-
Investigators examined Medicare and Optum Touchstone databases (2004–2010) to estimate costs attributed to nonvalvular AF versus propensity-matched control subjects in 2014 US dollars143:
For patients aged 18 to 64 years, average per capita medical spending was $38 861 (95% CI, $35 781–$41 950) versus $28 506 (95% CI, $28 409–$28 603) for matched patients without AF. Corresponding numbers for patients ≥65 years old were $25 322 with AF (95% CI, $25 049–$25 595) versus $21 706 (95% CI, $21 563–$21 849) for matched non-AF patients.
The authors estimated that the incremental cost of AF was $10 355 for commercially insured patients and $3616 for Medicare patients.
Estimating that the prevalence of diagnosed versus undiagnosed nonvalvular AF was 0.83% versus 0.07% for individuals 18 to 64 years of age and 8.8% versus 1.1% for those ≥65 years of age, the investigators estimated that the incremental cost of undiagnosed AF was $3.1 billion (95% CI, $2.7–3.7 billion).
-
Investigators examined Medicare and MarketScan databases (2004–2006) to estimate costs attributed to AF in 2008 US dollars142 (Chart 17-8):
Annual total direct costs for AF patients were ≈$20 670 versus ≈$11 965 in the control group, for an incremental per-patient cost of $8705.
Extrapolating to the US population, it is estimated that the incremental cost of AF was ≈$26 billion, of which $6 billion was attributed to AF, $9.9 billion to other cardiovascular expenses, and $10.1 billion to noncardiovascular expenses.
In individuals in a commercial claims data set (MarketScan) of nonrepeat stroke admissions, AF was associated with an adjusted $4905 higher cost (2012 US $) than in patients with stroke without AF. The higher cost was observed regardless of age (all <65 years), sex, urban versus rural setting, or region.144
Secular Trends
During 50 years of observation of the FHS (1958–1967 to 1998–2007), the age-adjusted prevalence and incidence of AF approximately quadrupled. However, when only AF that was ascertained on ECGs routinely collected in the FHS was considered, the prevalence but not the incidence increased, which suggests that part of the changing epidemiology was attributable to enhanced surveillance. Although the prevalence of most risk factors changed over time, the hazards associated with specific risk factors did not change. Hence, the PAR associated with BMI, hypertension treatment, and DM increased (consistent with increasing prevalence). Over time, the multivariable-adjusted hazards of stroke and mortality associated with AF declined by 74% and 25%, respectively.145
Between 2000 and 2010 in Olmsted County, MN, age- and sex-adjusted incidence rates and survival did not change over time.146 However, over a similar time frame in the United Kingdom (2001–2013), the incidence of nonvalvular AF increased modestly from 5.9 (95% CI, 5.8–6.1) per 1000 patient-years to 6.9 (95% CI, 6.8–7.1) per 1000 patient-years, with the largest increase observed in those >80 years of age.147
In data from the ARIC study, the prevalence of AF in the setting of MI increased slightly, from 11% to 15%, between 1987 and 2009. However, the increased risk of death (OR, 1.47; 95% CI, 1.07–2.01) in the year after MI accompanied by AF did not change over time.148
An increased incidence of nonvalvular AF has also been reported from the United Kingdom between 2001 and 2013.147
Risk Factors
(See Chart 17-9)
Chart 17-9. Population attributable fraction of major risk factors for atrial fibrillation in the ARIC study.
ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index (in kg/m2); cardiac disease, patients with history of coronary artery disease or heart failure; and smoking, current smoker.
Data derived from Huxley et al.150
-
Standard risk factors
Hypertension accounted for ≈14%149 to 22%150 of AF cases (Chart 17-9). In MESA, the population attributable fraction of AF attributable to hypertension appeared to be higher in US non-Hispanic blacks (33.1%), Chinese (46.3%), and Hispanics (43.9%) than in non-Hispanic whites (22.2%).151
ARIC,152 the FHS,21,153,154 and the Women’s Health Study155 have developed risk prediction models to predict new-onset AF. Predictors of increased risk of new-onset AF include advancing age, European ancestry, body size (greater height and BMI), electrocardiography features (LV hypertrophy, left atrial enlargement), DM, BP (SBP and hypertension treatment), and presence of CVD (CHD, HF, valvular HD).
More recently, the ARIC, CHS, and FHS investigators developed and validated a risk prediction model for AF in blacks and whites, which was replicated in 2 European cohorts.156 The CHARGE-AF model has been validated in a US multiethnic cohort including Hispanics157 and in a UK cohort (EPIC Norfolk).158
Other consistently reported risk factors for AF include clinical and subclinical hyperthyroidism,159,160 CKD,161 and moderate162 or heavy alcohol consumption.163
-
Family history
Although unusual, early-onset lone AF has long been recognized to cluster in families.164,165
-
In the past decade, the heritability of AF in the community has been appreciated. In studies from the FHS:
Adjusted for coexistent risk factors, having at least 1 parent with AF was associated with a 1.85-fold increased risk of AF in the adult offspring (multivariable-adjusted 95% CI, 1.12–3.06; P=0.02).166
A history of a first-degree relative with AF also was associated with an increased risk of AF (HR, 1.40; 95% CI, 1.13–1.74).152 The risk was greater if the first-degree relative’s age of onset was ≤65 years (HR, 2.01; 95% CI, 1.49–2.71) and with each additional affected first-degree relative (HR, 1.24; 95% CI, 1.05–1.46).167
Similar findings were reported from Sweden.168
-
Genetics
Mutations in genes coding channels (sodium and potassium), gap junction proteins, and signaling have been described, often in lone AF or familial AF series, but they are responsible for few cases of AF in the community.169
Meta-analyses of GWAS have revealed that single-nucleotide polymorphisms on chromosomes 4q25 (upstream of PITX2),170–172 16q22 (ZFHX3),171,173 and 1q21 (KCNN3),172 as well as 6 other novel susceptibility loci (near PRRX1, CAV1, C9orf3, SYNPO2L, SYNE2, and HCN4),174 are associated with AF in individuals of European and Japanese ancestry.175 Although an area of intensive inquiry, the causative single-nucleotide polymorphisms and the functional basis of the associations have not been revealed.
Some studies suggest that genetic markers of AF could improve risk prediction for AF over models that include clinical factors.155
-
Borderline risk factors
Data from the ARIC study indicated that having at least 1 elevated risk factor explained 50% and having at least 1 borderline risk factor explained 6.5% of incident AF cases. The estimated overall incidence rate per 1000 person-years at a mean age of 54.2 years was 2.19 for those with optimal risk, 3.68 for those with borderline risk, and 6.59 for those with elevated risk factors.150
Prevention
(See Charts 17-9 and 17-10)
Chart 17-10. Time-to-event analysis of incidence of atrial fibrillation by category of METs in the FIT Project between 1991 and 2009.
The P value was determined by a log-rank test. FIT indicates Henry Ford Exercise Testing; and METs, metabolic equivalents.
Reprinted from Qureshi et al.179 Copyright © 2015, American Heart Association, Inc.
On the basis of data from ARIC, the highest PAR for AF was hypertension, followed by BMI, smoking, cardiac disease, and DM (Chart 17-9).
Data from some studies suggested that vigorous-intensity exercise 5 to 7 days a week was associated with a slightly increased risk of AF (HR, 1.20; P=0.04).124 In contrast, a meta-analysis suggested that more intensive physical activity was not associated with excess risk of AF (RR, 1.0; 95% CI, 0.82–1.22), but the heterogeneity statistic was significant.176
Observational data from the CHS suggested that moderate-intensity exercise (such as regular walking) was associated with a lower risk of AF (HR, 0.72).177 Similar data have been reported from Sweden.178 A multiracial longitudinal study from Detroit reported a dose-response relation between objectively assessed exercise capacity and lower risk of new-onset AF.179 In unadjusted analyses, the incidence rates of AF over 5 years were 3.7%, 5.0%, 9.5%, and 18.8% for >11, 10 to 11, 6 to 9, and <6 metabolic equivalents, respectively. Every 1 higher peak metabolic equivalent was associated with an adjusted 7% lower risk of AF (HR, 0.93; 95% CI, 0.92–0.94). The protective association of fitness was observed in all subgroups examined but was particularly beneficial in obese individuals (Chart 17-10).
Although heterogeneous in their findings, modest-sized short-term studies suggested that the use of statins might prevent AF; however, larger longer-term studies do not provide support for the concept that statins are effective in AF prevention.180
Treatment of obstructive sleep apnea has been noted to decrease risk of recurrent AF, after car-dioversion181 and ablation,182 but its role in primary prevention is unproven.
In a national outpatient registry of AF patients (ORBIT-AF), 93.5% had indications for guideline-based primary or secondary prevention in addition to oral anticoagulant drugs; however, only 46.6% received all guideline-indicated therapies, consistent with an underutilization of evidence-based preventive therapies for comorbid conditions in individuals with AF.182 Predictors of not receiving all guideline-indicated therapies included frailty, comorbid illness, geographic region, and antiar-rhythmic drug therapy. Factors most strongly associated with the 17.1% warfarin discontinuation rate in the first year prescribed included hospitalization because of bleeding (OR, 10.9; 95% CI, 7.9–15.0), prior catheter ablation (OR, 1.8; 95% CI, 1.4–2.4), noncardiovascular/nonbleeding hospitalization (OR, 1.8; 95% CI, 1.4–2.2), cardiovascular hospitalization (OR, 1.6; 95% CI, 1.3–2.0), and permanent AF (OR, 0.25; 95% CI, 0.17–0.36).183
Prevention: Randomized Data
Intensive glycemic control was not found to prevent incident AF in the ACCORD study.103
In the Look AHEAD randomized trial of individuals with type 2 DM who were overweight to obese, an intensive lifestyle intervention associated with modest weight loss did not significantly affect the rate of incident AF (6.1 versus 6.7 cases per 1000 person-years of follow up; multivariable HR, 0.99; 95% CI, 0.77–1.28). However, AF was not prespecified as a primary or prespecified secondary outcome.184
In individuals with AF, there are increasing data supporting the importance of risk factor modification for secondary prevention of AF recurrence and improved symptoms. Randomized trials of overweight or obese patients referred for management of symptomatic paroxysmal or persistent AF to an Adelaide, Australia, arrhythmia clinic demonstrated that weight loss was associated with a dose-dependent greater likelihood of being arrhythmia free185 and reporting a lower symptom burden.185,186 Similarly, in individuals referred for catheter ablation, those who agreed to aggressive risk factor modification had lower symptom burden in follow-up.187
Meta-analyses have suggested that renin-angiotensin system blockers might be useful in primary and secondary (recurrences) prevention of AF in trials of hypertension, after MI, in HF, and after cardio-version.102,188 However, the studies were primarily secondary or post hoc analyses, and the results were fairly heterogeneous. Recently, in an analysis of the EMPHASIS-HF trial, in one of many secondary outcomes, eplerenone was nominally observed to reduce the incidence of new-onset AF.120
Awareness
In a US national biracial study of individuals with AF, compared with whites, blacks had approximately one third the likelihood (OR, 0.32; 95% CI, 0.20–0.52) of being aware that they had AF.189
Global Burden of AF
(See Chart 17-11)
-
The vast majority of research on the epidemiology of AF has been conducted in Europe and North America. Investigators from the GBD project noted that the global prevalence, incidence, mortality, and DALYs associated with AF increased from 1990 to 2010190 (Chart 17-11).
The 2010 worldwide prevalence of AF was estimated at 33.5 million: 20.9 million males (95% CI, 19.5–22.2 million) and 12.6 million females (95% CI, 12.0–13.7). In 2010, the age-adjusted AF prevalence per 100 000 people was estimated to be 596.2 (95% CI, 558.4–636.7) in males and 373.1 (95% CI, 347.9–402.2) in females.
The 2010 estimated annual AF incidence per 100 000 person-years was estimated to be 77.5 (95% CI, 65.2–95.4) in males and 59.5 (95% CI, 49.9–74.9) in females.
Although AF accounted for <1% of global deaths, the age-adjusted mortality rate was 1.6 (95% UI, 1.0–2.4) in males and 1.7 (95% UI, 1.4–2.2) in females in 2010.
The 2010 estimated DALYs per 100 000 population from AF were 64.5 (95% UI, 46.8–84.2) and 45.9 (95% UI, 35.7–58.5) in 2010; DALYs were higher in developed than in developing countries.
Tachycardia
ICD-9 427.0, 427.1, 427.2; ICD-10 I47.1, I47.2, I47.9.
Mortality—796. Any-mention mortality—6708. Hospital discharges—78 000.
Premature Ventricular Contractions
In the population-based CHS, a study of older adults without HF or systolic dysfunction studied by Holter monitor (median duration, 22.2 hours), 0.011% of all heart beats were PVCs, and 5.5% of participants had nonsustained VT. Over follow up, baseline PVC percentage was significantly associated with an adjusted increased odds of decreased LVEF (OR 1.13; 95% CI, 1.05–1.21) and an increased adjusted risk of incident HF (HR, 1.06; 95% CI, 1.02–1.09) and death (HR, 1.04; 95% CI, 1.02–1.06).191
Monomorphic VT
Prevalence and Incidence
The true prevalence and incidence of monomorphic VT in the US general population are not known.
Of 150 consecutive patients with wide-complex tachycardia subsequently studied by invasive electrophysiological study, 122 (81%) had VT; the remainder had SVT.192
Of patients with ventricular arrhythmias presenting for invasive electrophysiological studies, 11% to 21% had no structural HD, and the majority of those with structural HD had CAD.193,194
In 634 patients with implantable cardioverter-defibrillators who had structural HD (including both primary and secondary prevention patients) followed up for a mean 11±3 months, ≈80% of potentially clinically relevant ventricular tachyarrhythmias were attributable to VT amenable to antitachycardia pacing (which implies a stable circuit and therefore monomorphic VT).195 Because therapy may have been delivered before spontaneous resolution occurred, the proportion of these VT episodes with definite clinical relevance is not known.
Of those with VT in the absence of structural HD, RV outflow tract VT is the most common form.196
Among 2099 subjects (mean age, 52 years; 52.2% male) without known CVD, exercise-induced non-sustained VT occurred in nearly 4% and was not independently associated with total mortality.197
Complications
Polymorphic VT
Prevalence and Incidence
The true prevalence and incidence of PVT in the US general population are not known.
During ambulatory cardiac monitoring, PVT prevalence ranged from 0.01% to 0.15%202,203; however, among patients who developed SCD during ambulatory cardiac monitoring, PVT was detected in 30% to 43%.203–205
In the setting of AMI, the prevalence of PVT ranged from 1.2% to 2%.206,207
Out-of-hospital PVT is estimated to be present in ≈25% of all cardiac arrest cases involving VT.208,209
A prevalence range of 15% to 19% was reported during electrophysiological study in patients resuscitated from cardiac arrest.205,210,211
Complications
Risk Factors
PVT in the setting of a normal QT interval is most frequently seen in the context of acute ischemia or MI.214,215
Less frequently, PVT with a normal QT interval can occur in patients without apparent structural HD. Catecholaminergic PVT, which is discussed under inherited arrhythmic syndromes, is one such disorder.
A prolonged QT interval, whether acquired (drug induced) or congenital, is a common cause of PVT. Drug-induced prolongation of the QT interval that causes PVT is discussed under TdP, whereas congenital prolonged QT interval is discussed under inherited arrhythmic syndromes.
Torsade de Pointes
Prevalence and Incidence
The true incidence and prevalence of drug-induced TdP in the US general population are largely unknown.
By extrapolating data from non-US registries,216 it has been estimated that 12 000 cases of drug-induced TdP occur annually in the United States.206
A prospective, active surveillance, Berlin-based registry of 51 hospitals observed that the annual incidence of symptomatic drug-induced QT prolongation in adults was 2.5 per million males and 4.0 per million females. The authors reported 42 potentially associated drugs, including metoclopramide, amiodarone, melperone, citalopram, and levomethadaone. The mean age of patients with QT prolongation/TdP was 57±20 years, and the majority of the cases occurred in females (66%), out of the hospital (60%).217
The prevalence of drug-induced prolongation of QT interval and TdP is 2 to 3 times higher in females than in males.207
With the majority of QT-interval–prolonging drugs, drug-induced TdP can occur in 3% to 15% of patients.205
Antiarrhythmic drugs with QT-interval–prolonging potential carry a 1% to 3% risk of TdP over 1 to 2 years of exposure.218
Complications
Drug-induced TdP can result in morbidity that requires hospitalization and in mortality attributable to SCD in ≤31% of patients.206,219
Patients with advanced HF with a history of drug-induced TdP had a significantly higher risk of SCD during therapy with amiodarone than amiodarone-treated patients with no history of drug-induced TdP (55% versus 15%).220
Current use of antipsychotic drugs was associated with a significant increase in the risk of SCD attributable to TdP (OR, 3.3; 95% CI, 1.8–6.2).221
In a cohort of 459 614 Medicaid and Medicaid-Medicare enrollees aged 30 to 75 years who were taking antipsychotic medications, the incidence of sudden death or ventricular arrhythmia was 3.4 per 1000 person-years.222
Hospitalization was required in 47% and death occurred in 8% of patients with QT-interval prolongation and TdP caused by administration of methadone.223
Risk Factors
TdP is usually related to administration of QT-interval–prolonging drugs.224 An up-to-date list of drugs with the potential to cause TdP is available at a Web site maintained by the University of Arizona Center for Education and Research on Therapeutics.225
Specific risk factors for drug-induced TdP include prolonged QT interval, female sex, advanced age, bradycardia, hypokalemia, hypomagnesemia, LV systolic dysfunction, and conditions that lead to elevated plasma concentrations of causative drugs, such as kidney disease, liver disease, drug interactions, or some combination of these.206,226,227
Predisposition was also noted in patients who had a history of ventricular arrhythmia and who experienced a recent symptomatic increase in the frequency and complexity of ectopy.228
Drug-induced TdP rarely occurs in patients without concomitant risk factors. An analysis of 144 published articles describing TdP associated with non-cardiac drugs revealed that 100% of the patients had at least 1 risk factor, and 71% had at least 2 risk factors.229
Both common and rare genetic variants have been shown to increase the propensity to drug-induced QT interval prolongation.230,231
Prevention
Keys to reducing the incidence of drug-induced cardiac arrhythmias include increased awareness among the medical, pharmaceutical, and nursing professions of the potential problems associated with the use of certain agents.
Appropriate monitoring when a QT-interval–prolonging drug is administered is essential. Also, prompt withdrawal of the offending agent should be initiated.232
REFERENCES
- 1.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR for the Atherosclerosis Risk in Communities (ARIC) Study Investigators. Electrocardiographic findings in a healthy biracial population. Am J Cardiol. 1998;81:453–459. doi: 10.1016/S0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 2.Walsh JA, 3rd, Prineas R, Daviglus ML, Ning H, Liu K, Lewis CE, Sidney S, Schreiner PJ, Iribarren C, Lloyd-Jones DM. Prevalence of electrocardiographic abnormalities in a middle-aged, biracial population: Coronary Artery Risk Development in Young Adults study. J Electrocardiol. 2010;43:385.e1–385.e9. doi: 10.1016/j.jelectrocard.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, Reunanen A, Huikuri HV. Prognostic significance of prolonged PR interval in the general population. Eur Heart J. 2014;35:123–129. doi: 10.1093/eurheartj/eht176. [DOI] [PubMed] [Google Scholar]
- 4.Wolbrette D, Naccarelli G. Bradycardias: sinus nodal dysfunction and atrioventricular conduction disturbances. In: Topol EJ, editor. Textbook of Cardiovascular Medicine. 3. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojic EM, Hardarson T, Sigfusson N, Sigvaldason H. The prevalence and prognosis of third-degree atrioventricular conduction block: the Reykjavik study. J Intern Med. 1999;246:81–86. doi: 10.1046/j.1365-2796.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- 7.Ostrander LD, Jr, Brandt RL, Kjelsberg MO, Epstein FH. Electrocardiographic findings among the adult population of a total natural community, Tecumseh, Michigan. Circulation. 1965;31:888–898. doi: 10.1161/01.cir.31.6.888. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RL, Averill KH, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects, VII: atrioventricular block. Am J Cardiol. 1960;6:153–177. doi: 10.1016/0002-9149(60)90044-8. [DOI] [PubMed] [Google Scholar]
- 9.Rose G, Baxter PJ, Reid DD, McCartney P. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J. 1978;40:636–643. doi: 10.1136/hrt.40.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of third-degree atrioventricular block in patients with type II diabetes mellitus. Chest. 2005;128:2611–2614. doi: 10.1378/chest.128.4.2611. [DOI] [PubMed] [Google Scholar]
- 11.Bordachar P, Zachary W, Ploux S, Labrousse L, Haissaguerre M, Thambo JB. Pathophysiology, clinical course, and management of congenital complete atrioventricular block. Heart Rhythm. 2013;10:760–766. doi: 10.1016/j.hrthm.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Turner CJ, Wren C. The epidemiology of arrhythmia in infants: a population-based study. J Paediatr Child Health. 2013;49:278–281. doi: 10.1111/jpc.12155. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 16.Mymin D, Mathewson FA, Tate RB, Manfreda J. The natural history of primary first-degree atrioventricular heart block. N Engl J Med. 1986;315:1183–1187. doi: 10.1056/NEJM198611063151902. [DOI] [PubMed] [Google Scholar]
- 17.Barold SS, Ilercil A, Leonelli F, Herweg B. First-degree atrioventricular block: clinical manifestations, indications for pacing, pacemaker management & consequences during cardiac resynchronization. J Interv Card Electrophysiol. 2006;17:139–152. doi: 10.1007/s10840-006-9065-x. [DOI] [PubMed] [Google Scholar]
- 18.Goldberger JJ, Johnson NP, Gidea C. Significance of asymptomatic bradycardia for subsequent pacemaker implantation and mortality in patients >60 years of age. Am J Cardiol. 2011;108:857–861. doi: 10.1016/j.amjcard.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Soliman EZ, Alonso A, Misialek JR, Jain A, Watson KE, Lloyd-Jones DM, Lima J, Shea S, Burke GL, Heckbert SR. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) J Electrocardiol. 2013;46:702–706. doi: 10.1016/j.jelectro-card.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm W, Koehler U, Fus E, Hoffmann J, Menz V, Funck R, Peter JH, Maisch B. Outcome of patients with sleep apnea-associated severe bradyarrhythmias after continuous positive airway pressure therapy. Am J Cardiol. 2000;86:688–692. A9. doi: 10.1016/s0002-9149(00)01055-9. [DOI] [PubMed] [Google Scholar]
- 21.Glickstein JS, Buyon J, Friedman D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am J Cardiol. 2000;86:236–239. doi: 10.1016/s0002-9149(00)00867-5. [DOI] [PubMed] [Google Scholar]
- 22.Brignole M, Menozzi C, Lolli G, Oddone D, Gianfranchi L, Bertulla A. Pacing for carotid sinus syndrome and sick sinus syndrome. Pacing Clin Electrophysiol. 1990;13(pt 2):2071–2075. doi: 10.1111/j.1540-8159.1990.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 23.Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003;67:1725–1732. [PubMed] [Google Scholar]
- 24.Rodriguez RD, Schocken DD. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics. 1990;45:26–30. 33–36. [PubMed] [Google Scholar]
- 25.Sutton R, Kenny RA. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986;9(pt 2):1110–1114. doi: 10.1111/j.1540-8159.1986.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 26.Brignole M. Sick sinus syndrome. Clin Geriatr Med. 2002;18:211–227. doi: 10.1016/s0749-0690(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 27.Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–538. doi: 10.1016/j.jacc.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menozzi C, Brignole M, Alboni P, Boni L, Paparella N, Gaggioli G, Lolli G. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol. 1998;82:1205–1209. doi: 10.1016/s0002-9149(98)00605-5. [DOI] [PubMed] [Google Scholar]
- 29.Simon AB, Janz N. Symptomatic bradyarrhythmias in the adult: natural history following ventricular pacemaker implantation. Pacing Clin Electrophysiol. 1982;5:372–383. doi: 10.1111/j.1540-8159.1982.tb02245.x. [DOI] [PubMed] [Google Scholar]
- 30.Alt E, Völker R, Wirtzfeld A, Ulm K. Survival and follow-up after pacemaker implantation: a comparison of patients with sick sinus syndrome, complete heart block, and atrial fibrillation. Pacing Clin Electrophysiol. 1985;8:849–855. doi: 10.1111/j.1540-8159.1985.tb05904.x. [DOI] [PubMed] [Google Scholar]
- 31.Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NA, 3rd, Greenspon A, Goldman L Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854–1862. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- 32.Hesselson AB, Parsonnet V, Bernstein AD, Bonavita GJ. Deleterious effects of long-term single-chamber ventricular pacing in patients with sick sinus syndrome: the hidden benefits of dual-chamber pacing. J Am Coll Cardiol. 1992;19:1542–1549. doi: 10.1016/0735-1097(92)90616-u. [DOI] [PubMed] [Google Scholar]
- 33.Udo EO, van Hemel NM, Zuithoff NP, Doevendans PA, Moons KG. Prognosis of the bradycardia pacemaker recipient assessed at first implantation: a nationwide cohort study. Heart. 2013;99:1573–1578. doi: 10.1136/heartjnl-2013-304445. [DOI] [PubMed] [Google Scholar]
- 34.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) [published correction appears in Circulation. 2009;120:e34–e35] Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 35.Birnie D, Williams K, Guo A, Mielniczuk L, Davis D, Lemery R, Green M, Gollob M, Tang A. Reasons for escalating pacemaker implants. Am J Cardiol. 2006;98:93–97. doi: 10.1016/j.amj-card.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 36.Svendsen JH, Nielsen JC, Darkner S, Jensen GV, Mortensen LS, Andersen HR DANPACE Investigators. CHADS2 and CHA2DS2-VASc score to assess risk of stroke and death in patients paced for sick sinus syndrome. Heart. 2013;99:843–848. doi: 10.1136/heartjnl-2013-303695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Issa Z, Miller J, Zipes D. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- 38.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULA-TIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 39.Rosenqvist M, Obel IW. Atrial pacing and the risk for AV block: is there a time for change in attitude? Pacing Clin Electrophysiol. 1989;12(pt 1):97–101. doi: 10.1111/pace.1989.12.p1.97. [DOI] [PubMed] [Google Scholar]
- 40.Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 41.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 42.Orejarena LA, Vidaillet H, Jr, DeStefano F, Nordstrom DL, Vierkant RA, Smith PN, Hayes JJ. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–157. doi: 10.1016/s0735-1097(97)00422-1. [DOI] [PubMed] [Google Scholar]
- 43.Murman DH, McDonald AJ, Pelletier AJ, Camargo CA., Jr U.S. emergency department visits for supraventricular tachycardia, 1993–2003. Acad Emerg Med. 2007;14:578–581. doi: 10.1197/j.aem.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Still AM, Raatikainen P, Ylitalo A, Kauma H, Ikäheimo M, Antero Kesäniemi Y, Huikuri HV. Prevalence, characteristics and natural course of inappropriate sinus tachycardia. Europace. 2005;7:104–112. doi: 10.1016/j.eupc.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Maurer MS, Shefrin EA, Fleg JL. Prevalence and prognostic significance of exercise-induced supraventricular tachycardia in apparently healthy volunteers. Am J Cardiol. 1995;75:788–792. doi: 10.1016/s0002-9149(99)80412-3. [DOI] [PubMed] [Google Scholar]
- 46.Poutiainen AM, Koistinen MJ, Airaksinen KE, Hartikainen EK, Kettunen RV, Karjalainen JE, Huikuri HV. Prevalence and natural course of ectopic atrial tachycardia. Eur Heart J. 1999;20:694–700. doi: 10.1053/euhj.1998.1313. [DOI] [PubMed] [Google Scholar]
- 47.Wu EB, Chia HM, Gill JS. Reversible cardiomyopathy after radio-frequency ablation of lateral free-wall pathway-mediated incessant supraventricular tachycardia. Pacing Clin Electrophysiol. 2000;23:1308–1310. doi: 10.1111/j.1540-8159.2000.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang YS, Scheinman MM, Chien WW, Cohen TJ, Lesh MD, Griffin JC. Patients with supraventricular tachycardia presenting with aborted sudden death: incidence, mechanism and long-term follow-up. J Am Coll Cardiol. 1991;18:1711–1719. doi: 10.1016/0735-1097(91)90508-7. [DOI] [PubMed] [Google Scholar]
- 49.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, Devereux RB, Fink ME. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brembilla-Perrot B, Houriez P, Beurrier D, Claudon O, Burger G, Vançon AC, Mock L. Influence of age on the electrophysiological mechanism of paroxysmal supraventricular tachycardias. Int J Cardiol. 2001;78:293–298. doi: 10.1016/s0167-5273(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 51.Porter MJ, Morton JB, Denman R, Lin AC, Tierney S, Santucci PA, Cai JJ, Madsen N, Wilber DJ. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393–396. doi: 10.1016/j.hrthm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Bradley DJ, Fischbach PS, Law IH, Serwer GA, Dick M., 2nd The clinical course of multifocal atrial tachycardia in infants and children. J Am Coll Cardiol. 2001;38:401–408. doi: 10.1016/s0735-1097(01)01390-0. [DOI] [PubMed] [Google Scholar]
- 53.Kastor JA. Multifocal atrial tachycardia. N Engl J Med. 1990;322:1713–1717. doi: 10.1056/NEJM199006143222405. [DOI] [PubMed] [Google Scholar]
- 54.Anand RG, Rosenthal GL, Van Hare GF, Snyder CS. Is the mechanism of supraventricular tachycardia in pediatrics influenced by age, gender or ethnicity? Congenit Heart Dis. 2009;4:464–468. doi: 10.1111/j.1747-0803.2009.00336.x. [DOI] [PubMed] [Google Scholar]
- 55.De Bacquer D, De Backer G, Kornitzer M. Prevalences of ECG findings in large population based samples of men and women. Heart. 2000;84:625–633. doi: 10.1136/heart.84.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sano S, Komori S, Amano T, Kohno I, Ishihara T, Sawanobori T, Ijiri H, Tamura K. Prevalence of ventricular preexcitation in Japanese schoolchildren. Heart. 1998;79:374–378. doi: 10.1136/hrt.79.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCord J, Borzak S. Multifocal atrial tachycardia. Chest. 1998;113:203–209. doi: 10.1378/chest.113.1.203. [DOI] [PubMed] [Google Scholar]
- 58.Blomström-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Jr, Stevenson WG, Tomaselli GF. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias) Circulation. 2003;108:1871–1909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- 59.Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, Holmes DR, Jr, Gersh BJ. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation. 1993;87:866–873. doi: 10.1161/01.cir.87.3.866. [DOI] [PubMed] [Google Scholar]
- 60.Leitch JW, Klein GJ, Yee R, Murdock C. Prognostic value of electrophysiology testing in asymptomatic patients with Wolff-Parkinson-White pattern [published correction appears in Circulation. 1991;83:1124] Circulation. 1990;82:1718–1723. doi: 10.1161/01.cir.82.5.1718. [DOI] [PubMed] [Google Scholar]
- 61.Dagres N, Clague JR, Lottkamp H, Hindricks G, Breithardt G, Borggrefe M. Impact of radiofrequency catheter ablation of accessory pathways on the frequency of atrial fibrillation during long-term follow-up; high recurrence rate of atrial fibrillation in patients older than 50 years of age. Eur Heart J. 2001;22:423–427. doi: 10.1053/euhj.2000.2429. [DOI] [PubMed] [Google Scholar]
- 62.Goudevenos JA, Katsouras CS, Graekas G, Argiri O, Giogiakas V, Sideris DA. Ventricular pre-excitation in the general population: a study on the mode of presentation and clinical course. Heart. 2000;83:29–34. doi: 10.1136/heart.83.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of electrocardiographic preexcitation in men: the Manitoba Follow-up Study. Ann Intern Med. 1992;116:456–460. doi: 10.7326/0003-4819-116-6-456. [DOI] [PubMed] [Google Scholar]
- 64.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 65.Pappone C, Vicedomini G, Manguso F, Saviano M, Baldi M, Pappone A, Ciaccio C, Giannelli L, Ionescu B, Petretta A, Vitale R, Cuko A, Calovic Z, Fundaliotis A, Moscatiello M, Tavazzi L, Santinelli V. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130:811–819. doi: 10.1161/CIRCULATIONAHA.114.011154. [DOI] [PubMed] [Google Scholar]
- 66.Obeyesekere MN, Leong-Sit P, Massel D, Manlucu J, Modi S, Krahn AD, Skanes AC, Yee R, Gula LJ, Klein GJ. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125:2308–2315. doi: 10.1161/CIRCULATIONAHA.111.055350. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K, Igarashi H, Fukushige J, Ohno T, Joh K, Hara T. Long-term prospective study on the natural history of Wolff-Parkinson-White syndrome detected during a heart screening program at school. Acta Paediatr. 2000;89:542–545. doi: 10.1080/080352500750027817. [DOI] [PubMed] [Google Scholar]
- 68.Pappone C, Manguso F, Santinelli R, Vicedomini G, Sala S, Paglino G, Mazzone P, Lang CC, Gulletta S, Augello G, Santinelli O, Santinelli V. Radiofrequency ablation in children with asymptomatic Wolff-Parkinson-White syndrome. N Engl J Med. 2004;351:1197–1205. doi: 10.1056/NEJMoa040625. [DOI] [PubMed] [Google Scholar]
- 69.Cain N, Irving C, Webber S, Beerman L, Arora G. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am J Cardiol. 2013;112:961–965. doi: 10.1016/j.amjcard.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 70.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 71.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke [published correction appears in N Engl J Med. 2016;374:998] N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 72.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35:508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP Study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 74.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: a systematic review. Thromb Haemost. 2013;110:213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 75.Moran PS, Flattery MJ, Teljeur C, Ryan M, Smith SM. Effectiveness of systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2013;(4):CD009586. doi: 10.1002/14651858.CD009586.pub2. [DOI] [PubMed] [Google Scholar]
- 76.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies: the SEARCH-AF study. Thromb Haemost. 2014;111:1167–1176. doi: 10.1160/TH14-03-0231. [DOI] [PubMed] [Google Scholar]
- 77.McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, Harrington J, Mick E, Chon KH. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm. 2013;10:315–319. doi: 10.1016/j.hrthm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 79.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence [published correction appears in Circulation. 2006;114:e498] Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 80.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 81.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen AY, Contreras R, Sobnosky S, Shah AI, Ichiuji AM, Jorgensen MB, Brar SS, Chen W. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults: a cross-sectional study. J Natl Med Assoc. 2010;102:906–913. doi: 10.1016/s0027-9684(15)30709-4. [DOI] [PubMed] [Google Scholar]
- 83.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 85.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 86.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 87.Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GY. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–119. doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 88.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Centers for Disease Control and Prevention, National Center for Health Statistics. Mortality multiple cause micro-data files, 2013: public-use data file and documentation. [Accessed May 19, 2015];NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 90.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 91.Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 93.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S RE-LY Investigators. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 94.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 95.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 96.Cheng M, Lu X, Huang J, Zhang J, Zhang S, Gu D. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a meta-analysis. Eur J Heart Fail. 2014;16:1317–1322. doi: 10.1002/ejhf.187. [DOI] [PubMed] [Google Scholar]
- 97.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study [published correction appears in Circulation. 2013;128:e465] Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2011;141:1305–1312. doi: 10.1016/j.jtcvs.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 101.Phan K, Ha HS, Phan S, Medi C, Thomas SP, Yan TD. New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2015;48:817–824. doi: 10.1093/ejcts/ezu551. [DOI] [PubMed] [Google Scholar]
- 102.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation: the Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 103.Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study) Am J Cardiol. 2014;114:1217–1222. doi: 10.1016/j.amjcard.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis [published correction appears in Nephrol Dial Transplant. 2014;29:2152] Nephrol Dial Transplant. 2012;27:3816–3822. doi: 10.1093/ndt/gfs416. [DOI] [PubMed] [Google Scholar]
- 105.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124:289–296. doi: 10.1161/CIRCULATIONAHA.110.011130. [DOI] [PubMed] [Google Scholar]
- 108.Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of race on outcomes (stroke and death) in patients >65 years with atrial fibrillation. Am J Cardiol. 2015;116:230–235. doi: 10.1016/j.amjcard.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li CY, Lin CP, Lin YS, Wu LS, Chang CJ, Chu PH. Newly diagnosed atrial fibrillation is an independent factor for future major adverse cardiovascular events. PLoS One. 2015;10:e0123211. doi: 10.1371/journal.pone.0123211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frost L, Engholm G, Johnsen S, Møller H, Henneberg EW, Husted S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161:272–276. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]
- 111.Bekwelem W, Connolly SJ, Halperin JL, Adabag S, Duval S, Chrolavicius S, Pogue J, Ezekowitz MD, Eikelboom JW, Wallentin LG, Yusuf S, Hirsch AT. Extracranial systemic embolic events in patients with nonvalvular atrial fibrillation: incidence, risk factors, and outcomes. Circulation. 2015;132:796–803. doi: 10.1161/CIRCULATIONAHA.114.013243. [DOI] [PubMed] [Google Scholar]
- 112.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general Medicare population: a 10-year perspective (1992 to 2002) Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 113.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 114.Hayden DT, Hannon N, Callaly E, Ní Chróinín D, Horgan G, Kyne L, Duggan J, Dolan E, O’Rourke K, Williams D, Murphy S, Kelly PJ. Rates and determinants of 5-year outcomes after atrial fibrillation-related stroke: a population study [published correction appears in Stroke. 2015;46:e262] Stroke. 2015;46:3488–3493. doi: 10.1161/STROKEAHA.115.011139. [DOI] [PubMed] [Google Scholar]
- 115.Penado S, Cano M, Acha O, Hernández JL, Riancho JA. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med. 2003;114:206–210. doi: 10.1016/s0002-9343(02)01479-1. [DOI] [PubMed] [Google Scholar]
- 116.Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gamra H, Murin J, Chiang CE, Naditch-Brûlé L, Brette S, Steg PG RealiseAF Investigators. Use of antithrombotics in atrial fibrillation in Africa, Europe, Asia and South America: insights from the International RealiseAF Survey. Arch Cardiovasc Dis. 2014;107:77–87. doi: 10.1016/j.acvd.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study: the Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 119.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(pt 1):338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B EMPHASIS-HF Study Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 121.Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, Van Gelder IC, Ellinor PT, Benjamin EJ. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012;125:2933–2943. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rienstra M, Lyass A, Murabito JM, Magnani JW, Lubitz SA, Massaro JM, Ellinor PT, Benjamin EJ. Reciprocal relations between physical disability, subjective health, and atrial fibrillation: the Framingham Heart Study. Am Heart J. 2013;166:171–178. doi: 10.1016/j.ahj.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L, Gallagher R, Neubeck L. Health-related quality of life in atrial fibrillation patients over 65 years: a review. Eur J Prev Cardiol. 2015;22:987–1002. doi: 10.1177/2047487314538855. [DOI] [PubMed] [Google Scholar]
- 124.Giacomantonio NB, Bredin SS, Foulds HJ, Warburton DE. A systematic review of the health benefits of exercise rehabilitation in persons living with atrial fibrillation. Can J Cardiol. 2013;29:483–491. doi: 10.1016/j.cjca.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 125.O’Neal WT, Qureshi WT, Judd SE, Bowling CB, Howard VJ, Howard G, Soliman EZ. Effect of falls on frequency of atrial fibrillation and mortality risk (from the REasons for Geographic And Racial Differences in Stroke Study) Am J Cardiol. 2015;116:1213–1218. doi: 10.1016/j.amjcard.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing anti-thrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 127.Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612–617. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 128.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27:936–941. doi: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 129.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. doi: 10.1093/eurheartj/eht483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 131.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction [published correction appears in JAMA Intern Med. 2014;174:308] JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M, Alonso A. Atrial fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131:1843–1850. doi: 10.1161/CIRCULATIONAHA.114.014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Neal WT, Sangal K, Zhang ZM, Soliman EZ. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol. 2014;37:750–755. doi: 10.1002/clc.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–636. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 135.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. JAMA Intern Med. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bardai A, Blom MT, van Hoeijen DA, van Deutekom HW, Brouwer HJ, Tan HL. Atrial fibrillation is an independent risk factor for ventricular fibrillation: a large-scale population-based case-control study. Circ Arrhythm Electrophysiol. 2014;7:1033–1039. doi: 10.1161/CIRCEP.114.002094. [DOI] [PubMed] [Google Scholar]
- 138.Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, McGavigan AD. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37:1591–1602. doi: 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]
- 139.Rahman F, Wang N, Yin X, Ellinor PT, Lubitz SA, LeLorier PA, McManus DD, Sullivan LM, Seshadri S, Vasan RS, Benjamin EJ, Magnani JW. Atrial flutter: clinical risk factors and adverse outcomes in the Framingham Heart Study. Heart Rhythm. 2016;13:233–240. doi: 10.1016/j.hrthm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, Division of Health Care Statistics. [Accessed July 22, 2013];Distribution of first-listed diagnoses among hospital discharges with diabetes as any listed diagnosis, adults aged 18 years and older, United States. 2010 http://www.cdc.gov/diabetes/statistics/hosp/adulttable1.htm.
- 141.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 142.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 143.Turakhia MP, Shafrin J, Bognar K, Goldman DP, Mendys PM, Abdulsattar Y, Wiederkehr D, Trocio J. Economic burden of undiagnosed nonvalvular atrial fibrillation in the United States. Am J Cardiol. 2015;116:733–739. doi: 10.1016/j.amjcard.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 144.Wang G, Joo H, Tong X, George MG. Hospital costs associated with atrial fibrillation for patients with ischemic stroke aged 18–64 years in the United States. Stroke. 2015;46:1314–1320. doi: 10.1161/STROKEAHA.114.008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 Year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, Killian JM, Weston SA, Roger VL. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260–267.e1. doi: 10.1016/j.amjmed.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martinez C, Katholing A, Wallenhorst C, Granziera S, Cohen AT, Freedman SB. Increasing incidence of non-valvular atrial fibrillation in the UK from 2001 to 2013. Heart. 2015;101:1748–1754. doi: 10.1136/heartjnl-2015-307808. [DOI] [PubMed] [Google Scholar]
- 148.Bengtson LG, Chen LY, Chamberlain AM, Michos ED, Whitsel EA, Lutsey PL, Duval S, Rosamond WD, Alonso A. Temporal trends in the occurrence and outcomes of atrial fibrillation in patients with acute myocardial infarction (from the Atherosclerosis Risk in Communities Surveillance Study) Am J Cardiol. 2014;114:692–697. doi: 10.1016/j.amjcard.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 150.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC, Jr, Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76. 76.e1. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, Pencina MJ, D’Agostino RB, Sr, Levy D, Kannel WB, Wang TJ, Kronmal RA, Wolf PA, Burke GL, Launer LJ, Vasan RS, Psaty BM, Benjamin EJ, Gudnason V, Heckbert SR. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. [Accessed July 20, 2016];Framingham Heart Study AF score (10-year risk) 2016 http://www.framinghamheartstudy.org/risk-functions/atrial-fibrillation/10-year-risk.php.
- 155.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–2251. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shulman E, Kargoli F, Aagaard P, Hoch E, Di Biase L, Fisher J, Gross J, Kim S, Krumerman A, Ferrick KJ. Validation of the Framingham Heart Study and CHARGE-AF risk scores for atrial fibrillation in Hispanics, African-Americans, and non-Hispanic whites. Am J Cardiol. 2016;117:76–83. doi: 10.1016/j.amjcard.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 158.Pfister R, Brägelmann J, Michels G, Wareham NJ, Luben R, Khaw KT. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015;22:932–939. doi: 10.1177/2047487314544045. [DOI] [PubMed] [Google Scholar]
- 159.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D’Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 160.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281–289. doi: 10.1016/j.jacc.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 163.Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427–436. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 164.Wolff L. Familial auricular fibrillation. N Engl J Med. 1943;229:396–398. [Google Scholar]
- 165.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 166.Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 167.Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, Larson MG, Ellinor PT, Benjamin EJ. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zöller B, Ohlsson H, Sundquist J, Sundquist K. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. J Am Heart Assoc. 2013;2:e003384. doi: 10.1161/JAHA.112.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ellinor PT, MacRae CA. Ion channel mutations in AF: signal or noise? Heart Rhythm. 2008;5:436–437. doi: 10.1016/j.hrthm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 171.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, Schnabel RB, Bis JC, Boerwinkle E, Sinner MF, Dehghan A, Lubitz SA, D’Agostino RB, Sr, Lumley T, Ehret GB, Heeringa J, Aspelund T, Newton-Cheh C, Larson MG, Marciante KD, Soliman EZ, Rivadeneira F, Wang TJ, Eiríksdottir G, Levy D, Psaty BM, Li M, Chamberlain AM, Hofman A, Vasan RS, Harris TB, Rotter JI, Kao WH, Agarwal SK, Stricker BH, Wang K, Launer LJ, Smith NL, Chakravarti A, Uitterlinden AG, Wolf PA, Sotoodehnia N, Köttgen A, van Duijn CM, Meitinger T, Mueller M, Perz S, Steinbeck G, Wichmann HE, Lunetta KL, Heckbert SR, Gudnason V, Alonso A, Kääb S, Ellinor PT, Witteman JC. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Köttgen A, Moebus S, Newton-Cheh C, Li M, Möhlenkamp S, Wang TJ, Kao WH, Vasan RS, Nöthen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kääb S. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, Gulcher J, Mathiesen EB, Njølstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Kucera G, Stubblefield T, Carter S, Roden D, Ng MC, Baum L, So WY, Wong KS, Chan JC, Gieger C, Wichmann HE, Gschwendtner A, Dichgans M, Kuhlenbäumer G, Berger K, Ringelstein EB, Bevan S, Markus HS, Kostulas K, Hillert J, Sveinbjörnsdóttir S, Valdimarsson EM, Løchen ML, Ma RC, Darbar D, Kong A, Arnar DO, Thorsteinsdottir U, Stefansson K. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dörr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Völker U, Völzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JC, Kao WH, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjögren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BH, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kääb S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lubitz SA, Lunetta KL, Lin H, Arking DE, Trompet S, Li G, Krijthe BP, Chasman DI, Barnard J, Kleber ME, Dörr M, Ozaki K, Smith AV, Müller-Nurasyid M, Walter S, Agarwal SK, Bis JC, Brody JA, Chen LY, Everett BM, Ford I, Franco OH, Harris TB, Hofman A, Kääb S, Mahida S, Kathiresan S, Kubo M, Launer LJ, Macfarlane PW, Magnani JW, McKnight B, McManus DD, Peters A, Psaty BM, Rose LM, Rotter JI, Silbernagel G, Smith JD, Sotoodehnia N, Stott DJ, Taylor KD, Tomaschitz A, Tsunoda T, Uitterlinden AG, Van Wagoner DR, Völker U, Völzke H, Murabito JM, Sinner MF, Gudnason V, Felix SB, März W, Chung M, Albert CM, Stricker BH, Tanaka T, Heckbert SR, Jukema JW, Alonso A, Benjamin EJ, Ellinor PT. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol. 2014;63:1200–1210. doi: 10.1016/j.jacc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kwok CS, Anderson SG, Myint PK, Mamas MA, Loke YK. Physical activity and incidence of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2014;177:467–476. doi: 10.1016/j.ijcard.2014.09.104. [DOI] [PubMed] [Google Scholar]
- 177.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart. 2015;101:1627–1630. doi: 10.1136/heartjnl-2014-307145. [DOI] [PubMed] [Google Scholar]
- 179.Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al-Mallah MH. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation. 2015;131:1827–1834. doi: 10.1161/CIRCULATIONAHA.114.014833. [DOI] [PubMed] [Google Scholar]
- 180.Rahimi K, Emberson J, McGale P, Majoni W, Merhi A, Asselbergs FW, Krane V, Macfarlane PW PROSPER Executive. Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ. 2011;342:d1250. doi: 10.1136/bmj.d1250. [DOI] [PubMed] [Google Scholar]
- 181.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 182.Hess PL, Kim S, Piccini JP, Allen LA, Ansell JE, Chang P, Freeman JV, Gersh BJ, Kowey PR, Mahaffey KW, Thomas L, Peterson ED, Fonarow GC. Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med. 2013;126:625–632.e1. doi: 10.1016/j.amjmed.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O’Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, Thomas LE, Ezekowitz MD, Mahaffey KW, Chang P, Piccini JP, Peterson ED. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2014;168:487–494. doi: 10.1016/j.ahj.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 184.Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, Vetter M, Mantzoros CS, Jeffery RW, Soliman EZ Look AHEAD Research Group. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170:770–777.e5. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 186.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 187.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 188.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy: Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000;35:183–187. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 189.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–587. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–109. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Akhtar M, Shenasa M, Jazayeri M, Caceres J, Tchou PJ. Wide QRS complex tachycardia: reappraisal of a common clinical problem. Ann Intern Med. 1988;109:905–912. doi: 10.7326/0003-4819-109-11-905. [DOI] [PubMed] [Google Scholar]
- 193.Sacher F, Tedrow UB, Field ME, Raymond JM, Koplan BA, Epstein LM, Stevenson WG. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008;1:153–161. doi: 10.1161/CIRCEP.108.769471. [DOI] [PubMed] [Google Scholar]
- 194.Swerdlow CD, Winkle RA, Mason JW. Determinants of survival in patients with ventricular tachyarrhythmias. N Engl J Med. 1983;308:1436–1442. doi: 10.1056/NEJM198306163082402. [DOI] [PubMed] [Google Scholar]
- 195.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS, Volosin KJ PainFREE Rx II Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 196.Lemery R, Brugada P, Bella PD, Dugernier T, van den Dool A, Wellens HJ. Nonischemic ventricular tachycardia: clinical course and long-term follow-up in patients without clinically overt heart disease. Circulation. 1989;79:990–999. doi: 10.1161/01.cir.79.5.990. [DOI] [PubMed] [Google Scholar]
- 197.Marine JE, Shetty V, Chow GV, Wright JG, Gerstenblith G, Najjar SS, Lakatta EG, Fleg JL. Prevalence and prognostic significance of exercise-induced nonsustained ventricular tachycardia in asymptomatic volunteers: BLSA (Baltimore Longitudinal Study of Aging) J Am Coll Cardiol. 2013;62:595–600. doi: 10.1016/j.jacc.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 199.Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F, Jr, Crawford T, Ebinger M, Oral H, Morady F, Bogun F. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 200.Viskin S, Rosso R, Rogowski O, Belhassen B. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912–916. doi: 10.1111/j.1540-8167.2005.50040.x. [DOI] [PubMed] [Google Scholar]
- 201.Noda T, Shimizu W, Taguchi A, Aiba T, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol. 2005;46:1288–1294. doi: 10.1016/j.jacc.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 202.Denes P, Gabster A, Huang SK. Clinical, electrocardiographic and follow-up observations in patients having ventricular fibrillation during Holter monitoring: role of quinidine therapy. Am J Cardiol. 1981;48:9–16. doi: 10.1016/0002-9149(81)90566-x. [DOI] [PubMed] [Google Scholar]
- 203.Panidis IP, Morganroth J. Sudden death in hospitalized patients: cardiac rhythm disturbances detected by ambulatory electrocardiographic monitoring. J Am Coll Cardiol. 1983;2:798–805. doi: 10.1016/s0735-1097(83)80225-3. [DOI] [PubMed] [Google Scholar]
- 204.Kempf FC, Jr, Josephson ME. Cardiac arrest recorded on ambulatory electrocardiograms. Am J Cardiol. 1984;53:1577–1582. doi: 10.1016/0002-9149(84)90582-4. [DOI] [PubMed] [Google Scholar]
- 205.DiMarco JP, Haines DE. Sudden cardiac death. Curr Probl Cardiol. 1990;15:183–232. doi: 10.1016/0146-2806(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 206.Tisdale JE, Miller DA. Drug-Induced Diseases: Prevention, Detection and Management. 2. Bethesda, MD: American Society of Health System Pharmacists; 2010. [Google Scholar]
- 207.Lehmann MH, Timothy KW, Frankovich D, Fromm BS, Keating M, Locati EH, Taggart RT, Towbin JA, Moss AJ, Schwartz PJ, Vincent GM. Age-gender influence on the rate-corrected QT interval and the QT-heart rate relation in families with genotypically characterized long QT syndrome. J Am Coll Cardiol. 1997;29:93–99. doi: 10.1016/s0735-1097(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 208.White RD, Wood DL. Out-of-hospital pleomorphic ventricular tachycardia and resuscitation: association with acute myocardial ischemia and infarction. Ann Emerg Med. 1992;21:1282–1287. doi: 10.1016/s0196-0644(05)81767-6. [DOI] [PubMed] [Google Scholar]
- 209.Brady W, Meldon S, DeBehnke D. Comparison of prehospital monomorphic and polymorphic ventricular tachycardia: prevalence, response to therapy, and outcome. Ann Emerg Med. 1995;25:64–70. doi: 10.1016/s0196-0644(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 210.Roy D, Waxman HL, Kienzle MG, Buxton AE, Marchlinski FE, Josephson ME. Clinical characteristics and long-term follow-up in 119 survivors of cardiac arrest: relation to inducibility at electro-physiologic testing. Am J Cardiol. 1983;52:969–974. doi: 10.1016/0002-9149(83)90514-3. [DOI] [PubMed] [Google Scholar]
- 211.Stevenson WG, Brugada P, Waldecker B, Zehender M, Wellens HJ. Clinical, angiographic, and electrophysiologic findings in patients with aborted sudden death as compared with patients with sustained ventricular tachycardia after myocardial infarction. Circulation. 1985;71:1146–1152. doi: 10.1161/01.cir.71.6.1146. [DOI] [PubMed] [Google Scholar]
- 212.Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85:321–341. doi: 10.1016/s0025-7125(05)70318-7. [DOI] [PubMed] [Google Scholar]
- 213.Brady WJ, DeBehnke DJ, Laundrie D. Prevalence, therapeutic response, and outcome of ventricular tachycardia in the out-of-hospital setting: a comparison of monomorphic ventricular tachycardia, polymorphic ventricular tachycardia, and torsades de pointes. Acad Emerg Med. 1999;6:609–617. doi: 10.1111/j.1553-2712.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 214.Wolfe CL, Nibley C, Bhandari A, Chatterjee K, Scheinman M. Polymorphous ventricular tachycardia associated with acute myocardial infarction. Circulation. 1991;84:1543–1551. doi: 10.1161/01.cir.84.4.1543. [DOI] [PubMed] [Google Scholar]
- 215.Pellegrini CN, Scheinman MM. Clinical management of ventricular tachycardia. Curr Probl Cardiol. 2010;35:453–504. doi: 10.1016/j.cpcardiol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 216.Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;3(suppl K):K70–K80. [Google Scholar]
- 217.Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and torsade de pointes in Germany. Europace. 2014;16:101–108. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 218.Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Faber TS, Zehender M, Just H. Drug-induced torsade de pointes: incidence, management and prevention. Drug Saf. 1994;11:463–476. doi: 10.2165/00002018-199411060-00007. [DOI] [PubMed] [Google Scholar]
- 220.Middlekauff HR, Stevenson WG, Saxon LA, Stevenson LW. Amiodarone and torsades de pointes in patients with advanced heart failure. Am J Cardiol. 1995;76:499–502. doi: 10.1016/s0002-9149(99)80138-6. [DOI] [PubMed] [Google Scholar]
- 221.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, ‘t Jong GW, Kingma JH, Sturkenboom MC, Stricker BH. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164:1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 222.Leonard CE, Freeman CP, Newcomb CW, Bilker WB, Kimmel SE, Strom BL, Hennessy S. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiolog. 2013;(suppl 10):1–9. doi: 10.4172/2155-9880.S10-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14:747–753. doi: 10.1002/pds.1112. [DOI] [PubMed] [Google Scholar]
- 224.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Woosley RL, Romero KA AZCERT, Inc. QTDrugs List. http://www.crediblemeds.org.
- 226.Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–899. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 227.Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22:39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- 228.Lewis BH, Antman EM, Graboys TB. Detailed analysis of 24 hour ambulatory electrocardiographic recordings during ventricular fibrillation or torsade de pointes. J Am Coll Cardiol. 1983;2:426–436. doi: 10.1016/s0735-1097(83)80268-x. [DOI] [PubMed] [Google Scholar]
- 229.Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82:282–290. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 230.Jamshidi Y, Nolte IM, Dalageorgou C, Zheng D, Johnson T, Bastiaenen R, Ruddy S, Talbott D, Norris KJ, Snieder H, George AL, Marshall V, Shakir S, Kannankeril PJ, Munroe PB, Camm AJ, Jeffery S, Roden DM, Behr ER. Common variation in the NOS1AP gene is associated with drug-induced QT prolongation and ventricular arrhythmia. J Am Coll Cardiol. 2012;60:841–850. doi: 10.1016/j.jacc.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ramirez AH, Shaffer CM, Delaney JT, Sexton DP, Levy SE, Rieder MJ, Nickerson DA, George AL, Jr, Roden DM. Novel rare variants in congenital cardiac arrhythmia genes are frequent in drug-induced torsades de pointes. Pharmacogenomics J. 2013;13:325–329. doi: 10.1038/tpj.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Doig JC. Drug-induced cardiac arrhythmias: incidence, prevention and management. Drug Saf. 1997;17:265–275. doi: 10.2165/00002018-199717040-00006. [DOI] [PubMed] [Google Scholar]
- 233.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
18. SUDDEN CARDIAC ARREST
See Tables 18-1 through 18-11 and Charts 18-1 through 18-4
Table 18-1.
Utstein Classification of Cardiac Arrest Causes2
| Primary Causes | Description |
|---|---|
| Medical | Includes cases in which the cause of the cardiac arrest is presumed to be cardiac or other medical cause (eg, anaphylaxis, asthma, GI bleed) and in which there is no obvious cause of the cardiac arrest |
| Traumatic | Cardiac arrest directly caused by blunt, penetrating, or burn injury |
| Drug overdose | Evidence that the cardiac arrest was caused by deliberate or accidental overdose of prescribed medications, recreational drugs, or ethanol |
| Drowning | Victim is found submersed in water without an alternative causation |
| Asphyxia | External causes of asphyxia, such as foreign-body airway obstruction, hanging, or strangulation |
GI indicates gastrointestinal.
