Abstract
Our understanding of the role of cuticular hydrocarbons (CHC) in recognition is based largely on temperate ant species and honey bees. The stingless bees remain relatively poorly studied, despite being the largest group of eusocial bees, comprising more than 400 species in some 60 genera. The Meliponini and Apini diverged between 80–130 Myr B.P. so the evolutionary trajectories that shaped the chemical communication systems in ants, honeybees and stingless bees may be very different. The aim of this study was to study if a unique species CHC signal existed in Neotropical stingless bees, as has been shown for many temperate species, and what compounds are involved. This was achieved by collecting CHC data from 24 colonies belonging to six species of Melipona from North-Eastern Brazil and comparing the results with previously published CHC studies on Melipona. We found that each of the eleven Melipona species studied so far each produced a unique species CHC signal based around their alkene isomer production. A remarkable number of alkene isomers, up to 25 in M. asilvai, indicated the diversification of alkene positional isomers among the stingless bees. The only other group to have really diversified in alkene isomer production are the primitively eusocial Bumblebees (Bombus spp), which are the sister group of the stingless bees. Furthermore, among the eleven Neotropical Melipona species we could detect no effect of the environment on the proportion of alkane production as has been suggested for some other species.
Electronic supplementary material
The online version of this article (10.1007/s10886-017-0901-5) contains supplementary material, which is available to authorized users.
Keywords: Stingless bees, Melipona, Cuticular hydrocarbons, Alkenes, Dimethyldisulfide, Chemical communciation
Introduction
Chemical communication is the oldest form of communication across all forms of life (Wilson 1970). Pheromones are one of the most important chemical signals (Wyatt 2013), and are particularly well-studied in insects (Howard and Blomquist 2005). Short-range contact semiochemicals are used by many insects to identify other individuals of the same or different species (Wyatt 2013) and cuticular hydrocarbons (CHC) are an important group of compounds used in recognition (Blomquist and Bagnères 2010; Wyatt 2013). This lipid layer preserves the insect from desiccation, cuticle abrasion and infection, thus directly ensuring their survival (Lockey 1988). However, across many insect taxa, cuticular lipids have evolved to become part of their communication system by enabling them to differentiate between friend and foe or find a mate (Blomquist and Bagnères 2010; Prestwich and Blomquist 2014).
Currently much of our understanding of CHC’s is based on temperate ant species and honeybees (Breed and Bennett 1987; Kather and Martin 2015; Pradella et al. 2015; Tannure-Nascimento et al. 2008, 2009). The stingless bees (Meliponini) and the honey bees (Apini) are the only two groups of highly eusocial bees. The well-studied honey bees have only 11 species all within a single genus (Apis), whereas the stingless bees remain relatively poorly studied (Leonhardt 2017), despite being the largest group of eusocial bees, comprising more than 400 species in some 60 genera (Rasmussen and Cameron 2010) and are responsible for pollinating 40–90% of native flora in some regions of Brazil (Nascimento et al. 2000). The Apini and Meliponini diverged between 80 and 130 Myr B.P. (Michener and Grimaldi 1988; Rasmussen and Cameron 2010) and so ants, honeybees and stingless bees have very different evolutionary trajectories that will have shaped their chemical communication systems.
The majority of stingless bees occur in the Neo-tropics (Roubik 1989) with colonies typically containing 200–700 adults (Sakagami 1982) and they have a perennial life-cycle (Michener 2000). During their evolution, stingless bees have become adapted to inhabit a wide range of climatic environments. For example, in Brazil, Melipona fasciculata and M. mandacaia inhabit very hot and arid areas in Caatinga and Cerrado, whereas M. scutellaris and M. quadrifasciata occur in humid Atlantic forests. Therefore, this group are ideally suited to look for the effect of the environment on their CHC profiles.
The aim of this investigation was to collect new data on the CHC profiles in six species of Melipona from across North-eastern Brazil. This new data was compared with that from all published CHC studies on Melipona to investigate if a stable species CHC signal exists in this group. Finally, we used the entire dataset to determine which CHC groups might be used in species recognition and what role the environment is playing in shaping the CHC profiles in stingless bees in South America.
