Abstract
Multiple myeloma (MM) is an incurable hematological malignancy of plasma cells with an estimated 30,000 new cases diagnosed each year in the United States, signifying the need for new therapeutic approaches. We hypothesized that targeting MM using a bispecific antibody (biAb) to simultaneously engage both innate and adaptive cytolytic immune cells could present potent antitumor activity. We engineered a biAb by fusing an anti-CS1 single chain variable fragment (scFv) and an anti-NKG2D scFv (CS1-NKG2D biAb). Although NKG2D is a potent activation receptor ubiquitously expressed on mostly cytolytic immune cells including NK cells, CD8+ T cells, γδ T cells, and NKT cells, the CS1 tumor-associated antigen on MM represents a promising target. CS1-NKG2D biAb engaged human MM cell lines and NKG2D+ immune cells, forming immune synapses. In effector cells, CS1-NKG2D biAb triggered the phosphorylation of AKT, a downstream protein kinase of the activated NKG2D-DAP10 complex. The EC50 of CS1-NKG2D biAb for CS1high and for CS1low MM cell lines with effector PBMCs were 10−12 M and 10−9 M, respectively. CS1-NKG2D biAb acted through multiple types of immune cells, and this induced cytotoxicity was both CS1- and NKG2D-specific. In vivo, survival was significantly prolonged using CS1-NKG2D biAb in a xenograft NOD-SCIDIL2γc−/− (NSG) mouse model engrafted with both human PBMCs and MM cell lines. Collectively, we demonstrated that the CS1-NKG2D biAb facilitated an enhanced immune synapse between CS1+ MM cells and NKG2D+ cytolytic innate and antigen-specific effector cells, which, in turn, activated these immune cells for improved clearance of MM.
Keywords: Multiple myeloma, NKG2D, bispecific antibody, CS1, cytolytic immune cells
Introduction
Multiple myeloma (MM) is an incurable malignant disease of plasmocytes with an estimate of 30,770 new cases diagnosed in 2018 (1). Current therapeutics for MM include proteasome inhibitors (bortezomib and carfilzomib), immunomodulatory agents (thalidomide, lenalidomide, and pomalidomide), and stem cell transplantation (2). Both daratumumab, an antibody against CD138, and elotuzumab, an antibody against CS1 (SLAMF7), exert their anti-MM activity, at least in part, through enhanced Fc receptor-medicated cytotoxicity provided by both natural killer (NK) cells and phagocytes (3–5). Elotuzumab, when used together with lenalidomide, dexamethssone, or bortezomib has demonstrated improved clinical outcomes for refractory MM compared to therapy without elotuzumab (6). On the other hand, lenalidomide may exert its anti-MM activity, in part, through modulation of both T and NK cells (7,8).
NKG2D is a lectin-type receptor and one of the major activating NK cell receptors (9,10). NKG2D has also been reported as a co-stimulatory molecule on cytotoxic CD8+ T cells (11) and on invariant NKT cells (12,13), leading to TCR-independent cell activation. Successful activation via NKG2D relies on the expression of its cognate ligands, MICA and ULBP, on tumor cells, including MM cells (14,15). However, not all MM cells express NKG2D ligands, leading to evasion of NKG2D-mediated immune surveillance. Retargeting tumor-associated antigen and effector activation receptors could provide specific and safe antitumor effects. Bispecific antibodies (biAbs) and T/NK-cell engager (BiTE/BiKE) therapies fall into this new class of treatment, with the first BiTE targeting CD19 and CD3 having been FDA-approved in 2014 (16). Expanding the discovery and characterization of tumor-associated antigens explores the possibility of applying this antibody platform to other cancers.
MM cells express high CS1 (CD319 or SLAMF7), a surface lymphocytic activation molecule, whereas NK cells and a subset of activated T cells have low expression (17). Its expression, as determined by staining with the CS1 monoclonal antibody (mAb) elotuzumab, is found on nearly all tumor cells in 95% of MM patients and has become a target for therapy with elotuzumab (2,18). However, unless combined with other chemotherapies such as lenalidomide, dexamethasone, or proteasome inhibitor bortezomib, elotuzumab has no significant clinical benefits to MM patients (3). Our group reported that anti-CS1 chimeric antigen receptors (CAR) T and NK cells could effectively eradicate MM cells (19,20). This suggests that targeting CS1 while activating T or NK cells remains a promising therapeutic strategy for MM.
Here we describe the generation and therapeutic activity of a biAb targeting CS1 on MM cells and NKG2D on cytotoxic immune effector cells. This CS1-NKG2D biAb was derived from clones of high-affinity anti-CS1 (20) and anti-NKG2D (21). We hypothesized that by engaging CS1 on MM and simultaneously activating NKG2D on all NKG2D-expressing immune cells, including NK cells, CD8+ T cells, γδ T cells, and NKT cells, we could exert an additive cytotoxic effect against MM cells. Our in vitro data demonstrated that CS1-NKG2D biAb induced a dose-dependent increase in specific cytotoxicity of these effector cells against CS1+ MM cells, as well as IFNγ production, and significantly prolonged survival when administered in vivo to an NSG mouse model of human MM.
Materials and Methods
Bispecific antibody construction, expression, and purification
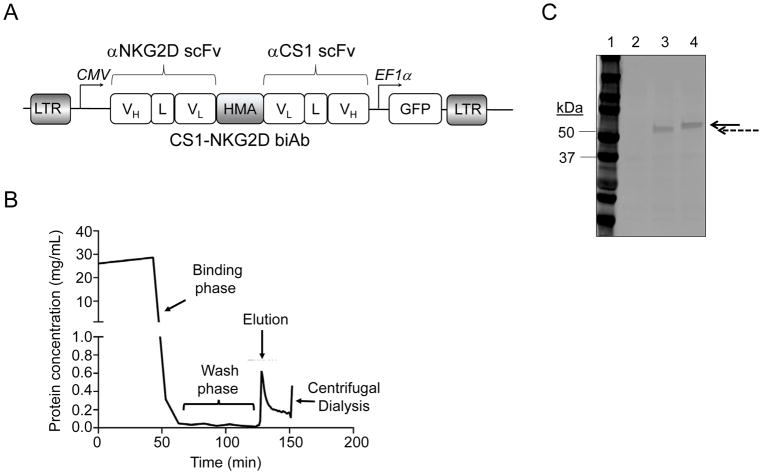
CS1- NKG2D biAbs were in silico designed (Fig. 2A), and single chain variable fragments (scFv) of the mouse anti-human NKG2D (21) and anti-human CS1 (19) mAbs were joined with non-immunogenic human muscle aldose protein linker (22). A six-histidine tag was added to the c-terminus of anti-CS1 scFv. A secretory signal peptide H7 was added in front of the entire sequence (23). The sequence was then codon-optimized (22), synthesized, and subcloned into a lentiviral vector pCDH-CMV-MCS-EF1α-GFP (SBI Bioscience, Palo Alto, CA, USA). The lentivirus generated was used to transduce a CHO-S cell line (Invitrogen, Waltham, MA, USA), which was authenticated by the manufacturer, passaged three times and mycoplasma-tested before used for transduction. A stable CHO-S biAbs-producing cell line was created by serial sorting the GFP high expressers using a BD Aria II (BD Biosciences, San Jose, CA, USA). A site-directed mutagenesis was performed to delete the CDR3 region of the heavy chain variable region of anti-NKG2D scFv for the creation of a negative-binding biAb control (Supplementary Fig. S1). A fed-batched CHO-S culture was set up with Freestyle CHO expression medium (Invitrogen) and maintained for no more than 30 passages. The culture supernatant of the fed-batched cultures was collected and purified with HisTrap excel columns (GE Healthcare Life Science, Pittsburgh, PA, USA), as per the manufacturer’s protocol. Briefly, the culture supernatant was first filtered through a 0.45 um filter and then dialyzed against a binding buffer (20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, pH 7.4) in a centrifugal filter unit with a 100 kDa cutoff (Millipore Sigma, Burlington, MA, USA). The dialyzed supernatant was then loaded to a nickel ion sepharose pre-packed column at 1 mL/minute using a peristatic pump. The column was then washed with 5 column volumes of wash buffer (20 mM sodium phosphate, 0.5 M NaCl, 40 mM imidazole, pH 7.4) at the same flow rate. The biAb was eluted with elution buffers (20 mM sodium phosphate, 0.5 M NaCl, pH 7.4) with a linear gradient of imidazole concentrations ranged from 50 mM to 100 mM. The eluted biAb was collected in fractions and analyzed by SDS-PAGE and Coomassie brilliant blue staining under the standard procedure to determine the presence of monomers and dimers. The eluted fractions containing monomer (CS1-NKG2D biAb or control biAb) fractions were sequentially dialyzed at pore size cutoffs of 100 kDa and 50 kDa against PBS before use in in vitro or in vivo studies. Purified biAbs were routinely analyzed by SDS-PAGE and stained with Coomassie brilliant blue for size estimation and quality control.
