Abstract
Objective
Advances in the field of metabolomics and the concomitant development of bioinformatics tools constitute a promising avenue towards the development of precision medicine and personalized profiling for numerous disease states. Studies in animal models have strengthened this concept, but the application in human subjects is scarce.
Methods
Utilizing high-throughput metabolomics, we have analyzed the metabolome levels of human serum and skeletal muscle in the morning and evening in response to divergent nutritional challenges in order to identify unique signatures present in serum and muscle.
Results
We reveal dynamic daily variation of human metabolome unique to serum and muscle. The overall effect of nutritional challenges on the serum and muscle metabolome results in a profound rewiring of morning-evening metabolic profiles in human participants in response to the timing and type of dietary challenge.
Conclusion
We highlight time-of-day and meal-composition dependence of reprogramming of human metabolome by nutritional challenges.
Keywords: Circadian clock, Human, Serum metabolome, Skeletal muscle metabolome, High fat diet, High carbohydrate diet
Abbreviations: 18S rRNA, 18S ribosomal RNA; ANOVA, analysis of variance; BHBA, β-hydroxybutyric acid; BMI, body mass index; Bmal1, brain and muscle ARNT-Like 1; CHO, carbohydrate; Dbp, D-box binding PAR bZIP transcription factor; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; FDR, false discovery rate; GC–MS, gas chromatography–mass spectrometry; HFD, high fat diet; HCD, high-carbohydrate diet; LC-MS, liquid-chromatography mass spectrometry; Pparδ, peroxisome proliferator-activated receptor-delta; Ppargc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; HK2, hexokinase 2; PCR, polymerase chain reaction; PCA, principal component analysis; Pdk4, pyruvate dehydrogenase kinase 4; Pdp1, pyruvate dehyrogenase phosphatase catalytic subunit 1; RMR, resting metabolic rate; SCN, suprachiasmatic nucleus; TEI, total energy intake; UPLC-MS/MS, ultra-high performance liquid chromatography-tandem mass spectrometry
Highlights
-
•
Human metabolome identifies the daily variation of metabolite levels.
-
•
Divergent nutritional challenges reprogram the daily variation of human serum/muscle metabolome.
-
•
Metabolomics delineates parallels between human serum and skeletal muscle.
1. Introduction
During the past decades, clinical chemistry has contemplated only a limited number of small metabolites such as glucose, cholesterol, creatinine and urea to evaluate an individual's health profile [1]. It is becoming clear that fingerprinting by metabolomics will play a greater role in tracking and diagnosing an individual's health status as it provides a direct functional read-out of the physiological status of an organism and a comprehensive analysis of low-molecular weight compounds in biological samples such as cells, body fluids and tissues [2], [3]. Recent technological advances, such as metabolomics measures through liquid-chromatography mass spectrometry (LC-MS) along with the subsequent development of sophisticated bioinformatics tools, have been valuable in unraveling the complexity and specificity of metabolic changes in a number of mouse and human tissues, as well blood and saliva [4], [5]. These approaches are especially useful in pathology, as metabolomics is well suited to identify and deliver biomarkers for a variety of clinical conditions [1]. The identification of specific metabolomics ‘signatures’ in response to challenges such as a high fat diet (HFD), exercise, drugs of abuse or distinctive of diseases is of utmost importance to guide the discovery of diagnostic and mechanistic biochemical biomarkers that monitor perturbations to individual metabolic homeostasis [6], [7], [8]. In this context, circadian rhythms have emerged as essential in the control of daily metabolic cycles [9]. Revealing how the time-of-the-day affects variations in an individual's metabolic state provides a robust tool to explore metabolomics signatures that are specific for a time of day or night, as well as the effect of the timing of meals, exercise and other ‘zeitgebers’.
Circadian rhythms govern a large variety of behavioral, physiological and metabolic processes, with a large fraction of mammalian metabolism undergoing circadian oscillations [10]. Such a notion is critical, as it raises awareness for increased consideration regarding the time of monitoring clinically relevant biomarkers in patient and other populations. Indeed, studies in humans show significant oscillations in the levels of key biomarkers, possibly leading to misleading readouts that may result in incorrect therapeutic outcomes [3], [4], [5], [11]. The human body comprises a series of circadian feedforward and feedback loops with cross-talks between tissues and organs. However, sampling of muscle, adipose tissue, liver and other tissues is highly invasive and prohibitive in the majority of cases. Accordingly, a comparative analysis of the circadian metabolome in the serum versus peripheral tissues (i.e., skeletal muscle) is critical to decipher circulating metabolites that constitute specific signatures associated with a given physiological state. In this context, human interventions remain scarce [12], and to date, there are no studies that have simultaneously measured metabolomics both serum and a peripheral tissue in humans.
Analyses of the serum metabolome in humans and mice have been performed under a variety of conditions. Davies et al. [13] studied the effect of sleep deprivation on the human plasma metabolome, revealing the implication of sleep/wake cycle with human metabolism. A more recent study describes the distinct link of gut microbiome to serum metabolome between lean and obese individuals [7]. However, it has been suggested that a ‘disrupted’ circadian rhythm as a result of dietary intake patterns is linked with metabolic diseases associated with obesity. In turn, obesity is fueled by inactivity and sedentary behavior, and has been highly related to detrimental glucose profiles (i.e. development of type 2 diabetes). Research in rodent models has shown the detrimental effect of a high-fat, low carbohydrate diet on glucose regulation and weight management [14]. Indeed, the majority of the studies demonstrating the intimate interplay between the circadian clock and cellular metabolism have used animal models [15], [16], [17], [18], [19], likely due to the ease of sampling of muscle, adipose tissue, liver and other tissues. To date, there are few studies in humans to assess the effects of a HFD on metabolic measures in the skeletal muscle.
As serum is the biological sample most easily obtained in human participants, as well as a critical link between peripheral tissues and the brain, we explored how the consumption of a HFD compared to a high-carbohydrate diet (HCD) affects the levels of both serum and skeletal muscle metabolites of humans in a time-of-day specific manner. A comparative analysis of the circadian metabolome in the serum versus peripheral tissues (i.e., skeletal muscle) is critical to decipher the circulating metabolites that constitute specific signatures associated with any given physiological state. Here we present the first comparative analysis of the metabolome at different time of day in the muscle and serum of human participants under nutritional challenges.
2. Materials and methods
2.1. Experimental model and subject details
As previously reported [20], overweight/obese (body mass index (BMI) 27–32.5 kg/m2) men (aged 30–45 yr) following a sedentary lifestyle (<150 min/wk exercise and >3 h/d sitting) were recruited to participate. The study was approved by the Human Research Ethics Committee of the Australian Catholic University (2016–77H) and registered with the Australian New Zealand Clinical Trials Registry (ACTRN12616000637448). All participants provided written informed consent and completed two experimental conditions in a randomized, crossover design with a 7 day wash out period between conditions.
An overview of the study design is outlined in Fig. S1A,B. Briefly, the study employed two experimental dietary conditions for 5-d, each following a 7-d habitual period where participants were monitored for dietary intake and physical activity patterns. The dietary conditions were A) a high fat, low carbohydrate diet (HFD) consisting of 65% total energy intake (TEI) from fat, 15% TEI from carbohydrate (CHO) and 20% TEI from protein and B) an isoenergetic high carbohydrate, low fat diet (HCD) consisting of 65% TEI from CHO, 15% TEI from fat and 20% TEI from protein. Skeletal muscle biopsies and blood samples were obtained in a fasted state in the morning (0730 h) and following dinner in the evening (1930 h) on Day 0 (end of habitual baseline period) and Day 5 of each experimental dietary condition, to compare the diet intervention to baseline and assess individual variability.