Methods and Materials
Stingless Bee Samples
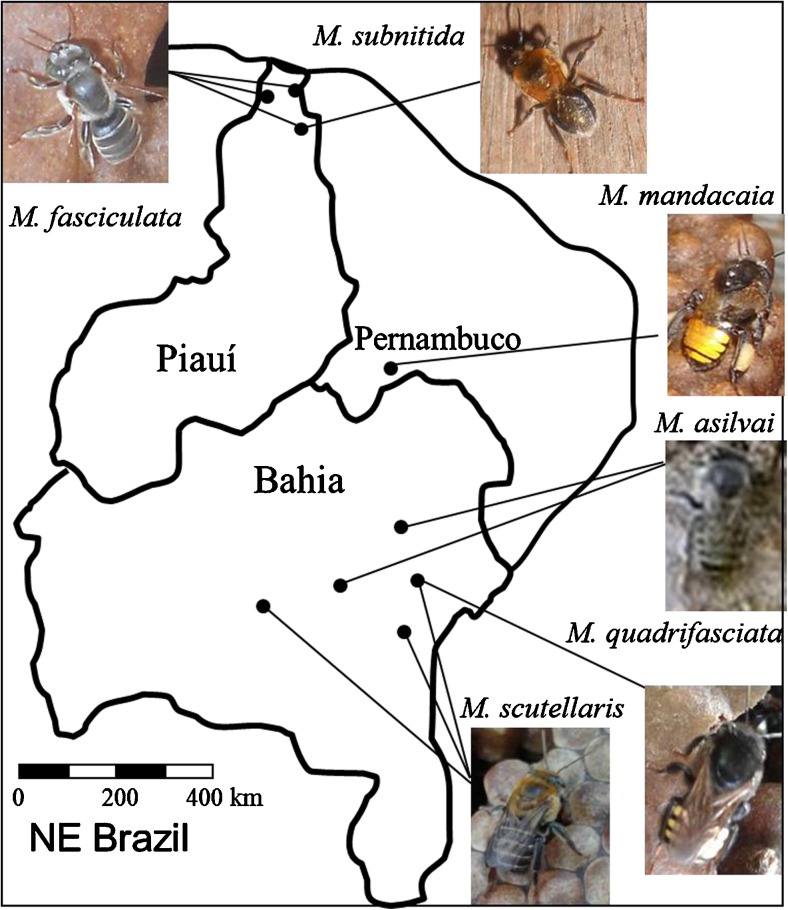
All bees were collected from meliponaries maintained by local people across NE Brazil in the states of Bahia, Pernambuco and Piauí (Fig. 1). Adult workers were collected by placing a clear tube over the colony entrance and tapping the nest box lightly. All colonies were sampled between October 2014 and March 2017.
Fig. 1.
Geographic location and images of the six species of Melipona collected across the states of Piauí, Pernambuco and Bahia in North-eastern Brazil
In order to determine the amount of intra- and inter-colony variation in CHC profiles we initially studied M. fasciculata by sampling 10 workers from each of the six colonies in October 2014. The colonies were located in three different meliponaries that were between 5 and 70 km apart (Fig. 1). All three locations were in the hot and arid area with average temperatures of 25 °C – 28 °C and low rainfall of around 20 to 40 cm a year. This established that very low levels of intra or inter-colony variation existed across the 53 individuals studied (Supplemental Fig. S1). Therefore, we subsequently collected five individuals from three to six colonies of M. scutellaris, M. asilvai, M. quadrifasciata and M. mandacaia, depending on their availability. From a single rare M. subnitida colony we collected ten individuals.
Chemical Analysis
As in previous CHC studies of stingless bees (e.g. Abdalla et al. 2003; Kerr et al. 2004) we removed both sets of wings from each bee and placed them in a glass vial with 50 μl of HPLC grade hexane. After 10 min, the wings were removed and the hexane evaporated to dryness. Vials were then sealed and stored at 5 °C until just prior to analysis when HPLC grade hexane (30 μl) was added to each vial.
Samples were analyzed on an Agilent 7890 gas chromatograph (GC) connected to an Agilent 5975 MSD quadruple mass spectrometer (Agilent, Stockport, Cheshire, UK) operated in electron impact mode at 70 eV. The GC was equipped with a Vf-5ht UltiMetal column (30 m × 0.25 mm i.d. × 0.1 μm film thickness; Agilent) and the oven temperature was programmed from 70 to 200 °C at 40 °C min−1, then from 200 to 320 °C at 25 °C min−1 and held for 2 min at 320 °C. Carrier gas was helium at a constant flow rate of 1.0 ml min−1. For each sample 2 μl were injected in the splitless mode.
The data were analyzed using Agilent ChemStation and standard MS databases, diagnostic ions and their Kovats Indices for compound identification. The positions of alkene double bond were determined using dimethyl disulfide (DMDS) derivatization (Carlson et al. 1989). For this the wing extracts were pooled by colony (M. asilvai, M. quadrifasciata, M. mandacaia, M. scutellaris and M. subnitida) or location (M. fasciculata) then subjected to DMDS derivatization and re-analyzed on the GC-MS under similar conditions to those above. This resulted in 21 derivatized samples.
The configuration of the double bonds in the alkenes was not determined, but was assumed to be (Z) due to their presumed biosynthesis via the action of desaturase enzymes on saturated fatty acids (Morgan 2010).