Figure 2. Design and purification of CS1-NKG2D biAb by metal-affinity chromatography.
(A) Schematic diagram of the lentiviral construct for mammalian expression of CS1-NKG2D biAb in CHO-S cells. (B) A typical profile of the protein eluted from immobilized metal-affinity chromatography column using stepwise imidazole gradient. (C) SDS-PAGE for eluted protein. Lane 1: molecular weight marker (kDa); Lane 2: protein lysate from mock transduced CHO-S cells; Lane 3: eluted control biAb (dashed arrow); Lane 4: eluted CS1-NKG2D biAb (solid arrow). Results shown are representative of at least ten independent experiments.
Multiple myeloma cell and cell isolation
The multiple myeloma cell lines MM.1S, NCI-H929, and RPMI-8226 were obtained from ATCC (Manassas, VA, USA) and cultured under the manufacturer’s instruction. U266, L363, and OPM2 (from Dr. Don Benson) were cultured in RPM 1640 with 10% FBS and 1% antibiotic-antimycotic (Invitrogen). These cell lines have not been authenticated since receipt but were not passaged for more than two months. All the cell lines used were routinely tested for mycoplasma using MycoAlert™ PLUS Mycoplasma Detection Kit from Lonza (Walkersville, MD).
Peripheral blood of 48 healthy donors and 3 MM patients were obtained from American Red Cross and The James Cancer Hospital, respectively. The study was approved by the Institutional Review Board at The Ohio State University and was conducted in accordance to Declaration of Helsinki. Informed and written consents were obtained from all 3 patients and no inclusion/exclusion criteria were specified for the study. Human peripheral blood mononuclear cells (PBMCs) were isolated from the healthy donors or MM samples within 6 hours after receipt by Ficoll-Paque Plus (GE Healthcare Life Science) gradient density. Human NK cells from peripheral blood were negatively enriched using a RosetteSep human NK cell enrichment cocktail (catalog #15065, Stem Cell Technology, Cambridge, MA, USA), whereas human T cells were enriched using a RosetteSep human T cell enrichment cocktail (catalog #15061, Stem Cell Technology), following the manufacturer’s instructions. In indicated experiments, CD56- or CD3-negative cells were collected from the flow-throughs of the PBMCs stained with CD56 or CD3 microbeads (Miltenyi Biotech, Auburn, CA, USA), respectively. To test the specificity of CS1-NKG2D biAbs, NKG2D-negative cells were sorted from IL2 (500 U/mL)-primed PBMCs.
Antibodies and flow cytometry
Antibodies used in this study include from BD Biosciences: anti-human CD3 (clone SK7, FITC and PE-CF594-conjugated), anti-human CD4 (clone RM4-5, FITC-conjugated), human anti-CD8 (clone RPA-T8, FITC-conjugated), anti-CD56 (clone N901, APC-conjugated), anti-CD14 (clone MϕP9, FITC-conjugated), anti-human CD45 (clone 2D1, APC-H7-conjuagted), anti- mouse CD45 (clone 30-F11, PE-conjugated), anti-human NKG2D (clone 1D11, PE-conjugated), anti-human TCRγδ (11F2, FITC-conjugated), anti-human CD19 (clone SJ25C1, FITC-conjugated), anti-human CD20 (clone L27, FITC-conjugated, and anti-human CD34 (clone 8G12, APC-conjugated); from Biolegend: San Diego, CA, USA: anti-mouse CD3 (clone 145-2C11; PE-conjugated), anti-phalloidin (APC-conjugated), and anti-human TCRVα24-Jα18 (clone 6B11, APC-conjugated); from Miltenyi Biotech: anti-CD38 (clone REA572, PE-Vio770-conjugated); from ThermoFisher Scientific: anti-CD319 (CS1/SLAMF7; clone 162, PE-conjugated), and anti-human CD138 (clone DL-101, APC-conjugated). For staining NKG2D ligands, a recombinant NKG2D-Fc chimera protein was used (catalog number 1299-NK-050, R&D Systems, Minneapolis, MN, USA). Anti-human MICA/B and human ULBP2 antibodies were from R&D Systems and both were PE-conjugated. To stain, 1 × 106 cells were first washed once with PBS, then stained with antibodies in staining buffer (PBS with 0.5% BSA and 2 mM EDTA) for 20 minutes at room temperature. All samples were analyzed with an LSRII flow cytometer (BD Biosciences).
Identification of immune effector cell subsets and hematopoietic stem cells
Total T cells in PBMCs were stained with antibodies directed against either CD3 alone, CD3 and CD4, CD3 and CD8, and CD3 and TCRγδ for γδT cells and CD3 and CD56 for total NKT cells. NK cells were identified as CD56+CD3−. Invariant (i) NKT cells were stained with antibodies directed against CD3 and TCRVα24-Jα18. Monocytes/macrophages were identified as CD14+LIN−. Hematopoietic stem cells were identified as CD34+LIN−. LIN− was defined using a cocktail of monoclonal antibodies directed against CD3, CD56, CD19, and CD20.
Flow-based cytotoxicity assay
The flow-based cytotoxicity assay was performed as previously described (24). Briefly, the multiple myeloma cell lines MM.1S, NCI-H929, and RPMI-8226 were transduced with a pCDH-GFP vector (System Biosciences) to establish corresponding target cell lines stably expressing GFP. For U266, OPM2 and L363 cells, they were labelled with Calcein-AM (BD Biosciences) per manufacturer’s instruction. Briefly, 1×106 target cells were stained with 5 μM Calcein-AM for 30 minutes at 37 °C water bath protected from light. The cells were then washed for 4 times with RPMI 1640 medium supplemented with 10% FBS before used as target cells. The target cells were then cocultured with IL2 (500 U/mL)-primed PBMCs at an effector-to-target (E:T) ratio of 20:1, 10:1, 5:1 and 1:1 for 4 hours in RPMI 1640 medium supplemented with 10% FBS. The cells were then harvested and stained for Sytox Blue (Invitrogen) before analysis by a BD LSRII flow analyzer (BD Biosciences). To determine the effect of CS1-NKG2D biAb on PBMCs cytotoxicity, MM cell lines were cocultured with IL2-primed PBMCs at an E:T ratio of 10:1 in the presence of control or CS1-NKG2D biAbs at indicated doses for 24 hours before harvested for flow cytometry analysis of induced cytotoxicity. Calcein-AM-labelled autologous PBMCs were also used as target cells and cocultured with autologous IL2-primed PBMCs at an E:T ratio of 10 in the presence of biAbs.