Table 18-11.
Quality-of-Care Parameters for IHCA: GWTG-Resuscitation 2015
| Adults ≥18 y | Children 0–18 y | Children 0–30 d | |
|---|---|---|---|
| IHCA event outside ICU | 46.8 | 13 | 25.2 |
| Shockable rhythm | 15.0 | 5.3 | 3.3 |
| All objective CPR data collected | 93.4 | 95.7 | 35.7 |
| PETCO2 monitoring during CPR | 5.5 | 35.4 | 19.4 |
| Percent who survived event who received therapeutic hypothermia | 5.9 | 5.4 | 7.0 |
Values are percentages. CPR indicates cardiopulmonary resuscitation; GWTG, Get With the Guidelines; ICU, intensive care unit; IHCA, in-hospital cardiac arrest; and PETCO2, end-tidal carbon dioxide concentration.
Chart 18-1. Detailed causes of cardiac arrest by age group in children and young adults in King County, WA (1980–2009).
CAD indicates coronary artery disease; DCM, dilated cardiomyopathy; and HCM, hypertrophic cardiomyopathy. “Other” corresponds to all other causes.
Reprinted with permission from Meyer et al.3 Copyright © 2012, American Heart Association, Inc.
Chart 18-4. Temporal trends in survival to hospital discharge after pulseless IHCA in GWTG-Resuscitation from 2000 to 2015.
GWTG indicates Get With the Guidelines; IHCA, in-hospital cardiac arrest; PEA, pulseless electrical activity; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Source: GWTG-Resuscitation unpublished data.
Cardiac Arrest (Including VF and Ventricular Flutter) (See Table 18-1)
ICD-9 427.4, 427.5; ICD-10 I46.0, I46.1, I46.9, I49.0.
2014: Mortality – 16 864. Any-mention mortality – 3 53 427. Cardiac arrest is defined as the cessation of cardiac mechanical activity, as confirmed by the absence of signs of circulation.1 Cardiac arrest is traditionally categorized as being of cardiac or noncardiac origin. An arrest is presumed to be of cardiac origin unless it is known or likely to have been caused by trauma, submersion, drug overdose, asphyxia, exsanguination, or any other noncardiac cause as best determined by rescuers.1 More recently, a consensus statement by the International Liaison Committee on Resuscitation recommended categorizing cardiac arrest into 6 primary causes.2 Table 18-1 describes the Utstein classifications of cardiac arrest origin.
Abbreviations Used in Chapter 18
| AED | automated external defibrillator |
| AF | atrial fibrillation |
| AMI | acute myocardial infarction |
| AV | atrioventricular |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CARES | Cardiac Arrest Registry to Enhance Survival |
| CI | confidence interval |
| CPC | Cerebral Performance Index |
| CPR | cardiopulmonary resuscitation |
| DCM | dilated cardiomyopathy |
| DNR | do not resuscitate |
| ECG | electrocardiogram |
| ED | emergency department |
| EMS | emergency medical services |
| GI | gastrointestinal |
| GWAS | genome-wide association studies |
| GWTG | Get With The Guidelines |
| HCM | hypertrophic cardiomyopathy |
| HD | heart disease |
| HF | heart failure |
| HR | hazard ratio |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| ICU | intensive care unit |
| IHCA | in-hospital cardiac arrest |
| IQR | interquartile range |
| LCL | lower confidence limit |
| LQTS | long-QT syndrome |
| LV | left ventricular |
| MD | doctor of medicine (medical doctor) |
| MI | myocardial infarction |
| NA | not applicable |
| NIS | Nationwide Inpatient Sample |
| OHCA | out-of-hospital cardiac arrest |
| OR | odds ratio |
| PA | physical activity |
| PAR | population attributable risk |
| PEA | pulseless electrical activity |
| PETCO2 | end-tidal carbon dioxide concentration |
| PVT | polymorphic ventricular tachycardia |
| ROC | Resuscitation Outcomes Consortium |
| RR | relative risk |
| RV | right ventricular |
| SCD | sudden cardiac death |
| SD | standard deviation |
| UCL | upper confidence limit |
| VF | ventricular fibrillation |
| VT | ventricular tachycardia |
In practice, the accuracy of this classification is difficult, and some data sets do not attempt to make the distinction. Because of fundamental differences in underlying causes and the system of care, epidemiological data for OHCA and IHCA are typically collected and reported separately. For similar reasons, data for adults and children (aged 1–18 years) are commonly reported separately.
There are a number of ongoing challenges to understanding the epidemiology of cardiac arrest in the United States. Despite being a leading cause of death, there are currently no nationwide standards for surveillance to monitor the incidence and outcomes of cardiac arrest. In addition, it is challenging to define what “unexpected” or “sudden” death is. SCD has been defined as unexpected death without an obvious noncardiac cause that occurs within 1 hour of symptom onset (witnessed) or within 24 hours of last being observed in normal health (unwitnessed)3; however, this definition is difficult to apply in the real-world setting. OHCA registries and clinical trials typically include patients in cardiac arrest who were either “attended/assessed” by EMS providers or “treated” by EMS providers. Regional and cultural differences in EMS system access and decision to treat are potential sources of variability in these data sets. Similar challenges exist related to the epidemiology of IHCA.
OHCA: Children
(See Table 18-2 and Chart 18-1)
Table 18-2.
Annual Incidence of OHCA in US Sites of the ROC, June 1, 2014 to May 31, 2015
| Incidence per 100 000 (95% CI) | Annual No. of US Cases | |||
|---|---|---|---|---|
| N | 95% LCL | 95% UCL | ||
| EMS assessed | ||||
| Any age | 110.8 (108.9–112.6) | 356 461 | 350 349 | 362 252 |
| Adults | 140.7 (138.3–143.1) | 347 322 | 341 397 | 353 246 |
| Children | 9.4 (8.3–10.5) | 7037 | 6214 | 7861 |
| EMS treated | ||||
| Any age | 57.3 (56.0–58.7) | 184 343 | 180 161 | 188 847 |
| Adults | 73.0 (71.2–74.7) | 180 202 | 175 759 | 184 399 |
| Children | 7.3 (6.3–8.3) | 5465 | 4716 | 6214 |
| VF* | ||||
| Any age | 12.1 (11.5–12.7) | 38 928 | 36 997 | 40 858 |
| Adults | 15.8 (15.0–16.6) | 39 003 | 37 028 | 40 978 |
| Children | 0.5 (0.3–0.8) | 374 | 225 | 599 |
| Bystander-witnessed VF | ||||
| Any age | 7.0 (6.5–7.5) | 22 520 | 20 912 | 24 129 |
| Adults | 9.2 (8.6–9.8) | 22 710 | 21 229 | 24 192 |
| Children | 0.3 (0.1–0.5) | 225 | 75 | 374 |
Assumes total US population is 321 716 000.10 CI indicates confidence interval; EMS, emergency medical services; LCL, lower confidence limit; OHCA, out-of-hospital cardiac arrest; ROC, Resuscitation Outcomes Consortium; UCL, upper confidence limit; and VF, ventricular fibrillation.
The estimated number of annual VF cases of any age is less than the estimated number of cases in adults alone because of rounding and missing information about patient age.
Source: ROC Investigators, unpublished data; data time frame is June 1, 2014, to May 31, 2015. Population growth of 0.93% per year has now been added from the 2010 population. In 2013, 23.27% of population was <18 years of age. This is used for annual number of case estimates for adults and children.
Incidence
The incidence of OHCA among individuals <18 years of age in the United States is best characterized by data from the ROC Registry. Extrapolation of the incidence of EMS-assessed OHCA reported by ROC (ROC Investigators, unpublished data, July 7, 2016) suggests that each year, 7037 (quasi CI, 6214–7861) children experience EMS-assessed OHCA in the United States (Table 18-2).
The underlying cause of OHCA varies by age group. Chart 18-1 illustrates the causes of OHCA by age group based on a retrospective cohort of OHCA patients 0 to 35 years of age treated in King County, WA, between 1980 and 2009.3
The incidence of SCD in high school athletes screened every 3 years between 1993 and 2012 with standard preparticipation evaluations during Minnesota State High School League activities was 0.24 per 100 000 athlete-years.4
A longitudinal study of students 17 to 24 years of age participating in National Collegiate Athletic Association sports showed that the incidence of nontraumatic OHCA was 1 per 22 903 athlete participant-years. The incidence of cardiac arrest tended to be higher among blacks than among whites and among males than among females.5
The most common causes of sudden death in competitive young athletes are HCM (26%), commotio cordis (20%), and coronary artery anomalies (14%).6
Mortality
(See Tables 18-3 through 18-6)
Table 18-3.
Annual Sudden Cardiac Arrest Mortality by Location (Any-Mention Cardiac Arrest Deaths)
| Place of Death | Any-Mention Cardiac Arrest Deaths |
|---|---|
| Medical facility, inpatient | 139 921 |
| Medical facility, outpatient or ED | 42 022 |
| Medical facility, dead on arrival | 2304 |
| Medical facility, status unknown | 0 |
| Decedent’s home | 82 629 |
| Hospice facility | 8021 |
| Nursing home/long-term care | 66 767 |
| Other | 11 530 |
| Place of death unknown | 233 |
| Total | 353 427 |
ED indicates emergency department.
Source: Centers for Disease Control and Prevention Wonder database.7
Table 18-6.
2015 Quality-of-Care Metrics for OHCA in ROC Epistry, January 1, 2015 to June 30, 2015
| Metrics | Overall | Adults | Children* |
|---|---|---|---|
| Prehospital metrics† | |||
| Bystander CPR, % | 45.7 (44.1–47.4) | 45.6 (43.9–47.2) | 53.5 (44.4–62.7) |
| Shocked by AED before EMS, % | 2.3 (1.8–2.7) | 2.2 (1.8–2.7) | 2.6 (0.0–5.6) |
| Chest compression fraction during first 5 min of CPR | 0.86 (0.12) | 0.86 (0.12) | 0.87 (0.11) |
| Compression depth, mm | 49.3 (10.2) | 49.3 (10.2) | 48.6 (10.0) |
| Preshock pause duration, s | 10.3 (10.5) | 10.4 (10.5) | 7.3 (5.8) |
| Time to first EMS defibrillator applied, min | 8.5 (4.3) | 8.5 (4.3) | 8.5 (5.9) |
| Dispatcher-assisted CPR, % | 11.3 (10.3–12.3) | 11.3 (10.3–12.4) | 10.5 (4.9–16.2) |
| Hospital-based metrics, % | |||
| Hypothermia induced after initial VT/VF‡ | 68.1 (62.1–74.2) | 68.1 (62.1–74.2) | NA |
| No order for withdrawal/DNR during first 72 h§ | 64.9 (60.4–69.3) | 64.9 (60.4–69.3) | NA |
| Implantable cardioverter-defibrillator assessment, initial VT/VF, no AMI per MD notes or final ECG interpretation| | 24.1 (15.1–33.1) | 24.1 (15.1–33.1) | NA |
Values are mean (95% confidence interval) or mean (SD). AED indicates automated external defibrillator; AMI, acute myocardial infarction; CPR, cardiopulmonary resuscitation; DNR, do not resuscitate; EMS, emergency medical services; MD, medical doctor; NA, not applicable; OHCA, out-of-hospital cardiac arrest; ROC, Resuscitation Outcomes Consortium; VF, ventricular fibrillation; and VT, ventricular tachycardia.
During the first 6 months of 2015, there were 6 pediatric cases with initial rhythm VT/VF that were admitted to a hospital; none had data collected on hospital-based metrics.
Prehospital metrics data are from EMS-treated cases.
Denominator is all cases with initial rhythm VT/VF and admitted to hospital.
Denominator is all cases admitted to hospital.
Denominator is all cases with initial rhythm VT/VF, no indication of AMI, no percutaneous coronary intervention, no bypass, and admitted to hospital.
Source: ROC Investigators, unpublished data, July 7, 2016.
In 2014, any-mention sudden cardiac arrest mortality for all ages in the United States was 353 427 (Table 18-3).7
The ROC Epistry for cardiac arrest is a prospective population-based registry of all EMS-attended calls for patients with OHCA in 8 US and 3 Canadian regions.8 In the ROC Epistry, survival to hospital discharge from January 1, 2015, to June 30 2015, after EMS-treated nontraumatic cardiac arrest was 13.2% (95% CI, 7.0%–19.4%) for children (ROC Investigators, unpublished data, July 7, 2016) (Table 18-4).
The CARES Registry reported survival to hospital discharge in 2015 of 6.2% (4.9%–8.0%) for children <1 year old, 16.6% (13.1%–20.7%) for children 1 to <12 years old, and 23.5% (19.05–28.6%) for children 12 to 18 years old (Table 18-5).
In 1 case series, long-term survival of pediatric OHCA patients surviving to hospital discharge was 92% at 1 year, 86% at 5 years, and 77% at 20 years.9
Table 18-4.
Survival After OHCA in US ROC Sites, January 1, 2015, to June 30, 2015
| Survival to Discharge (95% CI), % | |
|---|---|
| EMS assessed | |
| Any age | 5.8 (5.3–6.4) |
| Adults | 5.8 (5.3–6.4) |
| Children | 10.2 (5.3–15.1) |
| EMS treated | |
| Any age | 11.4 (10.4–12.4) |
| Adults | 11.4 (10.4–12.4) |
| Children | 13.2 (7.0–19.4) |
| VF | |
| Any age | 30.0 (26.7–33.2) |
| Adults | 29.5 (26.2–32.8) |
| Children | 77.8 (50.6–100) |
| Bystander-witnessed VF | |
| Any age | 37.4 (32.7–42.0) |
| Adults | 36.5 (31.8–41.1) |
| Children | 100 (100–100) |
CI indicates confidence interval; EMS, emergency medical services; OHCA, out-of-hospital cardiac arrest; ROC, Resuscitation Outcomes Consortium; and VF, ventricular fibrillation.
Source: ROC, unpublished data; time frame: January 1, 2015, to June 30, 2015.
Table 18-5.
Outcomes of EMS-Treated Nontraumatic OHCA in Children: CARES Registry 2015
| Age Groups, y | Survival to Hospital Admission, % (95% CI) | Survival to Hospital Discharge, % (95% CI) | Survival with Good Neurological Function (CPC 1 or 2), % (95% CI) | In-Hospital Mortality, % (95% CI) |
|---|---|---|---|---|
| <1 | 17.4 (15.1–20.0) | 6.2 (4.9–8.0) | 4.4 (3.3–5.9) | 64.2 (56.7–71.2) |
| 1–12 | 38.0 (33.2–43.1) | 16.6 (13.1–20.7) | 12.8 (8.6–15.1) | 56.4 (48.2–64.4) |
| 12–18 | 47.3 (41.6–53.0) | 23.5 (19.05–28.6) | 18.7 (14.7–23.6) | 50.4 (42.2–58.6) |
CARES indicates Cardiac Arrest Registry to Enhance Survival; CI, confidence interval; CPC, Cerebral Performance Category; EMS, emergency medical services; and OHCA, out-of-hospital cardiac arrest.
Derived from CARES Registry 2015.
Quality of Care
Quality-of-care metrics from the ROC Epistry for pediatric OHCA in 2015 are listed in Table 18-6 (ROC Investigators, unpublished data, July 7, 2016).
OHCA: Adult
Incidence
(See Tables 18-2 and 18-7 through 18-9 and Charts 18-2 and 18-3)
Table 18-7.
Range of Reported Estimates of Burden of OHCA in the United States
| Patient Population | Incidence per 100000 Person-Years | Total Incidence per Year* |
|---|---|---|
| CARES | ||
| EMS treated | 57 | 179 877 |
| ROC Epistry* | ||
| EMS treated | 63.8 | 201 690 |
| EMS treated and untreated† | 124.8 | 394 529 |
CARES indicates Cardiac Arrest Registry to Enhance Survival; EMS, emergency medical services; OHCA, out-of-hospital cardiac arrest; and ROC, Resuscitation Outcomes Consortium.
ROC Epistry incidence counts all cardiac arrests (with cardiac and noncardiac pathogenesis), whereas CARES incidence includes cardiac arrest of presumed cardiac origin only.
“Untreated” refers to cases that did not receive resuscitation treatment because patients were either dead on EMS arrival or had existing do-not-resuscitate orders.
Reprinted with permission from “Strategies to Improve Cardiac Arrest Survival: A Time to Act”85 (2015) by the National Academy of Sciences, Washington, DC. Copyright © 2015, National Academy of Sciences.
Table 18-9.
Trends in Bystander Response and OHCA Outcomes Between 2006 and 2015 in the ROC Epistry
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Survival EMS treated, % | 10.2 | 10.1 | 11.9 | 10.3 | 11.1 | 11.3 | 12.4 | 11.9 | 12.7 | 12.4 |
| Survival first rhythm shockable, % | 25.9 | 29.0 | 33.6 | 27.8 | 30.1 | 30.9 | 34.1 | 32.7 | 33.5 | 30.2 |
| First rhythm shockable, % | 23.7 | 21.7 | 21.9 | 20.9 | 20.8 | 21.4 | 21.7 | 20.2 | 20.8 | 21.3 |
| Bystander CPR, % | 36.5 | 37.9 | 37.4 | 39.1 | 38.6 | 38.6 | 42.8 | 43.0 | 44.5 | 43.6 |
| Lay use of AED, % | 3.2 | 3.3 | 3.9 | 4.5 | 4.0 | 3.9 | 5.1 | 6.0 | 6.6 | 6.7 |
| AED shock given, % | 2.0 | 1.6 | 1.8 | 1.8 | 2.0 | 1.8 | 2.0 | 2.2 | 2.2 | 2.3 |
| Time from call to AED use, mean (SD), min* | 8.0 (4.3) | 9.6 (8.6) | 8.4 (3.9) | 7.9 (3.8) | 8.1 (3.7) | 8.5 (3.9) | 8.3 (4.6) | 8.1 (3.5) | 8.1 (3.5) | 7.9 (3.6) |
| Chest compression fraction, mean (SD) | 0.68 (0.21) | 0.66 (0.18) | 0.66 (0.21) | 0.70 (0.19) | 0.71 (0.19) | 0.77 (0.18) | 0.81 (0.16) | 0.83 (0.14) | 0.83 (0.14) | 0.85 (0.14) |
| Chest compression depth, mean (SD), mm | 36.9 (11.4) | 37.4 (9.8) | 39.4 (10.8) | 40.0 (11.5) | 41.6 (24.9) | 43.0 (10.7) | 44.9 (11.8) | 47.2 (11.8) | 49.8 (11.6) | 50.6 (11.0) |
Annual results calculated using a subset of ROC EMS agencies from 7 US sites that participated continuously from 2008 to 2015. Previous calculations and those in Table 18-4 use all ROC agencies that participated during the respective year. AED indicates automated external defibrillator; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; OHCA, out-of-hospital cardiac arrest; and ROC, Resuscitation Outcomes Consortium.
Non–EMS-witnessed cases.
Source: ROC Investigators, unpublished data, July 7, 2016.
Chart 18-2. Location of out-of-hospital cardiac arrest, 2015.
Data derived from 2015 Cardiac Arrest Registry to Enhance Survival (CARES) National Summary Report.14
Chart 18-3. Out-of-hospital cardiac arrest witness status, 2015.
EMS indicates emergency medical services.
Data derived from 2015 Cardiac Arrest Registry to Enhance Survival National Summary Report.14
The incidence of EMS-assessed, EMS-treated non-traumatic cardiac arrest and bystander-witnessed VF among individuals of any age in the United States is characterized by an ongoing registry from ROC and the CARES registry (Tables 18-2 and 18-7).
The total resident population of the United States was 321 716 000 as of September 10, 2015.10 Extrapolation of the incidence of EMS-assessed OHCA reported by the ROC Investigators (ROC Investigators, unpublished data, July 7, 2016) to the total population of the United States suggests that each year, 110.8 individuals per 100 000 population (95% CI, 108.9–112.6), or 356 500 people of any age (quasi-CI, 350 000–362 000) or 347 000 adults (95% CI, 341 000–353 000), experience EMS-assessed OHCA (Table 18-2).
On the basis of extrapolation of data from the Oregon Sudden Unexpected Death Study, the estimated risk-adjusted incidence of sudden cardiac arrest was 76 per 100 000 per year (≈230 000 per year in the United States), and the estimated risk-adjusted incidence of SCD was 69 per 100 000 per year (≈210 000 per year in the United States).11 This data set excluded cases that were judged to have a noncardiac cause of arrest, which underestimates the overall burden of cardiac arrest. In the same study, the estimated societal burden of premature death was 2 million years of potential life lost for males and 1.3 million years of potential life lost for females.
Approximately 60% of OHCAs were treated by EMS personnel.12 Twenty-five percent of those with EMS-treated OHCA have no symptoms before the onset of arrest.13
The overall estimated incidence of EMS-treated OHCA in the ROC Registry increased from 47 per 100 000 to 66 per 100 000 between 2008 and 2015 (ROC Investigators, unpublished data, July 7, 2016) (Table 18-8).
Among EMS-treated patients with OHCA in 2015, 21.3% had an initial rhythm of VF or VT or had a rhythm that was shockable by an automated external defibrillator (Table 18-9).
In the data from the 2015 CARES summary report, the median age for OHCA was 65 years.
The majority of OHCAs occur at a home or residence (70%).14 In 2015, public settings (19.8%) and nursing homes (10.6%) were the second and third most common locations of OHCA (Chart 18-2).
In 2015, cardiac arrest was witnessed by a bystander in 37% of cases and by an EMS provider in 12% of cases and was unwitnessed in 51% of cases14 (Chart 18-3).
Among 10.9 million registered participants in 40 marathons and 19 half marathons, the overall incidence of cardiac arrest was 0.54 per 100 000 participants (95% CI, 0.41–0.70).15 Those with cardiac arrest were more often male and were running a marathon versus a half marathon. Seventy-one percent of those with cardiac arrest died; those who died were younger (mean 39±9 years of age) than those who did not die (mean 49±10 years of age), were more often male, and were more often running a full marathon.
Table 18-8.
Estimated Overall Annual Incidence of EMS-Treated OHCA Between 2008 and 2015 in the ROC Epistry
| Incidence Rates per 100 000 Person-Years | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
| EMS treated | 47.1 | 57.7 | 59.9 | 61.6 | 62.9 | 64.9 | 68.1 | 66 |
| Initial rhythm VT/VF | 9.8 | 11.9 | 12.4 | 13 | 13.8 | 13.2 | 14.4 | 13.5 |
| Bystander-witnessed VT/VF | 5.7 | 6.9 | 7.4 | 7.7 | 7.8 | 7.3 | 8.4 | 7.5 |
The Resuscitation Outcomes Consortium (ROC) maintained an epidemiological registry to describe the process and outcome of care for patients with OHCA, as well as to augment the efficiency of randomized trials of interventions in such patients. For several years, ROC has used data from this registry to estimate the burden of cardiac arrest for the American Heart Association. The methods used to estimate incidence in this registry have changed over time, as census data changed. As well, the methods changed as the completeness of reporting by census tract evolved over time. As such, previously reported incidence rates are not directly comparable from year to year nor with the rates presented here. The treated cardiac incidence rates shown in the Table were calculated using the subset of ROC EMS agencies from 7 US sites that participated continuously from 2008 to 2015. Previous calculations, and those in Table 18-2, used all ROC agencies that participated during the respective year. Methods of calculation have been modified over time.
Technical notes: The total yearly population of the ground footprint covered by these agencies has been adjusted from the 2000 and 2010 US census to account for yearly population increases. Arrest location is collected by ROC agencies as the location of the arrest, not the home residence of the patient who experiences the cardiac arrest. The population represents the number of people who live within the ground footprint and does not account for people who commute into the area during business hours. The ground footprint represents all census tracts where an ROC agency arrives and treats a patient even if there is not full ROC coverage within the tract.
EMS indicates emergency medical services; OHCA, out-of-hospital cardiac arrest; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Source: ROC Investigators, unpublished data, July 7, 2016.
Mortality
(See Tables 18-3, 18-4, 18-6, and 18-9)
In 2014, any-mention sudden cardiac arrest mortality in the United States was 353 427 (Table 18-3).
In the ROC Epistry, survival to hospital discharge (January 1, 2015, to June 30, 2015) after EMS-treated cardiac arrest was 11.4% (95% CI, 10.4%–12.4%) for patients of any age and 11.4% (95% CI 10.4%–12.4%) for adults (ROC Investigators, unpublished data, July 7, 2016) (Table 18-4). Survival after bystander-witnessed VF was 37.4% (95% CI, 32.7%–42.0%) for patients of any age. Contemporary survival data will be available on completion of ongoing randomized trials.
In the ROC Epistry between 2006 and 2010, unadjusted survival to hospital discharge after EMS-treated OHCA increased from 8.2% in 2006 to 10.4% in 2010.16 In 2015, survival to hospital discharge after EMS-treated OHCA in US ROC sites was 11.4% (unpublished data from ROC Investigators, July 7, 2016) (Table 18-4).
In CARES, risk-adjusted rates of OHCA survival to hospital discharge increased from 5.7% in 2005 to 2006 to 8.3% in 2012 (adjusted risk ratio, 1.47; 95% CI, 1.26–1.70; P<0.001).17
In CARES, 53 637 OHCAs were treated in 2015. Survival to hospital discharge was 10.6% (95% CI, 10.4%–10.9%), and survival with good neurological function (Cerebral Performance Category 1 or 2) was 8.56% (95% CI, 8.4%–8.8%). For bystander-witnessed arrest with a shockable rhythm, survival to hospital discharge was 33.6% (95% CI, 32.4%–34.7%), and survival with good neurological function was 30.1%.= (95% CI, 28.9%–31.2%).14
In a study using the US NIS data, in-hospital mortality for patients hospitalized after treatment for cardiac arrest declined 11.8%, from 69.6% in 2001 to 57.8% in 2009.18
A study conducted in New York City found the age-adjusted survival to 30 days after discharge was more than twice as poor for blacks as for whites, and survival among Hispanics was also lower than among whites.19
A study in Denmark of 1218 OHCA patients between 2002 and 2010 demonstrated that transport to a non–tertiary care center versus a tertiary care center after return of spontaneous circulation or with ongoing resuscitation was independently associated with increased risk of death (HR, 1.32; 95% CI, 1.09–1.59; P=0.004).20
Risk Factors
Prior HD is a major risk factor for cardiac arrest. A study of 1275 health maintenance organization enrollees 50 to 79 years of age who had cardiac arrest showed that the incidence of OHCA was 6.0 per 1000 person-years in subjects with any clinically recognized HD compared with 0.8 per 1000 person-years in subjects without HD. In subgroups with HD, incidence was 13.6 per 1000 person-years in subjects with prior MI and 21.9 per 1000 person-years in subjects with HF.21
A family history of cardiac arrest in a first-degree relative is associated with an ≈2-fold increase in risk of cardiac arrest.5,6
In a study of 81 722 females in the Nurses’ Health Study, the PAR of sudden death associated with 4 lifestyle factors (smoking, PA, diet, and weight) was 81% (95% CI, 52%–93%).22
A study conducted in New York City found the age-adjusted incidence of OHCA per 10 000 adults was 10.1 among blacks, 6.5 among Hispanics, and 5.8 among whites.19
Analysis of 9235 sudden cardiac arrests in the ROC Epistry revealed the incidence of sudden cardiac arrest in the lowest socioeconomic quartile was nearly double that in the highest quartile (incidence rate ratio, 1.9; 95% CI, 1.8–2.0).23
Analysis of data from the CARES registry revealed that patients who had a cardiac arrest in low-income black neighborhoods were less likely to receive bystander-initiated CPR than those who had a cardiac arrest in high-income white neighborhoods (OR, 0.49; 95% CI, 0.41–0.58).24
Quality of Care
Quality-of-care metrics from the ROC Epistry for adults with OHCA in 2015 are listed in Table 18-6.
Outcomes for EMS-treated nontraumatic OHCA in adults in the 2015 CARES Registry are listed in Table 18-10.
Annual trends in OHCA quality of care in the ROC Epistry are listed in Table 18-9.
Table 18-10.
Outcomes of EMS-Treated Nontraumatic OHCA in Adults (Age >18 Years), CARES Registry 2015
| Presenting Characteristics | Survival to Hospital Admission (95% CI) | Survival to Hospital Discharge (95% CI) | Survival With Good Neurological Function (CPC 1 or 2), (95% CI) | In-Hospital Mortality (95% CI) |
|---|---|---|---|---|
| All presentations | 29.0 (28.6–29.4) | 10.6 (10.4–10.9) | 8.6 (8.4–8.8) | 63.3 (62.5–64.0) |
| Home/residence | 27.0 (26.6–27.5) | 8.8 (8.5–9.1) | 6.9 (6.7–7.2 | 67.3 (66.4–68.2) |
| Nursing home | 18.9 (17.9–19.9) | 4.4 (3.9–4.9) | 2.2 (1.9–2.6) | 76.8 (74.2–79.2) |
| Public setting | 41.3 (40.4–42.3) | 20.4 (17.7–21.2) | 17.7 (17.0–18.4) | 50.5 (49.0–52.0) |
| Unwitnessed | 18.9 (18.4–19.3) | 4.6 (4.4–4.9) | 3.4 (3.2–3.6) | 75.4 (74.2–76.5) |
| Bystander witnessed | 38.6 (37.9–39.3) | 16.4 (15.8–16.9) | 13.7 (13.2–14.2) | 57.6 (56.5–58.7) |
| EMS provider witnessed | 42.5 (41.3–43.7) | 18.6 (17.7–19.6) | 15.0 (14.1–15.9) | 56.2 (54.4–58.1) |
| Shockable presenting rhythm | 48.6 (47.7–49.6) | 29.1 (28.3–30.0) | 25.8 (25.0–26.7) | 40.1 (38.8–41.4) |
| Nonshockable presenting rhythm | 24.0 (23.6–24.4) | 6.0 (5.8–6.2) | 4.2 (4.0–4.4) | 75.1 (74.2–75.9) |
| Bystander CPR | 29.6 (29.0–30.2 | 12.1 (11.7–12.6) | 10.1 (9.8–10.5) | 59.0 (57.8–60.2) |
| No bystander CPR | 25.0 (24.4–25.5 | 7.3 (7.0–7.5) | 5.6 (5.4–5.9) | 70.7 (69.5–71.8) |
Values are percentages. AED indicates automated external defibrillator; CI, confidence interval; CPC, Cerebral Performance Index; CPR, cardiopulmonary resuscitation; and OHCA, out-of-hospital cardiac arrest.
Modified with permission from 2015 Cardiac Arrest Registry to Enhance Survival (CARES) National Summary Report.14 Copyright © 2015, MyCARES.
IHCA: Children
(See Table 18-11)
Incidence
Among 1031 children at 12 hospitals in GWTG-Resuscitation between 2001 and 2009, the initial cardiac arrest rhythm was asystole and pulseless electrical activity in 874 children (84.8%) and VF and pulseless VT in 157 children (15.2%).
Mortality
Risk-adjusted rates of survival to discharge increased from 14.3% in 2000 to 43.4% in 2009 (adjusted rate ratio per year, 1.08; 95% CI, 1.01–1.16; P for trend=0.02) without an increased rate of neurological disability among survivors over time (unadjusted P for trend=0.32).25
In 2015, GWTG-Resuscitation reported 407 pulse-less IHCAs in children 0 to 18 years old. Survival to hospital discharge was 35.9% (95% CI, 31.4%–40.6%). Of 157 neonates (0–30 days old) with pulseless IHCA, 24.2% (95% CI, 18.2%–31.4%) survived to hospital discharge.
Quality of Care
Quality-of-care metrics from GWTG-Resuscitation for pediatric IHCA in 2015 are listed in Table 18-11.
IHCA: Adult
(See Chart 18-4 and Table 18-11)
Incidence
Extrapolation of the incidence of IHCA reported by GWTG-Resuscitation to the total population of hospitalized patients in the United States suggests that each year, 209 000 (quasi-CI, 192 000–211 000) people are treated for IHCA.26
Analysis of the UK National Cardiac Arrest Audit database between 2011 and 2013 (144 acute hospitals and 22 628 patients ≥16 years of age) revealed an incidence of IHCA of 1.6 per 1000 hospital admissions, with a median across hospitals of 1.5 (IQR, 1.2–2.2). The overall unadjusted survival rate was 18.4%.27
Mortality
In 2015, the GWTG-Resuscitation program reported 20 876 IHCAs in adults ≥18 years old. Survival to hospital discharge was 23.8% (95% CI, 23.2%–24.3%). Among survivors, 86.5% were discharged with good neurological function (Cerebral Performance Category 1 or 2).
Survival trends for pulseless IHCA between 2000 and 2015 in GWTG-R are illustrated in Chart 18-4.
In the UK National Cardiac Arrest Audit database between 2011 and 2013, the overall unadjusted survival rate was 18.4%. Survival was 49% when the initial rhythm was shockable and 10.5% when the initial rhythm was not shockable.27
Chan et al28 demonstrated that rates of survival to discharge were lower for black patients (25.2%) than for white patients (37.4%) after IHCA. Lower rates of survival to discharge for blacks reflected lower rates of both successful resuscitation (55.8% versus 67.4% for blacks versus whites, respectively) and postresuscitation survival (45.2% versus 55.5%, respectively). Adjustment for the hospital site at which patients received care explained a substantial portion of the racial differences in successful resuscitation (adjusted RR, 0.92; 95% CI, 0.88–0.96; P<0.001) and eliminated the racial differences in postresuscitation survival (adjusted RR, 0.99; 95% CI, 0.92–1.06; P=0.68).
Quality of Care
Quality-of-care metrics from GWTG-Resuscitation for adult IHCA in 2015 are listed in Table 18-11.
Inherited Syndromes Associated With SCD
Overview
The majority of OHCA occurs in the general population without an underlying inherited syndrome associated with SCD.29 A large proportion of patients with OHCA have coronary atherosclerosis.30 Approximately 5% to 10% of SCD cases occur in the absence of CAD or structural HD.31 The inheritable arrhythmic disorders constitute approximately one half of unexplained cardiac arrests.32
Long-QT Syndrome
The hereditary LQTS is a genetic channelopathy characterized by prolongation of the QT interval (typically >460 ms) and susceptibility to ventricular tachyarrhythmias that lead to syncope and SCD. Investigators have identified mutations in 15 genes leading to this phenotype (LQT1 through LQT15).33,34 LQT1 (KCNQ1), LQT2 (KCNH2), and LQT3 (SCN5A) mutations account for the majority (≈80%) of the typed mutations.35,36
Prevalence of LQTS is estimated at 1 per 2000 live births from ECG-guided molecular screening of ≈44 000 infants (mostly white) born in Italy.37 A similar prevalence was found among nearly 8000 Japanese school children screened by use of an ECG-guided molecular screening approach.38 LQTS has been reported among those of African descent, but its prevalence is not well assessed.39
There is variable penetrance and a sex-time interaction for LQTS symptoms. Risk of cardiac events is higher among males than females (21% among males and 14% among females by 12 years of age). Risk of events during adolescence is equivalent between sexes (≈25% for both sexes from ages 12–18 years). Conversely, risk of cardiac events in young adulthood is higher among females than males (39% among females from ages 18–40 years and 16% among males).35
Individuals may be risk stratified for increased risk of SCD40 according to their specific long-QT mutation and their response to β-blockers.41
Among 403 patients from the LQTS Registry from birth through age 40 years, multivariate analysis demonstrated that patients with multiple LQTS gene mutations had a 2.3-fold (P=0.015) increased risk for life-threatening cardiac events (comprising aborted cardiac arrest, implantable defibrillator shock, or SCD) compared with patients with a single mutation.42
Short-QT Syndrome
Short-QT syndrome is a recently described inherited mendelian condition characterized by shortening of the QT interval (typically QT <320 ms) and predisposition to AF, ventricular tachyarrhythmias, and sudden death. Mutations in 5 ion channel genes have been described (SQT1–SQT5).43
In a population of 41 767 young, predominantly male Swiss transcripts, 0.02% of the population had a QT interval shorter than 320 ms.44
Among 53 patients from the European Short QT Syndrome Registry (75% males, median age 26 years),45 a familial or personal history of cardiac arrest was present in 89%. Twenty-four patients received an implantable cardioverter-defibrillator, and 12 received long-term prophylaxis with hydroquinidine. During a median follow-up of 64 months, 2 patients received an appropriate implantable cardioverter-defibrillator shock, and 1 patient experienced syncope. Nonsustained PVT was recorded in 3 patients.45
In a cohort of 25 patients with short-QT syndrome ≤21 years of age followed up for 5.9 years, 6 patients had aborted sudden death (24%).46 Sixteen patients (84%) had a familial or personal history of cardiac arrest. A gene mutation associated with short-QT syndrome was identified in 5 of 21 probands (24%).
Brugada Syndrome
The Brugada syndrome is an acquired or inherited channelopathy characterized by persistent ST-segment elevation in the precordial leads (V1–V3), right bundle-branch block, and susceptibility to ventricular arrhythmias and SCD.47
Brugada syndrome is associated with mutations in at least 12 ion channel–related genes.47,48
Prevalence is estimated at 1 to 5 per 10 000 individuals. Prevalence is higher in Southeast Asian countries, including Thailand and the Philippines. There is a strong male predominance (80% male).47,49–53
Cardiac event rates for Brugada syndrome patients followed up prospectively in northern Europe (31.9 months) and Japan (48.7 months) were similar: 8% to 10% in patients with prior aborted sudden death, 1% to 2% in those with history of syncope, and 0.5% in asymptomatic patients.54,55 Predictors of poor outcome include clinical history of syncope or ventricular tachyarrhythmias, family history of sudden death, and a spontaneous early repolarization pattern on ECG.56,57
Among patients with Brugada syndrome, first-degree AV block, syncope, and spontaneous type 1 ST-segment elevation were independently associated with risk of sudden death or implantable cardioverter-defibrillator–appropriate therapies.58
Catecholaminergic PVT
Catecholaminergic PVT is a familial condition characterized by adrenergically induced ventricular arrhythmias associated with syncope and sudden death. It is associated with frequent ectopy, bidirectional VT, and PVT with exercise or catecholaminergic stimulation (such as emotion or medicines such as isoproterenol).
Mutations in genes encoding RYR259 (CPVT1) are found in the majority of patients and results in dominant pattern of inheritance. Mutations in genes encoding CASQ260,61 (CPVT2) are found in a small minority54 and result in a recessive pattern of inheritance. Mutations have also been described in KCNJ2 (CPVT3), TRDN, ANK2, and CALM1.62
Statistics regarding catecholaminergic PVT are primarily from case series. Of 101 patients with cat-echolaminergic PVT, the majority had experienced symptoms before 21 years of age.54
In small series (n=27–101) of patients followed up over a mean of 6.8 to 7.9 years, 27% to 62% experienced cardiac symptoms, and fatal or near-fatal events occurred in 13% to 31%.54,55,63
Risk factors for cardiac events included younger age at diagnosis and absence of β-blocker therapy. A history of aborted cardiac arrest and absence of β-blocker therapy were risk factors for fatal or near-fatal events.54
Arrhythmogenic RV Ventricular Cardiomyopathy
Arrhythmogenic RV cardiomyopathy is a form of genetically inherited structural HD that presents with fibrofatty replacement of the myocardium, with clinical presentation of palpitations, syncope, and sudden death.59
Twelve arrhythmogenic RV cardiomyopathy loci have been described (ARVC1–ARVC12). Disease-causing genes for 8 of these loci have been identified, the majority of which are in desmosomally related proteins.59
Prevalence is estimated at 2 to 10 per 10 000 individuals.64,65 Of 100 patients reported on from the Johns Hopkins Arrhythmogenic Right Ventricular Dysplasia Registry, 51 were males and 95 were white, with the rest being of black, Hispanic, or Middle Eastern origin. Twenty-two percent of index cases had evidence of the familial form of arrhyth-mogenic RV cardiomyopathy.60
The most common presenting symptoms were palpitations (27%), syncope (26%), and SCD (23%).60
During a median follow-up of 6 years, 47 patients received an implantable cardioverter-defibrillator, 29 of whom received appropriate implantable cardioverter-defibrillator shocks. At the end of follow-up, 66 patients were alive. Twenty-three patients died at study entry, and 11 died during follow-up (91% of deaths were attributable to sudden cardiac arrest).60 Similarly, the annual mortality rate was 2.3% for 130 patients with arrhythmogenic RV cardiomyopathy from Paris, France, who were followed up for a mean of 8.1 years.61
Hypertrophic Cardiomyopathy
(Please refer to Chapter 21, Cardiomyopathy and Heart Failure, for statistics regarding the general epidemiology of HCM.)
Over a mean follow-up of 8±7 years, 6% of HCM patients experienced SCD.66
Among 1866 sudden deaths in athletes between 1980 and 2006, HCM was the most common cause of cardiovascular sudden death (in 251 cases, or 36% of the 690 deaths that could be reliably attributed to a cardiovascular cause).6
The risk of sudden death increases with increasing maximum LV wall thickness,67,68 and the risk for those with wall thickness ≥30 mm is 18.2 per 1000 patient-years (95% CI, 7.3–37.6),68 or approximately twice that of those with maximal wall thickness <30 mm.67,68 Of note, an association between maximum wall thickness and sudden death has not been found in every HCM population.68
Nonsustained VT is a risk factor for sudden death,64,69 particularly in younger patients. Nonsustained VT in those ≤30 years of age is associated with a 4.35-greater odds of sudden death (95% CI, 1.5–12.3).64
A history of syncope is also a risk factor for sudden death in these patients,70 particularly if the syncope was recent before the initial evaluation and not attributable to a neurally mediated event.71
The presence of LV outflow tract obstruction ≥30 mm Hg appears to increase the risk of sudden death by ≈2-fold.72,73 The presence of LV outflow tract obstruction has a low positive predictive value (7%–8%) but a high negative predictive value (92%–95%) for predicting sudden death.73,74
The rate of malignant ventricular arrhythmias detected by implantable cardioverter-defibrillators appears to be similar between those with a family history of sudden death in ≥1 first-degree relative and those with at least 1 of the risk factors described above.75
The risk of sudden death increases with the number of risk factors.76
Early Repolarization Syndrome
Early repolarization, observed in ≈4% to 19% of the population77–80 (more commonly in young men77,79,81 and in athletes78) has conventionally been considered a benign finding.
A clinically relevant syndrome was initially described in which ≥1-mm positive deflections (sometimes referred to as “J waves”) in the S wave of ≥2 consecutive inferior or lateral leads was significantly more common among patients with idiopathic VF than among control subjects.77,78 Given an estimated risk of idiopathic VF in the general population (among those aged 35–45 years) of 3.4 per 100 000, the positive predictive value of such J-wave findings in a person 35 to 45 years of age increases the chances of having idiopathic VF to 11 of 100 000.78
In an analysis of the Social Insurance Institution’s Coronary Disease Study in Finland, J-point elevation was identified in 5.8% of 10 864 people.79 Those with inferior-lead J-point elevation more often were male and more often were smokers; had a lower resting heart rate, lower BMI, lower BP, shorter corrected QT interval, and longer QRS duration; and were more likely to have electrocardiographic evidence of CAD. Those with lateral J-point elevation were more likely to have LV hypertrophy. Before and after multivariable adjustment, subjects with J-point elevation ≥1 mm in the inferior leads (n=384) had a higher risk of cardiac death (adjusted RR, 1.28; 95% CI, 1.04–1.59; P=0.03) and arrhythmic death (adjusted RR, 1.43; 95% CI, 1.06–1.94; P=0.03); however, these patients did not have a significantly higher rate of all-cause mortality. Before and after multivariable adjustment, subjects with J-point elevation >2 mm (n=36) had an increased risk of cardiac death (adjusted RR, 2.98; 95% CI, 1.85–4.92; P=0.03), arrhythmic death (adjusted RR, 3.94; 95% CI, 1.96–7.90; P=0.03), and death of any cause (adjusted RR, 1.54; 95% CI, 1.06–2.24; P=0.03).
In CARDIA, 18.6% of 5069 participants had early repolarization restricted to the inferior and lateral leads at baseline; by year 20, only 4.8% exhibited an early repolarization pattern.80 Younger age, black race, male sex, longer exercise duration and QRS duration, and lower BMI, heart rate, QT index, and Cornell voltage were associated cross-sectionally with the presence of baseline early repolarization. Predictors of maintenance of the ECG pattern from baseline to year 20 were black race (OR, 2.62; 95% CI, 1.61–4.25), BMI (OR, 0.62 per 1 SD; 95% CI, 0.40–0.94), serum triglyceride levels (OR, 0.66 per 1 SD; 95% CI, 0.45–0.98), and QRS duration (OR, 1.68 per 1 SD; 95% CI, 1.37–2.06) at baseline.
Evidence from families with a high penetrance of the early repolarization syndrome associated with a high risk of sudden death suggests that the syndrome can be inherited in an autosomal dominant fashion.82 A meta-analysis of GWAS performed in population-based cohorts failed to identify any genetic variants that met criteria for statistical significance.83
Genome-Wide Association Studies
GWAS have been performed directly on cases of arrhythmic death to try to identify novel genetic variants associated with risk of sudden death. These are intended to discover previously unidentified genetic variants and biological pathways that contribute to potentially lethal ventricular arrhythmias. Limitations of these studies are the small number of samples available for analysis and the heterogeneity of case definition. The number of loci identified as having genome-wide significance for SCD is much smaller than for other complex diseases. In addition, studies to date have not consistently identified the same variants. A pooled analysis of case-control and cohort studies used GWAS to identify a rare (1.4% minor allele frequency) novel marker at the BAZ2B locus (bromo-domain adjacent zinc finger domain 2B) that was strongly associated with a risk of arrhythmic death (OR, 1.9; 95% CI, 1.6–2.3).84
REFERENCES
- 1.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; InterAmerican Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 2.Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, Deakin CD, Finn JC, Gräsner JT, Hazinski MF, Iwami T, Koster RW, Lim SH, Huei-Ming Ma M, McNally BF, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Eng Hock Ong M, Travers AH, Nolan JP Utstein Collaborators. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation [published correction appears in Circulation. 2015;132:e168–e169] Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 3.Meyer L, Stubbs B, Fahrenbruch C, Maeda C, Harmon K, Eisenberg M, Drezner J. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation. 2012;126:1363–1372. doi: 10.1161/CIRCULATIONAHA.111.076810. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WO, Stovitz SD. Incidence of sudden cardiac death in Minnesota high school athletes 1993–2012 screened with a standardized pre-participation evaluation. J Am Coll Cardiol. 2013;62:1298–1301. doi: 10.1016/j.jacc.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 5.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123:1594–1600. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC WONDER Online Database [database online] Atlanta, GA: Centers for Disease Control and Prevention; [Accessed May 25, 2016]. Compressed mortality file: underlying cause of death. Released 2015. http://wonder.cdc.gov/mortSQl.html. [Google Scholar]
- 8.Morrison LJ, Nichol G, Rea TD, Christenson J, Callaway CW, Stephens S, Pirrallo RG, Atkins DL, Davis DP, Idris AH, Newgard C ROC Investigators. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008;78:161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michiels EA, Dumas F, Quan L, Selby L, Copass M, Rea T. Long-term outcomes following pediatric out-of-hospital cardiac arrest*. Pediatr Crit Care Med. 2013;14:755–760. doi: 10.1097/PCC.0b013e31829763e2. [DOI] [PubMed] [Google Scholar]
- 10.US population data (population clock) [Accessed September 10, 2015];United States Census Bureau Web site. www.census.gov.
- 11.Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. 2014;7:212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Müller D, Agrawal R, Arntz HR. How sudden is sudden cardiac death? Circulation. 2006;114:1146–1150. doi: 10.1161/CIRCULATIONAHA.106.616318. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. [Accessed August 13, 2016];Cardiac Arrest Registry to Enhance Survival (CARES) National Summary Report. 2015 http://mycares.net/sitepages/uploads/2016/2015%20Non-Traumatic%20National%20Summary%20Report.pdf.
- 15.Kim JH, Malhotra R, Chiampas G, d’Hemecourt P, Troyanos C, Cianca J, Smith RN, Wang TJ, Roberts WO, Thompson PD, Baggish AL Race Associated Cardiac Arrest Event Registry (RACER) Study Group. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 16.Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, Brooks S, Christenson J, MacPhee R, Craig A, Rittenberger JC, Davis DP, May S, Wigginton J, Wang H Resuscitation Outcomes Consortium Investigators. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan PS, McNally B, Tang F, Kellermann A CARES Surveillance Group. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130:1876–1882. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, Rabinstein AA. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 19.Galea S, Blaney S, Nandi A, Silverman R, Vlahov D, Foltin G, Kusick M, Tunik M, Richmond N. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007;166:534–543. doi: 10.1093/aje/kwm102. [DOI] [PubMed] [Google Scholar]
- 20.Søholm H, Wachtell K, Nielsen SL, Bro-Jeppesen J, Pedersen F, Wanscher M, Boesgaard S, Møller JE, Hassager C, Kjaergaard J. Tertiary centres have improved survival compared to other hospitals in the Copenhagen area after out-of-hospital cardiac arrest. Resuscitation. 2013;84:162–167. doi: 10.1016/j.resuscitation.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Rea TD, Pearce RM, Raghunathan TE, Lemaitre RN, Sotoodehnia N, Jouven X, Siscovick DS. Incidence of out-of-hospital cardiac arrest. Am J Cardiol. 2004;93:1455–1460. doi: 10.1016/j.amjcard.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–69. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinier K, Thomas E, Andrusiek DL, Aufderheide TP, Brooks SC, Callaway CW, Pepe PE, Rea TD, Schmicker RH, Vaillancourt C, Chugh SS Resuscitation Outcomes Consortium Investigators. Socioeconomic status and incidence of sudden cardiac arrest. CMAJ. 2011;183:1705–1712. doi: 10.1503/cmaj.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasson C, Magid DJ, Chan P, Root ED, McNally BF, Kellermann AL, Haukoos JS CARES Surveillance Group. Association of neighborhood characteristics with bystander-initiated CPR. N Engl J Med. 2012;367:1607–1615. doi: 10.1056/NEJMoa1110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS American Heart Association Get With the Guidelines–Resuscitation Investigators. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PW American Heart Association Get With the Guidelines–Resuscitation Investigators. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan JP, Soar J, Smith GB, Gwinnutt C, Parrott F, Power S, Harrison DA, Nixon E, Rowan K National Cardiac Arrest Audit. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation. 2014;85:987–992. doi: 10.1016/j.resuscitation.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302:1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chelly J, Mongardon N, Dumas F, Varenne O, Spaulding C, Vignaux O, Carli P, Charpentier J, Pène F, Chiche JD, Mira JP, Cariou A. Benefit of an early and systematic imaging procedure after cardiac arrest: insights from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) registry. Resuscitation. 2012;83:1444–1450. doi: 10.1016/j.resuscitation.2012.08.321. [DOI] [PubMed] [Google Scholar]
- 31.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 32.Krahn AD, Healey JS, Chauhan V, Birnie DH, Simpson CS, Champagne J, Gardner M, Sanatani S, Exner DV, Klein GJ, Yee R, Skanes AC, Gula LJ, Gollob MH. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) [published correction appears in Circulation. 2010;121:e460] Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2016;61:51–55. doi: 10.1038/jhg.2015.74. [DOI] [PubMed] [Google Scholar]
- 34.Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circ Res. 2015;116:1919–1936. doi: 10.1161/CIRCRESAHA.116.304030. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg I, Zareba W, Moss AJ. Long QT syndrome. Curr Probl Cardiol. 2008;33:629–694. doi: 10.1016/j.cpcardiol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Wedekind H, Burde D, Zumhagen S, Debus V, Burkhardtsmaier G, Mönnig G, Breithardt G, Schulze-Bahr E. QT interval prolongation and risk for cardiac events in genotyped LQTS-index children. Eur J Pediatr. 2009;168:1107–1115. doi: 10.1007/s00431-008-0896-6. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P, Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi K, Fujino N, Uchiyama K, Ino H, Sakata K, Konno T, Masuta E, Funada A, Sakamoto Y, Tsubokawa T, Nakashima K, Liu L, Higashida H, Hiramaru Y, Shimizu M, Yamagishi M. Long QT syndrome and associated gene mutation carriers in Japanese children: results from ECG screening examinations. Clin Sci (Lond) 2009;117:415–424. doi: 10.1042/CS20080528. [DOI] [PubMed] [Google Scholar]
- 39.Fugate T, 2nd, Moss AJ, Jons C, McNitt S, Mullally J, Ouellet G, Goldenberg I, Zareba W, Robinson JL. U.S. portion of International Long QT Syndrome Registry Investigators. Long QT syndrome in African-Americans. Ann Noninvasive Electrocardiol. 2010;15:73–76. doi: 10.1111/j.1542-474X.2009.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jons C, JOU, Moss AJ, Reumann M, Rice JJ, Goldenberg I, Zareba W, Wilde AA, Shimizu W, Kanters JK, McNitt S, Hofman N, Robinson JL, Lopes CM. Use of mutant-specific ion channel characteristics for risk stratification of long QT syndrome patients. Sci Transl Med. 2011;3:76ra28. doi: 10.1126/scitranslmed.3001551. [DOI] [PubMed] [Google Scholar]
- 41.Barsheshet A, Goldenberg I, O-Uchi J, Moss AJ, Jons C, Shimizu W, Wilde AA, McNitt S, Peterson DR, Zareba W, Robinson JL, Ackerman MJ, Cypress M, Gray DA, Hofman N, Kanters JK, Kaufman ES, Platonov PG, Qi M, Towbin JA, Vincent GM, Lopes CM. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to β-blocker therapy in type 1 long-QT syndrome. Circulation. 2012;125:1988–1996. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullally J, Goldenberg I, Moss AJ, Lopes CM, Ackerman MJ, Zareba W, McNitt S, Robinson JL, Benhorin J, Kaufman ES, Towbin JA, Barsheshet A. Risk of life-threatening cardiac events among patients with long QT syndrome and multiple mutations. Heart Rhythm. 2013;10:378–382. doi: 10.1016/j.hrthm.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross B, Homoud M, Link M, Foote C, Garlitski AC, Weinstock J, Estes NA., 3rd The short QT syndrome. J Interv Card Electrophysiol. 2011;31:25–31. doi: 10.1007/s10840-011-9566-0. [DOI] [PubMed] [Google Scholar]
- 44.Kobza R, Roos M, Niggli B, Abächerli R, Lupi GA, Frey F, Schmid JJ, Erne P. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm. 2009;6:652–657. doi: 10.1016/j.hrthm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Giustetto C, Schimpf R, Mazzanti A, Scrocco C, Maury P, Anttonen O, Probst V, Blanc JJ, Sbragia P, Dalmasso P, Borggrefe M, Gaita F. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58:587–595. doi: 10.1016/j.jacc.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 46.Villafañe J, Atallah J, Gollob MH, Maury P, Wolpert C, Gebauer R, Watanabe H, Horie M, Anttonen O, Kannankeril P, Faulknier B, Bleiz J, Makiyama T, Shimizu W, Hamilton RM, Young ML. Long-term follow-up of a pediatric cohort with short QT syndrome. J Am Coll Cardiol. 2013;61:1183–1191. doi: 10.1016/j.jacc.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 47.Benito B, Brugada J, Brugada R, Brugada P. Brugada syndrome [published correction appears in Rev Esp Cardiol. 2010;63:620] Rev Esp Cardiol. 2009;62:1297–1315. doi: 10.1016/s1885-5857(09)73357-2. [DOI] [PubMed] [Google Scholar]
- 48.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP Heart Rhythm Society (HRS); European Heart Rhythm Association (EHRA) HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Europace. 2011;13:1077–1109. doi: 10.1093/europace/eur245. [DOI] [PubMed] [Google Scholar]
- 49.Baron RC, Thacker SB, Gorelkin L, Vernon AA, Taylor WR, Choi K. Sudden death among Southeast Asian refugees: an unexplained nocturnal phenomenon. JAMA. 1983;250:2947–2951. [PubMed] [Google Scholar]
- 50.Gilbert J, Gold RL, Haffajee CI, Alpert JS. Sudden cardiac death in a southeast Asian immigrant: clinical, electrophysiologic, and biopsy characteristics. Pacing Clin Electrophysiol. 1986;9(pt 1):912–914. doi: 10.1111/j.1540-8159.1986.tb06640.x. [DOI] [PubMed] [Google Scholar]
- 51.Hermida JS, Lemoine JL, Aoun FB, Jarry G, Rey JL, Quiret JC. Prevalence of the Brugada syndrome in an apparently healthy population. Am J Cardiol. 2000;86:91–94. doi: 10.1016/s0002-9149(00)00835-3. [DOI] [PubMed] [Google Scholar]
- 52.Miyasaka Y, Tsuji H, Yamada K, Tokunaga S, Saito D, Imuro Y, Matsumoto N, Iwasaka T. Prevalence and mortality of the Brugada-type electrocardiogram in one city in Japan. J Am Coll Cardiol. 2001;38:771–774. doi: 10.1016/s0735-1097(01)01419-x. [DOI] [PubMed] [Google Scholar]
- 53.Nademanee K, Veerakul G, Nimmannit S, Chaowakul V, Bhuripanyo K, Likittanasombat K, Tunsanga K, Kuasirikul S, Malasit P, Tansupasawadikul S, Tatsanavivat P. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–2600. doi: 10.1161/01.cir.96.8.2595. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 55.Sumitomo N, Harada K, Nagashima M, Yasuda T, Nakamura Y, Aragaki Y, Saito A, Kurosaki K, Jouo K, Koujiro M, Konishi S, Matsuoka S, Oono T, Hayakawa S, Miura M, Ushinohama H, Shibata T, Niimura I. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamakura S, Ohe T, Nakazawa K, Aizawa Y, Shimizu A, Horie M, Ogawa S, Okumura K, Tsuchihashi K, Sugi K, Makita N, Hagiwara N, Inoue H, Atarashi H, Aihara N, Shimizu W, Kurita T, Suyama K, Noda T, Satomi K, Okamura H, Tomoike H Brugada Syndrome Investigators in Japan. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1–V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 57.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, Borggrefe M, Haissaguerre M, Mabo P, Le Marec H, Wolpert C, Wilde AA. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 58.Maury P, Rollin A, Sacher F, Gourraud JB, Raczka F, Pasquié JL, Duparc A, Mondoly P, Cardin C, Delay M, Derval N, Chatel S, Bongard V, Sadron M, Denis A, Davy JM, Hocini M, Jaïs P, Jesel L, Haïssaguerre M, Probst V. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013;112:1384–1389. doi: 10.1016/j.amjcard.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton RM. Arrhythmogenic right ventricular cardiomyopathy. Pacing Clin Electrophysiol. 2009;32(suppl 2):S44–S51. doi: 10.1111/j.1540-8159.2009.02384.x. [DOI] [PubMed] [Google Scholar]
- 60.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 61.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 62.Lieve KV, Wilde AA. Inherited ion channel diseases: a brief review. Europace. 2015;17(suppl 2):ii1–ii6. doi: 10.1093/europace/euv105. [DOI] [PubMed] [Google Scholar]
- 63.Sy RW, Gollob MH, Klein GJ, Yee R, Skanes AC, Gula LJ, Leong-Sit P, Gow RM, Green MS, Birnie DH, Krahn AD. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8:864–871. doi: 10.1016/j.hrthm.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 64.Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873–879. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 65.Olivotto I, Gistri R, Petrone P, Pedemonte E, Vargiu D, Cecchi F. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:315–321. doi: 10.1016/s0735-1097(02)02713-4. [DOI] [PubMed] [Google Scholar]
- 66.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 67.Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 68.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 69.Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:697–704. doi: 10.1016/j.jacc.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 70.Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol. 2003;41:987–993. doi: 10.1016/s0735-1097(02)03004-8. [DOI] [PubMed] [Google Scholar]
- 71.Spirito P, Autore C, Rapezzi C, Bernabò P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NA, Barillà CS, Biagini E, Quarta G, Conte MR, Bruzzi P, Maron BJ. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 72.Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. doi: 10.1093/eurheartj/ehl041. [DOI] [PubMed] [Google Scholar]
- 73.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 74.Efthimiadis GK, Parcharidou DG, Giannakoulas G, Pagourelias ED, Charalampidis P, Savvopoulos G, Ziakas A, Karvounis H, Styliadis IH, Parcharidis GE. Left ventricular outflow tract obstruction as a risk factor for sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:695–699. doi: 10.1016/j.amjcard.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 75.Bos JM, Maron BJ, Ackerman MJ, Haas TS, Sorajja P, Nishimura RA, Gersh BJ, Ommen SR. Role of family history of sudden death in risk stratification and prevention of sudden death with implantable defibrillators in hypertrophic cardiomyopathy. Am J Cardiol. 2010;106:1481–1486. doi: 10.1016/j.amjcard.2010.06.077. [DOI] [PubMed] [Google Scholar]
- 76.Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 77.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O’Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clémenty J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 78.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 80.Walsh JA, 3rd, Ilkhanoff L, Soliman EZ, Prineas R, Liu K, Ning H, Lloyd-Jones DM. Natural history of the early repolarization pattern in a biracial cohort: CARDIA (Coronary Artery Risk Development in Young Adults) Study. J Am Coll Cardiol. 2013;61:863–869. doi: 10.1016/j.jacc.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viskin S, Rosso R, Rogowski O, Belhassen B. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912–916. doi: 10.1111/j.1540-8167.2005.50040.x. [DOI] [PubMed] [Google Scholar]
- 82.Gourraud JB, Le Scouarnec S, Sacher F, Chatel S, Derval N, Portero V, Chavernac P, Sandoval JE, Mabo P, Redon R, Schott JJ, Le Marec H, Haïssaguerre M, Probst V. Identification of large families in early repolarization syndrome. J Am Coll Cardiol. 2013;61:164–172. doi: 10.1016/j.jacc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 83.Sinner MF, Porthan K, Noseworthy PA, Havulinna AS, Tikkanen JT, Müller-Nurasyid M, Peloso G, Ulivi S, Beckmann BM, Brockhaus AC, Cooper RR, Gasparini P, Hengstenberg C, Hwang SJ, Iorio A, Junttila MJ, Klopp N, Kähönen M, Laaksonen MA, Lehtimäki T, Lichtner P, Lyytikäinen LP, Martens E, Meisinger C, Meitinger T, Merchant FM, Nieminen MS, Peters A, Pietilä A, Perz S, Oikarinen L, Raitakari O, Reinhard W, Silander K, Thorand B, Wichmann HE, Sinagra G, Viikari J, O’Donnell CJ, Ellinor PT, Huikuri HV, Kääb S, Newton-Cheh C, Salomaa V. A meta-analysis of genome-wide association studies of the electrocardiographic early repolarization pattern. Heart Rhythm. 2012;9:1627–1634. doi: 10.1016/j.hrthm.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arking DE, Junttila MJ, Goyette P, Huertas-Vazquez A, Eijgelsheim M, Blom MT, Newton-Cheh C, Reinier K, Teodorescu C, Uy-Evanado A, Carter-Monroe N, Kaikkonen KS, Kortelainen ML, Boucher G, Lagacé C, Moes A, Zhao X, Kolodgie F, Rivadeneira F, Hofman A, Witteman JC, Uitterlinden AG, Marsman RF, Pazoki R, Bardai A, Koster RW, Dehghan A, Hwang SJ, Bhatnagar P, Post W, Hilton G, Prineas RJ, Li M, Köttgen A, Ehret G, Boerwinkle E, Coresh J, Kao WH, Psaty BM, Tomaselli GF, Sotoodehnia N, Siscovick DS, Burke GL, Marbán E, Spooner PM, Cupples LA, Jui J, Gunson K, Kesäniemi YA, Wilde AA, Tardif JC, O’Donnell CJ, Bezzina CR, Virmani R, Stricker BH, Tan HL, Albert CM, Chakravarti A, Rioux JD, Huikuri HV, Chugh SS. Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet. 2011;7:e1002158. doi: 10.1371/journal.pgen.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Institute of Medicine (IOM) [Accessed September 19, 2015];Strategies to Improve Cardiac Arrest Survival: A Time to Act. Released June 30, 2015. http://iom.nationalacademies.org/Reports/2015/Strategies-to-Improve-Cardiac-Arrest-Survival.aspx.