Published Melipona CHC Studies
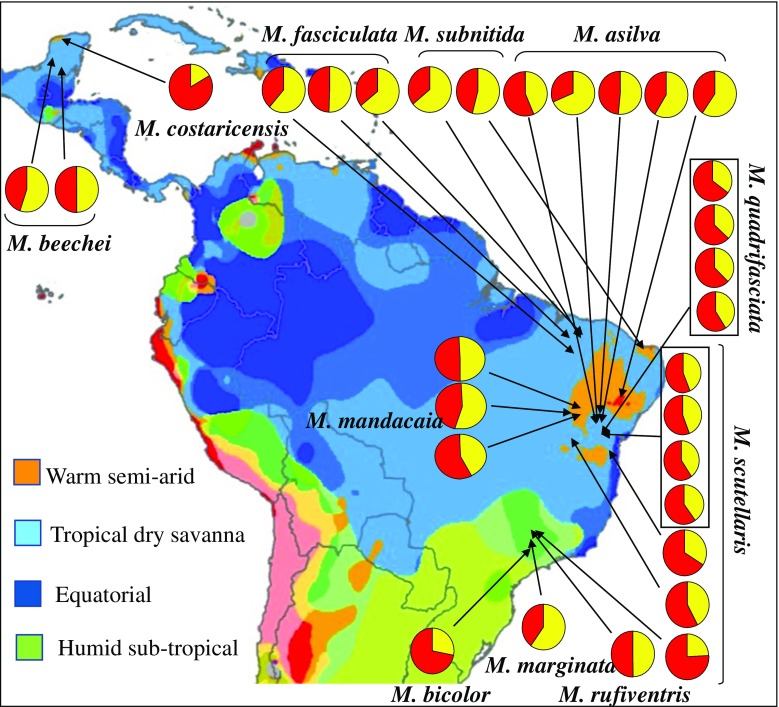
A review of the literature indicated only eight previous studies (Abdalla et al. 2003; Ferreira-Caliman et al. 2010; Kerr et al. 2004; Leonhardt et al. 2013; Nascimento and Nascimento 2012; Pianaro et al. 2007;dos Santos et al. 2015; Quezada-Euan et al. 2013;) that have investigated a total of ten species of Melipona, all which occur in South America (Fig. 2). The data from Kerr et al. (2004) was of limited value since the GC-MS column was only heated to 250 °C rather than the normal 320 °C, resulting in the CHC above nonacosane (C29) probably not being detected. Although Leonhardt et al. (2013) only reported the proportions and number of compound groups these authors kindly supplied the raw data so M. costaricensis and M. beecheii could be included in the analysis.
Fig. 2.
Proportion of alkanes (yellow) and olefins (red) for eleven Melipona species of stingless bees that have been sampled in four different climatic zones across South America. The prediction that the proportion of alkanes would increase from humid to semi-arid areas is not supported. See Table 1 for source data
Statistical Analysis
A total of 134 good quality profiles were obtained, for which the peak area (Total Ion Count, TIC) of each compound was converted into a percentage of the total TIC for each bee. The average and standard deviation was then calculated for each compound for each species. To estimate the proportions of the various alkene isomers which elute closely together, the presence or absence of each isomer was determined in analyses of the DMDS derivatives (Supplemental Fig. S2–S6), then using their relative strengths we were able to identify most of alkene isomers and integrate them in the analyses of the underivatized alkenes (Supplemental Fig. S7). Where it was not possible to separate isomers this is indicated.
Results
Species CHC Profiles
In all six species their profiles were dominated by a series of n-alkanes and olefins (alkenes and dienes) ranging in chain lengths from C23 to C33 depending on the species (Tables 1 and 2). Although all species produced basically the same alkanes, the number, chain length and positional isomers of the alkenes were unique to each species and shared by all individuals within that species, irrespective of location or time of collection. Across the six-species studied, ten alkene isomers (Tables 1 and 2) were detected with each species having a different number of alkene isomers (Table 2). Some species like M. fasciculata had only one positional isomer, i.e. (Z)-9, whereas M. asilvai had nine isomers. Furthermore, each alkene isomer including the more prevalent isomers, such as (Z)-9, has a species-specific distribution across the various change lengths (Table 1). Therefore, all six species of Melipona studied had their own very distinctive CHC profile even if this was just based on their alkene profile.
Table 1.