To test the effect of soluble NKG2D ligands or CS1 antigen on the cytotoxicity, 5 μg/mL NKG2D-Fc or 10 ng/mL recombinant CS1 protein (Biomatik, Wilmington, DE, USA) was added to the coculture of the IL2-primed PBMCs, MM.1S and biAbs. To test the specificity of the CS1-NKG2D biAb on the cytotoxicity, NKG2D neutralizing antibody (10 μg/mL; clone 1D11, BD Biosciences) was used to pretreat the effector cells before coculturing with target cells in the presence of biAbs. For cytotoxicity assay using primary MM patient sample as target, MM patient PBMCs were cocultured with allogeneic PBMCs from healthy donors at an E:T ratio of 10:1 in the presence of CS1-NKG2D biAb or control biAb for 24 hours. Cells were then stained with antibodies against CD38 and CD138. The CS1bright subset in MM patient samples was identified in CD38+/brightCD138dim/+ population. Dose of biAbs used was 50 μg/mL in most cytotoxicity experiments, except when particularly specified.
Imaging flow cytometric detection of immune-synapse
Isolated total CD3+ T or NK cells were activated at 1×106 cells/mL with IL2 (500 U/mL) overnight. The cells were harvested and labeled with CD3 or CD56 PE-conjugated monoclonal antibodies. These labeled effector cells were cocultured with GFP-transduced-MM.1S MM target cells at 1:1 E:T ratio for 30 minutes in the presence of PBS, control biAb (100 μg/mL), or CS1-NKG2D biAb (100 μg/mL). After coculture, cells were fixed with 2% paraformaldehyde in PBS (Millipore Sigma) for 20 minutes on ice, permeabilized with PBS containing 0.5% saponin (Millipore Sigma) and 2% FBS for 20 minutes on ice. The cells were washed and stained with Alexa Fluor 647-phalloidin and DAPI (ThermoFisher Scientific) for 20 minutes on ice. All the samples were washed twice and resuspended in 50 μL PBS.
The cells were analyzed with Amis ImageStream X Mark II imaging flow cytometer, and the data were analyzed with IDEAS (Amnis, Settle, WA, USA). Briefly, single cells were identified based on the cell size, focus attributes, and nucleus DAPI positivity stain. An actin valley mask was then created and defined in the co-localization of two cells (GFP and PE positive) by a high local pixel intensity formed with accumulation of phalloidin staining from actin in the immune-synapse. A valid synapse was defined by high Bright Detail Similarity Score (BDS) and high Bright Details Intensity (BDI) on the actin valley mask images. BDS was calculated based on the spatial location of the image pairs and determined the degree of overlapping between the two images. BDI was computed the intensity of bright spots that were 3 pixels in radius or less. A plot of BDS versus BDI identified the valid synapses, and the relative frequency of synapses formed in indicated conditions was calculated.
Immunoblotting
1 × 106 primary NK cells were stained with either CS1-NKG2D biAbs (100 μg/mL) or anti-NKG2D (BD Bioscience) for multiple time periods in a 37°C incubator. Cells were then harvested at indicated time points. The cells were lysed for 10 minutes on ice in a mammalian cell lysis buffer (ThermoFisher Scientific) supplemented with a protease inhibitor cocktail (cOmplete, Millipore Sigma), 1 mM sodium orthovanadate, and 1 mM PMSF. The protein lysates were quantified using a BCA protein assay kit (ThermoFisher Scientific). 30 μg of total protein was resolved with SDS-PAGE and transferred to the nitrocellulose membrane of a Trans-Blot Turbo transfer pack (Bio-Rad Laboratories, Hercules, CA, USA) using a Trans-Blot Turbo Transfer System (Bio-Rad). After the membrane was blocked with Odyssey blocking buffer/PBS (Li-Cor Biotechnology, Lincoln, NE, USA), it was immunoblotted with a rabbit anti–human phospho-AKT antibody (clone D9E, Cell Signaling, Danvers, MA) and a mouse-anti-human total β-actin (clone I-19; Santa Cruz Biotechnology, Dallas, TX, USA) as the loading control. A donkey anti-mouse IgG (H+L) IRDye 680RD antibody (Li-Cor) and a goat-anti-rabbit IgG (H+L) IRDye 680RD antibody (Li-Cor) were used as secondary antibodies. The stained membrane was washed 4 times with PBS plus 1% Tween 20 and 2 times with PBS alone before it was scanned with an Odyssey CLx imaging system (Li-Cor). The expression of the phospho-AKT was normalized with that of the loading control.
ELISA
To determine the IFNγ production of the biAb-induced PBMCs, cocultures of MM.1S (1 × 105) and IL2-primed PBMCs (1 × 105) were set up with or without adding biAbs (50 μg/mL). Cell-free supernatants were harvested after 48 hours, and the concentration of human IFNγ in the culture supernatants was quantified in ELISA assays with an IFNγ antibody pair (clones XMG1.2 and 2G1, ThermoFisher Scientific). For quantifying the biAb kinetics along time from different routes of administration in vivo, RAG2IL2rγ−/− mice were used. Briefly, 20 μg CS1-NKG2D biAb was injected into the mice via intravenous, intraperitoneal, or subcutaneous routes. 100 μL blood was collected from tail veins at indicated time points over 48 hours. Cell-free plasma samples were collected by centrifugation the blood samples. An in-house biAb ELISA was constructed to quantify the concentration of biAb. Briefly, biAb-containing plasma samples were first coated onto a 96-well EIA/RIA plate (Corning, Corning, NY, USA) at room temperature for 2 hours. A His-tagged protein (Biomatik) with known concentration was used as a standard. The plate was blocked with a PBS buffer containing 1% BSA and 0.05% Tween 20, washed with PBS supplemented with 0.05% Tween 20, and incubated with HisProbe-HRP conjugate (1 μg/mL; ThermoFisher Scientific) for 15 minutes. The plate was then washed, incubated with 100 μL per well tetramethylbenzidine substrate (Agilent Technologies, Santa Clara, CA, USA) for 15 minutes and read at 450 nm using a Synergy HT microplate reader (Biotek, Winooski, VT, USA).
In vivo experiment
All the animal studies were approved by The Ohio State University Institutional Animal Care and Use Committee. 8- to 12-week old NSG mice bearing a xenograft of MM.1S cells were used as a preclinical model to determine the efficacy of the CS1-NKG2D biAb. Mice were x-ray irradiated at 100 cGy the day before 5 × 106 MM.1S were intravenously (i.v.) injected. On the next day, 5 × 106 IL2-primed PBMCs were injected intraperitoneally. Human IL2 (100 U/mouse) was subcutaneously injected starting one day after effector cell injection and every other day for two weeks. CS1-NKG2D biAbs or control biAbs (200 μg/kg) were subcutaneously injected at the flank to the corresponding group twice a day for 2 weeks starting one day after effector cell injection. The progression of the disease was monitored by assessing body condition score (BCS) every day, and survival data were recorded. The mice were sacrificed when they reached an end-point at BCS<2, paralysis, or 20% body weight loss.