19. SUBCLINICAL ATHEROSCLEROSIS
See Table 19-1 and Charts 19-1 through 19-8
Table 19-1.
CAC Scores for the 75th Percentile of Males and Females of Different Race/Ethnic Groups, at Specified Ages
| Age, y | 75th Percentile CAC Scores* | |||
|---|---|---|---|---|
| Black | Chinese | Hispanic | White | |
| Females | ||||
| 45 | 0 | 0 | 0 | 0 |
| 55 | 0 | 2 | 0 | 1 |
| 65 | 26 | 45 | 19 | 54 |
| 75 | 138 | 103 | 116 | 237 |
| Males | ||||
| 45 | 0 | 3 | 0 | 0 |
| 55 | 15 | 34 | 27 | 68 |
| 65 | 95 | 121 | 141 | 307 |
| 75 | 331 | 229 | 358 | 820 |
CAC indicates coronary artery calcification.
The 75th percentile CAC score is the score at which 75% of people of the same age, sex, and race have a score at or below this level and 25% of people of the same age, sex, and race have a higher score.
Source: MESA (Multi-Ethnic Study of Atherosclerosis) CAC Tools Web site.71
Chart 19-1. Prevalence (%) of detectable coronary calcium in the CARDIA study: US adults 33 to 45 years of age (2000–2001).
P<0.0001 across race-sex groups. CARDIA indicates Coronary Artery Risk Development in Young Adults.
Data derived from Loria et al.9
Chart 19-8. Mean values of carotid IMT for different carotid artery segments in older adults, by race.
IMT indicates intima-media thickness.
Data derived from Manolio et al.44
Atherosclerosis, a systemic disease process in which fatty deposits, inflammation, cells, and scar tissue build up within the walls of arteries, is the underlying cause of the majority of clinical cardiovascular events. Atherosclerosis can develop in large and small arteries supplying a variety of end-organs, including the heart, brain, kidneys, and extremities. There can be significant variability in which size arteries and locations are affected in individual patients, although atherosclerosis is often a systemic disease. In recent decades, advances in imaging technology have allowed for improved ability to detect and quantify atherosclerosis at all stages and in multiple different vascular beds. Early identification of subclinical atherosclerosis could lead to more aggressive lifestyle modifications and medical treatment to prevent clinical manifestations of atherosclerosis such as MI, stroke, or renal failure. Two modalities, CT of the chest for evaluation of CAC and B-mode ultrasound of the neck for evaluation of carotid artery IMT, have been used in large studies with outcomes data and can help define the burden of atherosclerosis in individuals before they develop clinical events such as heart attack or stroke. Another commonly used method for detecting and quantifying atherosclerosis in the peripheral arteries is the ABI. Data on cardiovascular outcomes are beginning to emerge for additional modalities that measure anatomic and functional measures of subclinical disease, including brachial artery reactivity testing, aortic and carotid MRI, and to-nometric methods of measuring vascular compliance or microvascular reactivity. Further research could help to define the role of these techniques in cardiovascular risk assessment. Some guidelines have recommended that screening for subclinical atherosclerosis, especially by CAC or IMT, might be appropriate in people at intermediate risk for HD (eg, 10-year estimated risk of 10%–20%) but not for lower-risk general population screening or for people with preexisting HD or most other high-risk conditions.1,2 However, a recent guideline notes that those with DM who are ≥40 years of age may be suitable for screening of risk by coronary calcium.1
Abbreviations Used in Chapter 19
| ABI | ankle-brachial index |
| ACC | American College of Cardiology |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| ARIC | Atherosclerosis Risk in Communities Study |
| ASCVD | atherosclerotic cardiovascular disease |
| ATP III | Adult Treatment Panel III |
| BMI | body mass index |
| BNP | B-type natriuretic peptide |
| BP | blood pressure |
| CAC | coronary artery calcification |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CKD | chronic kidney disease |
| CI | confidence interval |
| CONFIRM | Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry |
| CRP | C-reactive protein |
| CT | computed tomography |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| ESRD | end-stage renal disease |
| FHS | Framingham Heart Study |
| FMD | flow-mediated dilation |
| FRS | Framingham Risk Score |
| HBP | high blood pressure |
| HD | heart disease |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein cholesterol |
| HF | heart failure |
| HR | hazard ratio |
| IMT | intima-media thickness |
| JUPITER | Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin |
| LDL-C | low-density lipoprotein cholesterol |
| MASALA | Mediators of Atherosclerosis in South Asians Living in America |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| MRI | magnetic resonance imaging |
| NHLBI | National Heart, Lung, and Blood Institute |
| NNT5 | 5-year number needed to treat |
| PAD | peripheral artery disease |
| QALY | quality-adjusted life-year |
| RR | relative risk |
| SBP | systolic blood pressure |
| SD | standard deviation |
| TIA | transient ischemic attack |
| TIPS | The Indian Polycap Study |
According to the latest ACC/AHA cholesterol management guidelines, when treatment decisions are uncertain after 10-year risk is estimated, then the patient and clinician should take into consideration additional factors that modify the risk estimate, including an elevated CAC score or an ABI <0.9.3 There are still limited data demonstrating whether screening with these and other imaging modalities can improve patient outcomes or whether it only increases downstream medical care costs. A recently published report in a large cohort randomly assigned to coronary calcium screening or not showed such screening to result in an improved risk factor profile without increasing downstream medical costs.4 In addition, a recent cost-effectiveness analysis based on data from MESA5 reported that CAC testing and statin treatment for those with CAC >0 was cost-effective (<$50 000 per QALY) in intermediate-risk scenarios (CHD risk 5%–10%) considering less favorable statin assumptions ($1.00 per pill). Furthermore, a recent MESA analysis compared these CAC-based treatment strategies to a “treat all” strategy and to treatment according to the ATP III guidelines, with clinical and economic outcomes modeled over both 5- and 10-year time horizons.6 The results consistently demonstrated that it is both cost-saving and more effective to scan intermediate-risk patients for CAC and to treat those with CAC ≥1 than to use treatment based on established risk assessment guidelines.6
Coronary Artery Calcification
Background
CAC is a measure of the burden of atherosclerosis in the heart arteries and is measured by CT. Other components of the atherosclerotic plaque, including fatty (eg, cholesterol-rich components) and fibrotic components, often accompany CAC and can be present even in the absence of CAC.
The presence of any CAC, which indicates that at least some atherosclerotic plaque is present, is defined by an Agatston score >0. Clinically significant plaque, frequently an indication for more aggressive risk factor management, is often defined by an Agatston score ≥100 or a score ≥75th percentile for one’s age and sex; however, although they predict short- to intermediate-term risk, absolute CAC cutoffs offer more prognostic information across all age groups in both males and females.7 An Agatston score ≥400 has been noted to be an indication for further diagnostic evaluation (eg, exercise testing or myocardial perfusion imaging) for CAD.
Prevalence
(See Table 19-1 and Charts 19-1 through 19-3)
Chart 19-3. Ten-year trends in coronary artery calcification in individuals without clinical cardiovascular disease in MESA.
MESA indicates Multi-Ethnic Study of Atherosclerosis.
Data derived from Bild et al.17
-
The NHLBI’s FHS reported CAC measured in 3238 white adults in age groups ranging from <45 years of age to ≥75 years of age.8
Overall, 32.0% of females and 52.9% of males had prevalent CAC.
Among participants at intermediate risk according to FRS, 58% of females and 67% of males had prevalent CAC.
-
The NHLBI’s CARDIA study measured CAC in 3043 black and white adults 33 to 45 years of age (at the CARDIA year 15 examination).9
Overall, 15.0% of males and 5.1% of females, 5.5% of those 33 to 39 years of age and 13.3% of those 40 to 45 years of age, had prevalent CAC. Overall, 1.6% of participants had an Agatston score that exceeded 100.
Chart 19-1 shows the prevalence of CAC by ethnicity and sex in adults 33 to 45 years of age. The prevalence of CAC was lower in black males than in white males but was similar in black and white females at these ages.
-
The NHLBI’s Jackson Heart Study recently reported outcomes with presence of elevated CAC (>100) in 4416 African American participants (mean age 54 years; 64% females) followed up for 6 years.10
CAC >100 was noted in 14% of those without any metabolic syndrome or DM, 26% of those with metabolic syndrome, and 41% of those with DM.
At 6-year follow-up, 265 CVD events were noted in this cohort.
High CAC scores were significantly associated with CVD events among those with neither metabolic syndrome nor DM (HR, 4.3; 95% CI, 2.0–9.5), those with metabolic syndrome (HR, 2.2; 95% CI, 1.1–4.4), and those with DM (HR, 3.4; 95% CI, 1.6–8.7).
In comparison, the presence of PAD was not predictive of CVD events in either group.
-
The NHLBI’s MESA measured CAC in 6814 participants 45 to 84 years of age, including white (n=2619), black (n=1898), Hispanic (n=1494), and Chinese (n=803) males and females.11
Chart 19-2 shows the prevalence of CAC by sex and ethnicity in US adults 45 to 84 years of age in MESA.
The prevalence and 75th percentile levels of CAC were highest in white males and lowest in black and Hispanic females. Significant ethnic differences persisted after adjustment for risk factors, with the RR of coronary calcium being 22% less in blacks, 15% less in Hispanics, and 8% less in Chinese than in whites.
Table 19-1 shows the 75th percentile levels of CAC by sex and race at selected ages in MESA.
In a comparison of MESA with the MASALA study, which is a community-based cohort of South Asians in the United States (mean age 58 years), the age-adjusted prevalence of CAC was similar among white (68.8%) and South Asian (67.9%) males, with these groups having a greater prevalence of CAC than Chinese (57.8%), African-American (51.2%), and Hispanic (57.9%) males. In contrast, the age-adjusted prevalence of CAC was lower in South Asian females (36.8%) than in white females (42.6%) and females of other races/ethnicities.12
To date, sparse research exists on the prevalence of subclinical atherosclerosis, including CAC, in rural areas of the United States. A recent study reported the distribution of CAC scores among 1607 (mean age 56 years; 56% females) community-dwelling asymptomatic individuals from central Appalachia. Overall, 44% had CAC of 0, whereas the prevalence of those with mild (1–99), moderate (100–399), and severe (>400) CAC was 29%, 15%, and 11%, respectively.13
The prevalence of CAC varies widely according to baseline risk profile, including global scores such as the FRS. In a report from MESA,14 the prevalence of CAC among individuals with a very low FRS (≤2.5%) was 22%, and it was 39% among those with an FRS 10-year risk for CHD of 2.5% to 5%. In recent studies from MESA, the prevalence of CAC in those with no lipid abnormalities was 42%,15 and nearly one fifth (22%) of people in MESA with no known traditional CVD risk factors had presence of CAC.16
In a 2005 update,17 the 10-year trends in CAC among individuals without clinical CVD in MESA were reported (Chart 19-3). After adjustment for age, sex, ethnicity, and type of CT scanner, the proportion of participants with no CAC decreased over time from 40.7% to 32.6% (P=0.007), and the proportions increased from 29.9% to 37.0% (P=0.01) for those with a CAC score ranging from 1 to 99 and from 14.7% to 17.7% (P=0.14) for those with a CAC score of 100 to 299, whereas the proportion with a CAC score ≥400 decreased from 9.1% to 7.2% (P=0.11). Trends in CAC among the 4 race/ethnicity groups revealed a significant trend toward increased prevalence of CAC in African Americans but not in any other group. Among African Americans, the CAC prevalence ratio (year 10 versus baseline) was 1.27 (P<0.001 for test for trend). Adjustment for risk factors made no notable difference in CAC trends in any ethnic group.17
Chart 19-2. Prevalence (%) of detectable coronary calcium in MESA: US adults 45 to 84 years of age.
P<0.0001 across ethnic groups in both males and females.
MESA indicates Multi-Ethnic Study of Atherosclerosis.
Data derived from Bild et al.11
CAC and Incidence of Cardiovascular Events
(See Charts 19-4 and 19-5)
Chart 19-4. HRs for CHD events associated with coronary calcium scores: US adults 45 to 84 years of age (reference group, CAC=0).
All HRs P<0.0001. Major CHD events included myocardial infarction and death attributable to CHD; any CHD events included major CHD events plus definite angina or definite or probable angina followed by revascularization.
CAC indicates coronary artery calcification; CHD, coronary heart disease; and HR, hazard ratio.
Data derived from Detrano et al.18
Chart 19-5. HRs for coronary heart disease events associated with coronary calcium scores: US adults (reference group, CAC=0 and Framingham Risk Score <10%).
Coronary heart disease events included nonfatal myocardial infarction and death attributable to coronary heart disease.
CAC indicates coronary artery calcification; and HR, hazard ratio.
Data derived from Greenland et al.20
-
The NHLBI’s MESA reported on the association of CAC scores with first CHD events over a median follow-up of 3.9 years among a population-based sample of 6722 males and females (39% white, 27% black, 22% Hispanic, and 12% Chinese).18
Chart 19-4 shows the HRs associated with CAC scores of 1 to 100, 101 to 300, and >300 compared with those without CAC (score=0), after adjustment for standard risk factors. People with CAC scores of 1 to 100 had ≈4 times greater risk and those with CAC scores >100 were 7 to 10 times more likely to experience a coronary event than those without CAC.
CAC provided similar predictive value for coronary events in whites, Chinese, blacks, and Hispanics (HRs ranging from 1.15–1.39 for each doubling of coronary calcium).
In MESA, CAC was noted to be highly predictive of CHD event risk across all age groups in a follow-up that extended to 8.5 years, which suggests that once CAC is known, chronological age has less importance. Compared with a CAC score of 0, CAC >100 imparted an increased multivariable-adjusted CHD event risk in the younger individuals (45–54 years old), with an HR of 12.4 (95% CI, 5.1–30.0). The respective risk was similar even in the very elderly (75–84 years of age), with an HR of 12.1 (95% CI, 2.9–50.2).19
-
In another report of a community-based sample, not referred for clinical reasons, the South Bay Heart Watch examined CAC in 1461 adults (average age 66 years) with coronary risk factors, with a median of 7.0 years of follow-up.20
Chart 19-5 shows the HRs associated with increasing CAC scores (relative to CAC=0 and <10% risk category) in low-risk (<10%), intermediate-risk (10%–15% and 16%–20%), and high-risk (>20%) FRS categories of estimated risk for CHD in 10 years. Increasing CAC scores further predicted risk in intermediate- and high-risk groups.
In a study of healthy adults 60 to 72 years of age who were free of clinical CAD, predictors of the progression of CAC were assessed. Predictors tested included age, sex, race/ethnicity, smoking status, BMI, family history of CAD, CRP, several measures of DM, insulin levels, BP, and lipids. Insulin resistance, in addition to the traditional cardiac risk factors, independently predicts progression of CAC.21 Clinically, however, it is not yet recommended to conduct serial scanning of CAC to measure effects of therapeutic interventions.
A recent publication from MESA also used CAC, in particular, and carotid IMT to stratify CHD and CVD event risk in people with metabolic syndrome and DM; those with low levels of CAC or carotid IMT have CHD and CVD event rates as low as many people without metabolic syndrome and DM. Those with DM who have CAC scores <100 have annual CHD event rates of <1%.22
It is noteworthy, as recently demonstrated in MESA in 5878 participants with a median of 5.8 years of follow-up, that the addition of CAC to standard risk factors resulted in significant improvement of classification of risk for incident CHD events, placing 77% of people in the highest or lowest risk categories compared with 69% based on risk factors alone. An additional 23% of those who experienced events were reclassified as high risk, and 13% with events were reclassified as low risk.23 The contribution of CAC to risk prediction has also been observed in other cohorts, including both the Heinz Nixdorf Recall Study23a and the Rotterdam Study.24
The prospective Dallas Heart Study recently reported the prognostic value of CAC scores in a relatively younger cohort (44.4±9.0 years of age). Among the 2084 participants who were followed up for a median of 9 years, compared with individuals with CAC=0, those with CAC scores of 10 to 99 and >100 were associated with an HR (95% CI) of 3.43 (1.36–8.56) and 5.64 (2.28–13.97) for CHD events, respectively. The addition of CAC to the traditional risk factor model resulted in significant improvement in the C statistic (Δ=0.03; P=0.003), as well as a net correct reclassification of 22%.25
In the Heinz Nixdorf Recall Study,26 CAC independently predicted stroke during a mean follow-up of 7.9 years. Cox proportional hazards regressions were used to examine CAC as a predictor of stroke in addition to established vascular risk factors (age, sex, SBP, LDL-C, HDL-C, DM, smoking, and AF). Study participants who had a stroke had significantly higher CAC values at baseline than the remaining subjects (median 104.8 [quartile 1, 14.0; quartile 3, 482.2] versus 11.2 [quartile 1, 0; quartile 3, 106.2]; P<0.001). In a multivariable Cox regression, log10(CAC+1) was an independent stroke predictor (HR, 1.52; 95% CI, 1.19–1.92; P=0.001). CAC discriminated stroke risk specifically in participants in the low (<10%) and intermediate (10%–20%) FRS categories.26
A meta-analysis27 also highlighted the utility of CAC testing in the diabetic population. In this meta-analysis, 8 studies were included (n=6521; 802 events; mean follow-up 5.18 years). The RR for all-cause mortality or cardiovascular events or both comparing a total CAC score ≥10 with a score <10 was 5.47 (95% CI, 2.59–11.53; I2=82.4%, P<0.001). For people with a CAC score <10, the posttest probability of the composite outcome was ≈1.8%, representing a 6.8-fold reduction from the pretest probability, which suggests that low or absent CAC could facilitate risk stratification by enabling the identification of people at low risk within this high-risk population.27
Recent studies have suggested CAC also predicts cardiac events beyond stroke and MI. In the Rotterdam Study, CAC independently predicted incident HF during a median follow-up of 6.8 years. Those with severe CAC (>400) after adjustment for risk factors had a 4.1-fold higher risk (95% CI, 1.7–10.1) of HF than those with CAC scores of 0 to 10.28 In addition, CAC substantially improved the risk classification of subjects (net reclassification index, 34.0%).
In MESA, during a median follow-up period of 8.5 years, after accounting for risk factors, higher CAC scores were associated with increased risk for AF (CAC=0: HR, 1.0 [referent]; CAC=1–100: HR, 1.4 [95% CI, 1.01–2.0]; CAC=101–300: HR, 1.6 [95% CI, 1.1–2.4]; CAC >300: HR, 2.1 [95% CI, 1.4–2.9]). The addition of CAC to the FHS AF risk score yielded relative integrated discrimination improvement of 0.10 (95% CI, 0.061–0.15).29
Investigators from MESA recently reported a higher CAC burden was also associated with non-CVD outcomes. During a median follow-up of 10.2 years, accounting for demographics and traditional risk factors, participants with severe CAC (>400) were at an increased risk of cancer (HR, 1.53; 95% CI, 1.18–1.99), CKD (HR, 1.70; 95% CI, 1.21–2.39), pneumonia (HR, 1.97; 95% CI, 1.37–2.82), chronic obstructive pulmonary disease (HR, 2.71; 95% CI, 1.60–4.57), and hip fracture (HR, 4.29; 95% CI, 1.47–12.50) compared with those with CAC=0.30
An absence of CAC, observed in 40% to 50% of individuals, confers a very low risk for future cardiovascular events. In a meta-analysis of 13 studies assessing the relationship of CAC with adverse cardiovascular outcomes that included 71 595 asymptomatic patients, 29 312 patients (41%) did not have any evidence of CAC.31 In a follow-up that averaged 3 to 5 years, 154 of 29 312 patients without CAC (0.47%) experienced a cardiovascular event compared with 1749 of 42 283 patients with CAC (4.14%). The cumulative RR was 0.15 (95% CI, 0.11–0.21; P<0.001). These findings were confirmed in MESA, which reported a rate of 0.52% for CHD events during a median of 4 years of follow-up among people with no detectable CAC.32
The value of CAC=0 has been confirmed in various high-risk groups. For example, in MESA, 38% of those with DM had CAC=0, and the annualized CHD and CVD event rates were 0.4% and 0.8%, respectively.22 A recent publication16 from MESA demonstrated a low hard CHD event rate per 1000 years during a median follow-up of 7.1 years across the entire spectrum of baseline FRS (0%–6%: 0.9; 6%–10%: 1.1; 10%–20%: 1.9; >20%: 2.5). Among high-risk individuals considered for various polypill criteria in MESA,33 based on age and risk factors, the prevalence of CAC=0 ranged from 39% to 59%, and the respective rate of CHD events varied from 1.2 to 1.9 events per 1000 person-years during a median follow-up of 7.6 years.
Furthermore, a recent study from MESA demonstrated that during a median 10-year follow-up, among 13 negative risk markers (CAC=0, carotid IMT <25th percentile, absence of carotid plaque, brachial FMD >5% change, ABI >0.9 and <1.3, high-sensitivity CRP <2 mg/L, homocysteine <10 μmol/L, N-terminal pro-BNP <100 pg/mL, no microalbuminuria, no family history of CHD (any/premature), absence of metabolic syndrome, and healthy lifestyle), CAC=0 had the lowest diagnostic likelihood ratio for all CHD (0.41) and CVD (0.54).34
CAC Progression and Risk
A recent report of 4609 individuals who had baseline and repeat cardiac CT found that progression of CAC provided incremental information over baseline score, demographics, and cardiovascular risk factors in predicting future all-cause mortality.35
More recently, data from 6778 people in MESA showed annual CAC progression was an average of 25 Agatston units, and among those without CAC at baseline, a 5-U annual change in CAC was associated with HRs of 1.4 and 1.5 for total and hard CHD events, respectively. Among those with CAC >0 at baseline, HRs per 100-U annual change in CAC were 1.2 and 1.3, respectively, and for those with annual progression ≥300 versus no progression, HRs were 3.8 and 6.3, respectively.36 Progression of CAC in MESA was also shown to be greater in those with metabolic syndrome and DM than in those with neither condition, and progression of CAC in each of these conditions was associated with a greater future risk of CHD events.37
Furthermore, a recent study from MESA specifically demonstrated association of CAC progression with incident AF. Presence of any CAC progression (>0 per year) in the 5-year follow-up was associated with 1.55-fold higher risk for AF (95% CI, 1.10–2.19). The risk of AF increased with higher levels of CAC progression: (1–100 per year: HR, 1.47; 95% CI, 1.03–2.09; 101–300 per year: HR, 1.92; 95% CI, 1.15–3.20; >300 per year: HR, 3.23; 95% CI, 1.48–7.05).38
In MESA, greater adherence to a healthy lifestyle based on a healthy lifestyle score was associated with slower progression of CAC and lower mortality rates relative to those with the most unhealthy lifestyle.39
Carotid IMT
Background
Carotid IMT measures the thickness of 2 layers (the intima and media) of the wall of the carotid arteries, the largest conduits of blood going to the brain. Carotid IMT is thought to be an even earlier manifestation of atherosclerosis than CAC, because thickening precedes the development of frank atherosclerotic plaque. Carotid IMT methods are still being refined, so it is important to know which part of the artery was measured (common carotid, internal carotid, or bulb) and whether near and far walls were both measured. This information can affect the average-thickness measurement that is usually reported.
Unlike CAC, everyone has some thickness to the layers of their arteries, but people who develop atherosclerosis have greater thickness. Ultrasound of the carotid arteries can also detect plaques and determine the degree of narrowing of the artery they may cause. Epidemiological data highlighted in the section “Prevalence and Association With Incident Cardiovascular Events” indicate that high-risk levels of thickening might be considered as those in the highest quartile or quintile for one’s age and sex, or ≥1 mm.
Although ultrasound is commonly used to diagnose plaque in the carotid arteries in people who have had strokes or who have bruits (sounds of turbulence in the artery), guidelines are limited as to screening of asymptomatic people with carotid IMT to quantify atherosclerosis or predict risk. However, some organizations have recognized that carotid IMT measurement by B-mode ultra-sonography may provide an independent assessment of coronary risk.40
Prevalence and Association With Incident Cardiovascular Events
(See Charts 19-6 and 19-7)
Chart 19-6. Mean values of carotid IMT for different carotid artery segments in younger adults by race and sex (Bogalusa Heart Study).
IMT indicates intima-media thickness.
Data derived from Urbina et al.41
Chart 19-7. Association between cardiovascular risk factors and mean common carotid intima-media thickness, by ethnicity.
Point estimates for betas, lines represent 95% confidence intervals.
HDL indicates high-density lipoprotein.
Reprinted from Gijsberts et al42. Copyright © 2015, Gijsberts et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
The Bogalusa Heart Study measured carotid IMT in 518 black and white males and females at a mean age of 32±3 years. These males and females were healthy but overweight.41
The mean values of carotid IMT for the different segments are shown in Chart 19-6 by sex and race. Males had significantly higher carotid IMT in all segments than females, and blacks had higher common carotid and carotid bulb IMTs than whites.
Even at this young age, after adjustment for age, race, and sex, carotid IMT was associated significantly and positively with waist circumference, SBP, DBP, and LDL-C. Carotid IMT was inversely correlated with HDL-C levels. Participants with greater numbers of adverse risk factors (0, 1, 2, 3, or more) had stepwise increases in mean carotid IMT levels.
Updates from individual participant meta-analysis involving 15 population-based cohorts worldwide that included 60 211 individuals (46 788 whites, 7200 blacks, 3816 Asians, and 2407 Hispanics) demonstrated differing associations between risk factors and burden of carotid IMT according racial/ethnic groups.42 Specifically, association between age and carotid IMT was weaker in blacks and Hispanics. SBP was more strongly associated with carotid IMT in Asians. HDL-C and smoking was associated less with carotid IMT in blacks (Chart 19-7).
In a subsequent analysis, the Bogalusa investigators examined the association of risk factors measured since childhood with carotid IMT measured in these young adults.43 Higher BMI and LDL-C levels measured at 4 to 7 years of age were associated with increased risk for being >75th percentile for carotid IMT in young adulthood. Higher SBP and LDL-C and lower HDL-C in young adulthood were also associated with having high carotid IMT. These data highlight the importance of adverse risk factor levels in early childhood and young adulthood in the early development of atherosclerosis.
Among both females and males in MESA, blacks had the highest common carotid IMT, but they were similar to whites and Hispanics in internal carotid IMT. Chinese participants had the lowest carotid IMT, in particular in the internal carotid, of the 4 ethnic groups44 (Chart 19-8).
The NHLBI’s CHS reported follow-up of 4476 males and females ≥65 years of age (mean age 72 years) who were free of CVD at baseline.45 Mean maximal common carotid IMT was 1.03±0.20 mm, and mean internal carotid IMT was 1.37±0.55 mm.
After a mean follow-up of 6.2 years, those with maximal combined carotid IMT in the highest quintile had a 4- to 5-fold greater risk for incident heart attack or stroke than those in the bottom quintile. After adjustment for other risk factors, there was still a 2- to 3-fold greater risk for the top versus the bottom quintile.
In MESA, during a median follow-up of 3.3 years, IMT rate of change of 0.5 mm/year was associated with an HR of 1.23 (95% CI, 1.02–1.48) for incident stroke. The upper quartile of IMT rate of change had an HR of 2.18 (95% CI, 1.07–4.46) compared with the lower 3 quartiles combined.46
A study of 441 individuals ≤65 years of age without a history of CAD, DM, or hyperlipidemia who were examined for carotid IMT found 42% had high-risk carotid ultrasound findings (carotid IMT ≥75th percentile, adjusted for age, sex, and race or presence of plaque). Among those with an FRS ≤5%, 38% had high-risk carotid ultrasound findings.47
Conflicting data have been reported on the contribution of carotid IMT to risk prediction. In 13 145 participants in the NHLBI’s ARIC study, the addition of carotid IMT combined with identification of plaque presence or absence to traditional risk factors reclassified risk in 23% of individuals overall, with a net reclassification improvement of 9.9%. There was a modest but statistically significant improvement in the area under the receiver operating characteristic curve, from 0.742 to 0.755.48 In contrast, data reported recently from the Carotid Atherosclerosis Progression Study observed a net reclassification improvement of −1.4% that was not statistically significant.49
In the Rotterdam Study, 3580 nondiabetic individuals aged 55 to 75 years were followed up for a median of 12.2 years. In older males, addition of carotid IMT to Framingham risk factors did not improve prediction of hard CHD or stroke. In older females, addition of carotid IMT to Framingham risk factors yielded a net reclassification improvement in females of 8.2% (P=0.03) for hard CHD and 8.0% (P=0.06) for stroke.50
A recent study from a consortium of 14 population-based cohorts consisting of 45 828 individuals followed up for a median of 11 years demonstrated little additive value of common carotid IMT to FRS for purposes of discrimination and reclassification as far as incident MI and stroke were concerned. The C statistics of the model with FRS alone (0.757; 95% CI, 0.749–0.764) and with addition of common carotid IMT (0.759; 95% CI, 0.752–0.766) were similar. The net reclassification improvement with the addition of common carotid IMT was small (0.8%; 95% CI, 0.1%–1.6%). In those at intermediate risk, the net reclassification improvement was 3.6% among all individuals (95% CI, 2.7%–4.6%).51
A recent study from the same consortium of a population-based cohort reported no added value of measurement of mean common carotid IMT in individuals with HBP for improving cardiovascular risk prediction. For those at intermediate risk, the addition of mean common carotid IMT to an existing cardiovascular risk score resulted in a small but statistically significant improvement in risk prediction.52
In a recent study, however, carotid plaque burden measured via 3-dimensional carotid ultrasound showed promise in improving CVD risk prediction. The prospective BioImage Study enrolled 5808 asymptomatic US adults (mean age 69 years, 56.5% females). Carotid plaque areas from both carotid arteries were summed as the carotid plaque burden. The primary end point was the composite of major adverse cardiac events (cardiovascular death, MI, and ischemic stroke). In a 2.7-year median follow-up, major adverse cardiac events occurred in 216 patients (4.2%), of which 82 (1.5%) were primary events. After adjustment for risk factors, the HRs for major adverse cardiac events were 1.45 (95% CI, 0.67–3.14) and 2.36 (95% CI, 1.13–4.92) with increasing carotid plaque burden tertile. Net reclassification improved significantly with carotid plaque burden (0.23).53
Two large population-based prospective studies have aimed to elucidate association of carotid ultrasound findings with outcomes with shared pathogenesis of atherosclerosis. Among 15 792 individuals aged 45 to 64 years (26% blacks, 56% females) followed up for a median of 22.7 years, mean carotid IMT in the fourth quartile (>0.81 mm) versus first quartile (<0.62) was significantly associated with ESRD.54 Investigators from the FHS demonstrated that additional information obtained from carotid ultrasound regarding the degree of carotid stenotic burden was predictive of cerebral microbleeds detected on brain MRI, which are recognized as a marker of stroke and dementia, in 1243 participants (aged 56.9±8.8 years; 53% females). Carotid stenosis ≥25% was associated with a 2.2-fold (95% CI, 1.10–4.40) increased risk of cerebral microbleed, whereas no association was noted with carotid IMT.55
CAC and Carotid IMT
-
In the NHLBI’s MESA, a study of white, black, Chinese, and Hispanic adults 45 to 84 years of age, carotid IMT and CAC were found to be commonly associated, but patterns of association differed somewhat by sex and race.44
Common and internal carotid IMT were greater in females and males who had CAC than in those who did not, regardless of ethnicity.
Overall, CAC prevalence and scores were associated with carotid IMT, but associations were somewhat weaker in blacks than in other ethnic groups.
In general, blacks had the thickest carotid IMT of all 4 ethnic groups, regardless of the presence of CAC.
Common carotid IMT differed little by race/ethnicity in females with any CAC, but among females with no CAC, IMT was higher among blacks (0.86 mm) than in the other 3 groups (0.76–0.80 mm).
-
In a more recent analysis from MESA, the investigators reported on follow-up of 6779 males and females in 4 ethnic groups over 9.5 years and compared the predictive utility of carotid IMT, carotid plaque, and CAC (presence and burden).56
CAC presence was a stronger predictor of incident CVD and CHD than carotid ultrasound measures.
Mean IMT ≥75th percentile (for age, sex, and race) alone did not predict events. Compared with traditional risk factors, C statistics for CVD (C=0.756) and CHD (C=0.752) increased the most by the addition of CAC presence (CVD, 0.776; CHD, 0.784; P<0.001) followed by carotid plaque presence (CVD, C=0.760; CHD, C=0.757; P<0.05).
Compared with risk factors (C=0.782), carotid plaque presence (C=0.787; P=0.045) but not CAC (C=0.785; P=0.438) improved prediction of stroke/TIA.
Investigators from the NHLBI’s CARDIA and MESA studies examined the burden and progression of subclinical atherosclerosis among adults <50 years of age. Ten-year and lifetime risks for CVD were estimated for each participant, and the participants were stratified into 3 groups: (1) those with low 10-year (<10%) and low lifetime (<39%) predicted risk for CVD; (2) those with low 10-year (<10%) but high lifetime (≥39%) predicted risk; and (3) those with high 10-year risk (>10%). The latter group had the highest burden and greatest progression of subclinical atherosclerosis. Given the young age of those studied, ≈90% of participants were at low 10-year risk, but of these, half had high predicted lifetime risk. Compared with those with low short-term/low lifetime predicted risks, those with low short-term/high lifetime predicted risk had significantly greater burden and progression of CAC and significantly greater burden of carotid IMT, even at these younger ages. These data confirm the importance of early exposure to risk factors for the onset and progression of subclinical atherosclerosis.57
Carotid IMT Progression and Risk
To date, few studies have comprehensively studied the impact of carotid IMT progression on CVD outcomes. Data from a comprehensive meta-analysis of individual participant data demonstrated that common carotid artery IMT progression in people with DM ranged between −0.09 and 0.04 mm per year in a follow-up of 3.6 years; however, this change was not associated with cardiovascular outcomes. The HR for a 1-SD increase in common carotid artery IMT progression was 0.99 (95% CI, 0.91–1.08).58
CT Angiography
CT angiography is widely used by cardiologists to aid in the diagnosis of CAD, particularly when other test results may be equivocal. It is also of interest because of its ability to detect and possibly quantitate overall plaque burden and certain characteristics of plaques that may make them prone to rupture, such as positive remodeling or low attenuation.
Compared with the established value of CAC scanning for risk reclassification in asymptomatic patients, there are limited data regarding the utility of CT angiography in asymptomatic people. This was recently assessed by the investigators of the CONFIRM registry,59 from which >7500 asymptomatic subjects with CAC and CT angiography were followed up for death and nonfatal MI for a median of 2 years. Overall, 2.2% either died or experienced nonfatal MI, and in multivariable models, compared with those without atherosclerosis, there was increasing risk across groups with increasing degrees of atherosclerosis measured by CT angiography. However, after the inclusion of CAC in the multivariable risk model, CT angiography did not provide incremental prognostic value over this short period of follow-up.59 In another study from the CONFIRM registry, it was noted that coronary CT angiography provided incremental prognostic utility for prediction of mortality and nonfatal MI for asymptomatic individuals with moderately high CAC scores but not for lower or higher CAC scores. The value of coronary CT angiography over the FRS was demonstrated in individuals with a CAC score >100 (increment in C statistic, 0.24; net reclassification index, 0.62; all P<0.001) but not among those with CAC scores ≤100 (all P>0.05).60
Because of the limited outcome data in asymptomatic people, as well as the associated expense and risk of CT angiography (including generally higher radiation levels than with CT scanning to detect CAC), current guidelines do not recommend its use as a screening tool for assessment of cardiovascular risk in asymptomatic people.2
Measures of Vascular Function and Incident CVD Events
Background
Measures of arterial tonometry (stiffness) are based on the concept that pulse pressure has been shown to be an important risk factor for CVD. Arterial tonometry offers the ability to directly and noninvasively measure central pulse wave velocity in the thoracic and abdominal aorta.
Brachial FMD is a marker for nitric oxide release from the endothelium that can be measured by ultrasound. Impaired FMD is an early marker of CVD.
Recommendations have not been specific, however, as to which, if any, measures of vascular function might be useful for CVD risk stratification in selected patient subgroups. Because of the absence of significant prospective data relating these measures to outcomes, the latest guidelines do not recommend measuring either FMD or arterial stiffness for cardiovascular risk assessment in asymptomatic adults.2
Arterial Tonometry and CVD
The Rotterdam Study measured arterial stiffness in 2835 elderly participants (mean age 71 years).61 They found that as aortic pulse wave velocity increased, the risk of CHD was 1.72 (second versus first tertile) and 2.45 (third versus first tertile). Results remained robust even after accounting for carotid IMT, ABI, and pulse pressure.
A study from Denmark of 1678 individuals aged 40 to 70 years found that each 1-SD increment in aortic pulse wave velocity (3.4 m/s) increased CVD risk by 16% to 20%.62
The FHS measured several indices of arterial stiffness, including pulse wave velocity, wave reflection, and central pulse pressure.63 They found that not only was higher pulse wave velocity associated with a 48% increased risk of incident CVD events, but pulse wave velocity additionally improved CVD risk prediction (integrated discrimination improvement of 0.7%, P<0.05).
FMD and CVD
MESA measured FMD in 3026 participants (mean age 61 years) who were free of CVD. As FMD increased (ie, improved brachial function), the risk of CVD was 16% lower.64 FMD also improved CVD risk prediction compared with the FRS by improving net reclassification by 29%.
A recent meta-analysis assessed relation of FMD with CVD events. Thirteen studies involving 11 516 individuals without established CVD, with a mean duration of 2 to 7.2 years and adjusted for age, sex, and risk factors, reported a multivariate RR of 0.93 (95% CI, 0.90–0.96) per 1% increase in brachial FMD.65
Comparison of Measures
In MESA, a comparison of 6 risk markers—CAC, ABI, high-sensitivity CRP, carotid IMT, brachial FMD, and family history of CHD—and their clinical utility over FRS was evaluated in 1330 intermediate-risk individuals. After 7.6 years of follow-up, CAC, ABI, high-sensitivity CRP, and family history were independently associated with incident CHD in multivariable analyses (HRs of 2.6, 0.79, 1.28, and 2.18, respectively), but carotid IMT and brachial FMD were not. CAC provided the highest incremental improvement over the FRS (0.784 for both CAC and FRS versus 0.623 for FRS alone), as well as the greatest net reclassification improvement (0.659).66
Similar findings were also noted in the Rotterdam Study, in which among 12 CHD risk markers, improvements in FRS predictions were most statistically and clinically significant with the addition of CAC scores.67
Utility for Risk Stratification for Treatment
CAC has been examined in multiple studies for its potential to identify those most likely and not likely to benefit from treatment.
In a study of 950 participants from MESA who met JUPITER clinical trial entry criteria (risk factors plus LDL-C <130 mg/dL and CRP ≥2 mg/L) were identified and stratified according to CAC scores of 0, 1 to 100, or >100; CHD event rates were calculated, and the number needed to treat was calculated by applying the benefit found in JUPITER to the event rates found in each of these groups. For CHD, the predicted NNT5 was 549 for those with CAC of 0, 94 for scores of 1 to 100, and 24 for scores >100.68
In a similar fashion, 2 studies extrapolated the NNT5 for LDL-C lowering by statins, applying the 30% RR reduction associated with a 1 mmol/L (39 mg/dL) reduction in LDL-C from a Cochrane meta-analysis of statin therapy in primary prevention across the spectrum of lipid abnormalities (LDL-C ≥130 mg/dL, HDL-C <40 mg/dL for males or <50 mg/dL for females, and triglycerides ≥150 mg/dL), as well as across 10-year FRS categories (0–6%, 6–10%, 10–20%, and >20%). The estimated NNT5 for preventing 1 CVD event across dyslipidemia categories in this MESA cohort ranged from 23 to 30 in those with CAC ≥100.15 The NNT5 was 30 in participants with no lipid abnormality and CAC >100, whereas the NNT5 was 154 in those with 3 lipid abnormalities and CAC=0.15 A very high NNT5 of 186 and 222, respectively, was estimated to prevent 1 CHD event in the absence of CAC among those with 10-year FRS of 11% to 20% and >20%. The respective estimated NNT5 were as low as 36 and 50 with the presence of a very high CAC score (>300) among those with 10-year FRS of 0% to 6% and 6% to 10%, respectively.33 These collective data show the utility of CAC in identifying those most likely to benefit from statin treatment across the spectrum of risk profiles with an appropriate number needed to treat.
Similarly, CAC testing also identified appropriate candidates who might derive the highest benefit with aspirin therapy. In MESA, individuals with CAC ≥100 had an estimated net benefit with aspirin regardless of their traditional risk status; the estimated NNT5 was 173 for individuals classified as having <10% FRS and 92 for individuals with ≥10% FRS, and the estimated 5-year number needed to harm was 442 for a major bleed.69 Conversely, individuals with zero CAC had unfavorable estimates (estimated NNT5 of 2036 for individuals with <10% FRS and 808 for individuals with ≥10% FRS; estimated 5-year number needed to harm of 442 for a major bleed). Sex-specific and age-stratified analyses showed similar results.
A study from MESA also examined the role of CAC testing to define the target population to treat with a polypill.33 The 5-year NNT5 to prevent 1 event was estimated by applying the expected 62% CHD event reduction associated with the use of the polypill (based on TIPS). The estimated NNT5 to prevent 1 CHD event ranged from 170 to 269 for patients with CAC=0, from 58 to 79 for those with CAC scores from 1 to 100, and from 25 to 27 for those with CAC scores >100,33 which enabled significant reductions in the population considered for treatment with more selective use of the polypill and, as a result, avoidance of treatment of those who were unlikely to benefit.
Within the scope of the new ACC/AHA guidelines, recent data from MESA demonstrated that among those for whom statins were recommended, 41% had CAC=0 and had 5.2 ASCVD events per 1000 person-years. Among 589 participants (12%) considered for moderate-intensity statin treatment, 338 (57%) had CAC=0, with an ASCVD event rate of 1.5 per 1000 person-years. Of participants eligible (recommended or considered) for statins, 44% (1316 of 2966) had CAC=0 at baseline and an observed 10-year ASCVD event rate of 4.2 per 1000 person-years. The study results highlighted that among the intermediate risk range of 5% to 20%, nearly half (48%) had CAC=0, and their 10-year ASCVD risk was below the threshold recommended for statin therapy (4.5%).70
REFERENCES
- 1.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:396] Circulation. 2014;129(suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 4.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, Thomson LE, Polk D, Min J, Budoff MJ, Berman DS. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pletcher MJ, Pignone M, Earnshaw S, McDade C, Phillips KA, Auer R, Zablotska L, Greenland P. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7:276–284. doi: 10.1161/CIR-COUTCOMES.113.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts ET, Horne A, Martin SS, Blaha MJ, Blankstein R, Budoff MJ, Sibley C, Polak JF, Frick KD, Blumenthal RS, Nasir K. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA) PLoS One. 2015;10:e0116377. doi: 10.1371/journal.pone.0116377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, Kondos G, Kronmal RA. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) [published correction appears in J Am Coll Cardiol. 2009;53:1474] J Am Coll Cardiol. 2009;53:345–352. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–1141. 1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Xanthakis V, Sung JH, Samdarshi TE, Hill AN, Musani SK, Sims M, Ghraibeh KA, Liebson PR, Taylor HA, Vasan RS, Fox ER. Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson Heart Study. Diabetes Care. 2015;38:1082–1088. doi: 10.2337/dc14-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya AM, Kandula NR, Ewing SK, Herrington D, Liu K, Blaha MJ, Srivastava S, Dave SS, Budoff MJ. Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies [published correction appears in Atherosclerosis. 2014;235:36–37] Atherosclerosis. 2014;234:102–107. doi: 10.1016/j.atherosclerosis.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamudu HM, Paul TK, Wang L, Veeranki SP, Panchal HB, Alamian A, Sarnosky K, Budoff M. The effects of multiple coronary artery disease risk factors on subclinical atherosclerosis in a rural population in the United States. Prev Med. 2016;88:140–146. doi: 10.1016/j.ypmed.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Okwuosa TM, Greenland P, Ning H, Liu K, Bild DE, Burke GL, Eng J, Lloyd-Jones DM. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol. 2011;57:1838–1845. doi: 10.1016/j.jacc.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, McClelland R, Kaufman JD, Blumenthal R, Burke GL, Carr JJ, Post WS, Register TC, Shea S, Szklo M. Ten-year trends in coronary calcification in individuals without clinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis [published correction appears in PLoS One. 2014;9:e103666] PLoS One. 2014;9:e94916. doi: 10.1371/journal.pone.0094916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Tota-Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, Blumenthal RS, Budoff MJ, Nasir K. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin Proc. 2014;89:1350–1359. doi: 10.1016/j.mayocp.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals [published correction appears in JAMA. 2004;291:563] JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 21.Lee KK, Fortmann SP, Fair JM, Iribarren C, Rubin GD, Varady A, Go AS, Quertermous T, Hlatky MA. Insulin resistance independently predicts the progression of coronary artery calcification. Am Heart J. 2009;157:939–945. doi: 10.1016/j.ahj.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–2290. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker-Preuss M, Mann K, Siegrist J, Jöckel K-H for the Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nix-dorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Elias-Smale SE, Proença RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JC. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56:1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Paixao AR, Ayers CR, El Sabbagh A, Sanghavi M, Berry JD, Rohatgi A, Kumbhani DJ, McGuire DK, Das SR, de Lemos JA, Khera A. Coronary artery calcium improves risk classification in younger populations. JACC Cardiovasc Imaging. 2015;8:1285–1293. doi: 10.1016/j.jcmg.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Hermann DM, Gronewold J, Lehmann N, Moebus S, Jöckel KH, Bauer M, Erbel R Heinz Nixdorf Recall Study Investigative Group. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44:1008–1013. doi: 10.1161/STROKEAHA.111.678078. [DOI] [PubMed] [Google Scholar]
- 27.Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ, Retnakaran R. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ. 2013;346:f1654. doi: 10.1136/bmj.f1654. [DOI] [PubMed] [Google Scholar]
- 28.Leening MJ, Elias-Smale SE, Kavousi M, Felix JF, Deckers JW, Vliegenthart R, Oudkerk M, Hofman A, Steyerberg EW, Stricker BH, Witteman JC. Coronary calcification and the risk of heart failure in the elderly: the Rotterdam Study. JACC Cardiovasc Imaging. 2012;5:874–880. doi: 10.1016/j.jcmg.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 29.O’Neal WT, Efird JT, Dawood FZ, Yeboah J, Alonso A, Heckbert SR, Soliman EZ. Coronary artery calcium and risk of atrial fibrillation (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2014;114:1707–1712. doi: 10.1016/j.amjcard.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handy CE, Desai CS, Dardari ZA, Al-Mallah MH, Miedema MD, Ouyang P, Budoff MJ, Blumenthal RS, Nasir K, Blaha MJ. The association of coronary artery calcium with noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:568–576. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification [published correction appears in JACC Cardiovasc Imaging. 2010;3:1089] JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittencourt MS, Blaha MJ, Blankstein R, Budoff M, Vargas JD, Blumenthal RS, Agatston AS, Nasir K. Polypill therapy, subclinical atherosclerosis, and cardiovascular events: implications for the use of preventive pharmacotherapy: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:434–443. doi: 10.1016/j.jacc.2013.08.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, Guerci A, Jacobs DR, Jr, Kronmal R, Liu K, Saad M, Selvin E, Tracy R, Detrano R. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5:358–366. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neal WT, Efird JT, Qureshi WT, Yeboah J, Alonso A, Heckbert SR, Nazarian S, Soliman EZ. Coronary artery calcium progression and atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e003786. doi: 10.1161/CIRCIMAGING.115.003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed HM, Blaha MJ, Nasir K, Jones SR, Rivera JJ, Agatston A, Blankstein R, Wong ND, Lakoski S, Budoff MJ, Burke GL, Sibley CT, Ouyang P, Blumenthal RS. Low-risk lifestyle, coronary calcium, cardiovascular events, and mortality: results from MESA. Am J Epidemiol. 2013;178:12–21. doi: 10.1093/aje/kws453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SC, Jr, Greenland P, Grundy SM. Prevention conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: executive summary. Circulation. 2000;101:111–116. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 41.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS Bogalusa Heart Study. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (the Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 42.Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJ, Asselbergs FW, Anderson TJ, Britton AR, Dekker JM, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kitagawa K, Kitamura A, de Kleijn DP, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Pasterkamp G, Peters SA, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Bots ML, den Ruijter HM. Race/ethnic differences in the associations of the Framingham risk factors with carotid IMT and cardiovascular events. PLoS One. 2015;10:e0132321. doi: 10.1371/journal.pone.0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study [published correction appears in JAMA. 2003;290:2943] JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 44.Manolio TA, Arnold AM, Post W, Bertoni AG, Schreiner PJ, Sacco RL, Saad MF, Detrano RL, Szklo M. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;197:132–138. doi: 10.1016/j.atherosclerosis.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 46.Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in Multi-Ethnic Study of Atherosclerosis. Stroke. 2011;42:3017–3021. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eleid MF, Lester SJ, Wiedenbeck TL, Patel SD, Appleton CP, Nelson MR, Humphries J, Hurst RT. Carotid ultrasound identifies high risk subclinical atherosclerosis in adults with low Framingham risk scores. J Am Soc Echocardiogr. 2010;23:802–808. doi: 10.1016/j.echo.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS) Eur Heart J. 2010;31:2041–2048. doi: 10.1093/eurheartj/ehq189. [DOI] [PubMed] [Google Scholar]
- 50.Elias-Smale SE, Kavousi M, Verwoert GC, Koller MT, Steyerberg EW, Mattace-Raso FU, Hofman A, Hoeks AP, Reneman RS, Witteman JC. Common carotid intima-media thickness in cardiovascular risk stratification of older people: the Rotterdam Study. Eur J Prev Cardiol. 2012;19:698–705. doi: 10.1177/1741826711414623. [DOI] [PubMed] [Google Scholar]
- 51.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis [published correction appears in JAMA. 2013;310:1739] JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 52.Bots ML, Groenewegen KA, Anderson TJ, Britton AR, Dekker JM, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Ikram MA, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Franco OH, Peters SA, den Ruijter HM. Common carotid intima-media thickness measurements do not improve cardiovascular risk prediction in individuals with elevated blood pressure: the USE-IMT collaboration. Hypertension. 2014;63:1173–1181. doi: 10.1161/HYPER-TENSIONAHA.113.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P, Garcia MJ, Gregson J, Pocock S, Falk E, Fuster V. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65:1065–1074. doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Pang Y, Sang Y, Ballew SH, Ballew SH, Grams ME, Heiss G, Coresh J, Matsushita K. Carotid intima-media thickness and incident ESRD: the Atherosclerosis Risk in Communities (ARIC) Study. Clin J Am Soc Nephrol. 2016;11:1197–1205. doi: 10.2215/CJN.11951115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero JR, Preis SR, Beiser A, DeCarli C, D’Agostino RB, Wolf PA, Vasan RS, Polak JF, Seshadri S. Carotid atherosclerosis and cerebral microbleeds: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002377. doi: 10.1161/JAHA.115.002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, Lloyd-Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the Coronary Artery Risk Development in Young Adults study and Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorenz MW, Price JF, Robertson C, Bots ML, Polak JF, Poppert H, Kavousi M, Dörr M, Stensland E, Ducimetiere P, Ronkainen K, Kiechl S, Sitzer M, Rundek T, Lind L, Liu J, Bergström G, Grigore L, Bokemark L, Friera A, Yanez D, Bickel H, Ikram MA, Völzke H, Johnsen SH, Empana JP, Tuomainen TP, Willeit P, Steinmetz H, Desvarieux M, Xie W, Schmidt C, Norata GD, Suarez C, Sander D, Hofman A, Schminke U, Mathiesen E, Plichart M, Kauhanen J, Willeit J, Sacco RL, McLachlan S, Zhao D, Fagerberg B, Catapano AL, Gabriel R, Franco OH, Bülbül A, Scheckenbach F, Pflug A, Gao L, Thompson SG. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: results from the PROG-IMT collaboration. Diabetes Care. 2015;38:1921–1929. doi: 10.2337/dc14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Callister TQ, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Maffei E, Cademartiri F, Kaufmann P, Shaw LJ, Raff GL, Chinnaiyan KM, Villines TC, Cheng V, Nasir K, Gomez M, Min JK CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multi-center Registry) Circulation. 2012;126:304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 60.Cho I, Chang HJ, Ó Hartaigh B, Shin S, Sung JM, Lin FY, Achenbach S, Heo R, Berman DS, Budoff MJ, Callister TQ, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Chow BJ, Dunning AM, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Leipsic J, Shaw LJ, Kaufmann PA, Cury RC, Feuchtner G, Kim YJ, Maffei E, Raff G, Pontone G, Andreini D, Min JK. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the COronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Eur Heart J. 2015;36:501–508. doi: 10.1093/eurheartj/ehu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 62.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULA-TIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 66.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, Krestin GP, Oudkerk M, de Maat MP, Leebeek FW, Mattace-Raso FU, Lindemans J, Hofman A, Steyerberg EW, van der Lugt A, van den Meiracker AH, Witteman JC. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156:438–444. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 68.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O’Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPI-TER population from MESA, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, Miedema MD, Sibley CT, Shaw LJ, Blumenthal RS, Budoff MJ, Krumholz HM. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) [published correction appears in J Am Coll Cardiol. 2015;66:2686] J Am Coll Cardiol. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 71.MESA CAC score reference values. [Accessed July 16, 2014];MESA CAC Tools Web site. http://www.mesa-nhlbi.org/Calcium/input.aspx.