Means and standard deviations of the percentages of each cuticular hydrocarbon in six species of Melipona stingless bees investigated in this study
| Compound | M. fasciculata (N = 53) | M. quadrifasciata (N = 18) | M. scutellaris (N = 23) | M. asilvai (N = 16) | M. subnitida(N = 10) | M. mandacaia (N = 14) |
|---|---|---|---|---|---|---|
| (Z)-9-Tricosene | 0.2 ± 0.1 | |||||
| Tricosane | 2.1 ± 0.8 | 2.9 ± 0.8 | 1.1 ± 0.3 | 0.8 ± 0.4 | 2.4 ± 0.4 | |
| (Z)-11-Pentacosene | 0.1 ± 0.1 | 0.1 ± 0.1 | ||||
| (Z)-10-Pentacosene | 1.5 ± 0.4 | |||||
| (Z)-9-Pentacosene | 2.1 ± 0.8 | 6.7 ± 2.5 | 0.5 ± 0.3 | 2.4 ± 0.6 | 33.6 ± 6.2 | |
| (Z)-7-Pentacosene | 0.5 ± 0.5 | 1.1 ± 0.7 | 1.3 ± 0.4 | |||
| (Z)-6-Pentacosene | 1.9 ± 0.5 | |||||
| Pentacosane | 14 ± 3.3 | 14.9 ± 1.7 | 21.2 ± 3.3 | 29 ± 5.8 | 36 ± 4.1 | 29.9 ± 5.9 |
| 9,11-Methylpentacosane | 0.6 ± 0.3 | |||||
| 5-Methylpentacosane | 0.5 ± 0.1 | 1 ± 0.2 | ||||
| 3-Methylpentacosane | 1.3 ± 0.1 | 1 ± 0.1 | ||||
| Heptacosadiene | 0.1 ± 0.0 | |||||
| (Z)-13-Heptacosene | 0.2 ± 0.3 | |||||
| (Z)-11-Hexacosene | 0.1 ± 0.1 | |||||
| (Z)-10-Heptacosene | 0.4 ± 0.4 | 0.8 ± 0.3 | ||||
| (Z)-9-Heptacosene | 6.2 ± 2.6 | 45 ± 2.2 | 22 ± 3.4 | 0.4 ± 0.3 | 1.9 ± 0.6 | 7.1 ± 1.1 |
| (Z)-8-Heptacosene | 0.7 ± 0.3 | |||||
| (Z)-7-Heptacosene | 1 ± 0.6 | 2.2 ± 1.5 | 0.4 ± 0.1 | |||
| (Z)-6-Heptacosene | 0.4 ± 0.3 | |||||
| Heptacosane | 32.9 ± 9.7 | 14.5 ± 1.4 | 11.9 ± 3 | 6.9 ± 1.4 | 22.3 ± 3.0 | 7.8 ± 1.7 |
| 9,11-Methylheptacosane | 0.1 ± 0.1 | 0.7 ± 0.1 | ||||
| 7-Methylheptacosane | 0.3 ± 0.1 | |||||
| 5-Methylheptacosane | 0.4 ± 0.1 | 0.8 ± 0.2 | ||||
| 3-Methylheptacosane | ||||||
| Nonacosadiene | 0.1 ± 0.1 | 0.5 ± 0.3 | 2.3 ± 0.5 | |||
| Nonacosadiene | 0.9 ± 0.5 | 0.7 ± 0.3 | ||||
| (Z)-13-Nonacosene | 0.1 ± 0.1 | |||||
| (Z)-12 + 13-Nonacosene | 1.2 ± 0.7 | |||||
| (Z)-10-Nonacosene | 7.9 ± 1.1 | 2.4 ± 0.6 | ||||
| (Z)-9-Nonacosene | 30.2 ± 11 | 12.1 ± 1.1 | 6.8 ± 2.2 | 14.6 ± 3.5 | 4.9 ± 0.8 | |
| (Z)-8-Nonacosene | 1.6 ± 0.4 | 0.3 ± 0.2 | ||||
| (Z)-7-Nonacosene | 0.6 ± 0.3 | 6.0 ± 3.3 | 0.7 ± 0.3 | 0.5 ± 0.1 | ||
| (Z)-6-Nonacosene | 2.3 ± 0.5 | |||||
| Nonacosane | 9.6 ± 2.9 | 4 ± 0.6 | 3.6 ± 1.1 | 12.8 ± 5.5 | 4.9 ± 0.8 | 6.1 ± 1.0 |
| 9,11-Methylnonocosane | 0.7 ± 0.2 | 1.2 ± 0.1 | ||||
| 7-Methylnonocosane | 5.7 ± 1.3 | |||||
| Hentriacontadiene | 1.1 ± 0.7 | 1.2 ± 0.4 | ||||
| Hentriacontadiene | 0.2 ± 0.1 | 2.2 ± 1.6 | 1.6 ± 0.4 | |||
| (Z)-15-Hentriacontene | 0.1 ± 0.1 | |||||
| (Z)-13-Hentriacontene | 0.3 ± 0.1 | |||||
| (Z)-12 > 14> > 15-Hentriacontene | 8.8 ± 2.7 | |||||
| (Z)-11-Hentriacontene | 0.1 ± 0.1 | |||||
| (Z)-10-Hentriacontene | 3.8 ± 1.1 | 1.6 ± 0.4 | ||||
| (Z)-9-Hentriacontene | 4.5 ± 1.6 | 1.9 ± 0.4 | 15.3 ± 3.5 | 0.1 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.3 |
| (Z)-7-Hentriacontene | 2.8 ± 1.3 | 3.2 ± 1.0 | ||||
| Hentriacontane | 2.2 ± 0.6 | 1.1 ± 0.4 | 0.9 ± 0.2 | 5.7 ± 3.4 | 1.2 ± 0.4 | 2.1 ± 0.4 |