2.2. Method details
2.2.1. Study protocol
At the beginning of the habitual period, participants attended the laboratory (Day −7) for baseline measures prior to a 7-d ‘habitual’ recording period. On Day 0, participants arrived at 0630 h after an overnight fast and a single forearm venipuncture was performed to collect of blood (5 mL Serum clot activator; 6 mL EDTA; Vacuette). The serum samples at room temperature for 30 min before centrifuging at 3000 g, for 10 min at 4 °C, while the plasma (EDTA) sample was immediately spun at 3000 g, for 10 min at 4 °C. Both samples were then aliquoted and stored at −80 °C for subsequent analysis. Following this at ∼0740 h, under local anesthesia (2–3 mL of 1% Xylocaine (lignocaine)), a ∼150 mg muscle sample from the vastus lateralis was obtained under sterile conditions, using a 6 mm Bergstrom needle modified with suction, and immediately frozen using liquid nitrogen before being stored at −80 °C for later analysis. Participants were provided with breakfast to be consumed within ±30 min of 0800 h, before leaving the laboratory with their lunch (also provided) to be consumed within ±30 min of 1300 h. Participants returned to the laboratory at ∼1830 h and then consumed an evening meal. Participants then underwent a forearm venipuncture and muscle biopsy sampling, as per the morning visit. On each day of measurement, morning and evening biopsies were obtained from the same leg. All pre-packaged food and fluid for the experimental condition was then provided to participants for the first experimental diet (Day 1–4).
On Day 5, Participants arrived in the lab at ∼0630 h after a 10 h overnight fast and following four days of the dietary experimental condition. All procedures were completed as per Day 0, including a forearm venipuncture, muscle biopsy sample, breakfast consumption and provision of lunch meal. Participants returned at ∼1830 h and, following the evening meal, a 11 mL blood sample and a muscle biopsy sample were obtained (∼1930 h). Following a 7-day washout, the procedure from Day −7 to Day 5 was repeated with the second dietary condition. Activity patterns were assessed using several activity monitors (ActivPAL3TM tri-axial physical activity monitor, PAL-technologies Ltd, Glasgow, Scotland; ActiGraph GTX3+ accelerometer, Pensacola, FL, USA [waking hours only]; SenseWear armband (SWA) Bodymedia, Pittsburgh, PA, USA) worn continuously throughout the baseline and experimental periods of each condition (i.e. 12 d).
2.2.2. Dietary control and adherence
Full detail of the dietary intervention can be found elsewhere [20]. Briefly, habitual intake was monitored for 7-d prior to each dietary intervention. On Day 0, participants were provided with meals matched with each participant's recorded ‘habitual’ intake while total energy intake provided was according to their baseline resting metabolic rate (RMR) × 1.4 activity factor. All food during each of the 5-d dietary experimental conditions was provided to participants and a daily food checklist was completed to maximize compliance. Participants were instructed to consume meals, each of 33.3% TEI, at standardized times (within ±30 min of 0800, 1300 and 1830 h) throughout both experimental conditions and to abstain from alcohol consumption, whilst habitual caffeine consumers were instructed to consume and record caffeine intake (i.e. black coffee/tea during experimental days) as usual on all days.
2.3. Biochemical analysis
Triglyceride and cholesterol concentrations were analyzed at time of collection from whole blood using a Cobas b 101 instrument (Roche Diagnostics Ltd, Basel, Switzerland). Blood glucose concentrations were determined using an YSI 2900 analyzer (YSI Life Sciences, Yellow Springs, OH, USA). Plasma insulin, connecting peptide (C-peptide), tGLP-1, leptin and TNF-alpha concentrations were determined using a Luminex Analyzer (MAGPIX®; Human Metabolic Hormone Magnetic Bead Panel, EMD Millipore, MA, USA) following manufacturer's instructions and sample preparation. Serum cortisol was determined using an enzyme-linked immunosorbent assay (ELISA; KA0918, Abnova Corporation, Tapei, Taiwan).
2.4. Real-time quantitative polymerase chain reaction (Real-time qPCR) analysis
Skeletal muscle (∼25–30 mg) tissue RNA extraction, reverse transcription and real-time PCR was performed for each subject samples from morning and evening, as previously described [21]. TaqMan-FAM labeled primer/probes (Applied Biosystems, Carlsbad, CA, USA) for Brain and Muscle ARNT-Like 1 (Bmal1; Cat No. Hs00154147), D-Box Binding PAR BZIP Transcription Factor (Dbp; Cat No. Hs00609747), CD36 (Cat No. Hs00354519), Peroxisome Proliferator Activated Receptor-delta (Pparδ; Cat No. Hs04187066), Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha (Ppargc1α; Cat No. Hs00173304), Hexokinase 2 (HK2; Cat No. Hs00606086), Pyruvate Dehydrogenase Kinase 4 (Pdk4; Cat No. Hs01037712), and Pyruvate Dehyrogenase Phosphatase Catalytic Subunit 1 (Pdp1; Cat No. Hs01081518) were used in a final reaction volume of 20 μL. 18S ribosomal RNA (18S rRNA, Hs99999901_s1) was used as the housekeeping gene for normalization of gene expression. The relative amounts of mRNAs were calculated using the relative quantification (ΔΔCT) method.
2.5. Mass spectrometry (MS) for metabolomics analysis
Metabolomics analysis was performed by Metabolon, Inc. (Durham, NC, USA), as previously described [22]. Both muscle and serum were methanol extracted and analyzed by ultra-high-performance liquid chromatography-tandem MS (UPLC-MS/MS; positive mode), UPLC-MS/MS (negative mode) and gas chromatography–MS (GC–MS). The UPLC-MS/MS platform utilized a Waters Acquity UPLC with Waters UPLC BEH C18-2.1 × 100 mm, 1.7 μm columns and a ThermoFisher LTQ MS, which included an electrospray ionization source and a linear ion-trap mass analyzer. Samples destined for analysis by GC–MS were dried under vacuum desiccation for a minimum of 18 h prior to being derivatized using bis(trimethylsilyl) trifluoroacetamide. Derivatized samples were separated on a 5% phenyldimethyl silicone column with helium as carrier gas and a temperature ramp from 60 °C to 340 °C within a 17-min period. All samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole MS operated at unit mass resolving power with electron impact ionization and a 50–750 atomic mass unit scan range. Metabolites were identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra and were curated by visual inspection for quality control using software developed at Metabolon, Inc [23].
2.6. Statistical analysis
2.6.1. Gene expression, and blood/plasma biochemical data
Statistical analyses were performed using Prism 5.0. Data from the two conditions were analyzed using two-way ANOVA, using time and diet condition as the grouping variable. Subsequent post-hoc comparisons between intervention groups were conducted when significant based on the Bonferroni test. Significance was set at P < 0.05 and data are presented as mean ± SEM.
2.6.2. Metabolome data
Untargeted metabolomic data do not follow the normal distribution. Therefore, we first used log transformation before calculating statistical significance. We applied False Discovery Rate (FDR) adjustment to modify the p-value across all metabolites. A metabolite was considered significantly gradient between morning and evening or changed by nutritional challenges based on an adjusted p-value cutoff of 0.05. Metabolome data analyses were carried out using R (www.R-project.org) and Bioconductor (www.bioconductor.org) [24].
3. Results and discussion
3.1. Participants and habitual diet analysis
Eight males were randomized and completed both conditions (Table S1). Habitual dietary analysis showed average intake of 41 ± 4% carbohydrate (including fiber), 35 ± 7% fat, 19 ± 2% protein and 5 ± 8% alcohol across both 7-d periods. Blood profiles before and after nutritional challenges are shown in Fig. S1C. The expression of core clock and metabolic genes exhibited robust morning-evening variations (Fig. S1D,E), providing the first validation of human serum and muscle metabolomics under nutritional challenges at different times of the day. Although the expression of some metabolic genes, including CD36, Pparδ, Hk2 and Pdp1, lost significant daily variation after HFD, we observed marginal effects of diet on daily muscle metabolic gene expression from pre- to post-nutritional challenge (Fig. S1D,E). These observations indicate that 5-d nutritional challenges might not induce robust metabolic response and adaptation at the levels of transcripts.