20. CORONARY HEART DISEASE, ACUTE CORONARY SYNDROME, AND ANGINA PECTORIS
See Tables 20-1 and 20-2 and Charts 20-1 through 20-11
Table 20-1.
Coronary Heart Disease
| Population Group | Prevalence, CHD, 2011–2014 Age ≥20 y | Prevalence, MI, 2011–2014 Age ≥20 y | New and Recurrent MI and Fatal CHD, Age ≥35 y | New and Recurrent MI, Age ≥35 y | Mortality,* CHD, 2014 All Ages | Mortality,* MI, 2014 All Ages | Hospital Discharges CHD, 2010 All Ages |
|---|---|---|---|---|---|---|---|
| Both sexes | 16 500 000 (6.3%) | 7 900 000 (3.0%) | 1 020 000 | 790 000 | 364 593 | 114 019 | 1 346 000 |
| Males | 9 100 000 (7.4%) | 4 700 000 (3.8%) | 590 000 | 465 000 | 207 412 (56.9%)† | 65 081 (57.1%)† | 828 000 |
| Females | 7 400 000 (5.3%) | 3 200 000 (2.3%) | 430 000 | 325 000 | 157 181 (43.1%)† | 48 938 (42.9%)† | 518 000 |
| NH white males | 7.7% | 4.0% | 505 000‡ | … | 166 752 | 52 767 | … |
| NH white females | 5.3% | 2.4% | 360 000‡ | … | 125 127 | 38 778 | … |
| NH black males | 7.1% | 3.3% | 85 000‡ | … | 20 883 | 6285 | … |
| NH black females | 5.7% | 2.2% | 70 000‡ | … | 17 960 | 5738 | … |
| Hispanic males | 5.9% | 2.9% | … | … | 12 594 | 4006 | … |
| Hispanic females | 6.1% | 2.1% | … | … | 9277 | 2957 | … |
| NH Asian males | 5.0% | 2.6% | … | … | 4862§ | 1455§ | … |
| NH Asian females | 2.6% | 0.7% | … | … | 3498§ | 1087§ | … |
| NH American Indian or Alaska Native | 6.0%||¶ | … | … | … | 2 009 | 598 | … |
CHD includes people who responded “yes” to at least 1 of the questions in “Has a doctor or other health professional ever told you that you had coronary heart disease, angina or angina pectoris, heart attack, or myocardial infarction?” Those who answered “no” but were diagnosed with Rose angina are also included (the Rose questionnaire is only administered to survey participants >40 years of age). CHD indicates coronary heart disease; ellipses (…), data not available; MI, myocardial infarction; and NH, non-Hispanic.
Mortality for Hispanic, NH American Indian or Alaska Native, and NH Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total CHD and MI mortality that is for males vs females.
Estimates include Hispanics and non-Hispanics. Estimates for whites include other nonblack races.
Includes Chinese, Filipino, Hawaiian, Japanese, and Other Asian or Pacific Islander.
National Health Interview Survey, National Center for Health Statistics 2014; data are weighted percentages for Americans ≥18 years of age.1
Estimate considered unreliable or does not meet standards of reliability or precision.
Sources: Prevalence: National Health and Nutrition Examination Survey 2011 to 2014 (National Center for Health Statistics) and National Heart, Lung, and Blood Institute. Percentages for racial/ethnic groups are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolated to the 2014 US population estimates. These data are based on self-reports. Incidence: Atherosclerosis Risk in Communities study (2005–2013), National Heart, Lung, and Blood Institute. Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death–United States. Mortality for NH Asians includes Pacific Islanders. Hospital discharges: National Hospital Discharge Survey, National Center for Health Statistics (data include those inpatients discharged alive, dead, or status unknown).
Table 20-2.
Angina Pectoris*
| Population Group | Prevalence, 2011–2014, Age ≥20 y | Incidence of Stable AP, Age ≥45 y | Hospital Discharges, 2010†, All Ages |
|---|---|---|---|
| Both sexes | 8 700 000 (3.4%) | 565 000 | 22 000 |
| Males | 4 200 000 (3.5%) | 370 000 | 12 000 |
| Females | 4 500 000 (3.3%) | 195 000 | 10 000 |
| NH white males | 3.7% | … | … |
| NH white females | 3.3% | … | … |
| NH black males | 3.5% | … | … |
| NH black females | 3.3% | … | … |
| Hispanic males | 2.7% | … | … |
| Hispanic females | 3.8% | … | … |
| NH Asian or Pacific Islander males | 2.0% | ||
| NH Asian or Pacific Islander females | 1.3% |
AP indicates angina pectoris; ellipses (…), data not available; and NH, non-Hispanic.
AP is chest pain or discomfort that results from insufficient blood flow to the heart muscle. Stable AP is predictable chest pain on exertion or under mental or emotional stress. The incidence estimate is for AP without myocardial infarction.
There were 56 000 days of care for discharges of patients with AP from short-stay hospitals in 2010.
Sources: Prevalence: National Health and Nutrition Examination Survey 2011 to 2014 (National Center for Health Statistics) and National Heart, Lung, and Blood Institute; percentages for racial/ethnic groups are age adjusted for US adults ≥20 years of age. AP includes people who either answered “yes” to the question of ever having angina or AP or who were diagnosed with Rose angina (the Rose questionnaire is only administered to survey participants >40 years of age). Estimates from National Health and Nutrition Examination Survey 2011 to 2014 (National Center for Health Statistics) were applied to 2014 population estimates (≥20 years of age). Incidence: AP uncomplicated by a myocardial infarction or with no myocardial infarction (Framingham Heart Study [the original cohort and the Offspring Cohort 1986–2009], National Heart, Lung, and Blood Institute). Hospital discharges: National Hospital Discharge Survey, National Center for Health Statistics; data include those inpatients discharged alive, dead, or status unknown.
Chart 20-1. Prevalence of coronary heart disease by age and sex (NHANES: 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 20-11. Incidence of angina pectoris (deemed uncomplicated on the basis of physician interview of patient) by age and sex (FHS 1986–2009).
FHS indicates Framingham Heart Study.
Data derived from the National Heart, Lung, and Blood Institute.
Coronary Heart Disease
ICD-9 410 to 414, 429.2; ICD-10 I20 to I25 (includes MI ICD-10 I21 to I22).
Abbreviations Used in Chapter 20
| ACC | American College of Cardiology |
| ACEI | angiotensin-converting enzyme inhibitor |
| ACS | acute coronary syndrome |
| ACTION | Acute Coronary Treatment and Intervention Outcomes Network |
| AHA | American Heart Association |
| AMI | acute myocardial infarction |
| AP | angina pectoris |
| ARB | angiotensin receptor blocker |
| ARIC | Atherosclerosis Risk in Communities study |
| ASCVD | atherosclerotic cardiovascular disease |
| BMI | body mass index |
| BP | blood pressure |
| BRFSS | Behavioral Risk Factor Surveillance System |
| CABG | coronary artery bypass graft |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| CRUSADE | Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines |
| CVD | cardiovascular disease |
| D2B | door-to-balloon |
| DBP | diastolic blood pressure |
| DES | drug-eluting stent |
| DM | diabetes mellitus |
| ECG | electrocardiogram |
| ED | emergency department |
| EMS | emergency medical services |
| FHS | Framingham Heart Study |
| GRACE | Global Registry of Acute Coronary Events |
| GWTG | Get With the Guidelines |
| HCUP | Healthcare Cost and Utilization Project |
| HDL-C | high-density lipoprotein cholesterol |
| HD | heart disease |
| HF | heart failure |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IHD | ischemic heart disease |
| JHS | Jackson Heart Study |
| LV | left ventricular |
| MEPS | Medical Expenditure Panel Survey |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| NAMCS | National Ambulatory Medical Care Survey |
| NCDR | National Cardiovascular Data Registry |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHDS | National Hospital Discharge Survey |
| NHIS | National Health Interview Study |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| NSTEMI | non–ST-segment–elevation myocardial infarction |
| OR | odds ratio |
| PCI | percutaneous coronary intervention |
| REGARDS | Reasons for Geographic and Racial Differences in Stroke |
| SBP | systolic blood pressure |
| STEMI | ST-segment–elevation myocardial infarction |
| TC | total cholesterol |
| TRIUMPH | Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status |
| UA | unstable angina |
| WISE | Women’s Ischemia Syndrome Evaluation |
| YLL | years of life lost |
Prevalence
(See Table 20-1 and Charts 20-1 and 20-2)
Chart 20-2. Prevalence of myo-cardial infarction by age and sex (NHANES: 2011–2014).
Myocardial infarction includes people who answered “yes” to the question of ever having had a heart attack or myocardial infarction.
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
-
On the basis of data from NHANES 2011 to 2014 (NHLBI tabulation), an estimated 16.5 million Americans ≥20 years of age have CHD (Chart 20-1). The prevalence of CHD was higher for males than females for all ages.
Total CHD prevalence is 6.3% in US adults ≥20 years of age. CHD prevalence is 7.4% for males and 5.3% for females.
Among non-Hispanic whites, CHD prevalence is 7.7% for males and 5.3% for females.
Among non-Hispanic blacks, CHD prevalence is 7.1% for males and 5.7% for females.
Among Hispanics, CHD prevalence is 5.9% for males and 6.1% for females.
Among non-Hispanic Asians, CHD prevalence is 5.0% for males and 2.6% for females.
On the basis of data from the 2014 NHIS1:
Among Asians ≥18 years of age, the CHD estimate is 3.3%.
Among American Indian/Alaska Natives ≥18 years of age, the estimate is 6.0%; however, this is not reliable.
-
According to data from NHANES 2011 to 2014 (NHLBI tabulation), the overall prevalence for MI is 3.0% in US adults ≥20 years of age. MI prevalence is 3.8% for males and 2.3% for females (Chart 20-2).
Among non-Hispanic whites, MI prevalence is 4.0% for males and 2.4% for females.
Among non-Hispanic blacks, MI prevalence is 3.3% for males and 2.2% for females.
Among Hispanics, MI prevalence is 2.9% for males and 2.1% for females.
Among non-Hispanic Asians, MI prevalence is 2.6% for males and 0.7% for females.
-
Data from NHANES indicate that between 2001 and 2012, the age-adjusted prevalence of CHD declined from 10.3% to 8.0%.
The prevalence of angina declined from 7.8% to 5.5%, and MI prevalence decreased from 5.5% to 4.7%.2
-
Data from the BRFSS 2014 survey indicated that 3.9% of respondents had been told that they had had an MI.
The highest prevalence was in West Virginia and Kentucky (6.0%), and the lowest was in the District of Columbia (2.7%), Maryland (2.8%), and Hawaii (2.8%).3
In the same survey, 3.9% of respondents had been told that they had angina or CHD. The highest prevalence was in West Virginia (6.5%), and the lowest was in Alaska (2.4%).
Incidence
(See Table 20-1 and Charts 20-3 through 20-5)
Chart 20-3. Annual number of adults per 1000 having diagnosed heart attack or fatal CHD by age and sex (ARIC surveillance: 2005–2013 and CHS).
These data include MI and fatal CHD but not silent MI.
ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; CHS, Cardiovascular Health Study; and MI, myocardial infarction.
Source: National Heart, Lung, and Blood Institute.
Chart 20-5. Incidence of myocardial infarction by age, sex, and race (ARIC Surveillance: 2005–2013).
ARIC indicates Atherosclerosis Risk in Communities.
Source: Unpublished data from ARIC, National Heart, Lung, and Blood Institute.
Approximately every 40 seconds, an American will have an MI (AHA computation).
-
On the basis of data from the ARIC study of the NHLBI4:
This year, ≈695 000 Americans will have a new coronary event (defined as first hospitalized MI or CHD death), and ≈325 000 will have a recurrent event. It is estimated that an additional 165 000 silent MIs occur each year. That assumes that ≈21% of the 790 000 first and recurrent MIs are silent.
The estimated annual incidence of MI is 580 000 new attacks and 210 000 recurrent attacks.
Average age at first MI is 65.3 years for males and 71.8 years for females.
-
On the basis of the NHLBI-sponsored FHS5:
CHD makes up more than half of all cardiovascular events in males and females <75 years of age.
The incidence of CHD in females lags behind males by 10 years for total CHD and by 20 years for more serious clinical events such as MI and sudden death.
In the NHLBI-sponsored ARIC study, among participants 35 to 84 years of age, the average age-adjusted first MI or fatal CHD rates per 1000 population were as follows: white males, 3.8; black males, 6.6; white females, 2.2; and black females, 4.3 (unpublished data from ARIC Surveillance 2005–2013, NHLBI).
Annual number and incidence rates for MI or fatal CHD in the NHLBI-sponsored ARIC study are displayed in Charts 20-3 and 20-4, stratified by age, race, and sex. The annual age-adjusted rates per 1000 population of first MI (2005–2013) were 5.7. in black males, 3.3 in white males, 3.9 in black females, and 2.0 in white females (unpublished data from ARIC Surveillance 2005–2013, NHLBI).
Incidence of MI by age, sex, and race in the NHLBI-sponsored ARIC study are displayed in Chart 20-5. Black males have a higher incidence of MI among all age groups.
Among 24 443 US adult participants in the REGARDS study, the incidence of CHD (nonfatal MI or fatal CHD) per 1000 person-years was 9.0 among non-Hispanic black males, 8.1 among non-Hispanic white males, 5.0 among non-Hispanic black females, and 3.4 among non-Hispanic white females. Non-Hispanic blacks had a higher risk for fatal CHD, which was explained by risk factors. No racial differences were present for nonfatal CHD.6
In the REGARDS study, 37% of adjudicated MIs had a primary hospital discharge diagnosis of MI, whereas 63% had a primary hospital discharge diagnosis other than MI, which suggests that most MIs that result in hospitalization might be occurring during hospitalization for other acute illnesses (eg, sepsis).7
In the ARIC study, silent MI (MI detected on ECG in the absence of a definite or probable hospitalized MI) accounted for 45% of incident MIs between 1987 to 1989 and 1996 to 1998. The incidence rate of silent MI was 3.84 per 1000 person-years compared with 4.68 per 1000 person-years for MI with clinical manifestations. The risk for all-cause mortality was similar after silent MI and MI with clinical manifestations (15.9 and 18.7 per 1000 person-years, respectively).8
Chart 20-4. Incidence of heart attack or fatal CHD by age, sex, and race (ARIC Surveillance: 2005–2013).
ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; and MI, myocardial infarction.
Source: National Heart, Lung, and Blood Institute.
Trends in Incidence
A number of studies have examined temporal trends in the incidence of MI. Geographic differences in patient populations, temporal changes in the criteria used to diagnosis MI, and differences in study methodology increase the complexity of interpreting these studies; however, the overall body of literature suggests that the incidence of MI has declined significantly over time, including over the past decade.9
In Olmsted County, MN, between 1995 and 2012, the population rate of MI declined 3.3% per year; however, these declines varied among types of MI, with the greatest declines occurring for prehospital fatal MI.10
Data from Kaiser Permanente Northern California showed that the age- and sex-adjusted incidence rate of hospitalizations for MI changed from 274 per 100 000 person-years in 1999 to 208 per 100 000 person-years in 2008. Furthermore, the age- and sex-adjusted incidence rate of hospitalizations for STEMI changed from 133 per 100 000 person-years in 1999 to 50 per 100 000 person-years in 2008 (P linear trend <0.001). The trajectory of the age- and sex-adjusted incidence rate of hospitalizations for NSTEMI did not change significantly over the entire study period, although it did show a significant decline after troponin became widely used to diagnose MI.11
From 1987 to 2011, the age- and biomarker-adjusted incidence rates of hospitalization for AMI or fatal CHD decreased by 5.0% per year (95% CI, −5.3% to −4.7%) among white males, 3.9% per year (95% CI, −4.4% to −3.5%) among white females, 2.2% per year (95% CI, −2.8% to −1.6%) among black males, and 3.4% per year (95% CI, −4.2% to −2.7%) among black females in the ARIC study (1987–2011).4
According to data from ARIC and the REGARDS study, between 1987 to 1996 and 2003 to 2009, the incidence of CHD declined from 3.9 to 2.2 per 1000 person-years in people without DM and 11.1 to 5.4 per 1000 person-years among those with DM.12
Among Medicare beneficiaries between 2002 and 2011, the incidence of MI hospitalization declined from 1485 to 1122 per 100 000 person-years. The incidence of MI as the primary reason for hospitalization decreased over time (from 1063 to 677 per 100 000 person-years between 2002 and 2011), whereas the percentage of MIs as a secondary reason for hospitalization increased (from 190 to 245 per 100 000 person-years). The percentage of MIs that were attributable to a secondary diagnosis increased from 28% to 40%.13
Among Medicare beneficiaries, the incidence of being hospitalized for MI (ie, the primary reason for hospitalization) between 2002 and 2011 declined by 36.6% among non-Hispanic whites (from 1057 to 670 per 100 000 person-years between 2002 and 2011) and by 26.4% among non-Hispanic blacks (from 966 to 711 per 100 000 person-years between 2002 and 2011).14
Predicted Risk
The percentage of US adults with a 10-year predicted ASCVD risk (using pooled-cohort risk equations) ≥20% decreased from 13.0% in 1999 to 2000 to 9.4% in 2011 to 2012. The proportion of US adults with 10-year predicted ASCVD risk of 7.5% to <20% was 23.9% in 1999 to 2000 and 26.8% in 2011 to 2012.15
For adults with “optimal” risk factors (TC of 170 mg/dL, HDL-C of 50 mg/dL, SBP of 110 mm Hg without antihypertensive medication use, no DM, and not a smoker), 10-year CVD risk ≥7.5% will occur at age 65 years for white males, 70 years for black males and females, and 75 years for white females.16
Individuals with atherosclerotic stroke should be included among those deemed to be at high risk (20% over 10 years) of further atherosclerotic coronary events. For primary prevention, ischemic stroke should be included among CVD outcomes in absolute risk assessment algorithms. The inclusion of atherosclerotic ischemic stroke as a high-risk condition has important implications because the number of people considered to be at high risk will increase over time.17
A survey of US family physicians, general internists, and cardiologists published in 2012 found that 41% of respondents reported using global CHD risk assessment at least occasionally.18 It is unclear whether physicians are using global CHD risk prediction more since the publication of the 2013 ACC/AHA cholesterol management guideline.18a
Lifetime risk for CHD varies drastically as a function of risk factor profile. With an optimal risk factor profile, lifetime risk for CHD is 3.6% for males and <1% for females; with ≥2 major risk factors, it is 37.5% for males and 18.3% for females.19
Mortality
-
Based on 2014 mortality data20:
CHD was an underlying cause of death in ≈1 of every 7 deaths in the United States in 2014.
CHD mortality was 364 593, and CHD any-mention mortality was 530 989.
MI mortality was 114 019. MI any-mention mortality was 150 590 (NCHS, NHLBI tabulation).
The overall age-adjusted CHD death rate per 100 000 was 98.8.
From 2004 to 2014, the annual death rate attributable to CHD declined 35.5% and the actual number of deaths declined 19.2% (NHLBI computation).
CHD death rates per 100 000 were 137.5 for white males, 150.6 for black males, and 98.4 for Hispanic males; for white females, the rate was 72.1; for black females, it was 89.4; and for Hispanic females, it was 57.2.
76% of CHD deaths occurred out of the hospital. According to NCHS mortality data, 277 995 CHD deaths occur out of the hospital or in hospital EDs annually (NCHS, AHA tabulation).
The estimated average number of YLL because of an MI death is 16.7 (NHLBI tabulation).
Approximately 36% of the people who experience a coronary event in a given year will die of it, and ≈14% who experience a heart attack (MI) will die of it (AHA computation).
Researchers investigating variation in hospital-specific 30-day risk-stratified mortality rates for patients with AMI found teaching status, number of hospital beds, AMI volume, cardiac facilities available, urban/rural location, geographic region, hospital ownership type, and socioeconomic status profile of the patients were all significantly associated with mortality rates. However, a substantial proportion of variation in outcomes for patients with AMI between hospitals remains unexplained by measures of hospital characteristics.21
Among 194 071 adults <65 years of age who were hospitalized for an AMI in the 2009 to 2010 NIS, in-hospital mortality was higher for Hispanic females (3.7%) than for black females (3.1%) and white females (2.5%). Differences were smaller for males <65 years of age. Among older adults (≥65 years), in-hospital mortality was 8.0% for white females and between 6% and 8% for other race-sex groups.22
Temporal Trends in Mortality
-
The decline in CHD mortality rates in part reflects the shift in the pattern of clinical presentations of AMI. In the past decade, there has been a marked decline in STEMI (from 133 to 50 cases per 100 000 person-years).11
In Olmsted County, MN, the age- and sex-adjusted 30-day case fatality rate decreased by 56% from 1987 to 2006.23
Among enrollees of the Kaiser Permanente Northern California healthcare delivery system, the age- and sex-adjusted 30-day mortality rate for MI dropped from 10.5% in 1999 to 7.8% in 2008, and the 30-day mortality rate for NSTEMI dropped from 10.0% in 1999 to 7.6% in 2008.11
Among Medicare fee-for-service beneficiaries, between 1999 and 2011, the 30-day mortality rate after hospitalized MI declined by 29.4%.24 Declines in 30-day mortality after MI occurred in all US census divisions between 2000 and 2008.25
Between 1999 and 2010, the risk for stroke in the year after hospital discharge for MI among Medicare beneficiaries decreased from 3.4% to 2.6%.26
In a community-based study of Worcester, MA, the percentage of patients dying after cardiogenic shock with MI declined from 47.1% in 2001 to 2003 to 28.6% in 2009 to 2011.27
Between 2001 and 2011 in the NIS, in-hospital mortality did not change for patients with STEMI with a PCI (3.52% and 3.40% in 2011 and 2001, respectively) or CABG (5.70% and 5.79% in 2011 and 2001, respectively) and increased for patients with no intervention (14.91% and 12.43% in 2011 and 2001, respectively). In-hospital mortality declined for patients with NSTEMI undergoing CABG (2.91% versus 4.97%) or no procedure (6.26% versus 8.87%) but did not change for patients with NSTEMI undergoing PCI (1.45% versus 1.73%).28
Among US males <55 years of age, CHD mortality declined an annual 5.5% per year between 1979 and 1989; a smaller decline was present in 1990–1999 (1.2% per year) and in 2000–2011 (1.8% per year). Among US females <55 years of age, CHD mortality declined an annual 4.6% per year in 1979–1989, with no decline between 1990 and 1999 and a decline of 1.0% in 2000–2011.29
Risk Factors
Risk factors for CHD act synergistically to increase CHD risk, as shown in the examples in Charts 20-6 and 20-7.
Chart 20-6. Estimated 10-year coronary heart disease risk in adults 55 years of age according to levels of various risk factors (FHS).
FHS indicates Framingham Heart Study; and HDL-C, high-density lipoprotein cholesterol.
Data derived from Wilson et al.70
Chart 20-7. Prevalence of low coronary heart disease risk, overall and by sex (NHANES: 1971–2006).
Low risk is defined as systolic blood pressure <120 mm Hg and diastolic blood pressure <80 mm Hg; cholesterol <200 mg/dL; body mass index <25 kg/m2; currently not smoking cigarettes; and no prior myocardial infarction or diabetes mellitus.
NHANES indicates National Health and Nutrition Examination Survey.
Source: Personal communication with the National Heart, Lung, and Blood Institute, June 28, 2007.
Awareness of Warning Signs and Risk Factors for HD
-
Women’s awareness that CVD is their leading cause of death increased from 30% in 1997 to 56% in 2012.30
Depending on age, 44% to 50% identified HD/heart attack as the leading cause of death for females, a significant increase from 16% to 34% in the original 1997 survey.
The percentages of females identifying warning signs for a heart attack were as follows: pain in the chest, neck, shoulder, and arm—56%; shortness of breath—38%; chest tightness—17%; nausea—18%; and fatigue—10%.
The 5 most commonly cited HD prevention strategies in 2012 were maintaining a healthy BP (78%), seeing the doctor (78%), and increasing fiber intake, eating food with anti-oxidants, and maintaining healthy cholesterol levels (each 66%).
Among online survey participants, 21% responded that their doctor had talked to them about HD risk. Rates were lower among His-panic females (12%) than whites (22%) or blacks (22%) and increased with age from 6% (25–34 years) to 33% (≥65 years).
Only a small percentage of US females and males have low CHD risk defined by SBP <120 mm Hg and DBP <80 mm Hg; cholesterol <200 mg/dL; BMI <25 kg/m2; currently not smoking cigarettes; and no prior MI or DM (Chart 20-7).
Among 2379 females and 1152 males <55 years of age hospitalized for MI, only 45.1% of females and 49.2% of males reported being told they were at risk for HD or a heart problem. Also, 45.9% of females and 54.7% of males reported their health-care provider discussing HD and things they could do to take care of their heart.31
Among US adults with CHD, between 1999 to 2000 and 2011 to 2012, the use of statins increased from 36% to 73%, aspirin use increased from 4% to 28%, ACEI or ARB use increased from 33% to 46%, and β-blocker use increased from 27% to 57%.32
Time of Symptom Onset and Arrival at Hospital
A meta-analysis of 48 studies enrolling >1.8 million patients showed that off-hours presentation for MI was associated with higher short-term mortality. In addition, those patients with STEMI who presented off hours had longer D2B times.33
Data from CRUSADE and the NCDR ACTION Registry–GWTG showed a longer median time to hospital presentation in males (3 hours) than in females (2.8 hours; P<0.001). From 2002 to 2007, presentation time did not change significantly in males or females.34
Individuals with documented CHD have 5 to 7 times the risk of having a heart attack or dying as the general population. Survival rates improve after a heart attack if treatment begins within 1 hour; however, most patients are admitted to the hospital 2.5 to 3 hours after symptoms begin. More than 3500 patients with a history of CHD were asked to identify possible symptoms of heart attack. Despite their history of CHD, 44% had low knowledge levels. Among these high-risk participants, 43% underestimated their risk for a future AMI (males 47%, females 36%).35
Data from Worcester, MA, indicate that the median time from symptom onset to hospital arrival did not improve from 2001 through 2011. In 2009 to 2011, 48.9% of patients reached the hospital within 2 hours of symptom onset compared with 45.8% in 2001 to 2003.36
An analysis of data from the NCDR ACTION Registry–GWTG showed that 60% of 37 634 STEMI patients used EMS to get to the hospital. Older adults, females, adults with comorbidities, and sicker patients were more likely to use EMS than their counterparts. Hospital arrival time was shorter for those who used EMS (89 minutes) than those who used self-transport (120 minutes).37
Among patients hospitalized for ACS between 2001 and 2011 in the NIS, those with STEMI admitted on the weekend versus on a weekday had a 3% higher odds of in-hospital mortality. Those admitted on the weekend versus weekday for non–ST-elevation ACS had a 15% higher odds of in-hospital mortality. The excess mortality associated with weekend versus weekday admission decreased over time.38
Complications
Depending on their sex and clinical outcome, people who survive the acute stage of an MI have a chance of illness and death 1.5 to 15 times higher than that of the general population. Among these people, the risk of another MI, sudden death, AP, HF, and stroke for both males and females is substantial (FHS, NHLBI).5
-
On the basis of pooled data from the FHS, ARIC, CHS, MESA, CARDIA, and JHS studies of the NHLBI (1995–2012), within 1 year after a first MI:
At ≥ 45 years of age, 18% of males and 23% of females will die.
At 45 to 64 years of age, 3% of white males, 5% of white females, 9% of black males, and 10% of black females will die.
At 65 to 74 years of age, 14% of white males, 18% of white females, 22% of black males, and 21% of black females will die.
At ≥75 years of age, 27% of white males, 29% of white females, 19% of black males, and 31% of black females will die.
In part because females have MIs at older ages than males, they are more likely to die of MI within a few weeks.
-
Within 5 years after a first MI:
At ≥45 years of age, 36% of males and 47% of females will die.
At 45 to 64 years of age, 11% of white males, 17% of white females, 16% of black males, and 28% of black females will die.
At 65 to 74 years of age, 25% of white males, 30% of white females, 33% of black males, and 44% of black females will die.
At ≥75 years of age, 55% of white males, 60% of white females, 61% of non-Hispanic black males, and 64% of black females will die.
-
Of those who have a first MI, the percentage with a recurrent MI or fatal CHD within 5 years is as follows:
At ≥45 years of age, 17% of males and 21% of females
At 45 to 64 years of age, 11% of white males, 15% of white females, 22% of black males, and 32% of black females
At 65 to 74 years of age, 12% of white males, 17% of white females, 30% of black males, and 30% of black females
At ≥75 years of age, 21% of white males, 20% of white females, 45% of black males, and 20% of black females
-
The percentage of people with a first MI who will have HF in 5 years is as follows:
At ≥45 years of age, 16% of males and 22% of females
At 45 to 64 years of age, 6% of white males, 10% of white females, 13% of black males, and 25% of black females
At 65 to 74 years of age, 12% of white males, 16% of white females, 20% of black males, and 32% of black females
At ≥75 years of age, 25% of white males, 27% of white females, 23% of black males, and 19% of non-Hispanic black females
-
The percentage of people with a first MI who will have an incident stroke within 5 years is as follows:
At ≥45 years of age, 4% of males and 7% of females
At ≥45 years of age, 5% of white males, 6% of white females, 4% of black males, and 10% of black females
-
The median survival time (in years) after a first MI is as follows:
At ≥45 years of age, 8.2 for males and 5.5 for females
At ≥45 years of age, 8.4 for white males, 5.6 for white females, 7.0 for black males, and 5.5 for black females
In the NCDR ACTION Registry–GWTG, cardiac rehabilitation referral after patients were admitted with a primary diagnosis of STEMI or NSTEMI increased from 72.9% to 80.7% between 2007 and 2012.39
An analysis of Medicare claims data revealed that only 13.9% of Medicare beneficiaries enroll in cardiac rehabilitation after an AMI, and only 31% enroll after CABG. Older people, females, nonwhites, and individuals with comorbidities were less likely to enroll in cardiac rehabilitation programs.40
In a community-based analysis of residents in Olmstead County, MN, discharged with first MI between 1987 and 2010, 52.5% participated in cardiac rehabilitation. The overall rate of participation did not change during the study period. Cardiac rehabilitation was associated with reductions in all-cause mortality and readmission.41
In 2007 to 2009, the age-adjusted mortality rates after hospital discharge for MI among Medicare beneficiaries was 224.8 per 1000 person-years and was higher among blacks versus whites.42
Between 2001 to 2003 and 2007 to 2009, the age-adjusted mortality rates after hospital discharge for MI among Medicare beneficiaries declined 7.4% per 3-year period among white males, but no change was observed for white females (−1.5%), black males (−3.0%), or black females (0.4%).42
In 2007 to 2009, the age-adjusted rate for recurrent CHD after hospital discharge for MI among Medicare beneficiaries was 124.9 per 1000 person-years and was higher among white males, black males, and black females than white females.42
Between 2001 to 2003 and 2007 to 2009, the age-adjusted rates of recurrent CHD declined among white males, white females, and black males but not black females.42
In a study of 3 central Massachusetts hospitals, the 90-day rehospitalization rate declined from 31.5% in 2001 to 2003 to 27.3% in 2009 to 2011. Crude 30-day rehospitalization rates decreased from 20.5% in 2001 to 2003 to 15.8% in 2009 to 2011.43,44
In a sample of >4000 US hospitals, core process measures for MI improved between 2006 and 2011. In 2011, >93% of hospitals provided each criterion for core process measures to >90% of their patients with MI. Core processes included aspirin at arrival, aspirin at discharge, ACEI or ARB for LV systolic dysfunction, smoking cessation advice/counseling, β-blocker at discharge, and PCI within 90 minutes of arrival.45
-
Adjusted 30-day mortality was similar for patients presenting to the hospital for MI during national cardiology meetings and not during meetings (40.4% versus 38.2% for high-risk patients and 7.7% versus 8.8% for low-risk patients, respectively). Mortality was lower among high-risk HF and cardiac arrest patients admitted during national cardiology meetings.46
Among 4316 patients from 24 hospitals in TRIUMPH, after adjustment for patient risk factors, 1-year mortality after MI varied across hospital from 4.9% to 8.6%.47
Hospital Discharges and Ambulatory Care Visits
(See Table 20-1 and Chart 20-8)
Chart 20-8. Hospital discharges for coronary heart disease by sex (United States: 1970–2010).
Hospital discharges include people discharged alive, dead, and “status unknown.”
Source: National Hospital Discharge Survey/National Center for Health Statistics and National Heart, Lung, and Blood Institute.
From 2000 to 2010, the number of inpatient discharges from short-stay hospitals with CHD as the first-listed diagnosis decreased from 2 165 000 to 1 346 000 (NCHS, NHLBI tabulation).1
From 1970 through 2010, the number of hospital discharges for CHD was higher for males than females (Chart 20-8).
In 2012, there were 8 953 000 physician office visits for CHD (NAMCS, NHLBI tabulation). In 2012, there were 480 000 ED visits, and in 2011, there were 691 000 outpatient department visits with a primary diagnosis of CHD (NHAMCS, NHLBI tabulation).
Total office visits for angina declined from 3.6 million per year in 1995 to 1998 to 2.3 million per year in 2007 to 2010 based on data from the NAMCS and NHAMCS.48
In a systematic review, secondary adherence (proportion of days covered ≥80%) in the year after ACS ranged from 54% to 62% for ACEI/ARBs, 67% to 69% for antiplatelet agent use, 64% to 65% for β-blockers, and 57% to 65% for statins.49
Among patients hospitalized for ACS between 2003 and 2008 in the GWTG-CAD registry, quality-of-care measures were lowest for ACEI/ARB use (88.2% and 72.8% for those undergoing PCI and CABG, respectively) and highest for aspirin at discharge (98.5% and 96.8% for PCI and CABG, respectively). The use of all 6 quality-of-care metrics improved between 2003 and 2008.50
Operations and Procedures
In 2010, an estimated 954 000 inpatient PCI procedures, 397 000 inpatient bypass procedures, 1 029 000 inpatient diagnostic cardiac catheterizations, 97 000 inpatient implantable defibrillator procedures, and 370 000 pacemaker procedures were performed for inpatients in the United States (NHLBI tabulation).
An analysis of data from HCUP showed that between 2001 and 2008, there had been a 15% decrease in the annual rate of coronary revascularization, primarily attributable to declines in CABG (1742 procedures per million in 2001–2002 versus 1081 procedures per million in 2007–2008). Rates of PCI did not change significantly over the same period.51
According to the US NIS, the number of PCI procedures declined by 38% between 2006 and 2011. Among patients with stable IHD, a 61% decline in PCI occurred over this time period.52
In Washington State, the overall number of PCIs decreased by 6.8% between 2010 and 2013, with a 43% decline in the number of PCIs performed for elective indications.53
However, in Massachusetts, age- and sex-adjusted rates of coronary revascularization (PCI or CABG) declined from 423 to 258 per 100 000 residents (39% decline) between 2003 and 2012. Rates of elective PCI declined by 50% over the period, whereas rates of PCI in the setting of MI declined by 16%.54,55
Among Medicare fee-for-service beneficiaries, the total number of revascularization procedures performed peaked in 2010 and declined by >4% per year through 2012. In-hospital and 90-day mortality rates declined after CABG surgery overall, as well as among patients presenting for elective CABG or CABG after NSTEMI.54
Among patients presenting for PCI after STEMI in the NCDR, D2B time decreased from a median of 86 to 63 minutes between 2005 and 2011. Between 2005 and 2011, in-hospital mortality declined from 5.3% to 4.7%, and 30-day mortality declined from 14.4% to 12.9%.56
Each 10-minute shorter D2B time was associated with an OR for in-hospital mortality of 0.92 (95% CI, 0.91–0.93) and an OR for 6-month mortality of 0.94 (95% CI, 0.93–0.95).56
Cost
(See Table 20-1)
The estimated direct and indirect cost of HD in 2012 to 2013 (average annual) was $199.6 billion (MEPS, NHLBI tabulation).
MI ($11.5 billion) and CHD ($10.4 billion) were 2 of the 10 most expensive hospital principal discharge diagnoses in 2011.57
Between 2013 and 2030, medical costs of CHD are projected to increase by ≈100%.58
A meta-analysis of 15 randomized trials estimated costs for patients with stable CAD as lowest for medical therapy ($3069 and $13 864 at 1 and 3 years, respectively) and highest for CABG ($27 003 and $28 670 at 1 and 3 years, respectively). PCI costs were between medical therapy and CABG costs and were higher with DES than with bare-metal stents and balloon angioplasty.59
Acute Coronary Syndrome
ICD-9 410, 411; ICD-10 I20.0, I21, I22.
The term ACS includes the diagnoses of AMI (STEMI or NSTEMI) and UA. UA is chest pain or discomfort that is accelerating in frequency or severity and may occur while at rest but does not result in myocardial necrosis. The discomfort may be more severe and prolonged than typical stable AP, or it may be the first time a person has had AP. UA, NSTEMI, and STEMI share common pathophysiological origins related to coronary plaque progression, instability, or rupture with or without luminal thrombosis and vasospasm.
One estimate for the number of discharges with ACS from hospitals in 2010 is 625 000. Of these, an estimated 363 000 are males and 262 000 are females. This estimate is derived by adding the first-listed inpatient hospital discharges for MI (595 000) to those for UA (30 000; NHDS, NHLBI).
When secondary discharge diagnoses in 2010 were included, the corresponding number of inpatient hospital discharges was 1 141 000 unique hospitalizations for ACS; 653 000 were males and 488 000 were females. Of the total, 813 000 were for MI alone, 322 000 were for UA alone, and 6000 hospitalizations received both diagnoses (NHDS, NHLBI).
Among commercially insured adults 18 to 64 years of age, the 1-year medical costs for an ACS event during 2004 to 2005 were $34 087 for those who were treated with medical management, $52 673 for those who were treated with percutaneous intervention, and $86 914 for those who had CABG. The 1-year short-term disability costs were $6048, $9221, and $17 335, respectively, and the 1-year absenteeism costs were $9826, $9460, and $14 960, respectively.60 Another study of the same database using adults 18 to 64 years of age who had a principal inpatient diagnosis of ACS during 2003 to 2006 estimated that the incremental annual direct cost was $40 671 and the incremental short-term disability cost was $999.61
In addition, the percentage of ACS or MI cases with ST-segment elevation appears to be declining. In an analysis of 46 086 hospitalizations for ACS in the Kaiser Permanente Northern California study, the percentage of MI cases with ST-segment elevation decreased from 47.0% to 22.9% between 1999 and 2008.11
Analysis of data from the GRACE multinational observational cohort study of patients with ACS found evidence of a change in practice for both pharmacological and interventional treatments in patients with either STEMI or non–ST-segment–elevation ACS. These changes have been accompanied by nonsignificant decreases in the rates of in-hospital death, cardiogenic shock, and new MI among patients with non–ST-segment–elevation ACS. The use of evidence-based therapies and PCI interventions increased in the STEMI population. This increase was matched by a statistically significant decrease in the rates of death, cardiogenic shock, and HF or pulmonary edema.62
A study of hospital process performance in 350 centers of nearly 65 000 patients enrolled in the CRUSADE National Quality Improvement Initiative found that ACC/AHA guideline–recommended treatments were adhered to in 74% of eligible instances.63 A better composite guideline adherence rate was significantly associated with decreased in-hospital mortality among all patients with ACS and those with NSTEMI.
After adjustment for clinical differences and the severity of CAD by angiogram, 30-day mortality after ACS is similar in males and females.64
According to data from the NIS, between 2001 and 2011, the use of PCI for patients with ACS declined by 15%.52
On the basis of administrative claims data for US Medicare beneficiaries, hospitalization for ACS (MI or UA) decreased from ≈2.5% in 1992 to 1.7% in 2009. UA decreased from 1.5% in 1997 to 0.6% in 2009.65
Stable AP
ICD-9 413; ICD-10 I20.1 to I20.9.
Prevalence
(See Table 20-2 and Charts 20-9 to 20-10)
Chart 20-9. Prevalence of angina pectoris by age and sex (NHANES: 2011–2014).
Angina pectoris includes people who either answered “yes” to the question of ever having angina or angina pectoris or were diagnosed with Rose angina.
NHANES indicates National Health and Nutrition Examination Survey.
Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 20-10. Secular trends in age- and sex-standardized prevalence rates of angina for adults aged =40 years in the United States, by race, for angina symptoms defined using the Rose questionnaire.
Reprinted from Will et al.67 Copyright © 2014, American Heart Association, Inc.
A study of 4 national cross-sectional health examination studies found that among Americans 40 to 74 years of age, the age-adjusted prevalence of AP was higher among females than males. Increases in the prevalence of AP occurred for Mexican American males and females and black females but were not statistically significant for the latter.66
The prevalence of AP increased with age from <1% among males and females 20 to 39 years of age to >10% among males and females ≥80 years of age (Chart 20-9).
On the basis of data from NHANES from 1998 to 2004 and the six 2-year surveys from 2001 to 2012, in 2009 to 2012, there were an average of 3.4 million people ≥40 years of age in the United States with angina each year compared with 4 million in 1988 to 1994. Declines in angina symptoms have occurred for non-Hispanic whites but not for non-Hispanic blacks.67
From 1988 to 1994 through 2009 to 2012, the prevalence of AP symptoms has declined among whites but not blacks (Chart 20-10).
Incidence
(See Table 20-2 and Chart 20-11)
Only 18% of coronary attacks are preceded by long-standing AP (NHLBI computation of FHS follow-up since 1986).
The annual rates per 1000 population of new episodes of AP for nonblack men are 28.3 for those 65 to 74 years of age, 36.3 for those 75 to 84 years of age, and 33.0 for those ≥85 years of age. For nonblack females in the same age groups, the rates are 14.1, 20.0, and 22.9, respectively. For black males, the rates are 22.4, 33.8, and 39.5, and for black females, the rates are 15.3, 23.6, and 35.9, respectively (CHS, NHLBI).68
In the FHS, the incidence of AP increased with age from 45 to 54 years of age to 75 to 84 years of age and was lower among participants ≥85 years of age versus 75 to 84 years of age (Chart 20-11).
Cost
For females with nonobstructive CHD enrolled in the WISE study of the NHLBI, the average lifetime cost estimate was ≈$770 000 and ranged from $1.0 to $1.1 million for females with 1- to 3-vessel CHD.69
REFERENCES
- 1.National Center for Health Statistics. [Accessed August 1, 2016];National Health Interview Survey. 2014 Public-use data file and documentation: NCHS tabulations. http://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm.
- 2.Yoon SS, Dillon CF, Illoh K, Carroll M. Trends in the prevalence of coronary heart disease in the U.S.: National Health and Nutrition Examination Survey, 2001–2012. Am J Prev Med. 2016;51:437–445. doi: 10.1016/j.amepre.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behavioral Risk Factor Surveillance System: prevalence and trends data. [Accessed August 1, 2016];Centers for Disease Control and Prevention Web site. http://www.cdc.gov/brfss/brfssprevalence/index.html.
- 4.Atherosclerosis Risk in Communities (ARIC) Study Website. [Accessed August 30, 2012];Community Surveillance Event Rates. http://www.cscc.unc.edu/aric/displaydata.php?pg_id=37.
- 5.Thom T, Kannel WB, Silbershatz H, D’Agostino RB., Sr . Cardiovascular diseases in the United States and prevention approaches. In: Fuster V, Alexander R, Schlant R, O’Rourke R, Roberts R, Sonnenblick E, editors. Hurst’s the Heart. 10. New York, NY: McGraw-Hill; 2001. pp. 3–7. [Google Scholar]
- 6.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G REGARDS Investigators. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levitan EB, Olubowale OT, Gamboa CM, Rhodes JD, Brown TM, Muntner P, Deng L, Safford MM. Characteristics and prognosis of acute myocardial infarction by discharge diagnosis: the Reasons for Geographic and Racial Differences in Stroke study. Ann Epidemiol. 2015;25:499–504.e1. doi: 10.1016/j.annepidem.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZM, Rautaharju PM, Prineas RJ, Rodriguez CJ, Loehr L, Rosamond WD, Kitzman D, Couper D, Soliman EZ. Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133:2141–2148. doi: 10.1161/CIRCULATIONAHA.115.021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Roger VL, Dunlay SM, Go AS, Rosamond WD. Challenges of ascertaining national trends in the incidence of coronary heart disease in the United States. J Am Heart Assoc. 2014;3:e001097. doi: 10.1161/JAHA.114.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber Y, Weston SA, Jiang R, Roger VL. The changing epidemiology of myocardial infarction in Olmsted County, Minnesota, 1995–2012. Am J Med. 2015;128:144–151. doi: 10.1016/j.amjmed.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 12.Carson AP, Tanner RM, Yun H, Glasser SP, Woolley JM, Thacker EL, Levitan EB, Farkouh ME, Rosenson RS, Brown TM, Howard G, Safford MM, Muntner P. Declines in coronary heart disease incidence and mortality among middle-aged adults with and without diabetes. Ann Epidemiol. 2014;24:581–587. doi: 10.1016/j.annepidem.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacks NC, Ash AS, Ghosh K, Rosen AK, Wong JB, Rosen AB. Trends in acute myocardial infarction hospitalizations: Are we seeing the whole picture? Am Heart J. 2015;170:1211–1219. doi: 10.1016/j.ahj.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks NC, Ash AS, Ghosh K, Rosen AK, Wong JB, Cutler DM, Rosen AB. Recent national trends in acute myocardial infarction hospitalizations in Medicare: shrinking declines and growing disparities. Epidemiology. 2015;26:e46–e47. doi: 10.1097/EDE.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Will JC, Mercado CI, Loustalot F. Trends in predicted risk for atherosclerotic cardiovascular disease using the pooled cohort risk equations among US adults from 1999 to 2012. JAMA Intern Med. 2015;175:299–302. doi: 10.1001/jamainternmed.2014.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmali KN, Goff DC, Jr, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–968. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 17.Lackland DT, Elkind MS, D’Agostino R, Sr, Dhamoon MS, Goff DC, Jr, Higashida RT, McClure LA, Mitchell PH, Sacco RL, Sila CA, Smith SC, Jr, Tanne D, Tirschwell DL, Touzé E, Wechsler LR American Heart Association Stroke Council; Council on Epidemiology and Prevention; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research. Inclusion of stroke in cardiovascular risk prediction instruments: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1998–2027. doi: 10.1161/STR.0b013e31825bcdac. [DOI] [PubMed] [Google Scholar]
- 18.Shillinglaw B, Viera AJ, Edwards T, Simpson R, Sheridan SL. Use of global coronary heart disease risk assessment in practice: a cross-sectional survey of a sample of U.S. physicians. BMC Health Serv Res. 2012;12:20. doi: 10.1186/1472-6963-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:396] Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 19.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. [Accessed July 15, 2016];Mortality multiple cause micro-data files, 2014: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 21.Bradley EH, Herrin J, Curry L, Cherlin EJ, Wang Y, Webster TR, Drye EE, Normand SL, Krumholz HM. Variation in hospital mortality rates for patients with acute myocardial infarction. Am J Cardiol. 2010;106:1108–1112. doi: 10.1016/j.amj-card.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez F, Foody JM, Wang Y, López L. Young Hispanic women experience higher in-hospital mortality following an acute myocardial infarction. J Am Heart Assoc. 2015;4:e002089. doi: 10.1161/JAHA.115.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh RW, Normand SL, Wang Y, Barr CD, Dominici F. Geographic disparities in the incidence and outcomes of hospitalized myocardial infarction: does a rising tide lift all boats? Circ Cardiovasc Qual Outcomes. 2012;5:197–204. doi: 10.1161/CIRCOUTCOMES.111.962456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Lichtman JH, Dharmarajan K, Masoudi FA, Ross JS, Dodson JA, Chen J, Spertus JA, Chaudhry SI, Nallamothu BK, Krumholz HM. National trends in stroke after acute myocardial infarction among Medicare patients in the United States: 1999 to 2010. Am Heart J. 2015;169:78–85.e74. doi: 10.1016/j.ahj.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-long trends (2001–2011) in the incidence and hospital death rates associated with the in-hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–125. doi: 10.1161/CIRCOUTCOMES.115.002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiyama T, Hasegawa K, Kobayashi Y, Takahashi O, Fukui T, Tsugawa Y. Differential time trends of outcomes and costs of care for acute myocardial infarction hospitalizations by ST elevation and type of intervention in the United States, 2001–2011. J Am Heart Assoc. 2015;4:e001445. doi: 10.1161/JAHA.114.001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ. Twelve-year follow-up of American women’s awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc QualOutcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, Krumholz HM, Lichtman JH. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO Study. J Am Coll Cardiol. 2015;66:1949–1957. doi: 10.1016/j.jacc.2015.08.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in myocardial infarction secondary prevention: the National Health and Nutrition Examination Surveys (NHANES), 1999–2012. J Am Heart Assoc. 2015;4:e001709. doi: 10.1161/JAHA.114.001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorita A, Ahmed A, Starr SR, Thompson KM, Reed DA, Prokop L, Shah ND, Murad MH, Ting HH. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ. 2014;348:f7393. doi: 10.1136/bmj.f7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diercks DB, Owen KP, Kontos MC, Blomkalns A, Chen AY, Miller C, Wiviott S, Peterson ED. Gender differences in time to presentation for myocardial infarction before and after a national women’s cardiovascular awareness campaign: a temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get with the Guidelines (NCDR ACTION Registry-GWTG) Am Heart J. 2010;160:80–87.e3. doi: 10.1016/j.ahj.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Dracup K, McKinley S, Doering LV, Riegel B, Meischke H, Moser DK, Pelter M, Carlson B, Aitken L, Marshall A, Cross R, Paul SM. Acute coronary syndrome: what do patients know? Arch Intern Med. 2008;168:1049–1054. doi: 10.1001/archinte.168.10.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makam RP, Erskine N, Yarzebski J, et al. Decade long trends (2001–2011) in duration of pre-hospital delay among elderly patients hospitalized for an acute myocardial infarction. J Am Heart Assoc. 2016;5:e002664. doi: 10.1161/JAHA.115.002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathews R, Peterson ED, Li S, Lessard D, Lau J, Allison J, Gore JM, Gurwitz J, McManus DD, Goldberg RJ. Use of emergency medical service transport among patients with ST-segment-elevation myocardial infarction: findings from the National Cardiovascular Data Registry Acute Coronary Treatment Intervention Oucomes Network Registry-Get With The Guidelines. Circulation. 2011;124:154–163. doi: 10.1161/CIRCULATIONAHA.110.002345. [DOI] [PubMed] [Google Scholar]
- 38.Khoshchehreh M, Groves EM, Tehrani D, Amin A, Patel PM, Malik S. Changes in mortality on weekend versus weekday admissions for acute coronary syndrome in the United States over the past decade. Int J Cardiol. 2016;210:164–172. doi: 10.1016/j.ijcard.2016.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty AL, Li S, Thomas L, Amsterdam EA, Alexander KP, Whooley MA. Trends in referral to cardiac rehabilitation after myocardial infarction: data from the National Cardiovascular Data Registry 2007 to 2012. J Am Coll Cardiol. 2014;63:2582–2583. doi: 10.1016/j.jacc.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 41.Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127:538–546. doi: 10.1016/j.amjmed.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown TM, Deng L, Becker DJ, Bittner V, Levitan EB, Rosenson RS, Safford MM, Muntner P. Trends in mortality and recurrent coronary heart disease events after an acute myocardial infarction among Medicare beneficiaries, 2001–2009. Am Heart J. 2015;170:249–255. doi: 10.1016/j.ahj.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Chen HY, Tisminetzky M, Lapane KL, Yarzebski J, Person SD, Kiefe CI, Gore JM, Goldberg RJ. Decade-long trends in 30-day re-hospitalization rates after acute myocardial infarction. J Am Heart Assoc. 2015;4:e002291. doi: 10.1161/JAHA.115.002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen HY, Tisminetzky M, Yarzebski J, Gore JM, Goldberg RJ. Decade-long trends in the frequency of 90-day rehospitalizations after hospital discharge for acute myocardial infarction. Am J Cardiol. 2016;117:743–748. doi: 10.1016/j.amjcard.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuti SV, Wang Y, Masoudi FA, Bratzler DW, Bernheim SM, Murugiah K, Krumholz HM. Improvements in the distribution of hospital performance for the care of patients with acute myocardial infarction, heart failure, and pneumonia, 2006–2011. Med Care. 2015;53:485–491. doi: 10.1097/MLR.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jena AB, Prasad V, Goldman DP, Romley J. Mortality and treatment patterns among patients hospitalized with acute cardiovascular conditions during dates of national cardiology meetings. JAMA Intern Med. 2015;175:237–244. doi: 10.1001/jamainternmed.2014.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigen R, Spertus JA, Maddox TM, Ho PM, Jones PG, Arnold SV, Masoudi FA, Bradley SM. Hospital-level variation in angina and mortality at 1 year after myocardial infarction: insights from the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) Registry. Circ Cardiovasc Qual Outcomes. 2014;7:851–856. doi: 10.1161/CIRCOUTCOMES.114.001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Will JC, Loustalot F, Hong Y. National trends in visits to physician offices and outpatient clinics for angina 1995 to 2010. Circ Cardiovasc Qual Outcomes. 2014;7:110–117. doi: 10.1161/CIRCOUTCOMES.113.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen HY, Saczynski JS, Lapane KL, Kiefe CI, Goldberg RJ. Adherence to evidence-based secondary prevention pharmacotherapy in patients after an acute coronary syndrome: a systematic review. Heart Lung. 2015;44:299–308. doi: 10.1016/j.hrtlng.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumbhani DJ, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Deedwania P, Grau-Sepulveda M, Schwamm LH, Bhatt DL Get With the Guidelines Steering Committee and Investigators. Temporal trends for secondary prevention measures among patients hospitalized with coronary artery disease. Am J Med. 2015;128:426.e1–426.e9. doi: 10.1016/j.amjmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–1776. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangalore S, Gupta N, Généreux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI-2D trials: insight from over 8.1 million percutaneous coronary interventions. Int J Cardiol. 2015;183:6–10. doi: 10.1016/j.ijcard.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 53.Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71–77. doi: 10.3122/jabfm.2013.01.120107. [DOI] [PubMed] [Google Scholar]
- 54.Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. doi: 10.1161/CIRCULATIONAHA.114.012485. [DOI] [PubMed] [Google Scholar]
- 55.Yeh RW, Mauri L, Wolf RE, Romm IK, Lovett A, Shahian D, Normand SL. Population trends in rates of coronary revascularization. JAMA Intern Med. 2015;175:454–456. doi: 10.1001/jamain-ternmed.2014.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nallamothu BK, Normand SL, Wang Y, Hofer TP, Brush JE, Jr, Messenger JC, Bradley EH, Rumsfeld JS, Krumholz HM. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. 2015;385:1114–1122. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfuntner A, Wier LM, Steiner C. Costs for Hospital Stays in the United States, 2011. Rockville, MD: Agency for Healthcare Research and Quality; 2006. HCUP Statistical Brief 168. [PubMed] [Google Scholar]
- 58.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 59.Caruba T, Katsahian S, Schramm C, Charles Nelson A, Durieux P, Bégué D, Juillière Y, Dubourg O, Danchin N, Sabatier B. Treatment for stable coronary artery disease: a network meta-analysis of cost-effectiveness studies. PLoS One. 2014;9:e98371. doi: 10.1371/journal.pone.0098371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z, Winget M. Economic burden of illness of acute coronary syndromes: medical and productivity costs. BMC Health Serv Res. 2011;11:35. doi: 10.1186/1472-6963-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston SS, Curkendall S, Makenbaeva D, Mozaffari E, Goetzel R, Burton W, Maclean R. The direct and indirect cost burden of acute coronary syndrome. J Occup Environ Med. 2011;53:2–7. doi: 10.1097/JOM.0b013e31820290f4. [DOI] [PubMed] [Google Scholar]
- 62.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 63.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Jr, Pollack CV, Jr, Newby LK, Harrington RA, Gibler WB, Ohman EM. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 64.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shroff GR, Heubner BM, Herzog CA. Incidence of acute coronary syndrome in the general Medicare population, 1992 to 2009: a real-world perspective. JAMA Intern Med. 2014;174:1689–1690. doi: 10.1001/jamainternmed.2014.3446. [DOI] [PubMed] [Google Scholar]
- 66.Ford ES, Giles WH. Changes in prevalence of nonfatal coronary heart disease in the United States from 1971–1994. Ethn Dis. 2003;13:85–93. [PubMed] [Google Scholar]
- 67.Will JC, Yuan K, Ford E. National trends in the prevalence and medical history of angina: 1988 to 2012. Circ Cardiovasc Qual Outcomes. 2014;7:407–413. doi: 10.1161/CIRCOUTCOMES.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 69.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G Women’s Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 70.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
21. CARDIOMYOPATHY AND HEART FAILURE
See Table 21-1 and Charts 21-1 through 21-5
Table 21-1.