| 9,11-Methylhentriacontane | 1.0 ± 0.3 | |||||
| Tritriacontadiene | 0.5 ± 0.1 | 0.3 ± 0.3 | ||||
| Tritriacontadiene | 0.9 ± 0.3 | |||||
| (Z)-16 > 14 > 12 > 10-Tritriacontene | 0.9 ± 0.9 | |||||
| (Z)-9-Tritriacontene | 0.1 ± 0.3 | 1.1 ± 0.4 |
The numbers of individual workers from which high quality total ion chromatograms were obtained are given in parenthesis. The compounds are given in retention order and those in bold are the n-alkanes. Were it is not possible to separate out closely eluting isomers the relative abundances of the isomers are given in order of abundance based on the DMDS ion counts
Table 2.
Comparison of the number of alkene and (diene) isomers at each chain length for each of the six Melipona species studied here and those in other published studies
| Species | C23:1 | C25:1 (C25:2) | C27:1 (C27:2) | C29:1 (C29:2) | C31:1 (C31:2) | C33:1 (C33:2) | Total |
|---|---|---|---|---|---|---|---|
| M. asilvai (This study) ([Z]-16,15,14,12,11,10,9,8,7) | 1 | 3 | 5(1) | 5(2) | 7(2) | 4(1) | 25(6) |
| M. aslivai (Nascimento and Nascimento 2012) | 2 | 3 | 3(2) | 4(2) | 15(9) | ||
| M.beecheii (Quezada-Euan et al. 2013) | 1 | 1 | 1 | 2(1) | 1(1) | 6(2) | |
| M.beecheii (Leonhardt et al. 2013) | 1 | 4 | 3(1) | 2 | 9(1) | ||
| M. bicolor (Abdalla et al. 2003) | 1 | 1 | 1 | 3 | |||
| M. costarisensis (Leonhardt et al. 2013) | 3 | 4(1) | 4(2) | 3(4) | 1(1) | 15(8) | |
| M. fasciculata (This study) ( Z )-9 | 1 | 1 | 1 | 3 | |||
| M. mandacaia (This study) ( Z )-9,7 | 2 | 2 | 2 | 1 | 7 | ||
| M. marginata (Ferreira-Caliman et al. 2010 ) | 1 | 3 | 3 | 2(2) | 4(2) | 13(4) | |
| M. quadrifasciata (This study) ( Z )-9,14 | 1 | 1 | 2 | 1 | 1 | 6 | |
| M. rufiventris (Pianaro et al. 2007) | 2 | 2 | 2 | 2(1) | 2(3) | 2(2) | 12(6) |
| M. scutellaris (Pianaro et al. 2007) | 2 | 2 | 2(1) | 1 | 1 | 8(1) | |
| M. scutellaris (This study) ( Z )-9, 11,13 | 1 | 2 | 3 | 3 | 2(1) | 1(2) | 12(3) |
| M. subnitida (dos Santos et al. 2015) | 1 | 3 | 3 | 3(2) | 2(2) | 12(4) | |
| M. subnitida (This study) ( Z )-10,9,8,7,6 | 3 | 2 | 3(2) | 2(2) | 10(4) | ||
| Totals | 12 | 34(1) | 30(3) | 37(15) | 30(17) | 11(7) |
The alkene isomers determined for the first time in this study are given in bold
These species-specific CHC profiles remained when we increased the data set to include all previously published data (Table 2). Despite maximum distances between study sites (M. asilvai 407 km; M. subnitida 751 km; M. scutellaris 1172 km) the same or similar species-specific patterns of olefins were consistently found in all populations in these three species.
We also investigated if we could find any colony-specific differences in their CHC profiles in any of the six species. Clear, quantitative colony-specific differences in some of the alkene-isomers (Z-7-C27, Z-10-C29, Z-12 + 14-C31) could be seen between colonies of M. asilvai, but not in any of the other species.