Statistical analysis
For data following normal distribution, or normal after transformation, Student’s t test or paired t-test was used to compare two independent groups or two matched groups. Linear model was used to compare multiple independent groups. Linear mixed model was applied to multiple group comparisons under the variance-covariance structure due to repeated measures. For survival data, Kaplan-Meier method was applied to estimate survival function and log-rank test was used to compare the survival between two groups. P values were adjusted for multiple comparisons by Holm’s procedure. A P value of 0.05 or less was considered statistically significant. All data were analyzed with R version 3.3.1 and SAS 9.4.
Results
Surface expression of NKG2D and its ligands on MM cells
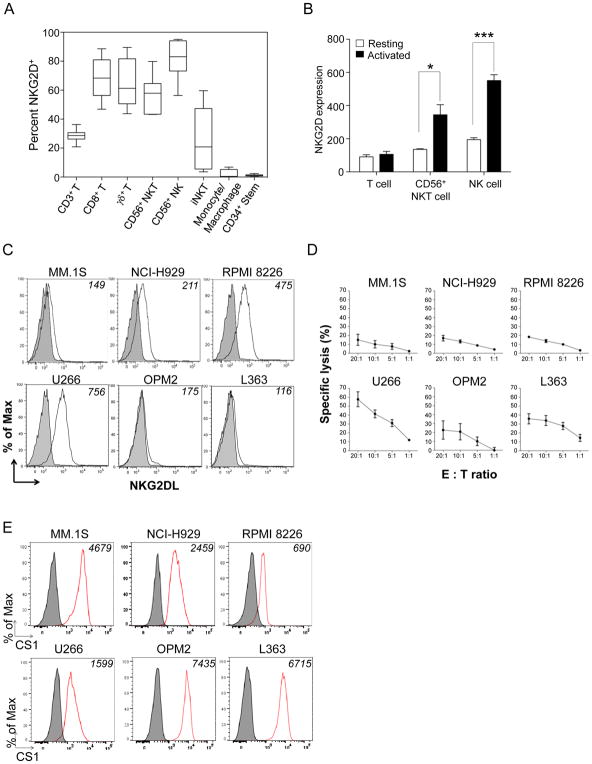
As previously reported (25), we found that NK cells, CD8+ T cells, δγ T cells, CD56+ NKT cells and TCRVα24-Jα18+ iNKT cells had high surface density expression of NKG2D (Fig. 1A). In contrast, CD14+LIN− monocyte/macrophage and CD34+ stem cells had low/no expression of NKG2D. Expression of NKG2D on CD56+ NKT cells and NK cells could be significantly increased in the presence of IL2 (Fig. 1B). The susceptibility of MM cells to NKG2D-mediated NK cell cytotoxicity relies on their expression of NKG2D ligands, namely MICs or ULBPs (14). We found that six human MM cell lines had low or absent expression of MICs or ULBPs (Fig. 1C). This likely contributes to the relative resistance to NK cell natural cytotoxicity seen in five of the six cell lines (Fig. 1D). Because the NKG2D receptor remains one of the major activation receptors for cytolytic effector cells, we attempted to trigger NKG2D activation on the NKG2D+ cytolytic immune cells and simultaneously engage MM cells via the MM-associated antigen, CS1. We, therefore, examined CS1 expression on the six MM cell lines. Each showed different surface density expression of CS1, with MM.1S, OPM2, and L363 showing high expression, H929 showing intermediate expression, and RPMI-8226 and U266 showing low expression (Fig. 1E).
Figure 1. NKG2D, NKG2D ligands, and CS1 expression.
(A) Box-and-whisker plot of percent NKG2D+ cells in peripheral blood immune cells. Data represent the median percent among 5 healthy donors. (B) Expression of NKG2D on T cells, CD56+ NKT cells, and NK cells at rest or overnight IL2-activated conditions. Results are mean MFI±S.E.M. from three independent experiments. *p<0.05; ***p<0.001 using Student’s t test. (C) The expression NKG2D ligands (white) on MM cell lines stained with NKG2D-Fc antibody compared to the isotype (grey). Numbers on the top right corner: mean fluorescent intensity (MFI). Results are from one representative of three independent staining experiments. (D) NK cell natural cytotoxicity against MM.1S, NCI-H929, RPMI-8226, OPM2, and L363 cells. Target cells were co-incubated with NK cells at indicated effector-to-target (E:T) ratios for 4 hours. Results are mean specific lysis with S.E.M. from three independent experiments and 3 healthy donors. (E) Expression of CS1 on different MM cells displayed as histograms compared to isotype. Numbers on the top right corner: MFI. Results are from one representative of three independent staining experiments.
A CS1-NKG2D bispecific antibody retargeting CS1 and NKG2D
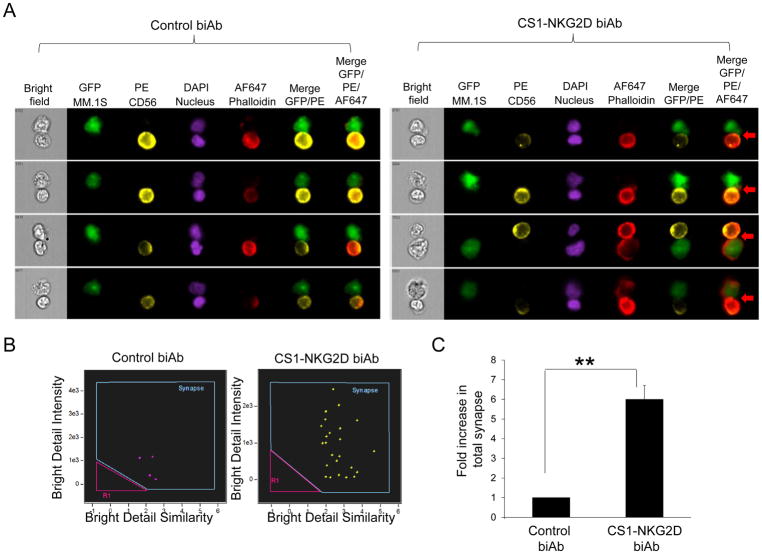
We designed the CS1-NKG2D bispecific antibody (biAb) in silico. It consisted of two codon-optimized single chain variable fragments (scFv) from a CS1 monoclonal antibody (20) and an NKG2D antibody (21) joined together by a non-immunogenic protein linker derived from human muscle aldose (26). Each scFv contained a corresponding heavy chain (VH) and light chain (VL) connected by a 15-amino acid long glycine-serine (GS) linker. The entire fragment was led by an IgG secretion signal peptide H7 (23) and expressed in a lentiviral vector (Fig. 2A). Culture supernatants from the stable CHO-S cell line carrying the biAb was purified as described. A typical elution profile is shown (Fig. 2B). A similar biAb with a CDR3 deletion on the VH of anti-NKG2D scFv was produced as our negative control biAb (termed control biAb hereafter, Supplementary Fig. S1). SDS-PAGE showed the CS1-NKG2D and control biAbs had the correct sizes of 56kDa and 50kDa, respectively, as expected from computational analysis (Fig. 2C). Using ImageStream imaging flow cytometry, we confirmed that functional immune-synapses were formed in the presence of the CS1-NKG2D biAb (Fig. 3A–B). A 1.92-fold increase in antigen-specific immune-synapses was observed in cocultures of NK cells and MM cells in the presence of CS1-NKG2D biAb but not with the control biAb (Fig. 3C).
Figure 3. Immune-synapses between the CS1+ MM.1S MM cells and NK cells increase in the presence of CS1-NKG2D biAb but not control biAb.