Heart Failure
| Population Group | Prevalence, 2011–2014, Age ≥20 y | Incidence (New Cases), 2013, Age ≥55 y | Mortality, 2014, All Ages* | Hospital Discharge, 2010, All Ages | Cost, 2012† |
|---|---|---|---|---|---|
| Both sexes | 6 500 000 (2.5%) | 960 000 | 68 626 | 1 023 000 | $30.7 billion |
| Males | 2 900 000 (2.4%) | 470 000 | 30 339 (44.2%)‡ | 501 000 | … |
| Females | 3 600 000 (2.6%) | 490 000 | 38 287 (55.8%)‡ | 522 000 | … |
| NH white males | 2.4% | 410 000§ | 25 316 | … | … |
| NH white females | 2.5% | 415 000§ | 32 206 | … | … |
| NH black males | 2.6% | 60 000§ | 3145 | … | … |
| NH black females | 3.9% | 75 000§ | 3817 | … | … |
| Hispanic males | 2.0% | … | 1256 | … | … |
| Hispanic females | 2.4% | … | 1486 | … | … |
| NH Asian males | 1.3% | … | 452|| | … | … |
| NH Asian females | 0.3% | … | 577|| | … | … |
| NH American Indian or Alaska Native | … | … | 233 | … | … |
Heart failure includes people who answered “yes” to the question of ever having congestive heart failure. Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality data for Hispanic, NH American Indian or Alaska Native, and NH Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
Cost data are from Heidenreich et al.15
These percentages represent the portion of total mortality attributable to heart failure that is for males vs females.
Estimates for whites include other nonblack races.
Includes Chinese, Filipino, Hawaiian, Japanese, and Other Asian or Pacific Islander.
Sources: Prevalence: National Health and Nutrition Examination Survey 2011 to 2014 (National Center for Health Statistics) and National Heart, Lung, and Blood Institute. Percentages are age adjusted for Americans ≥20 years of age. Age-specific percentages are extrapolated to the 2014 US population estimates. These data are based on self-reports. Incidence: Atherosclerosis Risk in Communities Study Community Surveillance, 2005 to 2013 from the National Heart, Lung, and Blood Institute. Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death–United States. Mortality for NH Asians includes Pacific Islanders.
Chart 21-1. Incidence of peripartum cardiomyopathy.
Reproduced from Blauwet et al.14 Copyright © 2011, BMJ Publishing Group Ltd and the British Cardiovascular Society, with permission from BMJ Publishing Group Ltd.
Chart 21-5. Number of patients receiving left ventricular assist devices in the United States, 2006 to 2014.
Data derived from Kirklin et al.77
Cardiomyopathy
ICD-9 425; ICD-10 I42.
2014: Mortality—23 126. Any-mention mortality—45 307. Hospital discharges—34 000.
Abbreviations Used in Chapter 21
| ABC | Health, Aging, and Body Composition Study |
| ACEI | angiotensin-converting enzyme inhibitor |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| ARB | angiotensin receptor blocker |
| ARIC | Atherosclerosis Risk in Communities Study |
| ASCEND-HF | Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure |
| BMI | body mass index |
| BNP | B-type natriuretic peptide |
| BP | blood pressure |
| CAD | coronary artery disease |
| CARDIA | Coronary Artery Risk Development in Young Adults Study |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DCM | dilated cardiomyopathy |
| DM | diabetes mellitus |
| ED | emergency department |
| EF | ejection fraction |
| FHS | Framingham Heart Study |
| GWTG | Get With the Guidelines |
| HbA1c | hemoglobin A1c (glycosylated hemoglobin) |
| HCM | hypertrophic cardiomyopathy |
| HD | heart disease |
| HF | heart failure |
| HR | hazard ratio |
| ICD-10 | International Classification of Diseases, 10th Revision |
| ICD-9 | International Classification of Diseases, 9th Revision |
| IHD | ischemic heart disease |
| INTERMACS | Interagency Registry for Mechanically Assisted Circulatory Support |
| LV | left ventricular |
| LVAD | left ventricular assist device |
| LVEF | left ventricular ejection fraction |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MI | myocardial infarction |
| MRI | magnetic resonance imaging |
| NAMCS | National Ambulatory Medical Care Survey |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHANES | National Health and Nutrition Examination Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| OR | odds ratio |
| PA | physical activity |
| PAR | population attributable risk |
| PPCM | peripartum cardiomyopathy |
| QALY | quality-adjusted life-year |
| REACH | Reduction of Atherothrombosis for Continued Health |
| RR | relative risk |
| SBP | systolic blood pressure |
| SCD | sudden cardiac death |
Youth
-
Since 1996, the NHLBI-sponsored Pediatric Cardiomyopathy Registry has collected data on all children with newly diagnosed cardiomyopathy in New England and the Central Southwest (Texas, Oklahoma, and Arkansas).1
The overall incidence of cardiomyopathy is 1.13 cases per 100 000 among children <18 years of age.
Among children <1 year of age, the incidence is 8.34, and among children 1 to 18 years of age, it is 0.70 per 100 000.
The annual incidence is lower in white than in black children, higher in boys than in girls, and higher in New England (1.44 per 100 000) than in the Central Southwest (0.98 per 100 000).
DCM is the most common form of cardiomyopathy among children. The Pediatric Cardiomyopathy Registry recently reported an annual incidence of DCM in children <18 years of age of 0.57 per 100 000 overall. The annual incidence was higher in boys than in girls (0.66 versus 0.47 cases per 100 000), in blacks than in whites (0.98 versus 0.46 cases per 100 000), and in infants (<1 year of age) than in children (4.40 versus 0.34 cases per 100 000). The majority of children (66%) had idiopathic disease. The most common known causes of DCM were myocarditis (46%) and neuromuscular disease (26%).2 Risk factors for death and transplantation in children varied according to cause of DCM. For idiopathic DCM, increased LV end-diastolic dimension was associated with increased risk for transplantation but not mortality. Short stature was significantly related to death but not transplantation.3 The 5-year incidence rate of SCD among children with DCM is 3%.4
HCM is the most common inherited heart defect, occurring in ≈1 of 500 individuals, although clinically expressed and HCM gene carrier prevalence could be as high as 1 in 200 individuals. In the United States, ≈500 000 people have HCM, yet most are unaware of it.5,6 In a recent report of the Pediatric Cardiomyopathy Registry, the overall annual incidence of HCM in children was 4.7 per 1 million children. There was a higher incidence in the New England than in the Central Southwest region, in boys than in girls, and in children diagnosed at <1 year of age than in older children.7 Long-term outcomes of children with HCM show that 9% progress to HF and 12% to SCD.8 See Chapter 17 (Disorders of Heart Rhythm) for statistics regarding sudden death in HCM.
Data from Kaiser Permanente indicate that the incidence of PPCM is 4.84 per 10 000 live births (95% CI, 3.98–5.83), and PPCM is associated with higher maternal and neonatal death rates and worse neonatal outcomes.9 There was a trend toward an increase in the incidence of PPCM in the United States from 1990 through 1993 to 2000 through 2002, which has been ascribed to a rise in maternal age.10 In a prospective cohort of PPCM, 13% of women had major events (death, cardiac transplantation, or implantation of an LVAD) or persistent severe cardiomyopathy at 12 months. Black females had worse LV dysfunction at presentation and at 6 and 12 months postpartum compared with “white or other” females.11
Global Burden of Cardiomyopathy
(See Chart 21-1)
Between 1990 and 2010, the global number of deaths attributed to cardiomyopathy and myocarditis increased 40.8%, from 286 800 to 403 900, but the age-standardized death rate decreased 9.8%, from 6.7 to 6.1 per 100 000.12 However, between 1990 and 2010, the global years lived with disability for cardiomyopathy and myocarditis increased 11.4%, from 5 to 6 years lived with disability per 100 000.13 The reported incidence of PPCM in the United States varies considerably, whereas the reported incidences in several African and Asian countries are similar14 (Chart 21-1).
Heart Failure
ICD-9 428; ICD-10 I50.
Prevalence
(See Table 21-1 and Chart 21-2)
Chart 21-2. Prevalence of heart failure for adults ≥20 years by sex and age (NHANES: 2011–2014).
NHANES indicates National Health and Nutrition Examination Survey. Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute.
On the basis of data from NHANES 2011 to 2014, an estimated 6.5 million Americans ≥20 years of age had HF (NHLBI tabulation).
Projections show that the prevalence of HF will increase 46% from 2012 to 2030, resulting in >8 million people ≥18 years of age with HF.15
Incidence
(See Table 21-1 and Chart 21-3)
Chart 21-3. First acute decompensated heart failure annual event rates per 1000 from ARIC Community Surveillance (2005–2013).
ARIC indicates Atherosclerosis Risk in Communities Study.
Source: ARIC and National Heart, Lung, and Blood Institute.
-
On the basis of data from the community surveillance component of the ARIC study of the NHLBI:
There are 960 000 new HF cases annually (ARIC 2005–2013; based on community trends in the occurrence of hospitalized HF and case fatality; unpublished report for the NHLBI) (Table 21-1).
At ages <75 years, HF incidence is higher in blacks than whites (Chart 21-3).
Data from the NHLBI-sponsored Chicago Heart Association Detection Project in Industry, ARIC, and CHS cohorts indicates that HF incidence approaches 21 per 1000 population after 65 years of age.16
-
In the FHS (1980–2003)
The annual rates per 1000 person-years of new HF events for white males were 9.2 for those 65 to 74 years of age, 22.3 for those 75 to 84 years of age, and 43.0 for those ≥85 years of age.
For white females in the same age groups, the rates were 4.7, 14.8, and 30.7, respectively.
Thus, HF incidence rates in males approximately double with each 10-year age increase from 65 to 85 years; however, the HF incidence rate triples for females between ages 65 to 74 and 75 to 84 years.17
In MESA, African Americans had the highest risk of developing HF, followed by Hispanic, white, and Chinese Americans (4.6, 3.5, 2.4, and 1.0 per 1000 person-years, respectively). This higher risk reflected differences in the prevalence of hypertension, DM, and low socioeconomic status.18
African Americans had the highest proportion of incident HF not preceded by clinical MI (75%).18
In the NHLBI’s ARIC study, the age-adjusted incidence rate per 1000 person-years was 3.4 for white women, less than for all other groups, that is, white males (6.0), black females (8.1), and black males (9.1). HF incidence rates in black females were more similar to those of males than those of white females. The greater HF incidence in blacks than in whites was explained largely by blacks’ greater levels of atherosclerotic risk factors.19
Data from Kaiser Permanente indicated an increase in the incidence of HF among the elderly and improved HF survival, resulting in increased HF prevalence, with both effects being greater in males.20
Data from Olmsted County, MN, indicate that the age- and sex-adjusted incidence of HF declined substantially from 315.8 per 100 000 in 2000 to 219.3 per 100 000 in 2010, with a greater rate reduction for HF with reduced EF (−45.1%; 95% CI, −33.0% to −55.0%) than for HF with preserved EF (−27.9%; 95% CI, −12.9% to −40.3%).21
In the CARDIA study, HF before 50 years of age was more common among blacks than whites. Hypertension, obesity, and systolic dysfunction are important risk factors that may be targets for prevention.22
Lifetime Risk
-
Data from the NHLBI-sponsored Chicago Heart Association Detection Project in Industry, ARIC, and CHS cohorts indicate the following16:
Overall, at age 45 years through age 95 years, lifetime risks for HF are high (20%–45%).
Lifetime risks for HF were 30% to 42% in white males, 20% to 29% in black males, 32% to 39% in white females, and 24% to 46% in black females.
Lifetime risk for HF was higher with higher BP and BMI at all ages.
The lifetime risk of HF occurring for people with BMI <25 kg/m2 is double that of those with BMI ≥30 kg/m2.
The lifetime risk of HF occurring for people with BP >160/90 mm Hg is 1.6 times that of those with BP <120/90 mm Hg.
Mortality
(See Table 21-1)
One in 8 deaths has HF mentioned on the death certificate (NCHS, NHLBI).23
In 2014, HF any-mention mortality was 308 976 (146 072 males and 162 904 females). HF was the underlying cause in 68 626 of those deaths in 2014.23 Table 21-1 shows the numbers of these deaths that were coded for HF as the underlying cause.
The 2014 overall any-mention death rate for HF was 84.0 per 100 000. Any-mention death rates in males were 103.7 for non-Hispanic whites, 108.0 for non-Hispanic blacks, 43.2 for non-Hispanic Asians or Pacific Islanders, 94.9 for non-Hispanic American Indians or Alaska Natives, and 61.8 for Hispanics. In females, the respective death rates were 75.3 for non-Hispanic whites, 80.4 for non-Hispanic blacks, 32.2 for non-Hispanic Asians or Pacific Islanders, 75.3 for non-Hispanic American Indians or Alaska Natives, and 47.0 for Hispanics.23
The number of any-mention deaths attributable to HF was higher in 2014 (309 000) than it was in 1995 (287 000) (NCHS, NHLBI).
Survival after HF diagnosis has improved between 1979 and 2000, as shown by data from the Rochester Epidemiology Project in Olmsted County, MN.24 However, the death rate remains high: ≈50% of people diagnosed with HF will die within 5 years.24,25
In older adults, data from Kaiser Permanente indicate that survival after the onset of HF has also improved.20
Five-year survival of HF diagnosis after an MI improved in 2001 to 2010 versus 1990 to 2000, from 54% to 61%.26
In the CHS, both the presence of depression and elevated N-terminal pro-BNP levels were independent risk factors that identified HF patients with a high risk of all-cause mortality.27 Correspondingly, a decrease in BNP levels has been shown to predict lower mortality.28
In the NHLBI’s ARIC study, the 30-day, 1-year, and 5-year case fatality rates after hospitalization for HF were 10.4%, 22%, and 42.3%, respectively. Blacks had a greater 5-year case fatality rate than whites (P<0.05).19
Among Medicare beneficiaries, the overall 1-year HF mortality rate declined slightly from 1998 to 2008 but remained high at 29.6%.29 Rates of mortality decline were uneven across states.
Recent data from Olmsted County, MN, reveal that among incident HF cases, 5-year mortality did not decline from 2000 to 2010. Five-year mortality remained high (52.6% overall; 24.4% for 60-year-olds and 54.4% for 80-year-olds) and was more frequently ascribed to noncardiovascular causes (54.3%); however, the risk of noncardiovascular death was greater in HF with preserved EF than in HF with reduced EF.21
Mortality declines have been primarily attributed to evidence-based approaches to treat HF risk factors and implementation of ACEIs, β-blockers, coronary revascularization, implantable cardioverter-defibrillators, and cardiac resynchronization therapeutic strategies.30 Contemporary evidence from the GWTG-HF registry suggests that ≈47% of individuals admitted to the hospital with HF should have had initiation of ≥1 new medication on discharge.31 In a large Swedish registry of patients with HF with preserved ejection fraction, statins improved 1-year cardiovascular hospitalization, mortality, and cardiovascular mortality,32 which could prompt further research with longer follow-up.
Risk Factors
-
Olmstead County found that CHD, hypertension, DM, obesity, and smoking are responsible for 52% of incident HF cases in the population with ORs and their PARs as follows33:
CHD: OR, 3.1; overall PAR, 20% (highest in males: 23% in males and 16% in females)
Cigarette smoking: RR, 1.4; PAR, 14%
Hypertension: RR, 1.4; PAR, 20% (highest in females: 13% in males and 28% in females)
Obesity: RR, 2.0; PAR, 12%
DM: OR, 2.7; PAR, 12%
Dietary sodium intake: RR, 1.4; PAR, not available34
Valvular HD: RR, 1.5; PAR, 2%35
One third of the US adult population has stage A HF, defined as people with predisposing conditions for HF.36
Among 20 900 male physicians in the Physicians’ Health Study, the lifetime risk of HF was higher in males with hypertension; healthy lifestyle factors (normal weight, not smoking, regular PA, moderate alcohol intake, consumption of breakfast cereals, and consumption of fruits and vegetables) were related to lower risk of HF.37
In older adults, both current and past cigarette smoking increase HF risk. In current smokers, this risk is high irrespective of pack-years of exposure, whereas in past smokers, there is a dose-effect association.38
Greater adherence to the AHA’s Life Simple 7 guidelines (better profiles in smoking, BMI, PA, diet, cholesterol, BP, and glucose) is associated with a lower lifetime risk of HF and better cardiac structure and functional parameters by echocardiography.39
The presence of DM conferred a greater risk for HF hospitalization despite contemporary management of CVD in 19 699 patients studied in the international REACH registry.40
-
Racial differences in risk factors for HF were observed in the ABC Study, a US cohort of 2934 adults aged 70 to 79 years followed up for 7 years.41
Among blacks, a greater proportion of HF risk (68% versus 49% among whites) was attributable to modifiable risk factors, including elevated SBP, elevated fasting glucose level, CHD, LV hypertrophy, and smoking. LV hypertrophy was 3-fold more prevalent in blacks than in whites.
Males and black participants were more likely to develop HF.
CHD (PAR 23.9% for white participants, 29.5% for black participants) and uncontrolled BP (PAR 21.3% for white participants, 30.1% for black participants) had the highest PARs in both races.41
Hispanics carry a predominance of HF risk factors and healthcare disparities, which suggests a high HF risk in this population.42
-
Nontraditional HF risk factors are as follows:
-
FHS
In the NHLBI-sponsored FHS, BNP, urinary albumin-to-creatinine ratio, elevated serum γ-glutamyl transferase, and higher levels of hematocrit were identified as risk factors for incident HF.43–45
In the Framingham Offspring Study, among 2739 participants, increased circulating concentrations of resistin were associated with incident HF independent of prevalent coronary disease, obesity, insulin resistance, and inflammation.46
Adiponectin was also associated with risk of HF (J-shaped relationship).47
Inflammatory markers (interleukin-6 and tumor necrosis factor-α), serum albumin levels, and cigarette smoking exposure were also associated with HF risk.38,48,49
In the CHS, baseline cardiac high-sensitivity troponin and changes in high-sensitivity troponin levels were significantly associated with incident HF.50 Circulating individual and total omega-3 fatty acid concentrations were associated with lower incidence of HF.51
In the ARIC study, white blood cell count, CRP, albuminuria, HbA1c among individuals without DM, cardiac troponin, ventricular premature complexes, and socioeconomic position over the life course were all identified as risk factors for HF.52–57
In MESA, plasma N-terminal pro-BNP provided incremental prognostic information beyond the traditional risk factors and the MRI-determined LV mass index for incident symptomatic HF.58
In the international ASCEND-HF clinical trial, a combination of clinical data including age, SBP, sodium, blood urea nitrogen, and dyspnea at rest predicted 30-day mortality risk.59
-
LV Function
-
Data from Olmsted County, MN, indicate the following:
Among all individuals (asymptomatic or with validated clinical HF), the prevalence of LV diastolic dysfunction was 21% for mild diastolic dysfunction and 7% for moderate or severe diastolic dysfunction. The prevalence of systolic dysfunction was 6%. The presence of any LV dysfunction (systolic or diastolic) was associated with an increased risk of overt HF, and asymptomatic diastolic dysfunction was predictive of all-cause death.60,61
After 4 years of follow-up, the prevalence of diastolic dysfunction increased to 39.2%.
Diastolic dysfunction was associated with development of clinical HF during 6 years of subsequent follow-up after adjustment for age, hypertension, DM, and CAD (HR, 1.81; 95% CI, 1.01–3.48).62
Among individuals with symptomatic HF, 55% had HF with preserved EF. The prevalence of LV diastolic dysfunction was 6% for mild and 75% for moderate or severe diastolic dysfunction. HF with preserved EF is associated with a high mortality rate, comparable to that of HF with reduced EF.63 Over a 15-year follow-up period, survival trends improved among individuals with HF with reduced EF but not among those with HF with preserved EF.64
The prevalence of HF with preserved EF has increased over a 15-year period, whereas the rate of death attributable to this disorder has remained unchanged.64 As a group, patients with HF with preserved EF are older, are more likely to be female, and have greater hypertension, obesity, and anemia than those with HF with reduced EF.65
In the NHLBI-sponsored FHS, among asymptomatic individuals, the prevalence of systolic dysfunction was 5%; the prevalence of LV diastolic dysfunction was 36%. LV systolic dysfunction and LV diastolic dysfunction were associated with increased risk of incident HF. Major organ system dysfunction (higher serum creatinine, lower ratios of FEV1 [forced expiratory volume in 1 second] to FVC [forced vital capacity], and lower hemoglobin concentrations) were also independently associated with increased risk of new-onset HF.60
In MESA, the overall prevalence of asymptomatic LV systolic dysfunction was higher in African Americans than in whites, Chinese, and Hispanics. After 9 years of follow-up, asymptomatic LV dysfunction was associated with incident clinical HF (HR, 8.69; 95% CI, 4.89–15.4) after adjustment for cardiac risk factors.61
In the Echocardiographic Study of Hispanic/Latinos, more than half (50.3%) of middle-aged or older Hispanics had some form of cardiac dysfunction (systolic and/or diastolic), yet fewer than 1 in 20 Hispanic/Latinos had symptomatic or clinically recognized HF.66
Hospital Discharges/Ambulatory Care Visits
(See Table 21-1 and Chart 21-4)
Chart 21-4. Hospital discharges for heart failure by sex (United States: 1980–2010).
Hospital discharges include people discharged alive, dead, and status unknown.
Source: National Hospital Discharge Survey/National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Hospital discharges for HF by sex (United States: 1980–2010): Hospital discharges include people discharged alive, dead, and status unknown (Chart 21-4).
Hospital discharges for HF were essentially unchanged from 2000 to 2010, with first-listed discharges of 1 008 000 and 1 023 000, respectively (NCHS, NHLBI tabulation).67
In 2012, there were 1 774 000 physician office visits with a primary diagnosis of HF (NAMCS, NHLBI tabulation). In 2012, there were 509 000 ED visits for HF, and in 2011, there were 257 000 hospital outpatient department visits for HF (NHAMCS, NHLBI tabulation).
Among 1077 patients with HF in Olmsted County, MN, hospitalizations were common after HF diagnosis, with 83% patients hospitalized at least once and 43% hospitalized at least 4 times. More than one half of all hospitalizations were related to noncardiovascular causes.68
Among Medicare beneficiaries, the overall HF hospitalization rate declined substantially from 1998 to 2008 but at a lower rate for black males.29 However, the temporal trend findings were uneven across states.
In the GWTG-HF Registry, only one tenth of eligible HF patients received cardiac rehabilitation referral at discharge after hospitalization for HF.69
Among Medicare part D coverage beneficiaries, HF medication adherence (ACEI/ARB, β-blockers, and diuretic agents) after HF hospitalization discharge decreased over 2 to 4 months after discharge, followed by a plateau over the subsequent year for all 3 medication classes.70
Rates of cardiovascular death or HF rehospitalization were greatest in those who have been previously hospitalized for HF.71
Although Hispanic patients hospitalized with HF were significantly younger than non-Hispanic whites, the prevalence of DM, hypertension, and overweight/obesity was higher. In multivariate analysis, a 45% lower in-hospital mortality risk was observed among Hispanics with HF with preserved EF compared with non-Hispanic whites but not among those with HF with reduced EF.72
-
On the basis of data from the community surveillance component of the ARIC study of the NHLBI73
The average incidence of hospitalized HF for those aged ≥55 years was 11.6 per 1000 people per year; recurrent hospitalized HF was 6.6 per 1000 people per year.
Age-adjusted annual hospitalized HF incidence was highest for black males (15.7 per 1000), followed by black females (13.3 per 1000), white males (12.3 per 1000), and white females (9.9 per 1000).
Of incident hospitalized HF events, 53% had HF with reduced EF and 47% had preserved EF. Black males had the highest proportion of hospitalized HF with reduced EF (70%); white females had the highest proportion of hospitalized HF with preserved EF (59%).
Age-adjusted 28-day and 1-year case fatality after hospitalized HF was 10.4% and 29.5%, respectively, and did not differ by race or sex.
Data from Olmsted County, MN, indicate that among those with HF, hospitalizations were particularly common among men and did not differ by HF with reduced EF versus preserved EF. Sixty-three percent of hospitalizations were for noncardiovascular causes. Among those with HF, hospitalization rates for cardiovascular causes did not change over time, whereas those for noncardiovascular causes increased (from 2000 to 2010).21
Cost
See Chapter 27 (Economic Cost of Cardiovascular Disease), for further statistics.
In 2012, total cost for HF was estimated to be $30.7 billion. Of this total, 68% was attributable to direct medical costs.15
Projections show that by 2030, the total cost of HF will increase almost 127% to $69.7 billion from 2012. This equals ≈$244 for every US adult.15
The costs associated with treating HF comorbidities and HF exacerbations in youths are significant, totaling nearly $1 billion in inpatient costs, and may be rising.74
Implantable cardioverter-defibrillators could be cost-effective in the guideline-recommended groups of individuals with HF with reduced EF; however, the benefit might not be as great in certain populations, particularly individuals with a high all-cause mortality (represented by risk factors including age ≥75 years, New York Heart Association functional class III, LVEF ≤20%, BNP ≥700, SBP ≤120 mm Hg, AF, DM, chronic lung disease, and CKD.75,76
Open Heart Transplant and LVAD Trends in the United States
(See Chart 21-5)
From September 1987 to December 2012, 40 253 people were waiting for heart transplants, with a median survival of 2.3 years; 26 943 received transplants, with median survival of 9.5 years. Life-years saved were 465 296; life-years saved per patient were 5.0.
-
The 7th INTERMACS report of >15 000 LVAD implantations from June 2006 to December 2014 revealed 80% survival at 1 year and 70% at 2 years.77
The number of patients receiving LVADs increased from 98 in 2006 to 2423 in 2014.
The proportion of LVADs as destination therapy increased from 14.7% in 2006 to 2007 to 19.6% in 2008 to 2010, 41.6% in 2011 to 2013, and 45.7% in 201477 (Chart 21-5).
The NIS reported 2038 LVAD implantations from 2005 to 2011, with 127 in 2005 and increasing to 506 procedures in 2011.78
In-hospital mortality with LVAD implantation decreased significantly from 47.2% in 2005 to 12.7% in 2011. An inflection point was seen with a sharp rise in LVAD implantation and decrease in the in-hospital mortality rate in 2008. Average hospital length of stay decreased from the pulsatile LVAD (pre-2008) to the continuous-flow LVAD (2008–2011) eras.79 The mean cost of LVAD-related hospitalization increased from $194 380 in 2005 to $234 808 in 2011.80
In a Markov model analysis, compared with nonbridged heart transplant recipients (who did not receive an LVAD bridge), receiving a bridge-to-transplantation LVAD increased survival, with greater associated cost (range $84 964 per life-year to $119 574 per life-year for high-risk and low-risk patients, respectively). Open heart transplantation increased life expectancy and was cost-effective (8.5 years with <$100 000 per QALY relative to medical therapy), but LVAD either for bridge to transplantation (12.3 years at $226 000 per QALY) or destination therapy (4.4 years at $202 000 per QALY) was not cost-effective.81
Elevated LVAD index admission costs could be related to procurement costs and length of stay. Hospital readmissions also contribute significantly to overall cost of LVAD therapy: with continuous-flow LVAD, 44% of patients were readmitted within 30 days of discharge, with a median cost of $7546. The most common causes of readmission were gastrointestinal bleeding, infection, and stroke, with device malfunction and arrhythmias the most costly causes of readmission. There was no difference in survival between patients who were and were not readmitted, although median follow up was only 11 months.82
LVAD and Open Heart Transplant Disparities
The 7th INTERMACS report did not specifically address the influence of race or ethnicity on mortality after LVAD procedures but did report that a higher mortality was seen in females (HR, 1.16; P=0.005).77
In the United Network for Organ Sharing Database of 18 085 patients who had open heart transplantation performed at 102 centers, blacks had a higher adjusted 1-year mortality, particularly at poor-performing centers (observed-to-expected mortality ratio >1.2; OR, 1.37 [95% CI, 1.12–1.69]; P=0.002).83 Compared with whites and Hispanics, a higher proportion of blacks were treated at centers with higher than expected mortality, which persisted after adjustment for insurance type and education level.
Global Burden of HF
HF is common throughout sub-Saharan Africa. Forty-four percent of patients with newly diagnosed CVD have HF, whereas only 10% have CAD.84 Common causes include nonischemic cardiomyopathies, rheumatic HD, congenital HD, hypertensive HD, and endomyocardial fibrosis; IHD remains relatively uncommon. HF strikes individuals in sub-Saharan Africa at a much younger age than in the United States and Europe.85
The prevalence estimates for HF across Asia range from 1.26% to 6.7%. Rheumatic HD is a major contributor to HF in certain parts of South Asia, such as India, but recently, trends toward an ischemic cause for HF have been observed in Asia, such as in China and Japan.86
For males, HF prevalence in 2010 was highest (>5 per 1000) in high-income North America, Oceania, and Eastern Europe. In females, HF prevalence in 2010 was highest (4.53 per 1000) in Oceania, followed by high-income North America and North Africa/Middle East. For both males and females, HF prevalence was lowest in west sub-Saharan Africa (0.74/1000 in males and 0.57/1000 in females).87 HF made the largest contribution to age-standardized years lived with disability among males in high-income North America, Oceania, Eastern and Western Europe, southern Latin America, and Central Asia.87
HF risk factors vary substantially across world regions, with hypertension being highly associated with HF in all regions but most commonly in Latin America, the Caribbean, Eastern Europe, and sub-Saharan Africa, and with a minimal association of IHD with HF in sub-Saharan Africa.88 IHD prevalence among HF patients is highest in Europe and North America but rare in sub-Saharan Africa, whereas hypertension prevalence among HF patients was highest in Eastern Europe and sub-Saharan Africa; valvular and rheumatic HD prevalence among HF patients was highest in East Asia and Asia-Pacific countries.88 Follow up from a multiethnic cohort composed of individuals from low- to middle-income countries in Africa, Asia, the Middle East, and South America will provide additional data regarding the global burden of HF.89
REFERENCES
- 1.Wilkinson JD, Landy DC, Colan SD, Towbin JA, Sleeper LA, Orav EJ, Cox GF, Canter CE, Hsu DT, Webber SA, Lipshultz SE. The pediatric cardiomyopathy registry and heart failure: key results from the first 15 years. Heart Fail Clin. 2010;6:401–413. vii. doi: 10.1016/j.hfc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez JA, Orav EJ, Wilkinson JD, Fleming LE, Lee DJ, Sleeper LA, Rusconi PG, Colan SD, Hsu DT, Canter CE, Webber SA, Cox GF, Jefferies JL, Towbin JA, Lipshultz SE Pediatric Cardiomyopathy Registry Investigators. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. Circulation. 2011;124:814–823. doi: 10.1161/CIRCULATIONAHA.110.973826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahl E, Sleeper LA, Canter CE, Hsu DT, Lu M, Webber SA, Colan SD, Kantor PF, Everitt MD, Towbin JA, Jefferies JL, Kaufman BD, Wilkinson JD, Lipshultz SE Pediatric Cardiomyopathy Registry Investigators. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: a report from the Pediatric Cardiomyopathy Registry. J Am Coll Cardiol. 2012;59:607–615. doi: 10.1016/j.jacc.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Bick AG, Flannick J, Ito K, Cheng S, Vasan RS, Parfenov MG, Herman DS, DePalma SR, Gupta N, Gabriel SB, Funke BH, Rehm HL, Benjamin EJ, Aragam J, Taylor HA, Jr, Fox ER, Newton-Cheh C, Kathiresan S, O’Donnell CJ, Wilson JG, Altshuler DM, Hirschhorn JN, Seidman JG, Seidman C. Burden of rare sarcomere gene variants in the Framingham and Jackson Heart Study cohorts. Am J Hum Genet. 2012;91:513–519. doi: 10.1016/j.ajhg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF, Lurie PR, Orav EJ, Towbin JA. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115:773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 8.Ziółkowska L, Turska-Kmieć A, Petryka J, Kawalec W. Predictors of long-term outcome in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2016;37:448–458. doi: 10.1007/s00246-015-1298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, Go AS. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118:583–591. doi: 10.1097/AOG.0b013e318229e6de. [DOI] [PubMed] [Google Scholar]
- 10.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–670. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 11.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J, 3rd, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD IPAC Investigators. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy) J Am Coll Cardiol. 2015;66:905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381:628] Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381:628] Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blauwet LA, Cooper LT. Diagnosis and management of peripartum cardiomyopathy. Heart. 2011;97:1970–1981. doi: 10.1136/heartjnl-2011-300349. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Fore-casting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 18.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 21.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. [Accessed July 15, 2016];Mortality multiple cause micro-data files, 2014: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 24.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. [Accessed May 25, 2016];Mortality multiple cause micro-data files, 2011: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/products/nvsr.htm.
- 26.Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. 2016;9:e002460. doi: 10.1161/CIRCHEARTFAILURE.115.002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Broek KC, Defilippi CR, Christenson RH, Seliger SL, Gottdiener JS, Kop WJ. Predictive value of depressive symptoms and B-type natriuretic peptide for new-onset heart failure and mortality. Am J Cardiol. 2011;107:723–729. doi: 10.1016/j.amjcard.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lourenço P, Ribeiro A, Pintalhão M, Silva S, Bettencourt P. Predictors of six-month mortality in BNP-matched acute heart failure patients. Am J Cardiol. 2015;116:744–748. doi: 10.1016/j.amjcard.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, Di Lenarda A, Sinagra G. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail. 2014;16:317–324. doi: 10.1002/ejhf.16. [DOI] [PubMed] [Google Scholar]
- 31.Allen LA, Fonarow GC, Liang L, Schulte PJ, Masoudi FA, Rumsfeld JS, Ho PM, Eapen ZJ, Hernandez AF, Heidenreich PA, Bhatt DL, Peterson ED, Krumholz HM American Heart Association’s Get With The Guidelines Heart Failure (GWTG-HF) Investigators. Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation. 2015;132:1347–1353. doi: 10.1161/CIRCULATIONAHA.115.014281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alehagen U, Benson L, Edner M, Dahlström U, Lund LH. Association between use of statins and mortality in patients with heart failure and ejection fraction of ≥50. Circ Heart Fail. 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 33.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–1028. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 35.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 36.Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One. 2015;10:e0132228. doi: 10.1371/journal.pone.0132228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. doi: 10.1016/j.ahj.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976.e2. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavender MA, Steg PG, Smith SC, Jr, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL REACH Registry Investigators. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–931. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 41.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the Health, Aging, and Body Composition Study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vivo RP, Krim SR, Cevik C, Witteles RM. Heart failure in Hispanics. J Am Coll Cardiol. 2009;53:1167–1175. doi: 10.1016/j.jacc.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Coglianese EE, Qureshi MM, Vasan RS, Wang TJ, Moore LL. Usefulness of the blood hematocrit level to predict development of heart failure in a community. Am J Cardiol. 2012;109:241–245. doi: 10.1016/j.amjcard.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhingra R, Gona P, Wang TJ, Fox CS, D’Agostino RB, Sr, Vasan RS. Serum gamma-glutamyl transferase and risk of heart failure in the community. Arterioscler Thromb Vasc Biol. 2010;30:1855–1860. doi: 10.1161/ATVBAHA.110.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, Vasan RS. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–1706. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frankel DS, Vasan RS, D’Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djoussé L, Wilk JB, Hanson NQ, Glynn RJ, Tsai MY, Gaziano JM. Association between adiponectin and heart failure risk in the Physicians’ Health Study. Obesity (Silver Spring) 2013;21:831–834. doi: 10.1002/oby.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, Bauer DC, Newman AB, Kim L, Harris TB, Kritchevsky SB, Butler J Health ABC Study. Serum albumin concentration and heart failure risk: the Health, Aging, and Body Composition Study. Am Heart J. 2010;160:279–285. doi: 10.1016/j.ahj.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain ω-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–170. doi: 10.7326/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal SK, Simpson RJ, Jr, Rautaharju P, Alonso A, Shahar E, Massing M, Saba S, Heiss G. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2012;109:105–109. doi: 10.1016/j.amjcard.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekwelem W, Lutsey PL, Loehr LR, Agarwal SK, Astor BC, Guild C, Ballantyne CM, Folsom AR. White blood cell count, C-reactive protein, and incident heart failure in the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2011;21:739–748. doi: 10.1016/j.annepidem.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, Coresh J. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011;58:47–55. doi: 10.1053/j.ajkd.2011.02.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, Selvin E. The association of hemoglobin a1c with incident heart failure among people without diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2010;59:2020–2026. doi: 10.2337/db10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD, Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172:717–727. doi: 10.1093/aje/kwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida AL, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JA. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–734. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khazanie P, Heizer GM, Hasselblad V, Armstrong PW, Califf RM, Ezekowitz J, Dickstein K, Levy WC, McMurray JJ, Metra M, Tang WH, Teerlink JR, Voors AA, O’Connor CM, Hernandez AF, Starling R. Predictors of clinical outcomes in acute decompensated heart failure: Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure outcome models. Am Heart J. 2015;170:290–297. doi: 10.1016/j.ahj.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community [published correction appears in Circulation. 2011;124:e458] Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, Carr JJ, Hundley WG, Herrington DM. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2012;126:2713–2719. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 64.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 65.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 66.Mehta H, Armstrong A, Swett K, Shah SJ, Allison MA, Hurwitz B, Bangdiwala S, Dadhania R, Kitzman DW, Arguelles W, Lima J, Youngblood M, Schneiderman N, Daviglus ML, Spevack D, Talavera GA, Raisinghani A, Kaplan R, Rodriguez CJ. Burden of systolic and diastolic left ventricular dysfunction among Hispanics in the United States: insights from the Echocardiographic Study of Latinos. Circ Heart Fail. 2016;9:e002733. doi: 10.1161/CIRCHEARTFAILURE.115.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention, National Center for Health Statistics. 2010 National Ambulatory Medical Care Survey and 2010 National Hospital Ambulatory Medical Care Survey. [Accessed July 17, 2013];Ambulatory health care data: questionnaires, datasets, and related documentation. For methodology. see National Center for Health Statistics, Public Use Data File Documentation: 2010 National Ambulatory Medical Care Survey and Public Use Data File Documentation: 2010 National Hospital Ambulatory Medical Care Survey. http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm.
- 68.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golwala H, Pandey A, Ju C, Butler J, Yancy C, Bhatt DL, Hernandez AF, Fonarow GC. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure Registry. J Am Coll Cardiol. 2015;66:917–926. doi: 10.1016/j.jacc.2015.06.1089. [DOI] [PubMed] [Google Scholar]
- 70.Sueta CA, Rodgers JE, Chang PP, Zhou L, Thudium EM, Kucharska-Newton AM, Stearns SC. Medication adherence based on part D claims for patients with heart failure after hospitalization (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2015;116:413–419. doi: 10.1016/j.amjcard.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vivo RP, Krim SR, Krim NR, Zhao X, Hernandez AF, Peterson ED, Piña IL, Bhatt DL, Schwamm LH, Fonarow GC. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get with the guidelines-heart failure. Circ Heart Fail. 2012;5:167–175. doi: 10.1161/CIRCHEARTFAILURE.111.963546. [DOI] [PubMed] [Google Scholar]
- 73.Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, Wruck LM, Rosamond WD. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2014;113:504–510. doi: 10.1016/j.amjcard.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nandi D, Rossano JW. Epidemiology and cost of heart failure in children. Cardiol Young. 2015;25:1460–1468. doi: 10.1017/S1047951115002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for Medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heidenreich PA, Tsai V, Curtis J, Wang Y, Turakhia MP, Masoudi FA, Varosy PD, Goldstein MK. A validated risk model for 1-year mortality after primary prevention implantable cardioverter defibrillator placement. Am Heart J. 2015;170:281–289.e2. doi: 10.1016/j.ahj.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 77.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Shah N, Agarwal V, Patel N, Deshmukh A, Chothani A, Garg J, Badheka A, Martinez M, Islam N, Freudenberger R. National trends in utilization, mortality, complications, and cost of care after left ventricular assist device implantation from 2005 to 2011. Ann Thorac Surg. 2016;101:1477–1484. doi: 10.1016/j.athoracsur.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 79.Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, Milano CA, Curtis LH, Hernandez AF. Trends in the use and outcomes of ventricular assist devices among Medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63:1395–1404. doi: 10.1016/j.jacc.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller LW, Guglin M, Rogers J. Cost of ventricular assist devices: can we afford the progress? Circulation. 2013;127:743–748. doi: 10.1161/CIRCULATIONAHA.112.139824. [DOI] [PubMed] [Google Scholar]
- 81.Alba AC, Alba LF, Delgado DH, Rao V, Ross HJ, Goeree R. Cost-effectiveness of ventricular assist device therapy as a bridge to transplantation compared with nonbridged cardiac recipients. Circulation. 2013;127:2424–2435. doi: 10.1161/CIRCULATIONAHA.112.000194. [DOI] [PubMed] [Google Scholar]
- 82.Marasco SF, Summerhayes R, Quayle M, McGiffin D, Luthe M. Cost comparison of heart transplant vs. left ventricular assist device therapy at one year. Clin Transplant. 2016;30:598–605. doi: 10.1111/ctr.12725. [DOI] [PubMed] [Google Scholar]
- 83.Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation. 2015;131:882–889. doi: 10.1161/CIRCULATIONAHA.114.011676. [DOI] [PubMed] [Google Scholar]
- 84.Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A, Stewart S. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–922. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 85.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, Kouam CK, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Davison BA, Cotter G, Sliwa K. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–1394. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 86.Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J. 2013;77:2209–2217. doi: 10.1253/circj.cj-13-0971. [DOI] [PubMed] [Google Scholar]
- 87.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dokainish H, Teo K, Zhu J, Roy A, Al-Habib K, ElSayed A, Palileo L, Jaramillo PL, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Dorairaj P, Huffman M, Badr A, Elmaghawry M, Damasceno A, Belley-Cote E, Harkness K, Grinvalds A, McKelvie R, Yusuf S. Heart failure in low- and middle-income countries: background, rationale, and design of the INTERnational Congestive Heart Failure Study (INTER-CHF) Am Heart J. 2015;170:627–634.e1. doi: 10.1016/j.ahj.2015.07.008. [DOI] [PubMed] [Google Scholar]
22. VALVULAR DISEASES
See Tables 22-1 through 22-3 and Charts 22-1 and 22-2
Table 22-1.
Pooled Prevalence of Valvular Heart Disease From CARDIA, ARIC, and CHS Cohorts
| Age, y | P Value for Trend | Frequency Adjusted to 2000 US Adult Population | |||||
|---|---|---|---|---|---|---|---|
| 18–44 | 45–54 | 55–64 | 65–74 | ≥75 | |||
| Participants, n | 4351 | 696 | 1240 | 3879 | 1745 | … | 209 128 094 |
| Male | 1959 (45) | 258 (37) | 415 (33) | 1586 (41) | 826 (47) | … | 100 994 367 (48) |
| Mitral regurgitation (n=449) | 23 (0.5) | 1 (0.1) | 12 (1.0) | 250 (6.4) | 163 (9.3) | <0.0001 | 1.7% (95% CI, 1.5%–1.9%) |
| Mitral stenosis (n=15) | 0 (0) | 1 (0.1) | 3 (0.2) | 7 (0.2) | 4 (0.2) | 0.006 | 0.1% (95% CI, 0.02%–0.2%) |
| Aortic regurgitation (n=90) | 10 (0.2) | 1 (0.1) | 8 (0.7) | 37 (1.0) | 34 (2.0) | <0.0001 | 0.5% (95% CI, 0.3%–0.6%) |
| Aortic stenosis (n=102) | 1 (0.02) | 1 (0.1) | 2 (0.2) | 50 (1.3) | 48 (2.8) | <0.0001 | 0.4% (95% CI, 0.3%–0.5%) |
| Any valve disease | … | … | … | … | … | … | … |
| Overall (n=615) | 31 (0.7) | 3 (0.4) | 23 (1.9) | 328 (8.5) | 230 (13.2) | <0.0001 | 2.5% (95% CI, 2.2%–2.7%) |
| Female (n=356) | 19 (0.8) | 1 (0.2) | 13 (1.6) | 208 (9.1) | 115 (12.6) | <0.0001 | 2.4% (95% CI, 2.1%–2.8%) |
| Male (n=259) | 12 (0.6) | 2 (0.8) | 10 (2.4) | 120 (7.6) | 115 (14.0) | <0.0001 | 2.5% (95% CI, 2.1%–2.9%) |
Values are n (%) unless otherwise indicated. ARIC indicates Atherosclerosis Risk in Communities study; CARDIA, Coronary Artery Risk Development in Young Adults; CHS, Cardiovascular Health Study; CI, confidence interval; and ellipses (…), not applicable.
Reprinted from The Lancet (Nkomo et al1), with permission from Elsevier. Copyright © 2006, Elsevier Ltd.
Table 22-3.
Incidence of IE and Valve Replacement From 2000 to 2011
| Year | Total IE Cases | IE Incidence per 100 000 | Valve Replacement per 1000 IE Cases |
|---|---|---|---|
| 2000 | 29 820 | 11 | 14 |
| 2001 | 31 526 | 11 | 16 |
| 2002 | 32 229 | 11 | 19 |
| 2003 | 35 190 | 12 | 18 |
| 2004 | 36 660 | 13 | 19 |
| 2005 | 37 508 | 13 | 23 |
| 2006 | 40 573 | 14 | 23 |
| 2007 | 38 207 | 12 | 30 |
| 2008 | 41 143 | 14 | 19 |
| 2009 | 43 502 | 14 | 27 |
| 2010 | 43 560 | 14 | 27 |
| 2011 | 47 134 | 15 | 26 |
IE indicates infective endocarditis.
Reprinted from Pant et al13 with permission from The American College of Cardiology Foundation. Copyright © 2015, The American College of Cardiology Foundation.
Mortality and any-mention mortality in this section are for 2014. “Mortality” is the number of deaths in 2014 for the given underlying cause based on ICD-10. Prevalence data are for 2006. Hospital discharge data are from the NHDS/NCHS; data included inpatients discharged alive, dead, or status unknown. Hospital discharge data for 2010 are based on ICD-9 codes.
Abbreviations Used in Chapter 22
| ACC | American College of Cardiology |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| ARIC | Atherosclerosis Risk in Communities study |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CHD | coronary heart disease |
| CHS | Cardiovascular Health Study |
| CI | confidence interval |
| DCM | dilated cardiomyopathy |
| GBD | Global Burden of Diseases, Injuries, and Risk Factors Study |
| HD | heart disease |
| HR | hazard ratio |
| ICD | International Classification of Diseases |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| ICE-PCS | International Collaboration on Endocarditis–Prospective Cohort Study |
| ICE-PLUS | International Collaboration on Endocarditis–PLUS |
| IE | infective endocarditis |
| LDL-C | low-density lipoprotein cholesterol |
| LV | left ventricular |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHDS | National Hospital Discharge Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| OR | odds ratio |
| REMEDY | Global Rheumatic Heart Disease Registry |
| RR | relative risk |
| SD | standard deviation |
| STS | Society of Thoracic Surgeons |
| TAVR | transcatheter aortic valve replacement |
| WHO | World Health Organization |
Valvular Heart Disease
(See Table 22-1)
ICD-9 424; ICD-10 I34 to I38.
Mortality—25 114. Any-mention mortality—51 055. Hospital discharges—85 000.
Prevalence
A large population-based epidemiological study with systematic use of echocardiography on 16 501 participants from Olmsted County, MN, showed an overall age-adjusted prevalence of clinically diagnosed (moderate or greater) valvular HD of 1.8%.
-
Prevalence of any valve disease increased with age1:
18 to 44 years: 0.3% (95% CI, 0.2%–0.3%)
45 to 54 years: 0.7% (95% CI, 0.6%–0.9%)
55 to 64 years: 1.6% (95% CI, 1.4%–1.9%)
65 to 74 years: 4.4% (95% CI, 3.9%–4.9%)
≥75 years: 11.7% (95% CI, 11.0%–12.5%)
-
Pooled echocardiographic data from 11 911 participants from CARDIA (4351), ARIC (2435), and CHS (5125) demonstrated a similar increase in prevalence with age (Table 22-1) 1:
18 to 44 years: 0.7% (95% CI, 0.5%–1.0%)
45 to 54 years: 0.4% (95% CI, 0.1%–1.3%)
55 to 64 years: 1.9% (95% CI, 1.2%–2.8%)
65 to 74 years: 8.5% (95% CI, 7.6%–9.4%)
≥75 years: 13.3% (95% CI, 11.7%–15.0%)
Adjusted to the entire US population, these data suggest that the prevalence of any valve disease is 2.5% (95% CI, 2.2%–2.7%), with no difference between males (2.4% [95% CI, 2.1%–2.8%]) and females (2.5% [95% CI, 2.1%–2.9%]). Within this sample, 0.4% had aortic stenosis, 0.5% had aortic regurgitation, 0.1% had mitral stenosis, and 1.7% had mitral regurgitation.1
Aortic Valve Disorders
ICD-9 424.1; ICD-10 I35.
Mortality—17 136. Any-mention mortality—34 408. Hospital discharges—55 000.
Prevalence
The prevalence of moderate or severe aortic stenosis in patients ≥75 years old is 2.8% (95% CI, 2.1%–3.7%), and the prevalence of moderate or severe aortic regurgitation in patients ≥75 years is 2.0% (95% CI, 1.4%–2.7%).1
Nationally representative data from Sweden demonstrate a lower age-adjusted incidence of aortic stenosis, from 15.0 to 11.4 per 100 000 males and from 9.8 to 7.1 per 100 000 females, between the years 1989 to 1991 and 2007 to 2009.2
Multiple single-nucleotide polymorphisms that encode for LDL-C have been combined to form a genetic risk score that has been associated with prevalent aortic valve calcification (OR, 1.38; 95% CI, 1.09–1.74 per genetic risk score increment) and incident aortic valve stenosis (HR, 2.78; 95% CI, 1.22–6.37 per genetic risk score increment) by use of a mendelian randomization design.3
Before US Food and Drug Administration approval of TAVR in 2011, ≈50% of patients with severe aortic stenosis were referred for cardiothoracic surgery, and ≈40% underwent aortic valve replacement according to data from 10 US centers of various sizes and geographic distribution. Reasons for not undergoing aortic valve replacement included high perioperative risk, age, lack of symptoms, and patient/family refusal.4
From 2011 through 2014, the STS/ACC Transcatheter Valve Therapy Registry recorded 26 414 TAVR procedures performed at 348 centers in 48 US states.5 Sixty-eight percent of patients were ≥80 years of age, and preoperative risk was high; in 2014, median STS risk was 6.7%, and 95% of patients were deemed to be at “extreme” or “high” risk.
In a cohort of 416 community-based participants from Olmsted County, MN, with bicuspid aortic valves followed up for a mean (SD) of 16 (7) years, the incidence of aortic dissection in individuals ≥50 years of age at baseline was 17.4 (95% CI, 2.9–53.6) cases per 10 000 patient-years. For patients aged ≥50 years with a bicuspid valve and a baseline aortic aneurysm, the incidence of aortic dissection was 44.9 (95% CI, 7.5–138.5) cases per 10 000 patient-years. In the remaining participants without baseline aortic aneurysm, the incidence of aneurysm was 84.9 (95% CI, 63.3–110.9) cases per 10 000 patient-years, for an age-adjusted RR of 86.2 (95% CI, 65.1–114) compared with the general population.6
Mitral Valve Disorders
ICD-9 424.0; ICD-10 I34.
Mortality—2344. Any-mention mortality—5366. Hospital discharges—22 000.
Prevalence
(See Table 22-1)
In pooled data from CARDIA, ARIC, and CHS, mitral valve disease was the most common valvular lesion. At least moderate mitral regurgitation occurred at a frequency of 1.7% as adjusted to the US adult population in 2000, increasing from 0.5% in participants aged 18 to 44 years to 9.3% in participants aged ≥75 years.1
-
A systematic review by de Marchena and colleagues7 found that in the US population, the prevalence of mitral regurgitation according to the Carpentier functional classification system was as follows:
Type I (congenital mitral regurgitation and endocarditis): <20 per 1 million
Type II (myxomatous mitral regurgitation): 15 000 per 1 million
Type IIIa (rheumatic HD, systemic lupus erythematosus, antiphospholipid syndrome): 10 520 per 1 million
Type IIIb (ischemic mitral regurgitation, LV dysfunction, DCM): 23 250 per 1 million
Pulmonary Valve Disorders
ICD-9 424.3; ICD-10 I37.
Mortality—15. Any-mention mortality—50.
Tricuspid Valve Disorders
ICD-9 424.2; ICD-10 I36.
Mortality—24. Any-mention mortality—105.
Rheumatic Fever/Rheumatic Heart Disease
(See Table 22-2 and Charts 22-1 and 22-2)
Table 22-2.
Rheumatic Fever/Rheumatic Heart Disease
| Population Group | Mortality, 2014: All Ages* | Hospital Discharges, 2010: All Ages |
|---|---|---|
| Both sexes | 3281 | 20 000 |
| Males | 1083 (33.0%)† | 5000 |
| Females | 2198 (67.0%)† | 15 000 |
| NH white males | 866 | … |
| NH white females | 1818 | … |
| NH black males | 100 | … |
| NH black females | 165 | … |
| Hispanic males | 81 | |
| Hispanic females | 125 | |
| NH Asian or Pacific Islander males | 27‡ | … |
| NH Asian or Pacific Islander females | 68‡ | |
| NH American Indian or Alaska Native | 22 | … |
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for American Indian or Alaska Native and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total mortality that is for males vs females.
Includes Chinese, Filipino, Hawaiian, Japanese, and Other Asian or Pacific Islander.
Sources: Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death–United States; data represent underlying cause of death only. Hospital discharges: National Hospital Discharge Survey, National Center for Health Statistics, and National Heart, Lung, and Blood Institute; data include those inpatients discharged alive, dead, or of unknown status.
Chart 22-1. Rheumatic heart disease prevalence trends per 1000 people for each WHO region.
A, the Americas; B, Europe; C, Africa; D, Eastern Mediterranean; E, Western Pacific; and F, Southeast Asia.
WHO indicates World Health Organization. Reprinted from Seckeler and Hoke.21 Copyright © 2011, Seckeler and Hoke, publisher and licensee Dove Medical Press Ltd. This is an Open Access article which permits unrestricted noncommercial use, provided the original work is properly cited.
Chart 22-2. Age and sex distribution of 3343 subjects with rheumatic heart disease participating in the REMEDY study.
REMEDY indicates Global Rheumatic Heart Disease Registry. Reprinted from Zühlke et al10 by permission of Oxford University Press. Copyright © 2014, The Authors.
ICD-9 390 to 398; ICD-10 I00 to I09.
Mortality—3281. Any-mention mortality—6220. Hospital discharges—20 000.
Rheumatic HD is uncommon in high-income countries such as the United States but remains endemic in some low- and middle-income countries. Data from the 2013 GBD study suggest that 275 100 people (95% CI, 222 600–353 900) died of rheumatic HD in 2013, which is a 27% decline from the number of global deaths estimated in 1990. The GBD also estimates an age-adjusted mortality rate of 4.4 deaths per 100 000 (95% CI, 3.5–5.6) in 2013 attributable to rheumatic HD, which is a 55% lower rate than in 1990.8
Mortality attributable to rheumatic HD remains exceptionally high in endemic settings. In a study from Fiji of 2619 people followed up during 2008 to 2012, the age-standardized death rate was 9.9 (95% CI, 9.8–10.0) per 100 000, or over twice the GBD 2013 estimate.8a
The 2014 overall age-adjusted death rate for rheumatic fever or rheumatic HD in the United States was 0.9 per 100 000. Death rates varied across race/ethnic groups but were generally low: non-Hispanic white, 0.9 per 100 000; non-Hispanic black or African American, 0.7 per 100 000; non-Hispanic Asian or Pacific Islander, 0.6 per 100 000; non-Hispanic American Indian or Alaska Native, 1.1 per 100 000; and Hispanic or Latino-origin individuals, 0.6 per 100 000.9
In 1950, ≈15 000 Americans (adjusted for changes in ICD codes) died of rheumatic fever/rheumatic HD compared with ≈3100 annually in the present era (NCHS/NHLBI).
The REMEDY study is a prospective registry of 3343 patients with rheumatic HD from 25 hospitals in 12 African countries, India, and Yemen. The age and sex distribution of the subjects are shown in Chart 22-2. A study of the baseline characteristics of this cohort10 highlights consistently poor access to recommended therapies: only 55% were taking penicillin prophylaxis, and only 3.6% of females of childbearing age were using contraception. Although 70% of those with indications (mechanical valve, AF, or severe mitral stenosis) were appropriately prescribed anticoagulant drugs, only a quarter of these had therapeutic international normalized ratios.
Rheumatic HD was twice as common among females in REMEDY,10 a finding consistent with prior studies across a variety of populations.
Underrecognition of acute rheumatic fever may contribute to delayed presentation of disease and poor outcomes. Of the REMEDY participants from low-income countries, only 22% reported a history of prior acute rheumatic fever.10
Bacterial Endocarditis
(See Table 22-3)
ICD-9 421.0; ICD-10 I33.0.
Mortality—1256. Any-mention mortality—2564. Hospital discharges—34 000, primary plus secondary diagnoses.