Effect of the Environment on CHC Profile
We used all available data to look for any association between the proportion of alkane production in a species and the climatic conditions where they were collected (Fig. 2). This indicated that no clear pattern emerged despite samples being analyzed for several species that live in several very different climatic zones (Fig. 2).
Discussion
Each CHC profile of the eleven Melipona species studied so is unique, even if based only on their alkene isomer production (Table 2). A remarkable number of alkene isomers were obtained from just the six species in which we determined the positional isomers. For example, M. asilvai had nine positional isomers (Z)-16, (Z)-15, (Z)-14, (Z)-12, (Z)-11, (Z)-10, (Z)-9, (Z)-8, and (Z)-7, which when combined with different carbon chain lengths resulted in 25 alkene isomers being produced (Table 1). This is the highest number isomers found in any species of Hymenoptera so far studied (Kather and Martin 2015). The only other group of insects to have really diversified in production of alkene isomers is the primitively eusocial Bumblebee (Bombus spp). In this group up to nine positional alkene isomers from (Z)-5 to (Z)-21 are produced, again in unique, species-specific, stable patterns (Martin et al. 2010). In evolutionary terms, bumblebees (Bombini) are the sister group of the stingless bees (Meliponini) (Koulianos et al. 1999) and this may help explain why these groups have diversified their alkene production relative to all other groups of Hymenoptera (Kather and Martin 2015).
Despite the diverse isomer patterns detected in this study, the reported number of alkenes in three species that had been previously studied were very similar to each of those species is this study (Table 2). This was despite being collected in totally different locations or climate zones (Fig. 2). This stability of the species specific CHC profile over large geographical distances has also been found in Formica (Martin et al. 2008) and Myrmica ants (Guillem et al. 2016). This helps explain why environmental factors appeared to have little or no impact on qualitative CHC profile. Leonhardt et al. (2013) also demonstrated that alkanes, alkenes and alkadienes showed no or little correlation with the geographical distribution of stingless bees from Southeast Asia, Australia and Central America. It has been suggested in Drosophila flies that the climate affects the proportions of alkanes produced (Rouault et al. 2001), but we were unable to detect any such effect among the Neotropical stingless bees (Fig. 2). Furthermore, in M. bicolor the proportion of alkanes in the CHC profile varied from 74% in nurse workers to just 28% in foraging workers (Abdalla et al. 2003), whereas, in M. marginata the opposite situation was reported (alkanes = 45% and 60% in nurses and foragers respectively) (Ferreira-Caliman et al. 2010). Thus results can be influenced by the age of bee sampled and task it is performing since this affects the proportion of alkanes produced (Kather et al. 2011; Martin and Drijfhout 2009).
The importance of alkenes as putative recognition cues is supported by the production of large proportions of alkenes, coupled with their diversification. Although beyond the scope of this study, colony-specific differences were seen in the some of the alkene-isomers in M. asilvai and may help explain the nest-mate recognition that has been shown in this species (Nascimento and Nascimento 2012) and merits further study. A central problem in chemical ecology is that the sensitivity of insects to chemical change can be much greater than we are able to detect with even our best methods (Angioy et al. 2003). Unless the variation in colony specific signals is large, as in Formica exsecta, for example (Martin et al. 2013) they are difficult to detect as has been the case in the honey bee (Apis mellifera) (Pradella et al. 2015).
In the Old-World tropics (Borneo) six species of stingless bees were studied by Leonhardt et al. (2009, 2011) and each species had a distinct chemical profile based on both genetically determined compounds (CHC’s) and environmentally derived terpenes, collected from tree resins that help protect social insect colonies from micro-organism and pathogens (Brütsch et al. 2017). Several of these Old-World species also produced a high proportion of alkenes although their isomeric composition has yet to be determined (Leonhardt 2017). Based on this study, we would predict a wide range of alkene-isomers to be found among stingless bees both in the Old and New World, since it appears that in general bees have specialized in alkene diversity, rather than in the diversity of methyl-branching found in the ants and wasps (Kather and Martin 2015).
Electronic supplementary material
(PDF 33 kb)
(PDF 104 kb)
(PDF 134 kb)
(PDF 93.2 kb)
(PDF 91.5 kb)
(PDF 127 kb)
(PDF 73 kb)
Acknowledgements
The authors thank Cherre S. B. da Silva for the helpful collaboration in collection of samples, all beekeepers for providing samples of bees, Maria E. C. de Oliveira for advice, Sara Leonhardt and Thomas Schmitt for providing their raw data. The Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) (Cod. SEG 02.11.01.029.00.00) provided financial support, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (MEC/MCTI/CAPES/CNPQ/FAPS - BOLSA PESQUISADOR VISITANTE ESPECIAL - PVE 2014) supported C. B. da S. lima- (200315/2015-3). Stephen Martin and Carlos A. L. de Carvalho were funded by CNPq via Special Visiting Researcher – PVE (400425/2014-9).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10886-017-0901-5) contains supplementary material, which is available to authorized users.