(A) Representative snapshot images of each conjugation between 1 × 105 MM.1S MM cell and 1 × 105 NK cell in the presence of 100 μg/mLcontrol biAb (left panel) or CS1-NKG2D (right panel). MM.1S MM cells: GFP; NK cells: PE; Nuclei: DAPI; and actin: phalloidin. Cell-to-cell conjugation shown in merged GFP and PE detection channels. Valid synapses were only identified by co-localization of actin (Alexa Fluor 647) at the interface of MM.1S MM (GFP) and NK (PE) in the merged image of the 3 detection channels (red arrows). B) Dot plots of immune-synapses formed between MM.1S and NK cells in the presence of control biAb (left panel) and CS1-NKG2D (right panel). Bright Detail Similarity (BDS): degree of overlapping between the two images of effector and target cells. Bright detail intensity (BDI): intensity of bright spots that were ≤3 pixels in radius. Blue gates: immune-synapses (high BDS and high BDI). Pink gates: low BDS and low BDI formed by coincidence. (C) Fold increase in immune-synapse formations between the MM.1S MM cells and NK cells in the presence of control biAb or the CS1-NKG2D biAb. Total percentage of synapses from control biAb was normalized as 1. Data are mean±S.D. from two independent experiments. **p<0.01 using Student’s t test.
Triggering NKG2D on NK cells by CS1-NKG2D biAb induces phosphorylation of AKT
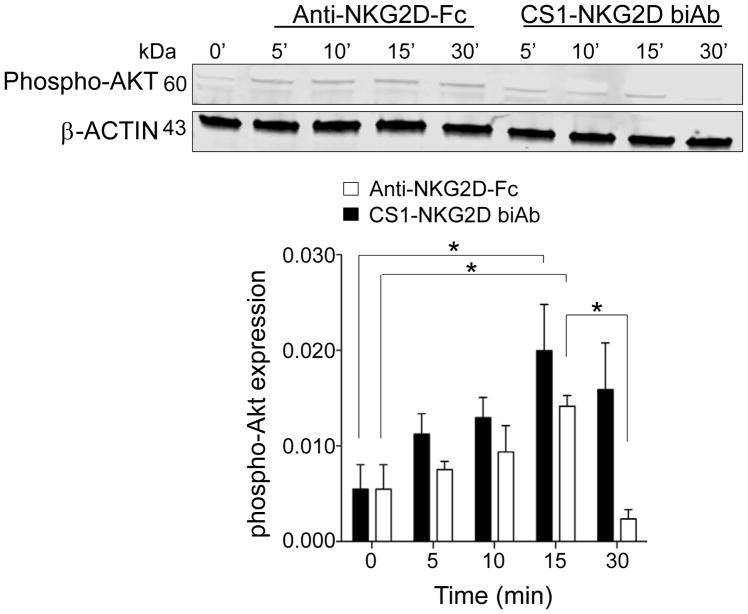
To determine the activation induced by the CS1-NKG2D biAb, we incubated IL2-primed NK cells with full-sized anti-NKG2D as a positive control or CS1-NKG2D biAb. AKT is a signaling molecule downstream of the NKG2D-DAP10 complex (27). We found a temporal increase in phosphorylated AKT that peaked at 15 minutes for both the positive control anti–NKG2D-Fc and the CS1-NKG2D biAb, whereas the signals from CS1-NKG2D biAb diminished at 30 minutes (Fig. 4). This demonstrated that the CS1-NKG2D biAb could bind to and trigger activation of effector target cells via NKG2D.
Figure 4. CS1-NKG2D biAb triggers the phosphorylation of the NKG2D-DAP10 complex signaling adaptor AKT.
(A) Upper panel: Immunoblot of AKT phosphorylation in protein lysates from IL2-primed PBMCs activated either with a full-sized anti–NKG2D-Fc (positive control) or CS1-NKG2D biAb at the indicated time points. Lower panel: Phosphorylated AKT, stimulated by anti–NKG2D-Fc (black) and CS1-NKG2D biAb (white), was normalized to β-actin and plotted. One representative blot of three independent experiments is shown in the upper panel, and the summary data of three experiments are shown in the bottom bar graph. Error bars denote S.D.
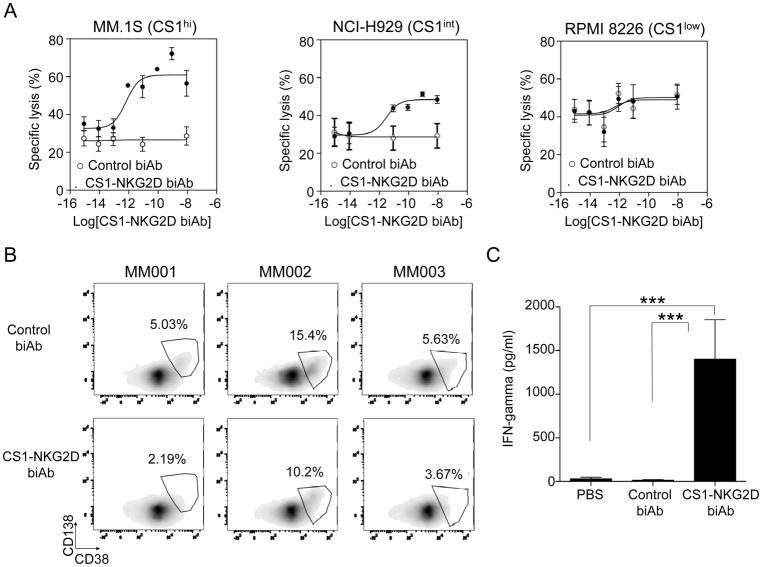
CS1-NKG2D biAb induces cytotoxicity and IFNγ production of PBMCs
To assess the functionality of the CS1-NKG2D biAb, we cocultured IL2-primed PBMCs as effectors with MM cell lines MM.1S, H929, or RPMI-8226 as targets, representing MM cells with high, intermediate, and low CS1 expression, respectively. Dose-dependent increases in specific lysis were observed in MM.1S and H929 cells in the presence of CS1-NKG2D biAb but not in the presence of RPMI-8226 or the negative control biAb (Fig. 5A). The EC50 for the CS1high MM.1S, CS1int H929, and CS1low RPMI-8226 MM cell lines were 10−12 M, 10−11 M, and 10−9 M, respectively. Primary MM patient peripheral blood samples were also used to validate the efficacy of the CS1-NKG2D biAb. All three samples contained the CS1bright subset in the CD38+/brightCD138dim/+ MM population (Supplementary Fig. S2). A reduction of the CS1bright population was observed when patient peripheral blood samples were treated with allogeneic PBMCs at an E:T ratio of 10:1 in the presence of the CS1-NKG2D biAb (Fig. 5B). We found that IFNγ production was significantly increased when CS1-NKG2D biAb was added to the coculture containing IL2-primed PBMCs and the CS1high MM.1S cell line (Fig. 5C). IL2 priming was observed to be required for this significant induction of IFNγ production (Supplementary Fig. S3). No significant increase in IFNγ production was observed in cocultures of primed PBMCs and CS1low RPMI-8226 cells in the presence of CS1-NKG2D biAb.
Figure 5. CS1-NKG2D biAb induces cytotoxicity against MM cells directly proportional to the expression of CS1 and IFNγ production.