3.2. Nutritional challenges impose daily variations in human serum metabolome
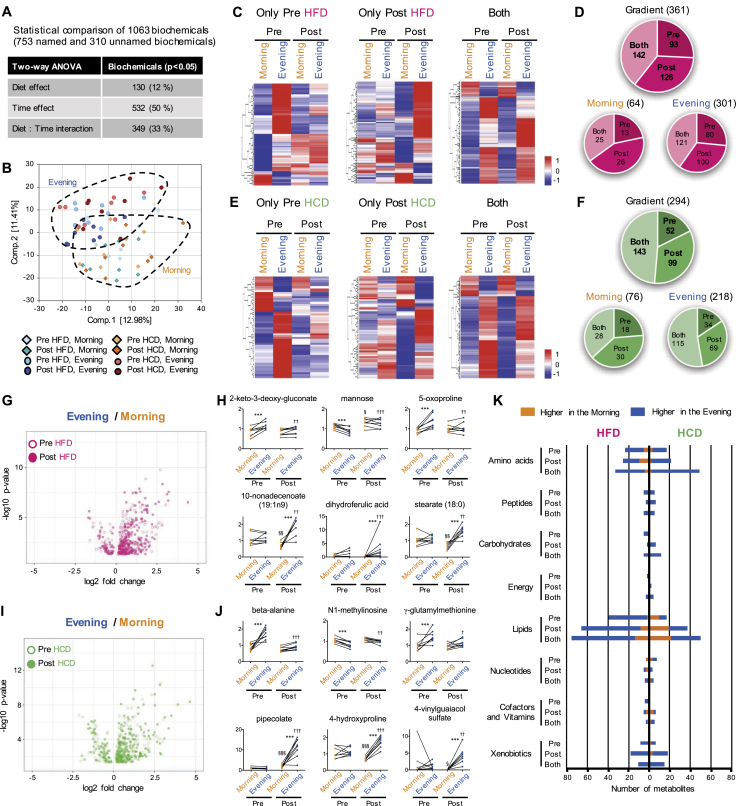
Mass spectrometry (MS) of serum samples detected over 1,000 biochemicals, with ∼70% of compounds of known identity and ∼30% of compounds of unknown structural identity (Figure 1A). More than half of serum metabolites exhibited time-of-day differences (Figure 1A). Principal component analysis (PCA) identified the separation of samples based on the time of collection between morning and evening in the serum (Figure 1B).
Figure 1.
Reprogramming of the daily variation of serum metabolome in human participants by nutritional challenges. (A) Number of serum metabolites affected by diet or time. (B) Separation of samples based on the timing of the collection of serum by PCA. (C) Heatmaps display significantly gradient serum metabolites exclusively in pre- (left panel) and post-HFD (middle panel), and in both pre- and post-HFD (right panel). (D) Pie chart shows the number and ratio of significantly gradient metabolites in the serum under HFD condition. Numbers within the pie chart indicate number of gradient metabolites. Gradient, metabolites significantly different between morning and evening, Morning, metabolites being significantly higher in the morning, and Evening, metabolites being significantly higher in the evening. (E) Heatmaps display gradient serum metabolites exclusively in pre- (left panel) and post-HCD (middle panel), and in both pre- and post-HCD (right panel). (F) Pie chart exhibits the number of gradient metabolites in the serum under HCD treatment. (G) Volcano plot exhibits the significance of gradient serum metabolites under pre- (open circle) and post- (closed circle) HFD condition. The x-axis and y-axis represent the fold change (evening/morning) and significance of gradient serum metabolites, respectively. (H) Top 3 gradient serum metabolites unique to pre-HFD (top) and post-HFD (bottom). *** indicates P < 0.001 between morning and evening, § and §§ indicate P < 0.05 and P < 0.01 between pre and post in the morning, †† and ††† indicate P < 0.01 and P < 0.001 between pre and post in the evening. (I) Volcano plot exhibits the significance of gradient serum metabolites under pre- (open circle) and post- (closed circle) HCD condition. (J) Top 3 gradient serum metabolites unique to pre-HCD (top) and post-HCD (bottom). *** indicates P < 0.001 between morning and evening, § and §§§ indicate P < 0.05 and P < 0.001 between pre and post in the morning, †, ††, ††† indicate P < 0.05, P < 0.01 and P < 0.001 between pre and post in the evening. (K) Histogram shows the biological classification of gradient metabolites in the serum under HFD (left) and HCD (right) conditions. Orange and blue bars indicate the number of metabolites being higher in the morning and evening, respectively, within each classification. Two-way ANOVA compares the mean differences of metabolites levels between morning and evening. Adjusted p-value cutoff of 0.05 was used. N = 8 biological replicates in each time of day were subjected to the metabolomics analysis.
To establish how a nutritional challenge influenced the daily variation of human serum metabolome, we used a two-way ANOVA to compare the different metabolite levels between morning and evening. The metabolites exhibiting the daily variation were termed “gradient” metabolites. The dynamic alteration of gradient serum metabolome after 5-d HFD and HCD treatments are displayed as heatmaps (Figure 1C,E, respectively). Following the HFD, ∼13% of total serum metabolites lost their daily variation (i.e. did not change between morning and evening), while ∼17% of total serum metabolites gained a new daily variation (Figure 1D). Most of the gradient metabolites were those detected to be higher in the evening following dinner, suggesting the HFD intake directly lead to the reprograming of serum metabolome (Figure 1D). Following the HCD, ∼7% of total serum gradient metabolites lost their oscillatory profile and induced 14% de novo gradient metabolites (Figure 1F). Similar to the effect of HFD, most of gradient serum metabolites were those higher in the evening after the HCD (Figure 1F).
The volcano plot (Figure 1G) supports the reprograming after the HFD, revealing that a larger number of metabolites were higher in the evening than the morning, with both greater significance and fold change (Table S2). The most significant metabolites that were exclusively gradient after HFD intervention were enriched by the metabolites related to lipid [10-nonadecenoate (19:1n9), P = 0.00000003; stearate (18:0), P = 0.00000004], in addition to xenobiotics derived from food components, i.e. organic or natural compounds contained in foods and as food additives (Figure 1H and Table S2). Given that food intake functions as an environmental ‘zeitgeber’ for the circadian clock and circadian control of metabolism [25], it is predictable that the levels of many serum metabolites are perturbed in response to a nutritional challenge. These results suggest that the robust alteration of the daily variation of human serum metabolome by HFD could lead to the remodeling of clock-controlled metabolic pathway.
Following the HCD, most of the serum gradient metabolites tended to heighten in the evening and lower in the morning (Figure 1I and Table S3). β-alanine (P = 0.0000006), N1-methylinosine (P = 0.00002) and γ-glutamylmethionine (P = 0.0003) were unique to gradient serum metabolites before HCD treatment, while xenobiotics derived from food components were predominant as the metabolites exclusively gradient after HCD (Figure 1J and Table S3). This was uniquely observed after HCD. Plasma metabolites such as glucose and insulin were robustly altered by the HCD, and not the HFD (Fig. S1C). These divergent responses highlight the sensitivity of metabolomics for detecting perturbations in body fluids that would otherwise be overlooked by traditional clinical diagnostic measures, underscoring the sensitivity and reliability of the analysis.
The biological classification of serum gradient metabolites showed an enrichment of metabolites being higher in the evening after consuming the dinner meal, within most classifications both before and after the HFD intervention (Figure 1K). It was expected that gradient metabolites related to lipid were likely to be affected by the HFD, especially in the fed state (i.e. evening). However, the HCD also heightened the number of gradient metabolites related to lipid metabolism, albeit to a lesser extent. Therefore, time-of-day-specific significance of lipid type under nutritional challenges was identified by scoring the number of metabolites related to lipid within each lipid sub-classification (Fig. S2). Although serum metabolites being higher in the evening after HFD were enriched with ketone bodies and many types of fatty acid, metabolites related to carnitine were selectively higher in the morning when fasted after HFD (Fig. S2A). Similar to the observation from the effect of HFD, ketone bodies and fatty acids gained daily gradient in the serum after HCD, but with relatively low enrichment rate (Fig. S2B). These results indicate that both short-term HFD and HCD challenges influence daily variations of serum metabolome but providing distinct metabolic signatures.