References
- Abdalla FC, Jones GR, Morgan ED, Cruz-Landim C. Comparative study of the cuticular hydrocarbon composition of Melipona bicolor Lepeletier, 1836 (hymenoptera, Meliponini) workers and queens. Gen Mol Res. 2003;2:191–199. [PubMed] [Google Scholar]
- Angioy AM, Desogus A, Barbarossa IT, Anderson P, Hansson BS. Extreme sensitivity in an olfactory system. Chem Senses. 2003;28:279–284. doi: 10.1093/chemse/28.4.279. [DOI] [PubMed] [Google Scholar]
- Blomquist GJ, Bagnères AG. Insect hydrocarbons: biology, biochemistry, and chemical ecology. New York: Cambridge University Press; 2010. [Google Scholar]
- Breed MD, Bennett B. Kin recognition in highly eusocial insects. In: Fletcher DJC, Michener CD, editors. Kin recognition in animals. Chichester: Wiley; 1987. pp. 243–285. [Google Scholar]
- Brütsch T, Jaffuel G, Vallat A, Turlings TCJ. Wood ants produce a potent antimicrobial agent by applying formic acid on tree-collected resin. Ecol Evol. 2017;7:2249–2254. doi: 10.1002/ece3.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DA, Roan C-S, Yost RA, Hector J. Dimethyl disulfide derivatives of long chain alkenes, alkadienes, and alkatrienes for gas chromatography/mass spectrometry. Anal Chem. 1989;61:1564–1571. doi: 10.1021/ac00189a019. [DOI] [Google Scholar]
- dos Santos CF, Ferreira-Caliman MJ, Nascimento FS. An alien in the group: eusocial male bees sharing nonspecific reproductive aggregations. J Insect Sci. 2015;15:157. doi: 10.1093/jisesa/iev107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Caliman MJ, Nascimento FS, Turatti IC, Mateus S, Lopes NP, Zucchi R. The cuticular hydrocarbons profiles in the stingless bee Melipona marginata reflect task-related differences. J Insect Physiol. 2010;56:800–804. doi: 10.1016/j.jinsphys.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Guillem RM, Drijfhout FP, Martin SJ. Species-specific cuticular hydrocarbon stability within European Myrmica ants. J Chem Ecol. 2016;42:1052–1062. doi: 10.1007/s10886-016-0784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RW, Blomquist GJ. Ecological, behavioural, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- Kather R, Martin SJ. Evolution of cuticular hydrocarbons in the hymenoptera: a meta-analysis. J Chem Ecol. 2015;41:871–883. doi: 10.1007/s10886-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather R, Drijfhout FP, Martin SJ. Task group differences in cuticular lipids in the honey bee Apis mellifera. J Chem Ecol. 2011;37:205–212. doi: 10.1007/s10886-011-9909-4. [DOI] [PubMed] [Google Scholar]
- Kerr WE, Jungnickel H, Morgan ED. Workers of the stingless bee Melipona scutellaris are more similar to males than to queens in their cuticular compounds. Apidologie. 2004;35:611–618. doi: 10.1051/apido:2004052. [DOI] [Google Scholar]
- Koulianos S, Schmid-Hempel R, Roubik DW, Schmid-Hempel P. Phylogenetic relationships within the corbiculate Apinae (hymenoptera) and the evolution of eusociality. J Evol Biol. 1999;12:380–384. doi: 10.1046/j.1420-9101.1999.00043.x. [DOI] [Google Scholar]
- Leonhardt SD. Chemical ecology of stingless bees. J Chem Ecol. 2017;43:385–402. doi: 10.1007/s10886-017-0837-9. [DOI] [PubMed] [Google Scholar]
- Leonhardt SD, Blüthgen N, Schmitt T. Smelling like resin: terpenoids account for species-specific cuticular profiles in southeast-Asian stingless bees. Insect Soc. 2009;56:157–170. doi: 10.1007/s00040-009-0007-3. [DOI] [Google Scholar]
- Leonhardt SD, Blüthgen N, Schmitt T. Chemical profiles of body surfaces and nests from six Bornean stingless bee species. J Chem Ecol. 2011;37:98–104. doi: 10.1007/s10886-010-9900-5. [DOI] [PubMed] [Google Scholar]
- Leonhardt SD, Rasmussen C, Schmitt T. Genes versus environment: geography and phylogenetic relationships shape the chemical profiles of stingless bees on a global scale. Proc R Soc B. 2013;95:131–140. doi: 10.1098/rspb.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol B-Biochem Mol Biol. 1988;89B:595–645. doi: 10.1016/0305-0491(88)90305-7. [DOI] [Google Scholar]