(A) Specific lysis of MM.1S (CS1high), NCI-H929 (CS1int), and RPMI-8226 (CS1low) MM cell lines in the presence of IL2-activated PBMCs at varying doses of CS1-NKG2D biAb or control biAb. Results are from five independent experiments. (B) 3 MM patient PBMCs were cocultured with the allogeneic PBMCs of healthy donors in the presence of biAbs (50 μg/mL). Effector cytolytic activity was assessed after 24 hours. Patient CS1+ tumor cells were identified as CD38brightCD138dim/+. Results of 3 MM patient samples are shown. (C) IFNγ production in coculture supernatants of IL2-activated PBMCs and MM.1S MM cells in the presence of PBS, control biAb, or CS1-NKG2D biAb. Results show the mean±S.E.M. of five independent experiments. ***p<0.001 using Linear mixed model.
The expression of CS1 on normal T, NKT, and NK cells was low and similar to that of the RPMI-8226 MM cell line (Supplementary Fig. S4A). Using autologous PBMCs as target cells, we found no significant increase in specific lysis against autologous T, NKT, or NK cells when cocultured with the CS1-NKG2D biAb compared to the negative control biAb (Supplementary Fig. S4B). We also found that neither soluble CS1 nor an NKG2D binding chimera protein, mimicking the circulating CS1 antigen (28) and shedded NKG2D ligands in the peripheral blood of MM patients (29), respectively, affected the CS1-NKG2D biAb-induced cytotoxicity against MM1.S (Supplementary Fig. S5).
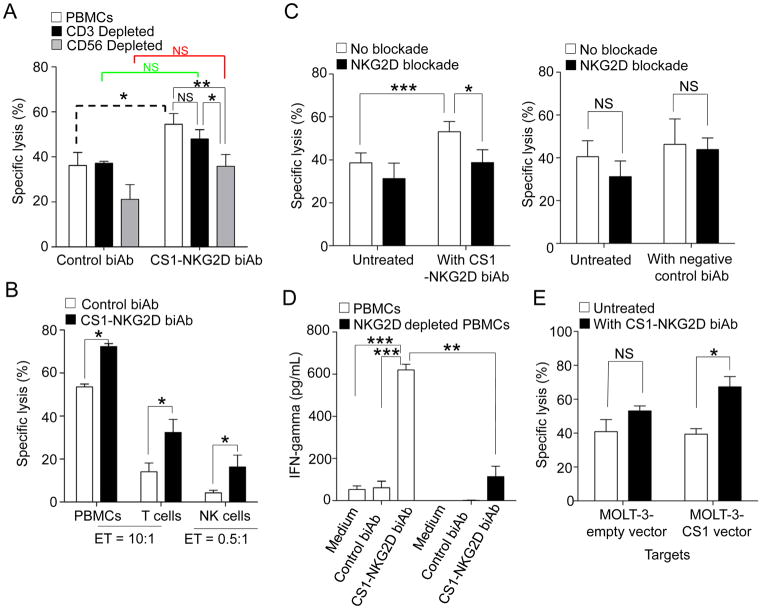
CS1-NKG2D biAb cumulatively induces specific cytolysis of MM
When IL2-activated PBMCs were used as effector cells in coculture with the CS1high MM.1S MM cell line, a significant increase in specific lysis was observed in the presence of CS1-NKG2D biAb compared to the control biAb (Fig. 6A; dashed bracket). When the same experiment was conducted with either CD3-depleted PBMCs (Fig. 6A; green bracket) or CD56-depleted PBMCs (Fig. 6A; red bracket), no significant increase in cytotoxicity was observed in the presence of CS1-NKG2D biAb compared to the same conditions with the control biAb. This suggests that T cells, as well as NK cells, each have a significant role in mediating the enhanced cytotoxic effect noted with total PBMCs plus the CS1-NKG2D biAb. When the cytotoxicity of total PBMCs plus the CS1-NKG2D biAb against the CS1high MM.1S MM cell line was compared to the same experiment using PBMCs depleted of T cells plus the CS1-NKG2D biAb against the CS1high MM.1S MM cell line, no significant difference compared to total PBMCs was seen, likely because at the same 10:1 cytotoxicity ratio, the absolute number of NK cells in the coculture with the CS1high MM.1S MM cell line was increased by approximately 100%. When the same experiment was performed using PBMCs depleted of NK cells plus the CS1-NKG2D biAb against the CS1high MM.1S MM cell line, significantly lower cytotoxicity compared to total PBMC was observed (Fig. 6A), demonstrating the critical role this minor population of cytolytic effector cells play in this process. To validate these results, we also used purified CD3+ T cells and NK cells as effectors in cocultures with MM1.S cells in the presence of control biAb or the CS1-NKG2D biAb. Similarly, we found that the CS1-NKG2D biAb, but not control biAb, induced a significant increase in cytotoxicity against the MM1.S target cells (Fig. 6B).
Figure 6. Assessment of cytotoxicity and IFNγ production in the presence of the CS1-NGK2D biAb.
(A) MM.1S cell line was cocultured with IL2-primed PBMCs, T cell- depleted PBMCs, or NK cell-depleted PBMCs at an E:T ratio of 10:1 for 24 hours in the presence of biAb. All depletions were from negative depletions. Specific lysis induced from the control biAb and CS1-NKG2D biAb on each effector was compared as shown in dashed (PBMCs), green (T cells), and red (NK cells). (B) IL2-primed PBMCs, purified T cells, and purified NK cells were cocultured with the MM.1S MM cell line for 24 hours in the presence of biAb. CD3+ T cells and CD56+CD3− NK cells were sorted. E:T ratio for NK cells: 0.5:1; E:T ratio for PBMCs and T cells: 10:1. (C) Effect of a NKG2D blocking antibody on the enhanced cytotoxicity of IL2-primed PBMCs induced by the CS1-NKG2D biAb against the MM.1S MM cell line at an E:T ratio of 10:1 for 24 hours. The effectors were pretreated with the blockade before coculturing the targets. (D) IFNγ production of the PBMCs and NKG2D-depleted PBMCs against the MM.1S MM cell line in the presence of biAbs. The E:T ratio is 1:1. (E) Cytotoxicity assay of the IL2-primed PBMCs against CS1− MOLT-3 cell line and CS1 overexpressed MOLT-3 cell line. The dose of biAbs for all experiments was 50 μg/mL. Results show specific lysis mean±S.E.M. of five independent experiments. NS: not significant; *p<0.05; **p<0.01; ***p<0.001 using Student’s t test for two-group comparison and linear mixed model for multiple group comparison.
To further confirm that the cytotoxicity was from the engagement of the NKG2D receptor, we pretreated the effector PBMC with an anti-NKG2D blocking antibody prior to the addition of the CS1-NKG2D biAb, and this significantly blunted the enhanced lysis resulting from the addition of the CS1-NKG2D biAb (Fig. 6C). IFNγ production was significantly increased when IL2-primed PBMCs were used as effector cells in coculture with the CS1high MM.1S MM cells and the CS1-NKG2D biAb compared to the negative control biAb. A profound decrease in IFNγ production when the same experiment was conducted with PBMCs depleted of NKG2D+ cells (Fig. 6D). Finally, we used an acute myeloid leukemia cell (AML) line, MOLT-3, as a negative target cell control because it does not express CS1. A significant increase in specific lysis was observed in the presence of the CS1-NKG2D biAb when the CS1 antigen was ectopically expressed on the MOLT-3 AML cell line (Fig. 6E).