3.3. Diverging effects of nutritional challenges on human metabolome in the morning versus evening
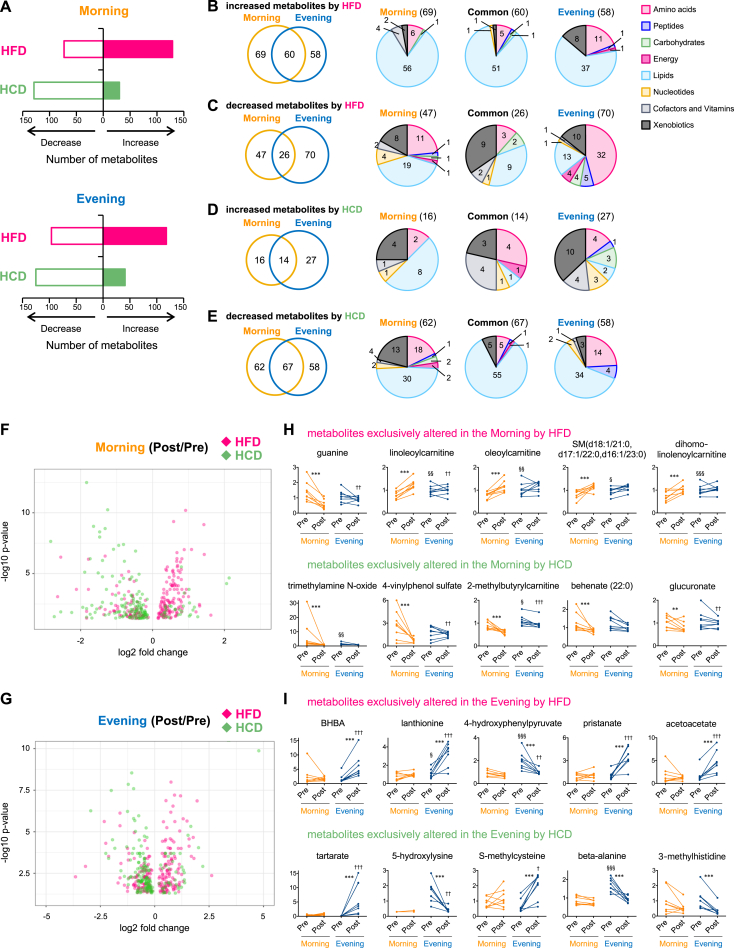
In addition to the time-of-day analysis, we compared the differences of serum metabolites levels before and after the two divergent dietary interventions. The levels of ∼18% and ∼16% of total serum metabolites were significantly changed at morning and evening measures, respectively, after 5-d of the HFD, whereas ∼18% and ∼17% of total serum metabolites were downregulated by the HCD in the morning and evening (Figure 2A). Although the proportion of increased or decreased serum metabolites were similar between morning and evening, the type of metabolites affected by nutritional challenges showed a specific time-of-day difference. The comparison of increased metabolites by HFD between morning and evening revealed that 37% of metabolites were exclusively increased in the morning when fasted and 31% of metabolites were only increased in the evening when fed, while the number of increased metabolites common to both morning and evening was only 32% (Figure 2B). However, the overlap of decreased metabolites by HFD between morning and evening was <20% (Figure 2C). Similarly, only 25% of increased metabolites overlapped between morning and evening after the HCD (Figure 2D). Following the HCD, 33% of metabolites were found to be exclusively decreased in the morning, 31% of metabolites were decreased only in the evening and only 36% of metabolites were commonly decreased at both times-of-day (Figure 2E).
Figure 2.
Time-of-day-specific impact of nutritional challenges on human serum metabolome. (A) Histograms exhibit the number of significantly increased or decreased serum metabolites by HFD (pink bar) and HCD (green bar) in the morning (top) and evening (bottom). (B–E) Venn diagrams show the overlap of the number of increased serum metabolites by HFD (B), decreased serum metabolites by HFD (C), increased serum metabolites by HCD (D), and decreased serum metabolites by HCD (E) between morning and evening. Pie charts exhibit the biological classifications of serum metabolites exclusively altered in the morning (left), evening (right) and common to morning and evening (middle). Numbers within Venn diagrams and pie charts indicate number of changed metabolites. (F) Volcano plots exhibit the significance of altered serum metabolite by HFD (pink) and HCD (green) in the morning. The x-axis and y-axis represent the fold change (post/pre) and significance of changed metabolites, respectively. (G) Volcano plots exhibit the significance of altered serum metabolite by HFD (pink) and HCD (green) in the evening. (H) Top 5 serum metabolites exclusively changed by HFD (top) and HCD (bottom) in the morning. (I) Top 5 serum metabolites exclusively changed by HFD (top) and HCD (bottom) in the evening. ** and *** indicates P < 0.01 and P < 0.001 between pre and post, §, §§ and §§§ indicate P < 0.05, P < 0.01 and P < 0.001 between morning and evening before nutritional challenges, †, ††, ††† indicate P < 0.05, P < 0.01 and P < 0.001 between morning and evening after nutritional challenges. Two-way ANOVA compares the mean differences of metabolites levels between pre- and post-nutritional challenges. Adjusted p-value cutoff of 0.05 was used. N = 8 biological replicates in each time of day were subjected to the metabolomics analysis.
To further probe the impact of the time-of-day and diet, the biological classification of serum metabolites changed by nutritional challenges in the morning versus evening is shown in Figure 2B–E. The HFD robustly increased the number of metabolites related to lipid metabolism, independent of the time-of-day (Figure 2B). In contrast, about half of the serum metabolites exclusively decreased in the evening after HFD were metabolites related to amino acid metabolism (Figure 2C). In support, reduced circulating amino acids and rates of skeletal muscle protein synthesis in response to increased fat availability has been demonstrated using intra-lipid infusions in humans [26] and from a HFD on selected muscle protein abundance [27]. There were few metabolites increased after the HCD at either morning or evening sampling times, but the HCD decreased the number of metabolites related to lipid, without time-of-day preference (Figure 2D,E). Since metabolites related to lipid seemed to be highly influenced by both nutritional challenges, the sub-classification of lipid was further explored. Evening-specific increase of ketone bodies and variety of fatty acids was observed after HFD (Fig. S3A), while HFD decreased metabolites related to carnitine, inositol and mevalonate selectively in the evening (Fig. S3B). From volcano plots, there was a mild bias between increased and decreased levels of serum metabolites by HFD in the morning but not the evening, whereas the HCD decreased the levels of metabolites both in the morning and evening (Figure 2F,G). Overall, and as would be expected, most of the serum metabolites whose levels changed under HFD both in the morning and evening were metabolites related to lipid metabolism (Tables S4,S5).
To further determine the time-of-day response of serum metabolites against nutritional challenges, we extracted several serum metabolites exclusively altered either in the morning or evening after the two different diet interventions (Figure 2H,I). Some acylcarnitines [linoleoylcarnitine (C18:2), P = 0.000001; oleoylcarnitine (C18:1), P = 0.00004; dihomo-linolenoylcarnitine (C20:2), P = 0.0001)] in the serum were selectively upregulated by the HFD in the morning (Figure 2H), suggesting that the HFD lead to a dysfunction of fatty acid β-oxidation and carnitine metabolism through the hyperaccumulation of long-chain fatty acids. The HCD intake decreased the levels of multiple types of metabolites such as lipid [trimethylamine N-oxide, P = 0.000008; behenate (22:0), P = 0.0006], xenobiotics (4-vinylphenol sulfate, P = 0.000009), amino acid (2-methylbutyrylcarnitine, P = 0.00002) and carbohydrate (glucuronate, P = 0.001) in the morning (Figure 2H). Moreover, ketone bodies, β-hydroxybutyric acid (BHBA, P = 0.0000001) and acetoacetate (P = 0.00003) were dramatically elevated after HFD intake in the evening (Figure 2I), demonstrating the daily variation of the activity of ketogenesis in humans after only 5-d exposure to the HFD. Previous studies have reported that both ketogenic diets (<50 g of carbohydrate per day), along with high fat and low-carbohydrate diets leads to the circadian oscillation of serum β-hydroxybutyric acid levels in the mouse [16], [28]. The observation of specific time-of-day effect of HFD on the levels of human serum ketone bodies in this intervention is consistent with the observation from animal studies [16]. In the evening, after the HCD dinner meal, there was also a decrease of the levels of several metabolites related primarily to lipid metabolism and to a lesser extent amino acid (Figure 2I, Fig. S3C,D, and Table S5). The consumption of carbohydrate-rich meals and the associated rise in plasma insulin concentrations suppresses lipolysis for several hours post-ingestion [29], and explains why lipid metabolites were downregulated following the HCD.