- Martin SJ, Drijfhout FP. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J Chem Ecol. 2009;35:368–374. doi: 10.1007/s10886-009-9612-x. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Helanterä H, Drijfhout FP. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol J Linn Soc. 2008;95:131–140. doi: 10.1111/j.1095-8312.2008.01038.x. [DOI] [Google Scholar]
- Martin SJ, Carruthers JM, Williams PH, Drijfhout FP. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J Chem Ecol. 2010;36:855–863. doi: 10.1007/s10886-010-9805-3. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Vitikainen E, Shemilt S, Drijfhout FP, Sundstrom L. Sources of variation in cuticular hydrocarbons in the ant Formica exsecta? J Chem Ecol. 2013;39:1415–1423. doi: 10.1007/s10886-013-0366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD. The bees of the world. London: John Hopkins University; 2000. [Google Scholar]
- Michener CD, Grimaldi DA. The oldest fossil bee: Apoid history, evolutionary stasis, and antiquity of social behavior. Proc Natl Acad Sci USA. 1988;85:6424–6426. doi: 10.1073/pnas.85.17.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Biosynthesis in insects, advanced edition Royal Society of Chemistry. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Nascimento DL, Nascimento FS. Acceptance threshold hypothesis is supported by chemical similarity of cuticular hydrocarbons in a stingless bee, Melipona asilvai. J Chem Ecol. 2012;38:1432–1440. doi: 10.1007/s10886-012-0194-7. [DOI] [PubMed] [Google Scholar]
- Nascimento VA, Matusita SH, Kerr WE. Evidence of hybridization between two species of Melipona bees. Genet Mol Biol. 2000;23:79–81. doi: 10.1590/S1415-47572000000100014. [DOI] [Google Scholar]
- Pianaro A, Flach A, Patricio EFLRA, Nogueira-Neto P, Marsaioli AJ. Chemical changes associated with the invasion of a Melipona scutellaris colony by Melipona rufiventris workers. J Chem Ecol. 2007;33:971–984. doi: 10.1007/s10886-007-9274-5. [DOI] [PubMed] [Google Scholar]
- Pradella D, Martin SJ, Dani FR. Using errors by guard honeybees (Apis mellifera) to gain new insights into nest-mate recognition signals. Chem Senses. 2015;40:649–653. doi: 10.1093/chemse/bjv053. [DOI] [PubMed] [Google Scholar]
- Prestwich GD, Blomquist GJ. Pheromone biochemistry. London: Academic Press; 2014. [Google Scholar]
- Quezada-Euan JJG, Ramirez J, Eltz T, Pokorny T, Medina R, Monsreal R. Does sensory deception matter in eusocial obligate food robber systems? A study of Lestrimelitta and stingless bee hosts. Anim Behav. 2013;85:817–823. doi: 10.1016/j.anbehav.2013.01.028. [DOI] [Google Scholar]
- Rasmussen C, Cameron SA. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol J Linn Soc. 2010;99:206–232. doi: 10.1111/j.1095-8312.2009.01341.x. [DOI] [Google Scholar]
- Rouault J, Capy P, Jallon JM. Variations of male cuticular hydrocarbons with geoclimatic variables: an adaptative mechanism in Drosophila melanogaster. Genetica. 2001;110:117–130. doi: 10.1023/A:1017987220814. [DOI] [PubMed] [Google Scholar]
- Roubik DW. Ecology and natural history of tropical bees. New York: Cambridge University Press; 1989. [DOI] [PubMed] [Google Scholar]
- Sakagami SF. Stingless bees. In: Hermann HR, editor. Social insects III. New York: Academic Press; 1982. pp. 361–423. [Google Scholar]
- Tannure-Nascimento IC, Nascimento FS, Zucchi R. The look of royalty: visual and odour signals of reproductive status in a paper wasp. Proc R Soc Lond B Biol Sci. 2008;275:2555–2561. doi: 10.1098/rspb.2008.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannure-Nascimento IC, Nascimento FS, Dantas JO, Zucchi R. Decision rules for egg recognition are related to functional roles and chemical cues in the queen less ant Dinoponera quadriceps. Naturwissenschaften. 2009;96:857–861. doi: 10.1007/s00114-009-0535-8. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Chemical communication within animal species. New York: Academic Press; 1970. [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour: communication by smell and taste. New York: Cambridge University Press; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 33 kb)
(PDF 104 kb)
(PDF 134 kb)
(PDF 93.2 kb)
(PDF 91.5 kb)
(PDF 127 kb)
(PDF 73 kb)