According to the 2013 GBD study, the age-standardized death rate attributable to endocarditis in 2013 was 1.0 per 100 000 (95% CI, 0.8–1.3), which represents a 13% median decrease since 1990. However, because of population growth and aging, the number of deaths attributable to endocarditis increased from 45 100 (95% CI, 35 600–58 600) in 1990 to 65 000 (95% CI, 48 600–79 400) in 2013.8
-
Although the absolute risk for acquiring IE from a dental procedure is impossible to measure precisely, the best available estimates are as follows: If dental treatment causes 1% of all cases of viridans group streptococcal IE annually in the United States, the overall risk in the general population is estimated to be as low as 1 case of IE per 14 million dental procedures. The estimated absolute risk rates for acquiring IE from a dental procedure in patients with underlying cardiac conditions are as follows11:
Mitral valve prolapse: 1 per 1.1 million procedures
CHD: 1 per 475 000
Rheumatic HD: 1 per 142 000
Presence of a prosthetic cardiac valve: 1 per 114 000
Previous IE: 1 per 95 000 dental procedures
Data collected between 2004 and 2010 from the Pediatric Health Information System database from 37 centers that included 1033 cases of IE demonstrate a mortality rate of 6.7% (n=45) and 3.5% (n=13) among children (0 to 19 years) with and without congenital HD, respectively.12
Data from the NIS (2000–2011)13 suggest no change in temporal trends in the incidence of IE before and after publication of the 2007 AHA guideline for antibiotic prophylaxis before dental procedures.11 These findings from referral centers were corroborated by a community-based review of adults in Olmsted County, MN.14 In this study, age- and sex-adjusted incidence of IE was 7.4 (95% CI, 5.3–9.4) cases per 100 000 person-years. In addition, these guideline changes do not appear to have altered rates of pediatric endocarditis. Using 2003 to 2010 data from 37 centers in the Pediatric Health Information Systems Database, Pasquali and colleagues15 did not demonstrate a significant difference in the number of IE hospitalizations after the guidelines were implemented in 2007 (1.6% difference after versus before guideline implementation; 95% CI, −6.4% to 10.3%; P=0.7).
A systematic review that included 160 studies and 27 083 patients from 1960 to 2011 demonstrated that in hospital-based studies (142 studies; 23 606 patients), staphylococcal endocarditis has increased over time (coagulase-negative Staphylococcus 2% to 10%, P<0.001), with recent increases in S aureus (21% to 30%, P<0.05) and enterococcal IE (6.8% to 10.5%, P<0.001) over the past decade and a corresponding decrease in streptococcal endocarditis (32% to 17%) over the same time period.16
Cardiac device IE appears to be present in 6.4% (95% CI, 5.5%–7.4%) of patients with definite IE, according to data from ICE-PCS (2000–2006). Nearly half (45.8%; 95% CI, 38.3%–53.4%) of such cases are related to healthcare-associated infection. In-hospital and 1-year mortality rates for these patients were 14.7% (26/177; 95% CI, 9.8%–20.8%) and 23.2% (41/177; 95% CI, 17.2%–30.1%), respectively.17
Prosthetic valve IE continues to be associated with high in-hospital and 1-year mortality, although early surgery is associated with improved outcomes compared with medical therapy alone (1-year mortality 22% versus 27%; HR, 0.68; 95% CI, 0.53–0.87), even in propensity-adjusted analyses (HR, 0.57; 95% CI, 0.49–0.67).18
Surgery was performed in 57% of cases of definite left-sided, non–cardiac device-related IE in the ICE-PLUS registry of 1296 patients from 16 countries; however, approximately one quarter of those with a surgical indication did not undergo surgery.19
Endocarditis, Valve Unspecified
ICD-9 424.9; ICD-10 I38.
Mortality—5595. Any-mention mortality—11 360.
Heart Valve Procedures
In 2013, for heart valve procedures:
The mean inflation-adjusted cost per hospitalization in 2013 dollars was $51 415, compared with $53 711 in 2005 and $43 829 in 2000.20
The number of discharges for which heart valve surgery was the principal operating room procedure was 102 425, which was an increase from 93 802 in 2005 and 79 719 in 2000.20
Total inflation-adjusted national costs in 2013 dollars (in millions) was $5264, which was an increase from the mean cost in millions of $5058 in 2005 and $3488 in 2000.20
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Martinsson A, Li X, Andersson C, Nilsson J, Smith JG, Sundquist K. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015;131:988–994. doi: 10.1161/CIRCULATIONAHA.114.012906. [DOI] [PubMed] [Google Scholar]
- 3.Smith JG, Luk K, Schulz CA, Engert JC, Do R, Hindy G, Rukh G, Dufresne L, Almgren P, Owens DS, Harris TB, Peloso GM, Kerr KF, Wong Q, Smith AV, Budoff MJ, Rotter JI, Cupples LA, Rich S, Kathiresan S, Orho-Melander M, Gudnason V, O’Donnell CJ, Post WS, Thanassoulis G Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Extracoronary Calcium Working Group. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach DS. Prevalence and characteristics of unoperated patients with severe aortic stenosis. J Heart Valve Dis. 2011;20:284–291. [PubMed] [Google Scholar]
- 5.Holmes DR, Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ STS/ACC TVT Registry. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813–2823. doi: 10.1016/j.jacc.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, 3rd, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 7.de Marchena E, Badiye A, Robalino G, Junttila J, Atapattu S, Nakamura M, De Canniere D, Salerno T. Respective prevalence of the different Carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg. 2011;26:385–392. doi: 10.1111/j.1540-8191.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2013 Mortality and Causes of Death Investigators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6737(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Parks T, Kado J, Miller AE, Ward B, Heenan R, Colquhoun SM, et al. Rheumatic heart disease-attributable mortality at ages 5–69 years in Fiji: a five-year, national, population-based record-linkage cohort study. PLoS Negl Trop Dis. 2015;9:e0004033. doi: 10.1371/journal.pntd.0004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics. [Accessed May 2016];Public use data sets for final US 2014 mortality tabulated by the National Heart, Lung, and Blood Institute: mortality multiple cause-of-death public use record. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 10.Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–1122a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377] Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 12.Ware AL, Tani LY, Weng HY, Wilkes J, Menon SC. Resource utilization and outcomes of infective endocarditis in children. J Pediatr. 2014;165:807–812.e1. doi: 10.1016/j.jpeds.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 14.DeSimone DC, Tleyjeh IM, Correa de Sa DD, Anavekar NS, Lahr BD, Sohail MR, Steckelberg JM, Wilson WR, Baddour LM. Temporal trends in infective endocarditis epidemiology from 2007 to 2013 in Olmsted County, MN. Am Heart J. 2015;170:830–836. doi: 10.1016/j.ahj.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquali SK, He X, Mohamad Z, McCrindle BW, Newburger JW, Li JS, Shah SS. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Am Heart J. 2012;163:894–899. doi: 10.1016/j.ahj.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slipczuk L, Codolosa N, Carlos D, Romero-Corral A, Pressman G, Figueredo V. Systematic review & meta-analysis of infective endocarditis microbiology over 5 decades. Circulation. 2012;126:A15138. Abstract. [Google Scholar]
- 17.Athan E, Chu VH, Tattevin P, Selton-Suty C, Jones P, Naber C, Miró JM, Ninot S, Fernández-Hidalgo N, Durante-Mangoni E, Spelman D, Hoen B, Lejko-Zupanc T, Cecchi E, Thuny F, Hannan MM, Pappas P, Henry M, Fowler VG, Jr, Crowley AL, Wang A ICE-PCS Investigators. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA. 2012;307:1727–1735. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- 18.Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante-Mangoni E, Fowler VG, Jr, Gordon D, Grossi P, Hannan M, Hoen B, Muñoz P, Rizk H, Kanj SS, Selton-Suty C, Sexton DJ, Spelman D, Ravasio V, Tripodi MF, Wang A International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis [published correction appears in JAMA Intern Med. 2013;173:1846] JAMA Intern Med. 2013;173:1495–1504. doi: 10.1001/jamainternmed.2013.8203. [DOI] [PubMed] [Google Scholar]
- 19.Chu VH, Park LP, Athan E, Delahaye F, Freiberger T, Lamas C, Miro JM, Mudrick DW, Strahilevitz J, Tribouilloy C, Durante-Mangoni E, Pericas JM, Fernández-Hidalgo N, Nacinovich F, Rizk H, Krajinovic V, Giannitsioti E, Hurley JP, Hannan MM, Wang A International Collaboration on Endocarditis (ICE) Investigators. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015;131:131–140. doi: 10.1161/CIRCULATIONAHA.114.012461. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2016. [Accessed Aug. 24, 2016]. http://www.cdc.gov/nchs/data/hus/hus15.pdf. [PubMed] [Google Scholar]
- 21.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
23. VENOUS THROMBOEMBOLISM (DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM), CHRONIC VENOUS INSUFFICIENCY, PULMONARY HYPERTENSION
Pulmonary Embolism
ICD-9 415.1; ICD-10 I26.
Mortality—8159. Any-mention mortality—32 099. Hospital discharges—186 000.
Deep Vein Thrombosis
ICD-9 451.1; ICD-10 I80.2.
Mortality—2621. Any-mention mortality—13 876.
Abbreviations Used in Chapter 23
| CDC | Centers for Disease Control and Prevention |
| CI | confidence interval |
| CT | computed tomography |
| CVI | chronic venous insufficiency |
| DM | diabetes mellitus |
| DVT | deep vein thrombosis |
| FHS | Framingham Heart Study |
| GWAS | genome-wide association study |
| HD | heart disease |
| HIV | human immunodeficiency virus |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| NIS | Nationwide Inpatient Sample |
| PE | pulmonary embolism |
| REGARDS | Reasons for Geographic and Racial Differences in Stroke |
| RR | relative risk |
| RV | right ventricular |
| VTE | venous thromboembolism |
| WHO | World Health Organization |
Incidence
Information on incidence is limited in the United States, but the CDC estimates an annual incidence of 300 000 to 600 000 VTE events; however, these data are derived from estimates that are ≥10 years old.1–3 Estimates are underestimates, because VTE can be missed or misdiagnosed, and fatal PE is underascertained because of low autopsy rates.
A modeling study estimated VTE incidence in 6 countries in Europe, accounting for factors such as missed diagnosis related to either lack of routine autopsy, misdiagnosis, or underdiagnosis because of lack of symptom recognition.4 The authors estimated annual numbers of DVT, PE, and VTE-related deaths as 465 715 (95% CI, 404 664–538 189), 295 982 (95% CI, 242 450–360 363), and 370 012 (95% CI, 300 193–483 108), respectively. Of these deaths, only 7% were diagnosed antemortem, 34% were sudden fatal PE, and 59% followed undiagnosed PE.
In the contemporary REGARDS cohort of 30 239 black and white adults ≥45 years old recruited from 2003 to 2007 and followed up for ≈2 years, age-standardized incidence rates of VTE were 1.4 to 2.2 per 1000 person-years.5
VTE incidence appears to be similar or higher among African Americans and lower among Asian Americans and Native Americans than among whites.6
Incidence rates increase exponentially with age for both males and females and for both DVT and PE.1,7,8
Incidence rates are higher in females than males during childbearing years, whereas incidence rates after age 45 years are higher in males.
PE accounts for an increasing proportion of VTE with increasing age in both sexes.
Patients with VTE are increasingly treated as outpatients, but data are not available documenting the frequency.
Lifetime Risk
In 2 US cohorts including 19 599 males and females aged 45 to 99 years at baseline and followed up for 288 535 person-years, the remaining lifetime risk of VTE at age 45 years was 8.1% (95% CI, 7.1%–8.7%) overall, 11.5% in African Americans, 10.9% in those with obesity, 17.1% in individuals with factor V Leiden, and 18.2% in people with sickle cell trait or disease.9
Mortality
Data from 1999 show that 30-day VTE survival is 72.0% (DVT alone, 94.5%, compared with PE with or without DVT, 55.6%).10
Data from a Worcester, MA, surveillance study11 from 1999 to 2009 suggested a decline in 3-year VTE-related mortality (from 41% to 26%). Because most PE deaths are sudden and may be attributed to other diseases (eg, cancer, other chronic lung disease, or HD), secular trends in fatal PE are unclear as a result of low autopsy rates.
Recurrence
VTE is a chronic disease with episodic recurrence; in the absence of long-term anticoagulation, ≈30% of patients develop recurrence within the next 10 years.6
Data from a Worcester, MA, surveillance study from 1999 to 2009 suggested a declining rate of recurrent VTE (from 17% to 9%), perhaps attributable to increased use of long-term anticoagulation.11
Independent predictors of early (within 180 days) recurrence include active cancer and inadequate anticoagulation. Two-week case fatality for recurrent DVT alone and recurrent PE with or without DVT is 2% and 11%, respectively.12
Data from a Worcester, MA, surveillance study from 1999 to 2009 suggested a declining 3-year rate of major bleeding after a VTE (from 12% to 6%).11
Complications
The 20-year cumulative incidence of postthrombotic syndrome/venous stasis syndrome and venous ulcer after proximal DVT is 40% and 3.7%, respectively.13
The incidence of chronic thromboembolic pulmonary hypertension is 6.5 per million person-years; ≈1400 incident cases occur annually among US whites.14
Costs
Grosse et al15 conducted a literature review that estimated the incremental direct medical costs per case among 1-year survivors (2014 US dollars) of acute VTE treatment at $12 000 to $15 000 and the cost of complications including recurrent VTE, postthrombotic syndrome, chronic thromboembolic pulmonary hypertension, and anticoagulation-related adverse events at $18 000 to $23 000 per case. Assuming 375 000 to 425 000 new cases annually, overall cost was estimated at $7 to 10 billion annually.15
Risk Factors
Approximately 20% of VTEs are provoked because of immobilization, trauma surgery, or hospitalization in the antecedent 3 months; 30% are associated with cancer; and 50% are unprovoked.7
Independent VTE risk factors include increasing patient age, surgery, trauma/fracture, hospital or nursing home confinement, active cancer, central venous catheter or transvenous pacemaker, prior superficial vein thrombosis, infection, varicose veins, inherited thrombophilia, kidney disease, neurological disease with leg paresis, sickle cell anemia and sickle cell trait, and among females, use of oral contraceptives, pregnancy/postpartum period, and hormone therapy.16–18
Among patients hospitalized for acute medical illness, independent risk factors for VTE include prior VTE, thrombophilia, cancer, age >60 years, leg paralysis, immobilization ≥7 days, and admission to an intensive care unit or coronary care unit.19
Pregnancy-associated VTE has an incidence of 200 per 100 000 female-years; compared with nonpregnant females of childbearing age, the RR for VTE is increased 4-fold. In pregnancy, 80% of events are DVT and 20% are PE.20 Approximately one third of the DVT events and one half of the PE events occur after delivery,21 with the RR being 21- to 84-fold increased within 6 weeks postpartum compared with the nonpregnant, nonpostpartum state.22 VTE risk appears to be higher for pregnancies after in vitro fertilization than for natural pregnancies, 23 as well as in association with the usual VTE risk factors and with multiple gestation and cesarean delivery.
VTE risk during the postpartum period is ≈5-fold higher than during pregnancy.
VTE is highly heritable,24,25 and a variety of coagulation genes have been implicated. Recently, a GWAS of >65 000 individuals implicated 2 new susceptibility loci, TSPAN15 and SLC44A2.26
Chronic Venous Insufficiency
ICD-10 I87.2.
Mortality—41. Any-mention mortality—461.
Prevalence
It is estimated that varicose veins affect 25 million US adults. More severe venous disease affects 6 million.27
Incidence
The FHS reported an annual incidence of varicose veins of 2.6% in females and 1.9% in males.28
Complications
Venous ulcer is a substantial morbidity of CVI. Estimated prevalence in adults is ≈0.3%, and incidence is ≈20% of those with CVI.
Analysis of NIS data for black and white Americans demonstrated declines in ulcer debridement, vein stripping, and sclerotherapy procedures from 1998 to 2011. Blacks presented at younger ages and more often had ulcer debridement and history of DVT than whites.29
Cost
Estimated cost in the United States to treat venous ulcers is $1 billion annually.30
Risk Factors
Moderate CVI increases with age, family history, hernia surgery, obesity, number of births, and presence of flat feet in females and is less likely in those with hypertension; risk factors for more severe CVI include smoking in males and leg injury in females.31 Blood coagulation disorders and inflammatory biomarkers, which are risk factors for DVT, are also associated with an increased risk of CVI, consistent with the hypothesis that venous thrombosis predisposes to CVI.7,32
Postthrombotic syndrome, a subset of CVI discussed above, has specific risk factors that can be identified at the time of or after DVT: recurrent ipsilateral DVT (RR, 2–6), obesity (RR, 2), more extensive DVT (RR, 2), poor quality of initial anticoagulation (RR, 2), ongoing symptoms or signs of DVT 1 month after diagnosis (RR, 4), and elevated D-dimer at 1 month (RR, 4.6–9.9).33,34
Varicose veins are more likely to occur in the setting of a positive family history, consistent with a heritable component. Although a number of genes have been implicated, the genetic factors predisposing to varicose veins have not been definitively identified.35
Pulmonary Hypertension
ICD-10 I27.0, I27.2, I27.9.
Mortality—6385. Any-mention mortality—20 084.
Prevalence and Incidence
The prevalence of WHO group 1 pulmonary hypertension (idiopathic, heritable, drug/toxin induced, or associated with other factors including connective tissue disease, infections [HIV, schistosomiasis], portal hypertension, and congenital HD) is estimated at 6.6 to 26.0 per million adults and the incidence at 1.1 to 7.6 per million adults annually.36
WHO group 2 pulmonary hypertension is attributable to left-sided HD.
The prevalence and incidence of WHO group 3 pulmonary hypertension (atrributable to lung disease or hypoxia) is difficult to estimate and would track with lung disease prevalence.
The prevalence of WHO group 4 pulmonary hypertension (chronic thromboembolic pulmonary hypertension and other pulmonary obstructions) ranges from 1.0% to 8.8% among those with PE.36 The incidence is 6.5 per million person-years; ≈1400 incident cases occur annually among US whites.14
The prevalence of WHO group 5 pulmonary hypertension (with multifactorial mechanisms) with sickle cell disease increases with age and has a prevalence of 6% to 10%, and the prevalence with thalassemia is 2.1%.36,37
Mortality
Mortality of pulmonary hypertension depends on the cause and treatment.
In sickle cell disease–related pulmonary hypertension, the 5-year survival rate in one study was 63% with pulmonary hypertension and 83% without it.38
An international prospective registry that included 679 patients with chronic thromboembolic pulmonary hypertension estimated 3-year survival was 89% with and 70% without pulmonary thromboendarterectomy. 39 Among the patients with chronic thromboembolic pulmonary hypertension, treatments for pulmonary hypertension did not affect survival; high New York Heart Association functional class, increased right atrial pressure, and history of cancer were associated with mortality regardless of surgery.
Risk Factors
Risk factors are implicit in the WHO disease classification of the 5 mechanistic subtypes of pulmonary hypertension described above. The most common risk factors are left-sided HD and lung disease.
In a study of 772 consecutive PE patients without major comorbidity such as cancer, the risk factors for chronic thromboembolic pulmonary hypertension were unprovoked PE, hypothyroidism, symptom onset >2 weeks before PE diagnosis, RV dysfunction on CT or echocardiography, DM, and thrombolytic therapy or embolectomy; a risk prediction score including these factors was able to predict a group with a chronic thromboembolic pulmonary hypertension incidence of 10% (95% CI, 6.5%–15%).40
Global Burden
80% of patients with pulmonary hypertension live in developing countries, and the cause of their pulmonary hypertension is HD and lung disease, but schistosomiasis, rheumatic HD, HIV, and sickle cell disease remain prominent compared with developed countries. In these countries, younger people are more often affected (average age of onset <40 years).36
REFERENCES
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, Becker RC, Goldberg RJ. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M VTE Impact Assessment Group in Europe (VITAE) Venous thromboembolism (VTE) in Europe: the number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 5.Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, Cushman M. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–1509. doi: 10.1161/CIRCULATIONAHA.113.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 9.Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd-Jones DM, Folsom AR. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129:339.e19–339.e26. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999;159:445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Goldberg RJ, Cohen AT, Anderson FA, Kiefe CI, Gore JM, Spencer FA. Declining long-term risk of adverse events after first-time community-presenting venous thromboembolism: the population-based Worcester VTE Study (1999 to 2009) Thromb Res. 2015;135:1100–1106. doi: 10.1016/j.thromres.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heit JA, Lahr BD, Petterson TM, Bailey KR, Ashrani AA, Melton LJ., 3rd Heparin and warfarin anticoagulation intensity as predictors of recurrence after deep vein thrombosis or pulmonary embolism: a population-based cohort study. Blood. 2011;118:4992–4999. doi: 10.1182/blood-2011-05-357343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc. 2000;75:1249–1256. doi: 10.4065/75.12.1249. [DOI] [PubMed] [Google Scholar]
- 14.Dunn WF, Heit JA, Farmer SA, Petterson TM, Ballman KV. The incidence of chronic thromboembolic pulmonary hypertension (CTEPH): a 21-year population-based study. Presented at: European Respiratory Society 13th Annual Congress; September 27, 2003–October 2, 2003; Vienna, Austria. [Google Scholar]
- 15.Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. doi: 10.1016/j.thromres.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkin L, Sweetland S, Balkwill A, Green J, Reeves G, Beral V Million Women Study Collaborators. Body mass index, surgery, and risk of venous thromboembolism in middle-aged women: a cohort study. Circulation. 2012;125:1897–1904. doi: 10.1161/CIRCULATIONAHA.111.063354. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoodi BK, Gansevoort RT, Næss IA, Lutsey PL, Brækkan SK, Veeger NJ, Brodin EE, Meijer K, Sang Y, Matsushita K, Hallan SI, Hammerstrøm J, Cannegieter SC, Astor BC, Coresh J, Folsom AR, Hansen JB, Cushman M. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964–1971. doi: 10.1161/CIRCULATIONAHA.112.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folsom AR, Tang W, Roetker NS, Kshirsagar AV, Derebail VK, Lutsey PL, Naik R, Pankow JS, Grove ML, Basu S, Key NS, Cushman M. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13:2–9. doi: 10.1111/jth.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spyropoulos AC, Anderson FA, Jr, Fitzgerald G, Decousus H, Pini M, Chong BH, Zotz RB, Bergmann JF, Tapson V, Froehlich JB, Monreal M, Merli GJ, Pavanello R, Turpie AG, Nakamura M, Piovella F, Kakkar AK, Spencer FA IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 20.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29:326–331. doi: 10.1161/ATVBAHA.109.184127. [DOI] [PubMed] [Google Scholar]
- 22.Jackson E, Curtis KM, Gaffield ME. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol. 2011;117:691–703. doi: 10.1097/AOG.0b013e31820ce2db. [DOI] [PubMed] [Google Scholar]
- 23.Henriksson P, Westerlund E, Wallén H, Brandt L, Hovatta O, Ekbom A. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ. 2013;346:e8632. doi: 10.1136/bmj.e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zöller B, Li X, Sundquist J, Sundquist K. A nationwide family study of pulmonary embolism: identification of high risk families with increased risk of hospitalized and fatal pulmonary embolism. Thromb Res. 2012;130:178–182. doi: 10.1016/j.thromres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Zöller B, Ohlsson H, Sundquist J, Sundquist K. Familial risk of venous thromboembolism in first-, second- and third-degree relatives: a nationwide family study in Sweden [published correction appears in Thromb Haemost. 2013;110:204] Thromb Haemost. 2013;109:458–463. doi: 10.1160/TH12-10-0743. [DOI] [PubMed] [Google Scholar]
- 26.Germain M, Chasman DI, de Haan H, Tang W, Lindström S, Weng LC, de Andrade M, de Visser MC, Wiggins KL, Suchon P, Saut N, Smadja DM, Le Gal G, van Hylckama Vlieg A, Di Narzo A, Hao K, Nelson CP, Rocanin-Arjo A, Folkersen L, Monajemi R, Rose LM, Brody JA, Slagboom E, Aïssi D, Gagnon F, Deleuze JF, Deloukas P, Tzourio C, Dartigues JF, Berr C, Taylor KD, Civelek M, Eriksson P, Psaty BM, Houwing-Duitermaat J, Goodall AH, Cambien F, Kraft P, Amouyel P, Samani NJ, Basu S, Ridker PM, Rosendaal FR, Kabrhel C, Folsom AR, Heit J, Reitsma PH, Trégouët DA, Smith NL, Morange PE Cardiogenics Consortium. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96:532–542. doi: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4:96–101. [PubMed] [Google Scholar]
- 29.Dua A, Desai SS, Heller JA. The impact of race on advanced chronic venous insufficiency. Ann Vasc Surg. 2016;34:152–156. doi: 10.1016/j.avsg.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130:333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 31.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg. 2007;46:331–337. doi: 10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cushman M, Callas PW, Denenberg JO, Bovill EG, Criqui MH. Risk factors for peripheral venous disease resemble those for venous thrombosis: the San Diego Population Study. J Thromb Haemost. 2010;8:1730–1735. doi: 10.1111/j.1538-7836.2010.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, Weitz JI American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association [published correction appears in Circulation. 2015;131:e359] Circulation. 2014;130:1636–1661. doi: 10.1161/CIR.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovich A, Cohen JM, Kahn SR. The predictive value of markers of fibrinolysis and endothelial dysfunction in the post thrombotic syndrome: a systematic review. Thromb Haemost. 2014;111:1031–1040. doi: 10.1160/TH13-11-0931. [DOI] [PubMed] [Google Scholar]
- 35.Anwar MA, Georgiadis KA, Shalhoub J, Lim CS, Gohel MS, Davies AH. A review of familial, genetic, and congenital aspects of primary varicose vein disease. Circ Cardiovasc Genet. 2012;5:460–466. doi: 10.1161/CIRCGENETICS.112.963439. [DOI] [PubMed] [Google Scholar]
- 36.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–322. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 37.Derchi G, Galanello R, Bina P, Cappellini MD, Piga A, Lai ME, Quarta A, Casu G, Perrotta S, Pinto V, Musallam KM, Forni GL Webthal Pulmonary Arterial Hypertension Group. Prevalence and risk factors for pulmonary arterial hypertension in a large group of β-thalassemia patients using right heart catheterization: a Webthal study. Circulation. 2014;129:338–345. doi: 10.1161/CIRCULATIONAHA.113.002124. [DOI] [PubMed] [Google Scholar]
- 38.Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, Xu D, Seamon C, Adams-Graves P, Castro OL, Minniti CP, Sachdev V, Taylor JG, 6th, Kato GJ, Machado RF. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187:840–847. doi: 10.1164/rccm.201207-1222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, Bresser P, Torbicki A, Mellemkjaer S, Lewczuk J, Simkova I, Barberà JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez-Sanchez MA, Kovacs G, Jaïs X, Ambroz D, Treacy C, Morsolini M, Jenkins D, Lindner J, Dartevelle P, Mayer E, Simonneau G. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 40.Klok FA, Dzikowska-Diduch O, Kostrubiec M, Vliegen HW, Pruszczyk P, Hasenfuß G, Huisman MV, Konstantinides S, Lankeit M. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thromb Haemost. 2016;14:121–128. doi: 10.1111/jth.13175. [DOI] [PubMed] [Google Scholar]
24. PERIPHERAL ARTERY DISEASE AND AORTIC DISEASES
ICD-9 440.20 to 440.24, 440.30 to 440.32, 440.4, 440.9, 443.9, 445.02; ICD-10 I70.2, I70.9, I73.9, I74.3, I74.4.
See Table 24-1 and Charts 24-1 24-2 24-3 24-4 24-5 24-6
Table 24-1.
Peripheral Artery Disease
| Population Group | Prevalence, Age ≥40 y | Mortality, 2014, All Ages* | Hospital Discharges, 2010, All Ages |
|---|---|---|---|
| Both sexes | ≥6.8 Million | 13 424 | 146 000 |
| Males | … | 6006 (44.7%)† | 84 000 |
| Females | … | 7418 (55.3%)† | 62 000 |
| NH white males | … | 4805 | … |
| NH white females | … | 6098 | … |
| NH black males | … | 706 | … |
| NH black females | … | 828 | … |
| Hispanic males | … | 338 | … |
| Hispanic females | … | 343 | … |
| NH Asian or Pacific Islander males | … | 95‡ | … |
| NH Asian or Pacific Islander females | … | 103‡ | … |
| NH American Indian/Alaska Native | … | 63 | … |
Ellipses (…) indicate data not available; and NH, non-Hispanic.
Mortality for Hispanic, American Indian or Alaska Native, and Asian and Pacific Islander people should be interpreted with caution because of inconsistencies in reporting Hispanic origin or race on the death certificate compared with censuses, surveys, and birth certificates. Studies have shown underreporting on death certificates of American Indian or Alaska Native, Asian and Pacific Islander, and Hispanic decedents, as well as undercounts of these groups in censuses.
These percentages represent the portion of total mortality attributable to peripheral artery disease that is for males vs females.
Includes Chinese, Filipino, Hawaiian, Japanese, and Other Asian or Pacific Islander.
Sources: Prevalence: Data derived from Allison et al.1 Prevalence of peripheral artery disease is based on an ankle-brachial index <0.9 or a previous revascularization for peripheral artery disease. Mortality: Centers for Disease Control and Prevention/National Center for Health Statistics, 2014 Mortality Multiple Cause-of-Death–United States.
Chart 24-1. Estimates of prevalence of peripheral artery disease in males by age and ethnicity.
Amer. indicates American; and NH, non-Hispanic.
Data derived from Allison et al.1
Chart 24-2. Estimates of prevalence of peripheral artery disease in females by age and ethnicity.
Amer. indicates American; and NH, non-Hispanic.
Data derived from Allison et al.1
Chart 24-3. Prevalence of peripheral artery disease by age in males and females in high-income countries and low-income or middle-income countries.
Data derived from Fowkes et al.34
Chart 24-4. Hazard ratios of cardiovascular mortality with 95% CI by ABI categories.
ABI indicates ankle-brachial index; and CI, confidence interval.
Data derived from Fowkes et al.7
Chart 24-5. Association between the diameter and the minimum and maximum risk of AAA rupture per year.
AAA indicates abdominal aortic aneurysm.
Data derived from Brewster et al.62
Chart 24-6. Numbers needed to screen to avoid an AAA-associated death and a ruptured AAA.
AAA indicates abdominal aortic aneurysm.
Data derived from Eckstein et al.44
Abbreviations Used in Chapter 24
| AAA | abdominal aortic aneurysm |
| ABI | ankle brachial index |
| AHA | American Heart Association |
| Amer. | American |
| CHD | coronary heart disease |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| ED | emergency department |
| GBD | Global Burden of Diseases, Injuries, and Risk Factors Study |
| HF | heart failure |
| HIC | high-income countries |
| HR | hazard ratio |
| ICD | International Classification of Diseases |
| ICD-9 | International Classification of Diseases, 9th Revision |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IRAD | International Registry of Acute Aortic Dissection |
| LIMC | low- and middle-income countries |
| MI | myocardial infarction |
| NCHS | National Center for Health Statistics |
| NH | non-Hispanic |
| NHAMCS | National Hospital Ambulatory Medical Care Survey |
| NHDS | National Hospital Discharge Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| NIS | Nationwide Inpatient Sample |
| OR | odds ratio |
| OVER | Open Versus Endovascular Repair |
| PA | physical activity |
| PAD | peripheral artery disease |
| REACH | Reduction of Atherothrombosis for Continued Health |
| RR | relative risk |
| YLL | years of life lost |
Peripheral Artery Disease
Prevalence and Incidence
(See Table 24-1 and Charts 24-1 and 24-3)
On the basis of data from several US cohorts during the 1970s to 2000s and the 2000 US Census, 6.5 million Americans aged ≥40 years (5.5%) are estimated to have low ABI (<0.9).1 Of these, one fourth have severe PAD (ABI <0.7).2
Further accounting for PAD cases with ABI >0.9 (after revascularization or false-negative results with ABI), PAD is estimated to affect ≈8.5 million Americans aged ≥40 years (7.2%).1
The highest prevalence of low ABI (<0.9) has been observed among older adults (22.7% among individuals aged ≥80 years versus 1.6% among those aged 40–49 years) and non-Hispanic blacks (≈9% in non-Hispanic blacks versus ≈5.5% in whites).1 The prevalence of low ABI (<0.9) is similar between females (5.9%) and males (5.0%).
Only ≈10% of people with PAD have the classic symptom of intermittent claudication. Approximately 40% do not complain of leg pain, whereas the remaining 50% have a variety of leg symptoms different from classic claudication.3,4
On the basis of ICD codes in nationwide claims data from large employers’ health plans and from Medicare and Medicaid programs between 2003 and 2008, among adults aged >40 years, the annual incidence and prevalence of PAD were 2.69% and 12.02%, respectively.5 The corresponding estimates for critical limb ischemia, the most severe form of PAD, were 0.35% and 1.33%, respectively.
Mortality
(See Table 24-1 and Chart 24-4)
The 2014 overall any-mention age-adjusted death rate for PAD was 16.2 per 100 000. Any-mention death rates in males were 19.9 for non-Hispanic whites, 24.8 for non-Hispanic blacks, 8.5 for non-Hispanic Asians or Pacific Islanders, 20.8 for non-Hispanic American Indians or Alaska Natives, and 15.4 for Hispanic males. In females, rates were 13.8 for non-Hispanic whites, 16.5 for non-Hispanic blacks, 6.8 for non-Hispanic Asians or Pacific Islanders, 16.1 for non-Hispanic American Indians or Alaska Natives, and 10.7 for Hispanic females.6
The number of any-mention deaths attributable to PAD declined from 81 765 in 2004 to 59 681 in 2014 (source: NCHS, AHA). PAD was the underlying cause in 13 424 of those deaths in 2014.6 Table 24-1 shows the numbers of these deaths that were coded for PAD as the underlying cause.
A 2008 meta-analysis of 24 955 males and 23 339 females from 16 cohorts demonstrated a reverse-J-shaped association between ABI and mortality in which participants with an ABI of 1.11 to 1.40 were at lowest risk for mortality. In males, low ABI (≤0.9) carried a 3-fold (RR, 3.33; 95% CI, 2.74–4.06) risk of all-cause death compared with a normal ABI (1.11–1.40), and a similar risk was observed in females (RR, 2.71; 95% CI, 2.03–3.62).7 A similar pattern was observed for cardiovascular mortality.
In-hospital mortality was higher in females than males, regardless of disease severity or procedure performed, even after adjustment for age and baseline comorbidities: 0.5% versus 0.2% after percutaneous transluminal angioplasty or stenting for intermittent claudication; 1.0% versus 0.7% after open surgery for intermittent claudication; 2.3% versus 1.6% after percutaneous transluminal angioplasty or stenting for critical limb ischemia; and 2.7% versus 2.2% after open surgery for critical limb ischemia (P<0.01 for all comparisons).8
Data from the GBD project suggest that the age-standardized death rate attributable to PAD was 1.7 (95% CI, 1.0–2.9) per 100 000 in 2010, with a 42% median increase since 1990. The YLL because of PAD was 21.2 (95% CI, 13.4–35.9) in 2010, with a 29% median increase since 1990.9
Progression of PAD as measured by a decline in ABI also carries prognostic value beyond single measurements.10 Among 508 patients (449 males) identified from 2 vascular laboratories in San Diego, CA, a decline in ABI of >0.15 within a 10-year period was associated with a subsequent increased risk of all-cause mortality (RR, 2.4; 95% CI, 1.2–4.8) and CVD mortality (RR, 2.8; 95% CI, 1.3–6.0) at 3 years’ follow-up.10
Among 400 patients with PAD confirmed with digital subtraction angiography, aortoiliac (proximal) disease was associated with an increased risk of mortality or cardiovascular events compared with infrailiac (distal) disease (adjusted HR, 3.28; 95% CI, 1.87–5.75).11 Compared with infrailiac PAD, aortoiliac PAD was associated with younger age, male sex, and smoking.
Complications
Pooled data from 11 studies in 6 countries found that PAD (defined by ABI <0.9) is a marker for systemic atherosclerotic disease and adverse clinical outcomes. The pooled age-, sex-, risk factor–, and CVD-adjusted RRs were 1.45 (95% CI, 1.08–1.93) for CHD and 1.35 (95% CI, 1.10–1.65) for stroke.12
From 2000 to 2008, the overall rate of lowerextremity amputation decreased significantly during the study period, from 7258 to 5790 per 100 000 Medicare beneficiaries with PAD. There was significant geographic variation in the rate of lower-extremity amputation, from 8400 amputations per 100 000 patients with PAD in the East South Central region to 5500 amputations per 100 000 patients with PAD in the Mountain region. After adjustment for clustering at the US Census Bureau level, geographic variation in lower-extremity amputations remained. Lower-extremity amputation was performed more often in the East South Central region (adjusted OR, 1.152; 95% CI, 1.131–1.174; P<0.001) and West South Central region (adjusted OR, 1.115; 95% CI, 1.097–1.133; P<0.001) and less often in the Middle Atlantic region (OR, 0.833; 95% CI, 0.820–0.847; P<0.001) than in the South Atlantic region.13
Among 186 338 older Medicare PAD patients undergoing major lower-extremity amputation, mortality was found to be 48.3% at 1 year.14
A study of Medicare beneficiaries reported that between 2006 and 2011, 39 339 required revascularization for PAD, and the annual rate of peripheral vascular intervention increased slightly from 401.4 to 419.6 per 100 000 persons.15
People with PAD have impaired function and quality of life. This is true even for people who do not report leg symptoms. Furthermore, patients with PAD, including those who are asymptomatic, experience a significant decline in lower-extremity function over time.16–18
Among patients with established PAD, higher PA levels during daily life are associated with better overall survival rate, a lower risk of death because of CVD, and slower rates of functional decline.19,20 In addition, better 6-minute walk performance and faster walking speed are associated with lower rates of all-cause mortality, cardiovascular mortality, and mobility loss.21,22
Interventions
-
A 2011 systematic review evaluated lower-extremity aerobic exercise against usual care and demonstrated a range of benefits, including the following23:
Increased time to claudication by 71 seconds (79%) to 918 seconds (422%)
Increased distance before claudication by 15 m (5.6%) to 232 m (200%)
Increased walking distance/time by 67% to 101% after 40 minutes of walking 2 to 3 times per week
Observational studies have found that the risk of death,24 MI,25 and amputation24 is substantially greater in individuals with PAD who continue to smoke than in those who stop smoking.
Several studies demonstrate that statins might reduce the risk of major adverse cardiovascular events and amputation among people with PAD.26
A meta-analysis of 42 trials demonstrated that antiplatelet therapy reduces the odds of vascular events by 26% among patients with PAD.27,28
Data from the REACH registry of 8273 PAD participants suggest that only 70% of PAD patients receive lipid-lowering therapy and only 82% receive antiplatelet therapy for secondary CVD prevention.29
In a study that randomized patients with PAD to 3 groups (optimal medical care, supervised exercise training, and iliac artery stent placement), supervised exercise resulted in superior treadmill walking distance compared with stenting. Results in the exercise group and stent group were superior to optimal medical care alone.30
Endovascular therapies for critical limb ischemia are being used with greater frequency in the United States. From 2003 to 2011, there was a significant increase in endovascular treatment of critical limb ischemia (from 5.1% to 11.0%), which was accompanied by lower rates of in-hospital mortality and major amputation, as well as shorter length of stay.31
Hospital Discharges and Ambulatory Care Visits
(See Table 24-1)
Hospital discharges for PAD slightly increased from 2000 to 2010, with first-listed discharges of 135 000 and 146 000, respectively (unreliable estimate, NHDS, NHLBI tabulation).32
In 2012, there were 1 126 000 physician office visits with a primary diagnosis of PAD.32 In 2012, there were 34 000 ED visits, and in 2011 there were 291 000 outpatient department visits for PAD (NHAMCS, NHLBI tabulation).32
Risk Factors
The risk factors for PAD are similar, but not identical, to those for CHD. DM and cigarette smoking are stronger risk factors for PAD than for CHD.26 ORs for associations of DM and smoking with symptomatic PAD are ≈3.0 to 4.0.
Among males in the Health Professionals Follow-up Study, smoking, type 2 DM, hypertension, and hypercholesterolemia accounted for 75% (95% CI, 64%–87%) of risk associated with development of clinical PAD.33
In a meta-analysis of 34 studies from HIC and LMIC, important risk factors for PAD included cigarette smoking (OR, HIC 2.72 versus LMIC 1.42), DM (OR, HIC 1.88 versus LMIC 1.47), hypertension (OR, HIC 1.55 versus LMIC 1.36), and hypercholesterolemia (OR, HIC, 1.19 versus LMIC 1.14).34
A study of 3.3 million people 40 to 99 years of age primarily self-referring for vascular screening tests in the United States showed that risk factor burden was associated with increased prevalence of PAD, and there was a graded association between the number of traditional risk factors and the prevalence of PAD.35
Other risk factors for PAD include sedentary lifestyle, elevated inflammation markers, hypertension in pregnancy, and CKD.35–38
A secondary analysis of a randomized feeding trial showed reduced risk of incident PAD with the Mediterranean diet compared with a control diet.39
Awareness
A cross-sectional, population-based telephone survey of >2500 adults ≥50 years of age, with oversampling of blacks and Hispanics, found that 26% expressed familiarity with PAD in contrast to >65% for CHD, stroke, and HF. Of these, half were not aware that DM and smoking increase the risk of PAD. One in 4 knew that PAD is associated with increased risk of MI and stroke, and only 14% were aware that PAD could lead to amputation. All knowledge domains were lower in individuals with lower income and education levels.40
In data concerning people aged ≥70 years or those aged 50 to 69 years with a history of DM or smoking and their physicians, 83% of patients with a prior diagnosis of PAD were aware of the diagnosis, but only half of their physicians had recognized the diagnosis.3
Global Burden of PAD
A systematic study of 34 studies reported that globally, 202 million people were living with PAD, and during the preceding decade, the number of people with PAD increased by 28.7% in LIMC and by 13.1% in HIC.34
Aortic Diseases
ICD-9 440 to 448; ICD-10 I70 to I78.
Aortic Aneurysm and Acute Aortic Dissection
(See Charts 24-5 and 24-6)
ICD-9 441; ICD-10 I71.
Prevalence and Incidence
The prevalence of abdominal aortic aneurysms (AAA) that are 2.9 to 4.9 cm in diameter ranges from 1.3% in males 45 to 54 years of age to 12.5% in males 75 to 84 years of age. For females, the prevalence ranges from 0% in the youngest to 5.2% in the oldest age groups.26
A meta-analysis of 15 475 individuals from 18 studies on small AAAs (3.0–5.4 cm) demonstrated that mean aneurysm growth rate was 2.21 mm per year and was independent of age and sex. Growth rates were higher in smokers (by 0.35 mm/y) and lower in patients with DM (by 0.51 mm/y).41
A study from Olmsted County, MN,42 demonstrated annual age- and sex-adjusted incidences per 100 000 persons of 3.5 (95% CI, 2.2–4.9) for thoracic aortic aneurysm rupture and 3.5 (95% CI, 2.4–4.6) for acute aortic dissection.
Mortality
Mortality—9863. Any-mention mortality—16 242.
According to the 2013 GBD study, the age-standardized death rate attributable to aortic aneurysm in 2013 was 2.6 per 100 000 (95% CI, 2.1–3.1), which represents a 15% median decrease since 1990. However, because of population growth and aging, the number of deaths attributable to AAAs increased from 99 600 (95% CI, 824 000–118,500) in 1990 to 151 500 (95% CI, 124 200–180 000) in 2013.43
Complications
Rates of aortic aneurysm rupture range from 0.71 to 11.03 per 1000 person-years, with higher rupture rates in smokers (pooled HR, 2.02; 95% CI, 1.33–3.06) and women (pooled HR, 3.76; 95% CI, 2.58–5.47).41
A 2015 systematic review that included 4 randomized trials of ultrasound screening demonstrated lower AAA-associated mortality, emergency operations, and rupture but with higher AAA-associated elective repair rates; however, there was no effect on all-cause mortality (Chart 24-6).44 Similar results were reported in a systematic review report prepared for the US Preventive Services Task Force.45
Data from IRAD demonstrated that the rate of mesenteric malperfusion in 1809 patients with type A acute dissections was 3.7%, with a higher mortality rate than for patients without malperfusion (63.2% versus 23.8%, P<0.001).46
Data from IRAD demonstrated that patients with acute type B aortic dissection have heterogeneous in-hospital outcomes. In-hospital mortality in patients with and without complications (such as mesenteric ischemia, renal failure, limb ischemia, or refractory pain) was 20.0% and 6.1%, respectively. In patients with complications, in-hospital mortality associated with surgical and endovascular repair was 28.6% and 10.1% (P=0.006), respectively.47
Seventeen-year trends in the IRAD database (1996–2013) demonstrate an increase in surgical repair of type A thoracic dissections (79%–90%) and a significant decrease in in-hospital and surgical mortality for type A dissections (31%–22% [P<0.001] and 25%–18% [P=0.003], respectively). Type B dissections were more likely to be treated with endovascular therapies, but no significant changes in mortality were observed.48
Interventions
Results from 4 trials (n=3314 participants) evaluating the effect of open or endovascular repair of small AAAs (4.0–5.5 cm) did not demonstrate an advantage to earlier intervention compared with routine ultrasound surveillance.49
Data from 23 838 patients with ruptured AAAs collected through the NIS (2005–2010) demonstrated in-hospital mortality of 53.2% (95% CI, 51.3%–54.9%), with 80.4% (95% CI, 79.0%–81.9%) undergoing intervention for repair. Of individuals who underwent repair, 20.9% (95% CI, 18.6%–23.2%) underwent endovascular repair, with a 26.8% (95% CI, 23.7%–30.0%) postintervention mortality rate, and 79.1% (95% CI, 76.8%–81.4%) underwent open repair, with a 45.6% (95% CI, 43.6%–47.5%) postintervention mortality rate.50
Data from the NIS suggest that the use of endovascular repair of AAA has risen substantially between 2000 and 2010 (5% versus 74% of all AAA repairs, respectively), whereas the overall number of AAAs (≈45 000 per year) has remained stable. In-hospital mortality and length of stay have declined during this period, but costs have risen.51
At least for the first 3 years after elective repair of AAA, individuals who have endovascular repair may have better outcomes than those who undergo open repair. After multivariable adjustment, Medicare patients who underwent open AAA repair had a higher risk of all-cause mortality (HR, 1.24; 95% CI, 1.05–1.47), AAA-related mortality (HR, 4.37; 95% CI, 2.51–7.66), and complications at 1 year than patients who underwent endovascular repair.52 However, after 8 years of follow-up, survival in the open repair group was similar to that in the endovascular repair group. Of note, individuals in the endovascular repair group had a higher rate of eventual aneurysm rupture (5.4%) than patients who underwent open repair (1.4%).53 Similar findings were observed in the OVER Veterans Affairs Cooperative trial, which compared open AAA repair to endovascular repair in 881 patients and demonstrated reductions in mortality from endovascular repair at 2 years (HR, 0.63; 95% CI, 0.40–0.98) and 3 years (HR, 0.72; 95% CI, 0.51–1.00).54 However, there was no survival difference between open and endovascular repair in individuals followed up for up to 9 years (mean, 5 years; HR, 0.97; 95% CI, 0.77–1.22).54
In ruptured AAA, implementation of a contemporary endovascular-first protocol was associated with decreased perioperative morbidity and mortality, a higher likelihood of discharge to home, and improved long-term survival in a retrospective analysis of 88 consecutive patients seen at an academic medical center.55
The data for surgery in thoracic aortic aneurysms are more mixed between open and endovascular repair. A sample of 12 573 and 2732 Medicare patients who underwent open thoracic aortic aneurysm and endovascular repair demonstrated higher perioperative mortality for open repair in both intact (7.1% versus 6.1%, P=0.07) and ruptured (46% versus 28%, P<0.01) thoracic aortic aneurysms but higher 1-year (87% versus 82%, P=0.001) and 5-year (72% versus 62%, P=0.001) survival rates.56 Perioperative mortality rates for open thoracic aortic aneurysms were higher for non-Hispanic black Medicare patients than for white Medicare patients (18% versus 10%, P<0.001), but rates were similar for endovascular repair (8% versus 9%, P=0.56). On the basis of data from the NIS (n=1400), weekend repair for thoracic aortic aneurysm rupture (n=322) was associated with higher mortality than weekday repair (n=1078; OR, 2.55; 95% CI, 1.77–3.68), likely because of delays in surgical intervention.57
Risk Factors
Many risk factors for atherosclerosis are also associated with increased risk for AAA.58 Of these, smoking is the most important modifiable risk factor for AAA.59
A 2014 systematic review of 17 community-based observational studies demonstrated a consistent, inverse association between DM and prevalent AAAs (OR, 0.80; 95% CI, 0.70–0.90).60
On the basis of nationally representative data from the United Kingdom, giant cell arteritis has been demonstrated to be associated with a 2-fold higher risk (sub-HR, 1.92; 95% CI, 1.52–2.41) after adjustment for competing risks for developing an AAA. These data also demonstrate an inverse association between DM and AAA.61
REFERENCES
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States [published correction appears in Am J Prev Med. 2014;47:103] Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Lower extremity disease among persons aged ≥40 years with and without diabetes: United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:1158–1160. [PubMed] [Google Scholar]
- 3.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 5.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e2. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. [Accessed May 17, 2016];Mortality multiple cause micro-data files, 2014: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 7.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo RC, Bensley RP, Dahlberg SE, et al. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. doi: 10.1016/j.jvs.2013.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Burden of Disease Collaborators. The state of US Health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboyans V, Desormais I, Lacroix P, Salazar J, Criqui MH, Laskar M. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. J Am Coll Cardiol. 2010;55:898–903. doi: 10.1016/j.jacc.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Heald CL, Fowkes FG, Murray GD, Price JF Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis. 2006;189:61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol. 2012;60:2230–2236. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, Peterson ED. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013;165:809–815. 815.e1. doi: 10.1016/j.ahj.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, Curtis LH. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. J Am Coll Cardiol. 2015;65:920–927. doi: 10.1016/j.jacc.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study [published correction appears in Ann Intern Med. 2003;139:306] Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmenter BJ, Raymond J, Dinnen P, Singh MA. A systematic review of randomized controlled trials: walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis. 2011;218:1–12. doi: 10.1016/j.atherosclerosis.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong EJ, Wu J, Singh GD, Dawson DL, Pevec WC, Amsterdam EA, Laird JR. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565–1571. doi: 10.1016/j.jvs.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 25.Jonason T, Bergström R. Cessation of smoking in patients with intermittent claudication: effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260. [PubMed] [Google Scholar]
- 26.Hirsch at, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 27.Westin GG, Armstrong EJ, Bang H, Yeo KK, Anderson D, Dawson DL, Pevec WC, Amsterdam EA, Laird JR. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63:682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, Sala J, Grau M, Martí R, Ponjoan A, Alves-Cabratosa L, Blanch J, Bolíbar B. Statins for prevention of cardiovascular events in a low-risk population with low ankle brachial index. J Am Coll Cardiol. 2016;67:630–640. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 30.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913. doi: 10.1016/j.jacc.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed May 24, 2016];2012 National Ambulatory Medical Care Survey and 2012 National Hospital Ambulatory Medical Care Survey. 2012 http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm.
- 33.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 35.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673–681.e1. doi: 10.1016/j.jvs.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 36.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 37.Garg PK, Biggs ML, Carnethon M, Ix JH, Criqui MH, Britton KA, Djoussé L, Sutton-Tyrrell K, Newman AB, Cushman M, Mukamal KJ. Metabolic syndrome and risk of incident peripheral artery disease: the Cardiovascular Health Study. Hypertension. 2014;63:413–419. doi: 10.1161/HYPERTENSIONAHA.113.01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissgerber TL, Turner ST, Bailey KR, Mosley TH, Jr, Kardia SL, Wiste HJ, Miller VM, Kullo IJ, Garovic VD. Hypertension in pregnancy is a risk factor for peripheral arterial disease decades after pregnancy. Atherosclerosis. 2013;229:212–216. doi: 10.1016/j.atherosclerosis.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Canela M, Estruch R, Corella D, Salas-Salvadó J, Martínez-González MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415–417. doi: 10.1001/jama.2013.280618. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch AT, Murphy TP, Lovell MB, Twillman G, Treat-Jacobson D, Harwood EM, Mohler ER, 3rd, Creager MA, Hobson RW, 2nd, Robertson RM, Howard WJ, Schroeder P, Criqui MH Peripheral Arterial Disease Coalition. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101. [DOI] [PubMed] [Google Scholar]
- 41.Sweeting MJ, Thompson SG, Brown LC, Powell JT RESCAN Collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 42.Clouse WD, Hallett JW, Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ., 3rd Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 43.GBD 2013 Mortality and Cause of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckstein H-H, Reeps C, Zimmermann A, Söllner H. Ultrasound screening for abdominal aortic aneurysms. Gefässchirurgie. 2015;20:1–12. [Google Scholar]
- 45.Guirguis-Blake J, Beil T, Sun XX, Senger C, Whitlock E. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2014. AHRQ Publication No. 14-05202-EF-1. [PubMed] [Google Scholar]
- 46.Di Eusanio M, Trimarchi S, Patel HJ, Hutchison S, Suzuki T, Peterson MD, Di Bartolomeo R, Folesani G, Pyeritz RE, Braverman AC, Montgomery DG, Isselbacher EM, Nienaber CA, Eagle KA, Fattori R. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145:385–390.e381. doi: 10.1016/j.jtcvs.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 47.Trimarchi S, Tolenaar JL, Tsai TT, Froehlich J, Pegorer M, Upchurch GR, Fattori R, Sundt TM, 3rd, Isselbacher EM, Nienaber CA, Rampoldi V, Eagle KA. Influence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J Cardiovasc Surg (Torino) 2012;53:161–168. [PubMed] [Google Scholar]
- 48.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K, Di Eusanio M, Bossone E, Montgomery DG, Eagle KA, Nienaber CA, Isselbacher EM, O’Gara P. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–358. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Filardo G, Powell JT, Martinez MAM, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015;(2):CD001835. doi: 10.1002/14651858.CD001835.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, Thompson MM. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383:963–969. doi: 10.1016/S0140-6736(14)60109-4. [DOI] [PubMed] [Google Scholar]
- 51.Dua A, Kuy S, Lee CJ, Upchurch GR, Jr, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–1517. doi: 10.1016/j.jvs.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA. 2012;307:1621–1628. doi: 10.1001/jama.2012.453. [DOI] [PubMed] [Google Scholar]
- 53.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, Landon BE. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med. 2015;373:328–338. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Jr, Kohler TR, Kougias P, Jean-Claude JM, Cikrit DF, Swanson KM OVER Veterans Affairs Cooperative Study Group. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–1997. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 55.Ullery BW, Tran K, Chandra V, Mell MW, Harris EJ, Dalman RL, Lee JT. Association of an endovascular-first protocol for ruptured abdominal aortic aneurysms with survival and discharge disposition. JAMA Surg. 2015;150:1058–1065. doi: 10.1001/jamasurg.2015.1861. [DOI] [PubMed] [Google Scholar]
- 56.Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, Stone DH. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124:2661–2669. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groves EM, Khoshchehreh M, Le C, Malik S. Effects of weekend admission on the outcomes and management of ruptured aortic aneurysms. J Vasc Surg. 2014;60:318–324. doi: 10.1016/j.jvs.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 59.Lederle FA. In the clinic: abdominal aortic aneurysm. Ann Intern Med. 2009;150:ITC5–ITC1. doi: 10.7326/0003-4819-150-9-200905050-01005. [DOI] [PubMed] [Google Scholar]
- 60.De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47:243–261. doi: 10.1016/j.ejvs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, Hamilton W, Emin A, Culliford D, Luqmani RA. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis. 2015;74:129–135. doi: 10.1136/annrheumdis-2013-204113. [DOI] [PubMed] [Google Scholar]
- 62.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms: report of a sub-committee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
25. QUALITY OF CARE
See Tables 25-1 through 25-13 and Chart 25-1
Table 25-1.
ACS Quality-of-Care Measures
| Quality-of-Care Measure | VHA* | ACTION-GWTG STEMI† | ACTION-GWTG NSTEMI† |
|---|---|---|---|
| Aspirin within 24 h of admission | 98 | 98.5 | 97.9 |
| Aspirin at discharge | 99 | 99.1 | 98.4 |
| β-Blockers at discharge | 99 | 98.2 | 97.2 |
| Lipid-lowering medication at discharge‡ | 99 | 99.4 | 98.9 |
| ARB/ACEI at discharge for patients with LVEF <40% | 96 | 93.0 | 89.8 |
| ACEI at discharge for AMI patients | NM | 69.4 | 56.5 |
| ARB at discharge for AMI patients | NM | 10.5 | 14.9 |
| Adult smoking cessation advice/counseling | Retired | 98.9 | 98.4 |
| Cardiac rehabilitation referral for AMI patients | NM | 84.5 | 75.9 |
Values are percentages. ACEI indicates angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ACTION-GWTG, Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; NM, not measured; NSTEMI, non–ST-segment–elevation myocardial infarction; STEMI, ST-segment–elevation myocardial infarction; and VHA, Veterans Health Administration.
VHA: AMI patients. Data reported include data from October 1, 2014, to September 30, 2015.
ACTION Registry: STEMI and NSTEMI patients are reported separately. Patients must be admitted with acute ischemic symptoms within the previous 24 hours, typically reflected by a primary diagnosis of STEMI or NSTEMI. Patients who are admitted for any other clinical condition are not eligible. Data reported include data from the first quarter of 2014 to the fourth quarter of 2014.
Denotes statin use at discharge. Use of nonstatin lipid-lowering agent was 5.2% for STEMI patients and 8.6% for NSTEMI patients in the ACTION registry.
Table 25-3.