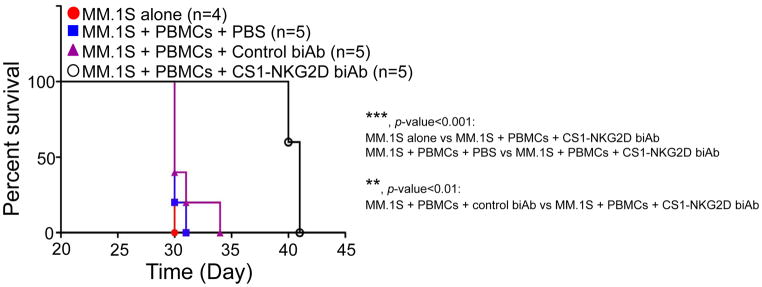
CS1-NKG2D biAb treatment prolongs survival of mice bearing human CS1high MM
To determine the efficacy of the CS1-NKG2D biAb in vivo, we employed NSG mice engrafted with the MM.1S (CS1high) or NCI-H929 (CS1int) MM cell line. Because the CS1-NKG2D biAb is small, approximately 56 kDa, we first elucidated the optimal protocol in dosing the biAb in vivo. We established an in-house ELISA for quantifying the amount of CS1-NKG2D biAb in mouse plasma, as shown in Supplementary Fig. S6A. Based on the calculated standard curve (Supplementary Fig. S6B), we determined that subcutaneous administration resulted in the longest half-life in vivo when compared to intravenous and intraperitoneal administration (Supplementary Fig. S6C). Based on these data, we injected the mice subcutaneously with 200 μg/kg of CS1-NKG2D biAb or the control biAb twice a day for 2 weeks. For NSG mice engrafted with human PBMCs and CS1high MM.1S MM cells and then treated with the CS1-NKG2D biAb, a significant prolongation of survival over mice engrafted with CS1high MM.1S MM cells alone (p<0.0001) or with PBMCs and CS1high MM.1S MM cells and then treated with the control biAb (p<0.0022, Fig. 7). By comparing groups receiving MM.1S MM cells and PBMCs, with or without CS1-NKG2D biAb, a prolonged survival in the group that received CS1-NKG2D biAb (p<0.01) was also seen. In contrast, there was no difference in survival between the NSG mice engrafted with human PBMCs and CS1high MM.1S MM cells that received PBS and the mice engrafted with human PBMCs and CS1high MM.1S MM cells that received the negative control biAb. When NSG mice were engrafted with human PBMCs and H929 MM cells, which have intermediate CS1 expression (Fig. 1E), a moderate prolongation of survival in mice that received the CS1-NKG2D biAb was observed, but statistical significance was only reached when compared to mice receiving PBS (p=0.04, Supplementary Fig. S7). This suggested that the prolonged mouse survival in this experimental MM model was dependent upon the presence of PBMCs, CS1 expression, as well as CS1-NKG2D biAb treatment.
Figure 7. Prolonged survival in MM bearing NSG mice received human effector cells and CS1-NKG2D biAb.
(A) Kaplan-Meier curve for NSG mice that received MM.1S MM cells alone (n=4), MM.1S MM cells+human PBMCs+PBS (n=5), MM.1S MM cells+PBMCs+control biAb (n=5), or MM.1S MM cells+PBMCs+CS1-NKG2D biAb (n=5). 5×106 MM.1S MM cells and 5×106 PBMCs were used. Dose of control biAb or CS1-NKG2D biAb was 200 μg/kg. Data from experiments using human PBMCs of two healthy donors. *p<0.05; **p<0.01; ***p<0.001 using Log-rank test.
Discussion
The use of bispecific antibody or bispecific T/NK cell engager (BiTE or BiKE) constructs is an attractive approach to treat cancer, as it not only simultaneously targets immune cells and tumor-associated antigens, it does so in a fashion that brings these two cell types into close proximity allowing for engagement. Here, we described the construction and functional assessment of a non-IgG–like, scFv-based biAb targeting CS1+ MM cells while activating a variety of innate and antigen specific NKG2D+ immune effector cells. We provided evidence to suggest that collective activation of NKG2D+ immune cells could enhance cytotoxicity promoted by CD3+CD8+ T cells or NK cells alone. This CS1-NKG2D biAb is distinct from other reported biAbs or BiTEs using an NKG2D mechanism (30,31) in that both redirecting subunits are scFvs, and the anti-NKG2D component not only redirects the molecule, but also activates its cognate immune effector cell once fully engaged with its tumor cell target. For human MM cell lines with high surface density expression of CS1, we were able to show that the addition of the CS1-NKG2D biAb, along with the MM target cell and activated human PBMCs, enhanced: (i) the immune synapse between MM target cells and NKG2D+ T and NK effector cells; (ii) the phosphorylation of AKT, the downstream signaling molecules of the NKG2D-DAP10 complex (9,27,32) within 15 minutes of engagement; (iii) the in vitro cytotoxicity and IFNγ production of NKG2D+ effector cells upon exposure to CS1+ MM cells; and (iv) the in vivo survival of mice bearing these cell populations, when compared to a negative control biAb.
To our knowledge, each of the currently available biAbs target MM function by redirecting T cells to the tumor cell via CD3. The ULBP2-BB4 bispecific protein construct described by Von Strandmann et al. fused an anti-CD138 scFv targeting this MM-associated antigen with ULBP2, a ligand of NKG2D (31). This bispecific protein activated NK cells, and when exposed to CD138+ MM cells, induced strong cytotoxicity and IFNγ production in vitro and in vivo. These data, along with our study here, suggest that activation of NKG2D provides a powerful means to eradicate MM cells. Importantly, NKG2D ligands such as MICA/B and ULBP1/2 have an affinity KD that is in the μM range (10). Here, we have shown that the anti-NKG2D component of the CS1-NKG2D biAb had a binding affinity in the nM range, thus, potentially leading to a more potent activation of NKG2D+ cytolytic effector cells (21). The possibility exists that CS1-NKG2D–activated effector cells could have bystander killing effects on the CS1− MM cells. However, in our experiment using MM patient samples as target cells, we found a reduction of the CS1+CD38bright/+CD138+ subset, but not of the CS1−CD38dim/+ subset. More experiments are needed to confirm the possible bystander effects from the CS1-NKG2D activated effector cells.
The ligands for NKG2D are often expressed by tumor cells, both as membrane bound and in secreted forms, with the latter proposed as a mechanism of tumor escape (33). Zhang T et al. proposed a biAb protein fusing an anti-CD3 scFv and the extracellular domain of NKG2D (30). This scFv-NKG2D fusion protein made use of the ligand binding ability of NKG2D to probe for NKG2D ligand-expressing tumor cells. However, there would be concern that this strategy, or others using similar NKG2D ligands as a binding domain (34), may be limited in efficacy because of the massive amounts of NKG2D ligands that can be shed from the tumor cell surface into both the microenvironment and the circulation, thereby, chronically desensitizing NKG2D+ effector cells (35,36). Thus, triggering NKG2D via the CS1-NKG2D biAb described herein may circumvent this potential limitation of effector cell desensitization. Previous reports suggest the NKG2D ligand MICA can shed from MM cell surfaces, which can desensitize NK cells (29). A low concentration of circulating soluble CS1 (no more than 10 ng/mL) is also detectable in serum of MM patients (28). These soluble targets may potentiate the effect of CS1-NKG2D biAb. However, our experiments show no significant difference in the effector-to-target synapse formation and effectors’ cytotoxicity against MM1.S cells in the presence of biAb with or without a soluble NKG2D-Fc chimera or a CS1 protein.