3.4. Distinct effect of HFD and HCD on the daily gradient metabolome of human skeletal muscle
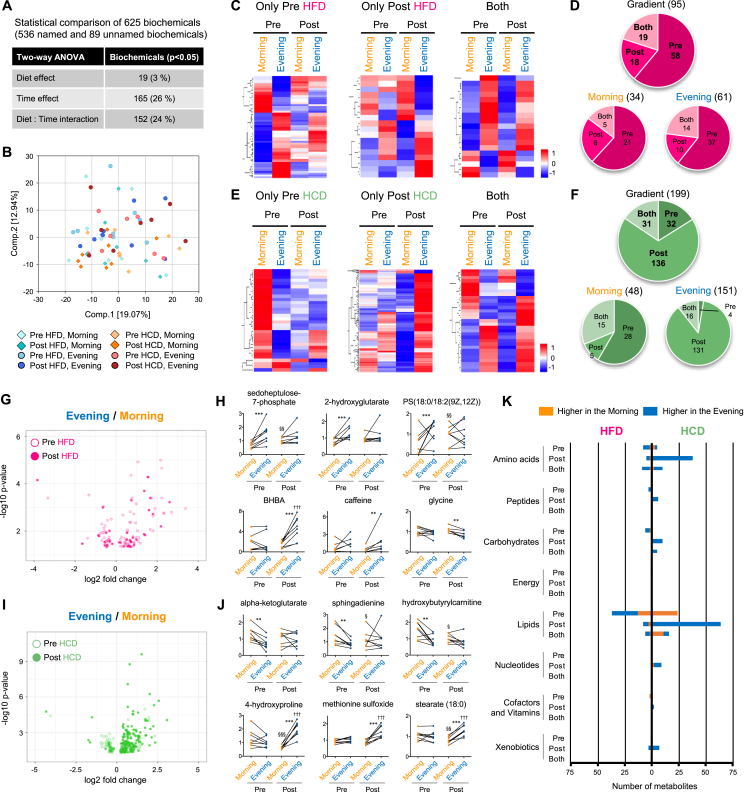
A total of 625 biochemicals (∼86% named and ∼14% unnamed biochemicals) were detected in skeletal muscle samples (Figure 3A), with 26% of muscle metabolites affected by the time-of-day (Figure 3A) compared with ∼50% of serum metabolites exhibiting daily variation (Figure 1A). The evening muscle and serum samples were collected 1 h after the dinner meal and, not surprisingly, the serum metabolome was more responsive to circulating (blood-borne) substrate availability than the muscle metabolome. The prevalence of an interaction (time × trial) effect was similar between serum and muscle (33% and 24%, respectively; Figure 1, Figure 3A), demonstrating that dietary intake functions as a strong zeitgeber for peripheral clocks in humans. As there was no significant separation of the muscle metabolome between morning and evening by PCA (Figure 3B), these observations underpin the robust daily variation of metabolome in the serum rather than skeletal muscle.
Figure 3.
Nutritional challenges reprogram daily variation of human skeletal muscle metabolome. (A) Number of muscle metabolites affected by diet or time. (B) Separation of samples based on the diet and collection time by PCA. (C) Heatmaps display significantly gradient muscle metabolites exclusively in pre (left panel) and post HFD (middle panel), and in both pre- and post-HFD (right panel). (D) Pie chart shows the number and ratio of significantly gradient metabolites in the muscle under HFD condition. Numbers within the pie chart indicate number of gradient metabolites. Gradient, metabolites significantly different between morning and evening, Morning, metabolites being significantly higher in the morning, and Evening, metabolites being significantly higher in the evening. (E) Heatmaps display gradient muscle metabolites exclusively in pre- (left panel) and post-HCD (middle panel), and in both pre and post HCD (right panel). (F) Pie chart exhibits the number of gradient metabolites in the muscle under HCD treatment. (G) Volcano plot exhibits the significance of gradient muscle metabolites under pre- (open circle) and post- (closed circle) HFD condition. The x-axis and y-axis represent the fold change (evening/morning) and significance of gradient muscle metabolites, respectively. (H) Top 3 gradient muscle metabolites unique to pre-HFD (top) and post-HFD (bottom). ** and *** indicates P < 0.01 and P < 0.001 between morning and evening, §§ indicate P < 0.01 between pre and post in the morning, ††† indicate P < 0.001 between pre and post in the evening. (I) Volcano plot exhibits the significance of gradient muscle metabolites under pre- (open circle) and post- (closed circle) HCD condition. (J) Top 3 gradient muscle metabolites unique to pre-HCD (top) and post-HCD (bottom). ** and *** indicates P < 0.01 and P < 0.001 between morning and evening, §, §§ and §§§ indicate P < 0.05, P < 0.01 and P < 0.001 between pre and post in the morning, ††† indicate P < 0.001 between pre and post in the evening. (K) Histogram shows the biological classification of gradient metabolites in the muscle under HFD (left) and HCD (right) conditions. Orange and blue bars indicate the number of metabolites being higher in the morning and evening, respectively, within each classification. Two-way ANOVA compares the mean differences of metabolites levels between morning and evening. Adjusted p-value cutoff of 0.05 was used. N = 8 biological replicates in each time of day were subjected to the metabolomics analysis.
Heatmaps display gradient muscle metabolites found exclusively pre-HFD, post-HFD and common to both measurement times (Figure 3C). More than 60% of muscle metabolites lost daily variation after the HFD treatment, while only 19% of metabolites gained a new daily gradient (Figure 3D). Volcano plots also highlight suppressive impact of the HFD on the daily variation of muscle metabolome (Figure 3G). Of note, and in accordance with the serum metabolomics, most of the muscle metabolites that lost the time-of-day difference after the HFD treatment were related to lipid metabolism (Figure 3H and Table S6). Few muscle metabolites such as BHBA (P = 0.00005), caffeine (P = 0.006) and glycine (P = 0.01), gained the time-of-day differences after 5-days of the HFD (Figure 3H and Table S6).
In addition to the effect of HFD, heatmaps show gradient muscle metabolites pre- HCD, post- HCD, and common to both measurement days (Figure 3E). However, 136 muscle metabolites gained time-of-day difference after the HCD (Figure 3F), with almost all of them being higher in the evening (Figure 3F), which was greater than the changes to the serum metabolites after HCD. Volcano plots exemplify the large impact of the HCD on muscle metabolome just after a meal (Figure 3I). Although some of the metabolites lost daily variation after the HCD, similar to the serum results, a number of metabolites prominently related to amino acid (4-hydroxyproline, P = 0.0000000002; methionine sulfoxide, P = 0.000000002) and lipid metabolism [stearate (C18:0), P = 0.00000008] gained daily variation after the HCD (Figure 3J and Table S7).
The analysis of the biological classification of gradient muscle metabolites showed that metabolites related to amino acid, peptide, carbohydrate and lipid metabolism lost daily variation after the HFD (Figure 3K). In contrast, de novo gradient muscle metabolites after HCD were seen within almost all biological classifications, but the most robust were observed for amino acid and lipid (Figure 3K). In contrast to the effect of HFD on the serum metabolome (Fig. S2A), muscle metabolites related to lipid such as phospholipids and fatty acids lost daily variation after HFD (Fig. S2C). More importantly, metabolites in the muscle within many of lipid sub-classifications became higher in the evening after HCD (Fig. S2D). Thus, both HFD and HCD challenges led to robust remodeling of the daily variation of the muscle metabolome, such that the HFD dampened the daily gradient of the muscle metabolome while the short-term HCD amplified the daily gradient of the muscle metabolome.