Time Trends in GWTG-ACS Quality-of-Care Measures, 2008 to 2014
| Quality-of-Care Measure | 2008 | 2009 | 2010* | 2011* | 2012* | 2013* | 2014* |
|---|---|---|---|---|---|---|---|
| Aspirin within 24 h of admission | 91.2 | 90.9 | 97 | 97.6 | 97.8 | 95.4 | 98.1 |
| Aspirin at discharge | 94.9 | 95.5 | 98 | 98.3 | 98.4 | 98.4 | 98.7 |
| β-Blockers at discharge | 94.5 | 94.9 | 96 | 96.7 | 97.1 | 97.1 | 97.6 |
| Lipid-lowering medication at discharge | 81.6 | 86.8 | 92† | 98.4† | 98.8† | 98.8 | 99.1 |
| Lipid therapy at discharge if LDL-C >100 mg/dL | 91.9 | 92.5 | NM | NM | NM | NM | NM |
| ARB/ACEI at discharge for patients with LVEF <40% | 91.9 | 91.9 | 86 | 87.8 | 89.7 | 90.0 | 91.2 |
| Adult smoking cessation advice/counseling | 98.4 | 98.4 | 98 | 98.4 | 98.4 | 98.4 | 98.6 |
| Cardiac rehabilitation referral for AMI patients | 52.0 | 49.1 | 75 | 76.5 | 77.3 | 77.2 | 79.4 |
Values are percentages. The American Heart Association’s Get With the Guidelines–Coronary Artery Disease (GWTG-CAD) program has merged into the ACTION registry. ACEI indicates angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; GWTGACS, Get With the Guidelines–Acute Coronary Syndrome; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; and NM, not measured.
Measures from 2008 to 2009 are from the American Heart Association’s GWTG-CAD registry. The 2010 to 2014 measures are from the American Heart Association’s ACTION registry.
Denotes statin use at discharge.
Chart 25-1. Survival rates after out-of-hospital cardiac arrest in US sites of the Resuscitation Outcomes Consortium, 2006 to 2014.
AED indicates automated external defibrillator; CPR, cardiopulmonary resuscitation; and EMS, emergency medical services.
The Institute of Medicine defines quality of care as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”1 The Institute of Medicine has defined 6 specific domains for improving health care, including care that is safe, effective, patient centered, timely, efficient, and equitable.
Abbreviations Used in Chapter 25
| ACC | American College of Cardiology |
| ACEI | angiotensin-converting enzyme inhibitor |
| ACS | acute coronary syndrome |
| ACTION | Acute Coronary Treatment and Intervention Outcomes Network |
| AED | automated external defibrillator |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| AMI | acute myocardial infarction |
| ARB | angiotensin receptor blocker |
| BMI | body mass index |
| BP | blood pressure |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CART | Clinical Assessment, Reporting, and Tracking |
| CHD | coronary heart disease |
| CHF | congestive heart failure |
| CI | confidence interval |
| COURAGE | Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation |
| CPR | cardiopulmonary resuscitation |
| CVD | cardiovascular disease |
| D2B | door-to-balloon |
| DES | drug-eluting stent |
| DM | diabetes mellitus |
| DNR | do not resuscitate |
| DVT | deep vein thrombosis |
| ECG | electrocardiogram |
| EF | ejection fraction |
| EMS | emergency medical services |
| ETco2 | end-tidal carbon dioxide |
| GBD | Global Burden of Diseases, Injuries, and Risk Factors Study |
| GWTG | Get With the Guidelines |
| HbA1c | hemoglobin A1c (glycosylated hemoglobin) |
| HF | heart failure |
| HMO | health maintenance organization |
| IHCA | in-hospital cardiac arrest |
| IHD | ischemic heart disease |
| IQR | interquartile range |
| IV | intravenous |
| LDL-C | low-density lipoprotein cholesterol |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| LVSD | left ventricular systolic dysfunction |
| MD | medical doctor |
| MI | myocardial infarction |
| N/A | not available or not applicable |
| NCDR | National Cardiovascular Data Registry |
| NM | not measured |
| NSTEMI | non–ST-segment–elevation myocardial infarction |
| OHCA | out-of-hospital cardiac arrest |
| OR | odds ratio |
| PCI | percutaneous coronary intervention |
| PINNACLE | Practice Innovation and Clinical Excellence |
| PPO | preferred provider organization |
| ROC | Resuscitation Outcomes Consortium |
| RR | relative risk |
| STEMI | ST-segment–elevation myocardial infarction |
| STS | Society of Thoracic Surgeons |
| TAVR | transcatheter aortic valve replacement |
| TIA | transient ischemic stroke |
| tPA | tissue-type plasminogen activator |
| TVR | target-vessel revascularization |
| UFH | unfractionated heparin |
| VHA | Veterans Health Administration |
| VT/VF | ventricular tachycardia/ventricular fibrillation |
| YLL | years of life lost |
In the following sections, data on quality of care will be presented across these 6 domains to highlight current care and to stimulate efforts to improve the quality of cardiovascular care nationally. Where possible, data are reported from recently published literature or as standardized quality indicators drawn from quality-improvement registries (ie, those consistent with the methods for quality performance measures endorsed by the ACC and the AHA).2 Additional data related to quality of care, such as adherence to ACC/AHA clinical practice guidelines, are also included to provide a spectrum of quality-of-care data. The data selected are meant to provide current examples of quality of care and are not meant to be comprehensive given the sheer number of publications yearly.
Safety
The safety domain has been defined as avoiding injuries to patients from the care that is intended to help them. The following recent publications have focused on safety issues related to cardiac care:
Using the NCDR CathPCI Registry, Tsai et al3 found that almost one fourth of dialysis patients undergoing PCI (n=22 778) received a contraindicated antithrombotic agent, specifically enoxaparin, eptifibatide, or both. Patients who received a contraindicated antithrombotic agent had an increased risk of in-hospital bleeding (OR, 1.63; 95% CI, 1.35–1.98).
Using data from the NCDR PINNACLE registry, Hira and colleagues4 showed that among 27 533 patients receiving prasugrel, 13.9% (3824) had a contraindication to prasugrel use (ie, history of TIA or strokes). This was considered inappropriate prasugrel use. A further 4.4% of patients (1210) were receiving it for a nonrecommended indication (age >75 years without history of DM or MI). Both inappropriate and nonrecommended prasugrel use showed wide practice-level variation (median rate ratio of 2.89 [95% CI, 2.75–3.03] and 2.29 [95% CI, 2.05–2.51], respectively).
In a random sample of medical and surgical care adult patients (not specifically patients with CVD) in Massachusetts hospitals, López et al5 assessed the association between disclosure of an adverse event and patients’ perception of quality of care. Overall, only 40% of adverse events were disclosed. Higher quality ratings were associated with disclosure of an adverse event. Conversely, lower patient perception of quality of care was associated with events that were preventable and with events that caused discomfort.
In an analysis from the ACC’s NCDR PINNACLE registry, the authors showed that among 68 808 patients receiving aspirin therapy for primary prevention, roughly 11.6% (7972 of 68 808) were receiving inappropriate aspirin therapy (10-year risk of CVD <6%). There was significant practice-level variation in inappropriate aspirin use (range, 0%–71.8%; median, 10.1%; IQR, 6.4%) for practices with an adjusted median rate ratio of 1.63 (95% CI, 1.47–1.77).6
Using Medicare Patient Safety Monitoring System data abstracted from medical records on 21 specific adverse events in 4 categories (adverse drug events, general events, hospital-acquired infection, and postprocedural events) for 61 523 patients hospitalized between 2005 and 2011 for AMI, CHF, pneumonia, or conditions requiring surgery, Wang et al7 reported that among patients with AMI, the rate of occurrence of adverse events declined from 5.0% to 3.7% (difference, 1.3%; 95% CI, 0.7%–1.9%). Among patients with CHF, the rate of occurrence of adverse events declined from 3.7% to 2.7% (difference, 1.0%; 95% CI, 0.5%–1.4%). Patients with pneumonia and those with conditions requiring surgery had no significant declines in adverse event rates.
Using aspirin dosing data from 221 199 patients with MI enrolled in the NCDR ACTION registry, Hall and colleagues8 showed a 25-fold variation in the use of high-dose aspirin (325 mg/d) across participating centers. Overall, 60.9% of patients were discharged on high-dose aspirin. High-dose aspirin was prescribed to 73% of patients treated with PCI and 44.6% of patients managed medically; 56.7% of patients with an in-hospital bleeding event were also discharged on high-dose aspirin. Among 9075 patients discharged on aspirin, thienopyridine, and warfarin, 44.0% were prescribed high-dose aspirin therapy.
Effectiveness
(See Tables 25-1, 25-2, 25-3, 25-4, 25-5, 25-6, 25-7, 25-8, 25-9 and Chart 25-1)
Table 25-2.
HF Quality-of-Care Measures, 2015
| Quality-of-Care Measure | AHA GWTG-HF | VHA |
|---|---|---|
| LVEF assessment | 98.3 | 100 |
| ARB/ACEI at discharge for patients with LVSD | 93.4 | 96 |
| Complete discharge instructions | 97.6 | Retired |
| β-Blockers at discharge for patients with LVSD, no contraindications | 95.8 | NM |
| Anticoagulation for AF or atrial flutter, no contraindications | 83.7 | Retired |
Values are percentages. ACEI indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AHA, American Heart Association; ARB, angiotensin receptor blocker; GWTG-HF, Get With the Guidelines–Heart Failure; HF, heart failure; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; NM, not measured; and VHA, Veterans Health Administration.
Table 25-4.
Time Trends in GWTG-HF Quality-of-Care Measures, 2008 to 2015
| Quality-of-Care Measure | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|
| LVEF assessment* | 96.4 | 98.0 | 98.0 | 96.6 | 96.5 | 99.0 | 99.0 | 98.3 |
| ARB/ACEI at discharge for patients with LVSD* | 91.5 | 92.9 | 94.2 | 95.2 | 95.4 | 95.6 | 95.3 | 93.4 |
| Postdischarge appointment (new for 2011)* | … | … | … | 16.3 | 47.4 | 61.3 | 67.7 | 72.9 |
| Complete discharge instructions | 97.2 | 97.7 | 99.3 | 93.8 | 93.4 | 94.1 | 95.7 | 97.6 |
| Evidence-based specific β-blockers* | 54.1 | 45.2 | 48.4 | 57.1 | 82.6 | 86.6 | 91.1 | 91.9 |
| β-Blockers at discharge for patients with LVSD, no contraindications | 92.6 | 92.5 | 94.8 | 96.0 | 97.2 | 97.7 | 97.9 | 95.8 |
| Anticoagulation for AF or atrial flutter, no contraindications | 60.5 | 68.8 | 70.2 | 75.9 | 78.7 | 80.1 | 82.2 | 83.7 |
Values are percentages. ACEI indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ellipses (…), data not available before 2011; GWTG-HF, Get With the Guidelines–Heart Failure; LVEF, left ventricular ejection fraction; and LVSD, left ventricular systolic dysfunction.
Indicates the 4 key achievement measures targeted in GWTG-HF. The composite quality-of-care measure for 2015 was 96.4%. The composite quality-of-care measure indicates performance on the provision of several elements of care. It is computed by summing the numerators for each key achievement measure across the population of interest to create a composite numerator (all the care that was given), summing the denominators for each measure to form a composite denominator (all the care that should have been given), and reporting the ratio (the percentage of all the needed care that was given). The composite performance measure includes β-blocker at discharge instead of evidence-based specific β-blockers and complete discharge instructions instead of postdischarge appointment until the data collection for the new achievement measures stabilizes.
Table 25-5.
Time Trends in GWTG-Stroke Quality-of-Care Measures, 2008 to 2015
| Quality-of-Care Measure | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|
| IV tPA in patients who arrived ≤2 h after symptom onset, treated ≤3 h* | 64.4 | 73.9 | 76.2 | 78.3 | 82.0 | 83.3 | 86.4 | 88.1 |
| IV tPA in patients who arrived <3.5 h after symptom onset, treated ≤4.5 h | … | … | 42.5 | 57.9 | 60.4 | 64.3 | 72.3 | 73.3 |
| IV tPA door-to-needle time ≤60 min | 25.9 | 28.0 | 29.5 | 33.8 | 39.9 | 59.3 | 66.2 | 70.3 |
| Antithrombotic agents <48 h after admission* | … | 96.1 | 96.3 | 96.7 | 96.9 | 97.0 | 97.2 | 97.1 |
| DVT prophylaxis by second hospital day* | … | 92.7 | 92.2 | 93.5 | 98.4 | 98.3 | 98.7 | 98.9 |
| Antithrombotic agents at discharge* | 97.0 | 97.8 | 97.7 | 98.1 | 97.8 | 97.6 | 98.0 | 98.2 |
| Anticoagulation for atrial fibrillation at discharge* | 93.1 | 93.5 | 93.5 | 93.1 | 93.4 | 93.6 | 94.8 | 95.1 |
| Therapy at discharge if LDL-C >100 mg/dL or LDL-C not measured or on therapy at time of admission* | 73.4 | 88.1 | 89.0 | 89.8 | 94.5 | 95.9 | 96.9 | 97.3 |
| Counseling for smoking cessation* | 94.3 | 96.3 | 96.7 | 97.0 | 96.8 | 96.3 | 96.5 | 96.8 |
| Composite quality-of-care measure | 89.7 | 94.7 | 94.2 | 94.4 | 96.3 | 96.4 | 96.9 | 97.3 |
Values are percentages. DVT indicates deep vein thrombosis; ellipses (…), data not available before 2010; GWTG, Get With the Guidelines; IV, intravenous; LDL-C, low-density lipoprotein cholesterol; and tPA, tissue-type plasminogen activator.
Indicates the 7 key achievement measures targeted in GWTG-Stroke.
Table 25-6.
Additional ACTION-GWTG Quality-of-Care Metrics for ACS Care, 2014
| Quality Metrics | Overall | STEMI | NSTEMI |
|---|---|---|---|
| ECG within 10 min of arrival | 65.4 | 75.6 | 60.9 |
| Aspirin within 24 h of arrival | 98.1 | 98.5 | 97.9 |
| Any anticoagulant use* | 94.2 | 96.4 | 92.7 |
| Dosing error | |||
| UFH dose | 45.7 | 44.6 | 45.7 |
| Enoxaparin dose | 9.9 | 7.6 | 10.0 |
| Glycoprotein IIb/IIIa inhibitor dose | 6.0 | 6.0 | 5.8 |
| Aspirin at discharge | 98.7 | 99.1 | 98.4 |
| Prescribed statins on discharge | 99.1 | 99.4 | 98.9 |
| Adult smoking cessation advice/counseling | 98.6 | 98.9 | 98.4 |
| Cardiac rehabilitation referral | 79.4 | 84.5 | 75.9 |
| In-hospital mortality† (95% CI) | 4.6 (4.5–4.7) | 6.4 (6.2–6.6) | 3.4 (3.3–3.5) |
Values are percentages. ACS indicates acute coronary syndrome; ACTION-GWTG, Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines; CI, confidence interval; ECG, electrocardiogram; NSTEMI, non–ST-segment–elevation myocardial infarction; STEMI, ST-segment–elevation myocardial infarction; and UFH, unfractionated heparin.
Data reported include data from the first quarter of 2014 to the fourth quarter of 2014.
Includes UFH, low-molecular-weight heparin, or direct thrombin inhibitor use.
Excludes transfer-out patients.
Table 25-7.
National Committee for Quality Assurance Health Plan Employer Data and Information Set Measures of Care, 2014
| Commercial | Medicare | Medicaid | |||
|---|---|---|---|---|---|
| HMO | PPO | HMO | PPO | HMO | |
| AMI | |||||
| β-Blocker persistence* | 84.4 | 81.8 | 90.5 | 89.2 | 83.3 |
| BP control† | 64.0 | 57.2 | 70.7 | 68.5 | 57.1 |
| DM | |||||
| HbA1c testing | 90.5 | 88.3 | 92.8 | 92.4 | 86.3 |
| HbA1c >9.0% | 31.1 | 37.3 | 24.9 | 24.9 | 43.6 |
| Eye examination performed | 56.2 | 48.7 | 68.5 | 68.4 | 54.4 |
| Monitoring nephropathy | 85.4 | 80.4 | 92.0 | 90.0 | 80.9 |
| BP <140/90 mm Hg | 64.6 | 58.7 | 65.0 | 60.1 | 61.9 |
| Advising smokers and tobacco users to quit | 77.0 | 70.8 | N/A | N/A | 75.8 |
| BMI percentile assessment in children and adolescents | 61.3 | 40.0 | N/A | N/A | 64.0 |
| Nutrition counseling (children and adolescents) | 59.2 | 40.5 | N/A | N/A | 60.5 |
| Counseling for physical activity (children and adolescents) | 56.0 | 38.2 | N/A | N/A | 53.5 |
| BMI assessment for adults | 75.9 | 49.4 | 92.9 | 90.0 | 79.9 |
| Physical activity discussion in older adults (≥65 y of age) | N/A | 55.9 | 56.6 | N/A | |
| Physical activity advice in older adults (≥65 y of age) | N/A | 51.3 | 49.4 | N/A | |
Values are percentages. AMI indicates acute myocardial infarction; BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HMO, health maintenance organization; N/A, not available or not applicable; and PPO, preferred provider organization.
β-Blocker persistence: Received persistent β-blocker treatment for 6 months after AMI hospital discharge.
Adults 18–59 years of age with BP <140/90 mm Hg, adults aged 60–85 with a diagnosis of DM and BP <140/90 mm Hg, adults aged 60–85 without a diagnosis of DM and BP <150/90 mm Hg.
Table 25-8.
Quality of Care for Patients With Out-of-Hospital Cardiac Arrest at US ROC Sites (January 1, 2014 to December 31, 2014)
| Overall | Adults | Children | |
|---|---|---|---|
| Bystander and EMS care* | |||
| Bystander CPR, % | 46.1 (45.0–47.3) | 45.7 (44.6–46.9) | 61.4 (54.9–67.9) |
| Shocked by AED before EMS, % | 2.0 (1.7–2.4) | 2.1 (1.7–2.4) | 1.4 (0.0–3.0) |
| Chest compression fraction during first 5 min of CPR, % | 0.85 (0.12) | 0.85 (0.12) | 0.83 (0.13) |
| Compression depth, mm | 48.1 (10.7) | 48.1 (10.7) | 47.2 (9.5) |
| Preshock pause duration, s | 10.8 (11.0) | 10.8 (10.9) | 16.2 (16.4) |
| Time to first EMS defibrillator applied, min | 8.8 (4.5) | 8.8 (4.5) | 8.7 (4.2) |
| Hospital-based metrics† | |||
| Hypothermia induced after initial VT/VF, %‡ | 66.3 (62.3–70.3) | 66.2 (62.1–70.2) | 100 (100–100) |
| No order for withdrawal/DNR during first 72 h, %§ | 45.0 (42.1–48.0) | 44.8 (41.9–47.8) | 100 (100–100) |
| Implantable cardioverter-defibrillator assessment, initial VT/VF, no AMI per MD notes or final ECG interpretation, %|| | 30.3 (24.8–35.8) | 30.0 (24.5–35.6) | 100 (100–100) |
Values are mean (95% confidence interval) or mean (standard deviation). Because age is missing for some cases, these cases are not included in either adults or children, thus explaining why overall rates equal the adult rates when rates for children are not available. AED indicates automated external defibrillator; AMI, acute myocardial infarction; CPR, cardiopulmonary resuscitation; DNR, do not resuscitate; ECG, electrocardiogram; EMS, emergency medical services; MD, medical doctor; ROC, Resuscitation Outcomes Consortium; and VT/VF, ventricular tachycardia/ventricular fibrillation.
Data are from EMS-treated cases.
During 2014, there was 1 pediatric case with initial rhythm VT/VF admitted to the hospital.
Denominator is all cases with initial rhythm VT/VF and admitted to the hospital.
Denominator is all cases admitted to the hospital.
Denominator is all cases with initial rhythm VT/VF, no indication of AMI, no percutaneous coronary intervention, no bypass, and admitted to the hospital.
Table 25-9.
Quality of Care of Patients With In-Hospital Cardiac Arrest Among GWTG-Resuscitation Hospitals, 2014*
| Adults | Children | |
|---|---|---|
| Event outside critical care setting | 48.1 | 11.2 |
| All objective CPR data collected | 78.6 | 82.0 |
| ETco2 used during arrest | 4.6 | 23.0 |
| Induced hypothermia after resuscitation from shockable rhythm | 7.6 | 13.6 |
Values are mean percentages. CPR indicates cardiopulmonary resuscitation; ETco2, end-tidal CO2; and GWTG, Get With the Guidelines.
Source: GWTG-Resuscitation Investigators, June 2014.
Effective care has been defined as providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit. It also encompasses monitoring results of the care provided and using them to improve care for all patients.1
Using data from the ACTION Registry among 202 213 patients discharged after AMI from 526 US participating sites between January 2007 and March 2011, Rao and colleagues9 showed that only 14.5% of the eligible patients without a documented contraindication received aldosterone antagonists. Fewer than 2% of the participating sites used aldosterone antagonists in ≥50% of eligible patients.
Data from the ACC PINNACLE outpatient registry of patients with CAD (n=38 775) showed that 77.8% of the patients (30 160) were prescribed statins, 5.3% (2042) were treated only with nonstatin lipid-lowering medications, and 17% (6573) were not taking any lipid-lowering medication. Lack of medical insurance (RR, 0.94; 95% CI, 0.89–1.00) was associated with a lower likelihood of statin treatment, whereas male sex (RR, 1.10; 95% CI, 1.07–1.13), coexisting hypertension (RR, 1.07; 95% CI, 1.02–1.12), prior CABG (RR, 1.09; 95% CI, 1.05–1.14), and prior PCI (RR, 1.11; 95% CI, 1.06–1.16) were associated with a higher likelihood of statin treatment.10 Another publication from the same registry showed that among 156 145 CAD patients in 58 practices, just over two thirds (n=103 830, 66.5%) of patients were prescribed the optimal combination of medications (β-blockers, ACEIs/ARBs, statins) for which they were eligible. After adjustment for patient factors, the practice median rate ratio for prescription was 1.25 (95% CI, 1.20–1.32), which indicates a 25% likelihood that any 2 practices would differ in treating identical CAD patients.11
A study from a national cohort of 972 532 CVD patients in the Veterans Health Administration showed that females with CVD (n=13 371) were less likely than males to receive statins (57.6% versus 64.8%, P<0.0001) or high-intensity statins (21.1% versus 23.6%, P<0.0001) as recommended in the 2013 ACC/AHA cholesterol management guidelines.11a In adjusted models, female sex was independently associated with a lower likelihood of receiving statins (OR, 0.68; 95% CI, 0.66–0.71) or high-intensity statins (OR, 0.76; 95% CI, 0.73–0.80). The authors concluded that although females with CVD are less likely to receive evidence-based statin and high-intensity statins than males, use of statins remains low in both sexes.12
In a study using a 5% random sample of 8762 Medicare beneficiaries (aged 65–74 years) discharged after a CHD event (MI or coronary revascularization) in 2007, 2008, or 2009 who filled a statin prescription within 90 days after the initial event, only 27% of first postdischarge statin fills were for a high-intensity statin. The proportion of patients filling a high-intensity statin after discharge was 23.1%, 9.4%, and 80.7% for those not taking statins before hospitalization, taking low- or moderate-intensity statins, and taking high-intensity statins before their CHD event, respectively. The authors concluded that a majority of Medicare beneficiaries do not receive a high-intensity statin after hospitalization for CHD.13
Stub et al14 reported a post hoc secondary analysis of a large, partial factorial trial of interventions for patients with OHCA. The quality of hospital-based postresuscitation care given to each patient was assigned an evidence-based quality score that considered (1) initiation of temperature management; (2) achievement of target temperature 32° to 34°C; (3) continuation of temperature management for >12 hours; (4) performance of coronary angiography within 24 hours; and (5) no withdrawal of life-sustaining treatment before day 3. These were aggregated as hospital-level composite performance scores, which varied widely (median [IQR] scores from lowest to highest hospital quartiles, 21% [20%–25%] versus 59% [55%–64%]). Adjusted survival to discharge increased with each quartile of composite performance score (from lowest to highest: 16.2%, 20.8%, 28.5%, and 34.8%; P<0.01). Adjusted rates of favorable neurological outcome also increased (from lowest quartile to highest: 8.3%, 13.8%, 22.2%, and 25.9%; P<0.01). Hospital score was significantly associated with outcome after risk adjustment for established baseline factors (highest versus lowest adherence quartile: adjusted OR of survival, 1.64; 95% CI, 1.13–2.38).
Using data from the Veterans Affairs CART Program, Maddox and colleagues15 studied outcomes associated with nonobstructive CAD. Among 37 674 veterans undergoing cardiac catheterization, 8384 (22.3%) had nonobstructive CAD. Compared with veterans with no CAD, 1-, 2-, and 3-vessel nonobstructive CAD was associated with 2 to 4.6 times higher odds of MI. The authors concluded that nonobstructive CAD appears to confer significant risks for MI, and therefore, appropriate measures for preventative therapies should be considered.
In a comparative effectiveness study of single-versus dual-chamber implantable cardioverter-defibrillators using data from the NCDR ICD (implantable cardioverter-defibrillator) Registry, Peterson and colleagues16 found that among patients receiving an implantable cardioverter-defibrillator for primary prevention without indications for pacing, the use of a dual-chamber device compared with a single-chamber device was associated with a higher risk of device-related complications and similar 1-year mortality and hospitalization outcomes. In a propensity-matched cohort, rates of complications were lower for single-chamber devices (3.51% versus 4.72%; P<0.001; risk difference, −1.20 [95% CI, −1.72 to −0.69]), but device type was not significantly associated with 1-year mortality (unadjusted rate, 9.85% versus 9.77%; HR, 0.99 [95% CI, 0.91–1.07]; P=0.79), 1-year all-cause hospitalization (unadjusted rate, 43.86% versus 44.83%; HR, 1.00 [95% CI, 0.97–1.04]; P=0.82), or hospitalization for HF (unadjusted rate, 14.73% versus 15.38%; HR, 1.05 [95% CI, 0.99–1.12]; P=0.19).
In 2013, investigators from the GBD 2010 study described their findings of a systematic analysis of disease burden, injuries, and leading risk factors in the United States and compared them with those of 34 countries in the Organization for Economic Co-operation and Development. They reported that the US life expectancy for both sexes combined increased from 75.2 years in 1990 to 78.2 years in 2010. During the same time period, healthy life expectancy (ie, the number of years that a person at a given age can expect to live in good health, taking into account mortality and disability) increased from 65.8 to 68.1 years in the United States. Despite declines in the YLLs because of premature mortality secondary to IHD and stroke, 15.9% of YLLs were related to IHD and 4.3% of YLLs were related to stroke in the United States in 2010, which highlights the continued dominance of CVD in causing premature death. Despite these absolute improvements, the US rank among 34 countries in the Organization for Economic Co-operation and Development changed from 18th to 27th for the age-standardized death rate, from 20th to 27th for life expectancy at birth, from 14th to 26th for healthy life expectancy, and from 23rd to 28th for the age-standardized YLL. These results indicate that improvements in population health in the United States have not kept pace with advances in population health in other wealthy nations.17
-
Outcome measures of 30-day mortality and 30-day readmission after hospitalization for AMI, HF, or ischemic stroke have been developed that adjust for patient mix (eg, comorbidities) so that comparisons can be made across hospitals.18 According to national Medicare data from July 2011 through June 2014:
The median (10th, 90th percentile) hospital risk-standardized mortality rate was 14.1% (12.6%, 15.7%) for AMI, 11.6% (9.9%, 13.5%) for HF, and 14.7% (12.8%,17.0%) for ischemic stroke.
The median risk-standardized 30-day readmission rate was 17.0% (15.7%, 18.4%) for AMI, 21.9% (20.2%, 24.1%) for HF, and 12.6% (11.5%, 14.9%) for ischemic stroke.
A study of 458 hospitals participating in the STS National Cardiac Database showed that an intervention of receiving quality-improvement educational material designed to influence the prescription rates of 4 medication classes (aspirin, β-blockers, lipid-lowering therapy, and ACEIs) after CABG discharge in addition to site-specific feedback reports led to a significant improvement in adherence for all 4 secondary prevention medications at the intervention sites compared with the control sites.19
Inpatient ACS, HF, and stroke quality-of-care measures data, including trends in care data, where available from national registries, are given in Tables 25-1 through 25-6.
Selected outpatient quality-of-care measures from the National Committee for Quality Assurance for 2014 appear in Table 25-7.
Quality-of-care measures for patients who had OHCA and were enrolled in the ROC cardiac arrest registry in 2014 (ROC Investigators, unpublished data, November 23, 2014) are given in Table 25-8. Longitudinal measures are also available (Chart 25-1).
Quality-of-care measures for patients who had IHCA and were enrolled in the AHA’s GWTG-Resuscitation quality-improvement project in 2014 (GWTG-Resuscitation Investigators, unpublished data, September 1, 2014) are given in Table 25-9.
Patient-Centered Care
Patient-centered care has been defined as the provision of care that is respectful of and responsive to individual patient preferences, needs, and values and that ensures that patient values guide all clinical decisions. Dimensions of patient-centered care include the following: (1) Respect for patients’ values, preferences, and expressed needs; (2) coordination and integration of care; (3) information, communication, and education; (4) physical comfort; (5) emotional support; and (6) involvement of family and friends. Studies that focused on some of these aspects of patient-centered care are highlighted below.
The COURAGE trial, which investigated a strategy of PCI plus optimal medical therapy versus optimal medical therapy alone, demonstrated that both groups had significant improvement in health status during follow-up. By 3 months, health status scores had increased in the PCI group compared with the medical therapy group, to 76±24 versus 72±23 for physical limitation (P=0.004), 77±28 versus 73±27 for angina stability (P=0.002), 85±22 versus 80±23 for angina frequency (P<0.001), 92±12 versus 90±14 for treatment satisfaction (P<0.001), and 73±22 versus 68±23 for quality of life (P<0.001). The PCI plus optimal medical therapy group had a small but significant incremental benefit compared with the optimal medical therapy group early on, but this benefit disappeared by 36 months.20
Peikes et al21 reported on 15 care-coordination programs as part of a Medicare demonstration project for patients with CHF, CAD, DM, and other conditions. Thirteen of the 15 programs did not show a difference in hospitalization rates, and none of the programs demonstrated net savings. The interventions tested varied significantly, but the majority of the interventions included patient education to improve adherence to medication, diet, exercise, and self-care regimens and improving care coordination through various approaches. These programs had favorable effects on none of the adherence measures and only a few of the many quality-of-care indicators examined. The authors concluded that programs with substantial in-person contact that target moderately to severely ill patients can be cost-neutral and improve some aspects of care.
Hernandez et al22 showed that patients with outpatient follow-up within 7 days of discharge for an HF hospitalization were less likely to be readmitted within 30 days in the GWTG-HF registry of patients who were ≥65 years of age. The median length of stay was 4 days (IQR, 2–6 days), and 21.3% of patients were readmitted within 30 days. At the hospital level, the median percentage of patients who had early follow-up after discharge from the index hospitalization was 38.3% (IQR, 32.4%–44.5%).
A randomized trial tested a multifaceted intervention to improve adherence to 4 cardioprotective medications (clopidogrel, statins, ACEIs/ARBs, and β-blockers) after ACS. A total of 253 patients were randomized to either a multifaceted intervention (including pharmacist-led medication reconciliation and tailoring; patient education; collaborative care between a pharmacist and a patient’s primary care provider and/or cardiologist; and 2 types of voice messaging for patient education and medication refill reminder) or to usual care. After a 1-year period, 89.3% of the patients in the intervention group were adherent to the 4 cardioprotective medications (mean proportion of days covered >0.8) compared with 73.9% in the usual care group (P=0.003). A greater proportion of patients in the intervention arm than in the usual care group were adherent to clopidogrel (86.8% versus 70.7%, P=0.03), statins (93.2% versus 71.3%, P<0.001), and ACEIs/ARBs (93.1% versus 81.7%, P=0.03) but not β-blockers (88.1% versus 84.8%, P=0.59). There were no statistically significant differences in the proportion of patients who achieved BP or LDL-C level goals.23
Reynolds et al reported results on health-related quality of life after TAVR in inoperable patients with severe aortic stenosis compared with those receiving standard therapy. Health-related quality of life was assessed at baseline and at 1, 6, and 12 months with the Kansas City Cardiomyopathy Questionnaire and the 12-item Short Form-12 General Health Survey. Although the Kansas City Cardiomyopathy Questionnaire summary scores improved in both groups, the extent of improvement was greater in the TAVR group than in the standard-care group at 1 month (mean between-group difference, 13 points; 95% CI, 8–19 points), with larger benefits at 6 months (mean difference, 21 points; 95% CI, 15–27 points) and 12 months (mean difference, 26 points; 95% CI, 19–33). At 12 months, TAVR patients also reported higher physical and mental health scores on the 12-item Short Form-12 General Health Survey, with a mean difference of 5.7 and 6.4 points, respectively (P<0.001 for both comparisons) compared with standard care.24
Timely Care
(See Table 25-10)
Table 25-10.
Timely Reperfusion for ACS and Stroke*
| Quality-of-Care Measure | VHA (for STEMI) or GWTG-Stroke (for Stroke) | ACTION-GWTG STEMI |
|---|---|---|
| STEMI | ||
| Thrombolytic agents within 30 min | 50† | 54.0† |
| PCI within 90 min | 65 | 95.9 |
| Stroke | ||
| IV tPA in patients who arrived <2 h after symptom onset, treated ≤3 h | 88.1 | N/A |
| IV tPA in patients who arrived <3.5 h after symptom onset, treated ≤4.5 h | 73.3 | N/A |
| IV tPA door-to-needle time ≤60 min | 70.3 | N/A |
Values are percentages. ACS indicates acute coronary syndrome; ACTION, Acute Coronary Treatment and Intervention Outcomes Network Registry; GWTG, Get With the Guidelines; IV, intravenous; N/A, not applicable; PCI, percutaneous coronary intervention; STEMI, ST-segment–elevation myocardial infarction; tPA, tissue-type plasminogen activator; and VHA, Veterans Health Administration.
VHA data reported include data from October 1, 2014, to September 30, 2015; GWTG-Stroke data include data from January 2015 to December 2015. ACTION Registry: data reported include data from first quarter of 2014 to the fourth quarter of 2014.
Indicates data based on low numbers.
The timely care domain relates to reducing waits and sometimes harmful delays for both those who receive and those who give care. Timeliness is an important characteristic of any service and is a legitimate and valued focus of improvement in health care and other industries.
Among patients who underwent primary PCI for STEMI and were enrolled in the CathPCI Registry (n=96 738) in a period that coincided with national efforts to reduce D2B times, median D2B times declined from 83 minutes in the 12 months from July 2005 to June 2006 to 67 minutes in the 12 months from July 2008 to June 2009. This improvement in processes of care was not associated with improved outcome (risk-adjusted in-hospital mortality 5.0% in 2005–2006 versus 4.7% in 2008–2009, P=0.34).25
Smolderen et al26 assessed whether health insurance status affects decisions to seek care for AMI. Uninsured and insured patients with financial concerns were more likely to delay seeking care during AMI and had prehospital delays of >6 hours (48.6% of uninsured patients and 44.6% of insured patients with financial concerns compared with 39.3% of insured patients without financial concerns). Lack of health insurance and financial concerns about accessing care among those with health insurance were each associated with delays in seeking emergency care for AMI.
Glickman et al27 showed that a year-long implementation of standardized protocols as part of a statewide regionalization program was associated with a significant improvement in median door-in–door-out times among 436 STEMI patients who presented at non-PCI hospitals who required transfer (before intervention: 97 minutes [IQR, 56–160 minutes]; after intervention: 58 minutes [IQR, 35–90 minutes]; P<0.0001).
Nallamothu et al28 evaluated the association between D2B times and mortality after primary PCI over time at both the hospital and the individual patient level among 150 116 STEMI patients from 423 hospitals who underwent primary PCI between January 1, 2005, and December 31, 2011, in the NCDR CathPCI Registry. Annual D2B times decreased significantly from a median of 86 minutes (IQR, 65–109 minutes) in 2005 to 63 minutes (IQR, 47–80 minutes) in 2011 (P<0.0001), with a concurrent rise in risk-adjusted in-hospital mortality (from 4.7% to 5.3%; P=0.06) and risk-adjusted 6-month mortality (from 12.9% to 14.4%; P=0.001). In multilevel models, shorter patient-specific D2B times were consistently associated at the individual level with lower in-hospital mortality (adjusted OR for each 10-minute decrease, 0.92; 95% CI, 0.91–0.93; P<0.0001) and 6-month mortality (adjusted OR for each 10-minute decrease, 0.94; 95% CI, 0.93–0.95; P<0.0001). By contrast, risk-adjusted in-hospital and 6-month mortality at the population level, independent of patient-specific D2B times, rose in the growing and changing population of patients undergoing primary PCI during the study period. These authors concluded that the absence of an association of annual D2B time and changes in mortality at the population level should not be interpreted as an indication of its individual-level relation in patients with STEMI undergoing primary PCI.
A study of 204 591 patients with ischemic and hemorrhagic strokes admitted to 1563 GTWG-Stroke participating hospitals between April 1, 2003, and June 30, 2010, showed that 63.7% of the patients arrived at the hospital by EMS. Older patients, those with Medicaid and Medicare, and those with severe strokes were more likely to activate EMS. Conversely, minority race/ethnicity (black, Hispanic, Asian) and living in rural communities were associated with a lower likelihood of EMS use. EMS transport was independently associated with an onset-to-door time ≤3 hours, a higher proportion of patients meeting door-to-imaging time of ≤25 minutes, more patients meeting a door-to-needle time of ≤60 minutes, and more eligible patients being treated with tPA if onset of symptoms was ≤2 hours. The authors concluded that although EMS use was associated with rapid evaluation and treatment of stroke, more than one third of stroke patients fail to use EMS.29
Data on time to reperfusion for STEMI or ischemic stroke are provided from national registries in Table 25-10.
Efficiency
Efficiency has been defined as avoiding waste, in particular waste of equipment, supplies, ideas, and energy. In an efficient healthcare system, resources are used to get the best value for the money spent.
Using data from the NCDR CathPCI registry from 2004 through 2010, Amin et al30 examined the association between risk of TVR and use of DES and the cost-effectiveness of lower use of DES in patients at low risk of TVR (<10% TVR risk). The authors showed a marked variation in physicians’ use of DES (range, 2%–100%). Even in groups with low TVR risk, 73.9% of the patients received DES. The authors projected that by reducing the use of DES by 50% in patients at low risk of TVR, US healthcare costs could be lowered by $205 million, whereas the overall TVR event rate would be increased by 0.5%.
A study of 35 191 CHD patients from the US Department of Veterans Affairs healthcare system showed that among 27 947 patients with LDL-C levels <100 mg/dL, 9200 (32.9%) received additional lipid assessments without any treatment intensification during the 11 months from the index lipid panel. Even among 13 114 patients with LDL-C <70 mg/dL, repeat lipid testing was performed in 8177 patients (62.4%) during 11 months of follow-up. These results show that redundant lipid testing is common in patients with CHD.31
In a recent study from the ACC’s PINNACLE registry, the authors compared the quality of care delivered by physicians and advanced practice providers (nurse practitioners and physicians assistants) for 459 669 patients with CAD, HF, or AF. Compliance with most CAD, HF, and AF measures was comparable, except for a higher rate of smoking cessation screening and intervention (adjusted rate ratio, 1.14; 95% CI, 1.03–1.26) and cardiac rehabilitation referral (rate ratio, 1.40; 95% CI, 1.16–1.70) among CAD patients receiving care from advanced practice providers. Compliance with all eligible CAD measures was low for both (12.1% and 12.2% for advanced practice providers and physicians, respectively), with no significant difference. The authors concluded that apart from minor differences, a collaborative care delivery model using both physicians and advanced practice providers could deliver an overall comparable quality of outpatient cardiovascular care compared with a physician-only model.32
Himmelstein et al33 analyzed whether more-computerized hospitals had lower costs of care or administration or better quality, to address a common belief that computerization improves healthcare quality, reduces costs, and increases administrative efficiency. They found that hospitals that increased computerization faster had more rapid administrative cost increases (P=0.0001); however, higher overall computerization scores correlated weakly with better quality scores for AMI (r=0.07, P=0.003) but not for HF, pneumonia, or the 3 conditions combined. In multivariate analyses, more-computerized hospitals had slightly better quality. The authors concluded that hospital computing might modestly improve process measures of quality but does not reduce administrative or overall costs.
In a retrospective cohort study of cases (111 707) submitted to the NCDR ICD (implantable cardioverter-defibrillator) Registry between January 1, 2006, and June 30, 2009, 25 145 (22.5%) received non–evidence-based implantable cardioverter-defibrillator therapy. Patients who received non–evidence-based implantable cardioverter-defibrillator therapy had a significantly higher risk of in-hospital death (0.57% versus 0.18%, P<0.001) and any postprocedure complication (3.23% versus 2.41%, P<0.001) than those who received evidence-based implantable cardioverter-defibrillator therapy.34
In a multicenter study of patients within the NCDR undergoing PCI, Chan et al35 reported results of the appropriateness of PCI for both acute and nonacute indications. Among patients undergoing PCI for acute indications (71.1% of the cohort), 98.5% of the procedures were classified as appropriate, 0.3% as uncertain, and 1.1% as inappropriate. Among patients undergoing PCI for nonacute indications (28.9% of the cohort), 50.4% of the procedures were classified as appropriate, 38% as uncertain, and 11.6% as inappropriate. There was also substantial variation for inappropriate nonacute PCI across hospitals (median hospital rate, 10.8%; IQR, 6.0%–16.7%).
Equitable Care
(See Tables 25-11 through 25-13)
Table 25-11.
Quality of Care by Race/Ethnicity and Sex in the ACTION Registry, 2014
| Quality-of-Care Measure | White | Black | Other | Males | Females |
|---|---|---|---|---|---|
| Aspirin at admission | 98.1 | 98.2 | 98.3 | 98.4 | 97.7 |
| Aspirin at discharge | 98.8 | 98.0 | 98.8 | 98.9 | 98.2 |
| β-Blockers at discharge | 97.6 | 97.2 | 97.5 | 97.9 | 97.0 |
| Time to PCI ≤90 min for STEMI patients | 96.1 | 94.3 | 96.0 | 96.2 | 95.2 |
| ARB/ACEI at discharge for patients with LVEF <40% | 91.2 | 91.7 | 88.5 | 91.5 | 90.5 |
| Statins at discharge | 99.1 | 98.9 | 99.4 | 99.3 | 98.8 |
Values are percentages. Data reported include data from first quarter of 2015 to fourth quarter of 2015. ACEI indicates angiotensinconverting enzyme inhibitor; ACTION, Acute Coronary Treatment and Intervention Outcomes Network; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and STEMI, ST-segment–elevation myocardial infarction.
Table 25-13.
Quality of Care by Race/Ethnicity and Sex in the GWTG-Stroke Program, 2014
| Quality-of-Care Measure | White | Black | Hispanic | Males | Females |
|---|---|---|---|---|---|
| IV tPA in patients who arrived ≤2 h after symptom onset, treated ≤3 h* | 86.2 | 85.4 | 88.1 | 87.1 | 85.6 |
| IV tPA in patients who arrived <3.5 h after symptom onset, treated ≤4.5 h | 64.7 | 65.6 | 69.9 | 66.6 | 64.1 |
| IV tPA door-to-needle time ≤60 min | 59.1 | 59.5 | 62.7 | 60.8 | 58.6 |
| Thrombolytic complications: IV tPA and life-threatening, serious systemic hemorrhage | 16.3 | 20.0 | 8.3 | 14.7 | 18.1 |
| Antithrombotic agents <48 h after admission* | 97.4 | 97.1 | 97.3 | 97.5 | 97.1 |
| DVT prophylaxis by second hospital day* | 98.4 | 98.2 | 98.2 | 98.3 | 98.4 |
| Antithrombotic agents at discharge* | 98.3 | 97.8 | 97.7 | 98.3 | 97.9 |
| Anticoagulation for atrial fibrillation at discharge* | 94.3 | 94.3 | 94.8 | 94.6 | 94.0 |
| Therapy at discharge if LDL-C >100 mg/dL or LDL-C not measured or on therapy at admission* | 96.0 | 96.4 | 96.0 | 96.7 | 95.5 |
| Counseling for smoking cessation* | 96.8 | 96.5 | 95.9 | 96.8 | 96.4 |
| Lifestyle changes recommended for BMI >25 kg/m2 | 55.5 | 52.6 | 57.5 | 55.2 | 54.6 |
| Composite quality-of-care measure | 96.9 | 96.8 | 96.7 | 97.1 | 96.6 |
Values are percentages. BMI indicates body mass index; DVT, deep vein thrombosis; GWTG, Get With the Guidelines; IV, intravenous; LDL-C, low-density lipoprotein cholesterol; and tPA, tissue-type plasminogen activator.
Indicates the 7 key achievement measures targeted in GWTG-Stroke.
Equitable care means the provision of care that does not vary in quality because of personal characteristics such as sex, ethnicity, geographic location, and socioeconomic status. The aim of equity is to secure the benefits of quality health care for all the people of the United States. With regard to equity in caregiving, all individuals rightly expect to be treated fairly by local institutions, including healthcare organizations.
Data on the quality of care by race/ethnicity and sex in the ACTION Registry (2014) are provided in Table 25-11.
Chan et al36 demonstrated that rates of survival to discharge were lower for black patients (25.2%) than for white patients (37.4%) after IHCA. Lower rates of survival to discharge for blacks reflected lower rates of both successful resuscitation (55.8% versus 67.4%) and postresuscitation survival (45.2% versus 55.5%). Adjustment for the hospital site at which patients received care explained a substantial portion of the racial differences in successful resuscitation (adjusted RR, 0.92; 95% CI, 0.88–0.96; P<0.001) and eliminated the racial differences in postresuscitation survival (adjusted RR, 0.99; 95% CI, 0.92–1.06; P=0.68). The authors concluded that much of the racial difference was associated with the hospital center in which black patients received care.
Davis et al evaluated data on 85 936 veterans (3181 females) undergoing initial cardiac catheterization between October 1, 2007, and September 30, 2012, in the Veterans Health Administration. Females had lower rates of obstructive CAD than males (22.6% versus 53.3%, respectively). Rates of procedural complications were similar in both sexes. Adjusted outcomes at 1 year showed females had lower mortality (HR, 0.74; 95% CI, 0.60–0.92) and less all-cause rehospitalization (HR, 0.87; 95% CI, 0.82–0.93), but there was no difference in rates of unplanned PCI.37
Kapoor et al38 evaluated 99 058 HF admissions from 244 sites between January 2005 and September 2009. Patients were grouped on the basis of payer status (private/health maintenance organization, no insurance, Medicare, or Medicaid). Compared with private/health maintenance organization group, the other 3 groups were less likely to receive evidence-based therapies (β-blockers, implantable cardioverter-defibrillators, anticoagulation for AF, ACEIs, or ARBs) and had longer hospital stays. Higher adjusted rates of in-hospital mortality were also seen in patients with Medicaid (OR, 1.22; 95% CI, 1.06–1.41) and in patients with reduced EF and no insurance (OR, 1.61; 95% CI, 1.15–2.25).
Data on the quality of care by race/ethnicity and sex in the GWTG-HF Program (2014) are provided in Table 25-12.
Cohen et al39 demonstrated that among hospitals engaged in a national quality monitoring and improvement program, evidence-based care for AMI appeared to improve over time for patients irrespective of race/ethnicity, and differences in care by race/ethnicity care were reduced or eliminated. They analyzed 142 593 patients with AMI (121 528 whites, 10 882 blacks, and 10 183 Hispanics) at 443 hospitals participating in the GWTG-CAD program. Overall, defect-free care was 80.9% for whites, 79.5% for Hispanics (adjusted OR versus whites, 1.00; 95% CI, 0.94–1.06; P=0.94), and 77.7% for blacks (adjusted OR versus whites, 0.93; 95% CI, 0.87–0.98; P=0.01). A significant gap in defect-free care was observed for blacks during the first half of the study but was no longer present during the remainder of the study. Overall, progressive improvements in defect-free care were observed regardless of race/ethnic groups.
Thomas et al40 analyzed data among hospitals that voluntarily participated in the AHA’s GWTG-HF program from January 2005 through December 2008. Relative to white patients, Hispanic and black patients hospitalized with HF were significantly younger (median age 78, 63, and 64 years, respectively) but had lower EFs (mean EF 41.1%, 38.8%, and 35.7%, respectively) with a higher prevalence of DM (40.2%, 55.7%, and 43.8%, respectively) and hypertension (70.6%, 78.4%, and 82.8%, respectively). The provision of guideline-based care was comparable for white, black, and Hispanic patients. Black (1.7%) and Hispanic (2.4%) patients had lower in-hospital mortality than white patients (3.5%). Improvement in adherence to all-or-none HF measures increased annually from year 1 to year 3 for all 3 racial/ethnic groups.
Al-Khatib et al41 analyzed implantable cardioverter-defibrillator use for primary prevention among 11 880 patients with a history of HF, LVEF <35%, and age >65 years enrolled in the GWTG-HF registry from January 2005 through December 2009. From 2005 to 2007, overall implantable cardioverter-defibrillator use increased from 30.2% to 42.4% and then remained unchanged in 2008 to 2009. After adjustment for confounders, implantable cardioverter-defibrillator use increased significantly in the overall study population during 2005 to 2007 (OR, 1.28; 95% CI, 1.11–1.48 per year; P=0.0008) and in black females (OR, 1.82; 95% CI, 1.28–2.58 per year; P=0.0008), white females (OR, 1.30; 95% CI, 1.06–1.59 per year; P=0.010), black males (OR, 1.54; 95% CI, 1.19–1.99 per year; P=0.0009), and white males (OR, 1.25; 95% CI, 1.06–1.48 per year; P=0.0072). The increase in implantable cardioverter-defibrillator use was greatest among blacks. They concluded that although previously described racial disparities in the use of implantable cardioverter-defibrillators were no longer present in their study by the end of the study period, a sex difference in their use persisted.
Data on the quality of care by race/ethnicity and sex in the GWTG-Stroke Program (2014) is provided in Table 25-13.
Table 25-12.
Quality of Care by Race/Ethnicity and Sex in the GWTG-HF Program, 2014
| Quality-of-Care Measure | White | Black | Hispanic | Males | Females |
|---|---|---|---|---|---|
| Postdischarge appointment (new for 2011)* | 62.6 | 64.8 | 62.7 | 63.8 | 62.5 |
| Complete set of discharge instructions† | 94.3 | 95.2 | 95.2 | 94.8 | 94.1 |
| Measure of LV function* | 99.4 | 99.3 | 99.3 | 99.4 | 99.2 |
| ACEI or ARB at discharge for patients with LVSD, no contraindications* | 95.6 | 96.6 | 96.1 | 96.0 | 96.0 |
| Smoking cessation counseling, current smokers† | 96.3 | 95.7 | 96.0 | 96.1 | 95.6 |
| Evidence-based specific β-blockers* | 89.2 | 92.0 | 89.2 | 90.6 | 89.3 |
| β-Blockers at discharge for patients with LVSD, no contraindications† | 97.8 | 98.2 | 98.3 | 98.0 | 97.9 |
| Hydralazine/nitrates at discharge for patients with LVSD, no contraindications‡ | … | 19.9 | … | 21.4 | 17.5 |
| Anticoagulation for AF or atrial flutter, no contraindications | 81.3 | 79.0 | 76.3 | 81.7 | 79.3 |
| Composite quality-of-care measure (using discharge instructions and β-blocker at discharge) | 96.8 | 97.0 | 97.1 | 96.8 | 96.6 |
Values are percentages. ACEI indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ellipses, data not available; GWTG-HF, Get With the Guidelines–Heart Failure; LV, left ventricular; and LVSD, left ventricular systolic dysfunction.
Indicates the 4 key achievement measures targeted in GWTG-HF.
Indicates historical key achievement measures in GWTG-HF.
For black patients only.
REFERENCES
- 1.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL American College of Cardiology; American Heart Association Task Force on Performance Measures. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–1712. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 3.Tsai TT, Maddox TM, Roe MT, Dai D, Alexander KP, Ho PM, Messenger JC, Nallamothu BK, Peterson ED, Rumsfeld JS National Cardiovascular Data Registry. Contraindicated medication use in dialysis patients undergoing percutaneous coronary intervention. JAMA. 2009;302:2458–2464. doi: 10.1001/jama.2009.1800. [DOI] [PubMed] [Google Scholar]
- 4.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63(pt A):2876–2877. doi: 10.1016/j.jacc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.López L, Weissman JS, Schneider EC, Weingart SN, Cohen AP, Epstein AM. Disclosure of hospital adverse events and its association with patients’ ratings of the quality of care. Arch Intern Med. 2009;169:1888–1894. doi: 10.1001/archinternmed.2009.387. [DOI] [PubMed] [Google Scholar]
- 6.Hira RS, Kennedy K, Nambi V, Jneid H, Alam M, Basra SS, Ho PM, Deswal A, Ballantyne CM, Petersen LA, Virani SS. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65:111–121. doi: 10.1016/j.jacc.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Eldridge N, Metersky ML, Verzier NR, Meehan TP, Pandolfi MM, Foody JM, Ho SY, Galusha D, Kliman RE, Sonnenfeld N, Krumholz HM, Battles J. National trends in patient safety for four common conditions, 2005-2011. N Engl J Med. 2014;370:341–351. doi: 10.1056/NEJMsa1300991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall HM, de Lemos JA, Enriquez JR, McGuire DK, Peng SA, Alexander KP, Roe MT, Desai N, Wiviott SD, Das SR. Contemporary patterns of discharge aspirin dosing after acute myocardial infarction in the United States: results from the National Cardiovascular Data Registry (NCDR) Circ Cardiovasc Qual Outcomes. 2014;7:701–707. doi: 10.1161/CIRCOUTCOMES.113.000822. [DOI] [PubMed] [Google Scholar]
- 9.Rao KK, Enriquez JR, de Lemos JA, Alexander KP, Chen AY, Mc-Guire DK, Fonarow GC, Das SR. Use of aldosterone antagonists at discharge after myocardial infarction: results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Am Heart J. 2013;166:709–715. doi: 10.1016/j.ahj.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SV, Spertus JA, Tang F, Krumholz HM, Borden WB, Farmer SA, Ting HH, Chan PS. Statin use in outpatients with obstructive coronary artery disease. Circulation. 2011;124:2405–2410. doi: 10.1161/CIRCULATIONAHA.111.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2014;63:539–546. doi: 10.1016/j.jacc.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:396] Circulation. 2014;129(suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 12.Virani SS, Woodard LD, Ramsey DJ, Urech TH, Akeroyd JM, Shah T, Deswal A, Bozkurt B, Ballantyne CM, Petersen LA. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115:21–26. doi: 10.1016/j.amjcard.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS, Kent ST, Brown TM, Farkouh ME, Levitan EB, Yun H, Sharma P, Safford MM, Kilgore M, Muntner P, Bittner V. Underutilization of high-intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65:270–277. doi: 10.1016/j.jacc.2014.09.088. [DOI] [PubMed] [Google Scholar]
- 14.Stub D, Schmicker RH, Anderson ML, Callaway CW, Daya MR, Sayre MR, Elmer J, Grunau BE, Aufderheide TP, Lin S, Buick JE, Zive D, Peterson ED, Nichol G ROC Investigators. Association between hospital post-resuscitative performance and clinical outcomes after out-of-hospital cardiac arrest. Resuscitation. 2015;92:45–52. doi: 10.1016/j.resuscitation.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP, Go AS, Greenlee RT, Magid DJ, Normand SL, Masoudi FA. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025–2034. doi: 10.1001/jama.2013.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. [Accessed September 6, 2016];Medicare Hospital Quality Chartbook: Performance Report on Outcome Measures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html.