We demonstrated that both CD3+ T cells and CD56+ NK cells contributed to the in vitro cytotoxicity against CS1high MM cells. This corroborated our hypothesis that the activation of different NKG2D+ immune effector cells provided improved cytotoxicity when compared with either effector population alone. We also observed that IL2 is required for the functional activation of NKG2D induced by the CS1-NKG2D biAb. Our group and others have previously proposed using IL2 combined with targeted therapy for the treatment of non-Hodgkin lymphoma (37,38). IL2 combined with zoledronate was demonstrated to be a feasible maintenance therapy for multiple myeloma in a phase II clinical trial (39). We foresee that the combination of IL2 and the CS1-NKG2D biAb deserves a future clinical exploration.
In our preclinical model of MM using NSG mice, those engrafted with CS1high MM.1S MM cells showed significantly prolonged survival, whereas those engrafted with CS1int MM NCI-H929 cells showed a less significant effect. This corroborates with the in vitro results, where the EC50 for MM.1S MM cells was one log lower than that for NCI-H929 MM cells. These data suggest that the efficacy of our CS1-NKG2D biAb in vivo is likely to be dependent not only on the fraction of cytolytic immune effector cells, but also on surface density expression of CS1 by MM. However, approximately 95% of MM patients have high CS1 expression, and the expression remains stable after common MM drug treatment such as VDTPACE or bortezomib (40). Thus, although the preclinical data appear promising in providing improved activity over the naked anti-CS1 mAb in MM, clinical efficacy might prove to be restricted to the CS1high subset of MM patients and might be enhanced by improved immune modulation of NKDG2+ effector populations in vivo.
Supplementary Material
Acknowledgments
Grant support: WKC was an NCI T32 fellow (5T32CA009338), MAC and JY received support from the NIH (AI129582, CA185301, NS106170, CA210087, CA163205, and CA068458).
This work was supported by NIH grants CA89341, AI129582, CA095426, CA163205, CA016058, and CA068458 (to MAC and/or JY); 5T32CA009338 (to WKC); the Leukemia & Lymphoma Society Translational Research Award, the American Cancer Society Scholar Award (RSG-14-243-01-LIB), and a grant from the Gabrielle’s Angel Cancer Research Foundation (all to J.Y.).
Footnotes
Conflict-of-Interest Statement
A patent application regarding this research has been submitted.
References
- 1.Cancer Stat Facts: Myeloma. 2018 < https://seer.cancer.gov/statfacts/html/mulmy.html>.
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Raje N, Longo DL. Monoclonal Antibodies in Multiple Myeloma Come of Age. N Engl J Med. 2015;373(13):1264–6. doi: 10.1056/NEJMe1509419. [DOI] [PubMed] [Google Scholar]
- 4.Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039–47. doi: 10.1038/leu.2017.60. [DOI] [PubMed] [Google Scholar]
- 5.van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia. 2012;26(2):199–213. doi: 10.1038/leu.2011.214. [DOI] [PubMed] [Google Scholar]
- 6.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373(7):621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 7.Chang X, Zhu Y, Shi C, Stewart AK. Mechanism of immunomodulatory drugs’ action in the treatment of multiple myeloma. Acta Biochim Biophys Sin (Shanghai) 2014;46(3):240–53. doi: 10.1093/abbs/gmt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacy MQ, McCurdy AR. Pomalidomide. Blood. 2013;122(14):2305–9. doi: 10.1182/blood-2013-05-484782. [DOI] [PubMed] [Google Scholar]
- 9.López-Larrea C, Suárez-Alvarez B, López-Soto A, López-Vázquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. 2008;14(4):179–89. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 11.Verneris MR, Karimi M, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103(8):3065–72. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 12.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118(12):3301–10. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 13.Kuylenstierna C, Björkström NK, Andersson SK, Sahlström P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. 2011;41(7):1913–23. doi: 10.1002/eji.200940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105(1):251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 15.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67(18):8444–9. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 16.Friberg G, Reese D. Blinatumomab (Blincyto®); lessons learned from the bispecific t-cell engager (BiTE®) in acute lymphocytic leukemia (ALL) Ann Oncol. 2017 doi: 10.1093/annonc/mdx150. [DOI] [PubMed] [Google Scholar]
- 17.Veillette A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol. 2013;88(1):168–77. doi: 10.1016/j.critrevonc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Kyle RA. Progress in Myeloma - A Monoclonal Breakthrough. N Engl J Med. 2016;375(14):1390–2. doi: 10.1056/NEJMe1609835. [DOI] [PubMed] [Google Scholar]
- 19.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–27. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(15):3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong KY, Baskar S, Zhang H, Mackall CL, Rader C. Generation, affinity maturation, and characterization of a human anti-human NKG2D monoclonal antibody with dual antagonistic and agonistic activity. J Mol Biol. 2008;384(5):1143–56. doi: 10.1016/j.jmb.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caligiuri MA, Chan WK, Yu J, inventors. Ohio State Innovation Foundation, assignee. WO2016134371A2. Bivalent antibody directed against nkg2d and tumor associated antigens International patent. 2016 Aug 25;
- 23.Haryadi R, Ho S, Kok YJ, Pu HX, Zheng L, Pereira NA, et al. Optimization of heavy chain and light chain signal peptides for high level expression of therapeutic antibodies in CHO cells. PLoS One. 2015;10(2):e0116878. doi: 10.1371/journal.pone.0116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan WK, Suwannasaen D, Throm RE, Li Y, Eldridge PW, Houston J, et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia. 2014 doi: 10.1038/leu.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res. 2005;11(10):3879–88. doi: 10.1158/1078-0432.CCR-04-2290. [DOI] [PubMed] [Google Scholar]
- 27.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7(5):524–32. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 28.Tai Y-T, Rice AG, Leiba M, Li X-F, Burger P, Song W, et al. Low Levels of Circulating CS1, a Newly Identified Multiple Myeloma (MM) Antigen for a Novel Humanized HuLuc63 Monoclonal Antibody, Is Detected in MM Patient Sera and Correlates with Active Disease. Blood. 2007;110(11):1509. [Google Scholar]
- 29.Rebmann V, Schütt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR, et al. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin Immunol. 2007;123(1):114–20. doi: 10.1016/j.clim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71(6):2066–76. doi: 10.1158/0008-5472.CAN-10-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood. 2006;107(5):1955–62. doi: 10.1182/blood-2005-05-2177. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 33.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 34.Stamova S, Cartellieri M, Feldmann A, Bippes CC, Bartsch H, Wehner R, et al. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia. 2011;25(6):1053–6. doi: 10.1038/leu.2011.42. [DOI] [PubMed] [Google Scholar]
- 35.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78(2):120–9. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 36.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111(7):3571–8. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 37.Friedberg JW, Neuberg D, Gribben JG, Fisher DC, Canning C, Koval M, et al. Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin’s lymphoma. Br J Haematol. 2002;117(4):828–34. doi: 10.1046/j.1365-2141.2002.03535.x. [DOI] [PubMed] [Google Scholar]
- 38.Khan KD, Emmanouilides C, Benson DM, Hurst D, Garcia P, Michelson G, et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12(23):7046–53. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- 39.Buda G, Fazzi R, Carulli G, Galimberti S, Sammuri P, Orciuolo E, et al. Phase II Study of the Combination of Interleukin-2 with Zoledronic Acid As Maintenance Therapy Following Autologous Stem Cell Transplant in Patients with Multiple Myeloma. Blood. 2016;128(22):5697. [Google Scholar]
- 40.Szmania S, Balasa B, Malaviarachchi P, Zhan F, Huang Y, Draksharapu A, et al. CS1 Is Expressed on Myeloma Cells from Early Stage, Late Stage, and Drug-Treated Multiple Myeloma Patients, and Is Selectively Targeted by the HuLuc63 Antibody. Blood. 2006;108(11):660. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.