3.5. Time-of-day-dependent impact of nutritional challenges on human muscle metabolome
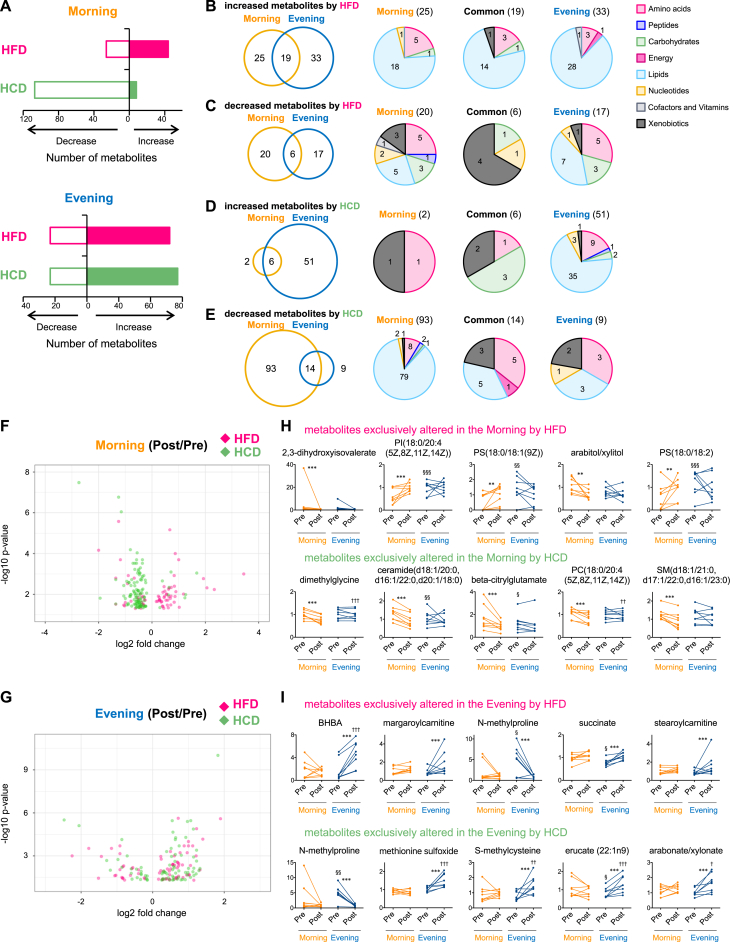
The HCD decreased the number of muscle metabolites specifically in the morning, whereas the HFD intervention had minimal impact on the muscle metabolome at this time (Figure 4A). The number of muscle metabolites were greatly increased in the evening by consumption of the HFD paralleled the HCD (Figure 4A). Comparison of the type of metabolites altered by nutritional challenges revealed the distinct impact of nutritional challenges on the muscle metabolome between morning (fasted) and evening (fed) (Figure 4B–E). Only 25% of metabolites increased by HFD were common to both morning and evening (Figure 4B). Independent of the time-of-day, metabolites related to lipid were predominantly increased by the HFD (Figure 4B), showing distinct types of lipid sub-classifications between morning and evening (Fig. S3E). The type of metabolites decreased by HFD were completely distinguished between morning and evening, and most of the common metabolites reduced by the HFD were the organic and natural compounds derived from food or plant (Figure 4C and Fig. S3B). The distinct impact of the HCD on muscle metabolome between morning and evening was confirmed (Figure 4D,E). The HCD increased the levels of metabolites related to lipid in the evening and decreased the lipid metabolites measured in the morning (Figure 4D,E, and Figs. S3G,H, respectively). Volcano plots illustrate the time-of-day difference of the effect of nutritional challenges (Figure 4F,G), where metabolites related to lipids, including phospholipidinositol [PI (18:0/20:4 (5Z, 8Z, 11Z, 14Z)), P = 0.0001] and phospholipidserine [PS (18:0/18:1 (9Z)), P = 0.001; PS (18:0/18:2), P = 0.002], tended to be increased by HFD in the morning, while the HCD decreased lipids such as ceramide (d18:1/20:0, d16:1/22:0, d20:1/18:0) (P = 0.0002), phospholipidcoline [PC (18:0/20:4 (5Z, 8Z, 11Z, 14Z)), P = 0.0003] and sphingomyelin [SM (d18:1/21:0, d17:1/22:0, d16:1/23:0), P = 0.0003] (Figure 4H). Metabolites exclusively altered in the evening by the HFD included BHBA (P = 0.000003) and acylacarnitines (margaroylcarnitine, P = 0.0002; stearoylcarnitine, P = 0.0006) (Figure 4I). In contrast to the effect of HFD, the top five metabolites exclusively changed in the evening by HCD were enriched by metabolites related to amino acids (N-methylproline, P = 0.000003; methionine sulfoxide, P = 0.000003; S-methylcysteine, P = 0.00005) (Figure 4I).
Figure 4.
Nutritional challenges have distinct impact on human muscle metabolome between morning and evening. (A) Histograms exhibit the number of significantly increased or decreased muscle metabolites by HFD (pink bar) and HCD (green bar) in the morning (top) and evening (bottom). (B–E) Venn diagrams show the overlap of the number of increased muscle metabolites by HFD (B), decreased muscle metabolites by HFD (C), increased muscle metabolites by HCD (D), and decreased muscle metabolites by HCD (E) between morning and evening. Pie charts exhibit the biological classifications of muscle metabolites exclusively altered in the morning (left), evening (right) and common to morning and evening (middle). Numbers within Venn diagrams and pie charts indicate number of changed metabolites. (F) Volcano plots exhibit the significance of altered muscle metabolite by HFD (pink) and HCD (green) in the morning. The x-axis and y-axis represent the fold change (post/pre) and significance of changed metabolites, respectively. (G) Volcano plots exhibit the significance of altered muscle metabolite by HFD (pink) and HCD (green) in the evening. (H) Top 5 muscle metabolites exclusively changed by HFD (top) and HCD (bottom) in the morning. (I) Top 5 muscle metabolites exclusively changed by HFD (top) and HCD (bottom) in the evening. ** and *** indicates P < 0.01 and P < 0.001 between pre and post, §, §§ and §§§ indicate P < 0.05, P < 0.01 and P < 0.001 between morning and evening before nutritional challenges, †, ††, ††† indicate P < 0.05, P < 0.01 and P < 0.001 between morning and evening after nutritional challenges. Two-way ANOVA compares the mean differences of metabolites levels between pre- and post-nutritional challenges. Adjusted p-value cutoff of 0.05 was used. N = 8 biological replicates in each time of day were subjected to the metabolomics analysis.
3.6. Tissue-specific metabolomic profiles
Examination of the serum metabolome is an expedient approach to identify metabolic properties related to human diseases and develop chrono-pharmacological treatments. As such, an understanding of tissue-specificity of the human circadian metabolome and how the serum metabolome may act as a surrogate for peripheral tissue profiles after various interventions is critical. Since the extraction methods for metabolites and the platform for the detection is different between serum and muscle metabolome, we compared the enrichment of biological classifications between serum and muscle metabolome under nutritional challenges. The ratio of gradient metabolites related to lipid metabolism was higher in muscle than serum before nutritional challenges (i.e. following the participants’ habitual diet; Fig. S4A,B). After a HFD, serum had newly gradient metabolites related to lipid rather than amino acid (Fig. S4A) whereas HCD dampened gradient metabolites related to lipids in muscle (Fig. S4B). The impact of HFD on lipid metabolism exhibited time-of-day-specific tissue-dependency where lipids in the serum and muscle seem to be sensitive to HFD in the morning and evening, respectively (Fig. S4C). Similar response of lipids against HCD was observed between serum and muscle regardless of the time-of-day (Fig. S4D). Of note, gradient and changed metabolites under nutritional challenges common to both serum and muscle exhibited a highly similar metabolic adaptation as serum- and muscle-specific responses of metabolome (Fig. S4A–D), indicating the possibility of common metabolites detected by both serum and muscle metabolome as tissue-independent diagnostic markers in human. Thus, the comparison analysis between serum and muscle metabolome revealed the synchronicity and heterogeneity of metabolic profiles under nutritional challenges, although further studies must be necessary to understand tissue-specificity of human circadian metabolome.
4. Conclusion
The past decade of metabolomics studies has been characterized by a dramatic increase in the quality of the analysis and an increased level of sophistication of consequent biocomputing tools. Using a combination of independent platforms and appropriate biocomputing analyses, a large number of metabolites have been identified by mass spectrometry and provided previously unappreciated insights into the physiology of single tissues as well as the degree of metabolic ‘crosstalk’ between tissues in numerous animal models. However, the application to human studies is scarce, especially with regard to the impact of various nutritional challenges and the timing of meals on the metabolome. The novel findings reported here illustrate the changes in metabolomics in human serum and skeletal muscle in response to nutritional challenges that are time-of-day dependent. Specifically, our study shows the remarkable consistency among the individuals participating in the study when taking into account their day–night metabolic profiles and responses to nutritional challenges. Finally, our results demonstrate that serum metabolomics constitutes an ideal approach towards the development of precision medicine, where personalized profiles would provide critical information for therapeutic strategies as well as help the design of appropriate chrono-pharmacological approaches.
Acknowledgments
We thank Dr. Selma Masri (UCI) and fellows in the Sassone-Corsi and Hawley laboratories for discussions. We gratefully acknowledge the technical assistance from Dr. Andrew Garnham, Mr. Marcus Callahan, Dr. Donny Camera and Dr. Jamie Whitfield (ACU). We thank the participants for their time and commitment to completing the challenging study protocols. This research was supported by funding to JAH and PSC from Novo Nordisk Foundation Challenge Grant (NNF14OC0011493). The funding source had no involvement in research conduct, article preparation, study design, data collection, analysis or interpretation, or in the decision to submit for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.06.008.