- 19.Williams JB, Delong ER, Peterson ED, Dokholyan RS, Ou FS, Ferguson TB, Jr Society of Thoracic Surgeons and the National Cardiac Database. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation. 2011;123(1):39–45. doi: 10.1161/CIRCULATIONAHA.110.981068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB COURAGE Trial Research Group. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 21.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 23.Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, Bosworth HB, Radcliff T, Davis R, Mun H, Weaver J, Barnett C, Barón A, Del Giacco EJ. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014;174:186–193. doi: 10.1001/jamainternmed.2013.12944. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ Placement of Aortic Transcatheter Valves (PARTNER) Investigators. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 25.Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. doi: 10.1056/NEJMoa1208200. [DOI] [PubMed] [Google Scholar]
- 26.Smolderen KG, Spertus JA, Nallamothu BK, Krumholz HM, Tang F, Ross JS, Ting HH, Alexander KP, Rathore SS, Chan PS. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303:1392–1400. doi: 10.1001/jama.2010.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glickman SW, Lytle BL, Ou FS, Mears G, O’Brien S, Cairns CB, Garvey JL, Bohle DJ, Peterson ED, Jollis JG, Granger CB. Care processes associated with quicker door-in-door-out times for patients with ST-elevation-myocardial infarction requiring transfer: results from a statewide regionalization program. Circ Cardiovasc Qual Outcomes. 2011;4:382–388. doi: 10.1161/CIRCOUTCOMES.110.959643. [DOI] [PubMed] [Google Scholar]
- 28.Nallamothu BK, Normand SL, Wang Y, Hofer TP, Brush JE, Jr, Messenger JC, Bradley EH, Rumsfeld JS, Krumholz HM. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. 2015;385:1114–1122. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekundayo OJ, Saver JL, Fonarow GC, Schwamm LH, Xian Y, Zhao X, Hernandez AF, Peterson ED, Cheng EM. Patterns of emergency medical services use and its association with timely stroke treatment: findings from Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2013;6:262–269. doi: 10.1161/CIRCOUTCOMES.113.000089. [DOI] [PubMed] [Google Scholar]
- 30.Amin AP, Spertus JA, Cohen DJ, Chhatriwalla A, Kennedy KF, Vilain K, Salisbury AC, Venkitachalam L, Lai SM, Mauri L, Normand SL, Rumsfeld JS, Messenger JC, Yeh RW. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch Intern Med. 2012;172:1145–1152. doi: 10.1001/archinternmed.2012.3093. [DOI] [PubMed] [Google Scholar]
- 31.Virani SS, Woodard LD, Wang D, Chitwood SS, Landrum CR, Urech TH, Pietz K, Chen GJ, Hertz B, Murawsky J, Ballantyne CM, Petersen LA. Correlates of repeat lipid testing in patients with coronary heart disease. JAMA Intern Med. 2013;173:1439–1444. doi: 10.1001/jamainternmed.2013.8198. [DOI] [PubMed] [Google Scholar]
- 32.Virani SS, Maddox TM, Chan PS, Tang F, Akeroyd JM, Risch SA, Oetgen WJ, Deswal A, Bozkurt B, Ballantyne CM, Petersen LA. Provider type and quality of outpatient cardiovascular disease care: insights from the NCDR PINNACLE Registry. J Am Coll Cardiol. 2015;66:1803–1812. doi: 10.1016/j.jacc.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himmelstein DU, Wright A, Woolhandler S. Hospital computing and the costs and quality of care: a national study. Am J Med. 2010;123:40–46. doi: 10.1016/j.amjmed.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Al-Khatib SM, Hellkamp A, Curtis J, Mark D, Peterson E, Sanders GD, Heidenreich PA, Hernandez AF, Curtis LH, Hammill S. Non-evidence-based ICD implantations in the United States. JAMA. 2011;305:43–49. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302:1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis MB, Maddox TM, Langner P, Plomondon ME, Rumsfeld JS, Duvernoy CS. Characteristics and outcomes of women veterans undergoing cardiac catheterization in the Veterans Affairs Healthcare System: insights from the VA CART Program. Circ Cardiovasc Qual Outcomes. 2015;8(suppl 1):S39–S47. doi: 10.1161/CIRCOUTCOMES.114.001613. [DOI] [PubMed] [Google Scholar]
- 38.Kapoor JR, Kapoor R, Hellkamp AS, Hernandez AF, Heidenreich PA, Fonarow GC. Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58:1465–1471. doi: 10.1016/j.jacc.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, Bonow RO, Smith SC., Jr Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010;121:2294–2301. doi: 10.1161/CIRCULATIONAHA.109.922286. [DOI] [PubMed] [Google Scholar]
- 40.Thomas KL, Hernandez AF, Dai D, Heidenreich P, Fonarow GC, Peterson ED, Yancy CW. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED Get With the Guidelines Steering Committee and Hospitals. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
26. MEDICAL PROCEDURES
(See Tables 26-1 and 26-2 and Charts 26-1, 26-2, 26-3, 26-4)
Table 26-1.
2013 National HCUP Statistics: Mean Hospital Charges, In-Hospital Death Rates, and Mean Length of Stay for Various Cardiovascular Procedures
| Procedure | Mean Hospital Charges, $ | In-Hospital Death Rate, % | Mean Length of Stay, d | ICD-9-CM Procedure Codes |
|---|---|---|---|---|
| Total vascular and cardiac surgery and procedures | 84 691 | 3.18 | 6.2 | 35–39, 00.50–00.51, 00.53–00.55, 00.61–00.66 |
| Cardiac revascularization (bypass) | 160 477 | 1.85 | 9.3 | 36.1–36.3 |
| PCI | 79 354 | 1.82 | 3.3 | 00.66 |
| Cardiac catheterization | 54 489 | 1.25 | 4.0 | 37.21–37.23 |
| Pacemakers | 79 616 | 1.45 | 5.0 | 37.7–37.8, 00.50, 00.53 |
| Implantable defibrillators | 159 283 | 0.61 | 5.5 | 37.94–37.99, 00.51, 00.54 |
| Endarterectomy | 41 873 | 0.38 | 2.6 | 38.12 |
| Valves | 192 703 | 3.08 | 10.2 | 35.1–35.2, 35.99 |
| Heart transplantations | 758 847 | 3.65 | 42.0 | 37.51 |
HCUP indicates Healthcare Cost and Utilization Project; ICD-9-CM, International Classification of Diseases, Clinical Modification, 9th Revision; and PCI, percutaneous coronary intervention.
Data derived from the Agency for Healthcare Research and Quality, HCUP Nationwide Inpatient Sample, 2013.
Table 26-2.
Estimated* Inpatient Cardiovascular Operations, Procedures, and Patient Data by Sex and Age: United States, 2010 (in Thousands)
| Operation/Procedure/Patients | ICD-9-CM Procedure Codes | All | Sex | Age, y | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | <15 | 15–44 | 45–64 | ≥65 | |||
| Valves | 35.1, 35.2, 35.99 | 106 | 64 | 42 | 4† | 5† | 32 | 65 |
| Angioplasty | 36.0, 0.66 | 955 | 642 | 313 | … | 44 | 421 | 489 |
| PCI (patients) | 36.06, 36.07, 0.66 | 492 | 330 | 162 | … | 23 | 216 | 253 |
| PCI | 0.66 | 500 | 334 | 166 | … | 23 | 220 | 257 |
| PCI with stents | 36.06, 36.07 | 454 | 308 | 146 | … | 21 | 201 | 233 |
| Cardiac revascularization‡ | 36.1–36.3 | 397 | 298 | 99 | … | 9† | 157 | 231 |
| Cardiac revascularization (patients) | 36.1–36.3 | 219 | 164 | 55 | … | 5† | 86 | 128 |
| Cardiac catheterization | 37.21–37.23 | 1029 | 638 | 391 | 7† | 64 | 456 | 502 |
| Pacemakers | 37.7, 37.8, 00.50, 00.53 | 370 | 196 | 174 | 3† | 6† | 57 | 305 |
| Pacemaker devices | 37.8, 00.53 | 159 | 81 | 78 | 1† | 3† | 20 | 135 |
| Pacemaker leads | 37.7, 00.50 | 212 | 115 | 96 | 1† | 3† | 36 | 171 |
| Implantable defibrillators | 37.94–37.99, 00.51, 00.54 | 97 | 71 | 26 | … | 8† | 31 | 58 |
| Endarterectomy | 38.12 | 100 | 55 | 45 | … | … | 29 | 71 |
| Total vascular and cardiac surgery and procedures§|| | 35–39, 00.50–00.51, 00.53–00.55, 00.61–00.66 | 7588 | 4397 | 3191 | 310 | 681 | 2706 | 3891 |
These data do not reflect any procedures performed on an outpatient basis. Many more procedures are being performed on an outpatient basis. Some of the lower numbers in this table compared with 2006 probably reflect this trend. Data include procedures performed on newborn infants.
Ellipses (…) indicate data not available; ICD-9-CM, International Classification of Diseases, Clinical Modification, 9th Revision; and PCI, percutaneous coronary intervention.
Breakdowns are not available for some procedures, so entries for some categories do not add to totals. These data include codes for which the estimated number of procedures is <5000. Categories with such small numbers are considered unreliable by the National Center for Health Statistics and in some cases may have been omitted.
Estimate should be used with caution because it may be unreliable or does not meet standards of reliability or precision.
Because ≥1 procedure codes are required to describe the specific bypass procedure performed, it is impossible from these (mixed) data to determine the average number of grafts per patient.
Totals include procedures not shown here.
This estimate includes angioplasty and stent insertions for noncoronary arteries.
Data derived from the National Hospital Discharge Survey/National Center for Health Statistics, 2010. Estimates are based on a sample of inpatient records from short-stay hospitals in the United States.
Chart 26-1. Trends in cardiovascular procedures, United States: 1979 to 2010; inpatient procedures only.
PCI indicates percutaneous coronary intervention.
Source: National Hospital Discharge Survey, National Center for Health Statistics, and National Heart, Lung, and Blood Institute.
Chart 26-2. Number of surgical procedures in the 10 leading diagnostic groups, United States: 2010.
Source: National Hospital Discharge Survey/National Center for Health Statistics and National Heart, Lung, and Blood Institute.
Chart 26-3. Trends in heart transplantations, 1975 to 2015.
Source: Organ Procurement and Transplantation Network data as of March 31, 2016.
Chart 26-4. Heart transplantations in the United States by recipient age, 2015.
Source: Organ Procurement and Transplantation Network data as of March 31, 2016.
Trends in Operations and Procedures
(See Tables 26-1 and 26-2 and Charts 26-1 through 26-4)
The total number of inpatient cardiovascular operations and procedures increased 28%, from 5 939 000 in 2000 to 7 588 000 in 2010 (NHLBI computation based on NCHS annual data).
Data from the NHDS were examined for trends from 1990 to 2010 for use of PCI and CABG for ages ≥18 years (discharge rates are age-adjusted) 1:
Abbreviations Used in Chapter 26
| ASD | atrial septal defect |
| CABG | coronary artery bypass graft |
| CAD | coronary artery disease |
| CDC | Centers for Disease Control and Prevention |
| DES | drug-eluting stent |
| GWTG | Get With the Guidelines |
| HCUP | Healthcare Cost and Utilization Project |
| HLHS | hypoplastic left heart syndrome |
| ICD-9-CM | International Classification of Diseases, 9th Revision, Clinical Modification |
| NCHS | National Center for Health Statistics |
| NHDS | National Hospital Discharge Survey |
| NHLBI | National Heart, Lung, and Blood Institute |
| PCI | percutaneous coronary intervention |
| PTCA | percutaneous transluminal coronary angioplasty |
| STEMI | ST-segment–elevation myocardial infarction |
| STS | Society of Thoracic Surgeons |
| VSD | ventricular septal defect |
Coronary Artery Bypass Grafting
Discharge rates for CABG (per 10 000 population) increased from 22.8 in 1990 to 31.2 in 1996 and declined to 16.0 by 2010.
In 1990, discharge rates (per 10 000 population) for CABG were 36.9 for males and 11.0 for females; these rates increased through the 1999, then declined to 26.2 and 7.4, respectively, by 2010.
Percutaneous Coronary Intervention
PCI discharge rates (per 10 000 population) increased from 23.8 for males and 8.5 for females to 44.2 and 18.8, respectively, over the 12-year time interval; then discharge rates declined to 29.2 and 12.4, respectively by 2010.
Discharge rates (per 10 000 population) for PCI increased from 15.6 in 1990 to 30.5 in 2002 and declined to 20.2 by 2010.
Data on Medicare beneficiaries undergoing a coronary revascularization procedure between 2008 and 2012 indicate that the rapid growth in nonadmission PCIs (from 60 405 to 106 495) has been more than offset by the decrease in PCI admissions (from 363 384 to 295 434).2
Cardiac Catheterization and PCI
(See Tables 26-1 and 26-2)
From 2000 to 2010, the number of cardiac catheterizations decreased slightly, from 1 221 000 to 1 029 000 annually (NHDS, NHLBI tabulation).
In 2010, an estimated 492 000 patients underwent PCI (previously referred to as percutaneous transluminal coronary angioplasty, or PTCA) procedures in the United States (NHDS, NHLBI tabulation).
In 2010, ≈67% of PCI procedures were performed on males, and ≈51% were performed on people ≥65 years of age (NHDS, NHLBI tabulation).
In-hospital death rates for PCI have remained stable, although comorbidities increased for patients who received the procedure.1
In 2010, ≈75% of stents implanted during PCI were DES compared with 25% that were baremetal stents (NHDS, NHLBI computation).
In a study of nontransferred patients with STEMI treated with primary PCI from July 2006 to March 2008, there was significant improvement over time in the percentage of patients receiving PCI within 90 minutes, from 54.1% from July to September 2006 to 74.1% from January to March 2008, among hospitals participating in the GWTG-CAD program. This improvement was seen whether or not hospitals joined the Door-to-Balloon Alliance during that period.3
The rate of any cardiac stent procedure rose by 61% from 1999 to 2006, then declined by 27% between 2006 and 2009.4
Cardiac Open Heart Surgery
The NHDS (NCHS/CDC) estimates that in 2010, in the United States, 219 000 patients underwent a total of 397 000 CABG procedures (defined by procedure codes). CABG volumes have declined nationally since 1998. Risk-adjusted mortality for CABG has declined significantly over the past decade.
Data from the STS Adult Cardiac Surgery Database, which voluntarily collects data from ≈80% of all hospitals that perform CABG in the United States, indicate that a total of 147 528 procedures involved isolated CABG in 2014.5
Congenital Heart Surgery, 2010 to 2014 (From STS)
Data from the STS Congenital Heart Surgery Database indicate that there were 117 038 procedures performed from January 2011 to December 2014. The in-hospital mortality rate was 3.3% during that time period. The 5 most common diagnoses were HLHS (6.4%), type 2 VSD (6.2%), patent ductus arteriosus (5.2%), secundum ASD (4.1%), and other cardiac (5.4%).6
The 5 most common primary procedures were delayed sternal closure (6.8%), patch VSD repair (6.4%), mediastinal exploration (3.5%), patch ASD repair (3.3%), and complete atrioventricular canal (atrioventricular septal defect) repair (2.9%).
Heart Transplantations (Organ Procurement and Transplantation Network, May 27, 2016)
(See Charts 26-3 and 26-4)
In 2015, 2804 heart transplantations were performed in the United States (Chart 26-3). There are 250 transplant hospitals in the United States, 135 of which performed heart transplantations (based on Organ Procurement and Transplantation Network data as of March 31, 2016).
Of the recipients in 2015, 71.4% were male, and 61.4% were white; 21.6% were black, whereas 11.1% were Hispanic. Heart transplantations by recipient age are shown in Chart 26-4.
For transplants that occurred between 2009 and 2010, the 1-year survival rate was 90.8% for males and 90.6% for females; the 5-year rates between 2005 and 2010 were 77.5% for males and 75.6% for females. The 1- and 5-year survival rates for white cardiac transplant patients were 91.2% and 79.1%, respectively. For black patients, they were 88.3% and 68.6%, respectively. For Hispanic patients, they were 91.9% and 76.3%, respectively. For Asian patients, they were 89.9% and 81.2%, respectively.
As of May 27, 2016, 4134 patients were on the transplant waiting list for a heart transplant, and 40 patients were on the list for a heart/lung transplant.
REFERENCES
- 1.National Center for Health Statistics. Health Data Interactive. [Accessed May 31, 2016];Centers for Disease Control and Prevention Web site. www.cdc.gov/nchs/hdi.htm.
- 2.Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. doi: 10.1161/CIRCULATIONAHA.114.012485. [DOI] [PubMed] [Google Scholar]
- 3.Nallamothu BK, Krumholz HM, Peterson ED, et al. Door-to-balloon times in hospitals within the Get-With-the-Guidelines registry after initiation of the Door-to-Balloon (D2B) Alliance. Am J Cardiol. 2009;103:1051–1055. doi: 10.1016/j.amjcard.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Auerbach D, Maeda J, Steiner C. Hospital Stays With Cardiac Stents, 2009. Rockville, MD: Agency for Health Care Research and Quality; Apr, 2012. [Accessed July 23, 2012]. HCUP Statistical Brief #128. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb128.pdf. [PubMed] [Google Scholar]
- 5.Adult Cardiac Surgery Database: Executive Summary: 10 Years: STS Period Ending 6/30/2015. [Accessed March 31, 2016];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/2015Harvest3_ExecutiveSummary.pdf.
- 6.STS Congenital Heart Surgery Executive Summary: All Patients: STS Period Ending 12/31/2014. [Accessed March 31, 2016];Society of Thoracic Surgeons Web site. http://www.sts.org/sites/default/files/documents/Congenital-STSExecSummary_AllPatients_0.pdf.
27. ECONOMIC COST OF CARDIOVASCULAR DISEASE
(See Tables 27-1 and 27-2 and Charts 27-1, 27-2, 27-3, 27-4, 27-5)
Table 27-1.
Estimated Direct and Indirect Costs (in Billions of Dollars) of CVD and Stroke: United States, Average Annual 2012 to 2013
| Heart Disease* | Stroke | Hypertensive Disease† | Other Circulatory Conditions | Total Cardiovascular Disease | |
|---|---|---|---|---|---|
| Direct costs‡ | |||||
| Hospital inpatient stays | 55.1 | 10.0 | 7.2 | 16.0 | 88.3 |
| Hospital ED visits | 5.4 | 1.0 | 1.7 | 1.1 | 9.2 |
| Hospital outpatient or office-based provider visits | 20.1 | 2.0 | 13.3 | 6.1 | 41.5 |
| Home health care | 8.3 | 3.9 | 5.4 | 0.7 | 18.3 |
| Prescribed medicines | 9.8 | 1.0 | 19.7 | 1.9 | 32.4 |
| Total expenditures | 98.7 | 17.9 | 47.3 | 25.8 | 189.7 |
| Indirect costs§ | |||||
| Lost productivity/mortality | 100.9 | 16.0 | 3.9 | 5.6 | 126.4 |
| Grand totals | 199.6 | 33.9 | 51.2 | 31.4 | 316.1 |
Numbers do not add to total because of rounding.
This category includes coronary heart disease, heart failure, part of hypertensive disease, cardiac dysrhythmias, rheumatic heart disease, cardiomyopathy, pulmonary heart disease, and other or ill-defined heart diseases.
Costs due to hypertensive disease are limited to hypertension without heart disease.
Medical Expenditure Panel Survey healthcare expenditures are estimates of direct payments for care of a patient with the given disease provided during the year, including out-of-pocket payments and payments by private insurance, Medicaid, Medicare, and other sources. Payments for over-the-counter drugs are not included. These estimates of direct costs do not include payments attributed to comorbidities. Total CVD costs are the sum of costs for the 4 diseases but with some duplication.
The Statistics Committee agreed to suspend presenting estimates of lost productivity due to morbidity until a better estimating method can be developed. Lost future earnings of persons who died in 2012 to 2013, discounted at 3%.
Sources: Estimates from the Household Component of the Medical Expenditure Panel Survey of the Agency for Healthcare Research and Quality for direct costs (average annual 2012–2013).1 Indirect mortality costs are based on 2012 to 2013 counts of deaths by the National Center for Health Statistics and an estimated present value of lifetime earnings furnished for 2010 by Wendy Max, PhD (Institute for Health and Aging, University of California, San Francisco, April 29, 2015) and inflated to 2012 from change in worker compensation reported by the US Bureau of Labor Statistics. The present value of lifetime earnings for 2013 was also furnished by Dr Max.
All estimates prepared by Michael Mussolino, National Heart, Lung, and Blood Institute.
Table 27-2.
Costs of Total CVD and Stroke in Billions of Dollars by Age and Sex: United States, Average Annual 2012 to 2013
| Total | Males | Females | Age <65 y | Age ≥65 y | |
|---|---|---|---|---|---|
| Direct | 189.7 | 98.8 | 90.9 | 91.6 | 98.1 |
| Indirect mortality | 126.4 | 94.1 | 32.3 | 107.9 | 18.5 |
| Total | 316.1 | 192.9 | 123.2 | 199.5 | 116.6 |
CVD indicates cardiovascular disease.
Numbers do not add to total because of rounding.
Source: Medical Expenditure Panel Survey, average annual 2012 to 2013 (direct costs) and mortality data from the National Center for Health Statistics and present value of lifetime earnings from the Institute for Health and Aging, University of California, San Francisco (indirect costs).
All estimates prepared by Michael Mussolino, National Heart, Lung, and Blood Institute.
Chart 27-1. Direct and indirect costs of CVD and stroke (in billions of dollars), United States, average annual 2012 to 2013.
CVD indicates cardiovascular disease.
Source: Prepared by the National Heart, Lung, and Blood Institute.1–4
Chart 27-2. The 23 leading diagnoses for direct health expenditures, United States, average annual 2012 to 2013 (in billions of dollars).
COPD indicates chronic obstructive pulmonary disease; and GI, gastrointestinal (tract).
Source: National Heart, Lung, and Blood Institute; estimates are from the Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality, and exclude nursing home costs.
Chart 27-3. Projected total costs of CVD, 2015 to 2030 (2012 dollars in billions) in the United States.
Unpublished data tabulated by the American Heart Association using methods described in Heidenreich et al.6
CHD indicates coronary heart disease; CHF, congestive heart failure; CVD, cardiovascular disease; and HBP, high blood pressure.
Chart 27-4. Projected total (direct and indirect) costs of total cardiovascular disease by age (2012 dollars in billions).
Unpublished data tabulated by the American Heart Association using methods described in Heidenreich et al.6
Chart 27-5. Projected direct costs of total cardiovascular disease by type of cost (2010 dollars in billions).
Unpublished data tabulated by the American Heart Association using methods described in Heidenreich et al.6
Using data from MEPS, the annual direct and indirect cost of CVD and stroke in the United States is an estimated $316.1 billion (Table 27-1; Chart 27-1). This figure includes $189.7 billion in expenditures (direct costs, which include the cost of physicians and other professionals, hospital services, prescribed medication, and home health care, but not the cost of nursing home care) and $126.4 billion in lost future productivity attributed to premature CVD and stroke mortality in 2012 to 2013 (indirect costs).
The direct costs for CVD and stroke are the healthcare expenditures for 2012 to 2013 (average annual) available on the Web site of the nationally representative MEPS of the AHRQ.1 Details on the advantages or disadvantages of using MEPS data are provided in the “Heart Disease and Stroke Statistics–2011 Update.”2 Indirect mortality costs are estimated for 2012 to 2013 (average annual) by multiplying the number of deaths for those years attributable to CVD and strokes, in age and sex groups, by estimates of the present value of lifetime earnings for those age and sex groups as of 2012 to 2013. Mortality data are from the National Vital Statistics System of the NCHS.3 The present values of lifetime earnings are unpublished estimates furnished by the Institute for Health and Aging, University of California, San Francisco, by Wendy Max, PhD, on April 29, 2015. Those estimates have a 3% discount rate, which is the recommended percentage.4 The discount rate removes the effect of inflation in income over the lifetime of earnings. The estimate is for 2010, inflated to 2012 to account for the 2010 to 2012 change in hourly worker compensation in the business sector reported by the US Bureau of Labor Statistics.5 The present value of lifetime earnings for 2013 was also furnished by Dr Max.
Abbreviations Used in Chapter 27
| AHA | American Heart Association |
| AHRQ | Agency for Healthcare Research and Quality |
| CHD | coronary heart disease |
| CHF | congestive heart failure |
| COPD | chronic obstructive pulmonary disease |
| CVD | cardiovascular disease |
| GI | gastrointestinal (tract) |
| HBP | high blood pressure |
| HD | heart disease |
| HF | heart failure |
| MEPS | Medical Expenditure Panel Survey |
| NCHS | National Center for Health Statistics |
The indirect costs exclude lost productivity costs attributable to CVD and stroke illness during 2012 to 2013 among workers, people keeping house, people in institutions, and people unable to work. Those morbidity costs were substantial in very old studies, but an adequate update could not be made.
Most Costly Diseases
(See Table 27-2 and Chart 27-2)
CVD and stroke accounted for 14% of total health expenditures in 2012 to 2013, more than any major diagnostic group.1 By way of comparison, CVD total direct costs shown in Table 27-1 are higher than the 2012 to 2013 AHRQ estimates for cancer, which were $81.2 billion (43% for outpatient or doctor office visits, 41% for inpatient care, and 10% for prescription drugs).1
Table 27-2 shows direct and indirect costs for CVD by sex and by 2 broad age groups. Chart 27-2 shows total direct costs for the 23 leading chronic diseases in the MEPS list. HD is the most costly condition.1
Projections
(See Charts 27-3 through 27-5)
The AHA developed methodology to project future costs of care for HBP, CHD, HF, stroke, and all other CVD.6
By 2030, 43.9% of the US population is projected to have some form of CVD.
Between 2012 and 2030, total direct medical costs of CVD are projected to increase from $396 billion to $918 billion (2012 $ in billions). Of this total, 60.5% is attributable to hospital costs, 15.6% to medications, 10.8% to physicians, 6.8% to nursing home care, 5.3% to home health care, and 1.1% to other costs.
Indirect costs (attributable to lost productivity) for all CVDs are estimated to increase from $183 billion in 2012 to $290 billion in 2030 (2012 $ in billions), an increase of 58%.
These data indicate that CVD prevalence and costs are projected to increase substantially.
REFERENCES
- 1.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey: household component summary tables. Table 7: Total expenses and percent distribution for selected conditions by type of service: United States, average annual 2012–2013. [Accessed August 9, 2016];Agency for Healthcare Research and Quality Web site. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HC2Y2013&Table=HC2Y2013%5FCNDXP%5FC&_Debug=
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: a report from the American Heart Association [published corrections appear in Circulation. 2011;124:e426 and Circulation. 2011;123:e240] Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. [Accessed July 15, 2016];Mortality multiple cause microdata files, 2013: public-use data file and documentation: NHLBI tabulations. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm#Mortality_Multiple.
- 4.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 5.Bureau of Labor Statistics, Office of Compensation Levels and Trends. Employment Cost Index, Historical Listing–Volume V: Continuous Occupational and Industry Series: September 1975–September 2016. [Accessed October 31, 2016];Table 4: employment cost index for total compensation, for civilian workers, by occupation and industry. http://www.bls.gov/web/eci/ecicois.pdf.
- 6.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
28. AT-A-GLANCE SUMMARY TABLES
See Tables 28-1, 28-2, 28-3
Table 28-1.
Males and CVD: At-a-Glance Table
| Diseases and Risk Factors | Both Sexes | Total Males | NH White Males | NH Black Males | Hispanic Males | NH Asian Males | NH American Indian/Alaska Native Males |
|---|---|---|---|---|---|---|---|
| Smoking | |||||||
| Prevalence, 2015* | 37.6 M (15.2%) | 20.1 M (16.7%) | 17.8% | 20.3% | 12.7% | 11.6% | 20.9% |
| PA† | |||||||
| Prevalence, 2015, %* | 21.6 | 25.3 | 23.5‡ | 19.9‡ | 16.8‡ | 19.1‡ | 18.8‡ |
| Overweight and obesity | |||||||
| Prevalence, 2011–2014 | |||||||
| Overweight and obesity, BMI >25.0 kg/m2§ | 164.9 M (69.4%) | 83.6 M (72.5%) | 73.0% | 69.1% | 79.6% | 46.6% | … |
| Obesity, BMI >30.0 kg/m2§ | 86.1 M (36.3%) | 39.5 M (34.3%) | 33.6% | 37.5% | 39.0% | 11.2% | 42.3%*‡ |
| Blood cholesterol | |||||||
| Prevalence, 2014 | |||||||
| Total cholesterol >200 mg/dL§ | 94.6 M (39.7%) | 42.3 M (37.0%) | 37.0% | 32.6% | 43.1% | 39.9% | … |
| Total cholesterol >240 mg/dL§ | 28.5 M (11.9%) | 12.1 M (10.6%) | 10.8% | 7.3% | 13.6% | 10.8% | … |
| LDL-C >130 mg/dL§ | 71.3 M (30.3%) | 34.0 M (30.0%) | 29.3% | 29.9% | 36.6% | 29.2% | … |
| HDL-C <40 mg/dL§ | 44.0 M (18.7%) | 32.1 M (27.9%) | 28.4% | 20.7% | 30.7% | 25.0% | … |
| HBP | |||||||
| Prevalence, 2011–2014§ | 85.7 M (34.0%) | 40.8 M (34.5%) | 34.5% | 45.0% | 28.9% | 28.8% | 26.4%*‡ |
| Mortality, 2011–2014, n|| | 73 345 | 34 688 | 23 232 | 7448 | 2627 | 916 | 440‡ |
| DM | |||||||
| Prevalence, 2011–2014 | |||||||
| Physician-diagnosed DM§ | 23.4 M (9.1%) | 11.4 M (9.4%) | 8.0% | 14.1% | 12.6% | 11.8% | … |
| Undiagnosed DM§ | 7.6 M (3.1%) | 4.5 M (3.8%) | 3.6% | 2.8% | 6.3% | 5.7% | … |
| Prediabetes§ | 81.6 M (33.9%) | 46.2 M (40.2%) | 39.6% | 32.8% | 45.9% | 42.0% | … |
| Incidence, diagnosed DM§ | 1.7 M | … | … | … | … | … | … |
| Mortality, 2014, n|| | 76 488 | 41 111 | 28 743 | 6369 | 4149 | 1208 | 908‡ |
| Total CVD | |||||||
| Prevalence, 2011–2014§ | 92.1 M (36.6%) | 44.3 M (37.4%) | 37.7% | 46.0% | 31.3% | 31.0% | … |
| Mortality, 2014, n||¶ | 807 775 | 408 747 | 320 859 | 49 210 | 24 875 | 9784 | 4054‡ |
| Stroke | |||||||
| Prevalence, 2014§ | 7.2 M (2.7%) | 3.1 M (2.4%) | 2.2% | 3.9% | 2.0% | 1.0% | 3.0%*‡ |
| New and recurrent strokes, n|| | 795.0 K | 370.0 K | 325.0 K | 45.0 K | … | … | … |
| Mortality, 2014, n|| | 133 103 | 55 471 | 41 410 | 7650 | 4092 | 1890 | 616‡ |
| CHD | |||||||
| Prevalence, CHD, 2011–2014§ | 16.5 M (6.3%) | 9.1 M (7.4%) | 7.7% | 7.1% | 5.9% | 5.0% | 6.0%*‡ |
| Prevalence, MI, 2011–2014§ | 7.9 M (3.0%) | 4.7 M (3.8%) | 4.0% | 3.3% | 2.9% | 2.6% | … |
| Prevalence, AP, 2011–2014§ | 8.7 M (3.4%) | 4.2 M (3.5%) | 3.7% | 3.5% | 2.7% | 2.0% | … |
| New and recurrent MI and fatal CHD, n#** | 1.02 M | 590.0 K | 505.0 K | 85.0 K | … | … | … |
| New and recurrent MI, n#** | 790.0 K | 465.0 K | … | … | … | … | … |
| Incidence, AP (stable angina), n†† | 565.0 K | 370.0 K | … | … | … | … | … |
| Mortality, 2014, CHD, n|| | 364 593 | 207 412 | 166 752 | 20 883 | 12 594 | 4862 | 2009‡ |
| Mortality, 2014, MI, n|| | 114 019 | 65 081 | 52 767 | 6285 | 4006 | 1455 | 598‡ |
| HF | |||||||
| Prevalence, 2011–2014§ | 6.5 M (2.5%) | 2.9 M (2.4%) | 2.4% | 2.6% | 2.0% | 1.3% | … |
| Incidence, 2013, n#‡‡ | 960.0 K | 470.0 K | 410.0 K | 60.0 K | … | … | … |
| Mortality, 2014, n|| | 68 626 | 30 339 | 25 316 | 3145 | 1256 | 452 | 233‡ |
AP indicates angina pectoris (chest pain); BMI, body mass index; CHD, coronary heart disease (includes heart attack, AP chest pain, or both); CVD, cardiovascular disease; DM, diabetes mellitus; ellipses (…), data not available; HBP, high blood pressure; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; K, thousands; LDL-C, low-density lipoprotein cholesterol; M, millions; MI, myocardial infarction (heart attack); NH, non-Hispanic; and PA, physical activity.
Age ≥18 years (National Health Interview Survey, 2014).
Met 2008 full federal PA guidelines for adults.
Both sexes.
Age ≥20 years.
All ages.
Total CVD mortality includes deaths of congenital heart disease.
Estimates include Hispanics and non-Hispanics. Estimates for whites include other nonblack races.
Age ≥35 years.
Age ≥45 years.
Age ≥55 years.
Table 28-2.
Females and CVD: At-a-Glance Table
| Diseases and Risk Factors | Both Sexes | Total Females | NH White Females | NH Black Females | Hispanic Females | NH Asian Females | NH American Indian/Alaska Native Females |
|---|---|---|---|---|---|---|---|
| Smoking | |||||||
| Prevalence, 2015* | 37.6 M (15.2%) | 17.4 M (13.7%) | 16.8% | 13.1% | 7.0% | 2.6% | 22.2% |
| PA† | |||||||
| Prevalence, 2015, %* | 21.6 | 18.0 | 23.5‡ | 19.9‡ | 16.8‡ | 19.1‡ | 18.8‡ |
| Overweight and obesity | |||||||
| Prevalence, 2014 | |||||||
| Overweight and obesity, BMI >25.0 kg/m2§ | 164.9 M (69.4%) | 81.3 M (66.4%) | 63.7% | 82.2% | 77.1% | 34.6% | … |
| Obesity, BMI >30.0 kg/m2§ | 86.1 M (36.3%) | 46.6 M (38.3%) | 35.5% | 56.9% | 45.7% | 11.9% | 42.3%*‡ |
| Blood cholesterol | |||||||
| Prevalence, 2014 | |||||||
| Total cholesterol >200 mg/dL§ | 94.6 M (39.7%) | 52.3 M (42.0%) | 43.4% | 36.1% | 41.2% | 40.5% | … |
| Total cholesterol >240 mg/dL§ | 28.5 M (11.9%) | 16.4 M (13.0%) | 13.8% | 9.6% | 12.5% | 11.2% | … |
| LDL-C >130 mg/dL§ | 71.3 M (30.3%) | 37.3 M (30.4%) | 32.1% | 27.9% | 28.7% | 25.0% | … |
| HDL-C <40 mg/dL§ | 44.0 M (18.7%) | 11.9 M (10.0%) | 10.3% | 8.0% | 11.8% | 6.7% | … |
| HBP | |||||||
| Prevalence, 2011–2014§ | 85.7 M (34.0%) | 44.9 M (33.4%) | 32.3% | 46.3% | 30.7% | 25.7% | 26.4%*‡ |
| Mortality, 2011–2014, n|| | 73 345 | 38 657 | 27 618 | 7276 | 2406 | 1025 | 440‡ |
| DM | |||||||
| Prevalence, 2011–2014 | |||||||
| Physician-diagnosed DM§ | 23.4 M (9.1%) | 12.0 M (8.9%) | 7.4% | 13.6% | 12.7% | 9.1% | … |
| Undiagnosed DM§ | 7.6 M (3.1%) | 3.1 M (2.3%) | 1.5% | 3.5% | 4.4% | 4.3% | … |
| Prediabetes§ | 81.6 M (33.9%) | 35.4 M (27.8%) | 29.2% | 24.1% | 25.0% | 25.5% | … |
| Incidence, diagnosed DM§ | 1.7 M | … | … | … | … | … | … |
| Mortality, 2014, n|| | 76 488 | 35 377 | 23 201 | 6895 | 3646 | 1115 | 908‡ |
| Total CVD | |||||||
| Prevalence, 2011–2014§ | 92.1 M (36.6%) | 47.8 M (35.9%) | 35.1% | 47.7% | 33.3% | 27.0% | … |
| Mortality, 2014, n||¶ | 807 775 | 399 028 | 316 843 | 48 573 | 21 571 | 9147 | 4054‡ |
| Stroke | |||||||
| Prevalence, 2014§ | 7.2 M (2.7%) | 4.1 M (2.9%) | 2.8% | 4.0% | 2.6% | 2.5% | 3.0%*‡ |
| New and recurrent strokes, n|| | 795.0 K | 425.0 K | 365.0 K | 60.0 K | … | … | … |
| Mortality, 2014, n|| | 133 103 | 77 632 | 60 916 | 9233 | 4621 | 2382 | 616‡ |
| CHD | |||||||
| Prevalence, CHD, 2011–2014§ | 16.5 M (6.3%) | 7.4 M (5.3%) | 5.3% | 5.7% | 6.1% | 2.6% | 6.0%*‡ |
| Prevalence, MI, 2011–2014§ | 7.9 M (3.0%) | 3.2 M (2.3%) | 2.4% | 2.2% | 2.1% | 0.7% | … |
| Prevalence, AP, 2011–2014§ | 8.7 M (3.4%) | 4.5 M (3.3%) | 3.3% | 3.3% | 3.8% | 1.3% | … |
| New and recurrent MI and fatal CHD, n#** | 1.02 M | 430.0 K | 360.0 K | 70.0 K | … | … | … |
| New and recurrent MI, n#** | 790.0 K | 325.0 K | … | … | … | … | … |
| Incidence, AP (stable angina), n†† | 565.0 K | 195.0 K | … | … | … | … | … |
| Mortality, 2014, CHD, n || | 364 593 | 157 181 | 125 127 | 17 960 | 9277 | 3498 | 2009‡ |
| Mortality, 2014, MI, n|| | 114 019 | 48 938 | 38 778 | 5738 | 2957 | 1087 | 598‡ |
| HF | |||||||
| Prevalence, 2011–2014§ | 6.5 M (2.5%) | 3.6 M (2.6%) | 2.5% | 3.9% | 2.4% | 0.3% | … |
| Incidence, 2013, n#‡‡ | 960.0 K | 490.0 K | 415.0 K | 75.0 K | … | … | … |
| Mortality, 2014, n|| | 68 626 | 38 287 | 32 206 | 3817 | 1486 | 577 | 233‡ |
AP indicates angina pectoris (chest pain); BMI, body mass index; CHD, coronary heart disease (includes heart attack, AP chest pain, or both); CVD, cardiovascular disease; DM, diabetes mellitus; ellipses (…), data not available; HBP, high blood pressure; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; K, thousands; LDL-C, low-density lipoprotein cholesterol; M, millions; MI, myocardial infarction (heart attack); NH, non-Hispanic; and PA, physical activity.
Age ≥18 years (National Health Interview Survey, 2014).
Met 2008 full federal PA guidelines for adults.
Both sexes.
Age ≥20 years.
All ages.
Total CVD mortality includes deaths of congenital heart disease.
Estimates include Hispanics and non-Hispanics. Estimates for whites include other nonblack races.
Age ≥35 years.
Age ≥45 years.
Age ≥55 years.
Table 28-3.
Children, Youth, and CVD: At-a-Glance Table
| Diseases and Risk Factors | Both Sexes | Total Males | Total Females | NH Whites | NH Blacks | Hispanic | NH Asian | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | ||||
| Smoking, % | |||||||||||
| Prevalence, ages 12–17 y, 2014* | |||||||||||
| Current cigarette smoking, 2014 | 4.9% | 5.1% | 4.6% | 6.3% | 2.2% | 3.8% | 1.3% | ||||
| Current cigar smoking, 2013 | 2.1% | 2.7% | 1.5% | 2.7% | 2.1% | 1.1% | 0.4% | ||||
| Current smokeless tobacco use, 2014 | 2.0% | 3.3% | 0.6% | 3.1% | 0.2% | 0.6% | 0.3% | ||||
| PA† | |||||||||||
| Prevalence, grades 9–12, 2015 | |||||||||||
| Met currently recommended levels of PA‡ | 27.1% | 36.0% | 17.7% | 38.5% | 19.5% | 30.8% | 16.6% | 34.2% | 14.7% | … | … |
| Overweight and obesity | |||||||||||
| Prevalence, 2013–2014§ | |||||||||||
| Children and adolescents, aged 2–19 y, overweight or obese | (33.4%) | 33.4% | 34.1% | … | … | … | … | … | … | … | … |
| Children and adolescents, aged 2–19 y, obese | 12.6 M (16.9%) | 6.4 M (16.7%) | 6.2 M (17.2%) | 15.9% | 14.6% | 16.8% | 20.9% | 20.6% | 22.1% | 12.1% | 5.0% |
| Blood cholesterol, mg/dL, 2011–2014 | |||||||||||
| Mean total cholesterol | |||||||||||
| Ages 6–11 y | 158.9 | 158.5 | 159.3 | 156.5 | 159.6 | 162.1 | 162.2 | 159.5 | 156.9 | 161.9 | 167.6 |
| Ages 12–19 y | 156.7 | 152.3 | 161.3 | 151.7 | 162.0 | 152.3 | 159.5 | 154.7 | 160.5 | 158.1 | 166.7 |
| Mean HDL-C | |||||||||||
| Ages 6–11 y | 54.3 | 55.6 | 52.9 | 55.1 | 52.8 | 60.0 | 56.3 | 54.3 | 51.3 | 55.8 | 54.5 |
| Ages 12–19 y | 51.0 | 49.1 | 52.9 | 48.3 | 52.0 | 52.4 | 55.7 | 48.8 | 52.8 | 52.0 | 57.1 |
| Mean LDL-C | |||||||||||
| Ages 12–19 y | 87.7 | 85.7 | 89.8 | 86.5 | 89.9 | 86.6 | 90.9 | 85.9 | 87.8 | 84.5 | 96.9 |
| Congenital cardiovascular defects | |||||||||||
| Mortality, 2014|| | 2921 | 1603 | 1318 | 989 | 783 | 259 | 230 | 277 | 233 | 51 | 48 |
“Overweight” indicates a body mass index in the 95th percentile of the Centers for Disease Control and Prevention 2000 growth chart. CVD indicates cardiovascular disease; ellipses (…), data not available; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; M, millions; NH, non-Hispanic; and PA, physical activity.
National Survey on Drug Use and Health; respondents were asked, “During the past 30 days, have you smoked part or all of a cigarette?”
Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, Queen B, Lowry R, Olsen EO, Chyen D, Whittle L, Thornton J, Lim C, Yamakawa Y, Brener N, Zaza S. Youth Risk Behavior Surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1–174. DOI: http://dx.doi.org/10.15585/mmwr.ss6506a1.
Physically active at least 60 min/d on all 7 days.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732.
All ages.
Sources: See the following summary tables and charts for complete details:
Smoking—Table 3-1
Physical activity—Table 4-1
Blood cholesterol—Table 8-1
High blood pressure—Table 9-1
Diabetes mellitus—Table 10-1
Total cardiovascular diseases—Table 13-1
Stroke—Table 14-1
Congenital heart defects—Table 16-1
Coronary heart disease—Table 20-1; Table 20-2
Heart failure—Table 21-1
29. GLOSSARY
| Age-adjusted rates | Used mainly to compare the rates of ≥2 communities or population groups or the nation as a whole over time. The American Heart Association (AHA) uses a standard population (2000), so these rates are not affected by changes or differences in the age composition of the population. Unless otherwise noted, all death rates in this publication are age adjusted per 100 000 population and are based on underlying cause of death. |
| Agency for Healthcare Research and Quality (AHRQ) | A part of the US Department of Health and Human Services, this is the lead agency charged with supporting research designed to improve the quality of health care, reduce the cost of health care, improve patient safety, decrease the number of medical errors, and broaden access to essential services. The AHRQ sponsors and conducts research that provides evidence-based information on healthcare outcomes, quality, cost, use, and access. The information helps healthcare decision makers (patients, clinicians, health system leaders, and policy makers) make more informed decisions and improve the quality of healthcare services. The AHRQ conducts the Medical Expenditure Panel Survey (MEPS; ongoing). |
| Bacterial endocarditis | An infection of the heart’s inner lining (endocardium) or of the heart valves. The bacteria that most often cause endocarditis are streptococci, staphylococci, and enterococci. |
| Body mass index (BMI) | A mathematical formula to assess body weight relative to height. The measure correlates highly with body fat. It is calculated as weight in kilograms divided by the square of the height in meters (kg/m2). |
| Centers for Disease Control and Prevention/National Center for Health Statistics (CDC/NCHS) | CDC is an agency within the US Department
of Health and Human Services. The CDC conducts the Behavioral Risk
Factor Surveillance System (BRFSS), an ongoing survey. The CDC/NCHS
conducts or has conducted these surveys (among others):
|
| Centers for Medicare & Medicaid Services, formerly Health Care Financing Administration | The federal agency that administers the Medicare, Medicaid, and Child Health Insurance programs. |
| Comparability ratio | Provided by the NCHS/CDC to allow time-trend analysis from one International Classification of Diseases (ICD) revision to another. It compensates for the “shifting” of deaths from one causal code number to another. Its application to mortality based on one ICD revision means that mortality is “comparability modified” to be more comparable to mortality coded to the other ICD revision. |
| Coronary heart disease (CHD) (ICD-10 codes I20–I25) | This category includes acute myocardial infarction (I21–I22), other acute ischemic (coronary) heart disease (I24), angina pectoris (I20), atherosclerotic cardiovascular disease (I25.0), and all other forms of chronic ischemic (coronary) heart disease (I25.1–I25.9). |
| Death rate | The relative frequency with which death occurs within some specified interval of time in a population. National death rates are computed per 100 000 population. Dividing the total number of deaths by the total population gives a crude death rate for the total population. Rates calculated within specific subgroups, such as age-specific or sex-specific rates, are often more meaningful and informative. They allow well-defined subgroups of the total population to be examined. Unless otherwise stated, all death rates in this publication are age adjusted and are per 100 000 population. |
| Diseases of the circulatory system (ICD codes I00–I99) | Included as part of what the AHA calls “cardiovascular disease” (“Total cardiovascular disease” in this Glossary). |
| Diseases of the heart | Classification the NCHS uses in compiling the leading causes of death. Includes acute rheumatic fever/chronic rheumatic heart diseases (I00–I09), hypertensive heart disease (I11), hypertensive heart and renal disease (I13), CHD (I20–I25), pulmonary heart disease and diseases of pulmonary circulation (I26–I28), heart failure (I50), and other forms of heart disease (I29–I49, I50.1–I51). “Diseases of the heart” are not equivalent to “total cardiovascular disease,” which the AHA prefers to use to describe the leading causes of death. |
| Health Care Financing Administration | See Centers for Medicare & Medicaid Services. |
| Hispanic origin | In US government statistics, “Hispanic” includes people who trace their ancestry to Mexico, Puerto Rico, Cuba, Spain, the Spanish-speaking countries of Central or South America, the Dominican Republic, or other Spanish cultures, regardless of race. It does not include people from Brazil, Guyana, Suriname, Trinidad, Belize, or Portugal, because Spanish is not the first language in those countries. Most of the data in this update are for Mexican Americans or Mexicans, as reported by government agencies or specific studies. In many cases, data for all Hispanics are more difficult to obtain. |
| Hospital discharges | The number of inpatients (including newborn infants) discharged from short-stay hospitals for whom some type of disease was the first-listed diagnosis. Discharges include those discharged alive, dead, or “status unknown.” |
| International Classification of Diseases (ICD) codes | A classification system in standard use in the United States. The International Classification of Diseases is published by the World Health Organization. This system is reviewed and revised approximately every 10 to 20 years to ensure its continued flexibility and feasibility. The 10th revision (ICD-10) began with the release of 1999 final mortality data. The ICD revisions can cause considerable change in the number of deaths reported for a given disease. The NCHS provides “comparability ratios” to compensate for the “shifting” of deaths from one ICD code to another. To compare the number or rate of deaths with that of an earlier year, the “comparability-modified” number or rate is used. |
| Incidence | An estimate of the number of new cases of a disease that develop in a population, usually in a 1-year period. For some statistics, new and recurrent attacks, or cases, are combined. The incidence of a specific disease is estimated by multiplying the incidence rates reported in community- or hospital-based studies by the US population. The rates in this report change only when new data are available; they are not computed annually. |
| Major cardiovascular diseases | Disease classification commonly reported by the NCHS; represents ICD codes I00 to I78. The AHA does not use “major cardiovascular diseases” for any calculations. See “Total cardiovascular disease” in this Glossary. |
| Metabolic syndrome | Metabolic syndrome is defined* as the presence of any 3 of the following 5 diagnostic measures: Elevated waist circumference (≥102 cm in males or ≥88 cm in females), elevated triglycerides (≥150 mg/dL [1.7 mmol/L] or drug treatment for elevated triglycerides), reduced high-density lipoprotein cholesterol (<40 mg/dL [0.9 mmol/L] in males, <50 mg/dL [1.1 mmol/L] in females, or drug treatment for reduced high-density lipoprotein cholesterol), elevated blood pressure (≥130 mm Hg systolic blood pressure, ≥85 mm Hg diastolic blood pressure, or drug treatment for hypertension), and elevated fasting glucose (≥100 mg/dL or drug treatment for elevated glucose). |
| Morbidity | Incidence and prevalence rates are both measures of morbidity (ie, measures of various effects of disease on a population). |
| Mortality | Mortality data for states can be obtained from the NCHS Web site (http://cdc.gov/nchs/), by direct communication with the CDC/NCHS, or from the AHA on request. The total number of deaths attributable to a given disease in a population during a specific interval of time, usually 1 year, are reported. These data are compiled from death certificates and sent by state health agencies to the NCHS. The process of verifying and tabulating the data takes ≈2 years. |
| National Heart, Lung, and Blood Institute (NHLBI) | An institute in the National Institutes of
Health in the US Department of Health and Human Services. The NHLBI
conducts such studies as the following:
|
| National Institute of Neurological Disorders and Stroke (NINDS) | An institute in the National Institutes of
Health of the US Department of Health and Human Services. The NINDS
sponsors and conducts research studies such as these:
|
| Physical activity | Any bodily movement produced by the contraction of skeletal muscle that increases energy expenditure above a basal level. |
| Physical fitness | The ability to perform daily tasks with vigor and alertness, without undue fatigue, and with ample energy to enjoy leisure-time pursuits and respond to emergencies. Physical fitness includes a number of components consisting of cardiorespiratory endurance (aerobic power), skeletal muscle endurance, skeletal muscle strength, skeletal muscle power, flexibility, balance, speed of movement, reaction time, and body composition. |
| Prevalence | An estimate of the total number of cases of a disease existing in a population during a specified period. Prevalence is sometimes expressed as a percentage of population. Rates for specific diseases are calculated from periodic health examination surveys that government agencies conduct. Annual changes in prevalence as reported in this Statistical Update reflect changes in the population size. Changes in rates can be evaluated only by comparing prevalence rates estimated from surveys conducted in different years. Note: In the data tables, which are located in the different disease and risk factor chapters, if the percentages shown are age adjusted, they will not add to the total. |
| Race and Hispanic origin | Race and Hispanic origin are reported separately on death certificates. In this publication, unless otherwise specified, deaths of people of Hispanic origin are included in the totals for whites, blacks, American Indians or Alaska Natives, and Asian or Pacific Islanders according to the race listed on the decedent’s death certificate. Data for Hispanic people include all people of Hispanic origin of any race. See “Hispanic origin” in this Glossary. |
| Stroke (ICD-10 codes I60–I69) | This category includes subarachnoid hemorrhage (I60); intra-cerebral hemorrhage (I61); other nontraumatic intracranial hemorrhage (I62); cerebral infarction (I63); stroke, not specified as hemorrhage or infarction (I64); occlusion and stenosis of precerebral arteries not resulting in cerebral infarction (I65); occlusion and stenosis of cerebral arteries not resulting in cerebral infarction (I66); other cerebrovascular diseases (I67); cerebrovascular disorders in diseases classified elsewhere (I68); and sequelae of cerebrovascular disease (I69). |
| Total cardiovascular disease (ICD-10 codes I00–I99, Q20–Q28) | This category includes rheumatic fever/rheumatic heart disease (I00–I09); hypertensive diseases (I10–I15); ischemic (coronary) heart disease (I20–I25); pulmonary heart disease and diseases of pulmonary circulation (I26–I28); other forms of heart disease (I30–I52); cerebrovascular disease (stroke) (I60–I69); atherosclerosis (I70); other diseases of arteries, arterioles, and capillaries (I71–I79); diseases of veins, lymphatics, and lymph nodes not classified elsewhere (I80–I89); and other and unspecified disorders of the circulatory system (I95–I99). When data are available, we include congenital cardiovascular defects (Q20–Q28). |
| Underlying cause of death or any-mention cause of death | These terms are used by the NCHS when defining mortality. Underlying cause of death is defined by the World Health Organization as “the disease or injury which initiated the chain of events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury.” Any-mention cause of death includes the underlying cause of death and up to 20 additional multiple causes listed on the death certificate. |
According to criteria established by the AHA/NHLBI and published in Circulation (Circulation. 2005;112:2735–2752).
Acknowledgments
We wish to thank our colleagues: Lucy Hsu and Michael Wolz, National Heart, Lung, and Blood Institute; Sheila Franco, National Center for Health Statistics; Lorena Egan, AHA; and the dedicated staff of the Centers for Disease Control and Prevention; the National Center for Health Statistics; and the National Heart, Lung, and Blood Institute for their valuable comments and contributions.
Footnotes
Disclosure: A portion of the data reported has been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
The views expressed in this document are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Department of Health and Human Services; or the US Department of Veterans Affairs.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
A copy of the document is available at http://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or e-mail kelle.ramsay@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:000-000. DOI: 10.1161/CIR.0000000000000485.
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at http://www.heart.org/HEARTORG/General/Copyright-Permission-Guidelines_UCM_300404_Article.jsp. A link to the “Copyright Permissions Request Form” appears on the right side of the page.
Disclosures
Writing Group Disclosures
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/Honoraria |
Expert Witness | Ownership Interest | Consultant /Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Emelia J. Benjamin | Boston University School of Medicine | NIH/NHLBI (R01HL128914)†; NIH/NHLBI (R01HL092577)†; NIH/AHA (P50 HL120163)† | None | None | None | None | None | American Heart Association (Associate Editor Circulation until June 2016)†; NIH/NCBI (CARDIA OSMB)* |
| Paul Muntner | University of Alabama at Birmingham | Amgen Inc.† | None | None | None | None | None | None |
| Heather M. Alger | American Heart Association | None | None | None | None | None | None | American Heart Association (Science & Medicine Advisor)† |
| Michael J. Blaha | Johns Hopkins University | FDA†; NIH/NHLBI†; AHA†; Aetna Foundation† | None | None | None | None | None | None |
| Stephanie E. Chiuve | Brigham and Women’s Hospital and Harvard Medical School | None | None | None | None | None | None | Shire (Associate Director of Epidemiology)† |
| Mary Cushman | University of Vermont | None | None | None | None | None | None | None |
| Sandeep R. Das | University of Texas Southwestern Medical Center | None | None | None | None | None | None | None |
| Rajat Deo | University of Pennsylvania | NIH† | None | None | None | None | Boehringer Ingelheim*; Janssen Pharmaceuticals* | None |
| Sarah D. de Ferranti | Children’s Hospital Boston | Pediatric Heart Network, NHLBI (coPI on upcoming pediatric prevention trial)* | None | None | None | None | None | None |
| James Floyd | University of Washington | None | None | None | None | None | None | None |
| Myriam Fornage | University of Texas Health Science Center at Houston | None | None | None | None | None | None | None |
| Cathleen Gillespie | Centers for Disease Control and Prevention | None | None | None | None | None | None | None |
| Carmen R. Isasi | Albert Einstein College of Medicine Epidemiology & Population Health | None | None | None | None | None | None | None |
| Monik C. Jiménez | Brigham and Women’s Hospital, Harvard Medical School | NIH (K01HL124391)† | None | None | None | None | None | None |
| Lori Chaffin Jordan | Vanderbilt University | NIH (Grants to study stroke prevention in children and young adults with sickle cell Disease)† | None | None | None | None | None | None |
| Suzanne E. Judd | University of Alabama at Birmingham | None | None | None | None | None | None | None |
| Daniel Lackland | Medical University of South Carolina | None | None | None | None | None | None | None |
| Judith H. Lichtman | Yale School of Public Health | None | None | None | None | None | None | None |
| Lynda Lisabeth | University of Michigan | NIH† | None | None | None | None | None | None |
| Simin Liu | Brown University | None | None | None | None | None | None | None |
| Chris T. Longenecker | Case Western Reserve University | None | None | None | None | None | Gilead Sciences* | None |
| Rachel H. Mackey | University of Pittsburgh | None | None | None | None | None | Amgen†; | None |
| Kunihiro Matsushita | Johns Hopkins University | AHA (14CRP20380886)†; Fukuda Denshi†; Kyowa Hakko Kirin†; NKF†; NIH (R21HL133694-01)† | None | Fukuda Denshi* | None | None | Kyowa Hakko Kirin* | None |
| Dariush Mozaffarian | Tufts University | NIH† | None | None | None | None | DSM*; UpToDate* | None |
| Michael E. Mussolino | NIH National Heart, Lung and Blood Institute | None | None | None | None | None | None | None |
| Khurram Nasir | Baptist Health South Florida | None | None | None | None | None | None | None |
| Robert W. Neumar | University of Michigan | None | None | None | None | None | None | None |
| Latha Palaniappan | Stanford University | None | None | None | None | None | None | None |
| Dilip K. Pandey | University of Illinois at Chicago | CDC† | None | None | None | None | None | None |
| Mathew J. Reeves | Michigan State University | None | None | None | None | None | None | None |
| Matthew Ritchey | Centers for Disease Control and Prevention | None | None | None | None | None | None | None |
| Carlos J. Rodriguez | Wake Forest University | None | None | None | None | None | None | None |
| Wayne D. Rosamond | University of North Carolina | None | None | None | None | None | None | None |
| Gregory A. Roth | University of Washington | None | None | None | None | None | None | None |
| Comilla Sasson | American Heart Association | None | None | None | None | None | None | American Heart Association (Vice President, Science & Medicine)† |
| Ravi R. Thiagarajan | Children’s Hospital, Boston | None | None | None | None | None | Bristol Myers Squibb*; Pfizer* | None |
| Amytis Towfighi | University of Southern California | None | None | None | None | None | None | None |
| Connie W. Tsao | Beth Israel Deaconess Medical Center | None | None | None | None | None | None | None |
| Melanie B. Turner | American Heart Association | None | None | None | None | None | None | American Heart Association (Senior Manager, Research Operations)† |
| Salim S. Virani | VA Medical Center, Baylor College of Medicine | None | None | None | None | None | None | None |
| Jenifer H. Voeks | Medical University of South Carolina | NINDS† | None | None | None | None | None | None |
| John T. Wilkins | Northwestern University | None | None | None | None | None | None | None |
| Joshua Z. Willey | Columbia University | NIH (NINDS K23 073104)†; NIH (StrokeNET, local PI of POINT trial aspirin and clopidogrel versus aspirin alone for minor stroke or TIA)*; Astra-Zeneca (Local PI for clinical trial SOCRATES aspirin versus ticagrelor)*; Genentech (Local PI for clinical trial PRISMS - alteplase versus placebo in minor stroke or TIA)* | None | None | None | None | Heartware* | American College of Physicians (Author: MKSAP 18)*; Uptodate (Peer reviewer: Uptodate online)* |
| Sally S. Wong | American Heart Association | None | None | None | None | None | None | American Heart Association (Science and Medicine Advisor)†; University of Illinois at Chicago (Clinical Associate Professor)† |
| Jason HY. Wu | University of Sydney (Australia) | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.