Contributor Information
John A. Hawley, Email: john.hawley@acu.edu.au.
Paolo Sassone-Corsi, Email: psc@uci.edu.
Conflict of interest
None declared.
Appendix A. Author contributions
SS analyzed and interpreted the data and wrote the initial draft of the manuscript. EBP and BLD conceived, designed and conducted the study, analyzed and interpreted the data, wrote the methodology section of the manuscript and participated in manuscript revision. JAH was involved in study design, data interpretation and critical revision of the manuscript. PSC assisted data interpretation and participated in critical revision of the manuscript. All authors approved the final version of this manuscript. JAH and PSC are the guarantors of this work and, as such, had full access to all the study data and take responsibility for data integrity and accuracy of the data analysis.
Appendix C. Supplementary data
The following are the supplementary data related to this article:
Figure S1: Experimental overview of human morning-evening metabolomics study. (A and B) Overview of the experimental design. (A) Each participant (n = 8) completed the experimental conditions in a random order. Human subjects consumed HFD and HCD in a randomized, crossover study with a 7-day washout between each dietary challenge. (B) Serum and muscle samples were taken in the morning (07:30 h, before breakfast) and at evening (19:30 h, after diner) before and after a 5-day nutritional challenge. (C) Blood parameters in the human subjects. GLP-1; glucagon-like peptide-1, TAG; triacylglycerol, NEFA; non-esterified fatty acid, Chol; cholesterol, HDL; high-density cholesterol, LDL; low-density cholesterol, TNFα; tumor necrosis factor-α. Data are mean ± SEM and was analyzed by two-way ANOVA using Bonferroni posttest. Two-way ANOVA compared significant difference between morning and evening (time-of-day) and between pre and post nutritional challenges (diet). *, ** and *** indicate p < 0.05, P < 0.01 and P < 0.001. (D and E) Daily variation of gene expression of core clock and metabolic genes in human skeletal muscle under HFD (D) and HCD (E) challenges was determined by qPCR. Data are mean ± SEM and was analyzed by two-way ANOVA using Bonferroni posttest. Two-way ANOVA compared significant difference between morning and evening (time-of-day). *, ** and *** indicate p < 0.05, P < 0.01 and P < 0.001. Figure S2: Sub-classification of lipid identified as gradient metabolites in the serum and muscle. (A) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HFD (left), post-HFD (middle) and both pre- and post-HFD (right) in the serum. (B) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HCD (left), post-HCD (middle) and both pre- and post-HCD (right) in the serum. (C) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HFD (left), post-HFD (middle) and both pre- and post-HFD (right) in the muscle. (D) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HCD (left), post-HCD (middle) and both pre- and post-HCD (right) in the muscle. Color scales indicate the enrichment of gradient metabolites related to lipid within a sub-classification of lipid. The numbers indicate the enrichment of metabolites within a lipid subclass. Top 10 lipid sub-classifications with the highest enrichment rates are shown. BA; bile acid, BCAA; branched chain amino acid, Cer; ceramide, CS; corticosteroid, DAG; diacylglycerol, eCB; endocannabinoid, FA; fatty acid, GL; glycerolipid, LPL; lysophospholipid, LyPl; lysoplasmalogen, MAG; monoacylglycerol, PC; phosphatidylcholine, PE; phosphatidylethanolamine, PG; phosphatidylglycerol, PI; phosphatidylinositol, PL; phospholipid, PS; phosphatidylserine, PUFA; polyunsaturated fatty acid, SL; Sphingolipid. Figure S3: Sub-classification of lipid in the serum and muscle altered by nutritional challenges at different time-of-day. (A) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HFD in the serum. (B) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HFD in the serum. (C) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HCD in the serum. (D) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HCD in the serum. (E) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HFD in the muscle. (F) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left) and evening (right) by HFD in the muscle. (G) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the evening by HCD in the muscle. (H) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HCD in the muscle. Color scales indicate the enrichment of altered metabolites related to lipid within a sub-classification of lipid. The numbers indicate the enrichment of metabolites within a lipid subclass. Top 10 lipid sub-classifications with the highest enrichment rates are shown. BA; bile acid, BCAA; branched chain amino acid, Cer; ceramide, CS; corticosteroid, DAG; diacylglycerol, eCB; endocannabinoid, FA; fatty acid, GL; glycerolipid, LPL; lysophospholipid, LyPl; lysoplasmalogen, MAG; monoacylglycerol, PC; phosphatidylcholine, PE; phosphatidylethanolamine, PG; phosphatidylglycerol, PI; phosphatidylinositol, PL; phospholipid, PS; phosphatidylserine, PUFA; polyunsaturated fatty acid, SL; Sphingolipid. Figure S4: Biological classification of gradient metabolites and changed metabolites in the serum and muscle under nutritional challenges. (A) Radar plots show the biological classification of gradient metabolites compared among serum (yellow circle), muscle (blue square) and both (green triangle) before (left panel) and after HFD (right panel). (B) Radar plots show the biological classification of gradient metabolites compared among serum (yellow circle), muscle (blue square) and both (green triangle) before (left panel) and after HCD (right panel). (C) Radar plots show the biological classification of changed metabolites by HFD in the morning (left panel) and evening (right panel) compared among serum (yellow circle), muscle (blue square) and both (green triangle). (D) Radar plots show the biological classification of changed metabolites by HCD in the morning (left panel) and evening (right panel) compared among serum (yellow circle), muscle (blue square) and both (green triangle). The plots indicate the enrichment of 8 different biological classes in each serum and muscle metabolome.
List of significant gradient serum metabolites under HFD.
List of significant gradient serum metabolites under HCD.
List of significantly changed serum metabolites in the morning by nutritional challenges.
List of significantly changed serum metabolites in the evening by nutritional challenges.
List of significant gradient muscle metabolites under HFD.
List of significant gradient muscle metabolites under HCD.
List of significantly changed muscle metabolites in the morning by nutritional challenges.
List of significantly changed muscle metabolites in the evening by nutritional challenges.
Characteristics of study participants at baseline.
References
- 1.Beger R.D., Dunn W., Schmidt M.A., Gross S.S., Kirwan J.A., Cascante M. Metabolomics enables precision medicine: "a white paper, community perspective". Metabolomics. 2016;12:149. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibney M.J., Walsh M., Brennan L., Roche H.M., German B., Ommen B.V. Metabolomics in human nutrition: opportunities and challenges. American Journal of Clinical Nutrition. 2005;82:497–503. doi: 10.1093/ajcn.82.3.497. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan G.M., Coffey V.G., Niere J.O., Hawley J.A., Adams M.J. Spectroscopic correlation analysis of NMR-based metabonomics in exercise science. Analytica Chimica Acta. 2009;652:173–179. doi: 10.1016/j.aca.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Dallmann R., Viola A.U., Tarokh L., Cajochen C., Brown S.A. The human circadian metabolome. Proceedings of the National Academy of Sciences of the U S A. 2012;109:2625–2629. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nature Reviews Molecular Cell Biology. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Q., Liu Z., Zhong S., Li R., Xia H., Jie Z. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Scientific Reports. 2016;6:22525. doi: 10.1038/srep22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nature Medicine. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 8.Krug S., Kastenmuller G., Stuckler F., Rist M.J., Skurk T., Sailer M. The dynamic range of the human metabolome revealed by challenges. FASEB J. 2012;26:2607–2619. doi: 10.1096/fj.11-198093. [DOI] [PubMed] [Google Scholar]
- 9.Sahar S., Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends in Endocrinology and Metabolism. 2012;23:1–8. doi: 10.1016/j.tem.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Top D., Young M.W. Coordination between differentially regulated circadian clocks generates rhythmic behavior. Cold Spring Harbor Perspectives in Biology. 2017 doi: 10.1101/cshperspect.a033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loizides-Mangold U., Perrin L., Vandereycken B., Betts J.A., Walhin J.P., Templeman I. Lipidomics reveals diurnal lipid oscillations in human skeletal muscle persisting in cellular myotubes cultured in vitro. Proceedings of the National Academy of Sciences of the U S A. 2017;114:E8565–E8574. doi: 10.1073/pnas.1705821114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriya T., Satomi Y., Kobayashi H. Metabolomics of postprandial plasma alterations: a comprehensive Japanese study. Journal of Biochemistry. 2017 doi: 10.1093/jb/mvx066. [DOI] [PubMed] [Google Scholar]
- 13.Davies S.K., Ang J.E., Revell V.L., Holmes B., Mann A., Robertson F.P. Effect of sleep deprivation on the human metabolome. Proceedings of the National Academy of Sciences of the U S A. 2014;111:10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamont B.J., Waters M.F., Andrikopoulos S. A low-carbohydrate high-fat diet increases weight gain and does not improve glucose tolerance, insulin secretion or beta-cell mass in NZO mice. Nutrition & Diabetes. 2016;6:e194. doi: 10.1038/nutd.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckel-Mahan K.L., Patel V.R., de Mateo S., Orozco-Solis R., Ceglia N.J., Sahar S. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tognini P., Murakami M., Liu Y., Eckel-Mahan K.L., Newman J.C., Verdin E. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metabolism. 2017;26:523–538.e525. doi: 10.1016/j.cmet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmers C., Gill S., DiTacchio L., Pulivarthy S.R., Le H.D., Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato S., Solanas G., Peixoto F.O., Bee L., Symeonidi A., Schmidt M.S. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170:664–677.e611. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parr E.B., Devlin B.L., Callahan M.J., Radford B.E., Blankenship J.M., Dunstan D.W. Effects of providing high-fat versus high-carbohydrate meals on daily and postprandial physical activity and glucose patterns: a randomised controlled trial. Nutrients. 2018;10 doi: 10.3390/nu10050557. pii: E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camera D.M., West D.W., Burd N.A., Phillips S.M., Garnham A.P., Hawley J.A. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. Journal of Applied Physiology (1985) 2012;113:206–214. doi: 10.1152/japplphysiol.00395.2012. [DOI] [PubMed] [Google Scholar]
- 22.Evans A.M., DeHaven C.D., Barrett T., Mitchell M., Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 23.Dehaven C.D., Evans A.M., Dai H., Lawton K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. Journal of Cheminformatics. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asher G., Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Stephens F.B., Chee C., Wall B.T., Murton A.J., Shannon C.E., van Loon L.J. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes. 2015;64:1615–1620. doi: 10.2337/db14-0961. [DOI] [PubMed] [Google Scholar]
- 27.Camera D.M., Burniston J.G., Pogson M.A., Smiles W.J., Hawley J.A. Dynamic proteome profiling of individual proteins in human skeletal muscle after a high-fat diet and resistance exercise. FASEB J. 2017;31:5478–5494. doi: 10.1096/fj.201700531R. [DOI] [PubMed] [Google Scholar]
- 28.Oishi K., Uchida D., Ohkura N., Horie S. PPARalpha deficiency augments a ketogenic diet-induced circadian PAI-1 expression possibly through PPARgamma activation in the liver. Biochemical and Biophysical Research Communications. 2010;401:313–318. doi: 10.1016/j.bbrc.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz J.F., Mora-Rodriguez R., Byerley L.O., Coyle E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. American Journal of Physiology. 1997;273:E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Experimental overview of human morning-evening metabolomics study. (A and B) Overview of the experimental design. (A) Each participant (n = 8) completed the experimental conditions in a random order. Human subjects consumed HFD and HCD in a randomized, crossover study with a 7-day washout between each dietary challenge. (B) Serum and muscle samples were taken in the morning (07:30 h, before breakfast) and at evening (19:30 h, after diner) before and after a 5-day nutritional challenge. (C) Blood parameters in the human subjects. GLP-1; glucagon-like peptide-1, TAG; triacylglycerol, NEFA; non-esterified fatty acid, Chol; cholesterol, HDL; high-density cholesterol, LDL; low-density cholesterol, TNFα; tumor necrosis factor-α. Data are mean ± SEM and was analyzed by two-way ANOVA using Bonferroni posttest. Two-way ANOVA compared significant difference between morning and evening (time-of-day) and between pre and post nutritional challenges (diet). *, ** and *** indicate p < 0.05, P < 0.01 and P < 0.001. (D and E) Daily variation of gene expression of core clock and metabolic genes in human skeletal muscle under HFD (D) and HCD (E) challenges was determined by qPCR. Data are mean ± SEM and was analyzed by two-way ANOVA using Bonferroni posttest. Two-way ANOVA compared significant difference between morning and evening (time-of-day). *, ** and *** indicate p < 0.05, P < 0.01 and P < 0.001. Figure S2: Sub-classification of lipid identified as gradient metabolites in the serum and muscle. (A) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HFD (left), post-HFD (middle) and both pre- and post-HFD (right) in the serum. (B) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HCD (left), post-HCD (middle) and both pre- and post-HCD (right) in the serum. (C) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HFD (left), post-HFD (middle) and both pre- and post-HFD (right) in the muscle. (D) Pie charts exhibit the enrichment of sub-classifications of lipid being different between morning and evening under pre-HCD (left), post-HCD (middle) and both pre- and post-HCD (right) in the muscle. Color scales indicate the enrichment of gradient metabolites related to lipid within a sub-classification of lipid. The numbers indicate the enrichment of metabolites within a lipid subclass. Top 10 lipid sub-classifications with the highest enrichment rates are shown. BA; bile acid, BCAA; branched chain amino acid, Cer; ceramide, CS; corticosteroid, DAG; diacylglycerol, eCB; endocannabinoid, FA; fatty acid, GL; glycerolipid, LPL; lysophospholipid, LyPl; lysoplasmalogen, MAG; monoacylglycerol, PC; phosphatidylcholine, PE; phosphatidylethanolamine, PG; phosphatidylglycerol, PI; phosphatidylinositol, PL; phospholipid, PS; phosphatidylserine, PUFA; polyunsaturated fatty acid, SL; Sphingolipid. Figure S3: Sub-classification of lipid in the serum and muscle altered by nutritional challenges at different time-of-day. (A) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HFD in the serum. (B) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HFD in the serum. (C) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HCD in the serum. (D) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HCD in the serum. (E) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the morning (left), evening (right) and both (middle) by HFD in the muscle. (F) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left) and evening (right) by HFD in the muscle. (G) Area charts exhibit the enrichment of sub-classifications of lipid exclusively increased in the evening by HCD in the muscle. (H) Area charts exhibit the enrichment of sub-classifications of lipid exclusively decreased in the morning (left), evening (right) and both (middle) by HCD in the muscle. Color scales indicate the enrichment of altered metabolites related to lipid within a sub-classification of lipid. The numbers indicate the enrichment of metabolites within a lipid subclass. Top 10 lipid sub-classifications with the highest enrichment rates are shown. BA; bile acid, BCAA; branched chain amino acid, Cer; ceramide, CS; corticosteroid, DAG; diacylglycerol, eCB; endocannabinoid, FA; fatty acid, GL; glycerolipid, LPL; lysophospholipid, LyPl; lysoplasmalogen, MAG; monoacylglycerol, PC; phosphatidylcholine, PE; phosphatidylethanolamine, PG; phosphatidylglycerol, PI; phosphatidylinositol, PL; phospholipid, PS; phosphatidylserine, PUFA; polyunsaturated fatty acid, SL; Sphingolipid. Figure S4: Biological classification of gradient metabolites and changed metabolites in the serum and muscle under nutritional challenges. (A) Radar plots show the biological classification of gradient metabolites compared among serum (yellow circle), muscle (blue square) and both (green triangle) before (left panel) and after HFD (right panel). (B) Radar plots show the biological classification of gradient metabolites compared among serum (yellow circle), muscle (blue square) and both (green triangle) before (left panel) and after HCD (right panel). (C) Radar plots show the biological classification of changed metabolites by HFD in the morning (left panel) and evening (right panel) compared among serum (yellow circle), muscle (blue square) and both (green triangle). (D) Radar plots show the biological classification of changed metabolites by HCD in the morning (left panel) and evening (right panel) compared among serum (yellow circle), muscle (blue square) and both (green triangle). The plots indicate the enrichment of 8 different biological classes in each serum and muscle metabolome.
List of significant gradient serum metabolites under HFD.
List of significant gradient serum metabolites under HCD.
List of significantly changed serum metabolites in the morning by nutritional challenges.
List of significantly changed serum metabolites in the evening by nutritional challenges.
List of significant gradient muscle metabolites under HFD.
List of significant gradient muscle metabolites under HCD.
List of significantly changed muscle metabolites in the morning by nutritional challenges.
List of significantly changed muscle metabolites in the evening by nutritional challenges.
Characteristics of study participants at baseline.